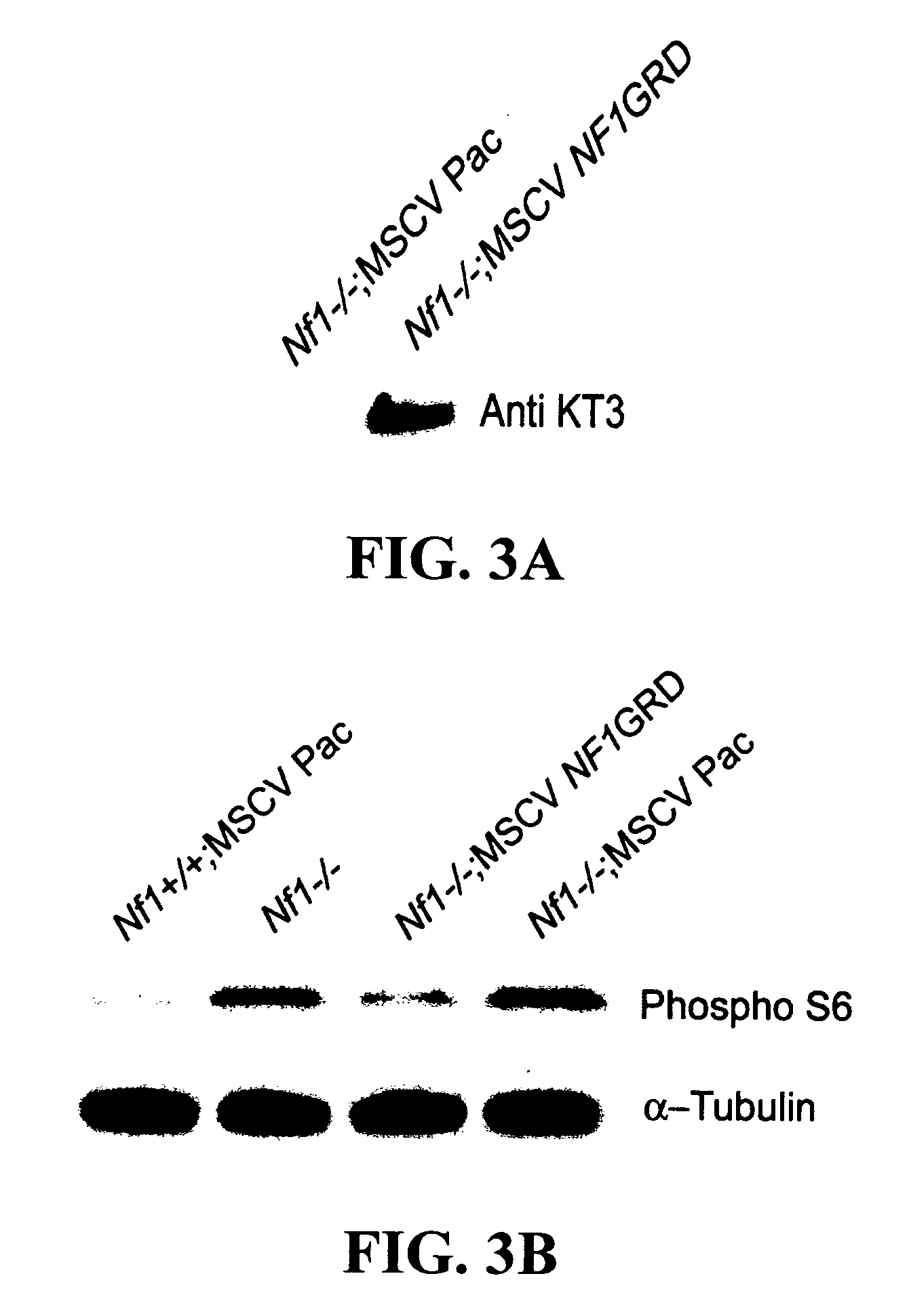
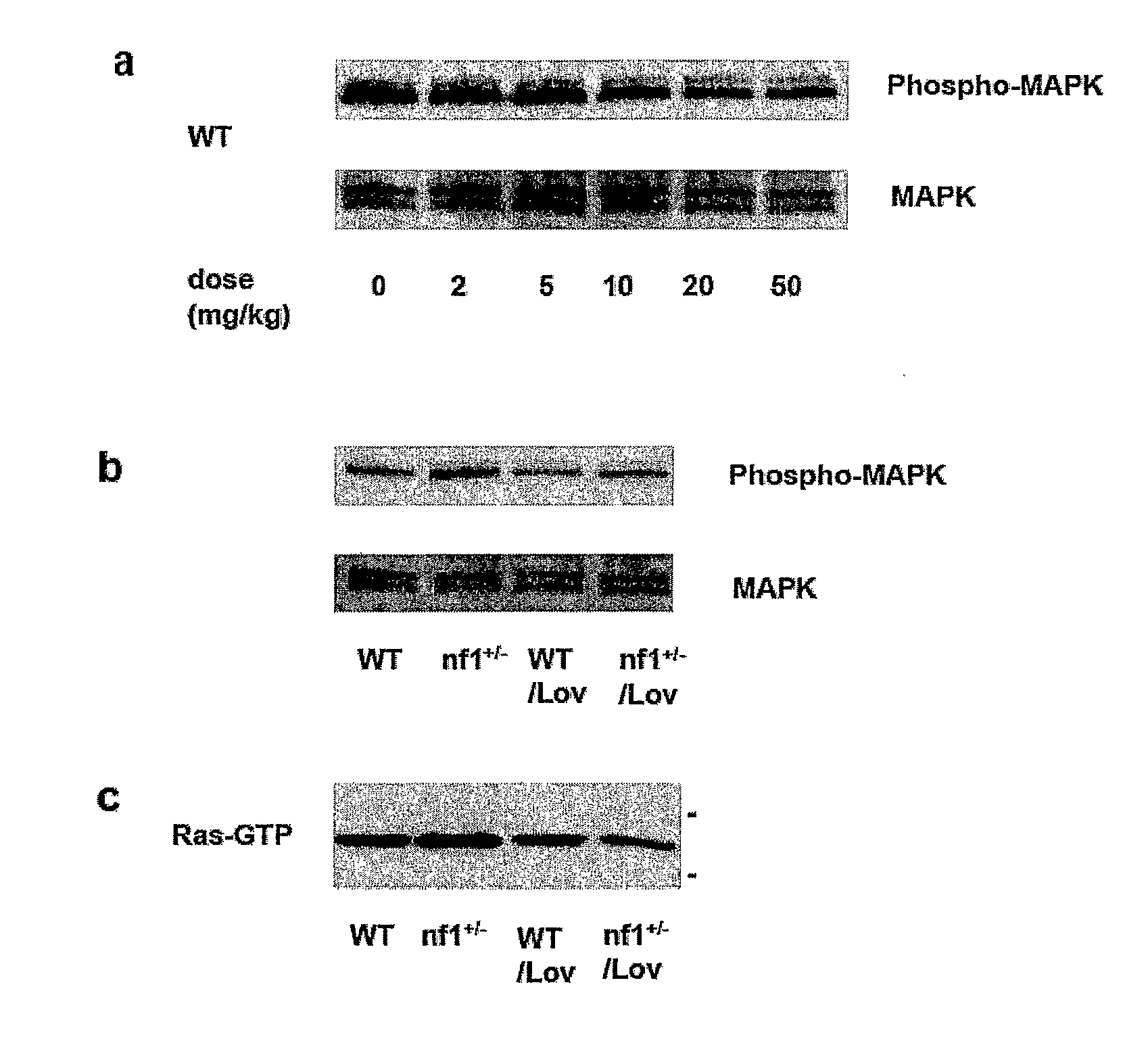
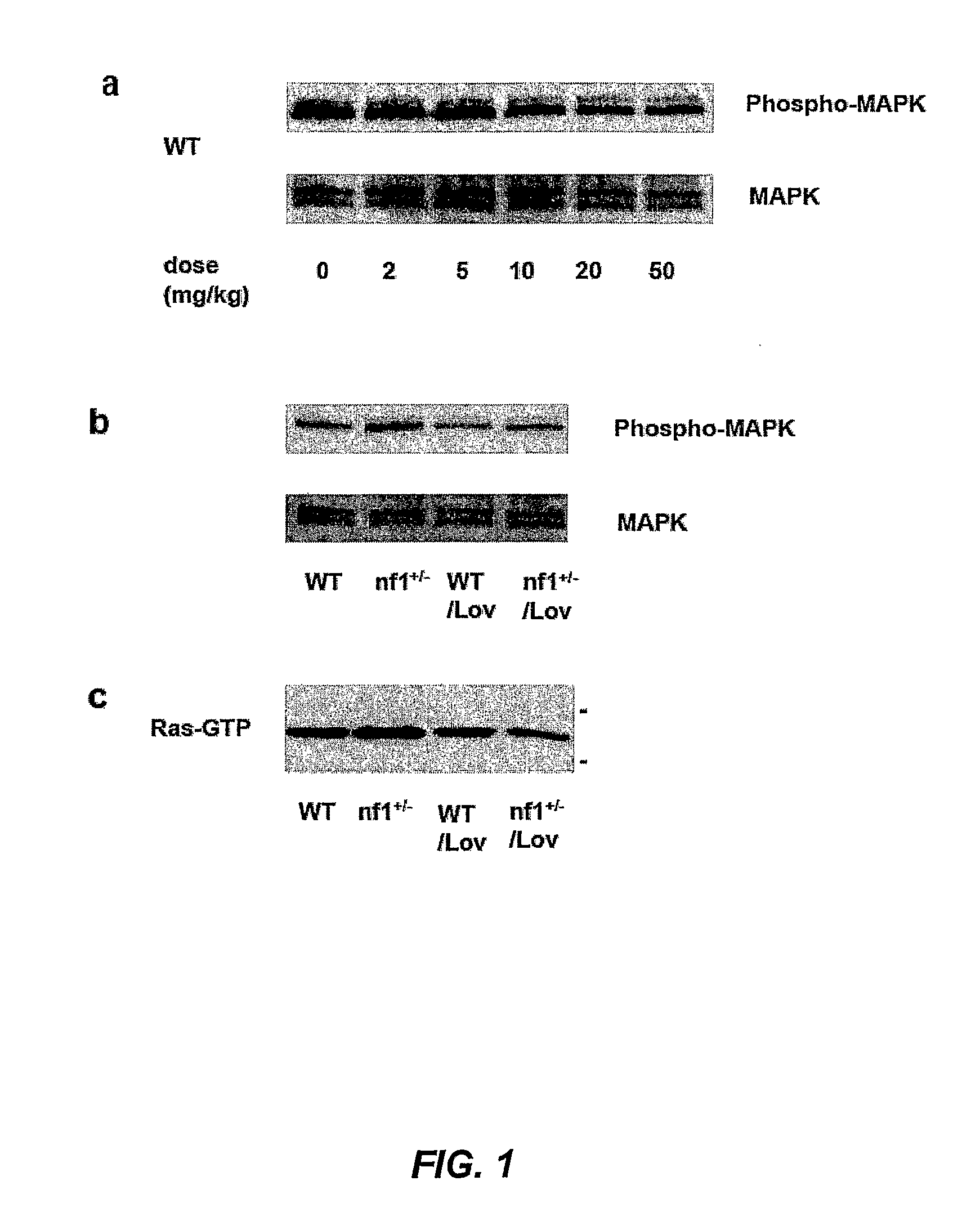
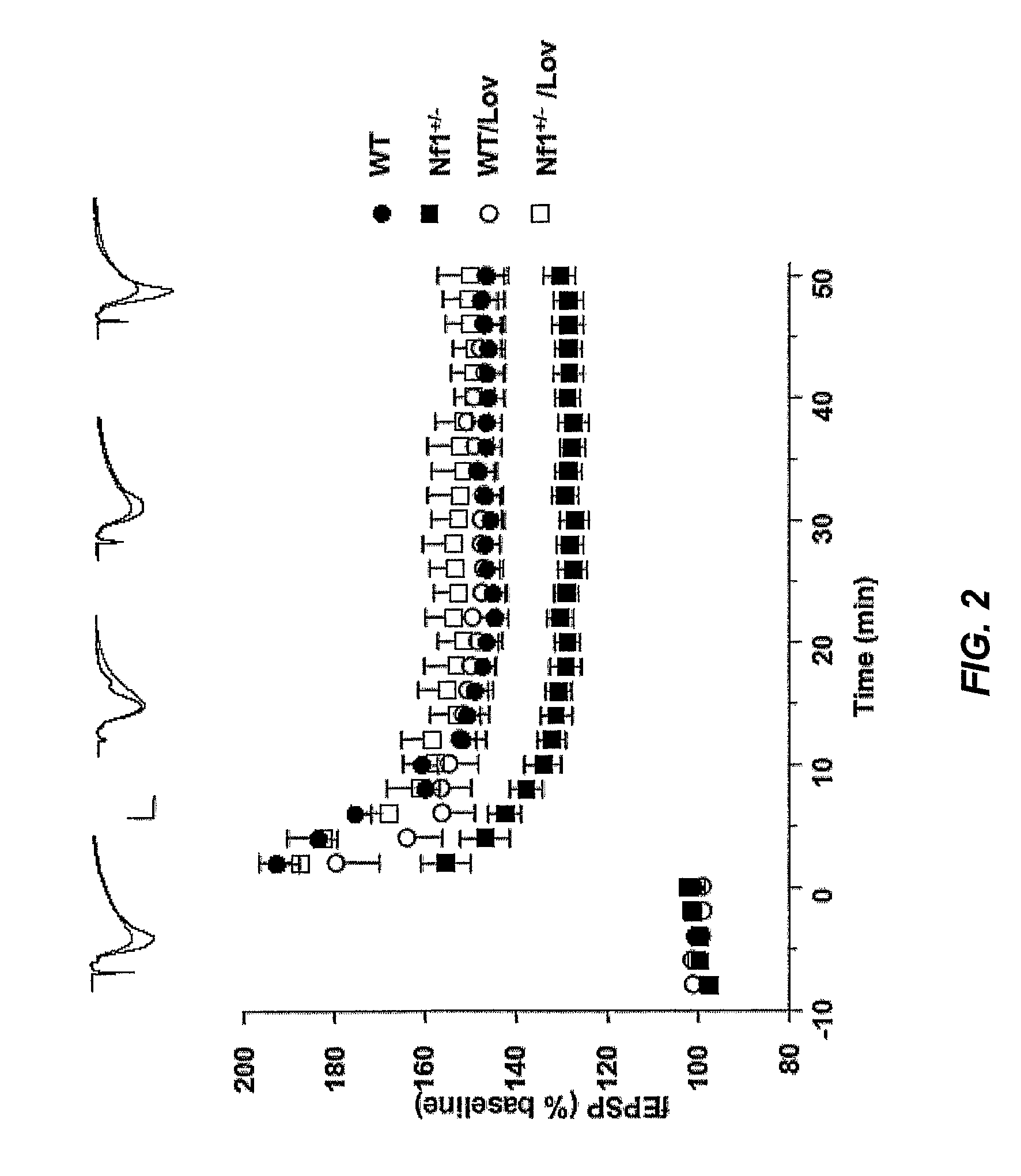
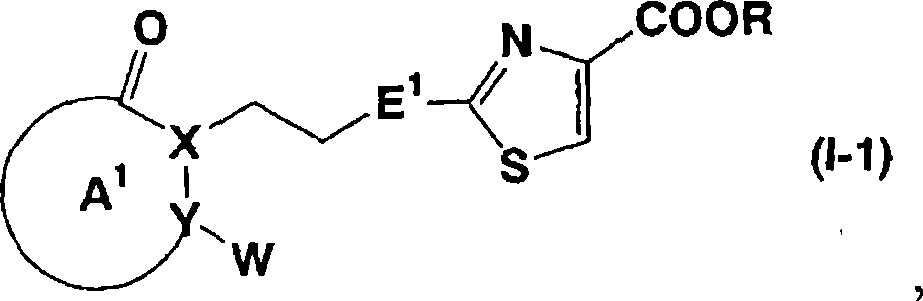
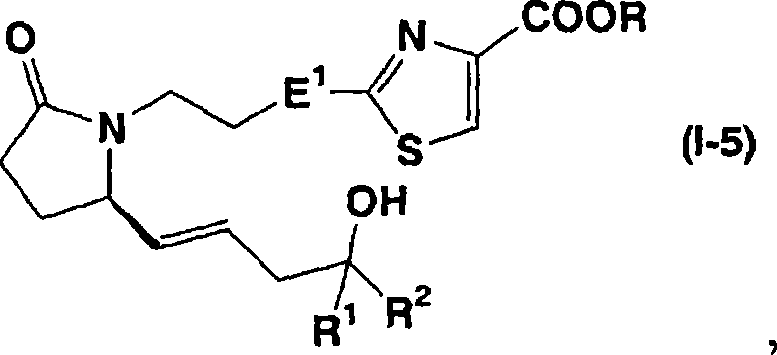
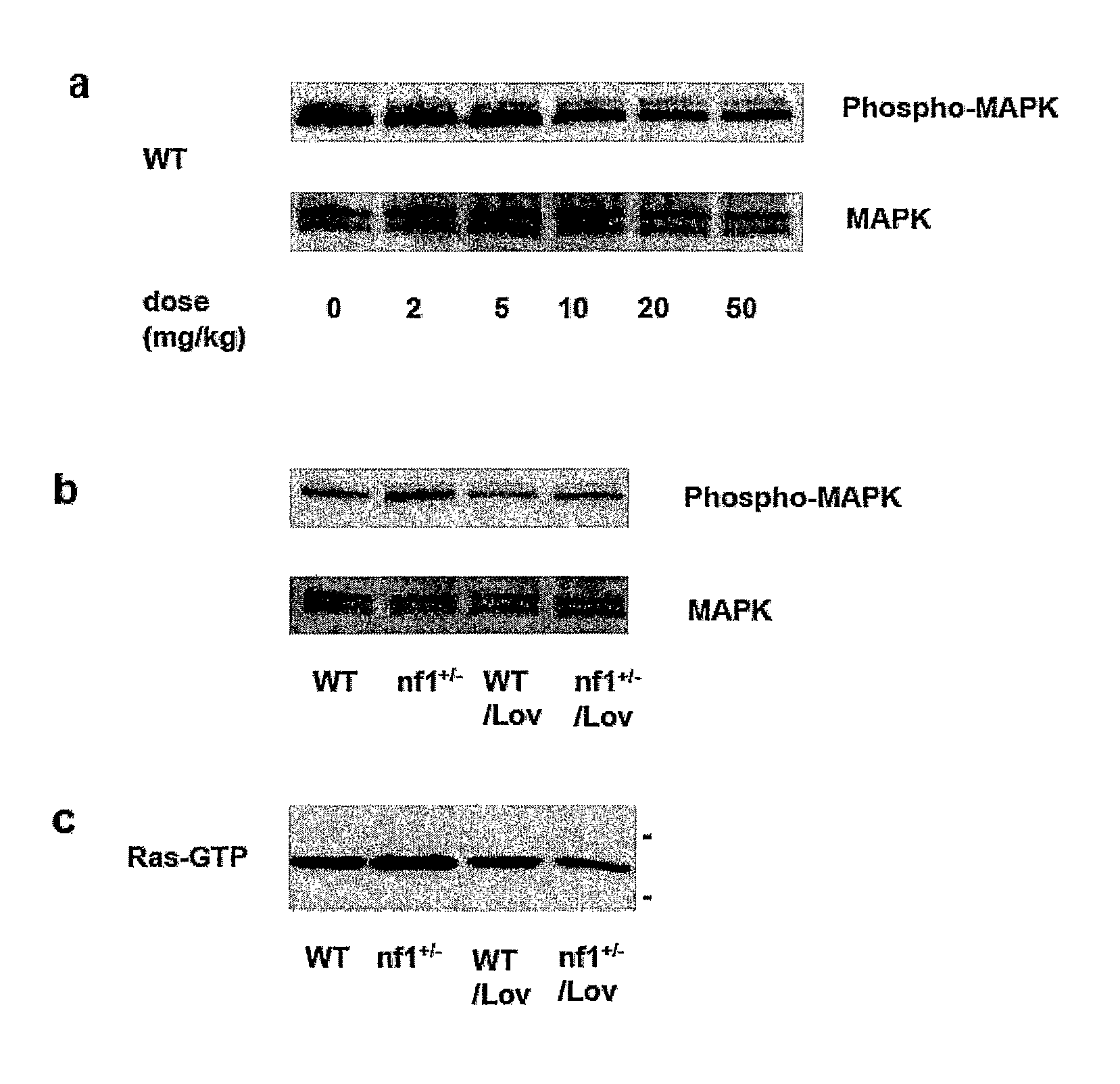
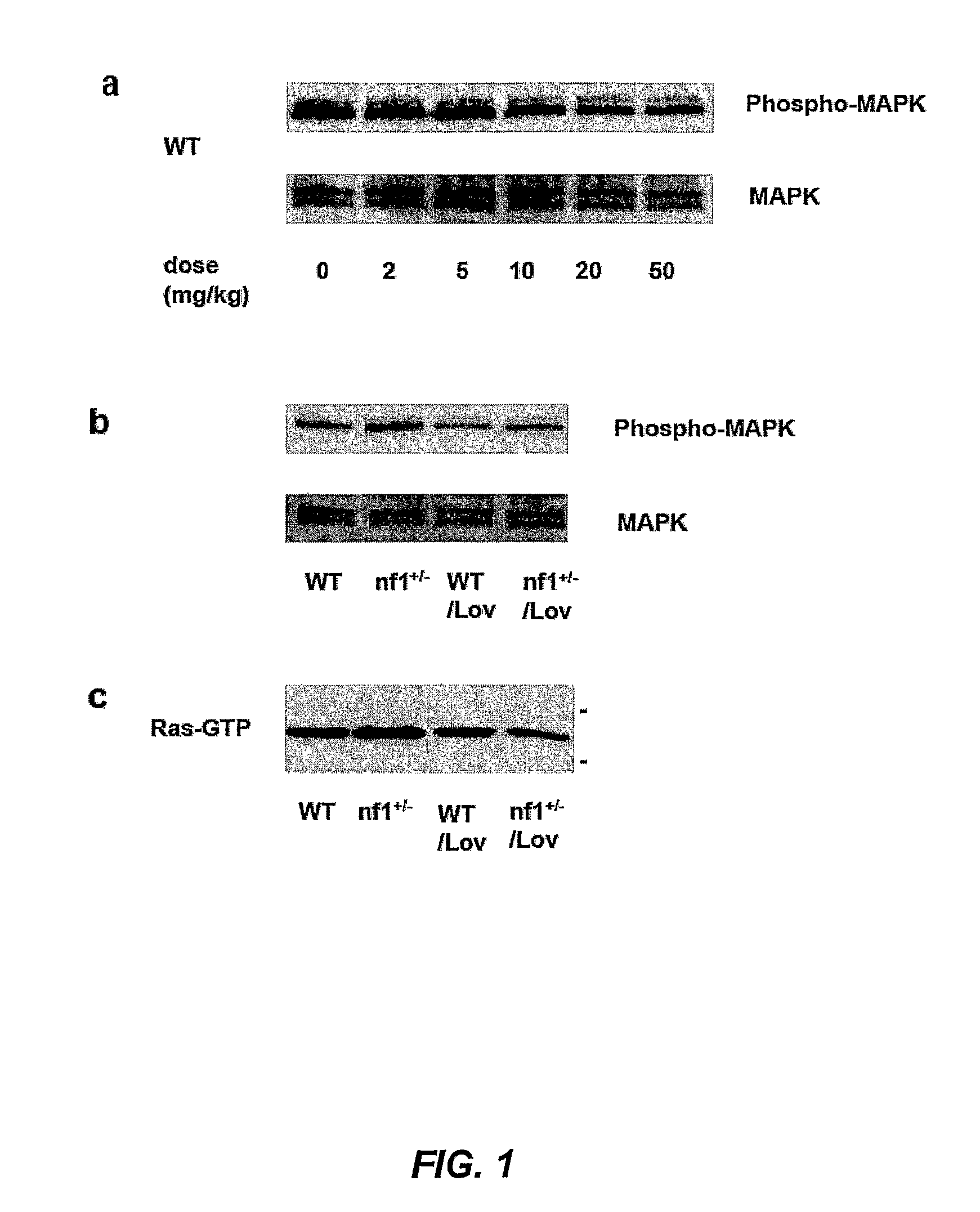
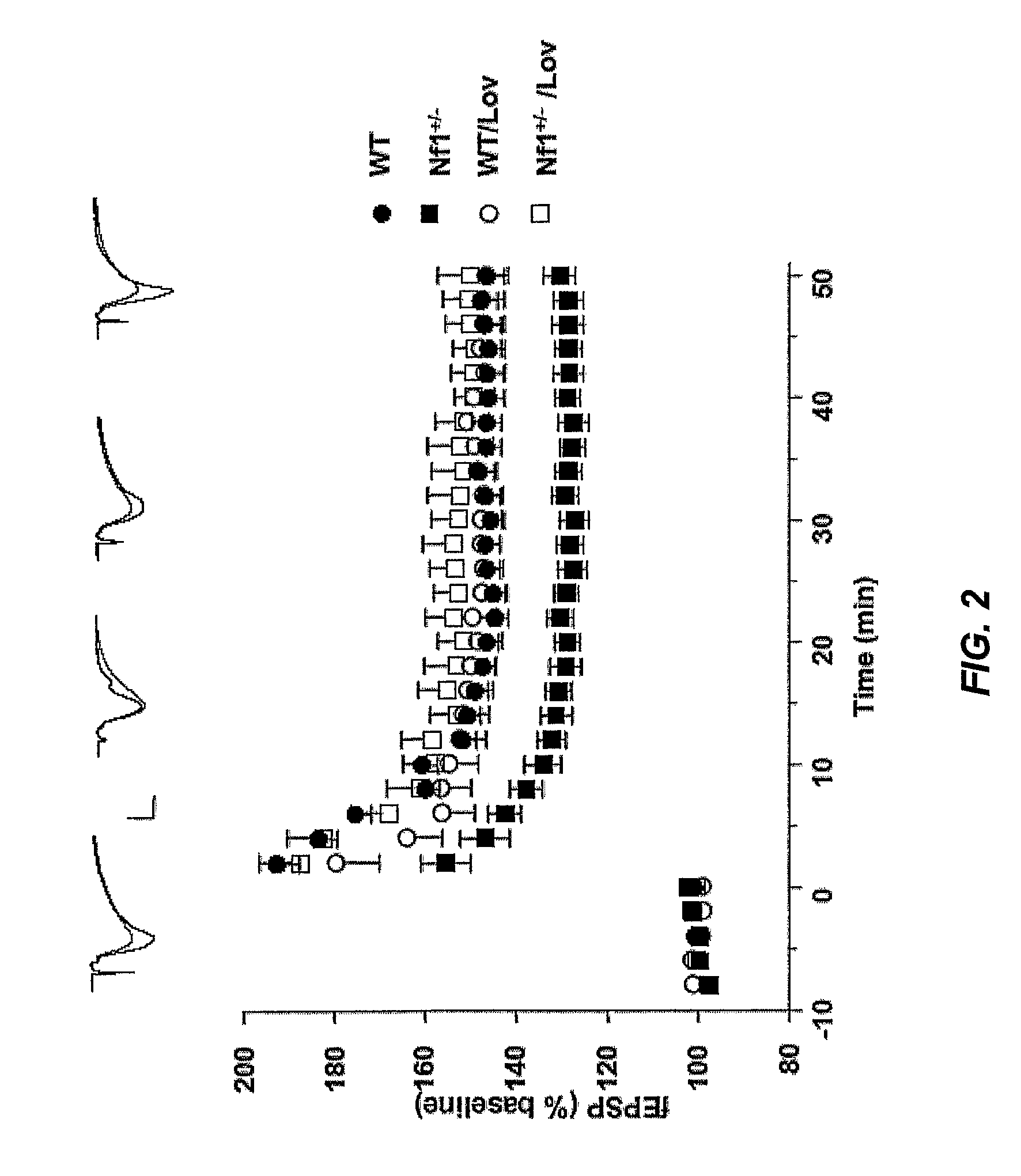
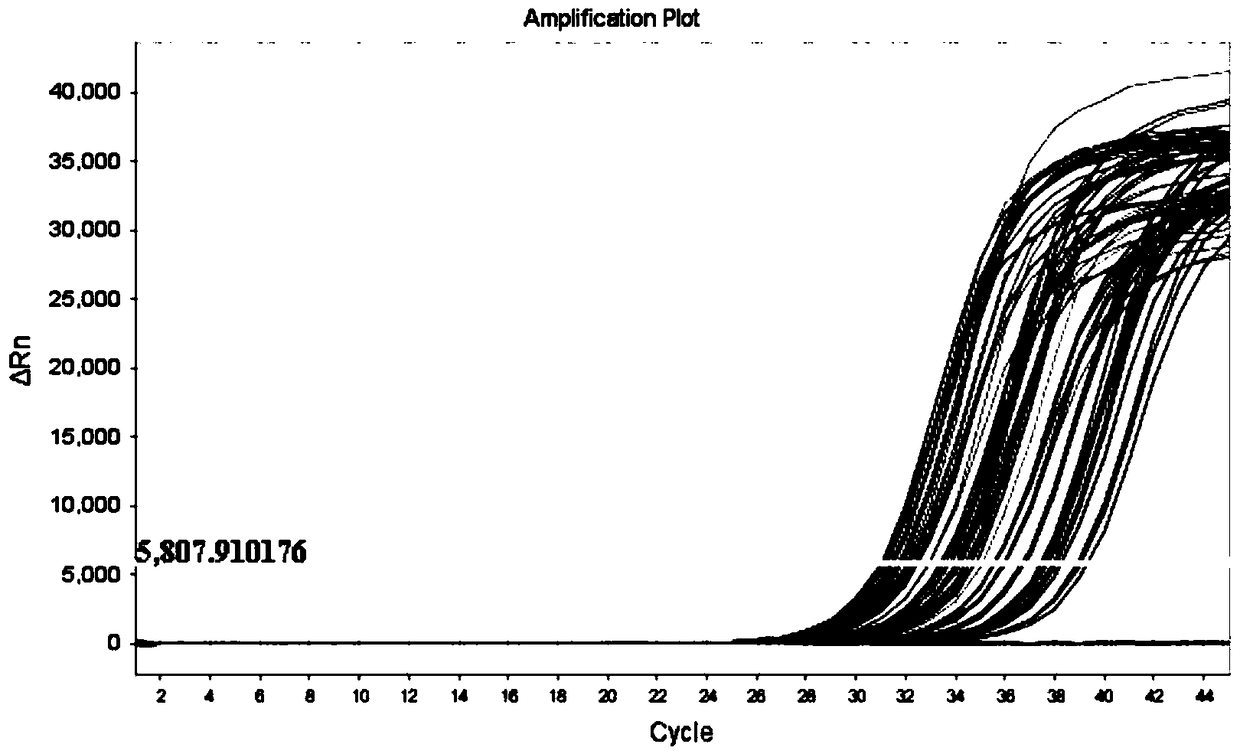
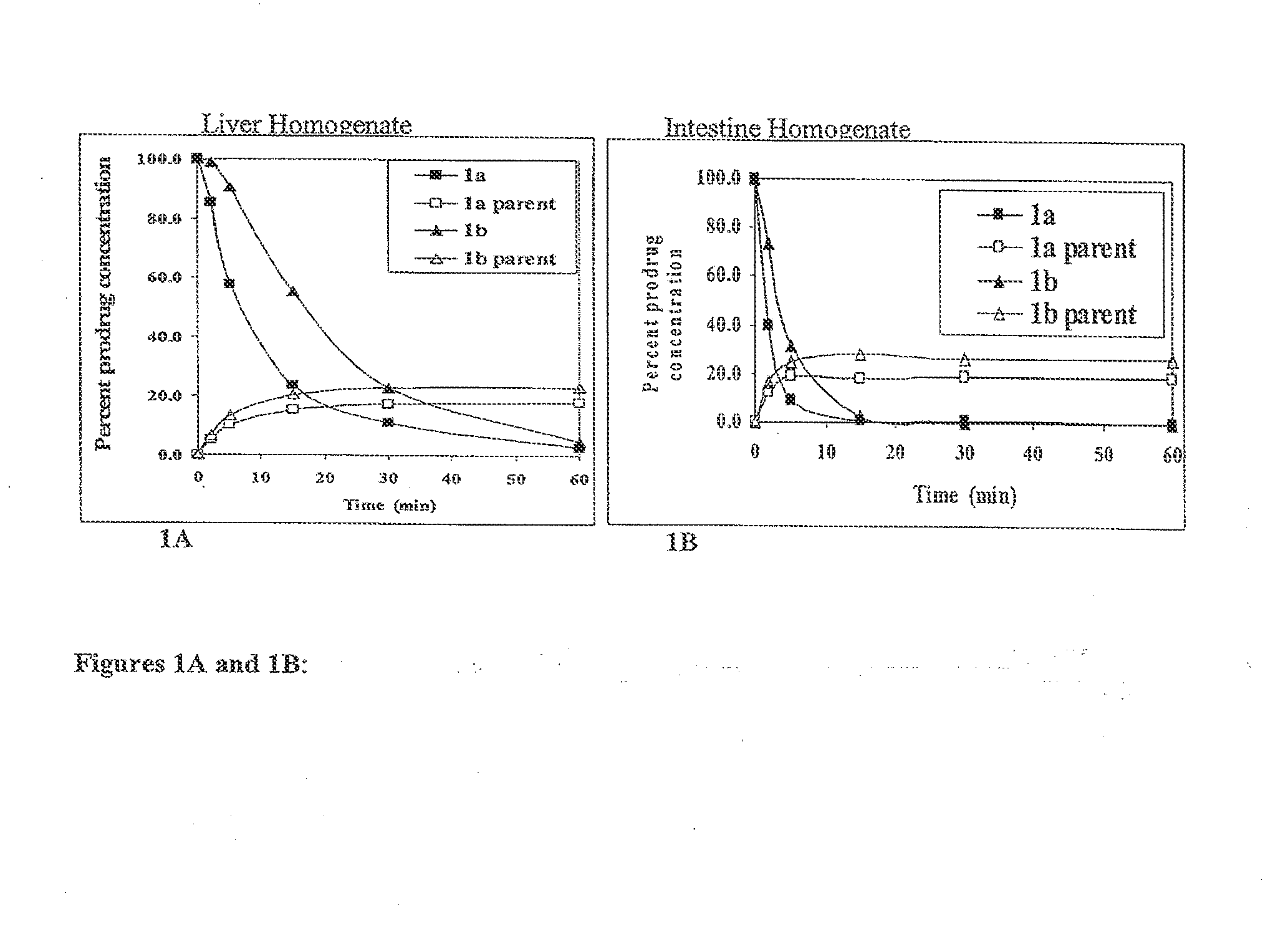
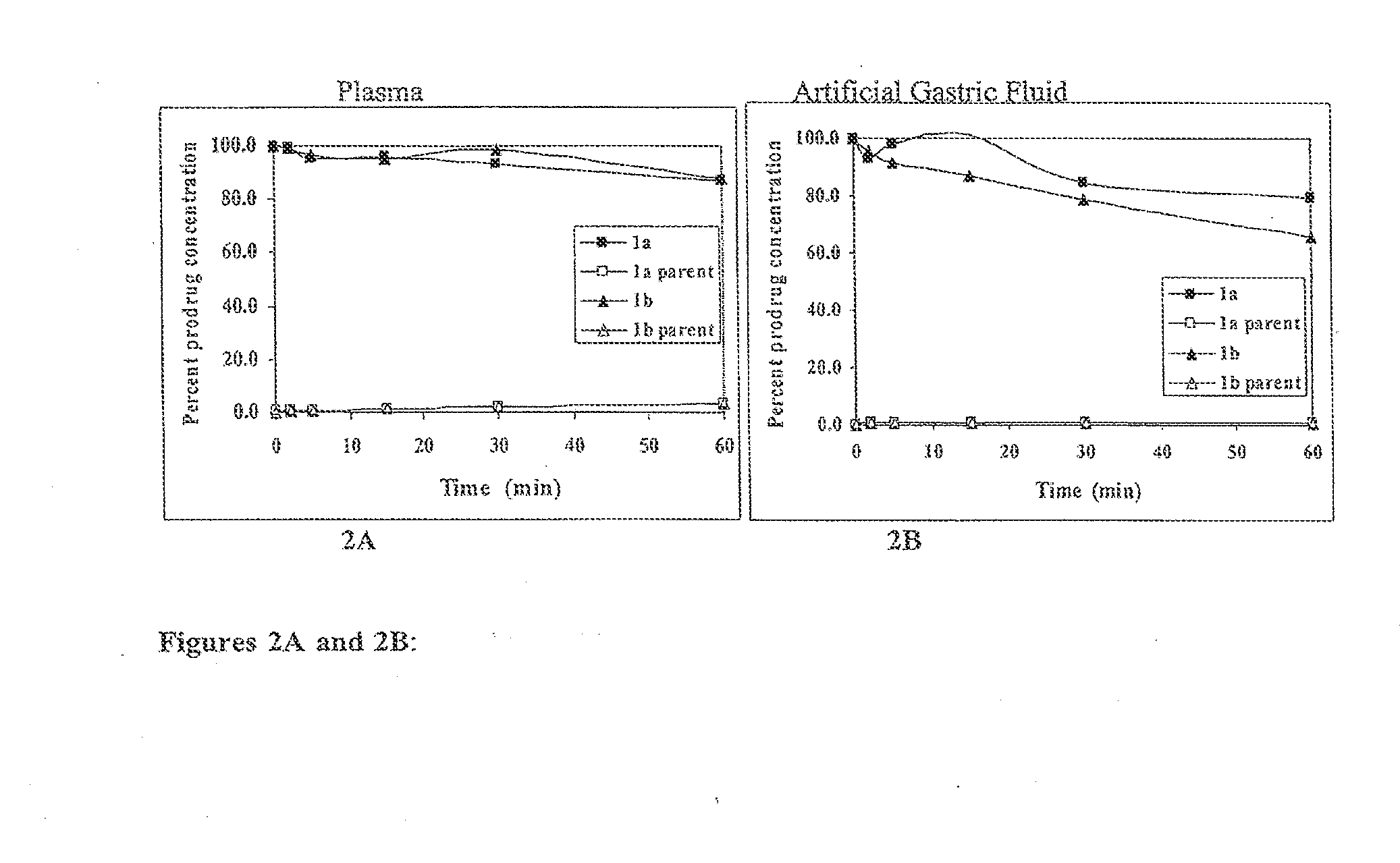
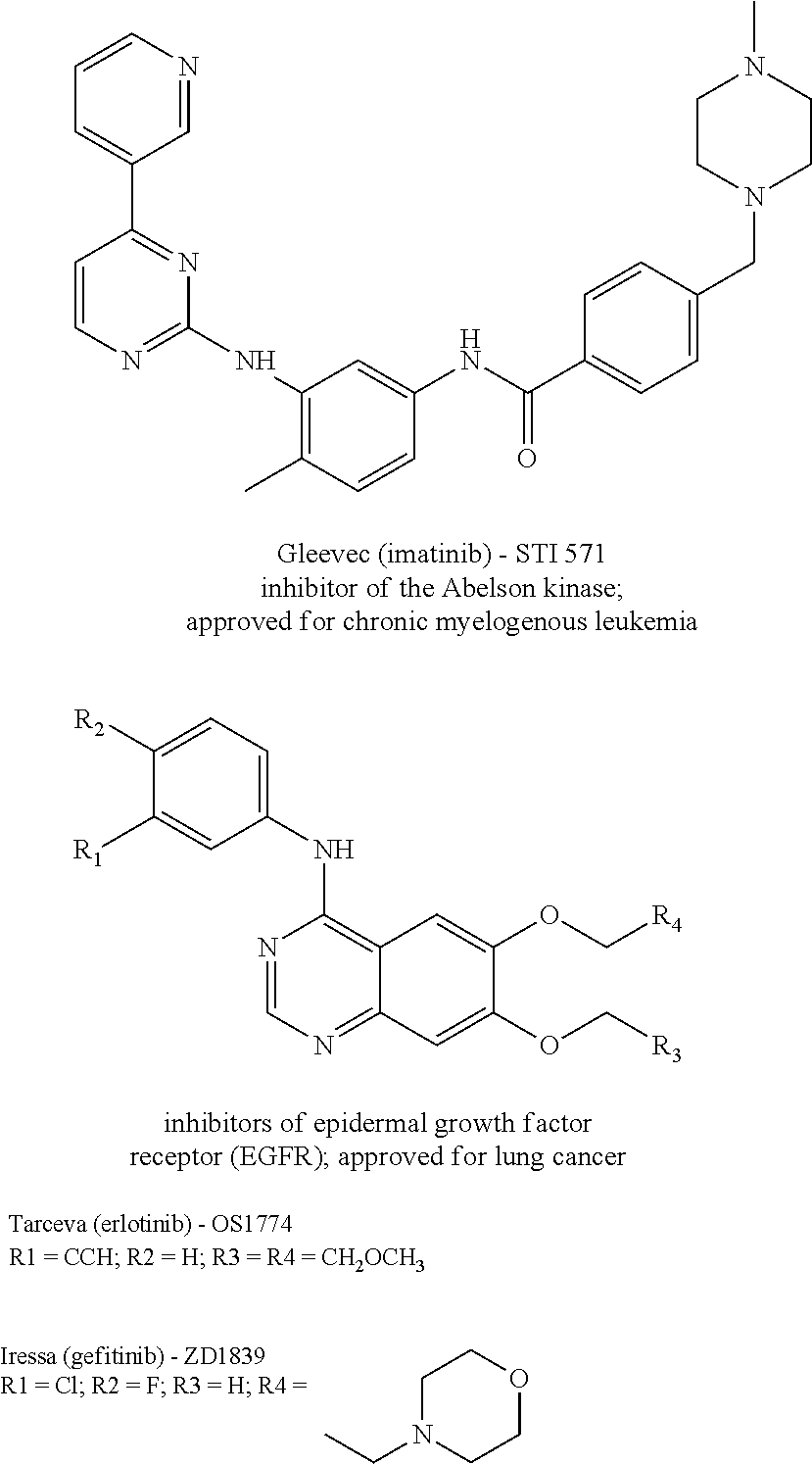
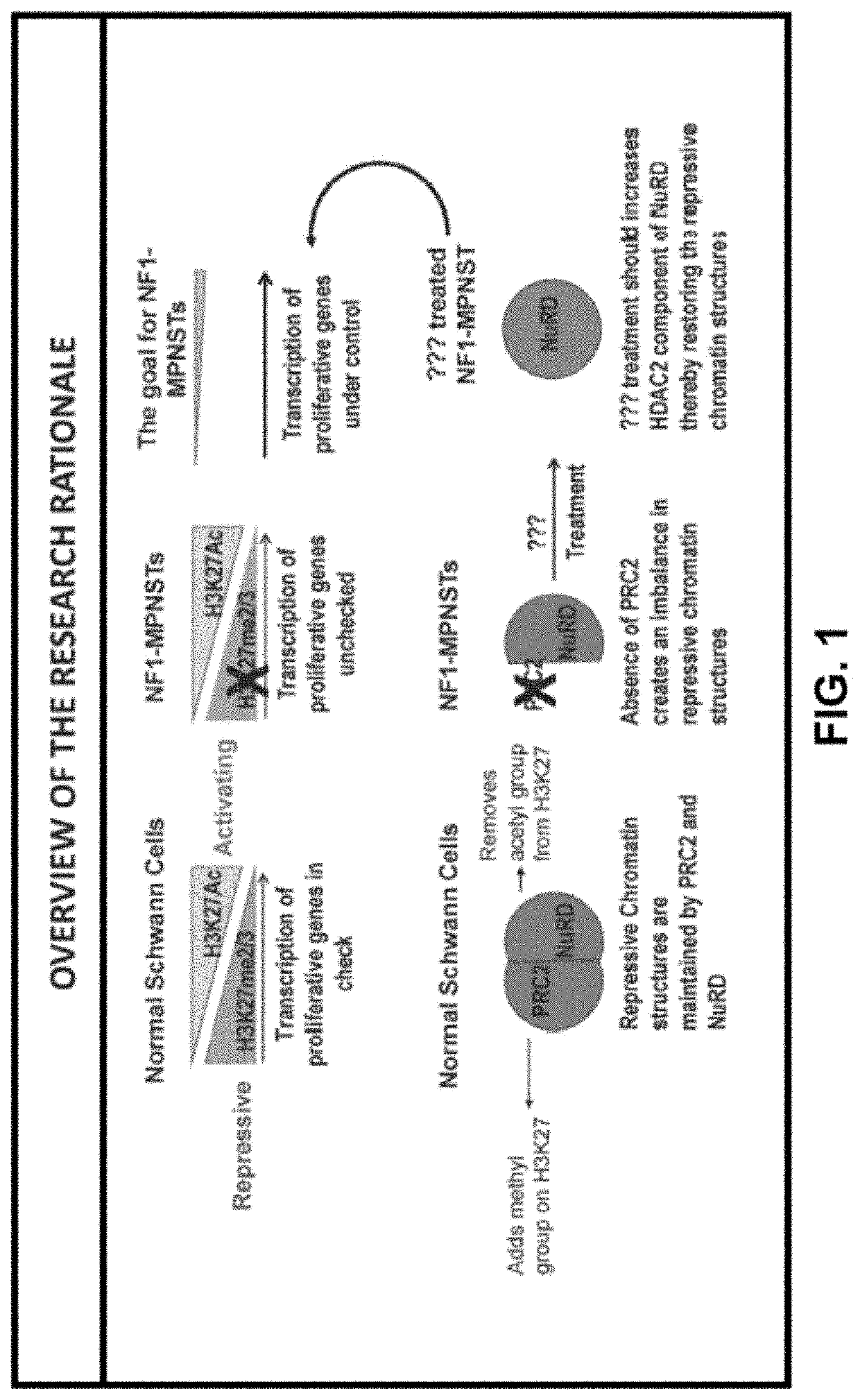
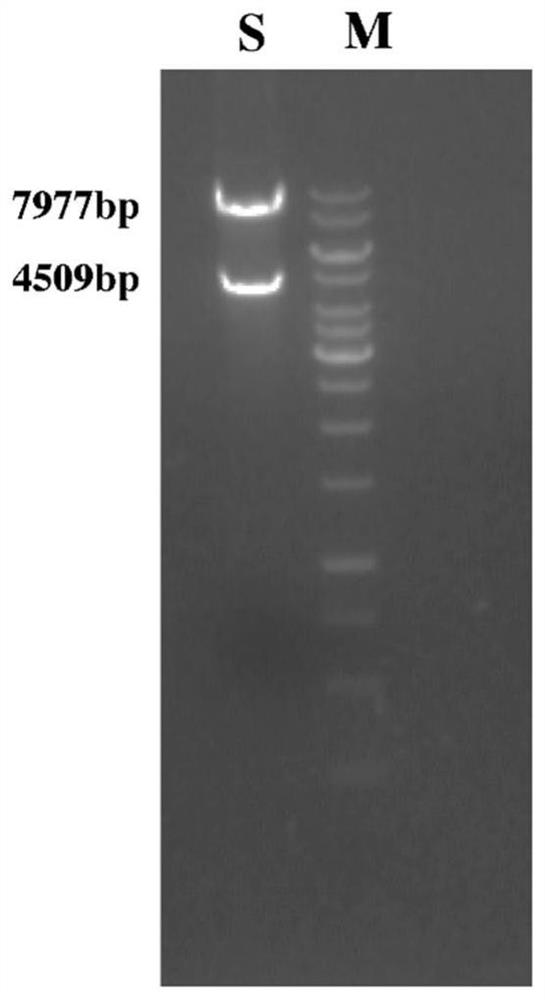
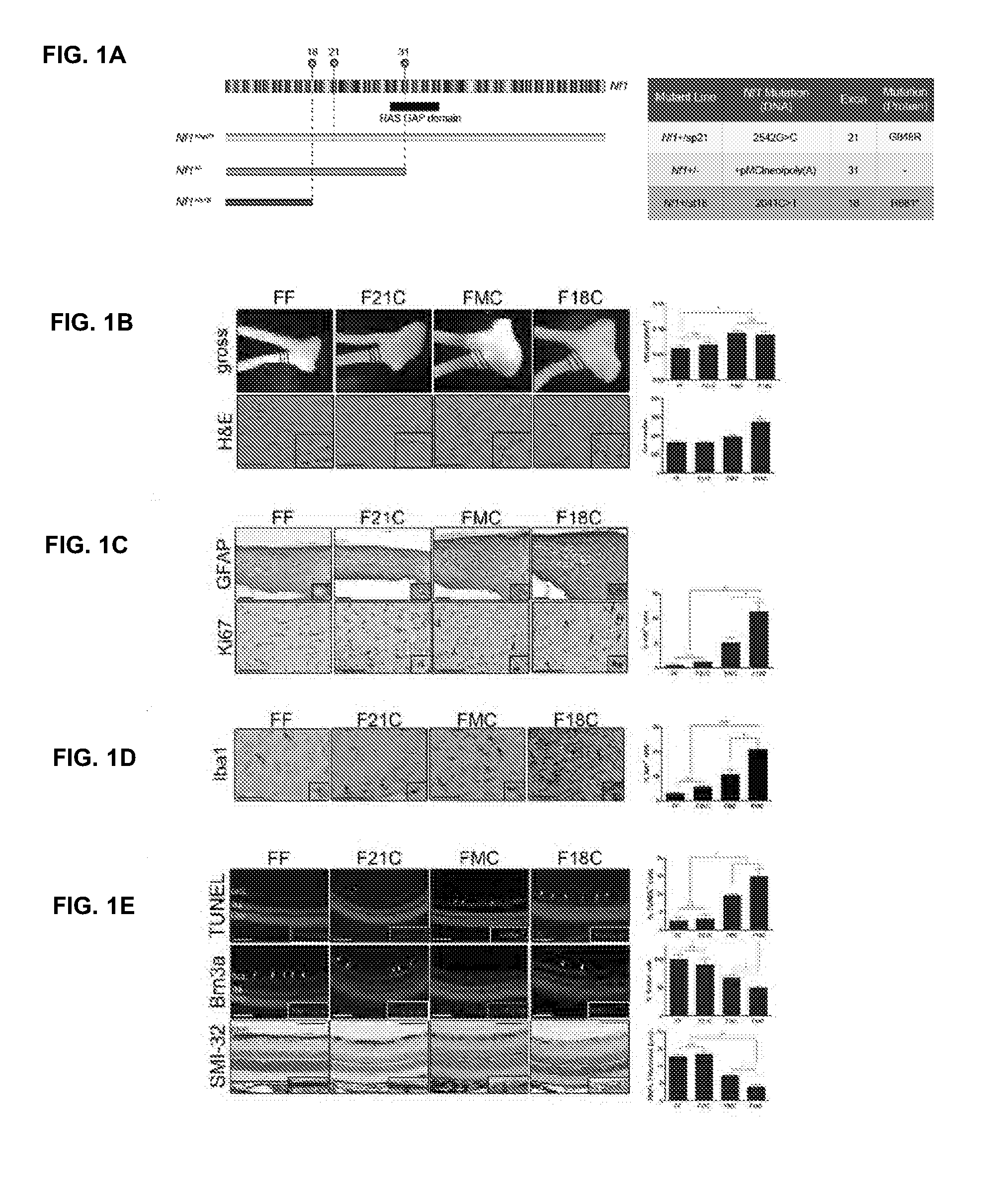
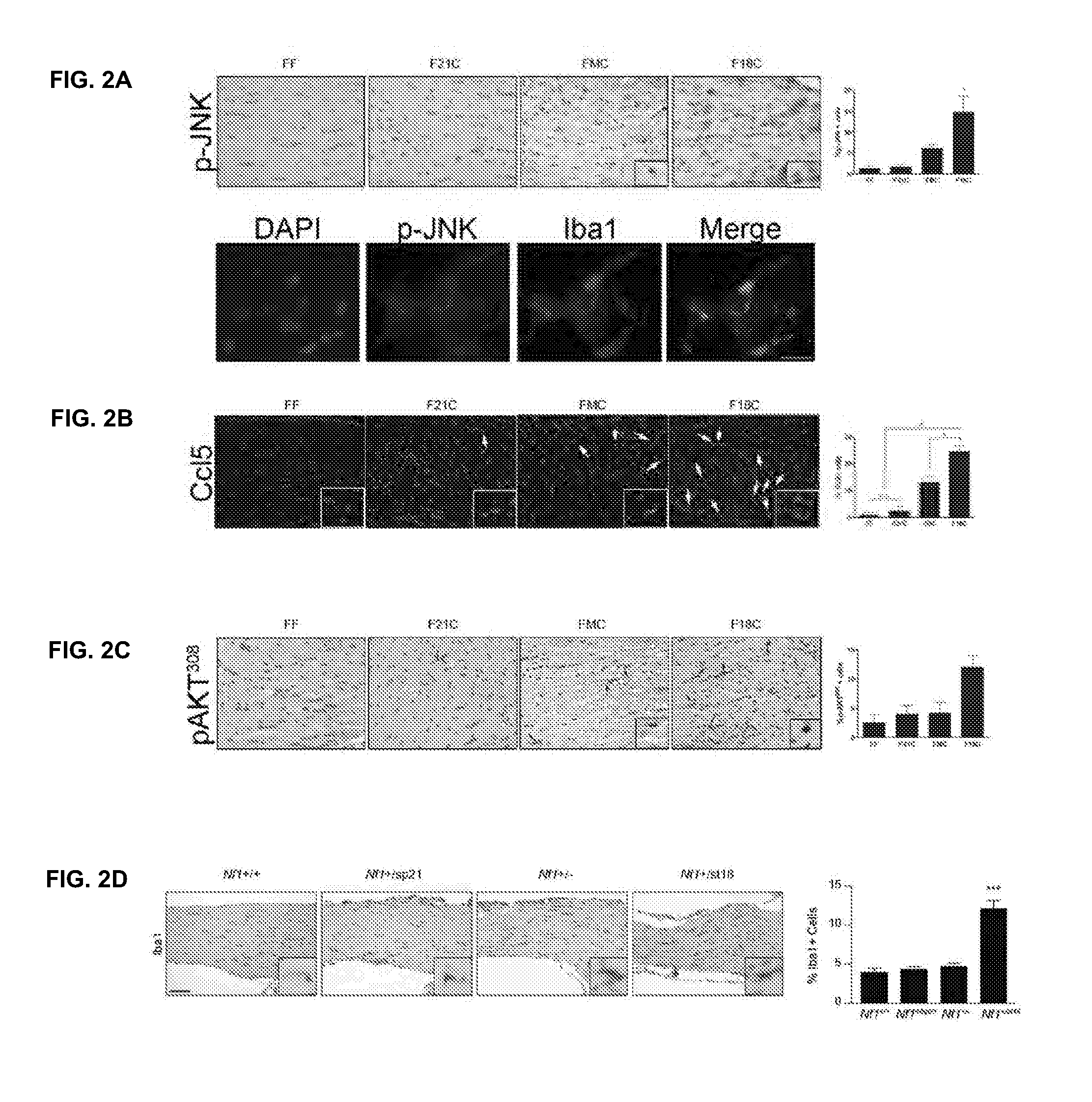
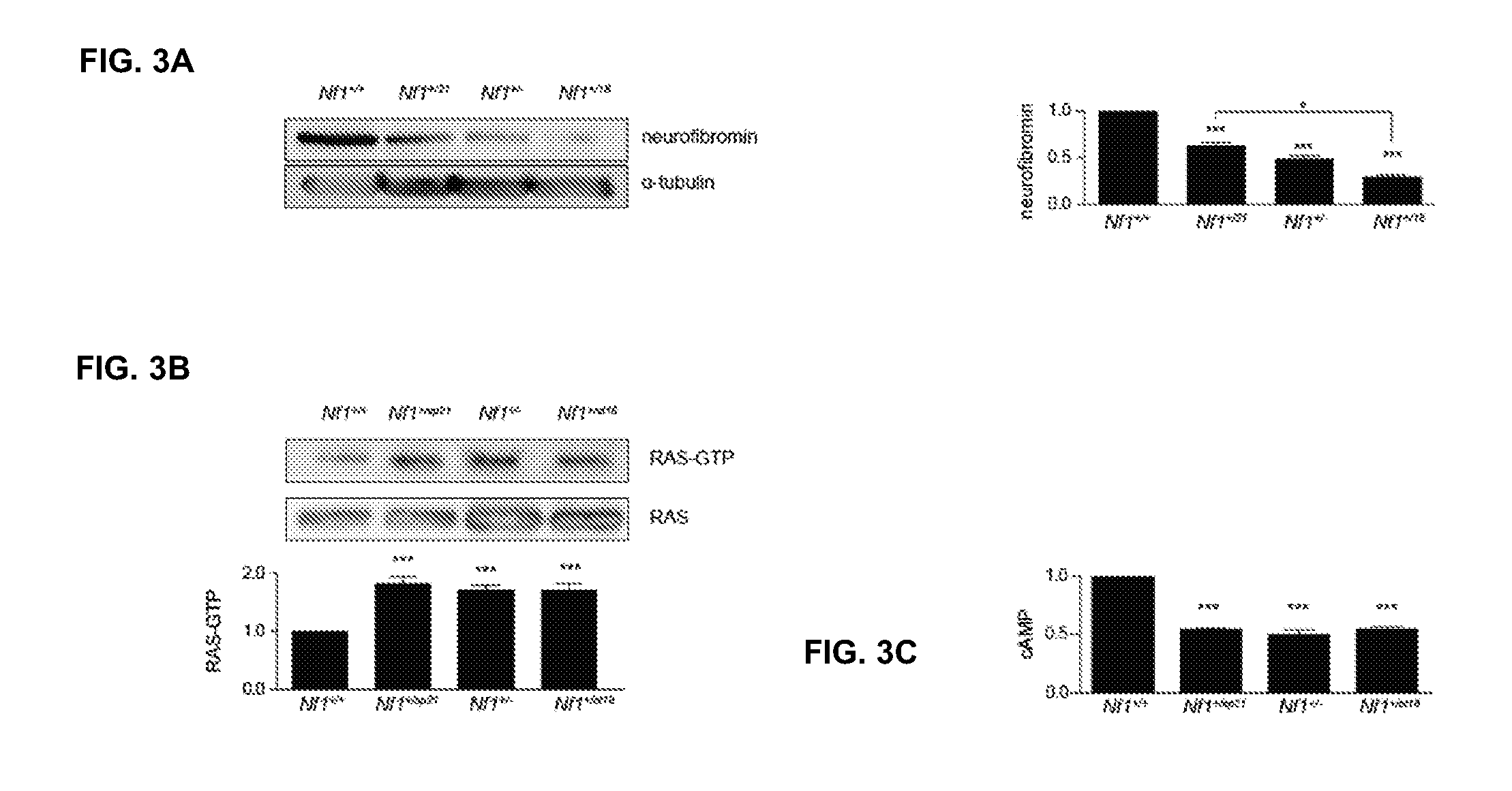
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Neurofibroma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
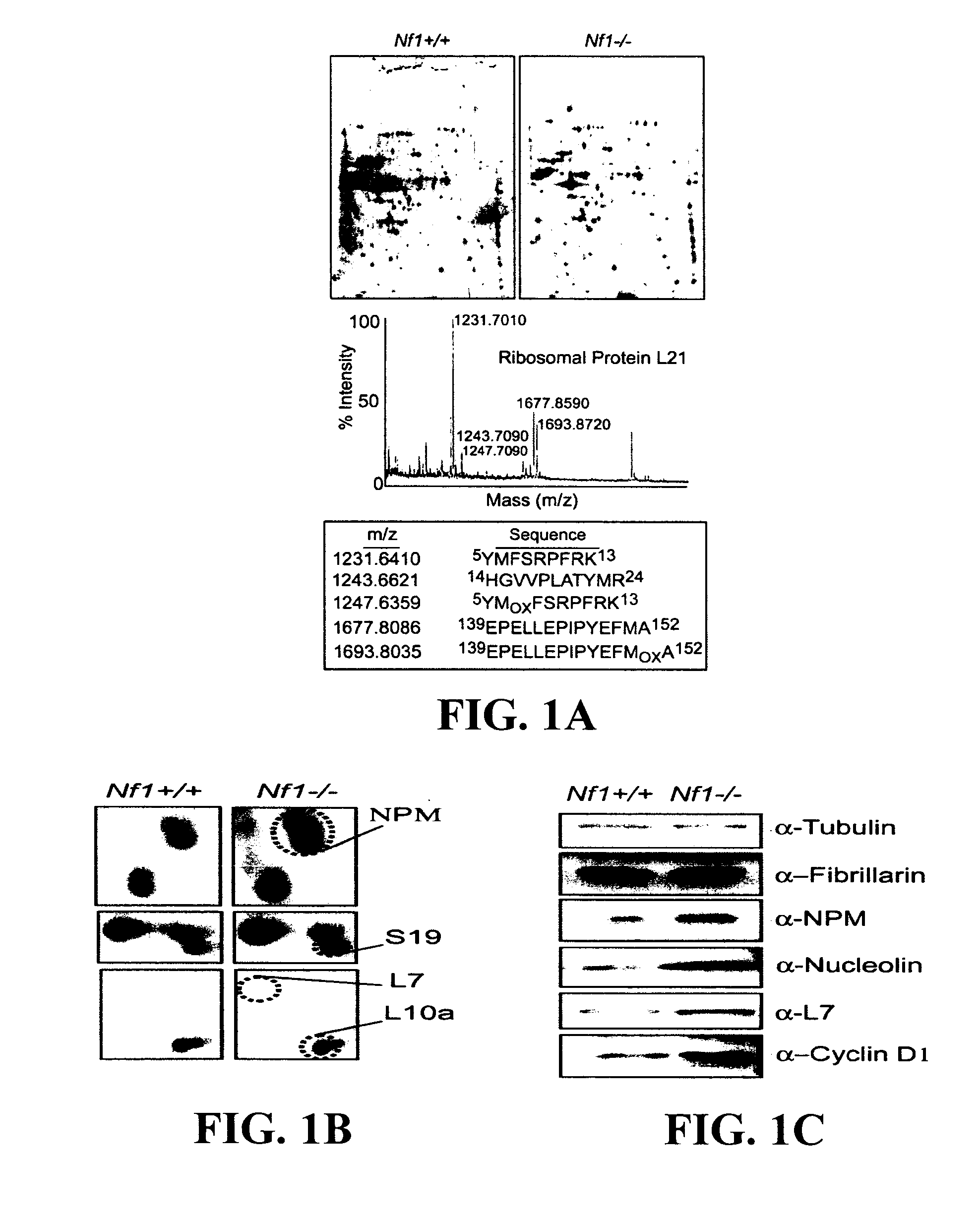
