Methods and compositions for treating hepatitis c virus
The technology of a composition and treatment scheme, applied in the field of compositions for the treatment of hepatitis C virus infection, can solve the problems that the treatment has shortcomings, and the resistance to NS3 protease inhibitors is not desired.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0051] A first embodiment relates to a method of treating a patient infected with hepatitis C virus comprising administering to the patient an effective amount of GS-7977 and an effective amount of ribavirin for a period of time.
[0052] In a first aspect of the first embodiment, the period of time is selected from about 2 weeks to about 12 weeks, about 3 weeks to about 12 weeks, about 4 weeks to about 12 weeks, about 5 weeks to about 12 weeks, about 6 weeks to about 12 weeks, From about 7 weeks to about 12 weeks, from about 8 weeks to about 12 weeks, from about 9 weeks to about 12 weeks, from about 10 weeks to about 12 weeks, from about 11 weeks to about 12 weeks, and about 12 weeks. In a subembodiment, the period of time is 12 weeks. In another subembodiment, the period of time is 8 weeks.
[0053] In a second aspect of the first embodiment, the effective amount of GS-7977 is selected from the group consisting of about 100 mg to about 800 mg, about 200 mg to about 800 mg, ...
Embodiment
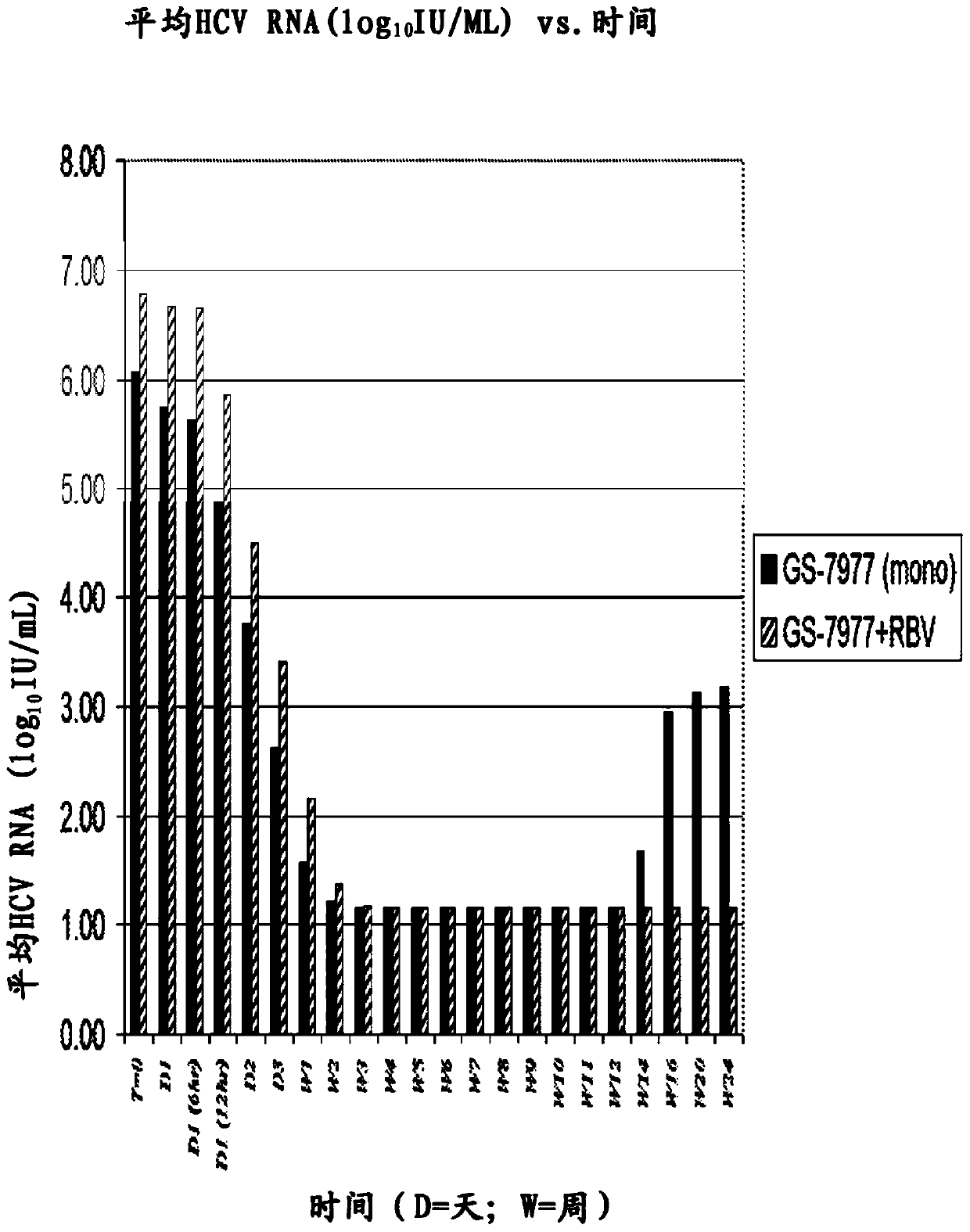
[0123] Using a standardized automated RNA extraction system with standardized controls and calibrators, the Roche trial, conducted a quantitative HCV RNA test for use in clinical trials. The LOD of the established assay was 15 IU / mL (defined by 95% hit rate using WHO criteria). Serum samples were used to measure HCV RNA levels.
