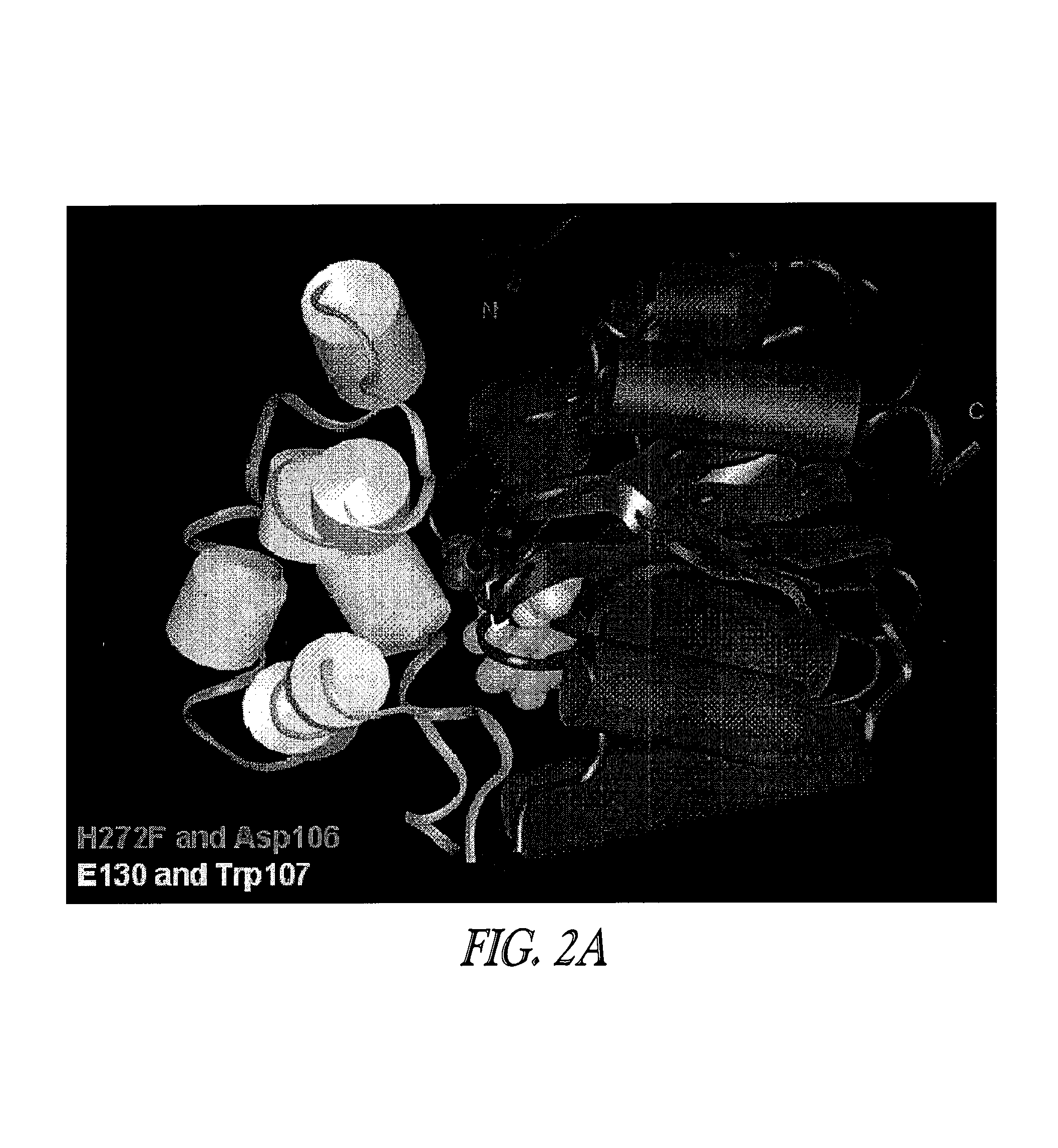
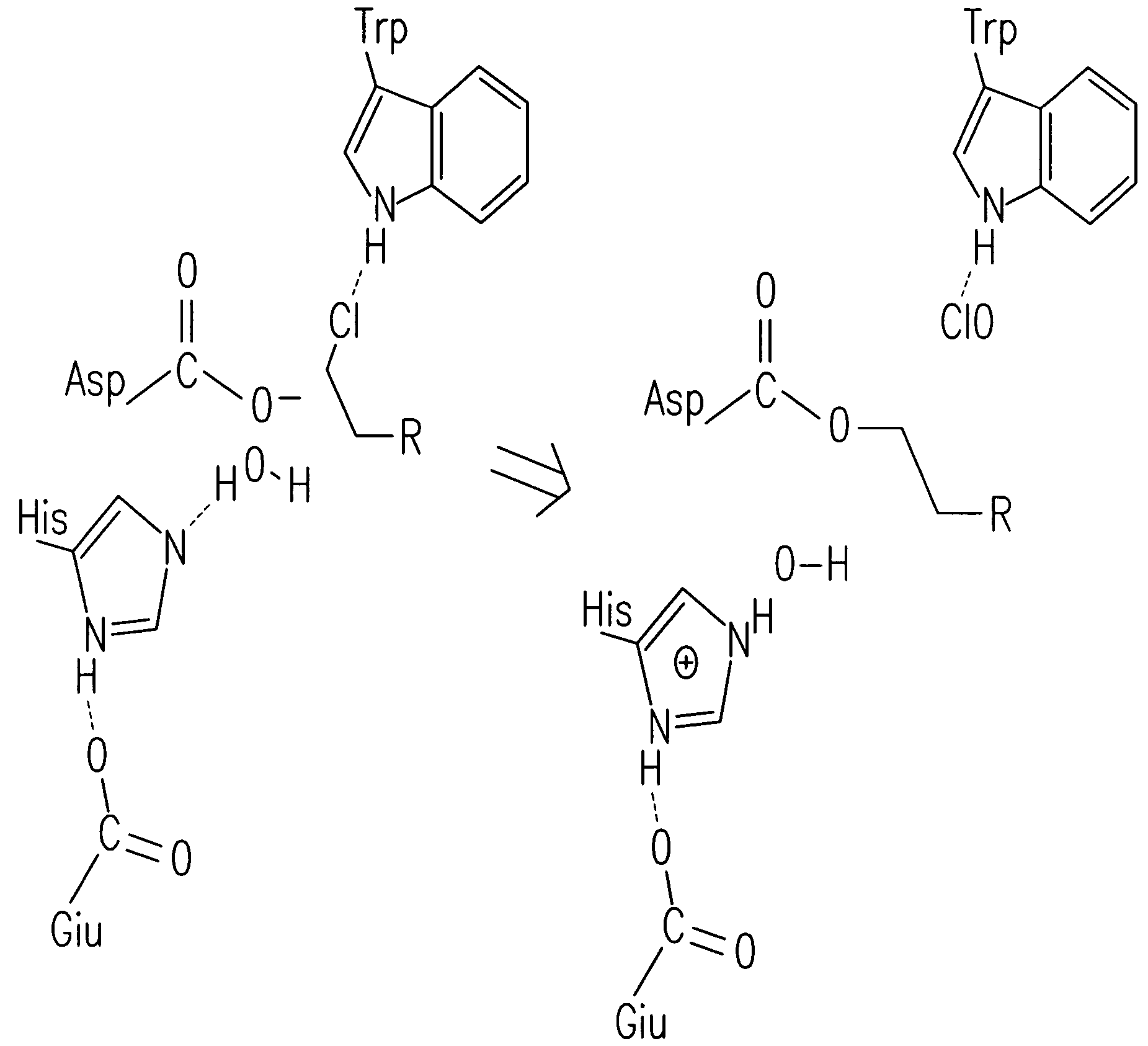
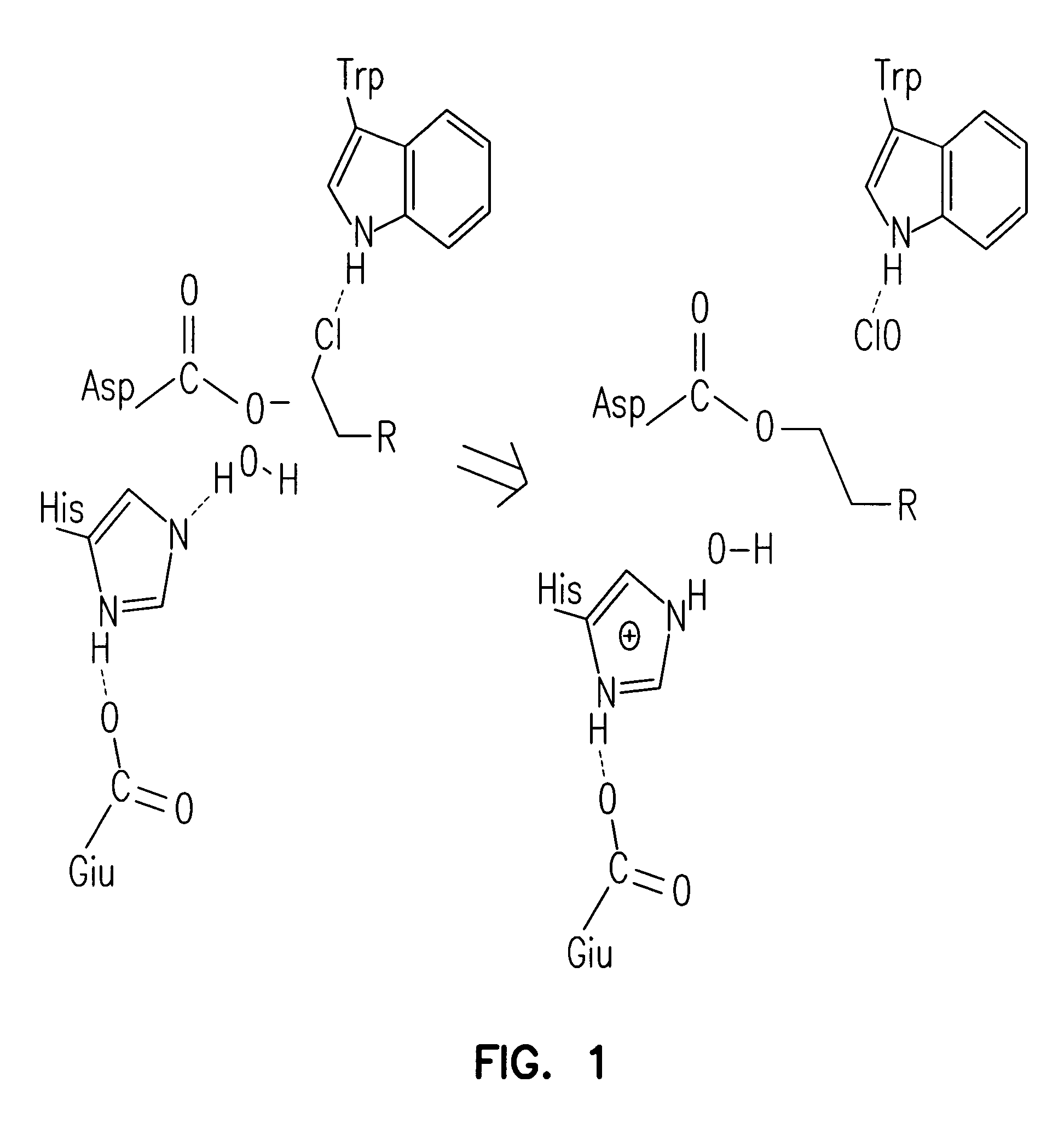
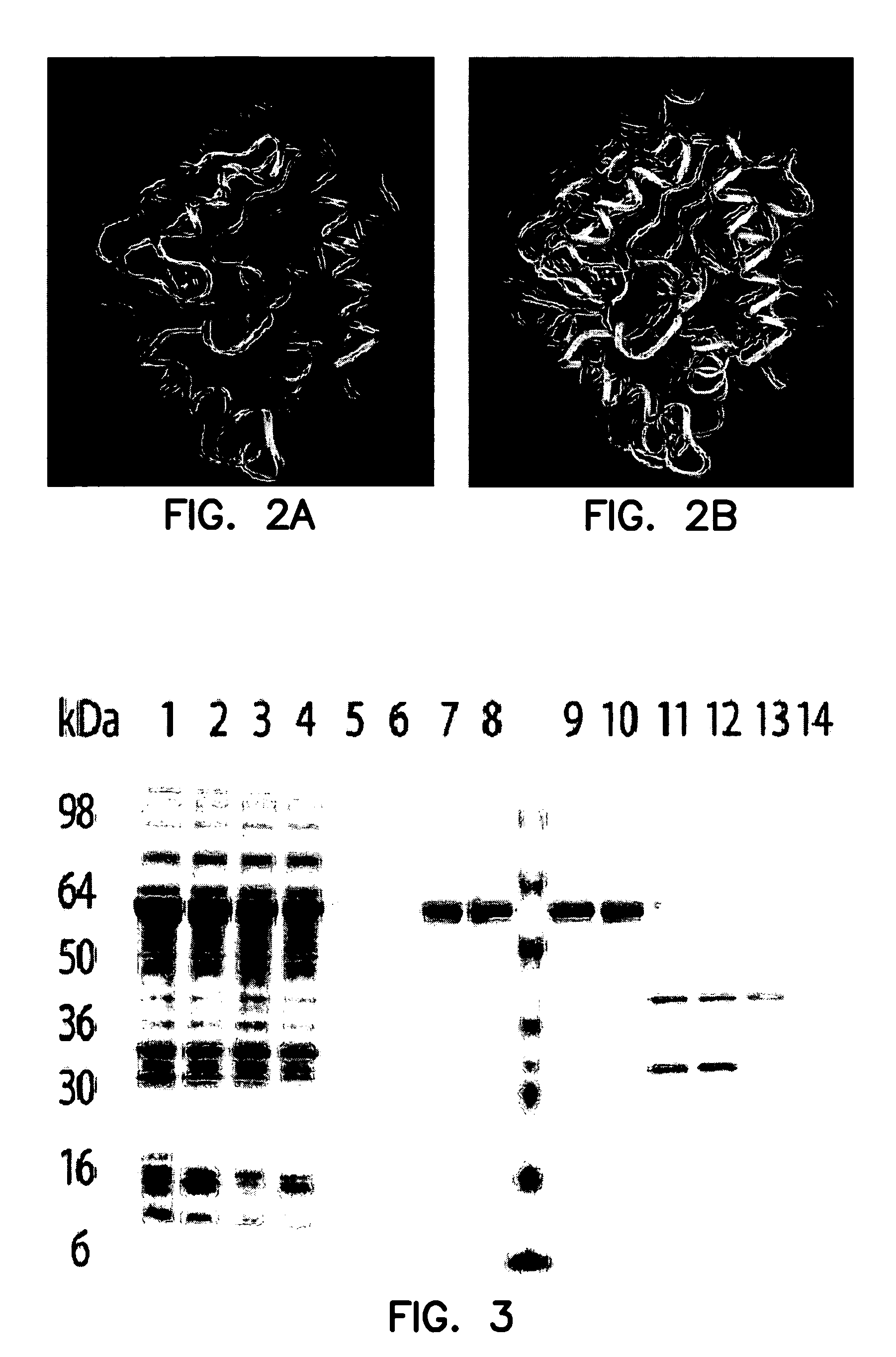
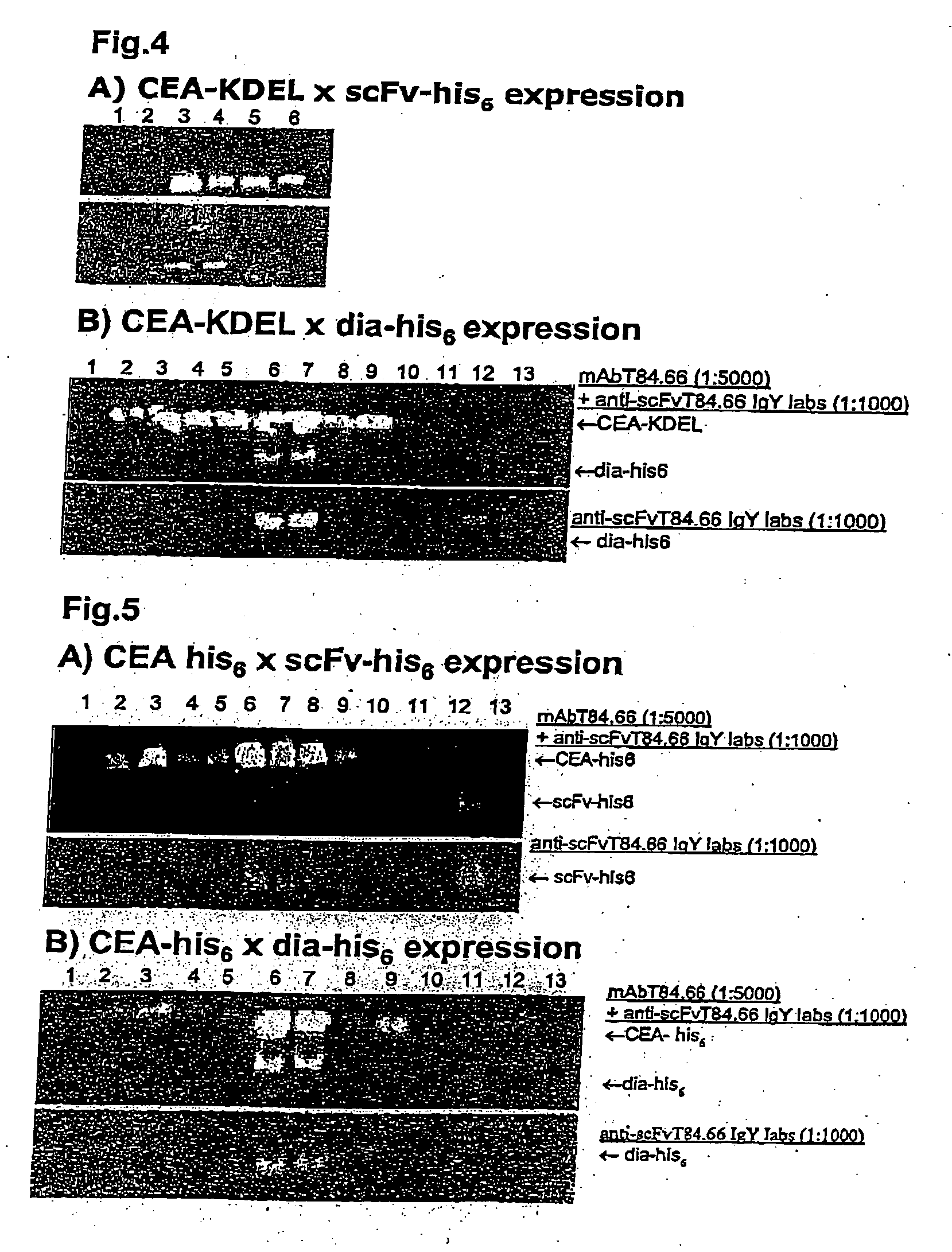
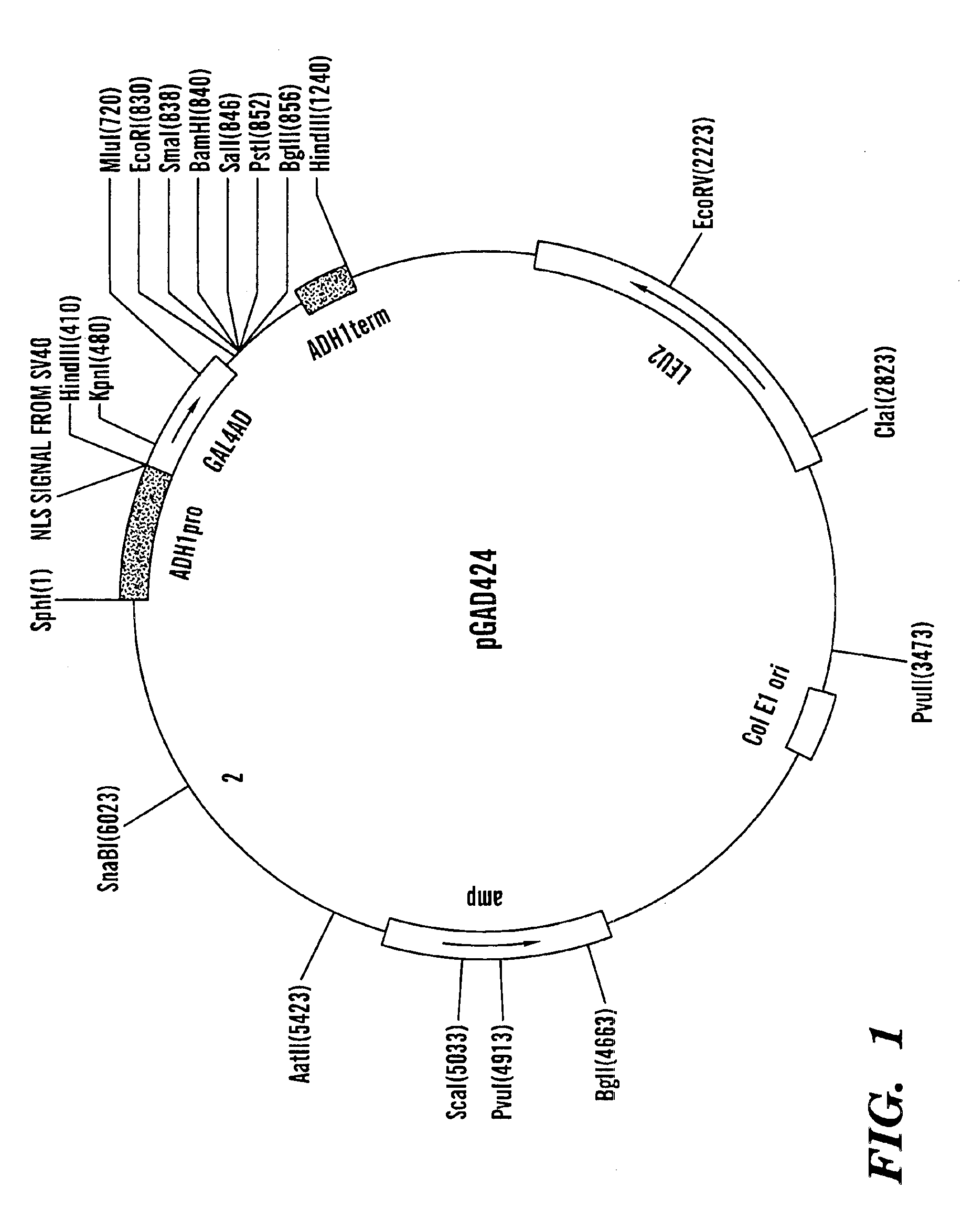
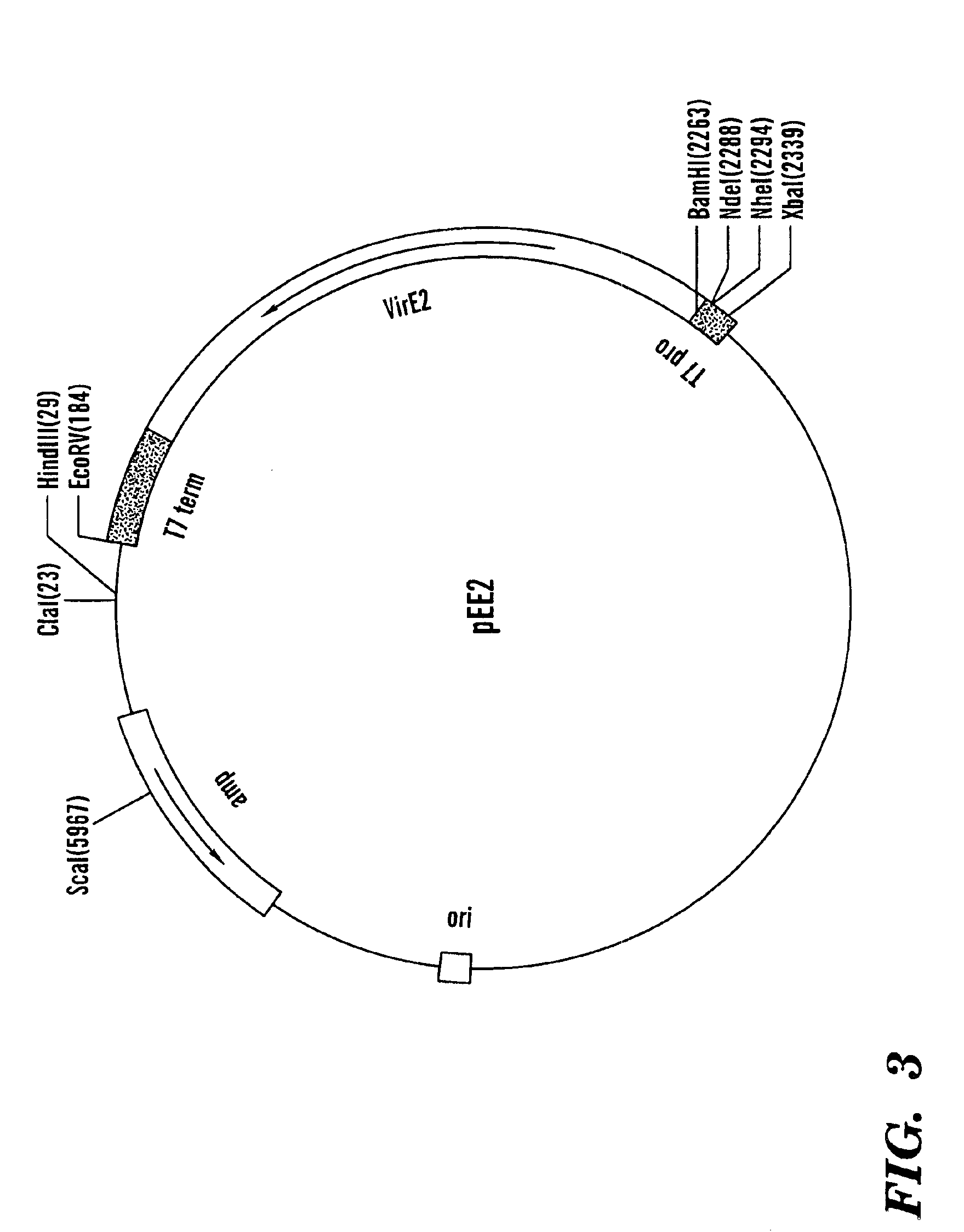
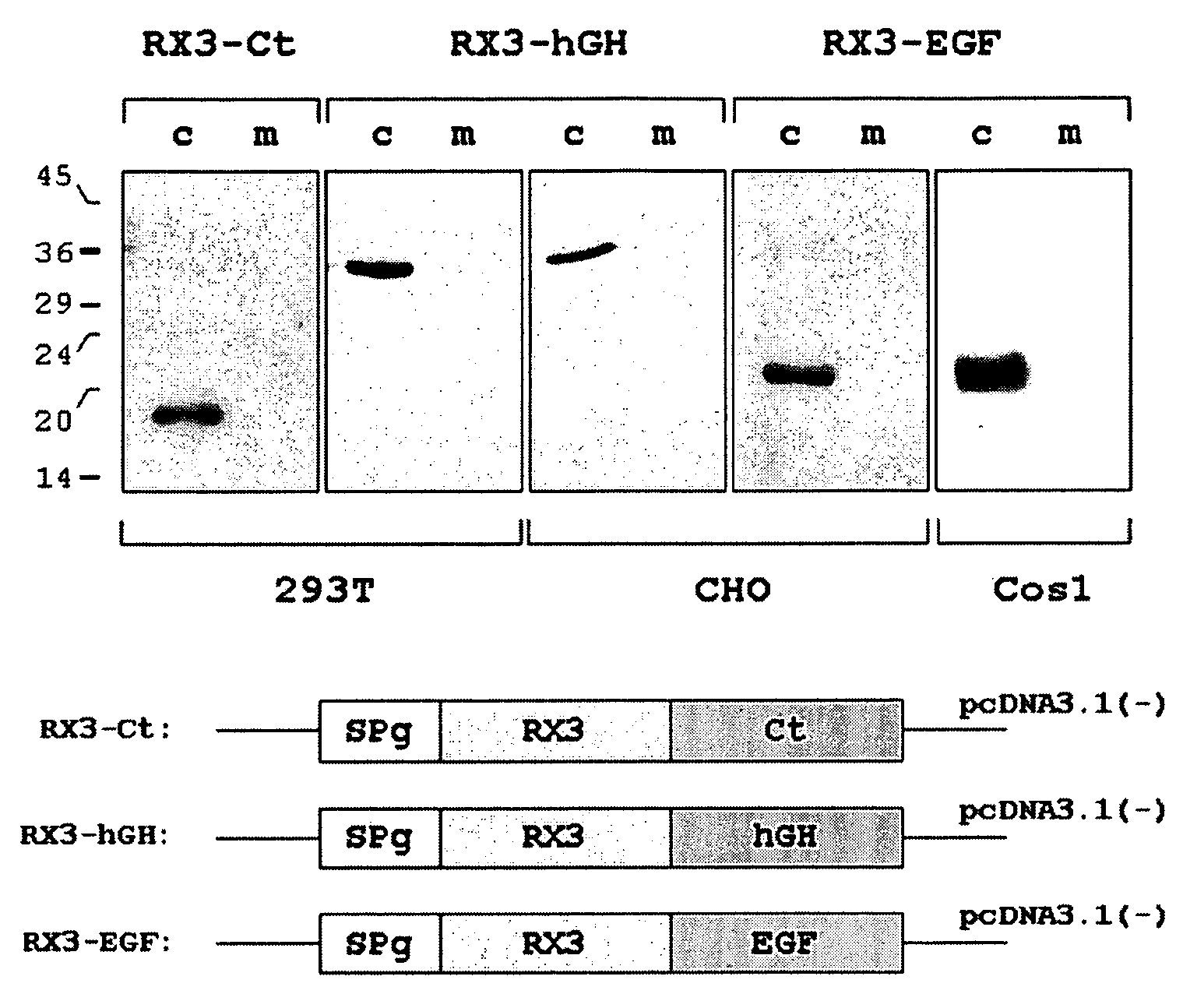
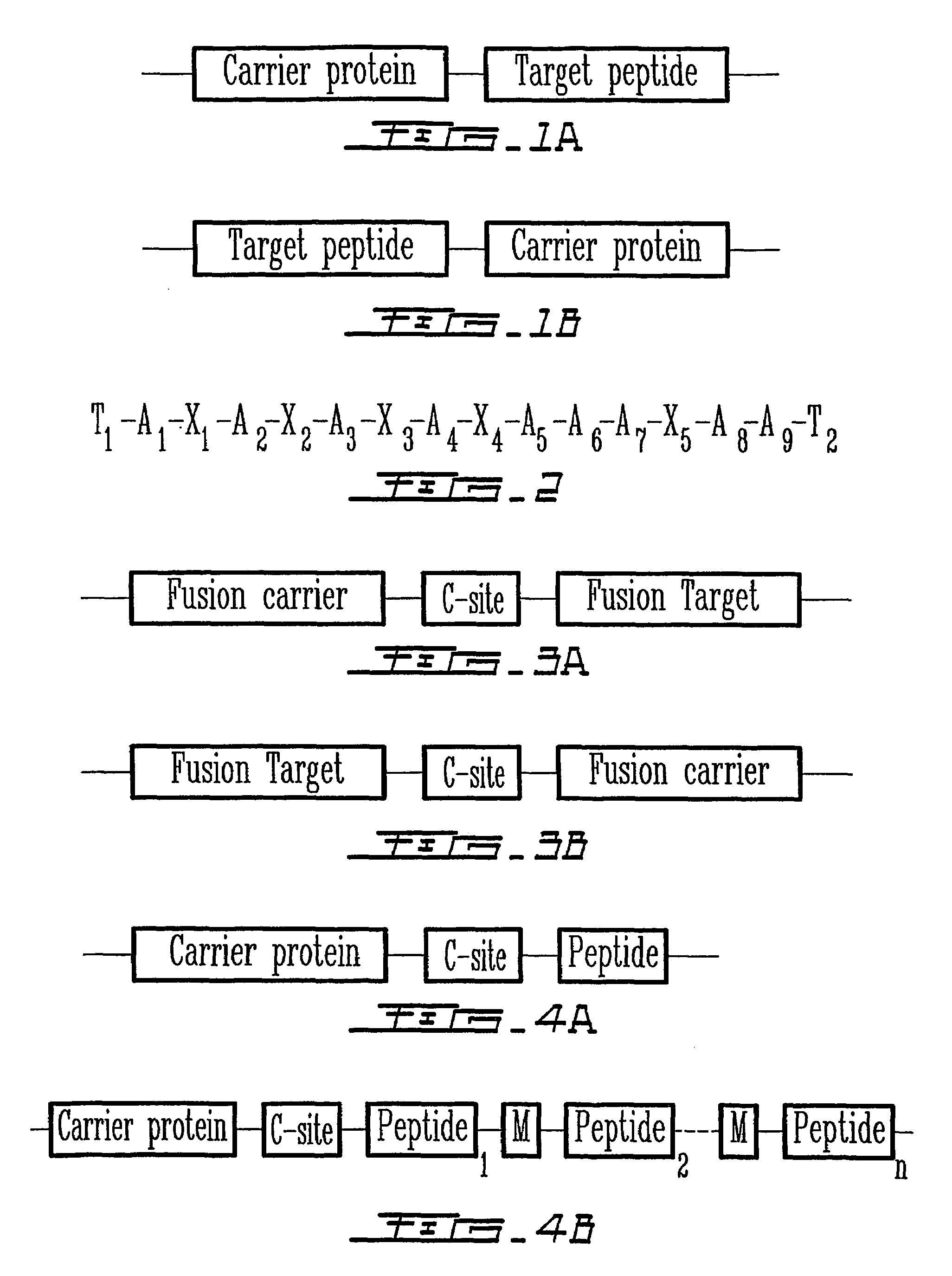
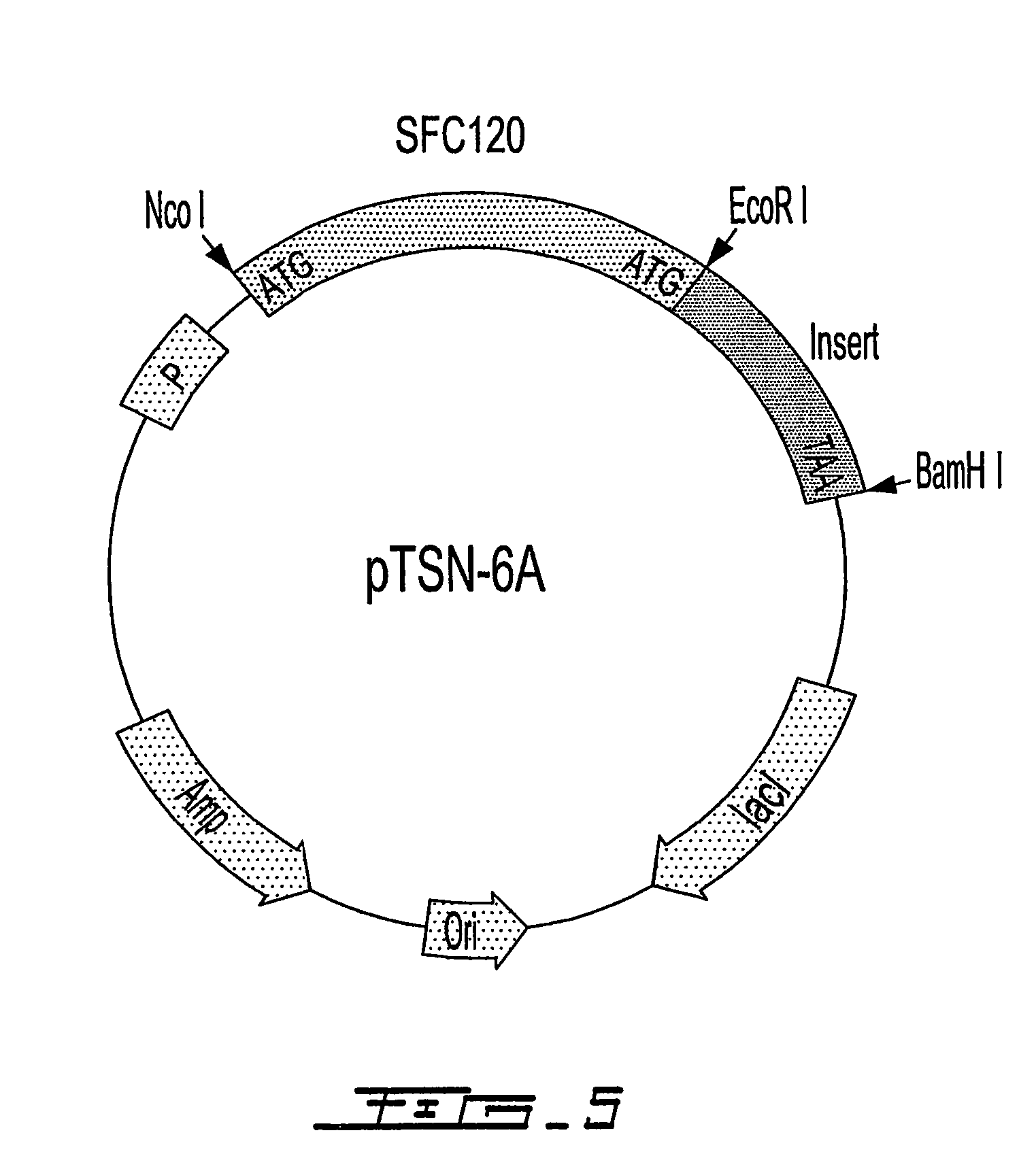
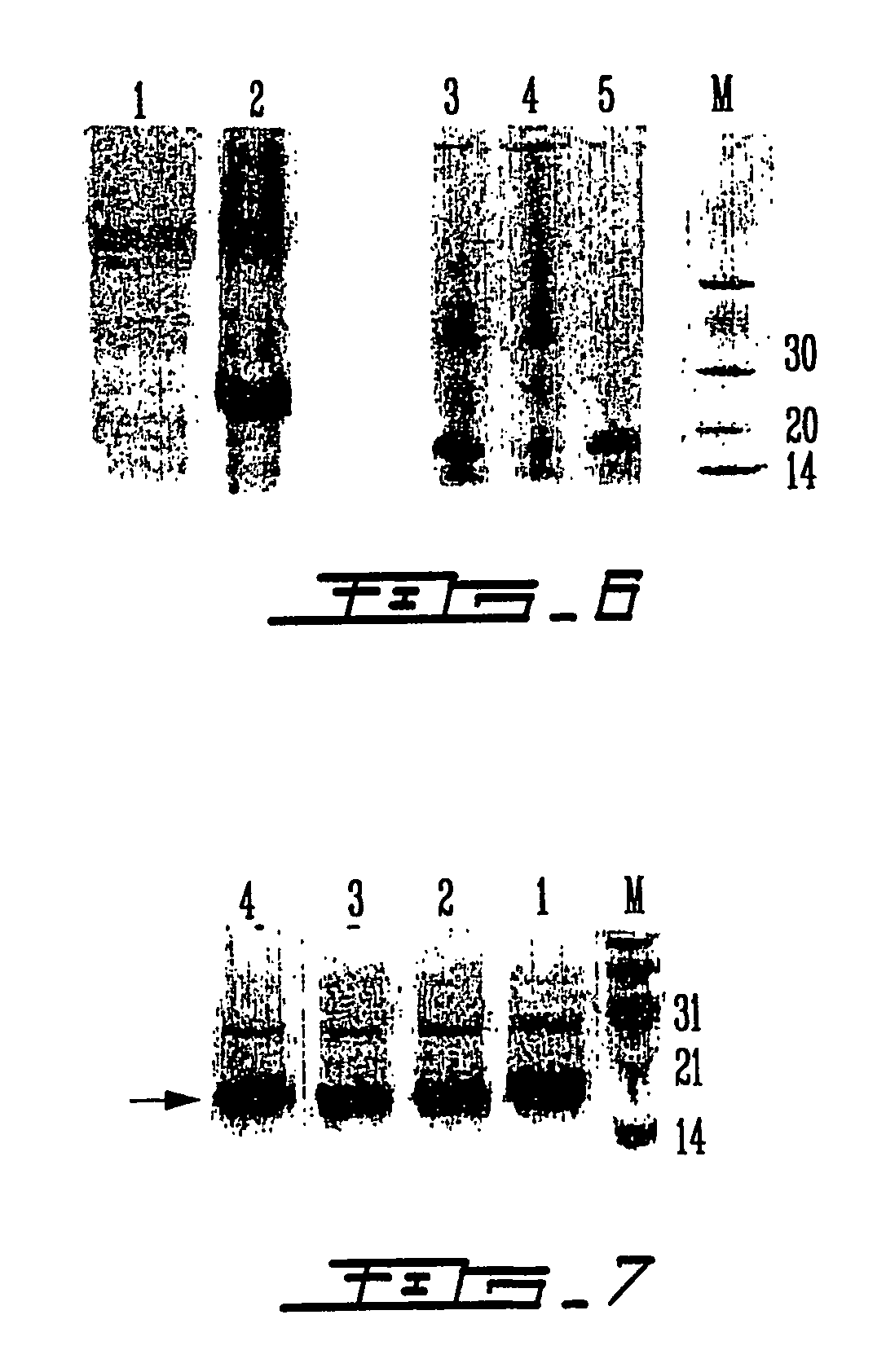
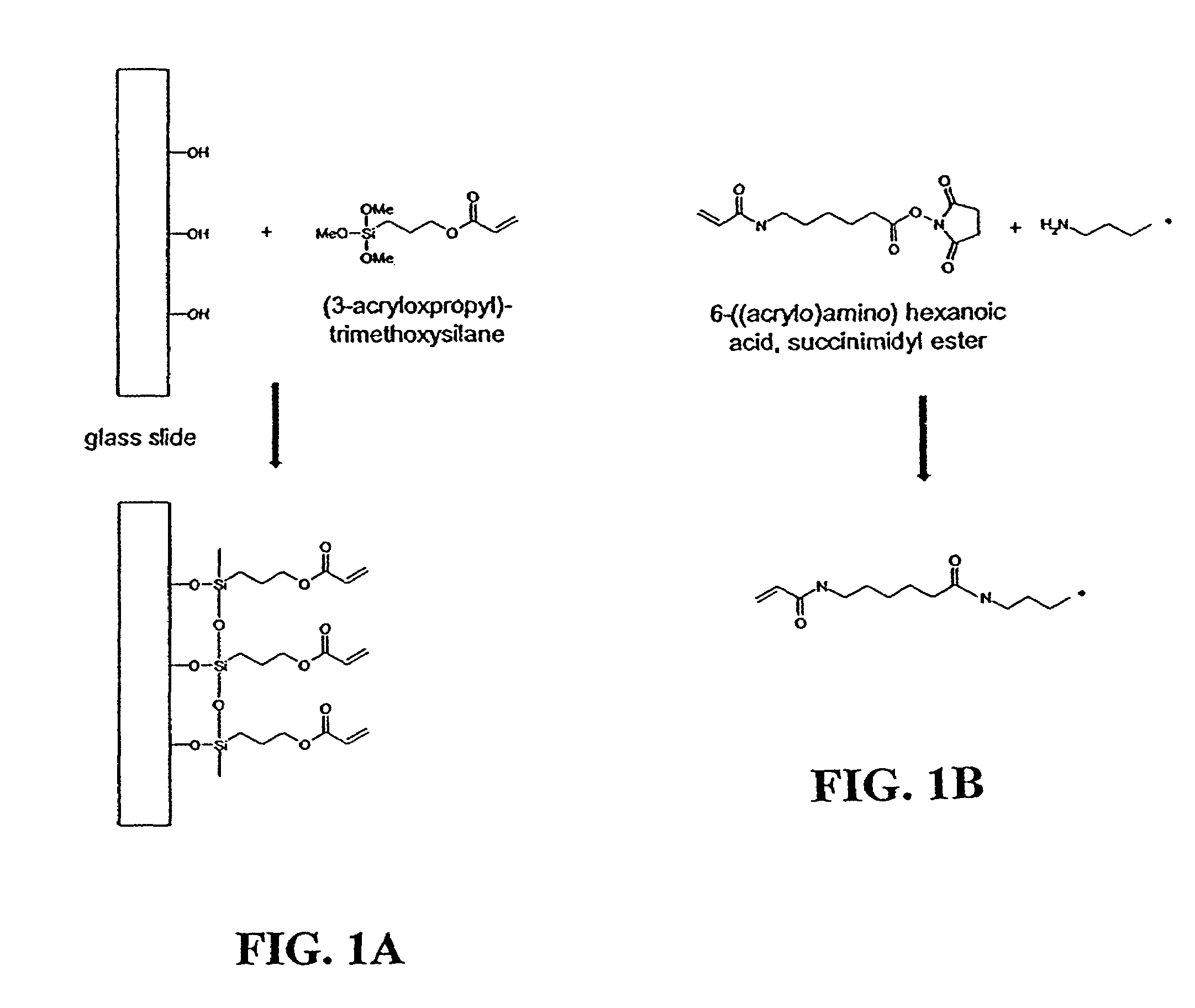
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94 results about "Protein neddylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
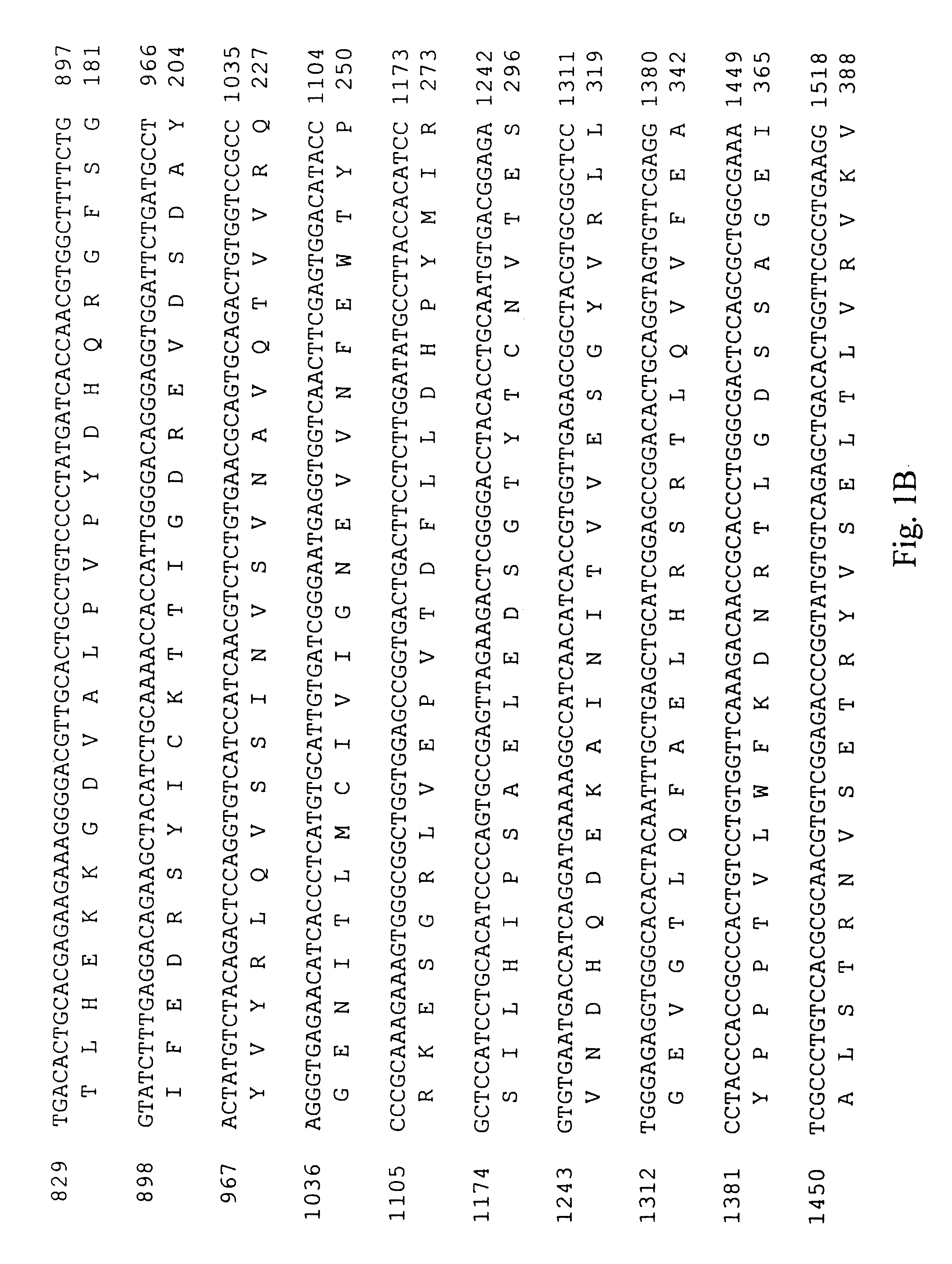
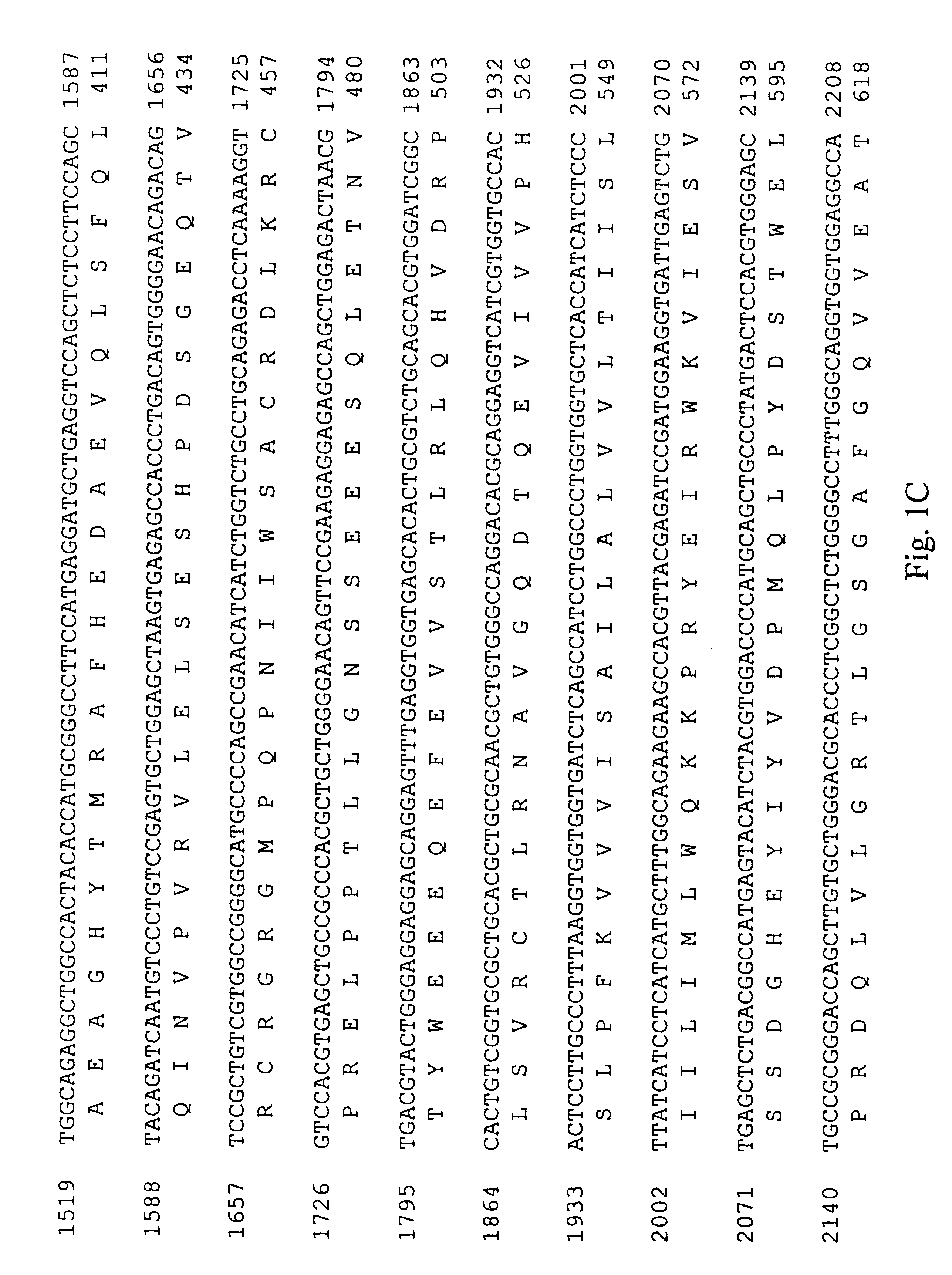
Covalent attachment of the ubiquitin-like protein NEDD8 (RUB1) to another protein. [PMID:11698580]
Biologically active dimerized and multimerized polypeptide fusions
InactiveUS6018026AImprove expression levelMass productionPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
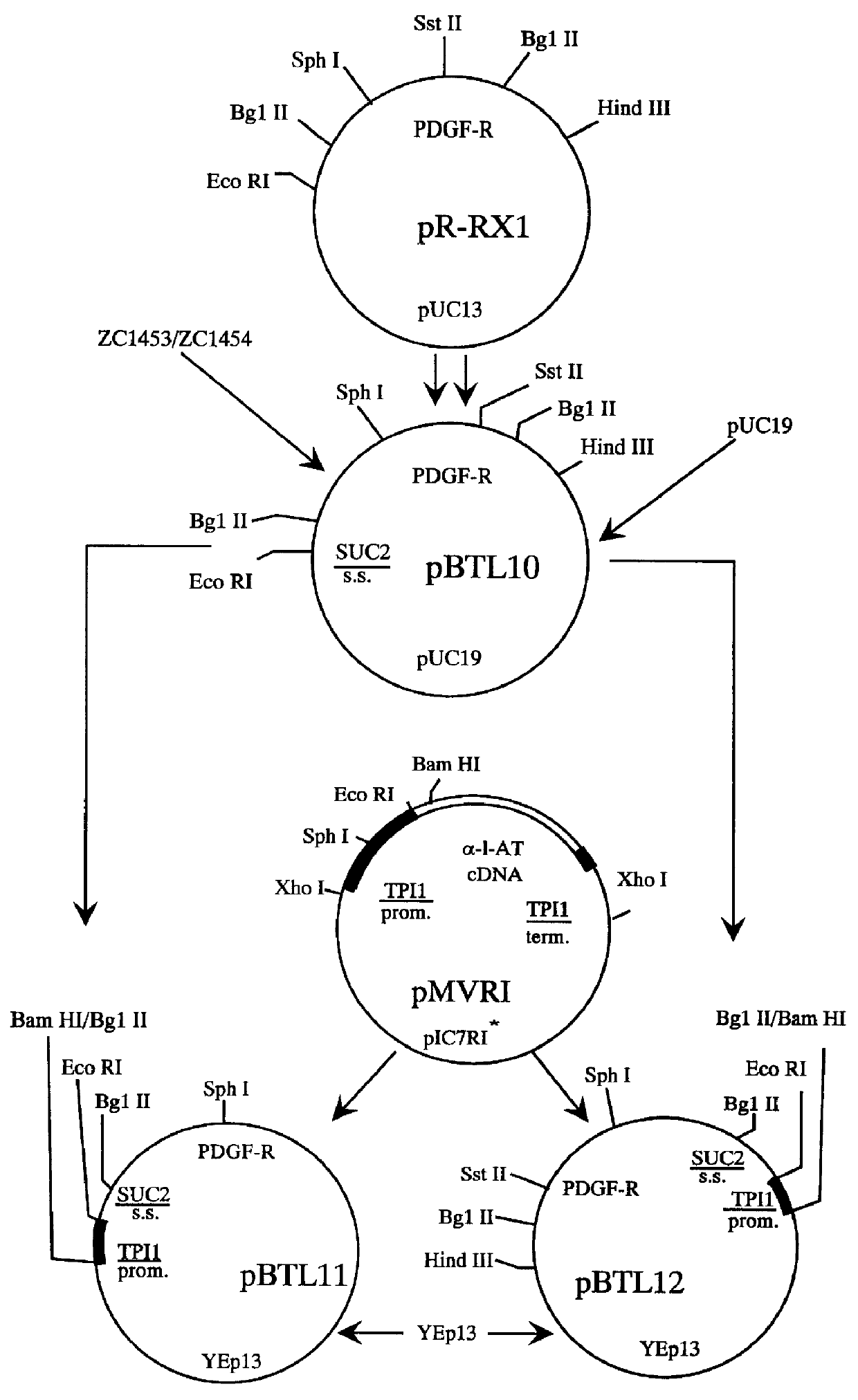
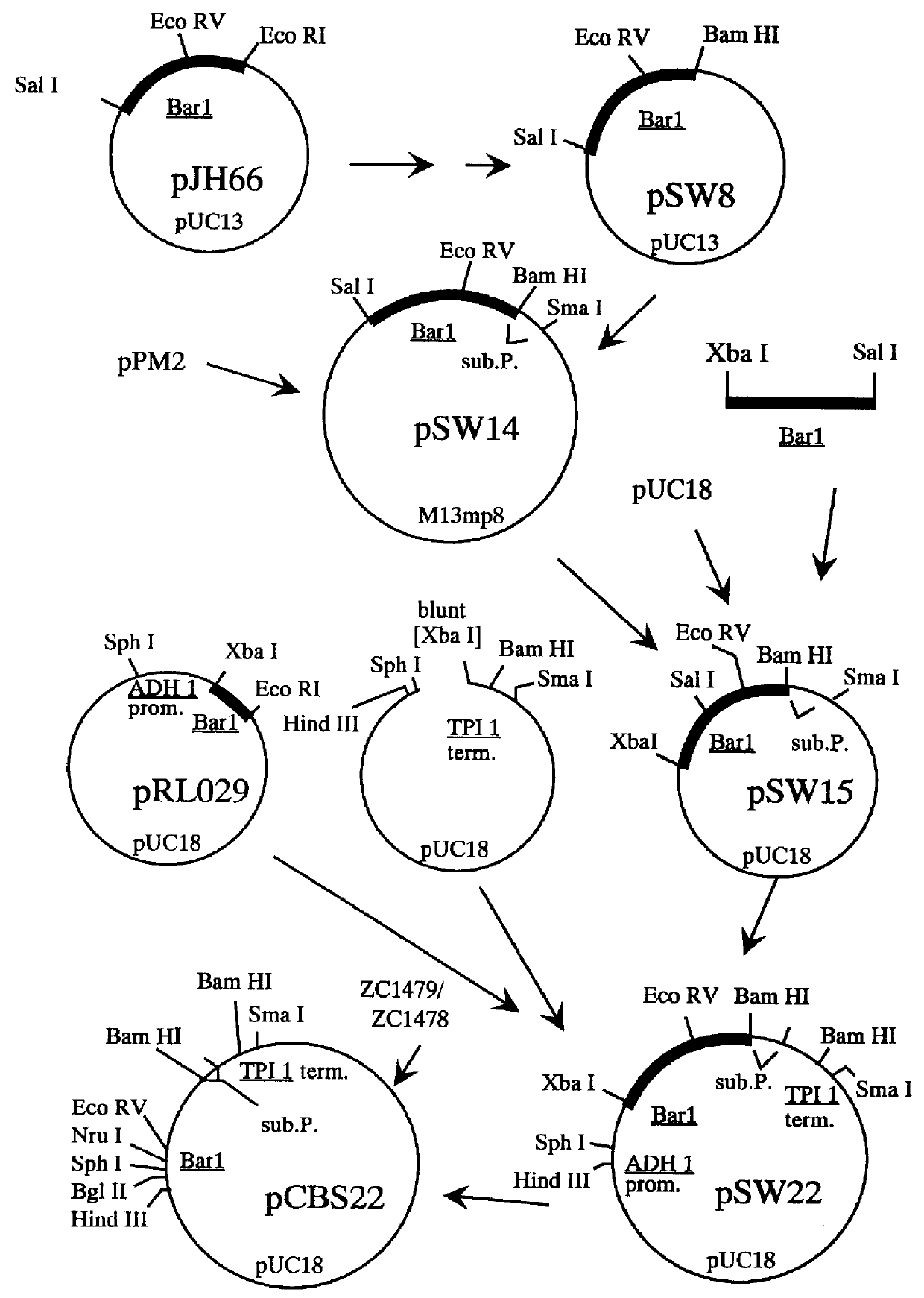
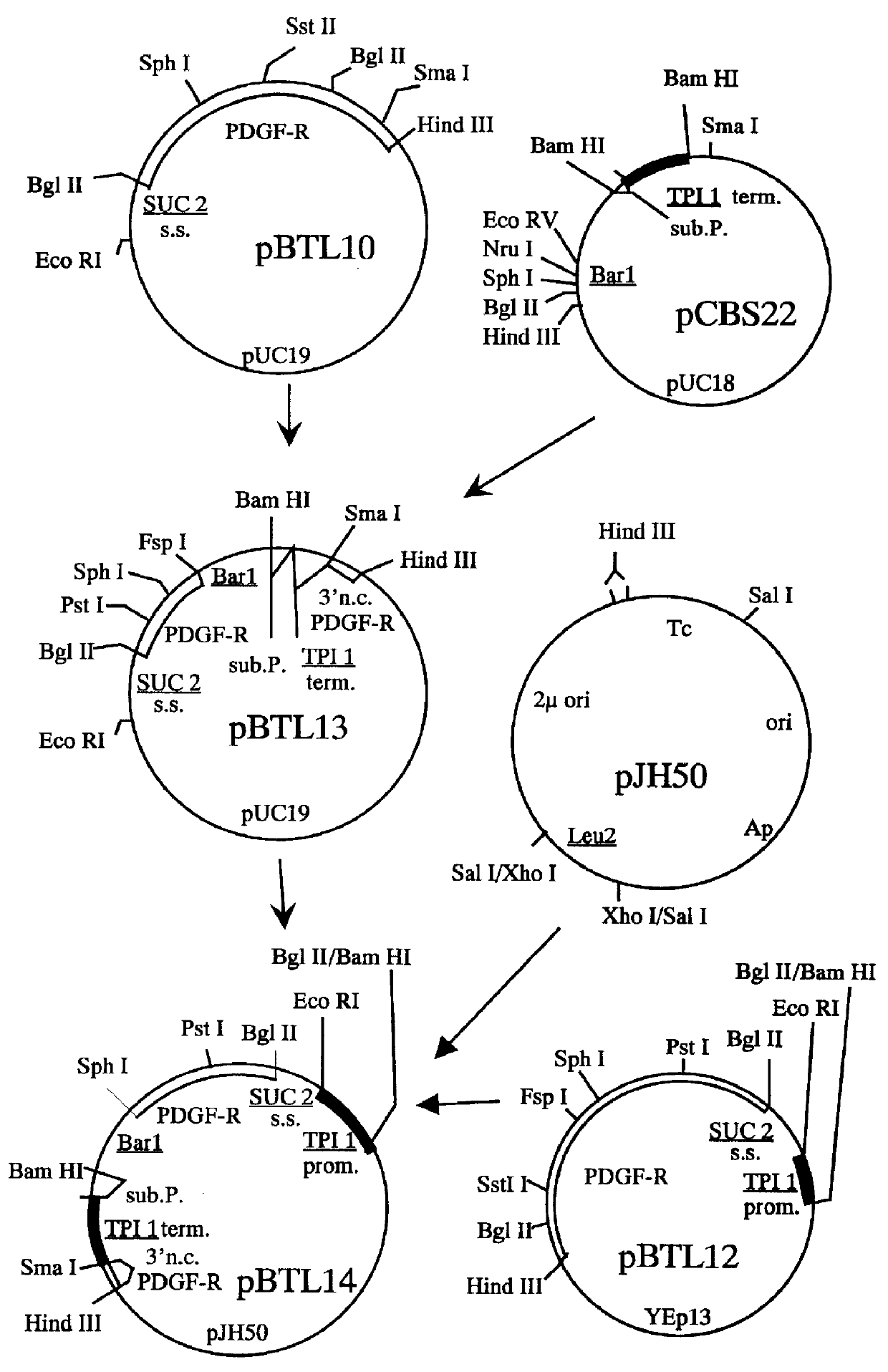
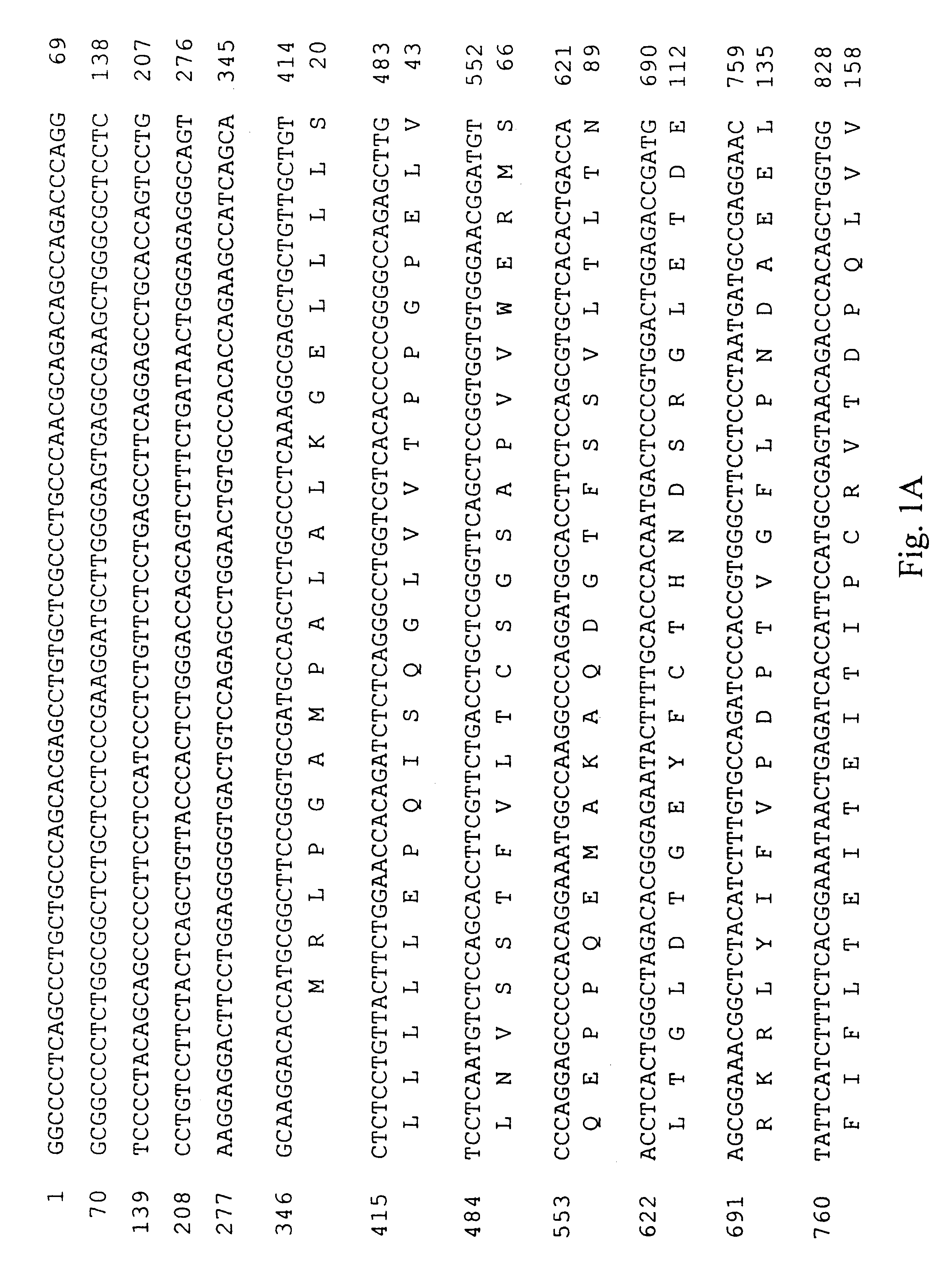
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Dimerized polypeptide fusions
InactiveUS6291646B1Mass productionImprove expression levelPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Identification and comparison of protein-protein interactions that occur in populations and identification of inhibitors of these interactors
