Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Peg interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
What PEG Interferon Is Used For: This medication is used to treat chronic hepatitis C. This medication is also being investigated for use in various cancers. Note: If a drug has been approved for one use, physicians sometimes elect to use this same drug for other problems if they believe it might be helpful.
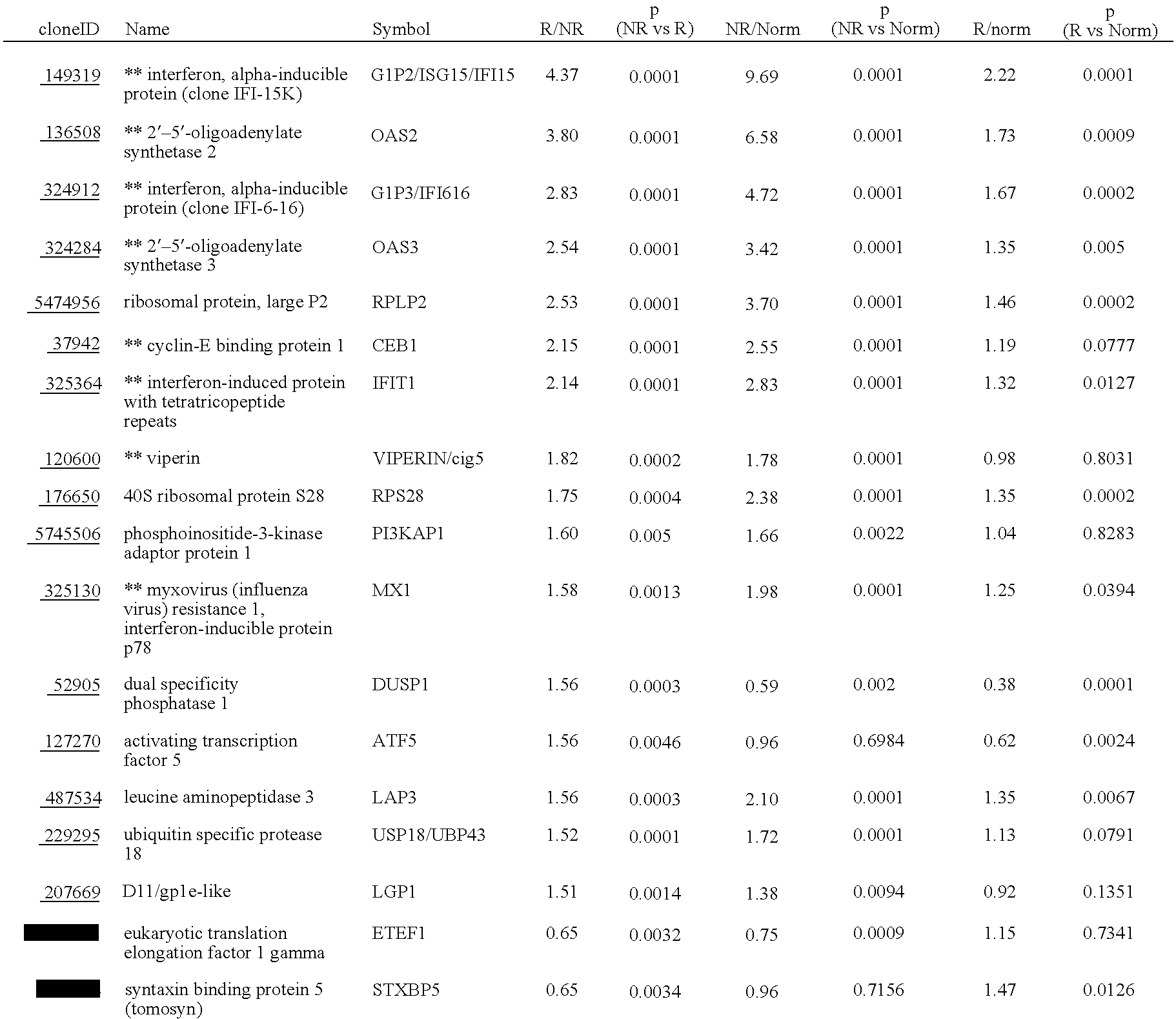
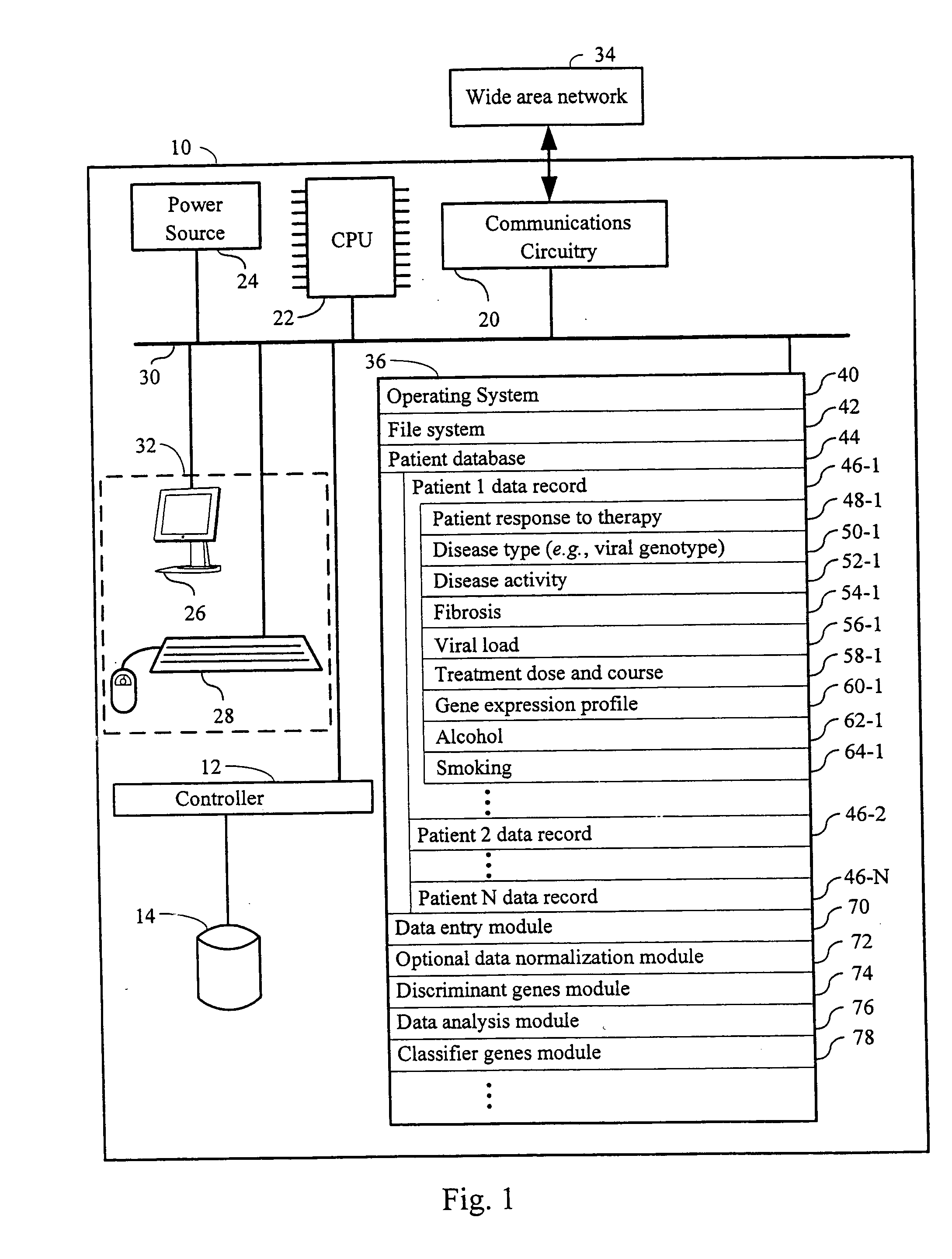
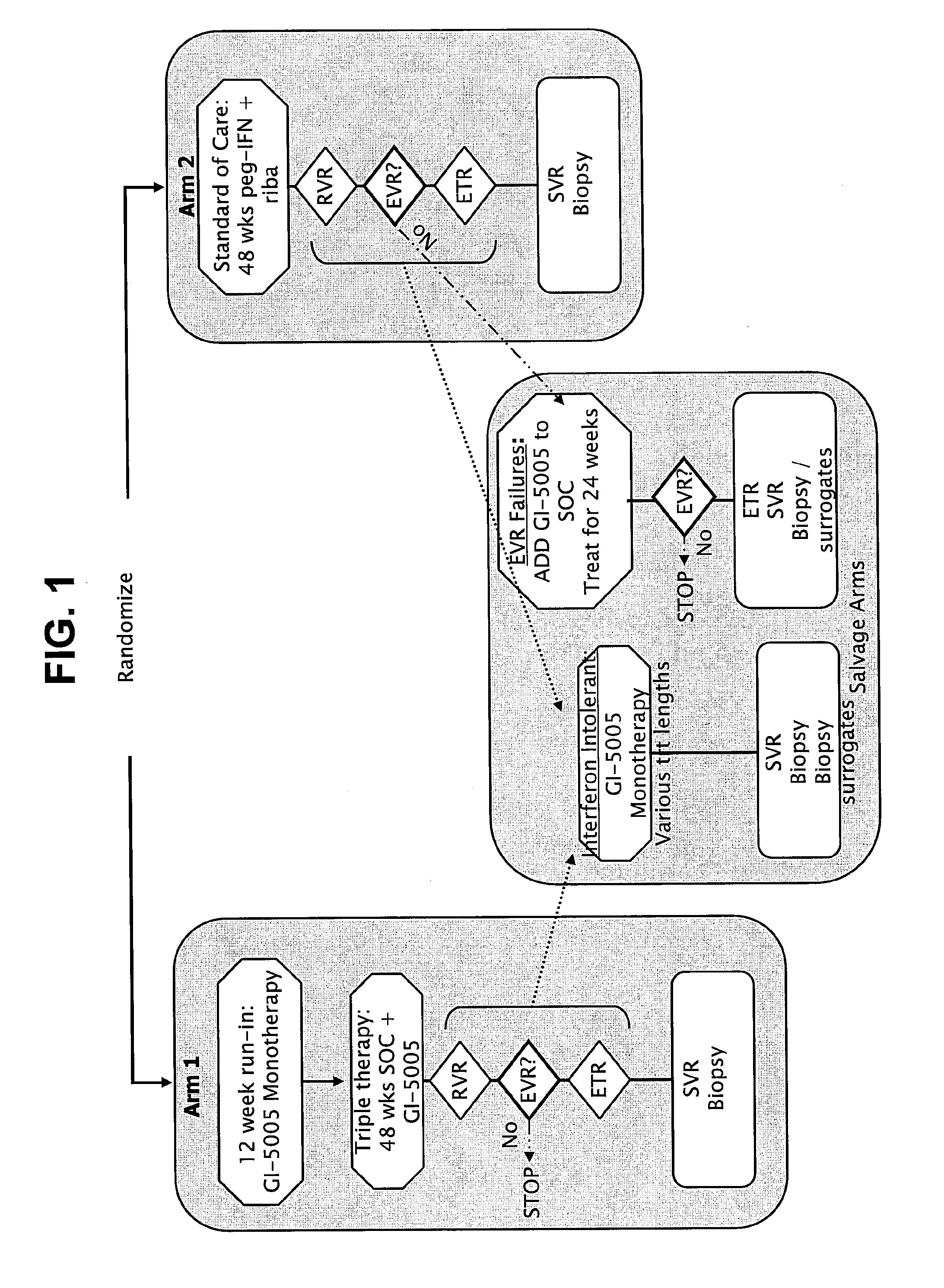
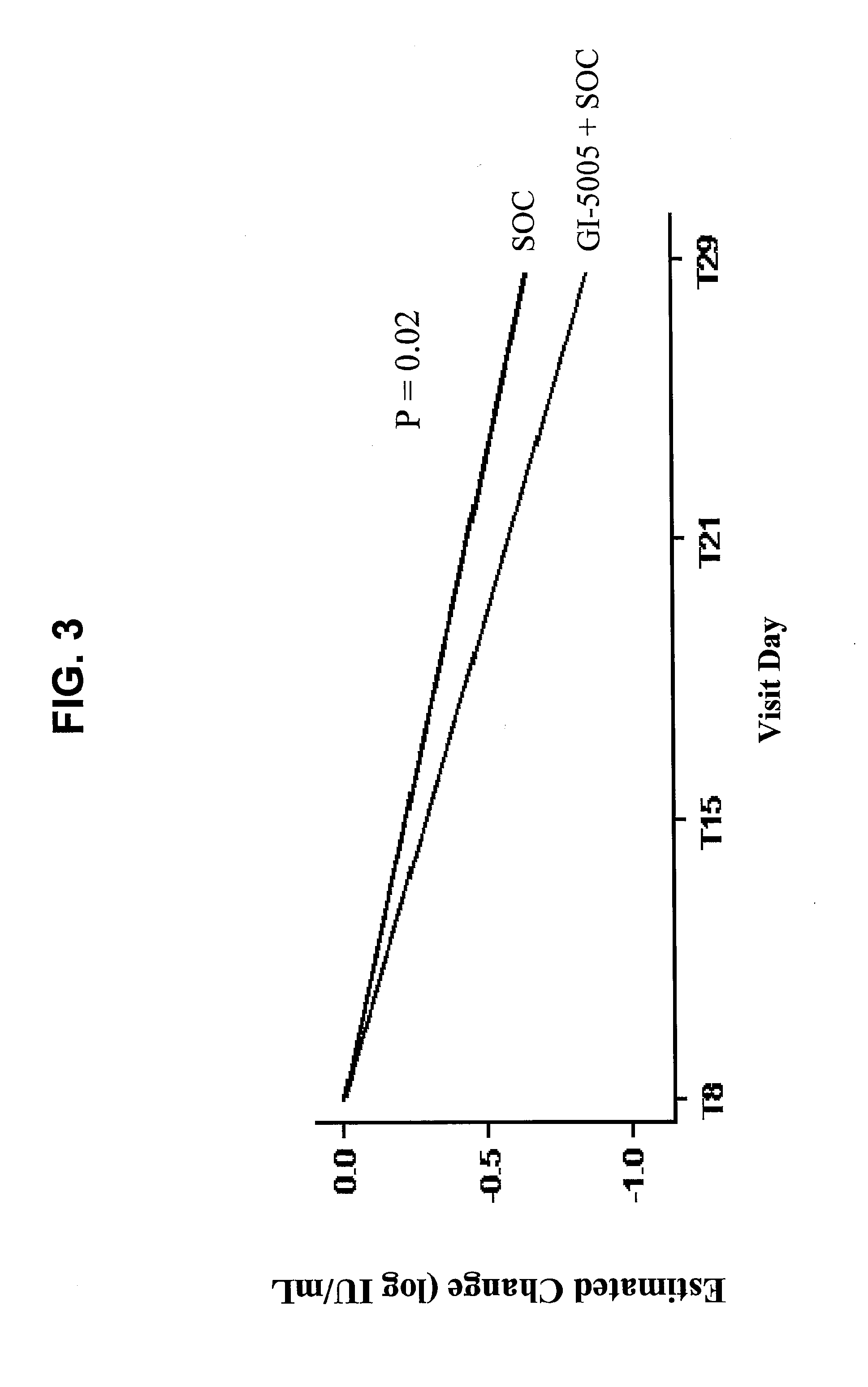
Systems and methods for identifying diagnostic indicators
InactiveUS20060177837A1Enhance and improve therapeutic effectReduces liver disease activityMicrobiological testing/measurementDrug and medicationsHepatitis c viralRegimen
Systems and methods are provided for predicting patient response to a therapy regimen for a liver disease or a disease that is treatable with an immunomodulatory disease therapy using gene expression classifiers. Systems and methods for screening for modulators of target gene expression are also provided. Systems and methods for developing therapeutics against one or more of the proteins coded for by genes of the present invention are also provided. Systems and methods for predicting a patient response to a regimen of pegylated interferon alpha and ribavirin in a therapy for hepatitis C viral infection are also provided.
