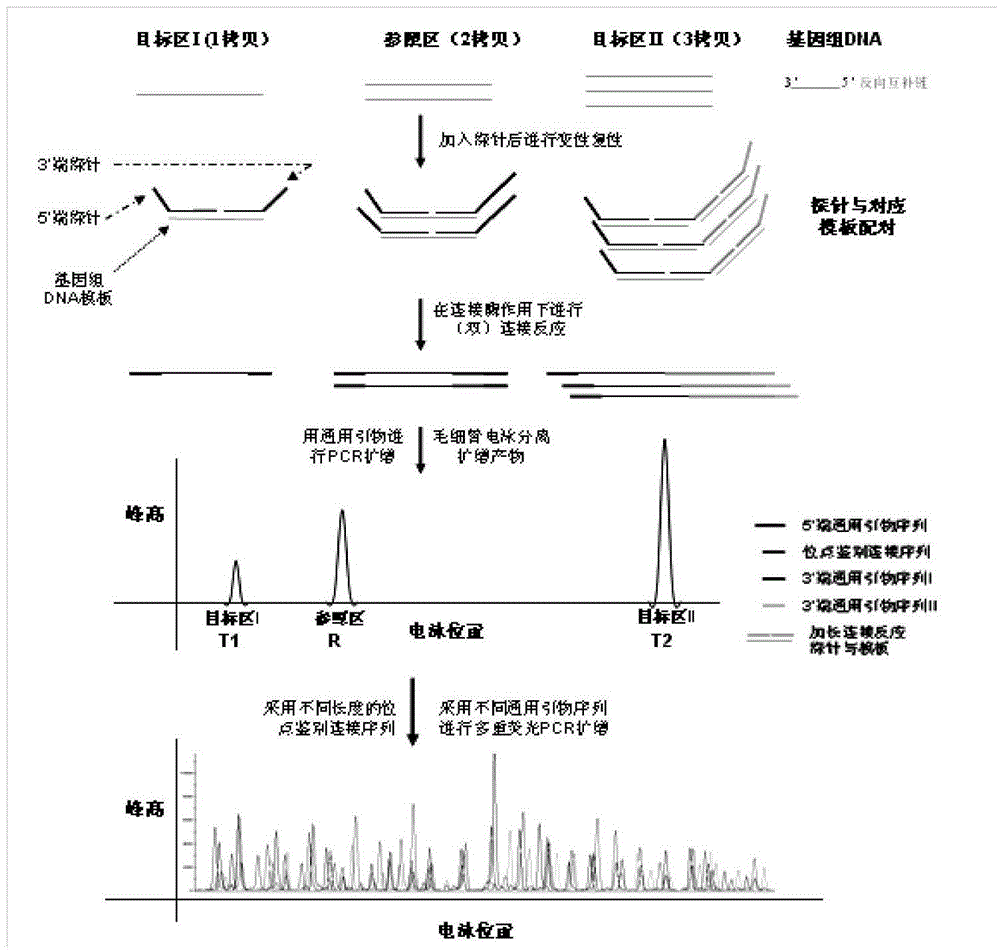
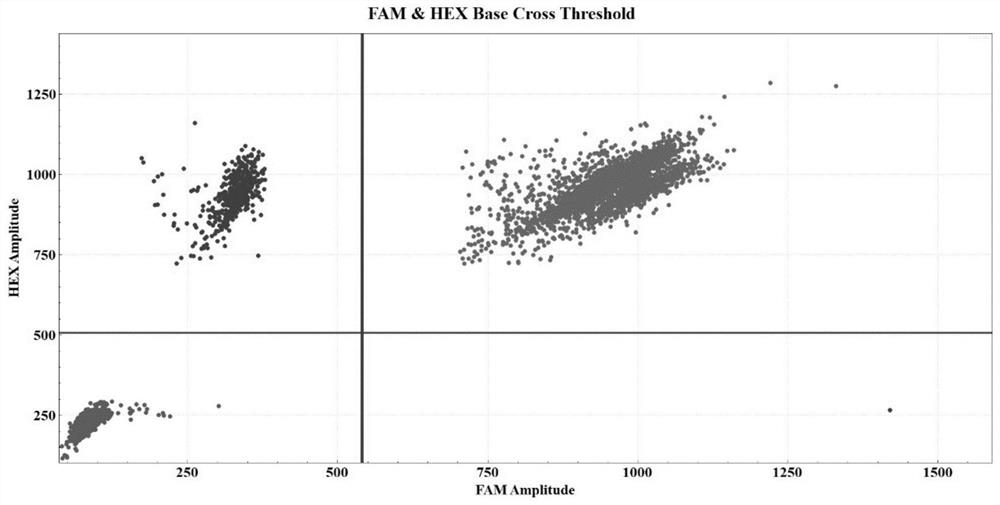
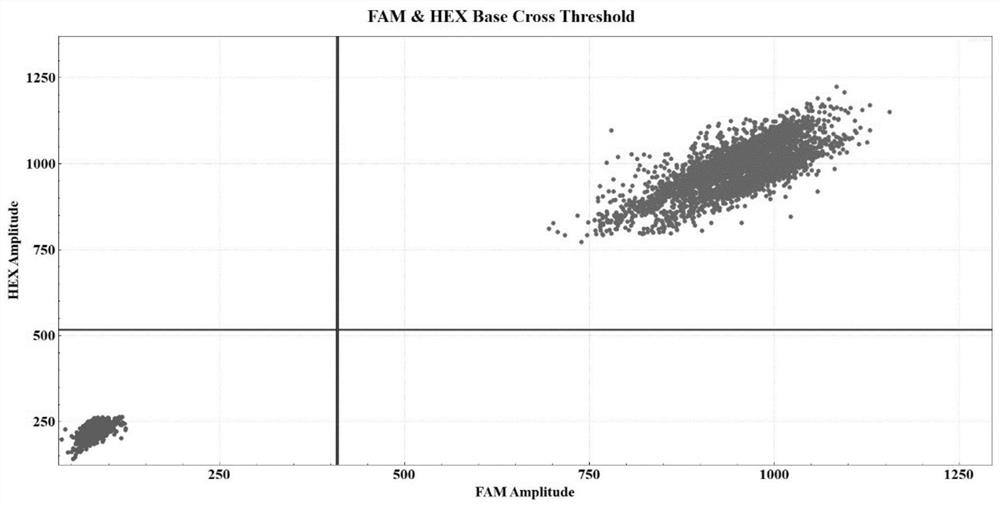
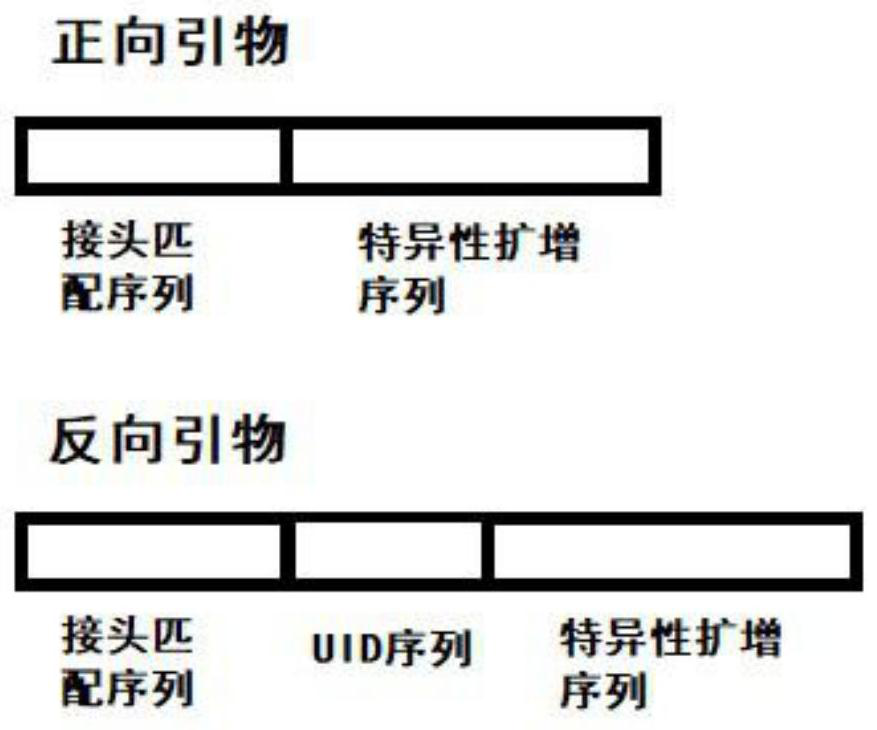
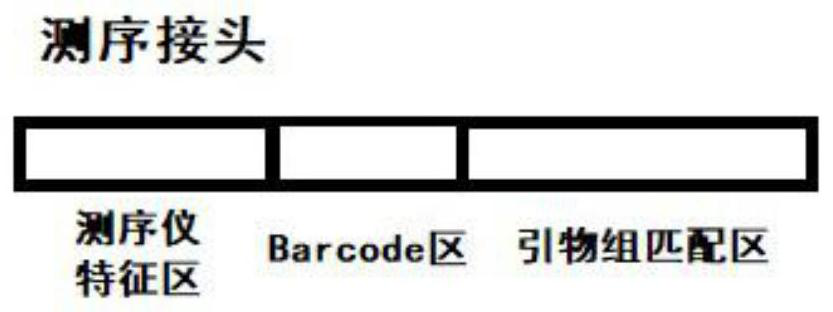
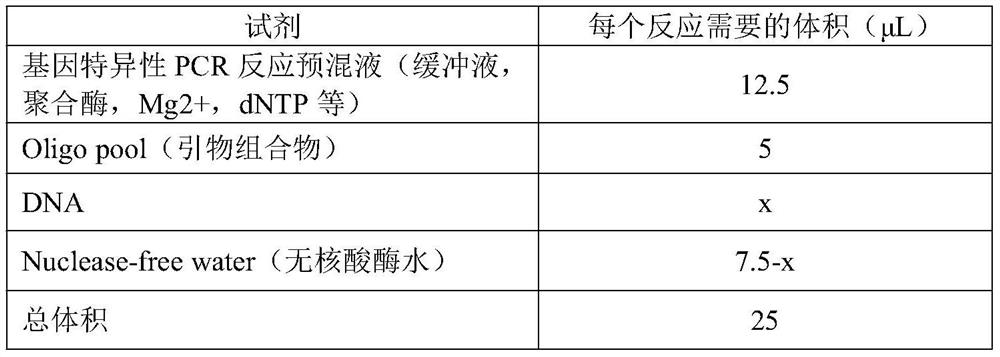
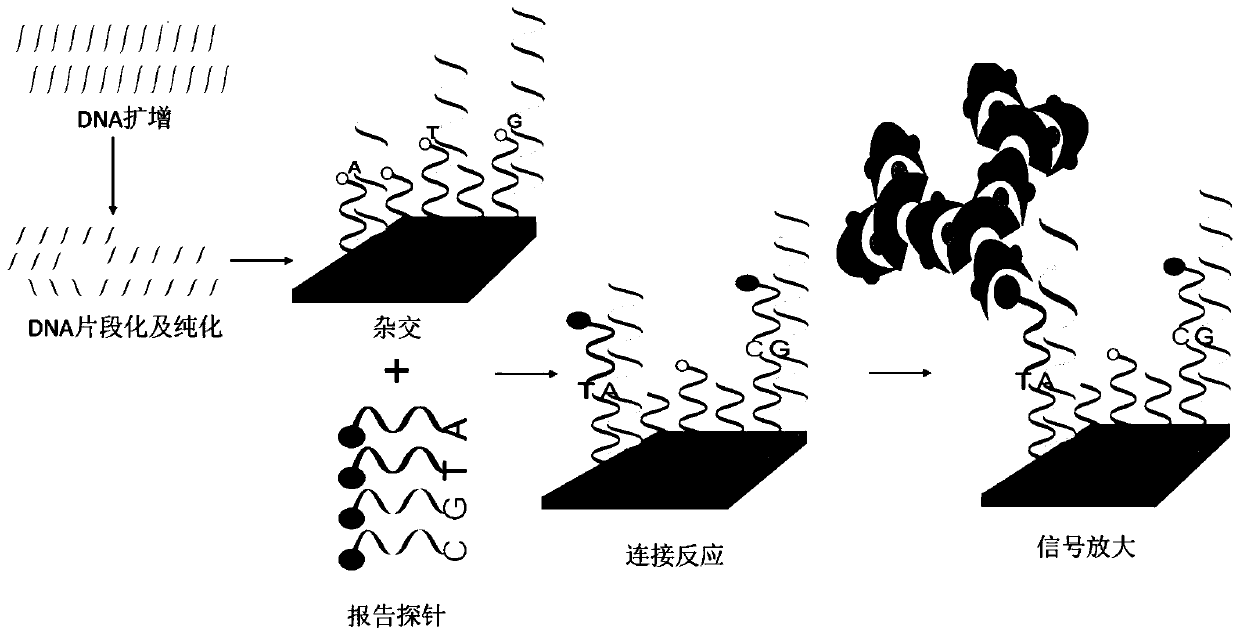
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Mutational hotspot" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
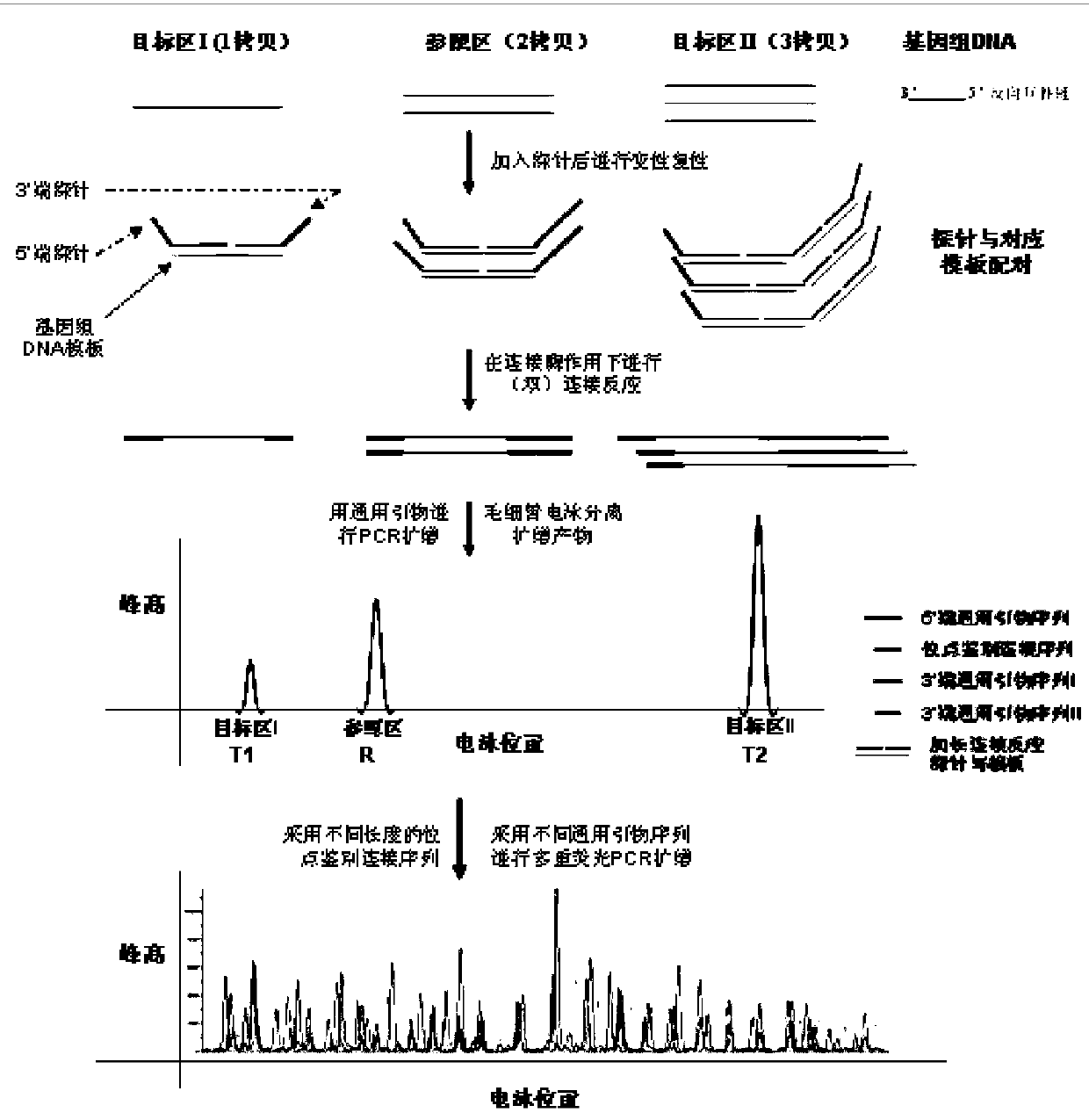
Mutations are concentrated at hotspots. KEY TERMS: A is a site in the genome at which the frequency of mutation (or recombination) is very much increased, usually by at least an order of magnitude relative to neighboring sites.
