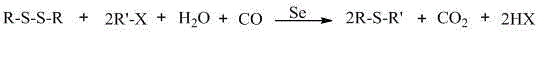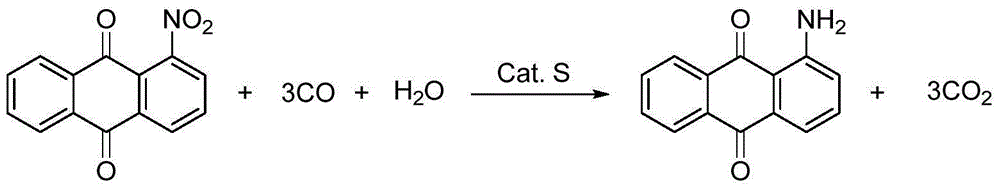Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "The difficulty of the reaction process is low" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
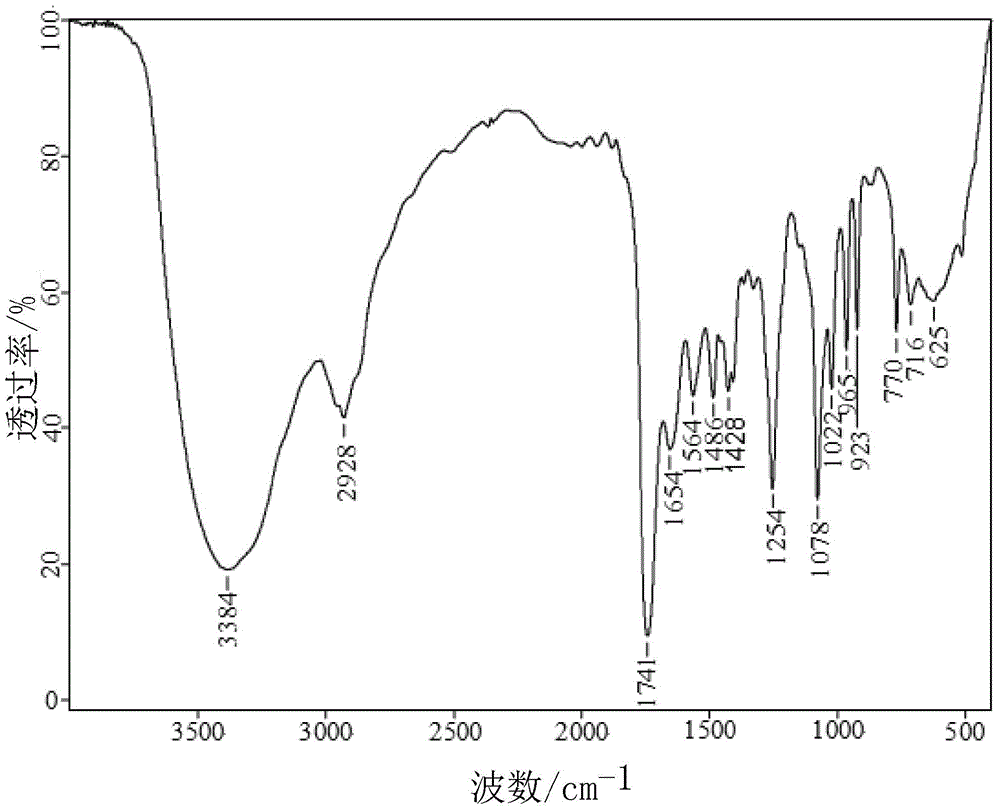
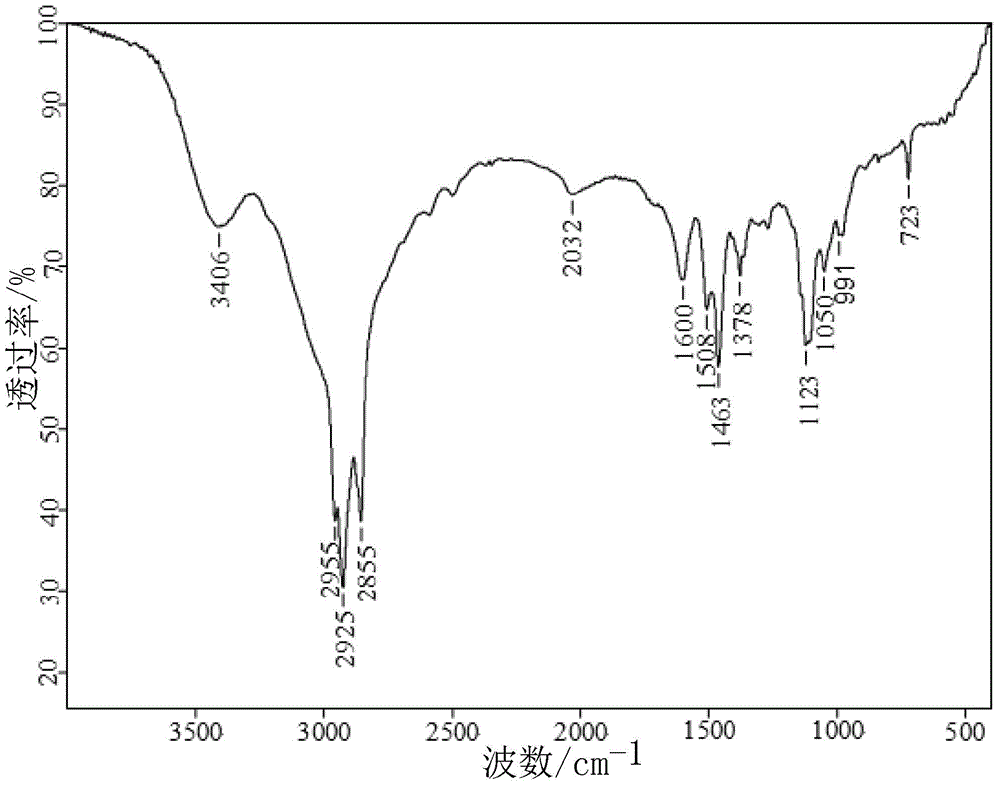
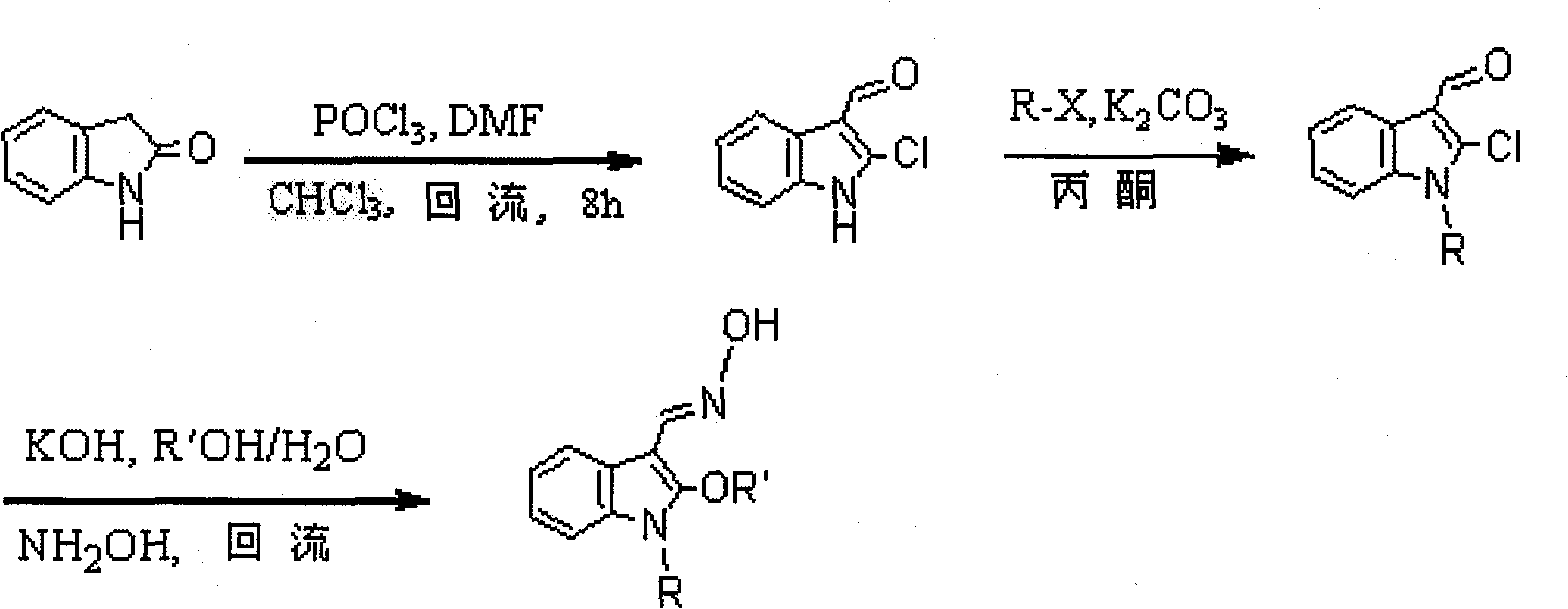
Method for synthesizing 1-alkyl-2-alkoxyl-3-indole aldoxime derivant
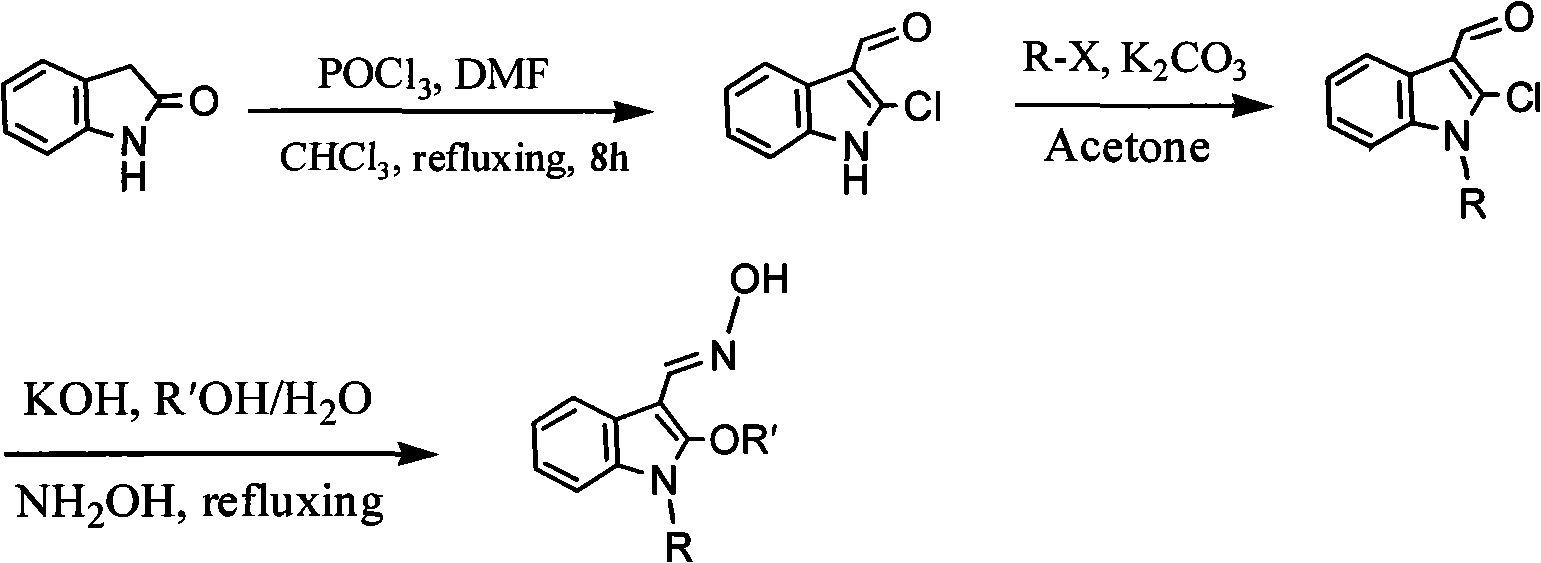
The present invention relates to a method used for synthesizing derivatives of 1-alkyl-2-alkoxy-3-indole aldehyde oxime. Indole-one is used as basic material and treated through Vilsmeier-Hacck formylation reaction and alkylation reaction to prepare 1-alkyl-2-chlorine-3-indole aldehyde; under the conditions with potassium hydroxide and fatty alcohol and at the temperature of 50 to 80 DEG C, the reaction is monitored by a thin chromatogram TLC; after the reaction is completed, no treatment is required and hydroxylamine hydrochloride is directly added into the mixture for oximation reaction; the reaction temperature is maintained; the reaction lasts for 1 to 2 hours; the ratio of the 1-alkyl-2-chlorine-3-indole aldehyde, the hydroxylamine hydrochloride, the potassium hydroxide, the fatty alcohol and water is equal to 1mmol to 2mmol to 2mmol to from 5ml to 8 ml to 1ml; finally water is added for sedimentation, and the novel compounds of the category of the 1-alkyl-2-chlorine-3-indole aldehyde can be separated through filtration and chromatography. The method has the advantages of mild reaction conditions, process under atmospheric pressure, simple operation, less investment in the equipment, low production costs, less pollution and high yield.
