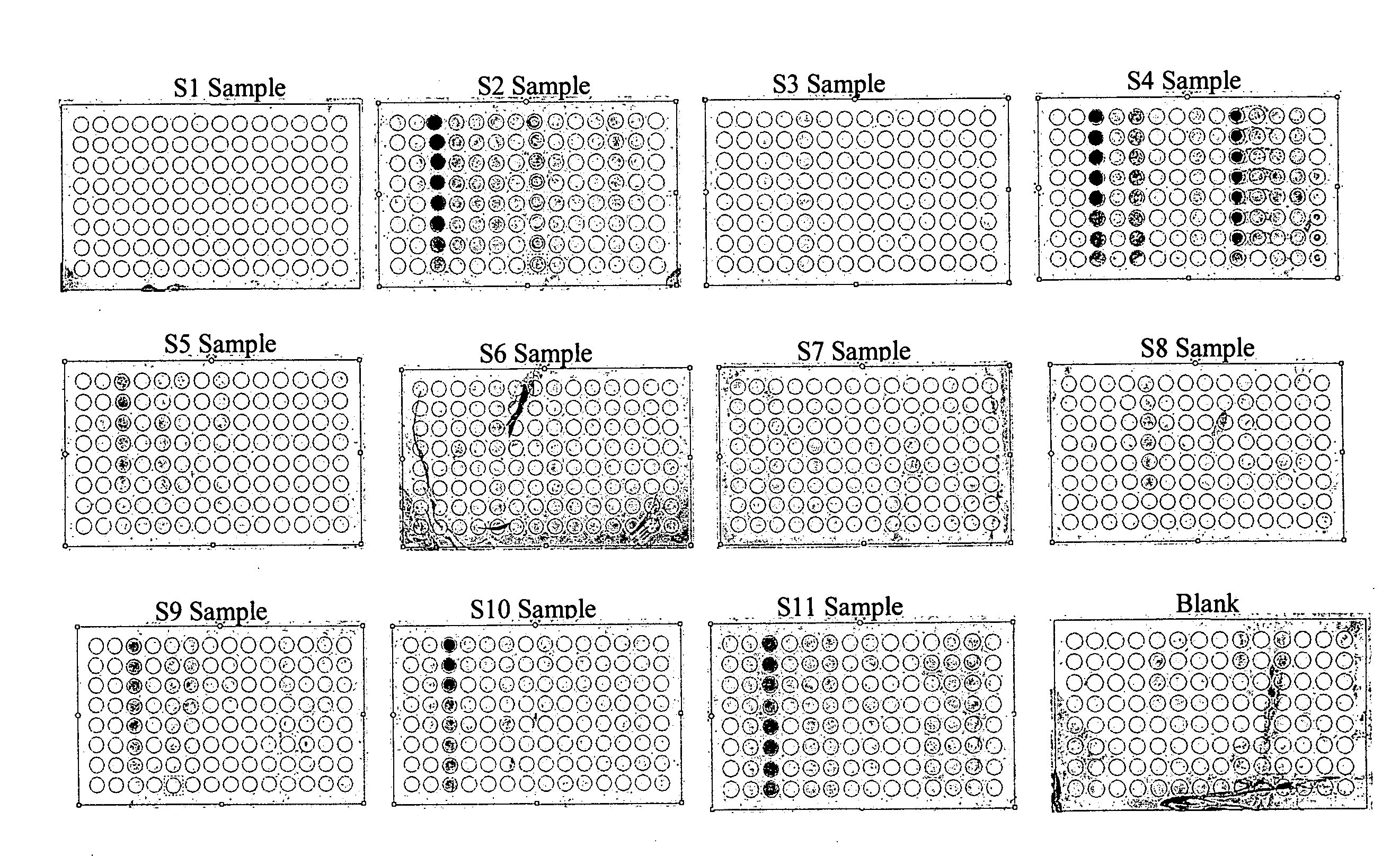
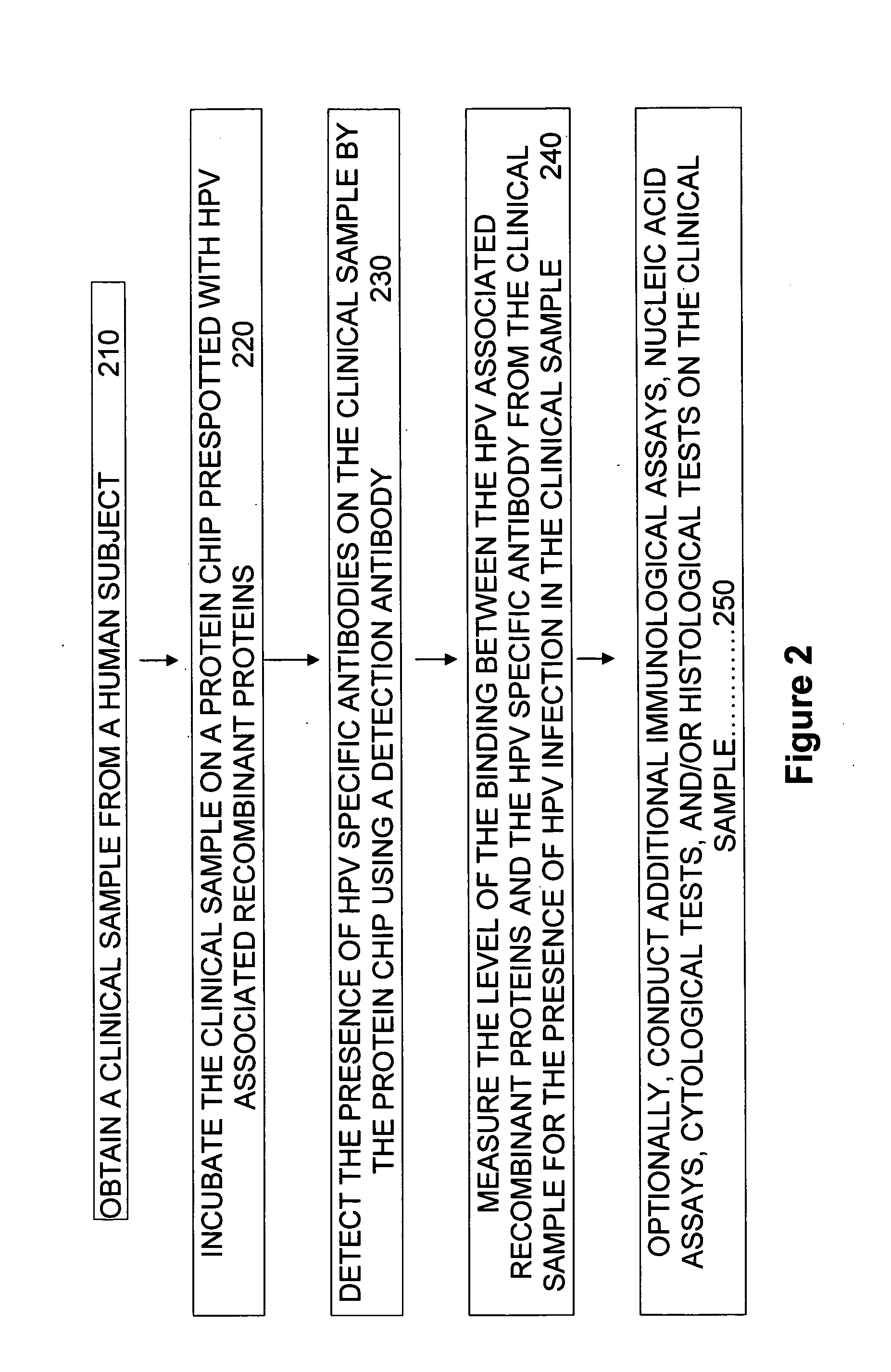
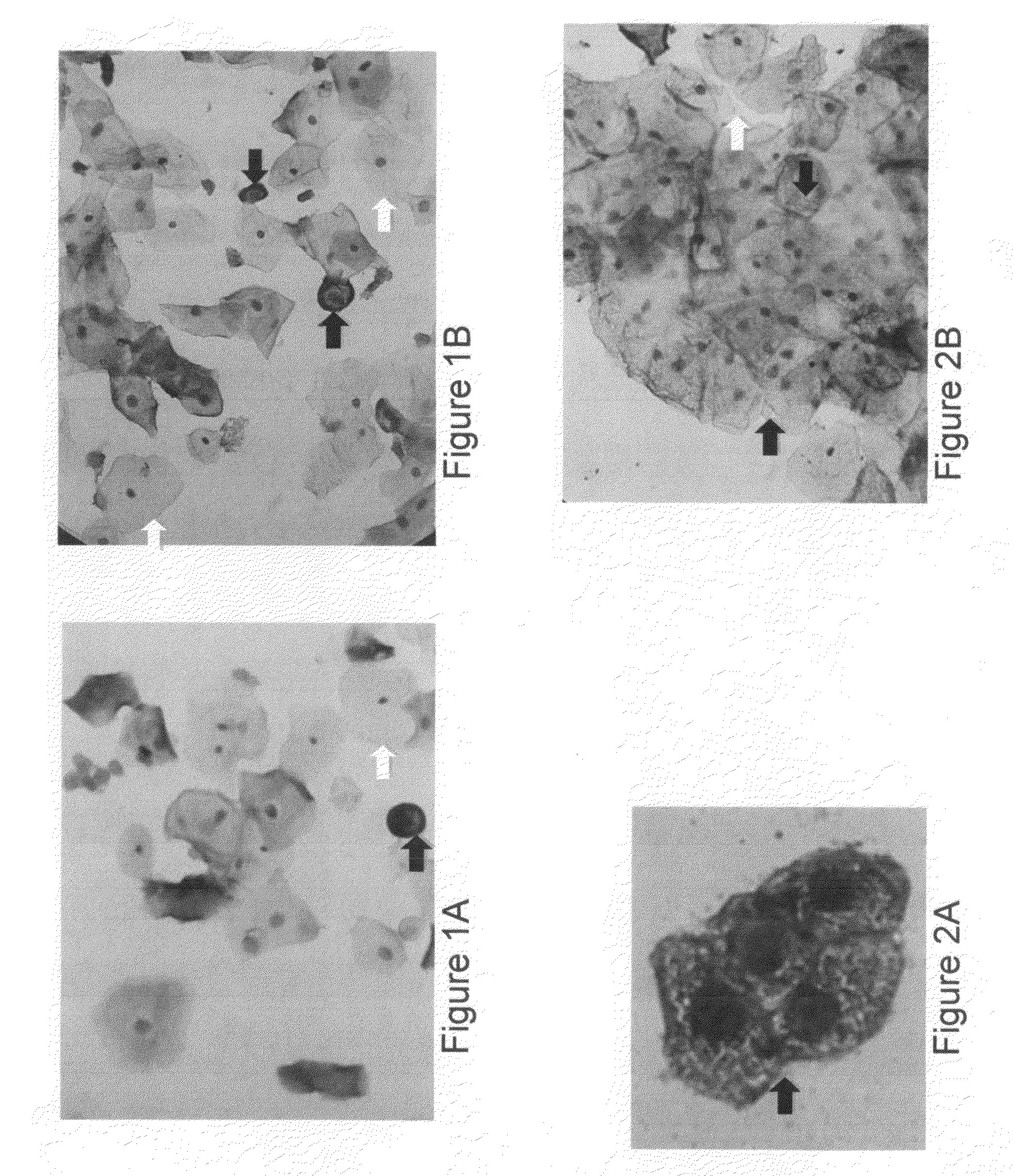
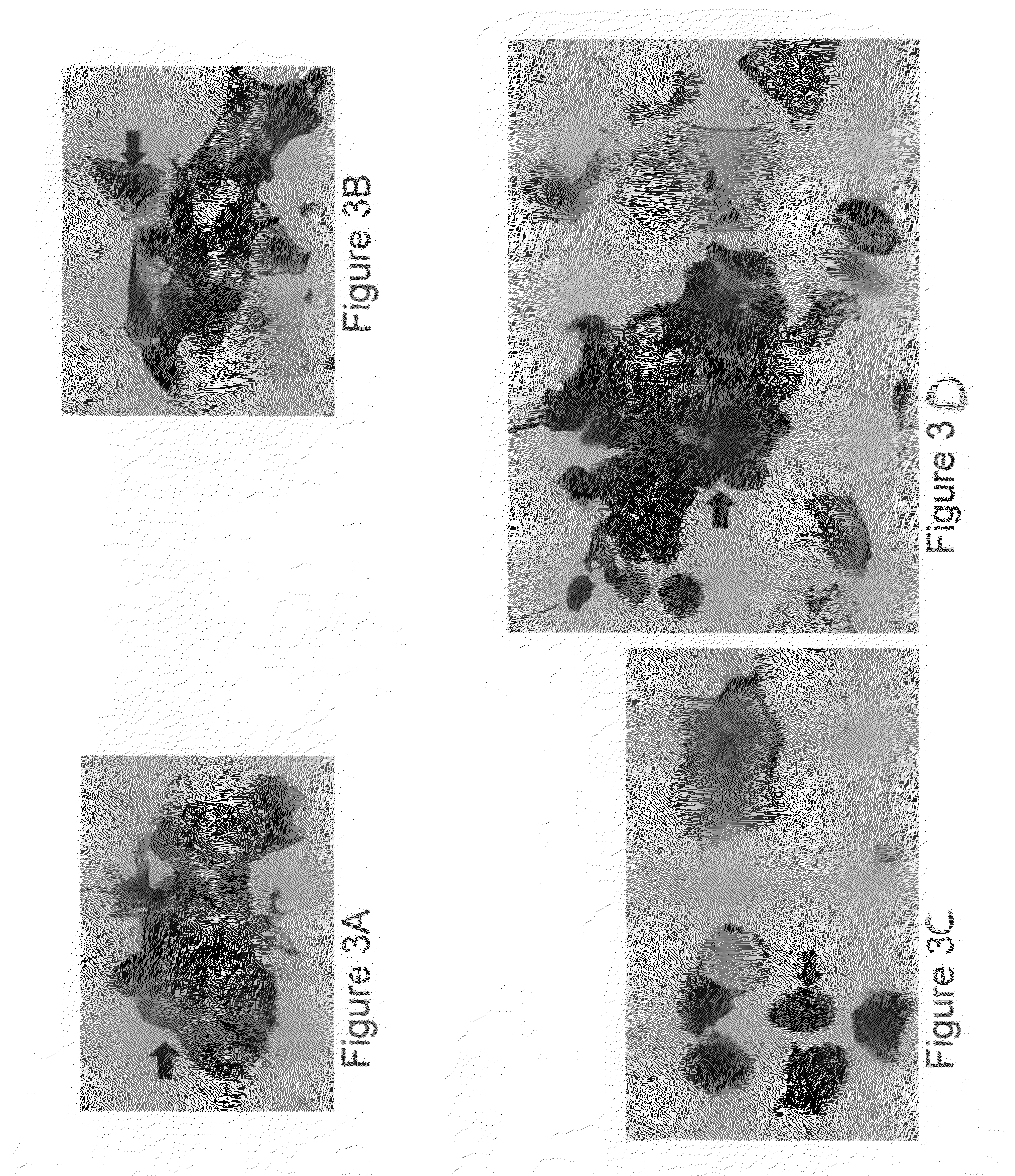
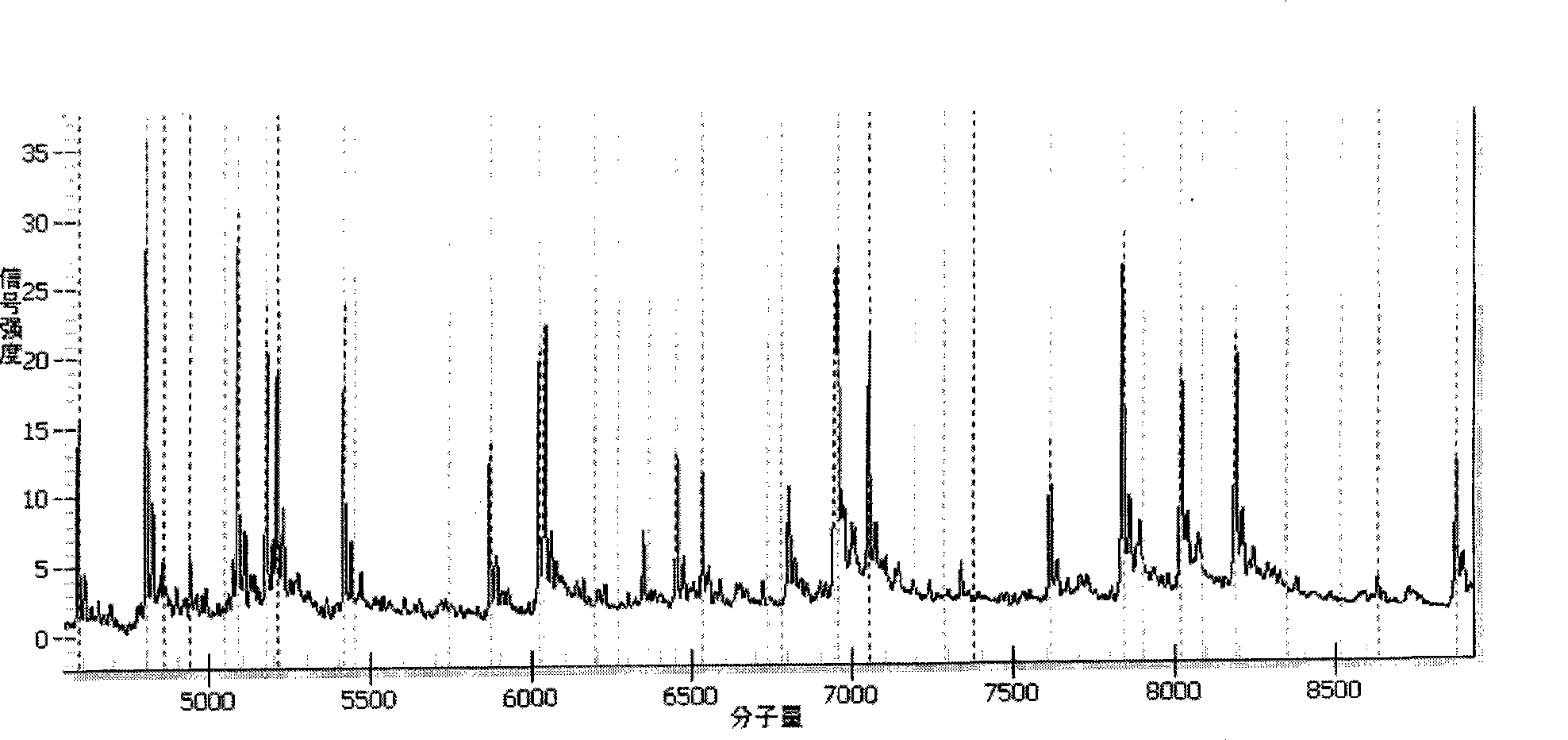
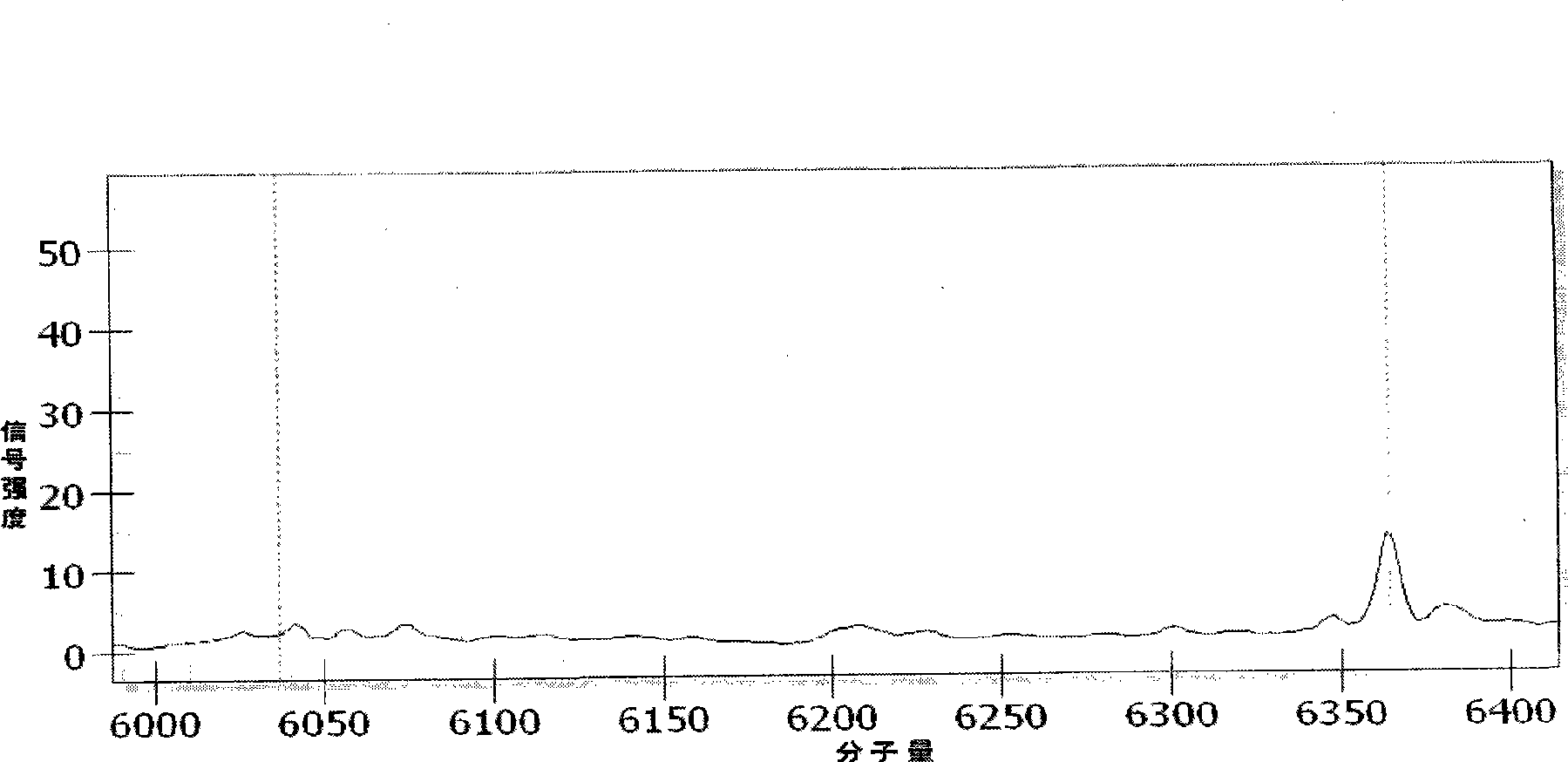
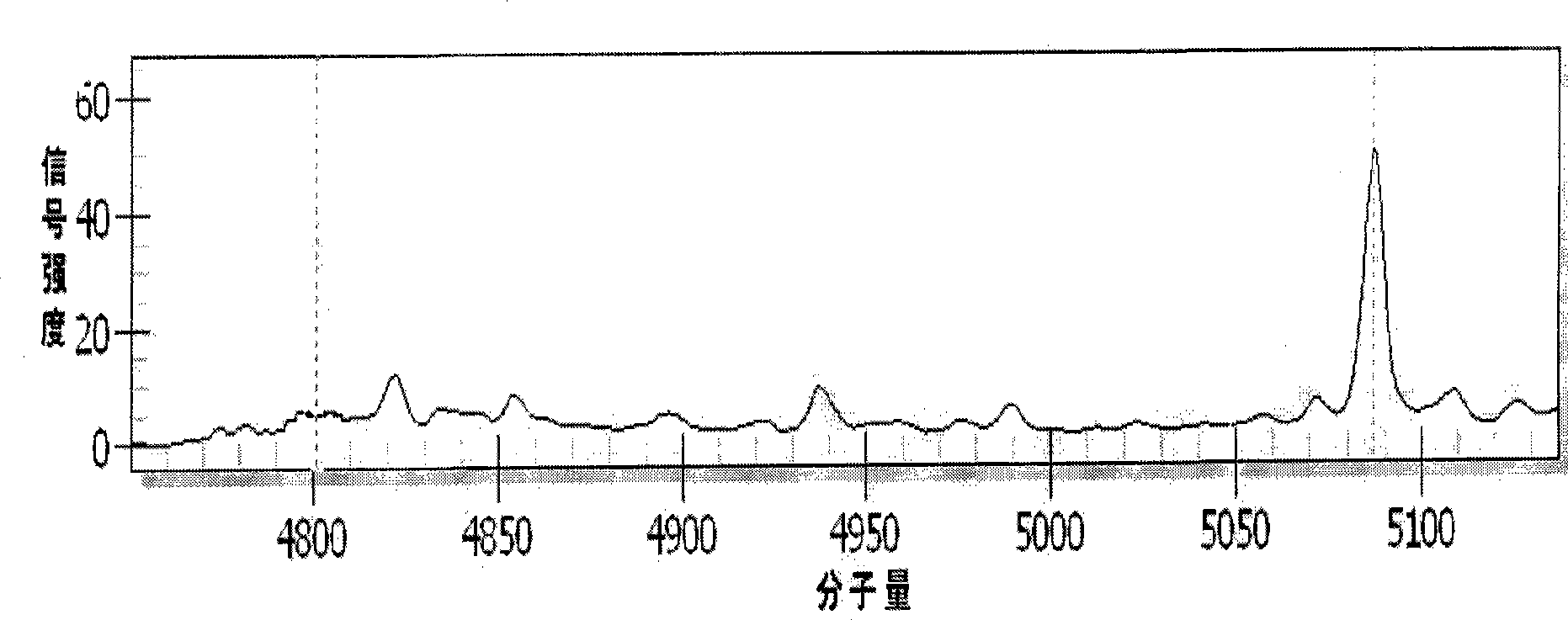
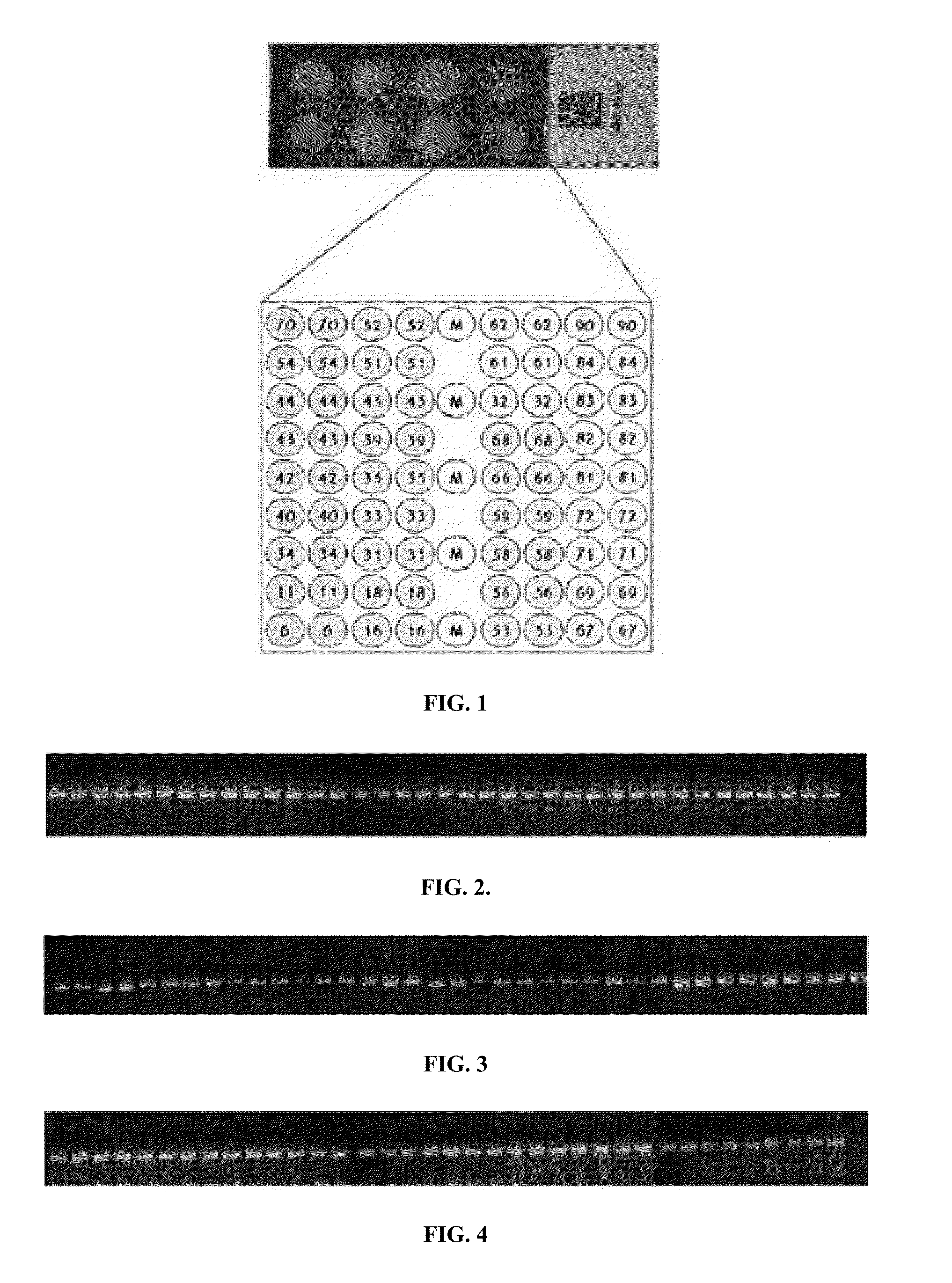
