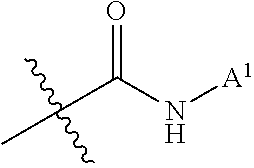
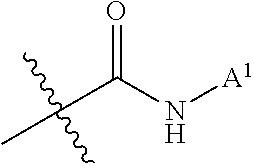
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "Phenylcarbamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbanilic acid; phenylcarbamic acid; from MeSH. Depositor-Supplied Synonyms. Chemical names and identifiers provided by individual data contributors and associated to PubChem Substance records. Synonyms of Substances corresponding to a PubChem Compound record are combined.
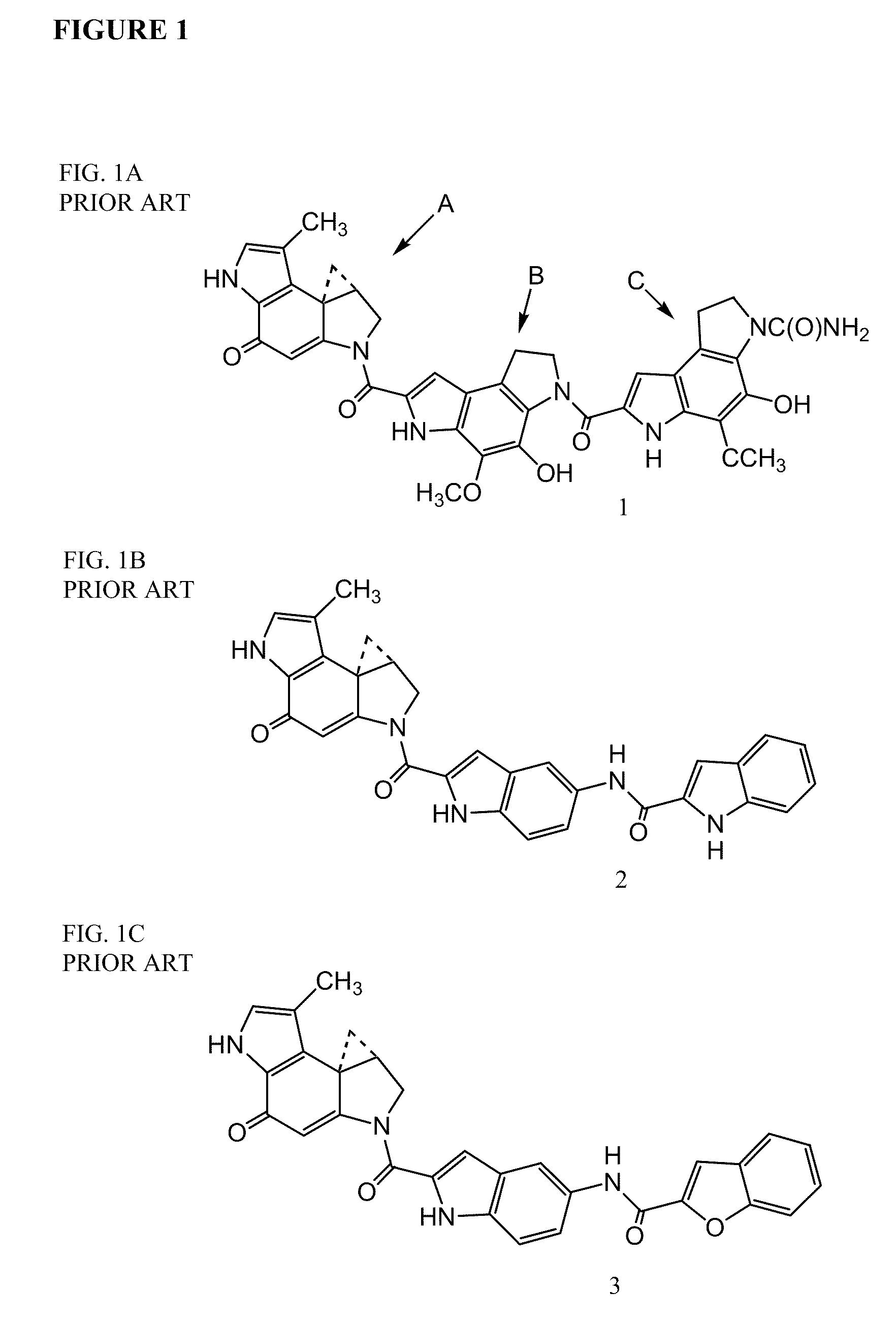
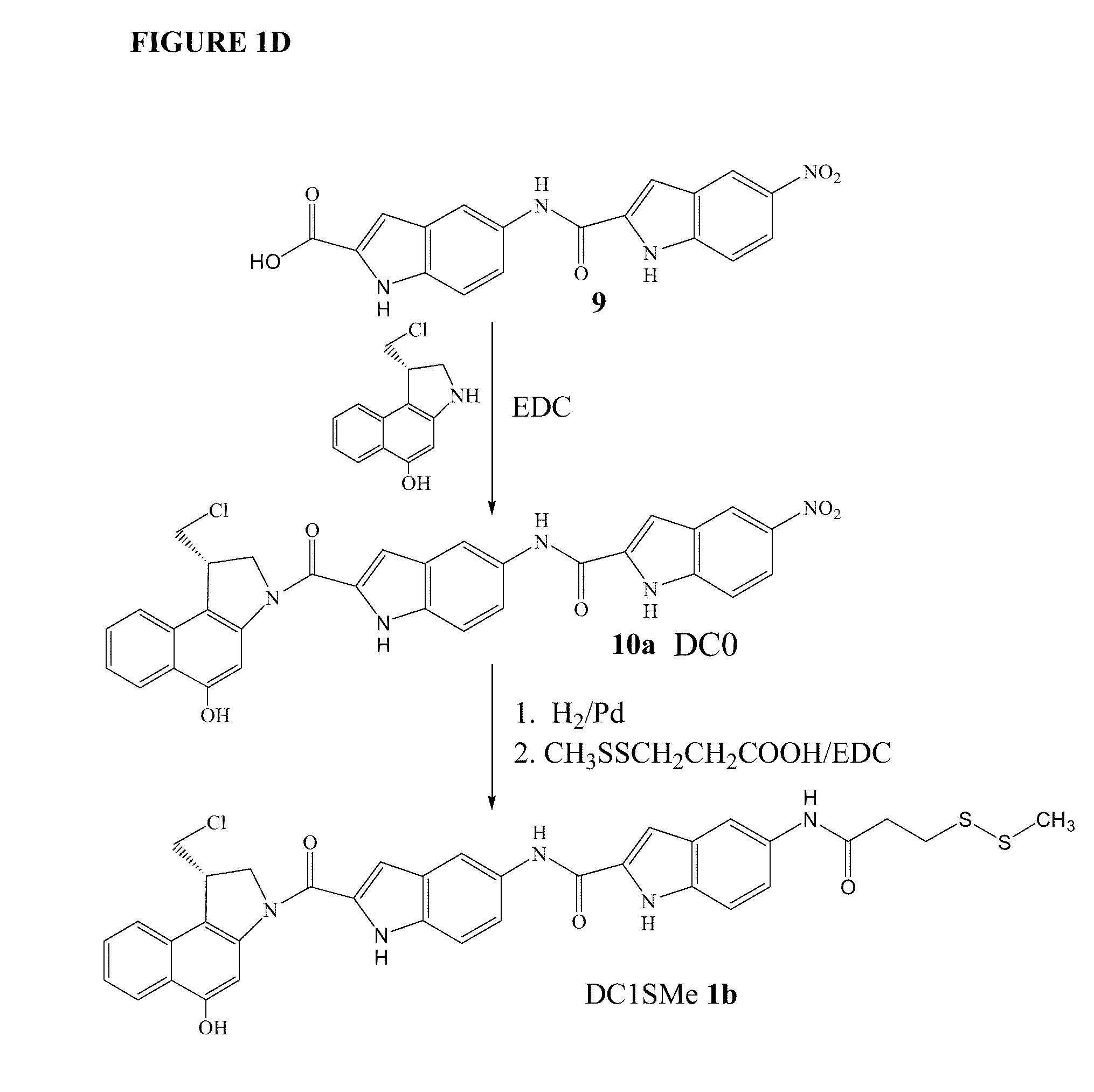
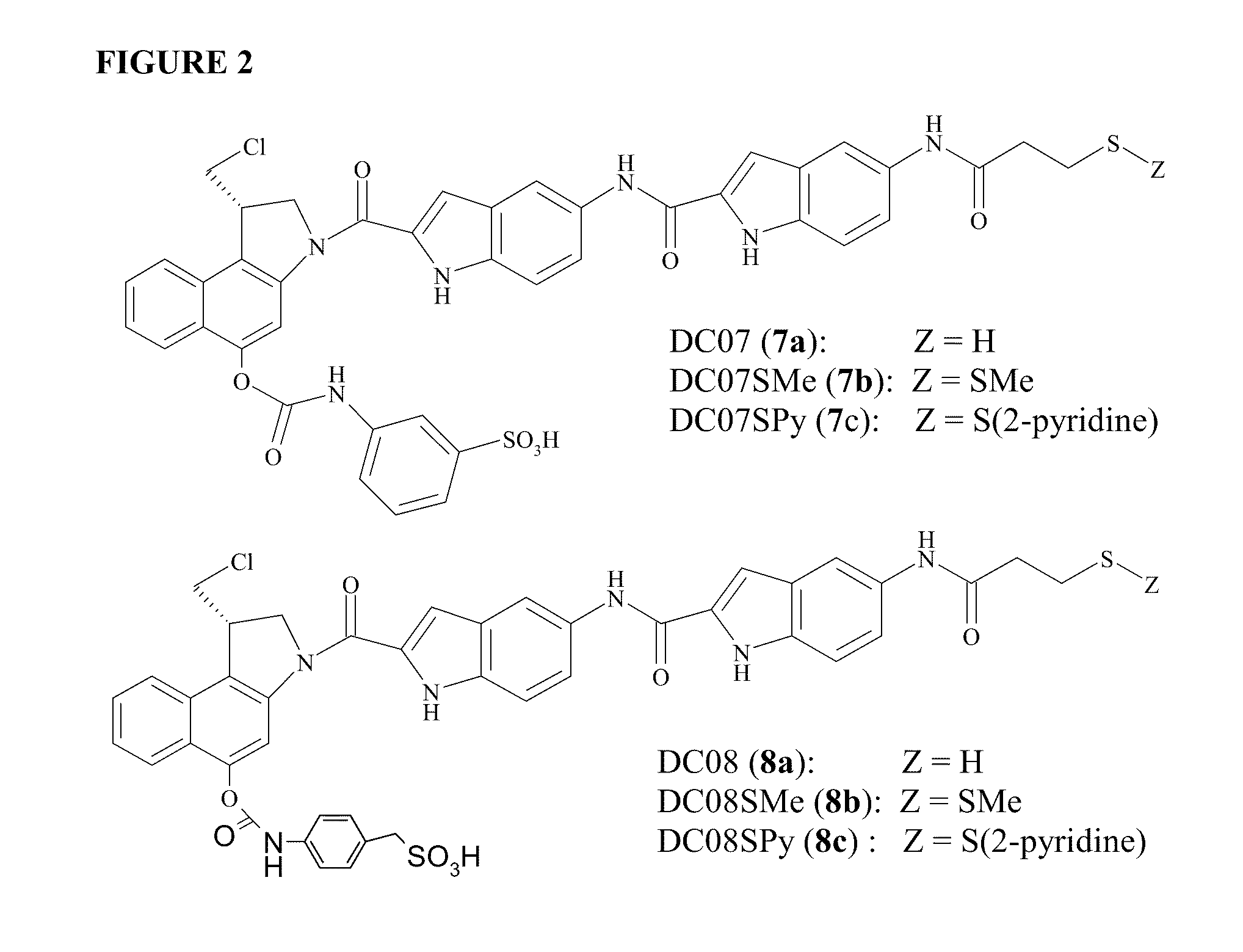
Prodrugs of CC-A1065 analogs
InactiveUS8012978B2Improve solubilityGood water solubilityAntibacterial agentsBiocideSolubilityCarbamate
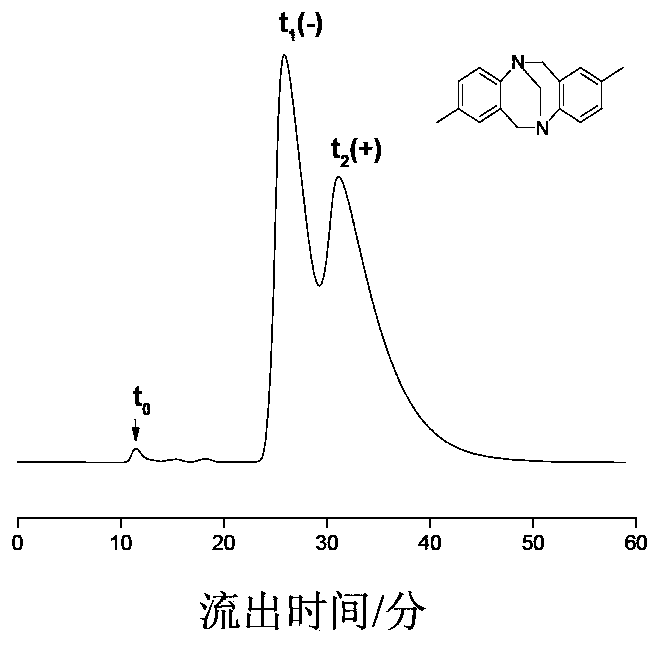
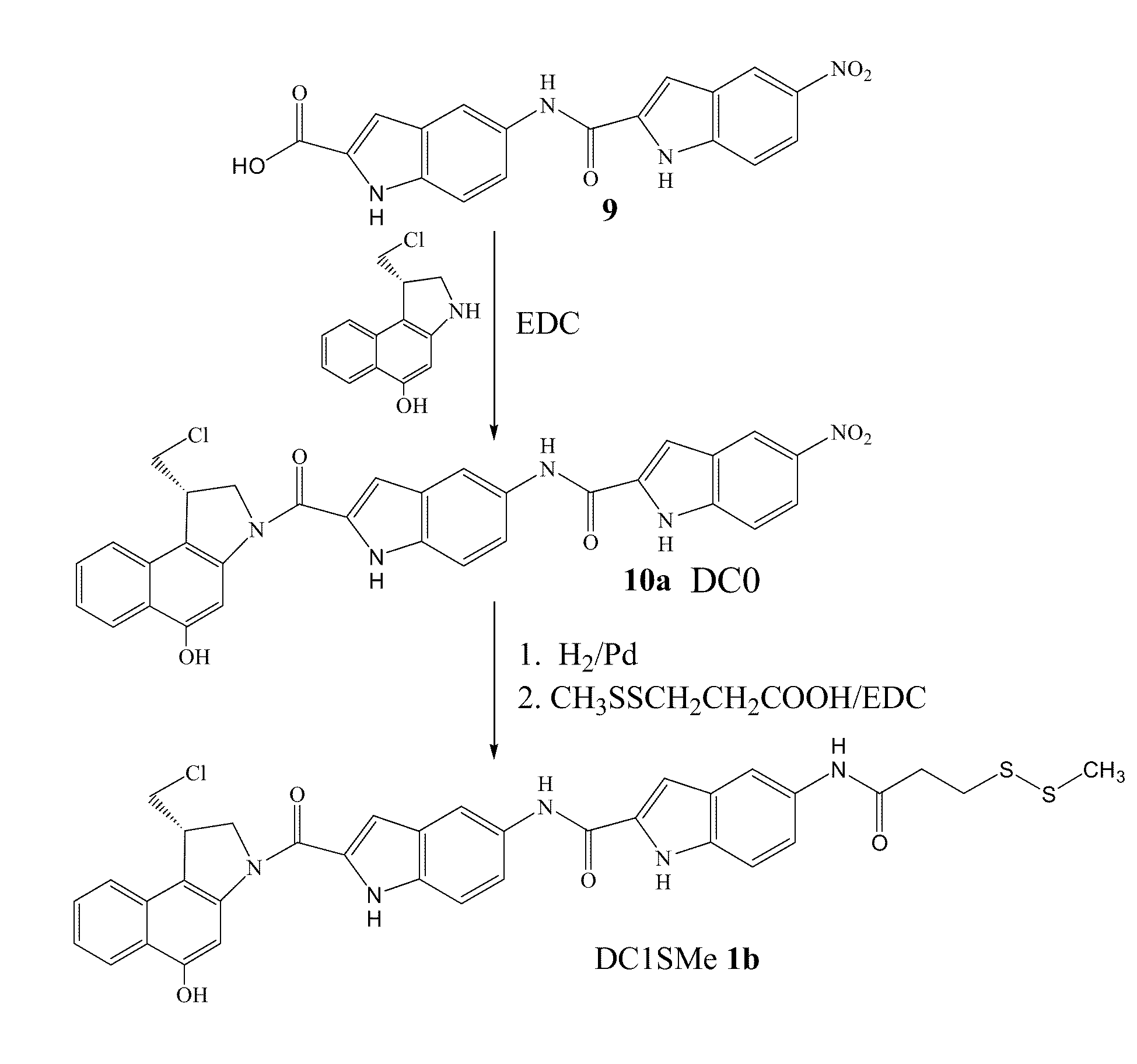
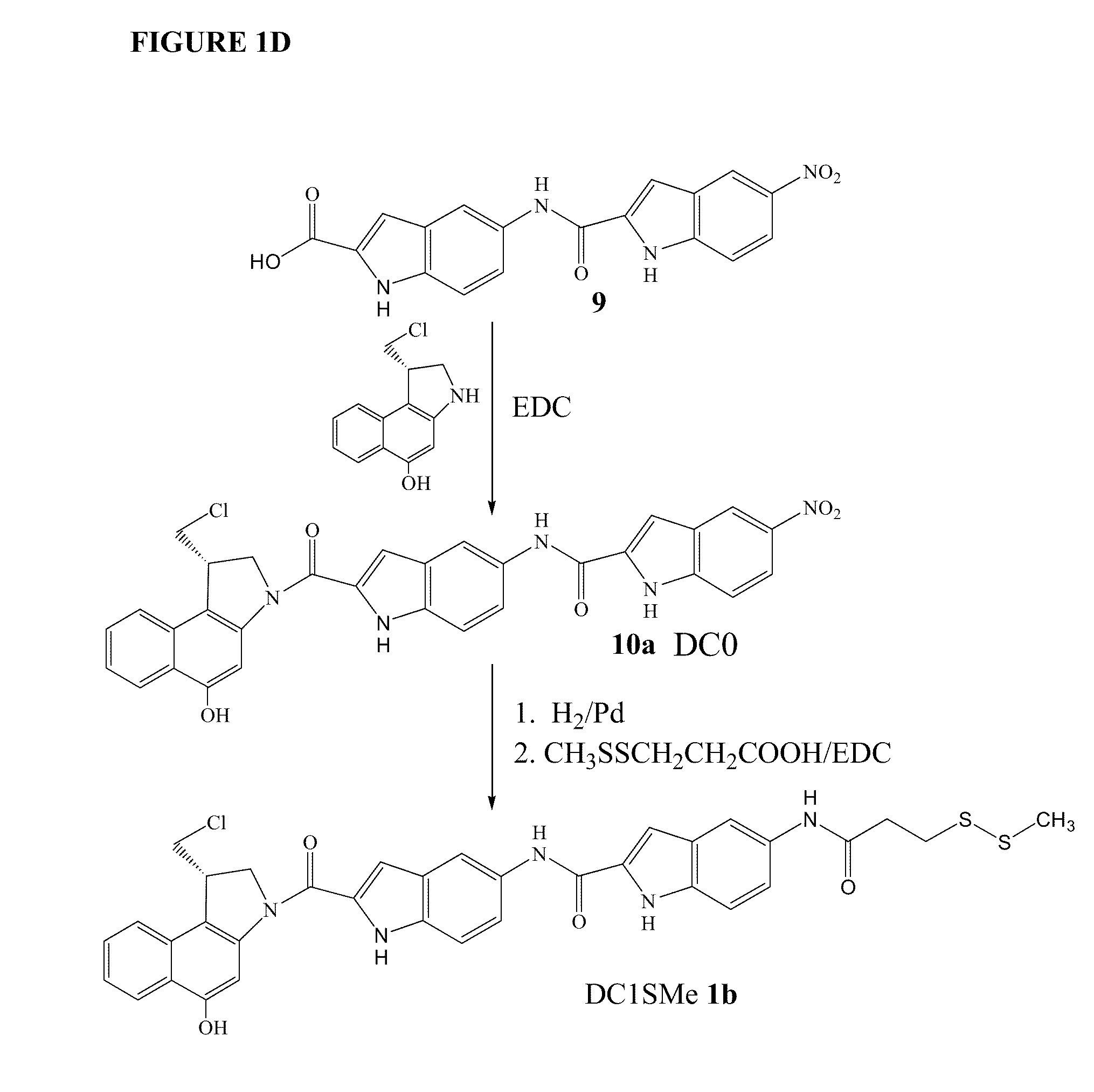
The present invention provides prodrugs of analogs of the anti-tumor antibiotic CC-1065 having a cleavable protective group containing a sulfonic acid containing phenyl carbamate, in which the protecting group confers enhanced water solubility upon the prodrug, and in which the prodrug also has a moiety, such as a sulfide or a disulfide, that can conjugate to a cell binding reagent such as an antibody, and for the therapeutic use of such prodrug and conjugates, and for processes for preparing such prodrugs and conjugates.
