Ribavirin-interferon alfa combination therapy for eradicating detectable HCV-RNA in patients having chronic hepatitis C infection
A technology for chronic hepatitis C and interferon, applied in the direction of drug combination, organic active ingredients, carbohydrate active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
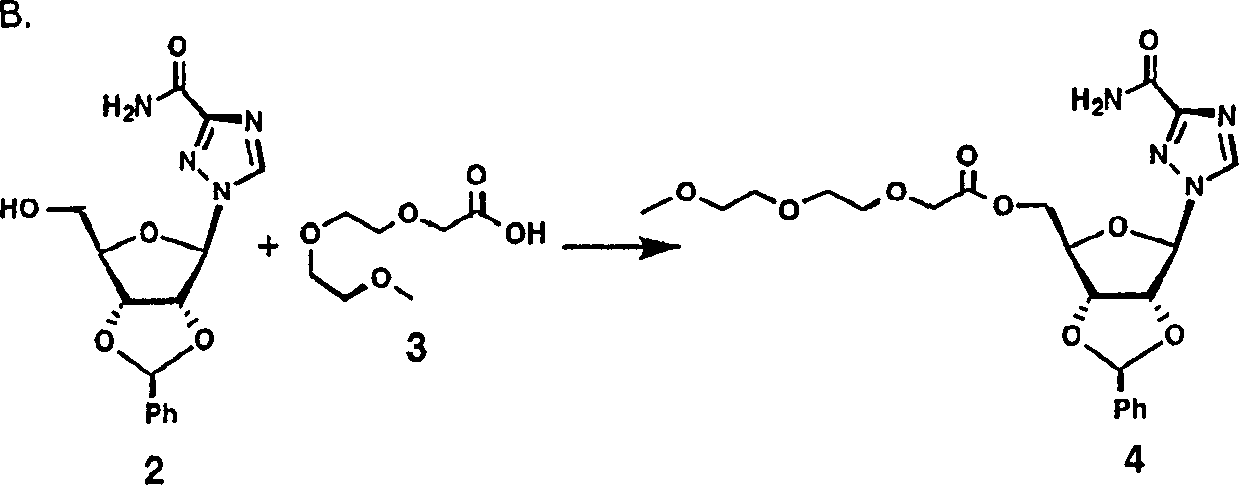
[0178] Example 1 A. Benzylidene Ribavirin
[0179] 20g ribavirin (1.87mmol), 200ml benzaldehyde and 20g ZnCl 2 mix. The resulting reaction mixture was stirred at room temperature for 24 hours. With stirring, the resulting solution was poured into 2.5L ether (Et 2 O). The resulting mixture was filtered with suction, and the solid precipitate was dried. The solid precipitate was mixed with 1.2 L of ice-cold 2N sodium hydroxide (NaOH) solution. The mixture was extracted with 2 x 0.75 L of cold ethyl acetate (EtOAc), and the organic layer was washed with brine. The organic layer was gravity filtered through a fluted filter, then concentrated in vacuo to a solid. With 0.5L Et 2 O grind the solid well, filter with suction, and wash with fresh Et 2 O washed the formed precipitate to give 23 g of compound 2 as a solid; C 15 h 16 N 4 o 5 Calculated value (332.32). MS (FAB) = 333.1.
[0180]
[0181] 0.5 g (2.8 mmol) of compound 2, 0.80 g (2.4 mmol)...
Embodiment 2
[0184] According to the method of Example 1A and 1B, but in Step B, with an equivalent amount of compound 6 (MeOCH 2 CH 2 OCHCO 2 H) Instead of compound 3, compound 8 is formed. Following the method of step C of Example 1, but substituting an equivalent amount of compound 7 for compound 4, compound 8 was formed.
Embodiment 3
[0186] At 5° C., a solution of 0.32 g (3.6 mmol) of compound 9 [2-(N, N’-dimethylamino) ethanol] in 10 mL of N, N-dimethylformamide (DMF) was treated with 0.58 g of carbonyldiimidazole ( 3.6 mmol) and the resulting solution was warmed to 20°C over 0.5 h. To the obtained reaction mixture was added 0.8 g (2.4 mmol) of compound 2 prepared according to Example 1A, and the resulting reaction mixture was stirred at room temperature for 24 hours. Concentrate in vacuo, add 50 mL of ether, and let the resulting mixture stand for 24 hours. The supernatant was decanted, and the residue was purified by column chromatography (silica gel, 10%-20% methanol-tetrahydrofuran gradient elution) to obtain 0.21 g of compound 10 as a solid; C 20 h 25 N 5 o 7 (447.44) calculated value. MS (FAB) = 448.1.
[0187] Following the procedure of Example 1C, but substituting an equivalent amount of compound 10 for compound 4, compound 11 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com