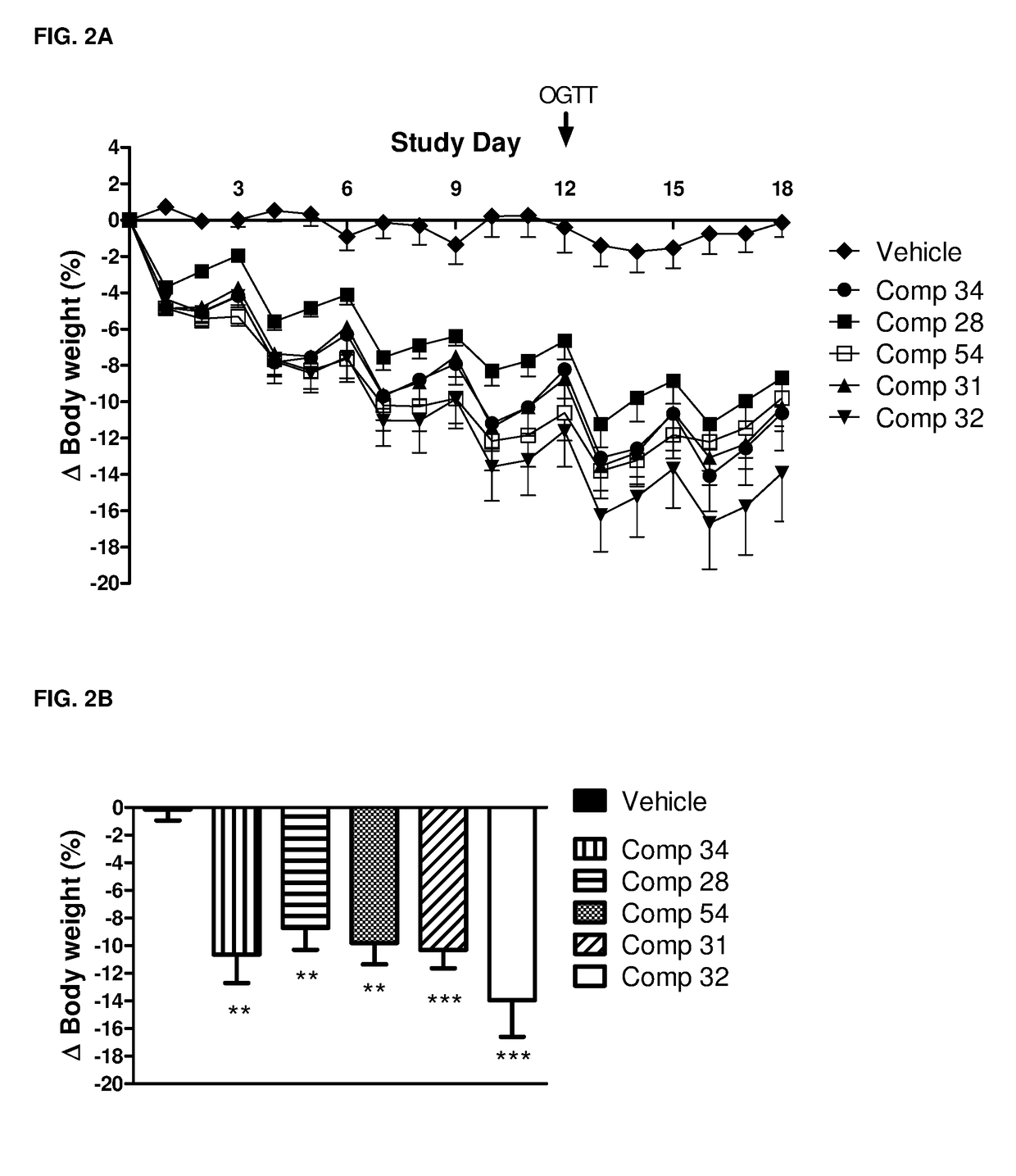
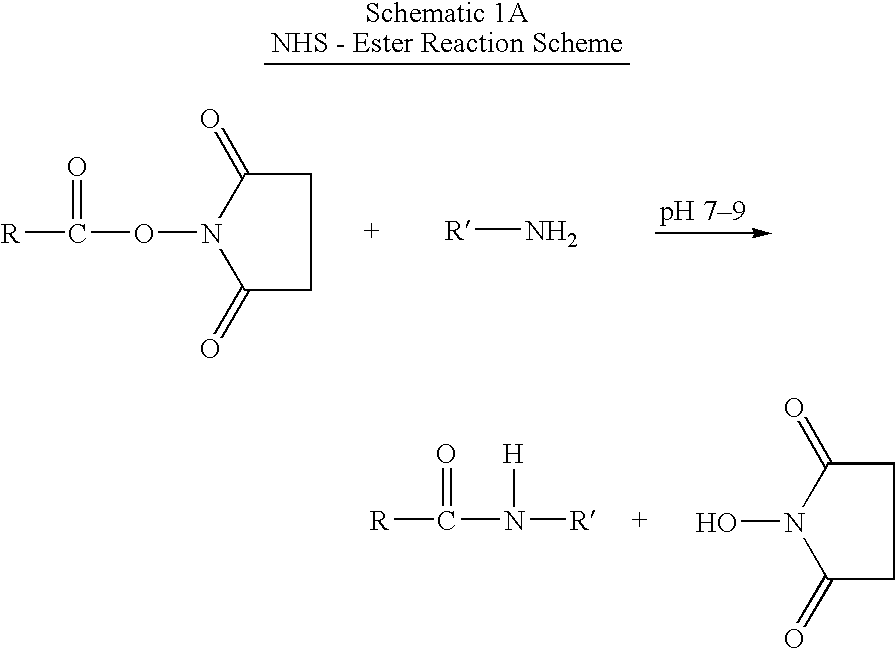
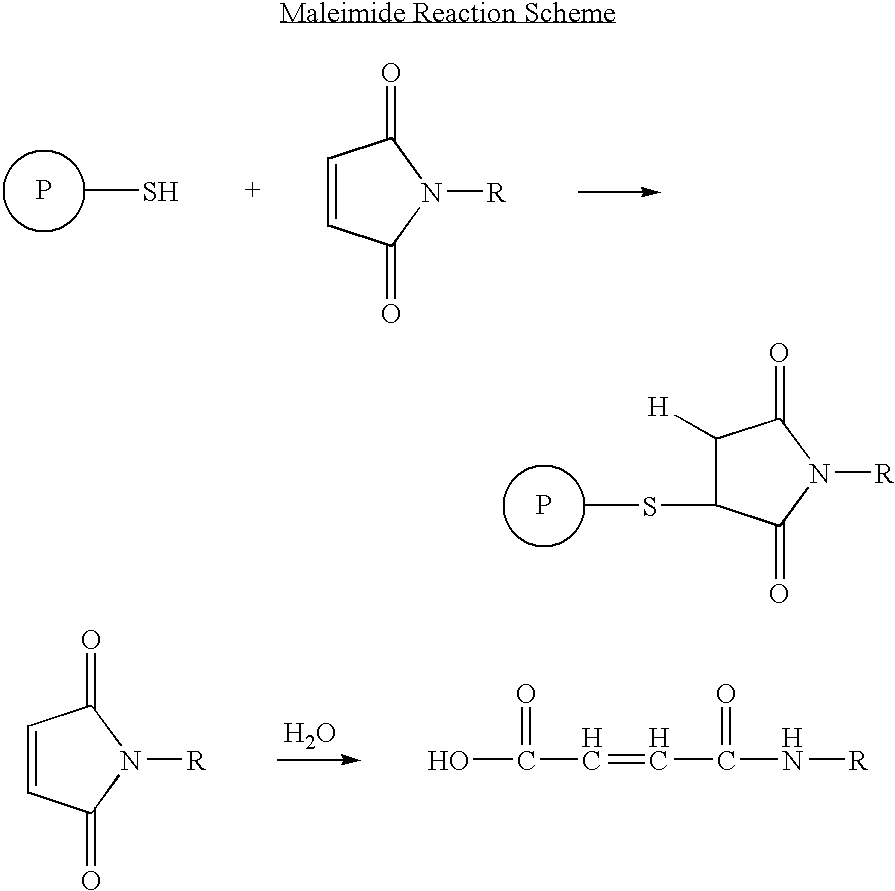
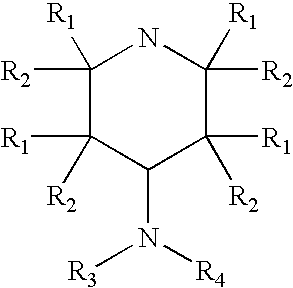
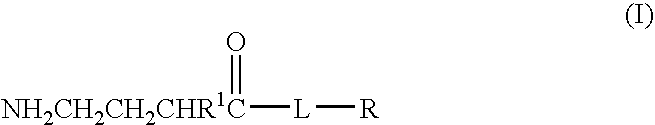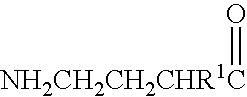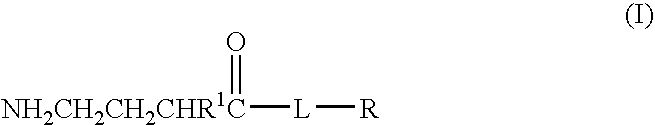Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54results about "Secretins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Activatable clostridial toxins
InactiveUS20080032931A1Minimize security riskEnhanced efficiency and rateHydrolasesPeptide/protein ingredientsClostridial toxinSaxitoxin
Owner:ALLERGAN INC
Methods for treatment of acute pancreatitis
The invention relates generally to methods for treating acute pancreatitis in patients. The methods comprise administering a therapeutically effective amount of a pharmaceutical composition comprising secretin and a pharmaceutically acceptable carrier.
