Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
686results about "Masers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
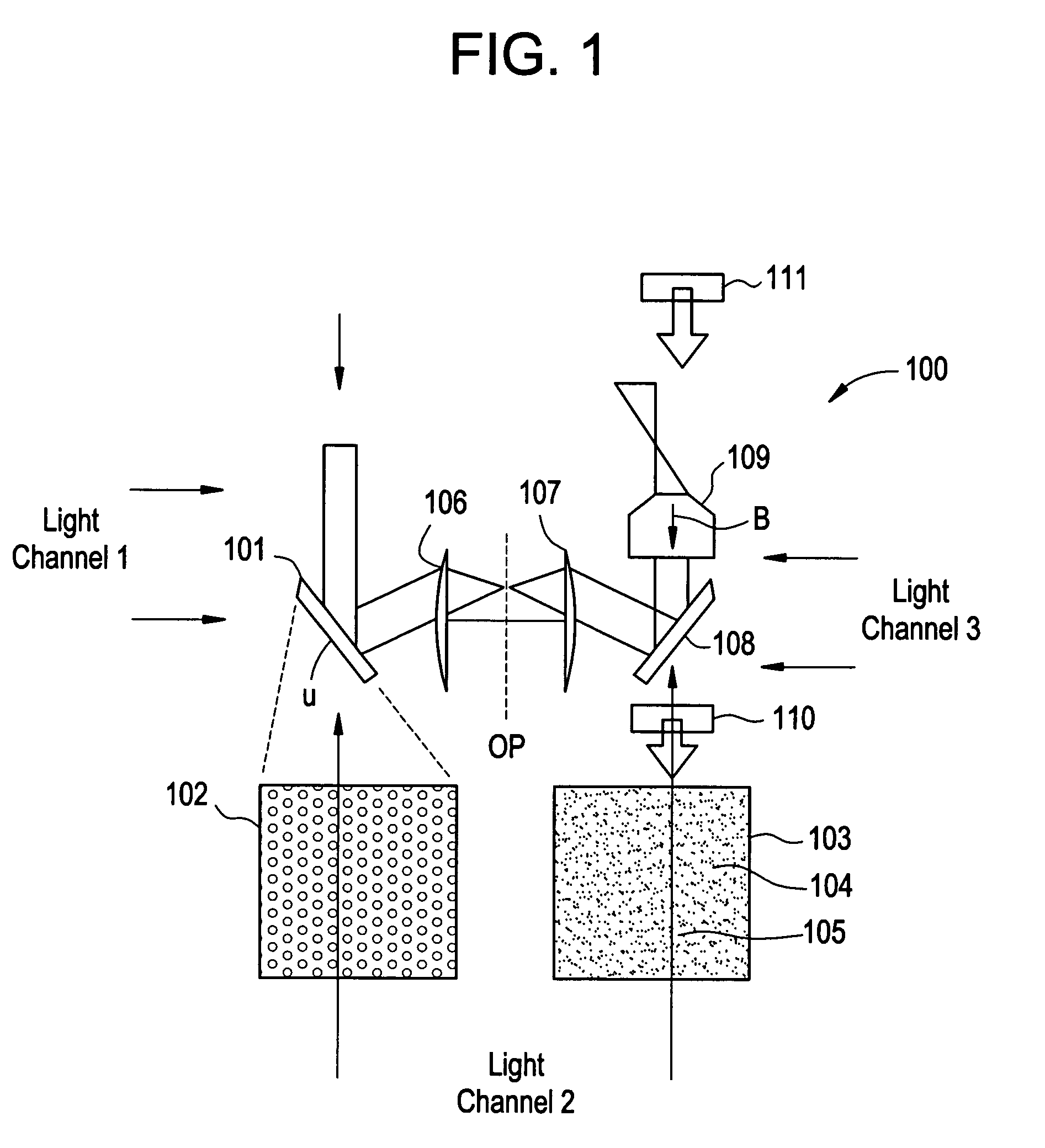
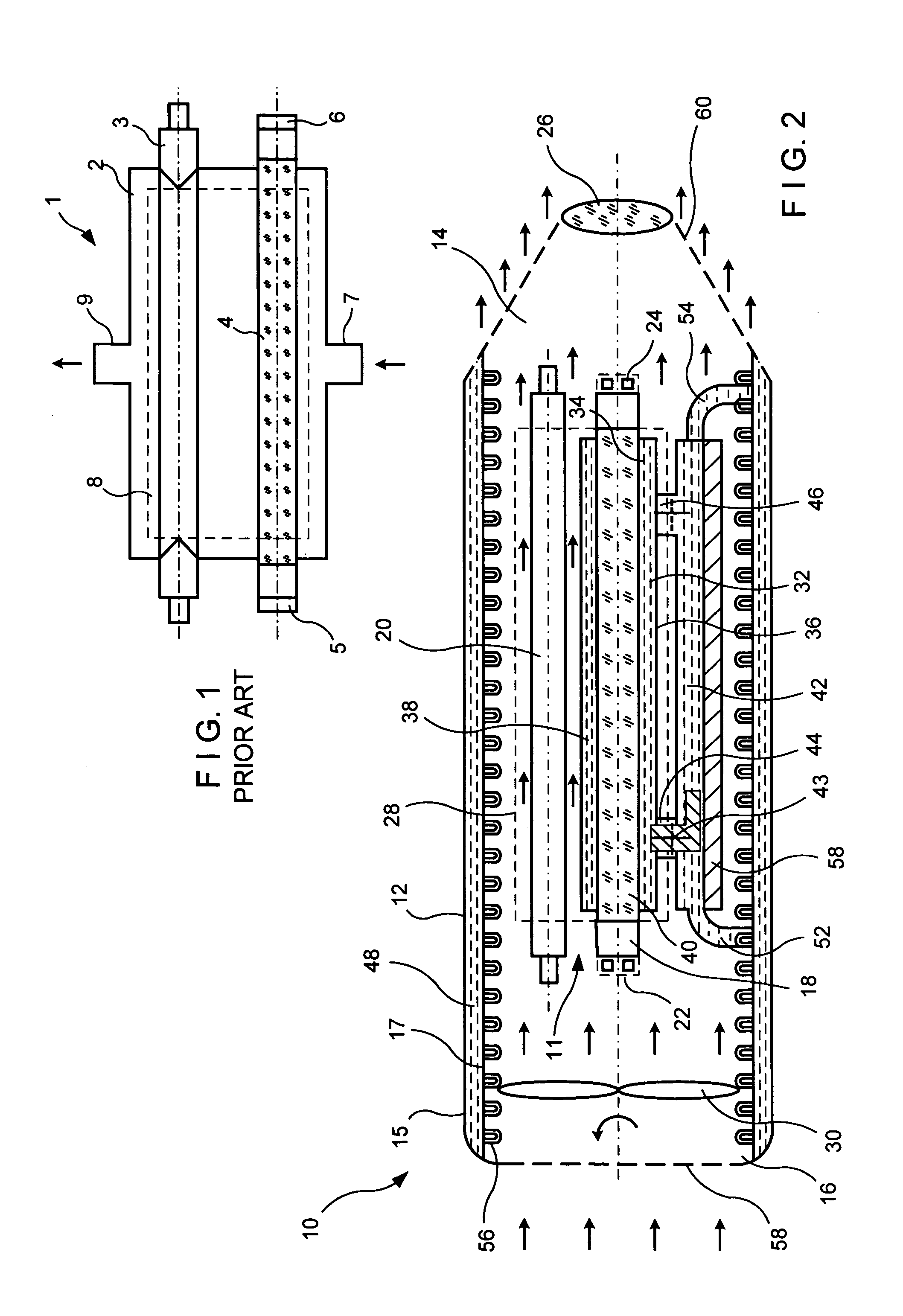
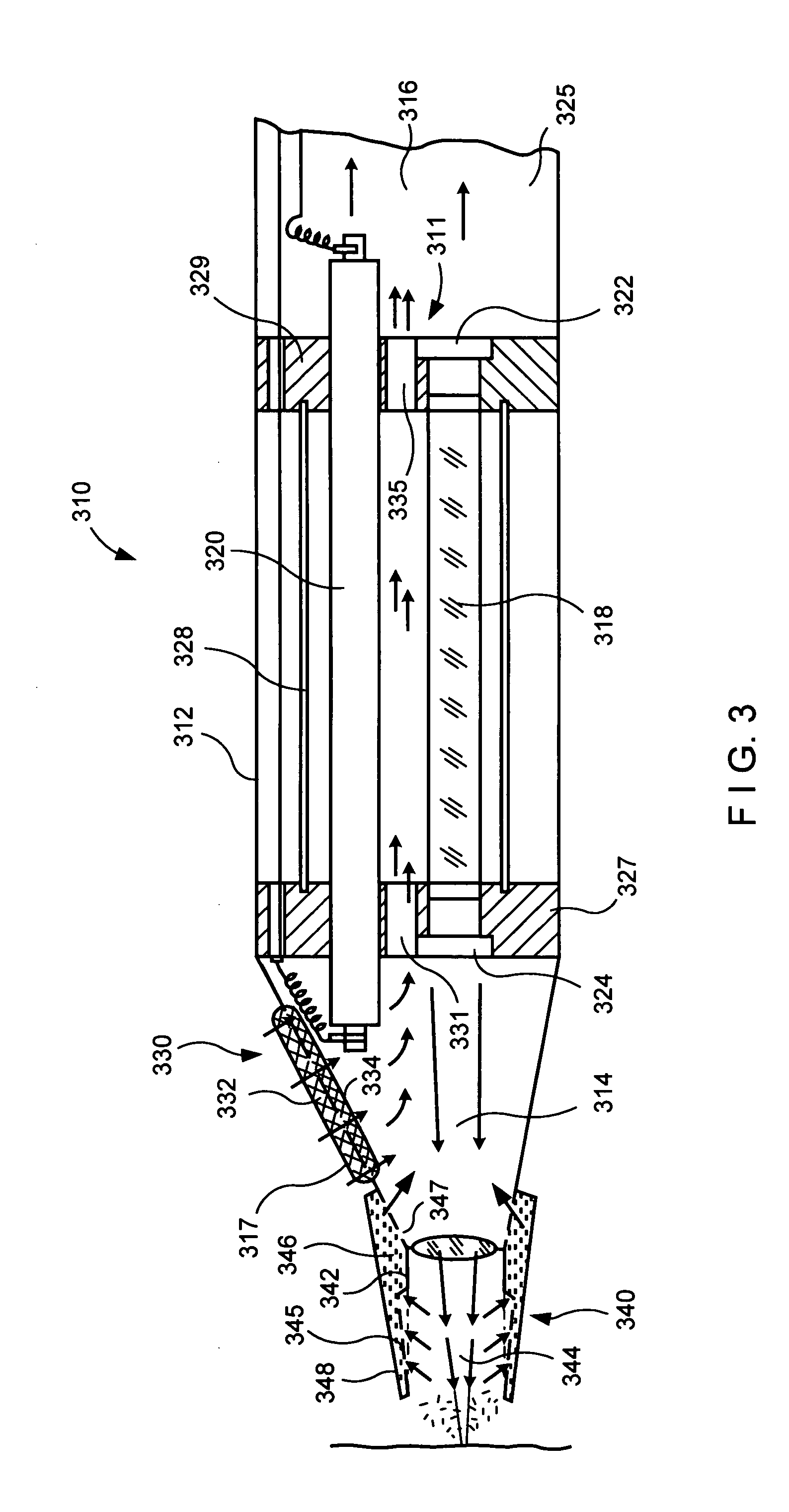
Apparatus and methods for optical analysis of molecules
ActiveUS7170050B2Small volumeEffective volumeRadiation pyrometryLaser detailsMolecular analysisChemical reaction
The present invention relates to optical confinements, methods of preparing and methods of using them for analyzing molecules and / or monitoring chemical reactions. The apparatus and methods embodied in the present invention are particularly useful for high-throughout and low-cost single-molecular analysis.
Owner:NANOFLUIDICS INC +1
Neutral particle beam processing apparatus
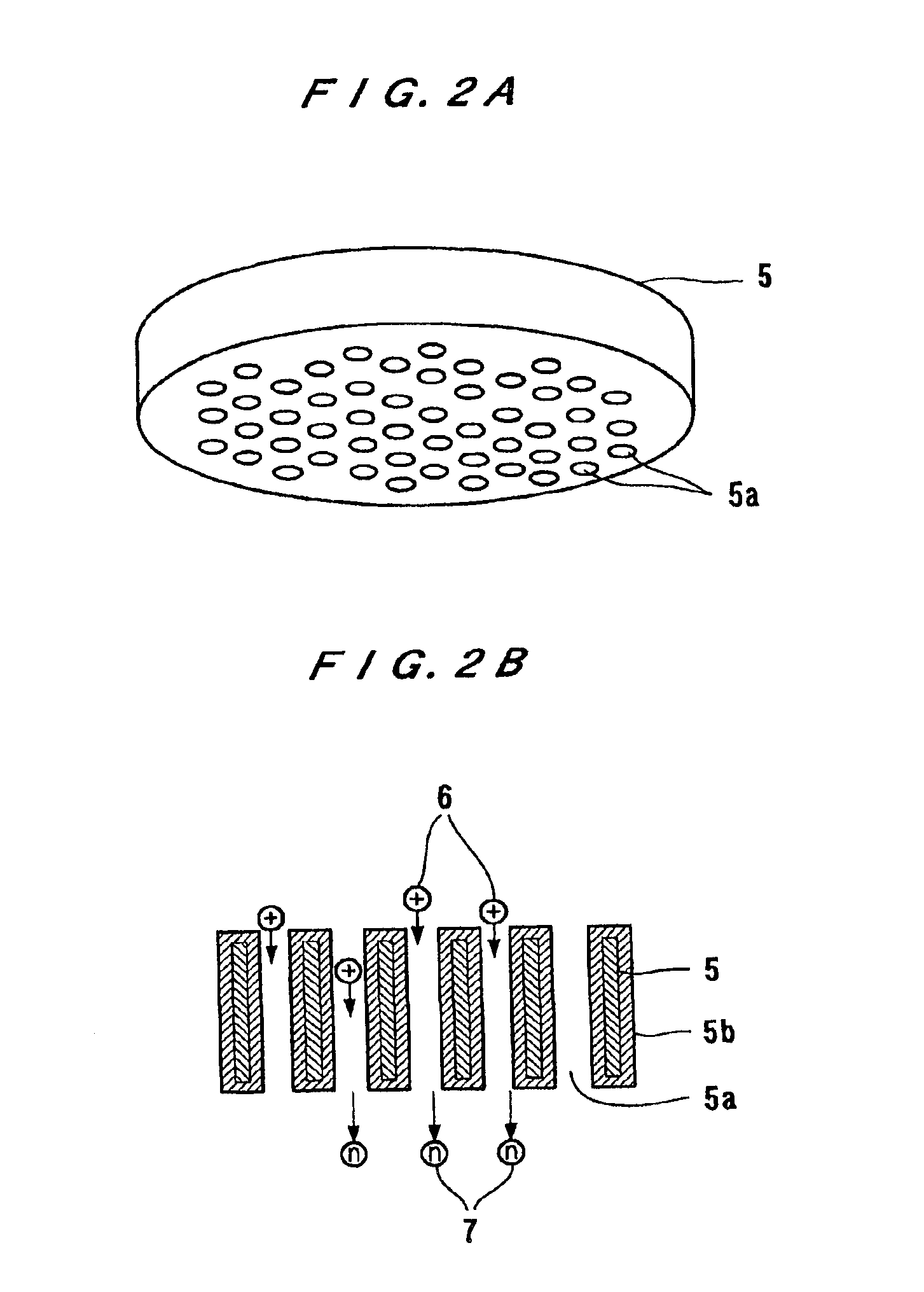
InactiveUS6861642B2Inexpensive and compact structureImprove neutralization efficiencyLaser detailsVacuum evaporation coatingDielectricPlasma generator
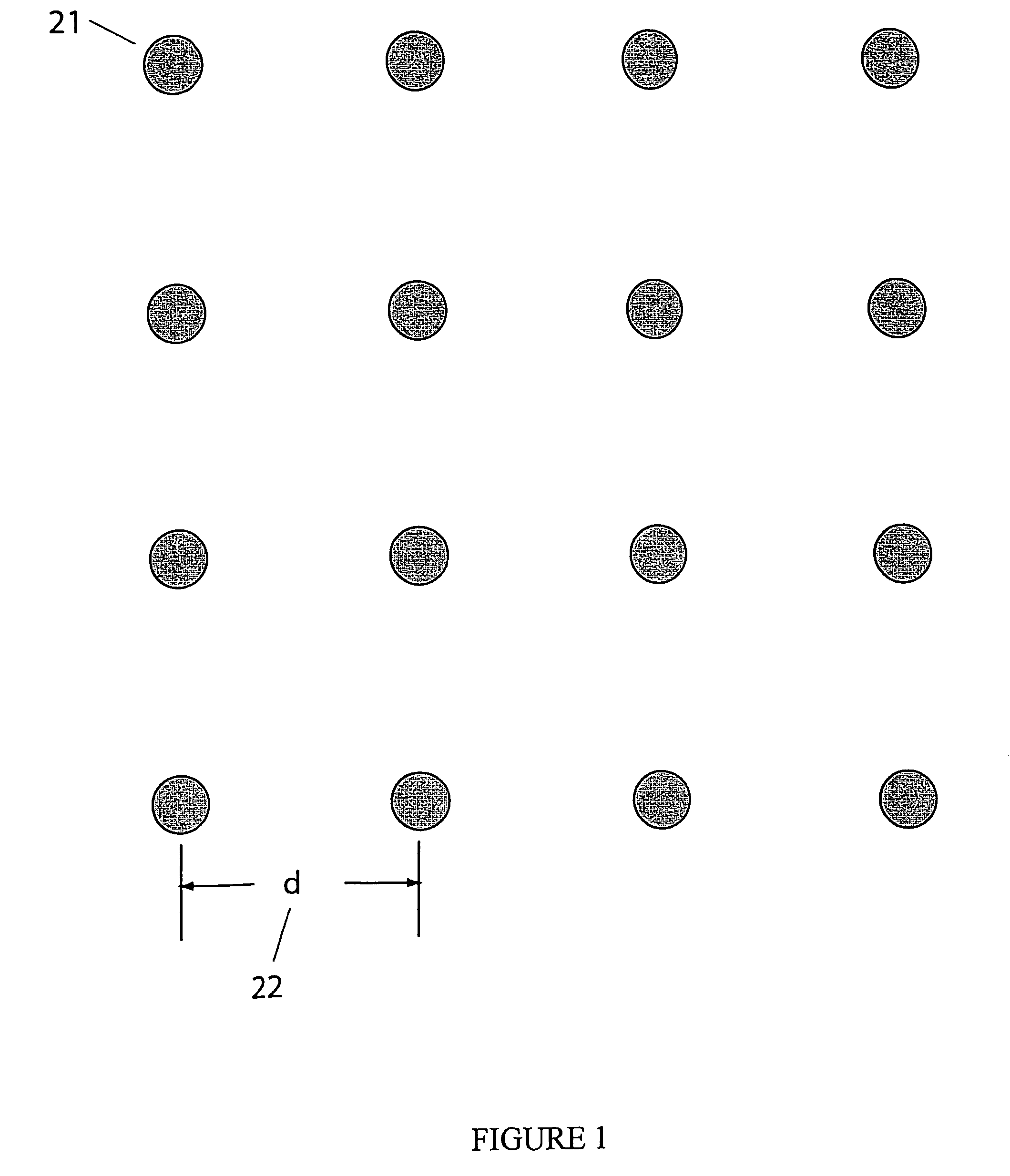
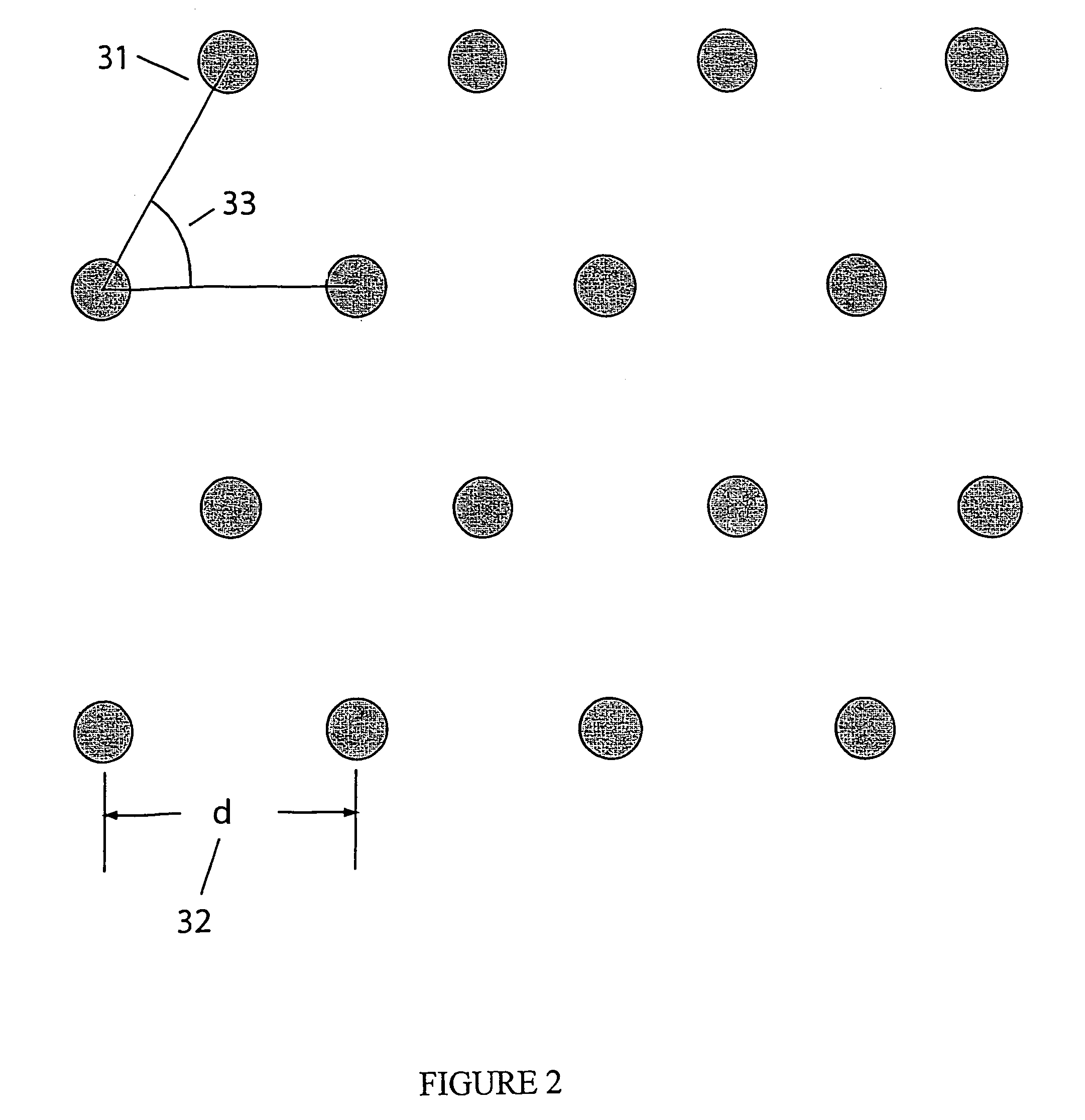
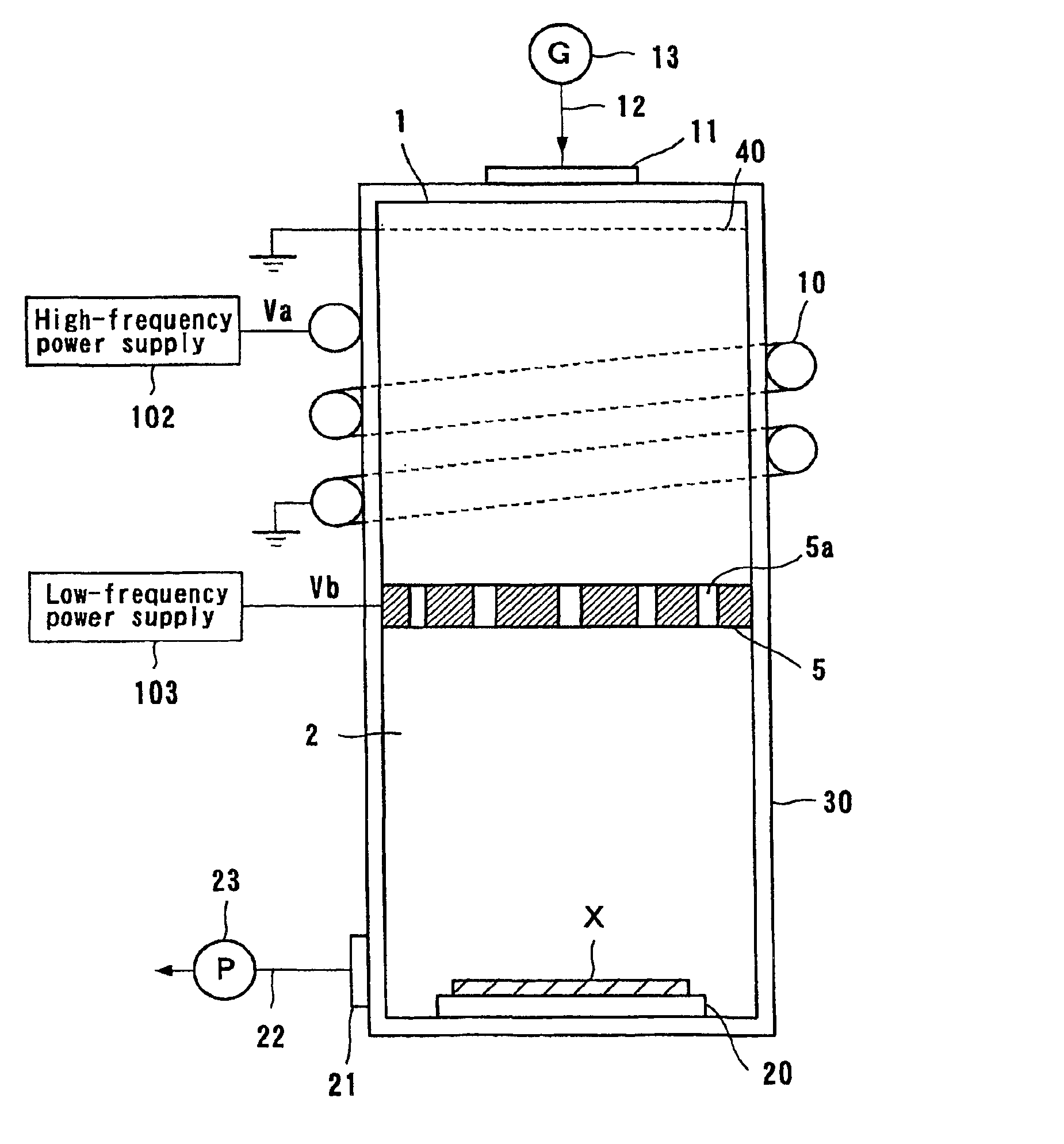
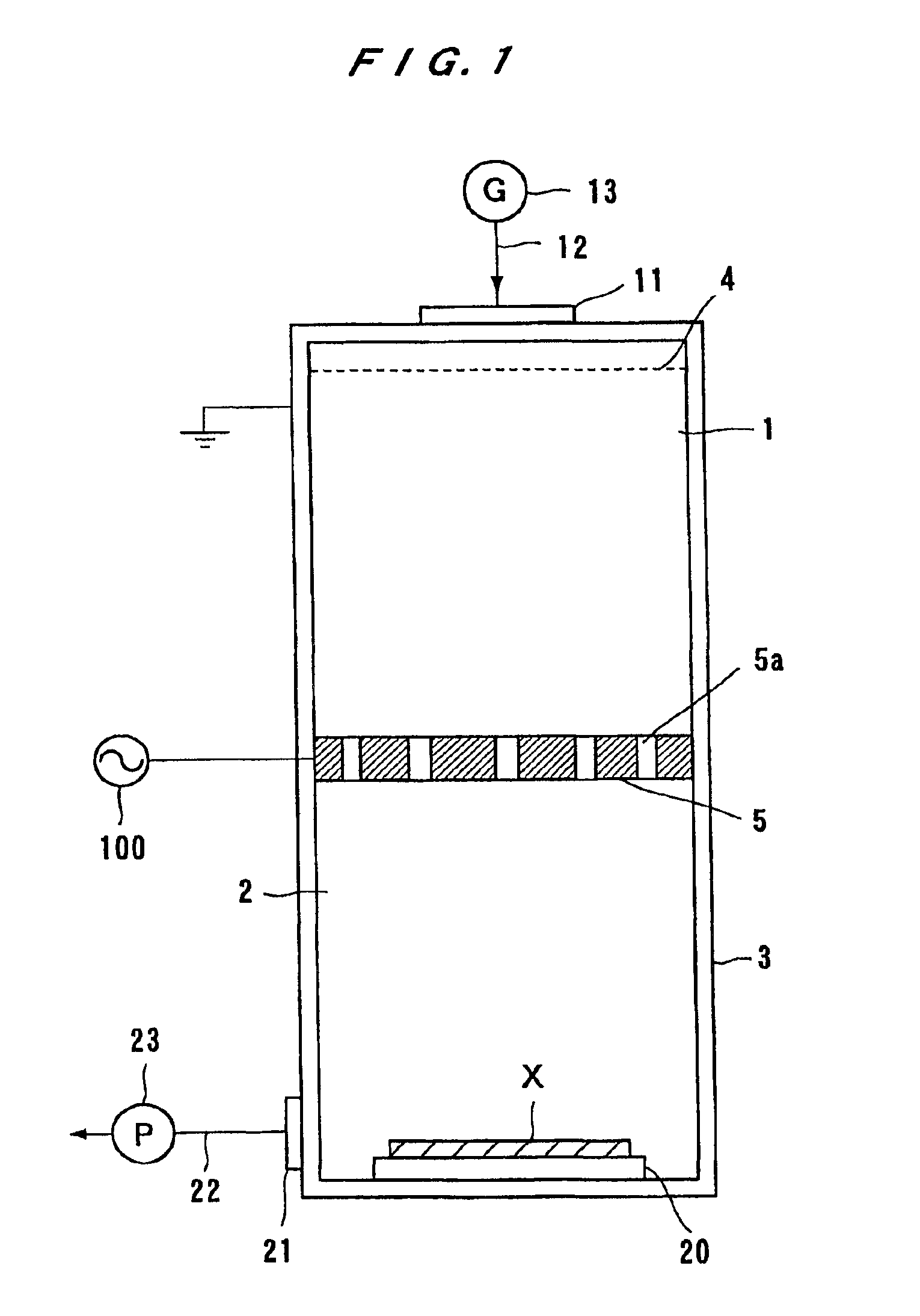
A neutral particle beam processing apparatus comprises a workpiece holder (20) for holding a workpiece (X), a plasma generator for generating a plasma in a vacuum chamber (3), an orifice electrode (5) disposed between the workpiece holder (20) and the plasma generator, and a grid electrode (4) disposed upstream of the orifice electrode (5) in the vacuum chamber (3). The orifice electrode (5) has orifices (5a) defined therein. The neutral particle beam processing apparatus further comprises a voltage applying unit for applying a voltage between the orifice electrode (5) and the grid electrode (4) via a dielectric (5b) to extract positive ions from the plasma generated by the plasma generator and pass the extracted positive ions through the orifices (5a) in the orifice electrode (5).