Owner:BOHAI UNIV
Method for synthesizing 1-amino naphthalenes
InactiveCN1600774ALess investmentSimple and safe operationOrganic compound preparationAmino compound preparationOrganic solventReaction temperature
A process for synthesizing amino naphthalene by nitro naphthalene with carbon monoxide and water, selenium as catalyst, organic alkali and inorganic alkali as assistant catalyst in organic solvent, the molar ratio of nitro naphthalene and water is 1:1 to 1:1000, the molar amount of selenium is 0.1-100% of the molar amount of nitro naphthalene; the molar amount of organic alkali and inorganic alkali is 1-36 hours; reaction temperature is 20-120 deg.C.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
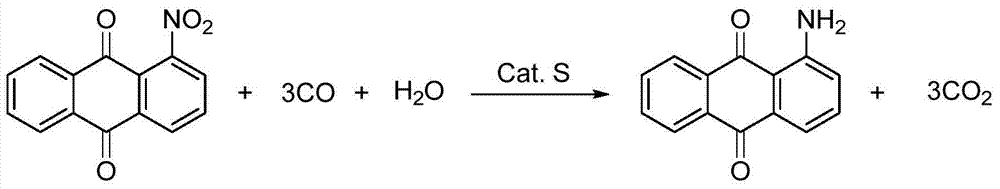
Method for synthesizing 1-aminoanthraquinone
ActiveCN103113245ALess investmentSimple and safe operationOrganic chemistryOrganic compound preparationAnthraquinonesPtru catalyst
The invention relates to a method for synthesizing 1-aminoanthraquinone. The technical scheme is as follows: in the presence of carbon monoxide and water, 1-nitroanthraquinone used as a raw material reacts by using selenium as a catalyst and inorganic alkali or organic alkali as a cocatalyst at high temperature under high pressure to synthesize the 1-aminoanthraquinone by one step. The invention is simple and safe to operate, and has the advantages of water-phase reaction, accessible raw material, no pollution, high selectivity and high yield; and after the reaction finishes, the catalyst can be recycled and utilized repeatedly.
