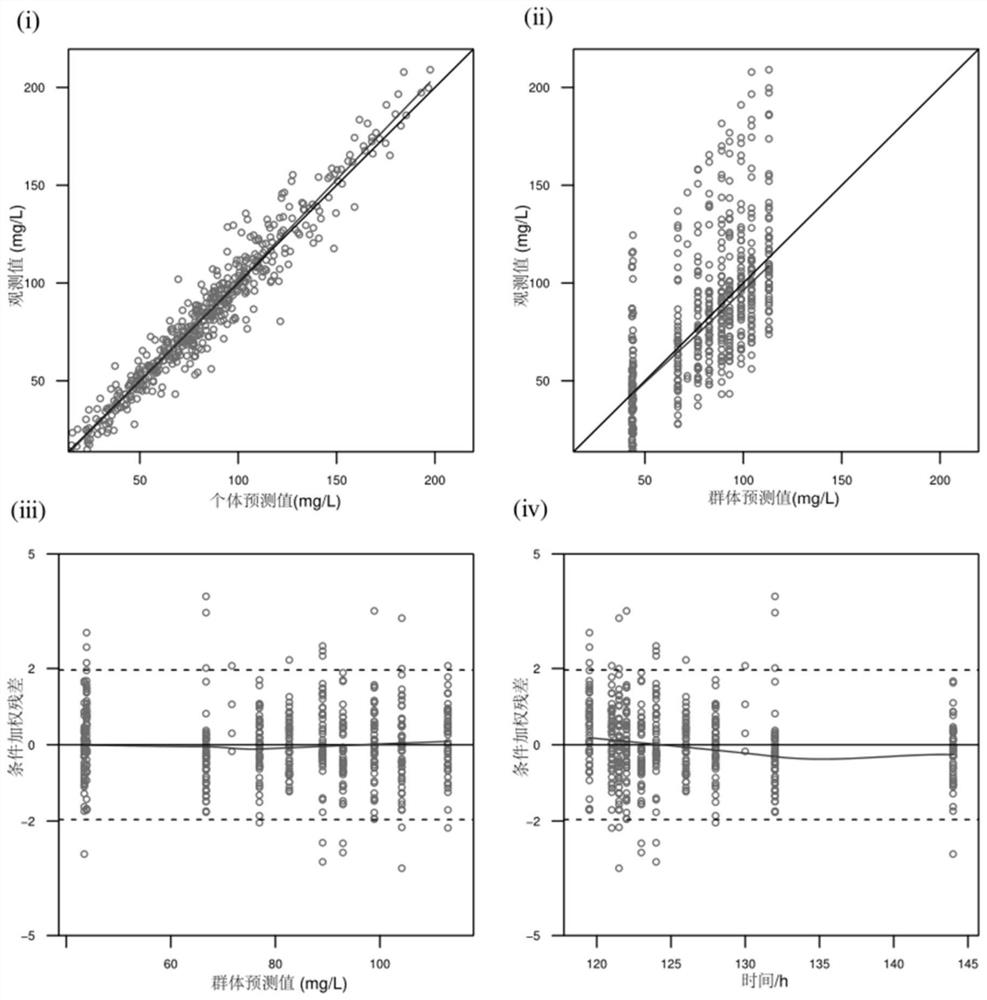
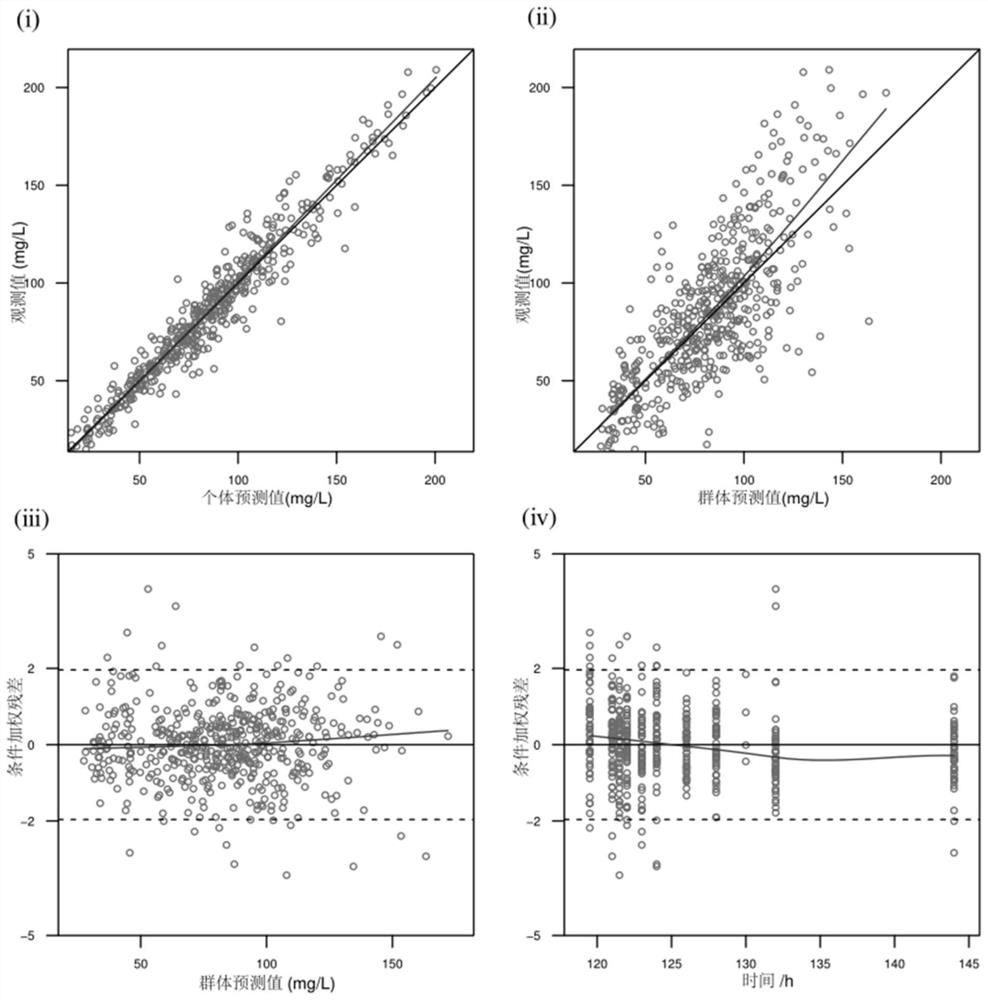
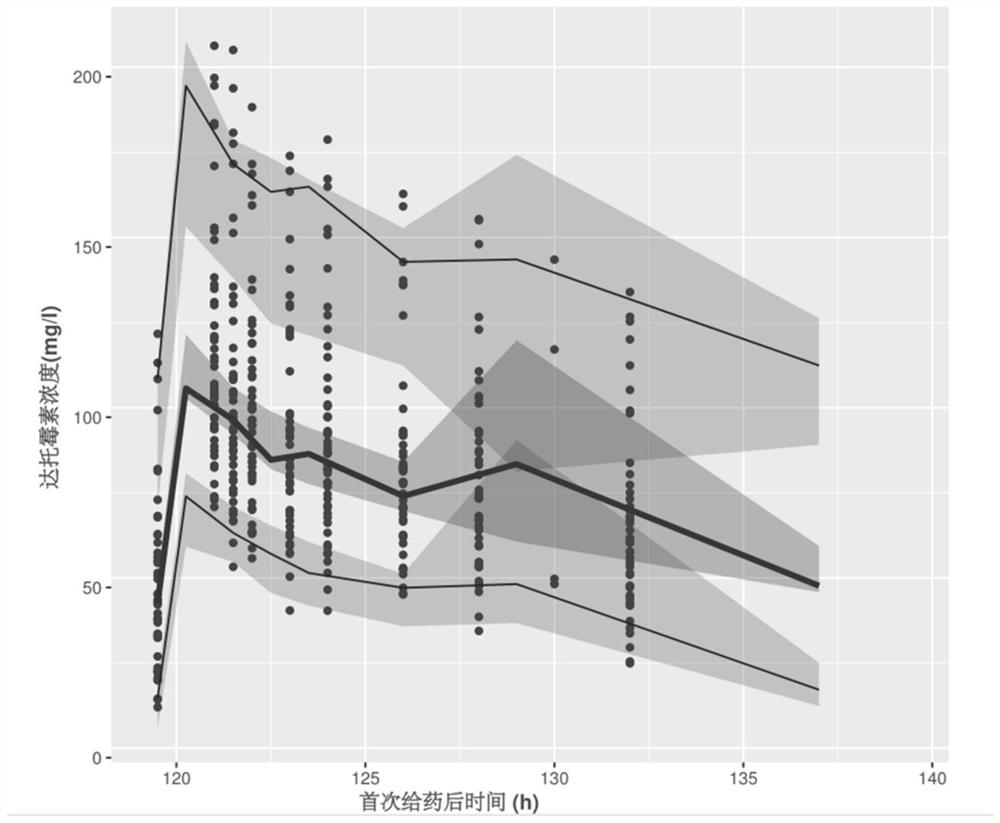
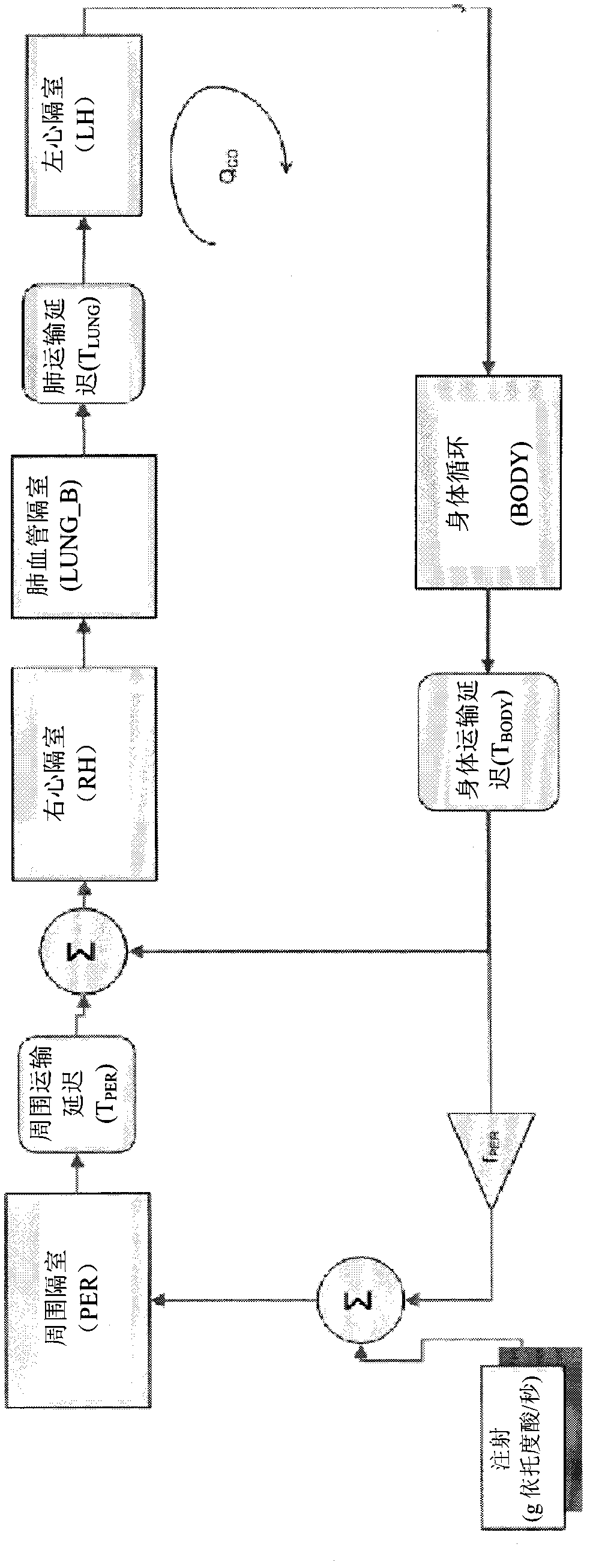
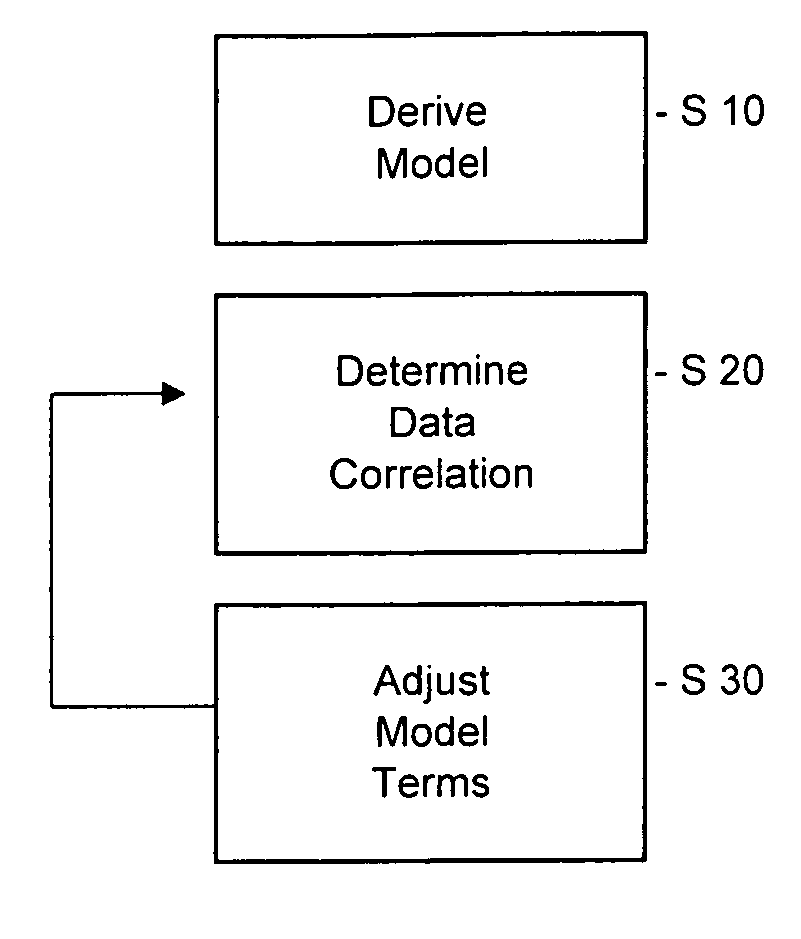
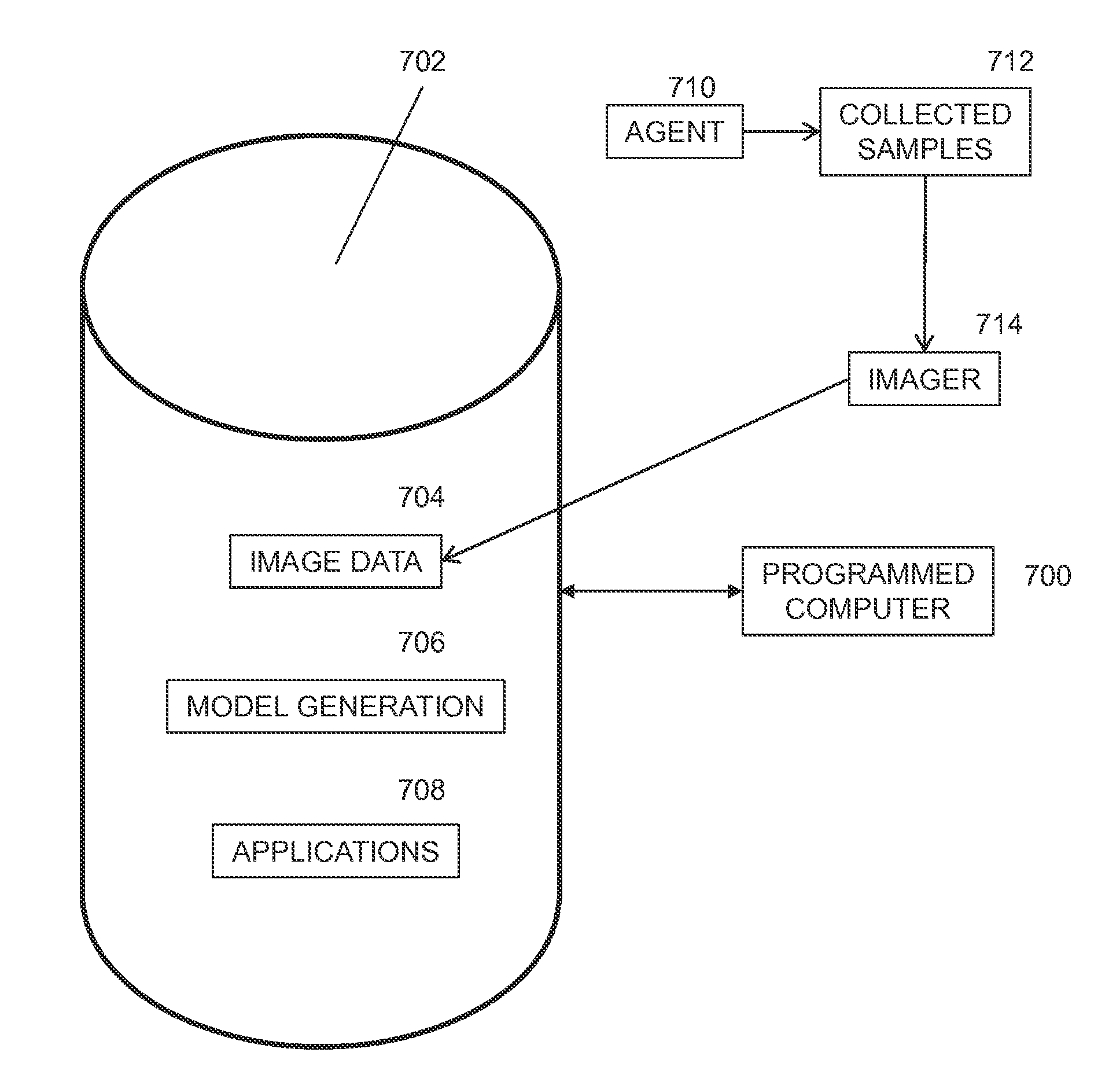
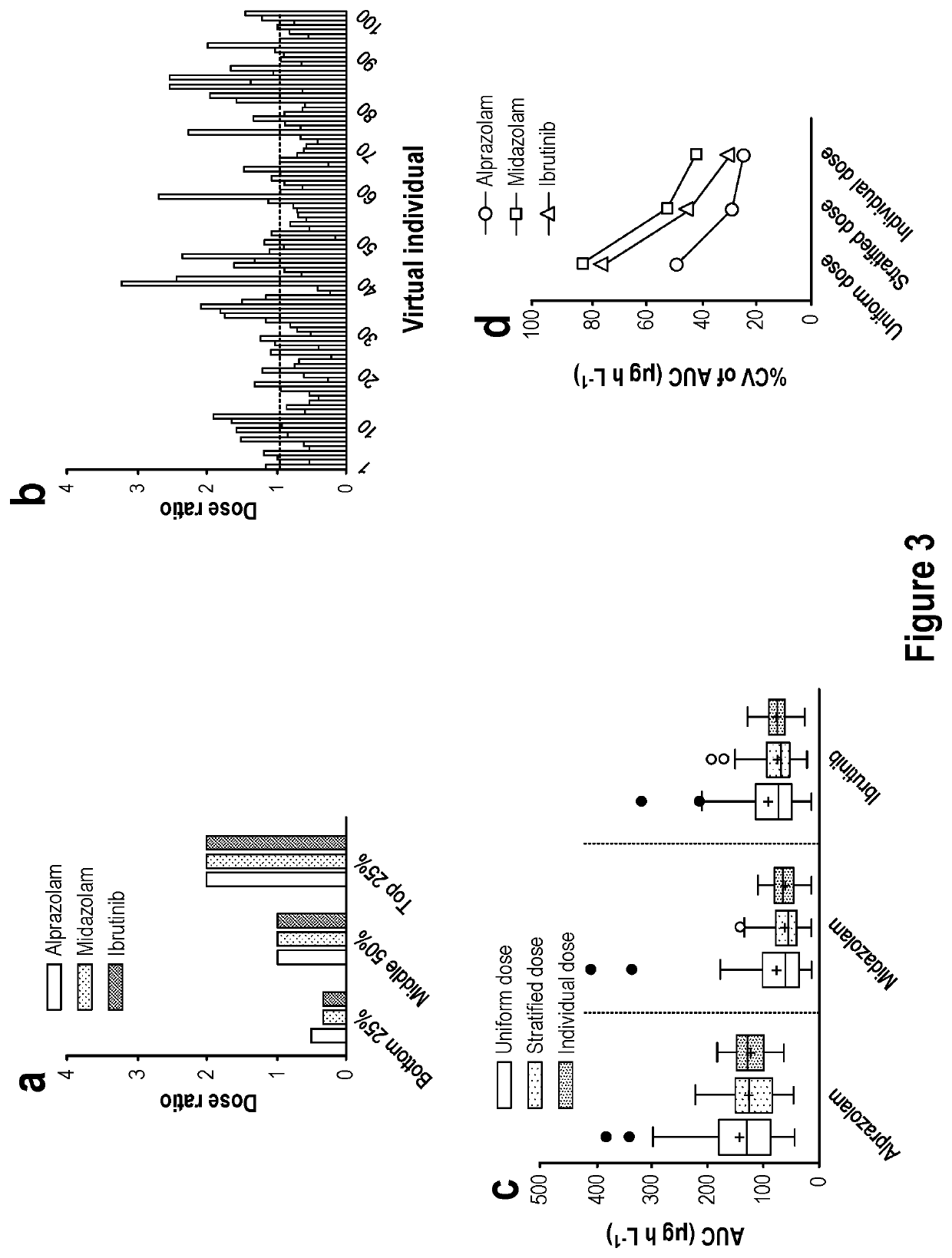
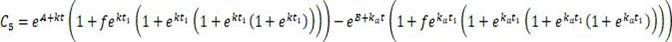Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Pharmacokinetic modeling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
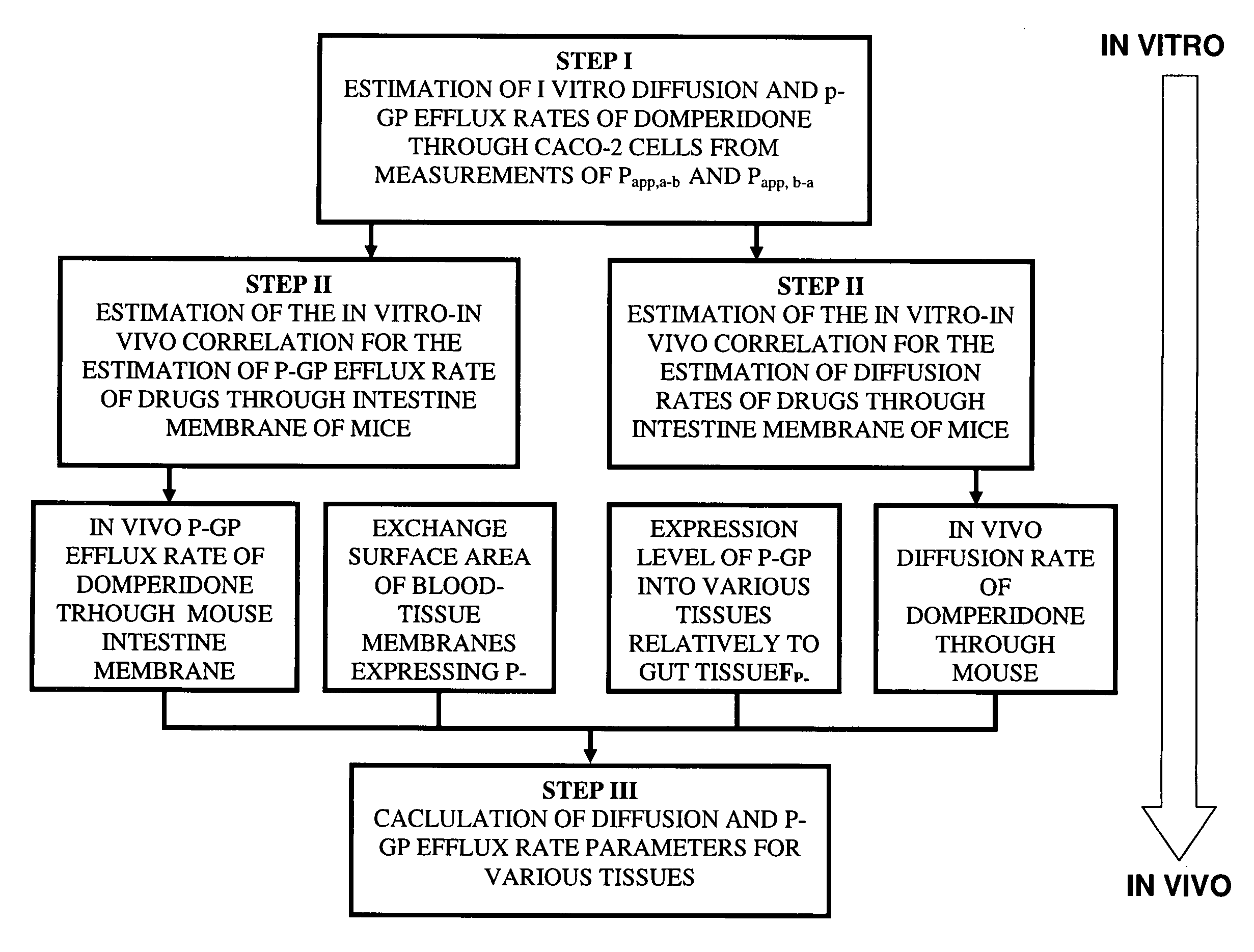
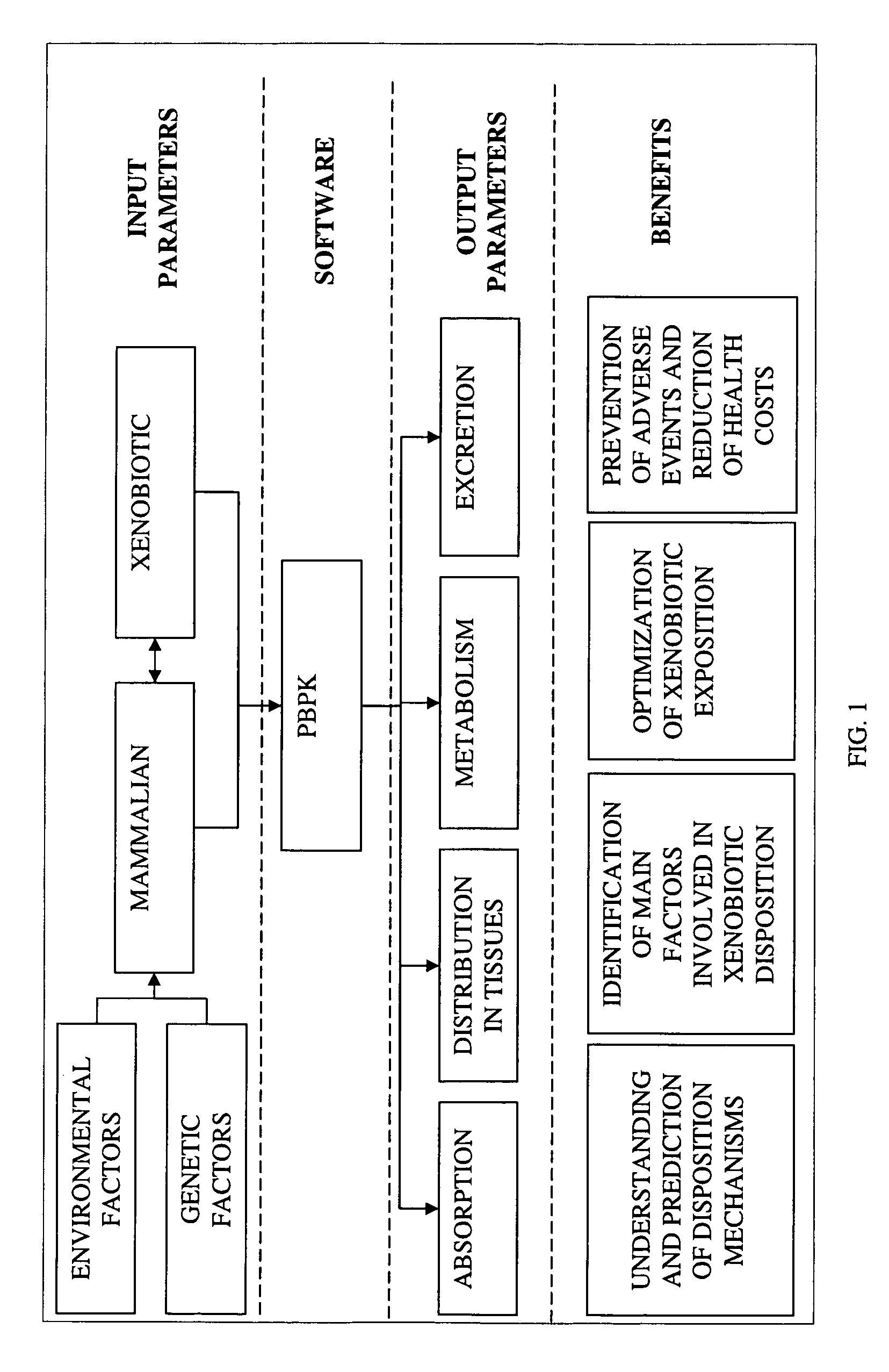
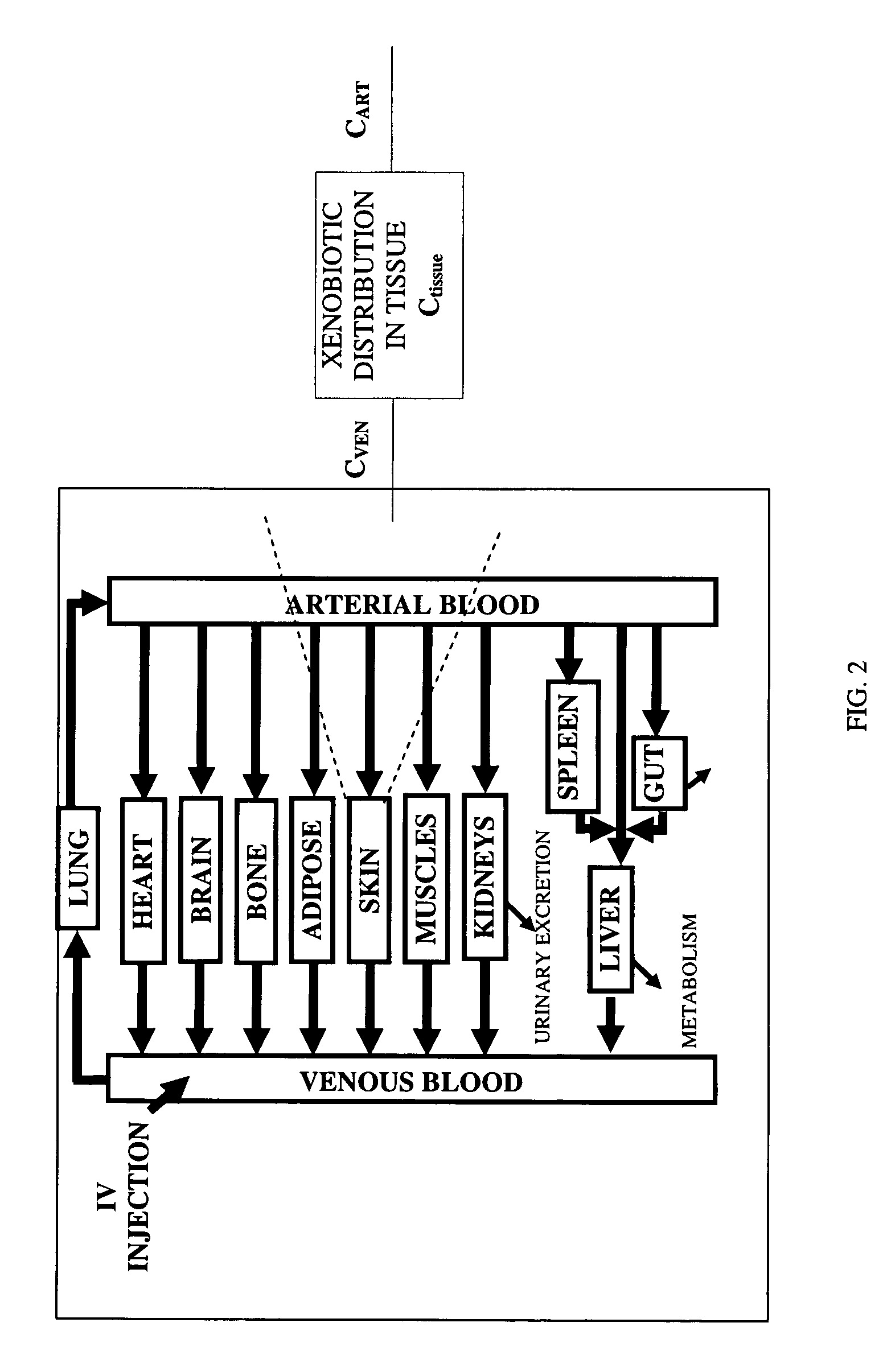
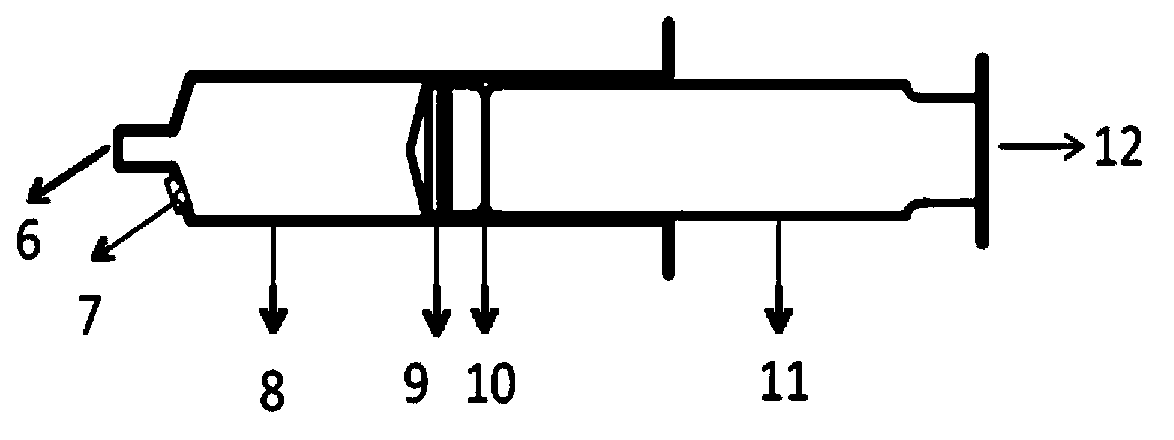
Method of developing a pharmacokinetic profile of a xenobiotic disposition in a mammalian tissue
InactiveUS20080221847A1Chemical property predictionAnalogue computers for chemical processesMammalian tissueBiology
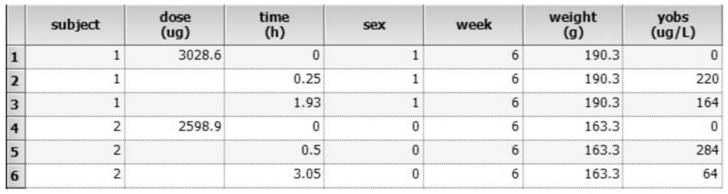
There is provided a method of developing a pharmacokinetic profile of an xenobiotic disposition in a mammalian tissue, the method comprising inputting mammalian-specific data into a physiologically based pharmacokinetic (PBPK) model, where said mammalian-specific data comprises tansporter properties related data, where said transporter properties related data reflect genetic and environmental factors associated with said mammalian; inputting xenobiotic-specific data into said PBPK model; and simulating, using said PBPK model, a pharmacokinetic profile of said xenobiotic disposition as a function of said inputted data.
