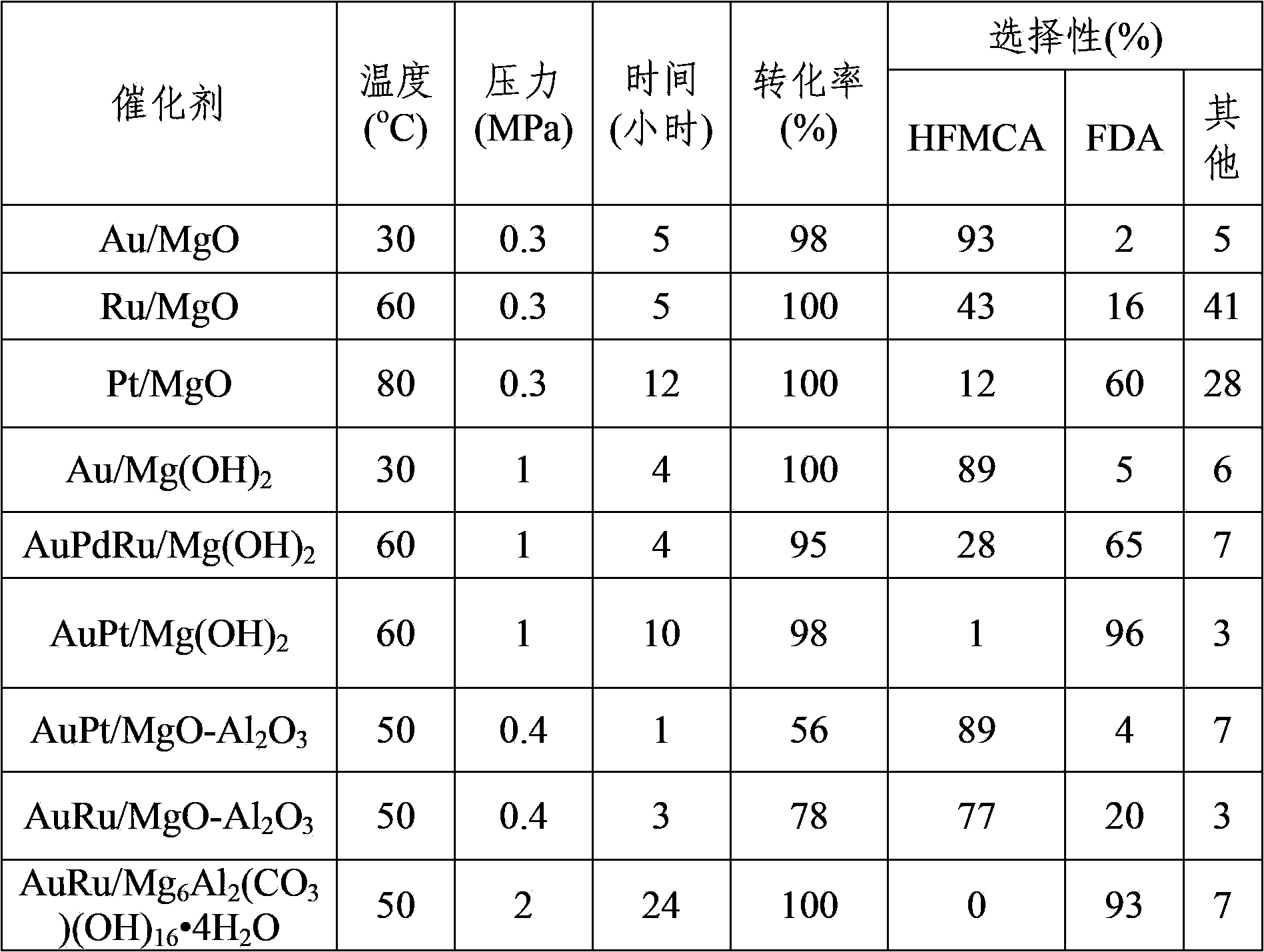
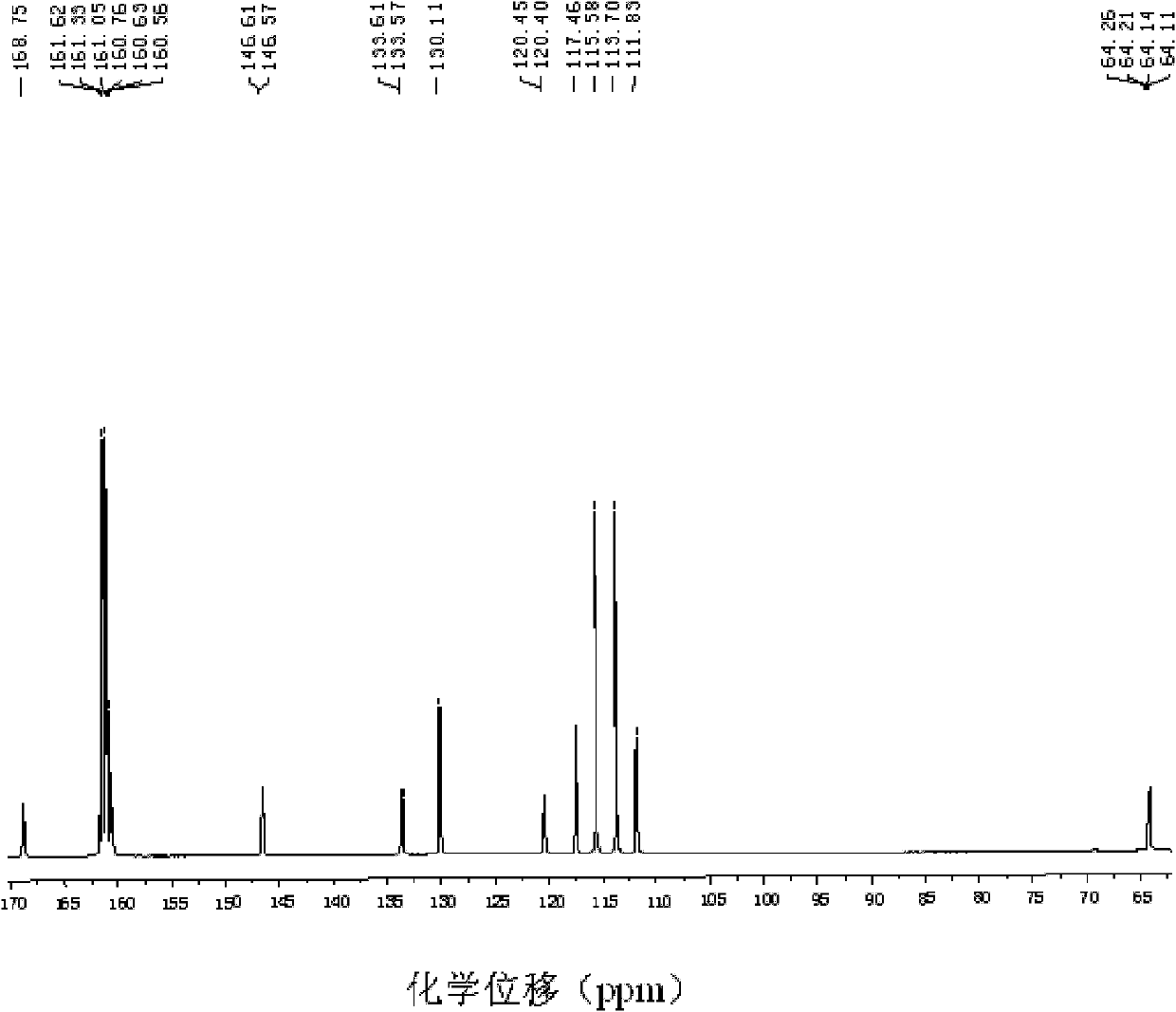
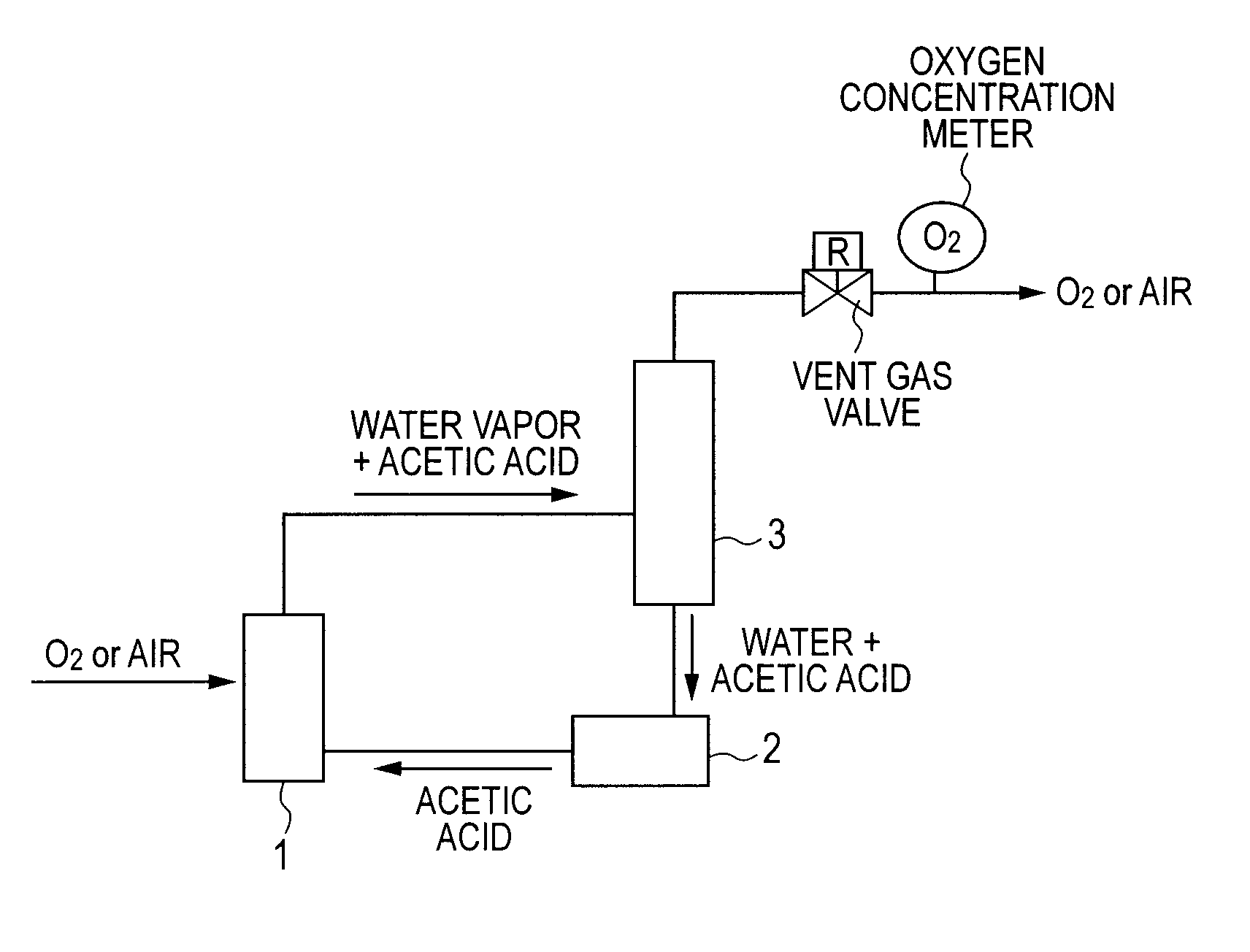
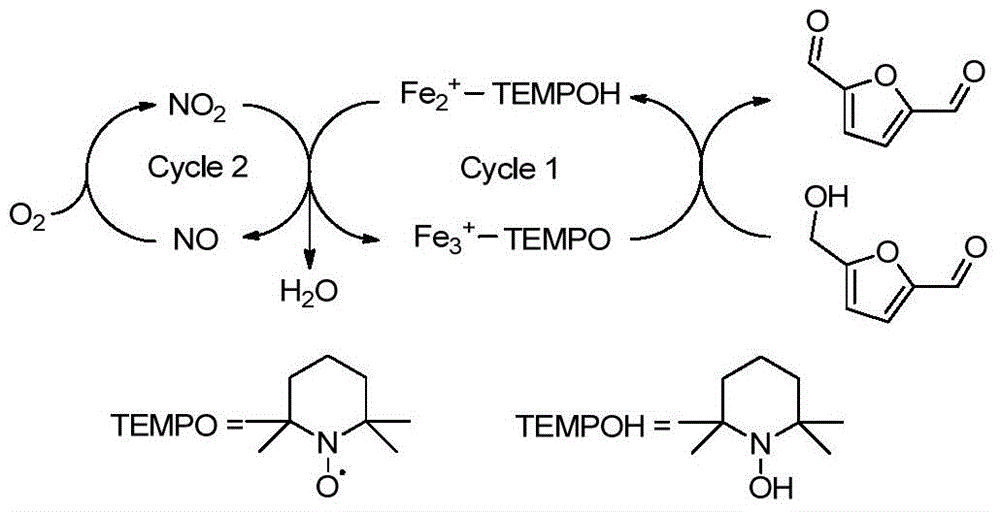
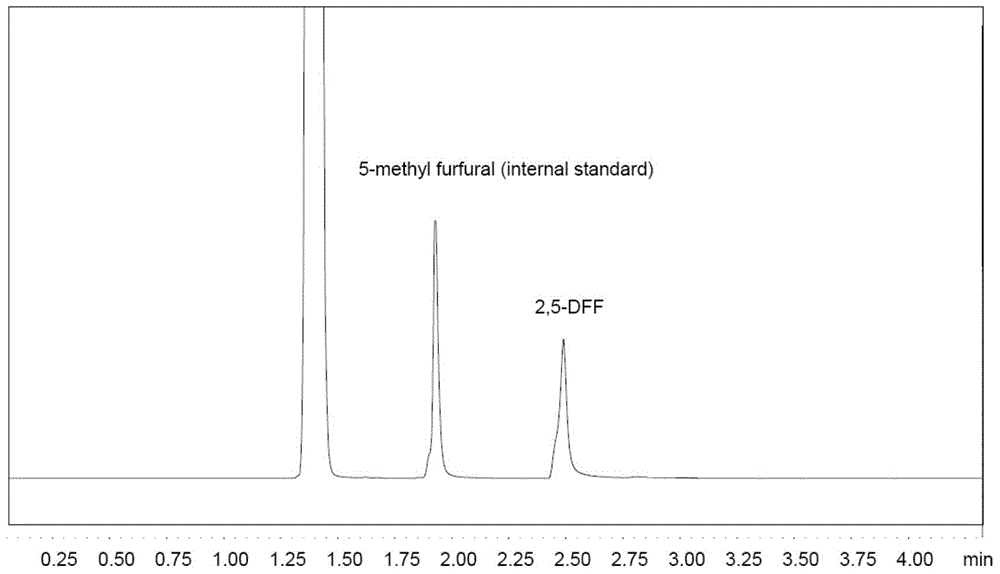
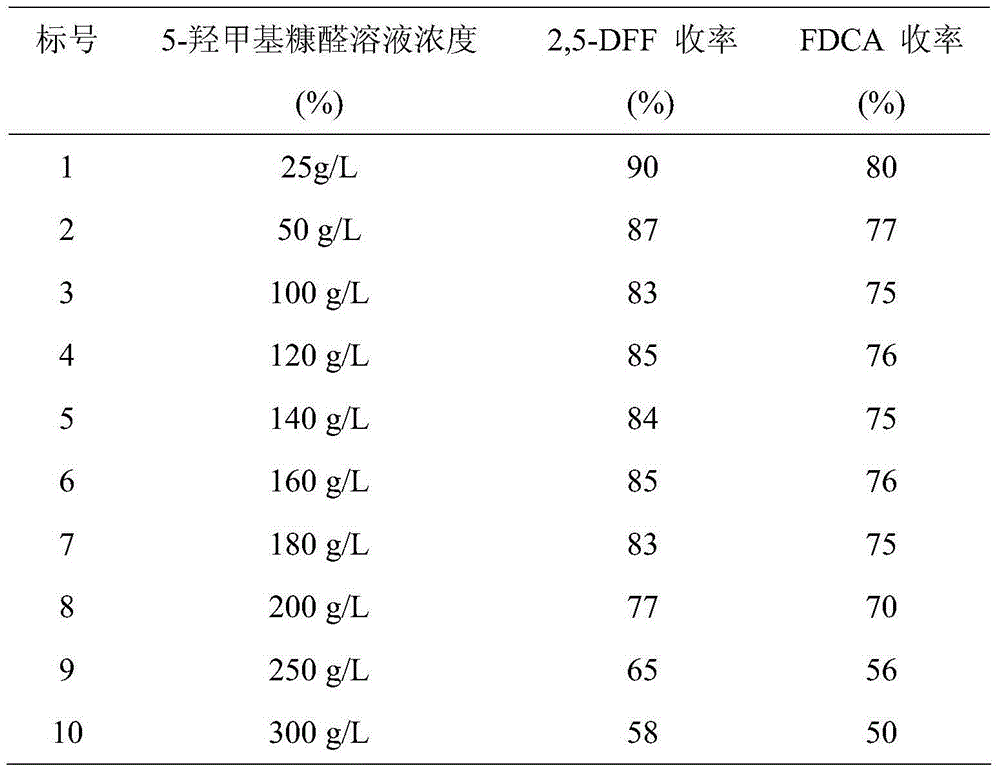
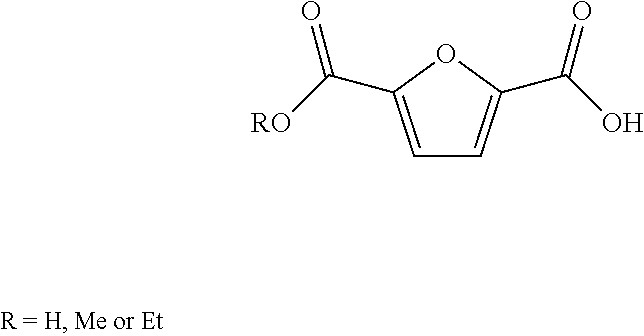
