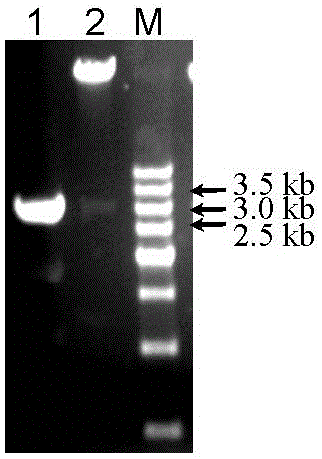
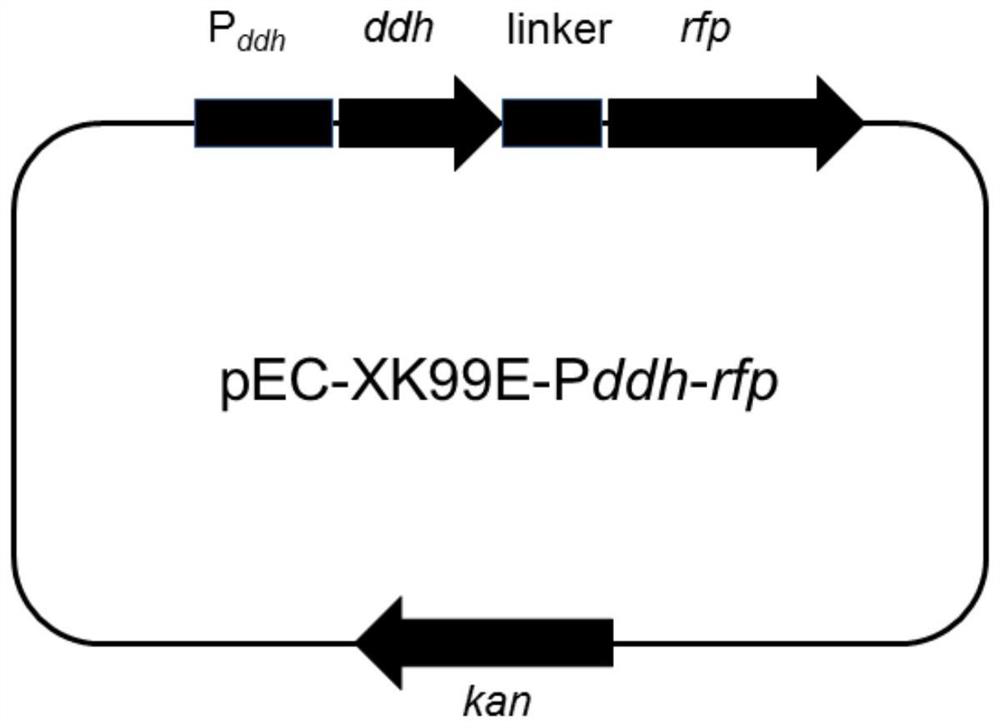
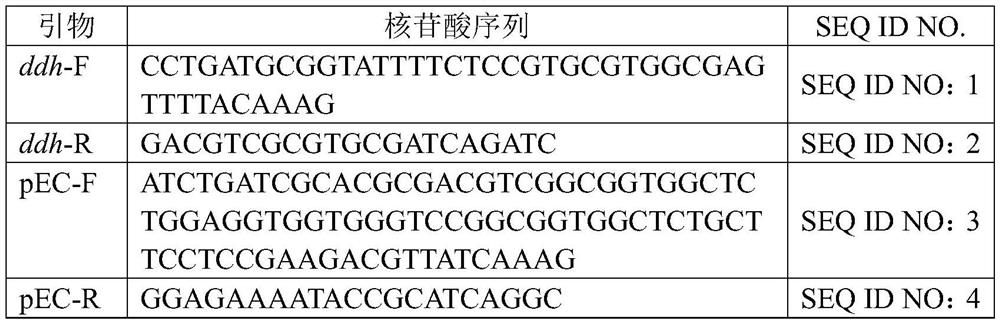
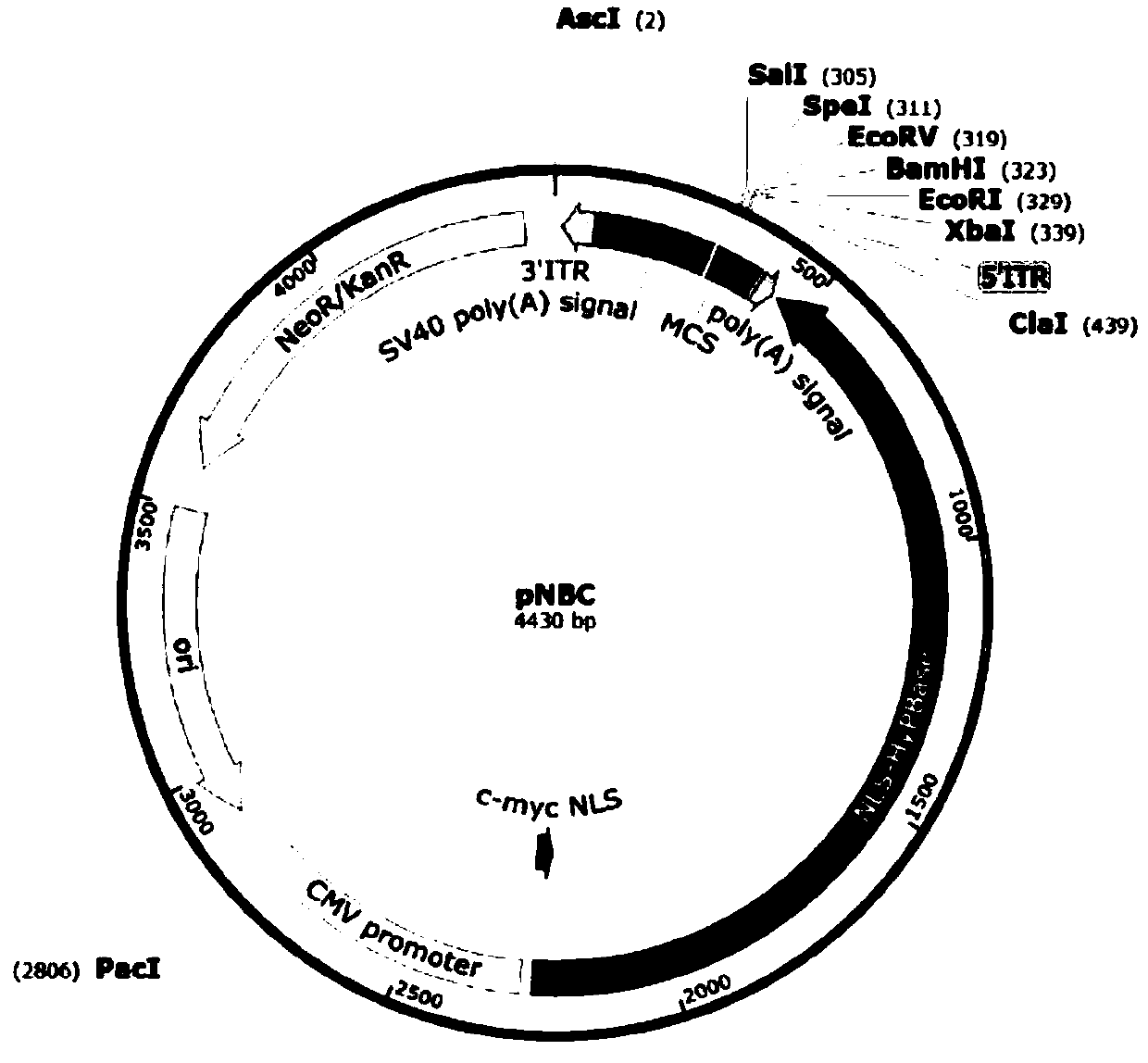
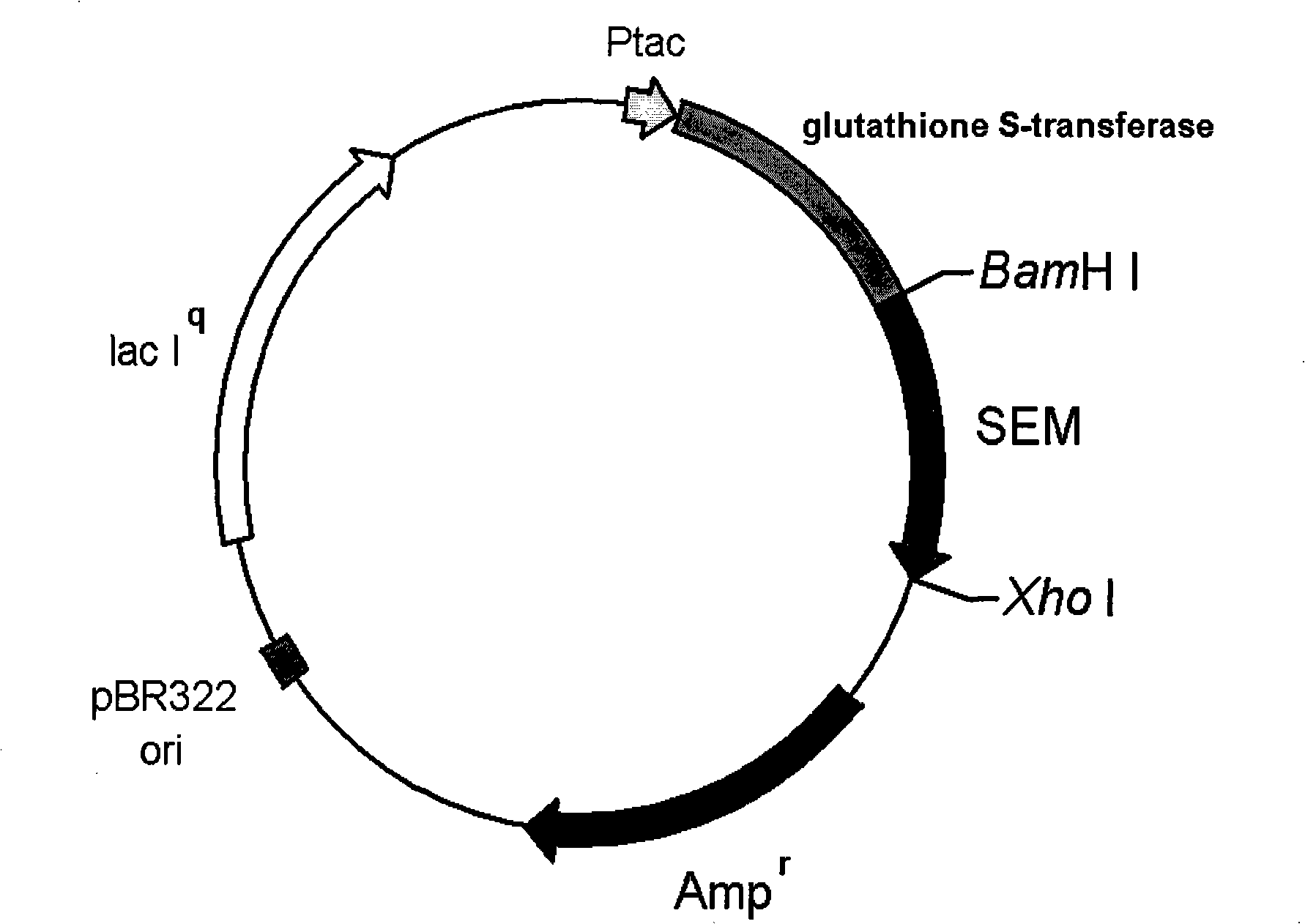
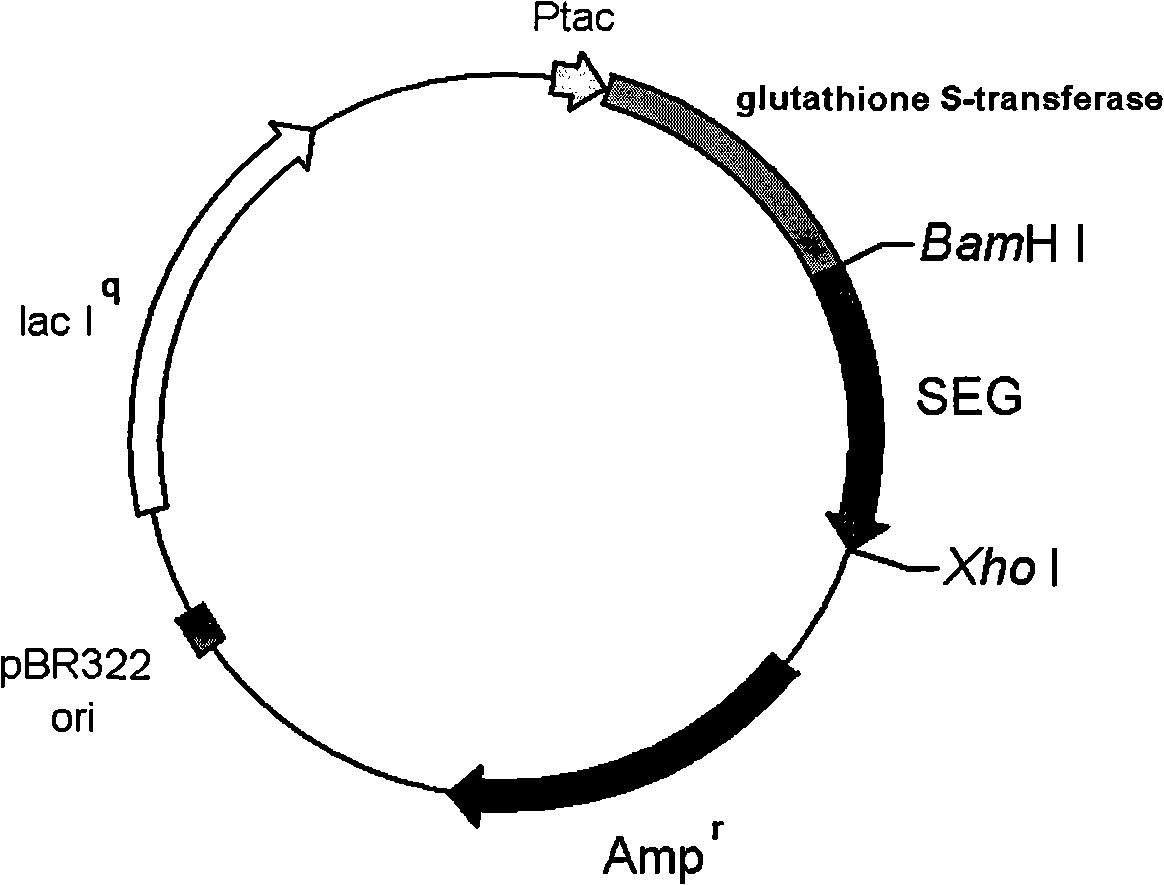
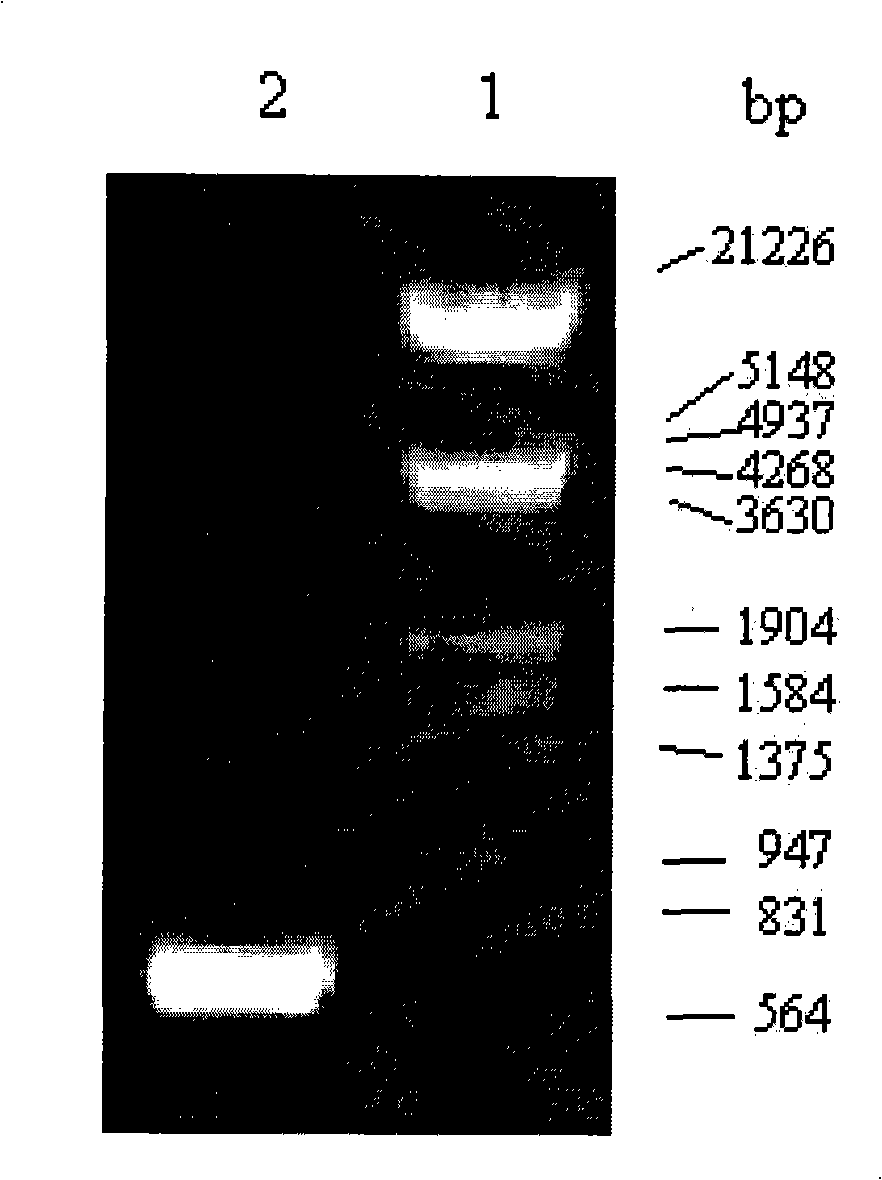
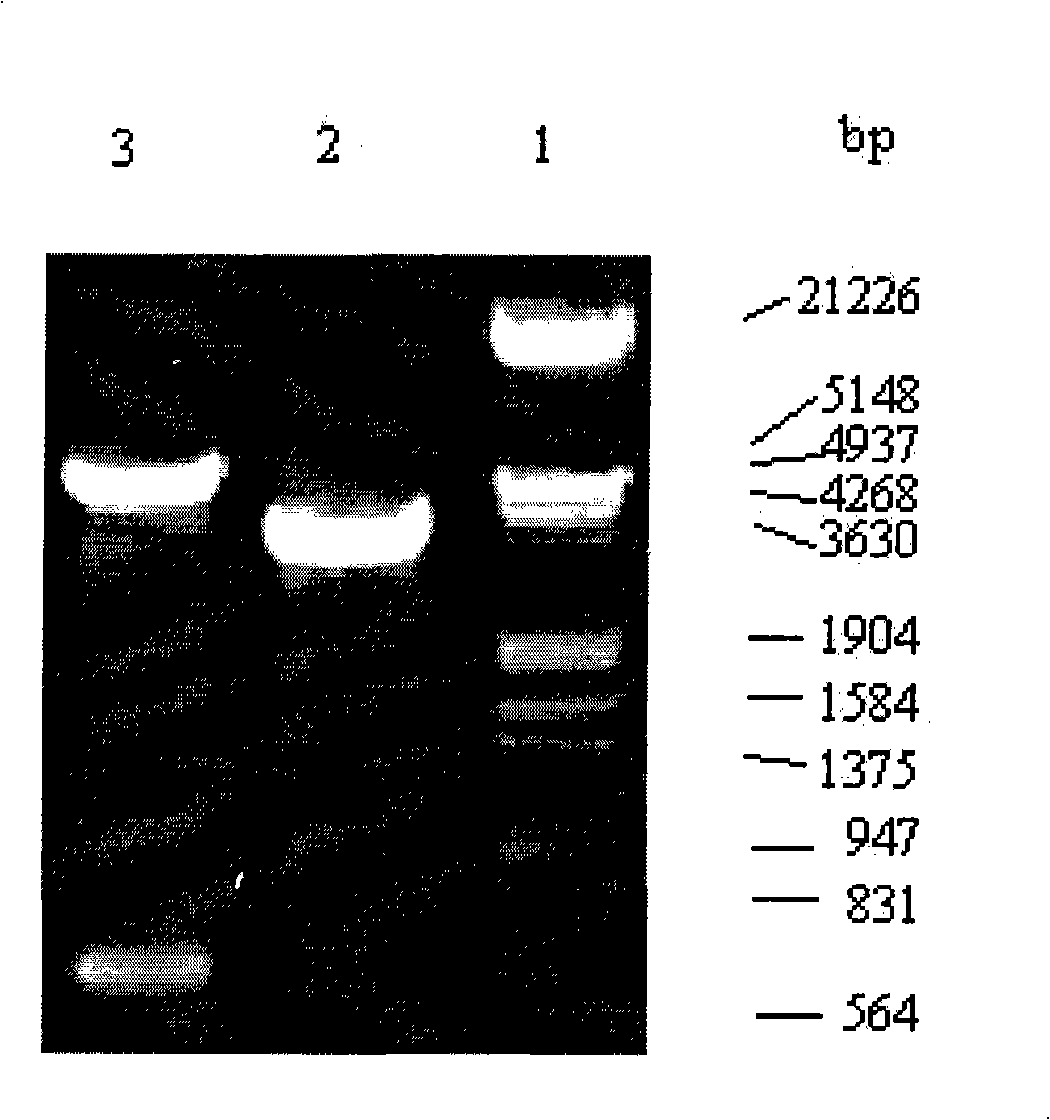
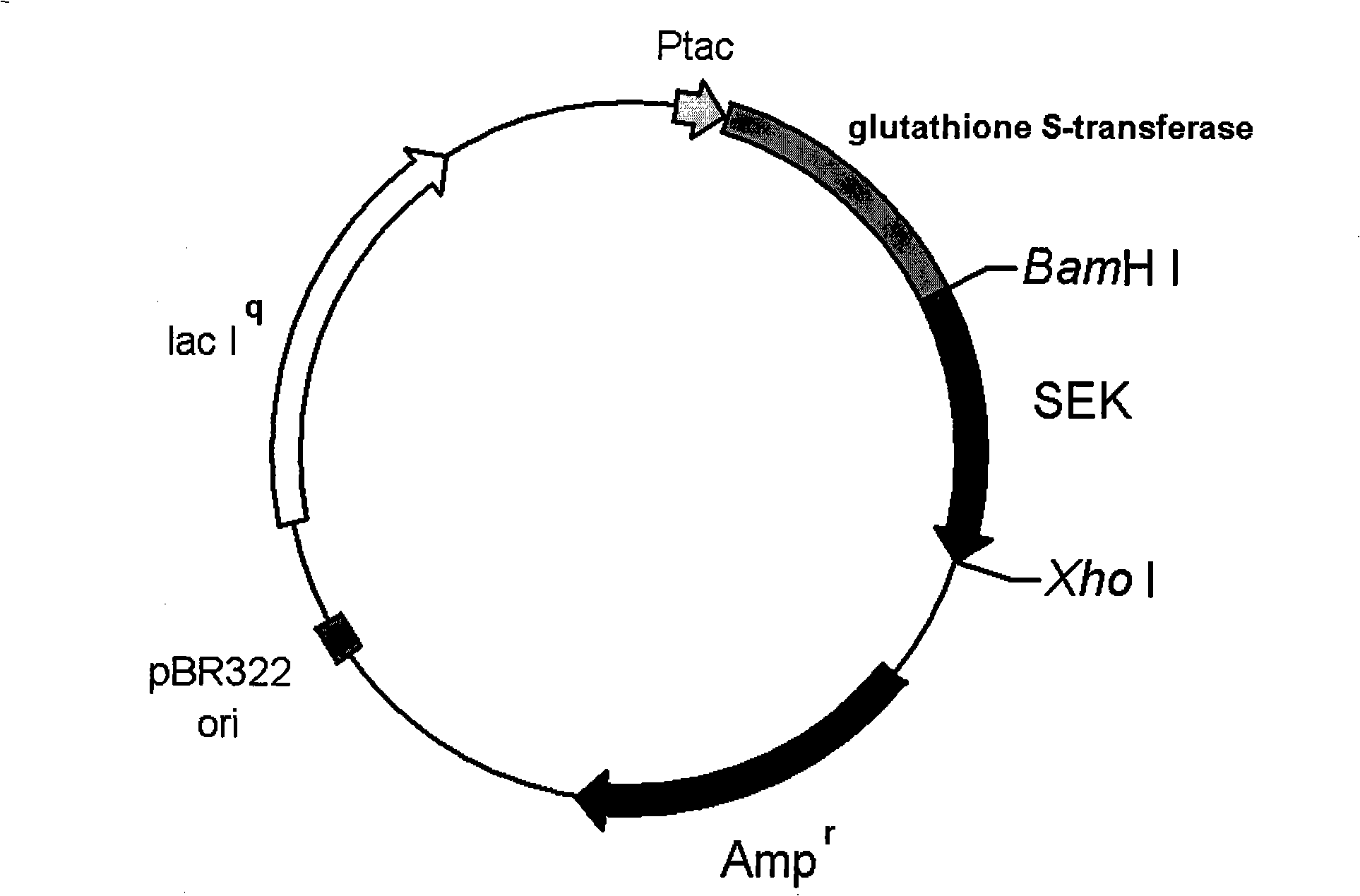
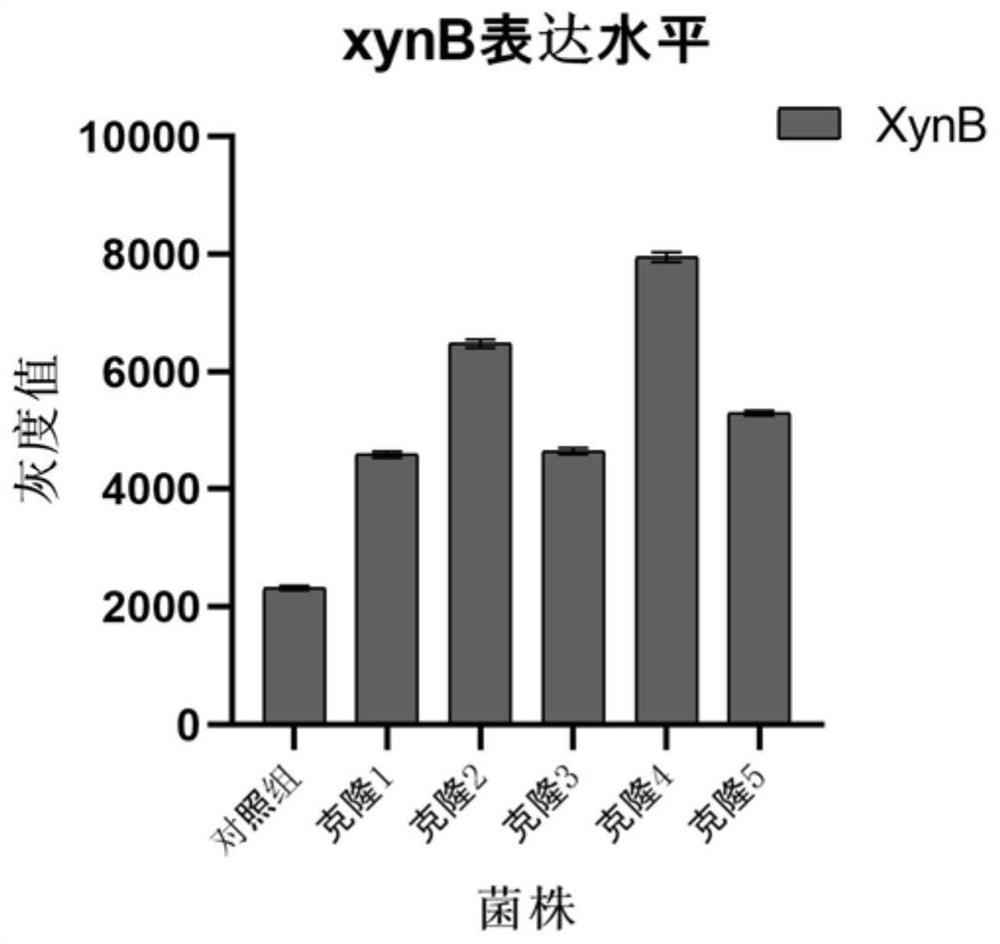
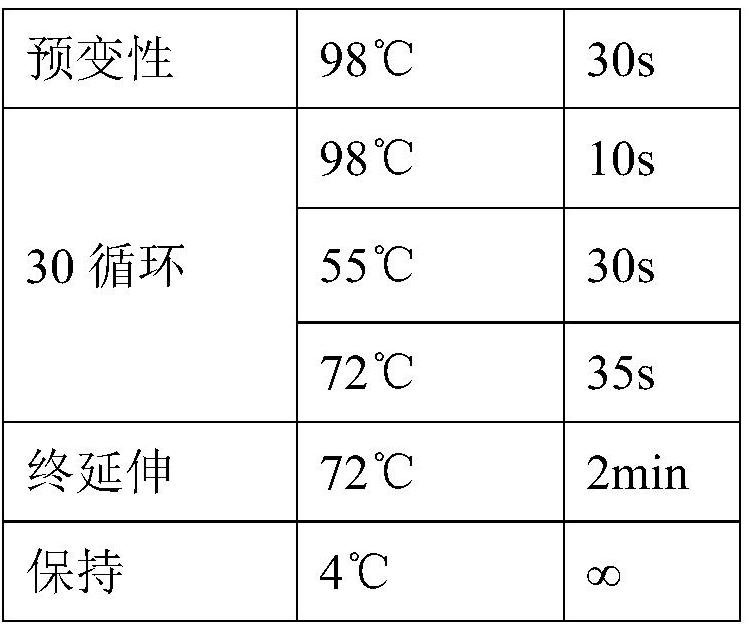
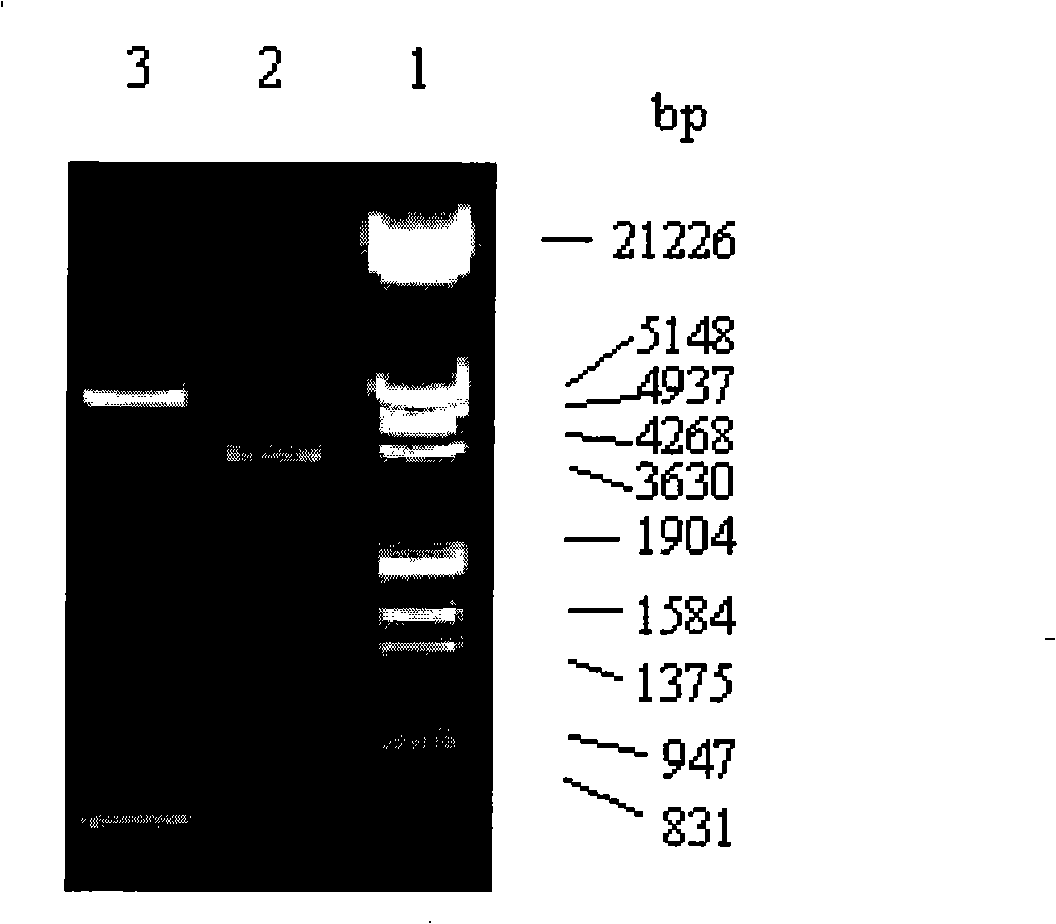
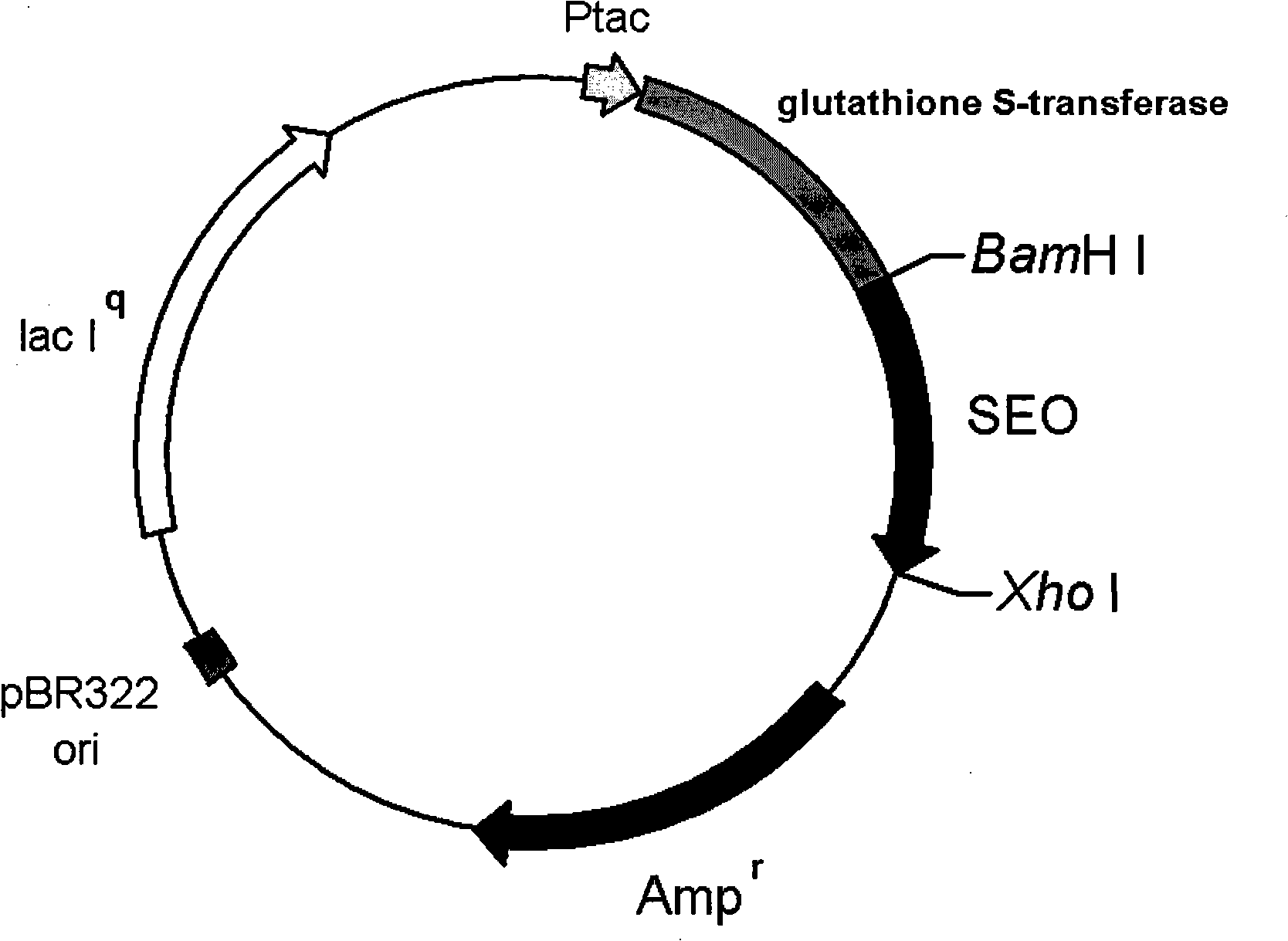
