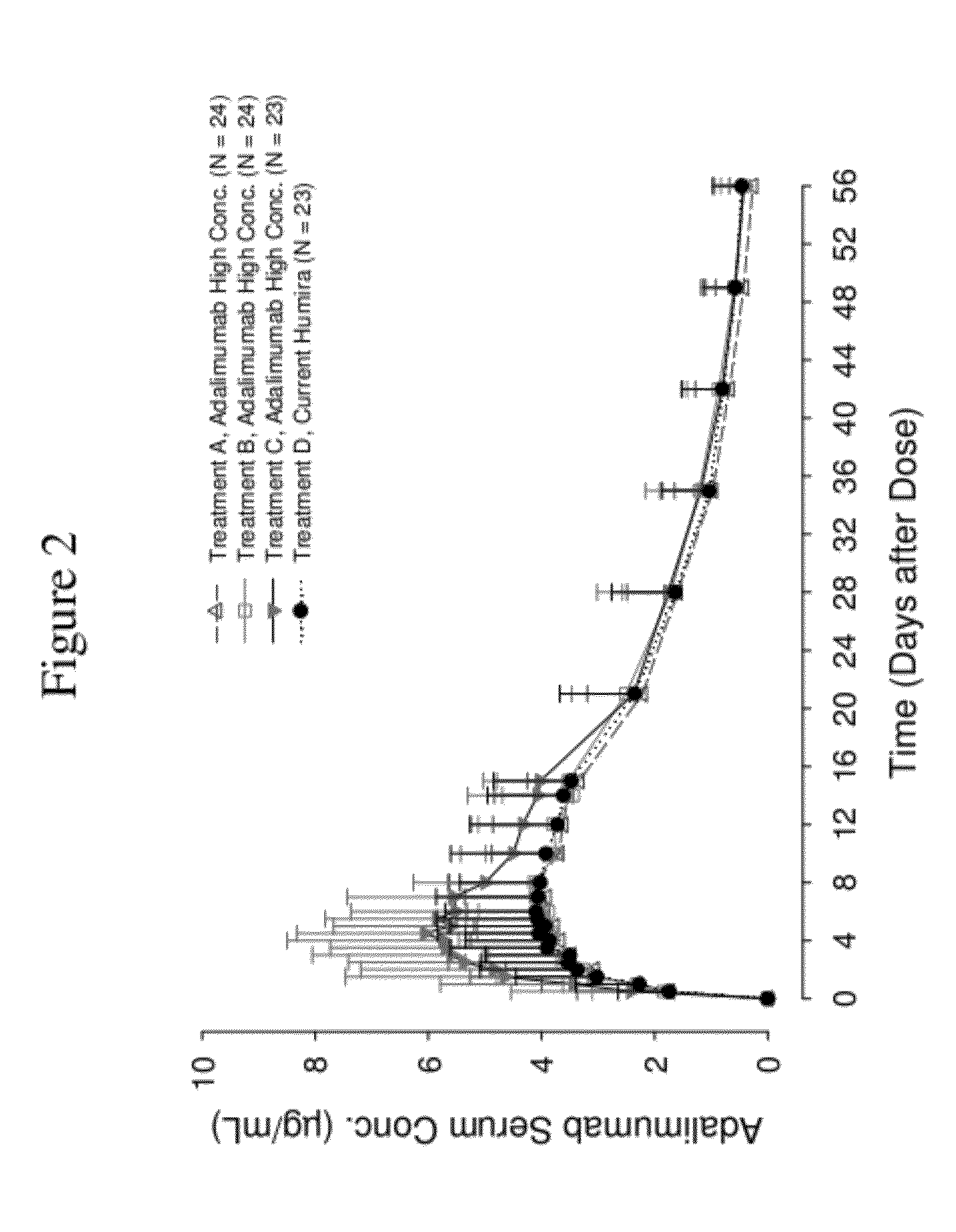
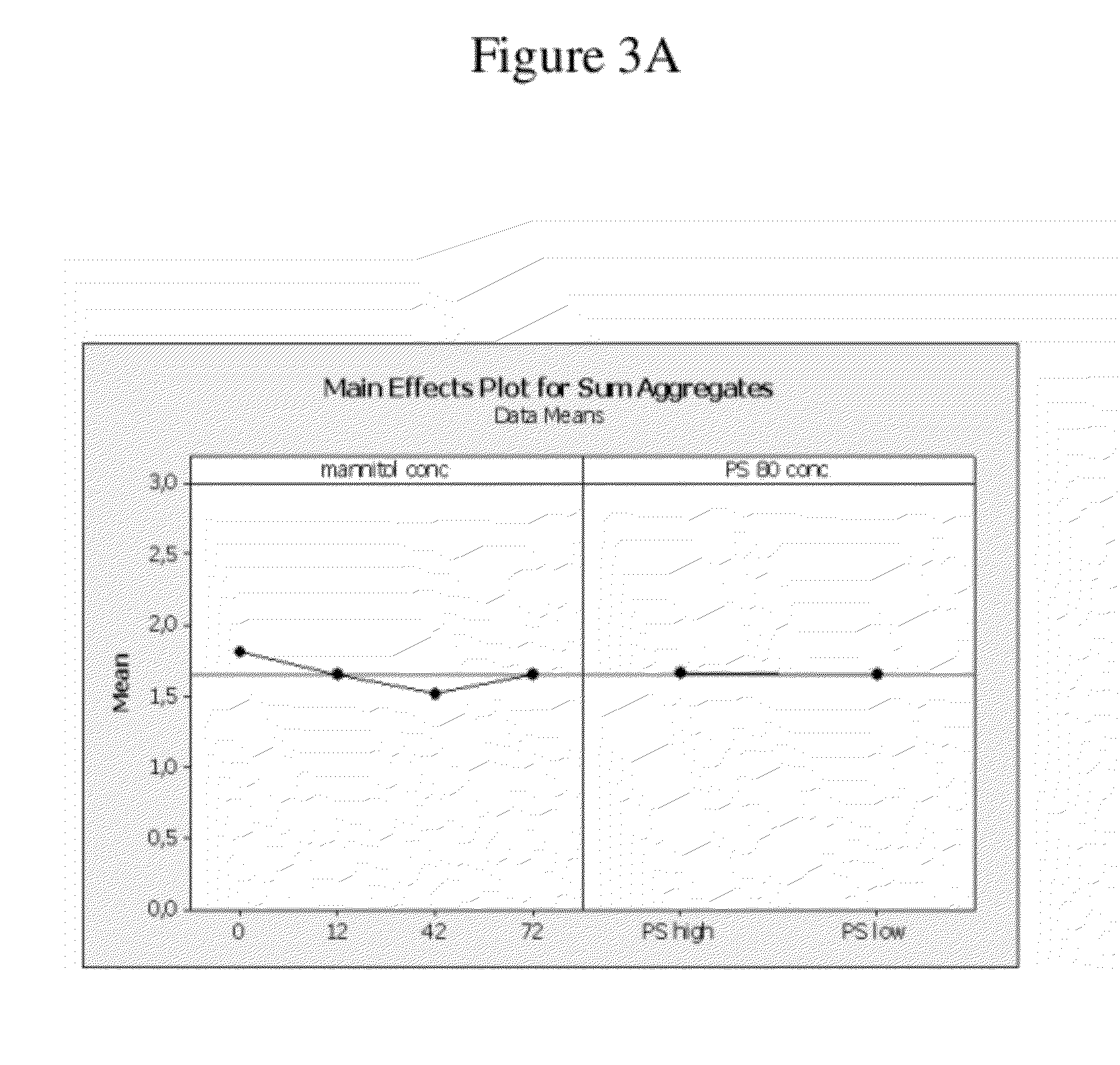
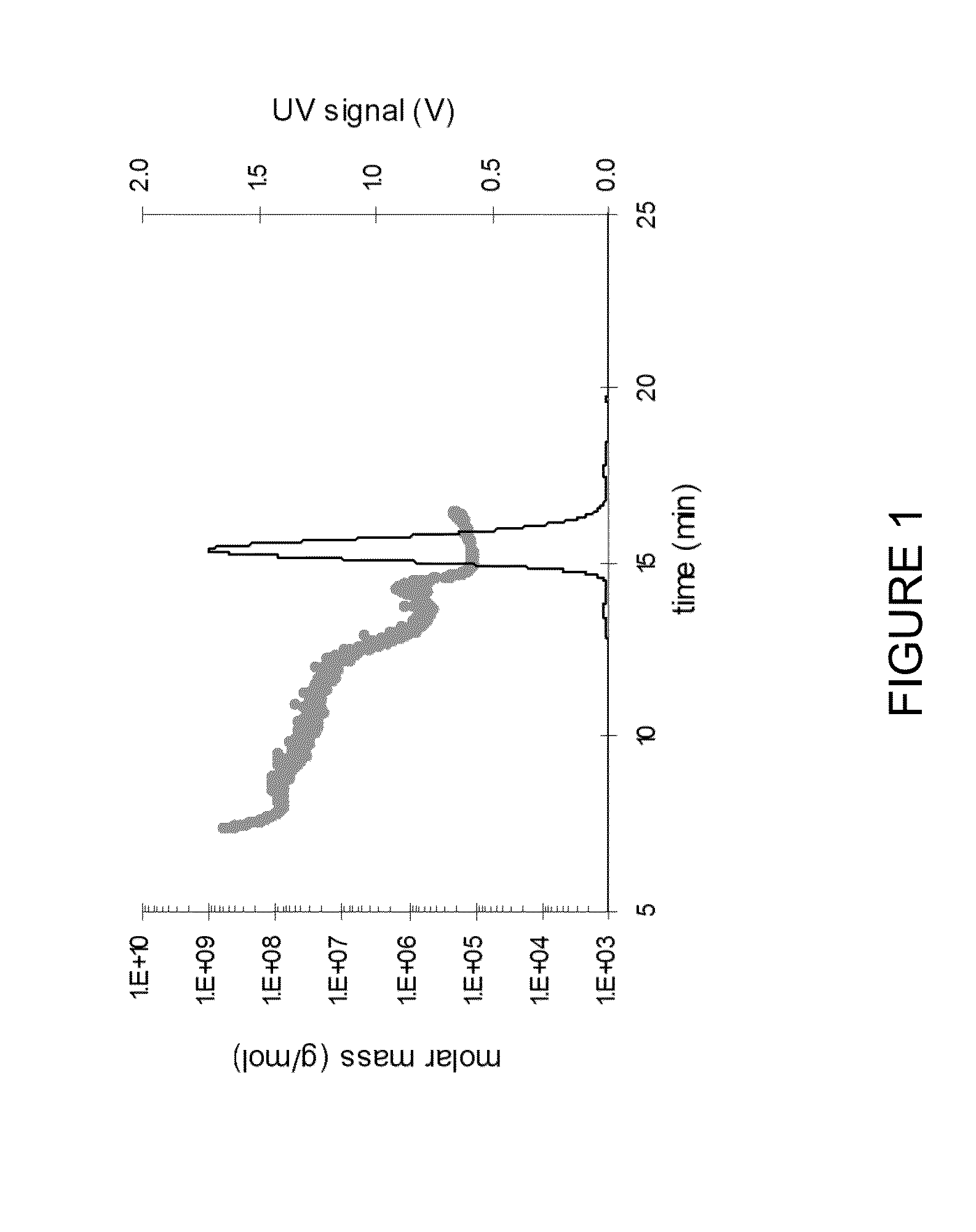
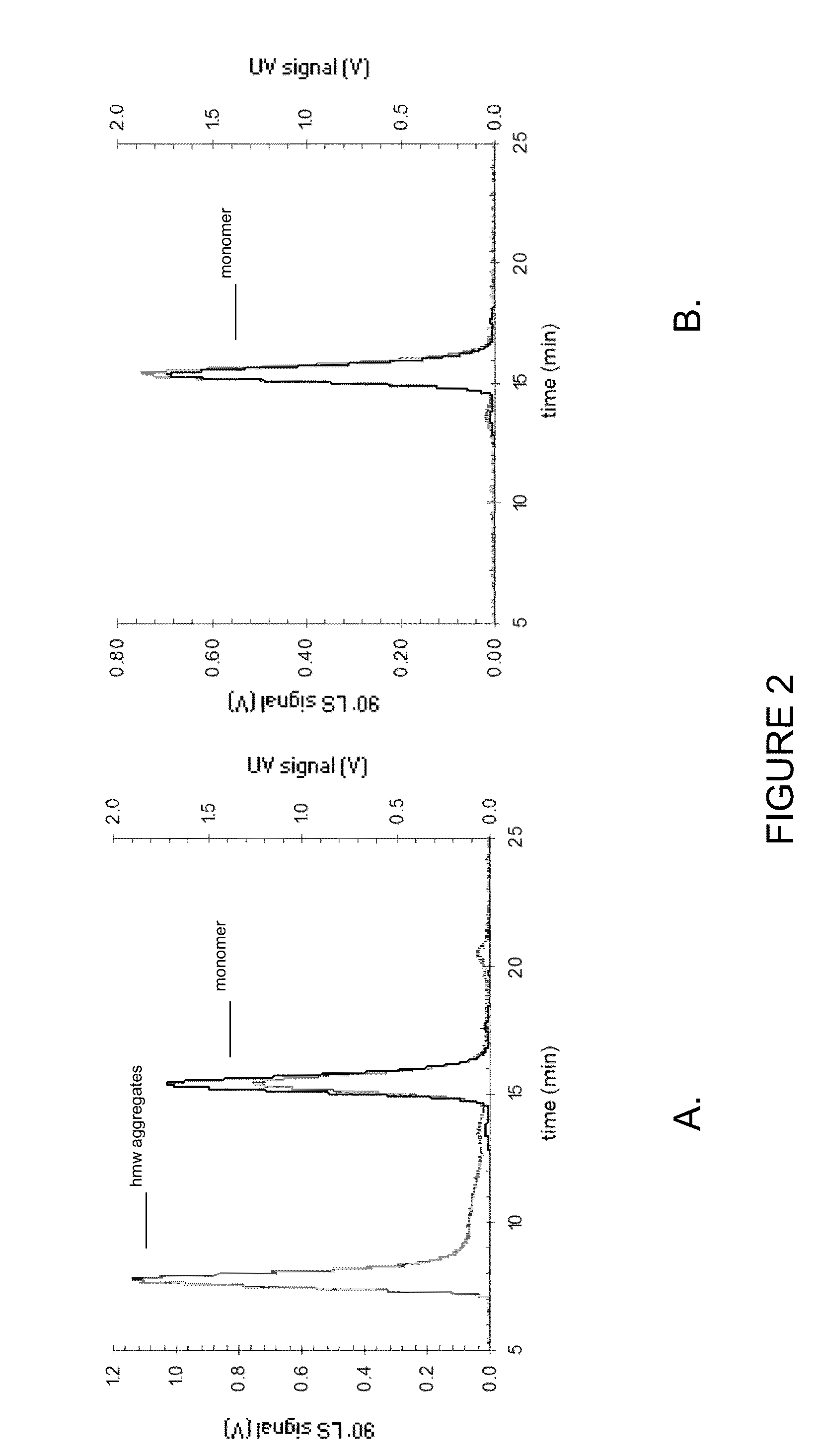
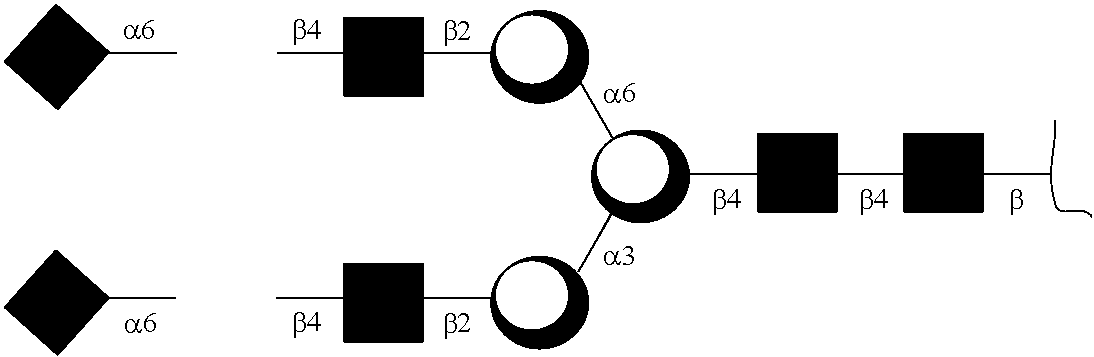
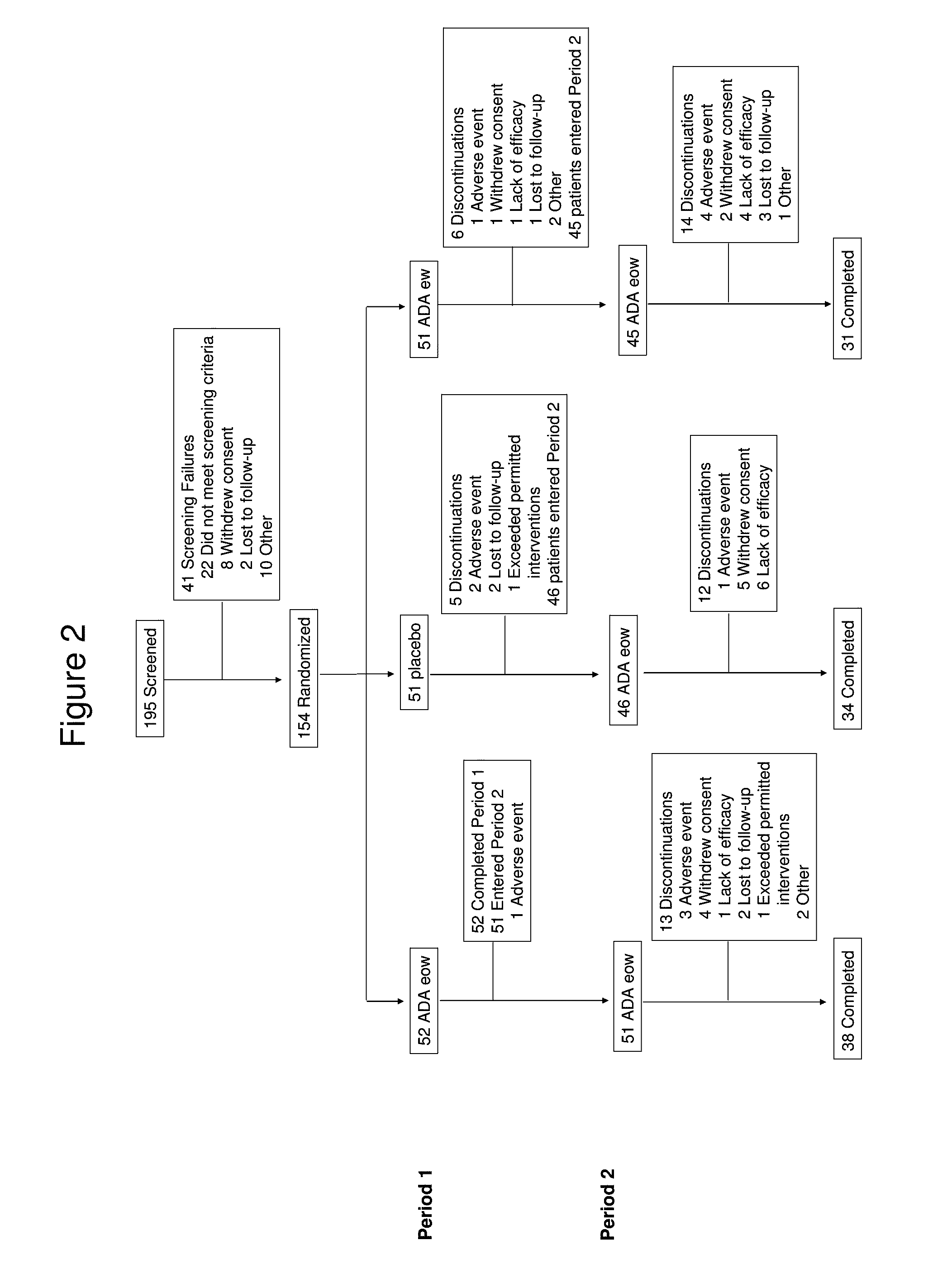
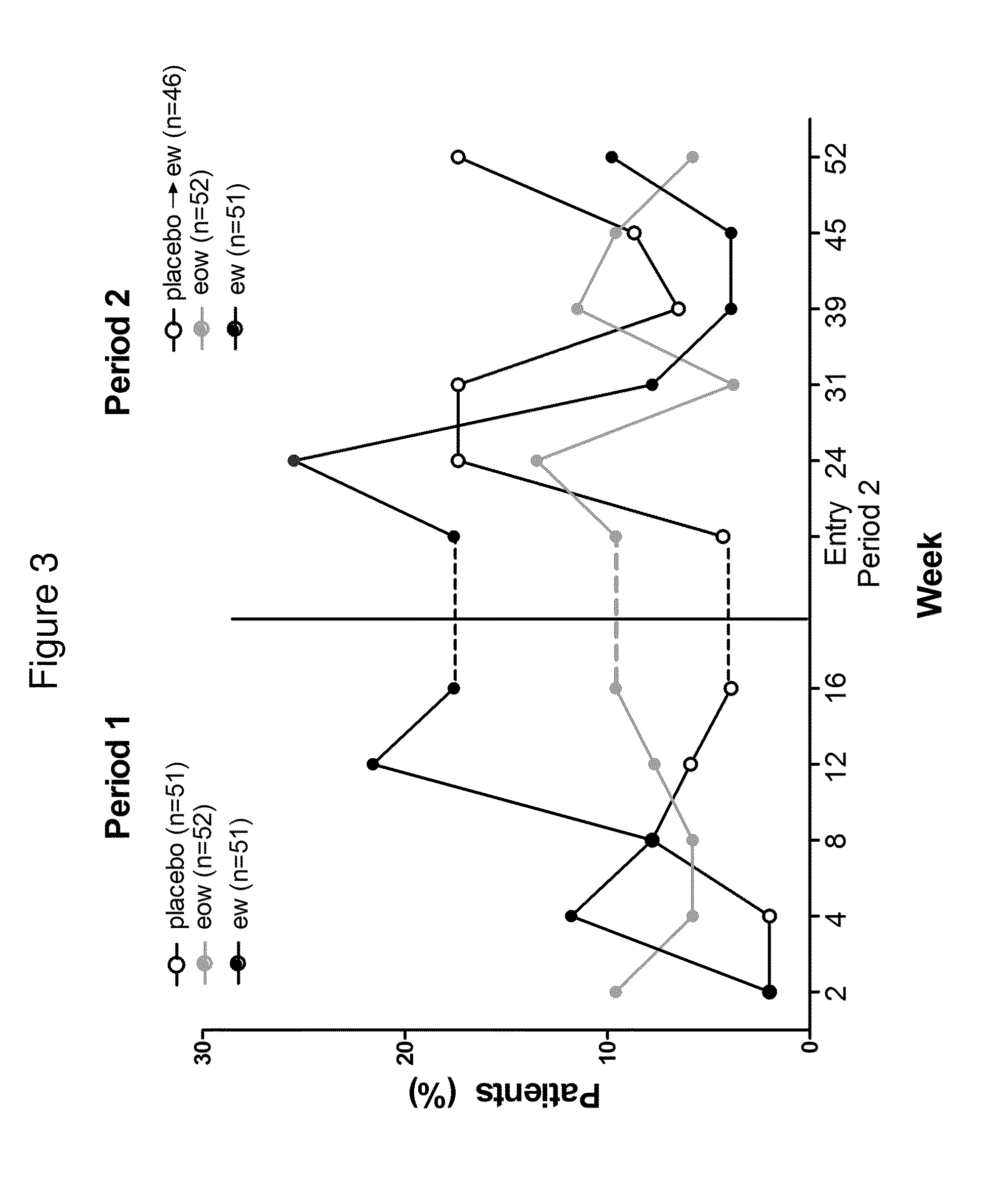
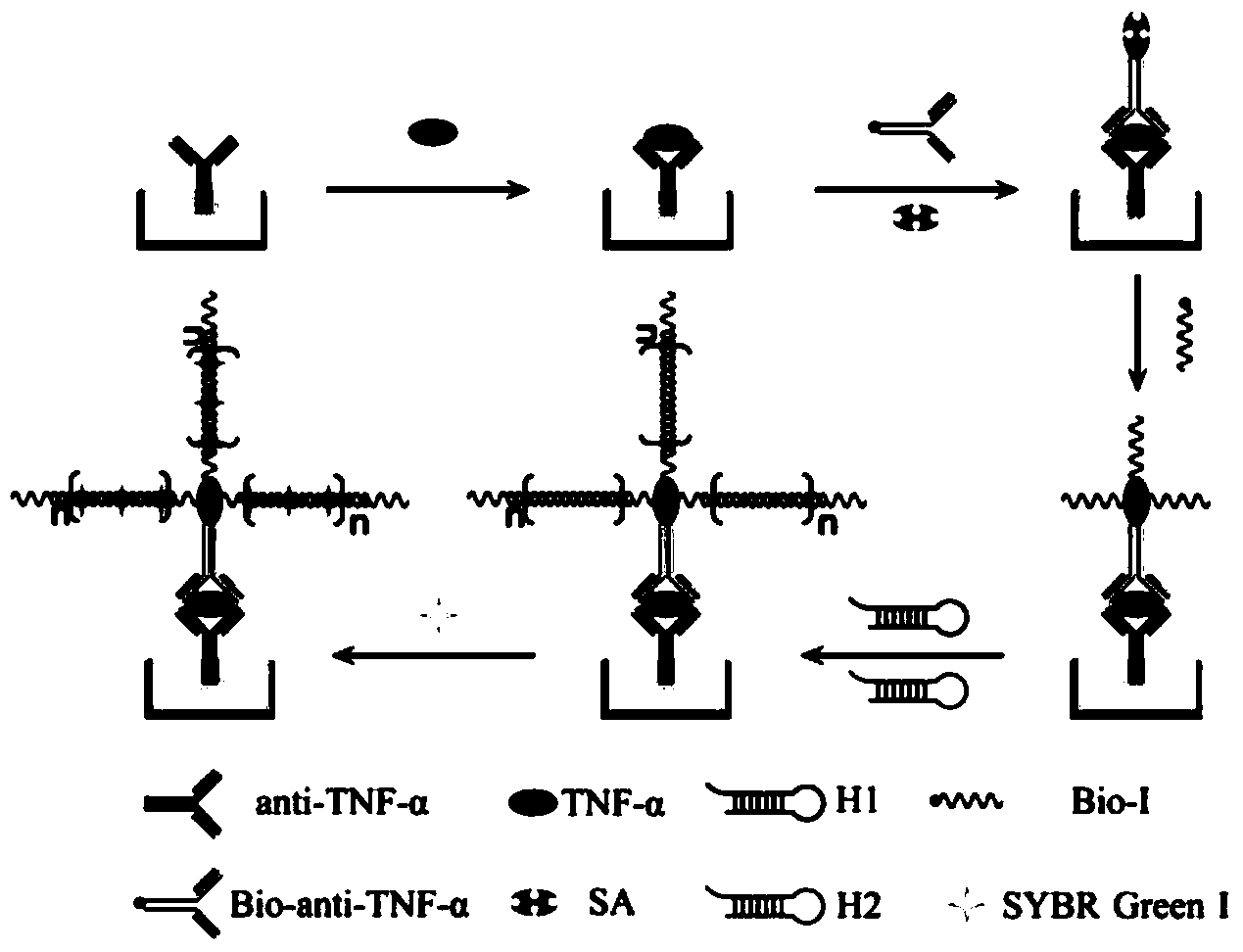
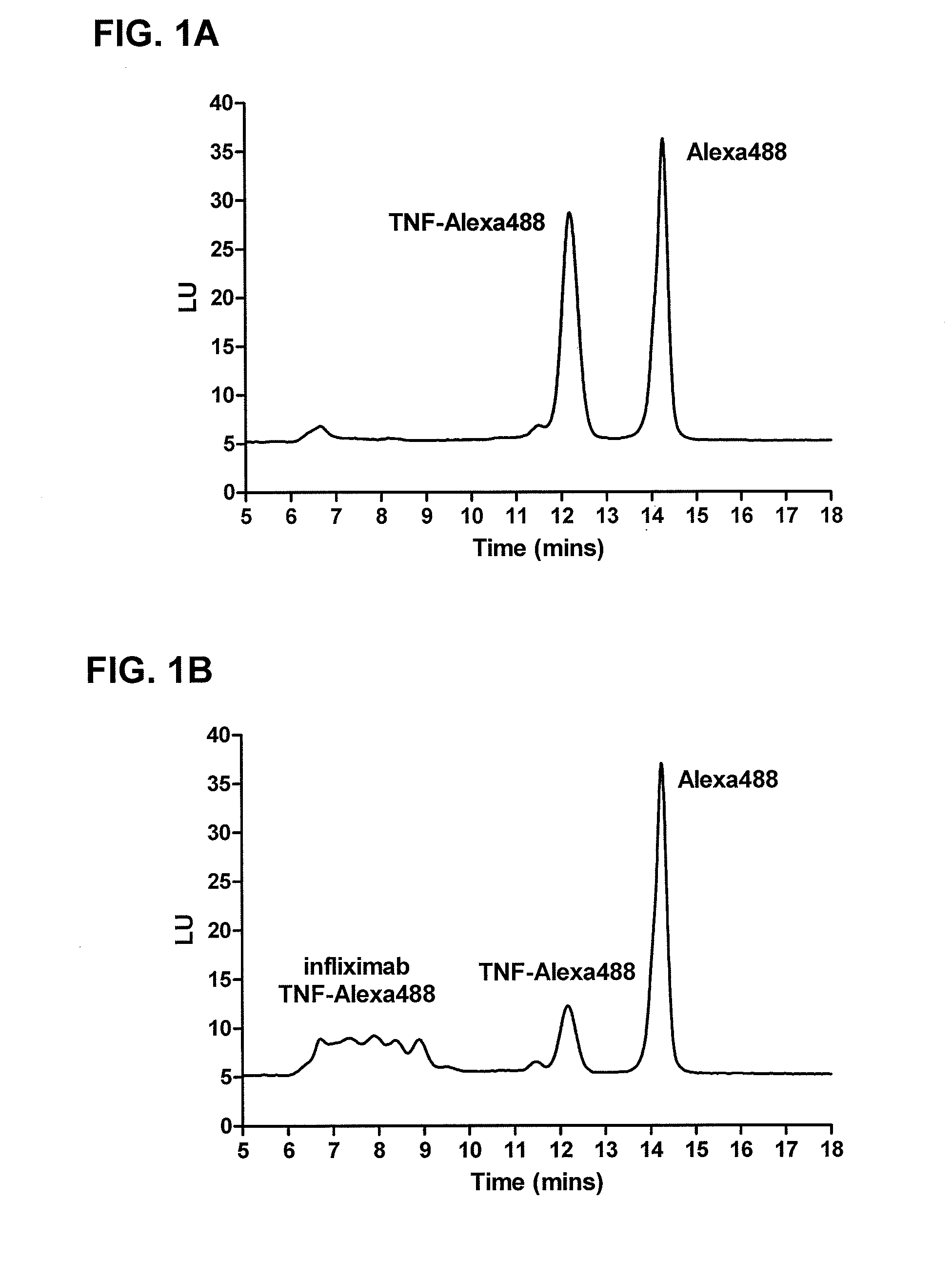
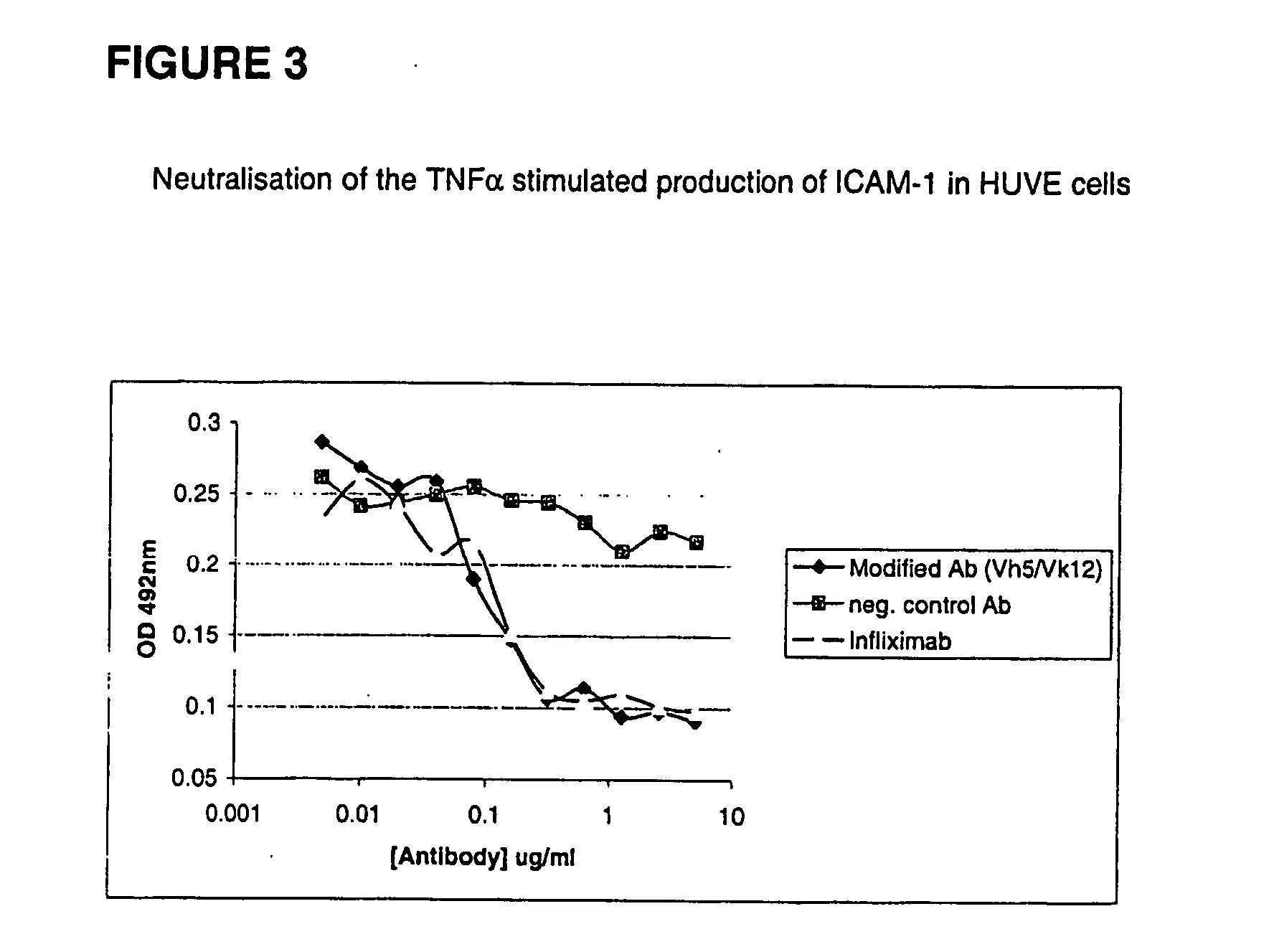
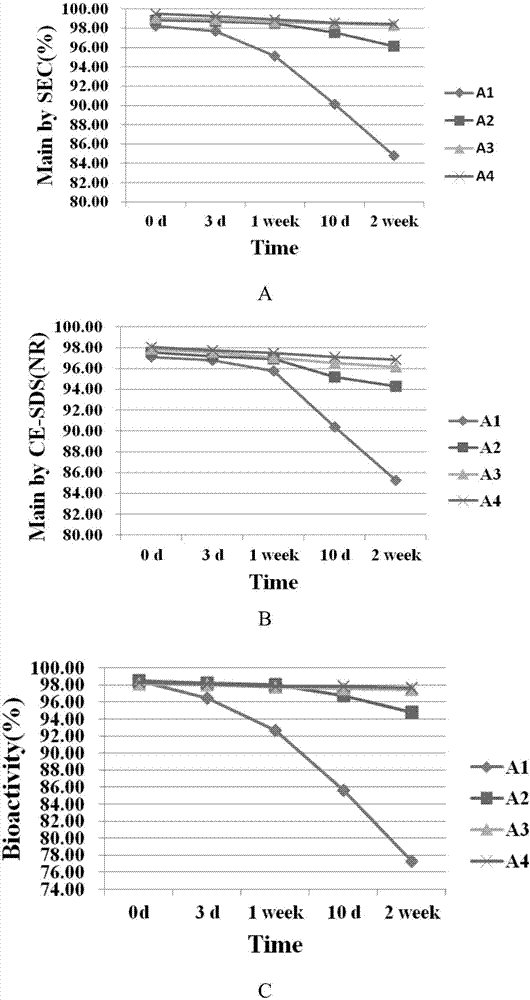
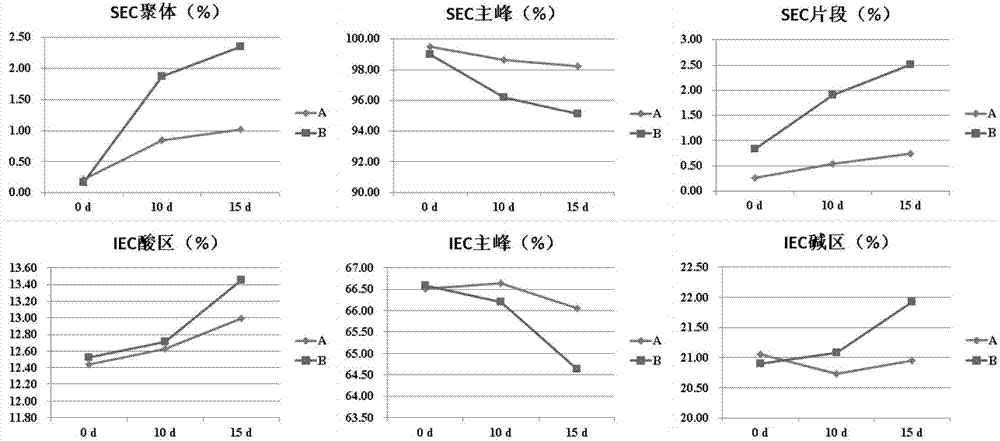
