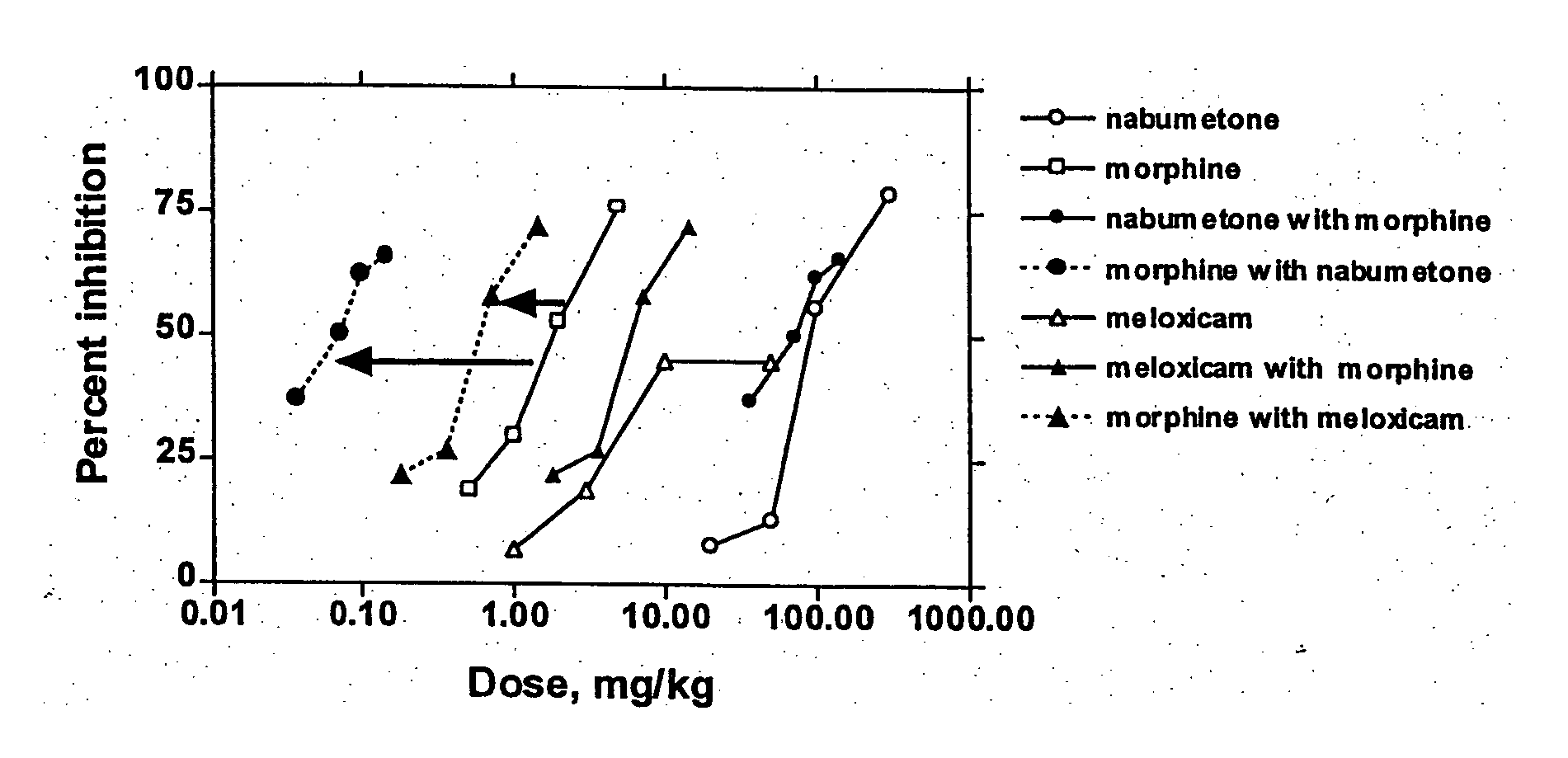
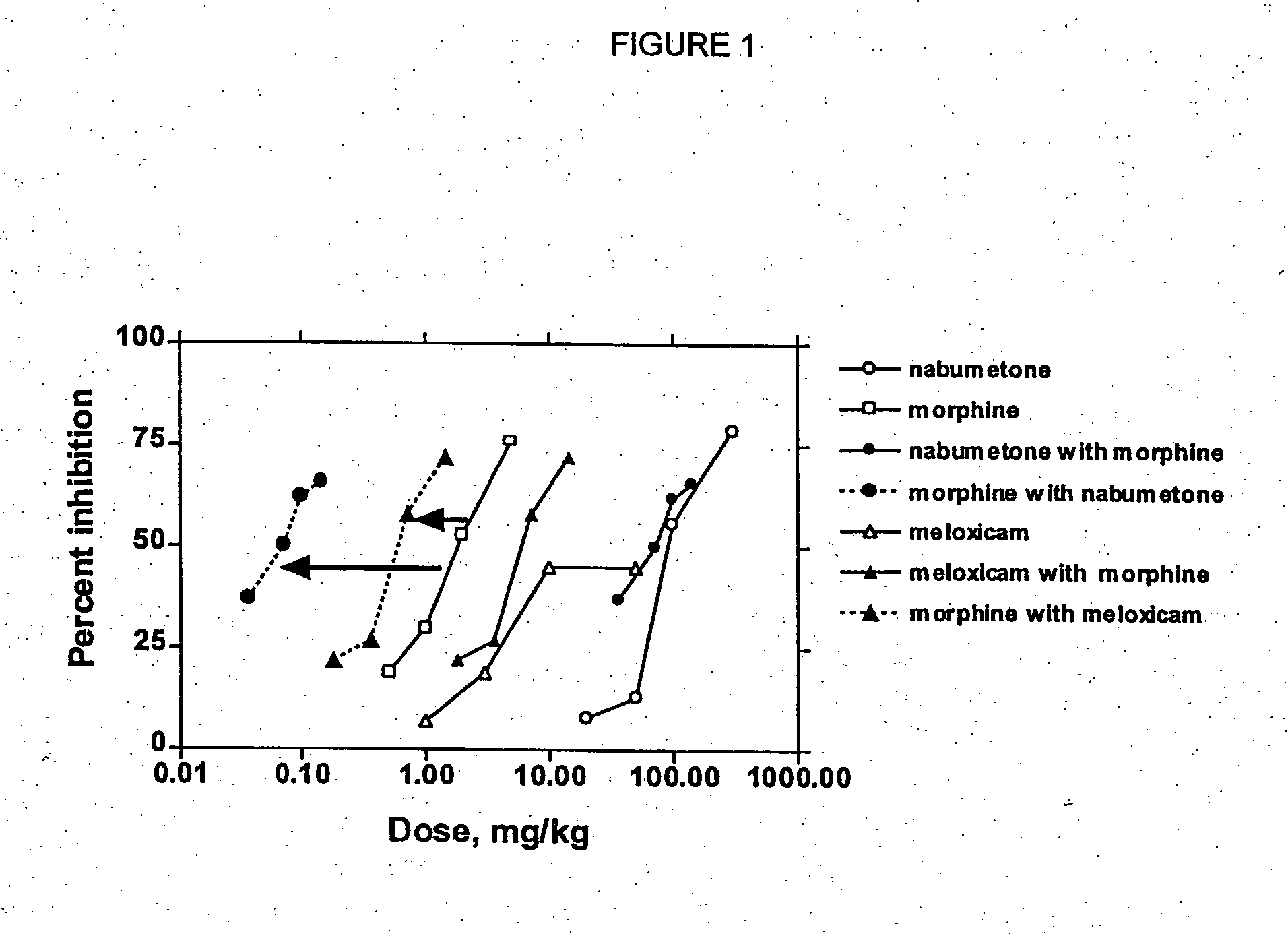
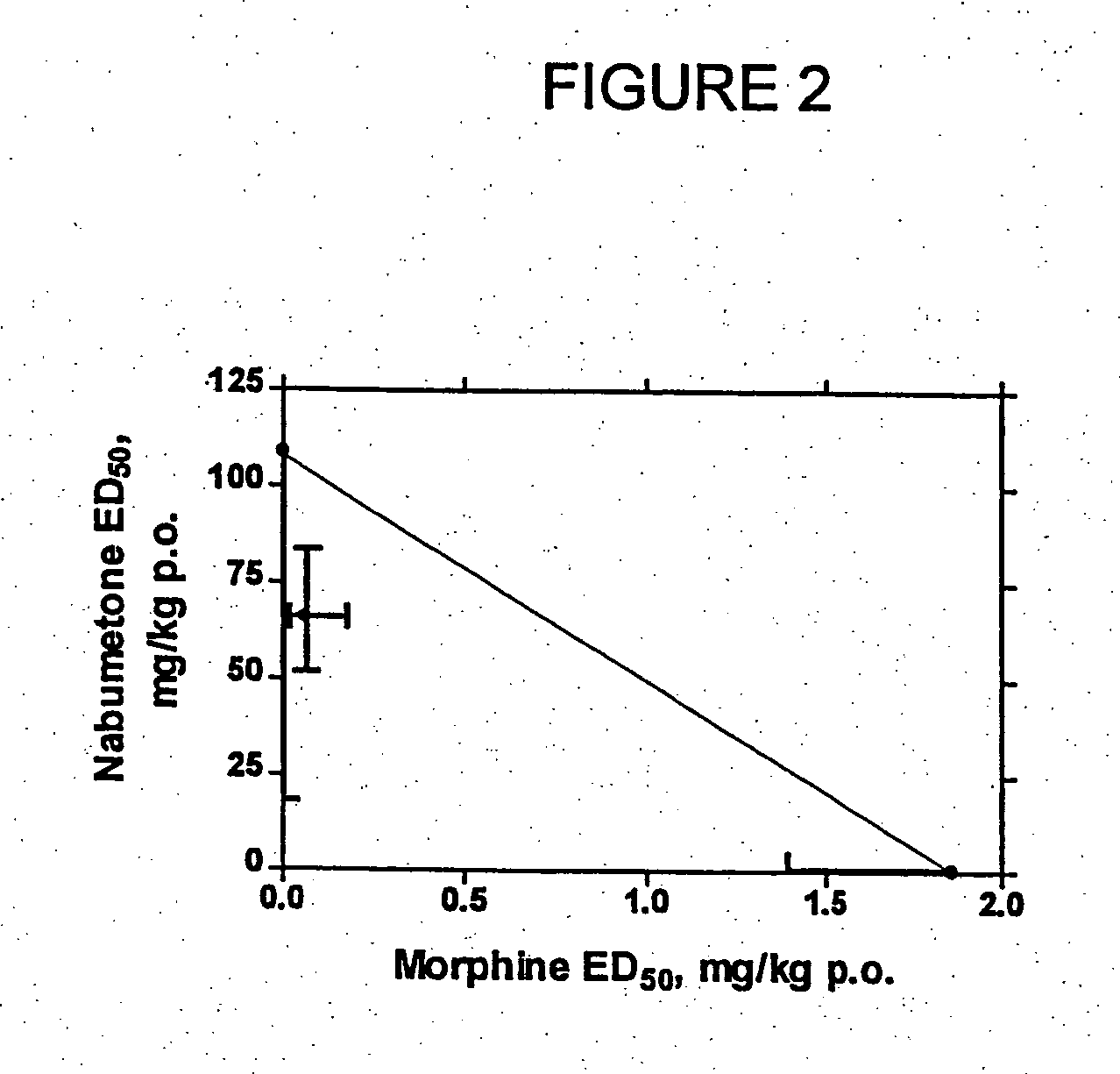
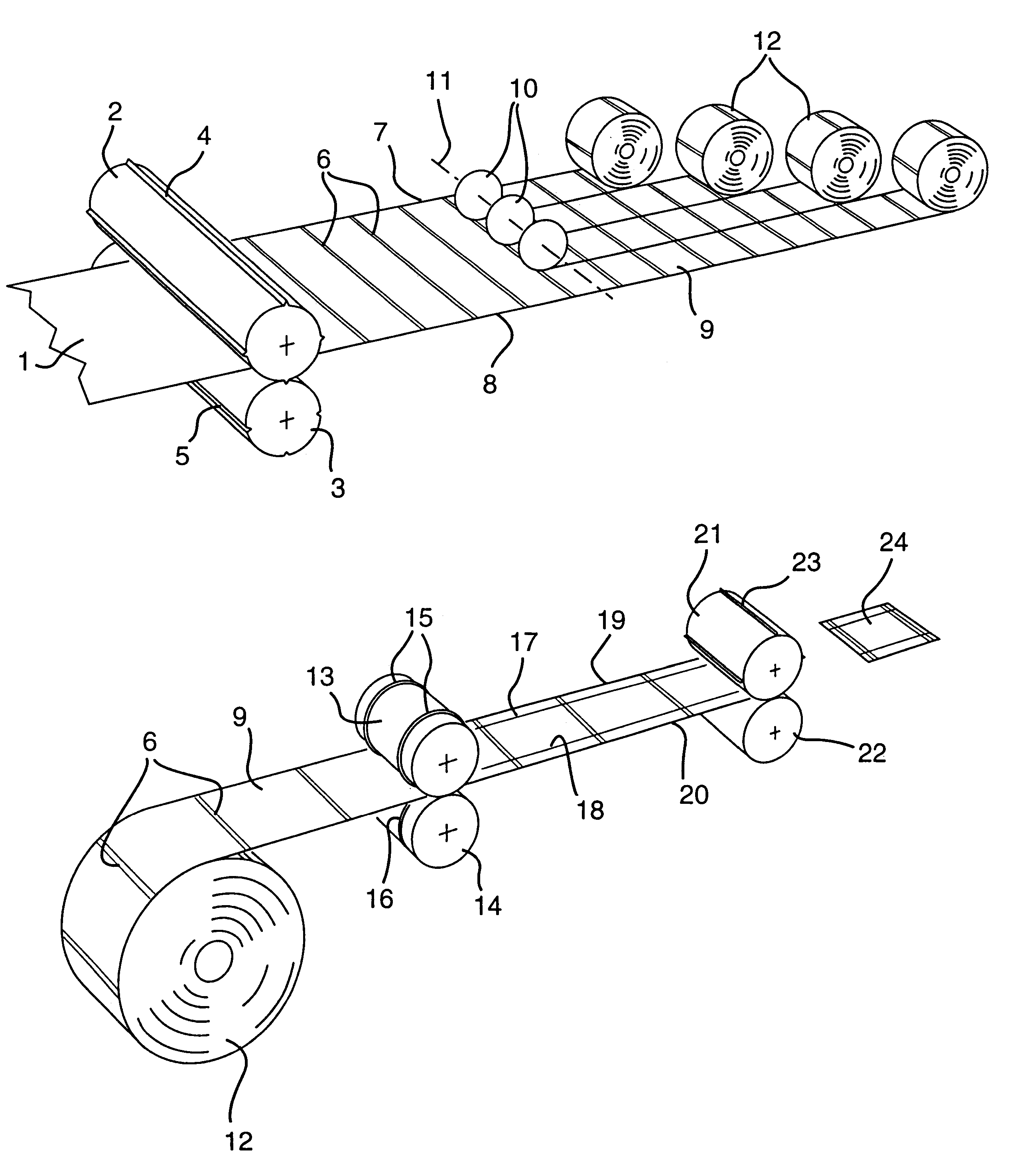
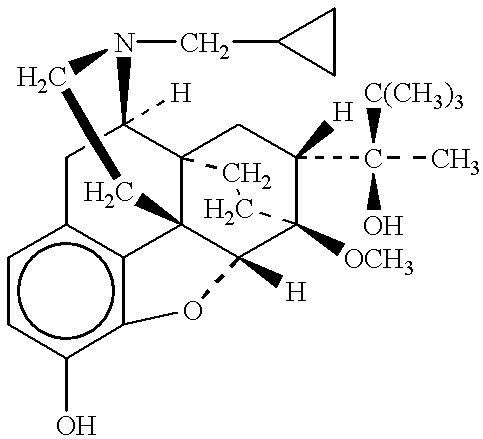
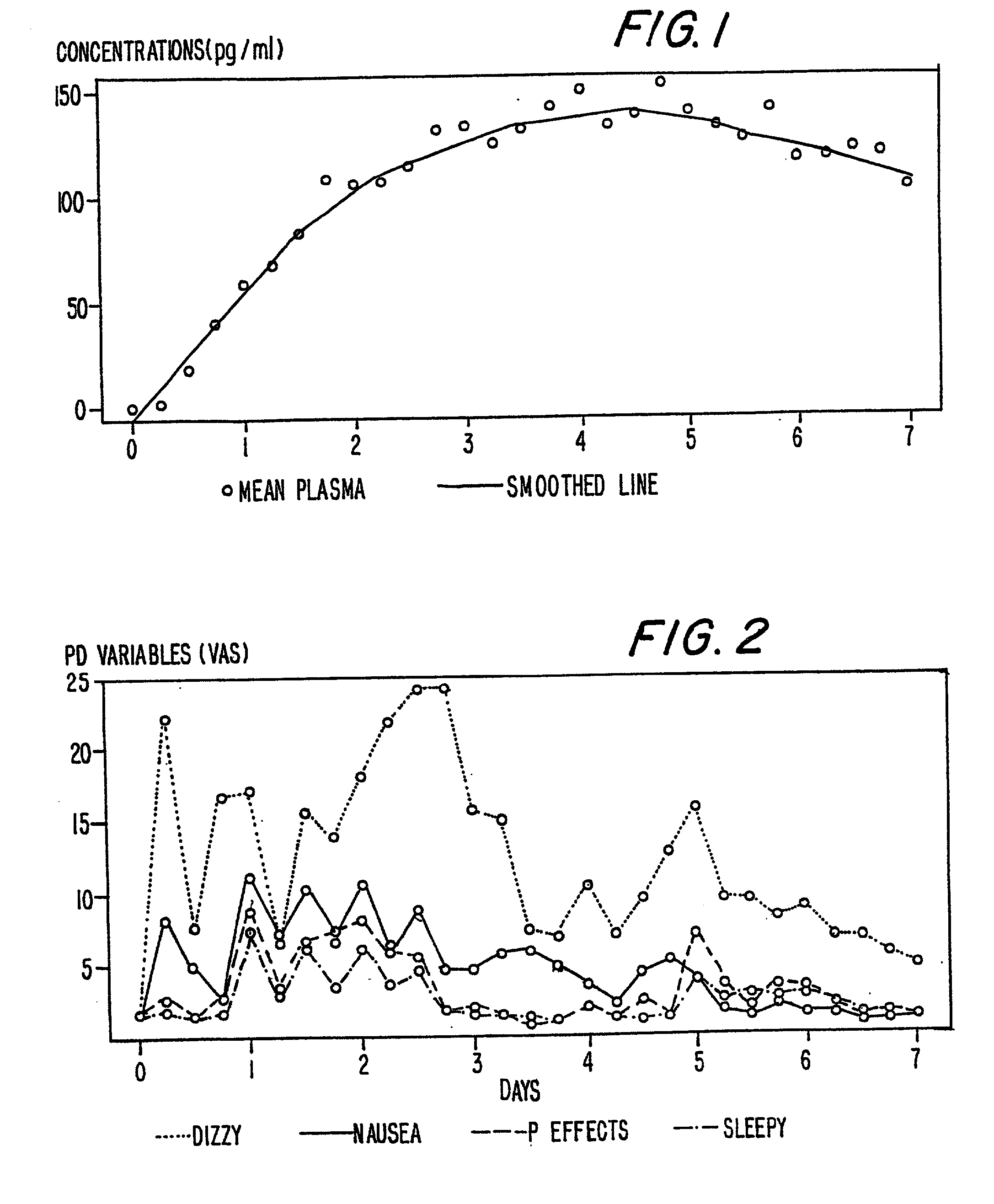
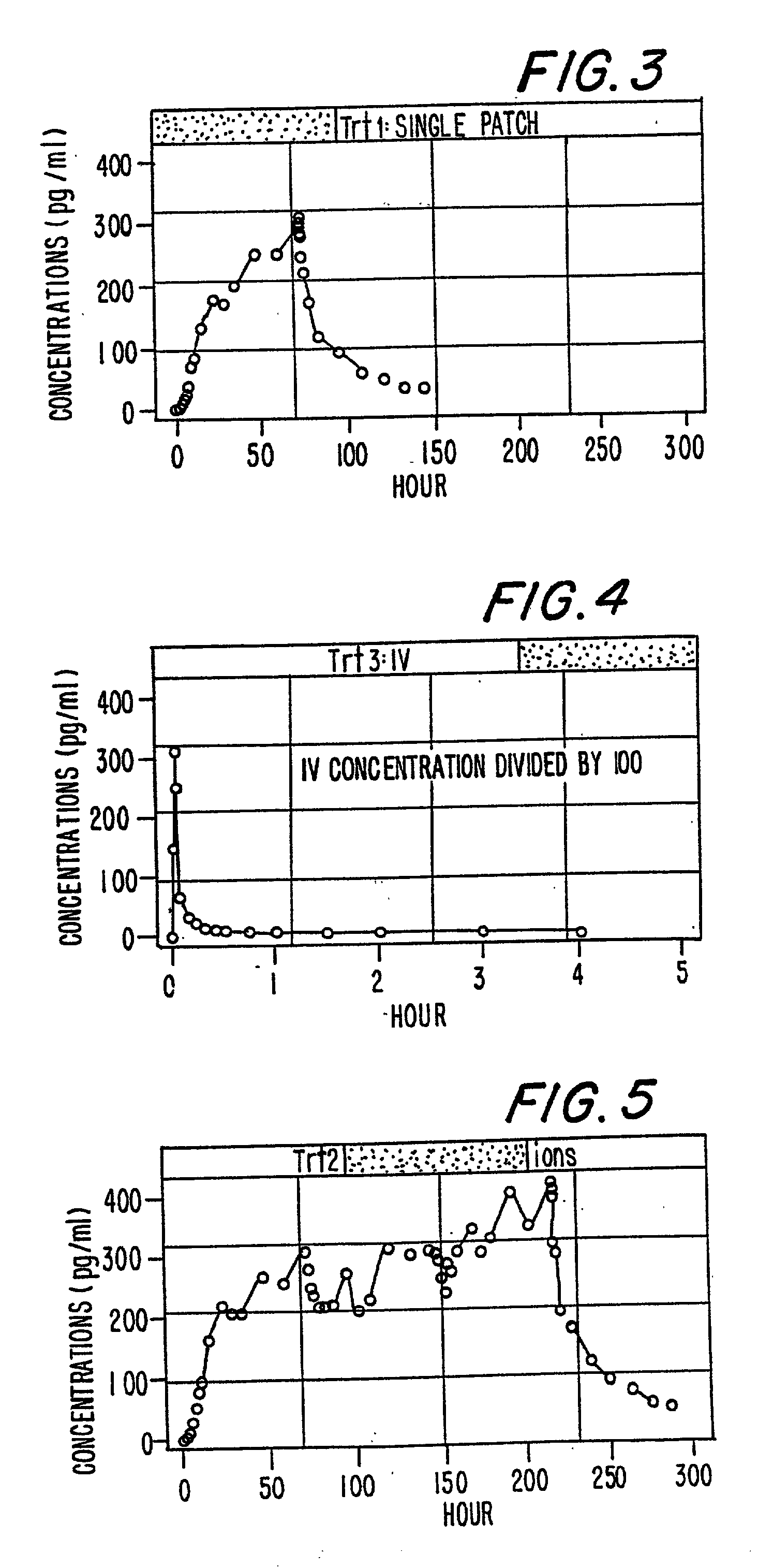
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
451results about How to "Reduced Tolerance Requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immersible thermal mass flow meter
ActiveUS6971274B2Quality improvementImprove measurement qualityVolume/mass flow by thermal effectsThin film sensorEngineering
Owner:SIERRA INSTR
System and method for mounting an image capture device on a flexible substrate
ActiveUS20050285973A1Facilitates fastImprove assembly efficiencyTelevision system detailsSolid-state devicesAdhesiveFlexible circuits
A digital camera module includes an image capture device mounted on a flexible circuit substrate. In one embodiment of the digital camera module, the image capture device is mounted directly (e.g., by an adhesive) on the flexible circuit substrate. A stiffener (e.g., a piece of circuit board material) is mounted to the back of the flexible circuit substrate to support wire bonding of the image capture device onto the flexible circuit substrate and / or to support the mounting of additional components (e.g., a lens housing).