Owner:LIAONING UNIVERSITY
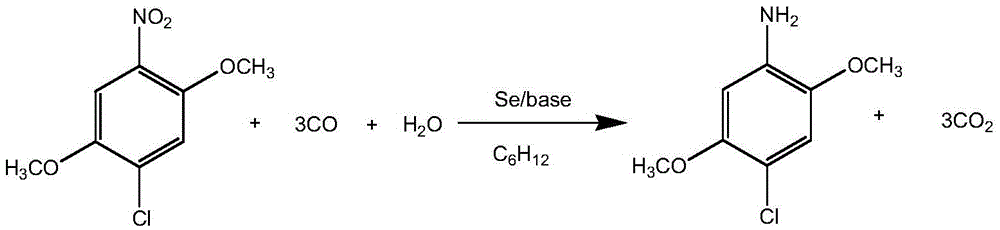
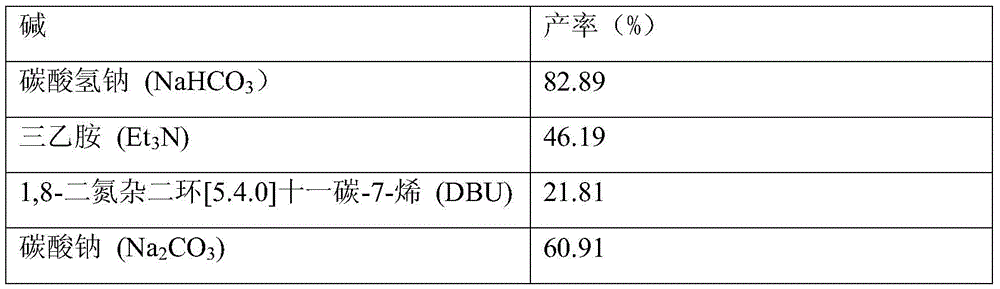
Synthesis of nitroarylamine compound
InactiveCN1403437ALess investmentSimple and safe operationOrganic compound preparationAmino compound preparationNitro compoundOrganic solvent
Nitroarylamine compound is prepared with aromatic dinitro compound and through single nitro selecting reduction reaction in organic solvent at normal pressure in the presence of CO and water by using Se as catalyst and alkali as cocatalyst. The said aromatic dinitro compound may have substituent radical in the phenyl radical and the substituent radical may be one or several eletron donating and / or accepting group. The reaction conditions includes the molar ratio between aromatic dinitro compound and water of 1 to 1-1000; molar amount of Se is 0.1-100 % of the aromatic dinitro compound; molar amount of alkali is 0-400 % of the aromatic dinitro compound; the weight ratio between aromatic dinitro compound and solvent of 1 to 2-1000; reaction time of 1-36 hr; and reaction temperature of 20-120 deg.c.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthetic method of ether amine flotation agent
InactiveCN104803862AOperational securitySimple processOrganic compound preparationAmino-hyroxy compound preparationDesiccantOrganic layer
The invention discloses a synthetic method of an ether amine flotation agent. The synthetic method is characterized by comprising the following steps: (1) at ordinary temperature and normal pressure, placing chlorinated alkyl amine hydrochloride and an acid-binding agent in a solvent to sufficiently react and separate out white acicular crystals, adding a drying agent, stirring, filtering and collecting the filtrate; (2) adding a hydride of alkali metals into the filtrate, increasing the temperature till the system is slightly boiled for sufficient reaction; (3) after finish of the reaction, filtering to remove the solvent in the filtrate; (4) adjusting the pH of the filtrate from which the solvent is removed, extracting, separating out an extraction agent layer, and drying to ensure constant weight; (5) adjusting the pH of the dried extraction agent layer, extracting, separating out the water layer, and drying to obtain ether amine hydrochloride; (6) using an inorganic base solution to adjust the pH of ether amine hydrochloride, extracting, separating out an organic layer and removing the solvent in the organic layer to obtain the product. According to the synthetic method, normal-pressure reaction is carried out, the operation is safe, the process is simple and the cost is low.
Owner:广东省石油化工研究院
Synthesis of arylamine compound
InactiveCN1187314CLess investmentSimple and safe operationOrganic compound preparationAmino compound preparationNitro compoundOrganic solvent
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Diselenide compound synthesis method
InactiveCN101619034BLess investmentSimple and safe operationOrganic chemistryHalohydrocarbonOrganic solvent
The invention relates to a diselenide compound synthesis method and the adopted technical proposal comprises the following steps: adopting halogenated hydrocarbon and selenium as raw materials and organic base or inorganic base as cocatalyst or no cocatalyst, continuously injecting carbon dioxide gas in organic solvent to react in the present of water under the temperature of 20-100 DEG C at normal pressure for 1-24h, cooling to the room temperature, switching carbon dioxide to air or oxygen to precipitate unreacted selenium, filtrating to collect filtrate, then adding 2-3 times of water, precipitating products and finally obtaining the diselenide compound by filtration. The method invention is normal press reaction with low cost, environmentally friend, low reaction process difficulty, convenient operation and easy following separation of products and catalyst.
Owner:LIAONING UNIVERSITY
Method for synthesizing 2,5-dimethoxy-4-chloroaniline
ActiveCN105601523ALess investmentEasy to operateOrganic compound preparationAmino-hyroxy compound preparationOrganic solventNitrobenzene
The invention relates to a method for synthesizing 2,5-dimethoxy-4-chloroaniline. The method comprises the steps: in the presence of carbon oxide and water, performing the reaction in an organic solvent under a high temperature and high pressure by adopting 2,5-dimethoxy-4-chloro-nitrobenzene as a raw material, selenium as a catalyst and organic alkali or inorganic alkali as an assistant catalyst, and reducing the nitro to amino to synthesize the 2,5-dimethoxy-4-chloroaniline. The method adopts high-pressure reaction and one-pot reaction, so that less equipment investment is needed, the operation is simple and convenient, and a product is relatively easy to separate and purify.
