Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Device removal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
To remove your device: On the Company Portal website, tap the menu button , then select My Devices. On the My Devices page, select the name of the device you want to remove. The device will open in a popup window. Tap the Remove button. Read the warning message, and then tap Remove to remove your device from the Company Portal.
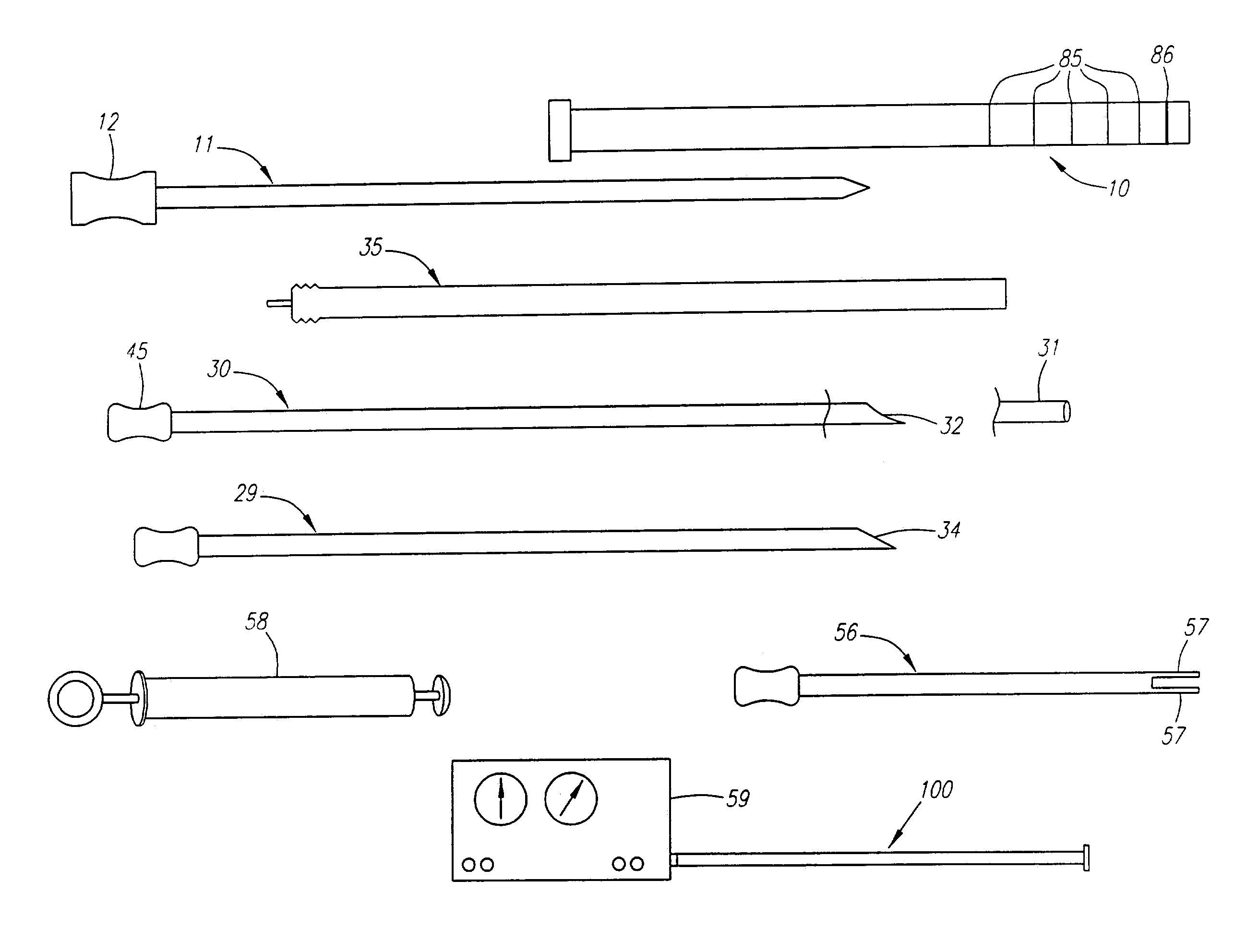
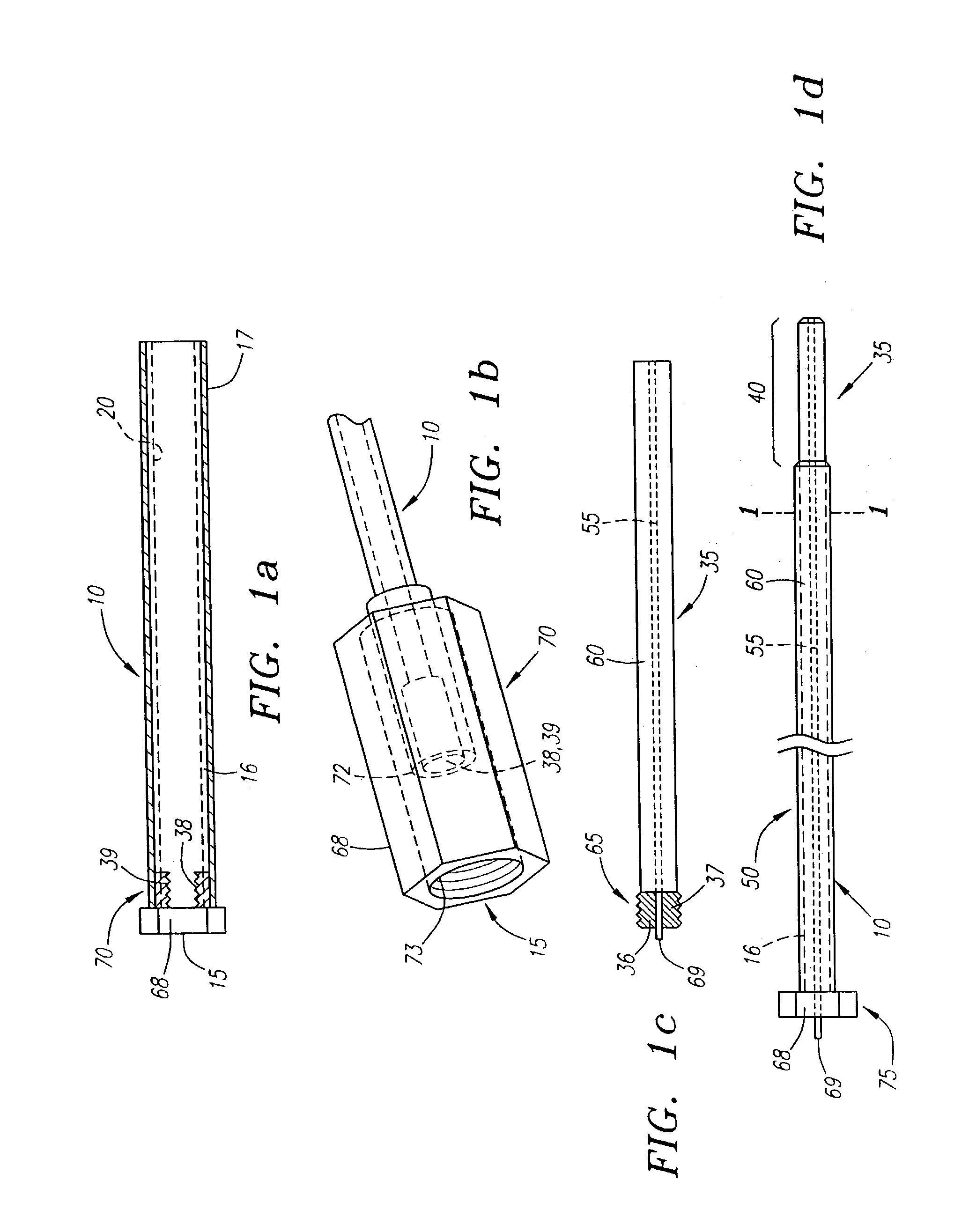
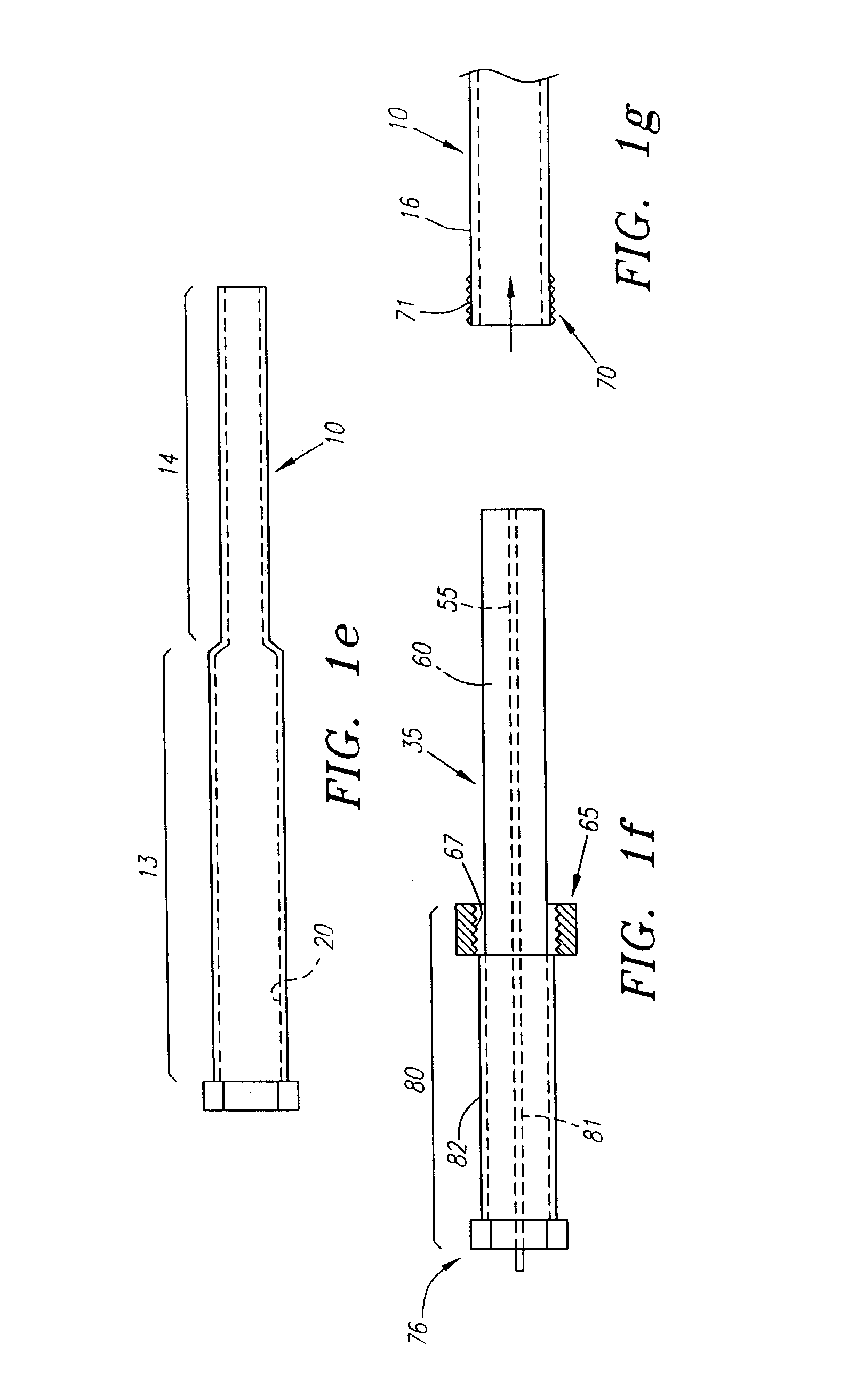
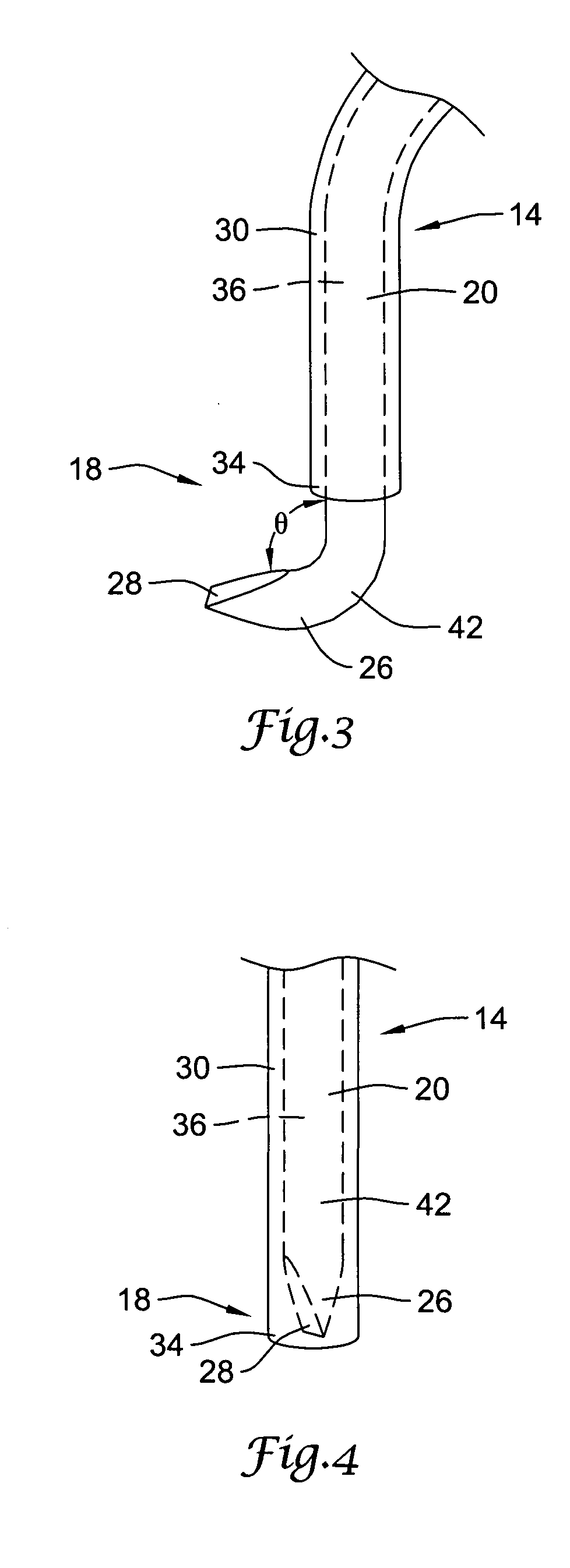
Needle kit and method for microwave ablation, track coagulation, and biopsy
InactiveUS7160292B2Minimize damageMinimize bleedingSurgical needlesVaccination/ovulation diagnosticsFungating tumourMicrowave ablation
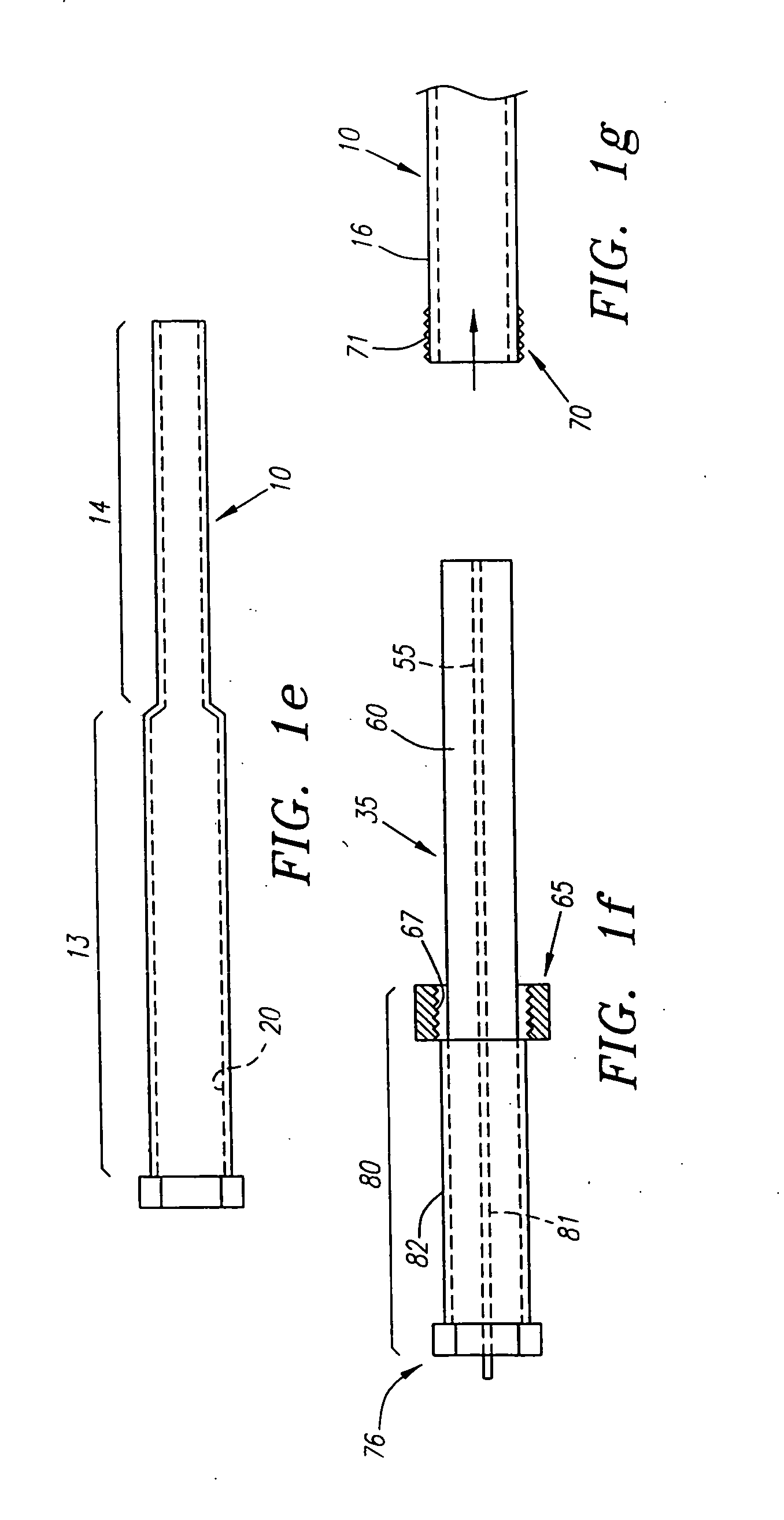
A modular biopsy, ablation and track coagulation needle apparatus is disclosed that allows the biopsy needle to be inserted into the delivery needle and removed when not needed, and that allows an inner ablation needle to be introduced and coaxially engaged with the delivery needle to more effectively biopsy a tumor, ablate it and coagulate the track through ablation while reducing blood loss and track seeding. The ablation needle and biopsy needle are adapted to in situ assembly with the delivery needle. In a preferred embodiment, the ablation needle, when engaged with the delivery needle forms a coaxial connector adapted to electrically couple to an ablating source. Methods for biopsying and ablating tumors using the device and coagulating the track upon device removal are also provided.
Owner:TYCO HEALTHCARE GRP LP
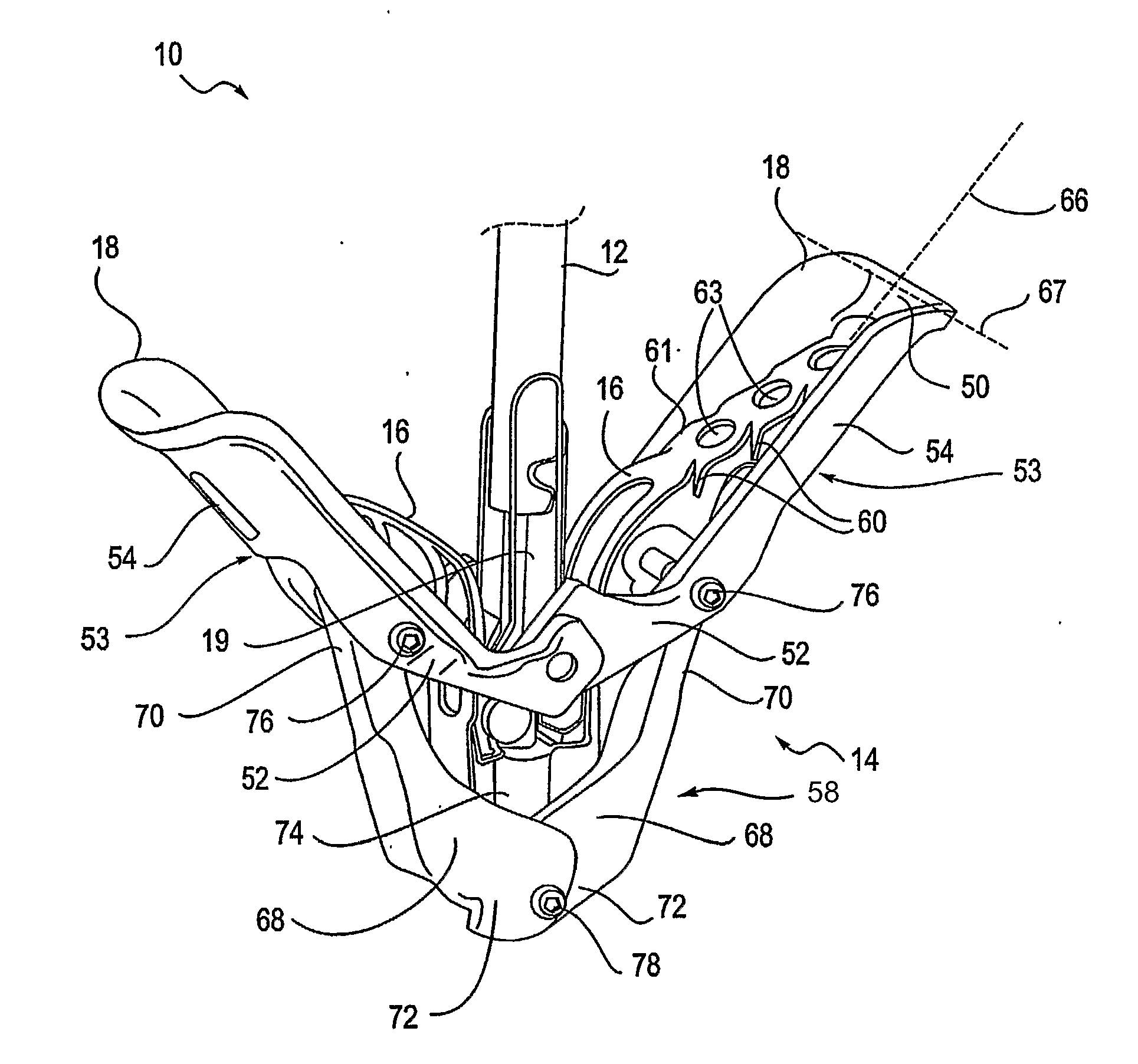
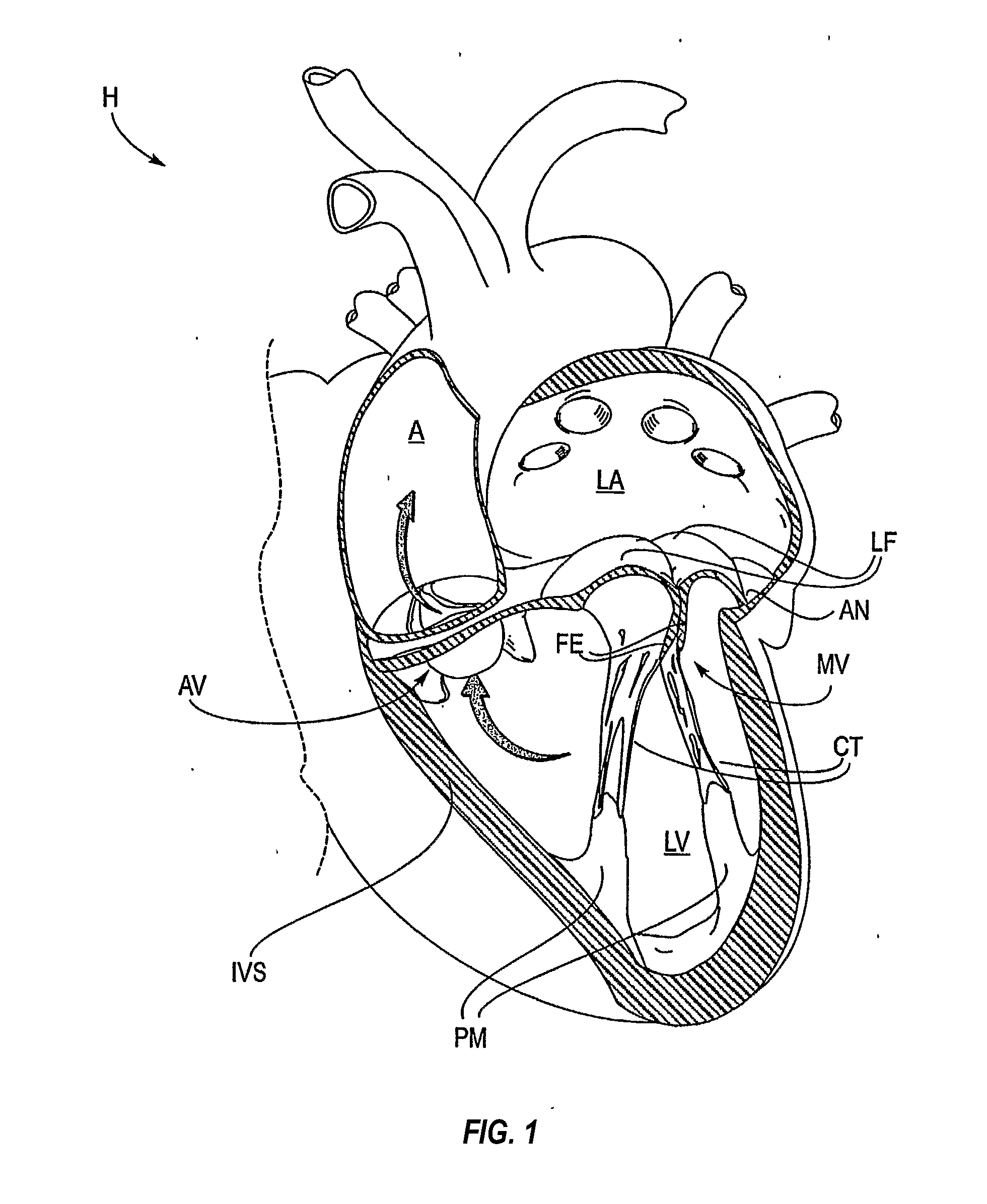
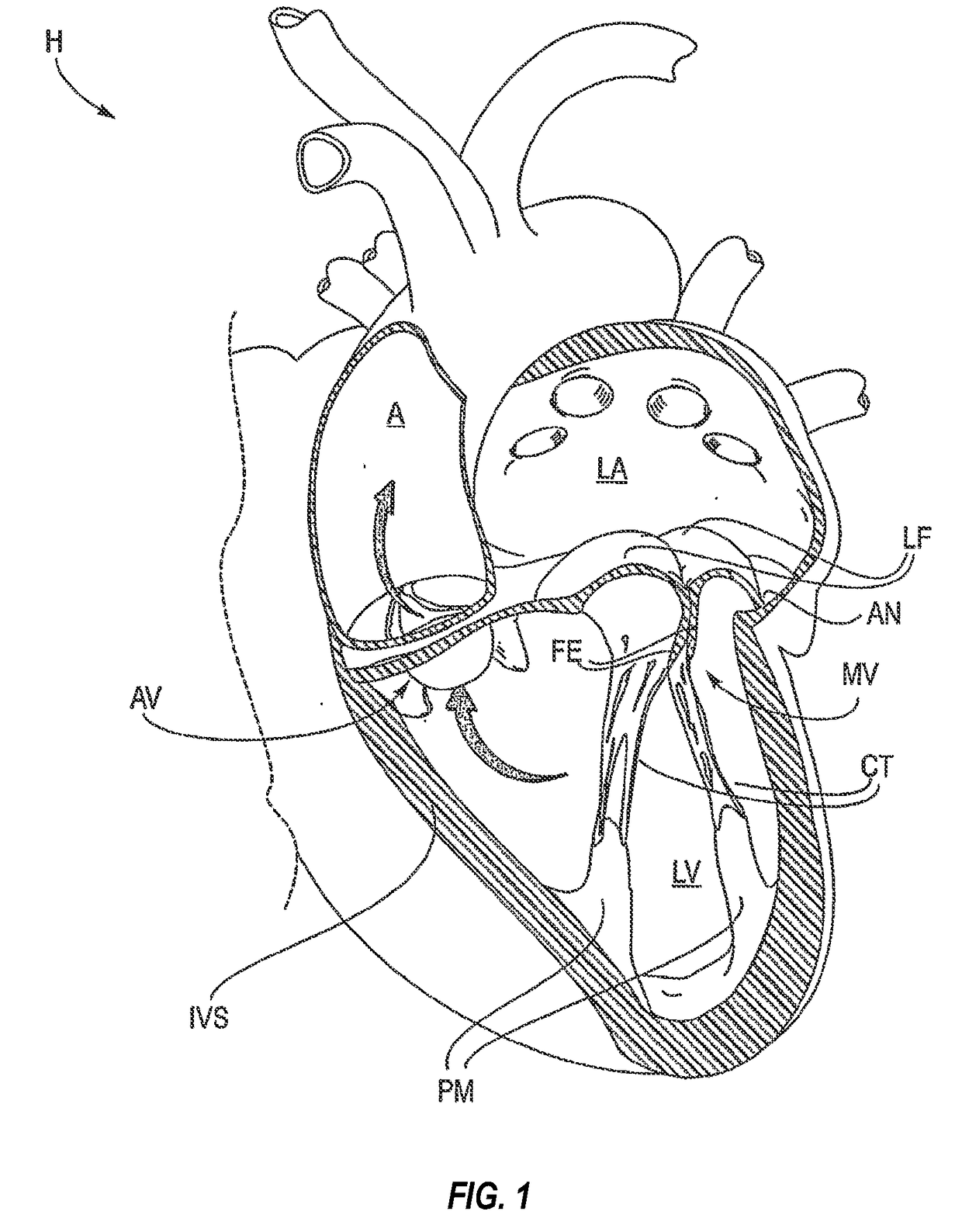
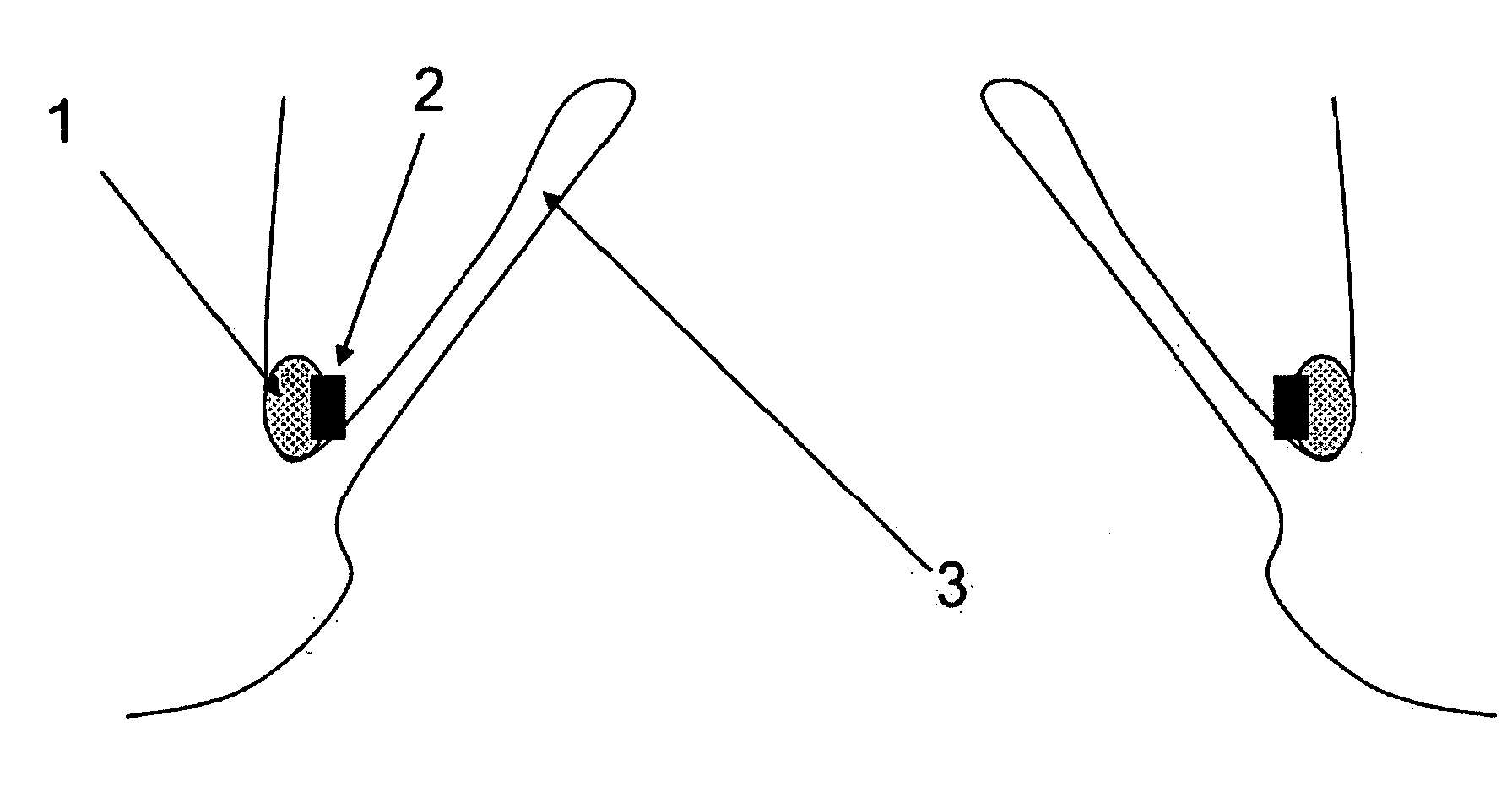
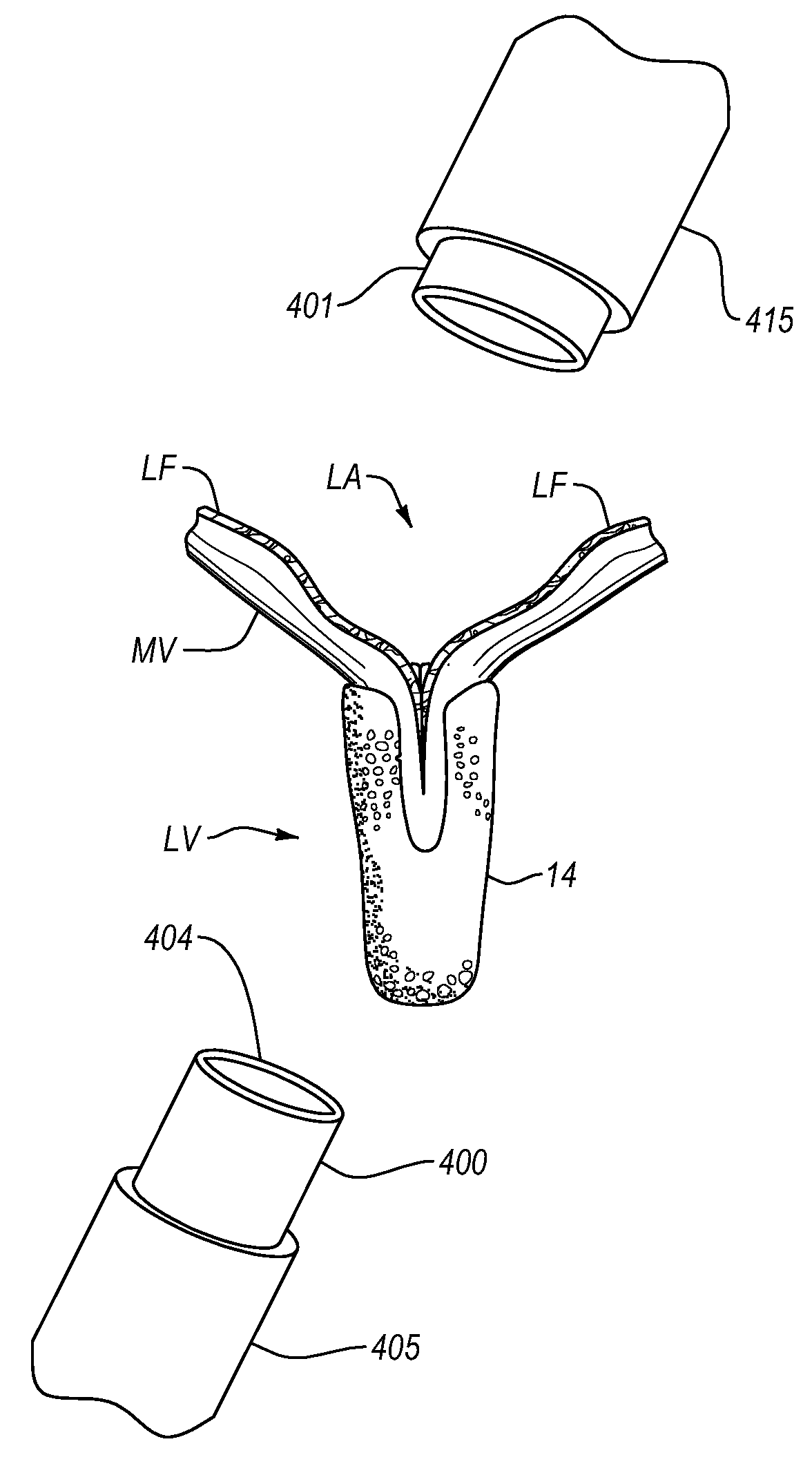
Mitral valve fixation device removal devices and methods
ActiveUS20150257883A1Easy accessSuture equipmentsHeart valvesEndovascular surgeryMitral valve leaflet
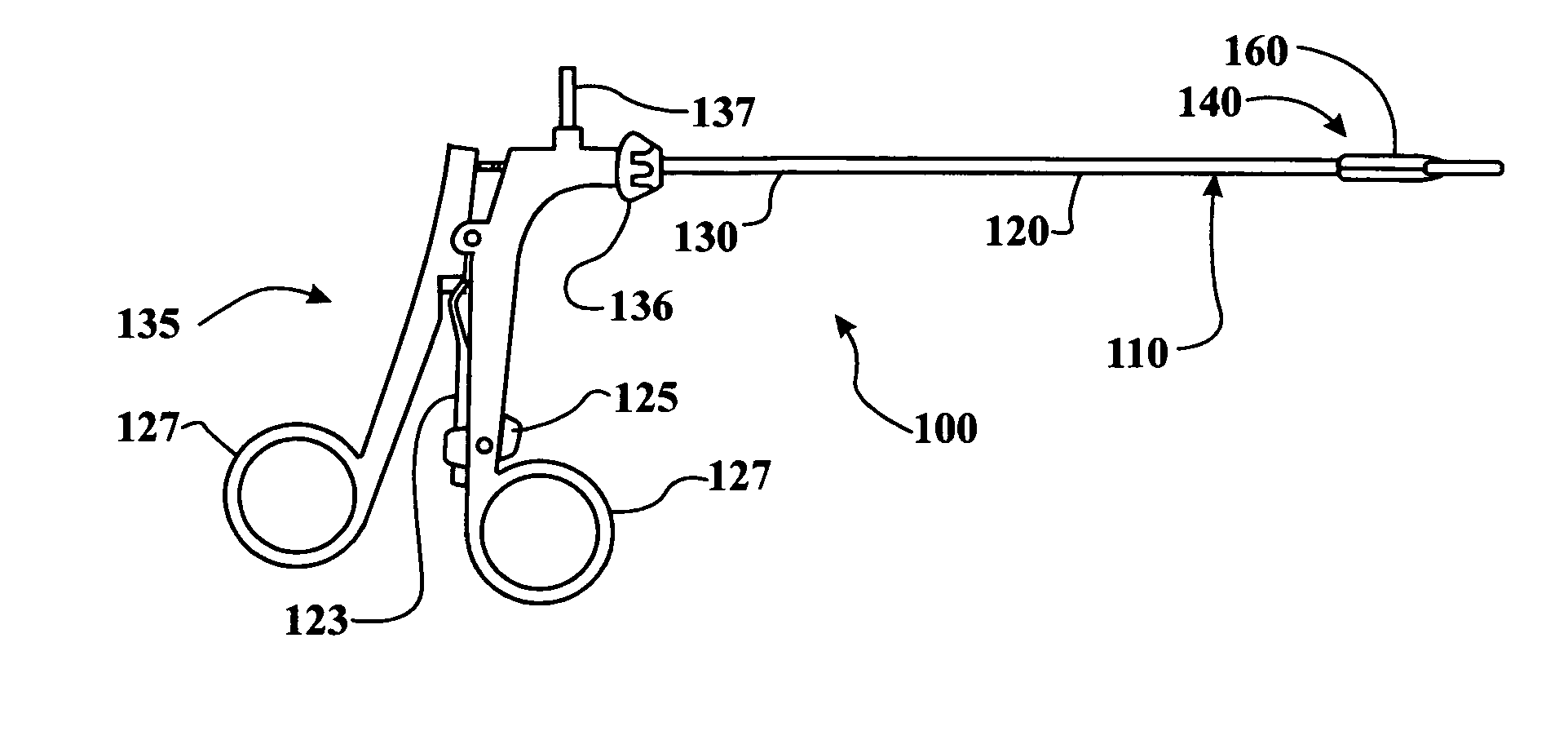
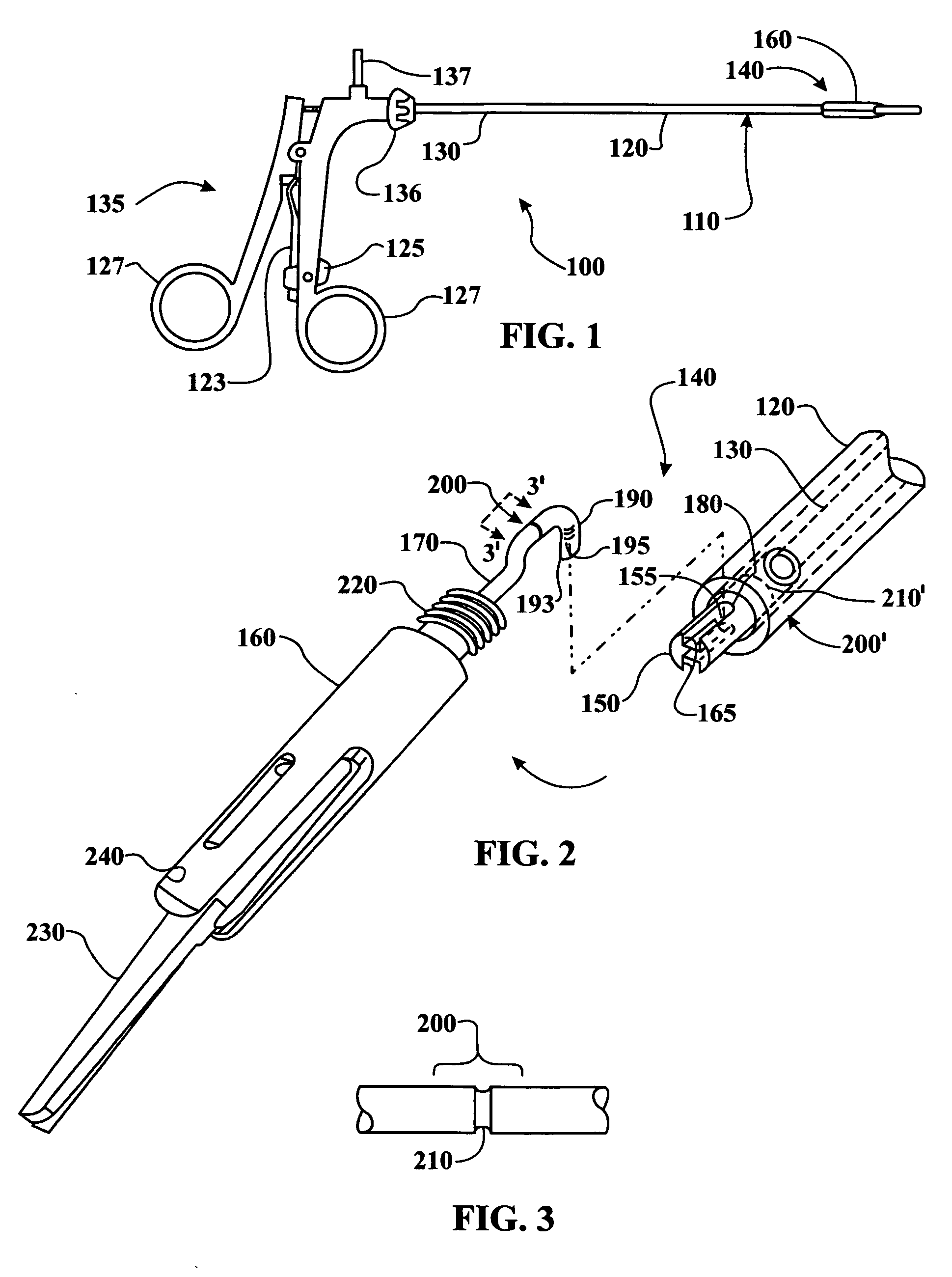
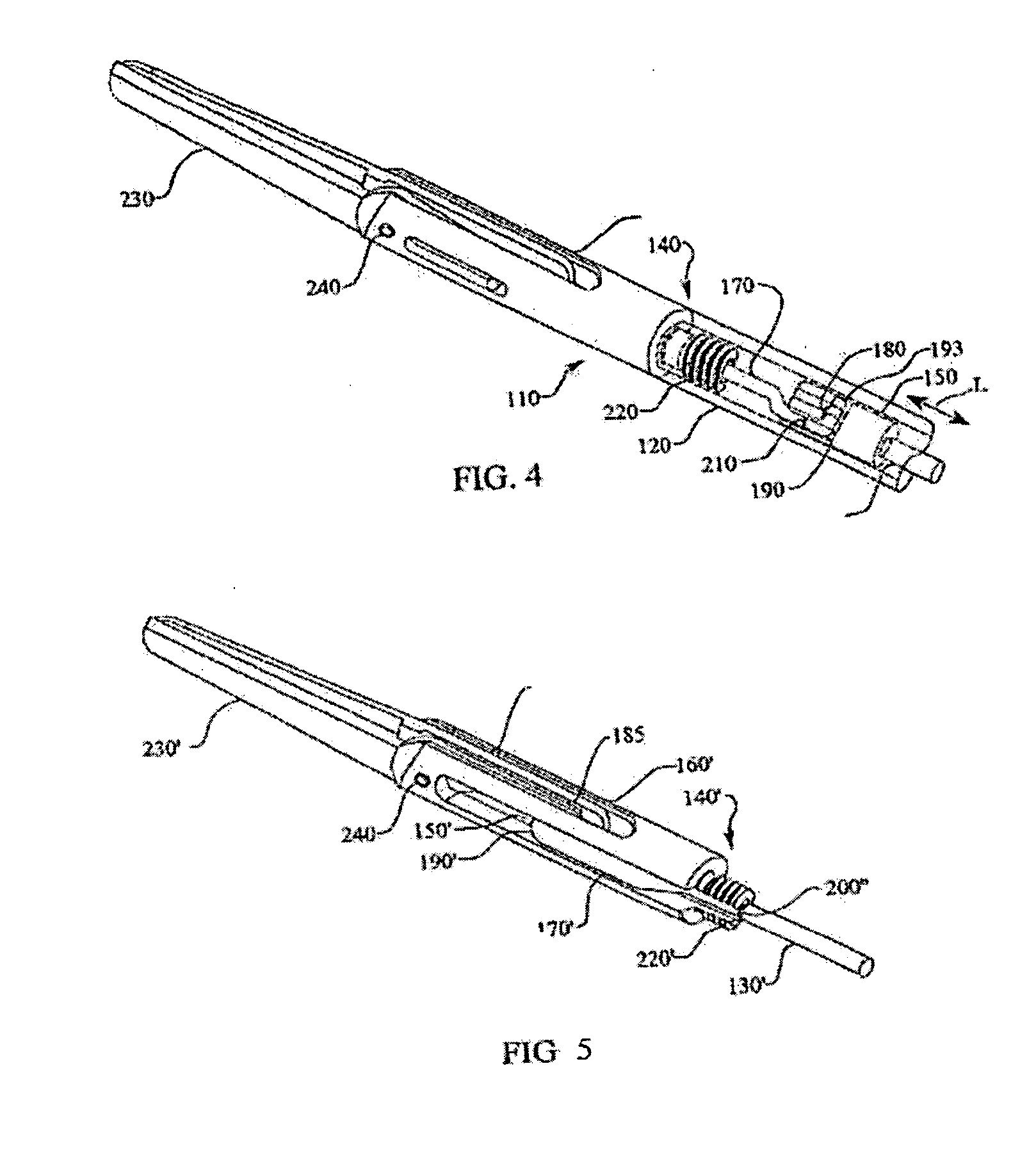
