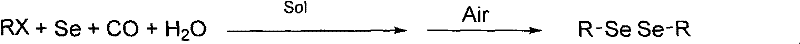Diselenide compound synthesis method
A synthesis method and technology of diselenide, applied in the direction of organic chemistry and the like, can solve the problems of complex operation, low yield, many steps, etc., and achieve the effects of high reaction selectivity, low equipment investment and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 12.7g (0.1mol) of benzyl chloride, 4g (0.05mol) of selenium powder, 18mL (1mol) of water, 4g (0.05mol) of sodium acetate and 400mL of solvent DMF into a 1000mL three-necked flask equipped with a stirring bar and a condenser , continue to feed pure carbon monoxide gas, then heat to 95 ° C and stir for 2 hours, after cooling to room temperature, switch carbon monoxide to air or oxygen, and stir for 0.5 to 1 hour. At this time, unreacted selenium is precipitated. Filter and collect the filtrate. Add water of 3 times the volume of the filtrate to the filtrate, at this time the product dissolved in the filtrate is precipitated, filtered, washed with water, and dried in vacuum to obtain the target product. The product has a purity of 100% and a yield of 98% as measured by proton nuclear magnetic resonance spectroscopy. (as dibenzyl diselenide).
Embodiment 2
[0023] Only change the raw material of halogenated hydrocarbon, experimental method and other conditions are the same as embodiment 1.
[0024] Halogenated hydrocarbons
Embodiment 3
[0026] Reaction temperature, selenium amount, reaction time are as follows, and experimental method and other conditions are with embodiment 1, and yield is as follows:
[0027] Selenium
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com