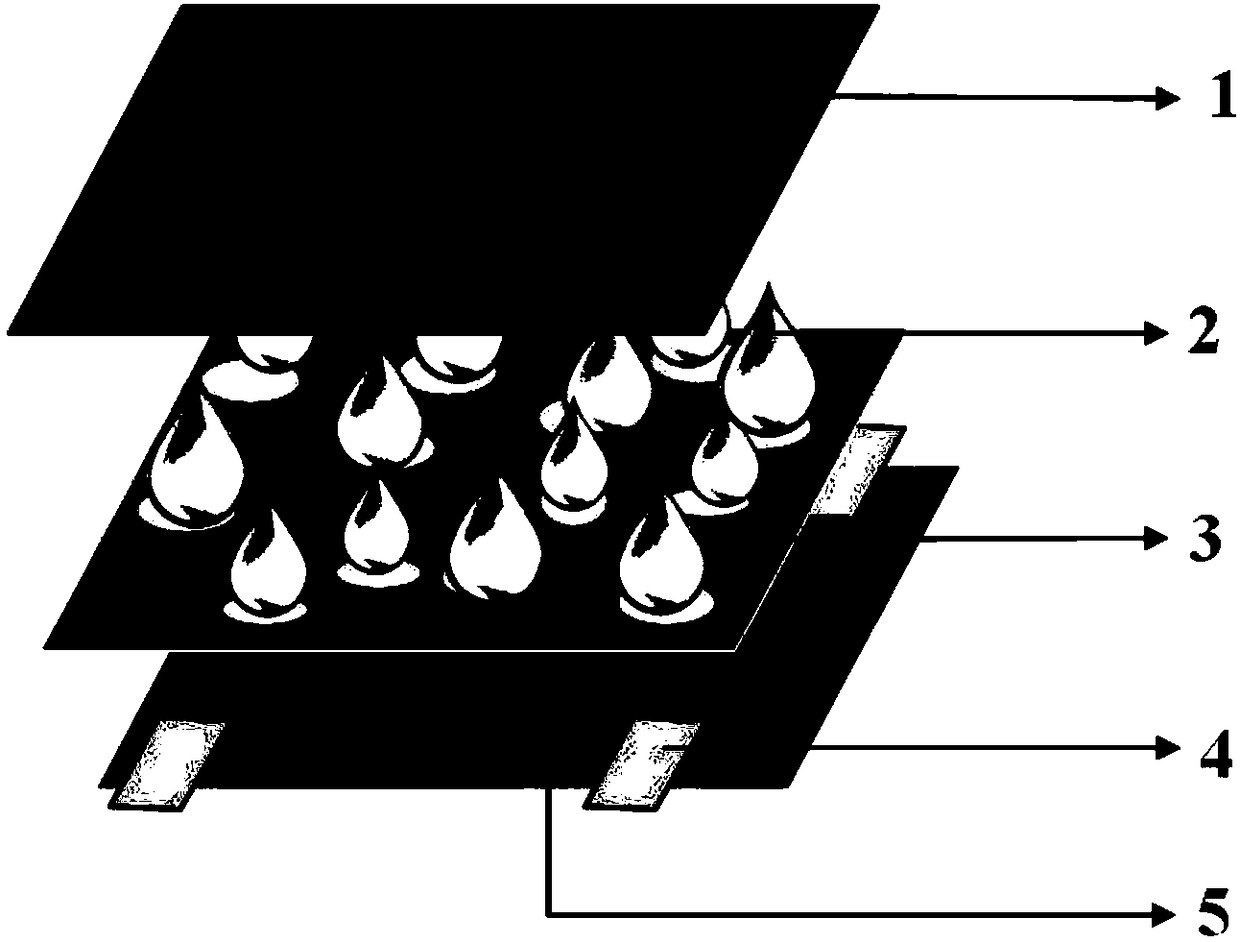
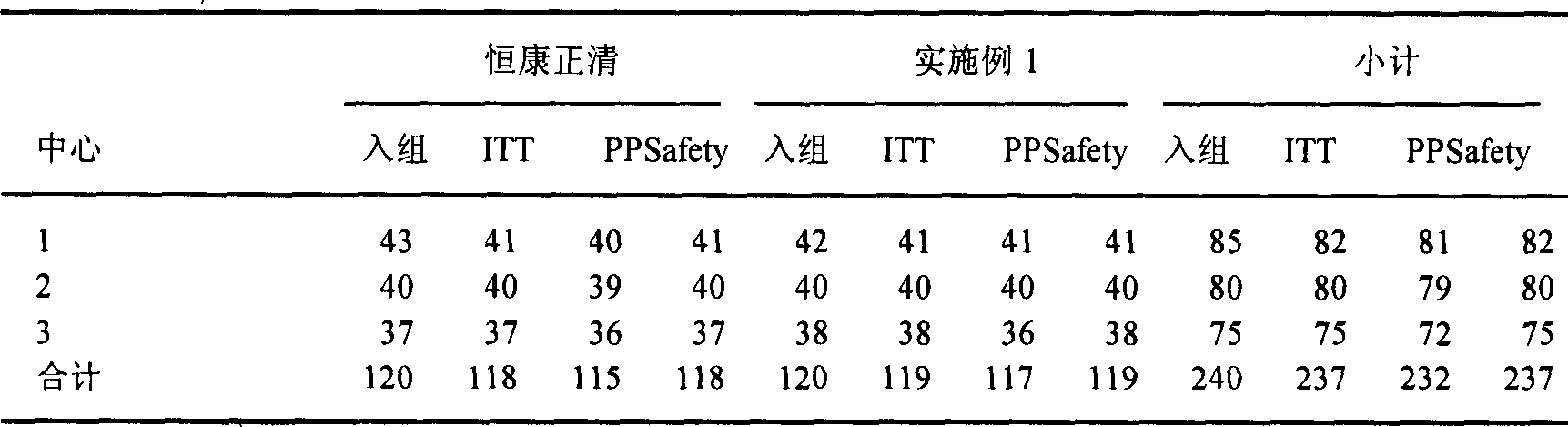
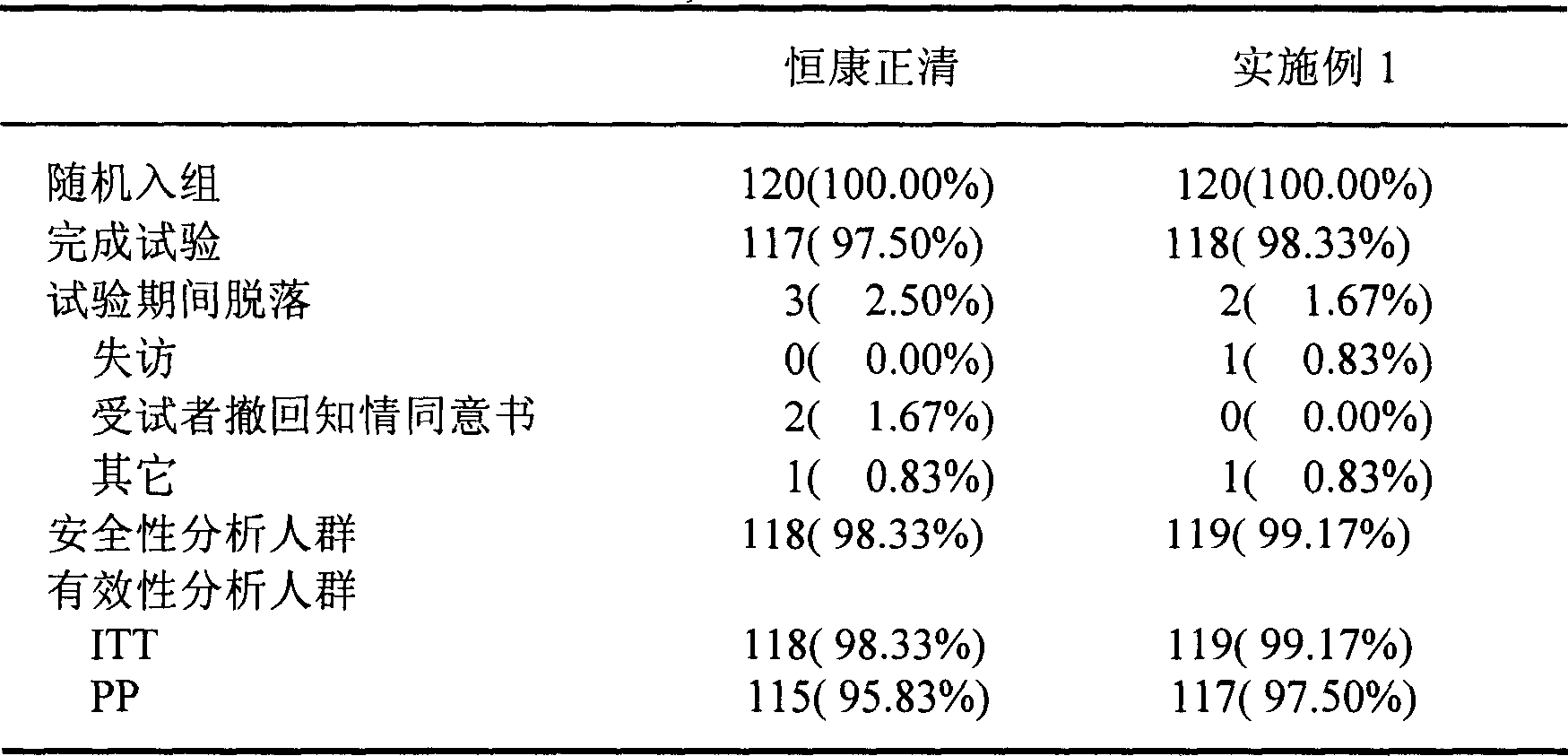
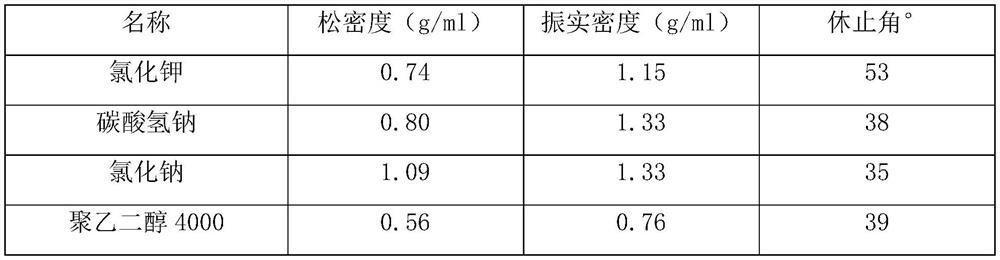
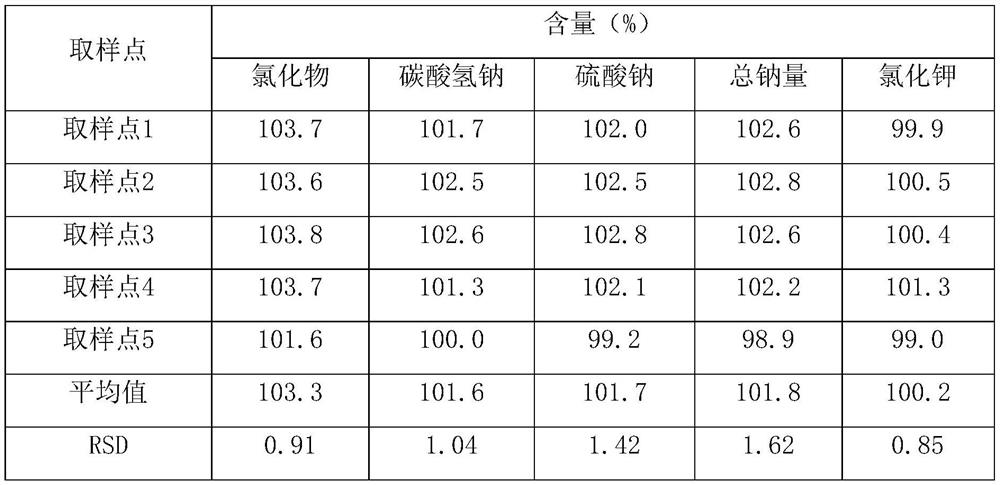
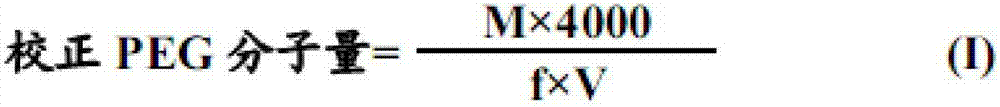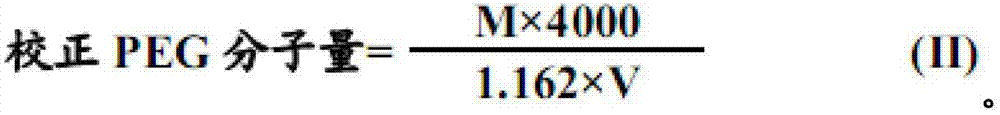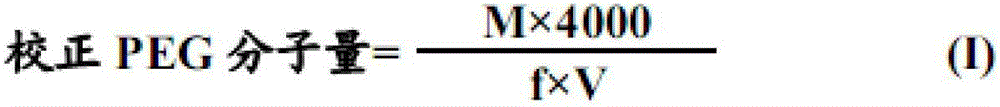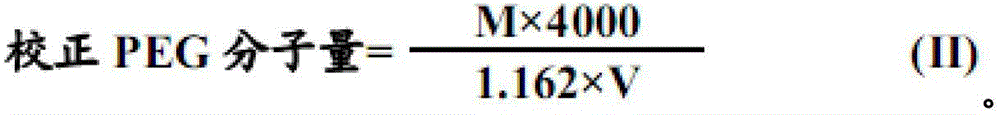Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Polyethylene glycol electrolyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
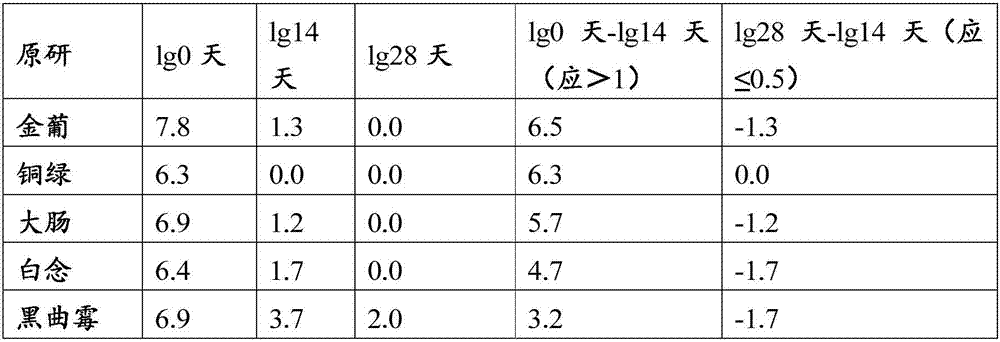
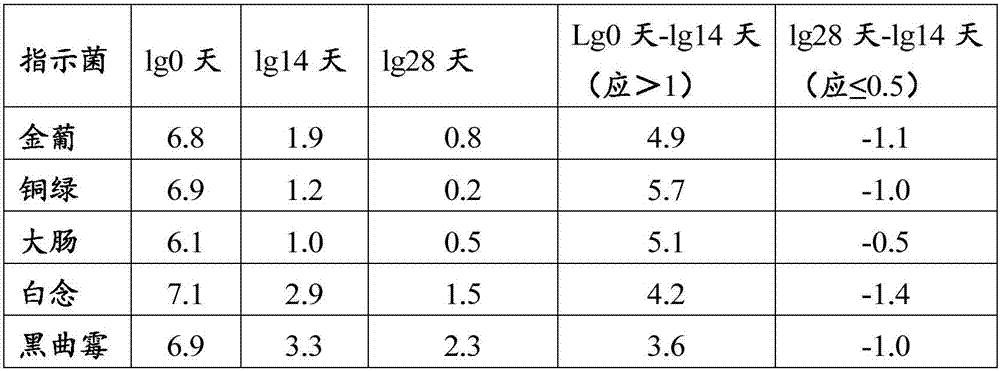
Compound polyethylene glycol electrolyte powder and preparation method thereof
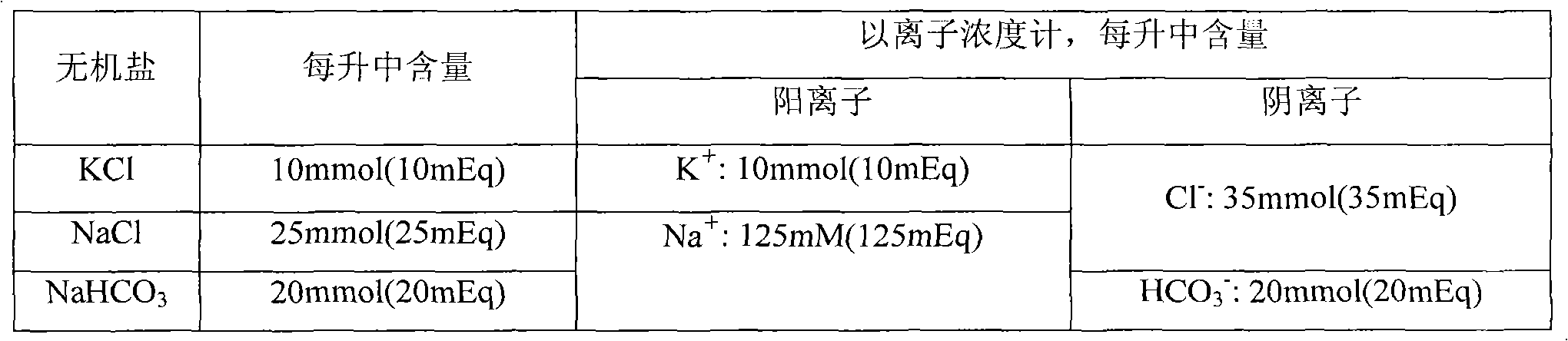
The invention relates to compound polyethylene glycol electrolyte powder and a preparation method thereof, in particular to a powder compound containing polyethylene glycol and electrolyte. The powder compound comprises, by weight, 6000 parts of polyethylene glycol, 118-176 parts of sodium chloride, 60-90 parts of potassium chloride, 137-205 parts of sodium bicarbonate, 461-692 parts of anhydrous sodium sulfate, 19-29 parts of powder essence, and / or 5-7.6 parts of aspartame. The compound polyethylene glycol electrolyte powder has fine micromeritic properties and taste.
Owner:SHENZHEN WANHE PHARMA
Compound polyethylene glycol electrolyte composition
The invention relates to a compound polyethylene glycol electrolyte composition, and in particular relates to a composition containing polyethylene glycol and electrolyte. The composition comprises the following components: polyethylene glycol, potassium chloride, sodium bicarbonate and anhydrous sodium sulfate. According to 6000 parts of polyethylene glycol, the composition comprises 118-178 parts of sodium chloride, 60-90 parts of potassium chloride, 137-205 parts of sodium bicarbonate and 461-692 parts of anhydrous sodium sulfate. The composition disclosed by the invention has good pharmaceutical properties.
