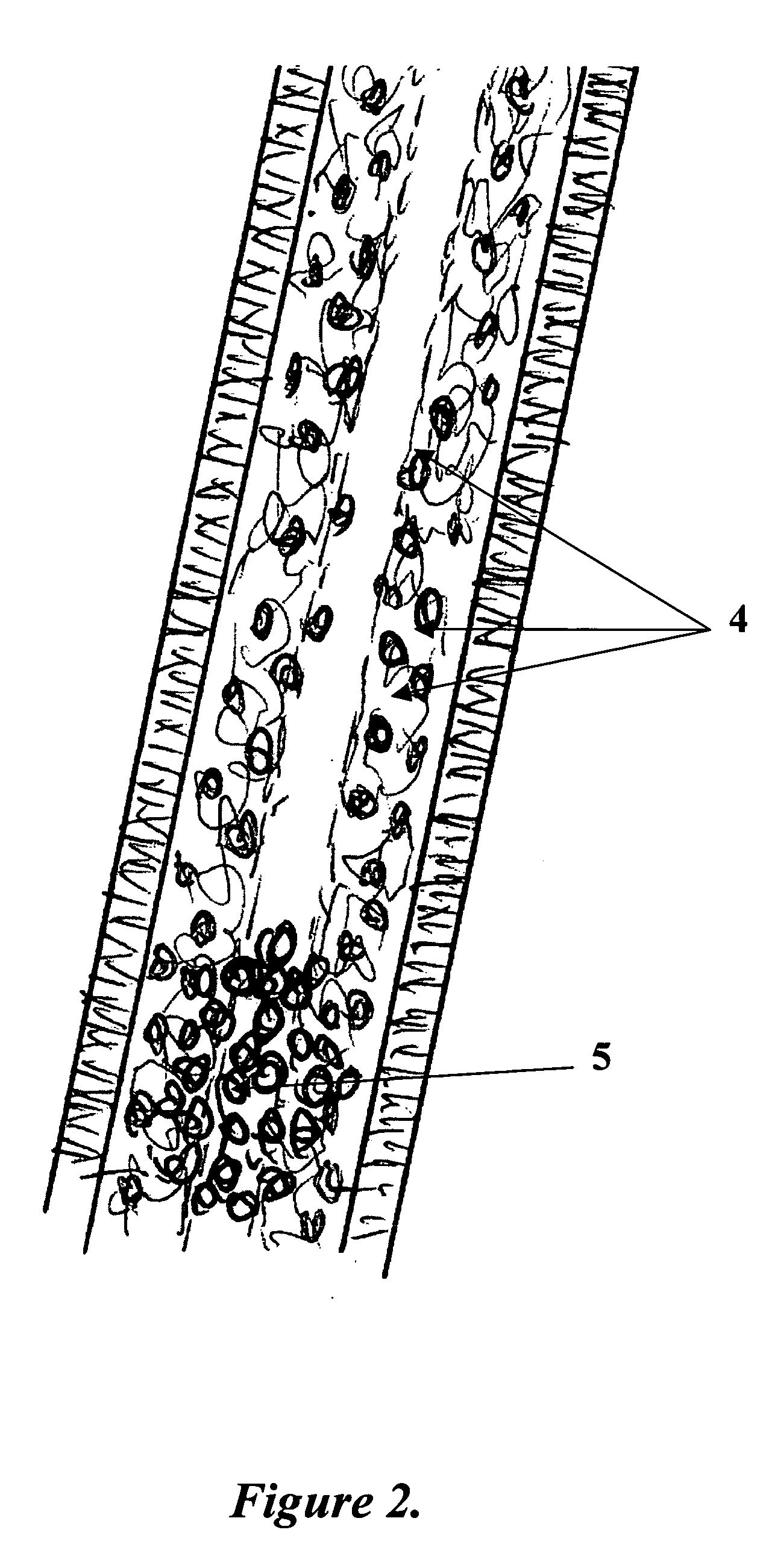
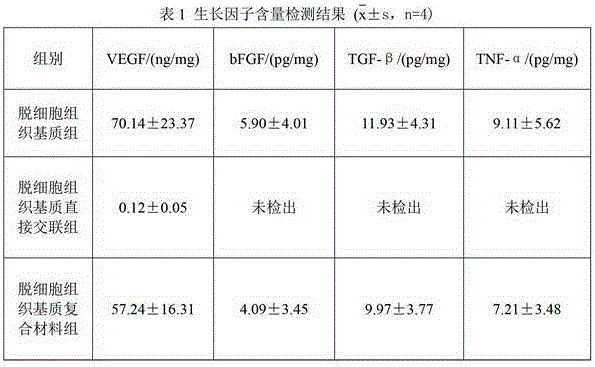
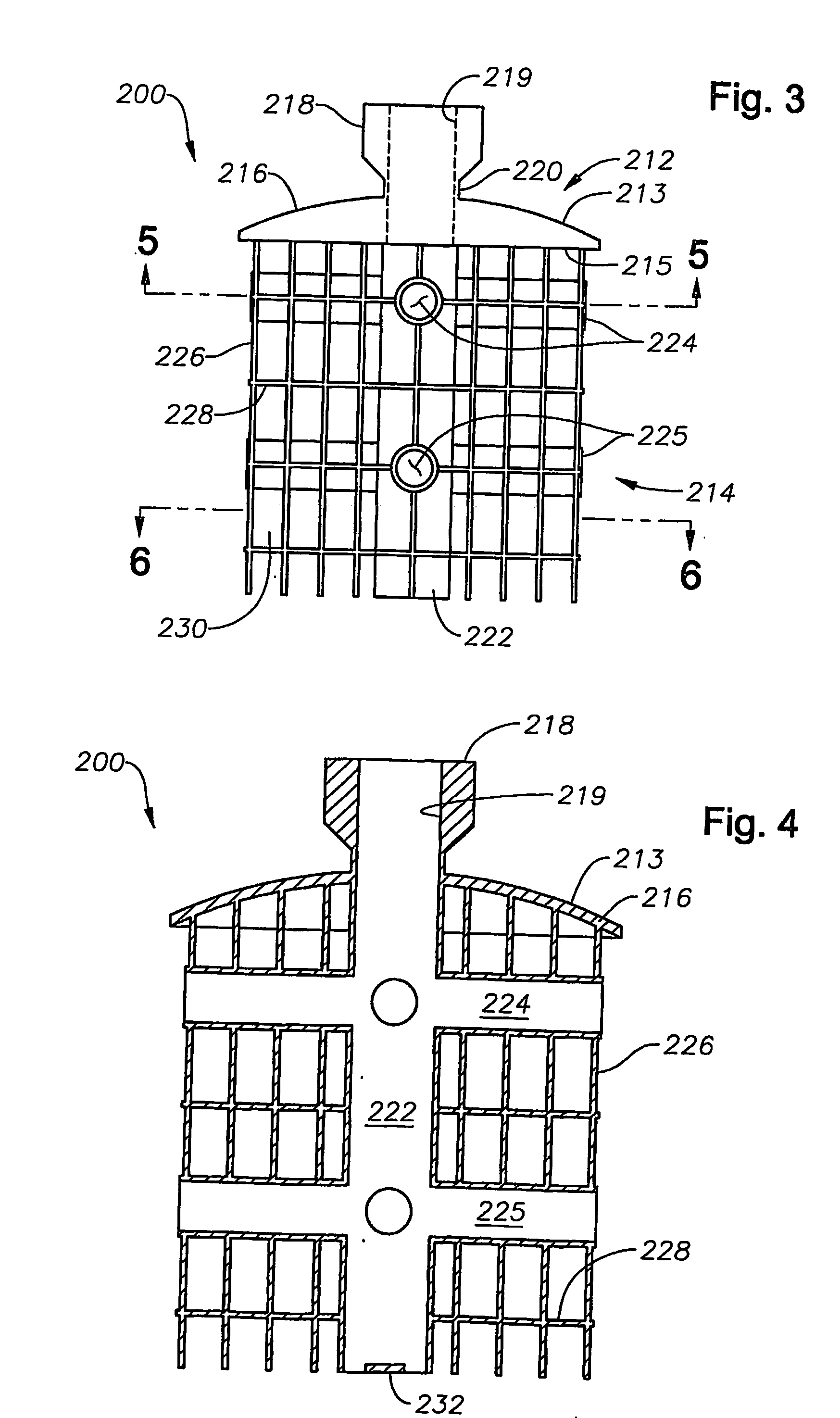
Patents
Literature
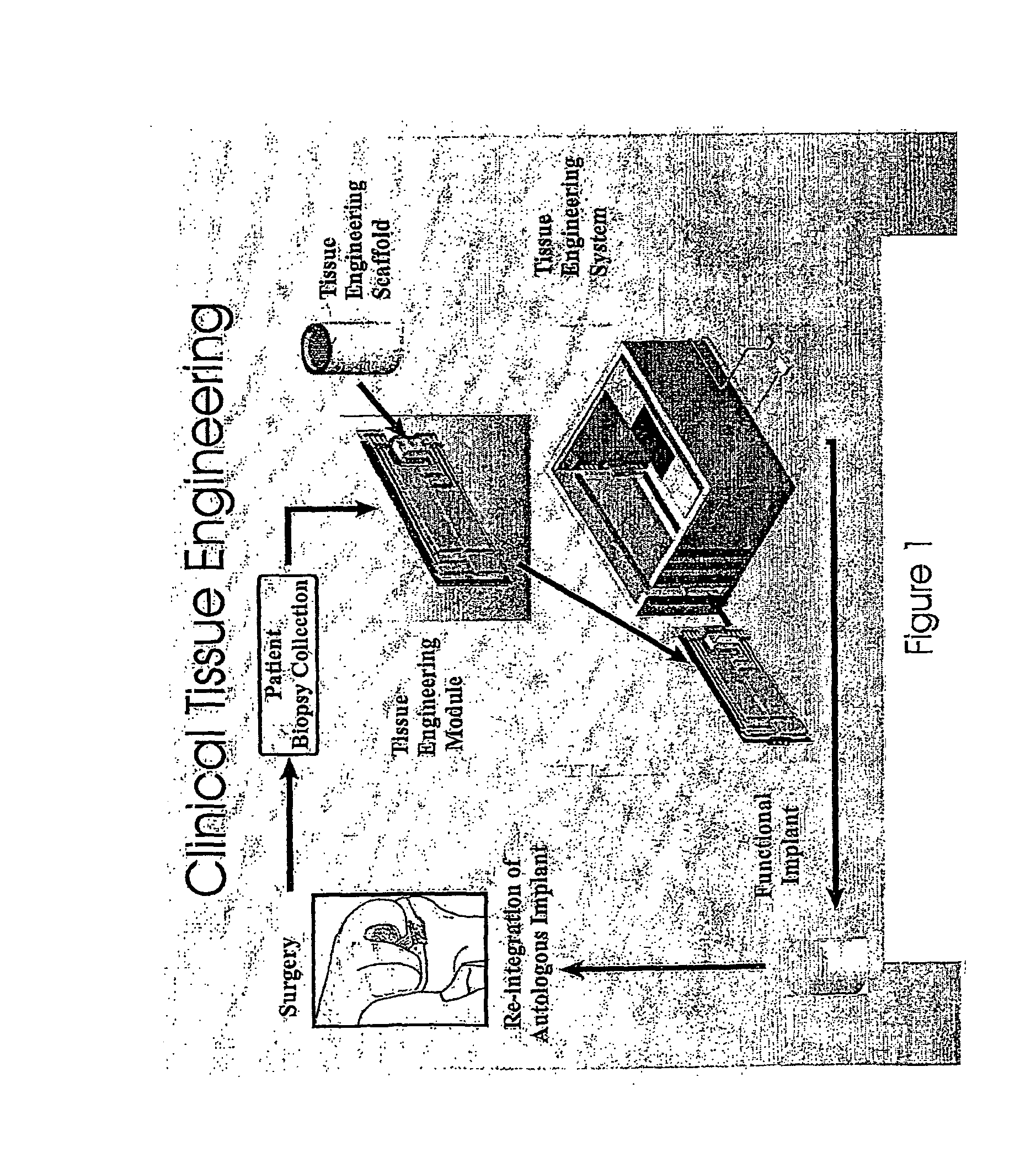
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
479 results about "Tissue engineer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Production of tissue engineered digits and limbs
ActiveUS20060257377A1Enhance tissue maturationImprove functionalityBiocidePeptide/protein ingredientsBone formingTissue construct
The invention pertains to methods of producing artificial composite tissue constructs that permit coordinated motion. Biocompatable structural matrices having sufficient rigidity to provide structural support for cartilage-forming cells and bone-forming cells are used. Biocompatable flexible matrices seeded with muscle cells are joined to the structural matrices to produce artificial composite tissue constructs that are capable of coordinated motion.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Decellularized tissue engineered constructs and tissues
InactiveUS6962814B2Low immunogenicityReduction in immuneBiocidePeptide/protein ingredientsGrowth phaseCell-Extracellular Matrix
New methods for producing tissue engineered constructs and engineered native tissues are disclosed. The methods include producing a tissue engineered construct by growing cells in vitro on a substrate and then decellularizing the construct to produce a decellularized construct consisting largely of extracellular matrix components. The construct can be used immediately or stored until needed. The decellularized construct can be used for further tissue engineering, which may include seeding the construct with cells obtained from the intended recipient of the construct. During any of the growth phases required for production of the construct, the developing construct may be subjected to various tissue engineering steps such as application of mechanical stimuli including pulsatile forces. The methods also include producing an engineered native tissue by harvesting tissue from an animal or human, performing one or more tissue engineering steps on the tissue, and subjecting the tissue to decellularization. The decellularized, engineered native tissue may then be subjected to further tissue engineering steps.