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Improve expression strength" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
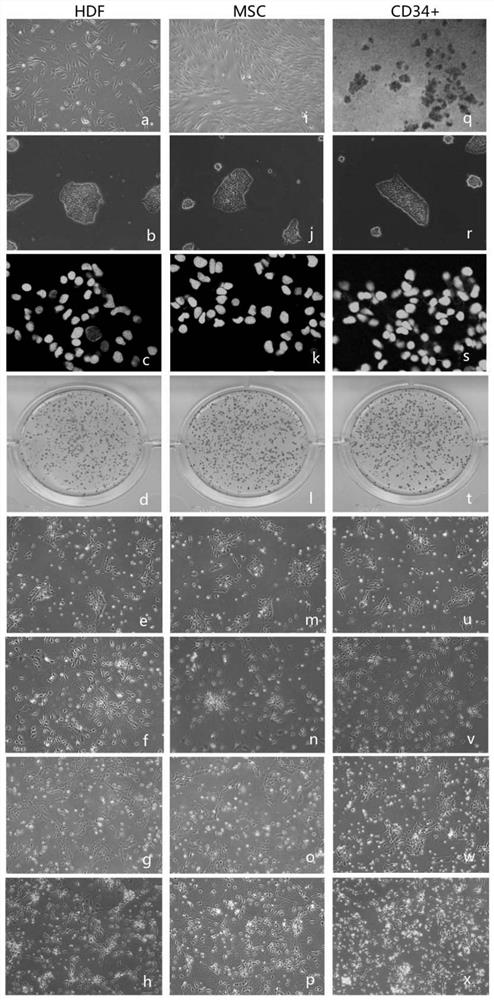
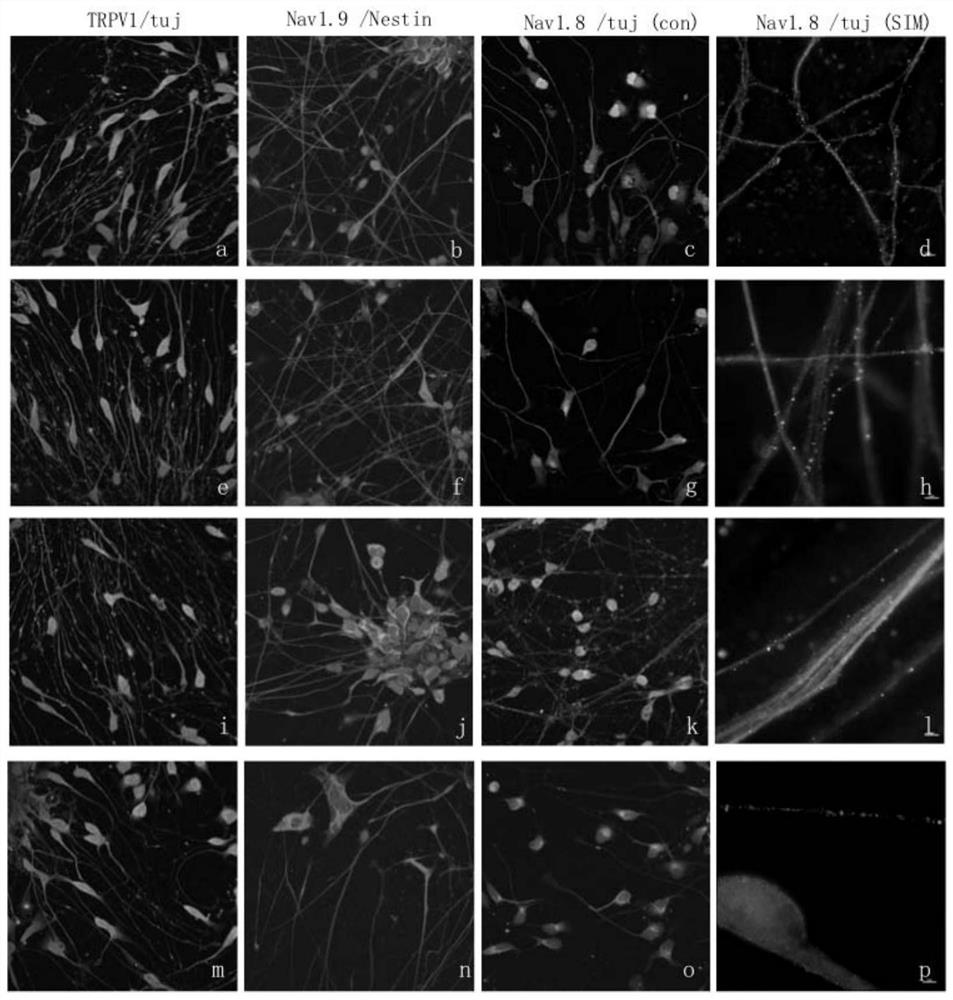
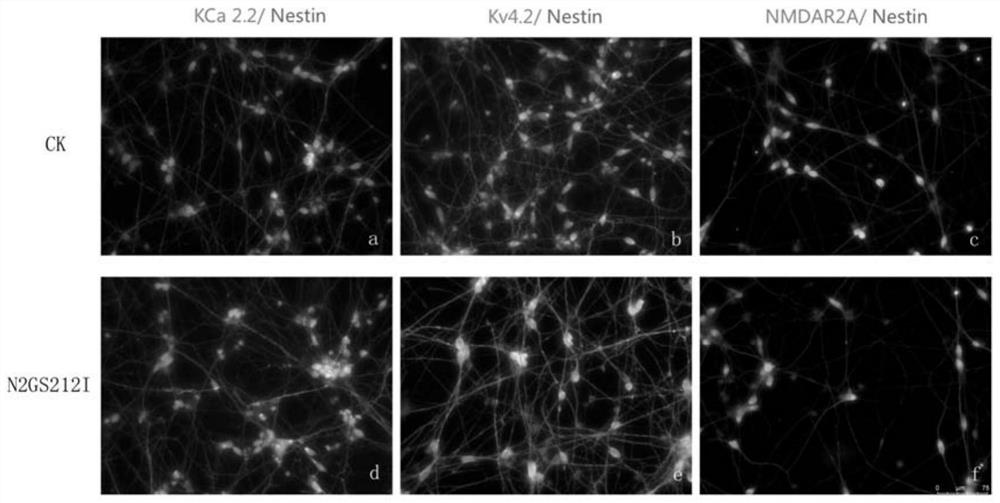
Serum-free induction method of sensory neuronal cells
PendingCN112055746AImprove expression strengthSolve pollutionCompound screeningNervous disorderIon Channel ProteinSerum free
According to the novel human sensory neuron induction culture system provided by the invention, the combination of the small-molecule inhibitor LY2157299 and the growth factor is added into the serum-free basal culture medium, so that compared with a serum-containing induction method, the efficiency of converting pluripotent stem cells into sensory neurons is greatly improved, and the induction efficiency of the pluripotent stem cells is improved. In addition, the expression of various ion channel proteins is obviously improved, so that various induced pluripotent stem cells with different sources are successfully induced into sensory neurons.