A neurofibroma is a benign nerve-sheath tumor in the peripheral nervous system. In 90% of cases, they are found as stand-alone tumors, while the remainder are found in persons with neurofibromatosis type I (NF1), an autosomal-dominant genetically inherited disease, they can result in a range of symptoms from physical disfiguration and pain to cognitive disability. Neurofibromas arise from nonmyelinating-type Schwann cells that exhibit biallelic inactivation of the NF1 gene that codes for the protein neurofibromin. This protein is responsible for regulating the RAS-mediated cell growth signaling pathway. In contrast to schwannomas, another type of tumor arising from Schwann cells, neurofibromas incorporate many additional types of cells and structural elements in addition to Schwann cells, making it difficult to identify and understand all the mechanisms through which they originate and develop.
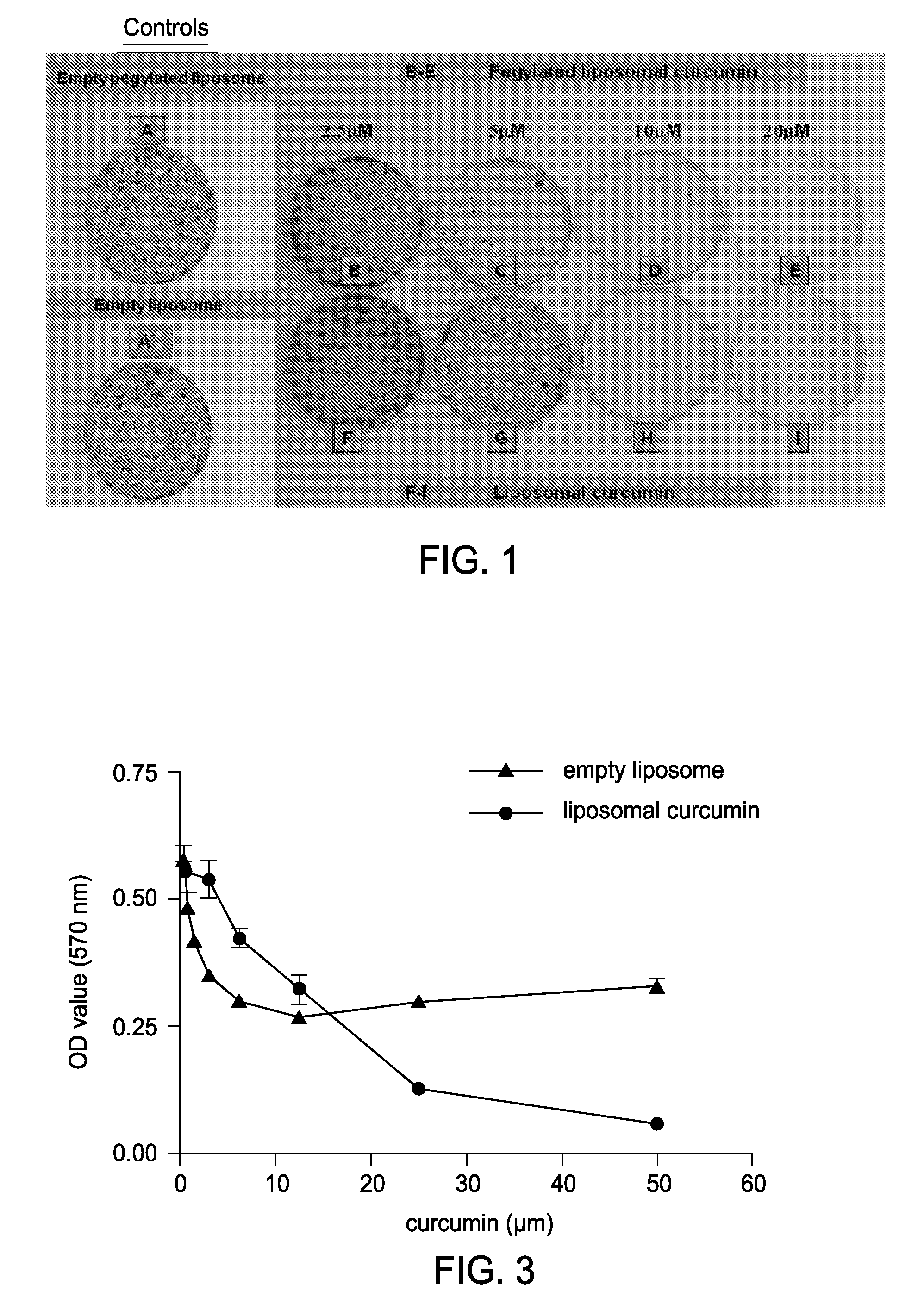
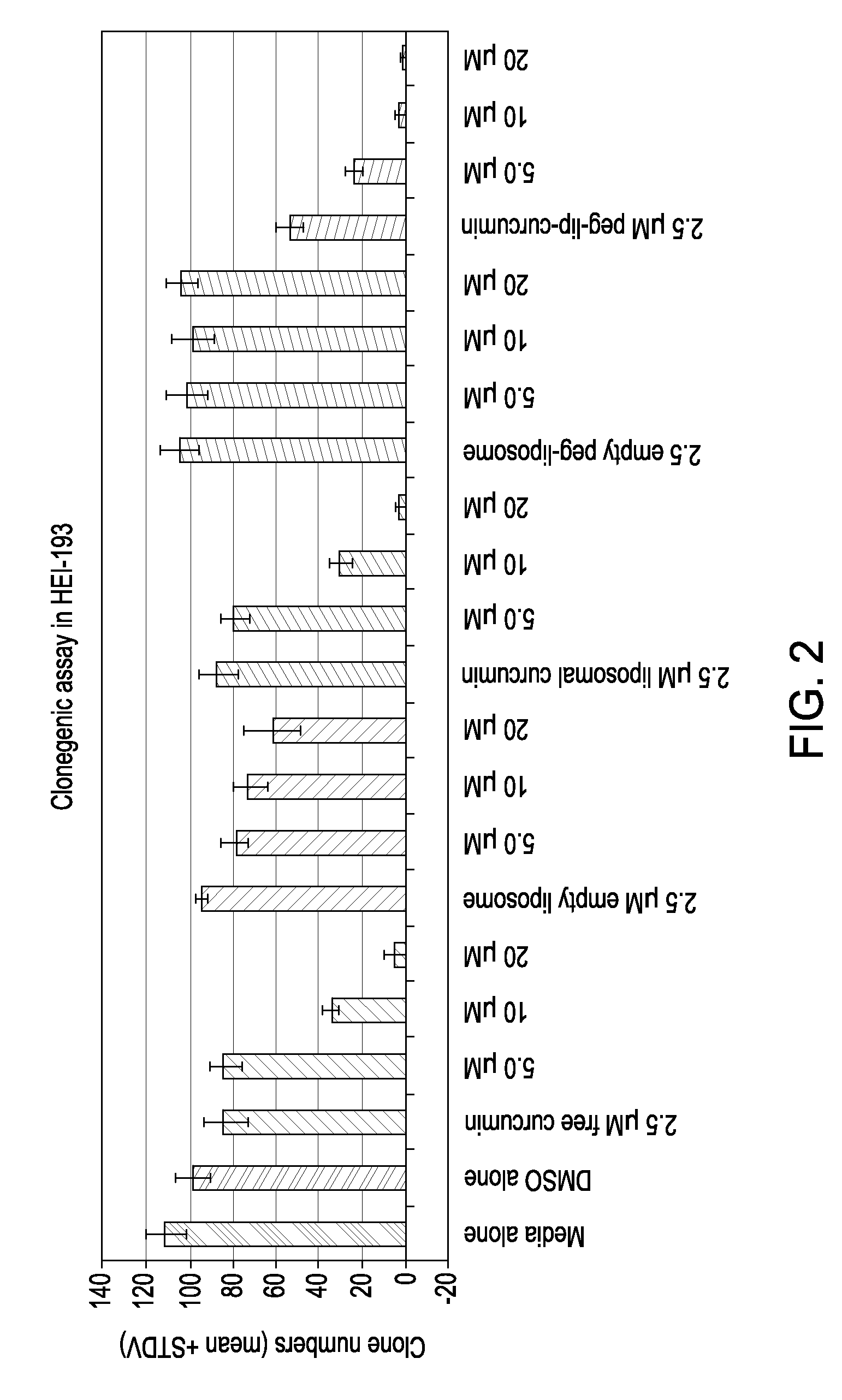
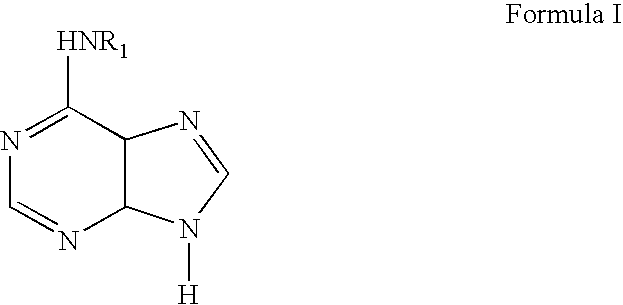
