Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "CD8A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD8a (Cluster of Differentiation 8a), is a human gene.
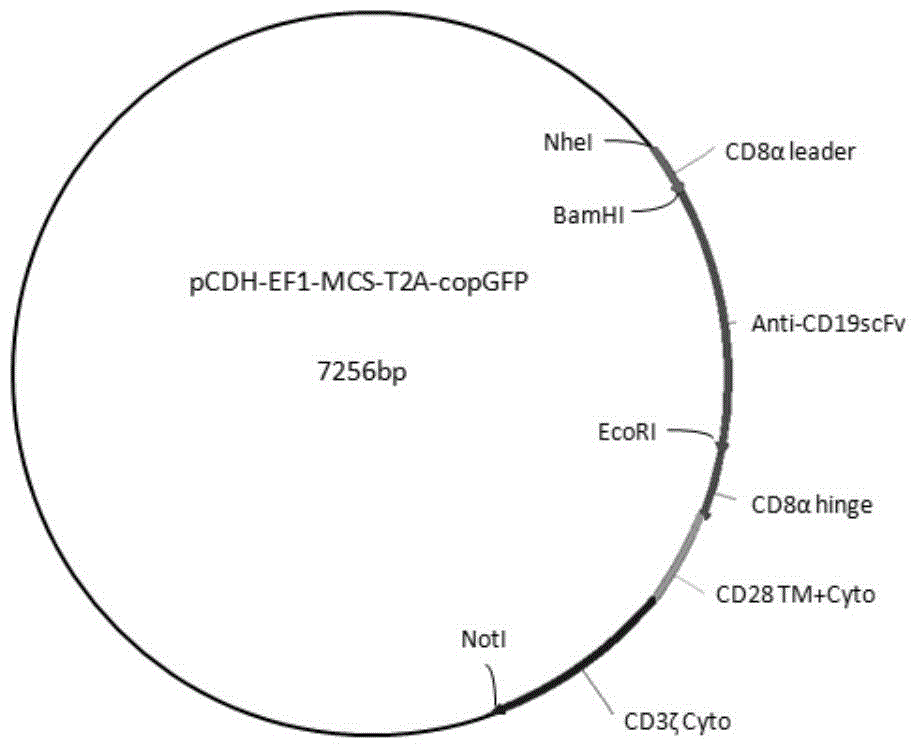
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
InactiveCN105837693AImprove anti-apoptotic abilityImprove bindingAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen
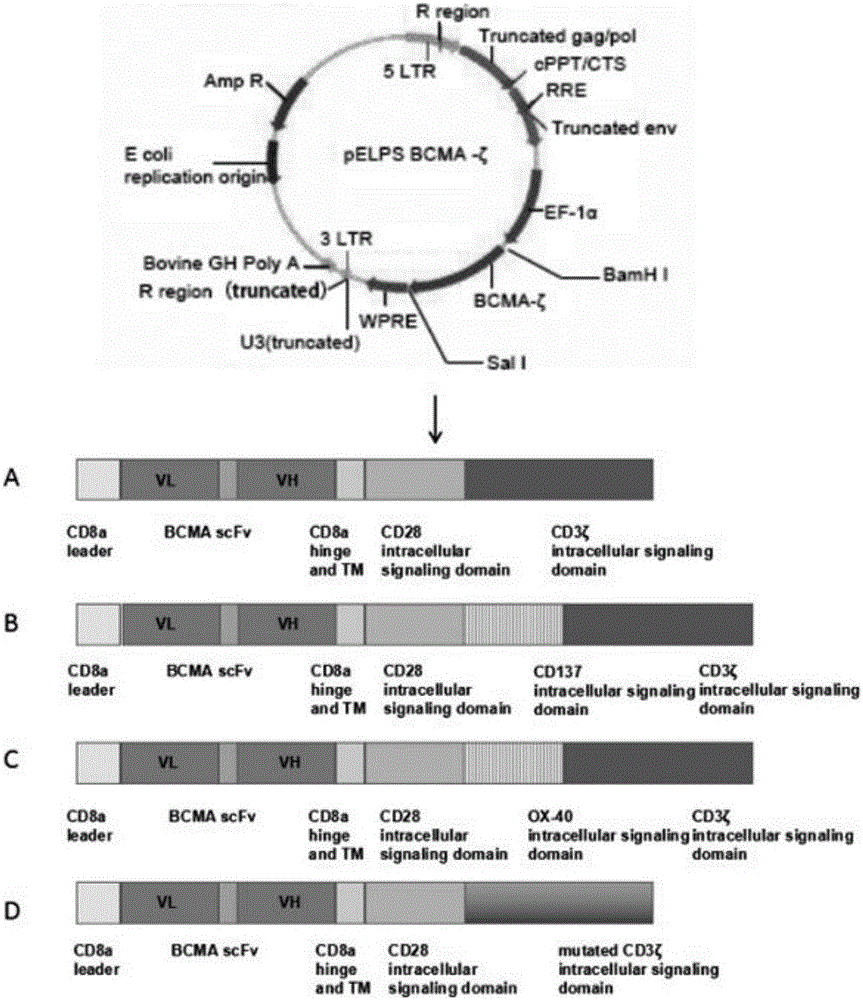
The invention provides a BCMA-based (B cell maturation antigen-based) chimeric antigen receptor, comprising following cis-form cascade domains: CD8a leader region, scFv fragment BCMA scFv of anti-BCMA antibody, CD8a hinge region and transmembrane region, CD28 intracellular signal domain and CD3 Zeta intracellular signal domain; the CD3 Zeta intracellular signal domain is wild CD3 Zetaintracellular signal domain or mutant CD3 Zeta mut intracellular signal domain; the BCMA-based chimeric antigen receptor can enhance anti-apoptotic capability of T-cells and enhance CAR T-cell and antigen bonding and signal conduction; T-cells with the BCMA-based chimeric antigen receptor show good tumor targeting performance in in-vivo experiments, tumors diminish significantly after two weeks of dosage, and the T-cells have excellent therapeutic effect in vivo.
Owner:李斯文 +1
Anti-tumor NK cell, and preparation method and application thereof
ActiveCN110964697AStrong cytotoxicityGood effectImmunoglobulinsBlood/immune system cellsSingle-Chain AntibodiesNatural Killer Cell Inhibitory Receptors
The invention provides an anti-tumor NK cell, and a preparation method and application thereof. The anti-tumor NK cell is an NK cell edited by a chimeric antigen receptor. The chimeric antigen receptor comprises an extracellular domain fragment of PD-1, an F2A peptide fragment, the extracellular region of a CD8a signal peptide, the extracellular variable region of a single-chain antibody capable of recognizing and binding to HER2 protein, a CD8 alpha Linker region, a CD28 transmembrane and intracellular synergistic co-stimulation region, and a CD3 zeta fragment for intracellular signal transmission. The NK cell disclosed by the invention can express a CAR element targeting HER2 on a cell membrane and express secretory PD-1 protein at the same time, so the binding of PD-1 and PD-L1 is blocked, and the killing function of the NK cell is increased; and the anti-tumor NK cell is used for preparing anti-tumor drugs, can target tumor cells and enhance cytotoxic effect, is low in immunogenicity, easily realizes response and activation and can achieve allogeneic reinfusion, and the tumor killing effect of the NK cell is remarkably improved.
Owner:OCEAN UNIV OF CHINA
BCMA specific chimeric antigen receptor T cell and application thereof
ActiveCN109748968AGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesT cell
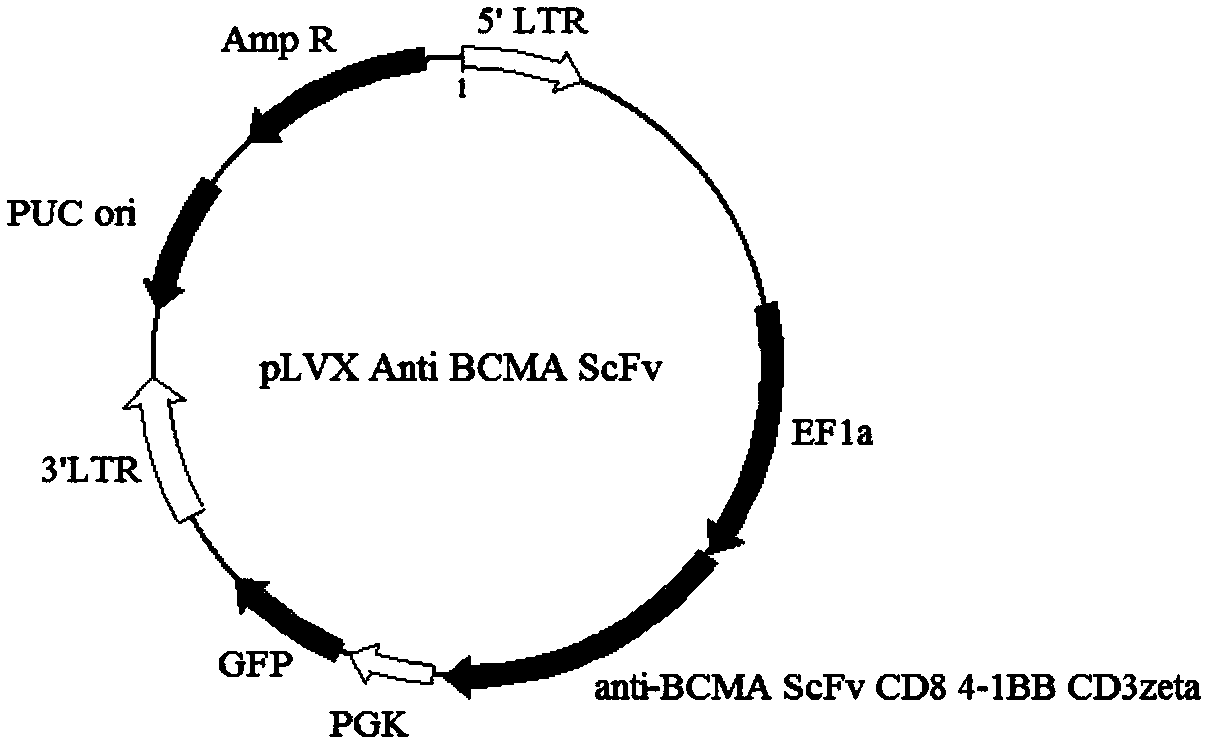
The invention discloses a specific chimeric antigen receptor (anti-BCMA scFv-CD8a-41BB-CD3 zeta) for human BCMA and application thereof. The chimeric antigen receptor is formed by a single-chain-antibody anti-BCMA scFv, a hinge region, a transmembrane region and an intracellular region in series. The chimeric antigen receptor is used for modifying human T lymphocyte, and the modified T lymphocyteis used for prevention and treatment of surface BCMA positive tumor and preparing of antitumor medicines.