Owner:NANCHANG O FILM OPTICAL ELECTRONICS TECH CO LTD
Directed delivery of agents to neural anatomy
InactiveUS20120310140A1Effective pain managementAdequate pain reliefSpinal electrodesPharmaceutical delivery mechanismDiseaseAutomatic control
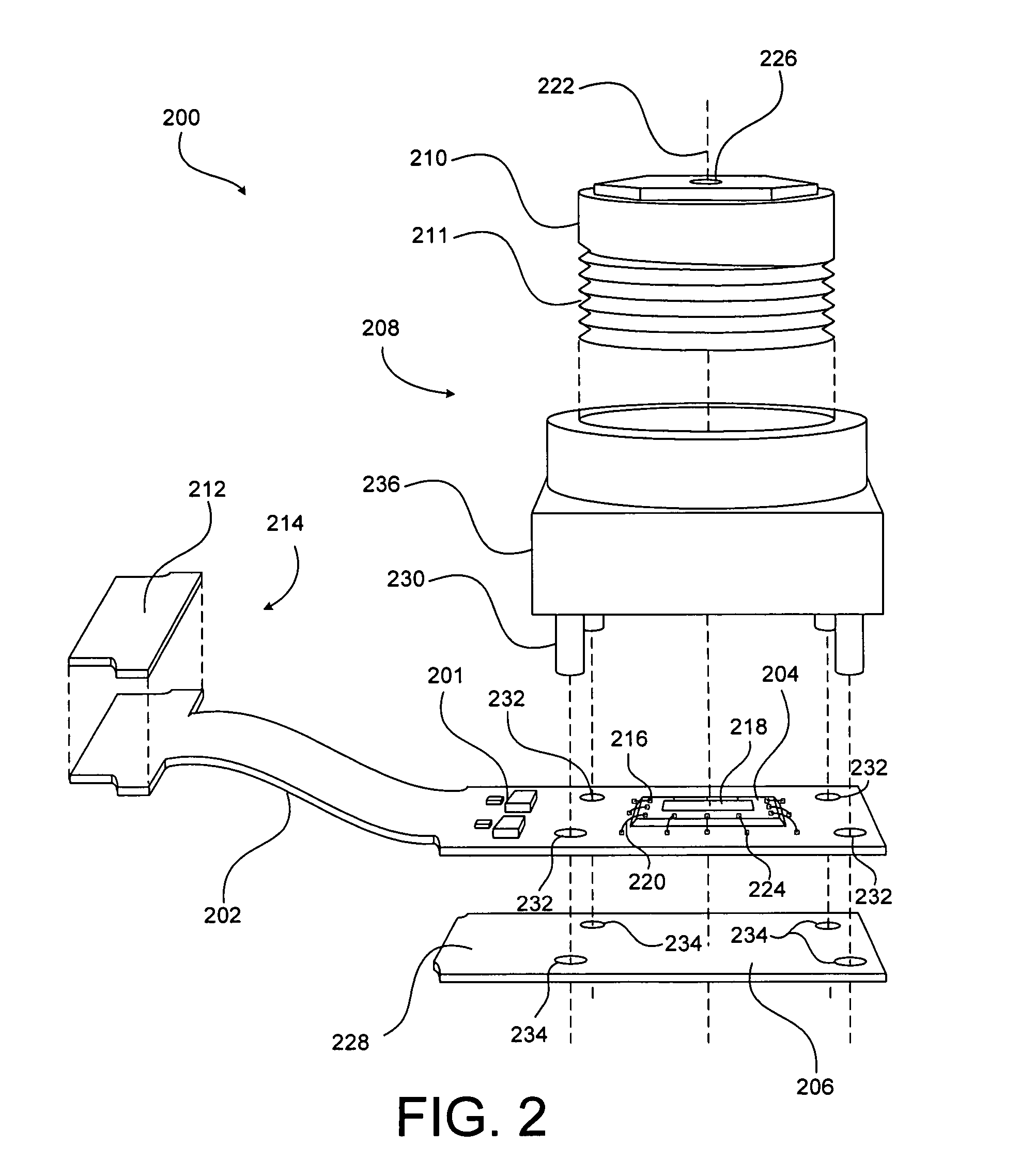
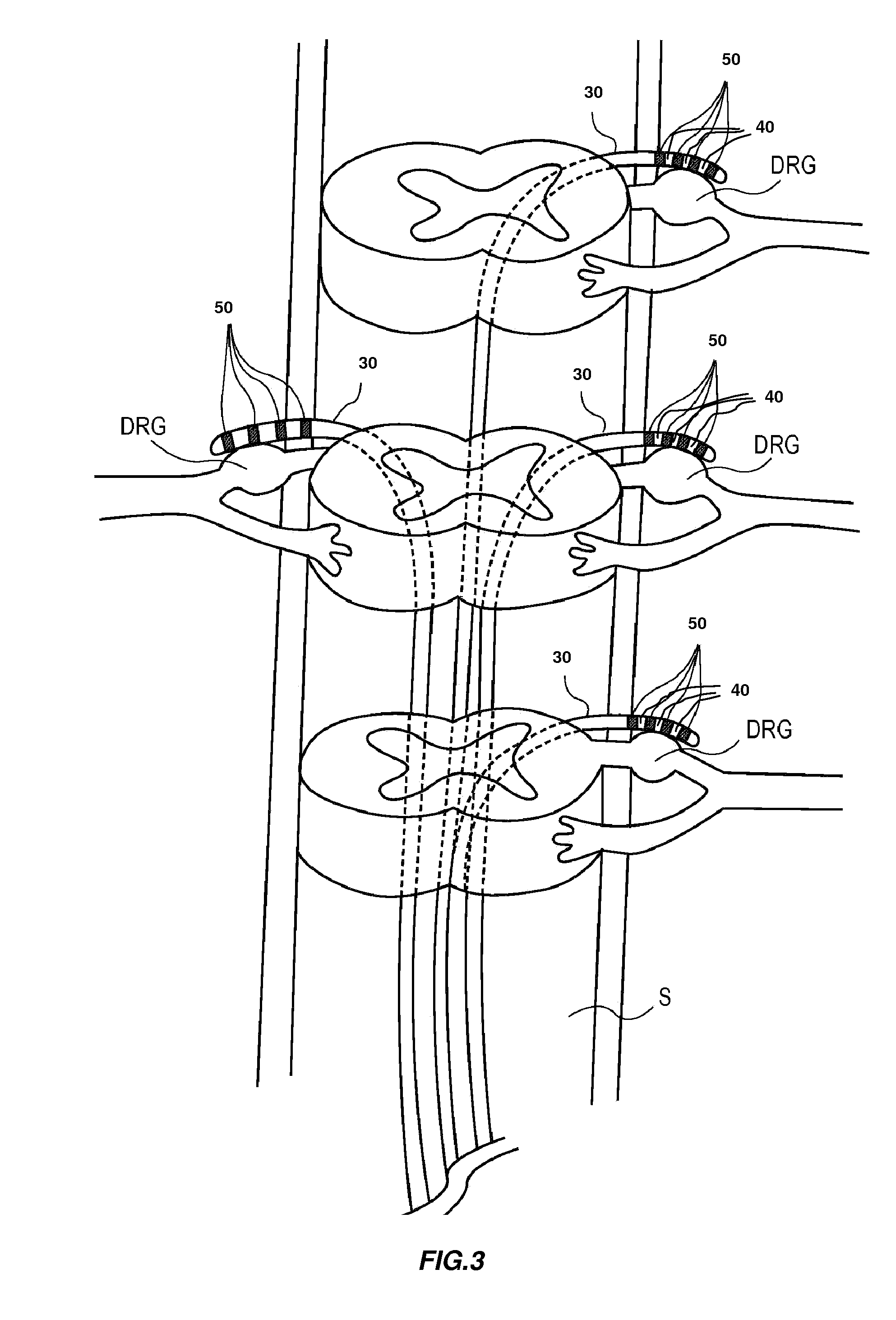
The present invention is directed generally to systems, devices and methods for direct delivery of agents, e.g., pharmaceutical agents, to target spinal and neuronal anatomies, e.g., the dorsal root ganglia (DRG), for the treatment of various disorders, particularly pain and pain related disorders, such as chronic itch, sensory disorders, multiple sclerosis, post-herpetic neuralgia and the like. The system, devices and methods of the invention encompass the agents to be delivered to the target anatomy alone or in combination with electrical stimulation. The delivery device and systems and methods as disclosed herein place the distal end of the delivery element, which comprises at least one agent delivery structure, and optionally at least one electrode, in close proximity, or in contact with or next to the target spinal anatomy, e.g., DRG. A variety of agents can be delivered using the device, including sodium channel blockers, biologics, neuroinflammatory modulators, toxins etc., to selectively neuromodulate the neurons. Agent delivery and / or electrical stimulation can be automated and / or can be controlled automatically or by a pre-determined program, or by a patient control pump (PCA).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Pre-equalized optical transmitter and pre-equalized optical transmission system
InactiveUS20090238580A1Reduced Tolerance RequirementsReduce consumptionDistortion/dispersion eliminationElectromagnetic transmittersEngineeringAnalog signal
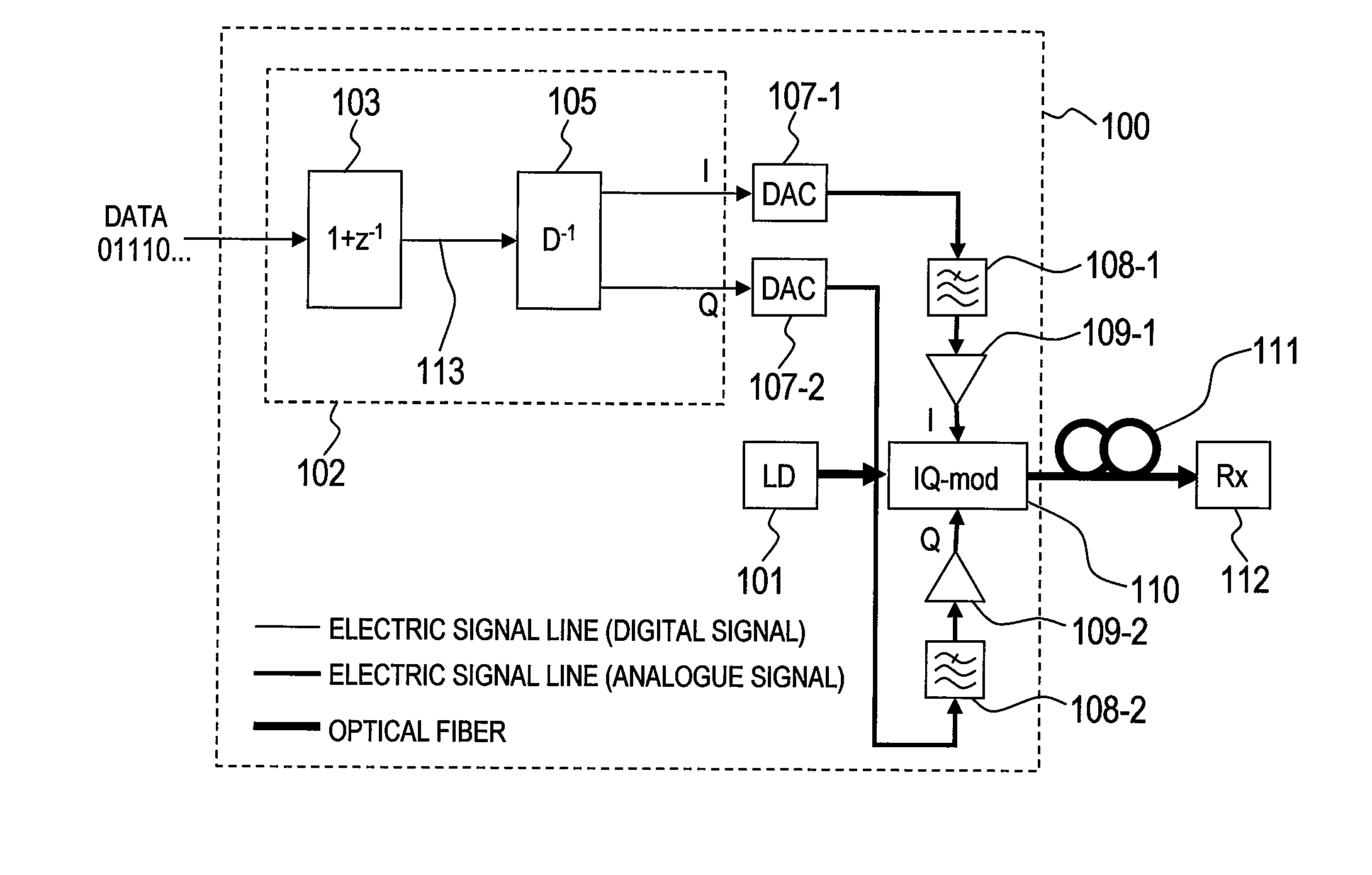
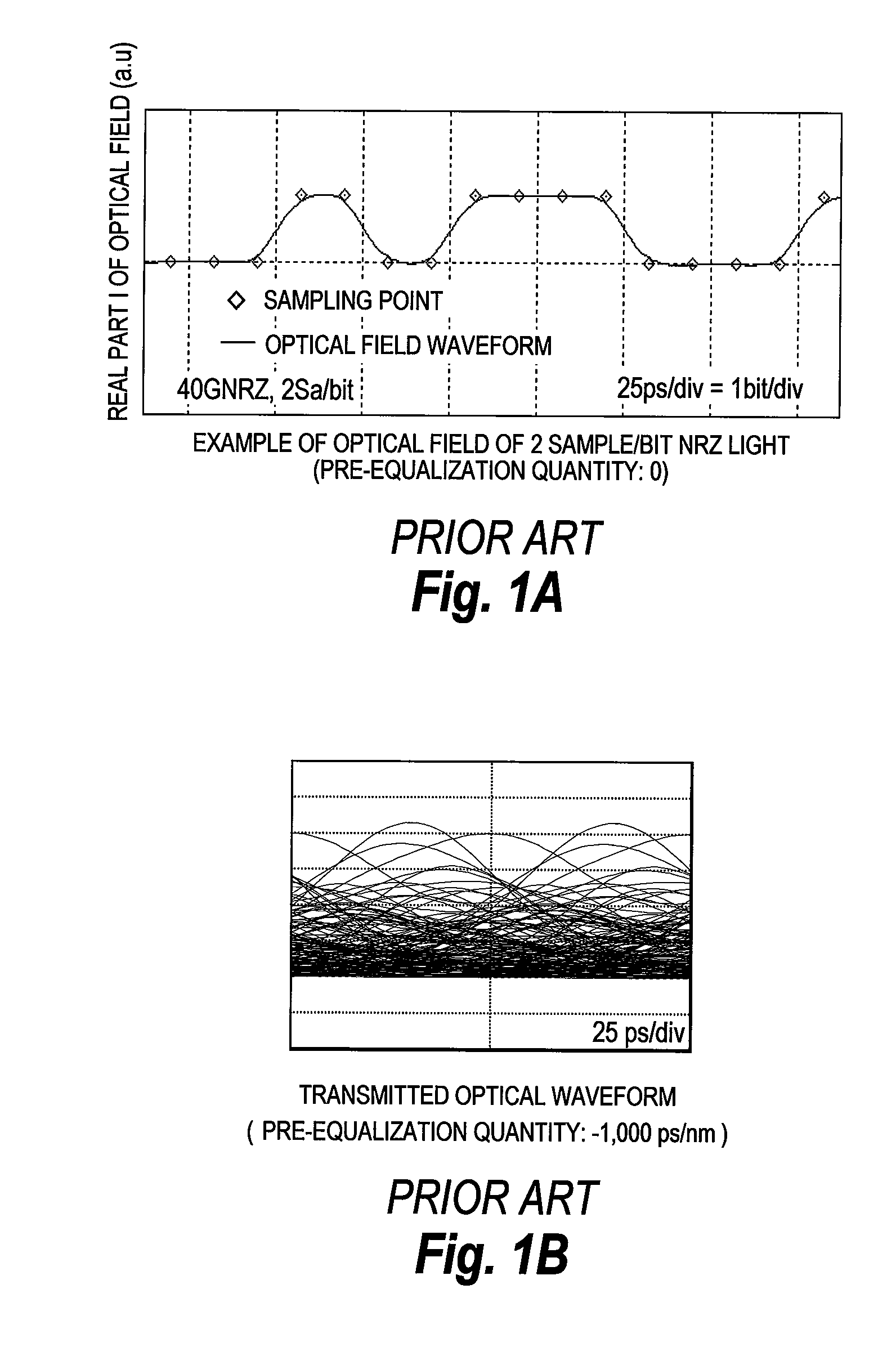
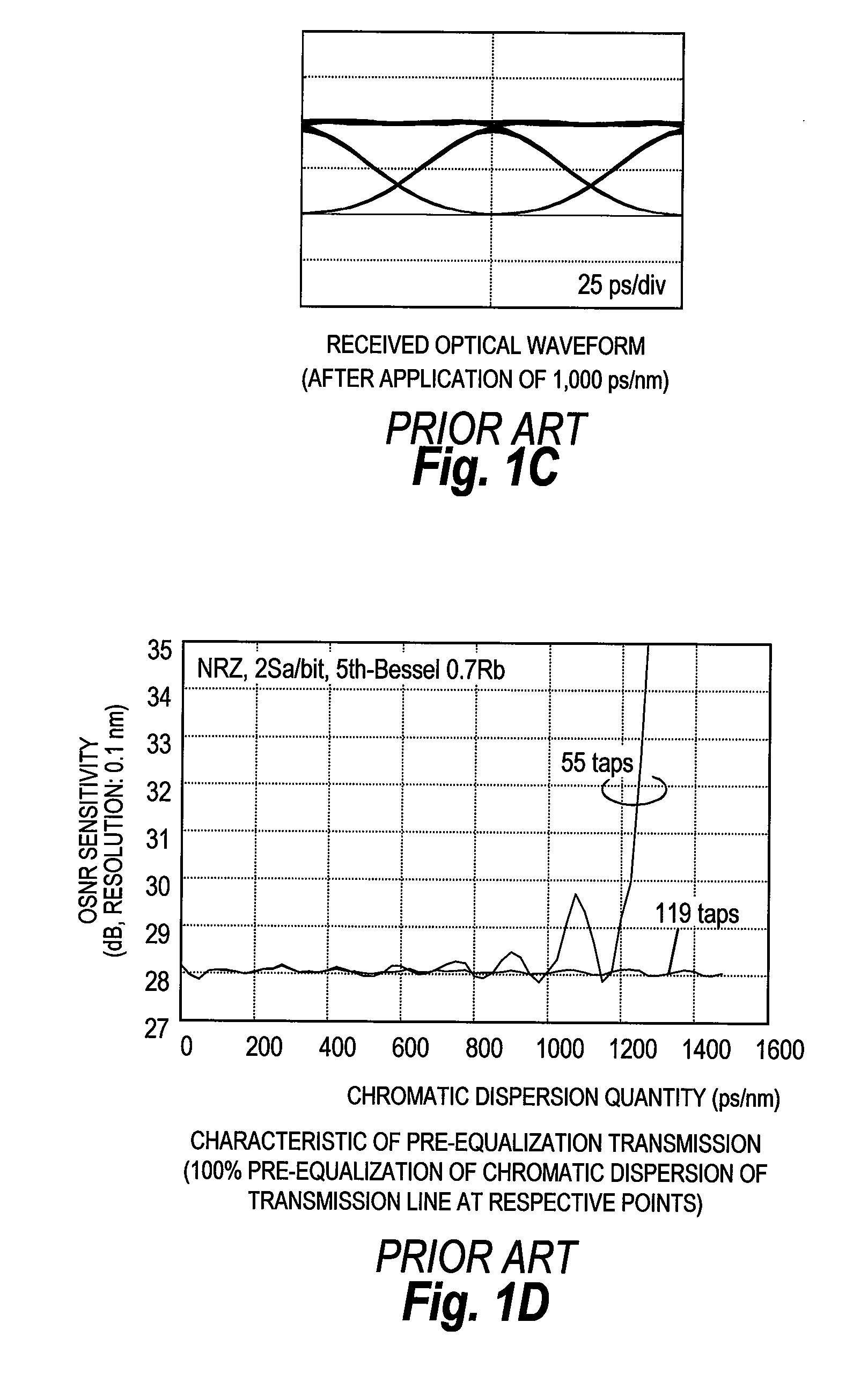
Provided is a pre-equalized optical transmitter, comprises: a laser source; a duo-binary pre-coder circuit; a pre-equalization circuit for applying an inverse function of chromatic dispersion; at least two D / A converters; and an optical field modulator comprising at least two input terminals for an electric signal. The pre-equalized optical transmitter: converts, by the duo-binary pre-coder circuit, a digital information signal of a predetermined symbol time to be transmitted into a digital complex signal including one sampling point per symbol; equalizes, by the pre-equalization circuit, degradation in transmission of the digital complex signal; converts, by the D / A converters, the equalized digital complex signal into an analogue signal; suppresses an analogue signal leaking outside a Nyquist bandwidth by at least 23 dB; modulates, by the optical field modulator, light output from the laser source with the analogue signal to generate a modulated optical field signal; and transmits the modulated optical field signal.
Owner:HITACHI LTD
Systems and methods for therapy of kidney disease and/or heart failure using chimeric natriuretic peptides
InactiveUS20120220528A1Lower elevated filing pressureImproves dyspneaPeptide/protein ingredientsPharmaceutical delivery mechanismNephrosisNephropathy
Medical systems and methods for treating kidney disease alone, heart failure alone, kidney disease with concomitant heart failure, or cardiorenal syndrome are described. The systems and methods are based on delivery of a chimeric natriuretic peptide to a patient. Methods for increasing peptide levels include direct peptide delivery via either an external or implantable programmable pump.
Owner:CAPRICOR THERAPEUTICS
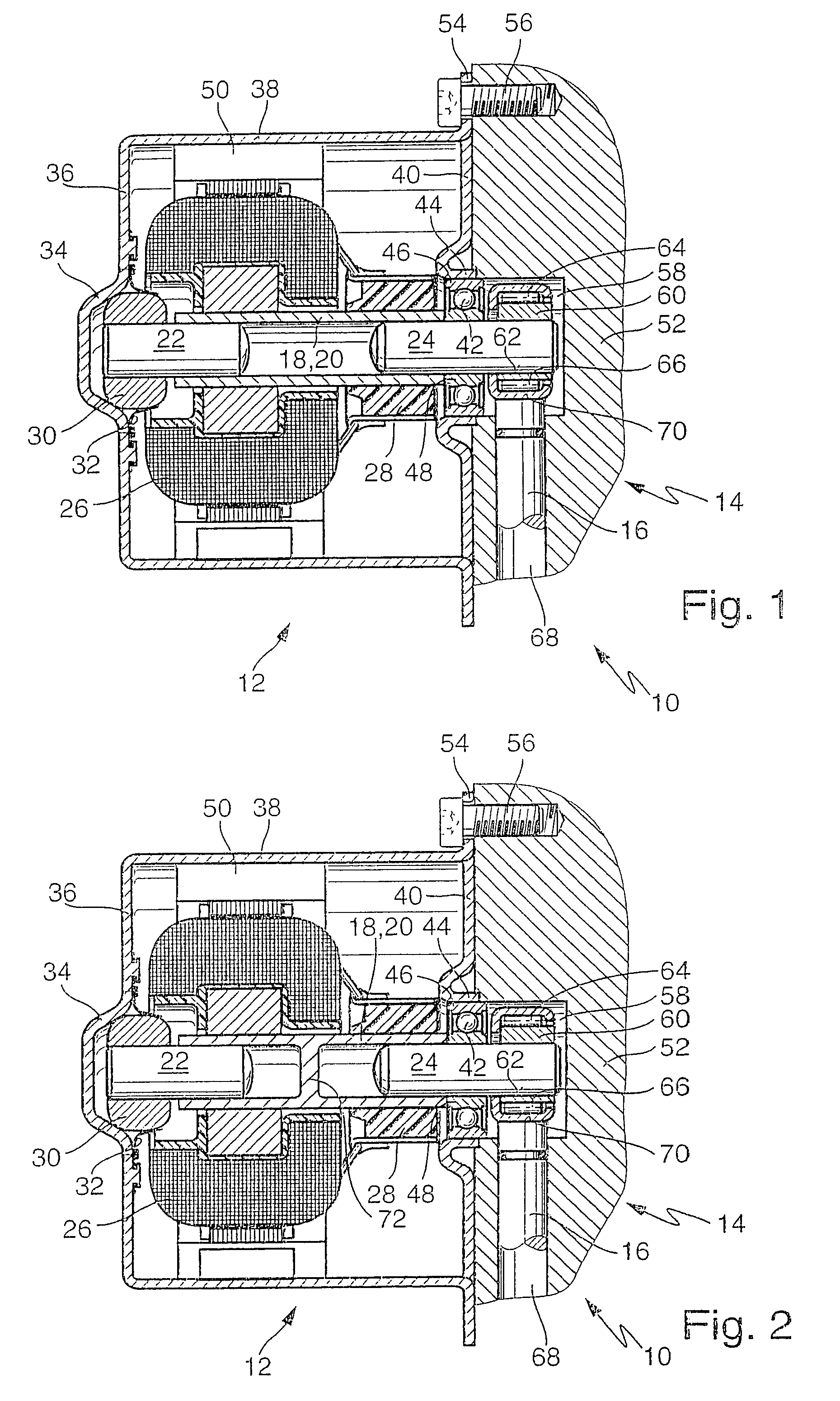
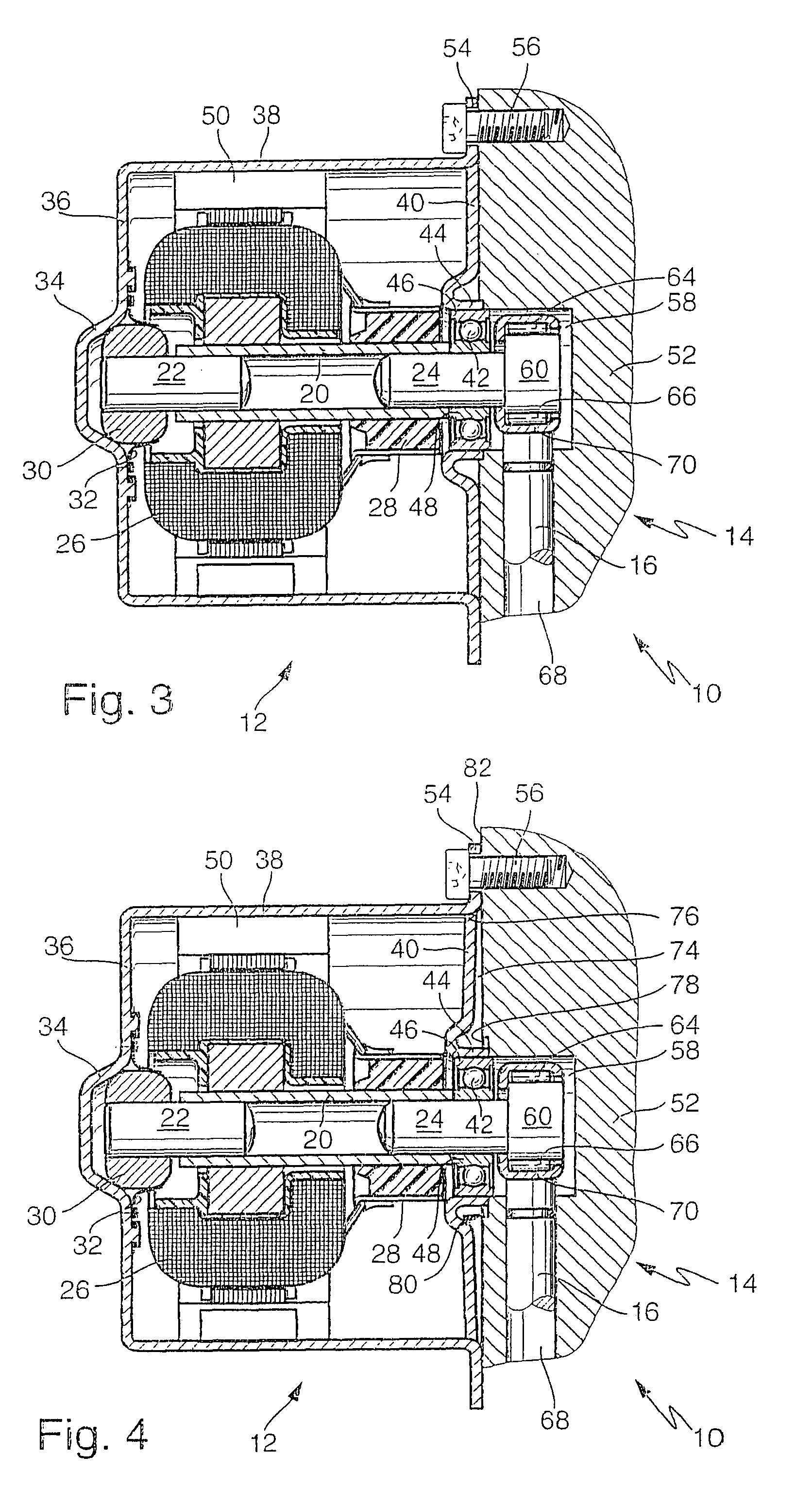
Multiple-clutch device
InactiveUS6464059B1Reduced Tolerance RequirementsReduce dependenceRotary clutchesFluid actuated clutchesMobile vehicleDrivetrain
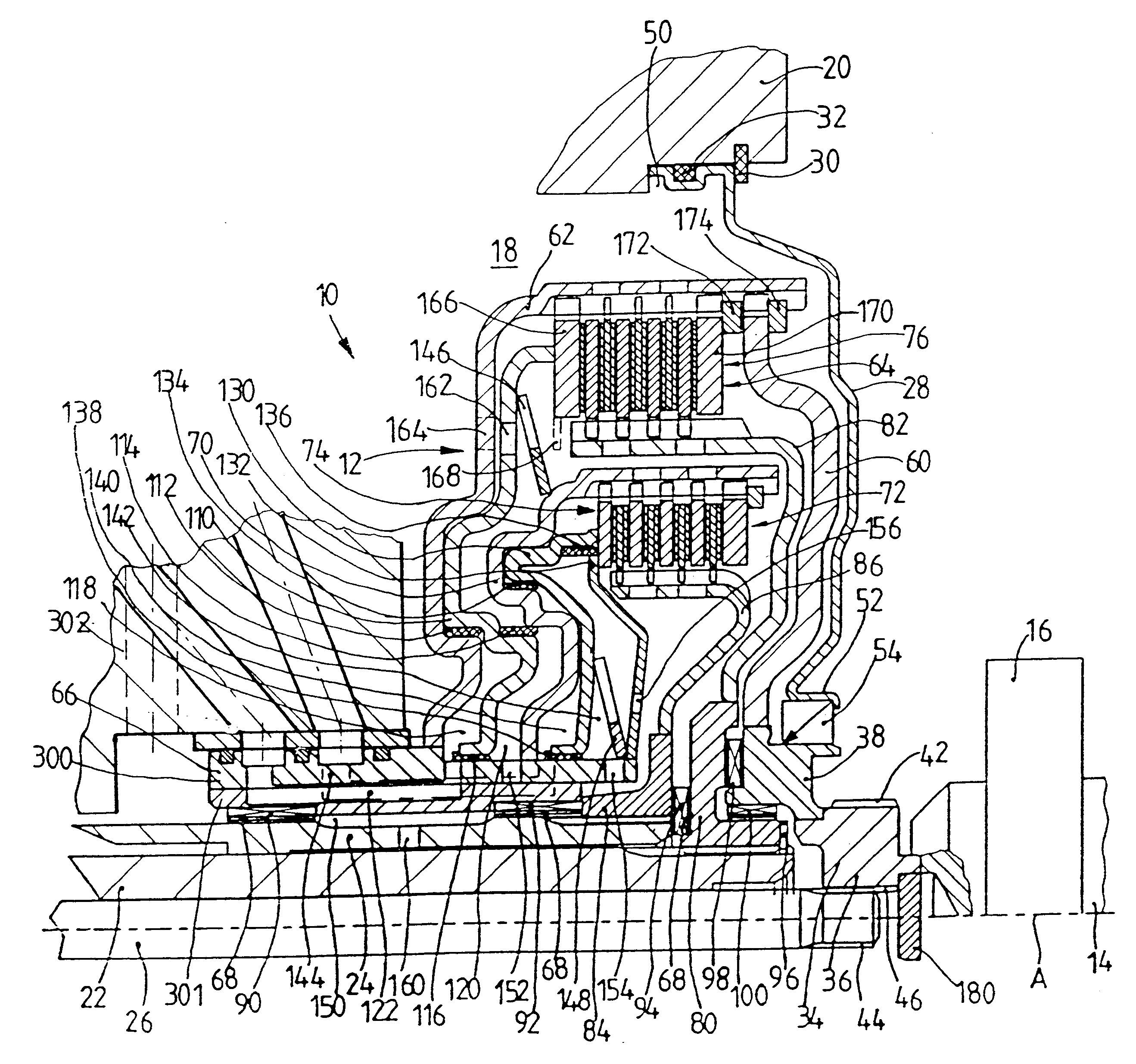
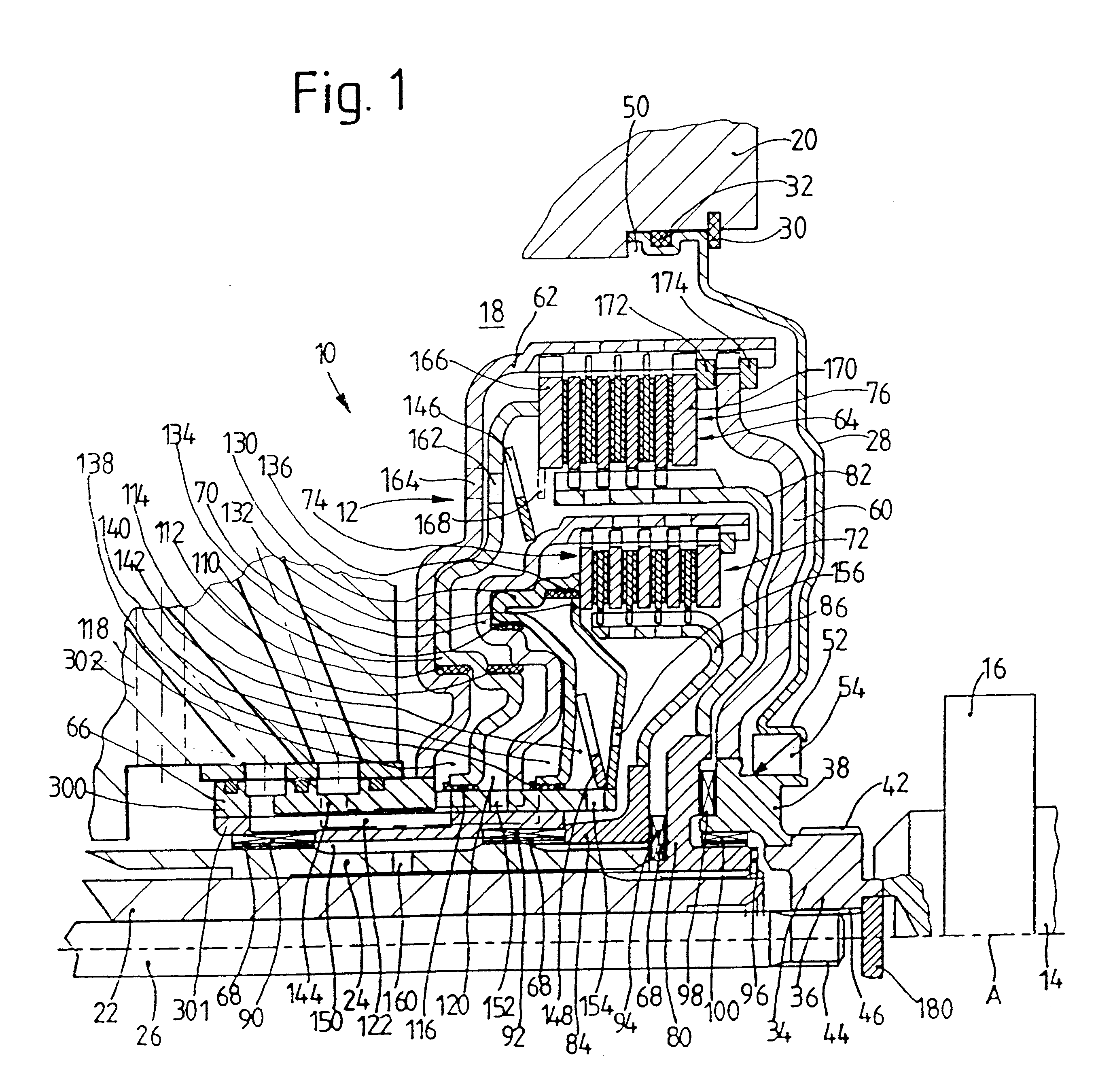
The invention is directed to a multiple-clutch device, such as a double-clutch device, for arranging in a drivetrain of a motor vehicle between a drive unit and a transmission, wherein the clutch device has a first clutch arrangement associated with a first transmission input shaft of the transmission and a second clutch arrangement associated with a second transmission input shaft of the transmission for transmitting torque between the drive unit and the transmission. It is suggested that a bearing arrangement is associated with at least one of the clutch arrangements, wherein the latter is supported or can be supported so as to be relatively rotatable by means of the bearing arrangement on at least one of the transmission input shafts, at least one of which is constructed as a hollow shaft and a shaft constructed as a hollow shaft encloses the other shaft, preferably at least at the radial outer transmission input shaft which is constructed as a hollow shaft.