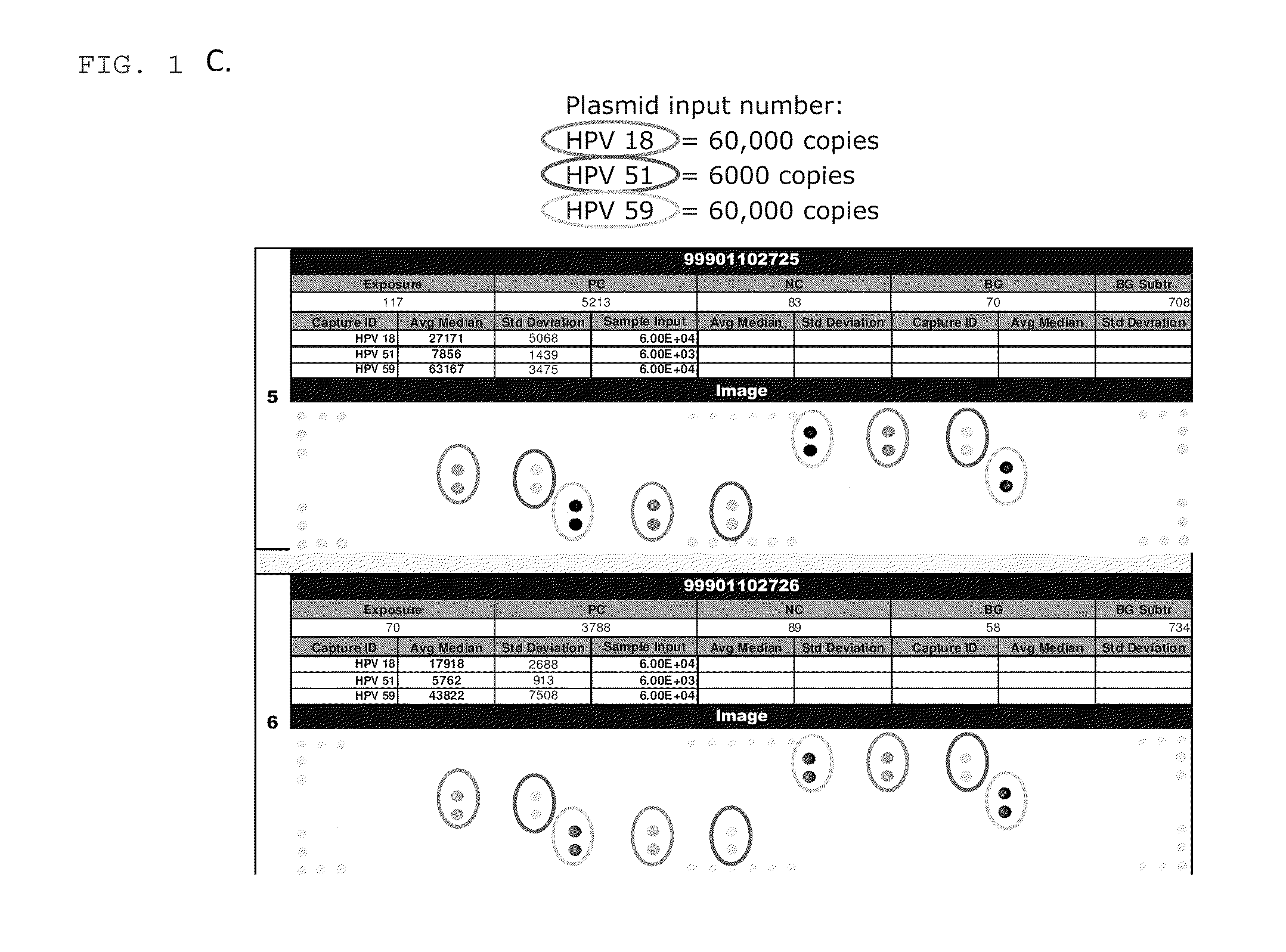
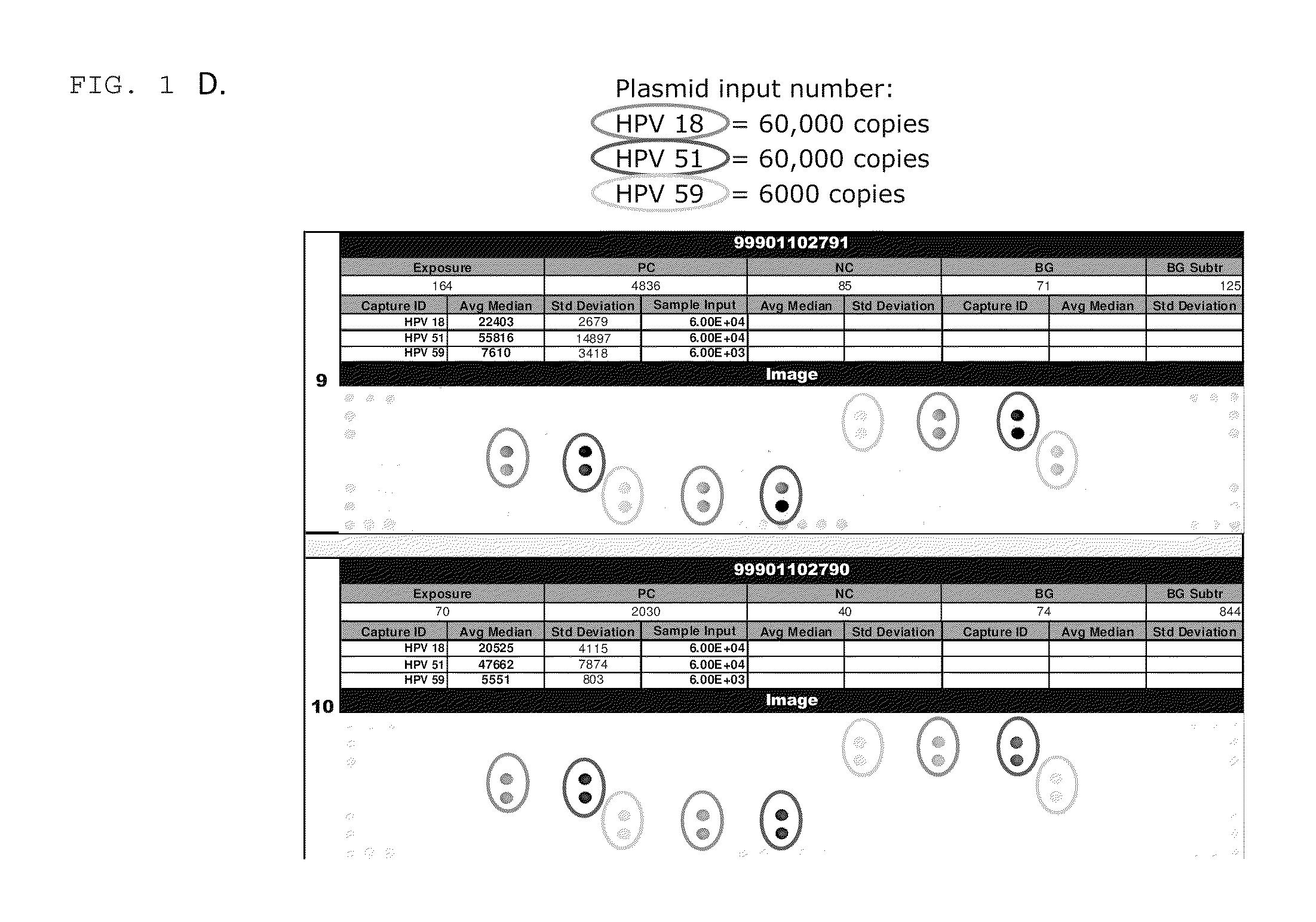
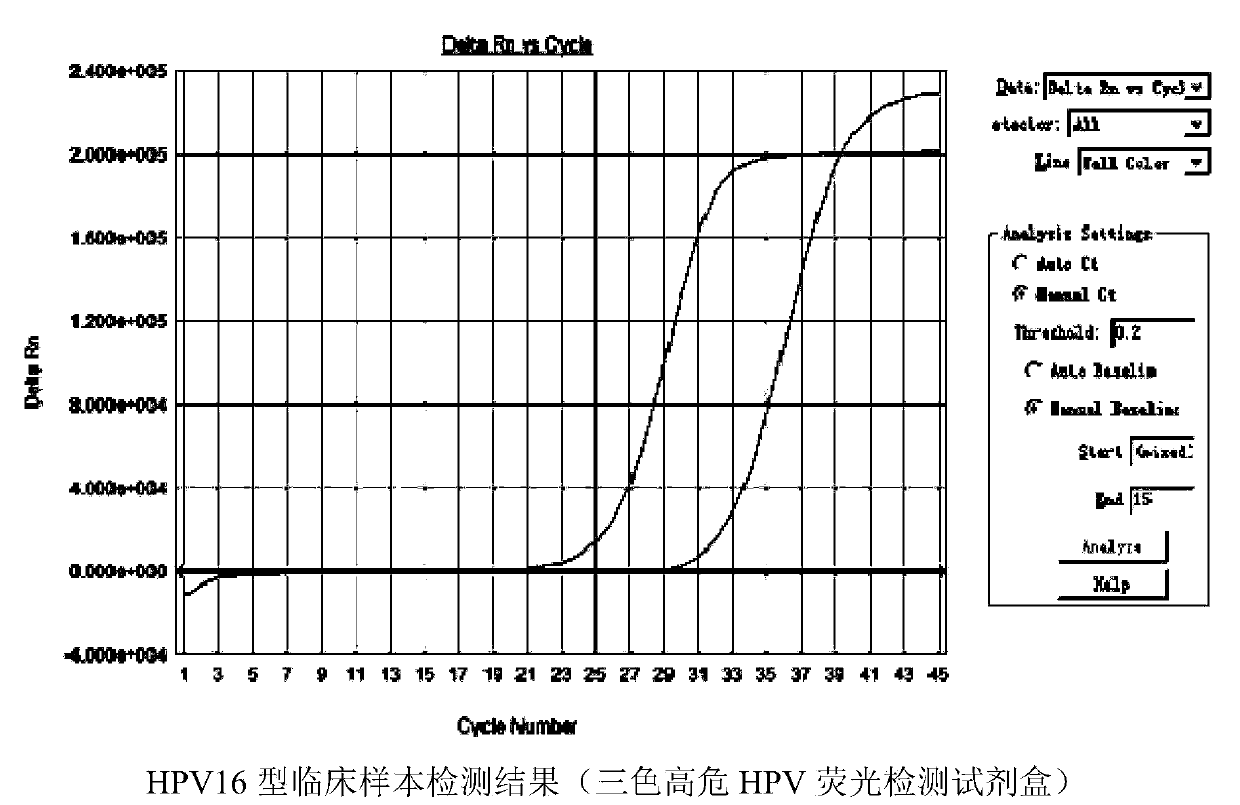
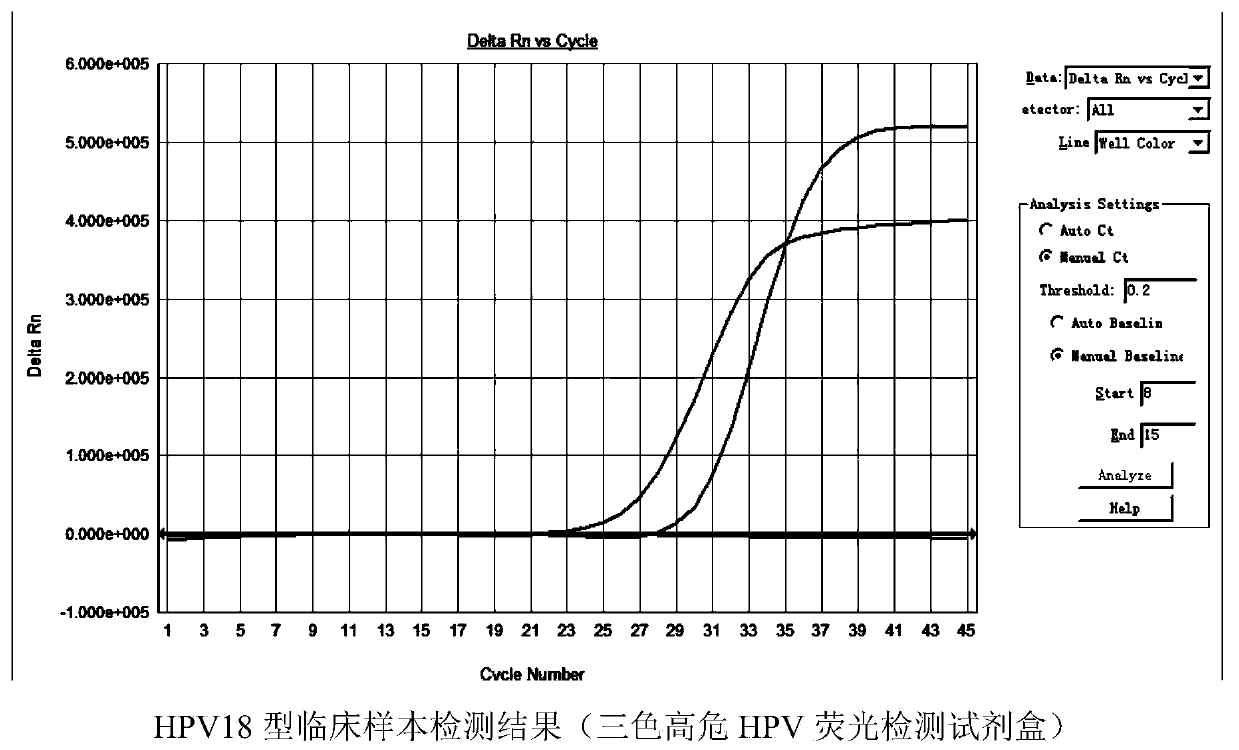
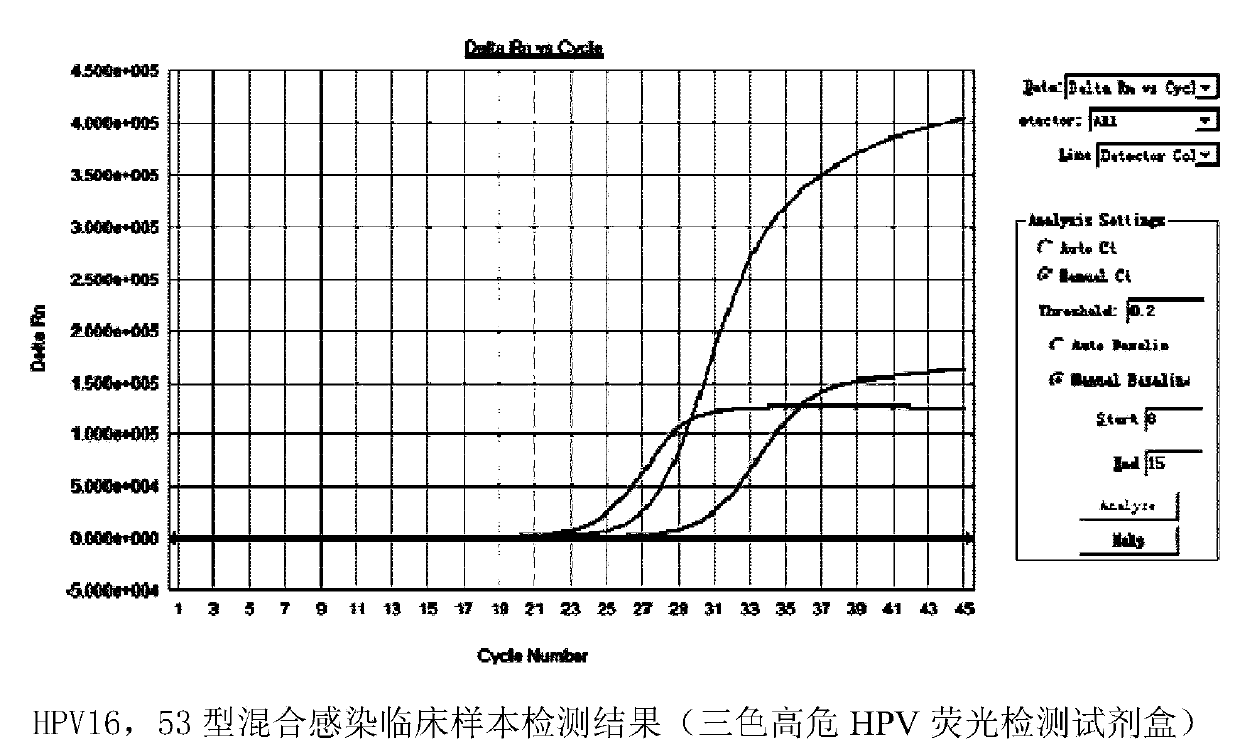
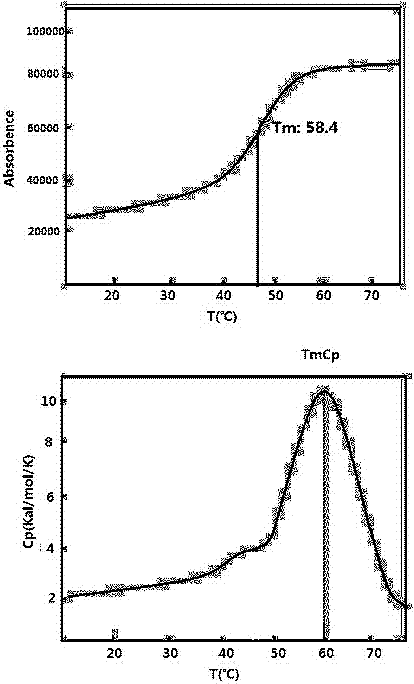
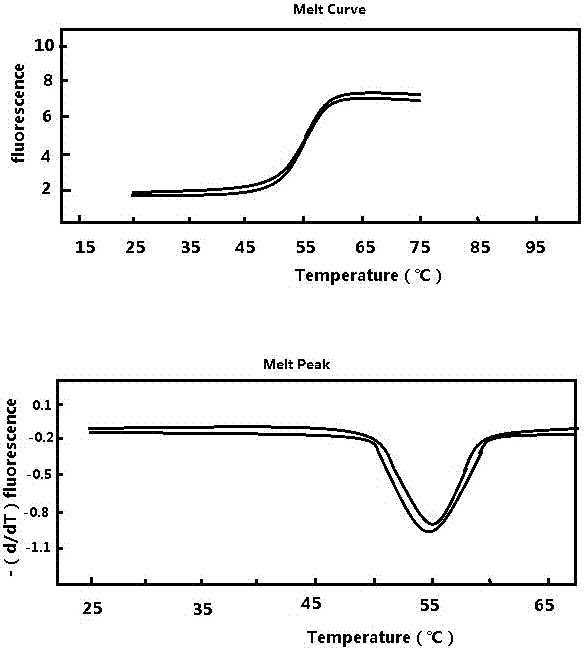
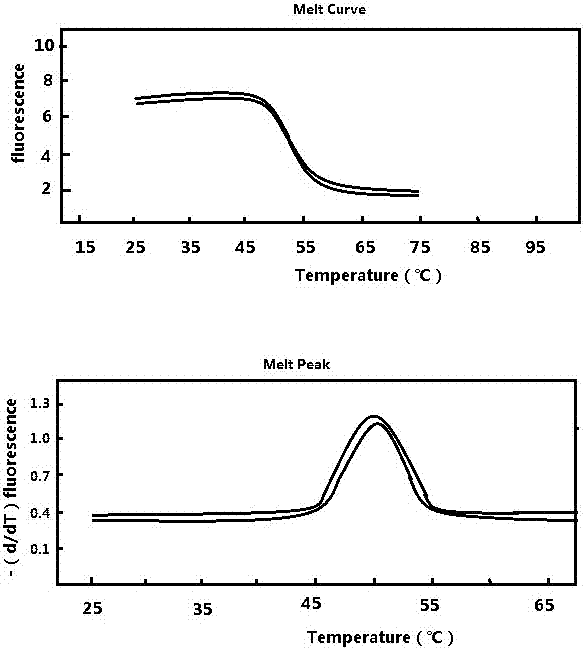