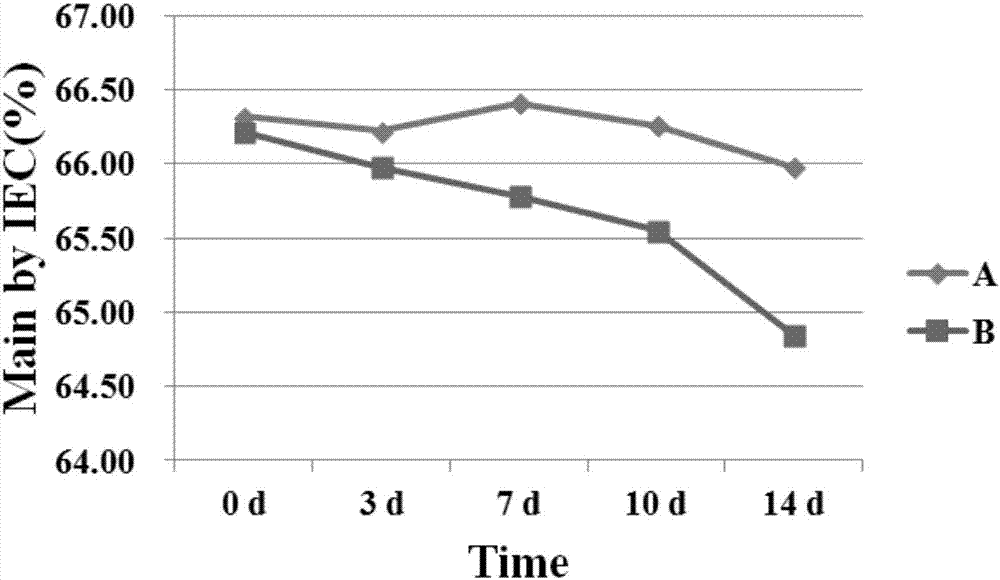
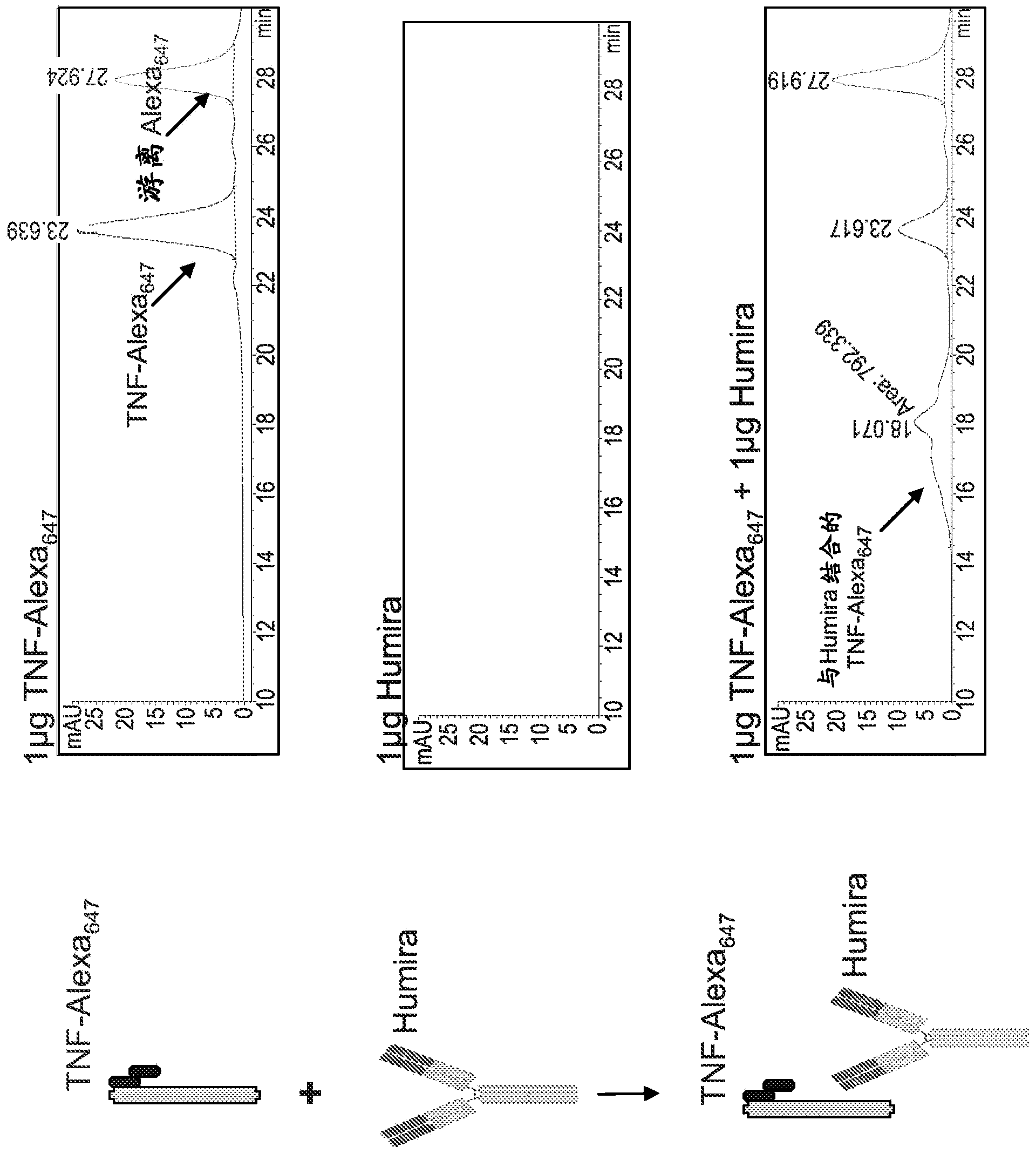
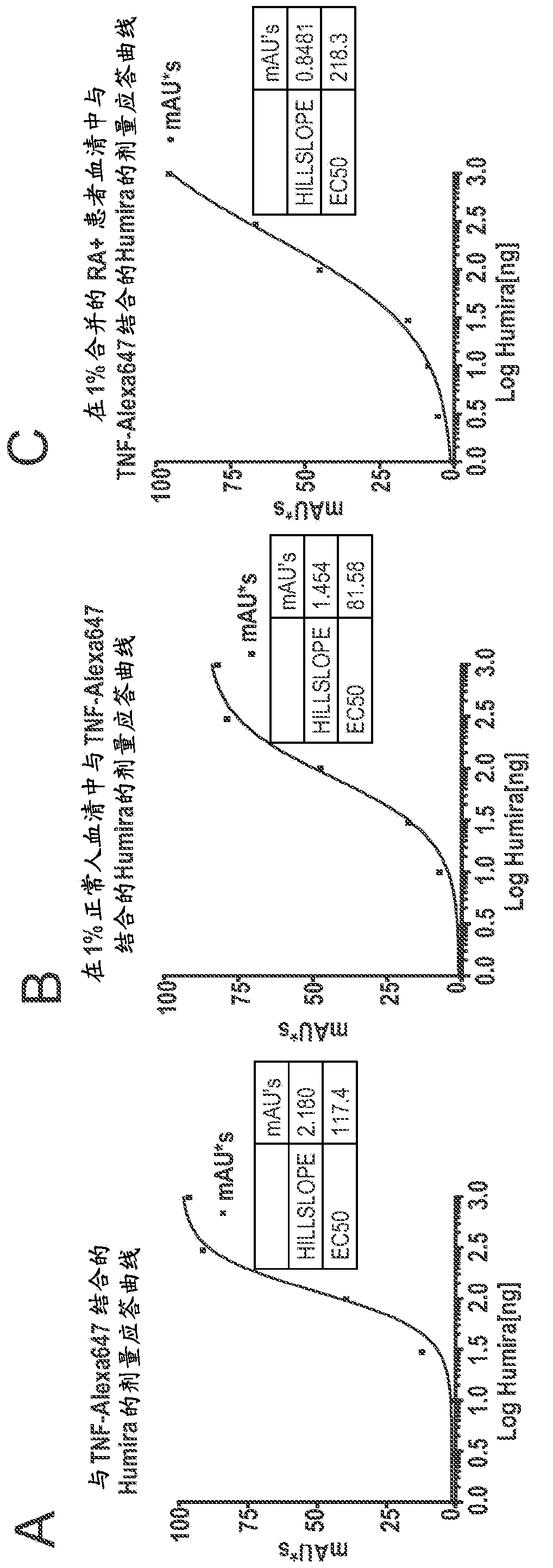
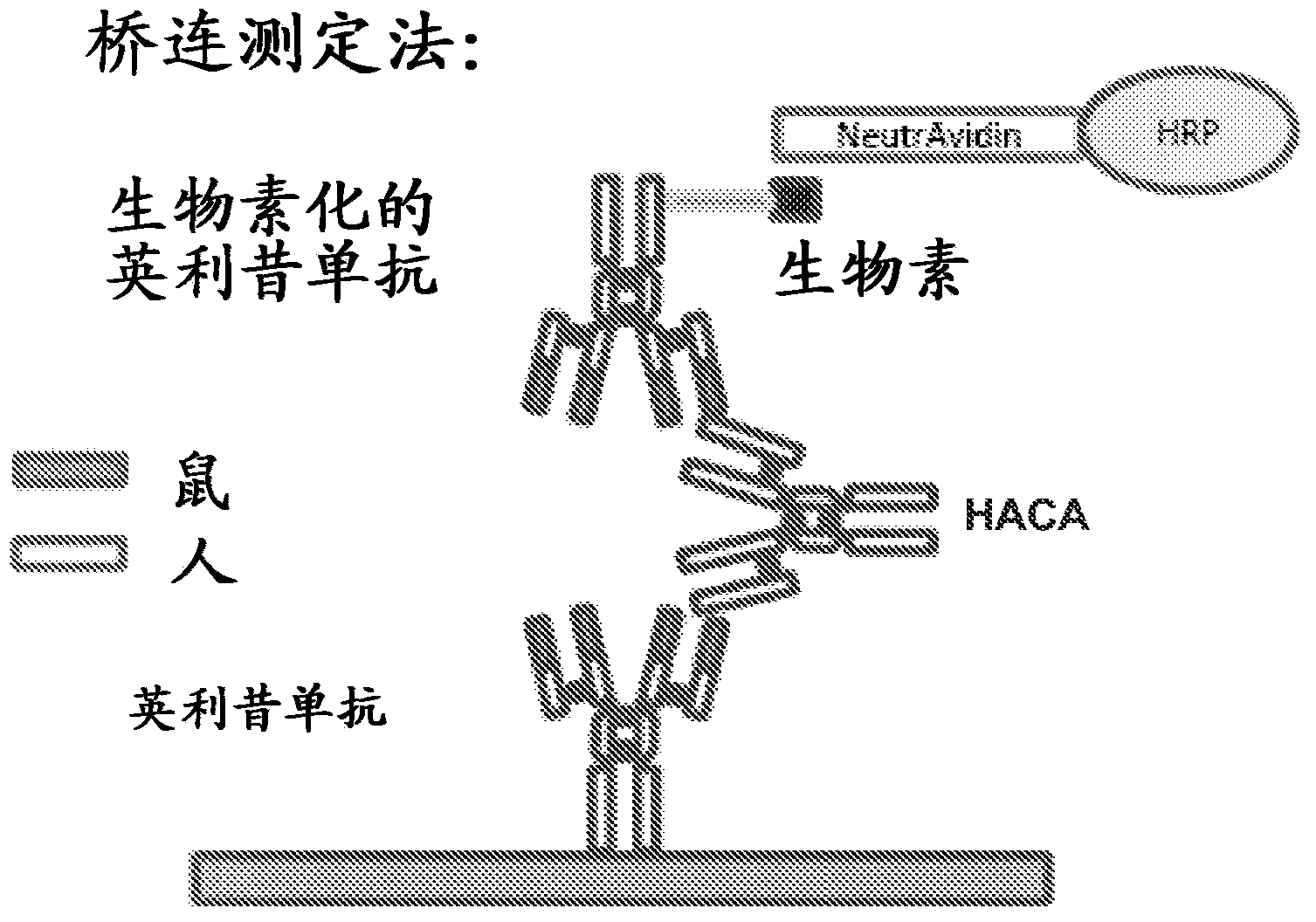
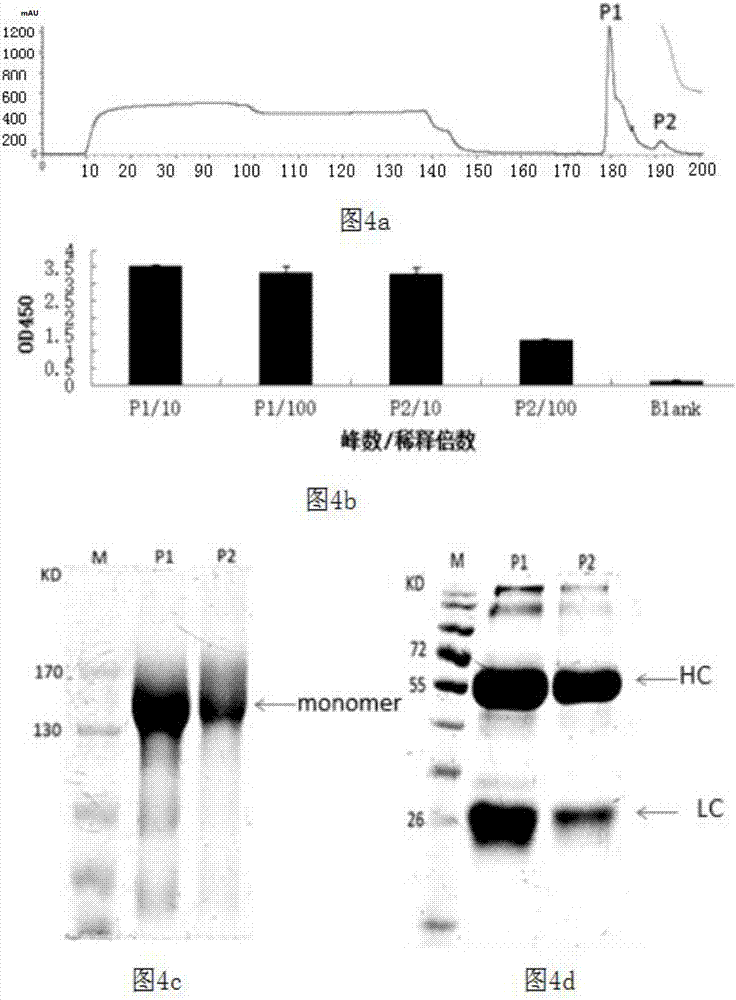
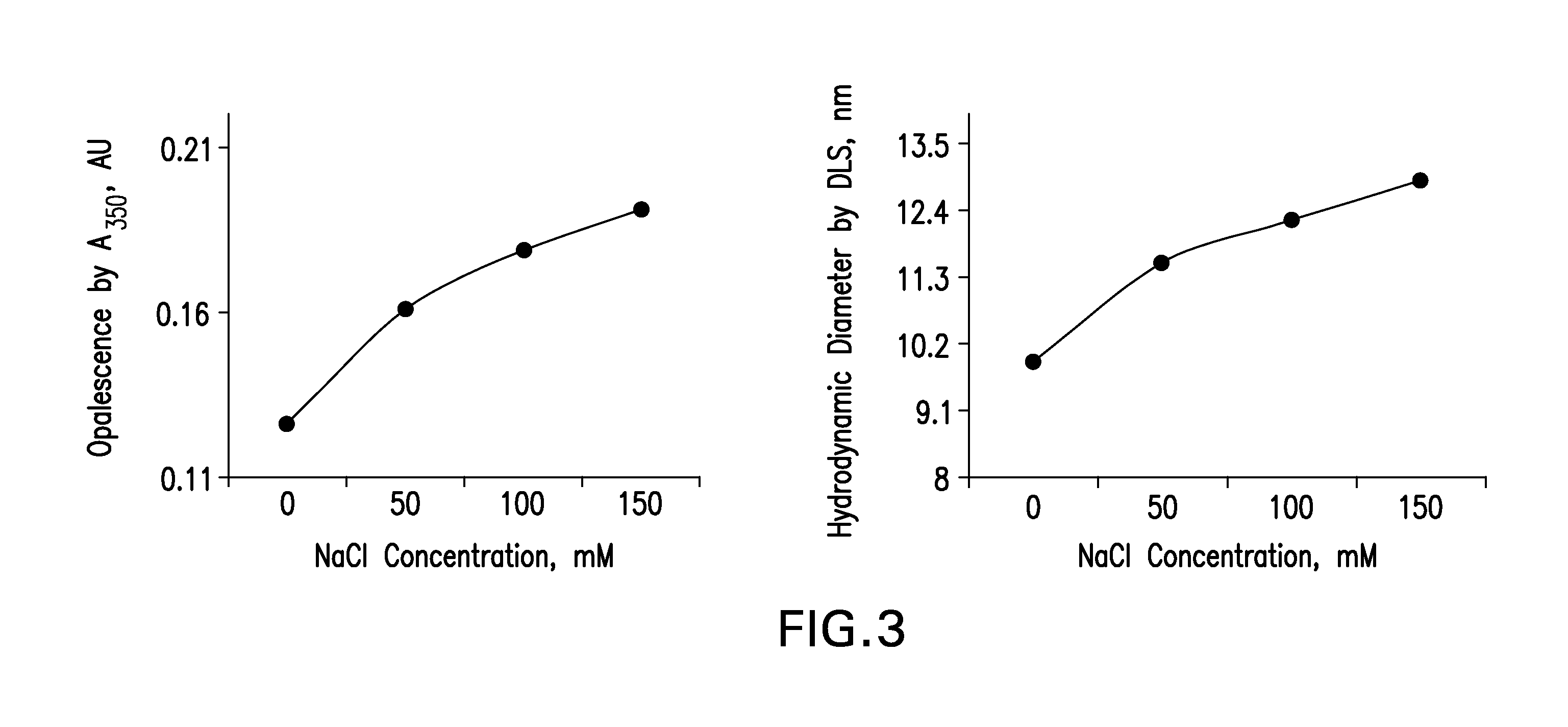
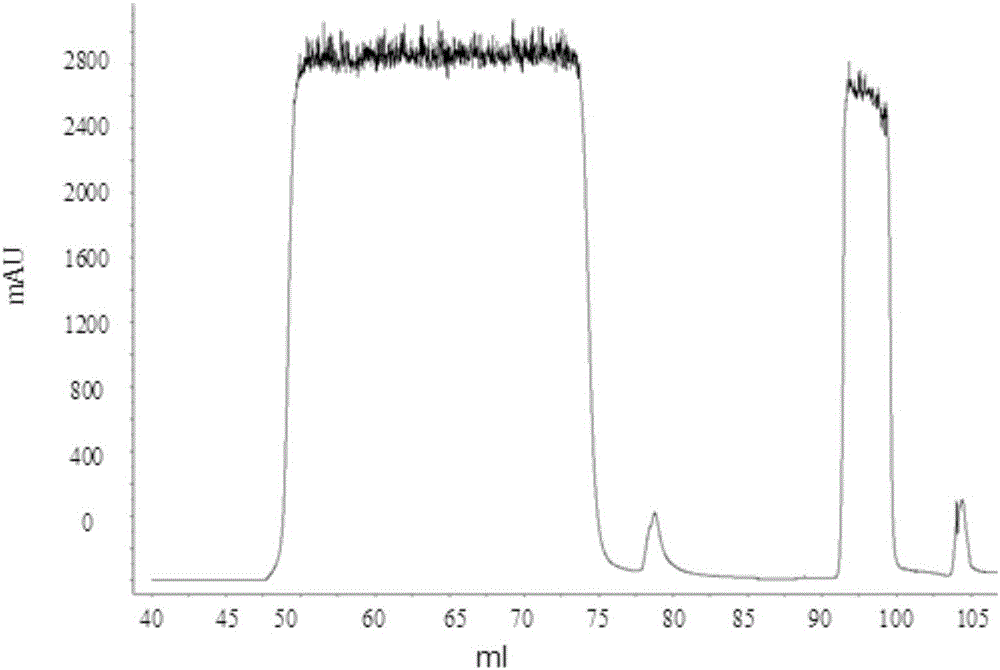
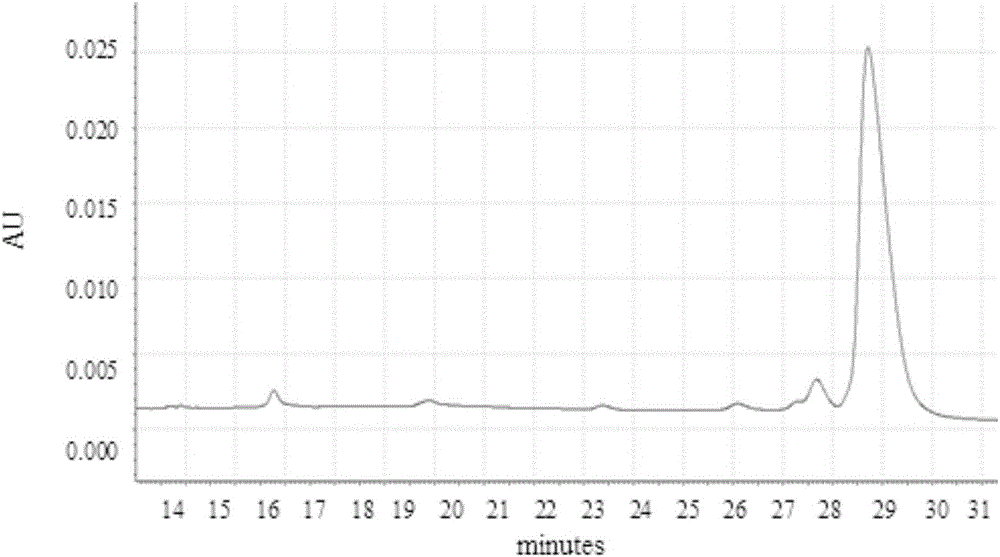
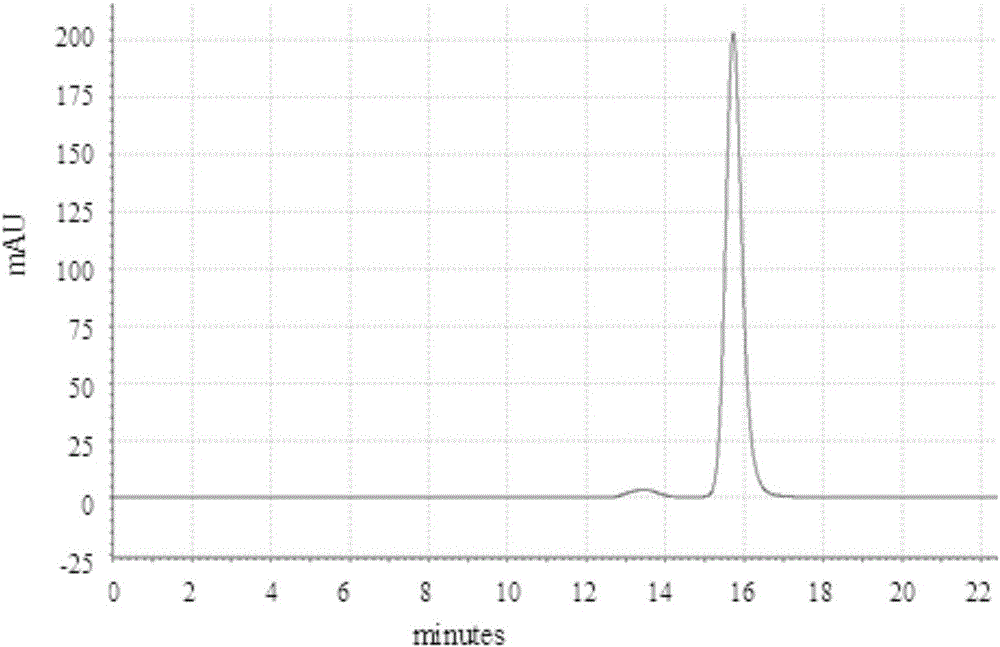
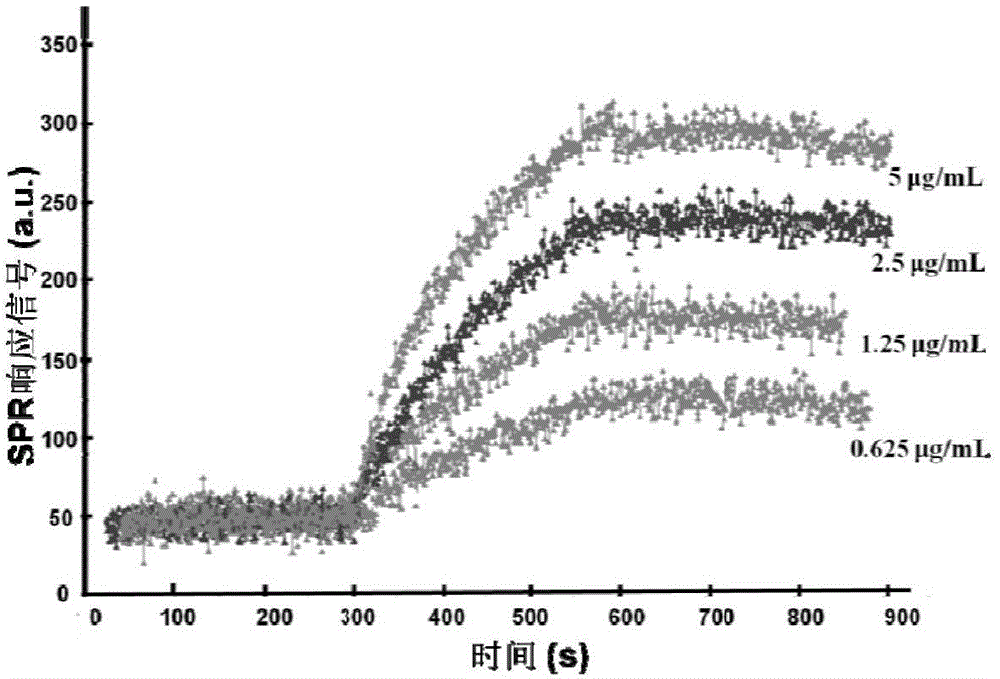
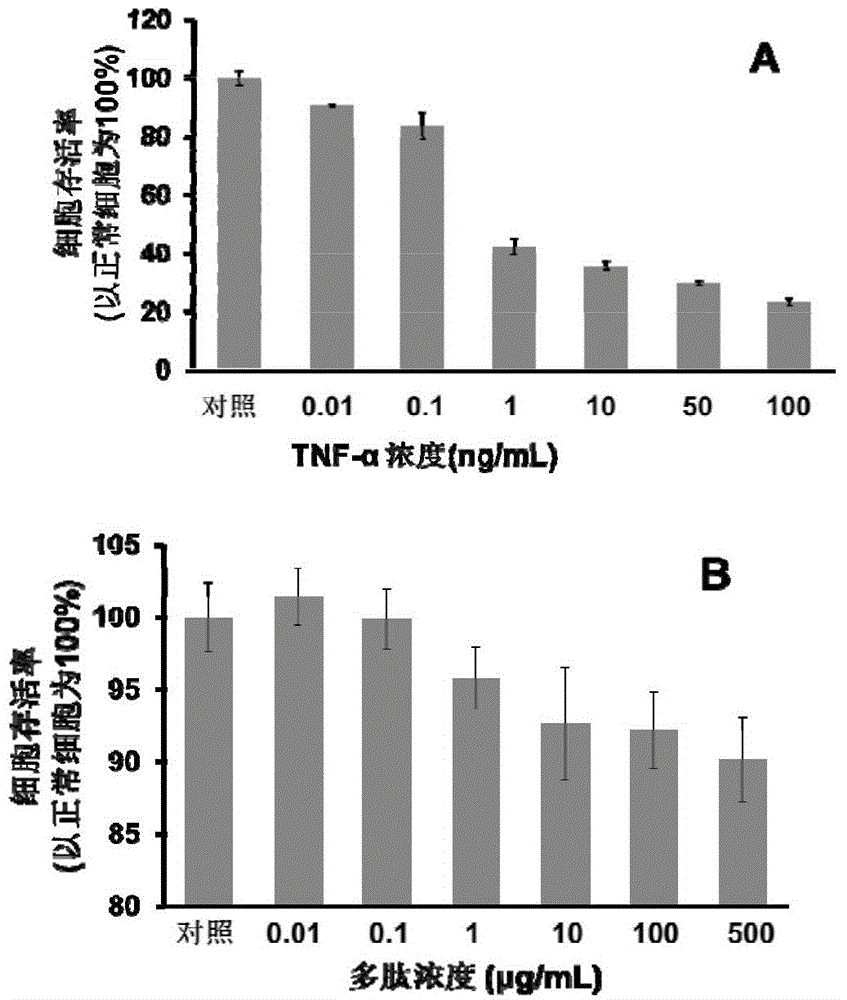