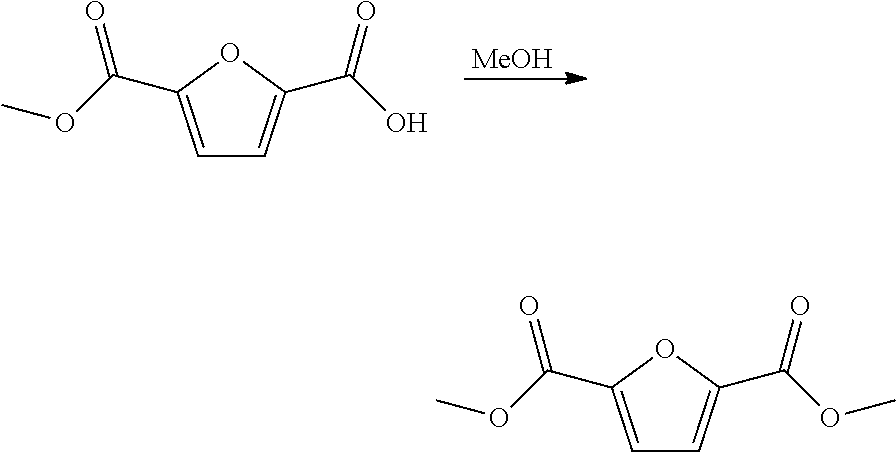
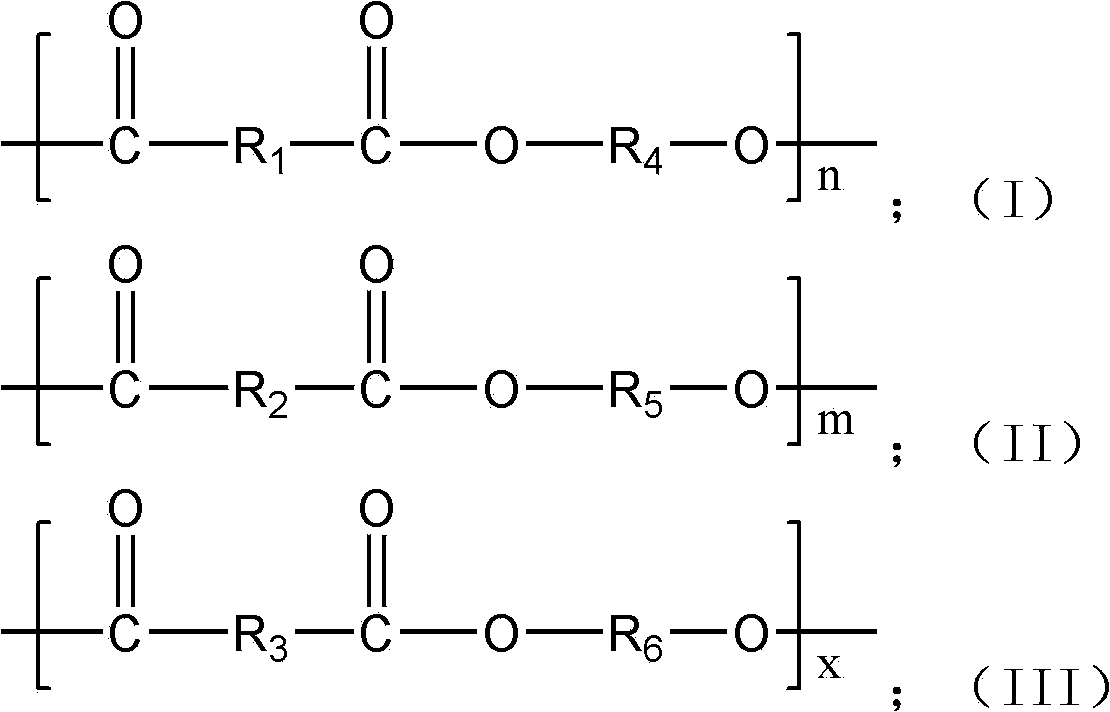Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
181 results about "2,5-Furandicarboxylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
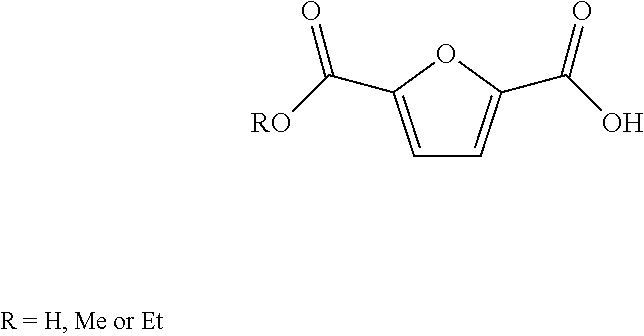
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.
