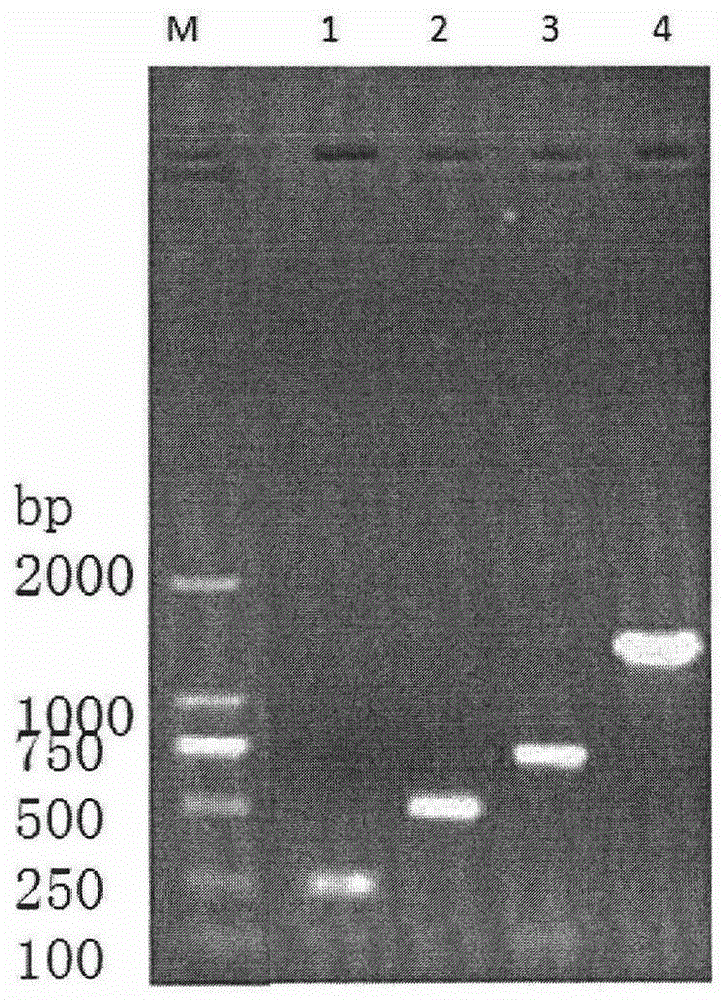
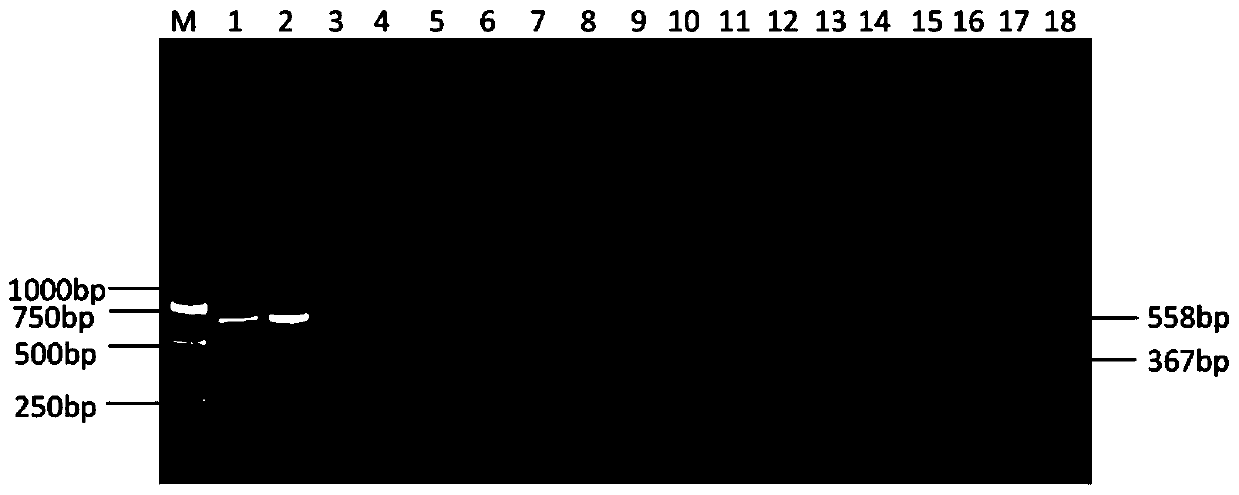
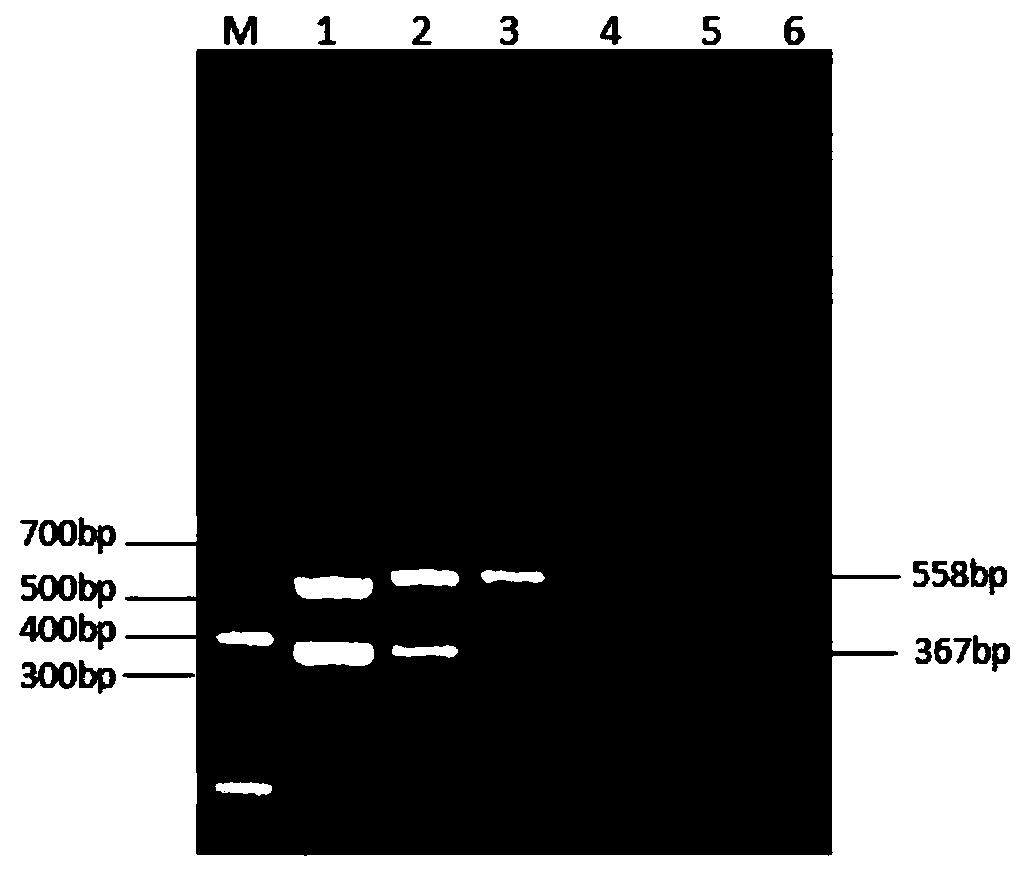
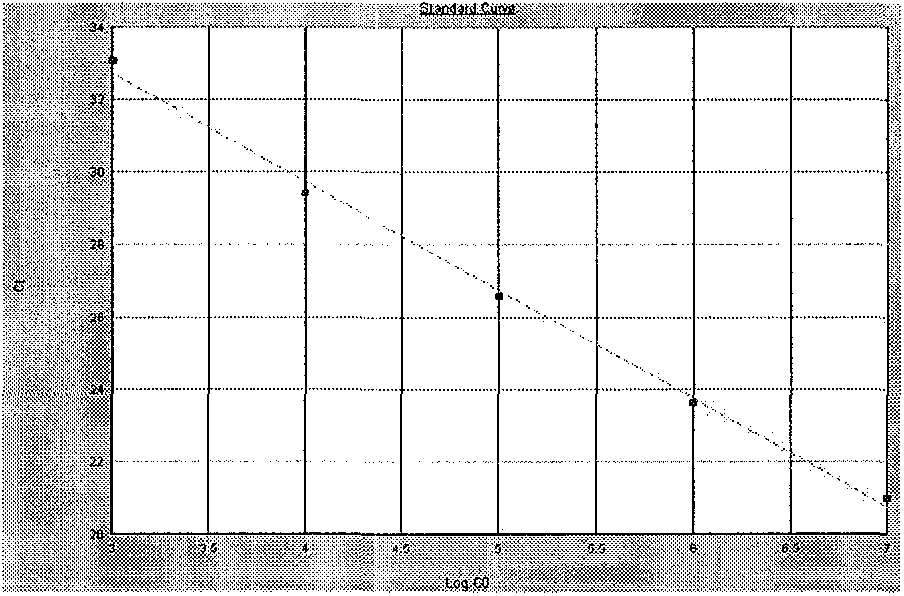
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Single infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Single Infection. A cell that has been adsorbed and consequently infected by only one virus. Typically when describing a single infection one disregards whether the bacterium being infected is or is not a lysogen and this is because it is assumed that the singly infecting phage and lysogen will only minimally interact.