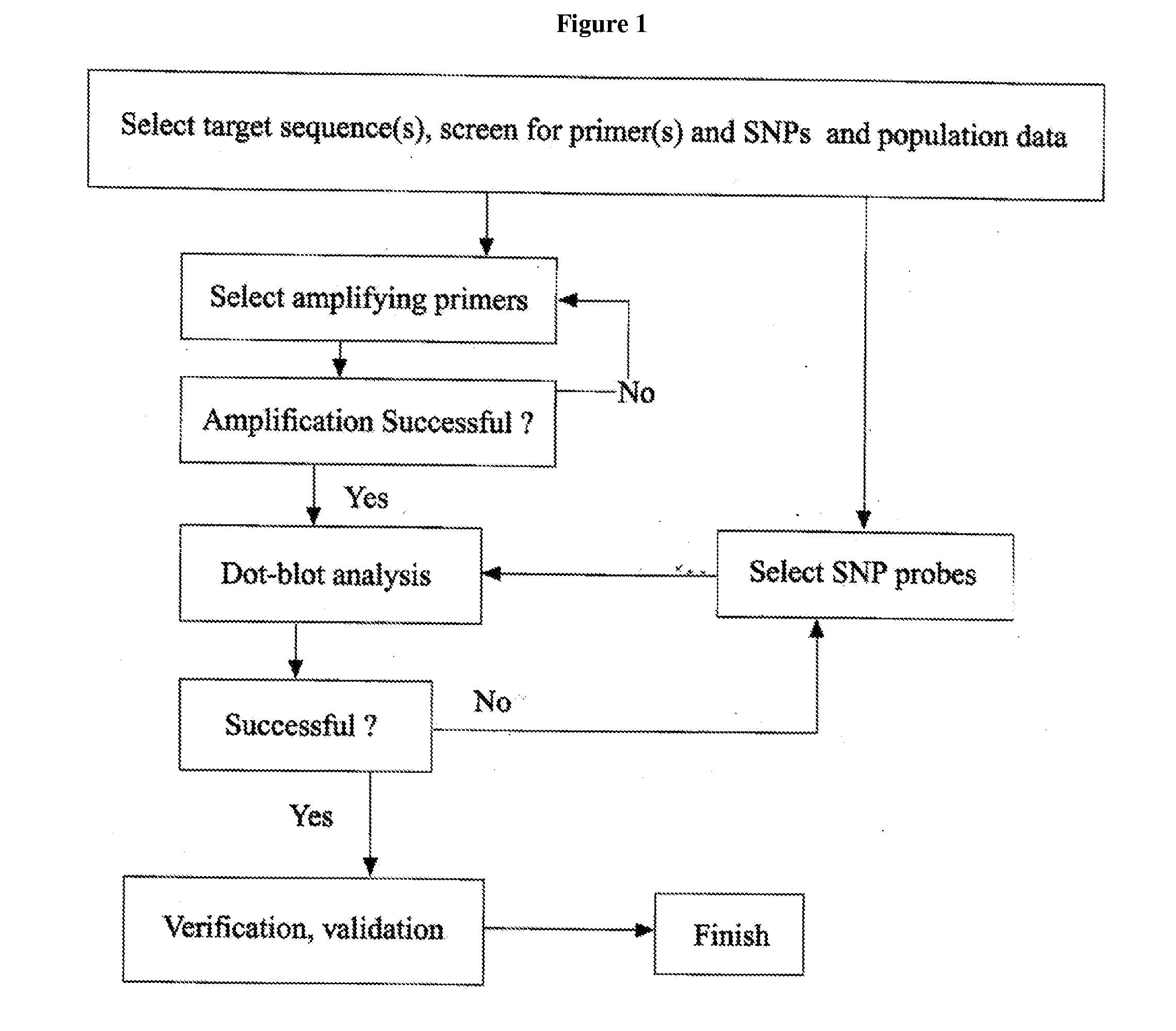
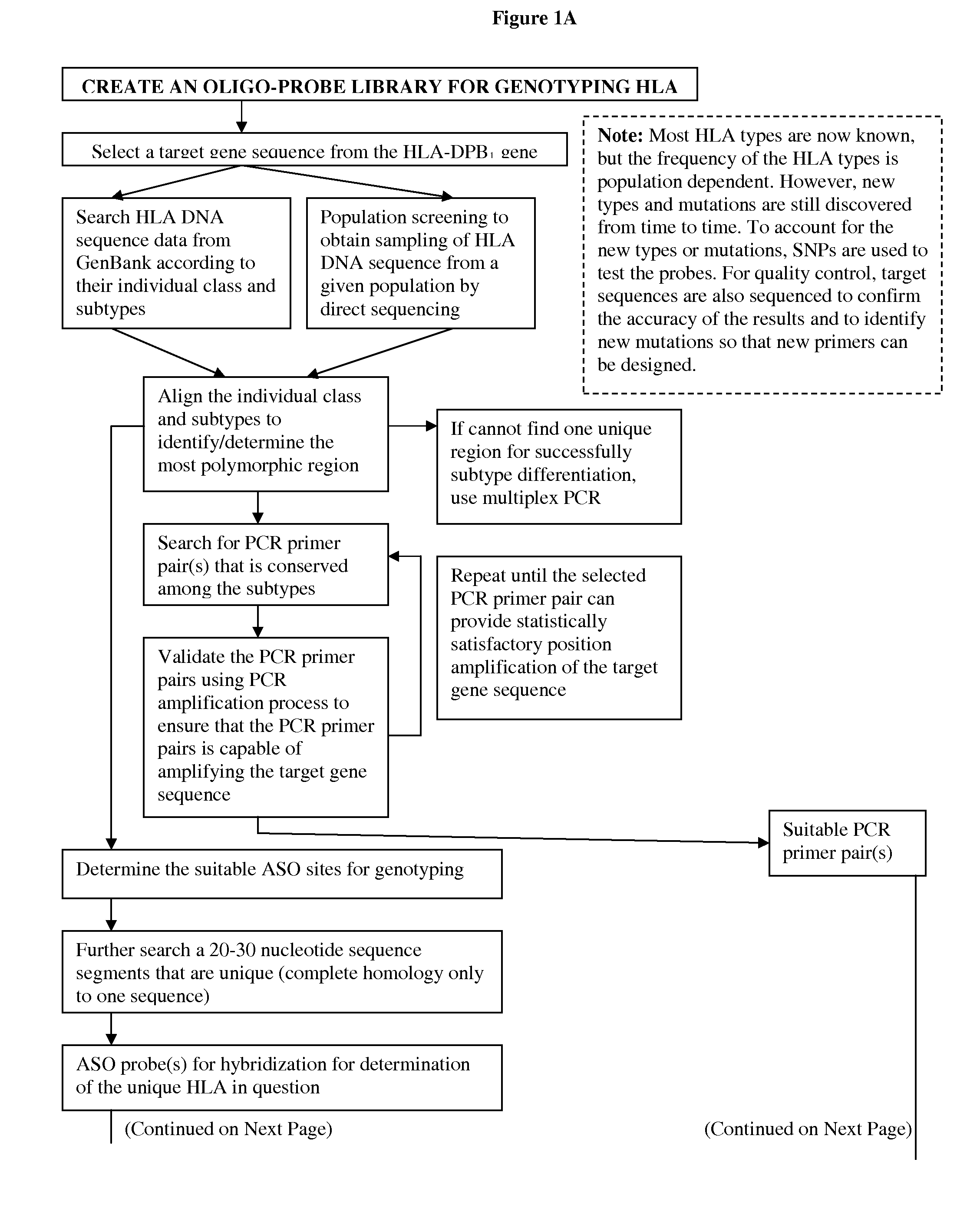
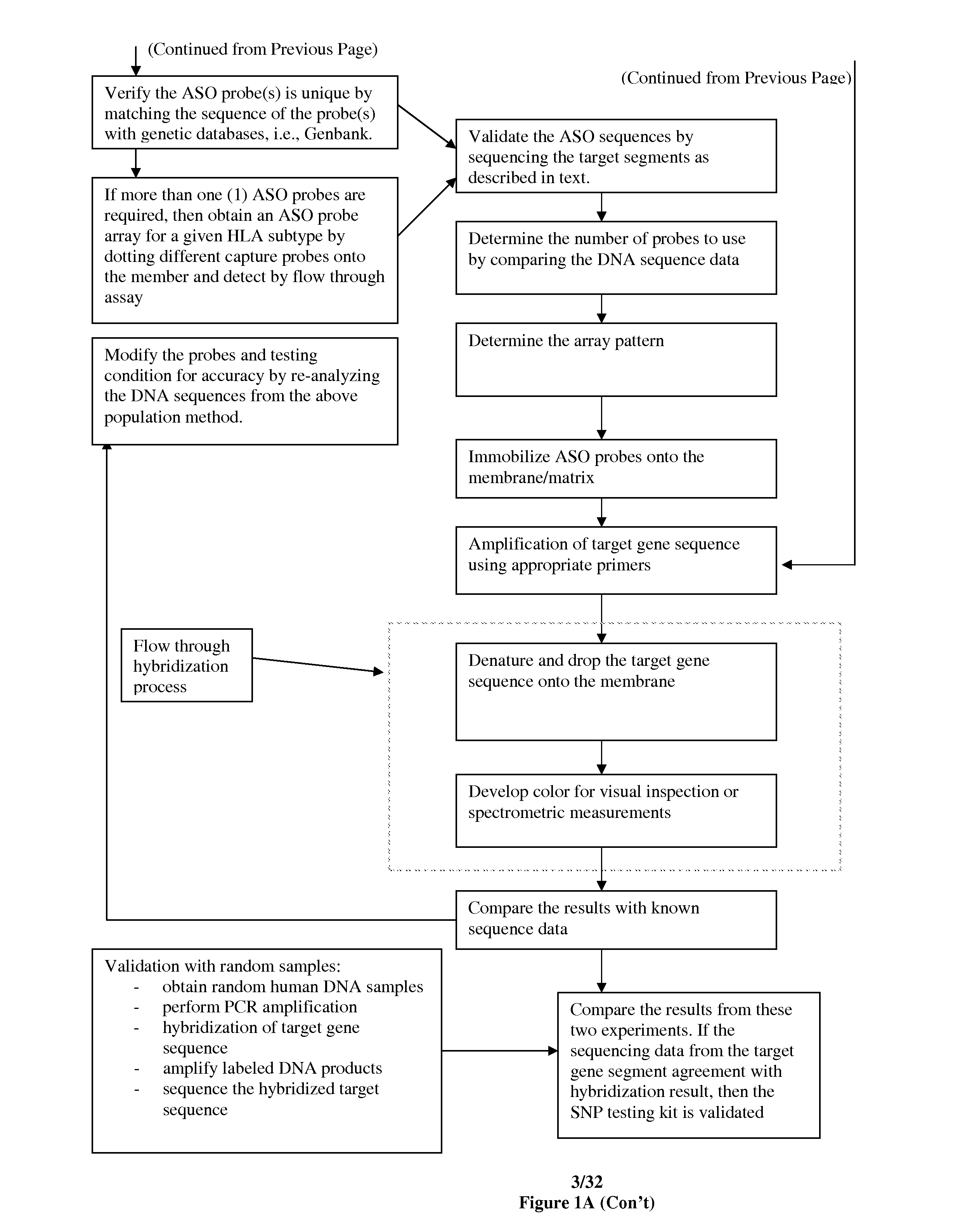
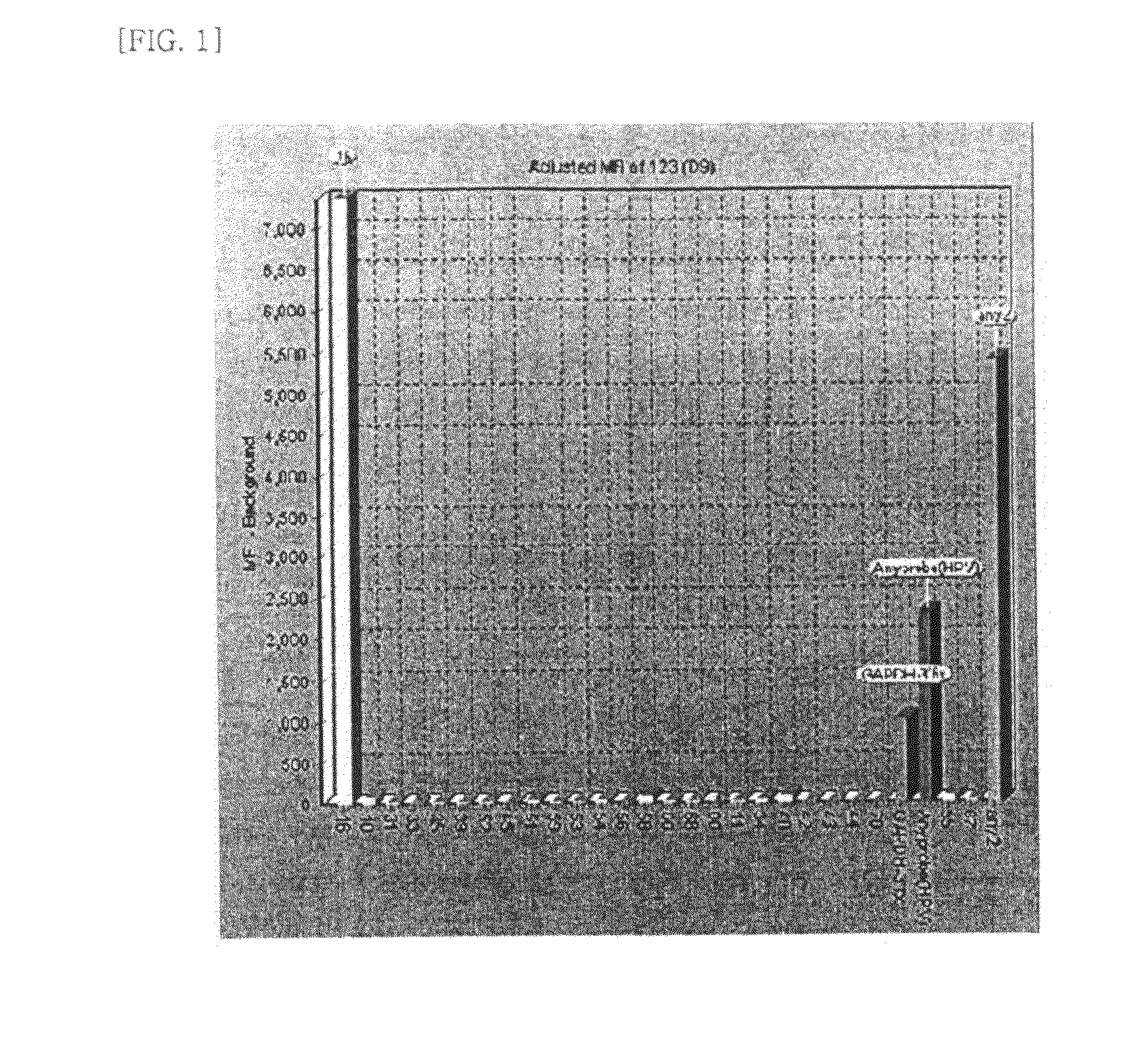
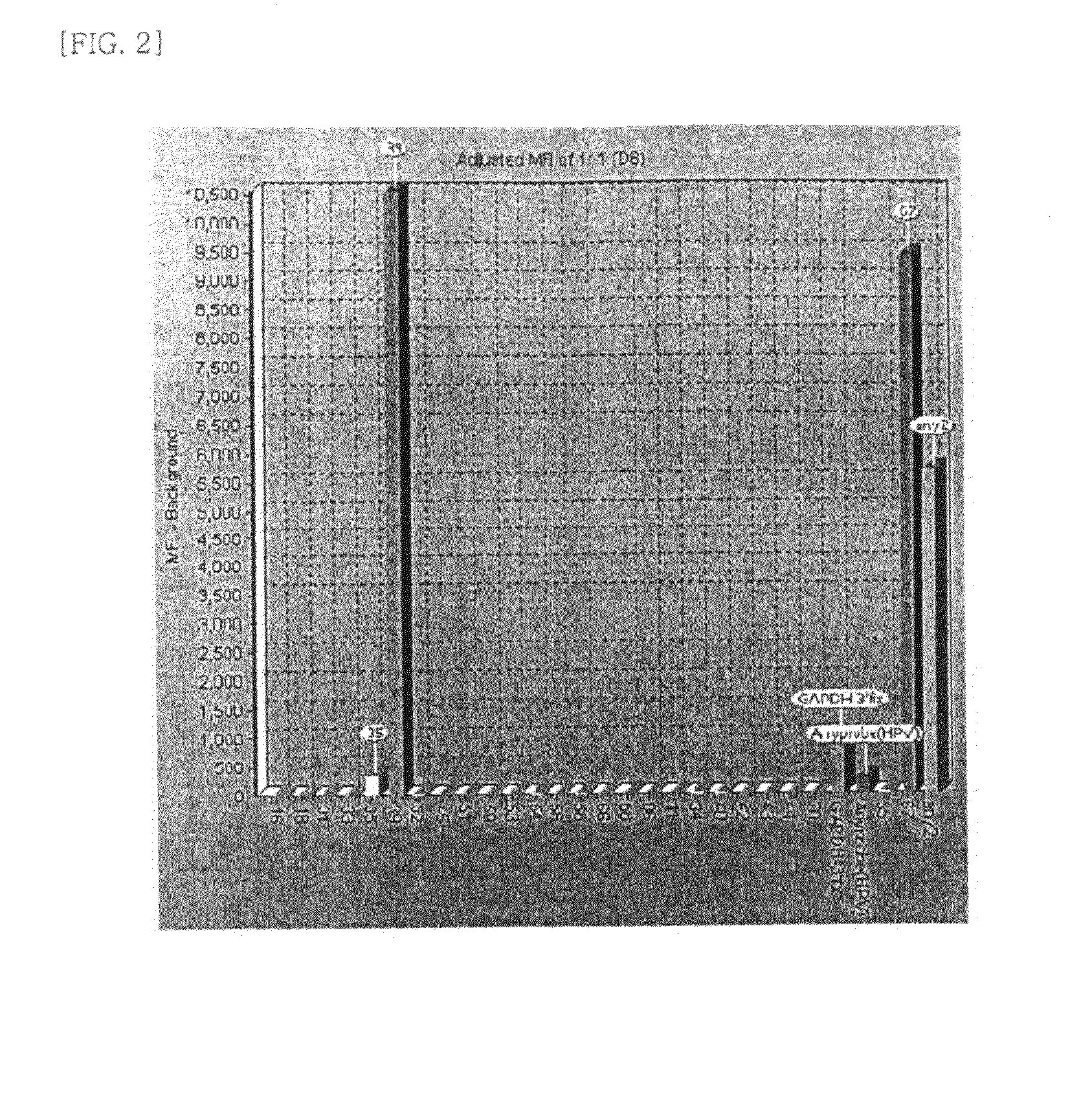
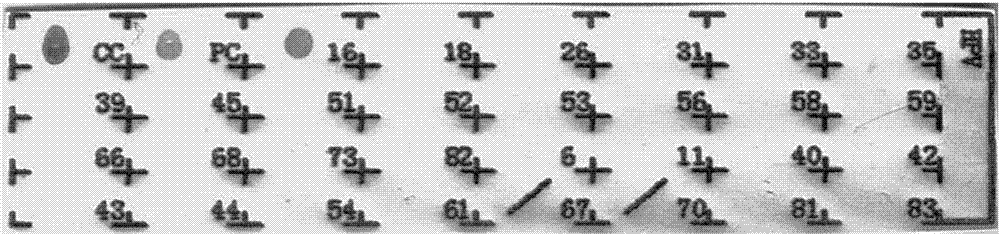
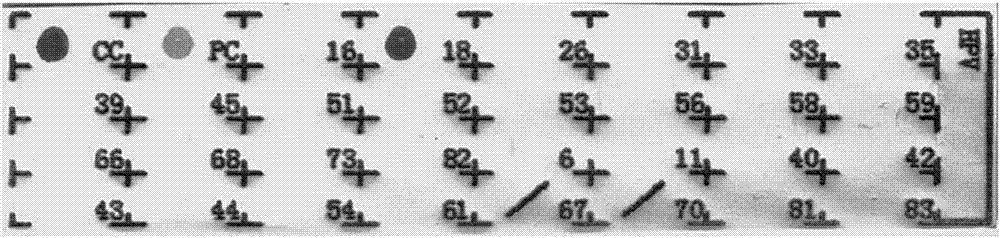