Owner:CHIRHOCLIN
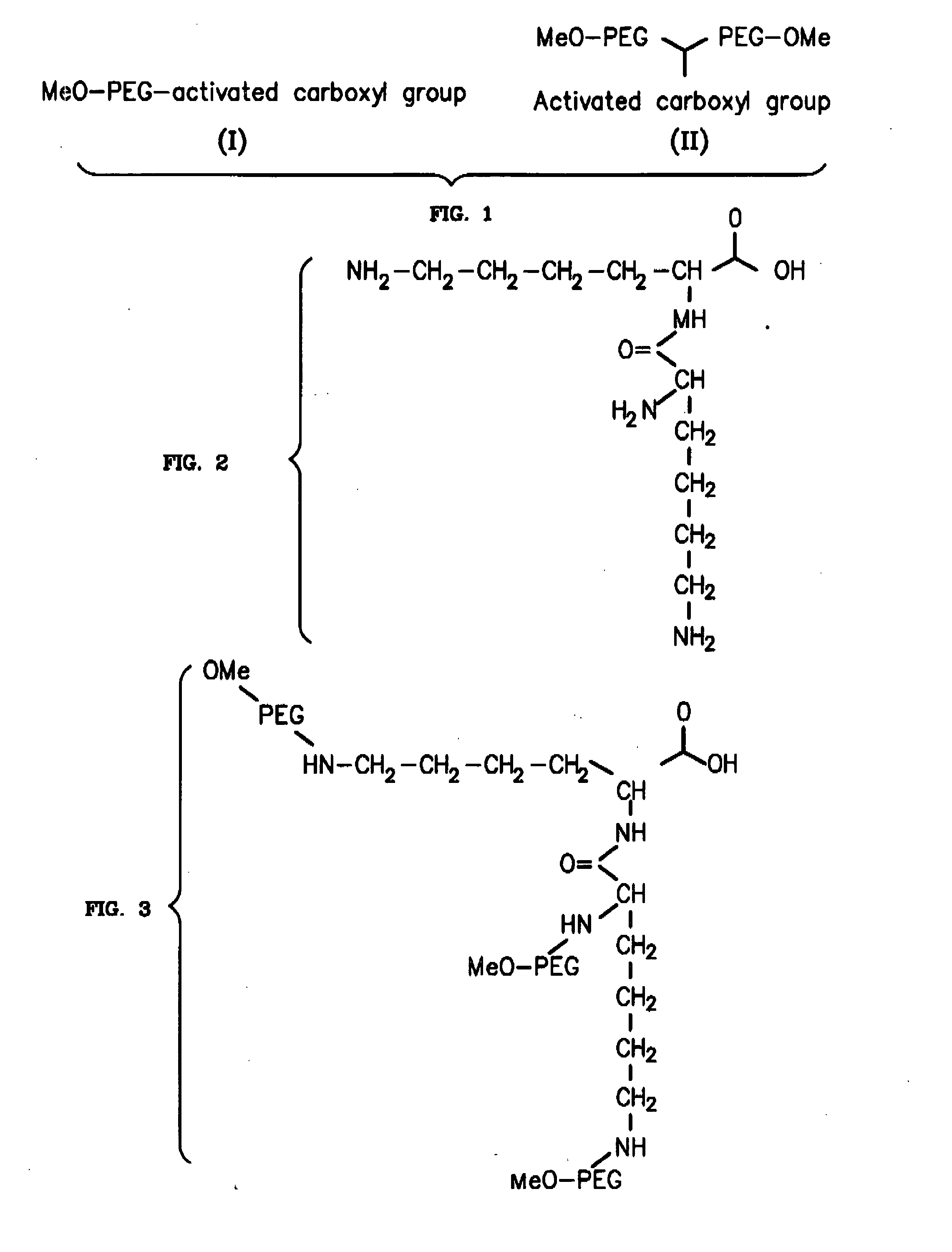
Novel PEGylation agent
InactiveUS20070184015A1Reduce compliancePromote degradationPeptide/protein ingredientsCalcitoninsHalf-lifeBlood plasma
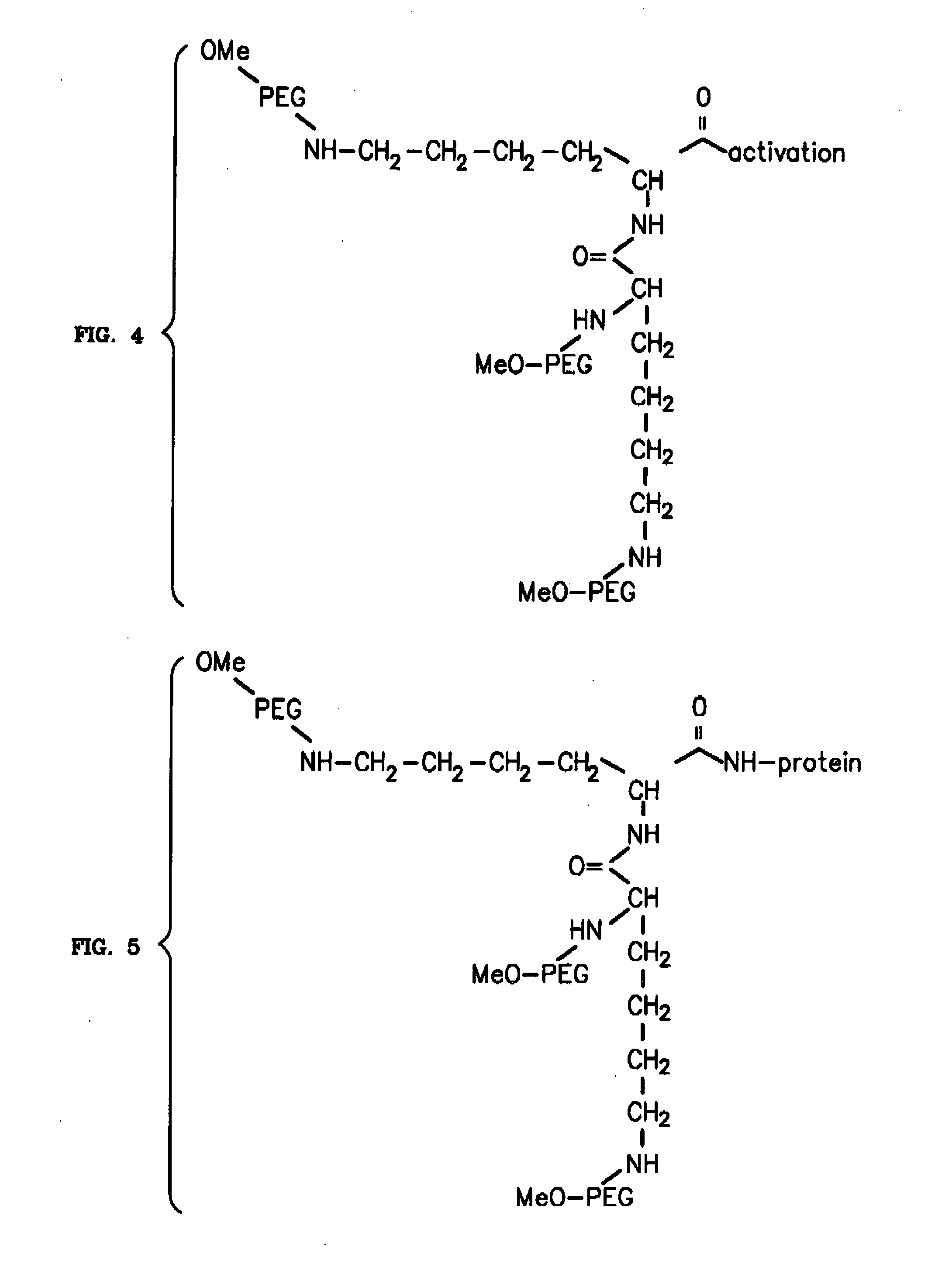
To address the issue of degradation by enzymatic reactions to proteins and peptides, polyethylene glycol (PEGylation) of the proteins and peptides has been established. PEGylated proteins and peptides have increased plasma half-lives and reduced immunogenicity. To further improve and extend the plasma half-life of desired protein or peptide therapeutics, a novel branched molecule of PEG possessing three PEGs with a single point of attachment is designed in this invention disclosure.