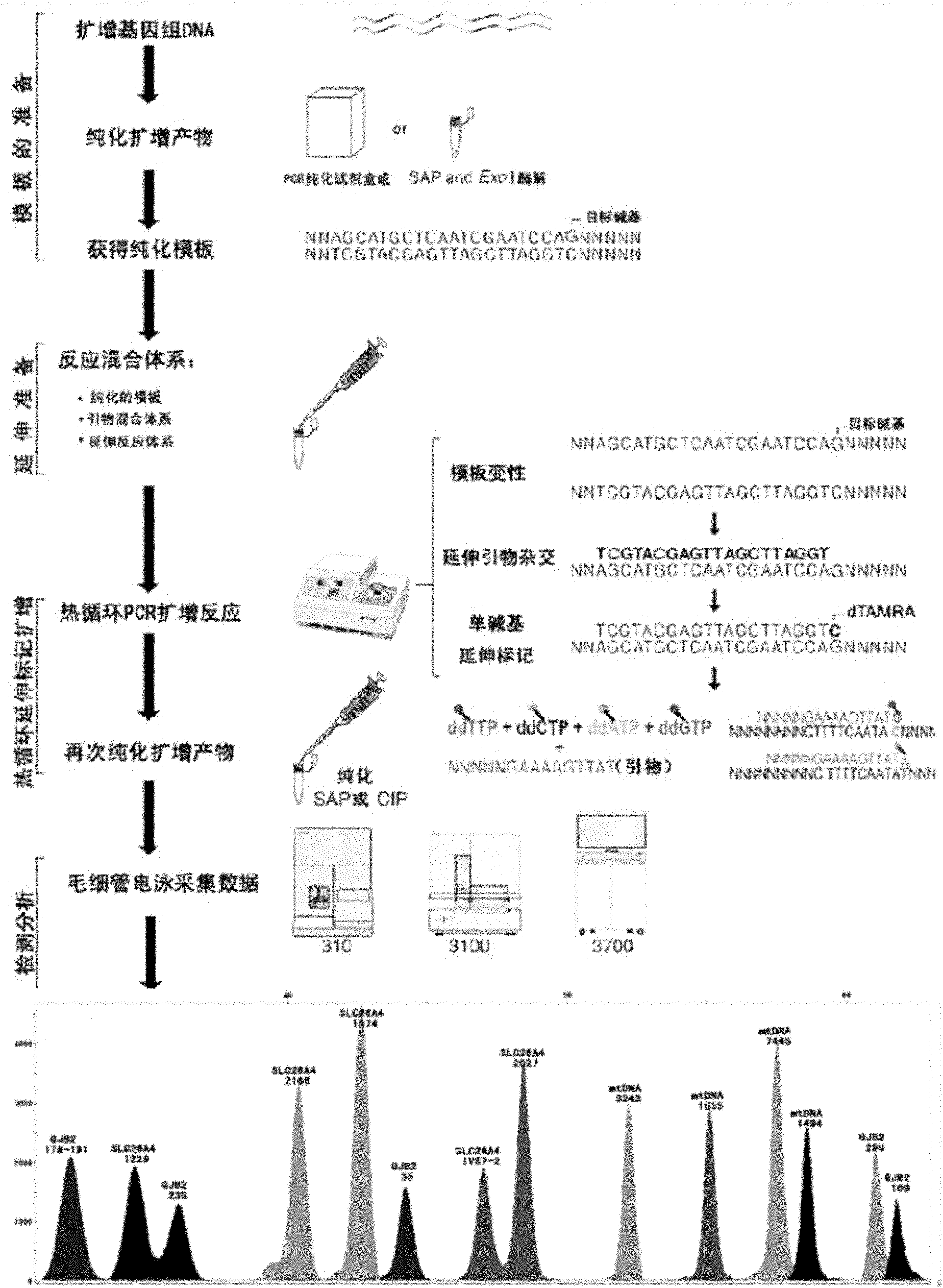
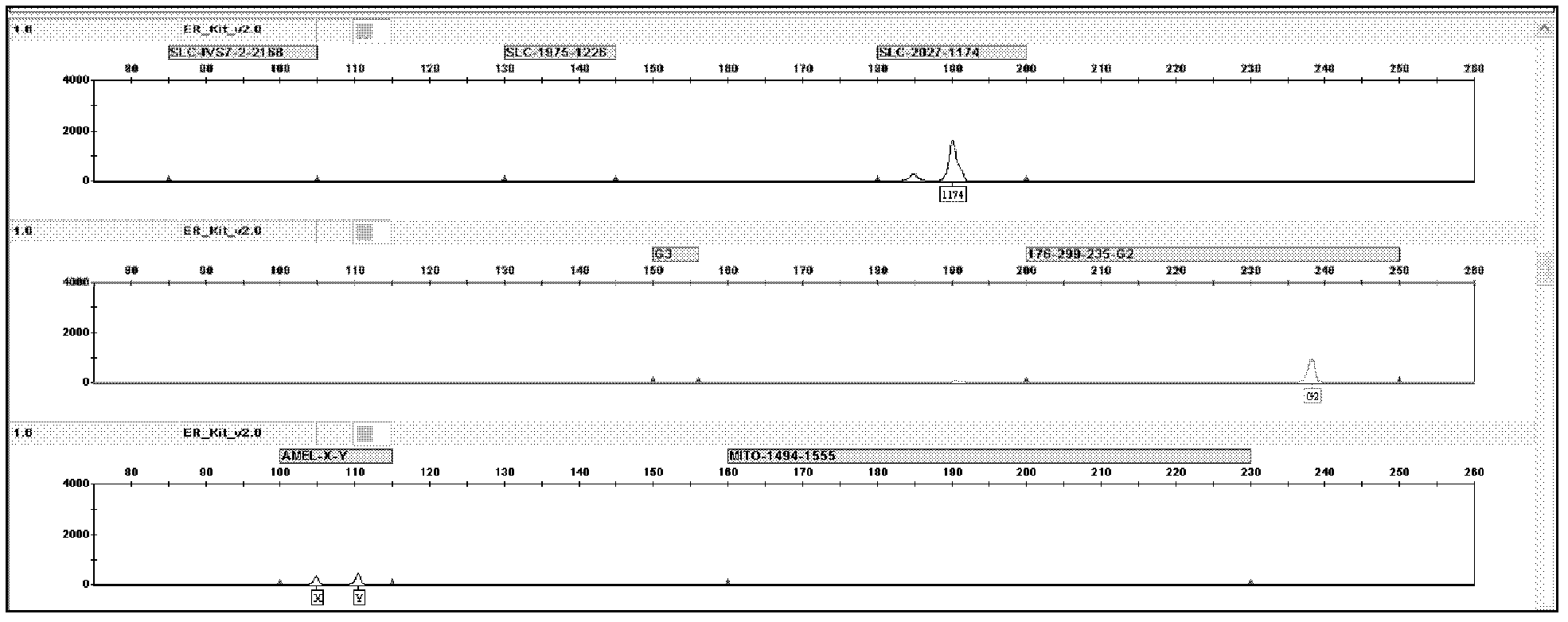
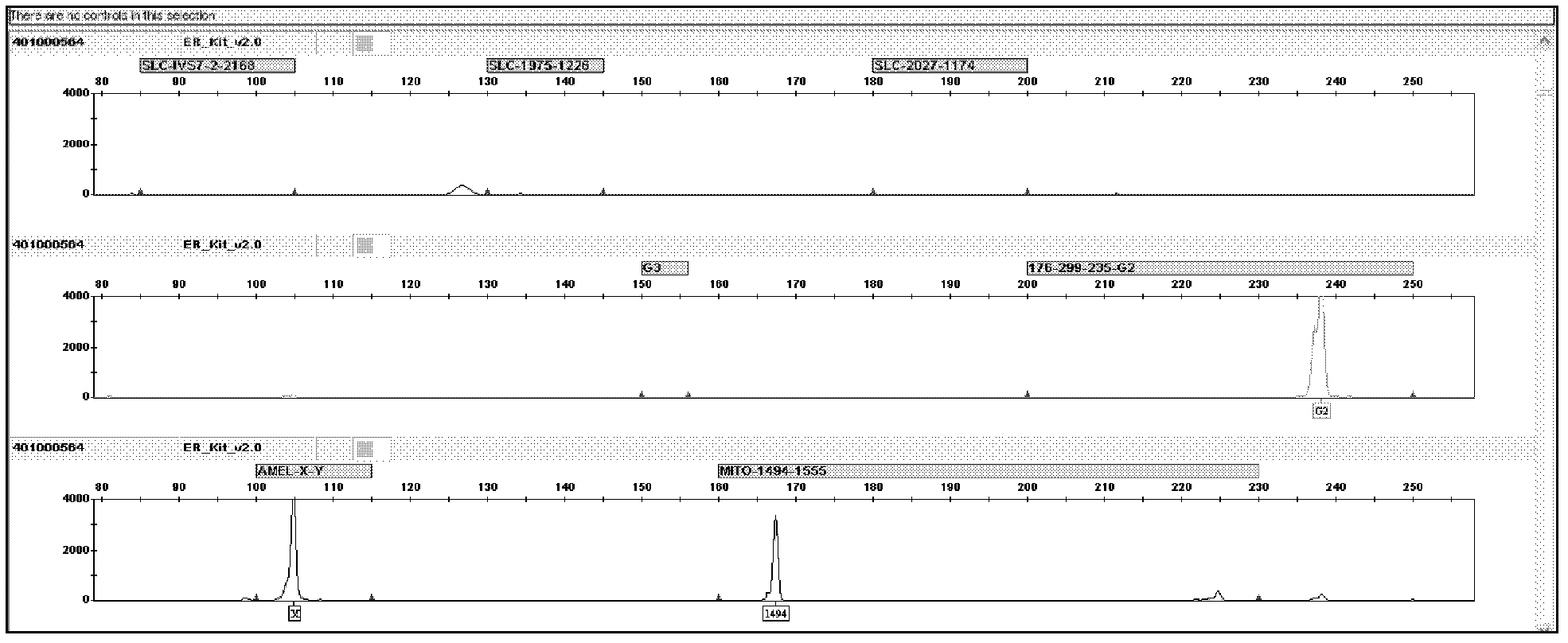
Kit for screening deaf gene of Chinese populations, and use method thereof
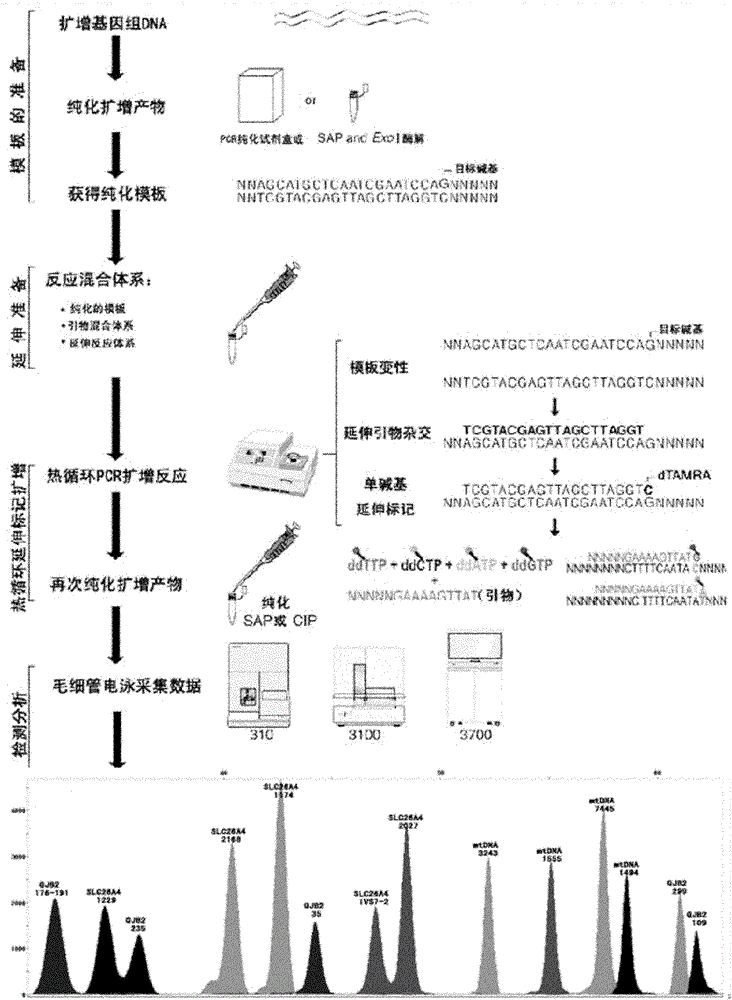
The invention discloses a kit for one-time qualitative screening of common fourteen mutational sites of deaf gene of Chinese populations and a use method thereof. The kit takes total fourteen sites of the deaf gene of the Chinese populations as detection objects. Application primers and extension primers are respectively designed for the mutation of each site, multiple PCR amplification and markup extension are simultaneously carried out on each target zone, and genotypes of above fourteen sites can be obtained at once through capillary electrophoretic analysis. The screening method and the kit of the invention have advantages of convenient use, simple operation, low cost, high flux, and direct and reliable detection result, and are suitable for large scale screening of the deaf gene mutation of the Chinese populations.
