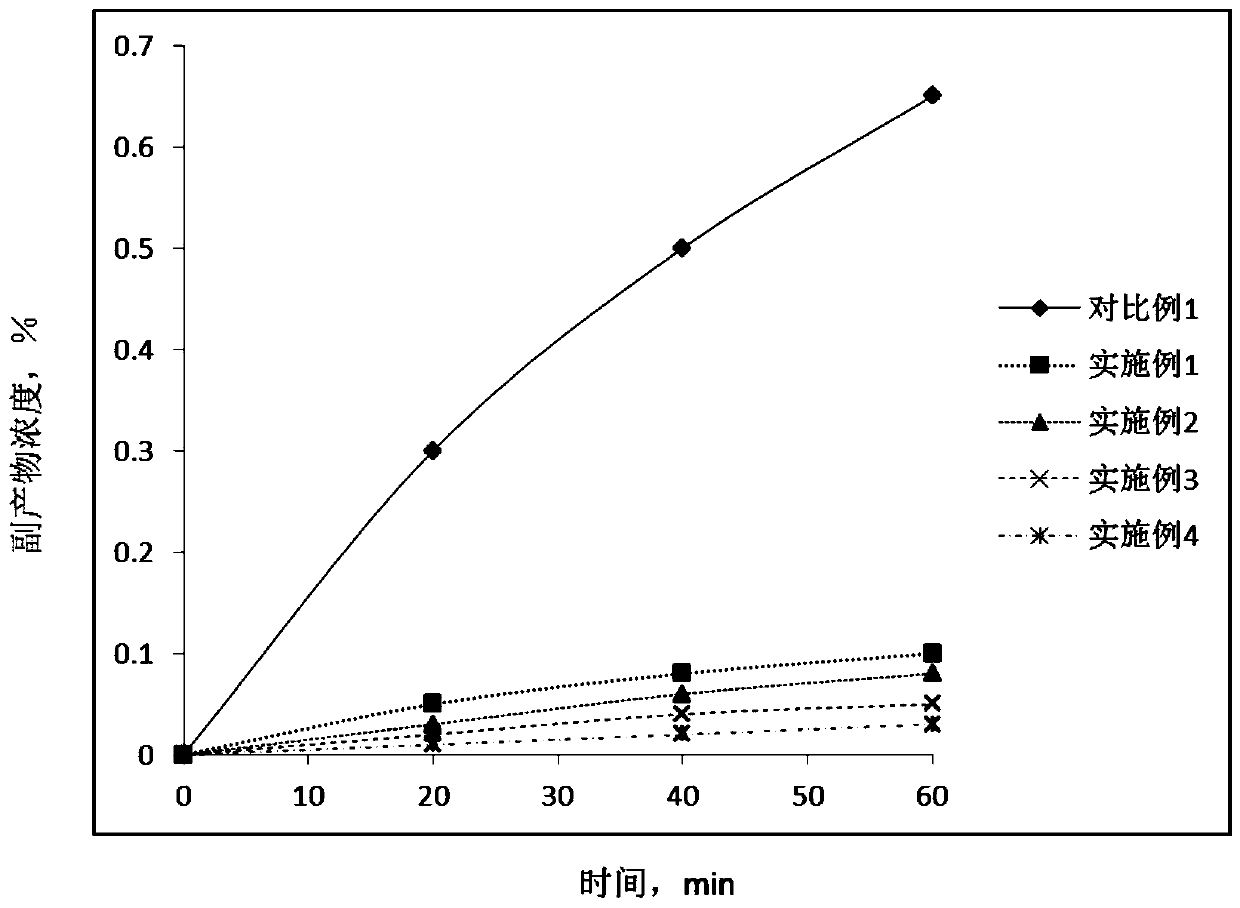
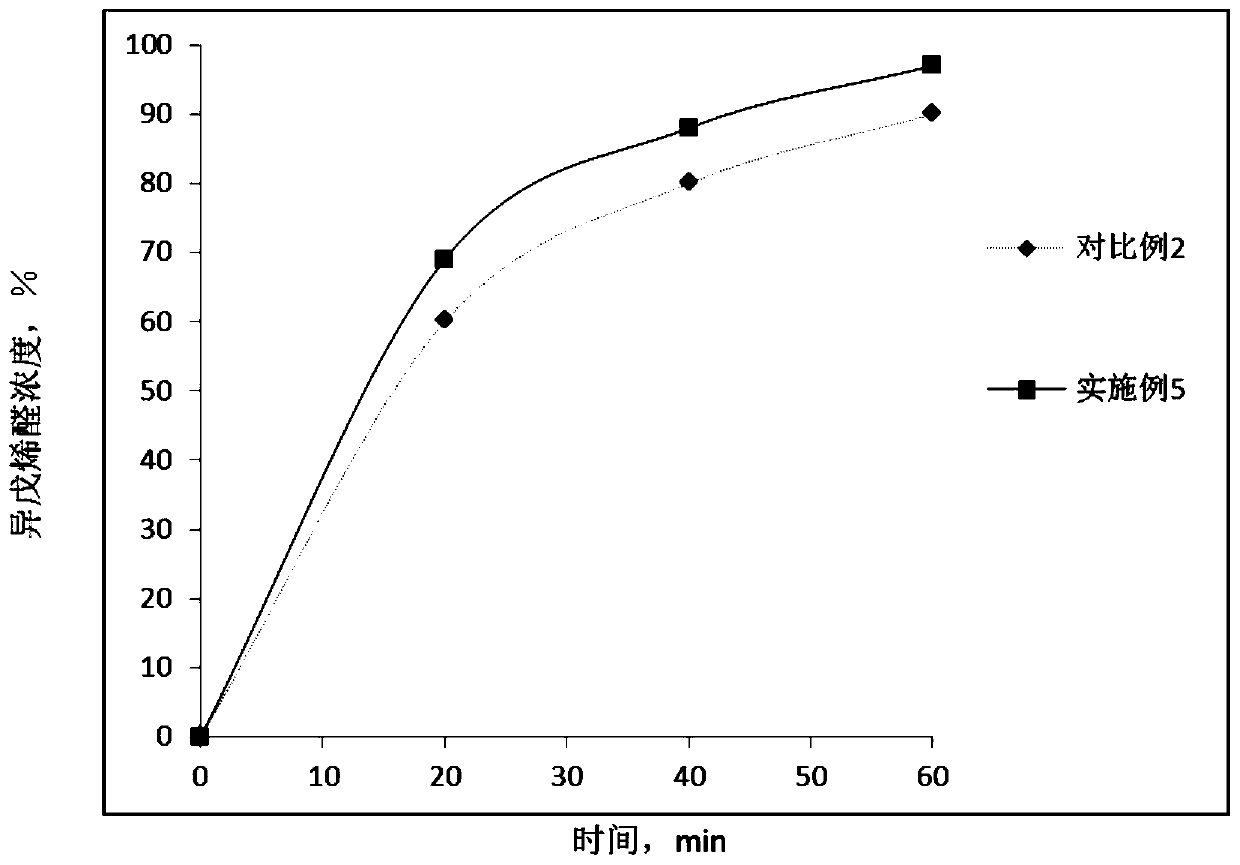
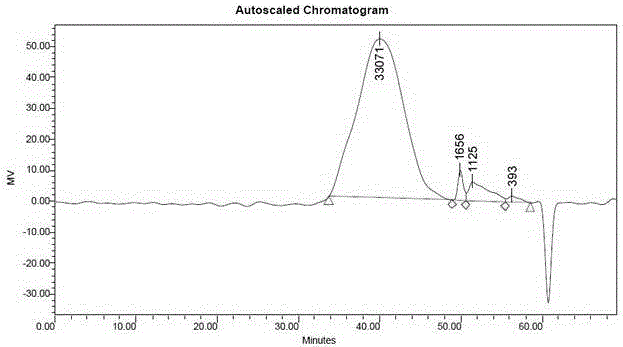
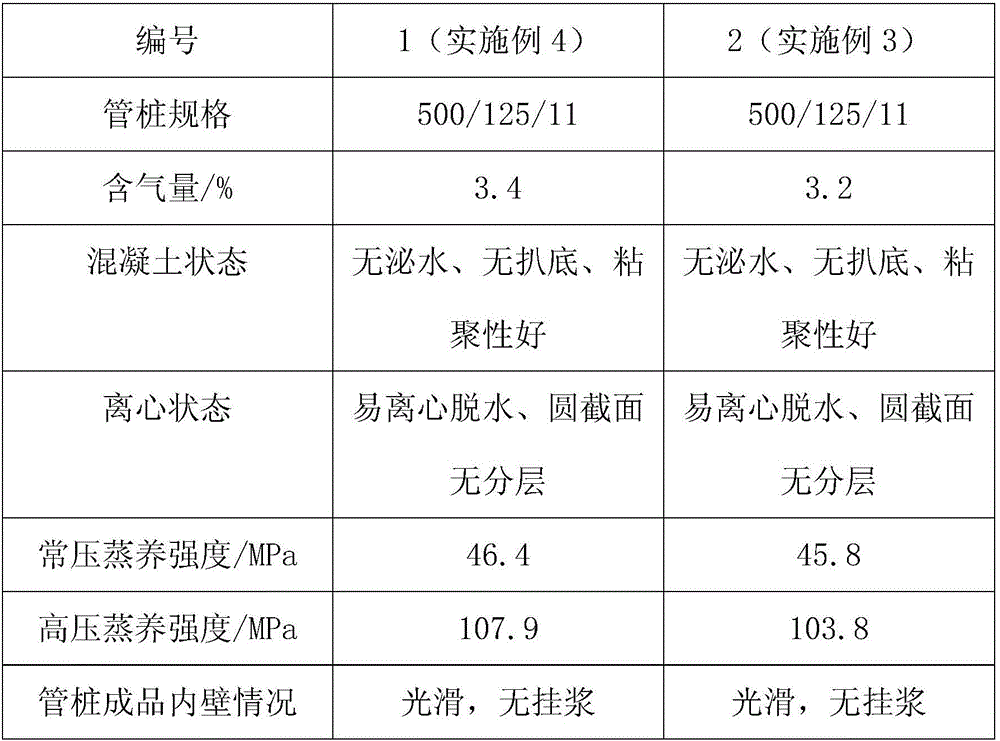
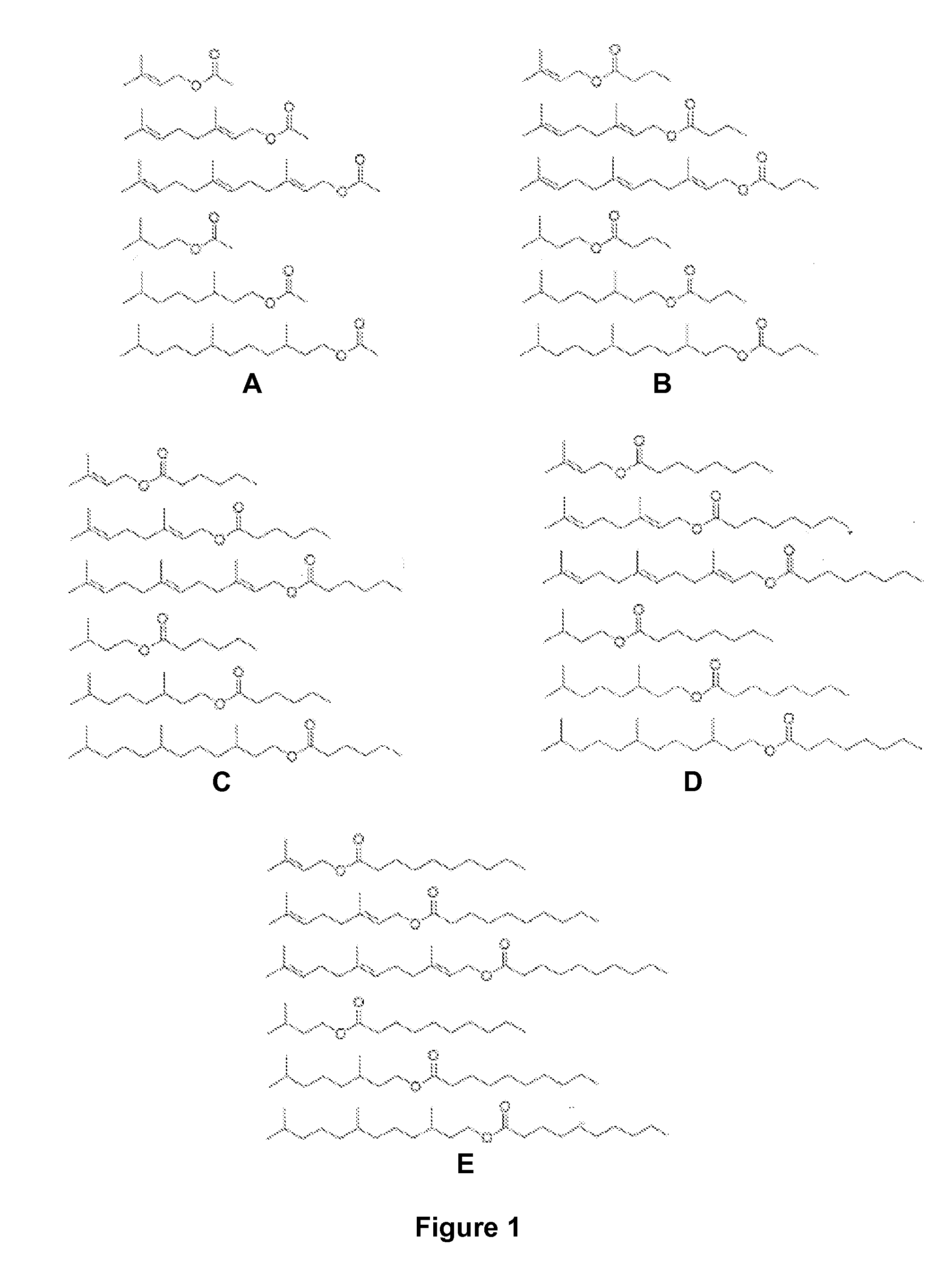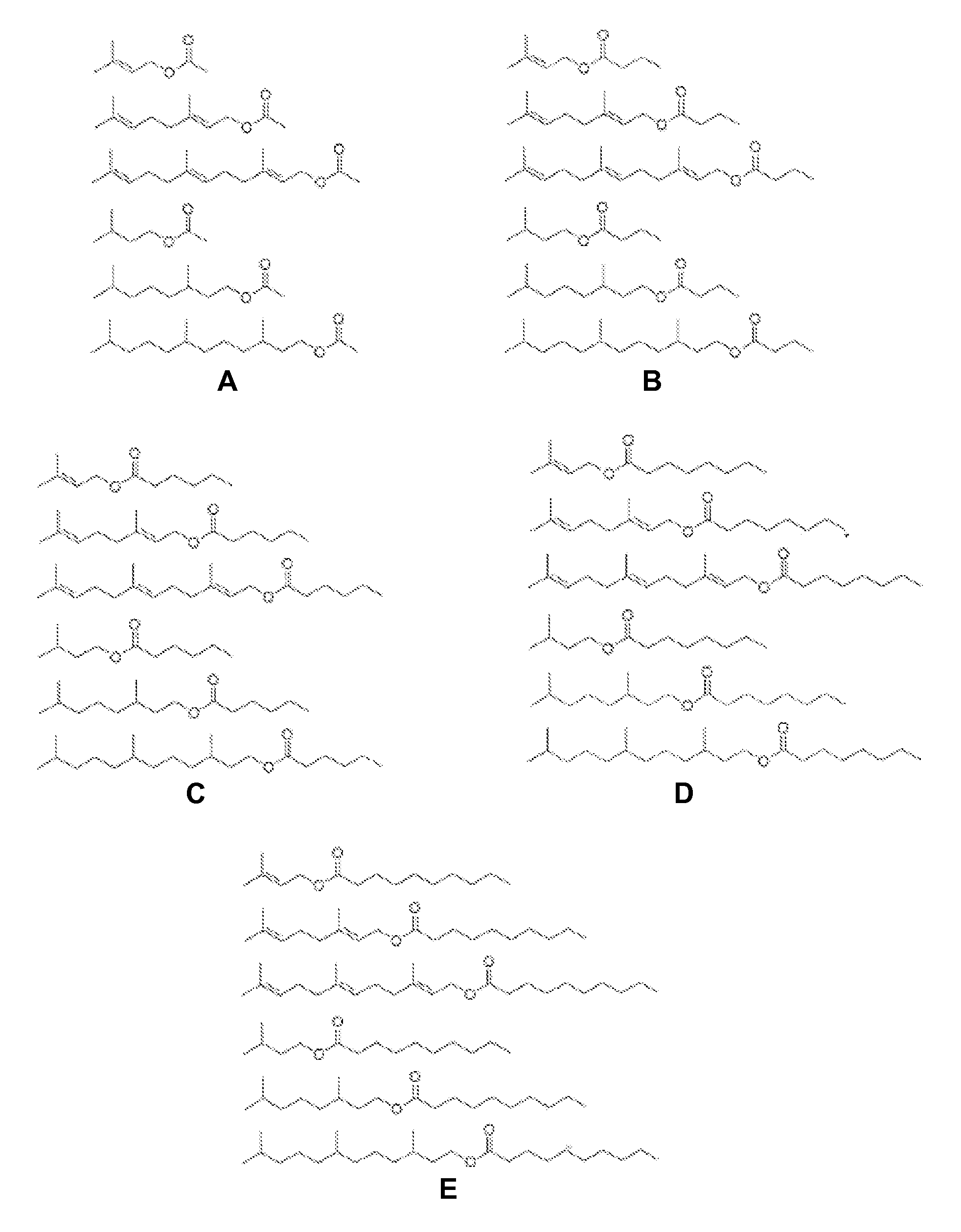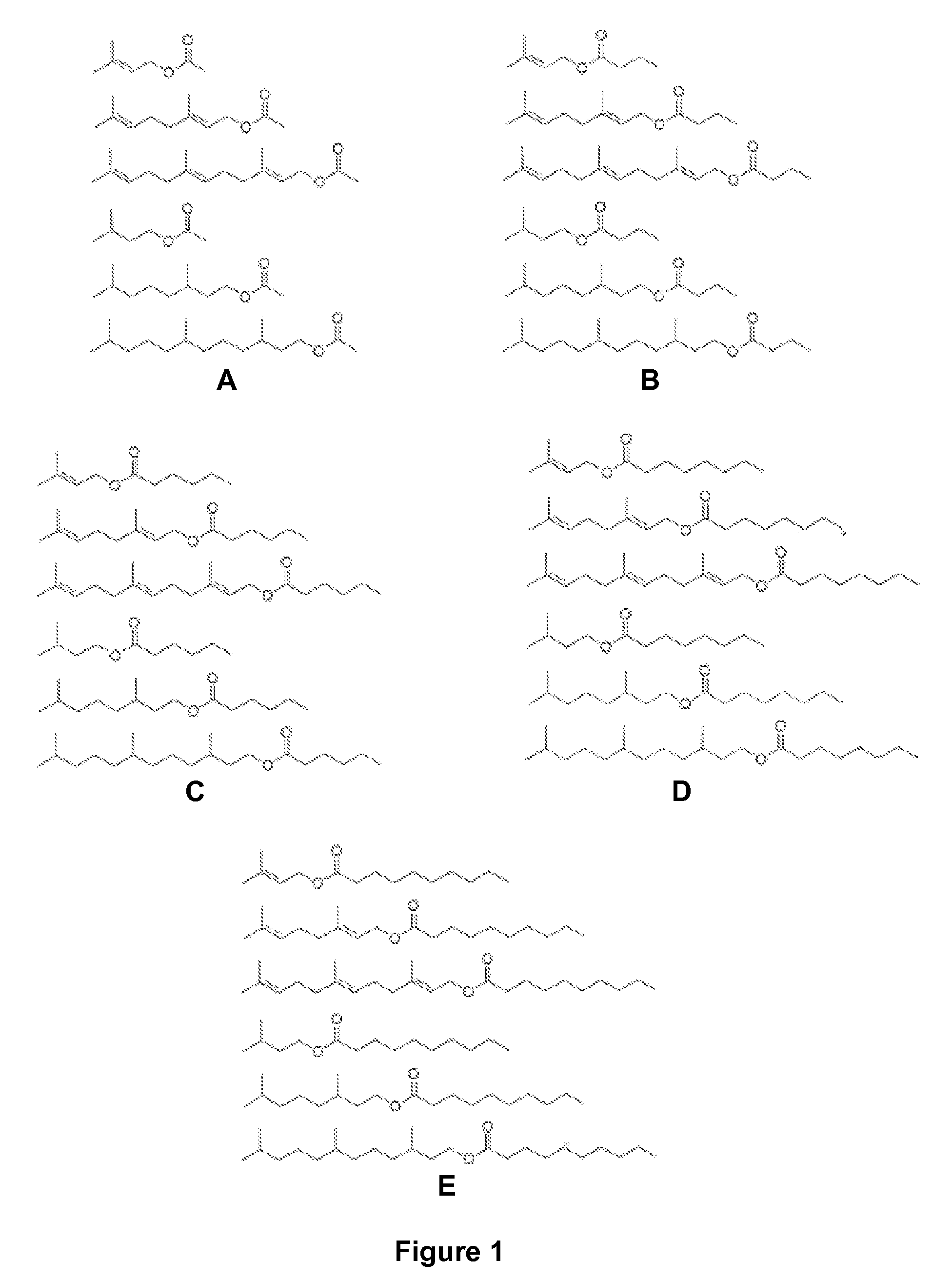Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Isoprenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
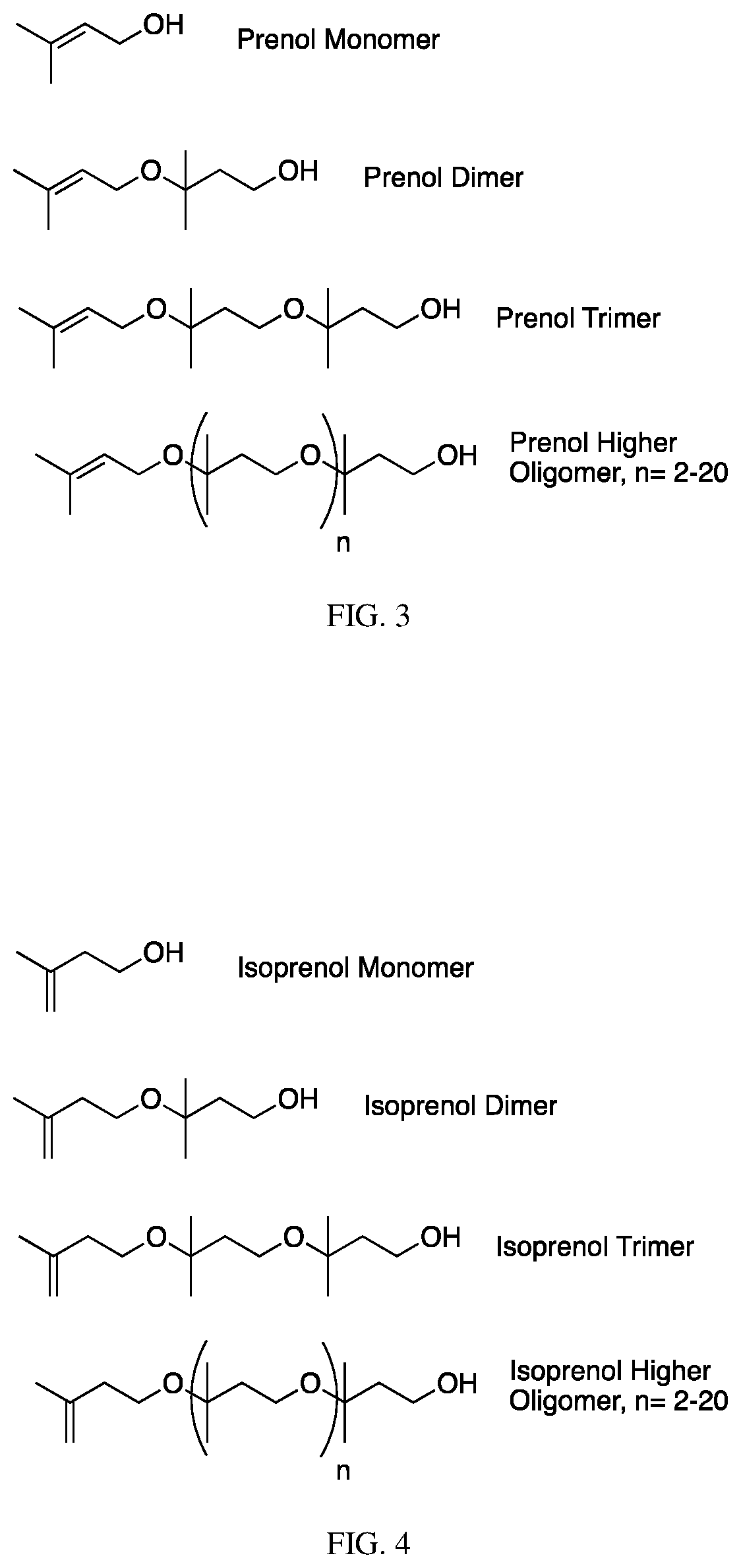
Isoprenol, also known as 3-methylbut-3-en-1-ol, is a hemiterpene alcohol. It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.
Production method of C5 enol
InactiveCN102381940AInhibit side effectsSimple processPreparation by hydrolysis2-methylbutaneOil phase
The invention relates to a production method of a C5 enol. The production method uses isoprene and hydrogen chloride as raw materials and comprises the following steps: 1) performing addition reaction on isoprene and hydrogen chloride to generate a mixture of 1-chloro-isopentene and 3-chloro-isopentene; and 2) performing a hydrolysis reaction on the product of the addition reaction, adding one or more of toluene, ethylbenzene, n-hexane, cyclohexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, 2,3-dimethylbutane and heptane as the solvent in the hydrolysis process, separating out the oil phase product after the hydrolysis, and rectifying to obtain products, namely methylbutenol and 3-methyl-2-buten-1-ol. Compared with the C5 enol production process of the prior art, the side reaction, in which dichloro-isopentane is generated, in the addition reaction process is effectively inhibited; after the solvent is added in the hydrolysis reaction, the yield of the hydrolysis reaction can be increased and the purification of the product can be facilitated; the yields of the addition reaction and the hydrolysis reaction are both more than 98% and are obviously increased as compared with the prior art; and the production cost is greatly reduced.
Owner:赵明江
Method for preparing 3-methyl-2-butenol
ActiveCN107141197AAvoid generatingHigh activityPreparation by isomerisationOrganic compound preparationIsomerizationOrganic base
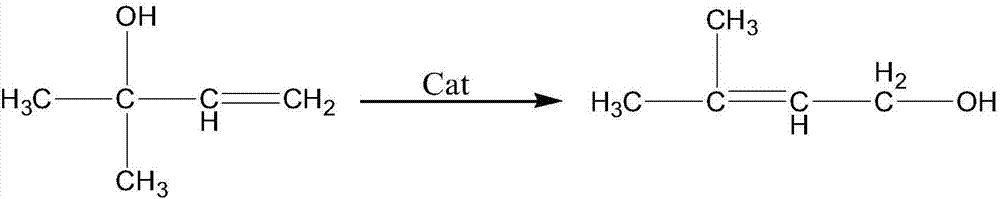