Process for preparing a polymer having a 2,5-furandicarboxylate moiety within the polymer backbone and such (CO)polymers
A process for preparing a polymer having a 2,5-furandicarboxylate moiety within the polymer backbone and having a number average molecular weight of at least 10,000 (as determined by GPC based on polystyrene standards) includes a first step where a prepolymer is made having the 2,5-furandicarboxylate moiety within the polymer backbone, followed in a second step by a polycondensation reaction. In the first step a 2,5-furandicarboxylate ester is transesterified with a compound or mixture of compounds containing two or more hydroxyl groups, in the presence of a tin(IV) based transesterification catalyst. In the second step at reduced pressure and under melt conditions the prepolymer prepared in the first step is polycondensed in the presence of a tin (II) based polycondensation catalyst until the polymer is obtained. This polymer may then be subjected to Solid State Polycondensation. Polymers so produced may have a 2,5-furandicarboxylate moiety within the polymer backbone, and having a number average molecular weight of at least 20,000 (as determined by GPC based on styrene standards), and an absorbance as a 5 mg / mL solution in a dichloromethane:hexafluoroisopropanol 8:2 at 400 nm of below 0.05.
Owner:FURANIX TECH BV
Terephthalic acid composition and process for the production thereof
InactiveUS7385081B1Reducing and eliminating resultantOrganic compound preparationCarboxylic compound preparationEtherCarbon oxide
Terephthalic acid is prepared by reacting a 2,5-furandicarboxylate with ethylene in the presence of a solvent to produce a bicyclic ether; and then dehydrating the bicyclic ether. The process of the present invention effectively produces terephthalic acid, while reducing or eliminating the impurities, color bodies and carbon oxides produced in commercial practice by the liquid-phase oxidation of methyl-substituted benzene feedstocks.
Owner:BP CORP NORTH AMERICA INC
Conversion of carbohydrates to hydroxymethylfurfural (HMF) and derivatives
InactiveUS20090156841A1Increase conversion rateStable formOrganic compound preparationCarboxylic compound preparationMANGANESE ACETATEFuran
A method of producing substantially pure HMF, HMF esters and other derivatives from a carbohydrate source by contacting the carbohydrate source with a solid phase catalyst. A carbohydrate starting material is heated in a solvent in a column and continuously flowed through a solid phase catalyst in the presence of an organic acid, or heated with the organic acid and a solid catalyst in solution to form a HMF ester. Heating without organic acid forms HMF. The resulting product is purified by filtration to remove the unreacted starting materials and catalyst. The HMF ester or a mixture of HMF and HMF ester may then be oxidized to 2,5-furandicarboxylic acid (FDCA) by combining the HMF ester with an organic acid, cobalt acetate, manganese acetate and sodium bromide under pressure. Alternatively, the HMF ester may be reduced to form a furan or tetrahydrofuran diol.
Owner:ARCHER DANIELS MIDLAND CO
Method of producing 2,5-furandicarboxylic acid
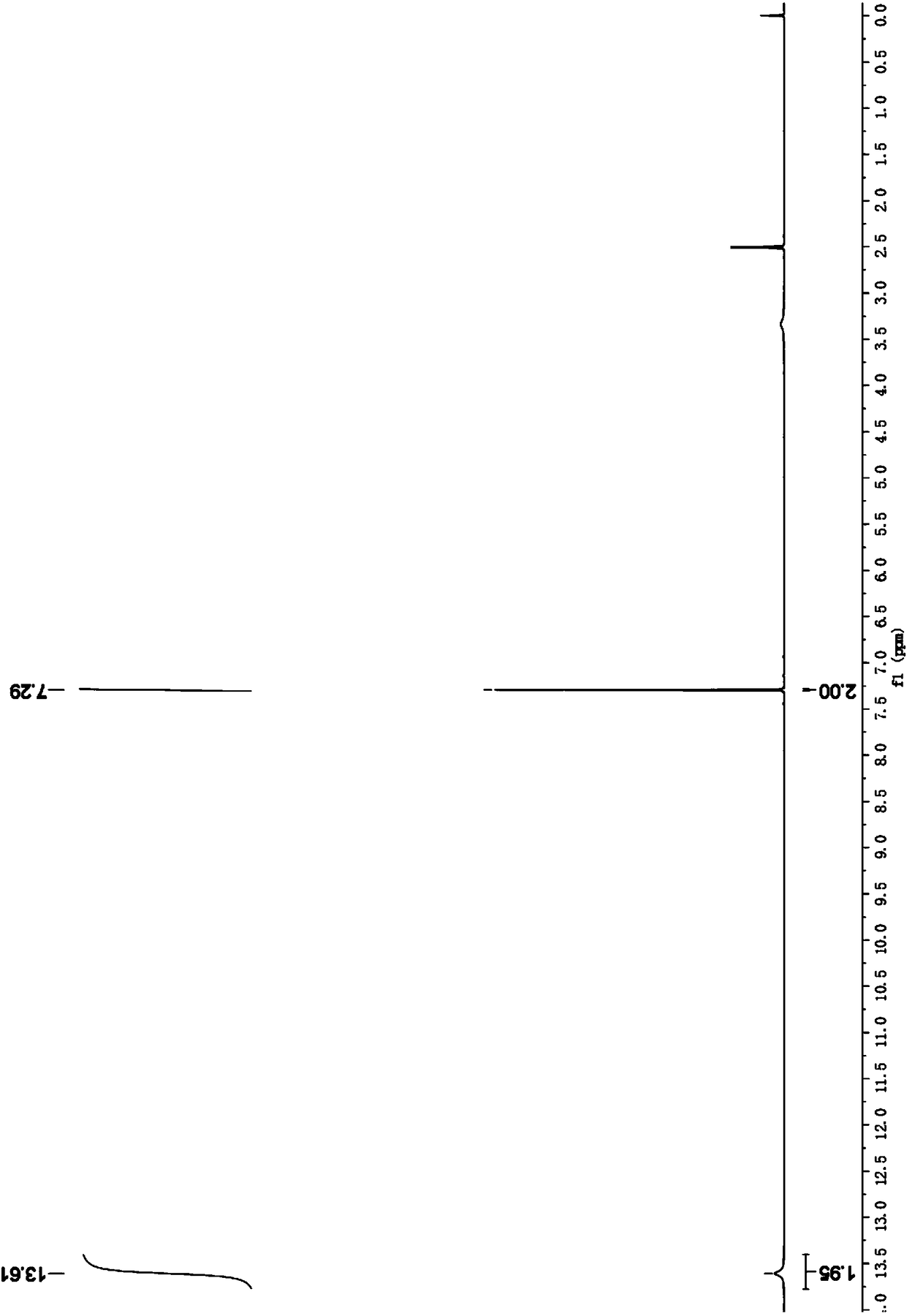
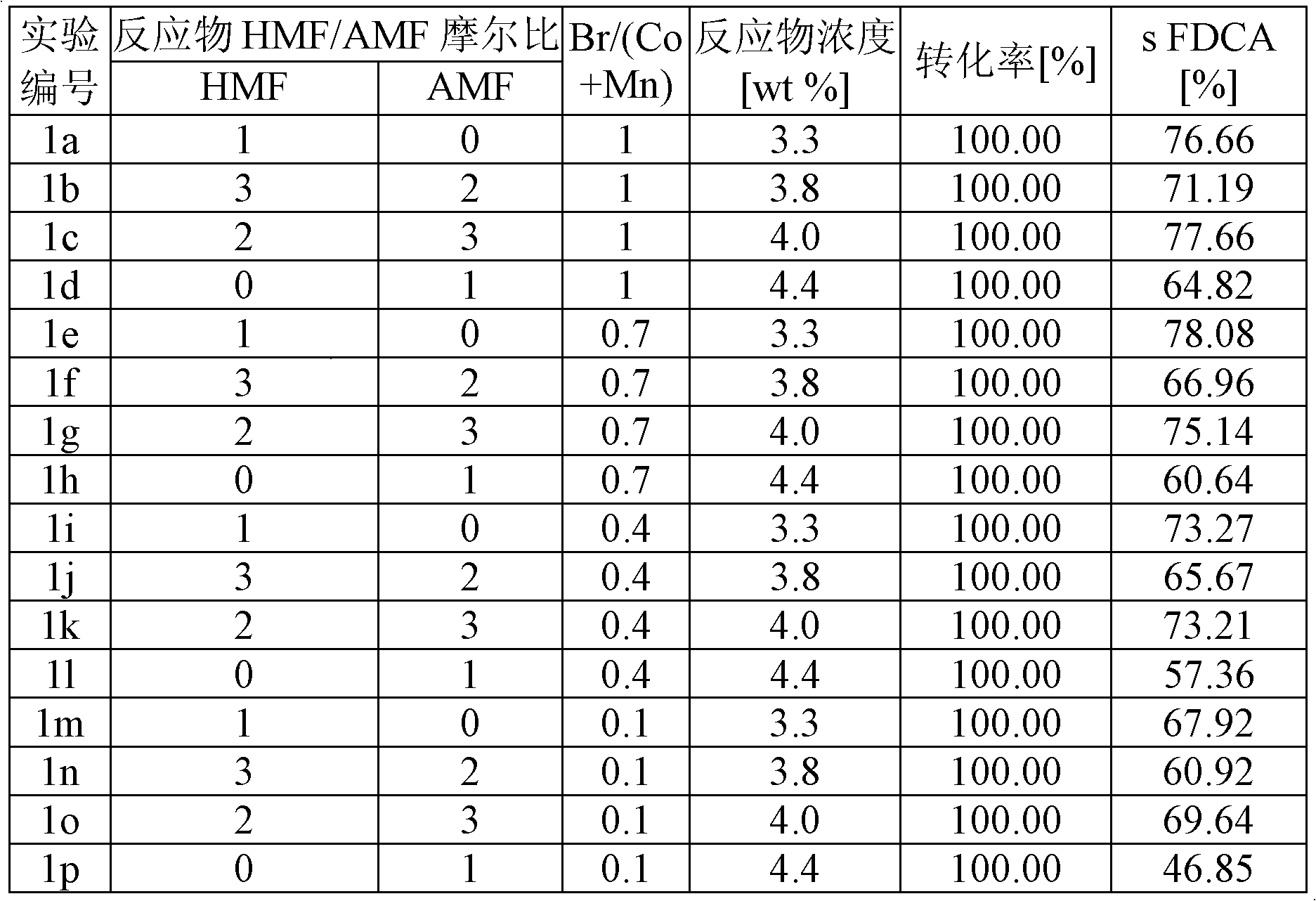
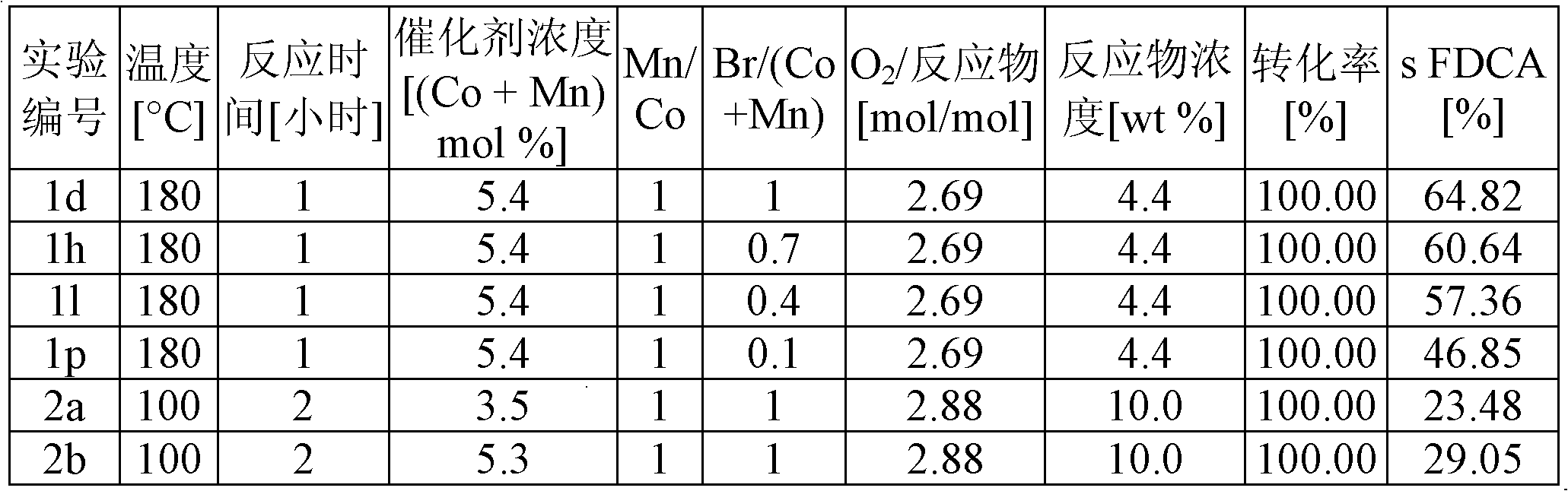
Provided is a method of producing FDCA by which high-purity FDCA can be produced in high yield. 2,5-furandicarboxylic acid is produced by: bringing 5-hydroxymethylfurfural into contact with an oxidant in an organic acid solvent in the presence of bromine and a metal catalyst; and allowing 5-hydroxymethylfurfural and the oxidant to react with each other while removing water produced by the reaction.
