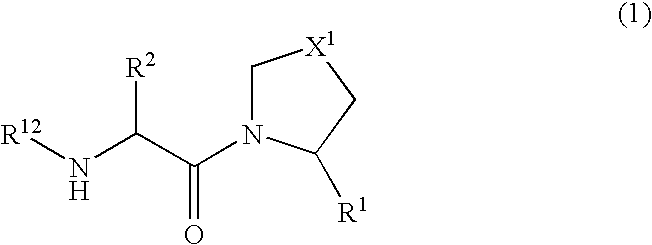
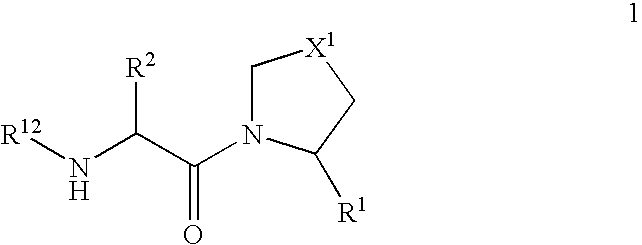
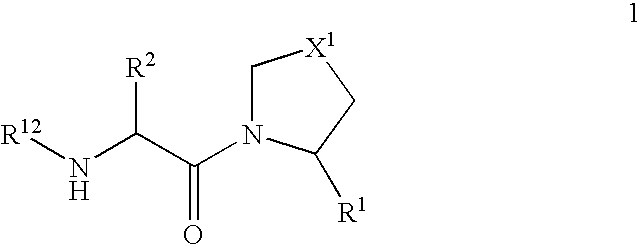
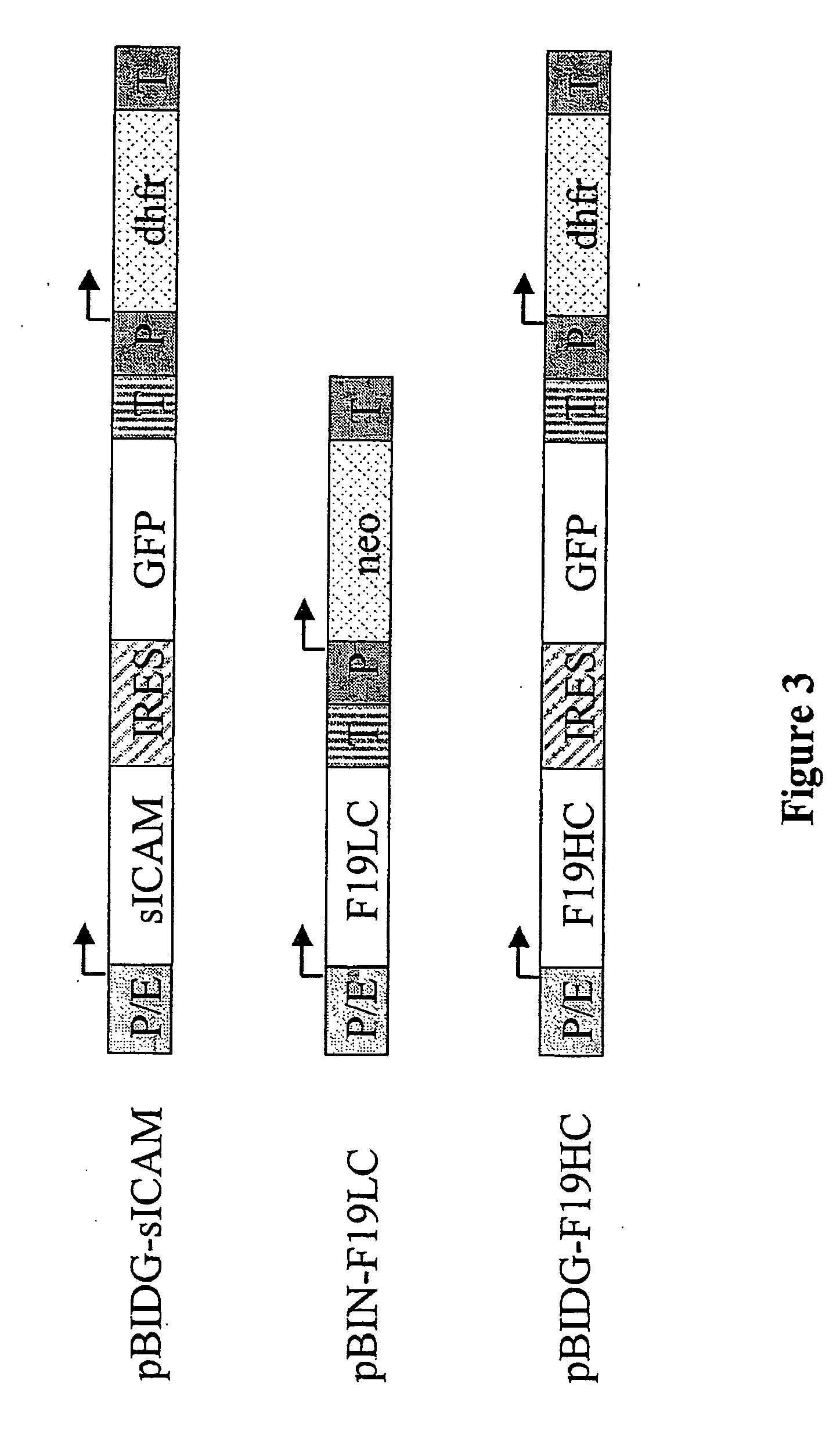
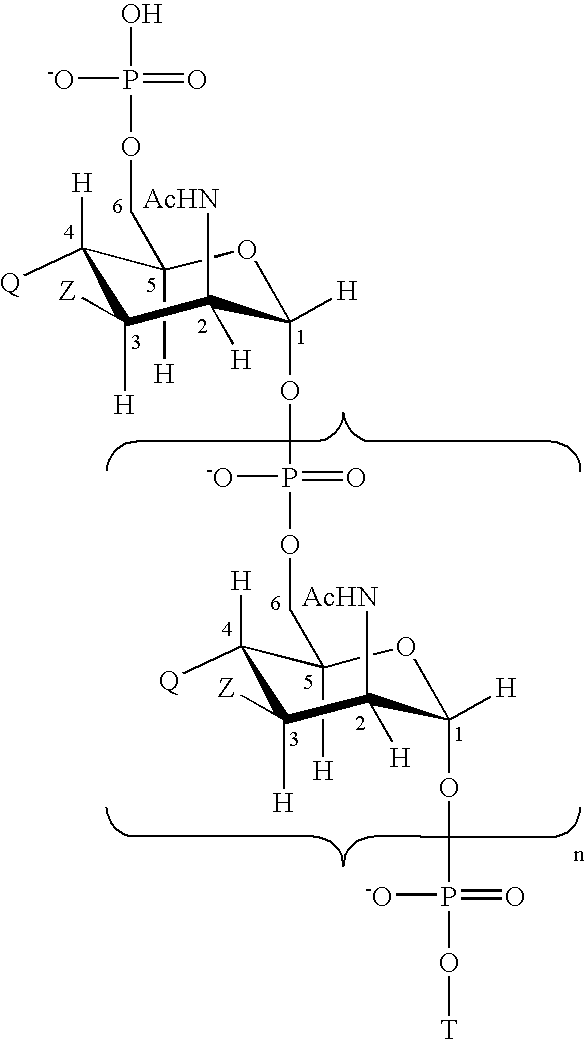
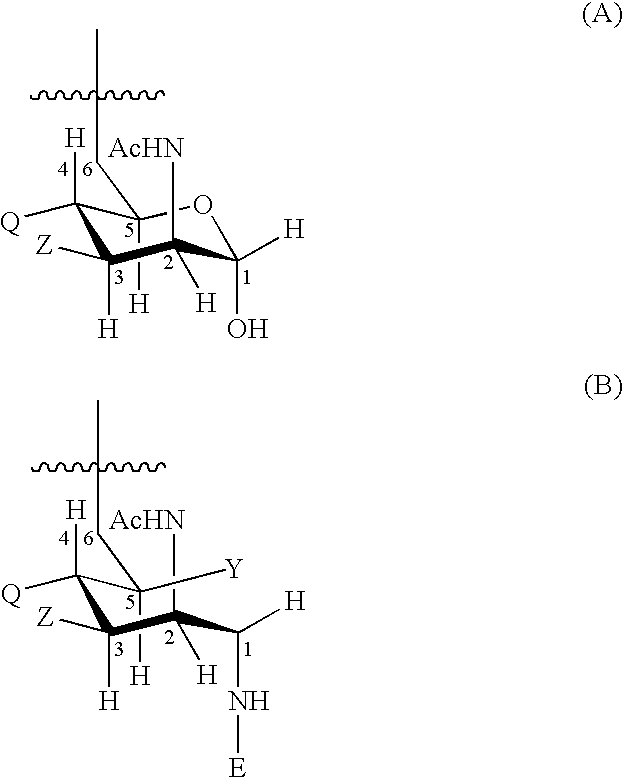
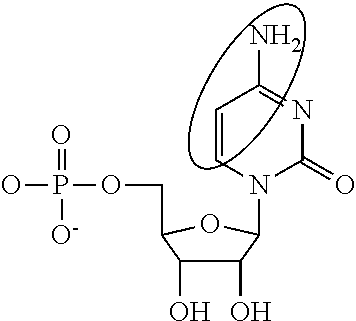
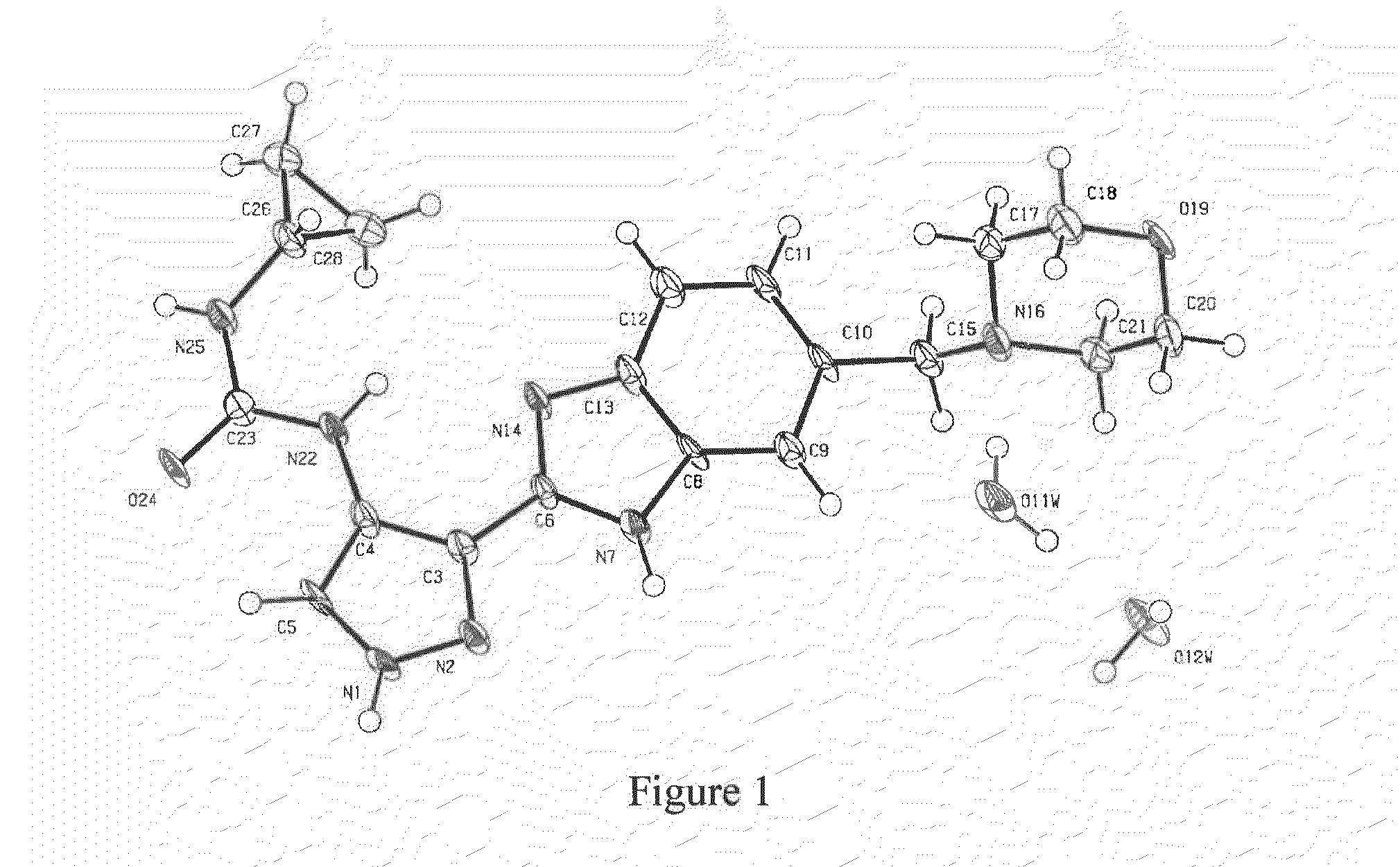
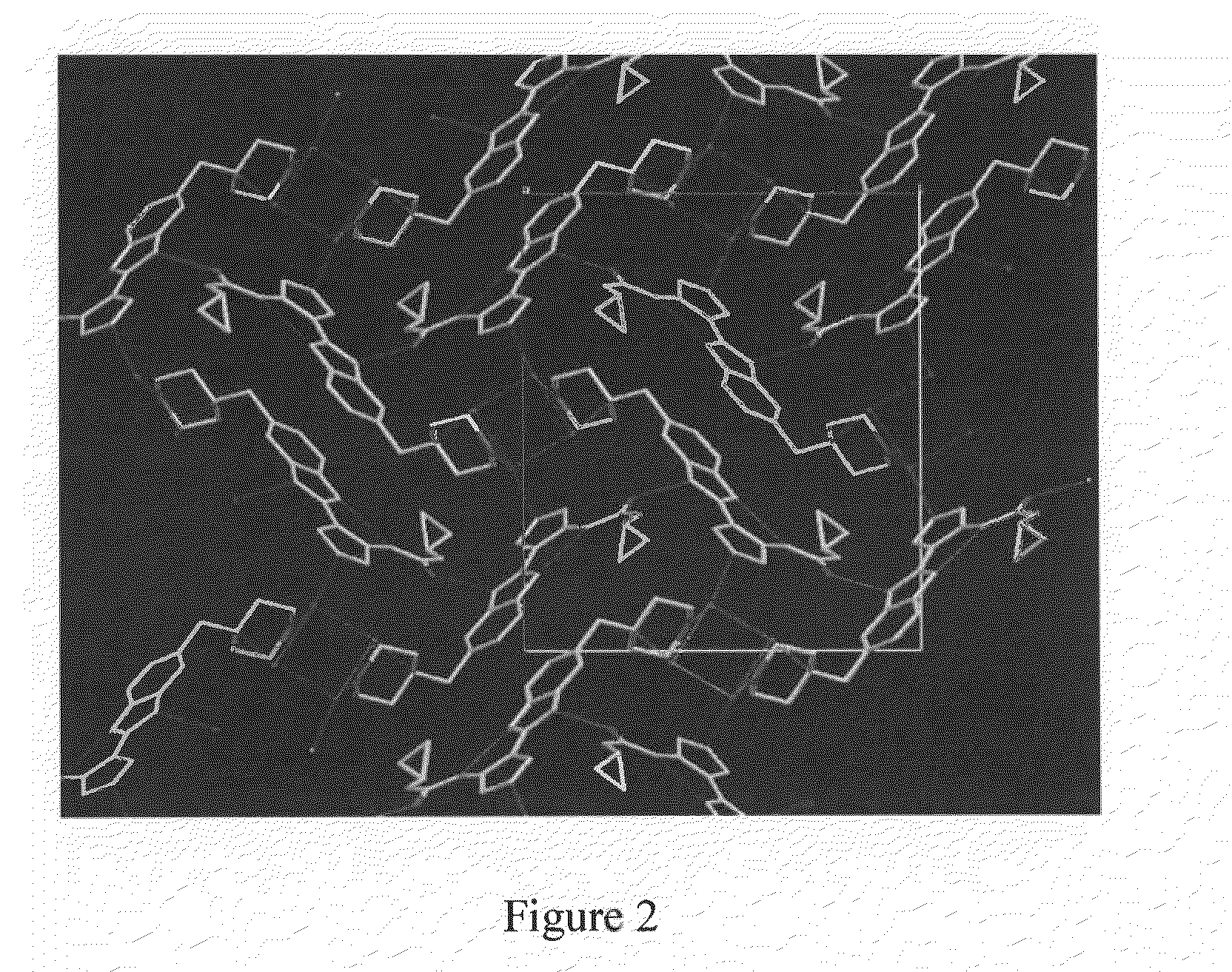
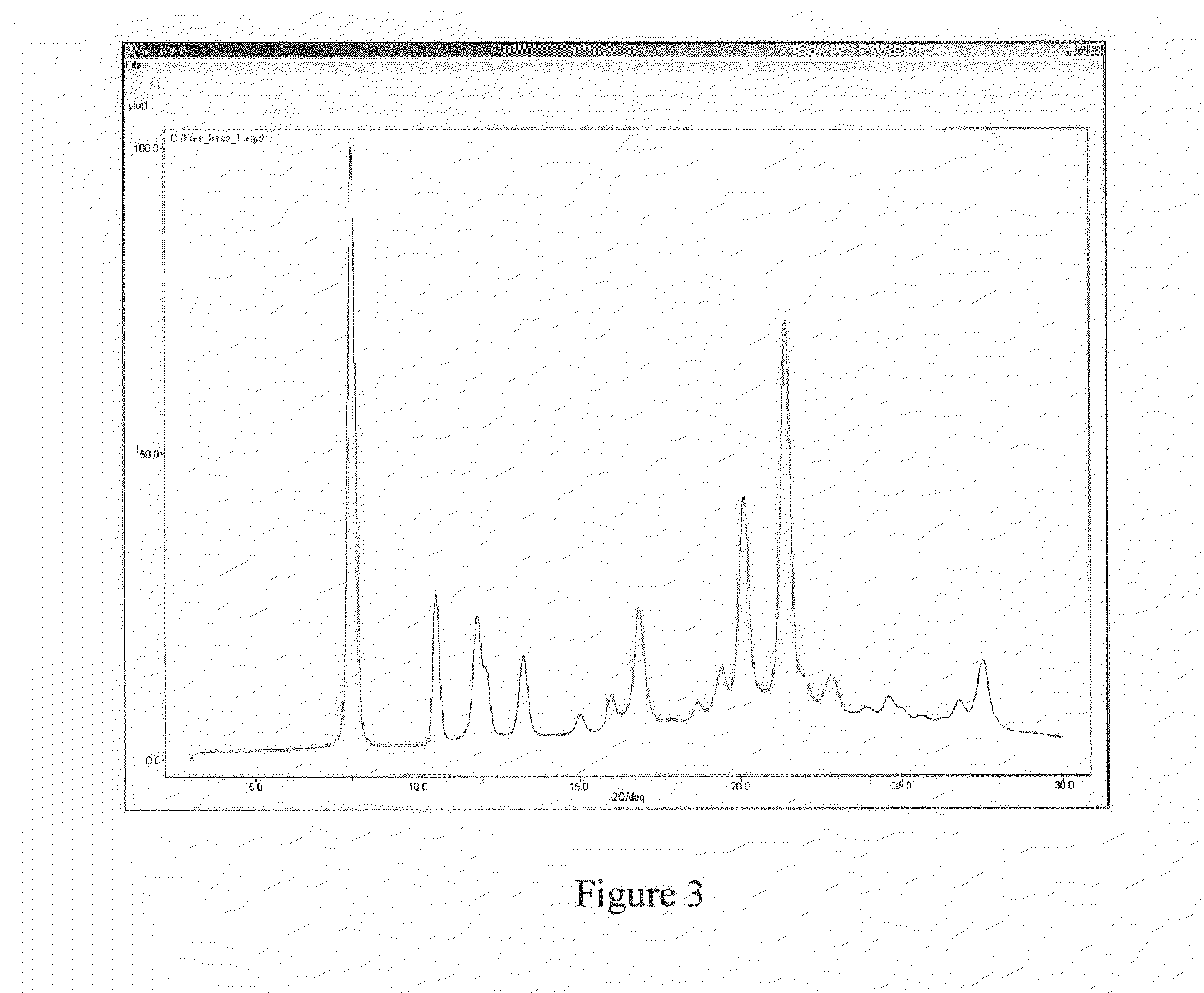
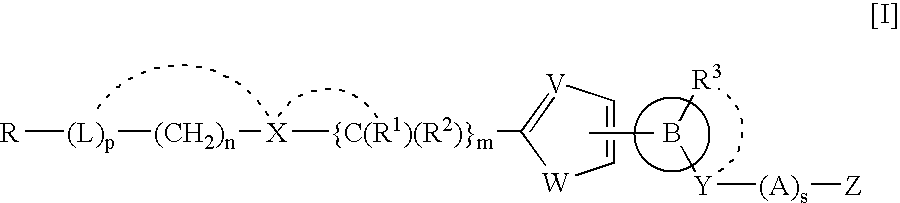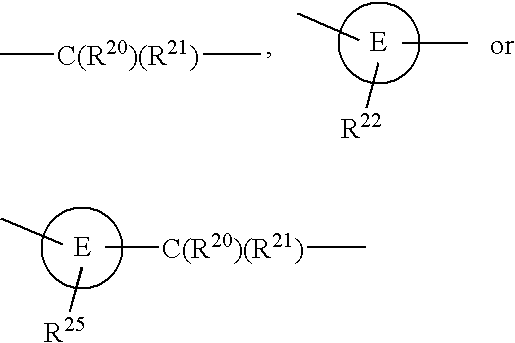Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
743 results about "TOLLIP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
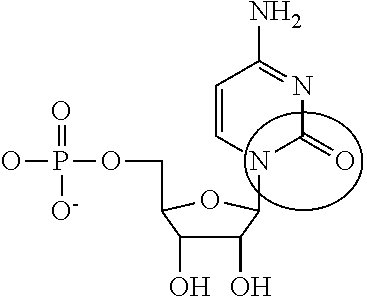
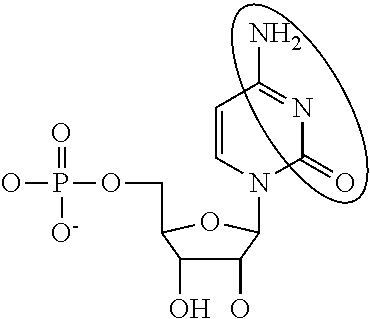
Toll interacting protein, also known as TOLLIP, is an inhibitory adaptor protein that in humans is encoded by the TOLLIP gene.