Owner:广州因明生物医药科技股份有限公司
Recombinant plasmid, recombinant malaria parasite and its application
ActiveCN106687592AConducive to long-term existenceFacilitated releaseTumor rejection antigen precursorsProtozoaBacteroidesMalaria
The present invention relates to the field of cellular immunotherapy for tumors and, more particularly, to a recombinant plasmid for the construction of recombinant malaria parasites and their use, wherein the recombinant plasmid is a tumorigenin-specific antigen gene inserted into the pL0017 plasmid, Recombinant malaria parasite, comprising the recombinant plasmid. Compared with plasmid DNA and RNA vector, the recombinant malaria parasite can be multiplied by the proliferation of Plasmodium, which is beneficial to the increase of the antigen in vivo. Compared with the defective virus and bacterial carrier, it survives in the red blood cells of the body longer, not short-term by the body's immune system to clear, long-term effective expression of exogenous tumor antigen, is conducive to long-term antigen and immune stimulation. The recombinant malaria parasite is capable of activating the high expression of Th1-related cytokines in vivo and increasing the proportion of CD8a + DCs in total CD11c + DCs and further activating specific cytotoxic T lymphocyte responses against tumor antigens, which is beneficial to the antitumor effect of the vaccine.
Owner:BLUE ELEGANT BIOTECH CO LTD
Chimeric antigen receptor, gene thereof, recombinant expression vector, CARHER2-NKT cell, and preparation method and application of CARHER2-NKT cell
PendingCN108395480AHigh infection efficiencyProlong survival timeMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilyNatural killer T cellWilms' tumor
The invention belongs to the technical field of tumor biological products, and discloses a chimeric antigen receptor, a gene thereof, a recombinant expression vector, an engineered HER2 targeting NKTcell, and an application of the NKT cell. The chimeric antigen receptor is HER2ScFv-CD8-CD137-CD3zeta and comprises a CD8a signal peptide, HER2ScFv, a hinge region and transmembrane region for shortening CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3zeta which are in series. The CARHER2-NKT cell provided by the invention has a certaintherapeutic effect on epithelial tumors when used for curing advanced HER2 positive epithelial tumors
Owner:GUANGZHOU BAINIFU BIOTECH CO LTD
Chimeric antigen acceptor DAP12-T2A-CD8a-CD19scfv-NKp44 and application thereof
InactiveCN109503717AMild release responseEnsure safetyAntibody mimetics/scaffoldsMammal material medical ingredientsAntiendomysial antibodiesAntigen receptors
The invention discloses an optimized chimeric antigen acceptor DAP12-T2A-CD8a-CD19scfv-NKp44 and application thereof. The chimeric antigen acceptor is formed by serially connecting an intracellular second conduction structural domain DAP12, T2A, a CD8a signal peptide, an anti-human CD19 monoclonal antibody FMC63 light-chain and heavy-chain variable region CD19scFv, and an intracellular first conduction structural domain. The chimeric antigen acceptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cells) can be used for treatment of CD19 positive hematologic malignancy. In a hematologic malignancy killing test, killing capacity of the CAR-T cells to leukemia tumor cells are obviously enhanced, and good safety and anti-tumor activity are shown in the clinical application.
Owner:NANJING CART MEDICAL TECH LTD
X-linked lymphoproliferative syndrome diagnostic kit and application thereof
InactiveCN107037219AQuick checkImprove throughputIndividual particle analysisBiological testingX-Linked Lymphoproliferative SyndromeFluorescence
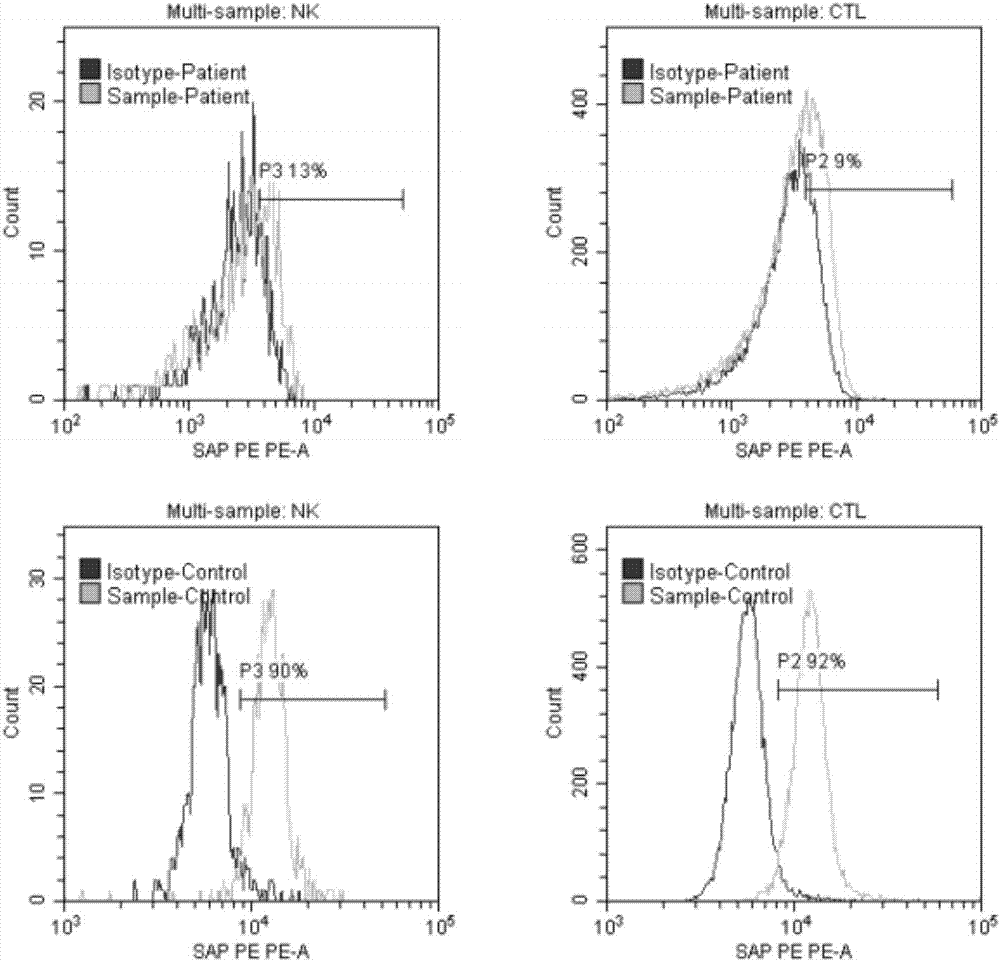
The invention relates to the technical field of medical diagnosis, and specifically relates to an X-linked lymphoproliferative syndrome diagnostic kit and application thereof. The kit comprises anti-human CD 3, CD56, CD8a and SAP flow fluorescent antibodies, an isotype control fluorescent antibody of the anti-human SAP flow fluorescent antibody, an anti-human SAP Western Blot antibody, and a secondary antibody corresponding to the anti-human SAP Western Blot antibody. A method for detecting SAP protein abundance in CTL and NK cells based on the diagnostic kit is quick to detect, high in flux, high in accuracy, and small in human factor influence.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Tumor cell dryness restriction-type CAR and application thereof
ActiveCN111423517AGood application effectEfficient killingAntibody mimetics/scaffoldsNucleic acid vectorDiseaseTumor cells
The invention belongs to the technical field of tumor cell immunotherapy, and particularly relates to a restriction-type CAR capable of inhibiting the dryness of tumor cells and application of the CAR. The chimeric antigen receptor is an amino acid sequence obtained by connecting a plurality of protein fragments, and is obtained by sequentially connecting a human CD8a molecule signal peptide CD8a,a human NKG2D extracellular region, a human CD8 molecule transmembrane region and a 41BB molecule intracellular region 41BB, and a human CD3z molecule intracellular region CD3Zeta. In the preferred design, the chimeric antigen receptor is further obtained by connecting a connecting sequence to an IL24 CDS region sequence IL24. By further optimizing the structure of the CAR and simultaneously in combination with SFN application, the chimeric antigen receptor has good technical effects on effectively killing tumor cells and tumor stem cells, reducing the recurrence of diseases and improving theapplication effect of CAR-T cells, so that the has good practical value and popularization and application significance.