Owner:CANON KK
Terephthalic Acid Composition and Process for the Production Thereof
ActiveUS20090124829A1Reducing and eliminating resultantOrganic compound preparationCarboxylic compound preparationEtherCarbon oxide
Terephthalic acid is prepared by reacting a 2,5-furandicarboxylate with ethylene in the presence of a solvent to produce a bicyclic ether; and then dehydrating the bicyclic ether. The process of the present invention effectively produces terephthalic acid, while reducing or eliminating the impurities, color bodies and carbon oxides produced in commercial practice by the liquid-phase oxidation of methyl-substituted benzene feedstocks.
Owner:BP CORP NORTH AMERICA INC
A process for preparing a polymer product having a 2,5-furandicarboxylate moiety within the polymer backbone to be used in bottle, film or fibre applications
A process for preparing a polymer having a 2,5-furandicarboxylate moiety within the polymer backbone, and having a number average molecular weight of at least 25,000, includes a transesterification step, a polycondensation step, a drying and / or crystallizing step, and a step where the polymer is subjected to post condensation conditions, and to a polyester-containing bottle or film or fibre-containing woven or non-woven object made from melt-processing poly(ethylene-2,5-furandicarboxylate), where the poly(ethylene-2,5-furandicarboxylate) is obtainable by the process of the invention.
Owner:FURANIX TECH BV
Production of Adipic Acid and Derivatives from Carbohydrate-Containing Materials
The present invention generally relates to processes for the chemocatalytic conversion of a carbohydrate source to an adipic acid product. The present invention includes processes for the conversion of a carbohydrate source to an adipic acid product via a furanic substrate, such as 2,5-furandicarboxylic acid or derivatives thereof. The present invention also includes processes for producing an adipic acid product comprising the catalytic hydrogenation of a furanic substrate to produce a tetrahydrofuranic substrate and the catalytic hydrodeoxygenation of at least a portion of the tetrahydrofuranic substrate to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Polyester compositions containing furandicarboxylic acid or an ester thereof, and 2,2,4,4-tetramethyl-1,3-cyclobutanediol
ActiveUS20130095263A1High glass transition temperatureBottlesSynthetic resin layered productsFiberPolyester
Described are polyesters comprising (a) a dicarboxylic acid component comprising 2,5-furandicarboxylic acid residues; optionally, aromatic dicarboxylic acid residues and / or modifying aliphatic dicarboxylic acid residues and 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues. The polyesters may be manufactured into articles such as fibers, films, bottles, coatings, or sheets.
Owner:EASTMAN CHEM CO
Method for preparing 2,5-furandicarboxylic acid through catalytic oxidation
ActiveCN103724303AImprove catalytic performanceLow costOrganic chemistryCatalytic oxidationReusability
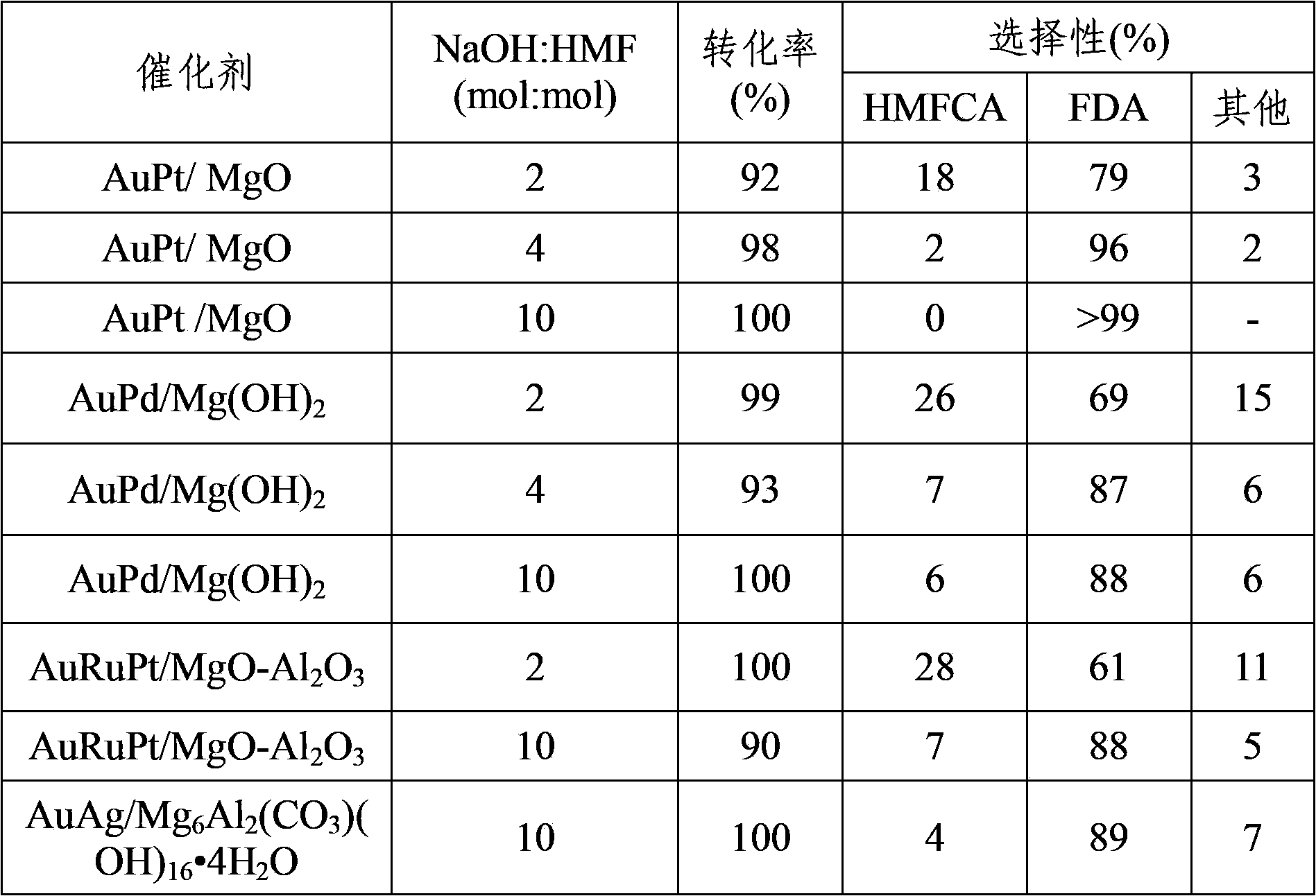
The invention discloses a method for synthesizing 2,5-furandicarboxylic acid through catalytic oxidation by using 5-hydroxymethylfurfural as a raw material. 5-hydroxymethylfurfural can be oxidized by using oxygen or air as an oxidant in the presence of a catalyst prepared by loading an alkaline carrier with noble metal with high efficiency and high selectivity to synthesize 2,5-furandicarboxylic acid. A catalytic reaction is easy to operate and mild in conditions; when 5-hydroxymethylfurfural is fully converted, the selectivity of the product-2,5-furandicarboxylic acid can reach more than 99%; the catalyst is good in reusability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing 2,5-furandicarboxylic acid
InactiveCN101891719ASimple processing methodHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranMass ratio
The invention discloses a method for synthesizing 2,5-furandicarboxylic acid, which comprises the following steps of: mixing furans and alkaline solution in a mass ratio of 1:5-50, adding a noble metal catalyst in an amount of 1 to 5 percent of the molar weight of the furans, continuously introducing oxygen at normal temperature, and reacting for 10 to 30 hours, wherein based on each gram of the furans, the oxygen introducing rate is 5 to 20ml / min; and adjusting the pH of the reaction liquid to less than 3 with concentrated acid, and precipitating the 2,5-furandicarboxylic acid, wherein the noble metal catalyst is Pt / C, Au / C, Pd / C, Pt / C / CuO-Ag2O, Au / C / CuO-Ag2O or Pd / C / CuO-Ag2O, and the concentrated acid is concentrated hydrochloric acid or concentrated sulfuric acid. The process method for synthesizing the 2,5-furandicarboxylic acid has the advantages of simple and direct route, energy conservation, environmental protection, high quality and low cost of the obtained product, and good application prospect.
Owner:SOUTH CHINA UNIV OF TECH
Method for the preparation of 2,5-furandicarboxylic acid and esters thereof
ActiveUS8519167B2Organic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranHydroxymethylfurfural
A method for the preparation of 2,5-furandicarboxylic acid (“FDCA”) and / or an alkyl ester of FDCA includes the step of contacting a feed comprising a starting material selected from 5-alkoxymethylfurfural, 2,5-di(alkoxymethyl)furan and a mixture thereof with an oxidant in the presence of an oxidation catalyst. The feed may also comprise 5-hydroxymethylfurfural as a further starting material.