Multifunctional and multispectral biosensor devices and methods of use
InactiveUS6743581B1Immobilised enzymesBioreactor/fermenter combinationsElectromagnetic radiationBiology
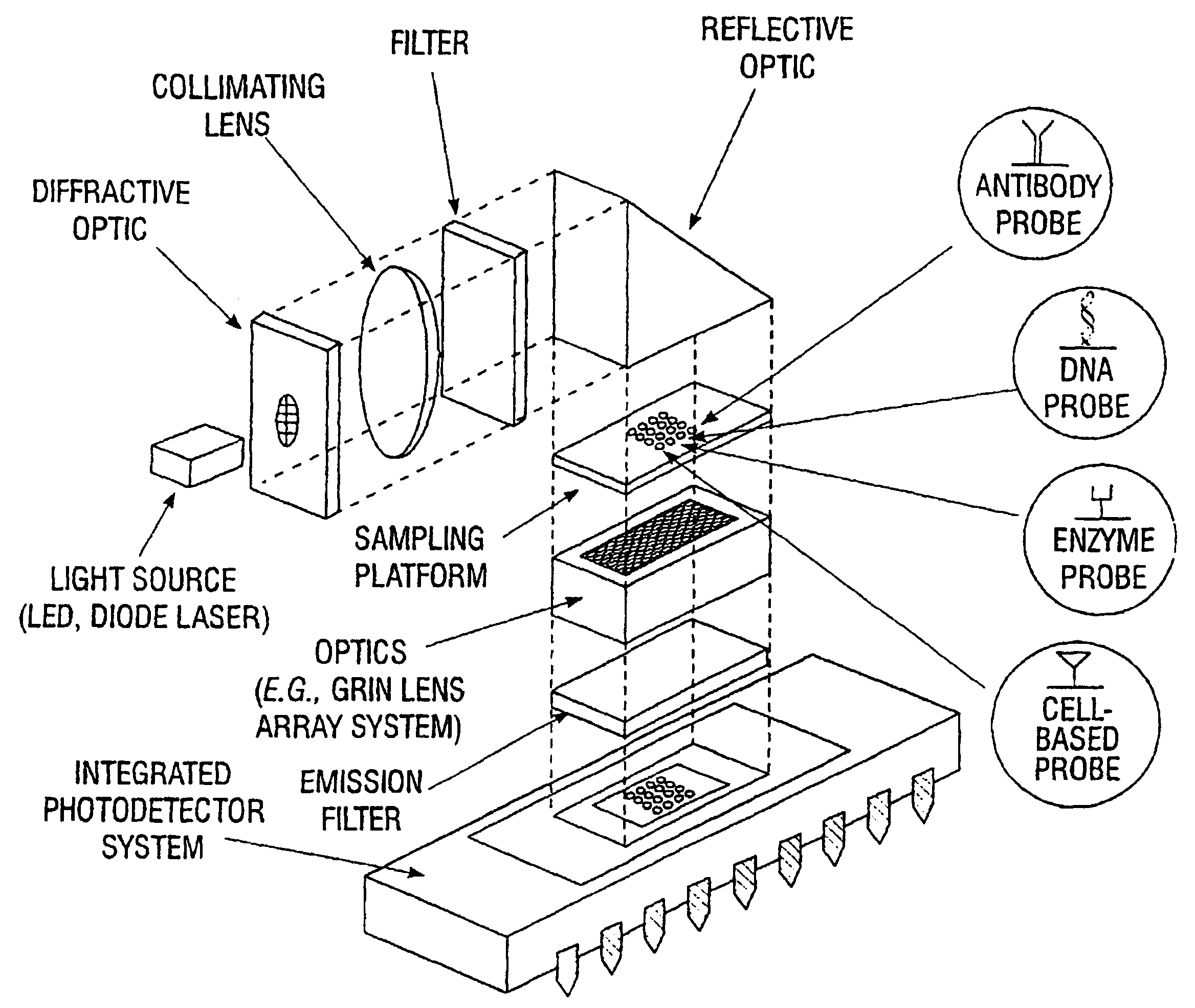
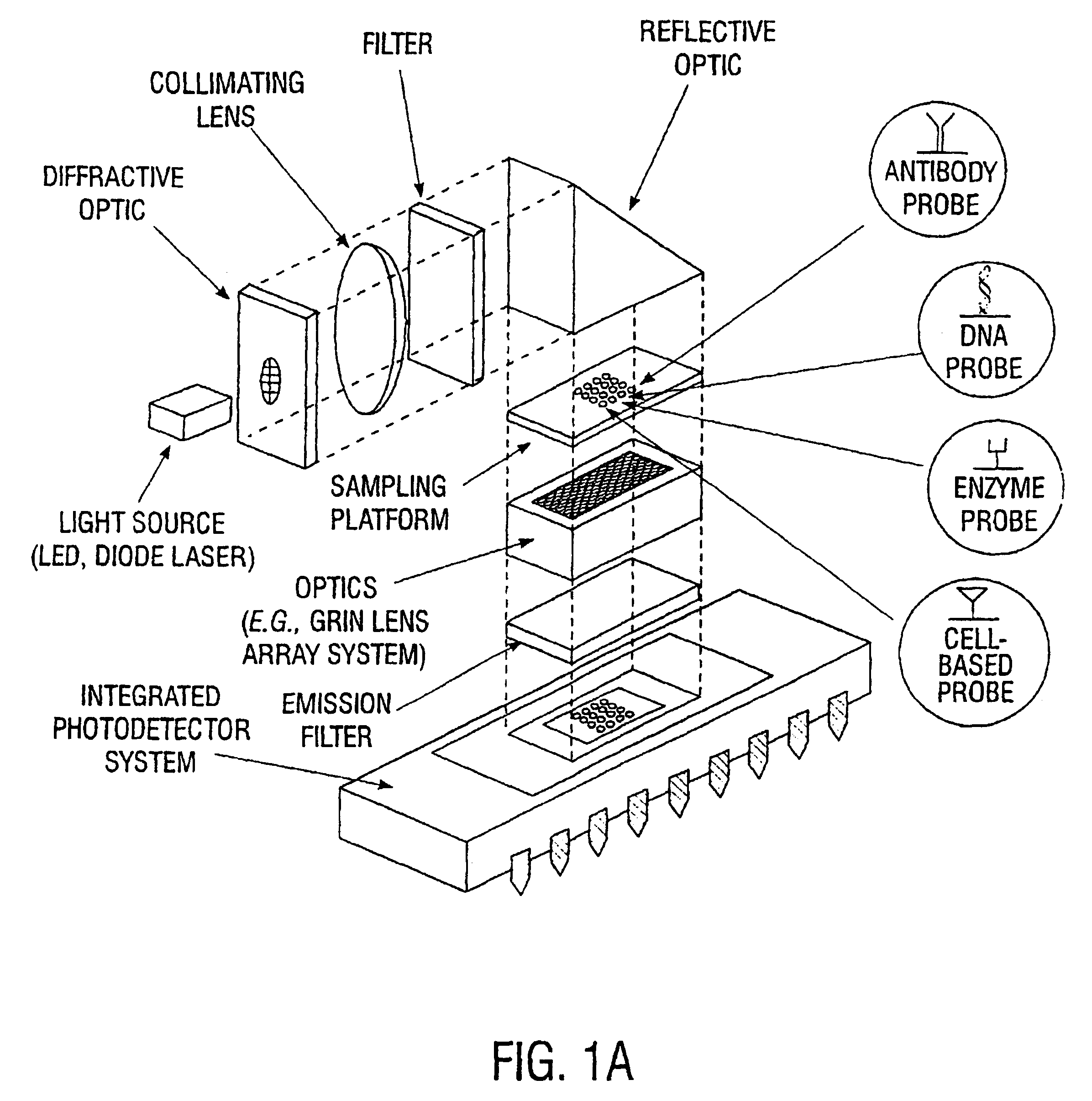
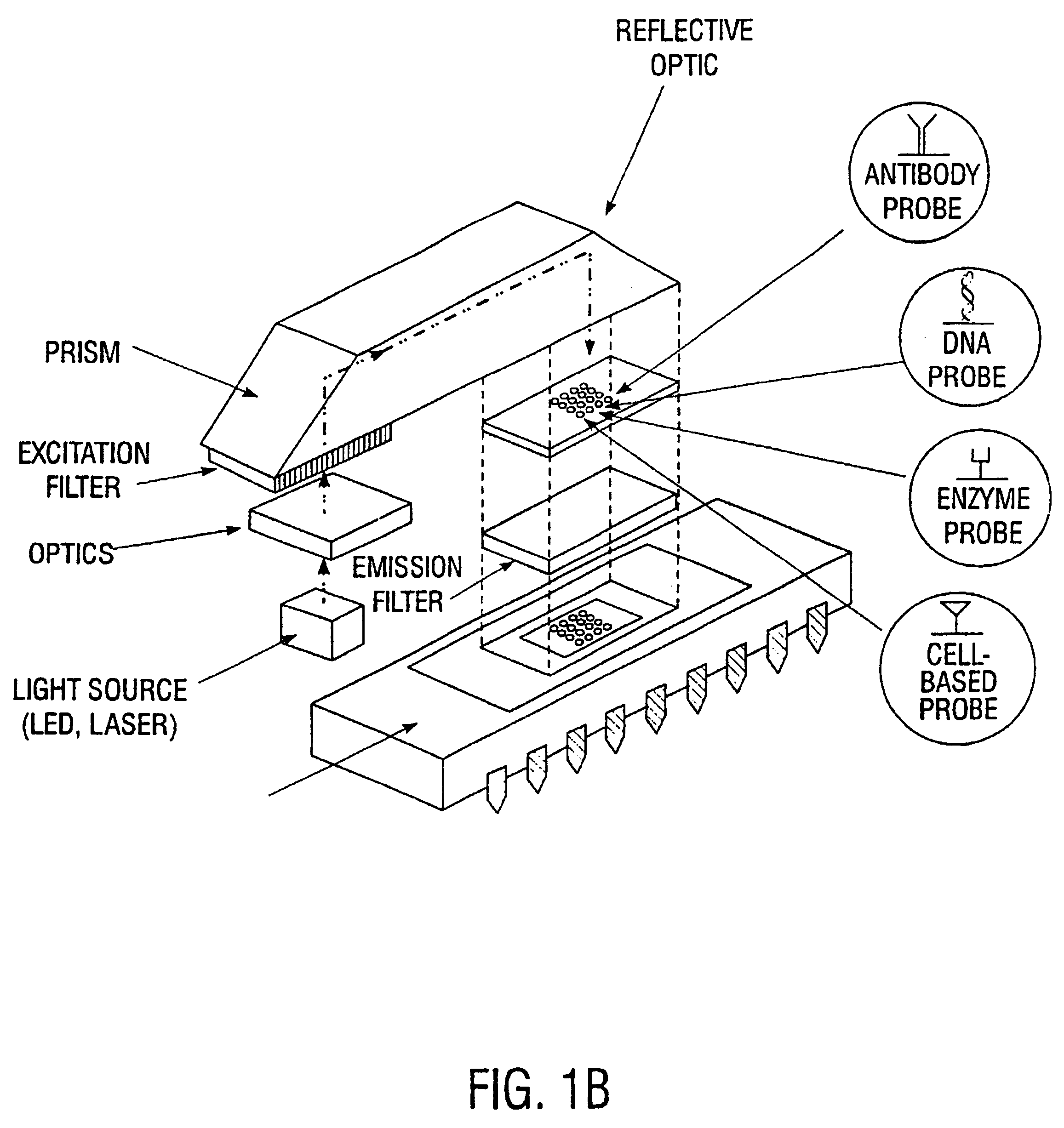
An integrated biosensor system for the simultaneously detection of a plurality of different types of targets includes at least one sampling platform, the sampling platform including a plurality of receptors for binding to the targets. The plurality of receptors include at least one protein receptor and at least one nucleic acid receptor. At least one excitation source of electromagnetic radiation at a first frequency is provided for irradiating the receptors, wherein electromagnetic radiation at a second frequency different from the first frequency is emitted in response to irradiating when at least one of the different types of targets are bound to the receptor probes. An integrated circuit detector system having a plurality of detection channels is also provided for detecting electromagnetic radiation at said second frequency, the detection channels each including at least one detector.