Owner:IREGENE THERAPEUTICS LTD
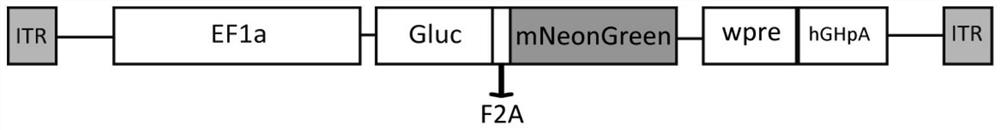
Recombinant adeno-associated virus, preparation method thereof and application thereof in antibody detection
ActiveCN112143712AEasy to collectAccurate detectionMicrobiological testing/measurementBiological material analysisNucleotideAdeno-associated virus
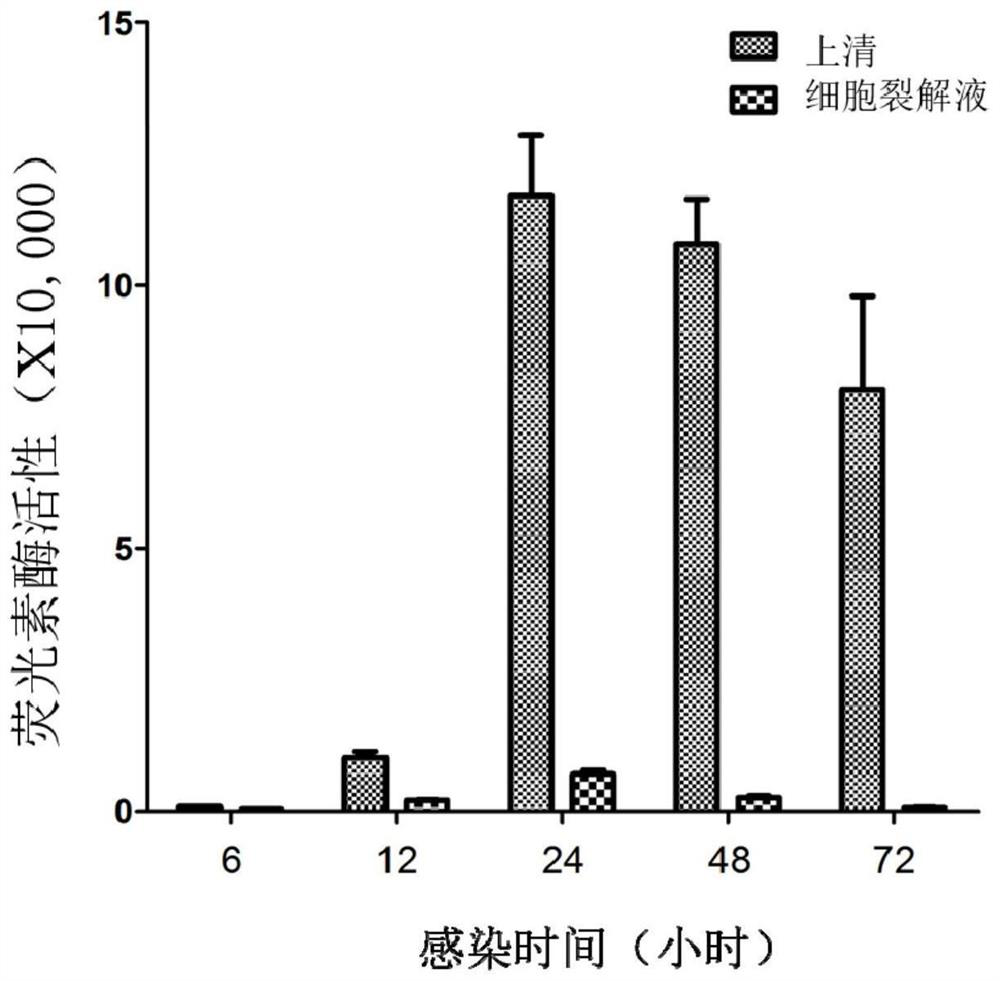
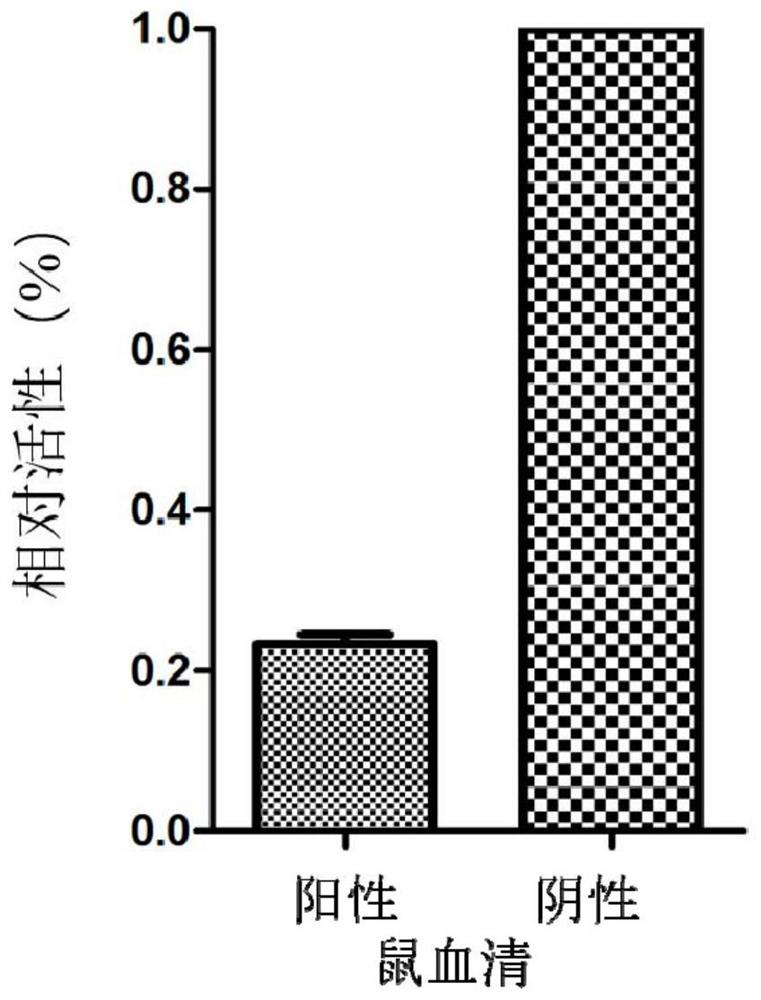
The invention relates to a recombinant adeno-associated virus, a preparation method thereof and an application thereof in antibody detection. An expression vector is constructed by utilizing an EF1[alpha] promoter and nucleotide sequences of Gaussia luciferase and green fluorescent protein mNeonGreen after base substitution, and the recombinant adeno-associated virus for simultaneously expressingthe Gaussia luciferase and the green fluorescent protein is prepared. The recombinant adeno-associated virus and serum to be detected are diluted and mixed, cells are added for culture, supernate is taken after infection, and the activity of the Gaussia luciferase is detected so as to detect whether adeno-associated virus antibodies exist in the serum or not. The recombinant adeno-associated virusalso has a wide application value in the aspect of neural circuit marking.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
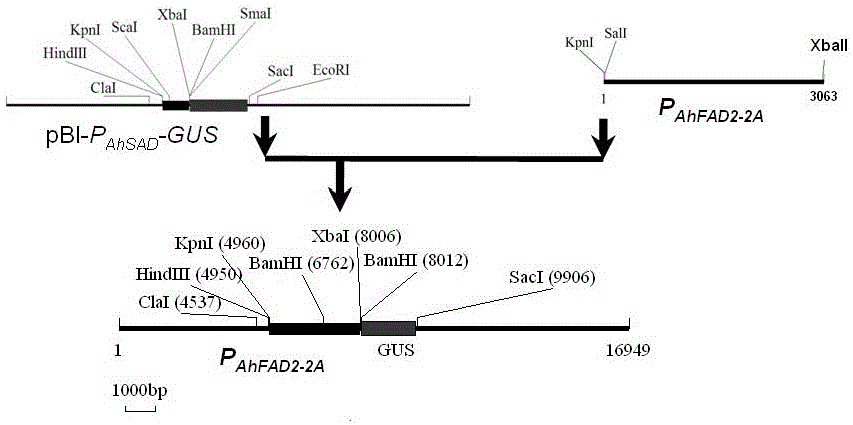
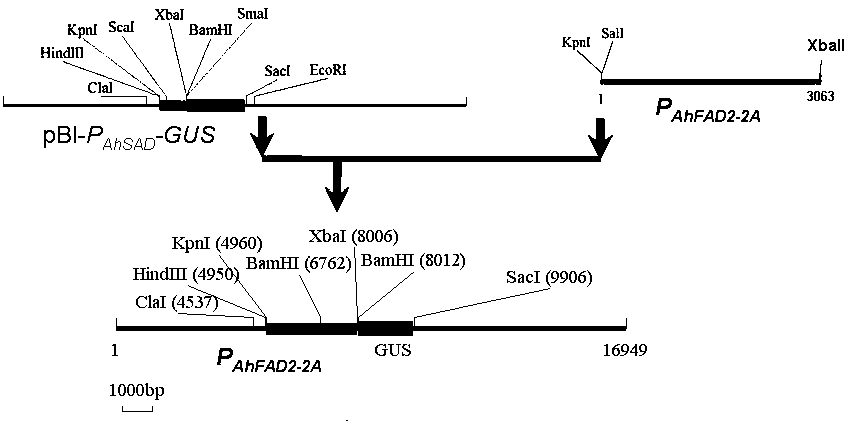
Peanut AhFAD2-2A gene promoter and application of peanut AhFAD2-2A gene promoter
ActiveCN105087587AAvoid wastingImprove expression efficiencyVector-based foreign material introductionDNA preparationBiotechnologyEnzyme Gene
The invention discloses an AhFAD2-2A gene promoter (P< AhFAD2-2A >) and an application of the AhFAD2-2A gene promoter, and belongs to the technical field of biology. A nucleotide sequence of the promoter is shown as SEQ ID NO.1. The AhFAD2-2A gene promoter is cloned from peanuts, and is used for building a recombinant expression vector; then, the recombinant expression vector is transformed into arabidopsis thaliana by an agrobacterium-mediated transformation method; a downstream recombinant gene is started to be expressed in seeds, roots and anthers, and is not expressed in other tissues; and the promoter belongs to a tissue specificity promoter, so that the promoter can be applied to transgenic engineering; a target gene expression product is accumulated in a certain organ or tissue; the expression amount in the tissue is increased; a good effect is achieved; meanwhile, the self energy waste of plants is avoided; and important application values are realized in the genetic engineering breeding and transgenic research process.
Owner:HENAN ACAD OF AGRI SCI
Polynucleotides having promoter activity and applications thereof in production of amino acids
ActiveCN113201536AHigh promoter activityImprove expression strengthBacteriaMicroorganism based processesPromoter activityTranscriptional expression
The present disclosure relates to polynucleotides having promoter activity and applications thereof in the production of amino acids. Specifically, the present disclosure relates to a polynucleotide having promoter activity, a transcription expression cassette containing the polynucleotide, a recombinant expression vector, a recombinant host cell, a method for enhancing expression of a target gene, a method for preparing a protein, and a method for producing an amino acid. The polynucleotide with the promoter activity is a mutant of the polynucleotide with the sequence as shown in SEQ ID NO: 9, compared with the polynucleotide with the sequence as shown in SEQ ID NO: 9, the promoter activity of the mutant is remarkably enhanced, stable and efficient expression of a target gene can be promoted, and then downstream products can be stably and efficiently produced.
