Method for building population pharmacokinetics model of multiple components of compound salvia miltiorrhiza drop pills in rats
A technology of pharmacokinetics and construction methods, applied in the field of construction of multi-component compound pharmacokinetic models of compound Danshen dripping pills in vivo in rats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
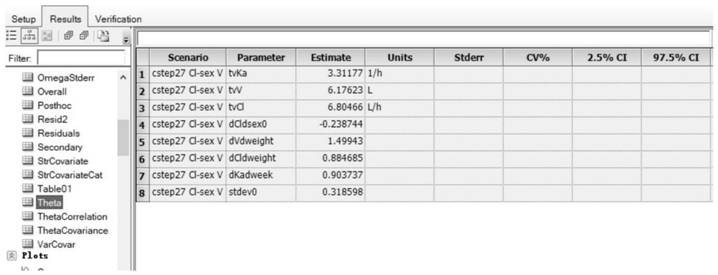
[0117] Example 1: Method for constructing the population pharmacokinetic model of compound Danshen dripping pills multi-components in rats
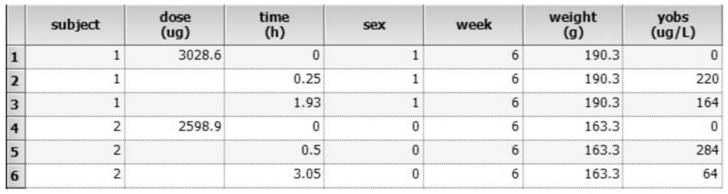
[0118] 1. Proposed sampling point time: Proposed sampling point time: Considering the long half-life of ginsenoside Rb1, the designed sampling points are: 5min, 15min, 30min, 1h, 1.5h, 2h, 3h, 4h, 6h, 8h, 10h, 12h, 24h, 48h, 72h. Divide the sampling points into 4 groups, (Group A: 5min, 1.5h, 6h) (Group B: 15min, 2h, 8h, 24h) (Group C: 30min, 3h, 10h, 48h) (Group D: 1h, 4h, 12h, 72h).
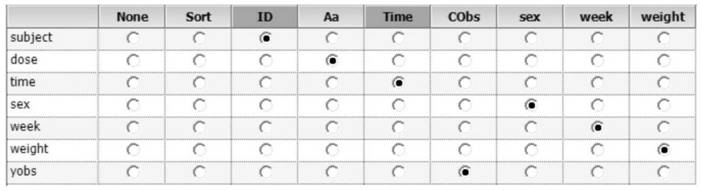
[0119] 2. Determine the influencing factors to be investigated (influencing factors are referred to as covariates hereinafter): the physiological and pathological information of the rats are used as the covariates to be investigated, and the influence of these factors on drug metabolism is investigated, and finally the sex, age, and body weight of the rats are determined. as a covariate to be investigated.
[0120] 3. A certain number of test samples we...
Embodiment 2
[0168] Example 2. Analytical method of danshensu, ginsenoside Rb1, Rg1 in rat plasma
[0169] 1. Chromatography and mass spectrometry conditions Liquid phase method: Shimadzu UFLC liquid phase system, using ACQUITY from Waters HSS T3column (1.8μm, 2.1mm×100mm) chromatographic column, equipped with pre-column VanGuard TM HSS T3pre-column (1.8μm, 2.1mm×5mm), flow rate 0.4mL / min, column temperature 40°C, mobile phase: A: 0.1% formic acid-water, B: acetonitrile. Gradient elution is used, taking the change of phase B as a reference, and the elution program is: 0→0.8min, 5%; 0.8→3.1min, 5%→90%; 3.1→3.3min, 90%→5%, 3.3→4.5 min, 5%.
[0170] Mass spectrometry conditions: Mass spectrometry detection is carried out synchronously according to the positive and negative ion modes.
[0171] Positive ion mode: curtain gas pressure (Curtain gas, abbreviated as GUR): 20psi, ion source voltage: 5500V, ion source temperature: 550°C, ion source gas 1: 55psi, ion source gas 2: 55psi; internal...
Embodiment 3
[0181] Embodiment 3: rat experiment
[0182] 1. Dosing and sampling plan
[0183] Compound Danshen dripping pills were given by intragastric administration, and the administration time was arranged at 8:00-9:00 am on the day of the experiment. The dosage of compound Danshen dripping pills was 1.5g / kg. The dissolution rate is 0.3g / mL, the administration volume is 1mL / 200g, and the specific drug volume is adjusted according to the body weight of the rat. Rats were fasted for 12 hours before administration, had free access to water, and ate uniformly 3 hours after administration.
[0184] Considering the long half-life of ginsenoside Rb1, the design sampling points are: 5min, 15min, 30min, 1h, 1.5h, 2h, 3h, 4h, 6h, 8h, 10h, 12h, 24h, 48h, 72h. Divide the sampling points into 4 groups, (Group A: 5min, 1.5h, 6h) (Group B: 15min, 2h, 8h, 24h) (Group C: 30min, 3h, 10h, 48h) (Group D: 1h, 4h, 12h, 72h), and then randomly divide rats of different sexes and ages into each group, coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com