Procedures may be performed on the heart after the installation of a mitral valve fixation device. In order to prepare the heart for such procedures, the fixation device may be removed or disabled in minimally invasive ways (e.g., through an endovascular procedure), without requiring open access to the heart. The fixation device may be partitioned so that one portion may remain attached to each leaflet of the mitral valve. In another example, the leaflets may be cut along the edges of the distal element(s) of the fixation device, so as to cut the fixation device from the leaflet(s). Systems and devices for performing such procedures endovascularly are disclosed. Fixation devices with improved access to a release harness are also disclosed.
Owner:EVALVE
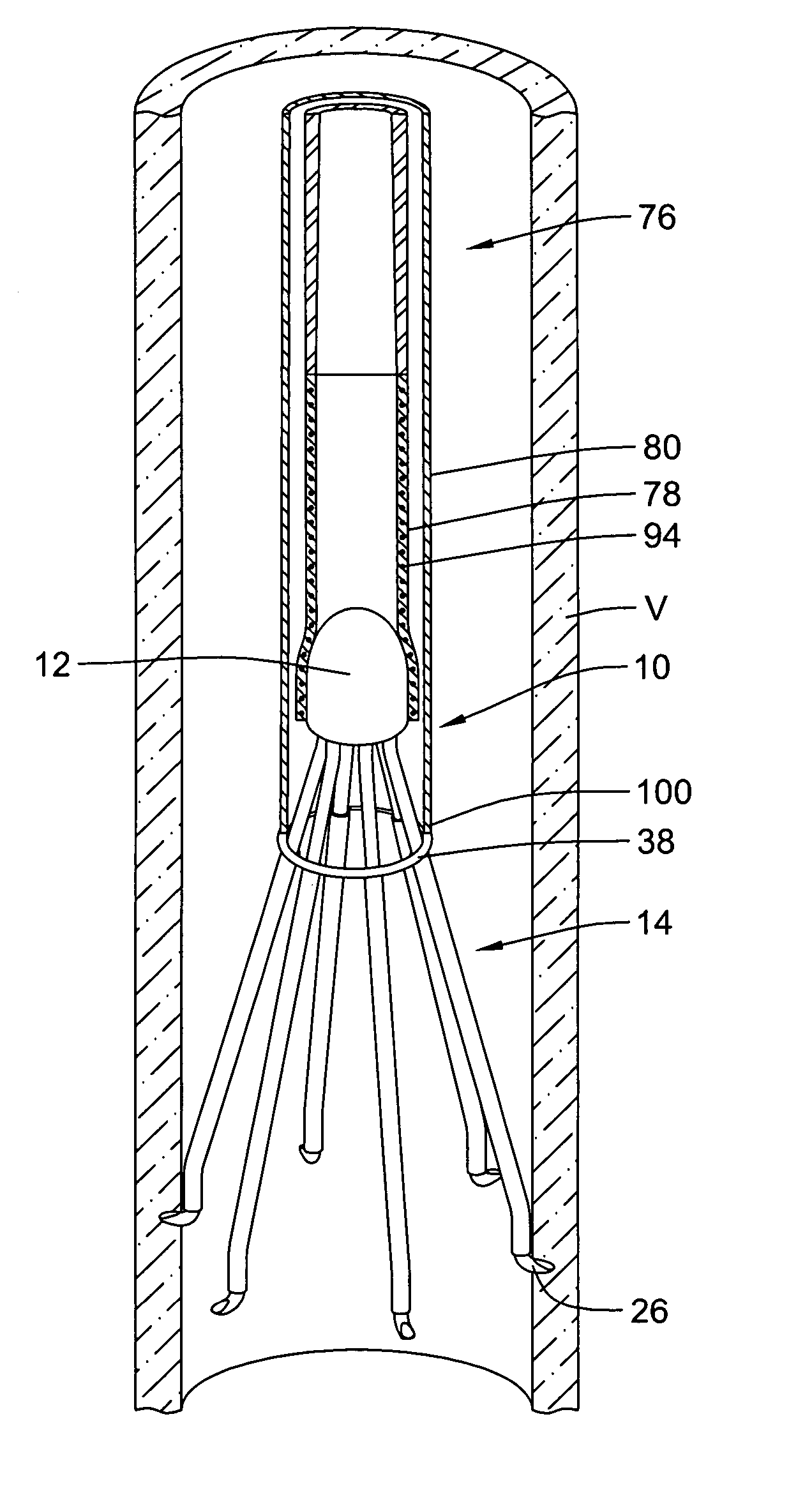
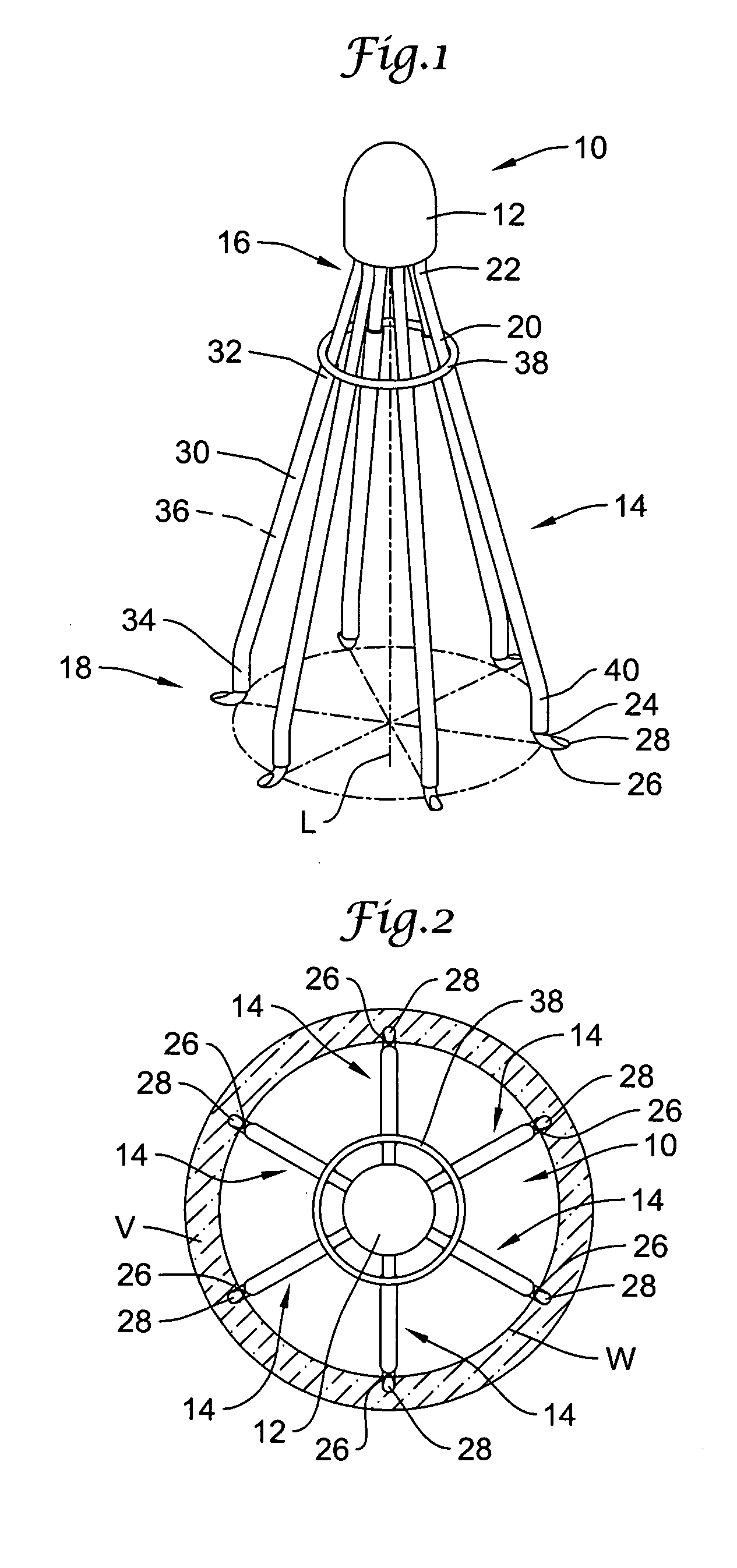
Retrievable blood clot filter with retractable anchoring members
Retrievable blood clot filter devices implantable within a blood vessel, including methods and apparatuses for retrieving such devices, are disclosed. The retrievable blood clot filter device can include an apical head, and a plurality of elongated filter legs configured to expand between a collapsed position and an expanded position within the blood vessel. A bendable anchoring member disposed on one or more filter support members can be used to secure the blood clot filter device along the inner wall of the blood vessel. The anchoring members can be configured to bend and retract into a number of filter tubes slidably disposed along the support members, allowing the blood clot filter device to be removed with a retrieval apparatus.
Owner:LIFESCREEN SCI
Carrying case for cell phone or other device with protective end cap and cushioning
InactiveUS20060052064A1Improve impact resistanceProvide tactile response structureTravelling carriersHoldersCushioningEngineering
The carrying case for a cell phone or other small electronic device includes a front, a rear, two opposing sides and a bottom. A resilient plastic bottom end cap forms a protective shield at the bottom. The end cap may include recessed grooves and / or raised lands to enhance impact resistance and / or provide a tactile response structure. The front side wall of the carrier may include a soft cushion edge pad along its upper edge to facilitate phone or device removal and insertion into the carrier. A carrier may also include bands of cushioning material about its sides which define shock resistant elements to protect the intermediate portions of the carried device. To better grip the device or phone, the case may include a swath of elastic with or without a releasable fastener system extending over the swath of elastic.