InactiveUS6057101AEfficient screeningLess experimentally significant and specific indicationMaterial nanotechnologyFungiDiseaseBinding site
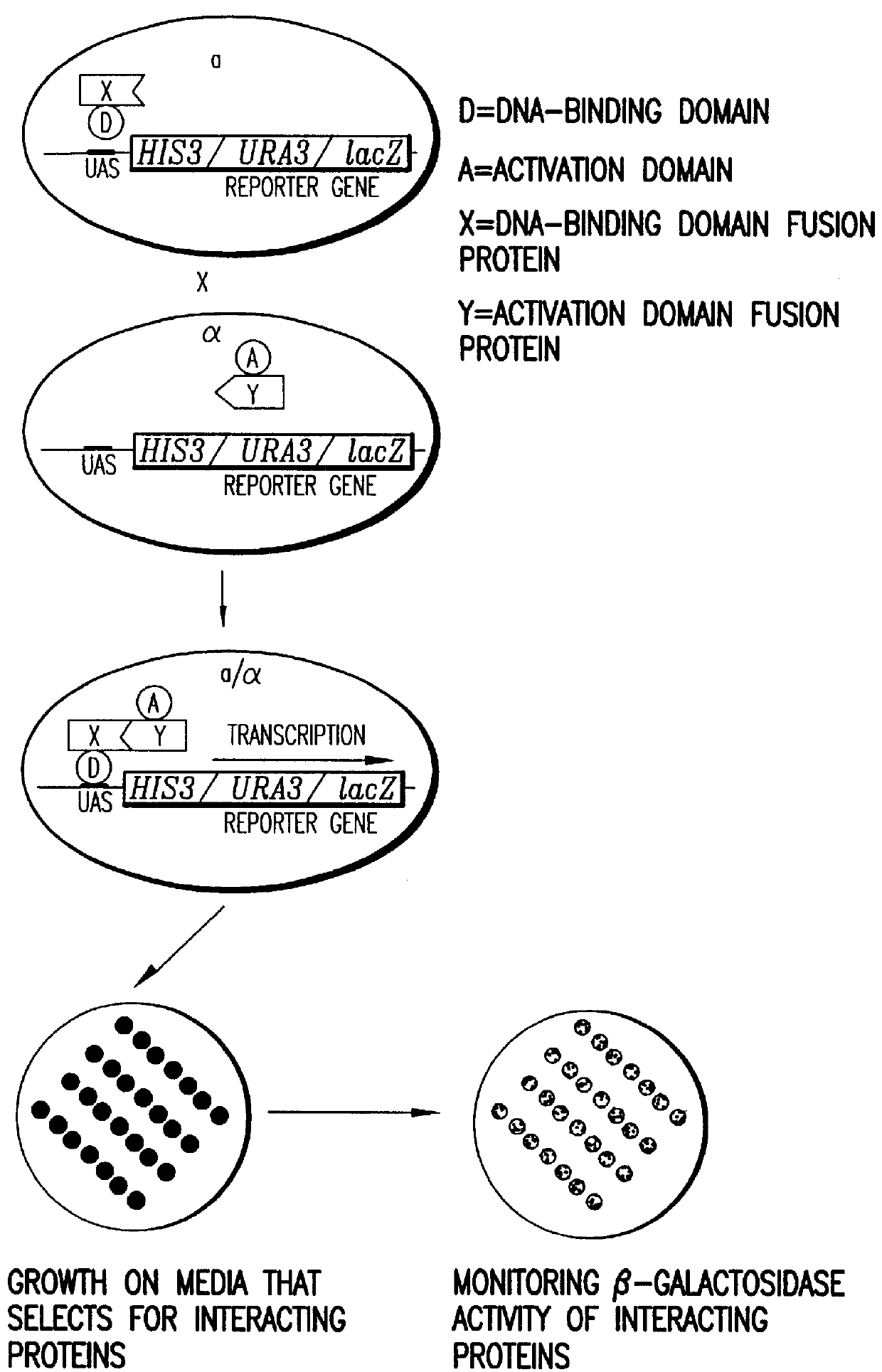
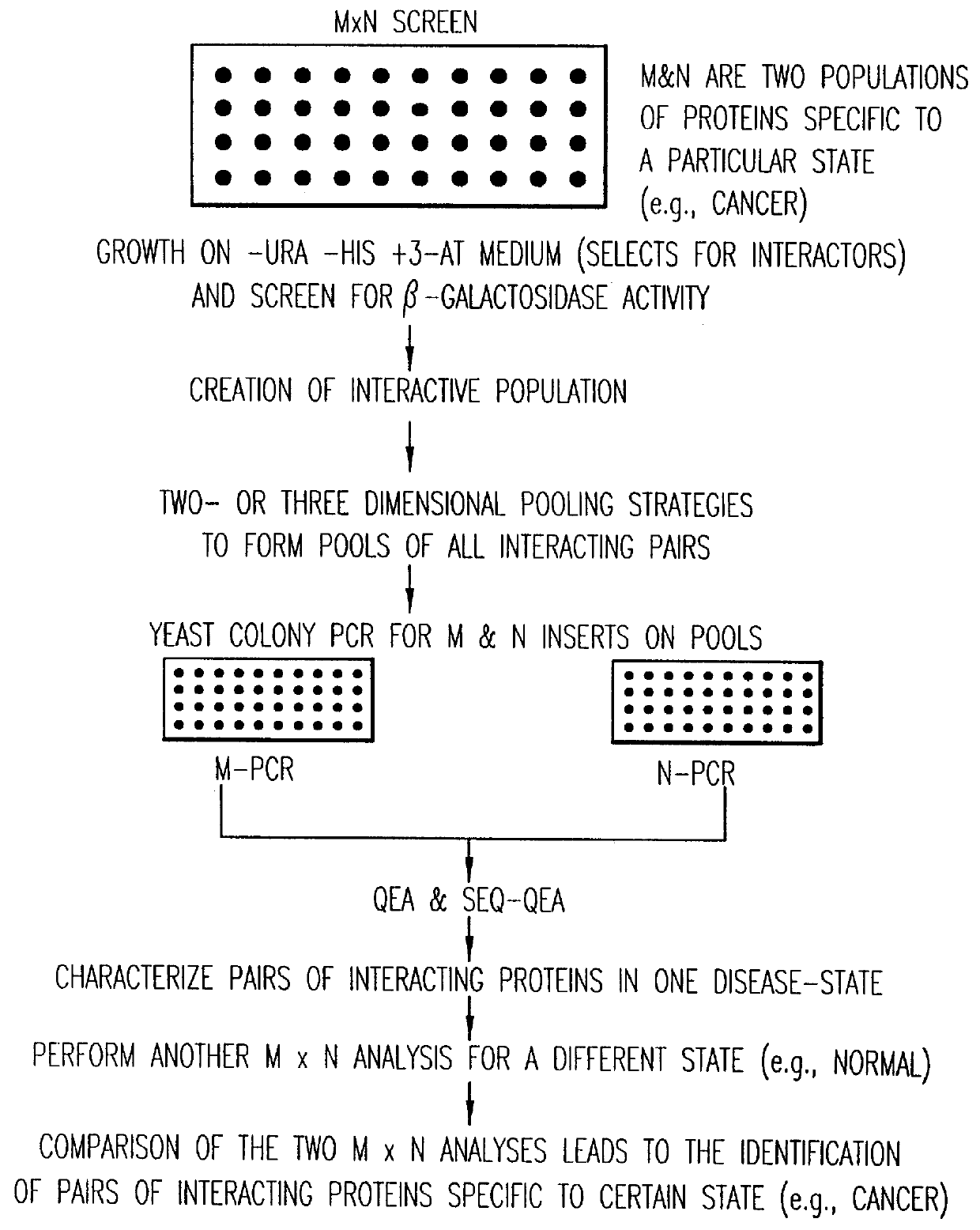
Methods are described for detecting protein-protein interactions, among two populations of proteins, each having a complexity of at least 1,000. For example, proteins are fused either to the DNA-binding domain of a transcriptional activator or to the activation domain of a transcriptional activator. Two yeast strains, of the opposite mating type and carrying one type each of the fusion proteins are mated together. Productive interactions between the two halves due to protein-protein interactions lead to the reconstitution of the transcriptional activator, which in turn leads to the activation of a reporter gene containing a binding site for the DNA-binding domain. This analysis can be carried out for two or more populations of proteins. The differences in the genes encoding the proteins involved in the protein-protein interactions are characterized, thus leading to the identification of specific protein-protein interactions, and the genes encoding the interacting proteins, relevant to a particular tissue, stage or