Owner:赛德特生物制药有限公司
Chimeric antigen receptor, gene thereof and recombinant expression vector of gene as well as CAR133-NKT cell and preparation method and application of CAR133-NKT cell
InactiveCN107793482AEnhance the ability to target and recognize CD133 antigen on the surface of cancer cellsEnhance specific killing activityAntibody mimetics/scaffoldsMammal material medical ingredientsAntigen receptorsTherapeutic effect
The invention belongs to the field of tumor biological products, and discloses a chimeric antigen receptor and a gene thereof and a recombinant expression vector of the gene as well as a CAR133-NKT cell and a preparation method and application of the CAR133-NKT cell. The chimeric antigen receptor is CD133ScFv-CD8-CD137-CD3 zeta including tandem a CD8a signal peptide, a CD133ScFv, CD8 hinge and transmembrane regions, a CD137 intracellular signal domain and a CD3 zeta intracellular signal domain. The CAR133-NKT cell provided by the invention has very good specific killing activity against a CD133-positive epithelial tumor cell when co-culture is performed with the CD133-positive epithelial tumor cell; and the CAR133-NKT cell provided by the invention has a certain therapeutic effect on an epithelial tumor when treating the CD133-positive epithelial tumor.
Owner:GUANGZHOU BAINIFU BIOTECH CO LTD
Diagnostic and therapeutic methods for cancer
PendingCN110621787AOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesEfficacy
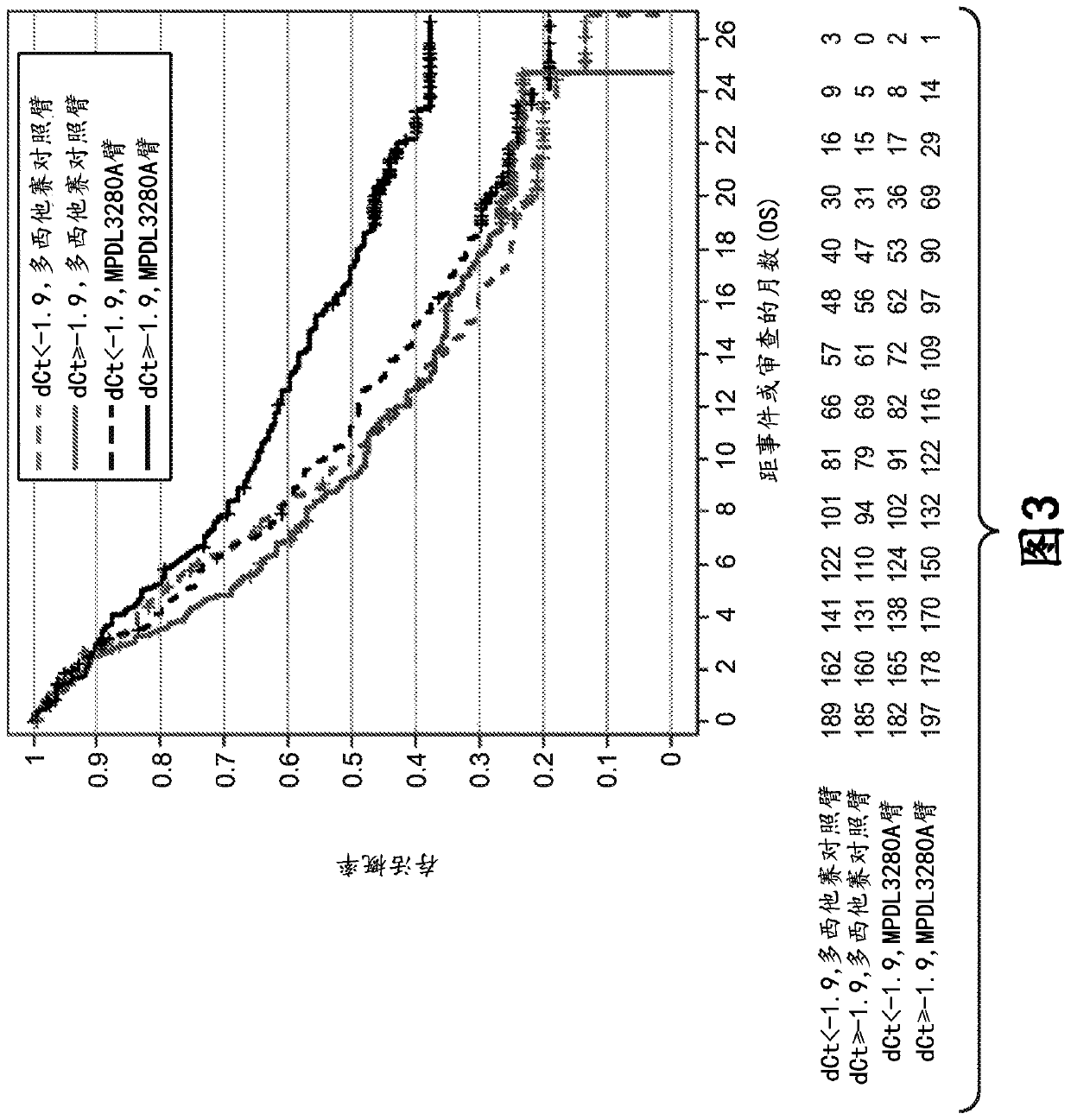
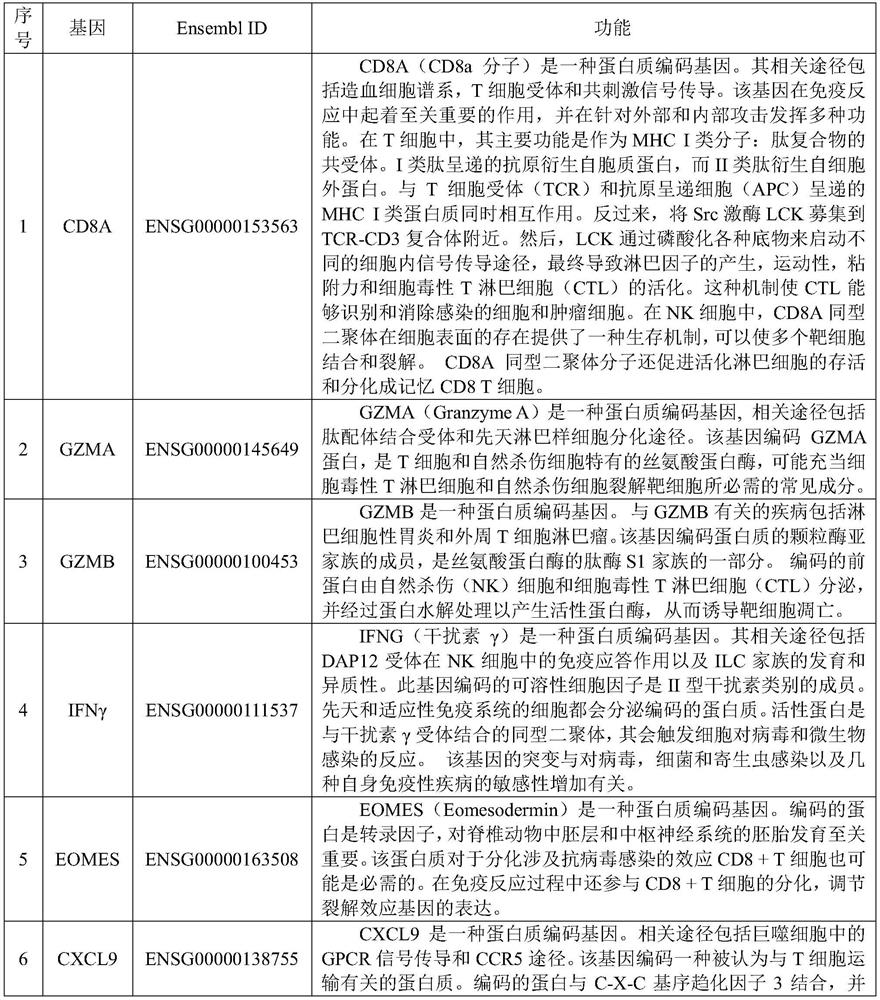
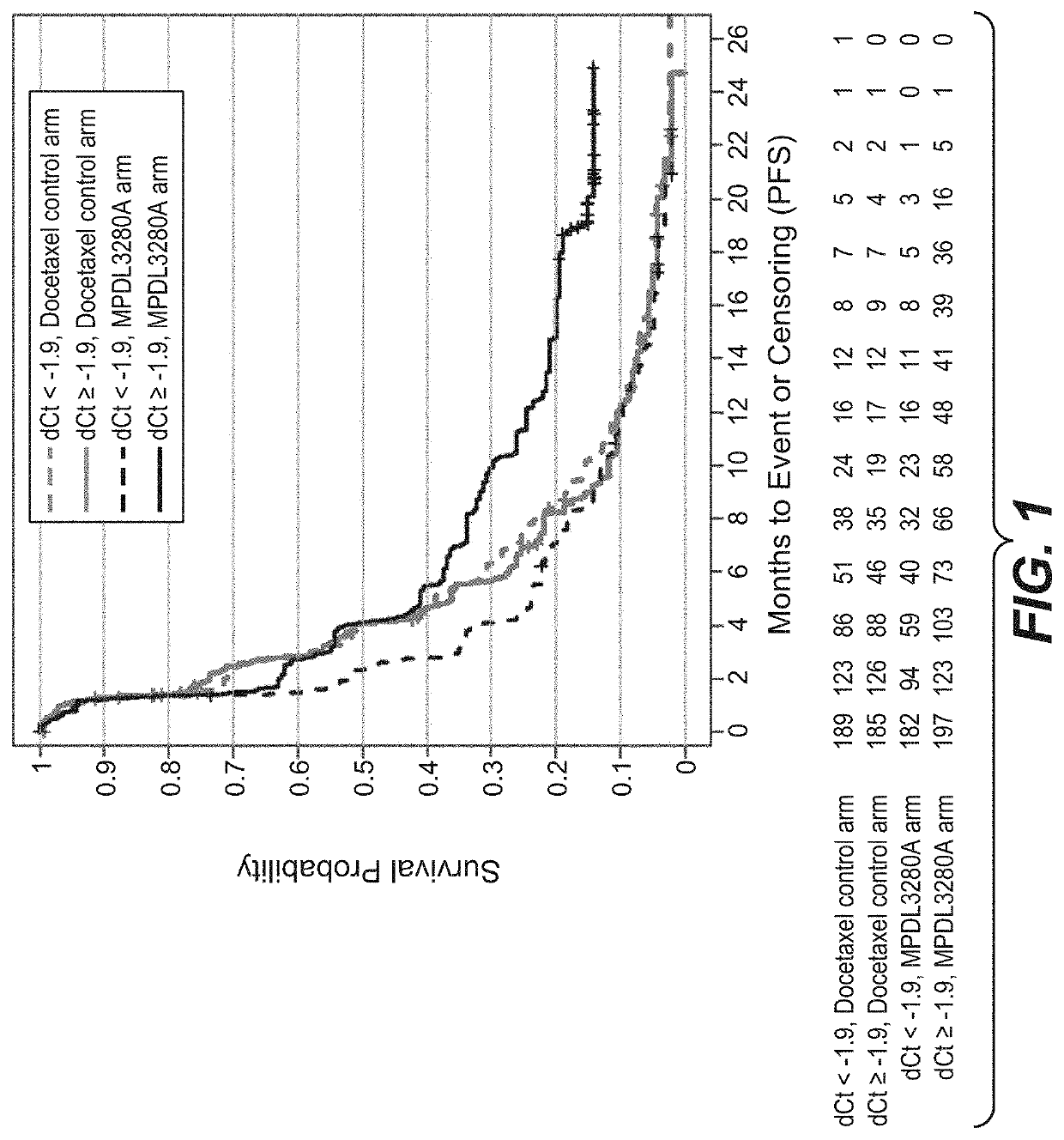
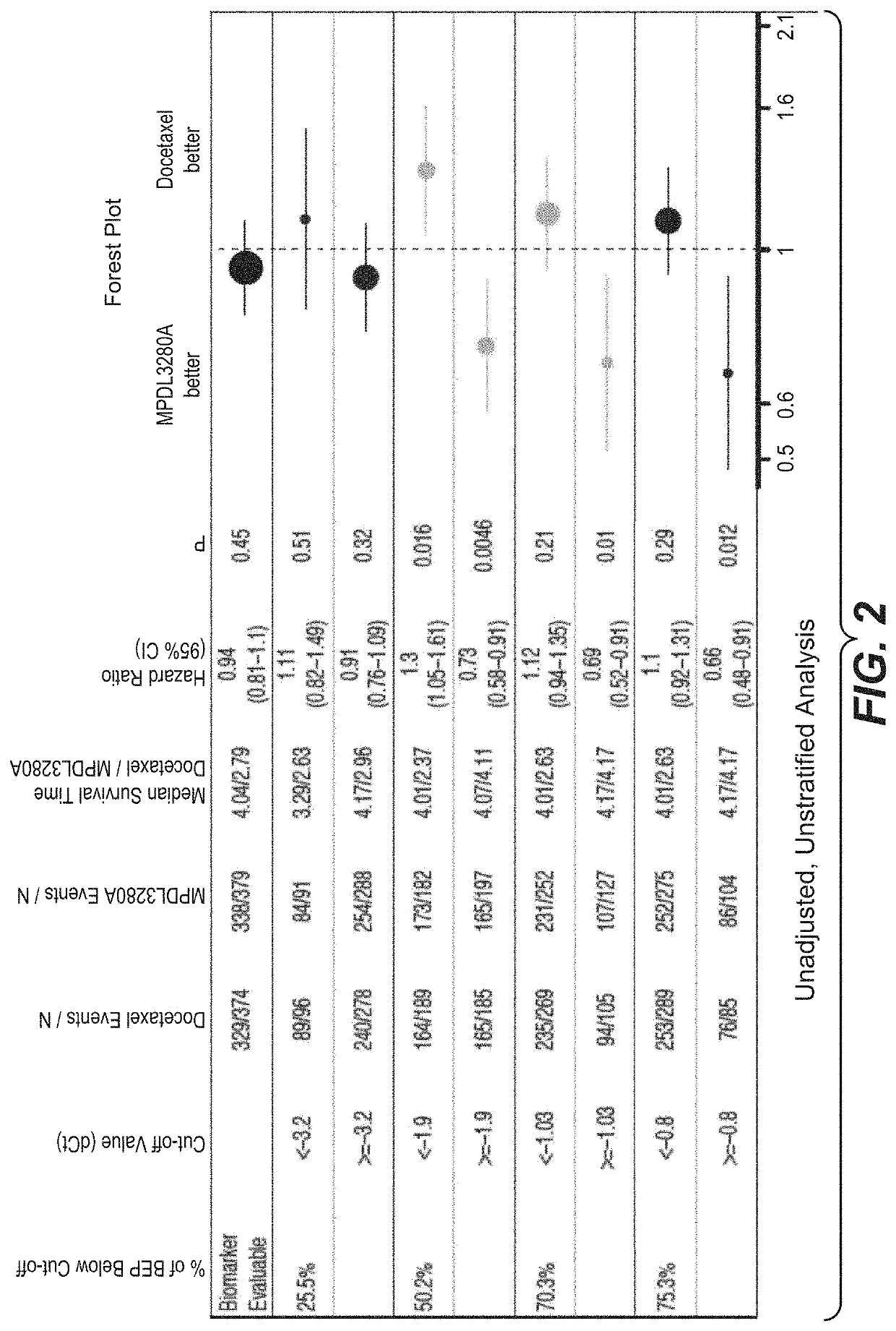
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:F HOFFMANN LA ROCHE & CO AG
Prognostic and treatment response predictive method
PendingUS20210139999A1Easy to sign forImprove predictive performanceMicrobiological testing/measurementBiostatisticsCCL2IL12A
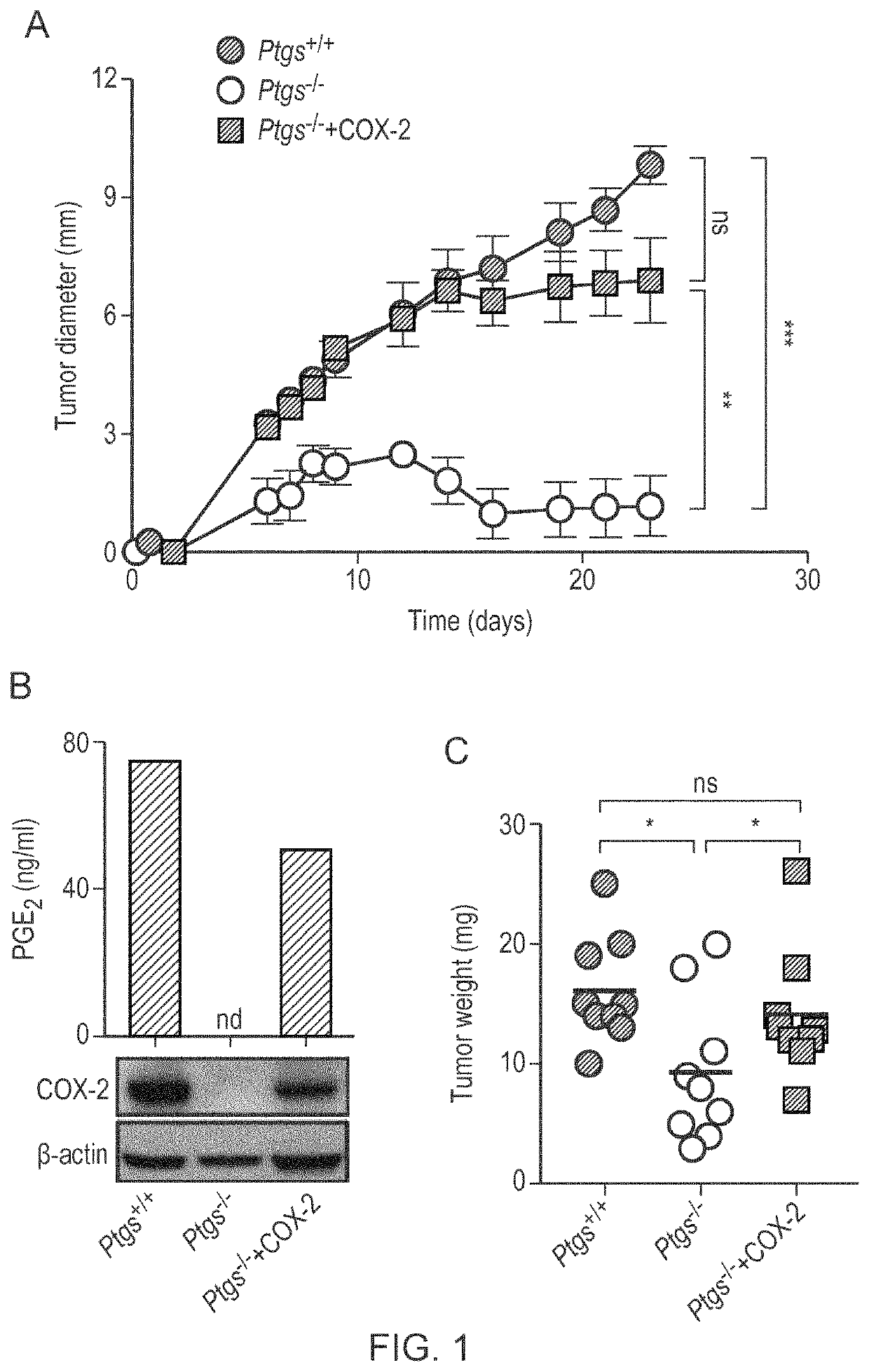
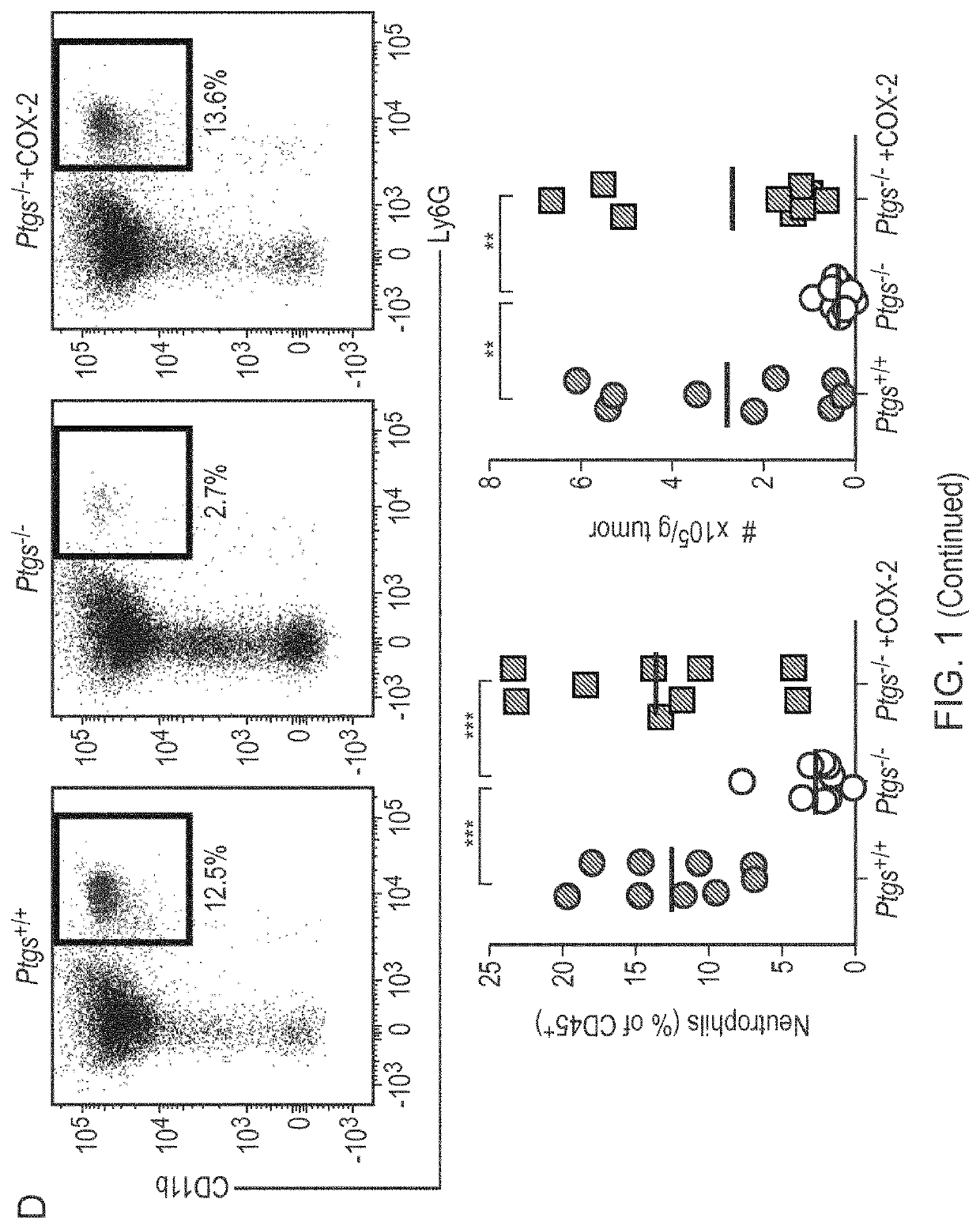
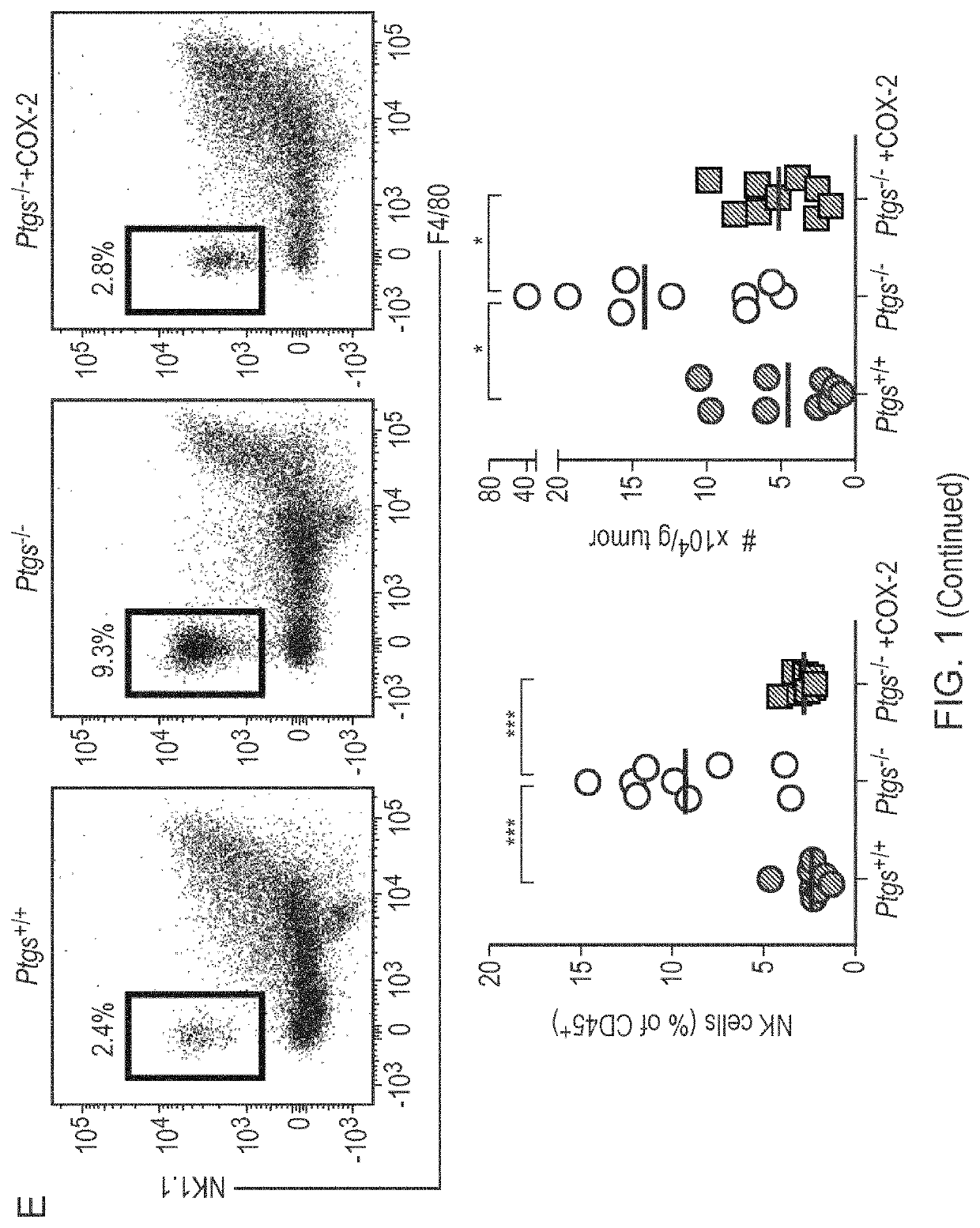
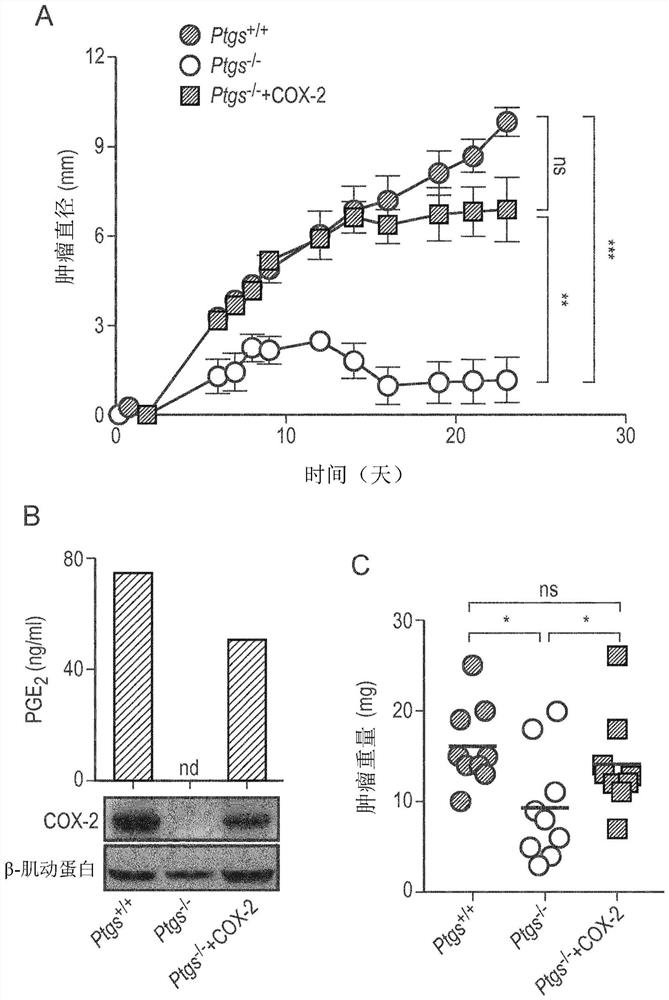
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of at least 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL A in a sample obtained from the tumour of the patient; b) measuring the gene expression of at least 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained from the tumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including with immune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Diagnostic methods and compositions for cancer immunotherapy