Owner:JAGUAR BIOSCI
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Treatments for viral infections using IFN cytokines and ribavirin, alone or in combination
InactiveUS20060024271A1Reduce dosageReduced pro-inflammatory responseBiocidePeptide/protein ingredientsCytokineViral infection
A treatment or prophylaxis for viral infection using interferon (IFN) cytokines alone or in combination with ribavirin is provided. The treatments and prophylaxis allow for lowered dosages of IFNs, reduced pro-inflammatory responses, and delays the initiation time and reduced frequency of the IFN treatment required.
Owner:AFG BIOSOLUTIONS
Formulations of peg-interferon alpha conjugates
Lyophilized and stabilized formulations of PEG-Interferon alpha conjugates and the process for their preparation that reduces lyophilization cycle time and are more cost competitive.
Owner:CADILA HEALTHCARE LTD
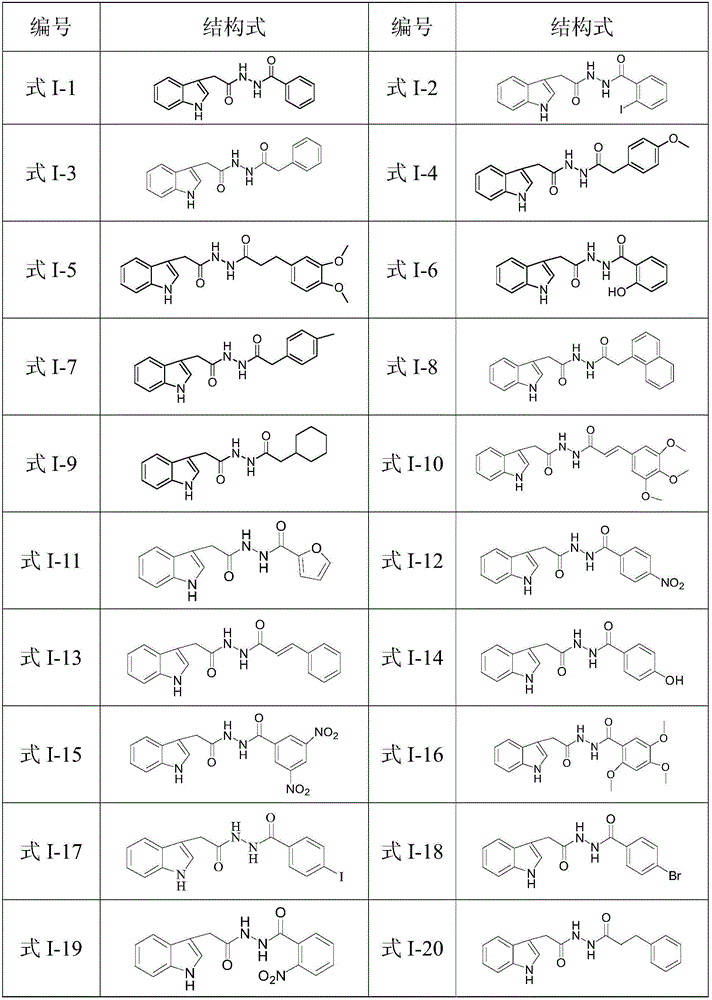
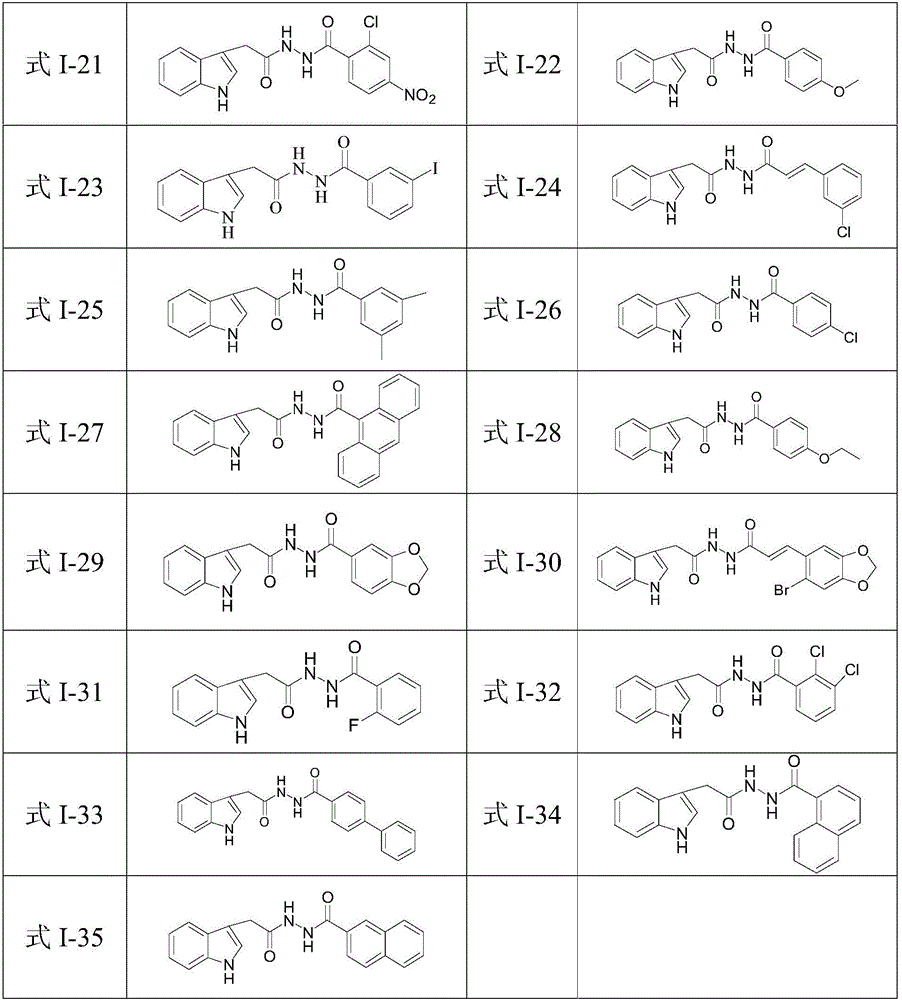
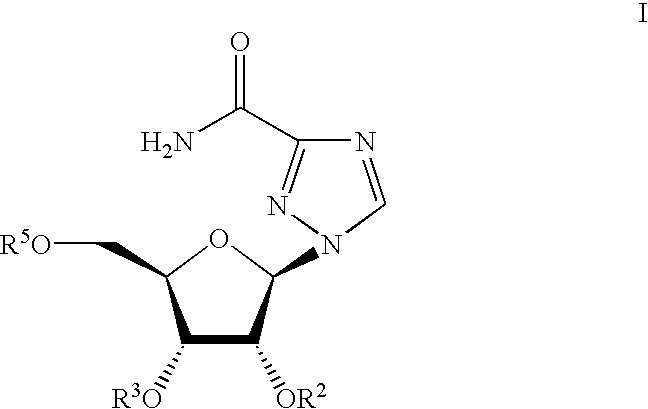
N'-(2-(1H-indole-3-yl)acetyl)arylhydrazide compound and preparation method and application thereof
InactiveCN105732468AEnhanced inhibitory effectImprove therapeutic indexOrganic chemistryAntiviralsVirusInhibitory effect
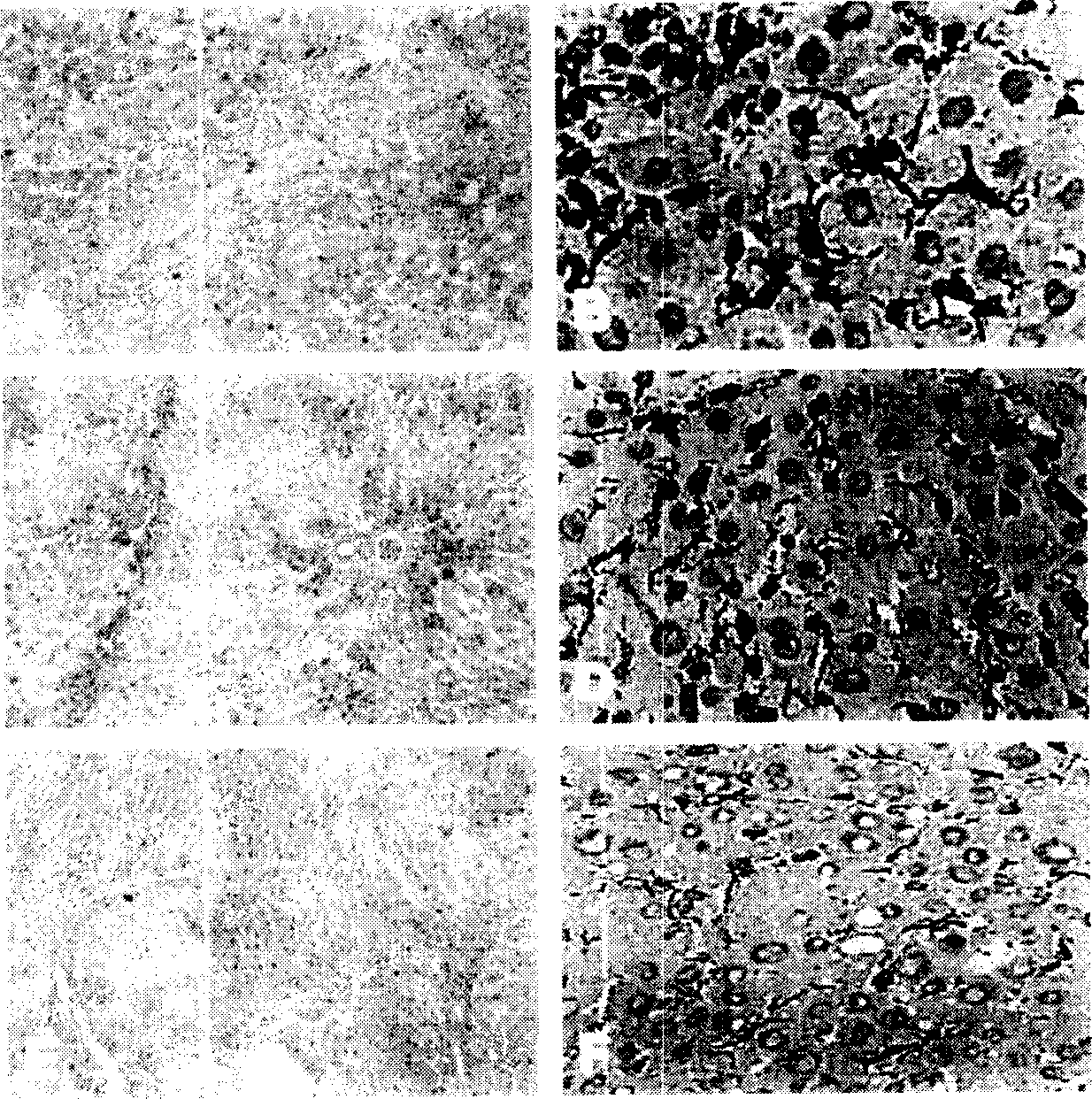
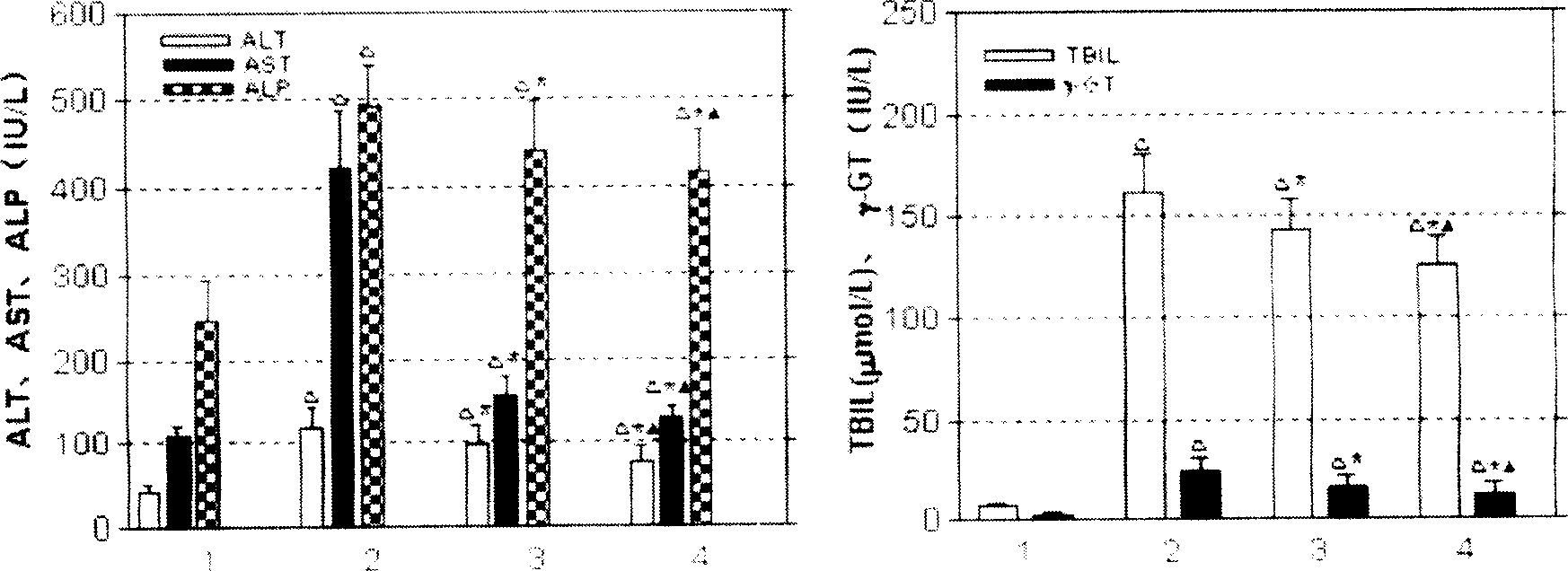
The invention provides a N'-(2-(1H-indole-3-yl)acetyl)arylhydrazide compound and a preparation method and application thereof.The N'-(2-(1H-indole-3-yl)acetyl)arylhydrazide compound has a structure shown as a formula I (please see the formula in the description) and has the obvious inhibiting effect on an HCV virus, the anti-HCV virus treatment index is higher than that of existing clinical drugs including alpha-1b(IFNalpha-1b) interferon and ribavirin, and the N'-(2-(1H-indole-3-yl)acetyl)arylhydrazide compound can serve as an anti-HCV drug candidate; in addition, the preparation method is simple, high in yield and suitable for industrial production.