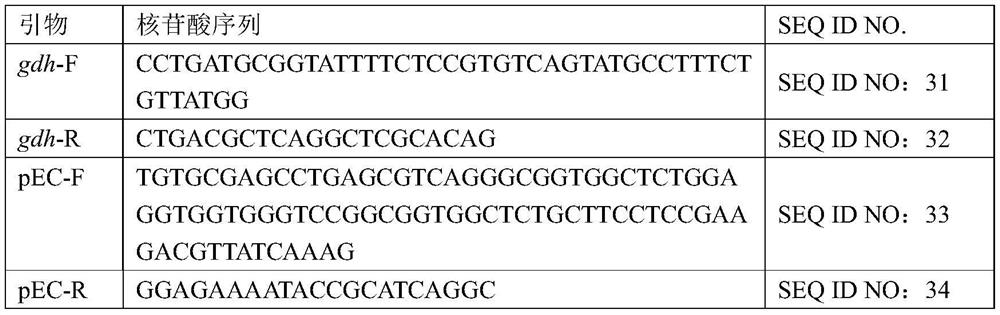
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
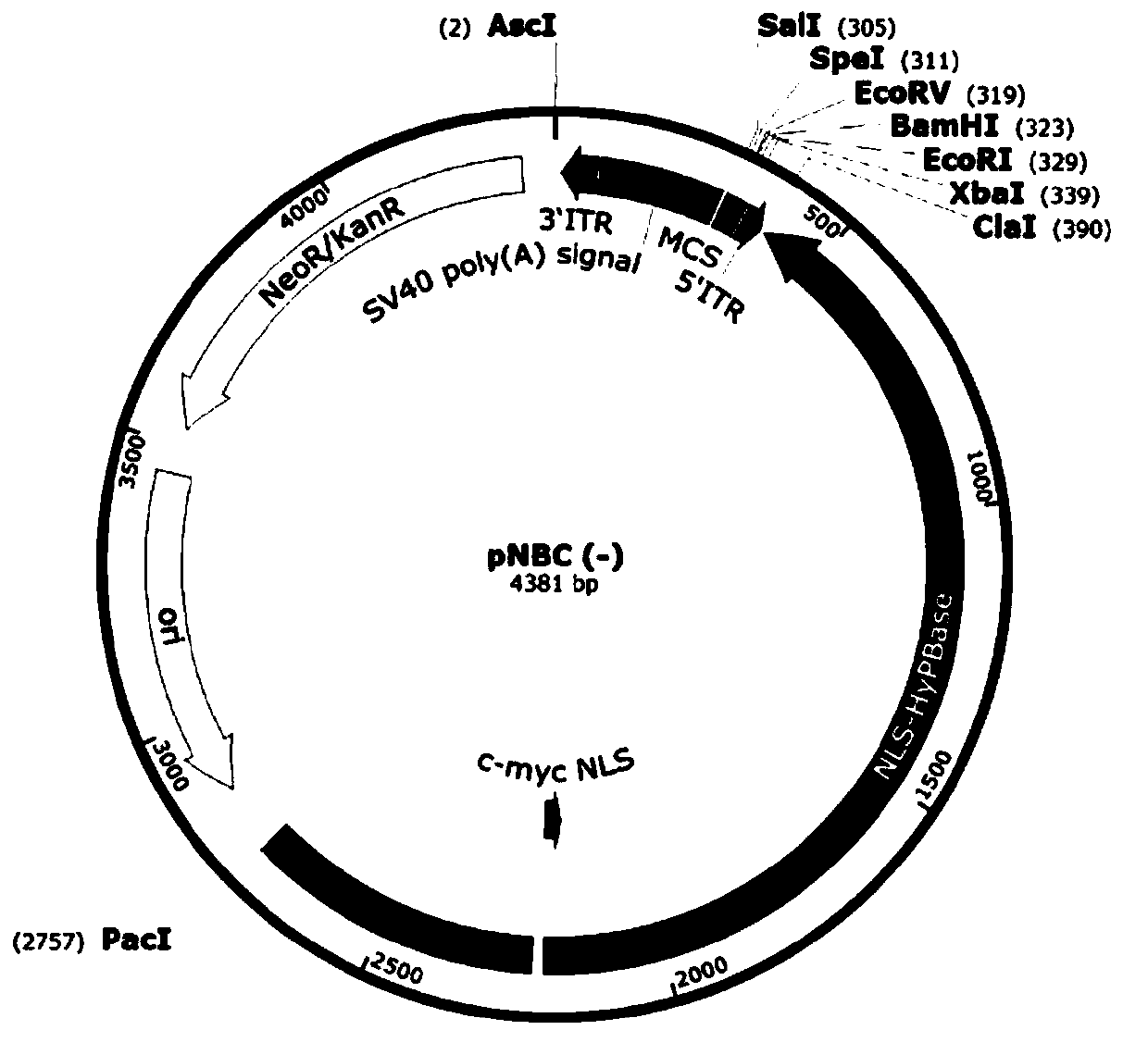
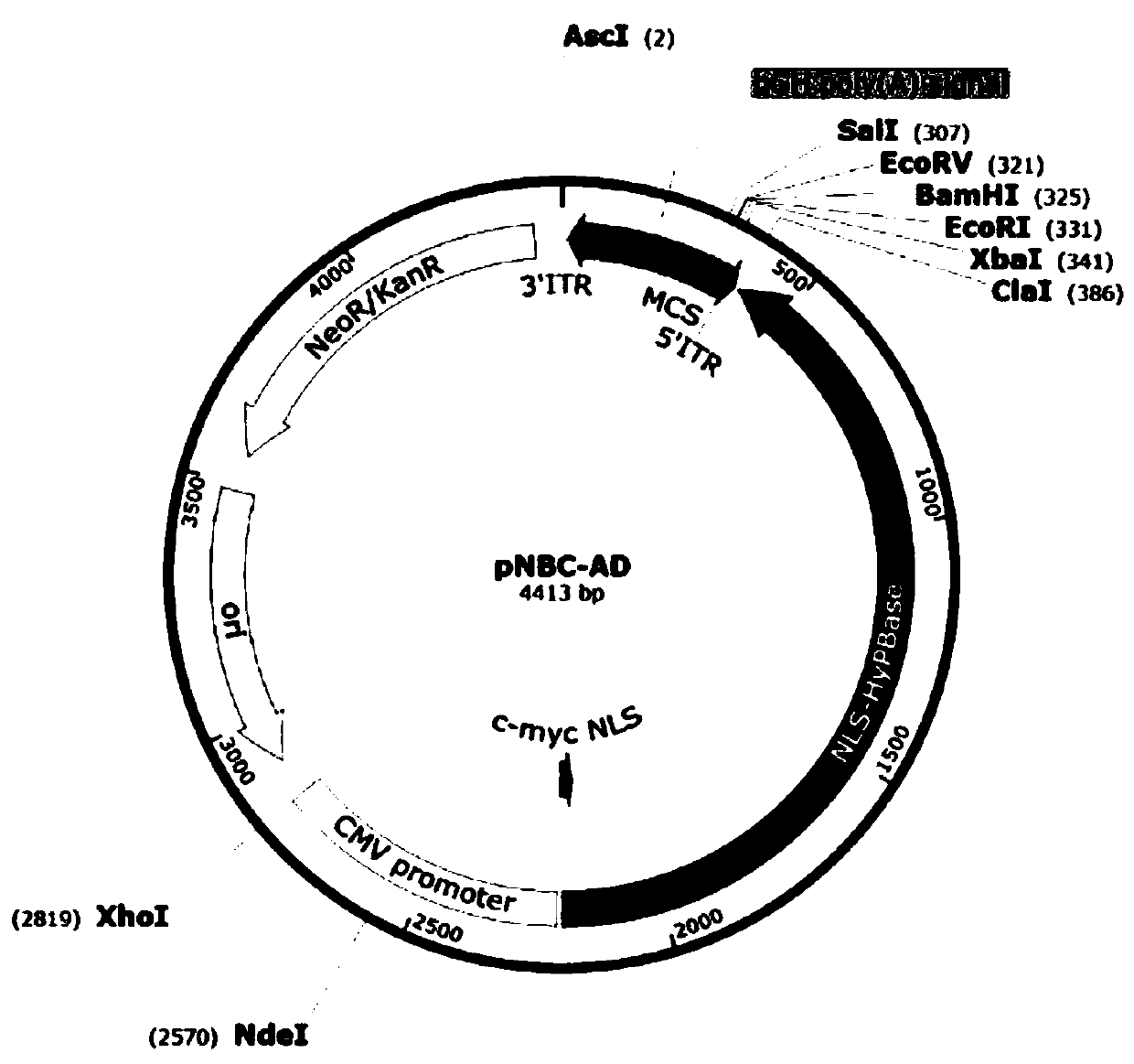
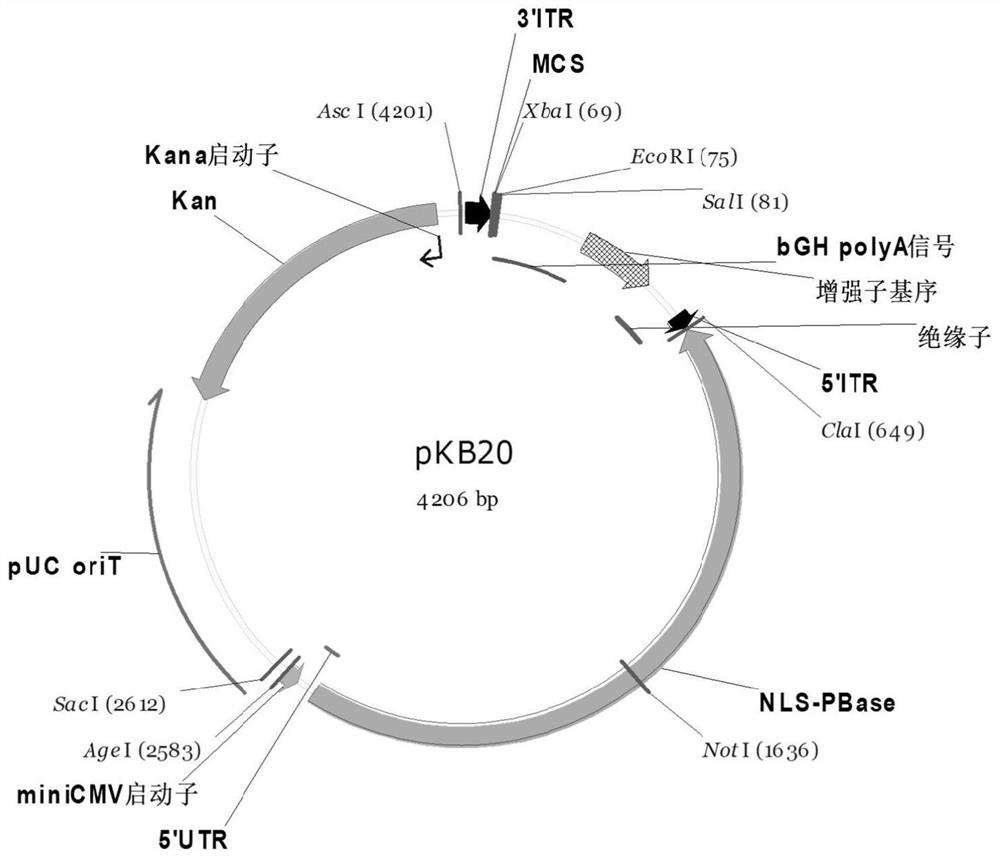
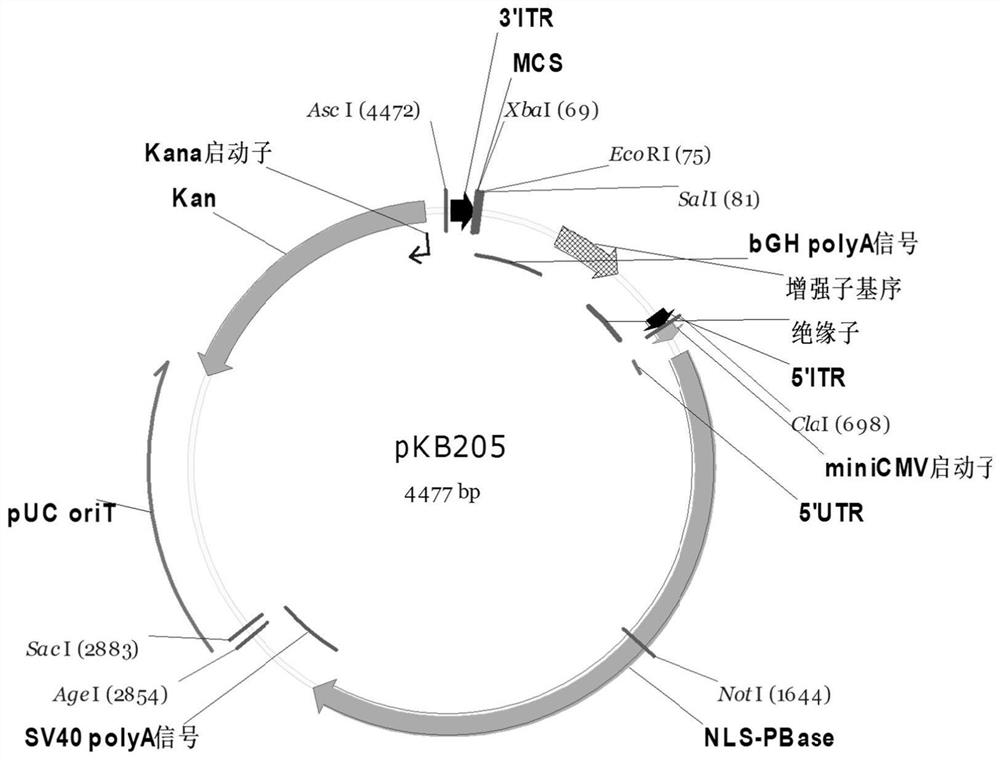
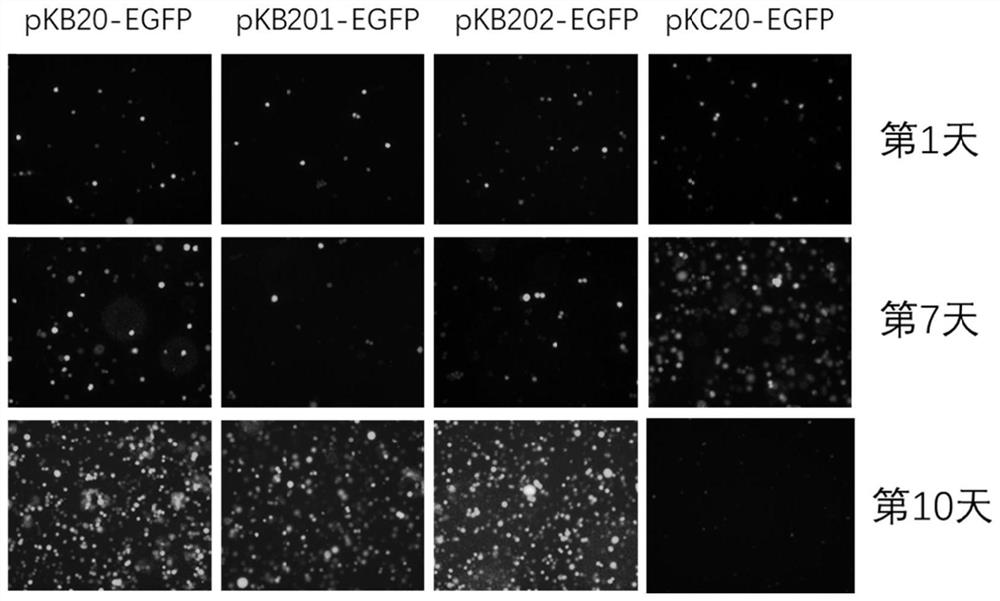
Improved PB transposon system and application thereof
PendingCN111206043ARaise the ratioImprove expression strengthGenetically modified cellsNucleic acid vectorPiggybac transposonInsertion site
The present invention relates to an improved PB transposon system and application thereof. Specifically, the present invention relates to a nucleic acid construct. The nucleic acid construct sequentially contains a promoter for controlling the expression of PiggyBac transposase, a PiggyBac transposase coding sequence, a terminal repeat at the 5'-end of the PiggyBac transposon, an optional polyA tailing signal sequence 1, a polyclonal insertion site, a polyA tailing signal sequence 2 and a terminal repeat at the 3'-end of the PiggyBac transposon. A recombinant vector constructed by using the nucleic acid construct provided by the invention has obviously improved integration efficiency.
Owner:SHANGHAI CELL THERAPY GRP CO LTD +1
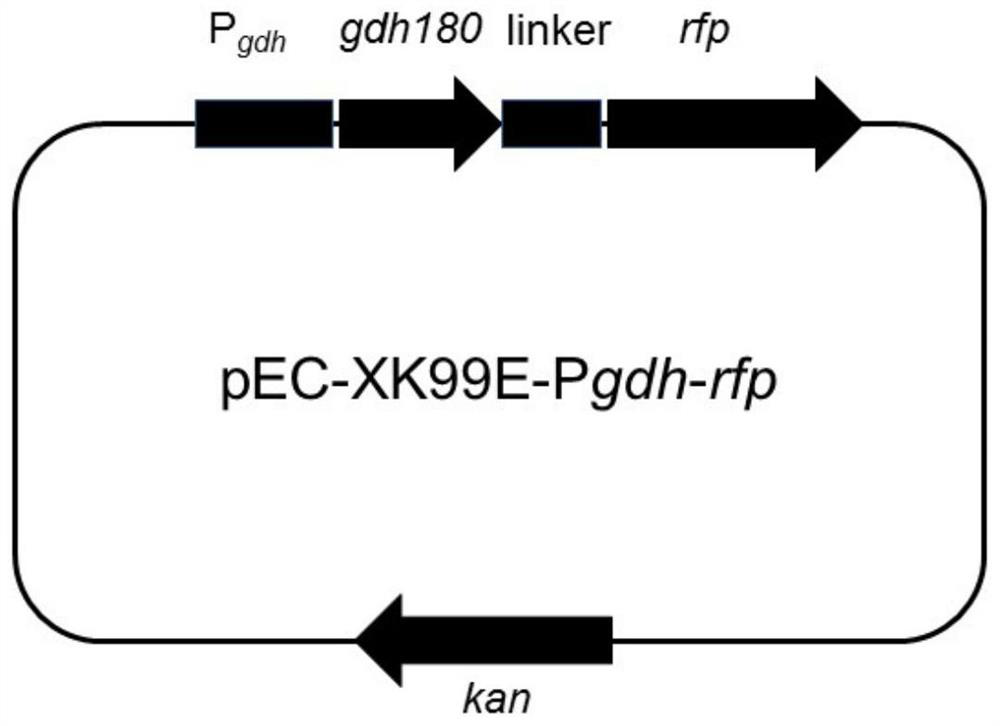
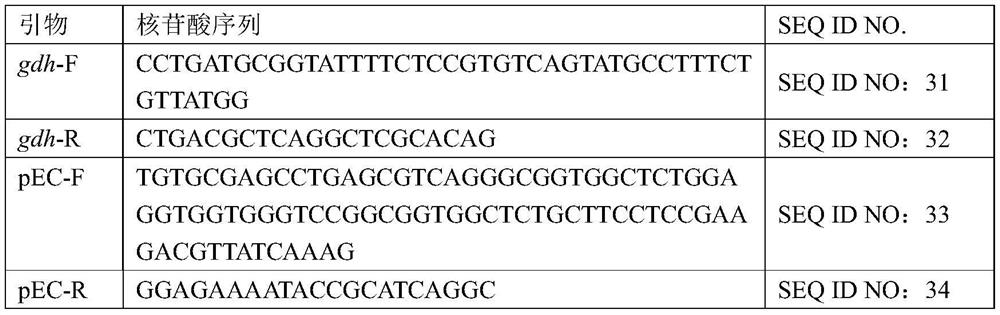
Mutant of glutamate dehydrogenase gene promoter and application thereof
ActiveCN113201535AHigh promoter activityImprove expression strengthBacteriaNucleotide librariesPromoter activityWild type
The invention discloses a mutant of a corynebacterium glutamicum glutamate dehydrogenase gene promoter and application of the mutant. The mutant has improved promoter activity compared with a wild type promoter. Therefore, the mutant can be used for enhancing the expression of a target gene, for example, the expression intensity of glutamate dehydrogenase can be enhanced by operably connecting the mutant with a glutamate dehydrogenase gene, so that the amino acid production efficiency of the recombinant strain is improved, and the application value is relatively higher.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
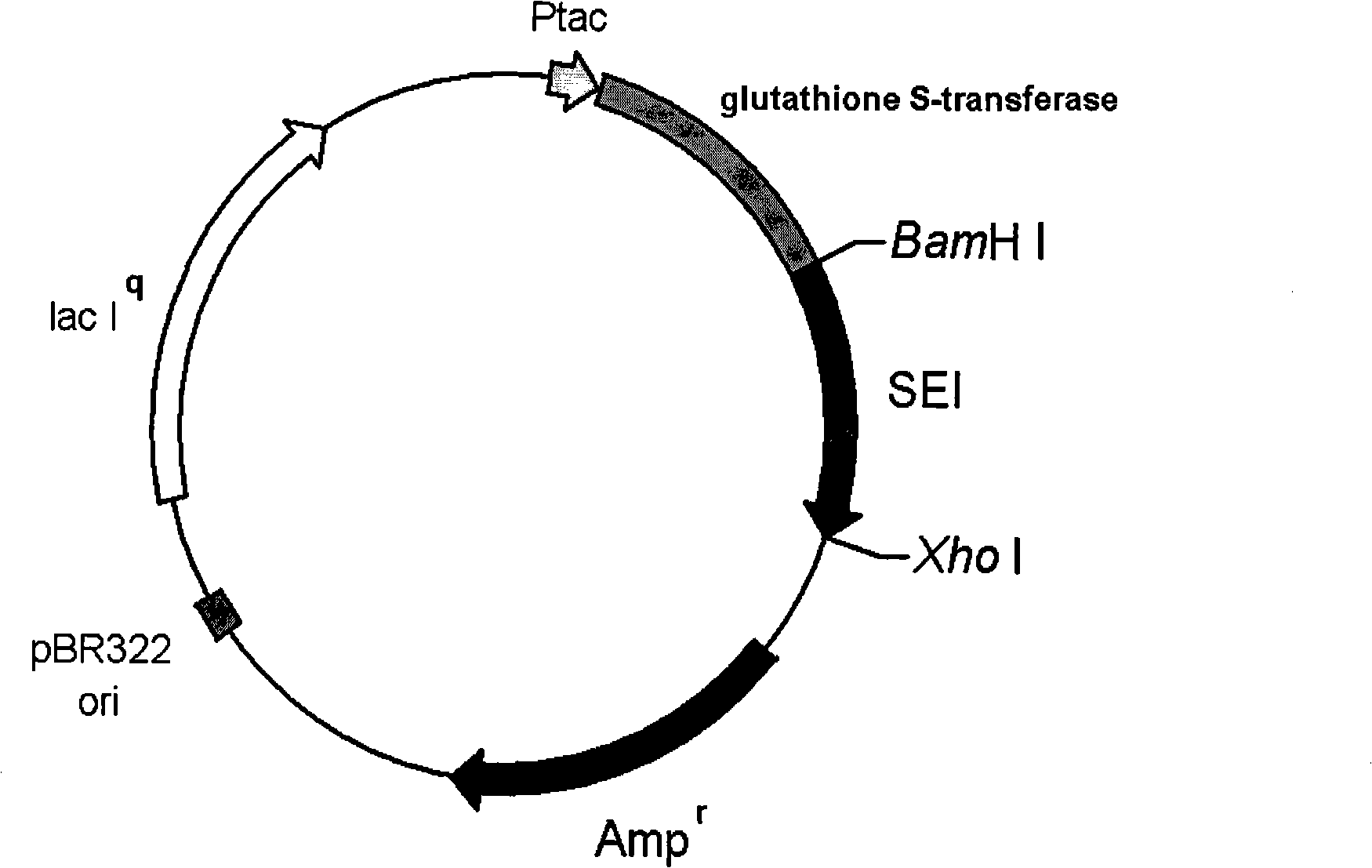
Recombined staphylococcus aureus enterotoxin I oral preparation and application thereof
InactiveCN101293092AHigh purityMaintain superantigen activityDepsipeptidesImmunological disordersAntigenSevere complication
The invention provides a recombinant staphylococcal enterotoxin I oral preparation, the recombinant staphylococcal enterotoxin I has SEQ ID NO.1 amino acid sequence, and the oral preparation further comprises a pharmaceutical allowable drug excipient or a carrier. The oral preparation proves that the protein can enter the systemic blood circulation by penetrating epithelial cells on small intestine with the form of complete molecules and maintain the super-antigen activity for promoting the spleen lymphocyte proliferation and inhibiting the growth of tumor cells, as well as the application in the preparation of drugs for treating malignant tumors and other serious complications by the Caco-2 monolayer cell transmembrane transport test.