Owner:SUZHOU MUNICIPAL HOSPITAL
High-specificity kit for detecting deafness predisposing genes
InactiveCN102534031AAvoid false positivesMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses a fluorescent detection kit for detecting 12 deafness predisposing genes simultaneously. The kit can detect 12 mutational hotspots in the most common deafness associated genes of the Chinese in 3 hours. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of detection sites at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:万戈江
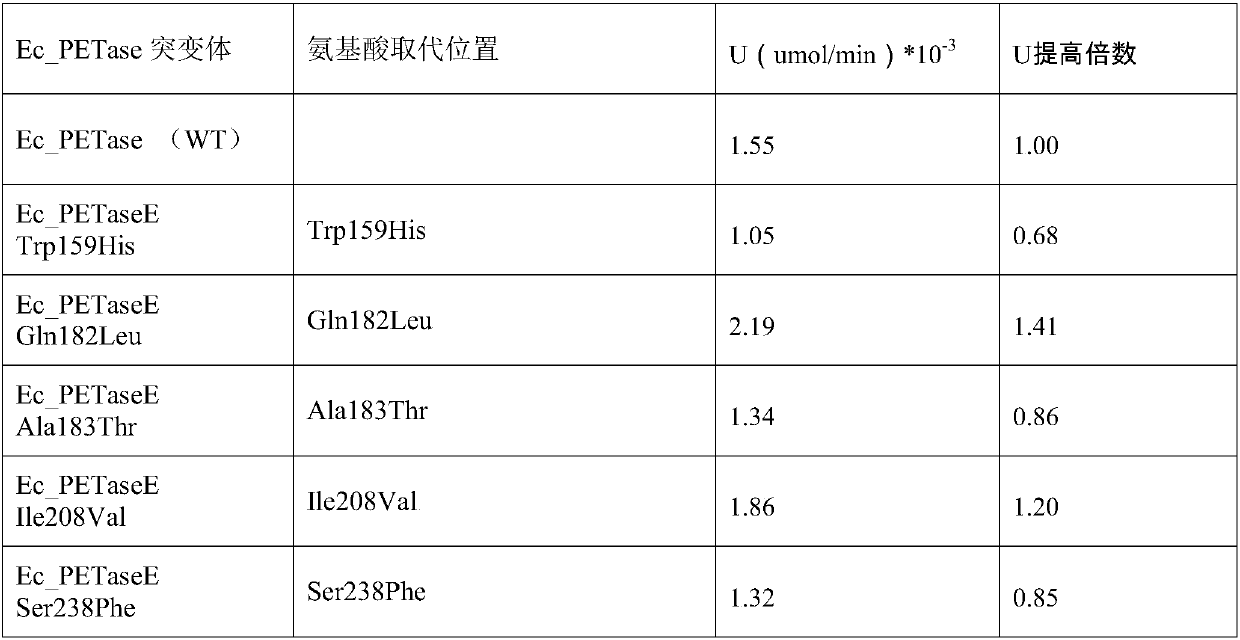
PET hydrolase mutant with high catalytic activity
ActiveCN107674866AIncrease enzyme activitySimplified degradation stepsHydrolasesFermentationWild typeSite-directed mutagenesis
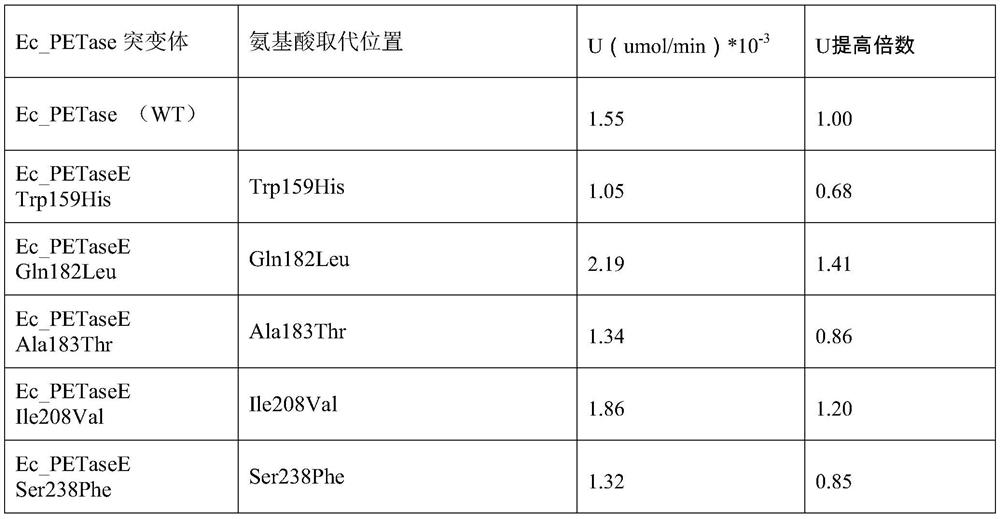
The invention belongs to the field of protein engineering, and specifically relates to a PET hydrolase mutant with high catalytic activity. The technical problem to be solved by the invention is thatthe activity of PET hydrolase (PETase) derived from Ideonella sakaiensis 201-F6 bacterial strains at present is still unsatisfactory. The technical scheme of the technical problem solved by the invention is to provide the PET hydrolase mutant. Five mutation hot spots are obtained through a large number of researches of PET hydrolase (PETase); fourteen mutants are constructed by adopting a site-specific mutagenesis technology, and finally the activity of ETase enzyme of two screened mutants are improved compared with that of wild type Ec_PETase, and the PET hydrolase mutant has preferable application prospects.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
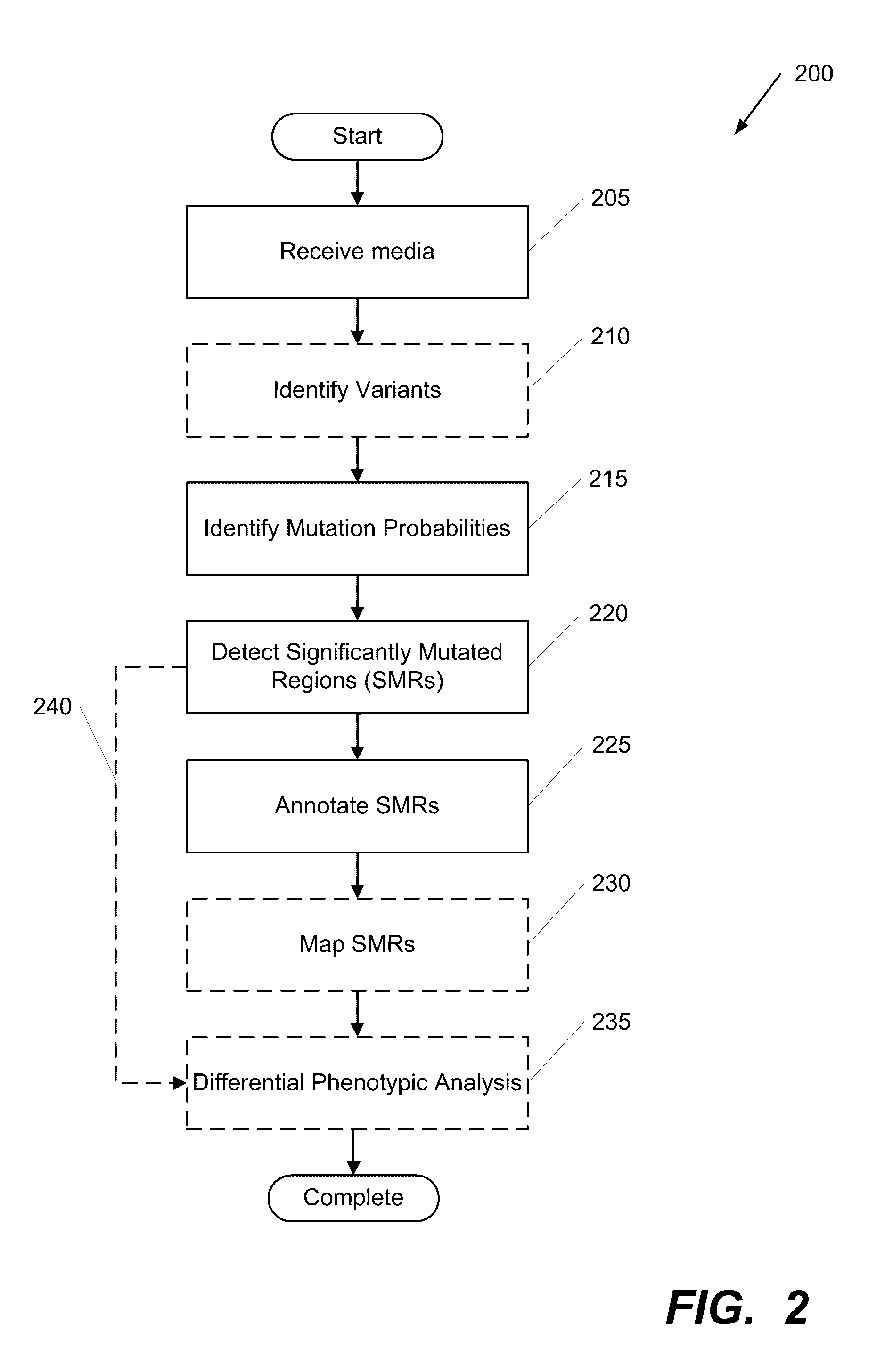
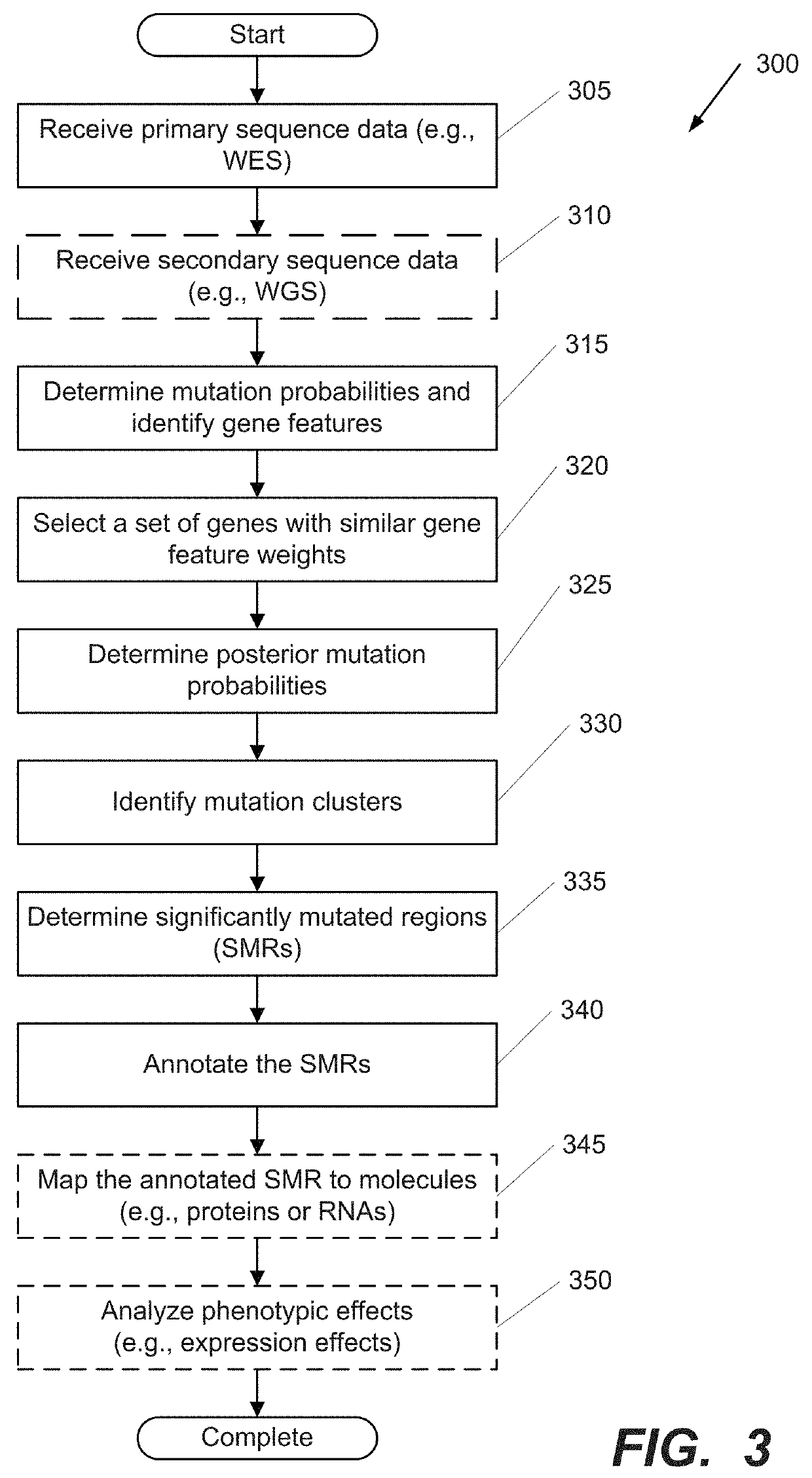
Systems and Methods for Multi-Scale, Annotation-Independent Detection of Functionally-Diverse Units of Recurrent Genomic Alteration
InactiveUS20160378915A1Useful in detectionMicrobiological testing/measurementBiostatisticsHuman DNA sequencingBinding site
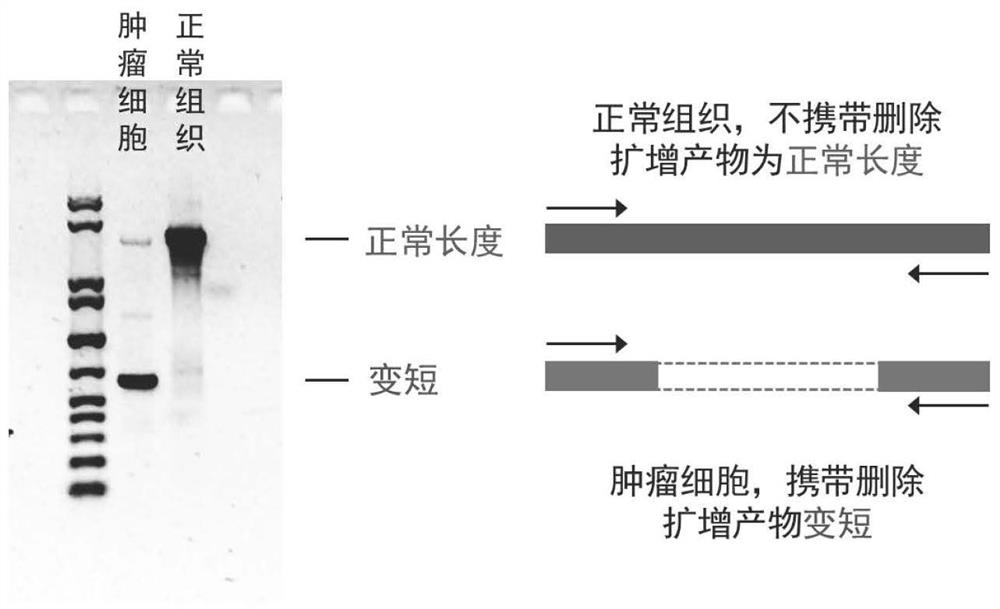
The functional interpretation of somatic mutations remains a persistent challenge in the interpretation of human genome data. Systems and methods for detecting significantly mutated regions (SMRs) in the human genome permit the discovery and identification of multi-scale cancer-driving mutational hotspot clusters. Systems and methods of SMR detection reveal differentially mutated genetic regions across various cancer types. SMR detection and annotation reveals a diverse spectrum of functional elements in the genome, including at least single amino acids, compete coding exons and protein domains, microRNAs, transcription factor binding sites, splice sites, and untranslated regions. Systems and methods of SMR detection optionally including protein structure mapping uncover recurrent somatic alterations within proteins. Systems and methods of SMR detection optionally including differential expression analysis reveal previously unappreciated connections between recurrent and somatic mutations and molecular signatures.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Novel chromosome microdeletion/microduplication syndrome detection system and kit
ActiveCN104651516ARealize detectionIncreased length rangeMicrobiological testing/measurementDECIPHERChromosome microdeletion
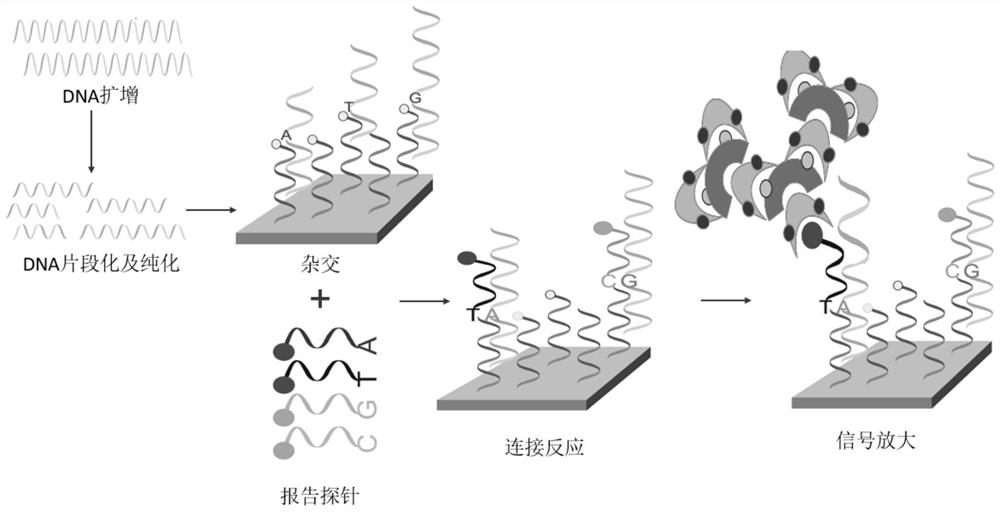
The invention discloses a novel chromosome microdeletion / microduplication syndrome detection system which mainly comprises 643 pairs of probes, multiplex PCR (polymerase chain reaction) amplification primers and correlation detection reaction reagents. The detection process comprises the following steps: hybridizing a probe set with target nucleic acid in the sample; connecting the hybridized probe; amplifying the connecting probe; and detecting the amplification product to determine the existence or quantity of the target nucleic acid in the sample. The platform can implement systematic screening and detection on 24 chromosome aneuploids, 24 chromosome telomere abnormities, more than 70 chromosome microdeletion / microduplication syndromes and multiple monogenic disease mutation hot spots at one time by using the optimized probe set and reaction system. Compared with the existing like detection techniques (such as MLPA and the like), the detection system covers nearly all definite pathogenic regions with abnormal number of copies in the DECIPHER database, has the advantages of wide detection range, high detection precision and low cost, and is simple to operate.