Owner:FINDINGS & MFG
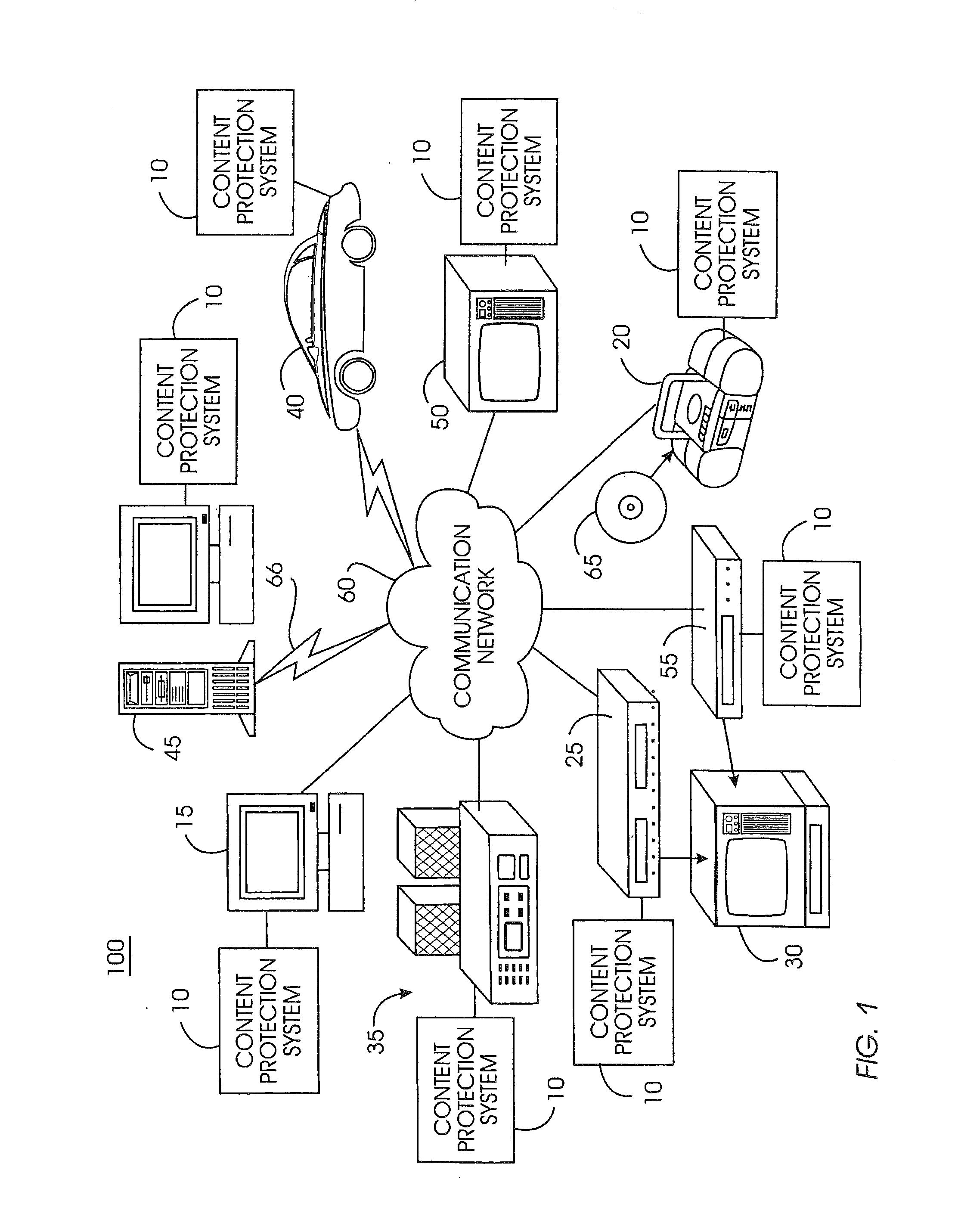
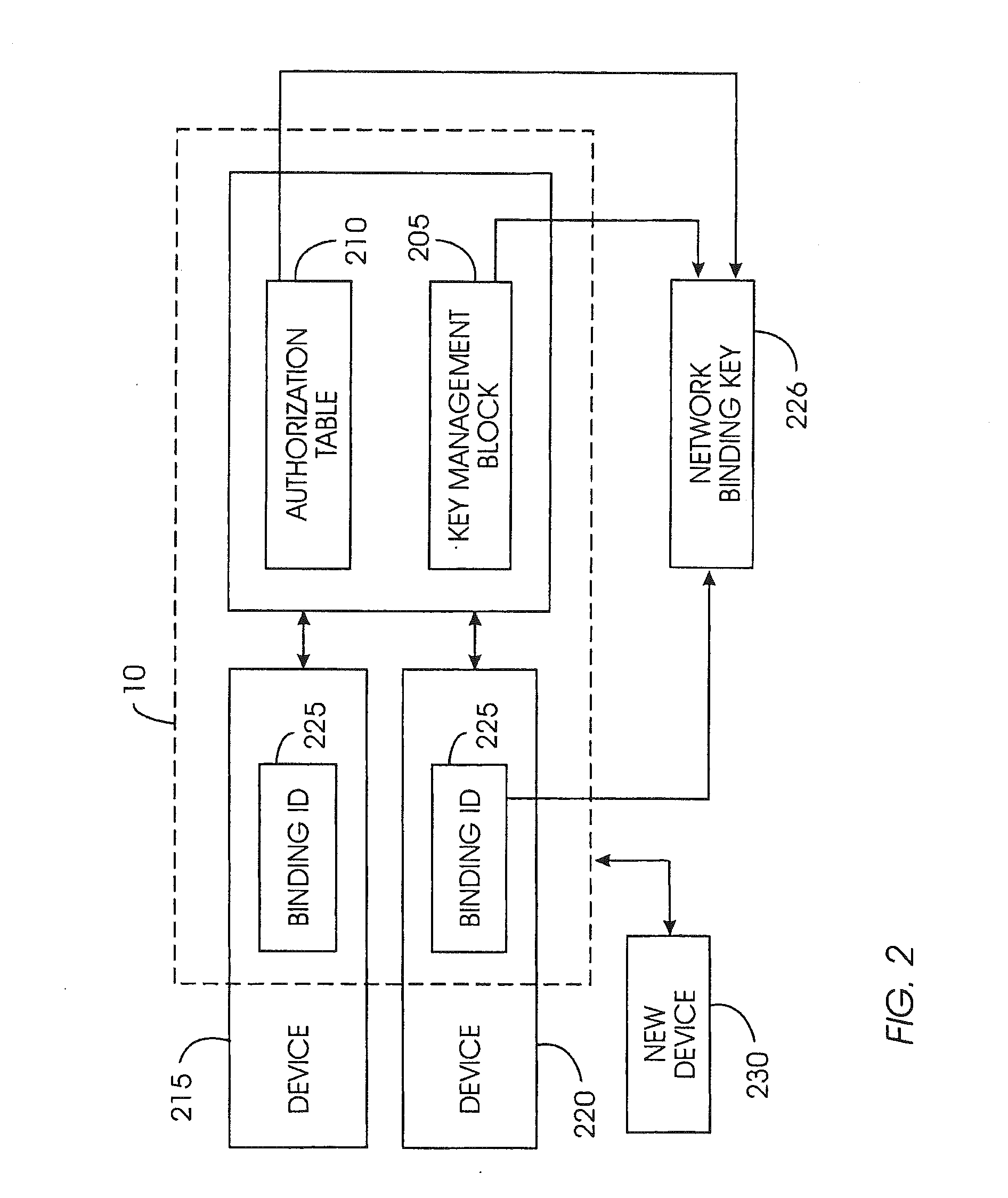
System and method for securely removing content or a device from a content-protected home network
InactiveUS20050086532A1Minimize storageDoubling sizeKey distribution for secure communicationDigital data processing detailsDevice failureAuthorization
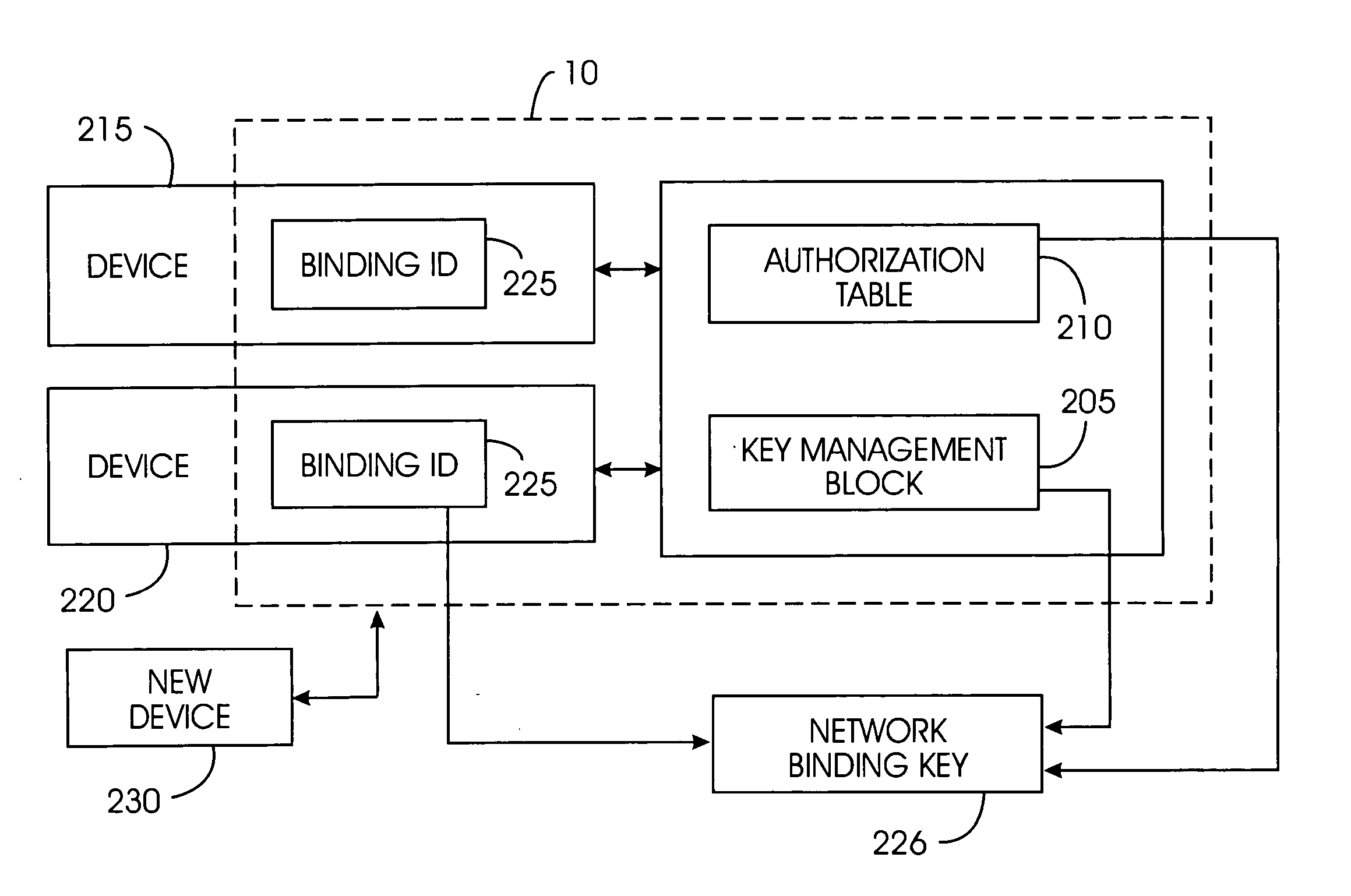
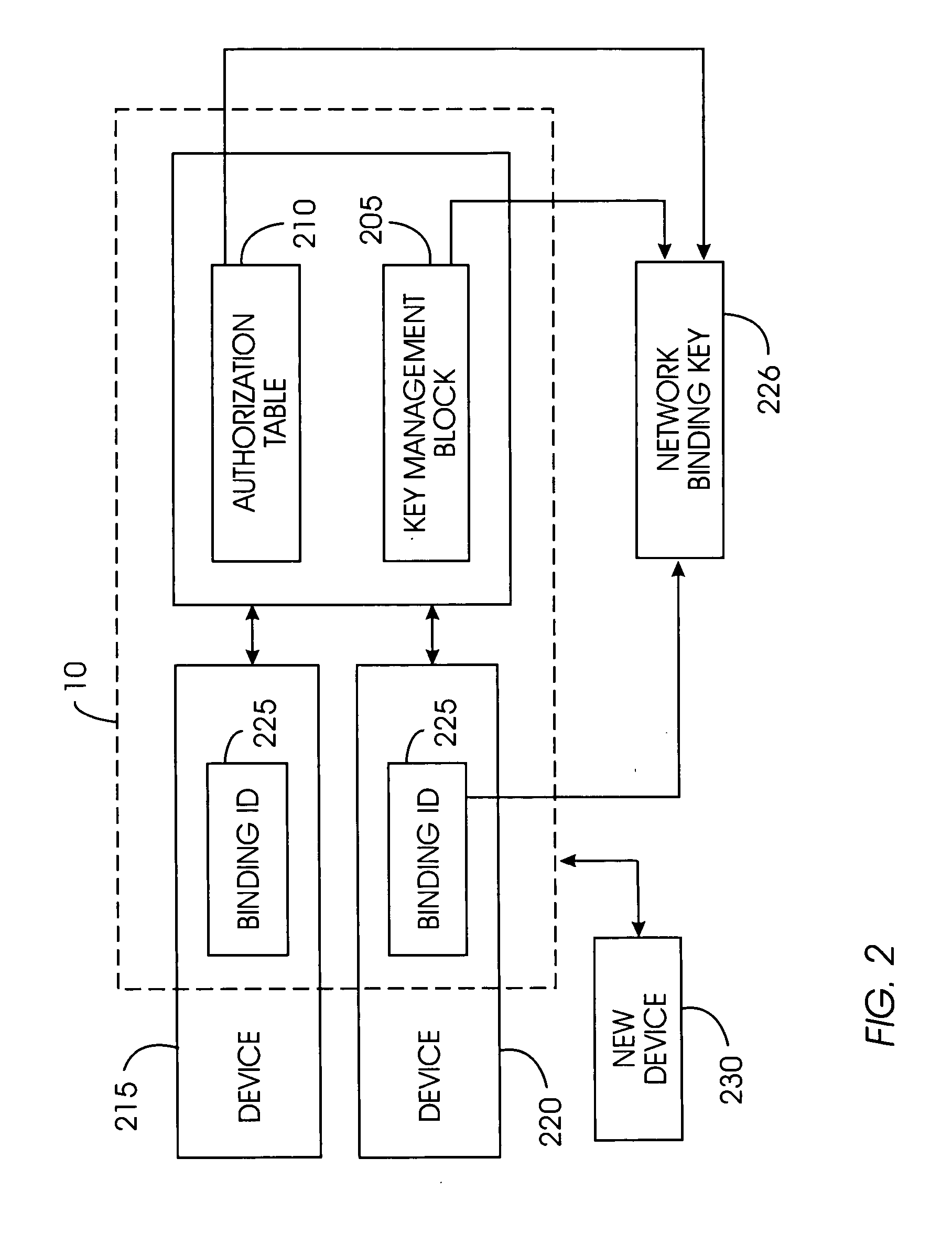
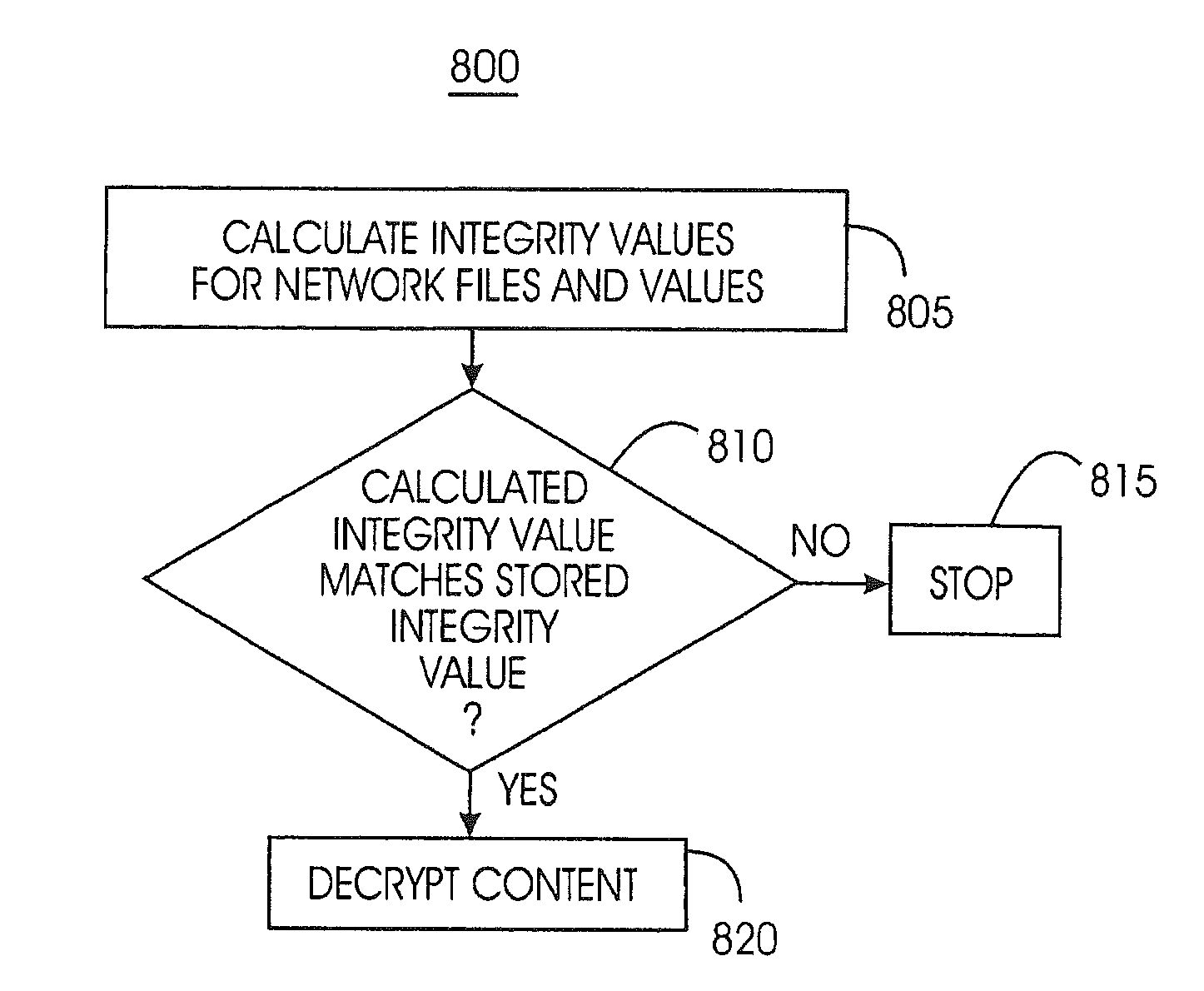
A device removal system securely removes an item of content or a device from a content-protected home network. An authorization table maintains a list of devices in the content-protected home network in addition to removed devices. The authorization table also maintains a list of deleted content. Through management of various cryptographic keys and techniques, devices and content will not play on a content-protected home network after they have been removed. A secret network ID reduces the possibility of unauthorized playing of content on the content-protected home network. A web server may join the content-protected home network as a device, providing backup for the secret network ID. Otherwise, the device manufacturer will provide the secret network ID in case of a device failure. Storing a verification value in each device ensures integrity of critical cryptographic values. This verification value is compared to network values to ensure network values have not been corrupted.
Owner:IBM CORP
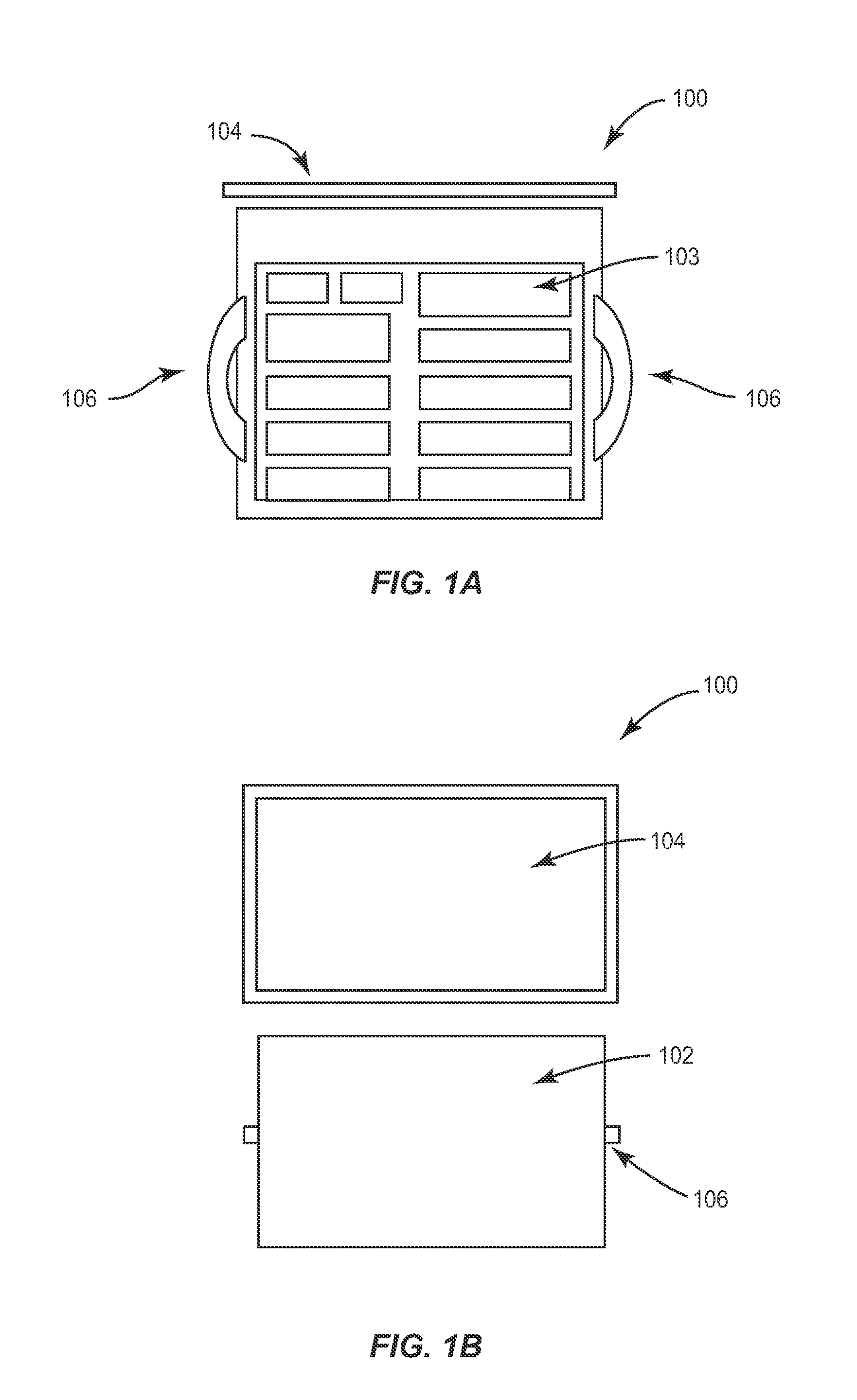
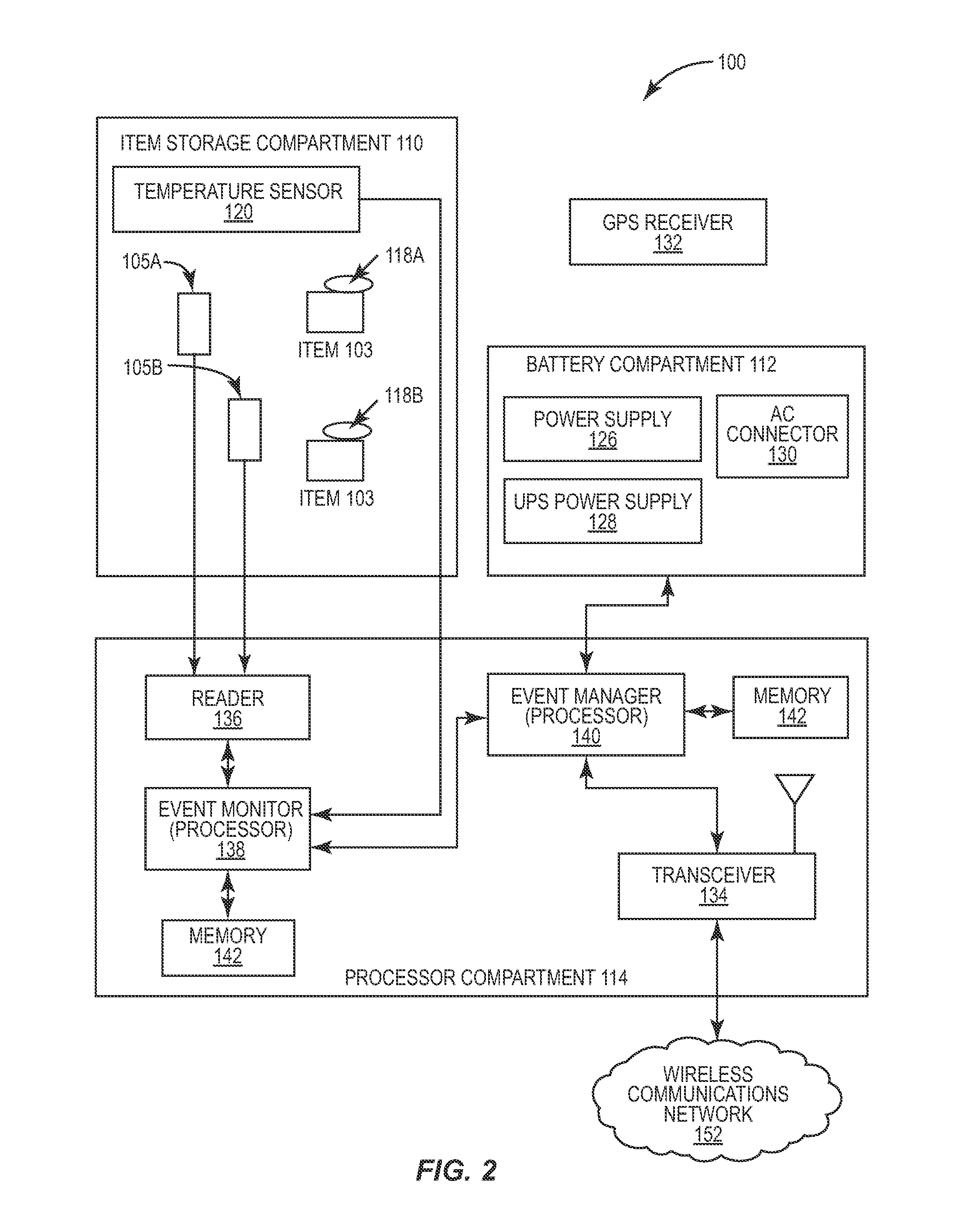
Portable RFID tagged carrier for sterile implants and biological products
ActiveUS20130106607A1Light weightVaccination/ovulation diagnosticsDead animal preservationEngineeringRadio frequency
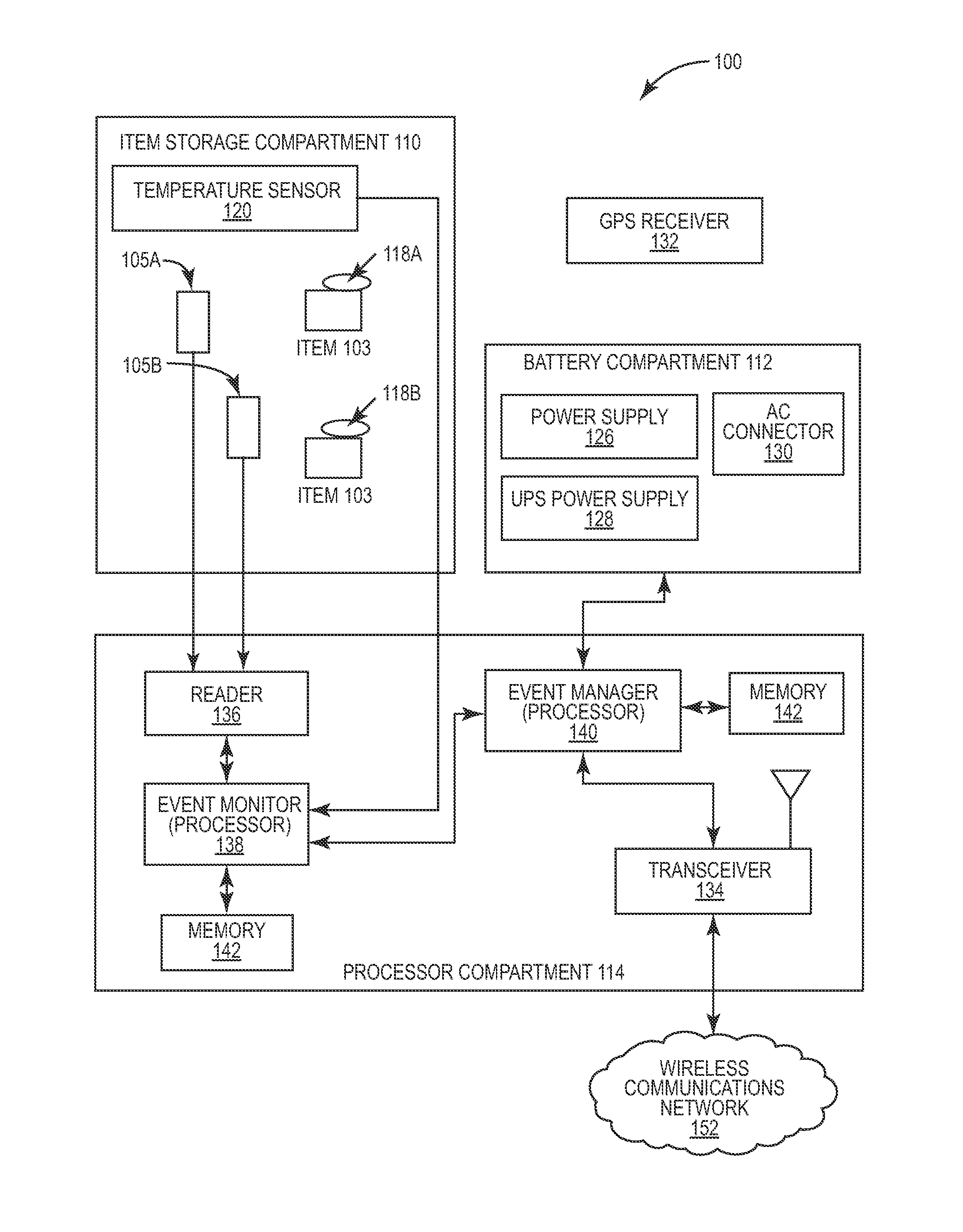
Intelligent portable carrier device for supporting movement in product tracking and monitoring of regulated products, such as tissue and biologics. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify the regulated products as they are added to or removed from the intelligent portable carrier device. Embodiments of the invention may also be configured to monitor and report temperature and other environmental conditions associated with the intelligent portable carrier device.