Owner:VOLKSWAGEN AG +1
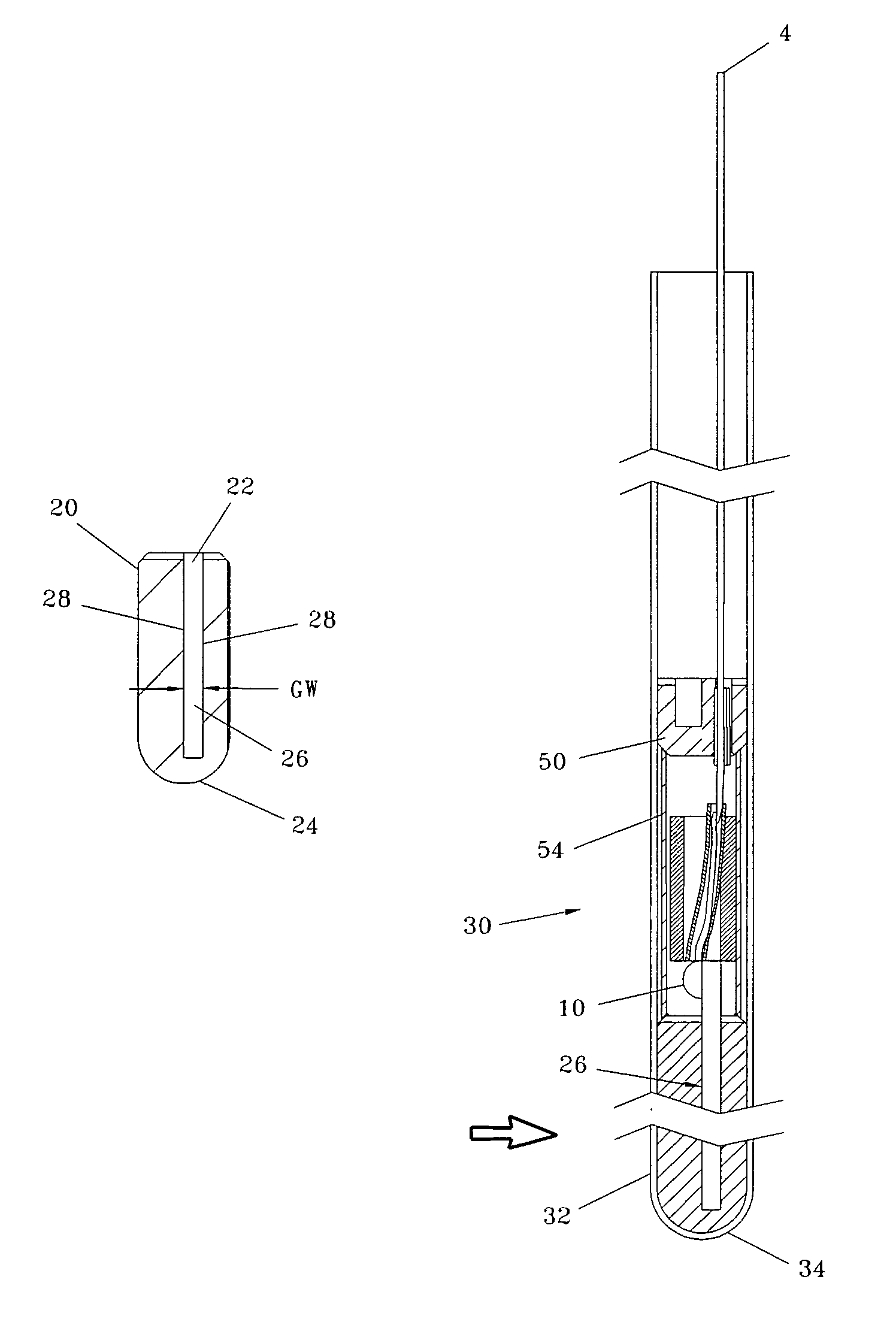
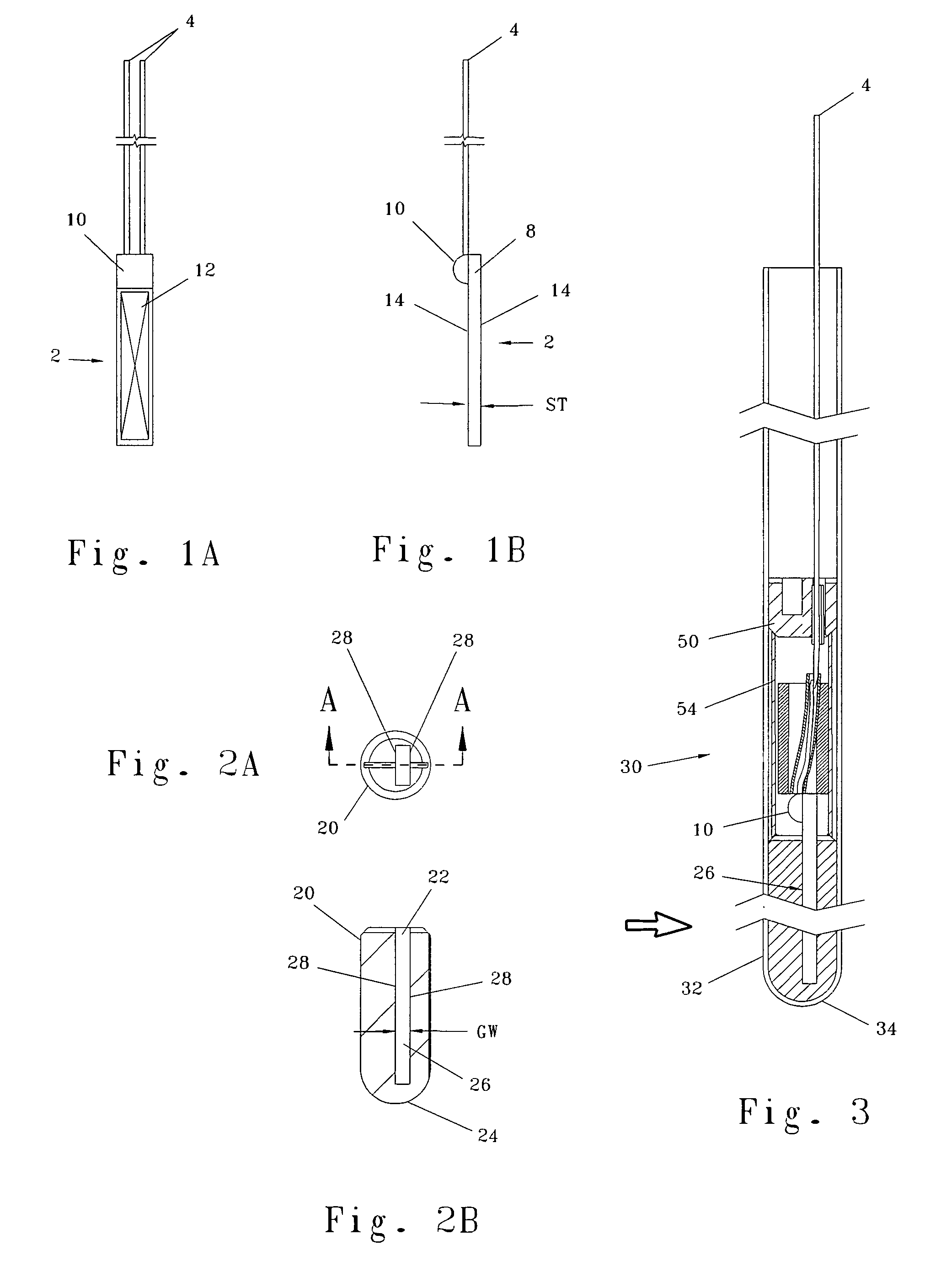
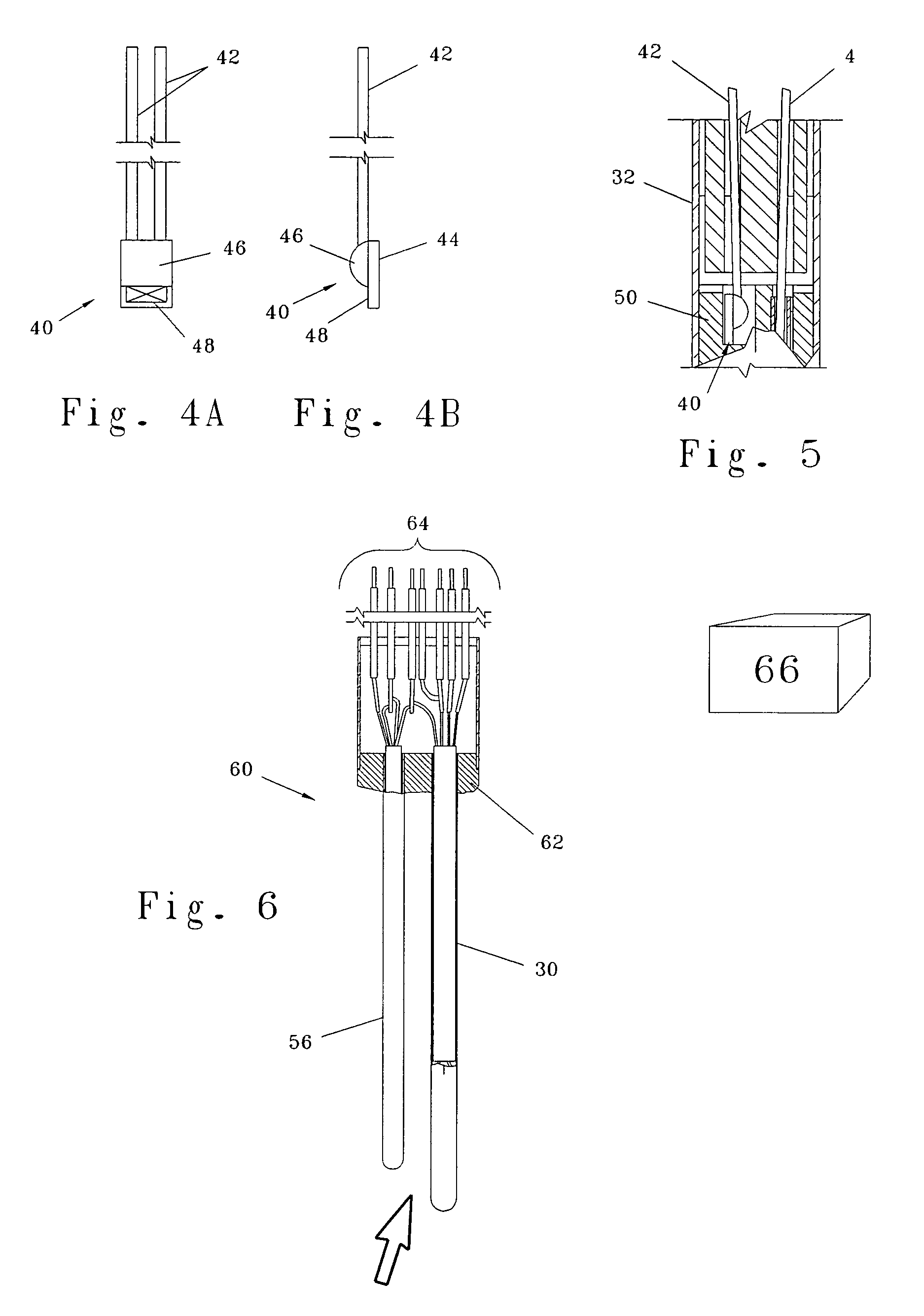
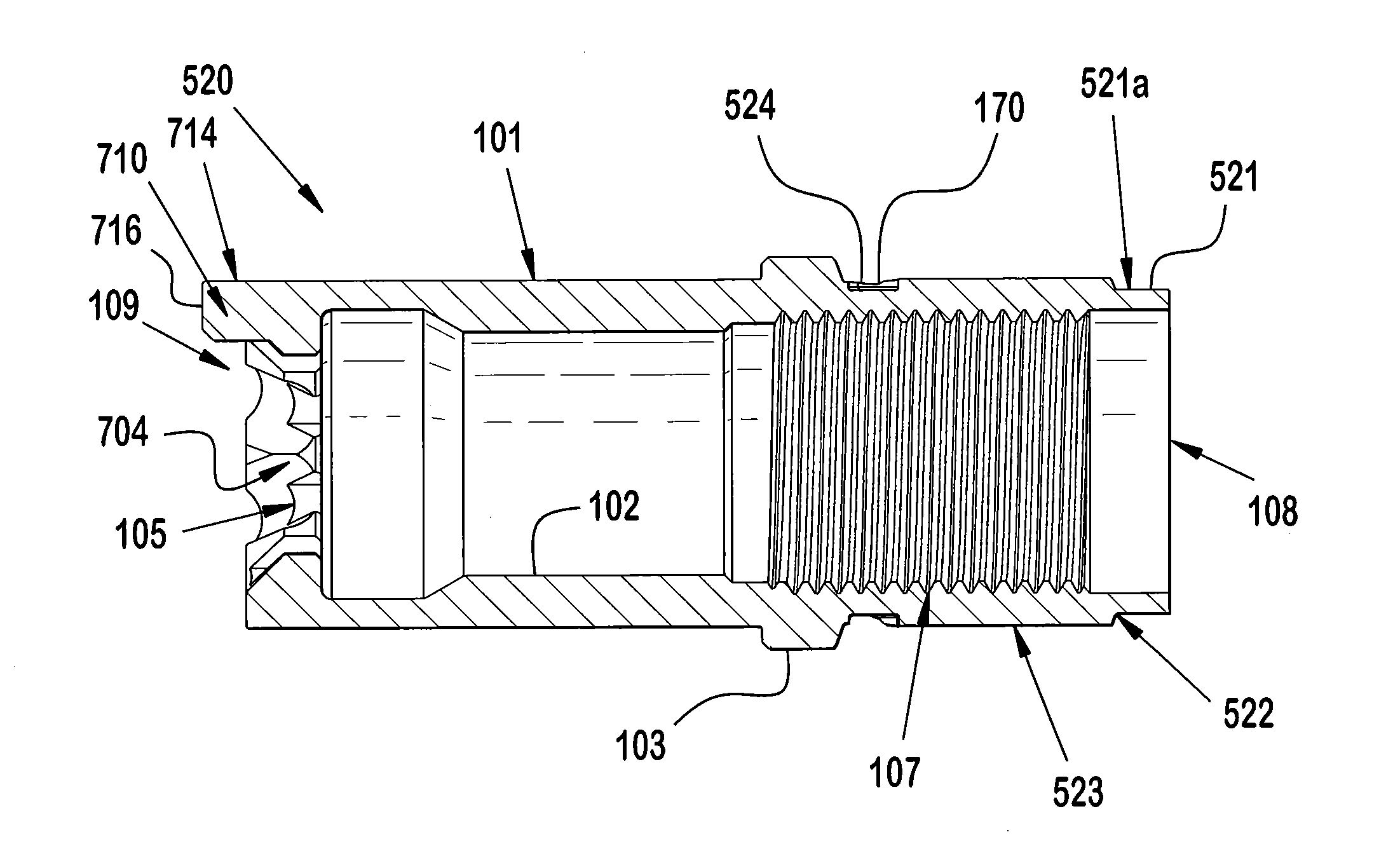
Injector
ActiveUS20070021720A1Low costReduce componentsAutomatic syringesIntravenous devicesBiomedical engineeringInjection device
The present invention relates to a method of operating an injection device, which device comprises a medicament cartridge and a needle attached to said cartridge, means for automatic penetration of needle, injection of medicament and withdrawal of needle, comprising the steps of initiating a penetration sequence, followed by an injection sequence and followed by a withdrawal sequence, wherein a previous sequence triggers a subsequent sequence, and wherein the subsequent sequence is triggered before the previous sequence has ended.
Owner:SHL MEDICAL AG
Polymer controlled induced viscosity fiber system and uses thereof
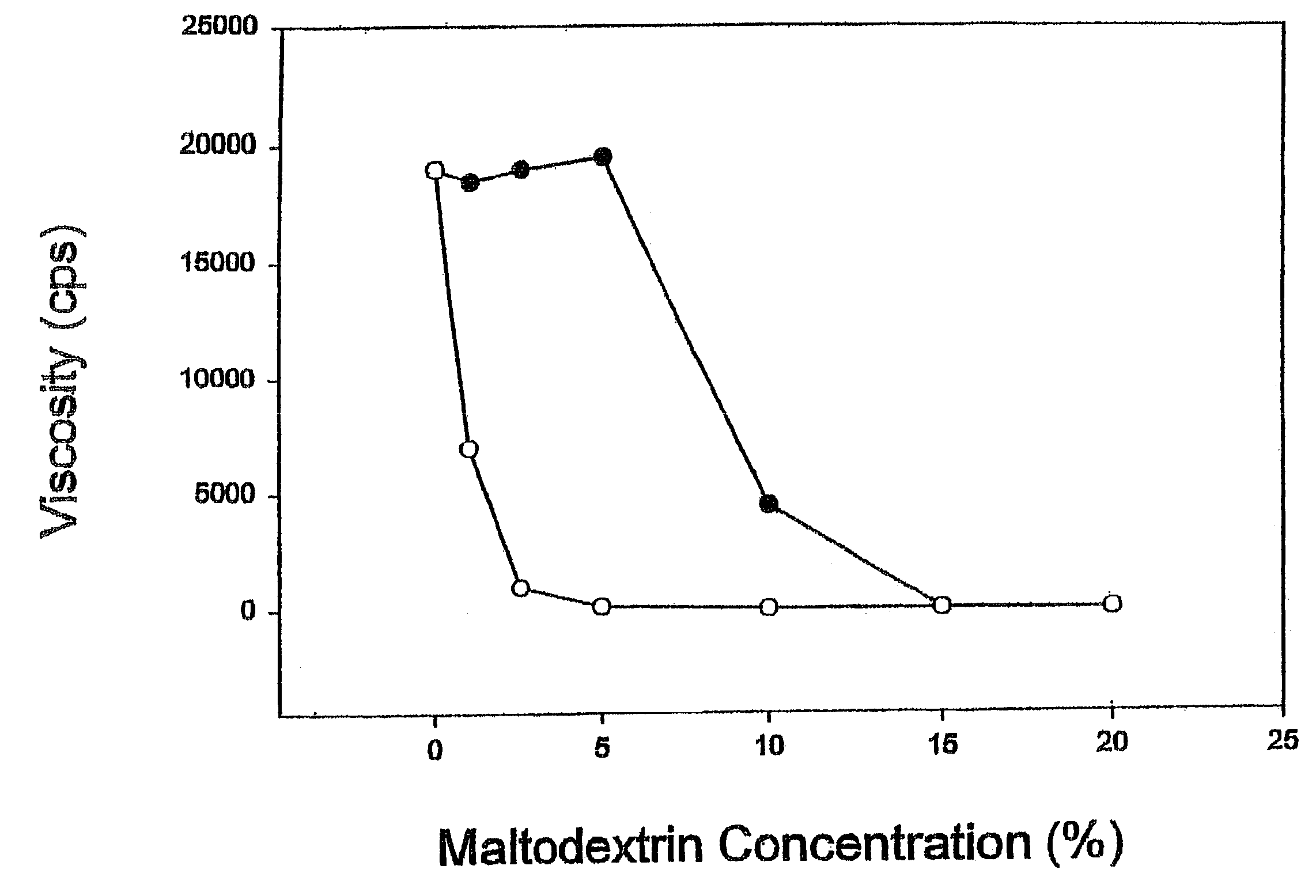
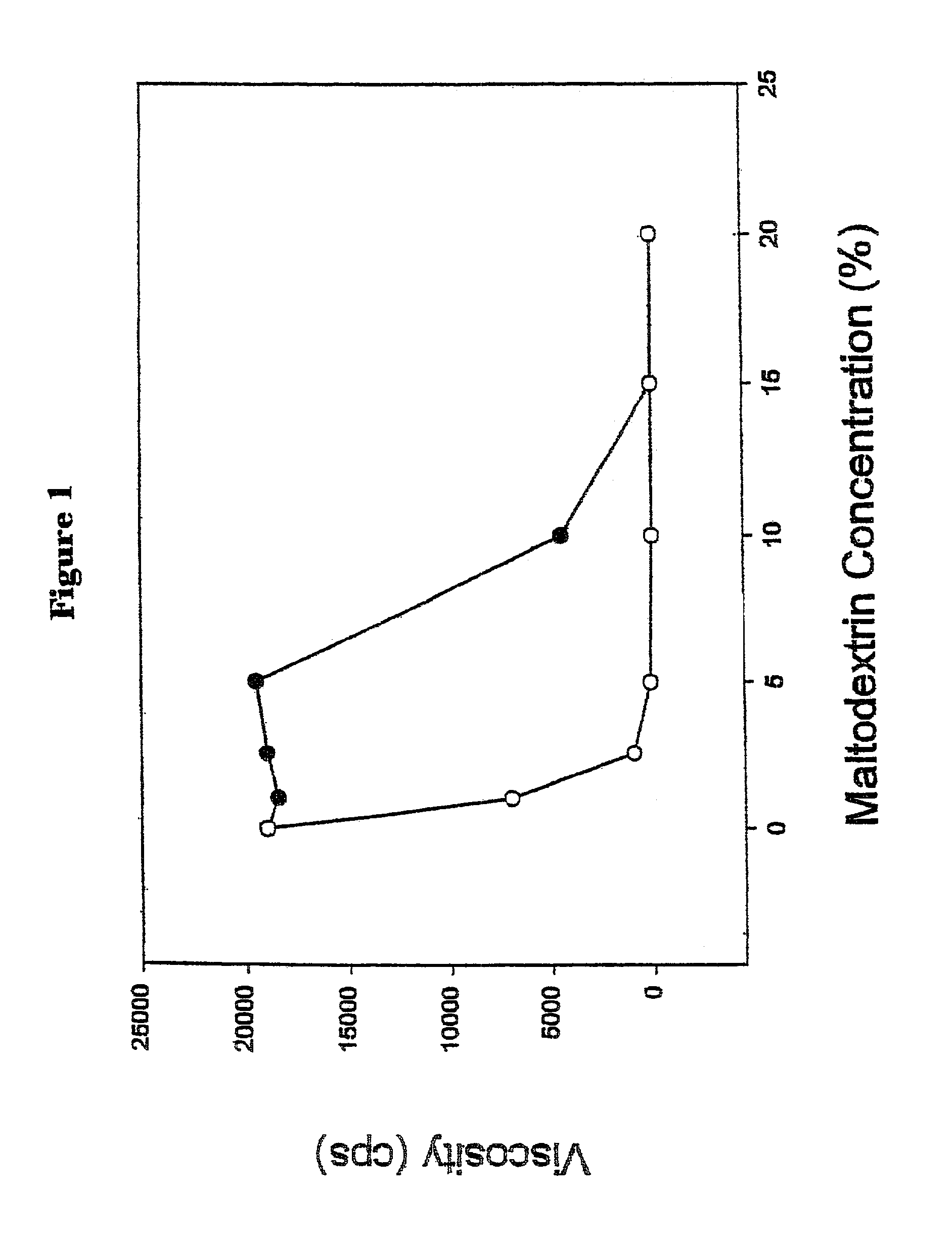
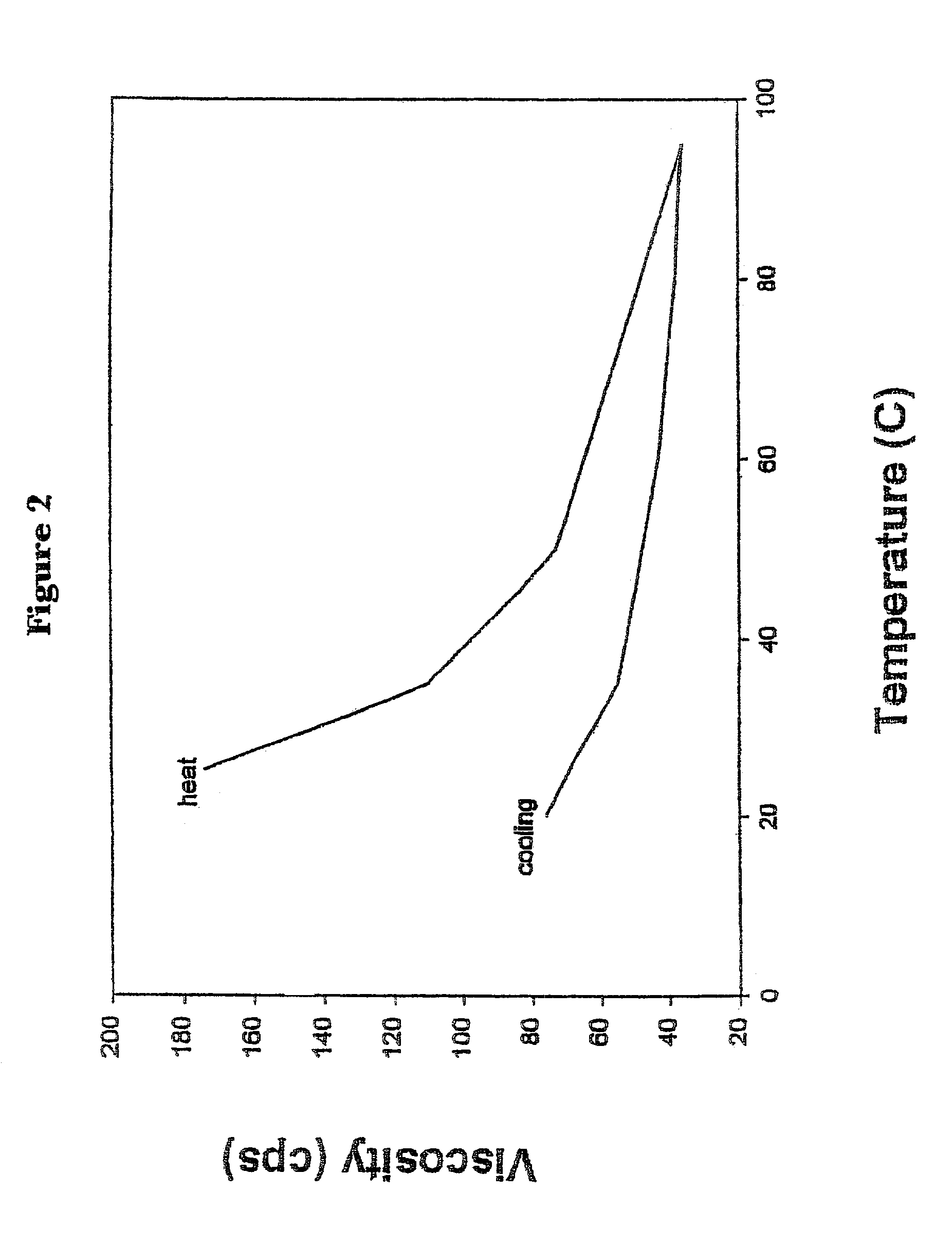
InactiveUS7067498B2Blunt glycemic responsePrevent dissolutionBiocideSugar food ingredientsPhysical stabilityChemistry
The present invention relates generally to a method of blunting the postprandial glycemic response in a human by feeding an induced viscosity fiber system. The invention also relates to an induced viscosity fiber system and the liquid products that incorporate the induced viscosity fiber system. Further, the invention relates to a method of incorporating soluble fiber into a liquid product without the typical negative organoleptic or physical stability issues. The invention also relates to a method of inducing the feeling of fullness and satiety by feeding the induced viscosity fiber system.