Liposomal curcumin for treatment of neurofibromatosis
The present invention provides a compositions and methods for the treatment of Neurofibromatosis Type 1 and 2, in a human patient. The methods and compositions of the present invention employ curcumin or a curcumin analogue encapsulated in a colloidal drug delivery system, preferably a liposomal drug delivery system to target Merlin and proteins of the Merlin pathway. Suitable colloidal drug delivery systems also include nanoparticles, nanocapsules, microparticles or block copolymer micelles. The colloidal drug delivery system encapsulating curcumin or a curcumin analogue is administered parenterally in a pharmaceutically acceptable carrier.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof
ActiveUS20130323244A1Reduced dose-dependent side effectPeptide/protein ingredientsHydrolasesDiseaseNeurofibromatosis type I
The present invention provides methods, compositions, and kits for the treatment of neurocutaneous syndromes, such as neurofibromatosis type I; disorders associated with overactivation of FGFR3, such as achondroplasia; bone or cartilage disorders; or vascular smooth muscle disorders; or for the elongation of bone. In some embodiments, the present invention provides polypeptides having an alkaline phosphatase peptide fused to an Fc domain of an immunoglobulin or a natriuretic peptide fused to an Fc domain of an immunoglobulin. Such polypeptides can be administered to subjects, e.g., subcutaneously, to treat a neurocutaneous syndrome, a disorder associated with overactivation of FGFR3, a bone or cartilage disorder, or a vascular smooth muscle disorder, or to elongate bone. The invention also features nucleic acid molecules encoding such polypeptides and the use of the nucleic acid molecules for treating neurocutaneous syndromes, disorders associated with overactivation of FGFR3, bone or cartilage disorders, or vascular smooth muscle disorders, or for elongating bone.