Owner:DUKE UNIV
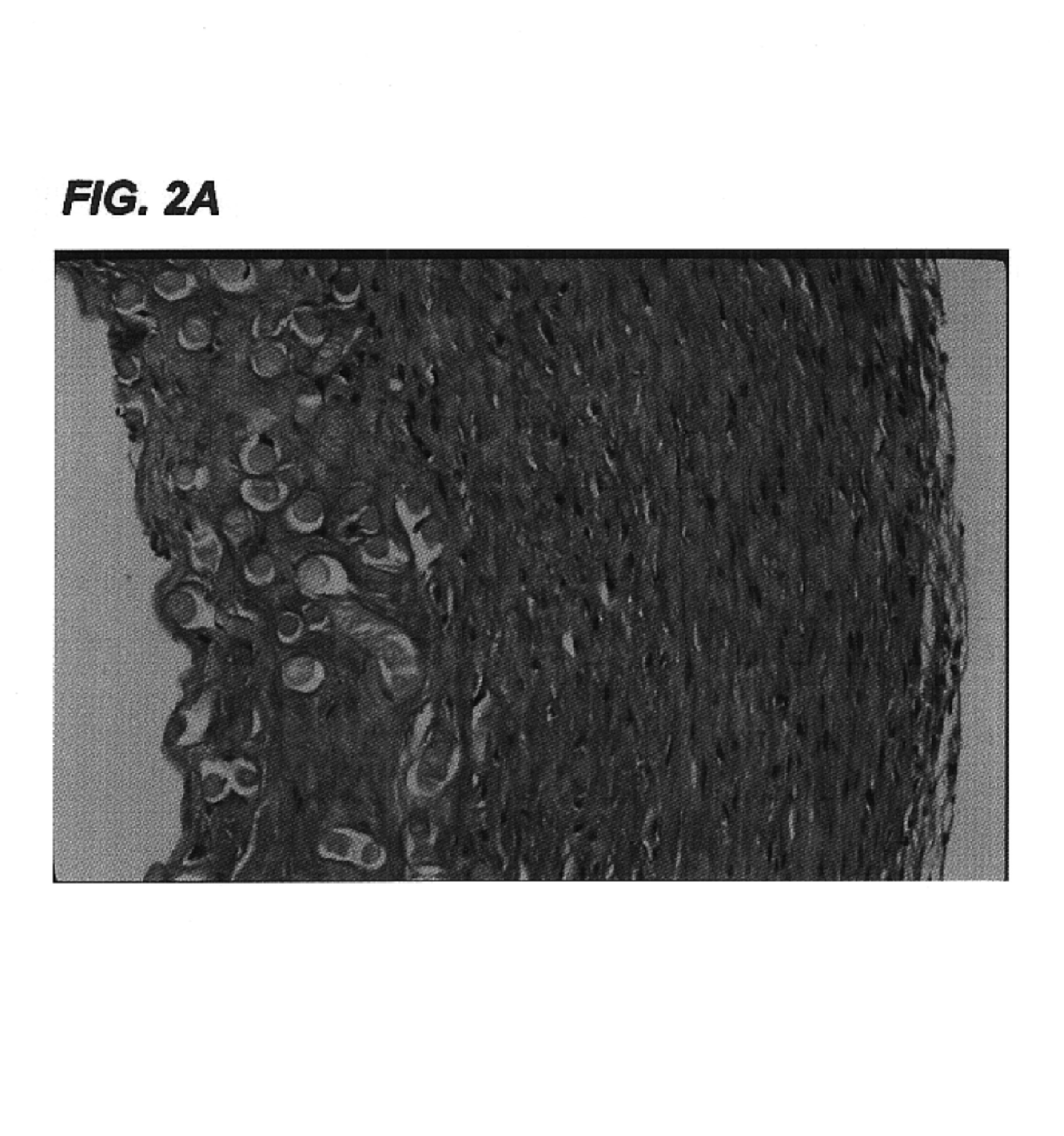
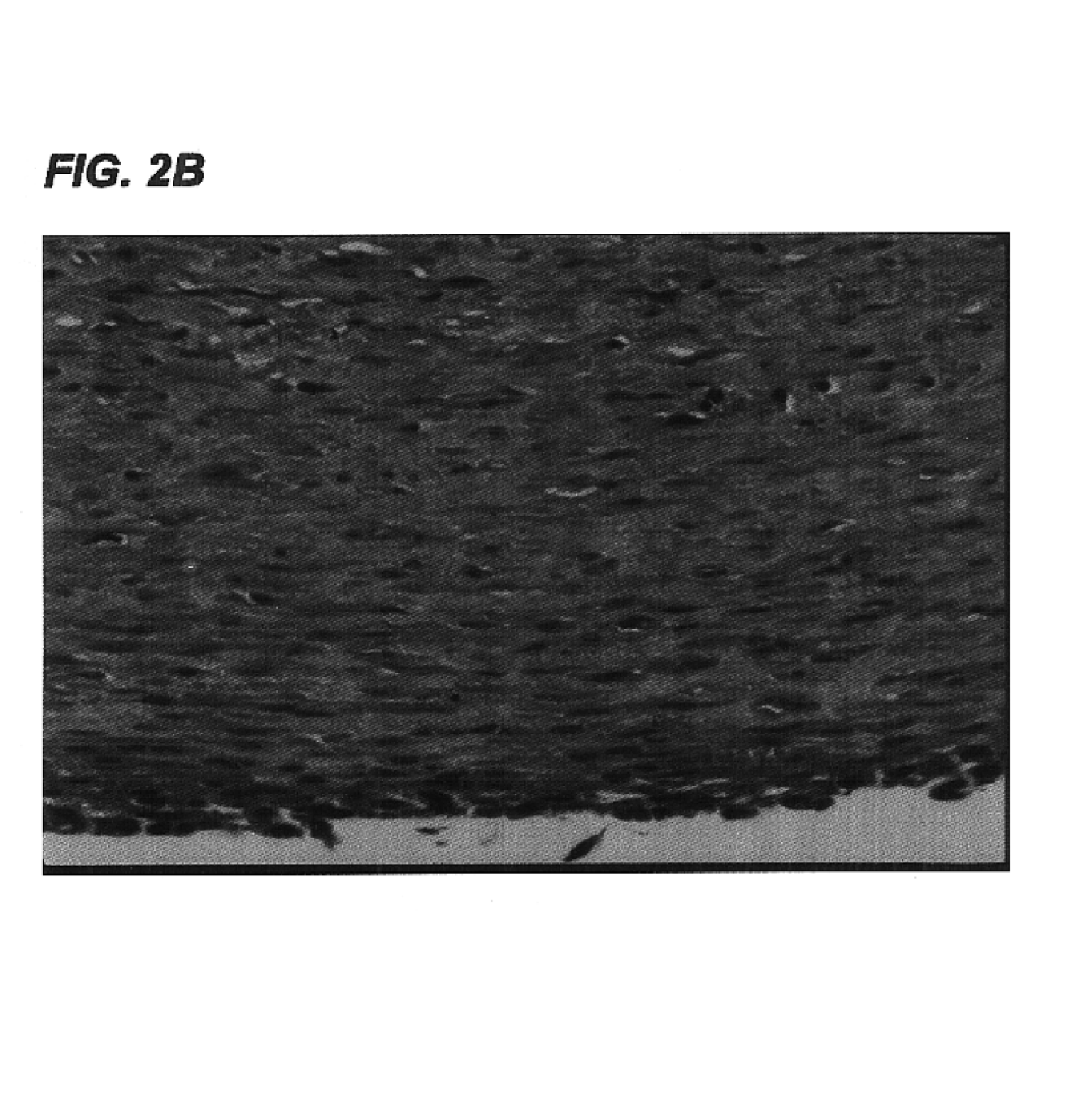
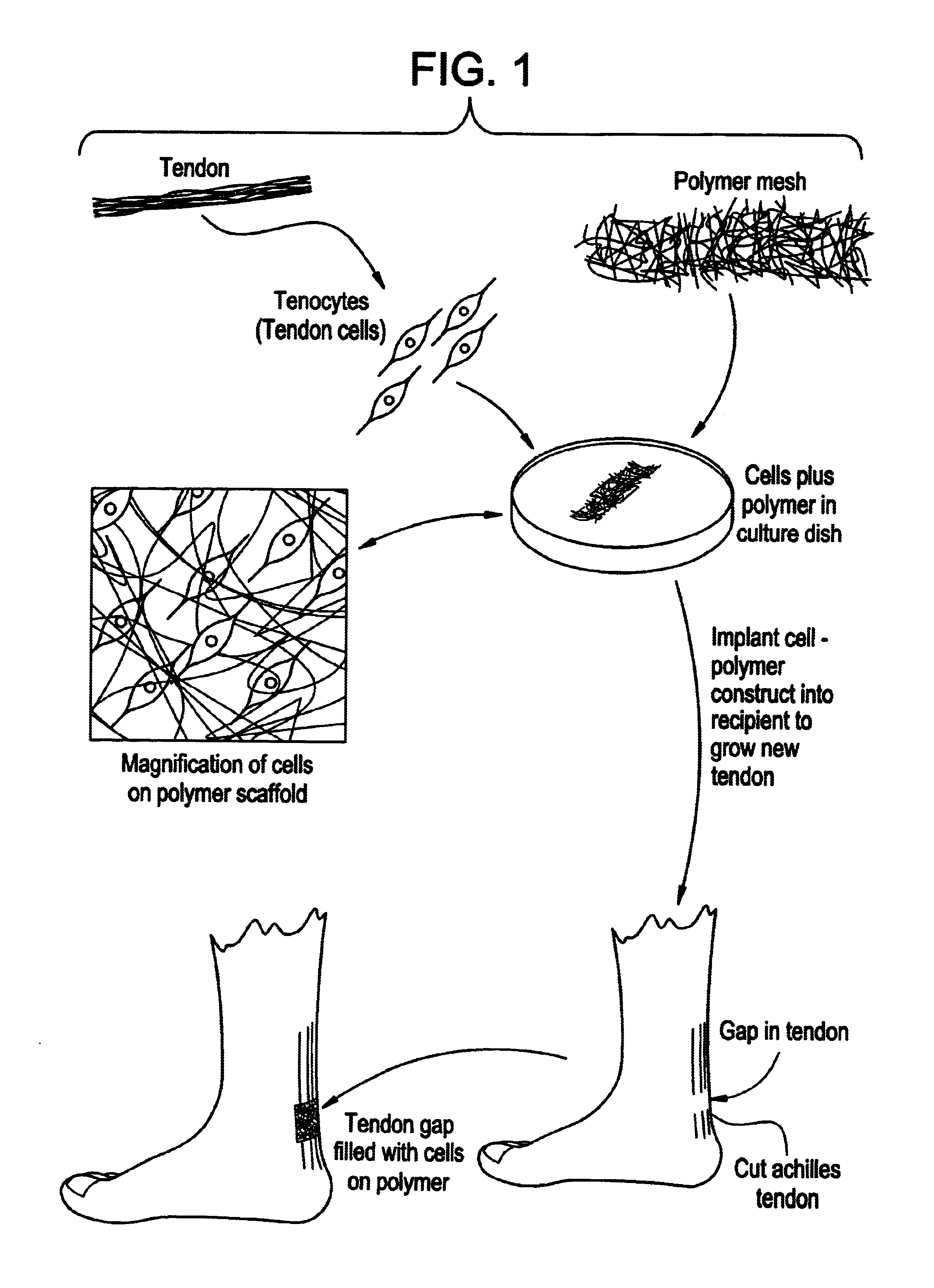
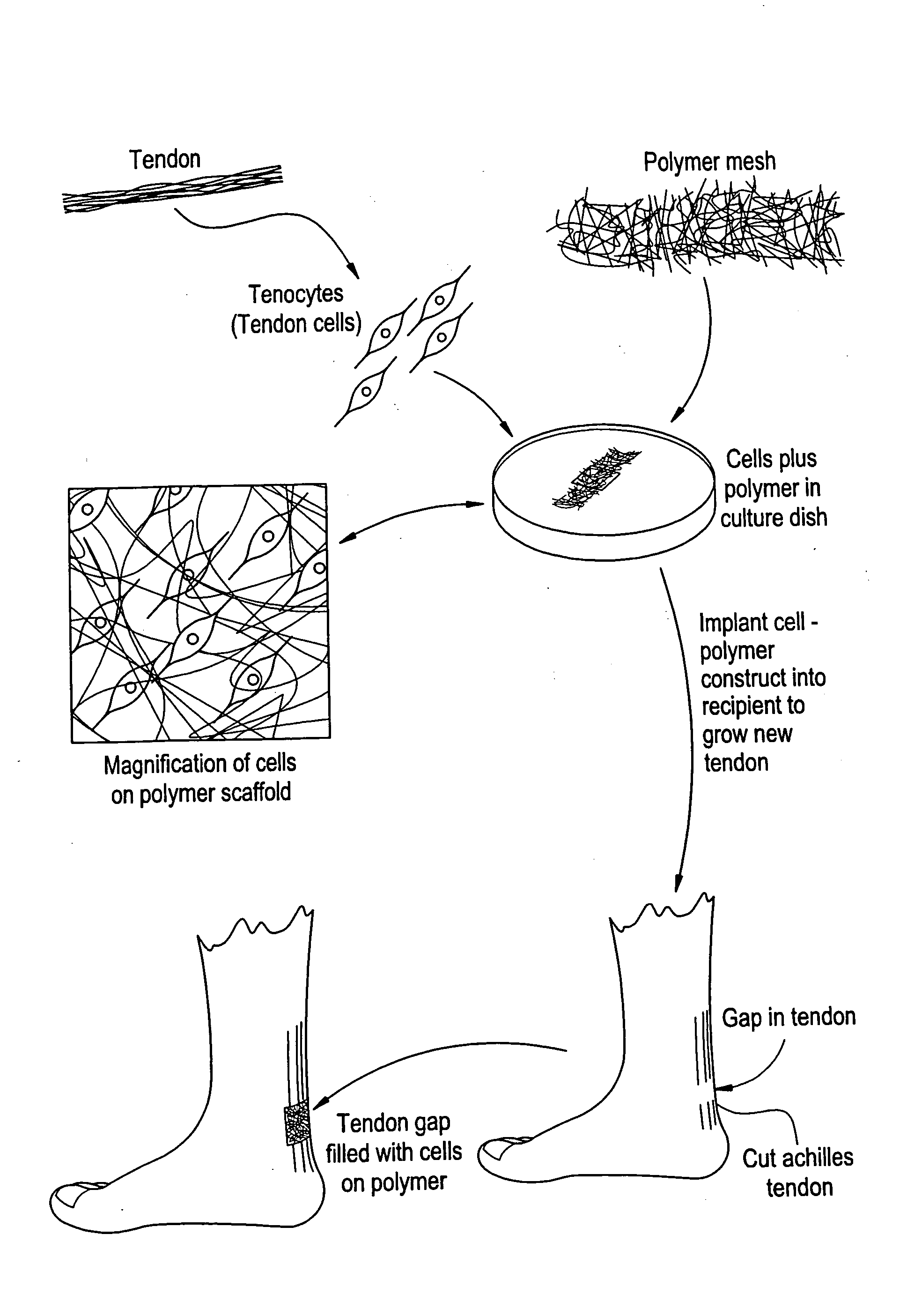
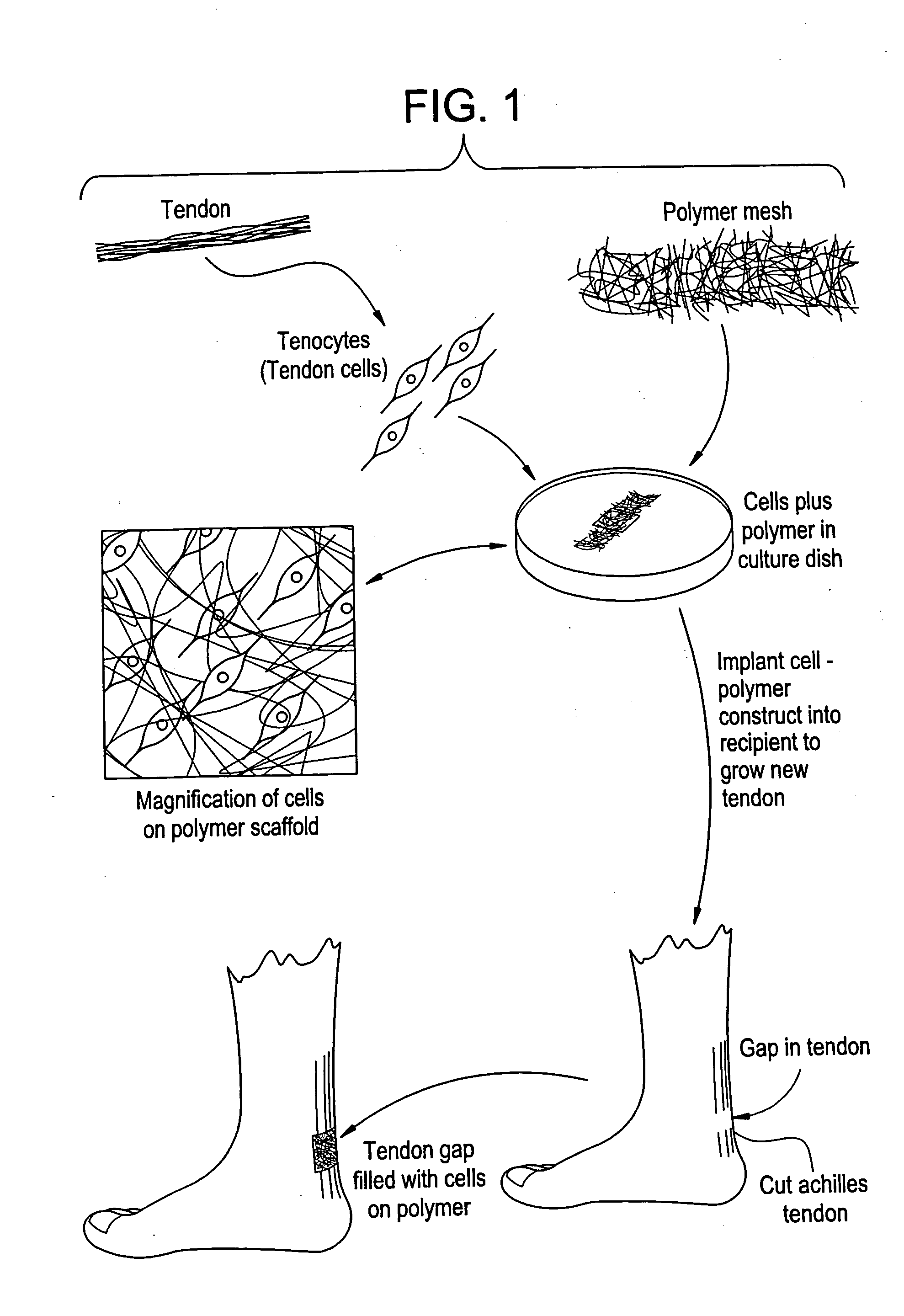
Tissue engineered tendons and ligaments
InactiveUS6840962B1Moderate strengthReduce inflammationLigamentsMusclesEnzymatic digestionLigament structure
Connective tissue, including neo-tendons and ligaments, has been constructed using biodegradable synthetic scaffolds seeded with tenocytes. The scaffolds are preferably formed from biodegradable fibers formed of a polymer such as polyglycolic acid-polylactic acid copolymers, and seeded with cells isolated from autologous tendon or ligament by means of enzymatic digestion or direct seeding into tissue culture dishes from explants. The cell polymer constructs are then surgically transplanted to replace missing segments of functioning tendon or ligament.
