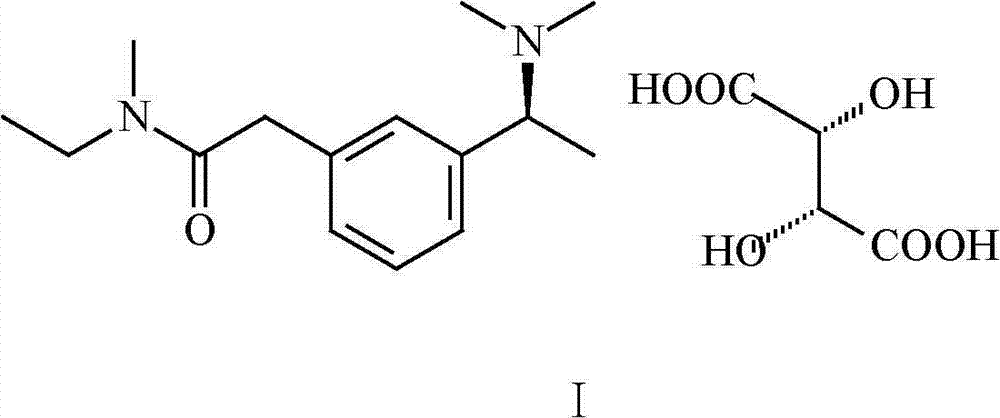
Preparation method of rivastigmine tartrate
A technology of rivastigmine bitartrate and tartaric acid, which is applied in the field of preparation of high-purity rivastigmine bitartrate, can solve the problems of high synthesis cost and high market price of rivastigmine bitartrate, and is beneficial to large-scale industrial production and eliminates the three wastes. Effects of reduced processing burden, easy separation, recovery, and recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
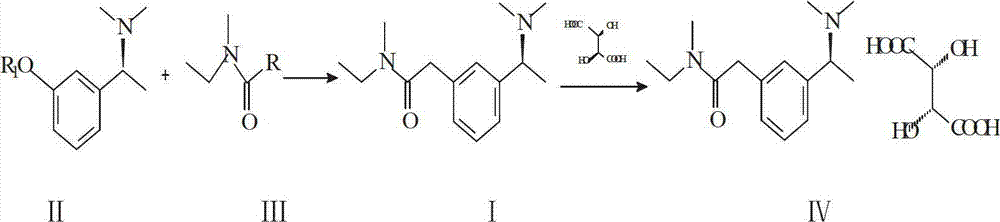
[0016] (1) Add (S)-3-(1-(dimethylamino)ethyl)phenol (19.8g, 0.12mol) and 250ml pyridine into a three-necked flask, and add methylethylcarbamoyl chloride (15.9g, 0.13mol) , reflux under reduced pressure for 3 hours, vacuum degree control 0.06MPa-0.08MPa, temperature 60°C-75°C, after recovering pyridine, add 100ml isopropyl ether for extraction, wash with 0.1mol / L NaOH solution, wash with water, dry over anhydrous magnesium sulfate, reduce The iso-ether was removed by pressure evaporation, and after vacuum drying, 29.2 g of rivastigmine free base was obtained as a yellow liquid, with a yield of 97.5%.
[0017] (2) Dissolve the product obtained in 1 in 180ml of 1.2-propanediol, add L-(+)-tartaric acid (18g, 0.12mol), heat and stir at 90°C, after the reaction liquid is clear, cool down to room temperature naturally under stirring, and precipitate The product was suction filtered and dried to obtain 42.5 g of rivastigmine bitartrate finished product, with a yield of 91%.
Embodiment 2
[0019] (1) Add (S)-3-(1-(dimethylamino)ethyl)phenol (19.8g, 0.12mol) and acetone 130ml into a three-necked flask, add 4.5g of anhydrous sodium carbonate to activate for a certain period of time, add Methylethylcarbamoyl chloride (15.9g, 0.13mol), react for 8 hours, after recovery of acetone, add 110ml of dichloromethane and 150ml of water, adjust the pH to 3 with hydrochloric acid, extract, discard the organic phase, add 150ml of dichloromethane to the water layer , add concentrated ammonia water to adjust the pH to 10, separate the aqueous layer, wash the organic phase with 100ml of water, separate the dichloromethane layer and evaporate the solvent under reduced pressure to obtain 29.3 g of rivastigmine free base as a yellow liquid with a yield of 97.6%.
[0020] (2) Dissolve the product obtained in 1 in 200ml of isopropanol, add L-(+)-tartaric acid (18g, 0.12mol), and heat to reflux. After the reaction solution is clear, it is cooled to room temperature naturally after stirr...
Embodiment 3
[0022] (1) Add (S)-3-(1-(dimethylamino)ethyl)potassium phenate (19.8g, 0.12mol) and 130ml of tetrahydrofuran into a three-necked flask, add methylethylcarbamoyl bromide (15.9g, 0.13 mol), reacted at 50°C for 1 hour, recovered the solvent, added 110ml of ethyl acetate and 150ml of water, adjusted the pH to 3 with hydrochloric acid, extracted, discarded the organic phase, added 150ml of ethyl acetate to the aqueous layer, and added concentrated ammonia water to adjust the pH to 10. Separate the water layer, wash the organic phase with 100 ml of water, separate the ethyl acetate layer and evaporate the solvent under reduced pressure to obtain 28.9 g of rivastigmine free base as a yellow liquid, with a yield of 96.3%.
[0023] (2) Dissolve the product obtained in 1 in 200ml of isopropanol, add L-(+)-tartaric acid (18g, 0.12mol), and heat to reflux. After the reaction solution is clear, it is cooled to room temperature naturally after stirring, and the weight is obtained after separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com