PendingCN113260633AOrganic active ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesAntigen Binding Fragment
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The compositions and methods described herein can be used, for example, to determine the propensity of a patient to benefit from treatment with a PD-L1 axis binding antagonist and to treat such patients accordingly. Using the compositions and methods of the disclosure, a patient, such as a human cancer patient, may be determined to be likely to benefit from treatment with a PD-L1 axis binding antagonist if the patient exhibits an elevated pre-treatment expression level of one or more of CST7, NKG7, GZMH, MT-ND4, HLA-H, CCL5, CD8A, CMC1, CD8B, HCST, MT-CYB, MT-ND4L, KLRG1, MT-CO2, MT-ATP6, PLEK, CTSW, HLA-C, LYAR, LITAF, GZMB, KLRD1, FGFBP2, KLRC4-KLRK1, KLRK1, B2M, GZMA, ID2, CX3CR1, PRSS23, GNLY, PRF1, and PATL2. Exemplary PD-L1 axis binding antagonists that may be used in conjunction with the compositions and methods of the disclosure are PD-L1 binding antagonists, such as anti-PD-L1 antibodies and antigen-binding fragments thereof, including atezolizumab, as well as PD-1 binding antagonists, such as anti-PD-1 antibodies and antigen-binding fragments thereof.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods for predicting the survival time and treatment responsiveness of a patient suffering from a solid cancer with a signature of at least 7 genes
ActiveUS11242564B2Increase productionEasier for the immune system to recognise and destroyMicrobiological testing/measurementAntineoplastic agentsCCL2Favorable prognosis
The present invention relates to a method for predicting the survival time of a patient suffering from a solid cancer comprising i) determining in a tumor sample obtained from the patient the gene expression level of at least 7 genes selected from the group consisting of CCR2, CD3D, CD3E, CD3G, CD8A, CXCL10, CXCL11, GZMA, GZMB, GZMK, GZMM, IL15, IRF1, PRF1, STAT1, CD69, ICOS, CXCR3, STAT4, CCL2, and TBX21, ii) comparing every expression level determined at step i) with their predetermined reference value and iii) providing a good prognosis when all expression levels determined at step i) are higher than their predetermined reference values, or providing a bad prognosis when all expression levels determined at step i) are lower than their predetermined reference values or providing an intermediate prognosis when at least one expression level determined value is higher than its predetermined value. The method is also particularly suitable for predicting the responsiveness of the patient to a treatment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
