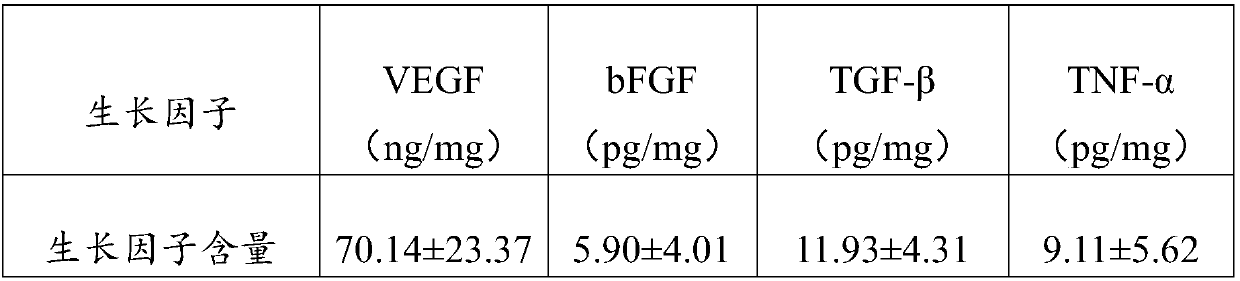
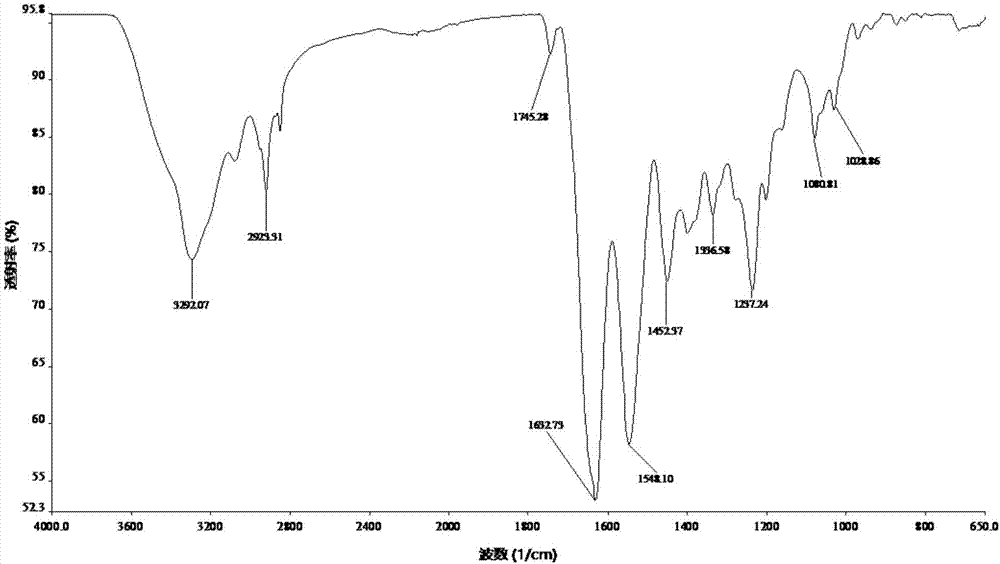
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73 results about "Small intestine submucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hybrid biologic-synthetic bioabsorbable scaffolds
ActiveUS8366787B2Increase surface areaGood mechanical integritySuture equipmentsBone implantBioabsorbable scaffoldCell-Extracellular Matrix
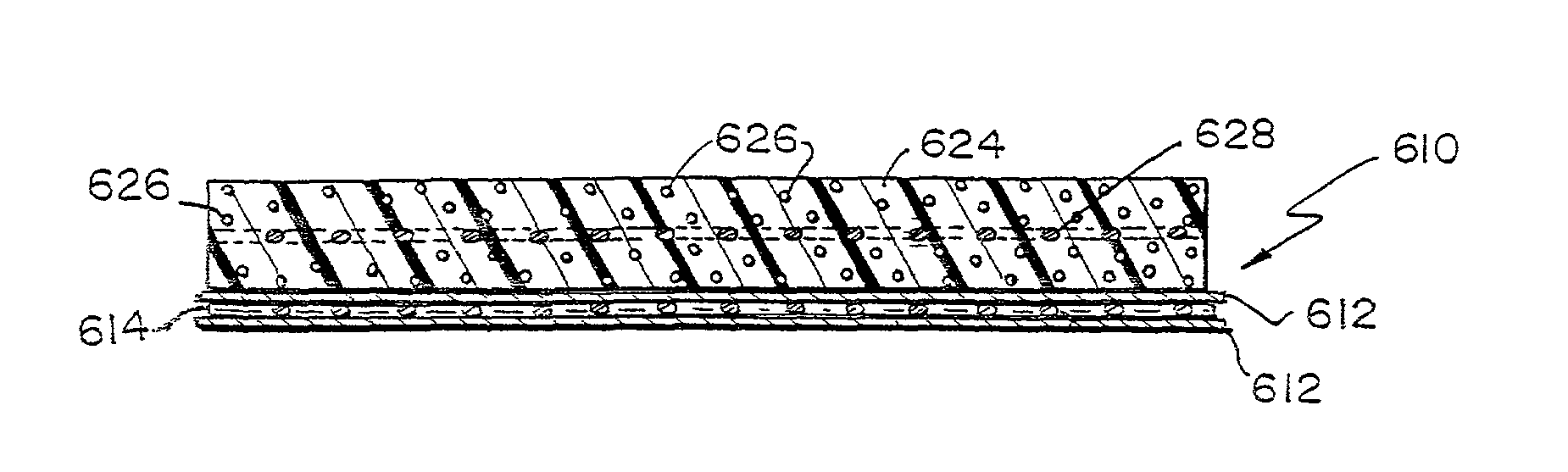
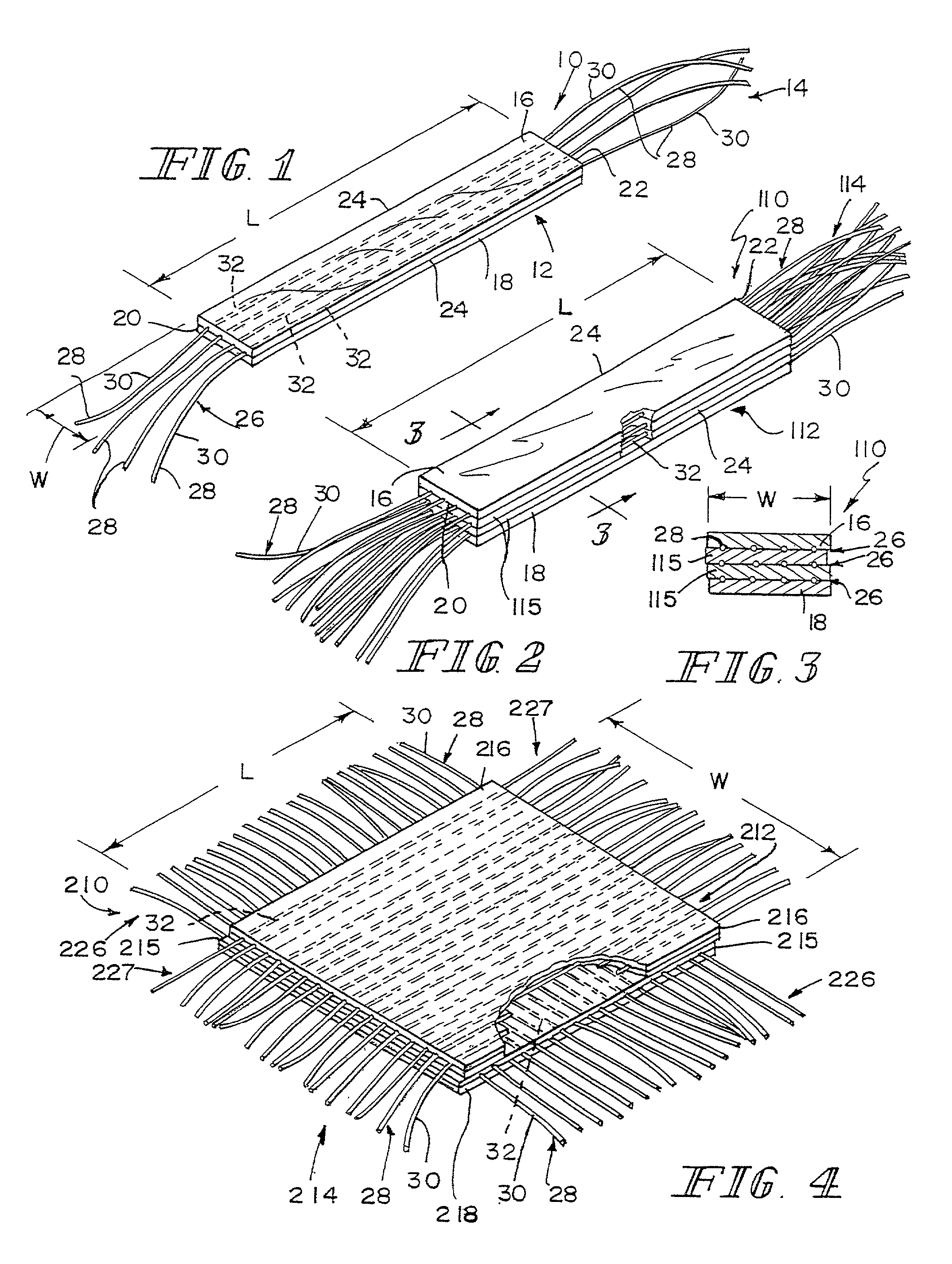
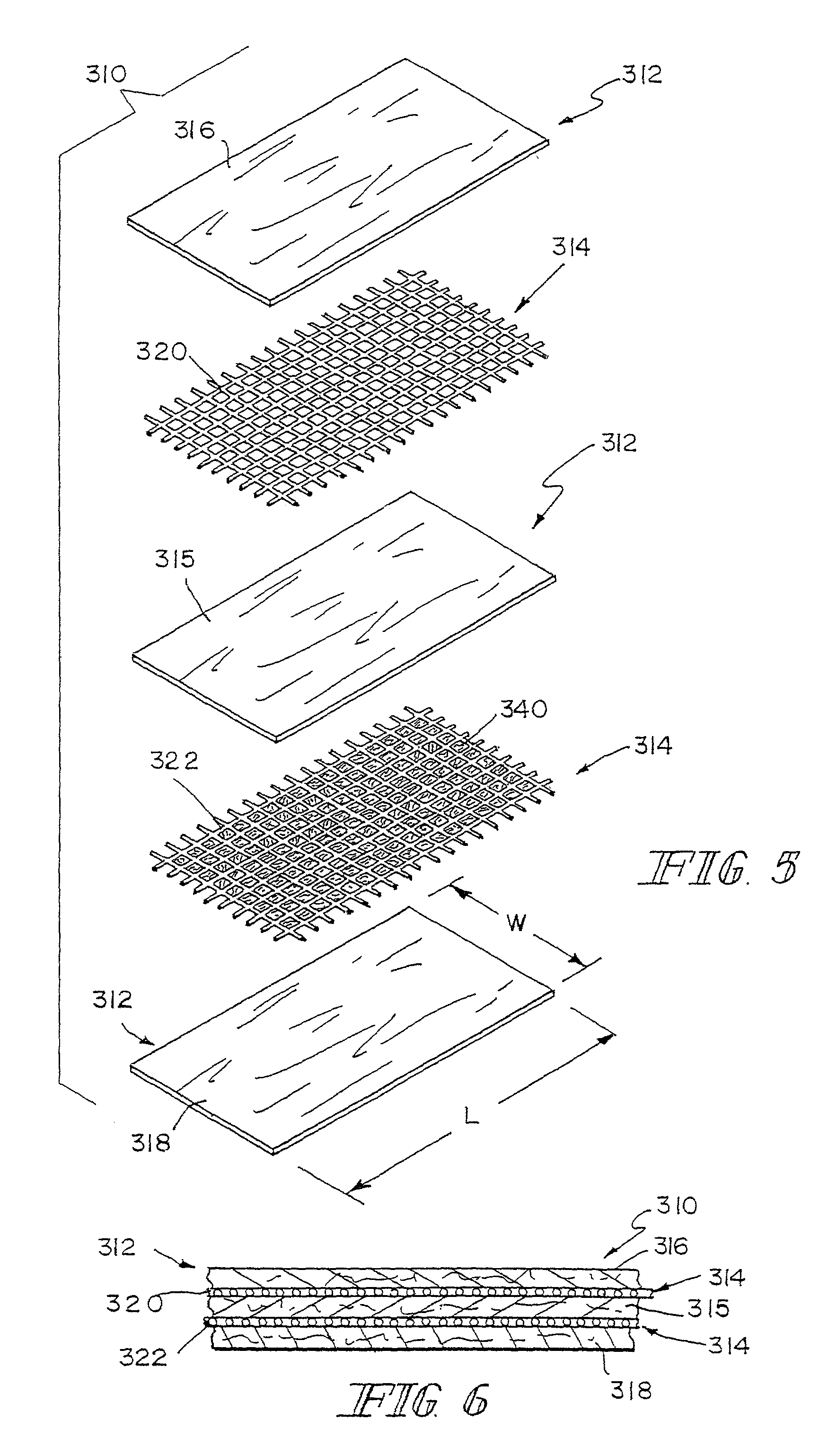
A bioprosthetic device is provided for soft tissue attachment, reinforcement, and or reconstruction. The device comprises a naturally occurring extracellular matrix portion and a three-dimensional synthetic portion. In illustrated embodiments, the naturally occurring extracellular matrix portion comprises layers of small intestine submucosa, and the three-dimensional synthetic portion comprises a foam or a three-dimensional mesh, textile, or felt.
