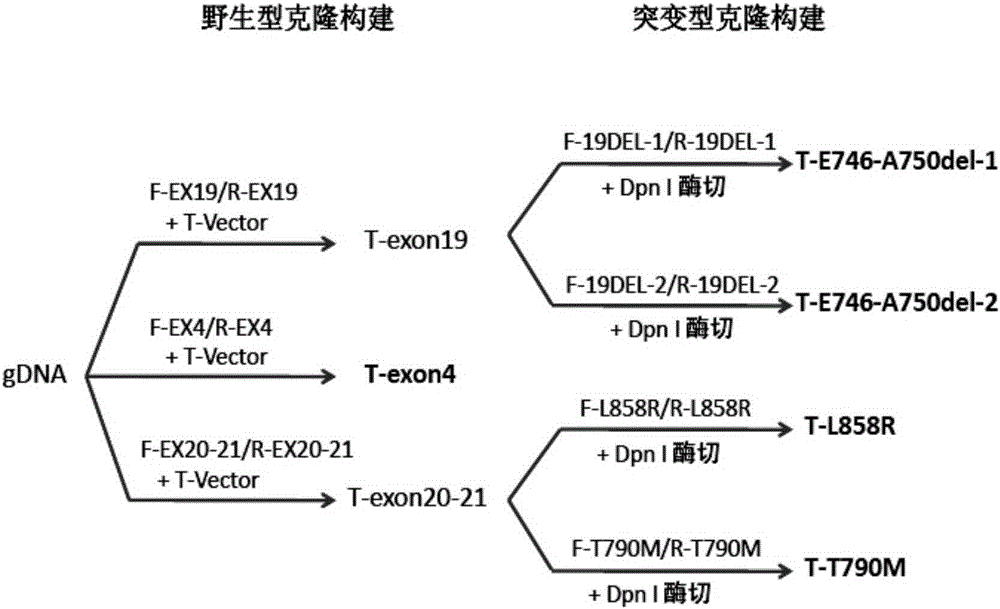
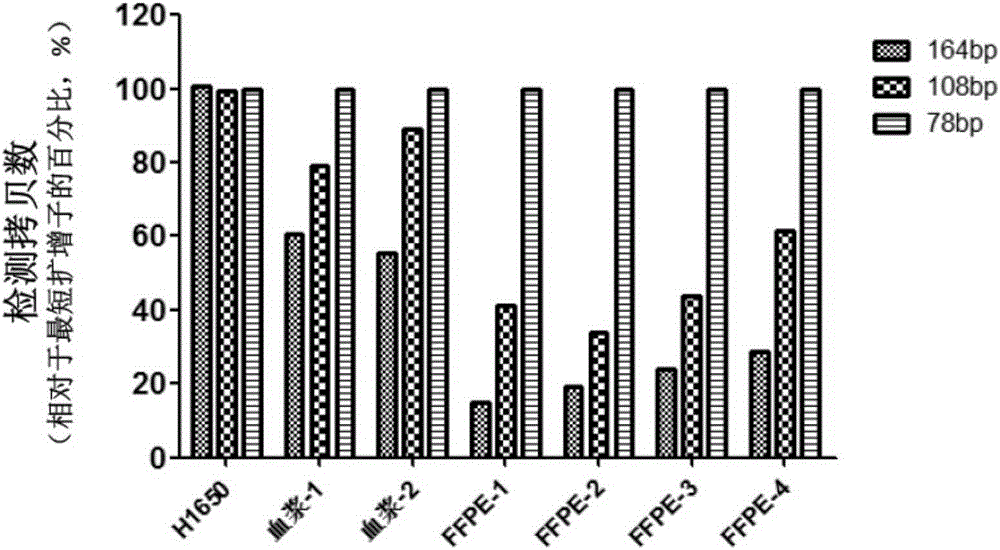
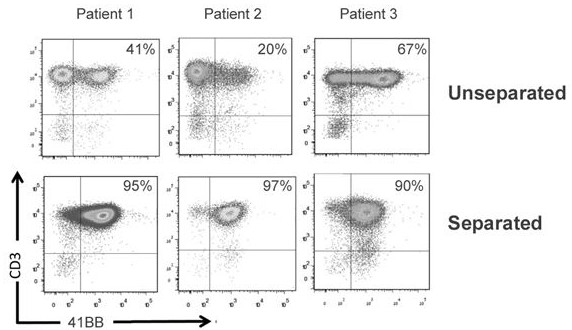
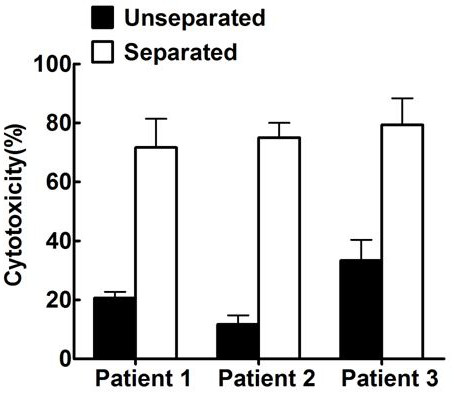
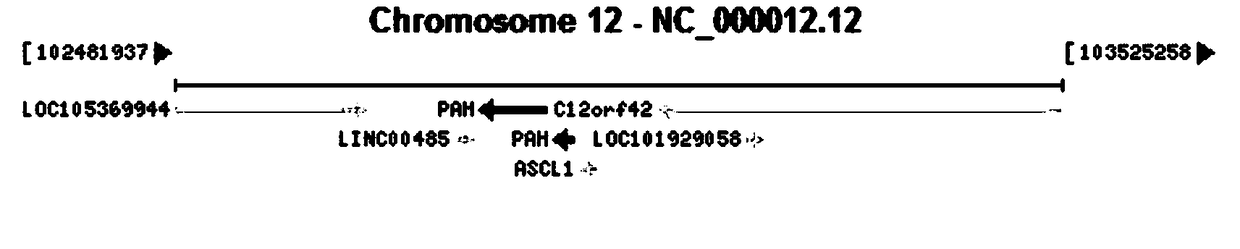
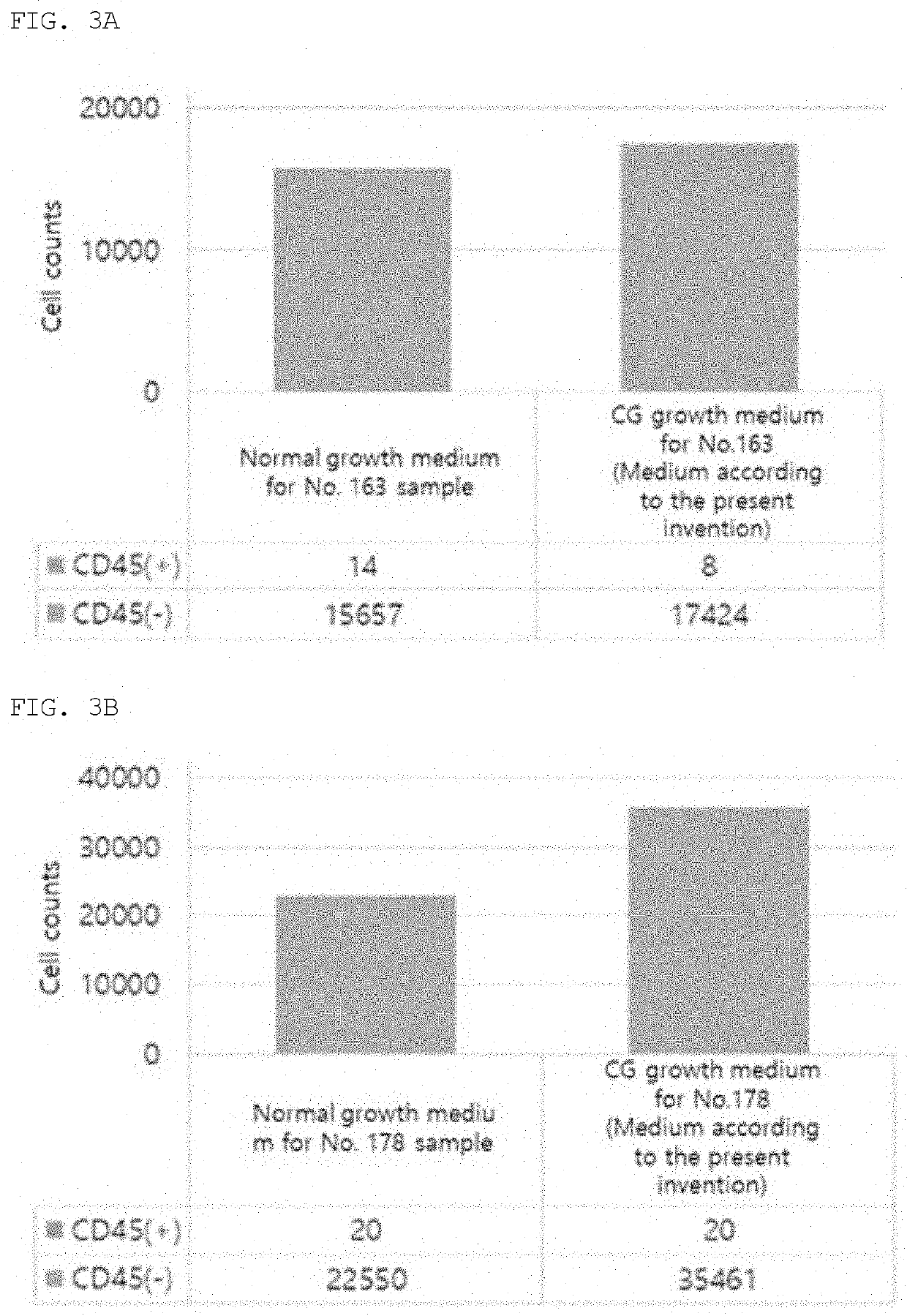
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Gene mutation analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene mutation analysis is now a fundamental part of the investigation and management of an individual and their family with an inherited bleeding disorder.
Primers for detecting ApoE gene polymorphism, kit and PCR (polymerase chain reaction) method for primers or kit
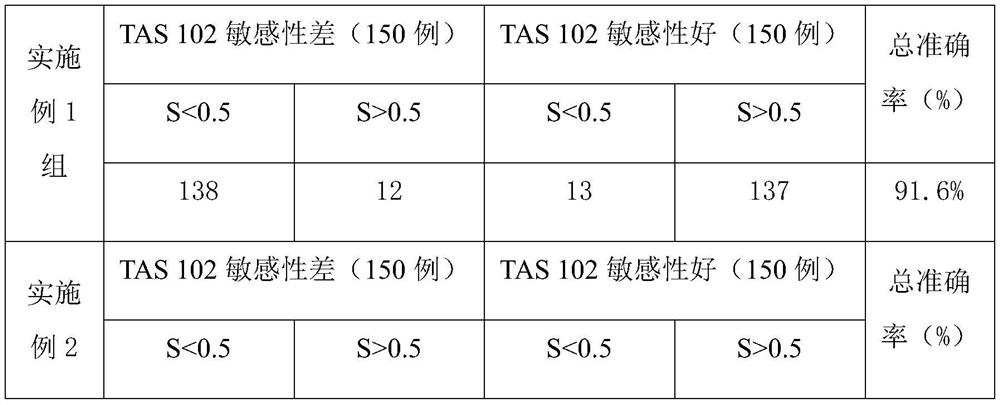
ActiveCN104862402ADifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationType specificMedicine
The invention discloses primers for detecting ApoE gene polymorphism, a kit and a PCR (polymerase chain reaction) method for the primers or the kit. The primers include two sets of primers for detecting an rs429358 site and an rs7412 site respectively; the first set of primers include a mutant-type specific upstream primer of the rs429358 site, a wild-type specific upstream primer of the rs429358 site and a first downstream primer shared by the mutant-type specific upstream primer and the wild-type specific upstream primer of the rs429358 site; the second set of primers include a mutant-type specific upstream primer of the rs7412 site, a wild-type specific upstream primer of the rs7412 site and a second downstream primer shared by the mutant-type specific upstream primer and the wild-type specific upstream primer of the rs7412 site. The kit has the advantages of simplicity, quickness, accuracy and low price for detection and the like, and provides a powerful tool for scientific research and clinical analysis of ApoE gene typing and gene mutation.
Owner:沈阳优吉诺生物科技有限公司
Kit for quantitatively detecting EGFR (Epidermal Growth Factor Receptor) gene mutation and application thereof
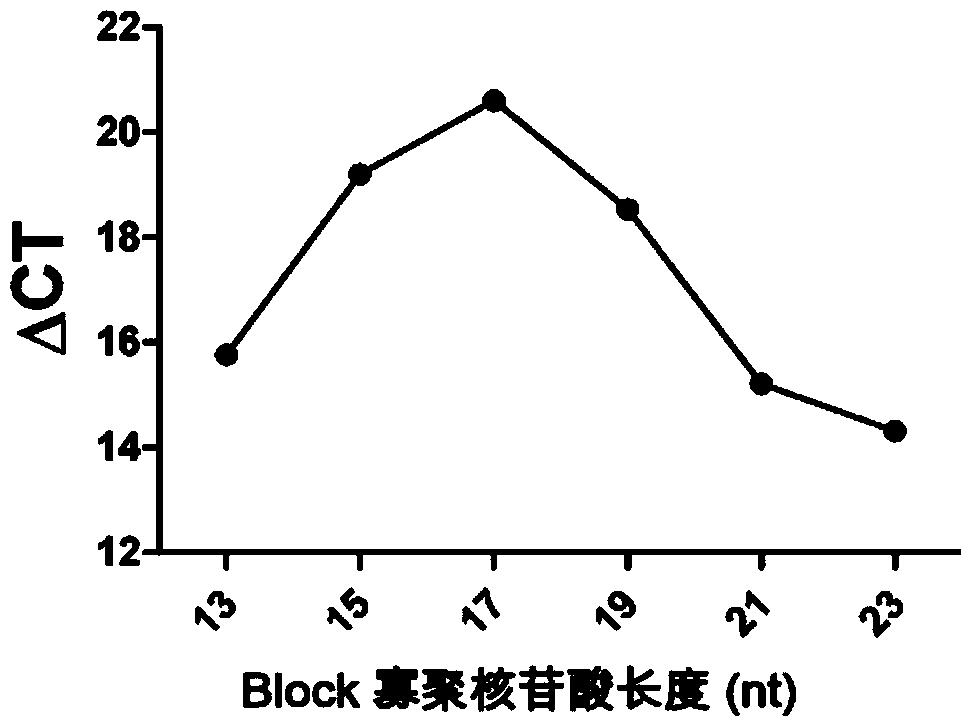
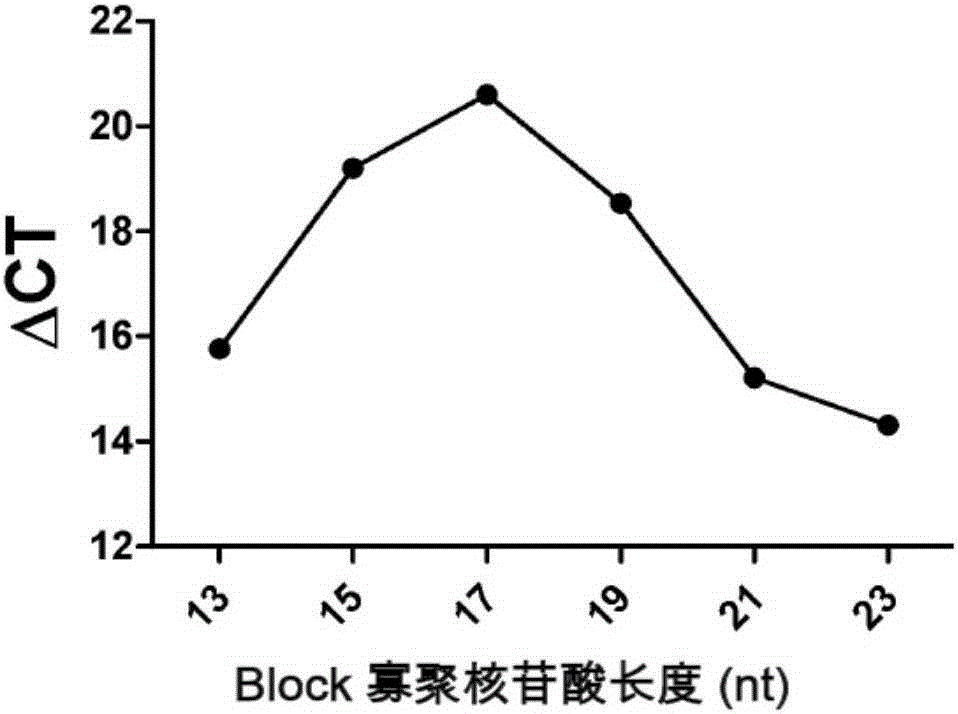
The invention relates to a high-sensitivity kit for quantitatively detecting EGFR (Epidermal Growth Factor Receptor) gene mutation and application thereof. The application comprises the following steps: respectively designing a forward primer and a backward primer in a sequence with the length of 50-100bp in a specific amplification gene mutation site area aiming at a to-be-detected gene template, designing the mutation sites at corresponding positions of the forward primer, and designing competitive Block oligonucleotides for inhibiting non-mutant amplification nearby the mutation sites. The detection is performed by utilizing the specificity aiming at a probe of the amplification product, and the gene mutation conditions are obtained by detecting the detectable markers on the probe. The invention provides a gene mutation analysis method which is stable in result, high in sensitivity and high in repeatability.
