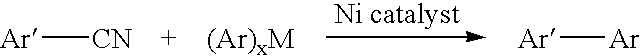Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83results about "Organic grignard reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
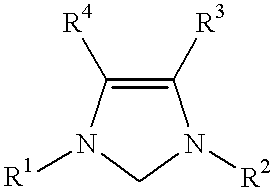
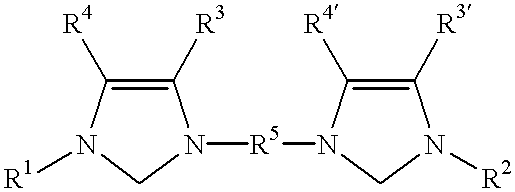
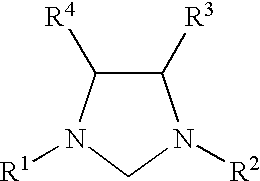
Catalyst system comprising transition metal and imidazoline-2-ylidene or imidazolidine-2-ylidene
InactiveUS6316380B1Inexpensive and readily synthesizedHigh yieldCarboxylic acid esters preparationOrganic grignard reactionsCarbeneImidazolidines
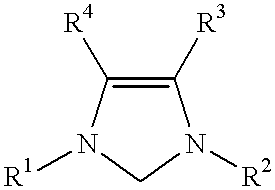
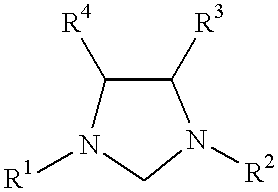
This invention provides a catalyst system useful in many coupling reactions, such as Suzuki, Kumada, Heck, and amination reactions. The catalyst system of the present invention makes use of N-heterocyclic carbenes or their protonated salts. The composition of the catalyst system comprises at least one transition metal compound and at least one N-heterocyclic carbene or its protonated salt. This invention further provides novel N-heterocyclic carbenes and their protonated salts. One type of N-heterocyclic carbene used in this invention is an imidazolinc-2-ylidene wherein the 1 and 3 positions are each, independently, substituted by an aromatic group in which each ortho position is, independently, substituted by a secondary or tertiary group which has at least three atoms.
Owner:NEW ORLEANS RES & TECH FOUND UNIV OF +1
Solvents containing cycloakyl alkyl ethers and process for production of the ethers
InactiveUS20050065060A1Moderate boiling pointPromote decompositionSurface-active detergent compositionsDetergent mixture composition preparationAlcoholChemical reaction
The present inventions are (A) a solvent comprising at least one cycloalkyl alkyl ether (1) represented by the general formula: R1-O-R2 (wherein R1 is cyclopentyl or the like; and R2 is C1-10 alkyl or the like); (B) a method of preparations the ethers (1) characterized by reacting an alicyclic olefin with an alcohol in the presence of an acid ion-exchange resin having a water content of 5 wt % or less. The solvent is useful as cleaning solvent for electronic components, precision machinery components or the like, reaction solvent using various chemical reactions, extraction solvent for extracting objective organic substances, solvent or remover for electronic and electrical materials, and so on. The process enables industrially advantageous production of the objective cycloalkyl alkyl ethers (1).