disease. Furthermore, inhibitors that interfere with these protein-protein interactions are identified by their ability to inactivate a reporter gene. The screening for such inhibitors can be in a multiplexed format where a set of inhibitors will be screened against a library of interactors. Further, information-processing methods and systems are described. These methods and systems provide for identification of the genes coding for detected interacting proteins, for assembling a unified database of protein-protein interaction data, and for processing this unified database to obtain protein interaction domain and protein pathway information.
Owner:CURAGEN CORP
Sensitive, multiplexed diagnostic assays for protein analysis
Disclosed herein are methods for detecting multiple compounds in a sample, involving: (a) contacting the sample with a mixture of binding reagents, the binding reagents being nucleic acid-protein fusions, each having (i) a protein portion which is known to specifically bind to one of the compounds and (ii) a nucleic acid portion which encodes the protein portion and which includes a unique identification tag; (b) allowing the protein portions of the binding reagents and the compounds to form complexes; (c) capturing the binding reagent-compound complexes; (d) amplifying the nucleic acid portions of the complexed binding reagents; and (e) detecting the unique identification tag of each of the amplified nucleic acids, thereby detecting the corresponding compounds in the sample. Also disclosed herein are kits for carrying out such methods.
Owner:BRISTOL MYERS SQUIBB CO
DNA-protein fusions and uses thereof
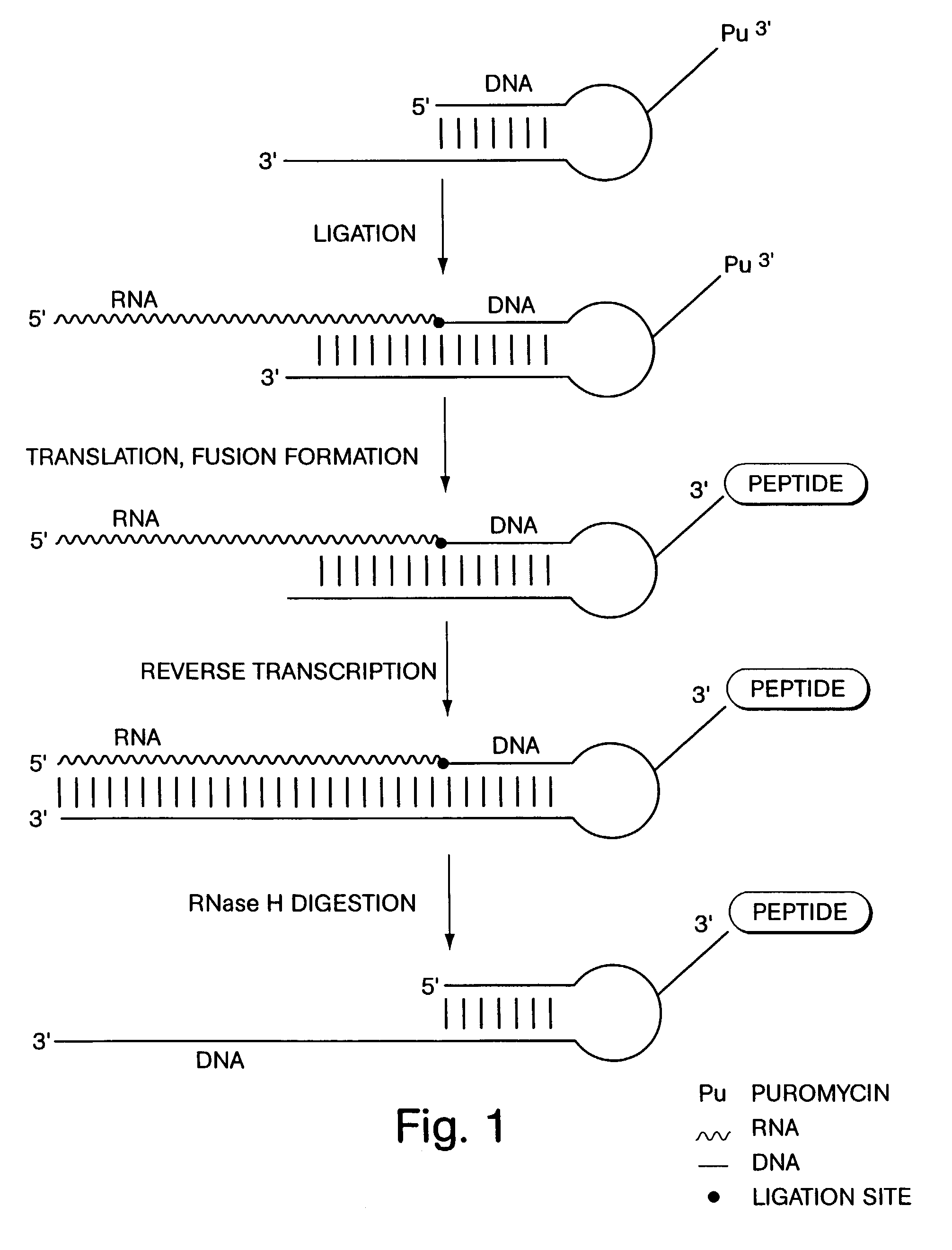
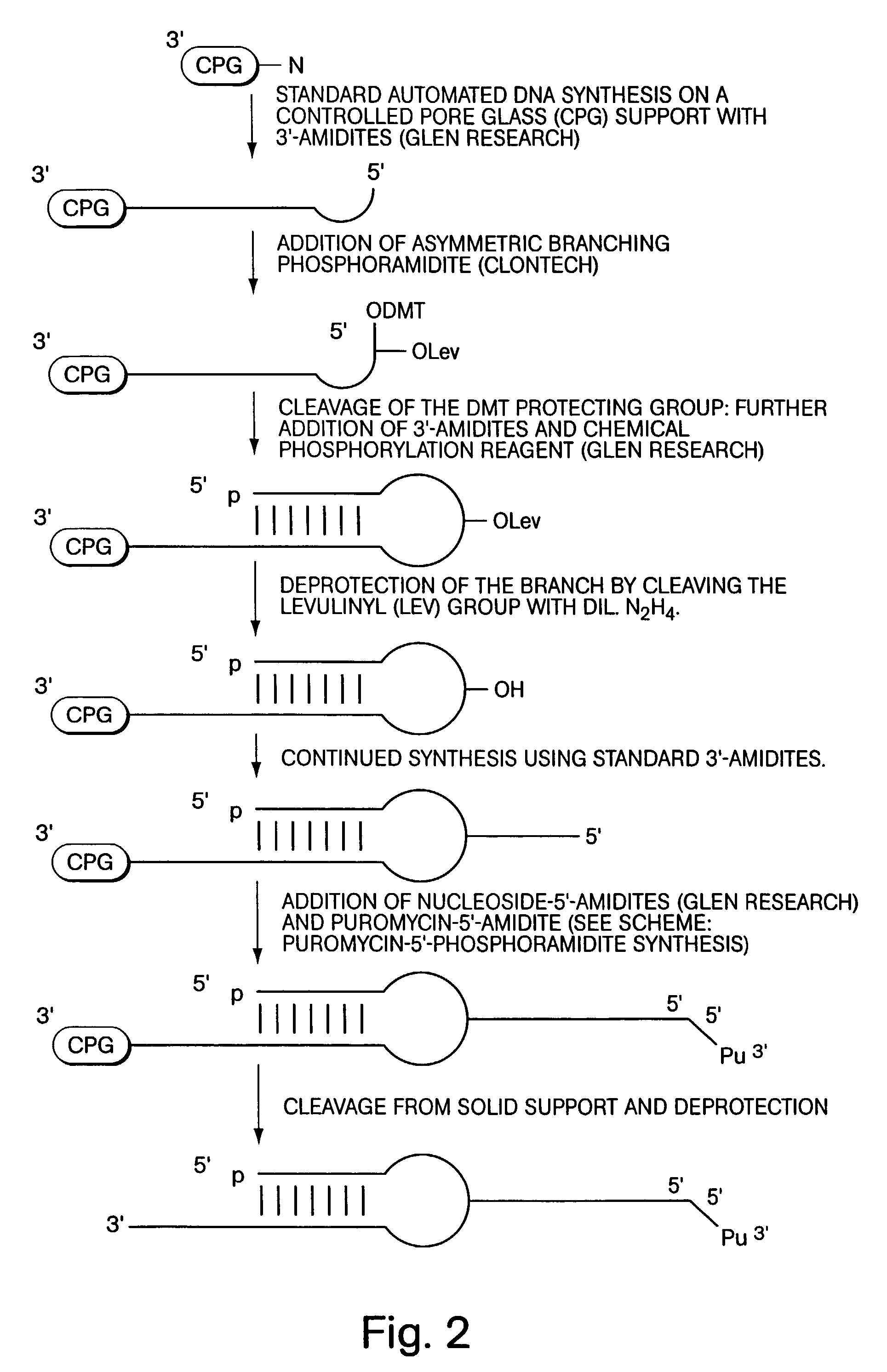
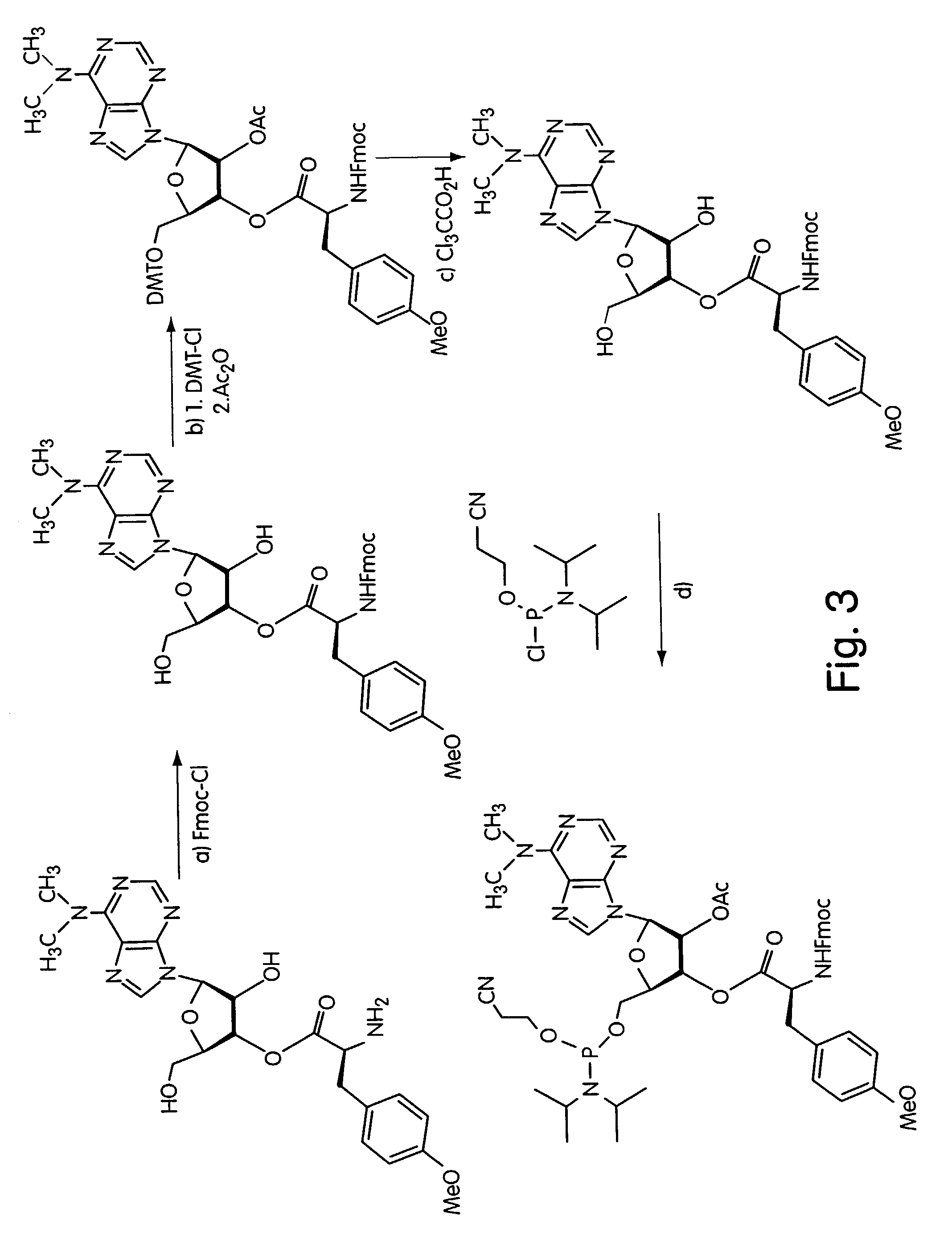
InactiveUS7195880B2Easy to handleGood chemical stabilityBioreactor/fermenter combinationsPeptide librariesProtein neddylationDna protein
Owner:BRISTOL MYERS SQUIBB CO
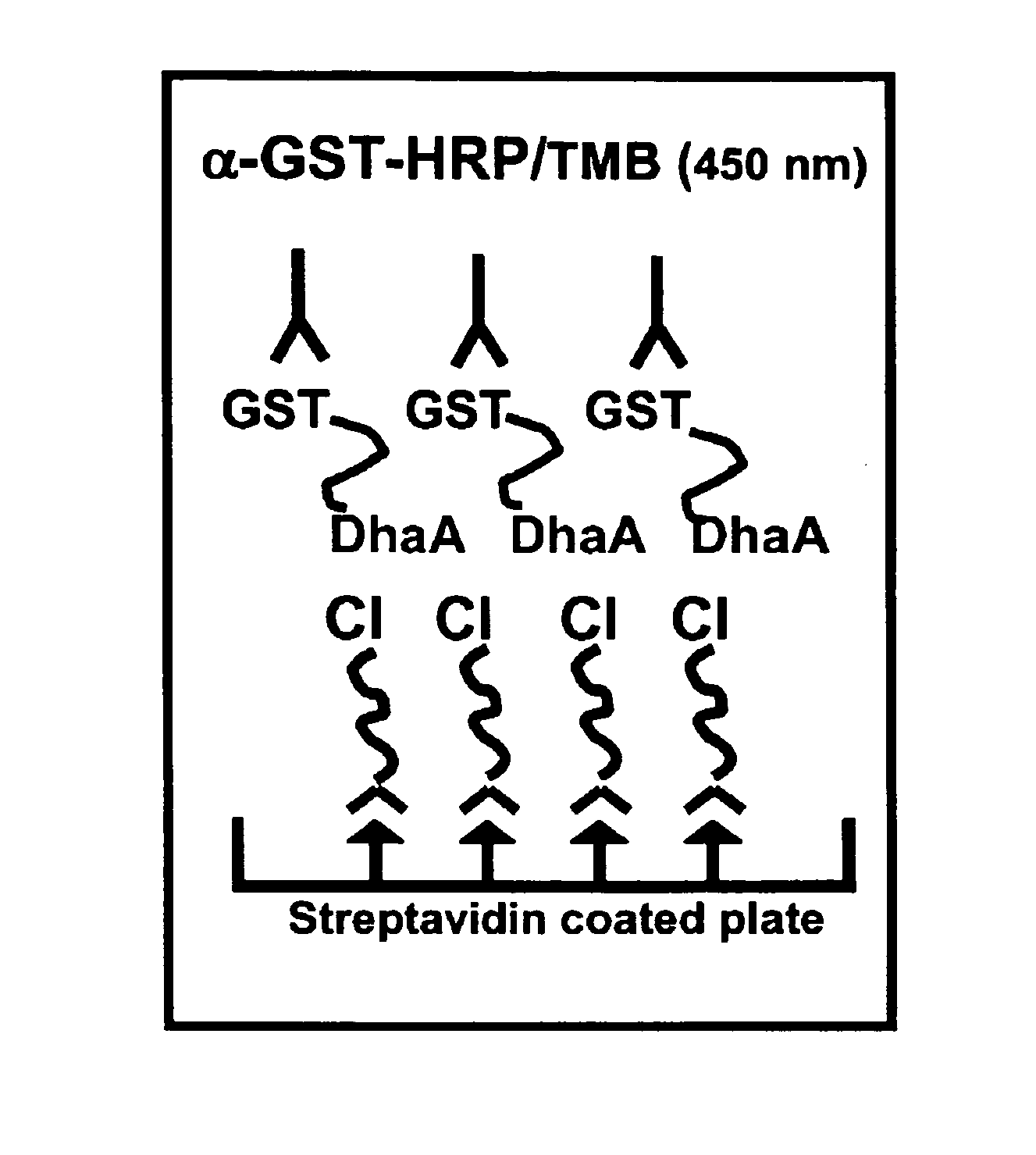
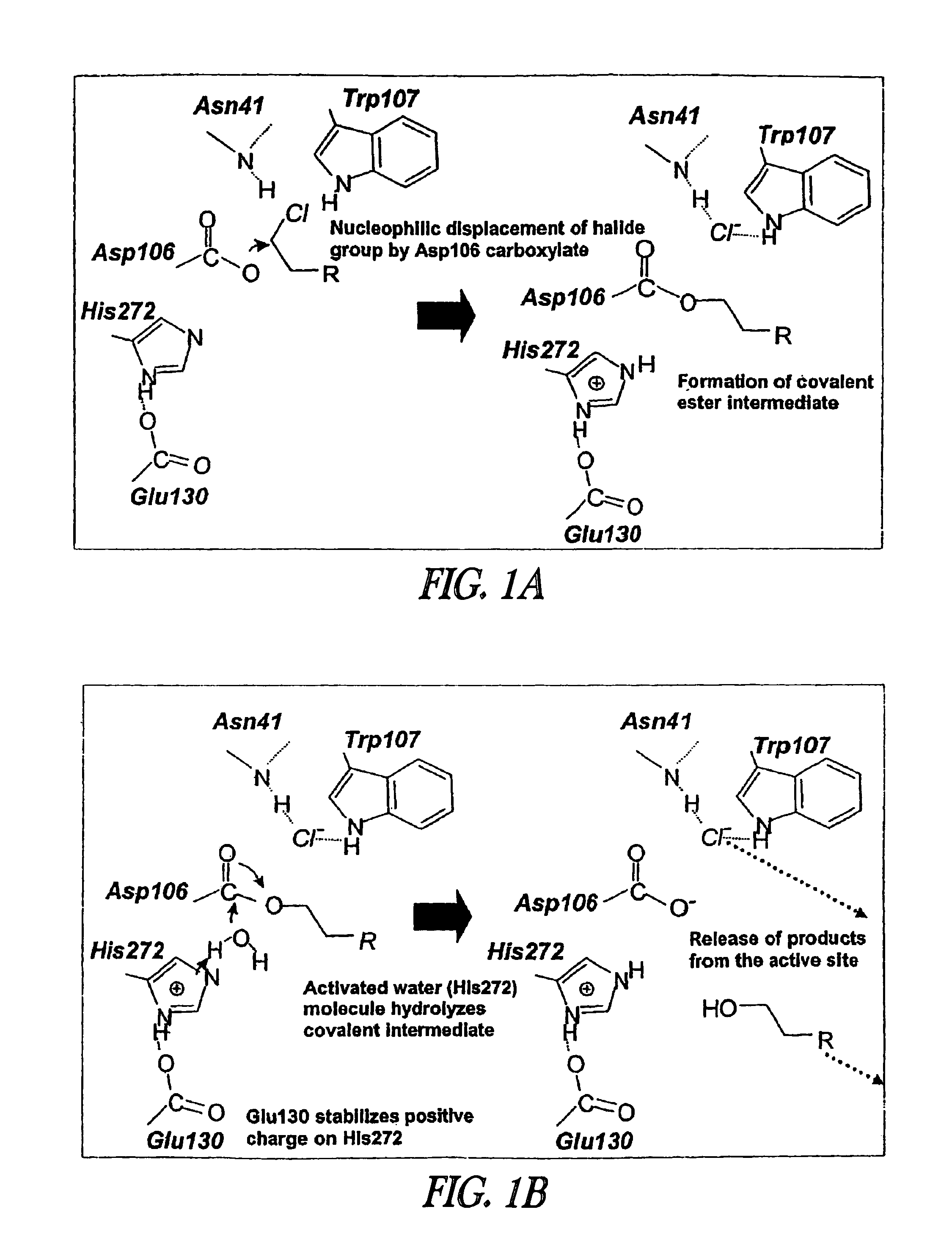
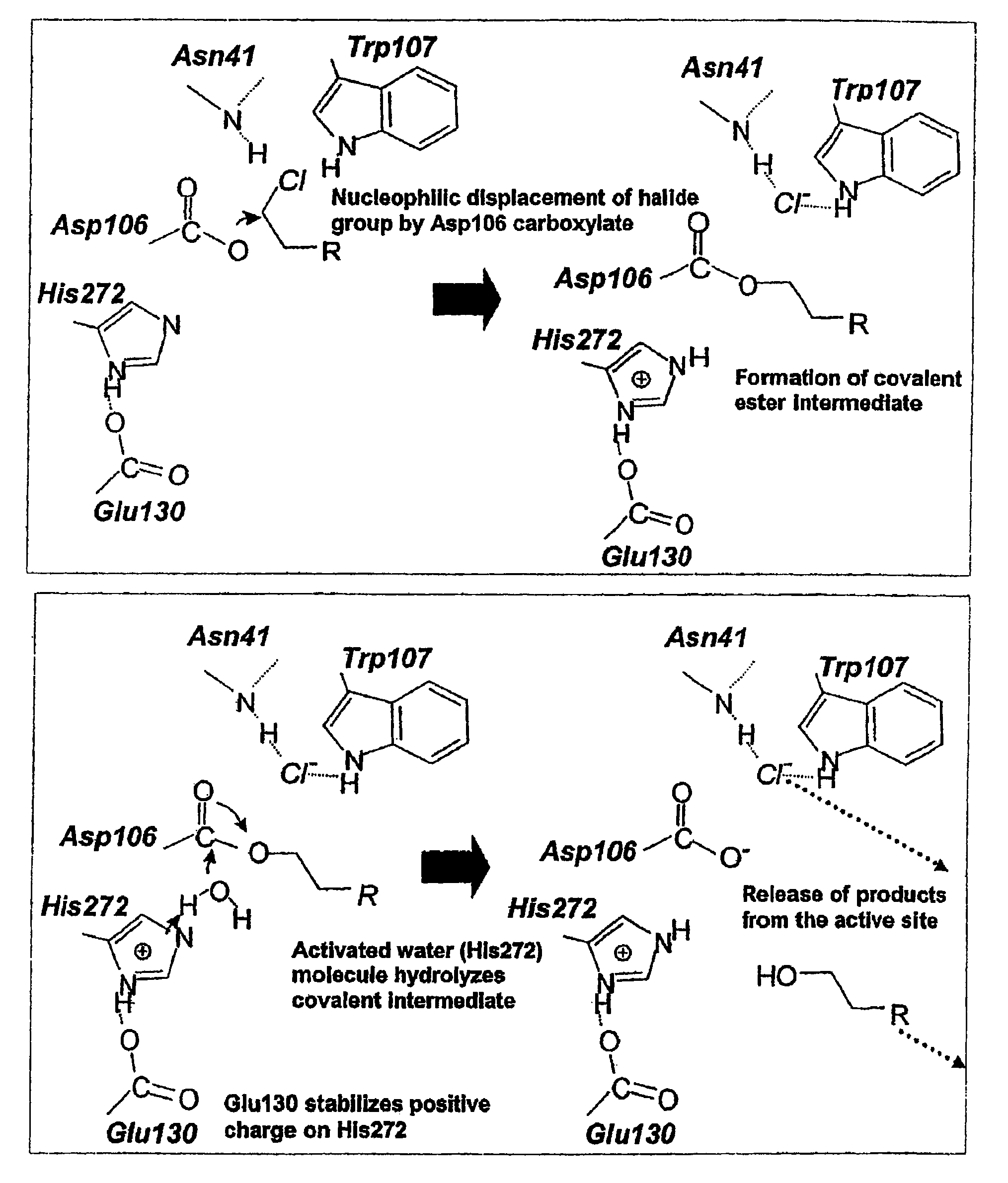
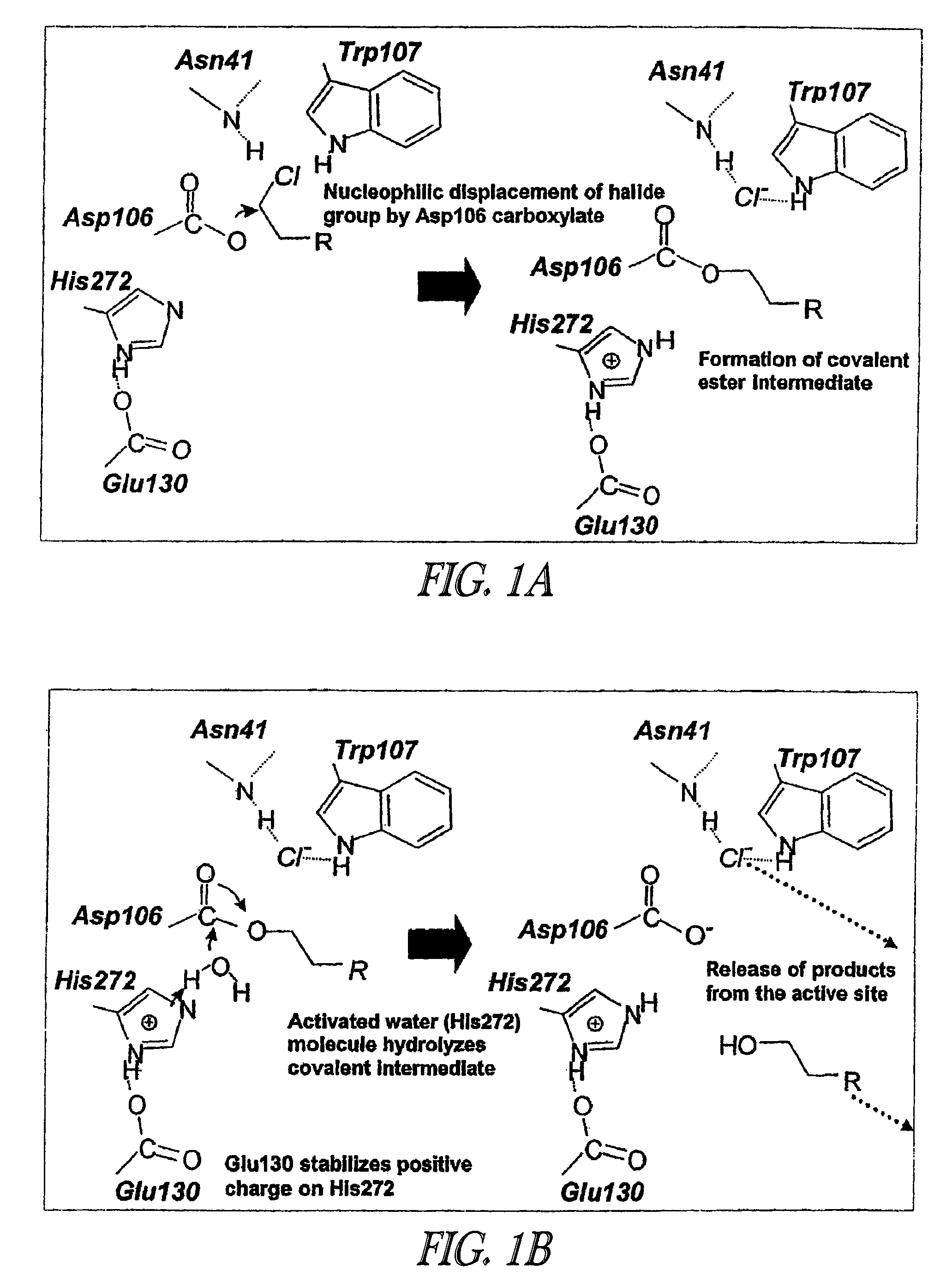
Method of immobilizing a protein or molecule via a mutant dehalogenase that is bound to an immobilized dehalogenase substrate and linked directly or indirectly to the protein or molecule
ActiveUS7429472B2Rapidly and efficiently loaded into and washedStable rateMaterial nanotechnologyPeptide/protein ingredientsAmino acid substitutionWild type
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
Covalent tethering of functional groups to proteins and substrates therefor
ActiveUS7425436B2Rapidly and efficiently loaded into and washedStable rateMethine/polymethine dyesBacteriaTetheringAmino acid substitution
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