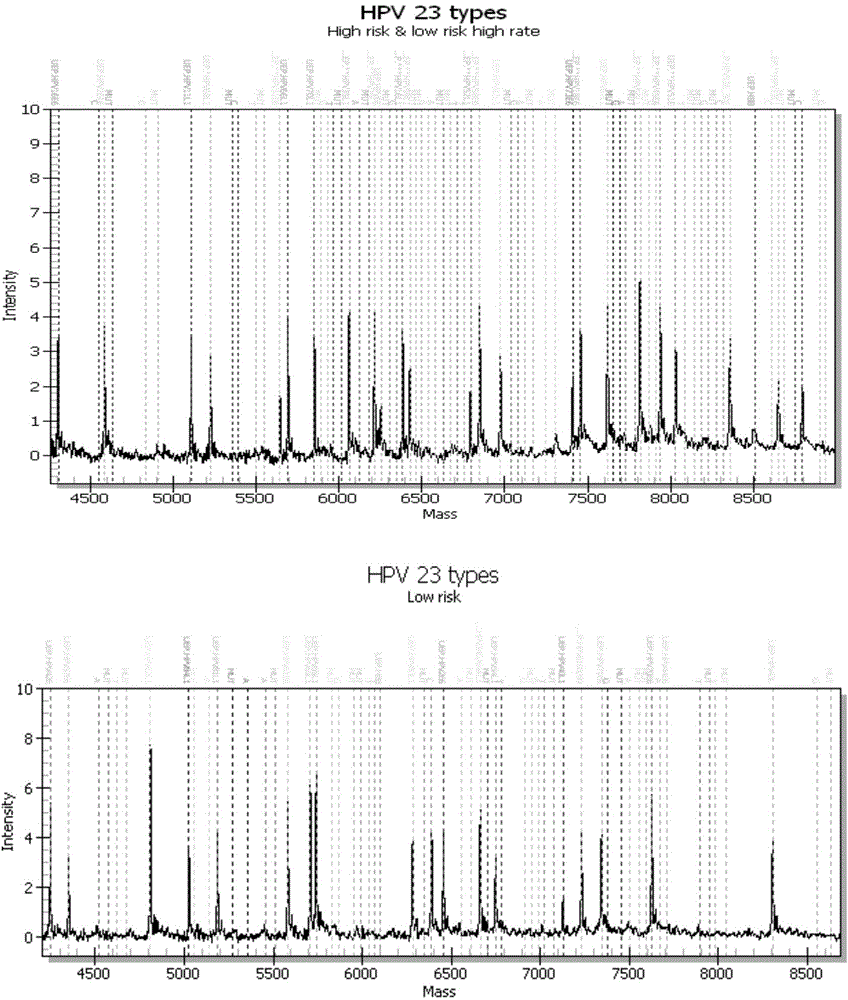
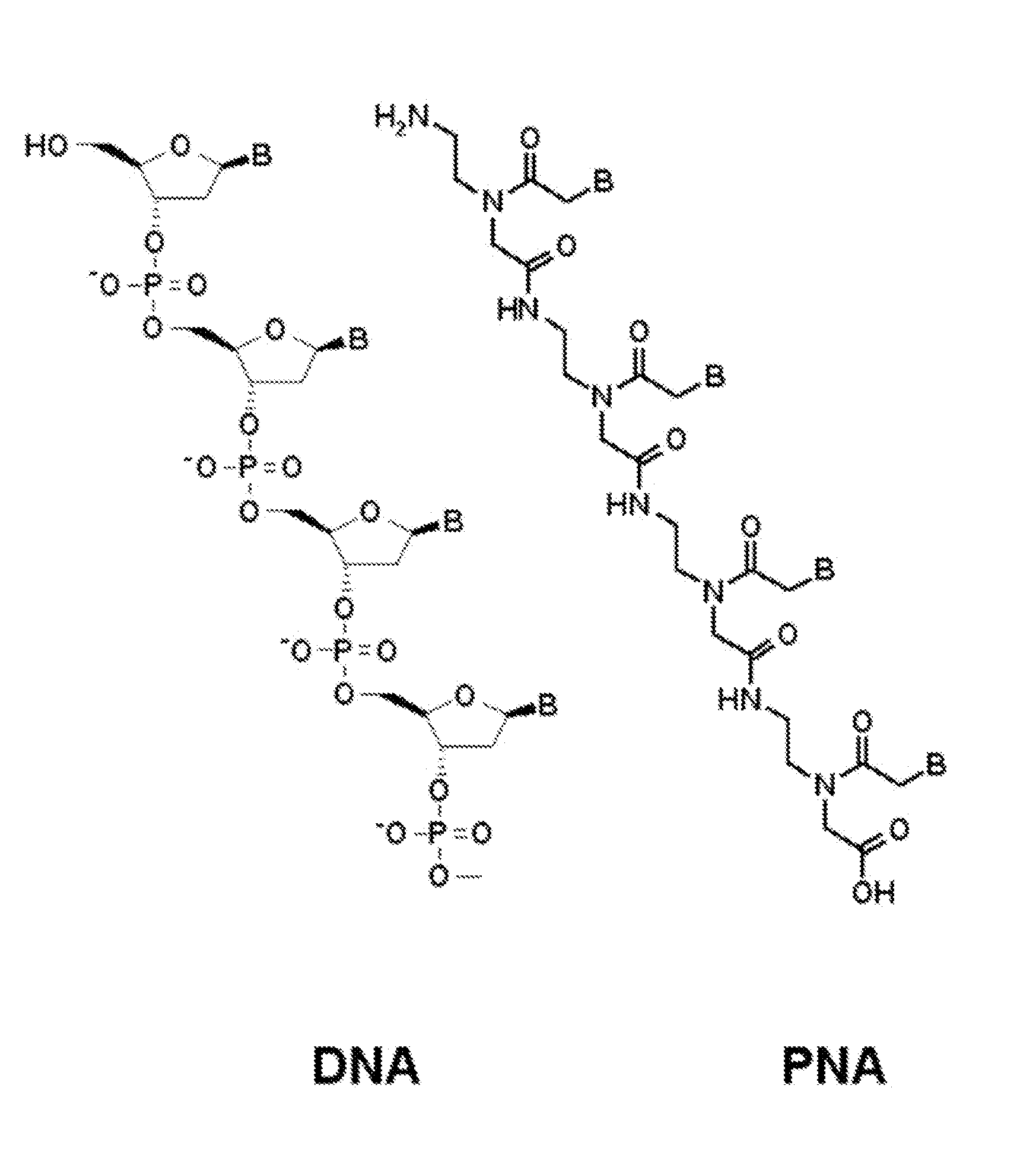
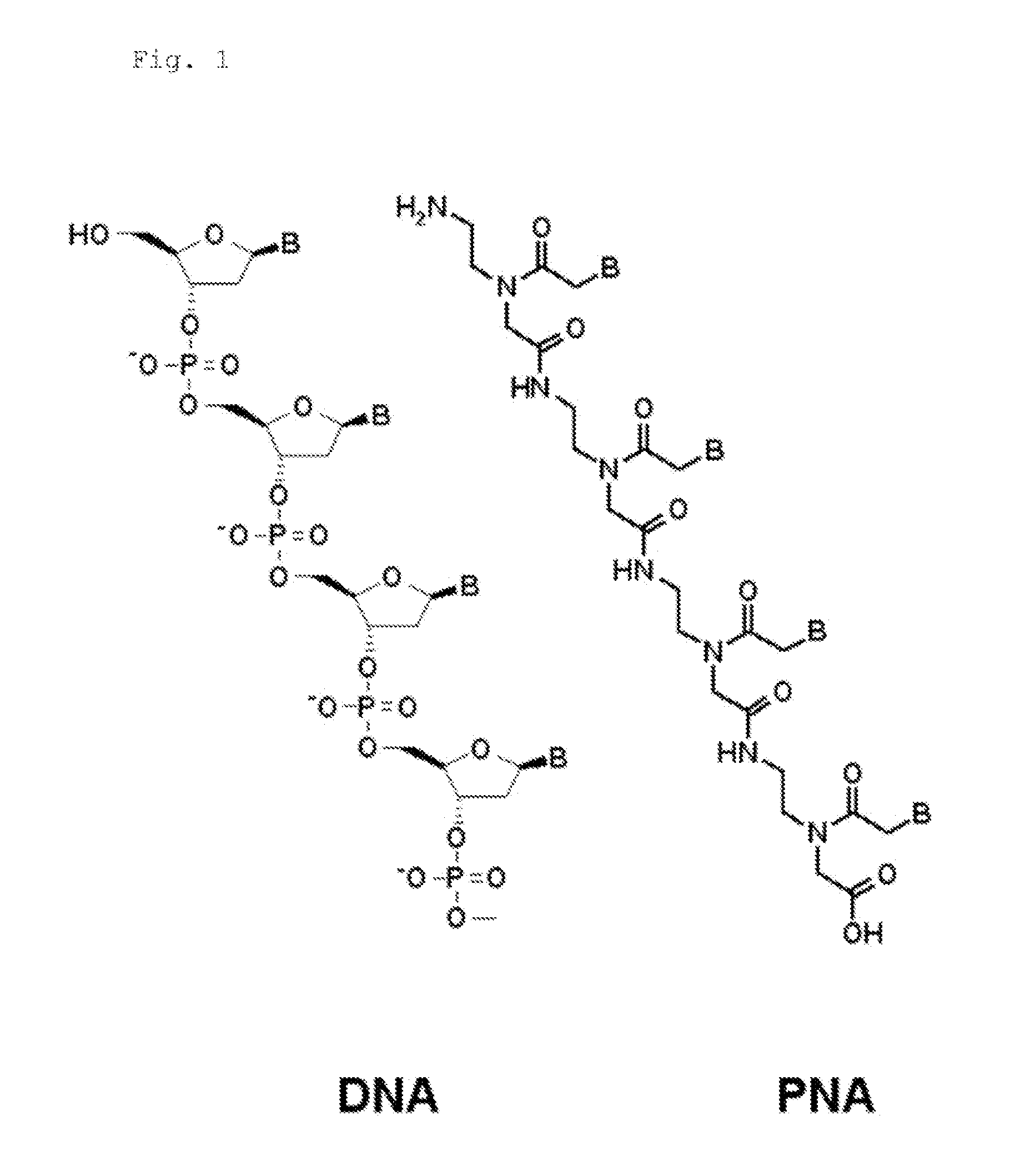
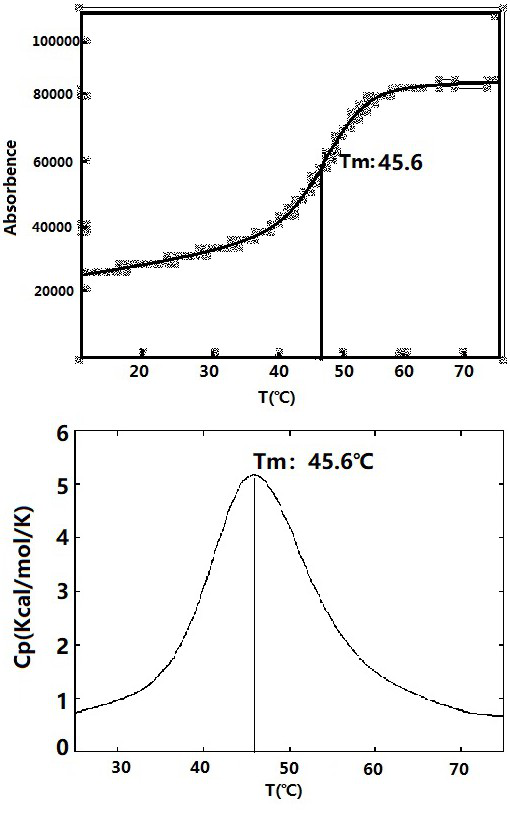
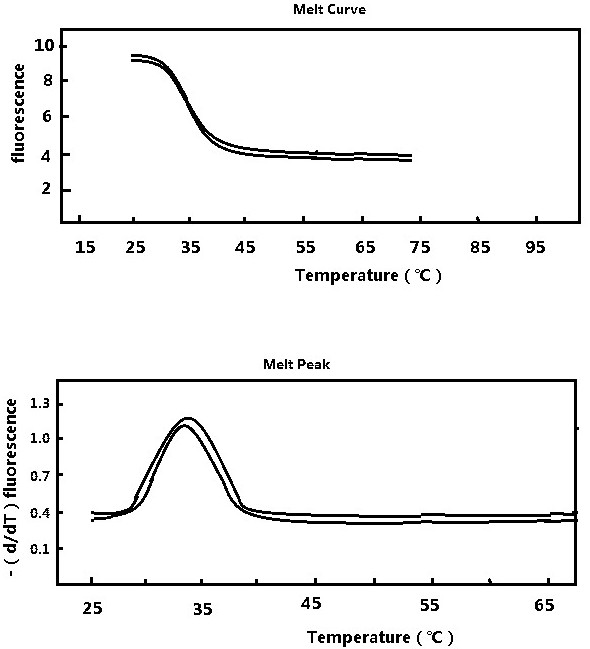
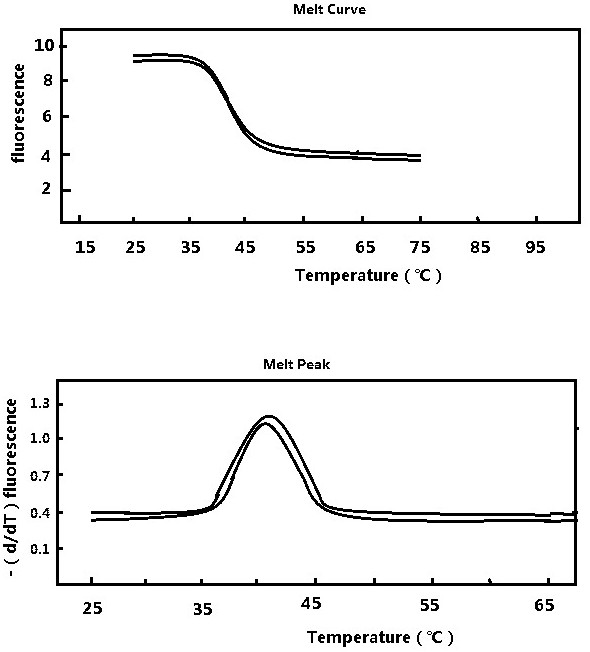
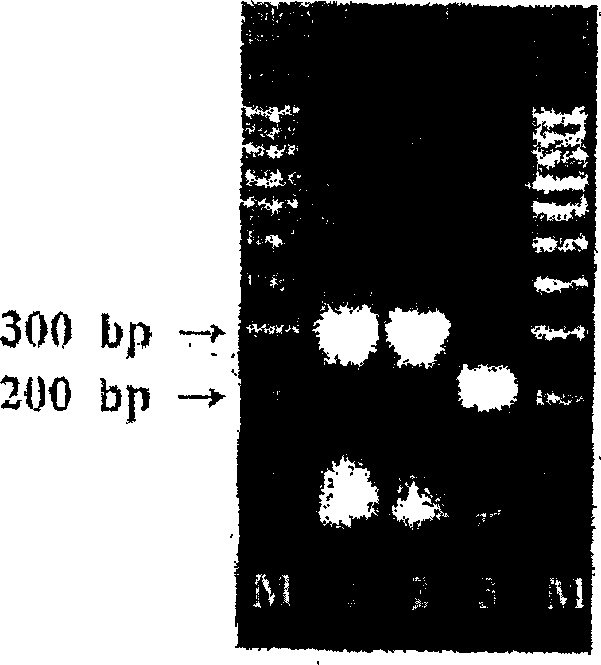Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Hpv genotyping" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HPV genotyping is other important test that can contribute to the prevention of invasive cervical cancer and is essential to the better understanding of the high-risk types of HPV for the introduction of an effective immunization program.