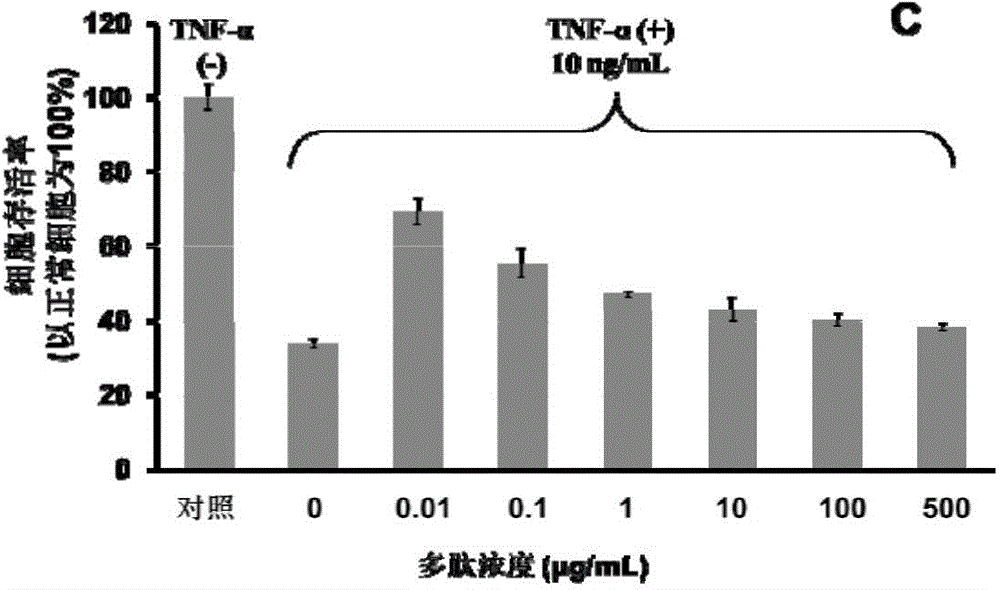
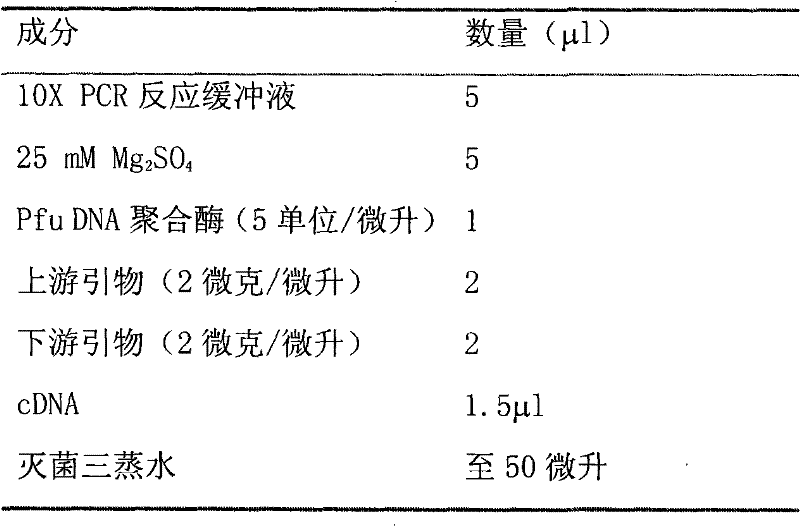
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Anti tnf alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human anti TNF Alpha (Infliximab Biosimilar) Anti-TNF Alpha Antibody is a non-therapeutic biosimilar of the monoclonal antibody drug infliximab (Remicade) for research use. It can be used in bioanalytical PK and ADA assays and for studying biological pathways affected by the drug.