Owner:ZEON CORP
Use of a catalyst system comprising nickel palladium or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in stille coupling reactions
InactiveUS6362357B1High yieldEliminate needHydrocarbon by isomerisationOrganic compound preparationLiquid mediumOxidation state
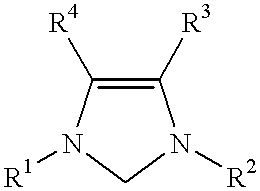
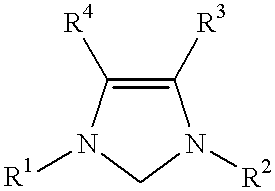
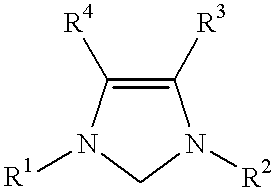
This invention provides a process for conducting Stille coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Stille couplings of aryl halides. A Stille coupling can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than stannyl groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one organotin compound wherein the tin atom is quaternary, wherein one group bound to the tin atom is unsaturated at the alpha or beta position, and wherein each of the remaining groups bound to the tin atom is a saturated group; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:RES & TECH FOUND UNIV OF NEW ORLEANS
Use of a catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in kumada coupling reactions
InactiveUS6369265B1Good yieldEliminate needOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsLiquid mediumGrignard reagent
This invention provides a process for conducting Kumada coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Kumada couplings of aryl halides. A Kumada coupling can be carried out by mixing, in a liquid medium, at least one aryl halide, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one Grignard reagent; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group. Homocoupling of aryl pseudohalides is also feasible using the processes of this invention.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Use of catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in amination reactions
InactiveUS6403802B1High yieldEliminate needGroup 5/15 element organic compoundsCarboxylic acid esters preparationLiquid mediumOxidation state
This invention provides a process for conducting amination reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in aminations of aryl halides and aryl pseudohalides. An amination can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than amino groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one primary amine and / or at least one secondary amine; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Ionic liquid type monophosphine monoimidazolium salt nickel (II) complex and preparation and application thereof
InactiveCN101591360ARich choiceVarious structural changesOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsGrignard reagentPhenyl group
The invention discloses an ionic liquid type monophosphine monoimidazolium salt nickel (II) complex and preparation and application thereof. The chemical molecular formula of the monophosphine monoimidazolium salt nickel (II) complex is (Ph3P)Ni{[(RNCHCHNR)CH]X}X2, wherein R is selected from one of C1-C4 saturated alkyl, phenmethyl, 2,6-diisopropylphenyl and mesitylene; and X is halogen and selected from one of chlorine, bromine or iodine. The monophosphine monoimidazolium salt nickel (II) complex has good catalytic activity on Kumada cross-coupling reaction between aryl Grignard reagent and aryl halide or halogenated pyridine.
Owner:SUZHOU UNIV
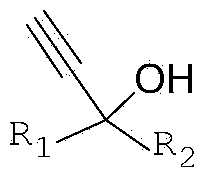
Method for preparing propargyl alcohol by using micro-structural reactor
InactiveCN103896737AGood choiceMild reaction conditionsOrganic compound preparationHydroxy compound preparationGrignard reagentProduct selection
The invention provides a method for preparing propargyl alcohol by using a micro-structural reactor, which comprises the steps of by taking a simple Grignard reagent as a raw material, carrying out gas-liquid Grignard exchange reaction on the simple Grignard reagent and acetylene gas to generate a high-quality acetylene-based magnesium bromide Grignard reagent, carrying out nucleophilic addition reaction on the obtained acetylene-based magnesium bromide Grignard reagent and an electrophilic reagent in a continuous flow condition, and carrying out hydrolysis, separation and refining on the product to obtain a substitution propargyl alcohol product. The method provided by the invention has the advantages that the operation is simple, the raw material and the reagent are simple, cheap and easily available, the process is continuous, rapid and controllable, the condition is mild, the product has good selectivity and industrial production can be realized.
Owner:NANJING UNIV OF TECH
Solvents containing cycloakyl alkyl ethers and process for production of the ethers
InactiveUS7494962B2Moderate boiling pointPromptly decomposed in the atmosphereDetergent mixture composition preparationHydroxy compound preparationChemical reactionAlcohol
Owner:ZEON CORP
Solvents containing cycloalkyl alkyl ethers and process for production of the ethers
InactiveUS20080312125A1Moderate boiling pointPromptly decomposed in the atmosphereSurface-active detergent compositionsDetergent mixture composition preparationChemical reactionIon exchange
The present inventions are (A) a solvent comprising at least one cycloalkyl alkyl ether (1) represented by the general formula: R1—O—R2 (wherein R1 is cyclopentyl or the like; and R2 is C1-10 alkyl or the like); (B) a method of preparations the ethers (1) characterized by reacting an alicyclic olefin with an alcohol in the presence of an acid ion-exchange resin having a water content of 5 wt % or less. The solvent is useful as cleaning solvent for electronic components, precision machinery components or the like, reaction solvent using various chemical reactions, extraction solvent for extracting objective organic substances, solvent or remover for electronic and electrical materials, and so on. The process enables industrially advantageous production of the objective cycloalkyl alkyl ethers (1).