Owner:IMMUNOGEN INC
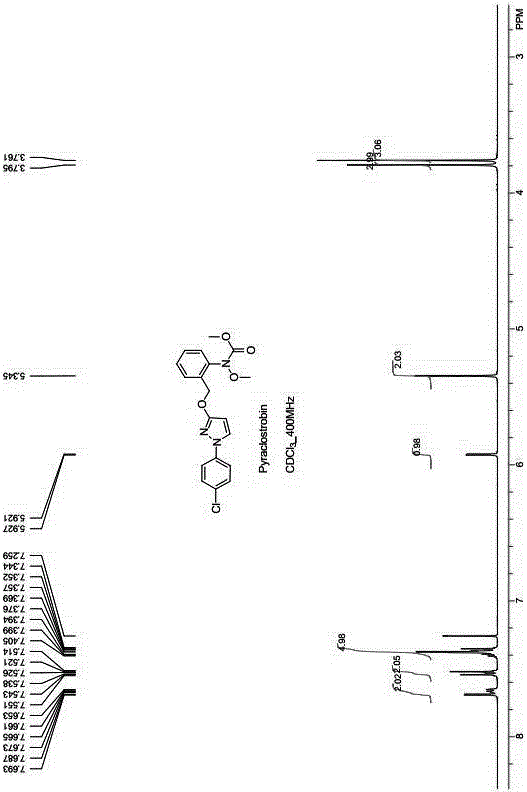
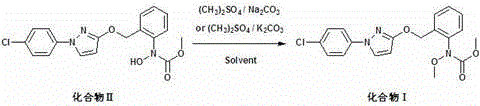
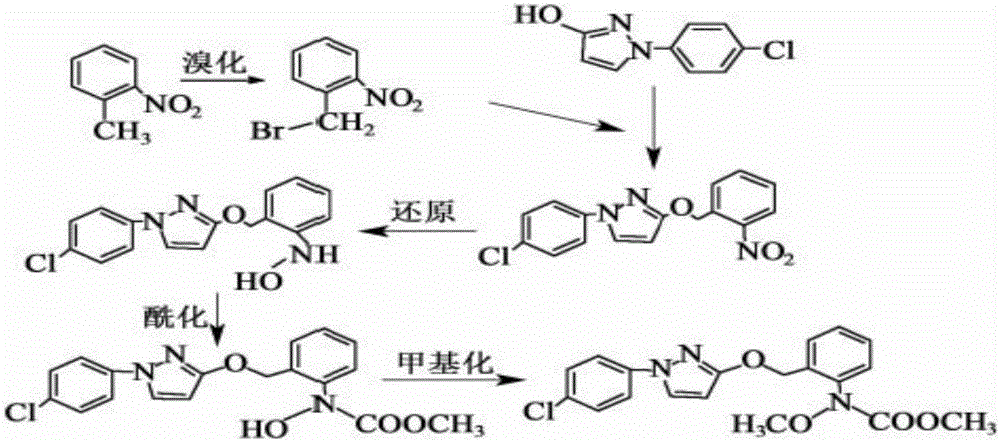
Pyraclostrobin and method for economically synthesizing same
InactiveCN102399190AIncrease profitReduce energy consumptionBiocideOrganic chemistryCarbamateSolvent
The invention aims to provide pyraclostrobin and a method for economically synthesizing the same. High purity pyraclostrobin can be synthesized at low cost. The method comprises the following steps of: adding N-hydroxy-N-2-[(N-parachlorobenzyl)-3-pyrazoloxymethyl]phenyl carbamate and an acid bonding agent into a polar solvent or non-polar solvent, uniformly mixing, adding dimethyl sulfate, and reacting at the temperature of between 20 and 30DEG C; and after the reaction is monitored to be finished through high performance liquid chromatography (HPLC), adding water to neutralize until the pH=7, removing the solvent, immersing in a recrystallization solvent, performing recrystallization, and removing a recrystallization solution to obtain the pyraclostrobin, wherein a molar ratio of the N-hydroxy-N-2-[(N-parachlorobenzyl)-3-pyrazoloxymethyl]phenyl carbamate to the acid bonding agent to the dimethyl sulfate is 1:(1-1.8):(1-1.8). By the invention, the energy consumption is low, the utilization rate of materials is high, the operation is simple, and the yield and purity of the product are high; and compared with the traditional method, the method is more energy-saving, environment-friendly and economic, and has great industrial application value.
Owner:HENAN UNIV OF CHINESE MEDICINE
Process for preparation of phenyl carbamate derivatives
ActiveUS20120184762A1Group 4/14 element organic compoundsPreparation by oxidation reactionsCarbamateCentral nervous system
Provided are a process for the preparation of phenyl carbamate derivatives, useful in the treatment of CNS (central nervous system) disorders, an intermediate in the synthesis of the phenyl carbamate derivatives, and a process for preparation of the intermediate.
Owner:BIO PHARM SOLUTIONS
Phenylcarbamate compound and muscle relaxant containing the same
A novel phenylcarbamate compound and a pharmaceutical composition containing the same are provided. More specifically, a novel phenylcarbamate compound, a composition for muscle relaxation containing the phenylcarbamate compound as an active ingredient, and a method of muscle relaxation comprising administering a therapeutically effective amount of the phenylcarbamate compound, are provided.
Owner:BIO PHARM SOLUTIONS
Chitosan carbanilate-carbamido derivative preparation method
ActiveCN104250312AImprove solubilitySmall structureOther chemical processesCarbamateChitosan phenylcarbamate
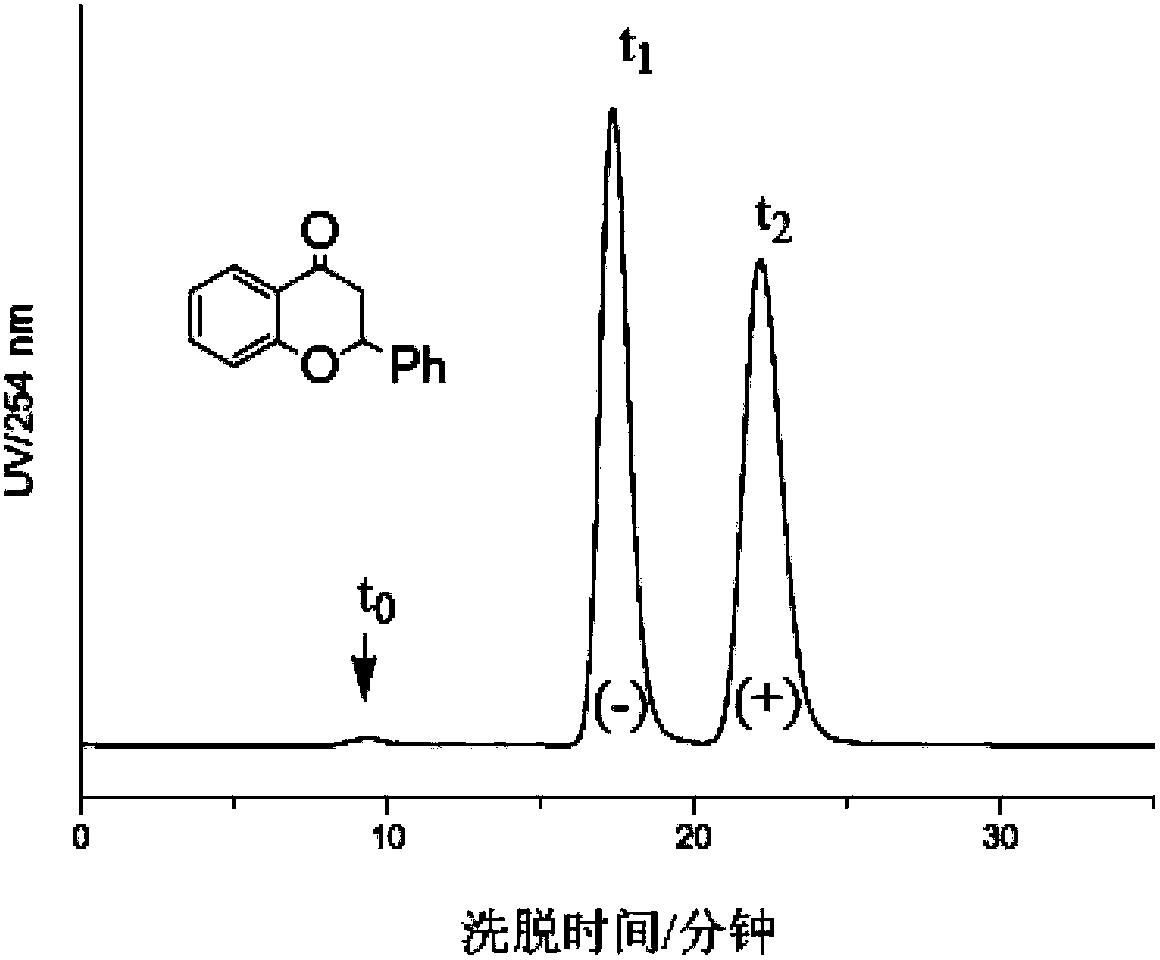
The invention provides a novel chitosan carbanilate-carbamido derivative synthetic method, the method employs chitosan and phenyl isocyanate with different groups to react, and then the hydroxy and amino on chitosan can be completely conversed to the chitosan carbanilate-carbamido derivative of carbamate and carbamido. According to the invention, a coating process is employed to prepare the derivative to a chiral stationary phase, and high performance liquid chromatography is used for resolution of various enantiomers, and the chiral stationary phase has high chiral identification capability.
Owner:DAICEL CHEM IND LTD
Preparation method of immobilized beta-cyclodextrin derivative type chiral stationary phase
InactiveCN103406113ASimple manufacturing methodMild reaction conditionsOther chemical processesSilanesSilicon oxygen
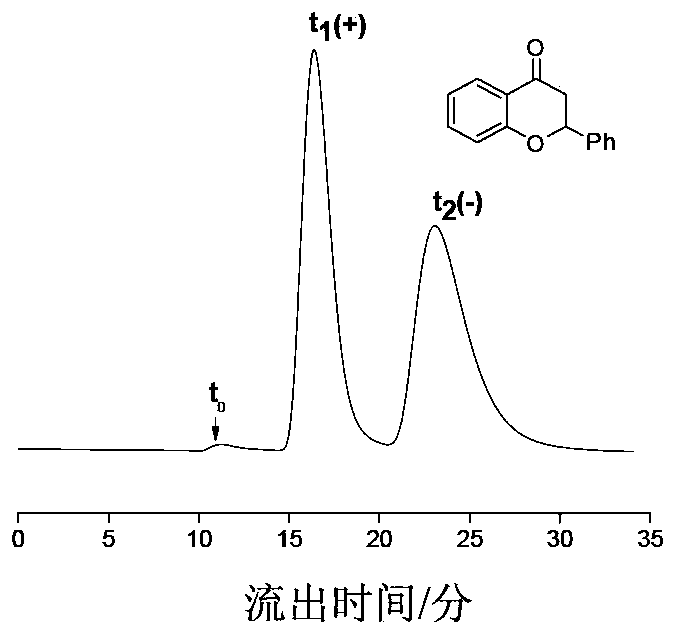
The invention provides a preparation method of an immobilized beta-cyclodextrin derivative type chiral stationary phase. The preparation method comprises the following steps: reacting isocyanate propyl triethoxy silane with beta-cyclodextrin; performing derivatization modification on a hydroxyl group on a beta-cyclodextrin glucose unit by using phenyl isocyanate to obtain a beta-cyclodextrin derivative; coating the beta-cyclodextrin derivative bonded with a small amount of silane coupling agent on the surface of aminopropyl silica gel, and performing condensation in ethanol / aqueous solution in the presence of trimethylchlorosilane by using the silane coupling agent in the beta-cyclodextrin derivative molecules to finally obtain the immobilized beta-cyclodextrin derivative type chiral stationary phase, wherein the phenylcarbamate beta-cyclodextrin derivative molecules are connected by using a silicon-oxygen-silicon bond to form an inclusion netlike structure which covers the surface of the aminopropyl silica gel. The preparation method has the characteristics of simplicity, few steps and high bonding efficiency, can be applied to the normal-phase high-performance liquid chromatographic condition, and has the advantages of high chromatographic column stability and high chiral separation capacity.
Owner:三亚哈尔滨工程大学南海创新发展基地
Prodrugs of cc-a1065 analogs
InactiveUS20090028821A1Improve solubilityGood water solubilityAntibacterial agentsBiocideSolubilityCarbamate
The present invention provides prodrugs of analogs of the anti-tumor antibiotic CC-1065 having a cleavable protective group containing a sulfonic acid containing phenyl carbamate, in which the protecting group confers enhanced water solubility upon the prodrug, and in which the prodrug also has a moiety, such as a sulfide or a disulfide, that can conjugate to a cell binding reagent such as an antibody, and for the therapeutic use of such prodrug and conjugates, and for processes for preparing such prodrugs and conjugates.
Owner:IMMUNOGEN INC
Method for catalytically synthesizing pyraclostrobin
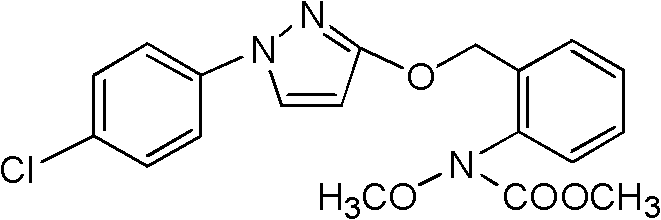
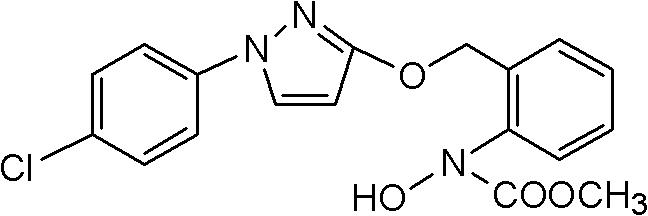
The invention discloses a method for catalytically synthesizing pyraclostrobin, relating to the technical fields of chemical industry and pesticide intermediate preparation. The method comprises the following steps: adding a compound II N-hydroxy-N-2-[(N-p-chlorphenyl)-3-pyrazolyloxymethyl]carbanilate into a two-phase solvent, adding a phase-transfer catalyst, and meanwhile, dropwisely adding an acid-binding agent water solution and dimethyl sulfate to carry out etherification reaction on the compound II N-hydroxy-N-2-[(N-p-chlorphenyl)-3-pyrazolyloxymethyl]carbanilate and dimethyl sulfate while keeping the pH value of the reaction system at 8-9, thereby obtaining the compound I pyraclostrobin. The method has the advantages of short reaction time and high product yield, lowers the production cost, and reduces the generated wastewater, thereby being beneficial to environment protection.