Owner:KUNMING UNIV OF SCI & TECH +1
Cyclic peptide containing arginine, glycine, asparagicacid-sequence and active target liposome
InactiveCN1687118ASmall molecular weightEasy to retouchDigestive systemPeptidesArginineTherapeutic effect
The present invention belongs to the field of pharmaceutical technology and clinical pharmacy, relates to a cyclopeptide containing arginine-glycine-aspartic acid (RGD) sequence and its active liposome targeting, its preparation and application. The described cyclopeptide structure meets the requirements as ligand, and is cyclized by amido bond, its spatial conformation is stable, and is not easily degraded, its one end contains active sulphydryl group, and is convenient for modifying artificially-synthetic function peptide, its molecular weight is small, so that it is not easy to result in immune reaction, and can be used as ligand, and can be combined with hepatic stellate cell (HSC) surface integrator acceptor to construct active liposome targeting and implement target therapy for experimental hepatic fibroid cell. Said invention can obtain good therapeutic effect for curing hepatitis fibrosis.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV +1
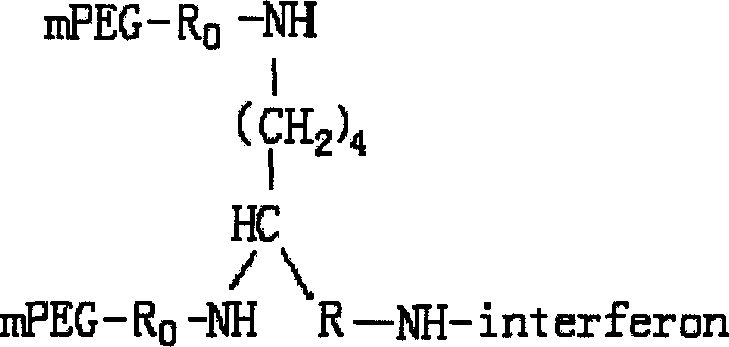
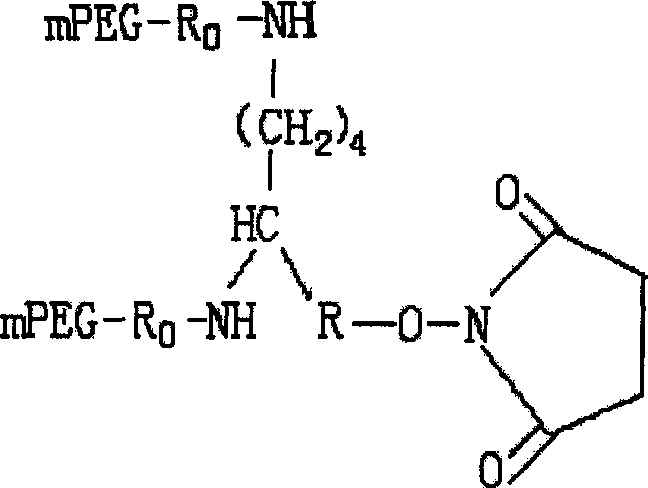
Conjugate of branched chair polymacrogol-interferon, and its preparing method
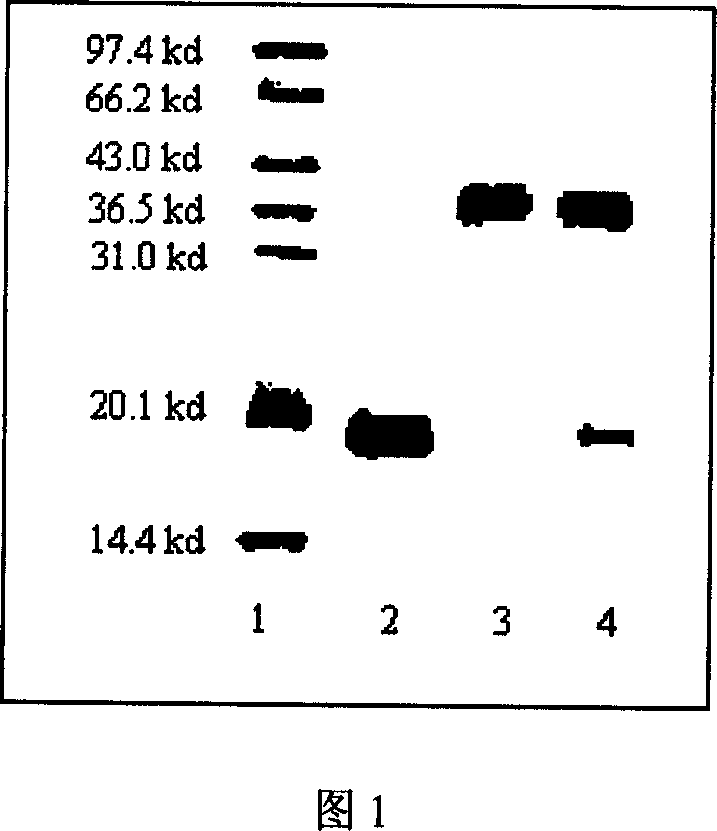
InactiveCN101002944ALong retention timeStrong antiviral activityPeptide/protein ingredientsAntiviralsHalf-lifePeg interferon
A compound of the branch chains PEG-interferon and its structural formula are disclosed. Said compound has high antiviral activity, long stay time in human body and long plasma half-life period (40-80 hr).
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Targeted hydrophilic polymer, binders with interferon and medical composite comprising above binders
ActiveUS20060014666A1Prolonged Circulatory Half-LifeImprove interferon concentrationIn-vivo radioactive preparationsPeptide/protein ingredientsDiseasePharmaceutical Substances
The present invention relates to an active targeted water-solubility macromolecule polymer, conjugate With interferon and pharmaceutical composition comprising the conjugate. The targeted agent includes, for example, glucose, galatose and the like, as well as their derivates. The conjugate of the present invention is well in water-solubility and havc long physiological cycle half-life period, and have specific recognition to pathology organize, improved and increased medication effect of interferon to Hepatitis B, Hepatitis C etc. infectivity sickness and cancer, infect complication etc.
Owner:JENKEM TECH
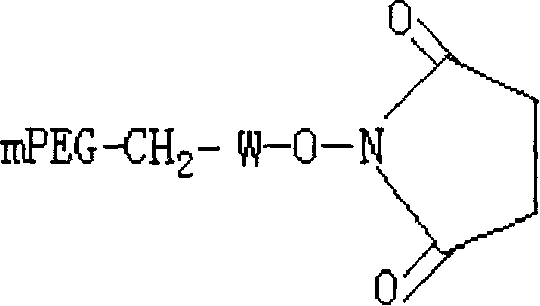
Polyethylene glycol-interferon coupler and its preparation method
InactiveCN1966527ALong retention timeStrong antiviral activityPeptide/protein ingredientsAntiviralsRetention timeHalf-life
This invention relates to a polyethylene glycol and interferon conjugate with physiological activity, which is a molecular weight of 5000~30000 Dalton of linear polyethylene glycol-modified interferon, as shown in the right formula, wherein mPEG is the polyethylene glycol chain and interferon is interferon. The PEG-interferon has high antiviral activity and long in vivo retention time, and the plasma half-life is 10~60 hours.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
HCV combination therapy containing ribavirin in association with antioxidants
Methods of treating patients having susceptible viral infections, especially chronic hepatitis C infection by administering to said patient a thereapeutically effective amount of a combination therapy of interferon-alfa and ribavirin for a time sufficient to lower HCV-RNA in association with a therapeutically effective amount of an antioxidant for a time sufficient to ameliorate ribavirin-related hemolysis are disclosed.
Owner:SCHERING AG
Use of 17-ketosteroid compounds, as well as derivatives, metabolites and precursors for treatment of hapatitis C type virus and other togavirus infections
The present invention provides 17-keto steroid compounds and their derivatives, metabolites and precursors and pharmaceutically acceptable salts thereof for the treatment or prevention of hepatitis C virus and / or hepatitis G virus in patients requiring such treatment, these compounds Collectively referred to as "compounds of the present invention". In addition, the present invention provides methods of treating or preventing togavirus infections including one or more of alphaviruses, flaviviruses (such as yellow fever virus), hepatitis C virus, and hepatitis G virus, Infection with rubella virus or pestiviruses (such as bovine viral diarrhea virus). In addition, the present invention provides combination therapy comprising the administration of one or more compounds described herein and the administration of one or more compounds selected from the group consisting of plasma concentration-enhancing compounds, macrophage stimulating factors, oxidative agents, ribavirin, and alpha interferon compounds and / or oxygen supply. The compounds of the invention may also be used to alleviate or alleviate one or more symptoms associated with togavirus infection.
Owner:HOLLIS EDEN PHARMA
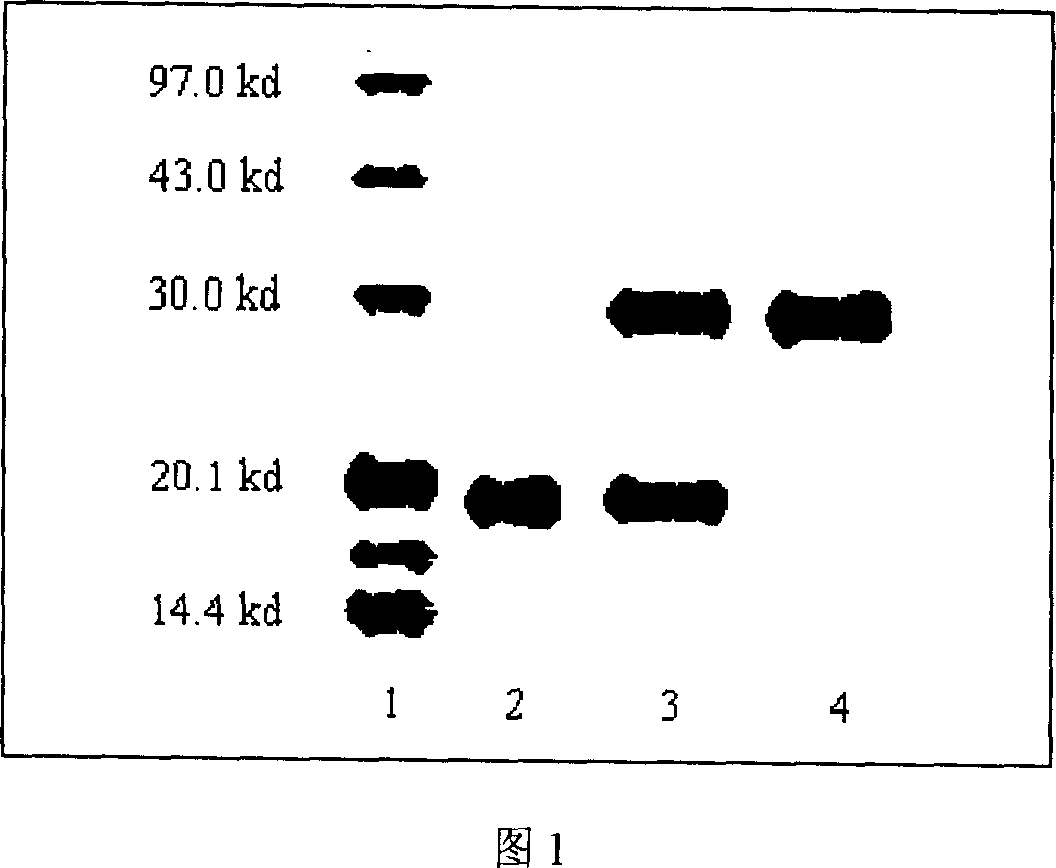
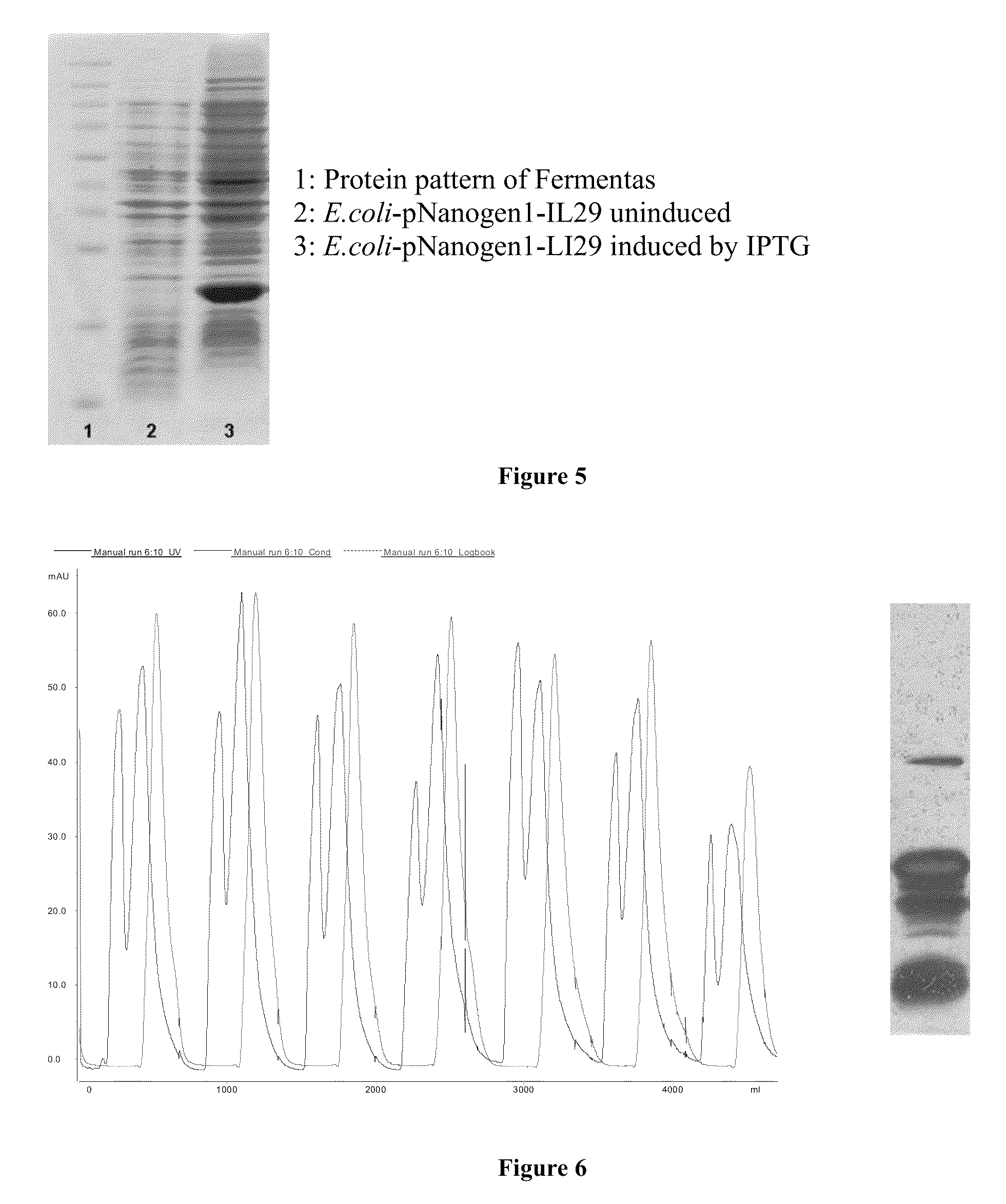
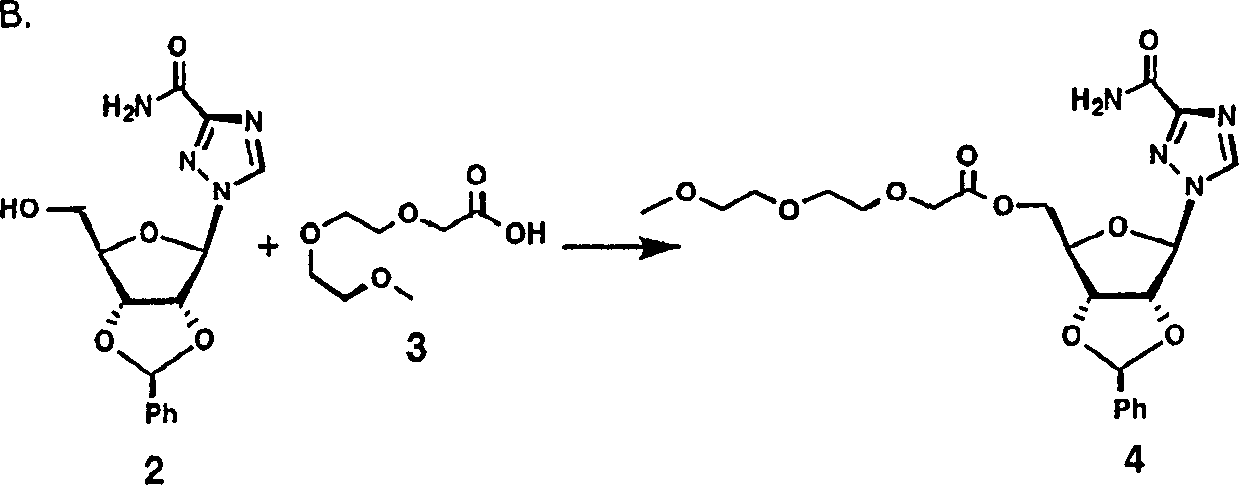
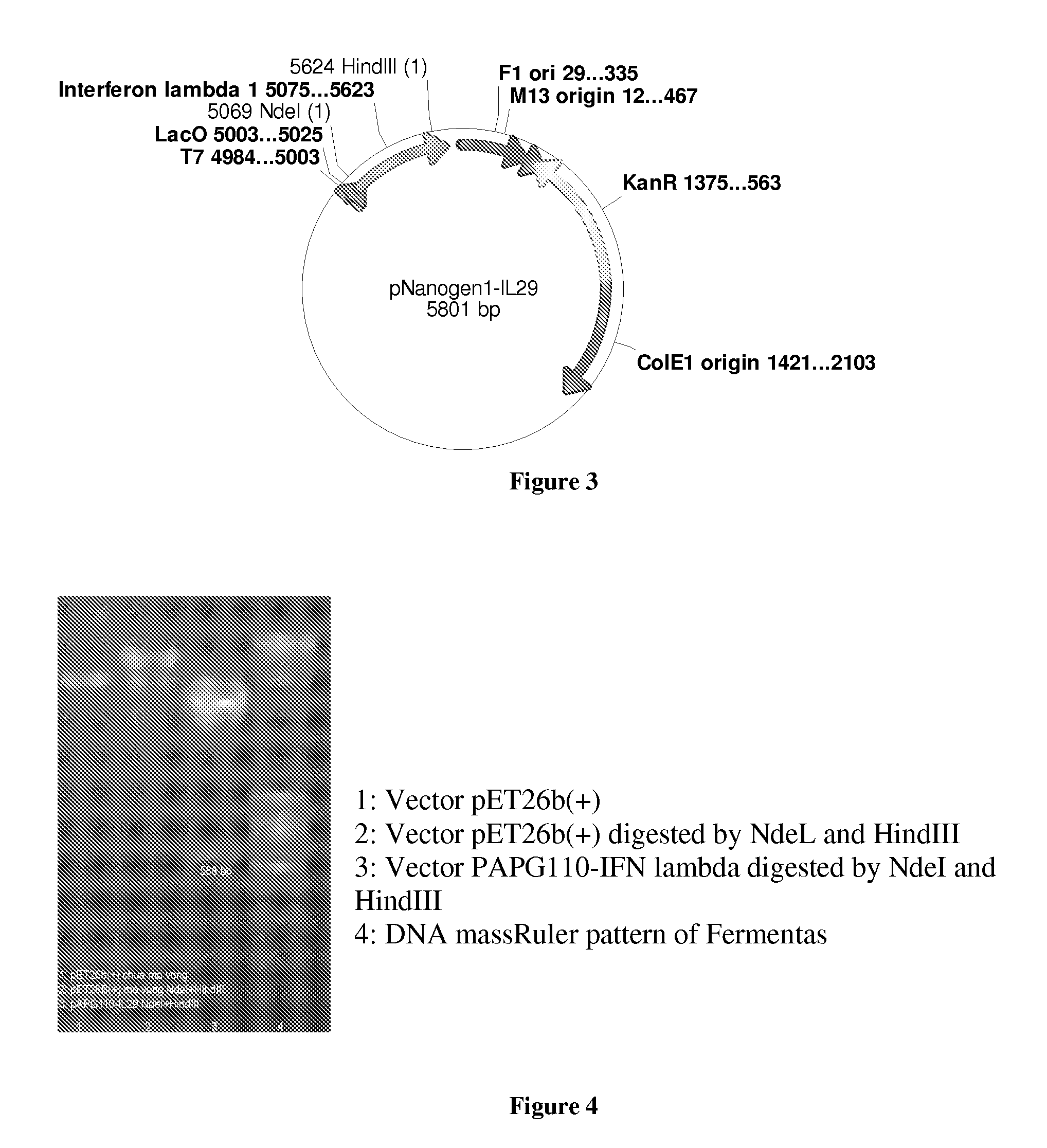
PEG-interferon lambda 1 conjugates
InactiveUS8454947B1Improve stabilityImprove solubilityPeptide/protein ingredientsDepsipeptidesHalf-lifeInterferon alpha
The present application discloses new PEG-interferon lambda 1 conjugates (PEG-IFNλ1), processes for their preparation, pharmaceutical compositions containing these conjugates and processes for making the same. These conjugates have increased blood half-lives and persistence time compared to IFNλ1 and are effective in the treatment of hepatitis B and hepatitis C.