Owner:GENOSABER BIOTECH CO LTD +1
New skin sampling kit which stores nucleic acids in stable status, genetic test methods by using the kit and their practical application
InactiveCN101990578AEx situ and stable acquisitionAccurate evaluationMicrobiological testing/measurementSurgical needlesStable statusSkin test results
Owner:GOODGENE
Reagent kit for detecting senility macular degeneration disease
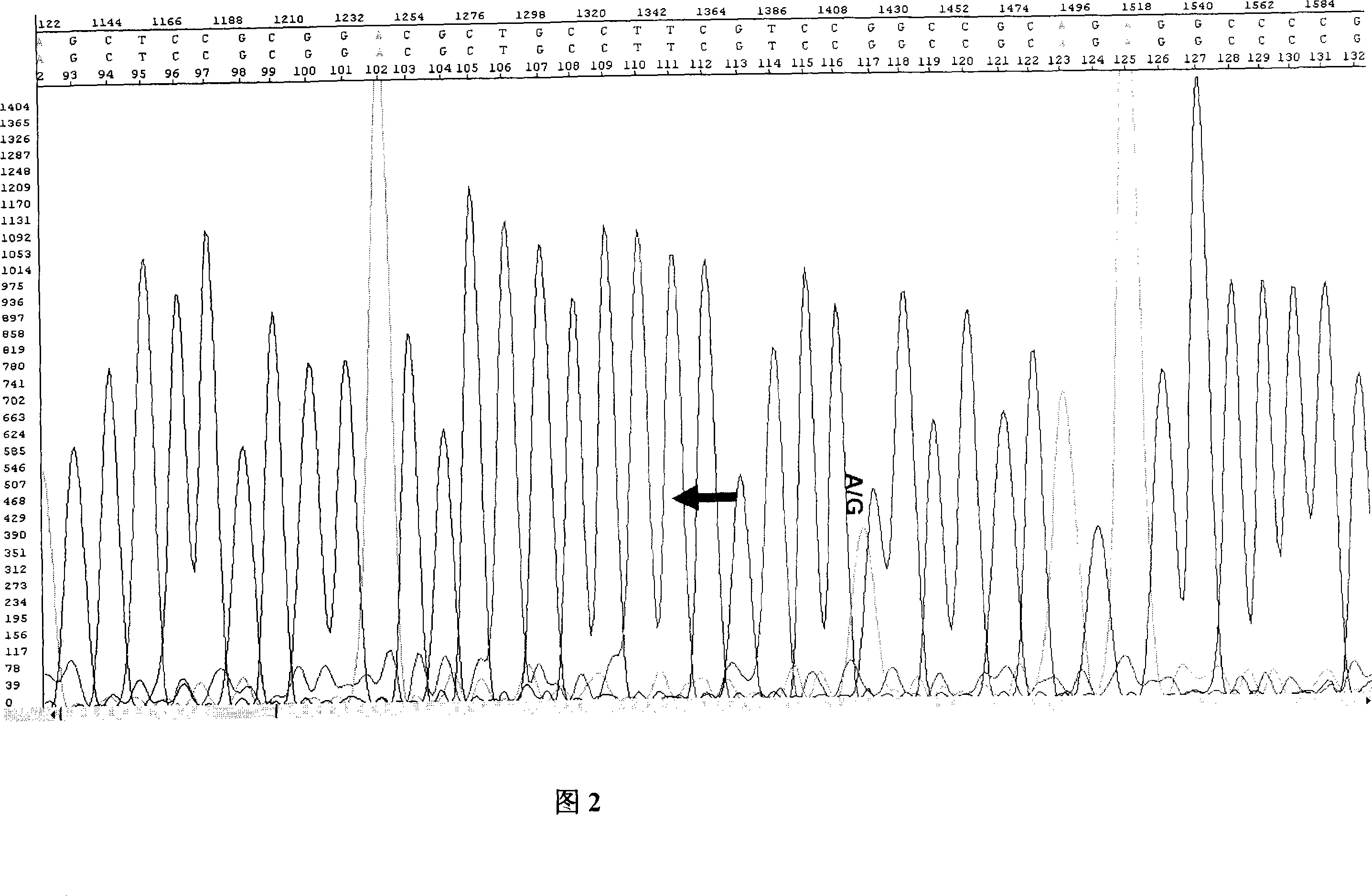
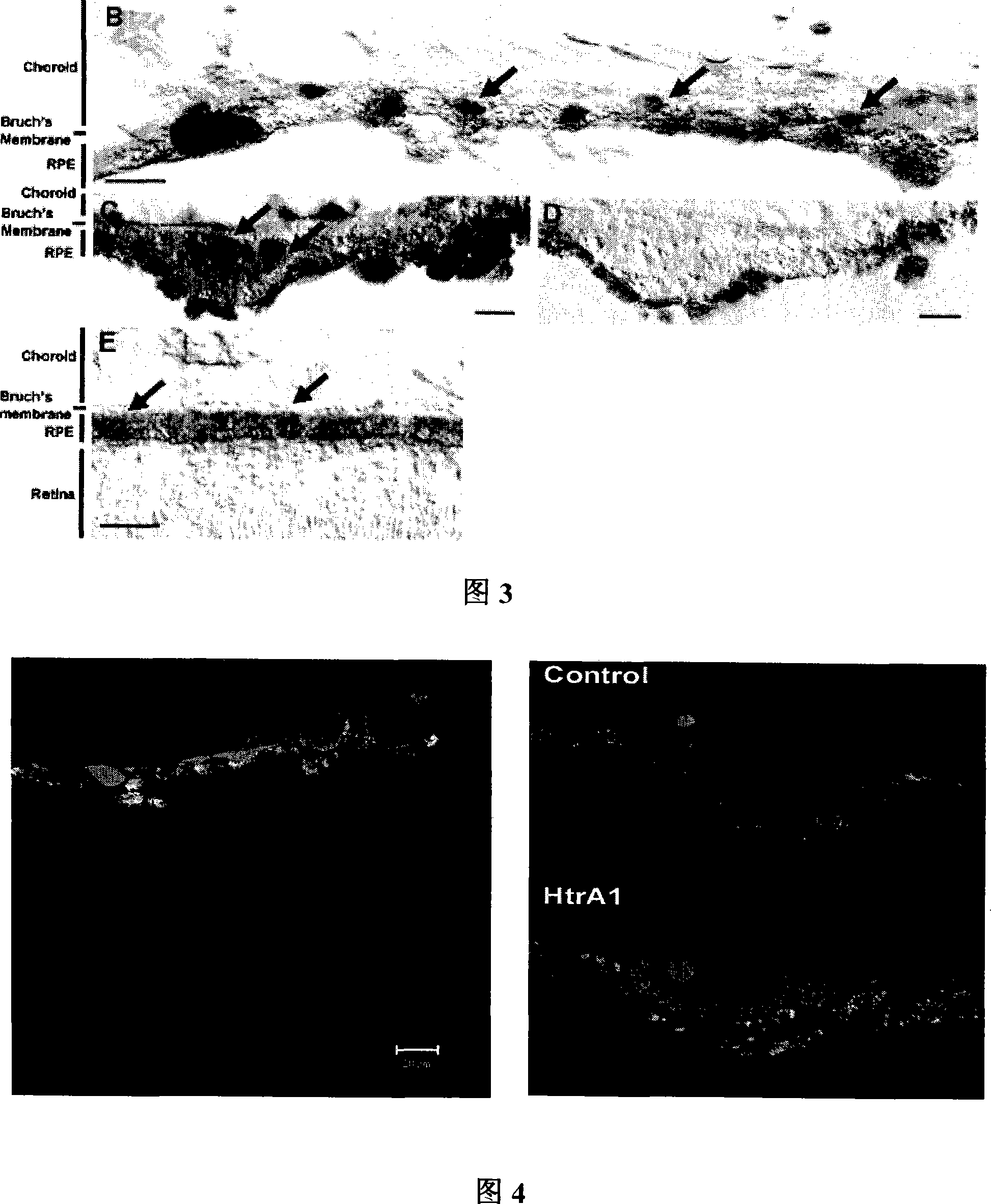
ActiveCN101173314AImprove relevanceLittle visual impairmentMicrobiological testing/measurementMaterial analysisHTRA1 GeneGene mutation analysis
The invention provides a kit for detecting age-related macular degeneration disease, which can be used for early monitoring and detection of AMD high-risk groups, implement early prevention and treatment, and reduce their visual impairment. The kit of the present invention targets the 512G→A mutation of the HTRA1 gene and its related sites / genes, has a high correlation with AMD, and cooperates with the analysis of the CHF gene mutation to improve the accuracy of detection.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
Gene detection kit for prognosing gastric cancer metastasis and use method of gene detection kit
InactiveCN106636366AThe result is objectiveOptimal treatment timeMicrobiological testing/measurementBreast cancer metastasisRAD51
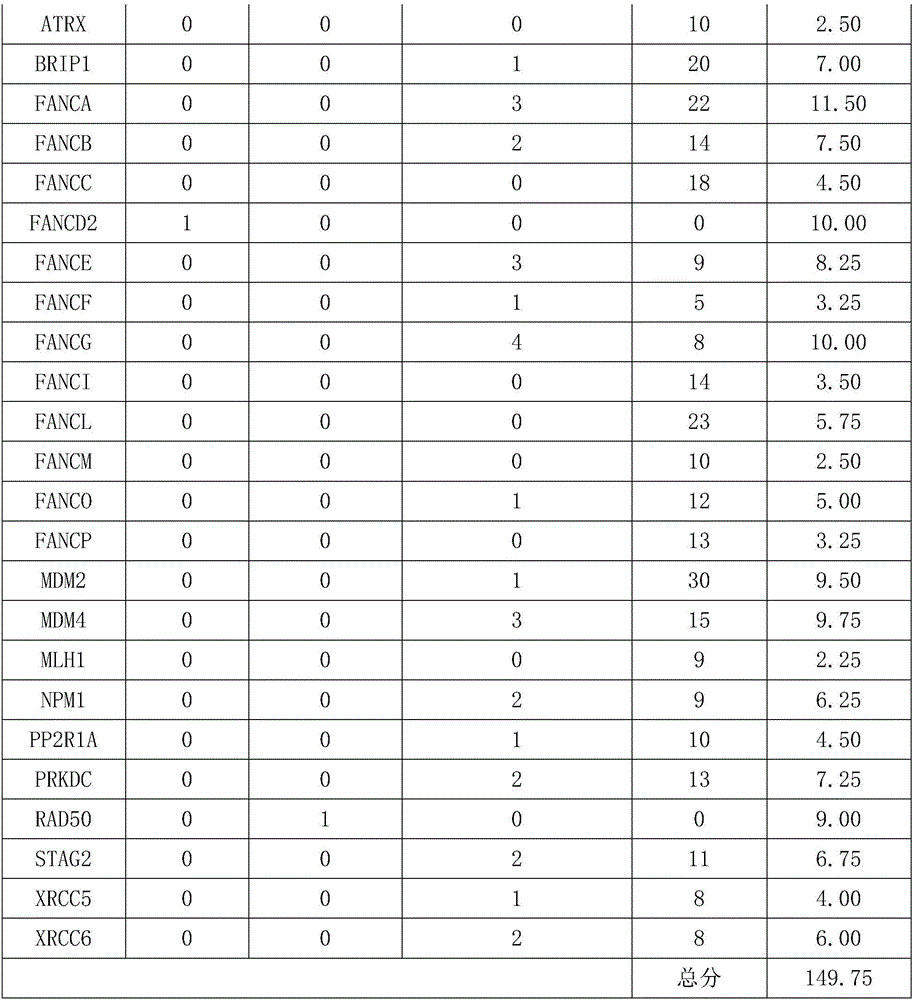
The invention relates to a gene detection kit for prognosing gastric cancer metastasis. The kit comprises a DNA library building kit, wherein the DNA library building kit comprises a high-risk gene probe and a low-risk gene probe; the high-risk gene probe comprises CDH1, CDH2, SNAIL, SLUG, MUC4, MUC6, PRSS3, USP6, MLH1, MSH2, MSH6, PMS2, TGFBR2, MMP2, MMP9, BRCA1, BRCA2, PALB2, ATM, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1 and XRCC1; the low-risk gene probe comprises ATRX, BRIP1, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FANCO, FANCP, MDM2, MDM4, MLH1, NPM1, PP2R1A, PRKDC, RAD50, STAG2, XRCC5 and CRCC6. The invention further discloses a use method of the kit. The use method comprises the following steps: extracting cfDNA in a blood sample; building a library for the cfDNA through the DNA library building kit, and then sequencing the DNA to obtain a gene overall length sequence; carrying out gene mutation analysis on the gene overall length sequence.