Owner:SHIJIAZHUANG SENTAY CHEM CO LTD
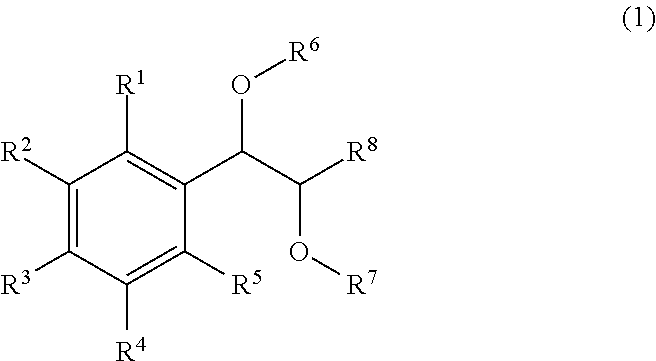
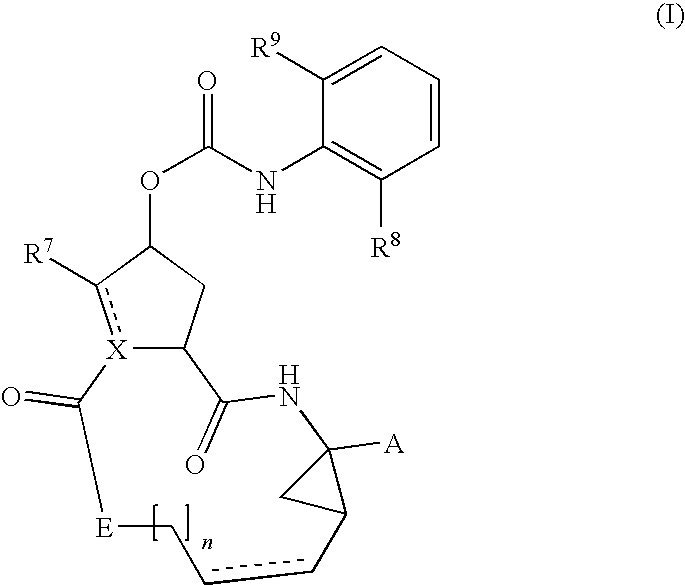
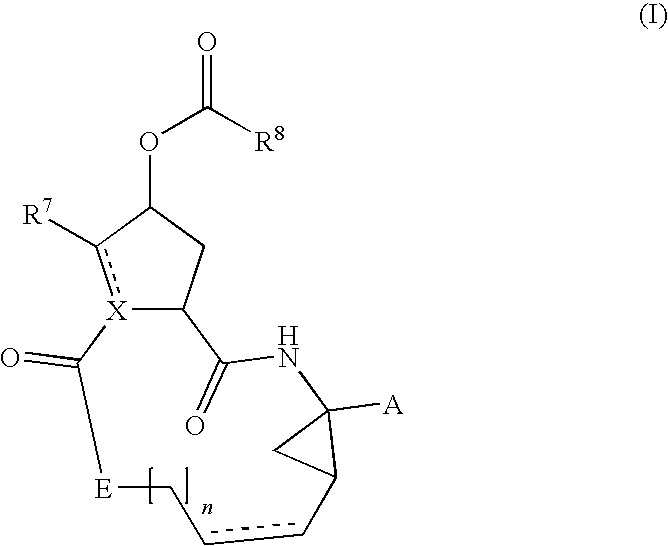
Hcv inhibiting macrocyclic phenylcarbamates
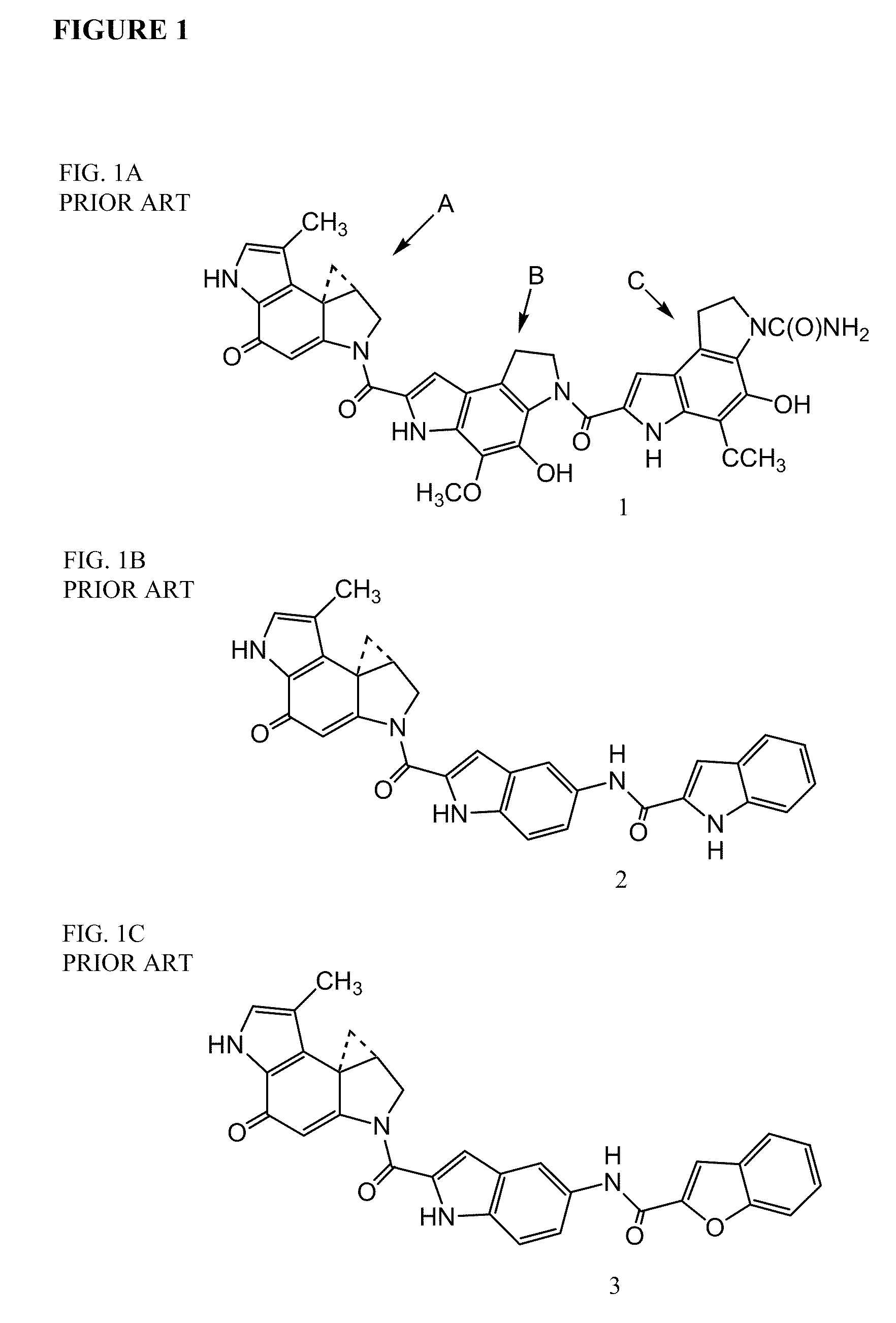
Compounds of the formula 1:including a stereoisomer thereof, or an N-oxide, a pharmaceutically acceptable addition salt, or a pharmaceutically acceptable addition solvate thereof; useful as HCV inhibitors; processes for preparing these compounds as well as pharmaceutical compositions comprising these compounds as active ingredient.
Owner:MEDIVIR AB +1
Phenylpropyl carbamate derivatives for use in preventing or treating multiple sclerosis
A phenyl carbamate compound, and a method of treating and / or preventing multiple sclerosis comprising administering a pharmaceutically effective amount of the phenyl carbamate compound to a subject in need of treating and / or preventing multiple sclerosis, are provided.
Owner:BIO PHARM SOLUTIONS
Process for the preparation of phenylcarbamates
InactiveUS7385076B2Easy to handleEasy to storeCarbamic acid derivatives preparationOrganic compound preparationHydrogenPhenylcarbamates
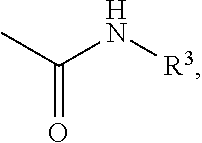
A process for the preparation of compound of formula (I); wherein R1 is hydrogen, linear, branched or cyclic lower alkyl, cyclohexyl, allyl, propargyl or benzyl; R2 is hydrogen, methyl, ethyl or propyl; or R1 and R2 together with the nitogen to which they are attached form a cyclic moiety of three to eight-membered ring, with or without a hetero atom like nitrogen or oxygen; R3 is hydrogen or lower alkyl; R4 and R5 are the same or different and each is a lower alkyl; comprising reacting compound of formula (II); wherein R3, R4 and R5 are as defined above, with compound of formula (III); wherein R1 and R2 are as defined above, in the presence of a base, and further resolving the compound of formula (I) to obtain (S)-isomer of compound of formula (I), substantially free of R-isomer
Owner:SUN PHARMA INDS
Phenylcarbamate compound and muscle relaxant containing the same
A novel phenylcarbamate compound and a pharmaceutical composition containing the same are provided. More specifically, a novel phenylcarbamate compound, a composition for muscle relaxation containing the phenylcarbamate compound as an active ingredient, and a method of muscle relaxation comprising administering a therapeutically effective amount of the phenylcarbamate compound, are provided.
Owner:BIO PHARM SOLUTIONS
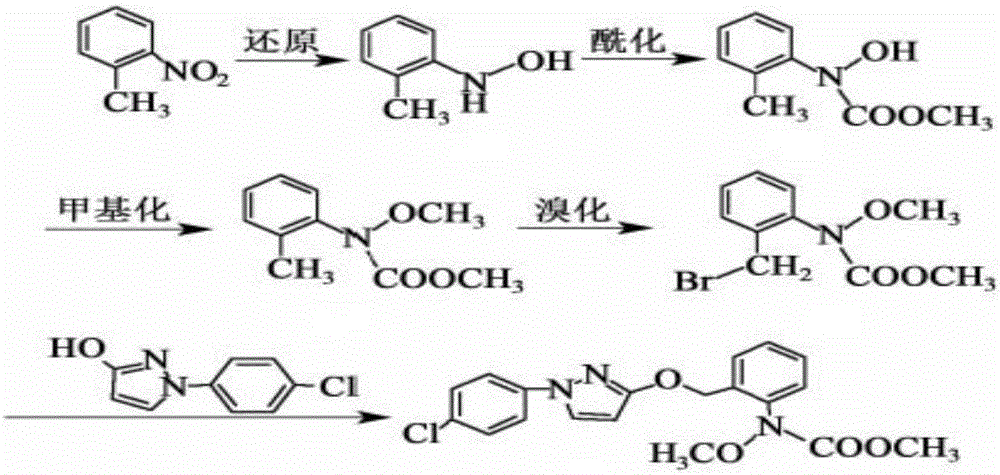
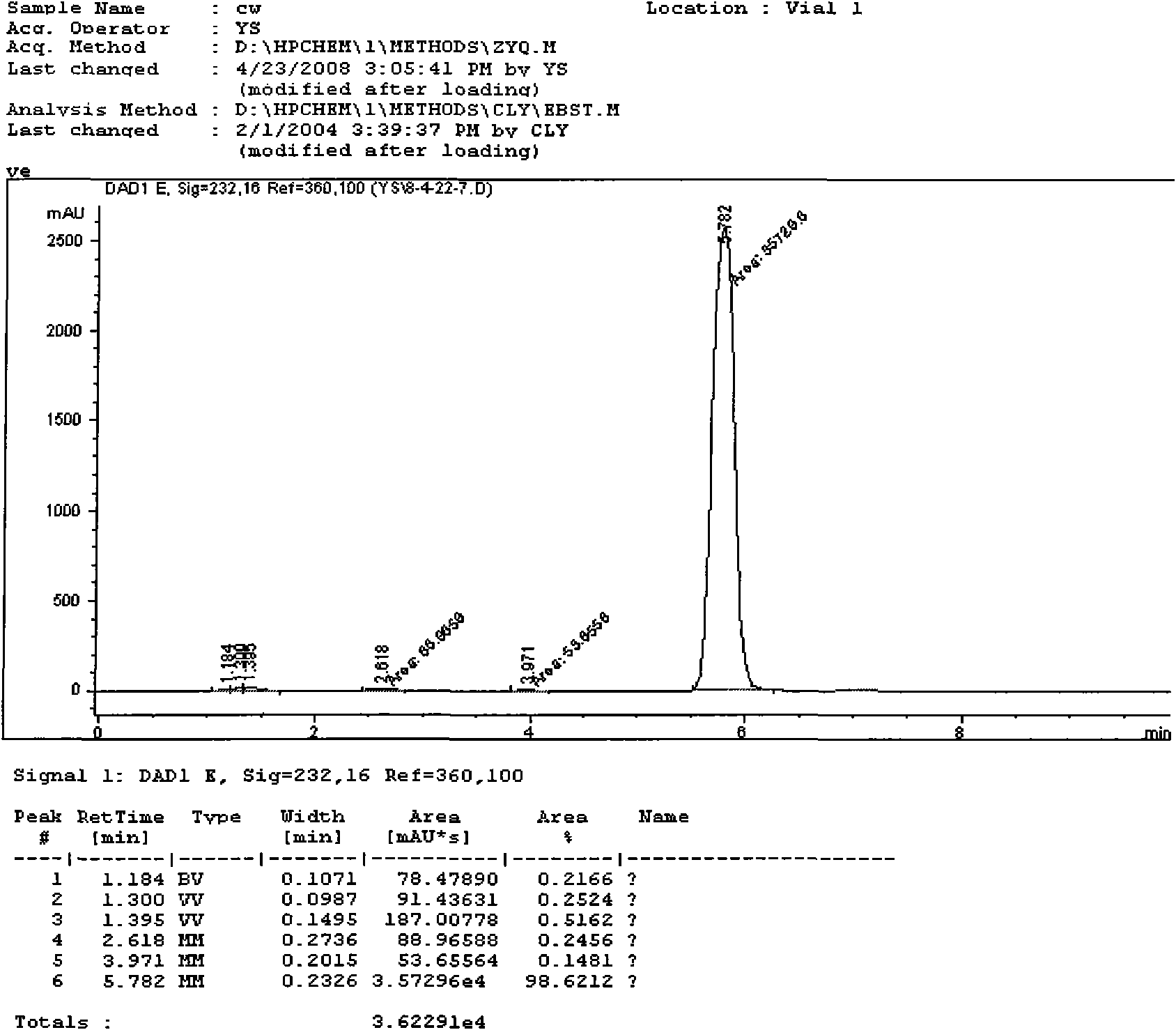
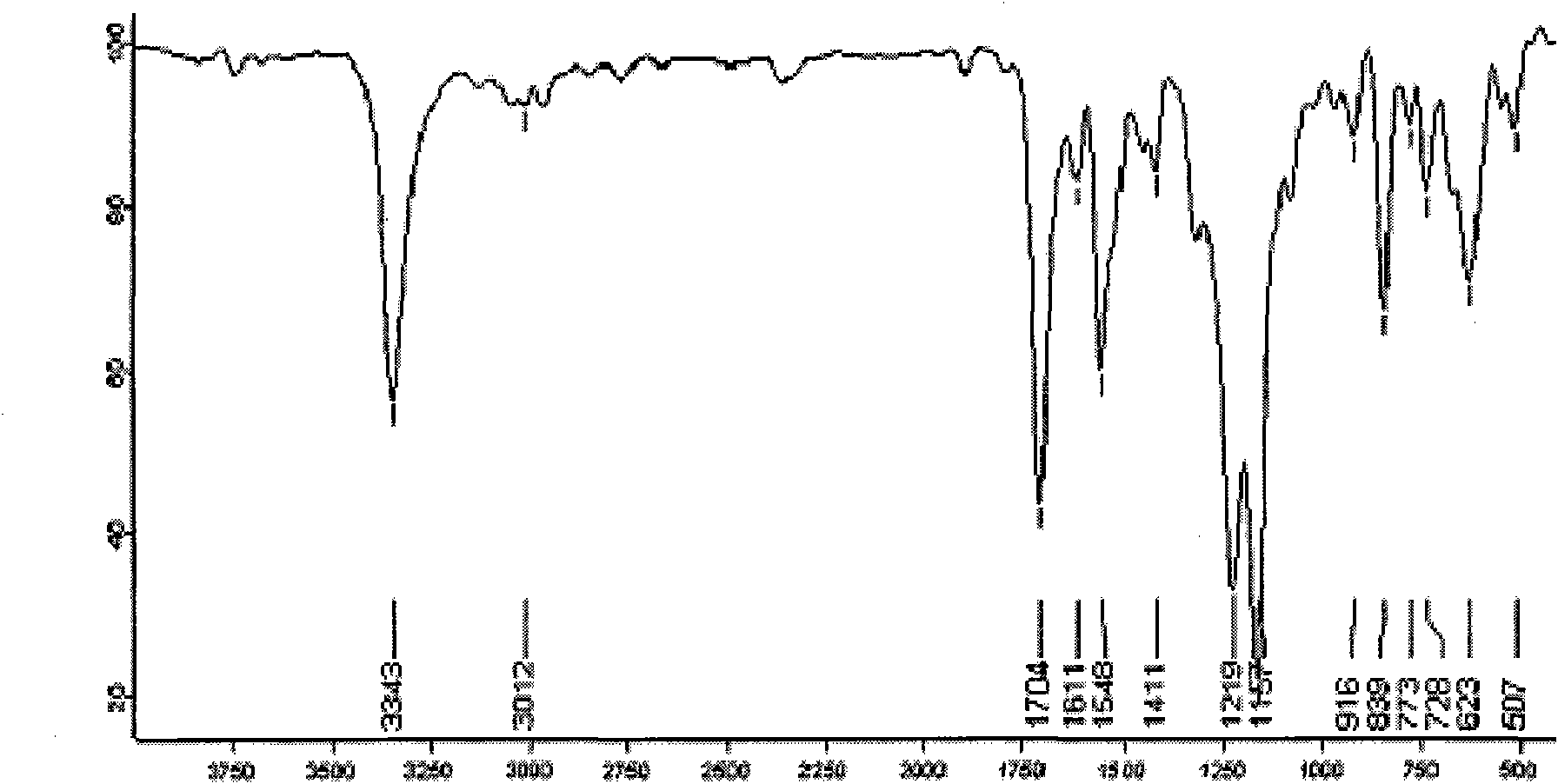
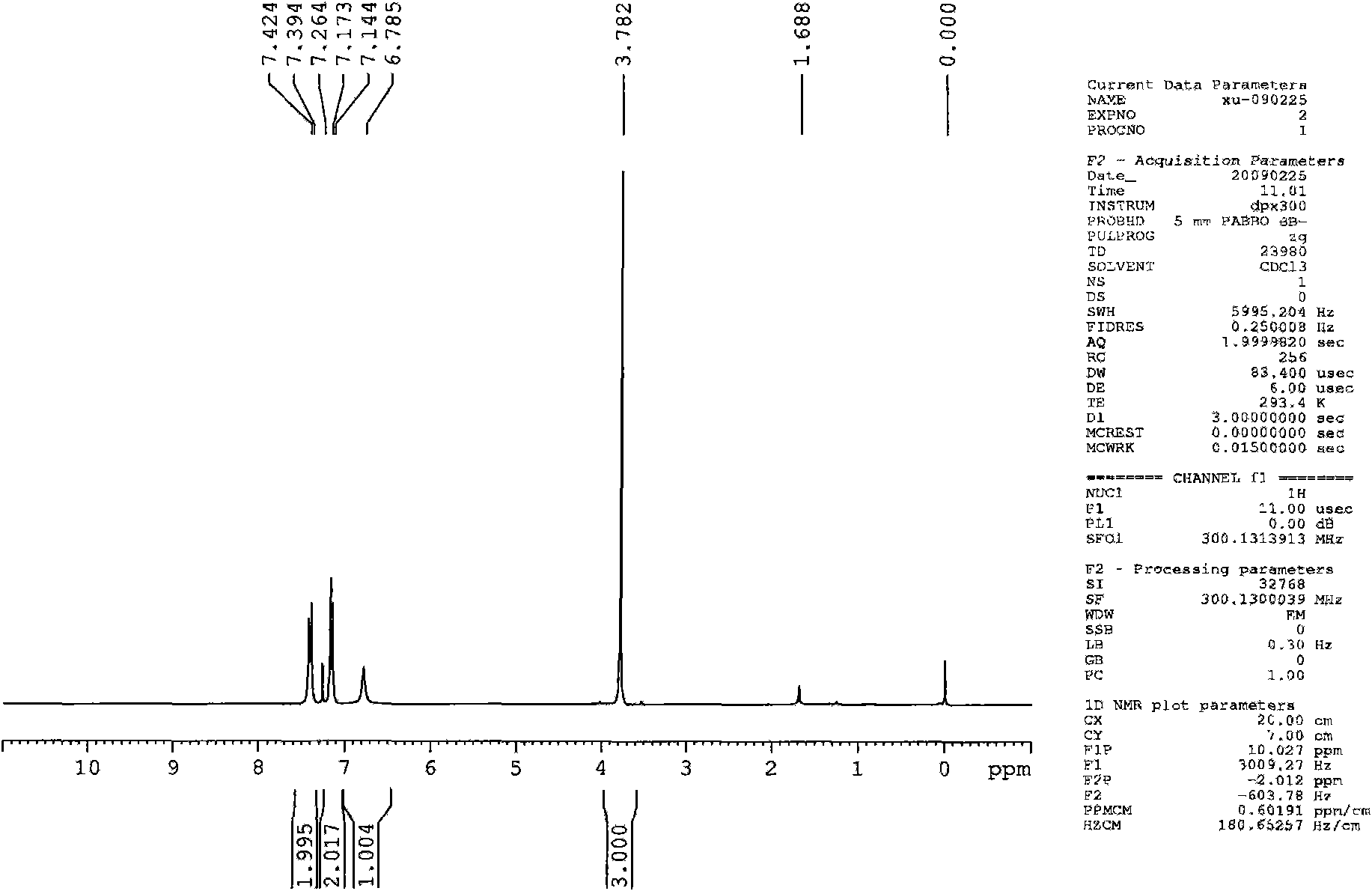
Synthesis technology of pyraclostrobin
InactiveCN106008347AImprove selective reducibilityHigh yieldOrganic chemistryMicro nanoHydroxylamine
The invention provides a synthesis process of pyraclostrobin, which can be divided into five steps: (1) o-nitrotoluene and NH 4 Cl reduction reaction occurs under the catalysis of zinc powder and alloy micronano powder; (2) acylation reaction of hydroxylamine; (3) methylation reaction; (4) N-methoxy-N -2-bromomethylphenyl methyl carbamate; (5) use DMF as a solvent to dissolve N-methoxy-N-2-bromomethyl phenyl methyl carbamate and make a solution for subsequent use, and 1-( 4‑chlorophenyl)‑pyrazolol, K 2 CO 3 , acetone and put them into the reactor together, after heating up and refluxing, slowly add N-methoxy-N-2-bromomethylphenyl carbamate solution in the reactor, and the product pyrazole ether is obtained after the reflux reaction is completed Strostrobin. Compared with the prior art, the preparation method of the present invention is simple, the raw materials are cheap and easy to obtain, the reaction conditions are mild, and the purity and yield of the obtained target product are high.