Owner:MASSACHUSETTS INST OF TECH +1
Tissue engineered tendons and ligaments
Connective tissue, including neo-tendons and ligaments, has been constructed using biodegradable synthetic scaffolds seeded with tenocytes. The scaffolds are preferably formed from biodegradable fibers formed of a polymer such as polyglycolic acid-polylactic acid copolymers, and seeded with cells isolated from autologous tendon or ligament by means of enzymatic digestion or direct seeding into tissue culture dishes from explants. The cell polymer constructs are then surgically transplanted to replace missing segments of functioning tendon or ligament.
Owner:VACANTI CHARLES A +5
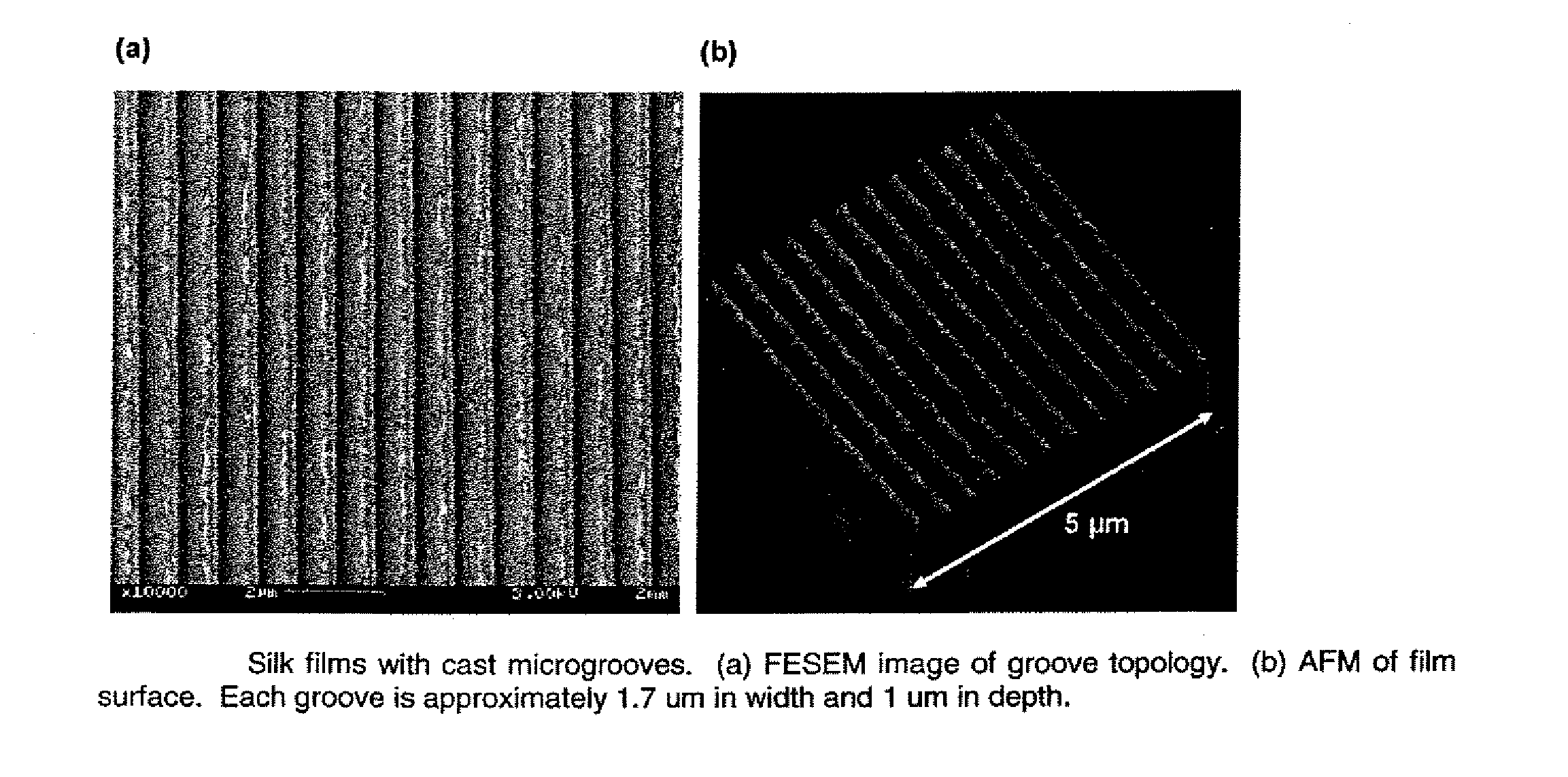
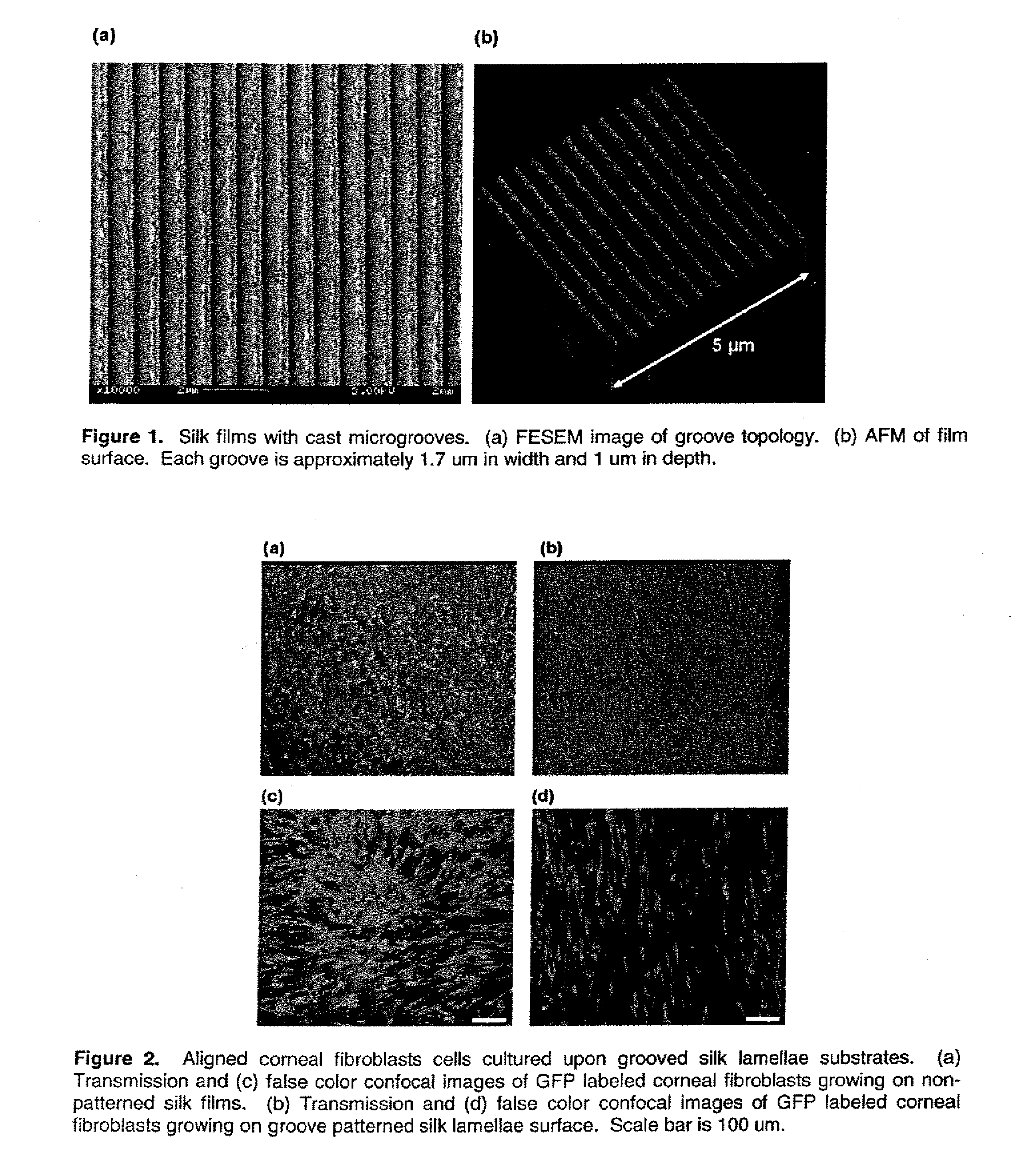
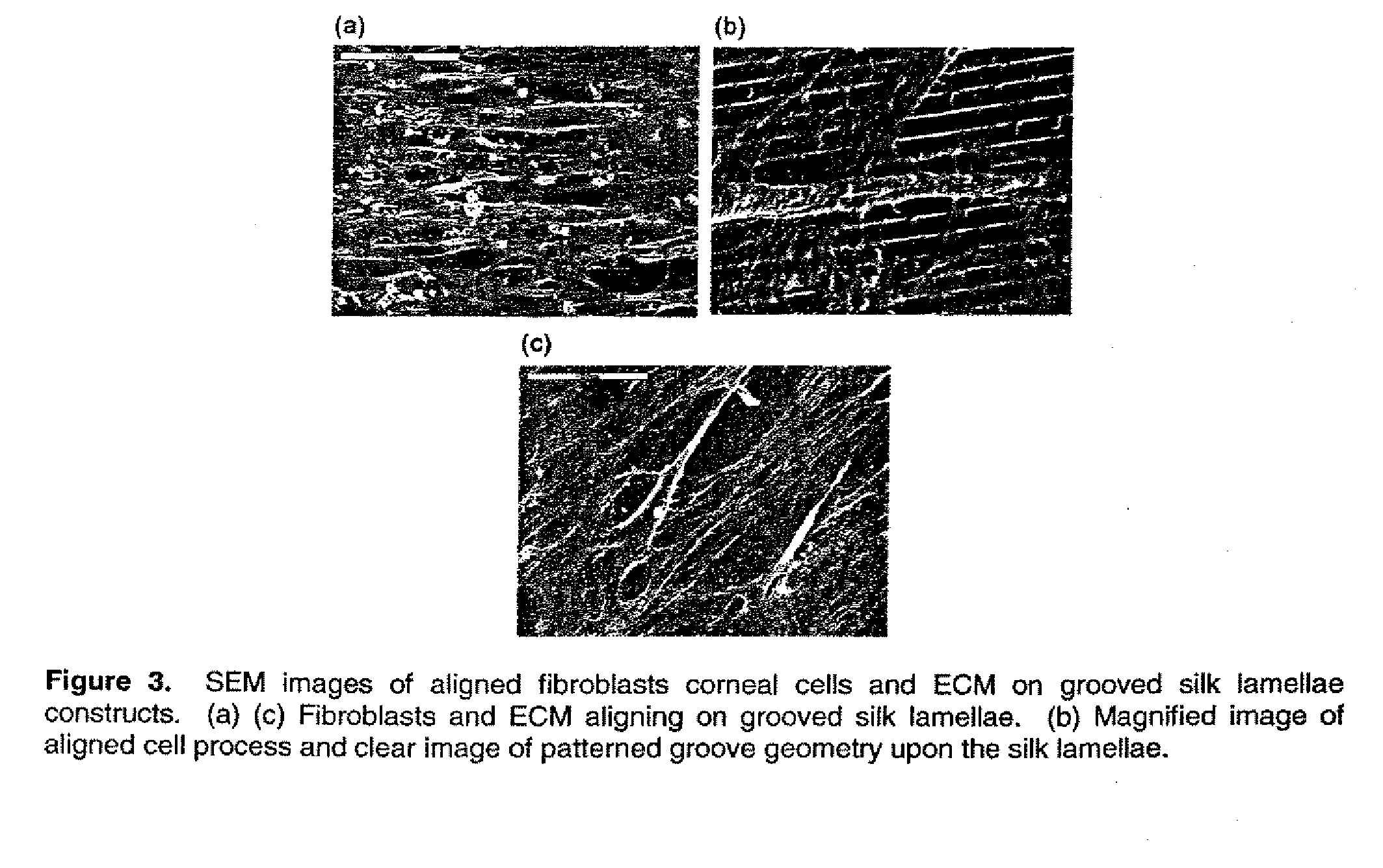
Tissue-engineered silk organs
ActiveUS20100191328A1Easy to controlEye implantsNervous system cellsCell-Extracellular MatrixCulture cell
This invention relates to a lamellae tissue layer, comprising a grooved silk fibroin substrate comprising tissue-specific cells. The silk fibroin substrates provides an excellent means of controlling and culturing cell and extracellular matrix development. A multitude of lamellae tissue layers can be used to create a tissue-engineered organ, such as a tissue-engineered cornea. The tissue-engineered organ is non-immunogenic and biocompatible.