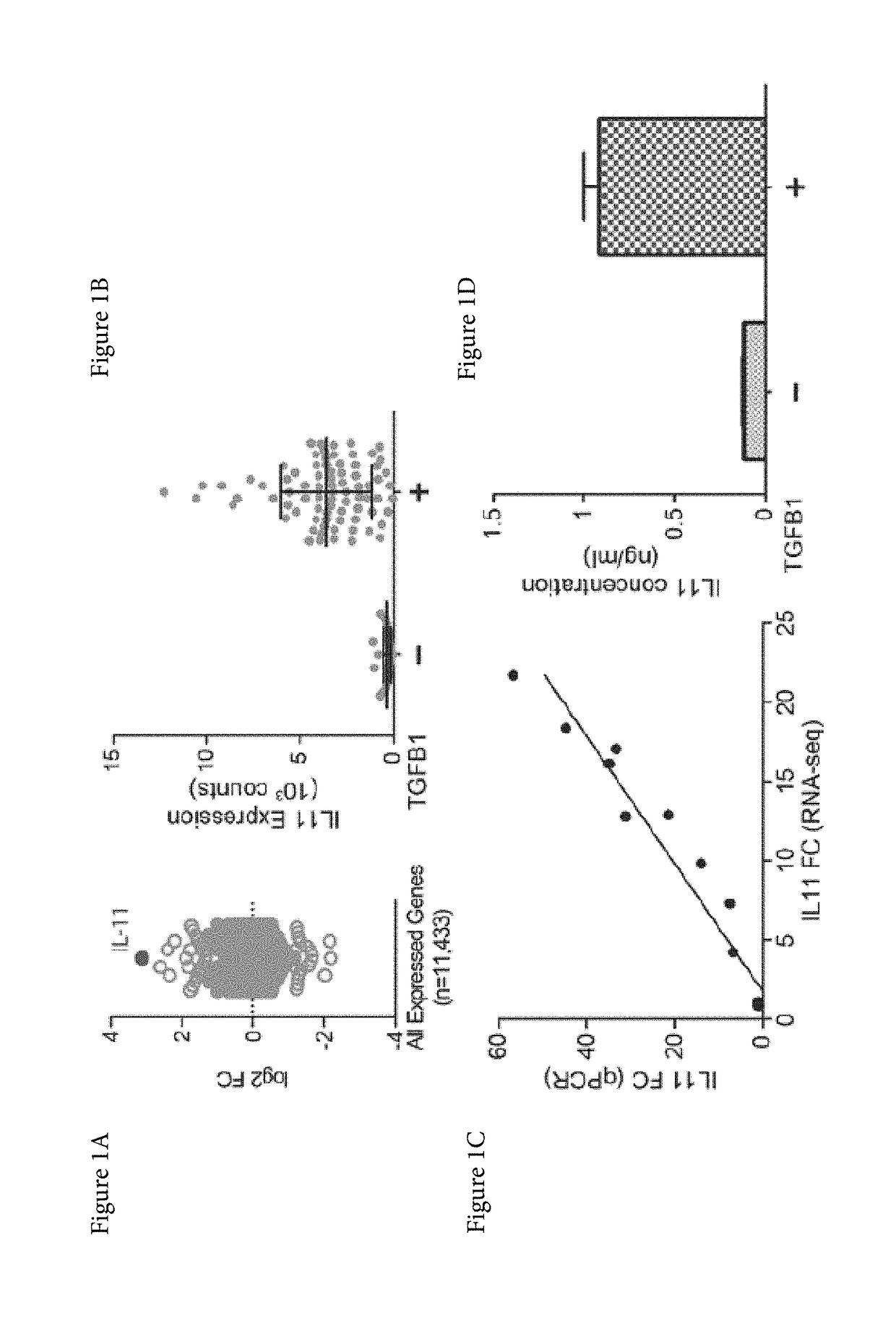