Owner:UT BATTELLE LLC
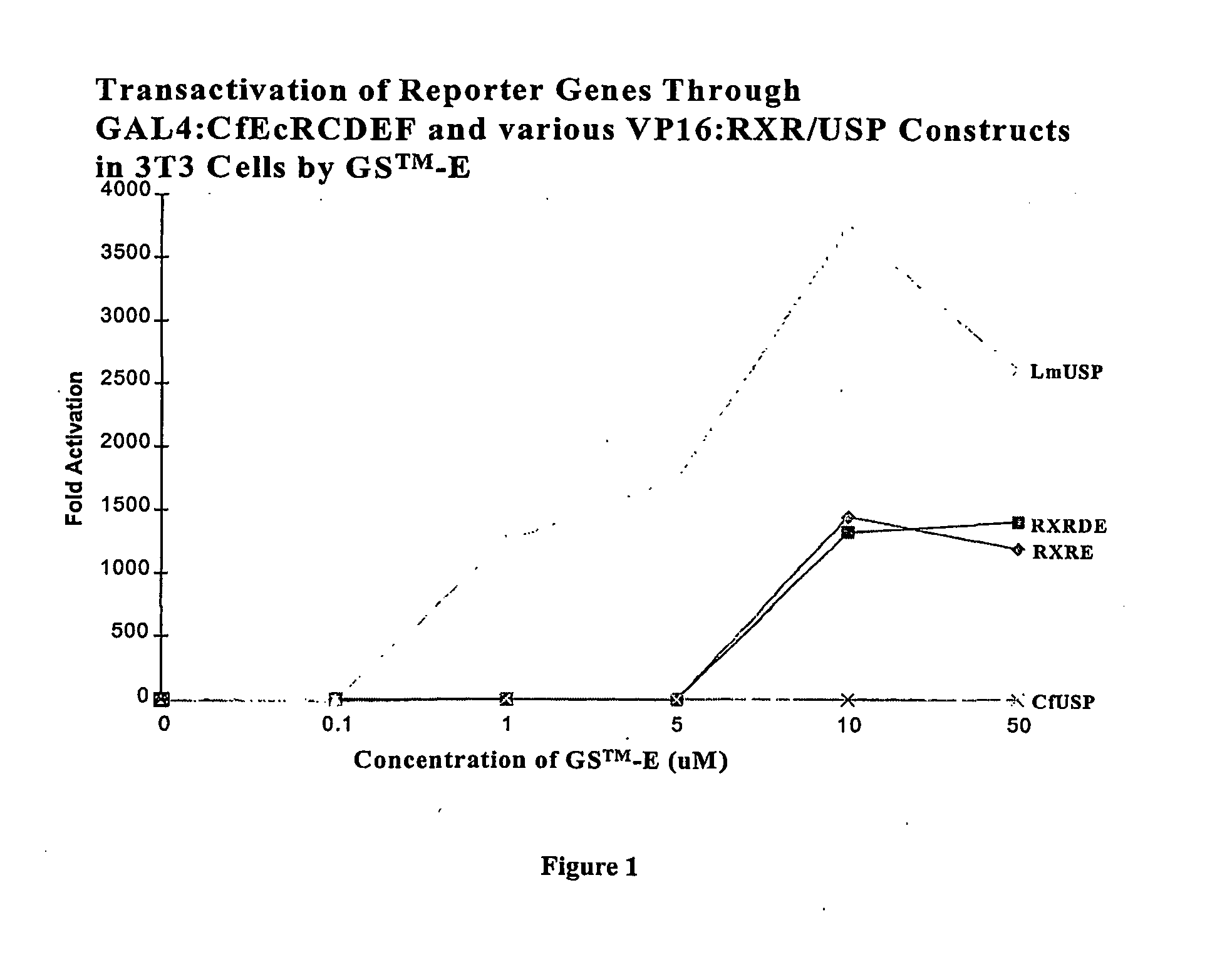
Ecdysone receptor-based inducible gene expression system
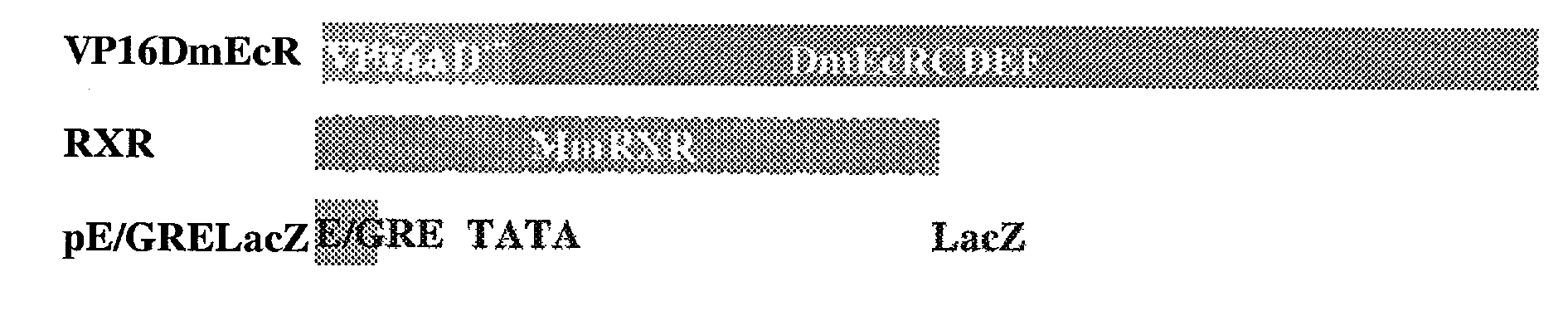
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to a novel inducible gene expression system and methods of modulating gene expression in a host cell for applications such as gene therapy, large-scale production of proteins and antibodies, cell-based high throughput screetng assays, functional genomics and regulation of traits in transgenic plants and animals.
Owner:PRECIGEN INC
Mutant receptors and their use in a nuclear receptor-based inducible gene expression system
InactiveUS20050266457A1Improve efficiencyGreat degree of sequence similarityBiocideBacteriaHigh-Throughput Screening AssaysGenomics
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to novel substitution mutant receptors and their use in a nuclear receptor-based inducible gene expression system and methods of modulating the expression of a gene in a host cell for applications such as gene therapy, large scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:PRECIGEN INC
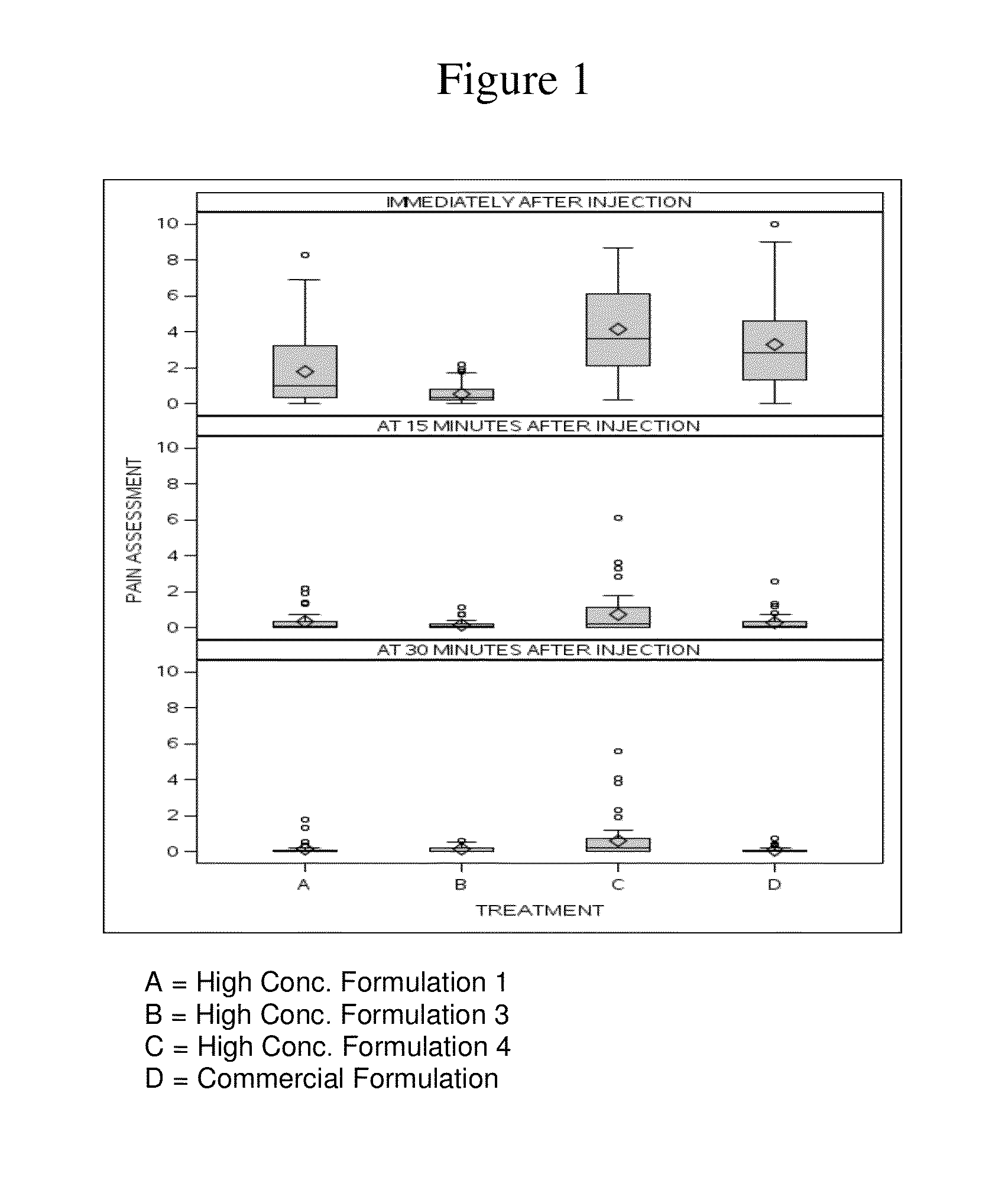
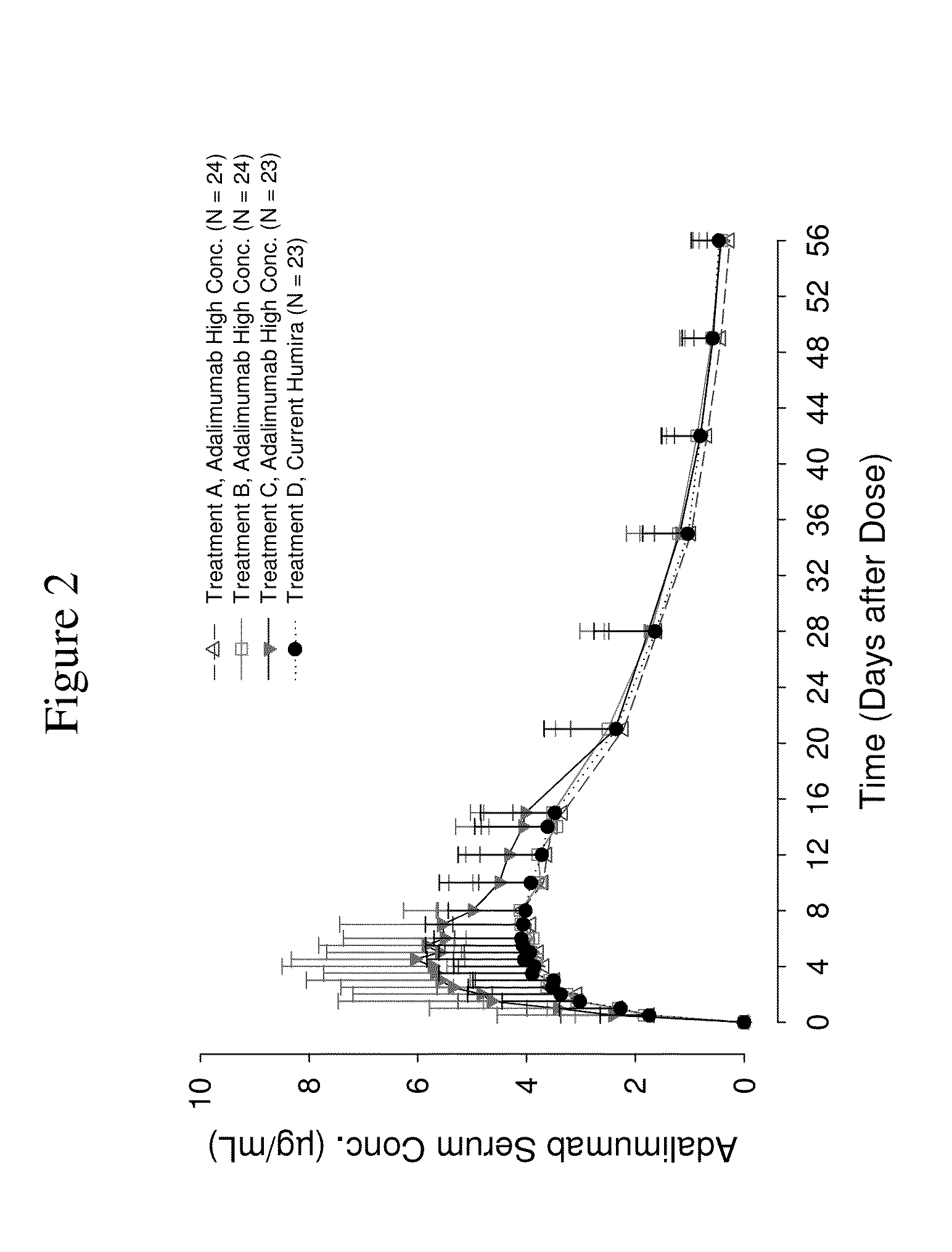
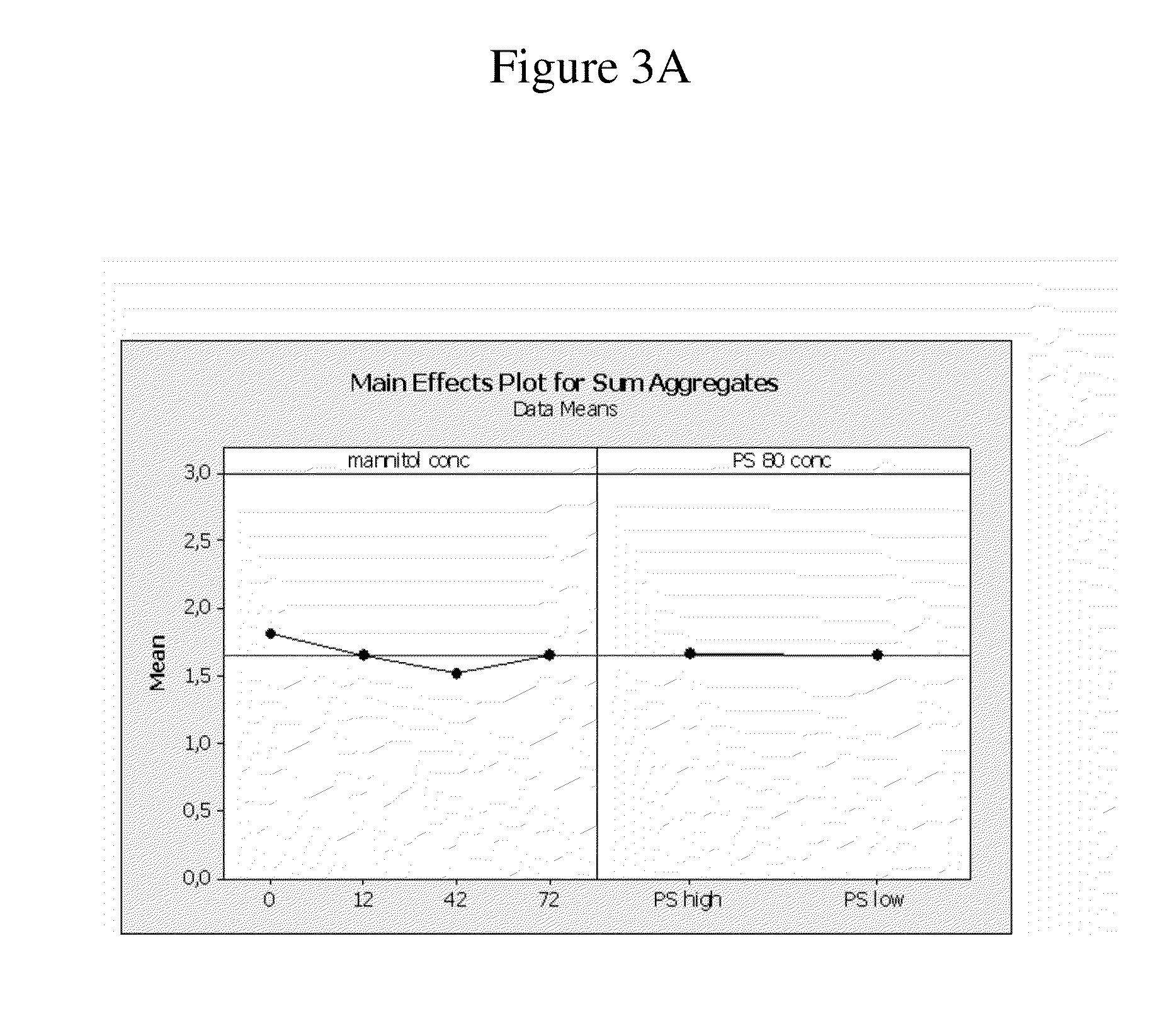
High concentration anti-TNFα antibody liquid formulations
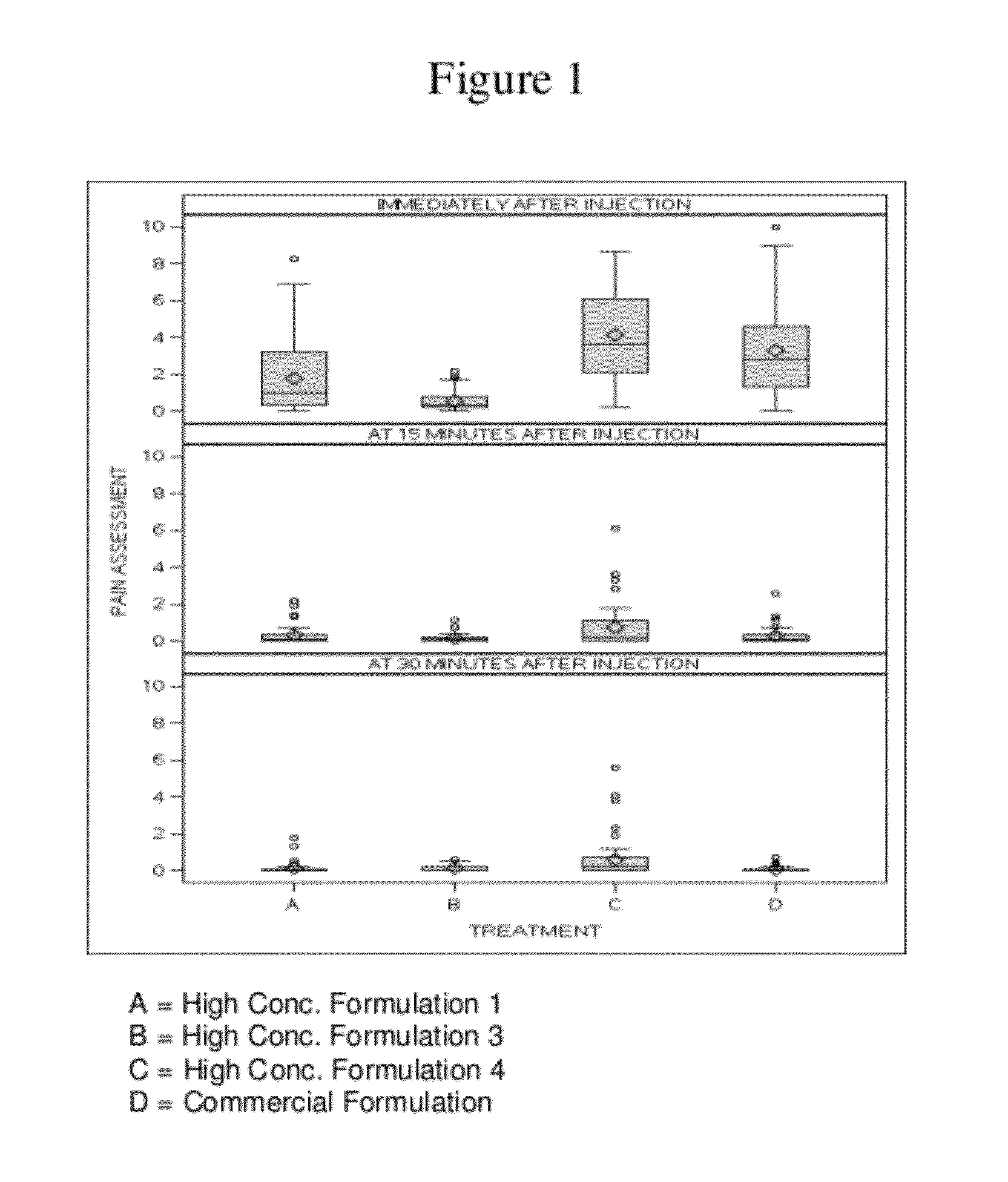
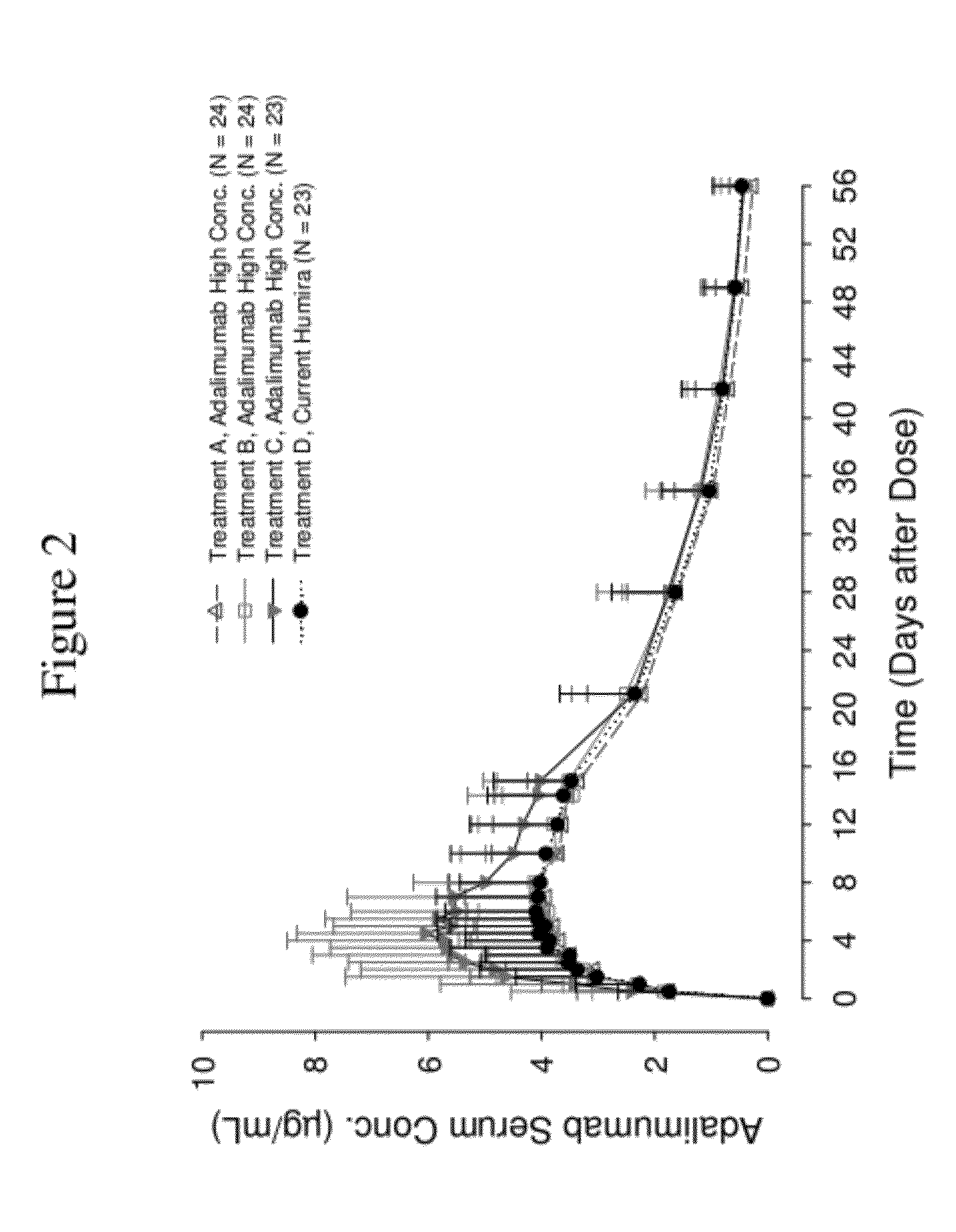
ActiveUS8821865B2Improve bioavailabilityRelieve painSenses disorderNervous disorderAntiendomysial antibodiesTherapeutic protein
The invention provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, which reduces pain associated with injection in a subject by at least about 50% when compared to injecting an otherwise identical formulation comprising at least one salt and / or at least one buffer. The invention also provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, having increased bioavailability upon subcutaneous administration into a subject. The formulation may comprise a therapeutic protein, such as a human anti-TNF-alpha antibody, or an antigen-binding portion thereof, or a biosimilar thereof.