Owner:FENETTEAU FREDERIQUE
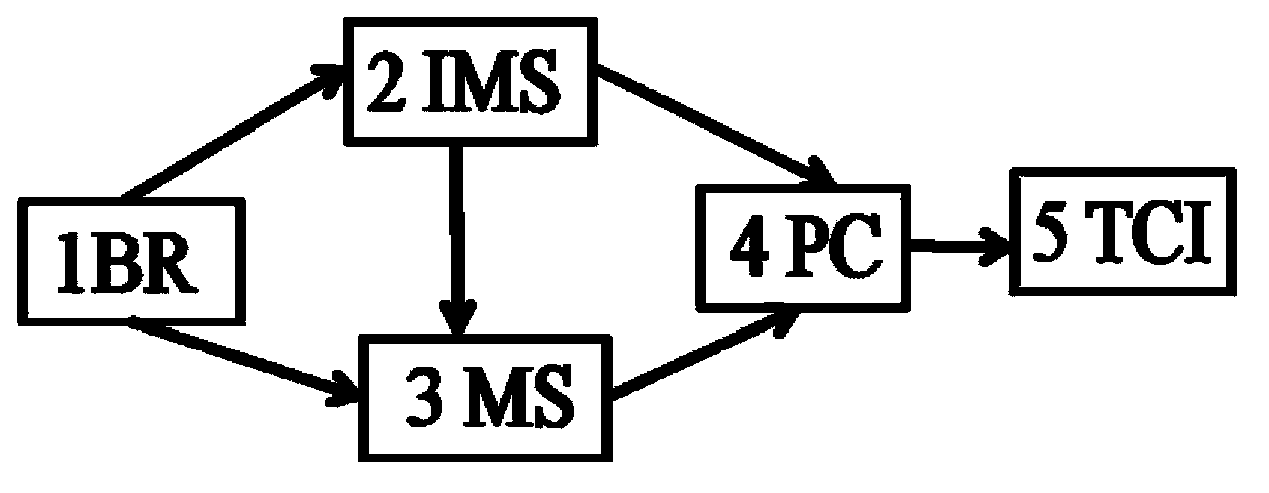
Detection control device for anesthetics in blood
InactiveCN103877645ARealize in situ detectionQualitatively accurateMaterial analysis by electric/magnetic meansFlow monitorsSoftware systemIon-mobility spectrometry
The invention discloses a detection control device for anesthetics in blood. The detection control device comprises an anesthetic patient blood receiver (BR). Detection limit and qualitative and quantitative analytical concentration of the blood are detected, analyzed and acquired by the aid of an ion mobility spectrometry (IMS) system or a mass spectrometry (MS) system or an ion mobility spectrometry and mass spectrometry (IMS-MS) combination system, so that the requirement of the human blood on an administration concentration analysis range can be met, and an analytical method for quickly detecting the anesthetics in the blood can be created; analysis results are accurately displayed via a computer software system and include pharmacokinetic models and parameters relevant to medicine infusion; a medical target control infusion (TCI) system can be directly guided by the output results, and hardware of the medical target control infusion system comprises medical infusion pumps, a personal computer (PC) and a safety system mechanism, the infusion pumps can run under the control of the personal computer, and the personal computer can be switched off by the safety system mechanism when the errors occur. The detection control device has the advantages that the detection control device can be used easily and conveniently, is speedy, is high in efficiency and good in reliability, can be automatically controlled, can be widely applied to clinical administration depth analysis and can be used for guiding doctors to accurately apply medicine to the blood in an online manner.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
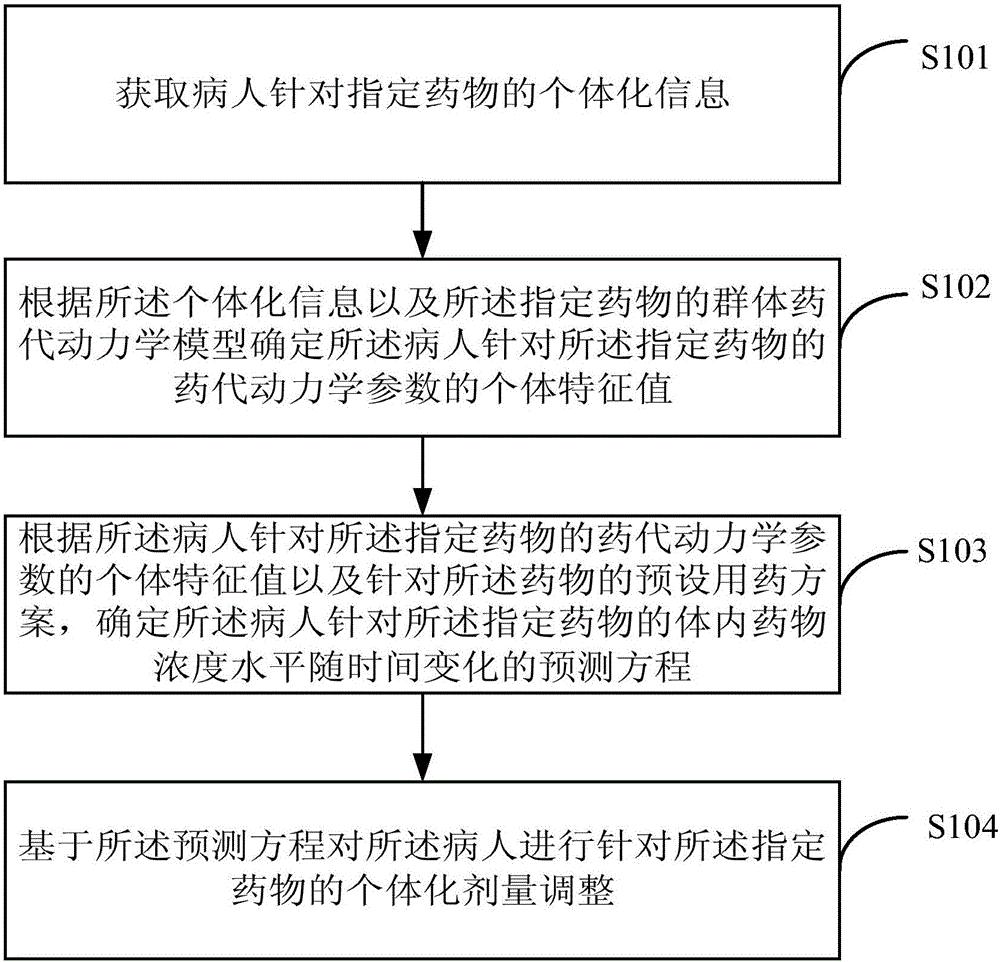
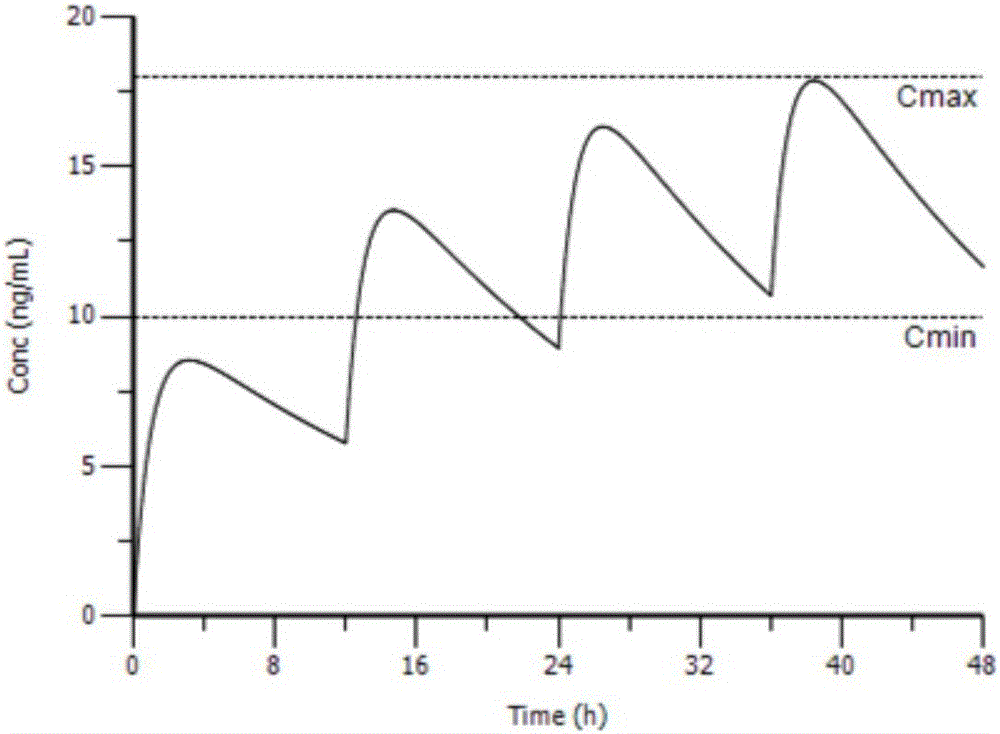
Individual drug dosage adjustment method and device
InactiveCN105844112AAvoid the risk of after-the-fact adjustmentsImprove medication safetyMolecular designComputer-assisted medicine prescription/deliveryDosage adjustmentMedicine
The invention provides an individual drug dosage adjustment method and device. The method comprises steps as follows: the individual feature value of a pharmacokinetic parameter of a designated drug for a patient is determined according to individual information and a group pharmacokinetic model of the designated drug; a time-variable prediction equation of the drug concentration level of the designated drug for the patient is determined according to the individual feature value of the pharmacokinetic parameter of the designated drug for the patient as well as a preset medication plan of the drug; individual dosage adjustment of the designated drug is performed on the patient on the basis of the prediction equation. The concentration of the drug in every patient corresponding to a preset medication plan is accurately predicted according to the individual information of the patient, accordingly, the preset medication plan is adjusted according to a prediction result, and risks caused by adjustment afterwards are avoided.
Owner:BEIJING DRYAS PHARMA TECH CO LTD
System and method for optimizing drug therapy for the treatment of diseases
The present invention concerns the optimization of hiv-1 therapy using the combination of a bioanalytical method, population pharmacokinetic models and phenotypic resistance testing.
Owner:GROEN KEES +1
Construction method of kidney transplantation anti-infection drug dosage prediction model
ActiveCN113035369ARapid MedicationAccurate MedicationMolecular designDrug referencesBlood drug concentrationPharmacology
The invention provides a construction method of a kidney transplantation anti-infection drug dosage prediction model. The method comprises the following steps: establishing a group pharmacokinetic model of an anti-infective drug by adopting a nonlinear mixed effect model, a two-atrioventricular model and a mixed residual model through plasma concentration analysis, and calculating pharmacokinetic parameters of the corresponding model; and carrying out related hypothesis testing and estimation on the obtained model pharmacokinetic parameters based on a bootstrap method, visual prediction testing and normalized prediction distribution errors, and completing the steps of stability and prediction capability evaluation and the like. According to the established pharmacokinetic model of the kidney transplantation patient population, the reasonable judgment on the medication scheme information is accurately realized, the reference of the optimal dosage is expected to be provided in the infection prevention and treatment process, the adverse reaction risk is reduced, the curative effect is improved, and finally the individualized administration of the anti-infection medicine of the kidney transplantation patient is realized.
Owner:ZHEJIANG UNIV
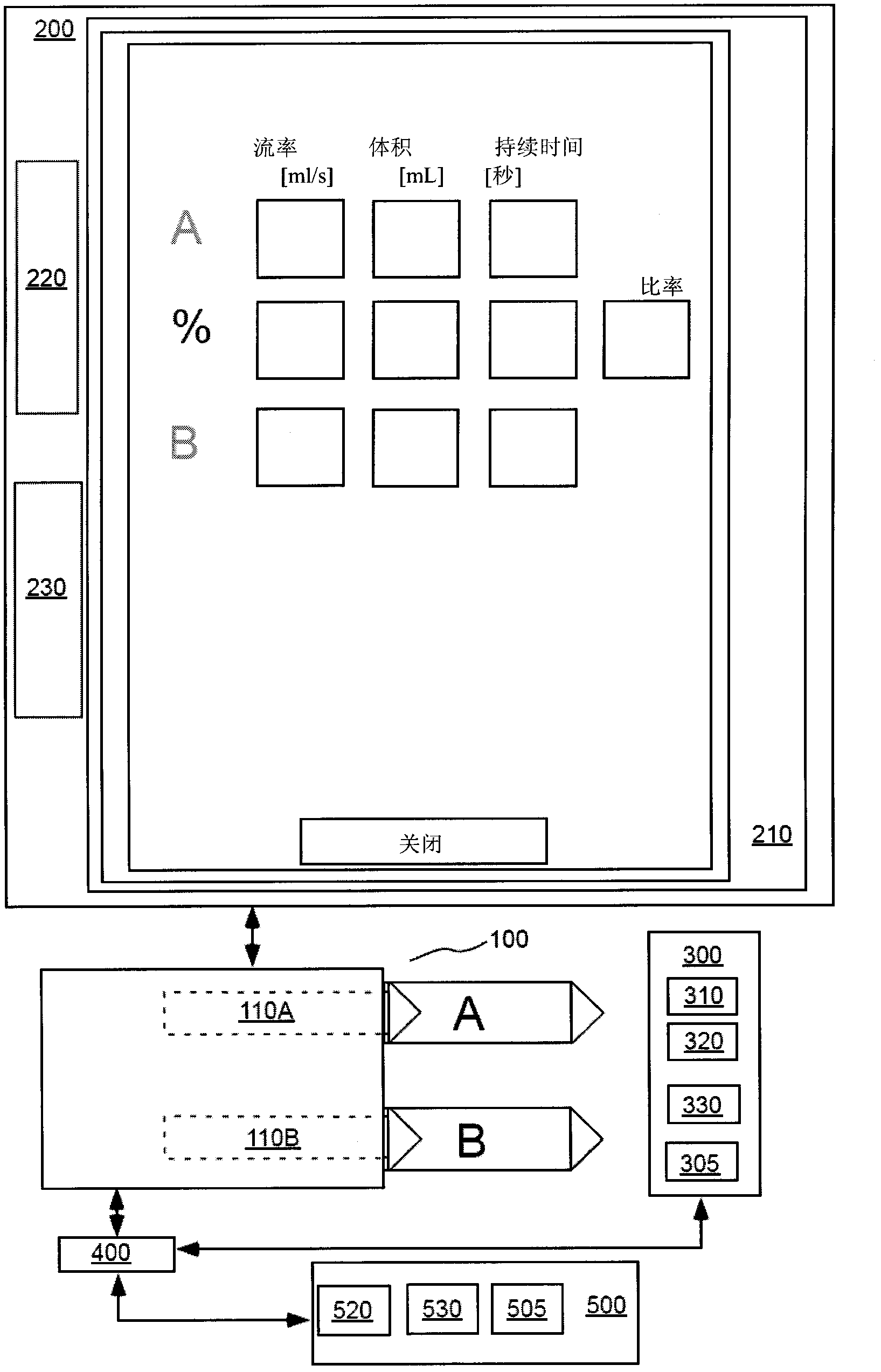
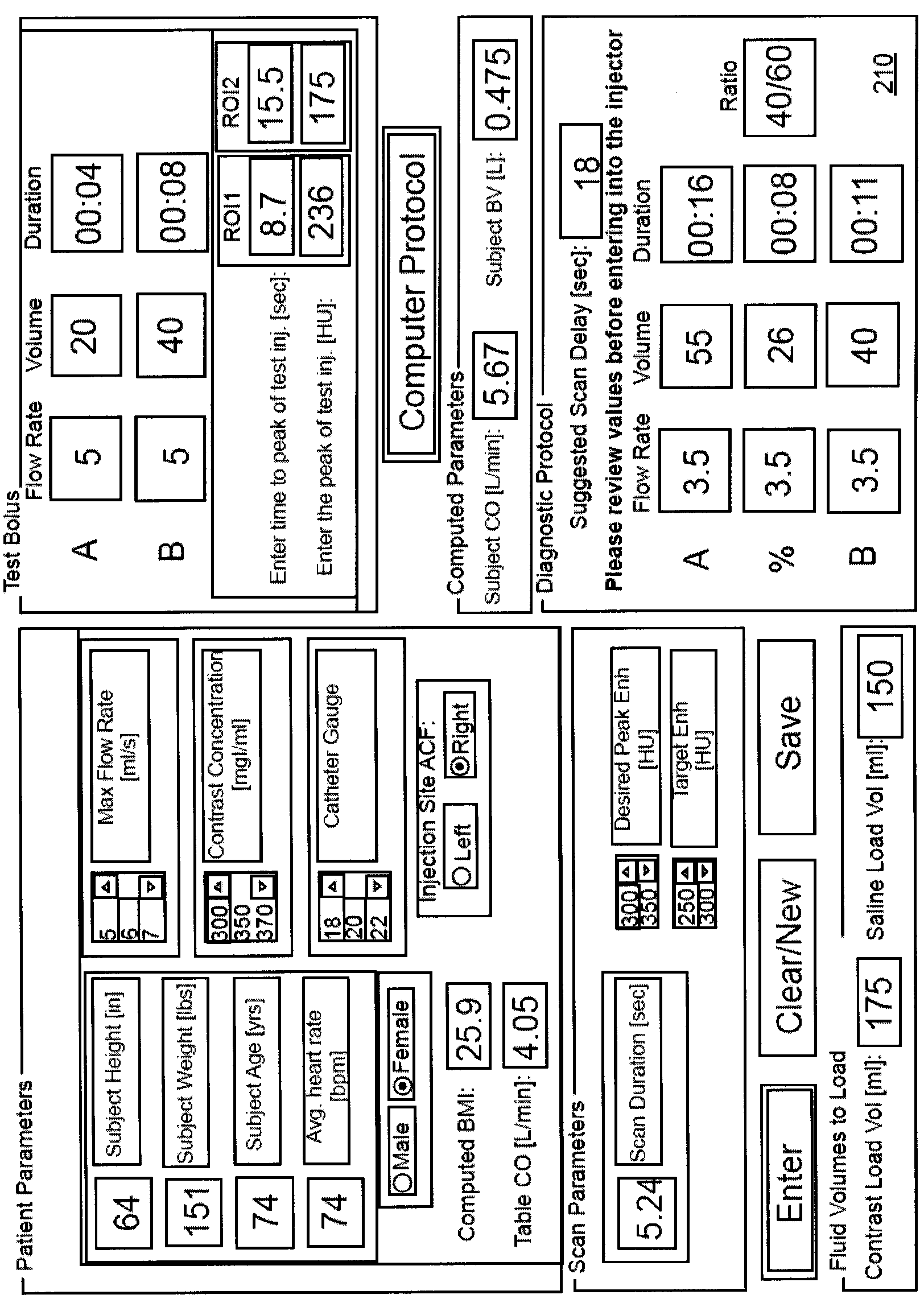
Modeling of pharmaceutical propagation and parameter generation for injection protocols
InactiveCN103221071AReduce loadImprove consistencyMedical simulationInfusion devicesPharmacokinetic modelingTransport delay
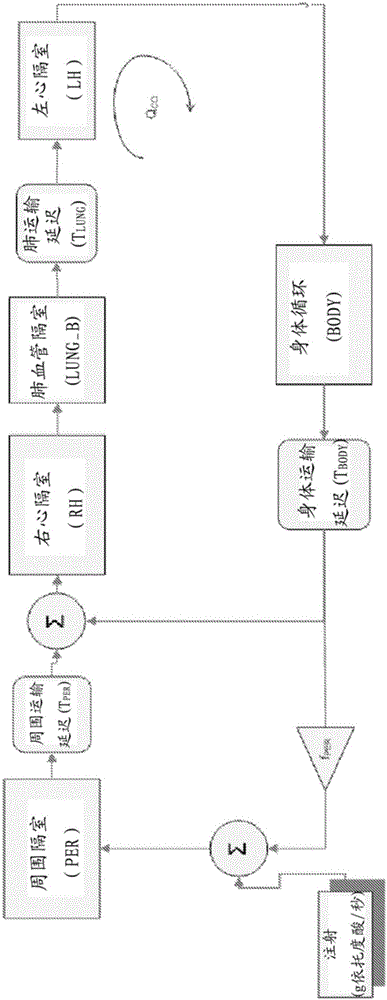
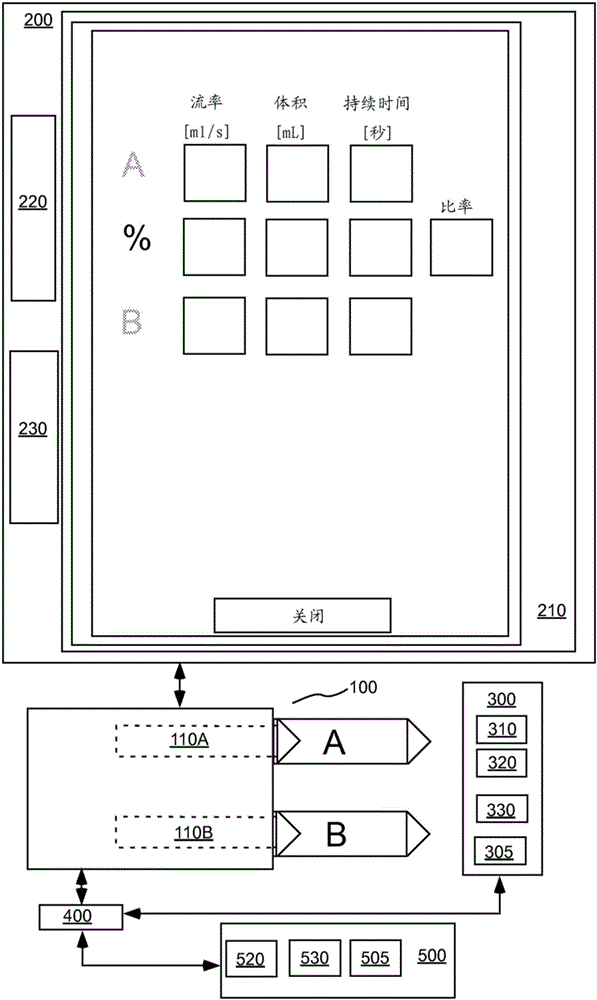
A system includes a parameter generation system to determine at least one parameter for an injection procedure (for example, a parameter of an injection protocol or an imaging system parameter), the parameter generator system includes a physiologically based pharmacokinetic model to model propagation of a contrast medium injected into a patient including at least one of a non-linear saturation term in a peripheral venous compartment, at least one configurable transport delay term through at least one compartment, or an adaptation to model volumetric flow rate of blood and an effect thereof on the propagation of contrast medium after injection of contrast medium ceases. The physiologically based pharmacokinetic model can, for example, be discretizable.