Owner:ABBOTT LAB INC
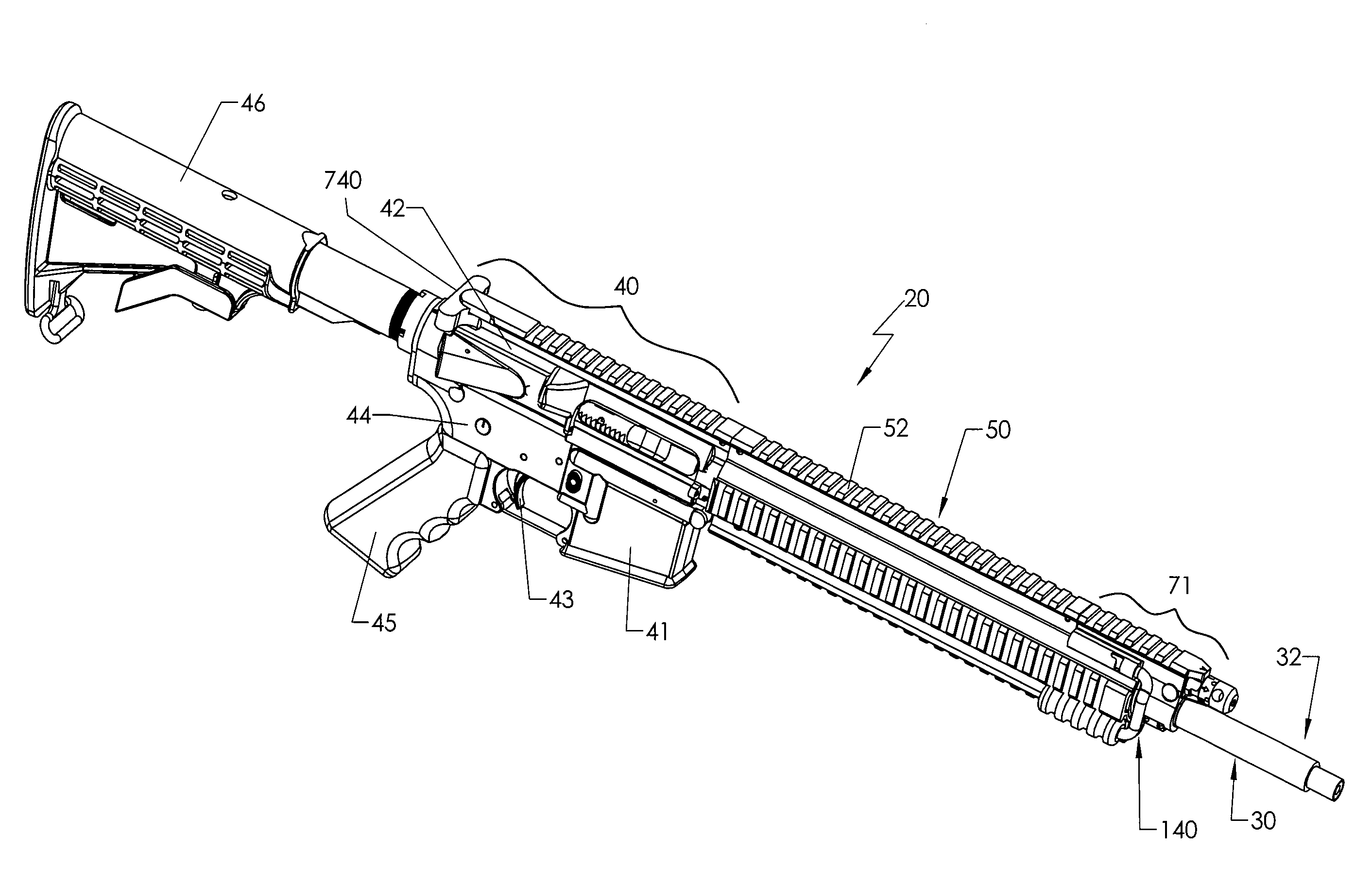
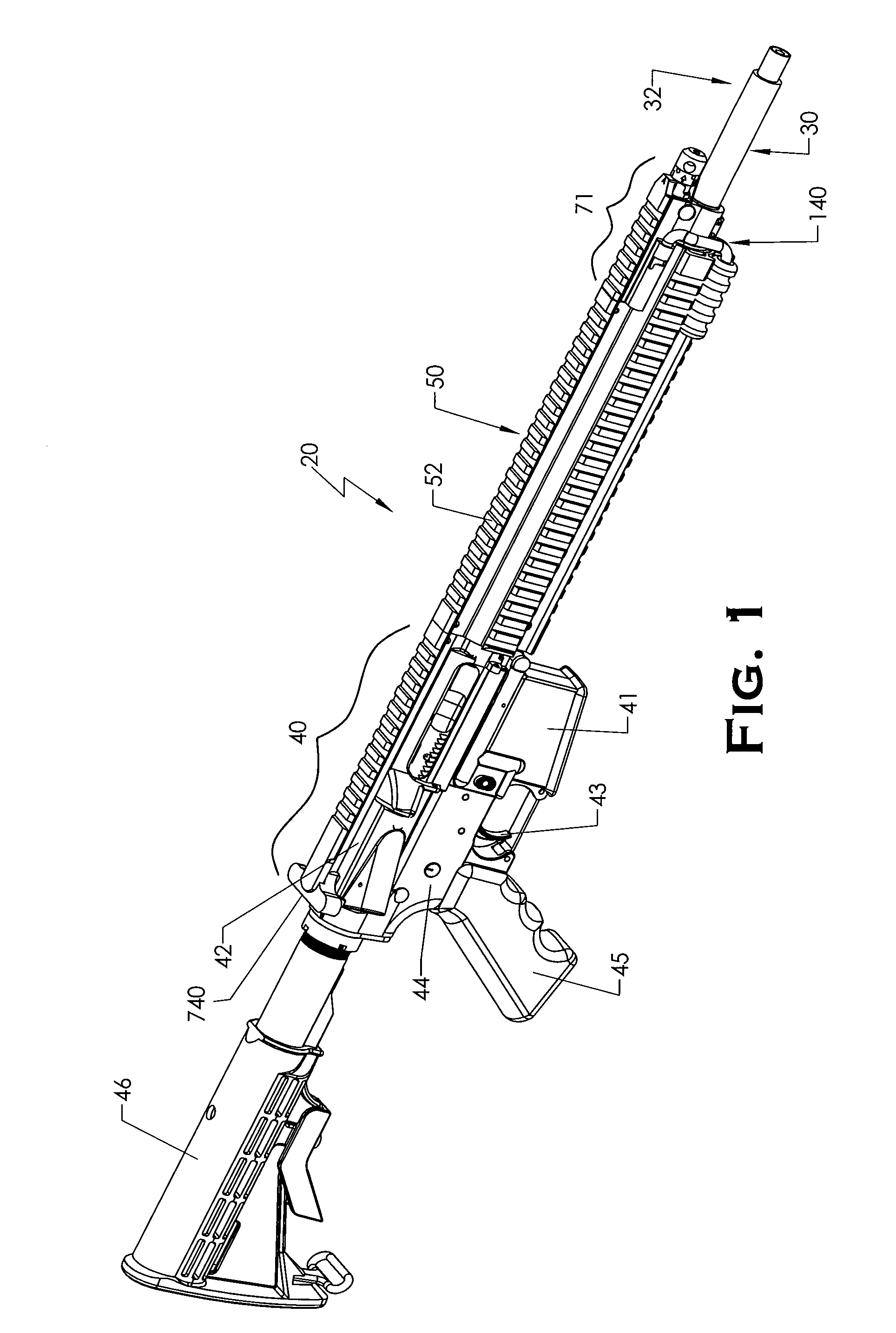
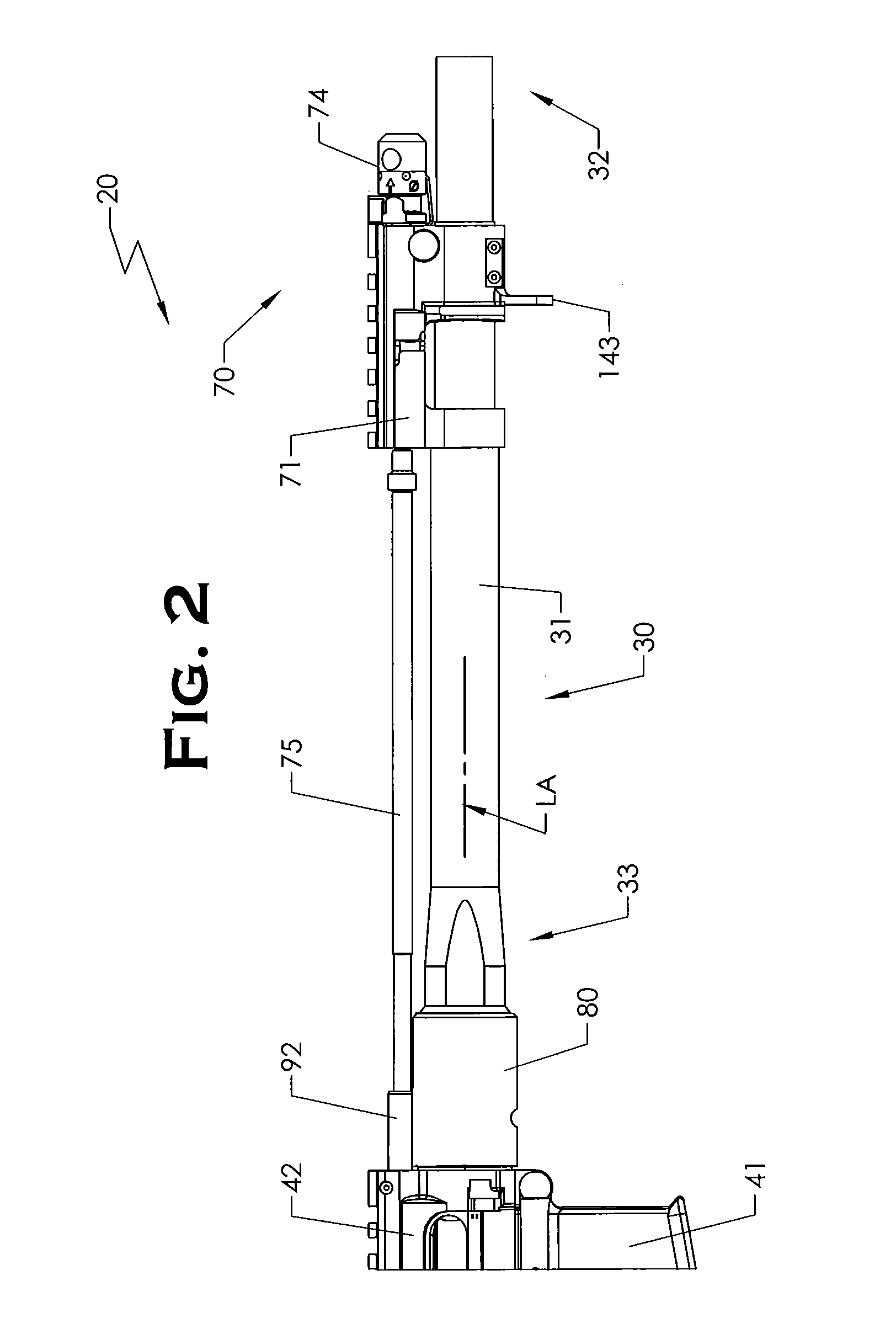
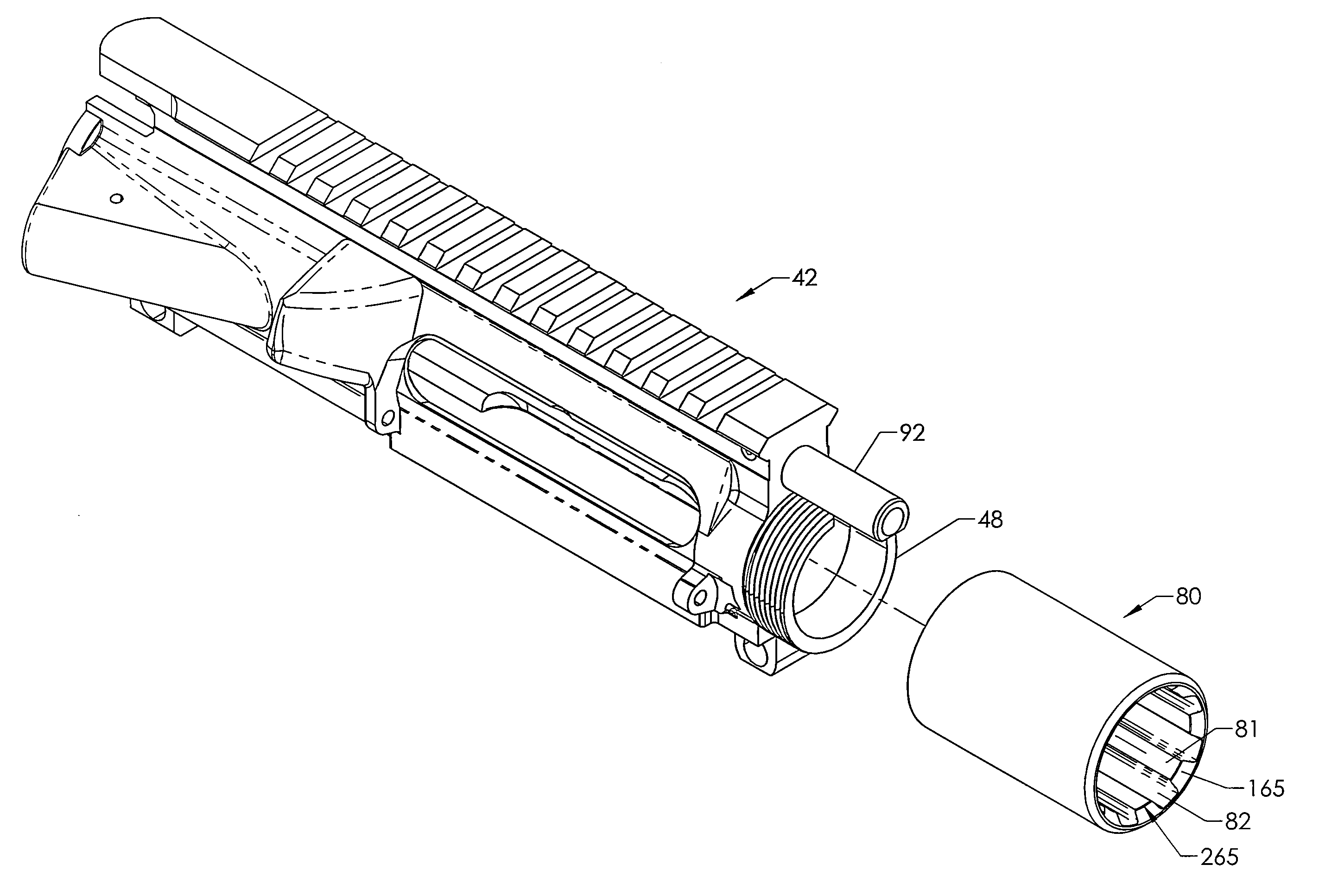
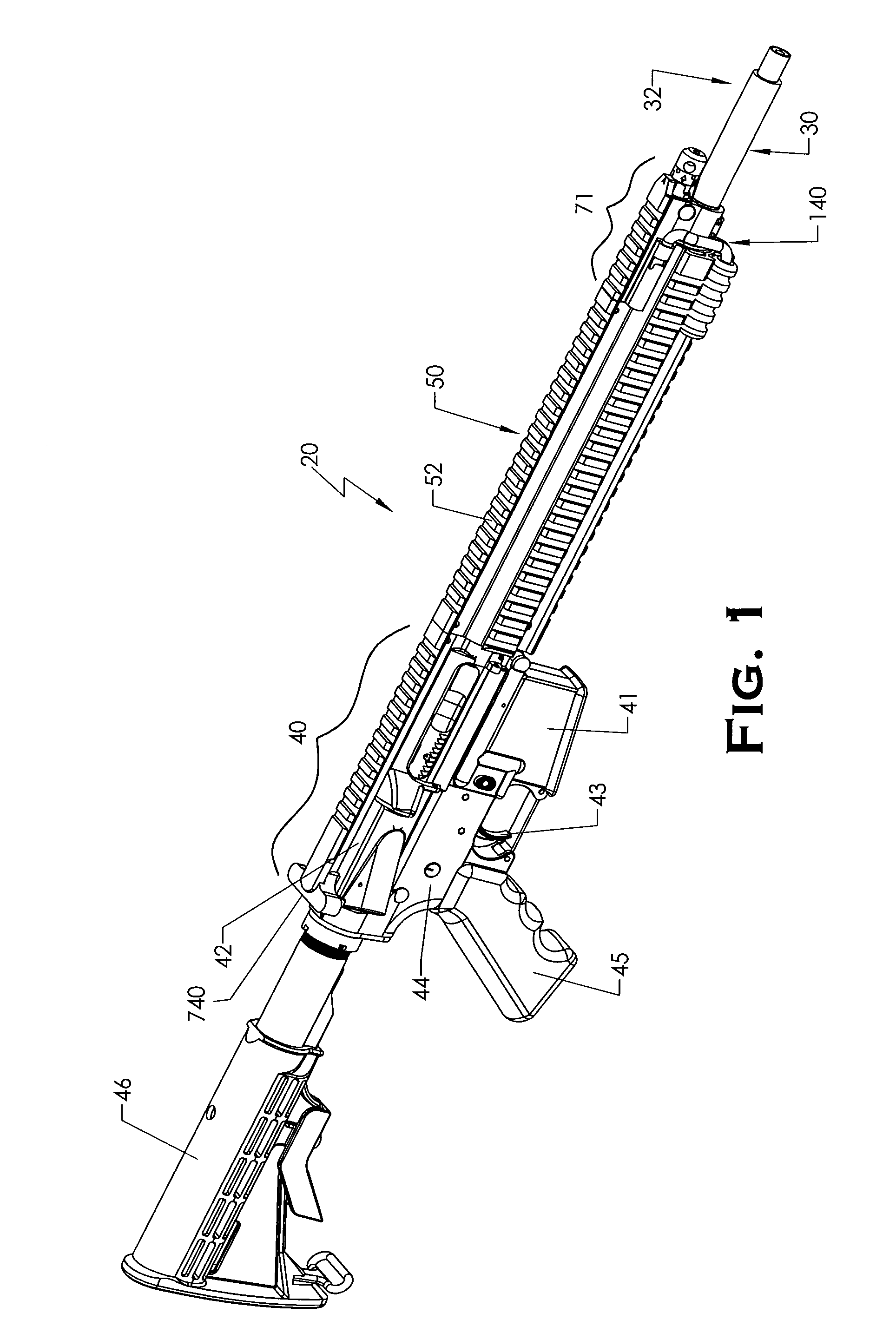
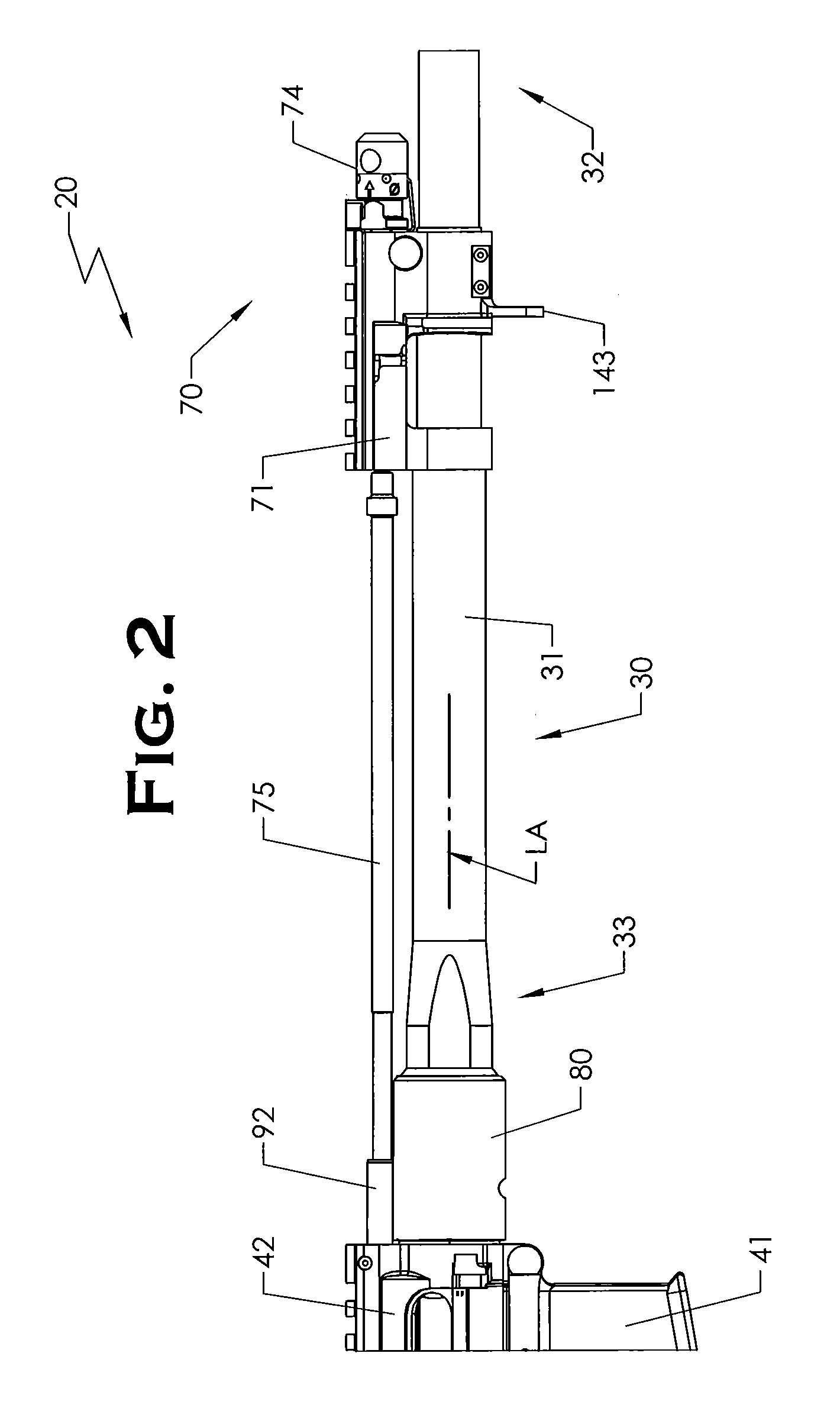
Quick coupling barrel system for firearm
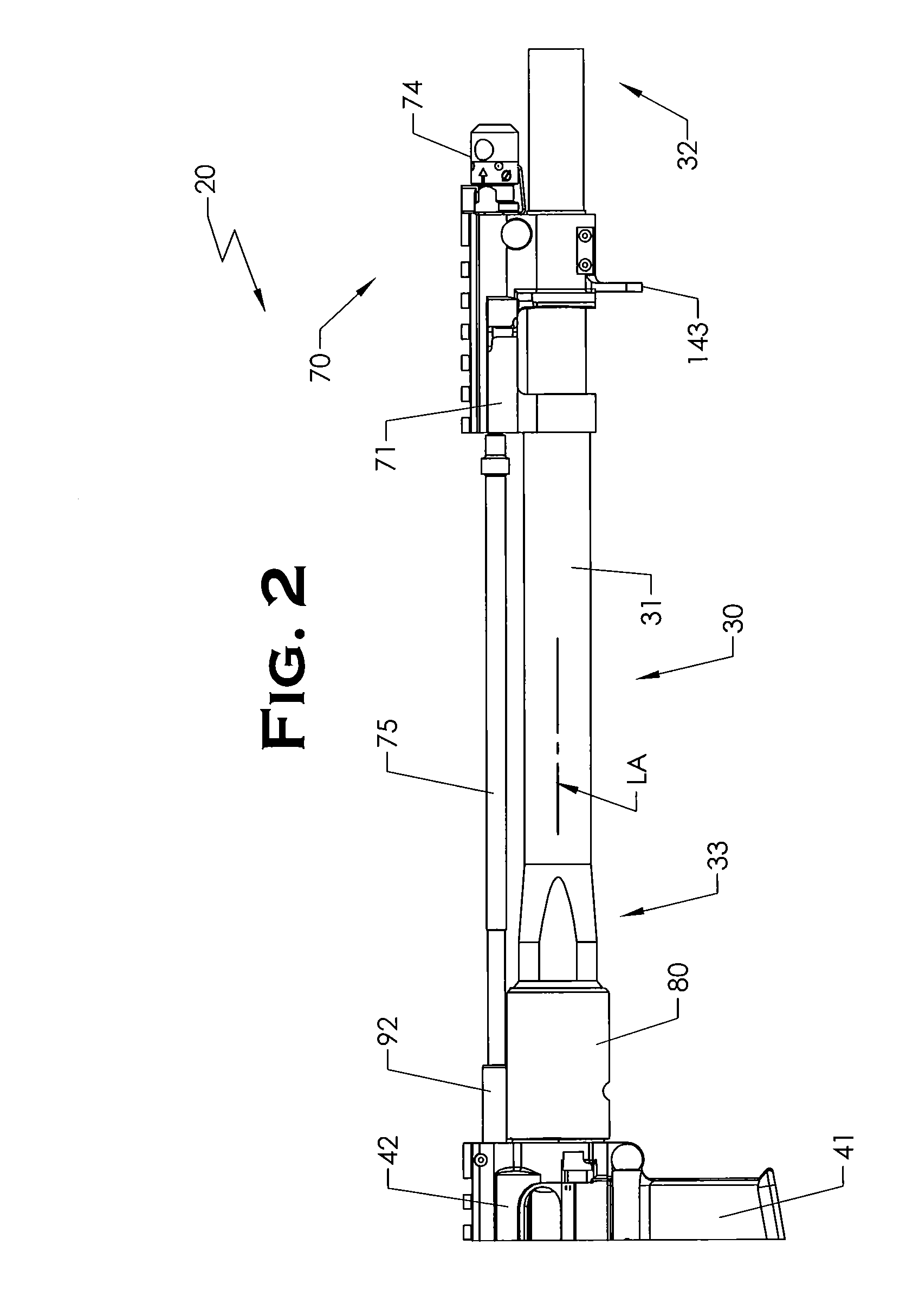
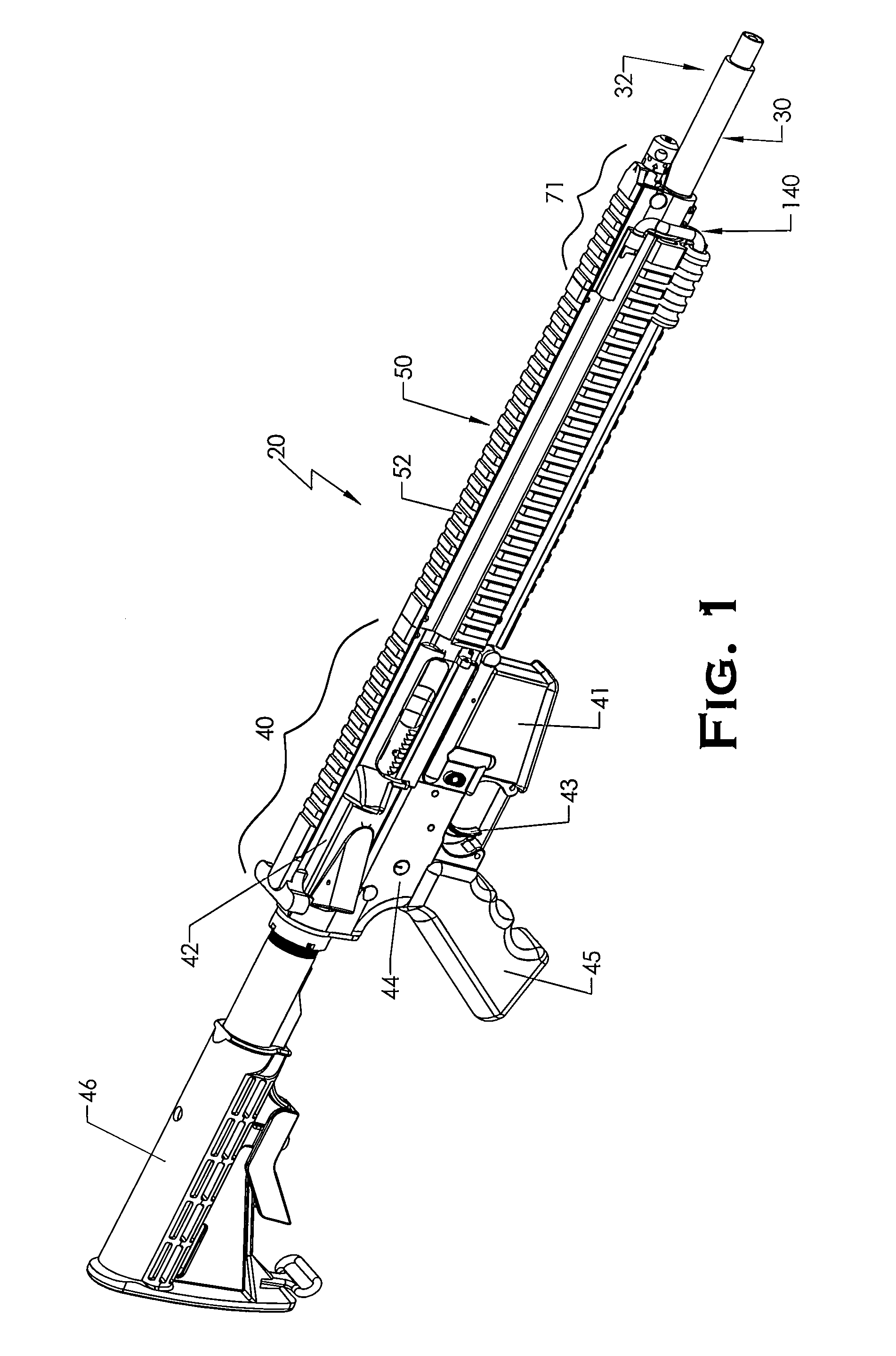
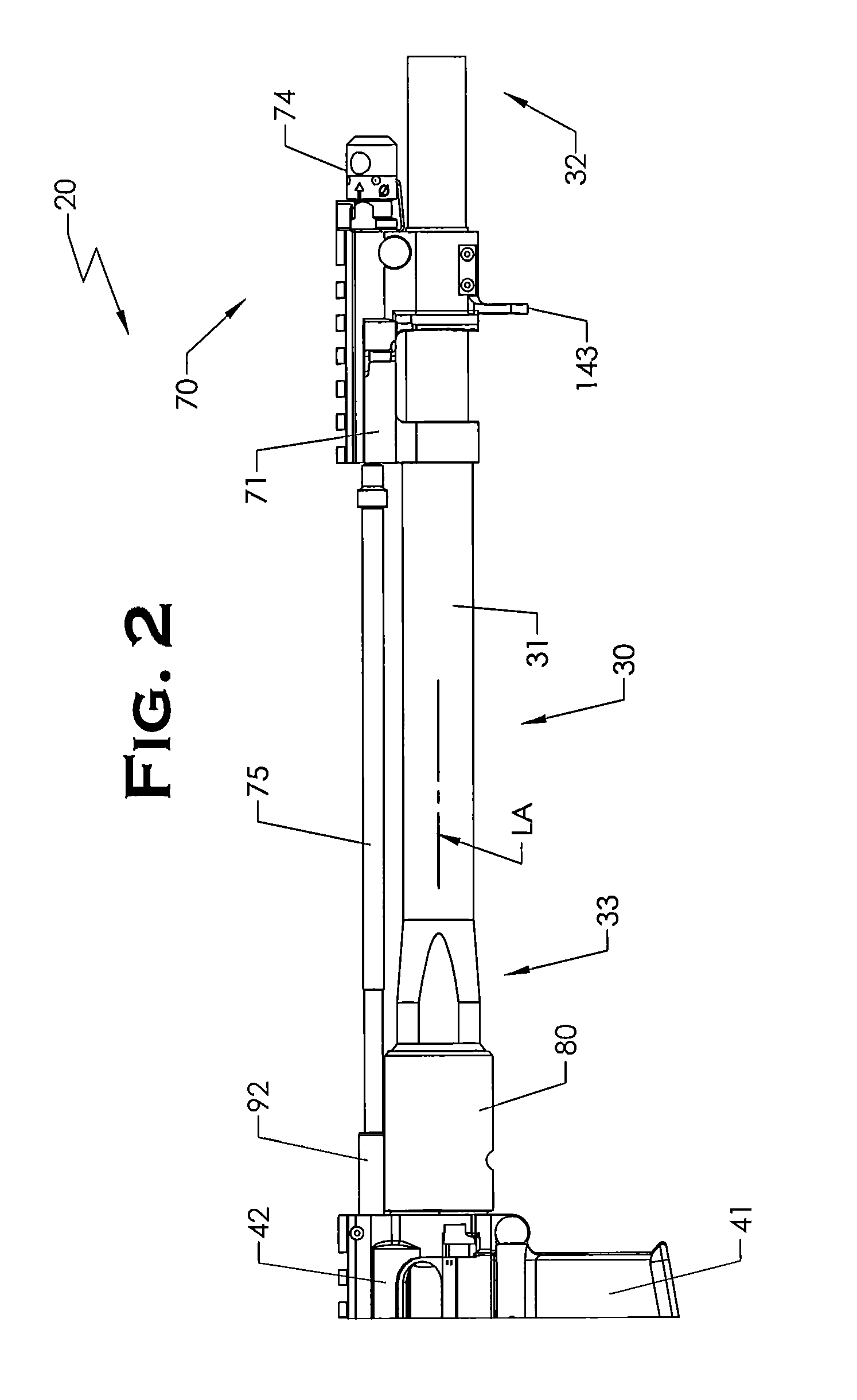
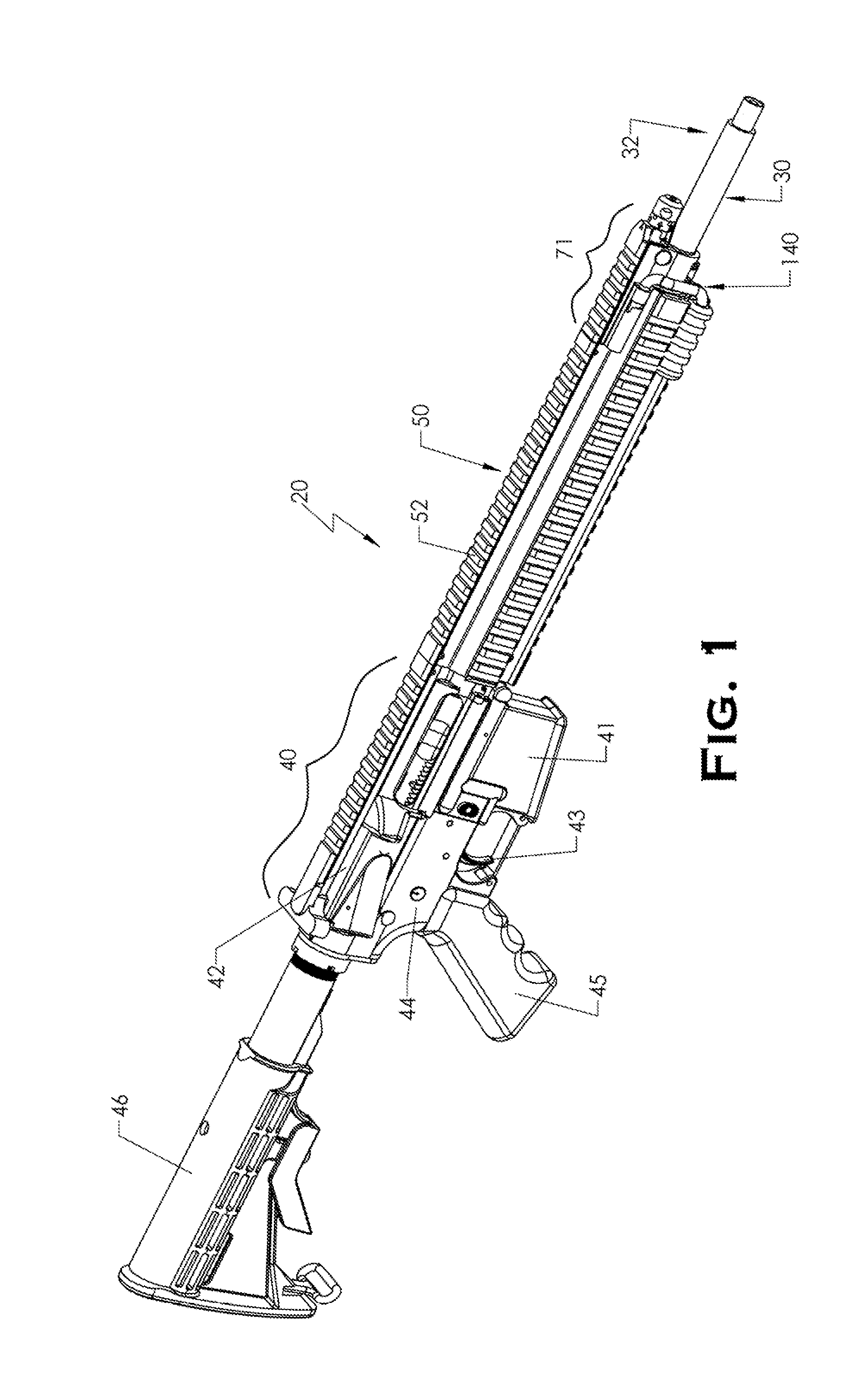
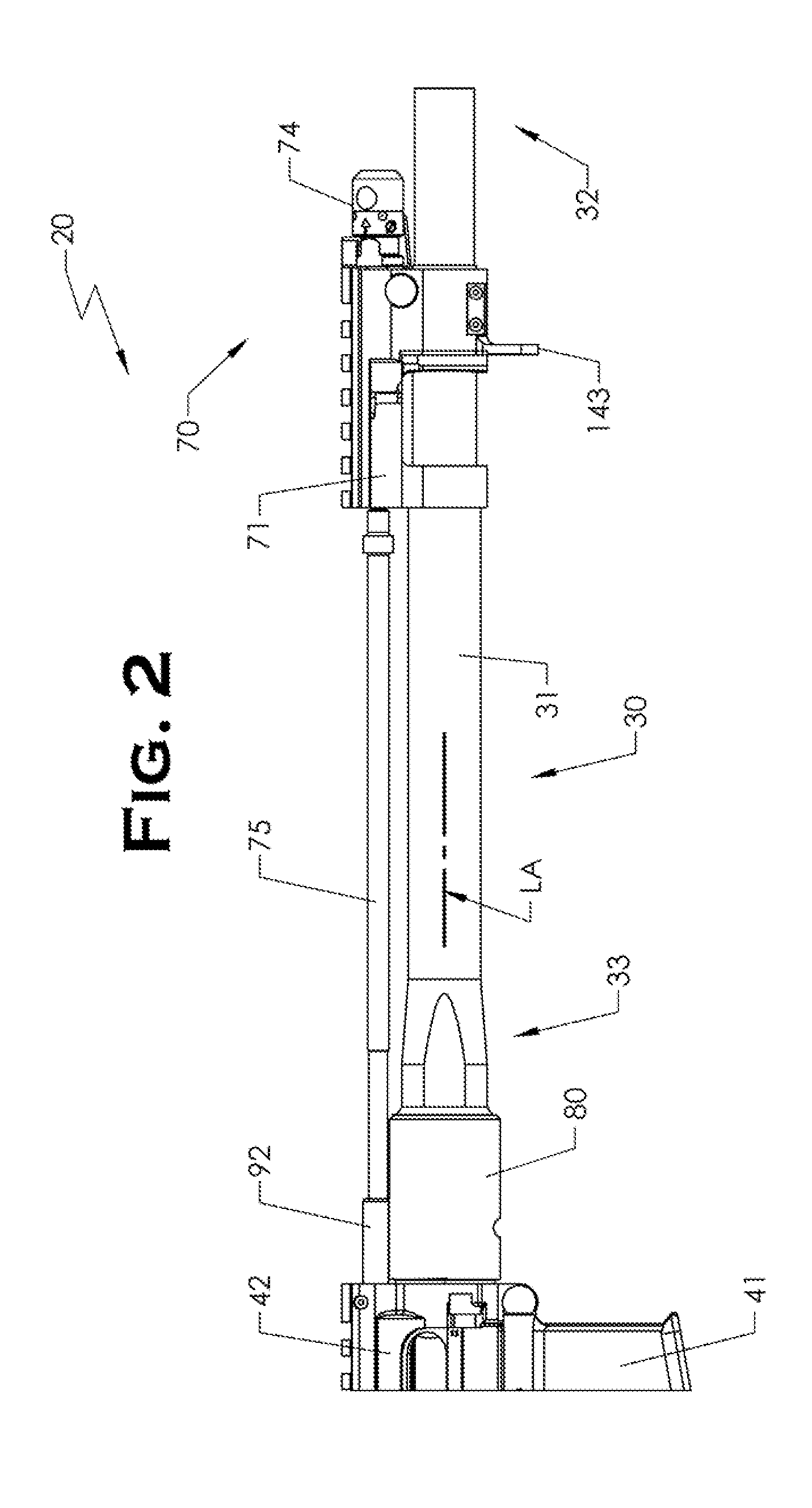
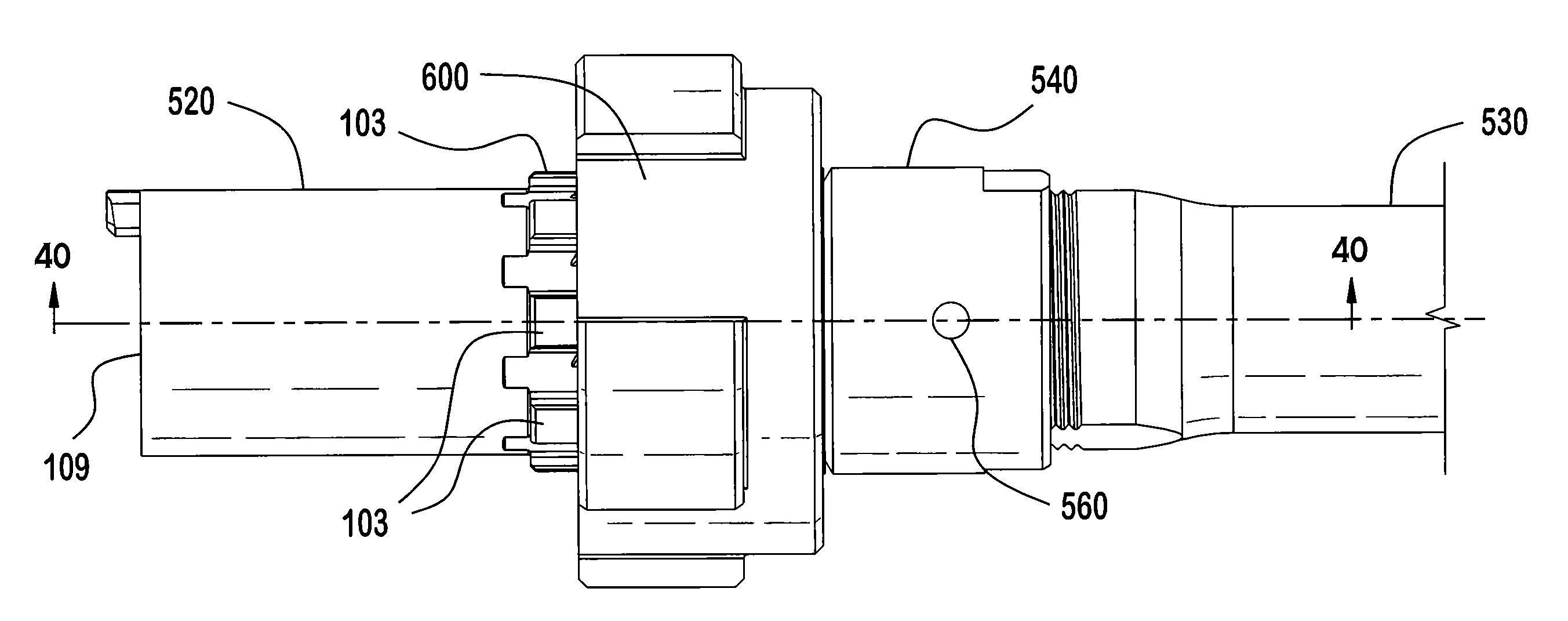
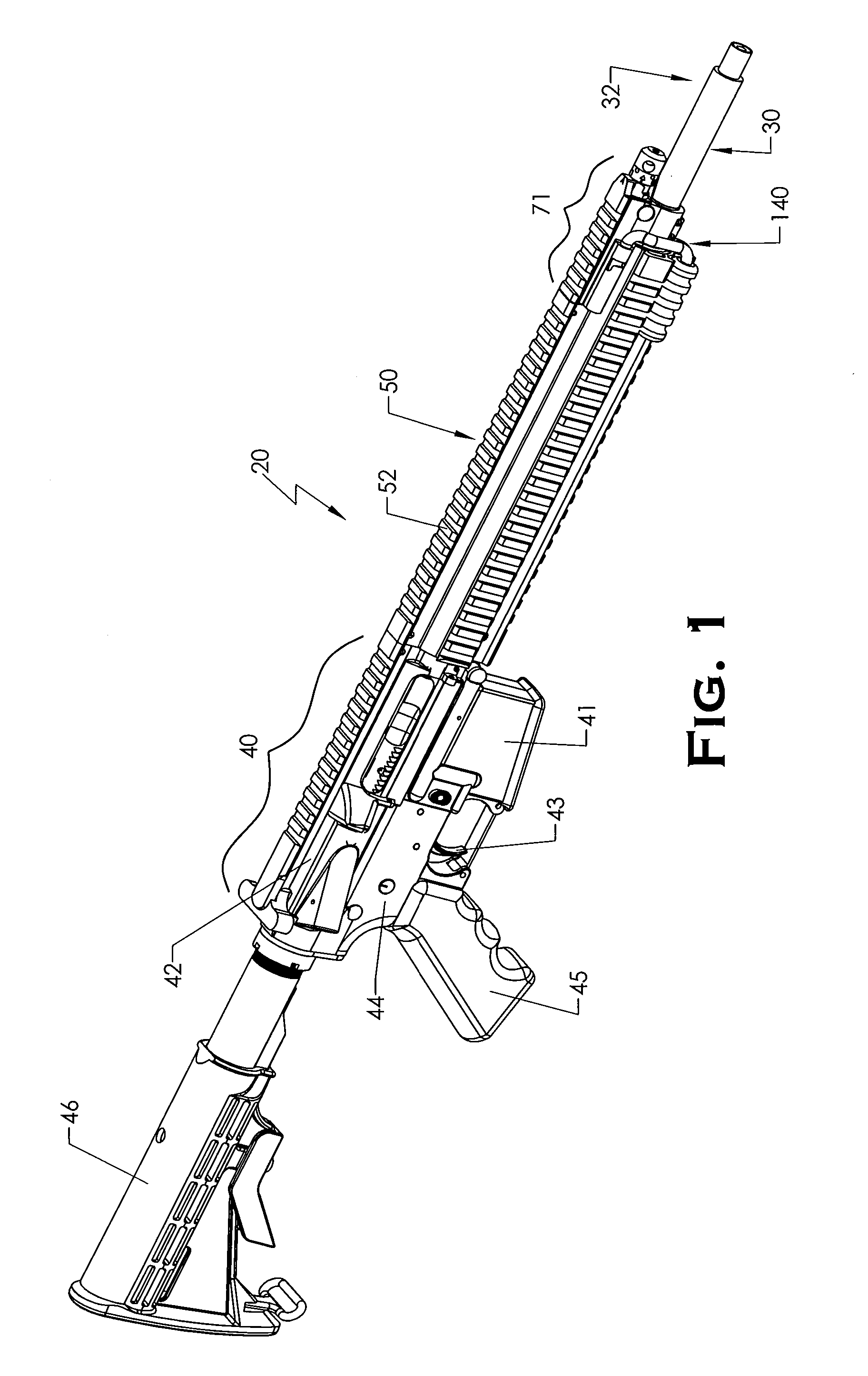
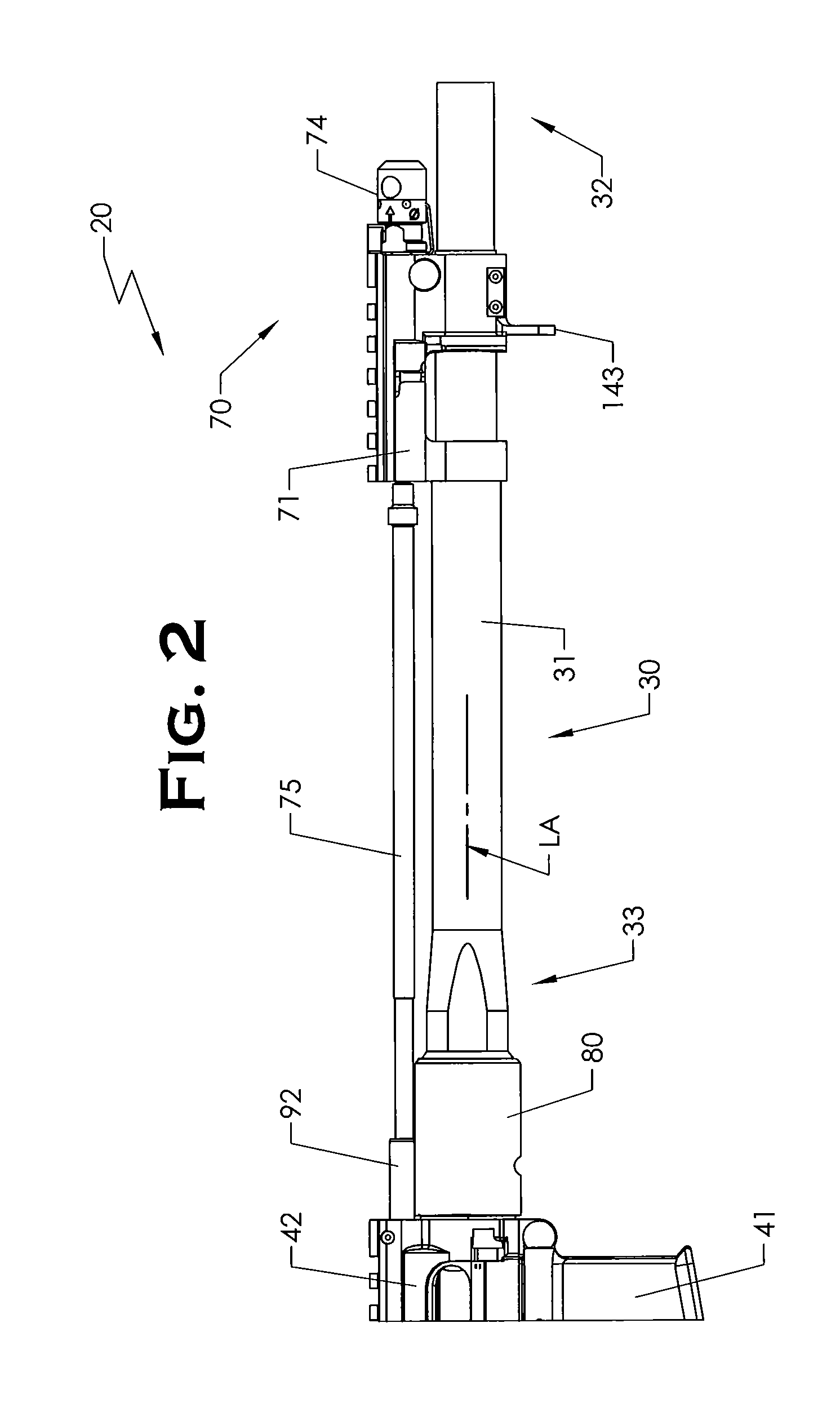
ActiveUS20120131835A1Quick changeImprove tightness and reliabilityBreech mechanismsMetal working apparatusCouplingSpring force
A spring-loaded quick coupling barrel retaining system for a firearm. The firearm includes a receiver, a barrel nut, and barrel assembly rotatably mounted thereto. In one embodiment, the barrel assembly may include barrel locking lugs which rotatably engage and interlock with corresponding locking elements disposed on the barrel nut such a splines. The barrel assembly further includes a spring member forming a flexible interface with