Owner:DEPUY SYNTHES PROD INC
Methods and devices for intervertebral augmentation using injectable formulations and enclosures
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SPINE INC (US) +1
Devices for intervertebral augmentation and methods of controlling their delivery
InactiveUS20070150063A1Promote tissue growthHigher compressive modulusBone implantLigamentsFilling materialsSmall intestine
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SYNTHES PROD INC +1
Methods and systems for modifying vascular valves
Described are methods and systems for modifying vascular valves in order to reduce retrograde blood flow through the valves. Preferred methods include connecting vascular valve leaflets with at least one remodelable material, such that the valve leaflets become fused by the ingrowth of the patient's native tissue. Preferred remodelable materials include collagenous extracellular matrix material, such as small intestine submucosa.
Owner:COOK MEDICAL TECH LLC
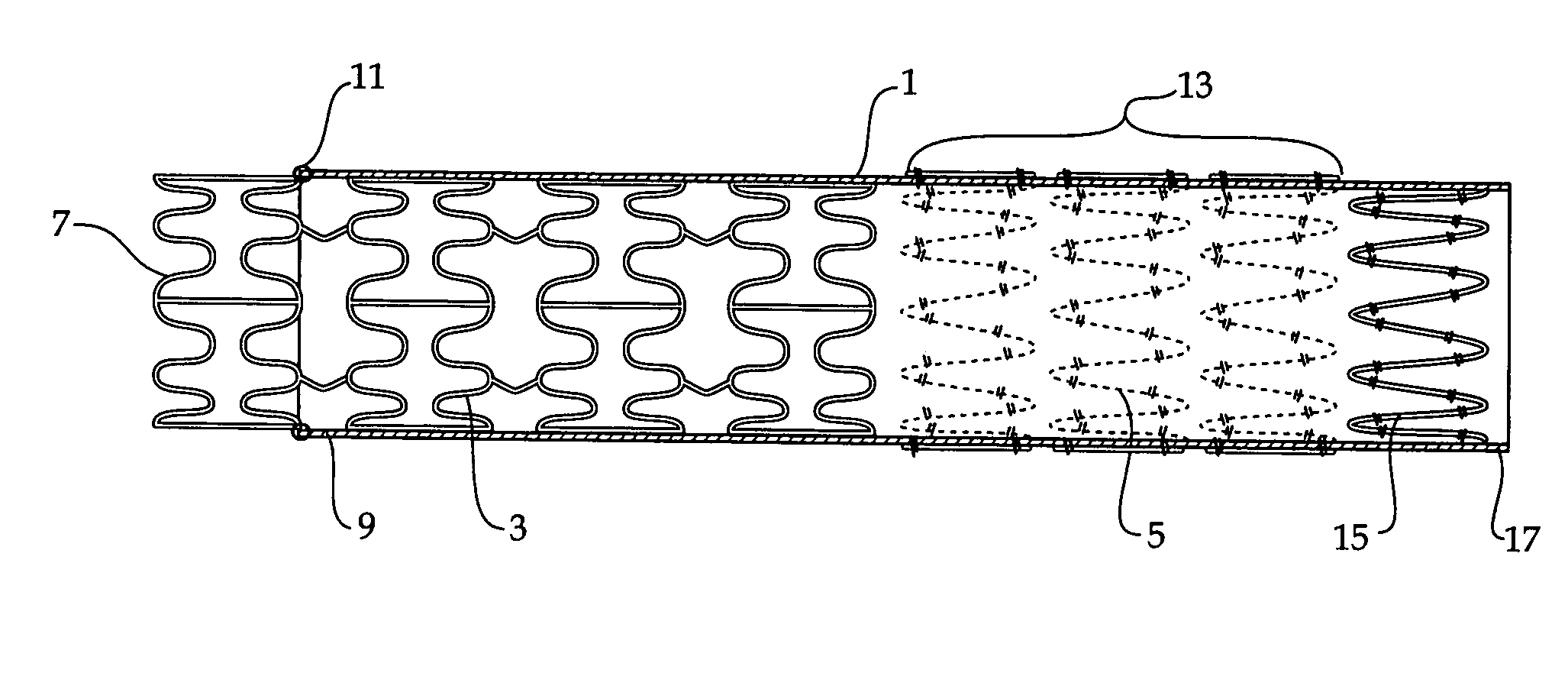
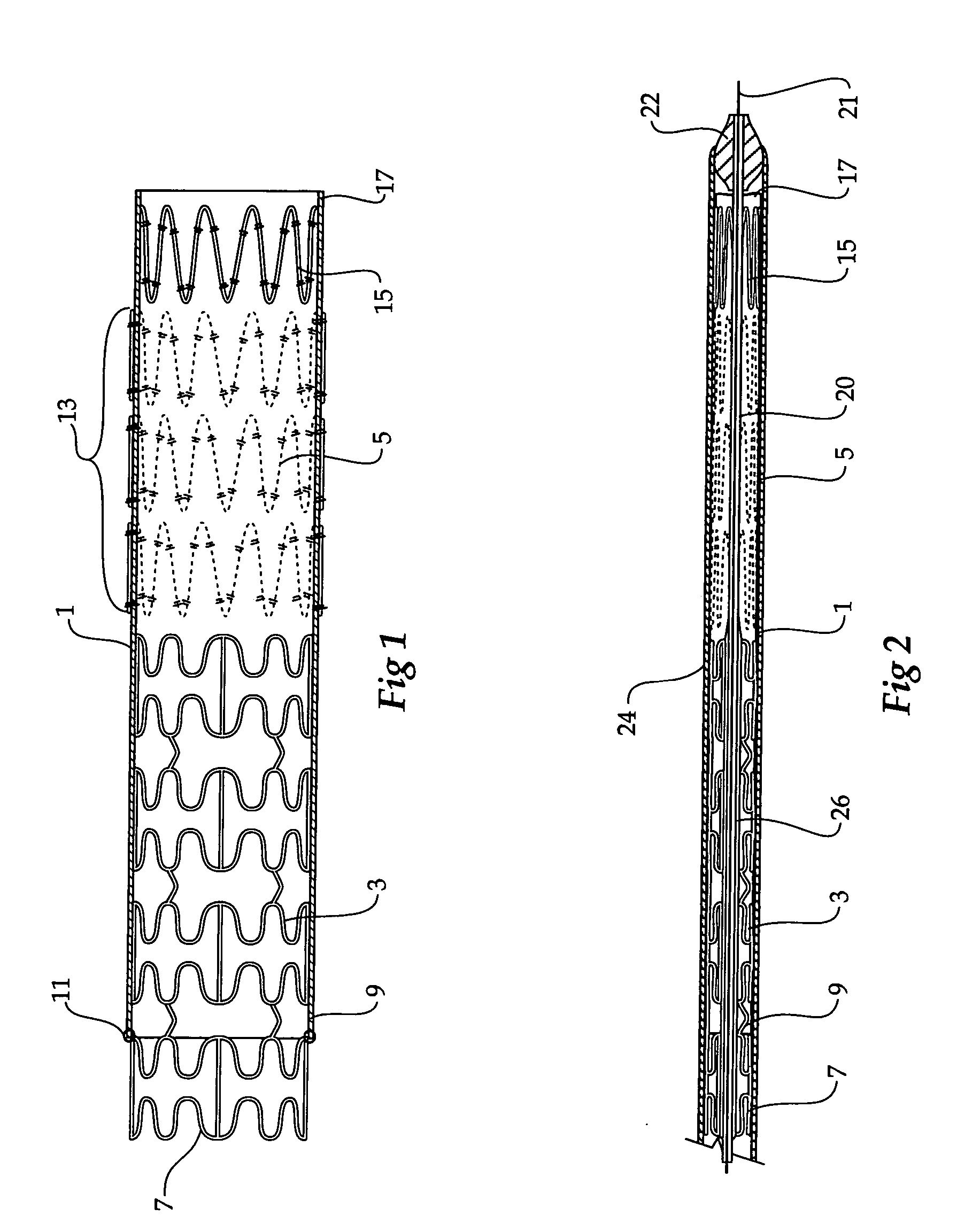
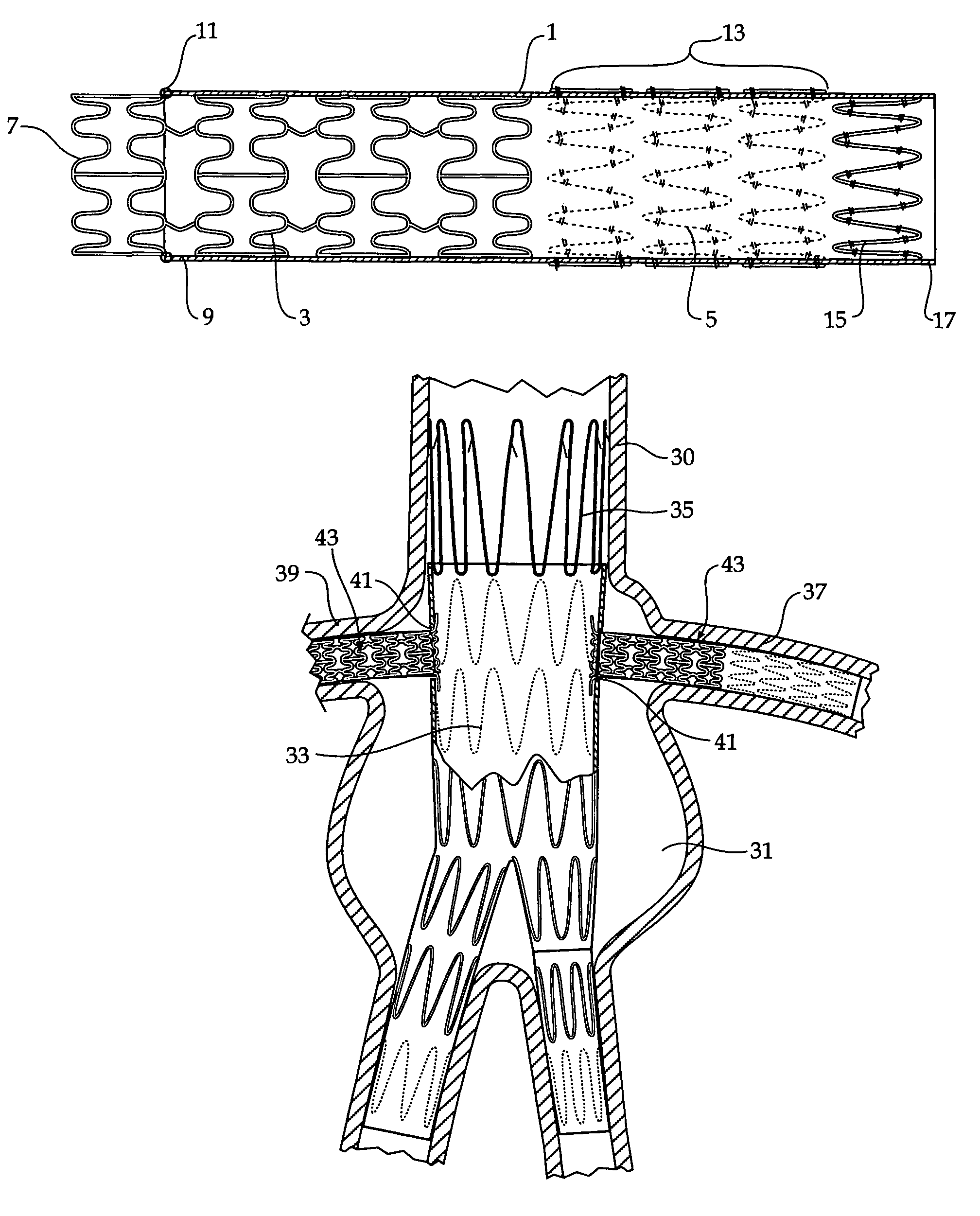
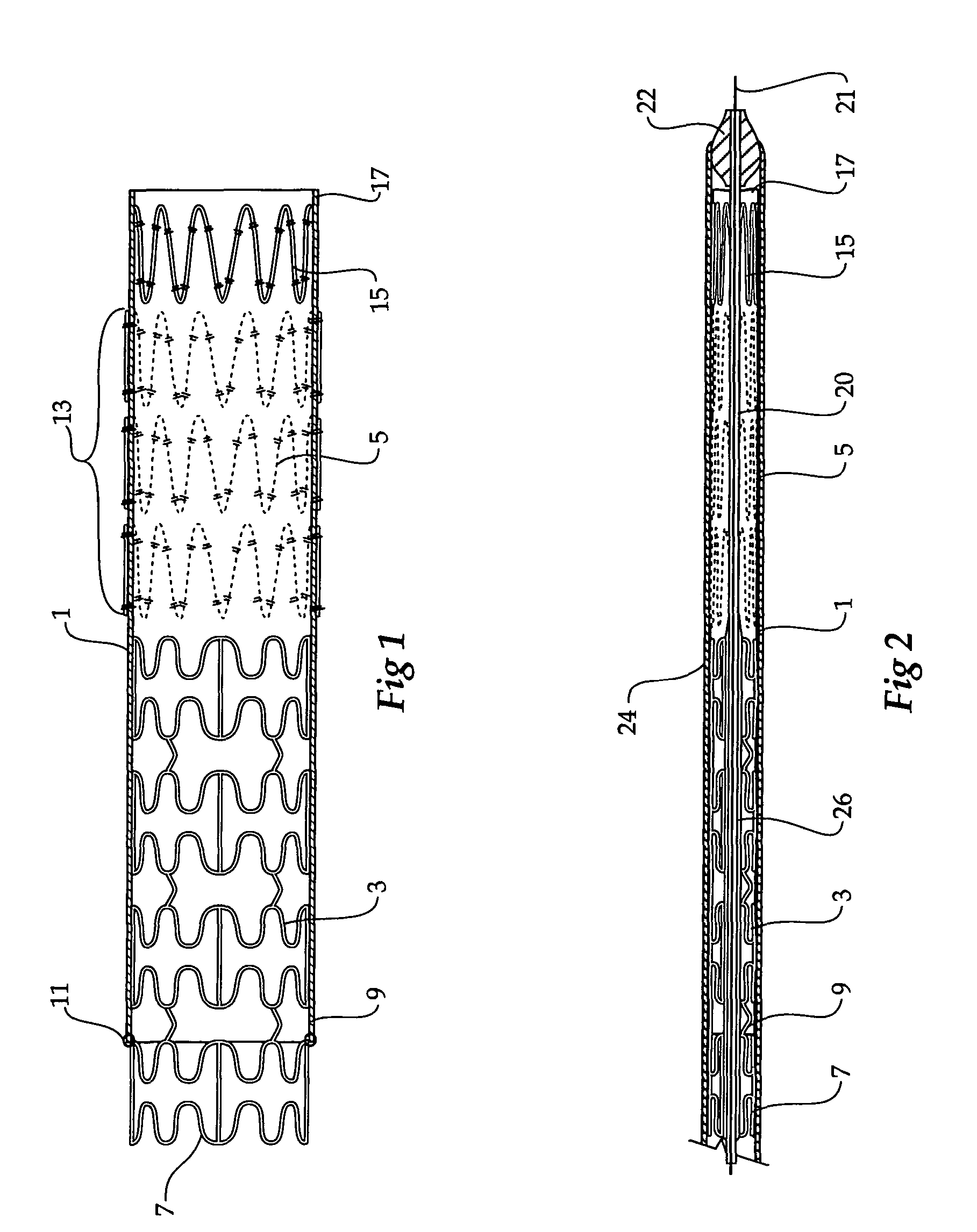
Composite stent graft
A composite stent graft has a balloon expandable stent portion ( 3 ), a tubular graft material portion ( 1 ) inside or outside of the balloon expandable stent portion and self expanding stents ( 5 ) associated with the tubular graft material portion. Part ( 7 ) of the balloon expandable stent portion can extend beyond the proximal end ( 9 ) of the tubular graft material portion. The tubular graft material can be polytetrafluoroethylene, Dacron, Thoralon(TM), polyamide, small intestine submucosa, collagenous extracellular matrix material, or any other suitable biocompatible material. A method of deploying which includes flaring a part ( 7 ) of the balloon expandable stent portion is also discussed.