Owner:ABBVIE BIOTECHNOLOGY LTD
HIGH CONCENTRATION ANTI-TNFalpha ANTIBODY LIQUID FORMULATIONS
ActiveUS20120263731A1Improve bioavailabilityRelieve painSenses disorderNervous disorderHigh concentrationBiosimilar Pharmaceuticals
The invention provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, which reduces pain associated with injection in a subject by at least about 50% when compared to injecting an otherwise identical formulation comprising at least one salt and / or at least one buffer. The invention also provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, having increased bioavailability upon subcutaneous administration into a subject. The formulation may comprise a therapeutic protein, such as a human anti-TNF-alpha antibody, or an antigen-binding portion thereof, or a biosimilar thereof.
Owner:ABBVIE BIOTECHNOLOGY LTD
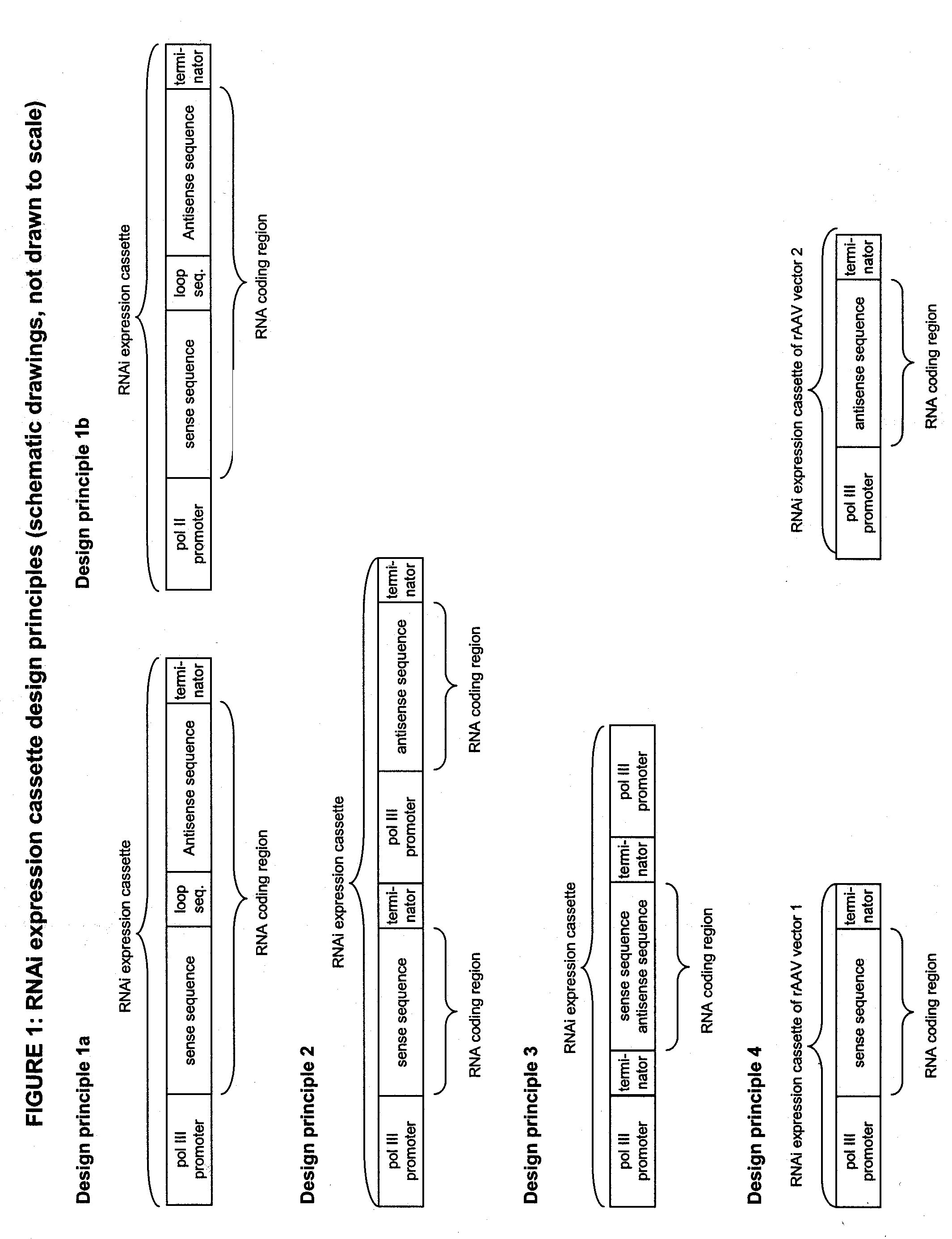
DECREASING GENE EXPRESSION IN A MAMMALIAN SUBJECT IN VIVO VIA AAV-MEDIATED RNAi EXPRESSION CASSETTE TRANSFER
InactiveUS20050019927A1High efficacyStrong specificityVectorsPeptide/protein ingredientsDecreased ConcentrationIn vivo
Decreasing the expression of genes in a mammalian subject has multiple applications ranging from cancer therapy to anti-infective therapy or treatment of autosomal dominant genetic disorders. Yet, there is still a lack of efficient technologies to achieve that goal in mammalian subjects in vivo. The present invention relates to methods for decreasing gene expression by administering to a mammalian subject a recombinant adeno-associated viral vector in vivo with said vector comprising an RNA interference (RNAi) expression cassette whose RNA expression products directly or indirectly lead to a decrease in expression of the corresponding RNAi target gene. Upon successful transduction with the recombinant adeno-associated viral vector, the RNA expression products of the RNAi expression cassette will decrease the cellular concentration of the mRNA transcripts of the RNAi target gene, thus resulting in decreased concentration of the protein encoded by the RNAi target gene.
Owner:HILDINGER MARKUS +1
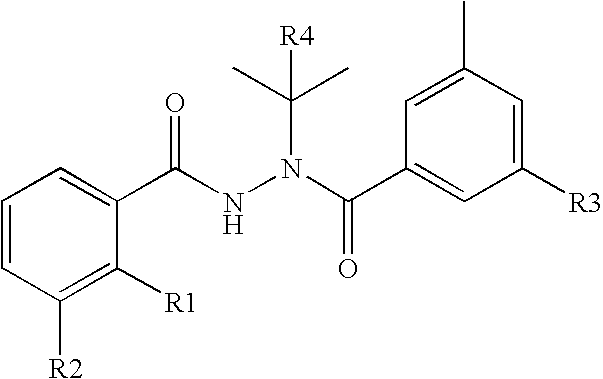
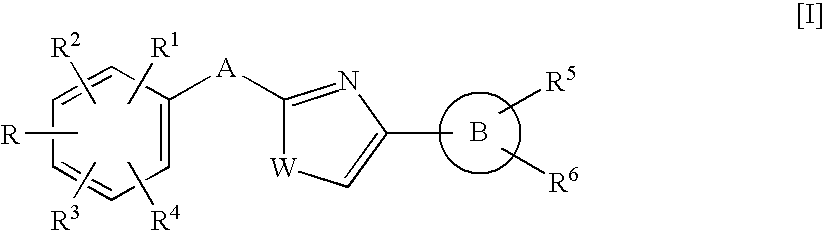
Heteroaromatic pentacyclic compound and medicinal use thereof
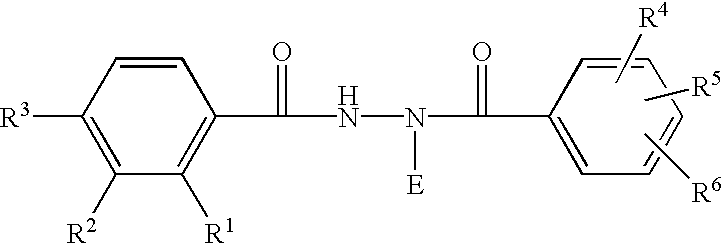
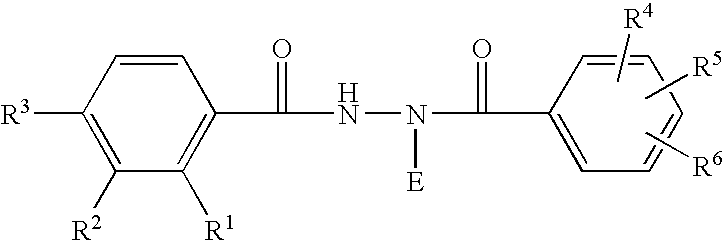
A 5-membered heteroaromatic ring compound represented by the formula [I]wherein V is CH or N; W is S or O; R1 and R2 are each H etc.; X is —N(R4)—, —O—, —S—, —SO2—N(R5)—, —CO—N(R7)— etc.; L is wherein R20, R21, R22 and R25 are each H etc.; E is aryl or heteroaromatic ring group; R is —COOH etc.; B is aryl etc.; R3 is H etc.; Y is —C(R13)(R14)—N(R12)—C(R13)(R14)—O—, —N(R11)—, —O— etc.; A is alkylene; and Z is aryl etc., a prodrug thereof and a pharmaceutically acceptable salt thereof. The compound [I] has a superior protein tyrosine phosphatase 1B inhibitory activity and is useful as a therapeutic agent for diabetes, diabetic complications, hyperlipidemia, obesity and the like.