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Method and device for treating osteoarthritis, cartilage disease, defects and injuries in the human knee
InactiveUS7022506B2Heart defibrillatorsMagnetotherapy using coils/electromagnetsHuman useCells transplantation
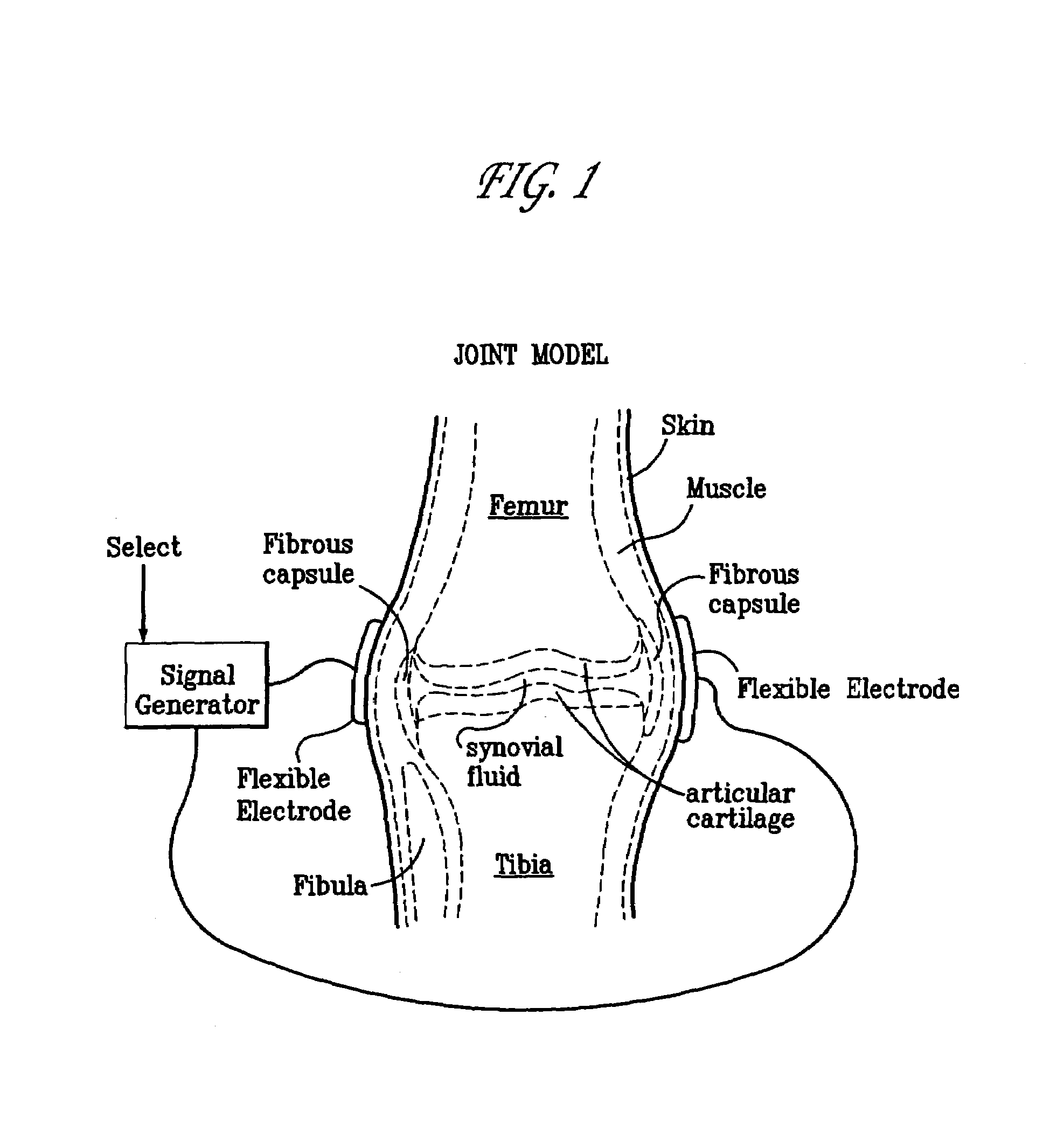
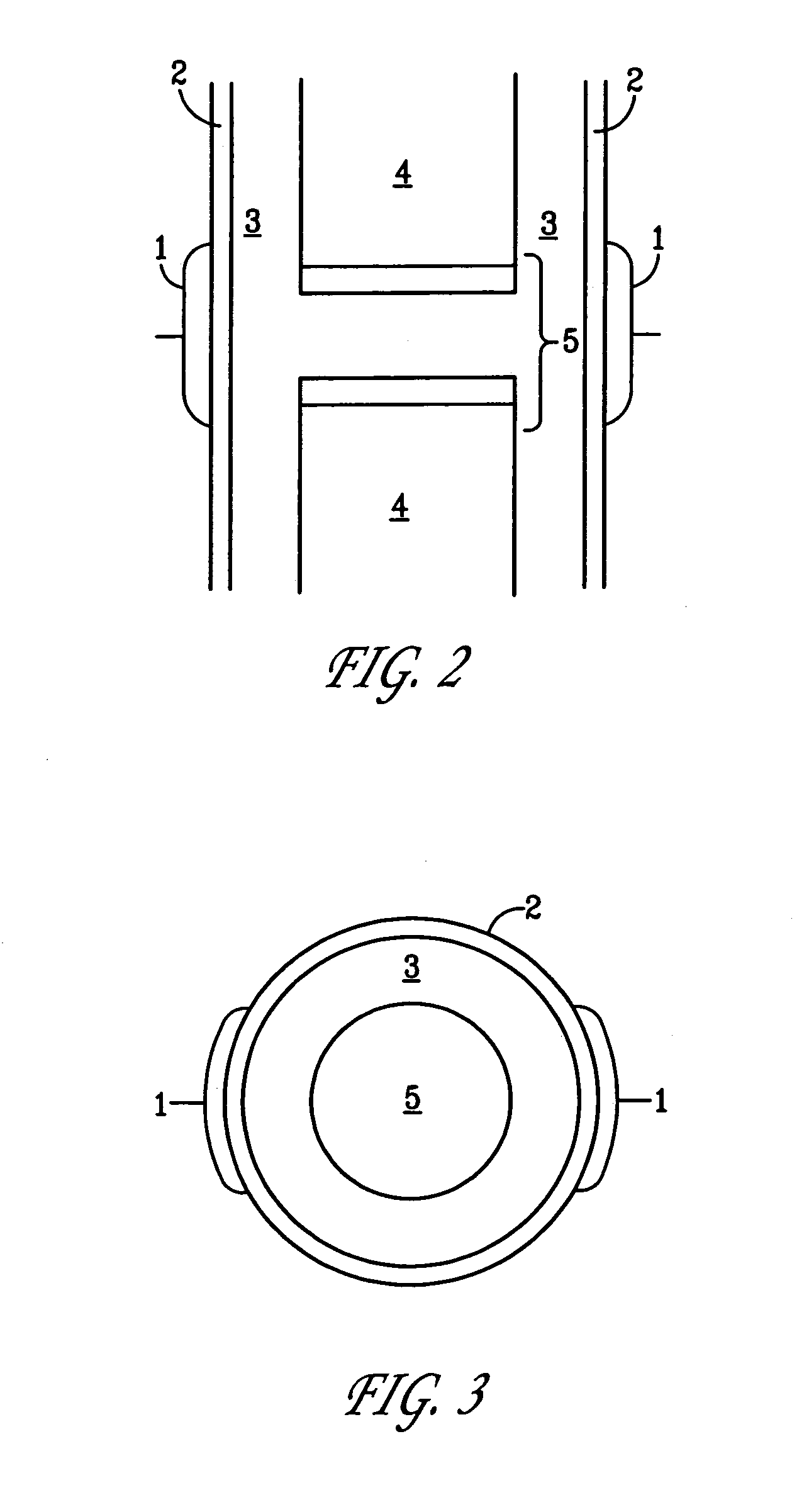
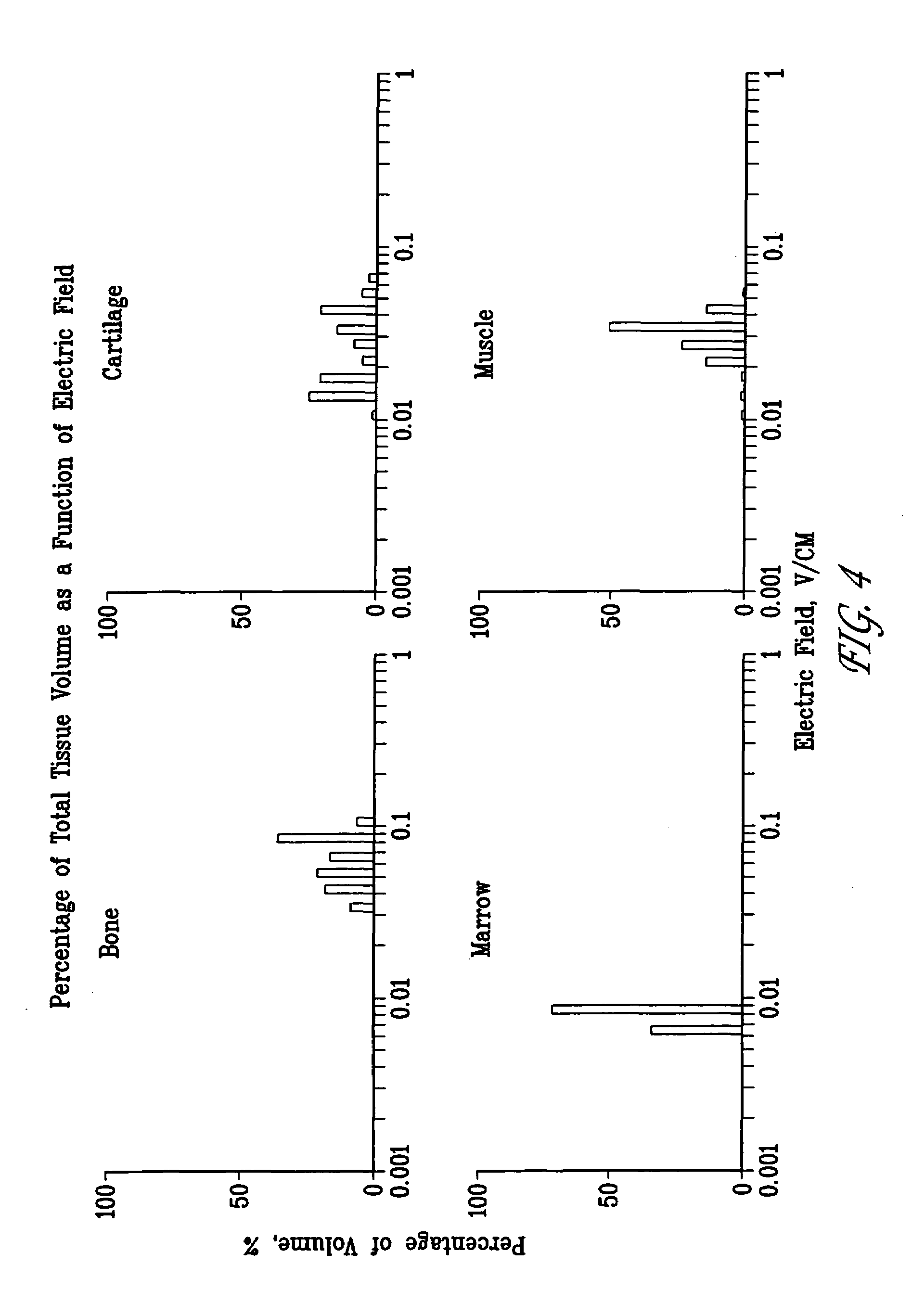
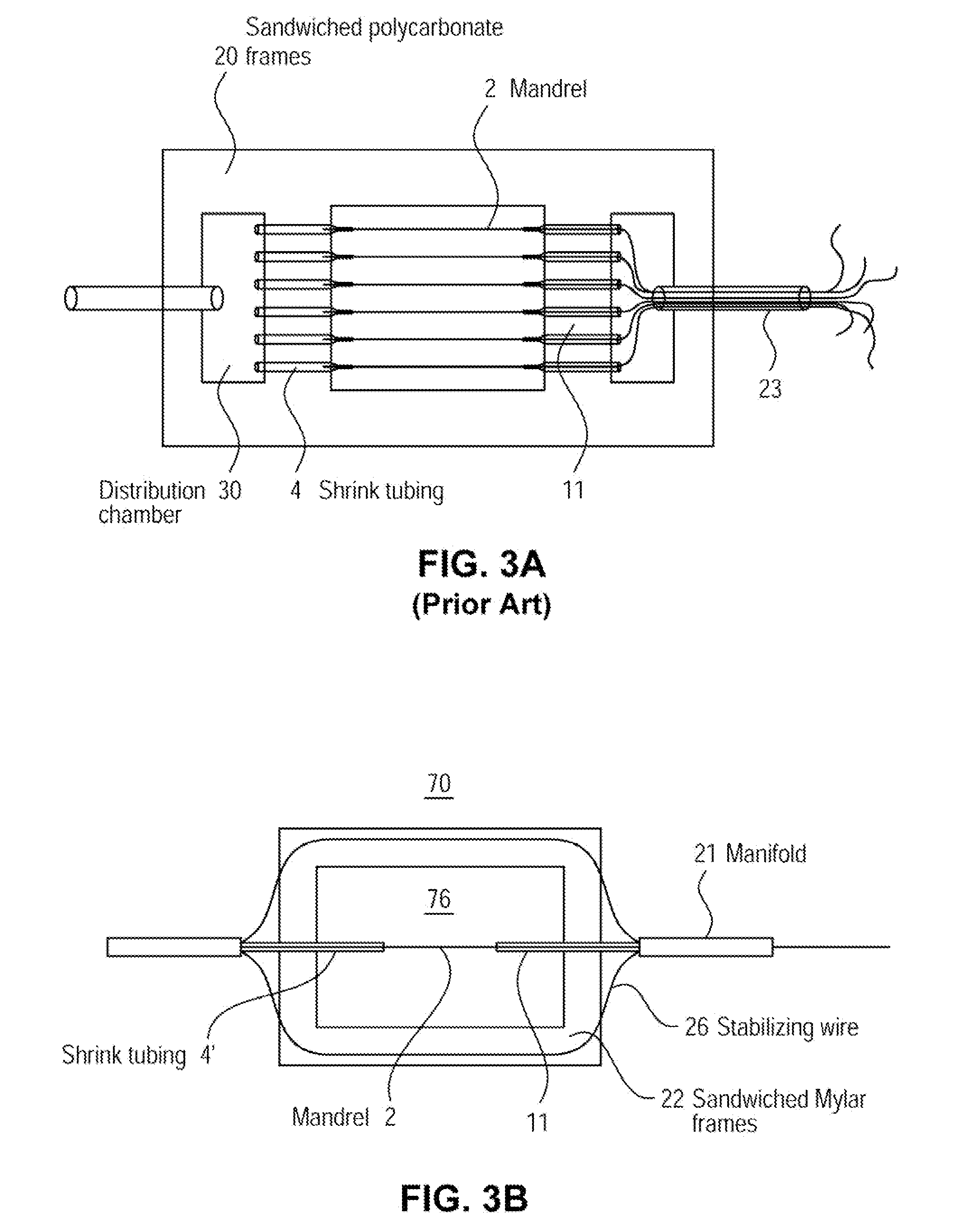
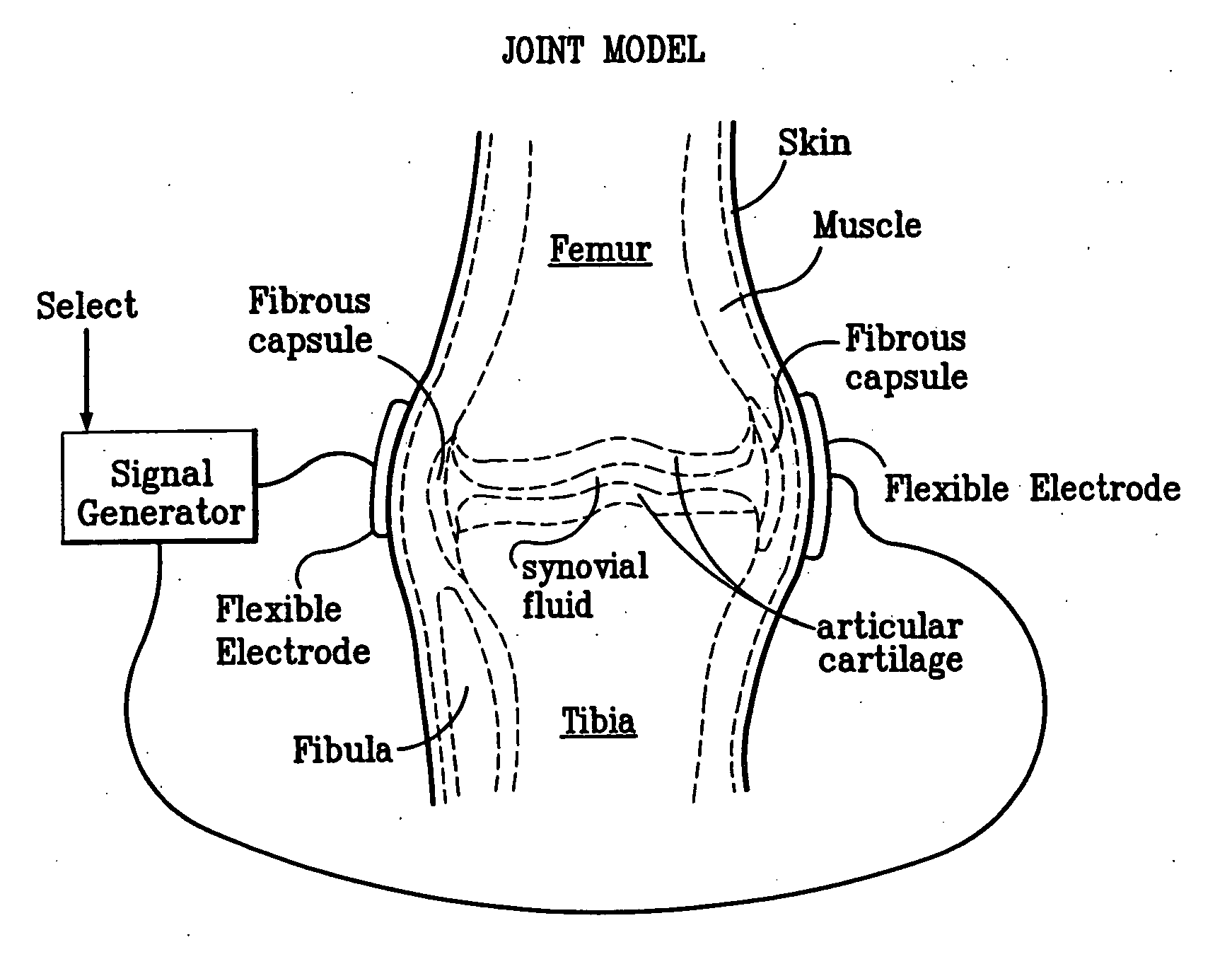
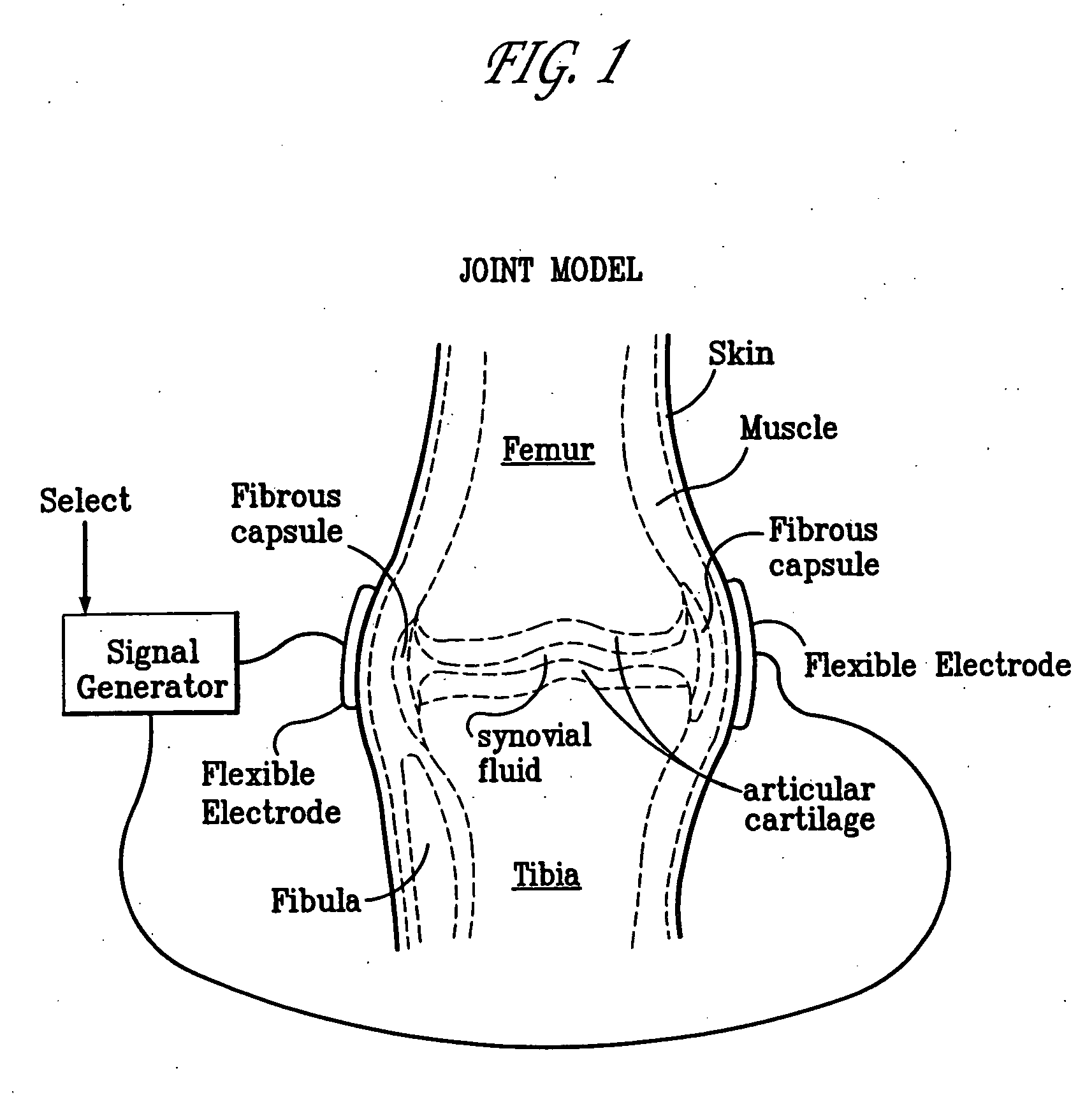
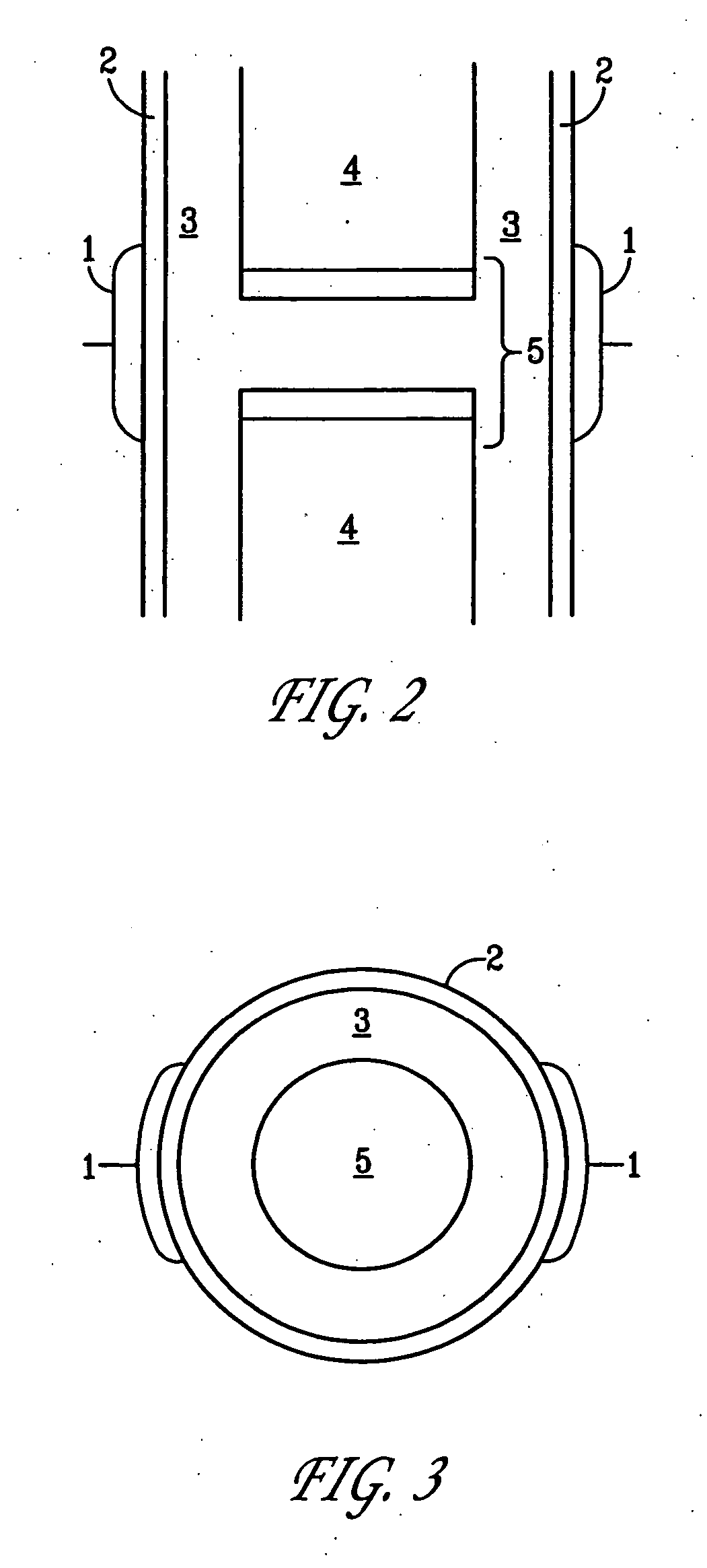
A method of determining the voltage and current output required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffolds, growth factors, etc.) for treating cartilage defects in the human knee joint and a device for delivering such signals to a patient's knee. An analytical model of the human knee is developed whereby the total tissue volume in the human knee may be determined for comparison to the total tissue volume of the diseased tissue in the animal model using electric field and current density histograms. The voltage and current output used in the animal model is scaled based on the ratio of the total tissue volume of the diseased tissue of the human to the total tissue volume of the diseased tissue in the animal model and the resulting field is applied to the diseased tissue of the human using at least two electrodes applied to the knee or a coil or solenoid placed around the knee. The voltage of the signal applied to the electrodes, coil or solenoid is varied based on the size of the knee joint; larger knee joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Use of three-dimensional microfabricated tissue engineered systems for pharmacologic applications
ActiveUS20060019326A1Additive manufacturing apparatusMicrobiological testing/measurementExperimental drugSide effect
The present invention generally relates to a combination of the fields of tissue engineering, drug discovery and drug development. It more specifically provides new methods and materials for testing the efficacy and safety of experimental drugs, defining the metabolic pathways of experimental drugs and characterizing the properties (e.g., side effects, new uses) of existing drugs. Preferably, evaluation is carried out in three-dimensional tissue-engineered systems, wherein drug toxicity, metabolism, interaction and / or efficacy can be determined.