Owner:ZEON CORP
Method of preparing organomagnesium compounds
ActiveUS20050218532A1High yieldAllows preparationCarboxylic acid nitrile preparationGroup 5/15 element organic compoundsSimple Organic CompoundsLithium
The present invention is directed to a reagent for use in the preparation of organomagnesium compounds as well as to a method of preparing such organomagnesium compounds. The present invention furthermore provides a method of preparing functionalized or unfunctionalized organic compounds as well as the use of the reagents of the present invention in the preparation of organometallic compounds and their reaction with electrophiles. Finally, the present invention is directed to the use of lithium salts—LiY in the preparation of organometallic compounds and their reactions with electrophiles and to an organometallic compound which is obtainable by the disclosed method.
Owner:LUDWIG MAXIMILIANS UNIV MUNCHEN
Grignard preparation of unsaturated organic compounds
InactiveUS6552237B1Low viscosityEnhanced mass transferSilicon organic compoundsOrganic compound preparationFiltrationEther
A Grignard-type process for the preparation of unsaturated organic compounds. The process comprises contacting an unsaturated organic halide with magnesium metal in a mixture of ether and a polar halogenated hydrocarbon co-solvent, filtering the reaction product from the reaction step and thereafter, treating the reaction product filtrate from the filtration step to obtain the desired unsaturated organic compounds.
Owner:DOW CORNING CORP
Clean generation of a fluoroaryl grignard reagent
Fluoroaryl Grignard reagents are produced from a hydrocarbyl Grignard reagent and fluoroaromatic compounds via separate additions of different fluoroaromatic compounds, such that the conversion of hydrocarbyl Grignard reagent to the desired fluoroaryl Grignard reagent is essentially complete, and thus the reaction product is free or essentially free of agents that may negatively affect subsequent reactions. The fluoroaryl Grignard reagents may be further reacted with boron trihalides in order to obtain tris(fluoroaryl)boranes or tetrakis(fluoroaryl)borates.
Owner:ALBEMARLE CORP
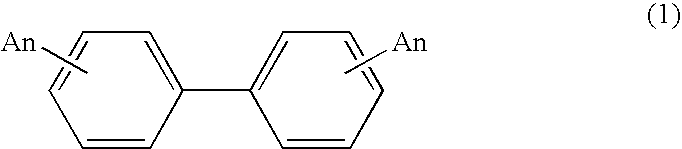
Process for production of biphenyl derivatives
InactiveUS7893306B2Low production costImprove productivityOrganic grignard reactionsOrganic substitutionBiphenyl derivativesGrignard reagent
A process for producing biphenyl derivatives represented by formula (1), including reacting a chlorine atom of a benzene derivative represented by formula (2) with metallic magnesium to form a Grignard reagent, and coupling two molecules of the Grignard reagent with each other in the presence of a catalyst.(wherein A represents at least one member selected from the group consisting of trifluoromethyl and fluoro, and n is an integer of 1 to 4.)(wherein A represents at least one member selected from the group consisting of trifluoromethyl and fluoro, and n is an integer of 1 to 4.)