Owner:ASM IP HLDG BV
Particle beam irradiation system
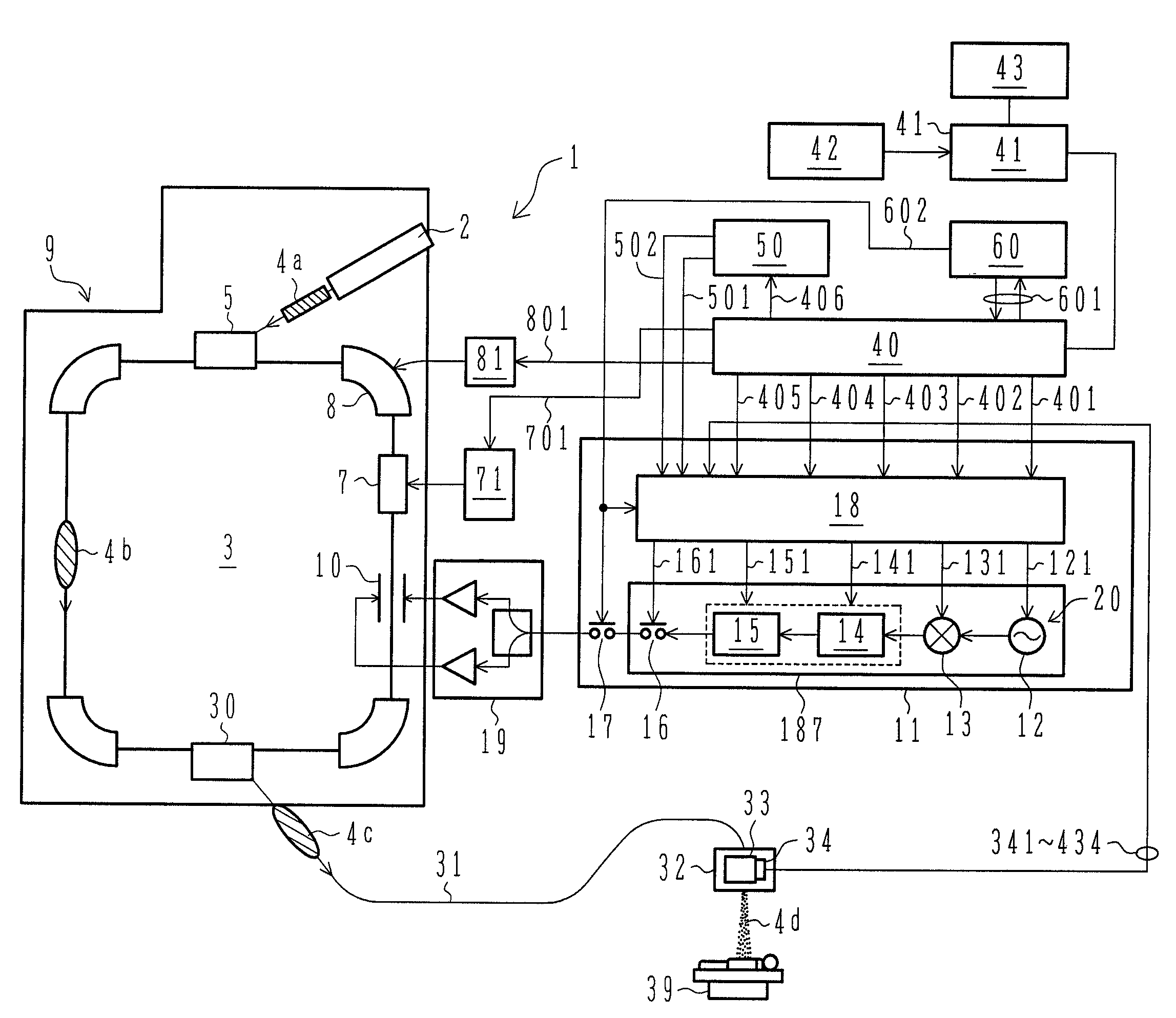
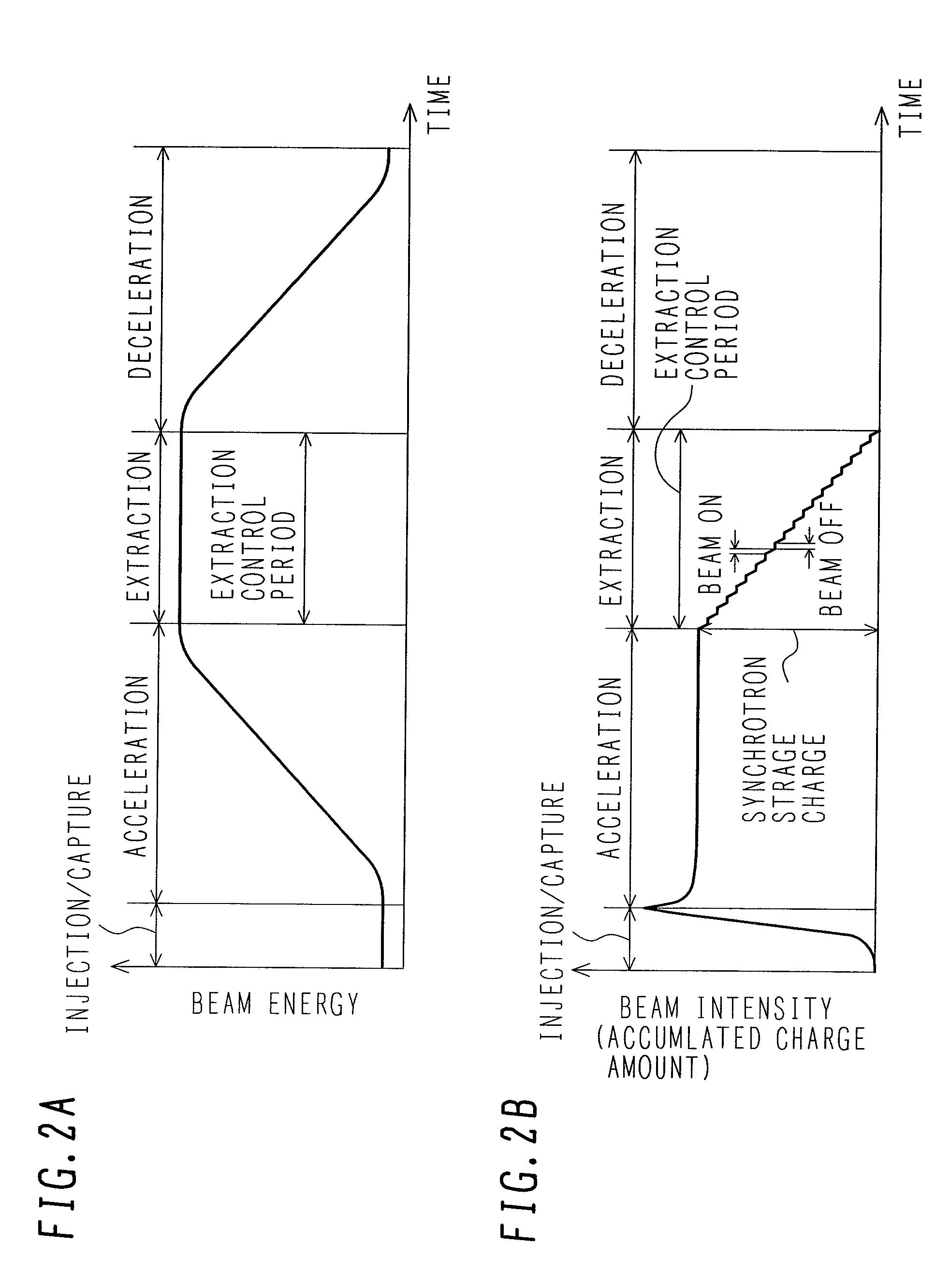
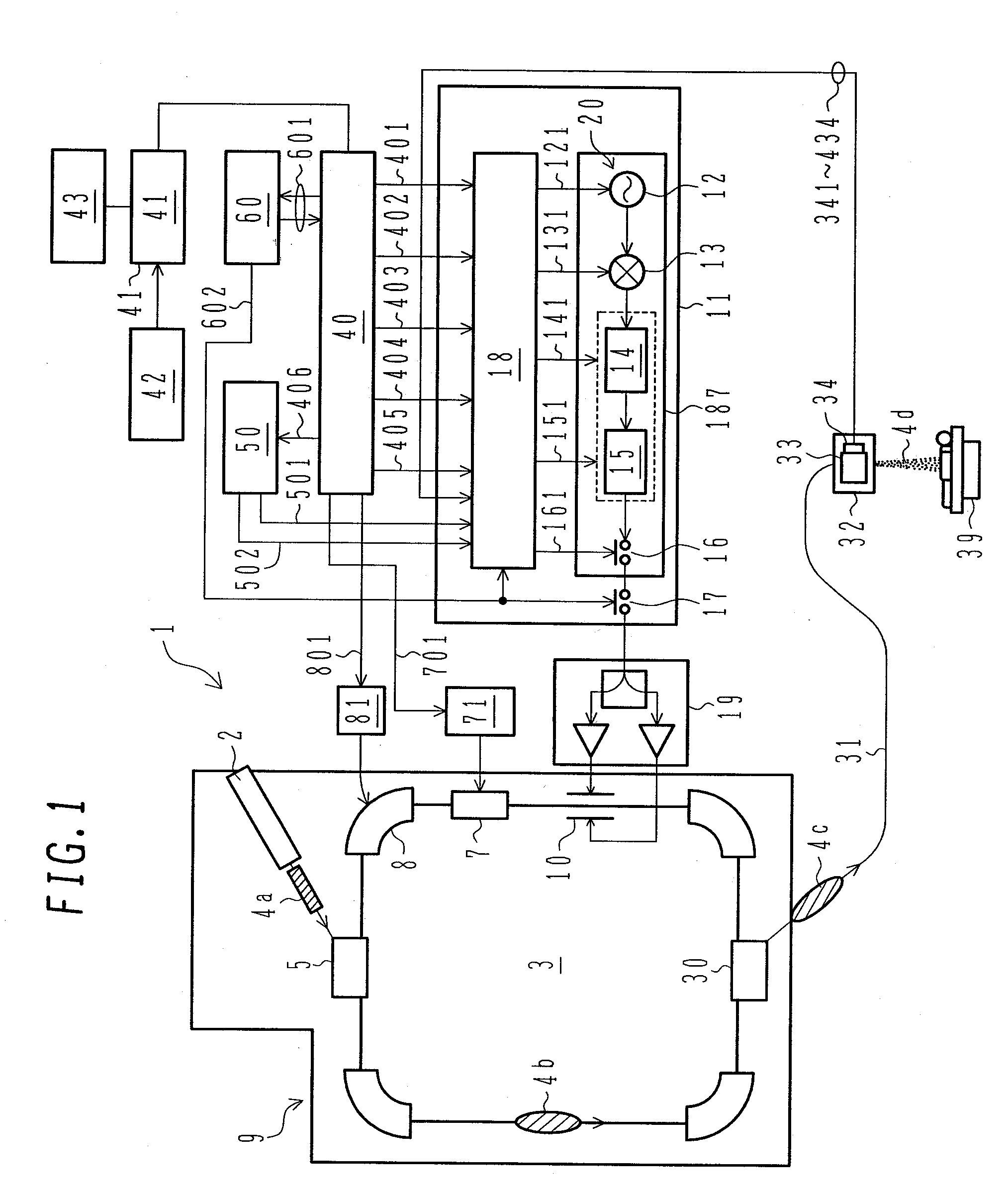
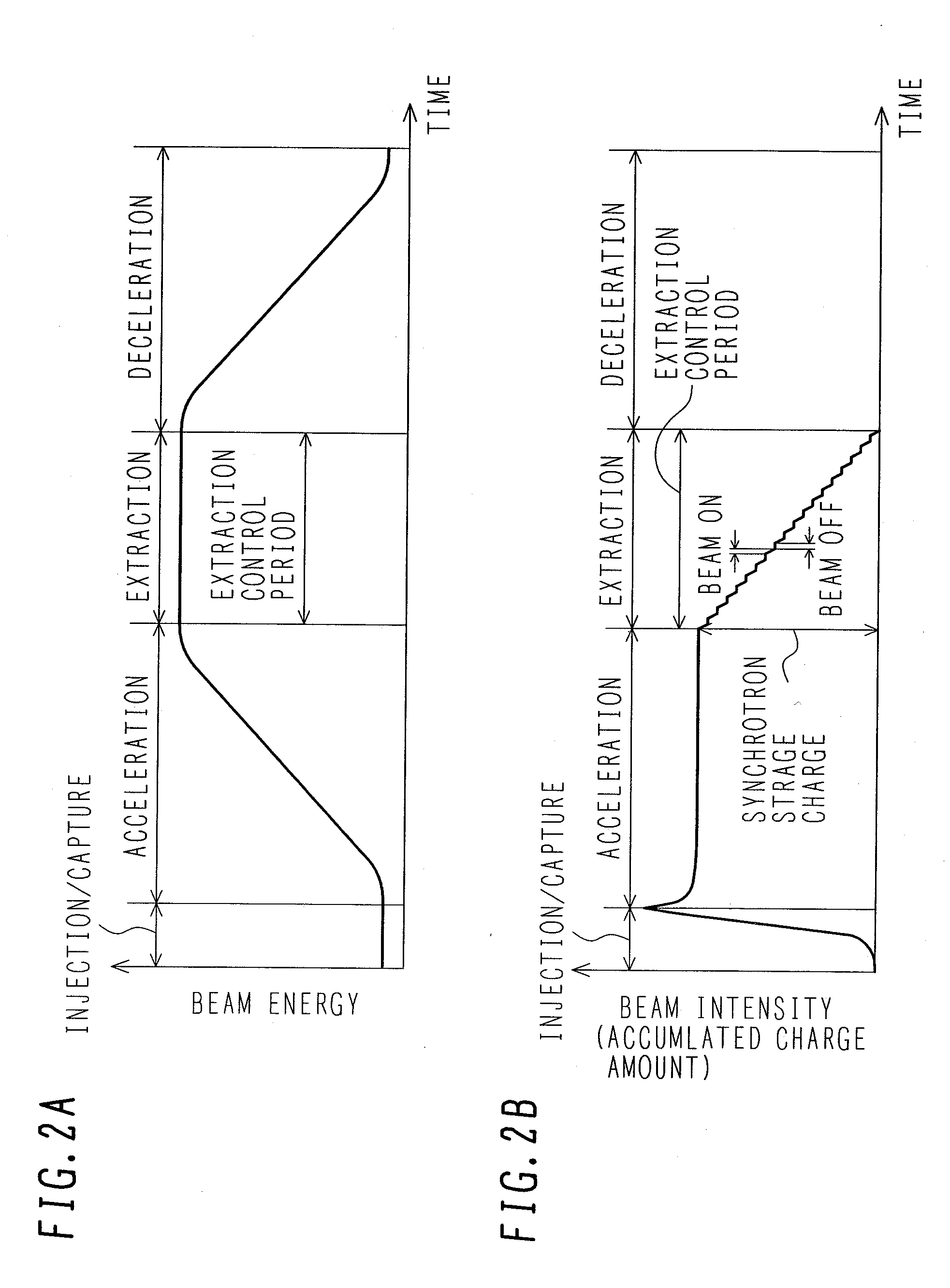
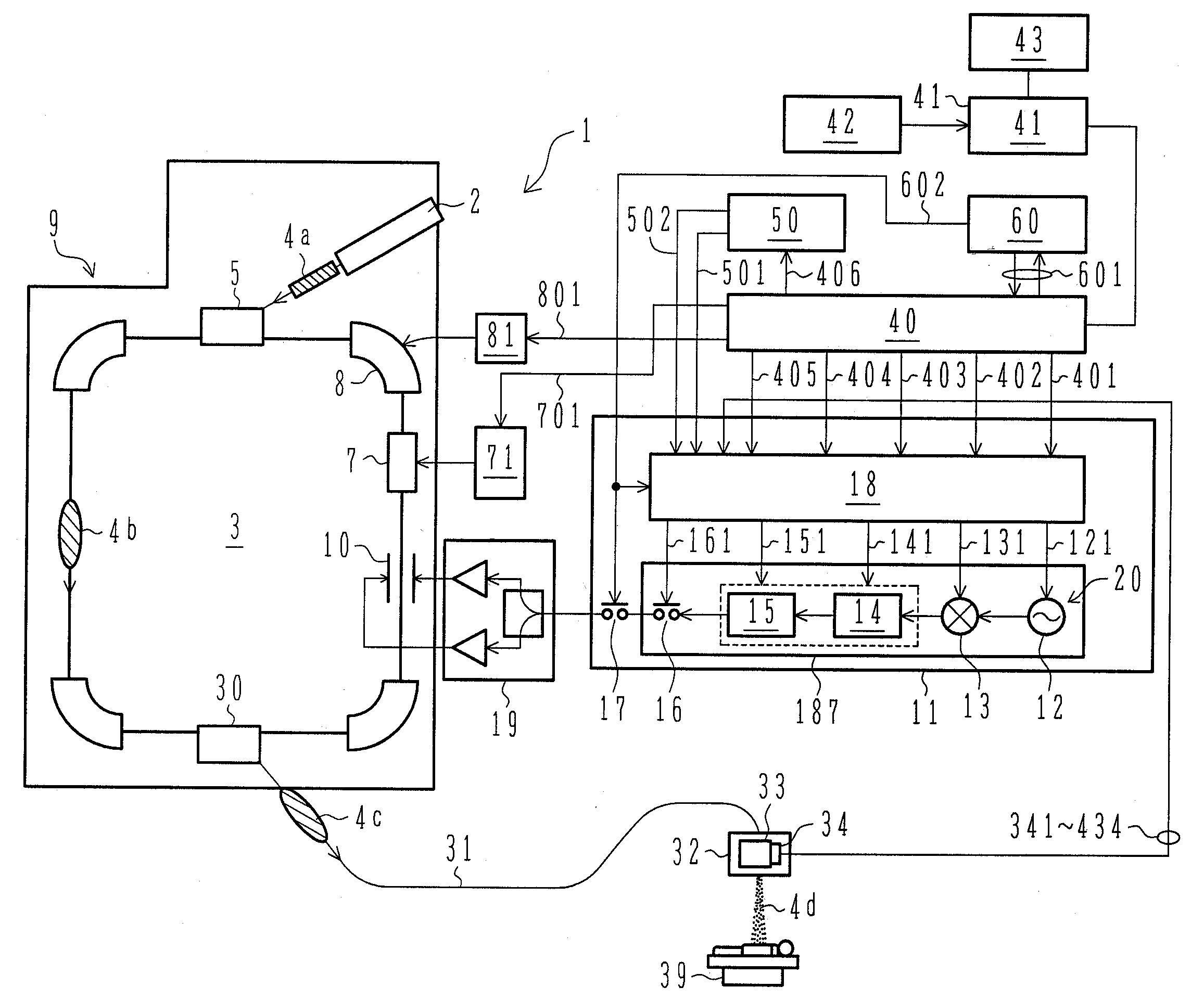
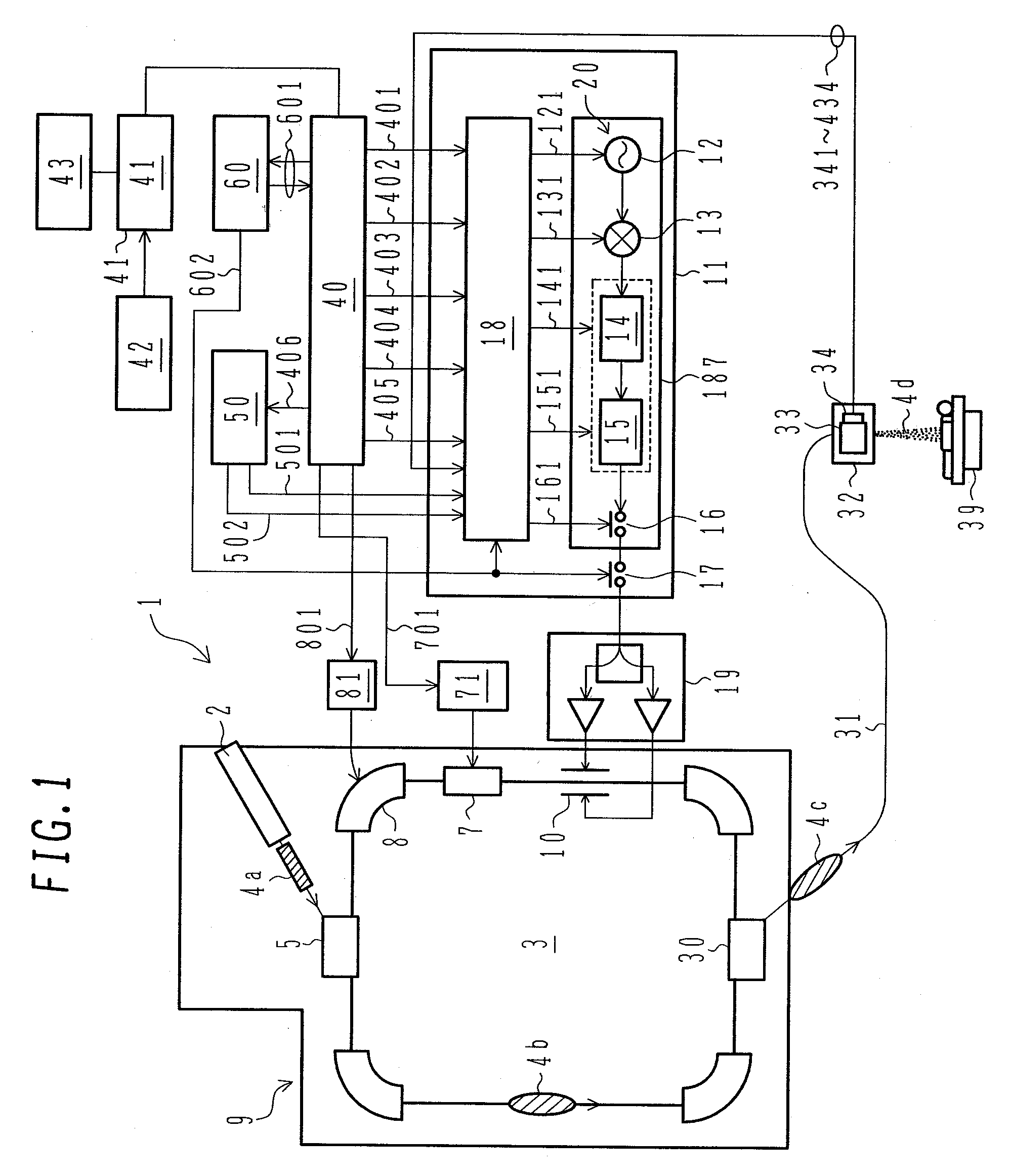
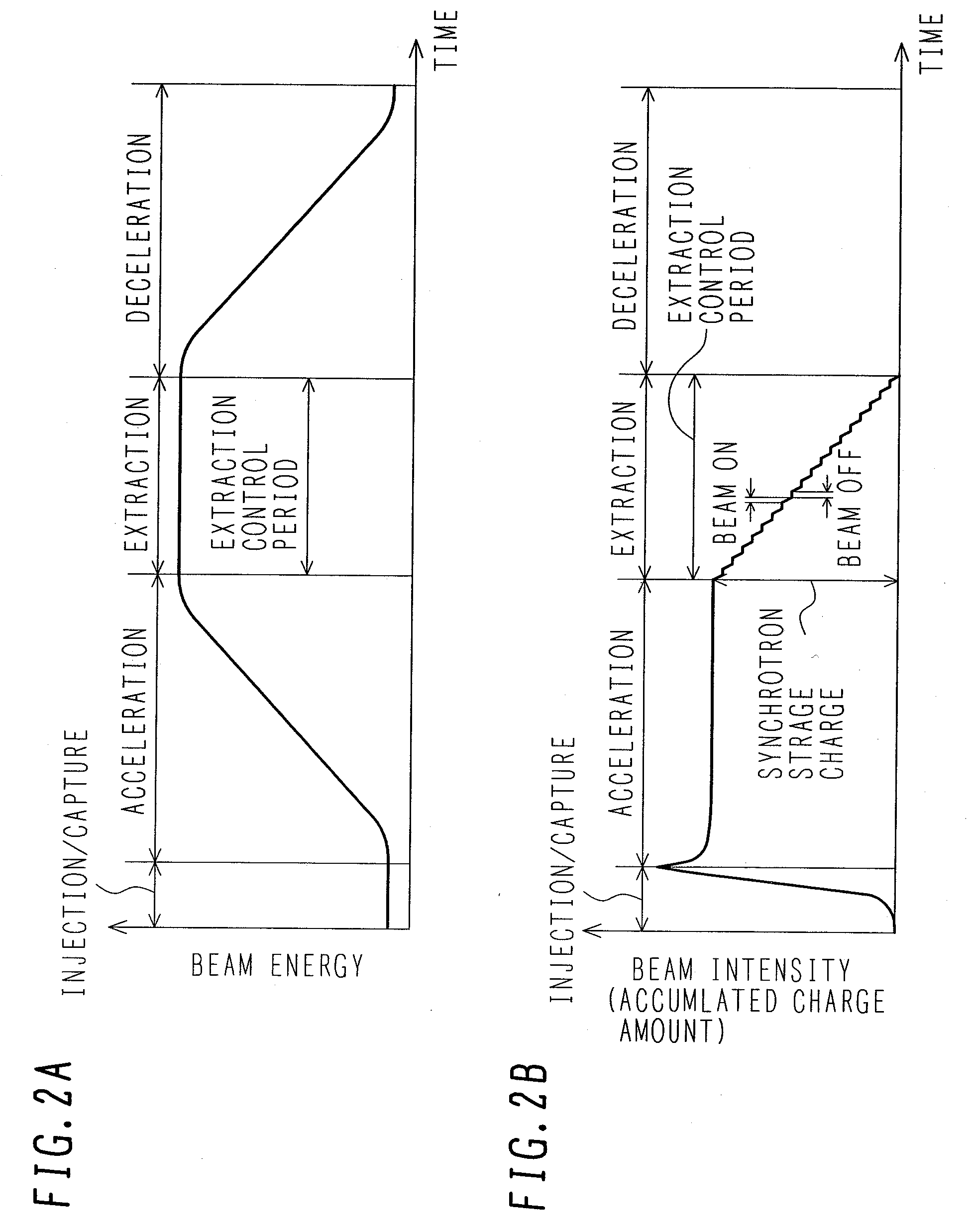
ActiveUS7807982B2Simplify device configurationEasy to controlLaser detailsParticle separator tubesIntensity controlSynchrotron
It is an object of the present invention to provide a charged particle beam extraction method and particle beam irradiation system that make it possible to exercise intensity control over an extracted ion beam while a simple device configuration is employed. To accomplish the above object, there is provided a particle beam irradiation system comprising: a synchrotron for accelerating and extracting an charged particle beam; an irradiation apparatus for extracting the charged particle beam that is extracted from the synchrotron; first beam intensity modulation means for controlling the beam intensity of the charged particle beam extracted from the synchrotron during an extraction control period of an operation cycle of the synchrotron; and second beam intensity modulation means for controlling the beam intensity during each of a plurality of irradiation periods contained in the extraction control period of the operation cycle.