Owner:VANDERBILT UNIV +1
Methods for treating neurofibromatosis 1
This invention is directed generally to methods for treating neurofibromatosis 1 (NF1), and, more particularly, to methods for treating NF1 by administering rapamycin, a rapamycin analog, a rapamycin prodrug, or a salt of rapamycin or the analog or prodrug. This invention also is directed generally to compositions and kits for treating NF1, and more particularly, to compositions and kits for treating NF1 that comprise rapamycin, a rapamycin analog, a rapamycin prodrug, or a salt of rapamycin or the analog or prodrug.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Treating Learning Deficits With Inhibitors of Hmg CoA Reductase
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
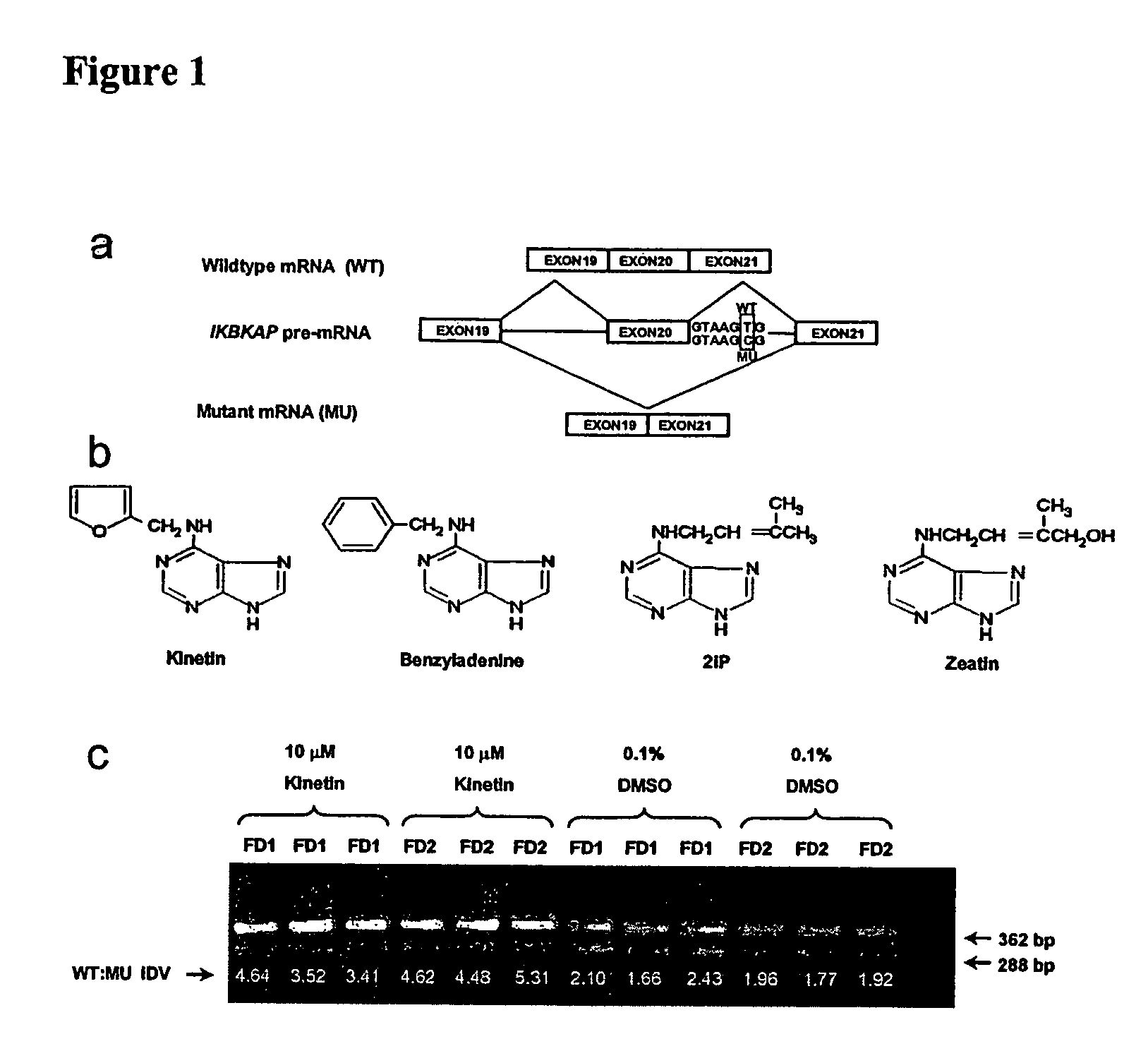
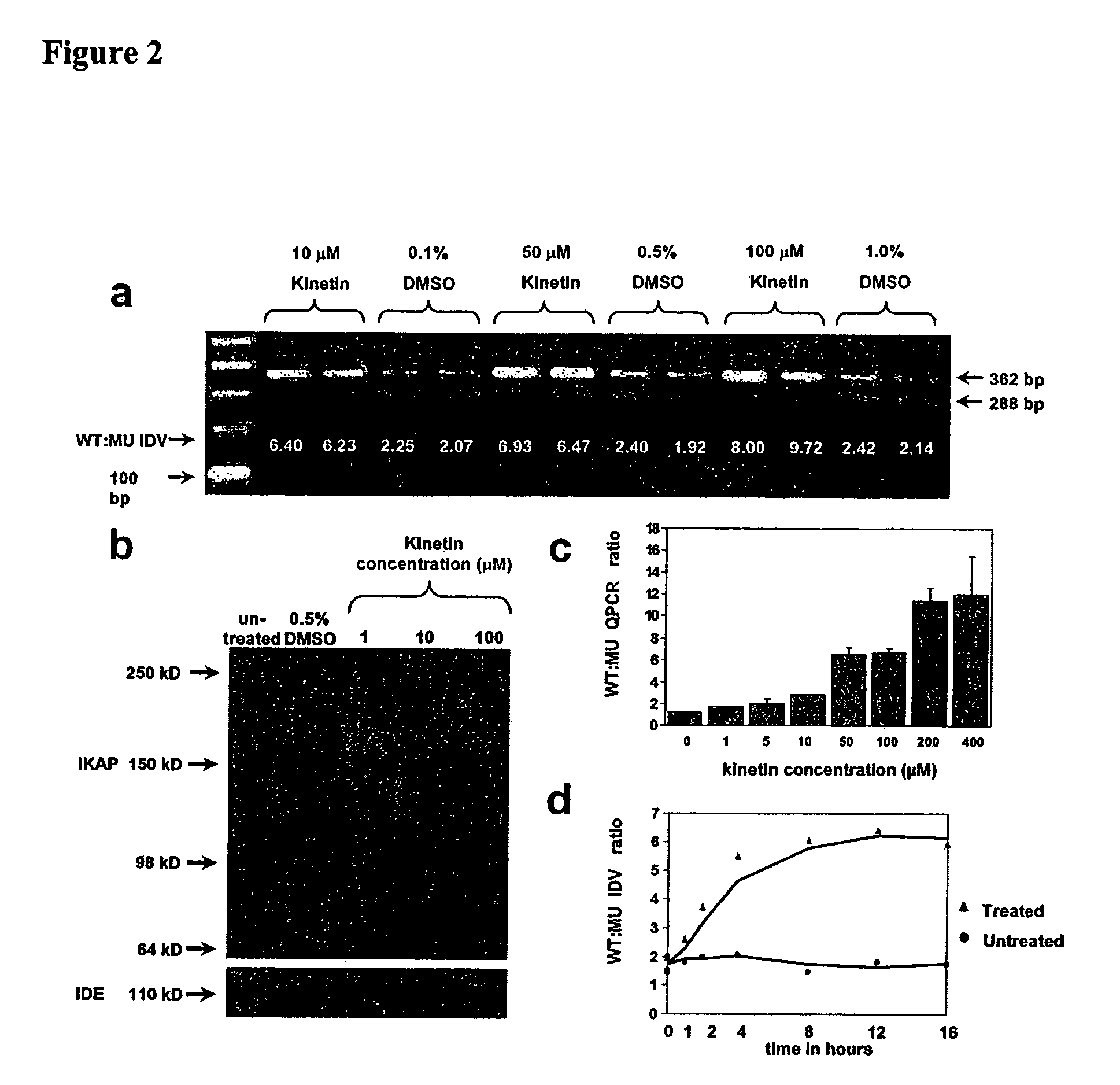
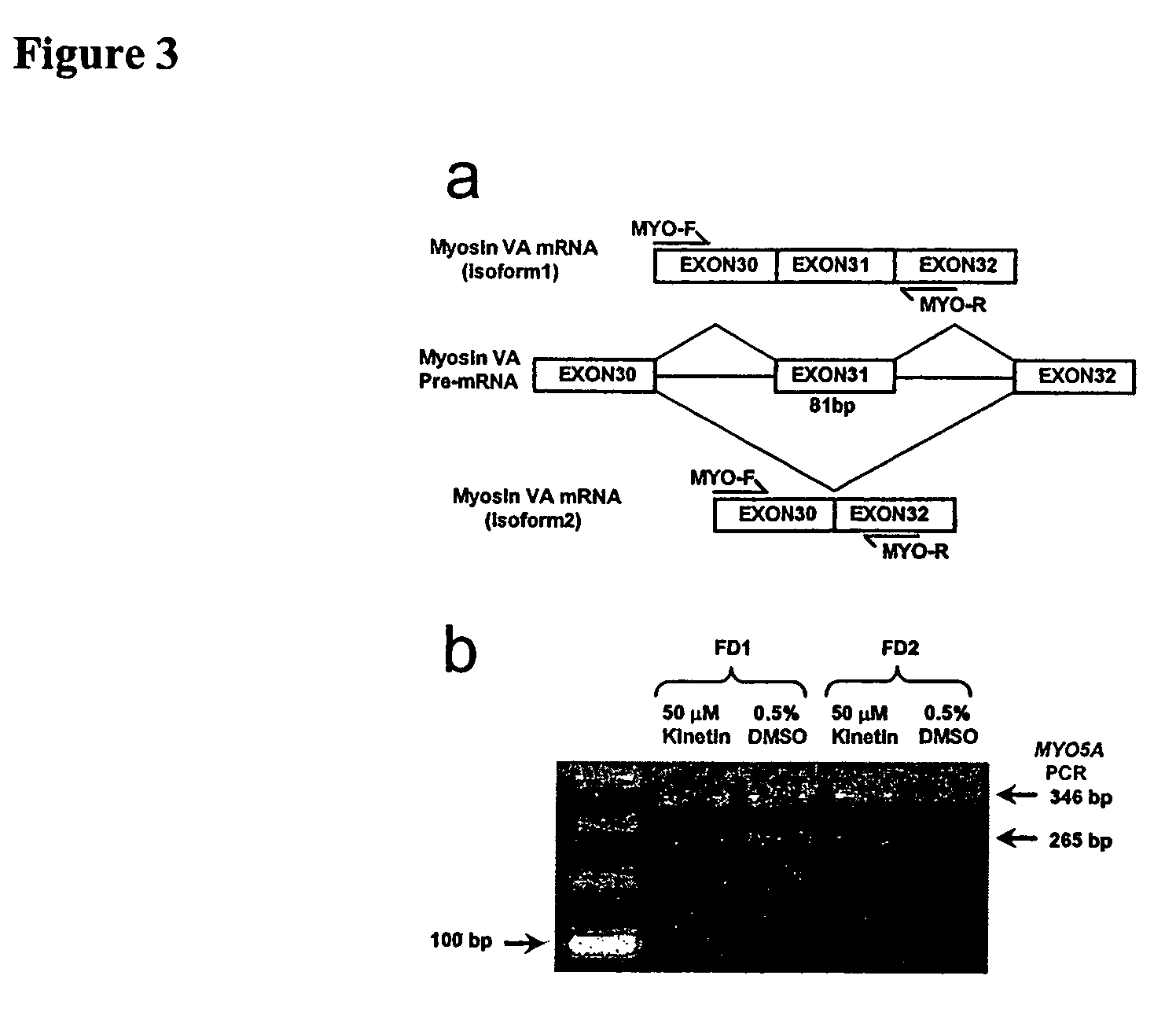
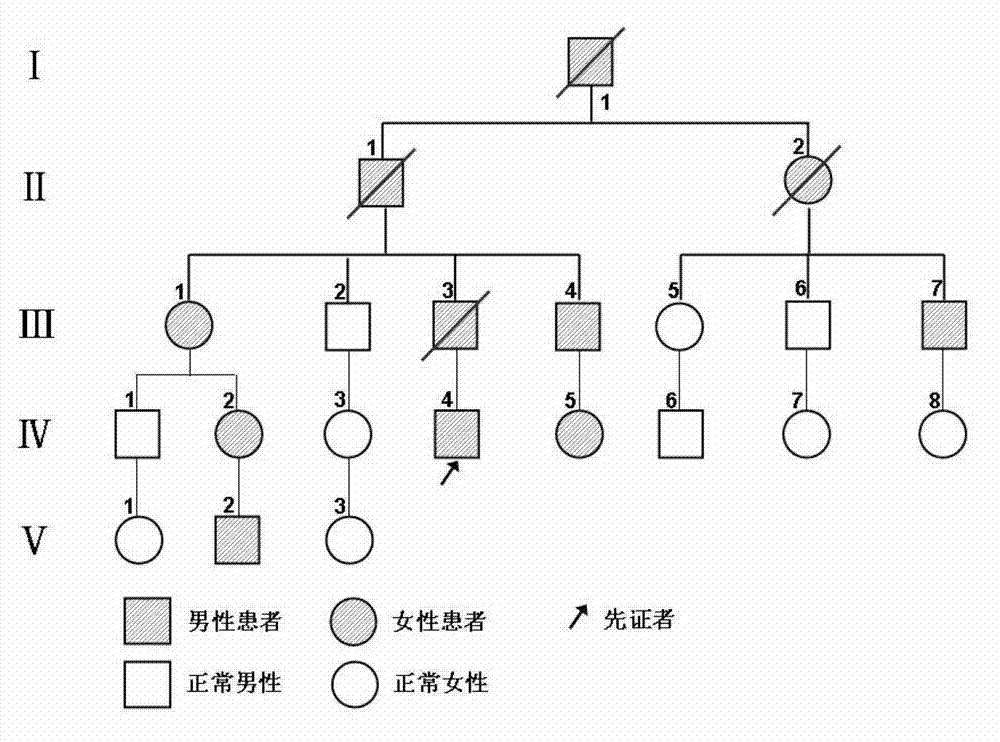
Methods for altering mRNA splicing and treating familial dysautonomia and other mechanistically related disorders
InactiveUS7737110B2Raise the ratioReduce misconnectionBiocideNervous disorderAutonomic bladder dysfunctionDisease
Owner:THE GENERAL HOSPITAL CORP
Methods for treating neurofibromatosis
InactiveUS20120157401A1Prevent proliferationControl growthBiocideNervous disorderFibromatosisCancer research
Methods for treating neurofibromatosis involving the administration of a compound that selectively inhibits pathological production of human VEGF are described. The compound can be administered as a single-agent therapy or in combination with one or more additional therapies to a human in need of such treatment.