Owner:HAYASHI TAMIO 50 OWNER +1
Process for preparing vinylaromatic compounds
InactiveUS6608212B1Efficient and effective processOrganic compound preparationHydrocarbons from unsaturated hydrocarbon additionGrignard reagentPalladium catalyst
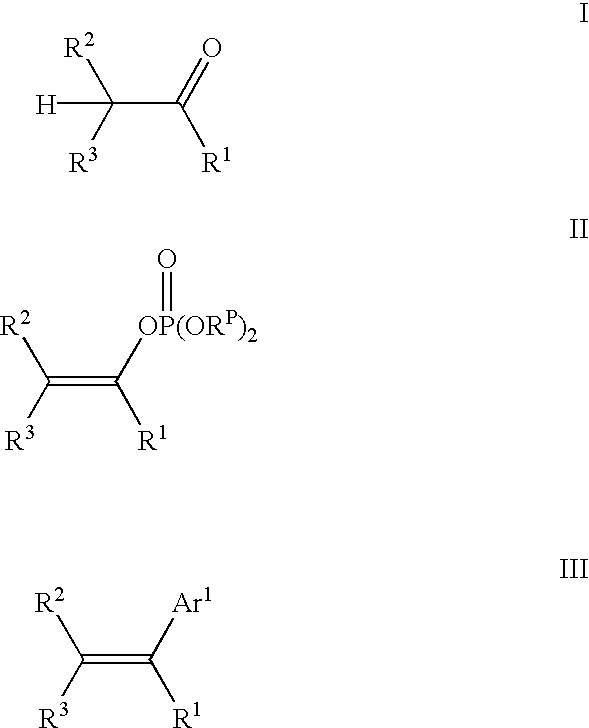
The present invention provides a process for preparing a vinylaromatic compound comprising reacting an arylmetal reagent selected from arylmagnesium reagents and aryllithium reagents with a vinylphosphate in the presence of a palladium catalyst. The present invention also provides a process for preparing a vinylphosphate comprising reacting an enolizable ketone with a sterically hindered Grignard reagent and a halophosphate diester.
Owner:DSM IP ASSETS BV +1
Ionized iron (III) coordination compound containing phenol-bridged imidazolium and application thereof
ActiveCN102603806AGenerate helpHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsIron organic compoundsHalohydrocarbonGrignard reagent
The invention discloses an ionized iron (III) coordination compound containing phenol-bridged imidazolium. The ionized iron (III) coordination compound containing the phenol-bridged imidazolium is characterized by having a chemical general formula as follows: [O-4-C(CH3)3-C6H2-2,6-di-{CH2[CH(NCHCHNR)]}FeX4, wherein R is selected from one of benzyl, 2,6-diisopropyl phenyl, mesityl and saturated alkyl from C1 to C4; and X is chlorine or bromine. Aryloxy-bridged bis(N-heterocyclic carbine) synthesized in situ in the catalysis process of the ionized iron (III) coordination compound containing the phenol-bridged imidazolium disclosed by the invention is not only beneficial to the generation of a low-valence-state iron catalytic active center, but also capable of more effectively stabilizing the low-valence-state iron catalytic active center, so that the ionized iron (III) coordination compound disclosed by the invention has extremely high catalytic activity for the cross-coupling reaction between an aryl grignard reagent and alkyl halohydrocarbon containing b-H and novel efficient catalysts are developed.
Owner:优标易站(苏州)电子商务有限公司
Synthetic method for 2,6-dimethy-5-heptenal
ActiveCN101016233ALow costMild reaction conditionsOrganic compound preparationOrganic grignard reactionsBenzeneAlcohol
The invention discloses a synthesizing method of 2, 6-dimethyl-5-heptene aldehyde, which comprises the following steps: 1) dissolving 6-methyl-5-heptene-2-ketone and aluminium isopropoxide with molar rate at 1:0.35-1 into benzene or toluene solution; reducing under 60-120 deg.c; hydrolyzing into 6-methyl-5-heptene-2-alcohol; 2) halogenating 6-methyl-5-heptene-2-alcohol and halogenating agent with molar rate at 1:0.35-2 under -10-80 deg.c to produce 2-chlorine-6-methyl-5-heptene or 2-bromine-6-methyl-t-heptene; 3) reacting 2-chlorine-6-methyl-5-heptene or 2-bromine-6-methyl-t-heptene and magnesium to produce Grignard reagent; allocating the composition and formic ether with molar rate at 0.5-1:1 under -10-30 deg.c to do Grignard additional reaction; hydrolyzing; decompressing; distilling; obtaining the product.