Owner:COOK MEDICAL TECH LLC
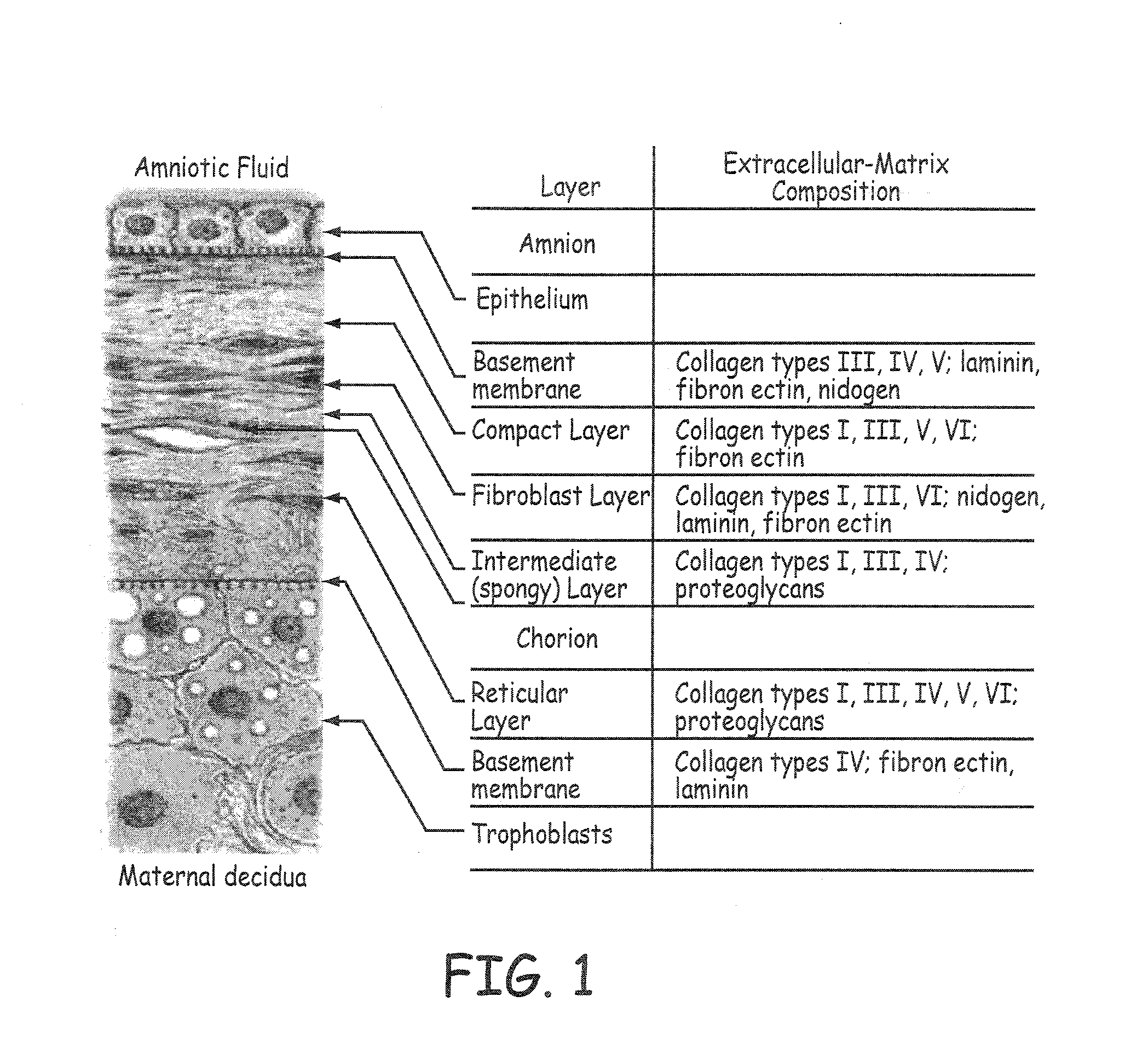
Stents modified with material comprising amnion tissue and corresponding processes
A stent scaffold combined with amniotic tissue provides for a biocompatible stent that has improved biocompatibility and hemocompatibility. The amnion tissue can be variously modified or unmodified form of amnion tissue such as non-cryo amnion tissue, solubilized amnion tissue, amnion tissue fabric, chemically modified amnion tissue, amnion tissue treated with radiation, amnion tissue treated with heat, or a combination thereof. Materials such as polymer, placental tissue, pericardium tissue, small intestine submucosa can be used in combination with the amnion tissue. The amnion tissue can be attached to the inside, the outside, both inside and outside, or complete encapsulation of the stent scaffold. In some embodiments, at least part of the covering or lining comprises a plurality of layers of amnion tissue. The method of making the biocompatible stent and its delivery and deployment are also discussed.
Owner:PEYTANT SOLUTIONS INC
Composite stent graft
A composite stent graft has a balloon expandable stent portion (3), a tubular graft material portion (1) inside or outside of the balloon expandable stent portion and self expanding stents (5) associated with the tubular graft material portion. Part (7) of the balloon expandable stent portion can extend beyond the proximal end (9) of the tubular graft material portion. The tubular graft material can be polytetrafluoroethylene, Dacron, Thoralon™, polyamide, small intestine submucosa, collagenous extracellular matrix material, or any other suitable biocompatible material. A method of deploying which includes flaring a part (7) of the balloon expandable stent portion is also discussed.
Owner:COOK MEDICAL TECH LLC
Surgical method and composition utilizing submucosal tissue to prevent incisional hernias
InactiveUS7105001B2Easy to adaptEliminate requirementsSuture equipmentsDiagnosticsAnatomical structuresAbdominal wall closure
A small intestine submucosa mesh is adapted for use in surgical abdominal closure routines. The submucosa mesh is incorporated into the normal surgical protocol for abdominal closure procedures. The submucosa mesh is applied to the abdominal incision area and maintains and enhances the stability and integrity of the abdominal wall closure, thereby preventing the onset or occurrence of incisional hernias. The submucosa mesh can also be applied to surgical closure procedures involving treatment of a hernia defect, such as an incisional hernia or other non-incisional hernia. The submucosa construct can also be adapted for use as a suturing component or bioretention suture. The submucosa construct can be adapted for use as part of a surgical strategy to facilitate wound healing, regeneration, reconstruction, and replacement of anatomical structures, such as tissues and organs.
Owner:MANDELBAUM JON A
Surgical implant
InactiveUS20050113855A1Suture equipmentsAnti-incontinence devicesSmall intestineSurgical Mesh Implant
An implant is disclosed including a bio-compatible implant body that can be provided with a tip, or interconnected by one or more filaments. The implant body can be formed of a variety of biocompatible materials, including bio-remodelable materials such as small intestine submucosa. Methods are disclosed for assembly of the implant. Additionally, a method is disclosed for delivering the implant to a desired location in a patient.
Owner:WILSONCOOK MEDICAL
Embolization device
InactiveUS7857825B2Promote remodelingIncreases Th- immune responseBiocideInorganic active ingredientsSmall intestineSmall intestine submucosa
A medical device (40) including a collagenous biomaterial (10) for causing embolus and treating an aneurysm. The collagenous biomaterial (10) includes a small intestine submucosa (12) that is configured into various components (16) for causing a vascular occlusion or a packing aneurysm lumen.