Method for manually inoculating and culturing root seedling of summer truffle
The invention discloses a method for manually inoculating and culturing a root seedling of a summer truffle. According to the steps of sterilized seedling culturing, microbial agent preparation of a strain, manual growth, seedling refining, root seedling culturing and determination by detecting, the difficulty of manually inoculating the summer truffle is overcome. Compared with the prior culturing method, according to the method, armand pine, chestnut and Yunan pine are taken as hosts, in the condition of relative sterilization, i.e. in the condition of eliminating competition of fungus and infection of majority moulds, sufficient spores of the summer truffle are contacted with tree root parts of summer truffle hosts, single infection advantage of the spores of the summer truffle is maintained in a certain period of time, and stable and a large amount of summer truffle roots are formed.
Owner:PANZHIHUA FORESTRY SCI & TECH PROMOTION STAND PANZHIHUA FORESTRY WORK
Development and application of multiple fluorescence RT-PCR detection method for foot-and-mouth disease, vesicular stomatitis and swine vesicular disease
InactiveCN103602757AMicrobiological testing/measurementMicroorganism based processesSwine vesicular diseaseAnimal virus
Based on a highly conserved domain of a foot-and-mouth disease virus 3D protein coding gene, a vesicular stomatitis virus N protein coding gene and a swine vesicular disease virus VP1 protein coding gene, the invention designs a specific primer and a probe and develops a multiple fluorescence RT-PCR detection method used for simultaneously detecting the three animal viruses. The detection method can detect 102 copied plasmids containing target amplification sequences in 1 h. The method has high sensitivity and good specificity, and can achieve rapid high-throughput fluorescence RT-PCR detection for single-virus infection or mixed infection by a plurality of viruses.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
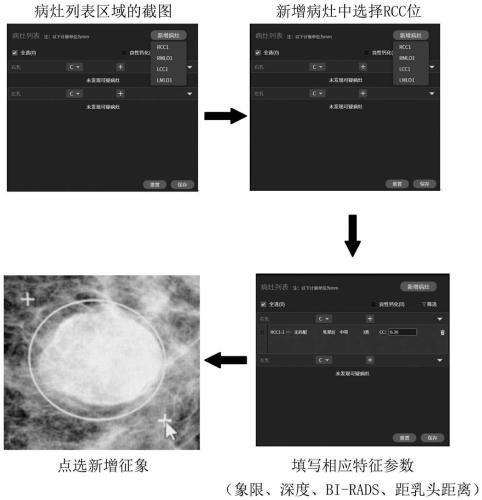
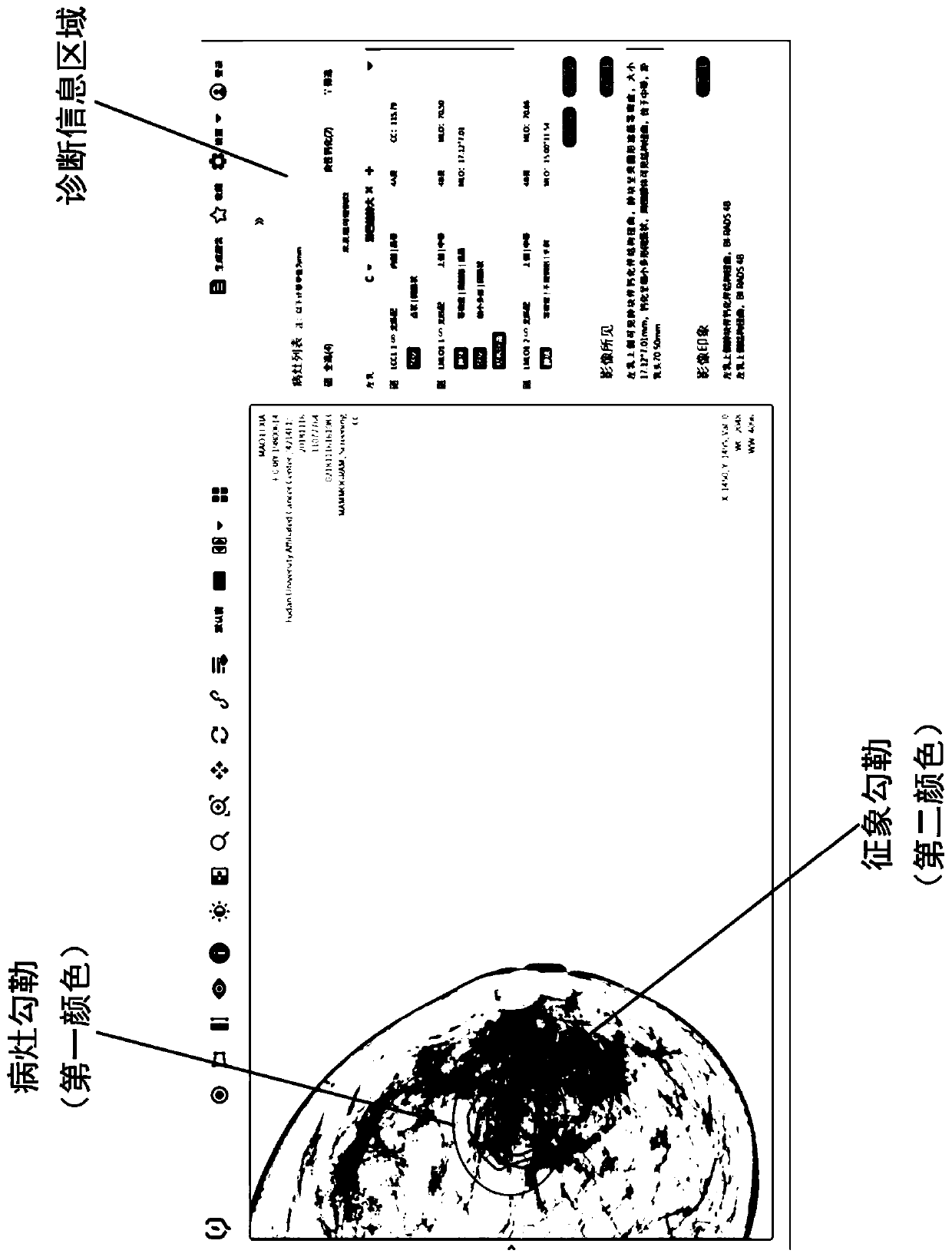
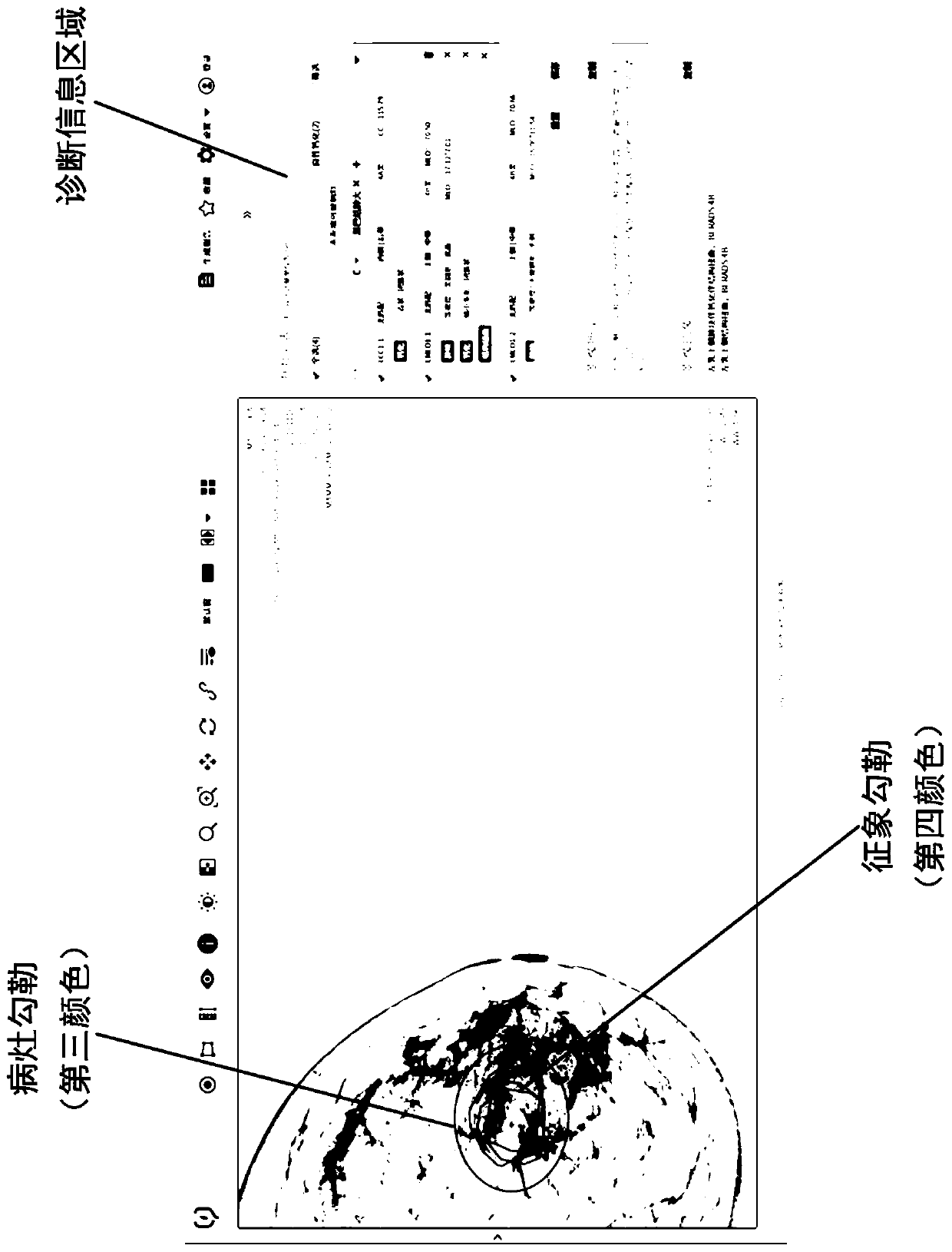
Gland medical image display method, interaction method, and storage medium
ActiveCN111430014ADaily film reading habits matchMedical imagesMedical reportsSurgeryImaging Procedures
The disclosure relates to a gland medical image display method, a gland medical image diagnosis interface interaction method, and a computer-readable storage medium. The display method includes: defining an infection focus area and representing a gland medical image containing the infection focus area in response to the input operation of infection focus feature parameters of the gland; in response to the selection operation of an operation body on the gland medical image, representing the infection focus and symptoms with different markers. The interaction method comprises: in response of theinput operation of the feature parameters, providing different interaction modes on the gland medical image containing the infection focus area; in response of the selection operation, representing the infection focus and symptoms with different markers. According to the technical scheme, under the application status of single infection focus corresponding to multiple symptoms, an infection focuslist includes the infection focus items and symptoms items, which are corresponded to the infection focus markers and symptom markers inside the infection focus in the image in a one-to-one manner, thereby greatly maintaining the usability of the system.
Owner:HANGZHOU YITU MEDIAL TECH CO LTD
Hepatitis B virus genotyping PCR (polymerase chain reaction) test kit