Owner:LIAONING UNIVERSITY
Method for synthesis of rivastigmine
InactiveCN100457722CLess investmentSimple and safe operationCarbamic acid derivatives preparationOrganic compound preparationMethylcarbamic acidRivastigmine
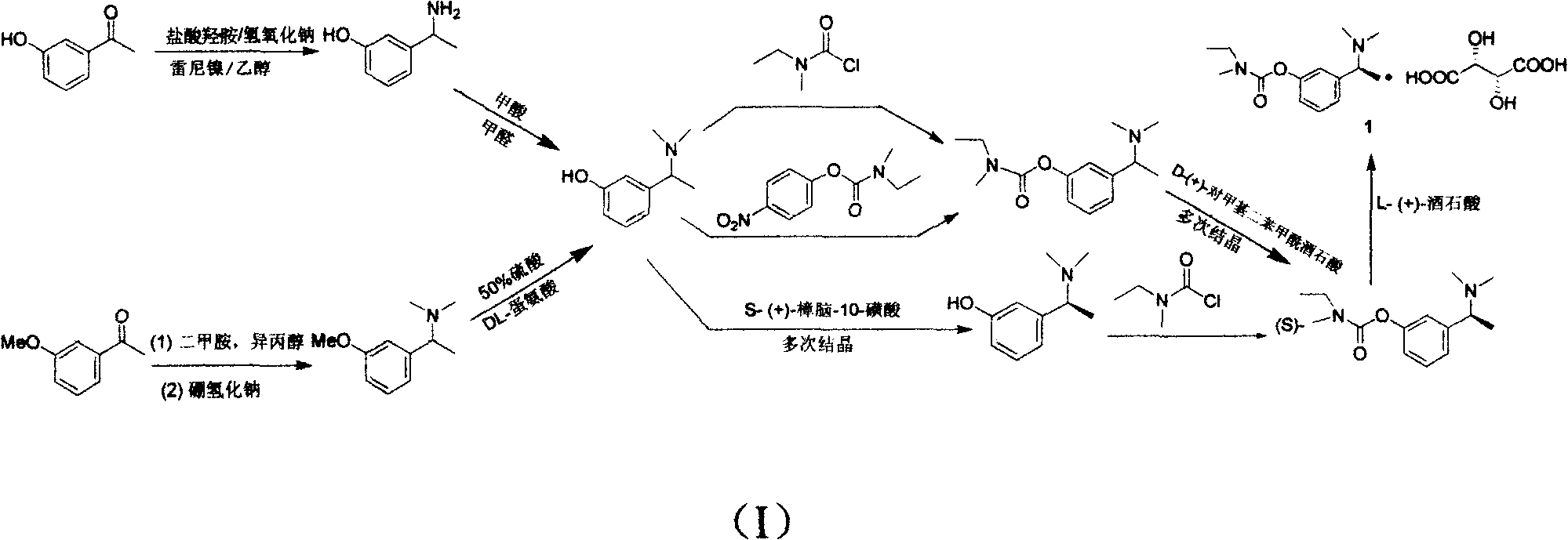
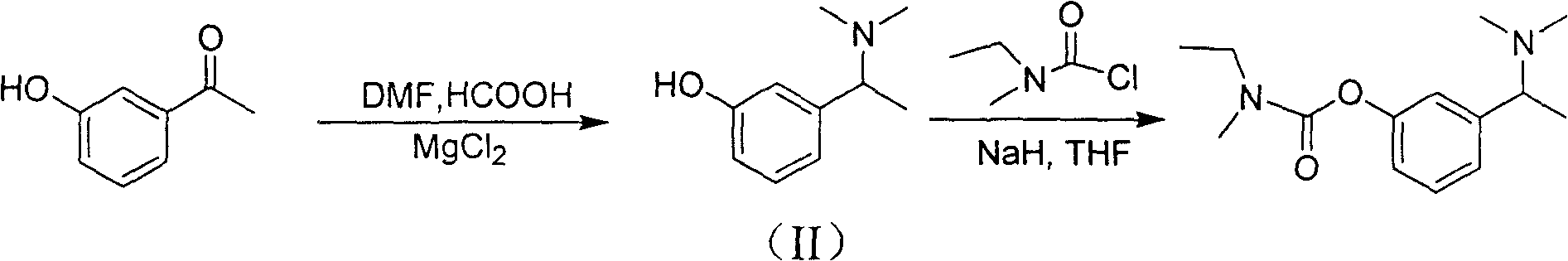
The invention discloses a synthesizing method of (S)-N-ethyl-3-[(1-dimethylamino) acetyl]-N-methyl amido phenyl formate, which is characterized by the following: adopting m-hydroxy acetophenone as raw material; synthesizing intermediate 3-(1-(dimethylamino) ethyl) phenol; condensing 3-(1-(dimethylamino) ethyl) phenol and formyl chloride to produce the product with high receiving rate.
Owner:JINAN UNIVERSITY
Process for synthesizing aryl substituted N-aryl amide compounds
InactiveCN1690045ALow costReduce stepsOrganic compound preparationCarboxylic acid amides preparationNitro compoundElectron donor
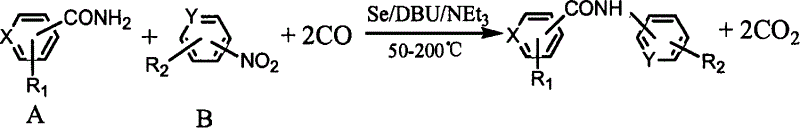
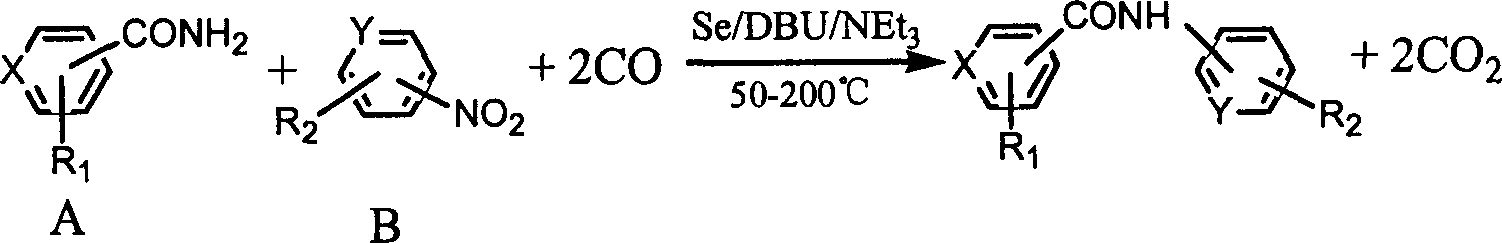
The present invention is synthesis process of N-aryl substituted aryl amide compound. Aryl amide and aromatic nitro compound are reacted in organic solvent inside high pressure reactor in the presence of CO with selenium as catalyst and DBU and triethylamine as co-catalyst, where, the substitutent on the aryl radical in the aromatic compound may be electron donor or acceptor group(s), the material molar ratio between aryl amide and aromatic nitro compound is 0.1-10, the molar consumption of selenium is 0.1-20 % of the less material, the molar consumption of triethylamine is 10-200 % of the less material, the molar ratio between the reactant and organic solvent is 0.02-1, the reaction period is 2-20 hr, reaction temperature is 50-200 deg.c, and CO pressure is 1-10 MPa gauge pressure. The present invention has simple operation, high selectivity, high yield, stable product quality and easy post separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
3-oxa acridone, derivatives thereof and preparation
InactiveCN101440095ALow temperature requirementLess investmentOrganic chemistryQuinolineStructural formula
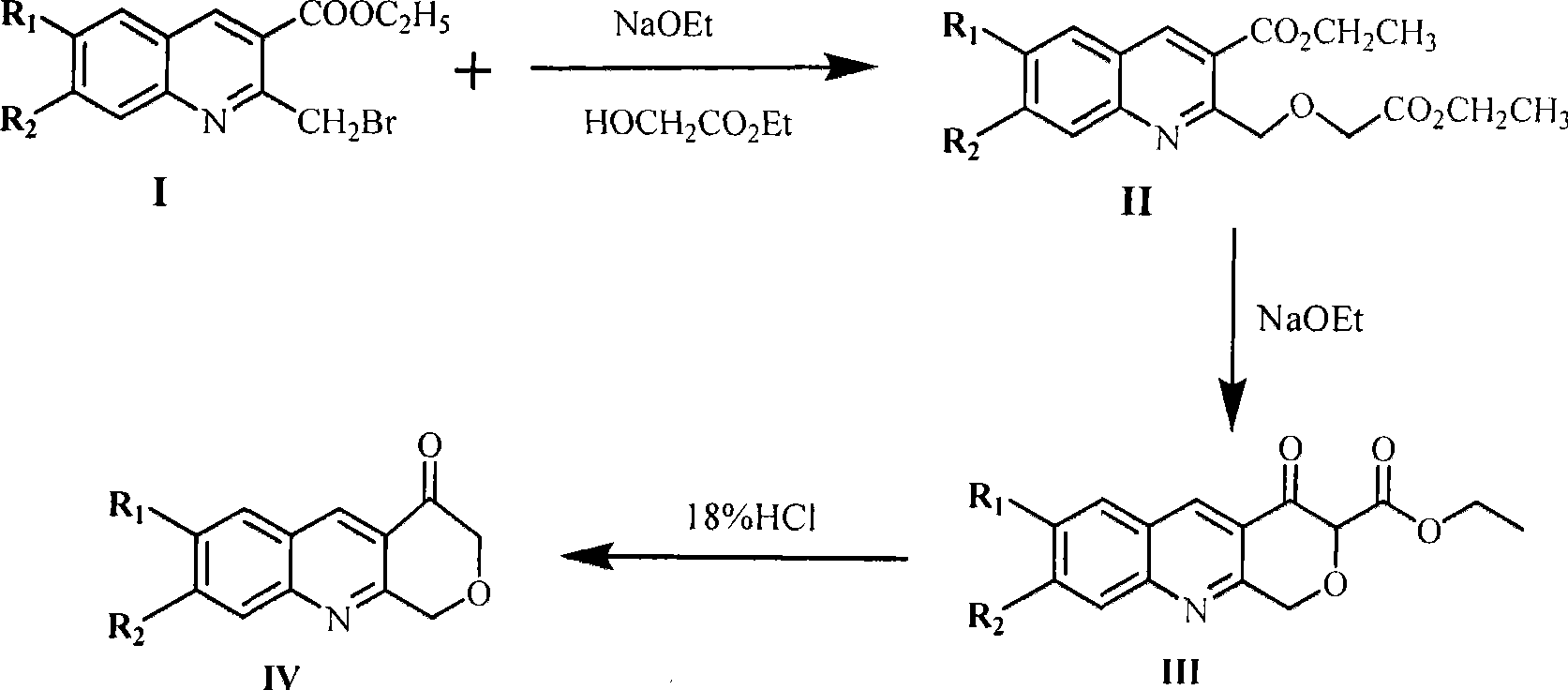
The invention provides 3-oxa-acridone, derivatives and a preparation method thereof. The 3-oxa-acridone and the derivatives thereof have the following structural formula: in the formula, A is the 3-oxa-acridone; and B is the derivatives of the 3-oxa-acridone. The reaction process of the method for preparing the 3-oxa-acridone and the derivatives thereof is shown in a figure, wherein R1 and R2 can respectively represent hydrogen or methylenedioxy. The 3-oxa-acridone, the derivatives and the preparation method thereof have the advantages of normal pressure reaction, low requirement on temperature, small equipment investment, simple and safe operation, low cost, simple raw materials, easy synthesis, low process difficulty, convenient operation, easy product separation, and can obtain a pure product only by column chromatography separation or recrystallization. The invention synthesizes a novel 3-oxa-acridone matrix ring containing a quinoline skeleton with novel structure but without a substituent and derivatives thereof, and can be applied to synthesis of antibiosis drugs, antiphlogosis drugs, anti-tumor drugs and the like, thereby providing a matrix compound to synthesize other novel compounds.