Owner:COOK BIOTECH
Stents modified with material comprising amnion tissue and corresponding processes
Owner:PEYTANT SOLUTIONS INC
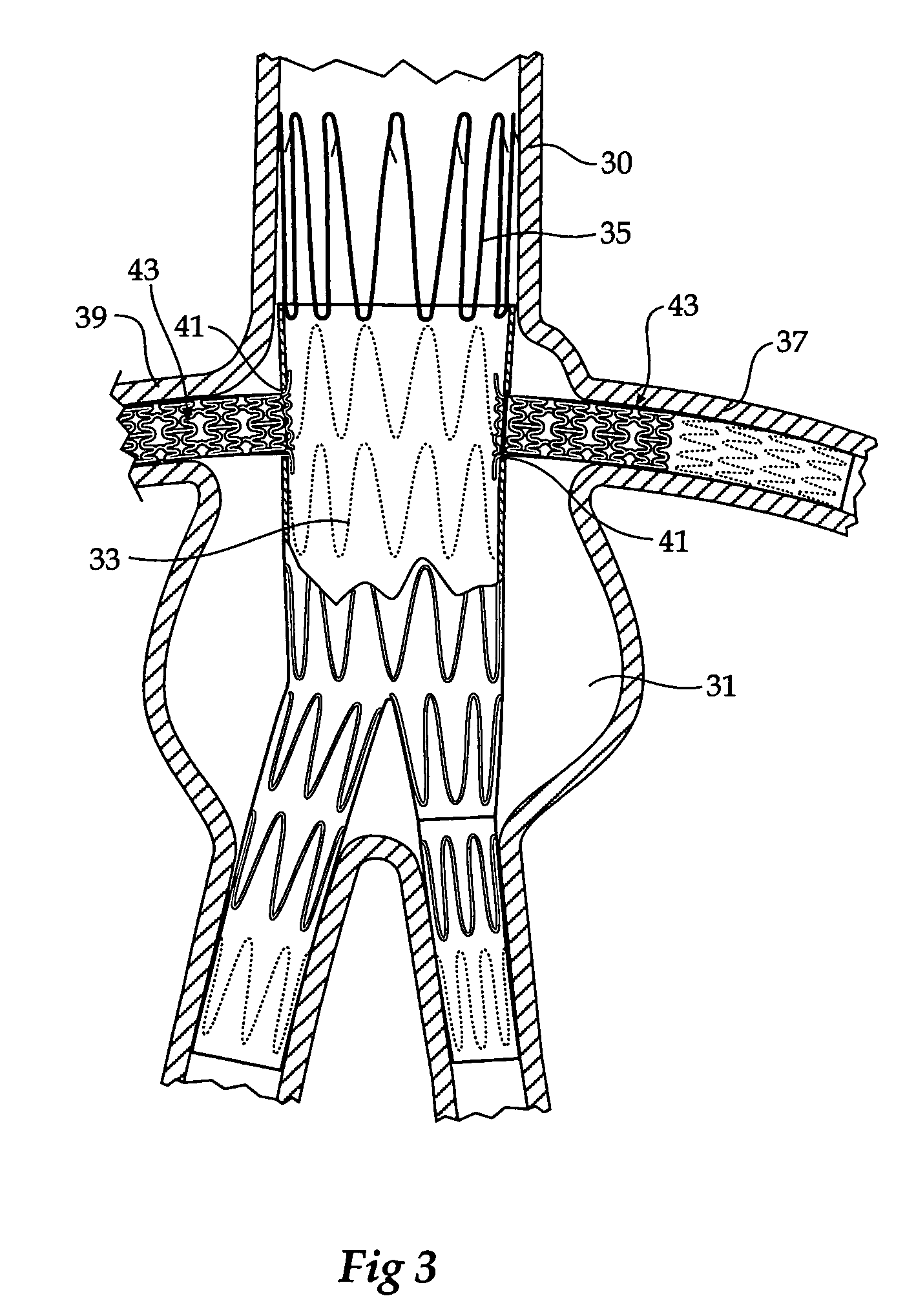
Stent tissue graft prosthesis
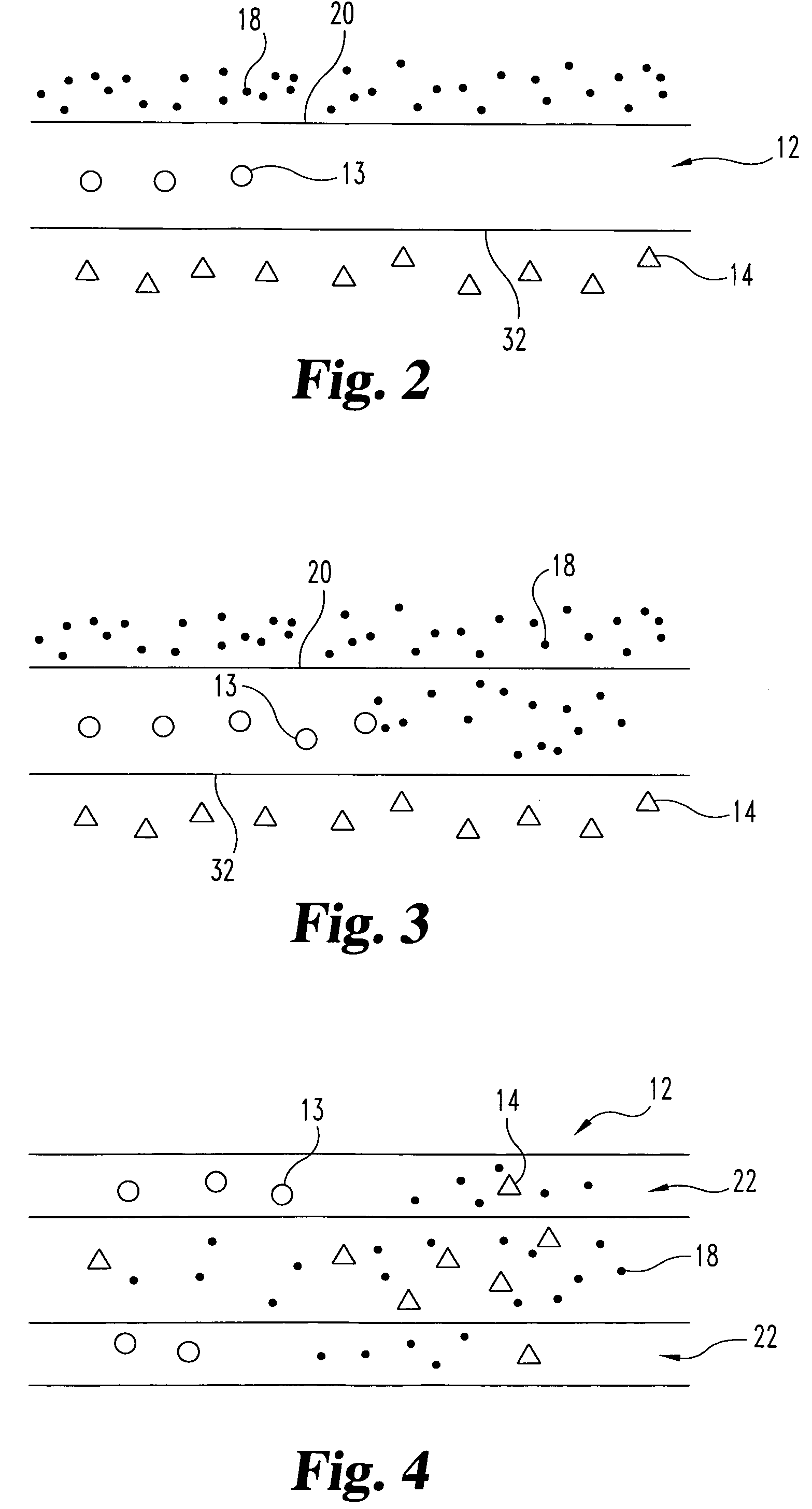
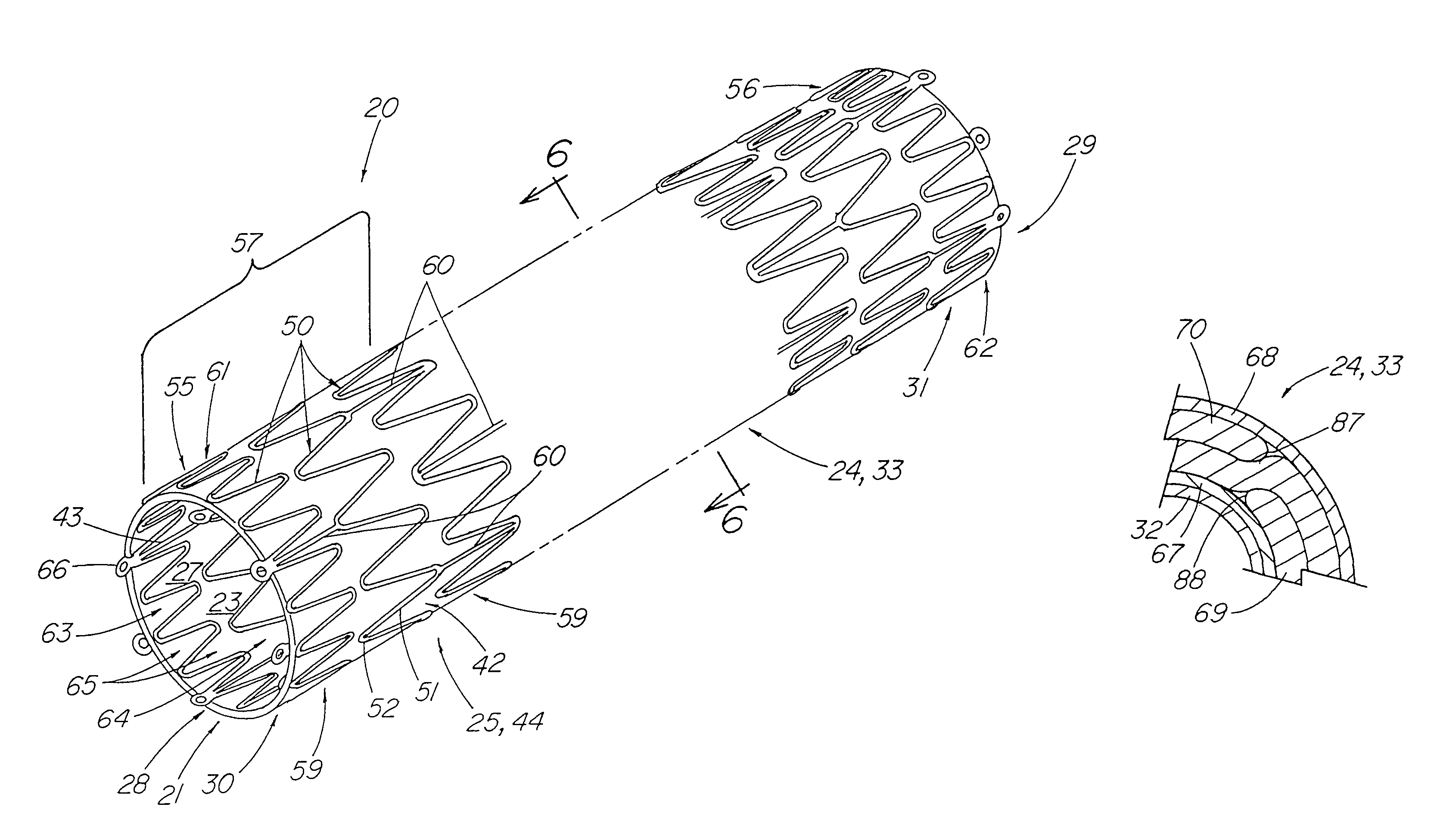
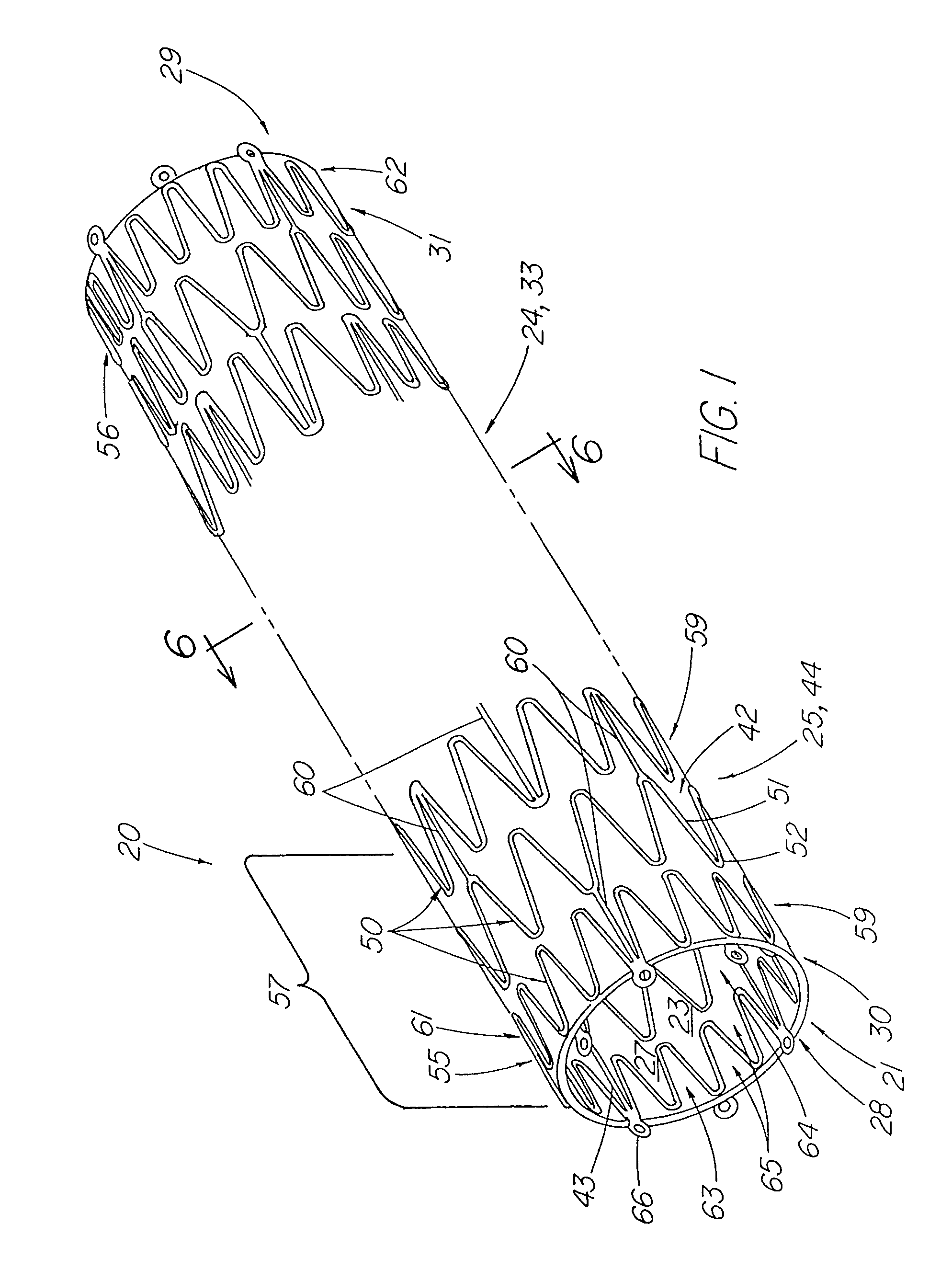
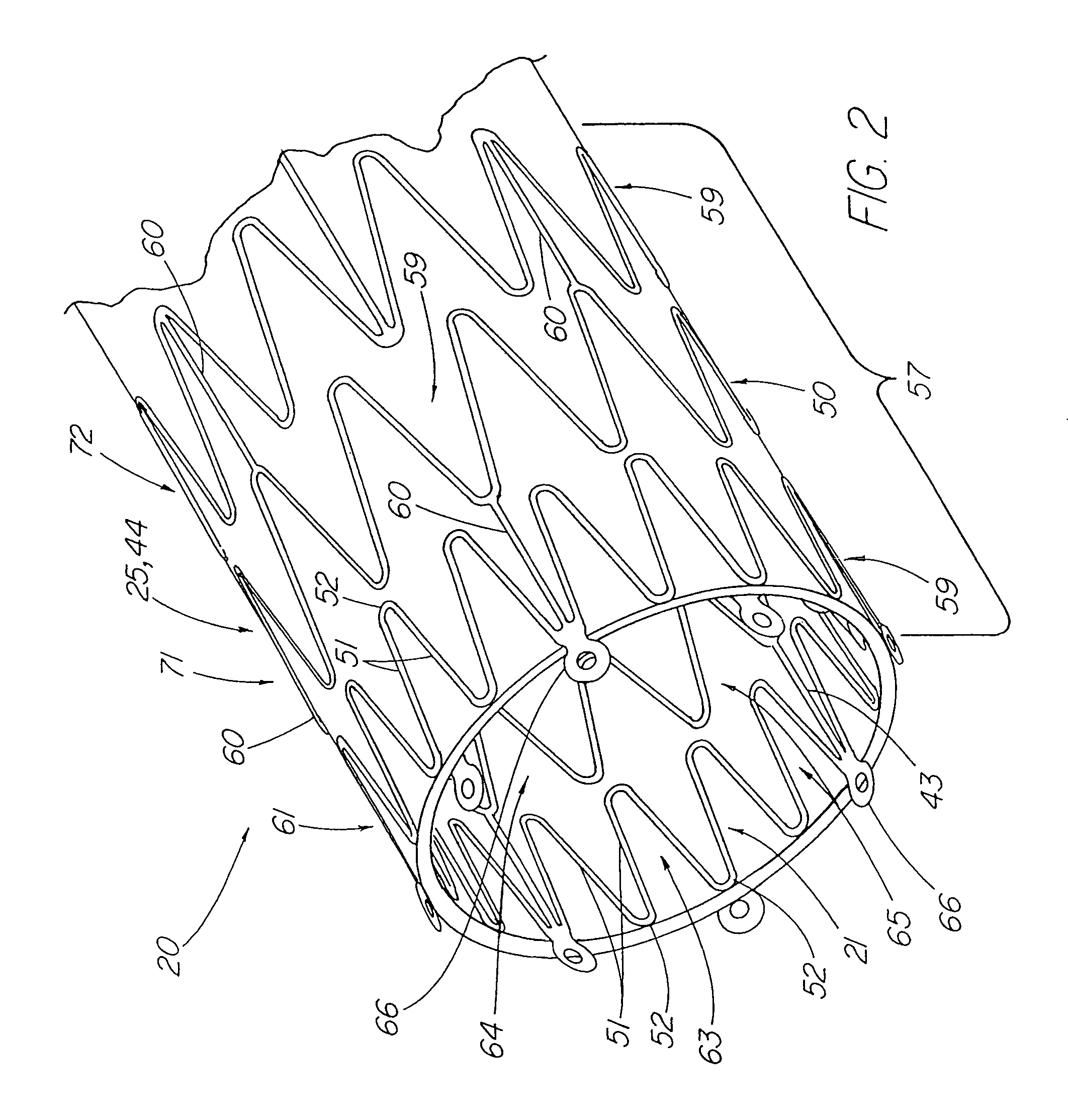
ActiveUS7914567B2Prevent eversionMinimize traumaStentsBlood vesselsCell-Extracellular MatrixECM Protein
A stent tissue graft prosthesis (20) for repairing, excluding and / or reinforcing a vessel, duct and the like in a patient. The prosthesis includes an inner expandable stent (21) of which a tissue graft (24) and preferably a multilayered tissue construct (33) is disposed thereon for application to the host tissue of a vessel, duct and the like. The tissue construct includes an extracellular matrix material (36) such as small intestine submucosa (37) for remodeling the host tissue into the prosthesis. The prosthesis further includes an outer tubular member (25) such as an outer expandable stent (44) for retaining the tissue graft on the inner stent. The ends of the inner and outer stents are coincident with or extend beyond the ends of the tissue graft to prevent eversion or fold back of the tissue graft during withdrawal of a delivery catheter in a placement procedure.
Owner:OREGON HEALTH & SCI UNIV +2
Mold for preparing accellular small intestine submucosa matrix material
ActiveCN103272277AGuaranteed porosityHigh porositySurgeryProsthesisPorosityCell-Extracellular Matrix
The invention provides a mold for preparing an accellular small intestine submucosa matrix material, which is composed of a base and a pressure screen, wherein the base is a square plate with pins on four sides, and the pins are perpendicular to the square plate; the pressure screen is a square frame with a square frame structure or reticular structure inside; and the length of the four sides of the pressure screen is 1-3 cm smaller than that of the base. When being used for preparing the accellular porcine small intestine submucosa matrix material, the mold can not compress the space structure of the accellular porcine small intestine submucosa matrix material or destroy the three-dimensional structure of the natural extracellular matrix, and can ensure the porosity of the prepared accellular porcine small intestine submucosa matrix material.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Biological repair tablet for herniae and preparation method thereof
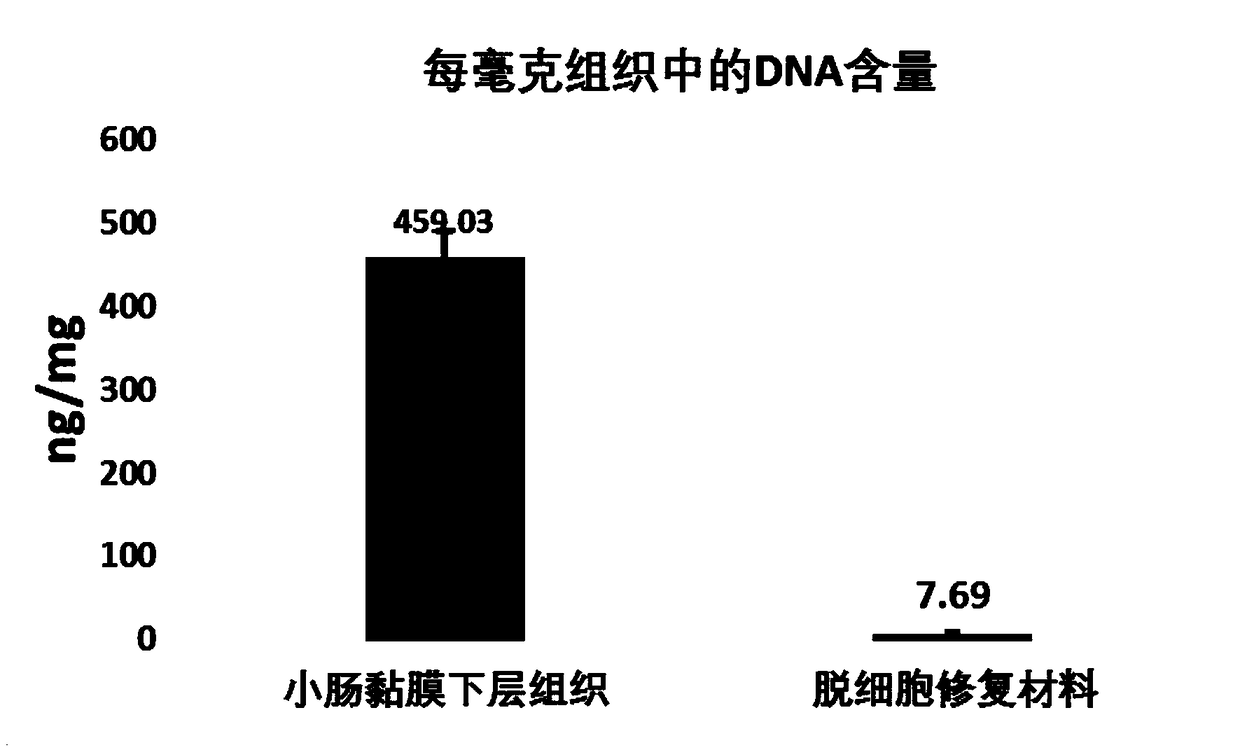
The invention provides a biological repair tablet for herniae and a preparation method thereof. The repair tablet uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the repair tablet comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The biological repair tablet for herniae prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the biological repair tablet for herniae has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Mold for preparing accellular small intestine submucosa matrix material
ActiveCN103272273AGuaranteed porosityHigh porositySurgeryProsthesisPorosityCell-Extracellular Matrix
The invention provides a mold for preparing an accellular small intestine submucosa matrix material, which is composed of a base, a pressure frame and a pressure screen, wherein the base is a square plate with bolt holes at four corners; the pressure frame is a hollow square frame with bolt holes at four corners; the pressure screen is a square frame with a square frame structure or reticular structure inside; the bolt holes in the base are corresponding to the bolt holes in the pressure frame one by one; the external diameter of the pressure frame is the same as the dimension of the base; and the inner diameter of the pressure frame is the same as the dimension of the pressure screen. When being used for preparing the accellular porcine small intestine submucosa matrix material, the mold can not compress the space structure of the accellular porcine small intestine submucosa matrix material or destroy the three-dimensional structure of the natural extracellular matrix, and can ensure the porosity of the prepared accellular porcine small intestine submucosa matrix material.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Tissue mending material with biological activity and preparation method thereof