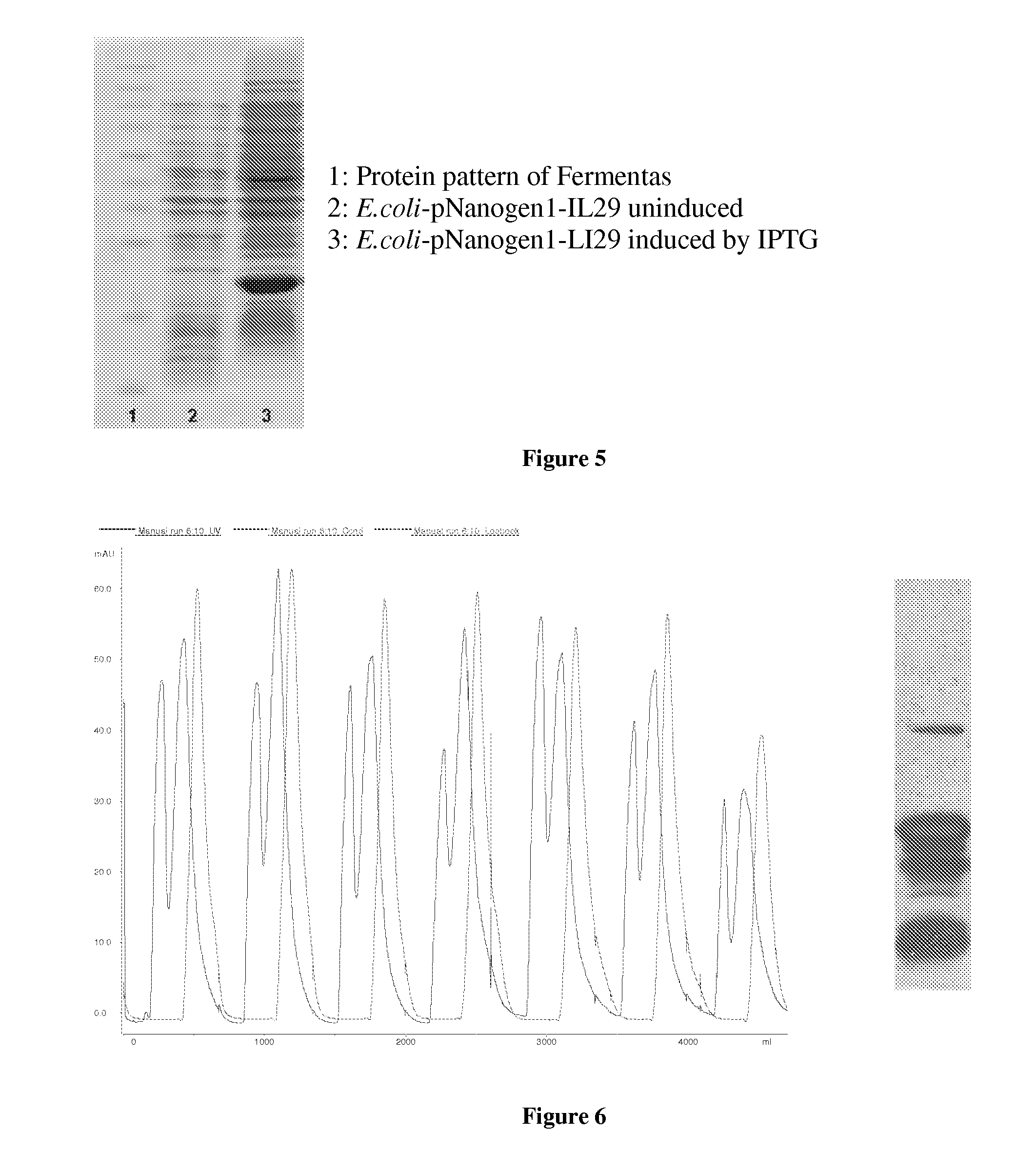
Owner:NANOGEN PHARMA BIOTECH CO LTD
HCV Combination Therapies
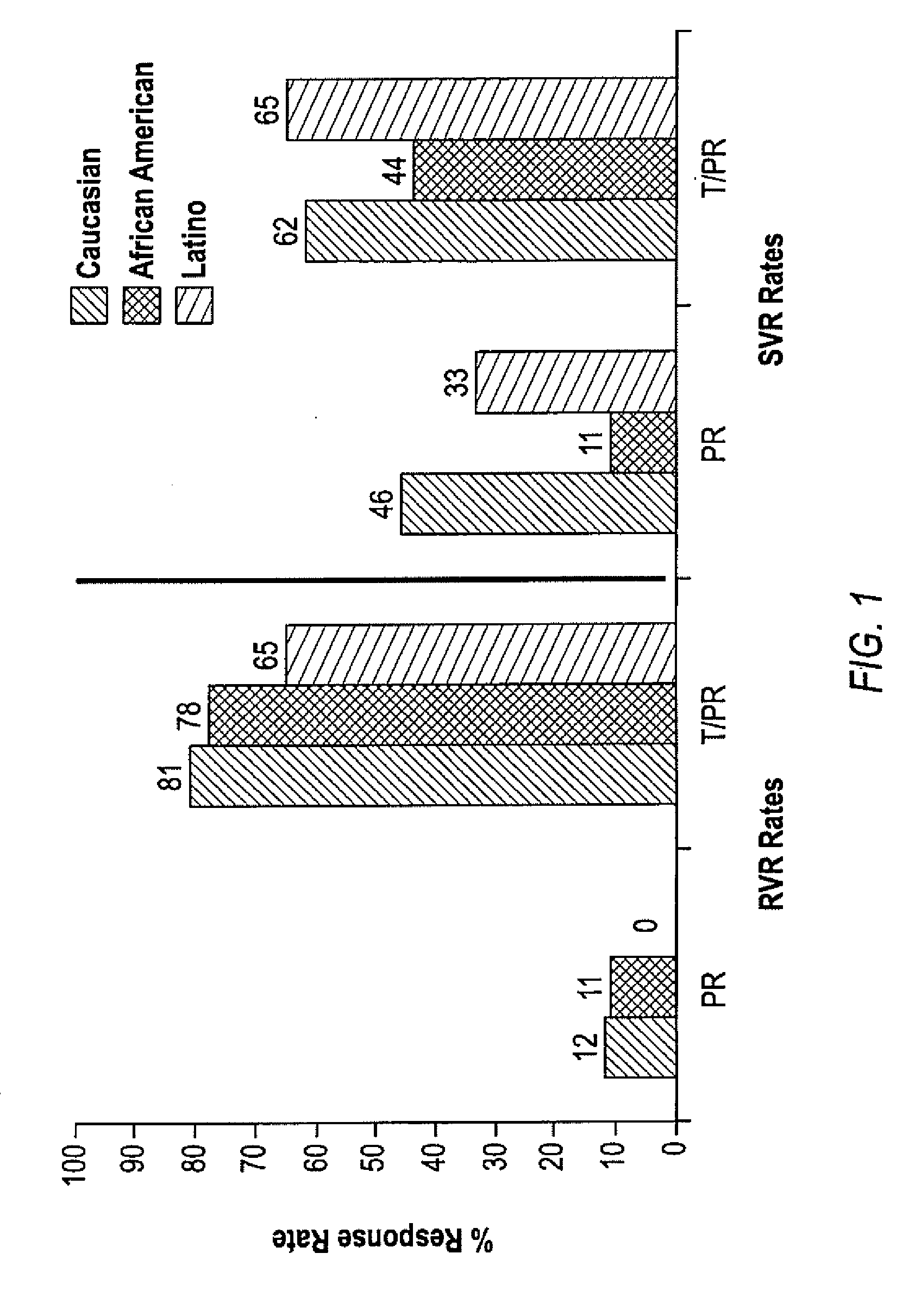
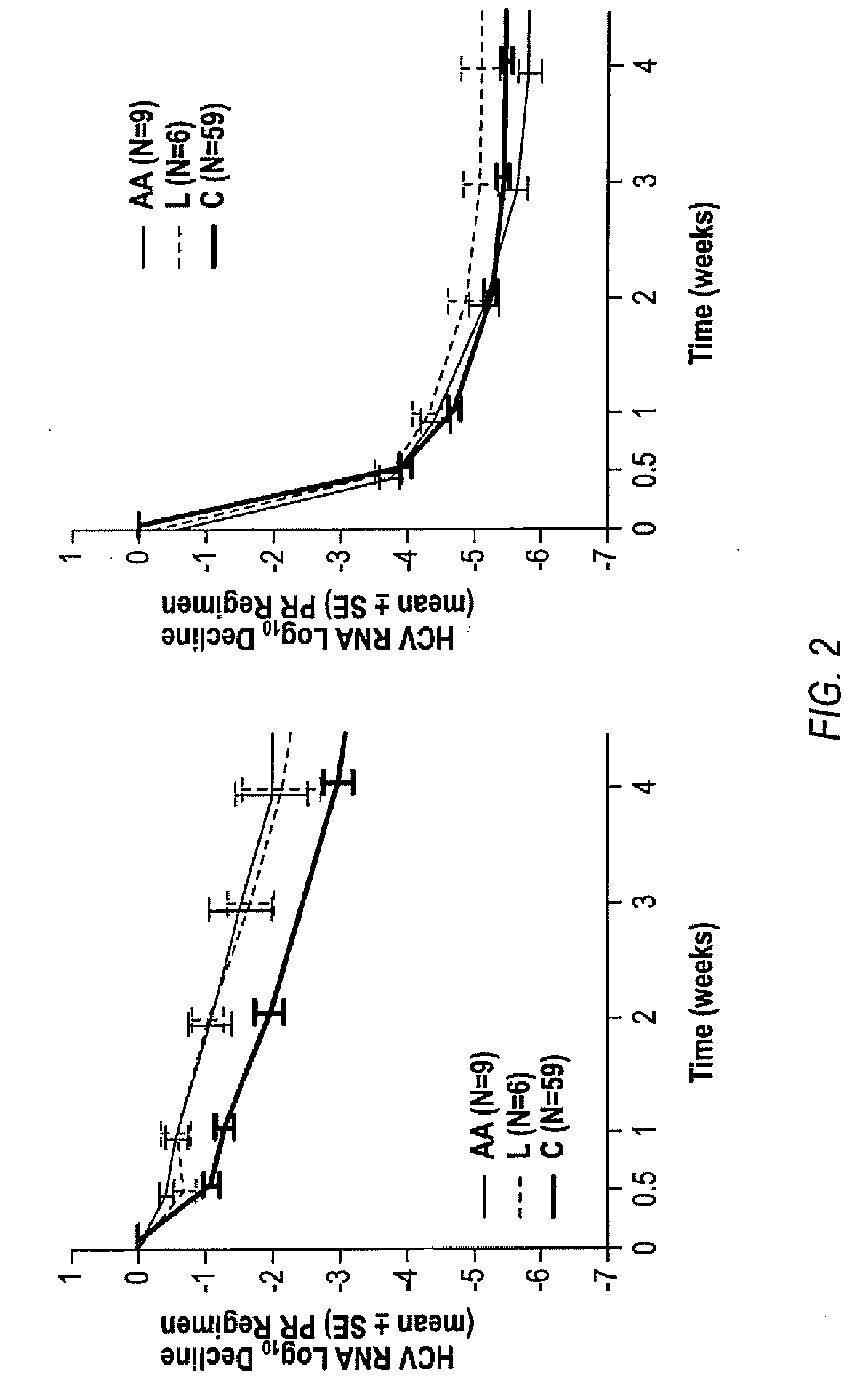
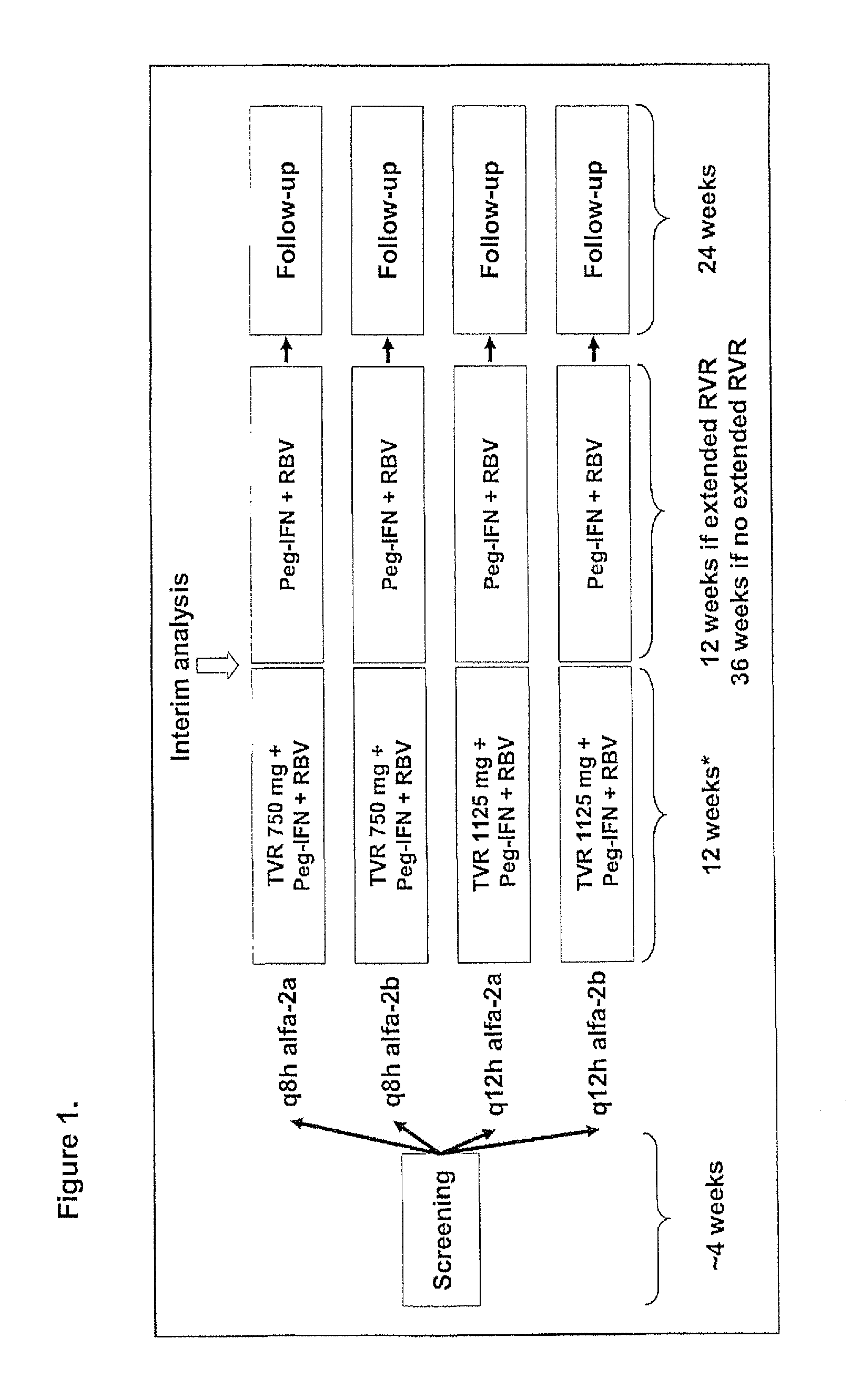
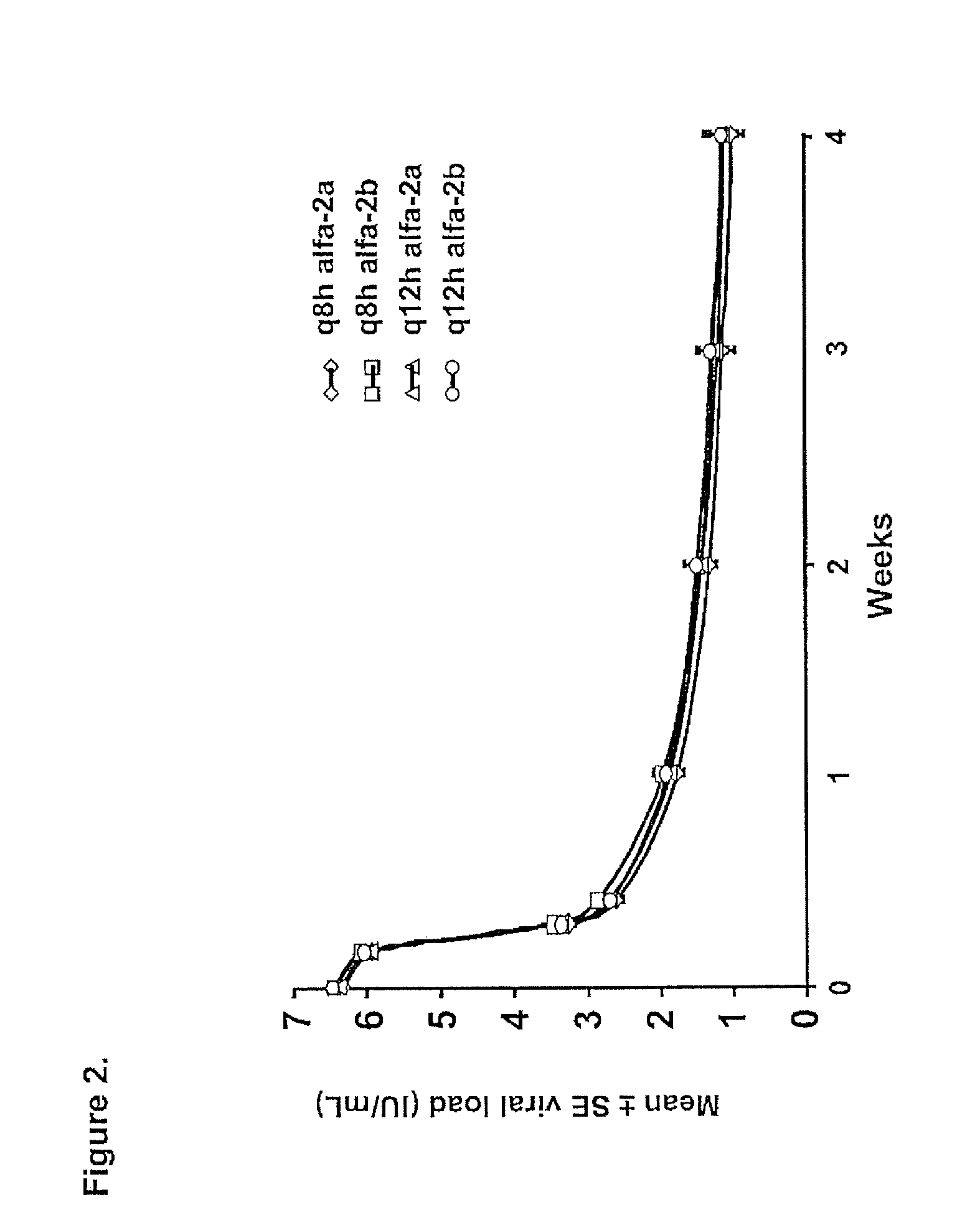
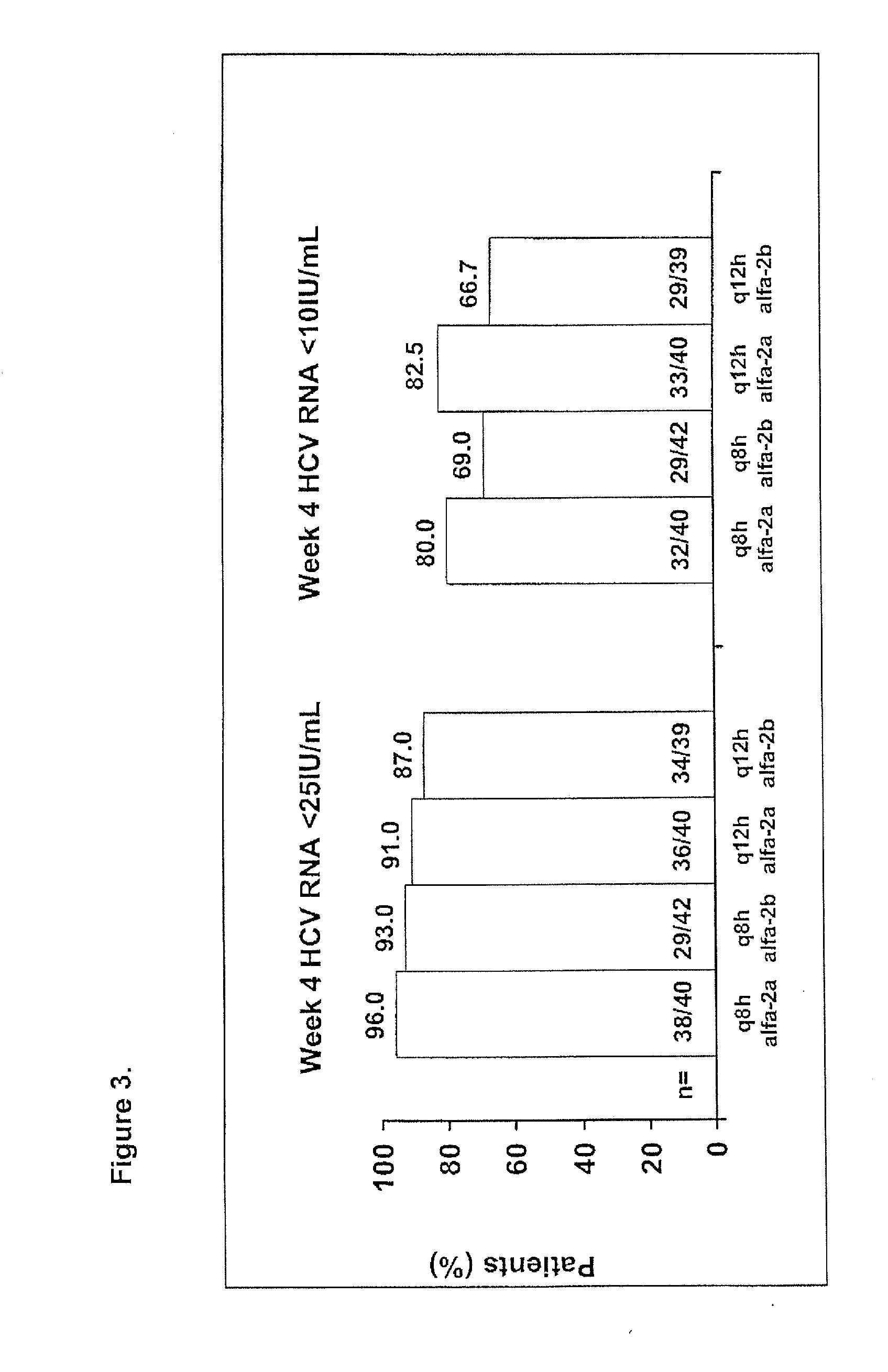
InactiveUS20100226889A1Reduce riskIncrease ratingsOrganic active ingredientsPeptide/protein ingredientsCombined Modality TherapyTelaprevir
The invention relates to combination therapies for the treatment of hepatitis C virus with telaprevir and pegylated interferon alfa-2a with or without ribavirin. The invention relates to the treatment of Latino and African American patients infected with HCV using the combination therapy.
Owner:VERTEX PHARMA INC
Ribavirin-interferon alfa combination therapy for eradicating detectable HCV-RNA in patients having chronic hepatitis C infection
A method for treating a patient having chronic hepatitis C infection to eradicate detectable HCV-RNA involving a combination therapy using a therapeutically effective amount of ribavirin derivative of formula (I) and a therapeutically effective amount of interferon-alpha for a time period of from 20 up to 80 weeks.
Owner:SCHERING AG
PEG-Interferon Lambda 1 Conjugates
InactiveUS20130230490A1Improve stabilityImprove solubilityPeptide/protein ingredientsDepsipeptidesMedicineHalf-life
The present application discloses new PEG-interferon lambda 1 conjugates (PEG-IFNλ1), processes for their preparation, pharmaceutical compositions containing these conjugates and processes for making the same. These conjugates have increased blood half-lives and persistence time compared to IFNλ1 and are effective in the treatment of hepatitis B and hepatitis C.