Owner:FURANIX TECH BV
2,5-furandicarboxylic-terephthalic-aliphatic copolyester and preparation method thereof
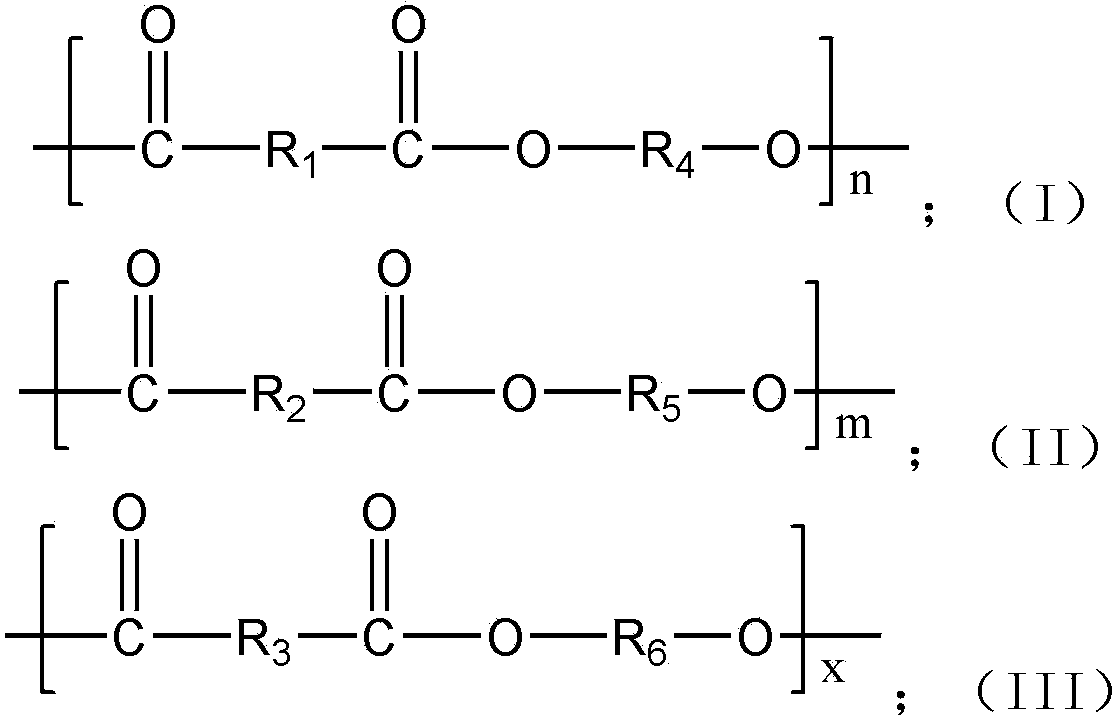
ActiveCN102432847ANot affected by resource depletionImprove performancePolymer scienceEngineering plastic
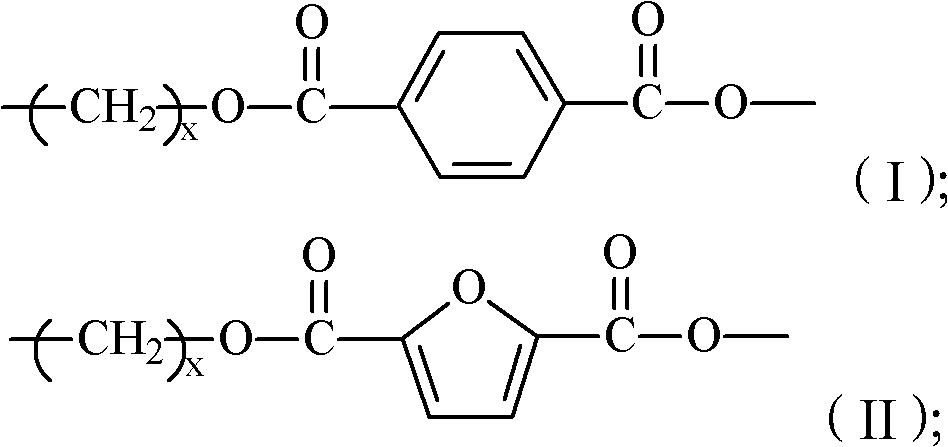
The invention provides 2,5-furandicarboxylic-terephthalic-aliphatic copolyester and a preparation method thereof. 2,5-furandicarboxylic-terephthalic-aliphatic copolyester has a first repetitive unit represented by a formula (I) and a second repetitive unit represented by a formula (II), wherein x=2 to 8. The molar ratio of the first repetitive unit to the second repetitive unit is 1:1000-1000:1. 2,5-furandicarboxylic-terephthalic-aliphatic copolyester provided by the invention has a structure and even a good performance similar to that of diol polyterephthalate. 2,5-furandicarboxylic-terephthalic-aliphatic copolyester can be used for preparing materials such as engineering plastics and membranes. As one of the raw materials, 2,5-furandicarboxylic acid can be obtained based on renewable resources, such that the resource is wide, and is not influenced by increasingly exhausted petroleum resources. The invention also provides a preparation method of 2,5-furandicarboxylic-terephthalic-aliphatic copolyester.
Owner:芜湖万隆新材料有限公司
Preparation method of 5-hydroxymethyl furoic acid and 2,5-furandicarboxylic acid
ActiveCN103626726AEasy to makeModification operation is simpleOrganic chemistryMolecular sieve catalystsHydroxymethylfurfuralHydroxymethyl
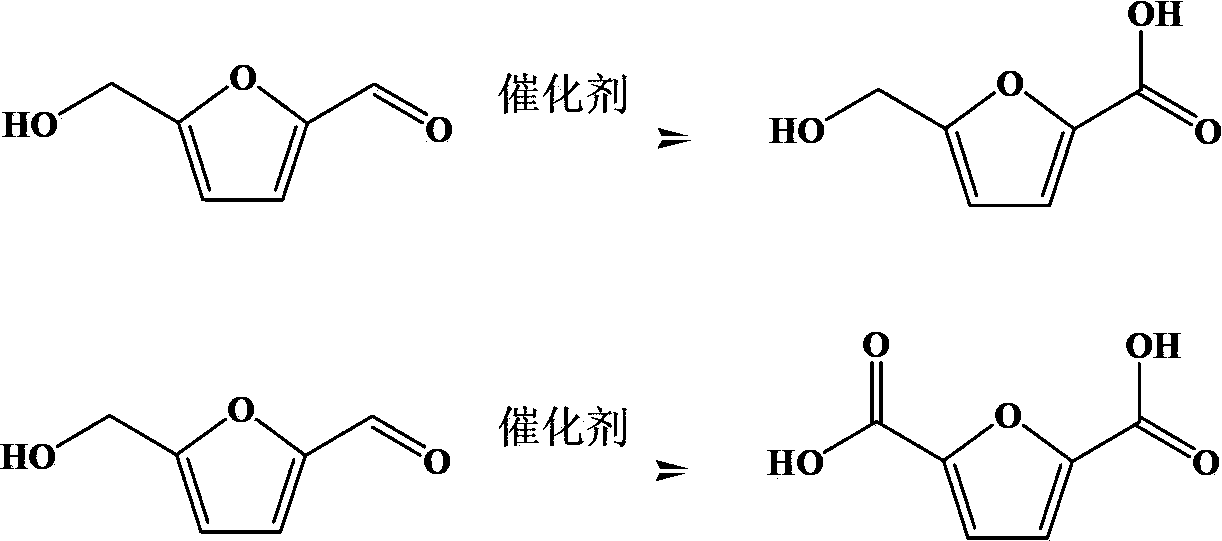
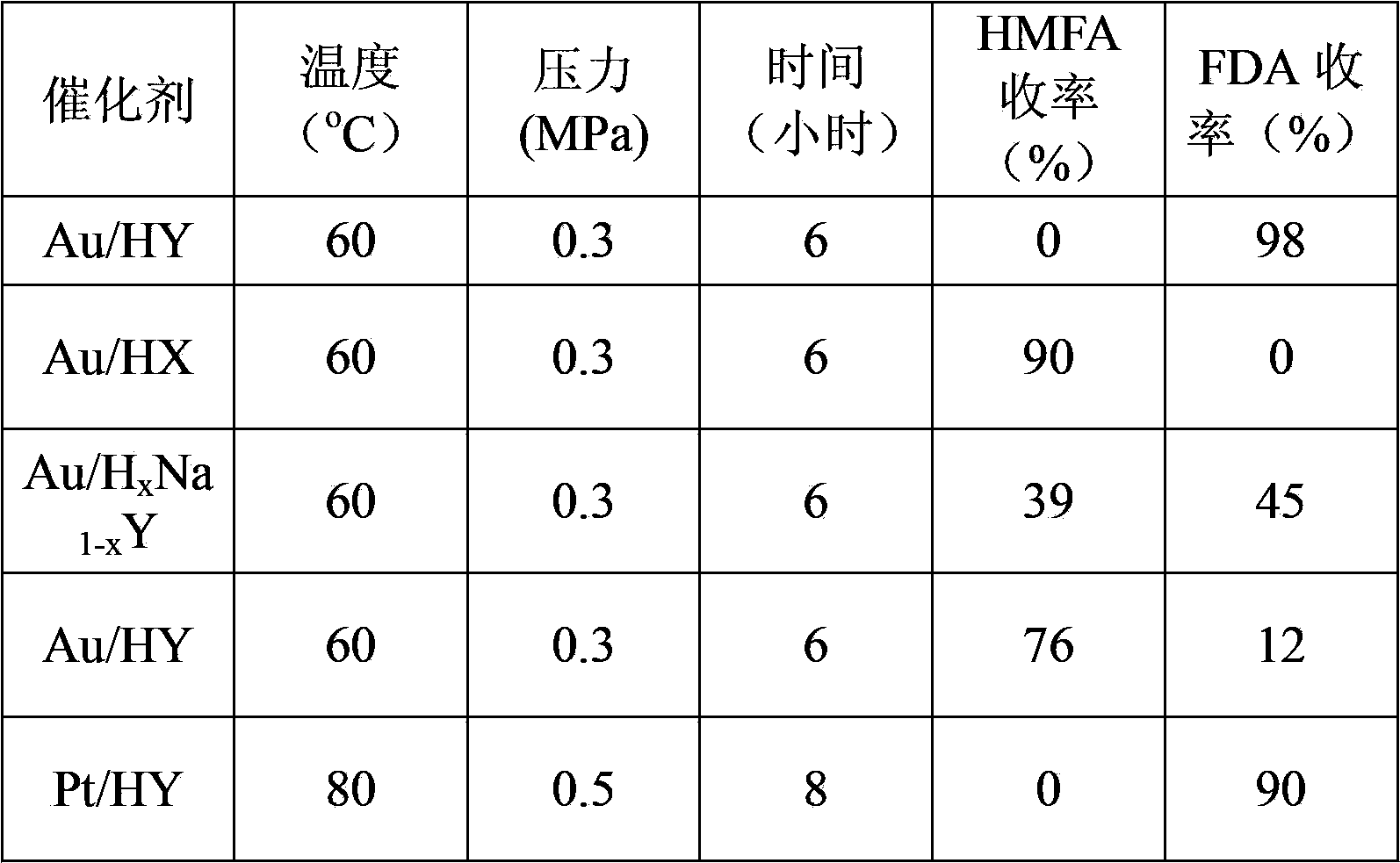
The invention discloses a preparation method of 5-hydroxymethyl furoic acid and 2,5-furandicarboxylic acid by selective oxidation of 5-hydroxymethylfurfural. The method comprises the following steps: taking a precious metal loaded acidic carrier as the catalyst, under mild conditions (temperature is in a range of 25 to 100 DEG C, and the oxygen pressure is in a range of 0.1 to 3.0 MPa), and modulating the temperature, pressure and reaction time so as to rapidly and high-efficiently oxidize 5-hydroxymethylfurfural to generate 5-hydroxymethyl furoic acid or 2,5-furandicarboxylic acid. The conversion rate of 5-hydroxymethylfurfural can reach 100%, the selectivity of 5-hydroxymethyl furoic acid reaches 98%, and the selectivity of 2,5-furandicarboxylic acid reaches 99%. The method has the advantages of high efficiency and environment-friendliness, and the products have a big application value and a wide application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
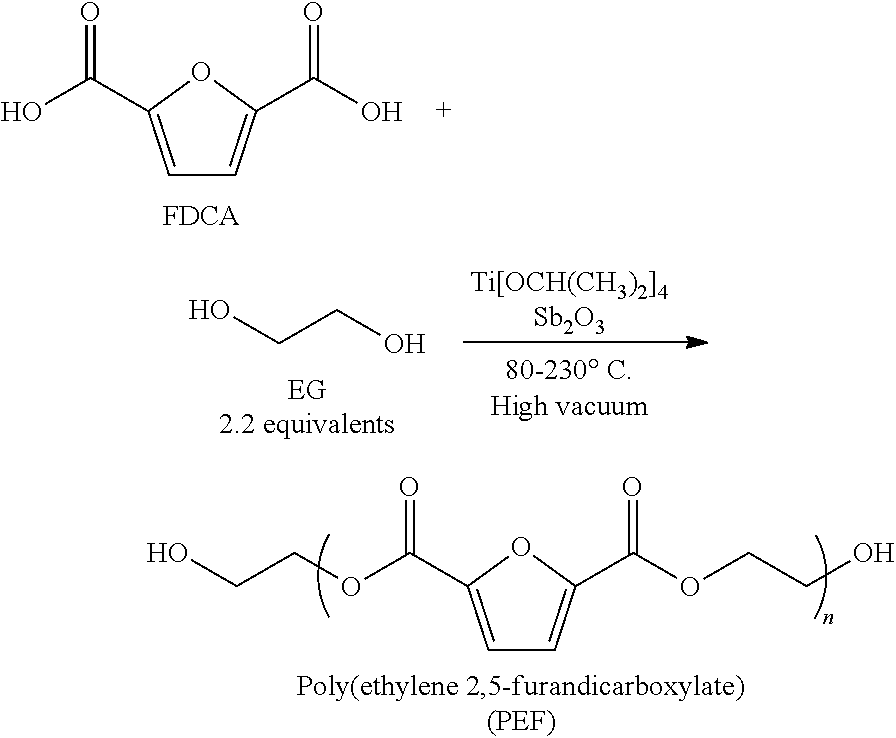