Owner:SUZHOU MUNICIPAL HOSPITAL +1
Kit for quantificationally detecting BRAF (Block Repeat Active Flag) mutation
InactiveCN102161990AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceBRAF Gene MutationEGFR Tyrosine Kinase Inhibitors
The invention relates to a method and kit for detecting BRAF (Block Repeat Active Flag) mutation relevant to the curative effect of a molecular-targeting anti-cancer medicament, in particular relating to a fluorescence quantificational PCR (Polymerase Chain Reaction) detecting method and kit for detecting mutation in BRAF gene mutation hot spot regions and application thereof. According to the invention, mutation at special positions of BRAF genes is detected, the curative effect of the molecular-targeting anti-cancer medicament such as an EGFR (Epidermal Growth Factor Receptor) tyrosine kinase inhibitor, and the like can be forecasted, and furthermore, the individual medicament use schemes of patients with tumors are directed.
Owner:BEIJING ACCB BIOTECH
Primer for detecting IDH1 and IDH2 gene polymorphism mutation sites, method and kit
InactiveCN103451314ASave the tedious processSave moneyMicrobiological testing/measurementDNA/RNA fragmentationMedicineExon
The invention discloses a primer for detecting IDH1 and IDH2 gene polymorphism mutation sites, a method and a kit. According to the primer, (1) an amplified IDH1 gene comprises a primer body of a 132nd amino acid sequence, and (2) an amplified IDH2 gene comprises primer bodies of 140th and 172nd amino acid sequences. A common PCR technique is adopted, the primer can be used for quickly detecting mutation situations of IDH1 and IDH2 gene polymorphism hot spots in the body of a patient suffering from acute granulocytic leukemia (AML). According to the primer, the method and the kit, the automation degree is effectively improved, complicated procedures and large detection expenses for expressed region sequencing of IDH1 and IDH2 are eliminated, the patient can be diagnosed quickly and precisely and expenses are low.
Owner:南京艾迪康医学检验所有限公司
Design method and application of probe combination for cancer detection
ActiveCN112951325AEasy to coverHigh sensitivityBiostatisticsProteomicsEarly Cancer DetectionCancer type
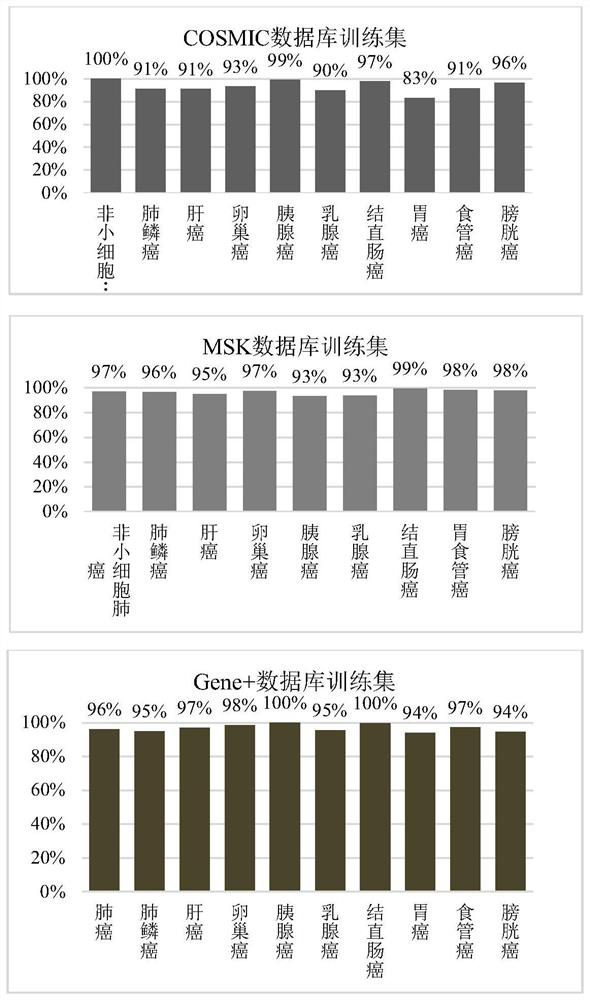
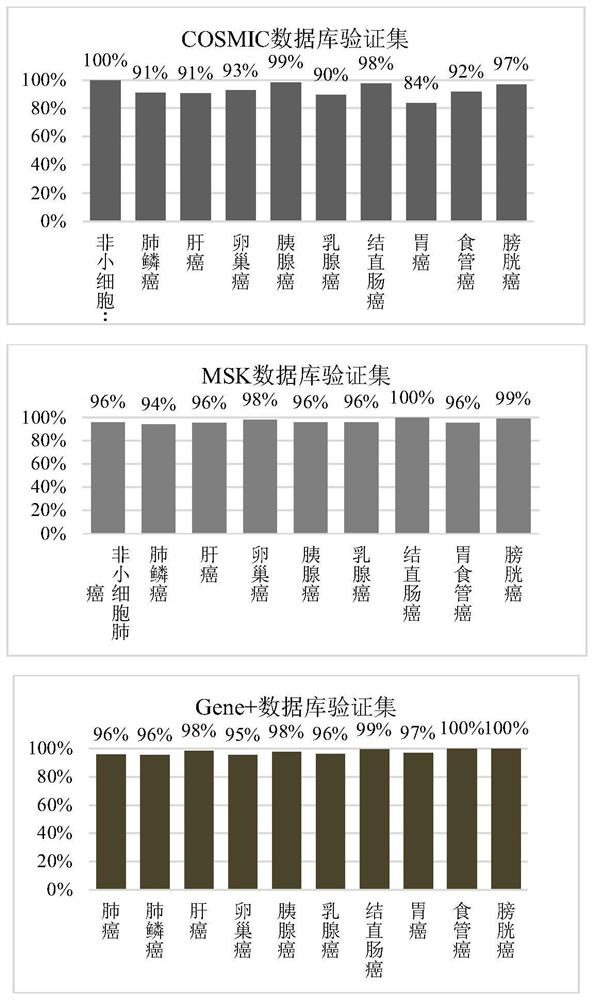
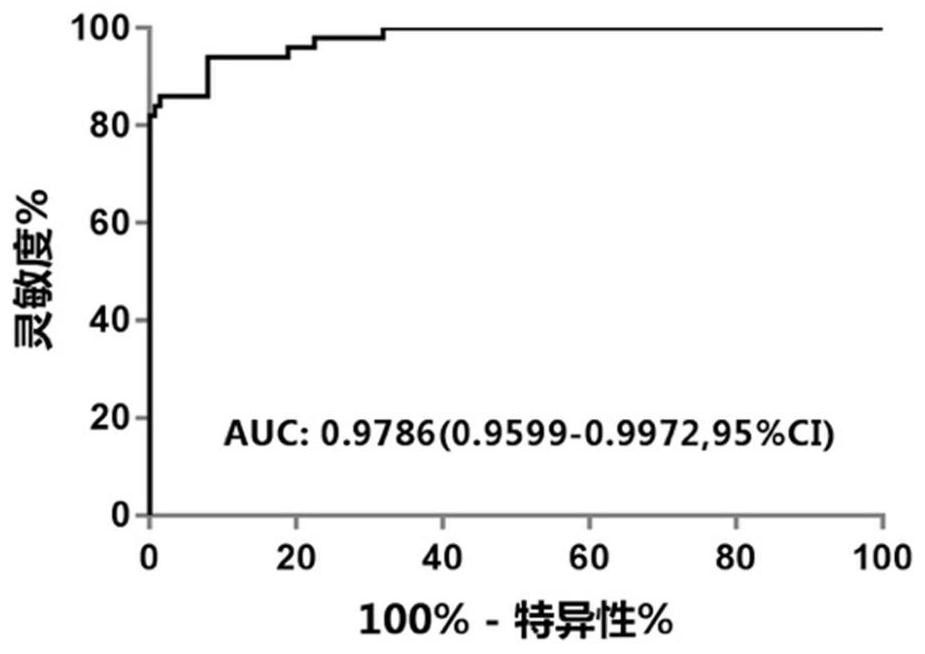
The invention relates to a design method and application of a probe combination for cancer detection. The design method comprises the following steps: extracting a mutation set of cancer in a database, dividing the mutation set into a training set and a verification set, and combining mutations with reference genome distance less than or equal to 80 in the training set so as to obtain a plurality of mutational hotspot intervals; and sequentially screening the multiple mutation hotspot intervals on the basis of the regional mutation density, and taking the mutation hotspot intervals meeting the following conditions as targets of the probe combination. The probe combination designed by the invention has excellent coverage on common cancers, a Gene+ database and MSK database verification set is adopted to simulate the coverage condition of the panel on nine cancers, and the result shows that the coverage degrees of nine cancer types are all greater than 93%; the early cancer detection based on the probe has high sensitivity and specificity, and the detection rate of liver cancer reaches 85%; and a ctDNA positive judgment method based on the probe can effectively perform prognosis layering on a patient.