Owner:NANOGEN PHARMA BIOTECH CO LTD
Methods for Treating HCV
InactiveUS20170360783A1Avoid side effectsEfficient managementOrganic active ingredientsShort durationInterferon alpha
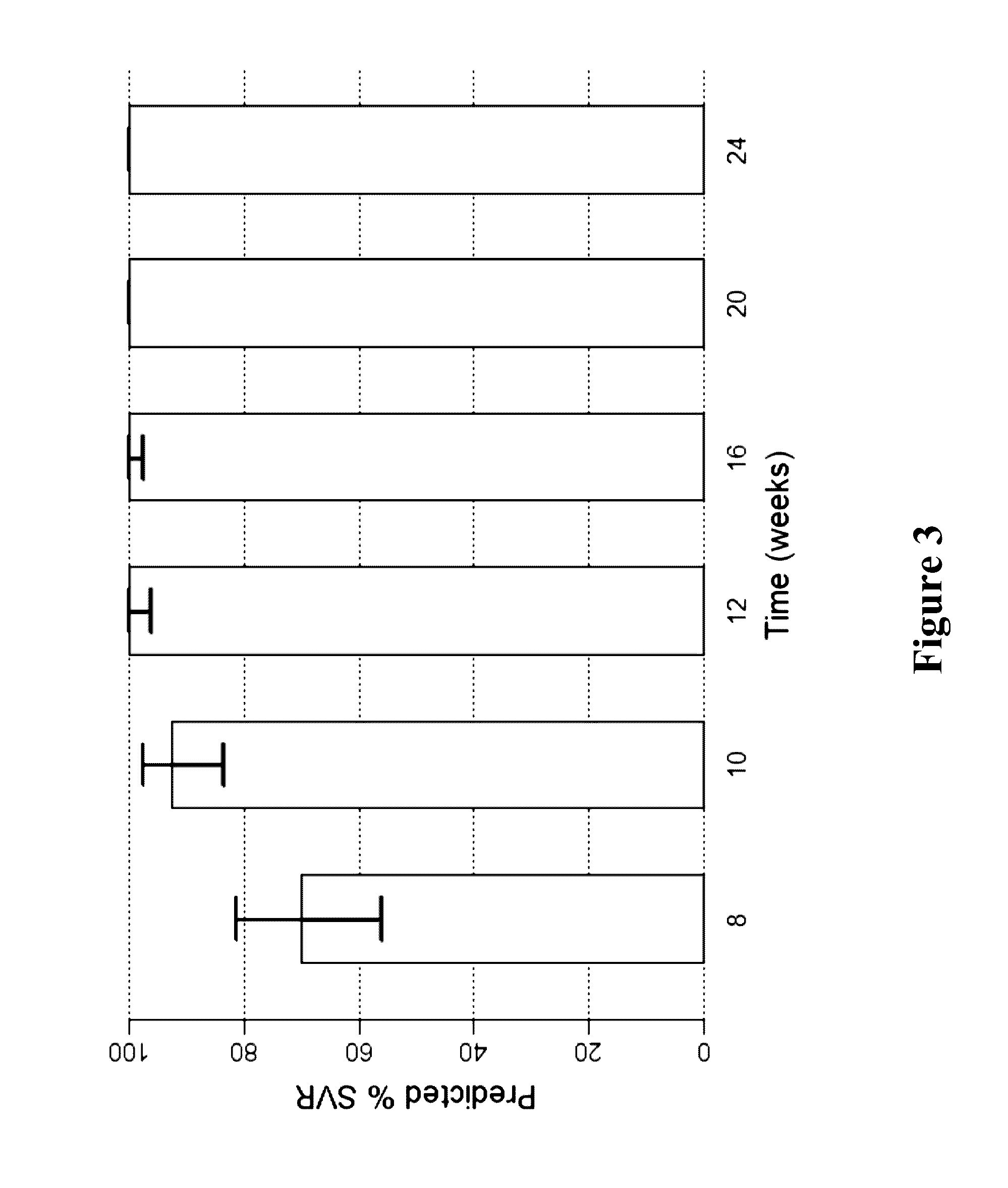
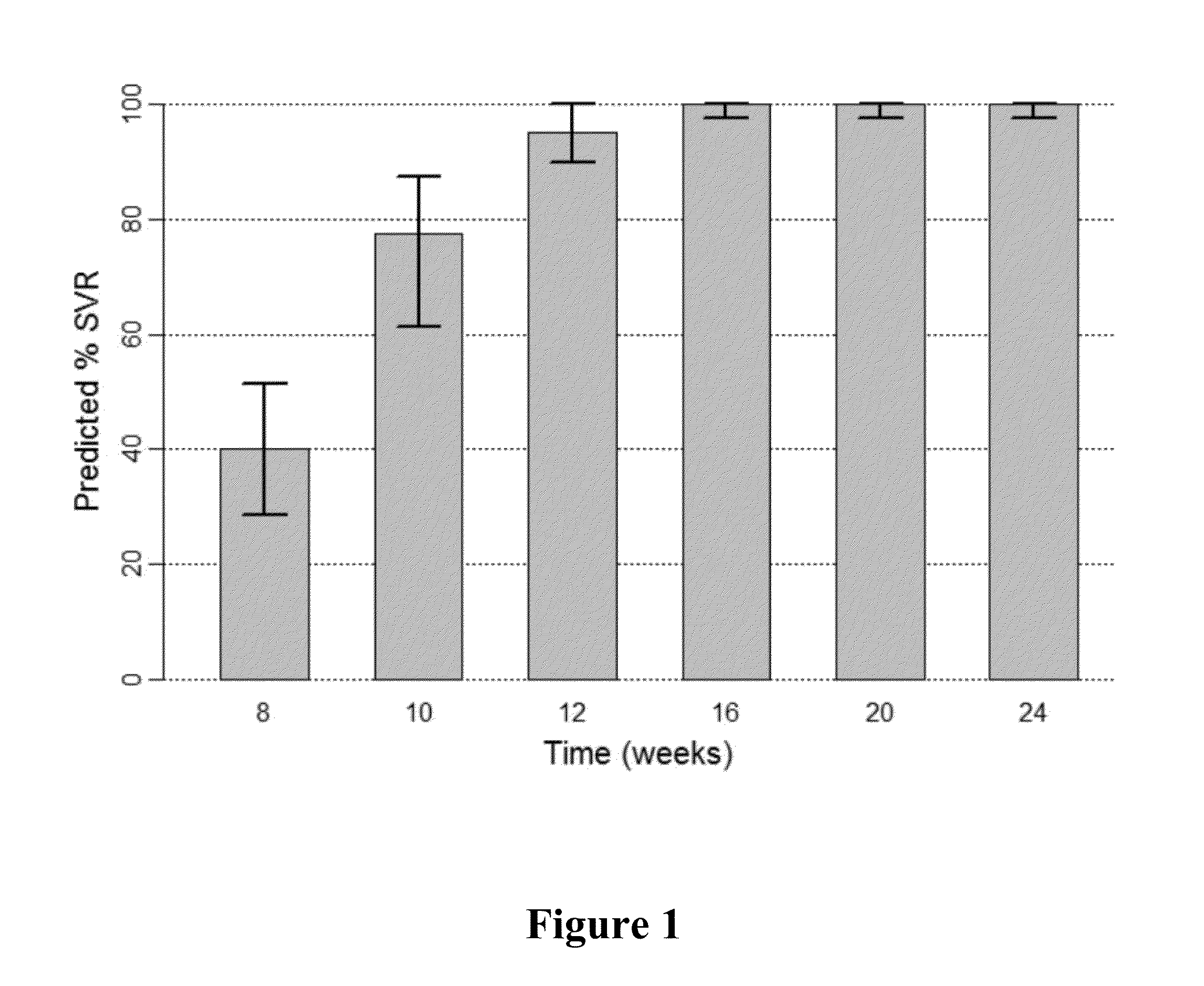
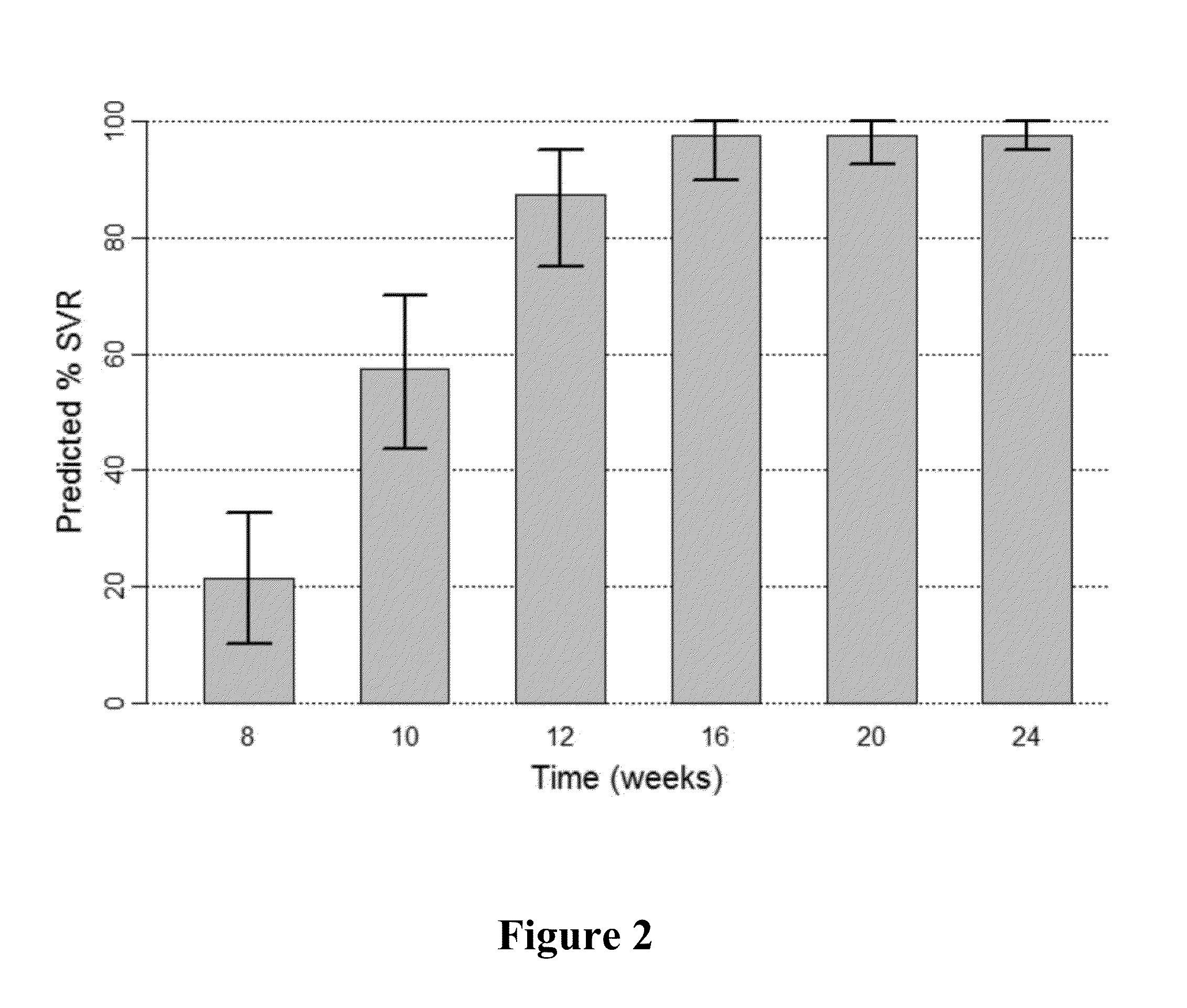
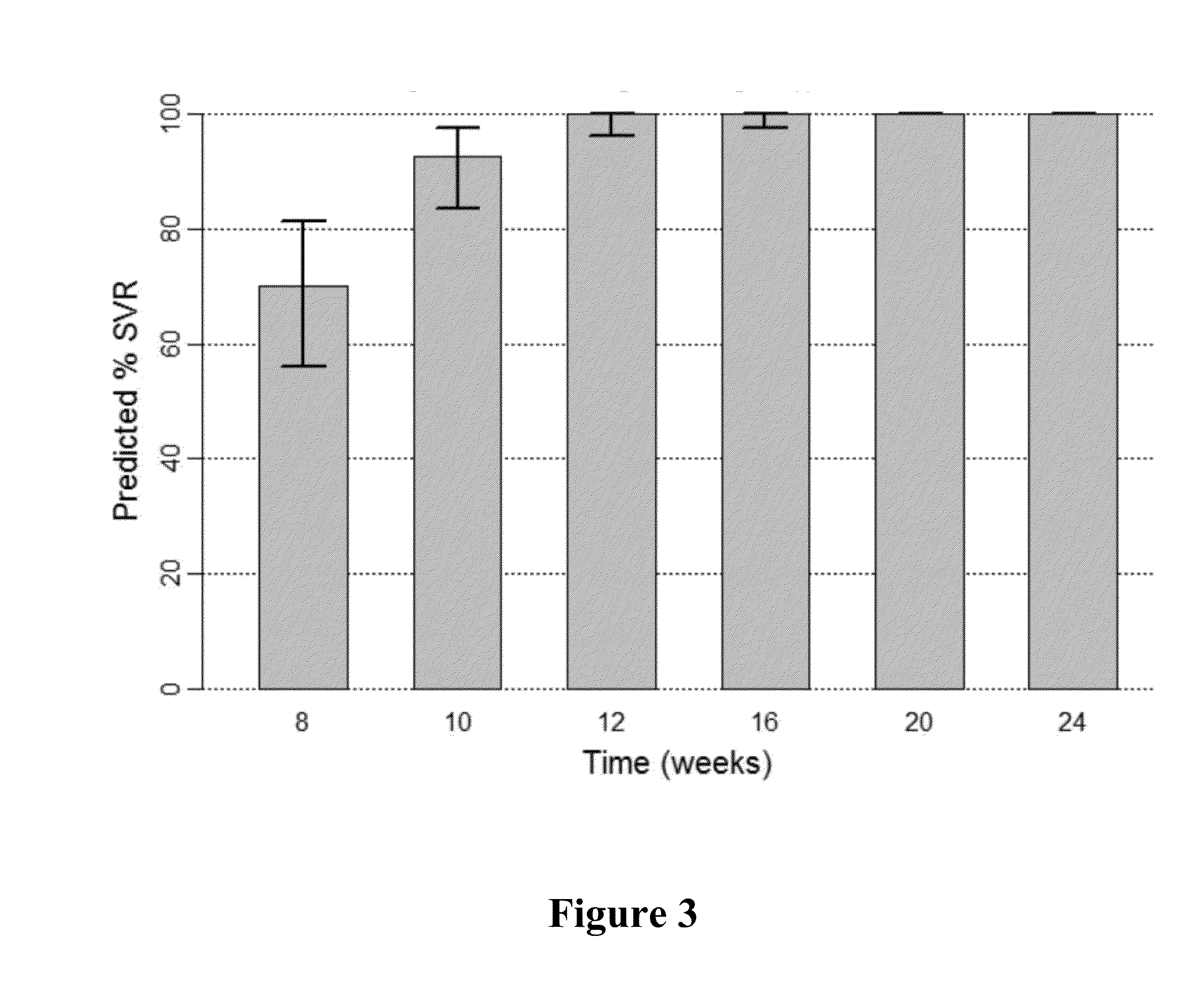
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 16 weeks, alternatively no more than 12 weeks, or alternatively no more than 8 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 16, 12, or 8 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Ribavirin-interferon alfa combination therapy for eradicating detectable HCV-RNA in patients having chronic hepatitis C infection
Ribavirin derivatives represented by the formula V, pharmaceutical compositions containing them as well as the use of the ribavirin derivatives represented by the formula V for the preparation of a medicament for the treatment of susceptible viral infections, for example, chronic hepatitis C infections administrating, the ribavirin derivatives being represented by formula V are disclosed.
Owner:SCHERING CORP
Oral ribavirin pharmaceutical compositions
InactiveUS20070166378A1Extension of timeMaintain bioavailabilityBiocideCarbohydrate active ingredientsReservoir typeImmediate release
The invention relates to oral pharmaceutical compositions for the prevention and / or the treatment of viral diseases. This invention also addresses methods of prevention and / or treatment of these viral diseases, using these oral compositions. One of the main problems considered in the present invention is to enhance the efficiency of anti-viral treatments, especially against Hepatitis C virus by means of ribavirin, for example in combination with interferon. The oral ribavirin antiviral composition according to the invention increases the bio-absorption time of ribavirin, and thus improves the treatment of patients. Said composition comprises at least one modified release form of ribavirin, the bio-absorption time BAT of which is greater than the bio-absorption time BAT* of a reference* immediate release form of ribavirin administered at the same dose; BAT being preferably comprised between 2 and 15 h and more preferably between 4 and 12 h. Said composition is a reservoir type form or a matrix type form. Said composition is a gastric retentive system or a multiparticulate form.
Owner:FLAMEL TECHNOLOGIES
Methods for Treating HCV
ActiveUS20160317602A9Organic active ingredientsDipeptide ingredientsChemical compoundPharmaceutical medicine
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Stable pharmaceutical composition of peginterferon alpha-2b
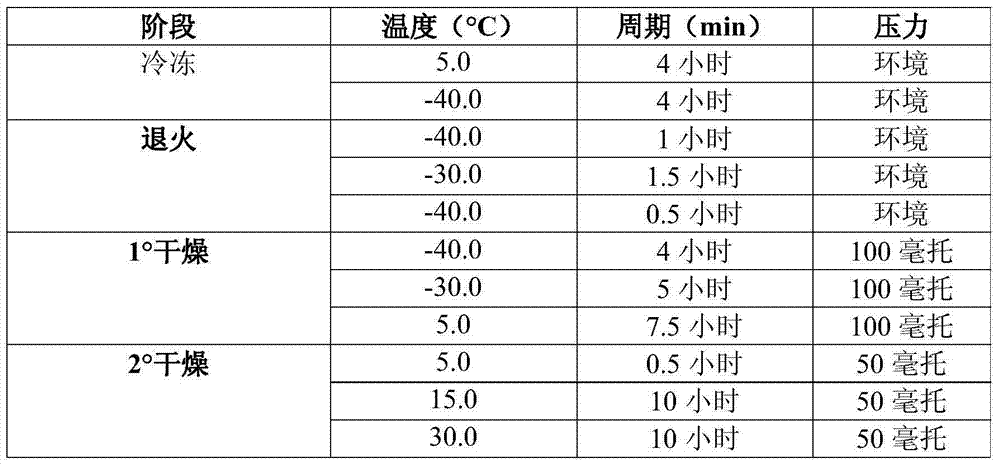
InactiveUS20150283252A1Peptide/protein ingredientsDigestive systemPeginterferon alpha-2bInterferon alpha
The present invention relates to the stable pharmaceutical compositions comprising PEG-interferon alpha-2b. More particularly, it relates to the stable pharmaceutical compositions comprising PEG-interferon alpha-2b and cryoprotectant selected from the group consisting of 2-Hydroxy propyl beta-cyclodextrin, sucralose, or polyvinylpyrrolidone 4000. It also relates to the methods of manufacturing the composition, method of administration and kits containing the same.
Owner:LUPIN LTD
Stable pharmaceutical composition of peginterferon alpha-2b
The present invention relates to the stable pharmaceutical compositions comprising PEG- interferon alpha-2b. More particularly, it relates to the stable pharmaceutical compositions comprising PEG-interferon alpha-2b and cryoprotectant selected from the group consisting of 2-Hydroxy propyl beta-cyclodextrin, sucralose, or polyvinylpyrrolidone 4000. It also relates to the methods of manufacturing the composition, method of administration and kits containing the same.