Owner:PTC THERAPEUTICS INC
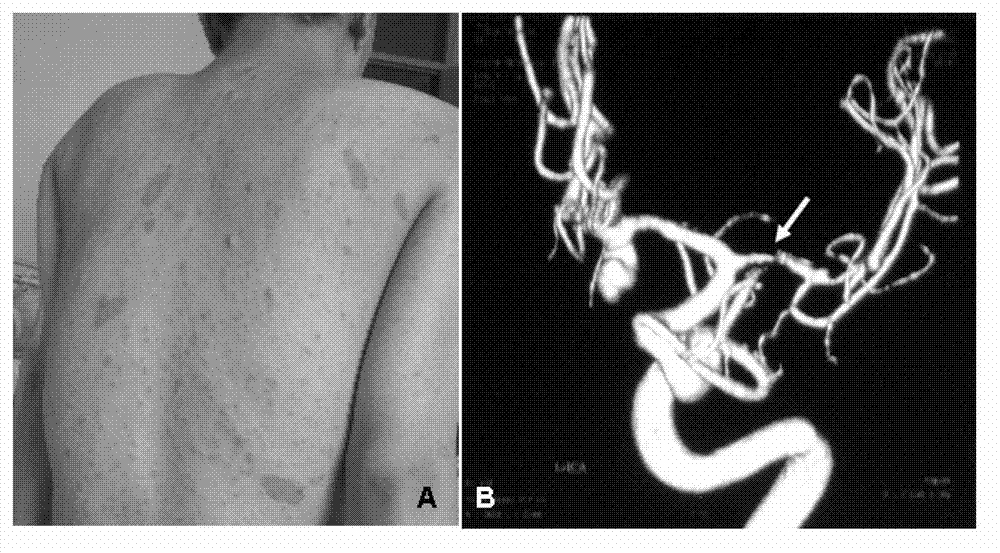
I type neurofibroma NF1 gene mutation nucleotide sequence related to cerebrovascular stenosis and application thereof
InactiveCN103045605AAt risk for cerebrovascular stenosisFungiBacteriaNormal peopleNucleotide sequencing
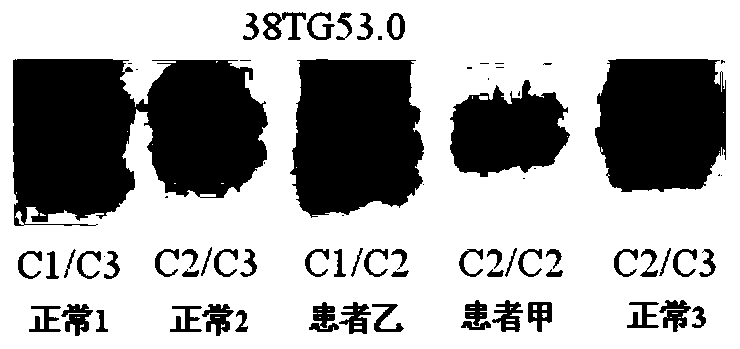
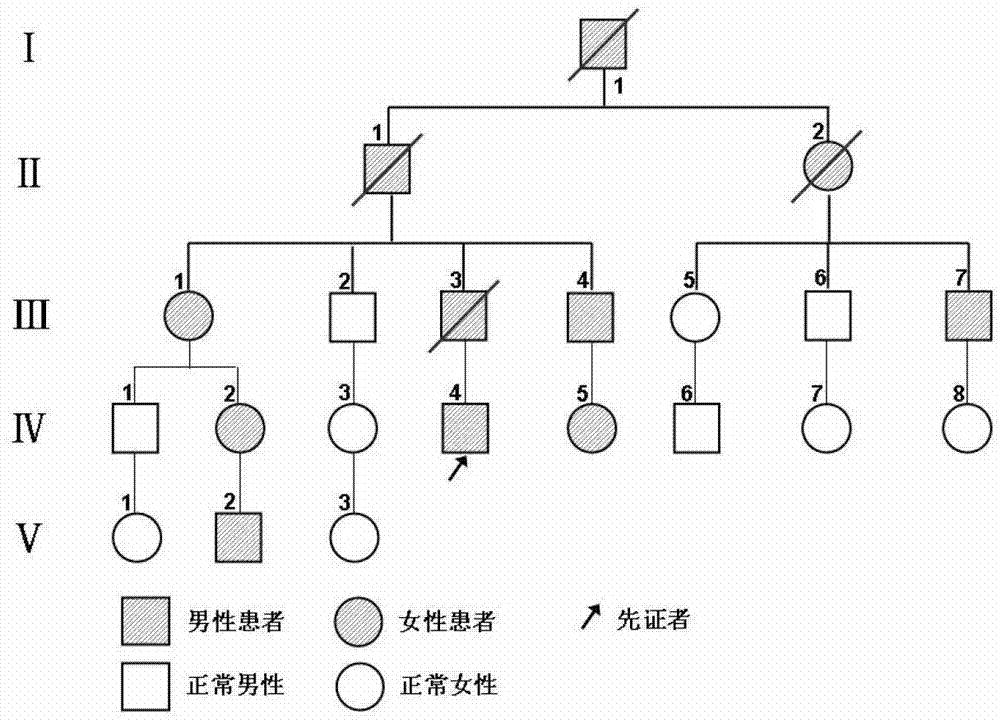
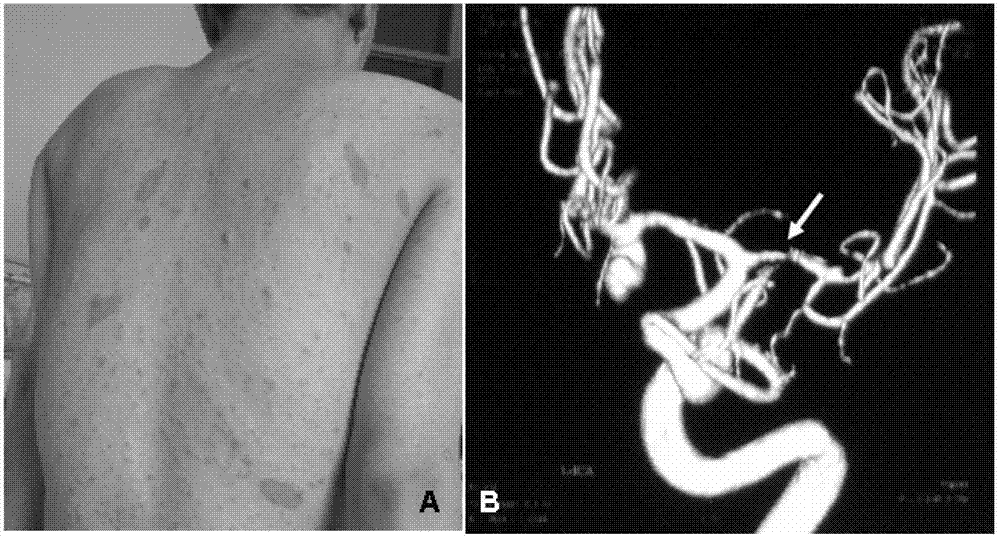
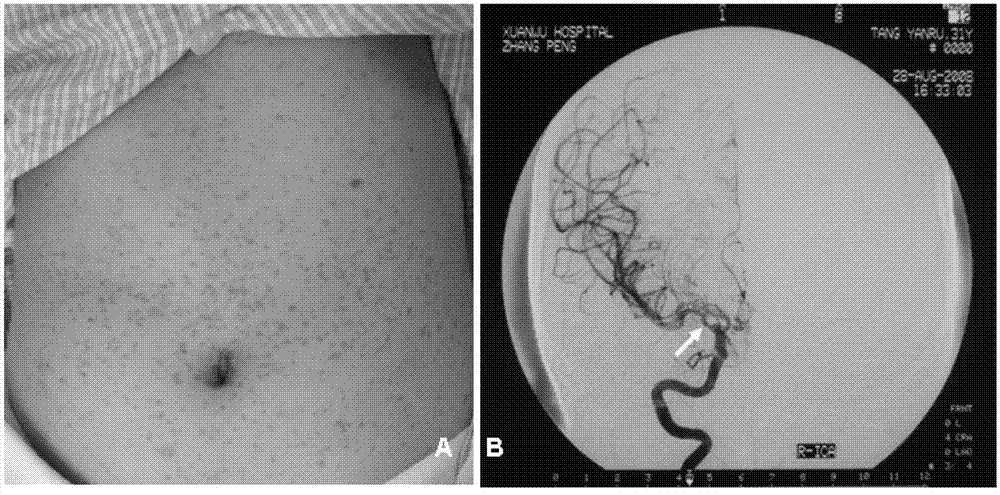
The invention relates to an I type neurofibroma NF1 gene mutation nucleotide sequence related to cerebrovascular stenosis and application thereof. Compared with a corresponding nucleotide sequence contained in NF1, the nucleotide sequence related to cerebrovascular stenosis, which is disclosed by the invention, is mutated at the following position: c.541C>T; the mutation position is started from a first base in a gene coding region of NF1; and the mutation causes a patient to suffer from cerebrovascular stenosis. Research finds that the nonsense mutation exists in all NF1 family patients suffering from cerebrovascular stenosis, but does not exist in normal people in the NF1 family. The mutational site can provide basis for establishing a similar clinical symptom cerebrovascular stenosis animal model and is further used for carrying out super early diagnosis and prevention on the sporadic potential cerebrovascular stenosis of NF1 patients.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Methods for altering mRNA splicing and treating familial dysautonomia and other mechanistically related disorders
InactiveUS20060014763A1Raise the ratioReduce misconnectionBiocideNervous disorderDiseaseMutated protein
This invention relates to methods for altering the splicing of mRNA in cells. In particular, this invention also relates to methods for increasing the ratio of wild type to misspliced forms of mRNA and corresponding encoded proteins in cells possessing a mutant gene encoding either the i) misspliced mRNA corresponding to the mutant protein or ii) a component in the splicing machinery responsible for processing the misspliced mRNA. In addition, this invention relates to treating individuals having a disorder associated with a misspliced mRNA, such as Familial Dysautonomia or Neurofibromatosis 1, by administering to such an individual a cytokinin such as kinetin.
Owner:THE GENERAL HOSPITAL CORP
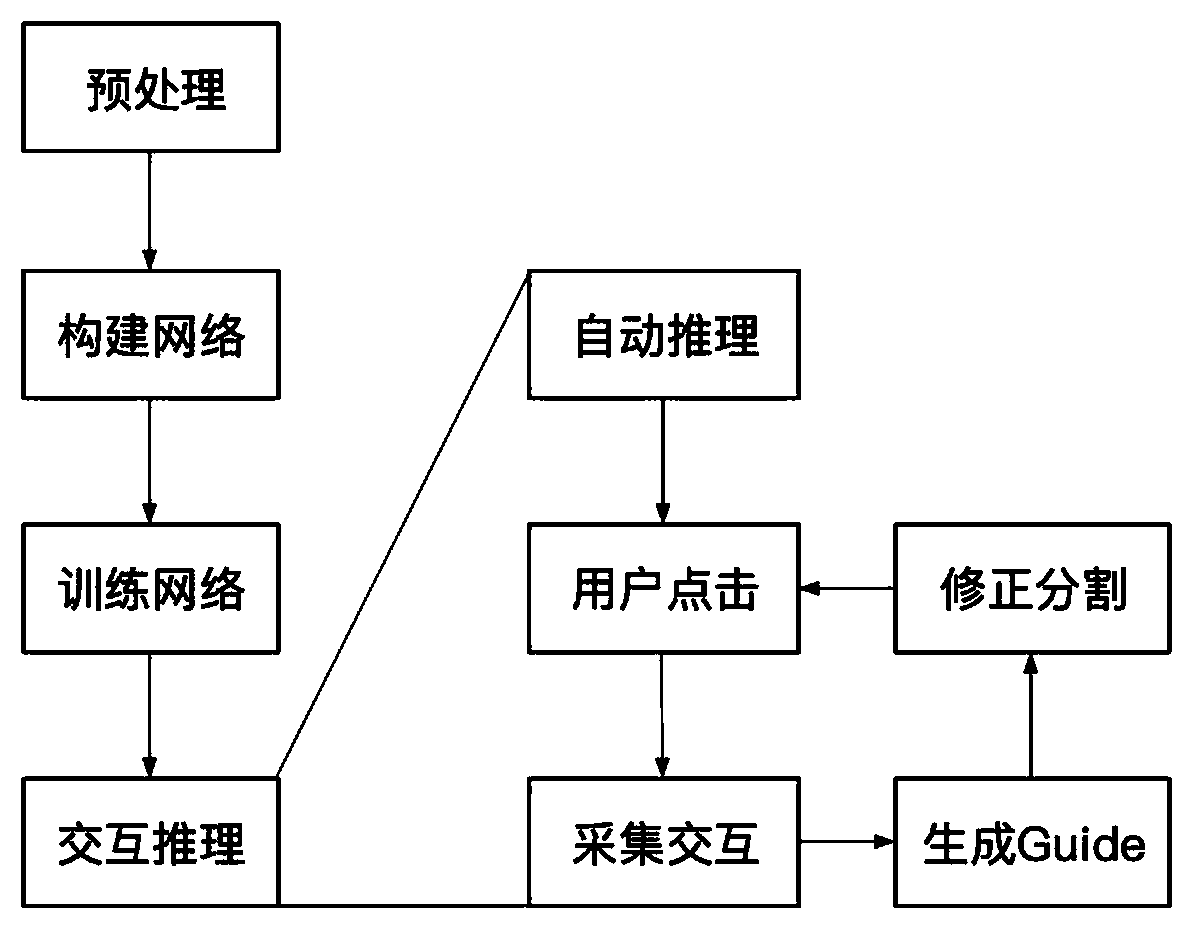
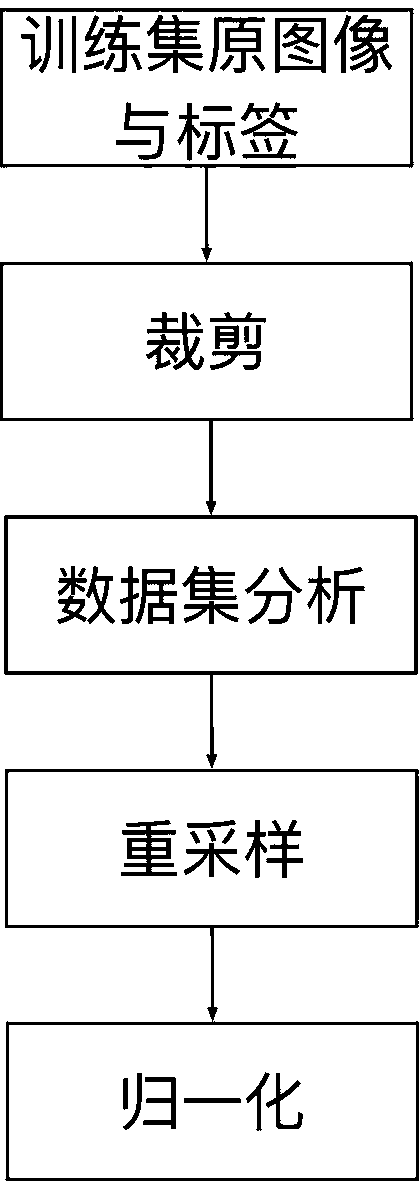
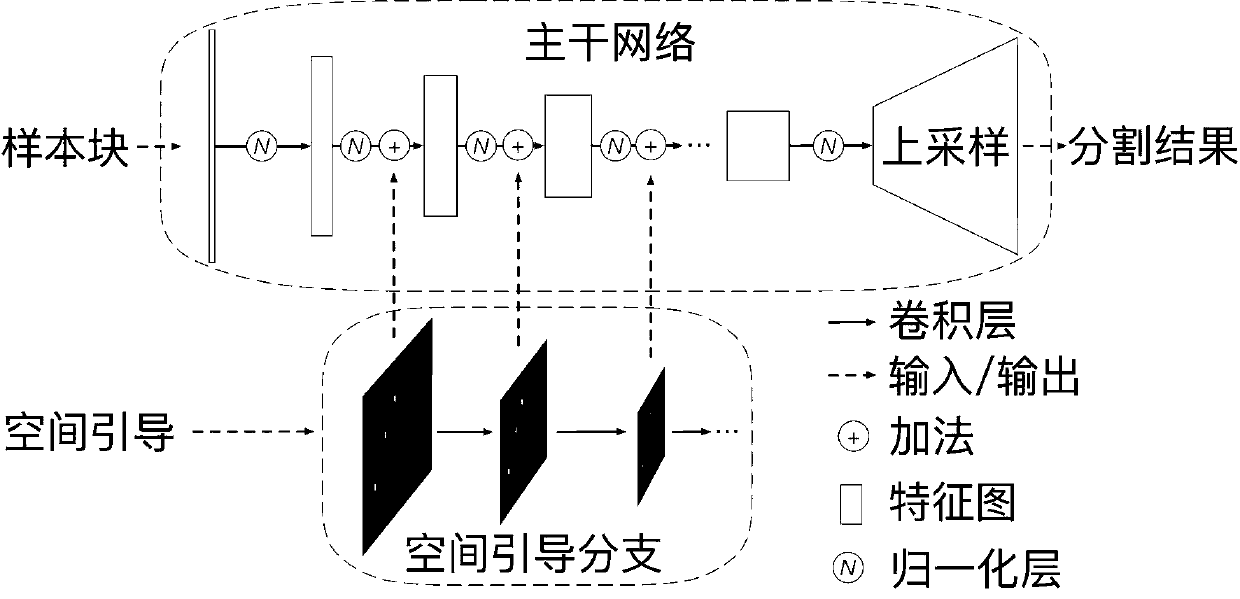
Neurofibroma segmentation method combined with space guidance
ActiveCN111260667AAutomatic segmentation results are goodImprove DiceImage enhancementImage analysisPattern recognitionAutomatic segmentation
The invention discloses a neural fibroma segmentation method combined with space guidance, and the method comprises the steps: taking nnU-Net as a trunk network, adding a space guidance branch, integrating user interaction information into a network such that the network is better segmented through user interaction on the basis of automatic segmentation; firstly, subjecting an original image to data preprocessing, and then guiding the image to be transmitted into a network with a certain probability according to label calculation space during training; during reasoning, making automatic segmentation, then enabling a user to click false positive and false negative areas to generate a guide label, generating space guidance according to the label, and transmitting the guide label and a test sample into a network together for prediction and cyclic reasoning until the user is satisfied. The deep neural network and the space guidance are combined, automatic segmentation can be completed, user guidance can also be accepted to correct segmentation, and a good segmentation result is obtained on the neurofibroma.
Owner:ZHEJIANG UNIV
Agent for regeneration and/or protection of nerves
An EP2 agonist which may have an EP3 agonistic effect has an effect of regenerating and / or protecting nerves, and is therefore useful as a therapeutic agent for a disease of the peripheral nervous system, such as a lower or upper motor neuron disease, a nerve root disease, plexopathy, thoracic outlet compression syndrome, peripheral neuropathy, neurofibromatosis and neuromuscular transmission disease. An EP2 agonist which has an EP3 agonistic effect is a safe and effective agent for the regeneration and / or protection of nerves which has little influence on the circulatory system.
Owner:ONO PHARMA CO LTD
Neurofibromatosis type I virulence gene mutation and etiological diagnosis agent based on neurofibromatosis type I virulence gene mutation
ActiveCN106755399AShorten detection timeReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationRNA extractionNeurofibromatosis type I
The invention relates to neurofibromatosis type I virulence gene mutation and an etiological diagnosis agent based on neurofibromatosis type I virulence gene mutation. The etiological diagnosis agent comprises point mutation analysis kits including a total RNA extraction system kit, a RNA reverse transcription and cDNA amplification system kit and a cDNA sequencing system kit. The total RNA extraction system kit is used for total RNA extraction of peripheral venous blood of a tested subject; the cDNA sequencing system kit is used for PCR amplification of cDNA obtained by reverse transcription and five primer pairs as shown in SEQ ID NO.7-16, nested PCR amplification and sequencing of cDNA five long segments obtained by amplification and primer pairs as shown in SEQ ID NO.17-60, and comparison with an original NF1 gene coding DNA sequence; if c.7106G>A and p.W2369X mutation exists in an amplified product, a neurofibromatosis type I patient is determined. The etiological diagnosis agent has advantages of time saving, accuracy and low test cost.
Owner:杭州艾诺医学检验所有限公司
Chinese medicinal composition for treating superficial tumor
InactiveCN101708299ASignificant effectHeavy metal active ingredientsMammal material medical ingredientsMyrrhFacial region
The invention discloses a Chinese medicinal composition for treating a superficial tumor, which comprises the following components by mass: 10 to 30 grams of java brucea fruit; 10 to 30 grams of walnut kernel; 8 to 15 grams of unprocessed rhizoma pinelliae; 8 to 15 grams of common burreed rhizome; 3 to 8 grams of calomel; 3 to 8 grams of frankincense; 3 to 8 grams of myrrh; and 1 to 2 grams of polythrinicium. The medicinal composition is prepared by grinding the raw material medicaments respectively, uniformly mixing the powders and putting the powder into bottles. When the Chinese medicinal composition is used, the raw material medicaments are prepared into a paste by adding the right amount of white vinegar; and the paste is externally applied to affected parts of the tumor to block blood supply around the tumor to make the tumor die naturally. Clinical application shows that the Chinese medicinal composition has obvious curative effect on hemangioma, thyroidtumor and neurofibroma; a few scars are left after healing, but can be faded out gradually in the future; and the facial region is recovered well.