Owner:苏州首度基因科技有限责任公司 +1
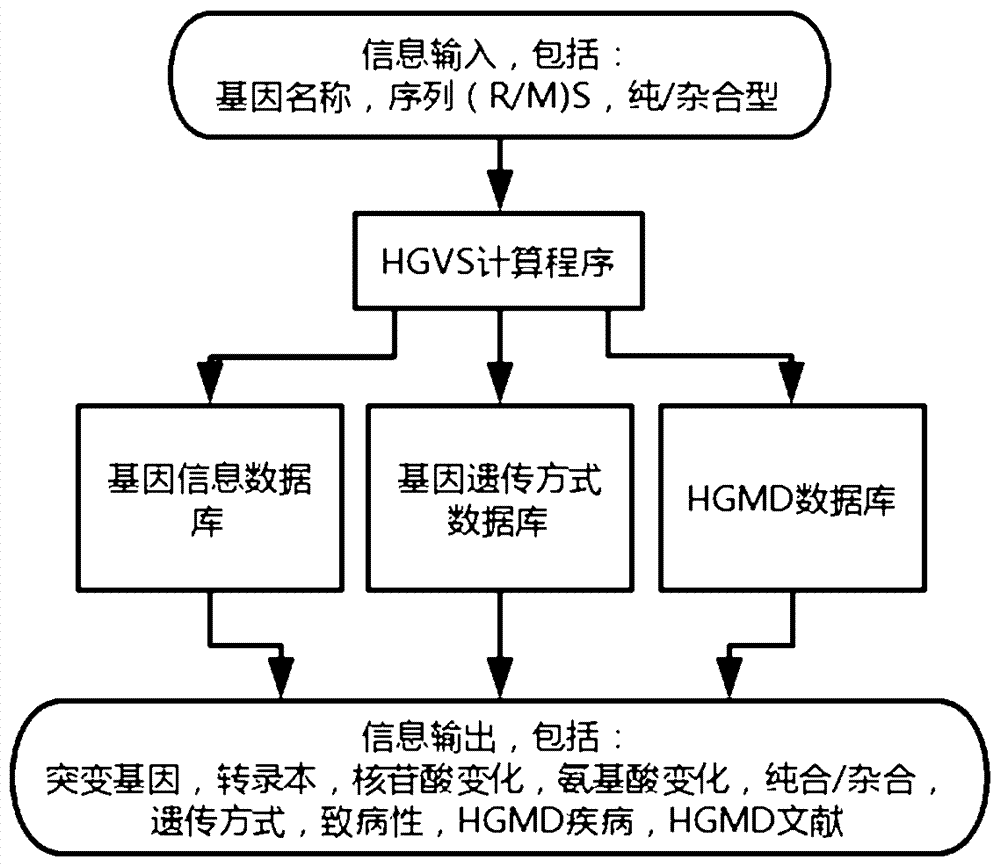
Implementation method for HGVS (Human Geome Variation Society) name generation and analysis system of human gene mutation
The invention discloses an algorithm for the automatic generation module of the HGVS (Human Geome Variation Society) name of gene mutation. The algorithm comprises the following elements: 1) describing a mutation site by a ''gene(R / M)Sz / c'' format, wherein the meaning of the gene(R / M)Sz / c is a gene name, one segment of sequence after a (mutation site reference sequence / mutation site mutation sequence) mutation site and whether the mutation site is a homozygotic type or a hybrid type, and the three parts are separated by a space so as to describe mutation discovered by sequencing; 2) the format is input into a mutation analysis system, a gene information database, a gene inheritance pattern way database, an HGMD database and the like are called through an HGVS calculation program for calculation; and 3) the HGVS name of the gene mutation and relevant information based on the HGVS information are obtained. Through tests, a human gene mutation system based on the algorithm can analyze of mutation of more than 6000 human genes so as to meet the analysis requirements of gene mutation including common genetic diseases, cancers and the like.
Owner:江阴检汇生物科技有限公司
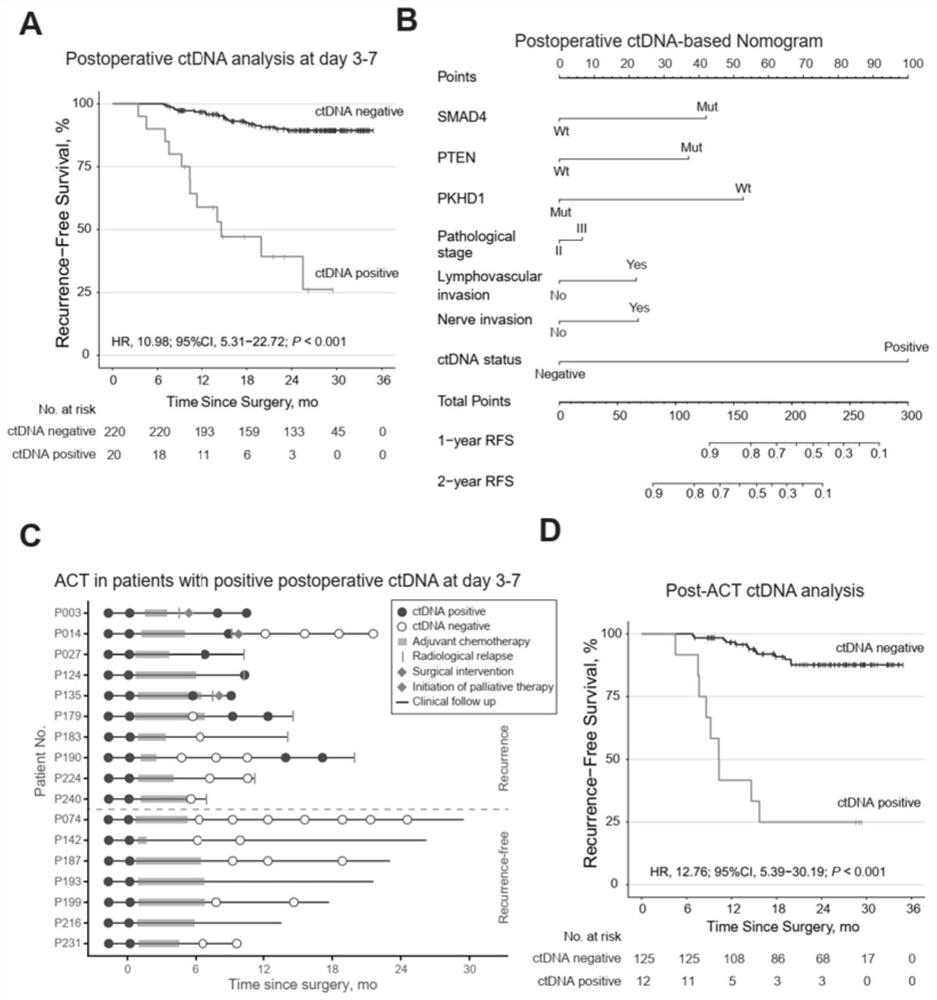
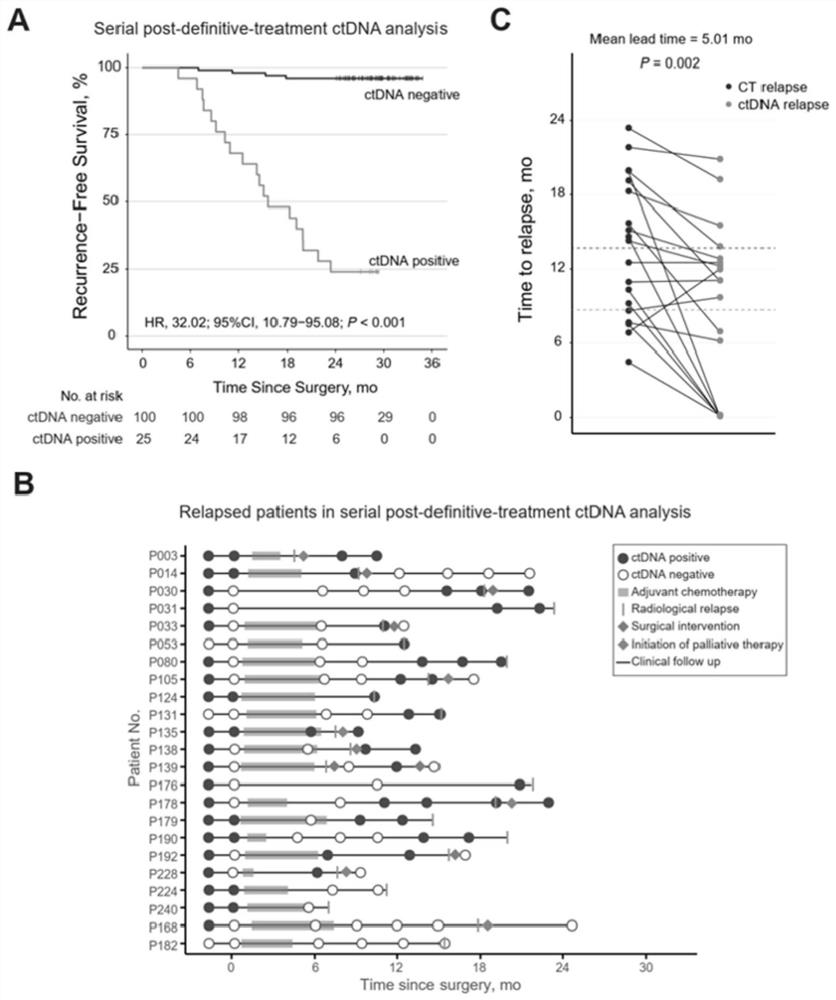
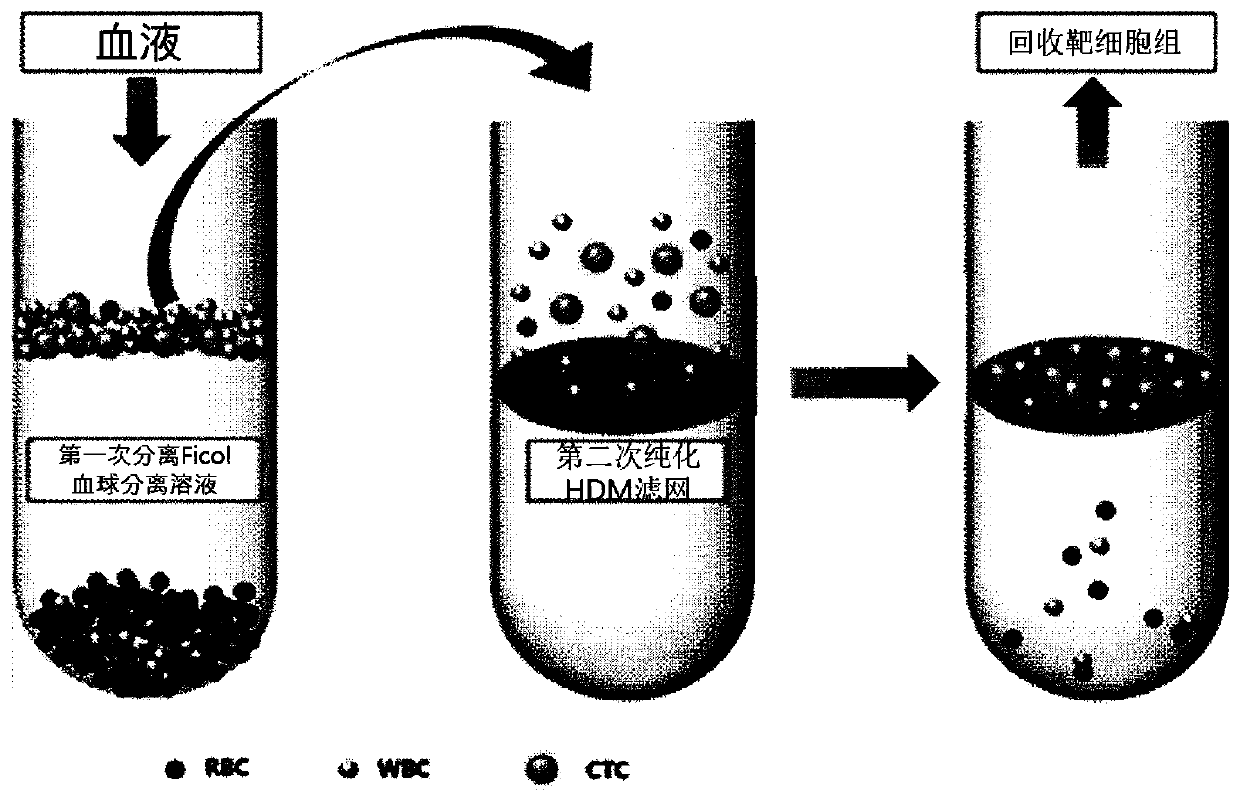
Circulating tumor DNA detection system for screening minimal residual focus after colorectal cancer operation and predicting recurrence risk and application
ActiveCN113284554AEasy to detectShort detection cycleMicrobiological testing/measurementProteomicsGenes mutationGene mutation analysis
The invention relates to a circulating tumor DNA detection system for screening existence of postoperative minimal residual focuses of colorectal cancer patients and predicting recurrence risk. The detection system comprises a colorectal cancer tissue gene mutation screening module, a plasma free DNA gene mutation analysis module and a circulating tumor DNA state judgment module. The system utilizes the same next-generation sequencing gene combination panel to detect primary tumor tissue and plasma free DNA, and considers all mutations detected in the primary tumor tissue of a patient instead of being limited to individual gene mutations, so that the system is more comprehensive. The system can be applied to dynamic and real-time monitoring of circulating tumor DNA, and can be used for evaluating the residual condition of minimal residual lesions after colorectal cancer radical operation, predicting the recurrence risk and guiding the postoperative treatment decision.
Owner:SUN YAT SEN UNIV CANCER CENT
Method for knocking out AAV receptor, HEK293 cell strain with AAV receptor knocked out and application
ActiveCN107245475AReduce lossesIncrease final yieldCell receptors/surface-antigens/surface-determinantsVectorsCytologyCell strain
The invention relates to the field of gene engineering and cytology, in particular to an AAV receptor knocking out method, an HEK293 cell strain with an AAV receptor being knocked out and an application. The knocked-out AAV receptor is KIAA0319L. The knocking out method comprises the following steps: establishing a sgRNA carrier of a targeted AAVR genome sequence; carrying out transfection for a 293 T-cell, and culturing to obtain each clonal cell; carrying out Suveyor gene mutation analysis test; selecting AAVR mutant monoclone; then selecting an AAVR mutant cell strain with high yield of AAV; identifying a mutation condition of other potential mutant site; and enlarging the cell strain without other mutant sites, and establishing a library for production. The final yield of the AAV produced by the HEK293 cell strain of the knocked-out AAV receptor is about 50 percent higher than the yield of the conventional HEK293.
Owner:GUANGZHOU PACKGENE BIOTECH CO LTD
Primer and kit for detecting BRAF gene V600E mutation sites, and PCR method of kit
ActiveCN104846106ADifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationForward primerBraf genes
The invention discloses a primer for detecting BRAF gene V600E mutation sites, and a PCR method of the kit. The primer comprises a wild type specific forward primer, a mutant type specific forward primer, and a reverse primer shared by the wild type specific forward primer and the mutant type specific forward primer, wherein the wild type specific forward primer has a sequence shown as SEQ No.17; the mutant type specific forward primer has a sequence shown as SEQ No.14; and the shared reverse primer has a sequence shown as SEQ No.16. The kit has the advantages of simple detection, rapidness, accuracy, low price and the like and provides a powerful tool for scientific research and clinical detection of BRAF gene V600E mutation sites and gene mutation analysis.