Owner:ZHEJIANG UNIV
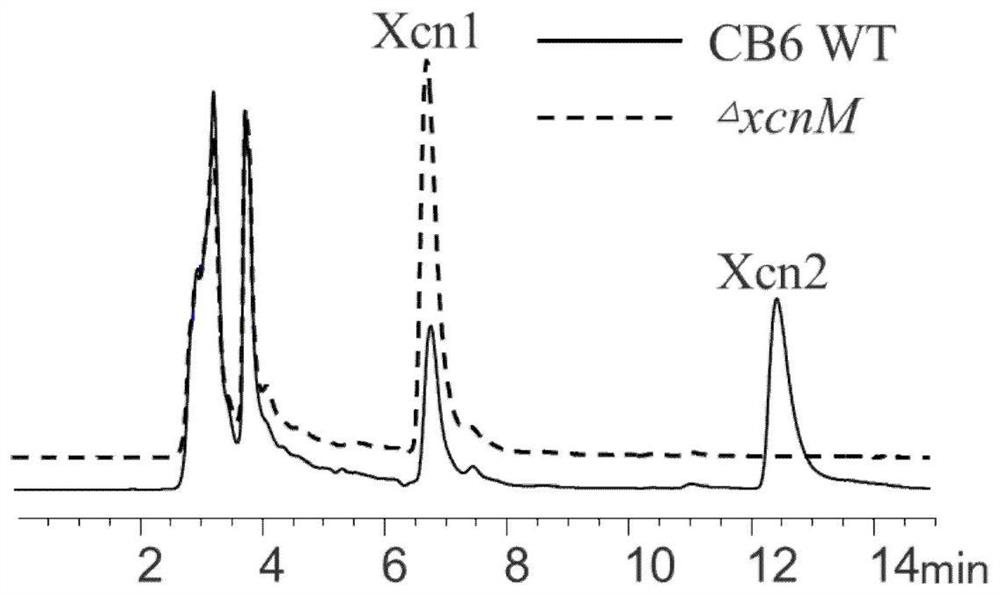
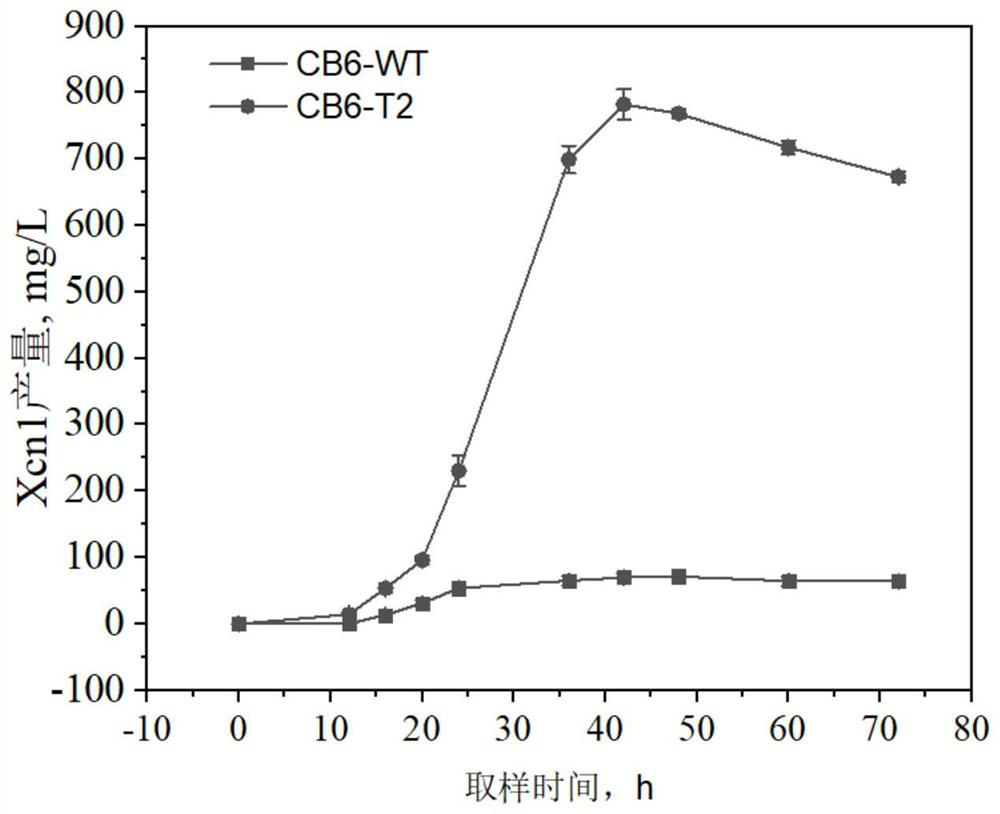
Xenorhabdus nematophila producing Xcn1 in high yield and application thereof
ActiveCN113881615AImprove expression strengthIncrease productionBacteriaMicroorganism based processesEnzyme GeneBiology
The invention relates to a strain of Xenorhabdus nematophila producing Xenocoumacin1 (Xcn1) in high yield. On the basis of a homologous recombination technology, genes related to anabolism of Xcn1 in a wild type strain of Xenorhabdus nematophila CB6 are subjected to engineering transformation to finally obtain an improved strain not producing Xcn2 but producing Xcn1 in high yield, named as CB6-T2. Studies show that an enzyme gene xcnM for mediating degradation and transformation of Xcn1 in the CB6-T2 strain is deleted and mutated, and a first gene xcnA promoter of an Xcn1 synthetic gene cluster is replaced with a promoter Promoter-g3509 that is derived from Xenorhabdus nematophila and has higher expression intensity. Tests show that the yield of Xcn1 obtained by fermenting the strain CB6-T2 in an LB culture medium is at least 782 mg / L or above, much higher than the fermentation yield of a wild strain of Xenorhabdus nematophila CB6. The strain can effectively reduce production cost in industrial development of Xcn1.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Recombined staphylococcus aureus enterotoxin M oral preparation and application thereof
InactiveCN101293093AImprove expression strengthAvoid degradationDepsipeptidesImmunological disordersAntigenSevere complication
The invention provides a recombinant staphylococcal enterotoxin M oral preparation, the recombinant staphylococcal enterotoxin M has SEQ ID NO.1 amino acid sequence, and the oral preparation further comprises a pharmaceutical allowable drug excipient or a carrier. The oral preparation proves that the protein can enter the systemic blood circulation by penetrating epithelial cells on small intestine with the form of complete molecules and maintain the super-antigen activity for promoting the spleen lymphocyte proliferation and inhibiting the growth of tumor cells, as well as the application in the preparation of drugs for treating malignant tumors and other serious complications by the Caco-2 monolayer cell transmembrane transport test.
Owner:ZHEJIANG UNIV
Recombinant Staphylococcus aureus enterotoxin G oral preparation and use
InactiveCN101322842AImprove expression strengthAvoid degradationDepsipeptidesImmunological disordersAbnormal tissue growthWhole body
The invention provides a recombinant staphylococcus aureus enterotoxin G oral preparation which has an amino acid sequence of SEQ ID NO.1 and also comprises excipients in medicine or carriers allowed by the preparation. By the experiment on the Caco-2 monolayer cell trans-membrane transport, the preparation of the invention proves that the proteins can enter the general blood circulation from intestinal epithelial cells in the form of complete molecules and keep promoting the splenic lymphocyte proliferation and preventing the superantigen activity for the growth of tumor cells, and can be applied to the preparation of drugs for curing the malignant tumor and other serious complications.
Owner:ZHEJIANG UNIV
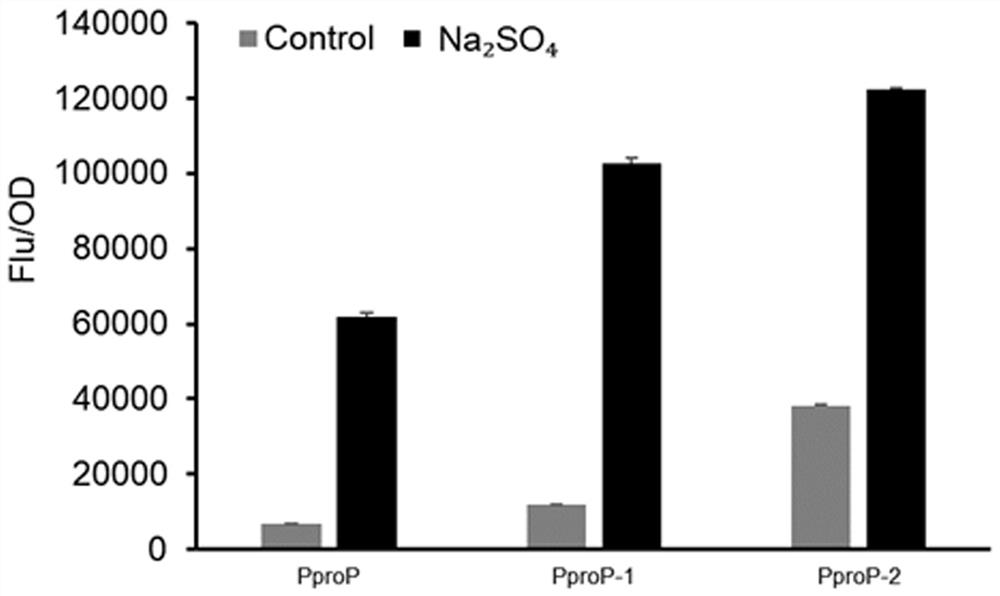
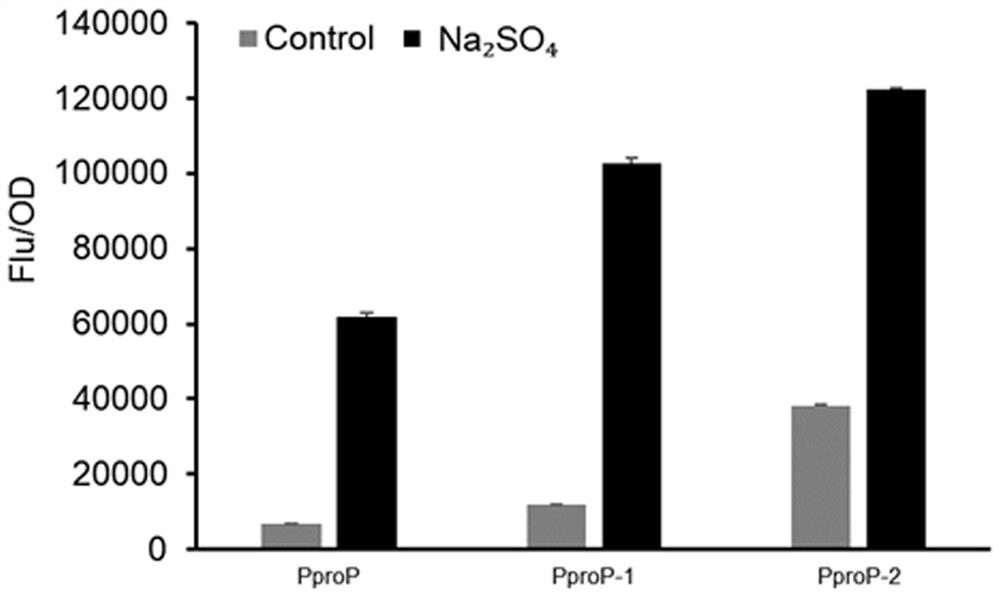
Mutated hypertonic inducible promoter PproP and application thereof
ActiveCN113278620AImprove expression strengthStable productionBacteriaMicroorganism based processesPromoter activityWild type
The invention provides a polynucleotide mutant with promoter activity. The polynucleotide mutant has higher promoter activity than a wild type promoter in an environment with increased salt concentration and osmotic pressure. Polynucleotide is operably connected with a target gene, so that the expression intensity of the target gene in a high-salt and high-osmotic-pressure stress environment can be remarkably improved, downstream products can be stably and efficiently produced, and the problems that expensive inducers such as IPTG are added currently, and toxicity is caused to strains are effectively solved.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Immune effector cell for chronic lymphocytic leukemia as well as preparation method and application thereof
ActiveCN111499766AHighly effective and specific treatmentWidely expressedPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsImmune effector cell
The invention discloses a chimeric antigen receptor (CAR) targeting CD32b, an immune effector cell modified by the CAR, a preparation method of the immune effector cell and application of the immune effector cell in inhibition of chronic lymphocytic leukemia for the first time. The invention provides a novel treatment scheme for refractory chronic lymphocytic leukemia.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Novel PiggyBac transposition subsystem and application thereof
PendingCN114317600AShorten the lengthLow toxicityGenetic material ingredientsMammal material medical ingredientsBiotechnologyPiggyBac Transposon System
The invention relates to a PiggyBac transposon system and application thereof, and particularly provides a nucleic acid construct which comprises the following elements: a transposon 3 '-terminal repetitive sequence, a first polyA sequence, an insulator sequence with a transcription termination function, a transposon 5'-terminal repetitive sequence, a transposase coding sequence and a promoter for controlling the expression of the transposase. The invention further provides a host cell or a pharmaceutical composition containing the nucleic acid construct and application of the host cell or the pharmaceutical composition.
Owner:SHANGHAI GENCELLS THERAPEUTICS CO LTD
Peanut δ9-stearyl-acp dehydrogenase ahsad promoter and its preparation method and application
ActiveCN104975027BAvoid wastingImprove expression efficiencyFermentationPlant genotype modificationAgricultural scienceEnzyme digestion
The invention discloses a peanut Δ9-stearyl-ACP dehydrogenase gene AhSAD promoter (PAhSAD), a preparation method and an application. The promoter has the nucleotide sequence shown in SEQ ID No.1. The PAhSAD sequence is cloned by using the genome walking method, the genomic DNA is extracted by the SDS cleavage method, and the DNA is digested with blunt-end endonuclease in different systems respectively. After ligation with the adapter fragment, the first round of PCR amplification was carried out with GSP1 and AP1 as primers, and the second round of amplification was carried out with GSP2 and AP2 as primers to obtain the DNA sequence containing PAhSAD. The promoter PAhSAD of the present invention has the function of driving the expression of exogenous genes, and its expression sites are in the roots, stems, leaves, fruit needles and seeds of plants. This promoter with tissue-specific expression has a role in plant genetic engineering. important application value.