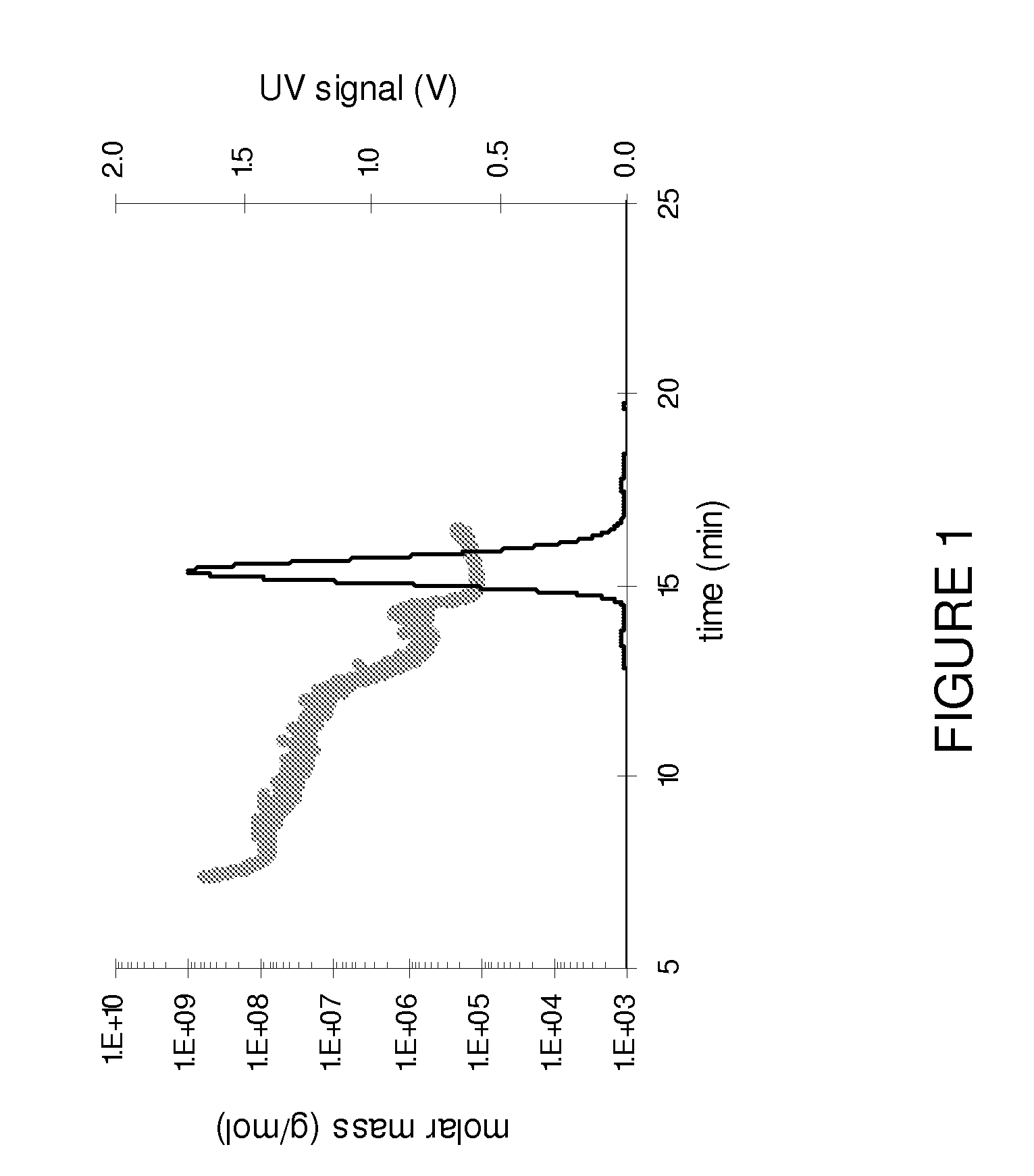
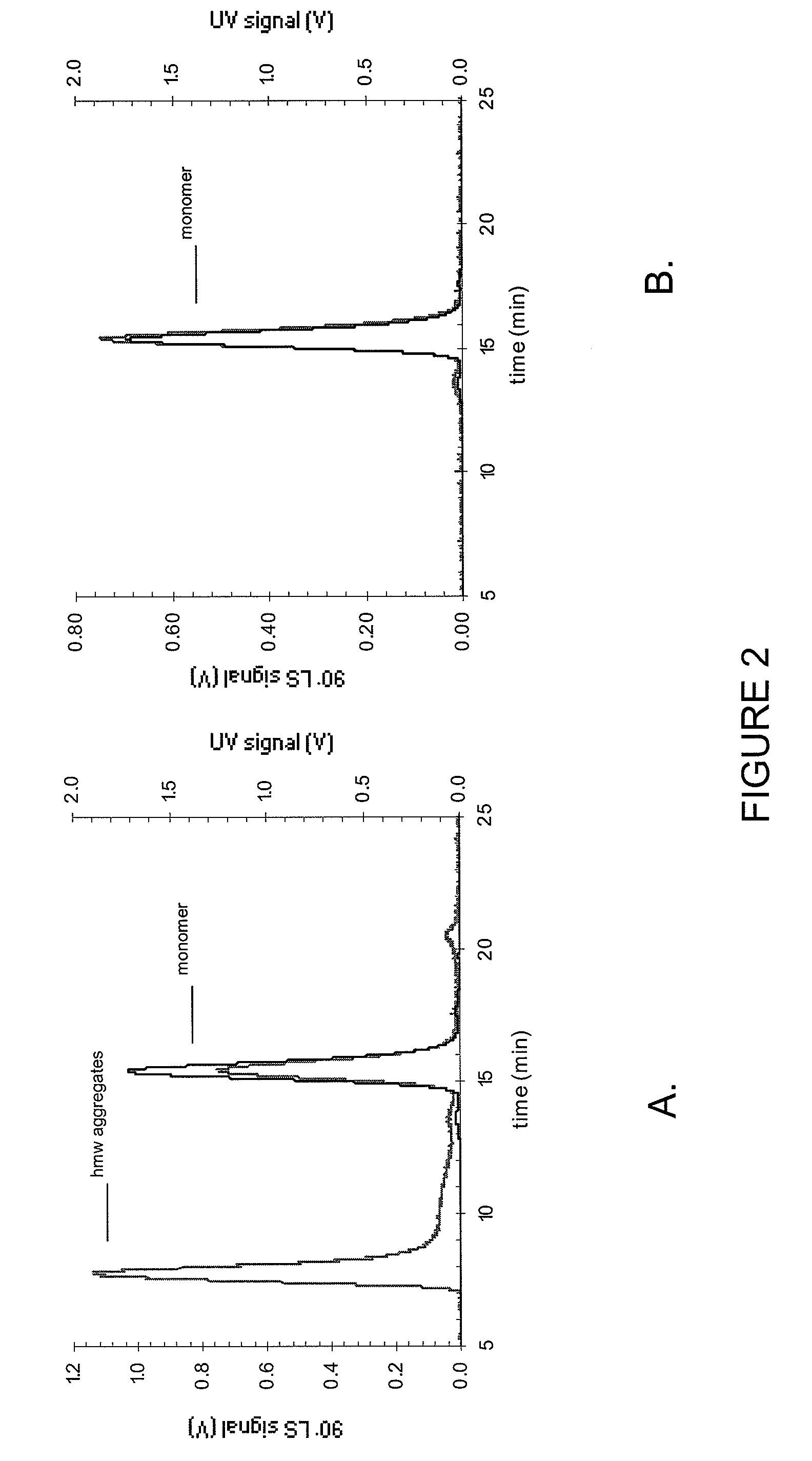
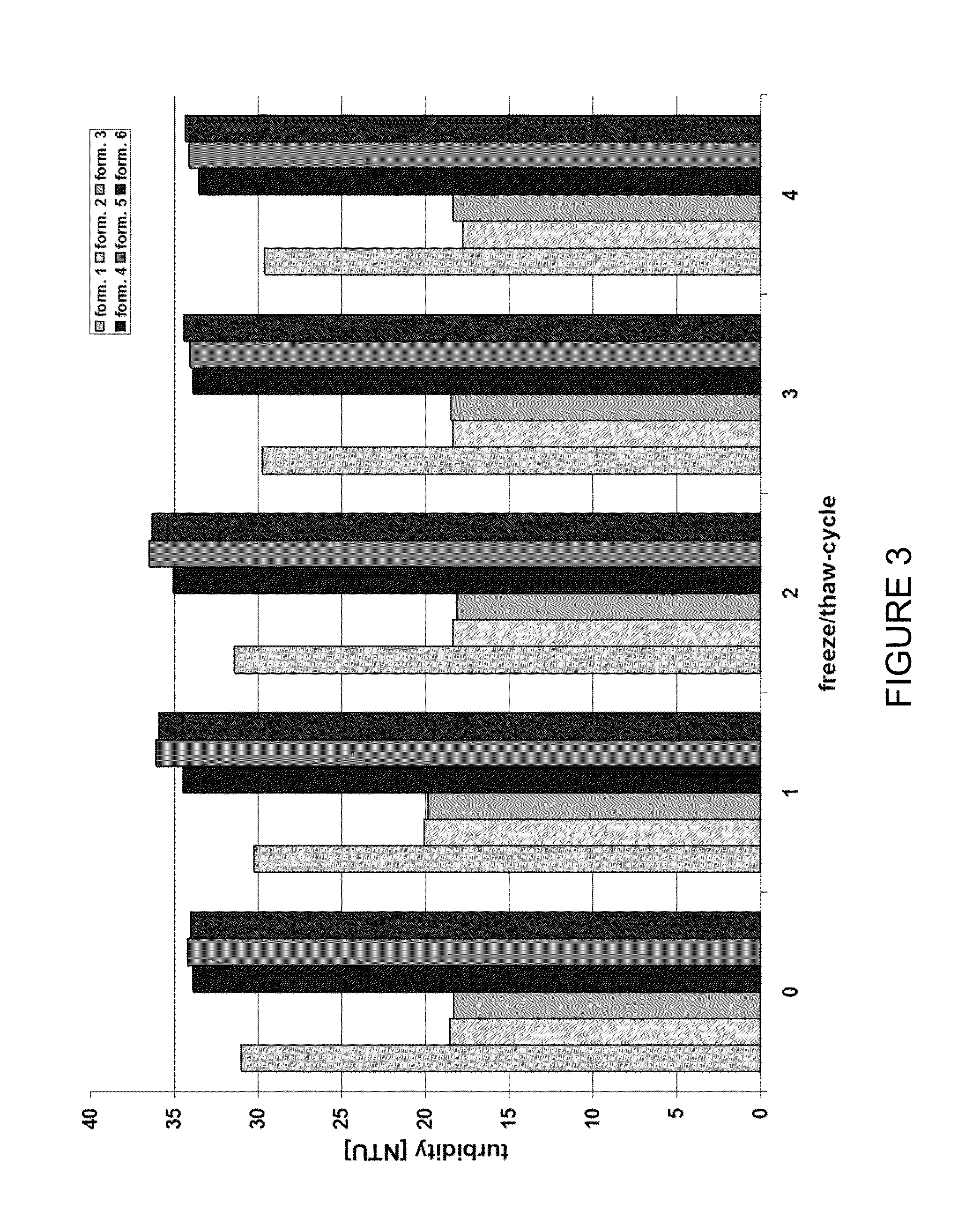
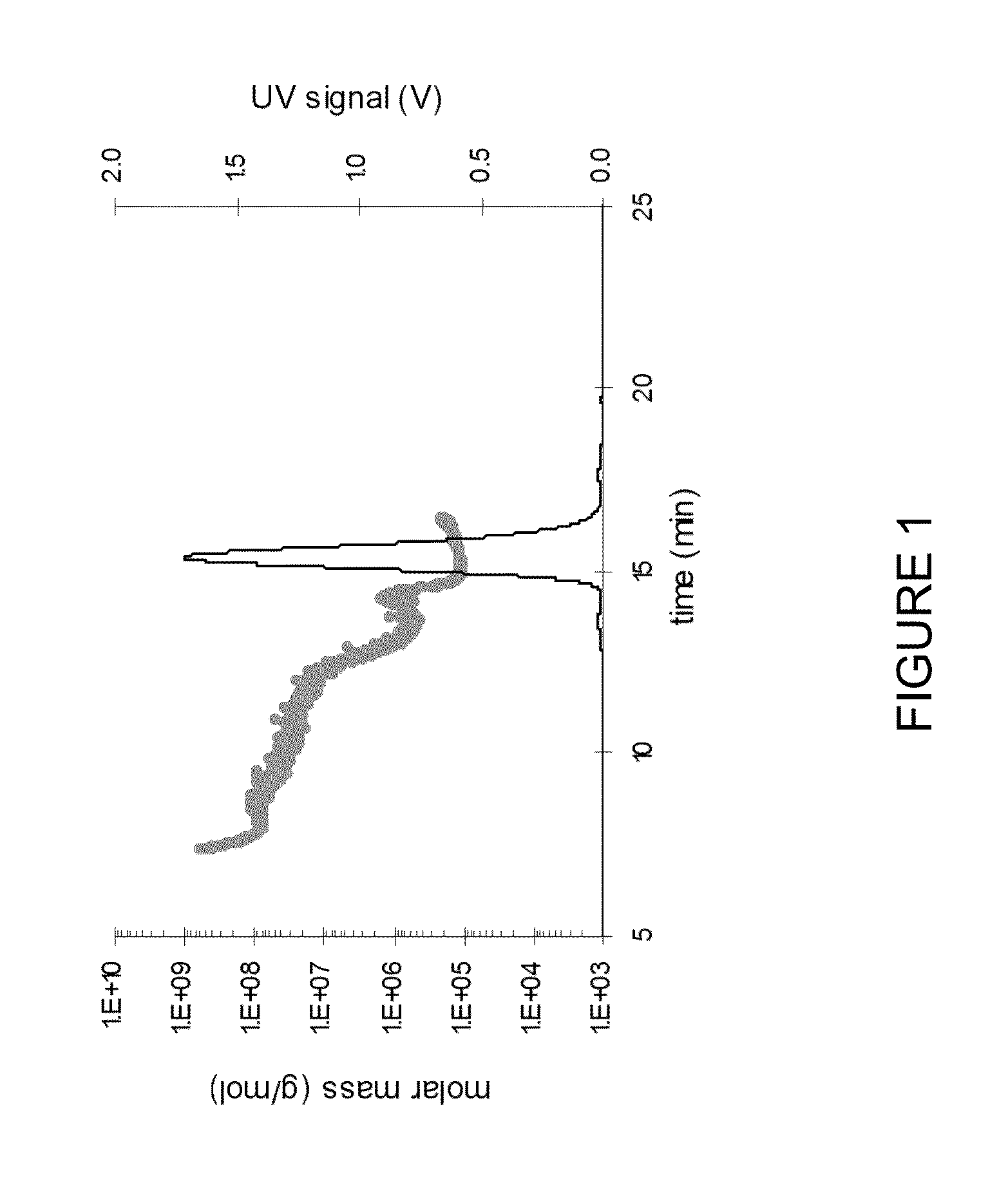
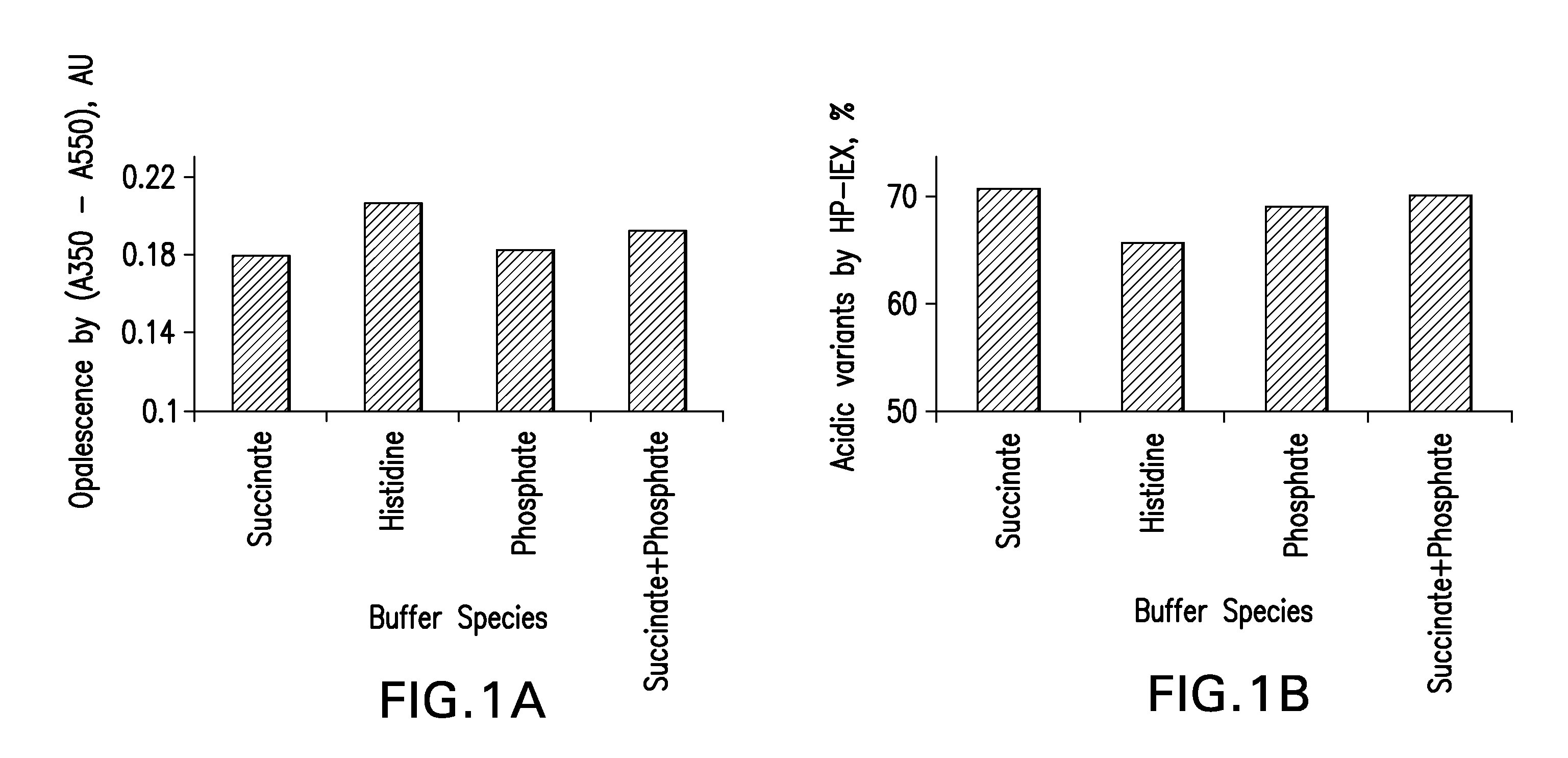
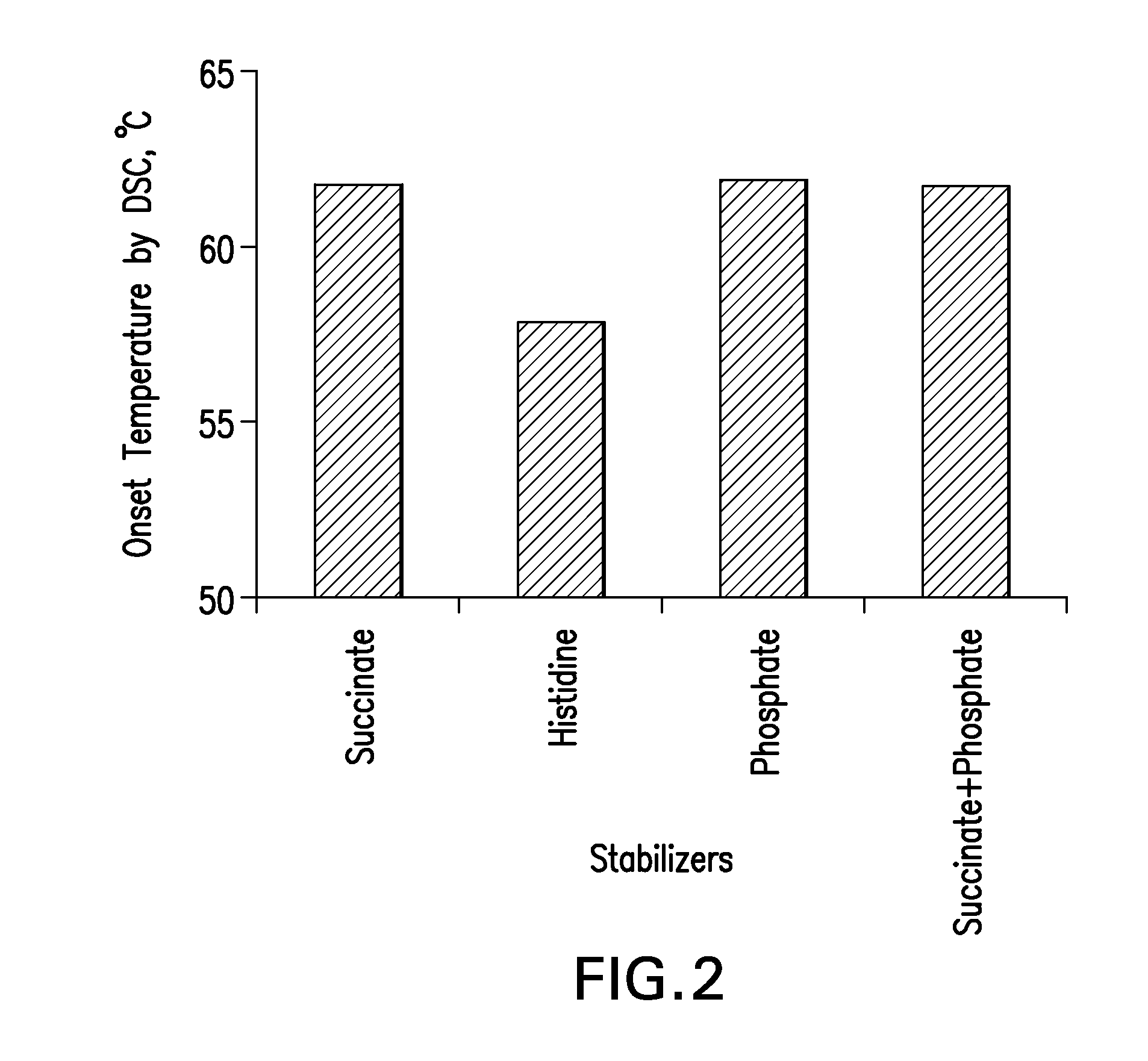
Stable high protein concentration formulations of human Anti-tnf-alpha-antibodies
InactiveUS20100278822A1Suitable viscosityIncrease concentrationAntibacterial agentsSenses disorderHigh concentrationPolyol
The invention provides a liquid pharmaceutical formulation which does not include NaCl and comprises more than 20 mg of a polyol and at least about 100 mg / mL of a human anti-TNF-alpha antibody, or antigen-binding portion thereof. The invention provides a high concentration antibody formulation having long-term stability and advantageous characteristics for subcutaneous administration.
Owner:ABBVIE BIOTECHNOLOGY LTD
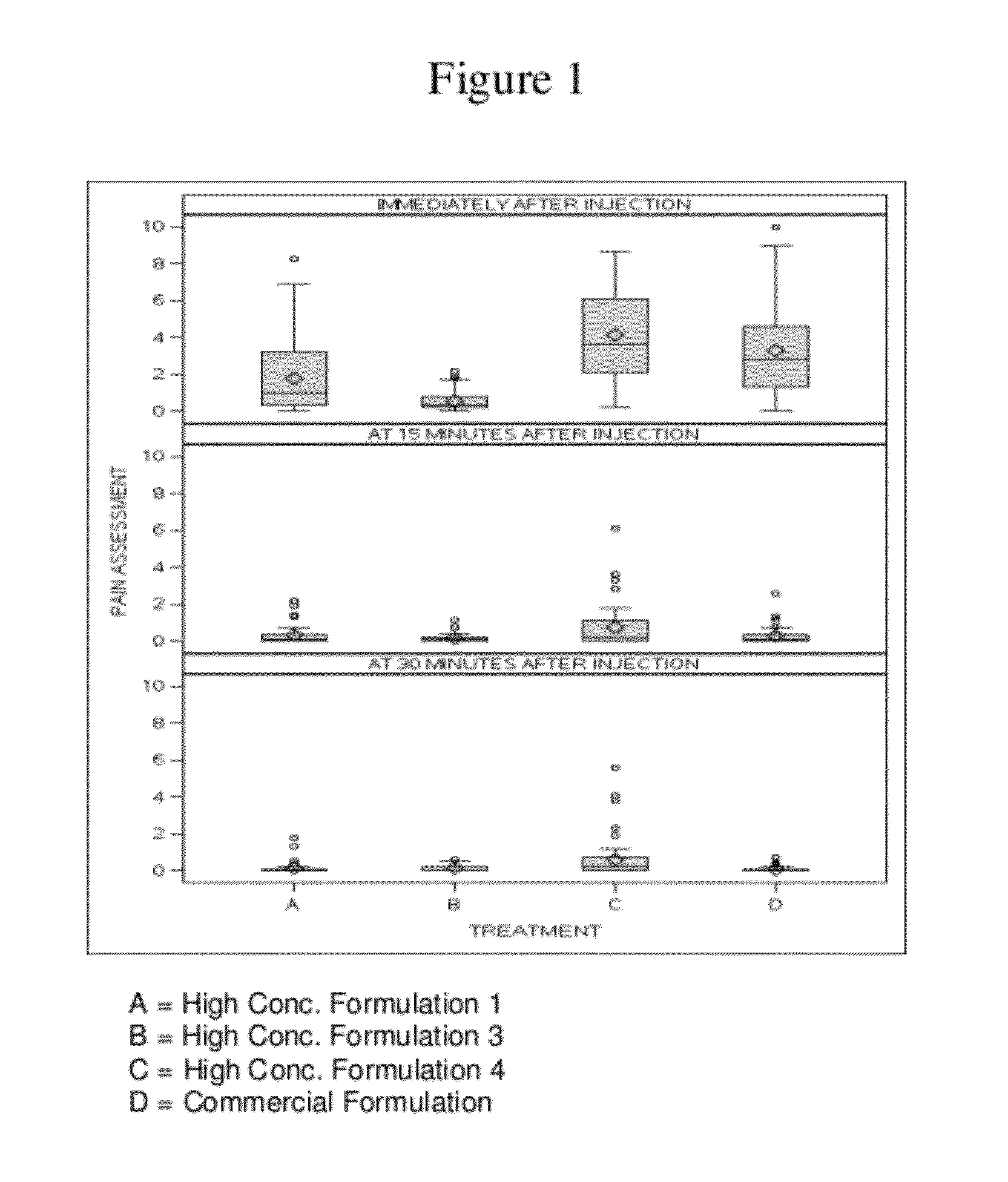
HIGH CONCENTRATION ANTI-TNFalpha ANTIBODY LIQUID FORMULATIONS
ActiveUS20120263731A1Improve bioavailabilityRelieve painSenses disorderNervous disorderHigh concentrationBiosimilar Pharmaceuticals
The invention provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, which reduces pain associated with injection in a subject by at least about 50% when compared to injecting an otherwise identical formulation comprising at least one salt and / or at least one buffer. The invention also provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, having increased bioavailability upon subcutaneous administration into a subject. The formulation may comprise a therapeutic protein, such as a human anti-TNF-alpha antibody, or an antigen-binding portion thereof, or a biosimilar thereof.