Owner:ANHUI GUANGXIN AGROCHEM
Technology for synthesizing pyraclostrobin by six-step method
The invention provides a technology for synthesizing pyraclostrobin by a six-step method. The synthesis technology comprises the following steps: 1) performing a reduction reaction of ortho-nitrotoluene; 2) performing an acylation reaction of hydroxylamine; 3) performing a methylation reaction; 4) obtaining N-methoxyl-N-2-bromomethyl phenyl methyl carbamate through a bromination reaction; and 5) performing synthesis of 1-(4-chlorophenyl)pyrazolidine-3-ketone; and 6) performing synthesis of pyraclostrobin. Compared with the prior art, the preparation method has the advantages of simple process, easily available raw materials with low cost, and mild reaction condition, and the purity and yield of the target products are high.
Owner:ANHUI GUANGXIN AGROCHEM
Method for preparing 4-trifluoro-methoxy phenyl carbamate
InactiveCN101602694ARaw materials are easy to getEasy to operateCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl carbonate
The invention belongs to the technical field of preparing pesticide intermediates and pharmaceutical intermediates, in particular to a method for preparing 4-trifluoro-methoxy phenyl carbamate. The method adopts 4-trifluoro-methoxyaniline as a starting raw material, and uses dimethyl carbonate as methoxycarbonyl reagent and solvent; and under normal pressure, the method utilizes catalyst to carry out carbonylation reaction to obtain the product. The invention aims at providing the method for preparing the 4-trifluoro-methoxy phenyl carbamate, which has low toxicity of raw materials, less pollution and easy reaction control. Compared with the prior art, the invention has the advantages of simple operation steps, higher yield, high product quality, low corrosion of raw materials, less environmental pollution, etc.