Owner:SHENZHEN WANHE PHARMA
Polyethylene glycol electrolyte granular preparation and production method
ActiveCN101766648AQuality is easy to controlGreat tasteOrganic active ingredientsDigestive systemPolyethylene glycolElectrolyte
The invention provides a polyethylene glycol electrolyte granular preparation and a production method. The polyethylene glycol electrolyte granular preparation comprises polyethylene glycol with the average molecular weight of 3000 to 4000 and electrolyte, and the granule diameter of the polyethylene glycol electrolyte granule is 180Mu m to 850Mu m. The invention at least solves the following problems of the prior polyethylene glycol electrolyte granular preparation that: the production cost is high, the mixing of components is not uniform, the weight cannot be controlled, use is complex, the granule diameters of medicinal powder are not uniform, fluidity is not good, and stability is poor.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Polyethylene glycol electrolyte oral solution
ActiveCN1850112AHigh clarityOsmolality ratio is stable and accurateOrganic active ingredientsMetabolism disorderGastrointestinal tract surgeryChronic constipation
The present invention provides a polyethylene glycol-electrolyte oral solution. Its is made up by using 59-60 g of polyethylene glycol, 1.0-1.5 g of sodium chloride, 0.5-1.0 g of potassium chloride, 1.5-2.0 g of sodium hydrogen carbonate, 5.5-6.0 g of sedium sulfate, 0.02-0.5 g of egtazide, 0.02-0.5 g of sweetener and 1-2 ml of edible essence, and adding deionized water to 1000 ml. It mainly is applicable to colonoscopy, barium enema X-ray examination and intestinal tract ablution, etc. and also can be used for curing chronic constipation.