Owner:HITACHI LTD
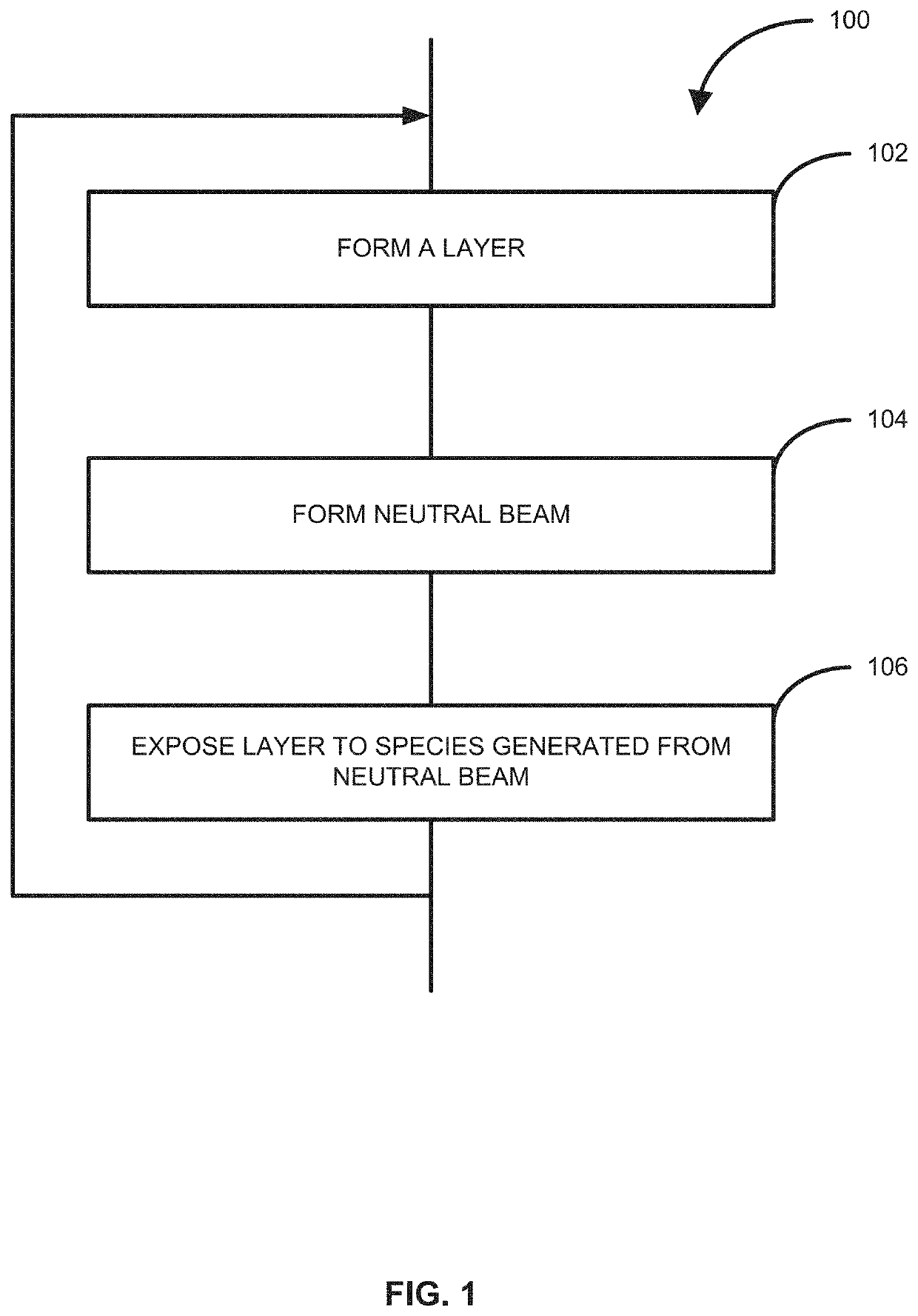
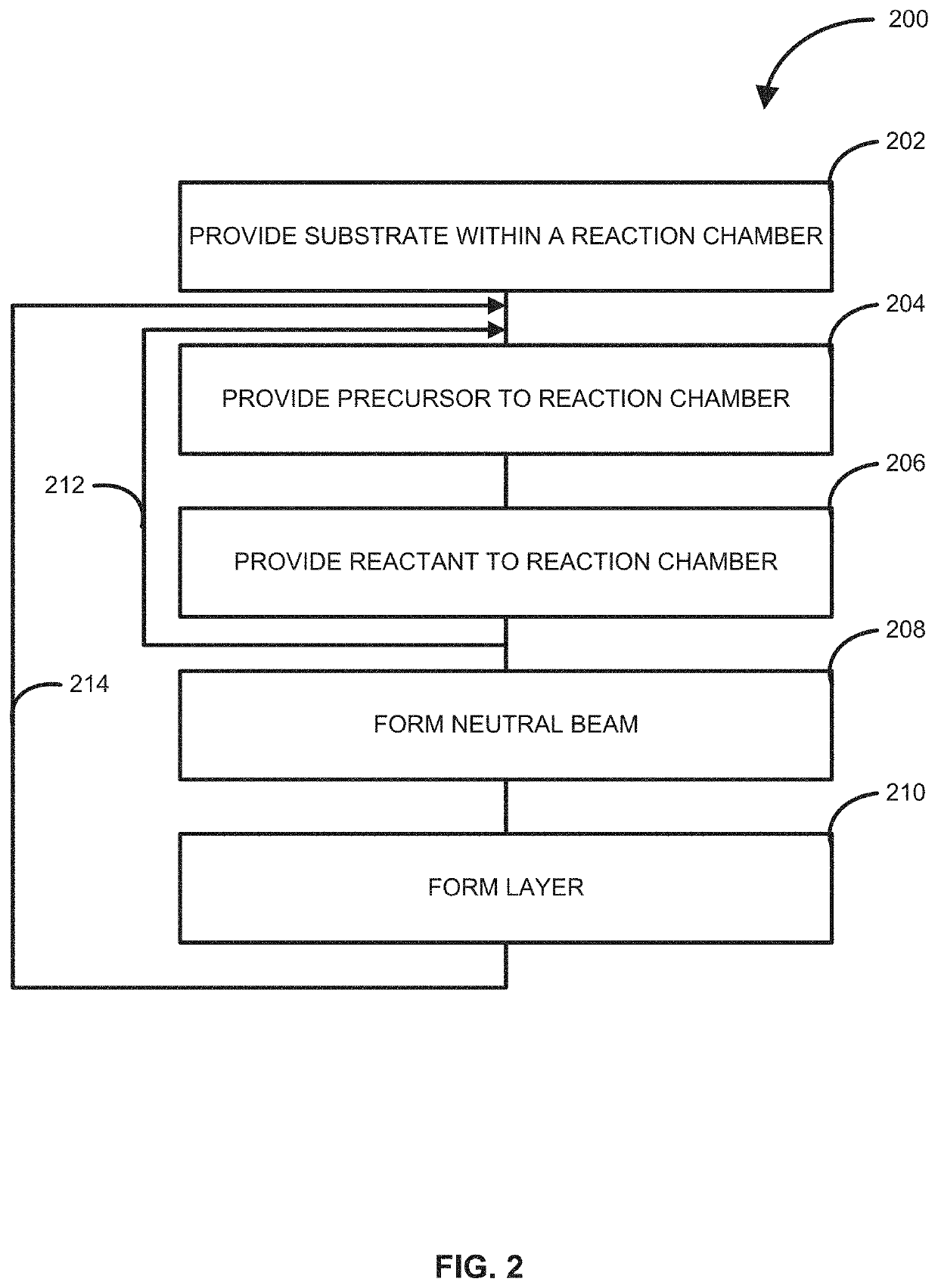
Method of forming structures using a neutral beam, structures formed using the method and reactor system for performing the method
ActiveUS20210017648A1Readily apparentMasersChemical vapor deposition coatingReactor systemEngineering
Owner:ASM IP HLDG BV
Arrays of optical confinements and uses thereof
ActiveUS20060061754A1Effective volumeHigh fill fraction arrayMaterial nanotechnologyLaser detailsMolecular analysisChemical reaction
The present invention relates to optical confinements, methods of preparing and methods of using them for analyzing molecules and / or monitoring chemical reactions. The apparatus and methods embodied in the present invention are particularly useful for high-throughput and low-cost single-molecular analysis.
Owner:PACIFIC BIOSCIENCES
Apparatus and methods for optical analysis of molecules
ActiveUS20060060766A1Reducing diffractive scatteringSmall volumeLaser detailsRadiation pyrometryMolecular analysisChemical reaction
The present invention relates to optical confinements, methods of preparing and methods of using them for analyzing molecules and / or monitoring chemical reactions. The apparatus and methods embodied in the present invention are particularly useful for high-throughout and low-cost single-molecular analysis.