Owner:HAHN SOONKAP
Methods of treating pervasive development disorders
InactiveUS20080219966A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsPervasive developmental disorderMethylphenidate
A therapeutic method for treating an individual diagnosed with PDD pervasive developmental disorder comprises determining the efficacy of digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level. A method for reducing the amount of methylphenidate (Ritalin) being taken by an individual with attention deficit disorder (ADD) or attention deficit hyperactivity disorder (ADHD) by administering a therapeutic amount of digestive enzymes is also provided.
Owner:CUREMARK
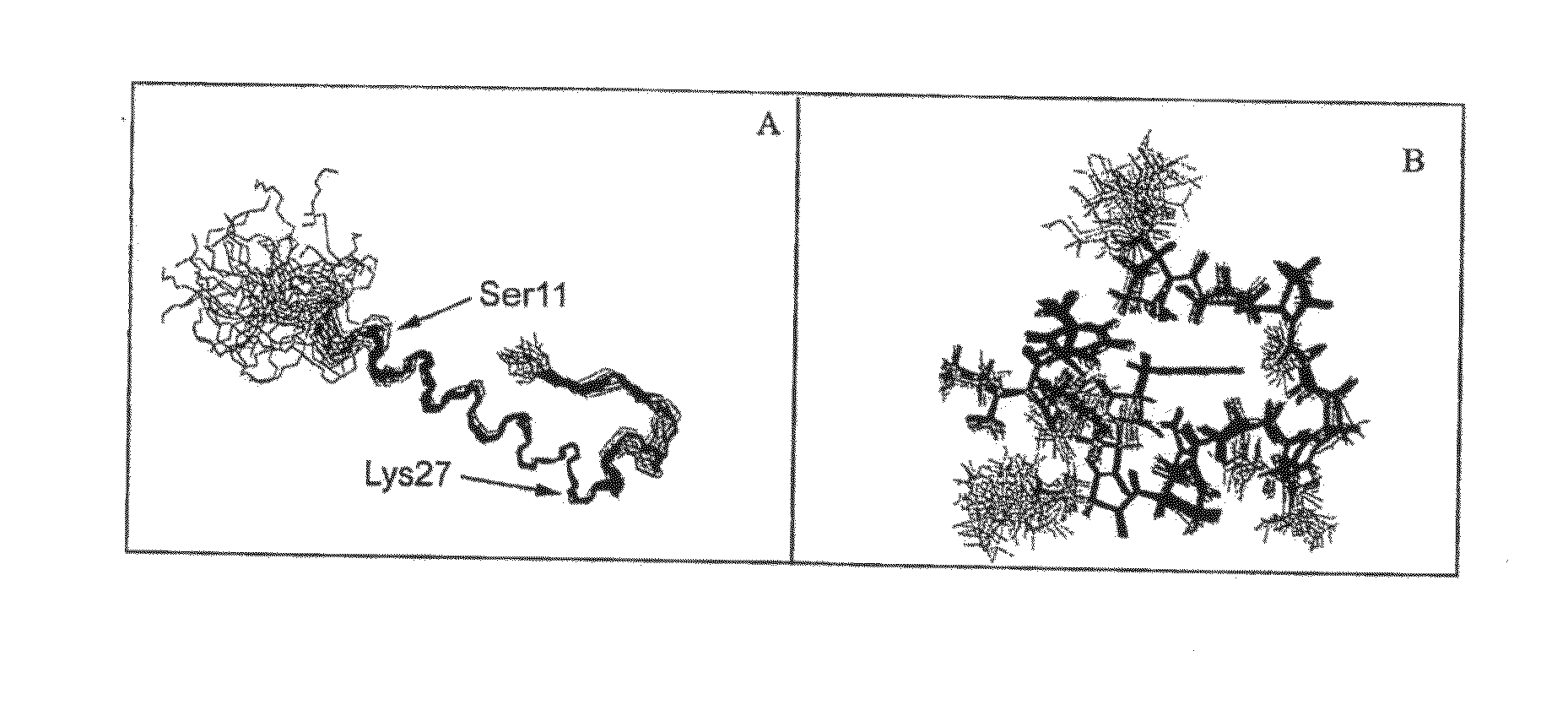
DPP-IV Resistant GIP Hybrid Polypeptides with Selectable Properties
ActiveUS20110136737A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaFeeding disability
The Present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
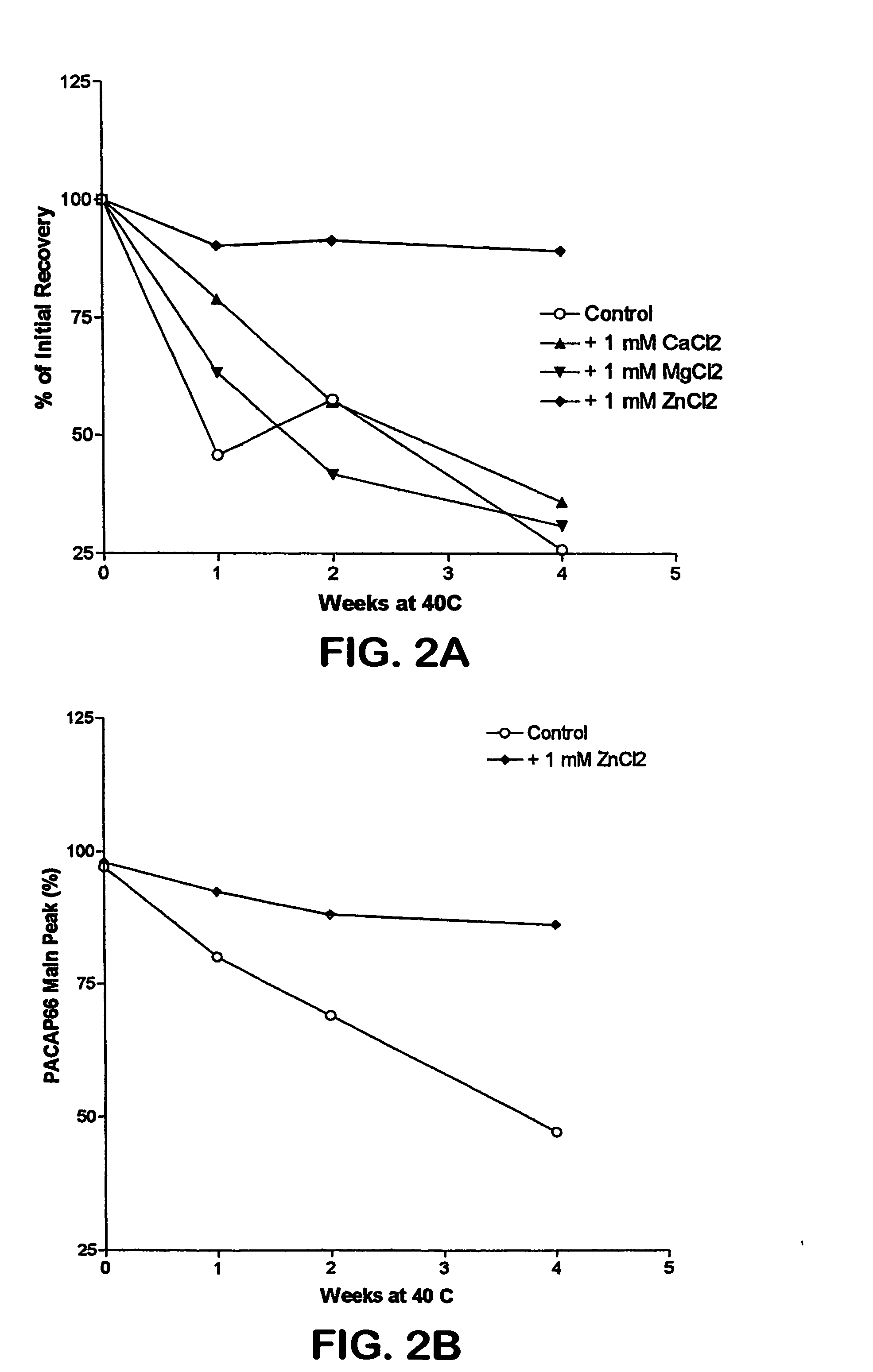
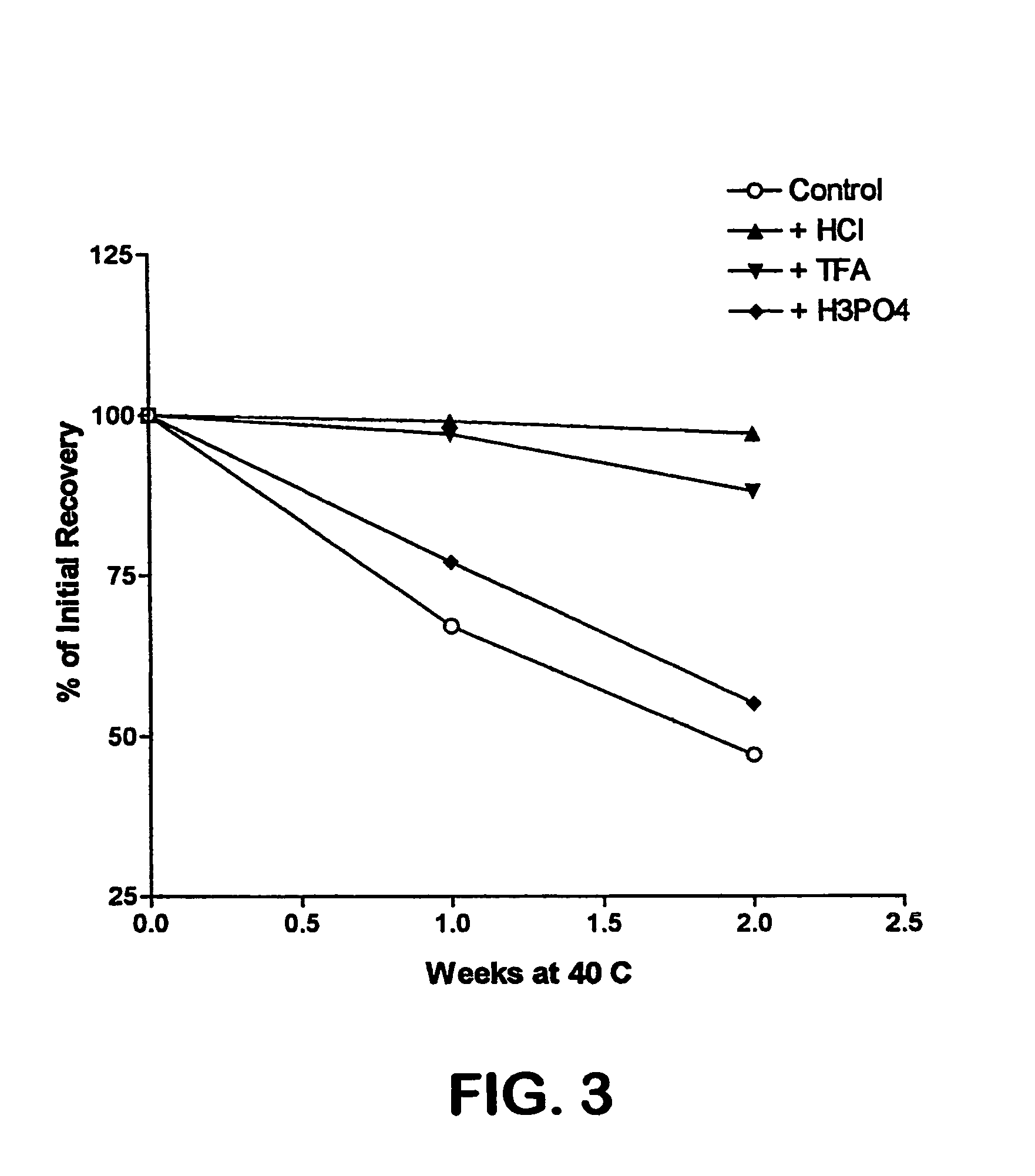
Formulation strategies in stabilizing peptides in organic solvents and in dried states
The invention relates to stabilized formulations of therapeutically active peptides, particularly PACAP 66. Formulations of the invention include a peptide containing at least one histidine residue, a transition metal salt and an organic solvent. The above formulations may contain peptides that have at least one asparagine residue and are acidified and dried (such as spray-dried or freeze-dried) before formulation preparation. Other formulations of the invention relate to stabilized formulations of PACAP 66 or peptides containing an asparagine residue, which are acidified and dried (such as spray-died or freeze-dried) with or without a transition metal salt.
Owner:BAYER HEALTHCARE LLC
Methods for treating autoimmune disorders, and reagents related thereto
InactiveUS20050070459A1Nervous disorderDipeptide ingredientsCrohn's diseaseInflammatory bowel disease