Covalent tethering of functional groups to proteins
ActiveUS7238842B2Rapidly and efficiently loaded intoRapidly and efficiently loaded into and washed outCarbamic acid derivatives preparationHydrolasesTetheringWild type
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA
Solid-phase immobilization of proteins and peptides
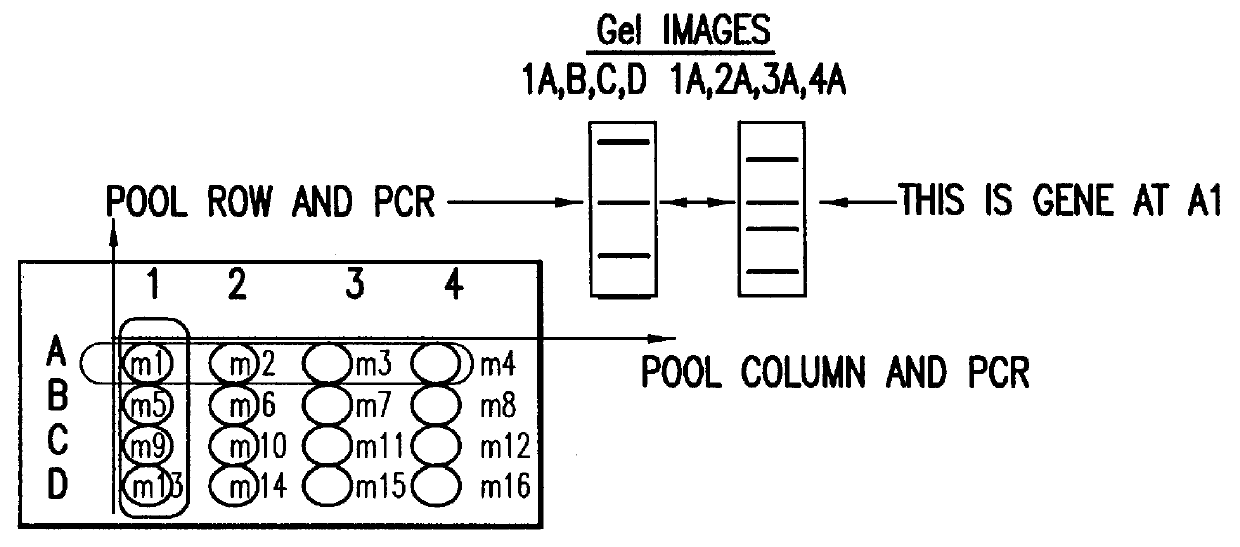
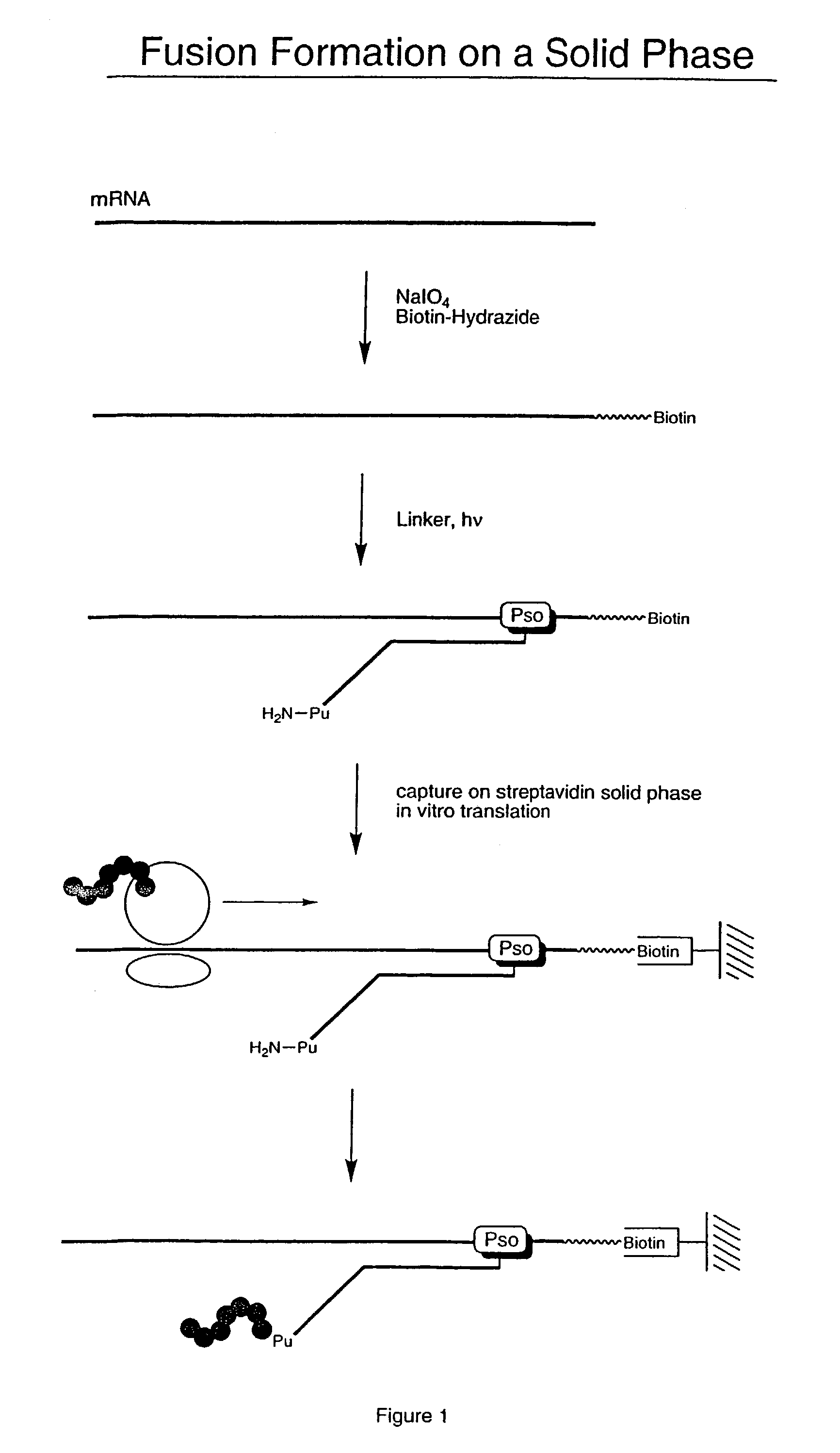
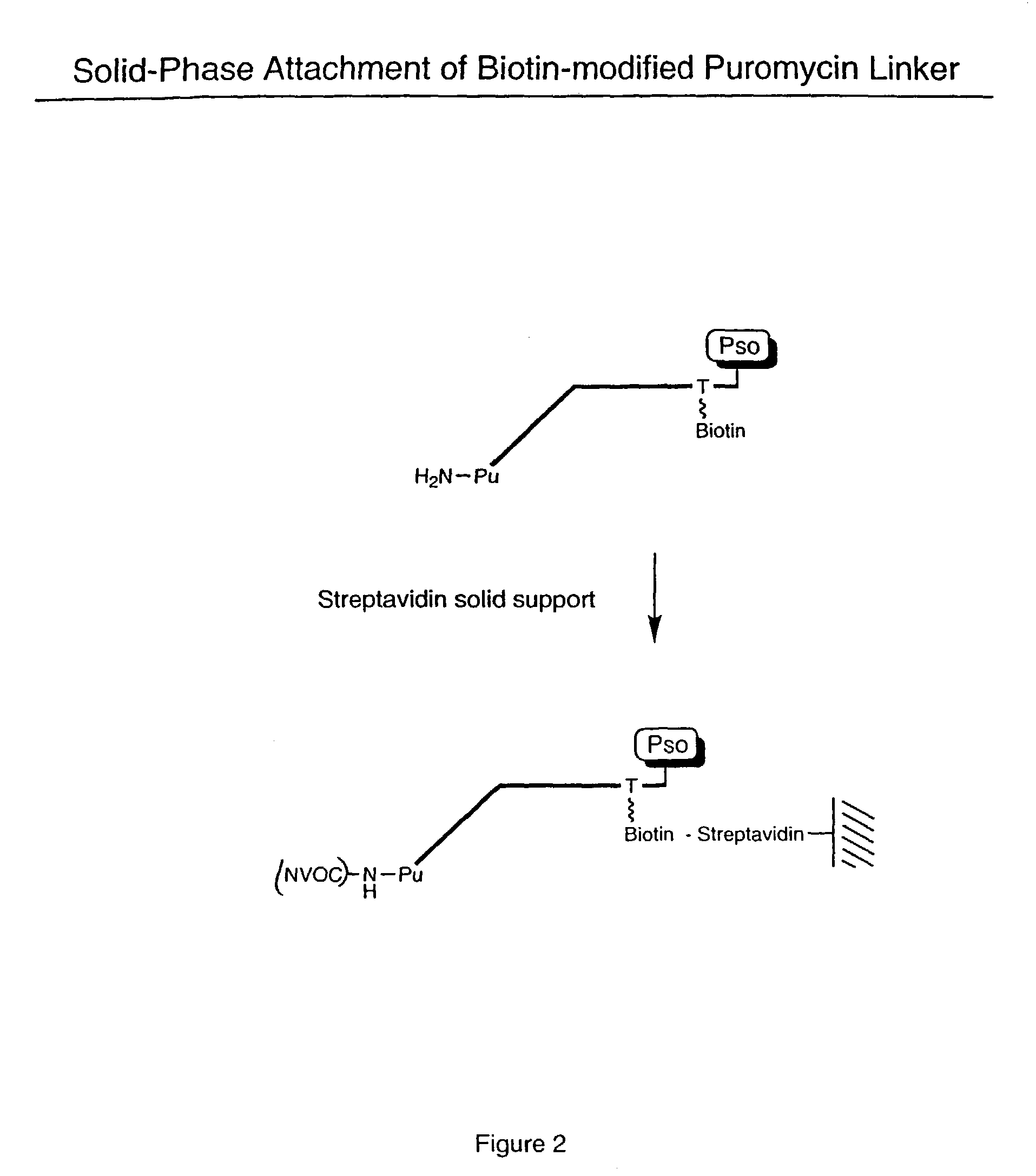
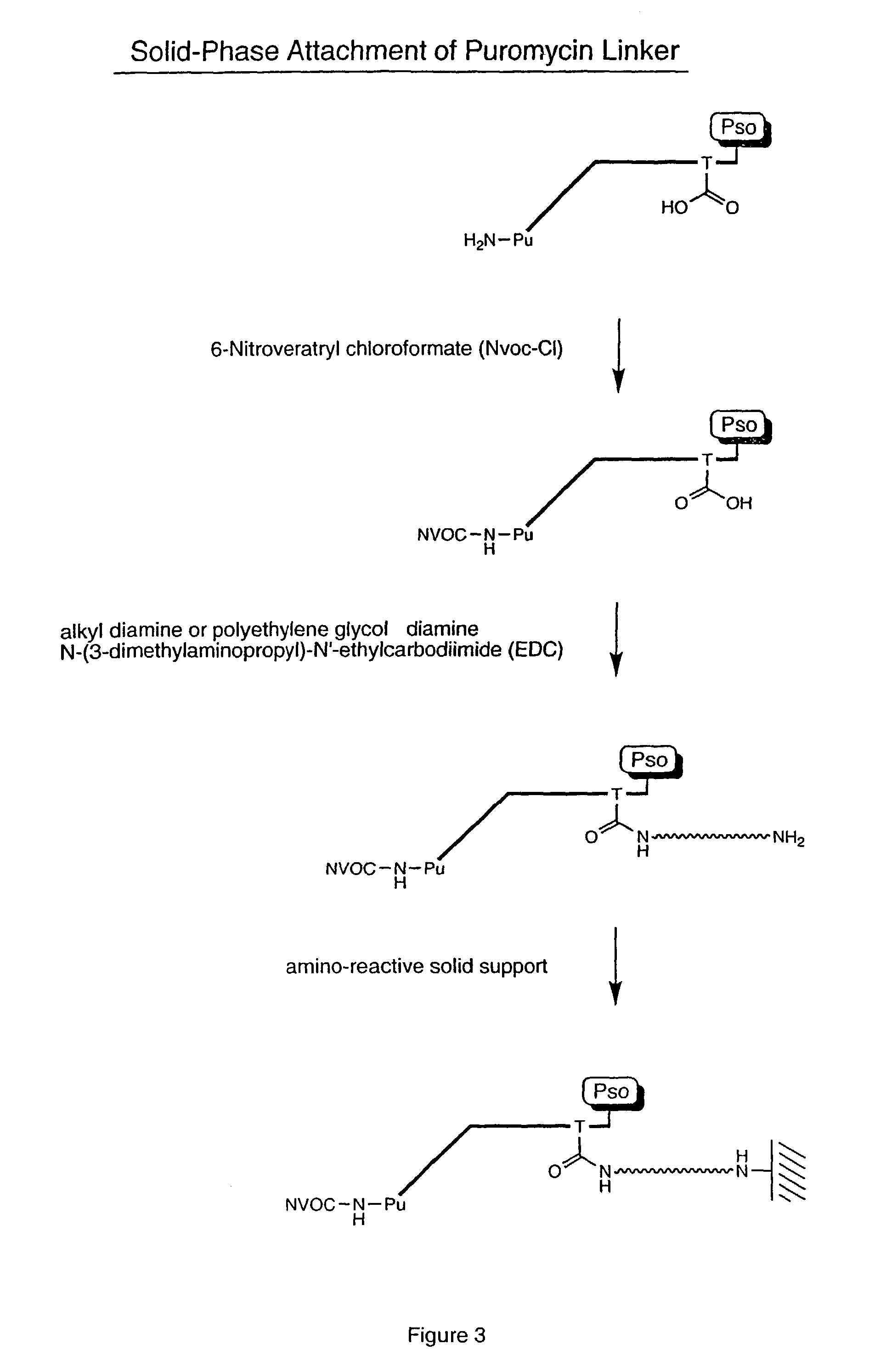
ActiveUS7125669B2Reduce solubilityNot easily purifiedBioreactor/fermenter combinationsBiological substance pretreatmentsRna proteinProtein neddylation
Disclosed herein are methods for immobilizing a peptide or protein on a solid support. The method generally includes the following steps: (a) providing one or more templates attached to a solid support, wherein the one or more templates include (i) an RNA encoding a peptide and (ii) a peptide acceptor-linker linked to the RNA; and (b) subjecting the one or more templates to conditions that support translation and attachment of said peptide to said peptide acceptor, thereby synthesizing the one or more peptides on the solid support. Also disclosed herein are solid supports having at least one RNA-protein fusion component immobilized thereon, methods for generating protein arrays, and methods for screening molecules using these arrays.