Process for the production of poly(ethylene 2,5-furandicarboxylate) from 2,5-furandicarboxylic acid and use thereof, polyester compound and blends thereof
Owner:NATURA COSMETICOS SA
Oxidation of furfural compounds
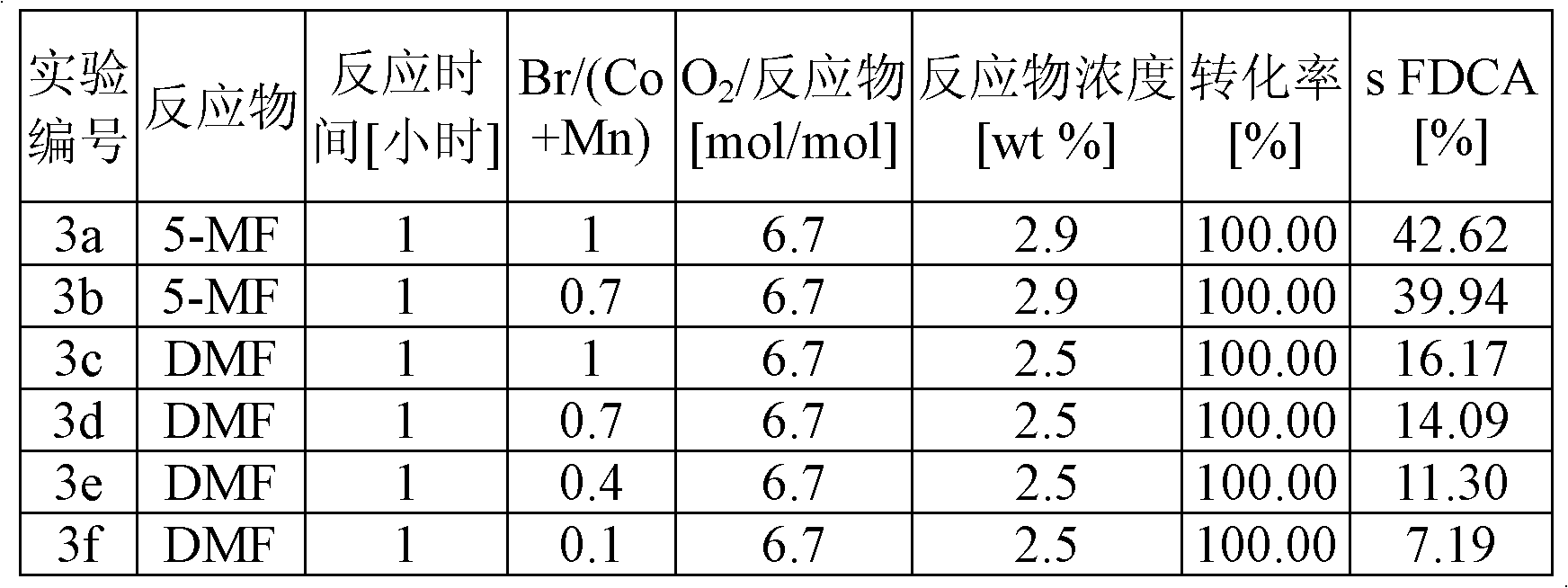
The disclosure pertains to a process for oxidation of furan aldehydes such as 5-hydroxymethyl)furfural (HMF) and derivatives thereof such as 5-(alkoxymethyl)furfural (AMF), 5-(aryloxymethyl)furfural, 5-(cycloalkoxy-methyl)furfural and 5-(alkoxycarbonyl)furfural compounds in the presence of dissolved oxygen and a Co(II), Mn(II), Ce(III) salt catalyst or mixtures thereof. The products from HMF can be selectively chosen to be predominantly 2,5-diformylfuran (DFF), particularly by inclusion of an aliphatic ketone, like methyl ethyl ketone, or can be further oxidized to 2,5-furandicarboxylic acid (FDCA) by the omission of methyl ethyl ketone and inclusion of bromide. When the reactant is an ether derivative of HMF the products are surprisingly ester derivatives where either both the ether and aldehyde functional groups have been oxidized or just the ether function group thereby producing one or both of 5-ester-furan-2-acids (i.e., 5-alkoxycarbonylfurancarboxylic acids) or 5-ester-furan aldehydes, (i.e., -alkoxycarbonylfurfurals a. k. a, 5-(alkoxycarbonyl)furfural).
Owner:ARCHER DANIELS MIDLAND CO
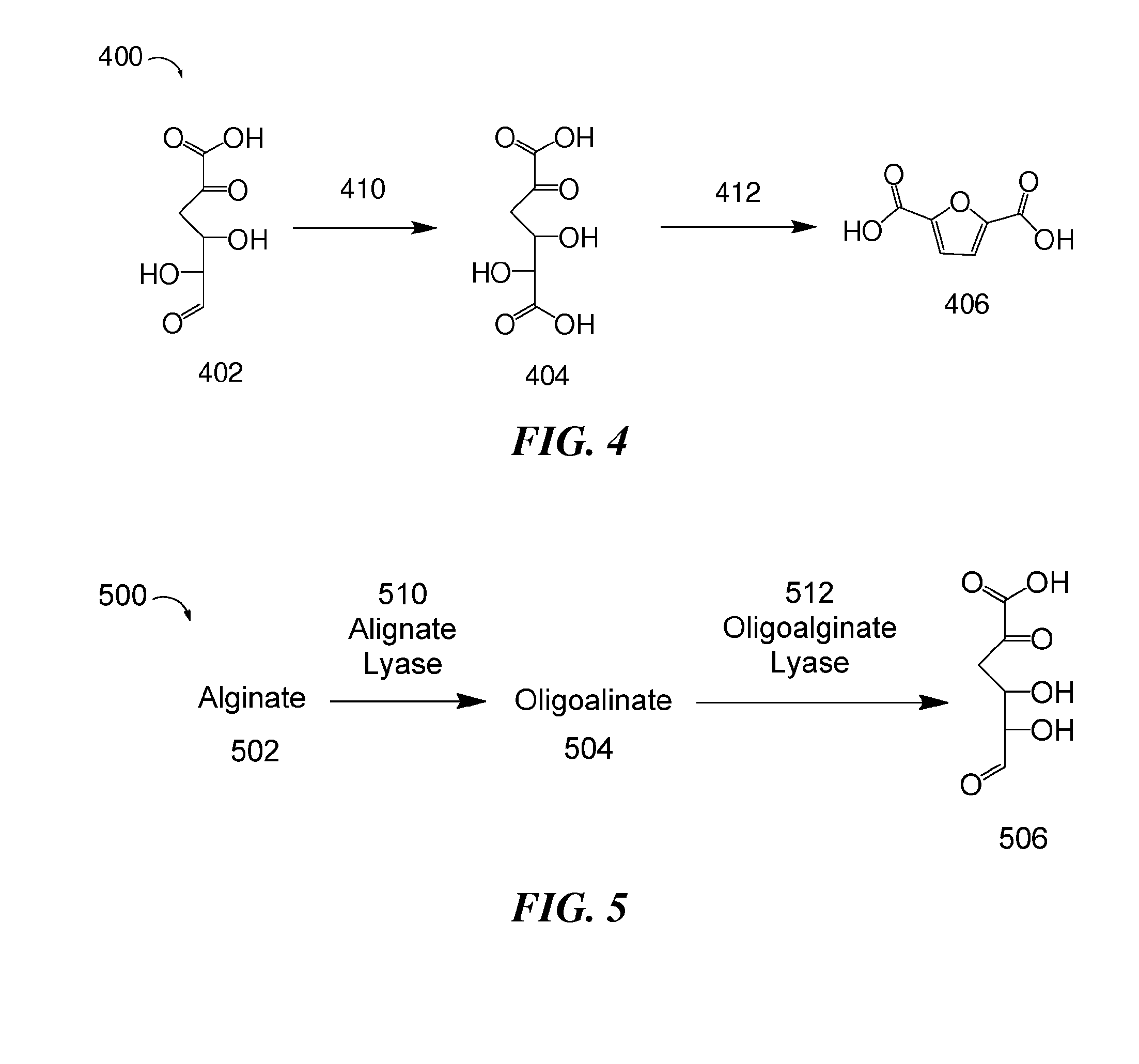
Methods for preparing 2,5-furandicarboxylic acid
Provided are methods of producing 2,5-furandicarboxylic acid (FDCA) from renewable sources such as seaweed, alginate, oligoalginate, pectin, oligopectin, polygalacturonate, galacturonate, and / or oligogalacturonate. The sugars in the renewable sources can be converted into one or more intermediates such as 4-deoxy-L-erythro-5-hexoseulose uronate (DEHU), 4-deoxy-L-threo-5-hexosulose uronate (DTHU), 5-hydroxymethyl furfural (HMF), 2,5-dihydroxymethyl furan (DHMF), and 5-formyl-2-furancarboxylic acid (FFA), which can be converted into FDCA by dehydration and cyclization to produce 5-formyl-2-furancarboxylic acid (FFA), followed by oxidation to produce FDCA. DEHU or DTHU may also be converted into FDCA by oxidation to produce 2,3-dihydroxy-5-oxohexanedioic acid (DOHA), which then undergoes dehydration and cyclization to produce FDCA.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Method of producing 2,5-furandicarboxylic acid
Provided is a method of producing FDCA by which high-purity FDCA can be produced in high yield. 2,5-furandicarboxylic acid is produced by: bringing 5-hydroxymethylfurfural into contact with an oxidant in an organic acid solvent in the presence of bromine and a metal catalyst; and allowing 5-hydroxymethylfurfural and the oxidant to react with each other while removing water produced by the reaction.