Owner:BAYER HEALTHCARE LLC
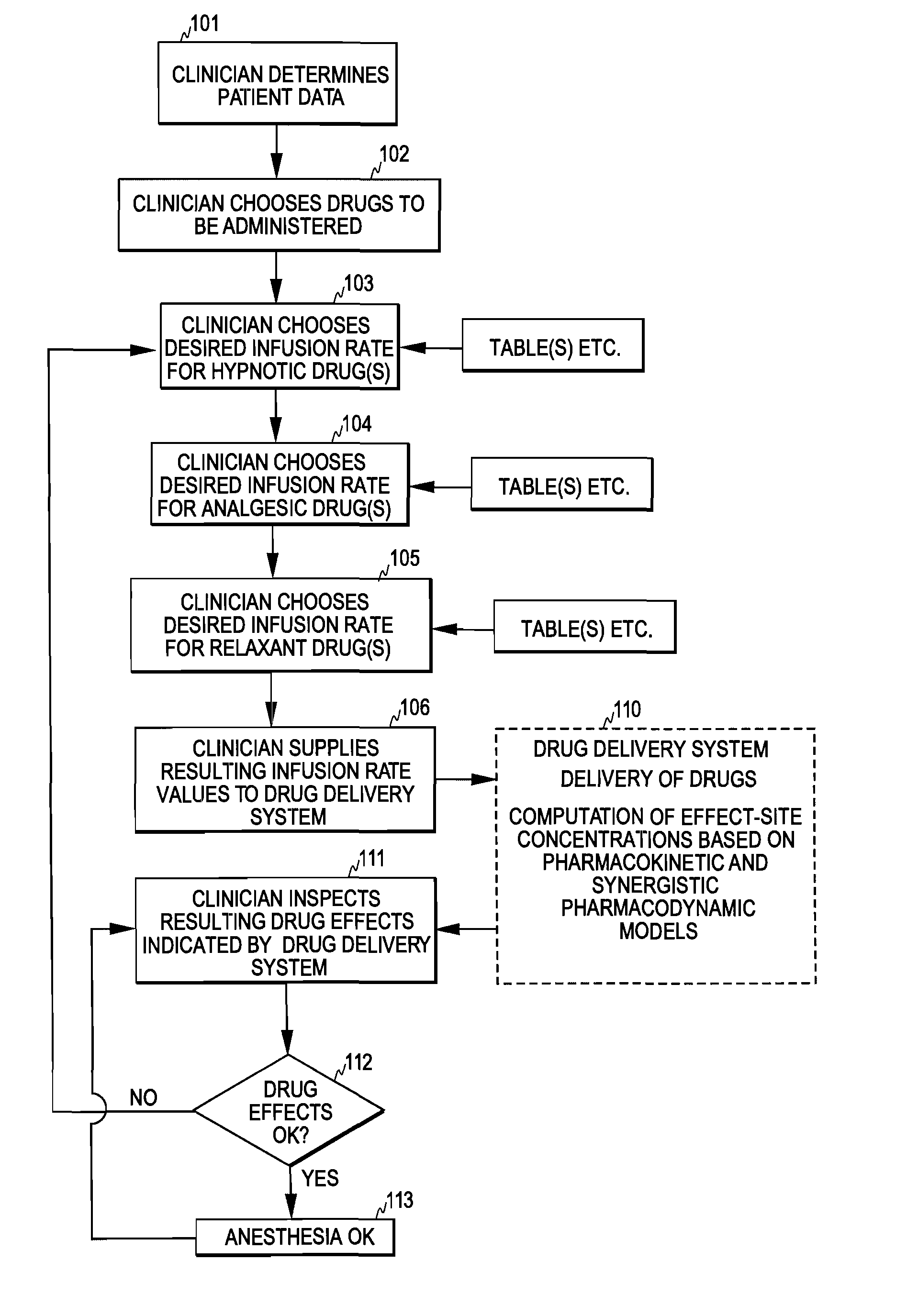
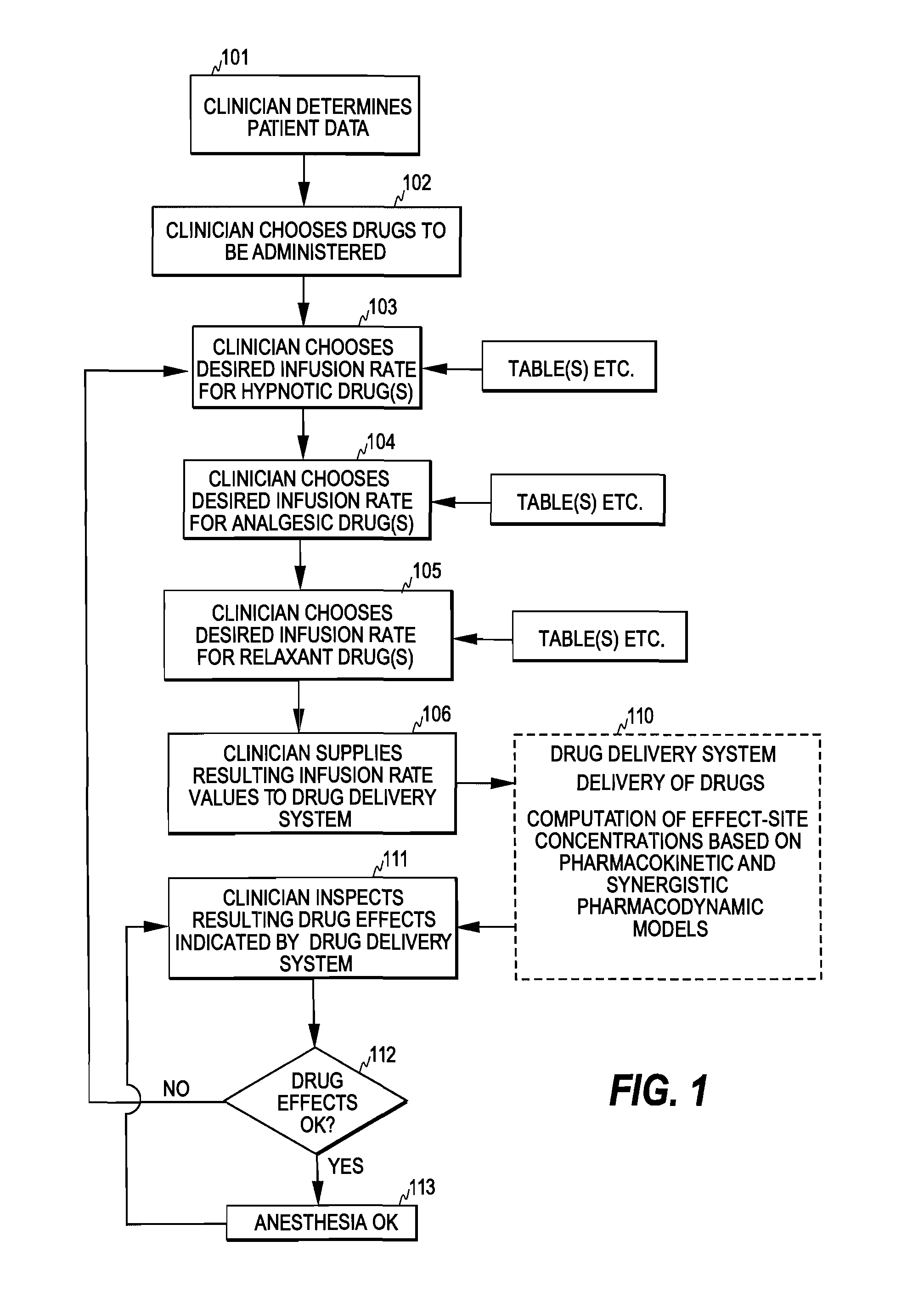
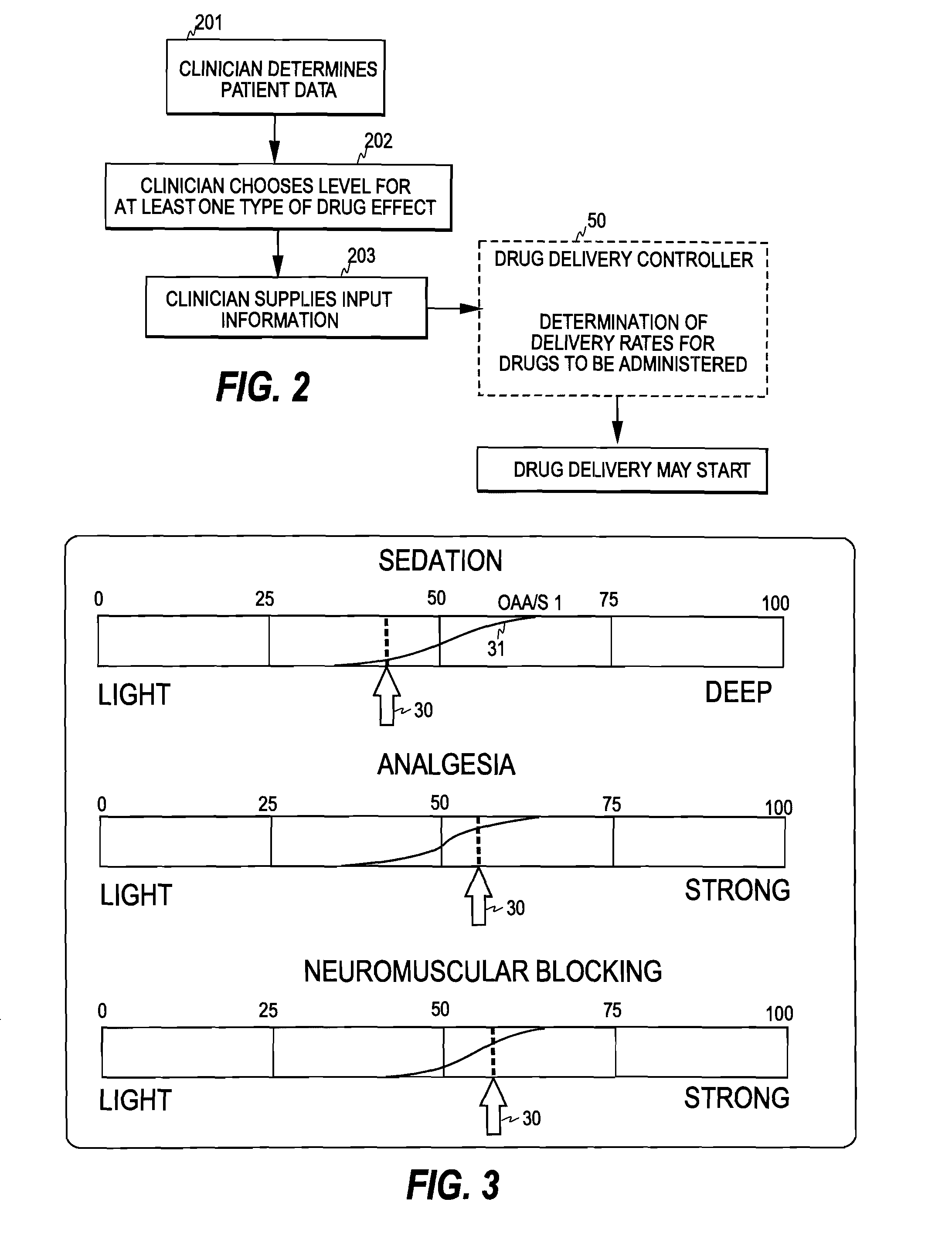
Control of Drug Administration
InactiveUS20080108970A1Optimization mechanismReliable technologyLocal control/monitoringMedical devicesPharmacokinetic modelingDrug administration
The invention relates to a method and apparatus for controlling drug administration. In order to achieve an easy-to-use and technically reliable control mechanism that can be implemented cost-effectively, input information including patient information and effect level information for at least one type of drug effect to be achieved in a patient is supplied to the control mechanism. Synergetic pharmacodynamic models are utilized to determine, based on the input information, effect-site concentration(s) for at least one drug to be administered. A pharmacokinetic model of each drug may then be utilized to define, based on the effect-site concentration of the drug, a delivery rate for the drug to be administered.
