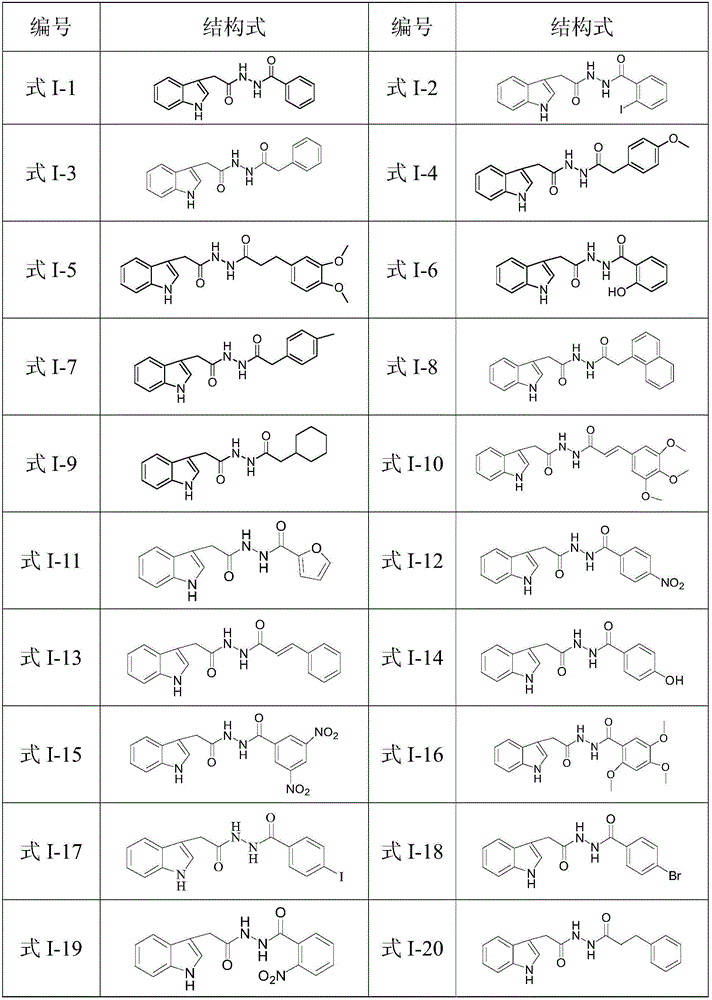
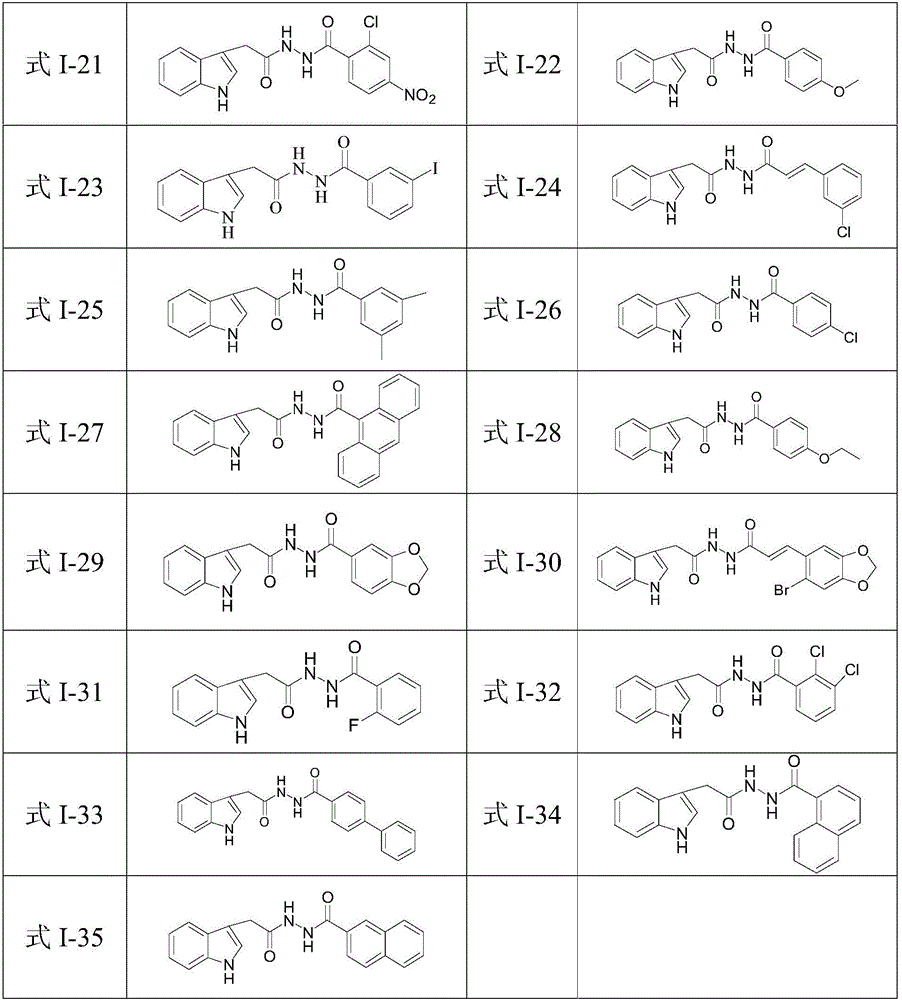
N'-(2-(1H-indole-3-yl)acetyl)arylhydrazide compound and preparation method and application thereof
A technology of acetyl group and aroyl hydrazide, applied in the field of drug preparation, can solve problems such as lack of preventive vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] In this example, the compound of formula I-1 is prepared by the following method, which specifically includes the following steps:
[0067] (1) Add 8.8g (50mmol) of indole acetic acid (50mmol), methanol (60mL), and concentrated sulfuric acid (3mL) to a 500ml round bottom flask, and react at 70°C for 1-3 hours. water (50mL), separate the organic phase, extract the aqueous phase with ethyl acetate (3×20mL), combine the organic phases, wash with saturated sodium bicarbonate solution and water successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain indole The crude product of methyl acetate was directly used in the next reaction without further purification.
[0068] (2) Add 9.46g (48mmol) of methyl indole acetate, ethylene glycol methyl ether (40mL), and 5mL of hydrazine hydrate into a 500ml round-bottomed flask, heat and reflux at 115°C for about 20 hours, thin-layer chromatography (TLC) After detecting the disappearance of the r...
Embodiment 2
[0074] In this example, the compounds of formula I-2 to formula I-35 were prepared. The only difference between the preparation method and the preparation of the compound of formula I-1 in Example 1 was that the aroyl chlorides used in step (4) were respectively Other than that, the preparation method is the same as that of the compound of formula I-1 in Example 1.
