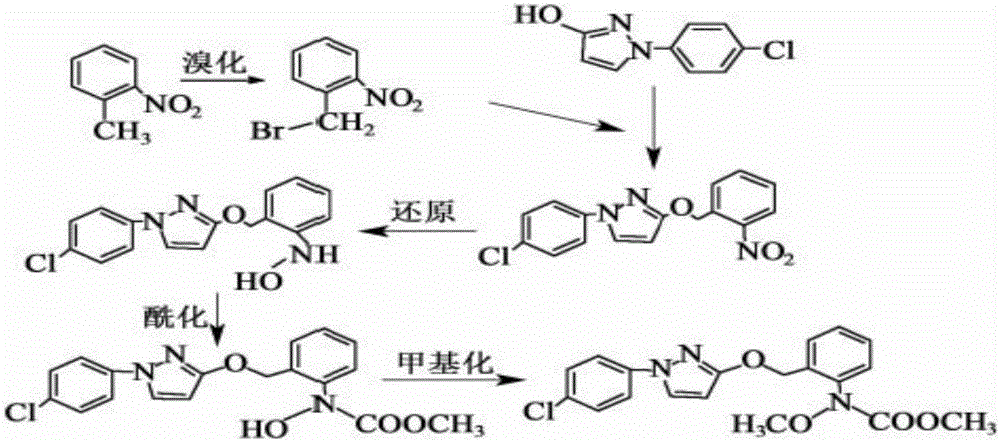
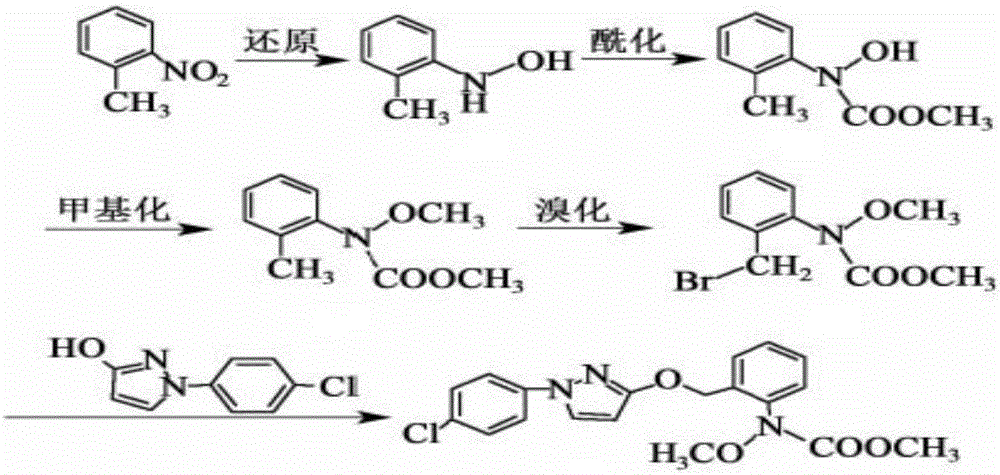
Synthesis technology of pyraclostrobin
A pyraclostrobin and synthesis process technology, which is applied in the field of pyraclostrobin synthesis process, can solve the problems of unsuitability for industrial production, high cost of raw materials, low yield, etc., and achieve high catalytic efficiency and selectivity, Effects of improving yield and purity, and improving selective reducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of synthetic technique of pyraclostrobin mainly comprises the following steps:
[0021] (1) Under the catalysis of 0.4mol zinc powder and its alloy micronano powder, 0.2mol o-nitrotoluene and 0.2mol NH 4 Cl aqueous solution undergoes a reduction reaction, and the reaction temperature is controlled at 60°C, and then 0.04mol of n-propylamine is added. After the reaction is complete, excess zinc powder is filtered, and the filtrate is extracted with ethyl acetate, washed with water and dried, and then collected by desolvation under reduced pressure. The orange material is N-(2-methylphenyl)hydroxylamine;
[0022] (2) Add 0.2mol N-(2-methylphenyl) hydroxylamine, 0.4mol NaHCO in the reactor 3 and dichloromethane solvent, slowly drop 0.2mol of methyl chloroformate at a temperature of 3°C, keep warm after the dropwise addition, filter, wash with water, dry, and crystallize after reduced pressure distillation, the white solid obtained is N -Methyl hydroxy-N-2-tolueneca...
Embodiment 2
[0028] A kind of synthetic technique of pyraclostrobin mainly comprises the following steps:
[0029] (1) Under the catalysis of 0.6mol zinc powder and its alloy micronano powder, 0.3mol o-nitrotoluene and 0.3mol NH 4 A reduction reaction occurs in the aqueous Cl solution, the reaction temperature is controlled at 55°C, and 0.06mol of n-propylamine is added. After the reaction is complete, the filtrate is extracted with ethyl acetate, washed with water and dried, and the orange material obtained by desolvation under reduced pressure is N-(2-methylphenyl)hydroxylamine;
[0030] (2) Add 0.2mol of N-(2-methylphenyl) hydroxylamine, 0.4mol of NaHCO in the reactor 3 and dichloromethane solvent, slowly drop 0.26mol of methyl chloroformate at a temperature of 8°C, keep warm after the dropwise addition is completed, filter, wash with water, dry, and crystallize after reduced pressure distillation, the white solid obtained is N -Methyl hydroxy-N-2-toluenecarbamate;
[0031] (3) Add 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com