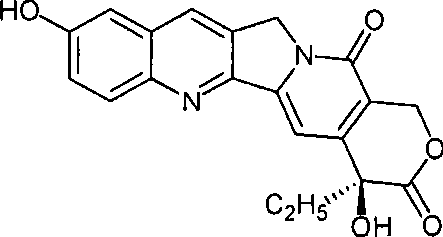
3-oxa acridone, derivatives thereof and preparation
A technology for oxacridone and derivatives, which is applied in the field of acridone derivatives, can solve the problems such as the unsubstituent parent ring structure that has not yet been found, and achieves the effects of low cost, simple operation and low equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
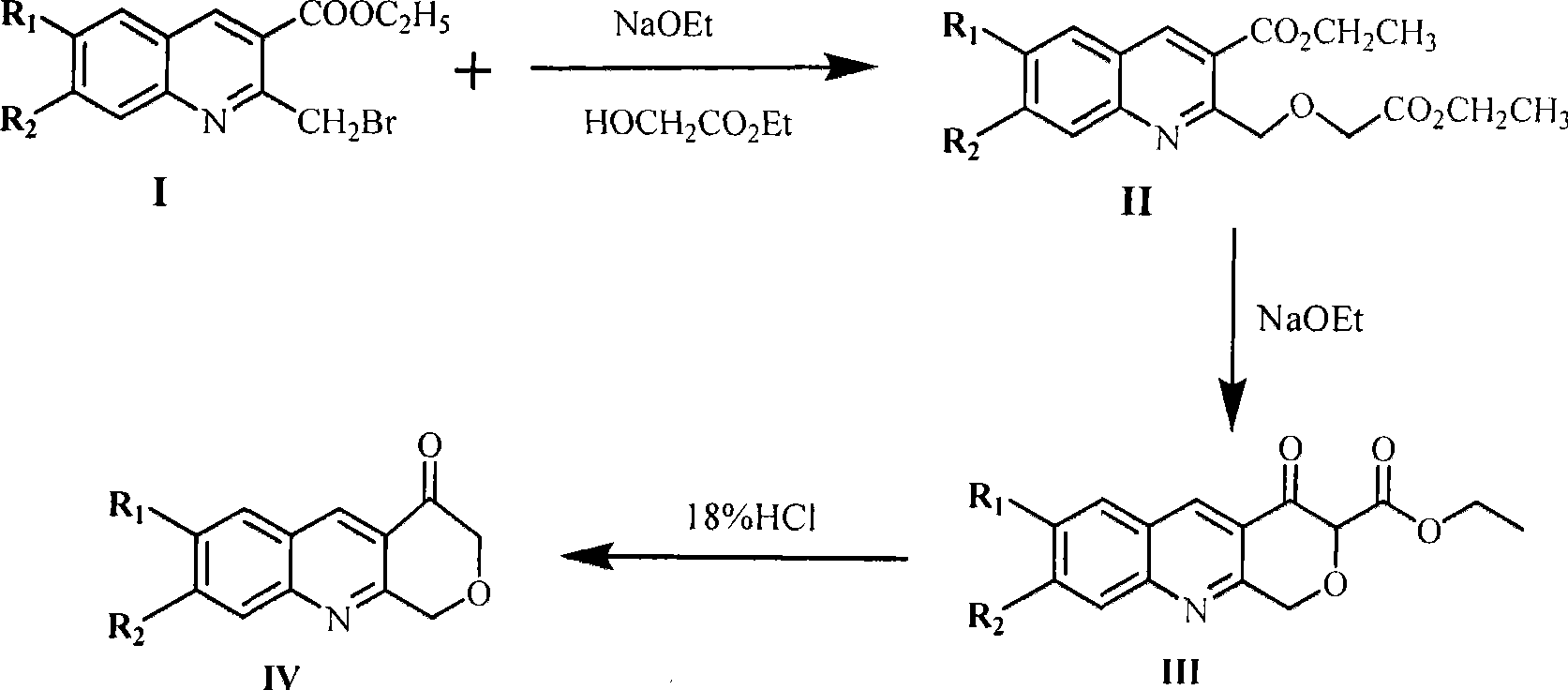
[0029] Add 0.023g (1mmol) sodium metal to 3mL of absolute ethanol, after it is completely dissolved, add 0.156g (1.5mmol) ethyl glycolate and react at 60°C for 5h, spin dry the white solid of the solvent, add dissolved 0.294g (1mmol) ethyl 2-bromomethyl-3-quinolinic acid in 2mL of dry benzene, react at 50°C for 12h, after the reaction is completed, cool to room temperature and add 3mL of cold water to decompose the excess sodium salt, separate the benzene layers The aqueous layer was extracted 4 times with ether, the benzene layer and the organic layer were combined and dried over anhydrous sodium sulfate, and then separated by column chromatography to obtain 0.254 g of diethyl o-carboxyquinoline methoxyacetate, with a yield of 80.1%. The melting point is 56.9-57.1°C.
[0030] Add 0.035g (1.5mmol) sodium metal to 8mL of absolute ethanol, spin the solvent after it is completely dissolved, add 2mL of dry toluene to it and raise the temperature to 120°C, then add 0.317g (1mmol) o...
Embodiment 2
[0033] Add 0.035g (1.5mmol) sodium metal to 6mL of absolute ethanol, after it is completely dissolved, add 0.321g (3mmol) ethyl glycolate and react at 40°C for 6h, spin dry the white solid of the solvent, add dissolved 0.294g (1mmol) ethyl 2-bromomethyl-3-quinolinic acid in 5mL of dry benzene, react at 60°C for 6h, after the reaction is completed, cool to room temperature and add 8mL of cold water to decompose the excess sodium salt, separate the benzene layers The aqueous layer was extracted 6 times with ether, the benzene layer and the organic layer were combined and dried with anhydrous sodium sulfate, and then separated by column chromatography to obtain 0.201 g of diethyl o-carboxyquinoline methoxyacetate, with a yield of 63.5%. The melting point is 56.7-57.1°C.
[0034] Add 0.023g (1mmol) sodium metal to 3mL of absolute ethanol, spin dry the solvent after it is completely dissolved, add 5mL of dry toluene to it and raise the temperature to 140°C, then add 0.317g (1mmol) ...
Embodiment 3
[0037] Add 0.046g (2mmol) sodium metal to 10mL of absolute ethanol, after it is completely dissolved, add 0.312g (4mmol) ethyl glycolate and react at 80°C for 4h, spin dry the white solid of the solvent, add 4mL of Dry 0.294g (1mmol) of ethyl 2-bromomethyl-3-quinolinic acid in benzene, react at 100°C for 6h, after the reaction is completed, cool to room temperature and add 6mL of cold water to decompose the excess sodium salt, and separate the benzene layer , the aqueous layer was extracted 3 times with ether, the benzene layer and the organic layer were combined and dried over anhydrous sodium sulfate, and then separated by column chromatography to obtain 0.241g of diethyl o-carboxyquinoline methoxyacetate, the yield was 76.1%, and the melting point 56.9~57.5℃.
[0038] Add 0.069g (3mmol) sodium metal to 12mL of absolute ethanol, spin the solvent after it is completely dissolved, add 3mL of dry toluene to it and raise the temperature to 90°C, then add 0.317g (1mmol) of o-carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com