c-Met-targeted single-chain antibody, chimeric antigen receptor, recombinant vector, CAR-T cell and application
ActiveCN112552404AGood tumor suppressor effectGood killing effectAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSingle-Chain Antibodies
The invention provides a c-Met-targeted single-chain antibody, a chimeric antigen receptor, a recombinant vector, a CAR-T cell and application, and belongs to the technical field of tumor treatment. The amino acid sequence of the single-chain antibody is shown as SEQ ID No.2; and the chimeric antigen receptor comprises a CD8a signal peptide, a Flag label, the single-chain antibody, a CD8a hinge region-transmembrane region, a 4-1BB functional region and a CD3 functional region which are connected in sequence. The c-Met-targeted chimeric antigen receptor can be highly and specifically combined with a c-Met antigen, T cells modified by the c-Met-targeted chimeric antigen receptor can efficiently kill c-Met high-expression tumor cells in a targeting manner, and relatively ideal curative effects are achieved in vivo and in vitro.
Owner:BEIJING DCTY BIOTECH CO LTD +1
Single-chain antibody targeting c-Met, chimeric antigen receptor, recombinant vector, CAR-T cell and application thereof
ActiveCN111848797AGood tumor suppressor effectGood killing effectAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSingle-Chain Antibodies
The invention provides a single-chain antibody targeting c-Met, a chimeric antigen receptor, a recombinant vector, a CAR-T cell and an application thereof, and belongs to the technical field of tumortherapy, and the amino acid sequence of the single-chain antibody is shown as one of SEQ ID No. 1 to SEQ ID No. 3; wherein the chimeric antigen receptor comprises a CD8a signal peptide, a Flag label,the single-chain antibody, a CD8a hinge region-transmembrane region, a 4-1BB functional region and a CD3 functional region which are connected in sequence; the chimeric antigen receptor targeting c-Met can be highly specifically combined with a c-Met antigen, T cells modified by the chimeric antigen receptor targeting c-Met can efficiently kill c-Met high-expression tumor cells in a targeting manner, and ideal curative effects are achieved in vivo and in vitro.