The invention provides a method for preparing 3-methyl-2-butenol (isoprenol) through isomerization of 3-methyl-3-butenol. A catalyst reaction system contains 1) a carbonyl iron compound, 2) an organic base, and 3) an epoxy group ligand. A solvent with an ether structure is added into an isomerization reaction, so that the activity of a catalyst can be improved, and the product selectivity is improved. During catalysis and separation, a mixed gas of carbon monoxide (CO) and nitrogen (N2) is introduced, wherein the volume content of the CO is 100-100000ppm. The catalyst and a raw material namely the 3-methyl-3-butenol are separated from a product namely isoprenol through rectification, and the catalyst does not need to be additionally separated. The catalyst keeps high activity and long life operation.
Owner:WANHUA CHEM GRP CO LTD
Host Cells and Methods for Producing Isoprenyl Alkanoates
The invention provides for a method of producing an isoprenyl alkanoate in a genetically modified host cell. In one embodiment, the method comprises culturing a genetically modified host cell which expresses an enzyme capable of catalyzing the esterification of an isoprenol and a straight-chain fatty acid, such as an alcohol acetyltransferase (AAT), wax ester synthase / diacylglycerol acyltransferase (WS / DGAT) or lipase, under a suitable condition so that the isoprenyl alkanoate is produced.
Owner:RGT UNIV OF CALIFORNIA
New synthetic method of carane aldehyde acid lactone, caronic acid, caronic anhydride and key intermediates thereof
ActiveCN102952011AMild conditionsImprove production safetyOxygen-containing compound preparationOrganic compound preparationGluconic acidDouble bond
The invention relates to a new synthetic method of carane aldehyde acid lactone, caronic acid, caronic anhydride and key intermediates thereof. The method comprises using hydroxy protected isoamyl alcohol as initial materials, performing an addition of a double bond to generate a key intermediate with a three-membered ring, hydrolyzing ethyl ester and protective groups, and then controlling oxidation conditions to obtain the carane aldehyde acid lactone and the caronic acid respectively. The method has advantages of mild condition, high production security, easy industrial production, no metal residues, and no other waste liquid, waste residue and exhaust gas that pollut the environment, and can effectively reduce cost.