The invention relates to a tissue repairing material with bioactivity and a preparation method thereof. The material is made by compounding human body living cells, and extracellular matrixes and cell growth factors synthesized and excreted by the human body living cells on the acellular small intestine submucosa. Natural antigen components are removed from the prepared tissue repairing material with bioactivity, and when the tissue repairing material is applied to a wound, the tissue repairing material can survive and directly take part in the repair of the wound to continuously synthesize and excrete growth factors, guide the cells around the wound to grow in and the creation of blood vessel, and induce the differentiation of stem cells to skin cells, thereby obviously promoting the wound healing; besides, the tissue repairing material not only has part of the characteristics of human body tissues and the good mechanical property of the acellular small intestine submucosa, but also has high biocompatibility to human body, obviously reduces the immune rejection; at the same time, the prepared tissue repairing material can cover the surface of wound, fill the defection of soft tissues, promote the growth and proliferation of the cells around the wound, repair the defection of the soft tissue organs, and promote the wound healing.
Owner:SHAANXI RUISHENG BIOTECH
Neural restoration material, and preparation method and application thereof
ActiveCN107007882AProtect the microenvironmentEffective isolationTissue regenerationProsthesisFibroblastIntestinal submucosa
The invention provides a neural restoration material, and a preparation method and application thereof. The neural restoration material is characterized in that the neural restoration material contains collagen, polysaccharose substances, active factors and nerve regeneration promoting factors; the neural restoration material is of a three-dimensional reticular porous structure; no immunogenicity exists; the degradation in vivo is realized; the neural restoration material can be in a flaky shape or a hollow tube shape. The neural restoration material is manufactured by small intestine submucosa tissues of pigs; the injured nerve can be effectively protected; the nerve regeneration protection microenvironment is formed; the injured nerve regeneration is promoted; a barrier effect can also be achieved; the injured nerve position is effectively isolated from peripheral tissues; fibroblasts in the peripheral tissues are prevented from invading the injured nerve position.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Stents modified with material comprising amnion tissue and corresponding processes
A stent scaffold combined with amniotic tissue provides for a biocompatible stent that has improved biocompatibility and hemocompatibility. The amnion tissue can be variously modified or unmodified form of amnion tissue such as non-cryo amnion tissue, solubilized amnion tissue, amnion tissue fabric, chemically modified amnion tissue, amnion tissue treated with radiation, amnion tissue treated with heat, or a combination thereof. Materials such as polymer, placental tissue, pericardium tissue, small intestine submucosa can be used in combination with the amnion tissue. The amnion tissue can be attached to the inside, the outside, both inside and outside, or complete encapsulation of the stent scaffold. In some embodiments, at least part of the covering or lining comprises a plurality of layers of amnion tissue. The method of making the biocompatible stent and its delivery and deployment are also discussed.