Owner:HENAN ACAD OF AGRI SCI
A mutant hypertonic inducible promoter pprop and its application
ActiveCN113278620BImprove expression strengthStable productionBacteriaMicroorganism based processesPromoter activityWild type
The present invention provides a polynucleotide mutant with promoter activity, which has enhanced promoter activity compared with wild-type promoter under the environment of elevated salt concentration and osmotic pressure. The polynucleotide is operably linked to the target gene, which can significantly increase the expression intensity of the target gene under the stress environment of high salt and high osmotic pressure, and then produce downstream products stably and efficiently, effectively solving the problem of adding expensive inducers such as IPTG. , and cause toxicity to the strain.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Recombined staphylococcus aureus enterotoxin K oral preparation and application thereof
InactiveCN101293094AHigh purityMaintain superantigen activityDepsipeptidesImmunological disordersAbnormal tissue growthAntigen
The invention provides a recombinant staphylococcal enterotoxin K oral preparation, the recombinant staphylococcal enterotoxin K has SEQ ID NO.1 amino acid sequence, and the oral preparation further comprises a pharmaceutical allowable drug excipient or a carrier. The oral preparation proves that the protein can enter the systemic blood circulation by penetrating epithelial cells on small intestine with the form of complete molecules and maintain the super-antigen activity for promoting the spleen lymphocyte proliferation and inhibiting the growth of tumor cells, as well as the application in the preparation of drugs for treating malignant tumors and other serious complications by the Caco-2 monolayer cell transmembrane transport test.
Owner:ZHEJIANG UNIV
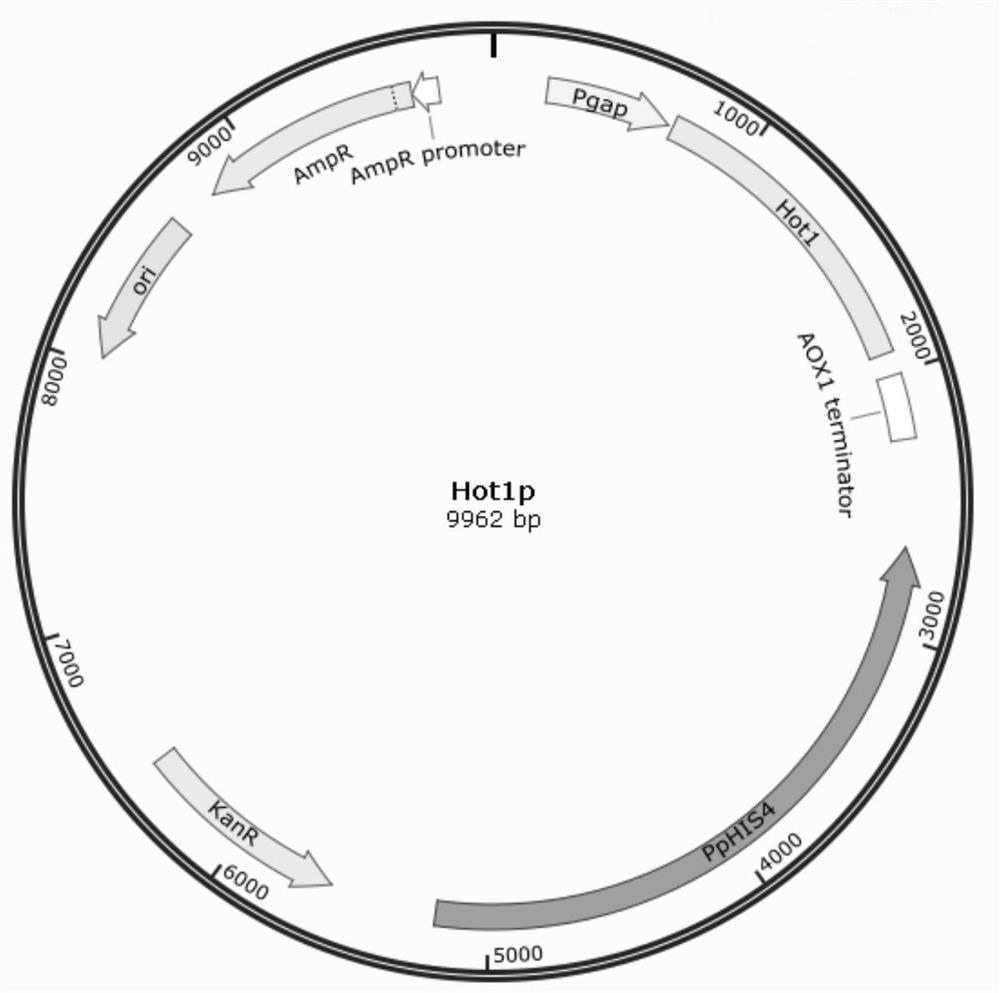
Application of Hot1p as positive regulatory factor in improvement of protein expression in host cells
ActiveCN114773441AImprove expression efficiencyImprove expression strengthFungiMicroorganism based processesNucleotidePromoter
The invention relates to application of a transcriptional regulation factor expressed by eukaryotic genes, in particular to application of a transcriptional regulation factor Hot1p of a constitutive promoter Pgap. The invention discloses application of Hot1p as a positive regulatory factor in improvement of protein expression in host cells. The amino acid sequence of the Hot1p is coded by an Hot1 gene with the nucleotide sequence of SEQ ID NO: 1; according to the application, an Hot1 gene is inserted behind a promoter Pgap, so that the expression of protein in host cells is improved. The application disclosed by the invention can enhance transcription of a constitutive promoter Pgap promoter of the pichia pastoris, so that a subsequent exogenous gene is efficiently expressed in the pichia pastoris, and the situation that the difference of expression quantities of different genes is too large due to a dilution effect generated by using the same promoter during multi-copy or multi-gene expression can be avoided.
Owner:JINAN UNIVERSITY
Promoter of peanut δ12 fatty acid dehydrogenase ahfad2-1b gene and its preparation method and application
ActiveCN105112419BAvoid wastingImprove expression efficiencyVector-based foreign material introductionDNA preparationNucleotideFatty acid
The invention discloses a promoter (PAhFAD2-1B) of the Δ12 fatty acid dehydrogenase AhFAD2-1B gene and an application thereof, belonging to the field of biotechnology. The nucleotide sequence of the promoter is shown in SEQ ID NO.1. The present invention clones the peanut Δ12 fatty acid dehydrogenase AhFAD2‑1B gene promoter from peanuts, uses the promoter provided by the present invention to construct a recombinant expression vector, and then transforms the expression vector into Arabidopsis thaliana using an Agrobacterium-mediated method, Promote the expression of downstream recombinant genes in seeds, leaves, calyx and silique peel, but not in other tissues. It is a tissue-specific promoter, so it can be applied to transgenic engineering, so that the target gene expression product can be expressed in a certain organ Or accumulate in the tissue, increase the expression level in the tissue, exert better effect, and avoid the waste of energy of the plant itself at the same time, so it has important application value in genetic engineering breeding and transgenic research.
Owner:HENAN ACAD OF AGRI SCI
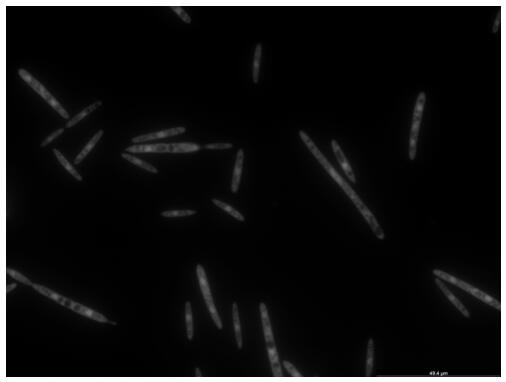
A kind of endogenous strong promoter phsp of smut smut and its expression vector and application
ActiveCN112725341BImprove expression strengthFungiMicrobiological testing/measurementUstilago esculentaNucleotide
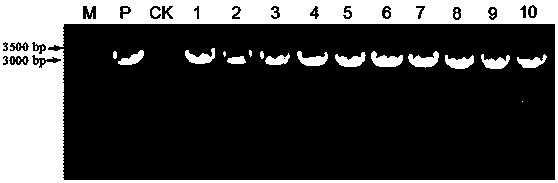
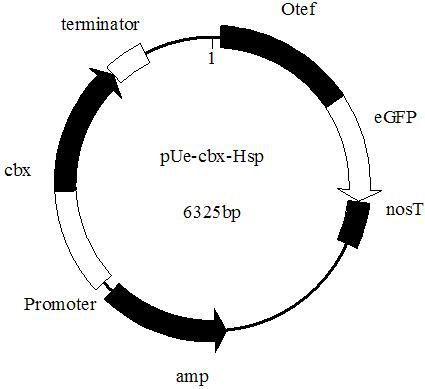
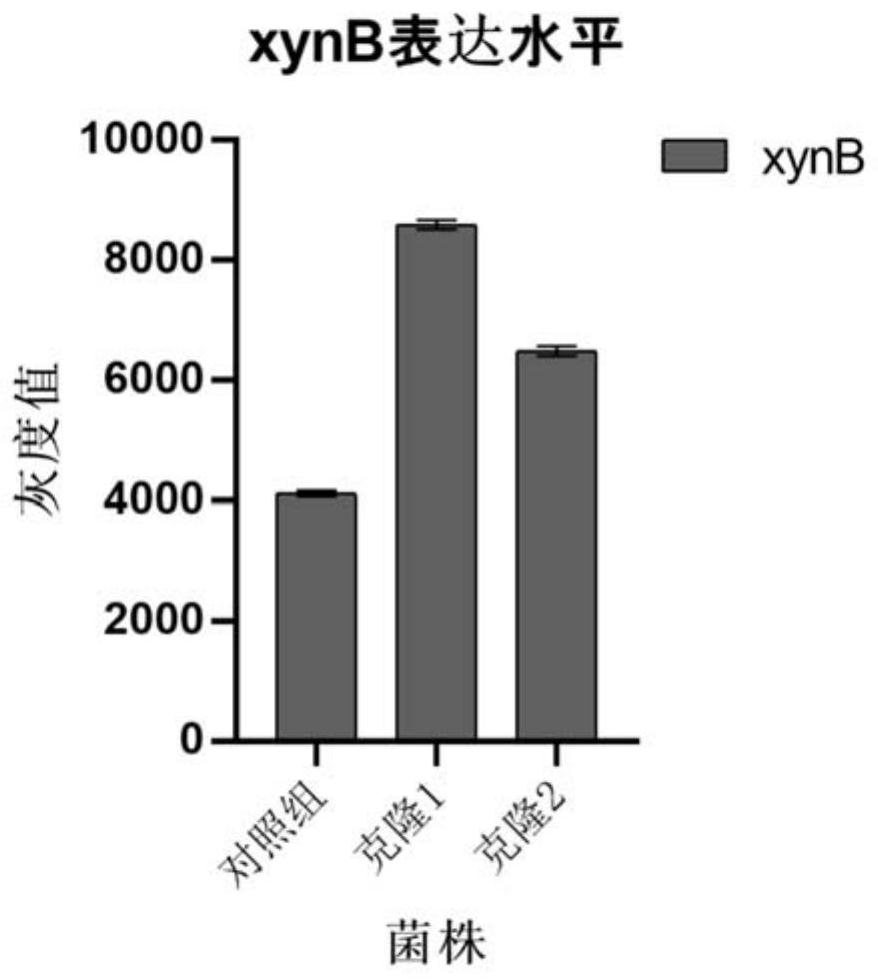
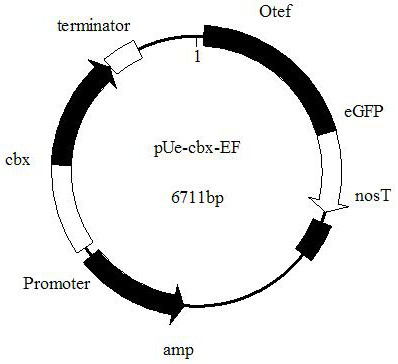
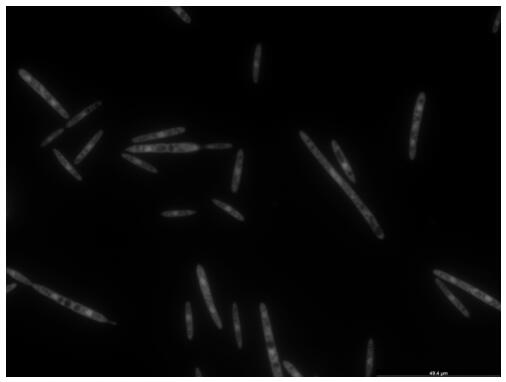
The invention discloses an endogenous strong promoter pHSP of smut smut and its expression vector and application, belonging to the technical field of genetic engineering. The present invention includes: a strong endogenous promoter pHSP of Ustilago smut, the nucleotide sequence of which is shown in SEQ ID NO.1; an expression vector containing strong endogenous promoter pHSP of Ustilago smut; Driving the transcription and expression of the eGFP gene, constructing a stable expression system and obtaining the application of engineering smut smut has improved eGFP expression intensity and fluorescence stability. The present invention combines endogenous pHSP promoter and strong terminator nosT of Ustilago smut with eGFP The genes are connected to construct an effective plasmid vector pUe-cbx-Hsp, which improves eGFP The expression intensity of the start eGFP Compared with otef and TFIID2, the gene transcription level was enhanced by more than 9 times and 11 times respectively.