Owner:谢足仔
Neurofibromin pathway modulators
Owner:WASHINGTON UNIV IN SAINT LOUIS
Treating learning deficits with inhibitors of Hmg CoA reductase
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
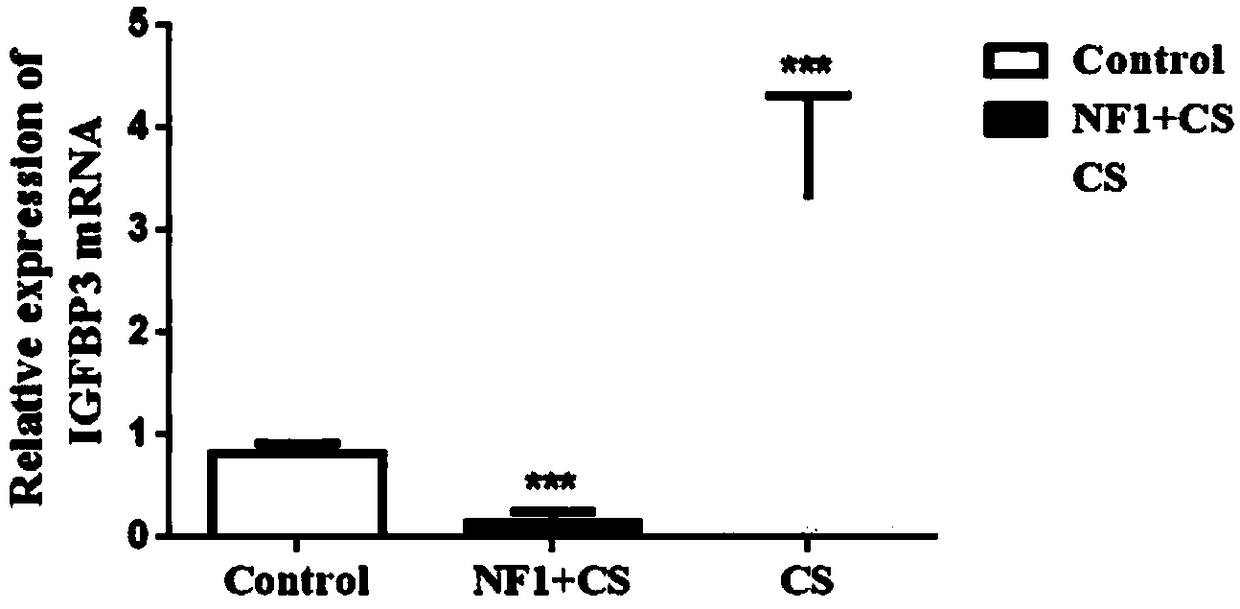
Application of IGFBP3 (insulin-like growth factor-binding protein 3) in preparation of products to diagnose type I neurofibromatosis with spinal deformity
ActiveCN108410990AMicrobiological testing/measurementSkeletal disorderMechanism of actionTranscriptome Sequencing
The invention discloses application of IGFBP3 (insulin-like growth factor-binding protein 3) in the preparation of products to diagnose type I neurofibromatosis with spinal deformity. Related markersIGFBP3 for NF1 with spinal deformity are acquired by screening via transcriptome sequencing; the role of IGFBP3 in genesis and development of bone healing defects in NF1 with spinal deformity is explored, and the acting mechanism of IGFBP3 is given to play; IGFBP3 as a novel biomarker of NF1 with spinal deformity provides important theoretical basis for early clinical intervention and targeted treatment of NF1 with spinal deformity.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Nitrogenous heterocyclic derivatives and their application in drugs
ActiveUS9434695B2Reduce severityOrganic active ingredientsNervous disorderNitrogenous heterocyclic compoundTissue fibrosis
The present invention relates to the field of medicine, provided herein are novel nitrogenous heterocyclic compounds, their preparation methods and their uses as drugs, especially for treatment and prevention of tissue fibrosis. Also provided herein are pharmaceutically acceptable compositions comprising the nitrogenous heterocyclic compounds and the uses of the compositions in the treatment of human or animal tissue fibrosis, especially for human or animal renal interstitial fibrosis, glomerular sclerosis, liver fibrosis, pulmonary fibrosis, peritoneal fibrosis, myocardial fibrosis, dermatofibrosis, postsurgical adhesion, benign prostatic hyperplasia, skeletal muscle fibrosis, scleroderma, multiple sclerosis, pancreatic fibrosis, cirrhosis, myosarcoma, neurofibroma, pulmonary interstitial fibrosis, diabetic nephropathy, alzheimer disease or vascular fibrosis.
Owner:SUNSHINE LAKE PHARM CO LTD
Methods of treating neurofibromatosis with perillyl alcohol
ActiveUS11147809B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismCarbamateNeurofibra
Owner:UNIV OF SOUTHERN CALIFORNIA
Treatments for social learning disorders
InactiveUS20140107222A1Reduced strengthShorten the durationBiocideOrganic active ingredientsLearning disabilityEndocrinology
Owner:INDIANA UNIV RES & TECH CORP
Traditional Chinese medicine preparation for treating neurofibroma
InactiveCN103156986AReduce dark spotsGood treatment effectUnknown materialsAntineoplastic agentsLiquoricesChinese drug
The invention discloses a traditional Chinese medicine preparation for treating neurofibroma, which is prepared from beautiful sweetgum fruit, trogopterus dung, corydalis tuber, root of common peony, angelica sinensis, tendril-leaved fritillary bulb, eucommia ulmoides and liquorice. The traditional Chinese medicine preparation disclosed by the invention can be clinically used for treating neurofibroma.
Owner:张冠珍
New mutant pathogenic gene of NF1 and application and kit of gene
ActiveCN110184275APerfect pathogenic variant geneTimely therapeutic interventionMicrobiological testing/measurementGenetic engineeringDiseaseMutated protein
The invention relates to a new mutant pathogenic gene of NF1 associated with neurofibromatosis type 1 disease, and belongs to the field of molecular biology. The nucleic acid sample of the new mutantpathogenic gene has a nucleotide change of c.738delA as compared with the nucleotide sequence of the NF1 gene shown in SEQ ID NO: 1. The invention also relates to a mutant protein encoded by the newmutant pathogenic gene. The present invention discloses that the c.738delA site mutation of the NF1 gene is associated with neurofibromatosis type 1 disease for the first time, and provides application of the new mutant pathogenic gene in preparing reagents or kits for detecting or screening the NF1 pathological change. Furthermore, screening kits for neurofibromatosis type1 disease are provided,more mutant genes of neurofibromatosis type 1 disease are improved, and more reliable guidance for timely detection and treatment of patients is provided.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Treatment Of Neurofibromatosis With Radicicol And Its Derivatives
ActiveUS20100292218A1Inhibiting and reducing growthInhibiting and reducing and numberBiocideAnimal repellantsDiseaseActive agent
The present invention provides compounds of formulae Ia, Ia′, IIa, IIa′, IIIa, IIIa′, IVa, or Va and the therapeutic use thereof. The present invention also includes methods of treating NF2-deficient or NF1-deficient cells or neurodegenerative diseases with radicicol or its derivatives, such as one or more compounds of formula I, II, III, IV, V, Ia, Ia′, IIa, IIa′, IIIa, IIIa′, IVa, or Va. Furthermore, the present invention is directed to methods of inhibiting the growth of NF2-deficient or NF1-deficient tumors. The methods comprise contacting NF2-deficient or NF1-deficient tumor cells with radicicol or its derivatives, such as one or more compounds of formula I, II, III, IV, V, Ia, Ia′, IIa, IIa′, IIIa, IIIa′, IVa, or Va. The present invention is also directed to the combinational use of radicicol or its derivatives, such as one or more compounds of formula I, II, III, IV, V, Ia, Ia′, IIa, IIa′, IIIa, IIIa′, IVa, or Va with at least one additional active agent, such as one or more HSP90 inhibitors.
Owner:UNIVERSITY OF STRASBOURG +1
Macrocyclic prodrug compounds useful as therapeutics
InactiveUS20140135290A1Inhibiting and reducing growthInhibiting and reducing and numberOrganic active ingredientsBiocideMedicineAutoimmune disease
The present invention includes macrocyclic prodrug compounds, pharmaceutical compositions containing them. The present invention also includes use of these compounds in the treatment of various diseases including an autoimmune disease, an inflammatory disease, a neurological or neurodegenerative disease, cancer, a cardiovascular disease, allergy, asthma, a hormone-related disease, and tumors or symptoms resulting from neurofibromatosis.
Owner:UNIVERSITY OF STRASBOURG +2
Chinese medicine for treating perineural fibroma disease
InactiveCN101347574AFormulation ScienceGood treatment effectNervous disorderAntineoplastic agentsDiseaseTreatment effect
The invention relates to a traditional Chinese medicine for treating neuroinomatosis, which effectively solves the problem of neurofibroma. The technical scheme of the invention is that: the traditional Chinese medicine is prepared by 72-88g of Chinese ephedra, 108-132g of semen perillae acutae, 108-132g of ballonflower, 135-165g of tansymustard seed, 108-132g of dried orange peel, 90-110g of immature bitter orange, 108-132g of tuckahoe, 90-110g of bulbus fritilariae, 108-132g of tuber pinellia, 90-110g of oarweed, 270-330g of oldenlandia diffusa, and 45-55g of liquorice. Firstly, the Chinese ephedra, the dried orange peel, the tuckahoe, the bulbus fritilariae, the tuber pinellia, the immature bitter orange and the liquorice are pulverized into fine powder, the grain diameter of which is at most 10 mum, and the fine powder is uniformly mixed for spare use; then, water is added in five medicines of the semen perillae acutae, the ballonflower, the tansymustard seed, the oarweed and the oldenlandia diffusa, the mixture is immerged for 30-40 minutes decocted for 1.5h two times; decoction is merged and concentrated into extractum, the relative density of which is 1.20-1.30; the fine powder and the extracted extractum are added in the mixture and mixed together to form medicine lumps; the medicine lumps are placed in a baking box and dried at 70-85 DEG C until the water content is less than 5%. The medicine has the advantages of scientific prescription, good curative effect and great economic and social benefit.
Owner:李勇
Pharmaceutical Composition for Treating Cancer
ActiveUS20210023158A1Organic active ingredientsNanomedicineNeural crest-derived tumorsCaffeoylquinic acid
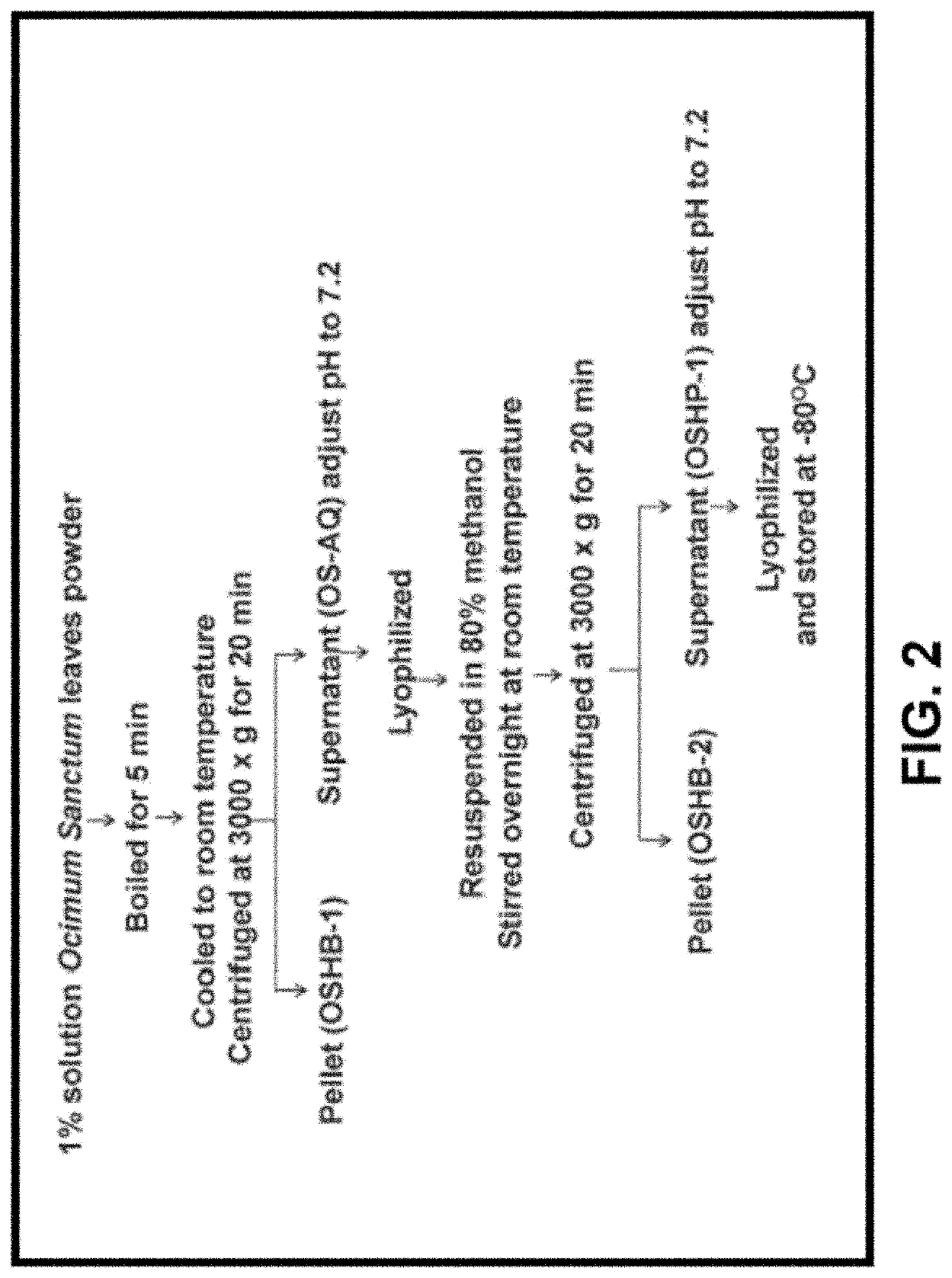
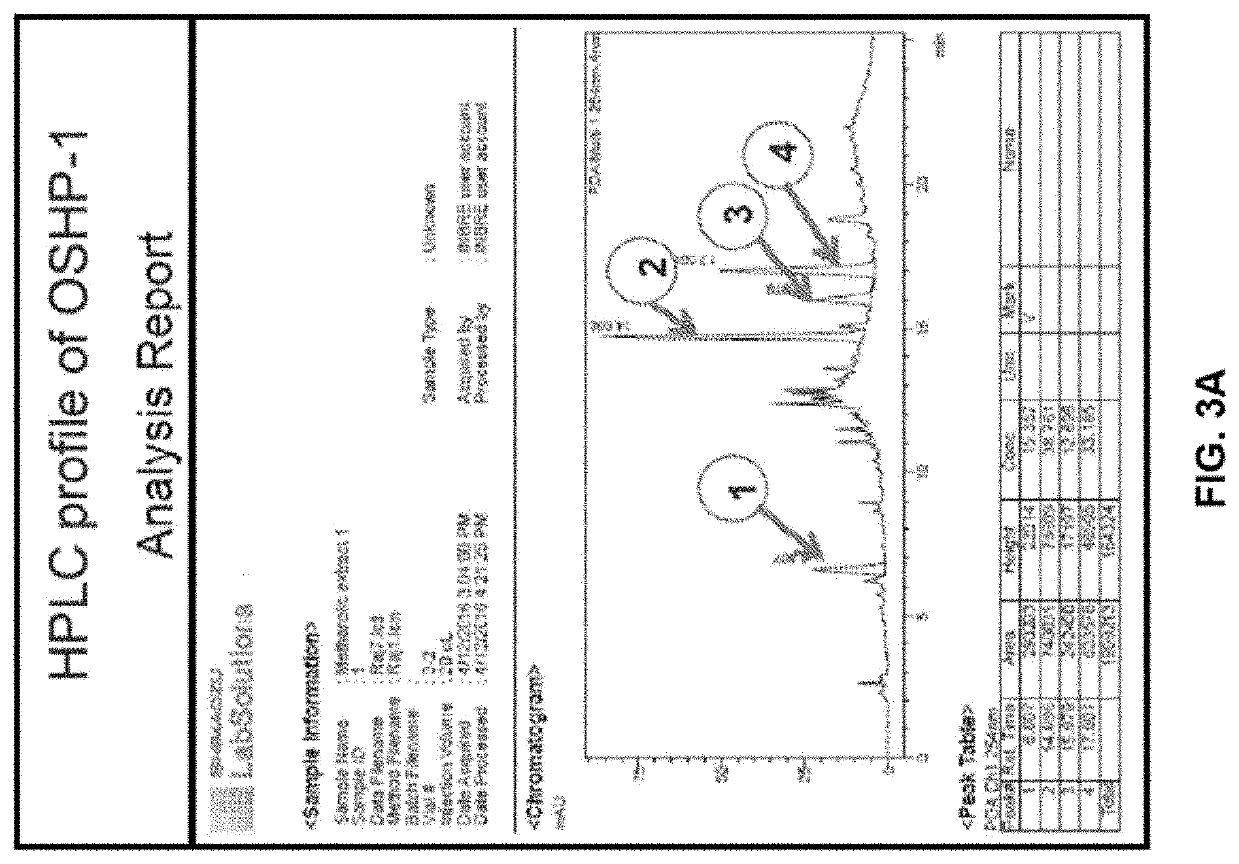
Presently claimed invention related to a pharmaceutical composition comprising ascorbic acid, caffeoylquinic acid, rosmarinic acid. and glycosyl sulfones obtained from Ocimum Sanctum and a process for isolating said composition from Ocimum Sanctum. Presently claimed invention also provides a method of treating neural crest derived tumors such as malignant neurofibroma and melanoma.