Owner:NANOFLUIDICS INC +1
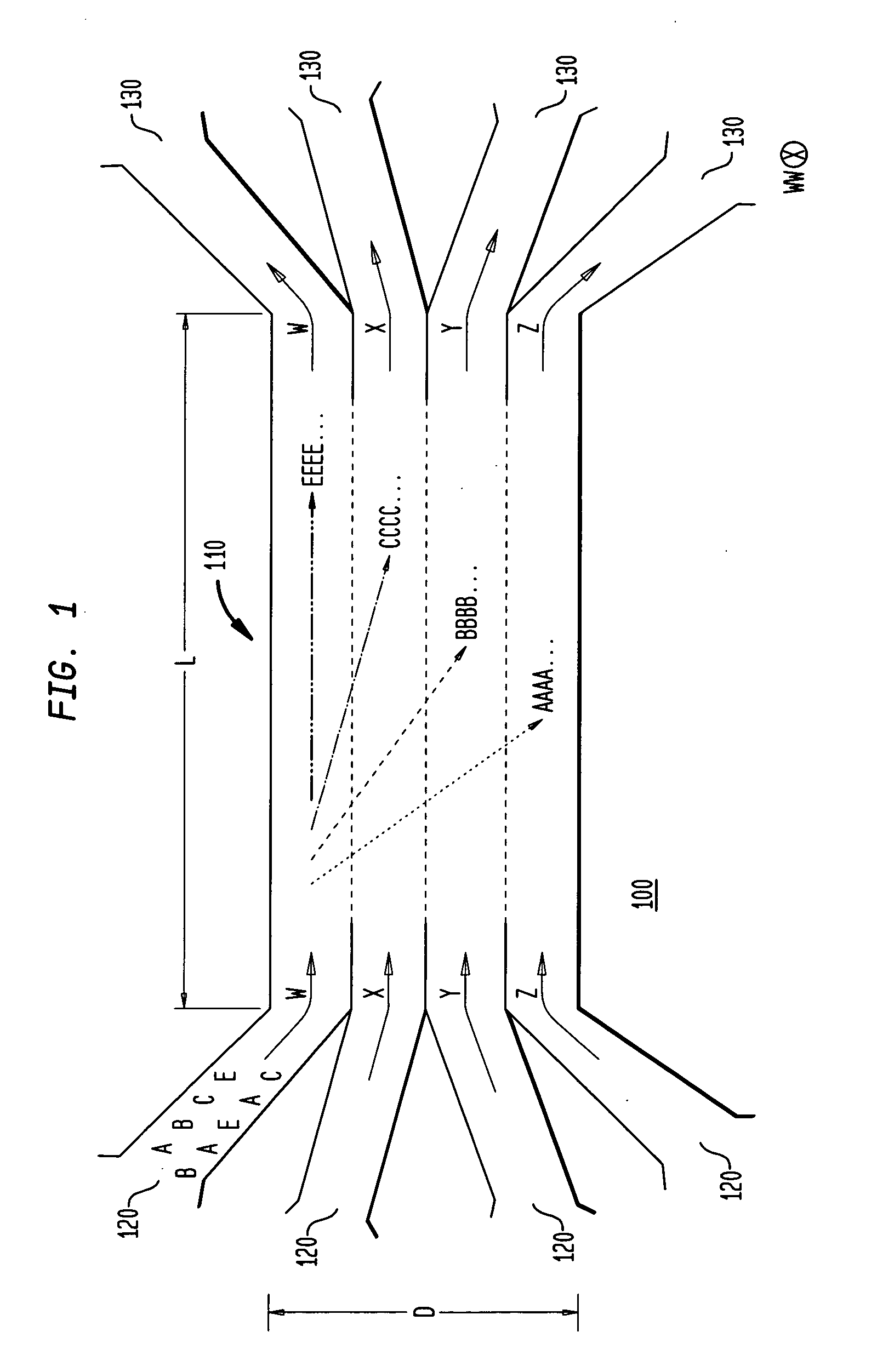
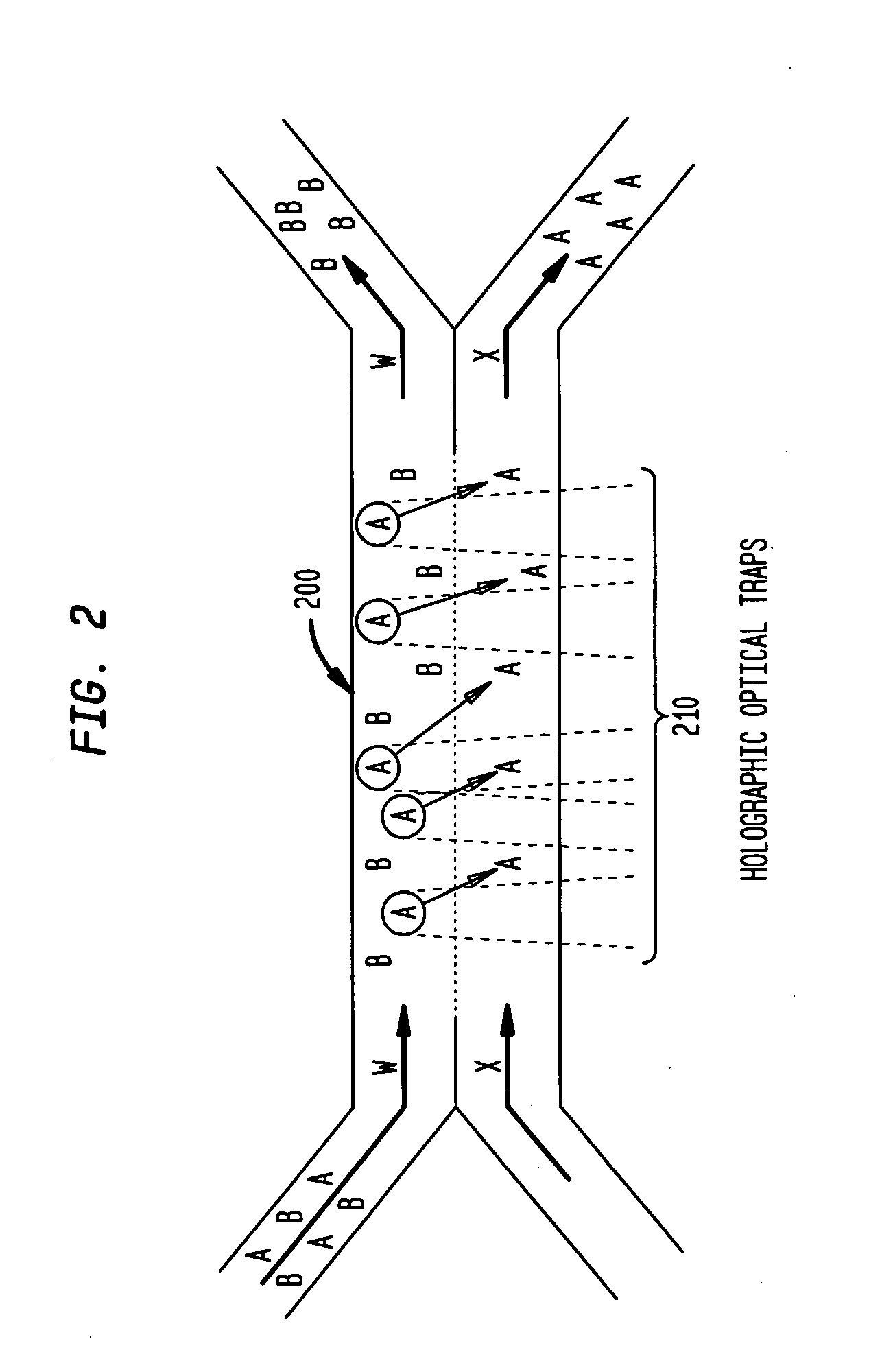
Multiple laminar flow-based particle and cellular separation with laser steering
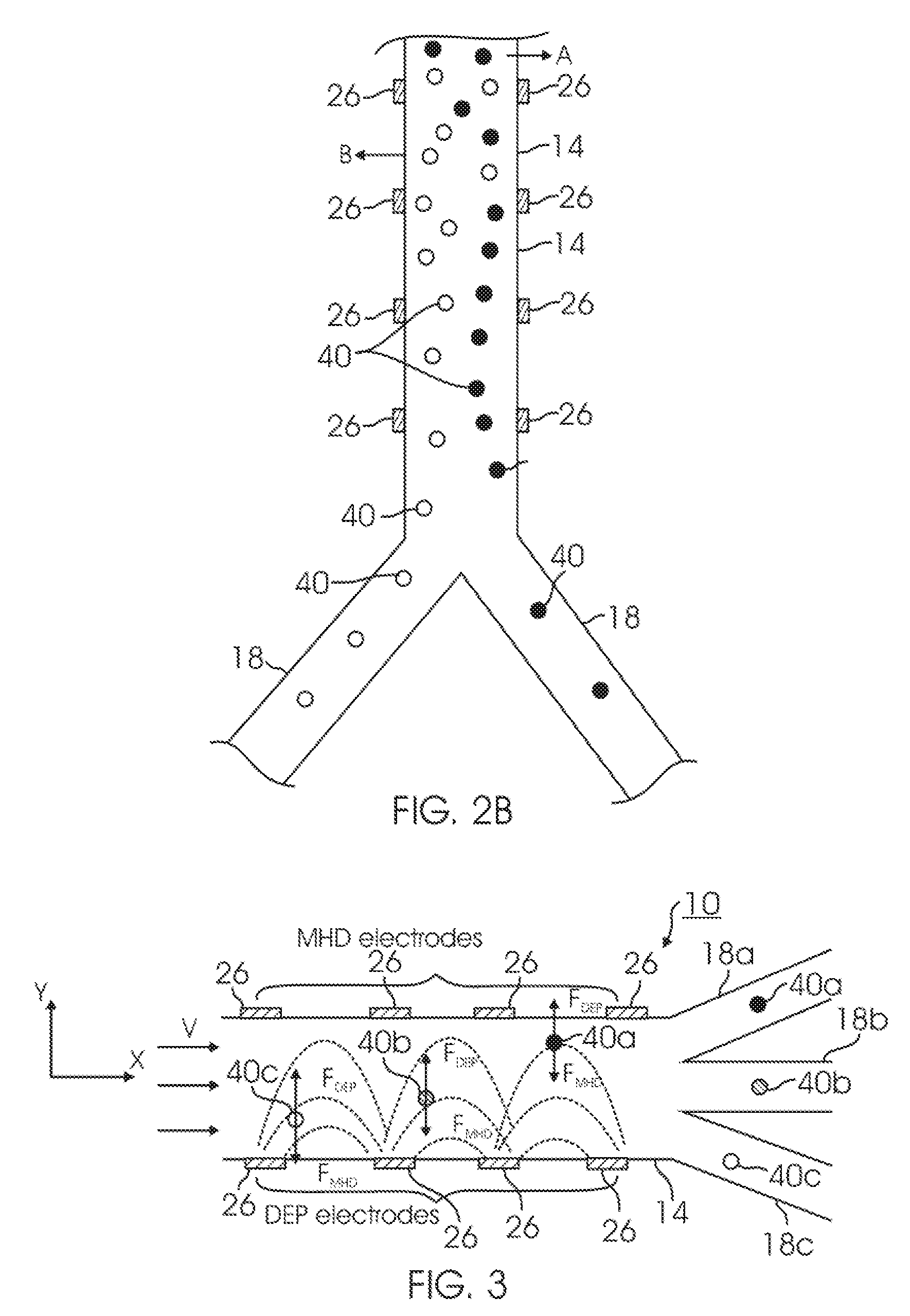
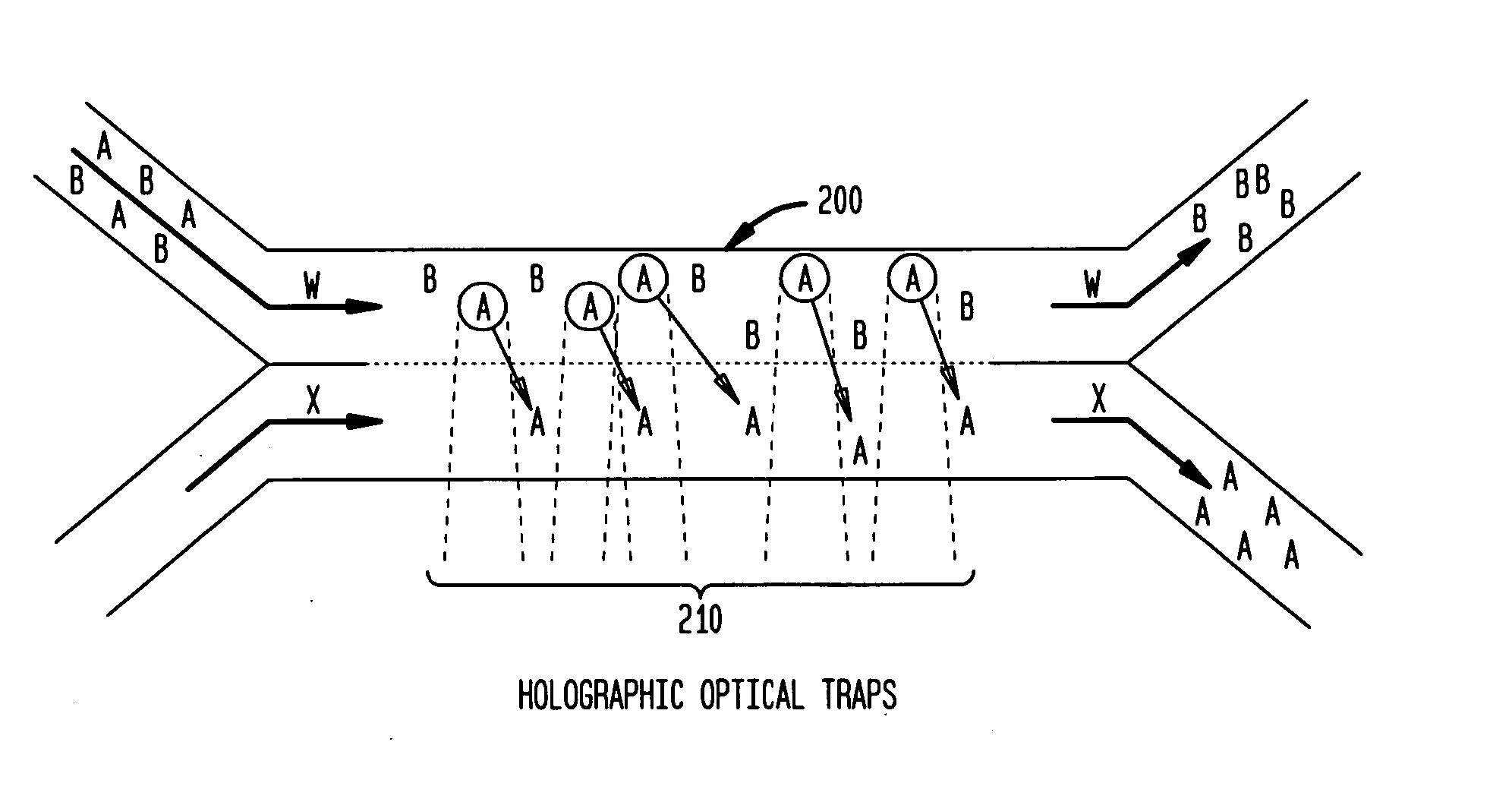
InactiveUS20050121604A1Save a lot of timeImprove throughputDielectrophoresisLaser detailsCellular componentBlood component
The invention provides a method, apparatus and system for separating blood and other types of cellular components, and can be combined with holographic optical trapping manipulation or other forms of optical tweezing. One of the exemplary methods includes providing a first flow having a plurality of blood components; providing a second flow; contacting the first flow with the second flow to provide a first separation region; and differentially sedimenting a first blood cellular component of the plurality of blood components into the second flow while concurrently maintaining a second blood cellular component of the plurality of blood components in the first flow. The second flow having the first blood cellular component is then differentially removed from the first flow having the second blood cellular component. Holographic optical traps may also be utilized in conjunction with the various flows to move selected components from one flow to another, as part of or in addition to a separation stage.
Owner:PREMIUM GENETICS UK
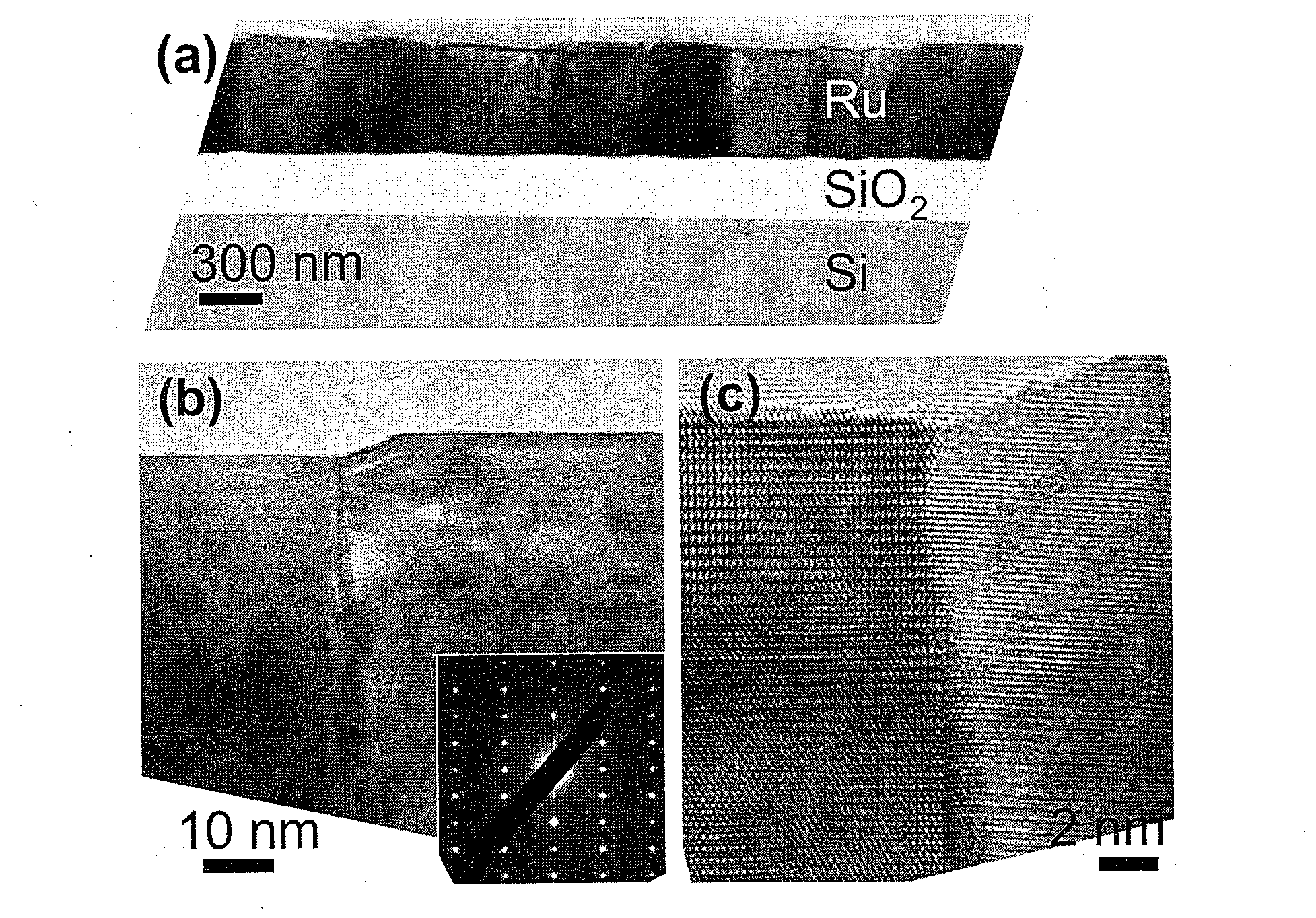
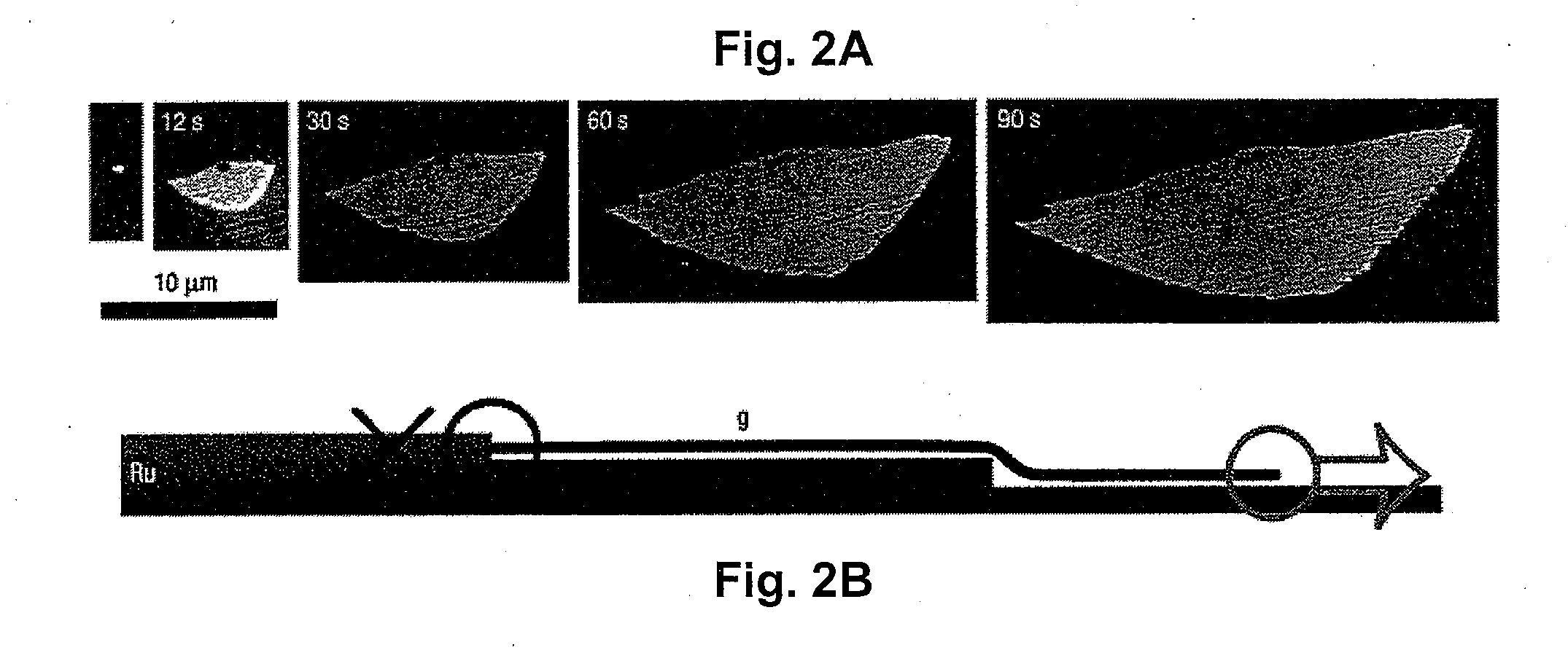
Monolayer and/or Few-Layer Graphene On Metal or Metal-Coated Substrates
InactiveUS20100255984A1Easy to disassembleMaterial nanotechnologyParticle separator tubesHigh concentrationIn plane
Graphene is a single atomic layer of sp2-bonded C atoms densely packed into a two-dimensional honeycomb crystal lattice. A method of forming structurally perfect and defect-free graphene films comprising individual mono crystalline domains with in-plane lateral dimensions of up to 200 μm or more is presented. This is accomplished by controlling the temperature-dependent solubility of interstitial C of a transition metal substrate having a suitable surface structure. At elevated temperatures, C is incorporated into the bulk at higher concentrations. As the substrate is cooled, a lowering of the interstitial C solubility drives a significant amount of C atoms to the surface where graphene islands nucleate and gradually increase in size with continued cooling. Ru(0001) is selected as a model system and electron microscopy is used to observe graphene growth during cooling from elevated temperatures. With controlled cooling, large arrays of macroscopic single-crystalline graphene domains covering the entire transition metal surface are produced. As the graphene domains coalesce to a complete layer, a second graphene layer is formed, etc. By controlling the interstitial C concentration and the cooling rate, graphene layers with thickness up to 10 atomic layers or more are formed in a controlled, layer-by-layer fashion.
Owner:BROOKHAVEN SCI ASSOCS
Particle beam irradiation system
ActiveUS20070228304A1Simplify device configurationEasy to controlLaser detailsHeart defibrillatorsIntensity controlSynchrotron
It is an object of the present invention to provide a charged particle beam extraction method and particle beam irradiation system that make it possible to exercise intensity control over an extracted ion beam while a simple device configuration is employed. To accomplish the above object, there is provided a particle beam irradiation system comprising: a synchrotron for accelerating and extracting an charged particle beam; an irradiation apparatus for extracting the charged particle beam that is extracted from the synchrotron; first beam intensity modulation means for controlling the beam intensity of the charged particle beam extracted from the synchrotron during an extraction control period of an operation cycle of the synchrotron; and second beam intensity modulation means for controlling the beam intensity during each of a plurality of irradiation periods contained in the extraction control period of the operation cycle.
Owner:HITACHI LTD
Particle beam irradiation system
ActiveUS20090283704A1Simplify device configurationEasy to controlLaser detailsRadiation/particle handlingParticle beamIon beam
It is an object of the present invention to provide a charged particle beam extraction method and particle beam irradiation system that make it possible to exercise intensity control over an extracted ion beam while a simple device configuration is employed. To accomplish the above object, there is provided a particle beam irradiation system comprising: a synchrotron for accelerating and extracting an charged particle beam; an irradiation apparatus for extracting the charged particle beam that is extracted from the synchrotron; first beam intensity modulation means for controlling the beam intensity of the charged particle beam extracted from the synchrotron during an extraction control period of an operation cycle of the synchrotron; and second beam intensity modulation means for controlling the beam intensity during each of a plurality of irradiation periods contained in the extraction control period of the operation cycle.
Owner:HITACHI LTD
Sensor instrument system including method for detecting analytes in fluids
ActiveUS8449824B2Novel and uniqueSimple designLaboratory glasswaresNanosensorsTransceiverHydrogen selectivity
A sensor instrument system for detecting and identifying analytes in fluids of a region contains a local sensor instrument and remote central station. The instrument includes a core technology employing a single sensor having two electrodes operated by an electrical frequency sweeping to generate two sets of patterned electrical information from a single measurement, a data transmission module and a GPS receiver module. The central station connects to a network means connected to a plurality of local receiving sites equipped with including the respective transceivers, so that the local analyte electrical information and geographic position information transmitted by the instrument can be wirelessly and remotely received and processed by the central station. The core technology further includes a reference sensor for eliminating background influence, a temperature programming to control adsorption and desorption of analytes, and usage of all kinds of adsorbent materials having chemical selectivities including the hydrogen selectivity to enhance detection and identification of analytes in fluids.
Owner:SUN YIZHONG
Microfluidic device for cell and particle separation
A microfluidic separation device includes a microchannel formed in a substrate and being defined at least by a bottom surface, a first side wall, and second side wall. Fluid containing particles or cells is flowed through the microchannel from an upstream end to a downstream end. The downstream end terminates in a plurality of branch channels. A plurality of vertically-oriented electrodes are disposed on the first wall and on the second wall opposite to the first wall. A voltage source is connected to the plurality of opposing electrodes to drive the electrodes. The opposing, vertically-oriented electrodes may be used to focus a heterogeneous population of particles or cells for subsequent downstream separation via additional electrodes placed on one of the side walls. Alternatively, the opposing, vertically-oriented electrodes may be used to spatially separate a heterogeneous population of particles or cells for later collection in one or more of the branch channels.
Owner:RGT UNIV OF CALIFORNIA
Optical microfluidic devices and methods
InactiveUS20030047688A1Optical radiation measurementElectrolysis componentsTrappingElectromagnetic radiation
The invention relates to microfluidic devices and methods that employ electromagnetic radiation to move a droplet of fluid on a fluid-transporting surface of a substrate. Typically, radiation of a particular wavelength is directed through a substantially transparent material, and the radiation imparts an optical trapping force to move the droplet. In addition, a means for reducing evaporative loss from the droplet may be provided.
Owner:SRI INTERNATIONAL
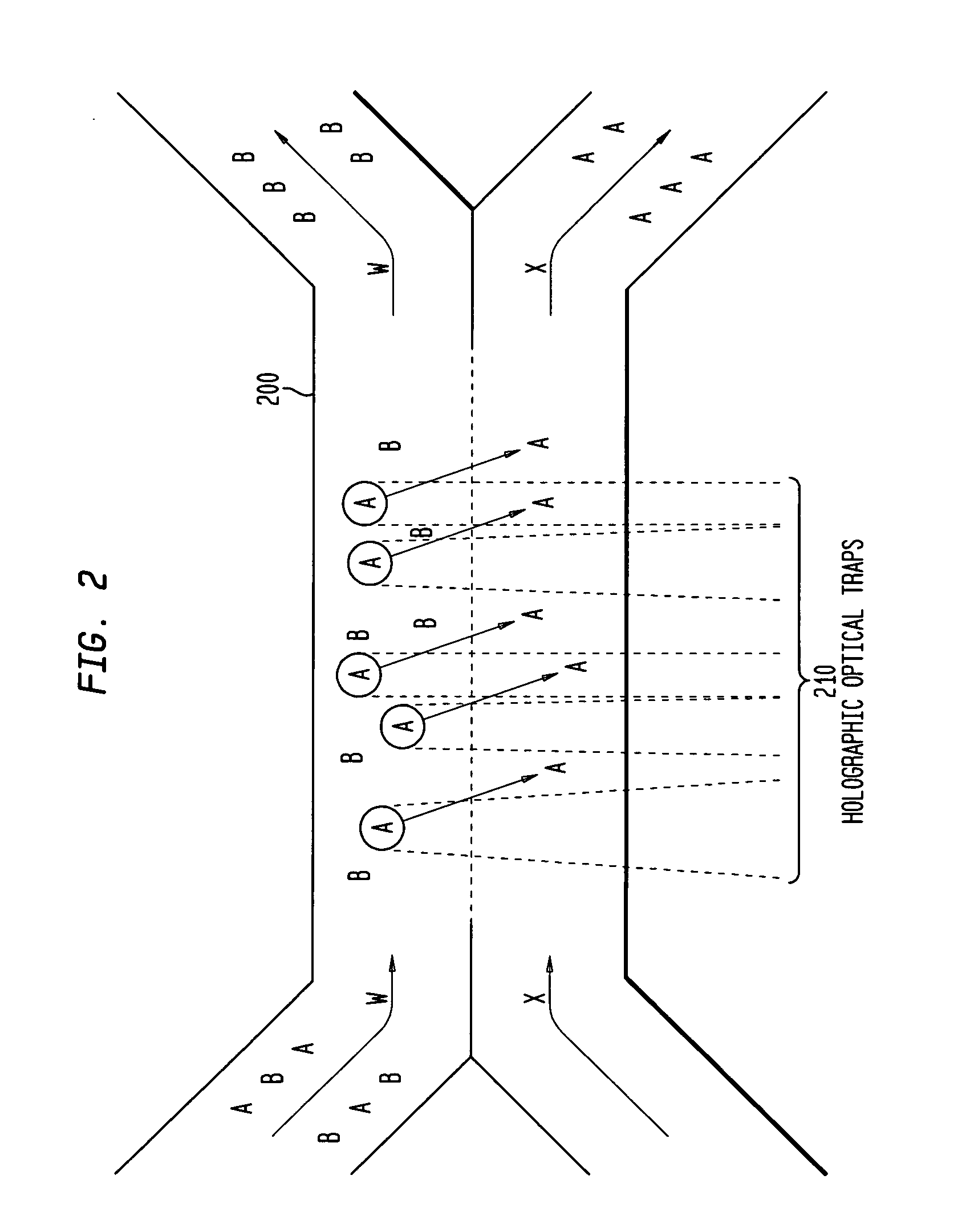
Multiple laminar flow-based rate zonal or isopycnic separation with holographic optical trapping of blood cells and other static components
ActiveUS20050061962A1Improve throughputSave a lot of timeDielectrophoresisOther blood circulation devicesCellular componentBlood component
The invention provides a method and apparatus for separating blood into components, may be expanded to include other types of cellular components, and can be combined with holographic optical manipulation or other forms of optical tweezing. One of the exemplary methods includes providing a first flow having a plurality of blood components; providing a second flow; contacting the first flow with the second flow to provide a first separation region; and differentially sedimenting a first blood cellular component of the plurality of blood components into the second flow while concurrently maintaining a second blood cellular component of the plurality of blood components in the first flow. The second flow having the first blood cellular component is then differentially removed from the first flow having the second blood cellular component. Holographic optical traps may also be utilized in conjunction with the various flows to move selected components from one flow to another, as part of or in addition to a separation stage.