the barrel nut. The spring member self-tensions and tightens the lockup between the barrel assembly and barrel nut to promote a tight fit. Some embodiments may include a lock nut and a setting tool for adjusting the spring force to promote consistently proper lockup from one replacement barrel assembly to the next.
Owner:STURM RUGER & CO INC
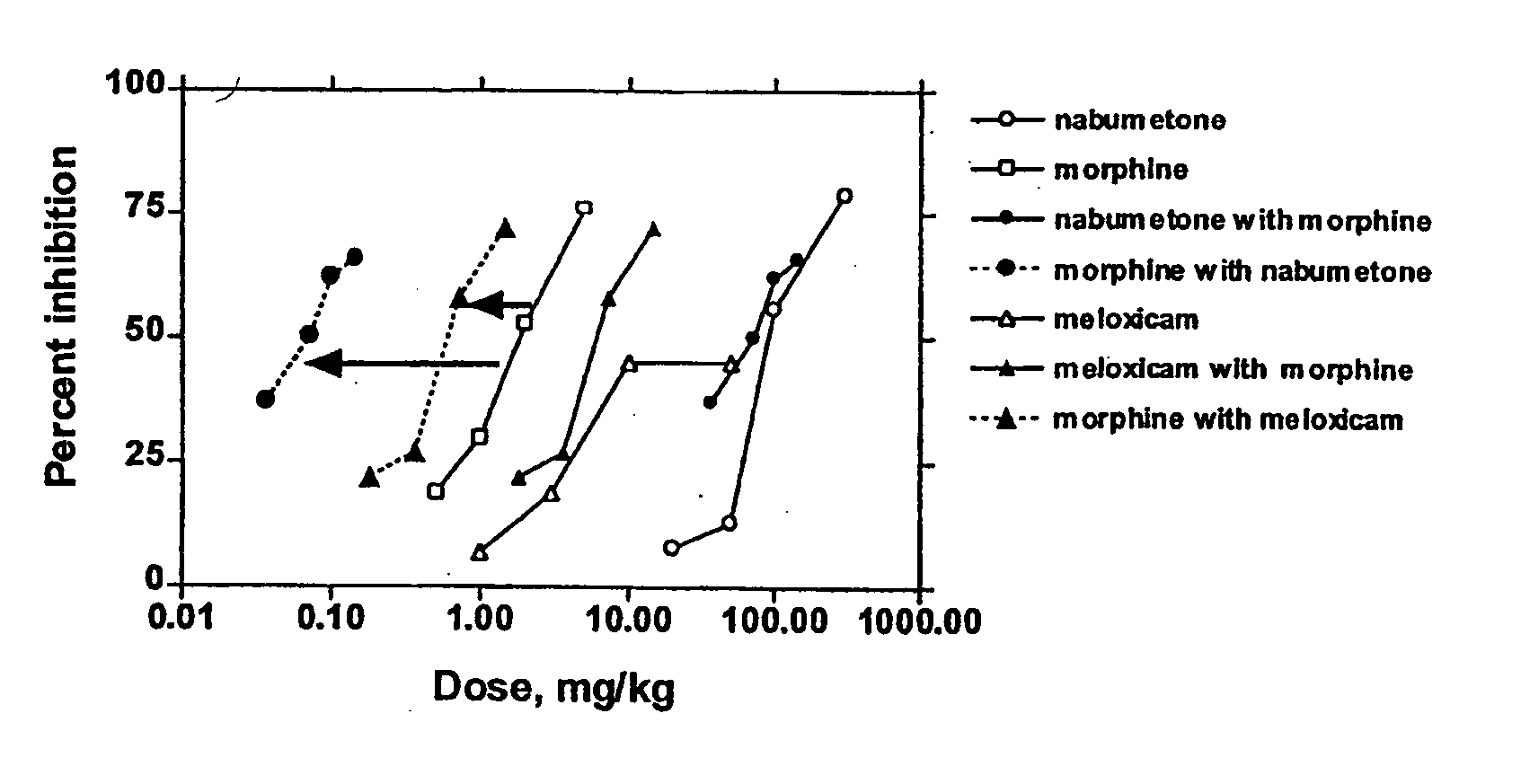
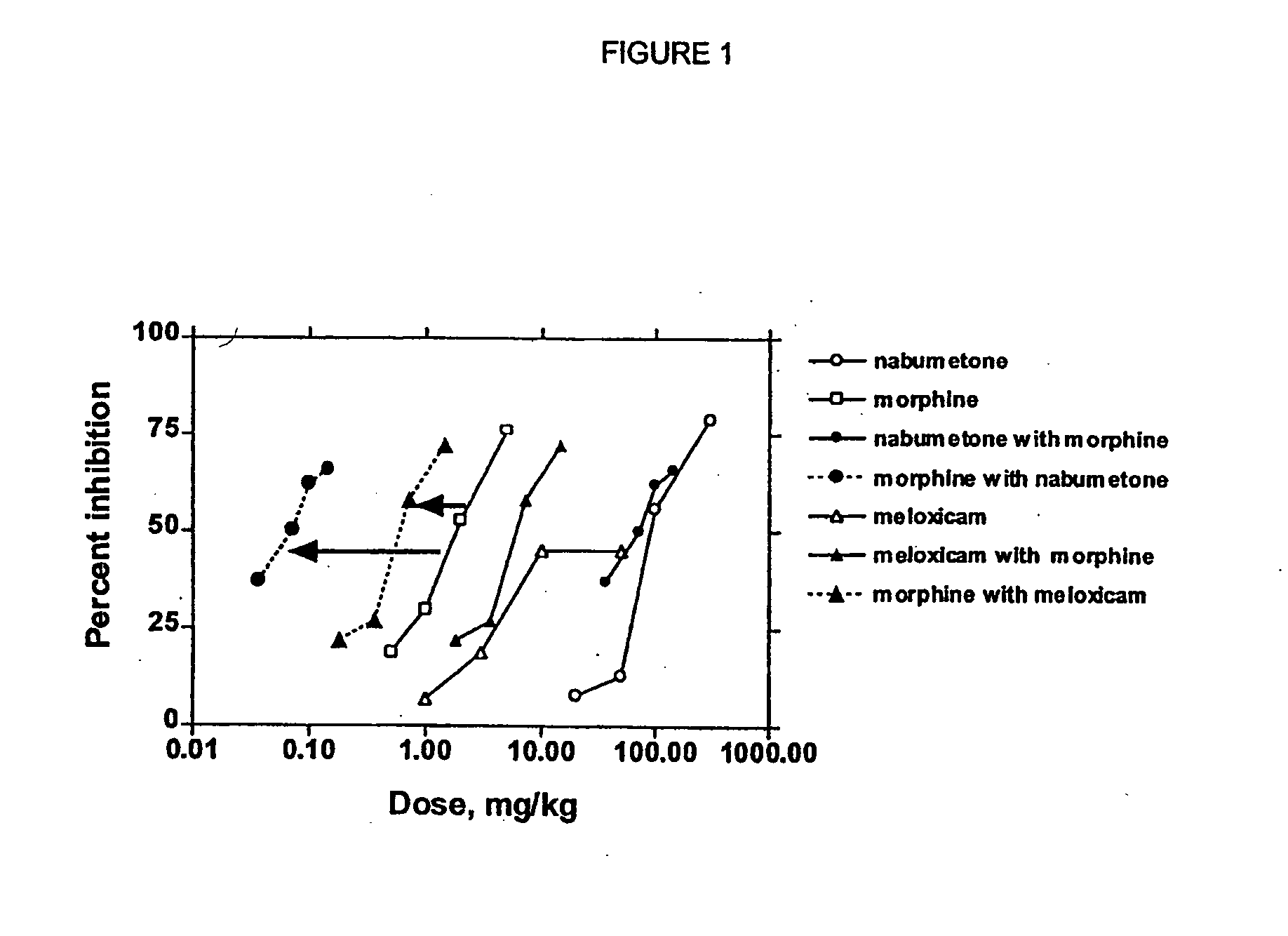
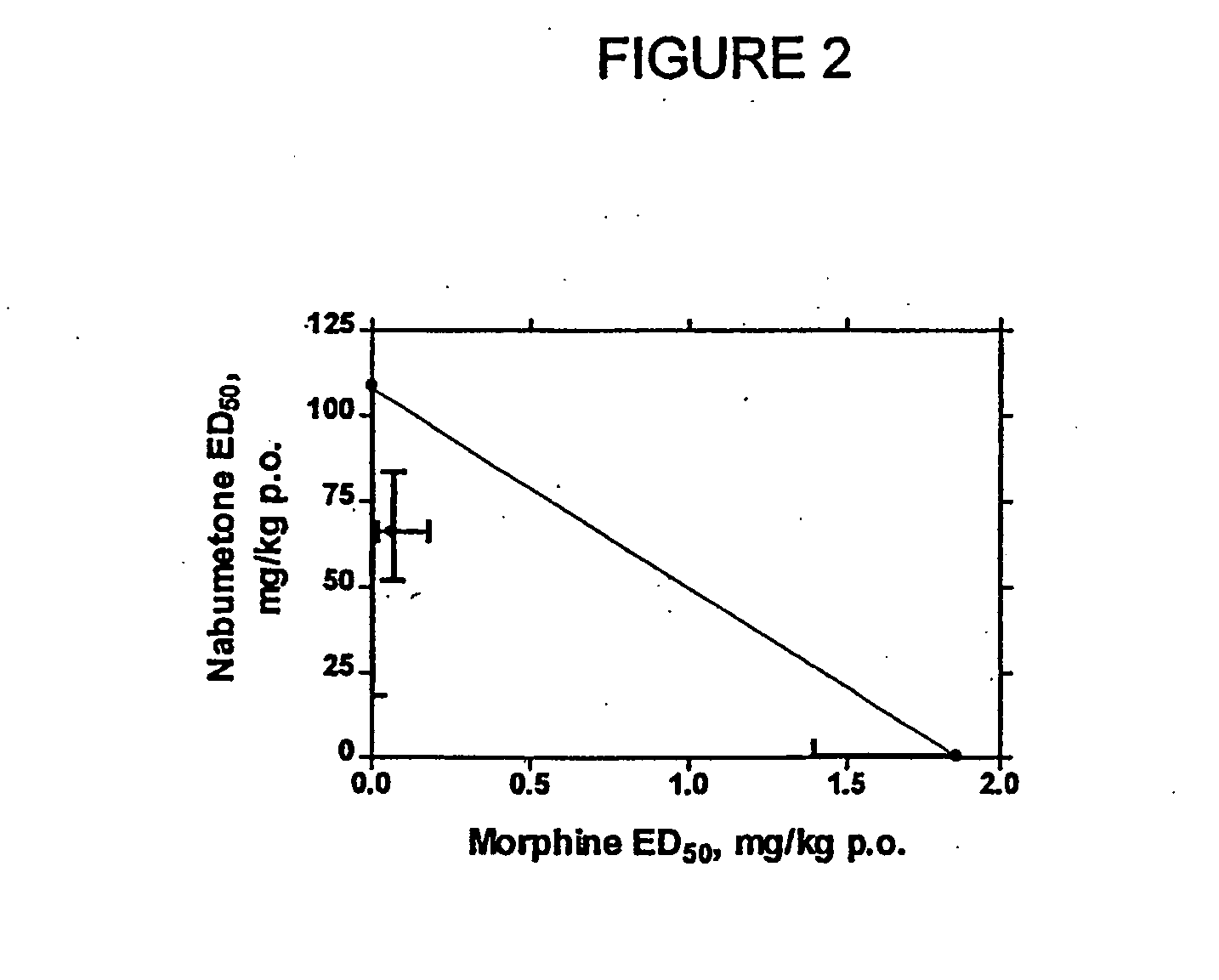
Analgesic combination of tramadol and meloxicam
InactiveUS20080050427A1Reduce concentrationEfficient managementBiocidePowder deliveryMeloxicamPharmaceutical drug
Disclosed is a pharmaceutical composition, comprising a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof, said combination in an amount sufficient to provide an analgesic effect in a human patient. Also disclosed is a method of effectively treating pain in humans or other mammals, comprising administering to the patient a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof such that the dosing interval of the meloxicam overlaps with the dosing interval of the oxycodone, said combination in an amount sufficient to provide an analgesic effect in a human patient.