Owner:JAPAN TOBACCO INC
Antigen delivery platforms
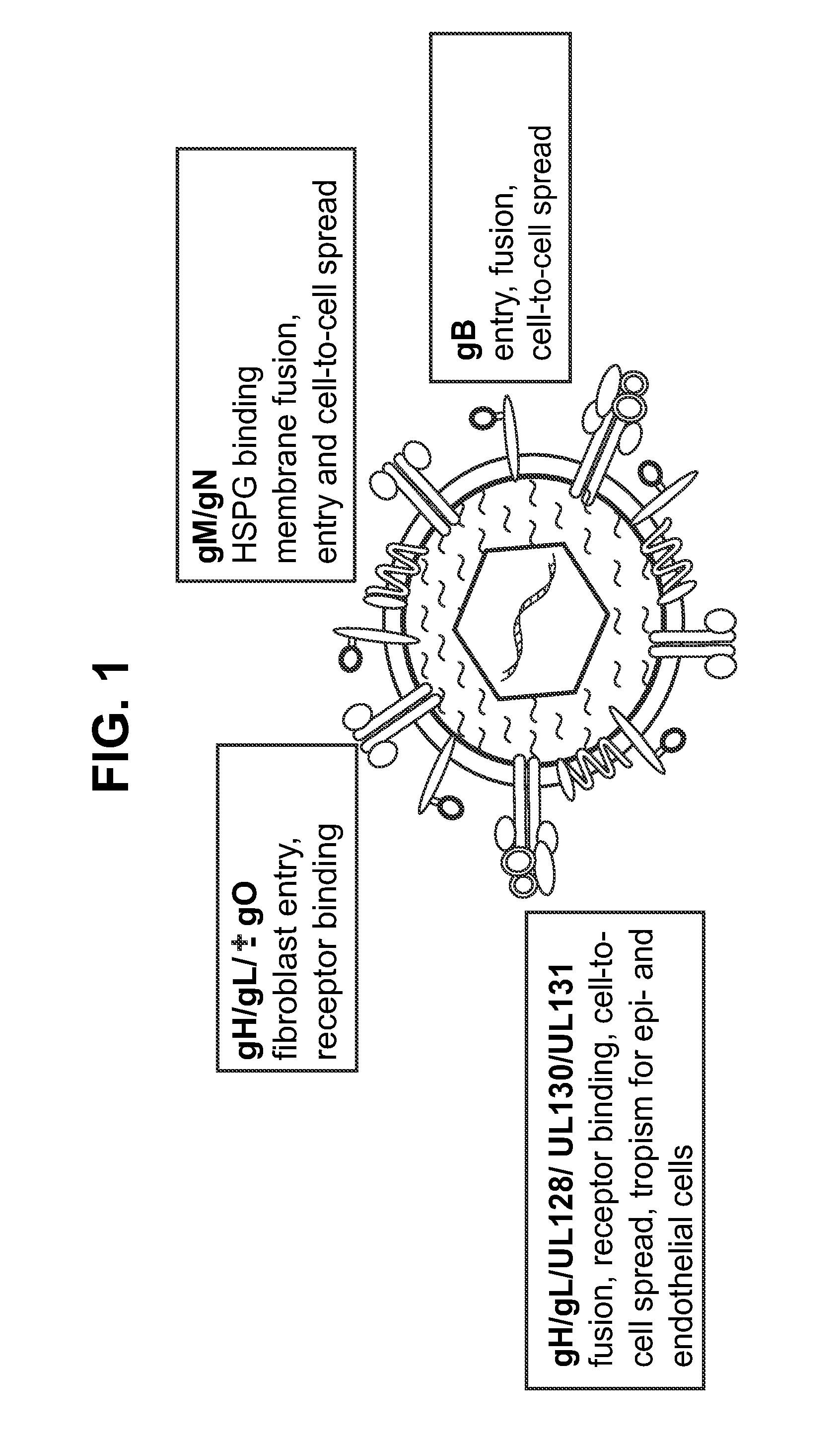
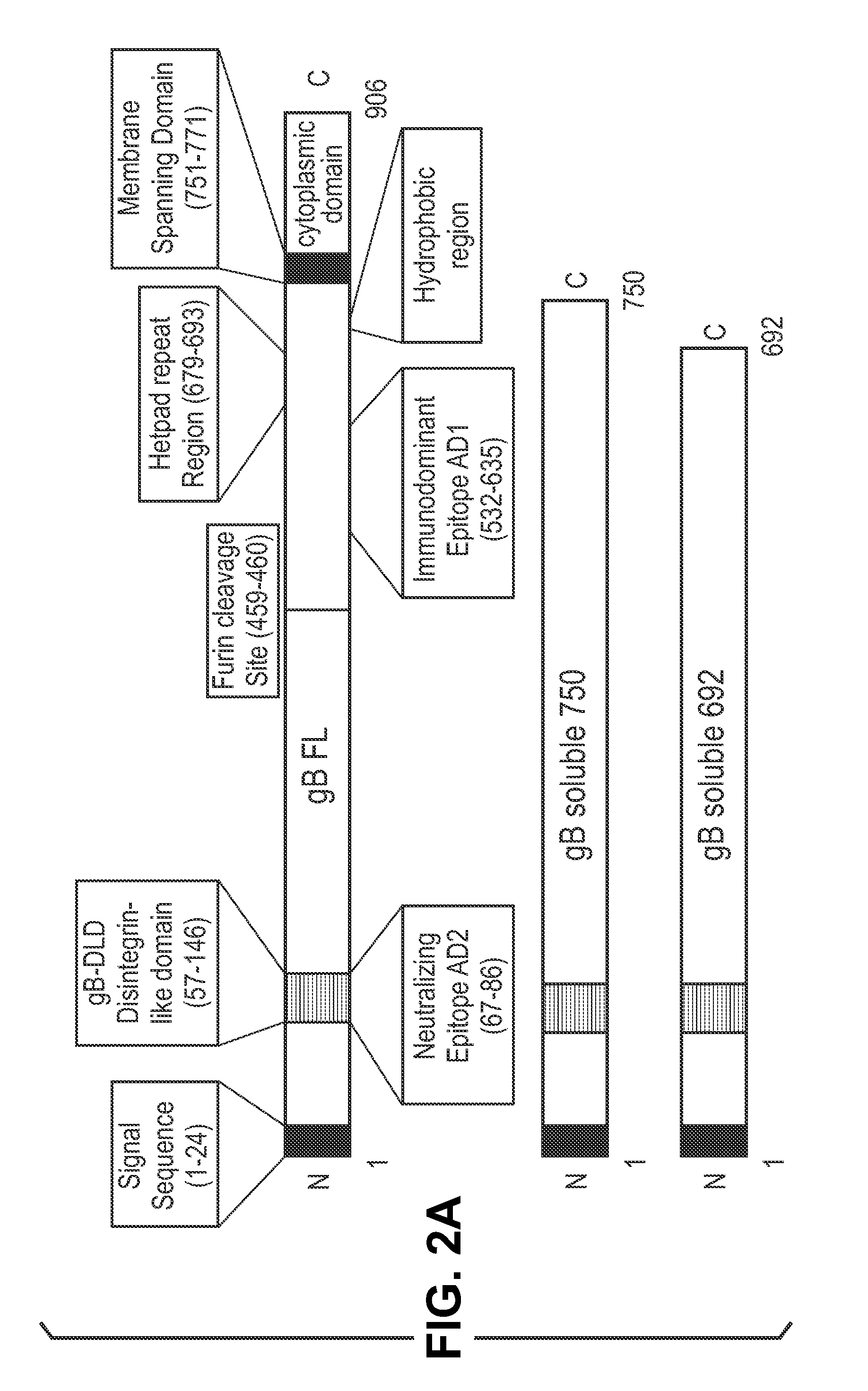
InactiveUS20140030292A1Improve stabilityAvoid reduce stimulationSsRNA viruses positive-senseFusion with post-translational modification motifAntigen deliveryHerpes simplex virus DNA
This disclosure provides platforms for delivery of herpes virus proteins to cells, particularly proteins that form complexes in vivo. In some embodiments these proteins and the complexes they form elicit potent neutralizing antibodies. Thus, presentation of herpes virus proteins using the disclosed platforms permits the generation of broad and potent immune responses useful for vaccine development.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Systems and methods for identifying diagnostic indicators
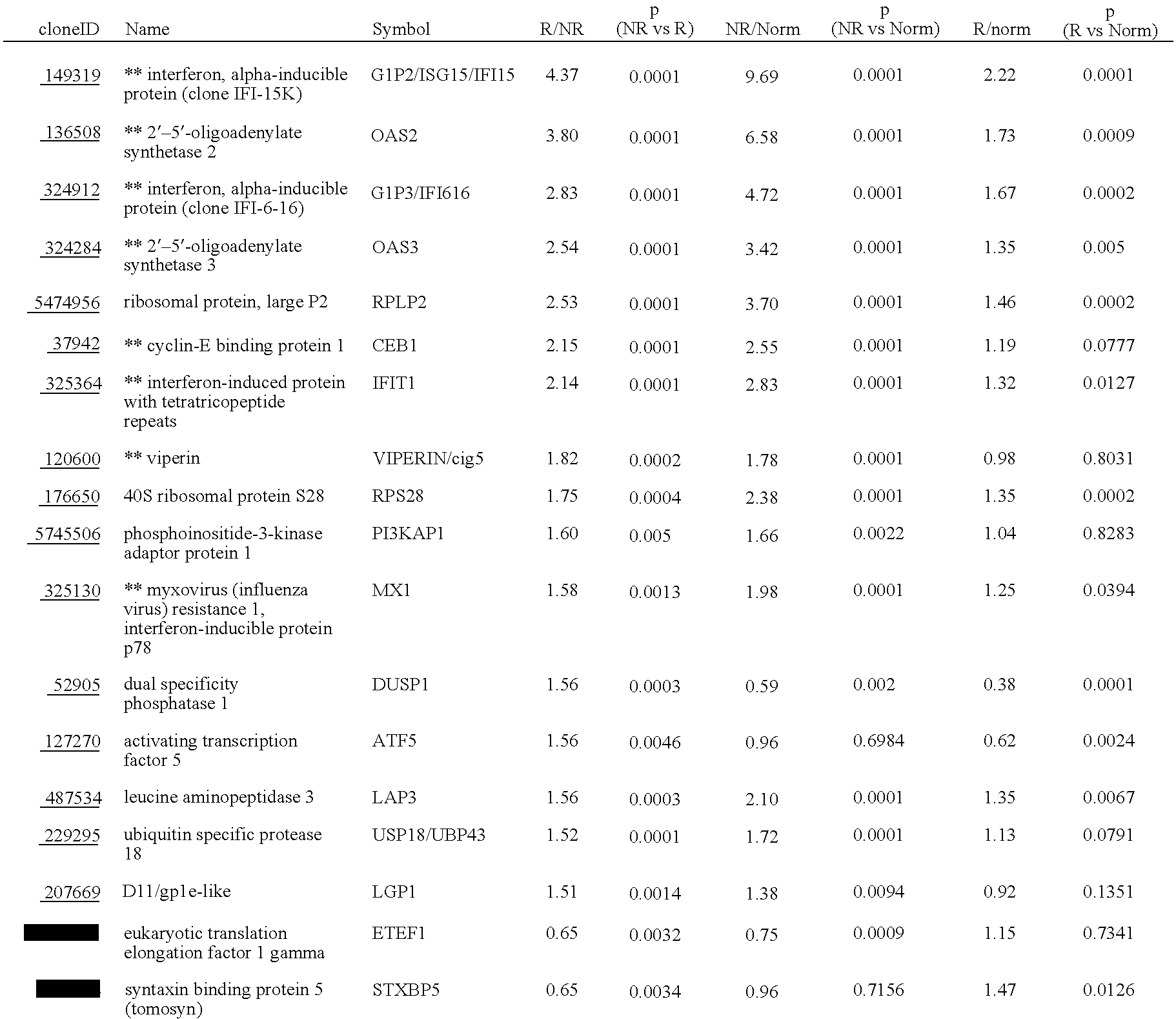
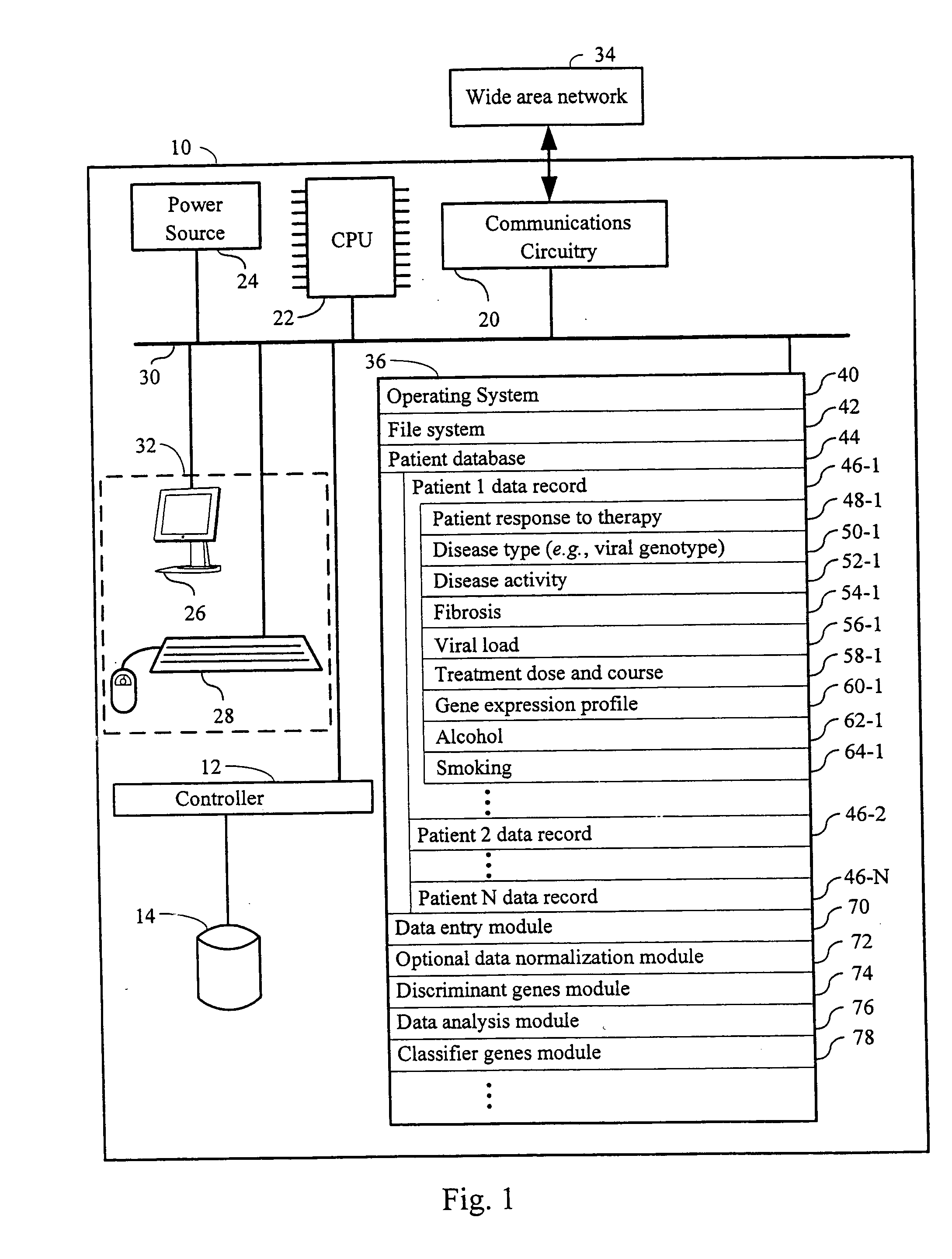
InactiveUS20060177837A1Enhance and improve therapeutic effectReduces liver disease activityMicrobiological testing/measurementDrug and medicationsHepatitis c viralRegimen
Systems and methods are provided for predicting patient response to a therapy regimen for a liver disease or a disease that is treatable with an immunomodulatory disease therapy using gene expression classifiers. Systems and methods for screening for modulators of target gene expression are also provided. Systems and methods for developing therapeutics against one or more of the proteins coded for by genes of the present invention are also provided. Systems and methods for predicting a patient response to a regimen of pegylated interferon alpha and ribavirin in a therapy for hepatitis C viral infection are also provided.
Owner:JAGUAR BIOSCI
Novel dipeptidyl peptidase iv (dp-iv) inhibitors as anti-diabetic agents
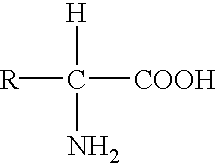
The present invention relates to a series of prodrugs of inhibitors of DP-IV with improved properties. The compounds can be used for the treatment of a number of human diseases, including impaired glucose tolerance and type II diabetes. The compounds of the invention are described by general formula (1); wherein R1 is H or CN; R2 is selected from CH2R5, CH2CH2R5 and C(R3)(R4)—X2—(CH2)aR5; R3 and R4 are each independently selected from H and Me; R5 is selected from CON(R6)(R7), N(R8)C(=0)R9, N(R8)C(═S)R9, N(R8)SO2R10 and N(R8)R10; R6 and R7 are each independently R11(CH2)b or together they are —(CH2)2-Z-(CH2)2— or CH2-o-C6H4-Z-CH2—; R8 is H or Me; R9 is selected from R11(CH2)b, R11(CH2)bO and N(R6)(R7); R10 is R11(CH2)b; R11 is selected from H, alkyl, optionally substituted aryl, optionally substituted aroyl, optionally substituted arylsulphonyl and optionally substituted heteroaryl; R12 is selected from H2NCH(R13)CO, H2NCH(R14)CONHCH(R15)CO, C(R16)═C(R17)COR18 and R19OCO; R13, R14 and R15 are selected from the side chains of the proteinaceous amino acids; R16 is selected from H, lower alkyl (C1-C6) and phenyl; R17 is selected from H and lower alkyl (C1-C6); R18 is selected from H, lower alkyl (C1-C6), OH, O-(lower alkyl (C1-C6)) and phenyl; R19 is selected from lower alkyl (C1-C6), optionally substituted phenyl and R20C(=0)OC(R21)(R22); R20, R21 and R22 are each independently selected from H and lower alkyl (C1-C6); Z is selected from a covalent bond, —(CH2)c—, —O—, —SOd— and —N(R10)—; X1 is S or CH2; X2 is O, S or CH2; a is 1, 2 or 3; b is 0-3; c is 1 or 2; and d is 0, 1 or 2.
Owner:FERRING BV
Tolerogenic synthetic nanocarriers to reduce immune responses to therapeutic proteins
ActiveUS20120276109A1Reduce frequencyHigh frequencyOrganic active ingredientsPowder deliveryAntigenIMMUNE SUPPRESSANTS
Disclosed are synthetic nanocarrier compositions, and related methods, comprising therapeutic protein APC presentable antigens and immunosuppressants that provide tolerogenic immune responses specific to therapeutic proteins.
Owner:SELECTA BIOSCI
Stable Protein Formulations
ActiveUS20100166774A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDiseaseTolerance induction
The present invention relates generally to stable formulations comprising CTLA4Ig molecules, including lyophilized, and liquid formulations for administration via various routes including, for example, routes such as intravenous (IV) and subcutaneous (SC) for treating immune system diseases and tolerance induction.
Owner:BRISTOL MYERS SQUIBB CO
Expression vector, methods for the production of heterologous gene products and for the selection of recombinant cells producing high levels of such products
ActiveUS20040148647A1Shorten the timeReduce development costsAnimal cellsMicrobiological testing/measurementHeterologousHamster
An expression vector for eukaryotic cells comprising a gene which codes for a protein of interest, functionally linked to a hamster-ubiquitin / S27a-promoter and a gene which codes for a fluorescent protein. Preferably the expression vector also contains an amplifiable selectable marker gene. The invention also describes host cells, preferably mammalian cells, which have been transfected with the expression vector, processes for producing heterologous gene products and a method of selecting high-producing cells.
Owner:BOEHRINGER INGELHEIM PHARM KG
Liquid Vaccines For Multiple Meningococcal Serogroups
Conjugated capsular saccharides from meningococcal serogroups C, W135 and Y are safe and immunogenic in humans when combined in a single dose. This effect is retained when a conjugated capsular saccharide from serogroup A is added. These conjugated antigens can be stably combined in a single aqueous dose without the need for lyophilisation. Broad protection against serogroup B infection can be achieved by using a small number of defined polypeptide antigens. These polypeptide antigens can be combined with the saccharide antigens without loss of protective efficacy for any of the five serogroups. Efficacy if retained even if a Hib conjugate is added. The efficacy of a serogroup W135 conjugate is enhanced by addition of protein antigens derived from a serogroup B strain. Addition of a Hib conjugate to meningococcal conjugates enhances the overall activity against meningococcus serogroup W135.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Modified mrnas encoding cell-penetrating polypeptides
InactiveUS20140371302A1Polypeptide with localisation/targeting motifSugar derivativesCell biologyNucleic acid
This invention relates to modified nucleic acid compositions encoding cell-penetrating polypeptides to provoke an innate immune response in a cell and methods of delivering protein-binding partners to target cells.
Owner:MODERNATX INC
Compositions and methods for treating inflammatory disorders
InactiveUS20050112118A1Preventing and ameliorating diseaseEfficient modulationHydrolasesPeptide/protein ingredientsAssayProtein-protein complex
Protein complexes are provided comprising at least one interacting pair of proteins. The protein complexes are useful in screening assays for identifying compounds effective in modulating the protein complexes, and in treating and / or preventing diseases and disorders associated with the protein complexes and / or their constituent interacting members.
Owner:MYRIAD GENETICS
Stable protein formulations
ActiveUS8476239B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTolerance inductionDisease
The present invention relates generally to stable formulations comprising CTLA4Ig molecules, including lyophilized, and liquid formulations for administration via various routes including, for example, routes such as intravenous (IV) and subcutaneous (SC) for treating immune system diseases and tolerance induction.
Owner:BRISTOL MYERS SQUIBB CO
Azole compound and medicinal use thereof
The present invention relates to an azole compound represented by the formula [I]wherein W is S or O; R is —COOR7, —X1-A1-COOR7 (R7 is H, alkyl) or tetrazolyl; R1, R2, R3 and R4 are H and the like; A is —(CH2)m—X— (X is —N(R8)—, —C(R9)(R10)—, —CO— or —CO—N(R8)—); B is aryl or aromatic heterocyclic group; R5 is H and the like; R6 is —(Y)s1-(A2)s-Z (Y is —O—, —S(O)t—, —N(R13)—, —N(R14)—CO—, —N(R14)—SO2—, —SO2—N(R14)— and the like, A2 is alkylene, and Z is cycloalkyl, aryl, aromatic heterocyclic group, indanyl, piperazinyl, a prodrug thereof or a pharmaceutically acceptable salt thereof. The compound [I] of the present invention has a protein tyrosine phosphatase 1B inhibitory activity, and is useful as a therapeutic agent for diabetes, a therapeutic drug for diabetic complications or a therapeutic drug for hyperlipidemia.