ActiveCN102586473AEliminate distractionsContinuation of fast and easy performanceMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceGenotype
The invention aims at fully utilizing the advantages of a Taqman probe technology, designing specific probes against various genotypes of HBV (hepatitis B virus), using the probes to mark different fluorescent dyes, detecting at different wavelengths and further achieving the genotyping purpose. A kit disclosed by the invention can detect B, C and D type hepatitis B virus in samples and detect B,C and D type single infection and mixed infection by designing primers and the specific probes against all the genotypes of the HBV. The B (or D) type hepatitis B virus is detected by adopting a double-color probe at FAM wavelength and the C-type hepatitis B virus is detected at HEX wavelength. The kit has the advantages that the B, C and D type hepatitis B virus can be simultaneously detected inone experiment, the operation is simple, dUTP and UNG enzymes are used in the kit, and the interference of a polluted amplified product on a detection result can be effectively eliminated.
Owner:泰普生物科学(中国)有限公司
Method for rapidly and synchronously detecting wheat yellow mosaic virus and Chinese wheat mosaic virus
ActiveCN103103288AMicrobiological testing/measurementMicroorganism based processesChinese wheat mosaic virusTriticeae
The invention discloses a method for rapidly and synchronously detecting wheat yellow mosaic virus and Chinese wheat mosaic virus. According to the method, three primers comprising a reverse primer CWWY-R2 of 5'-GGTTCCMGTTATCGTACT-3' and two positive primers of CW1-F2 and WY1-F2 of 5'-GGAAGGGATGCCATACAACT-3' and 5'-ACCCTACAAACAAACTCTGC-3' are designed according to similarities and differences of two virus gene sequences, the plant general RNA serves as a template, the CWWY-R2 serve as the primer, and reverse transcription is performed to synthesize c DNA. A reaction system for synchronously detecting two viruses is established through optimization, the system comprises total 20mu L of 1.6mu L of cDNA, respectively 0.4mu L of primers (10mu mol / L), 2mu L of 10*PCR Buffer (not containing Mg<2+>), 0.4mu L of dNTPs (10mmol / L each), 0.8mu L of Taq DNA polymerase (5U / mu L), 0.8mu L of MgCl2 (25mmol) and 13.2 mu L of ddH2O; the polymerase chain reaction (PCR) has the reaction conditions of initial denaturation within 5 minutes at the temperature of 94 DEG C, 50 seconds at the temperature of 94 DEG C, 50 seconds at the temperature of 50 DEG C, and 90 seconds at the temperature of 72 DEG C within 30 cycles totally, and extension within 10 minutes at the temperature of 72 DEG C; and the PCR amplification expected target fragments are respectively WYMV 508bp and CWMV 918bp. According to the method, samples in Gaoyou, Yangzhou and Dafeng in Jiangsu are detected and are subjected to WYMV, WYMV and CWMV single infection.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
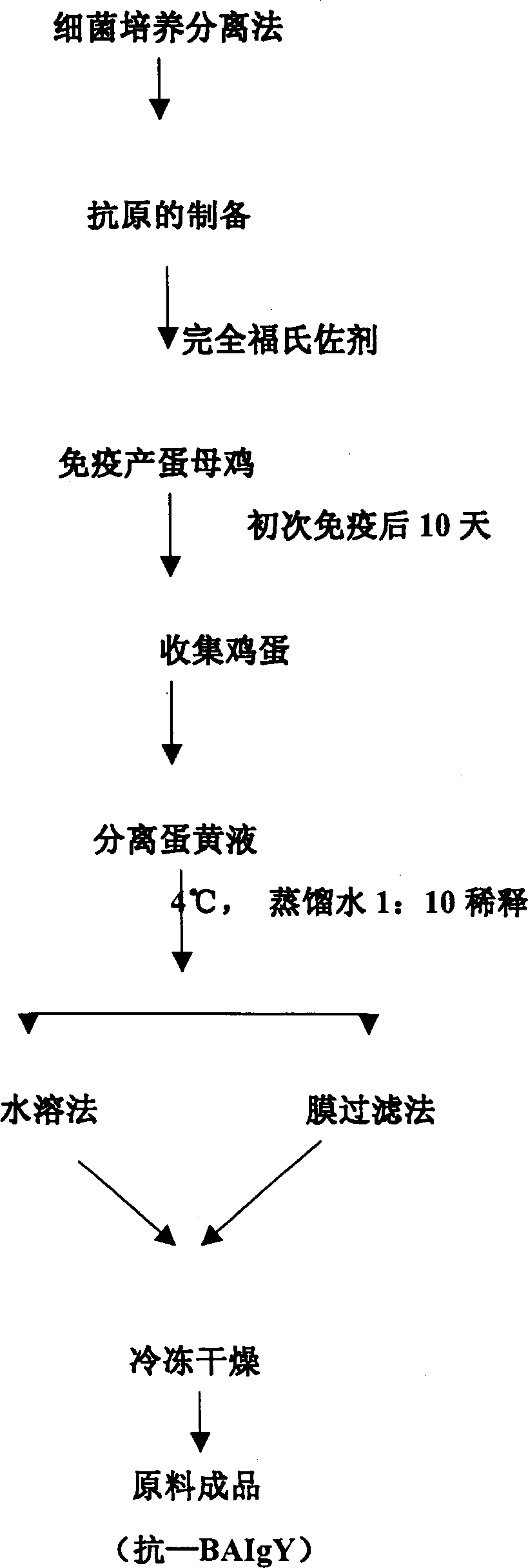
Compound yolk antibody capable of resisting fowl bacterial blight and its preparation and use
The present invention relates to a compound vitelline antibody (anti-BAIgY) with antibody activity for resisting salmonella gallinarum, bacterium multocidum and chicken colibacillus, method for preparing said antibody and application of said antibody in preparation of medicine or feed additive for curing relatied diseases which can be infected by above-mentioned bacteria. The preparation method of said compound vitelline antibody includes the following steps: preparing compound antigen of the above-mentioned bacteria, immunizing health hen with said antigen, collecting egg and extracting anti-BA IgY from egg.