Owner:ABS GLOBAL
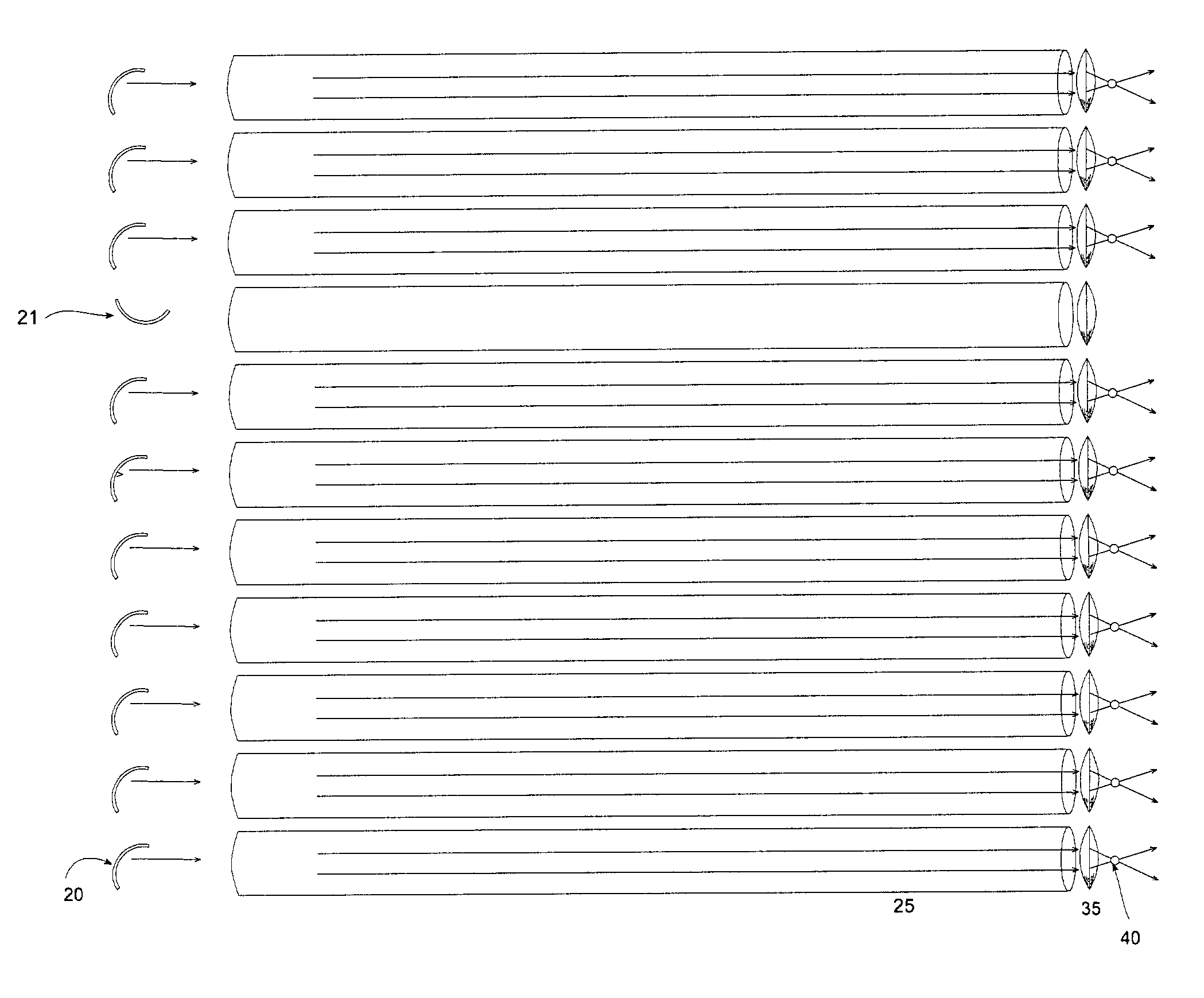
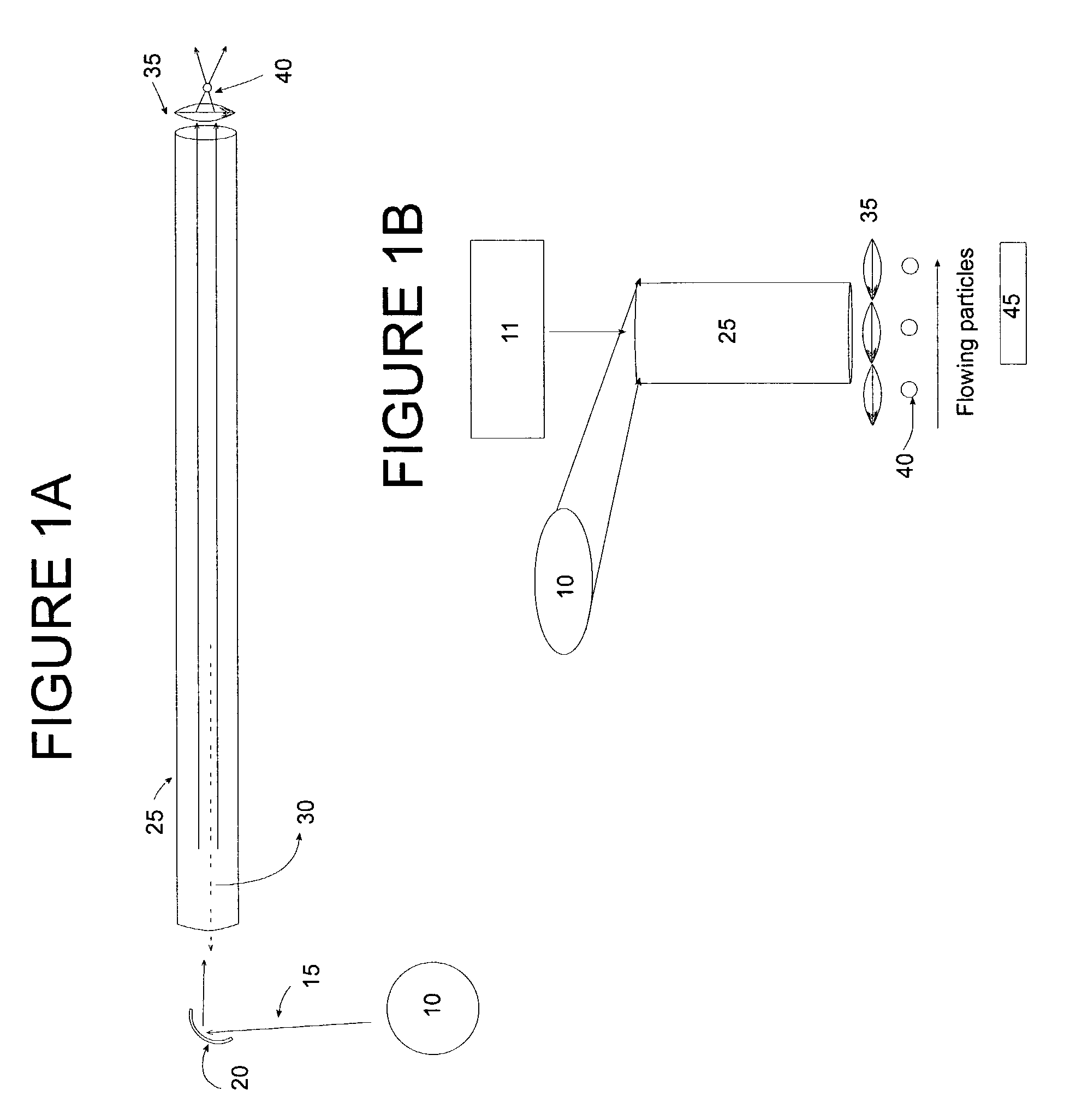
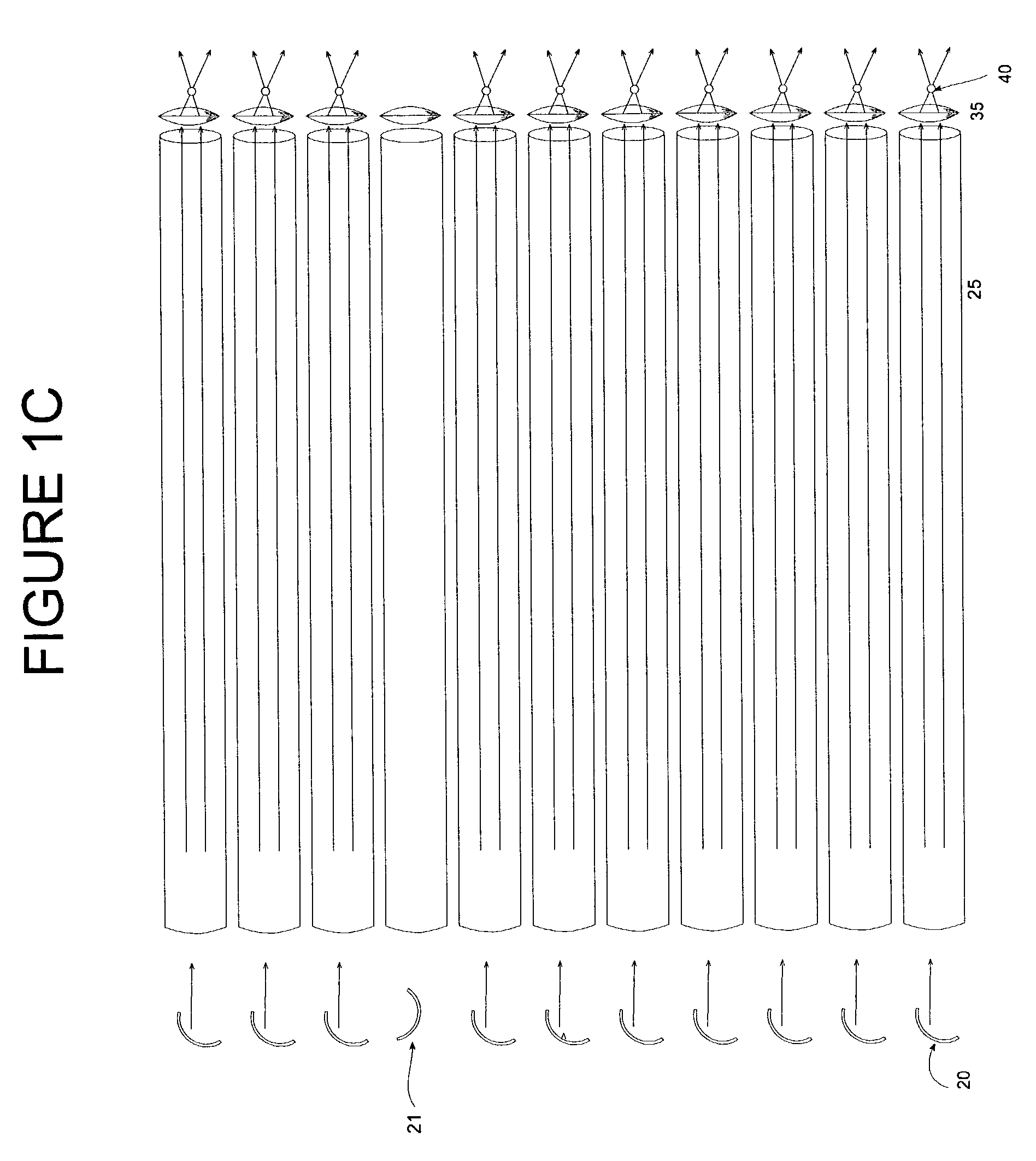
Optical array device and methods of use thereof for screening, analysis and manipulation of particles
InactiveUS6991939B2The process is simple and clearEasily addressableOptical radiation measurementBioreactor/fermenter combinationsFiberOptical property
Methods and devices are provided for the trapping, including optical trapping; analysis; and selective manipulation of particles on an optical array. A multi-channel device parcels a light source into many points of light transmitted through an optical array of fibers or conduits, preferably where the individual points of light are individually controllable through a light controlling device. Optical properties of the particles may be determined by interrogation with light focused through the optical array. The particles may be manipulated by immobilizing or releasing specific particles, separating types of particles, etc.
Owner:TUFTS UNIV
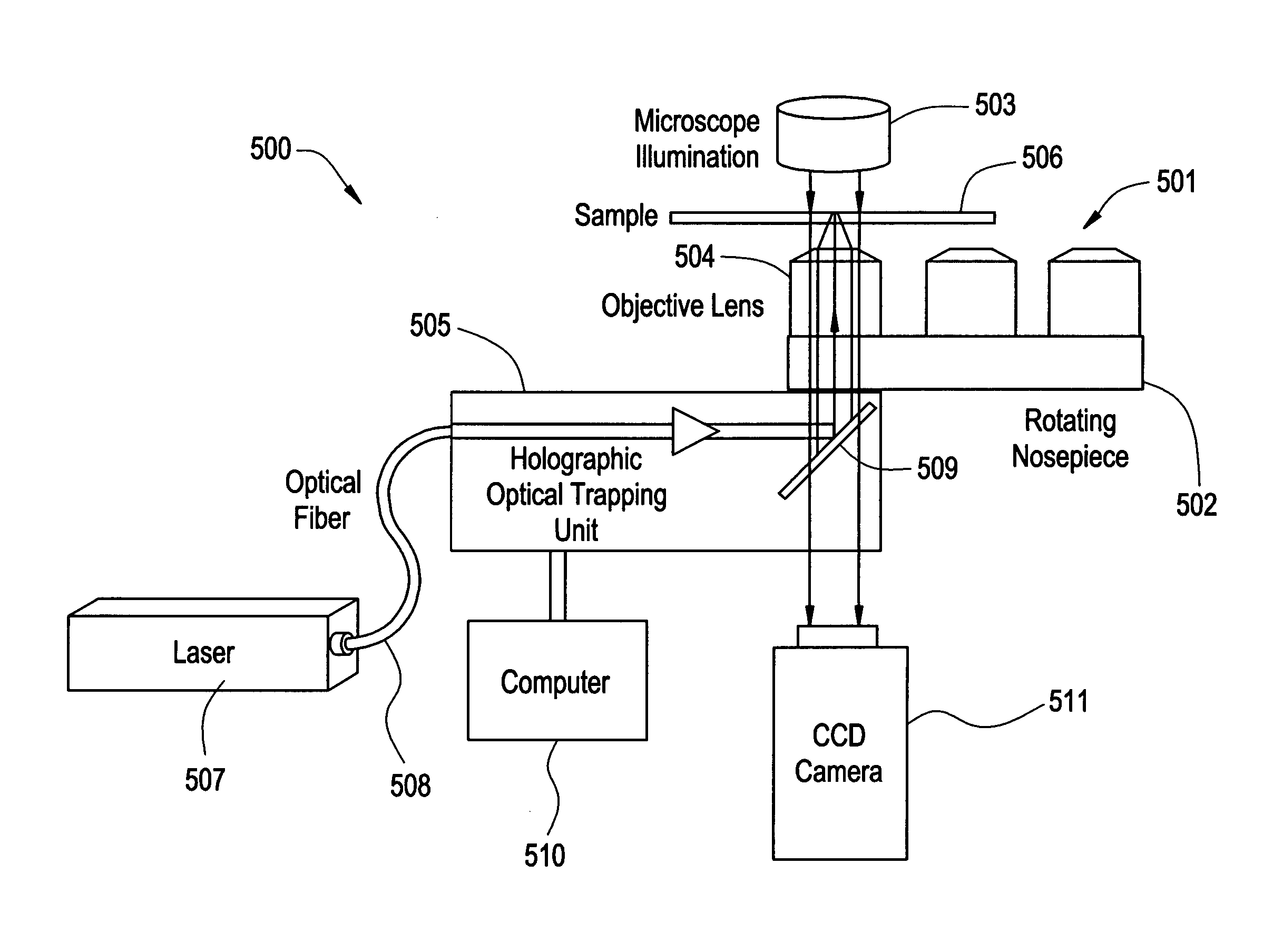
System and method for manipulating and processing materials using holographic optical trapping
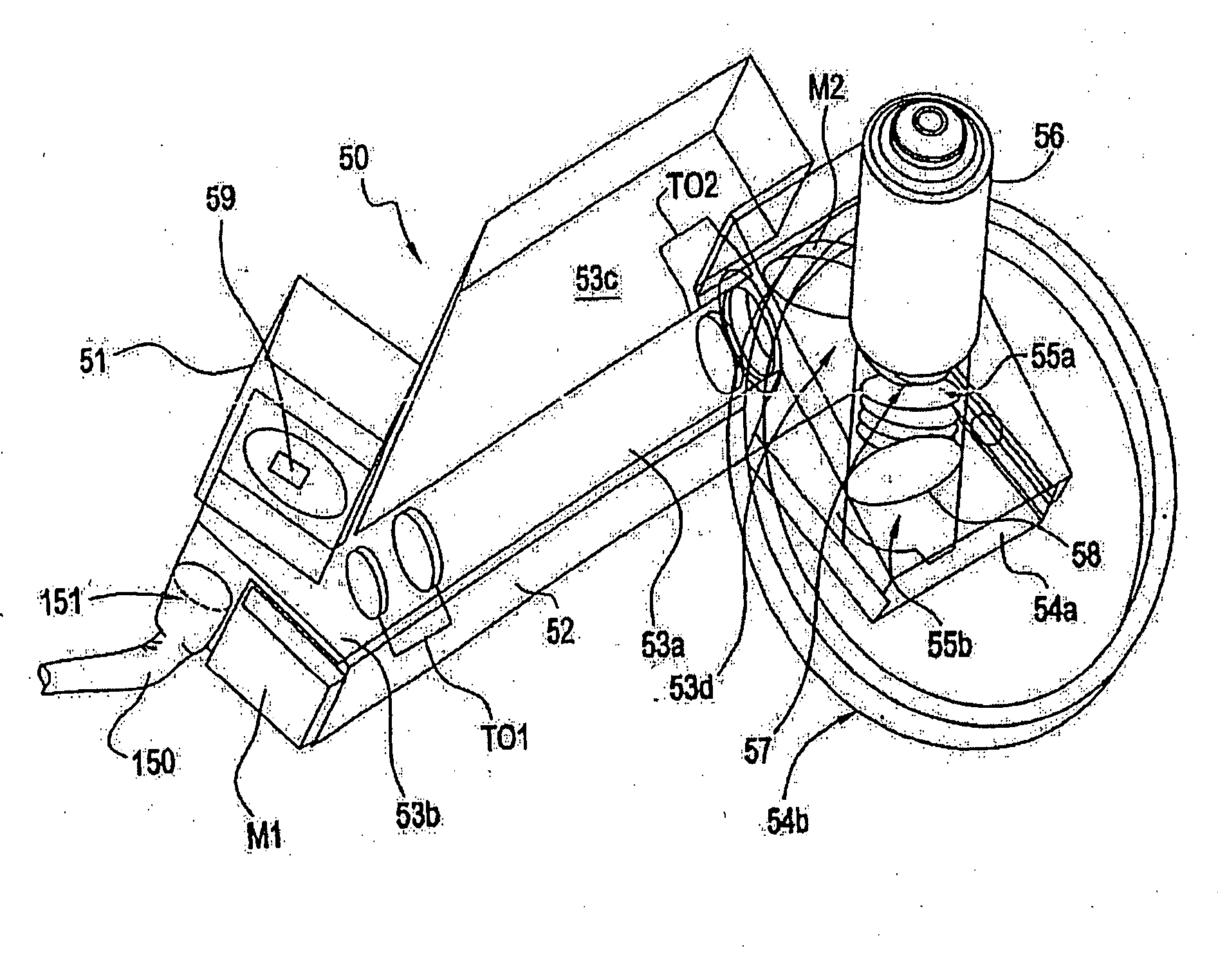
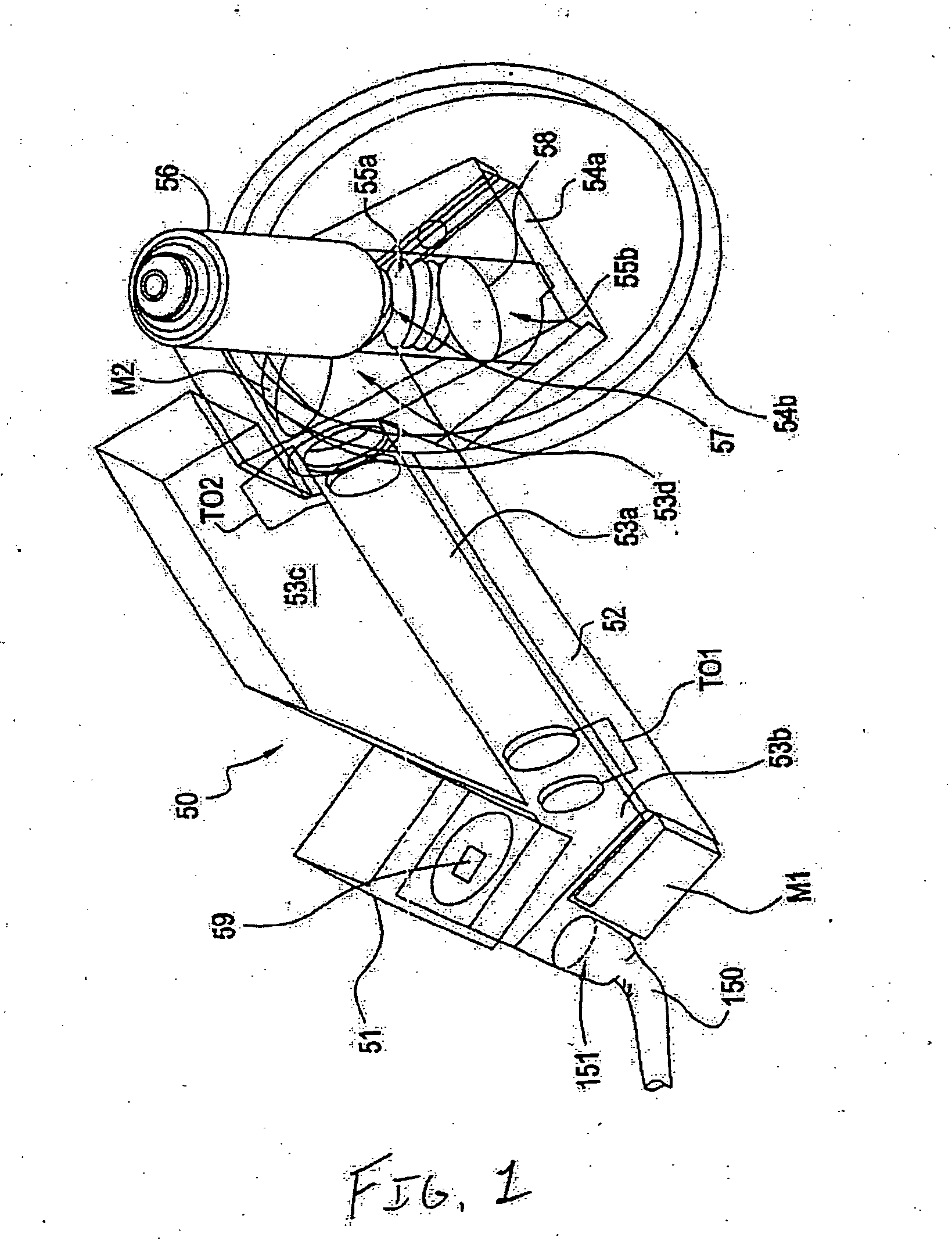
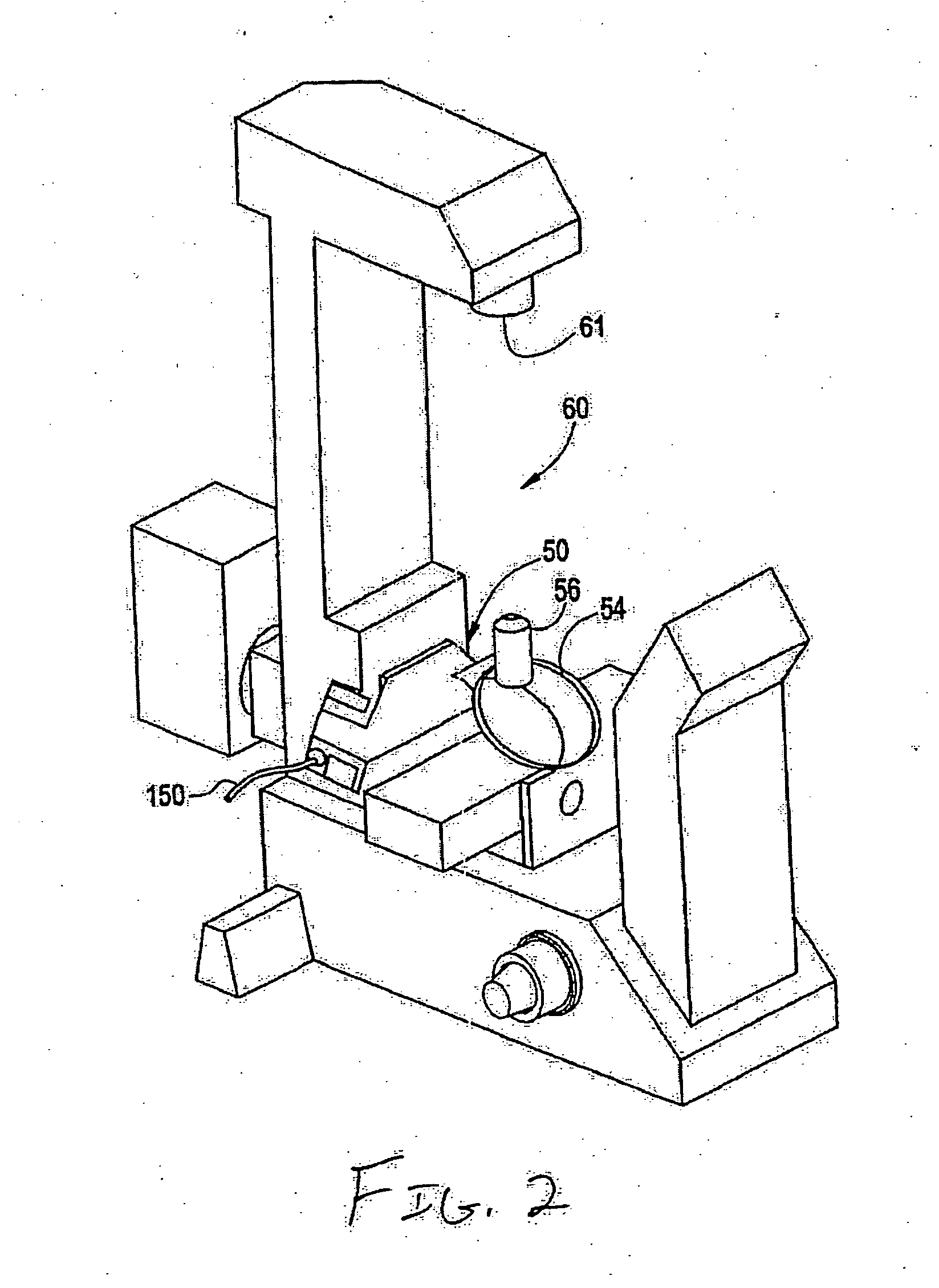
InactiveUS20050122550A1Enhance variety of techniqueFacilitate manipulationLaser detailsDiffraction gratingsTrappingLight beam
A method an apparatus for manipulating particles (micro, nano, and pico) having one or more characteristics with an optical trap formed by modulating a laser beam with a Diffractive Optical Element (DOE). At least one characteristic of the material is selected; and a laser beam having a selected wavelength corresponding to the at least one selected characteristic of the material is generated. Values of the DOE are calculated corresponding to the at least one selected characteristic of the material. The beam and the DOE are modulated to produce a holographic optical trap having properties corresponding to the at least one selected characteristic; the trap is focused to a beam focus or selected spot size; and the beam focus is located near a particle location for trapping the particle therein.