Owner:BOHAI UNIV
Method for synthesizing aliphatic amine compound
InactiveCN102070458ALess investmentSimple and safe operationPreparation by reductive alkylationHigh energyKetone
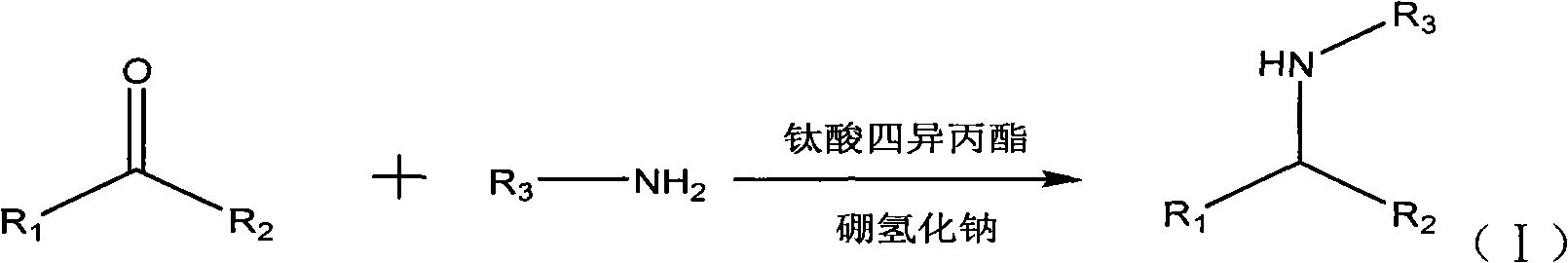
The invention discloses a method for synthesizing an aliphatic amine compound, which belongs to the field of synthesis of aliphatic compounds. In the technical scheme provided by the invention, aliphatic ketone and ammonia, or the aliphatic ketone and low-grade primary amine are used as raw materials; tetraisopropyl titanate is used as a catalyst; sodium borohydride is used as a reducing agent; one or a mixture of more than two of absolute ethanol, absolute methanol and isopropyl alcohol is used as a reaction solvent; and the aliphatic amine compound is synthesized by a one-step method. According to the technical scheme, a normal temperature and pressure reaction is adopted; the method is easily, conveniently and safely operated and low in cost, has simple and readily available raw materials, and is environmentally-friendly, and the discharge of three wastes (waste water, waste gas and industrial residues) is reduced; a reaction process has low difficulty, and the subsequent separation of a product is easy to operate; the method has the advantages of a small number of side reactions and high yield; the problems that the reaction is required to be performed at a high temperature under high pressure in the presence of a metal catalyst and has rigorous conditions, high danger, high energy consumption and high cost in the conventional industrial aliphatic amine production process are solved; and the method is suitable for large-scale industrial production.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
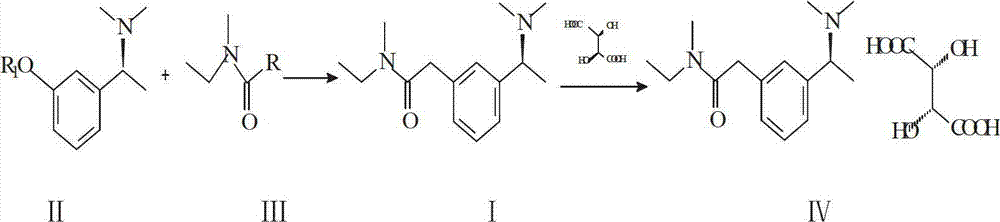
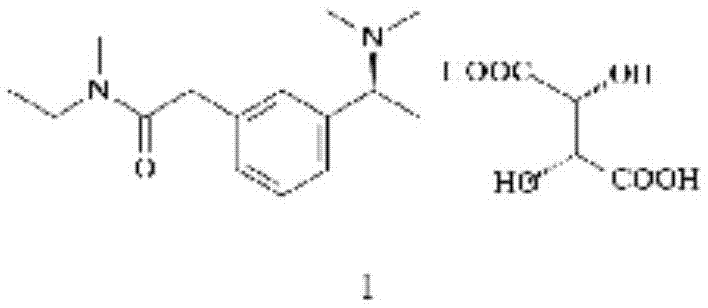
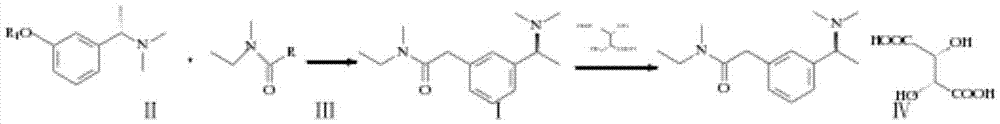
Preparation method of rivastigmine tartrate
InactiveCN102898333ALess investmentSimple and safe operationCarbamic acid derivatives preparationOrganic compound preparationSolventReagent
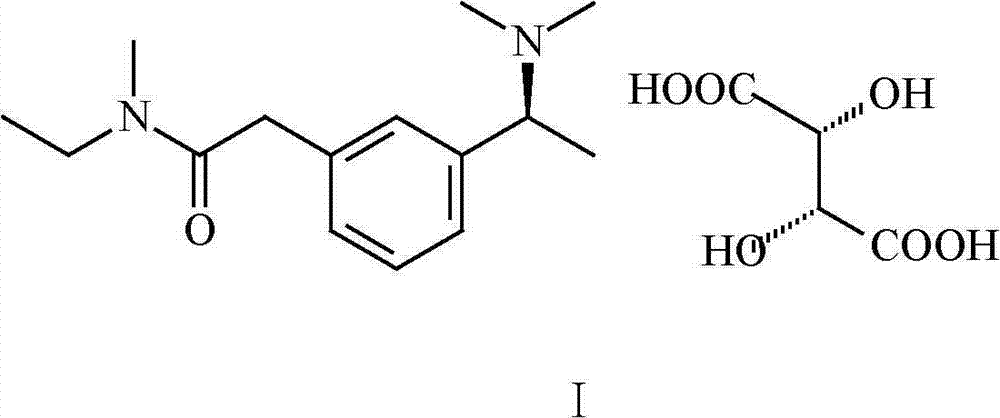
The invention relates to a preparation method of rivastigmine tartrate, which comprises the following steps of: carrying out condensation on compound II and compound III in proper solvent with catalyst or without catalyst to obtain component I; and salifying the compound I and L-(+)-tartaric acid in proper solvent to obtain the rivastigmine tartrate, wherein R1 is H, K or Na, and R is Cl, Br, I, CN or SCN. The method has the advantages of being short in process route, mild in reaction, easy in recovery of reagent, high in yield and the like, thus being suitable for industrial production.