Owner:ABA CHEM NANTONG
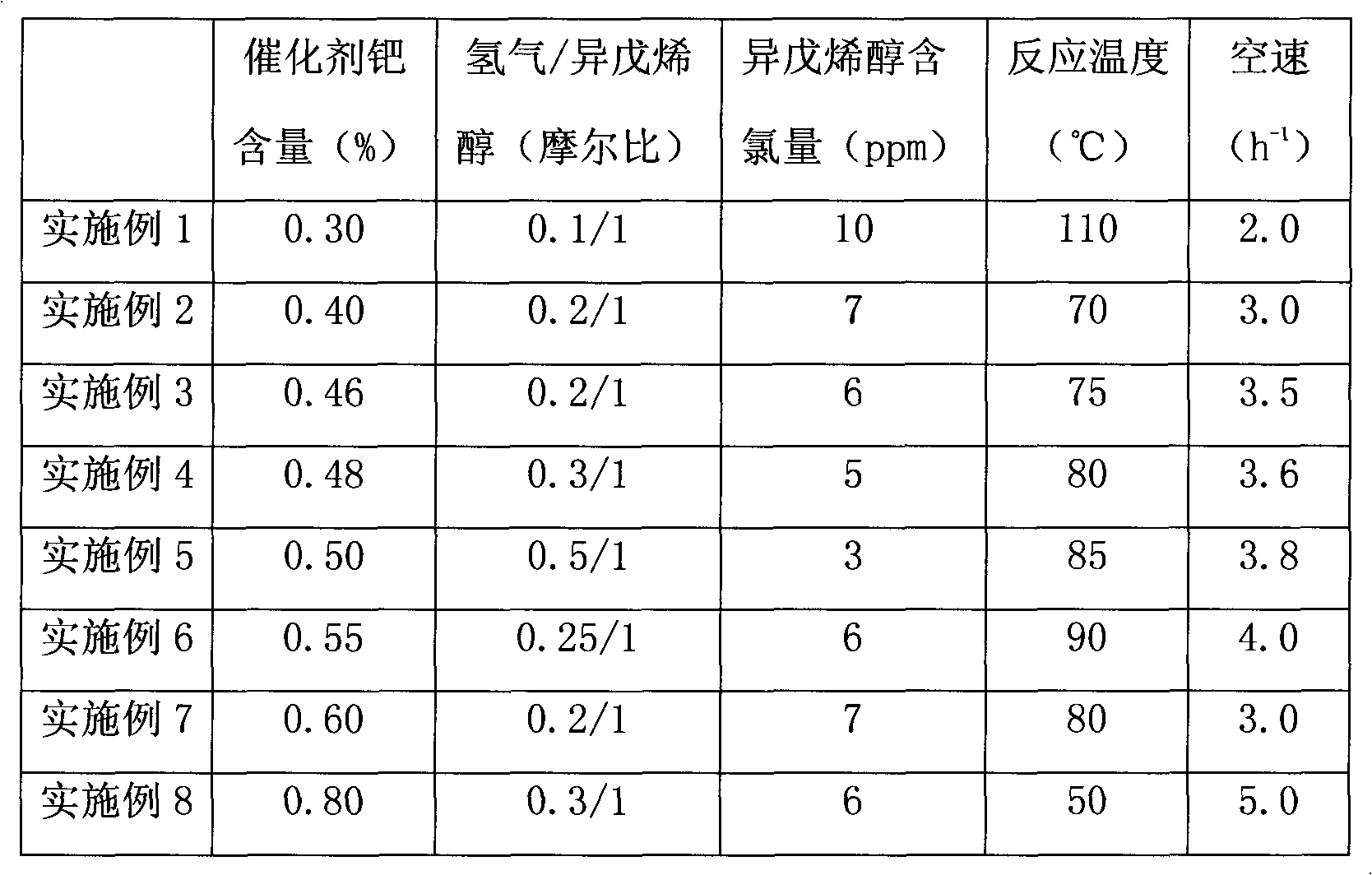
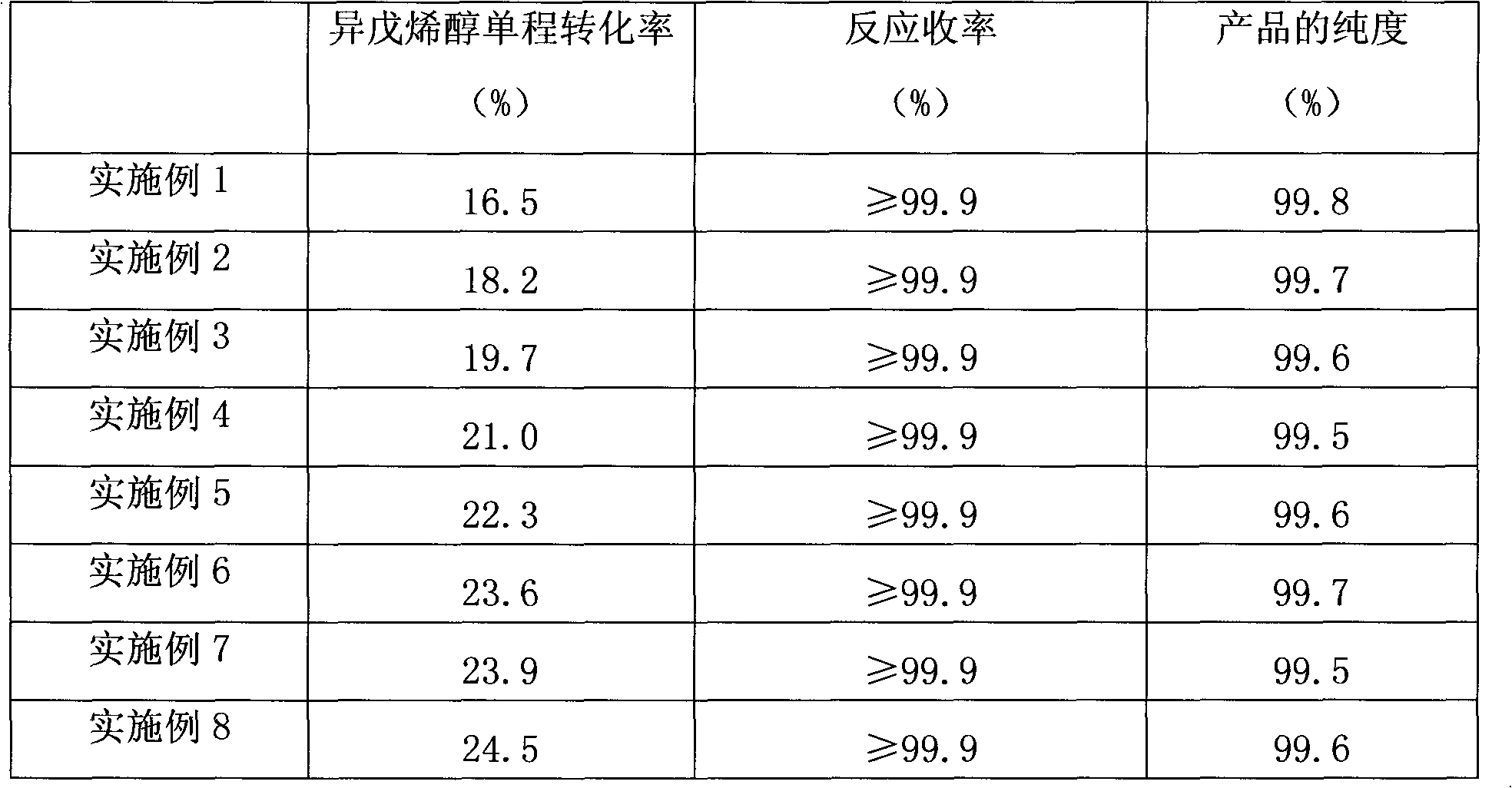
3-methyl-3-butenyl-1-alcohol production method
InactiveCN102367220AOvercome the problem of complex production processPreparation by isomerisationHydrogenIsomerization
The invention discloses a 3-methyl-3-butenyl-1-alcohol production method. The 3-methyl-3-butenyl-1-alcohol production method is characterized in that isoprenol as a raw material undergoes an isomerization reaction in a fixed bed reactor, wherein an isomerization catalyst is Pd / SiO2; Pd content is in a range of 0.3 to 0.8%; a reaction temperature is in a range of 50 to 110 DEG C; reaction pressure is ordinary pressure; a molar ratio of hydrogen to isoprenol is in a range of 0.1 to 0.5; a mass space velocity is in a range of 2 to 5h<-1>; and organic chlorine content of isoprenol is in a range of 3 to 10ppm. Compared with the existing 3-methyl-3-butenyl-1-alcohol production method, the 3-methyl-3-butenyl-1-alcohol production method provided by the invention solves the problems that the existing 3-methyl-3-butenyl-1-alcohol production method has a high raw material consumption rate and a high production cost and can cause severe environmental pollution, and has the advantages of simple processes, abundant raw material sources, low production cost, high reaction yield and low pollution on the environment.
Owner:赵明江
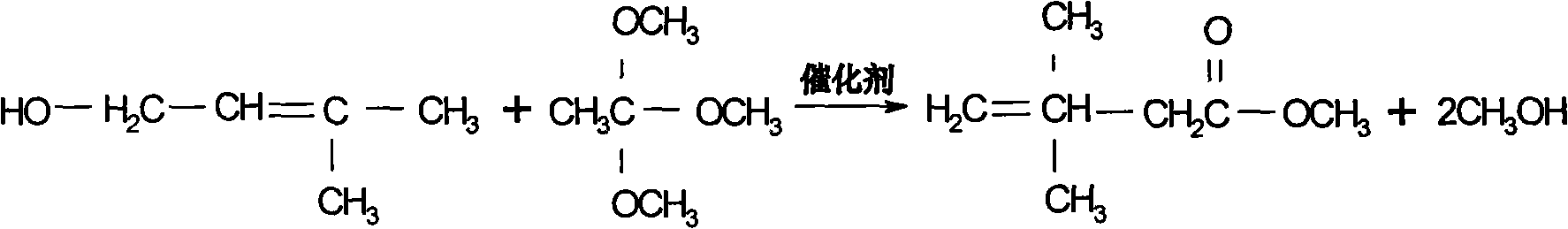
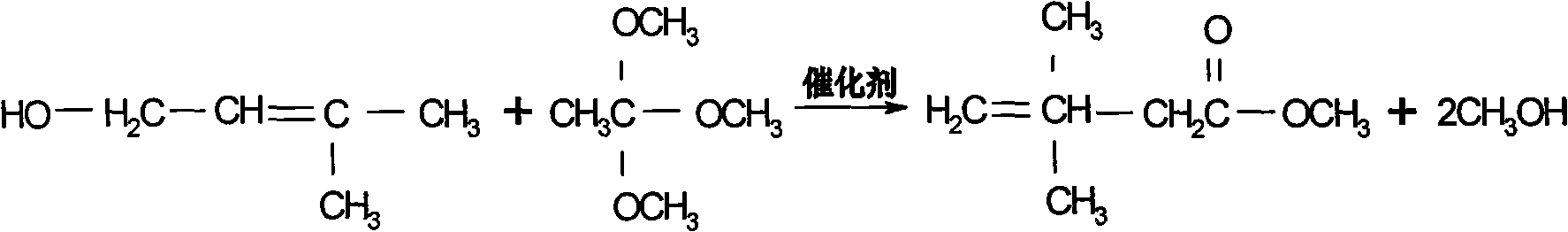
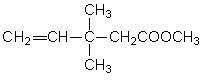
Preparation process of 3,3-dimethyl-4-pentenoic acid methyl ester
InactiveCN101565372AHigh yieldHigh purityPreparation by ester-hydroxy reaction4-pentenoic acidAcetic acid
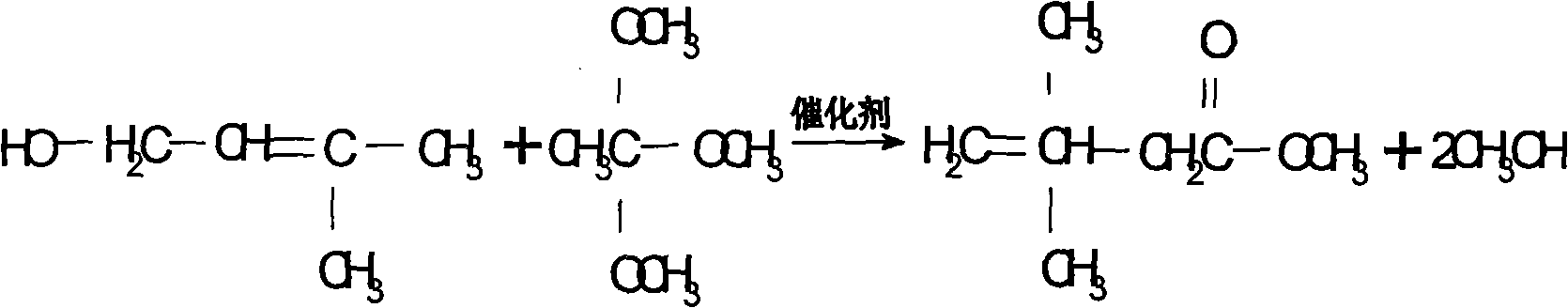
The invention discloses a preparation process of 3,3-dimethyl-4-pentenoic acid methyl ester, comprising the following steps: heating and reflowing prenol and trimethyl orthoacetate under the existence of a catalyst to obtain methyl alcohol and excessive trimethyl orthoacetate, and the then raising the temperature to produce the 3,3-dimethyl-4-pentenoic acid methyl ester after Clisen rearrangement reaction. The process adopts a novel composite catalyst, improves the yield of the 3,3-dimethyl-4-pentenoic acid methyl ester and has high product purity which is generally over 99 percent.
Owner:NANTONG TENDENCI CHEM
Catalytic hydrogenation synthesis method for prenyl alcohol
InactiveCN105175229AHigh activityAvoid inactivationPhysical/chemical process catalystsPreparation by isomerisationDistillationSynthesis methods
The invention provides a catalytic hydrogenation synthesis method for prenyl alcohol. The method comprises the following steps: under the action of a solid base catalyst, adding paraformaldehyde with a concentration of 93 to 97% and tert-butyl alcohol used as a solvent into a reaction vessel, injecting nitrogen into the reaction vessel to replace air in the reaction vessel, then adding isobutene into the reaction vessel, carrying out stirring, then carrying out a continuous reaction at 180 to 220 DEG C for 3 to 5 h and carrying out cooling, discharging and distillation; collecting prenyl alcohol obtained after distillation; and adding 3-methyl-buten-l-ol into a fixed-bed reactor and carrying out catalytic hydrogenation at 50 to 100 DEG C under the catalysis of a silica gel-loaded Pd catalyst promoted by Se and Ce so as to prepare prenyl alcohol. Thus, the method provided by the invention can improve reaction yield and selectivity of prenyl alcohol, overcomes the problems of corrosion and pollution existing in acidic catalysis, shortens reaction time and reduces production cost.
Owner:SHANDONG CHENGTAI CHEM IND
Method for improving yield of chloridization method preparation of prenyl alcohol
ActiveCN107082740ASolve problems such as destructionReduce generationOxygen-containing compound preparationOrganic compound preparationFormateOrganic layer
The invention discloses a method for improving a yield of chloridization method preparation of prenyl alcohol. The existing chlorination method for preparing prenyl alcohol produces about 10% of 3-chloroisopentene which is not a target product so that a prenyl alcohol yield is limited and more excess solid wastes are produced. The method comprises directly adding anhydrous formate or acetate into a mixture of 3-chloroisopentene and 1-chloroisopentene produced by a chloridization reaction, carrying out esterification, carrying out hydrolysis in an alkali solution so that iso-amylenol formate or iso-amylenol acetate is transformed into prenyl alcohol and 3-chloroisopentene is transformed into methyl butenol, carrying out rectification separation on the obtained organic layer to obtain methyl butenol, methylbenzene and prenyl alcohol and recycling methyl butenol and methylbenzene in a chloridization addition reaction. The method is free of the traditional chloroisopentene rectification so that the problems of equipment corrosion, chlorine substitute toxicity and high temperature-caused chlorine substitute destroy caused by chloroisopentene rectification are solved.