Owner:北京吉因加医学检验实验室有限公司 +1
Kit for screening deaf gene of Chinese populations, and use method thereof
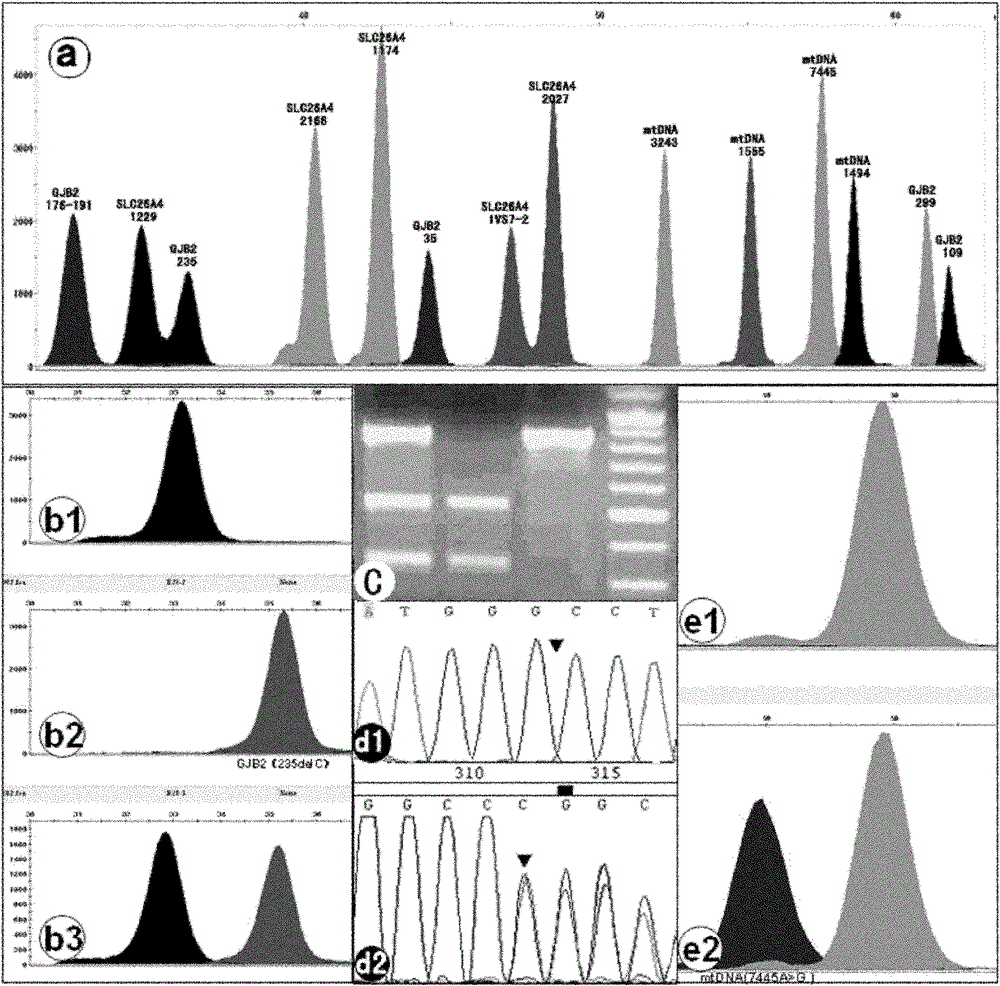
The invention discloses a kit for one-time qualitative screening of common seven mutational sites of deaf gene of Chinese populations, and a use method thereof. Seven mutational hotspots which comprise delC of a 235th site of GJB2 gene, delAT of 299-300th sites of the GJB2 gene, A-G mutation of a 2168th site of SLC26A4 gene, A-G mutation of an IVS7-2th site of the SLC26A4 gene, A-G mutation of a 1555th site of mitochondrial DNA gene, A-G mutation of a 3243th site of the mitochondrial DNA gene, and A-G mutation of a 7445th site of the mitochondrial DNA gene are treated as detection objects, application primers and extension primers are respectively designed for the mutation of each site, multiple PCR amplification and markup extension are simultaneously carried out on each target site, and genotypes of above seven sites can be obtained at once through capillary electrophoretic analysis. The screening method and the kit of the invention have advantages of convenient use, simple operation, low cost, high flux, and direct and reliable detection result, and are suitable for large scale screening of the deaf gene mutation of the Chinese populations.
Owner:SUZHOU MUNICIPAL HOSPITAL
Kit for quantitative detection of K-ras mutation
ActiveCN102115782AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The invention relates to a kit for quantitative detection of K-ras mutation. The invention specifically relates to a detecting method and a detecting kit for K-ras gene mutation relevant with the curative effect of molecular targeted anti-cancer drugs. The invention especially relates to fluorescence quantification PCR detecting method for K-ras gene mutational hotspot region detecting mutational, kit and the application of the fluorescence quantification PCR detecting method. The method of the invention detects the mutation of K-ras gene at a particular locus, predicts the curative effect of molecule targeting anticancer medicament EGFR tyrosine kinases inhibitor, furthermore provides clinic individualized medication scheme for a tumour patient with guidance.
Owner:BEIJING ACCB BIOTECH
Screening method of SARS-CoV2 potential mutation sites and application thereof
PendingCN113470745AImplement variant monitoringImprove packaging efficiencySsRNA viruses positive-senseVirus peptidesMutation frequencyProtein
The invention relates to the technical field of bioinformatics and biological medicine, in particular to a screening method of SARS-CoV2 potential mutation sites. The method comprises the following steps of: 1) downloading to obtain a gene sequence of SARS-CoV2, carrying out rapid file annotation and sequence comparison on the downloaded sequence, and extracting sequences of all coding genes from a whole genome sequence; (2) calculating the mutation frequency of each site, screening out high-frequency mutation hot spots, and screening out mutation sites with remarkable selection advantages in a population by combining the sampling time and geographical distribution information of strains; 3) downloading tertiary structure information of proteins corresponding to existing coding genes; and 4) according to the predicted B cell and T cell epitopes, screening mutation sites on or near the immune epitopes, evaluating the possible influence of the mutation sites on the host immune response, and identifying the potential key mutation sites possibly related to virus infection and host adaptation of the SARS-CoV-2 on the genome in epidemic propagation.
Owner:NANJING LEADING BIOMEDICAL TECH CO LTD
High-specificity kit for detecting deafness predisposing genes and uses
InactiveCN102534031BAvoid false positivesMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses a fluorescent detection kit for detecting 12 deafness predisposing genes simultaneously. The kit can detect 12 mutational hotspots in the most common deafness associated genes of the Chinese in 3 hours. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of detection sites at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:万戈江
Primer composition, kit and method for detecting EGFR gene mutations
InactiveCN107488729AEasy to detectGood choiceMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationMicrobiology
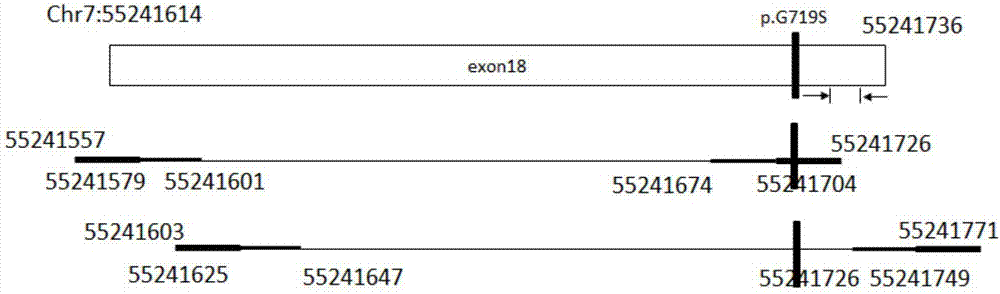
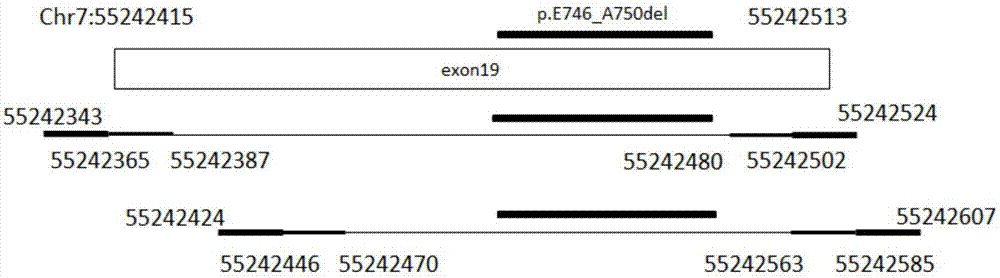
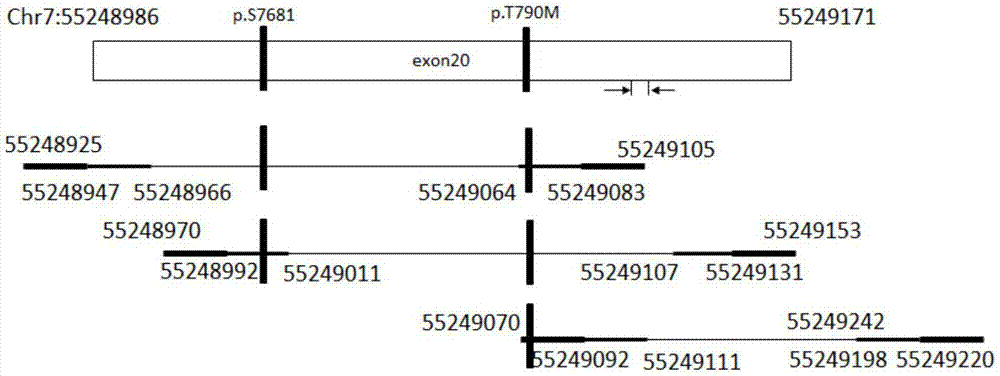
The invention discloses a primer composition, a kit and a method for detecting EGFR gene mutations, which aim to improve the convenience and the flexibility of EGFR gene mutation detection. The primer composition comprises a first composition A, wherein the first composition A comprises a first primer pair for amplifying a No.18 exon and a second primer pair for amplifying a No.19 exon; the first primer pair comprises A_E18-CS1-TS_1F as shown in SEQ ID NO:1 and A_E18-CS2-TS_1R as shown in SEQ ID NO:2. The primer composition simultaneously comprises the primer compositions for amplifying the No.18 exon and the No.19 exon, and the length of each amplicon obtained through amplification is smaller than 150bp, so that mutational hotspot areas in the two exons can be detected conveniently at the same time, an appropriate sample source is conveniently selected according to a sample state, and the convenience and the flexibility of EGFR gene mutation detection are improved.
Owner:LIAONING KEJUN BIOLOGICAL
Primers for detecting mutation of human B-raf gene V600E, primer probe composition and kit
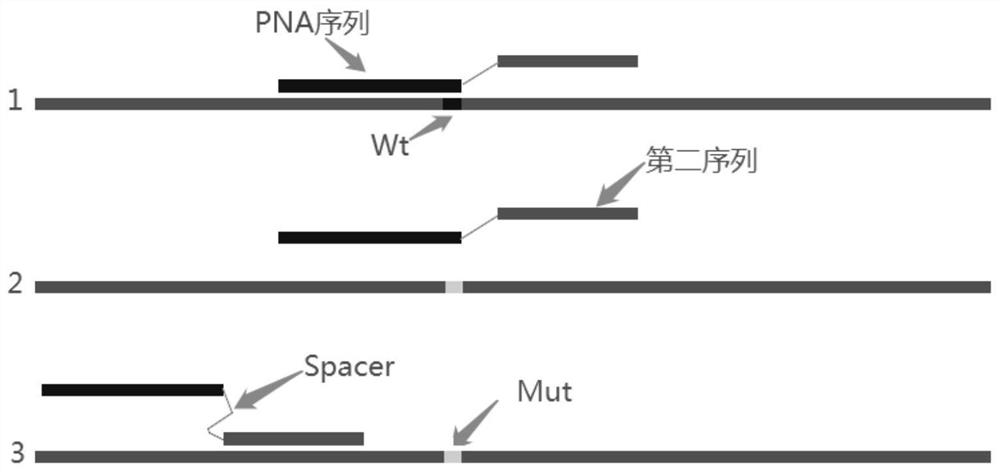
PendingCN111647650ANo mismatchHigh amplification efficiencyMicrobiological testing/measurementDNA/RNA fragmentationForward primerMutation detection
The invention discloses primers for detecting mutation of human B-raf gene V600E, primer probe composition and a kit. The kit comprises a B-raf gene V600E mutation detection primer pair and detectionprobe, an internal quality control primer pair and a quality control probe. A forward primer of the detection primer pair comprises three parts arranged in the following order from the 5' end to the 3' end: 1) a first sequence: the first sequence is used for identifying mutation hot spots, is a peptide nucleic acid PNA sequence hybridizing with a wild type template, is completely matched with thesequence of the wild type template and has a base mismatched with the sequence of a mutant template; 2) Spacer: the Spacer is connected with the 3' end of the first sequence and the 5' end of a secondsequence; 3) the second sequence: the second sequence is bound with a sequence upstream of a mutation site, 3-6bp of the sequence of the 3' end of the second sequence overlaps with the 5' end of thefirst sequence, Tm value of the first sequence is 6.3-11.3 DEG C higher than that of the second sequence. The kit is simple and quick, has sensitivity up to 1 permillage, and has high specificity, and the false positive rate is greatly reduced.
Owner:河南赛诺特生物技术有限公司
Detection kit and detection method for APL drug resistance gene mutation
InactiveCN106367503AAvoid missing detectionLow costMicrobiological testing/measurementSequence analysisDrug resistance
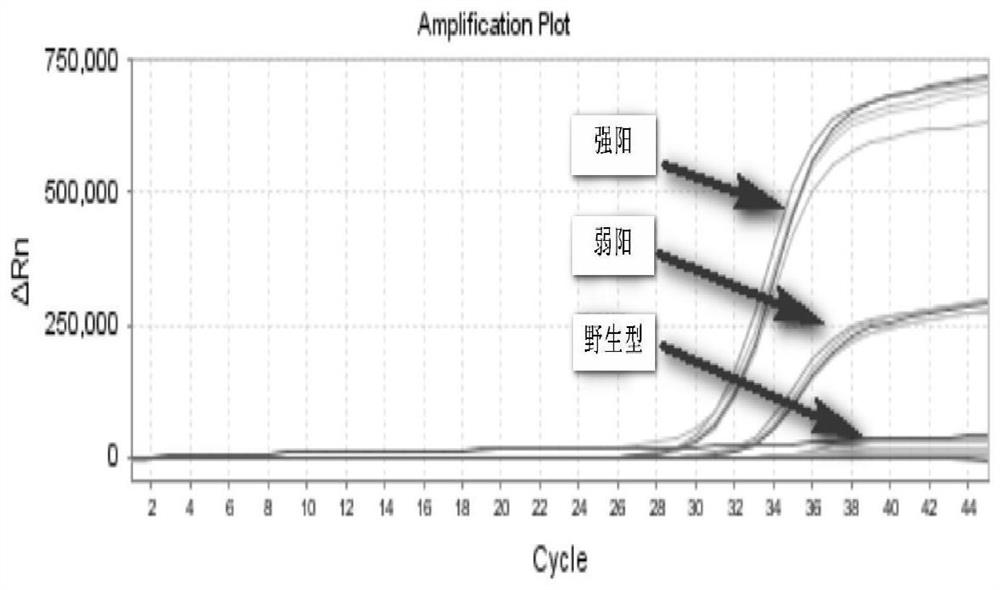
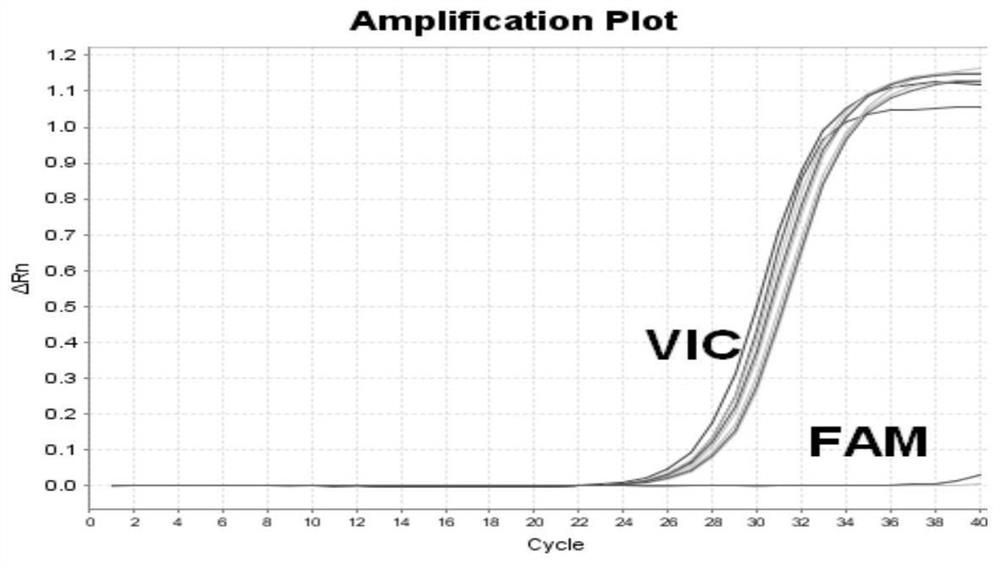
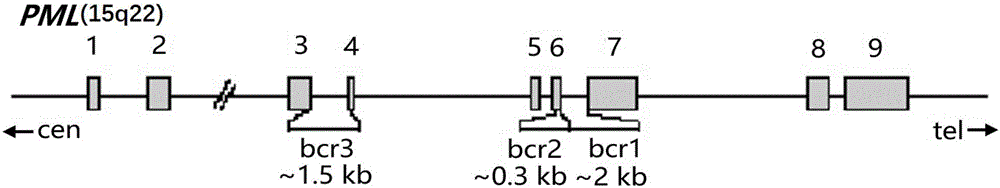
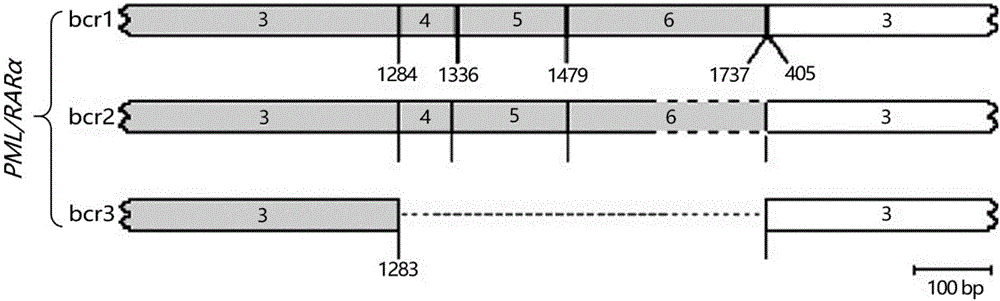
The embodiment of the invention provides a detection kit for APL drug resistance gene mutation and a detection method using the kit. The kit contains specific primers designed respectively for a PML / RARalpha fusion gene and protein mutational hotspot regains of PML and RARalpha, the primers are used for amplification of the PML / RARalpha fusion gene through PCR first and then respectively for amplification of the mutational hotspot regains of the PML and RARalpha, and finally sequencing analysis is carried out on target fragments in the APL sample amplification product to obtain the gene mutation condition of the APL sample. The kit and detection method provided by the invention can realize detection of mutation of three kinds of isomer PML / RARalpha fusion genes once, prevent S type and V type isomers from being undetected, and save the reagent cost at the same time; and by analyzing the gene mutation detection result acquired by the detection method provided by the embodiment of the invention, the suppression resistance caused by PML / RARalpha gene mutation to medicines such as As2O3 can be clinically revealed.
