PET hydrolase mutant with high catalytic activity
A technology of mutants and hydrolytic enzymes, applied in the direction of hydrolytic enzymes, biochemical equipment and methods, applications, etc., can solve the problems of low degradation efficiency of PET plastics, insufficient catalytic function of the key enzyme Ec_PETase, and difficulty in industrial application, etc., to achieve reduction Effects of degradation cost, high enzyme activity, and simplified degradation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1, Escherichia coli secreted often expressed PET hydrolase (Ec_PETase) mutation hotspot screening
[0035] 1. Construction of 3D model of PETase
[0036]Enter the amino acid sequence of PETase: (GenBank accession number, GAP38373.1) into the three websites I-TASSER, MULTICOM, and ROBETTA to obtain the 3D model of PETase, use Rampage to evaluate the obtained model, and select 3 models for follow-up Docking experiment.
[0037] 2. Use Auto Dock software for substrate docking
[0038] Since the pNPB method is a commonly used lipase activity detection method, pNPB, the product of lipase hydrolysis, has an absorption peak at 405nm under the conditions of 34°C and pH=7.4. Therefore, we used pNPB as the substrate for molecular docking. GaussView was used to draw its 3D structure, and dynamic optimization was performed to finally obtain the most stable conformation of pNPB in space. The best results were obtained after docking simulation analysis.
[0039] 3 mutati...
Embodiment 2
[0041] Example 2. Construction of site-directed mutants of mutation hotspots Trp159, Gln182, Ala183, Ile208 and Ser238.
[0042] The primers used in the experiment are listed in Table 1 below:
[0043] Table 1 Primers used for construction of site-directed mutants
[0044] Primer
serial number
Primer sequence (5'-3')
W159H-Forward
SEQ ID No.9
TATGGGTCATAGCATGGGTGGCGGTGGCAGC
W159H-Reverse
SEQ ID No.10
CACCCATGCTATGACCCATAACGCCCATACG
Q182L-F
SEQ ID No.11
GGCGCCGCTGGCGCCGTGGGACAGCAGCTTC
Q182L-R
SEQ ID No.12
CACGGCGCCAGCGGCGCCGCAGCTTTCAGGCT
A183T-F
SEQ ID No.13
CGCCGCAAACCCCCGTGGGACAGCAGCTTCAGC
A183T-R
SEQ ID No.14
TCCCACGGGGTTTGCGGCGCCGCAGCTTTCAG
I208V-F
SEQ ID No.15
ACGATAGCGTTGCGCCGGTGAACAGCAGCGCG
I208V-R
SEQ ID No.16
ACCGGCGCAACGCTATCGTTCTCGCACGCAAA
S238F-F
SEQ ID No.17
GCAGCCACTTCTGCGCGAACAGCGGTAACAGC
S238F-R
SEQ ID ...
Embodiment 3
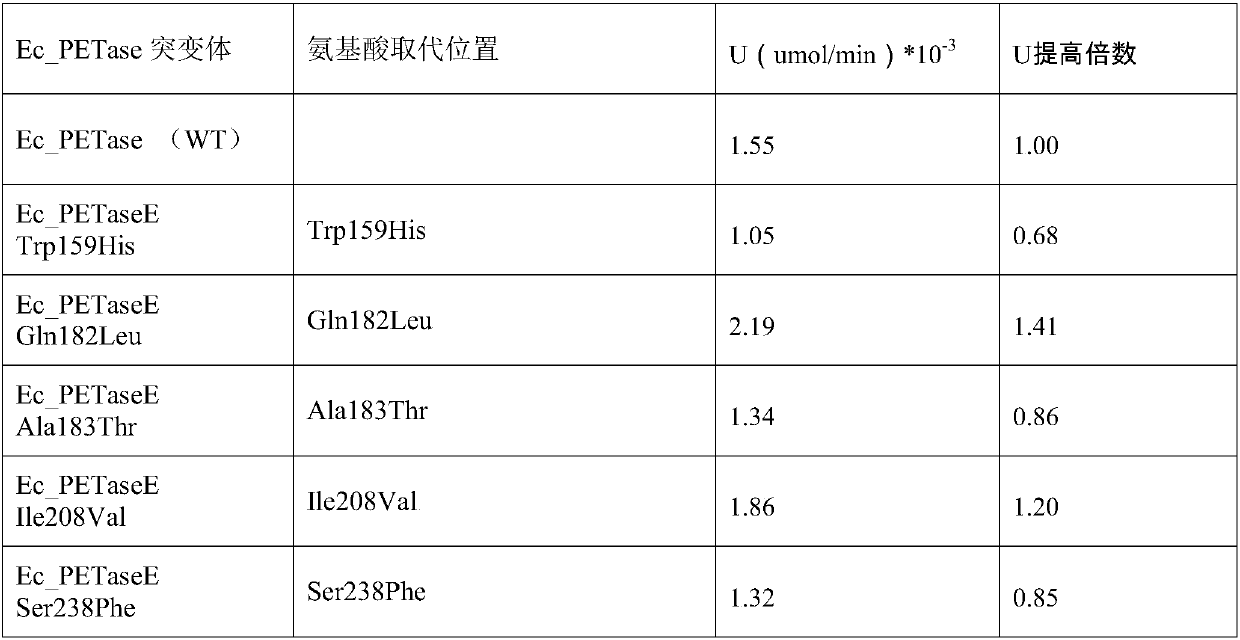
[0047] Example 3, EcPETase recombinant expression and activity evaluation
[0048] 1. Take pUC57-EcPETase genetically engineered Escherichia coli mutants respectively, culture them with shaking in LB liquid medium at 37°C and 180rpm for 4 hours (OD600=1.5), centrifuge to separate the protein secreted into the extracellular space and concentrate it to 0.025mg / ml is used for the determination of enzyme catalytic activity.
[0049] 2. Obtain a concentrated supernatant sample of the overnight culture product of the pUC57-EcPETase genetically engineered E. coli strain, using pNPB as the substrate. References: "oshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K , Miyamoto K, Kimura Y, Oda K.2016.A bacterium that degrades and assimilates poly(ethylene terephthalate).Science,351(6278):1196-1199."The experimental method disclosed in the test pUC57-EcPETase genetically engineered Escherichia coli strain Catalytic activity of EcPETase in culture medium. Add 100 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com