Owner:CHINA JILIANG UNIV
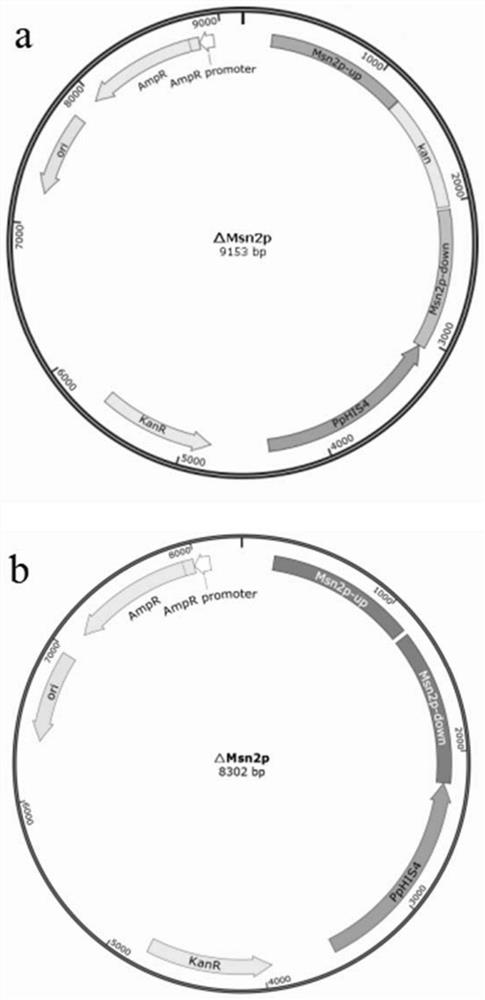
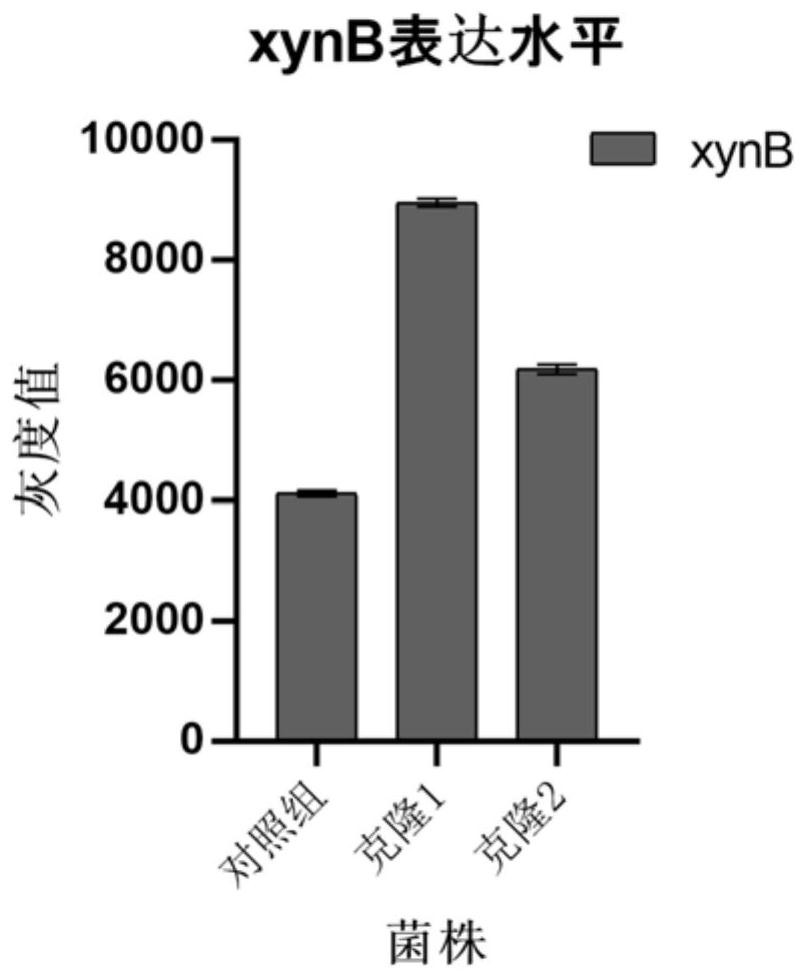
Application of Msn2p as negative regulatory factor in improvement of protein expression in host cells
ActiveCN114657190AAchieve non-induced bulk accumulationImprove expression strengthFungiMicroorganism based processesProtein targetNucleotide
The invention relates to an application of a transcriptional regulatory factor expressed by eukaryotic genes, in particular to an application of a transcriptional regulatory factor Msn2p of a constitutive promoter Pgap. The invention discloses application of Msn2p as a negative regulatory factor in improving protein expression in host cells. The amino acid sequence of the Msn2p is coded by an Msn2 gene with the nucleotide sequence of SEQ ID NO: 1; according to the application, expression of protein in host cells is improved by knocking out the Msn2 gene. According to the application disclosed by the invention, transcriptional regulation and control of the constitutive promoter Pgap in a pichia pastoris expression system can be enhanced by reducing a repression effect, so that the expression efficiency and the yield of target protein are improved.
Owner:JINAN UNIVERSITY
Promoter of peanut δ12 fatty acid dehydrogenase ahfad2-1b-m gene and its preparation method and application
ActiveCN105505930BAvoid wastingImprove expression efficiencyFermentationVector-based foreign material introductionCloned genesNucleotide
The invention discloses a Δ12 fatty acid dehydrogenase AhFAD2-1B-m gene promoter, a preparation method and an application thereof. The nucleotide sequence of the promoter is shown in SEQ ID NO.1. The present invention clones the peanut Δ12 fatty acid dehydrogenase AhFAD2‑1B‑m gene promoter from peanuts, uses the promoter provided by the present invention to construct a recombinant expression vector, and then transforms the expression vector into Arabidopsis by the method mediated by Agrobacterium Mustard, it can promote the expression of downstream recombinant genes in seeds, cotyledons, and hypocotyls, but not in other tissues. Accumulate in organs or tissues, increase the expression level in tissues, exert better effects, and avoid the waste of energy of the plant itself, so it has important application value in genetic engineering breeding and transgenic research.
Owner:HENAN ACAD OF AGRI SCI
Recombinant nitrile hydratase and application thereof in preparation of nicotinamide by coupling ion exchange resin
PendingCN114277023AIncreased substrate and product toleranceMild conditionsOrganic chemistryBacteriaIon exchangeGenetic engineering
The invention provides recombinant nitrile hydratase and application thereof in preparation of nicotinamide by coupling ion exchange resin, which comprises the following steps: by taking a concentrated solution of supernate obtained by ultrasonically crushing wet thalli obtained by fermentation culture of recombinant nitrile hydratase gene engineering bacteria as a catalyst and 3-cyanopyridine as a substrate, adding the ion exchange resin, and reacting at the temperature of between 20 and 30 DEG C to obtain the nicotinamide. The preparation method comprises the following steps: forming a reaction system by taking a phosphoric acid buffer solution with the pH value of 6-9 as a reaction medium, carrying out hydration reaction under the conditions of 20-30 DEG C and 100-1000rpm, and after the reaction is completed, separating and purifying the reaction liquid to obtain the product nicotinamide. The recombinant nitrile hydratase coupled ion exchange resin has the advantages of mild conditions, high efficiency, high chemical selectivity, regioselectivity and the like, the biological catalysis process has the characteristics of no toxicity, no pollution, low energy consumption and the like, the method is an environment-friendly synthesis method, 95% or more of nicotinic acid impurities are adsorbed, and the method is suitable for industrial production. The nicotinamide product with the purity of 99.99% or above is obtained.
Owner:ZHEJIANG UNIV OF TECH
Function identification of promoter of source sequence of gluconacetobacter xylinum and application of promoter in promotion of synthesis of bacterial cellulose
PendingCN114703185AEasy to synthesizeOvercome problems such as inability to expressTransferasesMicroorganism based processesPromoter activityMicrobiology
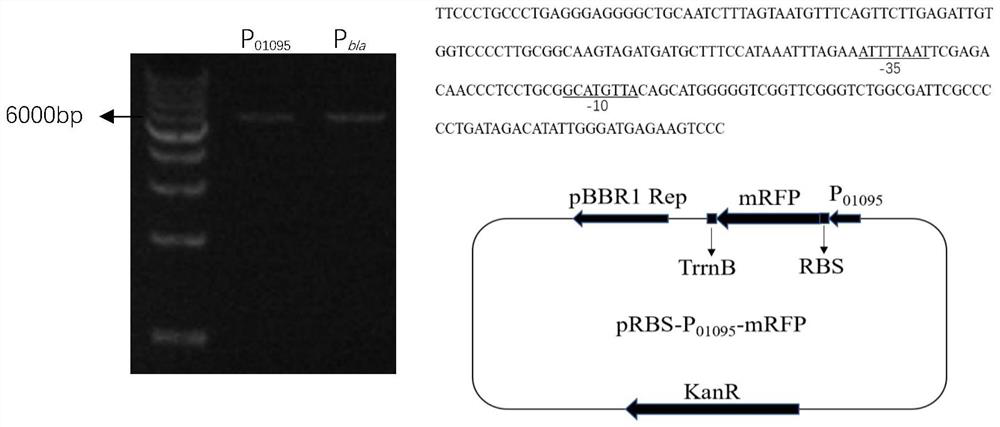
The invention discloses a sequence which is derived from gluconacetobacter xylinum and has a promoter function and application of the sequence in promoting synthesis of bacterial cellulose. The sequence has higher promoter activity than a pBla plasmid promoter. Therefore, the strain can be used for enhancing the expression of a target gene, for example, the expression intensity of phosphofructokinase pfkA is enhanced by operably connecting the strain with a phosphofructokinase gene, so that the bacterial cellulose production efficiency of the strain is improved.
Owner:TIANJIN UNIV OF SCI & TECH
Recombined staphylococcus aureus enterotoxin N oral preparation and application thereof
InactiveCN101293096AImprove expression strengthAvoid degradationDepsipeptidesImmunological disordersAntigenWhole body
The invention provides a recombinant staphylococcal enterotoxin N oral preparation, the recombinant staphylococcal enterotoxin N has SEQ ID NO.1 amino acid sequence, and the oral preparation further comprises a pharmaceutical allowable drug excipient or a carrier. The oral preparation proves that the protein can enter the systemic blood circulation by penetrating epithelial cells on small intestine with the form of complete molecules and maintain the super-antigen activity for promoting the spleen lymphocyte proliferation and inhibiting the growth of tumor cells, as well as the application in the preparation of drugs for treating malignant tumors and other serious complications by the Caco-2 monolayer cell transmembrane transport test.
Owner:ZHEJIANG UNIV
Mutant of glutamate dehydrogenase gene promoter and its application
ActiveCN113201535BHigh promoter activityImprove expression strengthBacteriaNucleotide librariesPromoter activityWild type
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
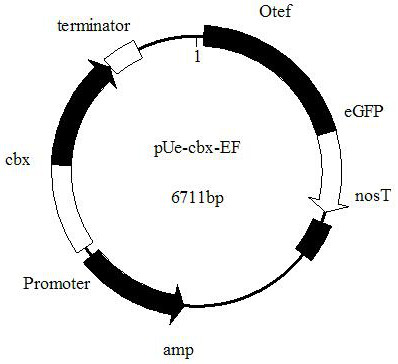
Ustilago esculenta endogenous promoter pEF as well as expression vector and application thereof
ActiveCN112680449AImprove expression strengthEnhanced gene transcription levelsFungiMicroorganism based processesNucleotideGenetic engineering
The invention discloses an ustilago esculenta endogenous promoter pEF as well as an expression vector and application thereof, and belongs to the technical field of gene engineering. The nucleotide sequence of the ustilago esculenta endogenous promoter pEF is shown as SEQ ID NO.1. The invention discloses the expression vector containing the ustilago esculenta endogenous promoter pEF, an application of the ustilago esculenta endogenous promoter pEF to driving transcription and expression of an eGFP gene, constructing a stable expression system and obtaining engineering ustilago esculenta, and an application of the ustilago esculenta endogenous promoter pEF to improving expression intensity and fluorescence stability of eGFP. The ustilago esculenta endogenous pEF promoter and a strong terminator nosT are connected with the eGFP gene, an effective plasmid vector pUe-cbx-EF is constructed, more choices are provided for construction of an ustilago esculenta genetic transformation vector, and a foundation is laid for functional gene research of the ustilago esculenta.
Owner:CHINA JILIANG UNIV
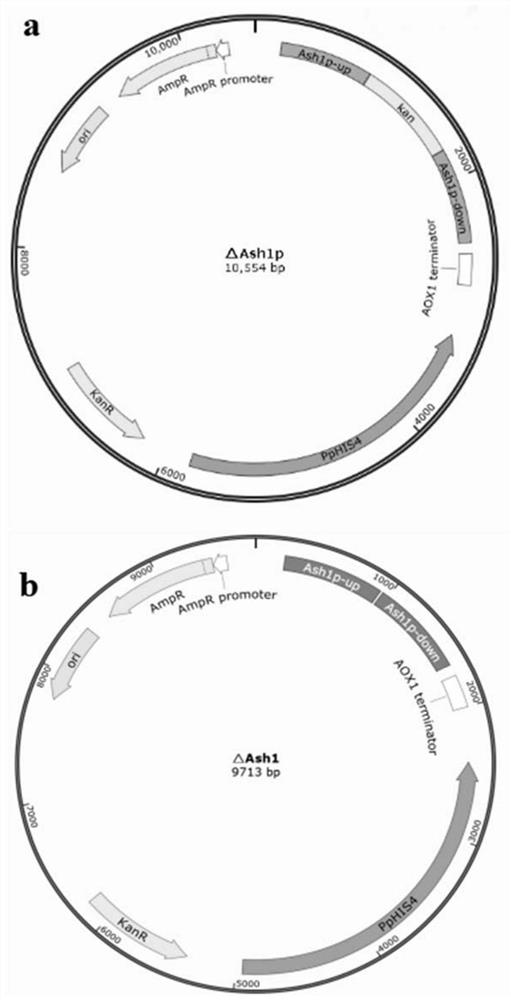
Application of Ash1p as negative regulatory factor in improvement of protein expression in host cells
PendingCN114774461AEfficient expressionImprove expression strengthFungiMicroorganism based processesRibosomal RNA GenesMolecular biology
The invention relates to application of a transcriptional regulatory factor expressed by eukaryotic genes, in particular to application of a transcriptional regulatory factor Ash1p of a constitutive promoter Pgap. The invention discloses application of Ash1p as a negative regulatory factor in improvement of protein expression in host cells. The amino acid sequence of the Ash1p is coded by an Ash1 gene with the nucleotide sequence of SEQ ID NO: 1; according to the application, expression of protein in host cells is improved by knocking out the Ash1 gene. According to the application disclosed by the invention, transcriptional regulation and control of the constitutive promoter Pgap in a pichia pastoris expression system can be enhanced by reducing a repression effect, so that the expression efficiency and the yield of target protein are improved.
Owner:JINAN UNIVERSITY
A kind of wild smut endogenous promoter pef and its expression vector and application
ActiveCN112680449BImprove expression strengthEnhanced gene transcription levelsFungiMicroorganism based processesBiotechnologyNucleotide
The invention relates to an endogenous promoter pEF of smut smut and its expression vector and application, which belong to the technical field of genetic engineering. The invention comprises: an endogenous promoter pEF of Ustilago smut, the nucleotide sequence of which is shown in SEQ ID NO.1; an expression vector containing the endogenous promoter pEF of Ustilago smut; eGFP The transcription and expression of the gene, the construction of a stable expression system and the application of the engineering smut fungus have improved the eGFP Application of expression intensity and fluorescence stability. The endogenous pEF promoter and the strong terminator nosT of the wild rice smut of the present invention and eGFP The genes were connected, and an effective plasmid vector pUe‑cbx‑EF was constructed, which provided more choices for the construction of the genetic transformation vector of Ustilago smut, and laid the foundation for the functional gene research of Ustilago smut.
Owner:CHINA JILIANG UNIV
Peanut delta 12 Promoter of fatty acid dehydrogenase ahfad2‑2a gene and its application
ActiveCN105087587BAvoid wastingImprove expression efficiencyVector-based foreign material introductionDNA preparationBiotechnologyEnzyme Gene
The invention discloses an AhFAD2-2A gene promoter (P< AhFAD2-2A >) and an application of the AhFAD2-2A gene promoter, and belongs to the technical field of biology. A nucleotide sequence of the promoter is shown as SEQ ID NO.1. The AhFAD2-2A gene promoter is cloned from peanuts, and is used for building a recombinant expression vector; then, the recombinant expression vector is transformed into arabidopsis thaliana by an agrobacterium-mediated transformation method; a downstream recombinant gene is started to be expressed in seeds, roots and anthers, and is not expressed in other tissues; and the promoter belongs to a tissue specificity promoter, so that the promoter can be applied to transgenic engineering; a target gene expression product is accumulated in a certain organ or tissue; the expression amount in the tissue is increased; a good effect is achieved; meanwhile, the self energy waste of plants is avoided; and important application values are realized in the genetic engineering breeding and transgenic research process.
Owner:HENAN ACAD OF AGRI SCI
Recombined staphylococcus aureus enterotoxin O oral preparation and application thereof
InactiveCN101293097AHigh purityMaintain superantigen activityDepsipeptidesImmunological disordersAntigenSevere complication
The invention provides a recombinant staphylococcal enterotoxin O oral preparation, the recombinant staphylococcal enterotoxin O has SEQ ID NO.1 amino acid sequence, and the oral preparation further comprises a pharmaceutical allowable drug excipient or a carrier. The oral preparation proves that the protein can enter the systemic blood circulation by penetrating epithelial cells on small intestine with the form of complete molecules and maintain the super-antigen activity for promoting the spleen lymphocyte proliferation and inhibiting the growth of tumor cells, as well as the application in the preparation of drugs for treating malignant tumors and other serious complications by the Caco-2 monolayer cell transmembrane transport test.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com