Owner:PURDUE PHARMA LP
Firearm with quick coupling barrel interlock system
ActiveUS20120216439A1Quick changeImprove tightness and reliabilityBreech mechanismsBarrel mountingCouplingEngineering
A firearm with barrel interlock system for a rotary mounted quick coupling barrel assembly. In one embodiment, the firearm includes a receiver and barrel assembly rotatably mounted thereto. A bolt carrier supporting a bolt is slidably disposed in the receiver and axially movable into and out of battery with the barrel assembly. The barrel interlock system may include a first barrel anti-rotation locking element disposed on the barrel assembly and a mating second barrel anti-rotation locking element disposed on the bolt carrier that is engageable with the first element. When mutually engaged, the first and second locking elements form a meshed relationship and prevent rotation and removal of the barrel assembly from the receiver. In some non-limiting embodiments, the locking elements may be in the form of a protrusion and complementary shaped recess.
Owner:STURM RUGER & CO INC
Firearm with quick coupling barrel system
ActiveUS8479429B2Quick changeImprove tightness and reliabilityBreech mechanismsMetal working apparatusCouplingSpring force
A spring-loaded quick coupling barrel retaining system for a firearm. The firearm includes a receiver, a barrel nut, and barrel assembly rotatably mounted thereto. In one embodiment, the barrel assembly may include barrel locking lugs which rotatably engage and interlock with corresponding locking elements disposed on the barrel nut such a splines. The barrel assembly further includes a spring member forming a flexible interface with the barrel nut. The spring member self-tensions and tightens the lockup between the barrel assembly and barrel nut to promote a tight fit. Some embodiments may include a lock nut and a setting tool for adjusting the spring force to promote consistently proper lockup from one replacement barrel assembly to the next.
Owner:STURM RUGER & CO INC
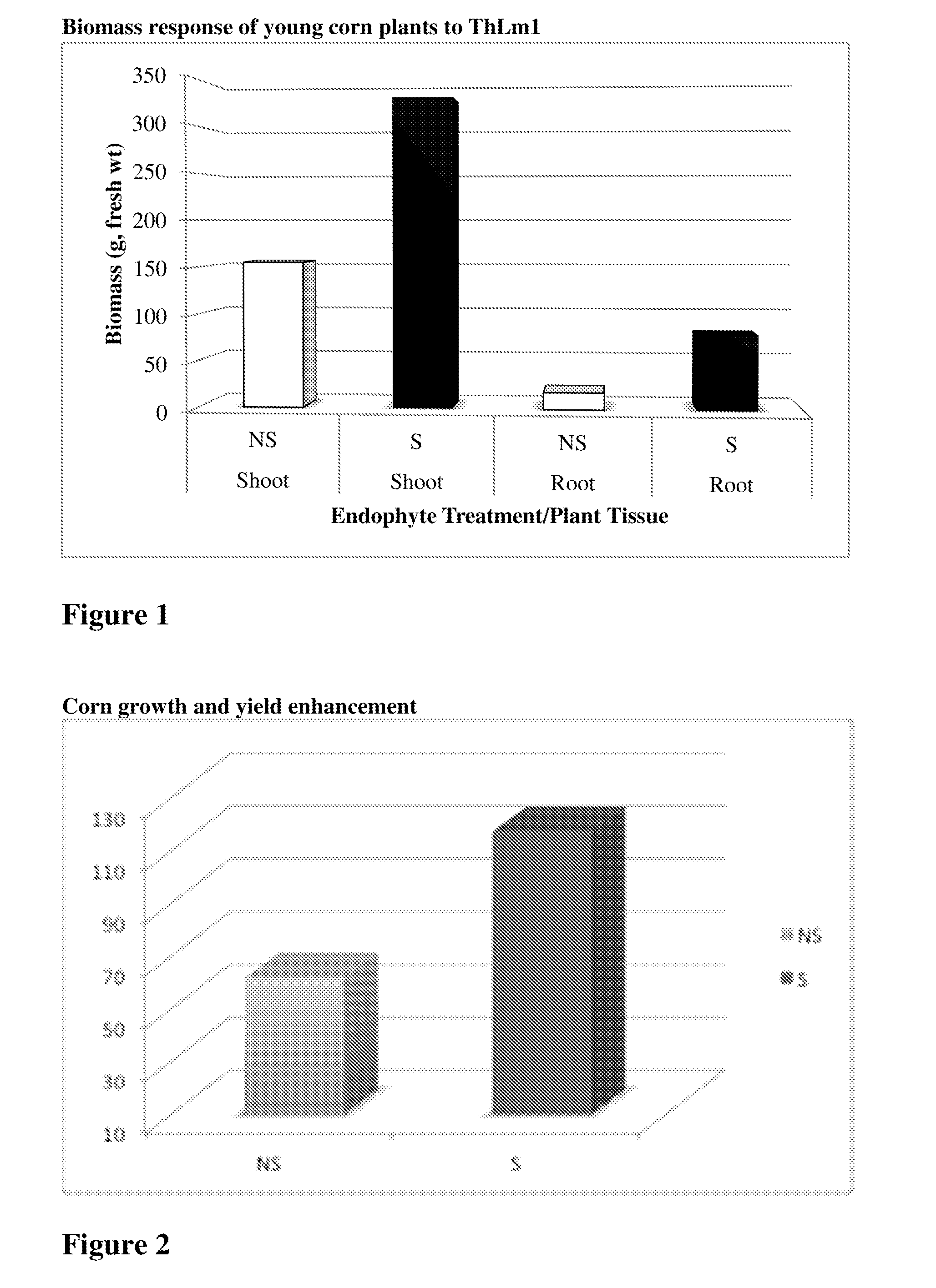
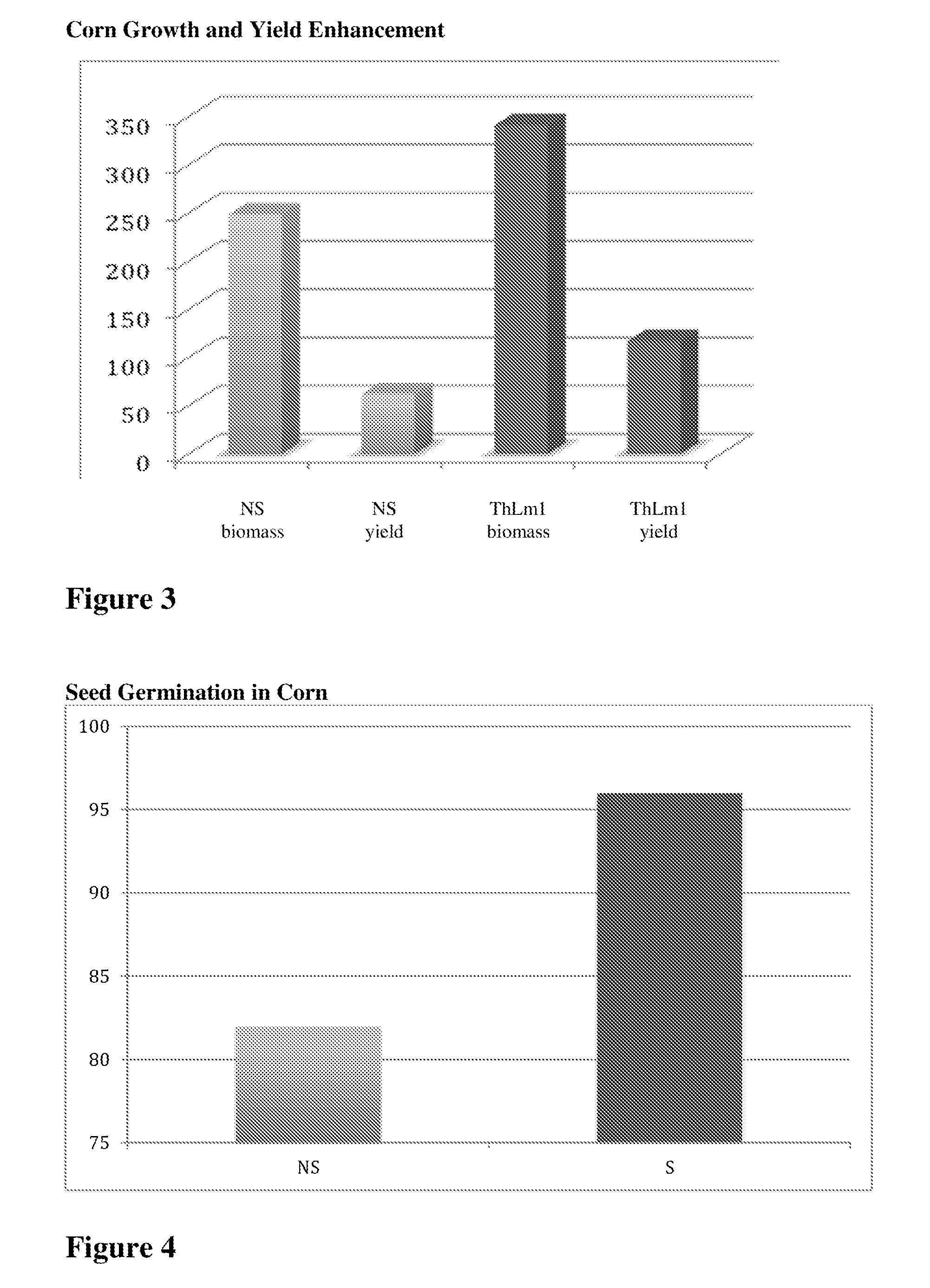
Compositions and methods related to isolated endophytes
ActiveUS20150033420A1Reduce agencyPromoting stress toleranceBiocideFungiBiotechnologyFungal endophyte
Described herein are compositions and methods related to isolated Trichoderma harzianum and strains thereof.
Owner:CRAFT3
Injector
ActiveUS7955304B2Low costReduce componentsAutomatic syringesIntravenous devicesBiomedical engineeringInjection device
The present invention relates to a method of operating an injection device, which device comprises a medicament cartridge and a needle attached to said cartridge, means for automatic penetration of needle, injection of medicament and withdrawal of needle, comprising the steps of initiating a penetration sequence, followed by an injection sequence and followed by a withdrawal sequence, wherein a previous sequence triggers a subsequent sequence, and wherein the subsequent sequence is triggered before the previous sequence has ended.
Owner:SHL MEDICAL AG
Pump aggregate for a hydraulic vehicle braking system
InactiveUS7168929B2Easy to bendImprove rigidityPositive displacement pump componentsBraking componentsRadial piston pumpTorsional strength
A pump unit for a hydraulic vehicle brake system with traction control has an electric motor driving a radial piston pump. A rotor shaft of the pump unit has a hollow shaft with two standardized, hardened cylindrical pins that are press-fitted into the ends of the hollow shaft. The rotor shaft can be produced simply, economically, and without metal-cutting machining. The hollow shaft has high bending and torsional strength. The rotor shaft has a small diameter at the bearing points, which makes a small bearing diameter and thus a small installation space for the pump unit possible.
Owner:ROBERT BOSCH GMBH
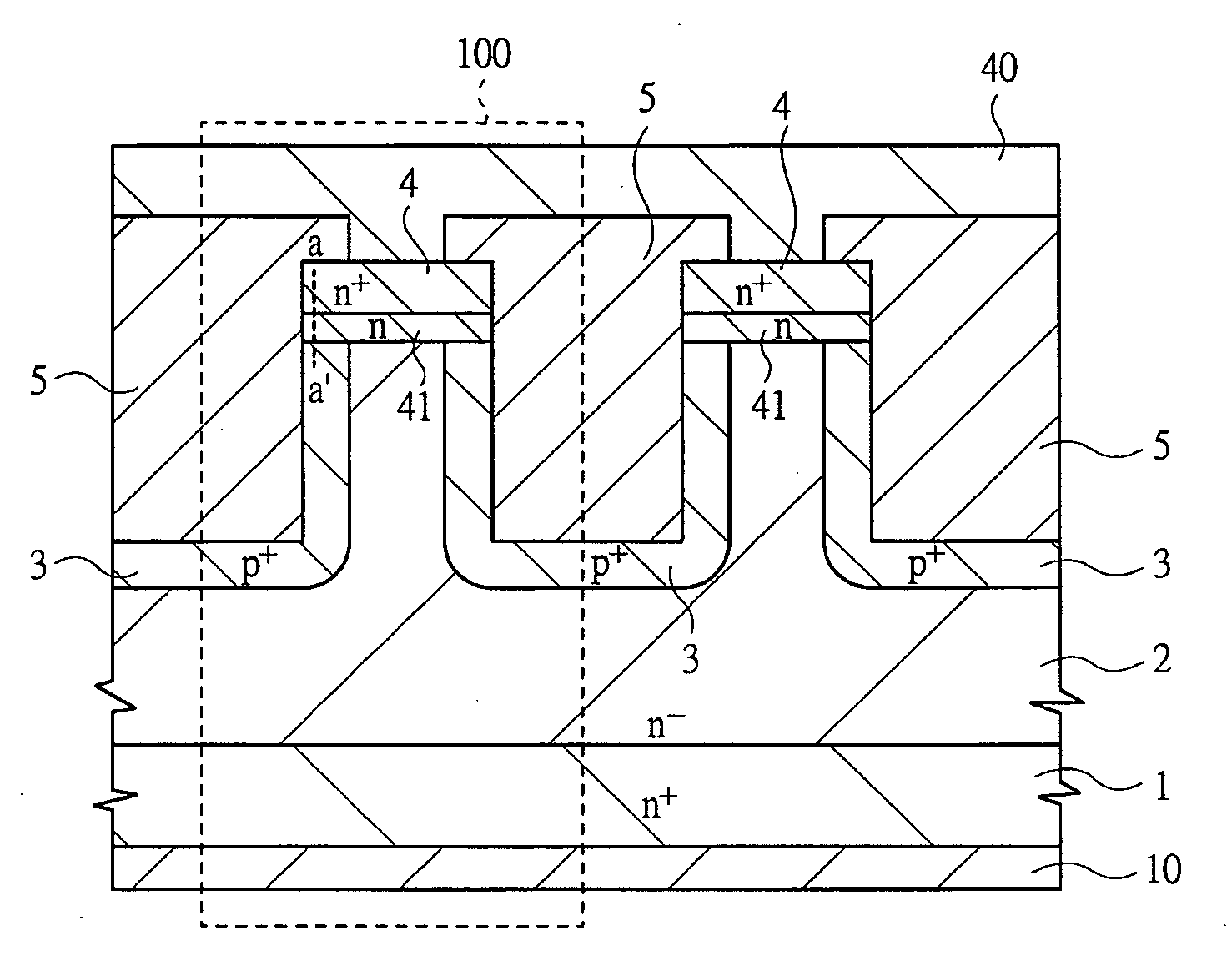
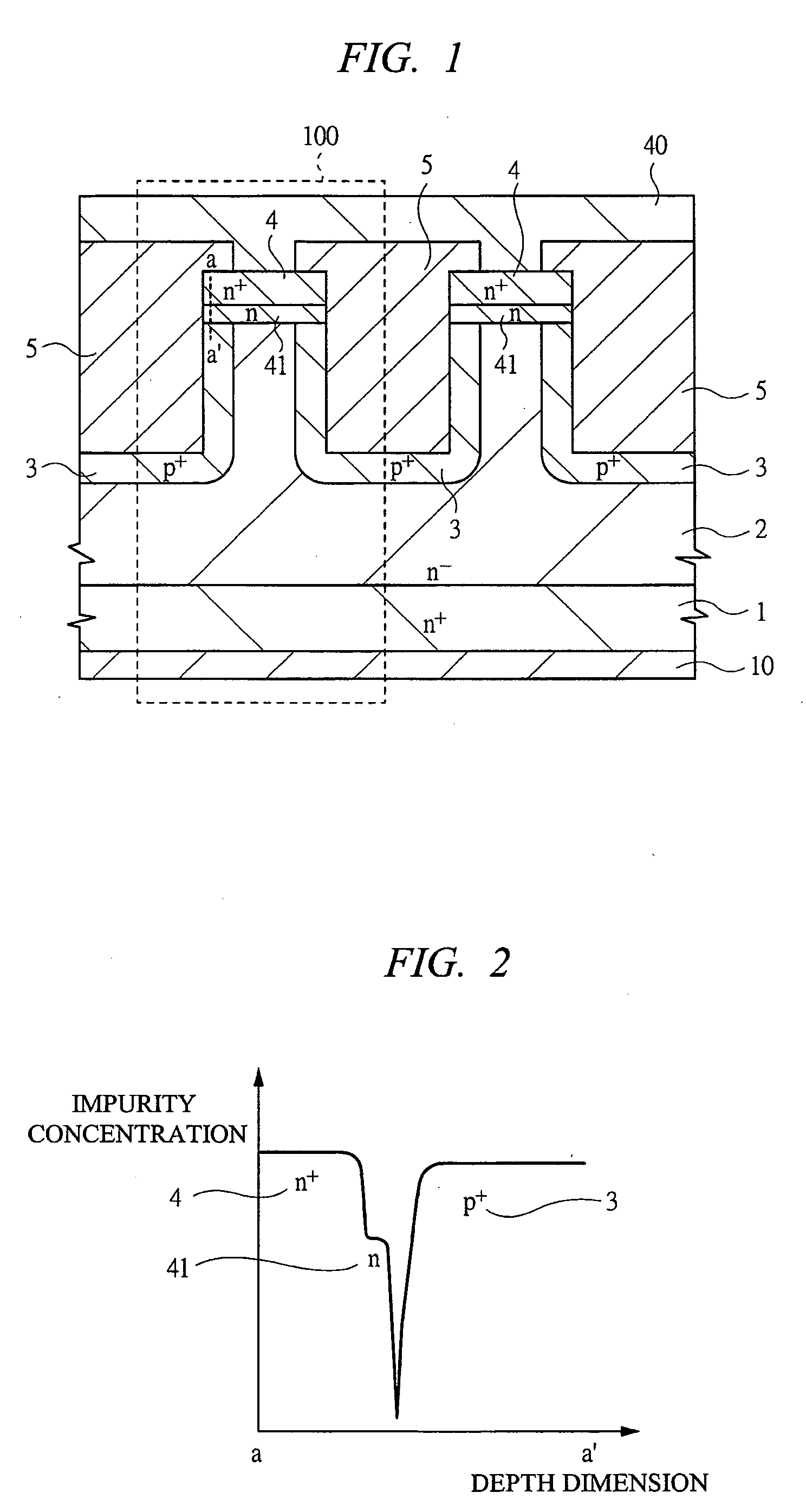
Switching semiconductor devices and fabrication process
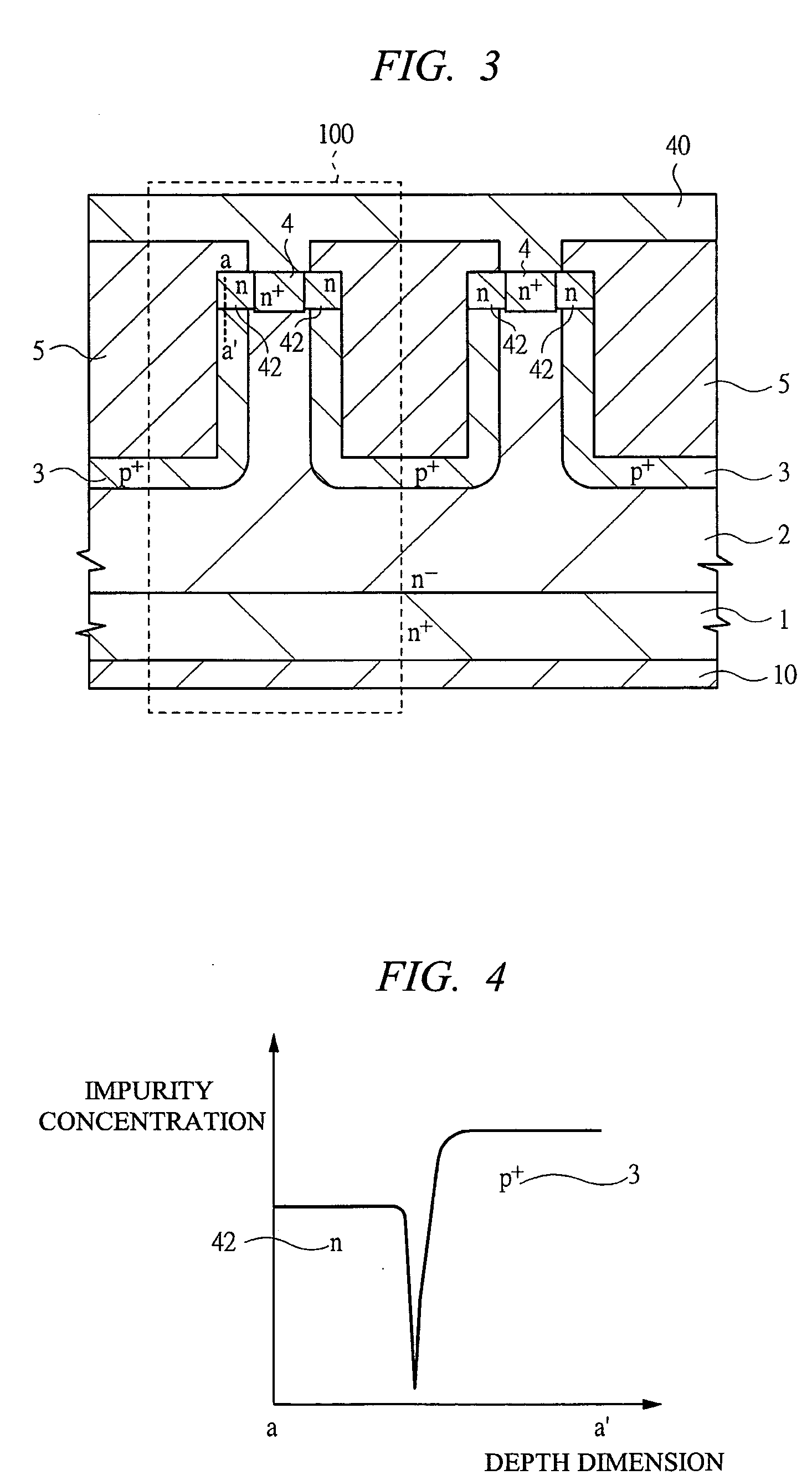
InactiveUS20070096145A1Avoid misuseImprove breakdown voltageSemiconductor devicesManufacturing technologyDevice material
A switching semiconductor device is provided, in which a negative gate voltage can be applied to the semiconductor device in an OFF state so as to increase a breakdown voltage of the gate junction without impairing a normally-off function of the semiconductor device and the ON-resistance. The switching semiconductor device is fabricated by using a semiconductor substrate with a band gap of 2.0 eV or more. In a JFET structure where a p+ type gate region and an n type source region are in contact so that a negative gate voltage can be applied, the p+ type gate region and an n+ type source region with a high impurity concentration are disposed with interposing an n type source region with an impurity concentration lower than that of the p+ type gate region and higher than that of a drift region of the JFET therebetween.
Owner:RENESAS ELECTRONICS CORP
Firearm with quick coupling barrel interlock system
ActiveUS8505227B2Quick changeImprove tightness and reliabilityBreech mechanismsBarrel mountingCouplingEngineering
A firearm with barrel interlock system for a rotary mounted quick coupling barrel assembly. In one embodiment, the firearm includes a receiver and barrel assembly rotatably mounted thereto. A bolt carrier supporting a bolt is slidably disposed in the receiver and axially movable into and out of battery with the barrel assembly. The barrel interlock system may include a first barrel anti-rotation locking element disposed on the barrel assembly and a mating second barrel anti-rotation locking element disposed on the bolt carrier that is engageable with the first element. When mutually engaged, the first and second locking elements form a meshed relationship and prevent rotation and removal of the barrel assembly from the receiver. In some non-limiting embodiments, the locking elements may be in the form of a protrusion and complementary shaped recess.
Owner:STURM RUGER & CO INC
Cyclonic separating apparatus for a cleaning appliance
InactiveUS8375509B2Improve sealingIncrease the areaCleaning filter meansSuction filtersCyclonic separationEngineering
A cyclonic separating apparatus for a cleaning appliance includes a plurality of cyclonic separators arranged in series for separating particles from a dirt- and dust-laden airflow, at least three collectors for collecting separated dirt and dust, and a closure member movable between a closed position in which the closure member closes an end of each collector and an open position in which separated dirt and dust can be emptied from the collectors. The ends of the collectors are separated by dividing walls. A seal is provided to seal between the closure member and the dividing walls when the closure member is in the closed position. This common seal between the dividing walls and the closure member is able to seal effectively even if the closure member is misaligned or incorrectly fitted.
Owner:DYSON TECH LTD
Firearm with quick coupling barrel system
ActiveUS20150007478A1Quick changeImprove tightness and reliabilityBreech mechanismsBarrel mountingCouplingSpring force
A spring-loaded quick coupling barrel retaining system for a firearm. The firearm includes a receiver, a barrel nut, and barrel assembly rotatably mounted thereto. In one embodiment, the barrel assembly may include barrel locking lugs which rotatably engage and interlock with corresponding locking elements disposed on the barrel nut such as splines. The barrel assembly further includes a spring member forming a flexible interface with the barrel nut. The spring member self-tensions and tightens the lockup between the barrel assembly and barrel nut to promote a tight fit. Some embodiments may include a lock nut and a setting tool for adjusting the spring force to promote consistently proper lockup from one replacement barrel assembly to the next.
Owner:STURM RUGER & CO INC
Firearm with quick coupling barrel system
ActiveUS20120131834A1Quick changeImprove tightness and reliabilityBreech mechanismsMetal working apparatusCouplingSpring force
A spring-loaded quick coupling barrel retaining system for a firearm. The firearm includes a receiver, a barrel nut, and barrel assembly rotatably mounted thereto. In one embodiment, the barrel assembly may include barrel locking lugs which rotatably engage and interlock with corresponding locking elements disposed on the barrel nut such a splines. The barrel assembly further includes a spring member forming a flexible interface with the barrel nut. The spring member self-tensions and tightens the lockup between the barrel assembly and barrel nut to promote a tight fit. Some embodiments may include a lock nut and a setting tool for adjusting the spring force to promote consistently proper lockup from one replacement barrel assembly to the next.