Owner:WARSAW ORTHOPEDIC INC
Needle kit and method for microwave ablation, track coagulation, and biopsy
InactiveUS20070161977A1Minimize damageMinimize bleedingSurgical needlesVaccination/ovulation diagnosticsAbnormal tissue growthBiomedical engineering
A modular biopsy, ablation and track coagulation needle apparatus is disclosed that allows the biopsy needle to be inserted into the delivery needle and removed when not needed, and that allows an inner ablation needle to be introduced and coaxially engaged with the delivery needle to more effectively biopsy a tumor, ablate it and coagulate the track through ablation while reducing blood loss and track seeding. The ablation needle and biopsy needle are adapted to in situ assembly with the delivery needle. In a preferred embodiment, the ablation needle, when engaged with the delivery needle forms a coaxial connector adapted to electrically couple to an ablating source. Methods for biopsying and ablating tumors using the device and coagulating the track upon device removal are also provided.
Owner:COVIDIEN LP
Failsafe reconfigurable surgical apparatus
InactiveUS20050131396A1Eliminate riskSurgical instrument detailsEndoscopic cutting instrumentsEngineeringActuator
A reconfigurable surgical apparatus that includes a surgical instrument assembly that is formed with a hollow manipulation shaft. A linearly or rotationally movable prime mover is received within the shaft and is activated by an actuator located at a proximal end. A coupler is formed about a distal end of the shaft to have a capture ledge that is configured to releasably engage an interchangeable surgical tool that is formed with an anchor adapted to releasably mate to the capture ledge. The coupler may optionally incorporate a frangible portion that severs a portion of the coupler when the interchangeable surgical tool is removed from the apparatus to ensure single use operation of the tool. The apparatus may also have a predetermined mode of failure ensuring a known point of failure upon exposure to a predetermined force. The apparatus may also be partially enclosed by a shroud.
Owner:STANCZAK GEORGE +1
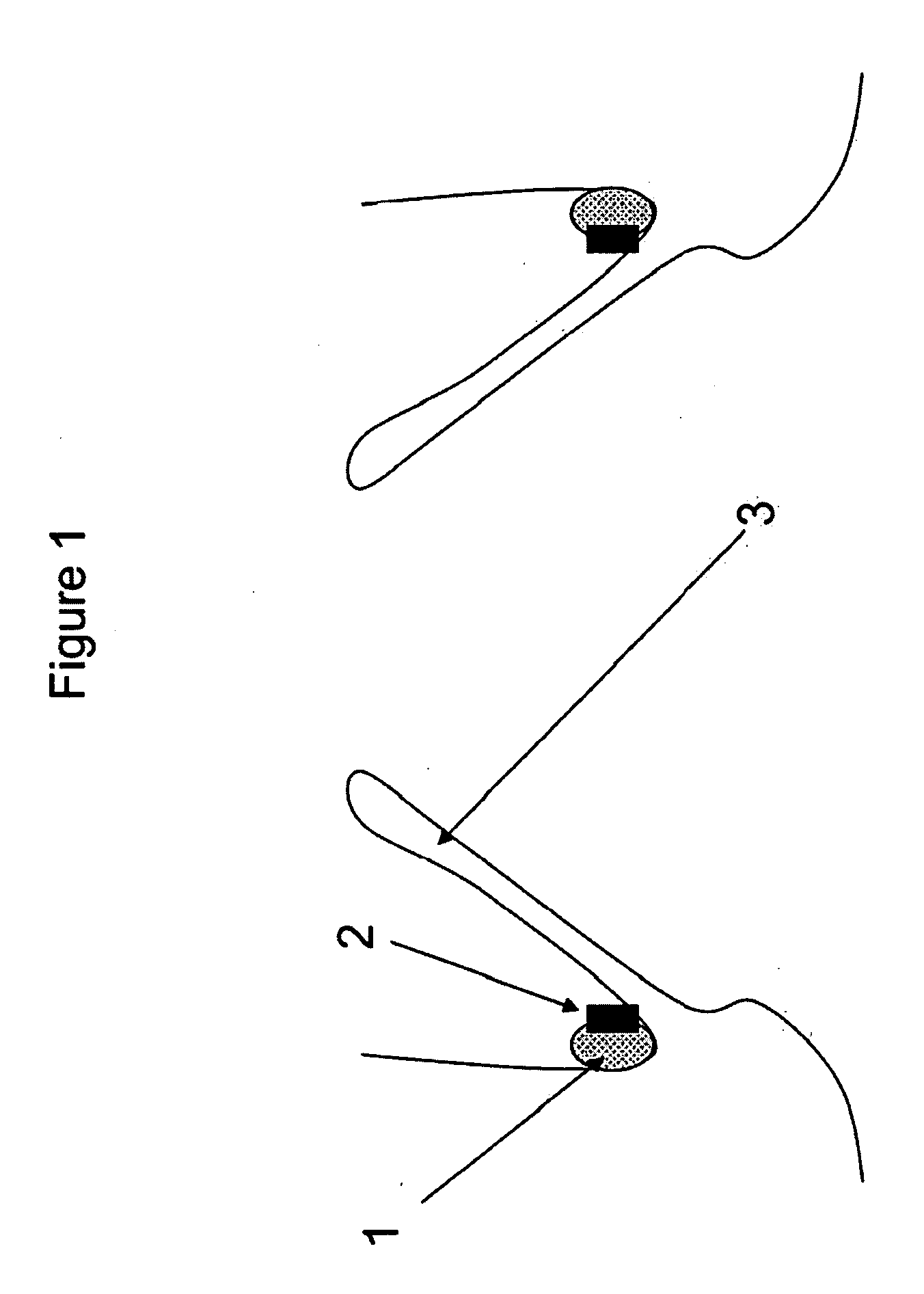
Mitral valve fixation device removal devices and methods
ActiveUS20170143330A1Easy accessSuture equipmentsHeart valvesEndovascular surgeryMitral valve leaflet
Procedures may be performed on the heart after the installation of a mitral valve fixation device. In order to prepare the heart for such procedures, the fixation device may be removed or disabled in minimally invasive ways (e.g., through an endovascular procedure), without requiring open access to the heart. The fixation device may be partitioned so that one portion may remain attached to each leaflet of the mitral valve. In another example, the leaflets may be cut along the edges of the distal element(s) of the fixation device, so as to cut the fixation device from the leaflet(s). Systems and devices for performing such procedures endovascularly are disclosed. Fixation devices with improved access to a release harness are also disclosed.
Owner:EVALVE
Method and system for coating tubular medical devices
InactiveUS20050147734A1Reduce the overall diameterPreventing “webbing” or “bridgingStentsPharmaceutical containersHigh volume manufacturingInsertion stent
A system and method for application of therapeutic and protective coatings to multiple tubular medical devices in a high volume production process. One or more tubular medical devices, such as stents, are placed on a coating-absorbent core, and coating is applied to the device(s), for example, as when the device-carrying core is passed through an extrusion coating machine to apply the coating in a uniform manner. Once coated, the medical device(s) may be quickly and efficiently removed from the core by causing the core diameter to decrease, such as by applying elongating tension to the core to cause the core diameter to radially contract, thereby allowing the coated device(s) to be simultaneously freed from the core. Improved coating uniformity, increased coated device removal ease and minimized bridging of openings in the tubular medical device may be obtained with a core that absorbs excess coating.
Owner:BOSTON SCI SCIMED INC
Facet replacement device removal and revision systems and methods
InactiveUS20080119845A1Improve the extraction forceIncrease forceInternal osteosythesisChiropractic devicesWaveguideTarsal Joint
A method and system for removing a portion of an artificial facet from a vertebra, and an adapter within the system that allows ultrasonic energy and extraction forces to be transmitted therethrough are provided. The method includes attaching an adapter to an ultrasonic waveguide and to a stem cemented into a vertebra, and applying ultrasonic energy and extraction force from the waveguide through the adapter to the stem. The system includes a handset that delivers ultrasonic energy, a waveguide attached to the handset to receive the energy therefrom, and an adapter attached to the waveguide to receive the energy therefrom. The adapter includes a first section attaching the adapter to the ultrasonic waveguide, and a second section attaching the adapter to a portion of the artificial facet joint having a stem embedded in a vertebra, the sections of the adapter transmitting energy and forces from the waveguide through the adapter to the attached stem.
Owner:GMEDELAWARE 2
Method and apparatus for anchoring cardiovascular implants
InactiveUS20090030435A1Reduce risk of migrationReduce intrusionSuture equipmentsStentsVascular implantTherapeutic Devices
Methods, devices and systems facilitate retention of a variety of therapeutic devices. Devices generally include an anchoring element, which has been designed to promote fibrotic ingrowth, and an anchored device, which has been designed to firmly engage the complementary region of the anchoring element. The anchoring element may be placed in a minimally invasive procedure temporally separated from the deployment of the anchored device. Once enough time has passed to ensure appropriate fixation of the anchoring element by tissue and cellular ingrowth at the site of placement, the anchored device may then be deployed during which it firmly engages the complementary region of the anchoring element. In this manner, a firm attachment to the implantation site may be made with a minimum of required hardware. Some embodiments are delivered through a delivery tube or catheter and while some embodiments may require laparoscopy or open surgery for one or more of the placement procedures. Some embodiments anchor devices within the cardiovascular tree while others may anchor devices within the gastrointestinal, peritoneal, pleural, pulmonary, urogynecologic, nasopharyngeal or dermatologic regions of the body. An alternative embodiment provides for the placement of the anchoring element and anchored device simultaneously, but allows for their removal separately. This embodiment allows the device, which may be placed only temporarily and be designed to be removed, to experience significant fibrotic ingrowth, but then to be easily detached from the ingrowth-anchored region to allow for simple and quick device removal.
Owner:THERANOVA LLC
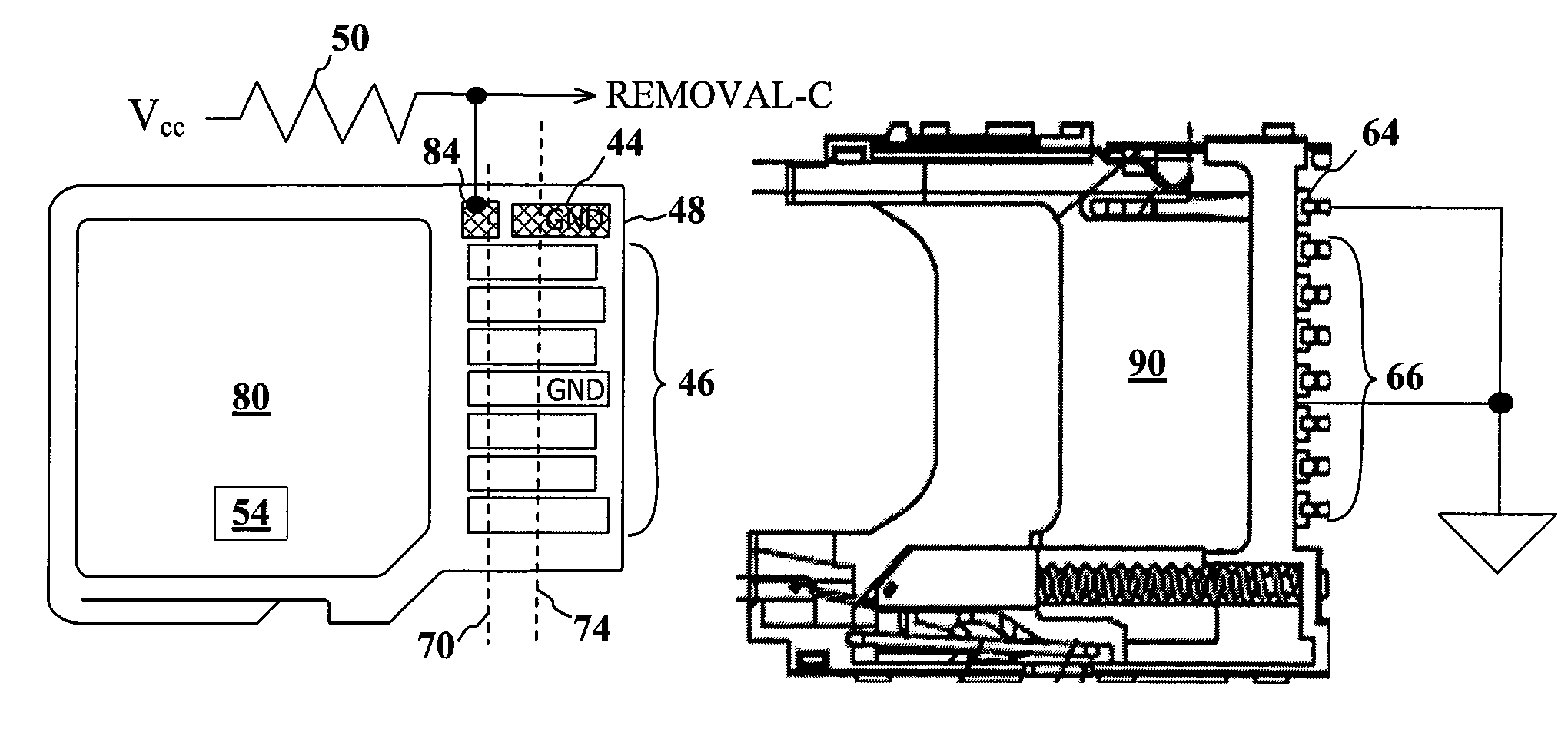
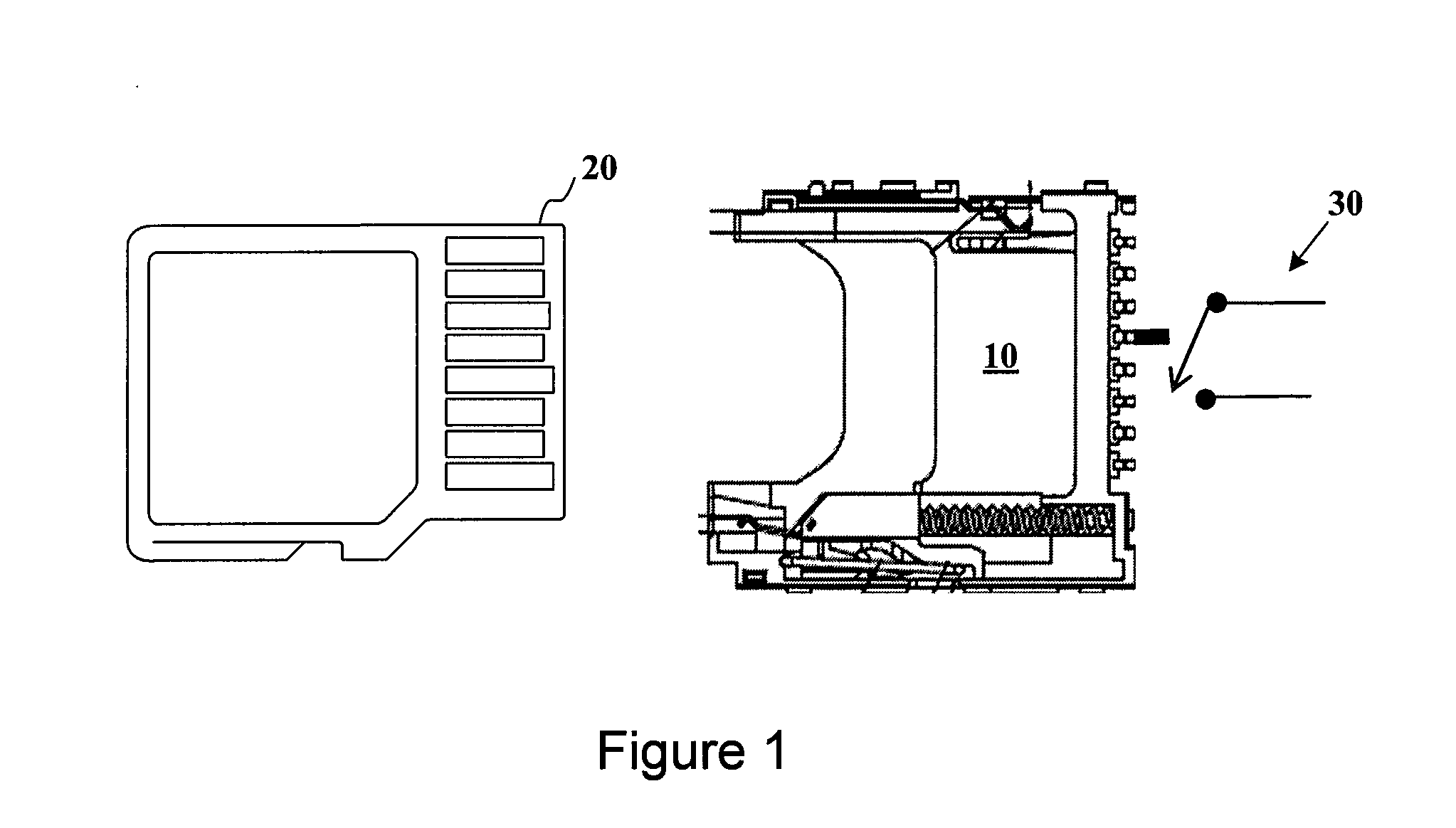
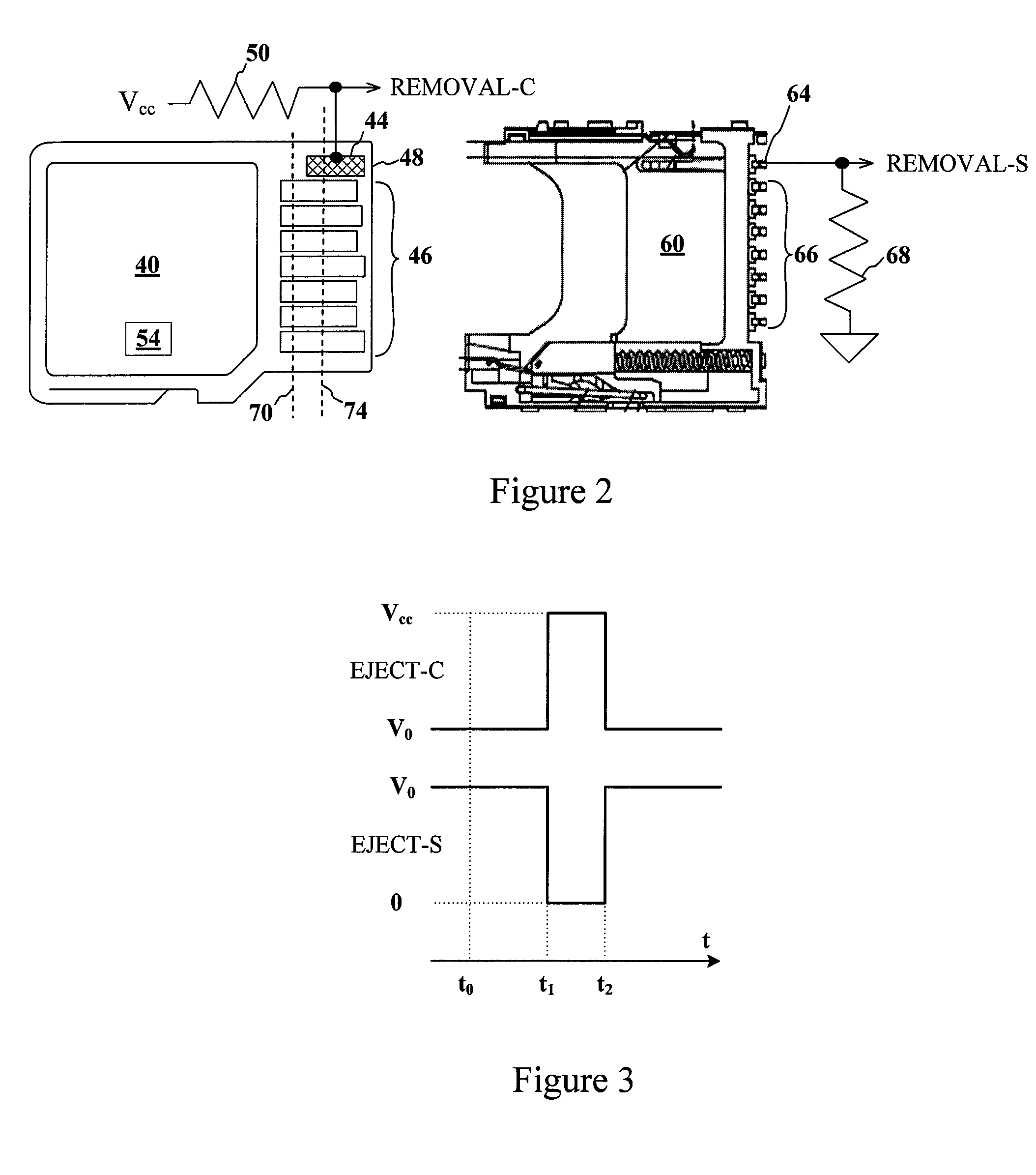
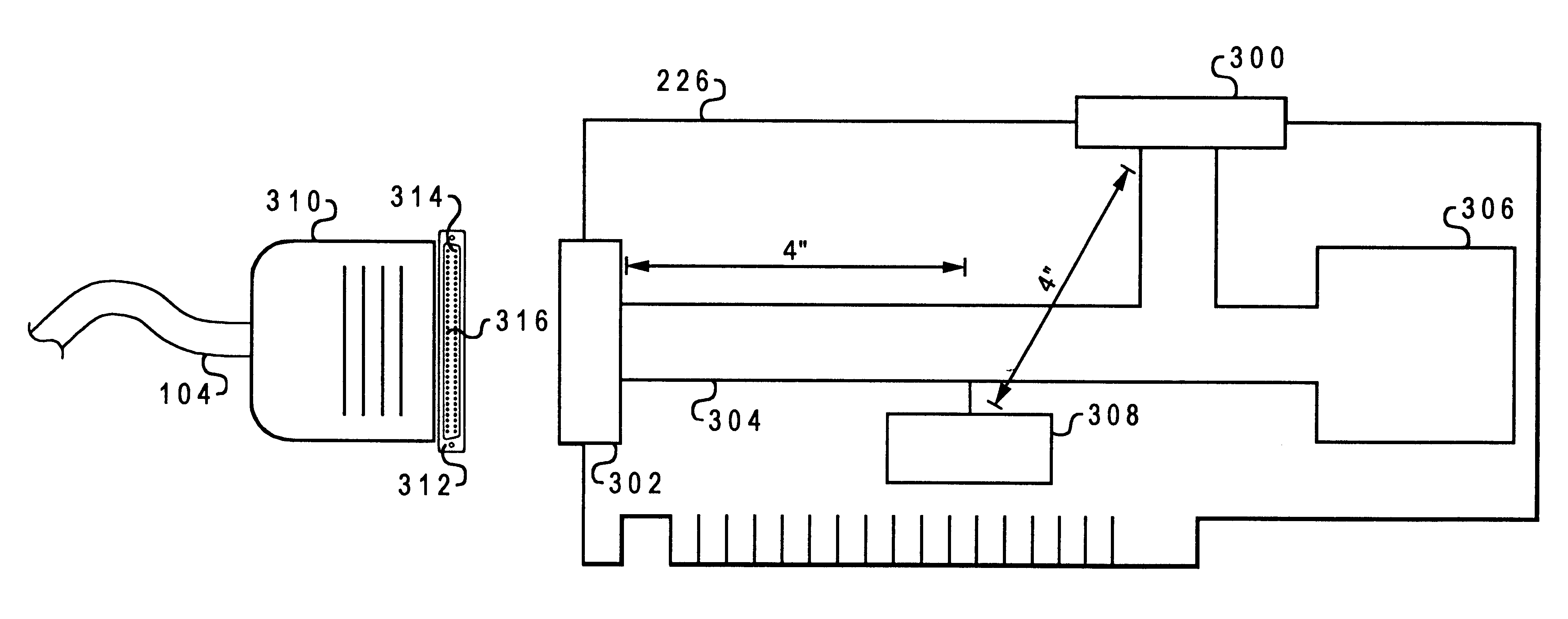
Advanced detection of memory device removal, and methods, devices and connectors
Memory devices, connectors and methods for terminating an operation are provided, including a memory device configured to terminate an internal operation such as a programming or erase operation responsive to receiving a signal during removal of the memory device from a connector, such as a socket. The memory device may be specially configured to generate the removal signal, such as by including a dedicated removal terminal. The memory card may respond to the signal by terminating a programming or erase operation before power is lost. The removal terminal may have a dimension that is different from a dimension of a power terminal through which the memory device receives power. Alternatively, the connector may be specially configured to generate a signal that causes a host to terminate programming or erase operations in the memory device prior to memory card removal, such as by including a switch that is actuated when the memory device moves to a pre-power loss position.
Owner:MICRON TECH INC
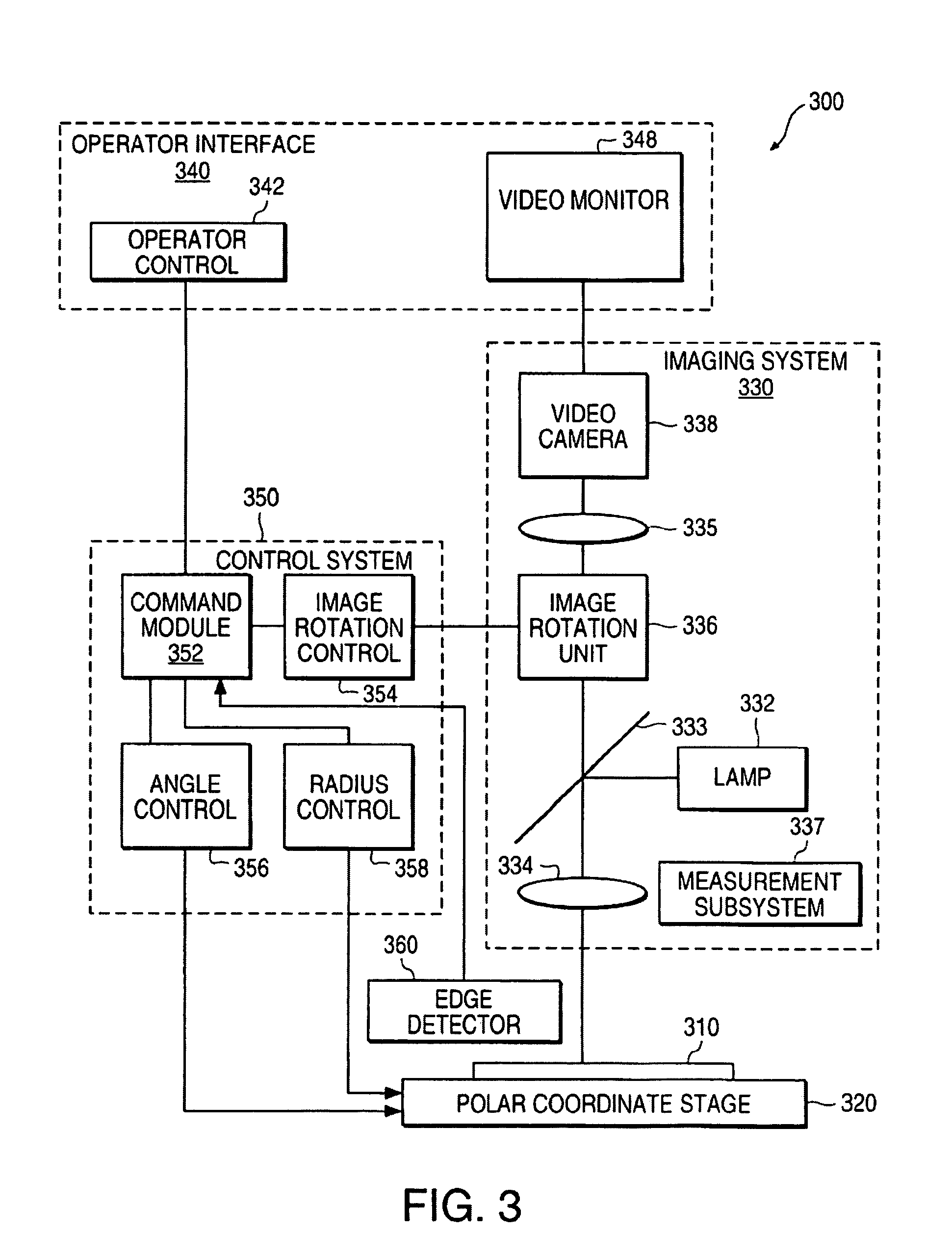
Metrology/inspection positioning system
InactiveUS7295314B1Avoid inaccuraciesEasy to identifyMaterial analysis by optical meansCharacter and pattern recognitionMetrologyEngineering
A metrology / inspection system moves the imaging and / or measuring equipment of the system relative to a wafer. Accordingly, measurement or inspection of the wafer does not require that the wafer be mounted on a precision stage. This allows the wafer to be at rest on any structure native in a processing apparatus when the system measures or inspects the wafer. Accordingly, measurement does not require removing the wafer from the processing apparatus and does not delay processing since the wafer can be measured, for example, during a required cool down period of device fabrication process. Alignment of an optical system includes pre-alignment base on edge detection using the optical system and more precise alignment using image recognition. An R-θ stage can position the optical system at inspection areas on the wafer. Image rotation can provide a fixed orientation for all images at the various inspection areas and can maintain the fixed orientation when moving from one inspection area to the next.