Owner:ARRYX INC
System and method of sorting materials using holographic laser steering
InactiveUS7241988B2Less powerEliminates hot spotBioreactor/fermenter combinationsLaser detailsLight beamEngineering
The present invention employs a beam steering apparatus to isolate valuable cells from other cells, tissues, and contaminants. In one embodiment, the system balances optical trapping against biasing flow to parallelize cell sorting under the flexible control of computer program-directed traps which differentially manipulate cells based on their composition or labels to direct separation.
Owner:PREMIUM GENETICS UK
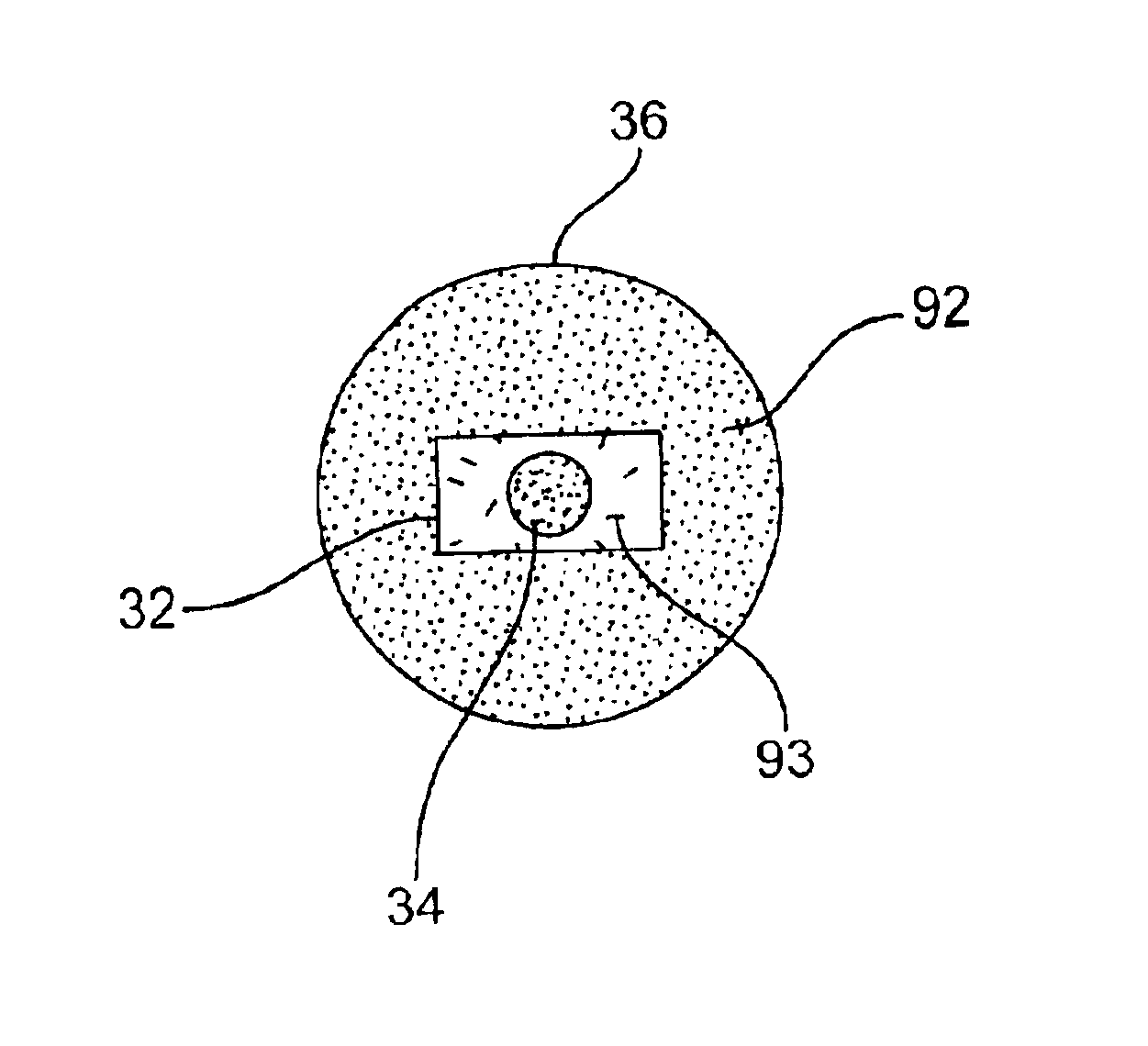
Cladding-pumped 3-level fiber laser/amplifier
InactiveUS6836607B2Optical wave guidanceLaser using scattering effectsThree levelAudio power amplifier
An optically active fiber (30) is disclosed for making a fiber laser (18) or an amplifier (16). This double-clad structured active fiber (30) has a core (34), doped with an optically excitable ion having a three-level transition. The core (34) has a core refractive index and a core cross-sectional area. An inner cladding (32) surrounds the core (34). The inner cladding (32) has an inner cladding refractive index less than the core refractive index, an inner cladding cross-sectional area between 2 and 25 times greater than that of the core cross-sectional area, and an aspect ratio greater than 1.5:1. An outer cladding (36) surrounds the inner cladding (32) and has an outer cladding refractive index less than the inner cladding refractive index.
Owner:CORNING INC
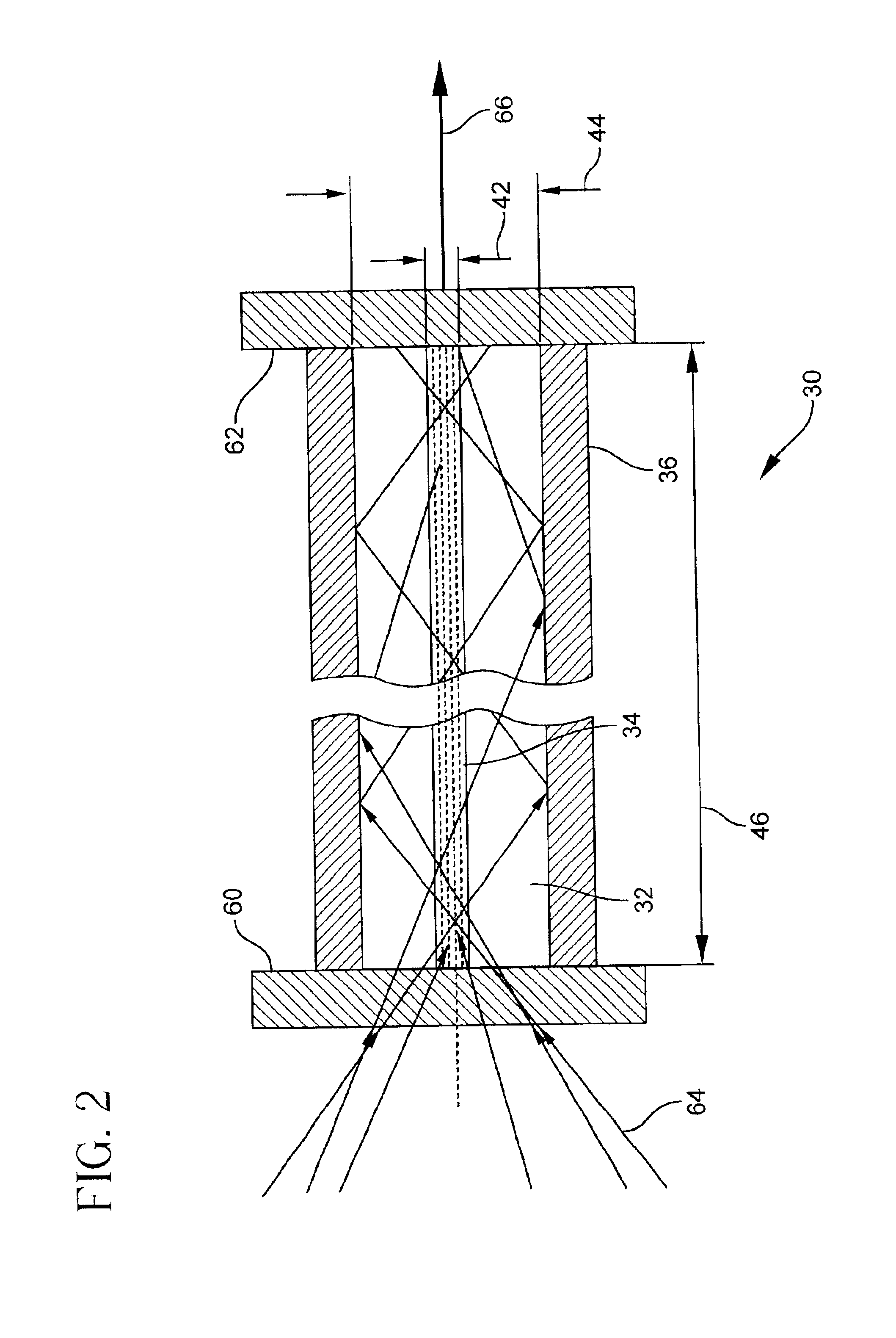
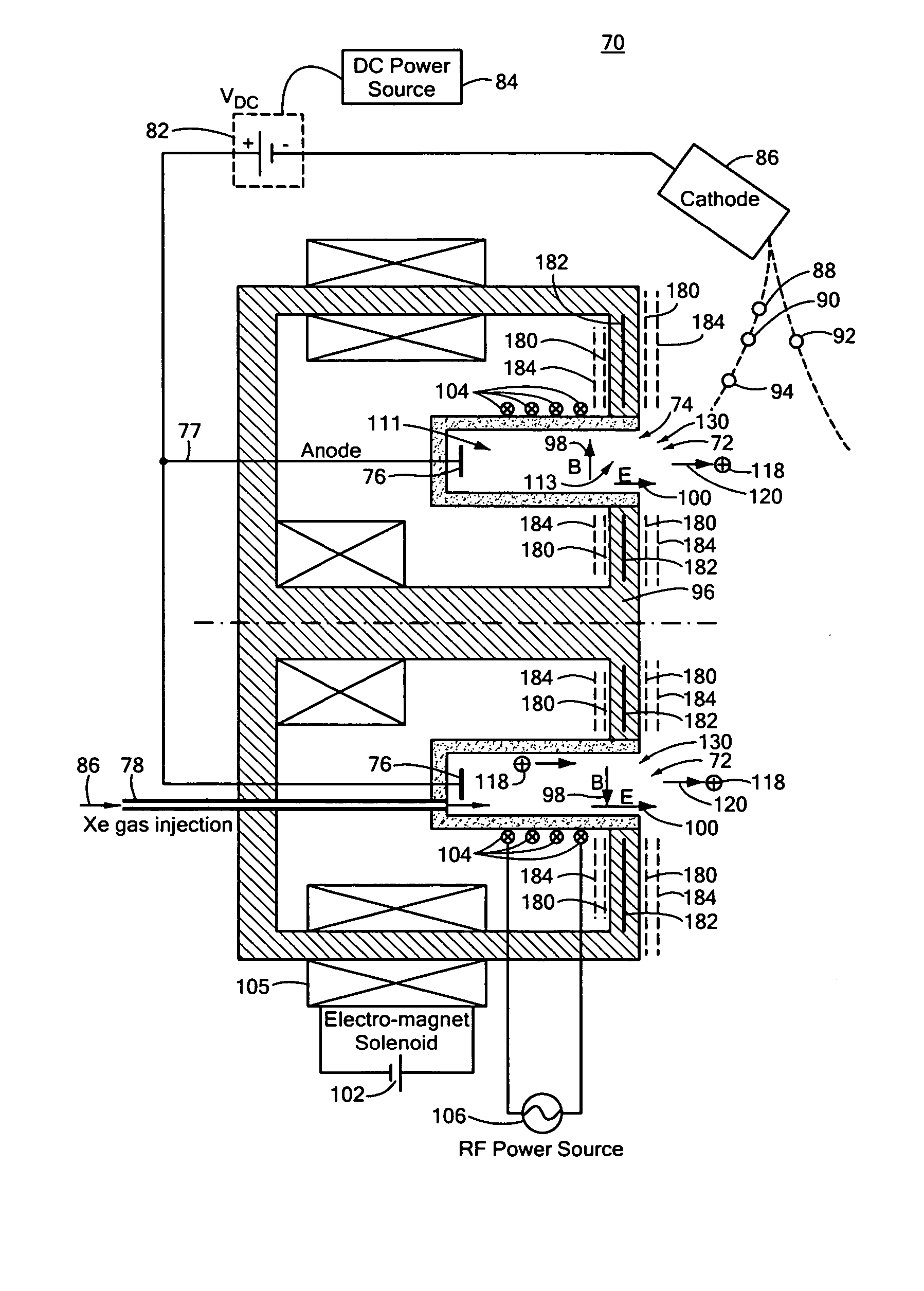
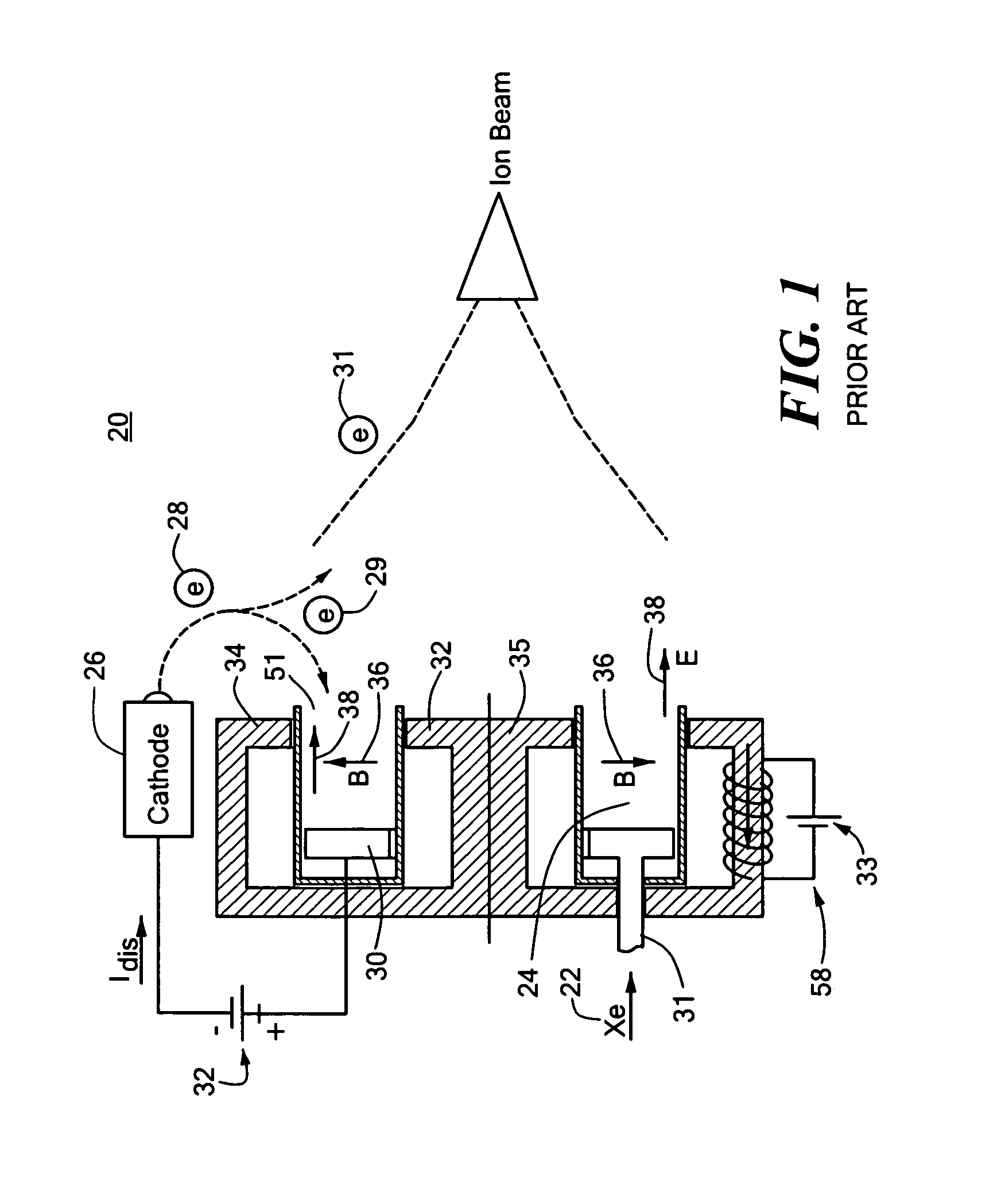
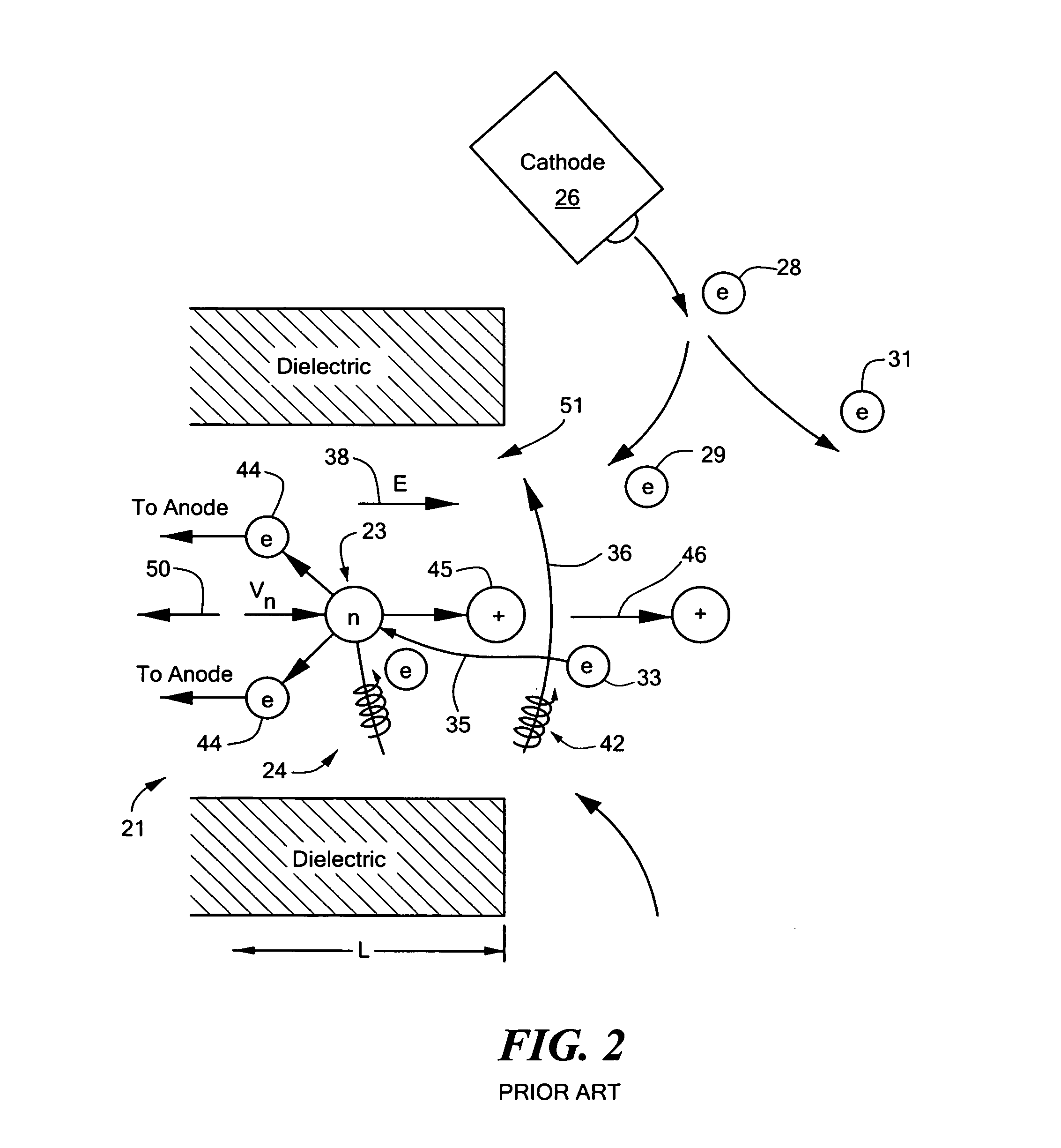
Combined radio frequency and hall effect ion source and plasma accelerator system
ActiveUS20060284562A1Improve efficiencyEliminate needLaser detailsMaterial analysis by optical meansTransverse magnetic fieldElectrical impedance
This invention features a combined radio frequency (RF) and Hall Effect ion source and plasma accelerator system including a plasma accelerator having an anode and a discharge zone, the plasma accelerator for providing plasma discharge. A gas distributor introduces a gas into the plasma accelerator. A cathode emits electrons attracted to the anode for ionizing the gas and neutralizing ion flux emitted from the plasma accelerator. An electrical circuit coupled between the anode and the cathode having a DC power source provides DC voltage. A magnetic circuit structure including a magnetic field source establishes a transverse magnetic field in the plasma accelerator that creates an impedance to the flow of the electrons toward the anode to enhance ionization of the gas to create plasma and which in combination with the electric circuit establishes an axial electric field in the plasma accelerator. An RF power source provides RF power to at least one electrode disposed about and / or inside the plasma accelerator that induces current for ionizing the gas to create the plasma such that the axial electric field accelerates ions through the plasma accelerator to provide ion flux.