The invention generally relates to improved methods for treatment or prophylaxis in animal subjects (including humans) of autoimmune disorders including Type I diabetes, septic shock, multiple sclerosis, inflammatory bowel disease (IBD) and Crohn's disease.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Nasal peptide pharmaceutical formulation
InactiveUS20050158247A1Optimal therapeutic dose levelConstant ratePeptide/protein ingredientsAerosol deliveryEpitheliumDiluent
The invention provides a pharmaceutical formulation comprising: (1) THAM, which is tris(hydroxymethyl)aminomethane, as a selective absorbefacient to enhance through the nasal mucosa-lined epithelium the absorption of substances of peptide nature; and (2) a therapeutically effective amount of active nasal peptide, its pharmaceutically acceptable salt or its peptidic fragment; in a pharmaceutically acceptable, aqueous liquid diluent or carrier, said formulation being in a form suitable for nasal administration.
Owner:THERAPICON SRL
Gip and glp-1 co-agonist compounds
The present invention relates to dual incretin peptide mimetic compounds that agonize receptors for both human glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), and may be useful for treating type 2 diabetes mellitus (T2D).
Owner:ELI LILLY & CO
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
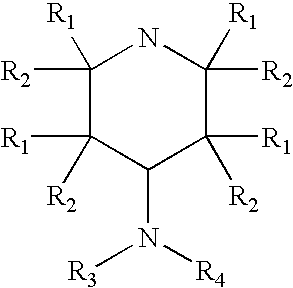
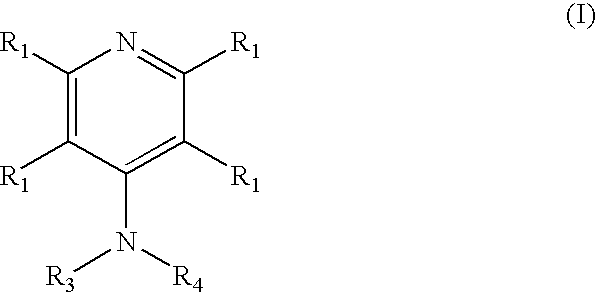
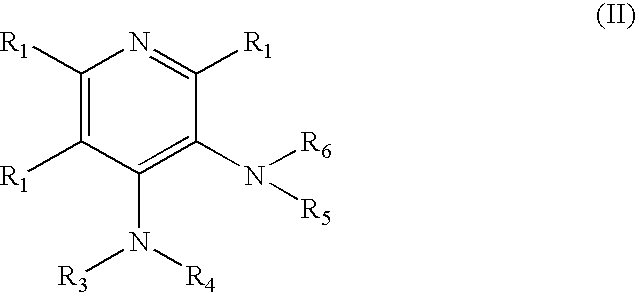
4-aminopyridine and a pharmaceutical composition for treatment of neuronal disorders
A composition is provided having the formulawhere R1 and R2 are each independently H or a C1-C4 hydrocarbon; R3 is H, and R4 is a moiety capable of crossing the blood brain barrier selected from the group consisting of: an amino acid, a peptide, transferrin, gluconate, lactate, citrate, malate, fumarate, benzoate, salicylate, pyruvate and propionate. The composition includes 4-aminopyridine and a transporter species which allows for improved transport of the aminopyridine across the blood brain barrier thereby reducing systemic side effects of aminopyridine administration.