Owner:JAPAN TOBACCO INC
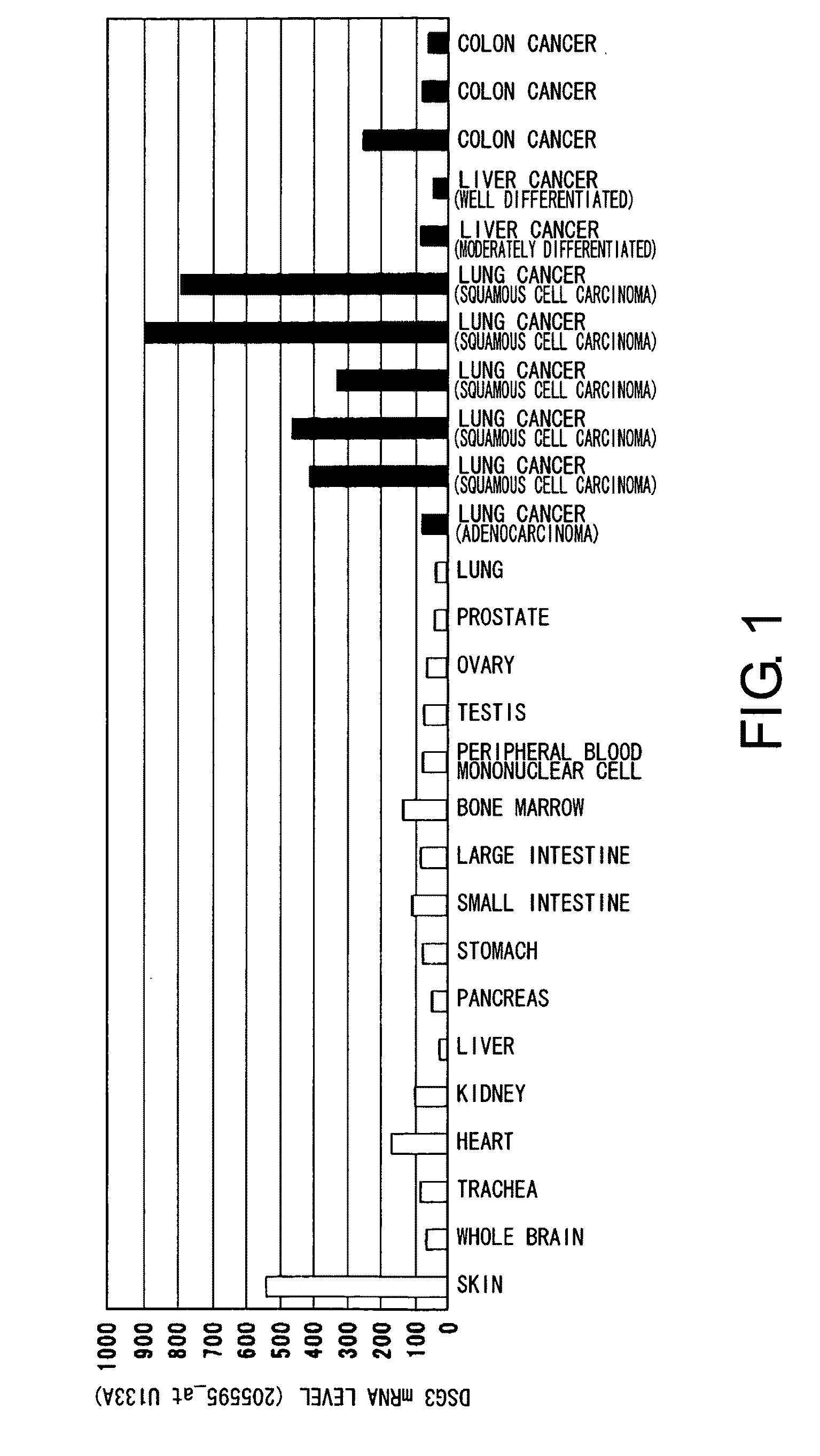
Diagnosis and Treatment of Cancer Using Anti-Desmoglein-3 Antibodies
InactiveUS20100092457A1High expressionAntibody mimetics/scaffoldsMicrobiological testing/measurementAntigen bindingBULK ACTIVE INGREDIENT
Methods that involve detection of a DSG3 protein for diagnosing cancer are disclosed. In lung cancer, the expression of DSG3 was found to be enhanced at very high frequency at the gene level and protein level. Methods of the present invention can be carried out using an antibody that recognizes a DSG3 protein. Pharmaceutical compositions, cell growth inhibitors, and anticancer agents containing a DSG3-binding antibody as an active ingredient are also disclosed. Methods of inducing cell damage in DSG3-expressing cells and methods of suppressing proliferation of DSG3-expressing cells by contacting the DSG3-expressing cells with DSG3-binding antibodies are also disclosed.
Owner:CHUGAI PHARMA CO LTD +1
Pluripotent therapeutic compositions and uses thereof
InactiveUS20090274660A1Reduce riskReduce significant riskBiocideOrganic active ingredientsSelf-healingAnticarcinogen
Synthetic Stem Cell-like Tissue Healing and Regeneration Medication with Anti-inflammatory, Protein Synthesis, Enzyme Deficiency Activation and Genetic Therapy, and Anti-cancer Agent derived from a series of inventions that include these products of Biomolecular Engineering, Drug Discovery from a Biologic Periodic Table of Applied Biochemistry and Biophysics. Tissue has a self healing effect promoting tissue healing and tissue regeneration. Not only does it maintain good health but also it has been observed that the patient's blood is withdrawn from the patient and applied to the ulcer has healing qualities. Cartilage placed in a wound promotes and accelerates wound healing. The anabolic biochemical and biophysical equivalent of tissue has been found in these embodiments to have the same pharmacologic qualities, when devoid of genetic DNA mismatch and other catabolic factors including the catabolic effects of microorganism overgrowth that lacks pro-biotic qualities. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
Method for Modeling a Disease
The invention described herein provides for methods of profiling cellular models of disease. Cellular systems biology is the investigation of the integrated and interacting networks of genes, proteins, and metabolites that are responsible for normal and abnormal cell functions. Methods and reagents for the profiling a disease state, the treatment of a disease state and assaying of treatments of a disease state are provided.