Owner:GENERAL ELECTRIC CO
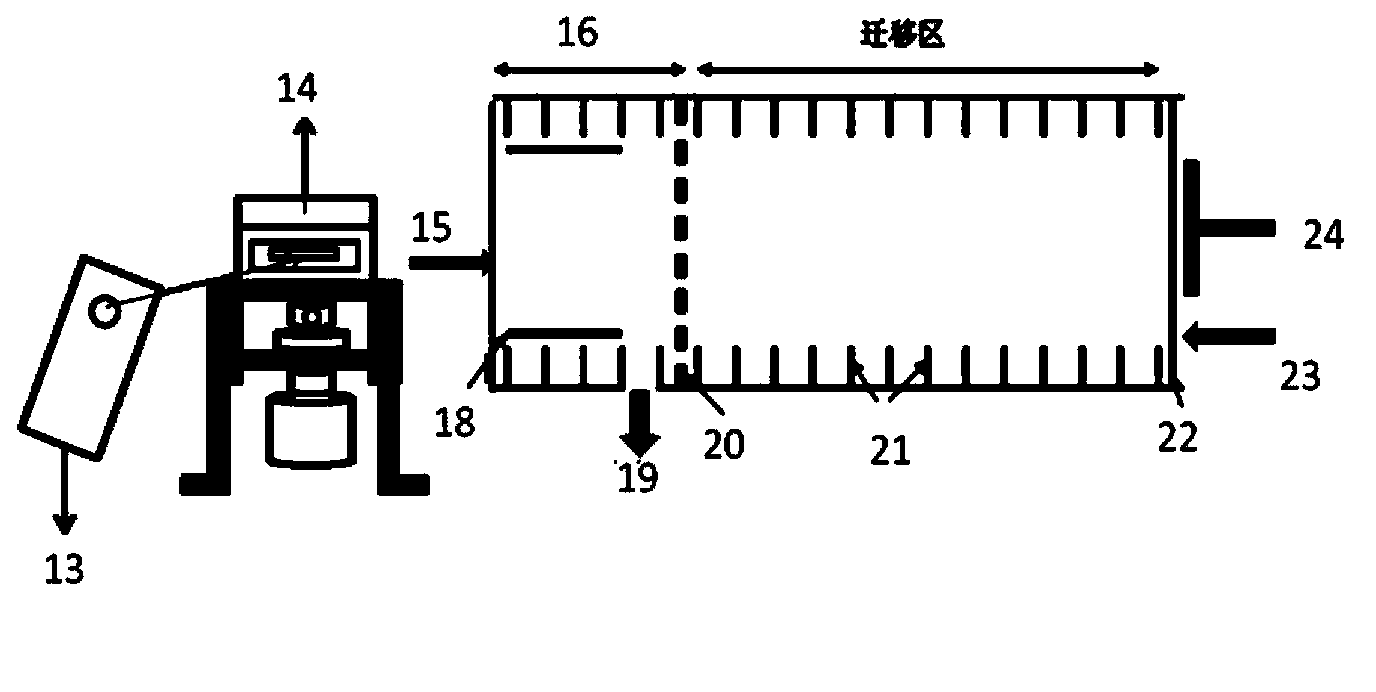
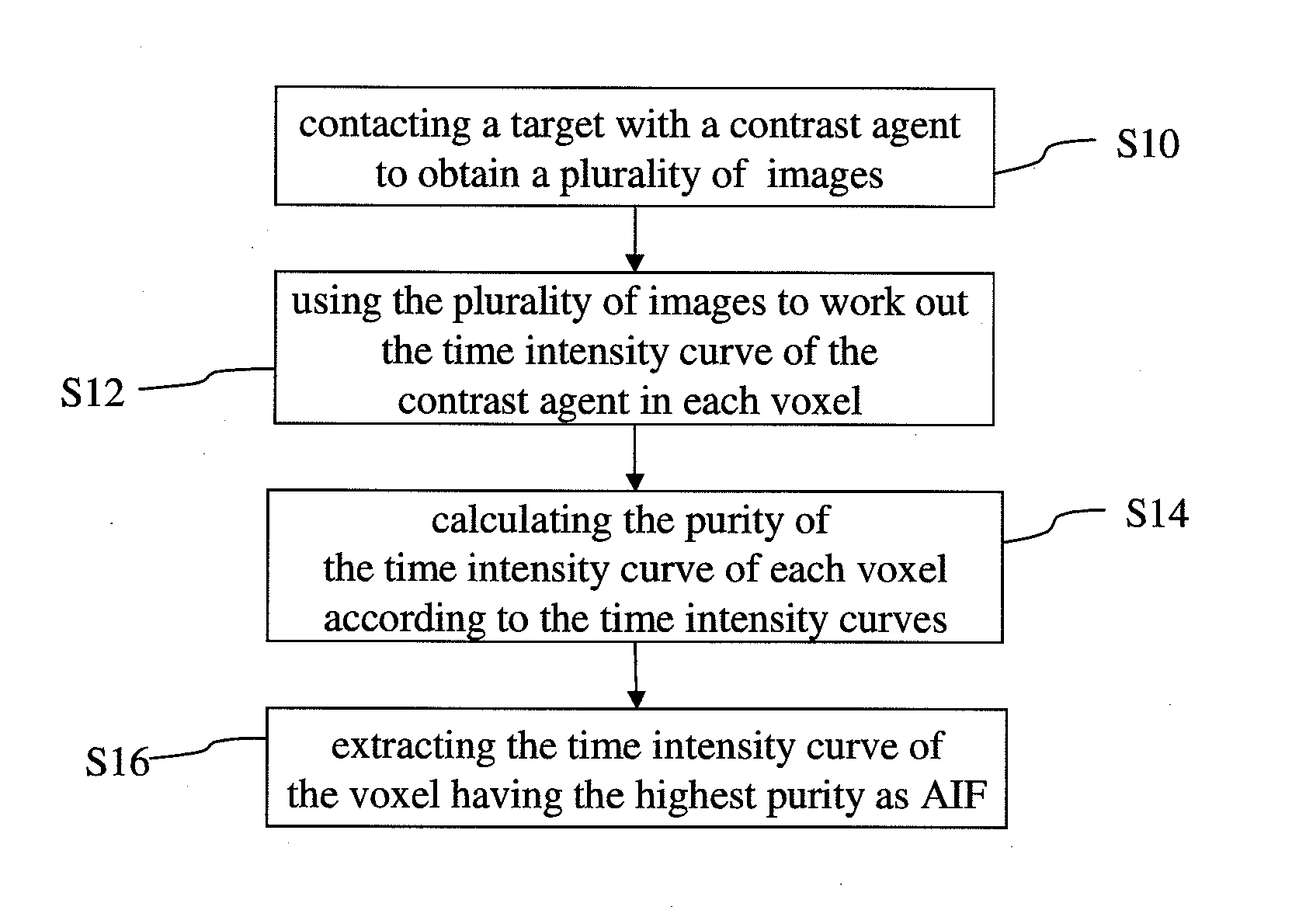
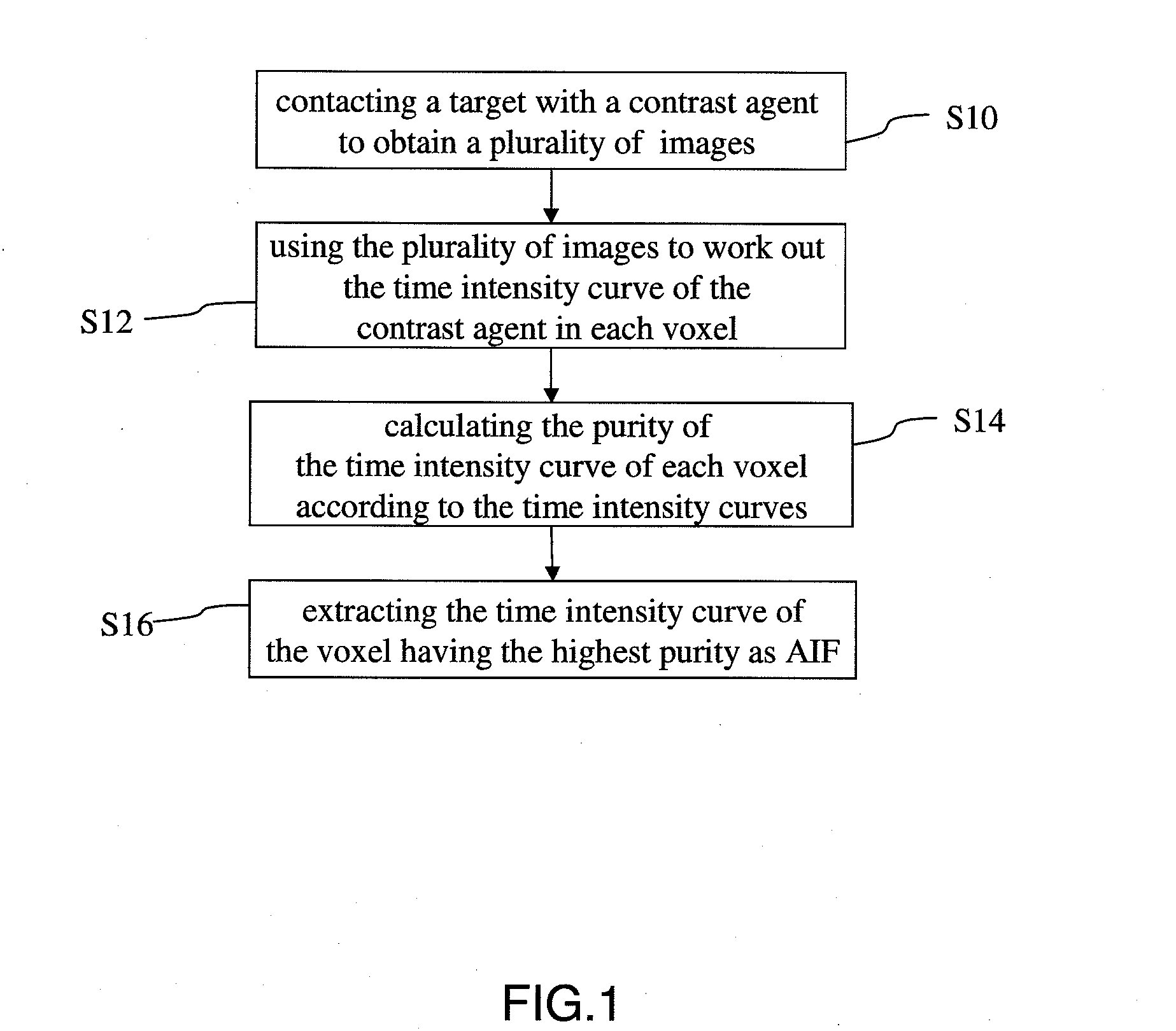
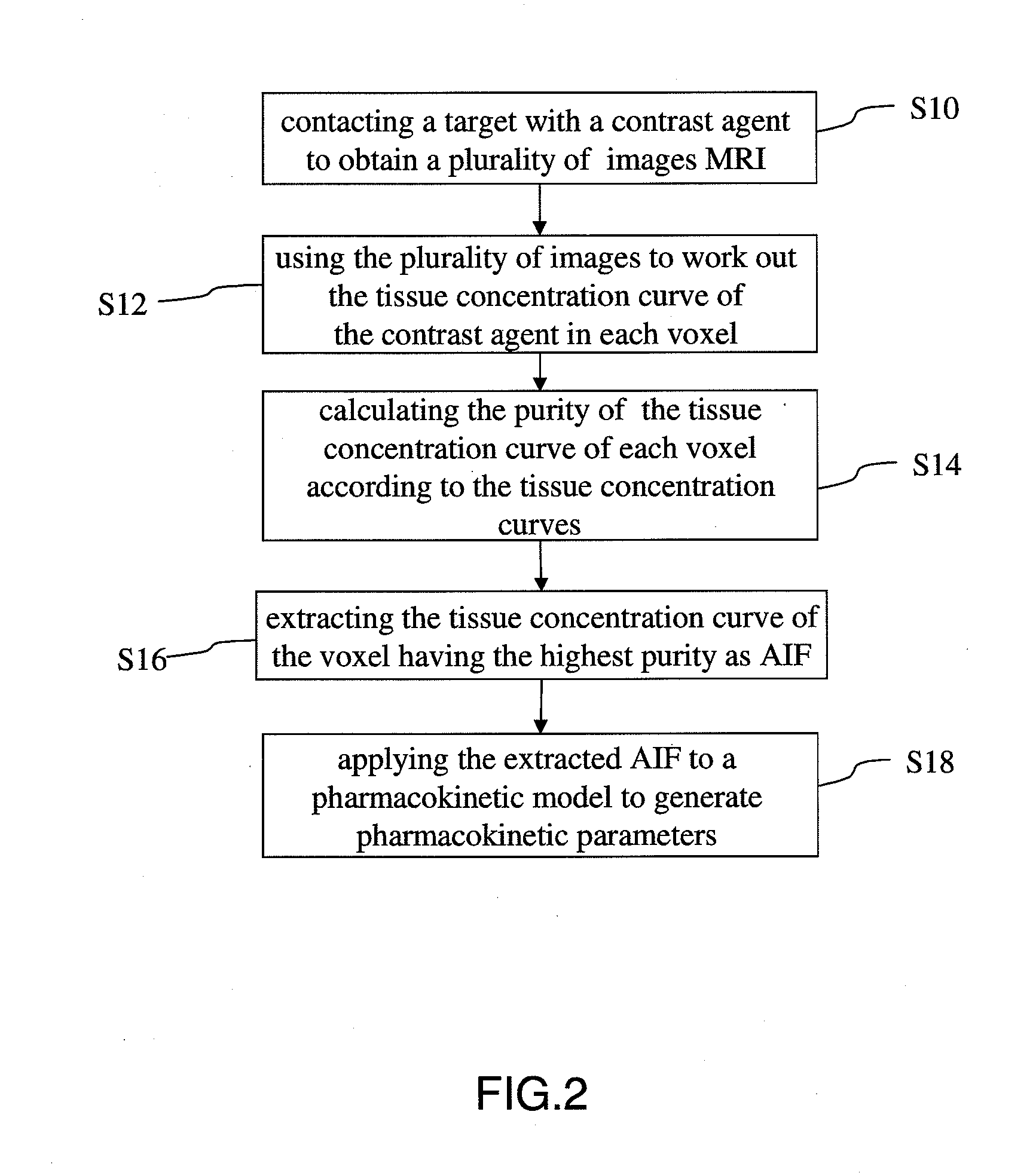
Method for extracting arterial input function and application thereof to dynamic contrast enhanced magnetic resonance imaging
ActiveUS20140241604A1Improve efficiencyImprove reliabilityImage enhancementImage analysisTissue concentrationsVoxel
The present invention discloses a method for extracting AIF and an application thereof to DCE-MRI. The method comprises steps: contacting a target tissue with a contrast agent to obtain a plurality of images; using the plurality of images to work out the tissue concentration curve of the contrast agent in each voxel; calculating the purity of the tissue concentration curve of each voxel according to the tissue concentration curves; and extracting the voxel having the highest purity as the optimized arterial location; and extracting the tissue concentration curve of the voxel having the highest purity as the arterial input function. The extracted AIF is applied to a pharmacokinetic model to obtain associated pharmacokinetic parameters. The present invention not only improves the accuracy and reliability of the derived quantitative indexes but also promotes the efficiency of the quantitative analysis.