Owner:BEIJING SHENGYONG PHARMA
Compound polyethylene glycol electrolyte granule and preparation method thereof
InactiveCN111419871AQuality is easy to controlEasy to control and useOrganic active ingredientsDigestive systemInorganic saltsPolythylene glycol
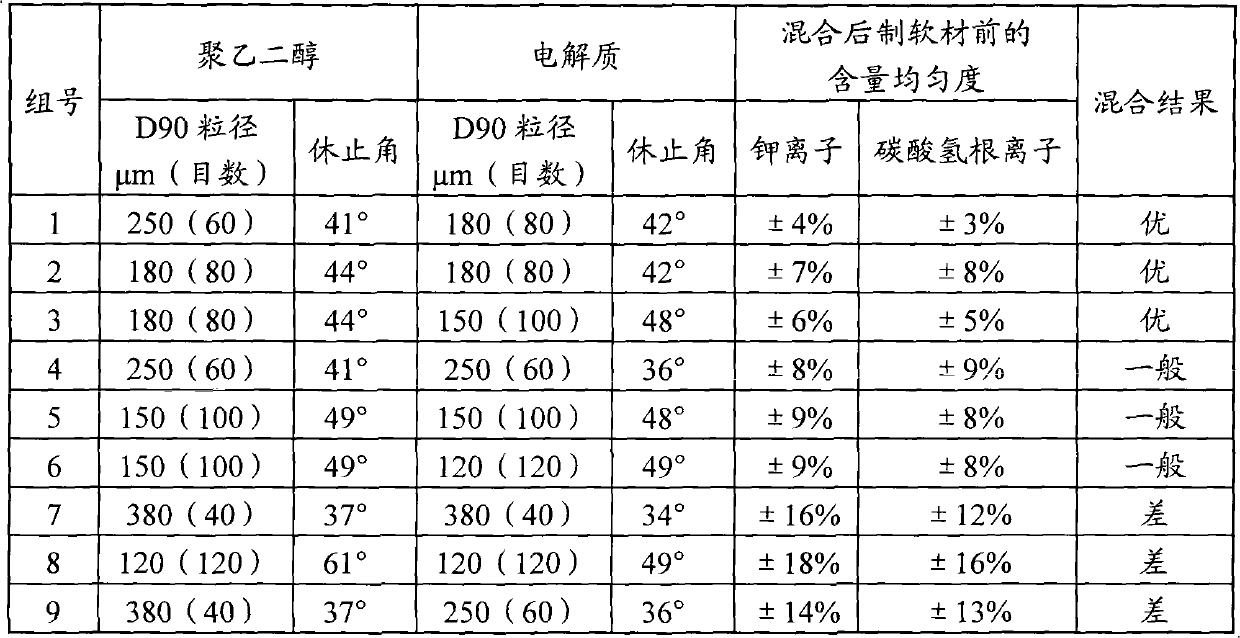
The invention discloses a compound polyethylene glycol electrolyte granule and a preparation method thereof. The compound polyethylene glycol electrolyte granule contains polyethylene glycol with an average molecular weight of 3400-4400 and an electrolyte inorganic salt, wherein the D90 particle size of the electrolyte granule is 40-80 meshes. The compound polyethylene glycol electrolyte granule is prepared by the following steps: mixing polyethylene glycol with an average molecular weight of 3400-4400 and the electrolyte inorganic salt, performing melting granulation by adopting a hot melt extruder, wherein the melting granulation temperature is 40-80 DEG C, and the screw speed is 60-140rpm; and then performing granulating by adopting a screen with a pore diameter of 0.40-4.00mm, and wrapping or packaging the granules in a packaging container in a unit dose mode to form a compound polyethylene glycol electrolyte granule product. Quality of the compound polyethylene glycol electrolytegranule provided by the invention is controllable, the problem of secondary layering in a production and preparation process of polyethylene glycol electrolyte powder is solved, and the production process is simple. The adopted melting granulation process is a continuous production process, so that production efficiency is improved, productivity is increased, and cost is reduced.
Owner:BEIJING MEDISAN TECH +1
Composite solid electrolyte material and preparation method thereof, and all-solid state electrochromic device
ActiveCN108254990AImprove ionic conductivityGood adhesionNon-linear opticsSolid state electrolyteAll solid state
The invention provides a composite solid electrolyte material. The composite solid electrolyte material comprises a highly viscous polymer porous membrane, and a polyethylene glycol electrolyte fillerplaced in pores of the highly viscous polymer porous membrane; the polyethylene glycol electrolyte filler comprises modified polyethylene glycol, transparent photocurable resin and lithium salt. Compared with the prior art, according to the composite solid electrolyte material, the highly viscous polymer porous membrane is introduced into the composite solid electrolyte material, so that the bonding performance is good; the polyethylene glycol electrolyte filler with high conductivity is placed in the pores of the porous membrane, so that the composite solid electrolyte material has responsetime comparable to that of a liquid device, and the ionic conductivity and bonding performance of an electrolyte is significantly improved to further prolong the cycle life of an all solid state electrochromic device.
Owner:UNIV OF SCI & TECH OF CHINA
Compound polyethylene glycol electrolyte composition
InactiveCN103690557AGood dispersionWell mixedPowder deliveryDigestive systemSodium bicarbonatePolyethylene glycol
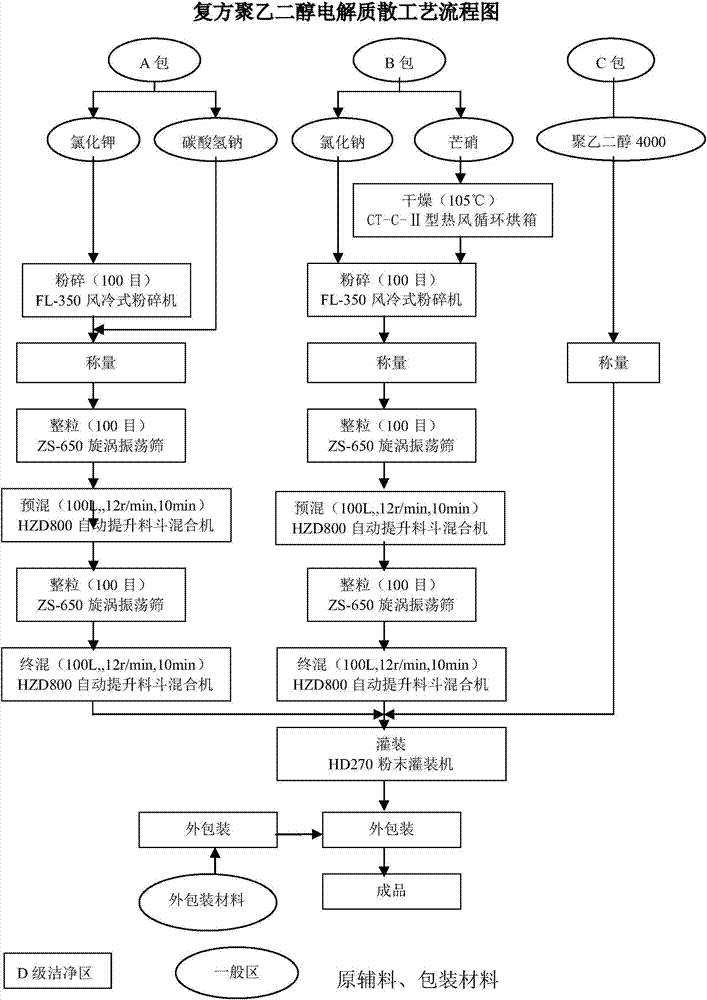
The invention discloses a compound polyethylene glycol electrolyte composition, which comprises the following components in parts by weight: 1 part of polyethylene glycol (4000), 0.01-0.05 part of potassium chloride, 0.03-0.06 part of sodium bicarbonate, 0.02-0.06 part of sodium chloride, and 0.9-1.2 parts of anhydrous sodium sulphate. The dosage form of the compound polyethylene glycol electrolyte composition disclosed by the invention is powder. The preparation method of the compound polyethylene glycol electrolyte composition comprises the following steps of respectively crushing polyethylene glycol 4000, potassium chloride, sodium bicarbonate, sodium chloride and anhydrous sodium sulphate, sieving through a 100-mesh sieve, and drying; weighing potassium chloride and sodium bicarbonate fine powder in prescription amount, uniformly mixing, and carrying out split charging in a packet A; weighing anhydrous sodium sulphate and sodium chloride fine powder in prescription amount, uniformly mixing, and split charging in a packet B; weighing polyethylene glycol 4000 fine powder in prescription amount, and split charging in a packet C; putting spared materials into a mixer to mix, and sampling to detect the content of a midbody; and carrying out split charging after calculating and loading according to the content of the midbody, thereby obtaining the compound polyethylene glycol electrolyte composition after sealing.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Polyethylene glycol electrolyte oral liquid and preparing method thereof
ActiveCN107028876AOverall small sizeImprove complianceOrganic active ingredientsDigestive systemSodium bicarbonatePatient compliance
The invention relates to polyethylene glycol electrolyte oral liquid and belongs to the field of medicine. The polyethylene glycol electrolyte oral liquid comprises polyethylene glycol, sodium bicarbonate, sodium chloride and potassium chloride. The polyethylene glycol electrolyte oral liquid has the advantages that the oral liquid is a solution and does not contain any preservative, patients can take the oral liquid directly without preparation, and the oral liquid is convenient to take and safe; the oral liquid is a concentrated preparation, required dosage is small, only 25 mL of the oral liquid is required for treating constipation, 50 mL is required for gut purge, adverse reaction like nausea and abdominal distension are avoided, and patient compliance is high.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Preparation method of polyethylene glycol electrolyte powder
InactiveCN109700827AGood dispersionWell mixedDigestive systemSulfur/selenium/tellurium inorganic active ingredientsSodium bicarbonateTreatment effect
The invention provides compound polyethylene glycol electrolyte powder and a preparation method thereof. The compound polyethylene glycol electrolyte powder consists of a bag A, a bag B and a bag C, wherein the bag A contains 1 part of potassium chloride and 2.0-2.5 parts of sodium bicarbonate, the bag B contains 1 part of sodium chloride and 3.5-4.0 parts of sodium sulphate, and the bag C contains 1 part of polyethylene glycol (4000). In the compound polyethylene glycol electrolyte powder provided by the invention, all the components are crushed into fine powder, the particle diameter of granules is very small, the specific surface area is large, all the components are easy to disperse, improvement of the dispersibility of the powder of the components is facilitated, uniform mixing of thecomponents in the powder is facilitated, the treatment effects and the medicine effects of the powder are improved, and accelerating the dissolving speed of the powder is also facilitated.