Owner:MILLER LANDON C G
Encapsulation of water soluble peptides
InactiveUS20070009605A1Peptide/protein ingredientsCalcitoninsControlled releaseBiodegradable microsphere
This invention relates to a process for preparing biodegradable microspheres and or nanospheres using an oil-in-water process for the controlled release of bioactive peptides.
Owner:SOC DE CONSEILS DE RECH & DAPPLICATIONS SCI SAS
Absorbable microparticles
This invention pertains to a sustained release complex of one or more peptides, one or more proteins or a combination thereof immobilized on an absorbable polymer microparticle optionally having an absorbable polymer coating. The microparticle complex of this invention comprises a peptide(s) and / or protein(s) which have at least one amino group and / or at least one carboxyl group per molecule and a solid absorbable polyester microparticle having surface and subsurface carboxylic group or amino groups in sufficient amounts to bind the peptide(s) and / or protein(s) so that the immobilized peptide(s) or protein(s) represent 0.1% to 30% of the total mass of the microparticle complex. The microparticle complex with immobilized peptide(s) and / or protein(s) are optionally further encased individually or in groups with an absorbable polymer to control, further, the release of the immobilized peptide(s) and / or protein(s). To control the release of the immobilized peptide(s) and / or protein(s) even further, the encased microparticles can be incorporated into a composition with an absorbable gel-forming liquid that transforms to a flexible gel or semi-solid upon contacting water in the biologic environment.
Owner:POLY MED
GIP-GLP-1 dual agonist compounds and methods
ActiveUS10093713B2Improve blood sugar controlReduce weightPeptide/protein ingredientsAntipyreticMetabolic disorder
Owner:ZEALAND PHARM AS
Long acting proteins and peptides and methods of making and using the same
ActiveUS20100121032A1Antibacterial agentsPeptide/protein ingredientsPolyethylene glycolGlucagon-like peptide-1
Disclosed is a method for refolding a protein or peptide that does not contain essential disulfides and that contains at least one free cysteine residue. Also disclosed are polymer IFN-γ conjugates that have been created by the chemical coupling of polymers such as polyethylene glycol moieties to IFN-γ, particularly via a free cysteine in the protein. Also disclosed are analogs of bioactive peptides that may be used to create longer acting versions of the peptides, including analogs of glucagon, glucagon-like peptide-1 (GLP-1), GLP-2, Gastric inhibitory peptide (GIP), PYY, exendin, ghrelin, gastrin, amylin, and oxyntomodulin.
Owner:BOLDER BIOTECH
Methods of making proteins and peptides containing a single free cysteine
ActiveUS8617531B2Antibacterial agentsPeptide/protein ingredientsCysteine thiolatePolyethylene glycol
Disclosed is a method for refolding a protein or peptide that does not contain essential disulfides and that contains at least one free cysteine residue. Also disclosed are polymer IFN-γ conjugates that have been created by the chemical coupling of polymers such as polyethylene glycol moieties to IFN-γ, particularly via a free cysteine in the protein. Also disclosed are analogs of bioactive peptides that may be used to create longer acting versions of the peptides, including analogs of glucagon, glucagon-like peptide-1 (GLP-1), GLP-2, Gastric inhibitory peptide (GIP), PYY, exendin, ghrelin, gastrin, amylin, and oxyntomodulin.
Owner:BOLDER BIOTECH
Protection of endogenous therapeutic peptides from peptidase activity through conjugation to blood components
InactiveUS20050187159A1Easy to testPeptide/protein ingredientsSecretinsAmino acid compositionOrganic chemistry
A method for protecting a peptide from peptidase activity in vivo, the peptide being composed of between 2 and 50 amino acids and having a C-terminus and an N-terminus and a C-terminus amino acid and an N-terminus amino acid is described. In the first step of the method, the peptide is modified by attaching a reactive group to the C-terminus amino acid, to the N-terminus amino acid, or to an amino acid located between the N-terminus and the C-terminus, such that the modified peptide is capable of forming a covalent bond in vivo with a reactive functionality on a blood component. In the next step, a covalent bond is formed between the reactive group and a reactive functionality on a blood component to form a peptide-blood component conjugate, thereby protecting said peptide from peptidase activity. The final step of the method involves the analyzing of the stability of the peptide-blood component conjugate to assess the protection of the peptide from peptidase activity.
Owner:CONJUCHEM
4-aminopyridine and a pharmaceutical composition for treatment of neuronal disorders
A composition is provided having the formula where R1 and R2 are each independently H or a C1-C4 hydrocarbon; R3 is H, and R4 is a moiety capable of crossing the blood brain barrier selected from the group consisting of: an amino acid, a peptide, transferrin, gluconate, lactate, citrate, malate, fumarate, benzoate, salicylate, pyruvate and propionate. The composition includes 4-aminopyridine and a transporter species which allows for improved transport of the aminopyridine across the blood brain barrier thereby reducing systemic side effects of aminopyridine administration.
Owner:MILLER LANDON C G
Treatment of inflammatory, autoimmune, or other disorders, using agents that reduce the sequestering of zinc by calprotectin
InactiveUS20070275095A1Reduce activity levelReduce concentrationBiocideNervous disorderDiseaseWhole body
Treatments are disclosed for inflammatory, autoimmune, or other disorders characterized by excessive activity of calprotectin, a protein that normally defends against microbial infections by sequestering available zinc, at a site of infection. Excessive calprotectin activity, which can cause zinc deficiencies in localized tissues, can create or aggravate various disorders. However, ingestion of systemic (oral) zinc supplements tends to activate offsetting mechanisms, and such supplements therefore usually are ineffective. Accordingly, targeted treatments are disclosed herein for suppressing and controlling excessive calprotectin activity, in local tissues. Such methods include targeted injections of zinc solutions, and plasmapheresis treatment. Screening tests also are described for identifying non-protein drugs that can either (i) bind specifically to the zinc-binding sites of calprotectin, or (ii) suppress the release of calprotectin by neutrophil cells.
Owner:KOSSOR DAVID C
Novel multipeptide regimen for the treatment of autistic spectrum, behavioral, emotional and visceral inflammation/autoimmune disorders
InactiveUS20050201998A1Effective treatmentReduce usageNervous disorderPeptide/protein ingredientsDisease irritable bowelRegimen
The present invention provides compositions and methods for preventing and treating gastrointestinal disorders by administering to a subject an effective amount of secretin either alone or in combination with an effective amount of oxytocin. The invention also provides compositions and methods for preventing and treating central nervous system disorders by administering to a subject an effective amount of secretin in combination with an effective amount of oxytocin. The invention further provides compositions and methods for treating and preventing a variety of autoimmune diseases by administering to a subject an effective amount of secretin in combination with an effective amount of oxytocin. Additionally, the invention provides compositions and methods for preventing and treating pain by administering to a subject using a combination of an effective amount of secretin and an effective amount of oxytocin. The invention also provides kits for use in treating and / or preventing gastrointestinal disorders, central nervous system disorders, autoimmune diseases and pain comprising a combination of secretin and oxytocin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Materials and Methods for Making Improved Micelle Compositions
InactiveUS20110142884A1Increase fat solubilityCosmetic preparationsNervous disorderDiseaseMedicinal chemistry
Provided are methods of treatment of many different diseases and disorders using micelle and sterically stabilized crystalline compounds of the invention.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Endocannabinoid conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound provided by the present invention includes an endocannabinoid, endocannabinoid derivative, or endocannabinoid analog moiety covalently bonded to a biologically active peptide. One example of an inventive compound described is a conjugate of an endocannabinoid, endocannabinoid derivative, or endocannabinoid analog moiety covalently coupled to an opioid peptide, such as an endorphin, enkephalin, dynorphin or endomorphin. Also detailed are processes for making the described conjugates and pharmaceutical compositions including such compounds.