Owner:北京海思特医学检验实验室有限公司
Drop-off ddPCR method and kit for quantitatively detecting U2AF1 gene mutation
ActiveCN113186283AStrong specificityHigh sensitivityMicrobiological testing/measurementGenes mutationAURKA Gene
The invention relates to a detection method and a kit for detecting U2AF1 gene mutation based on drop-ffdPCR. Primers and probes of the detection method are designed according to a U2AF1 gene DNA sequence, two wild probes are designed for two mutation hotspots Q157 and S34 of a U2AF1 gene respectively, one wild probe is located on the mutation hotspot, the other wild probe is located outside the mutation hotspot, and when mutation such as insertion, replacement and deletion exists in the mutation hotspot, the wild-type probe located at the mutation hotspot cannot be tightly combined with the template, so that the kit can detect multiple mutations of the two mutation hot spots Q157 and S34 of the U2AF1 gene only by using two pairs of primer probes, and the kit is high in sensitivity and can be used for MRD monitoring.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL
Drop-off ddPCR method and kit for quantitatively detecting NPM1 gene mutation
ActiveCN113249475AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAURKA GeneWild type
The invention relates to a detection method and a kit for detecting NPM1 gene mutation based on drop-offddPCR; primers and probes thereof are designed according to an NPM1 gene DNA sequence; two wild probes are designed aiming at a 12th exon mutation hot spot of an NPM1 gene; one wild probe is located on the mutation hot spot; the other wild probe is located outside the mutation hot spot; when mutation such as insertion, replacement and deletion exists in the mutation hot spot, the wild probe located at the mutation hot spot cannot be tightly combined with a template, so that the kit can detect various mutations of the mutation hot spot of the 12th exon of the NPM1 gene only by using two pairs of primer probes; and the kit is high in sensitivity and can be used for MRD monitoring.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL
Lung cancer or colorectal cancer mutant gene detection primer composition and application thereof
PendingCN112029856ABetter ratioImprove uniformityMicrobiological testing/measurementDNA/RNA fragmentationForward primerOncology
The invention provides a lung cancer or colorectal cancer mutant gene detection primer composition. The composition comprises 103 pairs of primers or a combination containing a part of the primers, and the forward primer sequence and the reverse primer sequence of each pair of primers comprise an amplification primer pair and other sequences connected to the 5' end of the amplification primer pair. Specific sequences in a forward primer and a reverse primer of each pair of primers are selected from sequences shown in SEQ ID NO.1-206, and preferably, the specific sequences are 18-25 base sequences in the sequences. According to the composition, 22 mutation hot spot regions of lung cancer or colorectal cancer related genes are carefully selected, a low-initial-quantity or low-quality FFPE sample can be detected, a ctDNA sample can also be detected, and general requirements of early screening, diagnosis, medication and prognosis of lung cancer and colorectal cancer are met.
Owner:PILLAR BIOSCI INC
Gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency disease
ActiveCN111304317AHigh degree of standardizationImprove diagnostic accuracyMicrobiological testing/measurementHereditary MutationDisease
The invention relates to a gene mutation detection kit for a human glucose-6-phosphate dehydrogenase deficiency disease. The gene mutation detection kit comprises probes with sequences represented bySEQ NO.1-382. By carrying out distribution analysis on positive mutation sites of 500 G6PD genes in the China area and combining with Human Gene Mutation Database (HGMD) and gene mutation hotspot dataof the European and American areas, gene mutation hotspots are more comprehensively summarized, so that the problems of genetic mutation detection of nearly 99% of patients with a G6PD deficiency disease are solved, and rapid and effective execution of genetic diagnosis of the G6PD deficiency disease is realized.
Owner:上海源庆生物科技有限公司
A kit for detecting thalassemia gene mutation
ActiveCN110577990BHigh sensitivityImprove stabilityMicrobiological testing/measurementNucleic acid detectionPrenatal diagnosis
The invention belongs to the technical field of nucleic acid detection, and particularly relates to a kit for detecting thalassaemia gene mutations. The kit includes amplification primers shown as SEQID NO:1-SEQ ID NO:10 and extension primers shown as SEQ ID NO:11-SEQ ID NO:28. The kit realizes the genotyping of various types of mutation hotspots of thalassaemia genes in the same reaction system,has both flexibility and scalability, is simple in operation and high in throughput and low in cost, and is of great significance for the screening, prenatal diagnosis and the like on people with thalassaemia.
Owner:SOUTHERN MEDICAL UNIVERSITY +1
PCR primer group, kit and application thereof
ActiveCN113881775AImprove responsePrecise positioningMicrobiological testing/measurementAgainst vector-borne diseasesRepetitive SequencesConserved sequence
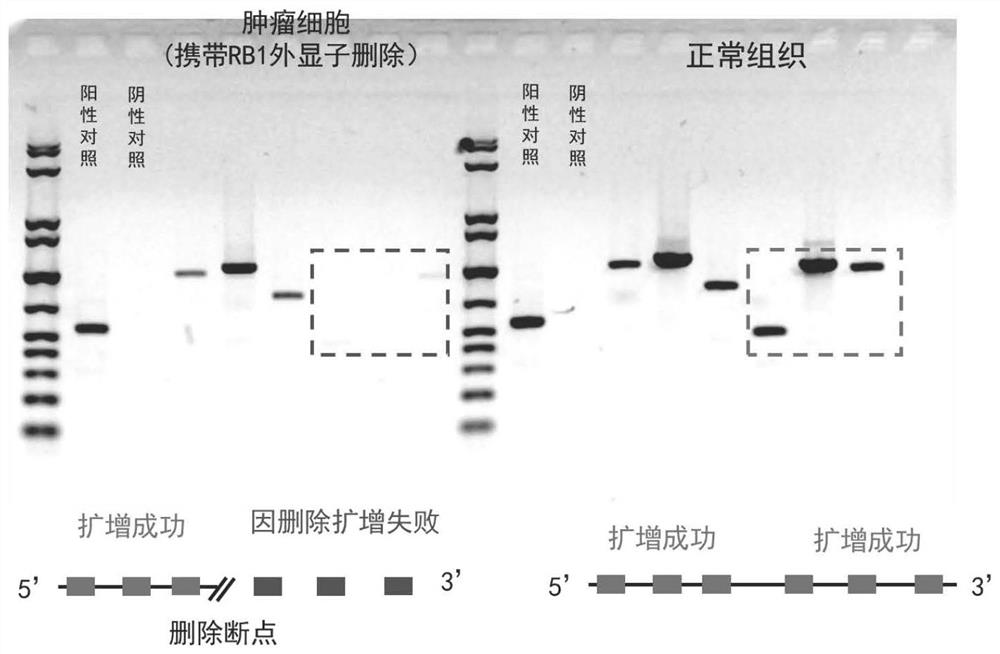