Owner:四川健能制药有限公司
Polyethylene glycol electrolyte oral solution
ActiveCN1850112BGood effectOrganic active ingredientsMetabolism disorderPolyethylene glycolIntestino-intestinal
Owner:BEIJING SHENGYONG PHARMA
Compound polyethylene glycol electrolyte and preparation method thereof
PendingCN114366757AOvercoming Difficulties of Inhomogeneous MixingFix storage stability issuesPowder deliveryDigestive systemPolythylene glycolPhysical chemistry
The invention discloses a compound polyethylene glycol electrolyte composition. The compound polyethylene glycol electrolyte composition is prepared from the following components in parts by mass: 1 part of polyethylene glycol (4000), 0.01 to 0.05 part of potassium chloride, 0.02 to 0.05 part of sodium bicarbonate, 0.02 to 0.05 part of sodium chloride, 0.07 to 1.0 part of anhydrous sodium sulfate and 0.001 to 0.01 part of saccharin sodium salt. The preparation method of the compound polyethylene glycol electrolyte composition comprises the following steps: (1) respectively crushing or screening sodium bicarbonate, anhydrous sodium sulfate, potassium chloride, sodium chloride and saccharin sodium salt; (2) weighing sodium bicarbonate, anhydrous sodium sulfate, potassium chloride, sodium chloride and saccharin sodium according to the prescription amount, and mixing in a wet granulation mixer; (3) weighing the polyethylene glycol 4000 and the premixed powder according to the prescription amount, mixing the polyethylene glycol 4000 and the premixed powder in a hopper type mixer, sampling and detecting the content of an intermediate; and (4) sub-packaging: calculating the packaging amount according to the content of the intermediate, and sub-packaging by adopting a composite membrane.
Owner:重庆健能医药开发有限公司
Polyethylene glycol-electrolyte oral solution
ActiveCN101804068AHigh clarityOsmolality ratio is stable and accurateOrganic active ingredientsMetabolism disorderBowel cleansingSodium bicarbonate
The invention provides polyethylene glycol-electrolyte oral solution, which comprises 59 to 60 grams of polyethylene glycol, 1.0 to 1.5 grams of sodium chloride, 0.5 to 1.0 gram of potassium chloride, 1.5 to 2.0 grams of sodium bicarbonate, 5.5 to 6.0 grams of sodium sulfate, 0.02 to 0.5 gram of edentate, 0.02 to0.5 gram of sweetener, 1 to 2 milliliters of flavoring essence and the balance of deionized water, wherein the solution is 1,000 milliliters. The polyethylene glycol-electrolyte oral solution is used in bowel cleansing and has the characteristics of quick response, safety, reliability and good cleansing effect. The oral solution is mainly suitable for cleansing bowels before colonoscopy, barium enema X-ray examination and gastrointestinal operation. The small-dose (about one eighth of a bowel cleansing dose) products of the oral solution can be used for treating chronic constipation.
Owner:BEIJING SHENGYONG PHARMA
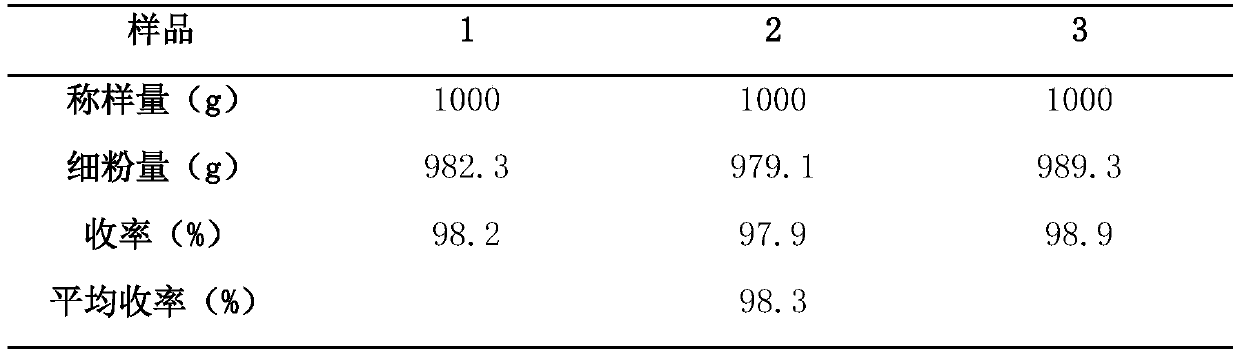
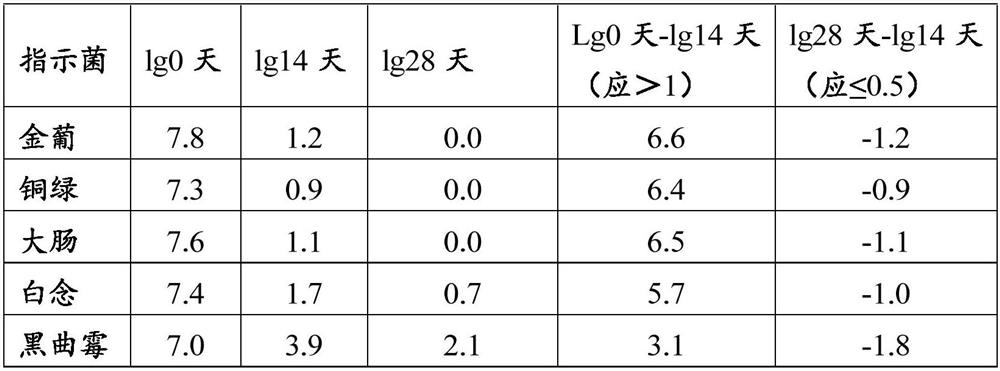
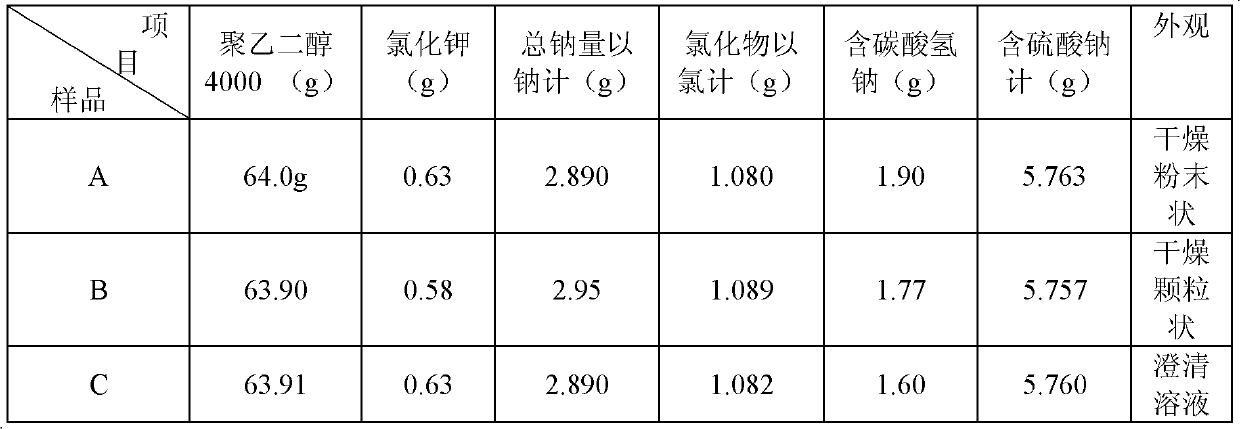
Method for determining content of chloride in compound polyethylene glycol electrolyte powder
PendingCN112326870AEliminate distractionsChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorSodium bicarbonatePolyethylene glycol
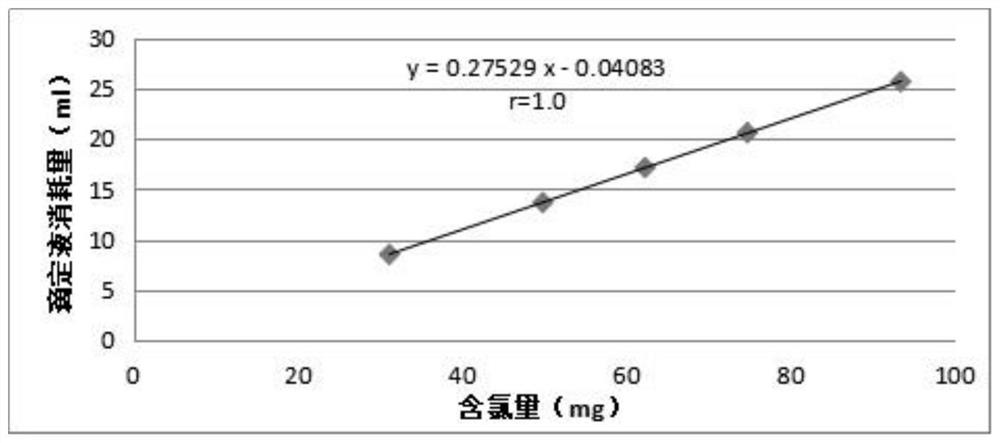
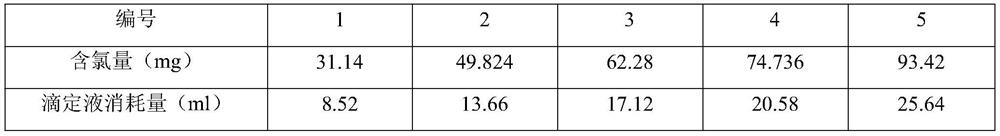
A fluorescein indicator and a silver precipitation method are selected to determine the content of chloride in s compound polyethylene glycol electrolyte powder, and dilute sulfuric acid is used to eliminate the interference of sodium bicarbonate in the compound polyethylene glycol electrolyte powder. A proper amount of a sample solution is taken, and is put into a conical flask, 1 drop of a phenolphthalein indicator, 5 ml of a 2% dextrin solution, 2 ml of a 2.5% borax solution and a proper amount of dilute sulfuric acid are added to disappear red, 5-8 drops of a fluorescein indicator solutionare added, and titrating is carried out with a silver nitrate titrating solution (0.1 mol / L) until the fluorescence color of the solution fades and becomes light pink.