Owner:PEYTANT SOLUTIONS INC
Polyurethane (PU)/small intestine submucosa (SIS) membrane composite material and application thereof
The invention discloses a polyurethane (PU) / small intestine submucosa (SIS) membrane composite material which is prepared according to the following steps: a, uniformly mixing anion waterborne PU emulsion and SIS membranes, and performing freeze-drying formation in vacuum, wherein the use amount of the SIS membranes is 0.01-0.03 times (w / w) of that of the PU emulsion, the average particle size of the PU emulsion is 15.18-42.86nm, and the solid content of the PU emulsion is 3-21%; and b, performing cross-linking, and freeze-drying in vacuum, thereby obtaining the PU / SIS membrane composite material. The PU / SIS composite has excellent properties of PU and SIS, has good rebound resilience property and mechanical property, also has biological activity and biocompatibility, can be used for inducing and promoting regeneration and remediation of tissue structures and overcoming the defects that a conventional soft tissue defect remediation material only has single property, the application is limited and the like, and has good application prospect.
Owner:睿博欣源(成都)生物科技有限责任公司
Self-assembling protein matrix prepared from natural extracellular matrices
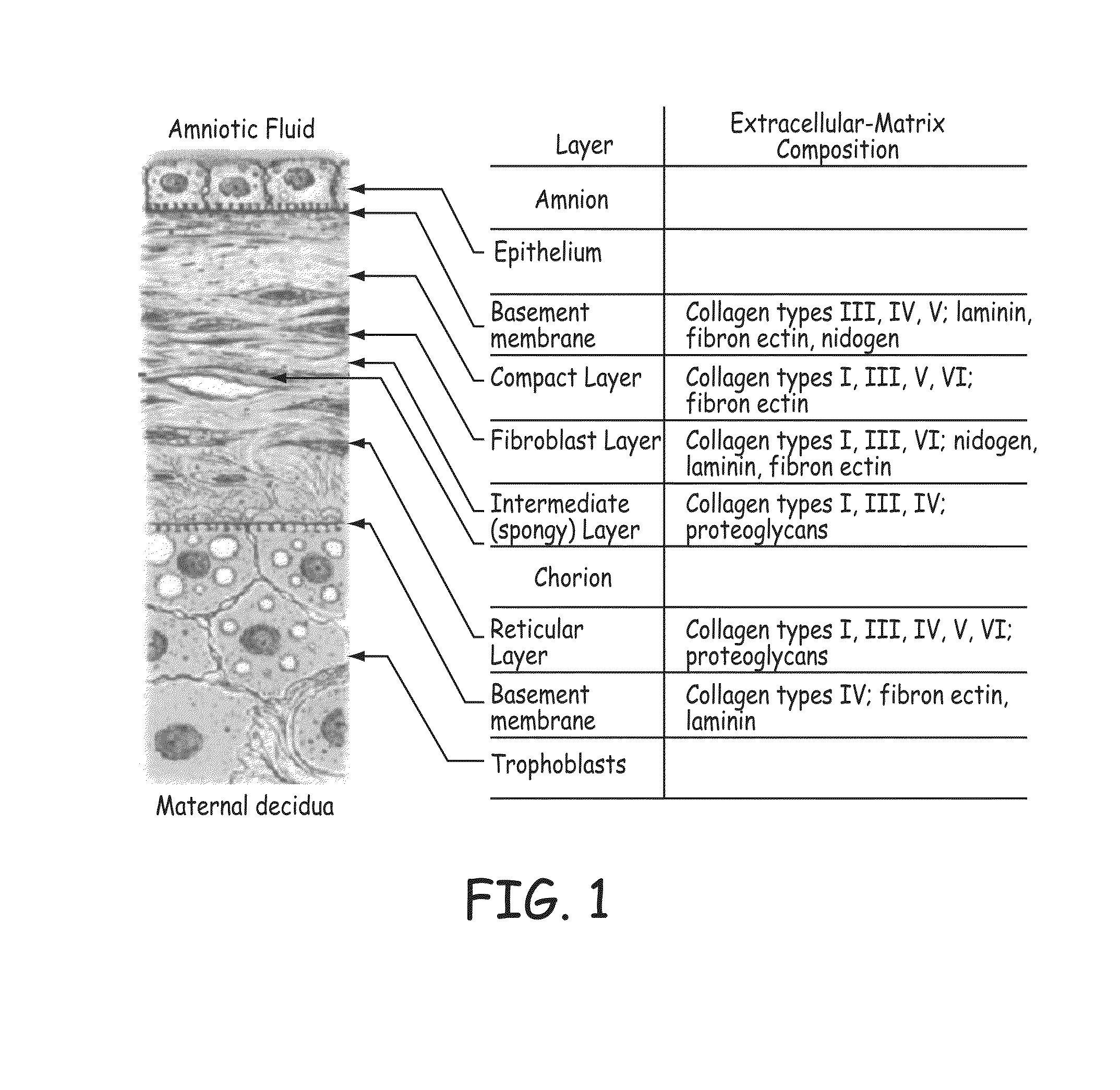
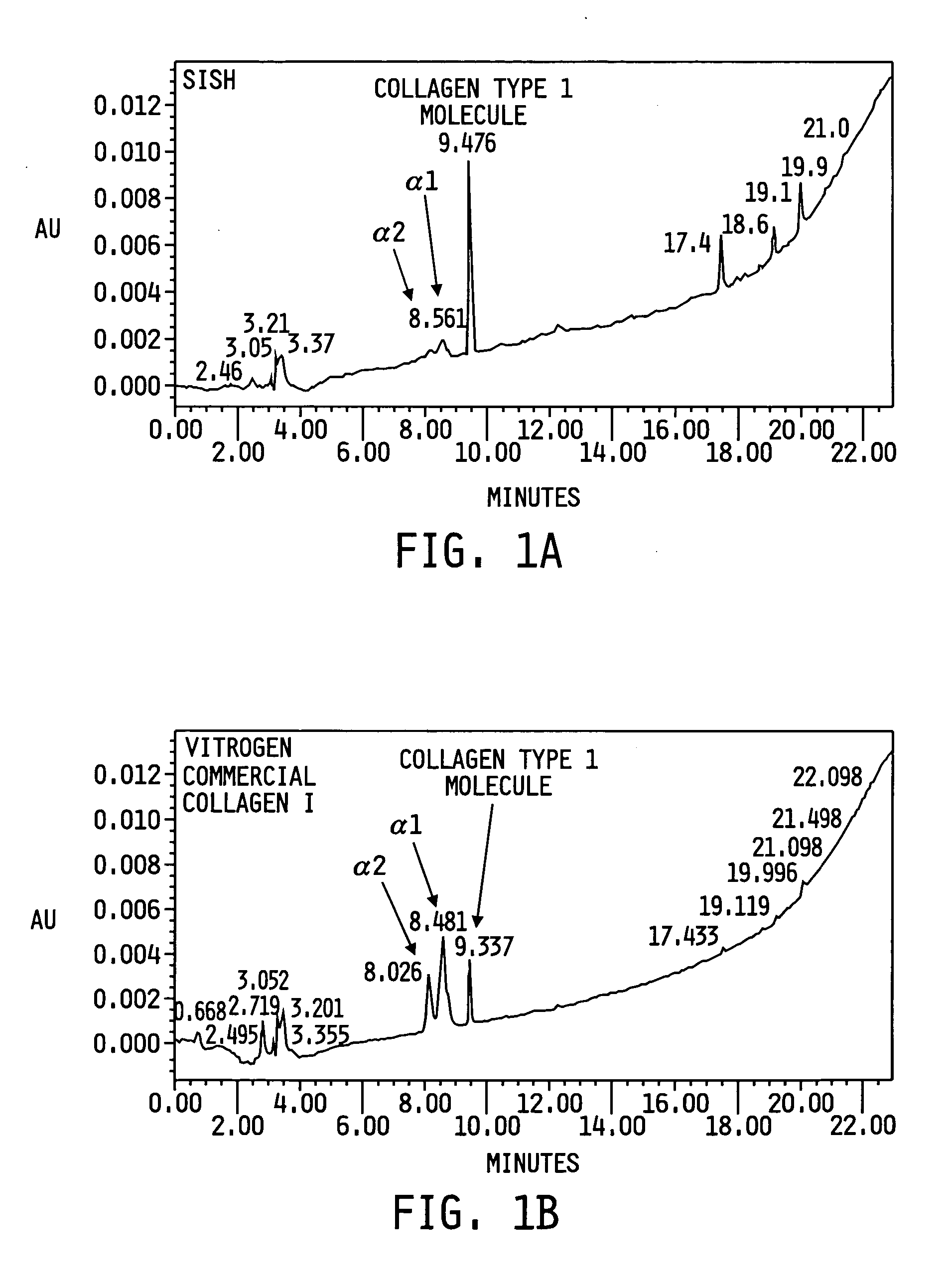
The present invention provides a method for preparing a biomaterial involving extracting Collagen Type I (Col I) protein from small intestine submucosa (SIS) under conditions that preserve the native helical configuration. The extract of the present method contains a substantial amount of the alpha helical form of Col I. Further the present method includes inducing the Col I protein to form a polymer material in solution or on a target surface.
Owner:DEPUY PROD INC
Biological repair tablet for endocranium and preparation method thereof
The invention provides a biological repair tablet for endocranium and a preparation method thereof. The repair tablet uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the repair tablet comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The biological repair tablet for endocranium prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the biological repair tablet for endocranium has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Preparation method of polyurethane/small intestine submucosa composite material
The invention discloses a preparation method of a polyurethane / small intestine submucosa composite material, which comprises the following steps: a. evenly mixing an anionic water-based polyurethane emulsion and small intestine submucosa, and carrying out vacuum freeze-drying to form, wherein the small intestine submucosa is 0.01-0.03 time of the polyurethane emulsion (w / w), the average particle diameter of the polyurethane emulsion is 15.18-42.86nm, and the solid content is 3-21%; and b. crosslinking, and carrying out vacuum freeze-drying. The composite material prepared by the method has favorable properties of the polyurethane and SIS (styrene-isoprene-styrene), has the advantages of favorable resilience, favorable mechanical properties, favorable bioactivity and favorable biocompatibility, can induce and promote the regeneration restoration of the tissue structure, overcomes the defect that the existing soft tissue defect restoration material only has the properties in a single aspect and is limited in application, and has favorable application prospects.