Owner:CHARLES STARK DRAPER LABORATORY +1
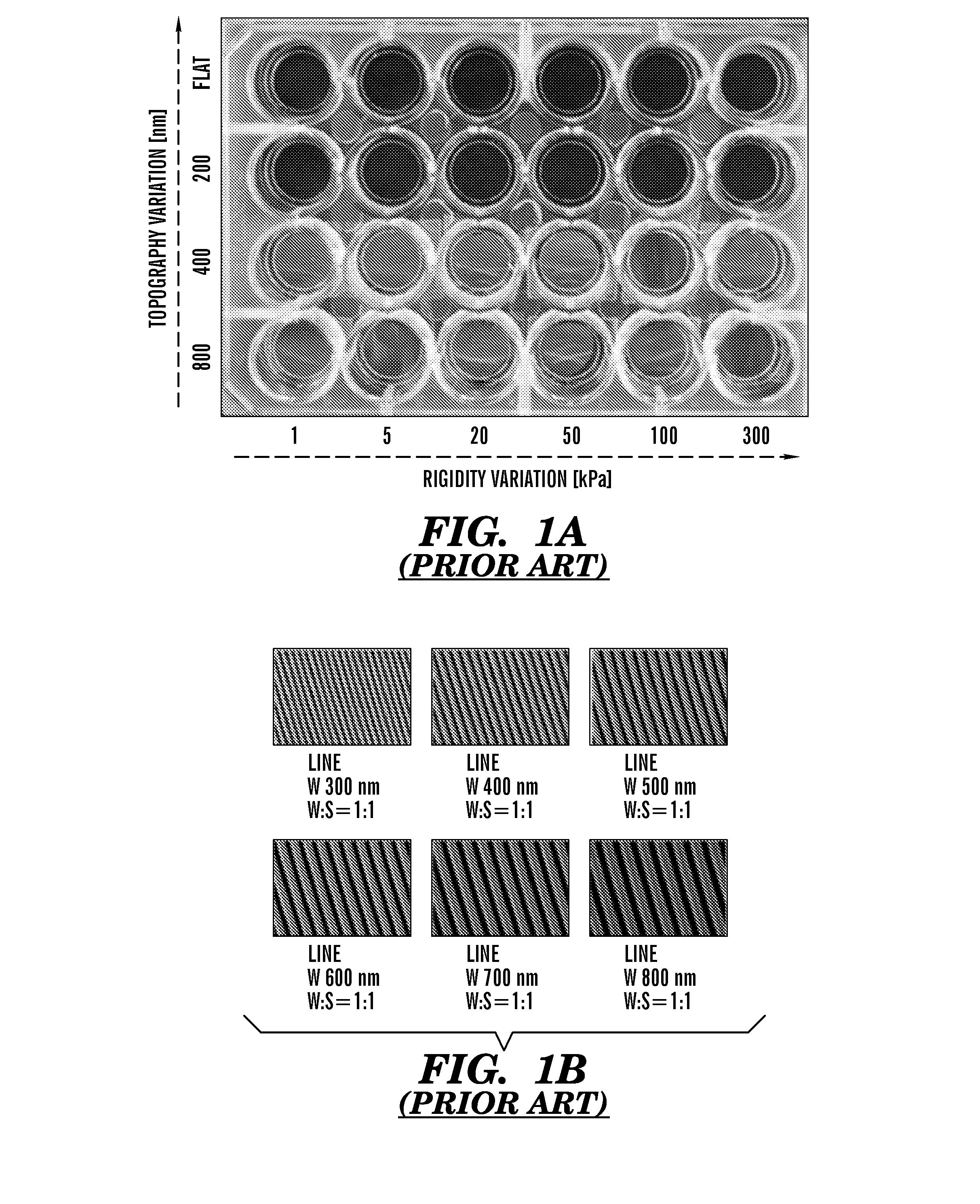
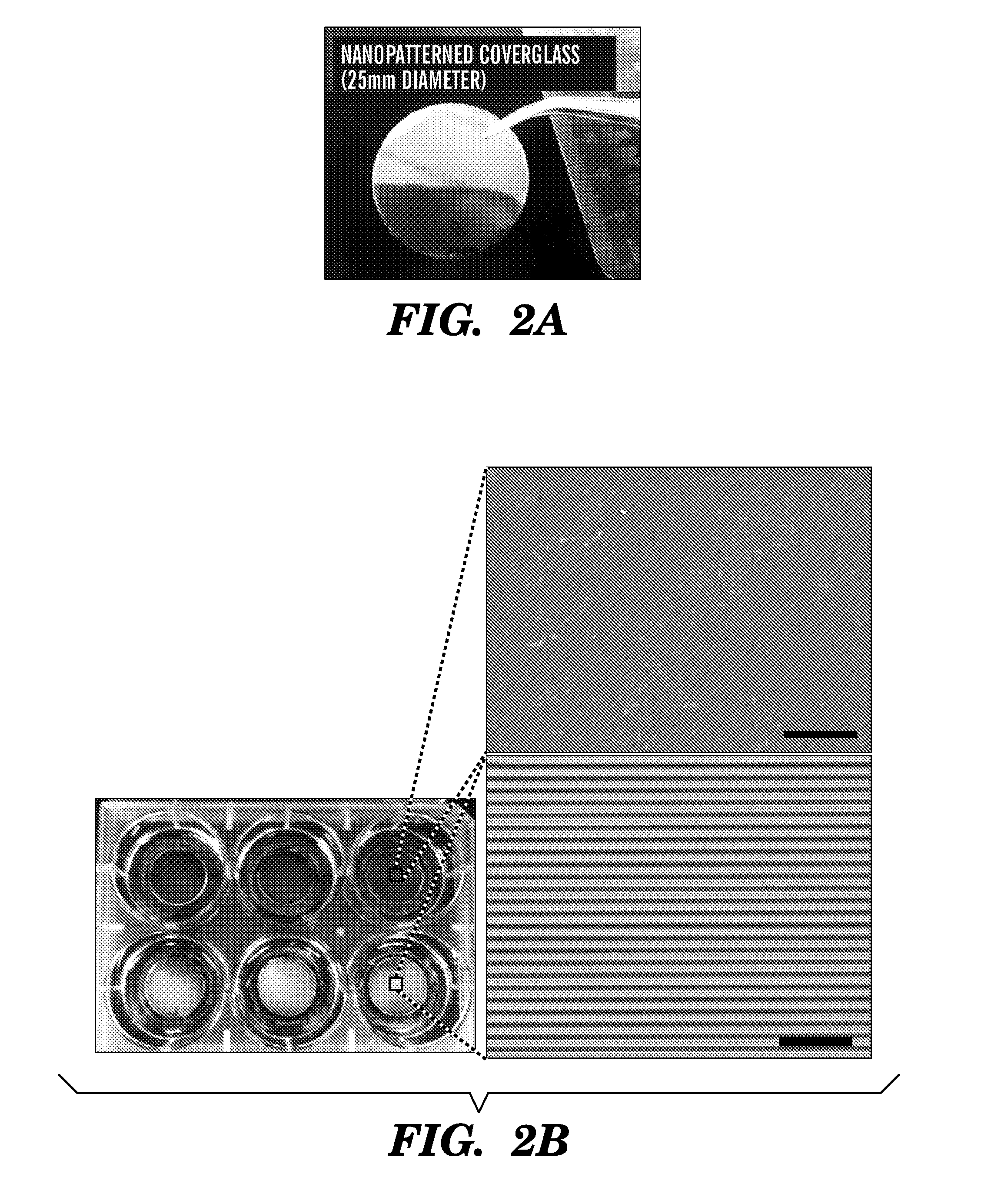
Nanotopographic Compositions and Methods for Cellular Organization in Tissue Engineered Structures
ActiveUS20080026464A1Additive manufacturing apparatusPharmaceutical delivery mechanismCell typeNanotopography
The present invention relates to tissue engineered compositions and methods comprising nanotopographic surface topography (“nanotopography”) for use in modulating the organization and / or function of multiple cell types.
Owner:THE GENERAL HOSPITAL CORP +1
Tissue engineered blood vessels
The invention is directed to methods for preparing artificial blood vessels by preconditioning a matrix seeded with endothelial cells to fluid flow conditions that mimic blood flow.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Automated tissue engineering system
ActiveUS20060141623A1Safe preparationImproving patient recoveryBioreactor/fermenter combinationsBiological substance pretreatmentsComputer moduleCellular functions
The invention provides systems, modules, bioreactor and methods for the automated culture, proliferation, differentiation, production and maintenance of tissue engineered products. In one aspect is an automated tissue engineering system comprising a housing, at least one bioreactor supported by the housing, the bioreactor facilitating physiological cellular functions and / or the generation of one or more tissue constructs from cell and / or tissue sources. A fluid containment system is supported by the housing and is in fluid communication with the bioreactor. One or more sensors are associated with one or more of the housing, bioreactor or fluid containment system for monitoring parameters related to the physiological cellular functions and / or generation of tissue constructs; and a microprocessor linked to one or more of the sensors. The systems, methods and products of the invention find use in various clinical and laboratory settings.
Owner:OCTANE BIOTECH +1
Three-dimensional fiber-based aerogel tissue engineering scaffold and preparation method thereof
The invention relates to a preparation method of a three-dimensional fiber-based aerogel tissue engineering scaffold and a product thereof. The preparation method comprises the following steps of: firstly dispersing fibers in solvents to form turbid liquid; secondly curing the turbid liquid to form cured pieces; thirdly removing cured solvents to form non-crosslinked fiber-based aerogel; finally carrying out crosslinking stabilization treatment and then carrying out sterilization treatment, thus obtaining the three-dimensional fiber-based aerogel tissue engineering scaffold. The product is a three-dimensional network-shaped material formed through mutual penetration and stagger of fibers. The fiber crossing points are effectively interconnected through non-hydrogen-bond bonding. The three-dimensional fiber-based aerogel tissue engineering scaffold has volume density of 0.1-500mg / cm<3>, average pore size of 0.01-2000mu m and specific surface area of 0.2-2000m<2> / g. The preparation method and the product have the advantages that the preparation process is simple; the raw material limitations are less; the aerogel tissue engineering scaffold product has good flexibility, connectivity and tissue growing environment and has broad application prospects in the tissue engineering field.
Owner:DONGHUA UNIV
Bioreactor and methods for tissue growth and conditioning
InactiveUS20050009179A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiomechanicsBiochemistry
A bioreactor and methods of using same for making tissue constructs and for conditioning tissue-engineered constructs and harvested tissues such as cryopreserved tissues. The bioreactor allows for static and dynamic culture / conditioning. The bioreactor is dual chambered (one chamber above and one below the cells or construct) to allow for application of biochemical and / or biomechanical stimuli to each side of the cells / construct.
Owner:GEORGIA TECH RES CORP
Method for creating perfusable microvessel systems
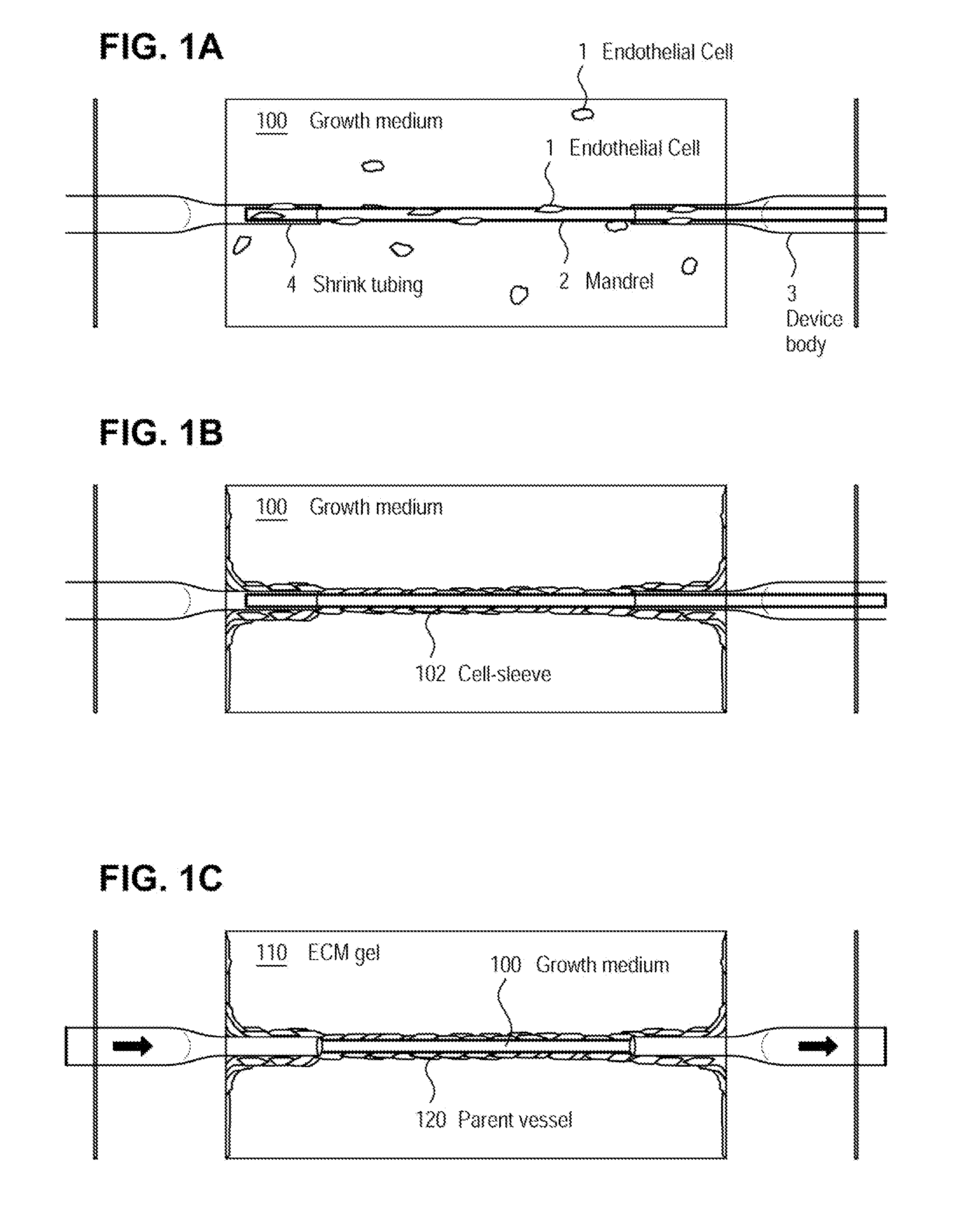
ActiveUS20070224677A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCell-Extracellular MatrixECM Protein
Microvessel networks are produced in vitro from tissue-engineered parent vessels sprouting into a supporting matrix, as for example gels, of extracellular matrix proteins. The microvessel systems are integrated into devices that allow for controlled perfusion with fluids. The vessels may include cells from one cell type, for example, endothelial cells, or from combinations of two or more cell types.
Owner:NORTIS
Method and device for treating osteoarthritis, cartilage disease, defects and injuries in the human knee
InactiveUS20060190043A1ElectrotherapyMagnetotherapy using coils/electromagnetsHuman useArticular cartilage
A method of determining the voltage and current output required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffolds, growth factors, etc.) for treating cartilage defects in the human knee joint and a device for delivering such signals to a patient's knee. An analytical model of the human knee is developed whereby the total tissue volume in the human knee may be determined for comparison to the total tissue volume of the diseased tissue in the animal model using electric field and current density histograms. The voltage and current output used in the animal model is scaled based on the ratio of the total tissue volume of the diseased tissue of the human to the total tissue volume of the diseased tissue in the animal model and the resulting field is applied to the diseased tissue of the human using at least two electrodes applied to the knee or a coil or solenoid placed around the knee. The voltage of the signal applied to the electrodes, coil or solenoid is varied based on the size of the knee joint; larger knee joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for making a porous calcium phosphate article
InactiveUS20050184417A1Improves bioresorbabilityPretreated surfacesCeramic shaping apparatusCalcium biphosphateCompressive strength
The present invention discloses a method for making a porous calcium phosphate article including i) preparing a shaped article from a paste containing a calcium phosphate cement, a pore-forming powder and a setting liquid; ii) immersing the shaped article in an immersing liquid for a period of time so that the pore-forming powder is dissolved in the immersing liquid, creating pores in said shaped article; and iii) removing the resulting porous shaped article from the immersing liquid, wherein the resulting porous shaped article has an improved compressive strength. The porous shaped calcium phosphate article of the present invention may be used as a tissue-engineered scaffold, medical implant or a reinforcing constituent of a composite.