Owner:哈药集团人民同泰医药股份有限公司
Synthesis process of benzoyl substituted carbamide compound
The synthesis process of benzoyl substituted carbamide compound is the reaction of benzamide and aryl nitro compound in organic solvent inside a sealed high pressure reactor in the presence of CO, Seas catalyst and triethylamine as cocatalyst. Where, the substitutent group of the phenyl group on the aryl nitro compound may be one or several electron donating group and / or electron withdrawing group; the molar ratio between benzamide and aryl nitro compound is 0.1-10; molar amount of Se is 0.1-20% of the minor reactant; molar amount of triethylamine is 10-200% of the minor reactant; the molar ratio between reactant and organic solvent is 0.021-1; the reactio period is 2-20 hr; the reaction temperature is 50-200 deg.C; and the CO pressure is 1-10 MPa gage pressure.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing rivastigmine hydrogen tartrate
InactiveCN105439906ALess investmentSimple and safe operationCarbamic acid derivatives preparationOrganic compound preparationSolventRivastigmine hydrogen tartrate
The invention provides a method for synthesizing rivastigmine hydrogen tartrate. The method includes the following steps that firstly, a compound II and a compound III are condensed into a compound I in a proper solvent without a catalyst or with a catalyst, and the compound I and L-(+)tartaric acid are salified to obtain rivastigmine tartrate in a proper solvent. The molecular formula of rivastigmine tartrate is shown in the specification, wherein R1 is H, K or Na, and R is Cl, Br, I, CN or SCN. The method has the advantages that a process route is short, reaction is moderate, reagents which are used are easy to recycle and the yield is high, and is suitable for industrial production.
Owner:HARBIN PHARM GROUP SANJING PHARMACEUTICAL CO LTD
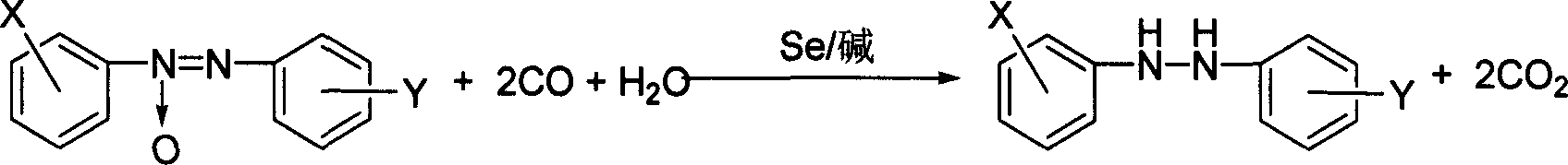
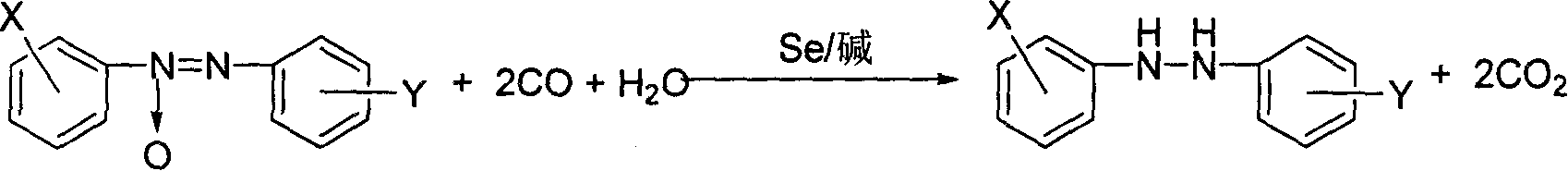
Method of synthesizing hydrodiazo kind compound
InactiveCN1295209CLess investmentSimple and safe operationOrganic chemistryPtru catalystOrganic base
The invention provides a process for synthesizing azocompound hydrogenated which comprises, reacting aromatic azocompound in organic solvent under normal pressure at the presence of carbon monoxide and water, using selenium as catalyst, organic bases or inorganic bases as catalyst promoter, synthesizing azocompound hydrogenated in one step, wherein the mol ratio of the aromatic azocompound and water is 1:1-20, the mol amount of selenium is 0.1-8% of the aromatic azocompound, the mol amount of organic base or inorganic base is 0-10% of the aromatic azocompound, the reacting time is 0.5-3 hours, the reaction temperature is 30-100 deg. C.
Owner:LIAONING UNIVERSITY
A kind of method for synthesizing 1-aminoanthraquinone
ActiveCN103113245BLess investmentSimple and safe operationOrganic chemistryOrganic compound preparationAnthraquinonesPtru catalyst
Owner:LIAONING UNIVERSITY
Method for synthesizing symmetric urea compounds from nitrocompounds
ActiveCN103113265BLess investmentSimple and safe operationUrea derivatives preparationOrganic compound preparationNitro compoundPtru catalyst
Owner:LIAONING UNIVERSITY
Rivastigmine precursor [1-(3-methoxyphenyl)ethyl]dimethylamine preparation method
InactiveCN103896787ALess investmentSimple and safe operationOrganic compound preparationAmino-hyroxy compound preparationHydrogenTetraisopropyl titanate
The invention relates to a rivastigmine precursor [1-(3-methoxyphenyl)ethyl]dimethylamine preparation method, which is characterized by comprising that: a dimethylamine hydrochloride reacts with an alkali to produce dimethylamine, the dimethylamine reacts with m-methoxyacetophenone for 5-24 h at a reaction temperature of 0-50 DEG C in the presence of titanium tetraisopropanolate, hydrogen gas is introduced in the presence of a catalyst, a reaction is performed for 12-72 h at a reaction temperature of 20-100 DEG C to prepare the [1-(3-methoxyphenyl)ethyl]dimethylamine. According to the present invention, the preparation method is the normal pressure reaction, and has characteristics of mild reaction conditions, low equipment investment, easy and safe operation, environmental pollution, less pollution and the like, and is easily subjected to large-scale industrial production.