Owner:ABBVIE BIOTECHNOLOGY LTD
Stable high protein concentration formulations of human Anti-tnf-alpha-antibodies
InactiveUS20140141007A1High concentrationIncrease concentrationAntibacterial agentsSenses disorderHigh concentrationPolyol
The invention provides a liquid pharmaceutical formulation which does not include NaCl and comprises more than 20 mg of a polyol and at least about 100 mg / mL of a human anti-TNF-alpha antibody, or antigen-binding portion thereof. The invention provides a high concentration antibody formulation having long-term stability and advantageous characteristics for subcutaneous administration.
Owner:ABBOTT LAB INC +1
Stable high protein concentration formulations of human Anti-tnf-alpha-antibodies
InactiveUS20140141008A1High concentrationIncrease concentrationAntibacterial agentsSenses disorderHigh concentrationPolyol
The invention provides a liquid pharmaceutical formulation which does not include NaCl and comprises more than 20 mg of a polyol and at least about 100 mg / mL of a human anti-TNF-alpha antibody, or antigen-binding portion thereof. The invention provides a high concentration antibody formulation having long-term stability and advantageous characteristics for subcutaneous administration.
Owner:ABBOTT LAB INC +1
Compositions and methods for regulation of active TNF- alpha
InactiveUS6020323AReduced activityReduced availabilityEsterified saccharide compoundsBiocideSulfationMedicine
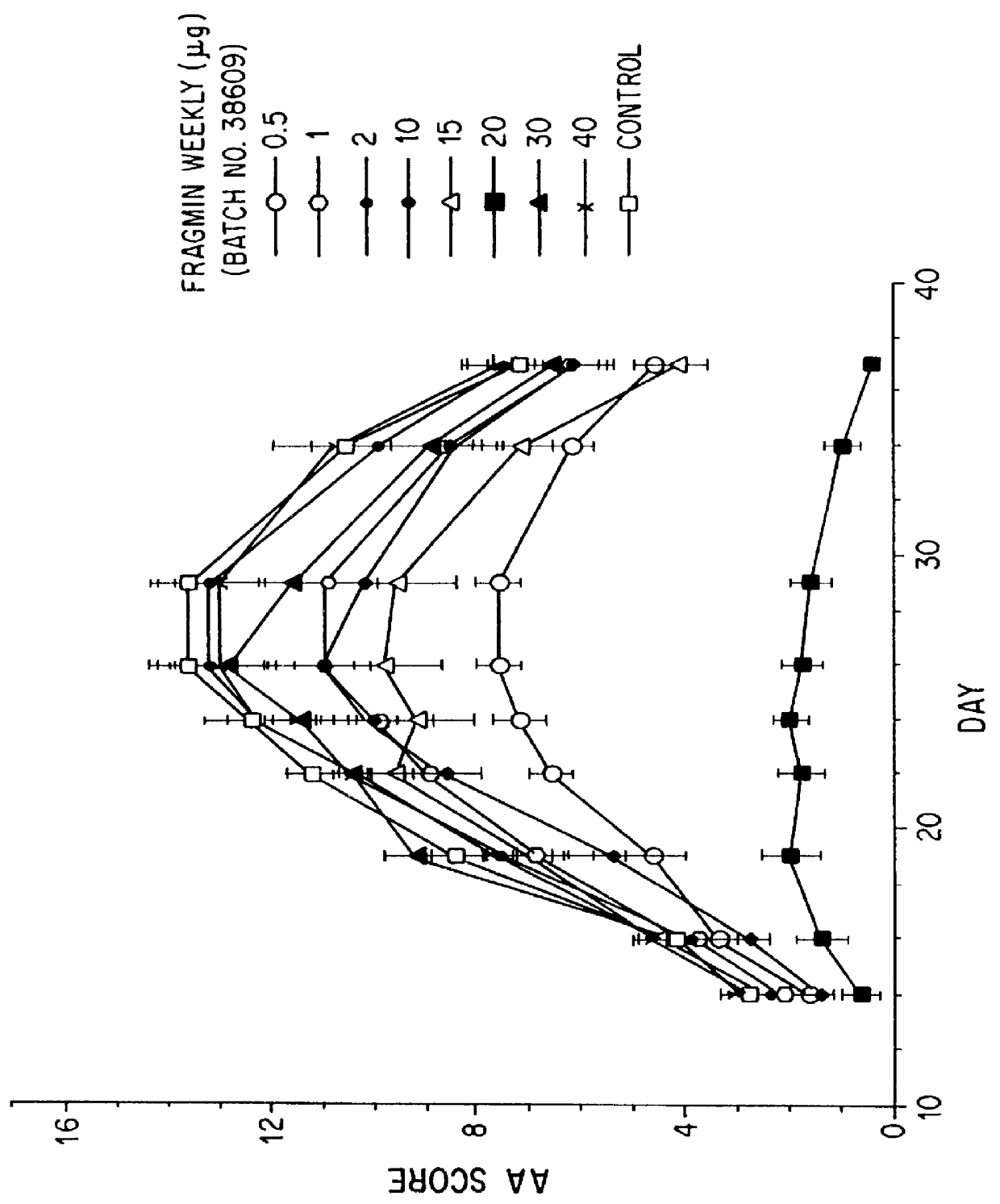
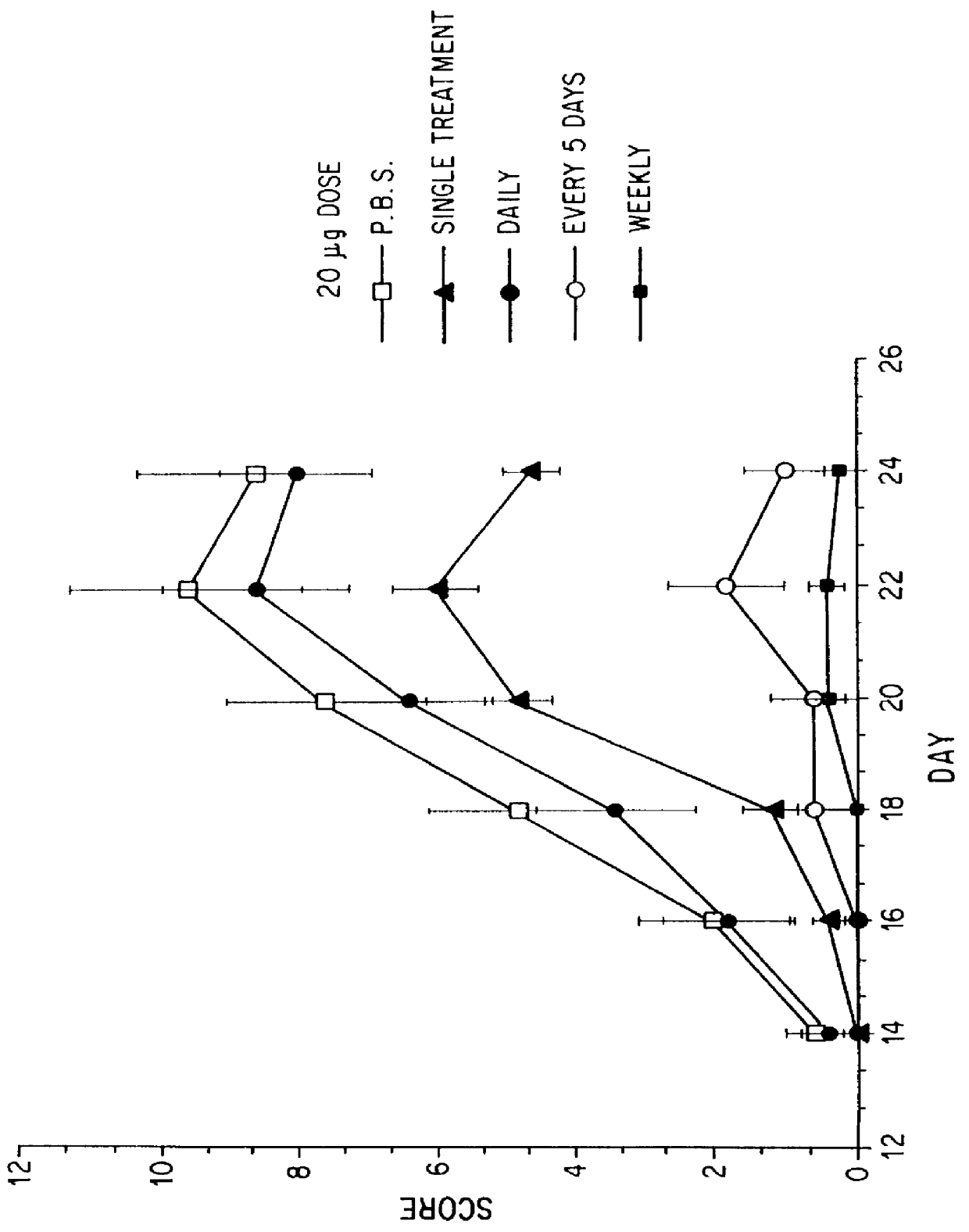
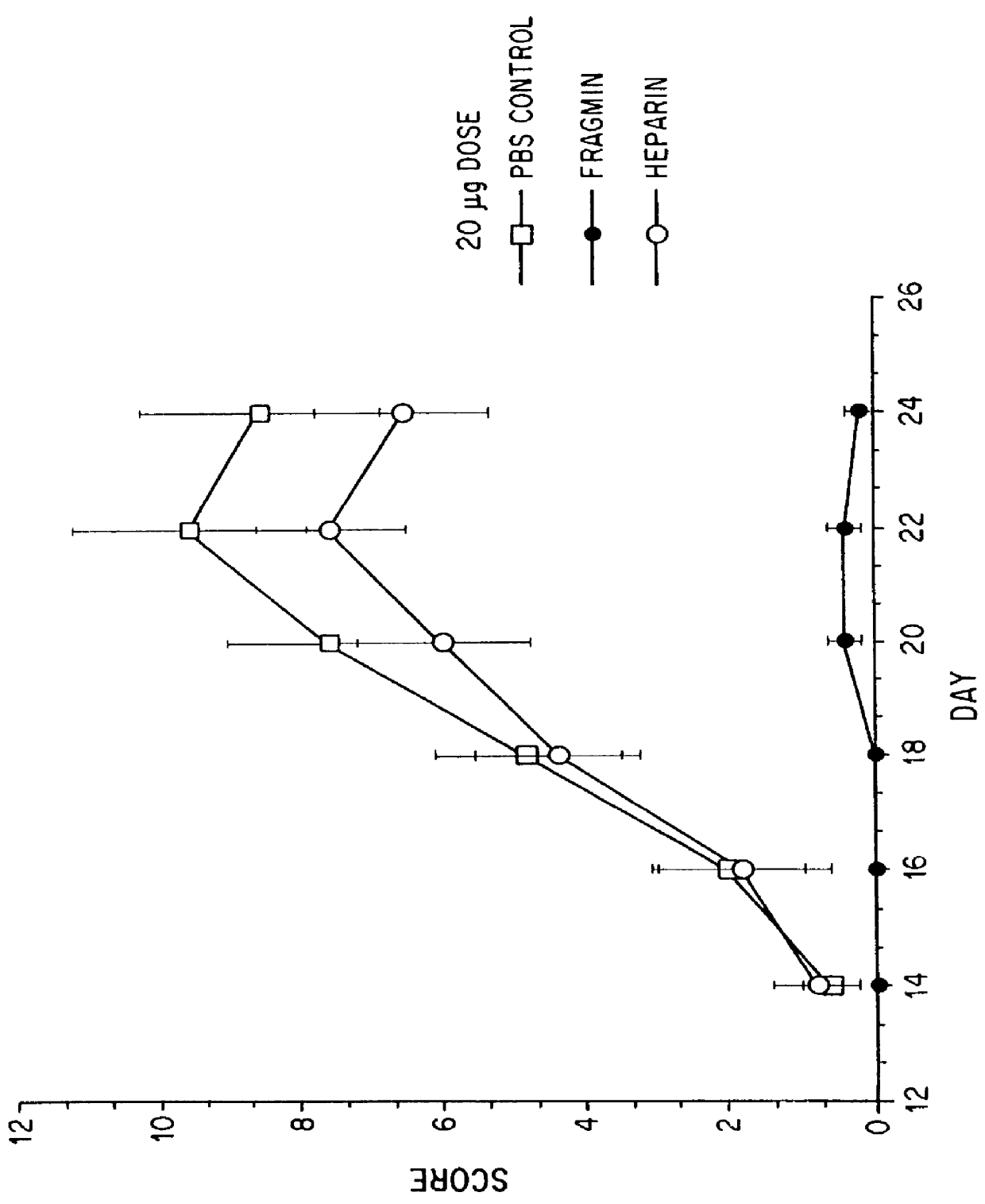
Substances comprising disaccharides and substances comprising carboxylated and / or sulfated oligosaccharides in substantially purified form, and methods of using same, are disclosed for the regulation of cytokine activity in a host. For instance, the secretion of active Tumor Necrosis Factor Alpha (TNF- alpha ) can be either inhibited or augmented selectively by administration to the host of an effective amount of a substance of the invention. Thus, the present invention also relates to pharmaceutical compositions and their use for the prevention and / or treatment of pathological processes involving the induction of active cytokine secretion, such as TNF- alpha . The invention also relates to the initiation of a desirable immune system-related response by the host to the presence of activators, including pathogens. The substances and pharmaceutical compositions of the present invention may be administered daily, at very low effective doses, typically below 0.1 mg / kg human, or at intervals of up to about 5-8 days, preferably once a week.
Owner:YEDA RES & DEV CO LTD
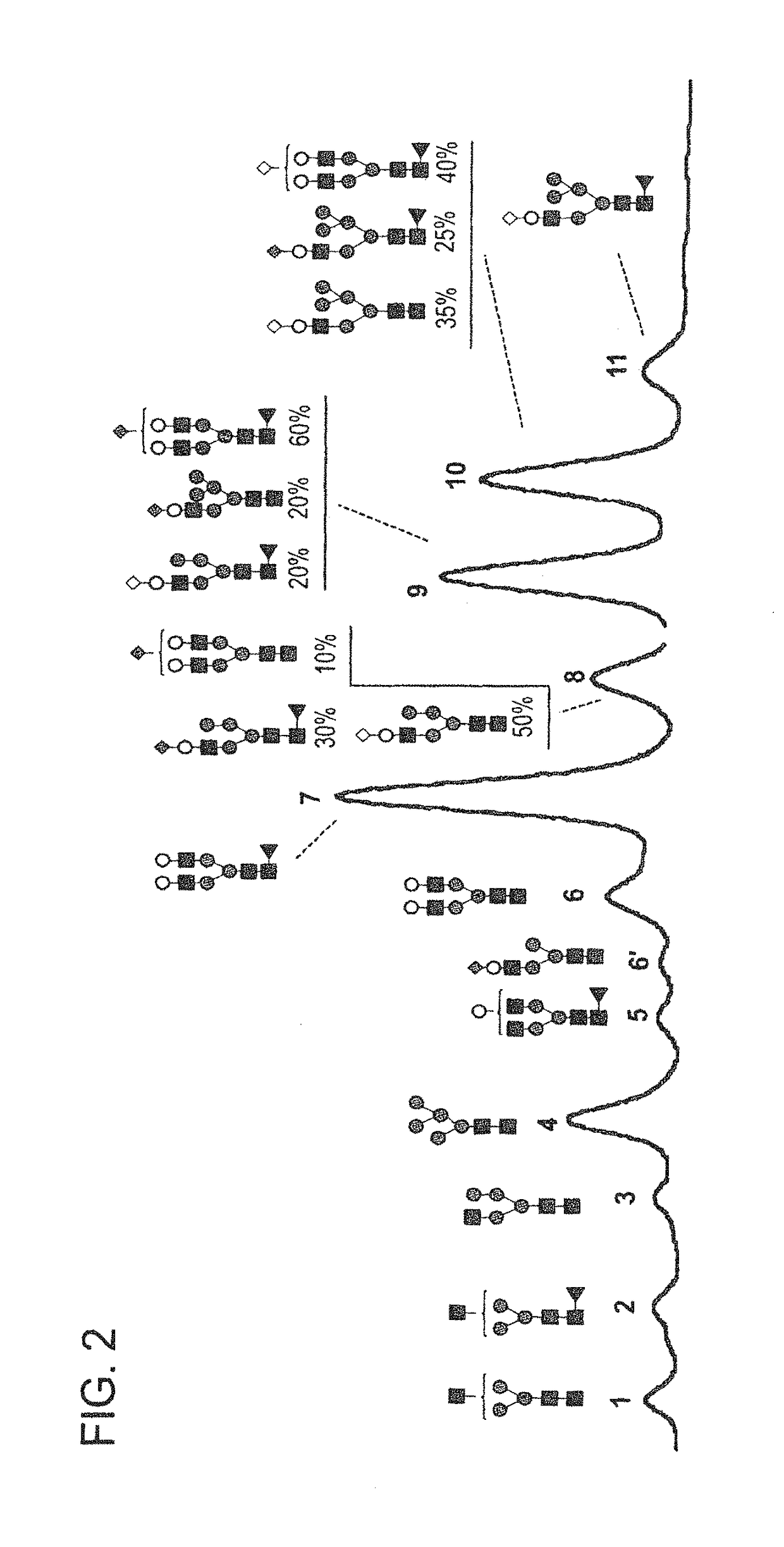
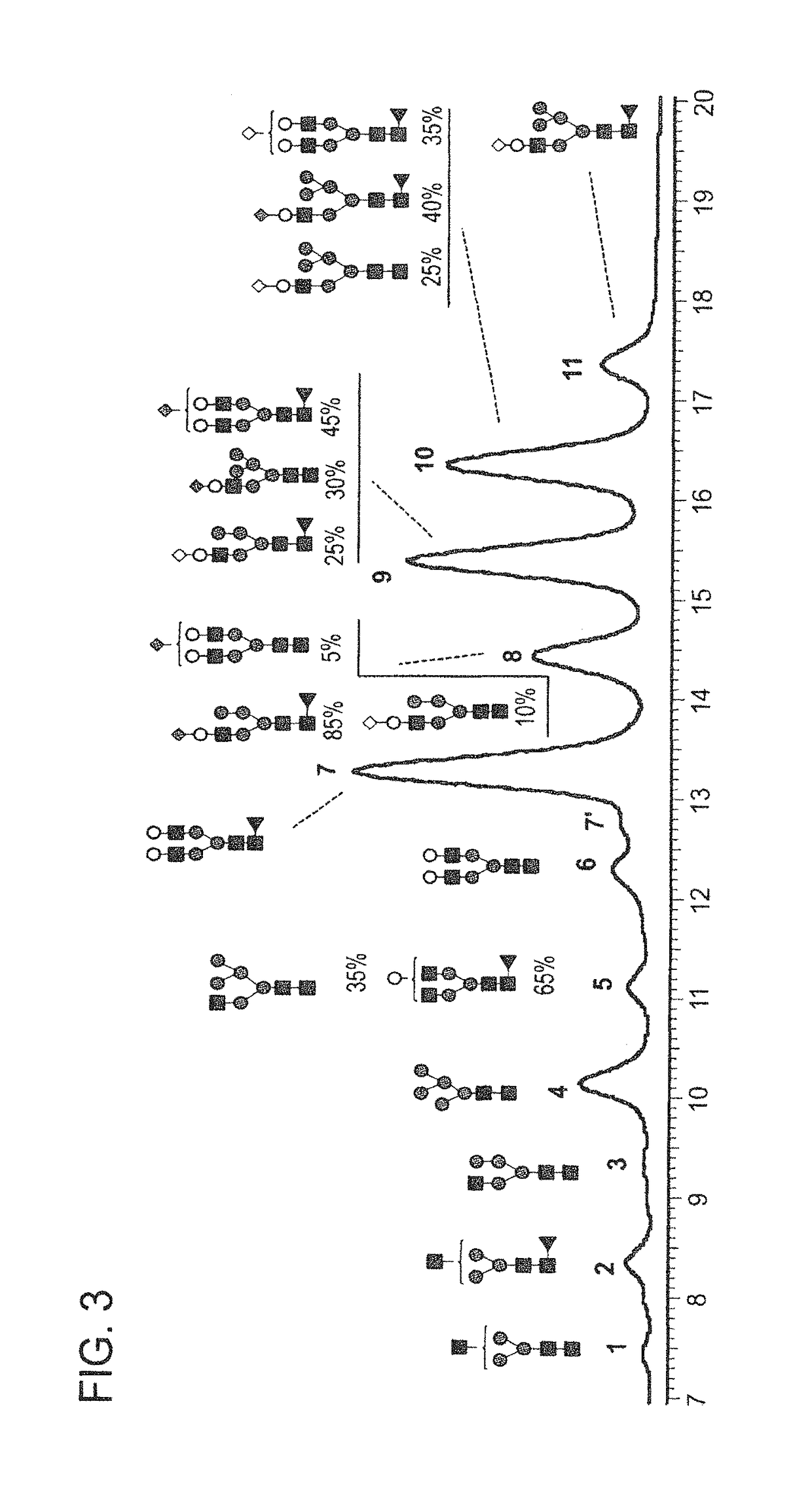
Anti-tnf-alpha glycoantibodies and uses thereof
ActiveUS20150344559A1Increase healing valueHigh binding affinityNervous disorderAntipyreticAntigen Binding FragmentGlycan
The present disclosure relates to a novel class of anti-TNFα monoclonal antibodies or antigen binding fragments comprising a homogeneous population of anti-TNFα IgG molecules having the same N-glycan on each of Fc. The antibodies of the invention can be produced from anti-TNFα monoclonal antibodies by Fc glycoengineering. The glycoantibodies of the invention may have improved therapeutic values compared to the corresponding monoclonal antibodies that have not been glycoengineered.