Owner:浙江可明生物医药有限公司
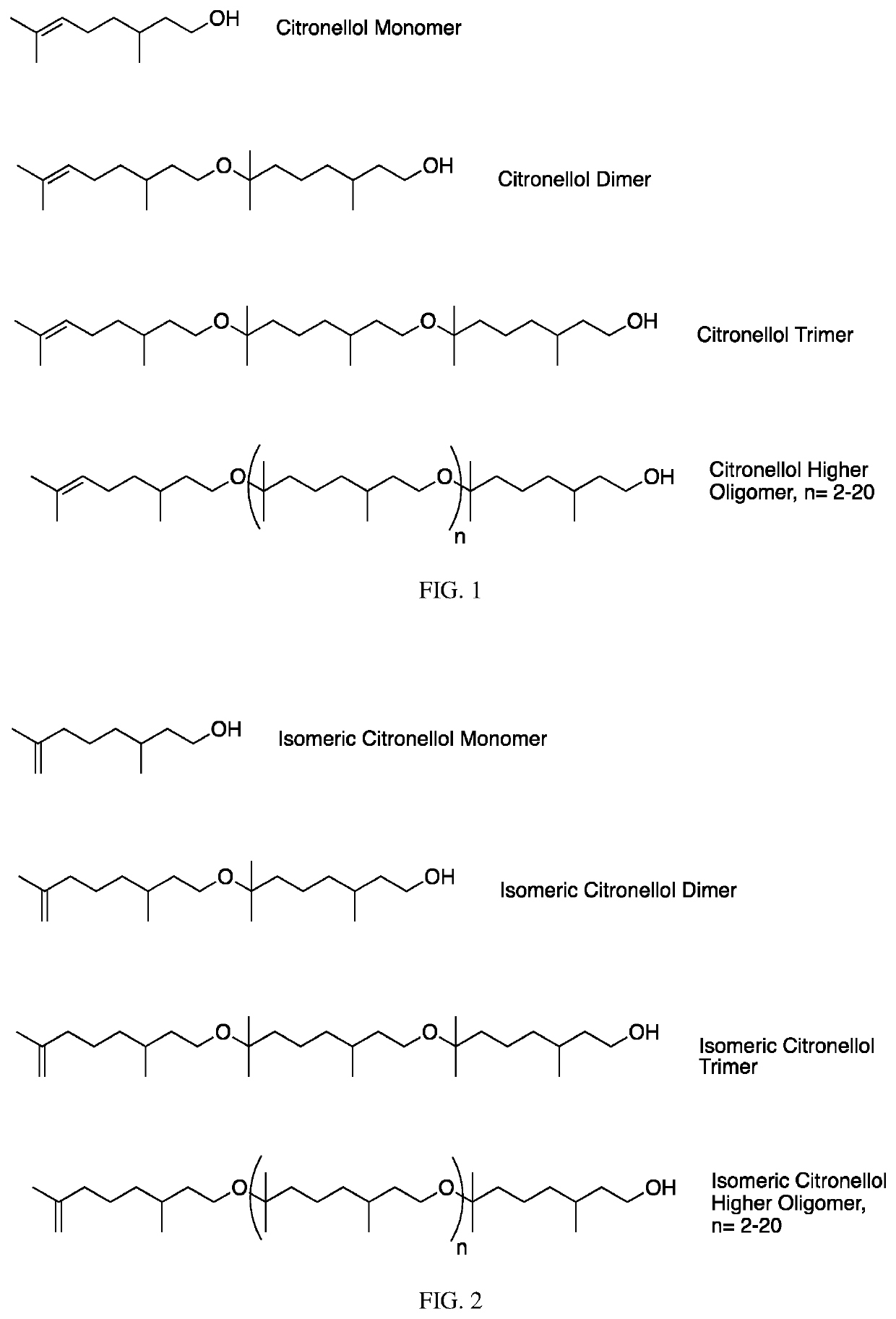
Polyether derivatives, uses, and methods of making the same
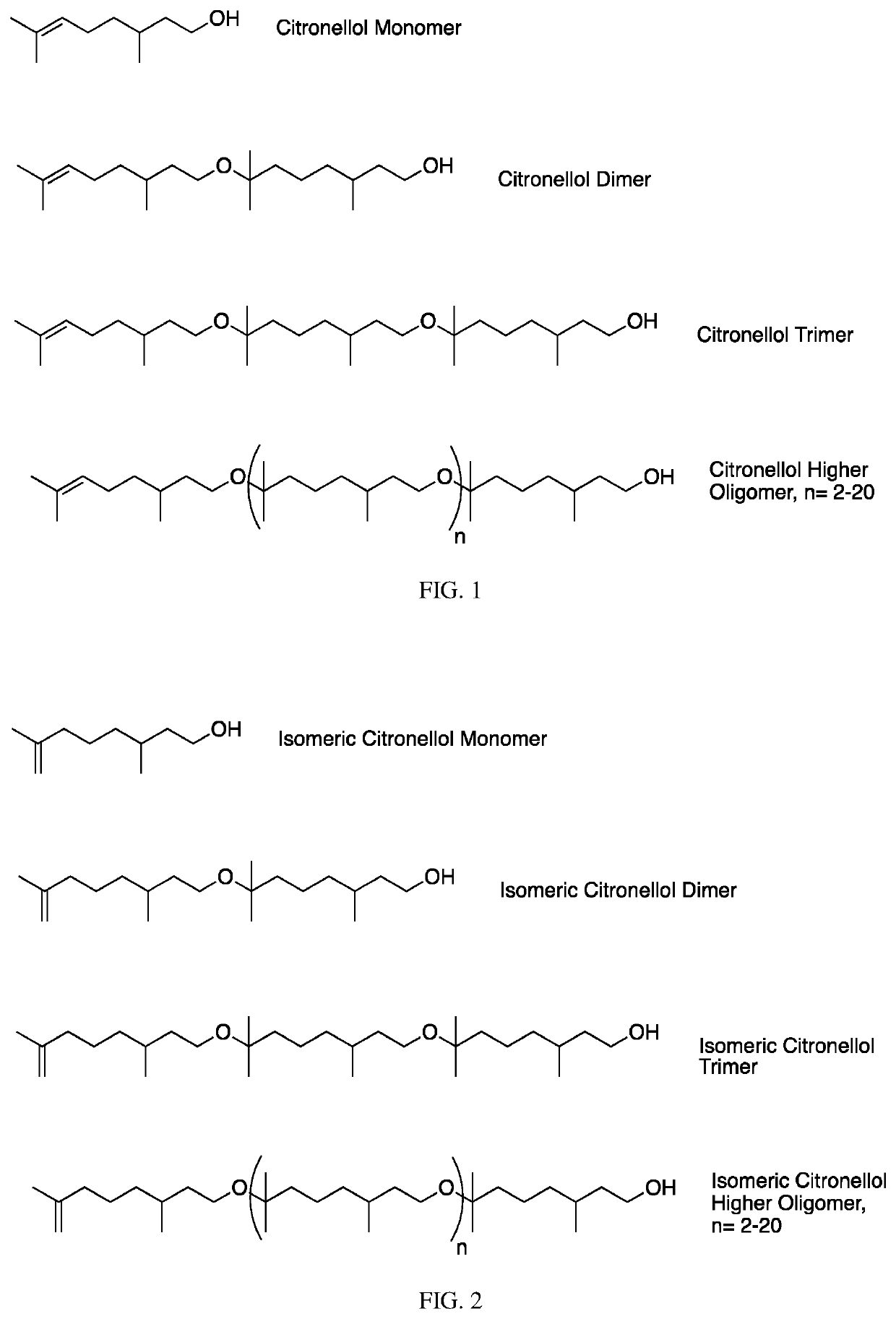
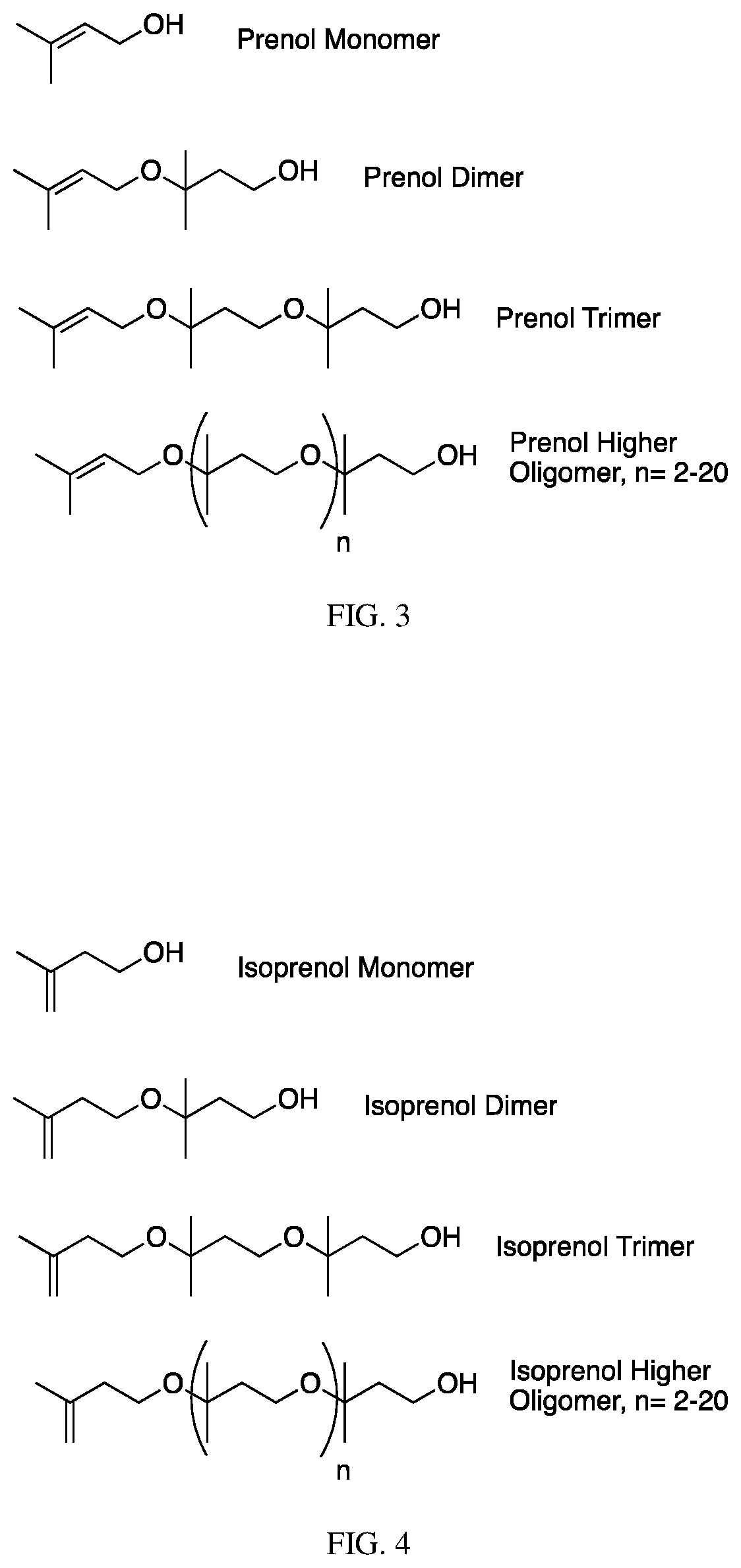
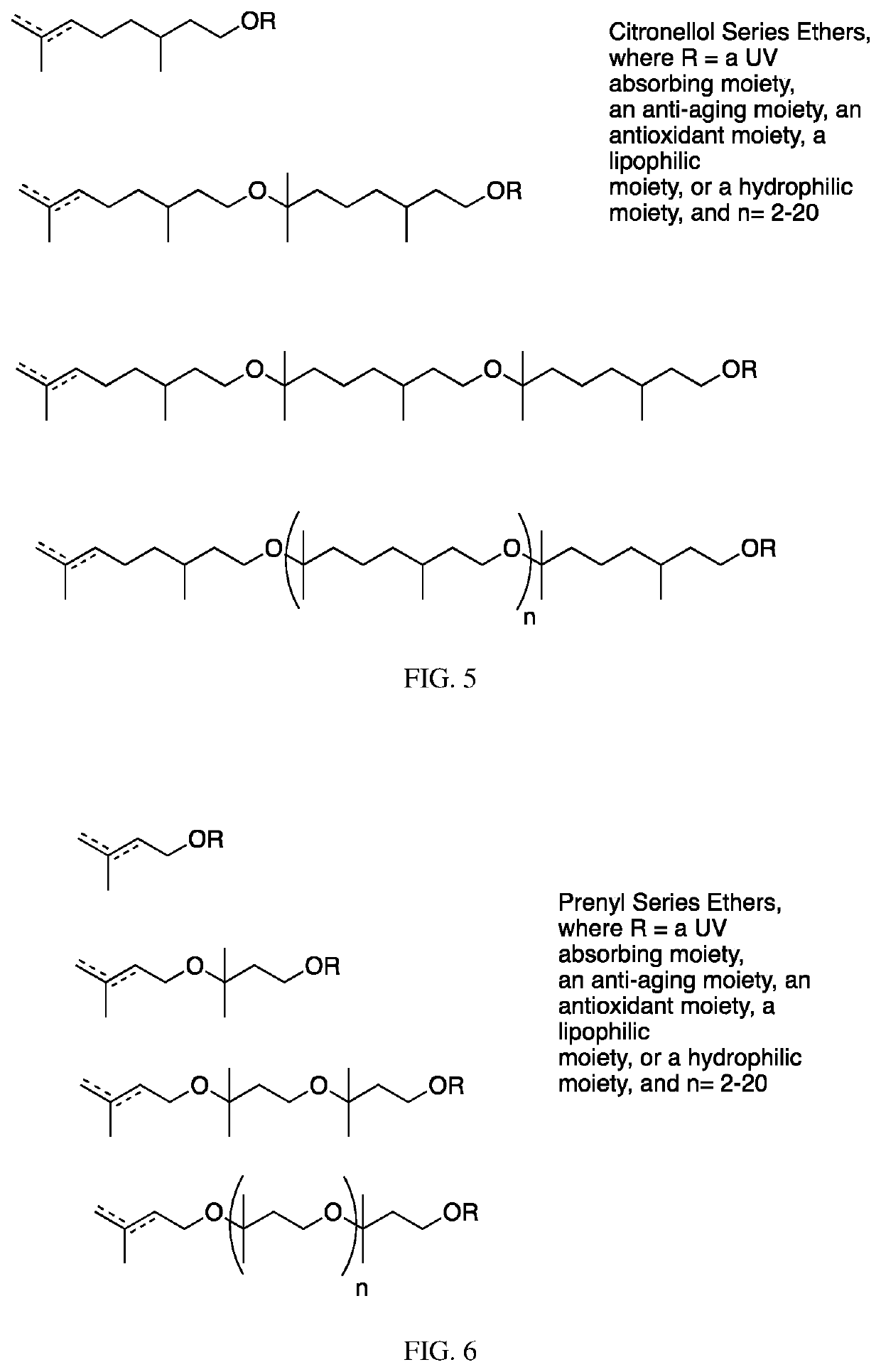
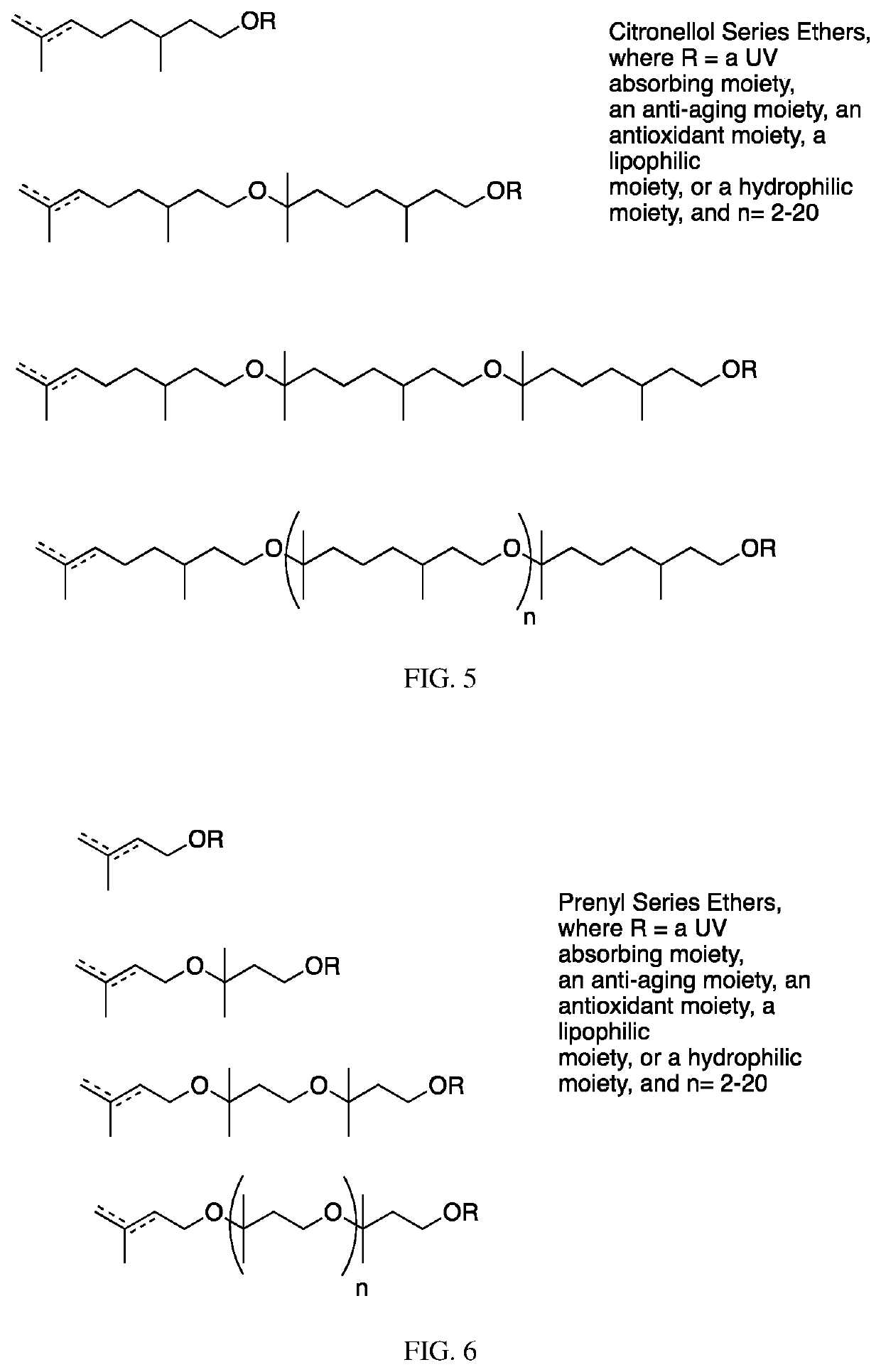
The invention contemplates certain polyethers, polyether derivatives, and methods of making and using those same polymers. For example, the starting materials can, e.g., citronellol, prenol, isocitronellol and isoprenol.