Owner:沈阳优吉诺生物科技有限公司
Method for detecting gene mutation detection results with relatively high credibility
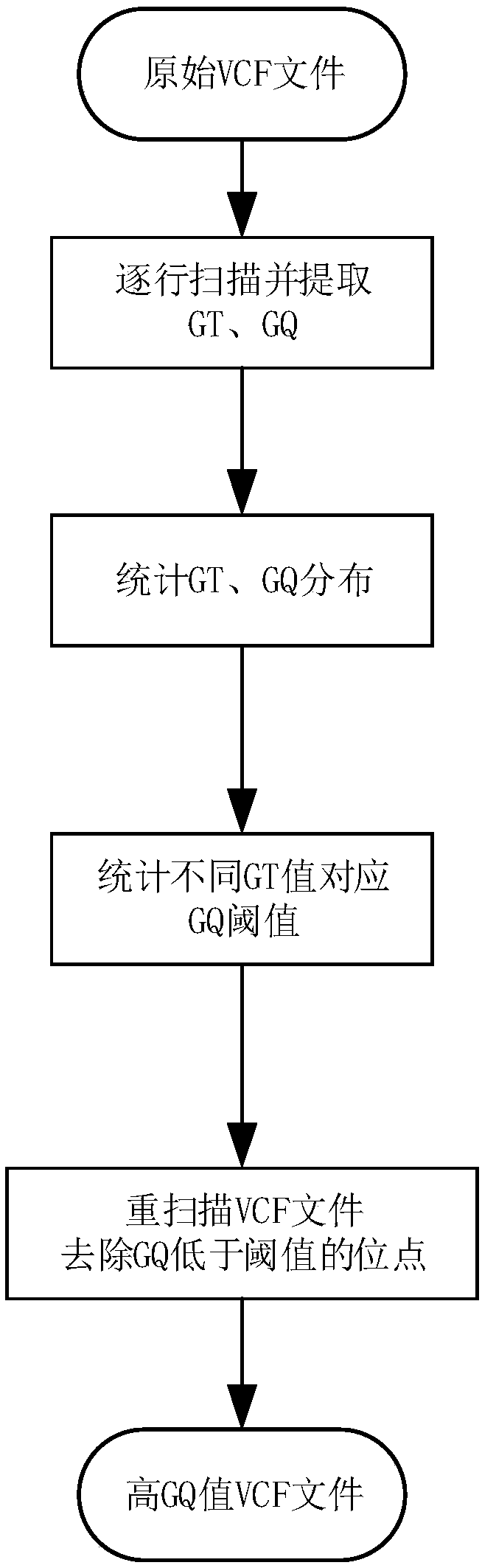
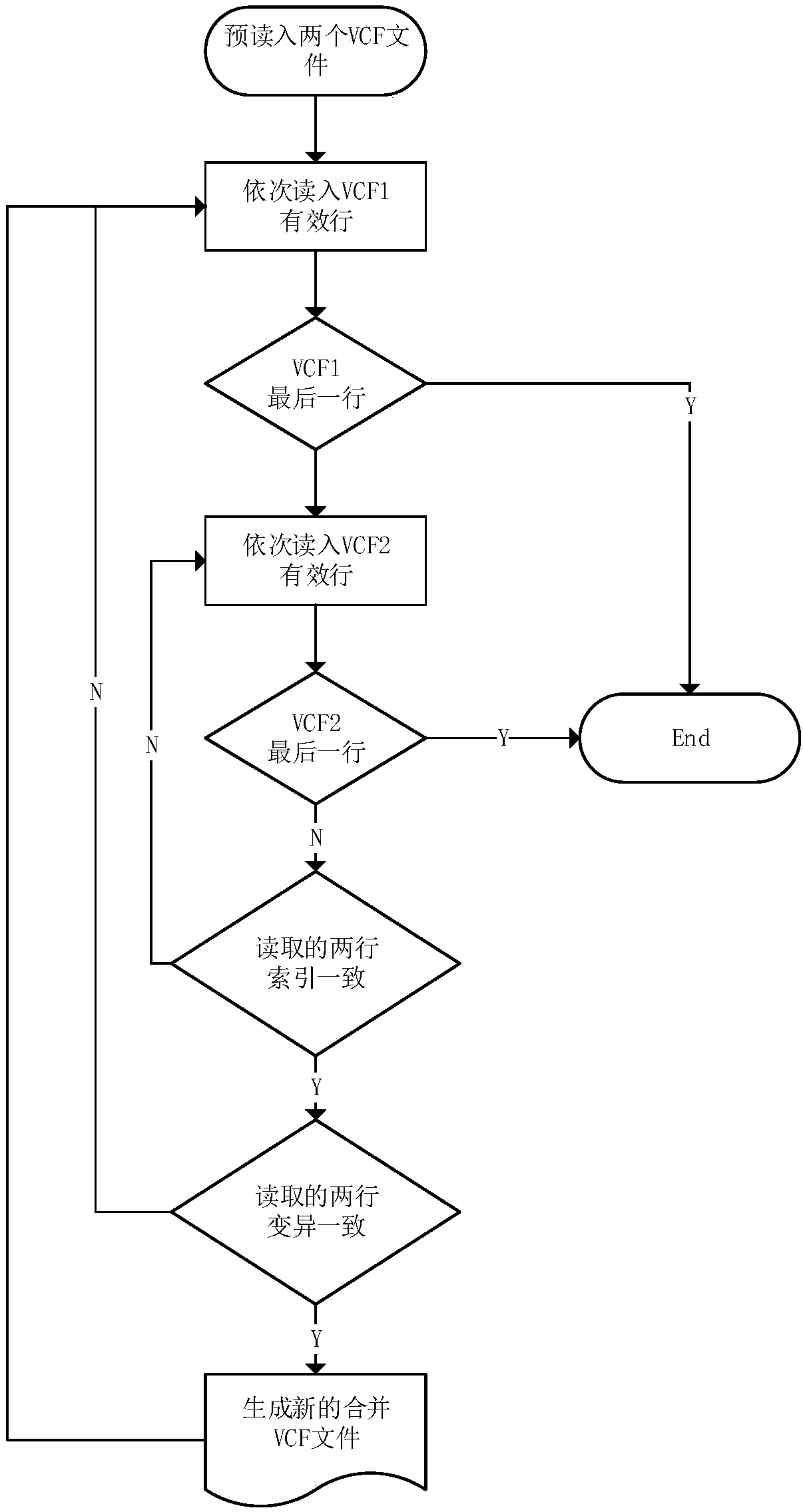
The invention discloses a method for generating gene mutation detection results with relatively high credibility by utilizing existing gene mutation detection analysis results. The method comprises the following steps of: carrying out statistical analysis on gene types recorded in variation points in each file and distribution condition of quality information by utilizing VCF-format point mutationdata files generated by gene mutation analysis software, and removing the points with low gene type quality; and synthesizing the mutation point data files generated by two software, extracting points with same variation at the same position so as to generate gene mutation data files with relatively high credibility when being compared with existing gene mutation detection analysis results, and using the gene mutation data files for the subsequent analysis process.
Owner:杭州米天基因科技有限公司 +1
Primer, kit as well as PCR method for detecting of D816V mutation site of C-KIT gene
ActiveCN104846108ADifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationForward primerType specific
The invention discloses a primer, a kit as well as a PCR method for detecting a D816V mutation site of a C-KIT gene. The primer comprises a wild-type specific forward primer, a mutant-type specific forward primer and a reverse primer which are shared by the wild-type specific forward primer and the mutant-type specific forward primer, wherein the wild-type specific forward primer has a sequence shown in SEQ No.17, the-mutant type specific forward primer has a sequence shown in SEQ No.14, and the shared reverse primer has a sequence shown in SEQ No.16. The kit has the advantages of being simple, rapid, accurate and cheap in detection; and a powerful instrument is provided for scientific research and clinical D816V site typing and gene mutation analysis of the C-KIT gene.
Owner:沈阳优吉诺生物科技有限公司
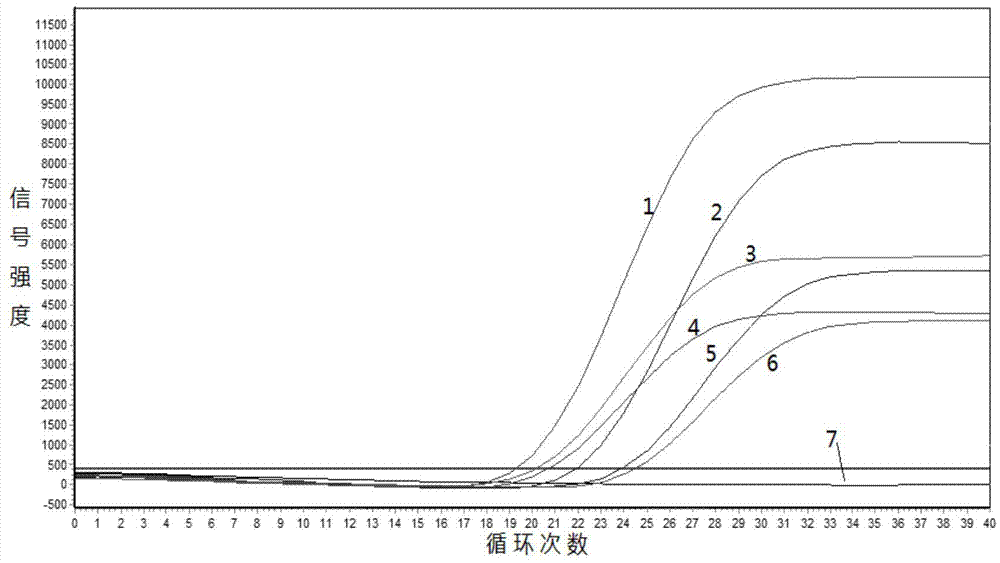
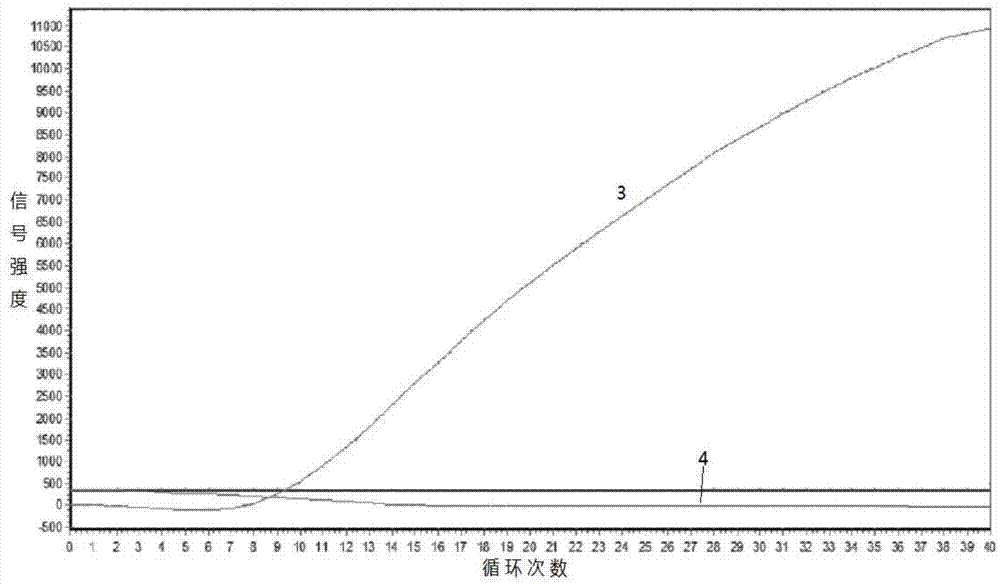
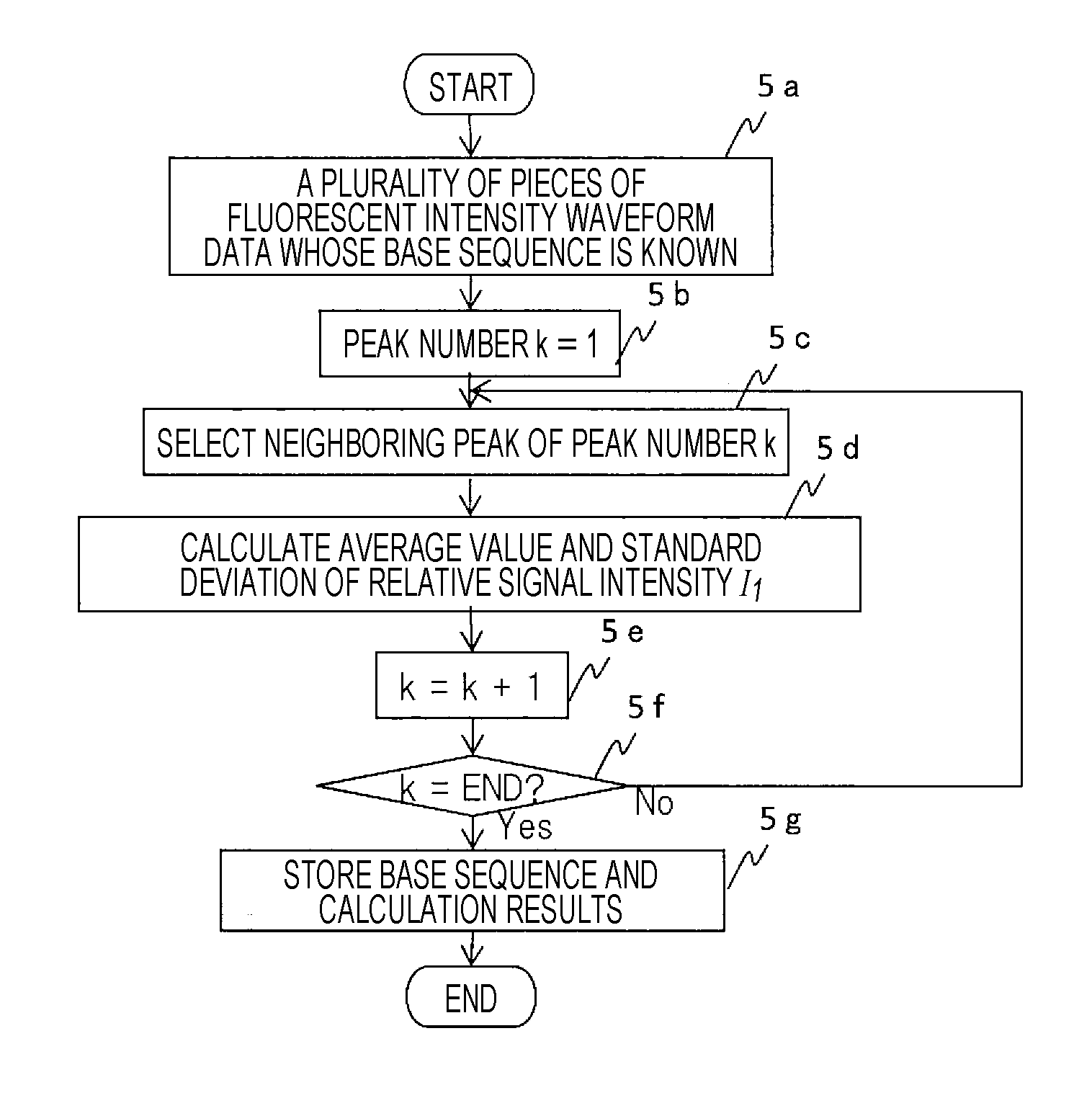
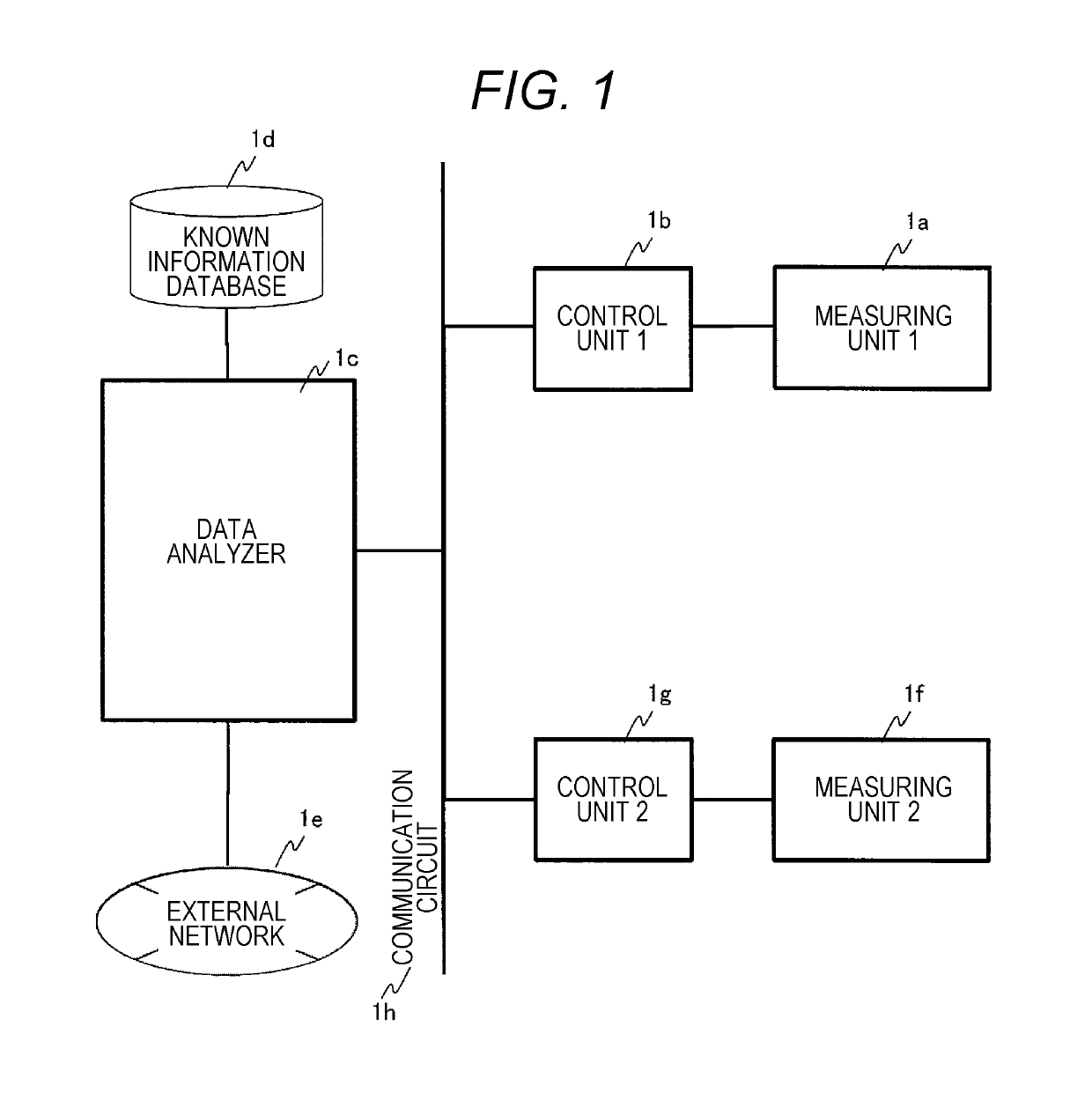
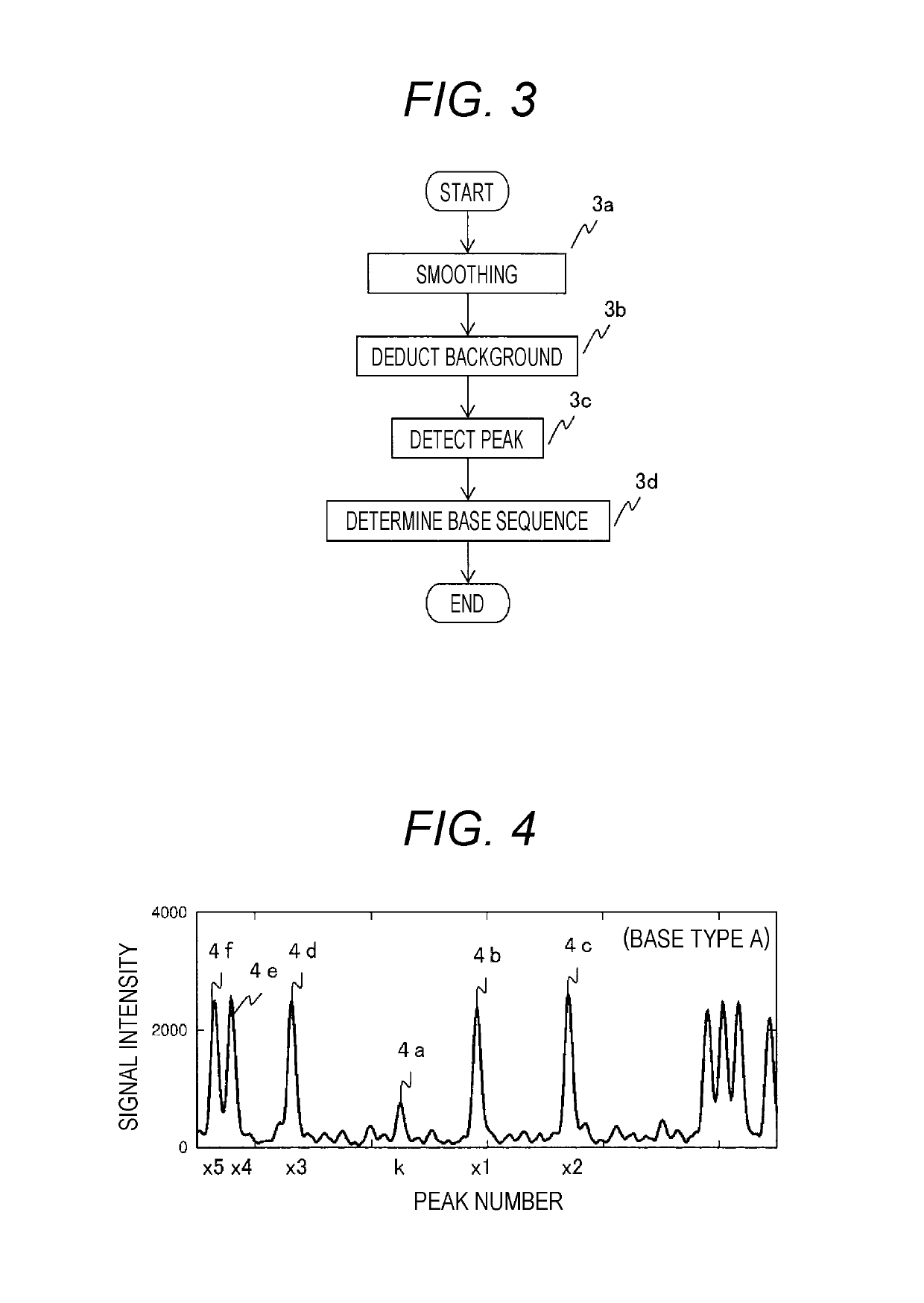
Gene Mutation Analyzer, Gene Mutation Analysis System, and Gene Mutation Analysis Method
ActiveUS20160187292A1Improve accuracySludge treatmentComponent separationGenes mutationElectrophoresis
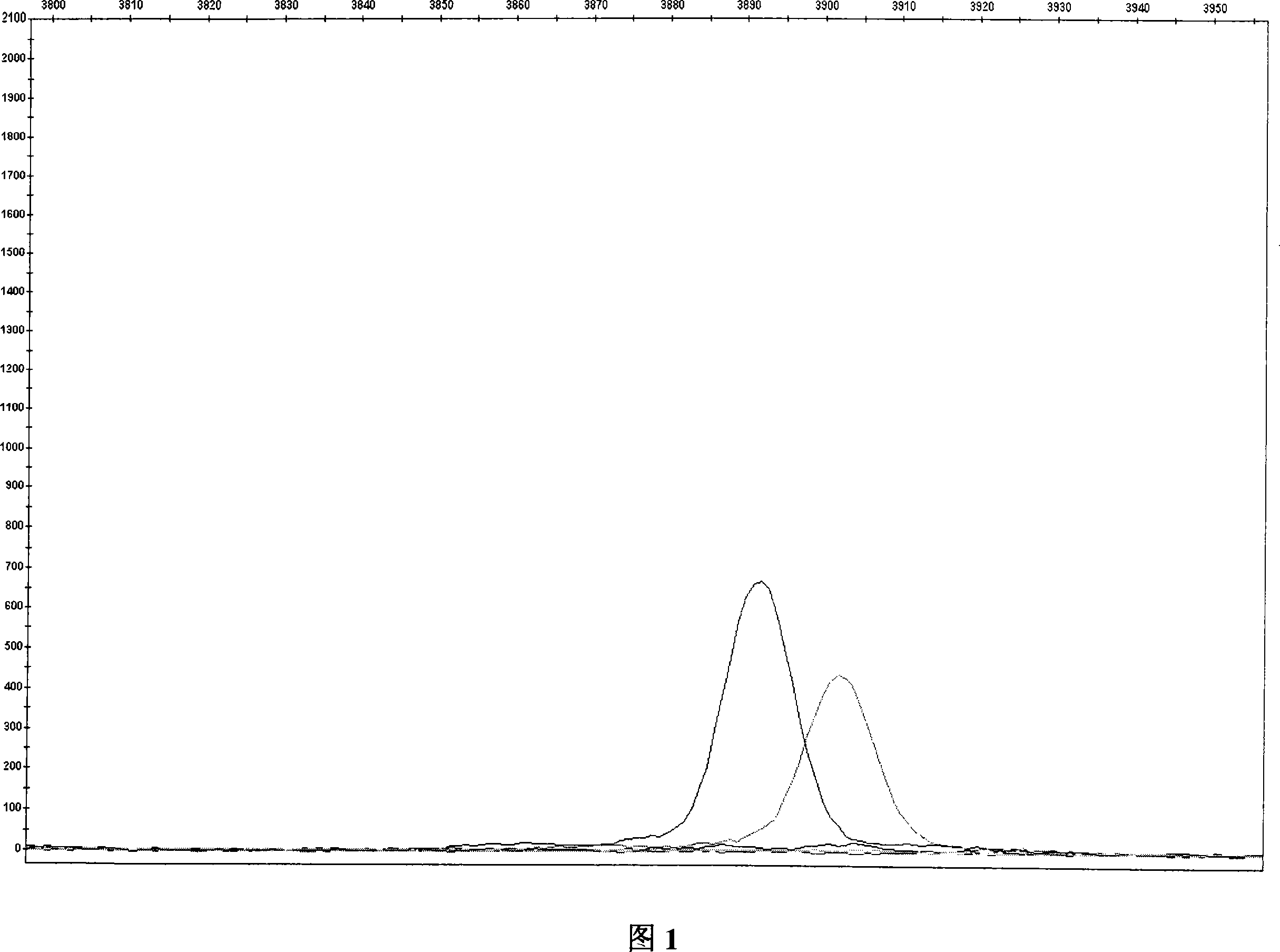
An aspect of the present invention includes performing electrophoresis of a nucleic acid sample to be analyzed labeled for each base type, generating waveform data of detected intensity by detecting a label signal for the each base type, selecting another peak position for each peak position of the waveform data for each base type, calculating relative signal intensity of signal intensity in each position relative to the signal intensity in the other selected position, and analyzing existence of each base type in a base sequence coordinate position of the nucleic acid sample by comparing the relative signal intensity of the nucleic acid sample to be analyzed and the relative signal intensity of a known nucleic acid sample in each peak position. Accordingly, acquiring information about a gene mutation in trace amounts existing in a target gene region highly sensitively with high precision is realized.
Owner:HITACHI LTD
Kit for quantitative detection of egfr gene mutation and use thereof