Owner:STURM RUGER & CO INC
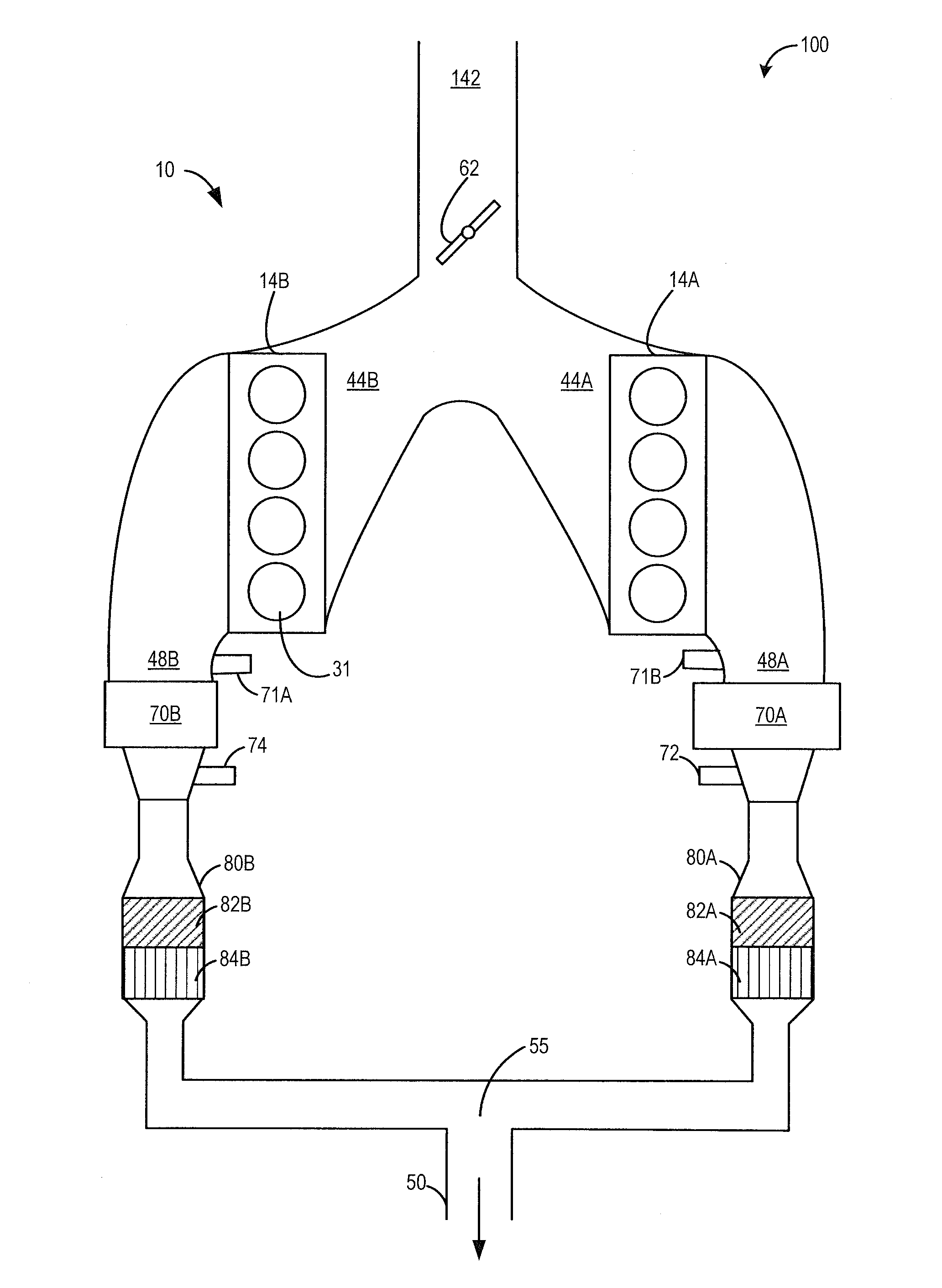
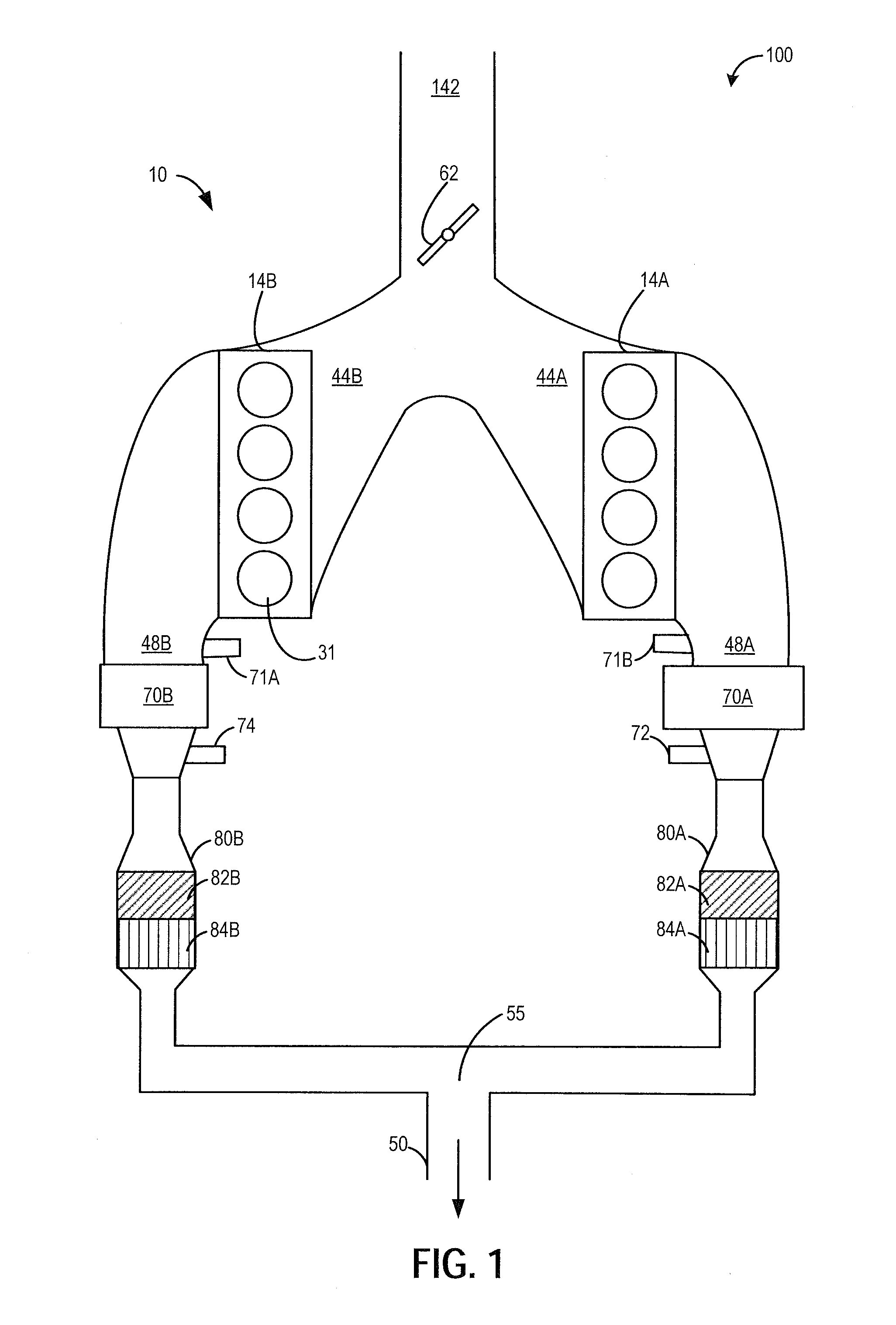
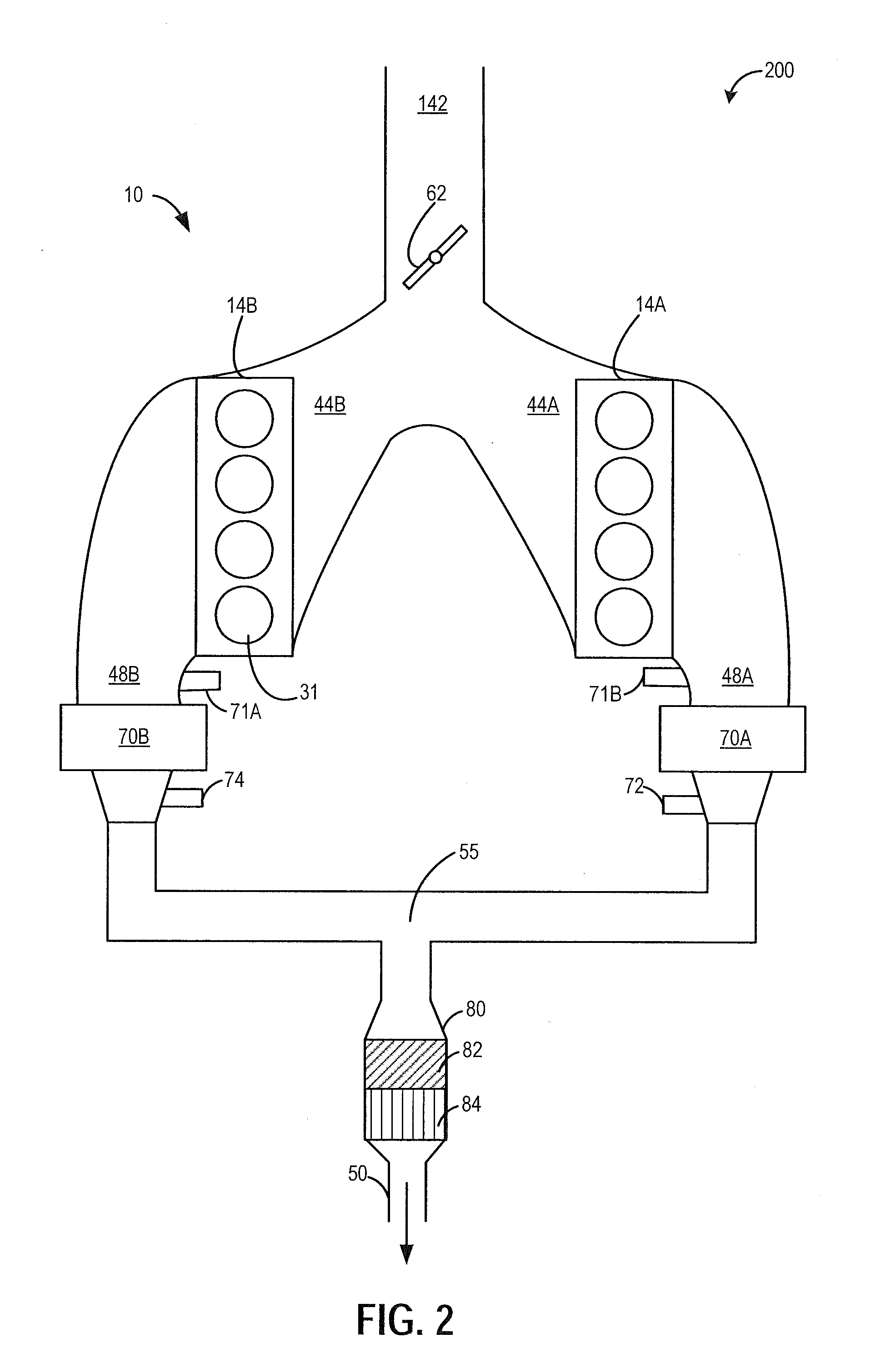
Variable displacement engine control system and method
ActiveUS20150051809A1Improve combustion stabilityDelay transitionElectrical controlInternal combustion piston enginesCombustionControl system
Methods and systems are provided for improving the performance of a variable displacement engine. Split injection and spark retard may be used in active cylinder during a VDE mode to heat an exhaust catalyst and extend the duration of VDE mode operation. Split injection and spark retard may also be used in reactivated cylinders at a time of cylinder reactivation to improve restart combustion stability.
Owner:FORD GLOBAL TECH LLC
Electronic oven with infrared evaluative control
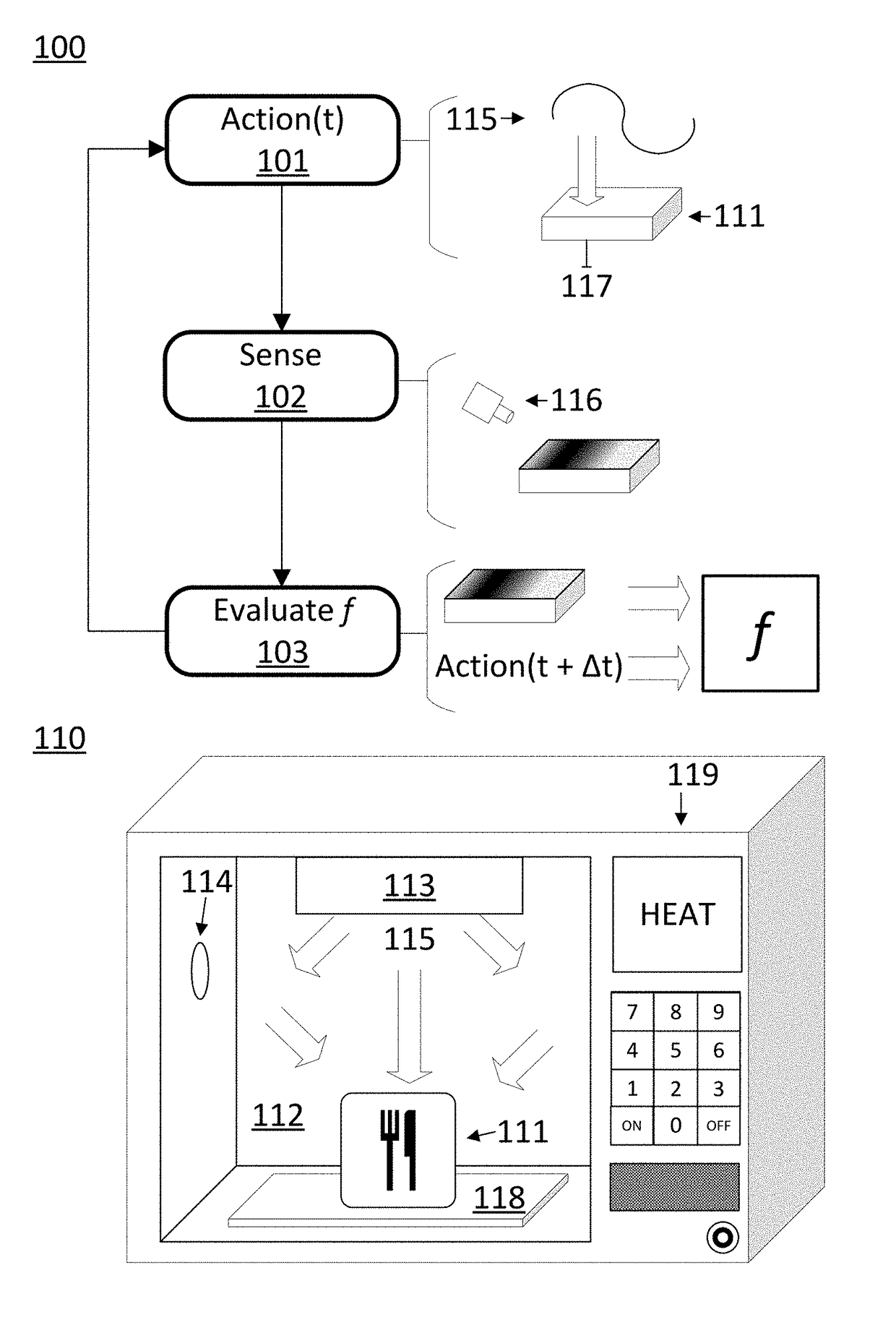
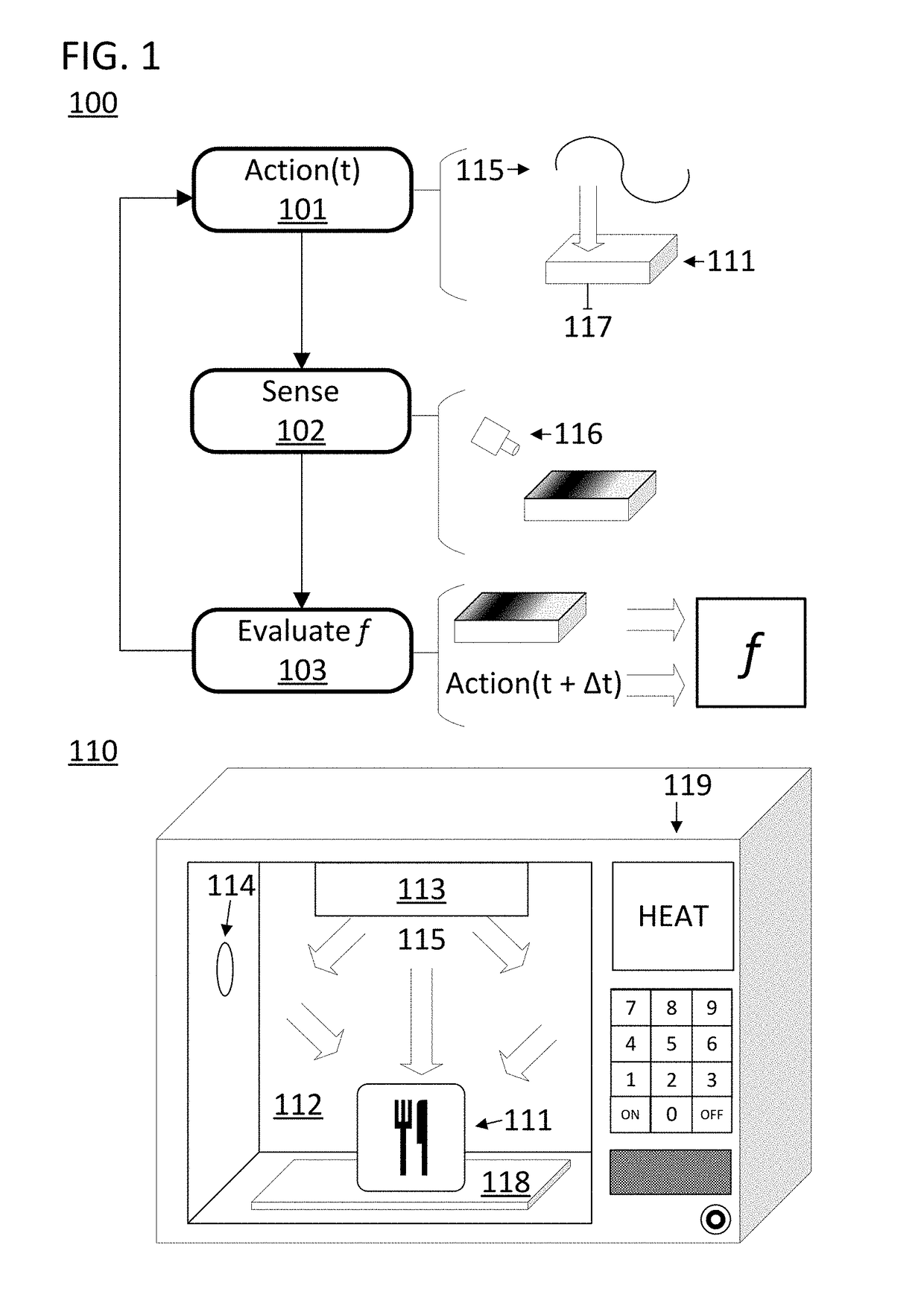
ActiveUS20170290095A1Reliable heatingImprove accuracyDomestic stoves or rangesLighting and heating apparatusControl systemProcess engineering
A disclosed computer-implemented method for heating an item in a chamber of an electronic oven towards a target state includes heating the item with a set of applications of energy to the chamber while the electronic oven is in a respective set of configurations. The set of applications of energy and respective set of configurations define a respective set of variable distributions of energy in the chamber. The method also includes sensing sensor data that defines a respective set of responses by the item to the set of applications of energy. The method also includes generating a plan to heat the item in the chamber. The plan is generated by a control system of the electronic oven and uses the sensor data.
Owner:THE MARKOV CORP
Medication delivery system with improved dose accuracy
InactiveUS20020107486A1Reduced Tolerance RequirementsMany calibrationAmpoule syringesAutomatic syringesDiseaseDiabetes mellitus
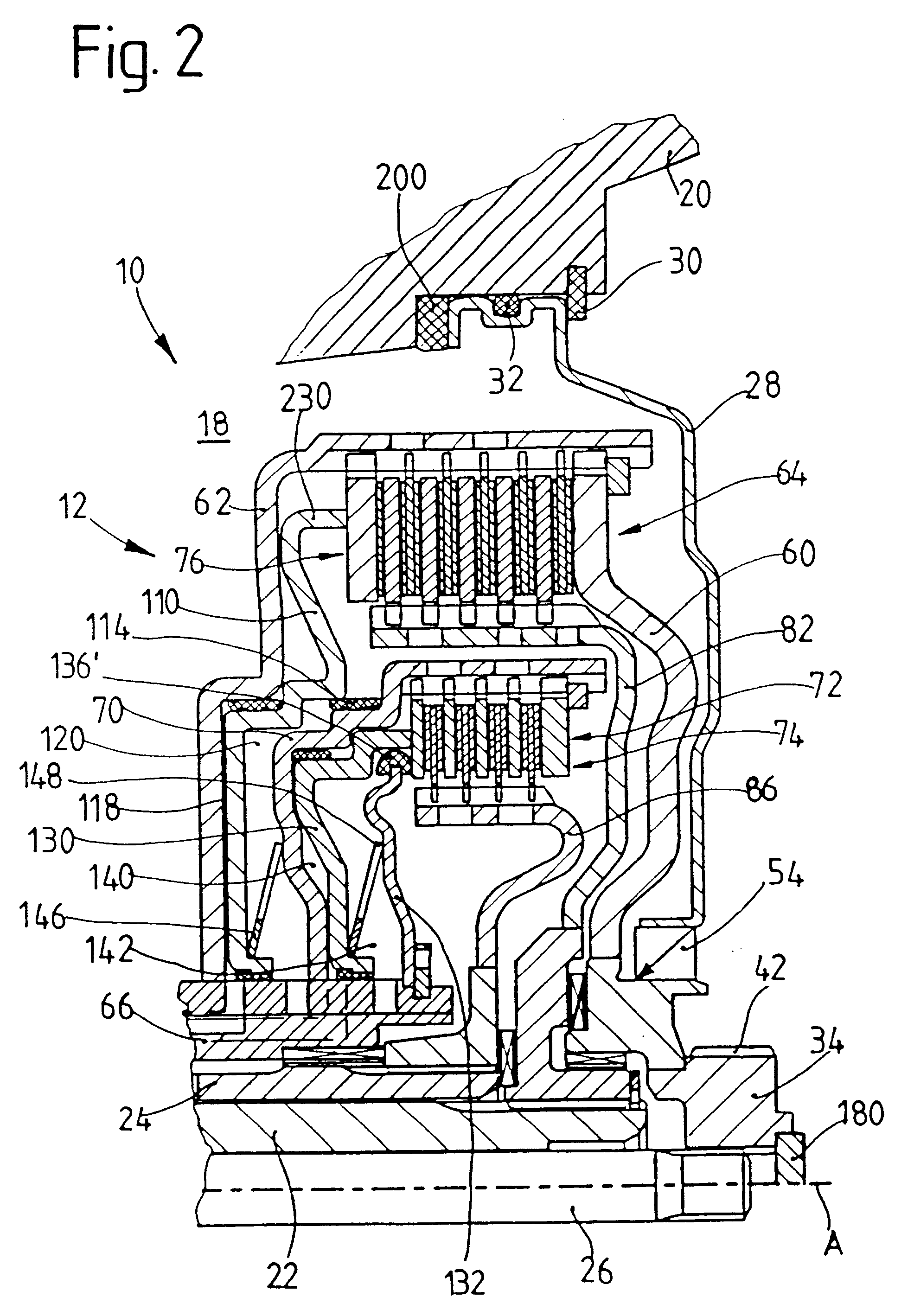
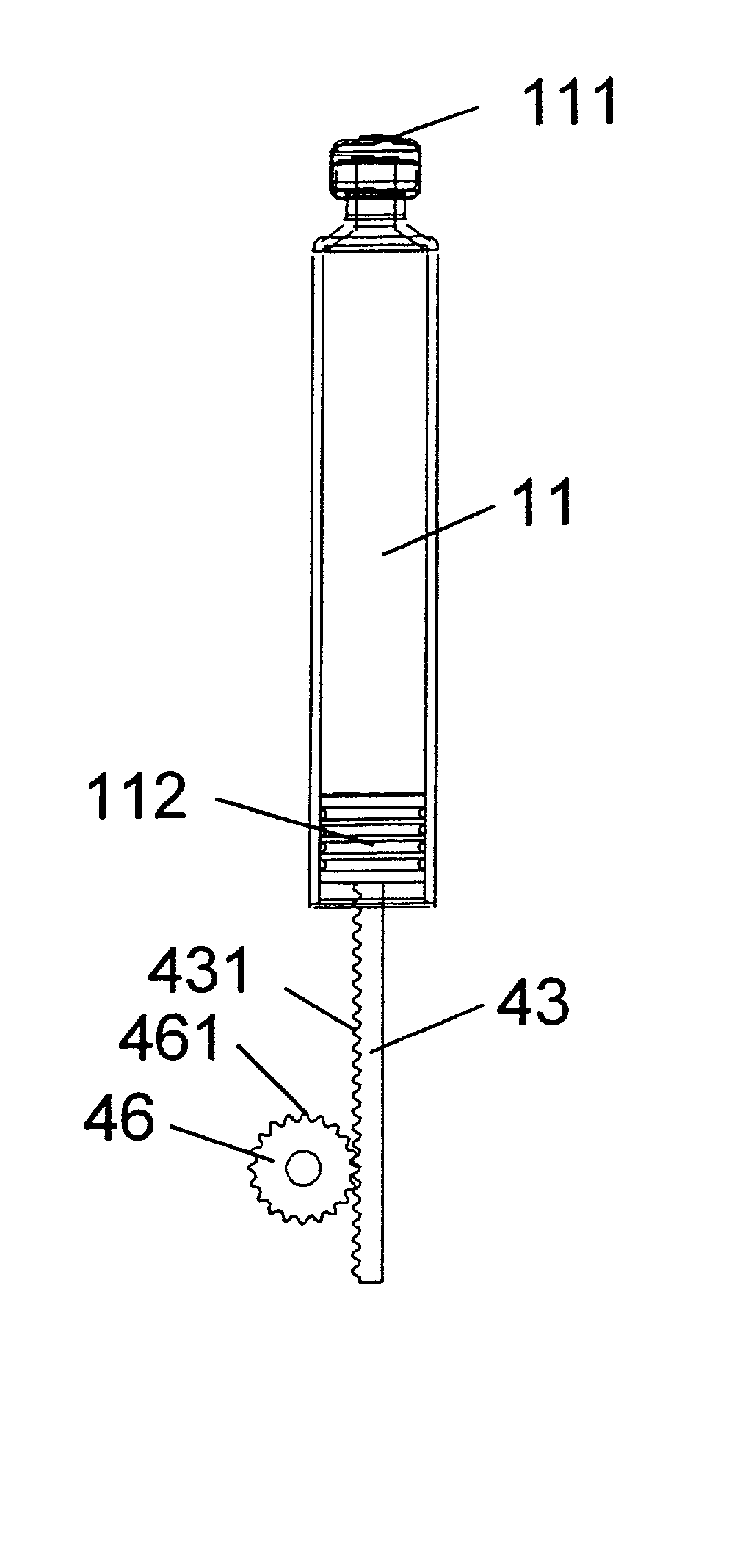
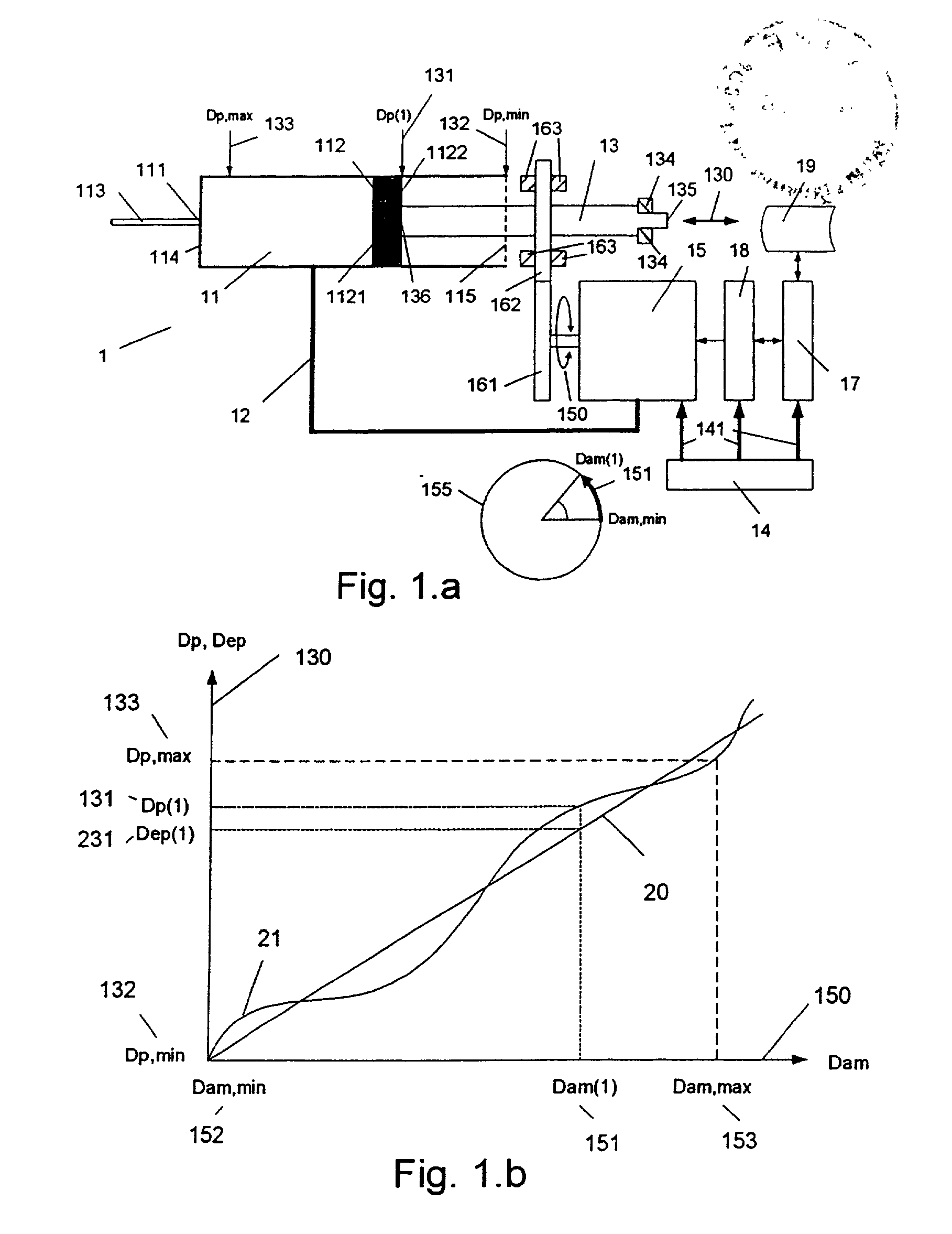
The invention relates to: A medication delivery device (1) for delivering a specific dose comprising a medication cartridge (11) having an outlet (111) and a piston (112), and means (12) for holding said cartridge, and a piston rod (13) being operable to engage and displace said piston, and electrically driven actuating means (15), and driving means (161, 162) for transferring movement from said actuating means to said piston rod, and memory means (17) for storing data, and processing means (18) for evaluating said data and for controlling said actuating means. The object of the present invention is to provide a medication delivery system that combines a relatively high dose accuracy with the use of relatively low quality mechanical components and which enables compensation for built-in non-linearities. The problem is solved in that a first set of data (19) describing the actual movement (130) of said piston rod (13) relative to said medication cartridge (11) as a function of the movement (150) of said actuating means (15) is stored in said memory means (17), and the movement (130) of the piston rod (13) governing the delivered dose is controlled by the processing means (18) on the basis of said first set of data (19). This has the advantage of allowing compensation for mechanical inaccuracies and built-in non-linearities linearities. The invention may e.g. be used in injection or infusion devices for a person's self-treatment of a disease such as diabetes.