Owner:P2 SCI INC
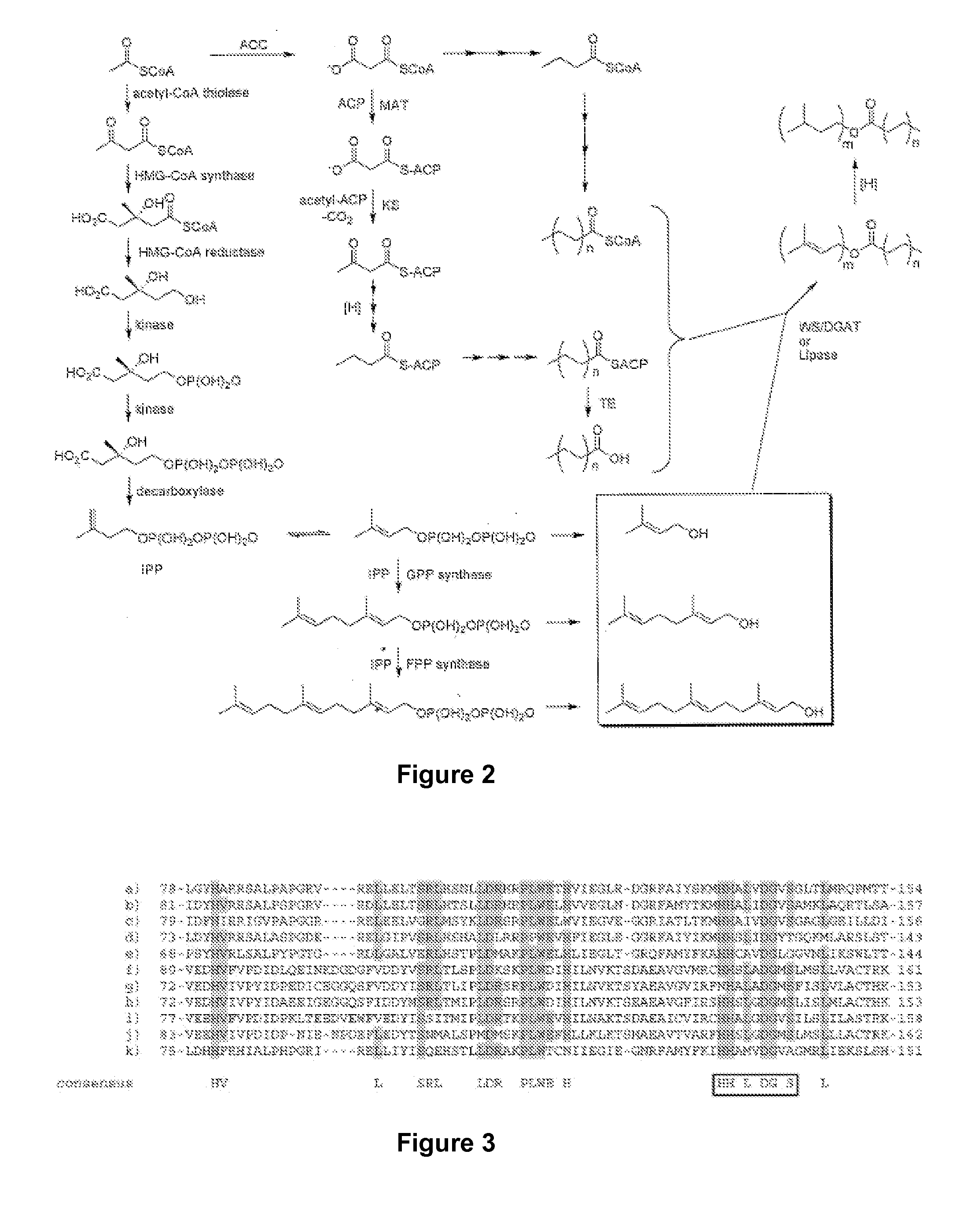
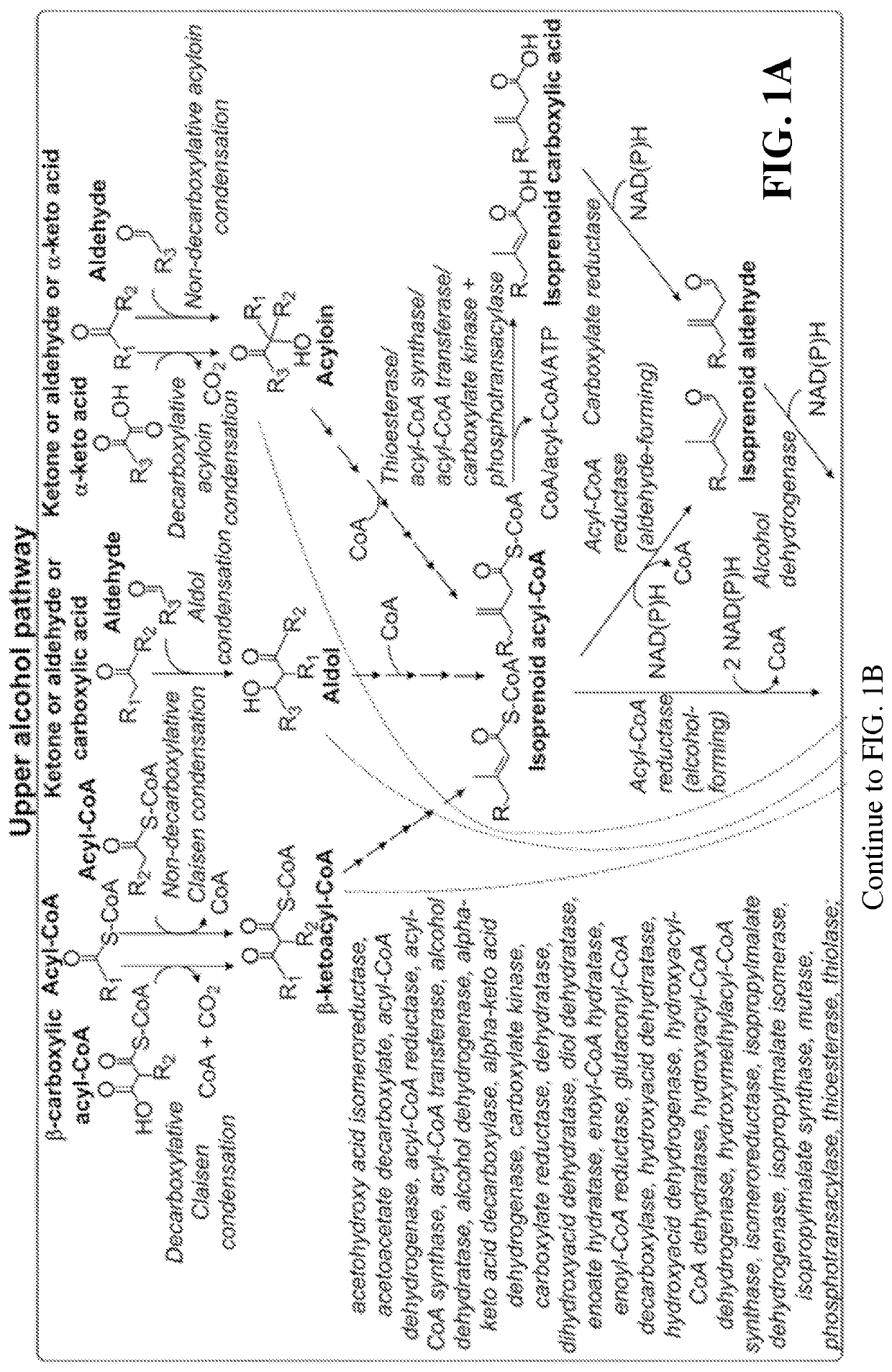
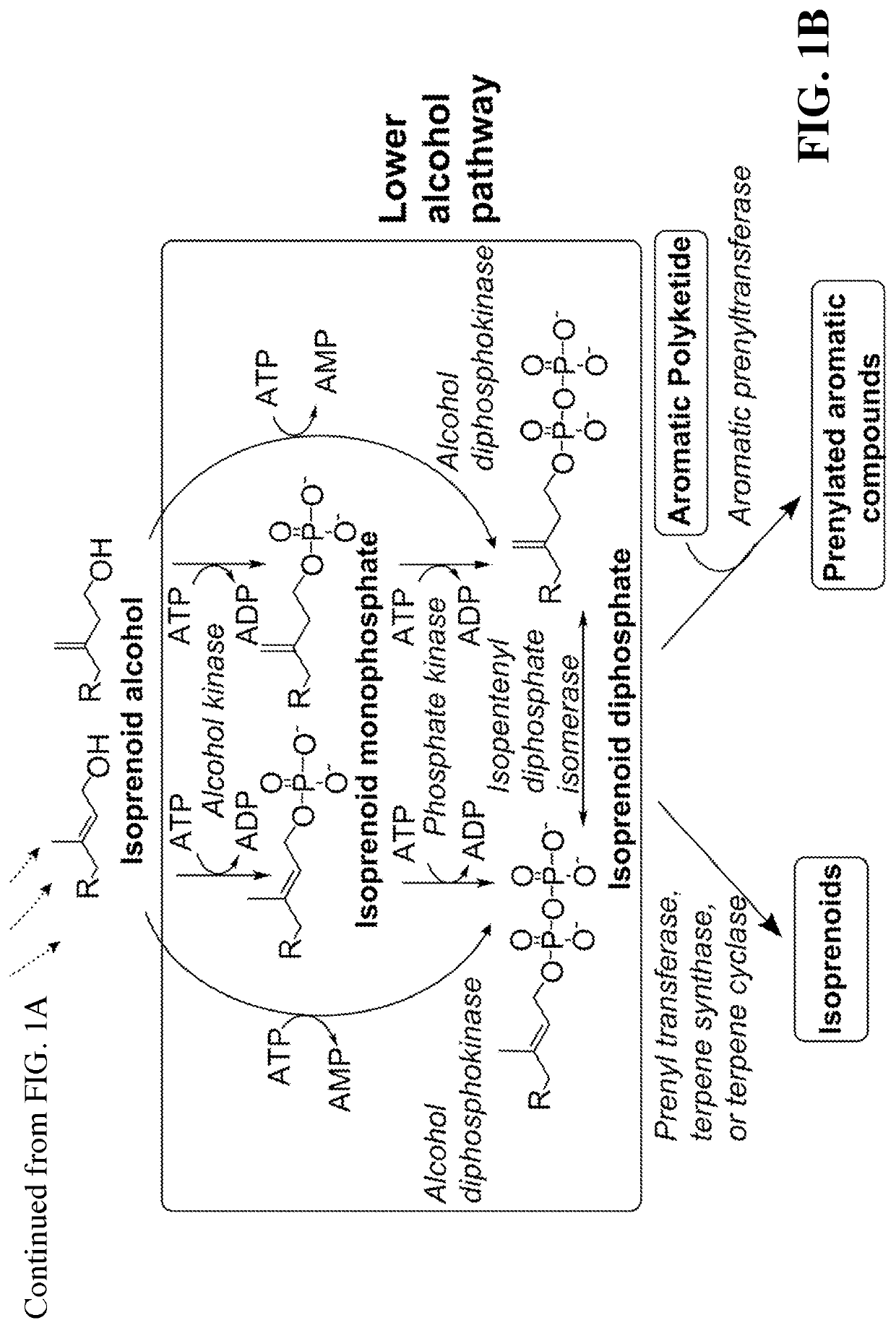
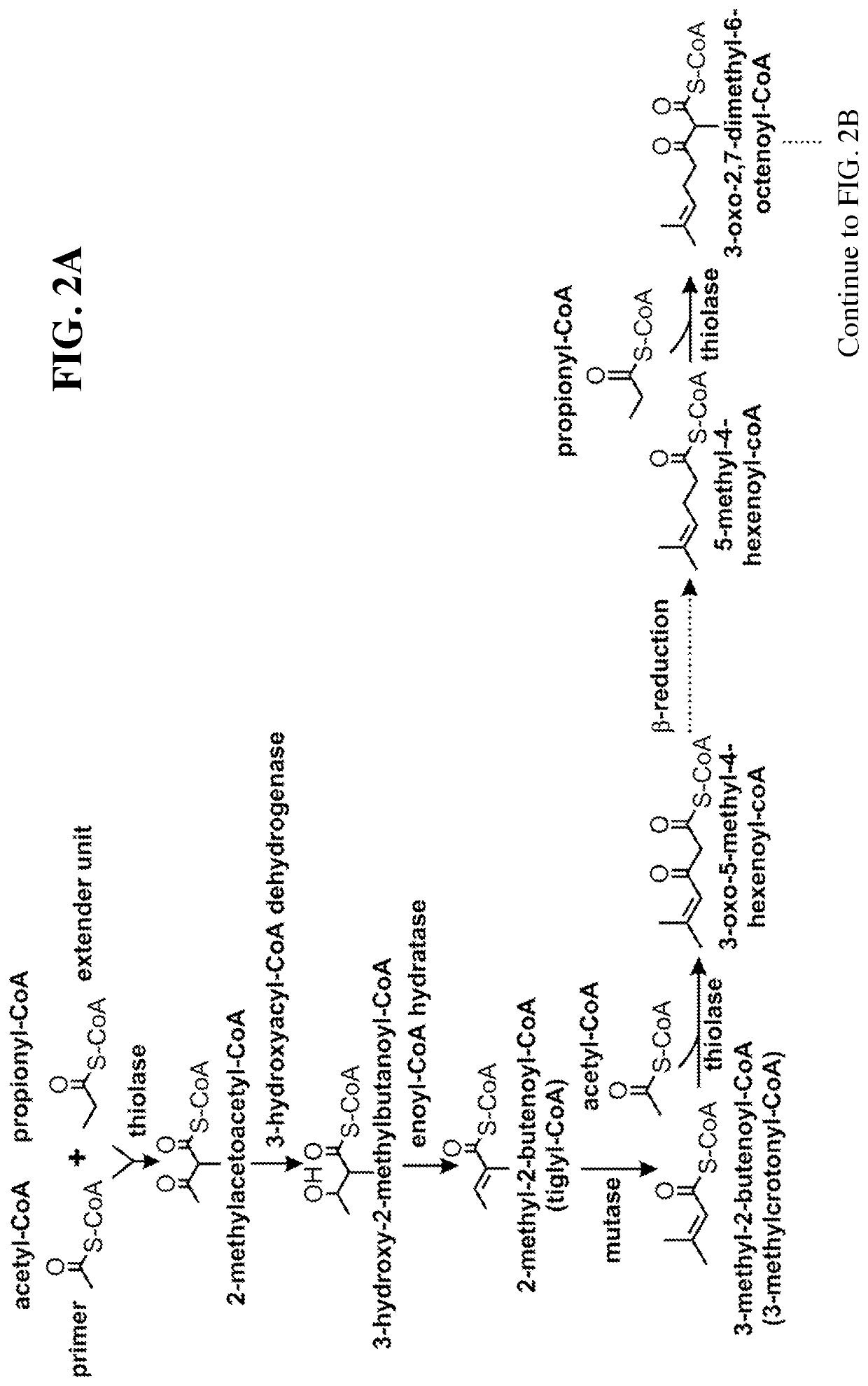
Microbial synthesis of isoprenoid precursors, isoprenoids and derivatives including prenylated aromatic compounds
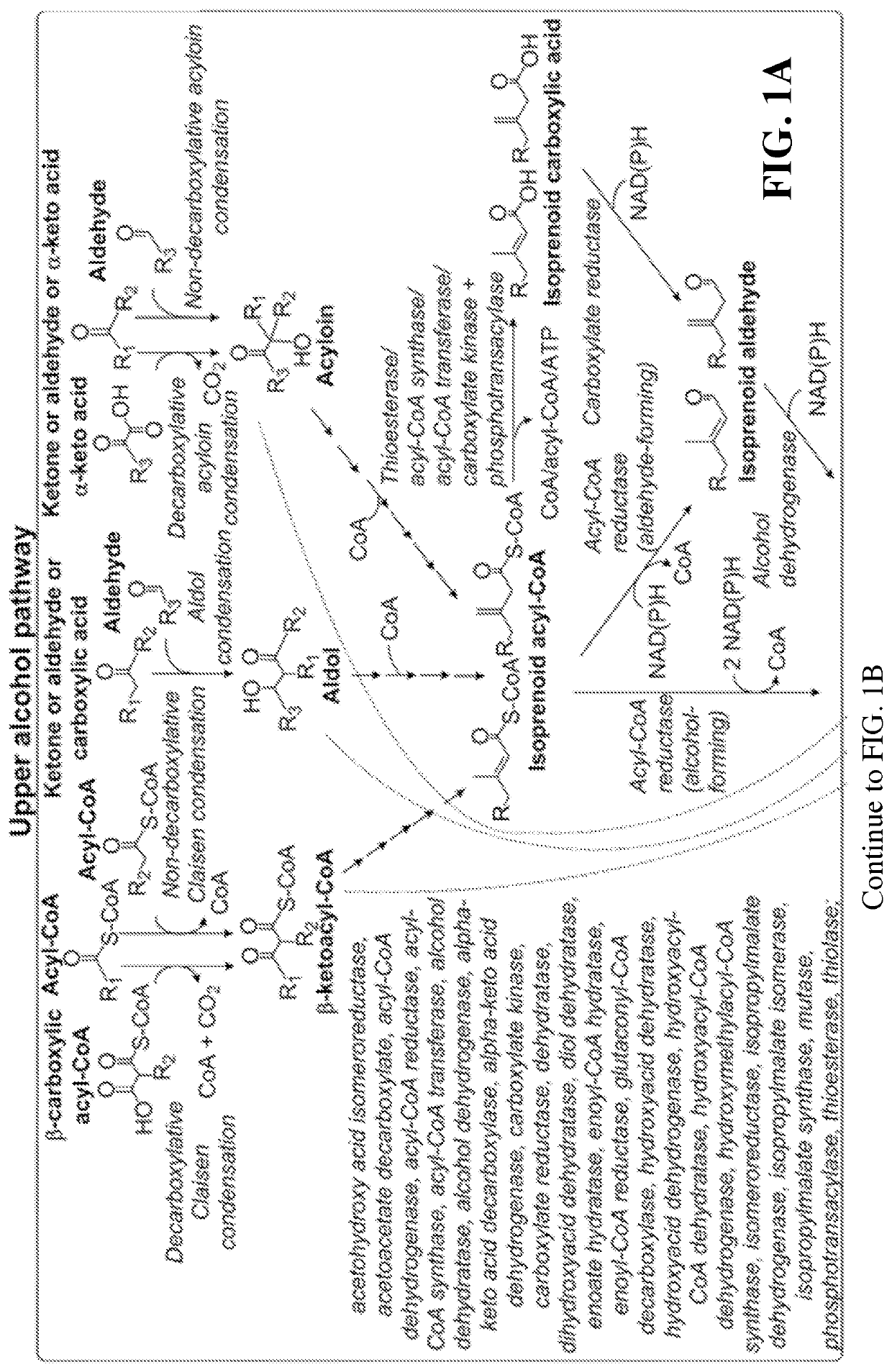
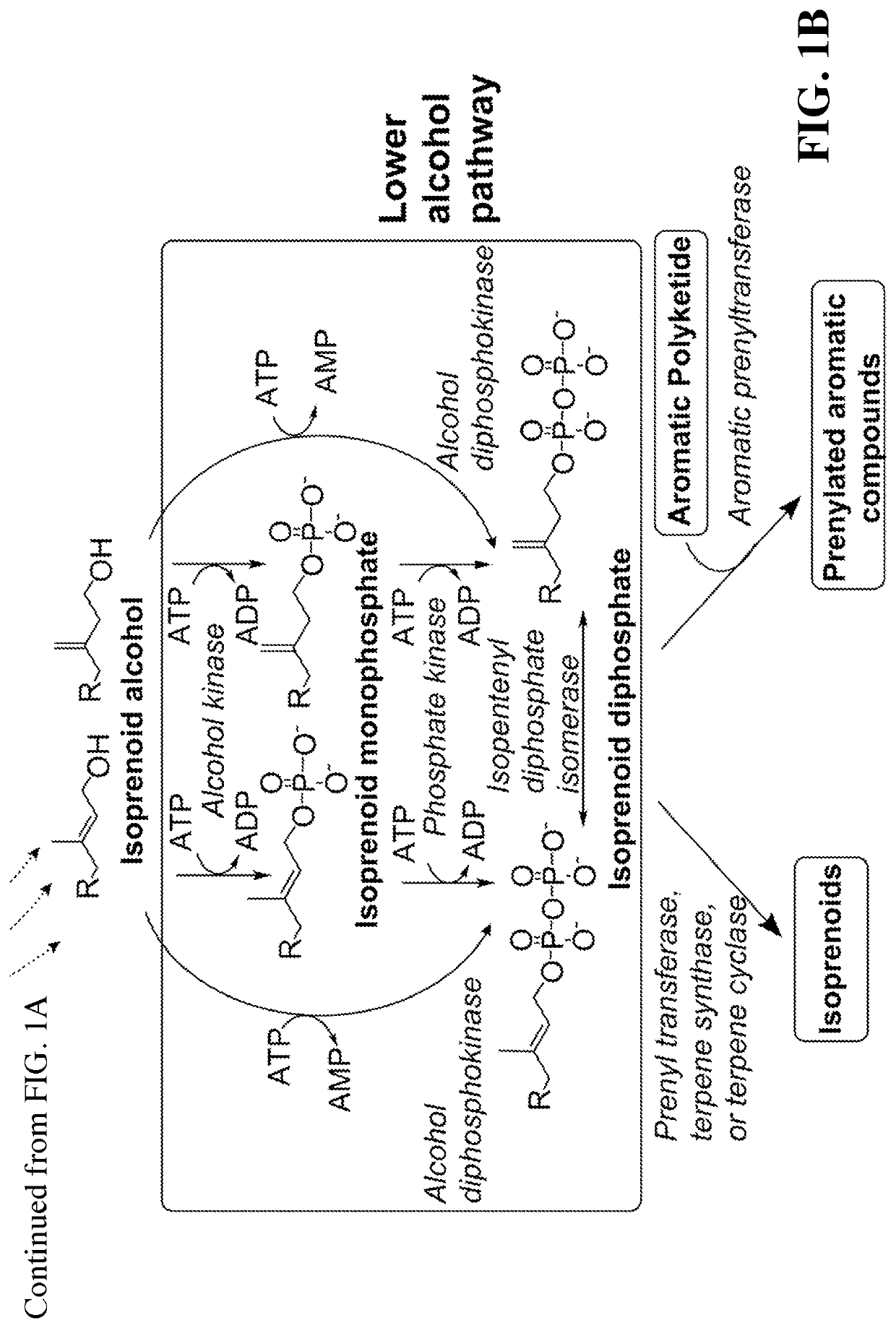
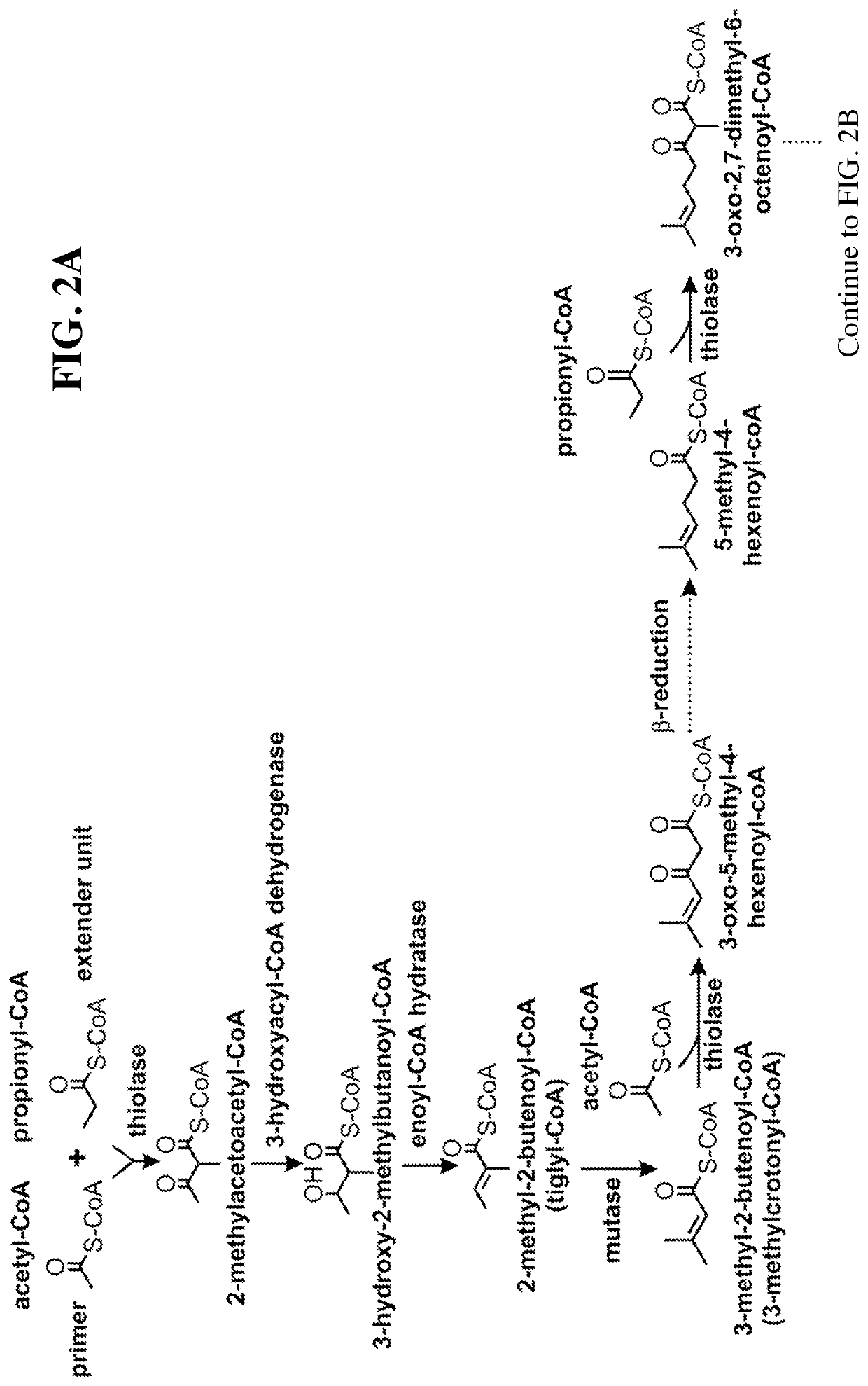
This disclosure generally relates to the use of enzyme combinations or recombinant microbes comprising same to make isoprenoid precursors, isoprenoids and derivatives thereof including prenylated aromatic compounds. Novel metabolic pathways exploiting Claisen, aldol, and acyioin condensations are used instead of the natural mevalonate (MVA) pathway or 1-deoxy-d-xylulose 5-phosphate (DXP) pathways for generating isoprenoid precursors such as isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), and geranyl pyrophosphate (GPP). These pathways have the potential for better carbon and or energy efficiency than native pathways. Both decarboxylative and non-carboxylative condensations are utilized, enabling product synthesis from a number of different starting compounds. These condensation reactions serve as a platform for the synthesis of isoprenoid precursors when utilized in combination with a variety of metabolic pathways and enzymes for carbon rearrangement and the addition / removal of functional groups. Isoprenoid alcohols are key intermediary products for the production of isoprenoid precursors in these novel synthetic metabolic pathways.
Owner:RICE UNIV
Method for synthesizing methyl heptenone from isopentenyl alcohol
ActiveCN109232212ALow costEasy to operateOrganic compound preparationCarbonyl compound preparationIsomerization2-Butene