Owner:CANON KK
Method for preparing 2,5-furandicarboxylic acid from 5-hydroxymethyl furfural
ActiveCN105037303AEffective control of selectivityEffective regulation of catalyst activityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTungstateHydroxymethylfurfural
The invention discloses a method for preparing 2,5-furandicarboxylic acid from 5-hydroxymethyl furfural. Quaternary ammonium molybdate and quaternary ammonium tungstate are taken as a catalyst; oxygen gas, hydrogen peroxide or air is taken as an oxidant; the catalyst and the oxidant are heated to 80-120 DEG C under an alkali environment so as to carry out selective oxidization on 5-hydroxymethyl furfural. According to the method, ammonium salt and molybdic acid or tungstic acidis utilized to structurally regulate the activity of the catalyst and the selectivity of a product; the used catalyst is high in activity, easy to recycle and good in stability; the oxidant adopted in the oxidization process is environmentally-friendly, green and pollution-free; high yield and high selectivity of the 2,5-furandicarboxylic acid can be obtained at a low temperature; and a conversion rate of the 5-hydroxymethyl furfural is higher than 90%.
Owner:TIANJIN POLYTECHNIC UNIV
Biodegradable polyester and preparation method thereof
InactiveCN103570925AReduce dependenceReduce usageSynthetic resin layered productsPolyester coatingsPolymer scienceCarboxylic acid
The present invention provides a biodegradable copolyester containing 2,5-furandicarboxylate, a product thereof, a preparation method therefor and a use thereof. The polyester has irregularly repeating structural units. The aromatic-aliphatic copolyester of the present invention integrates high crystallinity and high melting point of terephthalate copolyester, biomass sources of 2,5-furandicarboxylate, and biodegradability of aliphatic polyester.
Owner:SHANGHAI GENIUS ADVANCED MATERIAL (GRP) CO LTD
Preparation method of 2,5-furandicarboxylate
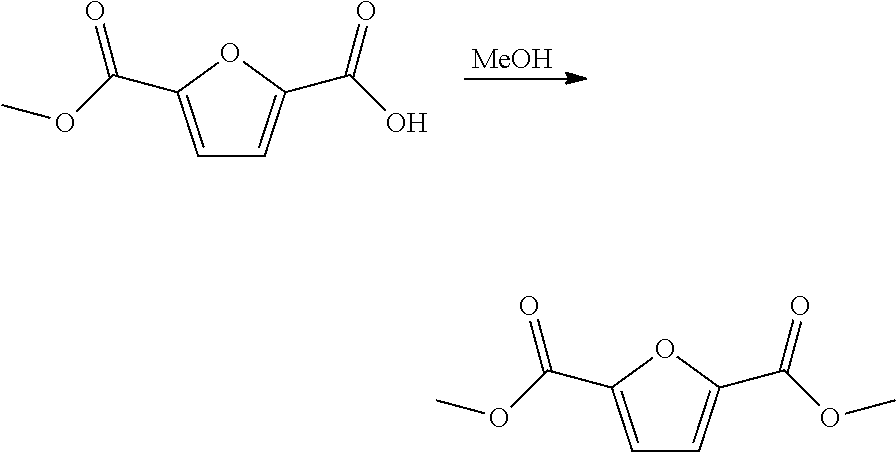
The invention discloses a method for preparing 2,5-furandicarboxylate. The preparation method is characterized by comprising the following steps of: mixing 2,5-dicarboxaldehyde with the aqueous solution of t-butylhydroperoxide, and stirring to enable 2,5-dicarboxaldehyde to react with the aqueous solution of t-butylhydroperoxide for 4-8 hours at the temperature of 100-120 DEG C, naturally cooling to the room temperature to obtain a reaction product, separating the reaction product centrifugally to obtain precipitate, and washing precipitate and drying to obtain the target product which is 2,5-furandicarboxylate. The method disclosed by the invention has the advantages of mild reaction condition, low cost, environment friendliness and high product yield.
Owner:INST OF ADVANCED TECH UNIV OF SCI & TECH OF CHINA +1
Poly(ethylene 2,5-furandicarboxylate), and preparation method thereof
InactiveCN102190785AImprove performanceWide variety of sourcesEsterification reactionGlass transition
The invention provides poly(ethylene 2,5-furandicarboxylate), which contains the repeating units shown in formula (I) and has a characteristic viscosity not smaller than 0.2 dL / g. The invention also provides a preparation method for poly(ethylene 2,5-furandicarboxylate) provided by the technical scheme, which comprises the following steps of: carrying out esterification reaction on 2,5-furandicarboxylic acid and ethylene glycol under the action of a tin catalyst to obtain a first intermediate product, wherein the esterification reaction pressure is 0.1-0.4 MPa; and carrying out condensation polymerization reaction on the first intermediate product under the pressure of 10-70 Pa to obtain poly(ethylene 2,5-furandicarboxylate). Experiments show that poly(ethylene 2,5-furandicarboxylate) provided by the invention is dark brown, and has a characteristic viscosity of not smaller than 0.2 dL / g, a glass transition temperature of 60-63 DEG C and a melting point of 210-215 DEG C.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for the preparation of 2,5-furandicarboxylic acid and for the preparation of the dialkyl ester of 2,5-furandicarboxylic acid
ActiveUS20120271060A1High yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranDicarboxylic acid
A method for the preparation of 2,5-furan dicarboxylic acid includes the step of contacting a feed comprising a compound selected from the group consisting of 5-hydroxymethylfurfural (“HMF”), an ester of 5-hydroxymethyl-furfural, 5-methylfurfural, 5-(chloromethyl)furfural, 5-methylfuroic acid, 5-(chloromethyl)furoic acid, 2,5-dimethylfuran and a mixture of two or more of these compounds with an oxidant in the presence of an oxidation catalyst at a temperature higher than 140° C.
Owner:FURANIX TECH BV
Method for the preparation of 2,5-furandicarboxylic acid and esters thereof
ActiveUS20120283452A1Organic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranHydroxymethylfurfural
A method for the preparation of 2,5-furandicarboxylic acid (“FDCA”) and / or an alkyl ester of FDCA includes the step of contacting a feed comprising a starting material selected from 5-alkoxymethylfurfural, 2,5-di(alkoxymethyl)furan and a mixture thereof with an oxidant in the presence of an oxidation catalyst. The feed may also comprise 5-hydroxymethylfurfural as a further starting material.
Owner:FURANIX TECH BV
Industrial preparation method of low-cost 2,5-furan dicarboxylic acid
The invention provides a preparation method of low-cost 2,5-furan dicarboxylic acid. The preparation method comprises the step of reacting furan formate, molten salt and a catalyst under the conditionof carbon dioxide gas to obtain the 2,5-furan dicarboxylic acid; a melting point of the molten salt is less than or equal to 400 DEG C; the catalyst comprises a metal salt catalyst and / or an organicalkali catalyst. The preparation method provided by the invention has the benefits that the 2,5-furan dicarboxylic acid is prepared by taking the furan formate as an initial raw material and the cheapand easy-to-obtain conventional metal salt as the catalyst and by specially utilizing a monomer or a mixture of organic salt or inorganic salt; both the used catalyst and the used molten salt are cheap chemical products, so that the reaction cost is greatly reduced; the preparation method is simple in process, low in reaction temperature, economic, environmentally-friendly and suitable for large-scale industrial production and pushes the industrialization progress of the 2,5-furan dicarboxylic acid.
Owner:芜湖万隆新材料有限公司
Method for the preparation of 2,5-furandicarboxylic acid and for the preparation of the dialkyl ester of 2,5-furandicarboxylic acid
ActiveCN102666521AOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranDicarboxylic acid
The invention describes a method for the preparation of 2,5-furan dicarboxylic acid comprising the step of contacting a feed comprising a compound selected from the group consisting of 5-hydroxymethylfurfural ("HMF"), an ester of 5-hydroxymethyl-furfural, 5-methylfurfural, 5-(chloromethyl)furfural, 5-methylfuroic acid, 5-(chloromethyl)furoic acid, 2,5- dimethylfuran and a mixture of two or more of these compounds with an oxidant in the presence of an oxidation catalyst at a temperature higher than 140 DEG C.
Owner:FURANIX TECH BV
Water-soluble copolyester and preparation method thereof
The invention discloses a water-soluble copolyester and a preparation method thereof. The invention is characterized in that the water-soluble copolyester is obtained by sequentially carrying out esterification or ester exchange reaction and polycondensation reaction on dicarboxylic acid or esters thereof, m-benzenesulfonate, 2,5-furyl-diformic acid and dibasic alcohol as raw materials; the glass transition temperature of the water-soluble copolyester is 30-60 DEG C; and under the condition of 35 DEG C and orthochlorophenol as a solvent, the intrinsic viscosity of the water-soluble copolyester is at least 0.25 dL / g. By partially or totally substituting terephthalic acid with biomass-base 2,5-furyl-diformic acid, the technique reduces the consumption of the fossil raw material, and the water-soluble polyester has better environment-friendly characteristic and sustainable development performance, and has higher water solubility.
Owner:苏州瀚海化学有限公司
Method for preparing 2,5-furandicarboxylic acid from biomass compound by electrocatalytic oxidation
ActiveCN108531936AWide range of resources and cheapLow costElectrolytic organic productionLiquid/solution decomposition chemical coatingCatalytic oxidationH shaped
The invention relates to a method for preparing 2,5-furandicarboxylic acid from a biomass compound by electrocatalytic oxidation. The method comprises the following steps: reacting by using an H-shaped electrolytic cell, in an anode compartment, taking a load type catalyst as a working electrode, and dissolving the biomass compound as a reaction substrate in an alkaline solution to obtain an anodesolution; and in a cathode compartment, carrying out electrocatalytic oxidation reaction by taking a platinum sheet as a counter electrode and taking the alkali solution as a cathode solution for 0.5-5 hours under the conditions that the temperature is 30-90 DEG C, the current is 5-50 mA and the cell voltage is 1-10 V, and after reaction is finished, carrying out aftertreatment to obtain the 2,5-furandicarboxylic acid. The technique is gentle in conditions of an electrocatalytic oxidation reaction process, green and pollution-free, high in conversion rate of the raw material and good in FDCAselectivity. Compared with a noble metal catalyst which is generally used in prior art, a metal vanadium compound catalyst used in the invention is low on cost; and consumption of rare precious metalraw materials is avoided.
Owner:ZHEJIANG UNIV OF TECH
Preparation methods of 2,5-furandicarboxylic acid and 2,5-furanyl polyester
InactiveCN108997278ALow costLow priceOrganic chemistryBulk chemical productionSodium bicarbonatePolyester
The invention provides a preparation method of 2,5-furandicarboxylic acid. The preparation method comprises the following steps: A), with non-grain biomass as a raw material, preparing furfural; B), oxidizing the furfural to obtain furoic acid; C), performing an addition reaction on the furoic acid and carbon dioxide under the action of a catalyst to obtain the 2,5-furandicarboxylic acid, whereinthe catalyst is one or more of potassium carbonate, sodium oxalate, sodium nitrate, potassium nitrate, sodium nitrite, potassium nitrite, potassium acetate, sodium acetate, potassium formate, sodium formate, sodium hydrogen carbonate, potassium hydrogen carbonate, sodium hydroxide and potassium hydroxide. The furfural is prepared from the non-grain biomass as the raw material, so that the raw material cost is low; the furoic acid reacts with the carbon dioxide under the action of the specific catalyst to obtain the 2,5-furandicarboxylic acid; the catalyst is low in price; the used carbon dioxide is environment-friendly; by the preparation method, the yield is high.
