Neurofibromatosis type I virulence gene mutation and etiological diagnosis agent based on neurofibromatosis type I virulence gene mutation
A technology of neurofibromatosis and diagnostic reagents, which is applied in the field of molecular biology and gene detection, and can solve the problems of high experimental conditions, high price, and easy missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Discovery of NF1 gene mutation in neurofibromatosis type I
[0118] The inventor has carried out gene mutation analysis to 13 routine patients, detailed steps are as follows:
[0119] 1) Detection by mutation analysis reagents: the reagents include 1) total RNA extraction system reagents; 2) RNA reverse transcription and cDNA amplification system reagents; 3) cDNA sequencing system reagents.
[0120] 2) Take 2ml of EDTA anticoagulated peripheral blood samples from suspected patients and normal controls, extract total RNA, perform reverse transcription PCR, and amplify cDNA to obtain 5 large fragments of cDNA1, 2, 3, 4, and 5 (for results, see Figure 4 ); Nested PCR was carried out to 5 large cDNA fragments, a total of 22 pairs of primers, PCR product agarose electrophoresis results ( Figure 5 ) and sequencing results ( Image 6 ).
[0121] Type I neurofibromatosis is an autosomal dominant inheritance. In this study, combining the actual situation of the family and ...
Embodiment 2
[0135] The usage method and application example of using the kit of the present invention for diagnosis.
[0136] 1. Obtain clinical samples and set up experimental and control groups
[0137] 1) Patient A
[0138] Patient A, a male, had samples from a tertiary hospital. He had 6 café-au-lait spots, axillary or inguinal freckles, and 9 neurofibromas. The initial diagnosis was type 1 neurofibroma.
[0139] 2) Patient B
[0140] Patient B’s sample came from a tertiary hospital. He had 8 or more café-au-lait spots, armpit or groin freckles, and 3 neurofibromas. The initial diagnosis was type 1 neurofibroma.
[0141] 3) Control group
[0142] Three normal subjects were selected as the control group.
[0143] 2. Experimental scheme and steps
[0144] The point mutation analysis kit of the present invention is used for detection: the kit includes 1) total RNA extraction system kit; 2) RNA reverse transcription and cDNA amplification system kit; 3) cDNA sequencing system kit.
...
Embodiment 3
[0180] The foregoing steps are basically the same as in Example 2, except that the detection of patient A and 3 normal persons with no detected mutation: c.7106G>A, p.W2369X continued as follows.
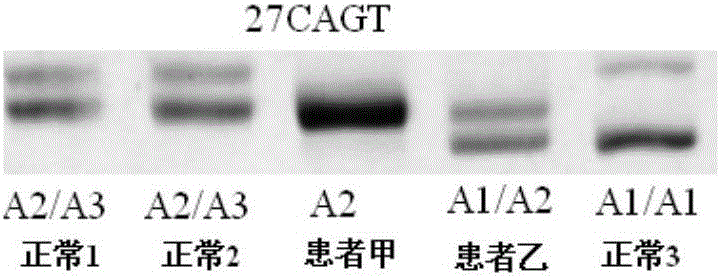
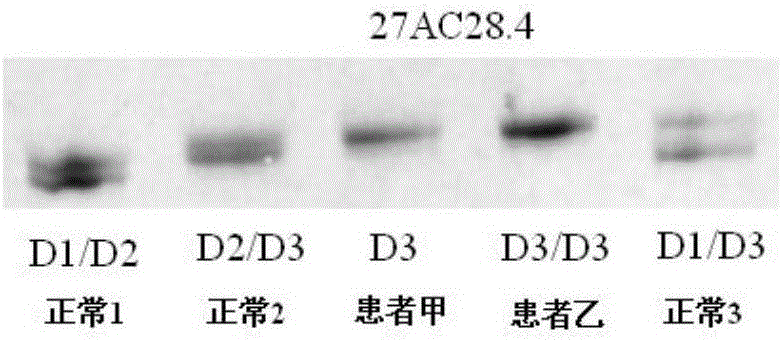
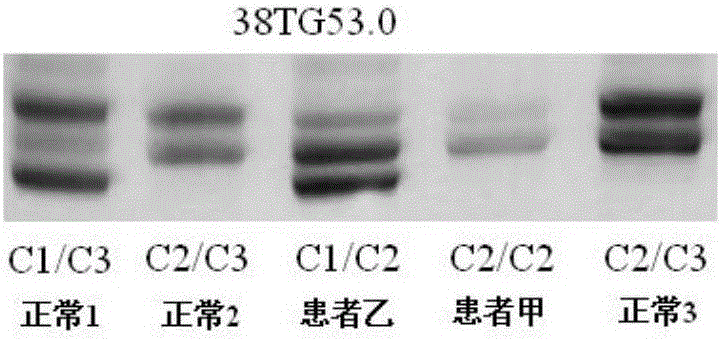
[0181] The NF1 whole gene large fragment deletion analysis kit of the present invention is used for detection, and the kit includes 1) a DNA extraction system kit; 2) an intragenic microsatellite polymorphic marker analysis kit.
[0182] The specific operation is as follows:
[0183] 5. Use the genomic DNA extraction kit to extract the peripheral blood DNA of suspected patient A and normal control (ensure that the extracted DNA storage concentration is >50ng / μl):
[0184] 5.1 Take 300 μl of anticoagulated whole blood, add 600 μl of cell lysate CL to the sample, mix by inversion, centrifuge at 10 000 rpm for 1 min, suck off the supernatant, and leave the cell nucleus pellet.
[0185] 5.2 Repeat the above steps once.
[0186] 5.3 Add 200 μl buffer GS to the cell nucleus pellet colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com