Owner:重庆和润实业(集团)有限公司
Method for breeding summer truffle root seedling through inoculation of suspension liquid
InactiveCN103070014ASolve the problem of manual vaccinationGuaranteed uniformityHorticultureSnow moldTuber aestivum
The invention discloses a method for breeding summer truffle root seedling through inoculation of suspension liquid, which is characterized in that the difficulty in artificial inoculation of summer truffle can be solved through steps of breeding of aseptic seedling, preparation of microbial inoculum, inoculation of suspension liquid, seedling exercising, breeding of fungus seedlings and test and confirmation. Compared with the existing breeding method, the method is characterized in that pinus armandi, castanea mollissima and pinus yunnanensis are adopted as hosts; under a relatively aseptic condition, i.e. the condition for preventing the competition of fungi and infection of most mycetes, the summer truffle spores sufficiently contact with roots of the host trees; the single infection predominance of the summer truffle is maintained within a given period of time; and a great number of firm summer truffle mycorrhizas can be formed.
Owner:PANZHIHUA FORESTRY SCI & TECH PROMOTION STAND PANZHIHUA FORESTRY WORK
Multi-PCR (Polymerase Chain Reaction) specific primer for detecting bacterial enteritis pathogens of pig and application thereof
InactiveCN106755529AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesEscherichia coliBacillus perfringens
The invention relates to the field of molecular biology and production of animal medicine, in particular to a multi-PCR (Polymerase Chain Reaction) specific primer for detecting bacterial enteritis pathogens, such as escherichia coli, salmonellae and clostridium perfringens, of a pig; nucleotide sequences are shown as SEQ ID NO:1 to SEQ ID NO:6; the specificity, the repeatability and the sensitivity are high. The invention also discloses a method which is established on the basis of the primer and is used for detecting the bacterial enteritis pathogens, such as the escherichia coli, the salmonellae and the clostridium perfringens, of the pig; the enteritis of the pig which is caused by single infection or mixed infection of the escherichia coli, the salmonellae and the clostridium perfringens can be distinguished quickly; the primer can be used to identify and diagnose quickly and effectively, and is beneficial to formulating targeted prevention and control measures to perform immune prevention and control on the bacterial enteritis pathogens of the pig in clinical parturition and ensure smooth parturition of the raised pig.
Owner:WENS FOODSTUFF GRP CO LTD
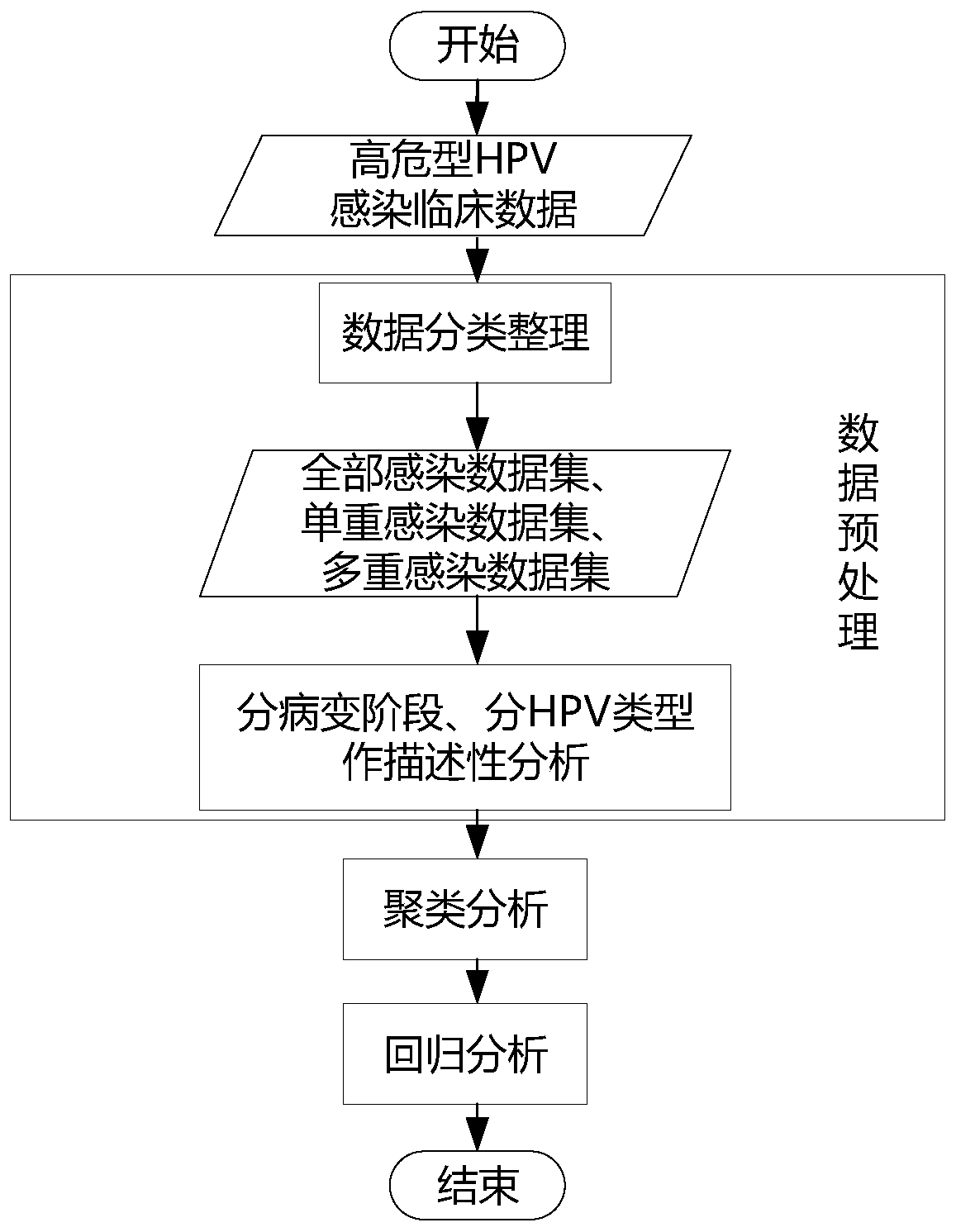
Method and device for calculating relationship between high-risk HPV types and cervical cancer precancerous lesion stages
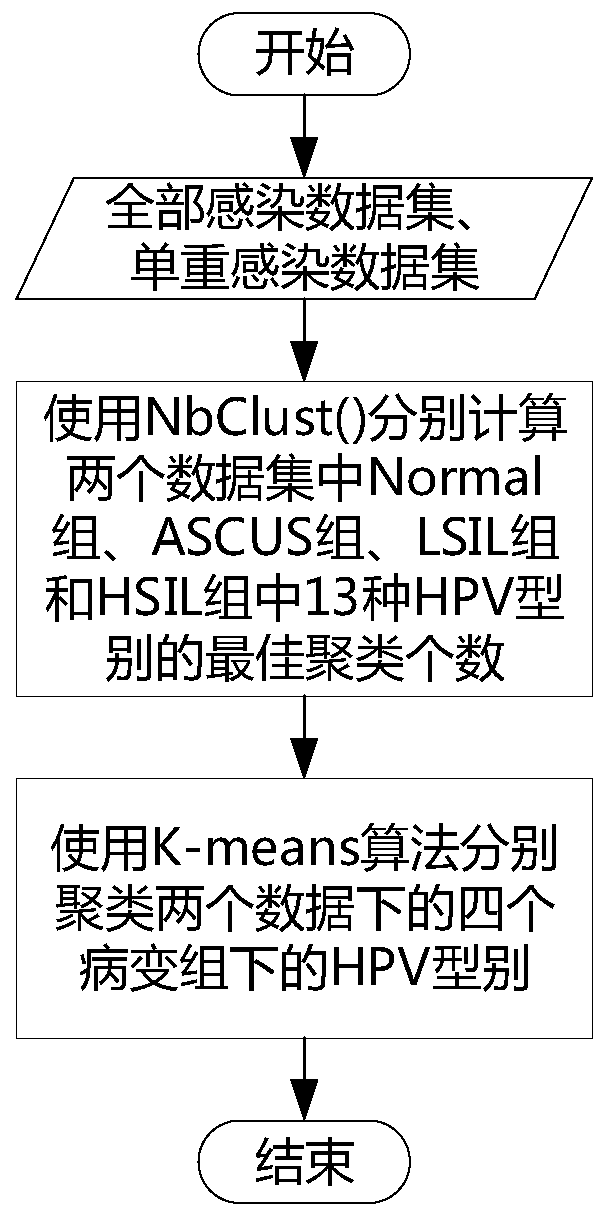
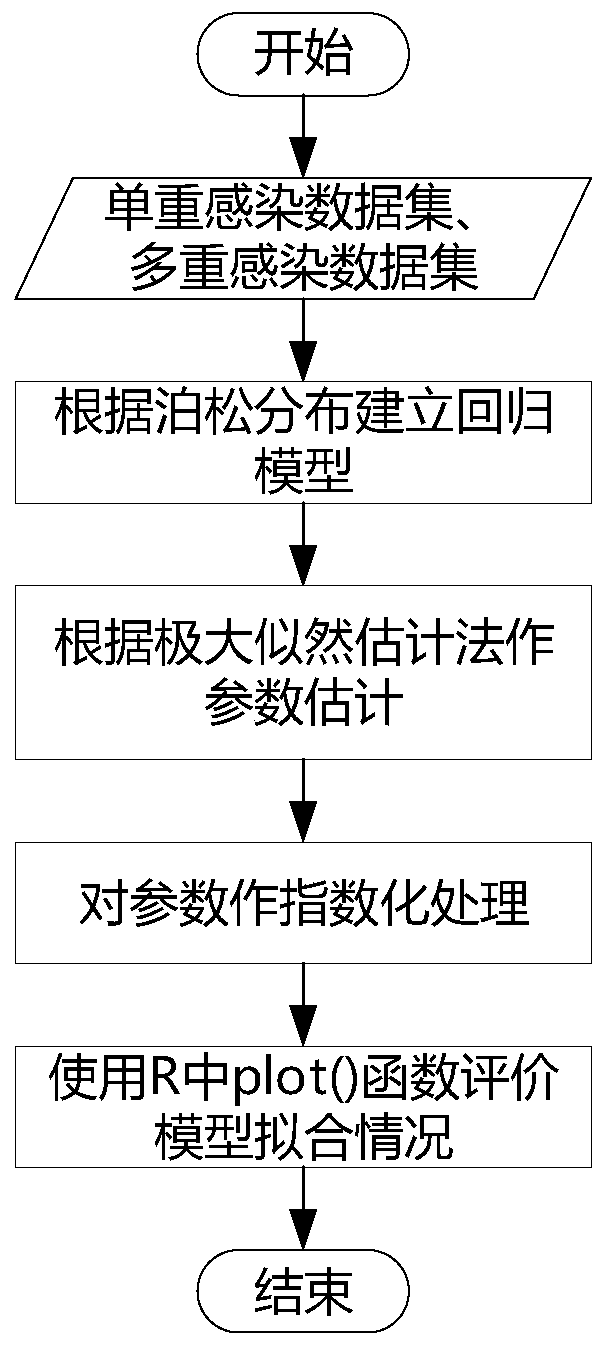
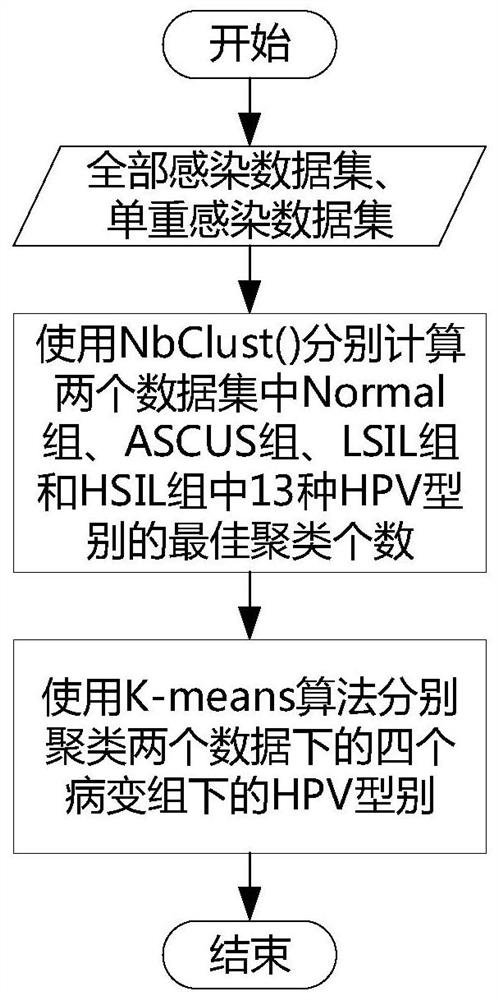
The invention provides a method and a device for calculating a relationship between high-risk HPV types and cervical cancer precancerous lesion stages. The method comprises the steps: carrying out classification on N kinds of high-risk HPV infection data, obtained through thin-layer cytological examination (TCT) and HPV genotyping detection, under M kinds of cervical cancer precancerous lesion stages, and obtaining HPV infection preprocessing data in different infection modes; performing clustering analysis based on the HPV preprocessing data, and obtaining the similarity of different high-risk HPVs based on a clustering analysis result; and carrying out modeling according to Poisson distribution based on the HPV preprocessing data under the single infection and multiple infection modes, and performing regression analysis to obtain the influence proportion of the HPV single infection and multiple infection on the cervical cancer precancerous lesion. According to the method, a clustering technology and a statistical analysis method are combined to mine biological data, and the relationship between different high-risk HPVs and different cervical cancer precancerous lesion stages is found.