Owner:CHANG GUNG UNIVERSITY
Method for inducing differentiation of stem cells into islet-like cells
InactiveCN102311940AMetabolism disorderMicrobiological testing/measurementIslet cellsCell engineering
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
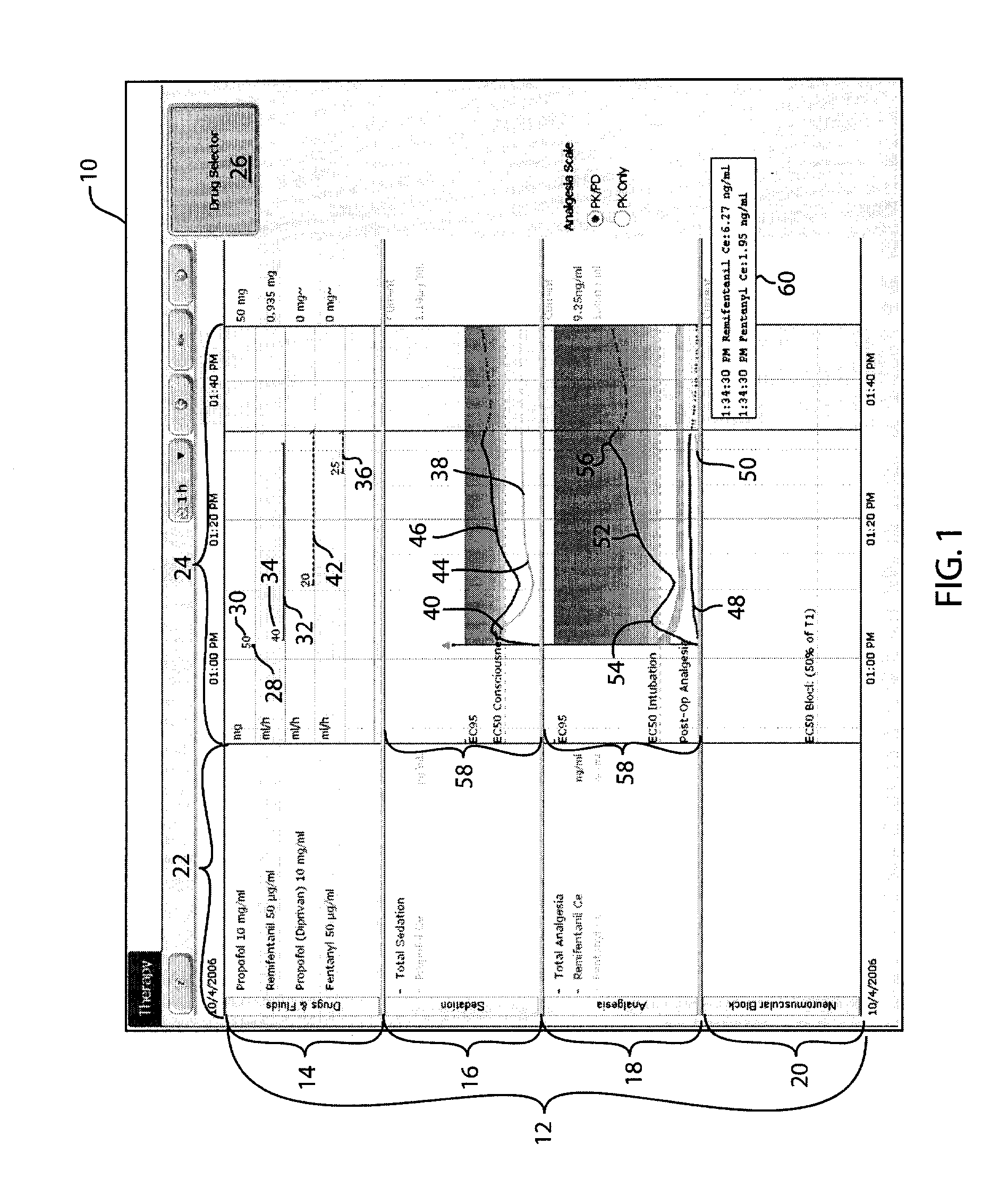
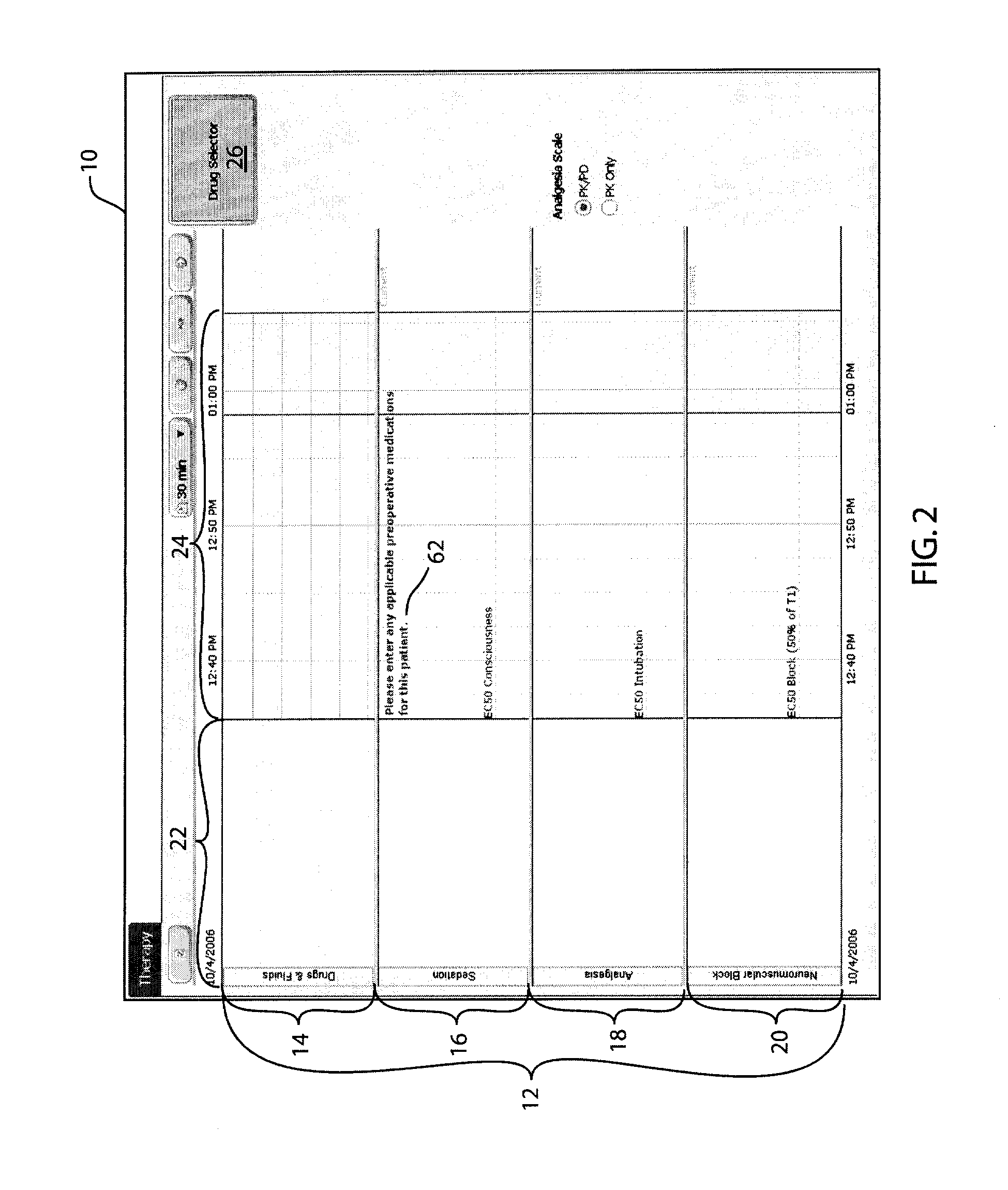
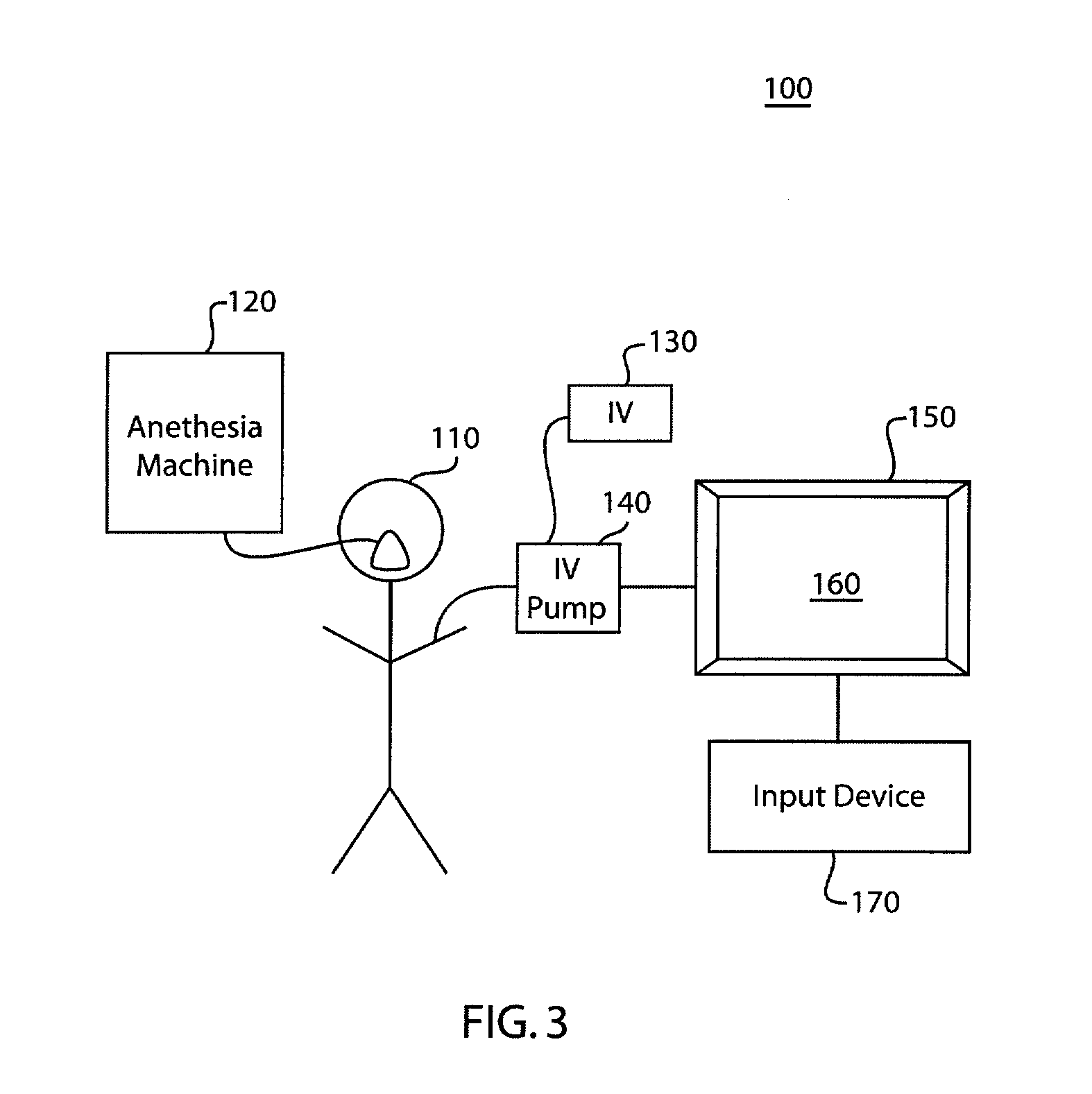
Anesthetic drug model user interface
A graphical user interface for the documentation of an administered anesthetic drug and the display of an associated pharmacokinetic model and an associated pharmacodynamic model. The graphical user interface comprises a first window that displays the drug administration data, and a second window displaying a pharmacokinetic model and a pharmacodynamic model, the second window being separate and distinct from the first window. The pharmacokinetic model and the pharmacodynamic models are overlaid and displayed and the pharmacokinetic model and the pharmacodynamic model are based on the drug administration data displayed in the first window.
Owner:GENERAL ELECTRIC CO
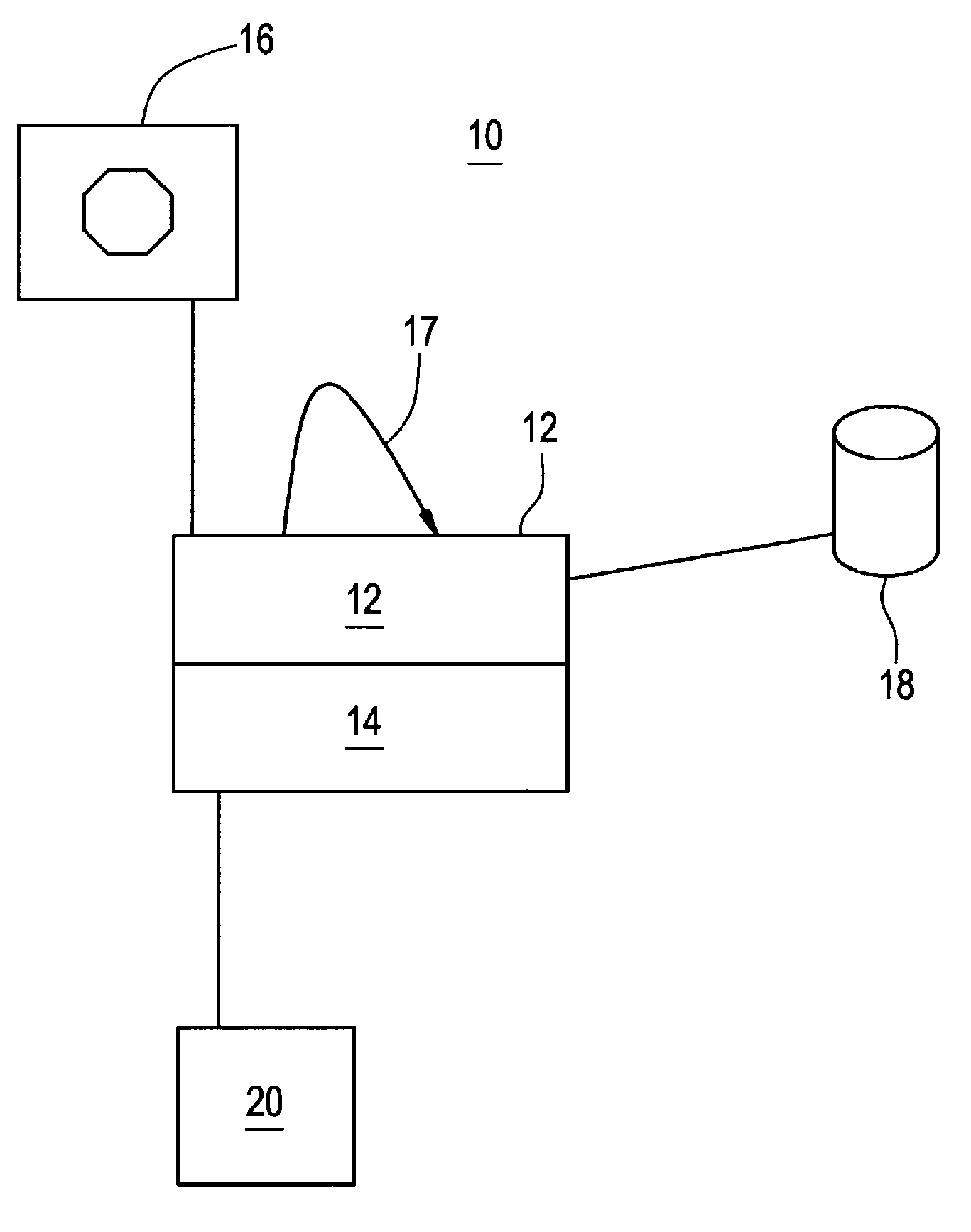
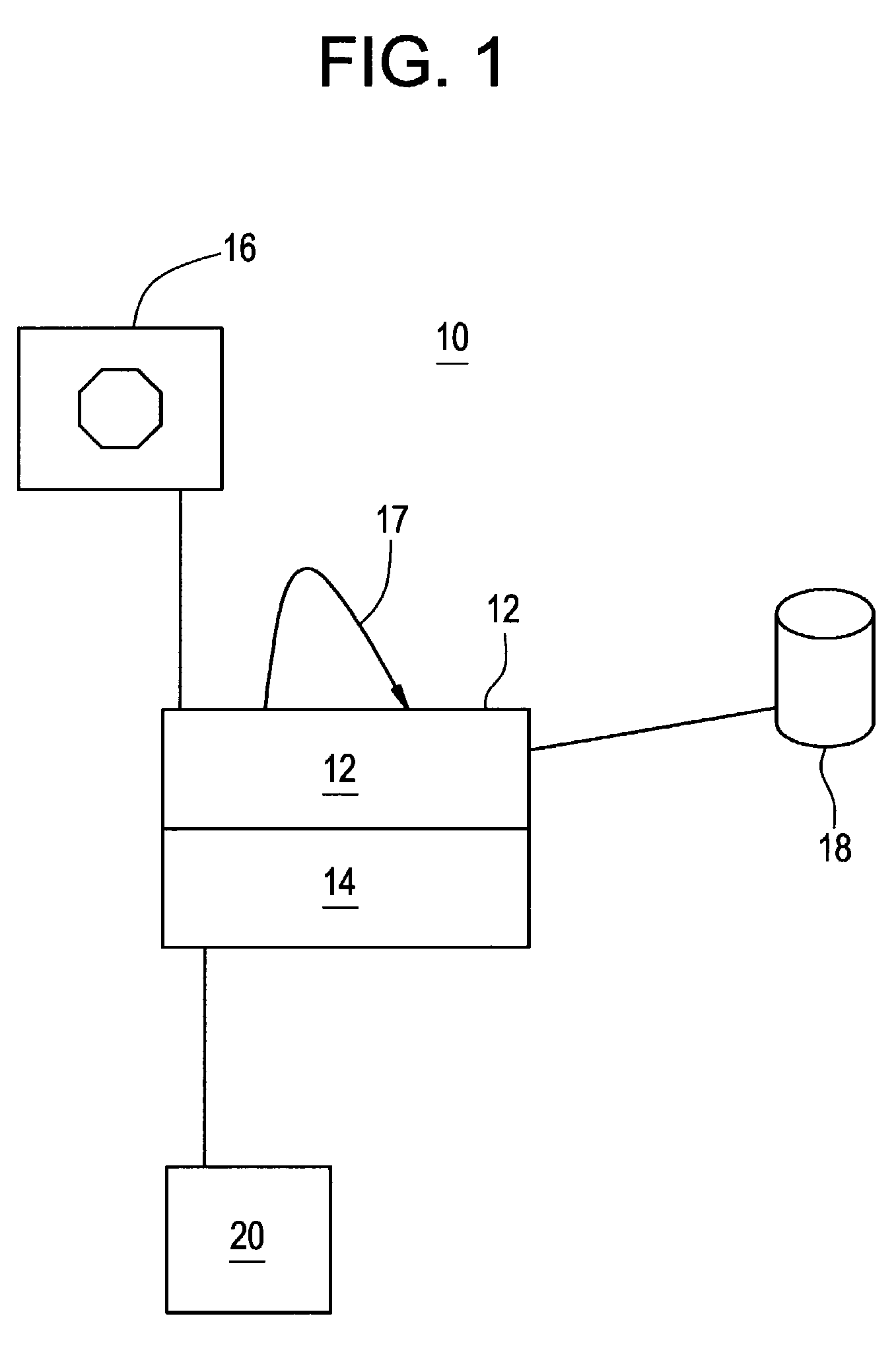
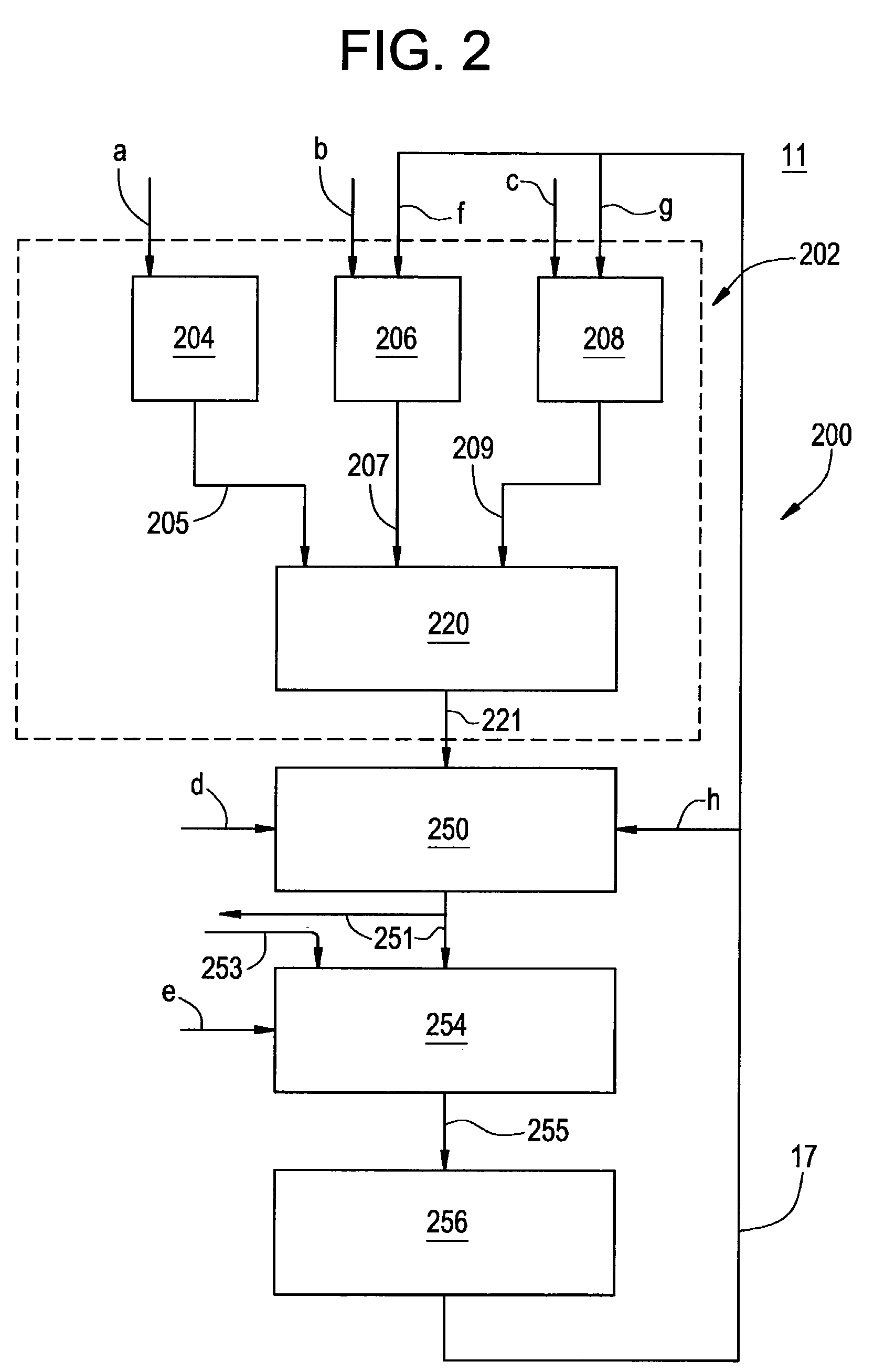
Simulation of nuclear medical imaging
InactiveUS7983735B2Ultrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsSensing dataDigital imaging
A nuclear medicine imaging simulator system is provided for simulating nuclear imaging of a target within a phantom using a selected pharmacokinetic model. The system includes a processor assembly having at least one processor receiving a digital phantom model and a digital pharmacokinetic model, and a dynamic integration module executable on the processor assembly for integrating the pharmacokinetic model with the phantom model to generate a dynamic phantom data representing activity of the pharmacokinetic model within the phantom model over simulated time. The system further includes an imager module executable on the processor assembly for generating a digital imager model representing a nuclear imager in accordance with at least one selectable imager parameter that controls activity of the imager model relative to simulated time, and an imager simulator module executable on the processor assembly for processing the dynamic phantom data with the imager model for simulating at least one imaging process of the dynamic phantom data in accordance with the at least one imager parameter and generating respective simulated sensed data in accordance with individual imaging processes of the at least one imaging process.