Owner:BEIJING DCTY BIOTECH CO LTD +1
CXCL9 modified chimeric antigen receptor T (CAR-T) structure and application thereof
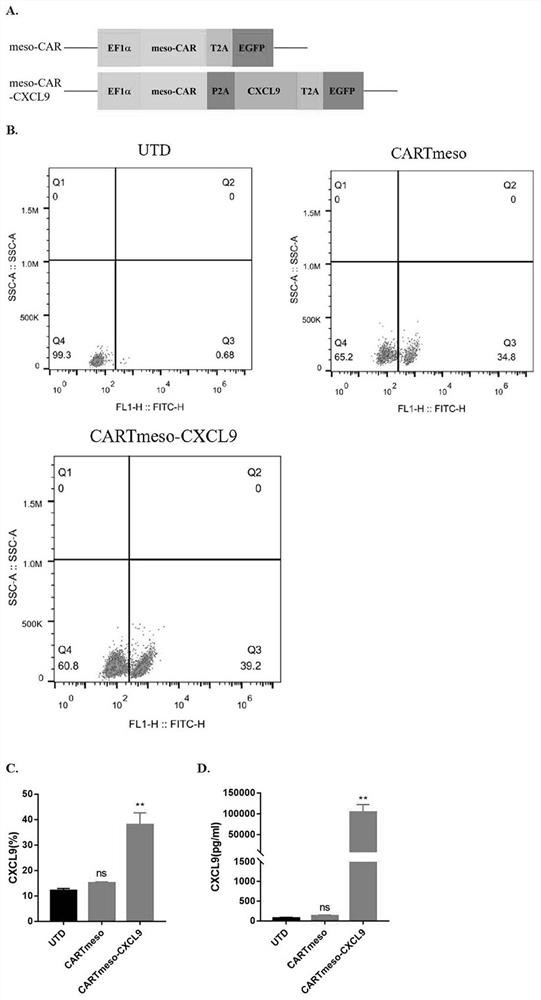
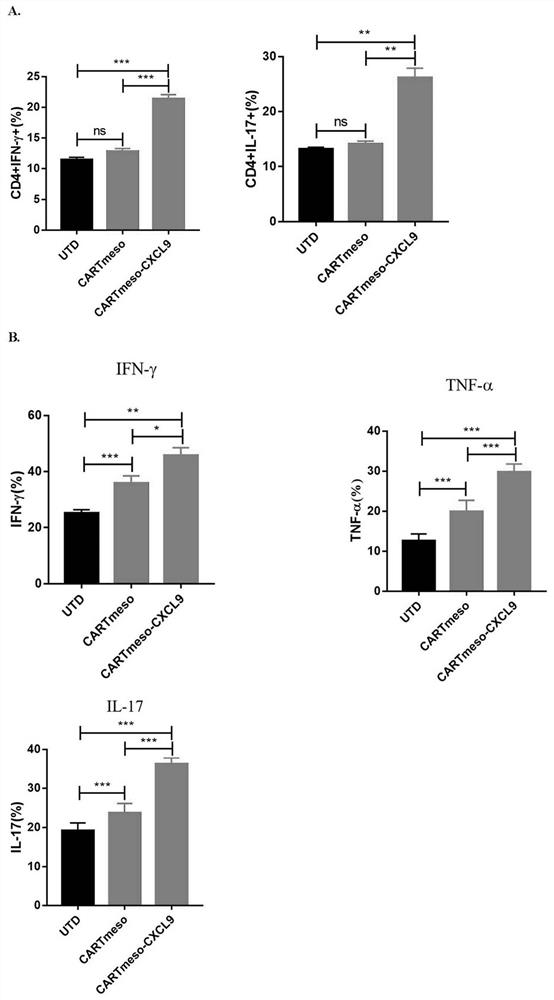
ActiveCN112625142APromote infiltrationPromote growthPolypeptide with localisation/targeting motifImmunoglobulin superfamilyOncologyCell therapy
The invention belongs to the technical field of tumor immunotherapy, and particularly relates to a CXCL9 modified chimeric antigen receptor T (CAR-T) structure and application patent application matter thereof. The CXCL9 modified CAR-T structure meso-CAR-CXCL9 has a specific structure: CD8a-meso-CD28-CD3Zeta-P2A-CXCL9. In the application, inventors obtain CAR-T-CXCL9 cells based on mesothelin design by taking a lung cancer model as an example. Preliminary experiment results show that, compared with conventional CAR-T cells, the modified cells have higher cytokine secretion and cytotoxicity capacities, can obviously increase immune cell infiltration of a tumor site, and inhibit angiogenesis, and further, the modified CAR-T-CXCL9 cell therapy slows down tumor growth, increases the survival rate of mice, and shows excellent anti-tumor activity.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Screening method of tumor immunotherapy prognosis marker and application
PendingCN111863130AAssessing immunogenicityGood treatment effectProteomicsGenomicsCandidate Gene Association StudyGZMB
The invention relates to a screening method of a tumor immunotherapy prognosis marker. The screening method comprises the following steps: acquiring expression data, mutation data and clinical curative effect data of a candidate gene group of cancer species to be analyzed; performing statistical independence test on the mutation condition of the candidate gene group and the expression condition ofan immune genome, finding out gene mutation related to the immune genome, and sorting genes in the candidate gene group according to association degrees from high to low, wherein the immune genome comprises CD8A, GZMA, GZMB, IFN [gamma], EOMES, CXCL9, CXCL10, and TBX21; dividing the first N genes into a mutation group and a non-mutation group according to whether the first N genes are mutated ornot, performing survival analysis, and selecting M genes with most significant differences for reservation according to log-rank changes; validating the resulting genes in independent datasets to determine mutation and prognostic correlation thereof.
