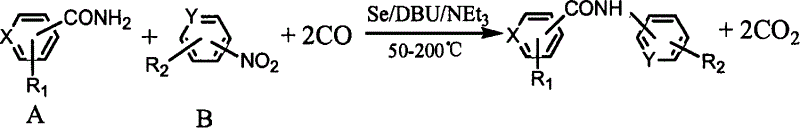
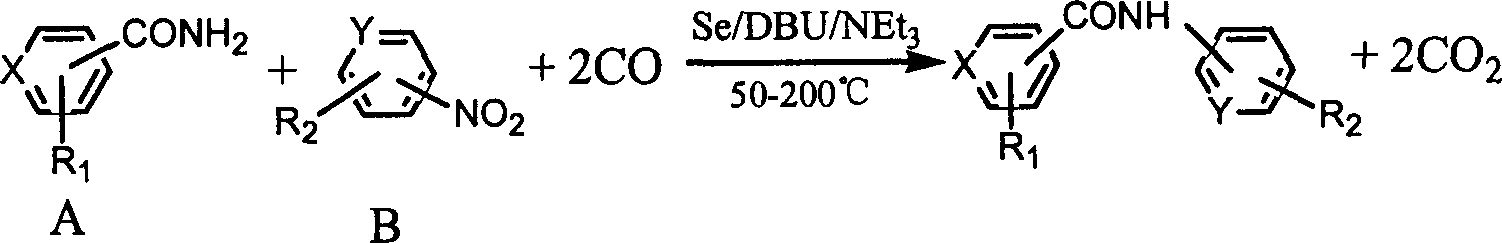
Process for synthesizing aryl substituted N-aryl amide compounds
A compound and arylamide technology, which is applied in the field of preparation of N-aryl substituted arylamide compounds, can solve the problems of difficult aryl substitution to synthesize N-aryl substituted arylamide compounds, etc., and achieves high reaction selectivity and low cost. Low, economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 100mL stainless steel autoclave, add benzamide (15mmol), Se (0.5mmol), nitrobenzene (10mmol), DBU (10mmol), Et 3 N (10mmol) and toluene (10ml), after replacing three times with CO, raise the CO pressure to 3MPa, put it into an oil bath that has risen to 150°C and stir for 4 hours, cool to room temperature, open the kettle, and filter The resulting solid and mother liquor concentrate were separated and purified by column chromatography, the eluent was petroleum ether: ethyl acetate (7:3), the product was obtained by concentrating and removing the eluent, the product was N-phenylbenzamide, HPLC The analytical purity is above 99%, and the yield is 90.1%. The content determination adopts Waters high-performance liquid chromatography system, including two 515 pumps, 486 type UV detector, Spherisorb ODS-2 column (5μm, 4.6×250mm), using methanol-water as mobile phase, flow rate: 1mL / min, detection The wavelength of the detector is λmax of each compound, column temperatur...
Embodiment 2
[0018] The organic solvent is benzene, and the consumption is 10ml. Other experimental methods and conditions are the same as in Example 1. The HPLC analysis purity is more than 99%, and the actual yield is 75.4% (in terms of nitrobenzene).
Embodiment 3
[0020] The organic solvent was tetrahydrofuran, and the consumption was 10 ml. Other experimental methods and conditions were the same as in Example 1. The HPLC analysis purity was more than 99%, and the actual yield was 61.6% (in terms of nitrobenzene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com