Owner:ACAD SINIC
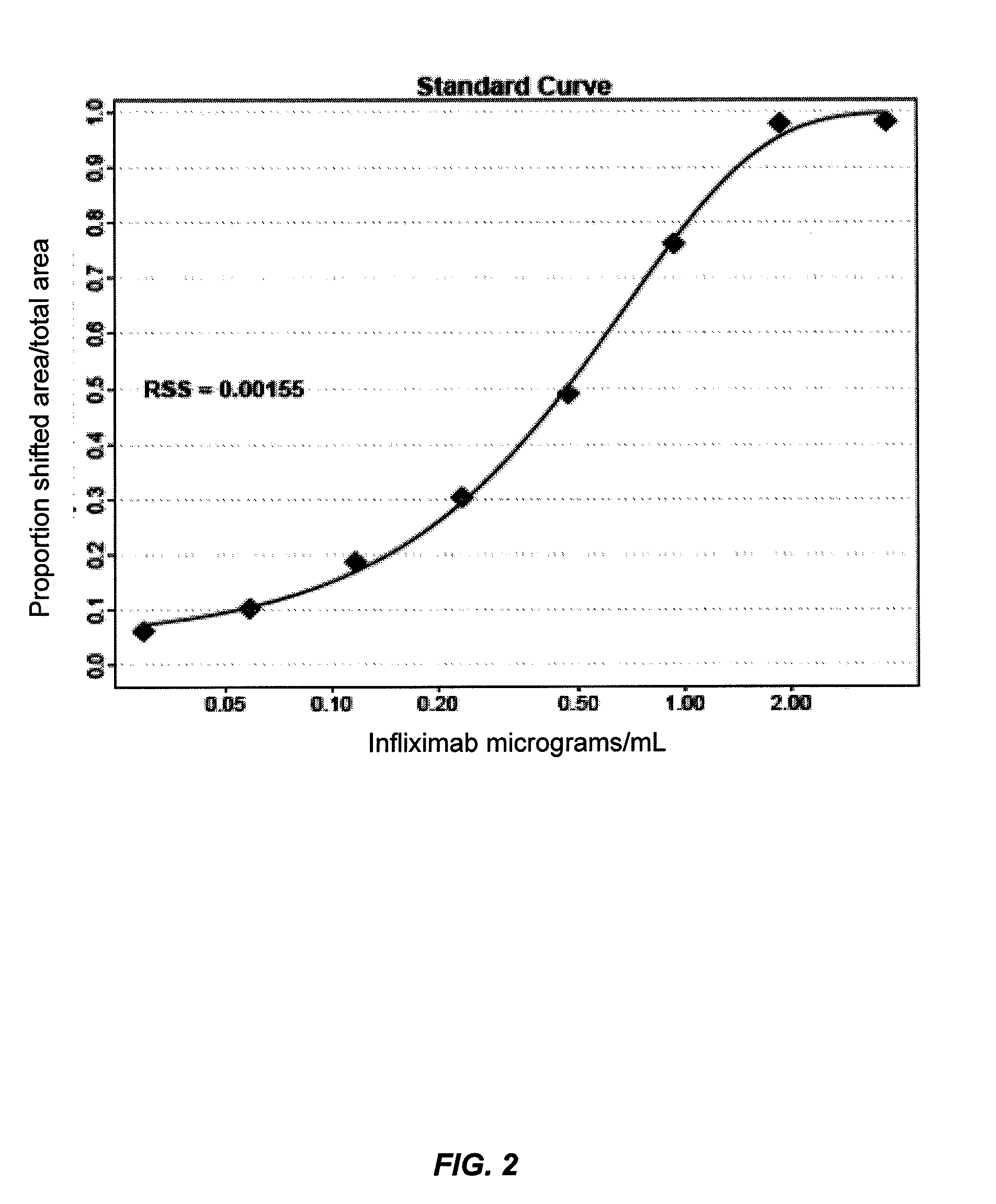
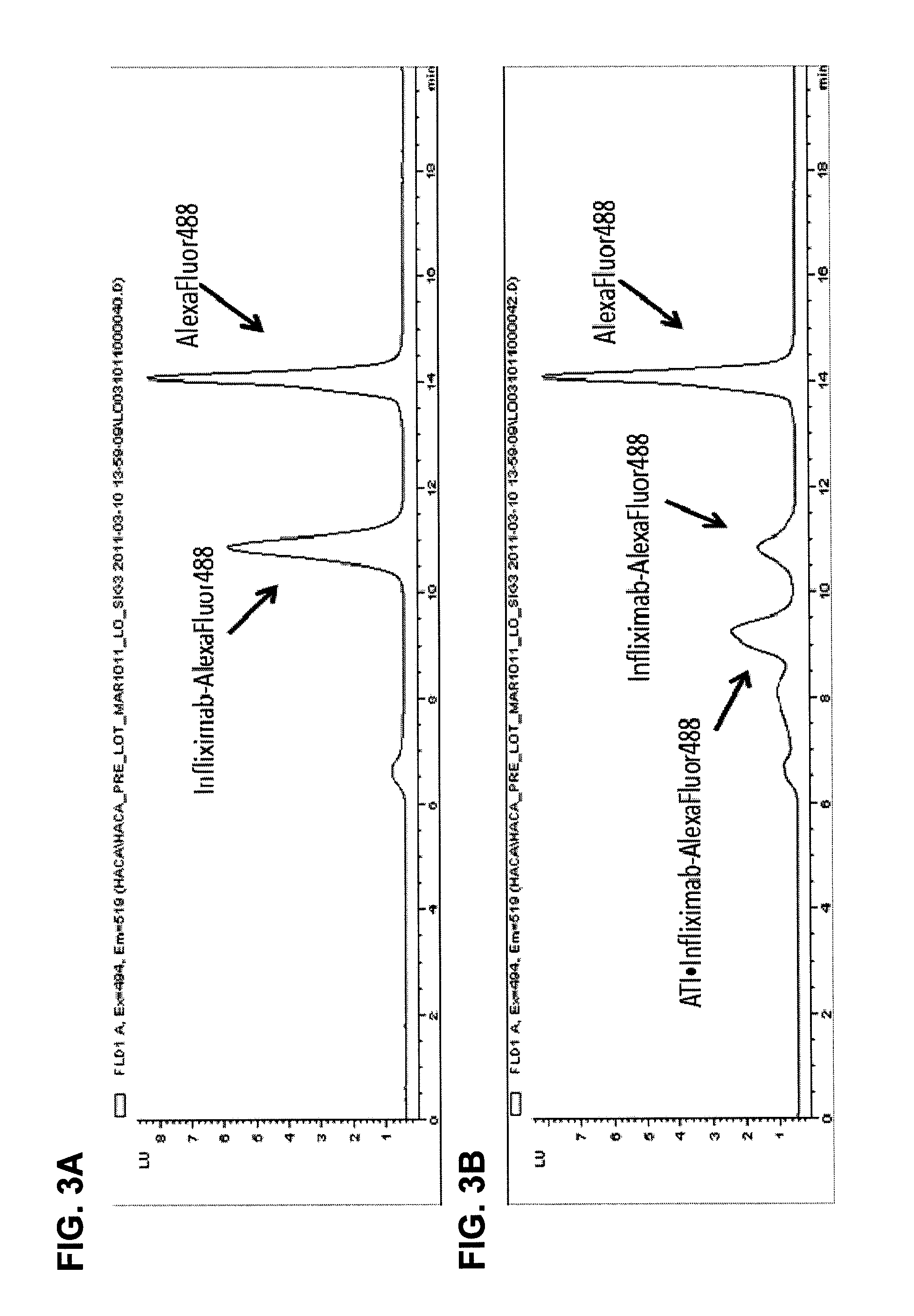
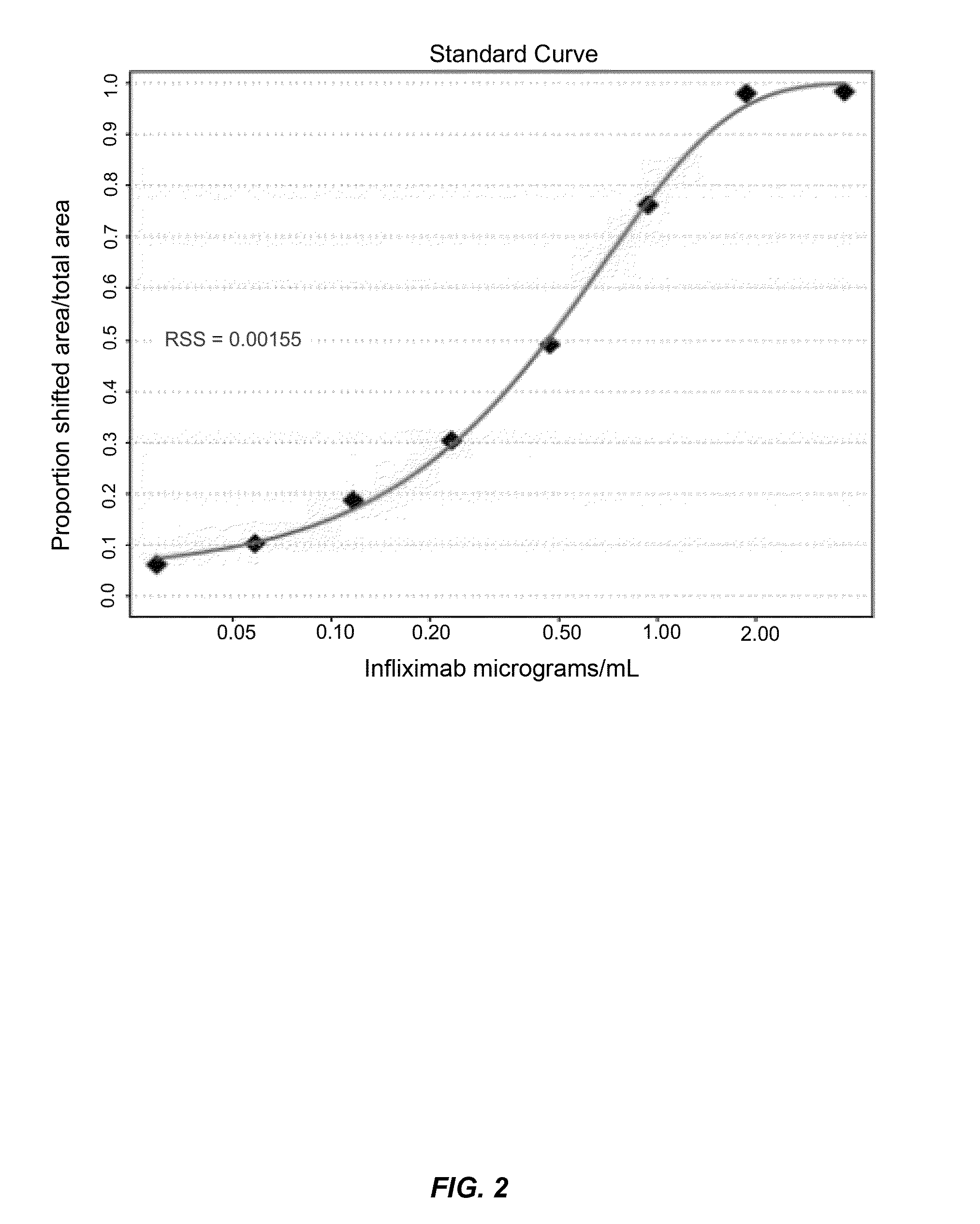
Mobility shift assays for detecting Anti-tnf alpha drugs and autoantibodies thereto
InactiveUS20140051184A1Low toxicityBiological material analysisBiological testingAutoantibody productionDrug treatment
The present invention provides assays for detecting and measuring the presence or level anti-TNFα drugs and / or the autoantibodies to anti-TNFα drugs in a sample. The present invention is useful for optimizing therapy and monitoring patients receiving anti-TNFα drug therapeutics to detect the presence or level of autoantibodies against the drug. The present invention also provides methods for selecting therapy, optimizing therapy, and / or reducing toxicity in subjects receiving anti-TNFα drugs for the treatment of TNFα-mediated disease or disorders.
Owner:NESTEC SA
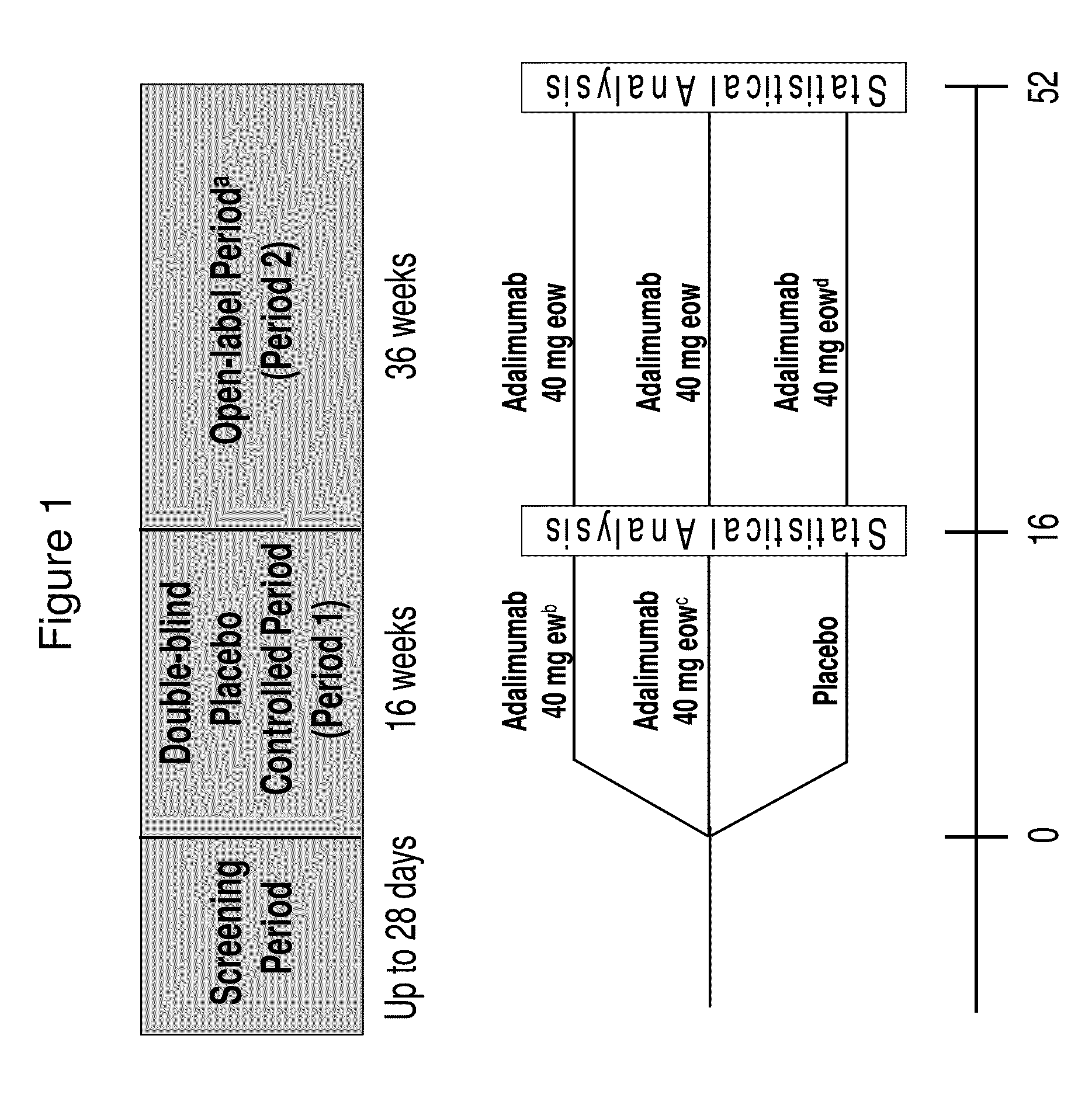
Methods of treating moderate to severe hidradenitis suppurativa with anti-TNF-alpha antibodies
ActiveUS8747854B2Treatment safetyStrong specificityAntipyreticAutomatic syringesAntigen bindingHidradenitis suppurativa
The invention provides methods, uses and compositions for the treatment of hidradenitis suppurativa. The invention describes methods and uses for treating hidradenitis suppurativa, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to treat hidradenitis suppurativa in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treating hidradenitis suppurativa in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
Immunosensor based on hybrid chain reaction and single molecule counting and application of immunosensor
InactiveCN103940989AHigh detection sensitivityReduce usageBiological material analysisFluorescence/phosphorescenceSmall sampleAnti tnf alpha
The invention discloses an immunosensor based on hybrid chain reaction and single molecule counting. The immunosensor consists of a capturing antibody, a detection antibody, streptavidin, a biotinylated primer and a hairpin probe, wherein the capturing antibody is anti-TNF-alpha; the detection antibody is Bio-anti-TNF-alpha; a base sequence of the biotinylated primer (Bio-I) is 5'-AGT CTA GGA TTC GGC GTG GGT TAA TTT TTT TTT-biotin-3'; the hairpin probe consists of a hairpin probe H1 and a hairpin probe H2; a base sequence of the hairpin probe H1 is 5'-TTA ACC CAC GCC GAA TCC TAG ACT CAA AGT AGT CTA GGA TTC GGC GTG-3'; a base sequence of the hairpin probe H2 is 5'-AGT CTA GGA TTC GGC GTG GGT TAA CAC GCC GAA TCC TAG ACT ACT TTG-3'. The invention also provides a preparation method of the immunosensor and application of the immunosensor in detection of the TNF-alpha. The immunosensor disclosed by the invention has the advantages of high detection sensitivity, small sample use amount, no need of enzyme amplification and the like, and quantitative detection on low-concentration TNF-alpha can be realized.
Owner:SHANDONG UNIV
Mobility shift assays for detecting Anti-tnf alpha drugs and autoantibodies
The present invention provides assays for detecting and measuring the presence or level of anti-TNFα drugs and / or the autoantibodies to anti-TNFα drugs in a sample. The present invention is useful for optimizing therapy and monitoring patients receiving anti-TNFα drug therapeutics to detect the presence or level of autoantibodies against the drug. The present invention also provides methods for selecting therapy, optimizing therapy, and / or reducing toxicity in subjects receiving anti-TNFα drugs for the treatment of TNFα-mediated disease or disorders.
Owner:NESTEC SA
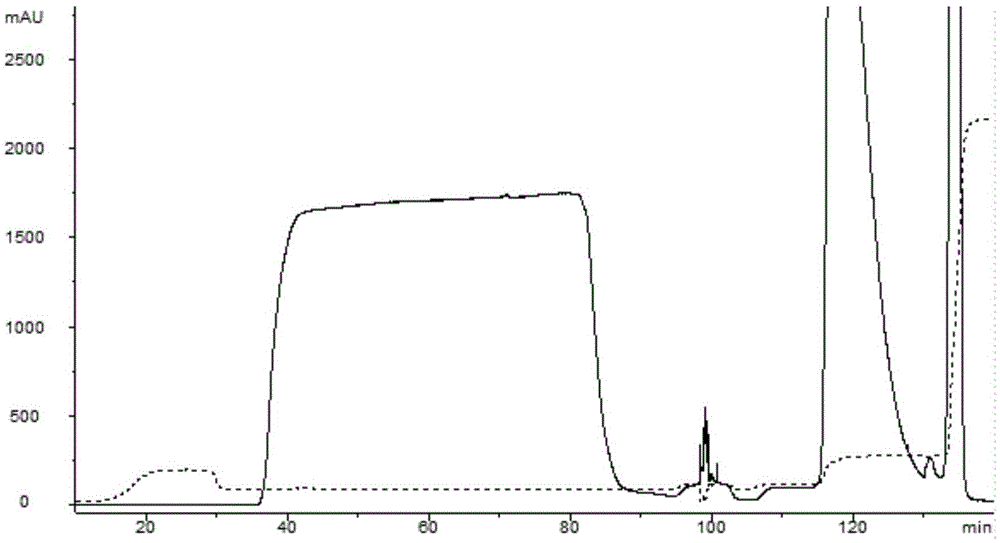
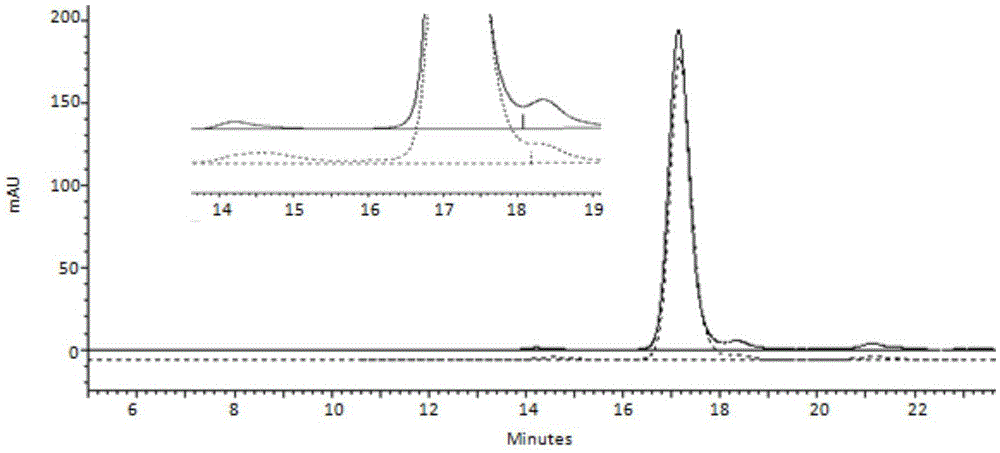
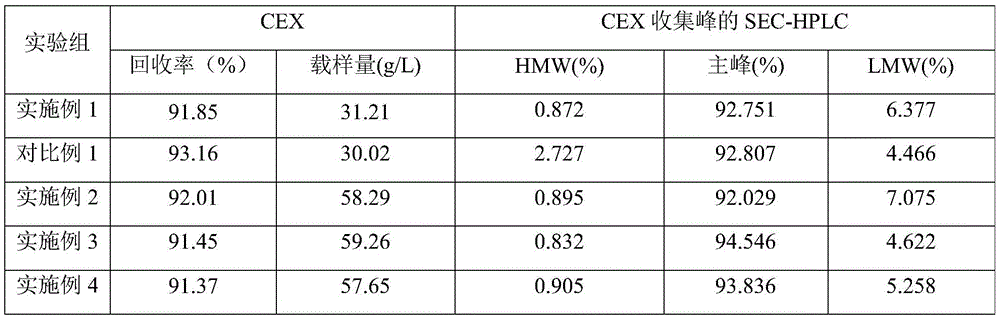
Cation exchange chromatographic purification method anti-TNF alpha-type monoclonal antibody
InactiveCN105777904AImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsPurification methodsMonoclonal antibody
The invention discloses a cation exchange chromatographic purification method an anti-TNF alpha-type monoclonal antibody. The method includes: adding urea and a betaine surfactant in the process of CEX multistep elution to remove pollutants in a mixture containing the antibody and the pollutants to separate a polymer from the antibody. The method has good pollutant separation effect, the chromatographic process is simple in sample adjusting, drastic changes of pH value are avoided, and content of the polymer in a final purified product is lowered remarkably.
