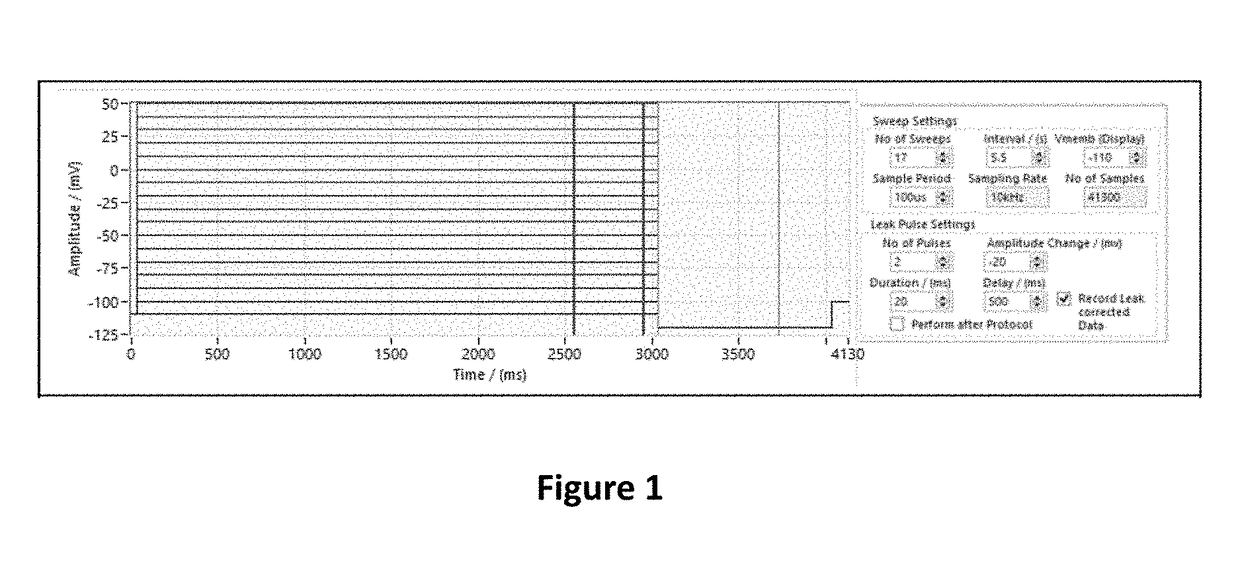
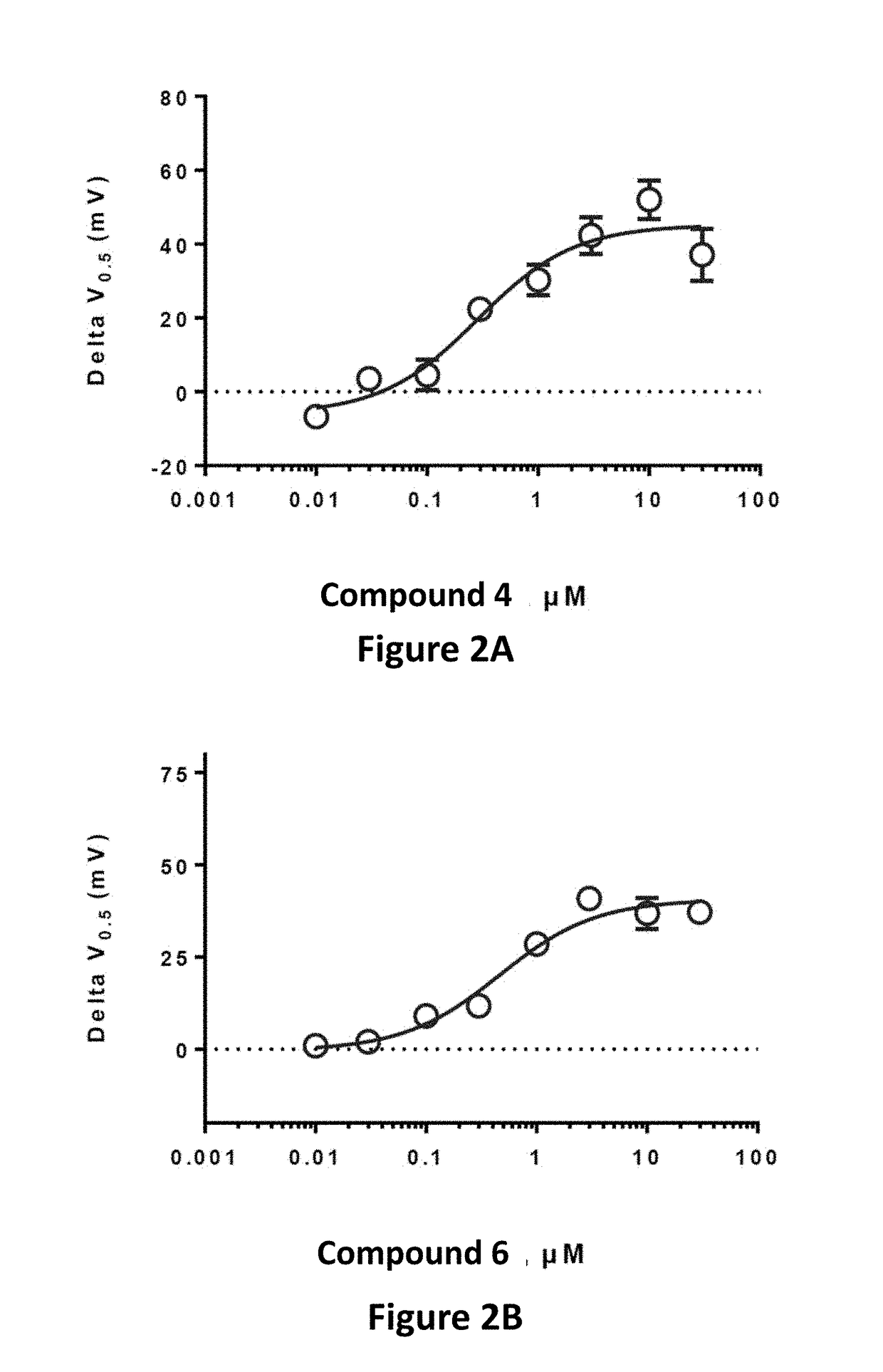
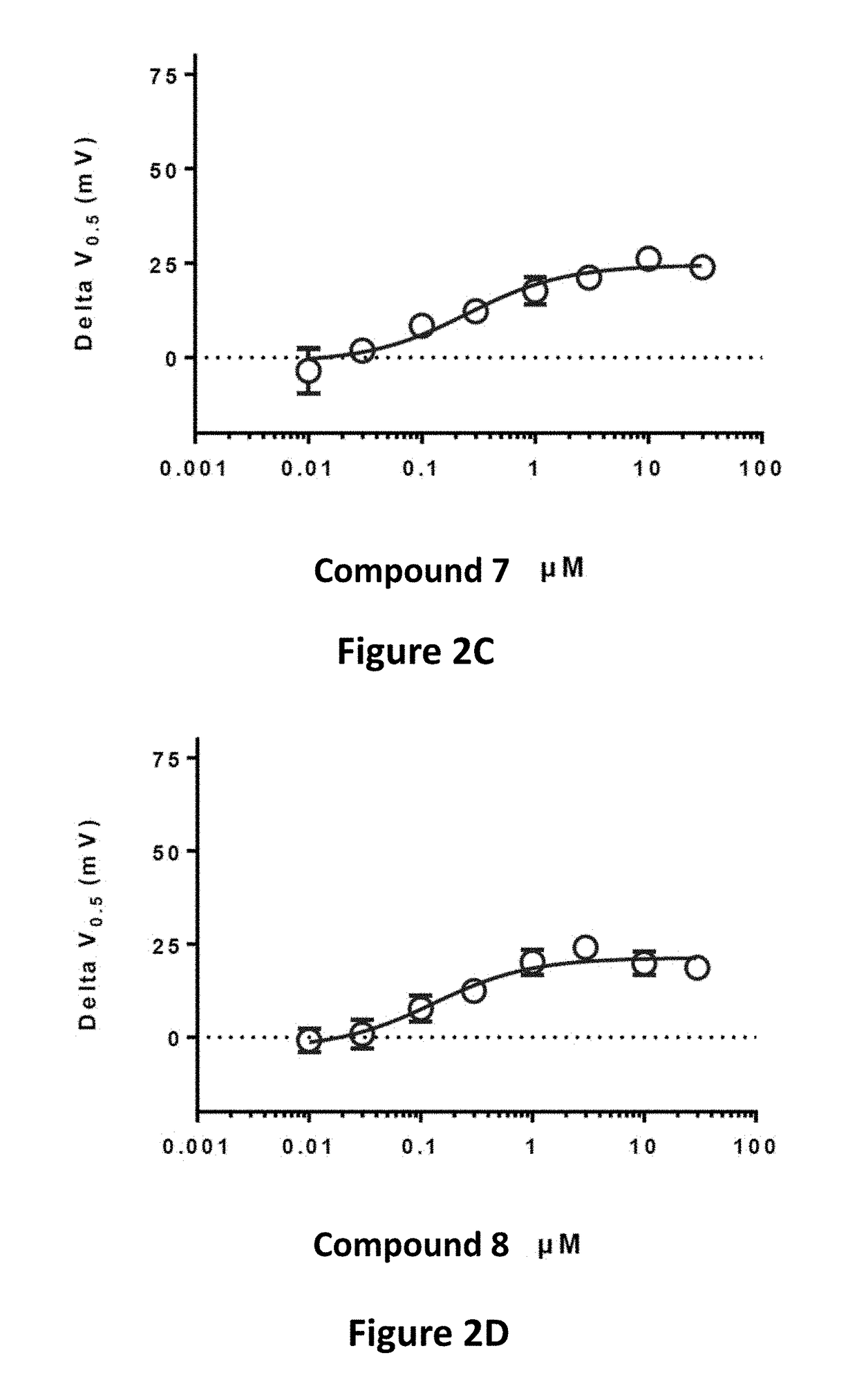
Fluorinated 2-amino-4-(substituted amino)phenyl carbamate derivatives
a technology of phenyl carbamate and substituted amino, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, organic chemistry, etc., can solve the problems of depression and sexual function, memory decline or worsening, depression and other lifestyle limitations, and many patients are not adequately treated with the current available options, so as to achieve the effect of treating or preventing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
[0314]
Step 1: Synthesis of Compound a
[0315]To a stirred solution of 4-fluorobenzylamine (1 equivalent (equiv)) dissolved in dimethylformamide (DMF) is added allyl bromide (1.5 equiv) and diisopropyl ethylamine (2 equiv) dropwise, and the resulting mixture is heated to 80° C. After 2 hours, the reaction mixture is cooled, diluted with water, and extracted with ethyl acetate (EtOAc). The organic layer is then washed with saturated brine, dried with anhydrous sodium sulfate, filtered, and concentrated under vacuum. The resulting residue is purified by silica gel liquid chromatography to provide Compound a.