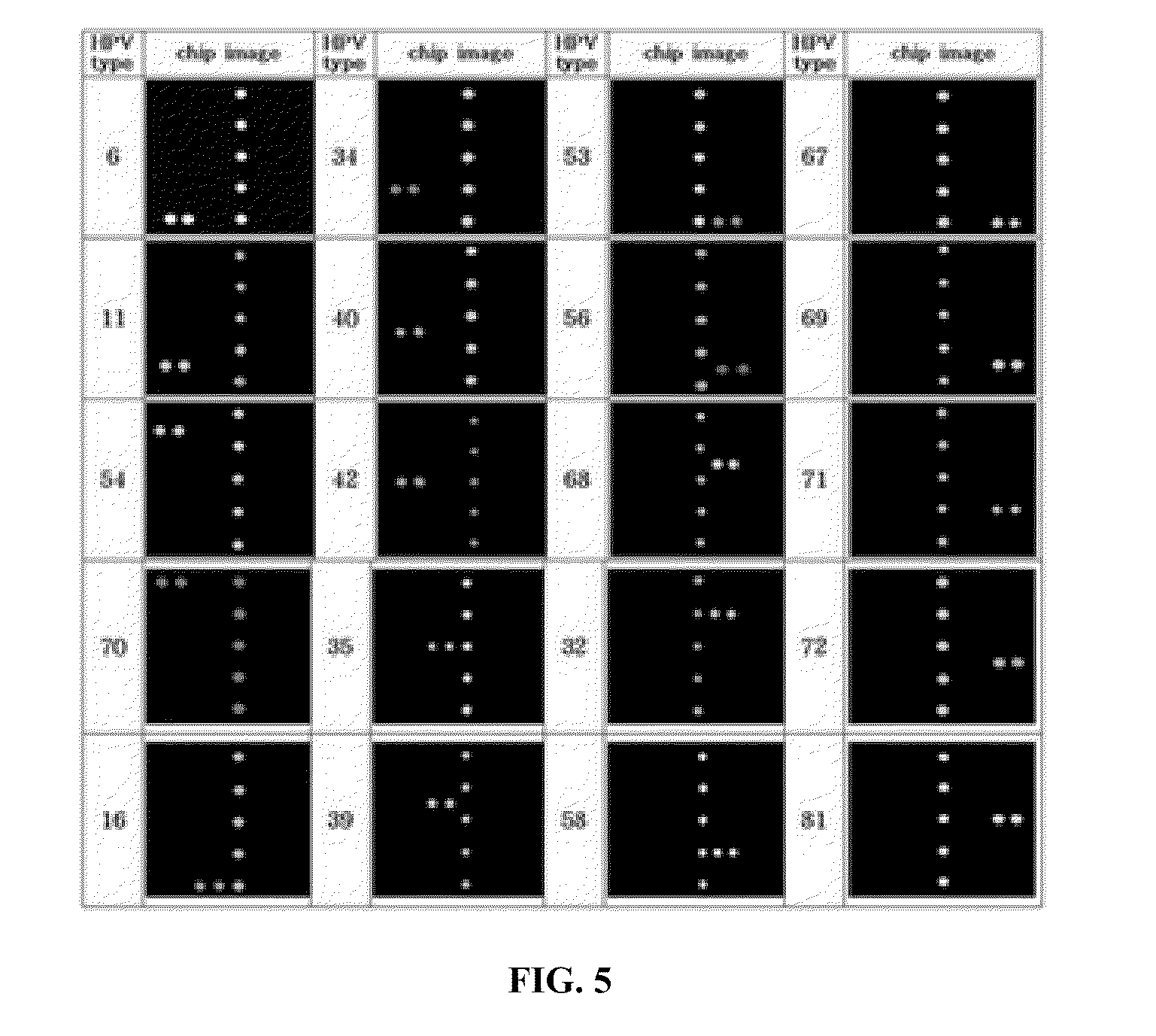
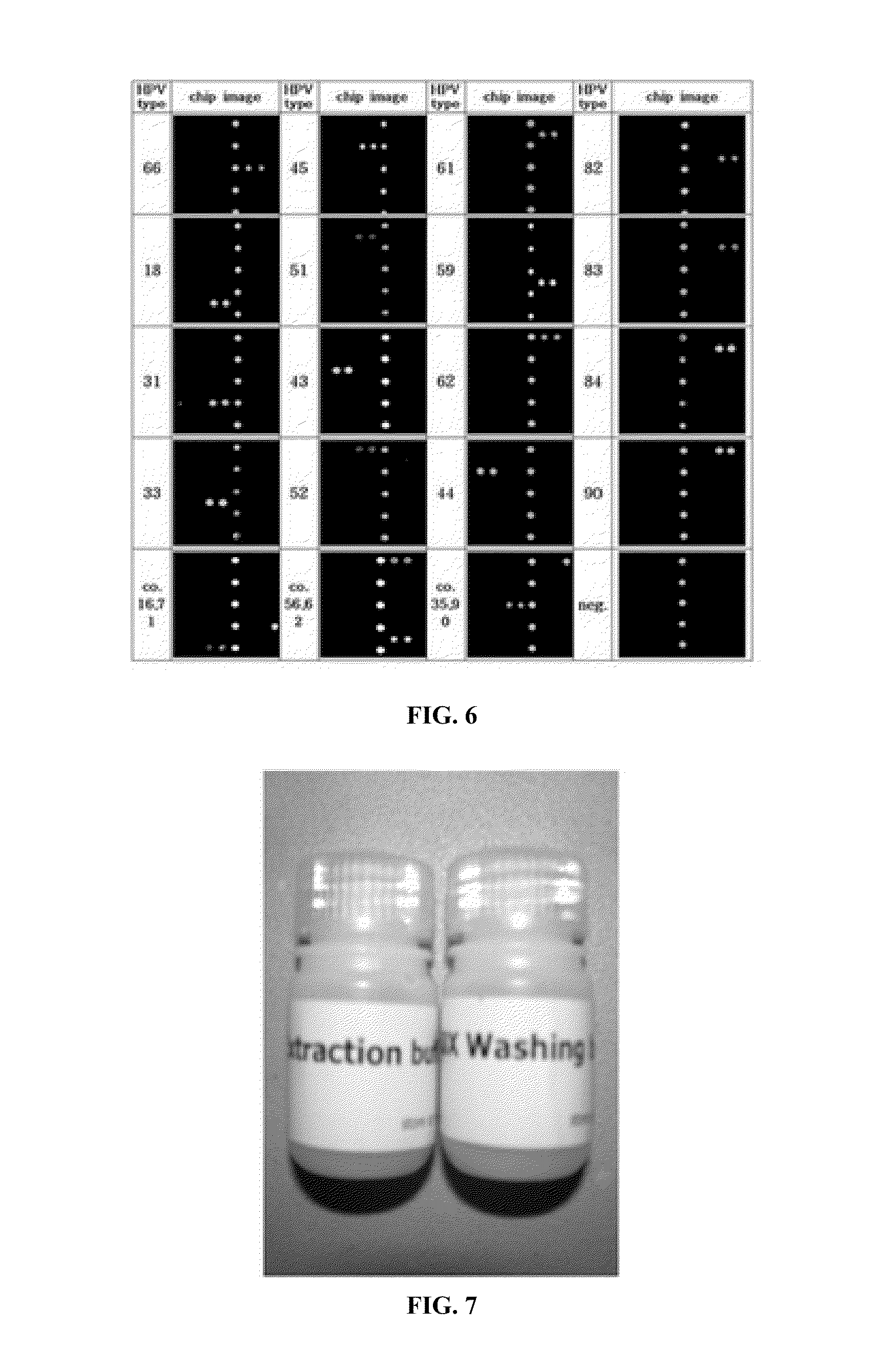
Protein chips for HPV detection
Embodiments of the invention provide methods, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Detection of HPV DNAs, genomes, and / or oncoproteins by protein chips immunological assays can be used in early clinical screening for HPV infection and general diagnosis for cervical cancer and can be advantageous performed in a multiplexed test. Comparative detection of altered levels of HPV proteins and host proteins can performed in one or more assays. The polypeptides, recombinant proteins, antibodies, nucleic acids, and various detection methods thereof are particularly useful for diagnosing carcinomas of the uterine cervix and those at risk of developing cervical cancer.
Owner:HEER MEDICAL TECH DEV CO LTD
In situ detection of early stages and late stages HPV einfection
ActiveUS20090311668A1Microbiological testing/measurementImmunoglobulinsCancers diagnosisMonoclonal antibody
Embodiments of the invention provide methods, monoclonal antibodies, polyclonal antibodies, assays, and kits for detecting HPV infection and HPV related cancer diagnosis, including infection by various HPV genotypes, early and / or late stage HPV-associated or HPV-specific cancers. The anti-HPV antibodies are used in performing immunological assays on clinical samples. Various immunological assays and kits for detecting HPV infection, cervical cancer, other HPV related cancers, early stage precancerous lesions as well as late stage cancer progression are also provided.
Owner:HEER MEDICAL TECH DEV CO LTD
Method for detecting human papilloma virogene type
ActiveCN101435002AReduce usageAvoid false positivesMicrobiological testing/measurementMaterial analysis by electric/magnetic meansThroughputHpv genotypes
The invention provides a method for detecting the HPV genotypes, with the gene to be detected being one or several types of the type 17 HPV, comprising the following steps: according to the variant sites of the selected HPV genotype universal primer sequence to be detected, an amplification primer aiming at each type is designed; the specific extension primer of each type is designed; (2) PCR amplification is conducted; (3) SAP enzyme treatment is conducted; (4) extension reaction is conducted, among the extending products and the extending primers, the difference of the molecular weight among the extending products of each type is not less than 9D; (5) resin is used to purify extension reaction products; and (6) mass spectrometry detection is conducted, and the type of HPV gene to be detected is determined. By using the method, the invention solves the problems of some of existing detection methods that the typing can not be realized, the multiple infections can not be detected, the accuracy is limited, the throughput is low, the cost is high, and the stability of reaction may be affected as the probe is RNA.
Owner:BGI SHENZHEN CO LTD
Primer, probe, DNA chip containing the same and method for detecting human papillomavirus and detection kit thereof
InactiveUS20100285485A1Strong specificityShorten the timeSugar derivativesMicrobiological testing/measurementHpv testingTrue positive rate
The present invention discloses a primer, a probe, a DNA chip, and a test kit for diagnosis and genotyping of Human Papilloma Virus (HPV) as well as a method for testing HPV genotype. According to the present invention, it is possible to screen HPV genotypes with high sensitivity and specificity, and to diagnose infection by multiple HPV types which was not possible in other conventional HPV testing methods
Owner:JUNG WOON WON +1
Human papilloma virus (24 types) detection (fluorescent PCR method) kit and detection method
InactiveCN103725792AAvoiding the False Negative ProblemSolve the problem of small fluxMicrobiological testing/measurementFluorescence/phosphorescenceForward primerHuman papilloma virus infection
Owner:JIANGSU MOLE BIOSCI
Novel monoclonal antibodies against HPV proteins
ActiveUS20090312527A1Microbiological testing/measurementImmunoglobulins against virusesCancers diagnosisDisease cause
Embodiments of the invention provide methods, monoclonal antibodies, polyclonal antibodies, assays, and kits for detecting HPV infection and HPV related cancer diagnosis, including infection by various HPV genotypes, early and / or late stage HPV-associated or HPV-specific cancers. Various monoclonal antibodies recognizing specific epitope for specific HPV protein or HPV type, common epitope for various HPV proteins or HPV types are obtained. These obtained monoclonal antibodies are useful tools in early clinical detection of HPV infection and general detection of HPV related diseases, specific detection of invasive cervical cancer, detection of other HPV related cancers, early stage precancerous lesions as well as late stage cancer progression.
Owner:HEER MEDICAL TECH DEV CO LTD
Gold nanoparticle HPV genotyping system and assay
InactiveUS20100304360A1High riskImprove hybridization efficiencySugar derivativesMicrobiological testing/measurementNanoparticleBiochemistry
Owner:NANOSPHERE INC
Detection, screening, and diagnosis of HPV-associated cancers
ActiveUS8968995B2Microbiological testing/measurementBiological material analysisScreening methodSpecific protein
Embodiments of the invention provide methods, polyclonal antibodies, monoclonal antibodies, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Mononoclonal antibodies are used to detect oncogenic high risk and low risk HPV types in a single assay, which is not limited to assay type or format. Useful tools for specific detection of various HPV associated cancers are provided. HPV associated cancer biomarkers are identified and can be used in a screening method for early stage precancerous lesions as well as late stage cancer progression.
Owner:HEER MEDICAL TECH DEV CO LTD
Multicolor high-risk HPV (human papilloma virus) fluorescence detection kit
ActiveCN104195247AHigh detection throughputReduce screening costsMicrobiological testing/measurementMultiplexFluoProbes
The invention discloses a multicolor high-risk HPV (human papilloma virus) fluorescence detection kit which comprises a warm-boot Taq enzyme system, universal primers, fluorescent probes, a negative control and a positive control, wherein the fluorescent probes are HPV-specific MGB probes. The kit is suitable for qualitatively detecting 18 high-risk HPV genotypes capable of causing cervical carcinoma in cervix exfoliated cell and genitourinary tract secretion samples. The kit uses the multiplex fluorescence PCR (polymerase chain reaction) technique to implement detection of 18 HPV genotypes in one tube amplification system. The kit can simultaneously detect 15 common high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 82) and can also detect 3 rare high-risk HPV genotypes (26, 53 and 73). The detection kit has the advantage of high detection flux and greatly lowers the detection cost.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Typing detection method and kit for high-risk human papilloma virus (HPV)
InactiveCN107043828ASimple designHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesHybridization probeFluorescence
The invention provides a typing detection method and kit for a high-risk human papilloma virus (HPV). According to the method, a method for performing fluorescence nucleic acid amplification by adopting multiple pairs of primers and multiple probes is combined with fusion curve analysis, so that multiple target nucleic acid sequences can be detected in a single-wavelength fluorescence channel; an L1 segment sequence of an HPV genome is used as a target sequence for designing an HPV specific primer, a TaqMan probe and a PCO hybridization probe which are not completely complementary with the TaqMan probe, and a 3' end of the hybridization probe can be subjected to fluorescence quenching group labeling or non-fluorescence quenching group labeling according to purposes and cannot be extended after the hybridization probe is labeled, so that an HPV gene typing kit containing a primer probe and other fluorescence PCR components is researched; and by combining fluorescence PCR amplification with single-wavelength fusion analysis, the types 16, 18, 31, 52 and 59 of the HPV can be identified.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD
Rapid genotyping analysis for human papillomavirus and the device thereof
InactiveUS20110111389A1Accurate measurementNot require expensiveMicrobiological testing/measurementProteomicsHuman papillomavirusReverse dot blot
The present invention discloses methods and devices for rapid genotyping. In one embodiment, the present invention is applied to human papillomavirus (HPV) genotyping, comprising the use of viral genotype-specific-oligonucleotide probes, reversed-dot-blotting genotype array format and flow through hybridization process, thereby providing a more efficient, faster and less expensive method for HPV genotyping. The genotyping method further comprises the use of generic probes to expand the detection of HPV genotypes.
Owner:DIAGCOR BIOSCI
IN SITU detection of early stages and late stages HPV infection
InactiveUS20100003704A1Microbiological testing/measurementImmunoglobulinsEarly carcinomaAntiendomysial antibodies
Embodiments of the invention provide methods, polyclonal antibodies, monoclonal antibodies, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Mononoclonal antibodies are used to detect oncogenic high risk and low risk HPV types in a single assay, which is not limited to assay type or format. Useful tools for specific detection of invasive cervical cancer are provided. Cervical cancer biomarkers are identified and can be used in a detection method for early stage precancerous lesions as well as late stage cancer progression.
Owner:ONCOHEALTH
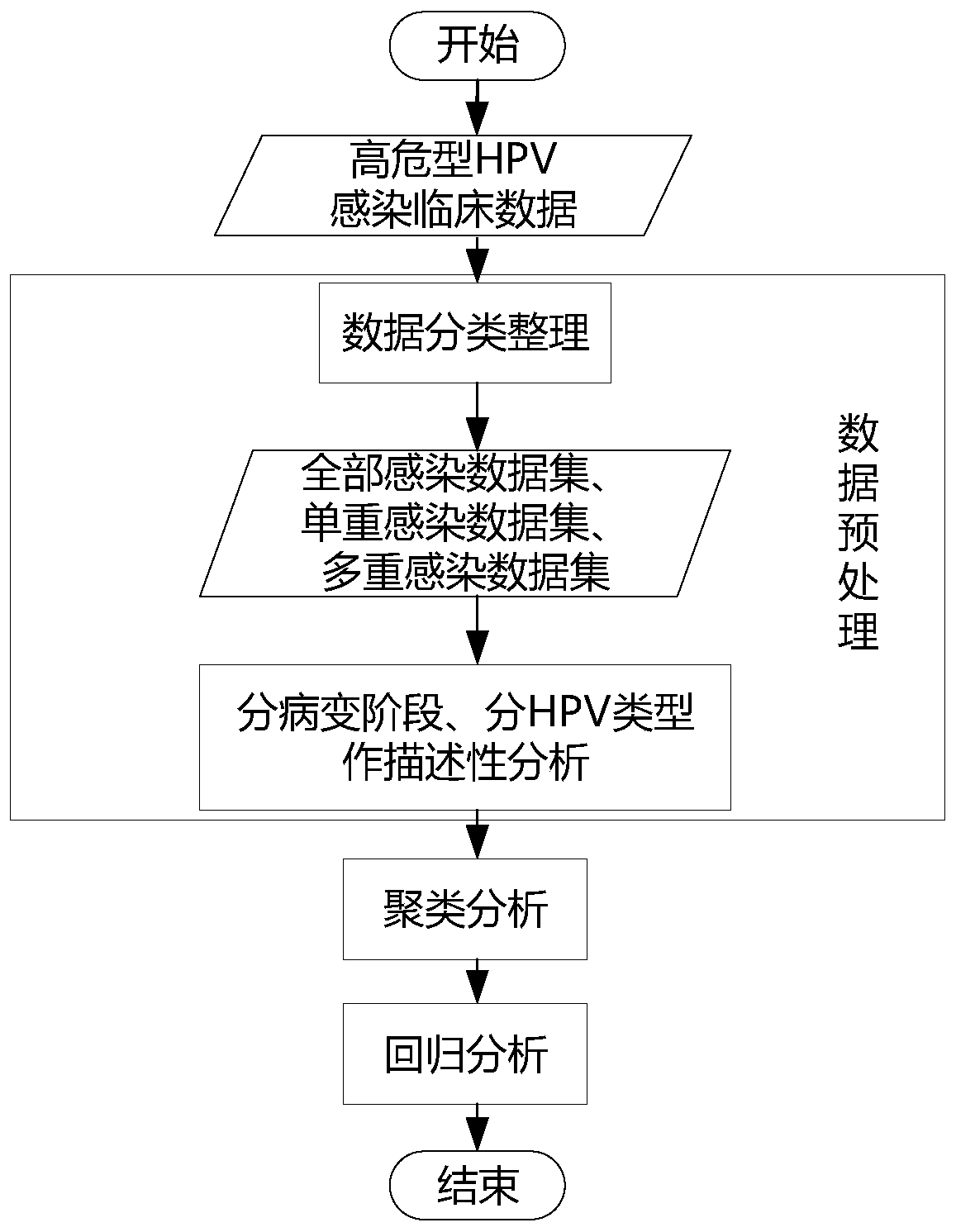
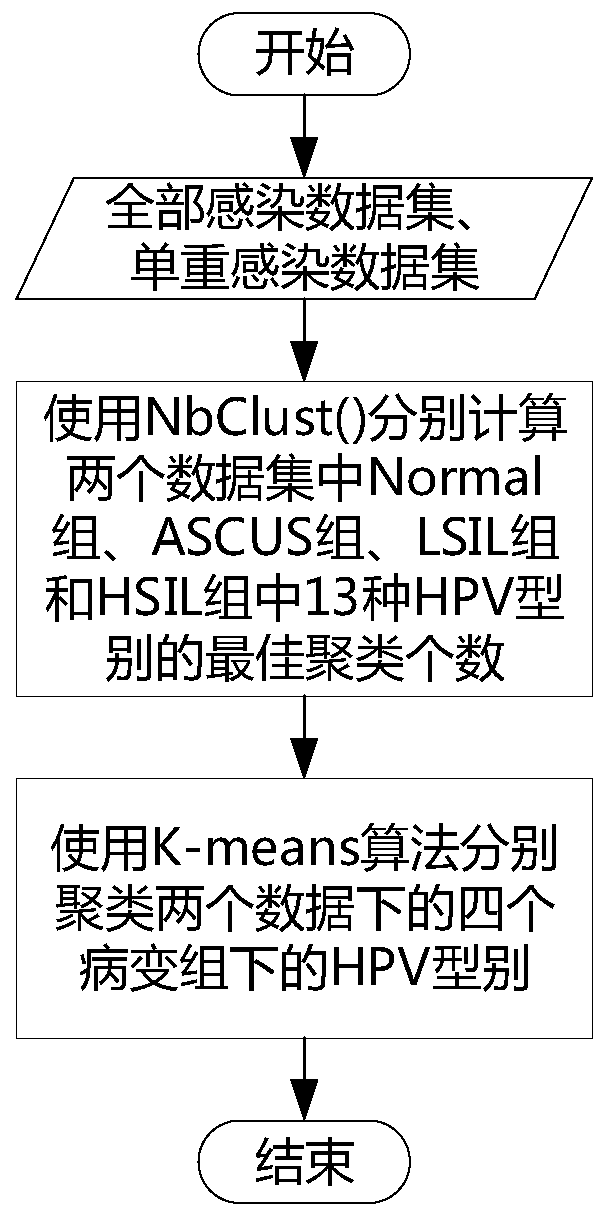
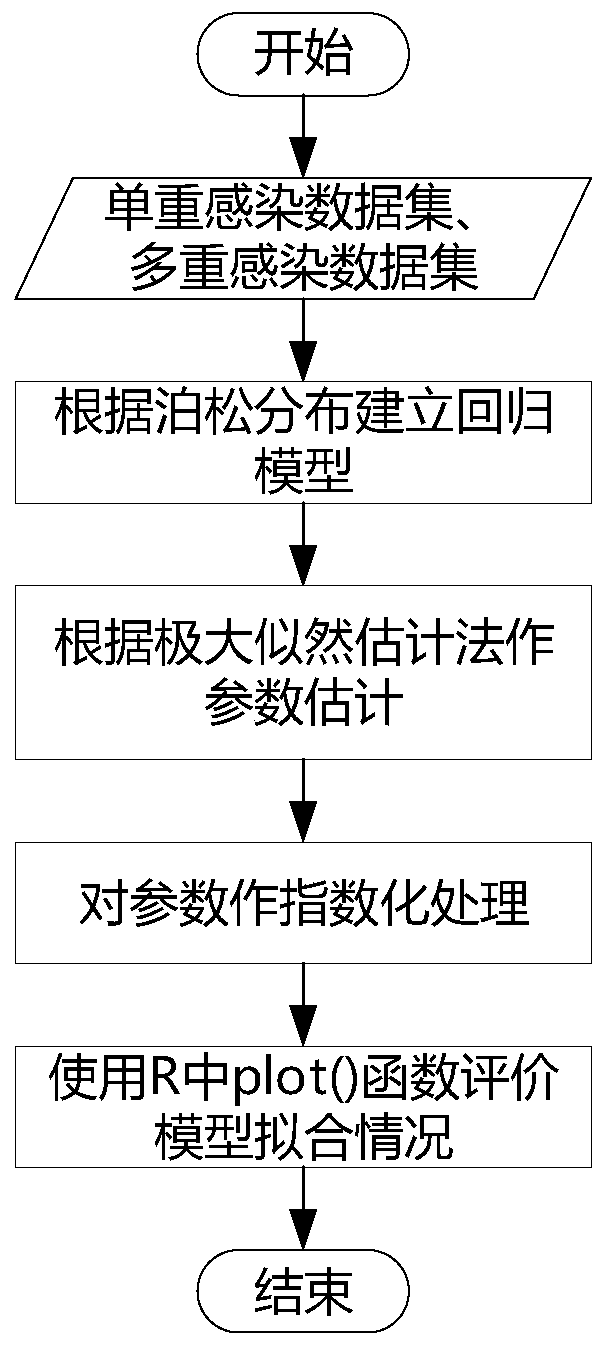
Method and device for calculating relationship between high-risk HPV types and cervical cancer precancerous lesion stages
The invention provides a method and a device for calculating a relationship between high-risk HPV types and cervical cancer precancerous lesion stages. The method comprises the steps: carrying out classification on N kinds of high-risk HPV infection data, obtained through thin-layer cytological examination (TCT) and HPV genotyping detection, under M kinds of cervical cancer precancerous lesion stages, and obtaining HPV infection preprocessing data in different infection modes; performing clustering analysis based on the HPV preprocessing data, and obtaining the similarity of different high-risk HPVs based on a clustering analysis result; and carrying out modeling according to Poisson distribution based on the HPV preprocessing data under the single infection and multiple infection modes, and performing regression analysis to obtain the influence proportion of the HPV single infection and multiple infection on the cervical cancer precancerous lesion. According to the method, a clustering technology and a statistical analysis method are combined to mine biological data, and the relationship between different high-risk HPVs and different cervical cancer precancerous lesion stages is found.
