Method for catalytically synthesizing pyraclostrobin
A technology for synthesizing pyrazole ether and strobilurin, which is applied in organic chemistry and other fields, can solve problems such as harsh reaction conditions, incomplete reaction, and long reaction time, and achieve short reaction time, control of process water consumption, and high product yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
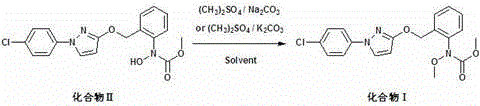
[0025] Add 373.8 g (1 mol) of compound II to a two-phase solvent consisting of 2250 mL of chloroform and 350 mL of water, add 11.2 g of tetrabutylammonium bromide, raise the temperature to reflux, and dropwise add 173 g of 30% (1.3 mol) of sodium hydroxide aqueous solution and 164g (1.3 mol) of dimethyl sulfate, keeping the pH of the reaction system = 8-9, adding dropwise in 2 hours, stirring at reflux for 2 hours, cooling down to room temperature, separating the aqueous phase, and subtracting the organic phase Concentrate under reduced pressure, add crystallization solvent isopropanol to the concentrate, and filter to obtain compound Ⅰ, 373 g of light yellow crystals, HPLC assay (external standard) content 98.8%, yield 96.2%, mp: 63-65°C.
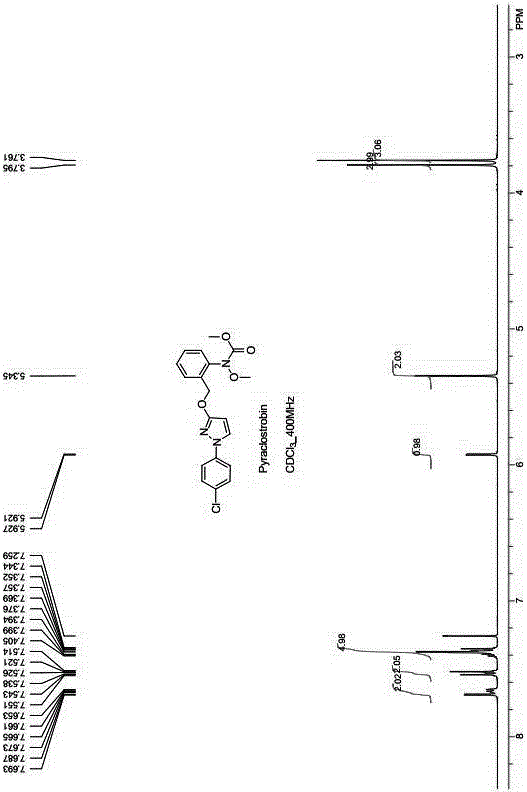
[0026] 1 H-NMR (400MHz, CDCl 3 ): δ = 3.761(3H, s, -CH 3 ), δ = 3.795(3H, s, -CH 3 ), δ = 5.345(2H, s, -CH 2 ), δ = 5.9215.927(1H, d, -CH), δ = 7.344~7.405(5H, m, Phenyl-H, -CH), δ = 7.5147.551(2H, d, Phenyl-H), δ = 7.6537.693(2H, m, ...
Embodiment 2
[0029] Add 373.8 g (1 mol) of compound II to a biphasic solvent consisting of 1699 mL of dichloromethane and 170 mL of water, add 22.4 g of tetrabutylammonium chloride, raise the temperature to 39-42 ° C, and drop 173 g of 30 % (1.3 mol) sodium hydroxide aqueous solution and 164g (1.3 mol) dimethyl sulfate, keep the pH of the reaction system = 8-9, add dropwise in 2 hours, keep stirring at 39-42°C for 2 hours, cool to room temperature, divide The aqueous phase was removed, and the organic phase was concentrated under reduced pressure. The crystallization solvent isopropanol was added to the concentrate, and compound Ⅰ was obtained by filtration, 370 g of light yellow crystals. The content determined by HPLC (external standard) was 98.3%, and the yield was 95.4%.
Embodiment 3
[0031] Add 373.8 g (1 mol) of compound II to a biphasic solvent composed of 2250 mL 1,2-dichloroethane and 350 mL water, add 11.2 g tetrabutylammonium bromide, and heat up to 60°C-65°C, At the same time, add 173 g of 30% (1.3 mol) sodium hydroxide aqueous solution and 164 g (1.3 mol) of dimethyl sulfate dropwise, keep the pH of the reaction system = 8-9, complete the dropwise addition in 2 hours, keep stirring at 60°C-65°C for 2 hours, lowered to room temperature, separated the water phase, concentrated the organic phase under reduced pressure, added crystallization solvent isopropanol to the concentrate, and filtered to obtain compound Ⅰ, 370 g of light yellow crystals, the content of which was determined by HPLC (external standard) was 98.5%, and the yield was The rate is 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com