Owner:GENERAL ELECTRIC CO
Pharmacokinetic modeling of mycophenolic acid
InactiveUS20090327175A1Reduce varianceGood correlationOrganic chemistryAnalogue computers for chemical processesPharmacokinetic modelingMycophenolic acid
A method of providing a pharmacokinetic model to provide optimize pharmacokinetic data associated with administering a drug to a patient and a method of optimising pharmacokinetic data associated with administering a drug to a patient, data processing apparatus, recording medium and a pharmacokinetic model are disclosed.
Owner:HE XIANG +2
Estimating Pharmacokinetic Parameters in Imaging
ActiveUS20140321723A1Expand selectionImprove stratificationChemical property predictionImage enhancementImage processing softwarePharmacokinetic modeling
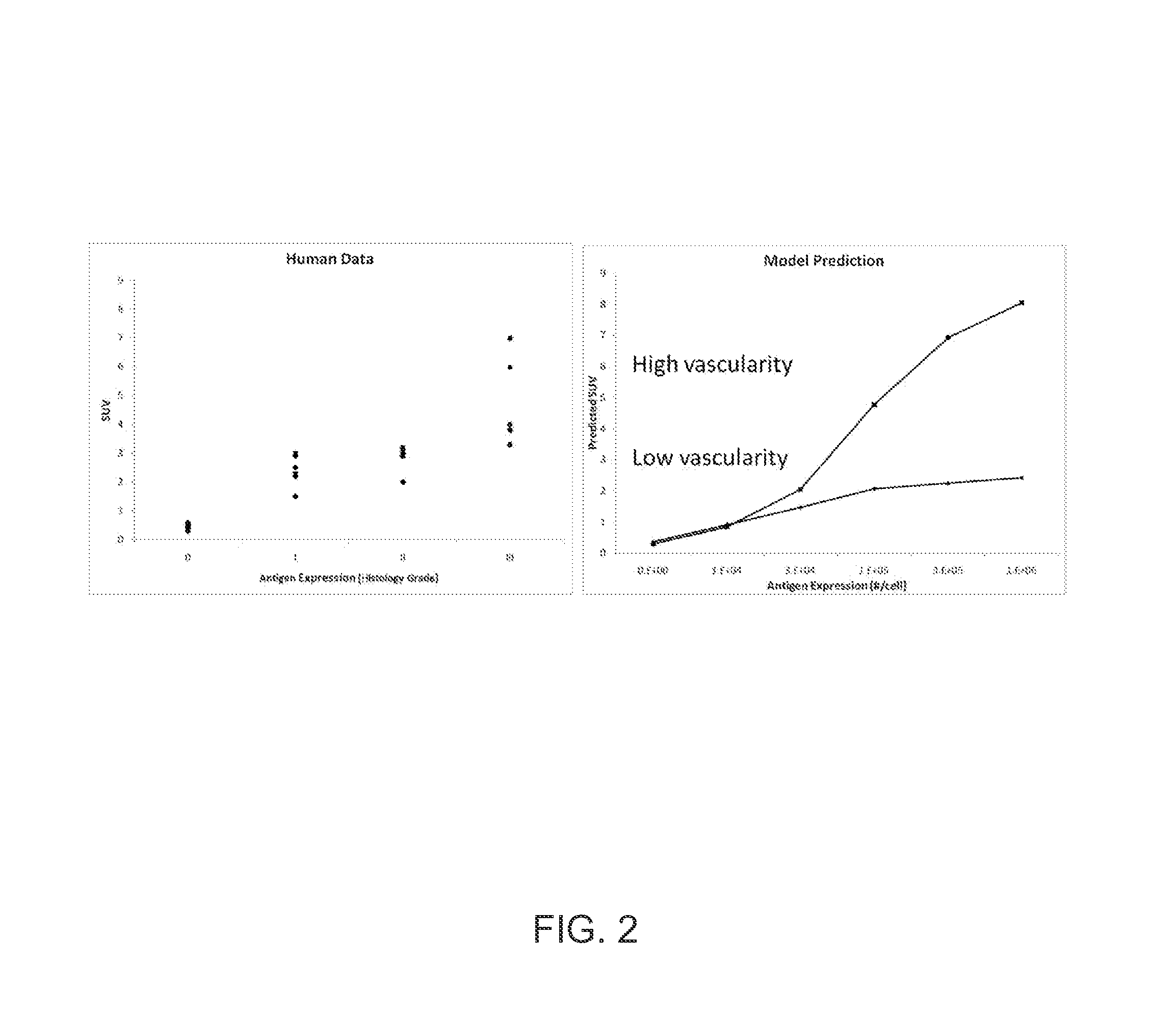
A method is provided for estimating a parameter of physiological significance. One or more images are provided of a tissue in a subject to whom a dose of a contrast agent (CA) has been administered, using a computer equipped with image processing software, the concentration or relative concentration of the agent in a region or regions of interest in the tissue is determined, thus generating concentration data. The time-based behavior of concentrations of CA within the tissue is determined using a pharmacokinetic model that is based on a set of pharmacokinetic model parameters. Using computer code, the pharmacokinetic model is fit to the concentration data, varying one or more parameters, such that a best fit estimate of a parameter of physiological significance is provided.
Owner:INVICRO
Pharmacokinetic analysis system and method thereof
InactiveUS20070239416A1Improve accuracyImprove estimation accuracyChemical property predictionData processing applicationsAlgorithmPharmacokinetic modeling
There are provided a system and a method therefor for analyzing pharmacokinetics in such a manner that the influence of genetic polymorphism of an individual is taken into consideration, and a system and a method therefor for allowing implementation of high-accuracy haplotype frequency estimation and diplotype configuration estimation even in a case where the number of individuals is small from which data is obtainable when making pharmacokinetic analysis. A diplotype configuration estimation step estimates diplotype configurations of individuals. Next, a pharmacokinetic model building step expresses pharmacokinetic parameters as functions of the diplotype configurations of the individuals, thereby configuring pharmacokinetic models. Moreover, a pharmacokinetic parameter estimation step estimates the pharmacokinetic parameters. Here, a diplotype configuration correction step corrects the estimation result of the diplotype configurations of the individuals, thereby implementing an enhancement in the estimation accuracy.
Owner:HITACHI LTD
Method of visualizing a bridge therapy process
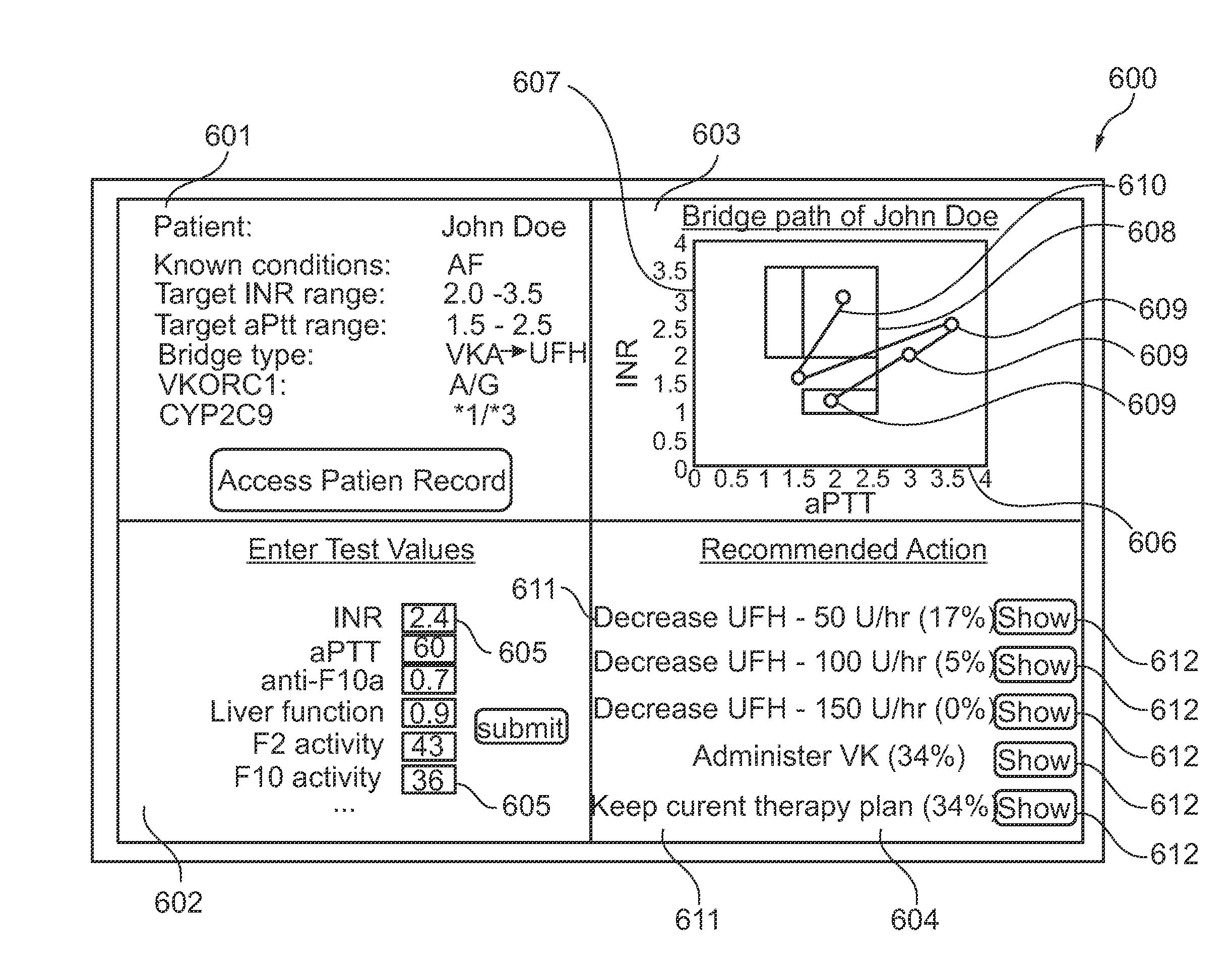
ActiveUS20140118356A1Good bridgingBalance securityMedical simulationDrawing from basic elementsThrombusPharmacokinetic modeling
The present invention provides for a simultaneous graphical representation, a risk of bleeding and a risk of thrombosis providing a visualized bridge therapy process. Furthermore, the present invention provides for a computer-based prediction of the haemostatic situation of the examined blood circulation by using a combination of a biochemical model and a pharmacokinetic model for calculation or another mathematical representation of the blood circulation.
Owner:KONINKLJIJKE PHILIPS NV
System that generates pharmacokinetic analyses of oligonucleotide total effects from full-scan mass spectra
System that automates analysis of mass spectrometry data for oligonucleotides to generate pharmacokinetic parameters and models. A user inputs an oligonucleotide sequence and a maximum number of nucleotides that may be lost during metabolism while retaining therapeutic effectiveness. The system calculates the possible active metabolites and develops a mass spectrum filter for the mass-to-charge ratio of ions for these metabolites. Full-scan spectra are analyzed to calculate the total concentration of these active molecules present in a time series of samples. Pharmacokinetic models and parameters are calculated from the time series of total concentration. Because full-scan spectra are captured, assumptions may be modified and analyses may be quickly rerun without collecting additional data. Overall pharmacokinetic analysis is therefore much more streamlined and efficient, reducing cost, delay, and the need for a mass spectrometrist who is highly skilled in spectral analysis.
Owner:BIOTUNE COMPUTATIONS LLC
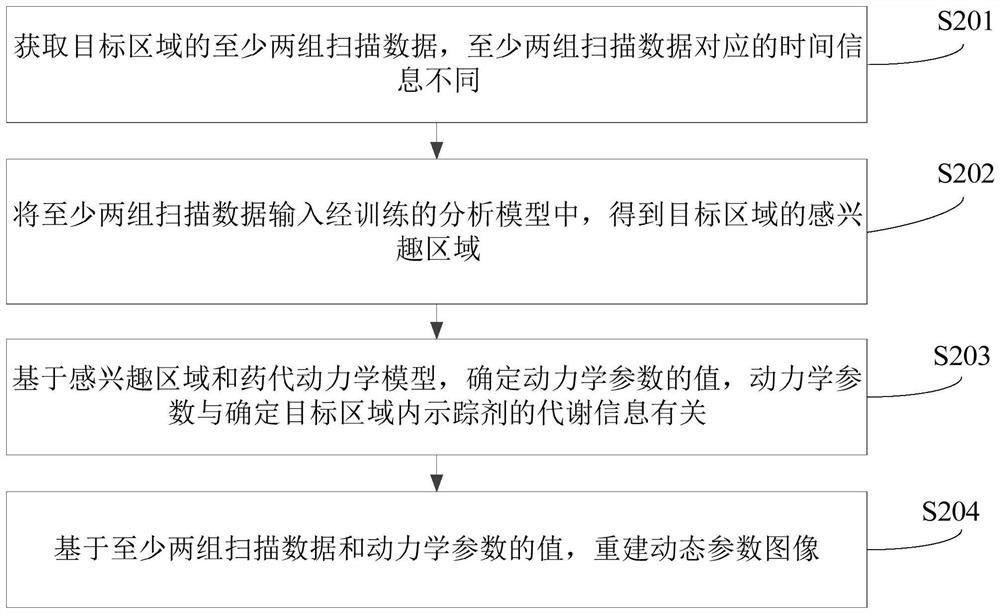
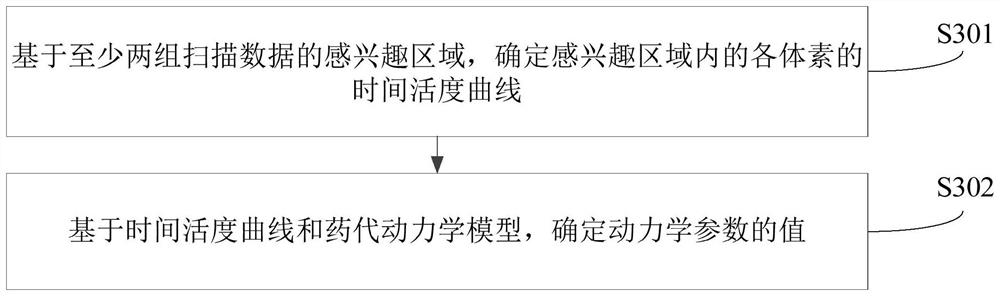
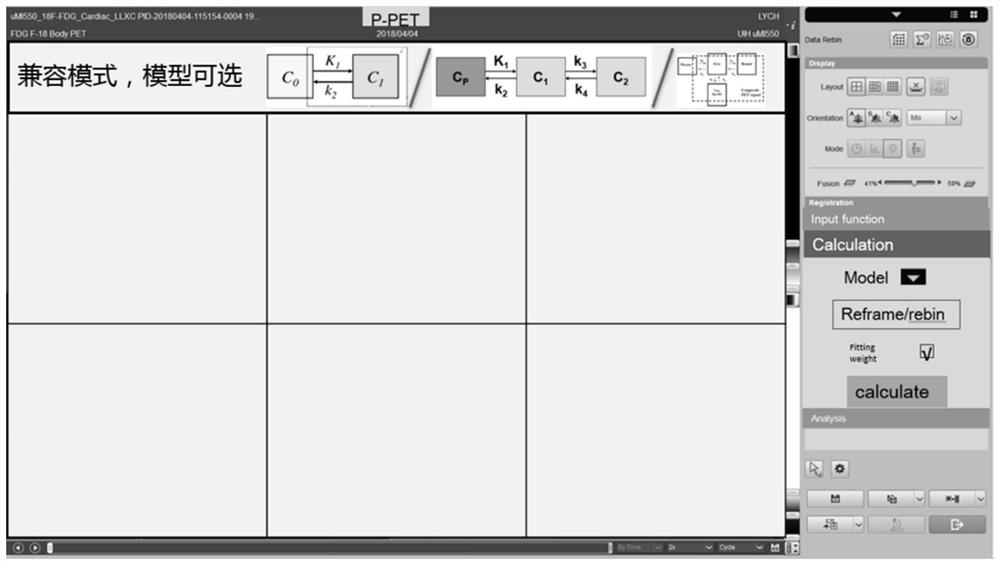
Dynamic parameter determination method and device, computer equipment and storage medium
The invention relates to a Dynamic parameter determination method and device, computer equipment and A storage medium. The method comprises the following steps: acquiring at least two groups of scanning data of a target area, wherein time information corresponding to the at least two groups of scanning data is different; inputting the at least two groups of scanning data into a trained analysis model to obtain a region of interest of the target region; determining values of kinetic parameters on the basis of the region of interest and a pharmacokinetic model, the kinetic parameters being related to the metabolic information of the tracer agent in the target region; and reconstructing a dynamic parameter image based on the at least two pieces of scanning data and the value of the kinetic parameter. By adopting the method, the efficiency of acquiring the PET kinetic parameters can be improved.
Owner:SHANGHAI UNITED IMAGING HEALTHCARE
Method for building population pharmacokinetics model of multiple components of compound salvia miltiorrhiza drop pills in rats
The invention relates to a method for building a population pharmacokinetics model of multiple components of compound salvia miltiorrhiza drop pills in rats. The method comprises the following steps of: (1) determining sampling point time: grouping sampling points at random on the basis of holographic sampling, with 3-4 sampling points in each group; (2) determining to-be-investigated covariates (the covariates are influence factors): taking physiological and pathological information of rats as the to-be-investigated covariates and investigating the influences of these factors on drug metabolism; (3) incorporating a certain number of tested samples: incorporating the tested samples for the covariates, and confirming that the incorporated samples are uniformly distributed for the covariatesand the test range is relatively wide and meets the later prediction range; (4) carrying out administration, sample acquisition, information collection and sample determination: determining an administration scheme of a compound salvia miltiorrhiza drop pill solution, acquiring blood samples within a predetermined sampling time range, recording corresponding acquisition time, recording individualrelated information for the predetermined covariates and completing sample determination work by using a detection method; and (5) building a model.