Owner:BUSEK
Repetitive circumferential milling for sample preparation
ActiveUS7442924B2Increase ratingsShorten the timeParticle separator tubesRecord information storageIon beamLight beam
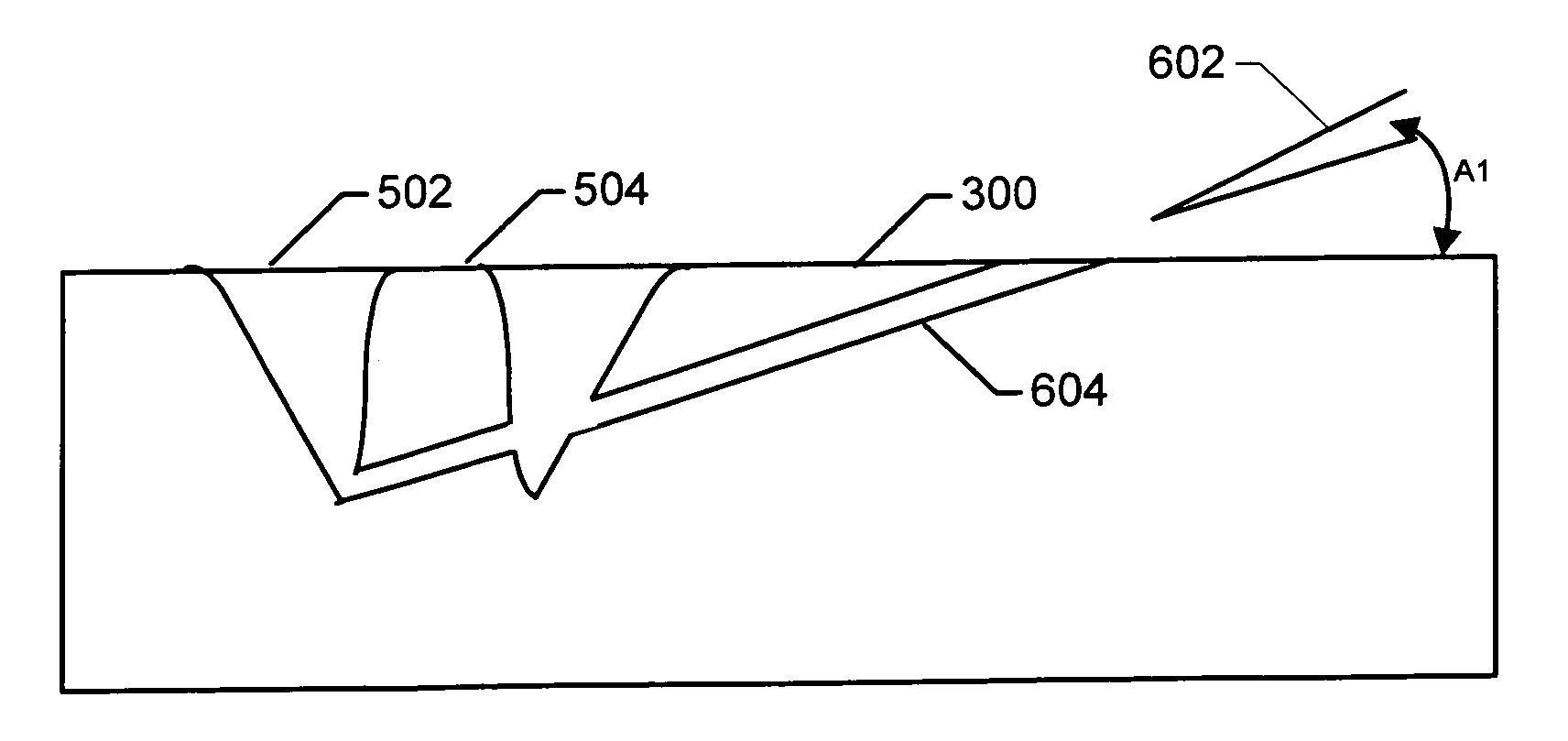
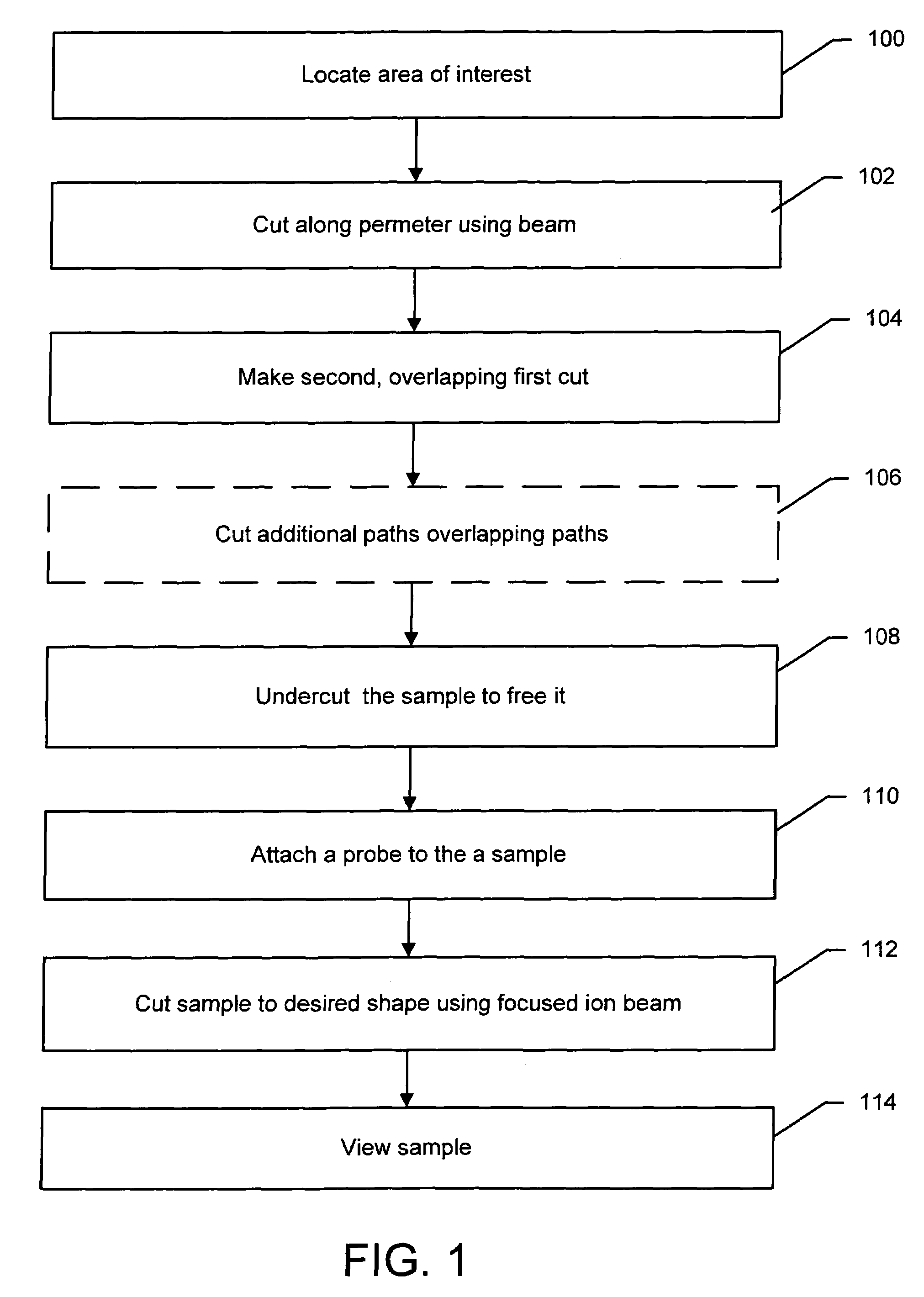
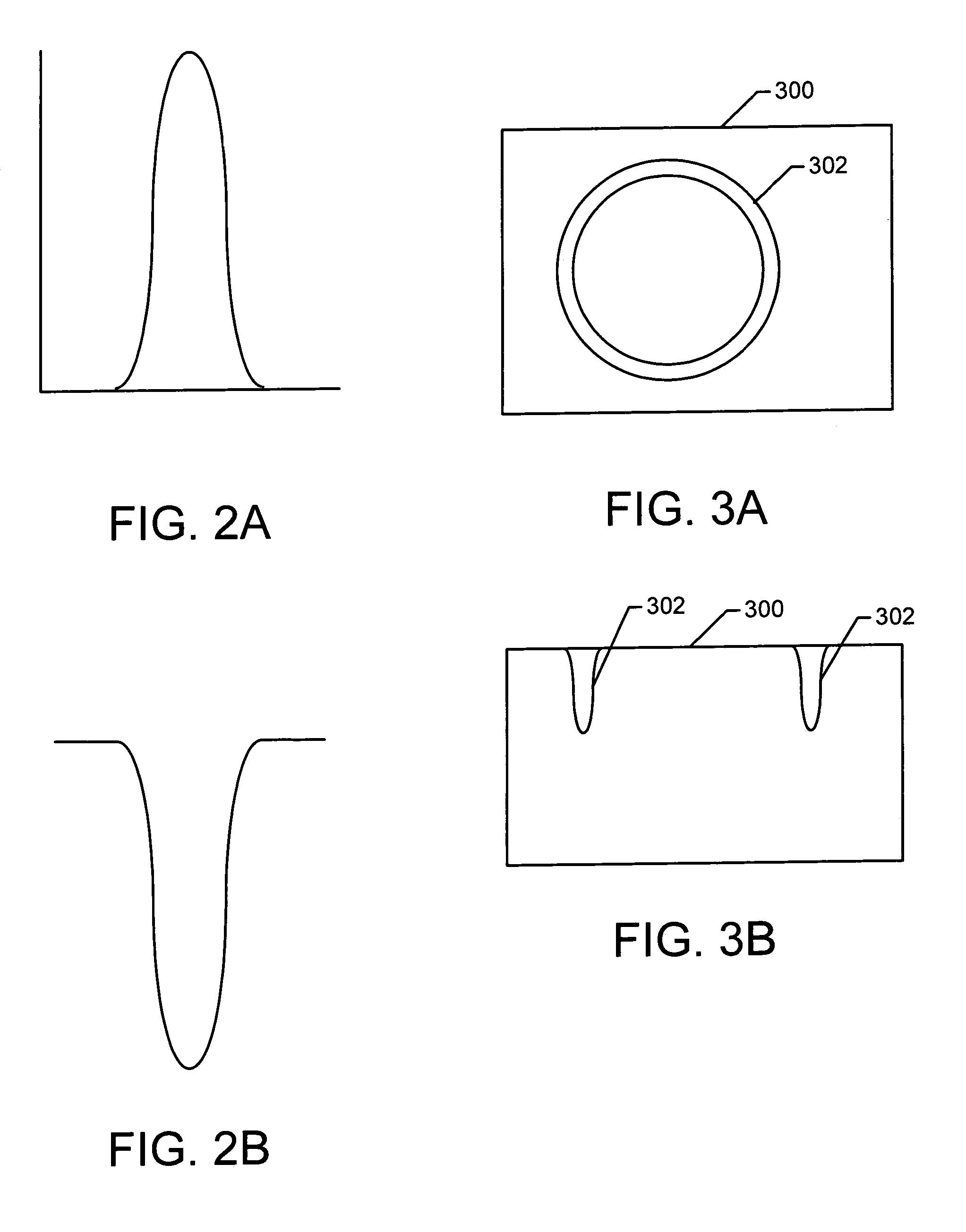
A method of sample extraction entails making multiple, overlapping cuts using a beam, such as a focused ion beam, to create a trench around a sample, and then undercutting the sample to free it. Because the sidewalls of the cut are not vertical, the overlapping cuts impinge on the sloping sidewalls formed by previous cuts. The high angle of incidence provides a greatly enhanced mill rate, so that making multiple overlapping cuts to produce a wide trench can requires less time than making a single, deep cut around the perimeter of a sample.
Owner:FEI CO
Thin film coated microwell arrays and methods of using same
InactiveUS7682816B2High densityFunction increaseBioreactor/fermenter combinationsNanotechOptoelectronicsFilm-coated tablet
Owner:454 LIFE SCIENCES CORP
Controlled fusion in a field reversed configuration and direct energy conversion
InactiveUS6852942B2Facilitates controlled fusionReduce eliminate anomalous transportLaser detailsParticle separator tubesNuclear forcePlasma electron
A system and apparatus for controlled fusion in a field reversed configuration (FRC) magnetic topology and conversion of fusion product energies directly to electric power. Preferably, plasma ions are magnetically confined in the FRC while plasma electrons are electrostatically confined in a deep energy well, created by tuning an externally applied magnetic field. In this configuration, ions and electrons may have adequate density and temperature so that upon collisions they are fused together by the nuclear force, thus forming fusion products that emerge in the form of an annular beam. Energy is removed from the fusion product ions as they spiral past electrodes of an inverse cyclotron converter. Advantageously, the fusion fuel plasmas that can be used with the present confinement and energy conversion system include advanced (aneutronic) fuels.
Owner:RGT UNIV OF CALIFORNIA +1
Photoelectrocatalytic fluid analyte sensors and methods of fabricating and using same
Fluid analyte sensors include a photoelectrocatalytic element that is configured to be exposed to the fluid, if present, and to respond to photoelectrocatalysis of at least one analyte in the fluid that occurs in response to impingement of optical radiation upon the photoelectrocatalytic element. A semiconductor light emitting source is also provided that is configured to impinge the optical radiation upon the photoelectrocatalytic element. Related solid state devices and sensing methods are also described.
Owner:VALENCELL INC
Automated system for formulating radiopharmaceuticals
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Apparatuses and methods for generating coherent electromagnetic laser radiation
The present disclosure is directed to laser apparatuses for generating coherent electromagnetic laser radiation having an electron beam generator, a diffraction grating element oriented such that a beam of electrons from the electron beam generator is directed over the diffraction grating element, and at least one wing element coupled to the diffraction grating element. In some embodiments, the wing element(s) can be coupled to a top portion of the diffraction grating element. While in others, the wing element(s) can be coupled to a side portion of the diffraction grating element. The present disclosure is also directed to methods of manufacturing diffraction grating elements involving placing at least one secondary conducting sheet having a first height on at least one primary conductive sheet having a different second height, and securing the primary and secondary conductive sheets together. The primary and secondary conductive sheets can be alternating and their thicknesses may also be different. Additionally, the primary and secondary conductive sheets may be secured via a clamping device.
Owner:VERMONT PHOTONICS
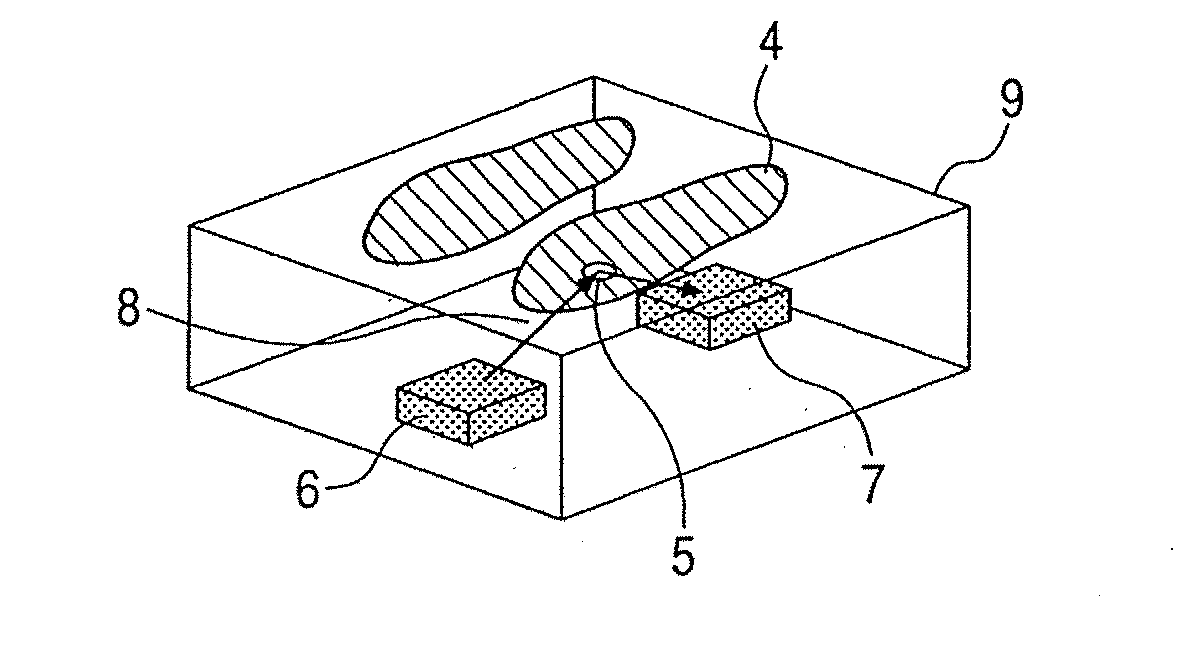
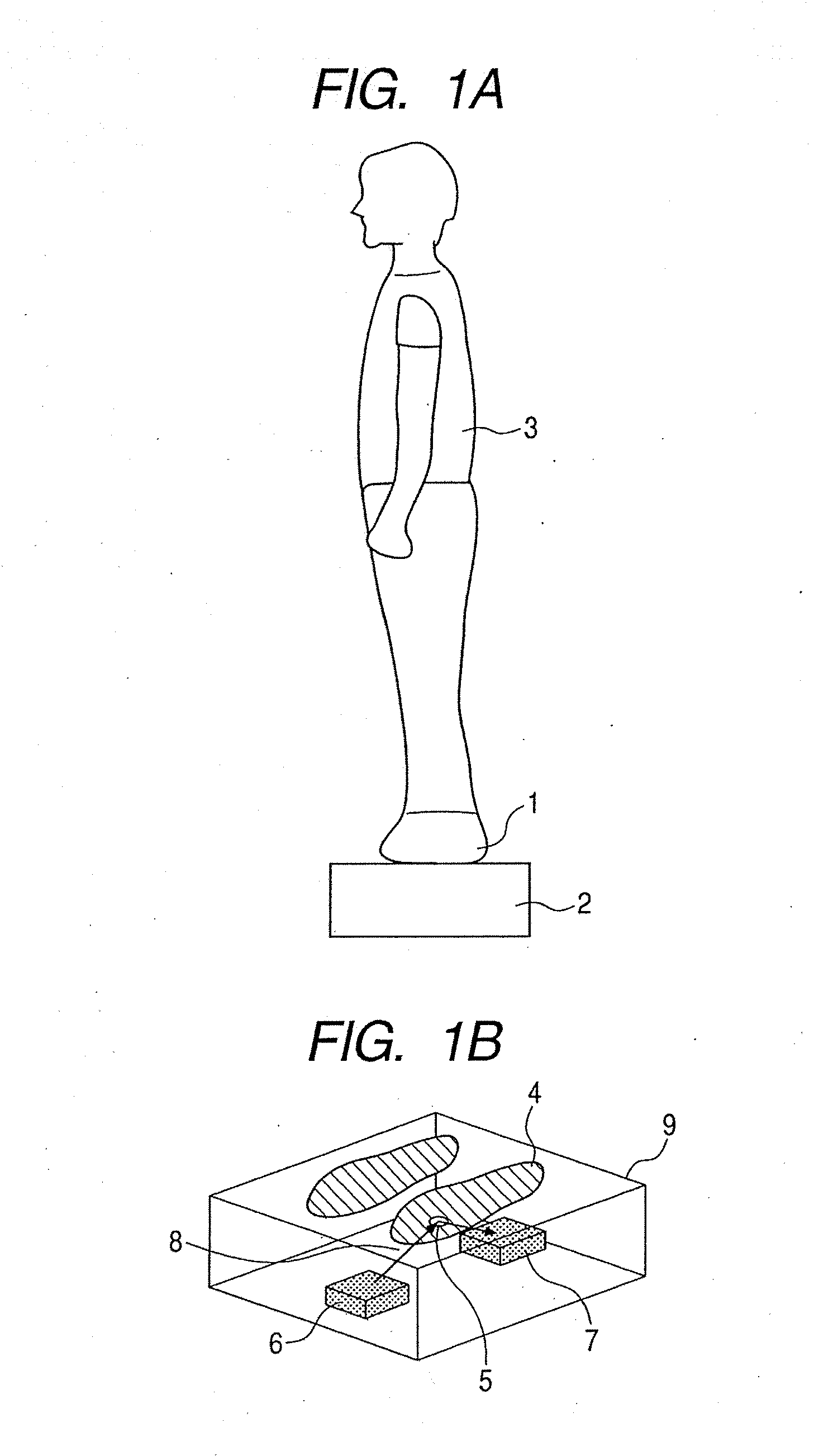
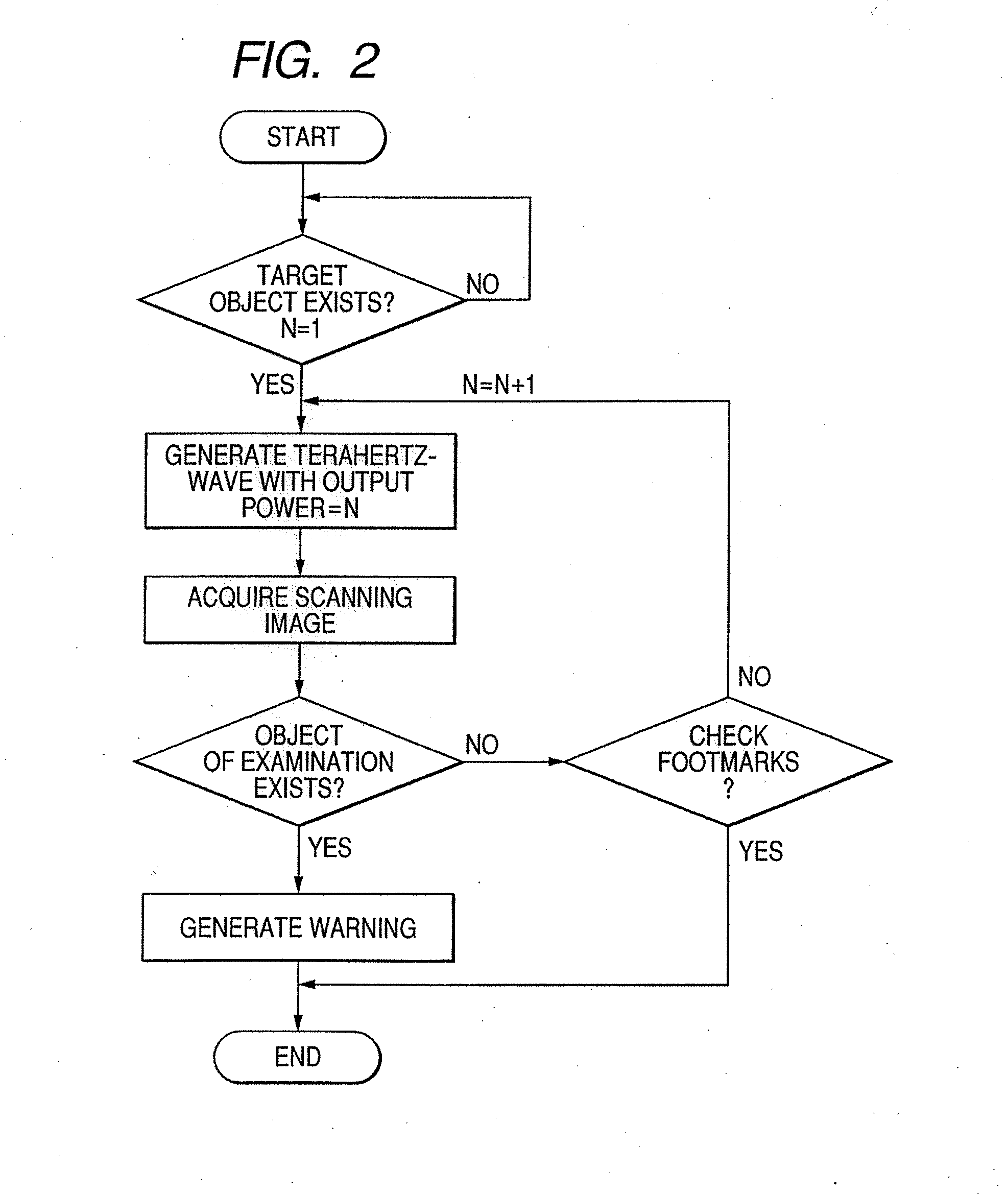
Ojbect information acquisition apparatus and object information acquisition method
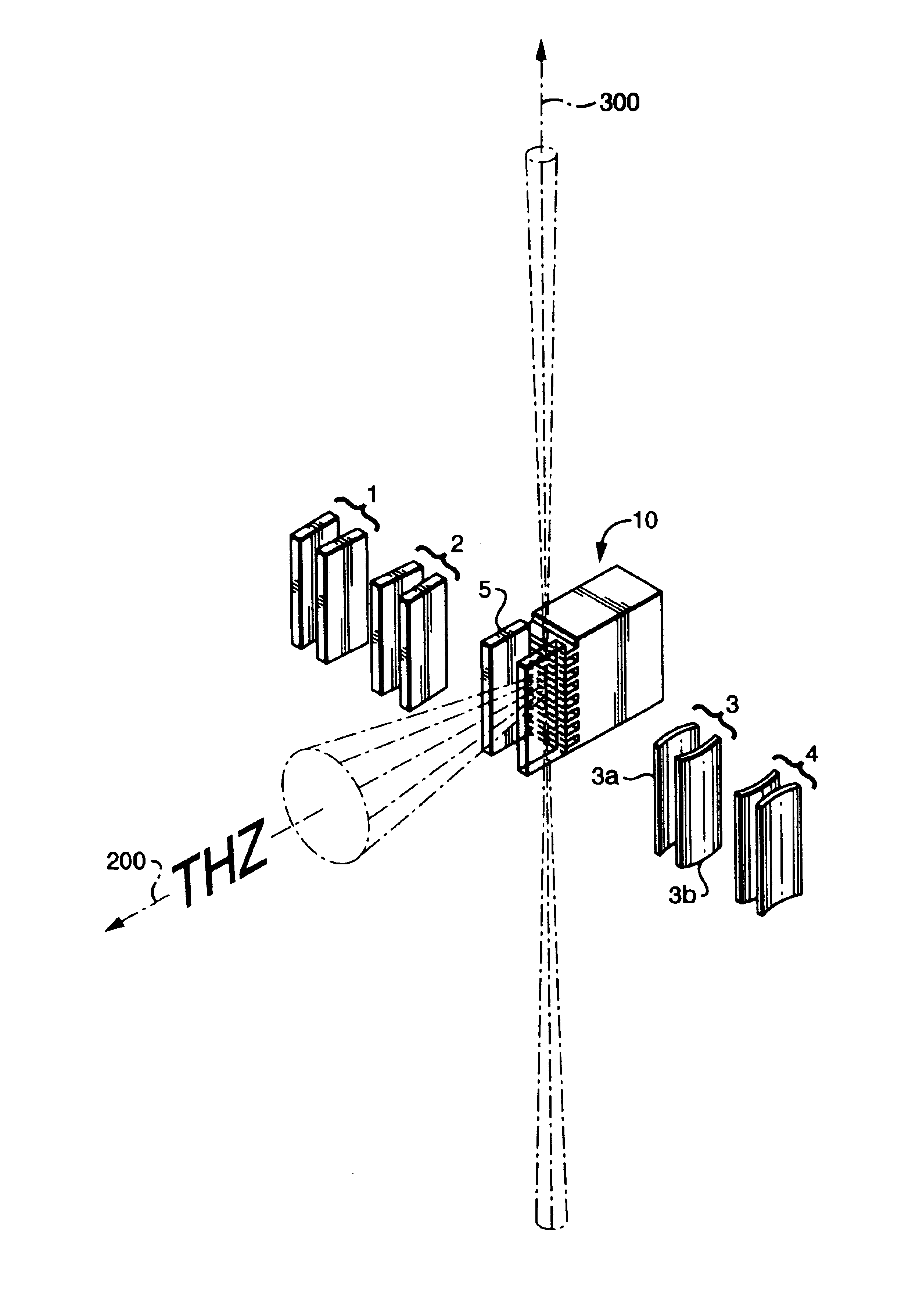
InactiveUS20080116374A1Appropriately convergeQuickly and relatively easily acquire informationLaser detailsMasersIrradiationInformation acquisition
An object information acquisition apparatus for acquiring information on the inside of an object includes an electromagnetic wave generation unit capable of outputting a terahertz-wave and of changing the output intensity, an irradiation unit that irradiates an electromagnetic wave onto an object, a scanning unit and a detection unit that detects the electromagnetic wave irradiated onto the object. The scanning unit changes the relative positions of the irradiated electromagnetic wave and the object. The detection unit detects the electromagnetic wave transmitted through or reflected by the object as a result of interaction of the object and the electromagnetic wave.