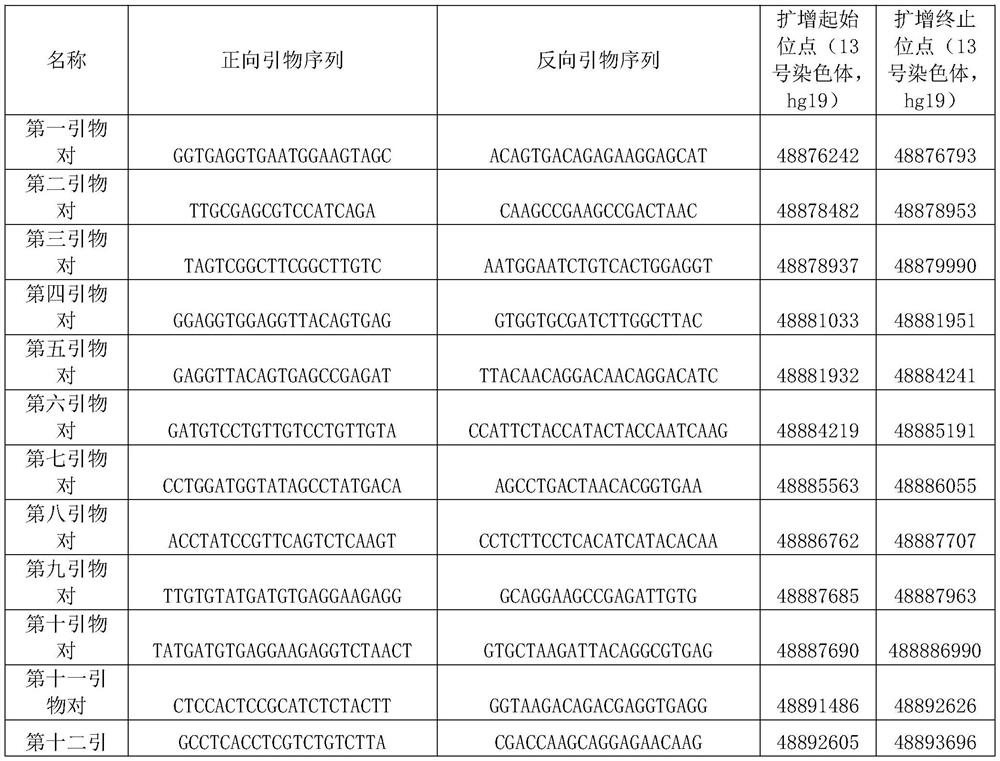
The invention discloses a PCR primer group, a kit and application thereof. The total number of the PCR primer group is 65. The primer pairs are uniformly distributed in the full length of the RB1 gene, and meanwhile, extra densification is carried out on a mutation hot spot region. The primer pair of the invention can be used for full-length amplification of exons which are short in part of the RB1 gene and are close to each other, and deletion in the amplification region can be found. For an intron region with a relatively long span, a primer pair design is additionally added, aiming at a relatively conserved sequence of an intron, a large-section repetitive sequence and a high-GC-ratio sequence are avoided, and a PCR reaction is easy to carry out. By using the method, the breakpoint of RB1 gene exon deletion can be simply and conveniently positioned with extremely low price, basic molecular biology experiment conditions and relatively low labor hours. The PCR primer group can be used for primary screening of RB1 gene exon deletion of small-scale tumor samples, and can also be used for accurately positioning breakpoints of RB1 gene exon deletion in combination with WES results.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
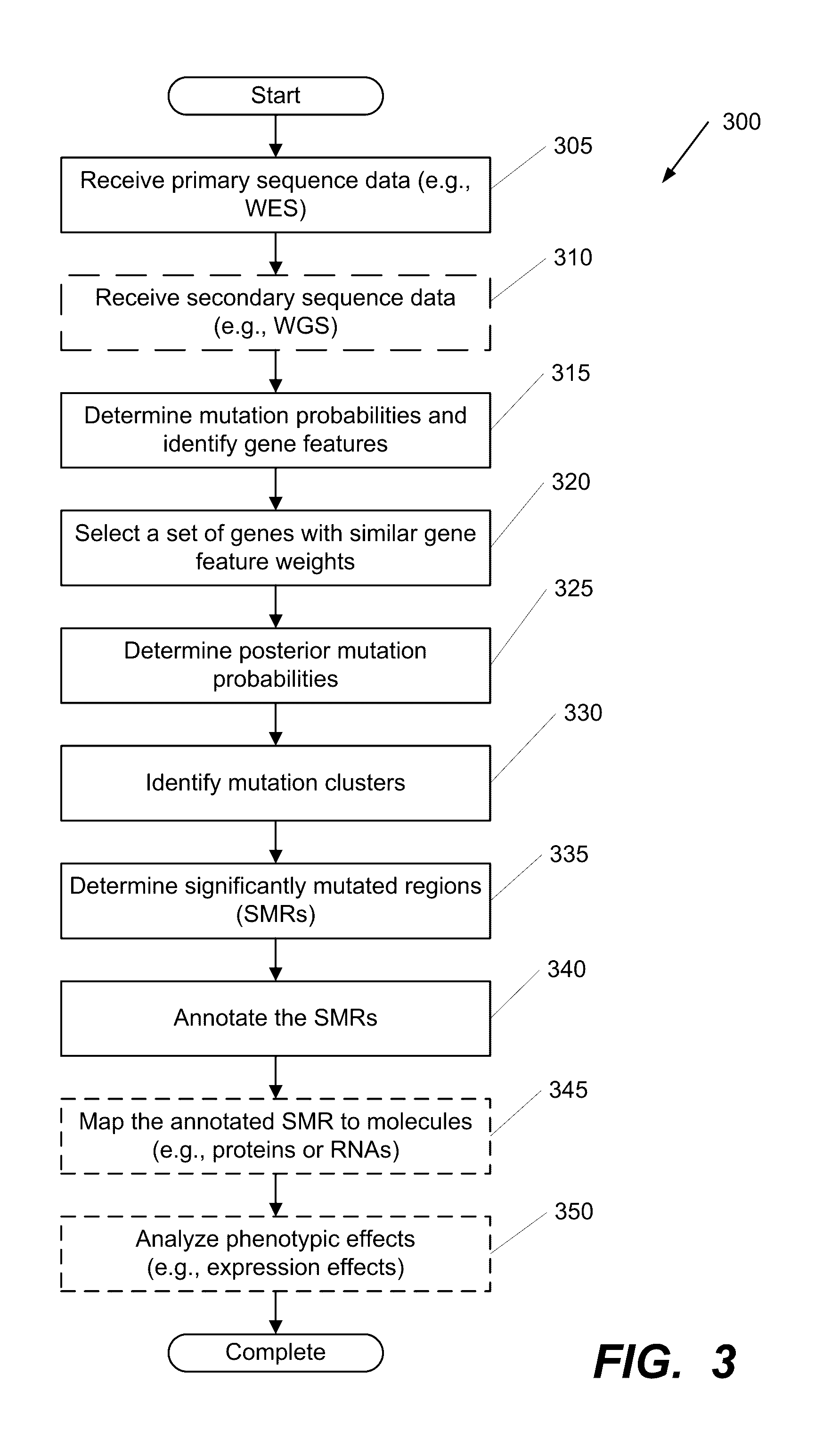
Systems and Methods for Multi-Scale, Annotation-Independent Detection of Functionally-Diverse Units of Recurrent Genomic Alteration
InactiveUS20190348151A1Microbiological testing/measurementBiostatisticsHuman DNA sequencingBinding site
The functional interpretation of somatic mutations remains a persistent challenge in the interpretation of human genome data. Systems and methods for detecting significantly mutated regions (SMRs) in the human genome permit the discovery and identification of multi-scale cancer-driving mutational hotspot clusters. Systems and methods of SMR detection reveal differentially mutated genetic regions across various cancer types. SMR detection and annotation reveals a diverse spectrum of functional elements in the genome, including at least single amino acids, compete coding exons and protein domains, microRNAs, transcription factor binding sites, splice sites, and untranslated regions. Systems and methods of SMR detection optionally including protein structure mapping uncover recurrent somatic alterations within proteins. Systems and methods of SMR detection optionally including differential expression analysis reveal previously unappreciated connections between recurrent and somatic mutations and molecular signatures.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
A drop-off ddPCR method and kit for quantitative detection of u2af1 gene mutation
ActiveCN113186283BStrong specificityHigh sensitivityMicrobiological testing/measurementGenes mutationAURKA Gene
The invention relates to a detection method and kit for detecting U2AF1 gene mutation based on drop-offddPCR. The primers and probes are designed according to the U2AF1 gene DNA sequence, and two wild One is located on the mutation hotspot, and the other is located outside the mutation hotspot. When there are insertions, substitutions, deletions and other mutations in the mutation hotspot, the wild-type probe located at the mutation hotspot cannot tightly bind to the template, so the kit is only used for Two pairs of primer probes can detect multiple mutations in the two mutation hotspots of U2AF1 gene Q157 and S34, and its sensitivity is high, which can be used for MRD monitoring.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL
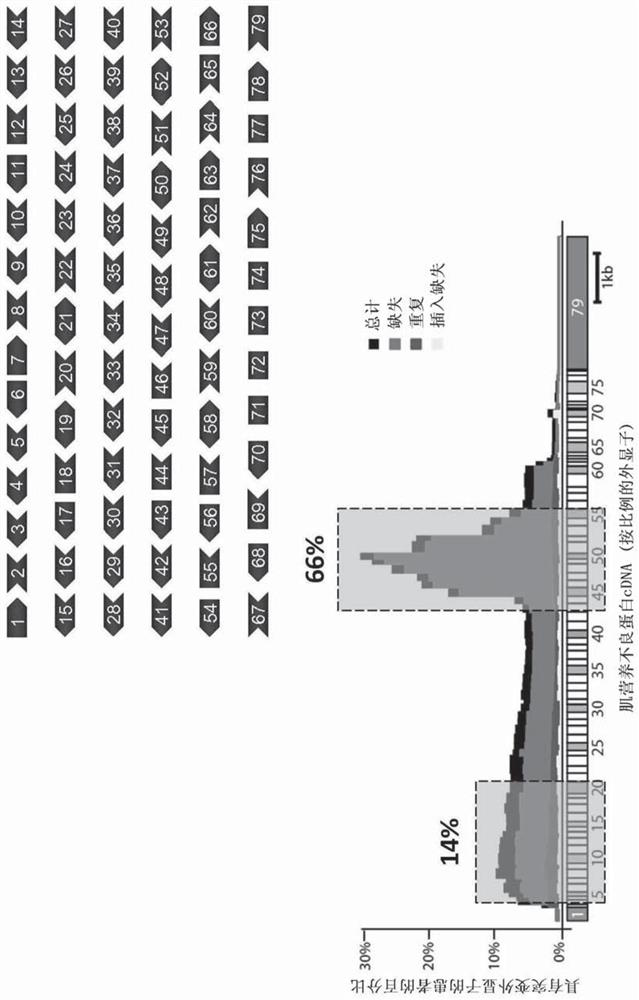
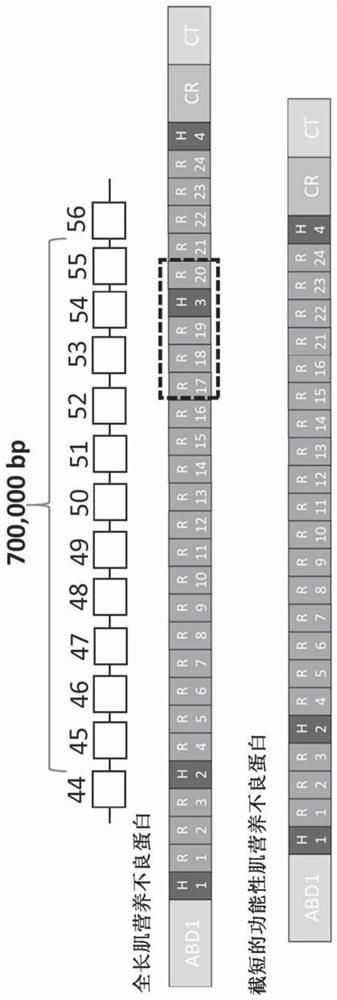
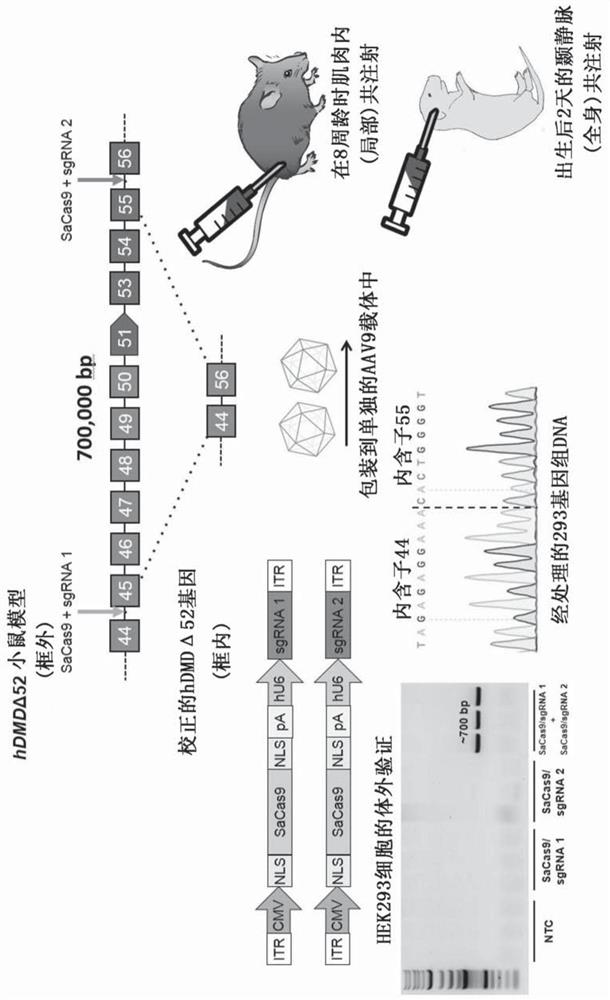
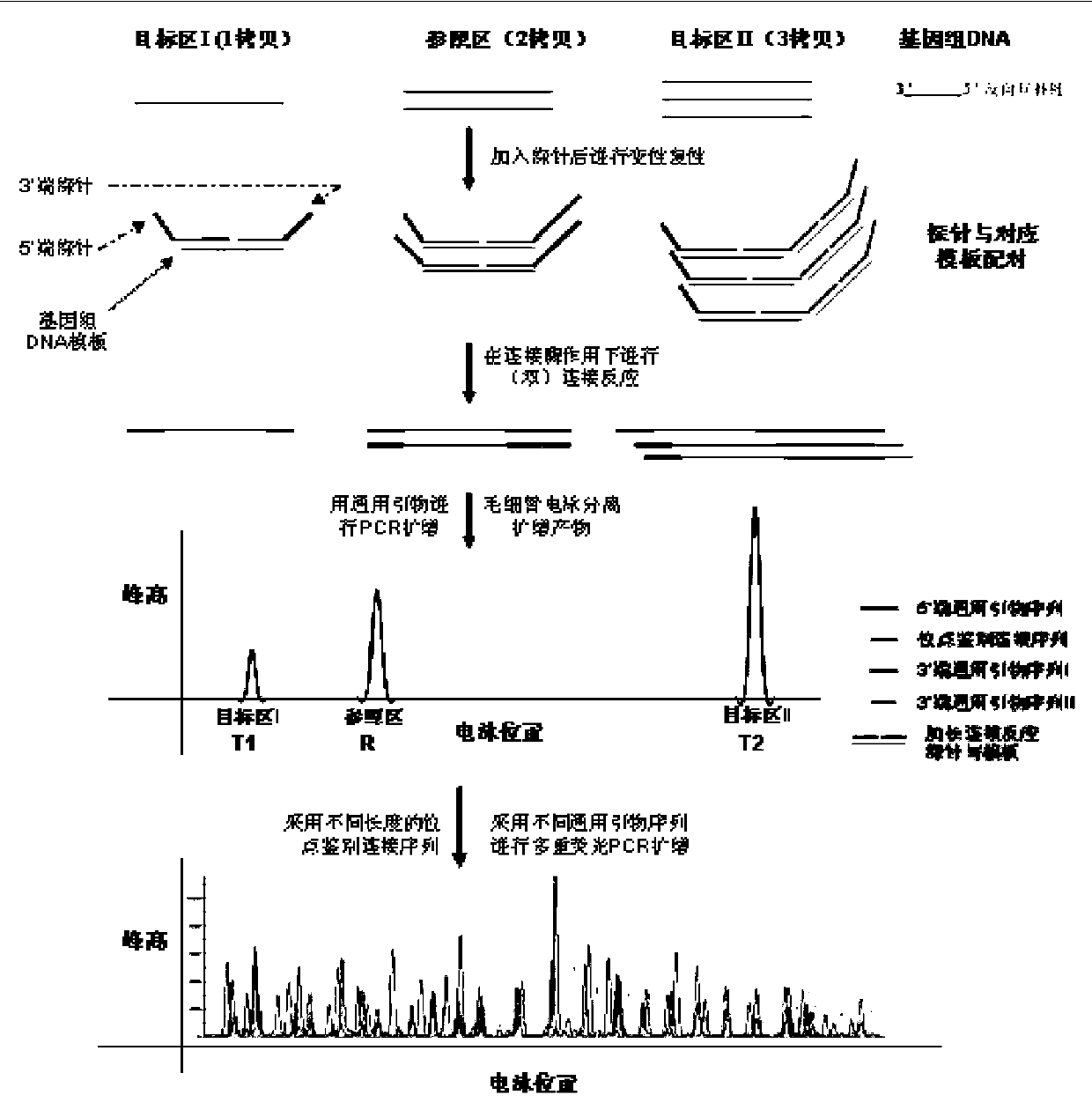
Aav vector-mediated deletion of large mutational hotspot for treatment of duchenne muscular dystrophy
Disclosed herein are therapeutic targets for the correction of the human dystrophin gene by gene editing and methods of use.
Owner:DUKE UNIV
New chromosomal microdeletion/microduplication syndrome detection system and kit
ActiveCN104651516BMultiple diseases involvedMultiple coverage precisionMicrobiological testing/measurementMultiplexDECIPHER
The invention discloses a novel chromosome microdeletion / microduplication syndrome detection system which mainly comprises 643 pairs of probes, multiplex PCR (polymerase chain reaction) amplification primers and correlation detection reaction reagents. The detection process comprises the following steps: hybridizing a probe set with target nucleic acid in the sample; connecting the hybridized probe; amplifying the connecting probe; and detecting the amplification product to determine the existence or quantity of the target nucleic acid in the sample. The platform can implement systematic screening and detection on 24 chromosome aneuploids, 24 chromosome telomere abnormities, more than 70 chromosome microdeletion / microduplication syndromes and multiple monogenic disease mutation hot spots at one time by using the optimized probe set and reaction system. Compared with the existing like detection techniques (such as MLPA and the like), the detection system covers nearly all definite pathogenic regions with abnormal number of copies in the DECIPHER database, has the advantages of wide detection range, high detection precision and low cost, and is simple to operate.