Owner:BRISTOL MYERS SQUIBB CO
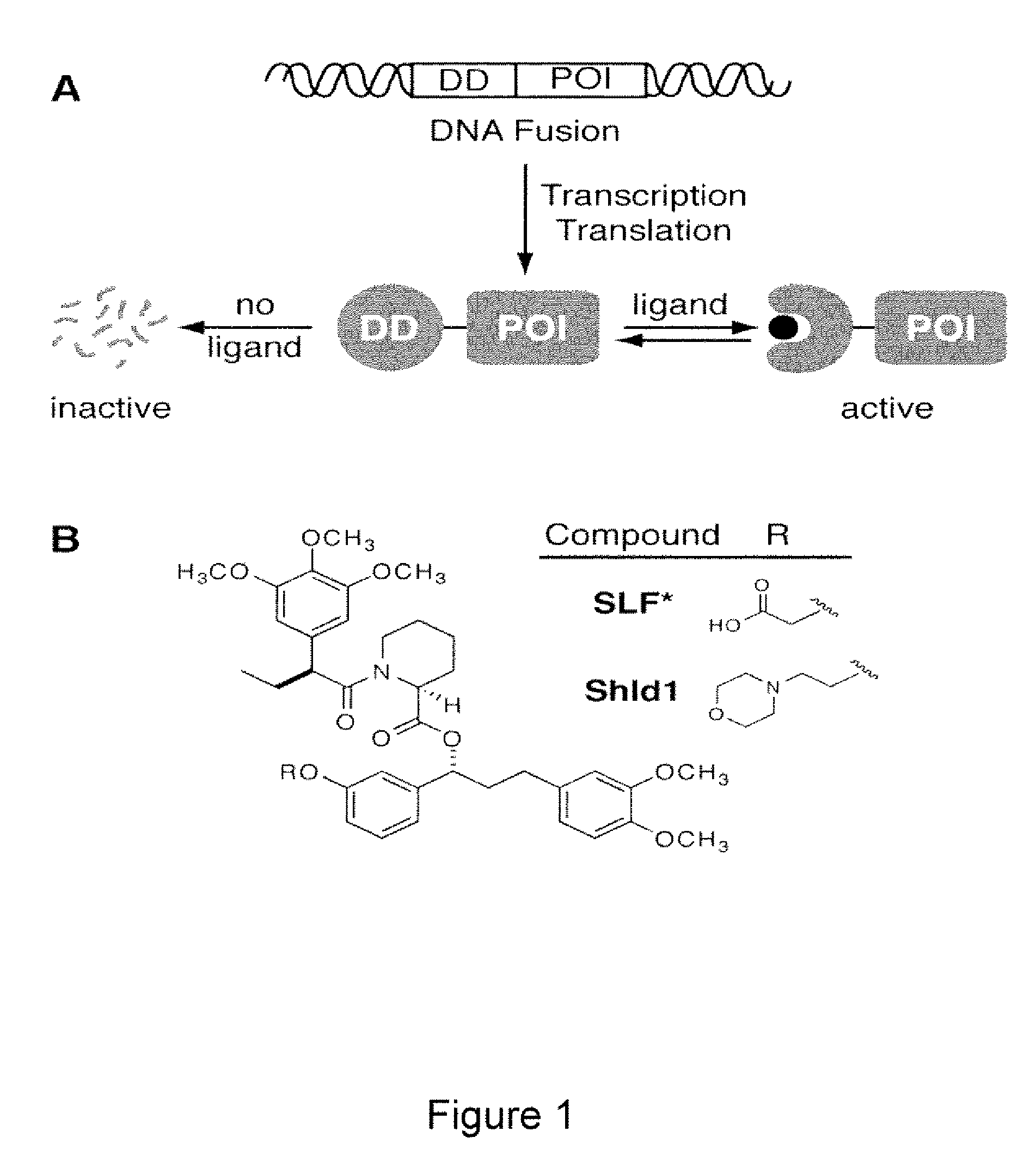
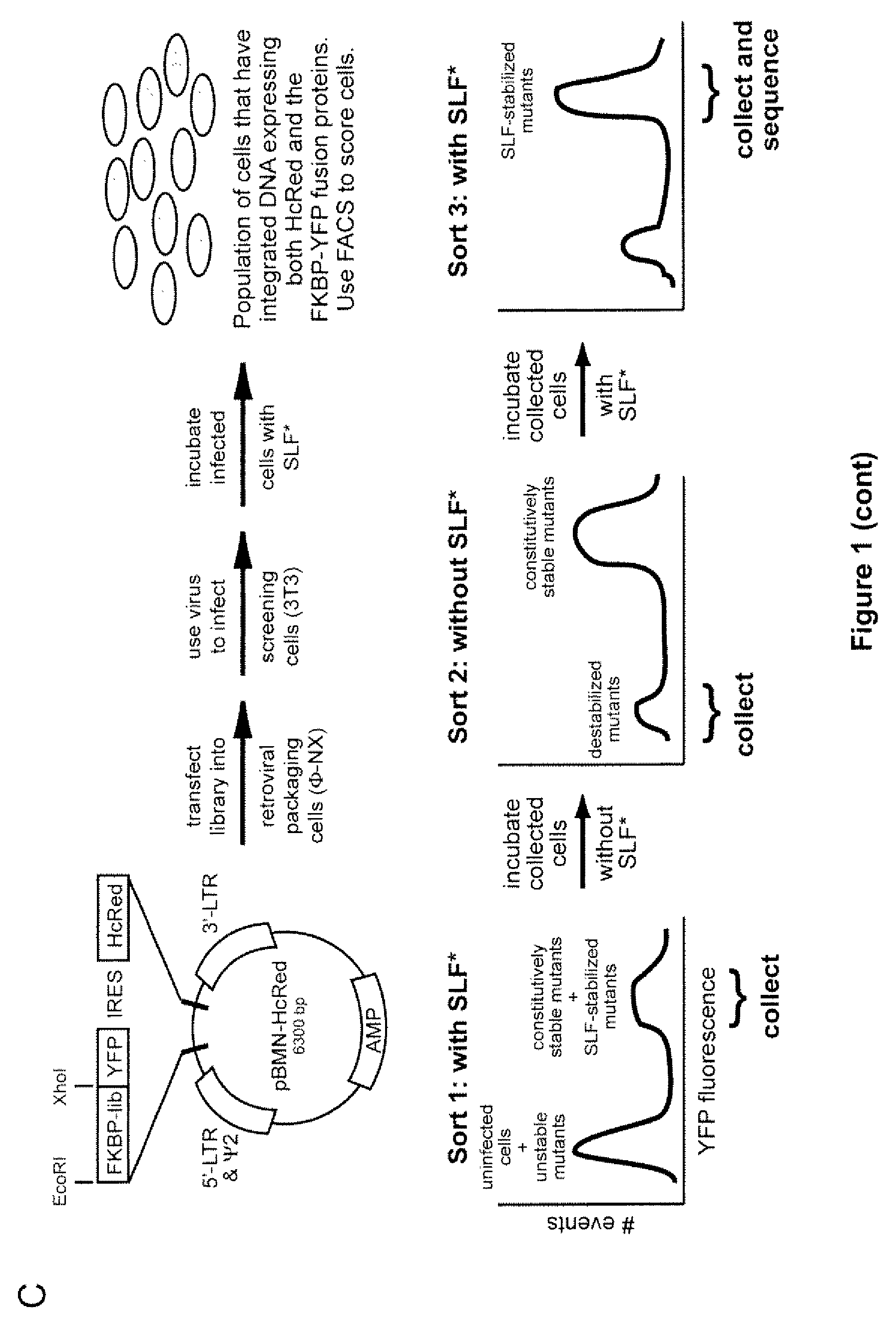
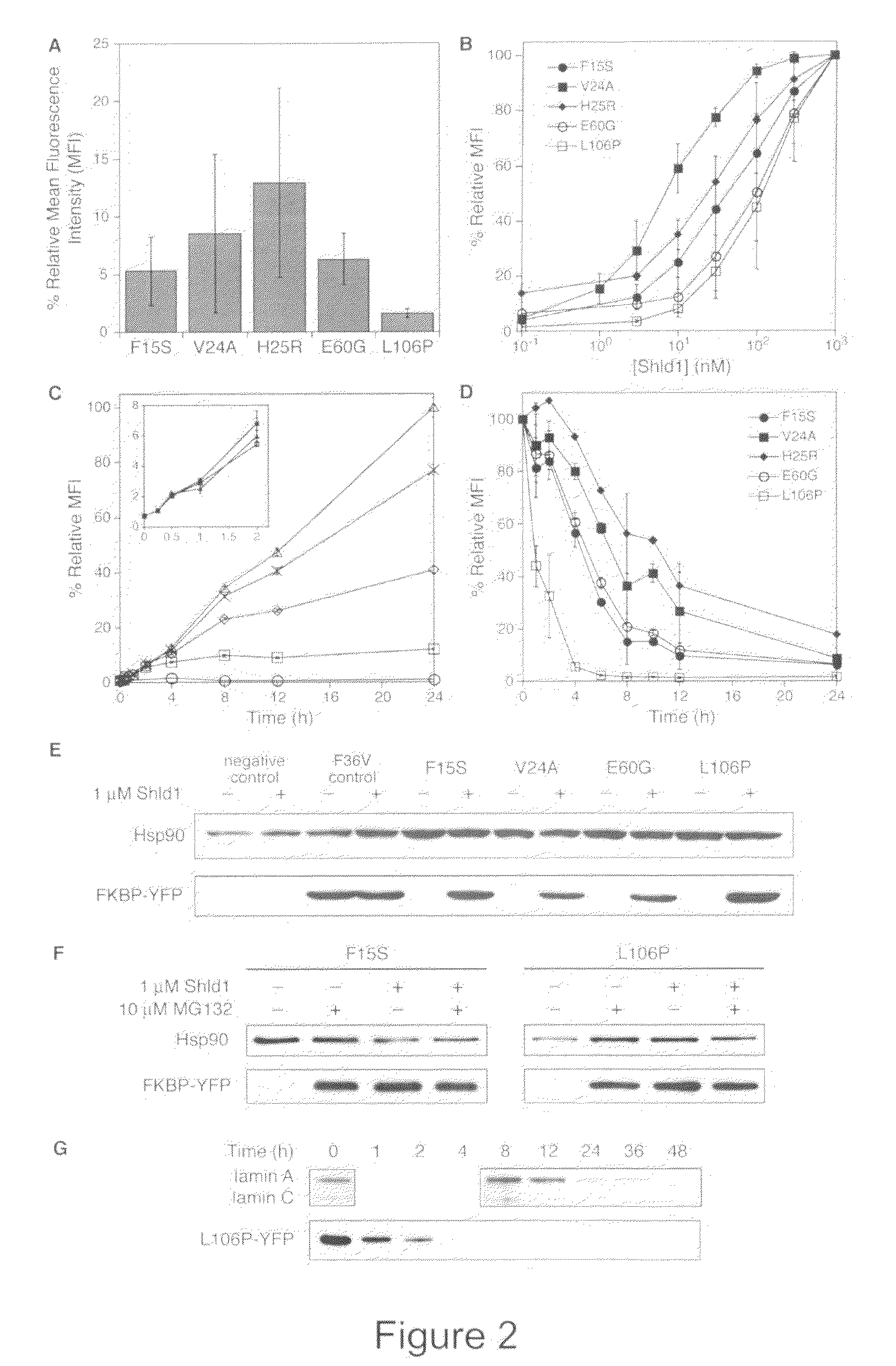
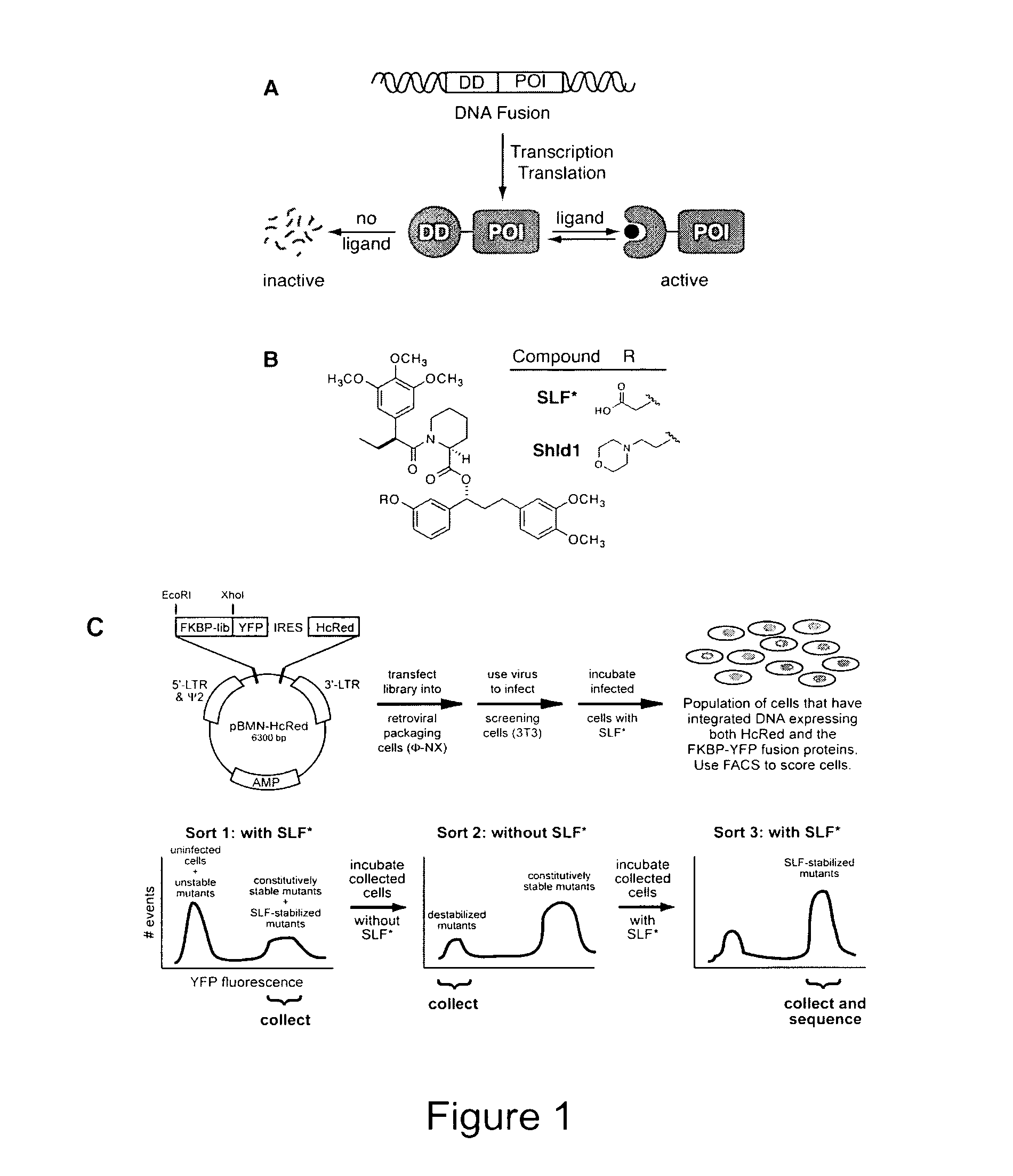
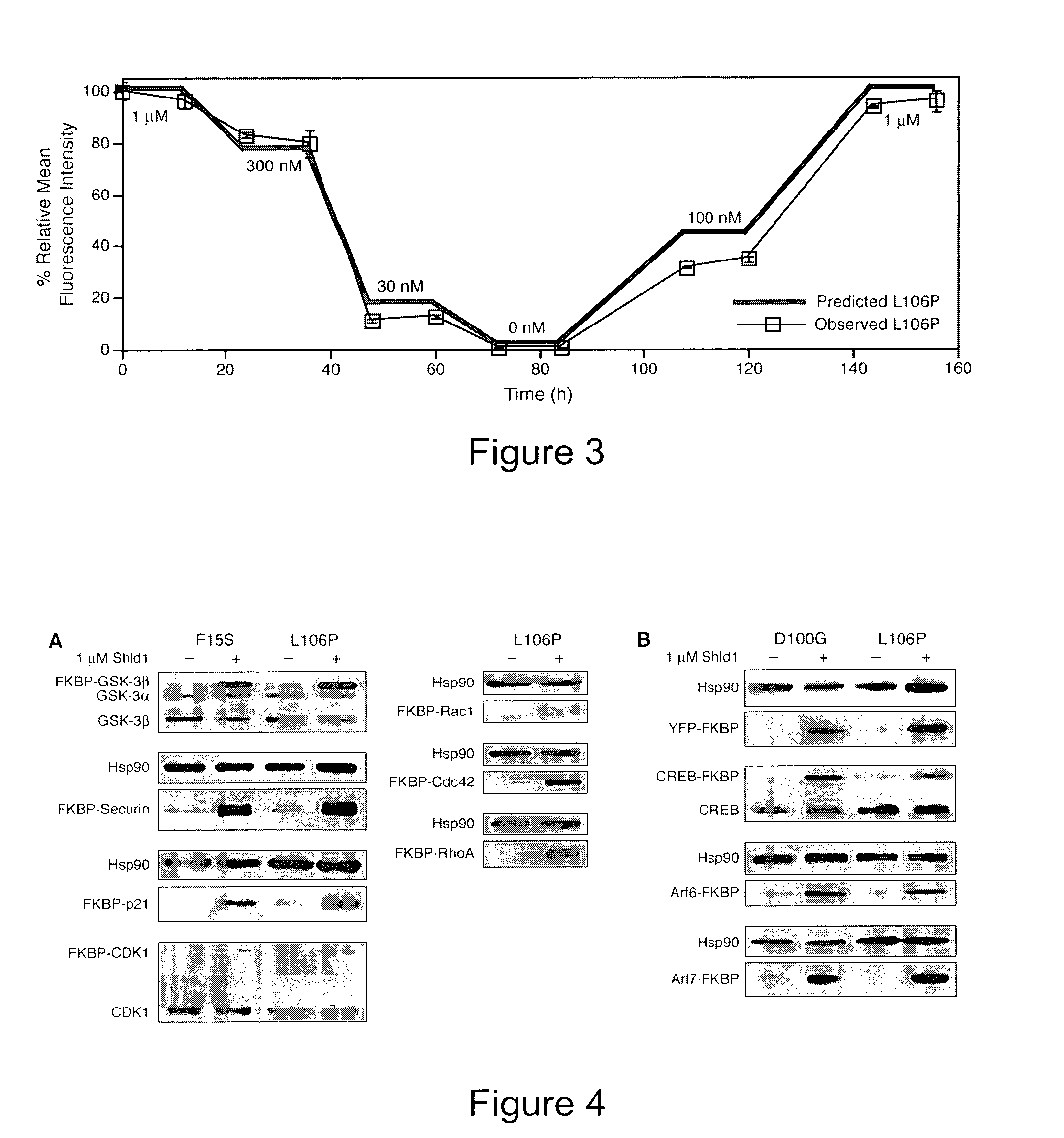
Method for regulating protein function in cells using synthetic small molecules
Methods and compositions for the rapid and reversible destabilizing of specific proteins using cell-permeable, synthetic molecules are described. Stability-affecting proteins, e.g., derived from FKBP and DHFR proteins are fused to a protein of interest and the presence or absence of the ligand is used to modulate the stability of the fusion protein.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for regulating protein function in cells using synthetic small molecules
Methods and compositions for the rapid and reversible destabilizing of specific proteins using cell-permeable, synthetic molecules are described. Stability-affecting proteins, e.g., derived from FKBP and DHFR proteins are fused to a protein of interest and the presence or absence of the ligand is used to modulate the stability of the fusion protein.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapeutic Anti-her2 antibody fusion polypeptides
InactiveUS20090226466A1Enhanced cell killing of cellImprove efficiencySugar derivativesAntibody mimetics/scaffoldsTherapeutic proteinAnti her2
Therapeutic protein fusions comprising anti-HER2 antibody and MicB sequences are described along with methods for their production and use
Owner:GENENTECH INC
Covalent tethering of functional groups to proteins and substrates therefor
ActiveUS20080274488A1Rapidly and efficiently loaded into and washedStable rateMethine/polymethine dyesBacteriaTetheringAmino acid substitution
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
Porcine DC-SIGN, ICAM-3 and LSECtin and uses thereof
The present invention relates to the cloning, identification and characterization of the unique and entire genomic sequences encoding new porcine DC-SIGN and LSECtin proteins, including the novel nucleotide sequences of the full-length cDNA and genes of both pDC-SIGN gene and pLSECtin. Also provided are the nucleic acid molecules encoding newly discovered porcine ICAM-3 isoforms from porcine monocyte-derived dendritic cells and the use thereof. Specifically, the invention is drawn to an isolated nucleic acid molecule comprising a nucleotide sequence encoding one or more of porcine DC-SIGN, porcine ICAM-3, porcine LSECtin, a complement of the nucleotide sequence or a functional, defined portion of the nucleotide sequence or a protein fusion product linked to a protein that may be of porcine or human origin. Methods for isolating and cloning the new porcine genes and for using the new nucleotide sequences in improved methods for propagating viruses, particularly enveloped viruses, are additionally described herein. The invention further includes new transfected cells or cell lines that can stably express the porcine proteins, new antibodies and the like.