Owner:TIANJIN TASLY PHARMA CO LTD
System and method for ranking options for medical treatments
ActiveUS20200152307A1Facilitate communicationEnhanced advantagePhysical therapies and activitiesMedical simulationEfficacyPharmacokinetic modeling
A computer system, computer program product and method for determining a probability of attaining a PK-PD target associated with efficacy for a patient that includes a processor obtaining information identifying an infection and based on the information, generating and displaying, by the one or more processors, a list comprising one or more pathogens consistent with the information, the processor then obtaining a first indication designating at least one pathogen from the list comprising one or more pathogens and based on at the obtaining of the least one pathogen, generating a list comprising one or more drug therapies utilized to treat the at least one pathogen. The method also includes the processor obtaining, descriptive information relating to a patient and based on the one or more drug therapies, selecting a pharmacokinetic model and the processor applying the pharmacokinetic model and utilizing the information relating to the patient to determine, for each of the one or more drug therapies, a probability of attaining a PK-PD target associated with efficacy for the patient with the infection.
Owner:PRXCISION LLC
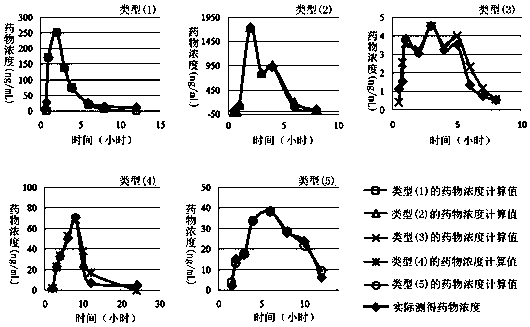
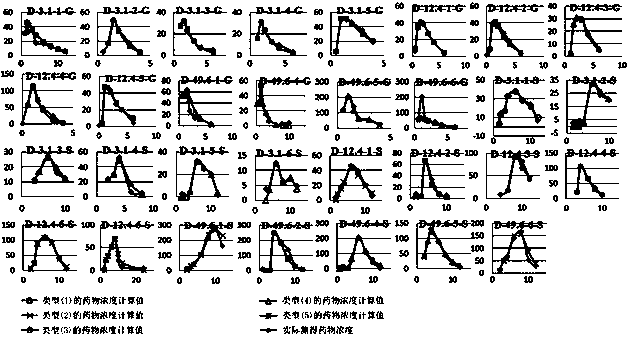
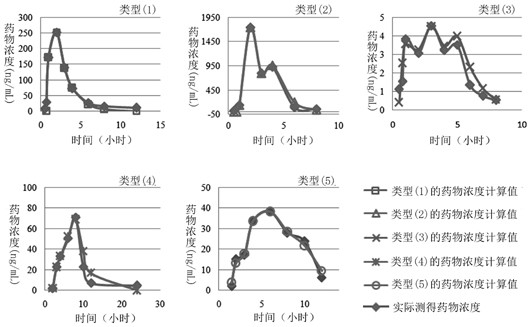
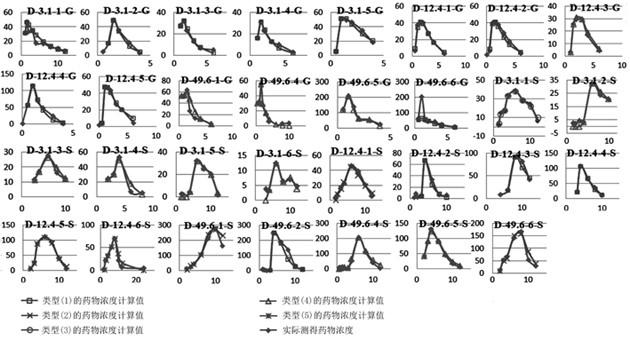
Method for creating animal pharmacokinetic model for drug multi-peak phenomenon
Owner:SUN YAT SEN UNIV
Method for amplifying NSCs and inhibiting it to neuroglial cell differentiation and its use
The invention relates to a method to induce nerve stem cell expanding and restrict differentiation toward gial cell. It adopts a serum-free culture system containing a new type grain legumes agglutinin to induce nerve stem cell expanding and restrict differentiation toward gial cell. It provides evidence to nerve cell renewable theory and supplies daughter cell to nerve cell transplant and micro-encapsulation preparation. It could be also used as extraneous pharmacokinetics model to selecting cell medicament. The invention would have great application prospect, and great social and economic benefits.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
System and method for ranking options for medical treatments
ActiveUS10534895B2Facilitate communicationDigital data information retrievalDrug and medicationsEfficacyPharmacokinetic modeling
A computer system, computer program product and method for determining a probability of attaining a PK-PD target associated with efficacy for a patient that includes a processor obtaining information identifying an infection and based on the information, generating and displaying, by the processor, a list comprising one or more pathogens consistent with the information, the processor then obtaining a first indication designating at least one pathogen from the list comprising one or more pathogens and based on at the obtaining of the least one pathogen, generating a list comprising one or more drug therapies utilized to treat the at least one pathogen. The method also includes the processor obtaining, descriptive information relating to a patient and based on the one or more drug therapies, selecting a pharmacokinetic model and the processor applying the pharmacokinetic model and utilizing the information relating to the patient to determine, for each of the one or more drug therapies, a probability of attaining a PK-PD target associated with efficacy for the patient with the infection.
Owner:PRXCISION INC
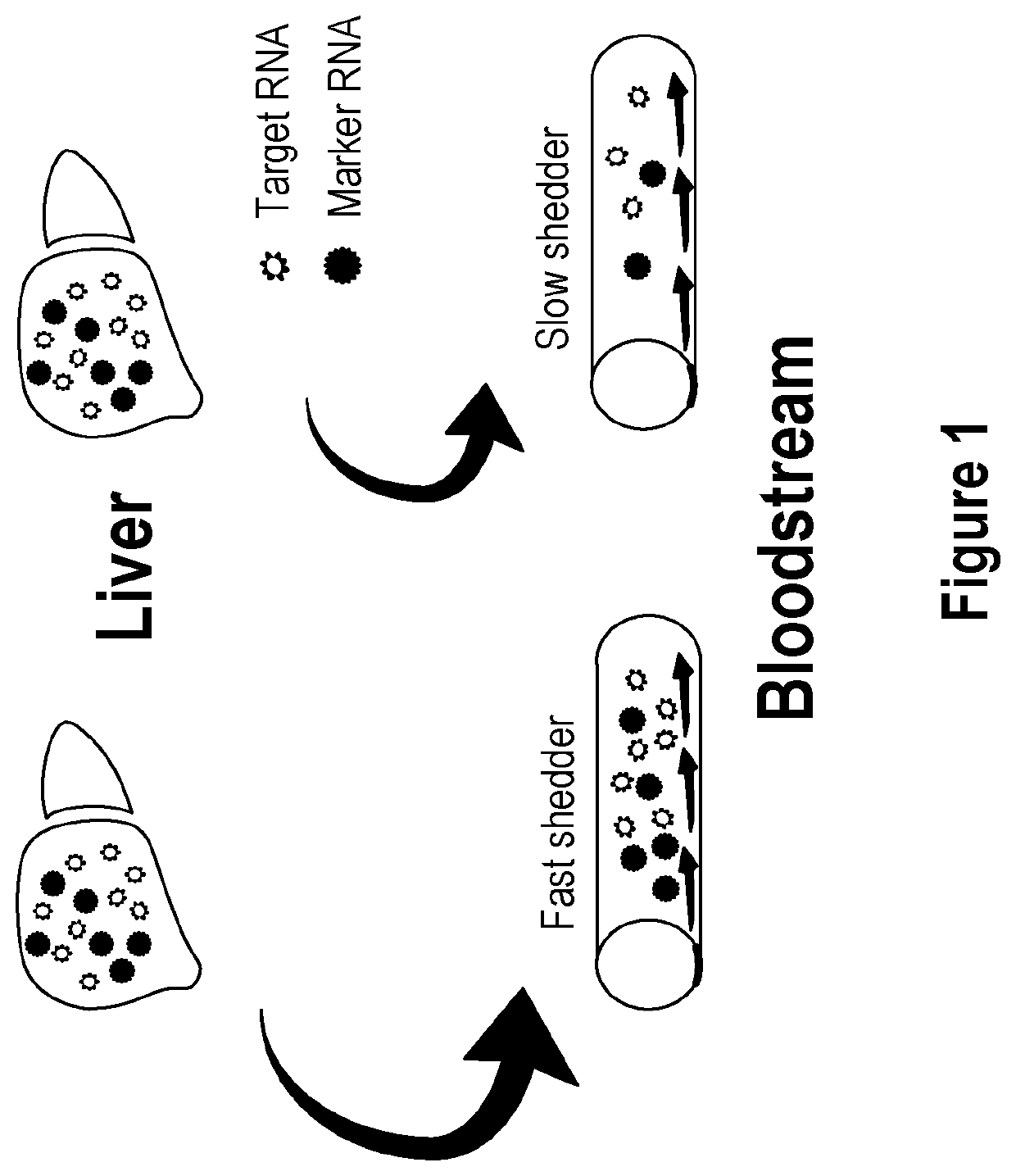
Methods and apparatus for generating a virtual model of xenobiotic exposure using transcriptomics analysis of liquid biopsy samples
PendingUS20220215898A1Improve the level ofReduce variationChemical property predictionMicrobiological testing/measurementCell freeProtein
Processes are provided for establishing a virtual physiologically based pharmacokinetic (PBPK) model in a population comprised of a plurality of individual subjects that has been or may be exposed to a xenobiotic molecule. The processes are derived from the identification of an abundance of a protein that is involved in absorption; distribution; localization; biotransformation; and excretion of the xenobiotic molecule from a liquid biopsy of corresponding cell free RNA. Personalised PBPK models for precision dosing, as well as methods of treatment are also provided.
Owner:CERTARA USA INC
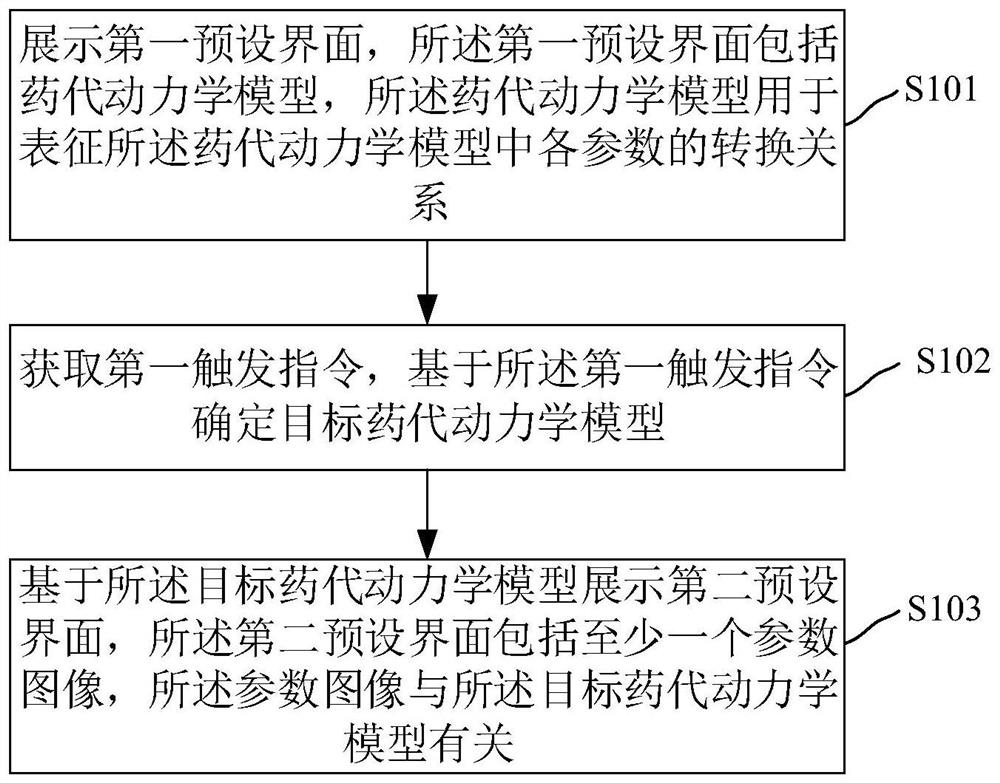
Dynamic parameter imaging visualization method and device, electronic device and storage medium
PendingCN114010212AWide applicabilityComputerised tomographsTomographyComputer graphics (images)Pharmacokinetic modeling
The invention relates to a dynamic parameter imaging visualization method and device, an electronic device and a storage medium, and the dynamic parameter imaging visualization method comprises the steps: displaying a first preset interface which comprises a pharmacokinetic model, wherein the pharmacokinetic model is used for representing a conversion relation of parameters in the pharmacokinetic model; obtaining a first trigger instruction, and determining a target pharmacokinetic model based on the first trigger instruction; displaying a second preset interface based on the target pharmacokinetic model, wherein the second preset interface comprises at least one parameter image, and the parameter images are related to the target pharmacokinetic model. According to the dynamic parameter imaging visualization method and device, the electronic device and the storage medium, the conversion relation of the parameters of the pharmacokinetic model is displayed on the display interface, a user can understand the conversion relation visually, and the application range is wider.
Owner:SHANGHAI UNITED IMAGING HEALTHCARE
Method for inducing stem cell to liver cell directional diferentiation and use of liver cell
The present invention relates to biological medicine field, concretely relates to a method for inducing stem cell to liver cell directional differentiation and use of liver cell. The invention combines the genetic engineering with the stem cell engineering, uses the latent energy of the stem cell multiway differentiation, chooses the liver stroma cell for stably expressing human liver cell growth factor, induces the stem cell to liver cell directional differentiation, and induces the fore-and-after stem cell to execute comparative identification. The build of the external induce system not only provides proof for the theory of 'adult stem cell plasticity ', but also can work as seed cell for preparing the stem cell transplant, biological artificial liver and microencapsulation stem cell preparations; the induced and differentiated stem cell can be as external pharmacokinetic model for cell drug screen selecting. The method will has excellent application foreground in regeneration medicine based on genetic engineering, cell engineering and organizing engineering, and will generate great social benefit and economic benefit.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
System that generates pharmacokinetic analyses of oligonucleotide total effects from full-scan mass spectra
ActiveUS20200402617A1More simplifiedMicrobiological testing/measurementBiostatisticsNucleotideTherapeutic effect
System that automates analysis of mass spectrometry data for oligonucleotides to generate pharmacokinetic parameters and models. A user inputs an oligonucleotide sequence and a maximum number of nucleotides that may be lost during metabolism while retaining therapeutic effectiveness. The system calculates the possible active metabolites and develops a mass spectrum filter for the mass-to-charge ratio of ions for these metabolites. Full-scan spectra are analyzed to calculate the total concentration of these active molecules present in a time series of samples. Pharmacokinetic models and parameters are calculated from the time series of total concentration. Because full-scan spectra are captured, assumptions may be modified and analyses may be quickly rerun without collecting additional data. Overall pharmacokinetic analysis is therefore much more streamlined and efficient, reducing cost, delay, and the need for a mass spectrometrist who is highly skilled in spectral analysis.
Owner:BIOTUNE COMPUTATIONS LLC
System and method for ranking options for medical treatments
ActiveUS11227680B2Facilitate communicationPhysical therapies and activitiesMedical simulationEfficacyPharmacokinetic modeling
Owner:PRXCISION INC
A method for creating a canine pharmacokinetic model for drug multimodality
The invention relates to a method for creating an animal pharmacokinetic model of drug multi-peak phenomenon. Include the following steps: select at least 6 dogs, half male and half female, administer according to the dose, collect blood samples after administration, and ensure that at least 2 blood collection points are collected before the first peak at the time point of blood collection, near the highest value of each peak At least 3 blood collection points were collected, and at least 3 blood collection points were collected after the last peak; blood drug concentration was measured by liquid chromatography mass spectrometry; the fitting constant was calculated according to 5 types of dogs ( DM), The minimum value is the pharmacokinetic standard model class for the dog. This model can establish a standard model in the dog body, and correctly calculate the pharmacokinetic parameters of this type of drug, and obtain a reasonable dosage regimen. The pharmacokinetic parameters of the multimodal drug calculated by the generation software are too small, which causes the defect of excessive dosage in dogs.
Owner:SUN YAT SEN UNIV
Modeling of drug delivery and parameter generation of injection protocols
InactiveCN103221071BReduce loadImprove consistencyMedical simulationInfusion devicesDiscretizationSystem parameters
A system includes a parameter generation system for determining at least one parameter of an injection procedure (eg, an injection protocol parameter or an imaging system parameter), the parameter generation system including a physiology for modeling the delivery of a contrast agent injected into a patient A pharmacokinetic model based on: at least one nonlinear saturation term in the peripheral venous compartment, at least one constructible transport delay term across at least one compartment, or a volume for modeling blood Improvements in flow rate and its effect on contrast medium delivery after contrast injection has ceased. The physiologically based pharmacokinetic model may, for example, be discretizable.
Owner:BAYER HEALTHCARE LLC
Pharmacokinetic analysis method for monitoring therapeutic drugs
PendingCN114155978AGood curative effectReduce the risk of adverse reactionsDrug referencesComputational theoretical chemistryMetaboliteMedication monitoring
A pharmacokinetic analysis method for monitoring therapeutic drugs comprises the following steps: (1) establishing a plasma concentration determination method: establishing the plasma concentration determination method capable of simultaneously monitoring the therapeutic drugs and main metabolites thereof in a plasma sample of a patient by adopting UPLC-MS / MS, and performing methodological verification on the established plasma concentration determination method; (2) establishing a pharmacokinetic model; and (3) monitoring treatment drugs. The pharmacokinetic analysis method for monitoring the therapeutic drug is reasonable in design, provides a basis for individualization of clinical medication of the therapeutic drug by taking the therapeutic drug as a monitoring target, taking blood concentration monitoring as a core and combining pharmacokinetic research data, and provides a basis for clinical medication individualization of the therapeutic drug by monitoring the therapeutic drug for a patient. The curative effect of the treatment medicine can be obviously improved, the occurrence risk of adverse reaction of the treatment medicine is reduced, and the application prospect is wide.
Owner:SUZHOU LEO BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com