Owner:WENZHOU UNIVERSITY +1
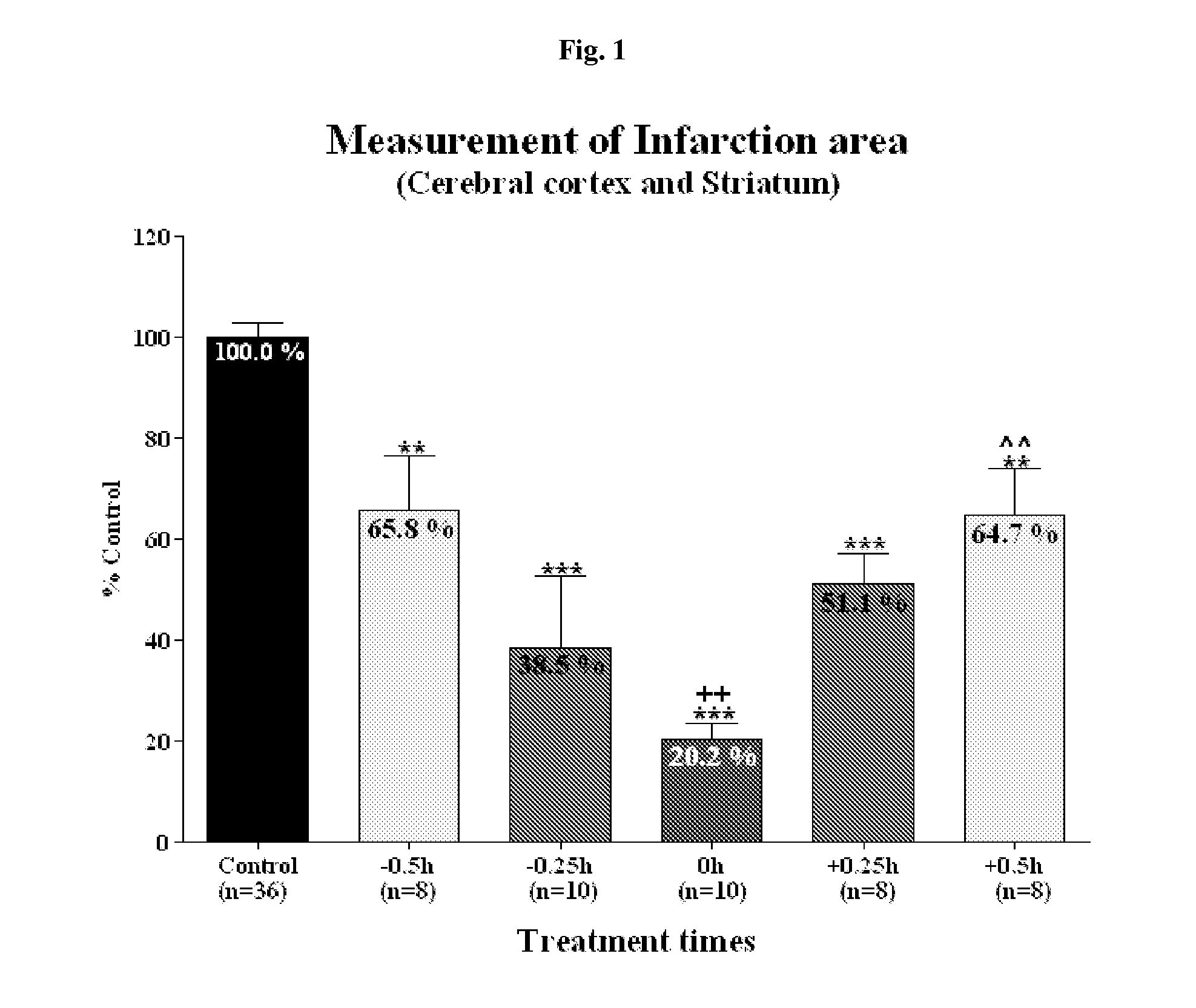
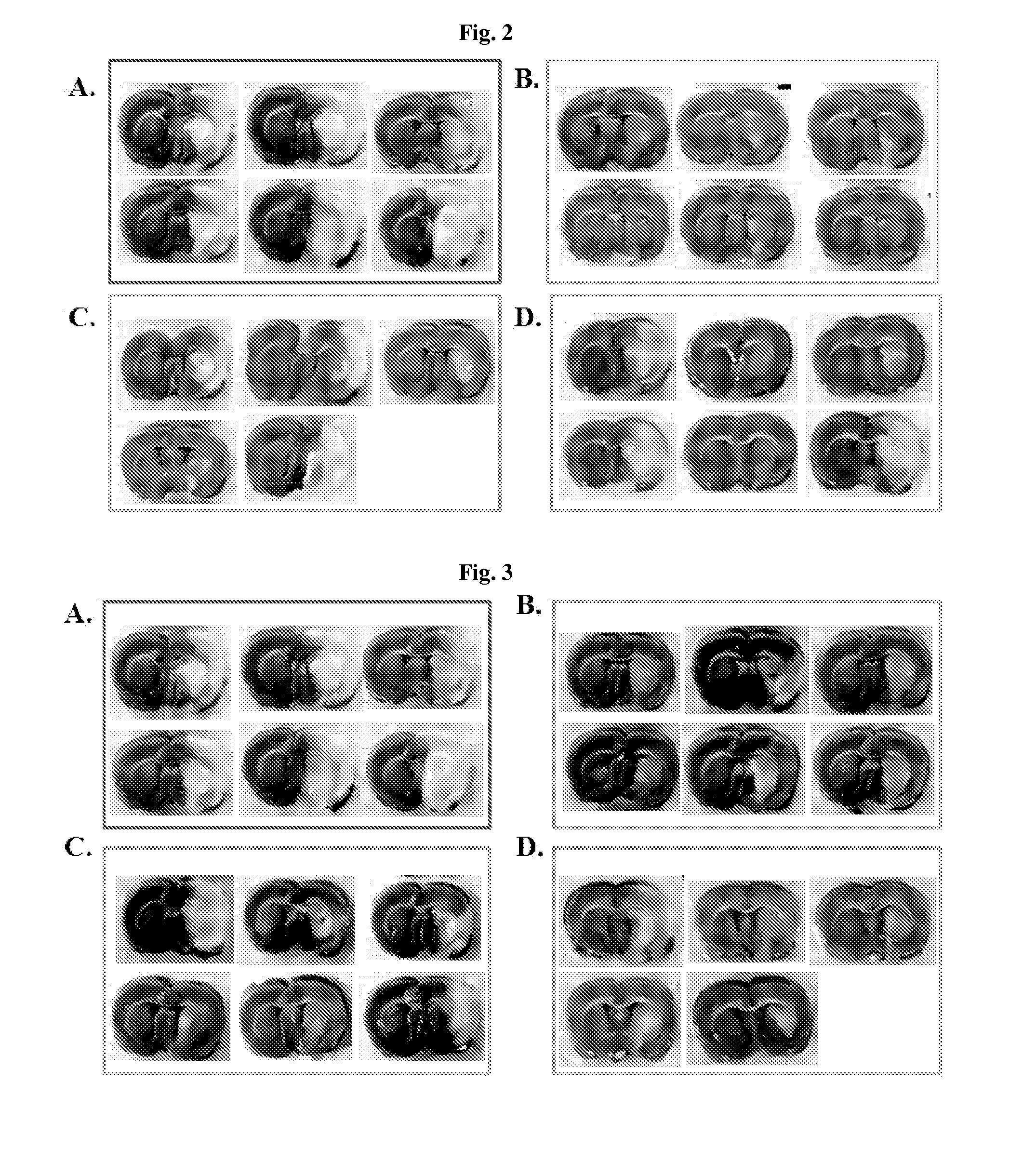
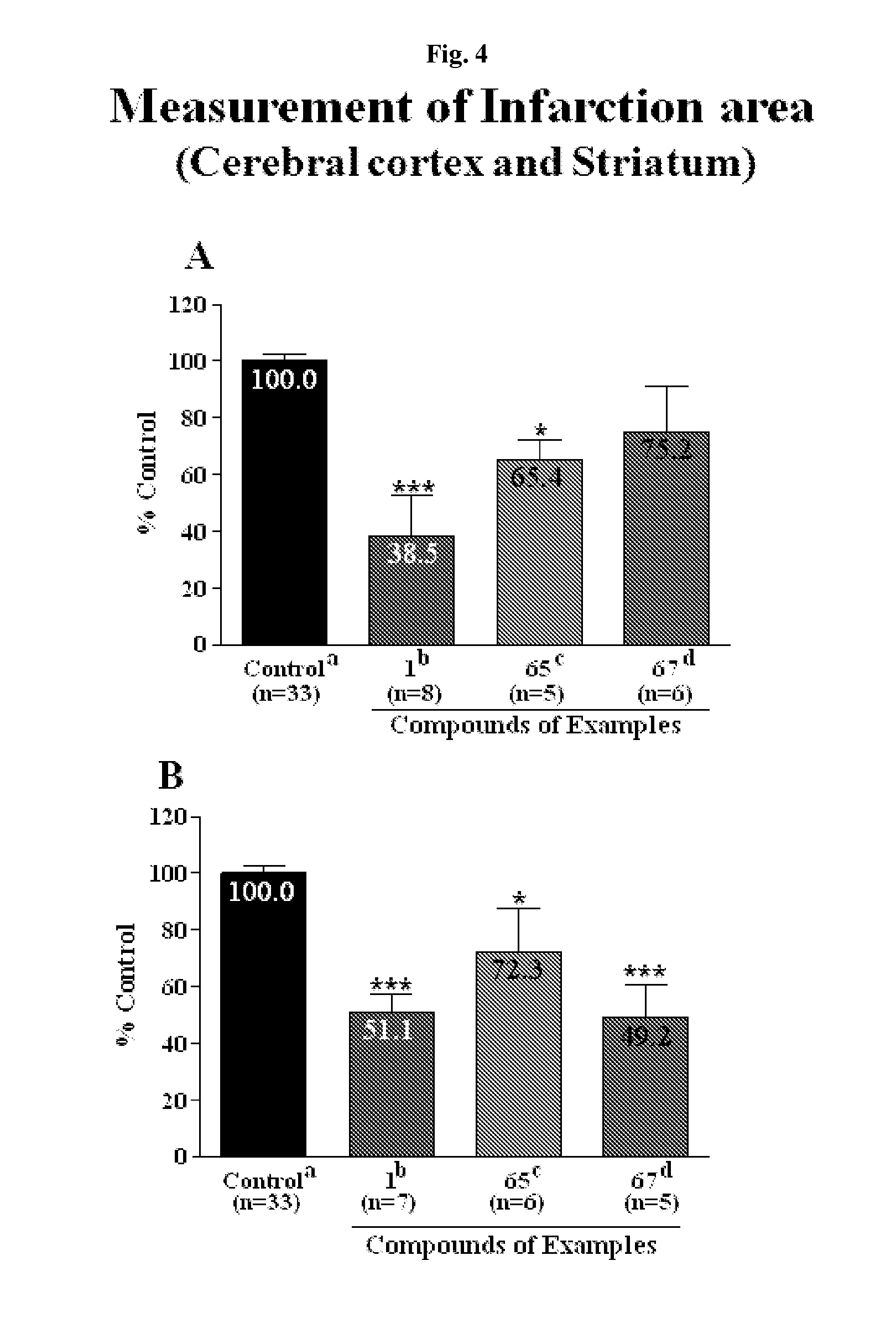
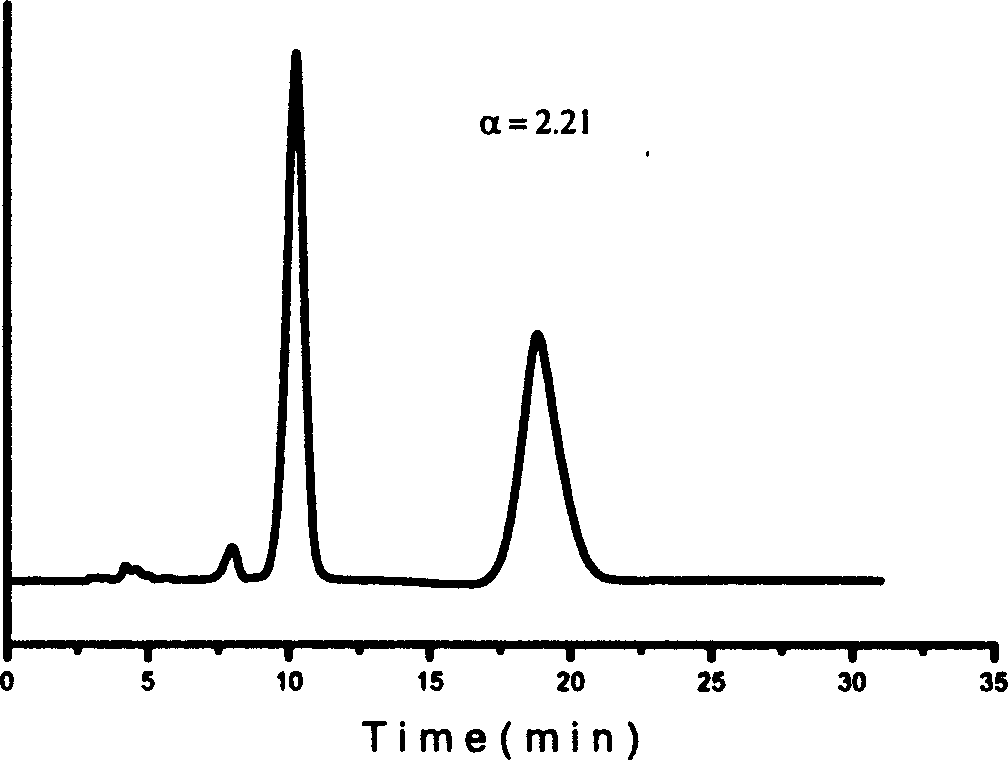
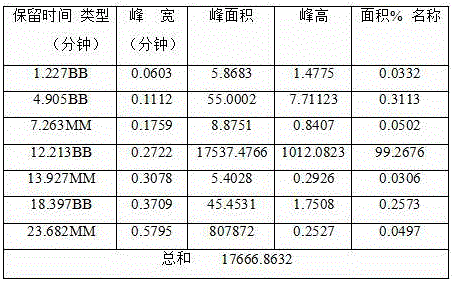
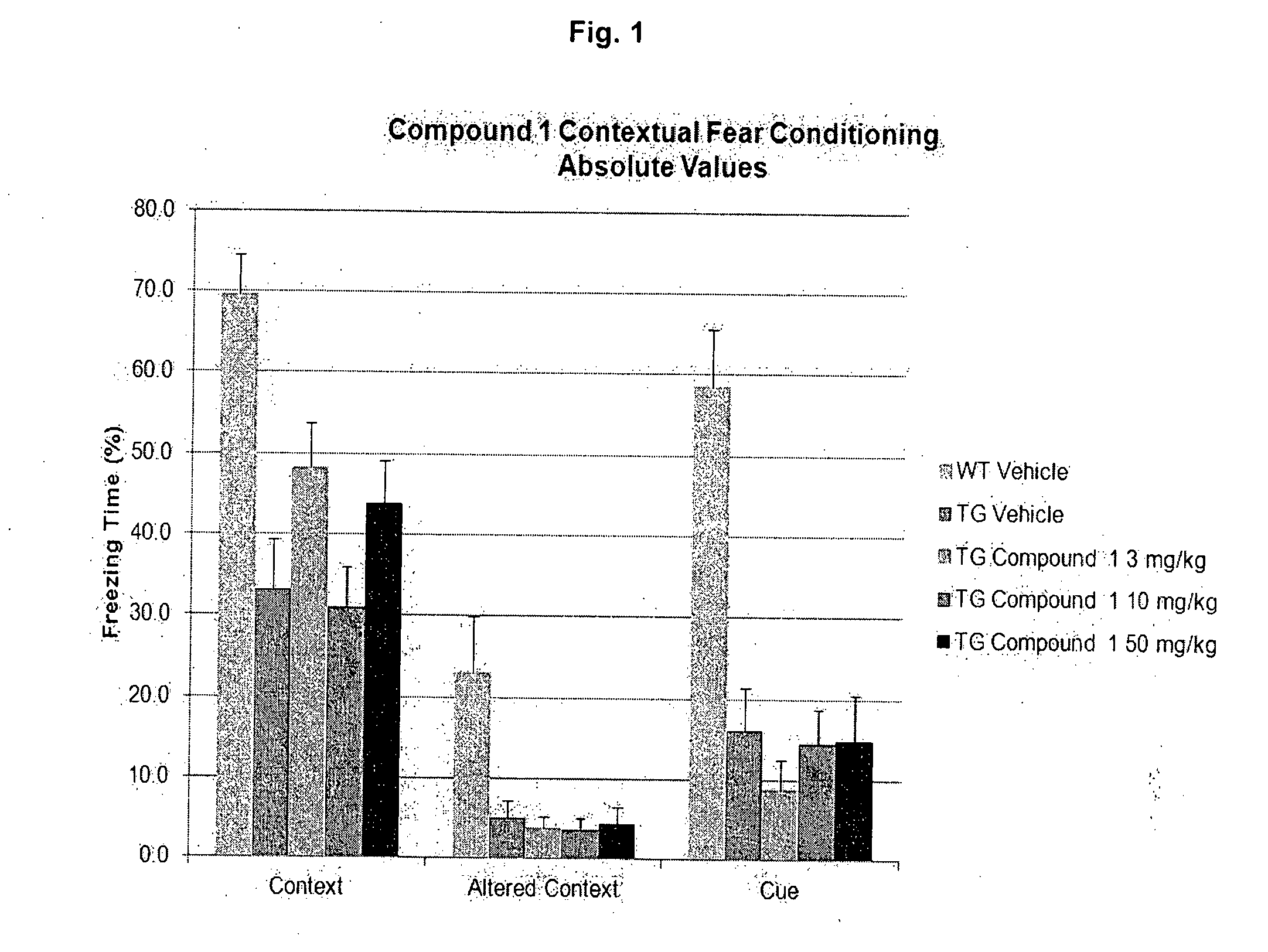
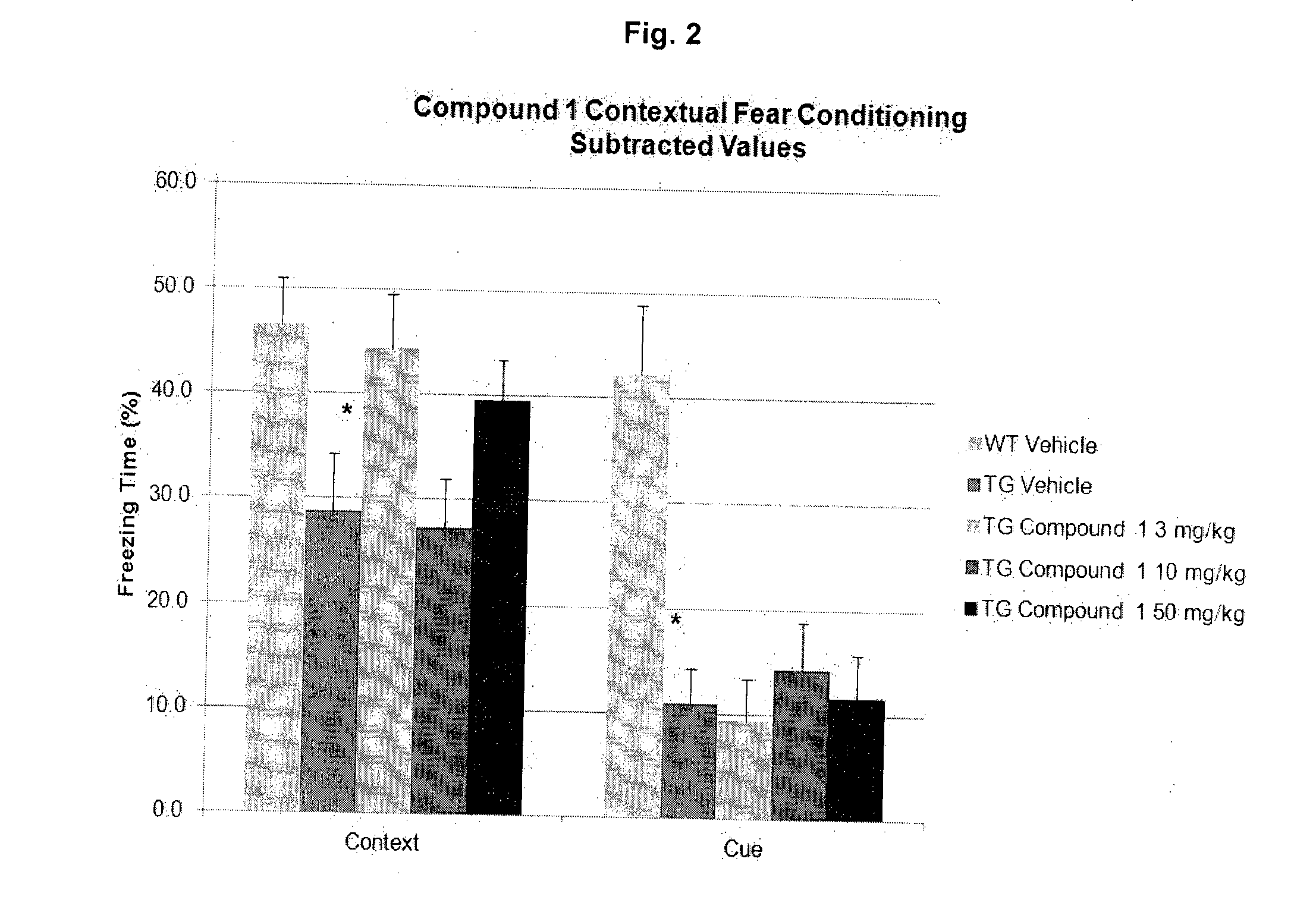
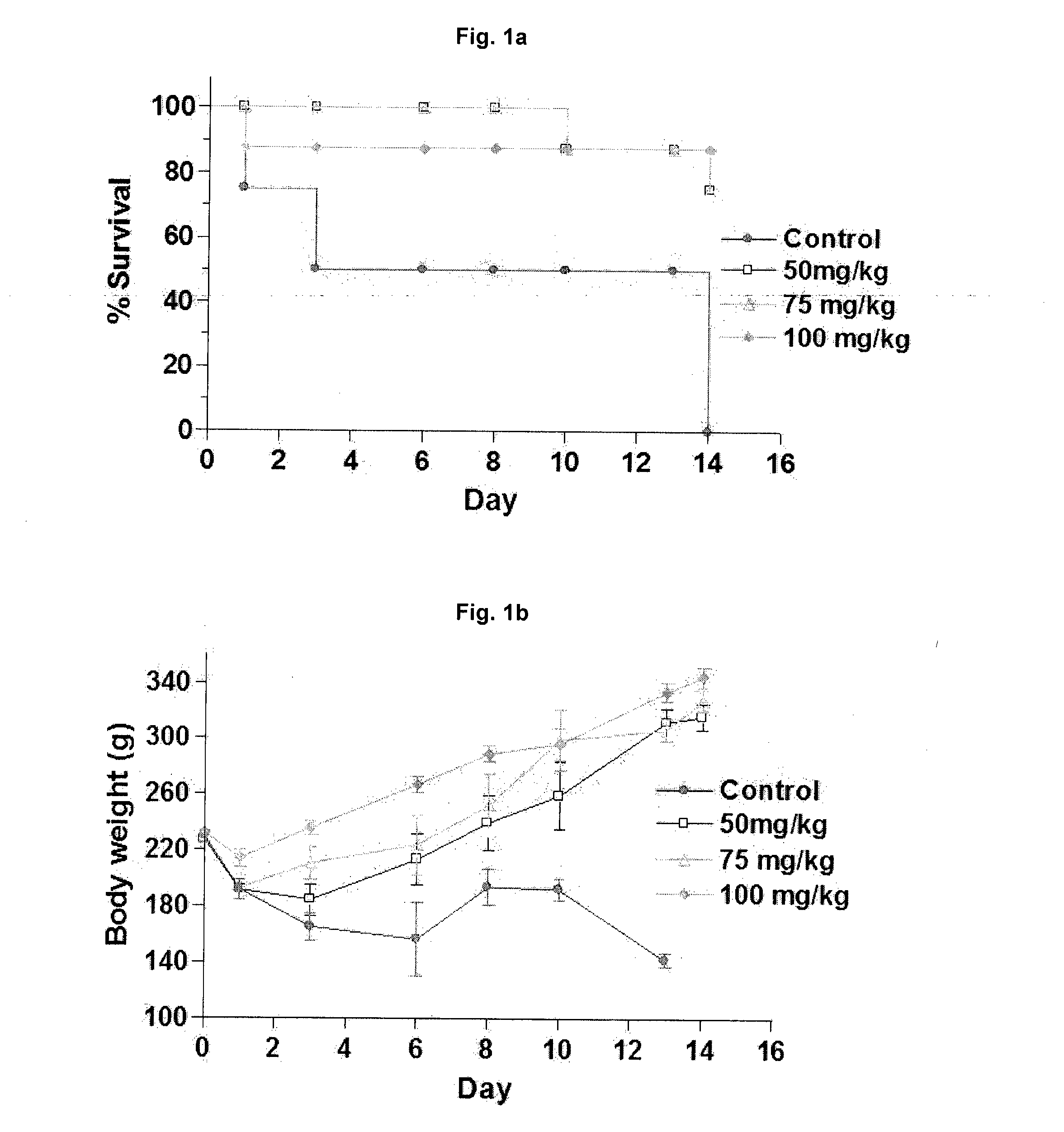
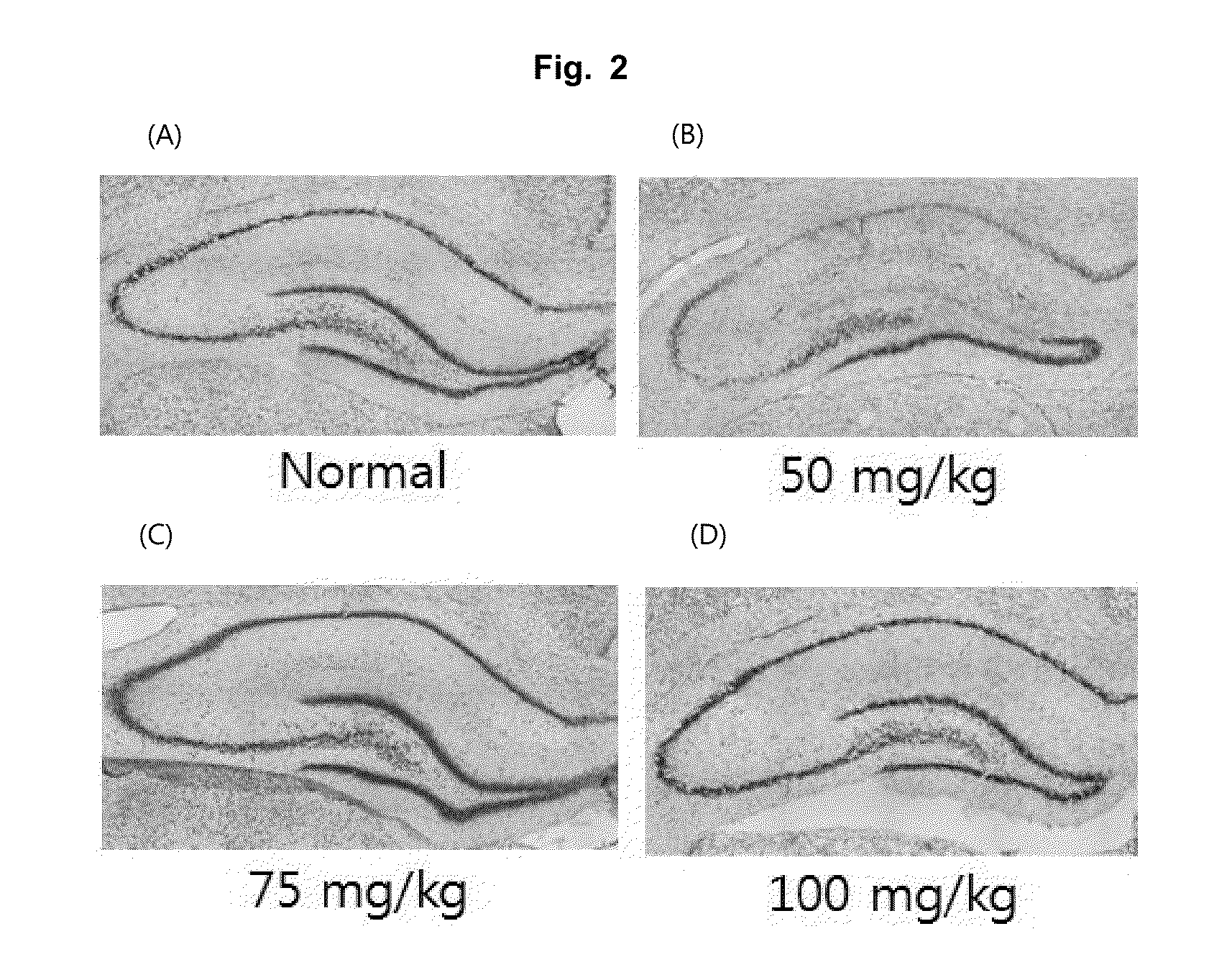
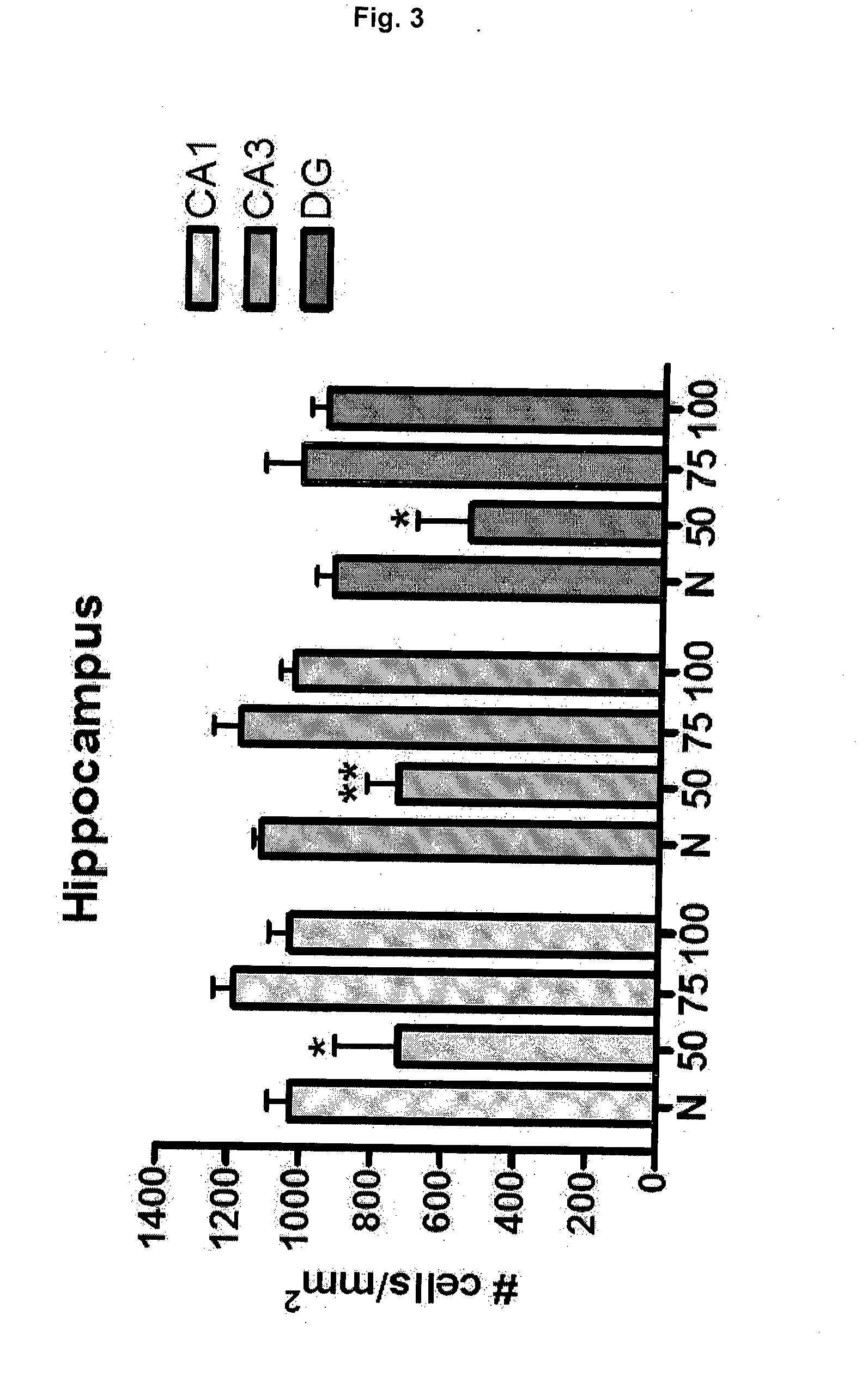
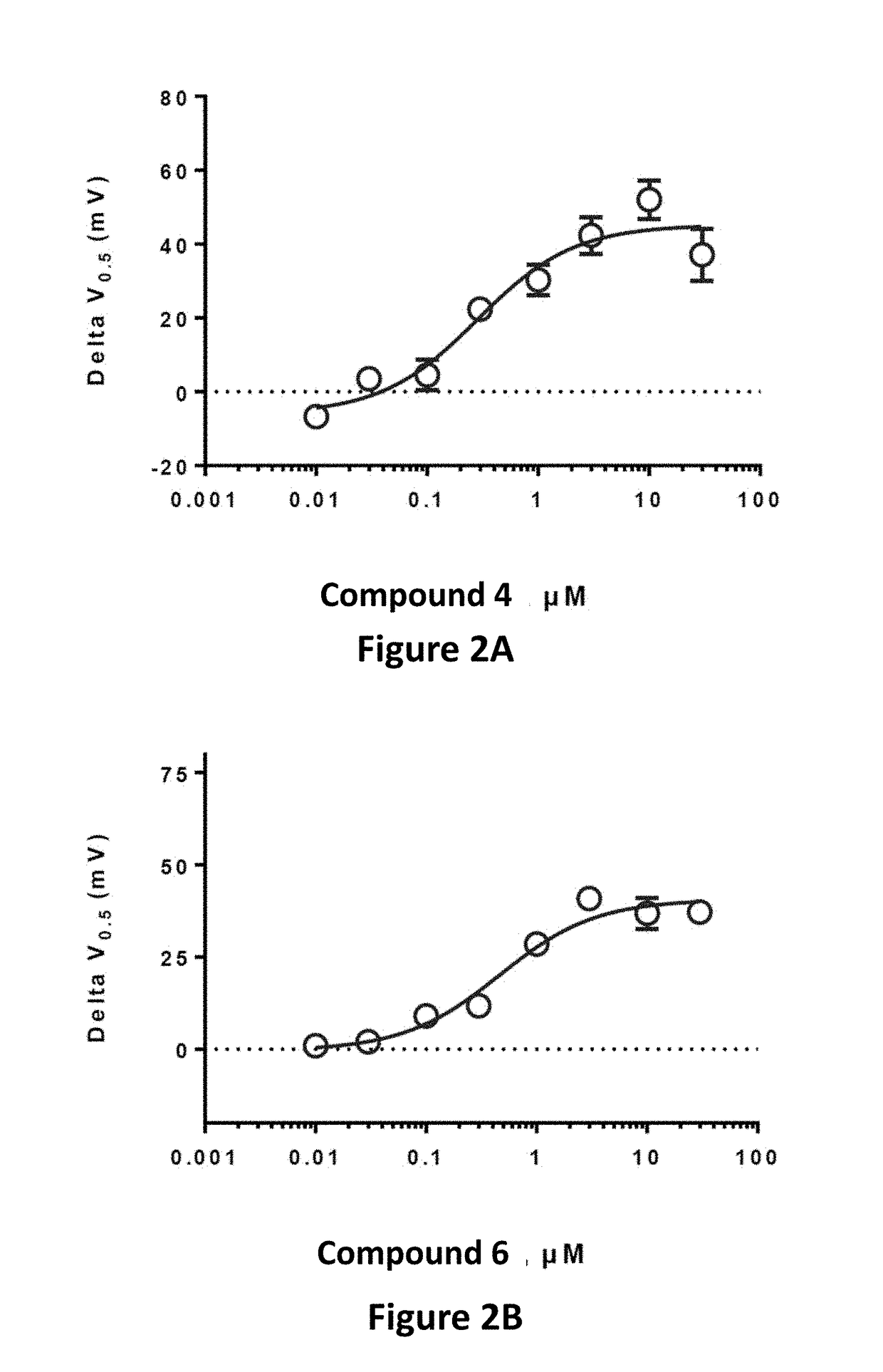
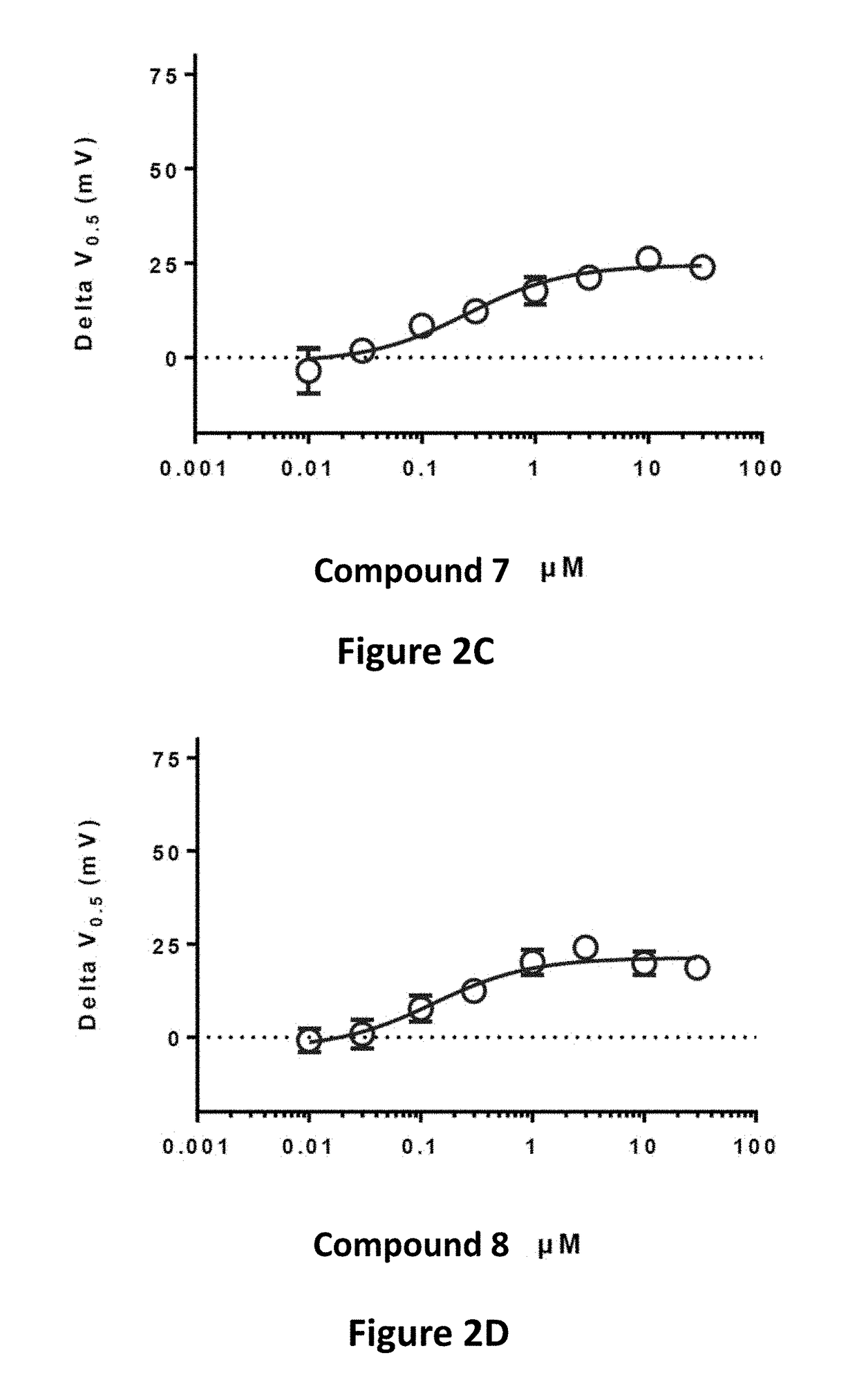
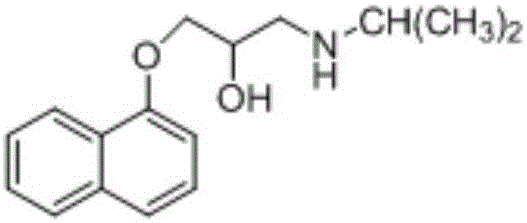
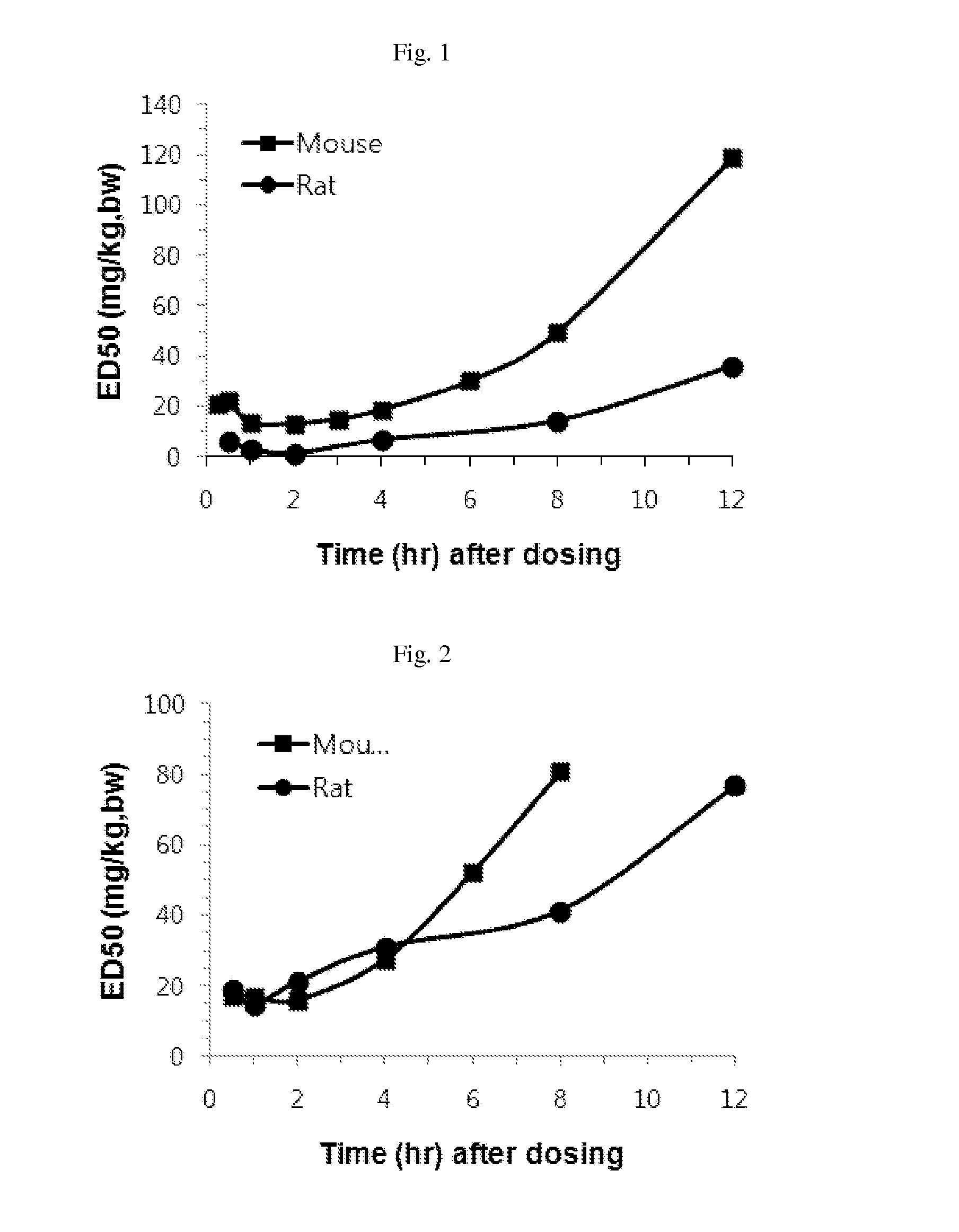
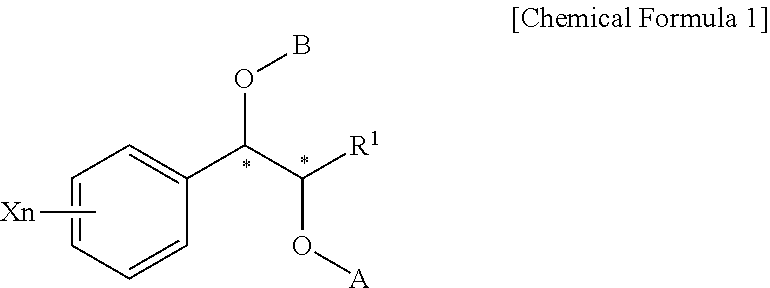
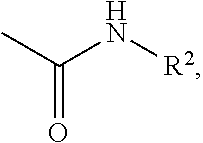
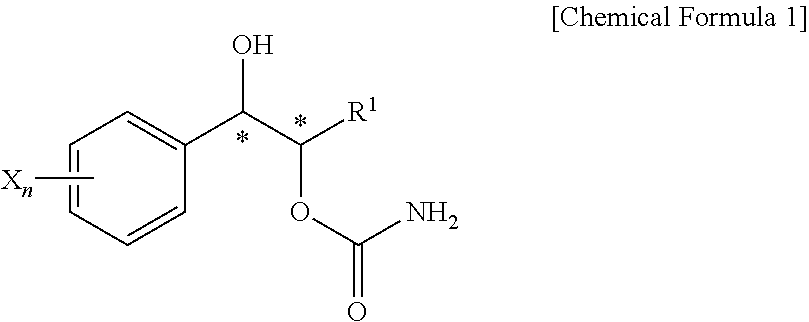
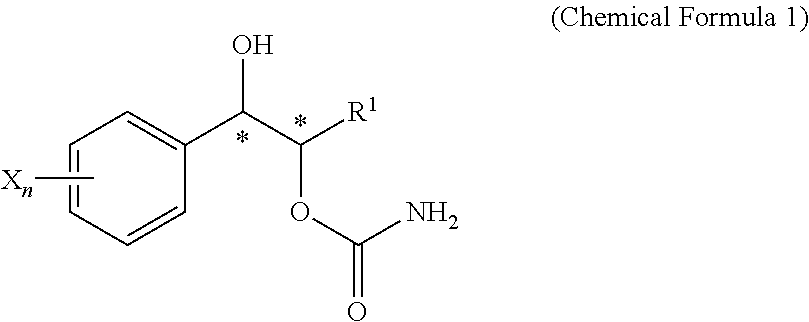
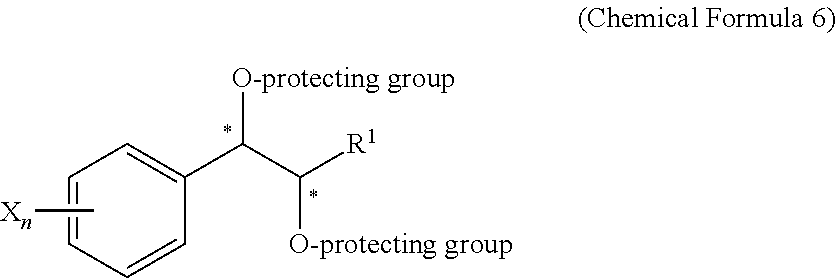
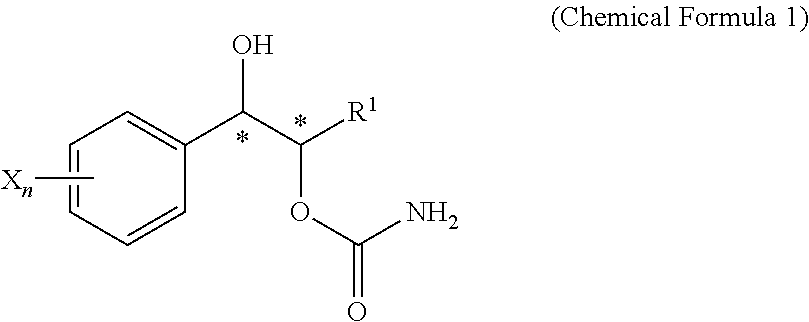
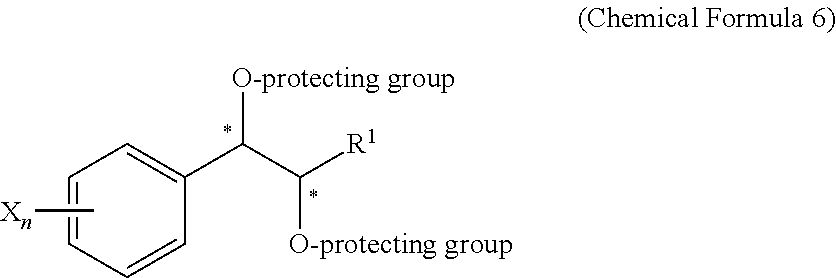
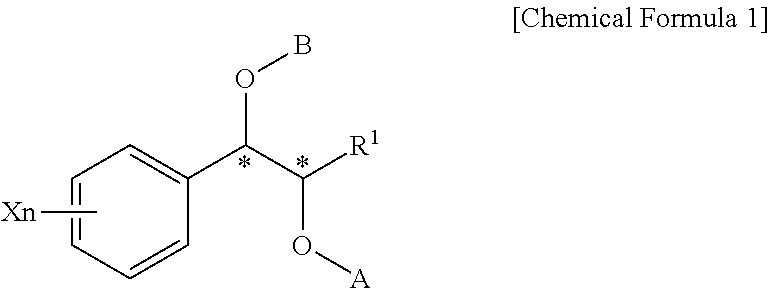
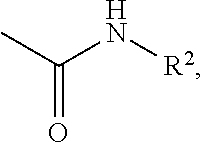
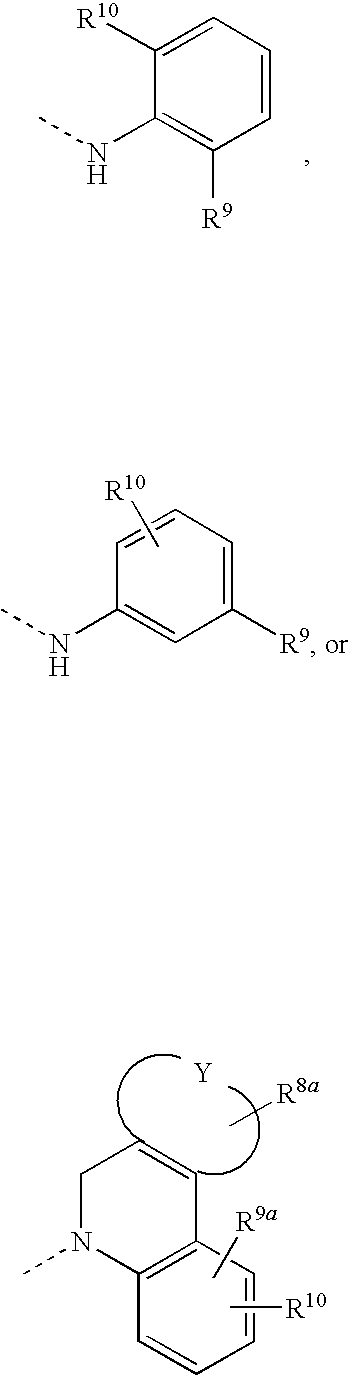
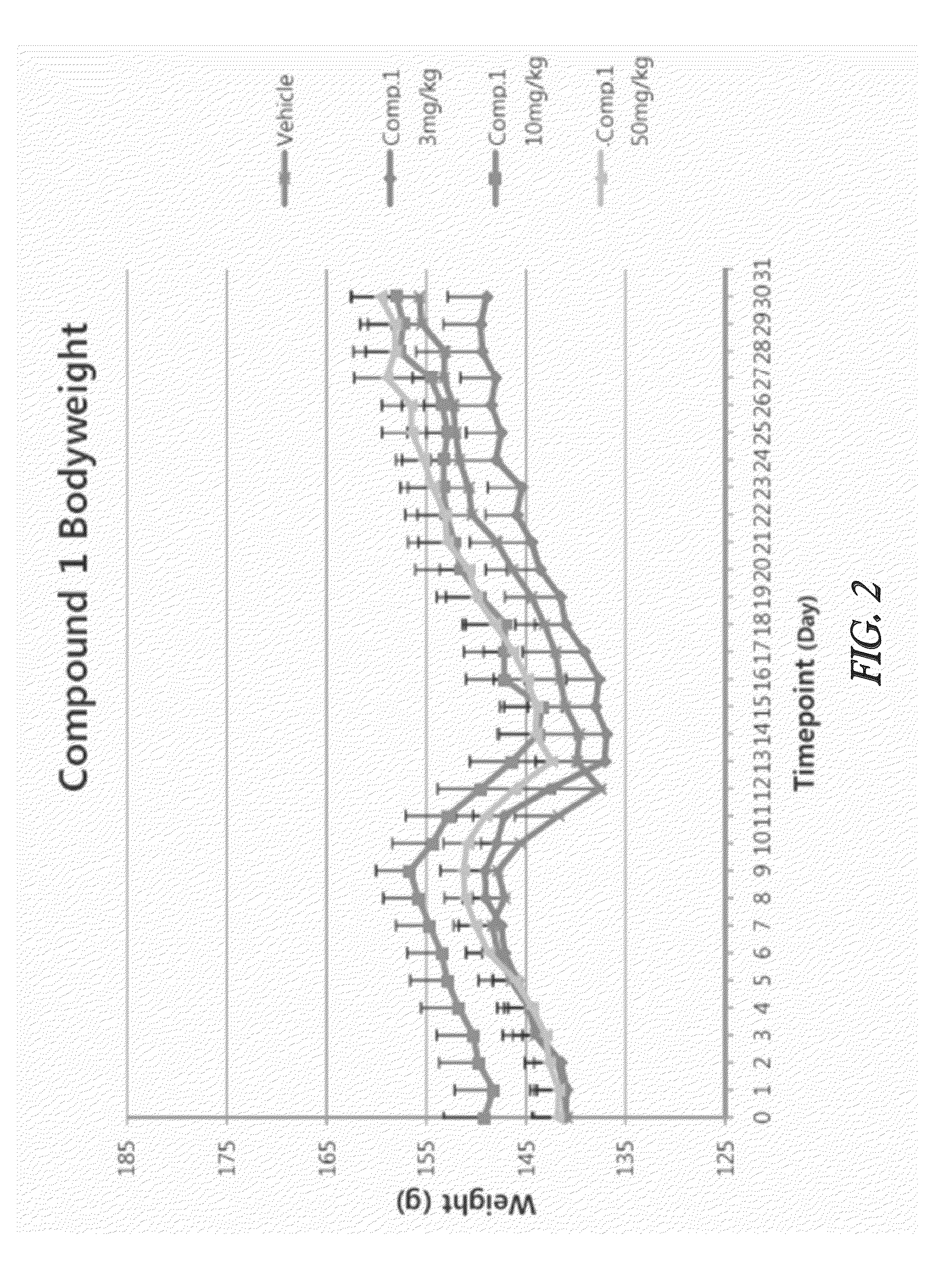
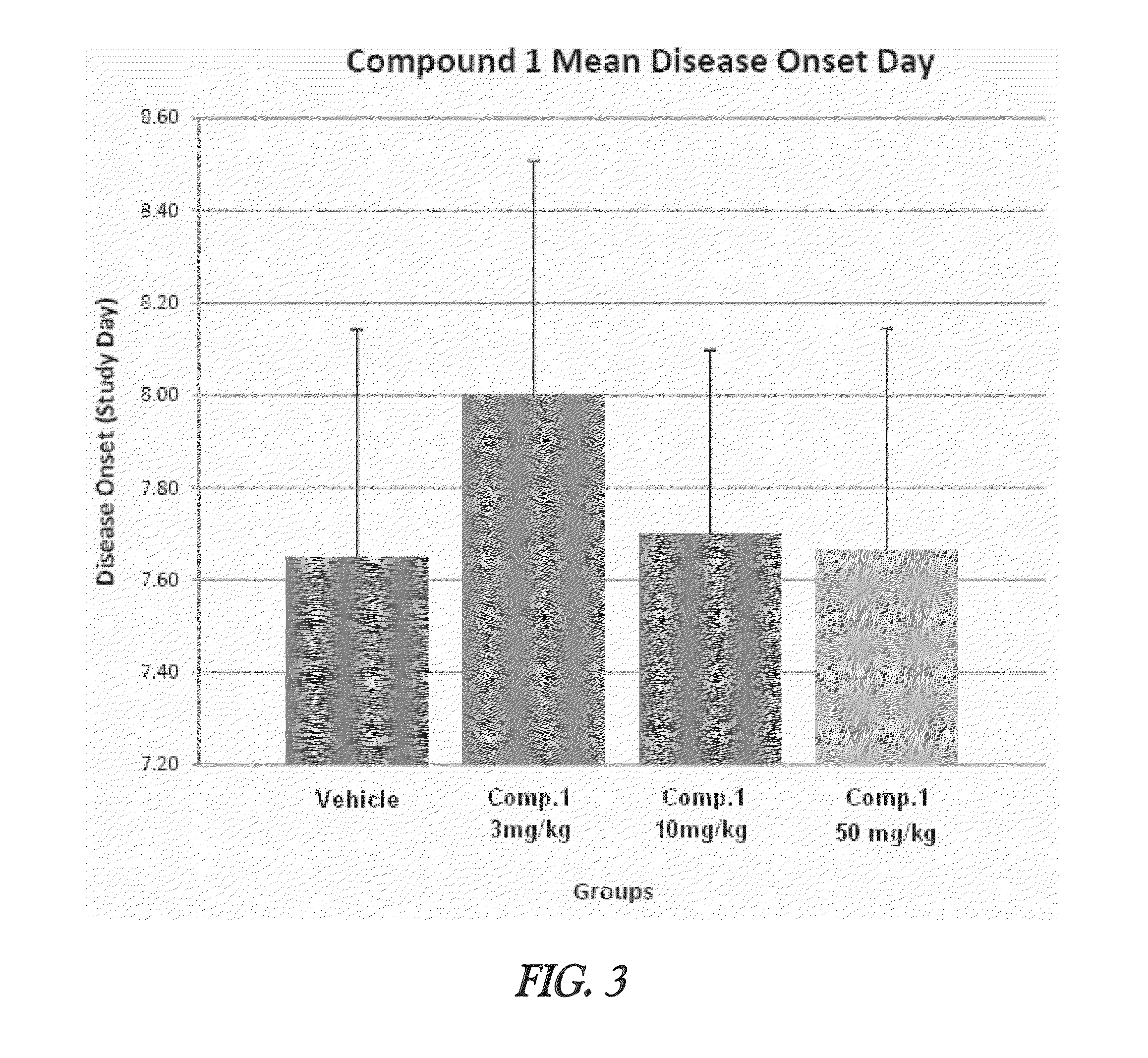
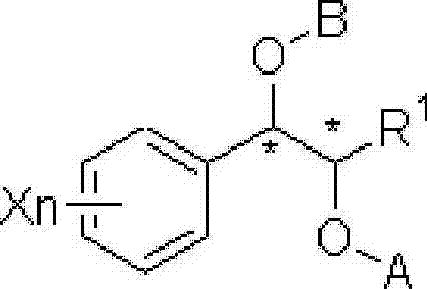
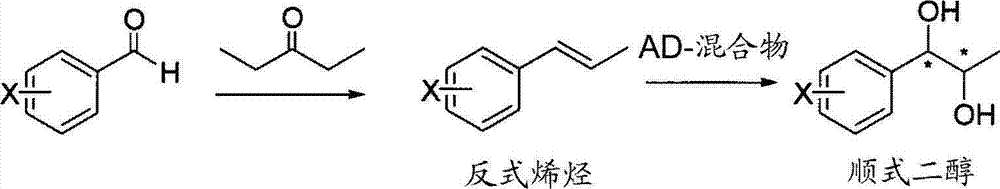
Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration
The invention includes an amount of (3aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl phenylcarbamate for administering to a subject and also a method of preventing or treating neurotoxicity or neurodegenerative processes in a subject in need thereof using the amount thereof.
Owner:QR PHARMA INC
Phenyl carbamate compounds for use in preventing or treating stroke