The invention provides a method for synthesizing methyl heptenone from isopentenyl alcohol. The method comprises the following steps: (1) reacting isopentenyl alcohol with concentrated hydrochloric acid in the presence of a catalyst A, splitting phase for the reacting liquid, washing and distilling the organic phase to obtain a chloro mixture of 1-chloro-3-methyl-2-butene and 3-chloro-3-methyl-1-butene; (2) directly condensing the chloro mixture with acetone in the presence of a catalyst B to obtain a single product methyl heptenone. According to the method, isopentenyl alcohol is efficientlychlorinated in the presence of metal inorganic salt functioning as a catalyst and concentrated hydrochloric acid functioning as a chlorinating agent; the chlorinating reaction liquid is split and simply distilled to obtain the chlorinated mixture, operation steps of isomerization, rectification and the like are not required; alkali solution is promoted by using N,N-dimethyl formamide as a condensation solvent, and a phase transfer catalyst is not required to be added, the condensation time is reduced, and the reaction yield is improved.
Owner:WANHUA CHEM GRP CO LTD
Methods for analysis of isomeric lipids
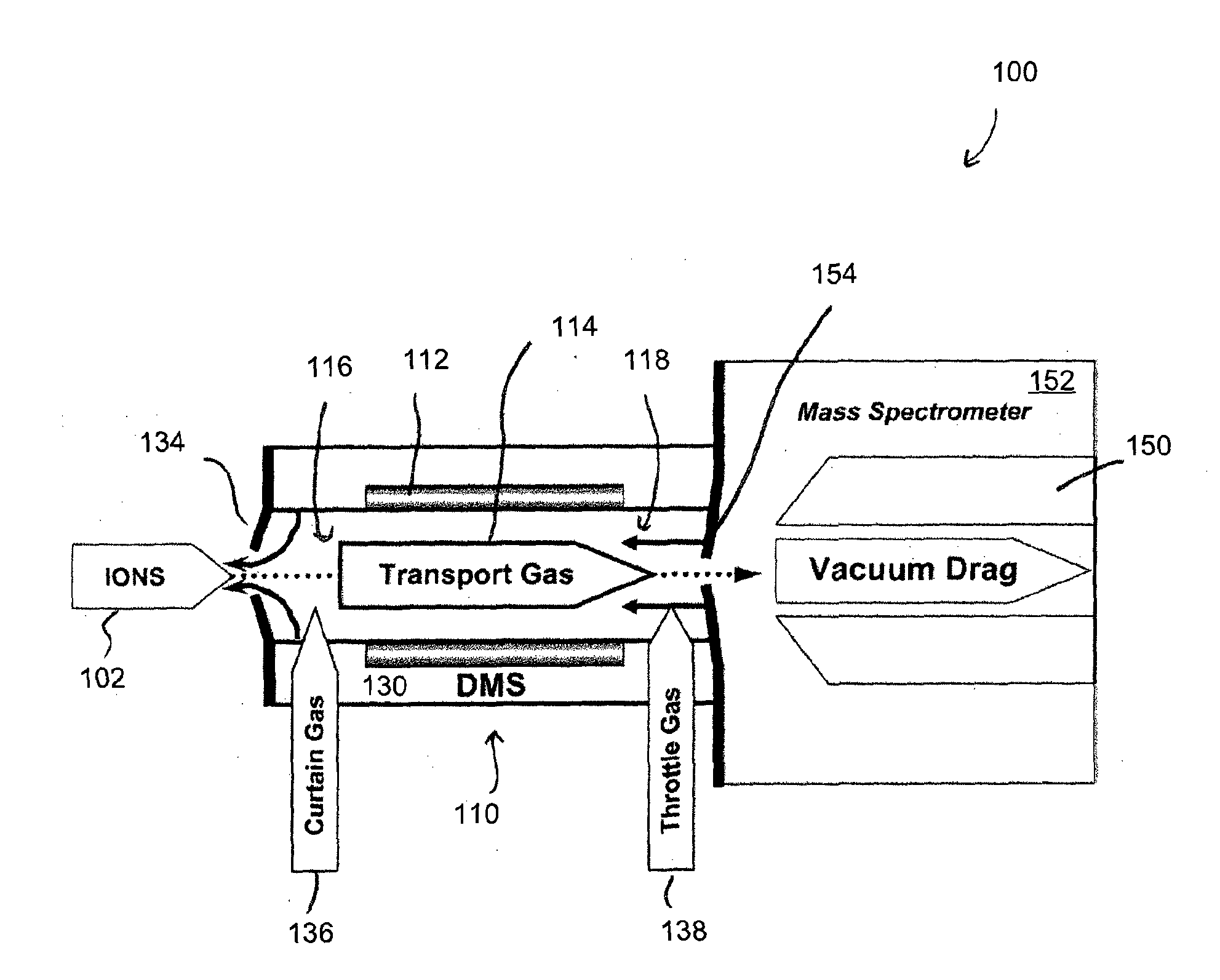
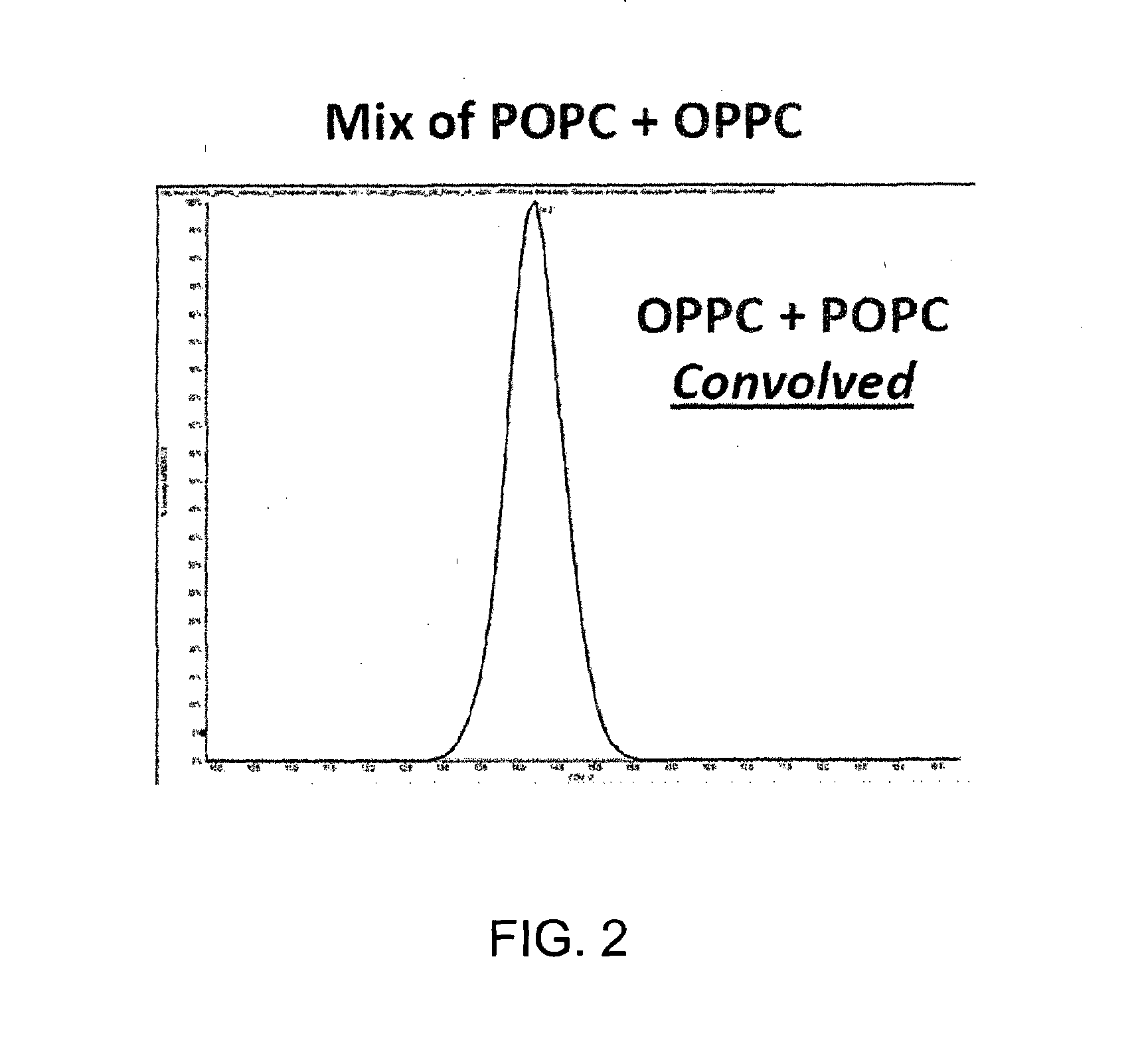
ActiveUS20150316507A1Allow separationEasy to separateSamples introduction/extractionMaterial analysis by electric/magnetic meansLipid formationPolyketide
A method for analyzing a sample that contains a plurality of lipid isomers is described that involves forming one or more lipid metal ion adducts and transporting the one or more lipid metal ion adducts through a Mix of POPC+OPPC differential mobility spectrometer to cause separation of the one or more lipid metal ion adducts from each other. The lipid isomers can be chosen, for example, from fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides, sterol lipids, and prenol lipids. Particular examples include phosphatidylcholine regioisomers such as 1-palmatoyl-2-oleoyl-sn-phosphatidylcholine (POPC) and 1-oleoyl-2-palmatoyl-sn-phosphatidylcholine and triacylglycerols containg palmetic and oleic acid groups. The metal chosen can include a cationization reagent that contains sodium, potassium, silver or lithium.
