Regioselective synthesis and application methods for amylose derivatives with different carbamate side groups
A carbamate, regioselective technology, applied in chemical instruments and methods, other chemical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
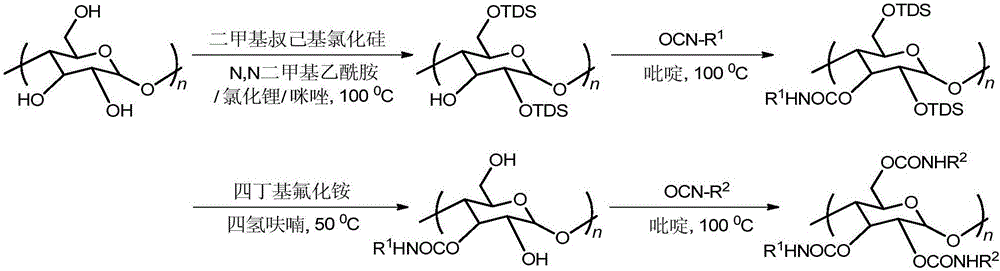
[0028] 1. Take 1g of amylose and vacuum-dry at 105°C for 4h, raise the temperature to 120°C, then stir and reflux in anhydrous N,N-dimethylacetamide for 4h; add 1.5g of lithium chloride after cooling to 100°C; Continue to stir for 2 hours, add 2.02 g of imidazole after cooling to room temperature; add excess dimethyl tert-hexyl silicon chloride after reflux for 20 minutes, reheat to 100 ° C, continue stirring and reflux for 24 hours to stop the reaction; cool to room temperature, add buffer ( Add 3.54 g of potassium dihydrogen phosphate and 7.14 g of dipotassium hydrogen phosphate to 1000 ml of distilled water), settle and filter, wash with ethanol and water (volume ratio 9:1), and vacuum-dry at 60° C. to constant weight. The yield is 80%.
[0029] 2. Vacuum-dry the above intermediate product at 80°C for 3h, then reflux in anhydrous pyridine for 6h, add excess 3,5-dichlorophenyl isocyanate after heating up to 100°C, continue to reflux at 100°C for 12h, then stop The reaction w...
specific Embodiment approach 2
[0034] 1. Take 1g of amylose and vacuum-dry at 105°C for 4h, raise the temperature to 120°C, then stir and reflux in anhydrous N,N-dimethylacetamide for 4h; add 1.5g of lithium chloride after cooling to 100°C; Continue to stir for 2 hours, add 2.02 g of imidazole after cooling to room temperature; add excess dimethyl tert-hexyl silicon chloride after reflux for 20 minutes, reheat to 100 ° C, continue stirring and reflux for 24 hours to stop the reaction; cool to room temperature, add buffer ( Add 3.54 g of potassium dihydrogen phosphate and 7.14 g of dipotassium hydrogen phosphate to 1000 ml of distilled water), settle and filter, wash with ethanol and water (volume ratio 9:1), and vacuum-dry at 60° C. to constant weight. The yield is 80%.
[0035] 2. Vacuum-dry the above intermediate product at 80°C for 4h, then reflux in anhydrous pyridine for 6h, heat up to 100°C, add excess 4-chlorophenyl isocyanate, continue to reflux at 100°C for 14h, then stop the reaction, use Wash tho...
specific Embodiment approach 3
[0040] 1. Take 1g of amylose and vacuum-dry at 105°C for 4h, raise the temperature to 120°C, then stir and reflux in anhydrous N,N-dimethylacetamide for 4h; add 1.5g of lithium chloride after cooling to 100°C; Continue to stir for 2 hours, add 2.02 g of imidazole after cooling to room temperature; add excess dimethyl tert-hexyl silicon chloride after reflux for 20 minutes, reheat to 100 ° C, continue stirring and reflux for 24 hours to stop the reaction; cool to room temperature, add buffer ( Add 3.54 g of potassium dihydrogen phosphate and 7.14 g of dipotassium hydrogen phosphate to 1000 ml of distilled water), settle and filter, wash with ethanol and water (volume ratio 9:1), and vacuum-dry at 60° C. to constant weight. The yield is 80%.
[0041] 2. Vacuum-dry the above intermediate product at 80°C for 4h, then reflux in anhydrous pyridine for 6h, add excess phenyl isocyanate after heating up to 100°C, stop the reaction after continuing to reflux at 100°C for 14h, and wash th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com