Owner:芜湖万隆新材料有限公司
Novel oxidation catalyst, the process for the preparation thereof and green process for selective aerobic oxidation
ActiveUS20150336090A1High yieldShort time spanAluminium compoundsMolecular sieve catalystsRecyclable catalystAlkaline earth metal
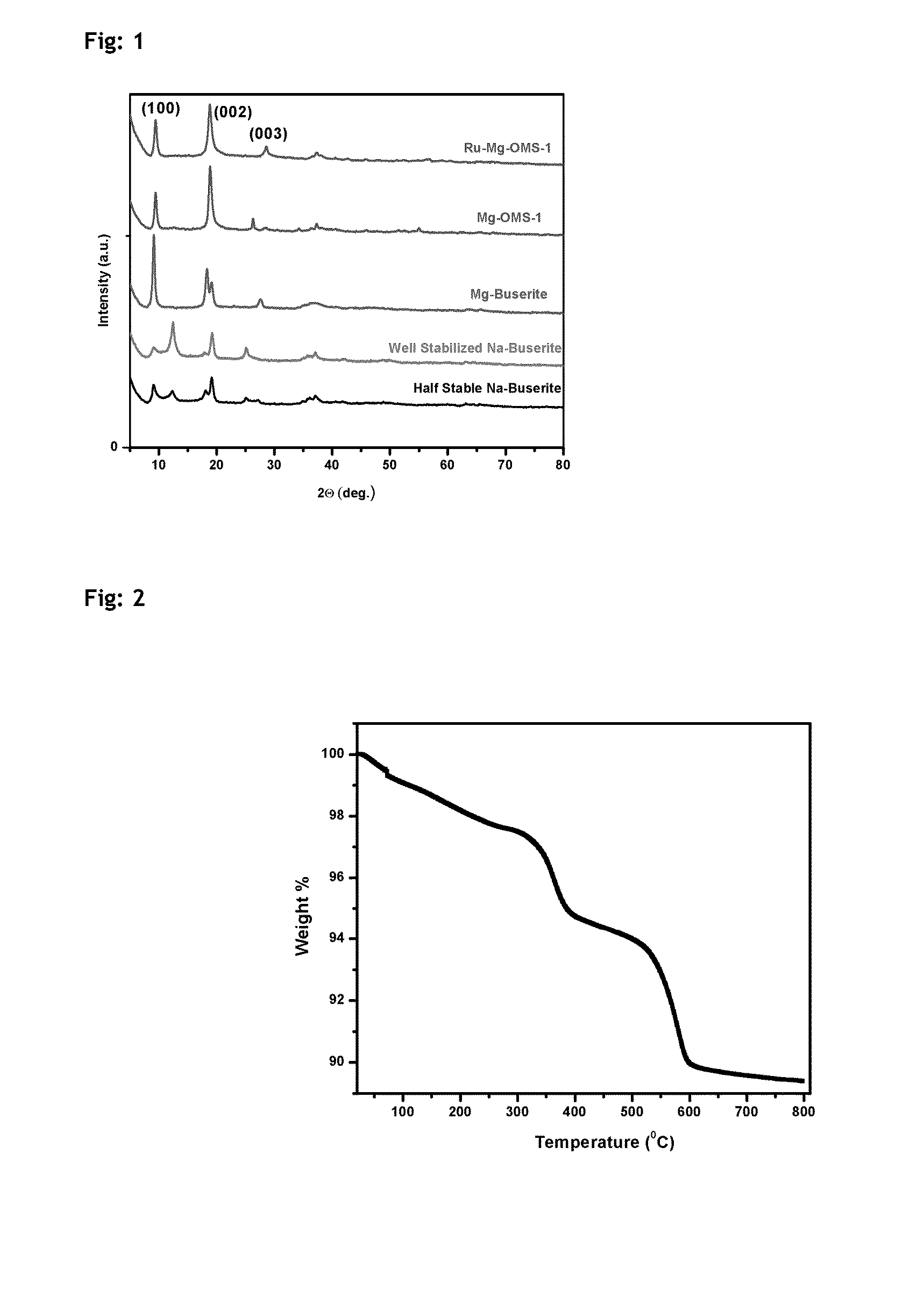
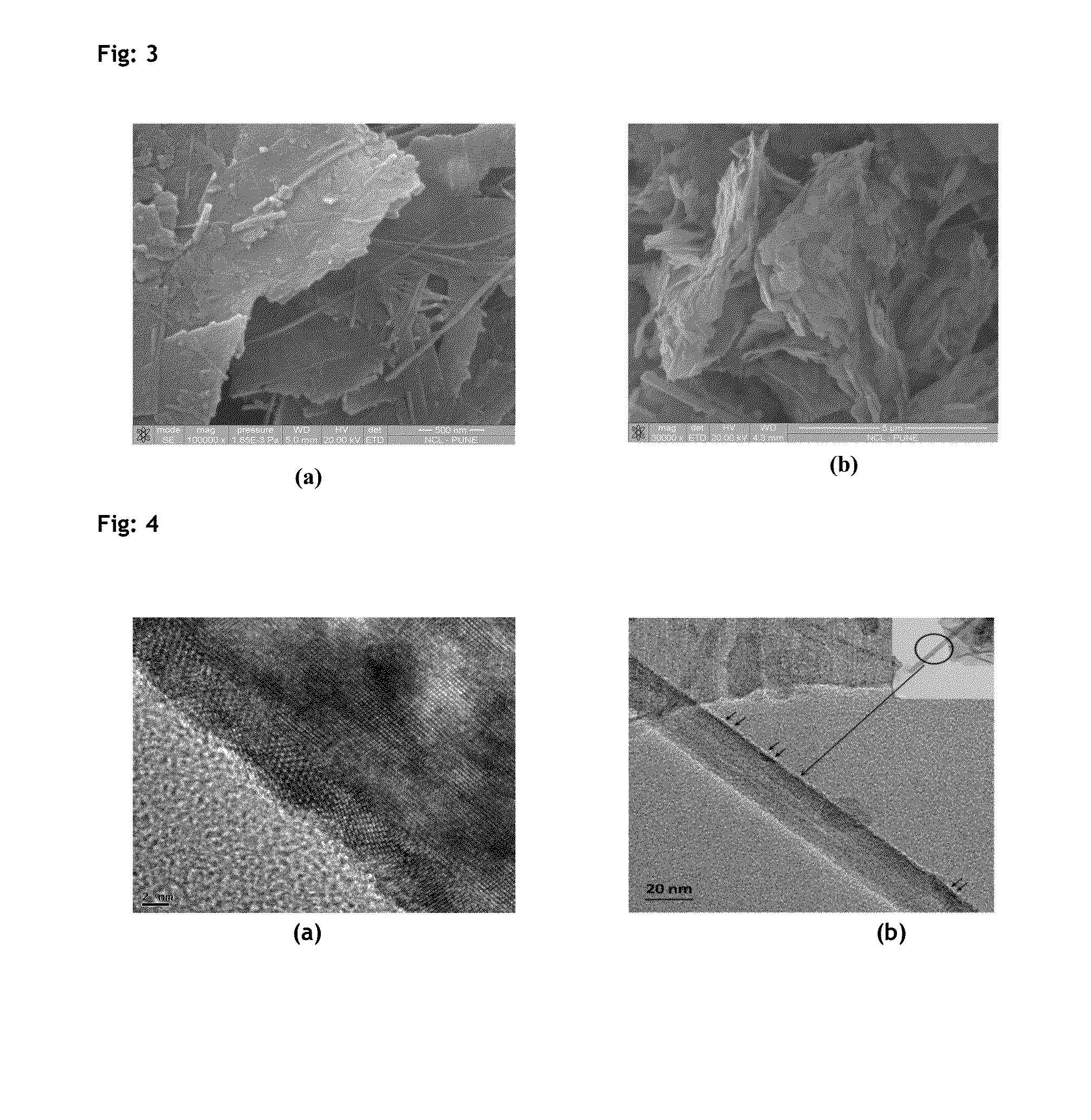
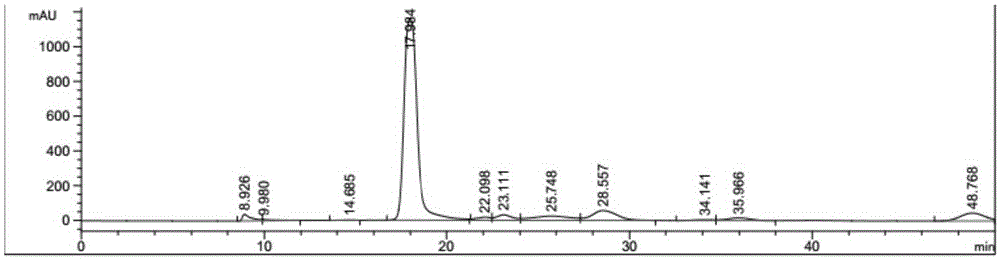
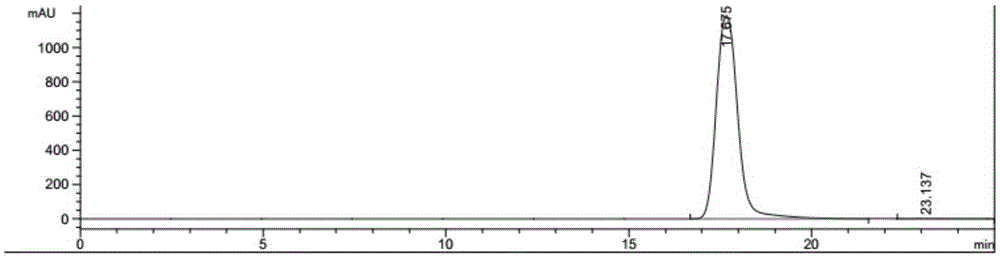
Described herein is a novel, cost effective, stable and recyclable catalyst composition i.e. A-B-OMS, wherein ‘A’ is selected from noble and transition metals; ‘B’ is selected from alkali or alkaline earth metals; and OMS is octahedral molecular sieve which includes synthetic todorokite (OMS-1) and cryptomelane (OMS-2); and characterization thereof. Further the invention provides base free green process for selective aerobic oxidation of 5-hydroxymethylfurfural (HMF) catalysed by said catalyst composition under optimized reaction conditions to obtain high yield of 2,5-furandicarboxylic acid (FDCA) in a shorter span of time. Invention also provides for selective oxidation of glucose to Gluconic Acid, furfural to furoic Acid and glycerol to Glyceric acid. Invention can also applicable for the selective oxidation of hexoses, pentoses, disaccharides to corresponding acids.
Owner:COUNCIL OF SCI & IND RES
Method for preparing 2,5-furandicarboxylic acid by supported bifunctional catalyst by catalyzing fructose
ActiveCN105418561AAcidicHas catalytic oxidation propertiesOrganic chemistryMolecular sieve catalystsFructoseCatalytic oxidation
The invention provides a method for preparing 2,5-furandicarboxylic acid by catalyzing fructose with a supported bifunctional catalyst. According to the method, a supported heteropolyacid salt is taken as a catalyst; and the catalyst disclosed by the invention is the bifunctional catalyst, and has acidity and catalytic oxidation performance. The fructose and biomass materials rich in the fructose can be efficiently catalyzed to dewater and prepare HMF; and meanwhile, 2,5-furandicarboxylic acid can be prepared by in-situ catalytic oxidation of the HMF at high selectivity. The catalyst is convenient to recycle and good in reusability, so that a foundation is laid for efficient preparation of the 2,5-furandicarboxylic acid.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com