[0124] US 2010 / 0226885 (US12 / 376,180), which is incorporated by reference, also discloses a method of measuring HCV RNA levels using RT-PCR to measure whether a patient has achieved HCV negative status.
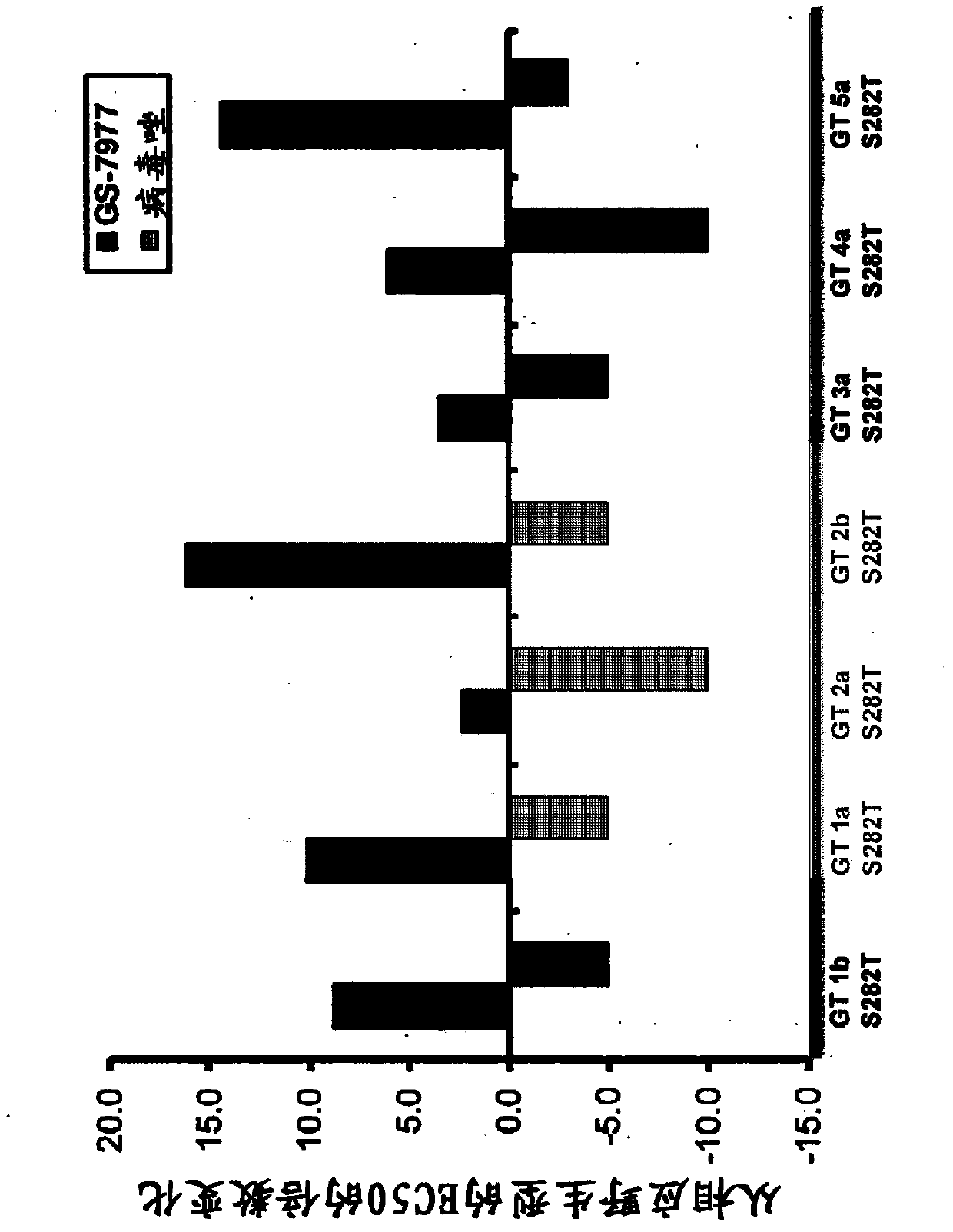
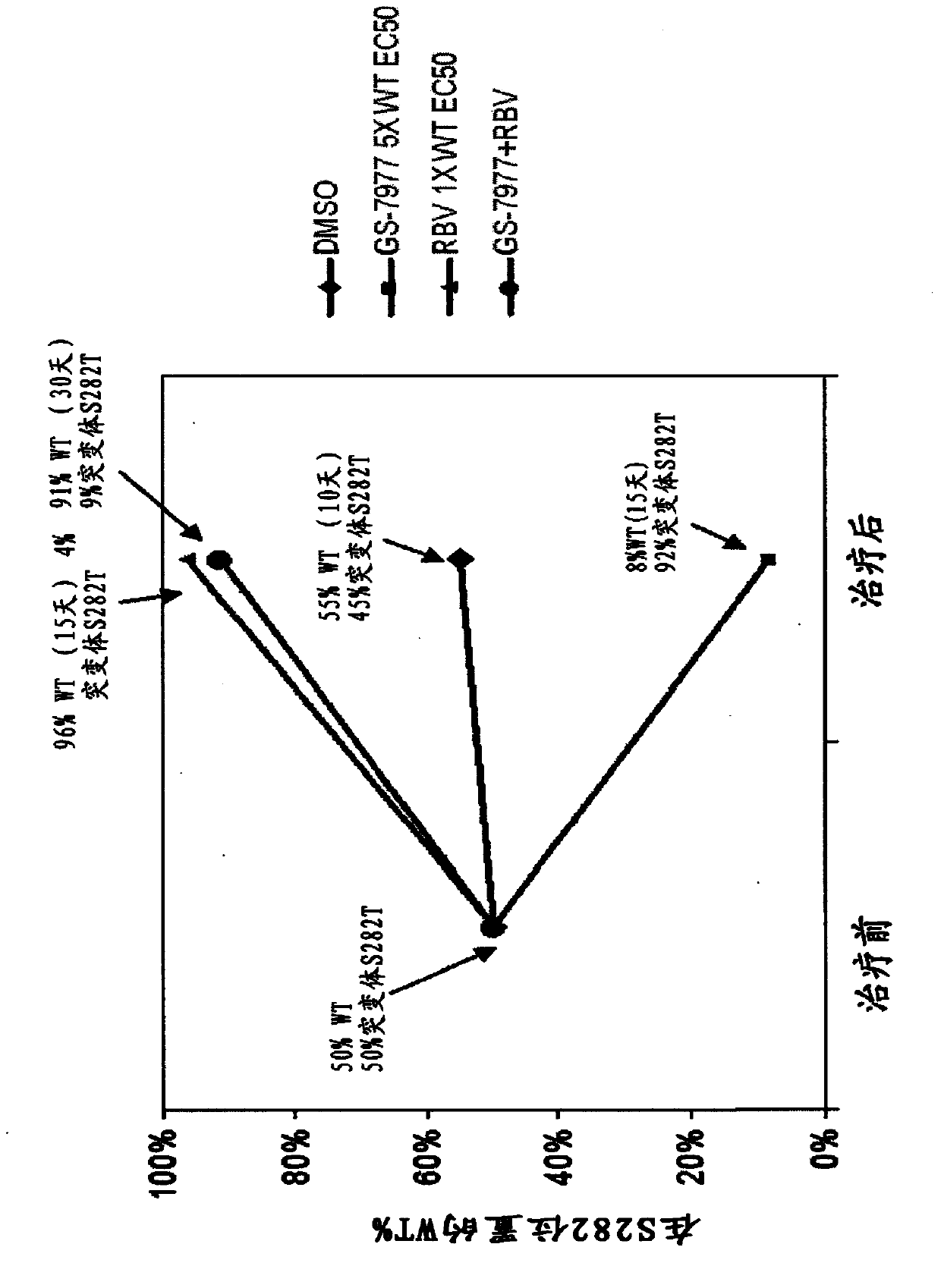
[0125] In Vitro Antiviral Synergy of Combination of GS-7977 and Ribavirin
[0126] The antiviral effect of GS-7977 in combination with ribavirin was assessed using the HCV genotype 1a replicon (Robinson et al., Antimicrob. Agents Chemother. (2010) 54(8):3099-3106). Cells were grown in cell culture medium containing Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% HyClone FBS, 100 units / mL penicillin, 100 μg / mL streptomycin, and 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com