Owner:LUPIN LTD
Methods for Treating HCV
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
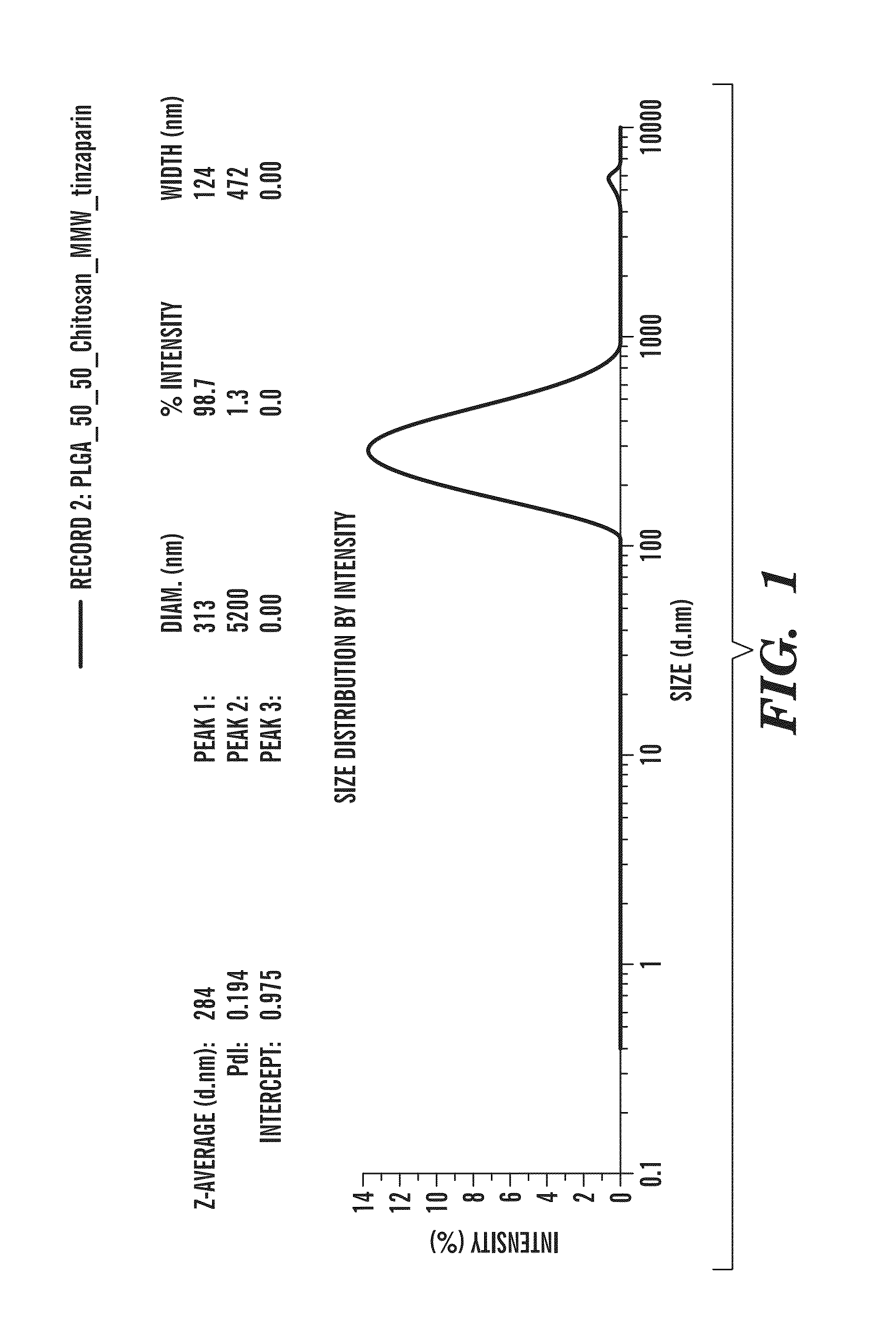
Nano-targeted delivery of protease, polymerase inhibitors with or without immune modulators in the treatment of hepatitis c
This disclosure concerns novel formulation and Nanoformulations as defined in the specification and compositions comprising combination of HCV protease and polymerase inhibitors, with or without interferon, along with anti-fibrotic / anti-hemolytic agents' combination of naturally driven Polyphenol / Thiols, and Non-anticoagulant GAGs. These compounds are effective antiviral agents, especially in inhibiting the function of the various genotypes of Hepatitis C virus (HCV). Thus, the disclosure also concerns a method of treating HCV related diseases or conditions by use of these novel compounds or a composition comprising nano-targeted delivery of novel nanoformulation containing combined composition for HCV and / or hepatic targeted delivery for improved efficacy and safety.
Owner:MOUSA SHAKER A
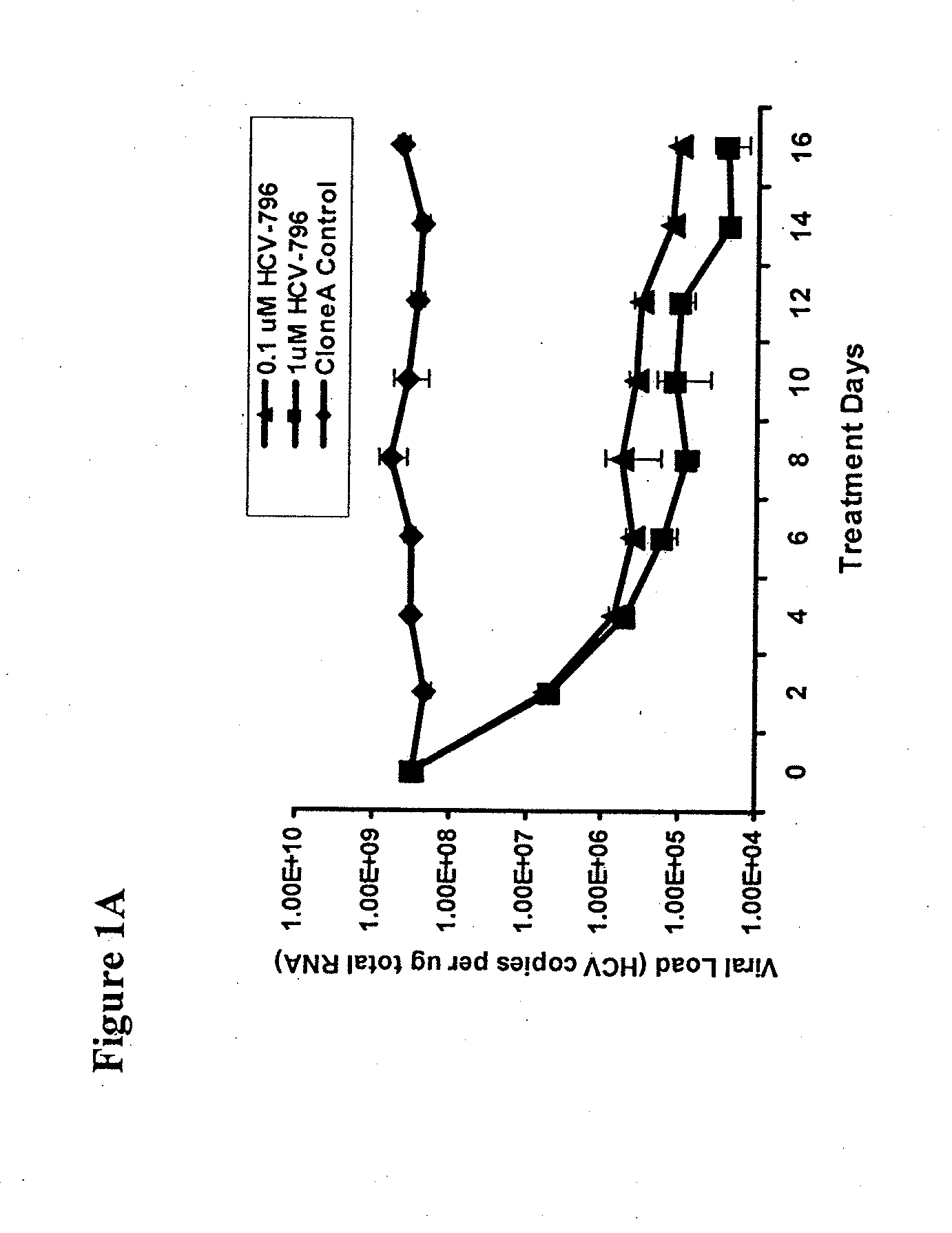
Identification and characterization of hcv replicon variants with reduced susceptibility to benzofurans, and methods related thereto
InactiveUS20100028922A1Reduced likelihoodHigh error rateOrganic active ingredientsMicrobiological testing/measurementHepatitis c viralHepatitis c rna
Owner:WYETH LLC +1
Methods and compositions for treating hepatitis c virus
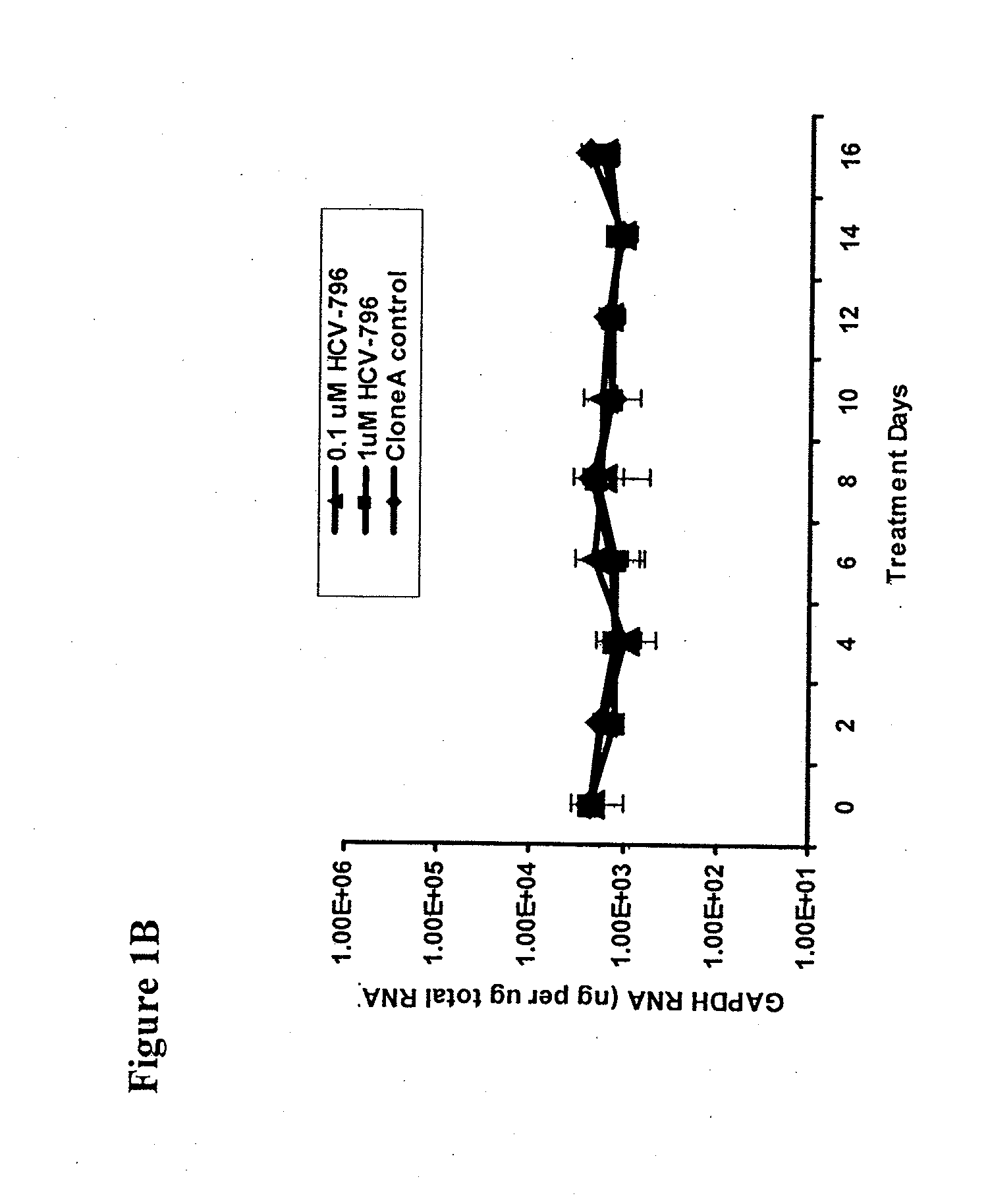
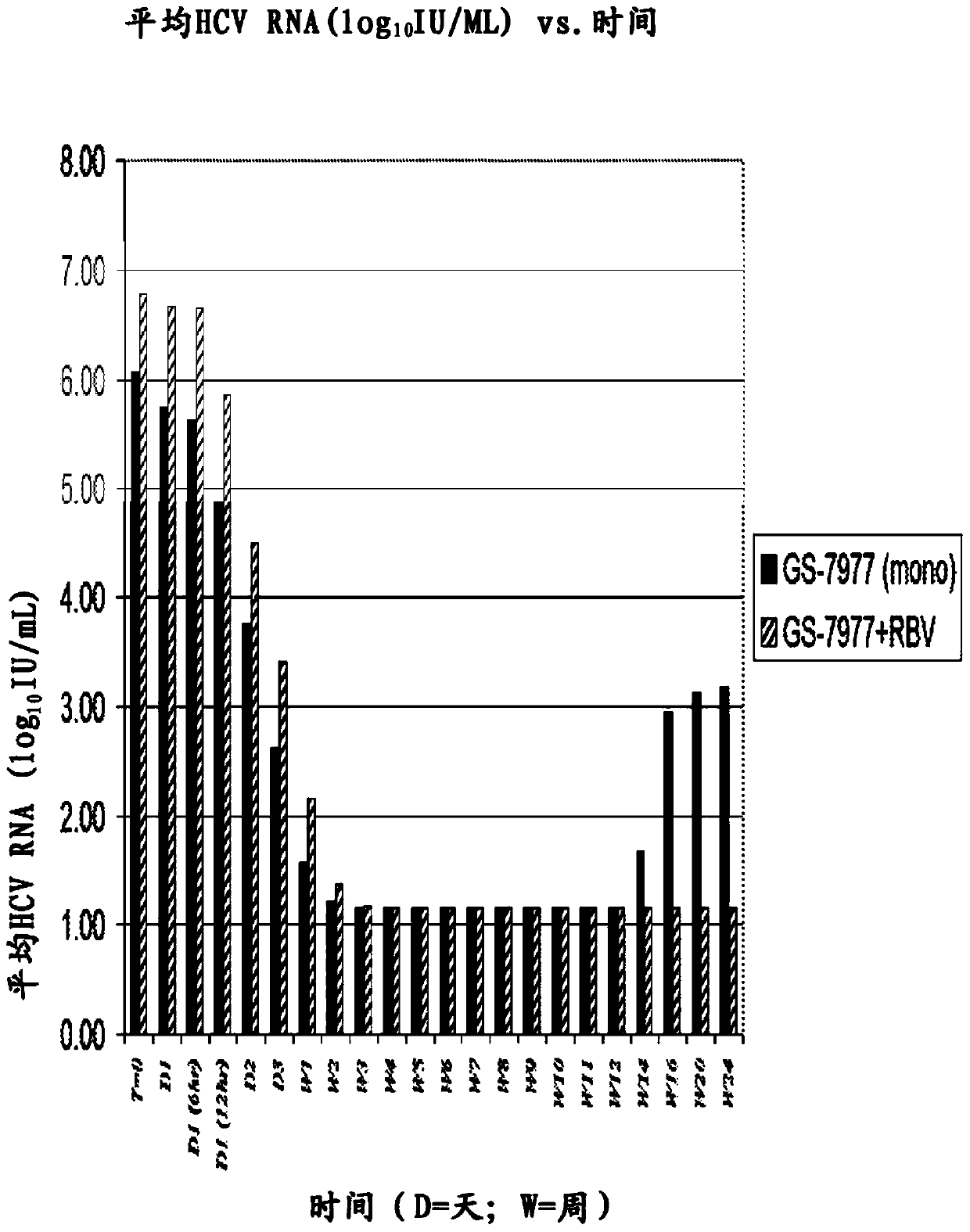
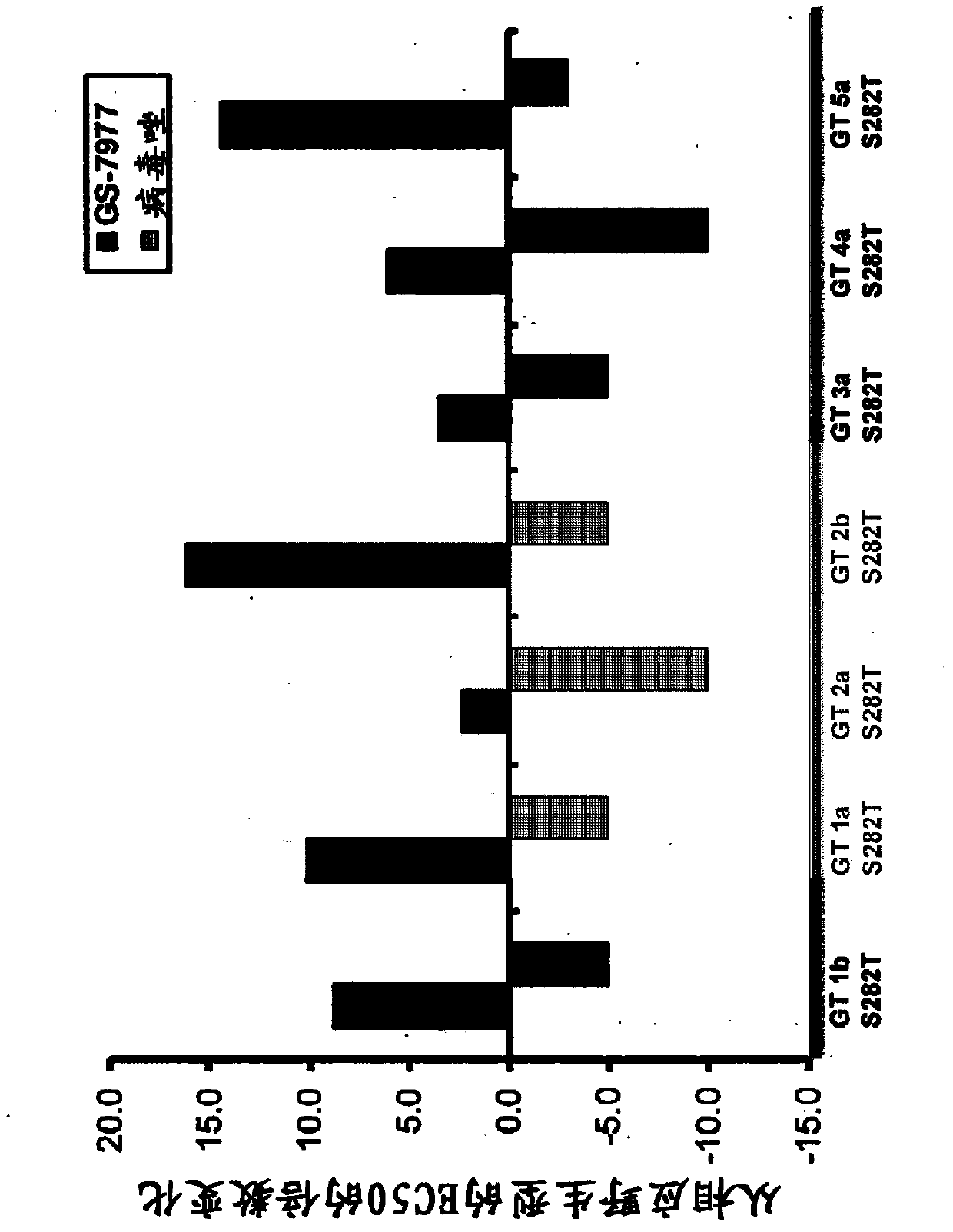
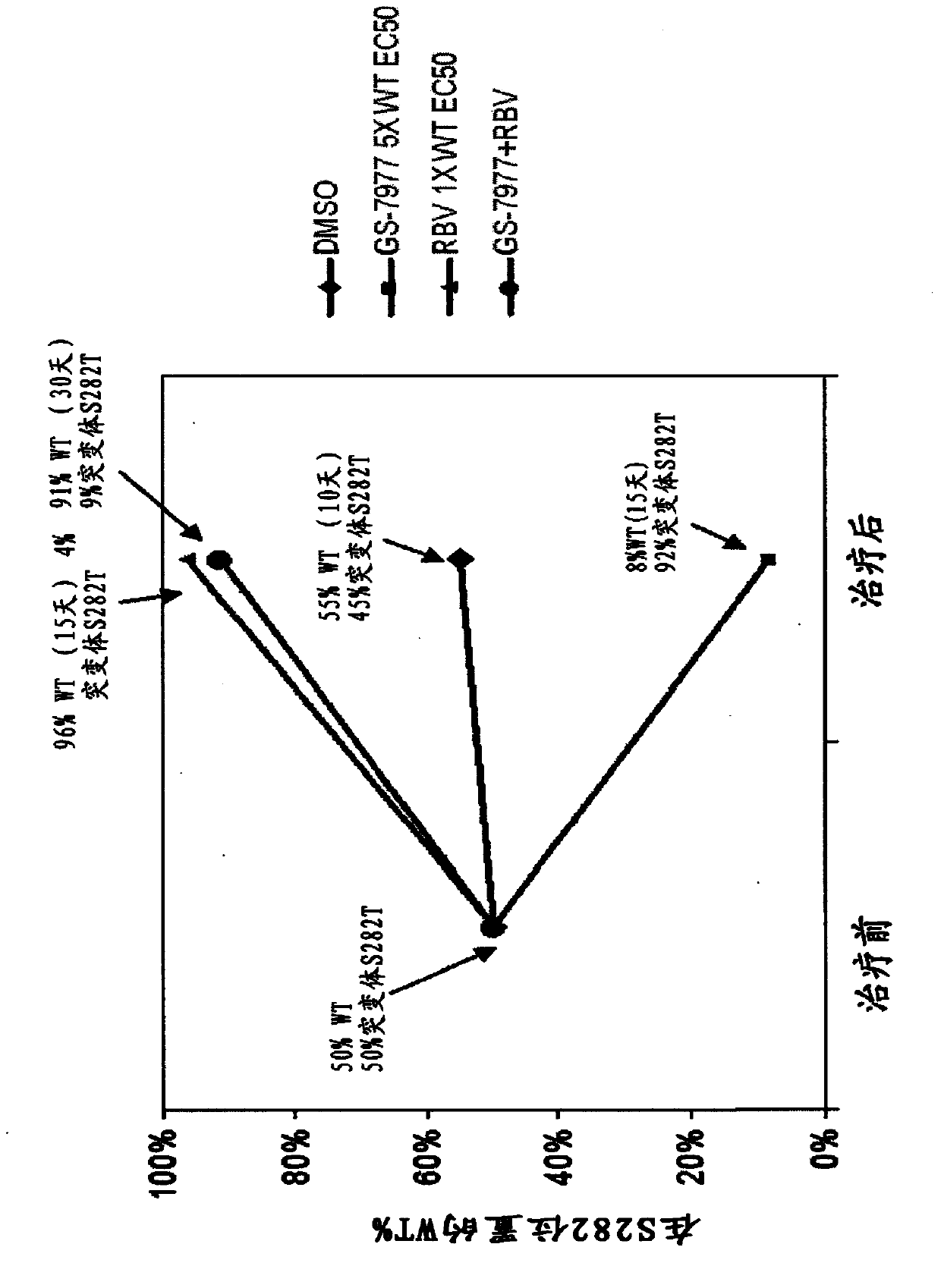
Disclosed herein is a method of treating a subject infected with hepatitis C virus, said method comprising administering to the subject for a time period an effective amount of GS-7977 and an effective amount of ribavirin. In one aspect, the method comprises administering to the subject an interferon-free treatment regimen comprising an effective amount of GS-7977 and an effective amount of ribavirin. In a particular aspect, the method is sufficient to produce an undetectable amount of HCV RNA in the subject for at least 12 weeks after the end of the time period. Also disclosed herein is a composition useful for the treatment of hepatitis C virus infection, said composition comprising an effective amount of GS-7977 and an effective amount of ribavirin.
Owner:GILEAD PHARMASSET LLC
Therapeutic regimen comprising peg-interferon, ribavirin and vx-950 for the treatment of hepatitis
The present invention relates to antiviral therapies and compositions for treating or preventing Hepatitis C infections in patients and relates to other methods disclosed herein. The invention also relates to kits and pharmaceutical packs comprising compositions and dosage forms.
Owner:JANSSEN PHARMA NV +1
Systematic novel anti-virus treatment method for coronavirus pneumonia
PendingCN113786478ASystematize the treatment processImprove treatment conditionsRespiratorsOrganic active ingredientsInitial treatmentDisease
The invention discloses a systematic novel anti-virus treatment method for coronavirus pneumonia. According to the systematic novel anti-virus treatment method, [alpha]-interferon, ibovitamide, ribavirin, chloroquine phosphate and arbidol are included. The whole novel anti-virus treatment method for coronavirus pneumonia is optimized, so that the treatment process is more systematized, a patient can be examined before suffering from a disease, and measures such as standard initial examination, initial treatment, anti-virus treatment and critical treatment are implemented when the patient suffers from the disease; the curative effect of antiviral treatment is improved to the greatest extent, a high-level medical service system is built, and high-level diagnosis and treatment services are provided for patients; and when the illness state of the patient deteriorates, the patient can be actively rescued and treated by selecting treatment schemes and measures for the critically ill patient, so that the life of the patient is actively saved, the rescue and treatment survival rate of the critically ill patient is improved, and the case fatality rate is reduced.