Owner:NANOMETRICS
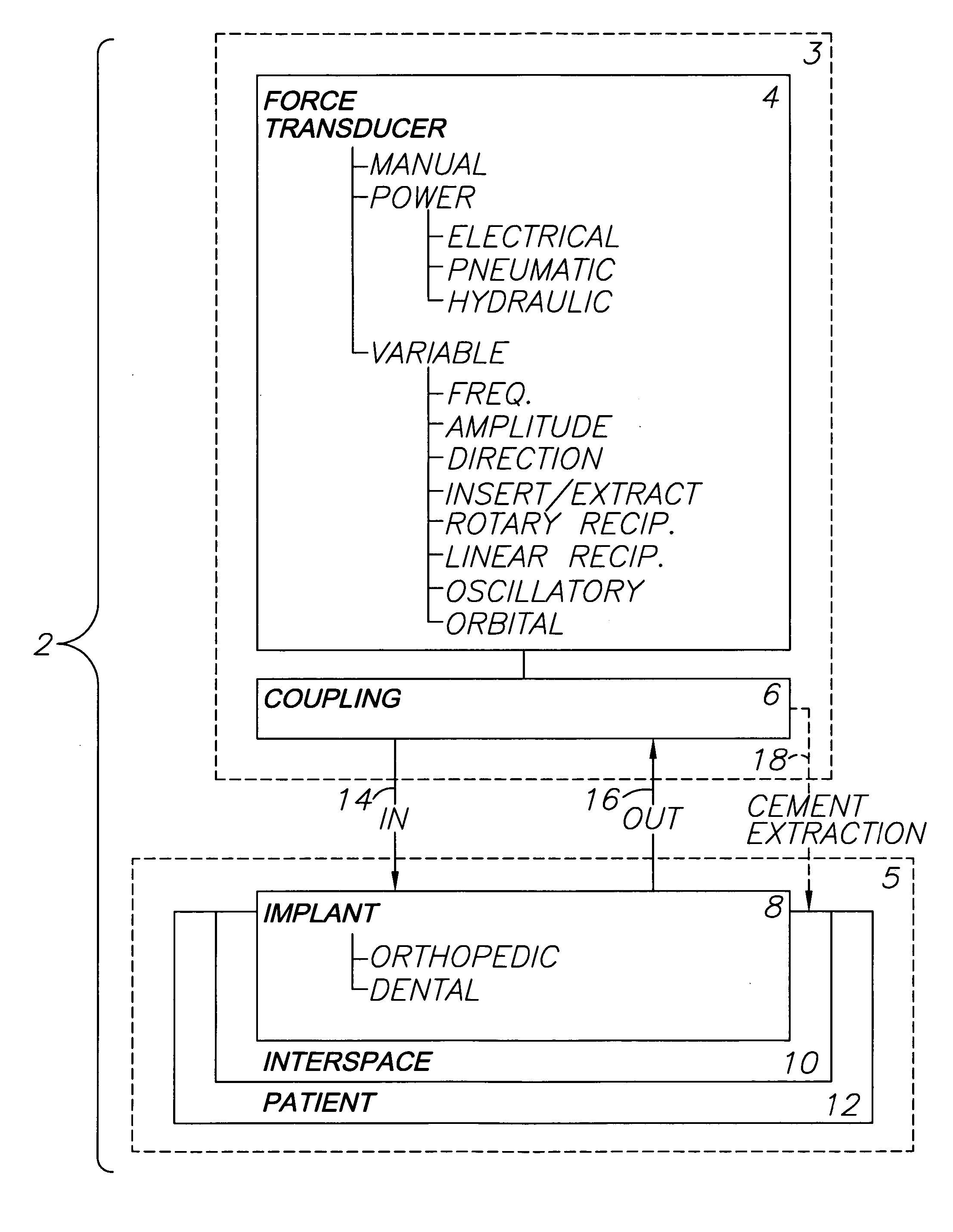
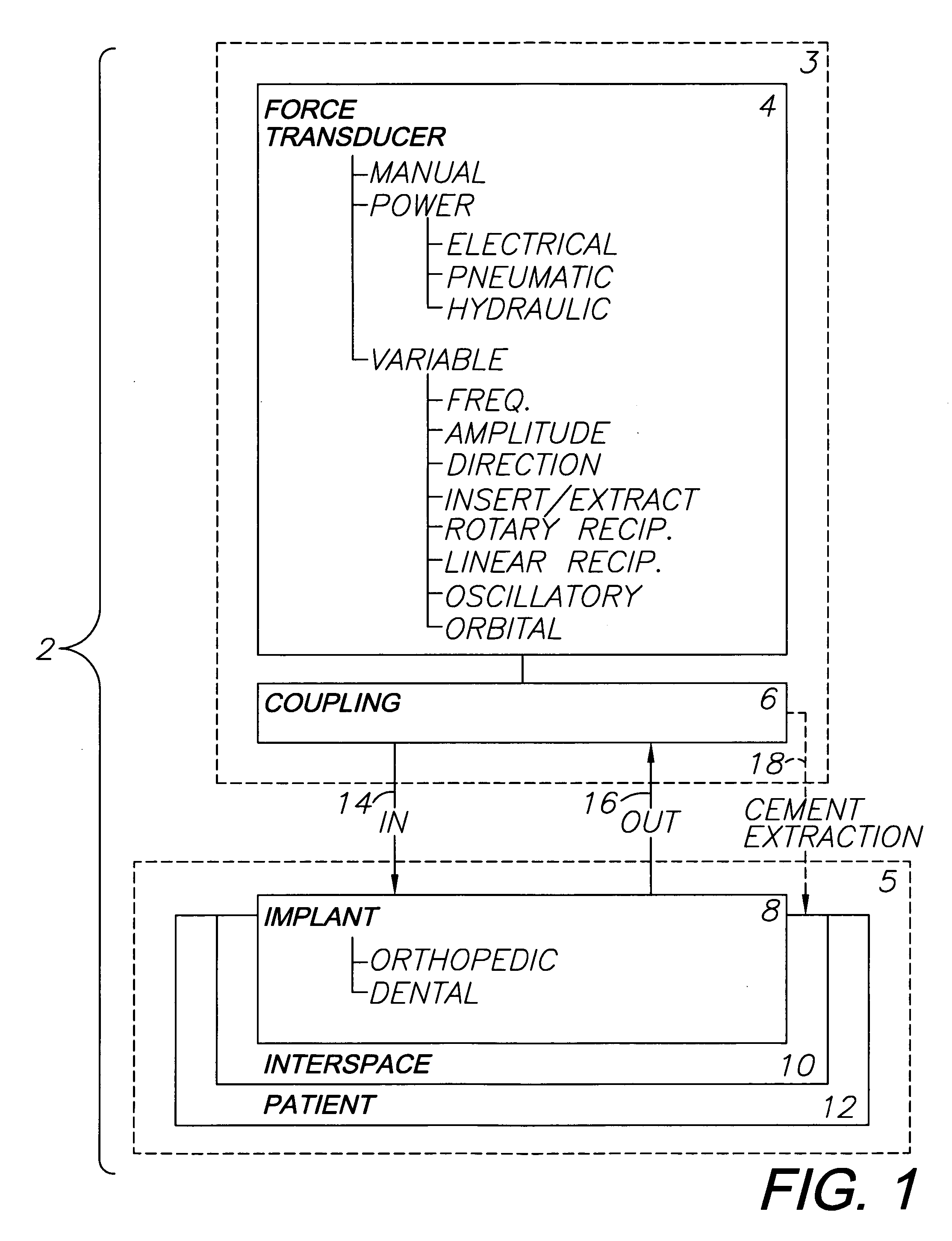
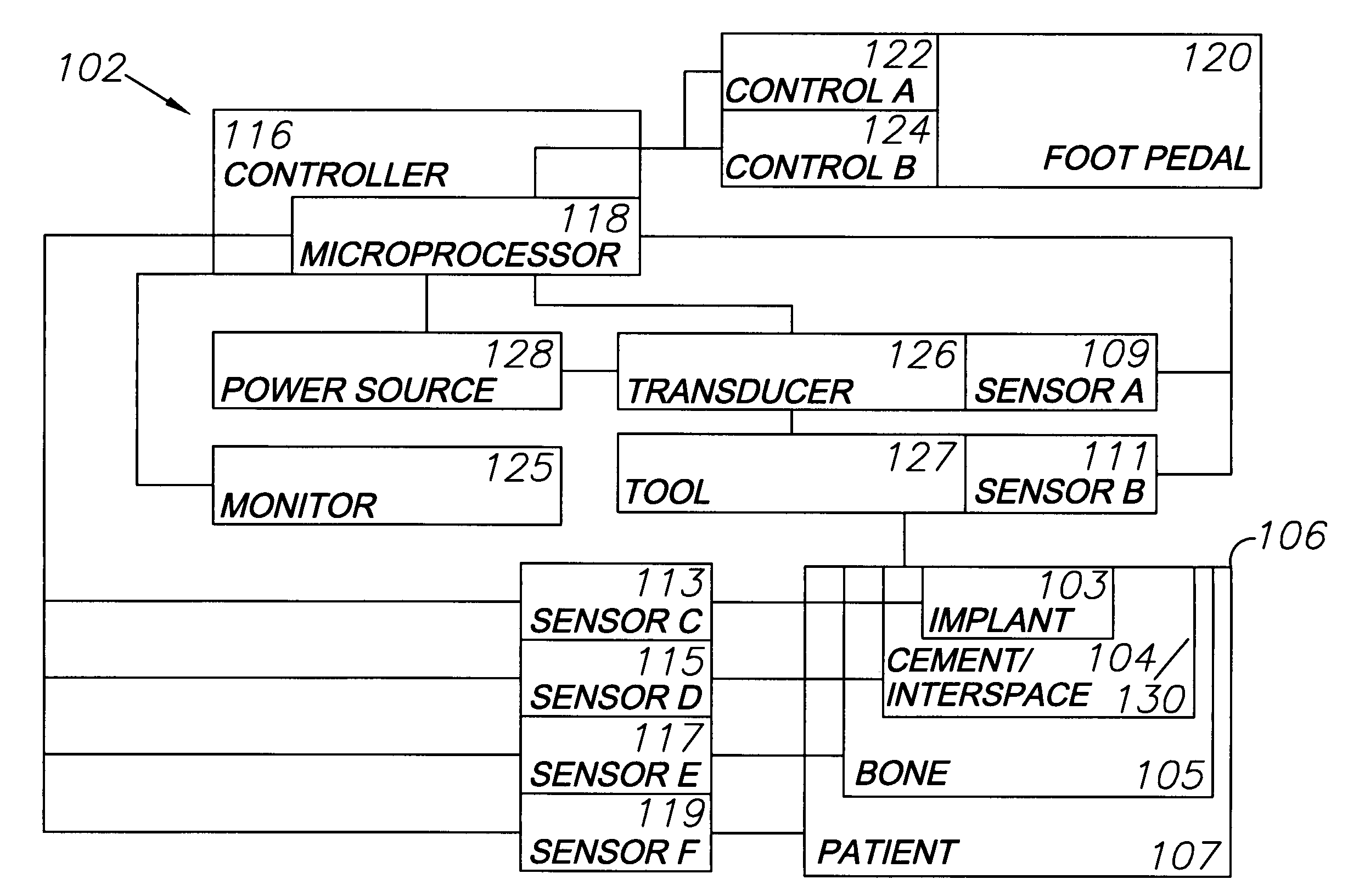
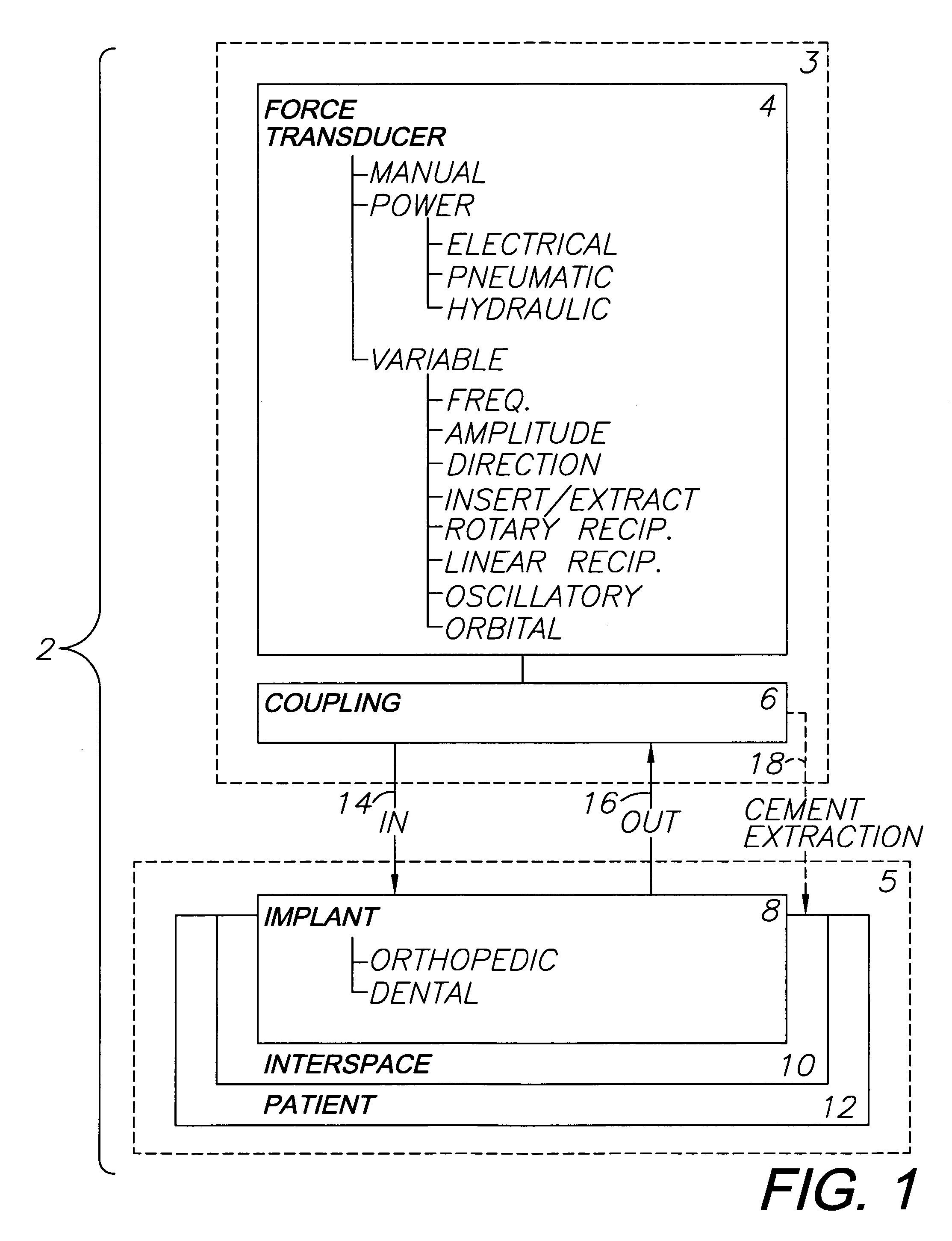
Orthopedic and dental implant system and method
A system for removing osteal cement and prosthetic joint components in connection with a prosthetic joint revision includes a controller connected to and controlling operation of a transducer, such as a surgical saw or drill. A tool mounted on the transducer is adapted for engaging the prosthetic joint cement mantel and melting an engagement portion of same. The cement in the engagement portion is resolidified with the tool tip embedded therein. The tool thus bonds to the cement mantel, and is used for vibrating, softening and breaking up same when operation of the transducer resumes. An osteal cement and prosthetic device removal method includes the steps of melting an engagement portion of the osteal cement mantel, bonding a transducer-mounted tool to the cement mantel by resolidifying the cement engagement portion and reactivating the transducer for vibrating, softening and breaking up the cement mantel whereby it can be removed from the patient.
Owner:BUBB STEPHEN K
Read-write access control method for plug-in memory device
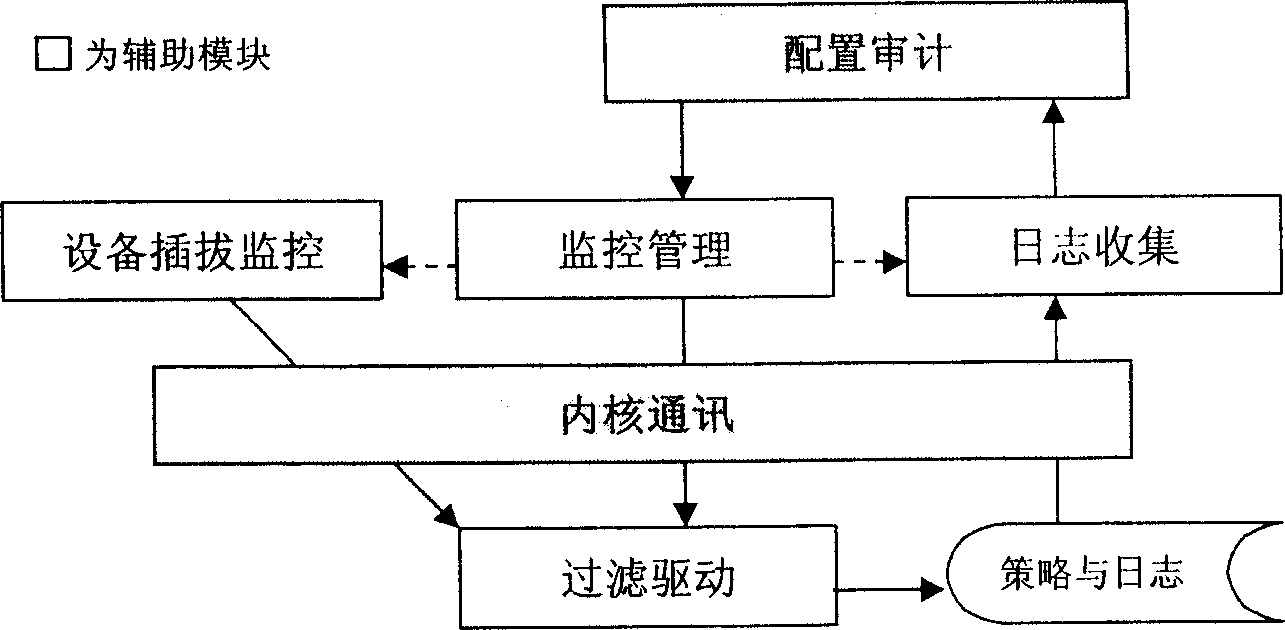
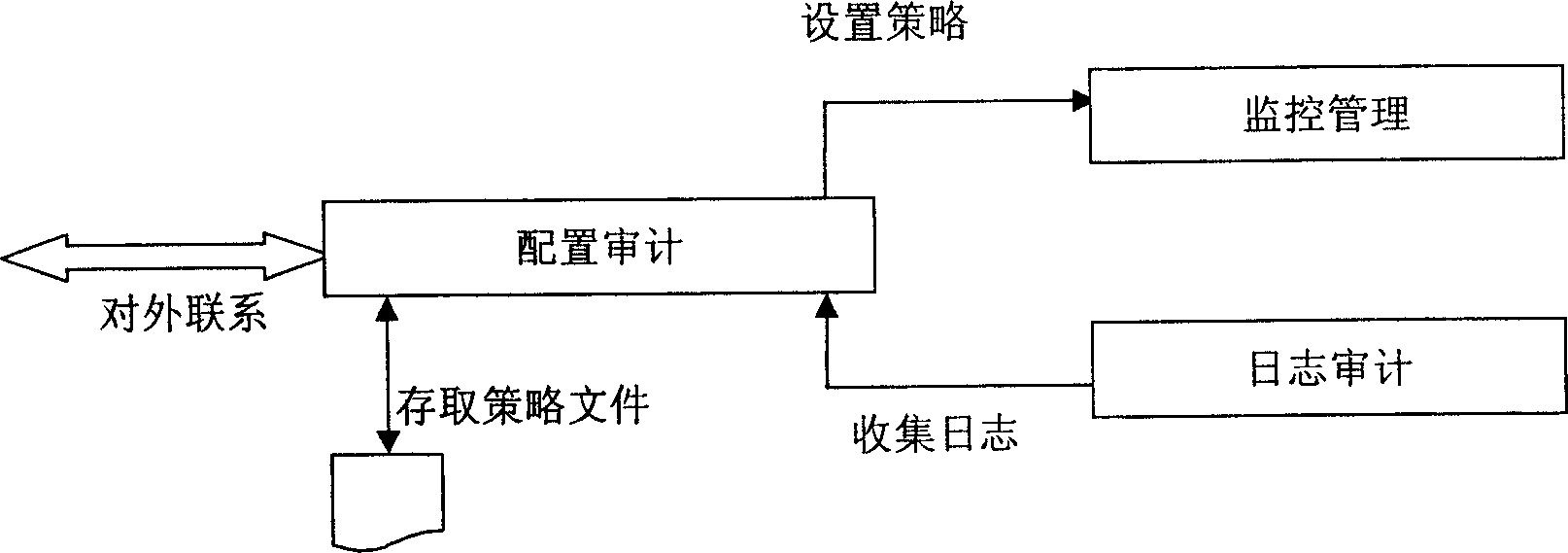
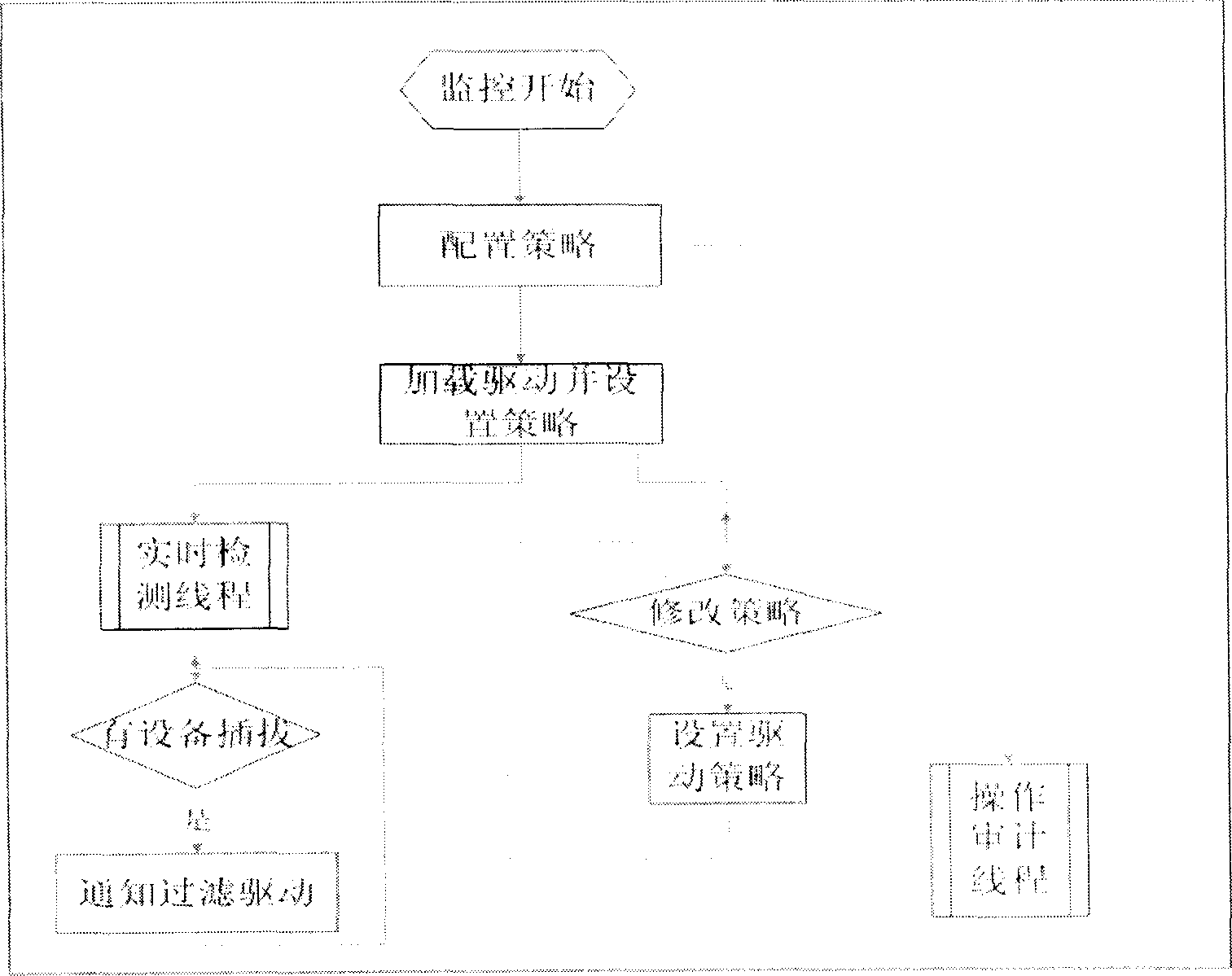
This invention relates to a read-write access control method for plug and play memory device. Wherein, configuring application security policy for the device by a control desk module; using a detection module to real-time detect the device and notify the kernel to start filter device for monitor; realizing the exact operation to access to monitor for the plug-play device according to strategy by a file system filter drive part module; detecting the device removal by the former detection module to notify kernel unload filter device and stop monitor. Besides, the system comprises an application layer for detecting device state and a kernel layer for monitor.