Owner:DH TECH DEVMENT PTE
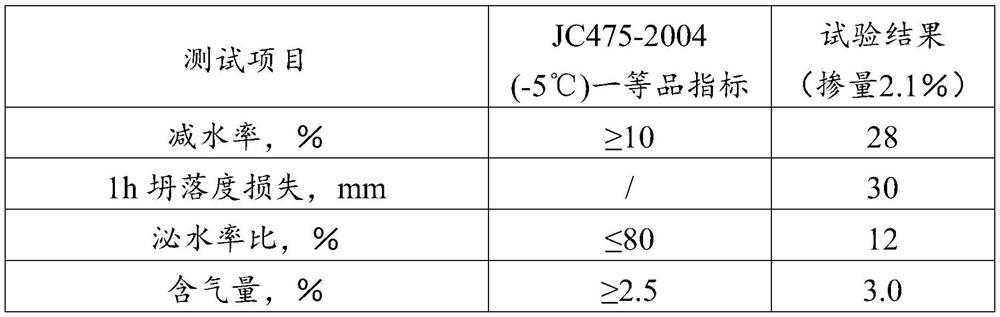
A kind of modified polycarboxylate water reducing agent and preparation method thereof
The invention relates to a modified polycarboxylate water reducer and a preparation method thereof. Isoprenol polyoxyethylene ether (TPEG), acrylic acid (AA) and modification monomer X are taken as monomers, and under the action of an initiator and a chain transfer agent, direct polymerization is performed to prepare the composite polycarboxylate water reducer which reduces water and retains slump. The modified polycarboxylate water reducer provided by the invention can be independently used or mixed with other water reducers for use, has an excellent slump retaining performance and good expansibility when the water reducer is used in concrete, has a dispersion delay effect on the concrete in an hour, and has a comprehensive performance exceeding the performances of existing products in the market.
Owner:CHONGQING ZHUYANG BUILDING MATERIAL CO LTD
Method for continuously synthesizing 3,3-dimethyl-4-pentenoic acid methylester
InactiveCN102557934AReduce contentNot corrosiveOrganic compound preparationCarboxylic acid esters preparation4-pentenoic acidAcetic acid
The invention discloses a method for continuously synthesizing 3,3-dimethyl-4-pentenoic acid methylester and relates to a compound synthesizing technology. Methyl-2-buten-1-ol and trimethyl orthoacetate are taken as raw materials, phenol is taken as a reaction catalyst, and the method comprises the following process steps of: (A) dispensing: continuously adding the trimethyl orthoacetate, the methyl-2-buten-1-ol and the catalyst phenol into a dispensing kettle, and uniformly mixing; (B) synthesizing and separating: continuously conveying the material in the dispensing kettle to a synthesis and separation tower for reaction, continuously separating out the generated byproduct methanol and excessive trimethyl orthoacetate on the top of the tower, and continuously extracting residue at the bottom of the tower; (C) distilling at reduced pressure: continuously injecting the residue of the synthesis and separation tower into a rectifying still, distilling at reduced pressure, and separating out the finished product 3,3-dimethyl-4-pentenoic acid methylester; and (D) storing: conveying the finished product 3,3-dimethyl-4-pentenoic acid methylester to a storage tank, and standing for later use. By the method, the synthetic yield of the 3,3-dimethyl-4-pentenoic acid methylester is improved, and full continuous operation of the process for synthesizing the finished product 3,3-dimethyl-4-pentenoic acid methylester is realized.
Owner:山东高新润农化学有限公司
Synthesis of isoprenoids and derivatives
This disclosure generally relates to the use of enzyme combinations or recombinant microbes comprising same to make isoprenoid precursors, isoprenoids and derivatives thereof including prenylated aromatic compounds. Novel metabolic pathways exploiting Claisen, aldol, and acyioin condensations are used instead of the natural mevalonate (MVA) pathway or 1-deoxy-d-xylulose 5-phosphate (DXP) pathways for generating isoprenoid precursors such as isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), and geranyl pyrophosphate (GPP). These pathways have the potential for better carbon and or energy efficiency than native pathways. Both decarboxylative and non-carboxylative condensations are utilized, enabling product synthesis from a number of different starting compounds. These condensation reactions serve as a platform for the synthesis of isoprenoid precursors when utilized in combination with a variety of metabolic pathways and enzymes for carbon rearrangement and the addition / removal of functional groups. Isoprenoid alcohols are key intermediary products for the production of isoprenoid precursors in these novel synthetic metabolic pathways.
Owner:RICE UNIV
Pentenoic acid methylester preparation process
The invention mainly discloses a pentenoic acid methylester preparation method, which is characterized in that prenol and trimethyl orthoacetate are in pressurized reaction in the existence of catalysts, and the by-product methanol is recovered by rectifying, so that pentenoic acid methylester is prepared. The pentenoic acid methylester preparation process has the advantages that reaction time is only 12-15 hours by means of pressurizing and catalyst synthesis, and the content of the prepared pentenoic acid methylester is higher than 99.8%.
Owner:NANTONG TENDENCI CHEM
Host cells and methods for producing isoprenyl alkanoates
The invention provides for a method of producing an isoprenyl alkanoate in a genetically modified host cell. In one embodiment, the method comprises culturing a genetically modified host cell which expresses an enzyme capable of catalyzing the esterification of an isoprenol and a straight-chain fatty acid, such as an alcohol acetyltransferase (AAT), wax ester synthase / diacylglycerol acyltransferase (WS / DGAT) or lipase, under a suitable condition so that the isoprenyl alkanoate is produced.
Owner:RGT UNIV OF CALIFORNIA
Process for producing prenol and prenal from isoprenol
ActiveUS20190077736A1High reactivityHigh selectivityPreparation by isomerisationOrganic compound preparationIsomerizationDehydrogenation
The present invention relates to a process for preparing 3-methyl-2-butenol (prenol) and 3-methyl-2-butenal (prenal) from 3-methyl-3-butenol (isoprenol), in which 3-methyl-3-butenol is subjected to a catalytic isomerization over a carbon-supported Pd catalyst in the presence of a gas mixture comprising 1% to 15% by volume of oxygen to obtain a first product mixture, and the first product mixture is subjected to an oxidative dehydrogenation over a Pd catalyst comprising SiO2 and / or Al2O3 as support material, or over a carbon-supported Pd / Au catalyst in the presence of a gas mixture comprising 5% to 25% by volume of oxygen.
Owner:BASF AG
Polyether derivatives, uses, and methods of making the same
The invention contemplates certain polyethers, polyether derivatives, and methods of making and using those same polymers. For example, the starting materials can, e.g., citronellol, prenol, isocitronellol and isoprenol.
Owner:P2 SCI INC
Method for synthesizing prenol from butenol
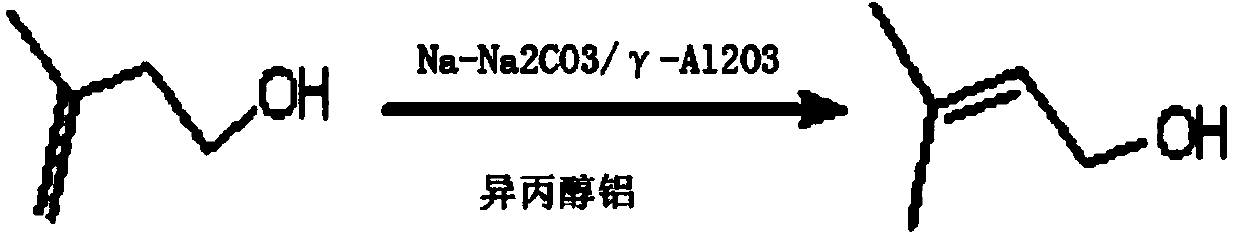
ActiveCN105111044BHigh activityMild reaction conditionsPreparation by isomerisationDouble bondAluminium isopropoxide
The invention is suitable for the technical field of a synthetic method for isopentenol and provides a method for synthesizing isopentenol from butenol. The method is a method for isomerizing double bonds of 3-methyl-3-butenol under the catalytic action of a catalyst composed of solid super base and aluminium isopropoxide, wherein the solid super base is Na-Na2CO3 / gamma-Al2O3, the dosage of the catalyst is 0.2-12% of 3-methyl-3-butenol, and the mass ratio of the solid super base and aluminium isopropoxide in the catalyst is 1: 1 to 1: 2. The reaction pressure is 0.5-4MPa, the temperature is 100-140 DEG C and the time is 2-3 hours. The method provided by the invention is mild in reaction condition, the catalyst is high in activity and easy to separate and can be repeatedly used, the method is low in production cost, environmental-friendly and safe in production, and the product is high in purity and selectivity and green and environmental-friendly, so that the cost is greatly lowered, and the method is an efficient and economical synthetic method and has a wide prospect.
Owner:山东成泰新材料有限公司
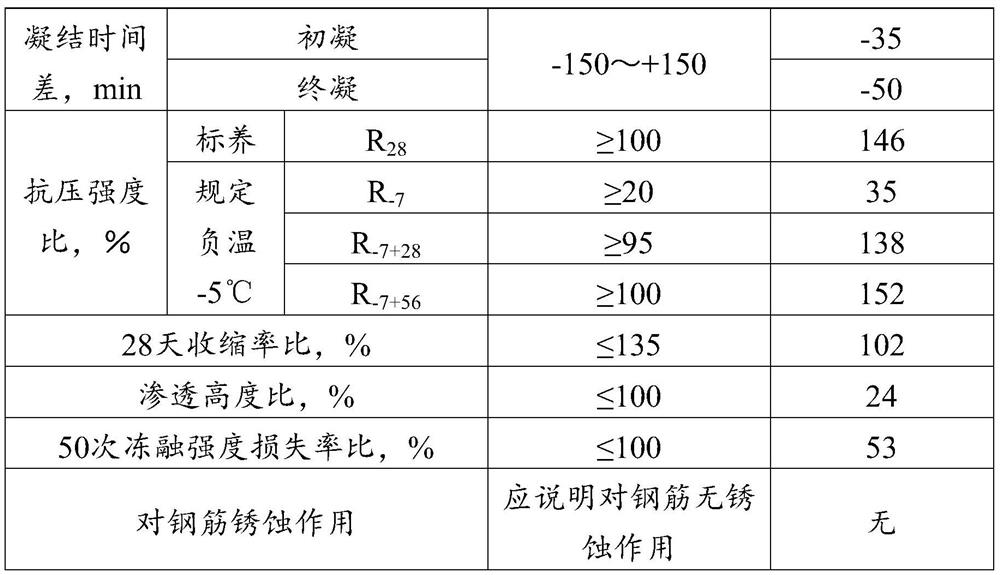
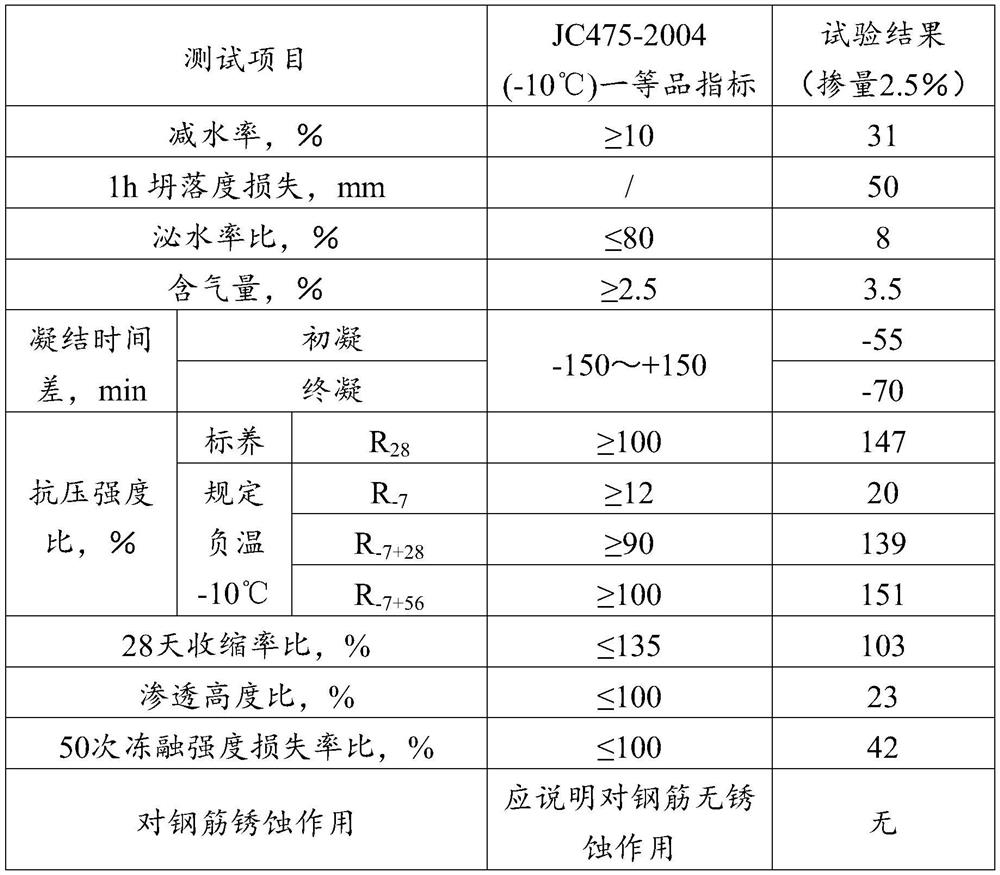
Water reducing agent and preparation method thereof, antifreeze agent and use thereof, and concrete
The invention provides a water reducing agent and a preparation method thereof, an antifreeze agent and its use, and concrete, and relates to the technical field of antifreeze. The water reducing agent includes the following raw materials: prenol polyoxyethylene ether and allyl alcohol polyoxyethylene ether, and at least one of acrylic acid and maleic anhydride, wherein the prenol polyoxyethylene ether and the The molar ratio of the allyl alcohol polyoxyethylene ether is 1:(0.5-1). Antifreezes include the aforementioned water reducers and non-oxidizing salts. In the water reducing agent, isoprenol polyoxyethylene ether and allyl alcohol polyoxyethylene ether are used to synthesize the water reducing agent. Workability, when the water reducer is used in antifreeze, because the water reducer has strong early strength properties, it can effectively reduce the use of antifreeze early strength components in antifreeze; antifreeze is safe and conducive to Reducing the alkali content in concrete can effectively reduce the risk of concrete bleeding.