Owner:CALCITEC
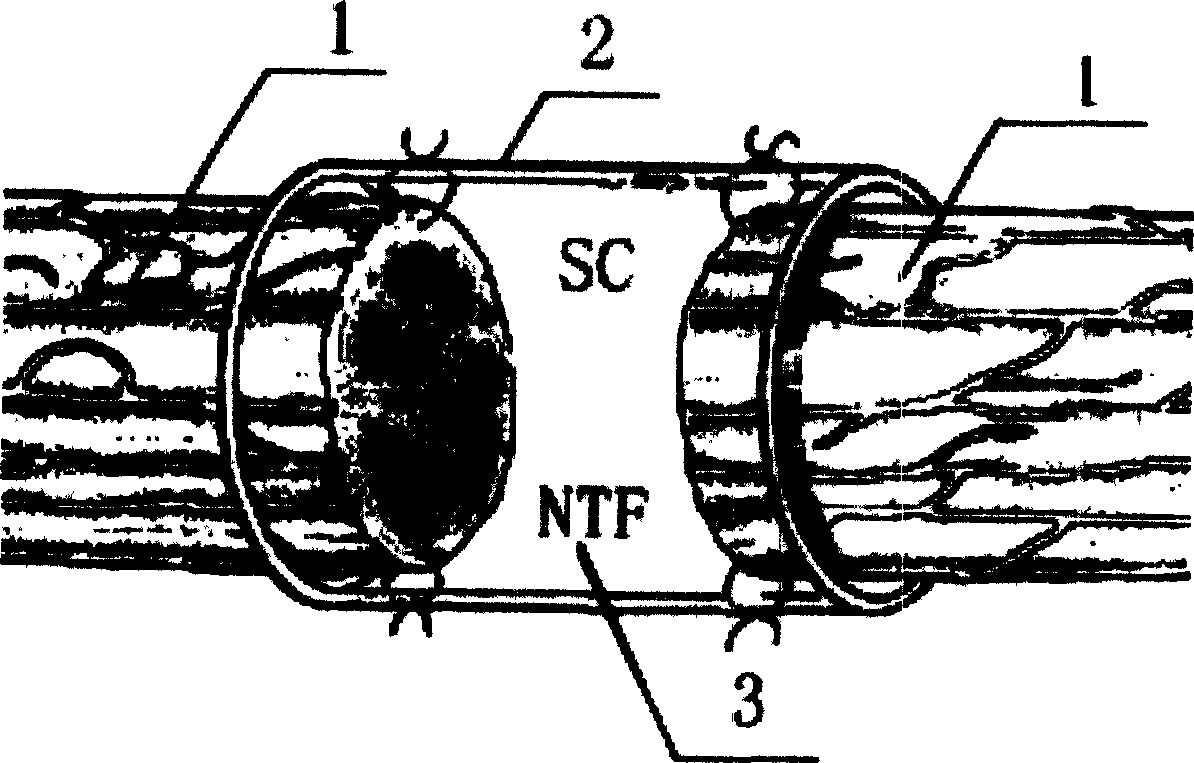
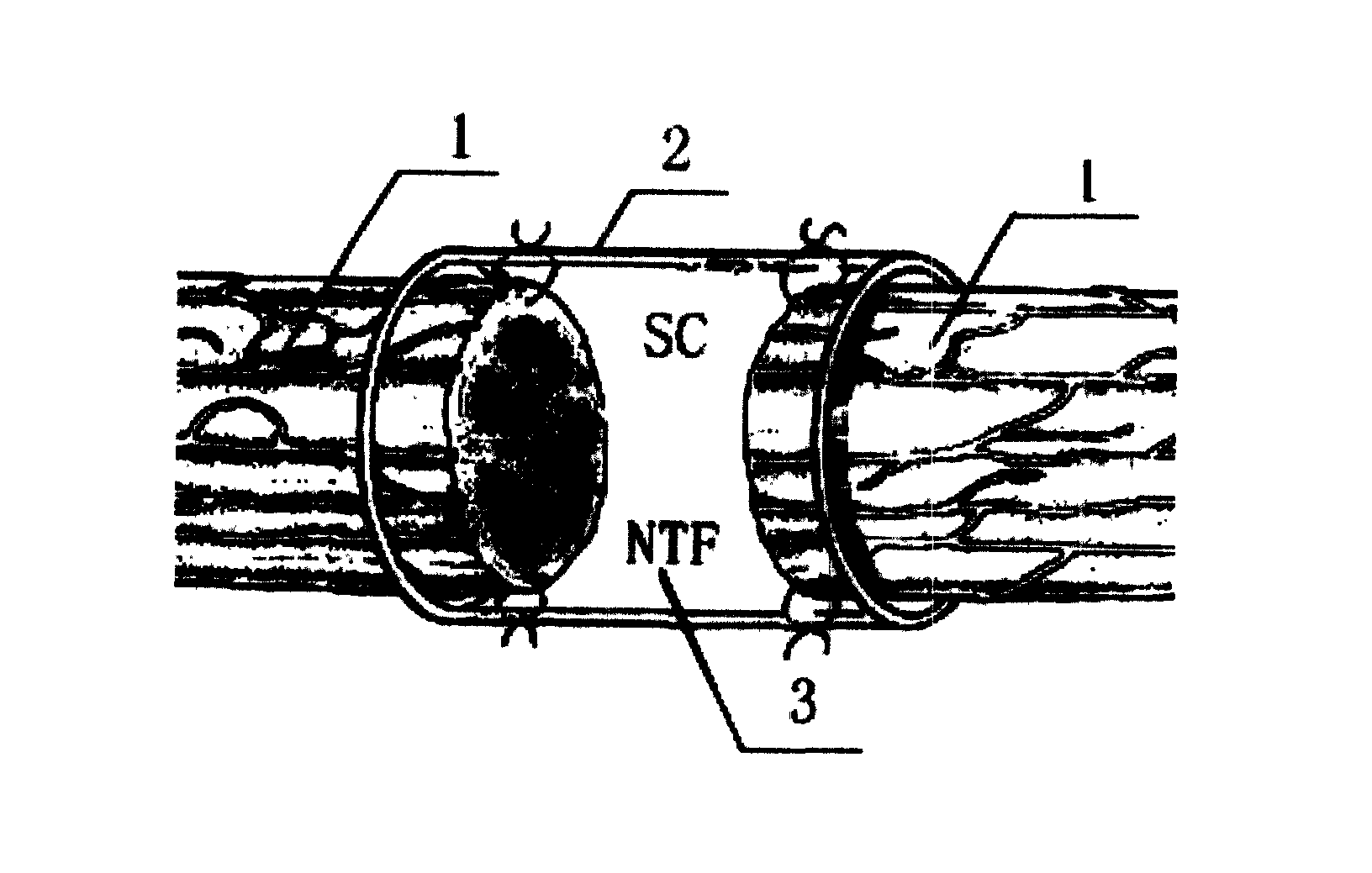
Tissue engineering peripheral nerve used for repairing peripheral nerve defect and its preparation method
A tissue-engineered peripheral nerve for reparing the demaged peripheral nerve is composed of neural canal and neuroglia cells or stem cells, and is prepared through preparing neural canal, preparing neurotrophic factor release controllable microspheres, and configuring the tissue-engineered peripheral nerve.
Owner:STOMATOLOGICAL HOSPITAL NO 4 ARMY MEDICAL COLLEGE PLA
Automated tissue engineering system
ActiveUS8492140B2Minimize risk of contaminationImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsTissue constructCellular functions
The invention provides systems, modules, bioreactor and methods for the automated culture, proliferation, differentiation, production and maintenance of tissue engineered products. In one aspect is an automated tissue engineering system comprising a housing, at least one bioreactor supported by the housing, the bioreactor facilitating physiological cellular functions and / or the generation of one or more tissue constructs from cell and / or tissue sources. A fluid containment system is supported by the housing and is in fluid communication with the bioreactor. One or more sensors are associated with one or more of the housing, bioreactor or fluid containment system for monitoring parameters related to the physiological cellular functions and / or generation of tissue constructs; and a microprocessor linked to one or more of the sensors. The systems, methods and products of the invention find use in various clinical and laboratory settings.
Owner:OCTANE BIOTECH +1
Method for making a porous calcium phosphate article
InactiveUS20050186354A1Improves bioresorbabilityBiocideInorganic phosphorous active ingredientsCalcium biphosphateCompressive strength
The present invention discloses a method for making a porous calcium phosphate article including i) preparing a shaped article from a paste containing a calcium phosphate cement, a pore-forming powder and a setting liquid; ii) immersing the shaped article in an immersing liquid for a period of time so that the pore-forming powder is dissolved in the immersing liquid, creating pores in said shaped article; and iii) removing the resulting porous shaped article from the immersing liquid, wherein the resulting porous shaped article has an improved compressive strength. The porous shaped calcium phosphate article of the present invention may be used as a tissue-engineered scaffold, medical implant or a reinforcing constituent of a composite.
Owner:CALCITEC
Production of tissue engineered heart valves
ActiveUS20060253192A1Avoid it happening againMore of their natural flexibilityHeart valvesNanoinformaticsProgenitorSmooth muscle
The invention is directed to methods for preparing artificial heart valves by preconditioning a matrix seeded with endothelial cells and smooth muscle cells differentiated from isolated progenitor cells. These cell seeded matrices are exposed to fluid conditions that mimic blood flow through the heart to produce tissue engineered heart valves that are analogous to native heart valves.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Tissue engineered biomimetic hair follicle graft
InactiveUS20050214344A1High degreeReduces and inhibits and cure lossBiocideAnimal repellantsBioabsorbable scaffoldAllogeneic cell
The present invention relates to an improved scaffold which is constructed to mimic the architecture of the native hair follicle. The present invention also relates to the use of specific compositions and methods of manufacture to produce scaffolds that combine biocompatibility with the desired rates of bioabsorption. In another embodiment, the present invention relates to a process for manufacturing a biomimetic hair follicle graft and a method for seeding the graft with cells and implanting the graft into the skin where the growth of a new hair shaft is desired. A further embodiment of the present invention relates to a method for hair multiplication in which cells are multiplied in culture and aliquoted into a multitude of bioabsorbable scaffolds in combination with cultured keratinocytes or other allogenic cells.
Owner:ADERANS RES INST
Decellularisation of matrices
InactiveUS7354749B2Low toxicityDifferent temperatureBiocideHydrolasesProteinase activityAnionic detergent
A method of preparing matrices or tissue engineered biomaterials for implantation, and in particular to a method of improving decellularisation of matrices or tissue engineered biomaterials prior to implantation. The method employs a single anionic detergent in combination with protease inhibitors.
Owner:TISSUE REGENIX +1
Method for making a porous calcium phosphate article
InactiveUS20050186353A1Improves bioresorbabilityPharmaceutical containersPretreated surfacesCalcium biphosphateCompressive strength
The present invention discloses a method for making a porous calcium phosphate article including i) preparing a shaped article from a paste containing a calcium phosphate cement, a pore-forming powder and a setting liquid; ii) immersing the shaped article in an immersing liquid for a period of time so that the pore-forming powder is dissolved in the immersing liquid, creating pores in said shaped article; and iii) removing the resulting porous shaped article from the immersing liquid, wherein the resulting porous shaped article has an improved compressive strength. The porous shaped calcium phosphate article of the present invention may be used as a tissue-engineered scaffold, medical implant or a reinforcing constituent of a composite.
Owner:CALCITEC
Novel tissue engineered scaffolds derived from copper capillary alginate gels
InactiveUS20050196423A1Excellent property conducive to growthPromote regenerationBiocideElectrotherapyIn vivoCopper
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for making a porous calcium phosphate article
InactiveUS20050186449A1Improves bioresorbabilityLayered productsPretreated surfacesCalcium biphosphateCompressive strength
The present invention discloses a method for making a porous calcium phosphate article including i) preparing a shaped article from a paste containing a calcium phosphate cement, a pore-forming powder and a setting liquid; ii) immersing the shaped article in an immersing liquid for a period of time so that the pore-forming powder is dissolved in the immersing liquid, creating pores in said shaped article; and iii) removing the resulting porous shaped article from the immersing liquid, wherein the resulting porous shaped article has an improved compressive strength. The porous shaped calcium phosphate article of the present invention may be used as a tissue-engineered scaffold, medical implant or a reinforcing constituent of a composite.
Owner:CALCITEC
Acellular tissue matrix composite and preparation method thereof
ActiveCN105999410APromote healingImprove adhesionTissue regenerationProsthesisPorosityPolyelectrolyte
The invention discloses an acellular tissue matrix composite and a preparation method thereof. The composite is formed by smashing an acellular tissue matrix, adding water or digestive juice for digestion, evenly mixing the product with polyelectrolyte and conducting freezing and drying, wherein the acellular tissue matrix is obtained by conducting acellular implantation on animal tissue. The product is in a porous sponge shape, in-situ implantation induction host vascularization and cell proliferation and growth are achieved with the composite as the support, and therefore tissue healing is promoted, and the composite can be used for tissue repairing such as wound surface repairing, subcutaneous filling and tissue-engineered muscle establishing. By means of the composite, active growth factors are kept, the support with complicated shape and adjustable size can also be established, and the micro-porous size, porosity and degradation time can be adjustable.
Owner:广州昕生医学材料有限公司
Pre-fabricated tissue-engineered plug
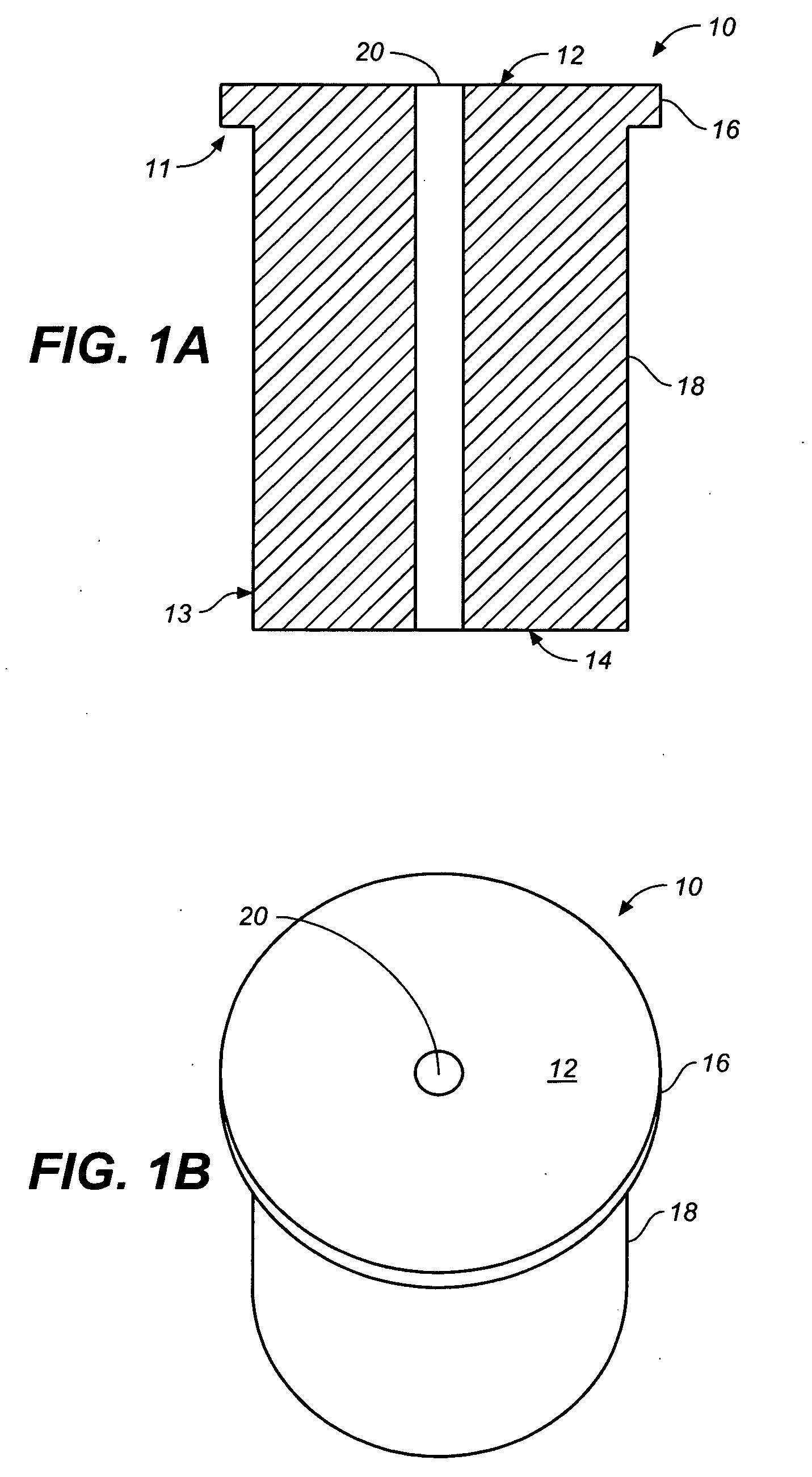
InactiveUS20050129726A1Promote growthFlat surfaceBioreactor/fermenter combinationsDental implantsDevice implantBone implant
Methods and apparatus for bone implants that allow for the directed application of an osteogenic compound. The implant is preferably constructed of a biodegradable polymer formed into a structure having micro-architectural features and includes features that allow for in-situ application of a liquid biodegradable polymer to securely attach the implant to the surrounding tissue. The implant is constructed with a nozzle connected to a fluid supply that can be injected through a central channel and one or more distribution channels. The implant is designed so as to provide structural support to the damaged area. The implant and the fluid supply are preferably biodegradable polymers that contain an osteogenic material.