Owner:ZHEJIANG UNIV +1
Method for preparing 2,6-dimethyl-2-heptanol
ActiveCN101125797AStable in natureEasy to storePreparation by hydrogenationOrganic grignard reactionsChemical industryGrignard reagent
The invention discloses a preparation method of an organism in chemical industry technical field, in particular to the preparation method of a 2, 6-Dimethyl-2-Heptanol. The invention is that: firstly methylic Grignard reagent is prepared through taking Benzyl magnesium Chloride or Benzyl magnesium Bromide as an initiator, then is carried out the Grignard reaction with 2-Methyl-2-Hepten-6-Ketone, and finally is catalyzed and hydrogenated to synthesize 2,6-Dimethyl-2-Heptanol. The initiator prepared by the invention has the advantages of relatively stable property, easy preservation, higher initiating speed compared with commonly used initiator, strong universality, low dosage, convenient storage and low toxicity, etc. The initiator is especially suitable for preparing Grignard Reagent in mass production. The product prepared by the invention has better security and higher yield. The prepared product can be widely used in essence prescription of daily chemistry.
Owner:格林生物科技股份有限公司
Process for preparing unsymmetrical biaryls and alkylated aromatic compounds from arylnitriles
Owner:PHARMACORE
Catalytic carbon-carbon bond formation
InactiveUS20050137382A1Stable structureGroup 8/9/10/18 element organic compoundsGroup 3/13 element organic compoundsCarbon–carbon bondPhenyl group
The present invention mainly relates to a carbon-carbon bond formation catalyzed by a complex comprising a novel and stable ligand and a metal center. The ligand uses a ring, particularly a phenyl group, or a hydrocarbon group to link an amino group and PR1R2, NR1R2, OR1, SR1, or AsR1R2 group for stabling the structure of the ligand.
Owner:NAT SUN YAT SEN UNIV
Method for synthesizing cis-alpha-allyl-beta, gamma-unsaturated carboxylic acid ester
InactiveCN101318896AEasy to separate and purifyEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAllyl acetatePlatinum
The invention relates to a cis-alpha-allyl-belta, gamma-unsaturated carboxylic ester and a method for synthesizing the same. The method comprises the following steps that: a1,4-addition of a Grignard reagent and an allenoic ester, is conducted to give a 1,3-conjugated diene magnesia salt at a temperature of below 78 DEG C under the catalytic action of ferric acetylacetonate. The 1,3-conjugated diene magnesia salt reacts with allyl acetates under the catalytic action of tetrakis(triphenylphosphine)platinum to give a series of cis-alpha-allyl-belta, gamma-unsaturated carboxylic ester compounds. The synthetic method has the advantages that the operation is simple, the raw materials and reagents are readily available, the reaction has high regional and vertical selectivity and can substitute two groups at the same time, the products can be separated and purified easily, and can be used to synthesize various cis-alpha-allyl-belta, gamma-unsaturated carboxylic esters.
Owner:ZHEJIANG UNIV
S-4- methyloctane derivate, synthetic method thereof and application thereof to (S)-3-methyl-heptanoate sythesis
InactiveCN101274891AEasy to manufactureSimple and fast operationOrganic compound preparationCarboxylic preparation by ozone oxidationSynthesis methodsSapogenin
The invention relates to an (S)-4-methyloctane compound and a synthesis method thereof. The compound can be used for the synthesis of optically pure (S)-3-methylheptanoic acid. The structural formula of the (S)-4-methyloctane compound is the formula and compared with the existing methods, the method for the synthesis of the optically pure (S)-3-methylheptanoic acid by utilizing the invention is easier and more convenient to operate, efficient and conducive to the utilization of steroidal sapogenins oxidative degraded waste.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Process of preparing grignard reagent
A novel process of preparing a Grignard reagent is disclosed. The process is effected by electrochemically reacting a Grignard precursor with an electrode which comprises a metal for forming the Grignard reagent, in the presence an electrolyte solution that comprises a room temperature ionic liquid (RTIL). Electrochemical cells and systems for performing the process, and uses thereof in various applications are also disclosed.