Owner:NANJING UNIV +1
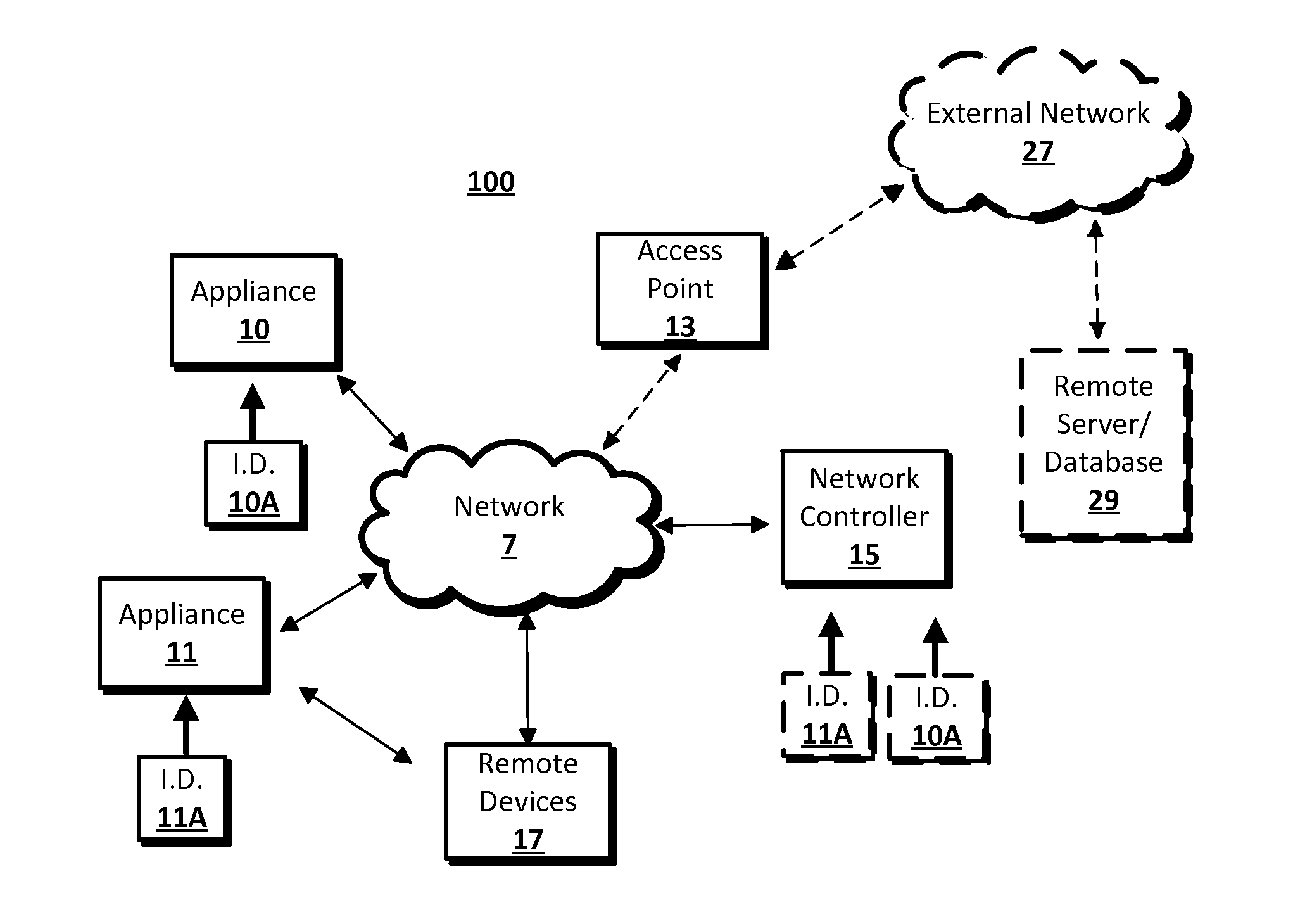
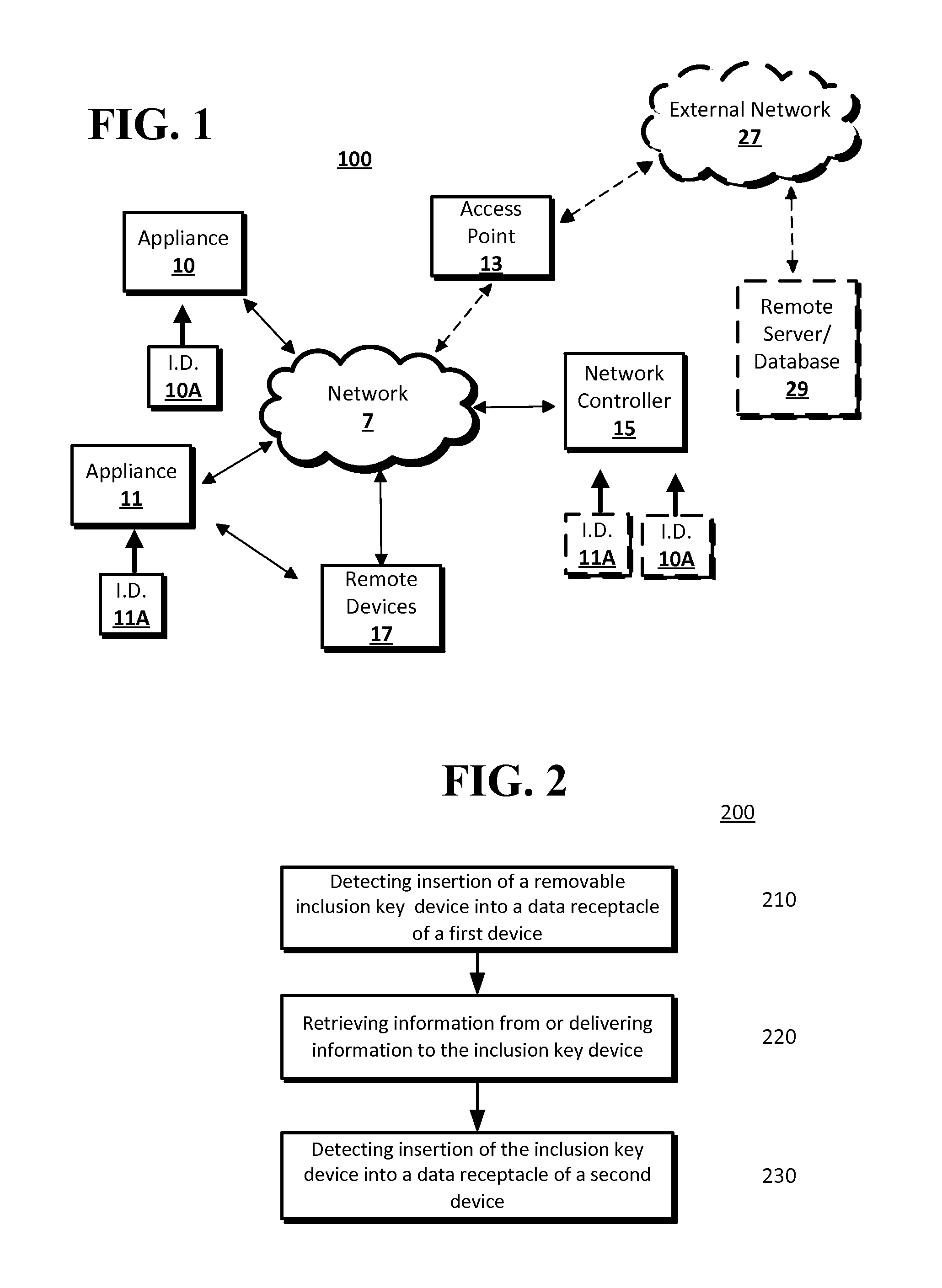
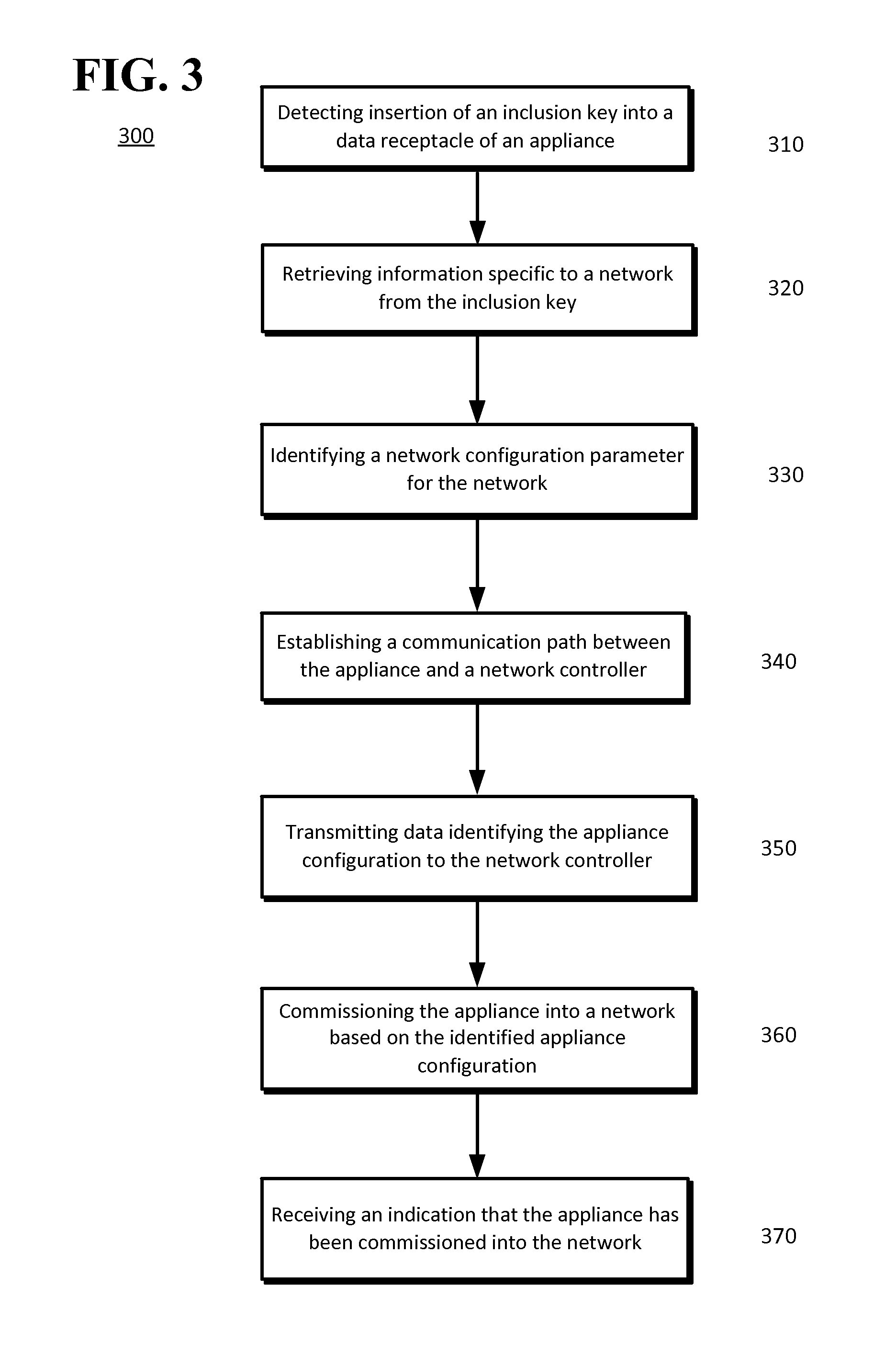
Device Commissioning
InactiveUS20140173059A1Easy to debugDigital computer detailsTransmissionNetwork controlSubject matter
The disclosed subject matter may provide a system and methods for commissioning a device or appliance into a network. A processor may detect the insertion of a removable device into a data receptacle of a first device. The first device may be an appliance or a network controller. Information may be retrieved from or delivered to the removable device. The information may be information specific to the appliance, such as model number, or may be information related to the network controller. For example, information delivered from the network controller to the removable device may include a network address assignment and network protocol to be used by an appliance when the appliance communicates with the network. The removable device may be removed from the first device and inserted into a data receptacle of a second device.
Owner:GOOGLE LLC
Orthopedic and dental implant system and method
A system for removing osteal cement and prosthetic joint components in connection with a prosthetic joint revision includes a controller connected to and controlling operation of a transducer, such as a surgical saw or drill. A tool mounted on the transducer is adapted for engaging the prosthetic joint cement mantel and melting an engagement portion of same. The cement in the engagement portion is resolidified with the tool tip embedded therein. The tool thus bonds to the cement mantel, and is used for vibrating, softening and breaking up same when operation of the transducer resumes. An osteal cement and prosthetic device removal method includes the steps of melting an engagement portion of the osteal cement mantel, bonding a transducer-mounted tool to the cement mantel by resolidifying the cement engagement portion and reactivating the transducer for vibrating, softening and breaking up the cement mantel whereby it can be removed from the patient.
Owner:BUBB STEPHEN K
Image forming apparatus with components removable in preselected direction and order
InactiveUS7082274B2Reduce loadAccurate replacementElectrographic process apparatusCorona dischargeReverse orderImage formation
An image forming apparatus of the present invention includes a plurality of components that form image forming means and should be mounted or dismounted in a preselected order. Following one of the components with respect to the preselected order cannot be dismounted from the apparatus until preceding one of the same has been dismounted. The components are mounted to the apparatus in the reverse order. The components unremovable from the apparatus and positioned above the removable components in a preselected dismounting direction are retractable.
Owner:RICOH KK
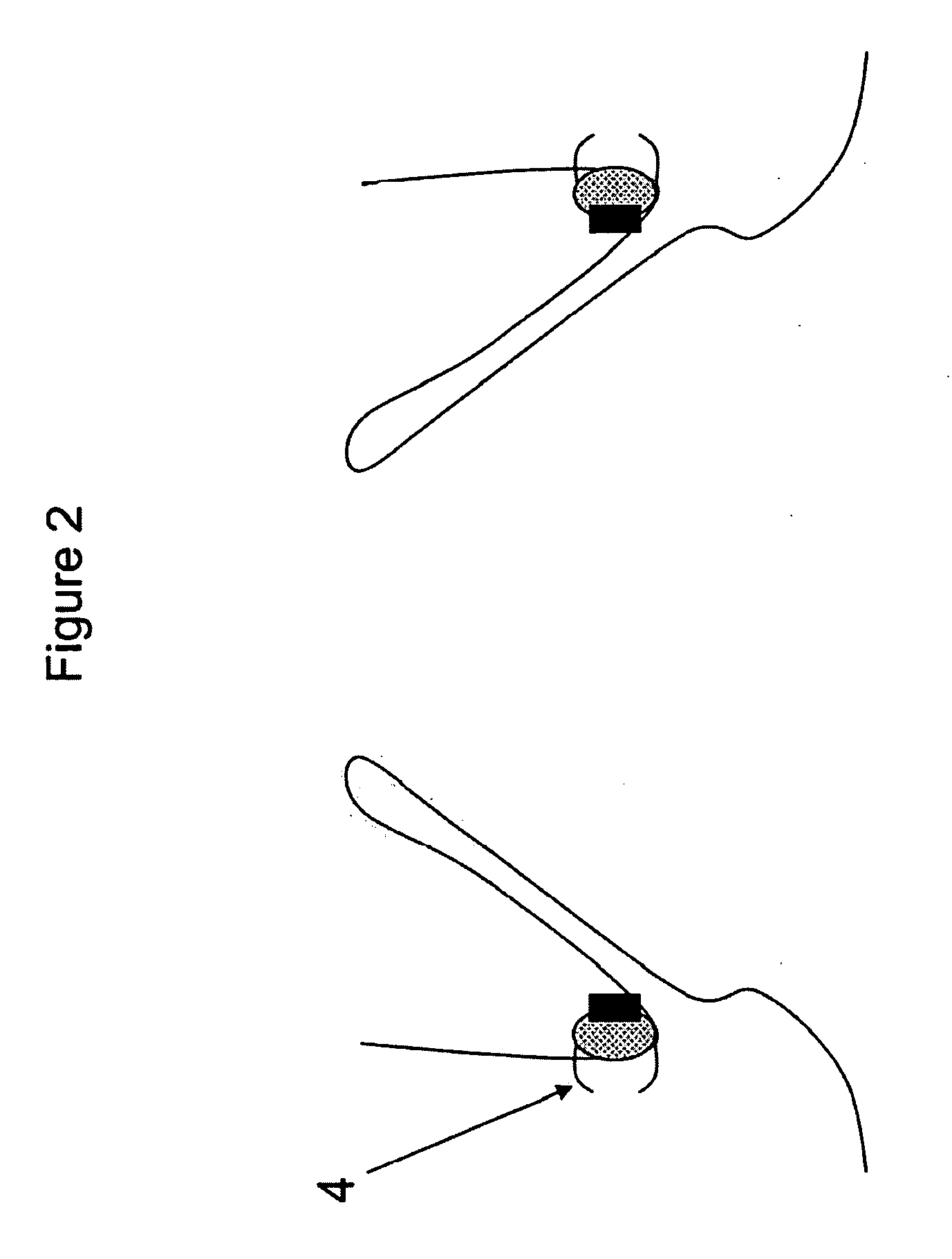
Mitral valve fixation device removal devices and methods
Procedures may be performed on the heart after the installation of a mitral valve fixation device. In order to prepare the heart for such procedures, the fixation device may be removed or disabled in minimally invasive ways (e.g., through an endovascular procedure), without requiring open access to the heart. The fixation device may be partitioned so that one portion may remain attached to each leaflet of the mitral valve. In another example, the leaflets may be cut along the edges of the distal element(s) of the fixation device, so as to cut the fixation device from the leaflet(s). Systems and devices for performing such procedures endovascularly are disclosed. Fixation devices with improved access to a release harness are also disclosed.
Owner:EVALVE
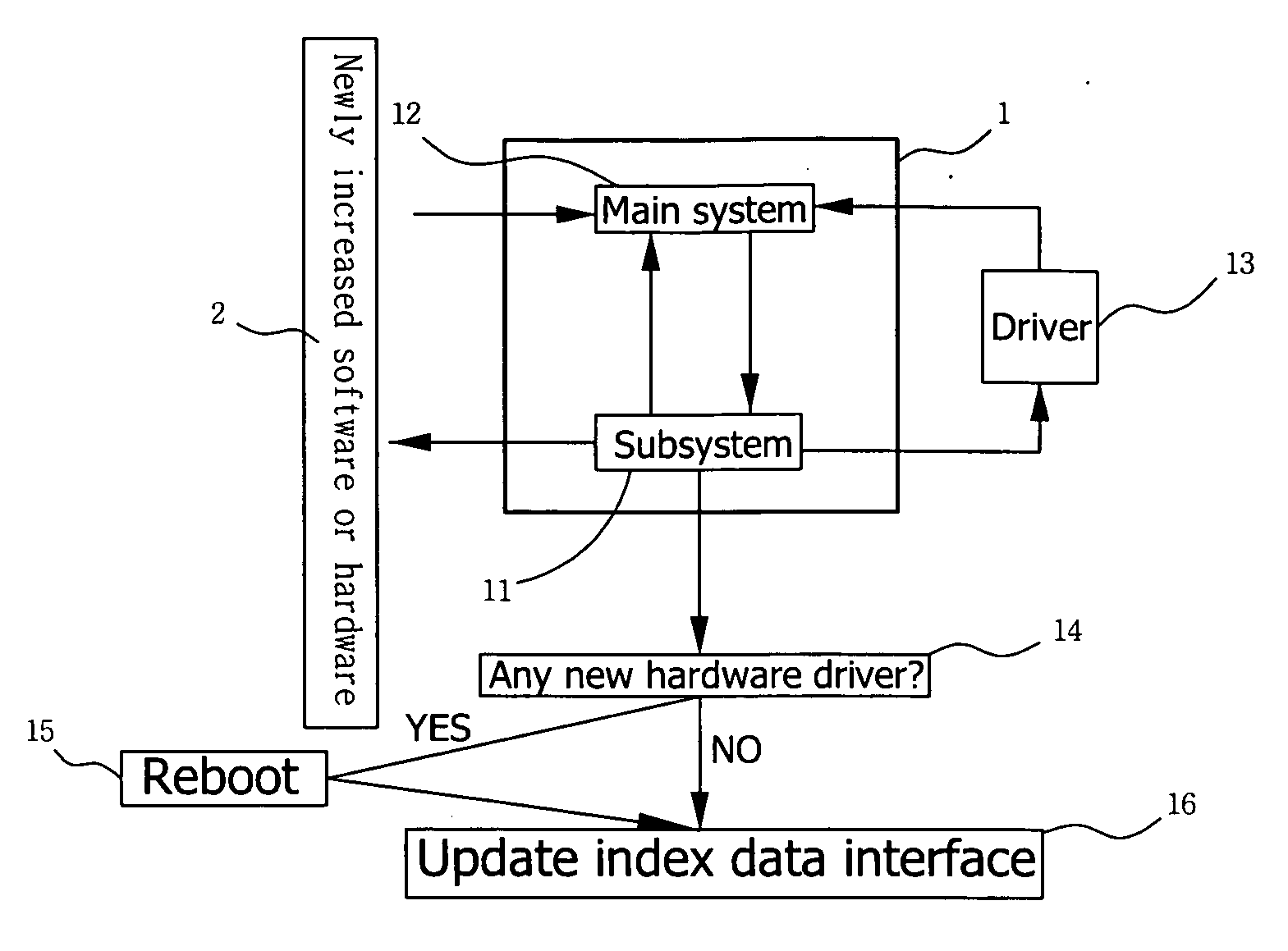
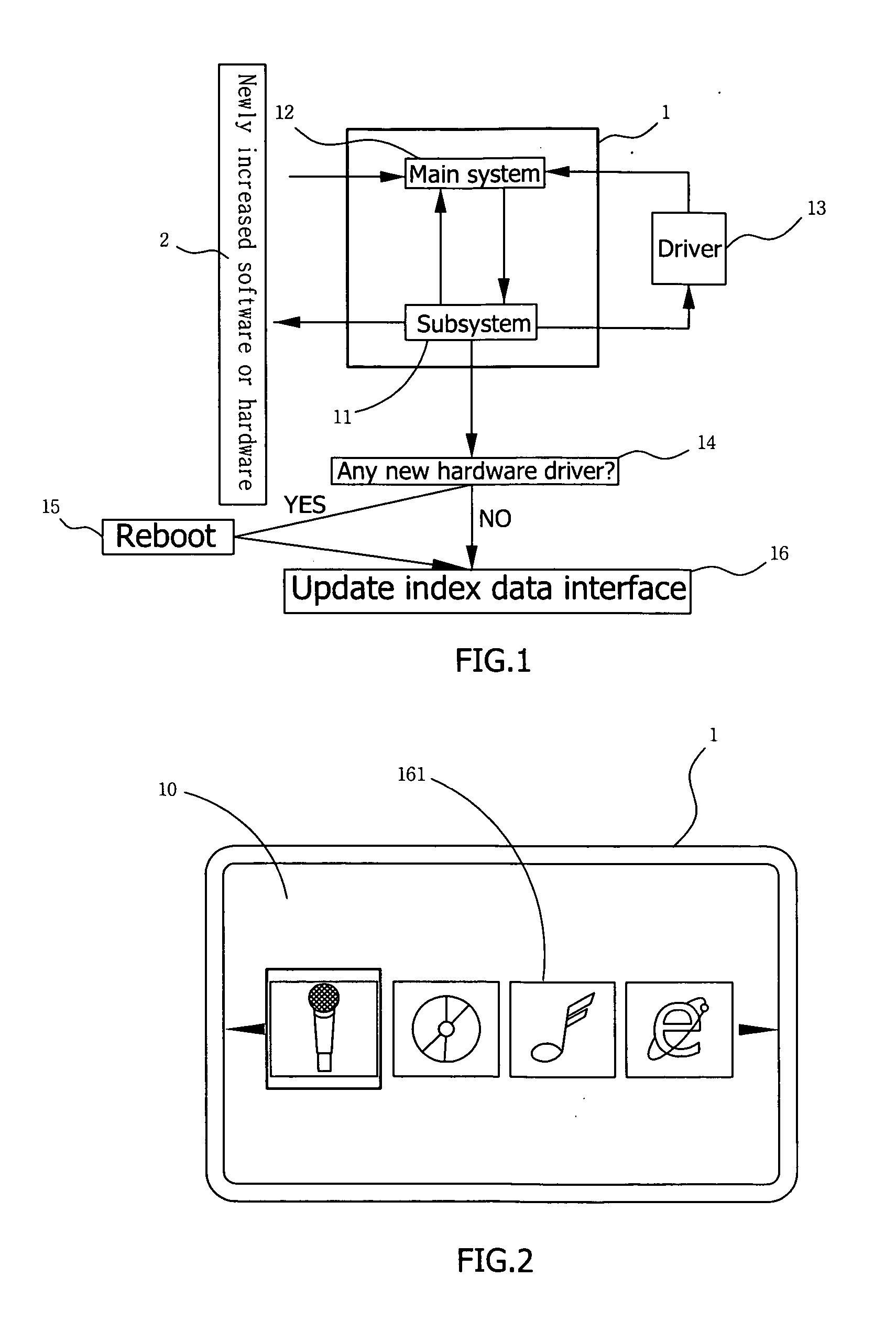
Method of controlling automatic updating of software or hardware kit for multimedia display device
InactiveUS20070169078A1Specific program execution arrangementsMemory systemsDisplay deviceData interface
In a method of controlling automatic updating of software or hardware kit for multimedia display device, when desired software or hardware is installed on or removed from a multimedia display device, the multimedia display device is caused to proceed with detection of newly increased software or hardware, and an end user is prompted to follow instructions shown on a screen of the display device to enter into a subsystem of the display device to proceed with procedures of driving the new software or hardware. The newly increased software kit may work immediately without the need of rebooting the display device. In the event a hardware driver is required, the display device is caused to reboot immediately, and an index data interface of the display device is automatically updated, so that a kit icon representing the new software or hardware is shown on or deleted from an index menu.