Owner:MILLER LANDON C G
Methods for treatment of acute pancreatitis
ActiveUS20050129675A1Peptide/protein ingredientsSecretinsAcute pancreatic inflammationDuodenal secretion
The invention relates generally to methods for treating acute pancreatitis in patients. The methods comprise administering a therapeutically effective amount of a pharmaceutical composition comprising secretin and a pharmaceutically acceptable carrier.
Owner:CHIRHOCLIN
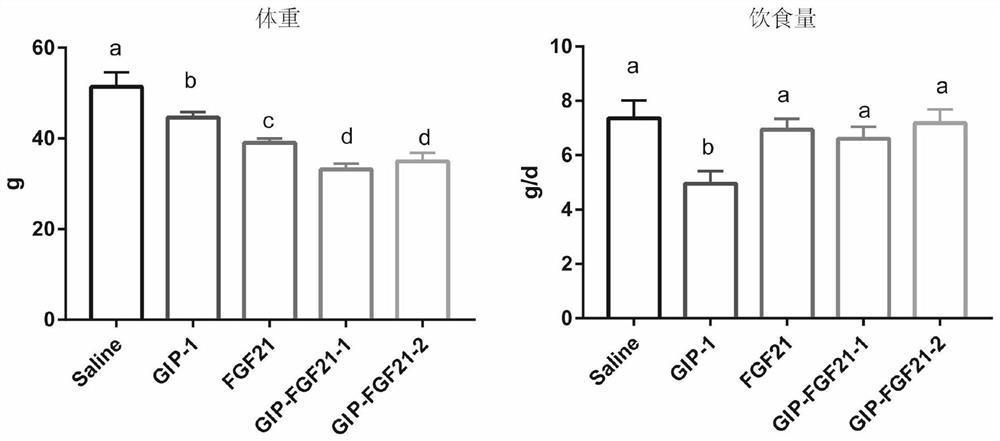
Fusion protein for treating metabolic diseases as well as preparation method and application thereof
ActiveCN113265007ALong-acting treatmentTreatment stablePeptide/protein ingredientsMetabolism disorderPharmaceutical drugTherapeutic effect
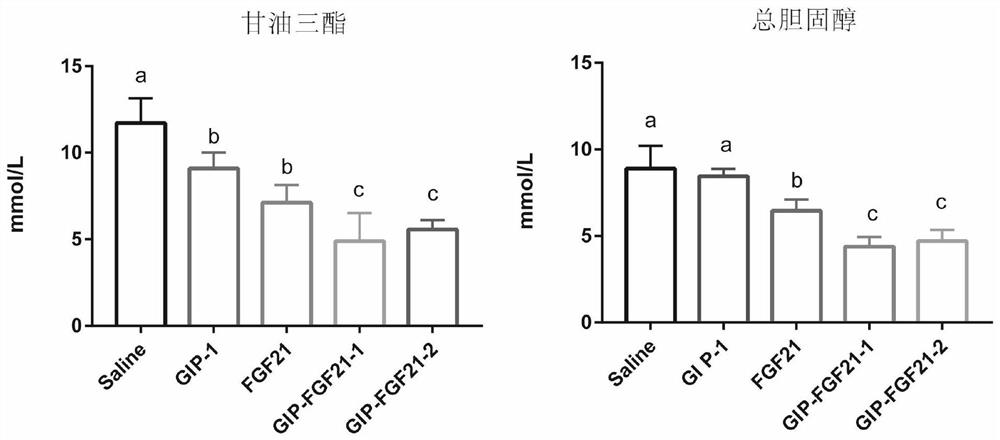
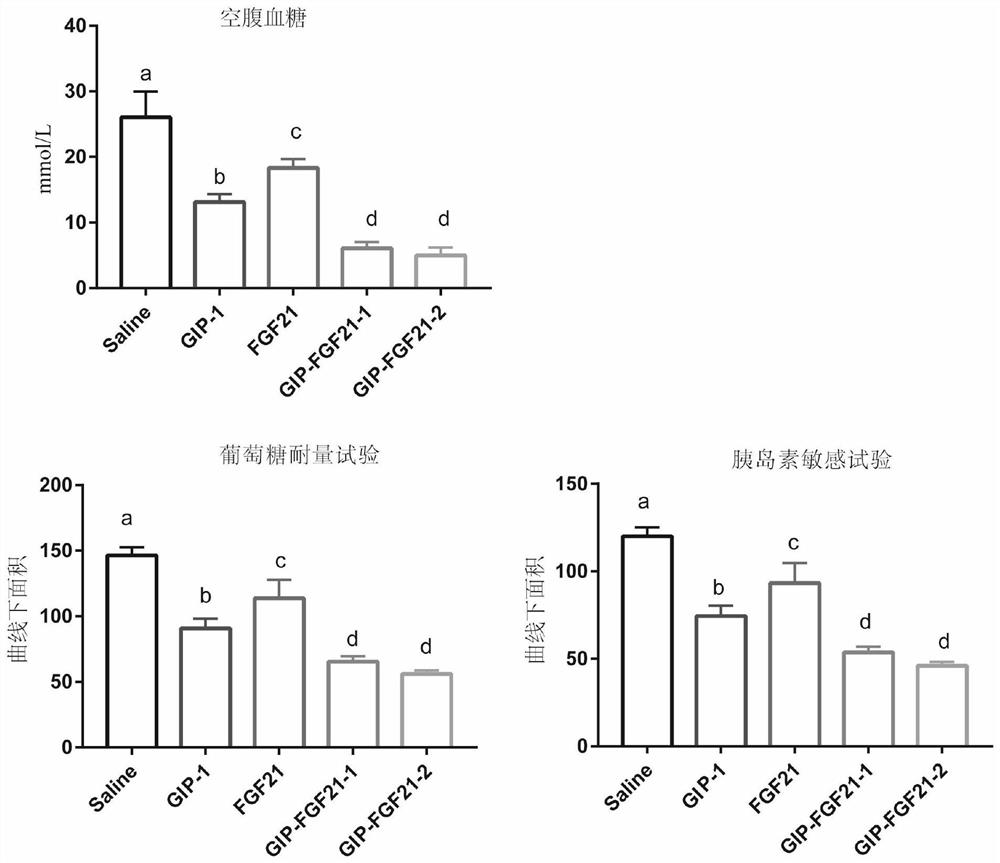
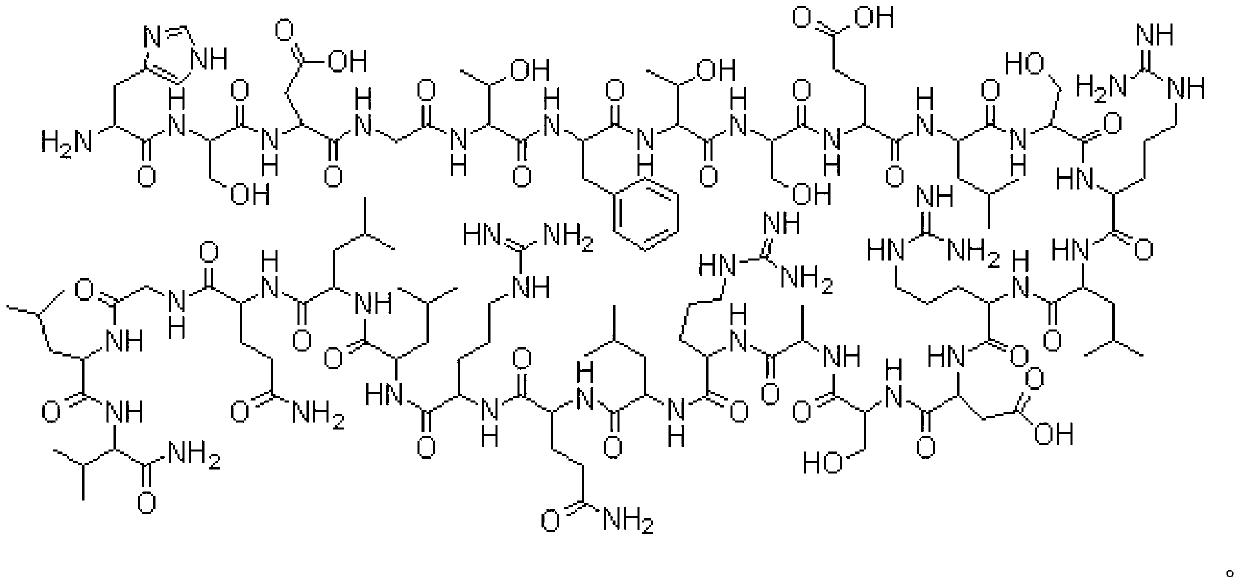
The invention belongs to the technical field of medicines, and relates to a fusion protein for treating metabolic diseases and a preparation method and application thereof. The general formula of the fusion protein is R1-L-R2 or R2-L-R1, wherein R1 is FGF21 protein, an FGF21 protein analogue or an analogue peptide with a FGF21 protein biological function, R2 is GIP, mutant GIP or an analogue peptide with a GIP biological function, and L is a connecting peptide. As a therapeutic drug or a pharmaceutical composition, the fusion protein disclosed by the invention can be used for treating diseases related to hyperglycemia and hyperlipidemia, such as diabetes, obesity, steatohepatitis or cardiovascular diseases, and the therapeutic effect is obviously superior to that of original FGF21 and GIP.