ActiveUS20130165410A1Inhibition effectAvoid treatmentBiocideNervous disorderCarbamateBULK ACTIVE INGREDIENT
A phenyl carbamate compound; a composition for treating and / or preventing stroke containing the phenyl carbamate compound or a pharmaceutically acceptable salt thereof as an active ingredient; a method of treating and / or preventing stroke comprising administering the phenyl carbamate compound or a pharmaceutically acceptable salt thereof to a patient in need of stroke treatment; and a use of the phenyl carbamate compound or a pharmaceutically acceptable salt thereof in treating and / or preventing stroke, are provided.
Owner:BIO PHARM SOLUTIONS
Method for splitting antipode of 3-butylbenzene phthalein
InactiveCN1539835ASimple and fast operationHigh yieldOrganic chemistryFormateChiral stationary phase
A process for splitting 3-n-butylphenyl phthaleine antipode features that the phenylamino formate or benzoate of polyose compound is used as chiral fixed phase, and the liquid-phase chromatography is directly used for said splitting. Its advantages are high speed and effect, high stability and long service life.
Owner:萍乡分水科技有限公司
Preparation method of oxazolidinone antibiotic intermediate
ActiveCN110938058ARaw materials are easy to getSimple processOrganic chemistryBiotechnologyCarbamate
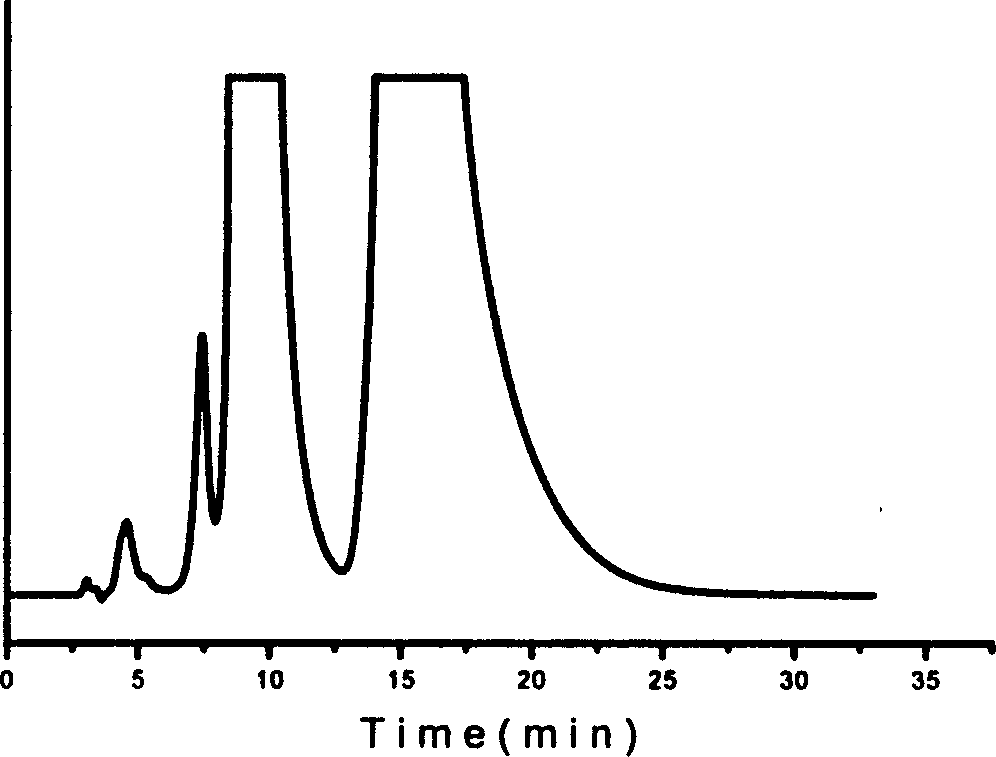
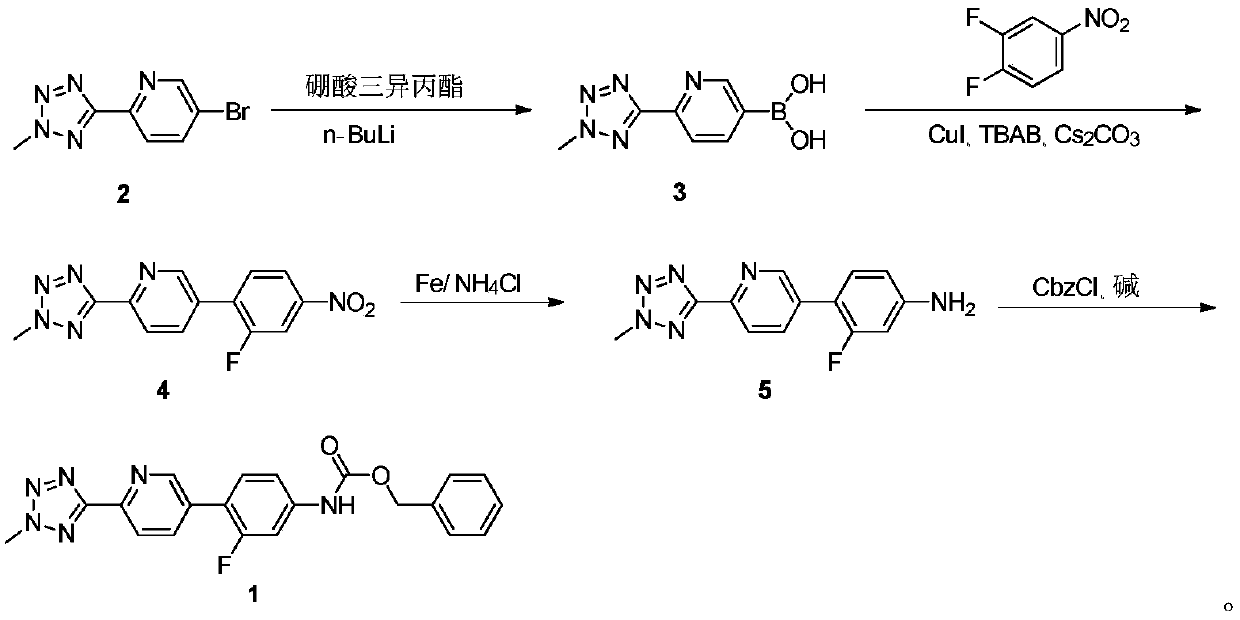
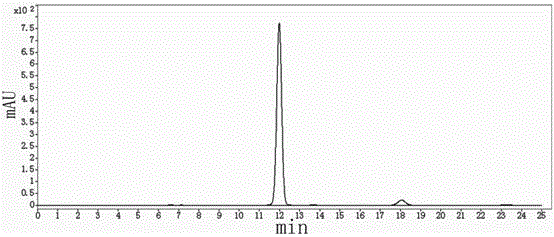
The invention provides a preparation method of a tedizolid phosphate intermediate compound 3-fluoro-4-(6-(2-methyltetrazole-5-yl)pyridine-3-yl)phenyl benzyl carbamate. The method comprises the following steps: carrying out a reaction on 5-bromo-2-(2-methyltetrazole-5-yl)pyridine as a raw material and triisopropyl borate under the action of n-butyllithium to prepare a boric acid