Stable pharmaceutical composition of peginterferon alpha-2b
A composition and drug technology, applied in the field of preparing the composition, can solve the problems of antigenic short pharmacological half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
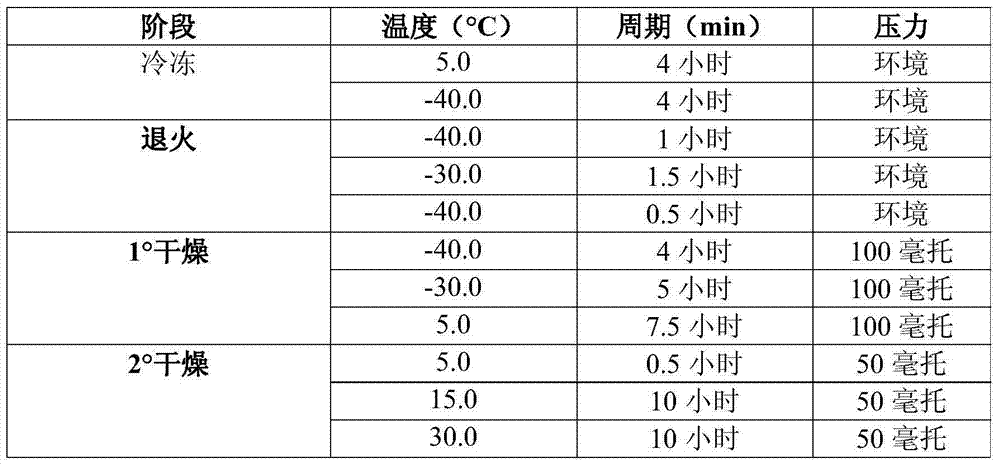
[0039] The formulation method for the Peg-IFN medicine includes 3 steps, namely preparation of the formulation body, filling into vials and freeze-drying. The formulated body is prepared by diluting the drug product with a formulation buffer to bring the formulated body to the desired concentration. Formulation buffer was prepared by adding disodium phosphate dihydrate and citric acid in required amounts (as mentioned in Table 1) to WFI followed by mixing. To this solution was added the required amount of cryoprotectant HPBCD, sodium chloride and polysorbate 80 in a stepwise manner, and after adjusting the pH the desired volume was adjusted with WFI. The formulation buffer was then sterile filtered through a 0.22μ sterile grade PES filter. Based on dosing calculations, the required amount of Peg-IFN (in the same formulation) was sterile diluted with filtered formulation buffer to bring the Peg-IFN composition to the desired concentration. The formulated bulk was filtered thr...
example 2
[0043] The method used to prepare the PEG-IFNα-2b composition is described in Example 1, wherein the cryoprotectant used was HPBCD. Excipients and amounts of PEG-IFNα-2b are provided in Table 2.
[0044] Table 2: Unit formulations of PEG-IFNα-2b compositions in stability studies
[0045] Element
example 3
[0047] The method used to prepare the PEG-IFNα-2b composition was the same as that described in Example 1, wherein the cryoprotectant used was sucralose. The amounts of excipients and PEG-IFNα-2b are provided in Table 3.
[0048] Table 3: Unit formulations of PEG-IFNα-2b compositions in stability studies
[0049] Element
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com