Owner:SICHUAN UNIV
Detection of early stages and late stages HPV infection
ActiveUS8278056B2Bioreactor/fermenter combinationsBiological substance pretreatmentsEpitopeCancers diagnosis
Embodiments of the invention provide methods, monoclonal antibodies, polyclonal antibodies, assays, and kits for detecting HPV infection and HPV related cancer diagnosis, including infection by various HPV genotypes, early and / or late stage HPV-associated or HPV-specific cancers. Various specific or pan monoclonal antibodies recognizing specific epitope for specific HPV protein or HPV type, or common epitope for various HPV proteins or HPV types are obtained. The invention also provides one or more solid surface to coat the testing cell lysate. Also, the anti-HPV antibody can be coated on the solid surface of the invention to capture HPV proteins and detect HPV infection.
Owner:HEER MEDICAL TECH DEV CO LTD
Kits and Method For Detecting Human Papilloma Virus With Oligo Nucleotide Bead Array
InactiveUS20080311561A1Sugar derivativesMicrobiological testing/measurementStreptolydiginFluorescence
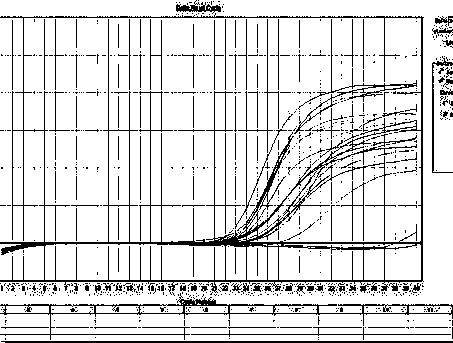
Provided are determining methods of human papillomavirus (HPV) genotypes with a high sensitivity. The method includes performing two-step PCRs on an HPV L1 gene in a sample to be analyzed as a biotin-labeled, single-stranded L1 gene, performing a hybridization reaction on the biotin-labeled, single-stranded L1 gene with a HPV genotype detection probe, reacting the hybridization reaction product with fluorescent substance combined with streptavidine, and measuring a fluorescent substance level to identify the HPV genotype. The detection method has high sensitivity enough to detect an extremely small amount of HPV in the sample. In addition, the high specificity exhibited by the detection method enables accurate diagnosis specific to HPV type.
Owner:GYNGEN BIO +1
Kit for rapid detection of HPV genotypes with reverse dot blotting method
InactiveCN107090520AHigh detection throughputReduce screening costsMicrobiological testing/measurementDNA/RNA fragmentationTypingHPV typing
The invention relates to a kit for rapid detection of HPV genotypes with a reverse dot blotting method. The kit for detection of 28 HPV genotypes which cover 18 HPV high-risk types and 10 HPV low-risk types in total is built. The kit comprises two parts of PCR reagents for HPV typing and reverse dot blotting reagents, the HPV types of clinical samples can be detected rapidly, accurately and in high throughput, the kit has a prediction effect on HPV clinical diagnosis and cervical cancer prognosis, and the problems that in the prior art, detection is time-consuming, specific typing cannot be carried out, and the genotypes classified are few, so that common HPV infection types of countrymen are not covered as many as possible are solved.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Set of Probes for the Detection and Typing of 46 Human Papillomavirus Mucosal Types
InactiveUS20130143751A1Accurate prevalence resultImprove automationNucleotide librariesMicrobiological testing/measurementHuman papillomavirusNorthern blot
We have developed a set of probes to detect and identify 46 types of mucosal human papillomaviruses (HPV). These probes recognize the variable region comprised between the 2 conserved regions of the published GP5+ / GP6+ set of PCR primers. The example described in this application, called NML Luminex genotyping method, uses a multiplex assay based on nested PCR amplification and the Luminex xMAP technology. The 46 probes have been shown to hybridize, as intended, to the DNA derived from the following HPV types: 6, 11, 13, 16, 18, 26, 30, 31, 32, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 74, 81, 82, 83, 84, 85, 86, 87, 89, 90, 91 and 97. The hybridization of each probe is specific for each type without any cross hybridization among types and it is sensitive enough to allow detection of PCR products for genotyping of HPV DNA contained in clinical samples. We also present a validation of the NML Luminex method against direct sequencing of HPV types and against the Roche Linear Array, a leading commercial method for HPV genotyping. The probes described here are suitable for use in other assays based on hybridization with labelled target HPV DNA, including, but not limited to, Southern and Northern blots, reverse line blot hybridization, DNA microarray, or ELISA.
Owner:SEVERINI ALBERTO +1
Detection, screening, and diagnosis of HPV-associated cancers
ActiveUS20100120019A1Access riskMicrobiological testing/measurementBiological material analysisSpecific detectionTumor Biomarkers
Embodiments of the invention provide methods, polyclonal antibodies, monoclonal antibodies, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Monoclonal antibodies are used to detect oncogenic high risk and low risk HPV types in a single assay, which is not limited to assay type or format. Useful tools for specific detection of various HPV associated cancers are provided. HPV associated cancer biomarkers are identified and can be used in a screening method for early stage precancerous lesions as well as late stage cancer progression.
Owner:HEER MEDICAL TECH DEV CO LTD
Method and kit for detecting HPV (Human Papillomavirus) genotype
ActiveCN103361442APrecise screeningIntuitively determinedMicrobiological testing/measurementMicroorganism based processesHuman papillomavirusA-DNA
The invention discloses a method and a kit for detecting an HPV (Human Papillomavirus) genotype. The method for detecting the HPV genotype comprises the following steps of: A, extracting DNA (Deoxyribonucleic Acid); b, carrying out PCR (Polymerase Chain Reaction) on a DNA template, and amplifying to obtain a PCR product of an HPV-infected DNA sample; and C, carrying out restricted segment length polymorphism analysis through restriction endonuclease. The method is low in detection cost and can be used for rapidly and intuitively determining the type of the infected HPV according to a special HPV type atlas.
Owner:HANGZHOU DIAGENS BIOTECH CO LTD
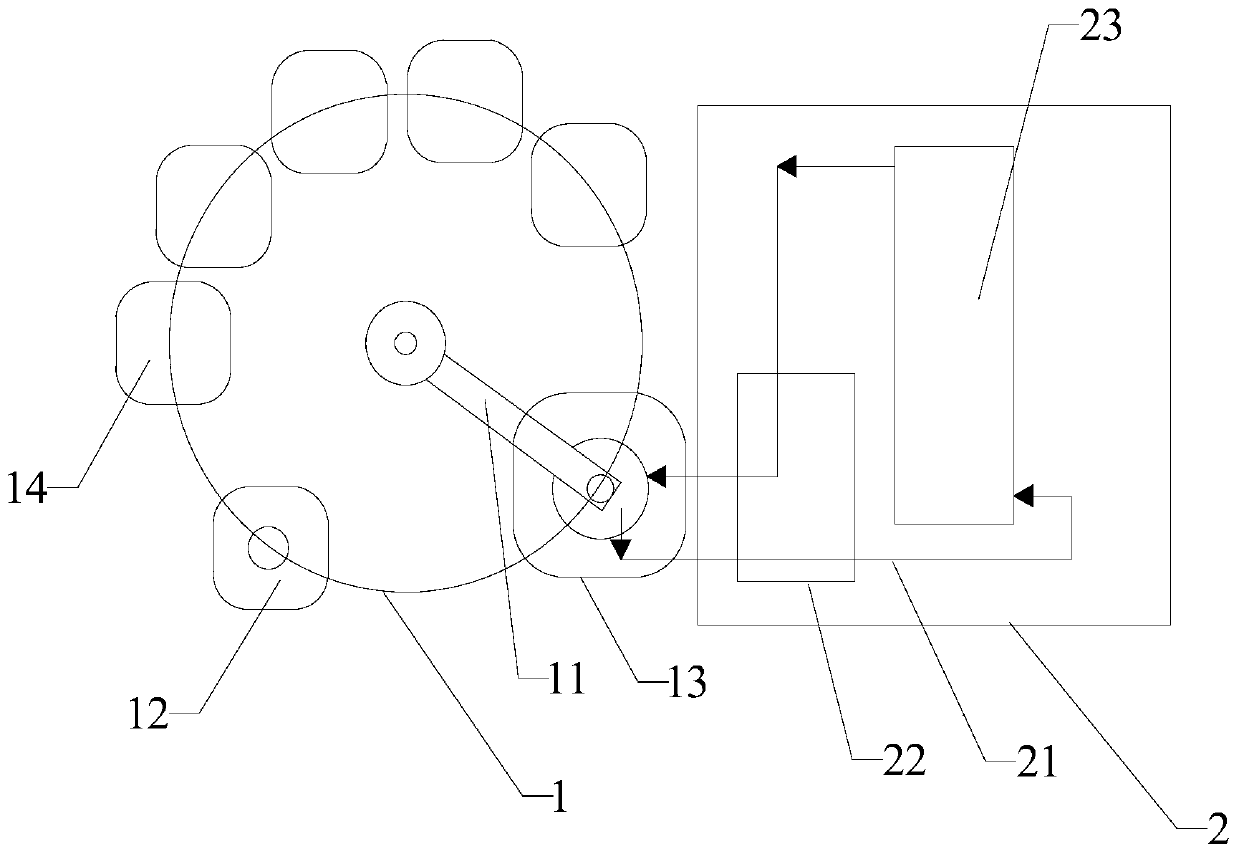
Full-automatic sample adding, hybridization and detection method for HPV genotyping chip
PendingCN110885907AGuaranteed continuitySmall manual intervention workloadBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringBiomedical engineering
The invention relates to the field of in-vitro diagnostic reagent detection, in particular to a full-automatic sample adding, hybridization and detection method for an HPV genotyping chip. The methodcomprises the following steps: dropwise adding a PCR product into a small test tube of a PCR product middle rotating disc, placing an HPV genotyping chip in a large test tube of a biochemical hybridization large rotating disc, preparing a reaction liquid required by hybridization color development and distilled water for cleaning a pipetting device, and carrying out hybridization detection. The detection method has the beneficial effects that rapid and convenient suction and sample adding are realized, the continuity of detection steps is ensured, the workload of manual intervention is relatively small and the detection efficiency is improved.
Owner:FUJIAN UNIV OF TECH
Method for flexibly detecting human papilloma virus genotypes in broad spectrum
InactiveCN104561349AEliminate residual contaminationEfficient detectionMicrobiological testing/measurementDiseaseGenotype
The invention provides a method for flexibly detecting human papilloma virus genotypes in a broad spectrum. The detected types contain 45 carcinogenic and pathogenic HPV genotypes (subtypes) described by the International Agency for Research on Cancer IARC and one giant wart-inducing type newly authenticated and reported by China CDC, and the coverage exceeds the sum of the types involved by the present various similar kits. According to the method provided by the invention, mass-spectrum scanning multiple PCR is taken as a platform, 21 carcinogenic and 2 high-incidence wart-inducing types with the highest clinic need frequency are detected as an independent reaction, and only two reactions are needed in total, thus further meeting the needs of clinic emphasis detection and difficult and complicated disease broad-spectrum screening; detection combinations are flexible and cost performance is high; several and even thousands of samples can be detected in one day; a result is automatically reported in real time, without the influences of human factors; tens of HPV types can be accurately detected simultaneously in one step. The method embodies the unique advantages of high flux, high flexibility, high automation and wide linear range, and provides an indispensable detection tool for comprehensive universal screening for pathogenic HPV infection.
Owner:赵宝慧 +1
Detection method and kit for human papilloma virus
InactiveCN104293974AMicrobiological testing/measurementFluorescence/phosphorescenceHuman papilloma virus infectionGenotype
The invention relates to a detection method for diagnosis of human papilloma virus (HPV) infection, and utilization of a kit prepare by the method for detecting HPV genotype DNA samples isolated from the patient in order to diagnose whether it belongs to one of the 24 types, and belongs to the technical field of life science. The method of the invention comprises a polymerase chain reaction based on PCR technology; the PCR reaction comprises simultaneous detection of DNA of 24 types including HPV6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 83, MM4 and cp8304 on a fluorescently-labeled upstream primers and unlabeled degenerated downstream primers of various types under particular PCR condition in a reaction tube, capillary denaturing gel electrophoresis, data read by sequenator, and division into specific types by a piece of provided software directly.
Owner:浦艳
Amplification-hybridisation method for detecting and typing human papillomavirus
The present invention provides amplification and hybridisation method for detecting and typing human papillomavirus (HPV), and the primers and hybridisation probes used in the method. The invention relates to a concrete part of the HPV genome, which is suitable for designing HPV genus-specific and HPV genotype-specific hybridisation oligonucleotide probes.
Owner:GENOID KFT
Amplification-hybridisation method for detecting and typing human papillomavirus
InactiveUS7294488B2Easy diagnosisStable supportSugar derivativesMicrobiological testing/measurementHuman papillomavirusHybridization probe
The present invention provides amplification and hybridisation method for detecting and typing human papillomavirus (HPV), and the primers and hybridisation probes used in the method. The invention relates to a concrete part of the HPV genome, which is suitable for designing HPV genus-specific and HPV genotype-specific hybridisation oligonucleotide probes.
Owner:GENOID KFT
PNA Probes, Kits, and Methods for Detecting Genotypes of Human Papillomavirus
InactiveUS20080248461A1Strong specificityHigh sensitivitySugar derivativesMicrobiological testing/measurementHuman papillomavirusCervix
Disclosed are PNA probes capable of genotype specifically binding with Human Paillomavirus (HPV) DNA, kits for detecting HPV genotypes comprising the probes, and methods for detecting HPV genotypes by using the kits, which enables the accurate detection of all 24 genotypes of HPV found in cervix, diagnosis of combined infection with more than one HPV genotype, and detection of HPV genotypes with high specificity and sensitivity.