[0075] The properties, yields and structural characterization results of the prepared compounds of formula I-2 to formula I-35 are as follows:
[0076] N'-(2-(1H-indol-3-yl)acetyl)-2-iodobenzohydrazide (Formula I-2): white solid, yield: 94%, mp: 188.1-189.0°C; 1 H-NMR (500MHz, DMSO-d 6 ), δ(ppm): 3.68(s,2H,CH 2 ),6.92-6.93(s,1H,Ar-H),6.98-7.24(m,4H,Ar-H),7.32-7.35(m,1H,Ar-H),7.43-7.44(m,1H,Ar-H -H),7.53-7.55(m,1H,Ar-H),7.80-7.82(m,1H,Ar-H),10.23(s,1H,NH),10.29(s,1H,NH),10.97( s,1H,indole-NH); m / z418.0[M + -1].
[0077] N'-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazide (Formula I-3): white solid, yield: ...
Embodiment 3
[0117] In this example, the in vitro anti-HCV activity of the compounds of formula I-1 to formula I-35 was evaluated, and the human liver cancer cell line Huh7.5.1 was used to evaluate the anti-HCV activity at the cellular level in vitro. The method is described as follows:
[0118] MTT method to detect drug cytotoxicity: Huh7.5.1 cells in the logarithmic growth phase were taken, and 9×10 3 Cells / well were spread on a 96-well plate, and after 5 hours of attachment, 2 μL DMSO was added to serially dilute the drug, 5-fold dilution, 5 dilutions, each gradient had three replicate wells, and a blank control (only medium) was set at the same time. , cell control, DMSO control and anti-HCV positive drug Ribavirin (Ribavirin) control, the final volume is 200 μL / well. Place the culture plate at 37°C, 5% CO 2 incubator for cultivation. On the third day, 20 μL of 5 mg / mL MTT solution was added to the experimental wells, at 37 °C, 5% CO 2 Incubate for 4 hours. Discard the supernatant,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com