Owner:JIANGSU KANGBEIDE PHARMA
Ether amine flotation agent synthesis method
InactiveCN104829469ALess investmentSimple and safe operationOrganic compound preparationAmino-hyroxy compound preparationEtherFatty alcohol
The invention discloses an ether amine flotation agent synthesis method. The method comprises that under normal pressure, fatty alcohol metal salt and halogenated alkylamine undergo a full reaction in a solvent. The method has the advantages of normal pressure reaction, less equipment investment, simple and safe operation, low cost, simple and easily available raw materials, no catalyst, use of fatty alcohol as a solvent, less by-product, low reaction technology difficulty, mild reaction condition, simple processes, high yield and easy separation and purification of products.
Owner:广东省石油化工研究院
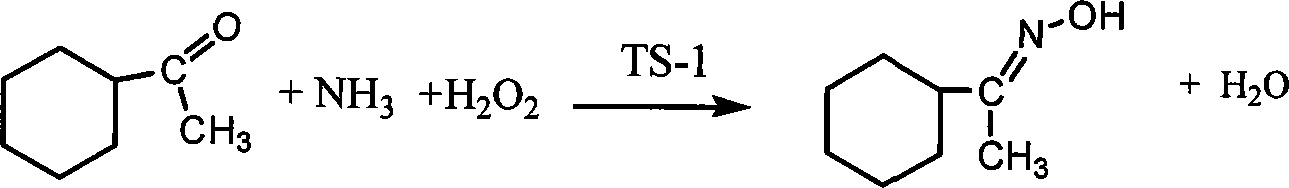
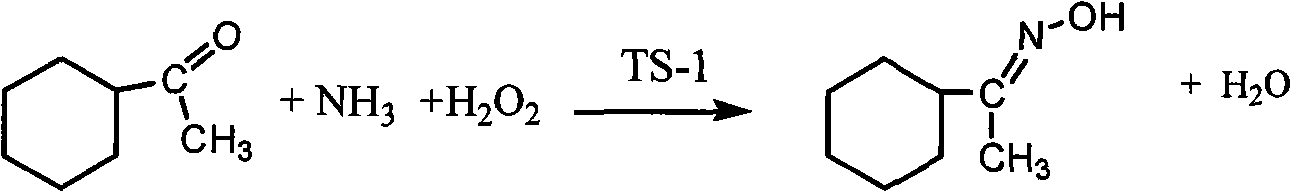
Method for synthesizing 1-alkyl-2-alkoxyl-3-indole aldoxime derivant
InactiveCN101270071BLow temperature requirementLess investmentOrganic chemistryFiltrationFormylation reaction
The present invention relates to a method used for synthesizing derivatives of 1-alkyl-2-alkoxy-3-indole aldehyde oxime. Indole-one is used as basic material and treated through Vilsmeier-Hacck formylation reaction and alkylation reaction to prepare 1-alkyl-2-chlorine-3-indole aldehyde; under the conditions with potassium hydroxide and fatty alcohol and at the temperature of 50 to 80 DEG C, the reaction is monitored by a thin chromatogram TLC; after the reaction is completed, no treatment is required and hydroxylamine hydrochloride is directly added into the mixture for oximation reaction; the reaction temperature is maintained; the reaction lasts for 1 to 2 hours; the ratio of the 1-alkyl-2-chlorine-3-indole aldehyde, the hydroxylamine hydrochloride, the potassium hydroxide, the fatty alcohol and water is equal to 1mmol to 2mmol to 2mmol to from 5ml to 8 ml to 1ml; finally water is added for sedimentation, and the novel compounds of the category of the 1-alkyl-2-chlorine-3-indole aldehyde can be separated through filtration and chromatography. The method has the advantages of mild reaction conditions, process under atmospheric pressure, simple operation, less investment in the equipment, low production costs, less pollution and high yield.
Owner:BOHAI UNIV
A kind of preparation technology of piperazine citrate
ActiveCN103159700BNo pollution in the processSimple processOrganic chemistryPiperazine CitrateSolvent
The present invention relates to a piperazine citrate preparation method, and further provides a piperazine citrate with characteristics of safe quality and good stability. The preparation method comprises that: citric acid and anhydrous piperazine are completely dissolved and filtered, and form a salt in a reaction kettle under an appropriate reaction condition, and cooling crystallization, centrifugation, washing and vacuum drying are performed after the complete reaction to obtain the product. The preparation process has characteristics of simpleness, easy operation, extremely small three-waste generation, high yield, low cost, high product purity, and easy industrial production, wherein the yield is up to more than 90%. In the prior art, the piperazine citrate preparation process comprises adopting citric acid and piperazine hexahydrate as raw materials, adopting water as a solvent, and carrying out dissolving, salt forming, alcohol precipitation, centrifugation and drying, and has disadvantages of complex process, large wastewater generation and low yield. With the present invention, the disadvantages in the prior art are overcome.
Owner:KAMP PHARMA
Method for synthesizing CLT acid
ActiveCN102675162BLess investmentSimple and safe operationChemical recyclingSulfonic acid preparationPtru catalystOrganic base
The invention relates to a method for synthesizing CLT acid, which has the technical scheme that 2-nitro-4-methyl-5-chlorobenzenesulfonic acid is taken as raw material; under the condition that carbon monoxide and water exist, selenium is taken as catalyst, inorganic base or organic base is taken as catalyst promoter; and reaction is carried out at high temperature and high pressure, so that the CLT acid can be synthesized by a one-step method. The method is simple, convenient and safe, realizes aqueous phase reaction, and is easy in obtaining of raw material, free from pollution, high in selectivity and high in yield; and the catalyst can be recycled after the reaction.
Owner:LIAONING UNIVERSITY
Method for synthesizing hydrogenated azo compounds
The present invention is the synthesis process of hydrogenating aromatic azo compound into hydrogenated azo compounds at normal pressure inside organic solvent in the presence of CO and water as well as Se as catalyst with or without organic or inorganic alkali as co-catalyst. Where, the aromatic azo compound may have substituted radical, and the reaction has molar ratio between aromatic azo compound and water of 1 to 1-20, molar ratio between aromatic azo compound and CO of 1 to 1.1-3, molar amount of Se in 0.1-8 % of aromatic azo compound, reaction time of 0.5-3 hr, and reaction time of 30-100 deg c. The synthesis process is simple and safe, and has easy to obtain material, no pollution, high selectivity, no influence on the sensitive radicals on the aromatic ring, high yield and reusability of the catalyst.