Owner:SUNSHINE LAKE PHARM CO LTD
Human tumour necrosin antibody, its preparation and medicinal composition
InactiveCN1613874AOvercome the disadvantage of high immunogenicityThe preparation procedure is advanced and simpleAntipyreticAnalgesicsFactor iiWilms' tumor
This invention relates to a full human anti-TNF-alpha monocloned antibody with strong specificity and low immunogenicity as well as its preparation and medicinal composition therewith.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
Application of substituted aryl hydrazone compound serving as anti-tumor necrosis factor inhibitor medicament
ActiveCN101766602AEffective pharmacological activityOrganic active ingredientsAntineoplastic agentsBone marrow fibrosisAnkylosing spondylitis
The invention relates to clinical application of a substituted aryl hydrazone compound and a derivative thereof serving as an anti-tumor necrosis factor inhibitor. A test result shows that the compound has a lower cytotoxicity in animal bodies, so the medical compound is expected to be used as an anti-TNF-alpha and TNF-beta inhibitor in the clinical application and is mainly used for treating some TNF related immunological diseases such as rheumatic arthritis, inflammatory bowel diseases, diabetes, hematosepsis, psoriasis, ankylosing spondylitis and some communicable diseases including HIV. A current TNF related research also shows that the medical compound can be used for treating clinical blood and solid tumor such as myelodysplastic syndrome, myelofibrosis, acute bone marrow cell leucocythemia, acute / chronic graft-versus-host reaction, ovarian cancer, nephrocyte cancer and the like.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
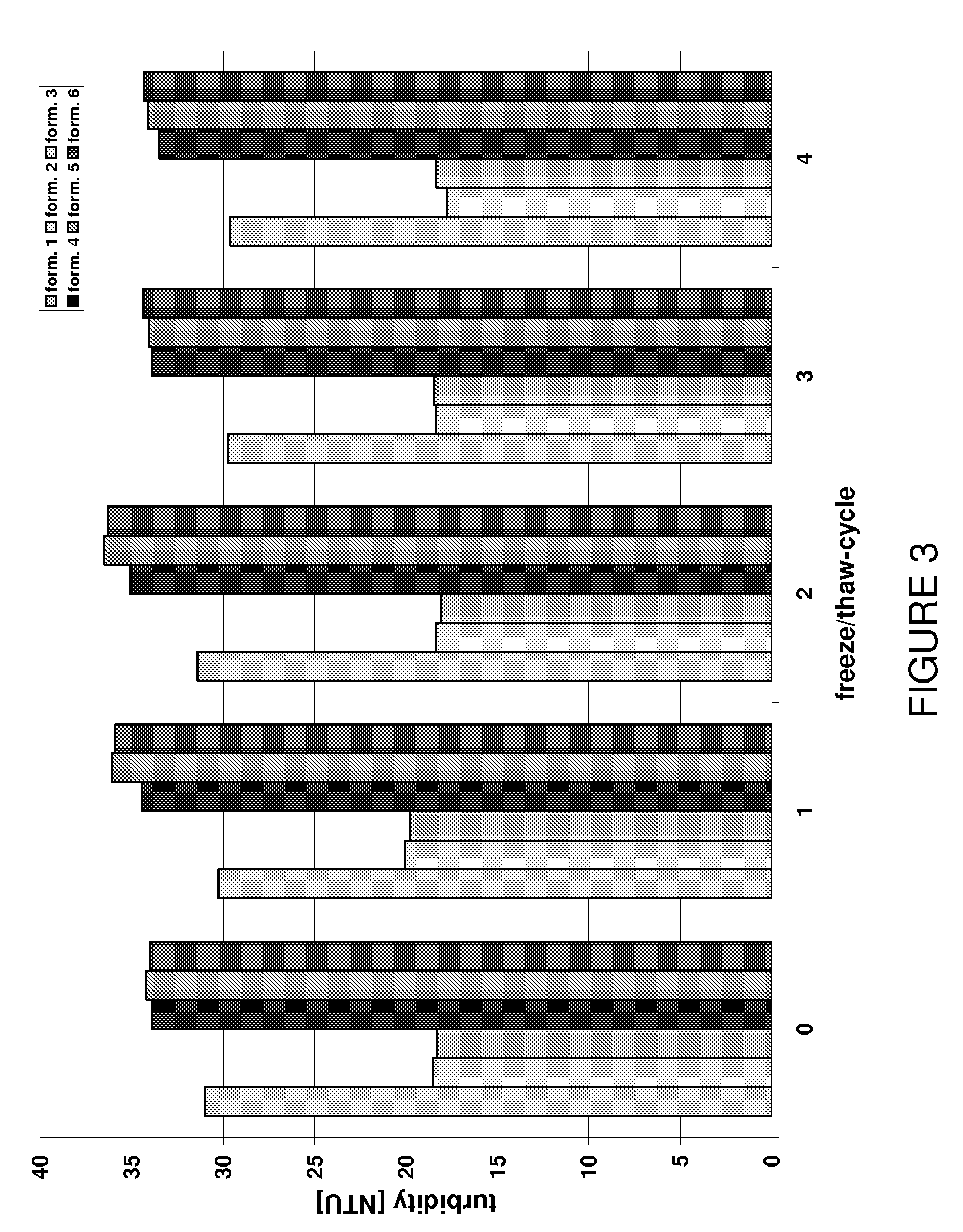
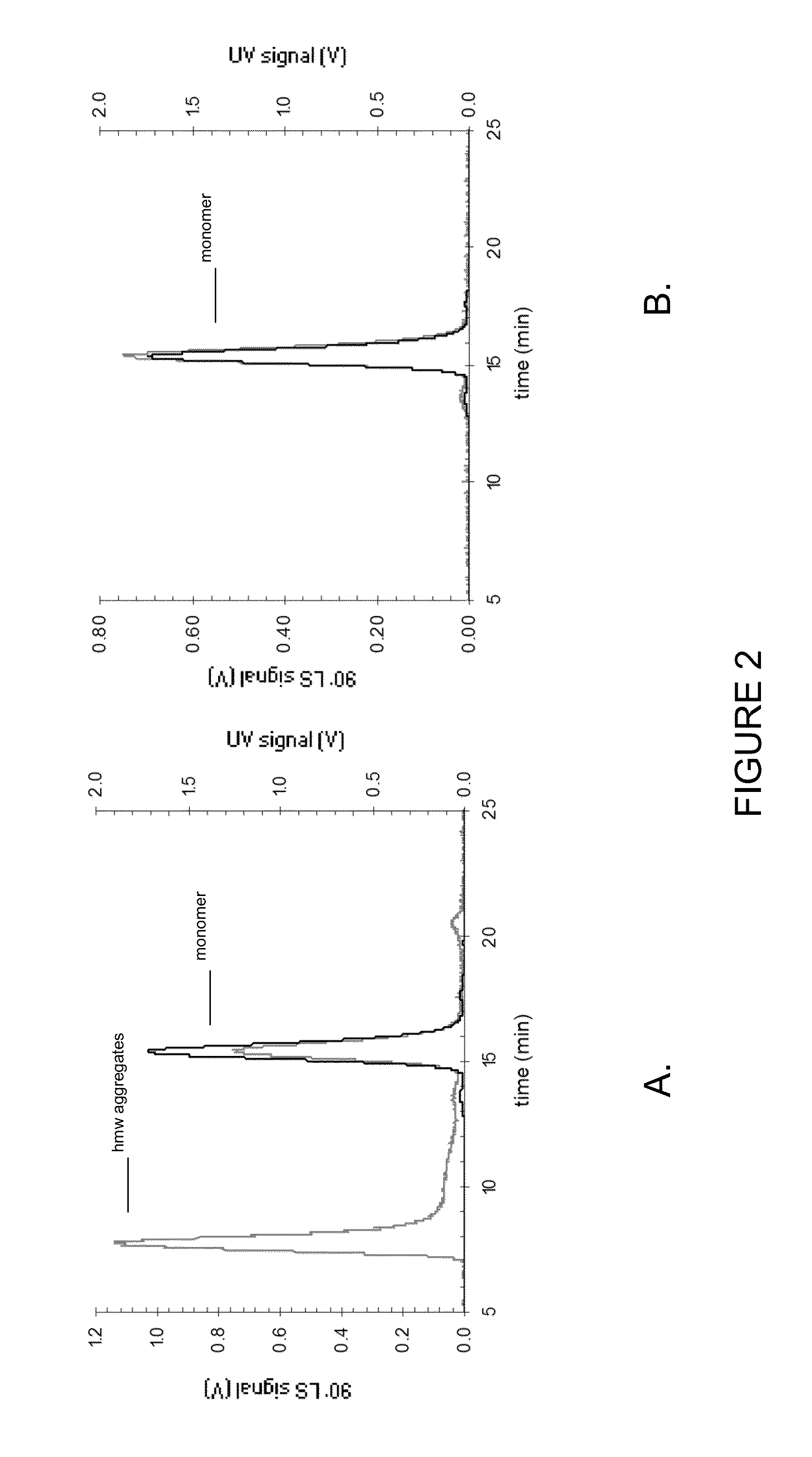
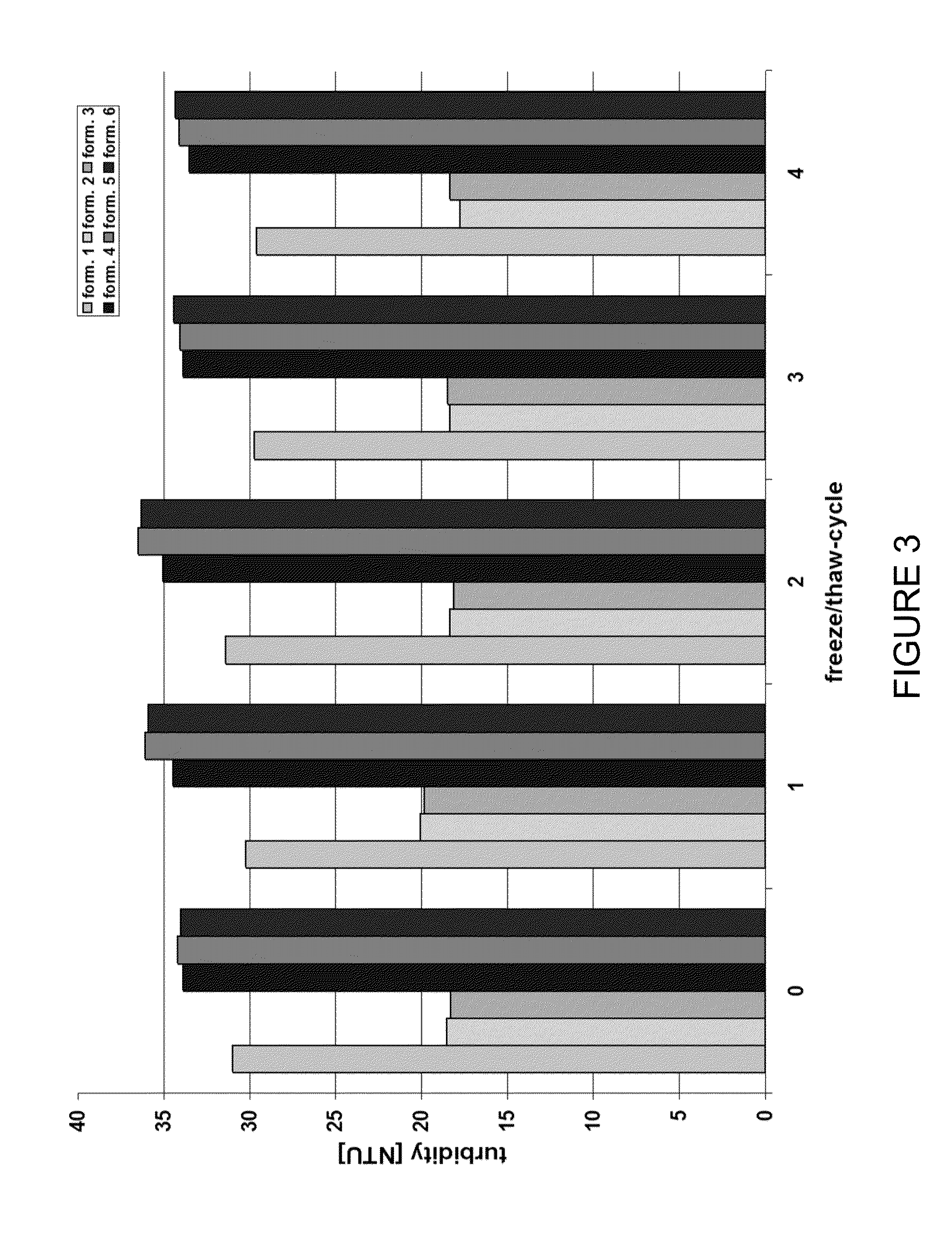
Stable anti-TNF-alpha antibody preparation and uses thereof
ActiveCN104666242AReduce the rate of chemical degradation reactionsGood chemical stabilityAntipyreticAnalgesicsSide effectInjection site
The present invention relates to a stable anti-TNF-alpha antibody preparation and uses thereof. Particularly the preparation comprises: (i) a therapeutically effective amount of an anti-TNF-alpha antibody, (ii) a buffer system containing 0.8-6.2 mg / ml histidine, (iii) an osmotic pressure adjusting agent, and (iv) a surfactant, wherein the pH value of the preparation is 5.5-6.5. According to the present invention, with the preparation, the chemical degradation reaction rate of the anti-TNF-alpha monoclonal antibody can be effectively reduced, the chemical stability of the antibody can be improved, the shelf life of the product can be prolonged, the side effect at the injection site of the patient can be eliminated or reduced, and the medication comfort of the patient can be improved. In addition, the present invention further discloses a method for stabilizing the antibody and the uses of the preparation.
Owner:INNOVENT BIOLOGICS (SUZHOU) CO LTD
Method for Predicting the Response of a Patient to Treatment with an Anti-TNF Alpha Antibody
InactiveUS20100196402A1Reduced suppressive capacityRestoring toleranceAntipyreticMicrobiological testing/measurementRegulatory T cellT cell
The present invention relates to a method for predicting the response of a patient to treatment with an anti-TNFα therapy, in particular an anti-TNFα antibody, the method comprising: (a) providing an in vitro sample of T cells from the patient; (b) exposing said T cells to an anti-TNFα therapy; and (c) determining whether regulatory T cells are induced in said sample of T cells wherein the induction of regulatory T cells indicates that the patient is likely to respond to treatment with said anti-TNFα therapy.
Owner:UCL BUSINESS PLC
Modified anti-tnf aplha antibody
InactiveUS20040260069A1Modify characteristicLow immunogenicityPeptide/protein ingredientsAntipyreticHuman tumorV region
The invention relates to the modification of antibodies reactive to human tumor necrosis factor alpha (TNF alpha) to result in anti-TNF alpha antibodies that are substantially non-immunogenic or less immunogenic than any non-modified parental antibody when used in vivo. The invention relates also to peptide molecules comprising T-cell epitopes of the V-regions of the parental antibody which are modified by amino acid alteration in order to reduce or eliminate said T-cell epitopes.
Owner:MERCK PATENT GMBH
Antibody composition for TNF-alpha, and application thereof
ActiveCN107485713AReduce pain and discomfortRealize the technical effect with a shelf life of 30 monthsAntipyreticAnalgesicsDiseaseSolvent
The invention provides an antibody composition for TNF-alpha, and an application thereof. The solvent of the composition comprises water, and the solute of the composition comprises an anti-TNF-alpha antibody; and the pH value of the composition is 4.0-8.0, and the pH value is adjusted through introducing carbon dioxide to the solvent and solute mixture of the composition. Compared with antibody compositions in the prior art, the antibody composition contains no common buffer systems, adopts carbon dioxide introduction to substitute a buffer system and keep the pH value of a preparation stable, reduces the pains and discomfort during patient injection, and is of great significance to treating TNF-alpha-related diseases.
Owner:BIO THERA SOLUTIONS
Assays for detecting autoantibodies to anti-TNF alpha drugs
The present invention provides assays for detecting and measuring the presence or level of autoantibodies to anti-TNFa drug therapeutics in a sample. The present invention is useful for optimizing therapy and monitoring patients receiving anti-TNFa drug therapeutics to detect the presence or level of autoantibodies against the drug. The present invention also provides methods for selecting therapy, optimizing therapy, and / or reducing toxicity in subjects receiving anti-TNFa drugs for the treatment of TNFa-mediated disease or disorders.
Owner:SOC DES PROD NESTLE SA
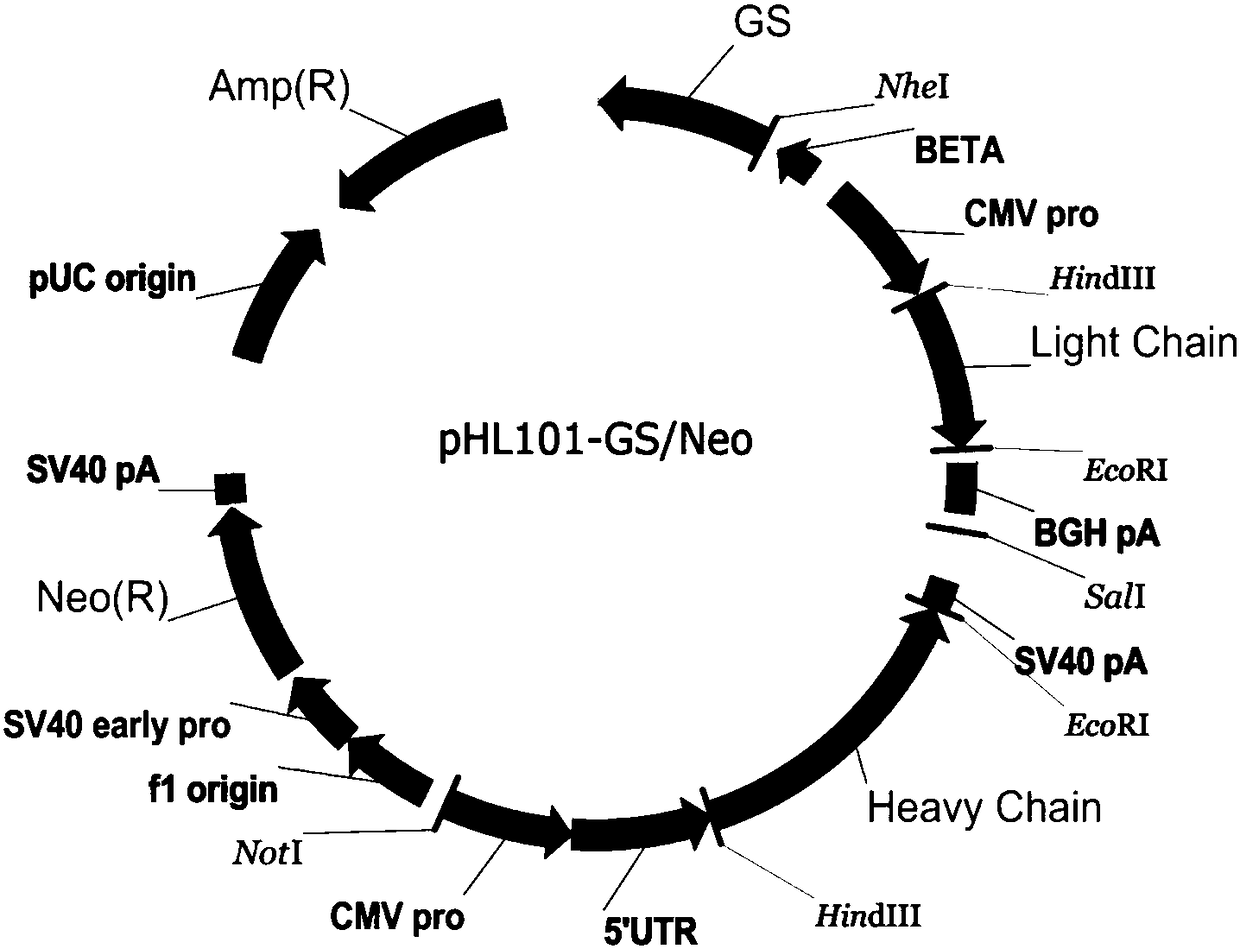
Cellular expression system for stably expressing anti-TNF-alpha monoclonal antibody
ActiveCN107236710AHigh genetic stabilityDoes not affect basic survivabilityStable introduction of DNANucleic acid vectorDiseaseHamster
The invention relates to a cellular expression system for stably expressing an anti-TNF-alpha monoclonal antibody, and a construction method and application of the cellular expression system. According to the cellular expression system, light and heavy chain encoding genes of the anti-TNF-alpha monoclonal antibody are optimized according to cell codon preference so as to construct anti-TNF-alpha human-mouse chimeric monoclonal antibody homologous recombination vector with a Bak1 gene and the like as a drone, meanwhile, a CRISPR / Case9 vector with a specific cutting effect is constructed for exon regions of drone genes such as Bak1, two vectors are commonly guided into Chinese hamster ovary cells (CHO) by lipofection transfection, and a positive cell strain capable of stably expressing the anti-TNF-alpha monoclonal antibody is obtained through screening. The cellular expression system can be applied to efficient production of the anti-TNF-alpha monoclonal antibody, and the anti-TNF-alpha monoclonal antibody can be used for treating diseases such as rheumatoid arthritis and ankylosing spondylitis.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Liquid formulations for an Anti-TNF alpha antibody
ActiveUS20150329628A1Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsMonoclonal antibodyAnti tnf alpha
The invention provides stable liquid formulations for a recombinant biopharmaceutical protein comprising a fully human anti-TNF monoclonal antibody.