Owner:SUZHOU MUNICIPAL HOSPITAL +1
Highly catalytically active pet hydrolase mutants
ActiveCN107674866BIncrease enzyme activitySimplified degradation stepsHydrolasesFermentationSite-directed mutagenesisProtein engineering
The invention belongs to the field of protein engineering, in particular to a PET hydrolase mutant. The technical problem to be solved by the present invention is that the activity of the PET hydrolase (PETase) currently derived from the Ideonella sakaiensis 201-F6 strain is not ideal. The technical solution of the present invention to solve the technical problem is to provide a PET hydrolase mutant. In the present invention, 5 mutation hotspots are obtained through a large number of researches on PET hydrolase (PETase); 14 mutants are constructed by applying site-directed mutation technology, and the activity of 2 mutant ETase enzymes is finally screened out compared with the wild type Ec_PETase. , has a good application prospect.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
A gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency
ActiveCN111304317BHigh degree of standardizationImprove diagnostic accuracyMicrobiological testing/measurementGenes mutationHereditary Mutation
The invention relates to a gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency, which comprises probes with sequences shown in SEQ NO.1-382. The present invention analyzes the distribution of 500 G6PD gene positive mutation sites in China, and combines the human gene mutation database (Human Gene Mutation Database, HGMD) database and gene mutation hotspot data in Europe and the United States to more comprehensively summarize the G6PD gene mutation hotspots, To solve the genetic mutation detection problem of nearly 99% of patients with G6PD deficiency, and realize the rapid and effective genetic diagnosis of G6PD deficiency disease.
Owner:上海源庆生物科技有限公司
Β-catenin oligonucleotide microchip and method for detecting β-catenin mutations employing same
InactiveUS7312070B2Fast and reliableBioreactor/fermenter combinationsBiological substance pretreatmentsGenes mutationOligonucleotide chip
The present invention relates to a β-catenin oligonucleotide microchip for detecting mutation in the mutational hot spot regions of β-catenin gene, a manufacturing process thereof and a method for detecting the β-catenin mutation employing same, wherein specific oligonucleotides are selectively designed to detect various missense mutations and in-frame deletion at the mutational hot spots of β-catenin gene. The β-catenin oligo chip of the present invention can be used in studies to detect β-catenin mutations and unravel the signal transduction mechanism and tumorigenesis related to β-catenin gene.
Owner:NAT CANCER CENT
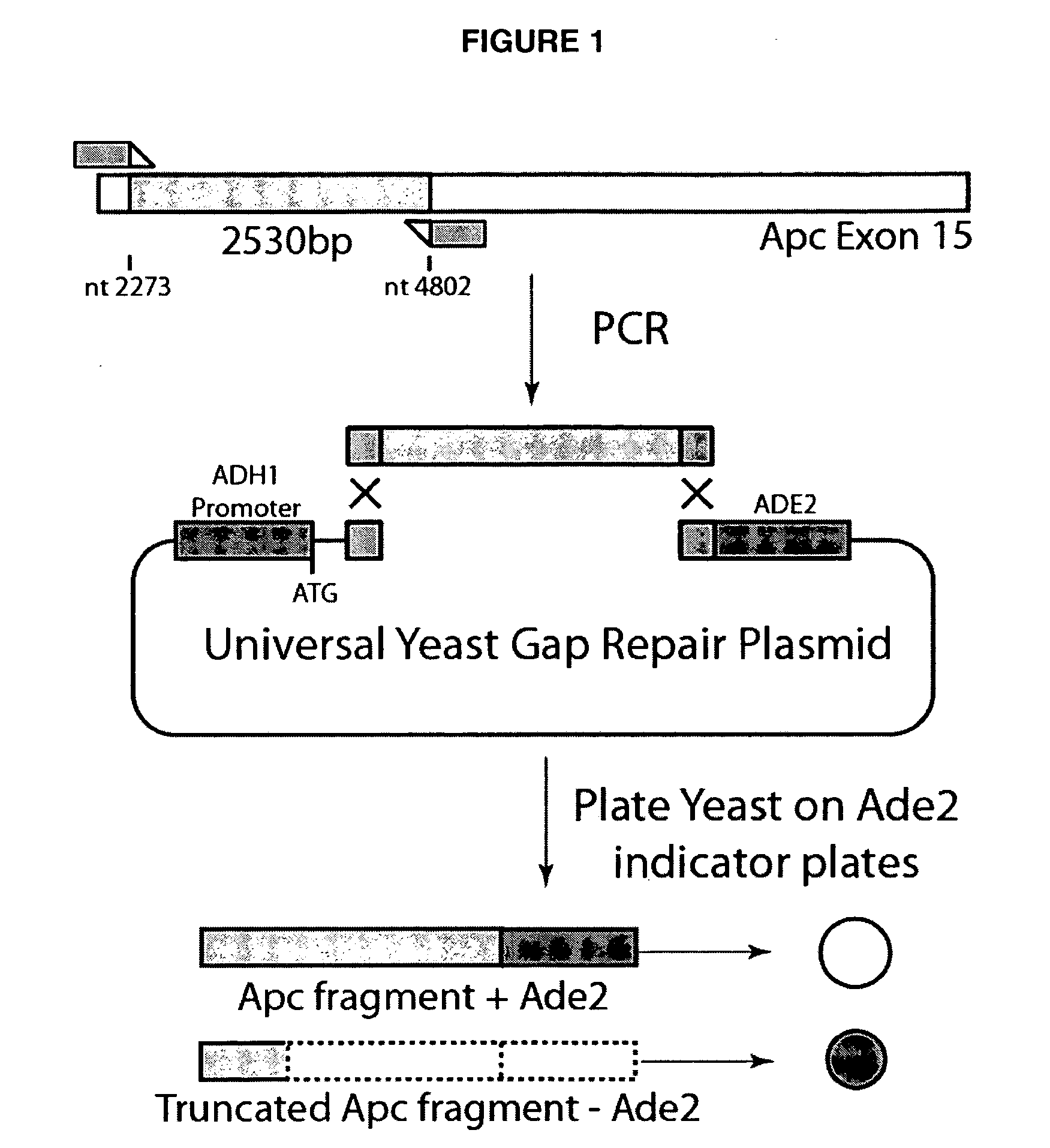
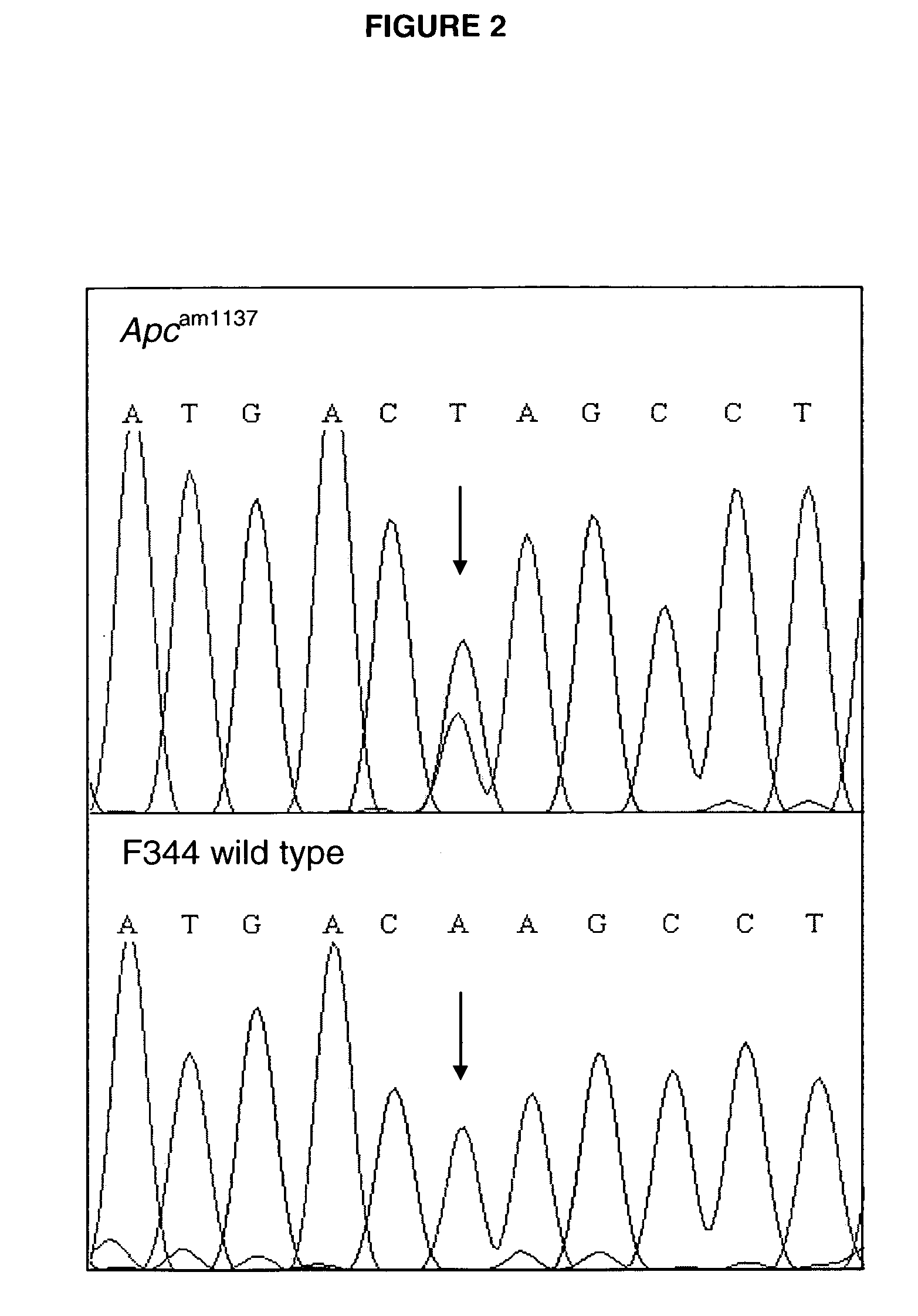
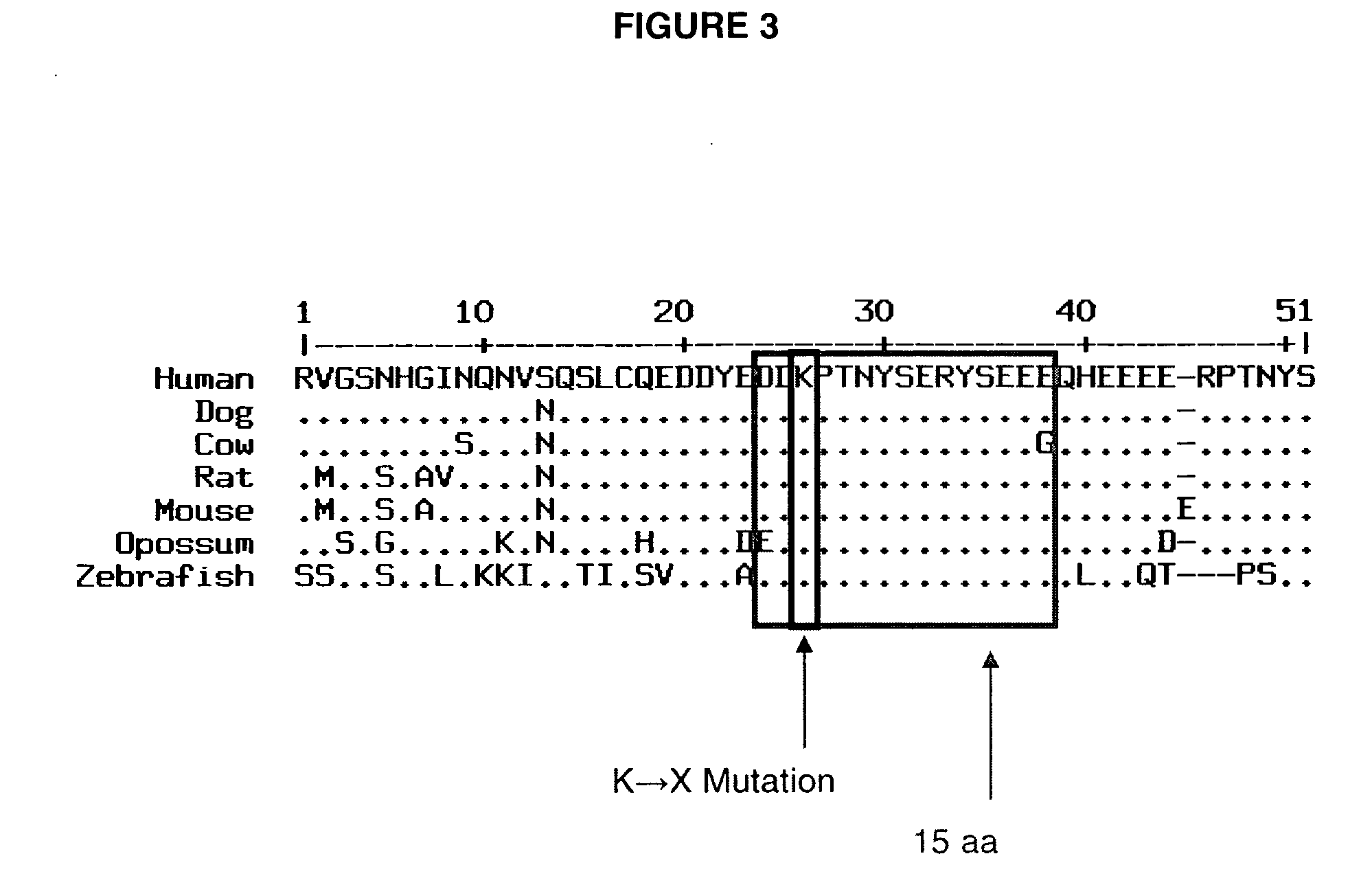
Mutation in the rat Adenomatous Polyposis Coli gene within the human mutation hotspot region
InactiveUS20070143867A1Increased susceptibilitySugar derivativesPeptidesCarcinogenAdenomatous polyposis coli
A rat with a disrupted Apc (adenomatous polyposis coli) gene is provided. The mutation can include an A to T transversion changing a lysine to a stop codon at codon 1137. Methods of generating the knockout rat are provided. Also provided is the offspring or progeny of that rat. In addition, methods of using these rats are provided, including methods for screening a carcinogen or a promoter of carcinogenesis, and methods for screening preventive and inhibitory agents of carcinogenesis.
Owner:WISCONSIN ALUMNI RES FOUND
Glioma mutant gene detection primer composition, kit and application of kit
InactiveCN113293209AMeet the diagnosisMeet medicationMicrobiological testing/measurementDNA/RNA fragmentationForward primerBase J
The invention is applicable to the technical field of gene engineering, and provides a glioma mutant gene detection primer composition, a kit and application of the kit. The primer composition comprises 242 pairs of primers or a combination comprising part of the primers, and specific sequences in a forward primer and a reverse primer of each pair of primers are selected from sequences shown as SEQ ID NO.1-484. The specific sequences in the forward primer and the reverse primer of each pair of primers are 18-25 base sequences in the sequence. A forward primer of the amplification primer pair comprises a sequence as shown in SEQ ID NO. 485. A reverse primer of the amplification primer pair comprises a sequence as shown in SEQ ID NO. 486. According to the invention, a plurality of authoritative databases are combined, mutation hot spot regions of 13 glioma related genes and conserved regions of 1p and 19q chromosomes are selected, a low-initial-quantity or low-quality FFPE sample can be detected, a cerebrospinal fluid sample can also be detected, and general requirements of diagnosis, medication and prognosis of glioma are met. And the comparison rate, the target rate and the uniformity of amplicons are relatively good.
Owner:上海贞固医学检验实验室有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com