Compositions and methods for detecting human papillomavirus
A technology for papilloma and composition, which is applied in the directions of biochemical equipment and methods, recombinant DNA technology, and microbial determination/examination, and can solve problems such as urethral swab pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
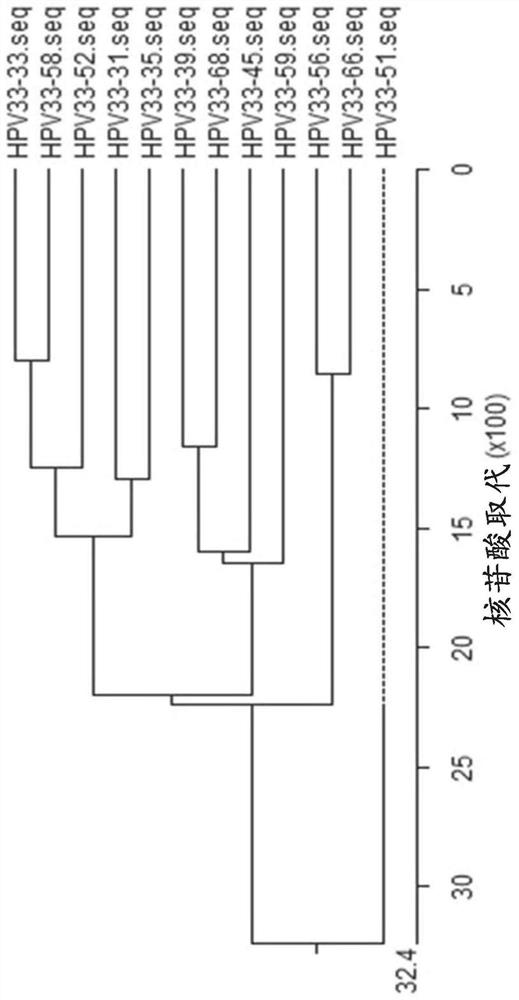
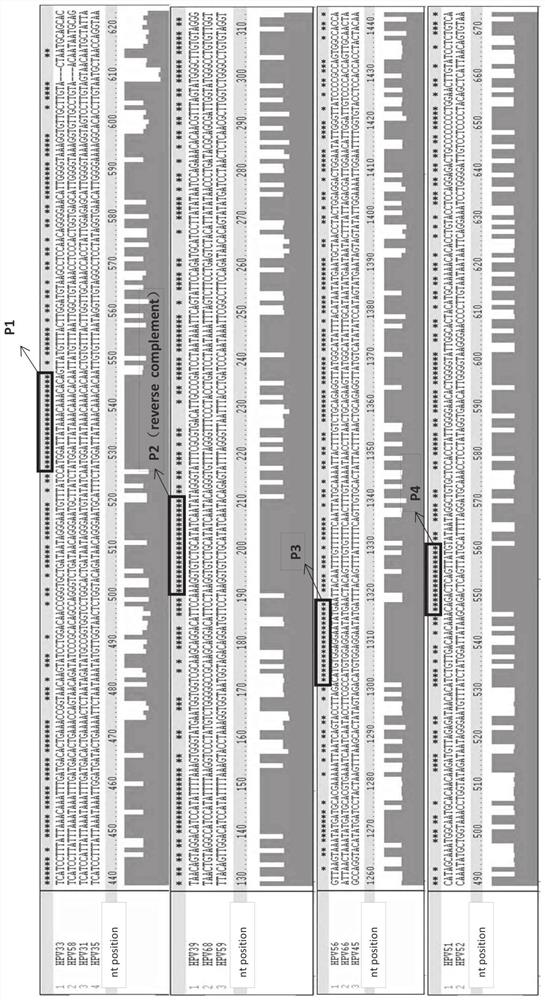
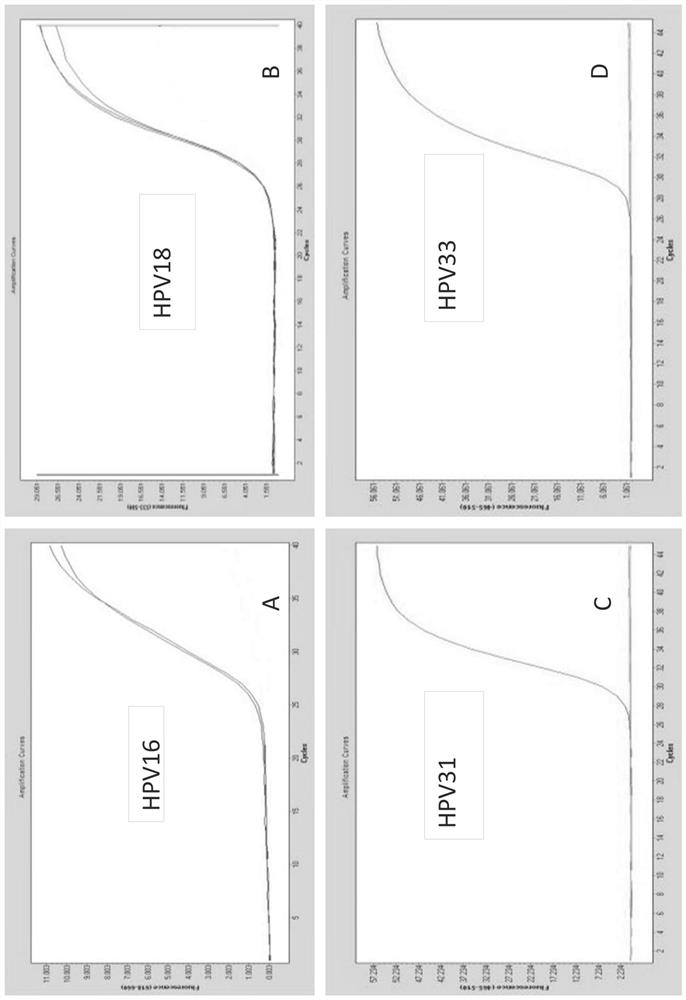
[0323] Example 1: Design and testing of primers / probes
[0324] Snijders et al. (J. Gen. Virol., 71 (1990), 173-181) and Surentheran et al. (J. Clin. Path., 51 (1998), 606-610) describe a method for detecting HPV L1 gene DNA PCR method. A disadvantage of both methods is that only a limited number of HPV types can be detected. For example, the primers described by Snijder et al. can only detect some HPV types, such as HPV30, HPV39, HPV51, but the sensitivity is greatly reduced. Furthermore, certain HPV types, such as HPV18, resulted in the formation of additional bands when using the primers described by Snijder et al. Therefore, existing assays can only detect a limited spectrum of HPV subtypes, and some rare HPV subtypes cannot be adequately detected. The compositions and methods provided herein can be used to detect the genotypes of up to 14 high-risk HPV subtypes and / or two low-risk HPV subtypes in one or two test tubes.
[0325] Design primers and probes
[0326] Mult...
Embodiment 2
[0334] Embodiment 2: high-risk HPV detection kit
[0335] As a non-limiting example, a typical kit may include the following parts:
[0336] (1) High-risk HPV qPCR mixture: 1×buffer (20mM Tris-HCl, 50mM KCl, pH8.4), 0.15-0.3mM dNTP, 2-4mM MgCl2, 0.2-1.2μM primers / probes (14 high-risk type), 0.1~1mg / ml BSA, 0.2%~2% (V / V) formamide, 0.2mM~2mM spermidine, 10mM~30mM tetramethylammonium chloride, 0.01mM~0.1mM DTT, 0.2% ~2% 2-pyrrolidone and H2O
[0337] (2) Taq enzyme: 1~6U / μl Taq enzyme
[0338] (3) Positive control: high-risk HPV16, HPV18, and HPV45 L1 gene plasmids (10 per template 3 copies / ml), and high-risk HPV DNA-negative urine
[0339] (4) Negative control: high-risk HPV DNA negative urine
Embodiment 3
[0340] Example 3: HPV detection kits for the detection of 14 high-risk subtypes and 2 low-risk subtypes
[0341] As a non-limiting example, a typical kit to detect 14 high-risk subtypes and two low-risk subtypes may include the following parts:
[0342] (1) HPV qPCR reaction solution I: 1×buffer (20mM Tris-HCl, 50mM KCl, pH 8.4), 0.2-0.3mM dNTP, 2-3mM MgCl2, 0.2-0.8μM primers and probes (HPV16, HPV18, HPV35, HPV39, HPV68, HPV59, HPV56, HPV66, HPV51), 0.1~1mg / ml BSA, 0.2%~2% (V / V) formamide, 0.2mM~2mM spermidine, 10mM~30mM tetramethyl chloride Ammonium, 0.01mM ~ 0.1mM DTT, 0.2% ~ 2% 2-pyrrolidone and H2O;
[0343] (2) HPV qPCR reaction solution II: 1×buffer (20mM Tris-HCl, 50mM KCl, pH 8.4), 0.2-0.3mM dNTP, 2-3mM MgCl2, 0.2-0.8μM primers and probes (HPV6, HPV11, HPV33 , HPV58, HPV31, HPV45, HPV52, β-actin), 0.1~1mg / ml BSA, 0.2%~2% (V / V) formamide, 0.2mM~2mM spermidine, 10mM~30mM tetramethyl chloride ammonium chloride, 0.01mM ~ 0.1mM DTT, 0.2% ~ 2% 2-pyrrolidone and H2O;
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com