Owner:四川健能制药有限公司
Preparation method of compound polyethylene glycol electrolyte particles
PendingCN114209714ASmall variationSolve for uniformityDigestive systemSynthetic polymeric active ingredientsPolyethylene glycolEngineering
The invention discloses a preparation method of compound polyethylene glycol electrolyte particles. The compound polyethylene glycol electrolyte granules are prepared by adopting a spray drying process. The preparation technology is simple, industrialization is easy to achieve, the quality of the compound polyethylene glycol electrolyte particles prepared through the technology is controllable, and the problems of mixing uniformity, secondary layering and packaging adsorption in the production and preparation process of the polyethylene glycol electrolyte particles are solved.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
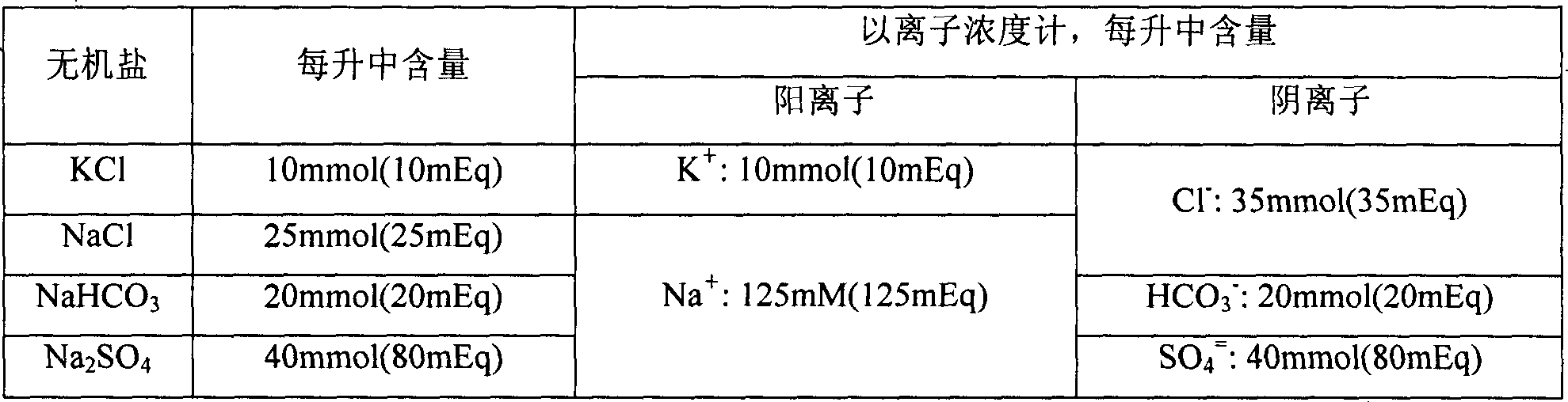
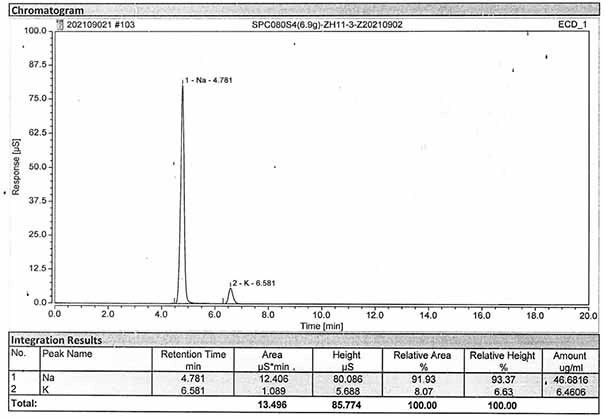
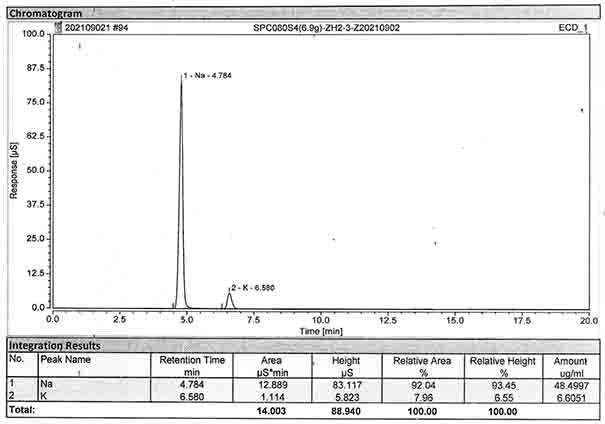
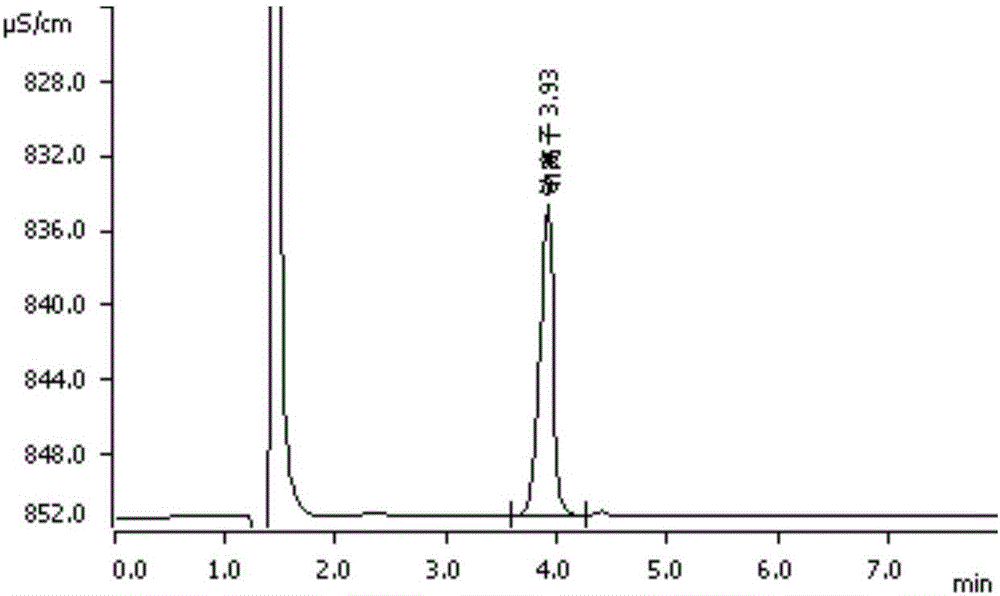
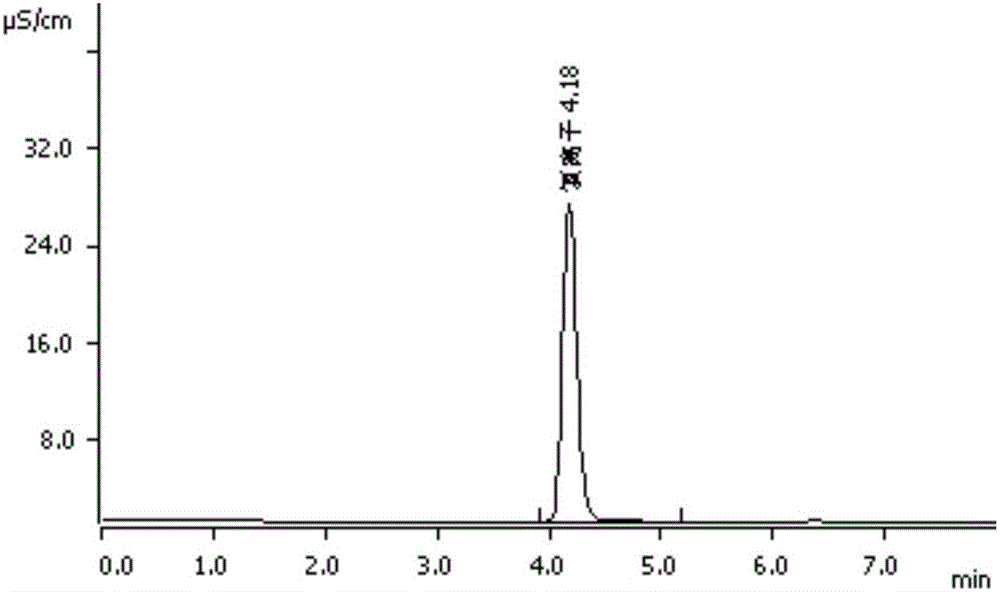
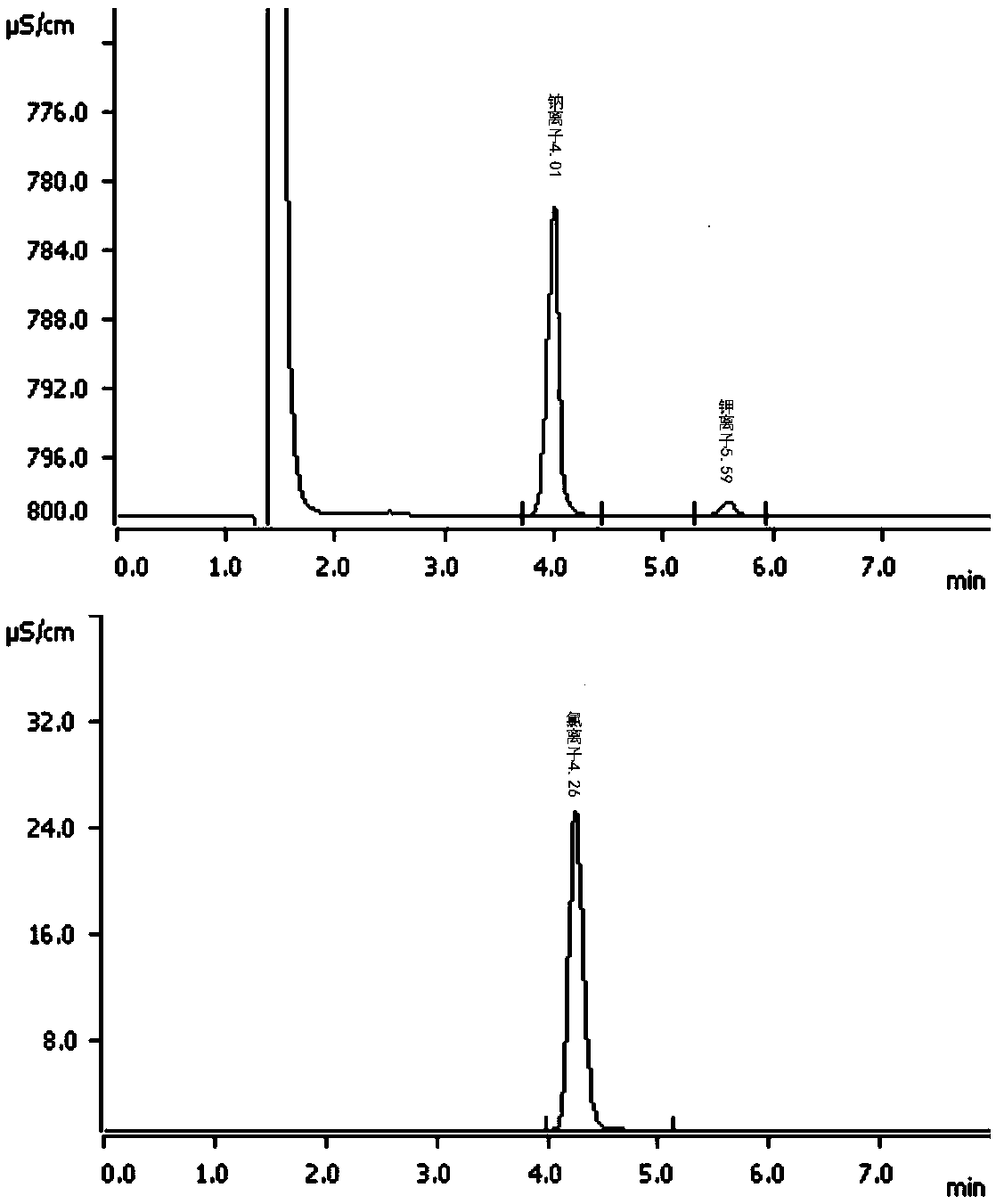
Method for detecting content of sodium, potassium and chloride ions in polyethylene glycol electrolyte preparation
InactiveCN106645546AImprove detection efficiencySimple methodComponent separationSodium bicarbonatePolyethylene glycol
The invention belongs to the field of medicine, and particularly provides a method for detecting the content of sodium, potassium and chloride ions in a polyethylene glycol electrolyte preparation by using a high performance liquid chromatography method. According to the specific chromatographic condition, the leacheate of cation sodium and potassium is an aqueous solution of 2.3-2.7mM nitric acid, having a flowing speed of 0.8-1.2ml / min; and the leacheate of anion chloride is an aqueous solution of 3.2-3.8mM sodium carbonate and 0.9-1.1mM sodium bicarbonate, having a flow speed of 0.6-0.8ml / min; the chromatographic column is an ion-exchange chromatographic column; and the detector is an electrical conductivity detector. The detecting method has the advantages of strong specificity, simple and quick operation, high sensitivity, relatively good repeatability, relatively good precision and the like.