Owner:睿博欣源(成都)生物科技有限责任公司
Diverticular remodling technique
InactiveUS20060235077A1Promote permanent closurePrevent egressBiocideOrganic active ingredientsMulti materialFibrosis
A diverticular remodeling technique comprises the introduction of a remodeling agent into the diverticulum to promote permanent closure of the diverticular sac. In disclosed exemplary embodiments, the diverticulum is accessed non-invasively and optionally cleaned to remove unwanted matter therefrom. The remodeling agent is introduced into the diverticular interior and the neck of diverticulum is closed (as necessary) to prevent egress of the remodeling agent. The remodeling agent can be any of a variety of materials, including small intestine submucosa (SIS) collagen or other absorbable fibrosis materials in gel, liquid or solid form. An endoscopic device may be used that includes a multi-lumen flexible catheter having a distal end adapted to be steered through a patient and a proximal end that remains outside the patient's body.
Owner:TAHERI SYDE A
Soft tissue repair material and method for preparing same
ActiveCN108888804AWide variety of sourcesConvenient sourceSurgerySkeletal disorderMammalSmall intestine mucous membrane
The invention relates to a soft tissue repair material and a method for preparing the same, and belongs to the technical field of biomedical material processing. The method includes steps of 1), removing small intestine mucous membranes, muscular layers and serous membranes on small intestine tissues of mammals and carrying out washing by the aid of sterile buffer solution with antibiotics by 3-5times to obtain washed lower-layer tissues of the small intestine mucous membranes; 2), clipping the lower-layer tissues to obtain sections, and splitting and spreading the sections along median linesto obtain pretreated lower-layer tissues of the small intestine mucous membranes; 3), placing the pretreated lower-layer tissues in sterile buffer solution with surfactants and nuclease and carryingout oscillation treatment at the temperatures of 37 DEG C for 1-4 h to obtain acellular lower-layer tissues of the small intestine mucous membranes; 4), carrying out oscillation cleaning, cutting anddrying to obtain dried central basement membranes; 5), carrying out irradiation sterilization to obtain the soft tissue repair material. The soft tissue repair material and the method have the advantage that the safe and effective soft tissue repair material with excellent bioactivity and biocompatibility can be prepared by the aid of the method.
Owner:SHANDONG EYE INST
Anal fistula suppository and preparation method thereof
The invention provides an anal fistula biological suppository and a preparation method thereof. The anal fistula biological suppository uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the anal fistula biological suppository comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The anal fistula biological suppository prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the anal fistula biological suppository has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Embolization device
InactiveUS20110077679A1Promote remodelingEnhance immune responseDilatorsDiagnostic markersSmall intestineSmall intestine submucosa
A medical device (40) including a collagenous biomaterial (10) for causing embolus and treating an aneurysm. The collagenous biomaterial (10) includes a small intestine submucosa (12) that is configured into various components (16) for causing a vascular occlusion or a packing aneurysm lumen.
Owner:COOK BIOTECH
Injectable decellularized small intestinal submucosal matrix particles and preparation method and application thereof
InactiveCN107929809APromote ingrowthReduce immune rejectionTissue regenerationProsthesisFreeze-dryingFreeze and thaw
The invention relates to injectable decellularized small intestinal submucosal matrix particles and a preparation method and the application thereof, and belongs to the field of regenerative medicine.The preparation method of the injectable decellularized small intestinal submucosal matrix particles comprises the following steps: (1), taking an animal small intestine tissue, and sequentially rinsing, sterilizing and repeatedly freezing and thawing; (2), shearing the animal small intestine tissue, scraping off a mucosal layer, a muscle layer and a serosa layer of the small intestine to obtaina stripped small intestine submucosa, and soaking the stripped small intestine submucosa in a sodium chloride solution; (3), decellularizing the small intestine submucosa, and then rinsing; (4), soaking the small intestine submucosa in a buffer solution, then putting into a descaling agent, extracting while shaking, and rinsing; (5), soaking the small intestine submucosa in the buffer solution, performing freeze drying, and grinding to obtain the injectable decellularized small intestinal submucosal matrix particles. The injectable decellularized small intestinal submucosal matrix particles prepared by the preparation method provided by the invention are rich in bioactive component and structurally loose and porous.
Owner:广州昕生医学材料有限公司
Vascular occlusion device
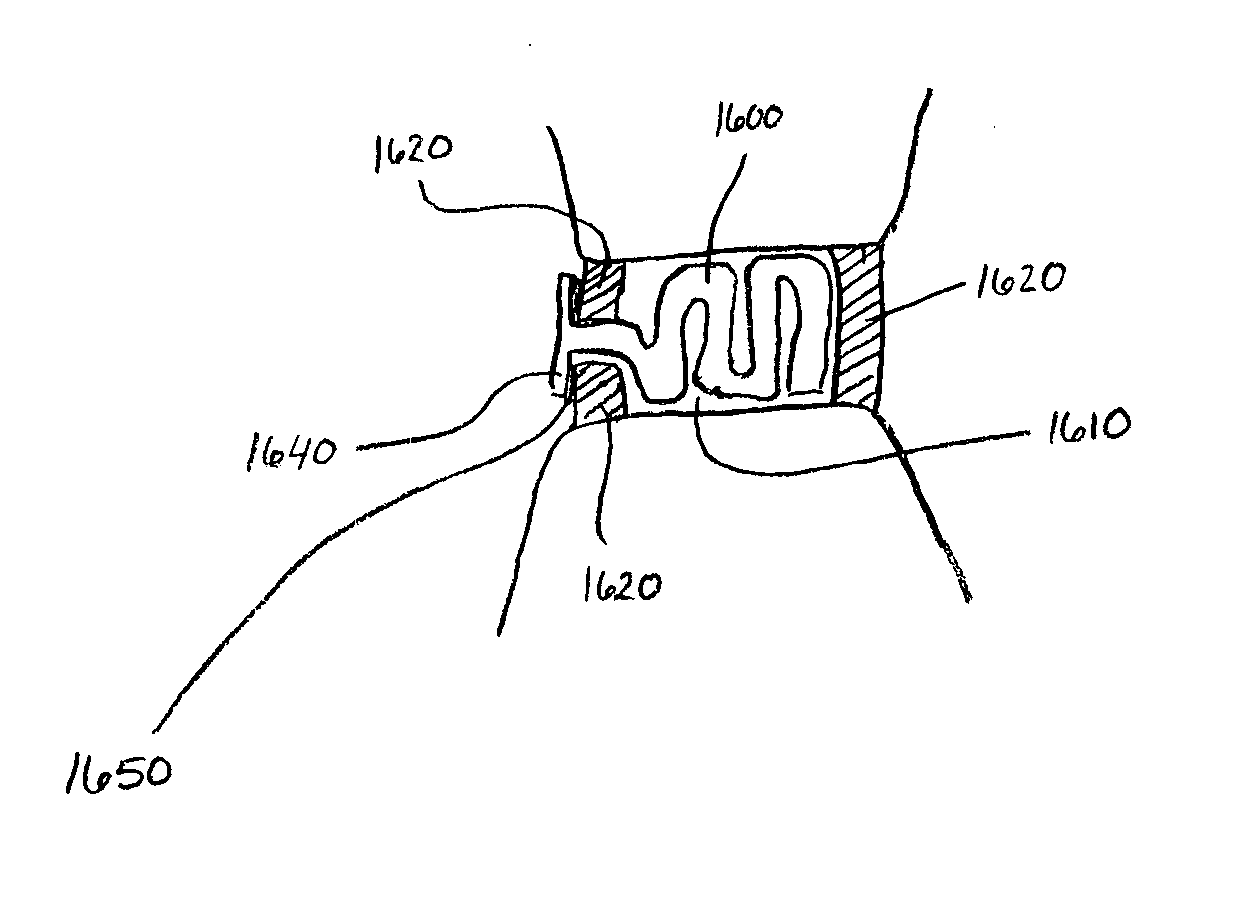
A vascular occlusion device for occluding a body cavity. The device includes an elongate flexible member having a proximal portion extending to a distal portion and a radially compressible substance being disposed therebetween. The substance is configured to promote growth of body tissue and may include an extracellular matrix such as small intestine submucosa. The proximal and distal end portions are anchored to the cavity walls such that the position of the substance within the body cavity promotes the growth of body tissue to occlude the body cavity. In one example, the body cavity includes a patent foramen ovale.
Owner:COOK INC
Biological sponge based on acellular small intestinal submucosa and preparation method thereof
InactiveCN110051884AGood effectPromote ingrowthPharmaceutical delivery mechanismTissue regenerationSubmucosaPepsin
The invention discloses a biological sponge based on the acellular small intestinal submucosa and a preparation method thereof. The preparation method comprises the steps of preparing the acellular porcine small intestinal submucosa, pepsin enzymatic hydrolysis, washing, lyophilization, pulverization and cross-linking. According to the preparation method, the porcine small intestinal submucosa istaken as a source; after the decellularization treatment, the immunological rejection is extremely small. At the same time, the cross-linking treatment is carried out by using a non-toxic cross-linking agent to ensure the biosafety of the obtained product. The product can be used not only for wound repair, but also as an excellent tissue engineering skin scaffold material. The product is sufficient in source, safe and reliable, cheap and easy to obtain. The composition and structure are similar to the humans. No ethical problems exist, and scale industrial preparation can be facilitated.
Owner:上海仁康科技有限公司
Uterine cavity implant as well as preparation method and application thereof
The invention relates to a uterine cavity implant as well as a preparation method and application thereof. The uterine cavity implant is characterized by having a bagged structure. The uterine cavity implant comprises a substrate material of animal small intestine submucosa subjected to decellularization treatment. An implantable medical device for preventing and treating intrauterine adhesion comprises a biological tissue substrate material. The present decellularization technology for a biological repairing material is improved; compared with the present product, the biological tissue substrate material provided by the invention has the advantages of lower DNA residual quantity, lower immunogenicity, higher anti-infection capacity and higher repairing capacity; and the uterine cavity implant is beneficial to the recovering of tissues, such as, uterine cavity base layer, submucosa and mucous layer. Besides, the invention prepares the biological repairing material into the uterine cavity built-in structure which is used for isolating wound surfaces, repairing uterine cavity tissues and reducing scar forming, so that the intrauterine adhesion problem is solved, and the accordingly generated sterility problem also can be solved.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com