Owner:TECHNION RES & DEV FOUND LTD
Process for preparing unsymmetrical biaryls and alkylated aromatic compounds from arylnitriles
Owner:PHARMACORE
Method of preparing 1,5-amino alcohol
ActiveCN104974049AAchieving Convergent SynthesisImprove resource utilization efficiencyOrganic compound preparationOrganic grignard reactionsSolventImine
The invention discloses a method of preparing 1,5-amino alcohol, wherein the method includes a following step of performing a three-component reaction to an imine represented as the formula II, a Grignard reagent represented as the formula III, a catalyst, an oxidation agent and an additive in a solvent to obtain the 1,5-amino alcohol which is represented as the formula I. The invention achieves simplified synthesis of the 1,5-amino alcohol, wherein the ratio of diastereoisomer of the 1,5-amino alcohol can reach 8:1 and the ratio can reach 99%. The raw materials are low in cost and easy to obtain. The method is mild in reaction conditions, is simple in operations and has an industrial production potential.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Synthesis of substituted methyl benzylketone
ActiveCN101367715ARaw materials are easy to getHigh yieldCarbonyl compound preparation by oxidationOrganic grignard reactionsArylPropanol
Owner:YIYUAN XINQUAN CHEM +1
Solutions of anhydrous lanthanide salts and its preparation
ActiveUS20090326235A1Easy to prepareEasy to addPhysical/chemical process catalystsCarboxylic acid nitrile preparationIndiumGrignard reagent
The present invention relates to anhydrous solutions of MX3-Z LiA in a solvent, wherein M is a lanthanide including lanthanum, or yttrium or indium; z>0; and X and A are independently or both monovalent anions, preferably Cl, Br or I. The solution is readily prepared by dissolving or suspending MX3 or its hydrate and z equiv LiA in water or hydrophilic solvents, or mixtures thereof, removing the solvent under vacuum and dissolving the resulting powder in another solvent. The solution of MX3-Z LiA can advantageously be used e.g. in addition reactions of Grignard reagents to ketones and imines. Even the catalytic use of MX3-Z LiA is possible.
Owner:LUDWIG MAXIMILIANS UNIV MUNCHEN
Deuterated cftr potentiators
This invention relates to compounds of Formula I:and pharmaceutically acceptable salts thereof, wherein each X and each R is defined within. This invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by administering a CFTR potentiator.
Owner:VERTEX PHARMA EURO
Method for manufacturing alcohols
InactiveCN101607857AImprove efficiencyExcellent productivityOrganic compound preparationHydroxy compound preparationThree levelOrganic solvent
The present invention provides a method for manufacturing alcohols having a good production character and having two level or three level carbon in a high yield. The method is that a Grignard reagent represented by the following chemical formula reacts with paraformaldehyde or formaldehyde with the existence of a mixed solvent of fatty ether and aromatic organic solvent, then the outcome yield is hydrolyzed. RnCH(3-n)-MgX, in the formula, R represents aromatic residue or fatty group residue or unsaturation fatty group, X represents a halogen atom, and n represents 2 or 3.
Owner:TORAY FINE CHEMICALS CO LTD
Grignard reagent synthesis reaction solvent in maltol production
InactiveCN101125840ACyclohexane is less toxicAppropriate adjustment of process indicatorsOrganic grignard reactionsGrignard reagentMaltol
The invention relates to a Grignard reagent synthesis solvent used in the production of maltol. The solvent is characterized in that the solvent is mixed by the raw material with the mixture ratio of: 2.6-3.2 of cyclohexane, 0.8-1.2 of tetrahydrofuran. The invention has the advantages that: cyclohexane is of little toxicity and is an allowed solvent listed in the safe foodstuffs by the European Union and U.S standards; experiment and application show that no change to the production equipment is made, only replacing the solvent and having a proper adjustment to the process index; the production operation is normal, the reaction can be easily controlled and the production cost is low.
Owner:ANHUI JINGHE IND
Process for the production of tertiary alcohols
Tertiary alcohols are prepared by reacting carboxylic esters with Grignard reagents in ethereal solvents in the presence of lanthanum trichloride and lithium chloride. The method is particularly suitable for the production of (aS)-a-[3-[(1E)-2-(7-chloro-2-quino- linyl)ethenyl]phenyl]-2-(1-hydroxy-1-methylethyl)benzenepropanol of formula (A) which is an intermediate in the production of montelukast.
Owner:LONZA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com