Owner:LIAONING UNIVERSITY
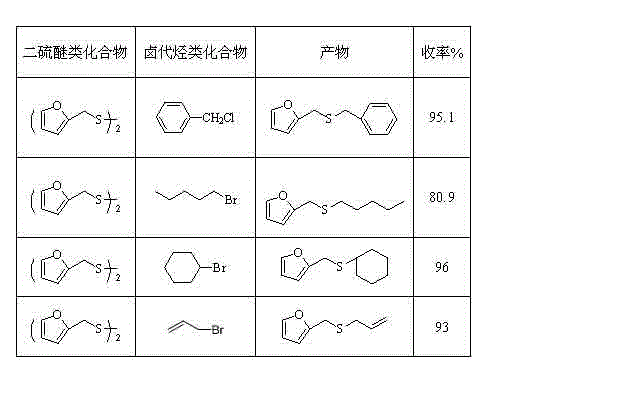
Method for synthetizing mono-thioether compound
InactiveCN102127038BLess investmentSimple and safe operationSulfide preparationHalohydrocarbonPtru catalyst
The invention relates to a method for synthetizing mono-thioether compound. The technical scheme adopted by the invention is as follows: in the presence of carbon monoxide and water, disulfide compound and halogenated hydrocarbon compound are used as raw materials, selenium is used as catalyst, organic base or inorganic base is used as cocatalyst or any cocatalyst is not added, the raw materials react in organic solvent at 20-100 DEG C under atmospheric pressure for 1-24 hours, the product is cooled to the room temperature, then carbon monoxide is displaced by air, stirring is performed for 0.2-2 hours, filtration is performed, distilled water and cyclohexane are used to extract the filtrate, and the solvent in the extract liquor is distilled out through reduced pressure distillation to obtain the target product. The method is convenient and safe to operate, adopts one-pot reaction, has common raw materials, no pollution, high selectivity and high yield; and the catalyst can be separated and recycled after the reaction.
Owner:LIAONING UNIVERSITY
Method for synthesizing disulfide compound
InactiveCN101613247ALess investmentSimple and safe operationOrganic-compounds/hydrides/coordination-complexes catalystsFunctional group formation/introductionOrganic baseOxygen
The invention relates to a method for synthesizing disulfide compound. The invention adopts the technical scheme as follows: using halogenated hydrocarbon and sulphur as materials, selenium as the catalyst, organic base or inorganic base as the auxiliary catalyst or without any auxiliary catalysts, leading in carbon monoxide gas into organic solvent, reacting for 1-24 hours at 20-100 DEG C under normal pressure with the presence of water, cooling to a room temperature, switching the carbon monoxide gas into air or oxygen to separate out unreacted selenium, filtering, collecting the filtrate, then adding water with a volume of 2-3 times of the filtrate, and separating out the product to obtain the disulfide compound. The method is realized under normal-pressure reaction, has little device investment, convenient and safe operation, low cost, environmental protection, low difficulty of the reaction process and favorable economical property, and has phase shift function.
Owner:LIAONING UNIVERSITY
Method for synthesizing hydrogenated azo compounds
The present invention is the synthesis process of hydrogenating aromatic azo compound into hydrogenated azo compounds at normal pressure inside organic solvent in the presence of CO and water as well as Se as catalyst with or without organic or inorganic alkali as co-catalyst. Where, the aromatic azo compound may have substituted radical, and the reaction has molar ratio between aromatic azo compound and water of 1 to 1-20, molar ratio between aromatic azo compound and CO of 1 to 1.1-3, molar amount of Se in 0.1-8 % of aromatic azo compound, reaction time of 0.5-3 hr, and reaction time of 30-100 deg c. The synthesis process is simple and safe, and has easy to obtain material, no pollution, high selectivity, no influence on the sensitive radicals on the aromatic ring, high yield and reusability of the catalyst.
Owner:LIAONING UNIVERSITY
A method for synthesizing 1-aminoanthraquinone
ActiveCN105085286BLess investmentSimple and safe operationOrganic chemistryOrganic compound preparationSulfurHigh pressure
The invention relates to a method for synthesizing 1-aminoanthraquinone. The method includes the following steps that 1-aminoanthraquinone is used as a raw material, in the existence of carbon monoxide and water, sulfur is used as a catalyst, inorganic alkali or organic alkali is used as a promoter, a reaction is carried out under the high-temperature and high-pressure condition, and accordingly the 1-aminoanthraquinone is synthesized at one step. Compared with a selenium-catalyzed reduction method, the sulfur-catalyzed reduction method is lower in cost, higher in productive rate and capable of greatly reducing water consumption.
Owner:YANCHENG OUHUA CHEM IND +1
Synthesis process of benzoyl substituted carbamide compound
The synthesis process of benzoyl substituted carbamide compound is the reaction of benzamide and aryl nitro compound in organic solvent inside a sealed high pressure reactor in the presence of CO, Se as catalyst and triethylamine as cocatalyst. Where, the substitutent group of the phenyl group on the aryl nitro compound may be one or several electron donating group and / or electron withdrawing group; the molar ratio between benzamide and aryl nitro compound is 0.1-10; molar amount of Se is 0.1-20% of the minor reactant; molar amount of triethylamine is 10-200% of the minor reactant; the molar ratio between reactant and organic solvent is 0.021-1; the reactio period is 2-20 hr; the reaction temperature is 50-200 deg.C; and the CO pressure is 1-10 MPa gage pressure.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing 1-aminoanthraquinone
The invention relates to a method for synthesizing 1-aminoanthraquinone. The method includes the following steps that 1-aminoanthraquinone is used as a raw material, in the existence of carbon monoxide and water, sulfur is used as a catalyst, inorganic alkali or organic alkali is used as a promoter, a reaction is carried out under the high-temperature and high-pressure condition, and accordingly the 1-aminoanthraquinone is synthesized at one step. Compared with a selenium-catalyzed reduction method, the sulfur-catalyzed reduction method is lower in cost, higher in productive rate and capable of greatly reducing water consumption.
Owner:YANCHENG OUHUA CHEM IND +1
Popular searches
Raw materials are easy to get Eliminate separation and purification Cost advantage Good repeatability Easy to separate The difficulty of the reaction process is low Simple and fast operation Reduce the burden on Meet the requirements of cleaner production Conducive to large-scale industrial production
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
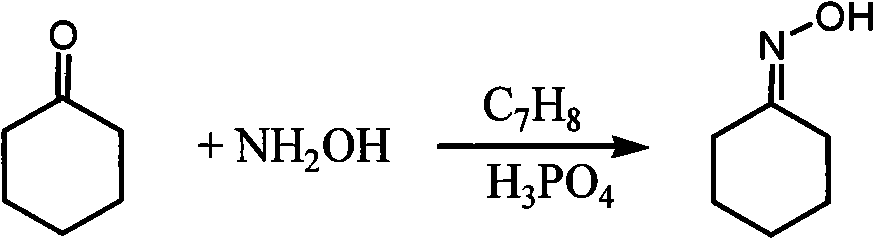





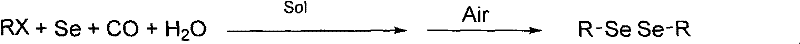




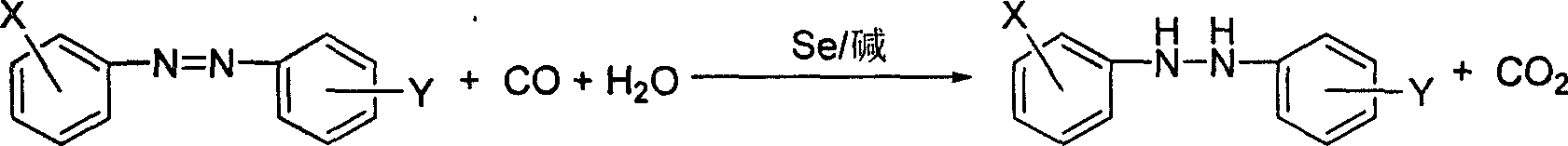

![Rivastigmine precursor [1-(3-methoxyphenyl)ethyl]dimethylamine preparation method Rivastigmine precursor [1-(3-methoxyphenyl)ethyl]dimethylamine preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34b2c63f-fc61-4bbd-91de-1e952b8f2a64/BSA00000829974800031.PNG)