intermediate, coupling the boric acid intermediate and 1,2-difluoro-4-nitrobenzene, and finally performing nitro reduction and amidation to obtain the required target product. Compared with the method in the prior art,the preparation method disclosed by the invention has the advantages of easily available raw materials, simple process, economy, environmental protection and suitability for industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
Preparation method of indoxacarb technical concentrate
InactiveCN107235926ANo pollution in the processHigh response rateOrganic chemistrySodium methoxideEthylenediamine
The invention discloses a preparation method of an indoxacarb mother drug, comprising the following steps: using 5-chloroindanone as a raw material to synthesize: (S)-5-chloro-2,3-dihydro-2-hydroxyl-1- Methyl oxo-1H-indene-2-carboxylate; from (S)-5-chloro-2,3-dihydro-2-hydroxy-1-oxo-1H-indene-2-methyl carboxylate and Benzyl carbazate is first condensed and then synthesized with diethoxymethane: 2‑benzyloxycarboxy‑7‑chloroindeno[1,2‑P][1,3,4]oxadiazine‑2, 4a-methyl carboxylate; use p-trifluoromethoxyaniline as raw material and methyl chloroformate, 1,2-diphenylethylenediamine as acid-binding agent to generate 4-(trifluoromethoxy)phenyl Methyl carbamate is then photoacylated with sodium methoxide to generate chloroformyl (4-trifluoromethoxyphenyl) methyl carbamate; by 2-benzyloxycarboxy-7-chloroindeno[1,2 ‑P][1,3,4]Oxadiazine‑2,4a‑carboxylate methyl ester is deprotected by hydrogenation, and then synthesized with methyl chloroformyl (4‑trifluoromethoxyphenyl) carbamate prestige. The invention has the advantages of high reaction yield, greatly improved production efficiency, high product purity and no pollution to the environment.