Owner:NOVO NORDISK AS
Injection Device
ActiveUS20110213314A1Reliable and controlled functionDampening shear forceAutomatic syringesInfusion needlesInjection deviceBiomedical engineering
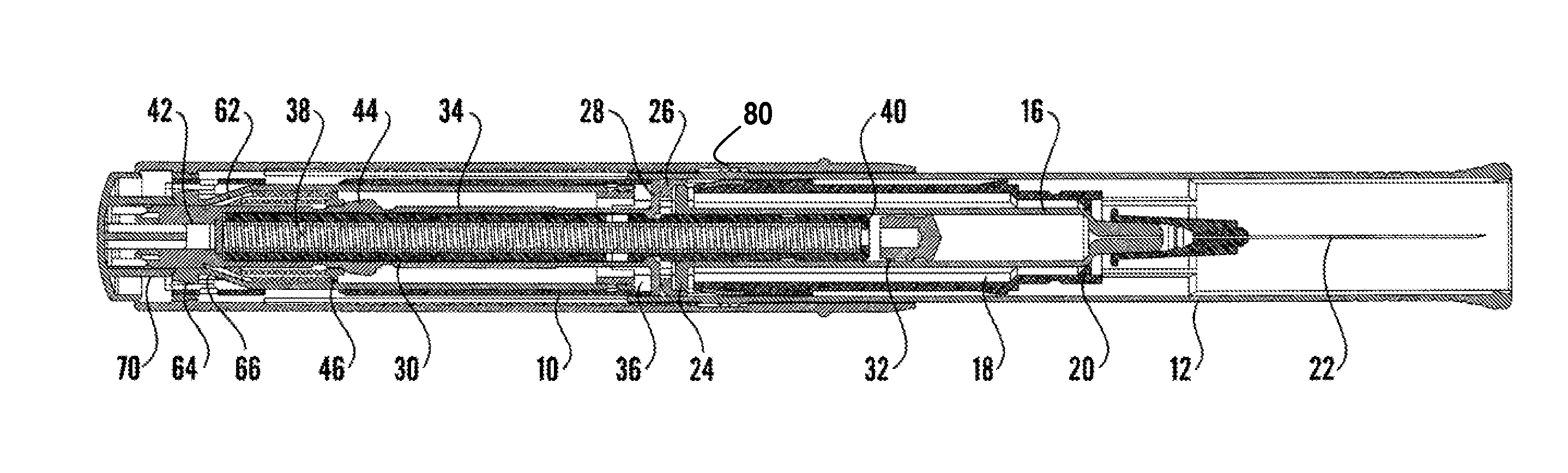
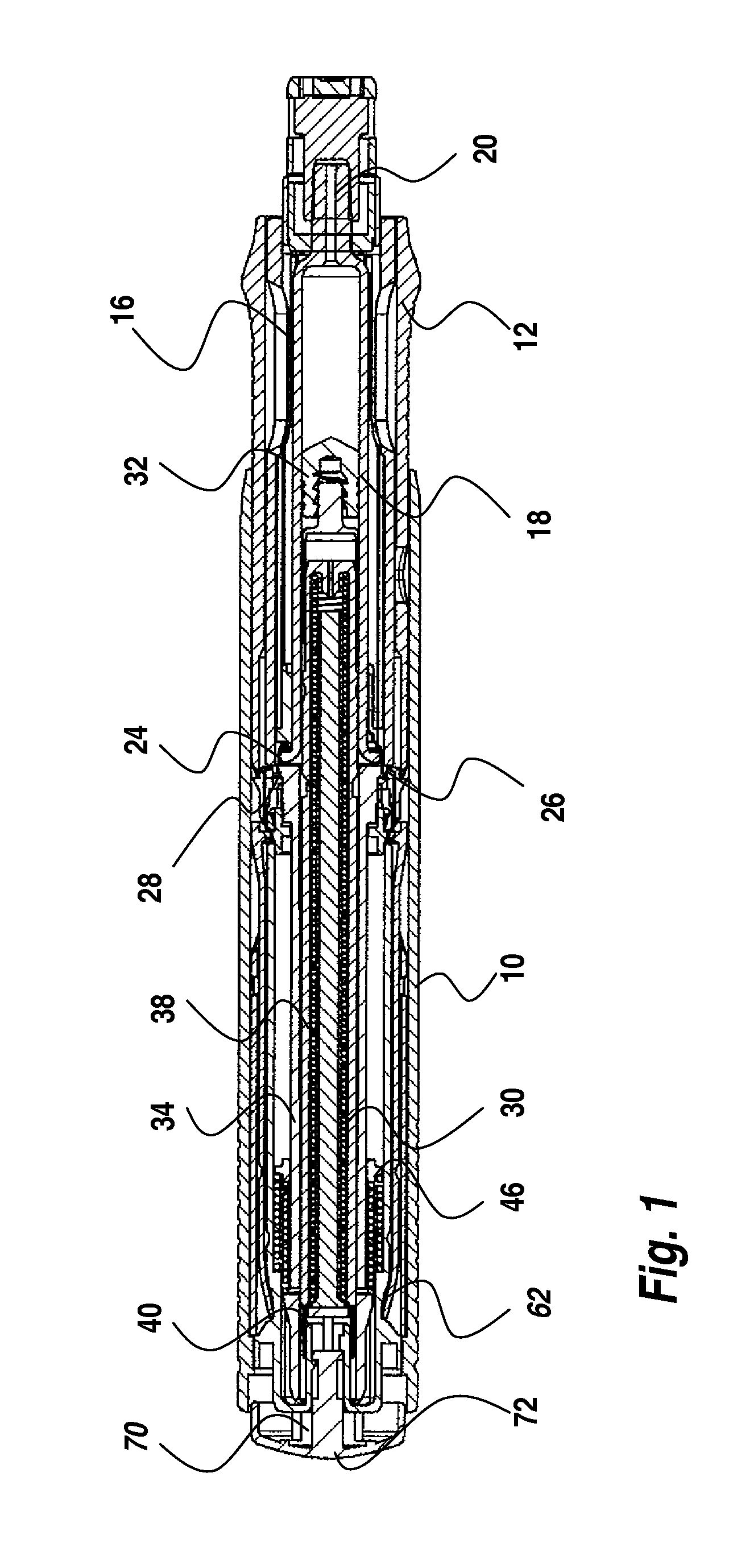
The present invention relates to an injection device comprising a medicament container (18) and a needle attached to said container, means (12,72) for automatic penetration of the needle, injection of medicament and withdrawal of the needle, comprising the steps of initiating a penetration sequence, followed by an injection sequence and followed by a withdrawal sequence, wherein a previous sequence triggers a subsequent sequence, and wherein said device further comprises a dampening means arranged and designed to dampen the movement of the injection means during the whole injection sequence.
Owner:SHL MEDICAL AG
Developer amount detector, and developer container, development device, and image forming apparatus incorporating same
ActiveUS20120189327A1High precisionAdverse effects caused by the fluctuations to the light transmission can be reducedElectrographic process apparatusEngineeringLight guide
A development device includes a development housing, a first developer conveyance member, a developer bearer, and a developer amount detector to detect an amount of developer in the development housing. The developer amount detector includes a light-emitting element, a right-receiving element, a first light guide including a first end from which light enters and a second end disposed inside the development housing, and a second light guide including a first end positioned inside the development housing across a predetermined distance from the second end of the first light guide and a second end from which the light exits. The second end of the first light guide and the first end of the second light guide are arranged in an axial direction of the first developer conveyance member with a light transmission path therebetween partly inside a locus of rotation of the first developer conveyance member.
Owner:RICOH KK
Synergistic analgesic combination of opioid analgesic and cyclooxygenase-2 inhibitor
InactiveUS20070191412A1Reduce plasma concentrationEffective pain managementBiocideNervous disorderDrugCyclooxygenase
Disclosed is a pharmaceutical composition, comprising two analgesic compounds and / or pharmaceutically acceptable salts thereof consisting of celecoxib and / or at least one pharmaceutically acceptable salt thereof and oxycodone and / or at least one pharmaceutically acceptable salt thereof, said two analgesic compounds in an amount sufficient to provide an analgesic effect in a human patient. Also disclosed is a method of effectively treating pain in humans or other mammals, comprising orally administering to the patient an oral dosage form comprising two analgesic compounds consisting of celecoxib and / or at least one pharmaceutically acceptable salt thereof and oxycodone and / or at least one pharmaceutically acceptable salt thereof, said two analgesic compounds in an amount sufficient to provide an analgesic effect in a human patient.
Owner:PURDUE PHARMA LP
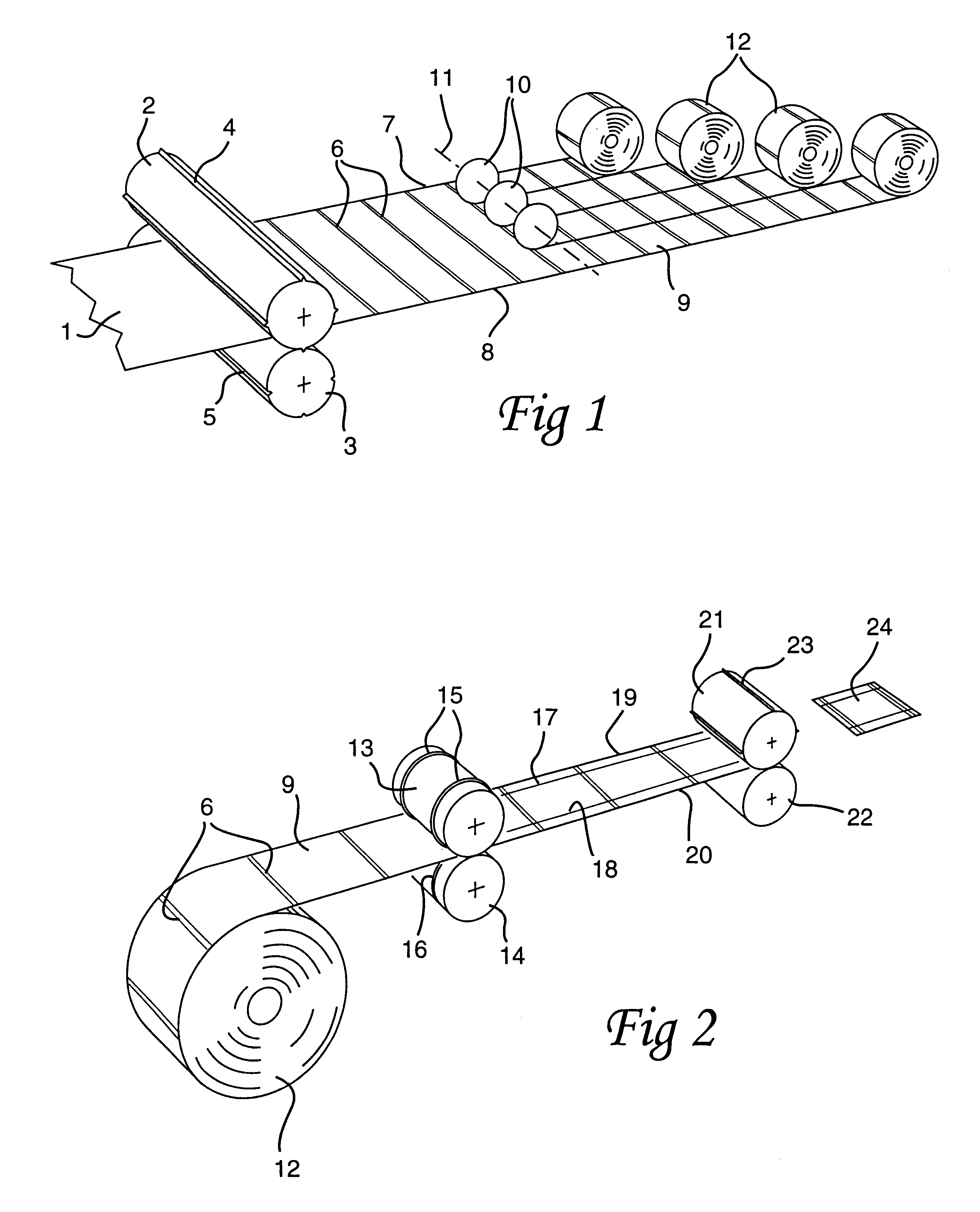
Method of producing crease-lined packaging material
InactiveUS6209291B1Reduction of such tolerance is limitedControl material weakeningMechanical working/deformationBox making operationsEngineering
The disclosure relates to a method of producing crease-lined packaging material with a high degree of accuracy as regards the placing and forming of longitudinal crease lines. A web-shaped starting material (1) is provided, in connection with the laminate production, with a pattern of crease lines (6) extending at an angle to the longitudinal direction of the web, whereafter the material is divided into part webs (9). In connection with the conversion of each part web (9) into individual packaging containers in a filling machine, each part web is provided with complementary, longitudinal crease lines (17, 18).
Owner:TETRA LAVAL HLDG & FINANCE SA
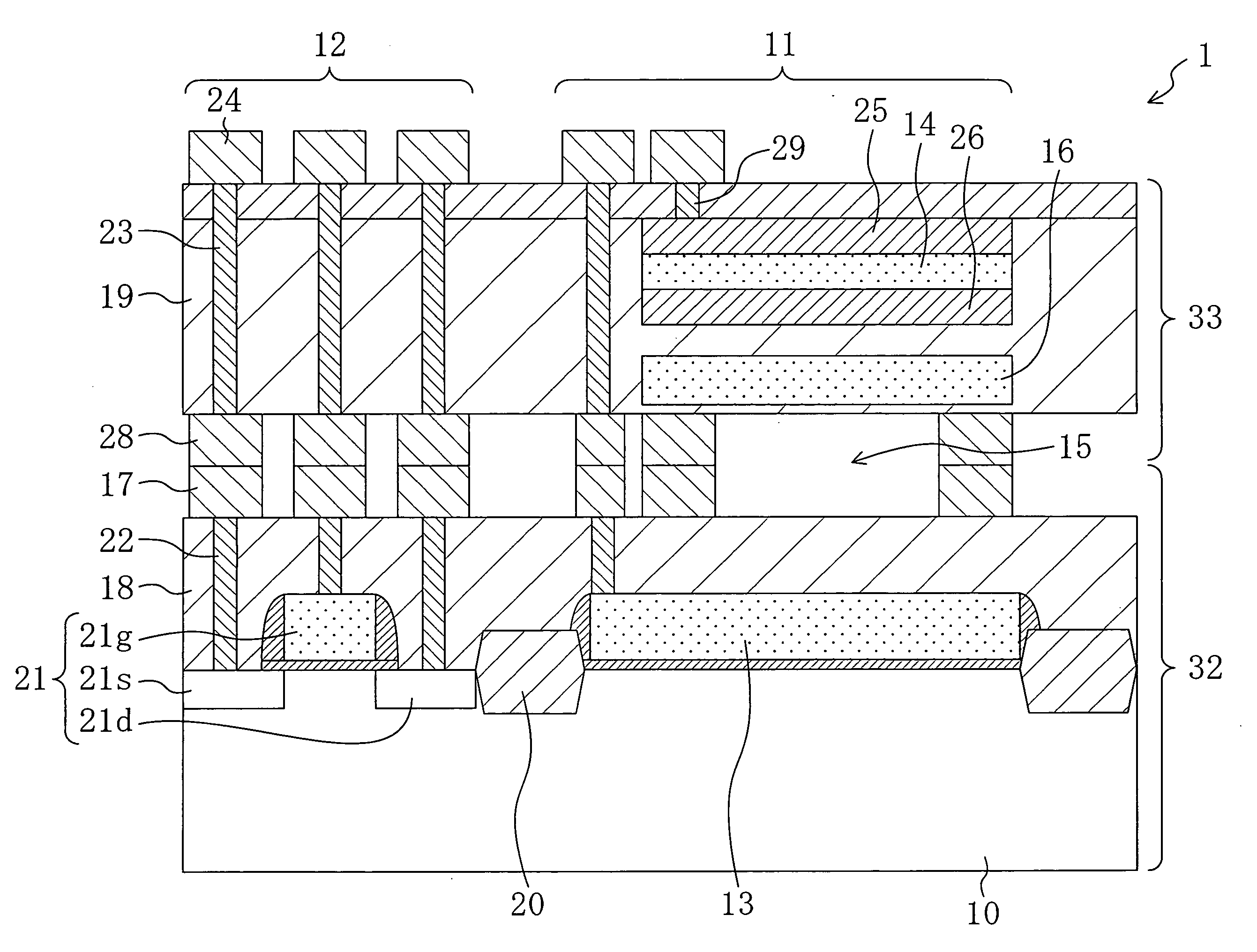
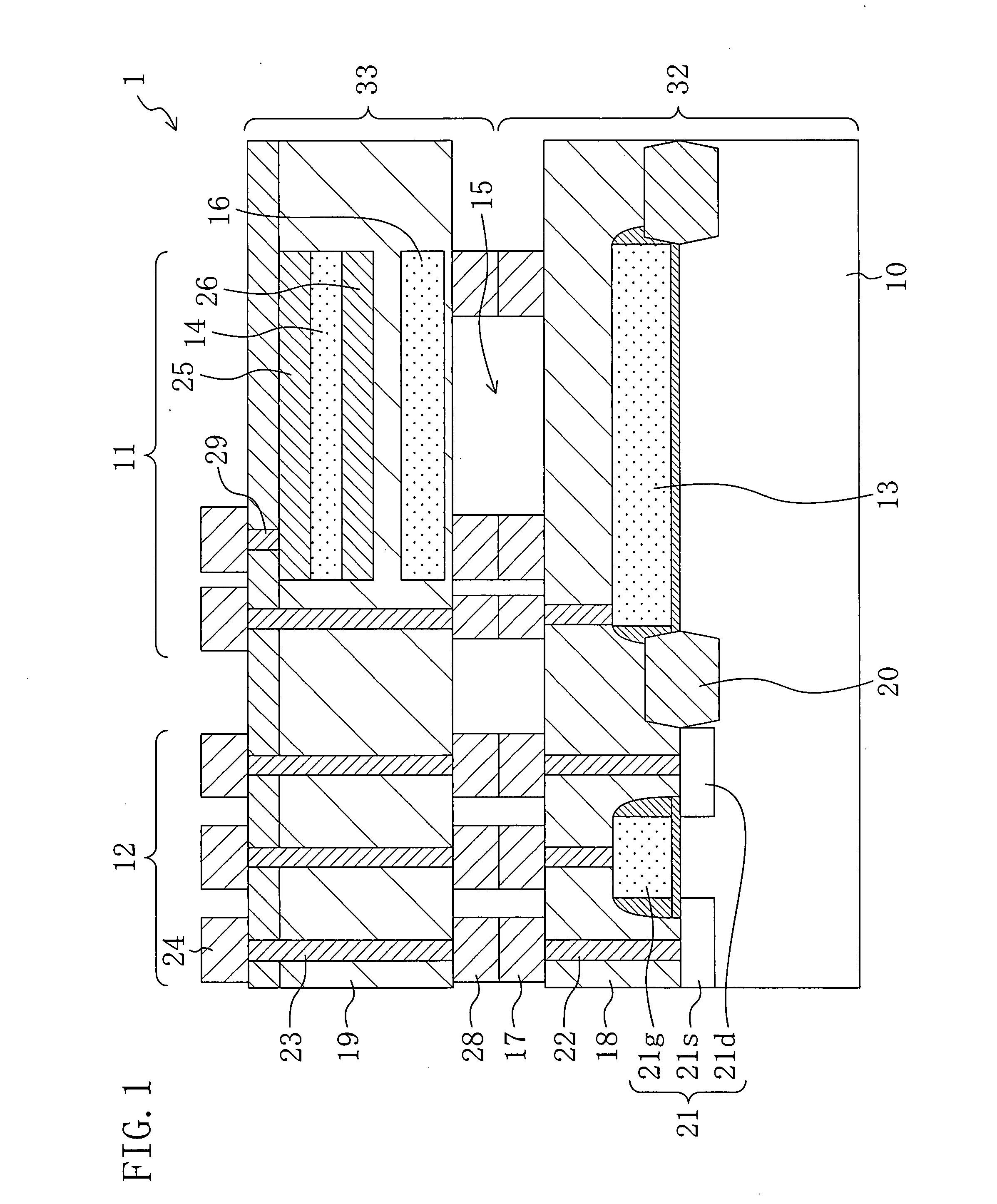
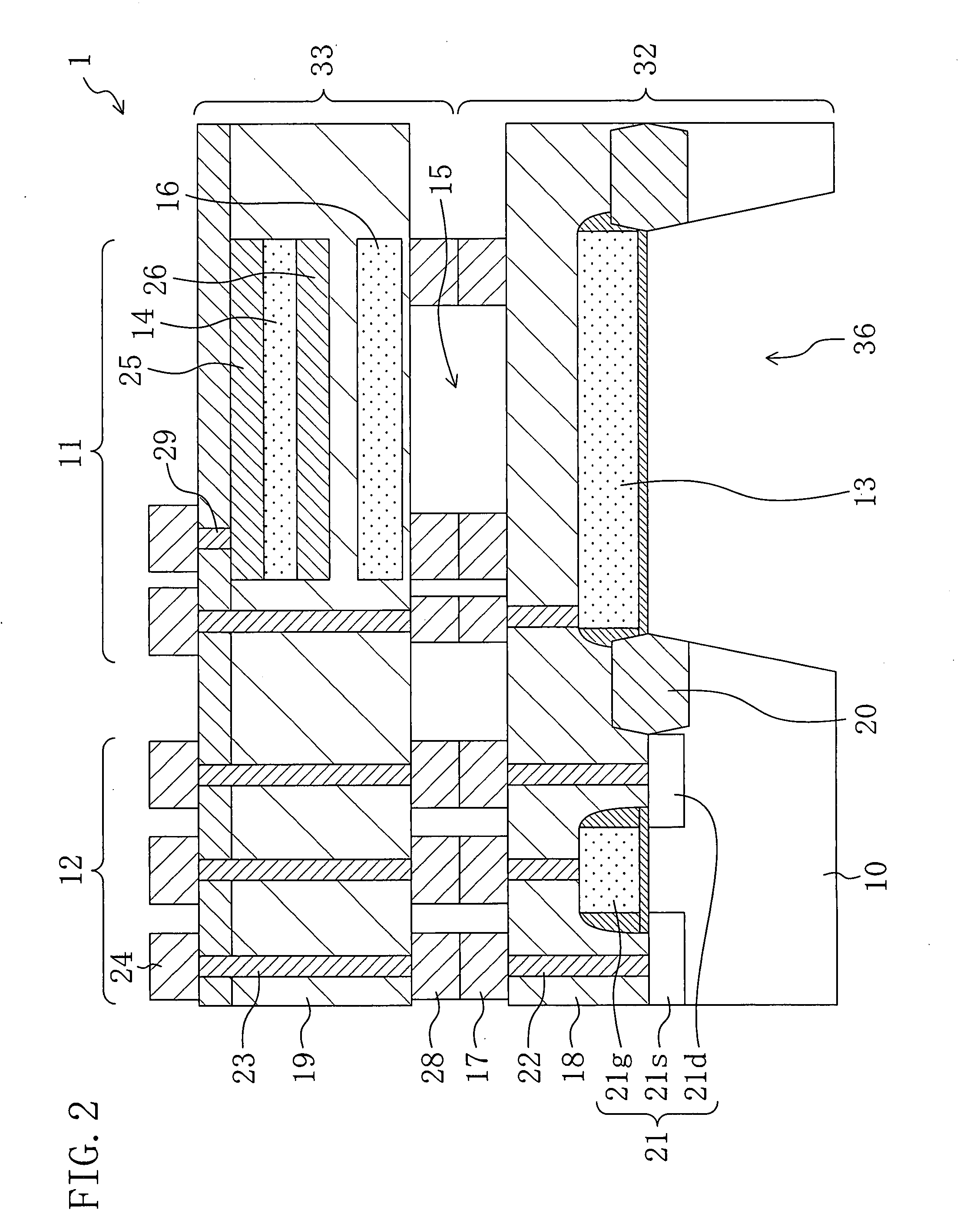
Method for fabricating condenser microphone and condenser microphone
ActiveUS20070272992A1Improve vibration resistanceReduce parasitic capacitanceTransistorSemiconductor/solid-state device detailsDielectricSemiconductor chip
A first semiconductor chip includes a fixed electrode formed on a first semiconductor substrate and a plurality of first metal spacers formed on a first interlayer dielectric. A second semiconductor chip includes a vibrating electrode formed on a second semiconductor substrate and a plurality of second metal spacers formed on a second interlayer dielectric. The first and second semiconductor chips are metallically bonded to each other using the first and second metal spacers. An air gap is formed in a region of the condenser microphone located between the first semiconductor chip and the second semiconductor chip except bonded regions of the first and second metal spacers.
Owner:PANASONIC CORP
Method of providing sustained analgesia with buprenorphine
InactiveUS20010002259A1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationZero order kinetics
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an additional two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
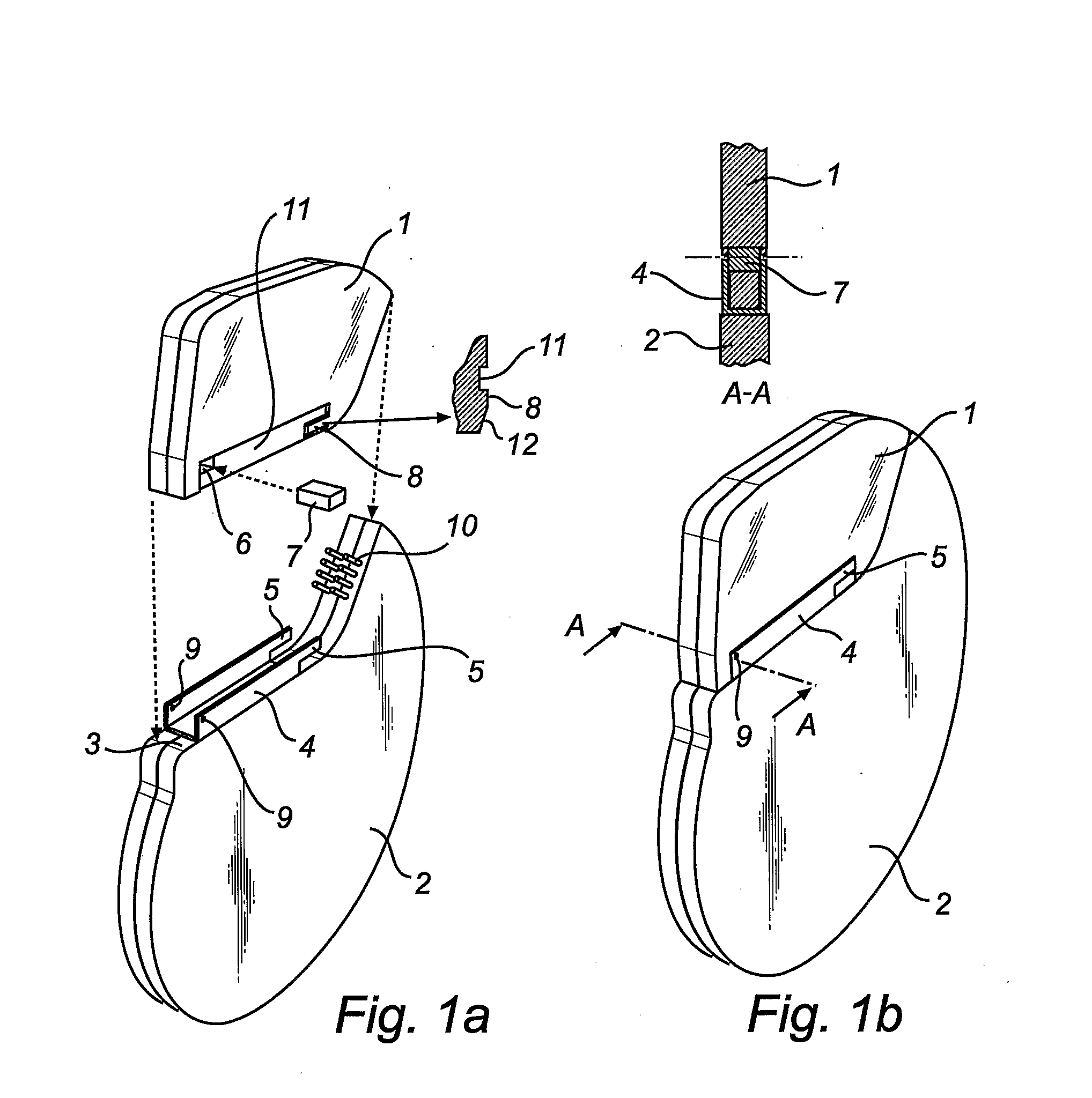
Implantable Medical Device
ActiveUS20080262563A1Strong jointImprove distributionElectrotherapyHermetically-sealed casingsHermetic sealMedical device
In an implantable medical device and a method for assembly thereof, a hermetically sealed housing encloses electronic circuitry and has a housing fastener part that is formed of metal and that protrudes from the housing. A per-fabricated header, for receiving conducting leads and for connecting the conducting leads to the electronic circuitry, has a header fastener part also formed of metal. The header is fastened to the housing by metal-to-metal welding of the housing fastener part and the header fastener part at a distance from the housing.
Owner:ST JUDE MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com