Owner:CANON KK
Portable laser device
InactiveUS20050085802A1Improve cooling effectSurgical instrument detailsMasersInterior spaceLaser transmitter
A hand-held laser device includes a casing formed with a substantially hollow interior space and having a laser emitter thereinside. The laser emitter is formed with an exciting lamp and a laser rod. A source generating a stream of gaseous coolant is provided within the interior space. A fluid cooling arrangement at least partially surrounding the laser rod is disposed within the stream of gaseous coolant for heat removal therefrom.
Owner:INNOTECH USA
Sensor instrument system including method for detecting analytes in fluids
ActiveUS20090261987A1Improves analyte detectionEasy to identifyElectric signal transmission systemsLaboratory glasswaresTransceiverDesorption
A sensor instrument system for detecting and identifying analytes in fluids of a region comprising a local sensor instrument and remote central station. The instrument is comprised of a core technology employing a single sensor having two electrodes operated by an electrical frequency sweeping to generate two sets of patterned electrical information from a single measurement, a data transmission module and a GPS receiver module. The central station connects to a network means connected to a plurality of local receiving sites equipped with including the respective transceivers, so that the local analyte electrical information and geographic position information transmitted by the instrument can be wirelessly and remotely received and processed by the central station. The core technology is further comprised of a reference sensor for eliminating background influence, a temperature programming to control adsorption and desorption of analytes, and usage of all kinds of adsorbent materials having chemical selectivity including the hydrogen selectivity to enhance detection and identification of analytes in fluids.
Owner:SUN YIZHONG
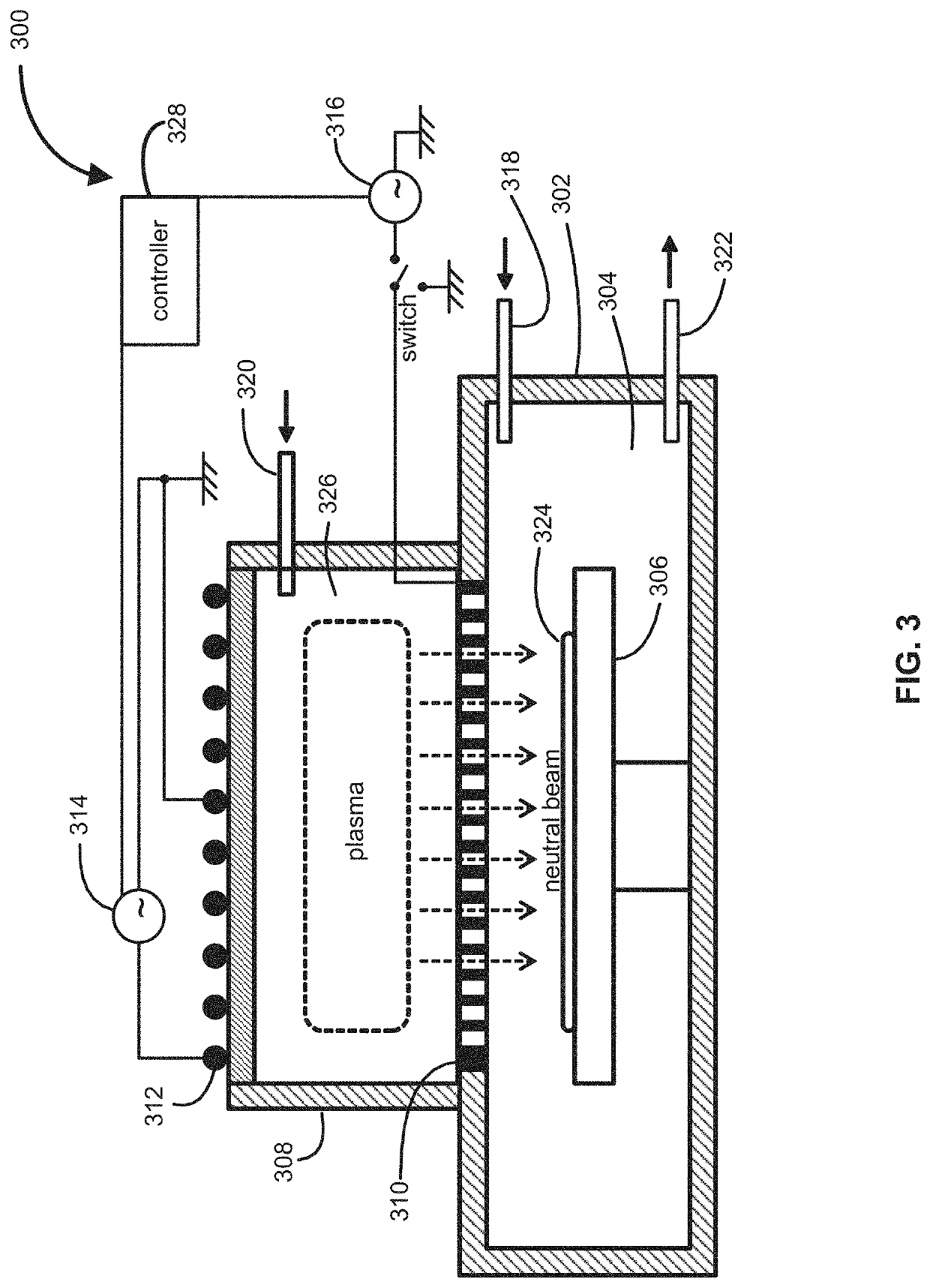
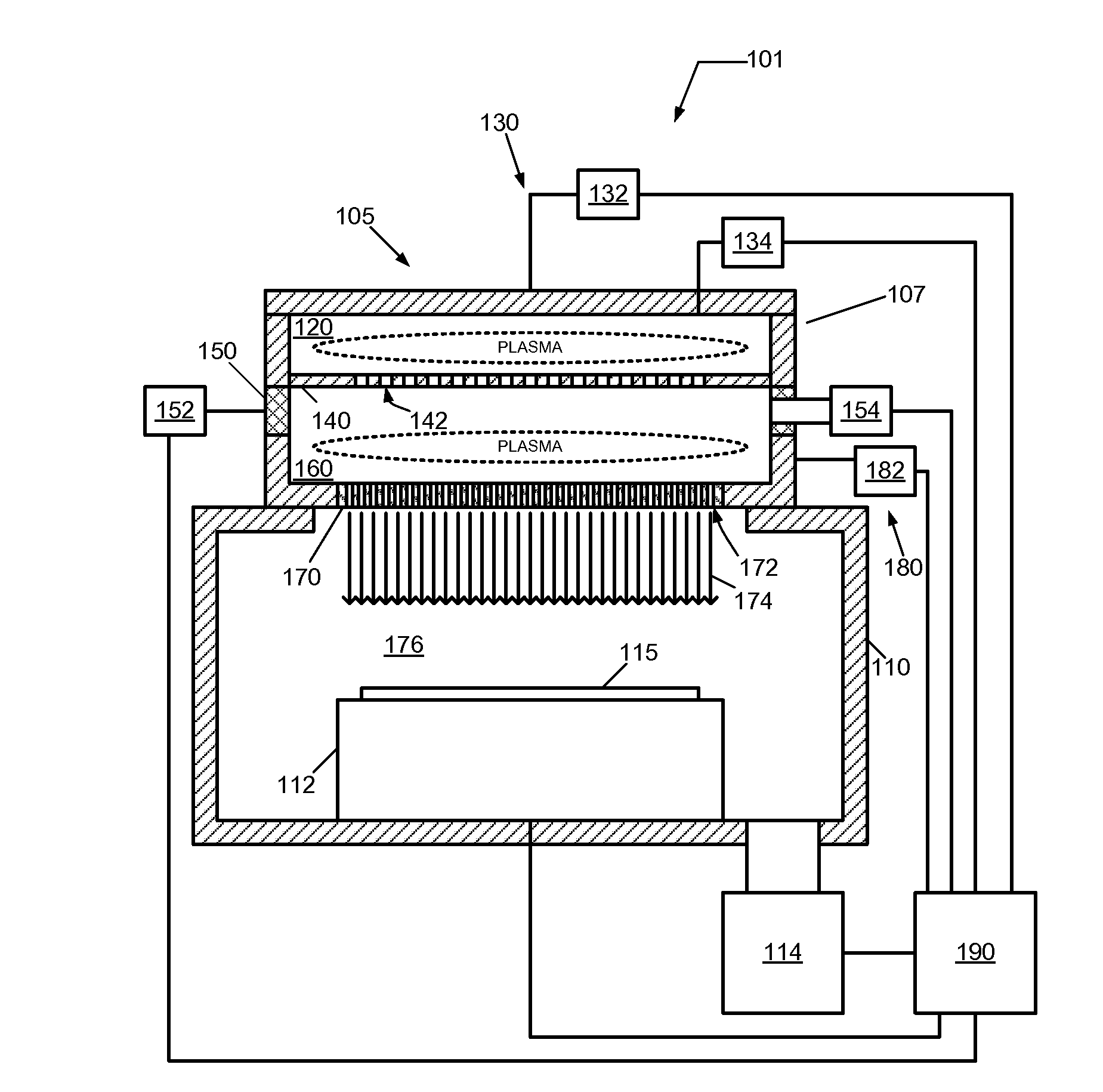
Multi-plasma neutral beam source and method of operating
Method and system for producing a neutral beam source is described. The neutral beam source comprises a plasma generation system for forming a first plasma in a first plasma region, a plasma heating system for heating electrons from the first plasma region in a second plasma region to form a second plasma, and a neutralizer grid for neutralizing ion species from the second plasma in the second plasma region. Furthermore, the neutral beam source comprises an electron acceleration member configured to accelerate the electrons from the first plasma region into the second plasma region. Further yet, the neutral beam source comprises a pumping system that enables use of the neutral beam source for semiconductor processing applications, such as etching processes.
Owner:TOKYO ELECTRON LTD
High Z material detection system and method
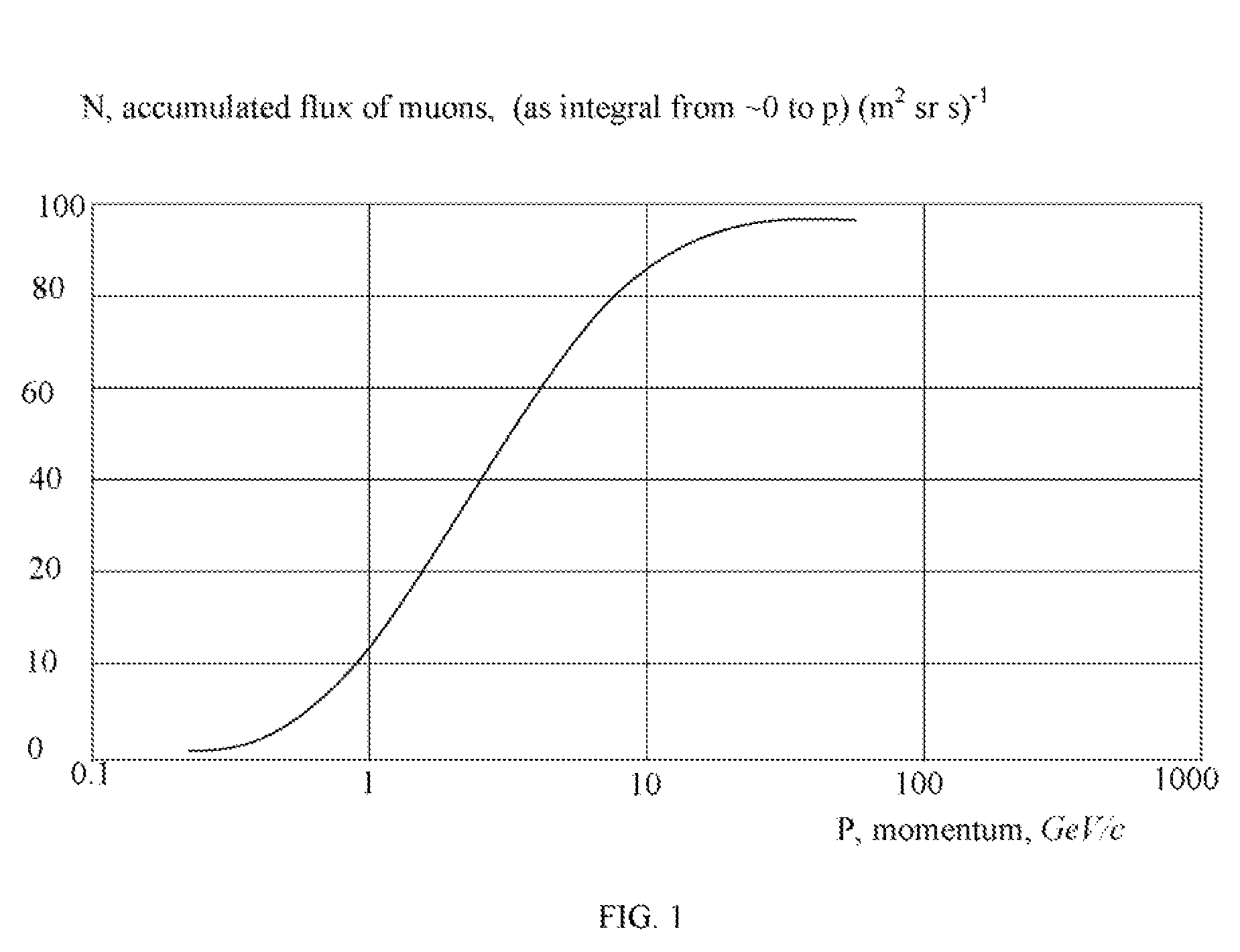
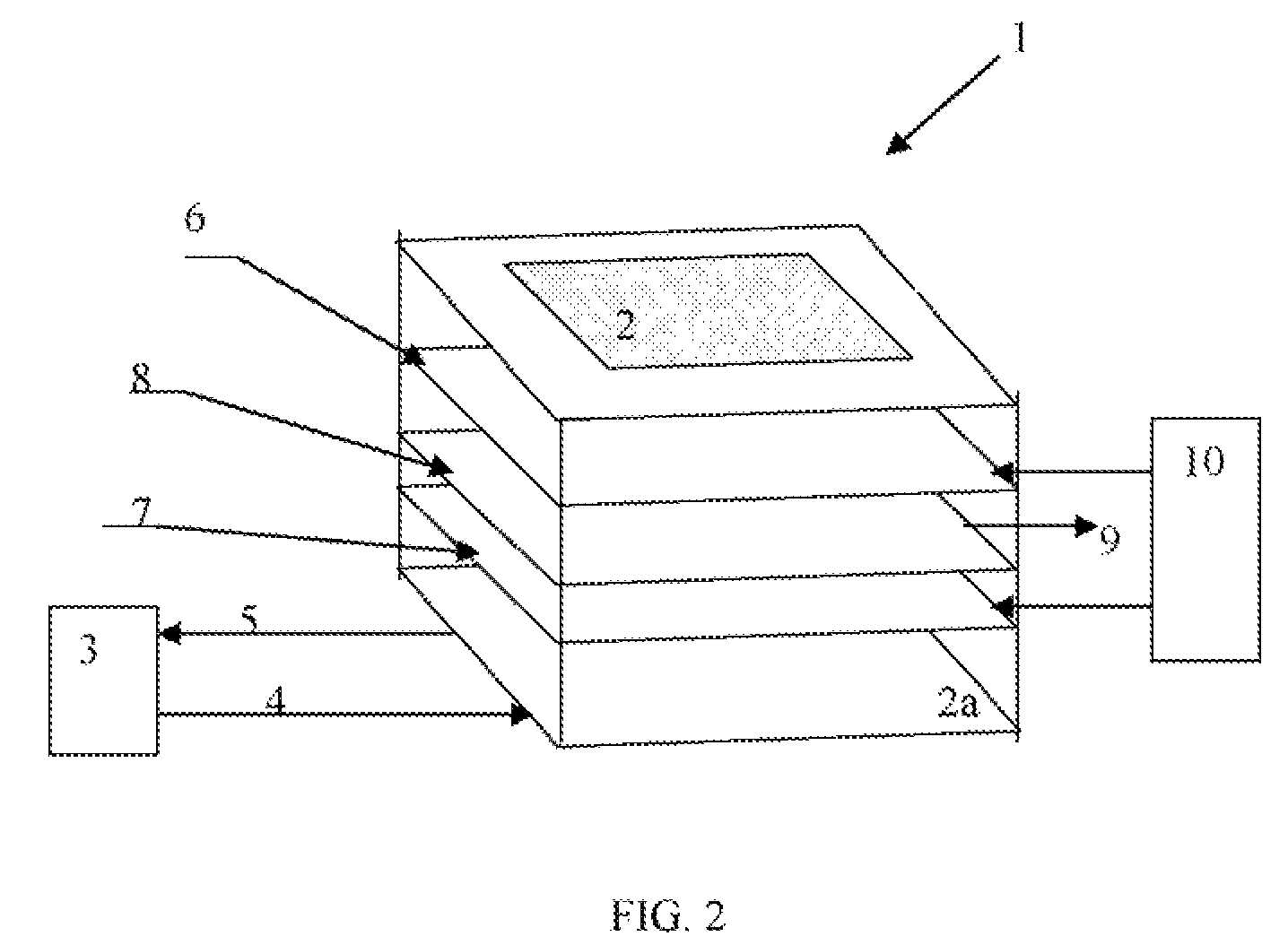
InactiveUS7470905B1Efficient measurementAccurate measurementThermometer detailsLaser detailsMuon detectorEngineering
A method and system for high Z substance revealing using muon detection technique is presented. Natural muon coordinate and incidence angle are measured above and below the interrogated volume. The data on muons trajectory change caused by the presence of high Z material and the muons time of flight between the upper and lower muon detectors are used for the decision making on the presence of a nuclear substance inside the volume. The system is adapted for performing measurements on moving objects such as moving trucks.
Owner:CELIGHT
Popular searches
Material analysis by observing effect on chemical indicator Microbiological testing/measurement Photoelectric discharge tubes Photometry using electric radiation detectors Optical waveguide light guide Accelerators Semiconductor/solid-state device manufacturing Sputtering coating Chemical conversion by chemical reaction Electric discharge tubes
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com