Owner:MERCK SHARP & DOHME CORP
Anti-TNF-alpha monoclonal antibody chromatographic method
InactiveCN105837687AImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsMonoclonal antibodyIon exchange
The invention relates to an anti-TNF-alpha monoclonal antibody chromatographic method, and belongs to the field of biotechnology. In particular, the method for removing host proteins and multimers during a process of purification of a TNF-alpha monoclonal antibody is disclosed. An ion exchange and hydrophobic composite chromatographic filler is used, a low-pH buffer solution is used for removing contaminants, and then a lower-pH elution buffer solution is used for eluting the target antibody. The method can remove a part of the multimers s and most of the host proteins, significantly improves the purity of the antibody, is cheaper in price, has no protein A falling off, is simple and convenient, has no need for adjusting the sample pH value and electric conductance, and is suitable for process amplification and industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Serum Markers Predicting Clinical Response to Anti-TNF Alpha Antibodies in Patients with Psoriatic Arthritis
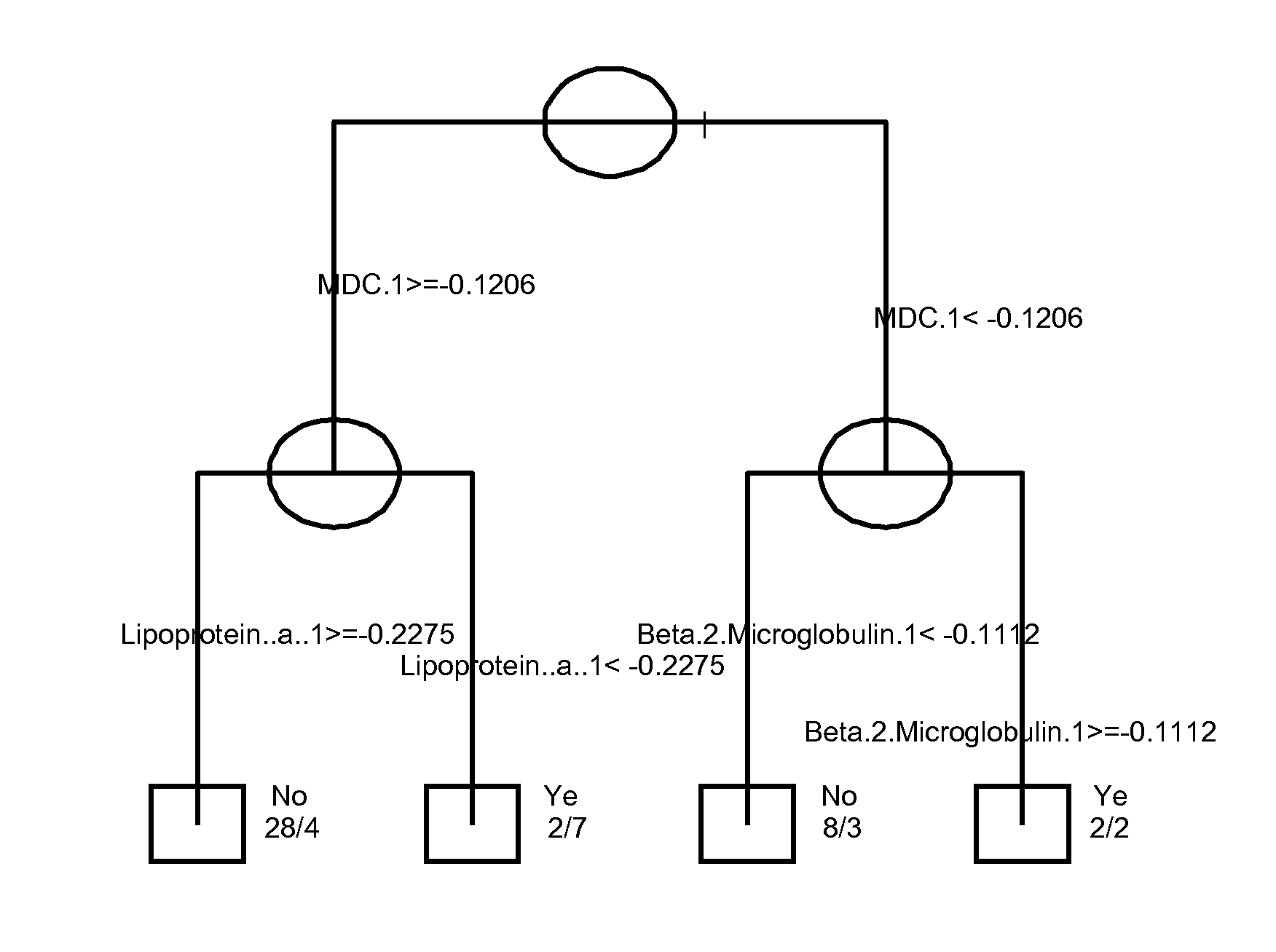
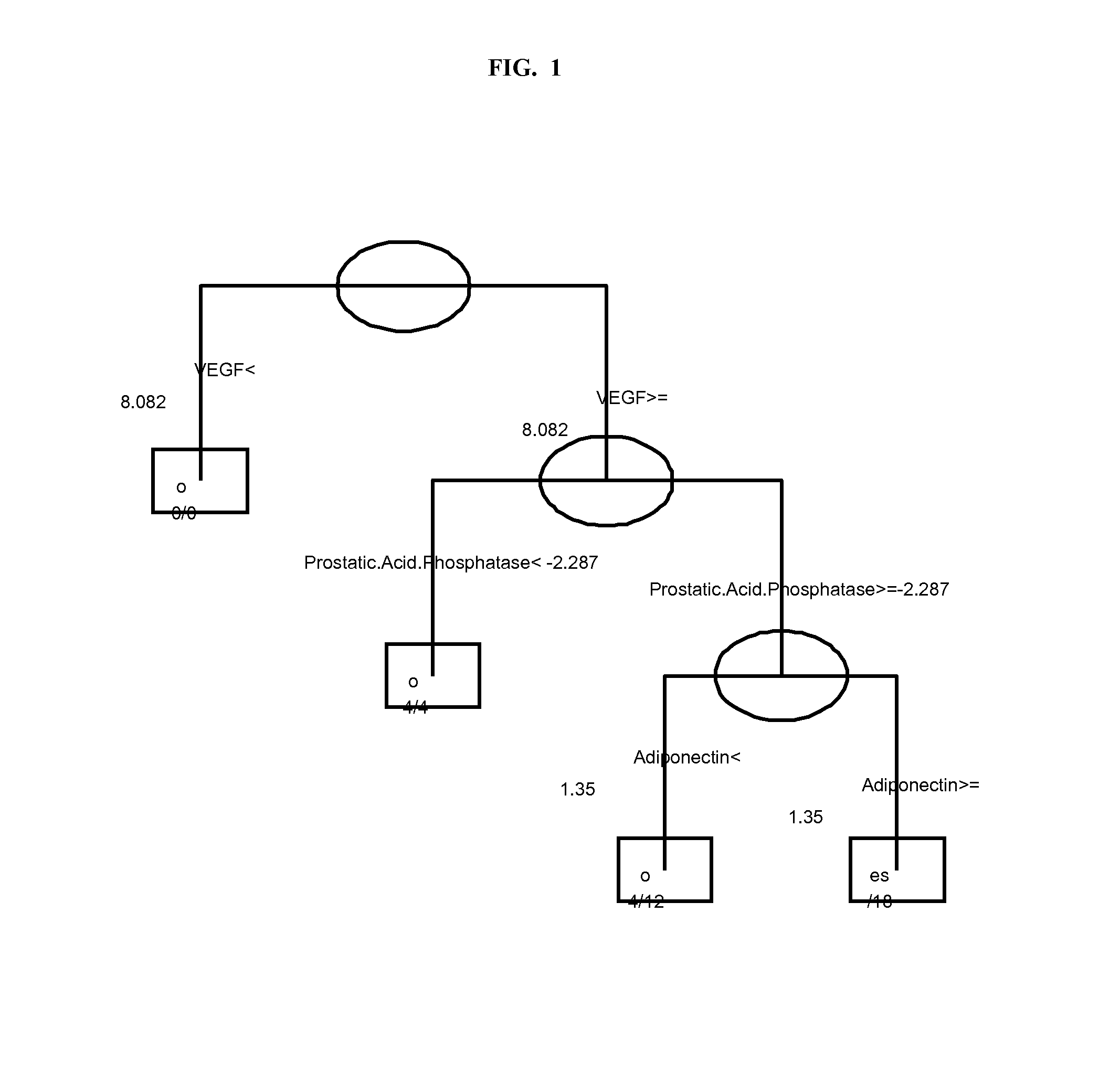
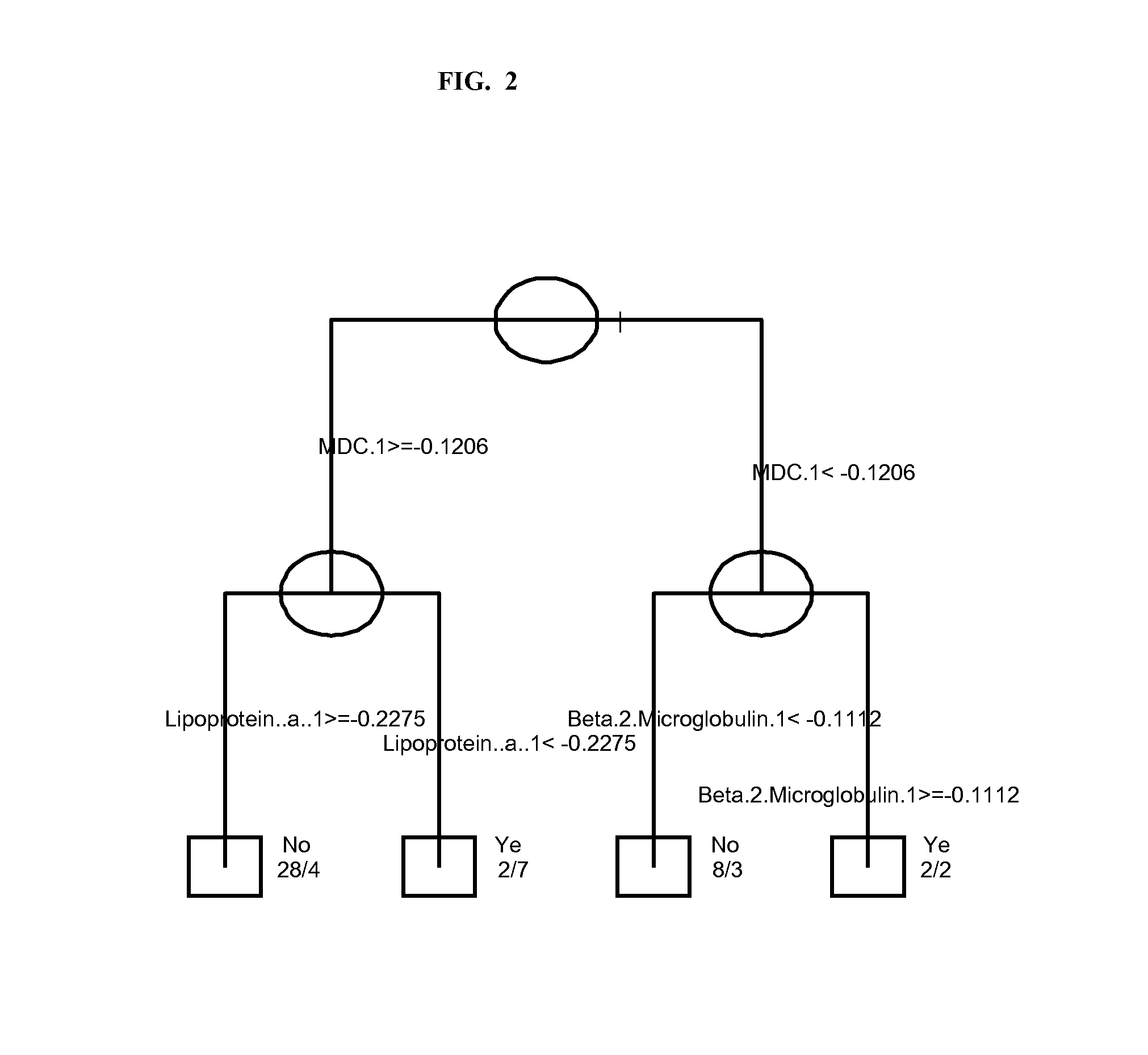
InactiveUS20120178100A1Sustained responseBioreactor/fermenter combinationsBiological substance pretreatmentsPsoriasis arthropathySerum protein
The invention provides tools for management of patients diagnosed with psoriatic arthritis, specifically, prior to the initiation of therapy with an anti-TNFα agent. The tools are specific markers and algorithms of predicting response to therapy based on standard clinical primary and secondary endpoints using serum marker concentrations. In one embodiment the baseline levels of VEGF, prostatic acid phosphatase, and adiponectin are used to predict the response at Week 14 after the initiation of therapy. In another embodiment, the change in a serum protein biomarker after 4 weeks of therapy is used such as MDC, lipoprotein a, and beta2-microglobulin.
Owner:CENTOCOR ORTHO BIOTECH
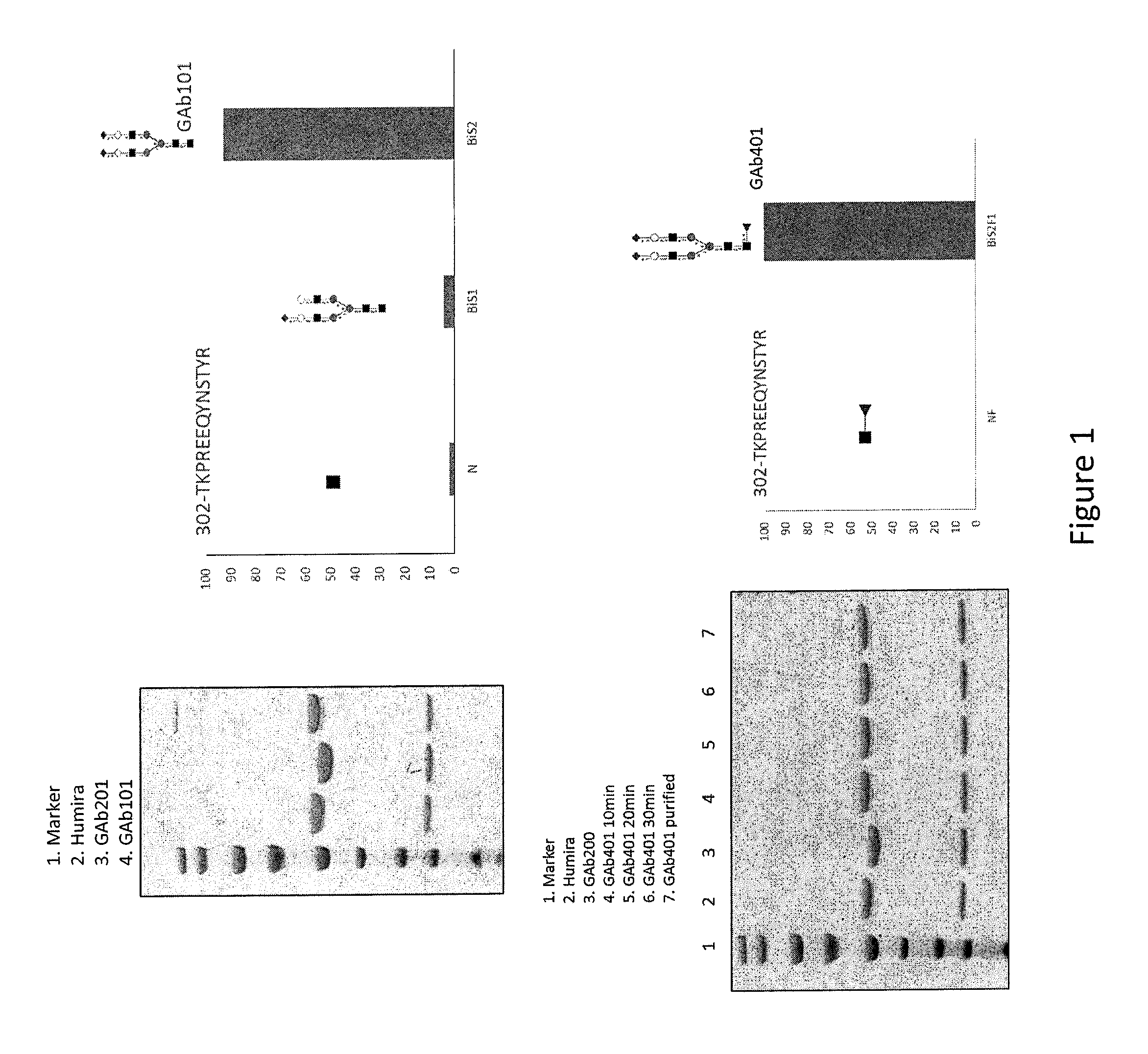
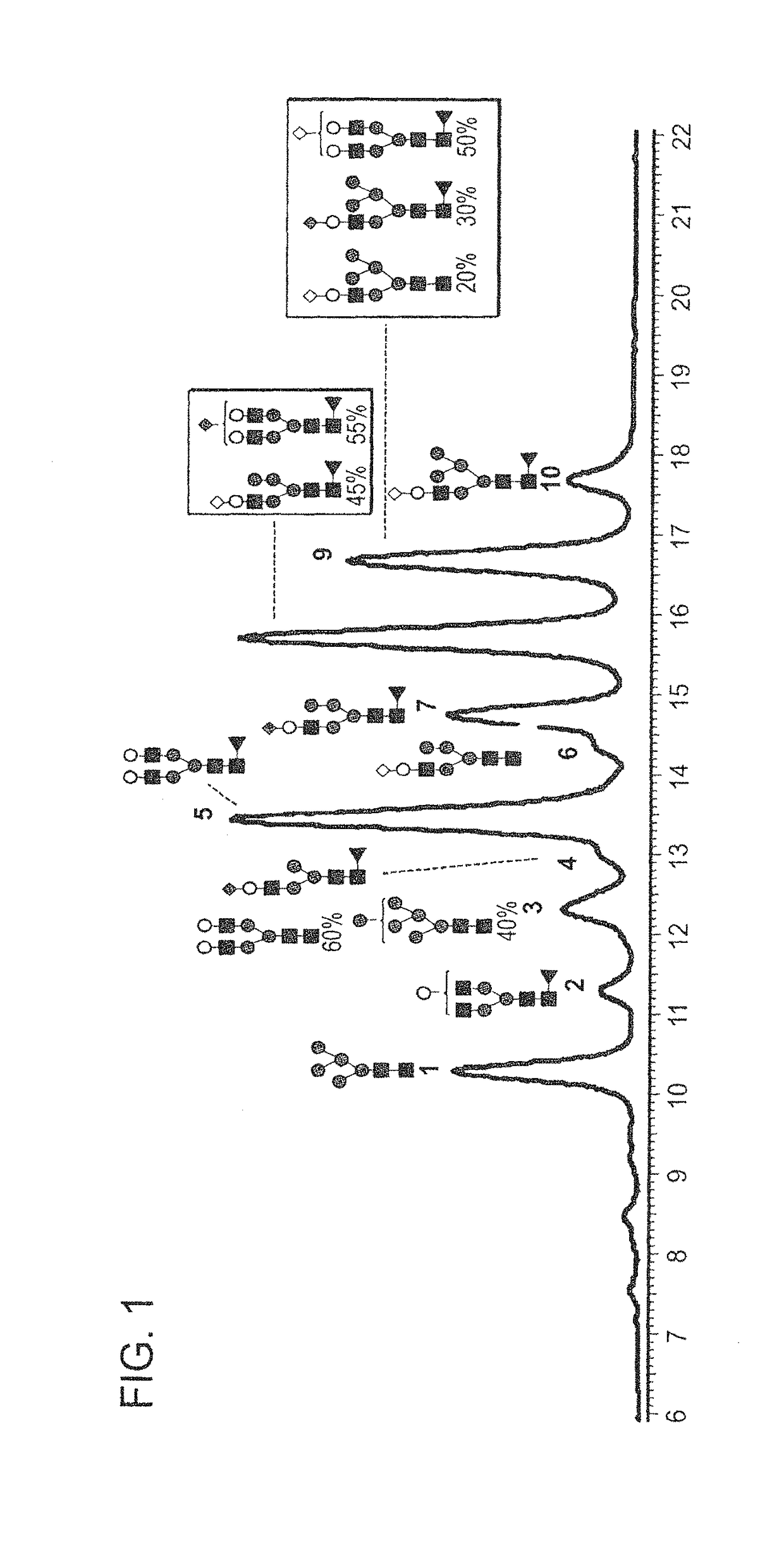
Highly galactosylated anti-TNF-α antibodies and uses thereof
InactiveUS10174110B2Strong cytotoxicityImprove the level ofAntipyreticMilk immunoglobulinsMethods of productionAnti tnf alpha
In one aspect, the disclosure relates to highly galactosylated anti-TNF-alpha antibodies and compositions thereof. In one aspect, the disclosure relates to populations of anti-TNF-alpha antibodies with a high level of galactosylation, and compositions thereof. In one aspect, the disclosure relates to methods of production and use of highly galactosylated anti-TNF-alpha antibodies and populations of anti-TNF-alpha antibodies with a high level of galactosylation. In some embodiments, the anti-TNF-alpha antibody is adalimumab.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Preparation method of recombinant anti-TNF-alpha completely humanized monoclonal antibody
InactiveCN108164601AIncrease productionImmunoglobulins against cytokines/lymphokines/interferonsVector-based foreign material introductionSerum igeSerum free
The invention discloses a preparation method of a recombinant anti-TNF-alpha completely humanized monoclonal antibody. The method comprises: synthesizing a nucleic acid sequence of a recombinant anti-TNF-alpha completely humanized monoclonal antibody, using the obtained nucleic acid sequence of the recombinant anti-TNF-alpha completely humanized monoclonal antibody to construct a carrier, transfecting a host cell, and screening high-expression cell clone to establish a cell bank; and using the obtained cell strain for culture, and performing separation and purification to obtain the recombinant anti-TNF-alpha completely humanized monoclonal antibody. Light chains and heavy chains of the recombinant anti-TNF-alpha completely humanized monoclonal antibody is designed and synthesized according to preferred codons of Chinese hamsters, an eukaryotic expression carrier is established, a host cell CHO-GS- is transfected, serum-free culture is adopted, and separation and purification are adopted to obtain the high-purity recombinant anti-TNF-alpha completely humanized monoclonal antibody. The preparation method is simple to operate, short in production period, and suitable for industrial production.
Owner:安徽未名生物医药有限公司
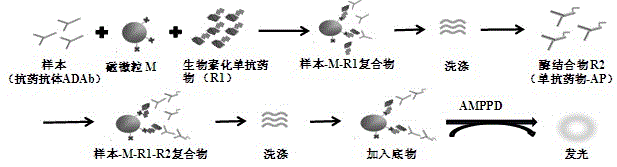
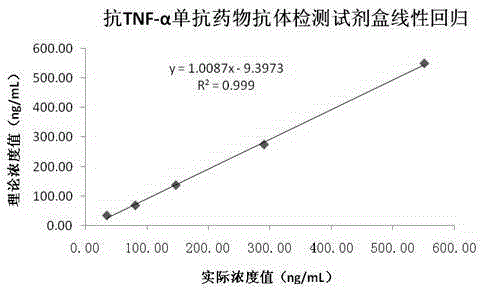
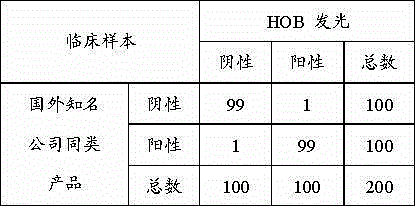
Anti-TNF-alpha monoclonal antibody drug antibody detection reagent kit
The invention discloses an anti-TNF-alpha monoclonal antibody drug antibody detection reagent kit. The reagent kit comprises biotinylation monoclonal antibody drugs, a calibrator, a quality control product, an enzyme conjugate, magnetic particle reagent, a chemiluminiscence substrate and cleaning fluid. In this way, the full-automatic magnetic particle chemiluminiscence detection method is adopted, an alkaline phosphatase (AP)-adamantane AMPPD system is selected, the method has the advantages that stability and repeatability are good, and sensitivity is high, meanwhile, detection time is greatly shortened, one test is completed within 50 minutes, operation is easy and convenient, and detection full automation is really achieved.
Owner:SUZHOU HAOOUBO BIOPHARML
Polypeptide binding with tumor necrosis factor, and applications thereof
ActiveCN104829697ALow cost of treatmentInhibit apoptosisPeptide/protein ingredientsAntipyreticApoptosis pathwaysMitophagy
The present invention relates to a polypeptide binding with tumor necrosis factor (TNF-alpha), and applications thereof. According to the present invention, the polypeptide and TNF-alpha have high affinity, and the polypeptide can be bound with the excess TNF-alpha to make the apoptosis promoting protein Bid in the apoptosis-related mitochondrion apoptosis pathway be down-regulated while make the anti-apoptosis protein Bcl-2 be up-regulated in the apoptosis-related mitochondrion apoptosis pathway so as to inhibit the apoptosis caused by TNF-alpha, such that the polypeptide can be used for preparation of TNF-alpha-related disease treating drugs. The present invention further provides a method for screening at the molecular and cellular level and researching the influence of the polypeptide antagonist on the TNF-alpha physiological effect, and applications thereof so as to provide the effective method for development of the low-cost anti-TNF-alpha drug.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Prognosis of response to treatment with Anti-tnf-alpha in patients with rheumatoid arthritis
The invention relates to the use of SNP rs3794271, and / or an SNP that is in total linkage disequilibrium with same, as a marker in predicting the response to treatment with anti-TNF in a patient with RA. The invention also relates to methods for predicting the response to treatment with anti-TNF, as well as for deciding on or recommending a treatment for a patient with RA, based on determining the genotype for rs3794271 and / or an SNP that is in total linkage disequilibrium with same.
Owner:FUNDACIO HOSPITAL UNIVERSITARI VALL DHEBRON INST DE RECERCA
Whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof
The invention relates to the technical field of biology, and discloses a whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof. The invention adopts a whole human antibody technology and acquires an anti-human TNF alpha whole human antibody. Preliminary analyses on the physical, chemical and biological activities of the antibody indicate that the antibody has better affinity with TNF, and can effectively neutralize the killing effect of the TNF on L929 cells in vitro. The whole human TNF alpha monoclonal antibody disclosed by the invention minimizes the immunogenicity of a human body. A PEG (polyethylene glycol) surface modification technology is adopted, thus avoiding the defect of short half life of small molecule antibody, and the whole human TNF alpha monoclonal antibody is more suitable for application in vivo while keeping the anti-TNF alpha activity.
Owner:优锐生物医药科技(深圳)有限公司
A kind of chromatographic method of anti-tnf-alpha monoclonal antibody
InactiveCN105837687BImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsIon exchangeMonoclonal antibody
The invention relates to an anti-TNF-alpha monoclonal antibody chromatographic method, and belongs to the field of biotechnology. In particular, the method for removing host proteins and multimers during a process of purification of a TNF-alpha monoclonal antibody is disclosed. An ion exchange and hydrophobic composite chromatographic filler is used, a low-pH buffer solution is used for removing contaminants, and then a lower-pH elution buffer solution is used for eluting the target antibody. The method can remove a part of the multimers s and most of the host proteins, significantly improves the purity of the antibody, is cheaper in price, has no protein A falling off, is simple and convenient, has no need for adjusting the sample pH value and electric conductance, and is suitable for process amplification and industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Method to predict the lack of response to Anti-tnf alpha therapies
This invention provides methods for predicting response to anti-TNFα biological agent treatment in a rheumatoid arthritis patient and methods for selecting a treatment for a rheumatoid arthritis patient, the methods comprising determining the level of expression of PIK3CD as a biomarker, and optionally also determining the level of expression of CX3CL1 as a second biomarker. The invention additionally provides kits for carrying out the methods described.
Owner:FUNDACIO HOSPITAL UNIVERSITARI VALL DHEBRON INST DE RECERCA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com