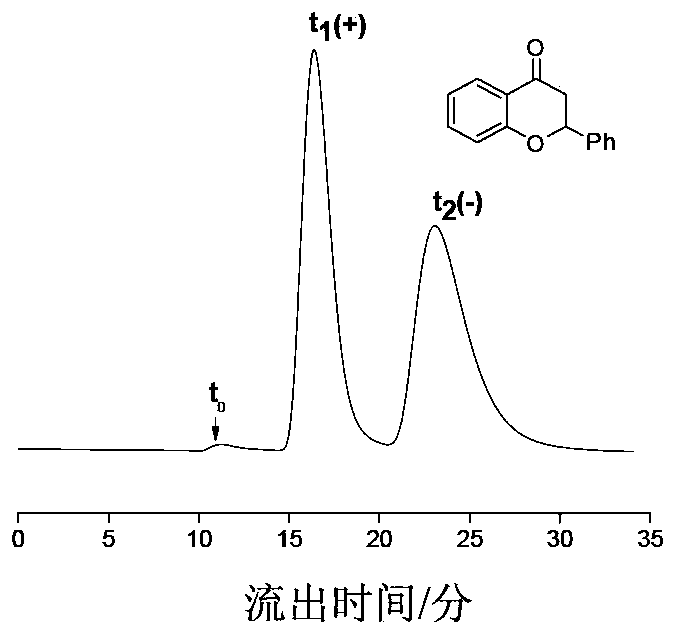
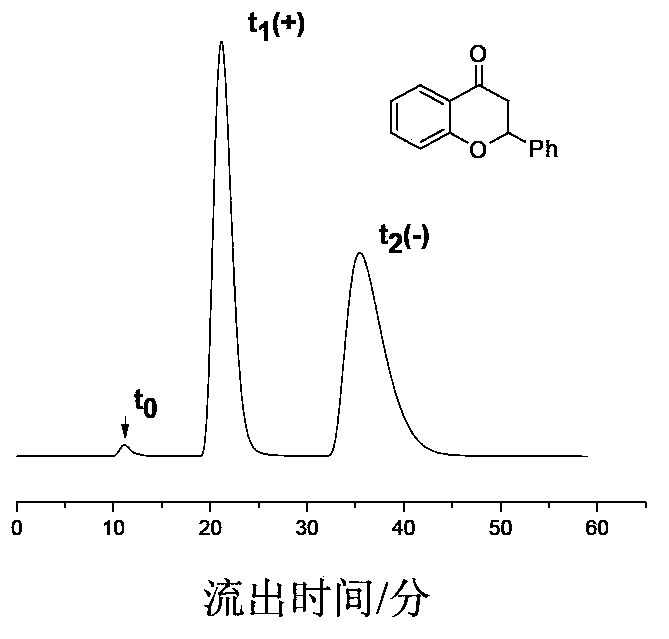
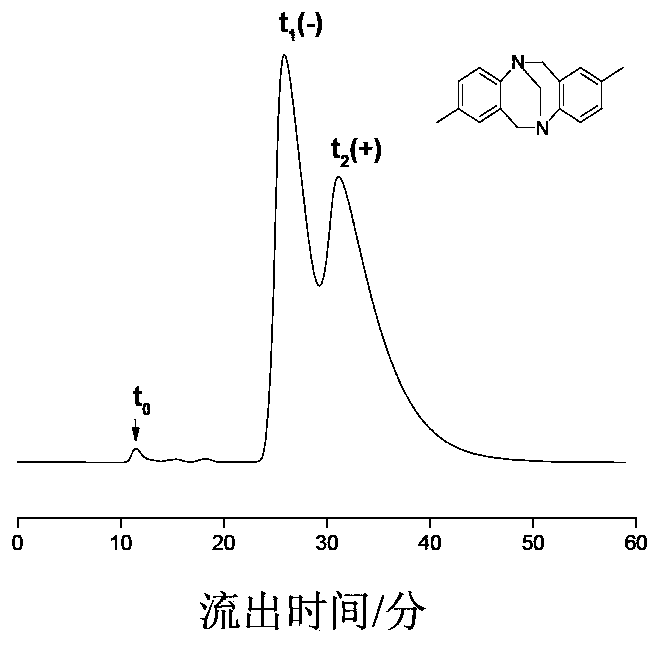
Preparation method of immobilized beta-cyclodextrin derivative type chiral stationary phase
A chiral stationary phase and cyclodextrin technology, applied in the field of high performance liquid chromatography, can solve the problem of reducing the chiral separation ability of the bonded cyclodextrin chiral stationary phase, increasing the achiral recognition effect, and low spatial regularity. The problem is to achieve the effect of an effective preparation method, simple post-processing and purification, and fast reaction rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Add 0.9g of completely dried β-cyclodextrin into a reactor containing 15ml of anhydrous pyridine, raise the temperature to 80°C under a nitrogen atmosphere, and add 0.6ml of propyl triethoxyisocyanate when all the solids are dissolved base silane, kept at a constant temperature of 80°C, and stirred for 12 hours. Then add 1.2ml of phenylisocyanate, keep the temperature at 80°C, stir for 24h, and stop heating. [0013] The cooled pyridine solution containing the product is added dropwise in the vigorously stirred 300ml aqueous methanol solution (methanol / water=4 / 1, v / v), producing a large amount of white precipitates. After continuing to stir vigorously for 15 minutes, stop stirring, and after standing still for 15 minutes, pour out the supernatant. The suspension containing a large amount of solids in the lower part was centrifuged at high speed to separate the solid product. After centrifugation, add 30ml of methanol aqueous solution (methanol / water=4 / 1, v / v) to wash, an...
Embodiment 2
[0029] The reaction steps and conditions are the same as those in Example 1, the substrate used is 4-methylphenylisocyanate, and its consumption is 1.4ml. Finally, a 4-methylphenylcarbamate β-cyclodextrin chiral column was obtained.
Embodiment 3
[0031] The reaction steps and conditions are the same as in Example 1, the substrate used is 4-chlorophenylisocyanate, and its consumption is 1.70g. Finally, a 4-chlorophenylcarbamate β-cyclodextrin chiral column was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com