Owner:STAIDSON BEIJING BIOPHARMACEUTICALS CO LTD +1
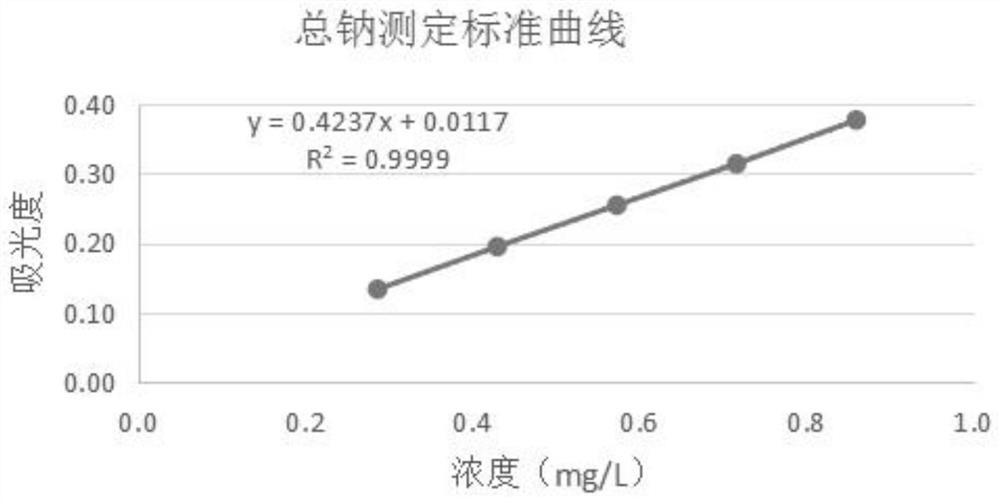
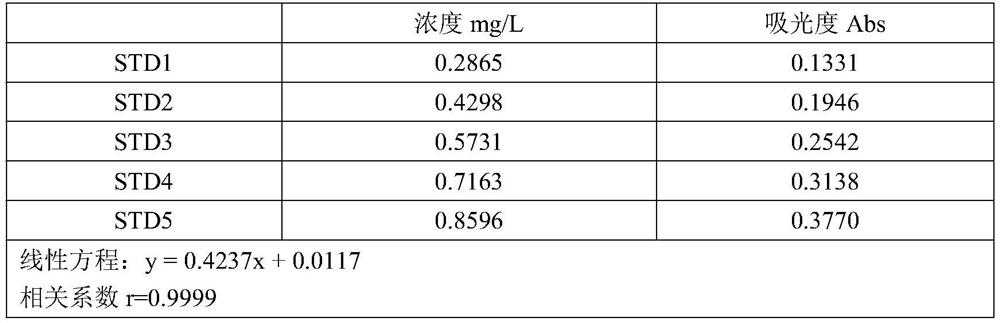
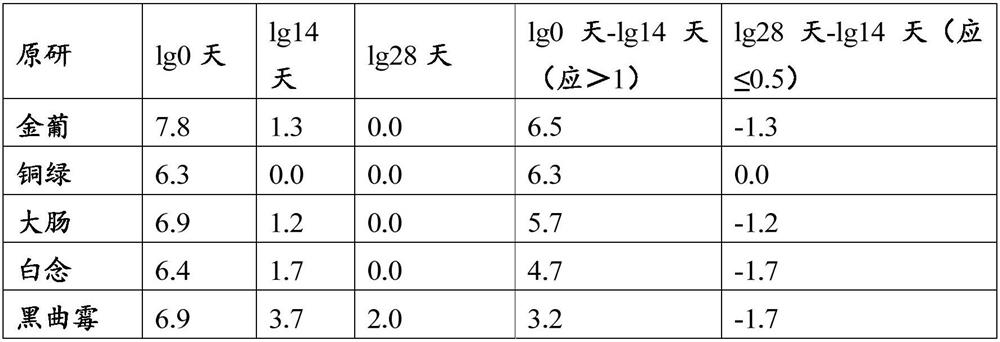
Method for determining total sodium content in compound polyethylene glycol electrolyte powder
PendingCN113029987AEliminate distractionsPlay an inhibitory roleColor/spectral properties measurementsPolyethylene glycolPolyethylene glycol electrolyte powder
The invention discloses a method for measuring the total sodium content in compound polyethylene glycol electrolyte powder. The method comprises the following steps: (1) preparing a reference substance solution, namely preparing the reference substance solution with the sodium element concentration of 14.33494 mg / L; (2) preparing a standard solution: precisely measuring the reference solution to prepare a sodium element series standard solution; (3) preparation of a test solution: taking the content of the compound polyethylene glycol electrolyte powder, and preparing the content with the concentration matched with the sodium element series standard solution; (4) preparation of a blank solution: precisely weighing a proper amount of polyethylene glycol, and preparing a solution with a concentration matched with that of the test solution; and (5) respectively taking the blank solution, the standard solution and the test solution, measuring absorbance at the wavelength of 589.0 nm, drawing a regression equation according to the concentration of the standard solution and the absorbance, and calculating the concentration of sodium in the test solution. The method disclosed by the invention is simple and rapid to operate, high in sensitivity and good in repeatability, the solution stability in the method is good, and the measured data is more accurate.
Owner:上海耀大生物科技有限公司
Polyethylene glycol electrolyte granular preparation and production method
ActiveCN101766648BQuality is easy to controlGreat tasteOrganic active ingredientsDigestive systemPolyethylene glycolElectrolyte
The invention provides a polyethylene glycol electrolyte granular preparation and a production method. The polyethylene glycol electrolyte granular preparation comprises polyethylene glycol with the average molecular weight of 3000 to 4000 and electrolyte, and the granule diameter of the polyethylene glycol electrolyte granule is 180Mu m to 850Mu m. The invention at least solves the following problems of the prior polyethylene glycol electrolyte granular preparation that: the production cost is high, the mixing of components is not uniform, the weight cannot be controlled, use is complex, thegranule diameters of medicinal powder are not uniform, fluidity is not good, and stability is poor.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Polyethylene glycol electrolyte oral liquid and preparation method thereof
ActiveCN107028876BOverall small sizeImprove complianceOrganic active ingredientsDigestive systemSodium bicarbonatePatient compliance
The invention relates to polyethylene glycol electrolyte oral liquid and belongs to the field of medicine. The polyethylene glycol electrolyte oral liquid comprises polyethylene glycol, sodium bicarbonate, sodium chloride and potassium chloride. The polyethylene glycol electrolyte oral liquid has the advantages that the oral liquid is a solution and does not contain any preservative, patients can take the oral liquid directly without preparation, and the oral liquid is convenient to take and safe; the oral liquid is a concentrated preparation, required dosage is small, only 25 mL of the oral liquid is required for treating constipation, 50 mL is required for gut purge, adverse reaction like nausea and abdominal distension are avoided, and patient compliance is high.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Determination method of anion and cation content in polyethylene glycol electrolyte preparation
ActiveCN106568886BImprove detection efficiencySimple methodComponent separationSodium bicarbonateOrganic solvent
The invention belongs to the field of medicine, and specifically provides a method of using HPLC (High Performance Liquid Chromatography) to measuring the contents of cations and anions in a polyethylene glycol electrolyte preparation. The chromatographic conditions are as follows: the cation eluent is a nitric acid water solution (2.3-2.7 M) containing a water soluble organic solvent accounting for 5 to 20 vol.% of the eluent; the flowing speed is 0.8 to 1.2 mL / min; the anion eluent is a water solution of sodium carbonate (3.2-3.8 mM) and sodium bicarbonate (0.9-1.1 mM), the flowing speed is 0.6 to 0.8 mL / min, and the detector is an electrical conductivity detector. The measuring method has the advantages of strong specificity, simple and quick operation, capability for prolonging the service life of chromatographic column, and high sensitivity.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Compound polyethylene glycol electrolyte composition
ActiveCN102772427BPowder deliveryMetabolism disorderSODIUM SULFATE ANHYDROUSPolyethylene glycol electrolyte
Owner:SHENZHEN WANHE PHARMA
Polyethylene glycol-electrolyte oral solution
ActiveCN101804068BGood effectOrganic active ingredientsMetabolism disorderSodium bicarbonateBowel cleansing
The invention provides polyethylene glycol-electrolyte oral solution, which comprises 59 to 60 grams of polyethylene glycol, 1.0 to 1.5 grams of sodium chloride, 0.5 to 1.0 gram of potassium chloride, 1.5 to 2.0 grams of sodium bicarbonate, 5.5 to 6.0 grams of sodium sulfate, 0.02 to 0.5 gram of edentate, 0.02 to0.5 gram of sweetener, 1 to 2 milliliters of flavoring essence and the balance of deionized water, wherein the solution is 1,000 milliliters. The polyethylene glycol-electrolyte oral solution is used in bowel cleansing and has the characteristics of quick response, safety, reliabilityand good cleansing effect. The oral solution is mainly suitable for cleansing bowels before colonoscopy, barium enema X-ray examination and gastrointestinal operation. The small-dose (about one eighth of a bowel cleansing dose) products of the oral solution can be used for treating chronic constipation.