Owner:JIANGNAN UNIV
Solid phase method of secretin
InactiveCN103214568AHigh yieldHigh puritySecretinsPeptide preparation methodsCombinatorial chemistrySecretin
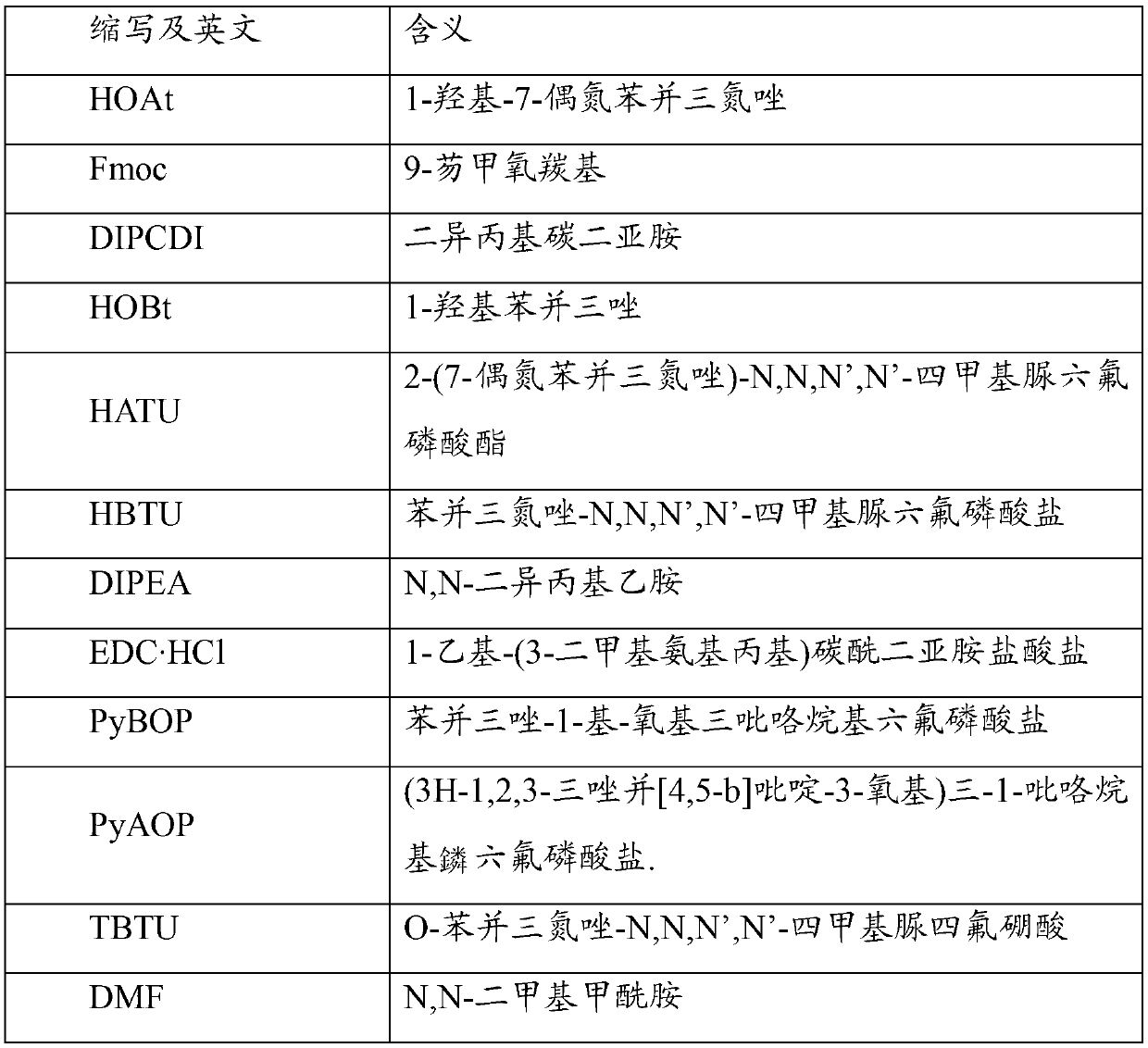
The invention provides a solid phase method of secretin, which comprises the following steps: 1) selecting an appropriate solid phase carrier; 2) coupling amino acids one by one according to a solid phase synthetic method; 3) splitting for obtaining a crude peptide; 4) obtaining the secretin by purifying the crude peptide, wherein, the solid phase synthesis employs Fmoc-strategy, and false proline is used for replacing parts of serine in the peptide chain during the solid phase synthesis. The solid phase method of secretin provided by the invention has the advantages of simple operation, small impurity, easy purifying and high yield, and is beneficial to realization of industrialization.
Owner:HYBIO PHARMA
Methods for preventing post endoscopic retrograde cholangiopancreatography pancreatitis
The invention relates generally to methods for preventing post endoscopic retrograde cholangiopancreatography pancreatitis (ERCP). The method comprises administering a therapeutically effective amount of a pharmaceutical composition comprising secretin and a pharmaceutically acceptable carrier.