Owner:PANAGENE INC
Compositions and methods for detecting human papillomavirus
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
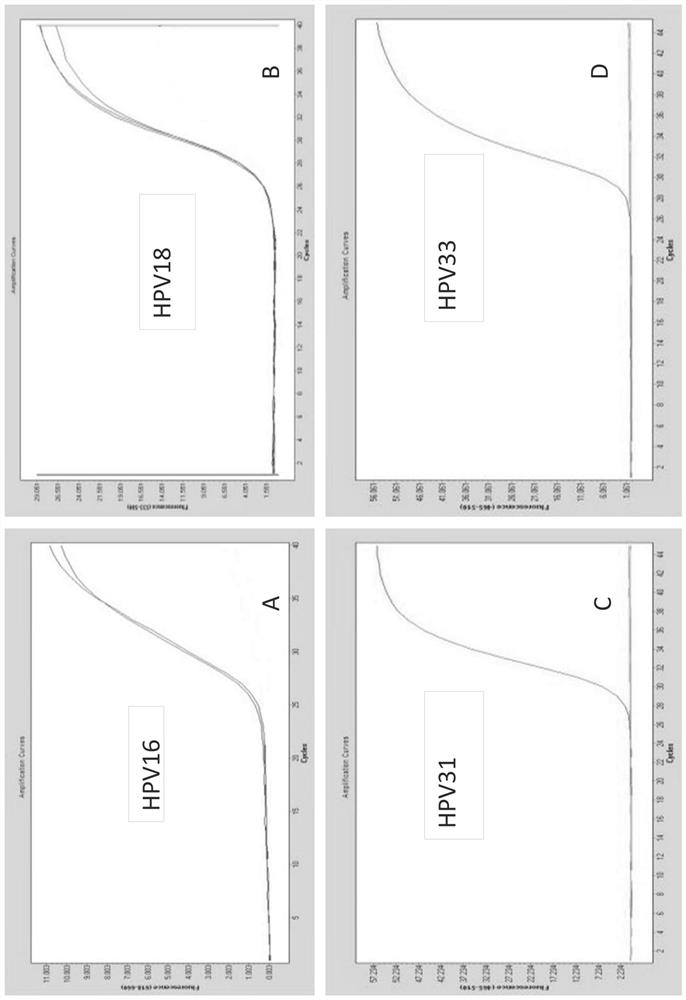
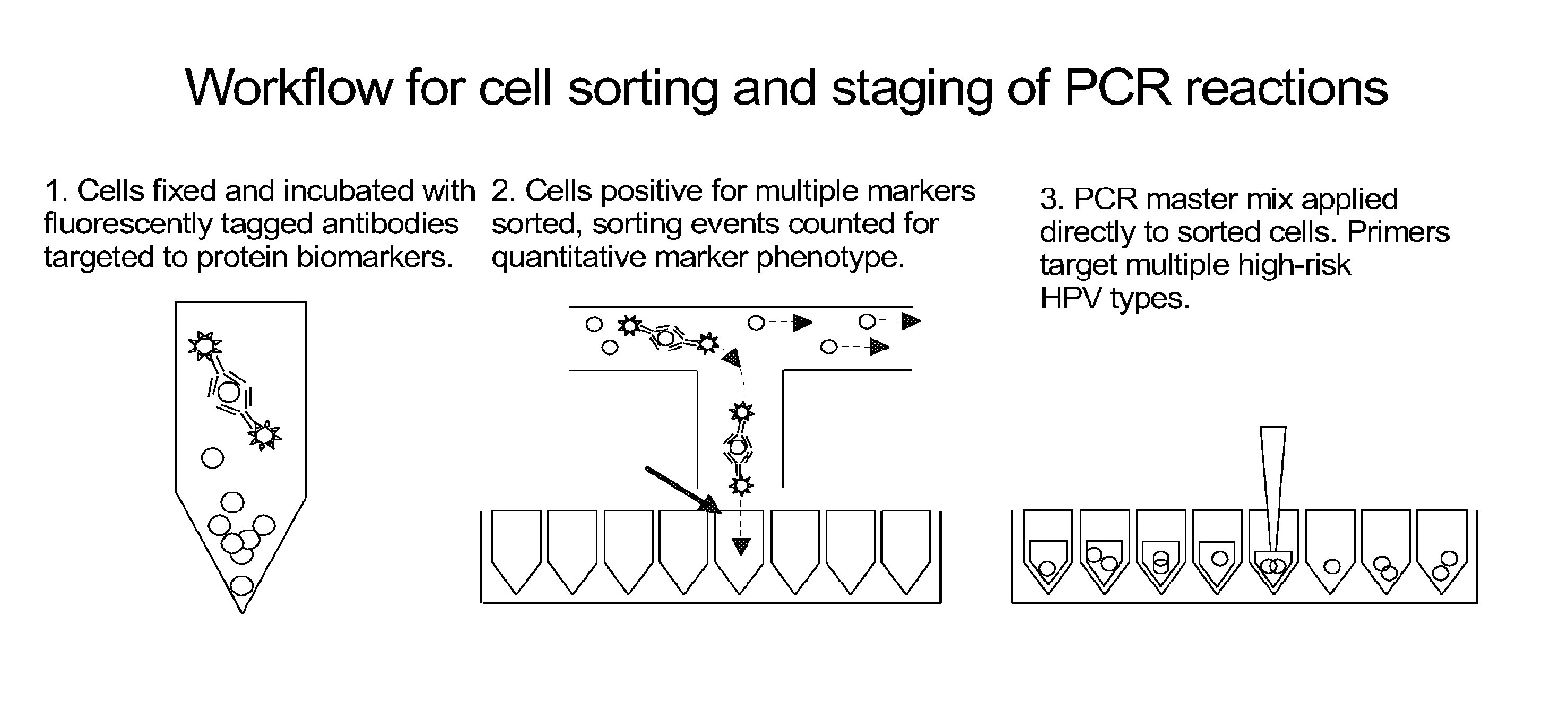
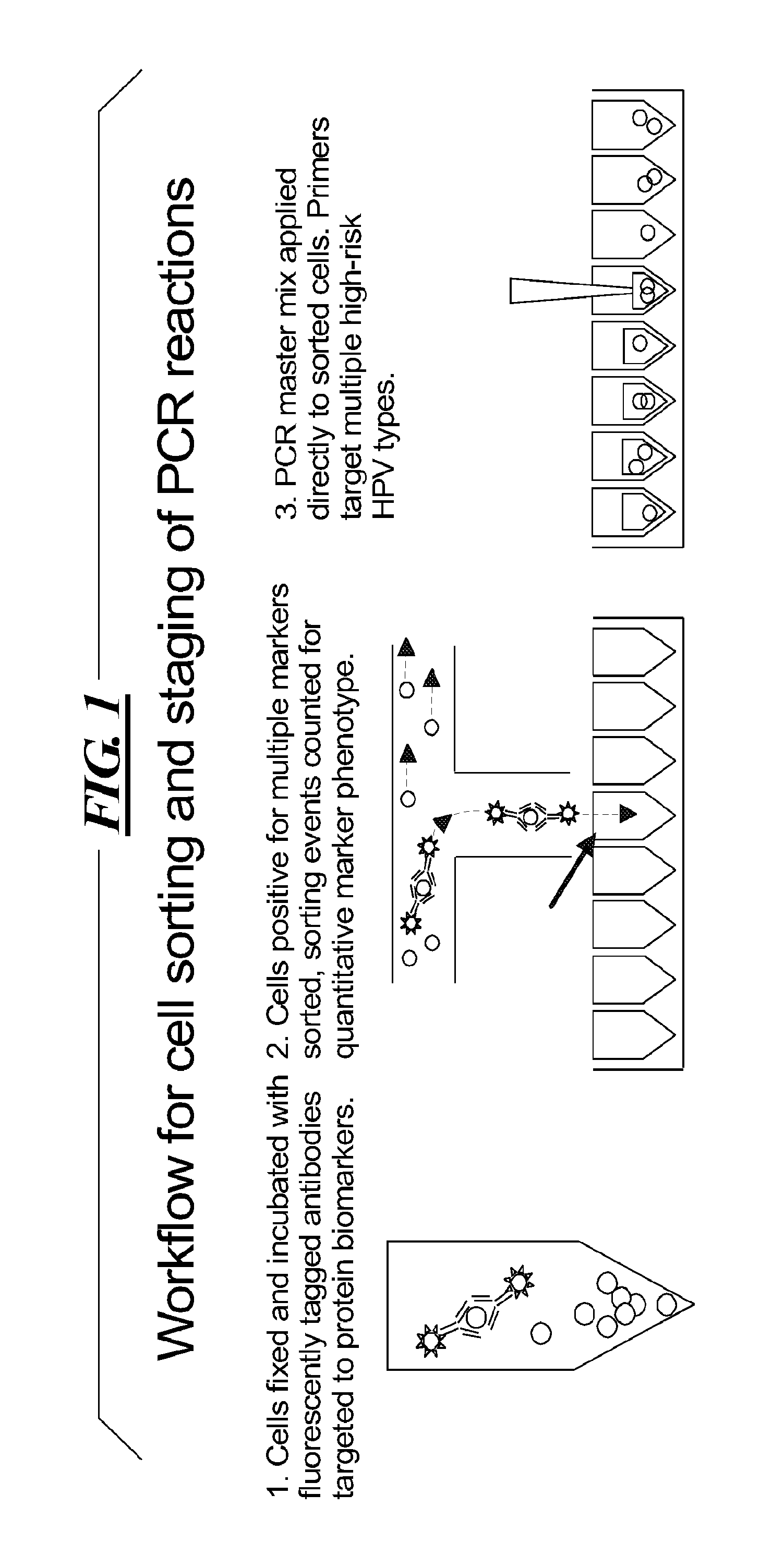
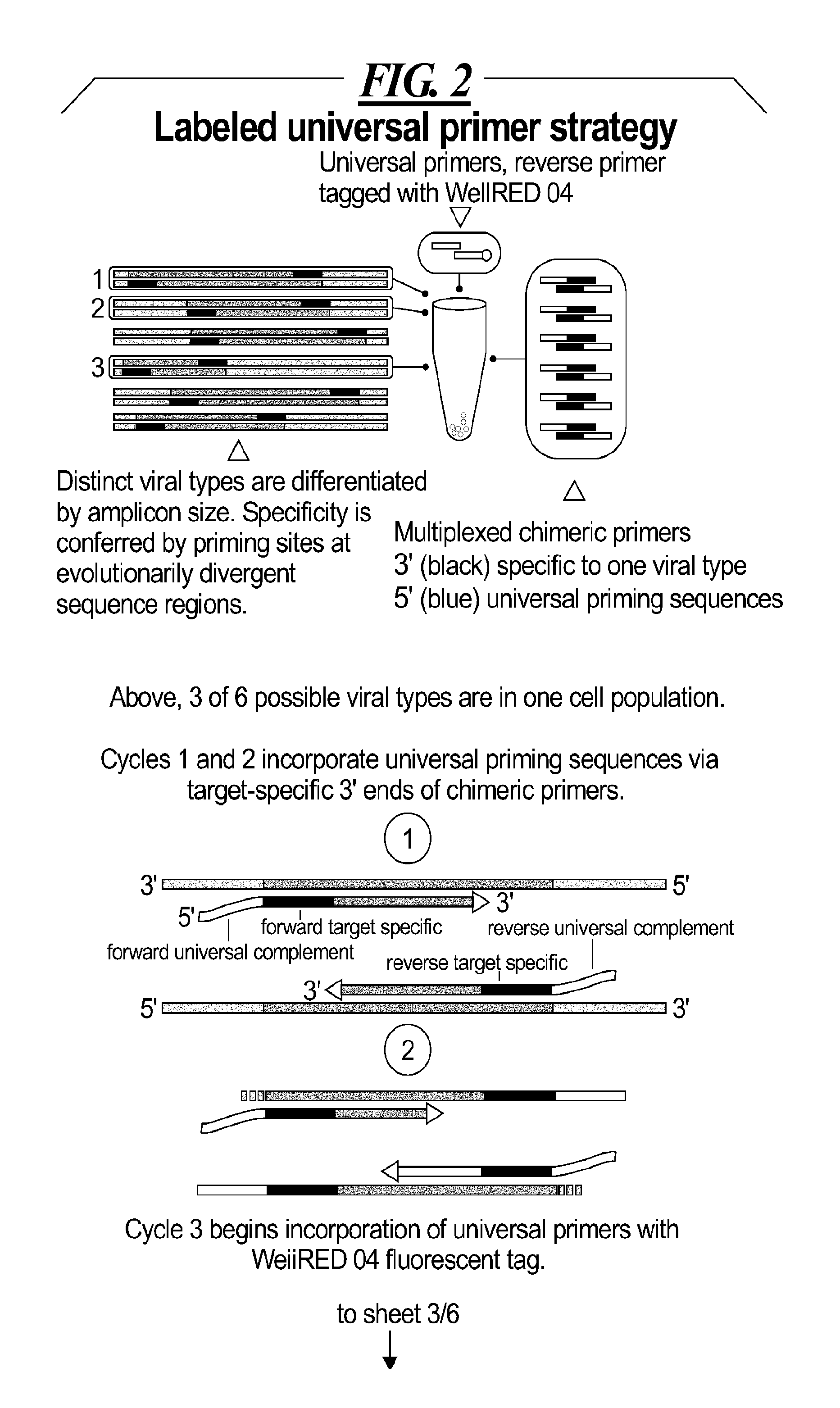
Integrated assay that combines flow-cytometry and multiplexed HPV genotype identification
InactiveUS20130165334A1Improve accuracyStrong specificitySugar derivativesMicrobiological testing/measurementCytometryOrganism
A two part assay is disclosed that enables collection of both protein biomarker phenotype and specific HPV genotype data from within a clinically derived population of cervical epithelial cells. Presence of multiple transformation-associated protein biomarkers acts as a gating criterion for cell sorting, followed by application of a PCR protocol sensitive enough to detect and identify individual HPV types from within the cells captured during sorting. The workflow has been optimized to work with cells conventionally fixed in PreservCyt (Cytyc), and it can be performed on residual cells remaining in a stored sample after a Pap test has been performed.
Owner:PURDUE RES FOUND INC
High-risk human papilloma virus typing detection method and kit
InactiveCN112391495ASimple designHigh detection sensitivityMicrobiological testing/measurementHybridization probeFluorescent quenching
The invention provides a high-risk human papilloma virus (HPV) typing detection method and a kit. The method adopts a fluorescent nucleic acid amplification method of multiple pairs of primers and multiple probes, combines melting curve analysis, and can realize detection of multiple target nucleic acid sequences in a single-wavelength fluorescence channel. According to the method, an HPV specificprimer and a TaqMan probe are designed by taking an L1 segment sequence of an HPV genome as a target sequence, and a PCO hybridization probe which is incompletely complementary with the TaqMan probeis designed; and fluorescent quenching group labeling or non-fluorescent quenching group labeling can be performed on the 3'end of the hybridization probe according to the purpose, and the 3 'end of the hybridization probe cannot extend again after labeling, so that the HPV genotyping kit containing the primer probe and other fluorescent PCR components is developed, and the HPV33, 35, 45, 51, 56 and 66 types can be identified by combining fluorescent PCR amplification with single-wavelength melting curve analysis.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
General primers and process for detecting diverse genotypes of human papillomavirus by PCR
The presence of diverse human Papillomavirus genotypes in a sample can be detected by 1 cycle of polymerase chain reaction using primers. The primers used in the method are general primers which can bind complementarily to specific nucleic acid sequences of all HPV genotypes related to cervical carcinoma and its pre-stage lesions and can be used to amplify cervical-neoplasia related HPV genotypes including oncogenic high-risk groups and low-risk groups.
Owner:CHA WILYINS
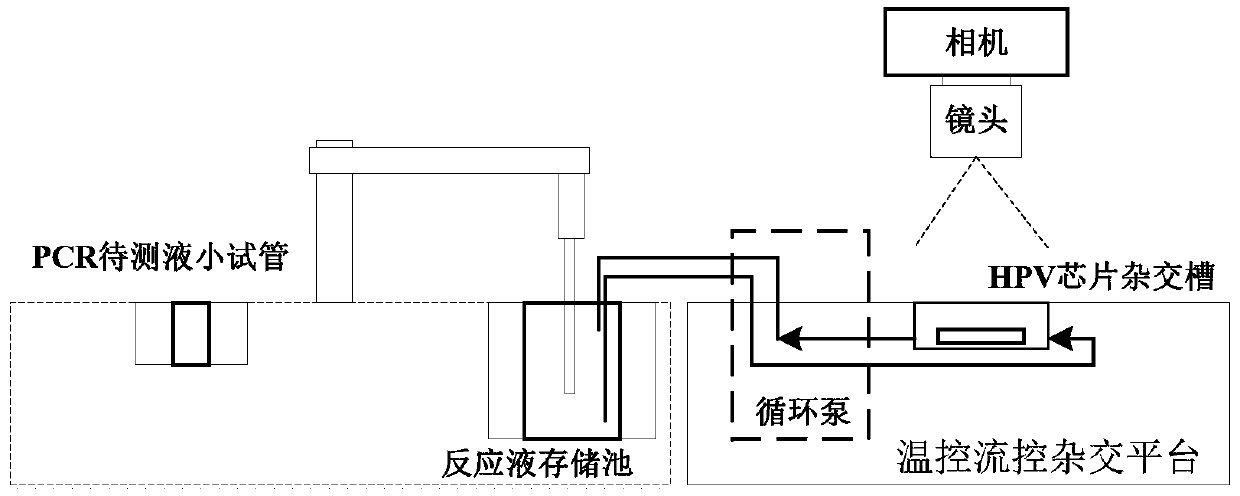
Fluidic HPV genotyping chip detection method
PendingCN110904274AGuaranteed continuityEfficient automatic detectionMicrobiological testing/measurementCirculator pumpBiomedical engineering
The invention relates to the field of in vitro diagnostic reagent detection, more specifically, relates to a fluidic HPV genotyping chip detection method comprising the following steps: dropwise adding a PCR product of a sample to be detected into PCR to-be-detected liquid small test tubes of an instrument, placing an HPV genotyping chip in an HPV chip hybridization tank, preparing reaction liquidrequired by hybridization, coupling and color development and distilled water required by cleaning, and carrying out automatic detection. The beneficial effects of the invention are that: a biochemical reaction model in a flow control mode is adopted; under the cooperation of a pipetting device for vertical sample adding and a circulating pump, the processes of multi-step hybridization, coupling,color development and cleaning required by HPV detection can share one set of pipetting device, storage pool, fluid pipeline, circulating pump and biochemical reaction tank, so that the continuity ofbiochemical detection steps is ensured, and automatic detection is easier to realize.
Owner:FUJIAN UNIV OF TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com