Owner:CELLUMEN INC
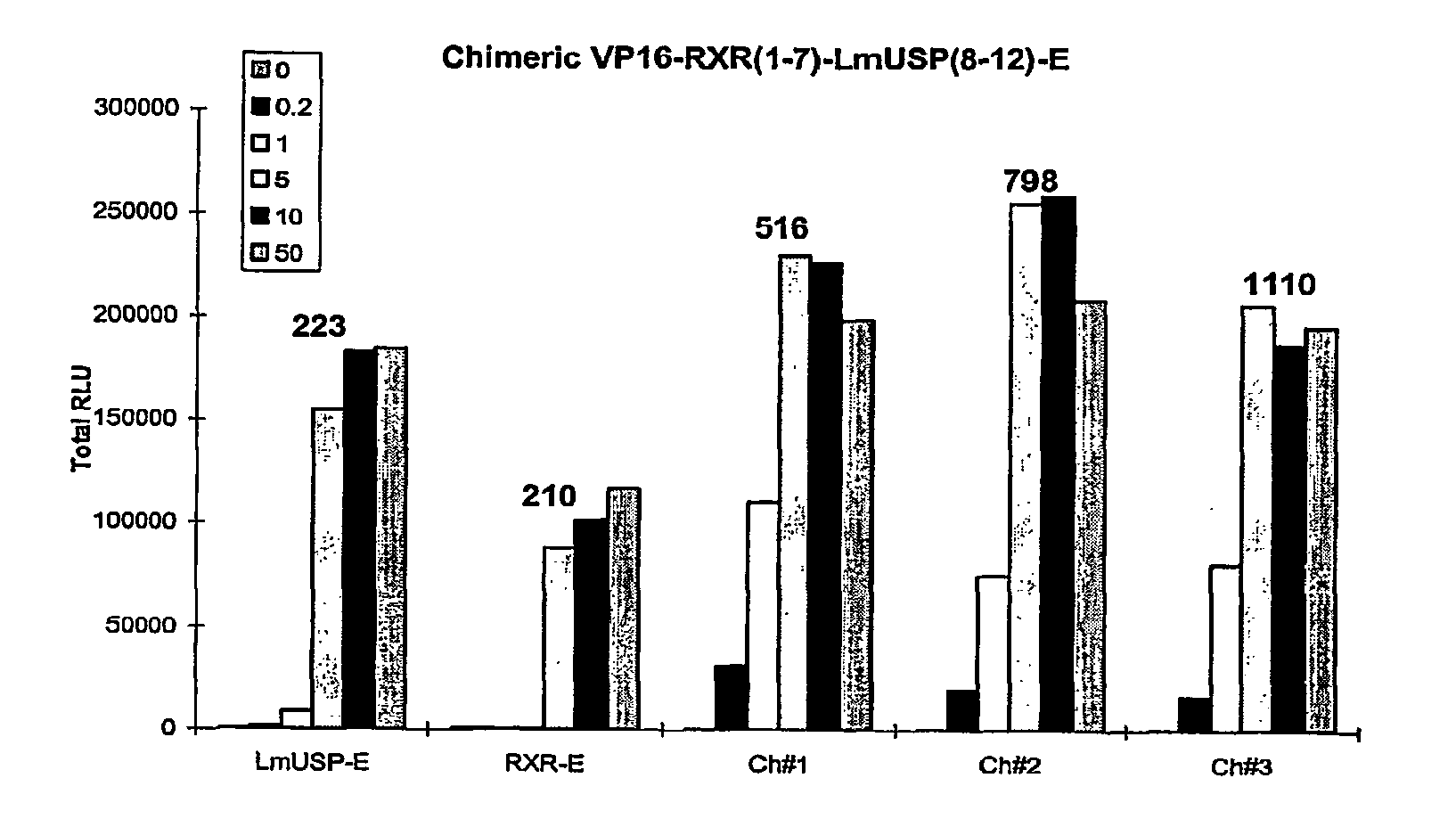
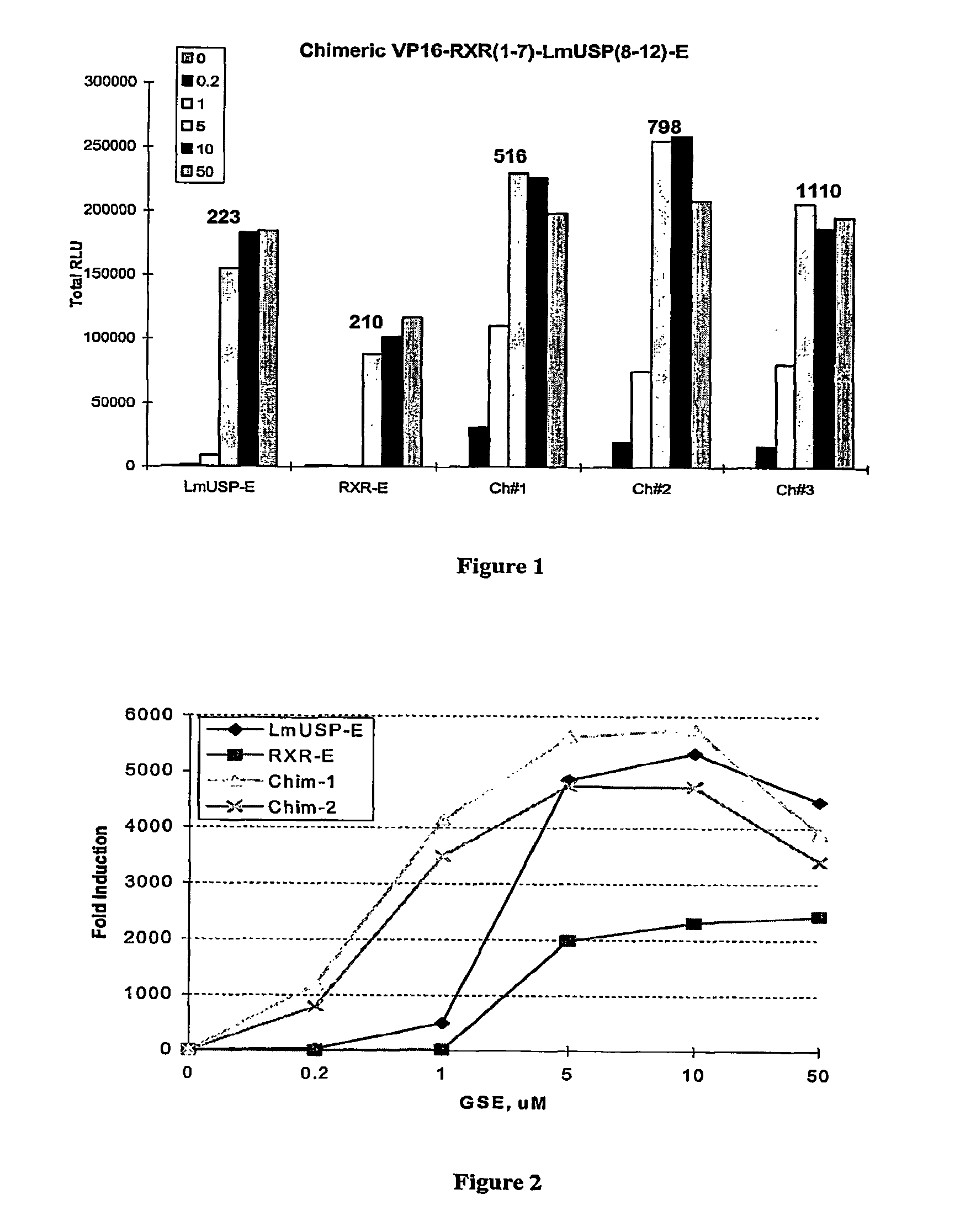
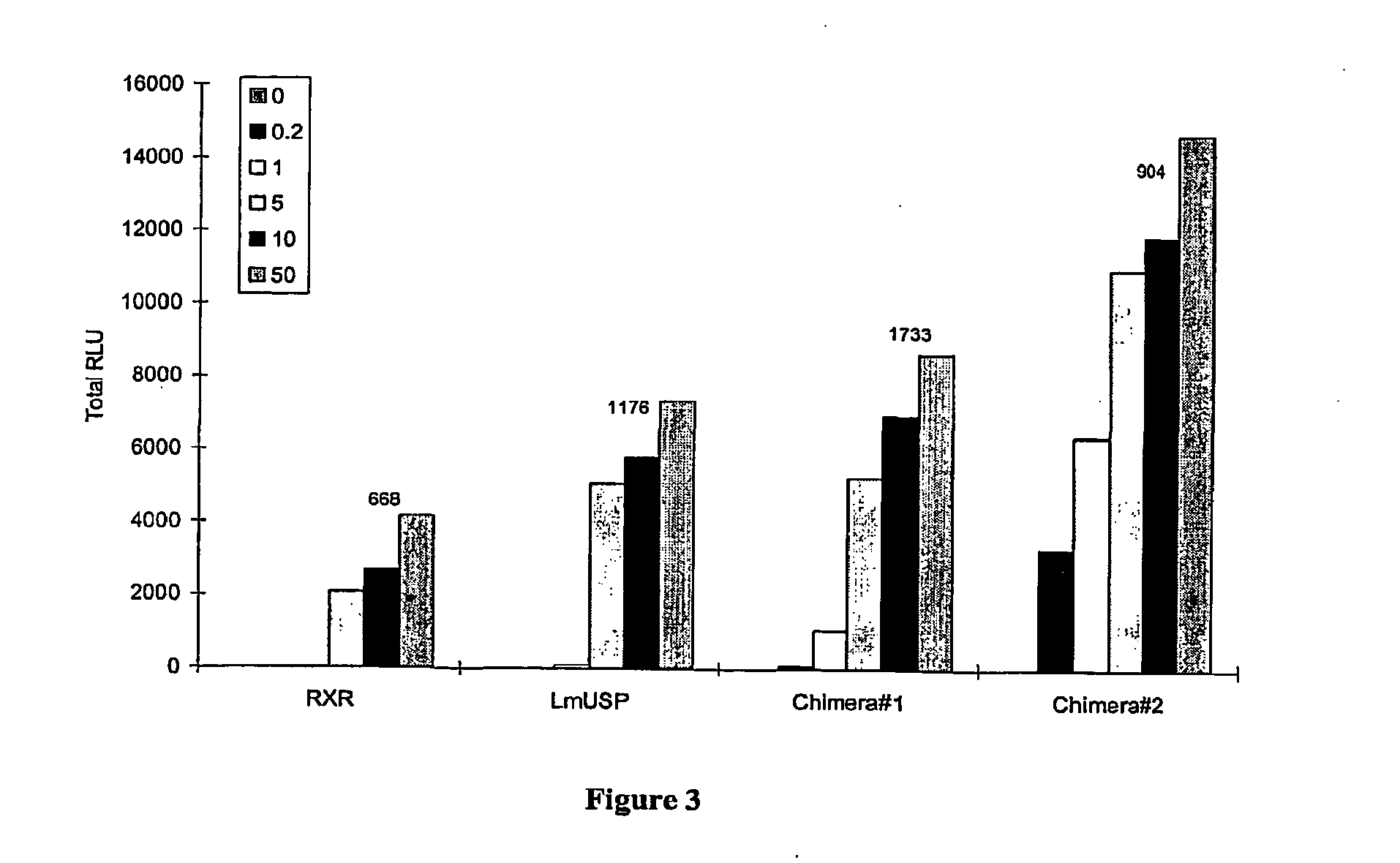
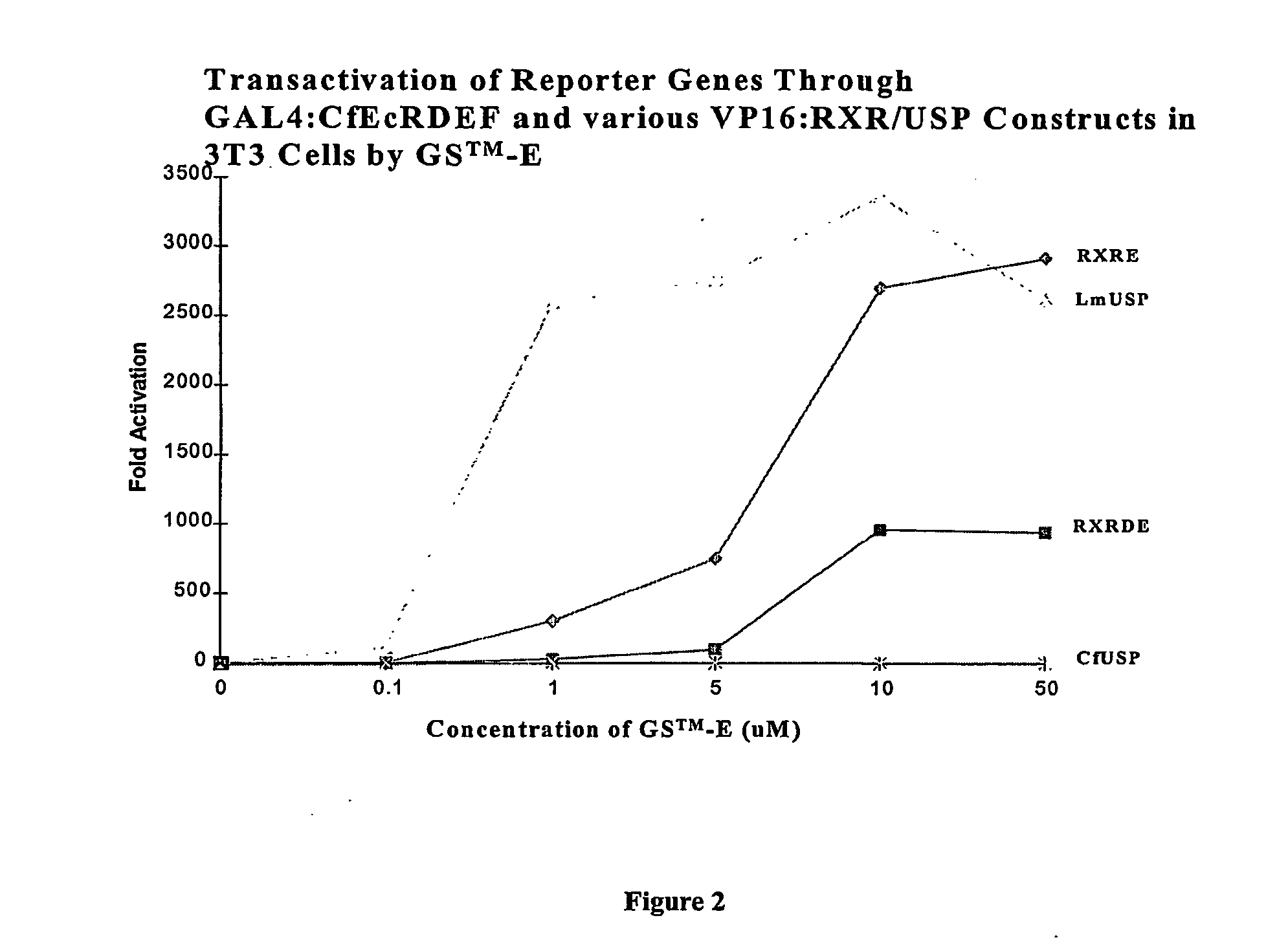
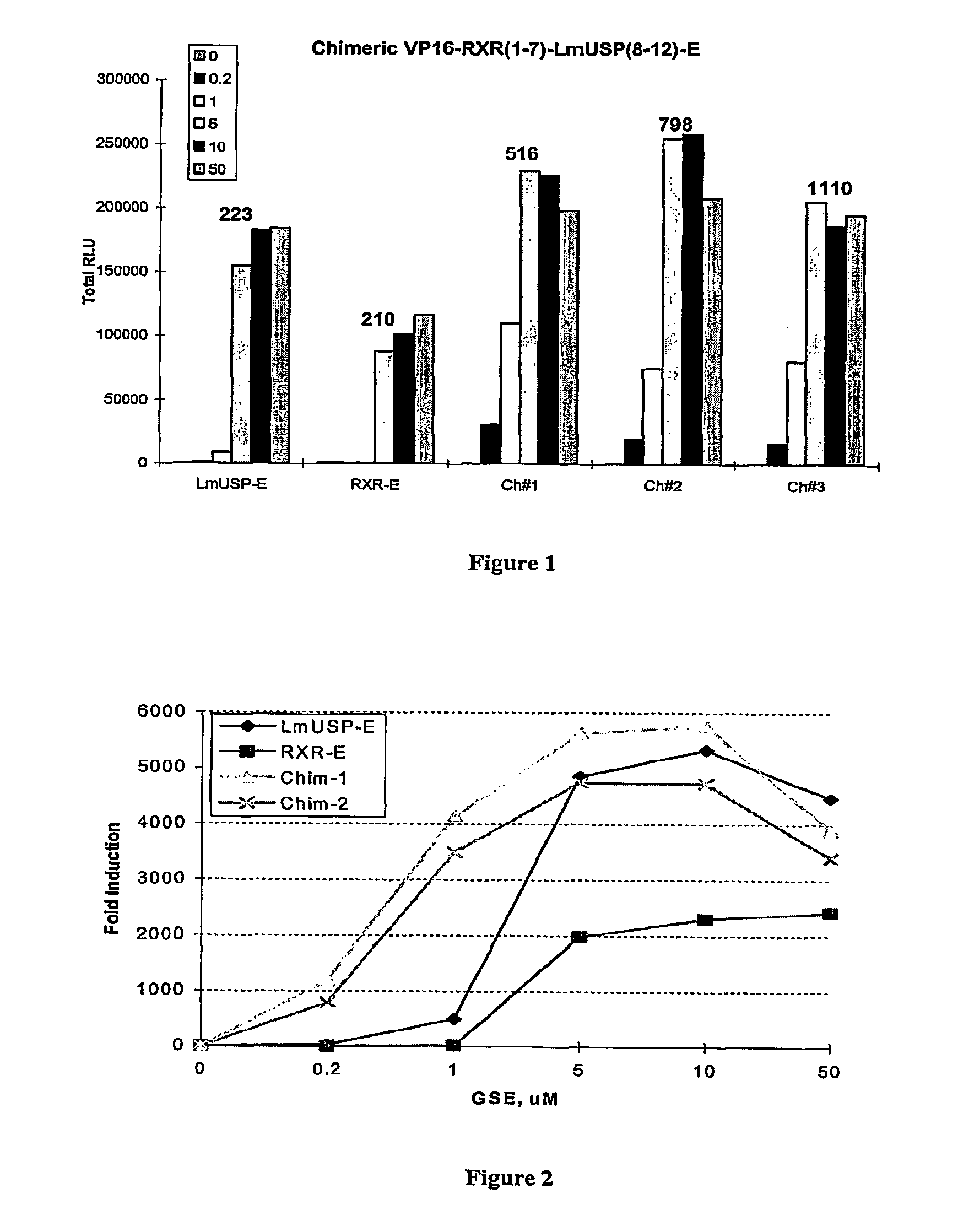
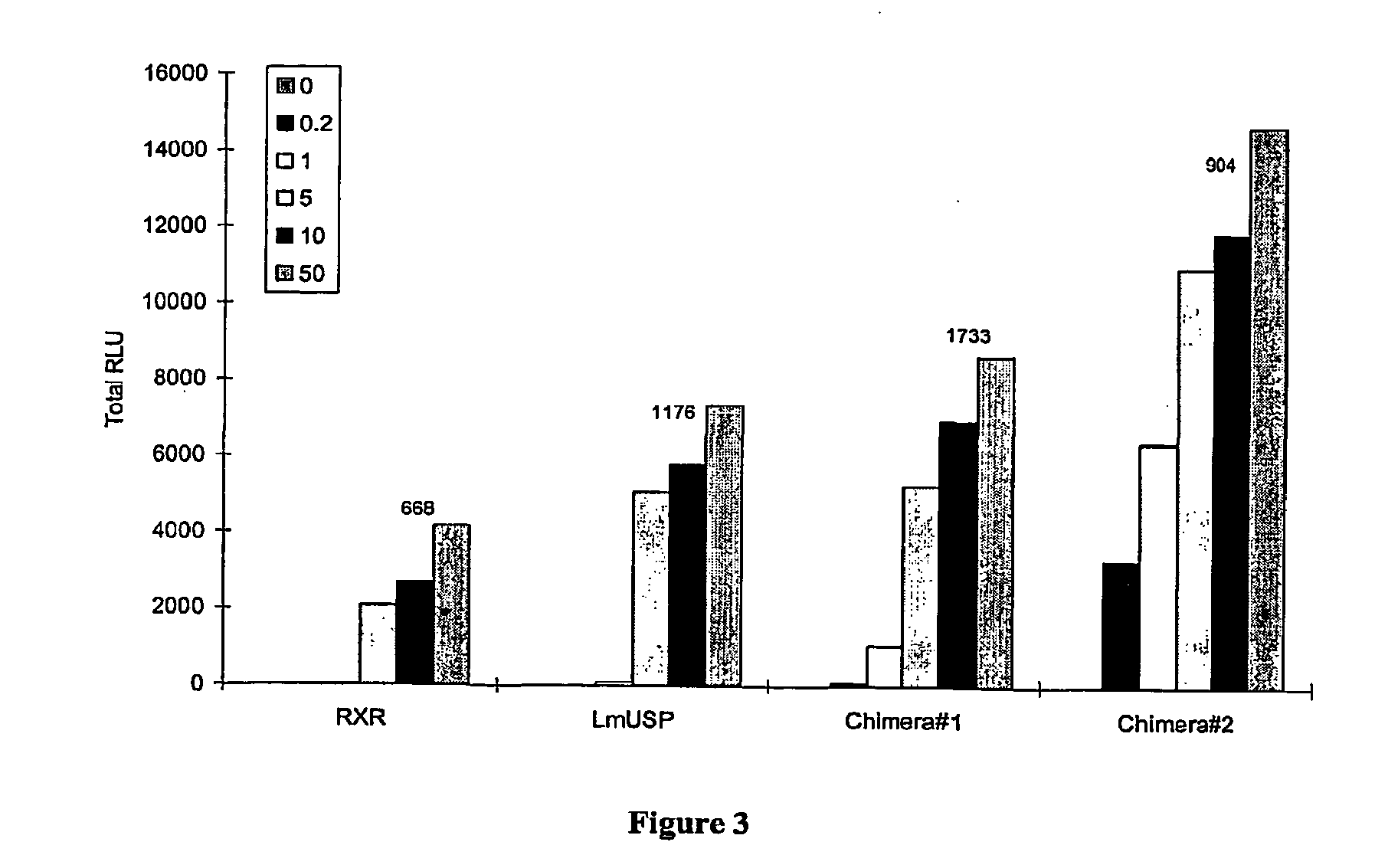
Chimeric retinoid x receptors and their use in a novel ecdysone receptor-based inducible gene expression system
InactiveUS20080263687A1FungiFusion with DNA-binding domainHigh-Throughput Screening AssaysBiotechnology
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to a novel ecdysone receptor / chimeric retinoid X receptor-based inducible gene expression system and methods of modulating gene expression in a host cell for applications such as gene therapy, large-scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:RHEOGENE INC DE
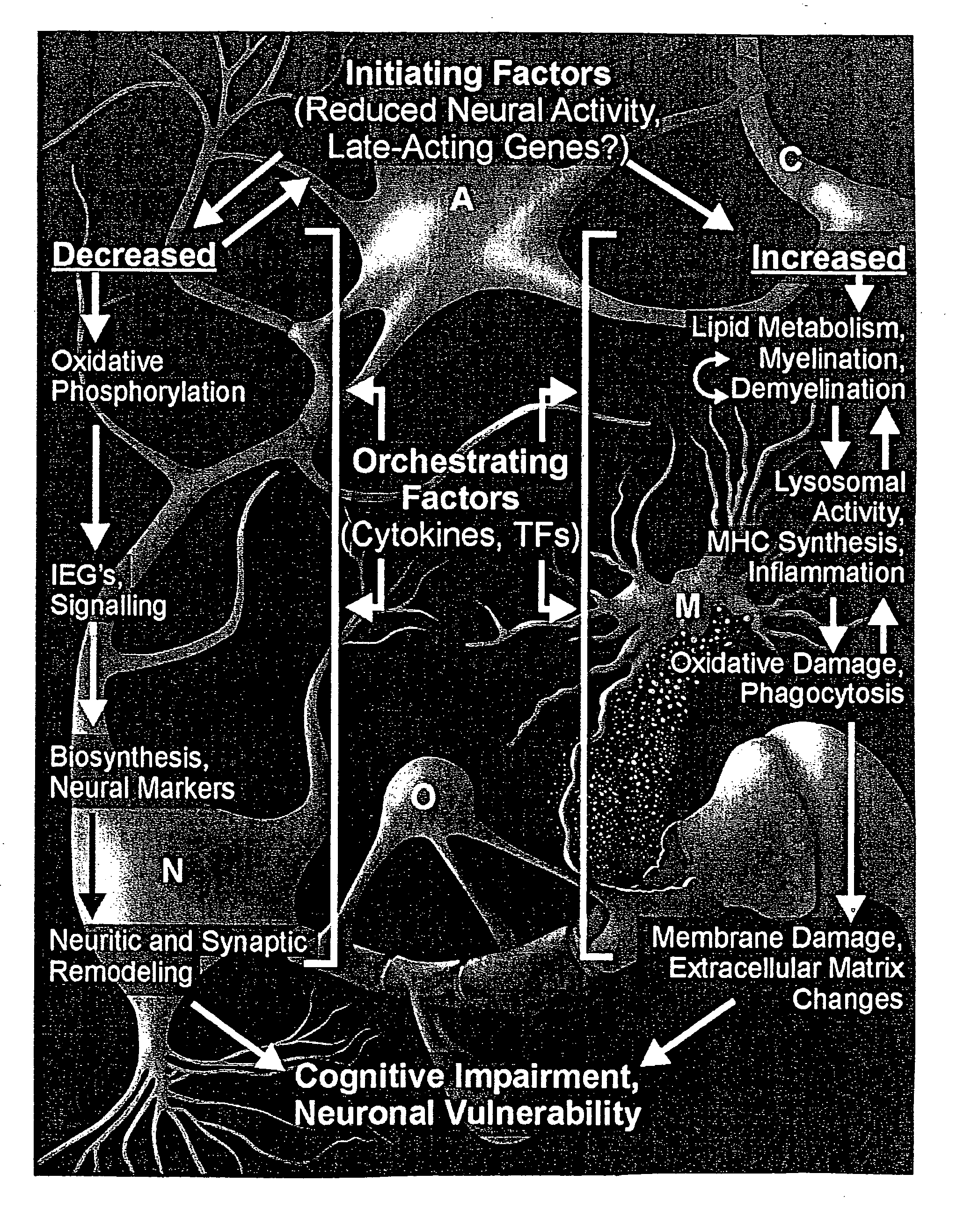
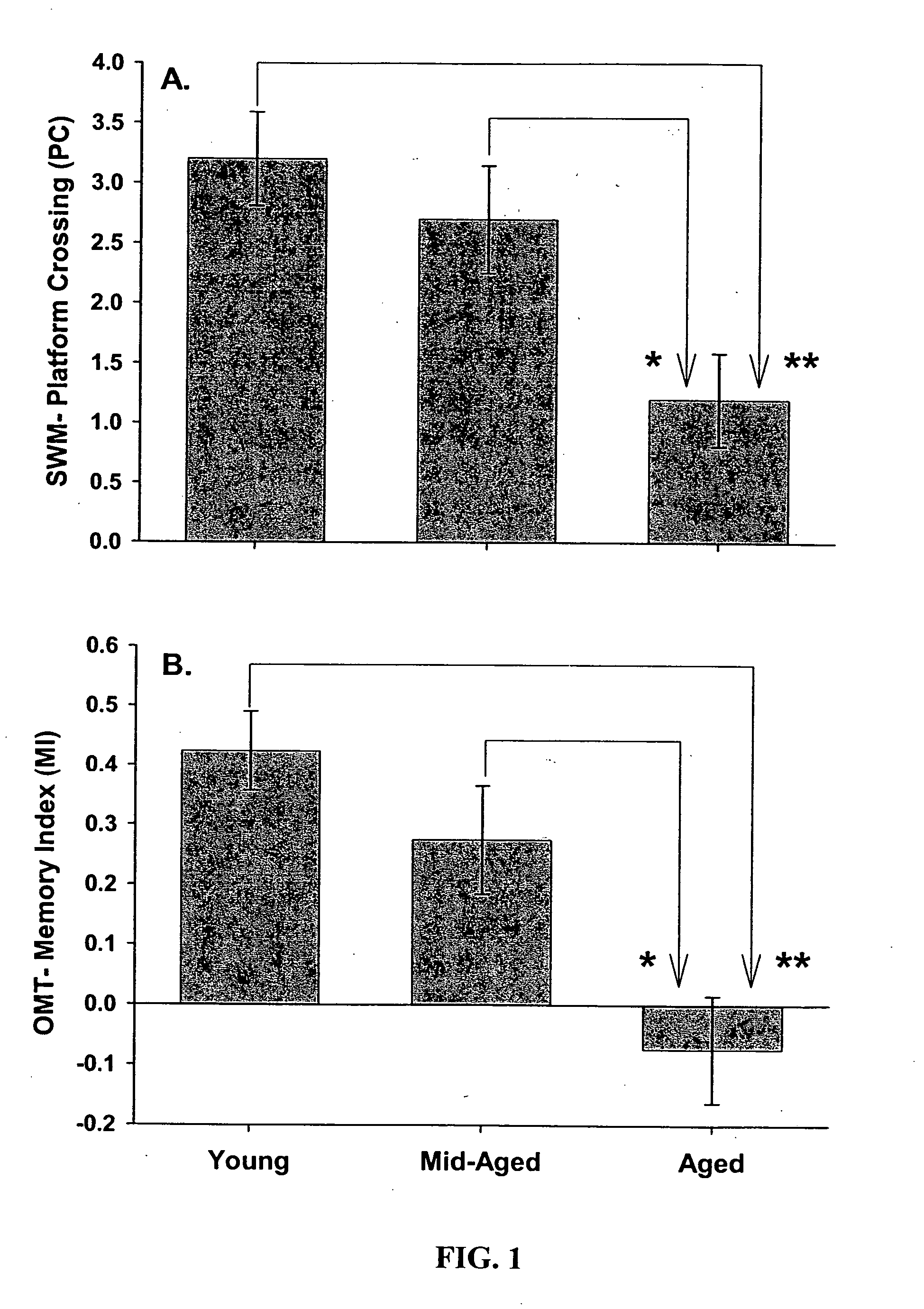
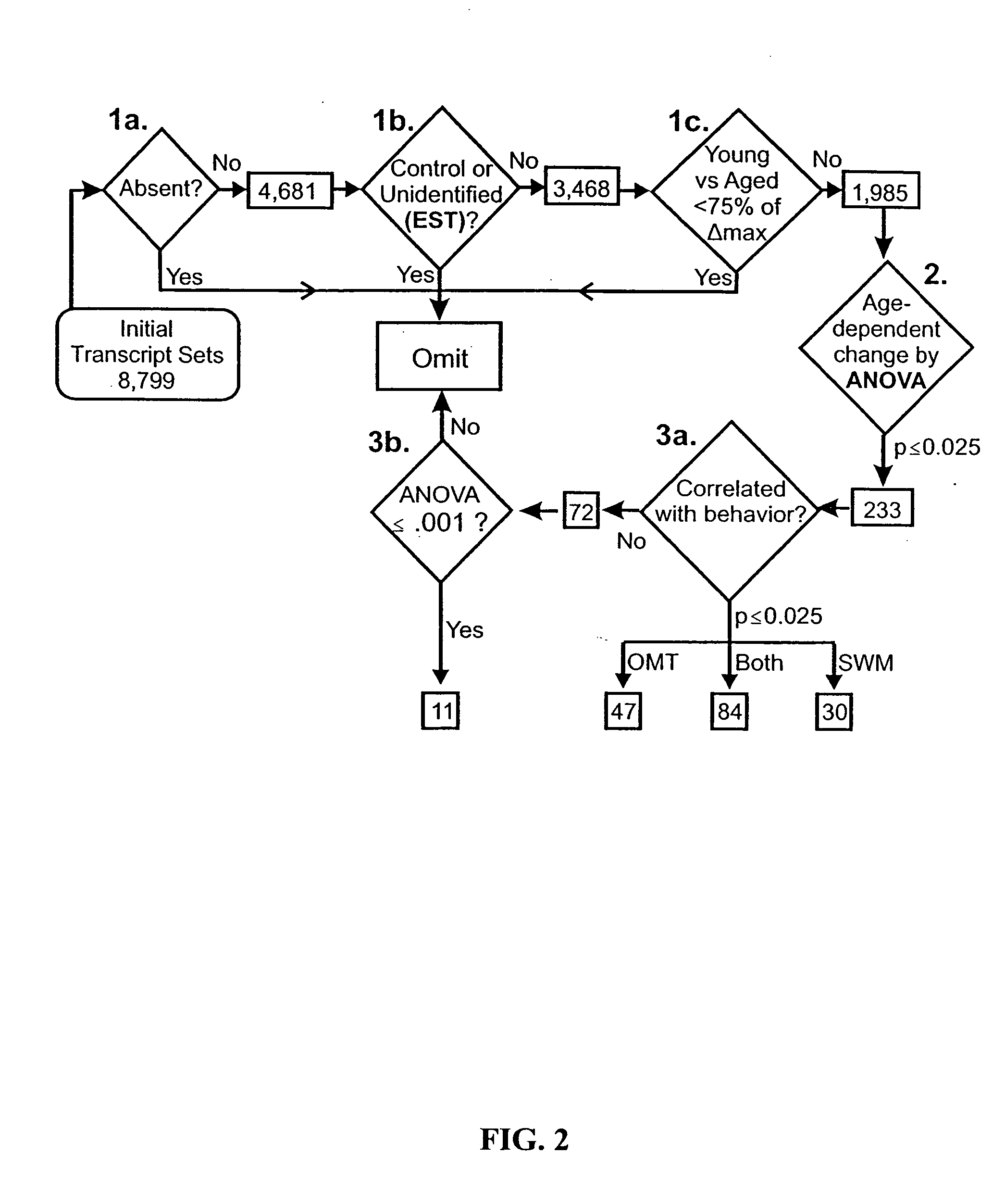
Gene expression profile biomarkers and therapeutic targets for brain aging and age-related cognitive impairment
InactiveUS20050071088A1Increase neuronal vulnerabilityImprove lipid metabolismMicrobiological testing/measurementProteomicsAntigenDisease cause
A statistical and functional correlation strategy to identify changes in cellular pathways specifically linked to impaired cognitive function with aging. Analyses using the strategy identified multiple groups of genes expressed in the hippocampi of mammals, where the genes were expressed at different levels for several ages. The aging changes in expression began before mid-life. Many of the genes were involved in specific neuronal and glial pathways with previously unrecognized relationships to aging and / or cognitive decline. The processes identified by the strategy suggest a new hypothesis of brain aging in which initially decreased neuronal activity and / or oxidative metabolism trigger separate but parallel genomic cascades in neurons and glia. In neurons, the cascade results in elevations in calcium signaling and reductions of immediate early gene signaling, biosynthesis, synaptogenesis and neurite remodeling. In contrast, glia undergo increased lipid metabolism and mediate a cycle of demyelination and remyelination that induces antigen presentation, inflammation, oxidative stress and extracellular restructuring. These identified genes and the proteins they encode can be used as novel biomarkers of brain aging and as targets for developing treatment methods against age-related cognitive decline, Alzheimer's Disease and Parkinson's Disease.