Owner:BEIJING SHENGYONG PHARMA
Compound polyethylene glycol electrolyte powder and preparation method thereof
The invention relates to compound polyethylene glycol electrolyte powder and a preparation method thereof, in particular to a powder compound containing polyethylene glycol and electrolyte. The powder compound comprises, by weight, 6000 parts of polyethylene glycol, 118-176 parts of sodium chloride, 60-90 parts of potassium chloride, 137-205 parts of sodium bicarbonate, 461-692 parts of anhydrous sodium sulfate, 19-29 parts of powder essence, and / or 5-7.6 parts of aspartame. The compound polyethylene glycol electrolyte powder has fine micromeritic properties and taste.
Owner:SHENZHEN WANHE PHARMA
A kind of electrolyte powder containing polyethylene glycol and preparation method thereof
ActiveCN111617102BSimilar bulk densityReduced instances of dysphagiaOrganic active ingredientsPowder deliveryInorganic saltsPolyethylene glycol
The invention discloses an electrolyte powder containing polyethylene glycol. The electrolyte powder contains polyethylene glycol, polylactic acid, inorganic salts and auxiliary agents. The content of each component is: polyethylene glycol 70~80%, polylactic acid 10~20%, inorganic salts 0.5~1%, and additives 1~3%. The invention also discloses a preparation method of the polyethylene glycol electrolyte powder. The polyethylene glycol electrolyte powder of the invention has good effect and few adverse reactions, and is suitable for patients. Moreover, the preparation method is simple, the raw materials are easy to obtain, and the cost is low.
Owner:南京金凯木纳米材料有限公司
MHR stepped immunity recombination therapy
InactiveCN105617518AMinimally invasive and painless treatmentThe whole treatment is safe and reliableGastroscopesOesophagoscopesSide effectMedicine
The invention discloses an MHR stepped immunity recombination therapy, comprising the following steps: orally taking 4000 ML of compound polyethylene glycol electrolyte powder the day before treatment until clear stool is present in defecation and contains little solid; subjecting a focus to fine examination, diagnosis and typing by an imported digital ultrasonic electronic gastroscopic device; targeting and focusing ulcers under acute and fine high-definition images to subject enteric cavity ulcers, erosion area and distribution to targeted spot injection repairing, and precisely repairing at ulcer mucous layers, from spot to area.The therapy of the invention is a safe and effective green therapy with no side effect.
Owner:BEIJING ZHONGSHAN HOSPITAL CO LTD
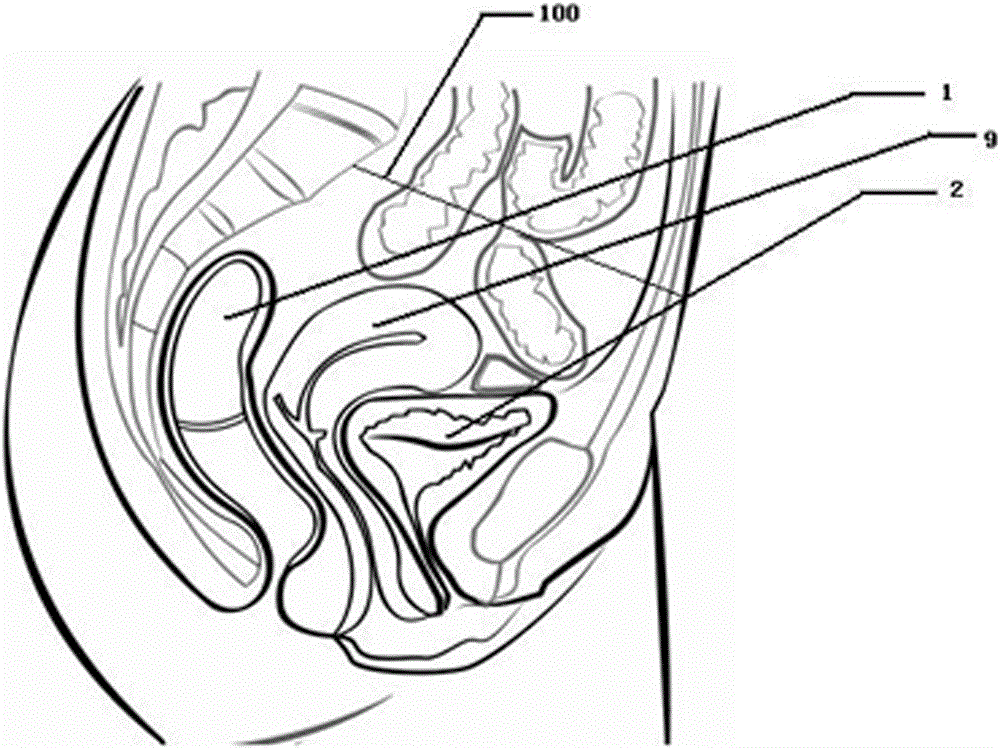
Method for detecting pelvic organs by ultrasound intracavitary probe after filling of transrectal enteric cavity
InactiveCN106264619AEasy to acceptEasy Early DiagnosisOrgan movement/changes detectionSurgeryDiseaseTargeted therapy
The invention discloses a method for detecting pelvic organs by an ultrasound intracavitary probe after filling of a transrectal enteric cavity contrast agent, and belongs to the technical field of medical uses. The method comprises the following steps: 1, giving an oral dose of compound polyethylene glycol electrolyte powder for a patient; 2, preparing an ultrasound contrast agent; 3, carrying out a colorectal ultrasound contrast examination. The method disclosed by the invention has the advantages of easy and simple operation, safety and capability of being repeatedly examined, meets requirements of noninvasive diagnosis and treatment, is more easily accepted by the patient, and further conducive to the early diagnosis of pelvic diseases and targeted therapies of local regions, thereby being specially suitable for the examinations of unmarried adult women; the method enables the pelvic organs to be visualized and standardized, and improves the scientificity, furthermore, the transrectal enteric cavity contrast agent of the invention has the advantages of being a gastric filling contrast agent for oral use, non-toxic and harmless to a human body, reasonable in design, safer and more effective in use and suitable for the early diagnosis of the pelvic diseases, differential diagnosis and the diagnosis and treatment of the targeted therapies of the local regions, thereby having stronger market popularization values.
Owner:SHAOXING UNIVERSITY
Compound polyethylene glycol electrolyte pulvis and preparation method thereof
InactiveCN102133225BSimilar bulk densityOvercoming Difficulties of Inhomogeneous MixingOrganic active ingredientsPowder deliverySodium bicarbonatePolyethylene glycol
The invention relates to a compound polyethylene glycol electrolyte pulvis and a preparation method thereof. The compound polyethylene glycol electrolyte pulvis comprises the following components: 1 part of polyethylene glycol (4000), 0.01-0.06 part of sodium chloride, 0.05-0.12 part of anhydrous sodium sulfate, 0.007-0.04 part of potassium chloride, and 0.009-0.046 part of sodium bicarbonate. The preparation method of the compound polyethylene glycol electrolyte pulvis comprises the following steps: respectively smashing, screening and drying the polyethylene glycol 4000, the sodium chloride, the anhydrous sodium sulfate, the potassium chloride and the sodium bicarbonate; weighting the polyethylene glycol 4000, the sodium chloride, the anhydrous sodium sulfate, the potassium chloride andthe sodium bicarbonate according to the prescription for later use; putting the materials into a mixer for mixing; sampling and detecting the content of intermediates; calculating the package quantity according to the content of the intermediates and then packaging in batch; and sealing, thereby acquiring the compound polyethylene glycol electrolyte pulvis.
Owner:HAINAN JINRUI PHARMA CO LTD
A composite solid electrolyte material and its preparation method, all-solid-state electrochromic device
ActiveCN108254990BImprove ionic conductivityGood adhesionNon-linear opticsSolid state electrolyteAll solid state
The invention provides a composite solid electrolyte material. The composite solid electrolyte material comprises a highly viscous polymer porous membrane, and a polyethylene glycol electrolyte fillerplaced in pores of the highly viscous polymer porous membrane; the polyethylene glycol electrolyte filler comprises modified polyethylene glycol, transparent photocurable resin and lithium salt. Compared with the prior art, according to the composite solid electrolyte material, the highly viscous polymer porous membrane is introduced into the composite solid electrolyte material, so that the bonding performance is good; the polyethylene glycol electrolyte filler with high conductivity is placed in the pores of the porous membrane, so that the composite solid electrolyte material has responsetime comparable to that of a liquid device, and the ionic conductivity and bonding performance of an electrolyte is significantly improved to further prolong the cycle life of an all solid state electrochromic device.