Owner:SICHUAN UNIV
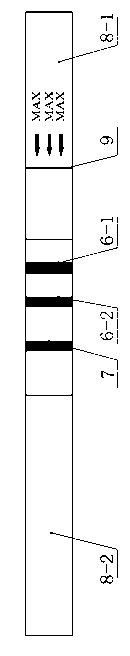
Marek's disease virus and subgroup-J avian leukosis virus rapid combined-detection test strip
ActiveCN103235129ARapid serology methodSerological method is simpleMicroorganism based processesImmunoglobulins against virusesLeucosisAvian leukosis viruses
The invention discloses a test strip used in one-step rapid detection of Marek's disease virus (MDV) and subgroup-J avian leukosis virus (ALV-J). The test strip comprises a non-absorbing supporting layer, and an absorption layer adhered to the supporting layer. The absorption layer is formed by sequentially spliced components of an absorption fiber layer, a gold-labeled antibody fiber layer, a cellulose film layer, and a water absorption layer. The cellulose film layer is marked with anti-goat IgG or anti-mouse IgG control blot, and a detection blot comprising anti-MDV and anti-ALV-J antibody. The anti-MDV and anti-ALV-J antibody is an anti-MDV and anti-ALV-J antibody monoclonal or polyclonal antibody. An anti-MDV and anti-ALV-J mixed polyclonal antibody or monoclonal antibody marked by colloidal gold and corresponding to the detection blot is adhered to the gold-labeled antibody fiber layer. The test strip provided by the invention has the advantages of high detection specificity, high sensitivity, simple operation, fast detection, and intuitive result. The test strip is suitable for MDV and ALV-J virus on-site rapid combined detection, and can be used in identification and diagnosis of single infection or mixed infection of the two viruses. The test strip can be widely applied, and is suitable for popularization.
Owner:HENAN ACAD OF AGRI SCI
Reticuendotheliosis virus and subgroup-J avian leukosis virus rapid combined-detection test strip
ActiveCN103235128ARapid serology methodSerological method is simpleMaterial analysisLeucosisAvian leukosis viruses
The invention discloses a test strip used in one-step rapid detection of reticuendotheliosis virus (REV) and subgroup-J avian leukosis virus (ALV-J). The test strip comprises a non-absorbing supporting layer, and an absorption layer adhered to the supporting layer. The absorption layer is formed by sequentially spliced components of an absorption fiber layer, a gold-labeled antibody fiber layer, a cellulose film layer, and a water absorption layer. The cellulose film layer is marked with anti-goat IgG or anti-mouse IgG control blot, and a detection blot comprising anti-REV and anti-ALV-J antibody. The anti-REV and anti-ALV-J antibody is an anti-REV and anti-ALV-J antibody monoclonal or polyclonal antibody. An anti-REV and anti-ALV-J mixed polyclonal antibody or monoclonal antibody marked by colloidal gold and corresponding to the detection blot is adhered to the gold-labeled antibody fiber layer. The test strip provided by the invention has the advantages of high detection specificity, high sensitivity, simple operation, fast detection, and intuitive result. The test strip is suitable for REV and ALV-J virus on-site rapid combined detection, and can be used in identification and diagnosis of single infection or mixed infection of the two viruses. The test strip can be widely applied, and is suitable for popularization.