Owner:UNIV OF KENTUCKY RES FOUND
Mutant receptors and their use in a nuclear receptor-based inducible gene expression system
ActiveUS20080145935A1Improve efficiencyGreat degree of sequence similarityPeptide/protein ingredientsTissue cultureHigh-Throughput Screening AssaysGenomics
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to novel substitution mutant receptors and their use in a nuclear receptor-based inducible gene expression system and methods of modulating the expression of a gene in a host cell for applications such as gene therapy, large scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:PRECIGEN INC
Novel ecdysone receptor/invertebrate retinoid X receptor-based inducible gene expression system
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to a novel ecdysone receptor / invertebrate retinoid X receptor-based inducible gene expression system and methods of modulating gene expression in a host cell for applications such as gene therapy, large-scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:PRECIGEN INC
Chimeric retinoid x receptors and their use in a novel ecdysone receptor-based inducible gene expression system
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to a novel ecdysone receptor / chimeric retinoid X receptor-based inducible gene expression system and methods of modulating gene expression in a host cell for applications such as gene therapy, large-scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:RHEOGENE INC DE
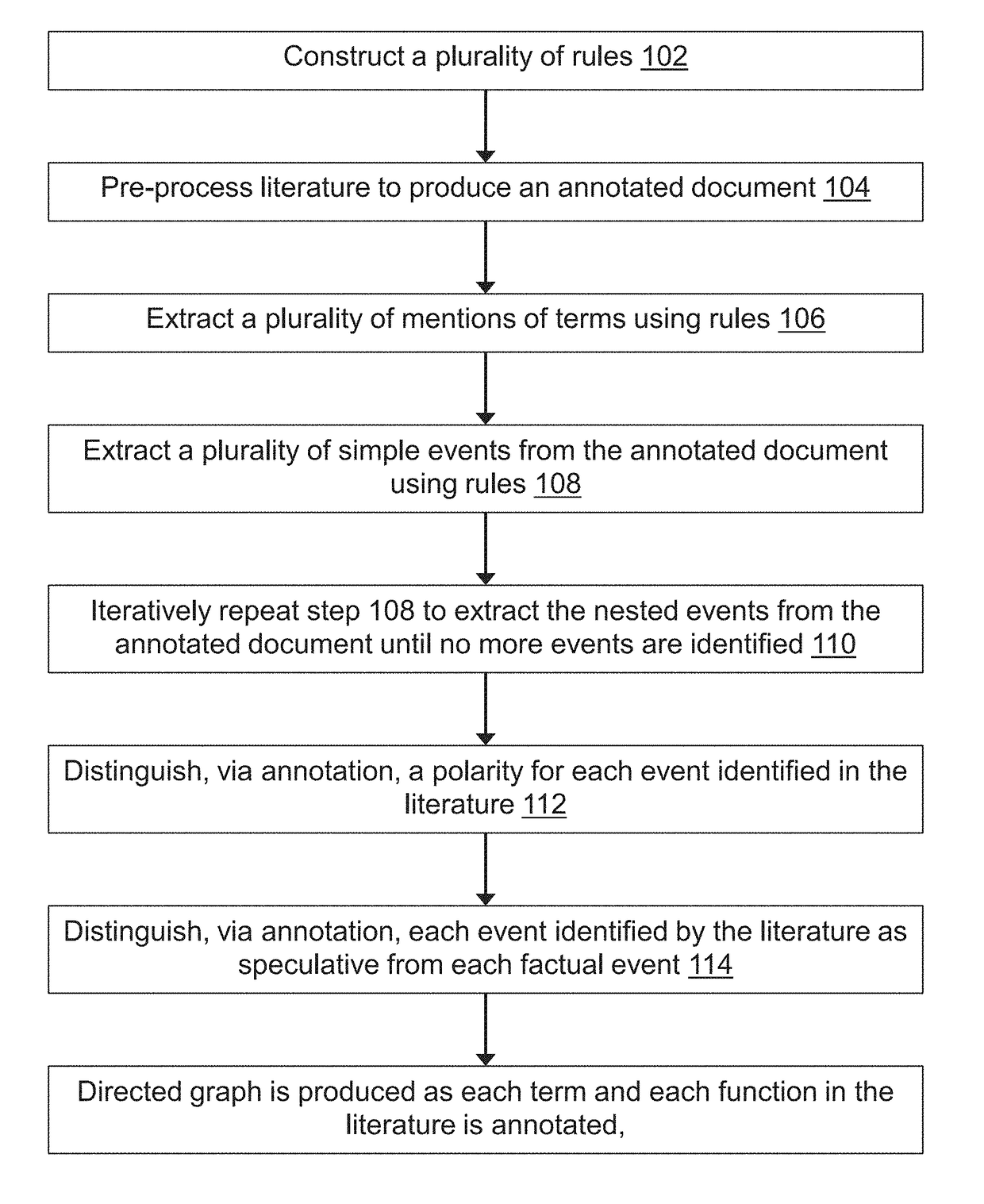
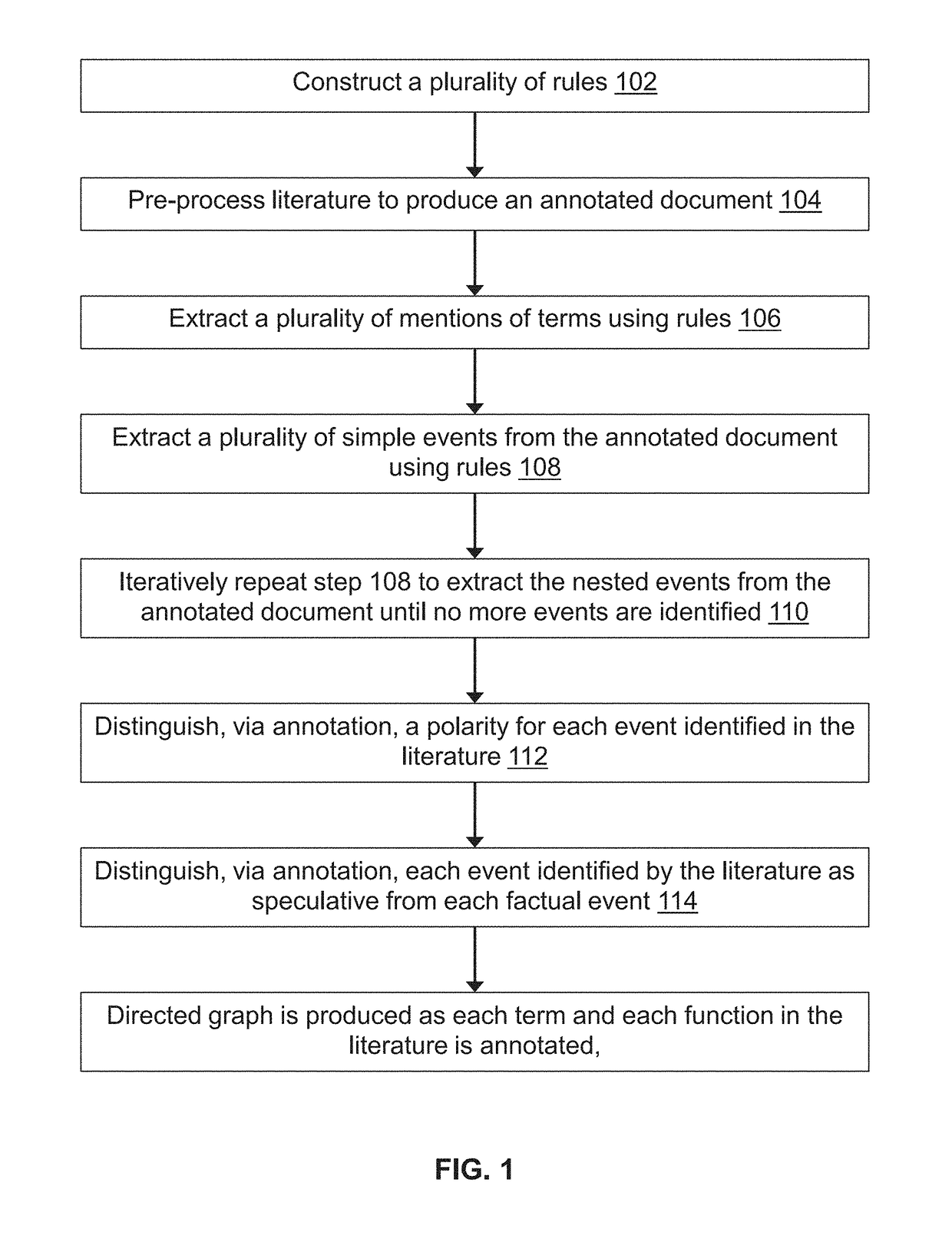
Methods for extracting and assessing information from literature documents
InactiveUS20180260474A1Complex structureWeb data indexingNatural language data processingSignalling pathwaysSemantics
A machine reading system is described herein that includes a framework in which grammar rules can be developed using a concise language that combines syntax and semantics. The resulting technology thus reduces the development time for new grammars in a new domain. An enormous amount of information appears in the form of natural language across millions of academic papers and other literature sources. For example, in the biological domain, there is a tremendous ongoing effort to extract individual chemical interactions from these texts, but these interactions are only isolated fragments of larger causal mechanisms such as protein signaling pathways. The proposed rule-based event extraction framework can model underlying syntactic representations of events in order to extract signaling pathway fragments. Though application to the biomedical domain is herein described, the framework is domain-independent and is expressive enough to capture most complex events annotated by domain experts.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Therapeutic Anti-her2 antibody fusion polypeptides
InactiveUS20090226466A1Enhanced cell killing of cellImprove efficiencySugar derivativesAntibody mimetics/scaffoldsTherapeutic proteinAnti her2
Therapeutic protein fusions comprising anti-HER2 antibody and MicB sequences are described along with methods for their production and use
Owner:GENENTECH INC
Protein markers for cardiovascular events
InactiveCN101889205AUse to reduce or preventReduce or prevent the risk of cardiovascular eventsDisease diagnosisBiological testingCathepsin SMacrophage migration inhibitory factor
The present invention relates to a method for predicting the risk of a subject developing a cardiovascular event comprising detecting at least one biomarker in (a sample of) the cardiovascular system from said subject, wherein said biomarker comprises at least one protein selected from the group consisting of Tumor Necrosis Factor Alpha Precursor; Lysosomal-associated Membrane Protein (1); Interleukin-5 Precursor; Interleukin-6 Precursor; C-C Motif Chemokine (2) Precursor; C-C Motif Chemokine (5) Precursor RANTES; Cathepsin L1 Precursor; Adenylate Kinase (1); Leukotriene B4 Receptor (1); Complement Factor D; Secreted Phosphoprotein (1); Small Inducible Cytokine A17 Precursor; C-X-C Motif Chemokine (10) Precursor; Tumor Necrosis Factor Ligand Superfamily Member (11)(RANKL); C-C Motif Chemokine (18) Precursor; 72 kDa Type IVCollagenase Precursor; Neutrophil Collagenase Precursor; fatty acid binding protein (4); calpain (2), (m / II) large subunit; Macrophage Migration Inhibitory Factor; Cathepsin S Precursor; Interleukin (13) Precursor; and soluble ICAM-1.
Owner:卡瓦迪斯有限责任公司
Pharmaceutical Compounds
InactiveUS20100004232A1Reduce morbidityPatient compliance is goodBiocideSenses disorderDiseaseImatinib resistant
The use of a compound for the manufacture of a medicament for the prophylaxis or treatment of: A. a disease state or condition mediated by a kinase which is BCR-abl, VEGFR, PDGFR, EGFR, Flt3, JAK (e.g. JAK2 or JAK3), C-abl, PDK1, Chk (e.g. Cbk1 or Chk2), FGFR (e.g. FGFR3), Ret, Eph (e.g. EphB2 or EphB4), or Src (e.g. cSrc); or B. a cancer in which the cancer cells thereof contain a drug resistant kinase mutation which is: (a) a threonine gatekeeper mutation; or (b) a drug-resistant gatekeeper mutation; or (c) an imatinib resistant mutation; or (d) a nilotinib resistant mutation; or (e) a dasatinib resistant mutation; or (f) a T670I mutation in KIT; or (g) a T674I mutation in PDGFR; or (h) T790M mutation in EGFR; or (i) a T315I mutation in abl; or C. a cancer which expresses a mutated molecular target which is a mutated form of BCRabl, c-kit, PDGF, EGF receptor or ErbB2; or D. a disease mediated by a kinase containing a mutation in a region of the protein that binds to or interacts with other cancer agents but does not bind to or interact with the compounds of formula (I) or (I′), for example a mutated kinase selected from c-abl, c-kit, PDGFR including PDGFR-beta and PDGFR-alpha, and ErbB family members such as EGFR (ErbB1), HER2 (ErbB2), ErbB3, and ErbB4, members of the Ephrin receptor family including EphA1, EphA2, EphA3, EphA4, EphA5, EphA8, EphA10, EphB1, EphB2, EphB3, EphB5, EphB6, c-Src and kinases of the JAK family such as TYK2; wherein the compound is a compound of the formula (I or I′): or a salt, solvate, tautomer or N-oxide thereof wherein R0′, R1, R1′, R2′, R3′, R4′, A′, X′, E, A and M are as defined in the claims.
Owner:ASTEX THERAPEUTICS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com