Owner:VIRGINIA TECH INTPROP INC
Biotinylation tag peptides
Biotinylation peptides are provided which can be fused with other peptides or proteins of interest using recombinant DNA techniques to provide efficient methods for biotinylating the resulting fusion proteins in vivo or in vitro.
Owner:PACIFIC BIOSCIENCES
PTD-modified proteins
InactiveUS20050112640A1Good effectPolypeptide with localisation/targeting motifSugar derivativesNucleotideMammal
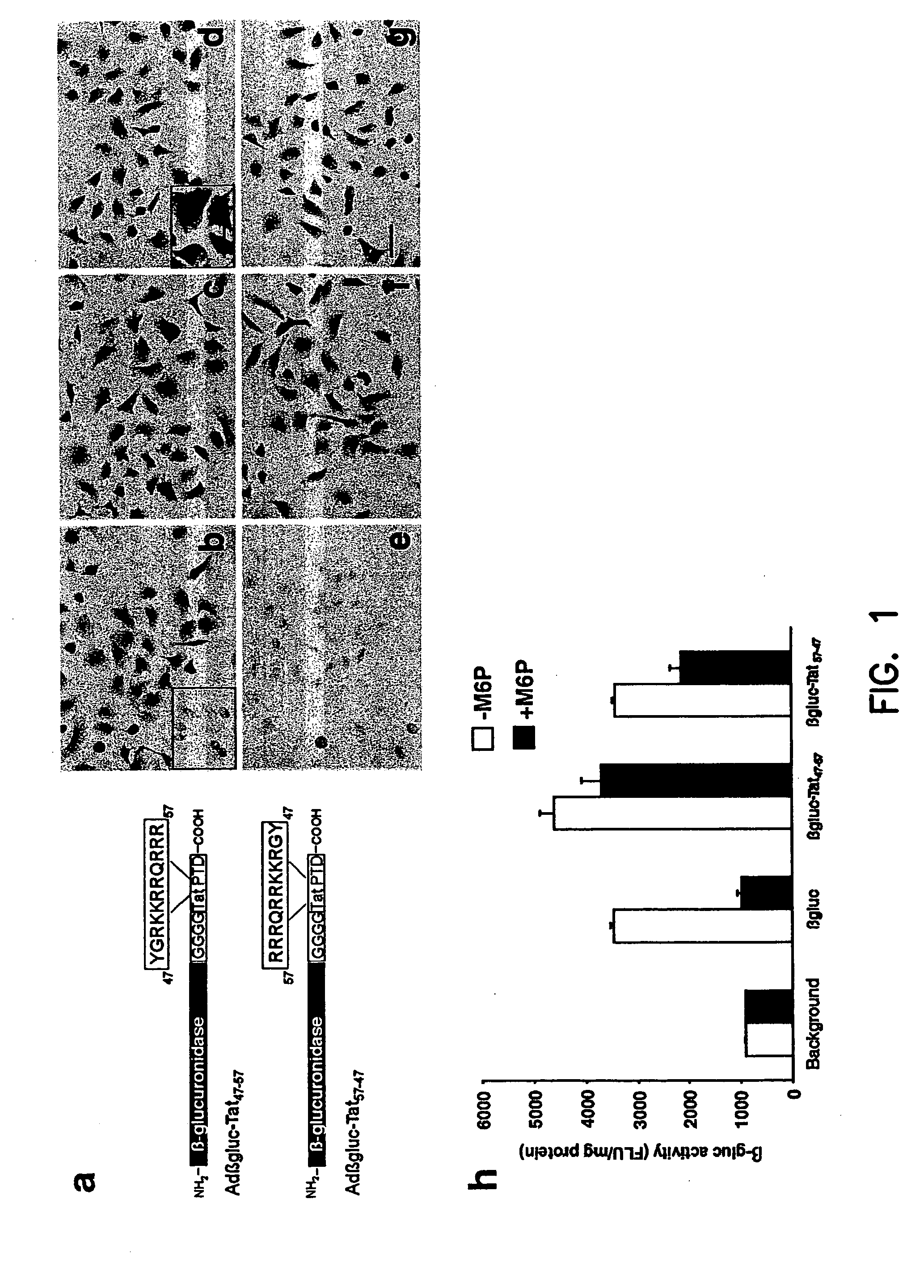
The present invention provides polynucleotides and expression vectors containing a sequence encoding a soluble lysosomal enzyme and a sequence encoding Tat protein transduction domain (PTD), and the corresponding polypeptides. The present demonstrates the utility of these protein fusions in altering the bioavailability of proteins for use in treating genetic diseases or acquired diseases. The invention further provides cell expression systems, and methods of treating a genetic disease or cancer in a mammal using the polynucleotides, polypeptides, or expression system of the present invention.
Owner:UNIV OF IOWA RES FOUND
Complex formation for the stabilisation and purification of proteins of interest
InactiveUS20050059053A1Increase volumeReduce degradation rateAntibody mimetics/scaffoldsVaccinesAntigenPost translational
A method is described for altering the properties such as the accumulation, the stability and / or integrity, the subcellular localisation, the post-translational modifications, the ability to get purified, and the phase partitioning behaviour of natural or recombinant target proteins expressed in a host organism. The method involves the co-expression of natural or recombinant proteins along with a specific binding partner that sequesters the target recombinant protein into a complex. The binding partner is supplied as a separate protein allowing formation of intermolecular complexes or is fused to the protein of interest, allowing the formation of intramolecular complexes. The binding partner can also be used to alter the subcellular localisation without modifying the sequence or structure of the target protein itself. This can be achieved by either incorporating appropriate targeting signals into the binding ligand, which are then linked to the target protein through complex formation, or complex formation itself may alter the subcellular localisation. The same strategy can be used to provide an affinity tag to facilitate protein purification. The principle of the invention is demonstrated by the coexpression of an unstable antibody and its cognate antigen.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Genetic assay for protein nuclear transport
InactiveUS6902886B1Decreasing reporter gene expression levelFunction increaseSugar derivativesMicroorganismsProtein insertionNuclear transport
The invention provides methods of determining the presence of a nuclear localization signal and / or the presence of a nuclear export signal in a protein of interest. The invention further provides chimeric nucleic acids and recombinant host cells for use in such methods. Additionally provided is a nucleic acid molecule encoding a modified LexA protein, wherein the modified LexA protein has no nuclear localization signal, as well as the modified LexA protein itself. In the nuclear import assay, if a protein of interest fused to a mLexA-Gal4AD hybrid contains a functional NLS, the fusion product will enter the yeast cell nucleus and activate the expression of reporter genes. In the nuclear export assay, if a protein of interest fused to a mLexA-SV40 NLS-Gal4AD hybrid contains a functional NES, the fusion product localized to the cell nucleus will exit into the cytoplasm, decreasing the reporter gene expression levels.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Staphylococcus saprophyticus nucleic acids and polypeptides
Isolated nucleic acid molecules which encode proteins from Staphylococcus saprophyticus are described. The invention also provides antisense nucleic acid molecules, recombinant expression vectors containing nucleic acid molecules, and host cells into which the expression vectors have been introduced. The invention still further provides isolated proteins, mutated proteins, fusion proteins, antigenic peptides and methods for the treatment, prevention or detection of an S. saprophyticus-associated disease or disorder. Furthermore, the nucleic acid molecules and polypeptide molecules of the invention may be used for the identification of agents which modulate S. saprophyticus activity, e.g., a small molecules.
Owner:ASTRAZENECA AB
Production of proteins
A method for forming a fusion protein that is expressed as a recombinant protein body-like assembly in host eukaryotic cells and organisms other than higher plants as host systems is disclosed. More particularly, peptides and proteins are fused to protein sequences that mediate the induction of recombinant protein body-like assembly (RPBLA) formation, are stably expressed and accumulated in these host cells after transformation with an appropriate vector. Methods for preparing the fusion protein are also disclosed.
Owner:ERA BIOTECH SA
Process for partitioning of proteins
InactiveUS7060669B1Minimize impactEfficient purificationFungiBacteriaProtein targetAqueous two-phase system
The present invention relates to isolation and purification of proteins in aqueous two-phase systems (ATPS). Specifically the invention provides processes for partitioning of proteins of interest in ATPS by fusing said proteins to targeting proteins which have the ability of carrying said protein into one of the phases.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS
Staphylococcal nuclease fusion proteins for the production of recombinant peptides
InactiveUS7390639B2Easy to disassembleNot complicate subsequent stepSugar derivativesBacteriaInclusion bodiesHplc method
Peptides are produced as fusions with a suitable carrier protein. The carrier protein disclosed herein are adapted from the N-terminal domain of staphylococcus nuclease. This novel carrier protein acts to promote the over-expression of the peptide-protein fusion in the form of inclusion bodies, which minimizes in-cell proteolysis of desired peptides. The fusion protein is readily purified by conventional procedures or His-tag affinity chromatography when His-tag is inserted into the fusion protein. The target peptide is released from the purified fusion protein by a simple cleavage step and separated from the librated carrier protein by use of a reverse-phase HPLC process or by repeating the same affinity purification method. A particular advantage of the disclosed method, in addition to the obvious advantage of high yields, is its use for producing isotopically labeled peptides for NMR characterization of bioactive peptides and their interactions with target proteins.
Owner:NAT RES COUNCIL OF CANADA
