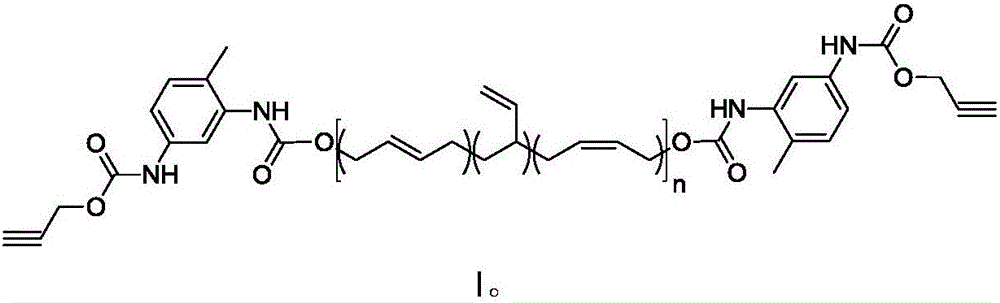
Synthesis method of alkynyl-terminated polybutadiene containing carbamate element
A technology of alkyne-terminated polybutadiene and hydroxyl-terminated polybutadiene, which is applied in the field of polymer materials, can solve the problems of poor mechanical properties and affect the mechanical properties of polytriazole elastomers, and achieve the effect of excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
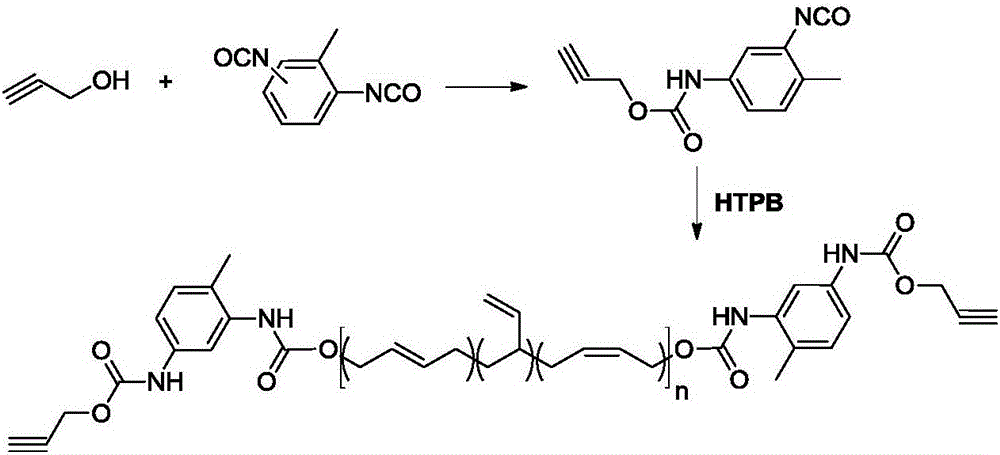
[0017] (1) Preparation of propargyl (3-isocyanato-4-methyl)phenylcarbamate
[0018] Under nitrogen protection, 5.6 g (0.1 mol) propargyl alcohol was added dropwise to a mixture of 52.2 g (0.3 mol) toluene-2,4-diisocyanate and 100 mL of toluene, and the temperature was maintained at 40°C during the dropwise addition. After the completion of the dropwise addition, the reaction was completed at 60°C for 6 hours. After cooling down, a solid was precipitated, which was filtered and dried to obtain 14 g of a white solid with a yield of 60.9%.
[0019] Characterization data:
[0020] 1 H NMR (DMSO-d 6 , 500MHz): δ=9.90(s, 1H), 7.31(s, 1H), 7.19(m, 2H), 4.76(d, 2H, J=2.0Hz), 3.56(t, 1H, J=2.0Hz) ,2.23(s,3H); 13 C NMR (DMSO-d 6 , 125MHz): δ=153.0, 138.2, 132.3, 131.3, 127.2, 124.6, 116.4, 114.9, 79.3, 78.0, 52.5, 17.6.
[0021] IR(KBr,cm -1 ): ν=3331, 3294, 2281, 2124, 1714, 1623, 1553, 1516, 1388, 1314, 1283, 1227, 1075, 916;
[0022] Elemental Analysis: C 12 H 10 N 2 O 3...