Owner:FEIN SEYMOUR H +3
N- and C- terminal substituted antagonistic analogs of GH-RH
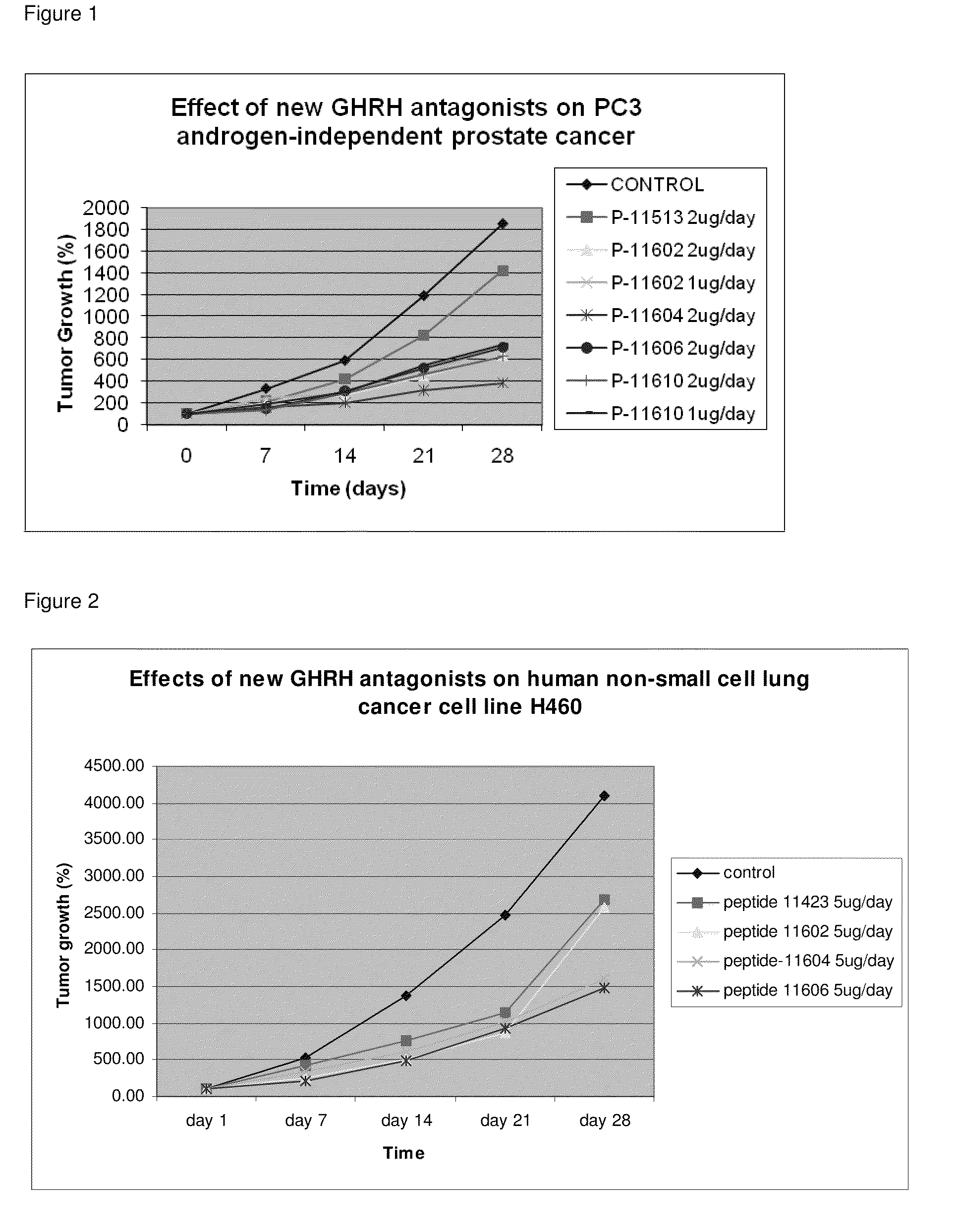
ActiveUS8691942B2Inhibition releaseEnhanced inhibitory effectPeptide/protein ingredientsSecretinsHuman cancerSynthetic analogue
There is provided a novel series of synthetic analogs of hGH-RH(1-29)NH2 (SEQ ID NO: 1) and hGH-RH(1-30)NH2. Of particular interest are those carrying PhAc, N-Me-Aib, Dca, Ac-Ada, Fer, Ac-Amc, Me-NH-Sub, PhAc-Ada, Ac-Ada-D-Phe, Ac-Ada-Phe, Dca-Ada, Dca-Amc, Nac-Ada, Ada-Ada, or CH3—(CH2)10—CO-Ada, at the N-Terminus and β-Ala, Amc, Apa, Ada, AE2A, AE4P, ε-Lys(α-NH2), Agm, Lys(Oct) or Ahx, at the C-terminus. These analogs inhibit the release of growth hormone from the pituitary in mammals as well as inhibit the proliferation of human cancers, and inhibit the hyperplastic and benign proliferative disorders of various organs, through a direct effect on the cancerous and non-malignant cells. The stronger inhibitory potencies of the new analogs, as compared to previously described ones, result from replacement of various amino acids.
Owner:MIAMI UNIVERISTY OF +2
Protein and protein conjugate for diabetes treatment, and applications thereof
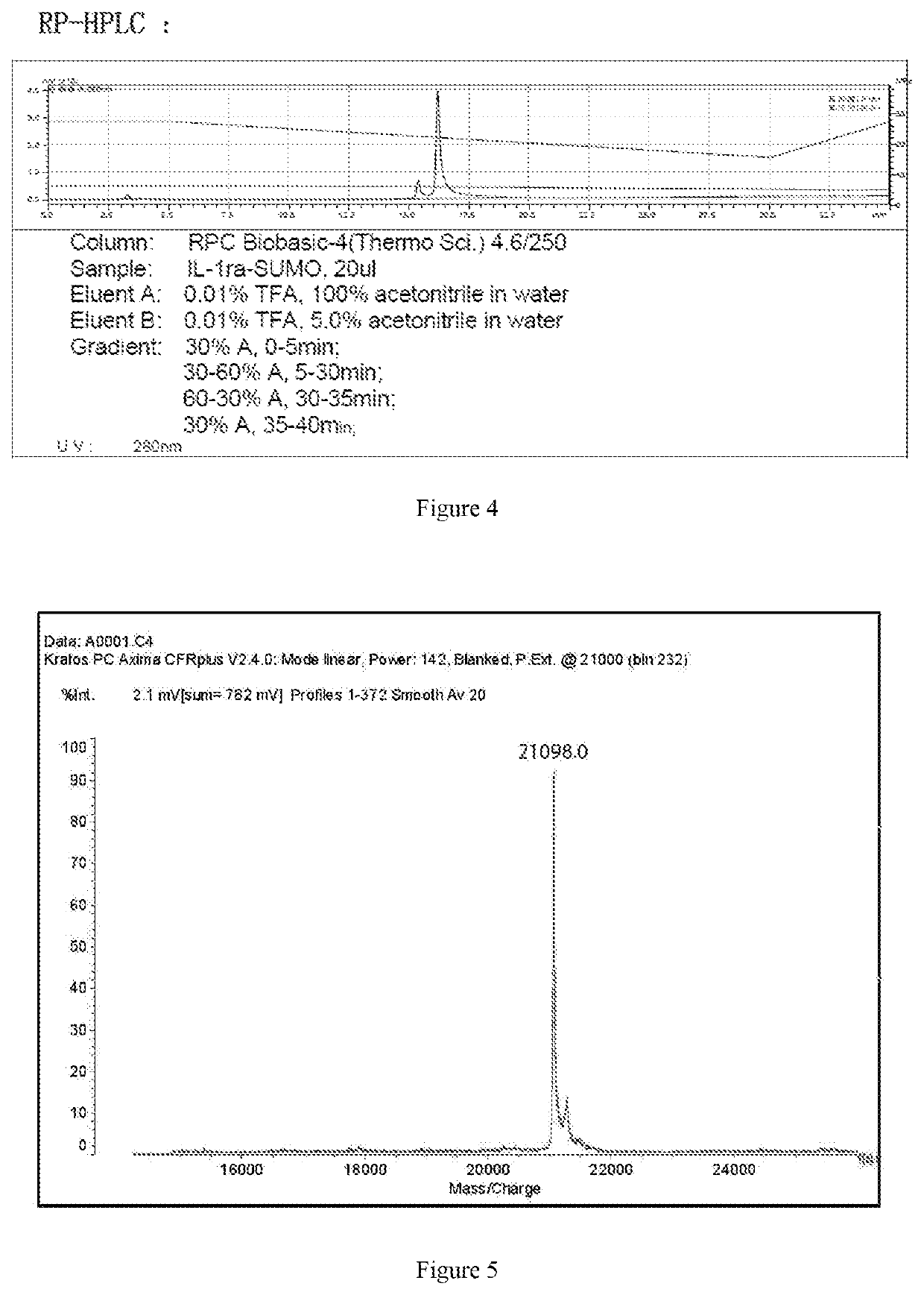
ActiveUS20200002397A1Maximum efficacyReduce dosePeptide/protein ingredientsMetabolism disorderReceptorEfficacy
The present invention relates to the field of biopharmaceuticals, and in particular to a protein, a protein conjugate, a pharmaceutical composition and its use for treating diabetes. The fusion protein of the present invention is obtained by linking two polypeptides, wherein one polypeptide is an interleukin-1 receptor antagonistic protein or an analogue thereof, and another polypeptide is GLP-1 receptor binding polypeptide or an analogue thereof, or an insulin receptor binding polypeptide or an analogue thereof, or a GIP receptor binding polypeptide or an analogue thereof. The fusion proteins of the present invention and conjugates thereof have a significant efficacy in treating diabetes, and can be used in a lower dose, resulting in marked reduction in side effects.
Owner:ADDA BIOTECH
Methods for preventing post endoscopic retrograde cholangiopancreatography pancreatitis
The invention relates generally to methods for preventing post endoscopic retrograde cholangiopancreatography pancreatitis (ERCP). The method comprises administering a therapeutically effective amount of a pharmaceutical composition comprising secretin and a pharmaceutically acceptable carrier.
Owner:FEIN SEYMOUR H +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com