Step 2: Synthesis of Compound b
[0316]To a stirred suspension of 2,3-difluoro-6-nitroaniline (1 equiv) in dry dimethyl sulfoxide (DMSO) is added Compound a (3 equiv) followed by Et3N (1.2 equiv) and I2 (catalytic amount). The resulting mixture is heated to 120° C. and stirred at 120° C. for 24 hours. Upon complete consumption of the starting material (as determined by thin layer chroma...
example 1b
Step 1: Synthesis of 2-Fluoro-N1-(4-fluorobenzyl)-4-nitrobenzene-1,3-diamine
[0318]2,3-Difluoro-6-nitroaniline (10.0 g, 79.9 mmole) was dissolved in anhydrous dimethylsulfoxide (90 mL). 4-fluorobenzylamine (9.3 g, 53.3 mmole) was added triethylamine (17.7 mL) and solid iodine (80 mg) were added and the mixture was heated at reflux for 4 h. under argon. The reaction was dissolved in ethyl acetate (200 mL) and extracted with water (3×100 mL). A yellow solid precipitated out of the organic layer to give 2-fluoro-N1-(4-fluorobenzyl)-4-nitrobenzene-1,3-diamine (13.6 g, 91% yield).
Step 2: Synthesis of di-tert-butyl (3-fluoro-4-((4-fluorobenzyl)amino)-1,2-phenylene)dicarbamate