The invention relates to a high-sensitivity kit for quantitatively detecting EGFR (Epidermal Growth Factor Receptor) gene mutation and application thereof. The application comprises the following steps: respectively designing a forward primer and a backward primer in a sequence with the length of 50-100bp in a specific amplification gene mutation site area aiming at a to-be-detected gene template, designing the mutation sites at corresponding positions of the forward primer, and designing competitive Block oligonucleotides for inhibiting non-mutant amplification nearby the mutation sites. The detection is performed by utilizing the specificity aiming at a probe of the amplification product, and the gene mutation conditions are obtained by detecting the detectable markers on the probe. The invention provides a gene mutation analysis method which is stable in result, high in sensitivity and high in repeatability.
Owner:GENOSABER BIOTECH CO LTD +1
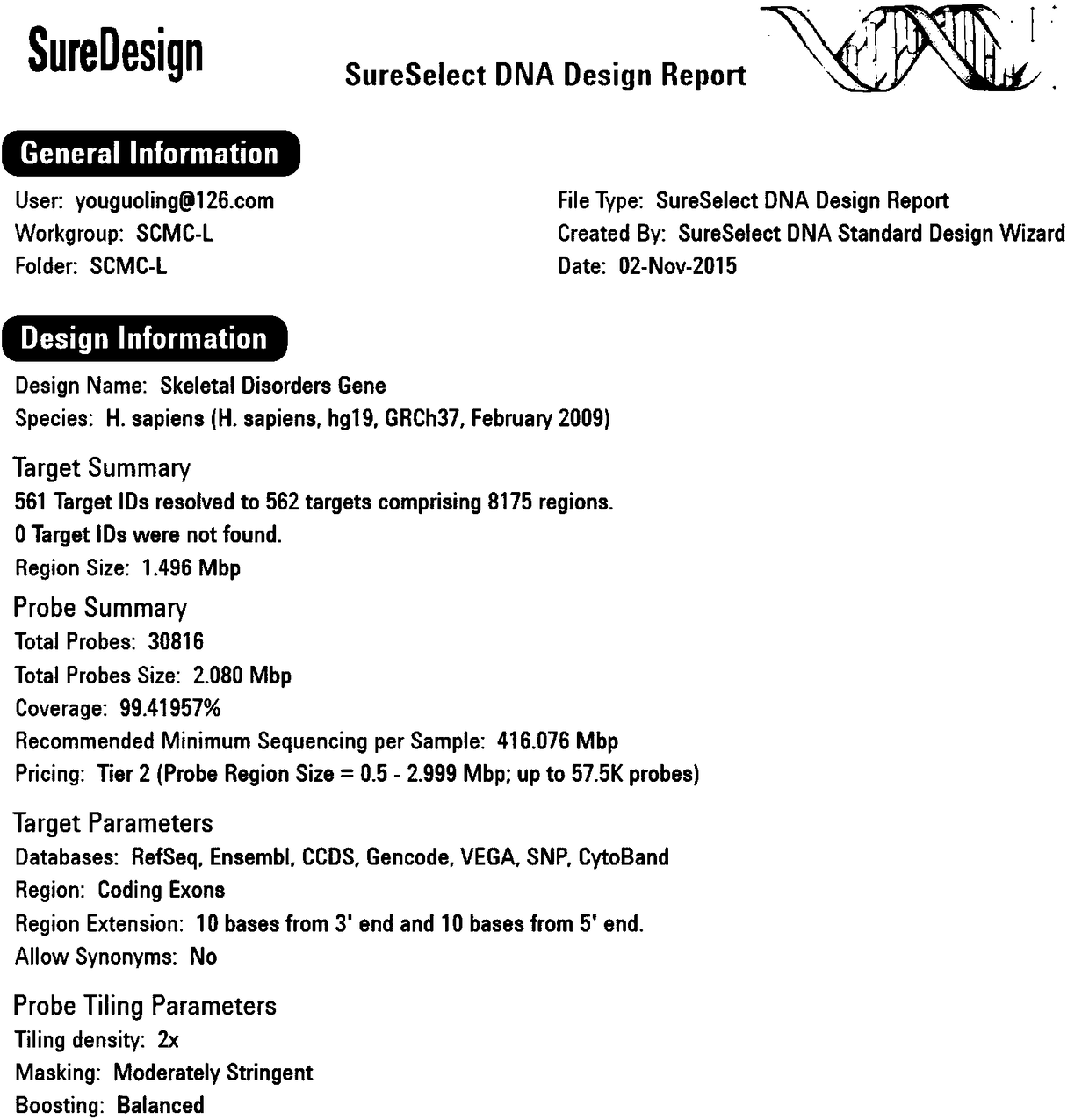
Molecular diagnostic method and kit of genetic bone diseases
InactiveCN108570496ALow costAccurate diagnosisMicrobiological testing/measurementPrenatal diagnosisGene mutation analysis
The invention relates to a molecular diagnostic method and kit of genetic bone diseases. The method includes using a capture probe aiming at 561 genetic bone disease related genes and applying a target gene high throughput parallel targeting sequencing method to determine the sequences of the corresponding genes of subjects. The kit includes the capture probe which aims at 561 genetic bone diseaserelated genes. The method has advantages of high throughput, high sensitivity and high singularity; the cost of analyzing pathogenic gene mutation of the genetic bone diseases can be rapidly reduced;and the method has practical clinical significance, is helpful for the drawing of genetic bone disease gene mutation spectrums and the explication of the molecular pathogenesis of the genetic bone diseases, and provides theoretical foundations of early accurate diagnosis, precision treatment, prenatal diagnosis and genetic counseling for the genetic bone diseases.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
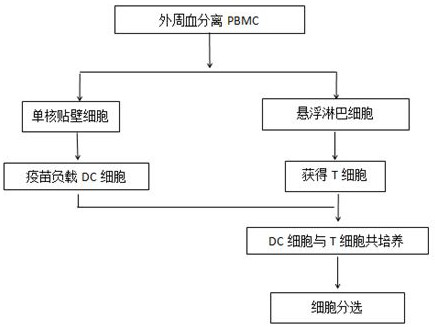
Tumor neoantigen-based T cell sorting preparation technology and application thereof
PendingCN112300988ACell dissociation methodsMammal material medical ingredientsGenes mutationAntigen
The invention discloses a tumor neoantigen-based T cell sorting preparation technology and application thereof in the technical field of molecular biology, and the T cell sorting preparation technology based on tumor neoantigen and application thereof comprise the following steps: S1: collecting a tumor specimen, analyzing tumor gene mutation through gene sequencing, and collecting tumor tissues of a patient by means of surgery, puncture, intervention and the like; performing sequencing and detecting gene mutation through methods such as full-exon sequencing and probe recovery method sequencing, S2: analyzing the tumor neoantigen according to the gene mutation, and analyzing the tumor neoantigen according to information such as the gene mutation, HLA affinity and gene expression. The T cell sorting preparation technology based on the tumor neoantigen and the application of the T cell sorting preparation technology are characterized in that NRT cells are sorted out based on tumor neoantigens, pure NRT cells are obtained, and preparations are made for subsequent cell treatment in the aspects of safety, cost and effectiveness.