Owner:KATDC
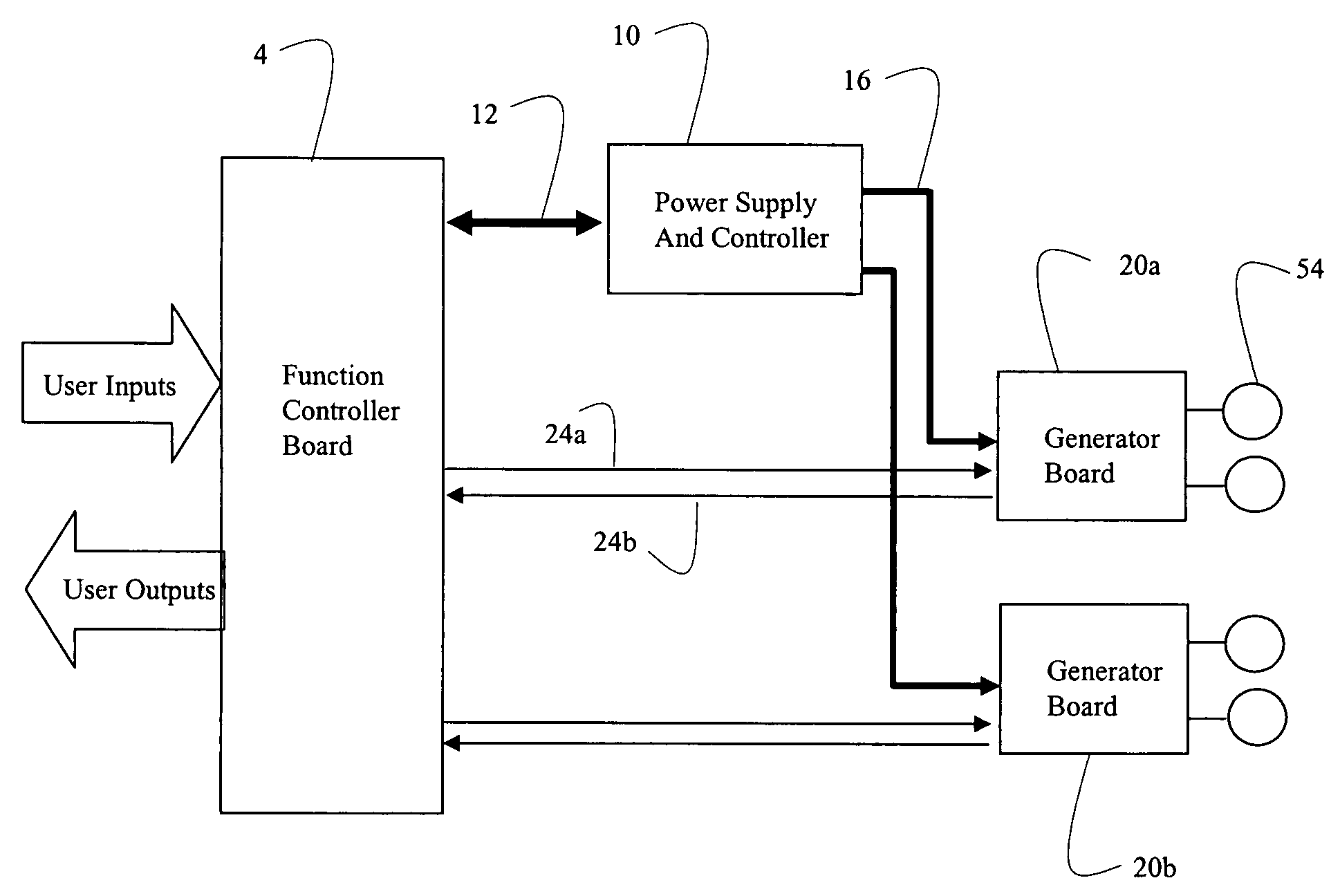
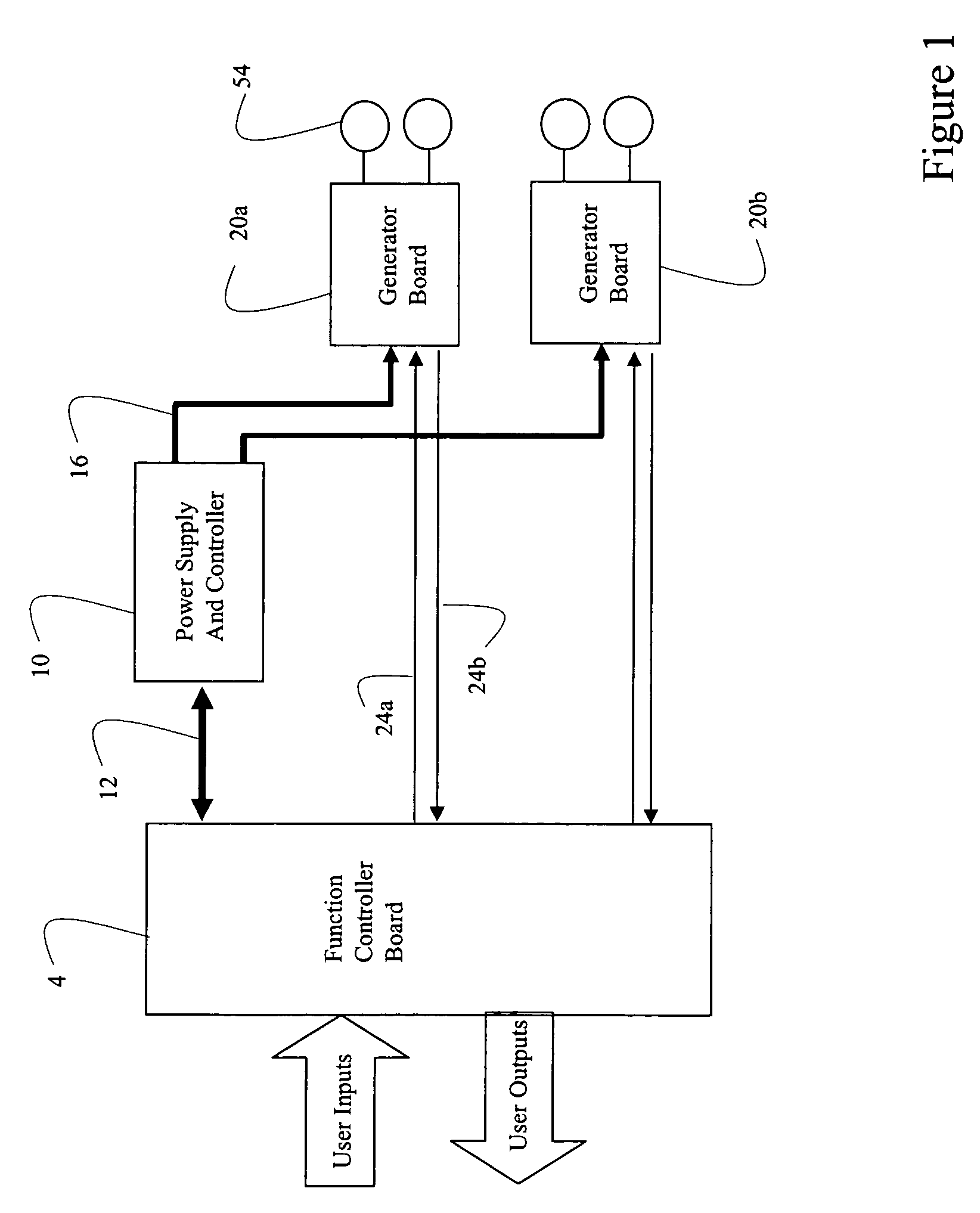
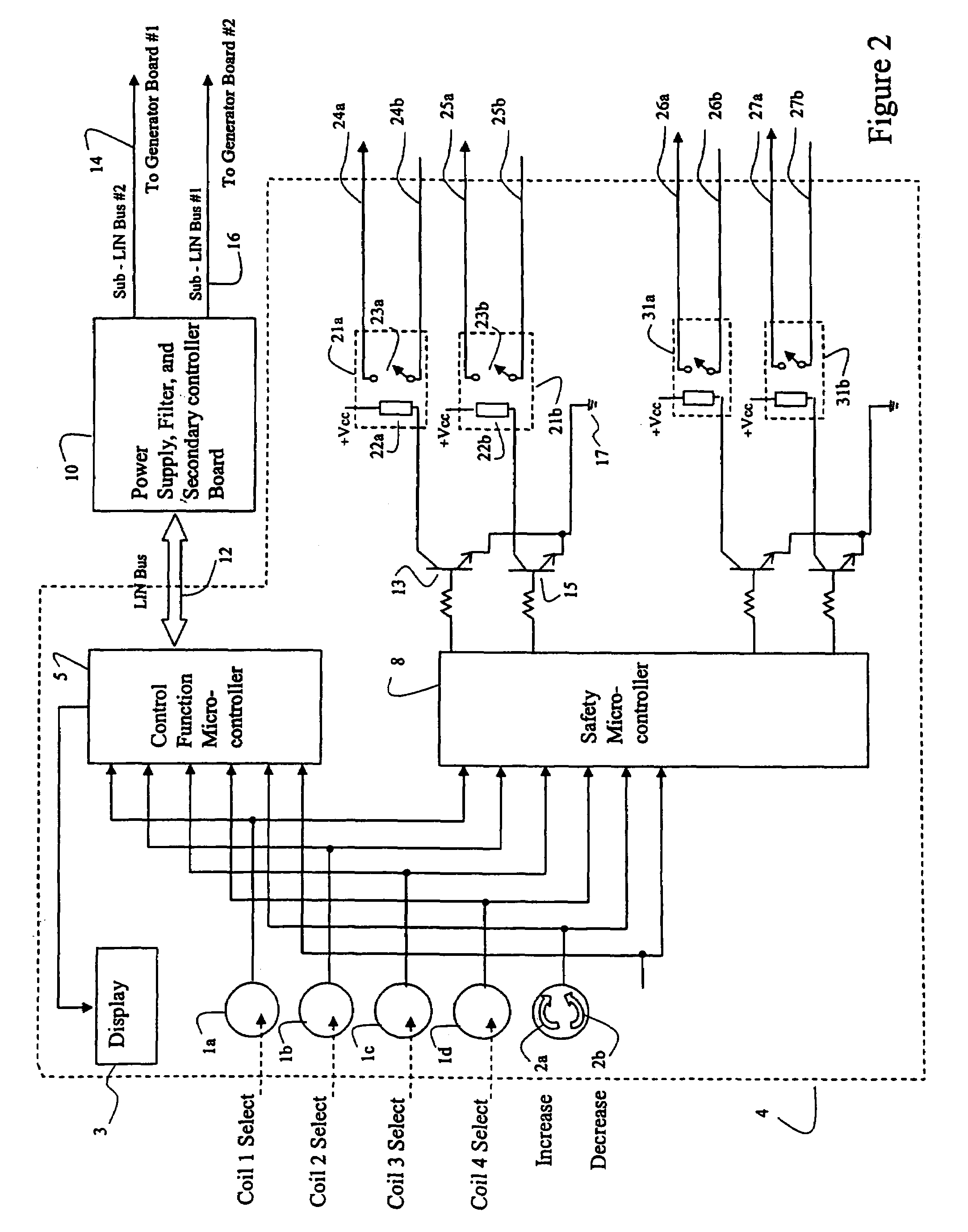
Systems and methods of using multiple microcontrollers for fail-safe control and enhanced feature operation of an appliance
Multiple microcontrollers are used in controlling operation of an appliance thereby providing enhanced safety. User input deactivating the appliance is received and processed by a first and second microcontrollers, which separately and independently act to remove power from the appliance upon receipt of user input. Failure of one microcontroller in processing user input does not result in the appliance entering into an unsafe mode. Further, a third processor in a power supply module is used to control power into, and out of, the power supply module, so that under certain conditions, power may be remove from the system entirely or to certain components.
Owner:E G O ELEKTRO GERAETEBAU GMBH
Separable in-line automatic terminator for use with a data processing system bus
InactiveUS6321277B1Simple methodError detection/correctionInput/output processes for data processingData processing systemSCSI
A method and system for automatically providing termination to a SCSI I / O bus within a data processing system is disclosed. An inline terminator is provided for use within a data processing system. The inline terminator provides a connection between a first controller and an I / O bus utilizing a plurality of connection pins including a device removal detection pin. The inline terminator includes a terminator circuit and a control circuit coupled to the device removal detection pin to detect whether the controller and bus are connected. In one embodiment, the detection pin is a short ground pin on the SCSI bus that is not associated with a signal or differential signal pair. The terminator circuit is activated when the controller and bus are disconnected and deactivated otherwise to automatically terminate the I / O bus.
Owner:IBM CORP
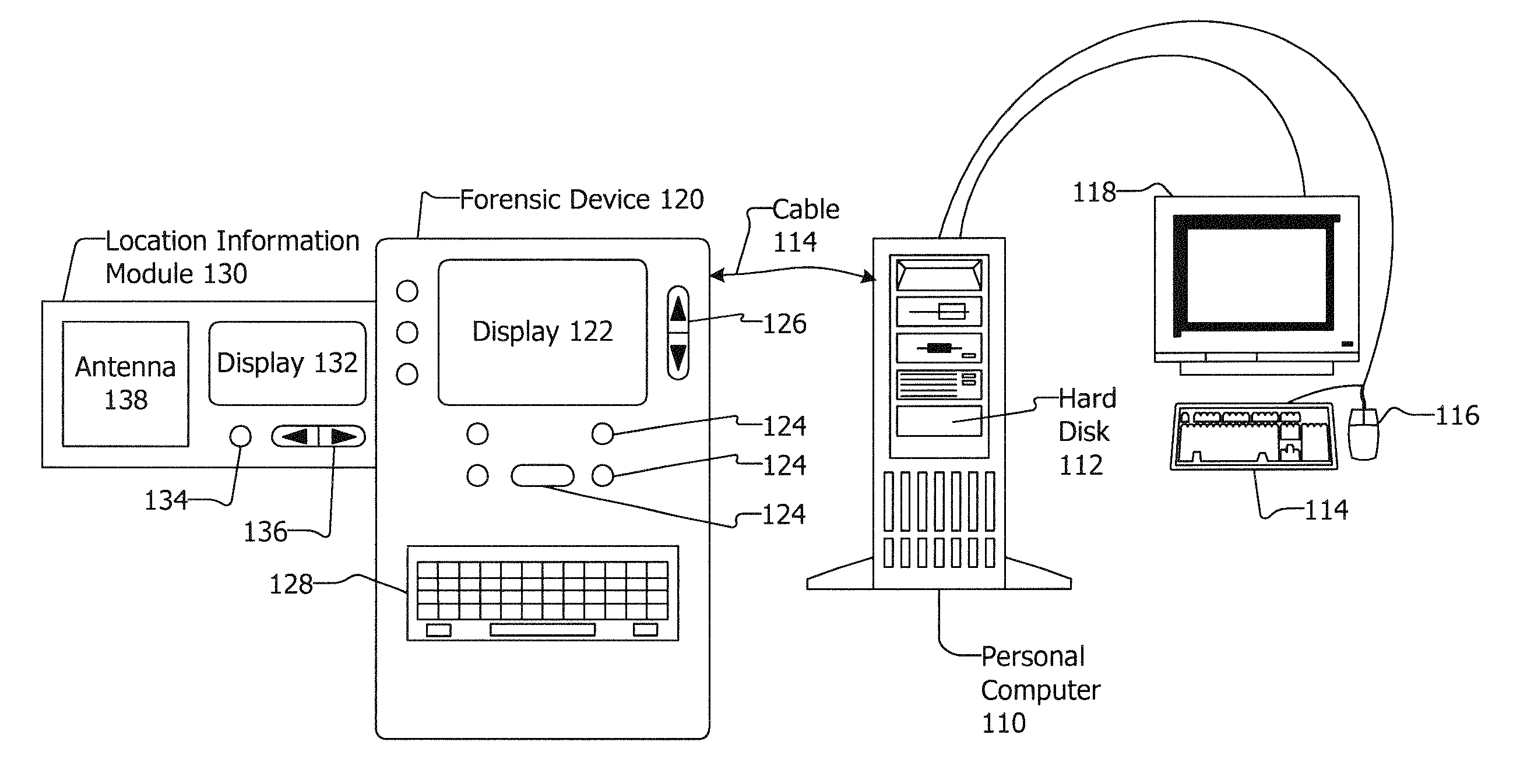
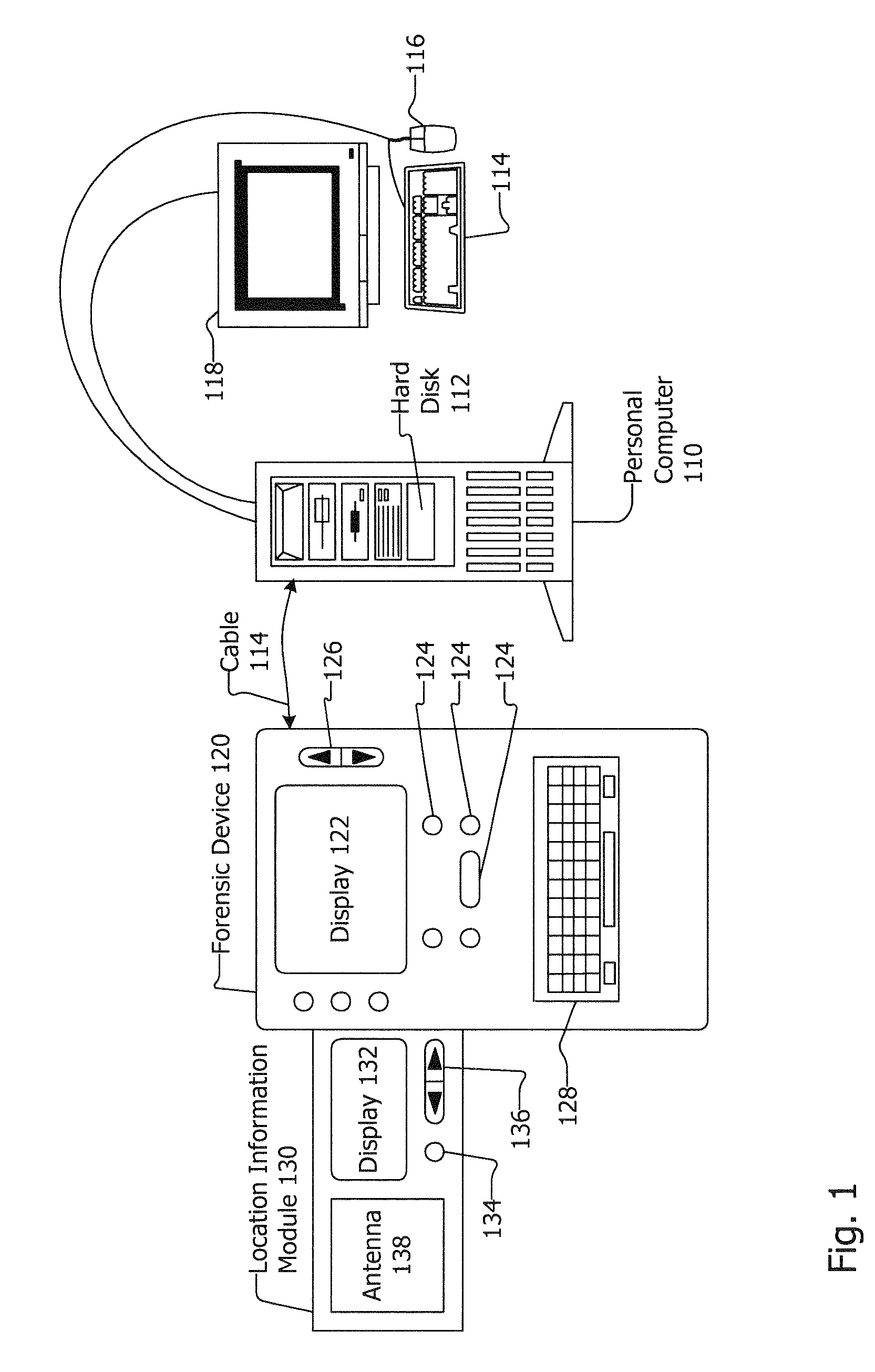
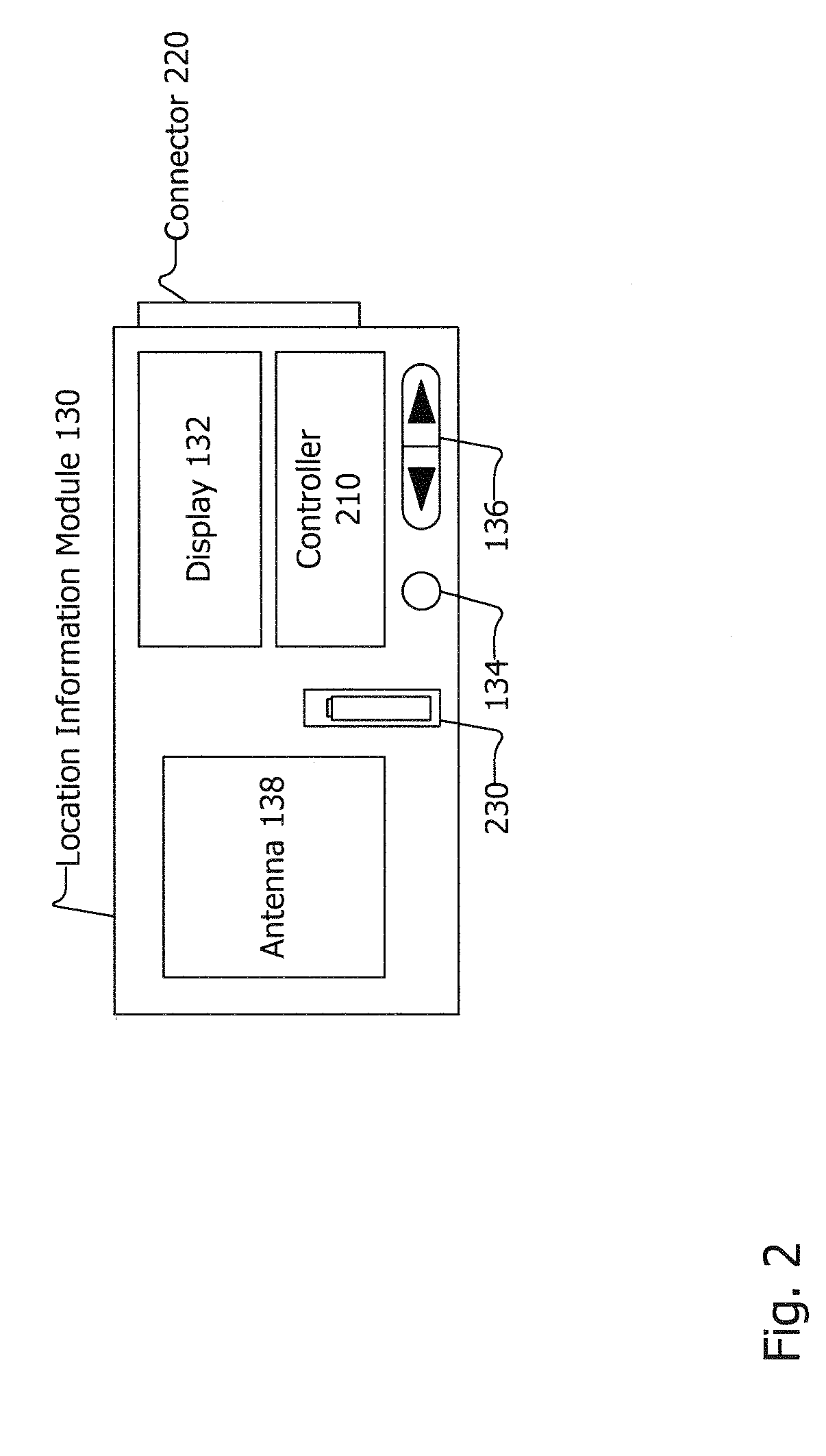
Associating Location Information with Forensic Data
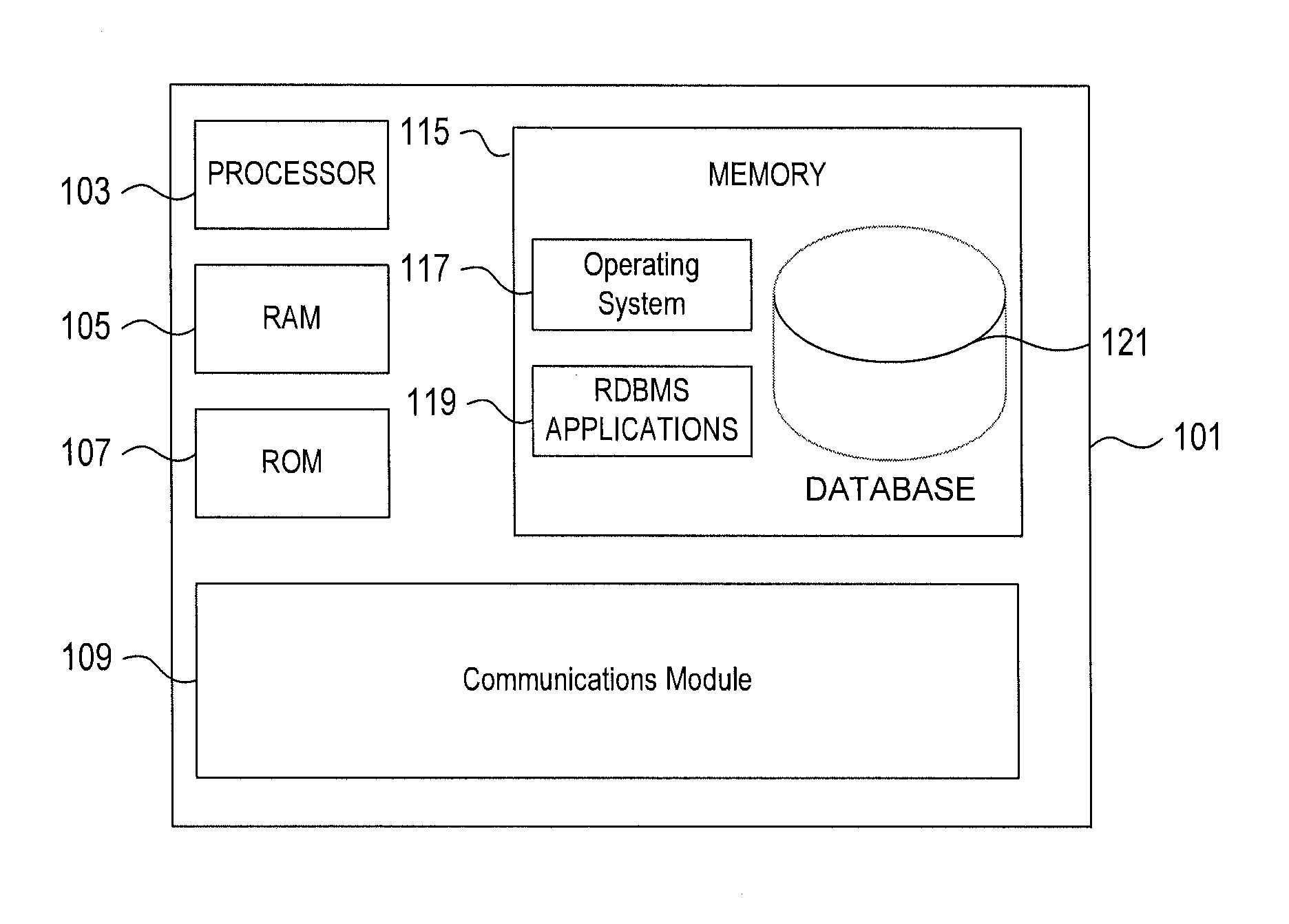
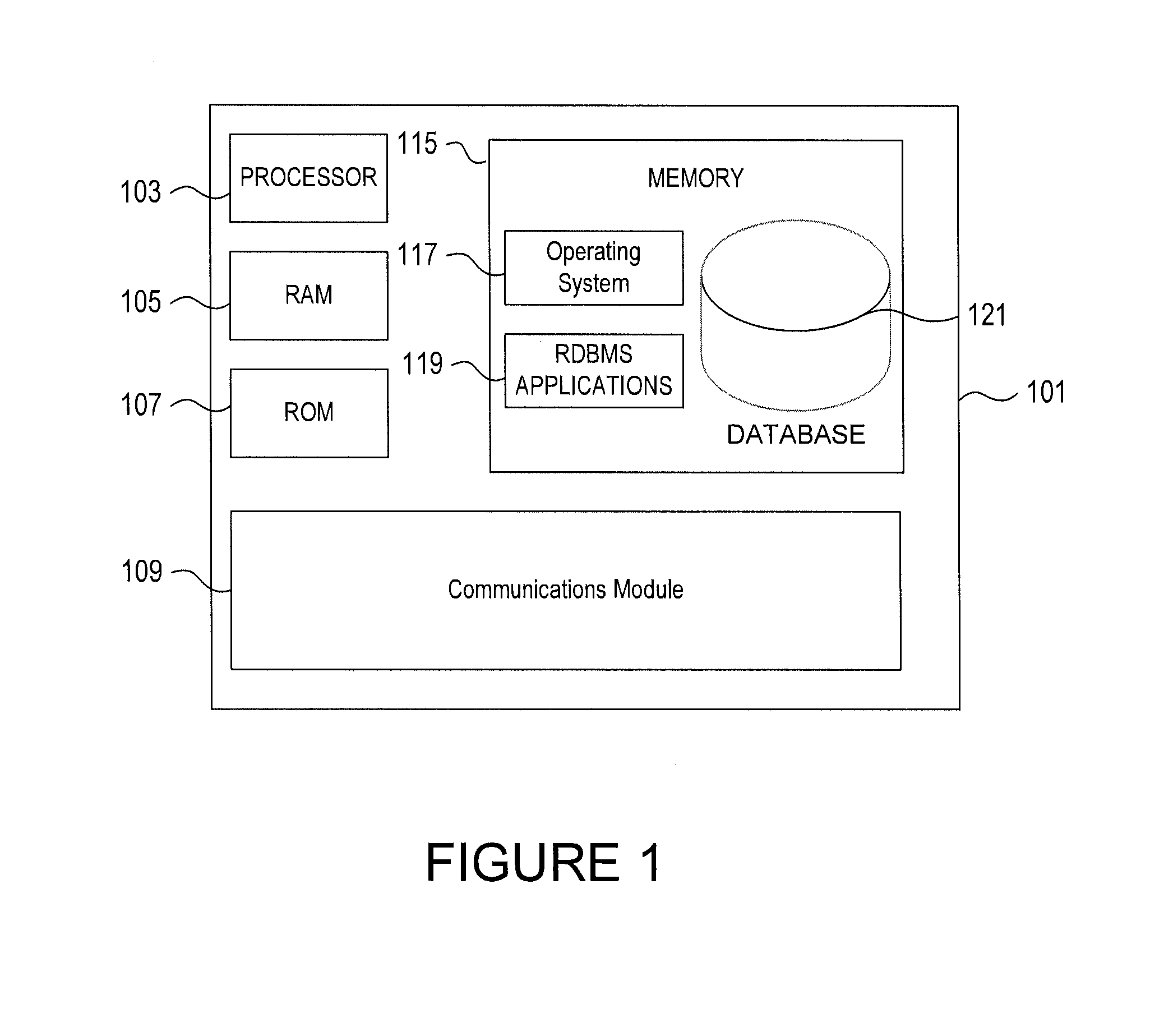
InactiveUS20080065315A1Road vehicles traffic controlNavigation instrumentsDisplay devicePositioning system
Associating location information with forensic data is disclosed. A location information module may include an antenna, a controller and a display. The location information module may be included in or be removably coupled to a forensic device, and may be removably coupled with a computing device or other device. The location information module may obtain location information from a location system such as a global position system (GPS) or other system. The location information module may prompt a user to remove the location information module from a forensic device, computing device or other device and instruct the user to go to a place with good reception. The location information may be transferred to the forensic device, computing device, or other device where it may be paired with forensic data, hard disk data and / or other data and / or information.