[0319]2-Fluoro-N1-(4-fluorobenzyl)-4-nitrobenzene-1,3-diamine (13.55 g, 48.53 mmole) was dissolved in methanol (60 mL) and tetrahydrofuran (60 mL). The mixture was cooled in an ice bath and zinc powder (31.70 g, 485.3 mmole) was added followed by ammonium chloride (26.0 g, 485.3 mmole) in DI water (64 mL) over 30 min. ...
example 2a
[0324]
Step 1: Synthesis of Compound c
[0325]To a stirred solution of 4-trifluoromethylbenzylamine (1 equiv) dissolved in DMF is added allyl bromide (1.5 equiv) and diisopropyl ethylamine (2 equiv) dropwise, and the resulting mixture is heated to 80° C. After 2 hours, the reaction mixture is cooled, diluted with water, and extracted with ethyl acetate. The organic layer is then washed with saturated brine, dried with anhydrous sodium sulfate, filtered, and concentrated under vacuum. The resulting residue is purified by silica gel liquid chromatography to give Compound c.
Step 2: Synthesis of Compound d
[0326]To a stirred suspension of 2, 3-difluoro-6-nitroaniline (1 equiv) in dry DMSO is added Compound c (3 equiv) followed by Et3N (1.2 equiv) and I2 (catalytic amount). The reaction mixture is heated to 120° C. and stirred at 120° C. for 24 h. Upon complete consumption of the starting material (as determined by TLC), the reaction mixture is cooled to RT, diluted with water (25 mL), and e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com