Owner:浙江格源致善生物医药科技有限公司
Kit for detecting drug sensitivity of rectal cancer cells based on gene mutation analysis
The invention discloses a kit for detecting drug sensitivity of rectal cancer cells based on gene mutation analysis, which comprises the following substances: (1) a rectal cancer cell drug reagent, (2) a magnetic nanoparticle indicating liquid A with a green fluorescent protein labeling a wild P53 protein antibody for surface modification, (3) a magnetic nanoparticle indicating liquid B with a redfluorescent protein labeling an anti-mutation P53 protein antibody for surface modification and (4) a substrate medium and a dilution buffer. The sensitivity of a patient to the drug is measured through simultaneous calculation of the expression levels of the wild P53 gene protein and the mutant P53 gene protein, the individualization is more prominent, the detection result is more accurate, a sensitive drug is screened for the treatment of the disease, and an optimal chemotherapy regimen is selected.
Owner:THE AFFILIATED HOSPITAL OF GUIZHOU MEDICAL UNIV
Detection of mispairing ratio of DNA polyase in PCR by denatured gradient gel electrophoresis
InactiveCN1438483AMicrobiological testing/measurementMaterial analysis by electric/magnetic meansElectrophoresisGene mutation analysis
The method incldues following steps. (1) The specific strain is selected to be as the strain used in experiments. (2) After purifying and separating the strains, the selected single colonly liquid is used to cultivate the thallus. (3) The purified gene group DNA of the distilled specific strain is utilized as the template of PCR reaction. (4) After selecting the specificity primer, using different DNa polymerase to be tested carries out the PCR amplification. (5) The denaturation gradient gel electrophoresist separates the PCR products. (6) With the electrophoresis being finished, dyeing bands and counting the number of the each band of specimens obtains the mismatching rate for different DNA polymerases. The invention provides the means for testing the mismatching rate so as to assure the accuracy of the experiments.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Specific primer for detecting phenylalanine hydroxylase gene mutation spectrums
PendingCN108330190AMicrobiological testing/measurementDNA/RNA fragmentationExonGene mutation analysis
The invention discloses a specific primer for detecting phenylalanine hydroxylase gene mutation spectrums, and belongs to the technical field of molecular biology. The specific primer comprises 13 primers. An amplification region includes 13 exons and flanking regions thereof. The genetic primer has corresponding specificity and proper length, efficient amplification is realized, secondary structures and hairpin structures are not formed, and fake triggering is avoided. Propositus PAH (phenylalanine hydroxylase) gene mutation analysis is performed through the primer according to a layering research method, and the method is simple, efficient, economical and practical. By the specific primer, an approach is provided for research on PKU (phenylketonuria) pathogenesis molecular mechanisms.
Owner:XUZHOU MATERNITY & CHILD HEALTH CARE HOSPITAL
Primers, kit and pcr method for detecting the v600e mutation site of braf gene
ActiveCN104846106BDifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationForward primerAgricultural science
The invention discloses a primer for detecting BRAF gene V600E mutation sites, and a PCR method of the kit. The primer comprises a wild type specific forward primer, a mutant type specific forward primer, and a reverse primer shared by the wild type specific forward primer and the mutant type specific forward primer, wherein the wild type specific forward primer has a sequence shown as SEQ No.17; the mutant type specific forward primer has a sequence shown as SEQ No.14; and the shared reverse primer has a sequence shown as SEQ No.16. The kit has the advantages of simple detection, rapidness, accuracy, low price and the like and provides a powerful tool for scientific research and clinical detection of BRAF gene V600E mutation sites and gene mutation analysis.
Owner:沈阳优吉诺生物科技有限公司
Eml4-alk gene mutation analysis method
PendingCN110997942AEasy to confirm dosingMicrobiological testing/measurementDisease diagnosisAnticarcinogenOncology
According to an embodiment of the present invention, a qRT-PCR primer capable of detecting blood-borne circulating cancer cell-based EML4-ALK gene mutations at a higher sensitivity detection limit than existing methods is provided. According to another embodiment of the present invention, EML4-ALK gene mutations can be detected using blood-borne circulating lung cancer tumor cells even in the caseof lung cancer patients for whom ALK-FISH testing has been impossible due to the difficulty of solid biopsy collection of lung cancer tissue, and thus a lung cancer patient screening method for determining whether an anticancer drug targeted to EML4-ALK gene mutations can be applied to a lung cancer patient is provided.
Owner:CYTOGEN CORP
A circulating tumor dna detection system and its application for screening minimal residual lesions after colorectal cancer surgery and predicting recurrence risk
ActiveCN113284554BReduce testing costsEasy to detectMicrobiological testing/measurementProteomicsGenes mutationPlasma cell
The invention relates to a circulating tumor DNA detection system for screening the existence of postoperative minimal residual lesions in colorectal cancer patients and predicting the risk of recurrence. The detection system includes a colorectal cancer tissue gene mutation screening module, a plasma cell-free DNA gene mutation analysis module, and a circulating tumor DNA status judgment module. The system uses the same next-generation sequencing gene panel to detect primary tumor tissue and plasma cell-free DNA, and considers all mutations detected in the patient's primary tumor tissue, rather than being limited to individual gene mutations, which is more comprehensive. This system can be applied to the dynamic and real-time monitoring of circulating tumor DNA, and can be used to assess the residual status of minimal residual lesions after radical resection of colorectal cancer, predict the risk of recurrence, and guide postoperative treatment decisions.