Owner:PRIME BIO INC
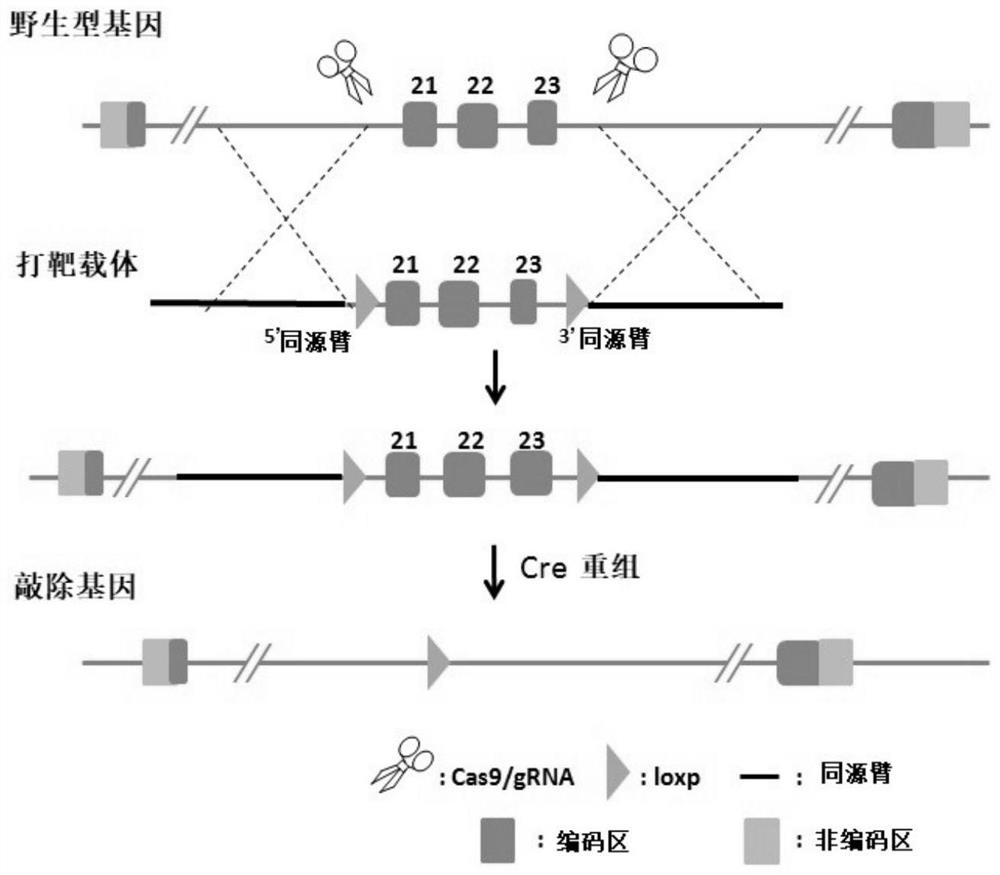
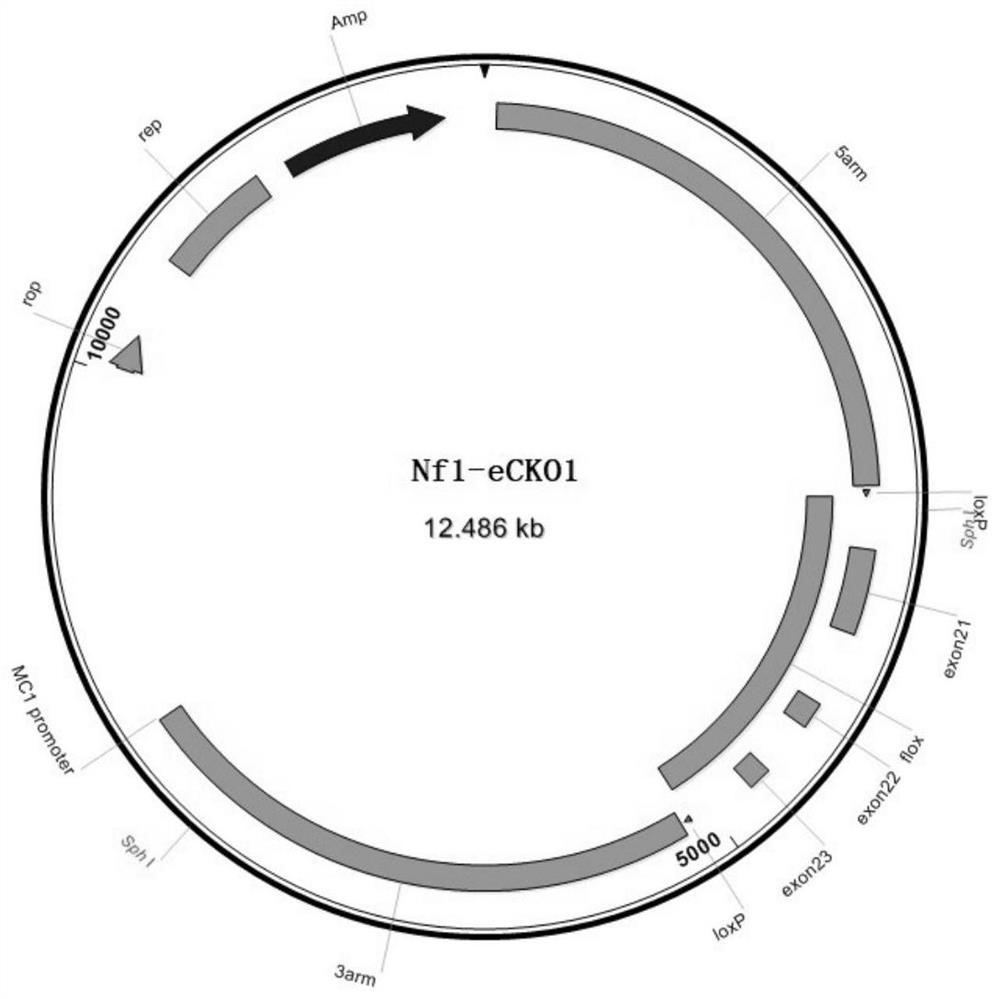
Construction method of Nf1 gene knockout animal model
PendingCN113957096AEasy to operateGood value for moneyFermentationAnimals/human peptidesAnimal scienceWild type
The invention discloses a construction method of an Nf1 gene knockout animal model. The construction method comprises the following steps of designing gRNA for identifying an Nf1 gene; carrying out in vitro transcription to obtain Cas9mRNA and gRNA (guide Ribonucleic Acid); constructing a homologous recombinant vector; microinjecting the Cas9mRNA, the gRNA and the homologous recombinant vector into fertilized eggs of animals, and obtaining F0-generation animals; and mating a positive F0-generation animal with a wild-type animal to obtain an F1-generation animal, namely the Nf1 gene knockout animal model. The animal model is the first successfully tumorigenic I-type neurofibroma transgenic animal model in China, provides a stable and reliable in-vivo model platform for research of the occurrence and development mechanism of I-type neurofibroma and exploration of therapeutic schedules such as targeted therapy, and makes up for the deficiency of lack of related models at present.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Neurofibromin/dopamine signaling as a biomarker for cognitive and behavioral problems in children with neurofibromatosis type 1 (NF1)
InactiveUS20160370383A1Microbiological testing/measurementDisease diagnosisDopaminergicClinical psychology
The present disclosure is generally related to neurofibromatosis type 1. More particularly, disclosed herein are methods for detecting behavioral disorders, methods for detecting cognitive impairment, and methods for detecting brain neurofibromin-dependent dopaminergic signaling associated with neurofibromatosis type 1.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Nitrogenous Heterocyclic Derivatives And Their Application In Drugs
ActiveUS20150087639A1Reduce severityBiocideNervous disorderNitrogenous heterocyclic compoundTissue fibrosis
The present invention relates to the field of medicine, provided herein are novel nitrogenous heterocyclic compounds, their preparation methods and their uses as drugs, especially for treatment and prevention of tissue fibrosis. Also provided herein are pharmaceutically acceptable compositions comprising the nitrogenous heterocyclic compounds and the uses of the compositions in the treatment of human or animal tissue fibrosis, especially for human or animal renal interstitial fibrosis, glomerular sclerosis, liver fibrosis, pulmonary fibrosis, peritoneal fibrosis, myocardial fibrosis, dermatofibrosis, postsurgical adhesion, benign prostatic hyperplasia, skeletal muscle fibrosis, scleroderma, multiple sclerosis, pancreatic fibrosis, cirrhosis, myosarcoma, neurofibroma, pulmonary interstitial fibrosis, diabetic nephropathy, alzheimer disease or vascular fibrosis.
Owner:SUNSHINE LAKE PHARM CO LTD
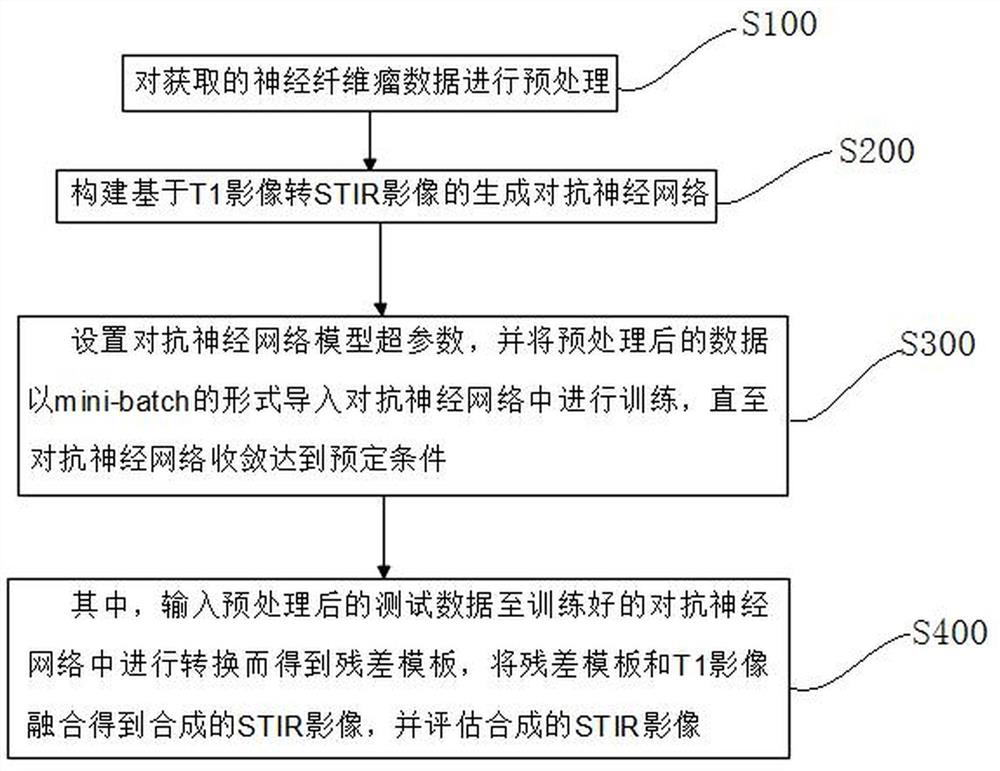
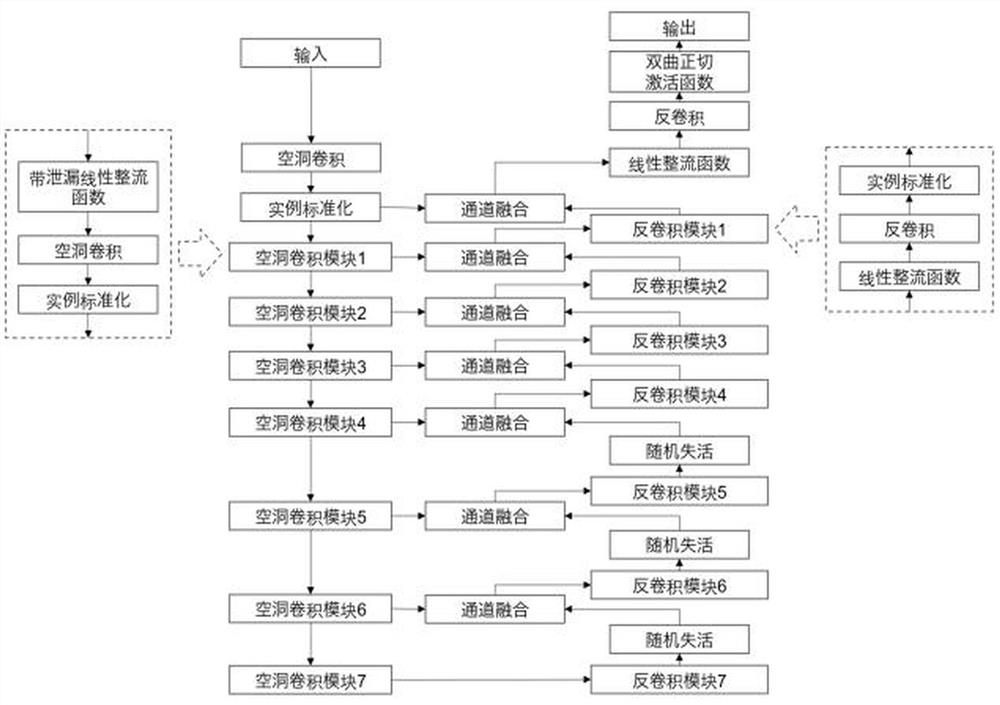
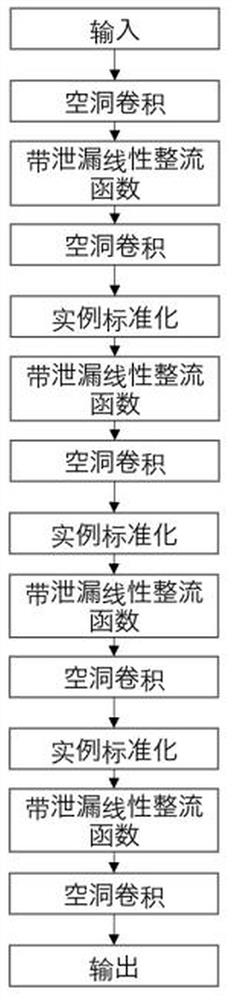
Detail enhancement-based T1 to STIR image conversion method
PendingCN112085687AImprove clarityHighlight the differenceImage enhancementImage analysisPattern recognitionNeurofibra
The invention discloses a detail enhancement-based T1 to STIR image conversion method, which comprises the following steps: preprocessing acquired neurofibroma data, constructing a generative adversarial neural network based on T1 image to STIR image conversion, setting hyper-parameters of an adversarial neural network model, and establishing an adversarial neural network model; importing the preprocessed data into an adversarial neural network in a minibatch form for training until convergence of the adversarial neural network reaches a predetermined condition, inputting the preprocessed testdata into the trained adversarial neural network for conversion to obtain a residual template, fusing the residual template and a T1 image to obtain a synthesized STIR image, and evaluating the synthesized STIR image. According to the conversion method, the details and features of the image can be restored to a greater extent by training the residual template, so that the conversion effect of theimage details is improved.
Owner:ZHEJIANG UNIV
A type I neurofibromatosis pathogenic mutation gene and an etiological diagnostic reagent based on the mutation gene
ActiveCN106755399BShorten detection timeReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationEtiologyNeurofibromatosis type I
The invention relates to neurofibromatosis type I virulence gene mutation and an etiological diagnosis agent based on neurofibromatosis type I virulence gene mutation. The etiological diagnosis agent comprises point mutation analysis kits including a total RNA extraction system kit, a RNA reverse transcription and cDNA amplification system kit and a cDNA sequencing system kit. The total RNA extraction system kit is used for total RNA extraction of peripheral venous blood of a tested subject; the cDNA sequencing system kit is used for PCR amplification of cDNA obtained by reverse transcription and five primer pairs as shown in SEQ ID NO.7-16, nested PCR amplification and sequencing of cDNA five long segments obtained by amplification and primer pairs as shown in SEQ ID NO.17-60, and comparison with an original NF1 gene coding DNA sequence; if c.7106G>A and p.W2369X mutation exists in an amplified product, a neurofibromatosis type I patient is determined. The etiological diagnosis agent has advantages of time saving, accuracy and low test cost.
Owner:杭州艾诺医学检验所有限公司
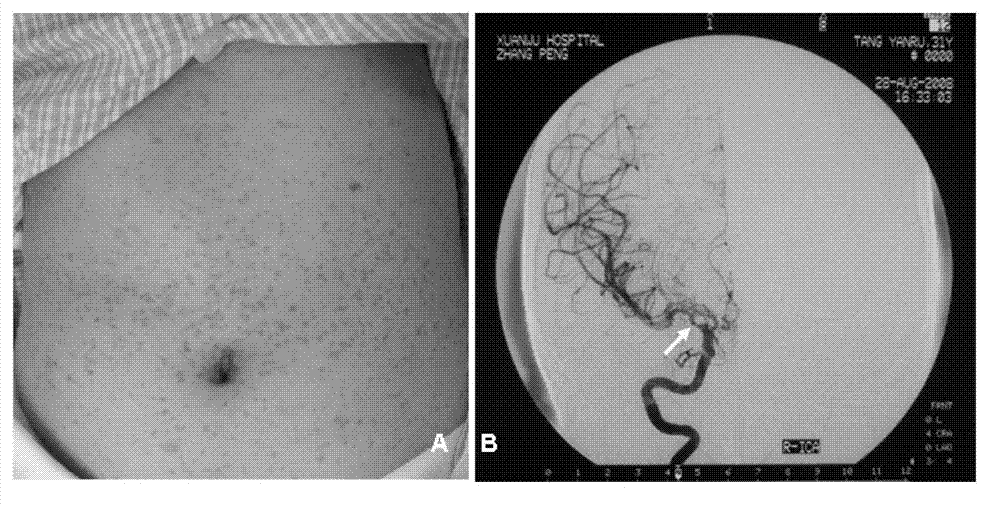
I type neurofibroma NF1 gene mutation nucleotide sequence related to cerebrovascular stenosis and application thereof
InactiveCN103045605BAt risk for cerebrovascular stenosisFungiBacteriaNormal peopleNucleotide sequencing
The invention relates to an I type neurofibroma NF1 gene mutation nucleotide sequence related to cerebrovascular stenosis and application thereof. Compared with a corresponding nucleotide sequence contained in NF1, the nucleotide sequence related to cerebrovascular stenosis, which is disclosed by the invention, is mutated at the following position: c.541C>T; the mutation position is started from a first base in a gene coding region of NF1; and the mutation causes a patient to suffer from cerebrovascular stenosis. Research finds that the nonsense mutation exists in all NF1 family patients suffering from cerebrovascular stenosis, but does not exist in normal people in the NF1 family. The mutational site can provide basis for establishing a similar clinical symptom cerebrovascular stenosis animal model and is further used for carrying out super early diagnosis and prevention on the sporadic potential cerebrovascular stenosis of NF1 patients.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com