Owner:RICE UNIV
Method for making a porous calcium phosphate article
InactiveUS20050184418A1Improves bioresorbabilityPretreated surfacesCeramic shaping apparatusCalcium biphosphateCompressive strength
The present invention discloses a method for making a porous calcium phosphate article including i) preparing a shaped article from a paste containing a calcium phosphate cement, a pore-forming powder and a setting liquid; ii) immersing the shaped article in an immersing liquid for a period of time so that the pore-forming powder is dissolved in the immersing liquid, creating pores in said shaped article; and iii) removing the resulting porous shaped article from the immersing liquid, wherein the resulting porous shaped article has an improved compressive strength. The porous shaped calcium phosphate article of the present invention may be used as a tissue-engineered scaffold, medical implant or a reinforcing constituent of a composite.
Owner:CALCITEC
Device and method for allograft and tissue engineered osteochondral graft surface matching, preparation, and implantation
ActiveUS20090209962A1Precise surface matchingEasy constructionBone implantOsteosynthesis devicesOsteochondral graftingSacroiliac joint
Owner:JAMALI AMIR
Systems and method for engineering muscle tissue
ActiveUS20150125952A1Accurately recapitulateHigh throughput formatGenetic material ingredientsDrug screeningMuscle tissueNanostructure
The present invention generally relates to the field of cell growth and tissue engineering, in particular, tissue engineered compositions comprising a nanotextured substrate which is structurally configured for growth of cells in an anatomically correct adult phenotype in vitro. In particular, described herein are nanotextured substrates which are structurally configured for the anisotropic organization, maturation, and growth of in vitro-differentiated muscle cells, such as cardiomyocytes, and methods for the production and use thereof in varying sizes, nanotextures and substrate rigidities. In vitro-differentiated cardiomyocytes grown on the nanotextured substrates described herein are better-differentiated and more closely mimic adult cardiac tissue than the same cells grown on a non-textured substrate of the same composition. The nanotextured substrate / cell constructs provide a platform for screening to predict the effect of test agents or drugs on, for example, human cardiac tissue, including patient-derived tissue, or for the identification of agents that effect various cardiac functional parameters.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
Tissue engineered blood vessels and apparatus for their manufacture
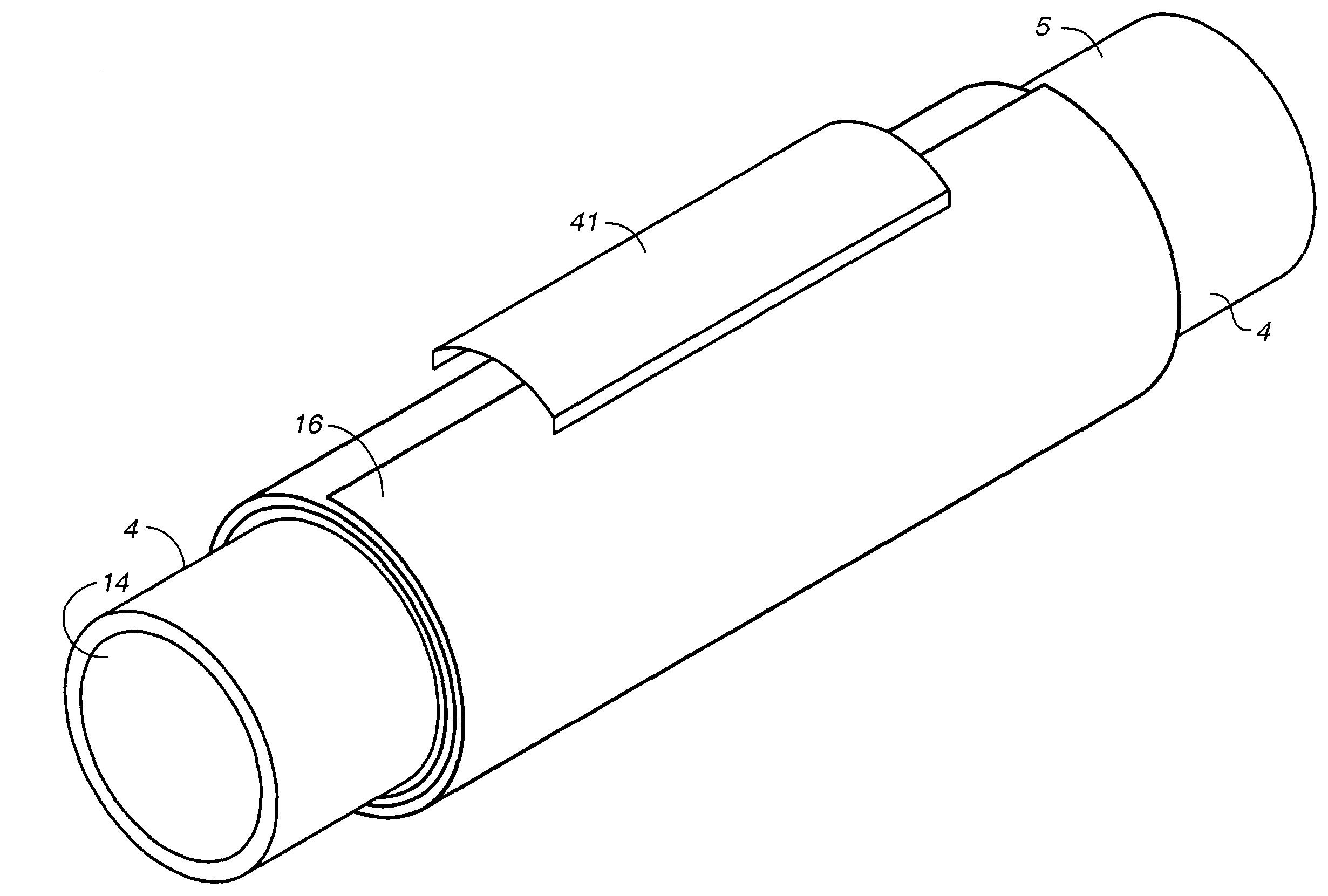
InactiveUS7112218B2Eliminate the problemBioreactor/fermenter combinationsBiological substance pretreatmentsBursting strengthFiber
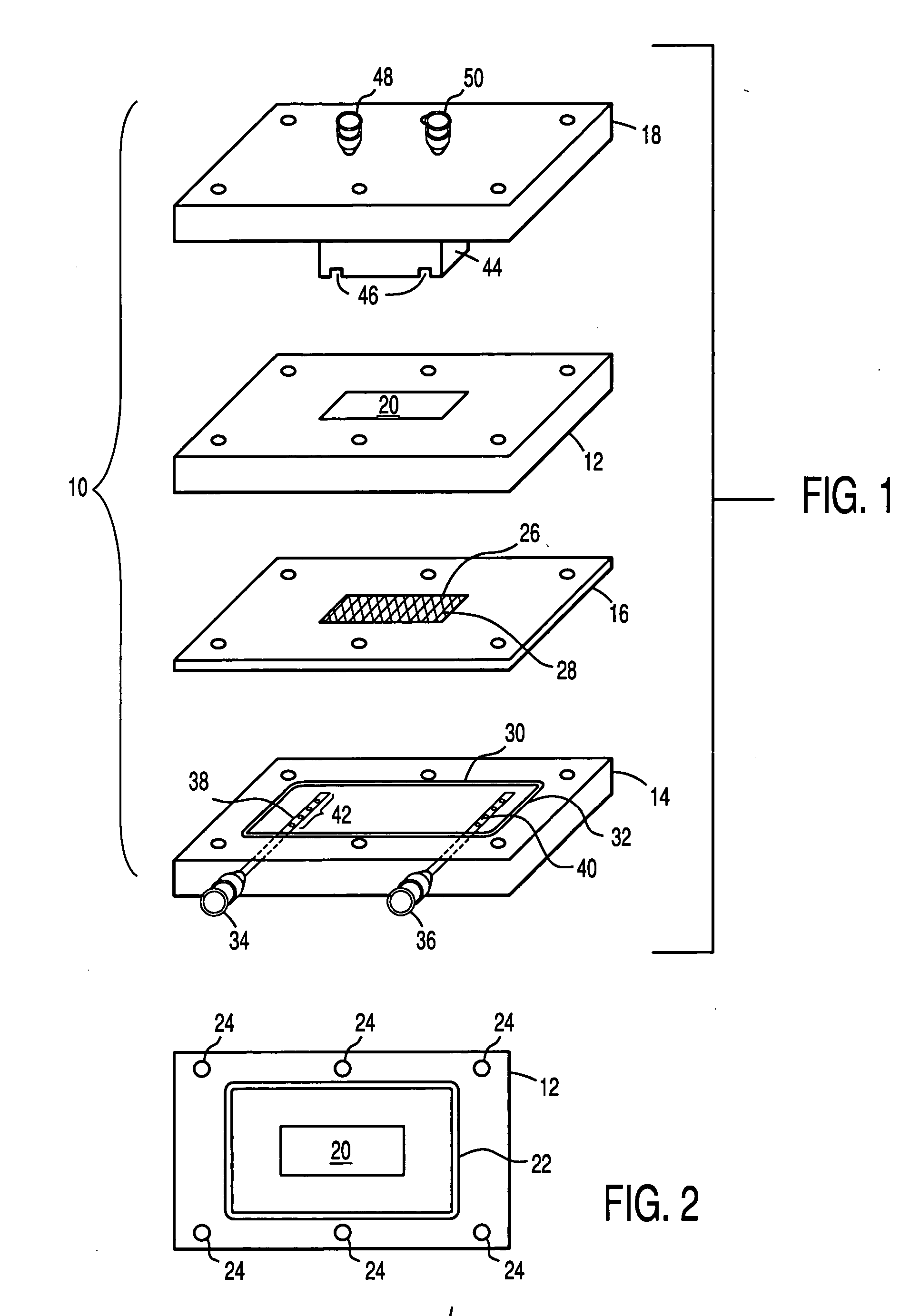
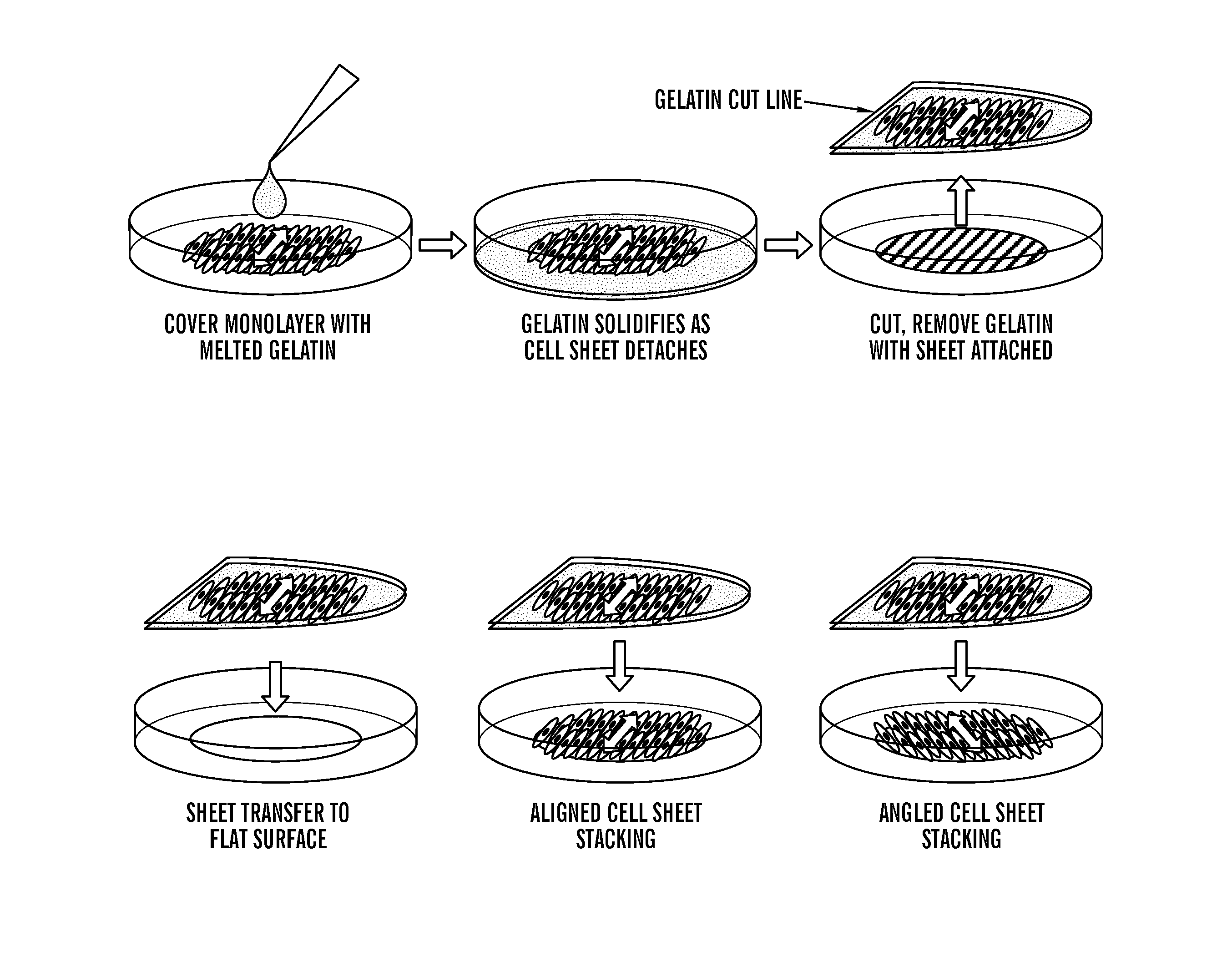
The invention is a tissue engineered blood vessel (TEBV) made from a cultured fibroblast sheet rolled into a multilayer vessel which has sufficient burst strength to withstand physiological blood pressure without the inclusion of smooth muscle cells or synthetic scaffolding. The TEBV is made in a bioreactor having an enclosed chamber, a sheet growth module, a rollable mandrel, and a clamp for holding the sheet to the mandrel for rolling.
Owner:NIGHTINGALE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com