Owner:北京金隅节能科技有限公司 +1
Catalyst for preparing prenyl alcohol from isopentenyl acetate through transesterification and application of catalyst
ActiveCN108927171ASolve problems such as difficult separationHigh selectivityPreparation by ester-hydroxy reactionOrganic compound preparationAlcoholTransesterification
The invention relates to a catalyst and reaction process for preparing prenyl alcohol from isopentenyl acetate through transesterification. The reaction process comprises the step: carrying out transesterification under the action of a solid acid serving as a catalyst by taking isopentenyl acetate and n-alkanol as reaction raw materials to generate prenyl alcohol and certain acetate. The n-alkanolcomprises methanol, ethanol, propanol and butanol, and corresponding certain acetate is methyl acetate, ethyl acetate, propyl acetate and butyl acetate. The solid acid catalyst adopts Ir-Fe / CeO2 / SiO2as a catalyst system. It is particularly noted that an important reaction process step for obtaining a target product with high yield (larger than or equal to 90%) is to firstly introduce H2 for a period of time in a reaction process, and then, turning to introduce N2. The reaction process has the remarkable advantages such as low economic and environmental costs as well as simplicity in productseparation and aftertreatment so as to have a good application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
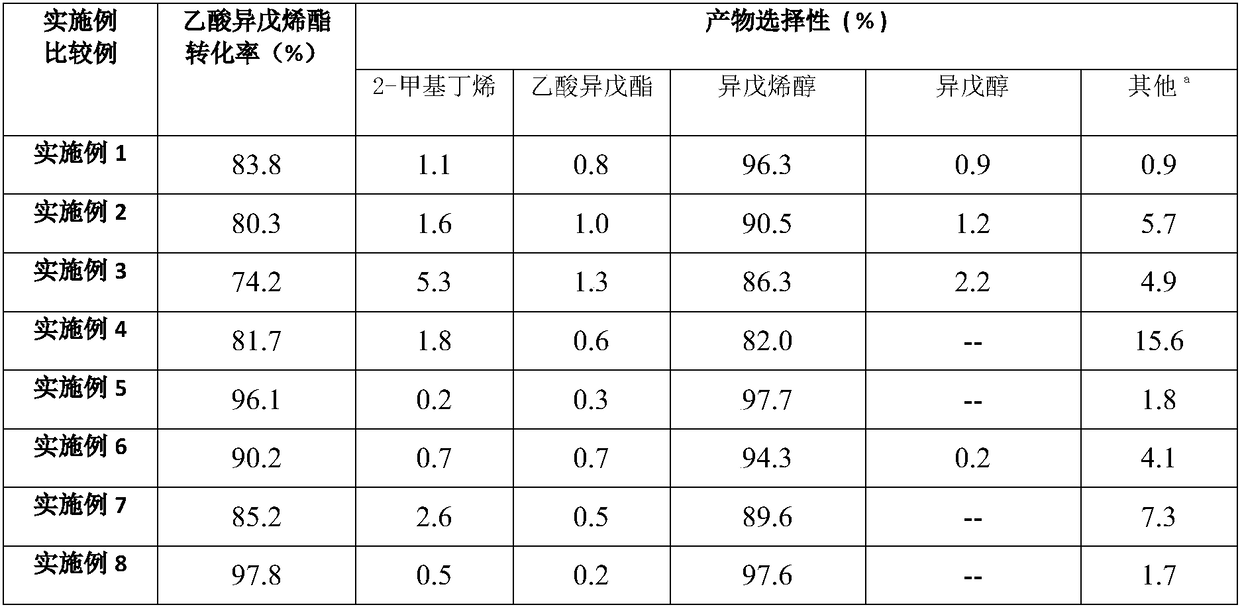
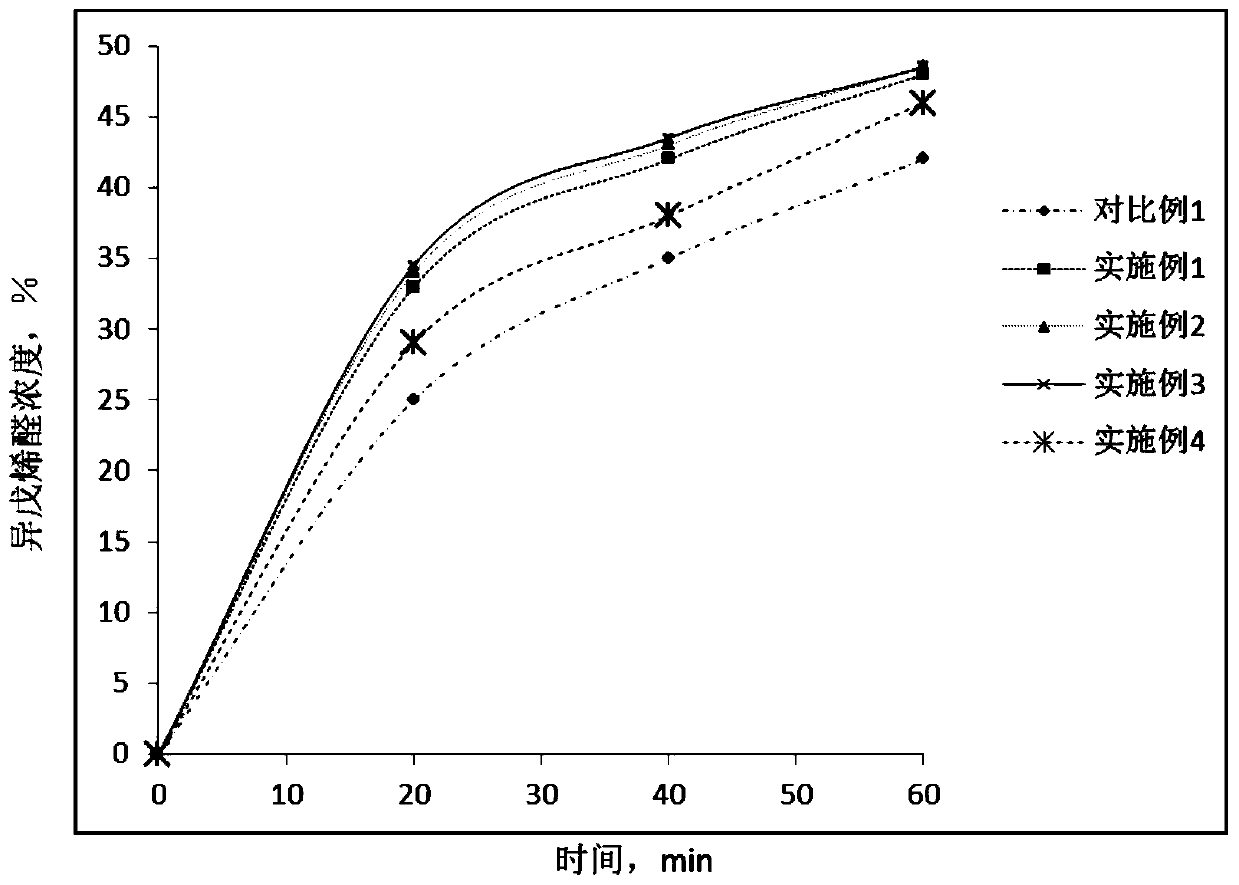
Method for preparing methyl-2-butenal
ActiveCN110407680AHigh selectivityInhibitors inhibit the production ofOrganic compound preparationCarbonyl compound preparationButeneAlcohol
The invention discloses a method for preparing methyl-2-butenal. The method comprises the steps of oxidizing iso-isopentenyl alcohol to obtain an organic phase containing the iso-isopentenyl alcohol and isomethyl-2-butenal, optionally separating the iso-isopentenyl alcohol from the isomethyl-2-butenal in the organic phase, adding isopentenyl alcohol and / or 2-methyl-3-butene-2-ol into the organic phase containing the isomethyl-2-butenal or the separated isomethyl-2-butenal to serve as a rearrangement reaction aid, and optionally adding a trace amount of citral to serve as an inhibitor. The method can significantly improve the selectivity of the methyl-2-butenal and inhibit the occurrence of a side reaction of generating tar.
Owner:WANHUA CHEM GRP CO LTD
A polycarboxylate water reducer for prestressed high-strength concrete pipe piles and its application
The invention discloses a polycarboxylic acid water reducer for a pre-stressed high-strength concrete pipe pile. The water reducer comprise the following components: methallyl polyoxyethylene ether 2400, isoprenol polyoxyethylene ether 2400, acrylic acid, maleic anhydride, sodium methacryl sulfonate, N-hydroxymethyl acrylamide, a 30% sodium hydroxide solution, a chain transfer agent, an oxidant, a reductant, a water softener and a 30% sodium hydroxide solution used for adjusting the pH value of the solution, wherein the mass ratio of methallyl polyoxyethylene ether 2400 to isoprenol polyoxyethylene ether 2400 is 1:(0.5-2) and the mass ratio of acrylic acid to maleic anhydride is 1:(0-1). The polycarboxylic acid water reducer disclosed by the invention is reliable in quality and when the polycarboxylic acid water reducer is applied in concrete, the concrete has the advantages of low entrained air, short setting time, high strength, good cohesiveness, easiness for centrifugal dehydration and less in possibility of pulp hanging.
Owner:太仓新亚逊生物科技有限公司
Process for producing prenol and prenal from isoprenol
ActiveUS10414707B2Increase conversion ratePreparation by isomerisationOrganic compound preparationIsomerizationDehydrogenation
The present invention relates to a process for preparing 3-methyl-2-butenol (prenol) and 3-methyl-2-butenal (prenal) from 3-methyl-3-butenol (isoprenol), in which 3-methyl-3-butenol is subjected to a catalytic isomerization over a carbon-supported Pd catalyst in the presence of a gas mixture comprising 1% to 15% by volume of oxygen to obtain a first product mixture, and the first product mixture is subjected to an oxidative dehydrogenation over a Pd catalyst comprising SiO2 and / or Al2O3 as support material, or over a carbon-supported Pd / Au catalyst in the presence of a gas mixture comprising 5% to 25% by volume of oxygen.
Owner:BASF AG
Method for extraction of high purity solanocupsin and synthesis of isodecyl dienol and isoprenol
InactiveCN100417632CHigh yieldLow costOrganic compound preparationHydroxy compound separation/purificationIsopreneEthyl fumarate
The invention provides a process for preparing high-purity solanesol, which is used as the raw material for halogenating, and reacting with acetoacetic ester to obtain solanesol propanone, then is subject to reaction with the chlorovinyl magnesium to obtain isoprene alcohol, wherein the overall yield rate of solanesol is 48%.
Owner:玉溪健坤生物药业有限公司
A kind of high molecular polymer, its preparation method and electrolyte solution containing it
ActiveCN109400786BThe synthesis method is pureHigh purityElectrolytic capacitorsElectrolytic agentHigh temperature storage
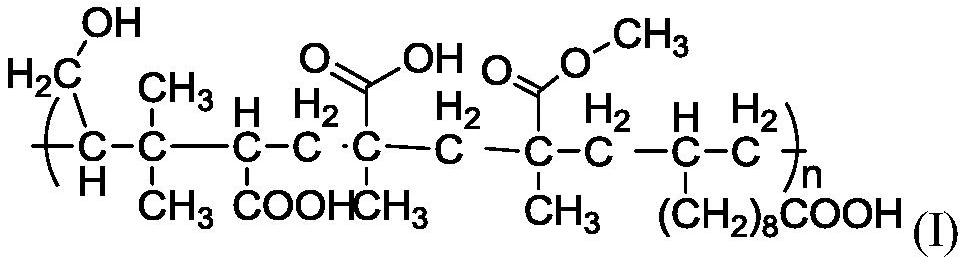
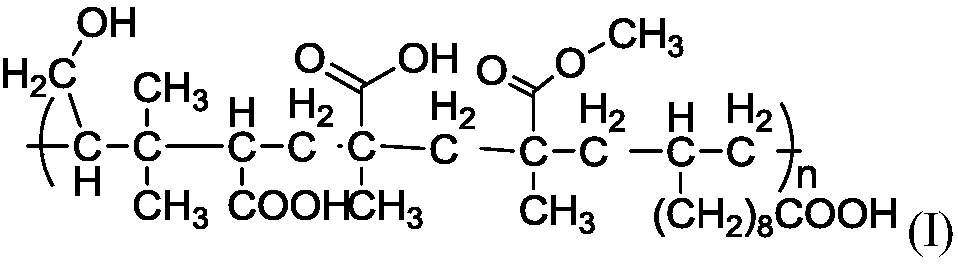
Theinvention provides a high molecular polymer, a preparation method thereof and an electrolyte containing the same. The high molecular polymer is shown in a formula (I), and is polymerized from monomeric isoprenol, methyl methacrylate, methacrylic acid, undecylenic acid and acrylic acid. The electrolyte uses the high molecular polymer shown in the formula (I) as a main solute. The 650V ultrahighvoltage capacitor prepared from the electrolyte meets the use requirements, and is more excellent in leakage current, loss and high temperature storage than similar capacitor products.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
A method for the selective hydrogenation of prenyl aldehyde to synthesize prenol
ActiveCN104387235BEasy to separateSimple and fast operationOrganic compound preparationHydroxy compound preparationHydrogenation reactionNitrogen
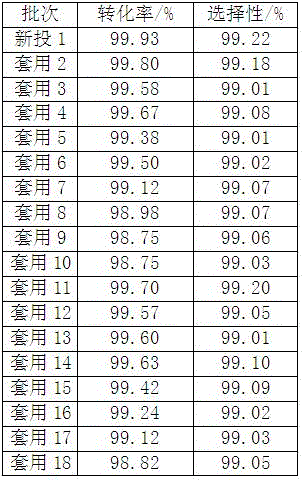
The invention discloses a method for synthesizing prenol employing selective hydrogenation of 3-methylcrotonaldehyde. The method comprises the following steps: feeding, nitrogen and hydrogen substitution, hydrogenation reaction, and separation. The used raw materials comprise 1 part of 3-methylcrotonaldehyde, 0.1-10 parts of water and 0.02-0.2 part of a catalyst; and the used catalyst is a water-soluble complex which is formed by a water-soluble salt of the group VIII metal and a water-soluble ligand. The 3-methylcrotonaldehyde conversion ratio and the selectivity of prenol of the synthetic reaction are high; a water phase of the catalyst can be applied repeatedly for over 20 times; the unit consumption of the catalyst is low; the production cost is greatly reduced; and the method is suitable for industrial production.
Owner:SHANDONG NHU PHARMA
Synthesis of Prenyl Alcohol by Isomerization of Aqueous Methyl Butenol
ActiveCN105967978BHigh reactivityHigh selectivityPreparation by isomerisation3-methyl-2-buten-1-olIsomerization
The invention belongs to the field of chemical engineering and particularly relates to isomerization synthesis of 3-methyl-2-buten-1-ol using methyl butenol in a water-containing condition. In the invention, the methyl butenol is adopted as a raw material for isomerization synthesis of 3-methyl-2-buten-1-ol; in the reaction materials, the water content is 0.5-6.5%; the catalyst is vanadium oxide or vanadium-containing metal salt; the catalyst concentration is 0.1-5.0%, the reaction temperature is 120-190 DEG C, and the reaction time is 20-180min; the purity of the obtained 3-methyl-2-buten-1-ol product is higher than or equal to 98%; and the 3-methyl-2-buten-1-ol selectivity in the reaction process can be controlled at 95% or above. The invention has the following advantages: the adopted catalyst does not need to be prepared in advance and is relatively cheap; the raw material methyl butenol can be adopted for isomerization synthesis of 3-methyl-2-buten-1-ol in a water-containing condition; and the reaction activity and selectivity are relatively high.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
High molecular polymer, preparation method thereof and electrolyte containing same
ActiveCN109400786AThe synthesis method is pureHigh purityElectrolytic capacitorsHigh temperature storagePolymer science
Theinvention provides a high molecular polymer, a preparation method thereof and an electrolyte containing the same. The high molecular polymer is shown in a formula (I), and is polymerized from monomeric isoprenol, methyl methacrylate, methacrylic acid, undecylenic acid and acrylic acid. The electrolyte uses the high molecular polymer shown in the formula (I) as a main solute. The 650V ultrahighvoltage capacitor prepared from the electrolyte meets the use requirements, and is more excellent in leakage current, loss and high temperature storage than similar capacitor products.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com