Owner:SUN YAT SEN UNIV CANCER CENT
A method for knocking out aav receptor, hek293 cell line knocking out aav receptor and application thereof
ActiveCN107245475BReduce lossesIncrease final yieldVectorsCell receptors/surface-antigens/surface-determinantsCytologyCell strain
The invention relates to the field of gene engineering and cytology, in particular to an AAV receptor knocking out method, an HEK293 cell strain with an AAV receptor being knocked out and an application. The knocked-out AAV receptor is KIAA0319L. The knocking out method comprises the following steps: establishing a sgRNA carrier of a targeted AAVR genome sequence; carrying out transfection for a 293 T-cell, and culturing to obtain each clonal cell; carrying out Suveyor gene mutation analysis test; selecting AAVR mutant monoclone; then selecting an AAVR mutant cell strain with high yield of AAV; identifying a mutation condition of other potential mutant site; and enlarging the cell strain without other mutant sites, and establishing a library for production. The final yield of the AAV produced by the HEK293 cell strain of the knocked-out AAV receptor is about 50 percent higher than the yield of the conventional HEK293.
Owner:GUANGZHOU PACKGENE BIOTECH CO LTD
Gene mutation analyzer, gene mutation analysis system, and gene mutation analysis method
ActiveUS10274459B2Improve accuracyComponent separationMicrobiological testing/measurementGenes mutationElectrophoresis
An aspect of the present invention includes performing electrophoresis of a nucleic acid sample to be analyzed labeled for each base type, generating waveform data of detected intensity by detecting a label signal for the each base type, selecting another peak position for each peak position of the waveform data for each base type, calculating relative signal intensity of signal intensity in each position relative to the signal intensity in the other selected position, and analyzing existence of each base type in a base sequence coordinate position of the nucleic acid sample by comparing the relative signal intensity of the nucleic acid sample to be analyzed and the relative signal intensity of a known nucleic acid sample in each peak position. Accordingly, acquiring information about a gene mutation in trace amounts existing in a target gene region highly sensitively with high precision is realized.
Owner:HITACHI LTD
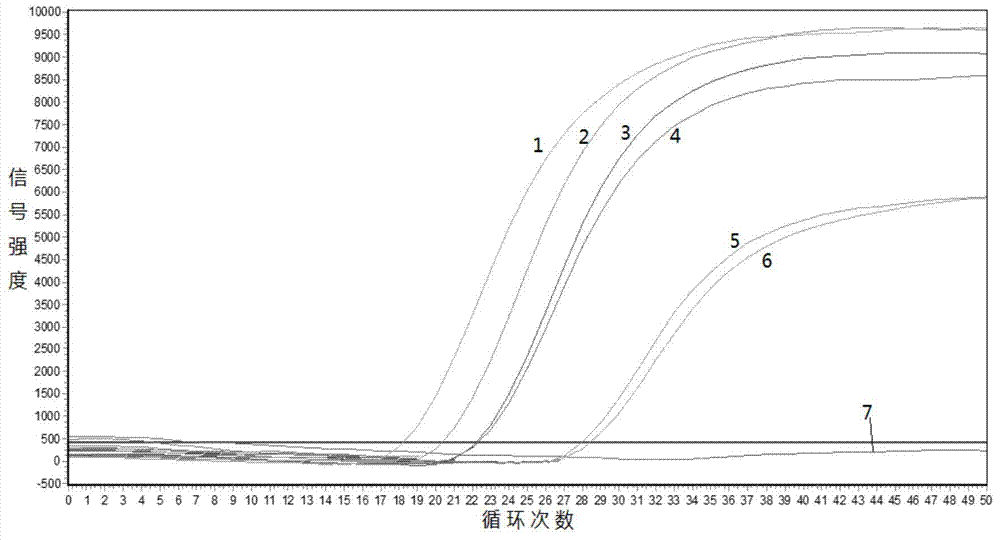
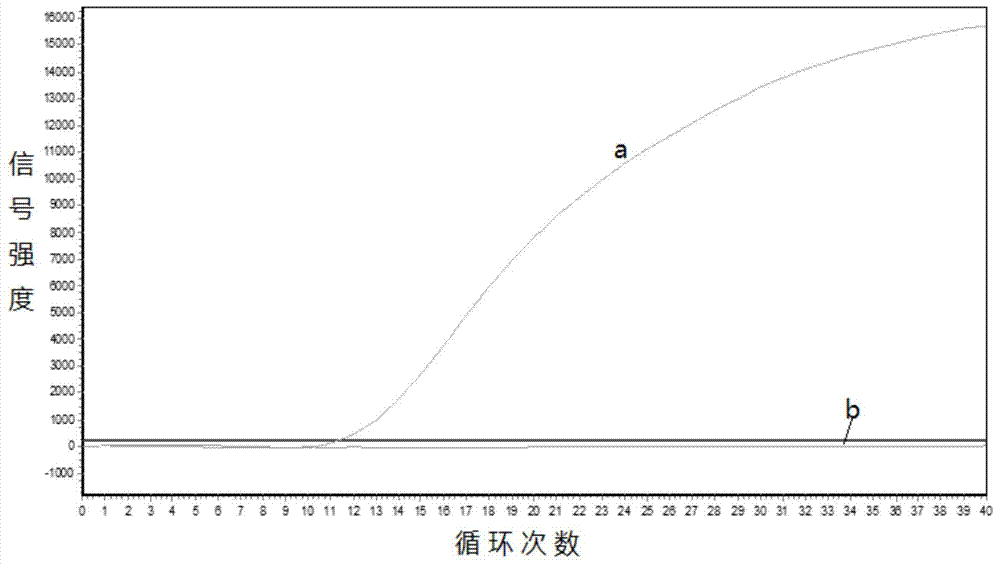
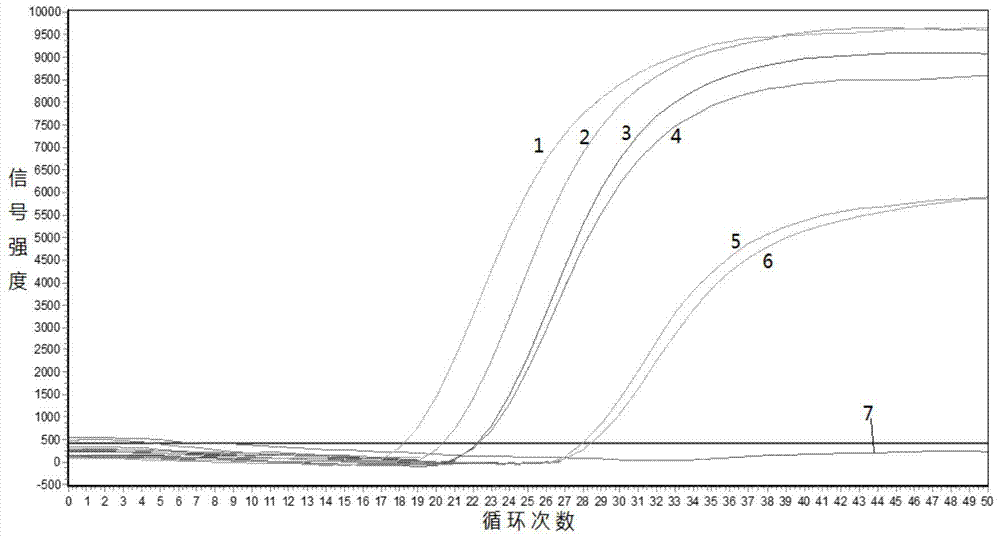
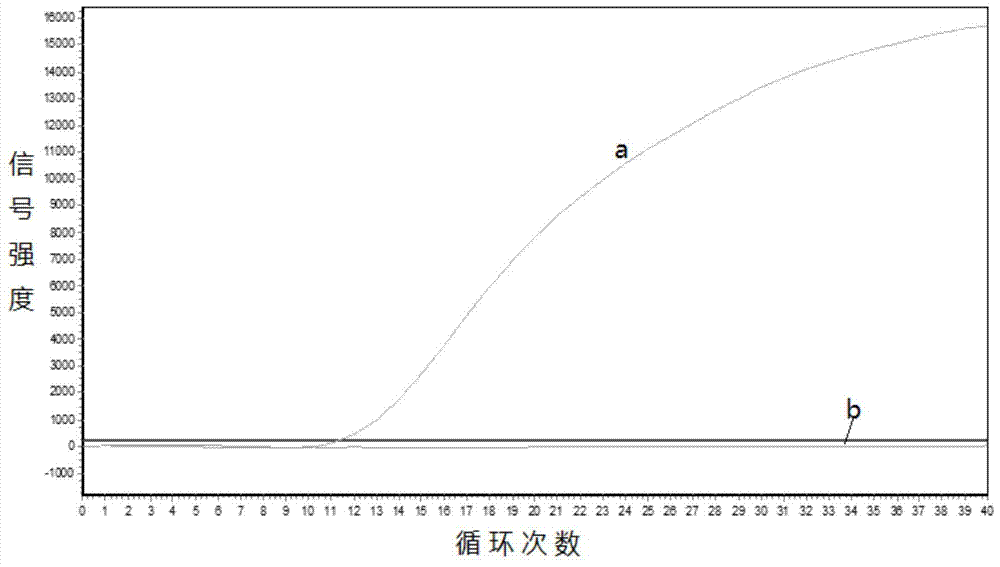
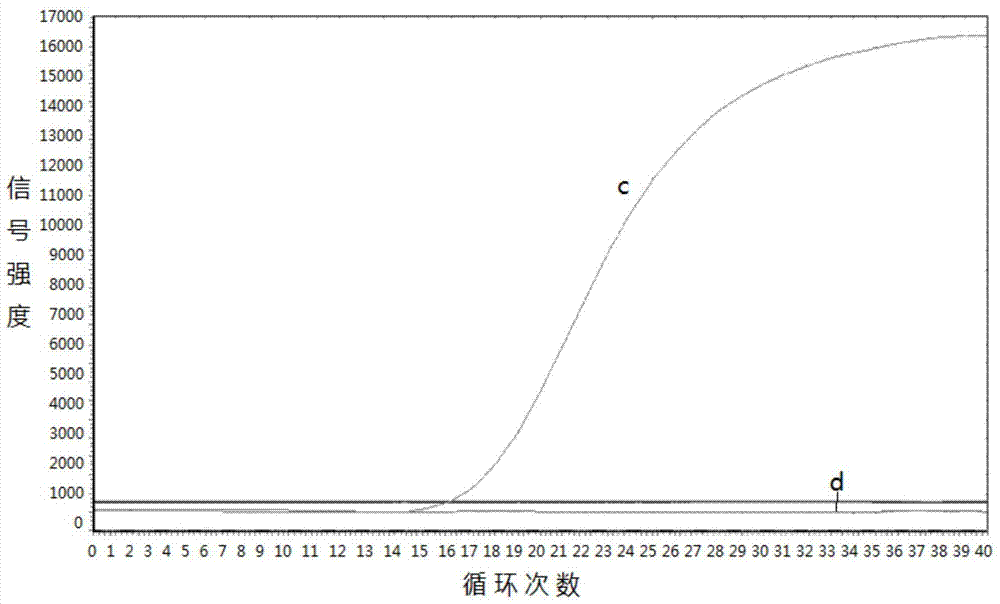
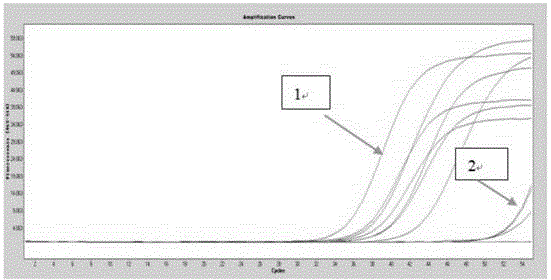
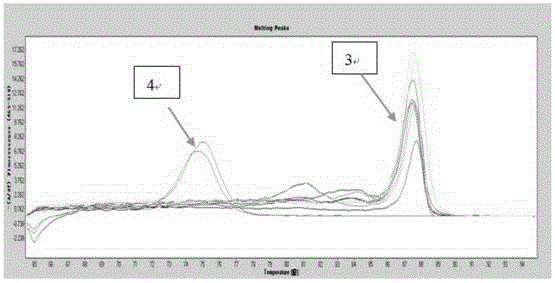
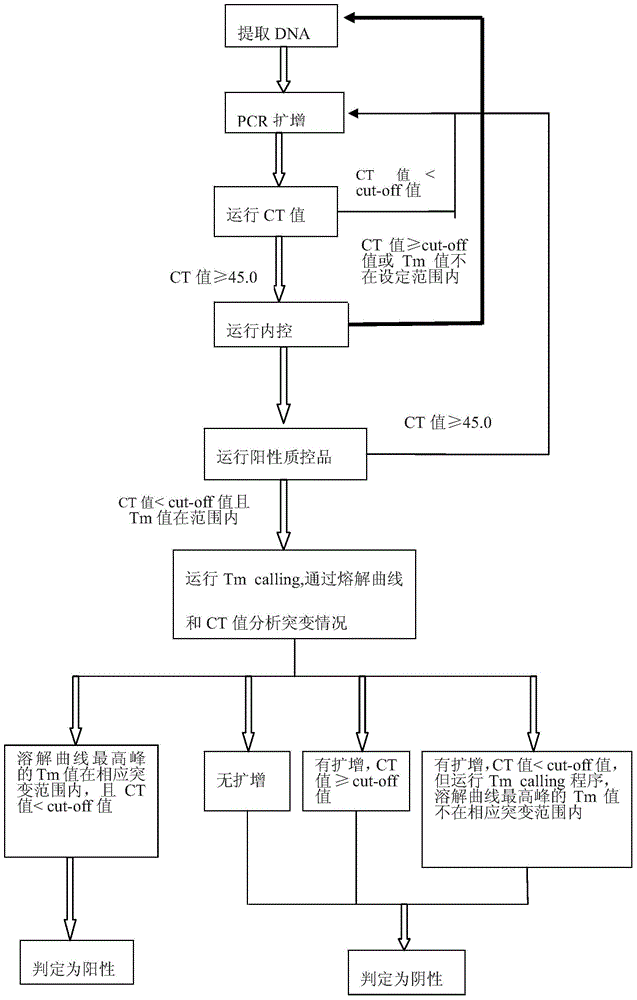
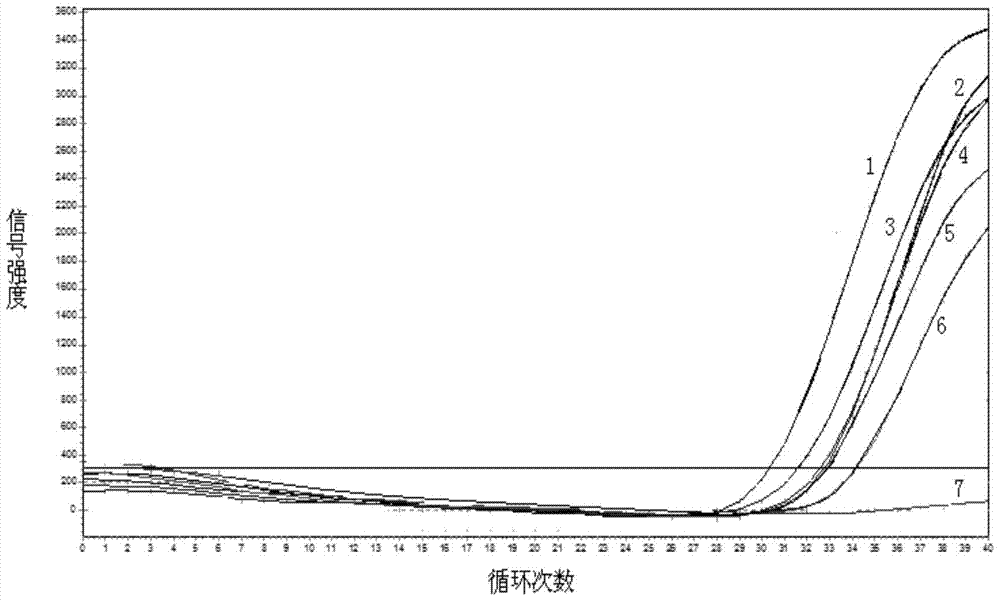
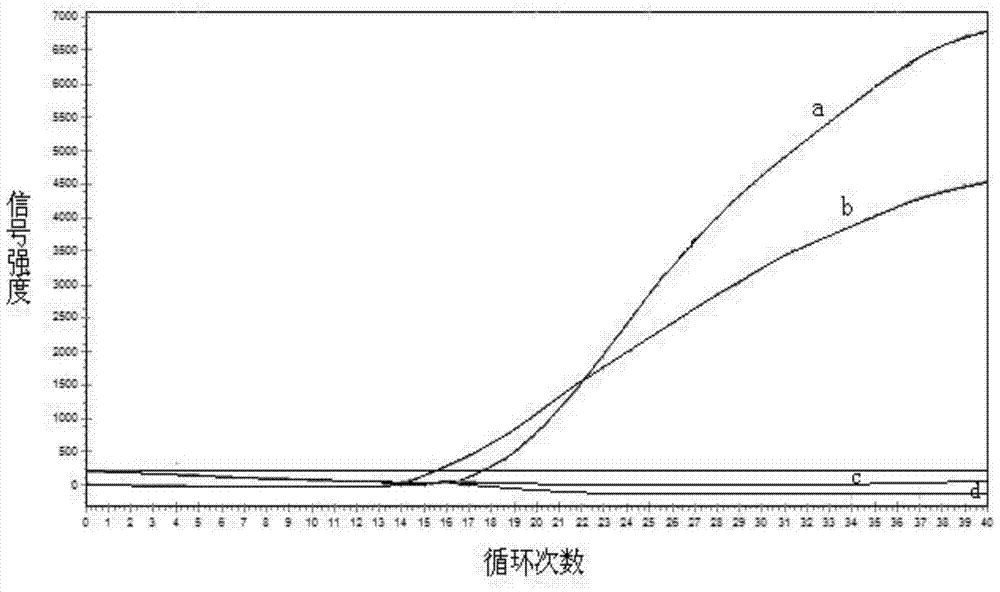
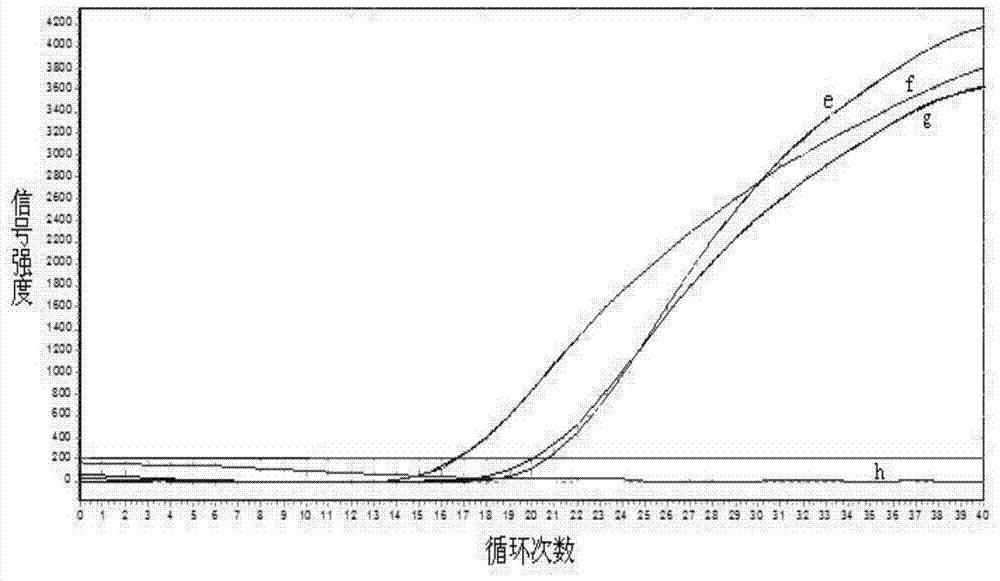
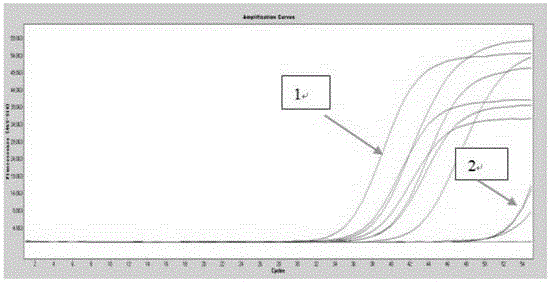
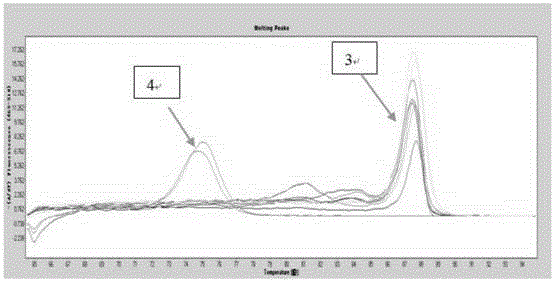
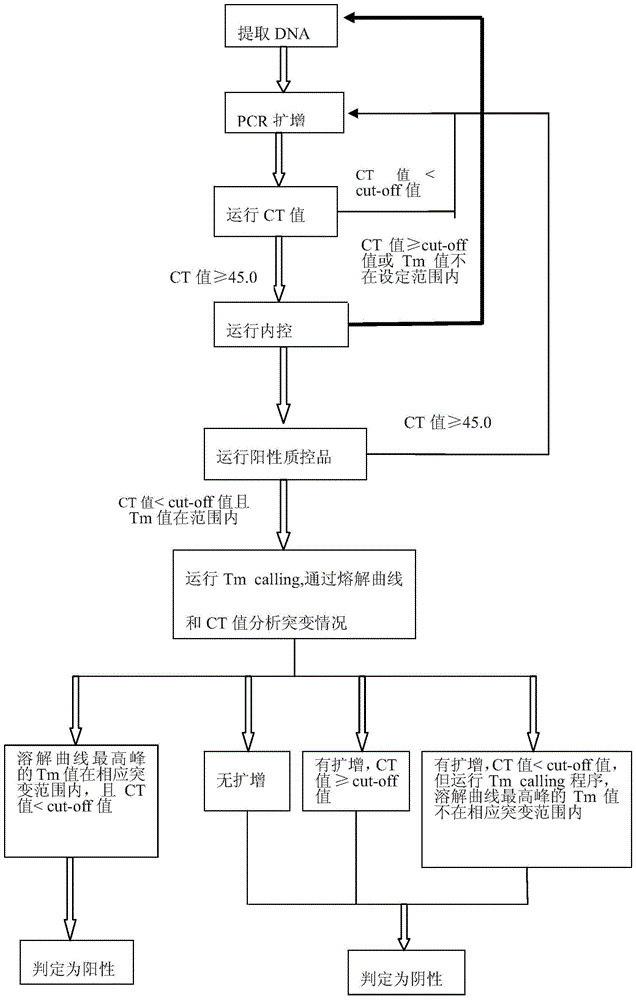
Gene mutation analysis judgment method based on real-time fluorescence quantification PCR (polymerase chain reaction) platform
ActiveCN104099421AAnalysis is clear and objectiveReduce false positive rateMicrobiological testing/measurementFluorescenceQuality control
The invention discloses a gene mutation analysis judgment method based on a real-time fluorescence quantification PCR (polymerase chain reaction) platform. A fluorescent quantitation PCR amplification result of a target gene is used for analysis judgment. The analysis judgment method comprises steps as follows: step one, after a PCR experiment ends, an experimental result is subjected to CT (computerized tomography) value calculation, and a CT value is judged to determine whether the experiment condition is continuously analyzed; step two, internal control is operated, and whether a DNA (deoxyribonucleic acid) sample is qualified is judged according to the internal control result; step three, a positive quality control product is operated, and whether an experiment system is qualified is judged according to the CT value of the positive quality control product; and step four, if the CT value is qualified through check analysis, a Tm calling program is operated, and whether the sample has mutation is judged through melting curve analysis and CT value analysis. According to gene mutation analysis judgment method, a PCR result is clearly and objectively judged, sample gene types are judged according to amplification data and a melting curve, and the misjudgment rate is low.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
True and false gene mutation analysis method based on high-throughput sequencing and application
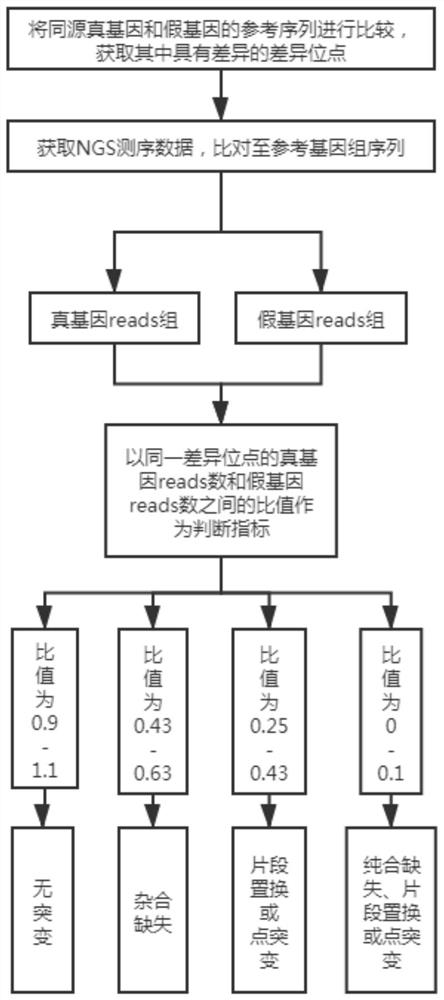
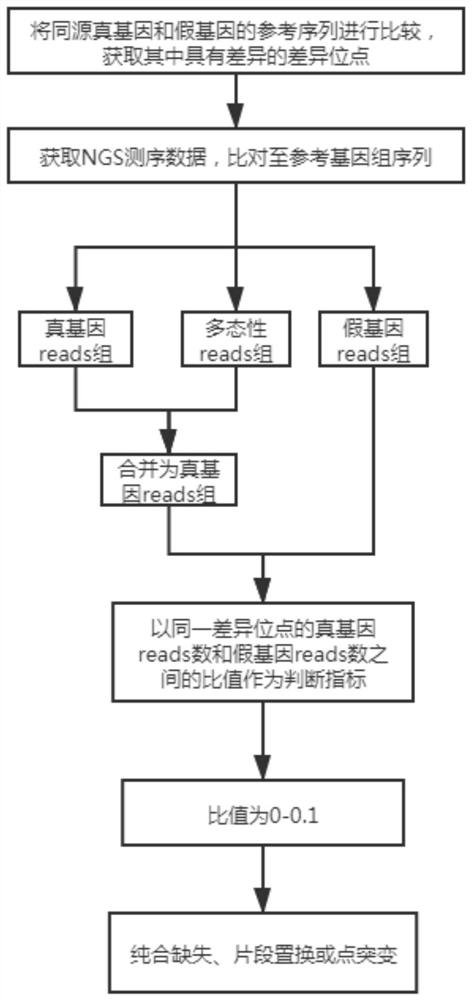
PendingCN112201306ASave experiment costSave experiment timeProteomicsGenomicsPseudogeneGene mutation analysis
The invention relates to a true and false gene mutation analysis method based on high-throughput sequencing and application, and belongs to the technical field of bioinformatics. The true and false gene mutation analysis method comprises the following steps: acquiring difference sites in reference sequences of homologous true genes and false genes; comparing the NGS sequencing data with the difference sites to respectively obtain the true gene reads number and the false gene reads number corresponding to the same difference site, taking the ratio of the true gene reads number to the false genereads number of the same difference site as a judgment index, and carrying out mutation analysis and judgment on the true gene according to a predetermined strategy. According to the kit, mutation oftrue and false genes can be preliminarily screened, genes which possibly have problems are found out, and judgment is carried out in combination with clinical actual conditions. MLPA or sanger sequencing experiments carried out on genes one by one are avoided, and the experiment cost and time are greatly saved.