Owner:HENAN ACAD OF AGRI SCI
Quick detection method for mastitis
InactiveCN106434855ARapid prevention and controlEffective prevention and controlMicrobiological testing/measurementMicroorganism based processesEscherichia coliGenomic DNA
The invention discloses a quick detection method for mastitis. The method comprises the following steps that genomic DNA to be detected is extracted; a mutiplex PCR reaction system is set; a designed primer group is added into the mutiplex PCR reaction system for reacting, and the reaction procedures that predegeneration is conducted for 5 min at 94 DEG C, degeneration is conducted for 45 s at 94 DEG C, annealing is conducted for 30 s at 57 DEG C, and extending is conducted for 90 s at 72 DEG C are included and conducted 32 cycles; extending is conducted for 10 min at 72 DEG C, and then a mutiplex PCR amplification product is obtained; gel electrophoresis is conducted on the mutiplex PCR amplification product, and whether the sample to be detected is subjected to single infection or mixed infection of staphylococcus aureus, streptococcus agalactiae, escherichia coli and mycoplasma bovis or not is judged according to electrophoretic bands generated in the sample to be detected. According to the method, the good sensitivity and specificity are achieved, four kinds of cow mastitis caused by the staphylococcus aureus, the streptococcus agalactiae, the escherichia coli and the mycoplasma bovis can be detected simultaneously, and then the cow mastitis is quickly and effectively prevented and controlled.
Owner:CHINTEM TECH CONSULTING BEIJING CO LTD
Mycoplasma hyorhinis strain, vaccine composition, preparation method and application thereof
ActiveCN104250623AImprove the effect of prevention and controlImmunity overAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
The invention provides a Mycoplasma hyorhinis strain LYH, and a vaccine composition prepared from the Mycoplasma hyorhinis strain LYH, in particular to a vaccine composition comprising the Mycoplasma hyorhinis and Mycoplasma hyopneumoniae. The vaccine composition can be effective in prevention and treatment of swine enzootic pneumonia caused by Mycoplasma hyorhinis, Mycoplasma hyopneumoniae single infection or mixed infection. Especially in the circumstance of mixed infection, immune effect of the vaccine composition significantly exceeds that of each single vaccine.
Owner:PU LIKE BIO ENG
H9 and H10 subtype avian influenza virus triple RT-PCR detection primer group, kit and method
PendingCN110669872AStrong specificityHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesHypotypeVirus
The invention provides an H9 and H10 subtype avian influenza virus triple RT-PCR detection primer group, a kit and a method. The design is directed at specific primers of H9 and H10 subtype AIV HA genes and M genes, different primer combinations are tested and screened to obtain a primer combination with a relatively good amplification effect; the concentration of the primers, the proportion amongthe primers, the annealing temperature and the amplification time in a reaction system are continued to be optimized; and finally, a method for simultaneously detecting H9 and H10 subtype AIV is established. The primers and the detection method can be used for simultaneously determining the situation of mixed infection and single infection of the H9 and H10 subtype AIV in samples through one-timePCR reaction; and the method has the advantages of strong specificity, high detection sensitivity and the like, is quick, convenient and easy to operate, is suitable for application and popularization in grass-roots departments, and provides technical support to supervision, prevention and control of the H9 and H10 subtype AIV.
Owner:GUANGXI VETERINARY RES INST
Method and system for improving infection prevention and control management quality
InactiveCN113873196AQuality improvementImprove quality and safetyClosed circuit television systemsHealthcare resources and facilitiesVideo monitoringEngineering
The invention discloses a method and system for improving infection prevention and control management quality, and the method comprises the steps: obtaining first monitoring information, second monitoring information and third monitoring information of a first hospital through a plurality of video monitoring devices; obtaining association degree information of a first department, a second department and a third department; screening and sorting the association degree information of the first department, the second department and the third department according to a predetermined association degree threshold to obtain a first interlocking monitoring sequence; obtaining a first interlocking monitoring video according to the first interlocking monitoring sequence, the first monitoring information, the second monitoring information and the third monitoring information; and performing infection prevention and control management on the first hospital according to the first interlocking monitoring video. The technical problems that the medical prevention and control management quality is low and the infection risk cannot be reduced due to single infection prevention and control management means in the prior art are solved.
Owner:THE FIRST PEOPLES HOSPITAL OF NANTONG
Primer group for carrying out dual nanometer PCR (Polymerase Chain Reaction) detection on H7 and N2 subtype avian influenza virus, kit and method
PendingCN111518953AStrong specificityHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationHypotypeVirus
The invention provides a primer group for carrying out dual nanometer PCR (Polymerase Chain Reaction) detection on an H7 and N2 subtype avian influenza virus, a kit and a method. The invention aims atthe specific primers of H7 and N2 subtype AIV (avian influenza virus) genes to experiment and screen different primer combinations to obtain a primer combination with a good amplification effect; then, a ratio, an annealing temperature and amplification time between primer concentration and a primer in a reaction system can be simultaneously optimized; and finally, a method capable of simultaneously detecting the H7 and N2 subtype avian influenza virus is finally established. The primer and the detection method can simultaneously determine a mixed infection and single infection situation of the H7 and N2 subtype AIV in a sample through one-time nanometer PCR reaction, especially, and a novel PCR technology, i.e., the nanometer PCR, is adopted and is more sensitive than common PCR and moreconvenient and quicker than real-time fluorescent quantitation PCR. The method simultaneously has the advantages of high specificity, high detection sensitivity, high speed, convenience and the like,is easy to operate, is very suitable to be popularized in grassroots units, and provides technical support for monitoring, preventing and controlling the H7 and N2 subtype avian influenza virus.
Owner:GUANGXI VETERINARY RES INST
Triple PCR detection kit for duck circovirus, duck adenovirus and duck tembusu virus
PendingCN114107556AQuick checkReduce workloadMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTembusu virus
The invention relates to a triple PCR (Polymerase Chain Reaction) detection primer group for duck circovirus, duck adenovirus and duck tembusu virus. The triple PCR detection primer group comprises a first primer pair, a second primer pair and a third primer pair, wherein the first primer pair has a nucleotide sequence pair as shown in SEQ ID NO.1 and SEQ ID NO.2; the second primer pair has a nucleotide sequence pair as shown in SEQ ID NO.3 and SEQ ID NO.4; the third primer pair has a nucleotide sequence pair as shown in SEQ ID NO. 5 and SEQ ID NO. 6. The invention further provides a triple PCR detection kit for the duck circovirus, the duck adenovirus and the duck tembusu virus, the detection kit disclosed by the invention can be used for simultaneously detecting the mixed infection or single infection condition of the duck circovirus, the duck adenovirus and the duck tembusu virus, the workload is reduced, and the detection time is greatly shortened; and the purpose of rapidly detecting pathogens is achieved.
Owner:LIAOCHENG UNIV
Triplex PCR detection primer group, kit and method for chicken parvovirus (ChPV) and H9 subtype avian influenza virus (AIV)
PendingCN110777219AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationChicken parvovirusHypotype
The invention discloses a triplex PCR detection primer group, kit and method for chicken parvovirus (ChPV) and H9 subtype avian influenza virus (AIV). Specific primers for the chicken ChPV and the H9subtype AIV are designed, a primer combination with a better amplification effect is obtained through testing and screening of different primer combinations, then, the primer concentration in a reaction system, ratios of primers, annealing temperature and amplification time are continuously optimized, and finally, the method capable of detecting the chicken ChPV and the H9 subtype AIV simultaneously is established. The primers and the detection method can determine mixed infection and single infection conditions of the chicken ChPV and the H9 subtype AIV in samples at the same time by one-timePCR reaction, and the method has the advantages of being high in specificity, high in sensitivity, rapid, convenient, easy to operate and the like, is quite suitable for application and promotion ingrassroots units and provides technical support for monitoring, preventing and controlling the chicken ChPV and the H9 subtype AIV.
Owner:GUANGXI VETERINARY RES INST
Hepatitis B virus genotyping PCR (polymerase chain reaction) test kit
ActiveCN102586473BEliminate distractionsContinuation of fast and easy performanceMicrobiological testing/measurementFluorescenceGenotype
The invention aims at fully utilizing the advantages of a Taqman probe technology, designing specific probes against various genotypes of HBV (hepatitis B virus), using the probes to mark different fluorescent dyes, detecting at different wavelengths and further achieving the genotyping purpose. A kit disclosed by the invention can detect B, C and D type hepatitis B virus in samples and detect B, C and D type single infection and mixed infection by designing primers and the specific probes against all the genotypes of the HBV. The B (or D) type hepatitis B virus is detected by adopting a double-color probe at FAM wavelength and the C-type hepatitis B virus is detected at HEX wavelength. The kit has the advantages that the B, C and D type hepatitis B virus can be simultaneously detected in one experiment, the operation is simple, dUTP and UNG enzymes are used in the kit, and the interference of a polluted amplified product on a detection result can be effectively eliminated.