Owner:NANTONG SHI ZHUANG CHEM
Method for simply synthesizing pyraclostrobin
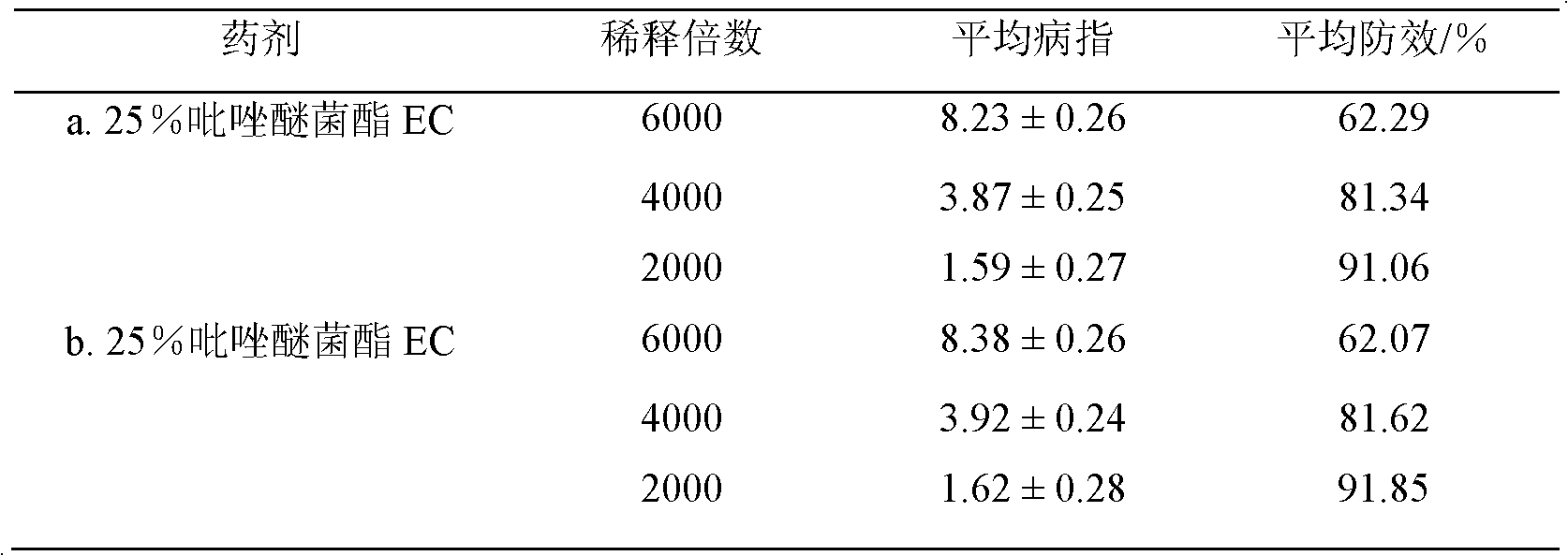
The invention discloses a method for simply synthesizing pyraclostrobin. The method comprises steps as follows: N-hydroxyl-N-2-[1-(4-chlorophenyl)-3-pyrazoloxy methyl]phenyl carbamate and a phase transfer catalyst are dissolved in an organic solvent, dimethyl sulfate is added, an acid-binding agent is dropwise added at the system temperature kept in a range of 5-70 DEG C, the components react for 1-5 h, and pyraclostrobin is obtained. Under the condition of reasonable control of feeding sequence of reaction raw materials and the system temperature, the problems of many reaction by-products as well as potential safety hazard and the like due to the many reaction by-products in an existing pyraclostrobin synthesis process are effectively solved, and a plurality of advantages such as reduction of production cost, reduction of wastewater quantity in a production process and the like can be provided for industrial production, so that the method is beneficial to energy conservation, emission reduction and environment protection of pesticide chemical enterprises and is more suitable for large-scale industrial production.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
Phenyl Carbamate Compound and a Composition for Preventing or Treating a Memory Loss-Related Disease Comprising the Same
ActiveUS20160016896A1Excellent neuroprotecting activityGood curative effectBiocideSenses disorderCarbamateRelated disorder
The present invention relates to a composition for preventing or treating a memory loss related disease comprising a phenyl carbamate compound and a method for preventing or treating various diseases related to loss of memory therewith. The present invention ensures the enhancement of neuroprotection, such that it is promising for preventing or treating memory loss-related diseases such as dementia and Alzheimer's disease.
Owner:BIO PHARM SOLUTIONS
Phenyl Carbamate Compound and a Composition for Neuroprotection Comprising the Same
ActiveUS20160015680A1Excellent neuroprotecting activityHigh activityBiocideSenses disorderDiseaseCarbamate
The present invention relates to a composition for neuroprotection comprising a phenyl carbamate compound and a method for providing neuroprotection therewith. The present invention ensures the enhancement of neuroprotection, such that it is promising for preventing or treating various diseases associated with neurological injury.
Owner:BIO PHARM SOLUTIONS
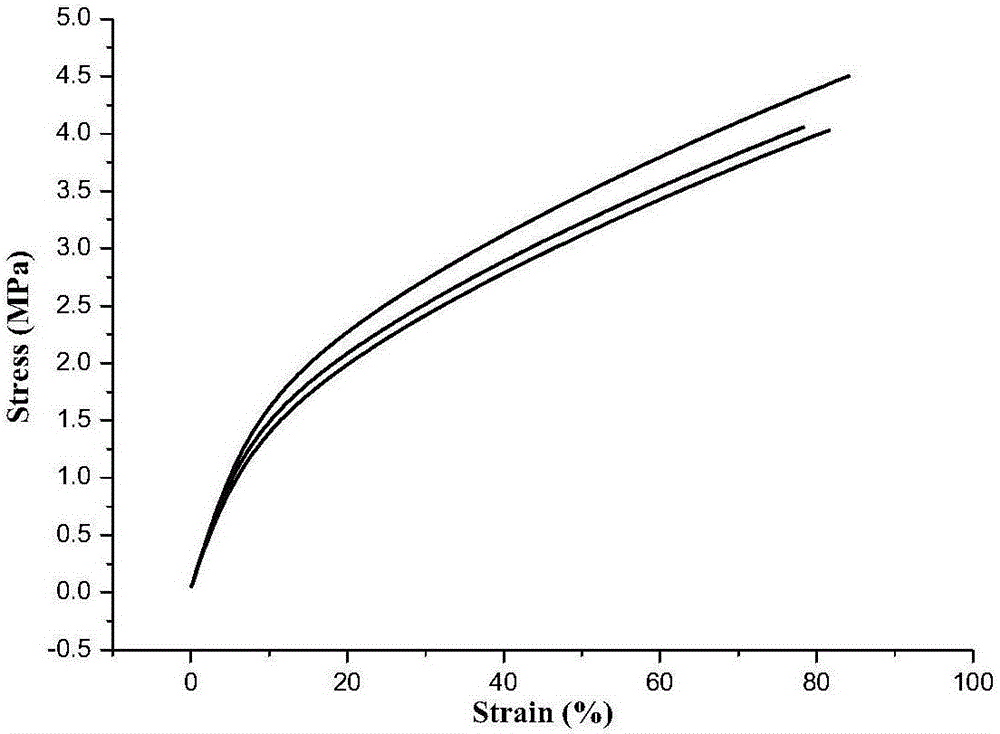
Acetylene-terminated ethylene oxide tetrahydrofuran copolyether containing carbamic acid ester units and synthesis method thereof
The invention discloses an acetylene-terminated ethylene oxide tetrahydrofuran copolyether containing carbamic acid ester units. The structural formula is shown in the picture I. A synthesis process includes the steps that 1, propargyl alcohol reacts with an excessive amount of toluene-2,4-diisocyanate, and then propargyl (3-isocyanate group-4-methyl) phenyl carbamate is obtained; 2, propargyl (3-isocyanate group-4-methyl) phenyl carbamate and terminal hydroxyl ethylene oxide tetrahydrofuran copolyether generate the acetylene-terminated ethylene oxide tetrahydrofuran copolyether containing the carbamic acid ester units through an additive reaction. The acetylene-terminated ethylene oxide tetrahydrofuran copolyether containing the carbamic acid ester units has a specific chain structure, is free of side reactions, and is applied to a polytriazole elastomer so that the polytriazole elastomer can have excellent mechanical properties.
Owner:XIAN MODERN CHEM RES INST
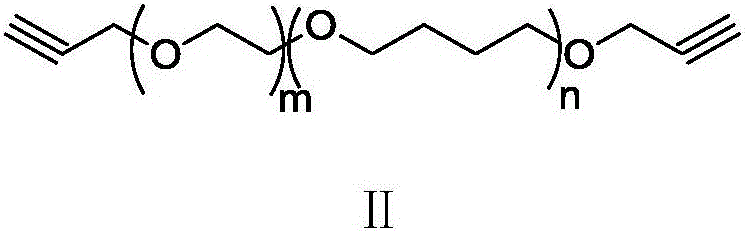
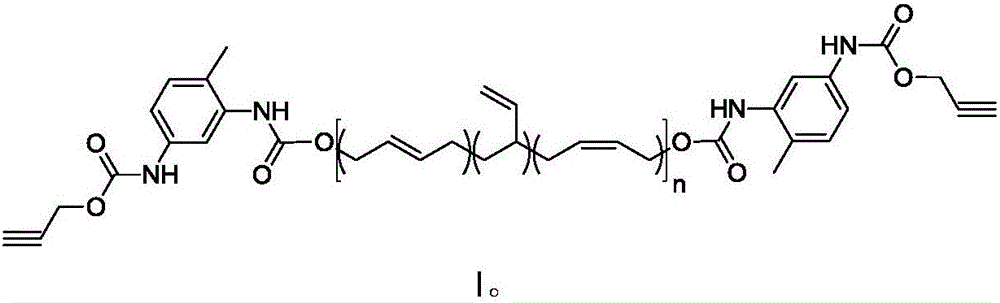
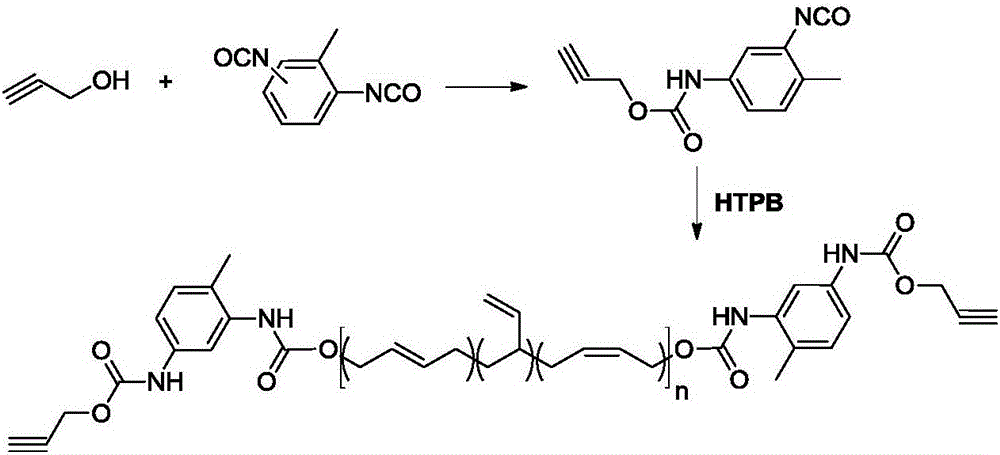
Synthesis method of alkynyl-terminated polybutadiene containing carbamate element
The invention discloses a synthesis method of alkynyl-terminated polybutadiene containing a carbamate element. The structural formula of alkynyl-terminated polybutadiene is shown in formula I. The synthesis process of alkynyl-terminated polybutadiene comprises steps as follows: (1) propynol and excessive toluene-2,4-diisocyanate react to produce propargyl(3-isocyanate-4-methyl)phenyl carbamate; (2) propargyl(3-isocyanate-4-methyl)phenyl carbamate and hydroxyl-terminated polybutadiene are subjected to an addition reaction to produce alkynyl-terminated polybutadiene containing the carbamate element. The synthesized alkynyl-terminated polybutadiene containing the carbamate element adopts a specific chain structure, has no side reaction and enables polytriazole elastomers to have excellent mechanical performance when applied to the polytriazole elastomers.
Owner:XIAN MODERN CHEM RES INST
Fluorinated 2-amino-4-(substituted amino)phenyl carbamate derivatives
InactiveUS20170355679A1Nervous disorderCarbamic acid derivatives preparationPotassium channelPhenylcarbamic acid
Owner:SCIFLUOR LIFE SCI
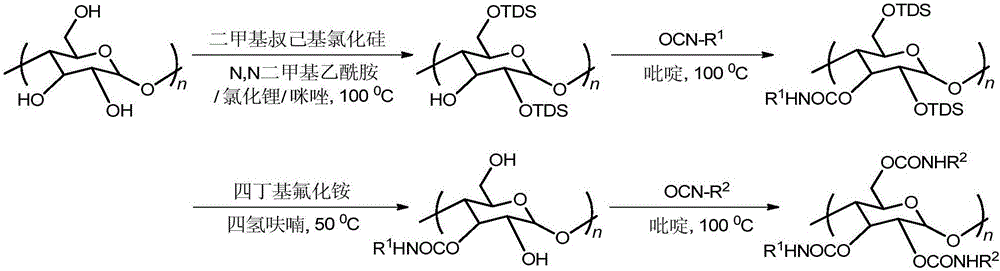
Regioselective synthesis and application methods for amylose derivatives with different carbamate side groups
InactiveCN105017437AExcellent chiral recognition performanceAchieve separationOther chemical processesCarbamateSynthesis methods
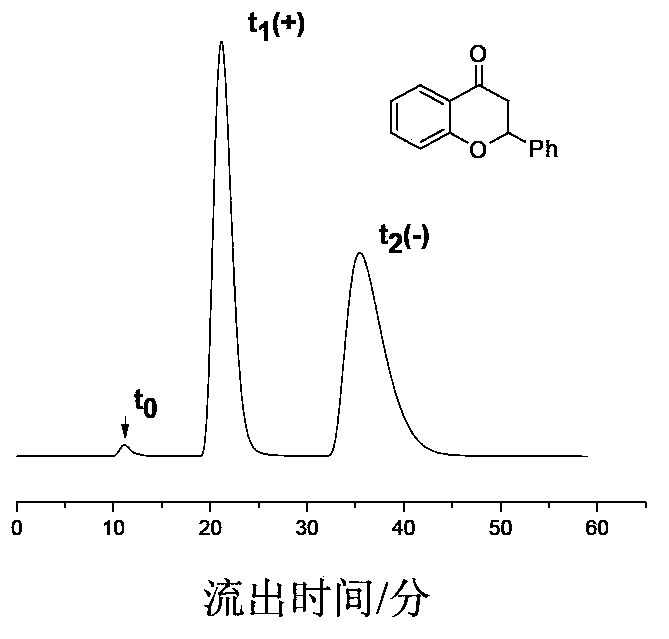
The invention provides regioselective synthesis and application methods for amylose derivatives with different carbamate side groups. The synthesis method comprises taking amylose as an initial raw material, firstly introducing a tert-butyldimethylsilyl ester at 2- and 6- positions of a sugar unit of amylase as a protective group, then introducing a phenylcarbamic acid ester group at 3- position of the sugar unit, then in an alkali solution, removing the protective group and reducing hydroxyl at 2- and 6- positions, and finally introducing another kind phenylcarbamic acid ester group at 2- and 6- positions at the same time so as to finally realize regioselective synthesis of the amylose derivatives. On the basis, the synthesized novel amylose derivative is fully dissolved, the surface of silica gel is uniformly coated with the polymer through a coating process, and a column is packed by employing a homogenating process, so that a novel amylose chiral stationary phase is prepared. high performance liquid chromatography is applicable to detailed evaluation on the chiral recognition performance of the prepared chiral stationary phase.
Owner:HARBIN ENG UNIV
Process for preparation of phenyl carbamate derivatives
ActiveUS8859817B2Group 4/14 element organic compoundsPreparation by oxidation reactionsCarbamateCentral nervous system
Provided are a process for the preparation of phenyl carbamate derivatives, useful in the treatment of CNS (central nervous system) disorders, an intermediate in the synthesis of the phenyl carbamate derivatives, and a process for preparation of the intermediate.
Owner:BIO PHARM SOLUTIONS
Phenyl Carbamate Compound and a Composition for Preventing or Treating a Psychiatric Disorder Comprising the Same
The present invention relates to a composition for preventing or treating a psychiatric disorder comprising a phenyl carbamate compound and a method for preventing or treating a psychiatric disorder therewith. The present invention provides anti-anxiety activity and protections against seizure and bipolar disorder, such that it may be effectively used for preventing or treating various psychiatric disorders related to mood disorder or resulting convulsion.
Owner:BIO PHARM SOLUTIONS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00000.png)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00001.png)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00002.png)