Owner:GUANGZHOU KINGMED DIAGNOSTICS GRP CO LTD +2
Primers, kit and pcr method for detecting polymorphism of apoe gene
ActiveCN104862402BDifficulty of SimplificationReduce errorsMicrobiological testing/measurementDNA/RNA fragmentationType specificMedicine
The invention discloses primers for detecting ApoE gene polymorphism, a kit and a PCR (polymerase chain reaction) method for the primers or the kit. The primers include two sets of primers for detecting an rs429358 site and an rs7412 site respectively; the first set of primers include a mutant-type specific upstream primer of the rs429358 site, a wild-type specific upstream primer of the rs429358 site and a first downstream primer shared by the mutant-type specific upstream primer and the wild-type specific upstream primer of the rs429358 site; the second set of primers include a mutant-type specific upstream primer of the rs7412 site, a wild-type specific upstream primer of the rs7412 site and a second downstream primer shared by the mutant-type specific upstream primer and the wild-type specific upstream primer of the rs7412 site. The kit has the advantages of simplicity, quickness, accuracy and low price for detection and the like, and provides a powerful tool for scientific research and clinical analysis of ApoE gene typing and gene mutation.
Owner:沈阳优吉诺生物科技有限公司
Eml4-alk gene mutation analysis method
ActiveUS20200140959A1Microbiological testing/measurementDisease diagnosisOncologyGene mutation analysis
qRT-PCR primers capable of detecting EML4-ALK gene variant based on circulating tumor cells at a more sensitive detection limit than a conventional method. Also disclosed is a lung cancer patient screening method which is capable of detecting EML4-ALK gene variant using circulating tumor cells even in a lung cancer patient on whom ALK-FISH testing has been difficult to perform, due to difficulty in collecting a solid lung cancer tissue sample, and is able to determine whether an anticancer drug targeting the EML4-ALK gene variant may be applied to the lung cancer patient.
Owner:CYTOGEN CO LTD
A gene mutation analysis and judgment method based on real-time fluorescent quantitative PCR platform
ActiveCN104099421BReduce concentrationInterpretation is clear and objectiveMicrobiological testing/measurementPositive controlThe Internet
The invention discloses a gene mutation analysis and judgment method based on a real-time fluorescent quantitative PCR platform, which uses the fluorescent quantitative PCR amplification result of the target gene to analyze and judge, and the steps of the analysis and judgment method are as follows: the first step, after the PCR experiment ends Afterwards, calculate the CT value of the experimental results and judge the CT value to determine whether to continue to analyze the experimental situation; the second step is to run the internal control, and determine whether the DNA sample is qualified according to the internal control results; the third step is to run the positive quality control product, according to The CT value of the positive quality control product determines whether the experimental system is qualified; the fourth step, if the CT value is qualified by the above-mentioned steps, run the Tm calling program, and judge whether there is a mutation in the sample through melting curve analysis and CT value analysis. The invention has a clear and objective interpretation of the PCR results, completes the interpretation of the gene type of the sample according to the amplification data and the melting curve, and has a low misjudgment rate.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
A kit for detecting drug sensitivity of colorectal cancer cells based on gene variation analysis
The invention discloses a kit for detecting drug sensitivity of rectal cancer cells based on gene variation analysis, which consists of the following materials: (1) drug reagents for rectal cancer cells; Modified magnetic nanoparticle indicator solution A; (3) magnetic nanoparticle indicator solution B containing red fluorescent protein-labeled anti-variant P53 protein antibody for surface modification; (4) substrate medium and dilution buffer. In the present invention, the expression of wild-type P53 gene protein and mutant P53 gene protein is simultaneously calculated to measure the patient's sensitivity to drugs, the individualization is more prominent and the detection results are more accurate, and more sensitive drugs are screened out for the treatment of the disease. Choose the best chemotherapy regimen.
Owner:THE AFFILIATED HOSPITAL OF GUIZHOU MEDICAL UNIV
Method for calculating error and error rate of gene mutation detection analysis process
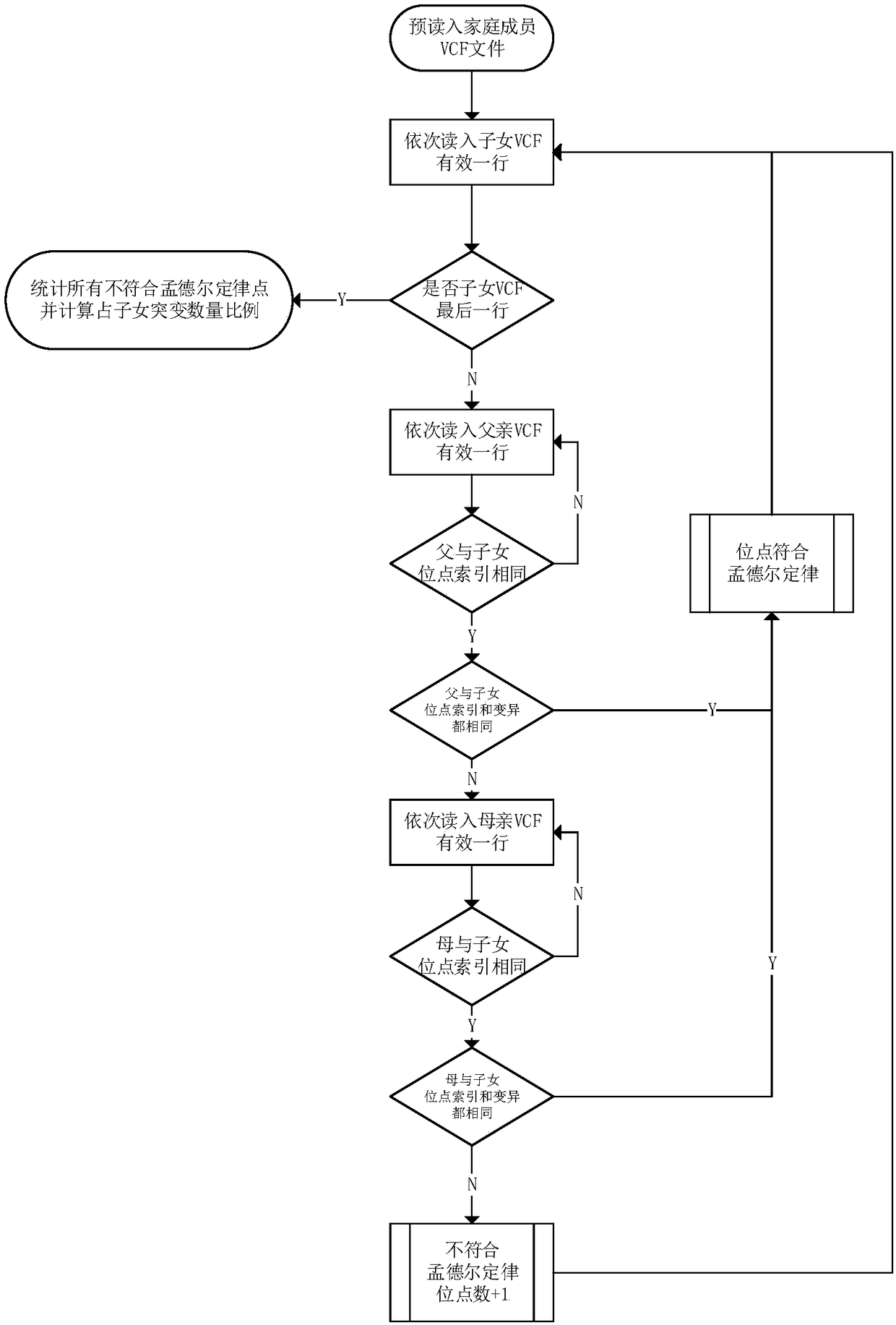
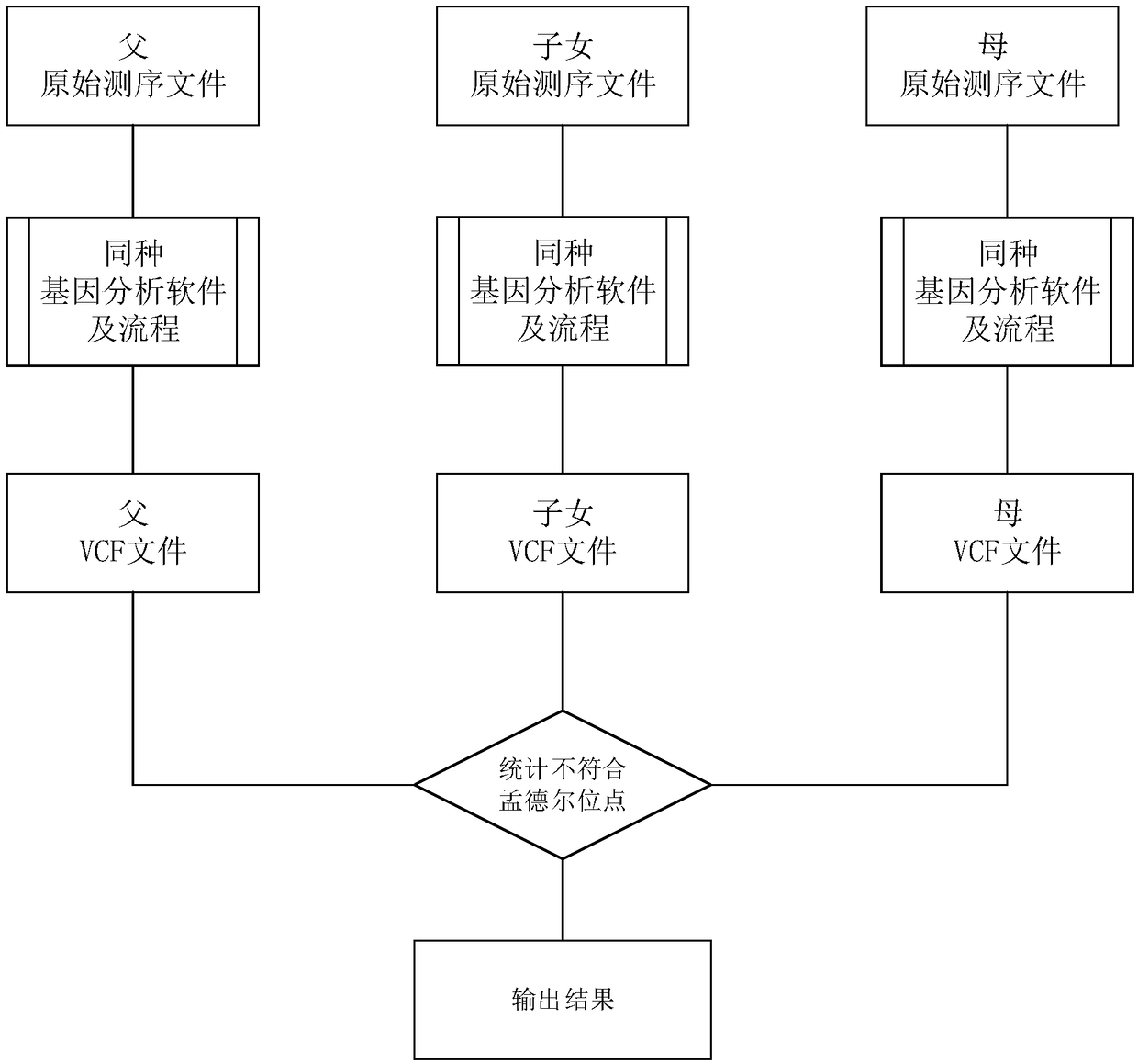
The invention discloses a method for calculating an error and an error rate of a gene mutation detection analysis process. The method comprises the steps of preprocessing gene original data of a family member to obtain gene sample data; by utilizing gene mutation detection software, analyzing the gene sample data to obtain a gene site mutation data file in a VCF format; and scanning the mutation data file to detect whether mutation site information of a child mutation file in a family meets a Mendelian inheritance law or not. The actual reproductive genetic mutation rate is extremely low, so that sites not meeting the Mendelian inheritance law are mainly caused by the error of the analysis process. A quantity and a ratio of the sites not meeting the Mendelian inheritance law are namely theerror and the error rate of the gene mutation detection analysis process, and provide a basis for subsequent algorithm optimization and software design.
Owner:杭州米天基因科技有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com