Owner:SHANGHAI ORIGIMED CO LTD
PSMA-targeted macrophage membrane-coated bionic nano-drug carrier and application thereof
PendingCN114306277AOvercoming exogenousOvercome the two major problems of weak targetingEnergy modified materialsSkeletal disorderSingle-Chain AntibodiesCell membrane
The invention discloses a PSMA-targeted macrophage membrane-wrapped bionic nano-drug carrier and application, a nanogold carrier is wrapped by a macrophage membrane, and a PSMA-targeted antibody based on a sequence as shown in SEQ.ID.NO.1 is expressed on the macrophage membrane in a transmembrane manner; the antibody is a gy-1 single-chain antibody, and is expressed on a macrophage cell membrane in a transmembrane manner through fusion expression with a CD8a hinge region and a CD8a transmembrane region. The preparation method comprises the following steps: constructing a lentiviral vector for expressing a transmembrane single-chain antibody by utilizing a genetic engineering method, packaging into lentivirus, infecting macrophages to express the transmembrane single-chain antibody, and extracting a cell membrane to carry out bionic modification on a nano-vector; the prepared nano-carrier is expected to overcome the two problems of strong exogenous property and weak targeting property of the traditional nano-drug carrier, and can be used for preparing targeted diagnosis and treatment reagents and drugs for prostatic cancer.
Owner:AIR FORCE MEDICAL UNIV
Prognostic and treatment response predictive method
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of atleast 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL1A in a sample obtained from the tumour of the patient; b)measuring the gene expression of atleast 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained fromthetumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including withimmune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Diagnostic and therapeutic methods for cancer
PendingUS20200157635A1Improve responsivenessResponsiveness to treatmentOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:GENENTECH INC
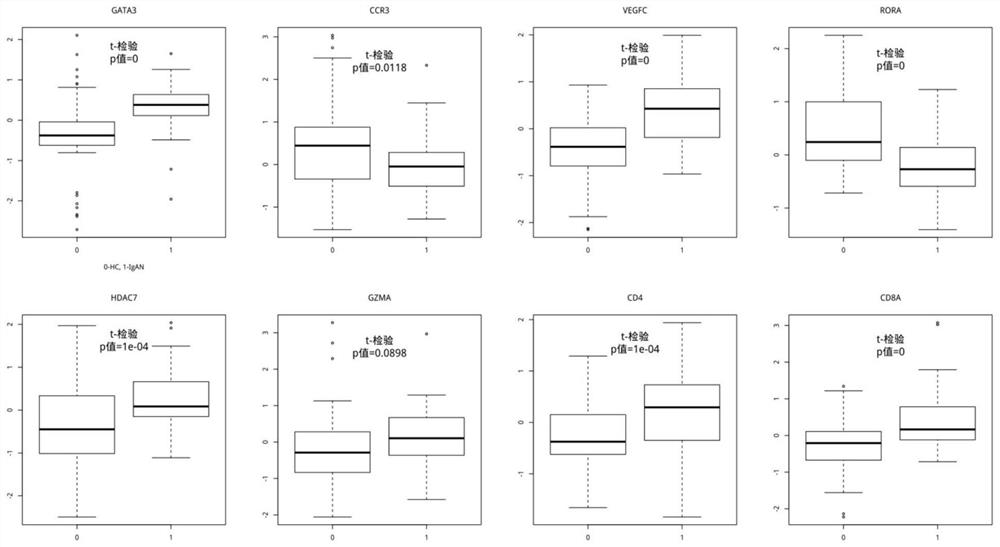
Immunoglobulin A nephropathy T cell diagnostic marker
PendingCN114324887AEfficient and accurate diagnosisImprove featuresMicrobiological testing/measurementBiostatisticsGATA3Cellular immunity
The invention discloses an immunoglobulin A nephropathy T cell diagnostic marker. On the first aspect, the invention provides application of a reagent for quantitatively detecting at least one of the following markers in preparation of the diagnostic kit for IgA nephropathy: CCR3, CD4, CD8A, GATA3, GZMA, HDAC7, RORA and VEGFC. According to the application disclosed by the embodiment of the invention, the application at least has the following beneficial effects that the pathogenesis of the immunoglobulin A nephropathy is related to five gene axes (Axis), screening is carried out from related mRNA gene expression data based on a specific gene or protein of a T cell from the T cell immune axis (T Cell Immunity Axis) to obtain the eight markers, and the immunoglobulin A nephropathy can be used for detecting the immunoglobulin A nephropathy. Whether a subject suffers from IgA nephropathy or not can be efficiently and accurately diagnosed through quantitative detection on the basis of at least one of the eight markers, and good specificity and sensitivity are achieved.
Owner:SHENZHEN LUWEI BIOTECHNOLOGY (BIOMANIFOLD TECH CO) LTD
Methods of detecting and treating subjects with checkpoint inhibitor-responsive cancer
Disclosed herein are methods of detecting and treating checkpoint inhibitor responsive cancers comprising calculating, determining, or obtaining PD-L1 expression, CD8A expression, and tumor content from a cancer specimen.
Owner:STRATA ONCOLOGY INC
Methods of treating tumor
PendingUS20220041733A1Shrink tumorSmall sizeMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesTumor Sample
The disclosure provides a method for treating a subject afflicted with a tumor comprising administering to the subject a therapeutically effective amount of an anti-PD-1 antibody or antigen-binding portion thereof or an anti-PD-L1 antibody or antigen-binding portion thereof, wherein the subject is identified as having a high inflammatory gene signature score. In some embodiments, the high inflammatory gene signature score is determined by measuring the expression of a panel of inflammatory genes in a tumor sample obtained from the subject, wherein the inflammatory gene panel comprises CD274 (PD-L1), CD8A, LAG3, and STAT1.
Owner:BRISTOL MYERS SQUIBB CO
Fibronectin type III domain that binds CD8A
ActiveCN110225770BConnective tissue peptidesGeneral/multifunctional contrast agentsPolynucleotideCD8A
Fibronectin type III domain (FN3) specifically binding to CD8A, related polynucleotides capable of encoding CD8A-specific FN3 domains, cells expressing FN3 domains, and related vectors, and detectably labeled FN3 domains Can be used in therapeutic and diagnostic applications.
Owner:JANSSEN BIOTECH INC
BCMA-specific chimeric antigen receptor T cells and their applications
ActiveCN109748968BGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesAntiendomysial antibodies
The present invention discloses a human BCMA-specific chimeric antigen receptor (anti-BCMA scFv-CD8a-41BB-CD3ζ) and an application thereof. The chimeric antigen receptor consists of a single-chain antibody anti-BCMA scFv, a hinge region, a transmembrane region, and an intracellular region in tandem. The chimeric antigen receptor is used to modify human T lymphocytes, and the modified T lymphocytes are used for the prevention and treatment of surface BCMA positive tumors and the preparation of tumor drugs.
Owner:广州因明生物医药科技股份有限公司
Chimeric antigen receptor based on human mesothelin antibody, lentiviral expression vector and its application
ActiveCN109608549BFunction increaseIncrease heightPolypeptide with localisation/targeting motifImmunoglobulin superfamilySingle-Chain AntibodiesAntiendomysial antibodies
The invention belongs to the field of biotechnology, and specifically relates to a chimeric antigen receptor based on human mesothelin antibody, a lentiviral expression vector and applications thereof. The chimeric antigen receptor consists of human CD8a molecular signal peptide, human mesothelin The single-chain antibody of corticosteroid, the flexible fragment of human CD8a molecule, the transmembrane region and intracellular region of human CD28 molecule, the intracellular region of human 41BB molecule, and the intracellular region of human CD3z molecule are sequentially connected in series. The chimeric antigen receptor prepared by the present invention can target mesothelin and improve the therapeutic effect of CAR-T cells.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Method for preparing CAR-T cells
ActiveCN114149978AGood technical effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyLentivirusCD8A
The invention relates to a method for preparing CAR-T (chimeric antigen receptor T) cells, which comprises the following steps of: synthesizing plasmids which are composed of a CD19 single-chain variable region, a CD8a hinge region, a CD8a transmembrane region, a 4-1BB signal, a CD3 zeta inclusion signal, an IL-7 sequence and a CCL17 sequence; packaging the virus to obtain a lentivirus concentrated solution, and measuring the virus titer of the lentivirus concentrated solution; and preparing T cells, namely activating, transducing and amplifying the prepared T cells which are CD8 < + > T cells, and harvesting CAR-T cells. The invention also relates to a method for preparing the T cell. The invention has excellent effects as described in the specification.
