Universal pharmaceutical formulation for recombined human serum albumin fusion proteins for injection
A technology of human serum albumin and fusion protein, which is applied in the field of production and manufacture of fusion protein formed by recombinant human serum albumin and therapeutic protein, and clinical therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
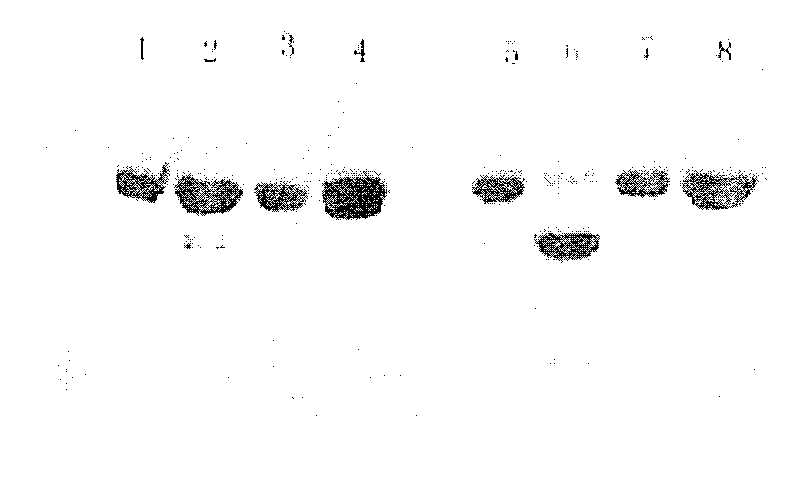
[0035] Example 1: Performance of recombinant human serum albumin fusion protein in aqueous injection formulation
[0036] The formulations of different recombinant protein drugs when made into water preparations are obviously different, and form their own patented formulations. For example, the formulations of recombinant interferon α2a and interferon α2b are different, and the recombinant granulocyte stimulating factor injection water The formulation formulation is also different from that of recombinant interferon. The recombinant human granulocyte-stimulating factor injection NEUPOGEN finished water injection formulation of Amgen Company of the United States was used as a reference for formulation screening and comparison. Specifications of NEUPOGEN injection of American Amgen Company 0.5ml: each tube contains rhGCSF: 0.3mg; acetic acid: 0.295mg; mannitol: 25.0mg; polysorbate 80: 0.02mg; sodium salt: 0.0175mg. Therefore, on this basis, taking human serum albumin / granulocyt...
Embodiment 2
[0045] Example 2: Freeze-dried dosage form-research on the type of sugar added in the formula (sucrose, glucose, lactose, trehalose):
[0046] Aiming at the specificity of the molecular structure of the human serum albumin fusion protein, the present invention adds different kinds of sugars on the basis of the selected mannitol selected in preliminary prescription screening to investigate whether the stability of the finished freeze-dried preparation can be increased.
[0047] Select qualified human serum albumin / interferon α2a stock solution, and make 10mM PB, 0.3mg fusion protein, 4% mannitol, 1% sucrose (or trehalose, glucose, lactose respectively), 0.004% Tween 80, The preparation of pH 6.5 has a filling volume of 0.5 ml; at the same time, 10 mM PB, 0.3 mg fusion protein, 5% mannitol, and 0.004% Tween 80 are prepared in a loading volume of 0.5 ml for comparison. After lyophilization, a purity analysis was performed. The results showed that, for the protein purity of the f...
Embodiment 3
[0050] Embodiment 3: research on polysorbate 80 content in freeze-dried dosage form-formulation
[0051] Aggregates are readily formed against recombinant human serum albumin fusion proteins. Taking recombinant human serum albumin / granulocyte stimulating factor fusion protein (rHSA / GCSF) as an example, a detailed prescription screening study was carried out. There is a free sulfhydryl group on the GCSF molecule. When two GCSF molecules meet, they can form a dimer, which cannot be separated by conventional methods, but can only be separated by complete denaturation. The formation of aggregates greatly reduces the biological activity of the prepared protein. During the production and preparation of rhGCSF, this kind of polymer is completely denatured and then refolded (of course, the yield will decrease). After the human serum albumin fusion protein is formed, it can no longer be resolved by denaturation-refolding. Recombinant human serum albumin fusion protein itself has 17 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com