Hydrogels for biomolecule analysis and corresponding method to analyze biomolecules
InactiveUS7588906B2Eliminate needSamples introduction/extractionMicrobiological testing/measurementPhosphorylationFluorescence
Owner:WISCONSIN ALUMNI RES FOUND
Hydrogels for biomolecule analysis and corresponding method to analyze biomolecules
InactiveUS20060121535A1Eliminate needSamples introduction/extractionMicrobiological testing/measurementPhosphorylationBinding peptide
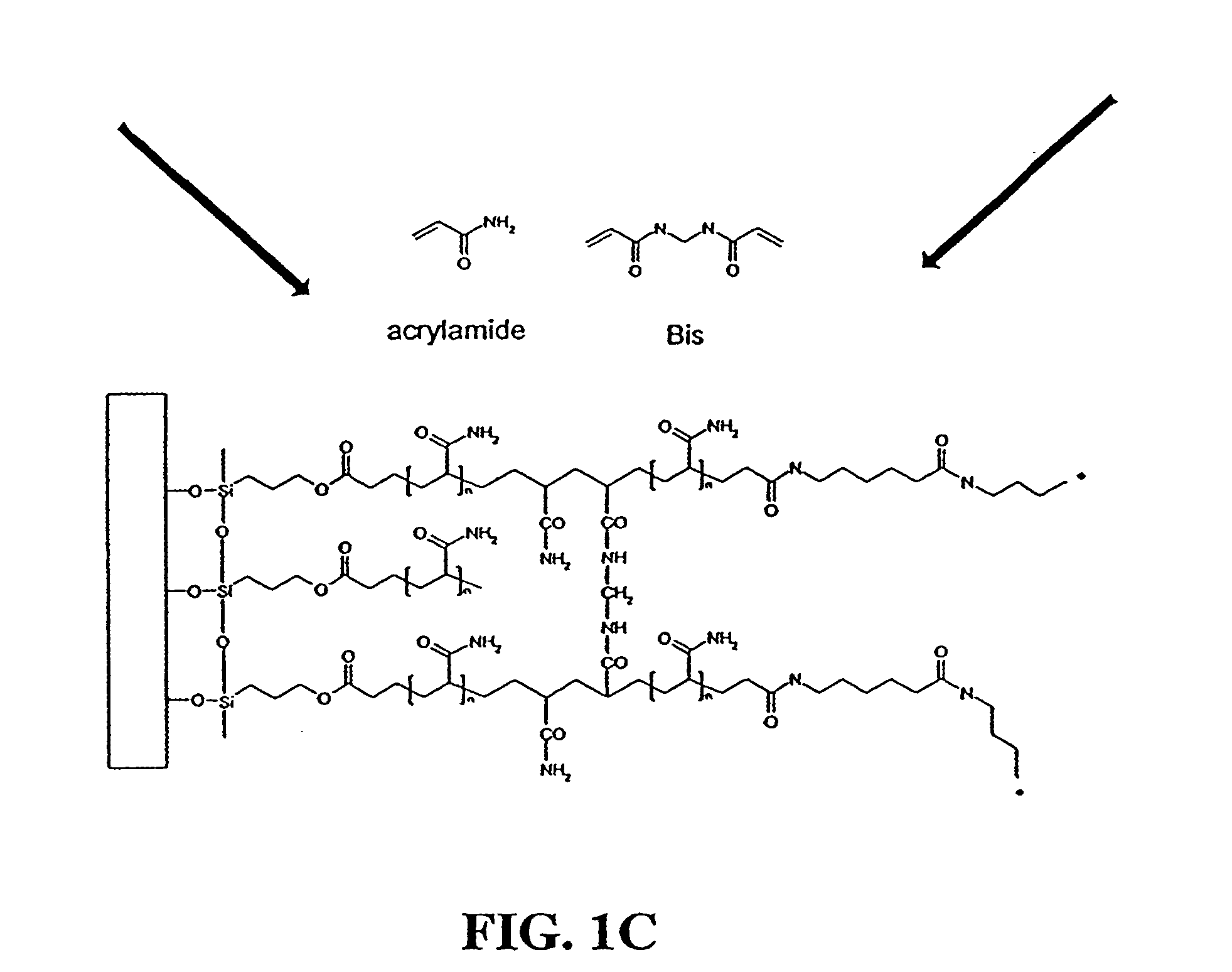
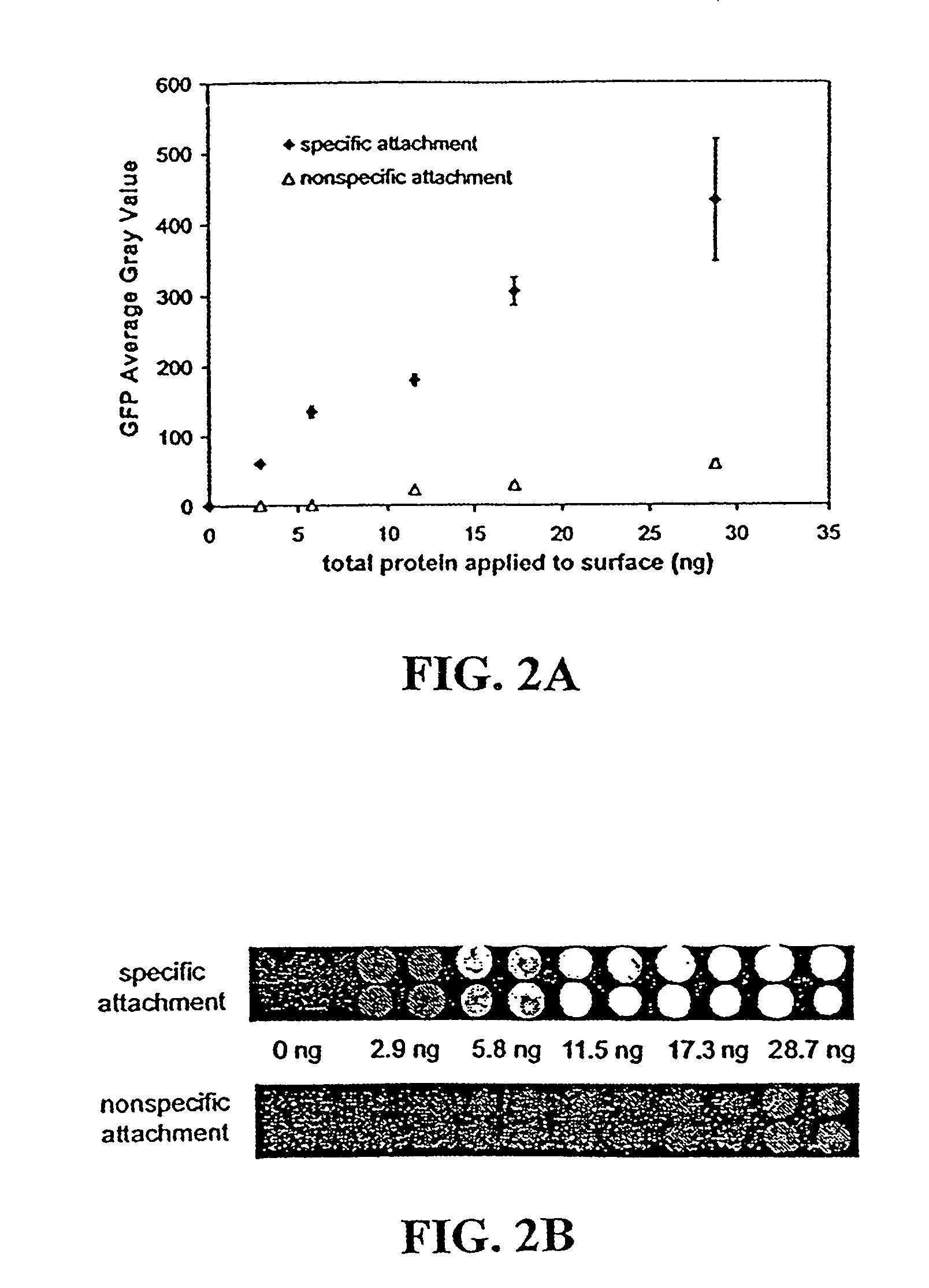
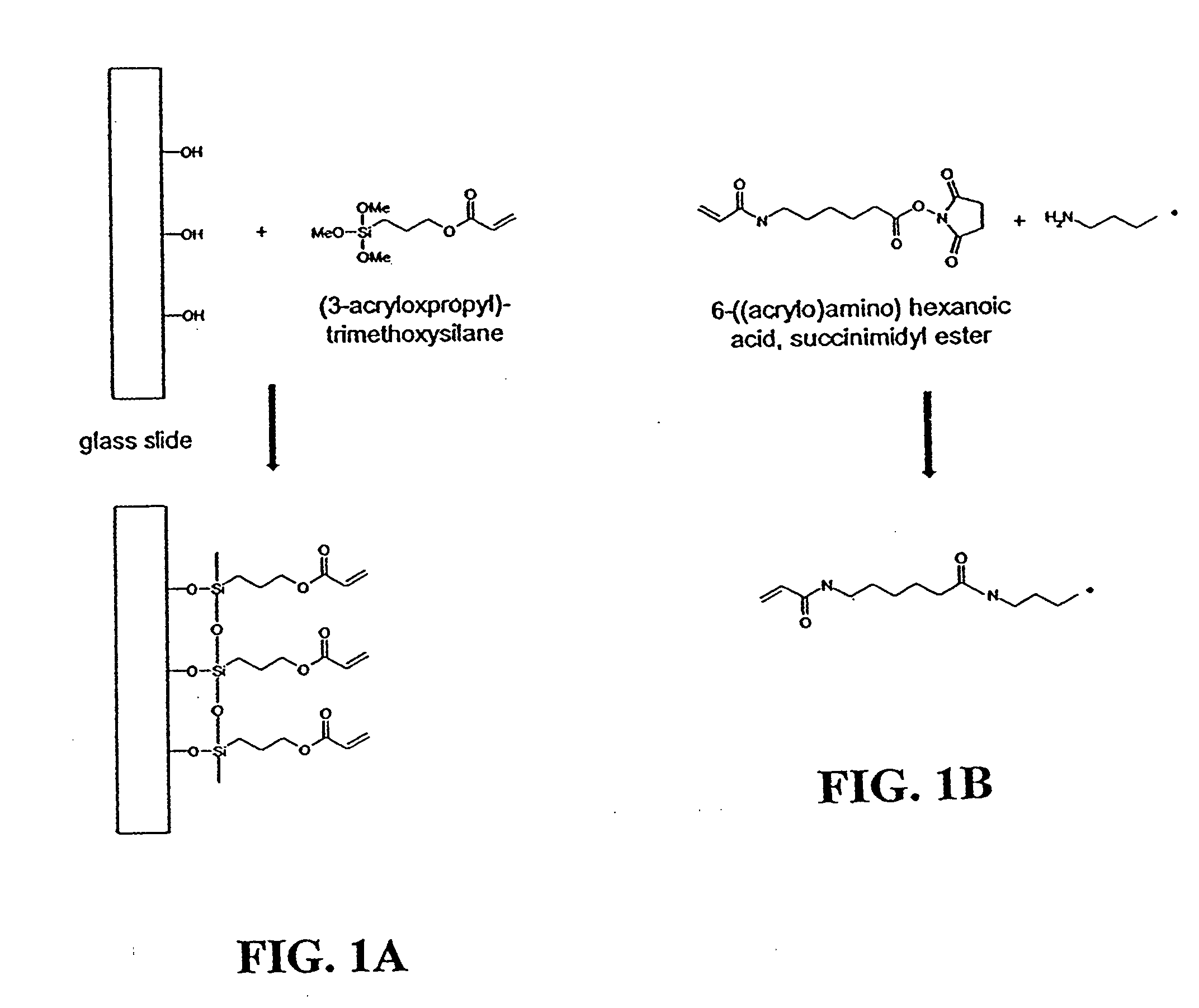
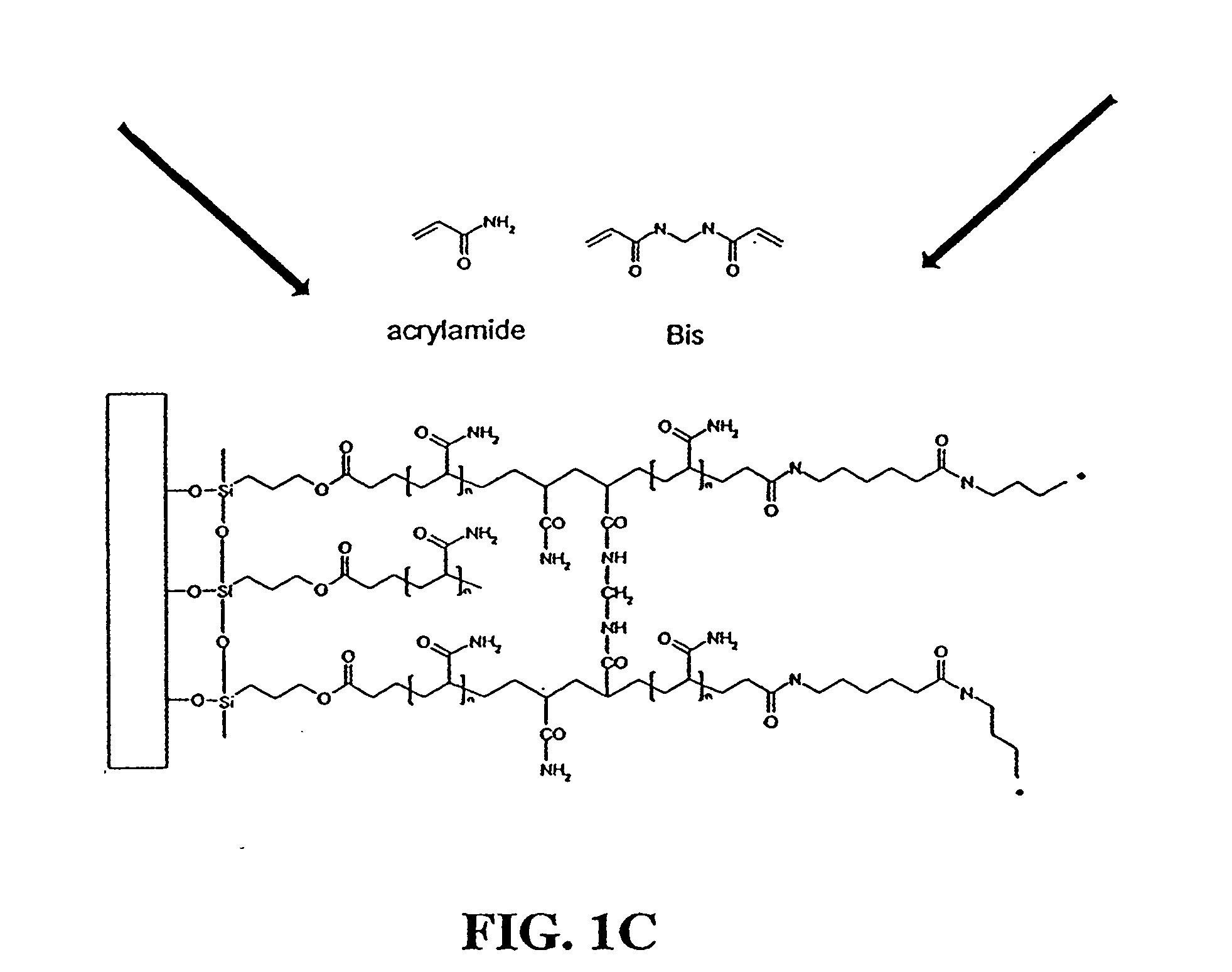
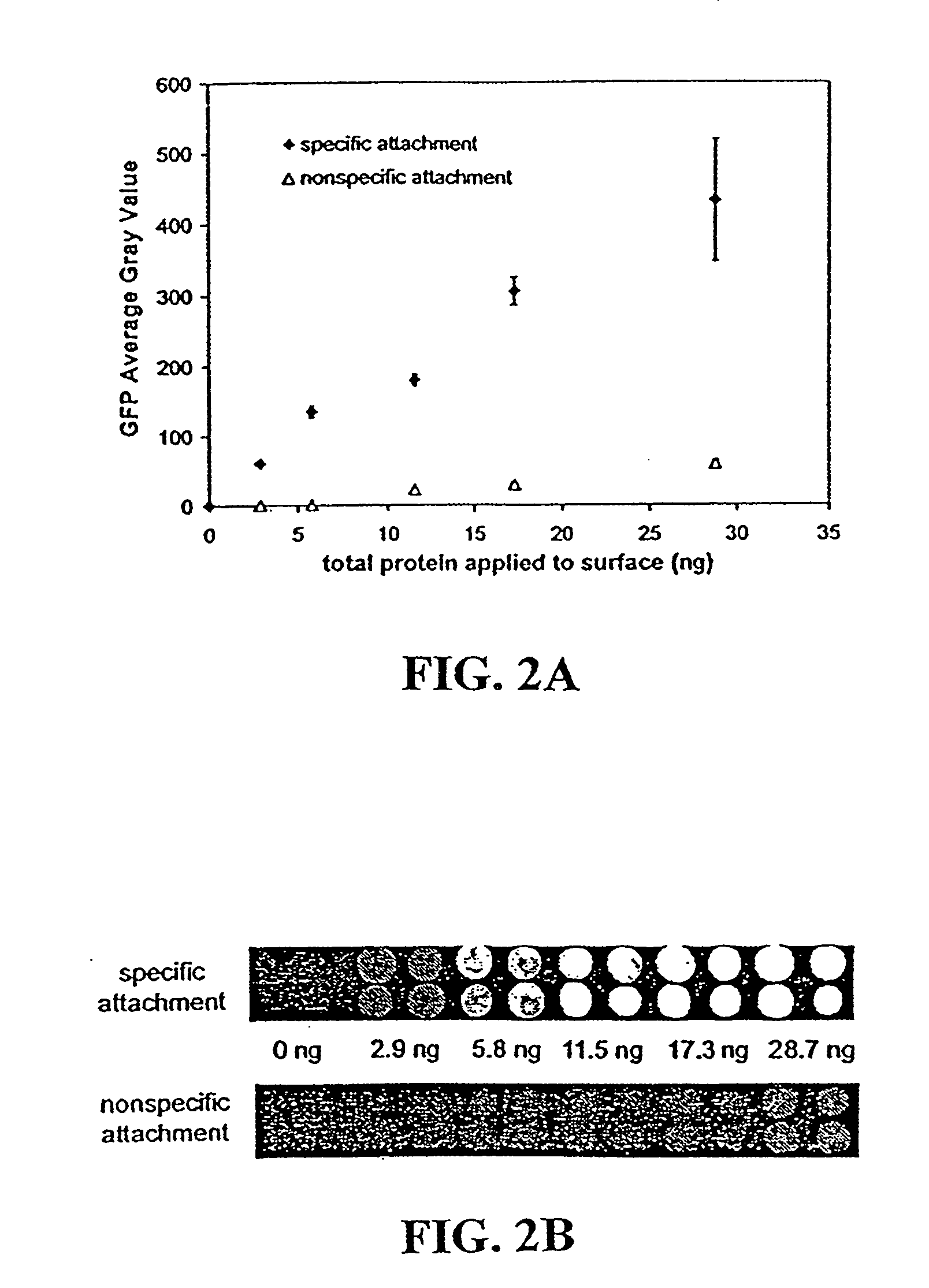
Disclosed are a polyacrylamide-based method of fabricating surface-bound peptide and protein arrays, the arrays themselves, and a method of using the arrays to detect biomolecules and to measure their concentration, binding affinity, and kinetics. Peptides, proteins, fusion proteins, protein complexes, nucleic acids, and the like, are labeled with an acrylic moiety and attached to acrylic-functionalized glass surfaces through a copolymerization with acrylic monomer. The specific attachment of GST-green fluorescent protein (GFP) fusion protein was more than 7-fold greater than the nonspecific attachment of non-acrylic labeled GST-GFP. Surface-attached GST-GFP (0.32 ng / mm2) was detectable by direct measurement of GFP fluorescence and this lower detection limit was reduced to 0.080 ng / mm2 using indirect antibody-based detection. The polyacrylamide-based surface attachment strategy was also used to measure the kinetics of substrate phosphorylation by the kinase c-Src. The surface attachment strategy is applicable to the proteomics field and addresses denaturation and dehydration problems associated with protein microarray development.
Owner:WISCONSIN ALUMNI RES FOUND
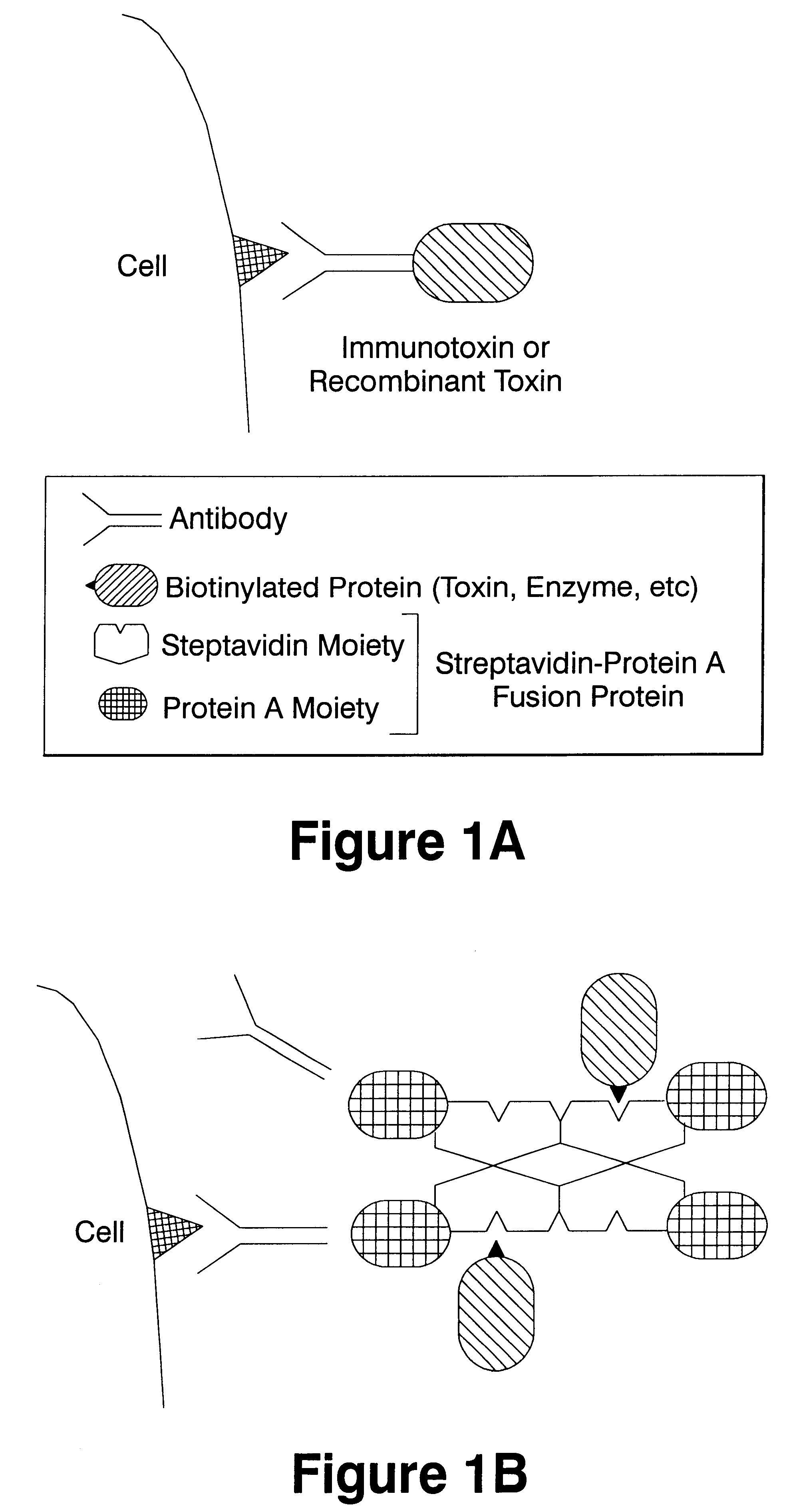
High efficiency tissue specific compound delivery system using streptavidin-protein a fusion protein
InactiveUS6497881B1Peptide/protein ingredientsAntibody mimetics/scaffoldsStreptavidinDelivery system
The present invention relates to methods and compositions that can be employed to introduce toxins and nucleic acids into the cytoplasm or nucleus of a eukaryotic cell, particularly a cell of a higher vertebate. The invention particularly concerns the use of a fusion protein of streptavidin and protein A sequences to form a non-covalent complex of a toxin or nucleic acid and an antibody.
Owner:NEW YORK UNIV
Mesothelin antibody protein fusions and methods of use
Owner:THE GENERAL HOSPITAL CORP
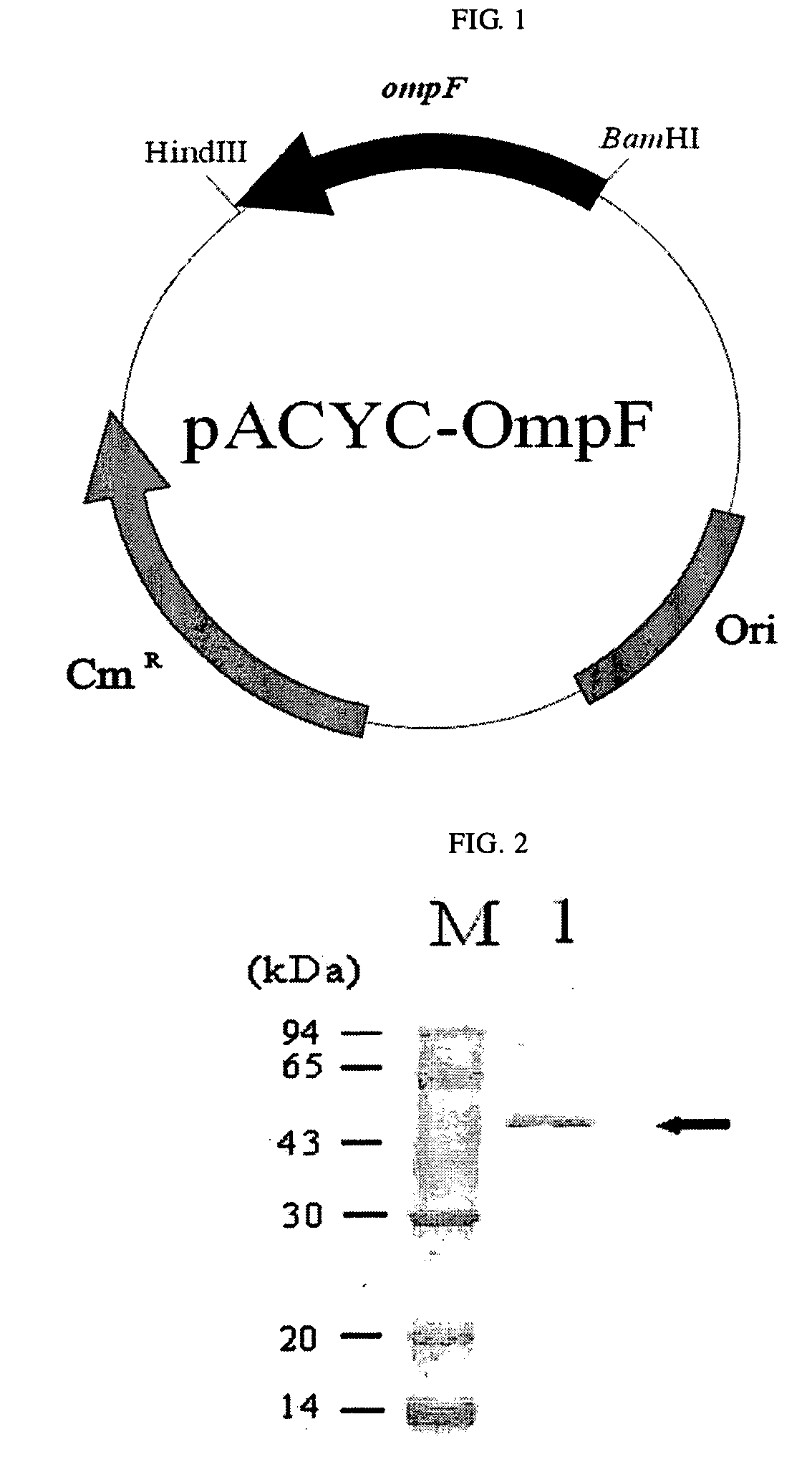
Method For Extracellular Production of Target Proteins By Co-Expression of Ompf and Target Proteins
ActiveUS20080206814A1Efficient secretionImprove efficiencyBacteriaPeptidesMicroorganismEscherichia coli
The present invention relates to a method for secreting and producing a target protein into cell culture broth. More particularly, the invention relates to a microorganism co-transformed with a recombinant expression vector containing E. coli outer membrane protein F (OmpF) and a recombinant expression vector containing a target protein to be secreted into cell culture broth, as well as a method of secreting and producing the target protein into cell culture broth by culturing the microorganism. According to the invention, the target protein can be secreted into cell culture broth in a pure form without fusion with other proteins so that the efficient isolation and purification of the target protein is possible.
Owner:KOREA ADVANCED INST OF SCI & TECH
Universal pharmaceutical formulation for recombined human serum albumin fusion proteins for injection
The invention provides a pharmaceutical formulation for manufacturing a clinical medicament for recombined human serum albumin / therapeutic protein fusion proteins for injection, which is universal, simple and extremely effective. The pharmaceutical formulation and a freeze-drying process include that a clinical therapeutic injecting injection is formed by using single effective formulation to prepare, freeze and dry different recombined human serum albumin fusion proteins. Frozen and dried injection is capable of decreasing the possibility of forming fractured dissociative human serum albumins, dissociative therapeutic protein fragments and fusion protein polymers. Products can be stored in a long period of time and are not easy to be decomposed or degenerated; the products have accurate dose, fine appearance, good stability and high safety and can greatly improve the safety of pharmacy. The pharmaceutical formulation and production process steps are simple and unique, high in quality and easy in industrialization, and pharmaceutics has the greatest advantages that no allergy or toxic and side effects are produced to human bodies and the like.
Owner:BEIJING MEIFUYUAN BIO PHARM TECH
Nanometer molecular biosensor, preparation method and application thereof
InactiveCN101624568AReduced preparation stepsAssembly SynchronizationBioreactor/fermenter combinationsBiological substance pretreatmentsNanowireFluorescence
The invention discloses a nanometer molecular biosensor and a preparation method thereof. The biosensor is mainly characterized in that a molecular biosensor is self-assembled into a nanometer molecular biosensor by adopting nanowire protein, and compared with an unassembled molecular biosensor, the detection sensitivity is greatly improved. The preparation method adopts the protein fusion technology, and fuses nanowire protein with self-assembled function, special enzyme molecule used as a biology identification element and fluorescence protein used as a transducer element, therefore, functional protein with self-assembled nanowire, identification and transduction output signal functions simultaneously, and after the functional protein is expressed and purified, the functional protein is self-assembled into a nanometer molecular biosensor which can be used for inspecting object molecules in various clinical samples and environment samples. The invention simultaneously discloses an application of the nanometer molecular biosensor prepared by the preparation method in pesticide methyl parathion detection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
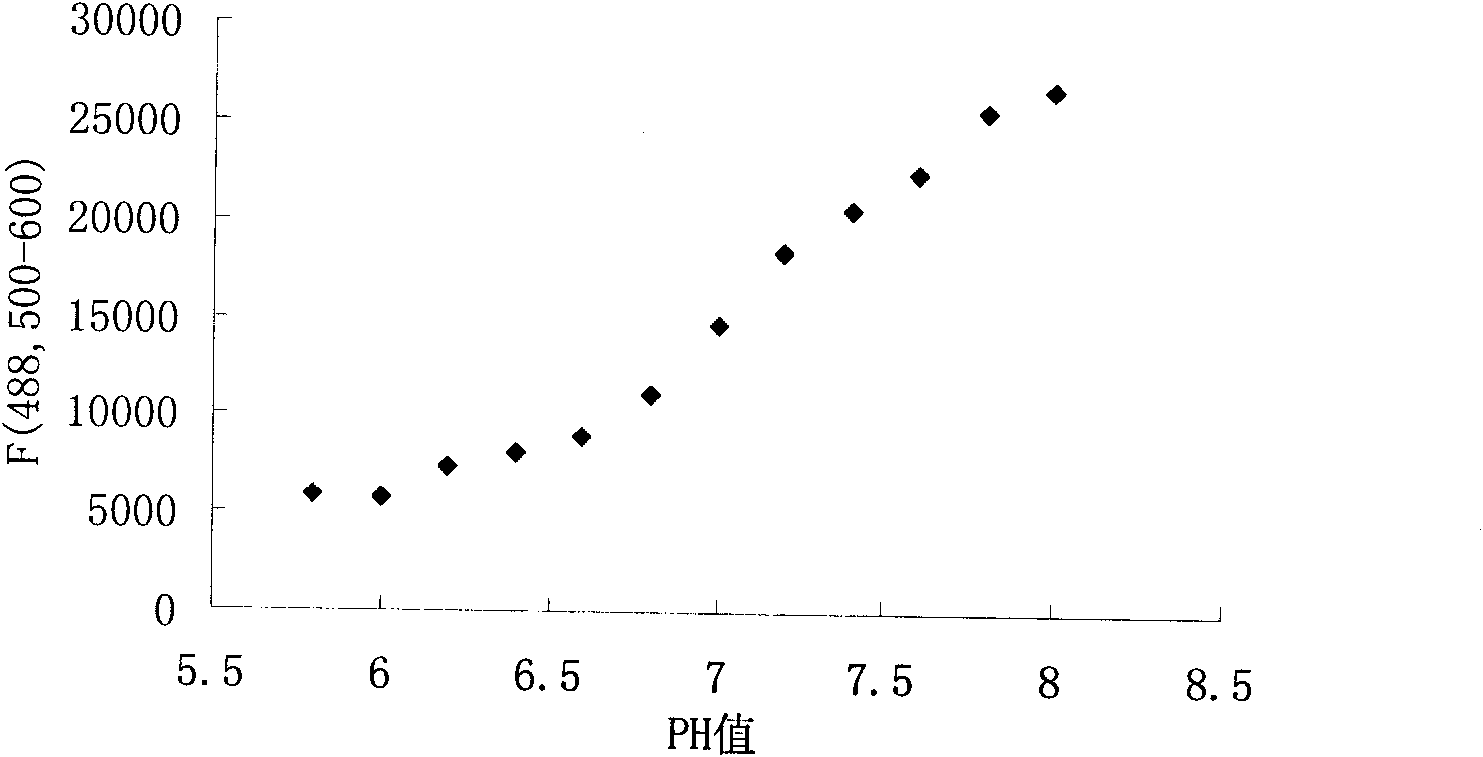
Tyrosine kinse substrate
InactiveUS20080003630A1Antibody mimetics/scaffoldsMicrobiological testing/measurementTyrosineKinase substrate
A tyrosine kinase substrate is described. The tyrosine kinase substrate comprises a fusion protein which comprises a protein for labeling and a specific peptide fused with the protein for labeling. The specific peptide has an amino acid sequence including a glutamic acid residue and a tyrosine residue. The tyrosine kinase substrate is capable of being phosphorylated by a plurality of kinds of tyrosine kinases.
Owner:SYSMEX CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com