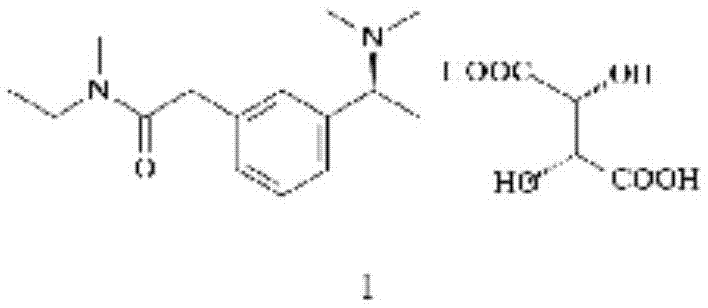
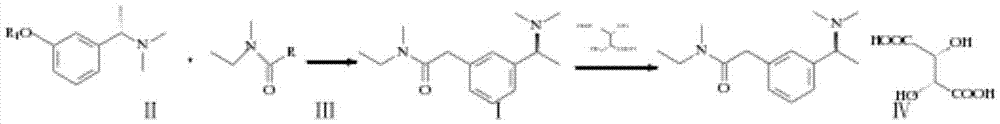
Method for synthesizing rivastigmine hydrogen tartrate
A technology of rivastigmine bitartrate and heavy tartaric acid, which is applied in the field of preparation of high-purity rivastigmine bitartrate, can solve the problems of high synthesis cost and high market price of rivastigmine bitartrate, and is beneficial to large-scale industrial production, The effect of reducing the burden of three wastes treatment and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Add 3-(1-(dimethylamino)ethyl)phenol (19.8g, 0.12mol) and 250ml pyridine in a three-necked flask, add methylethylcarbamoyl chloride (15.9g, 0.13mol), and reflux under reduced pressure 3h, vacuum control 0.06MPa-0.08MPa, temperature 60°C-75°C, after recovering pyridine, add 100ml of isopropyl ether for extraction, wash with 0.1mol / L NaOH solution, wash with water, dry over anhydrous magnesium sulfate, evaporate under reduced pressure to remove isopropyl ether Combined with ether and dried in vacuo, 29.2 g of rivastigmine free base was obtained as a yellow liquid with a yield of 97.5%.
[0016] (2) Dissolve the product obtained in 1 in 180ml of 1.2-propylene glycol, add L-(+) tartaric acid (18g, 0.12mol), heat and stir at 90°C, and after the reaction solution is clear, naturally cool down to room temperature under stirring, and the product precipitates , suction filtration, and dried to obtain 42.5 g of rivastigmine bitartrate finished product, with a yield of 91%.
Embodiment 2
[0018] (1) Add 3-(1-(dimethylamino)ethyl)phenol (19.8g, 0.12mol) and 130ml of acetone into a three-necked flask, add 4.5g of anhydrous sodium carbonate to activate for a certain period of time, add methylethylcarbamoyl chloride (15.9g, 0.13mol), react for 8 hours, after recovering acetone, add 110ml of dichloromethane and 150ml of water, adjust the pH to 3 with hydrochloric acid, extract, discard the organic phase, add 150ml of dichloromethane to the water layer, and add concentrated ammonia Adjust the pH to 10, separate the water layer, wash the organic phase with 100 mi of water, separate the dichloromethane layer and evaporate the solvent under reduced pressure to obtain 29.3 g of rivastigmine free base as a yellow liquid with a yield of 97.6%.
[0019] (2) Dissolve the product obtained in 1 in 200ml of isopropanol, add L-(+) tartaric acid (18g, 0.12mol), and heat to reflux. After the reaction solution is clear, it is naturally cooled to room temperature while stirring, and ...
Embodiment 3
[0021] (1) Add 3-(1-(dimethylamino)ethyl)potassium phenate (19.8g, 0.12mol) and 130ml of tetrahydrofuran in a three-necked flask, add methylethylcarbamoyl bromide (15.9g, 0.13mol), 50 React at ℃ for 1 hour, after recovering the solvent, add 110ml of ethyl acetate and 150ml of water, adjust the pH to 3 with hydrochloric acid, extract, discard the organic phase, add 150ml of ethyl acetate to the aqueous layer, add concentrated ammonia water to adjust the pH to 10, and separate the water layer, washed the organic phase with 100 ml of water, separated the ethyl acetate layer and evaporated the solvent to dryness under reduced pressure to obtain 28.9 g of rivastigmine free base as a yellow liquid with a yield of 96.3%.
[0022] (2) Dissolve the product obtained in 1 in 200ml of isopropanol, add L-(+) tartaric acid (18g, 0.12mol), and heat to reflux. After the reaction solution is clear, it is naturally cooled to room temperature while stirring, and after separation and drying, hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com