Embodiment 2
[0031] (1) Preparation of propargyl (3-isocyanato-4-methyl)phenylcarbamate
[0032] Under nitrogen protection, 5.6 g (0.1 mol) propargyl alcohol was added dropwise to a mixture of 69.6 g (0.4 mol) toluene-2,4-diisocyanate and 100 mL toluene, and the temperature was maintained at 50°C during the dropwise addition. After the completion of the dropwise addition, the reaction was completed at 60°C for 6 hours. After cooling, a solid was precipitated, which was filtered and dried to obtain 14.2 g of a white solid with a yield of 62.0%.
[0033] (2) Synthesis of alkynyl-terminated polybutadiene containing carbamate units
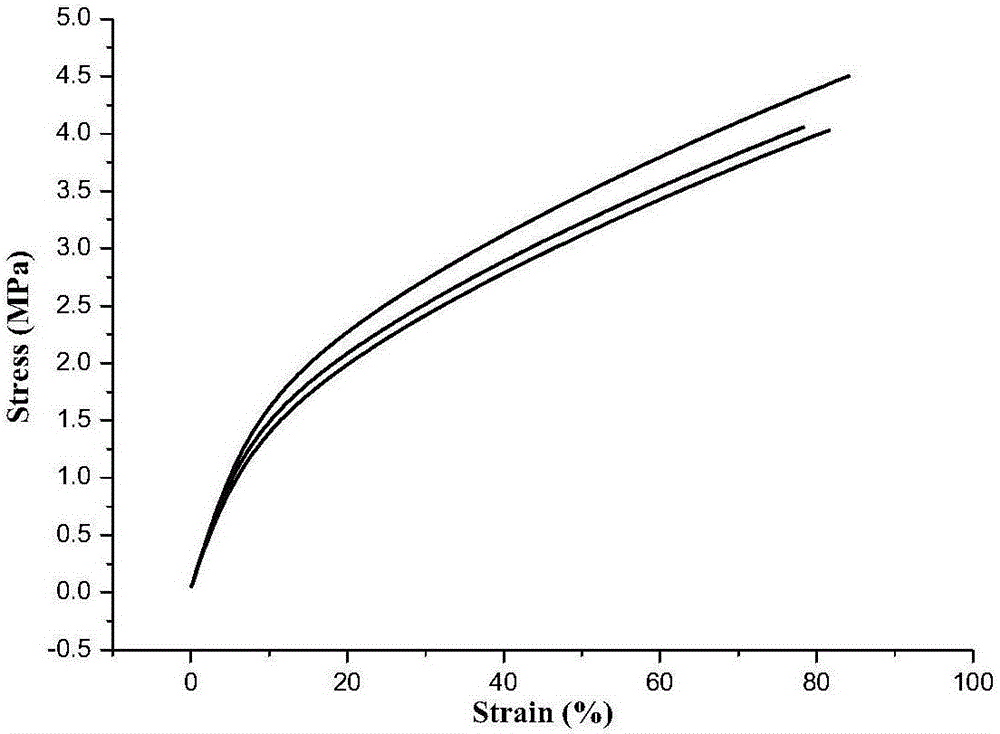
[0034] Under nitrogen protection, 9.4g (40.9mmol) propargyl (3-isocyanato-4-methyl)phenylcarbamate, 60g hydroxyl terminated polybutadiene (40.9mmol OH) and 250mL dry toluene were added to the reaction bottle. After stirring at 70° C. for 24 hours, the reaction was terminated, and the toluene was removed by concentration to obtain 68.0 g of a pale yellow solid w...
Embodiment 3
[0036] (1) Preparation of propargyl (3-isocyanato-4-methyl)phenylcarbamate
[0037] Under nitrogen protection, 5.6 g of propynol (0.1 mol) was added dropwise to a mixture of 87 g (0.5 mol) of toluene-2,4-diisocyanate and 100 mL of toluene, and the temperature was maintained at 50°C during the dropwise addition. After completion of the dropwise addition, the reaction was completed at 70°C for 5 hours. After cooling, a solid was precipitated, which was filtered and dried to obtain 15.6 g of a white solid with a yield of 68.1%.
[0038] (2) Synthesis of alkynyl-terminated polybutadiene containing carbamate units
[0039] Under nitrogen protection, 9.4g (40.9mmol) propargyl (3-isocyanato-4-methyl)phenylcarbamate, 60g hydroxyl terminated polybutadiene (40.9mmol OH) and 250mL dry toluene were added to the reaction bottle. The reaction was terminated after stirring at 60° C. for 24 hours, and 67.0 g of light yellow solid was obtained by concentrating to remove toluene, and the yie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com