Owner:UNIV OF SCI & TECH OF CHINA
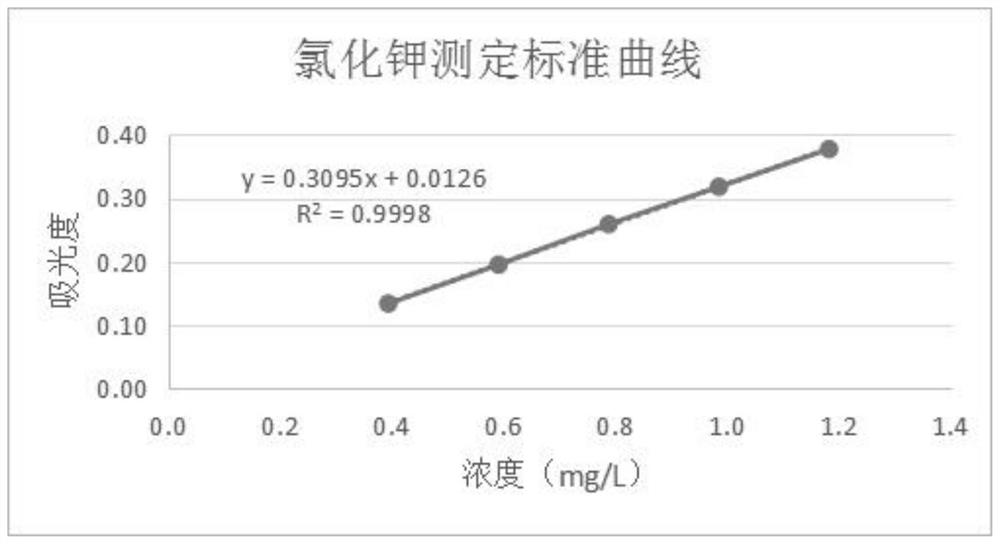
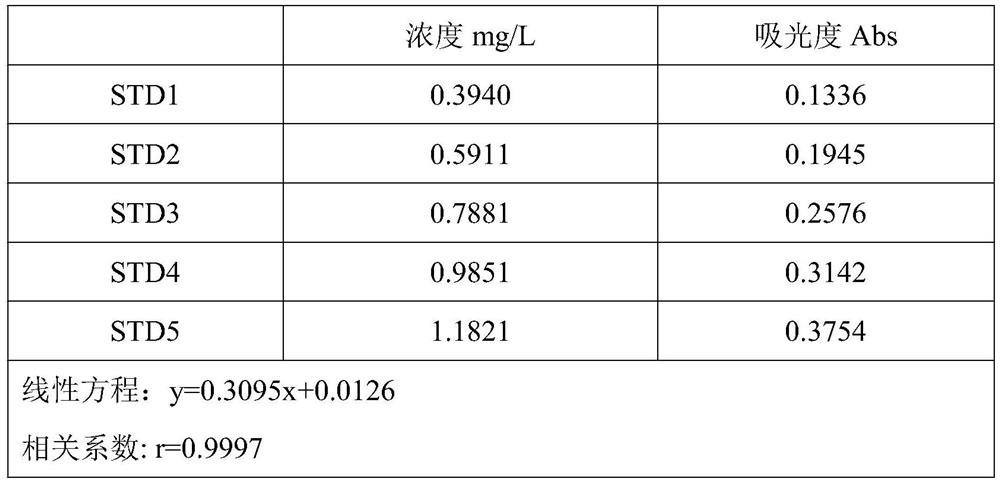
Method for determining content of potassium chloride in compound polyethylene glycol electrolyte powder
PendingCN113466149AEliminate distractionsPlay an inhibitory rolePreparing sample for investigationColor/spectral properties measurementsPolyethylene glycolPolyethylene glycol electrolyte powder
The invention discloses a method for determining the content of potassium chloride in compound polyethylene glycol electrolyte powder. The method comprises the following steps: (1) preparing a reference substance solution: preparing a reference substance solution of potassium element; (2) preparing a standard solution: precisely measuring the reference solution to prepare a sodium element series standard solution; (3) preparing a test solution: taking the content of the compound polyethylene glycol electrolyte powder, and preparing a concentration matched with the potassium element series standard solution; (4) preparing a blank solution: precisely weighing a proper amount of polyethylene glycol, and preparing a solution with a concentration matched with that of the test solution; and (5) respectively taking the blank solution, the standard solution and the test solution, measuring absorbance at the wavelength of 766.5 nm, drawing a regression equation according to the concentration of the standard solution and the absorbance, and calculating the concentration of potassium chloride in the test solution. The method disclosed by the invention is simple and rapid to operate, high in sensitivity and high in repeatability, the solution stability in the method is good, and the measured data is more accurate.
Owner:上海耀大生物科技有限公司 +1
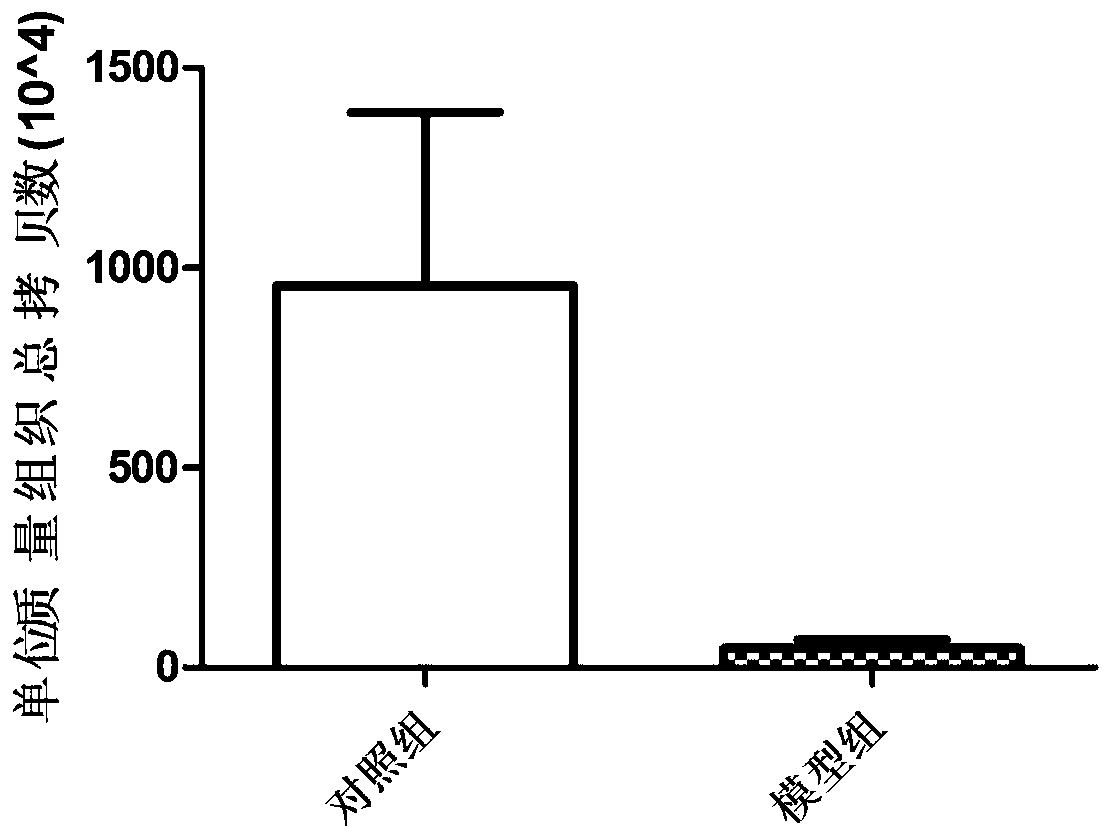
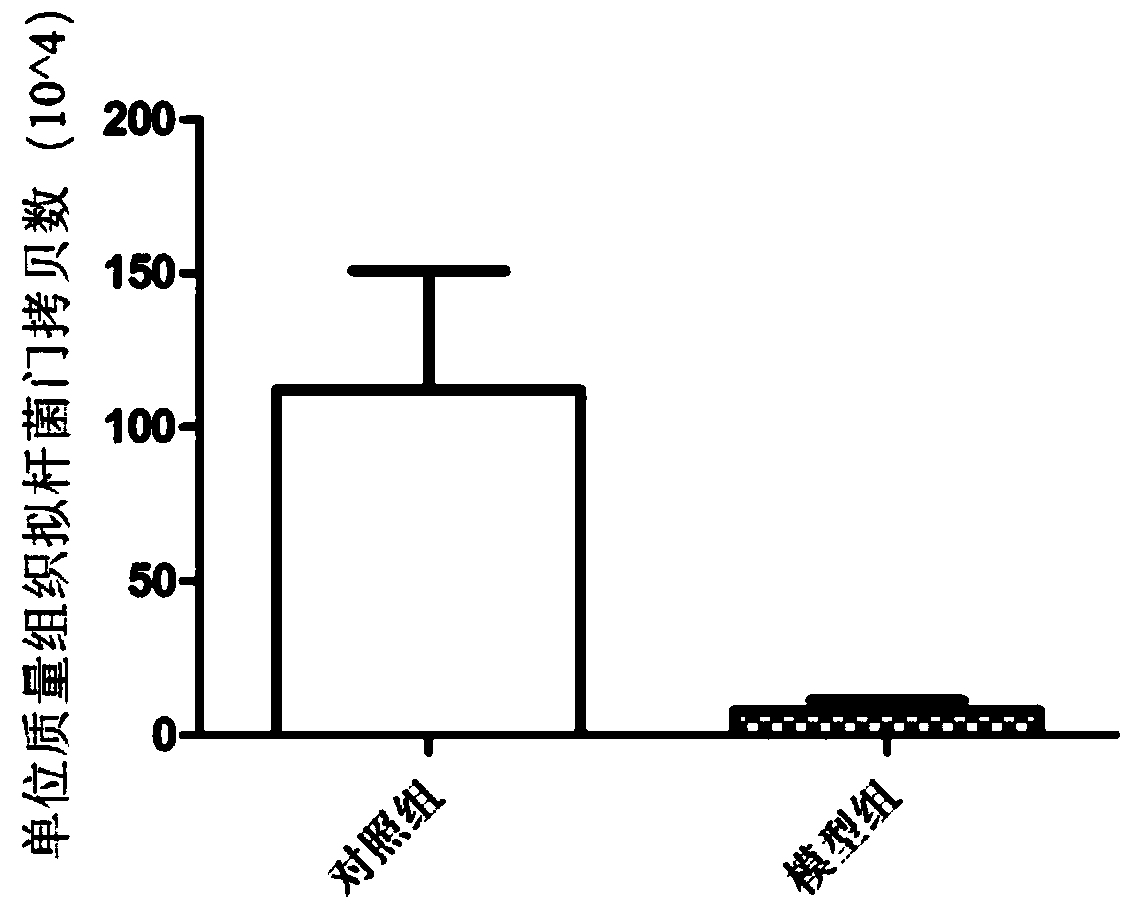
Method for constructing rodent model for intestinal flora
The invention discloses a method for constructing a rodent model for intestinal flora, and relates to the technical field of research on the intestinal flora. According to the method, a mouse is usedas an experimental object, and is fed with polyethylene glycol electrolyte powder through gavage to obtain the rodent model for the research on the intestinal flora; the polyethylene glycol electrolyte powder is used twice every day at an interval of 4 hours. The method has the advantages of short model construction time that is only 8 hours for a whole process, quickness, simplicity, feasibility,remarkable effect, no side effect and high repetition rate.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Electrolyte powder containing polyethylene glycol and preparation method of electrolyte powder
ActiveCN111617102ASimilar bulk densityReduced instances of dysphagiaOrganic active ingredientsPowder deliveryInorganic saltsPolyethylene glycol
The invention discloses electrolyte powder containing polyethylene glycol. The electrolyte powder contains polyethylene glycol, polylactic acid, inorganic salts and auxiliaries, and the electrolyte powder comprises components in percentage by weight as follows: 70%-80% of polyethylene glycol, 10%-20% of polylactic acid, 0.5%-1% of inorganic salts and 1%-3% of the auxiliaries. The invention furtherdiscloses a preparation method of the electrolyte powder containing polyethylene glycol. The electrolyte powder containing polyethylene glycol has a good effect, has few adverse reactions and is suitable for being used by patients. Besides, the preparation method is simple, the raw materials are easily available, and the cost is relatively low.
Owner:南京金凯木纳米材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com