Owner:泰普生物科学(中国)有限公司
Triple RT-PCR detection kit
InactiveCN104789698AStrong specificityGood repeatabilityMicrobiological testing/measurementMicroorganism based processesElectrophoresesRotavirus
The invention belongs to the field of biological detection and in particular relates to a triple RT-PCR detection kit. Three pairs of specific primers are respectively designed according to PEDV M gene, TGEV N gene and GAR VP 7 gene, wherein the sizes of the amplified fragments are 199bp, 499bp and 333bp respectively; a user can directly judge by naked eyes according to electrophoretic results. The kit is high in specificity, high in repeatability and convenient and quick to operate; single infection or mixed infection can be definitely diagnosed in 4-6 hours generally; the double purposes of diagnosing and identifying can be achieved. The kit is higher in sensitivity; the minimum detection concentration of porcine epidemic diarrhea virus is 4.3 ng per microliter; the minimum detection concentration of porcine transmissible gastroenteritis virus is 3.2 ng per microliter; the minimum detection concentration of rotavirus diarrhea of porcine group A is 6.1 ng per microliter.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES +1
Calculation method and device for relationship between high-risk hpv type and cervical precancerous lesion stage
ActiveCN111180071BMedical data miningHealth-index calculationStatistical analysisRegression analysis
Owner:SICHUAN UNIV
Rapid joint test strip for avian reticuloendotheliosis virus and subgroup j avian leukosis virus
ActiveCN103235128BRapid serology methodSerological method is simpleMaterial analysisLeucosisAntiendomysial antibodies
Owner:HENAN ACAD OF AGRI SCI
Triple PCR kit for diagnosing FHT/MP/HPS and detection method of triple PCR kit
ActiveCN111172304ASolve difficult to cultivateSolving the diagnostic problem of unculturable pathogensMicrobiological testing/measurementMicroorganism based processesEperythrozoon suisMycoplasma pneumonia
The invention belongs to the field of veterinary animal epidemic disease molecular biological diagnosis and discloses a triple PCR kit for diagnosing FHT / MP / HPS and a detection method of the triple PCR kit. The kit comprises 3 pairs of specific primers, wherein FHT-F / FHT-R is an eperythrozoon suis specific amplification primer group, MP-F / MP-R is a mycoplasma hyopneumoniae specific amplification primer group, and HPS-F / HPS-R is a haemophilus parasuis specific amplification primer group; and corresponding specific genome segments can be amplified from epidemic materials of a sick pig, single infection or mixed infection of three pathogens including eperythrozoon suis, mycoplasma hyopneumoniae and haemophilus parasuis can be confirmed in once detection. The kit has high detection sensitivity, good specificity, high stability and intuitive result, and the detection method is easy to operate, is convenient and quick, can greatly shorten the detection time and provides technical support toclinical infection control.
Owner:XIANYANG VOCATIONAL TECHN COLLEGE
Rapid joint test strip for Marek's disease virus and subgroup j avian leukosis virus
ActiveCN103235129BRapid serology methodSerological method is simpleImmunoglobulins against virusesMicroorganism based processesLeucosisAntiendomysial antibodies
The invention discloses a test strip used in one-step rapid detection of Marek's disease virus (MDV) and subgroup-J avian leukosis virus (ALV-J). The test strip comprises a non-absorbing supporting layer, and an absorption layer adhered to the supporting layer. The absorption layer is formed by sequentially spliced components of an absorption fiber layer, a gold-labeled antibody fiber layer, a cellulose film layer, and a water absorption layer. The cellulose film layer is marked with anti-goat IgG or anti-mouse IgG control blot, and a detection blot comprising anti-MDV and anti-ALV-J antibody. The anti-MDV and anti-ALV-J antibody is an anti-MDV and anti-ALV-J antibody monoclonal or polyclonal antibody. An anti-MDV and anti-ALV-J mixed polyclonal antibody or monoclonal antibody marked by colloidal gold and corresponding to the detection blot is adhered to the gold-labeled antibody fiber layer. The test strip provided by the invention has the advantages of high detection specificity, high sensitivity, simple operation, fast detection, and intuitive result. The test strip is suitable for MDV and ALV-J virus on-site rapid combined detection, and can be used in identification and diagnosis of single infection or mixed infection of the two viruses. The test strip can be widely applied, and is suitable for popularization.
Owner:HENAN ACAD OF AGRI SCI
Double PCR detection primer combination, detection kit and method for chicken parvoviruses and H9 subtype avian influenza viruses
PendingCN110846437AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationChicken parvovirusMonitoring and control
The invention discloses a double PCR detection primer combination, detection kit and method for chicken parvoviruses and H9 subtype avian influenza viruses. Specific primers targeting the chicken parvoviruses and the H9 subtype avian influenza viruses are designed, different primer combinations are tested and screened to obtain the primer combination good in amplification effect, and primer concentration, primer proportion, annealing temperature and amplification time in a reaction system are further optimized to finally build the method capable of simultaneously detecting the chicken parvoviruses and the H9 subtype avian influenza viruses. The primer combination and the detection method have the advantages that the mixed infection or single infection condition of the chicken parvovirusesand the H9 subtype avian influenza viruses in a sample can be determined at the same time through one PCR reaction; the method is high in specificity and sensitivity, fast and simple, easy to operate,quite suitable for being applied and popularized in grassroots units, capable of providing technical support for the monitoring and control of the chicken parvoviruses and the H9 subtype avian influenza viruses and the like.
Owner:GUANGXI VETERINARY RES INST
Establishment and application of multiplex fluorescent RT-PCR detection method for foot-and-mouth disease, vesicular stomatitis and porcine vesicular disease
InactiveCN103602757BMicrobiological testing/measurementMicroorganism based processesAnimal virusSwine vesicular disease
Based on a highly conserved domain of a foot-and-mouth disease virus 3D protein coding gene, a vesicular stomatitis virus N protein coding gene and a swine vesicular disease virus VP1 protein coding gene, the invention designs a specific primer and a probe and develops a multiple fluorescence RT-PCR detection method used for simultaneously detecting the three animal viruses. The detection method can detect 102 copied plasmids containing target amplification sequences in 1 h. The method has high sensitivity and good specificity, and can achieve rapid high-throughput fluorescence RT-PCR detection for single-virus infection or mixed infection by a plurality of viruses.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
A kind of vaccine composition and its preparation method and application
ActiveCN104338128BMixed infection works wellPreserve immune efficiencyAntibacterial agentsAntiviralsAntigenImmune effects
The invention provides a vaccine composition, which contains immunological amount of SIV, Mhp, APP antigen and veterinary acceptable carrier. The immunization procedure of the vaccine composition is simple, and can effectively prevent and treat the single infection or mixed infection of SIV, Mhp and APP. When mixed infection occurs, the immune effect is significantly higher than that of single vaccine injections respectively. The vaccine composition has few side effects, long immunization period, less time-consuming and labor-intensive production process, low immunization cost and strong practicability.