Owner:深圳博雅感知药业有限公司
Trophoblast, preparation method thereof and application of trophoblast in massive rapid amplification of gamma delta T cells
ActiveCN111206052AAchieve multiplicationEasy to operateGenetically modified cellsNucleic acid vectorAntiendomysial antibodiesCD86
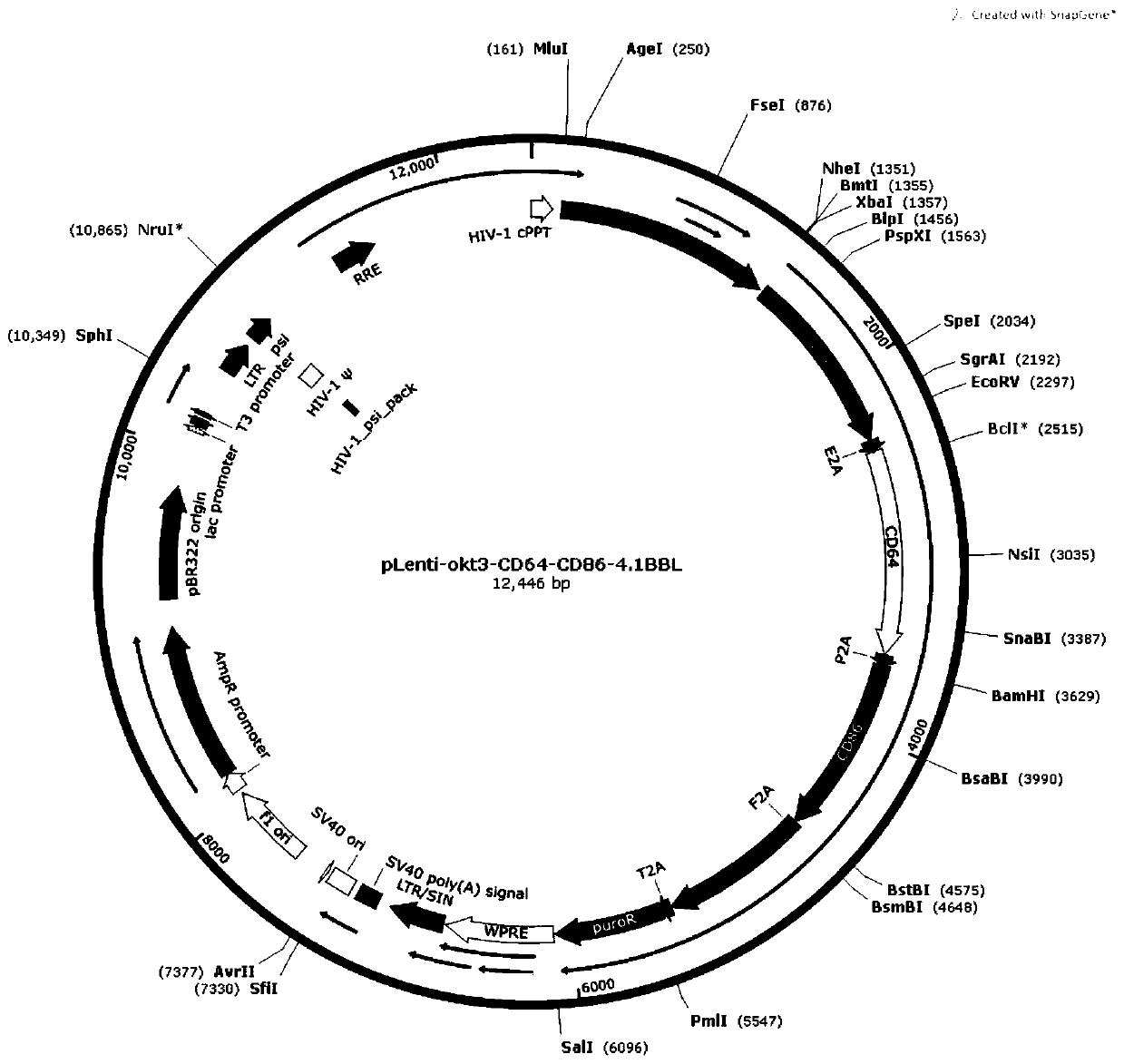
The invention relates to a trophoblast, a preparation method thereof and application of the trophoblast in massive rapid amplification of gamma delta T cells. The preparation method comprises the following steps: S1, constructing a pLenti-okt3-CD64-CD86-4.1BBL lentiviral plasmid vector; S2, packaging lentiviral particles by using the pLenti-okt3-CD64-CD86-4.1BBL plasmid vector; S3, preparing K562-okt3-CD64-CD86-4.1BBL trophoblast; S4, obtaining buffy coat cells of umbilical cord blood; and S5, carrying out in vitro amplification on gamma delta T cells of the umbilical cord blood. According tothe preparation method, an okt3 single-chain antibody is expressed on a trophoblast membrane through a CD8A transmembrane region, especially, the gamma delta T cells can be rapidly and massively amplified from the umbilical cord blood at low cost, the amplification multiple of the gamma delta T cells can reach about 10000 times after a 24-day amplification period, and the purity of the prepared gamma delta T cells can reach 90% or above. Compared with stimulation by a soluble okt3 antibody, the membrane-bound okt3 has the amplification multiple over 2 times to the gamma delta T cells, and hasa good clinical application prospect.
Owner:中邦干细胞科技有限公司
Kit for simultaneously detecting various cell subpopulations and functional changes and application of kit
PendingCN113866409AEasy to useEasy to operateBiological particle analysisIndividual particle analysisCD80Cell subpopulations
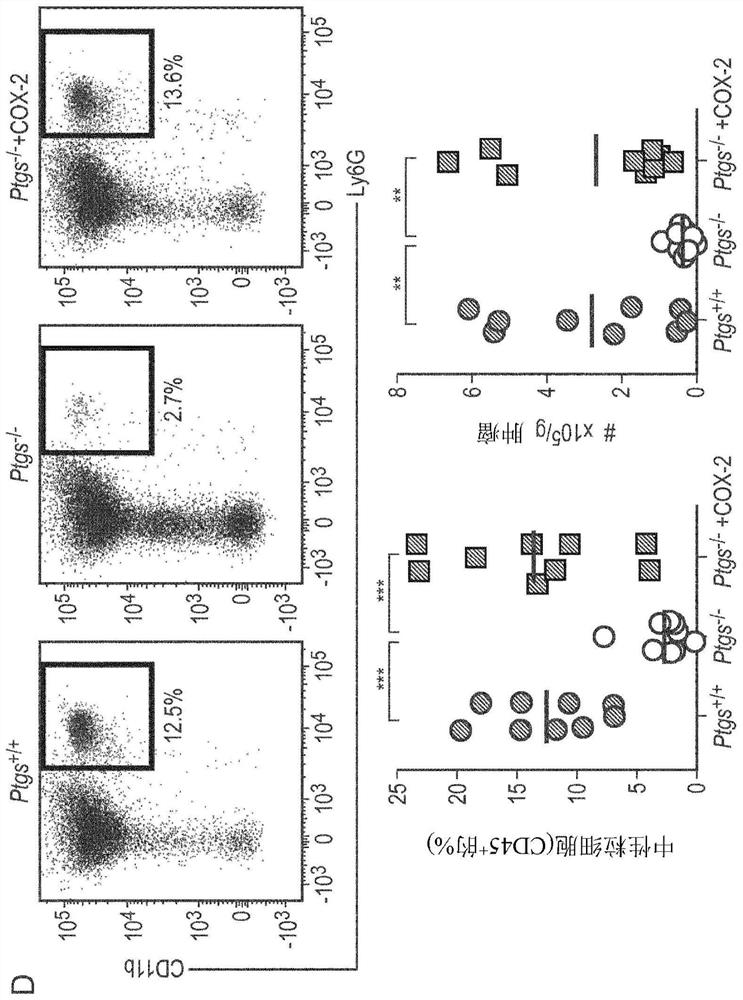
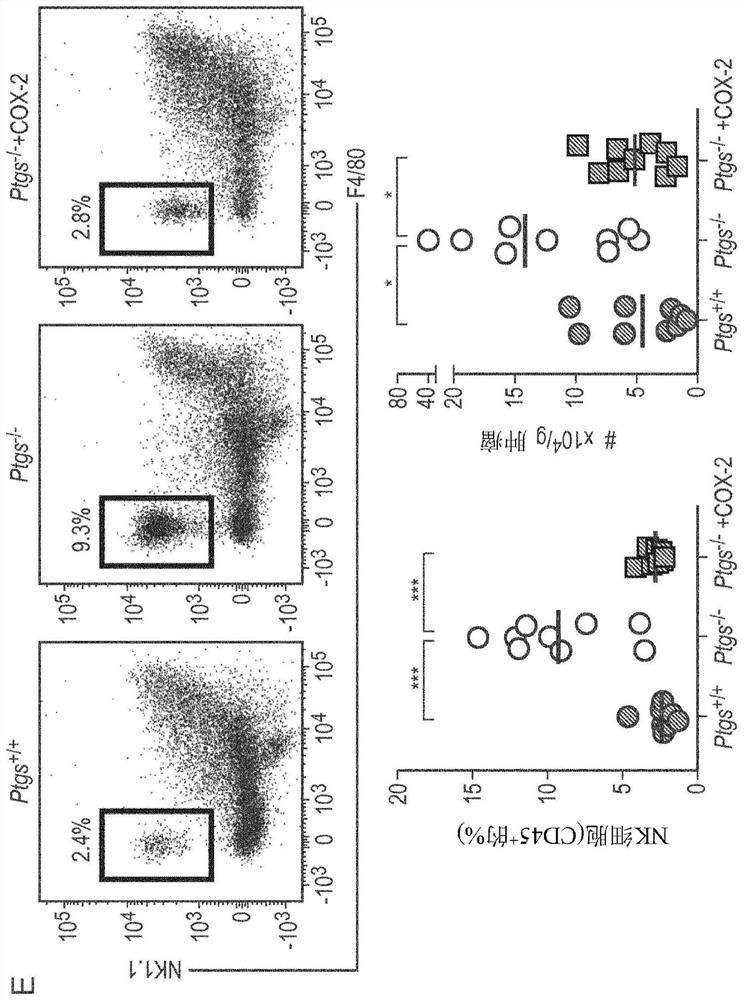
The invention provides a kit for simultaneously detecting various cell subpopulations and functional changes and application of the kit. The kit comprises an antibody composition, and the antibody composition comprises a surface antibody composition and an intracellular antibody composition. The surface antibody composition comprises a combination of at least two of Zombie NIR, CD45, Ly6G, CD11b, F4 / 80, Ly6c, NK1.1, CD3, CD4, CD25, CD8a, CD19, CD11c, CD80 and CD206. The intracellular antibody composition comprises a combination of at least two of FoxP3, IL-10, Granzyme B, Perforin and IFNgamma. The invention also provides a method for detecting the change of the immune cell subpopulations. The kit can be used for simultaneously detecting changes of a plurality of immune cell subgroups and functions, and the result is comprehensive and specific.
Owner:BLUE ELEGANT BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com