Application of substituted aryl hydrazone compound serving as anti-tumor necrosis factor inhibitor medicament
A technology of aromatic hydrazone and necrosis factor, which is used in antitumor drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Preparation of tablets each containing 100 mg of active ingredient:
[0116] mg / tablet
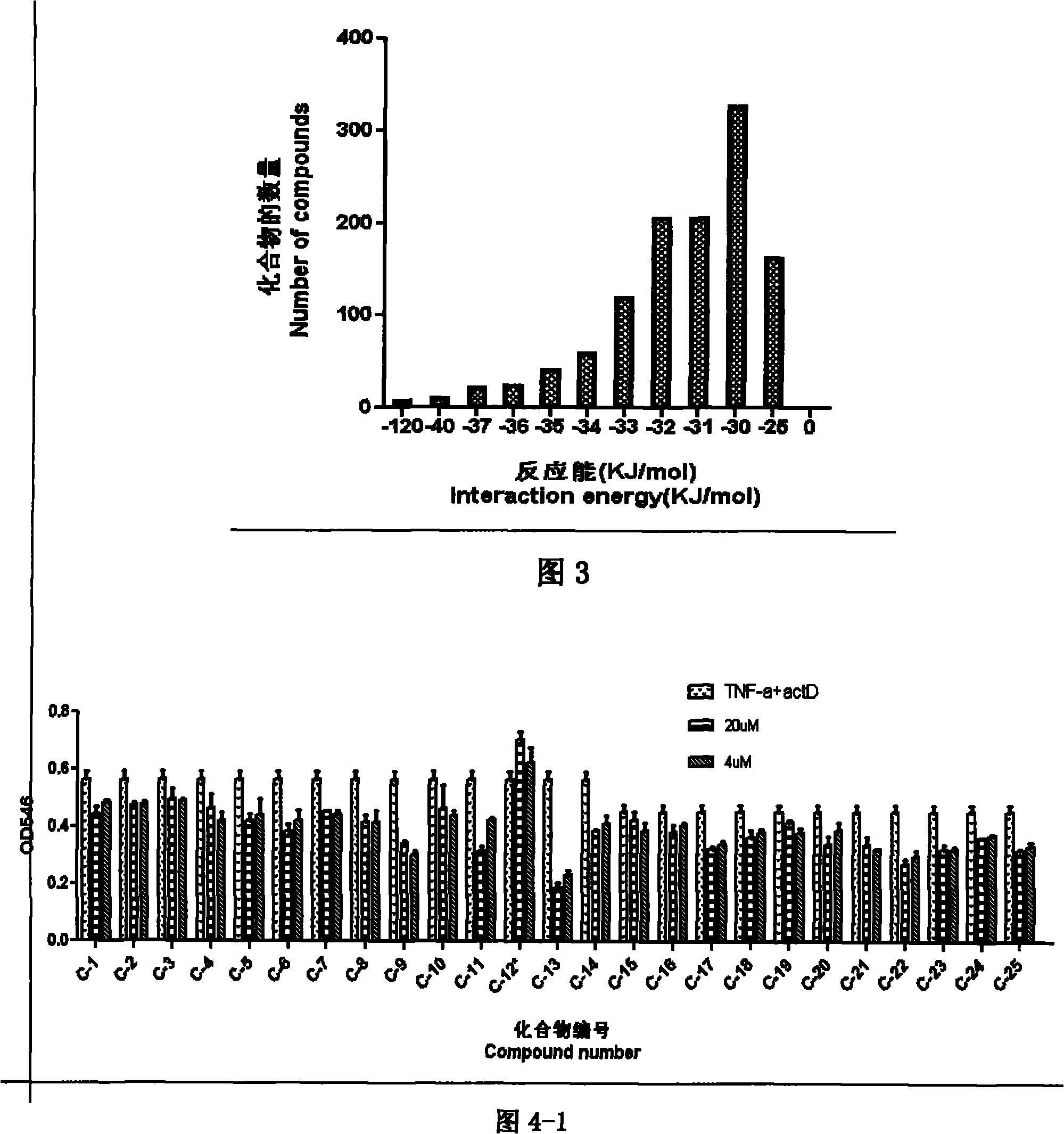
[0117] C-12 100
[0118] Lactose 50
[0119] Microcrystalline Cellulose 80
[0120] Starch 50
[0121] Hydroxymethylcellulose 40
[0123] Pass the active ingredients, lactose, starch, and microcrystalline cellulose through a 100-mesh sieve, and mix thoroughly, add 2% carboxymethylcellulose aqueous solution to the above mixed powder and mix, pass through a 20-mesh sieve to make a soft material, and obtain wet granules Dry at 45-55°C, add sodium carboxymethyl starch and magnesium stearate to the above-mentioned dry granules and press into tablets.
Embodiment 2
[0125] Capsules containing 100 mg of active ingredient per capsule are prepared as follows:
[0126] Dosage / Capsule Weight Concentration (%)
[0127] C-12 100mg 30.0
[0128] Polyoxyethylene sorrel 0.05mg 0.02
[0129] Sugar Alcohol Monooleate
[0130] Starch 250mg 69.98
[0131] Total 350.05mg 100.00
Embodiment 3
[0133] Preparation of Injections
[0134] C-12 200mg
[0135] Mannitol 700mg
[0136] PEG3000 10mg
[0137] Distilled water 100ml
[0138] Make the pH value 7.0-7.5 and filter the filtrate concentration to be 3 mg / ml, divide into 2 ml per ampoule, freeze-dry to obtain the injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com