Owner:成都市公共卫生临床医疗中心
PEG-interferon lambda 1 conjugates
The present application discloses novel PEG-interferon lambda 1 conjugates (PEG-1FN[lambda]1), preparation method thereof, pharmaceutical compositions containing these conjugates and processes for making the same. These conjugates have increased blood half-lives and persistence time compared to 1FN[lambda]1 and are effective in the treatment of hepatitis B and hepatitis C.
Owner:NANOGEN PHARMA BIOTECH CO LTD
Combined use of ribavirin derivatives and alpha-interferon in the treatment and/or prevention of viral infections and related diseases caused by viral infections
ActiveCN109481669BNo side effectsOrganic active ingredientsPeptide/protein ingredientsPharmaceutical drugViral infection
The present invention relates to the field of ribavirin anti-derivative viruses, and discloses a ribavirin derivative (as shown in formula (I)) combined with α-interferon in the manufacture of treatment and / or prevention of viral infection and Use in medicine for related diseases caused by viral infection, pharmaceutical composition containing ribavirin derivatives and α-interferon and use of the pharmaceutical composition for preventing viral infection and related diseases caused by viral infection. The combined use of the ribavirin derivative represented by the general formula (I) of the present invention and α-interferon has a significantly better virus-inhibiting effect, and has no side effects.
Owner:江西诺立医药科技有限公司
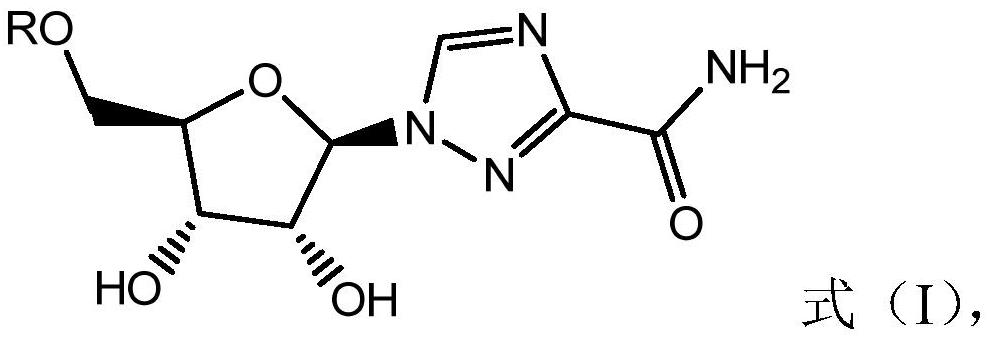
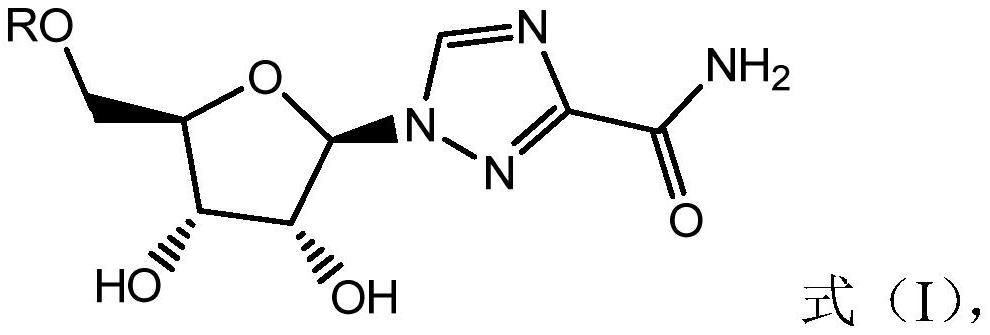
Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof
ActiveCN105985356AHigh activityNo cross resistanceOrganic active ingredientsOrganic chemistryCross-resistanceCross-tolerance
The invention belongs to the field of chemical pharmaceuticals, and particularly relates to an imidazole [2, 1-b] thiazole derivative as well as a preparation method and an application thereof. The structure of the imidazole [2, 1-b] thiazole derivative is shown by a formula I. The invention also provides a preparation method and an application of the imidazole [2, 1-b] thiazole derivative. The compound provided by the invention has the advantages of having good activity, having no cross tolerance with an NS3 / 4A inhibitor, an NS5A inhibitor, and nucleoside and non-nucleoside type NS5B inhibitors, and being capable of cooperatively resisting viruses when applied in combination with the NS3 / 4A inhibitor, the NS5A inhibitor, the nucleoside and non-nucleoside type NS5B inhibitors, and can be used for treating HCV (hepatitis C virus) infected patients independently or in a manner of forming a pharmaceutical composition together with one or more of ribavirin, PEG-interferon-[alpha], the NS3 / 4A inhibitor, the NS5A inhibitor, and the nucleoside and non-nucleoside type NS5B inhibitors.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







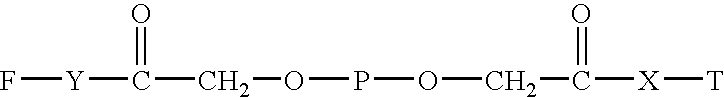
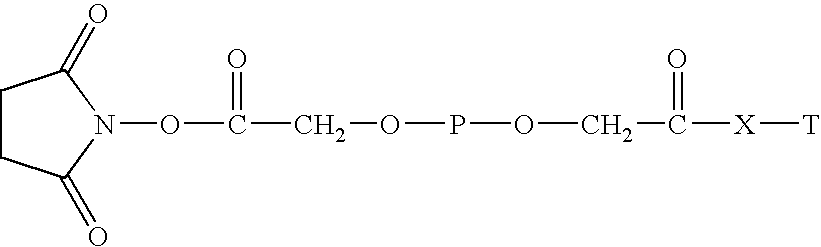














![Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0cc137aa-7d91-4d7c-a0e8-4bbeab44cee5/BDA0000667485740000021.PNG)
![Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0cc137aa-7d91-4d7c-a0e8-4bbeab44cee5/BDA0000667485740000031.PNG)
![Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof Imidazole [2, 1-b] thiazole derivative as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0cc137aa-7d91-4d7c-a0e8-4bbeab44cee5/BDA0000667485740000041.PNG)