Owner:LOGICUBE
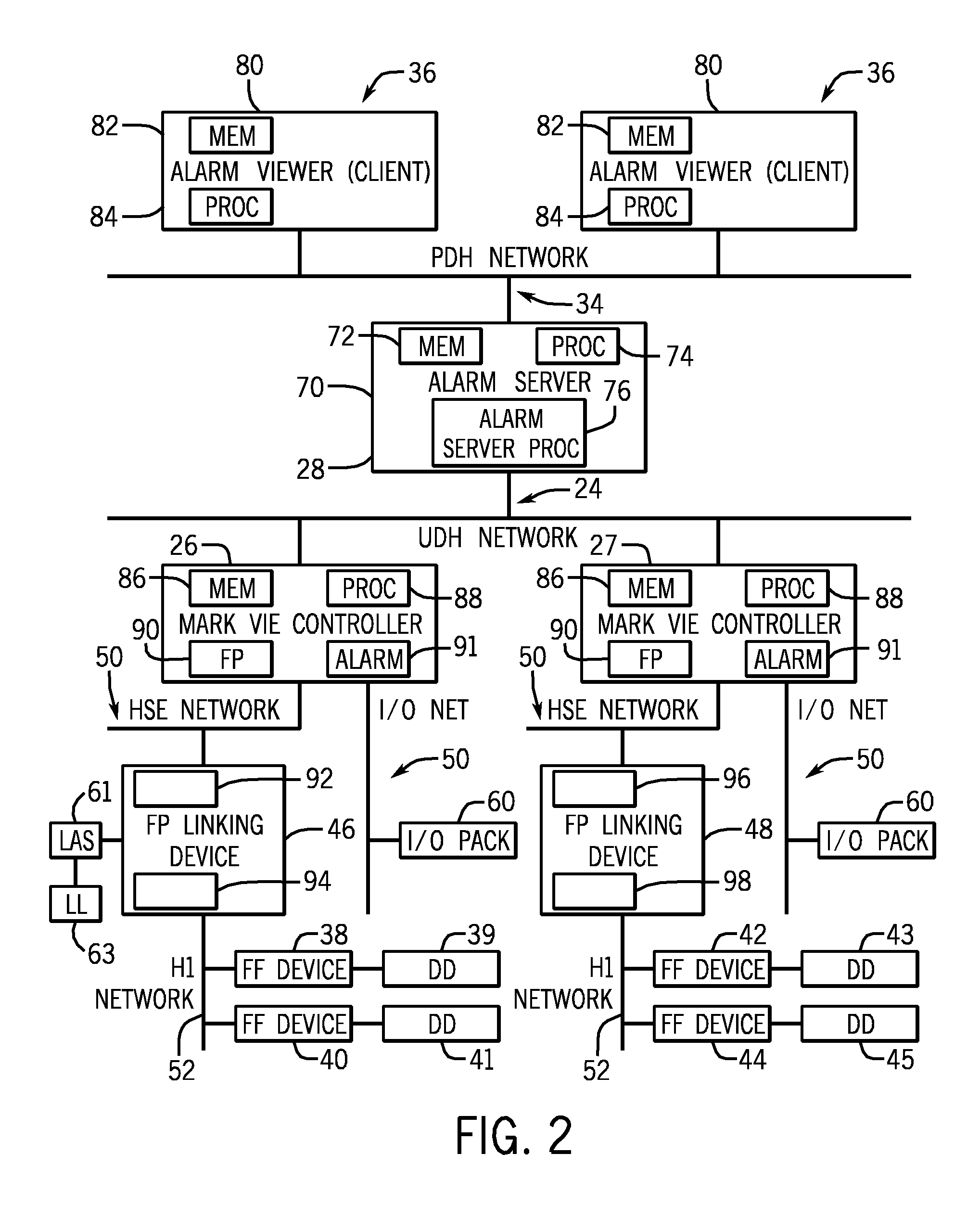
Systems and methods for alert device removal
ActiveUS20120306658A1Minimize or eliminate human involvementMinimizing or eliminating human involvementProgramme controlAlarmsControl systemField device
The embodiments described herein include systems and methods for removal of alerts for a device. In one embodiment, an industrial process control system includes a controller a controller coupled to a field device. The industrial process control system further includes an alert server coupled to the controller. The controller is configured to detect, via a first protocol, removal of the field device and to communicate, in a second protocol, the removal of the field device to the alert server.
Owner:GENERAL ELECTRIC CO
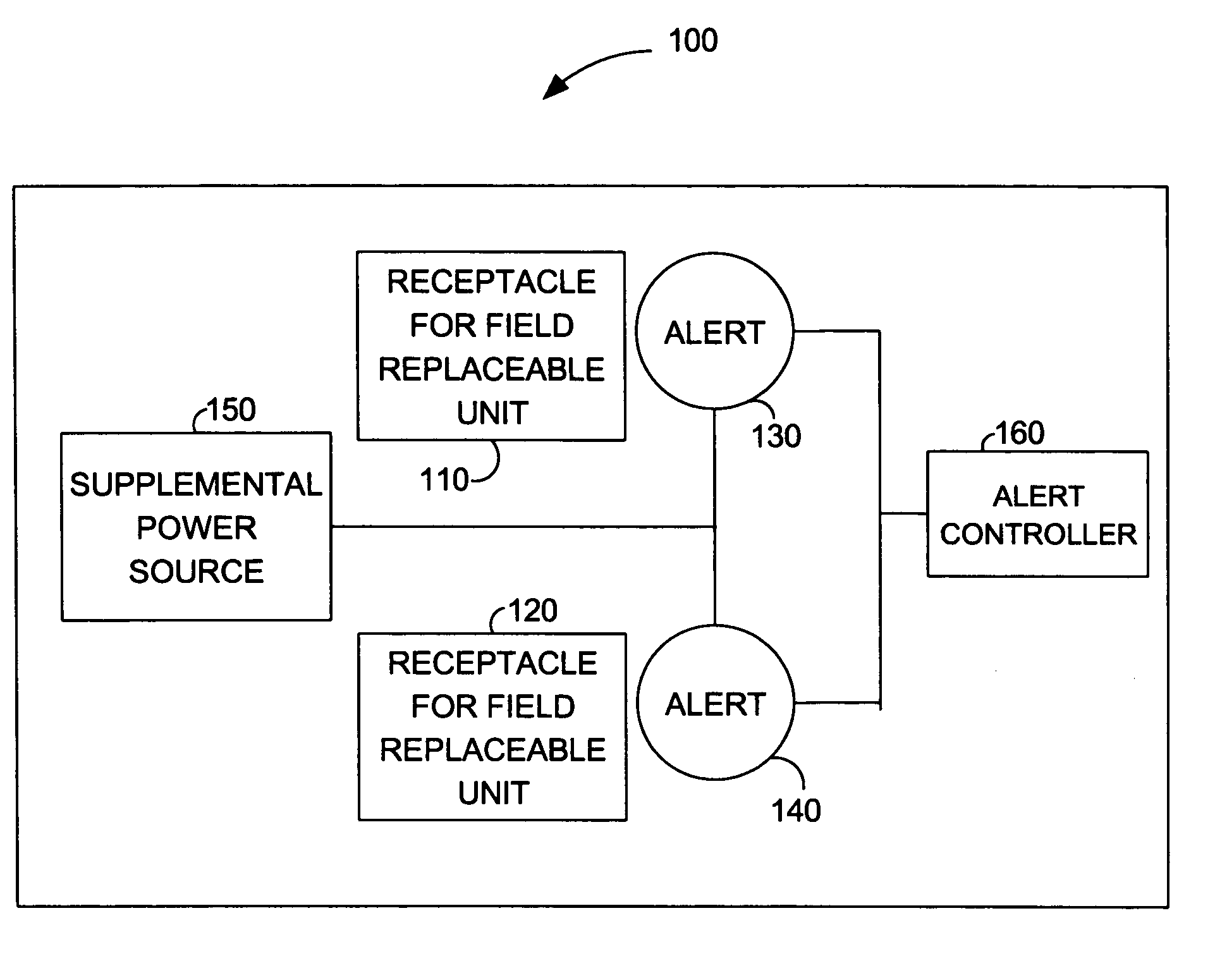
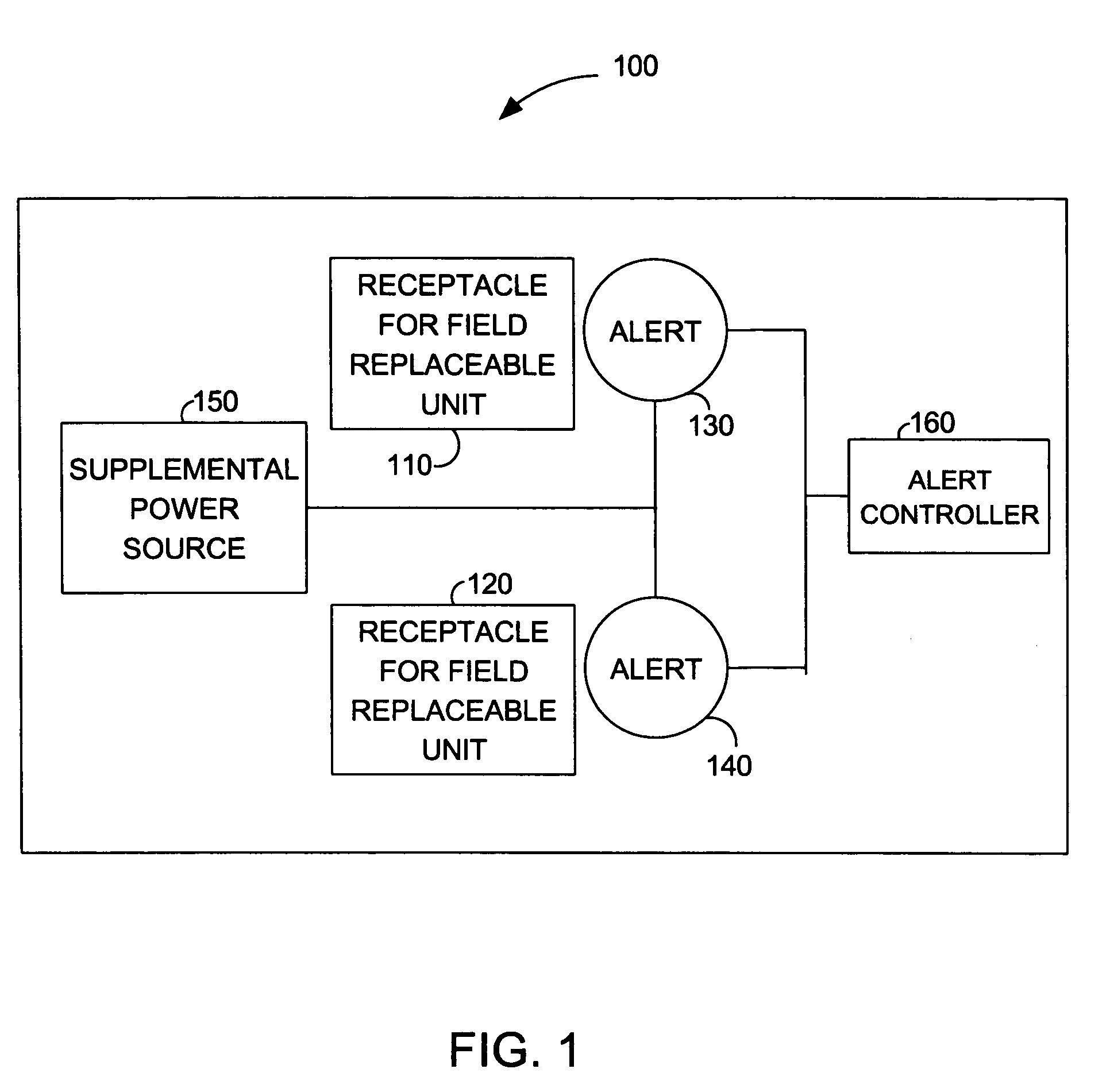
Alert for indicating field replaceable unit status and configuration
ActiveUS7315251B1Error detection/correctionPower supply for data processingField replaceable unitEngineering
The present invention is a method and system for indicating field replaceable unit status and configuration. A visible alert may be mounted on a card, in proximity to a location for a field replaceable unit. The visible alert may indicate a failed replaceable unit or a location for installation of a new replaceable unit. Supplemental power may be available on the card whereby a visible alert may be operable when an appliance, which contains the field replaceable unit, is powered down or when the card has been removed from the appliance. Additionally, an appliance in accordance with the present invention may include cards with visible alerts associated with field replaceable units. The visible alerts may be visible to an administrator without opening the chassis of the appliance.
Owner:NETWORK APPLIANCE INC
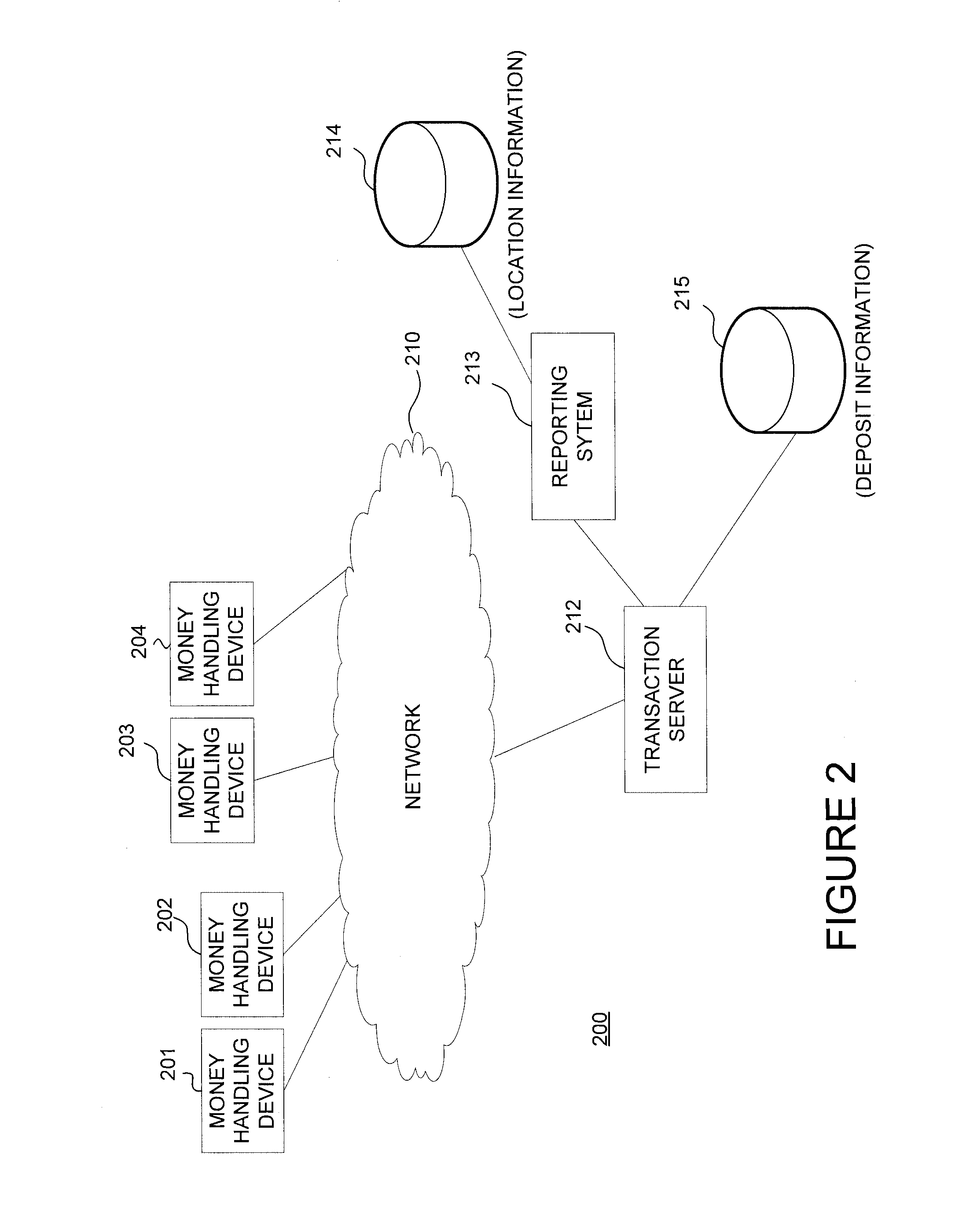
Forecasting of Deposits for a Money Handling Machine
InactiveUS20140074708A1Improve convenienceReduce adverse effectsComplete banking machinesFinanceNatural disasterComputerized system
A computer system obtains deposit information from a money handling device and projects a number of deposited checks at the money handling device at a subsequent time. The transaction computer determines whether the projected number of deposited checks exceeds a check threshold and generates an indicator whether deposits should be removed from the money handling device based on the determination. The number of deposited checks may be tracked at the money handling device and, if the tracked deposits are trending differently from the projected number of deposited checks, the generated indicator may be adjusted. The projected number of deposited checks at a money handling device may be compensated based on an event such as a special event or holiday. Also, the projected number of deposited checks at the money handling device may be modified when a natural disaster such as a hurricane occurs.
Owner:BANK OF AMERICA CORP
Network integrity maintenance
InactiveUS20110238983A1Minimize storageDoubling sizeKey distribution for secure communicationComputer hardwareWeb service
A device removal system securely removes an item of content or a device from a content-protected home network. An authorization table maintains a list of devices in the content-protected home network in addition to removed devices. The authorization table also maintains a list of deleted content. Through management of various cryptographic keys and techniques, devices and content will not play on a content-protected home network after they have been removed. A secret network ID reduces the possibility of unauthorized playing of content on the content-protected home network. A web server may join the content-protected home network as a device, providing backup for the secret network ID. Otherwise, the device manufacturer will provide the secret network ID in case of a device failure. Storing a verification value in each device ensures integrity of critical cryptographic values. This verification value is compared to network values to ensure network values have not been corrupted.
Owner:INT BUSINESS MASCH CORP
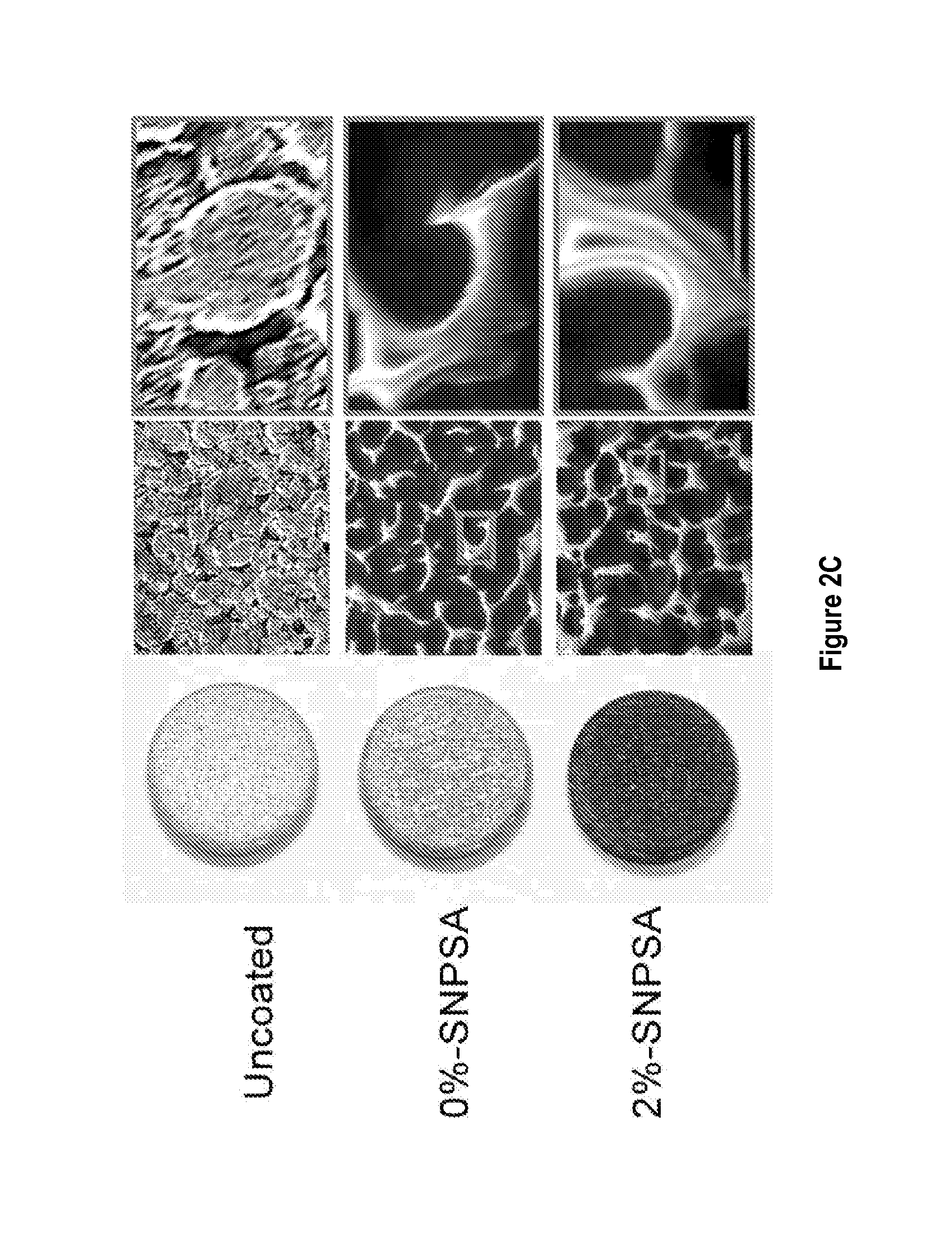
Nanoparticle-based scaffolds and implants, methods for making the same, and applications thereof
ActiveUS20140287018A1Reducing and eliminating microbial infectionEliminate and reduce sourceBiocideDental implantsAntibacterial activityAlloy
Implant-associated bacterial infections are one of the most serious complications in orthopedic surgery. Treatment of these infections often requires multiple operations, device removal, long-term systemic antibiotics, and extended rehabilitation, and is frequently ineffective, leading to worse clinical outcomes and increased financial costs. Silver nanoparticle / poly(DL-lactic-co-glycolic acid) (PLGA)-coated stainless steel alloy (SNPSA) was evaluated as a potential antimicrobial implant material. It was found that SNPSA exhibited strong antibacterial activity in vitro and ex vivo, and promoted MC3T3-E1 pre-osteoblasts proliferation and maturation in vitro. Furthermore, SNPSA implants induced osteogenesis while suppressing bacterial survival in contaminated rat femoral canals. The results indicate that SNPSA has simultaneous antimicrobial and osteoinductive properties that make it a promising therapeutic material in orthopedic surgery.
Owner:RGT UNIV OF CALIFORNIA
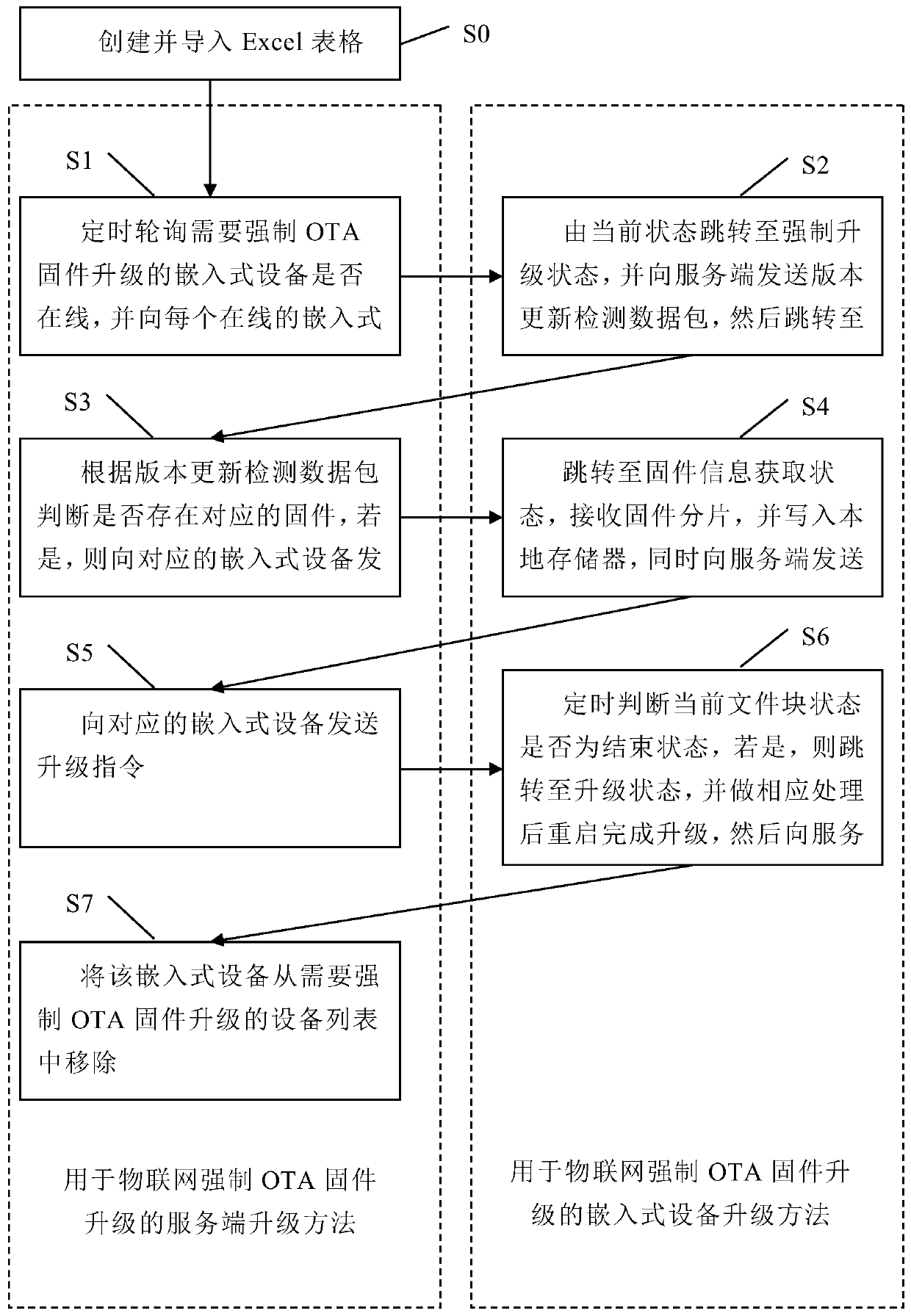
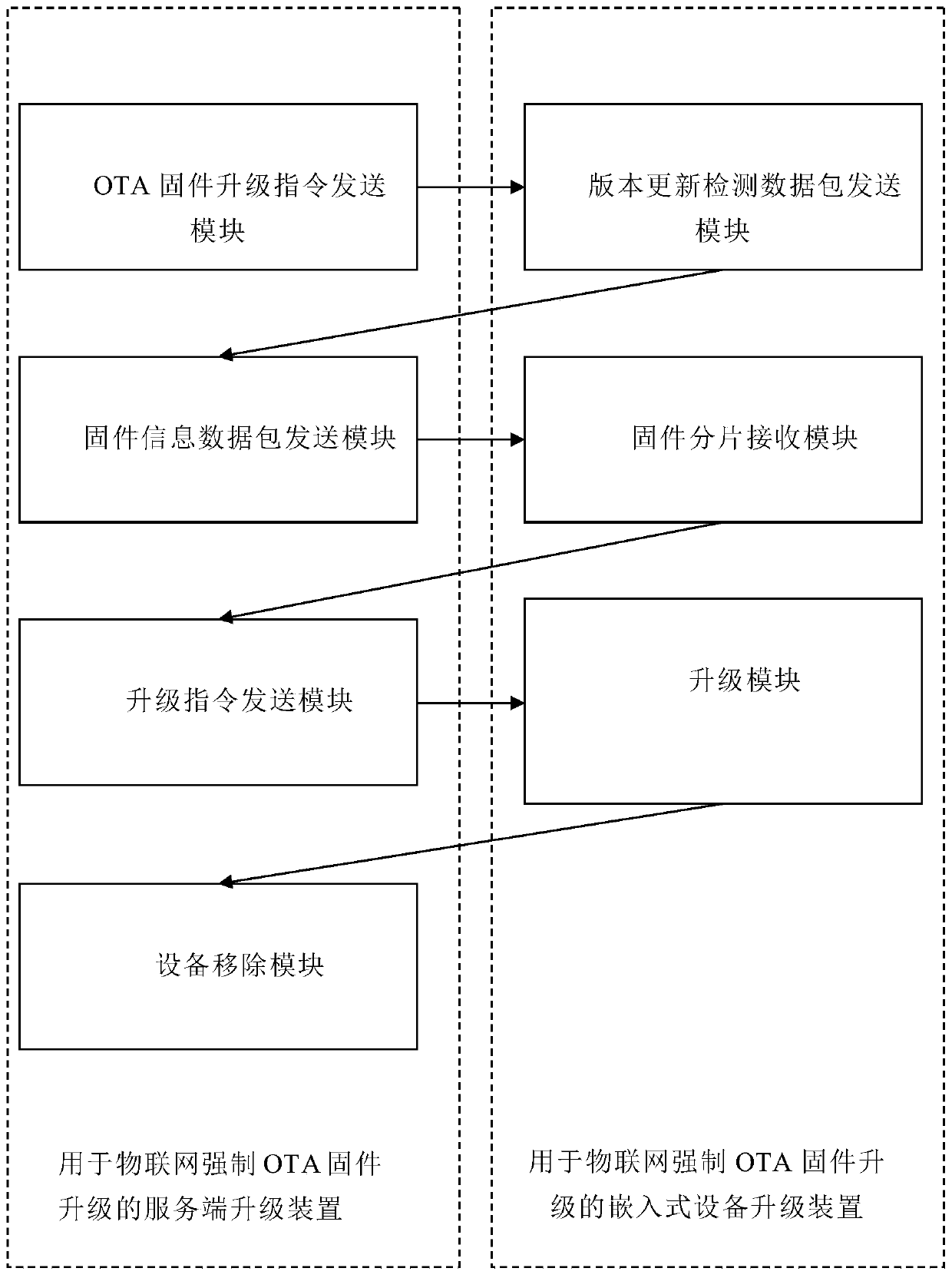
Server for OTA upgrading and embedded device upgrading method and device
PendingCN110633092ATroubleshoot upgrade errorsReduce maintenance costsVersion controlTransmissionNetwork packetInformation data
The invention discloses a server for OTA upgrading and an embedded device upgrading method and device. The method comprises the following steps: a server sends a forced OTA firmware upgrading instruction to each online embedded device according to a list, and the embedded device sends a version updating detection data packet to the server, the server returns a firmware information data packet to the corresponding embedded device, the embedded device receives the firmware fragments, and after upgrading is completed, the server deletes the device from the list. The server upgrading device comprises an OTA firmware upgrading instruction sending module; a firmware information data packet sending module; an upgrade instruction sending module; and a device removal module. The embedded device upgrading device comprises a version updating detection data packet sending module, a firmware fragmentation receiving module; and an upgrade module. The traditional OTA firmware upgrading process is bypassed, the problem of firmware upgrading errors caused by human negligence can be effectively solved, and the maintenance cost is reduced.
Owner:北京方研矩行科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com