Owner:PU LIKE BIO ENG
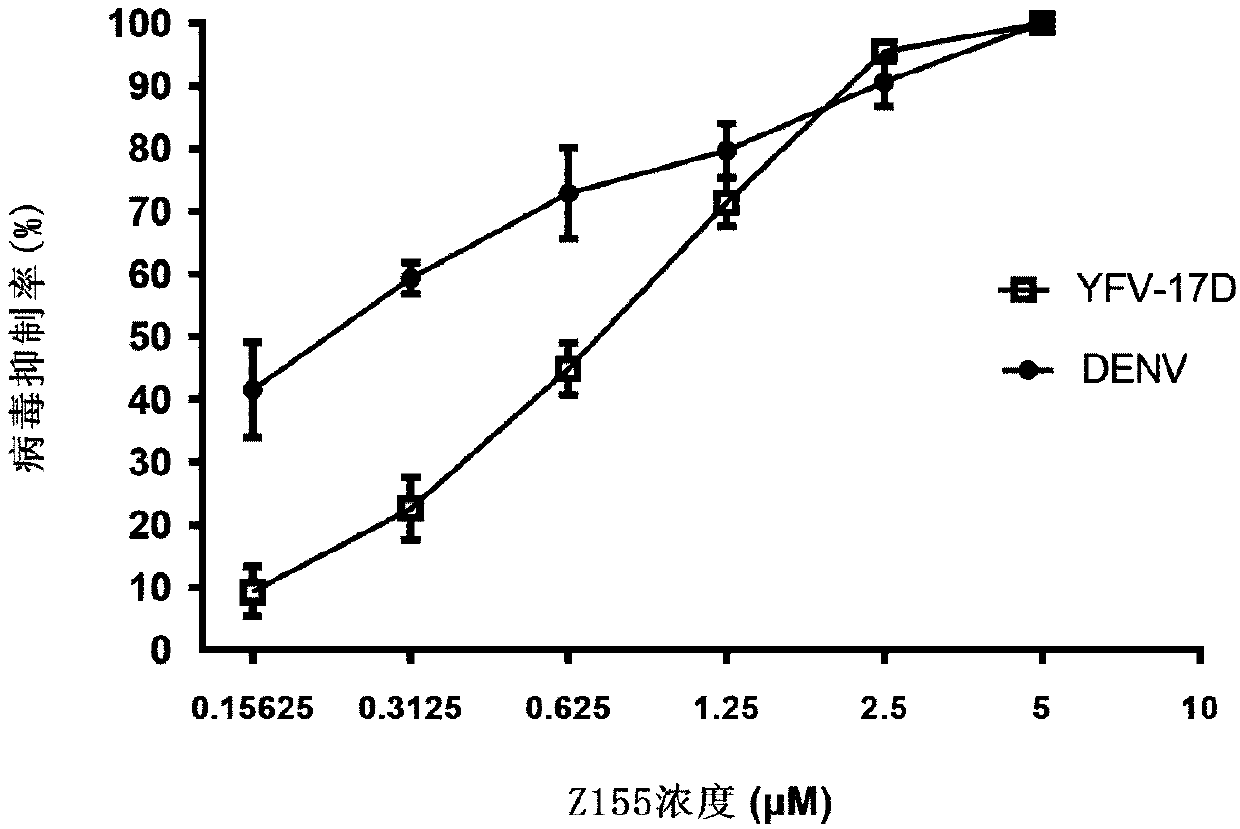
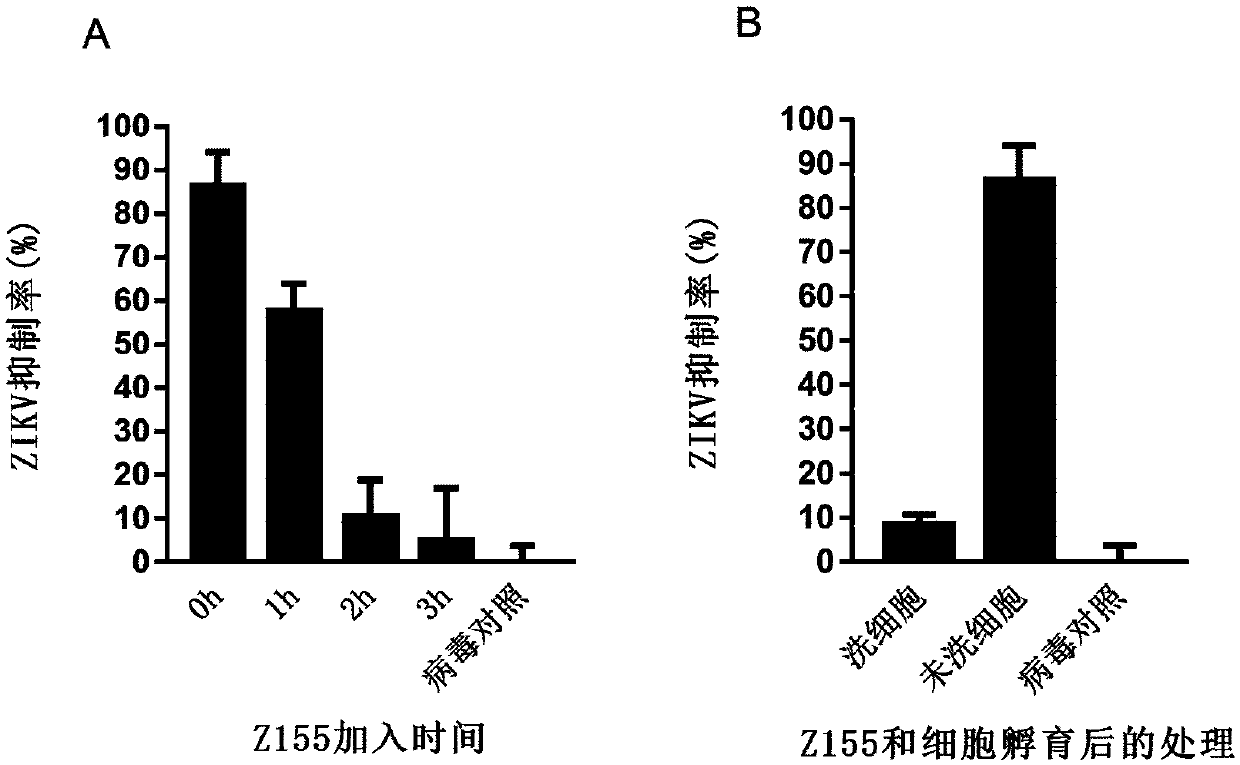
Polypeptide capable of inhibiting infection of Zika virus, dengue virus and yellow fevervirus and application of polypeptide
ActiveCN111320670AGood antiviral effect in vivoImprove securityPeptide/protein ingredientsAntiviralsYellow feverCytotoxicity
The invention belongs to the technical field of biotechnology and biological medicines, relates to a virus inhibitor, and in particular to a polypeptide Z155 and an application thereof in preparing apolypeptide inhibitor for single infection or co-infection of Zika virus (ZIKV), dengue virus (DENV) and yellow fevervirus (YFV). Experiments show that the polypeptide Z155 can be combined with Zika virus particles and further inhibits virus infection cells through combinination with virus membrane-melting peptides. The polypeptide has a broad-spectrum anti-virus effect on flavivirus, and the cytotoxicity of the polypeptide is extremely low. The polypeptide inhibitor can be prepared into a medicament for treating ZIKV infection, is beneficial to treatment of single infection or co-infection ofZIKV, DENV and YFV, and is particularly important to treatment of Zaika infected persons, particularly infected pregnant women.
Owner:FUDAN UNIV
Method for breeding summer truffle root seedling through inoculation of suspension liquid
InactiveCN103070014BSolve the problem of manual vaccinationGuaranteed uniformityHorticultureSnow moldTuber aestivum
The invention discloses a method for breeding summer truffle root seedling through inoculation of suspension liquid, which is characterized in that the difficulty in artificial inoculation of summer truffle can be solved through steps of breeding of aseptic seedling, preparation of microbial inoculum, inoculation of suspension liquid, seedling exercising, breeding of fungus seedlings and test and confirmation. Compared with the existing breeding method, the method is characterized in that pinus armandi, castanea mollissima and pinus yunnanensis are adopted as hosts; under a relatively aseptic condition, i.e. the condition for preventing the competition of fungi and infection of most mycetes, the summer truffle spores sufficiently contact with roots of the host trees; the single infection predominance of the summer truffle is maintained within a given period of time; and a great number of firm summer truffle mycorrhizas can be formed.
Owner:PANZHIHUA FORESTRY SCI & TECH PROMOTION STAND PANZHIHUA FORESTRY WORK
A kind of external medicine for treating skin eczema and eczema-like dermatitis and preparation method thereof
ActiveCN105497043BInhibition formationPromote hydrationAntibacterial agentsOrganic active ingredientsDiseaseAdditive ingredient
The invention provides an externally-applied drug for treating skin eczema and eczematous dermatitis and a preparation method thereof. The externally-applied drug is prepared from miconazole nitrate, chloramphenicol, urea, triamcinolone acetonide acetate, stearic acid, Vaseline, glycerol monostearate, glycerol, purified water, triethanolamine, lauryl sodium sulfate and ethylparaben. The externally-applied drug can effectively treat and prevent fungus and bacterium single infection and concurrent infection diseases. The externally-applied drug can effectively treat and prevent skin fungus and bacterium single infection and concurrent infection, eczema, eczematous dermatitis and other skin diseases, and the recovery process of various skin diseases is accelerated. Meanwhile, the skin layer rapidly and fully absorbs all the ingredients of the externally-applied drug, all the ingredients are in compatibility in corresponding proportions, the treatment effect of the externally-applied drug prepared through the preparation method is remarkably improved, and the externally-applied drug has the effects of relieving itching, diminishing inflammation, achieving sterilization and eliminating skin surface symptoms, and especially has the obvious effect on various kinds of fungal infection and severe infantile eczema.
Owner:汶上县皮肤病防治站
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com