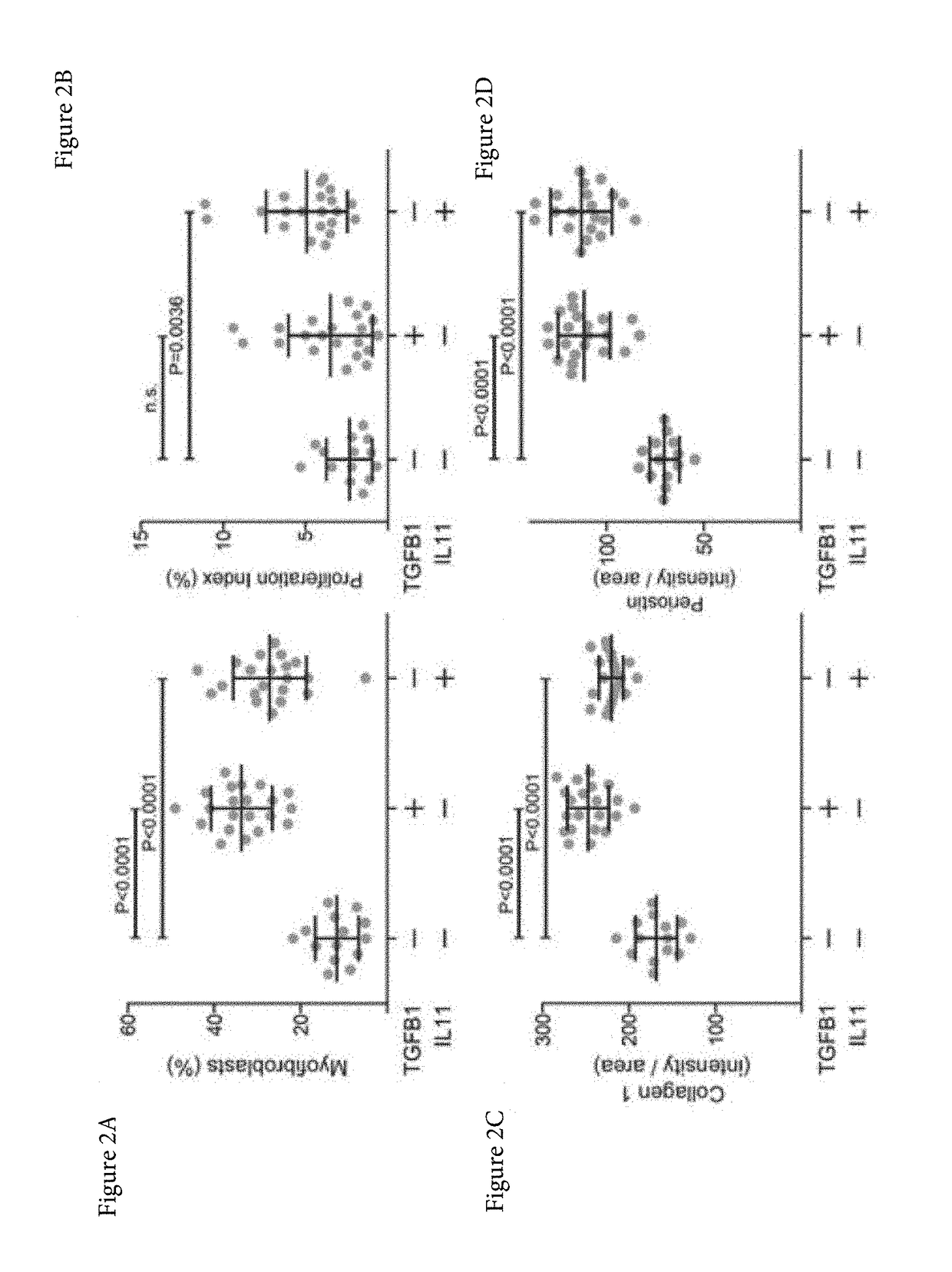
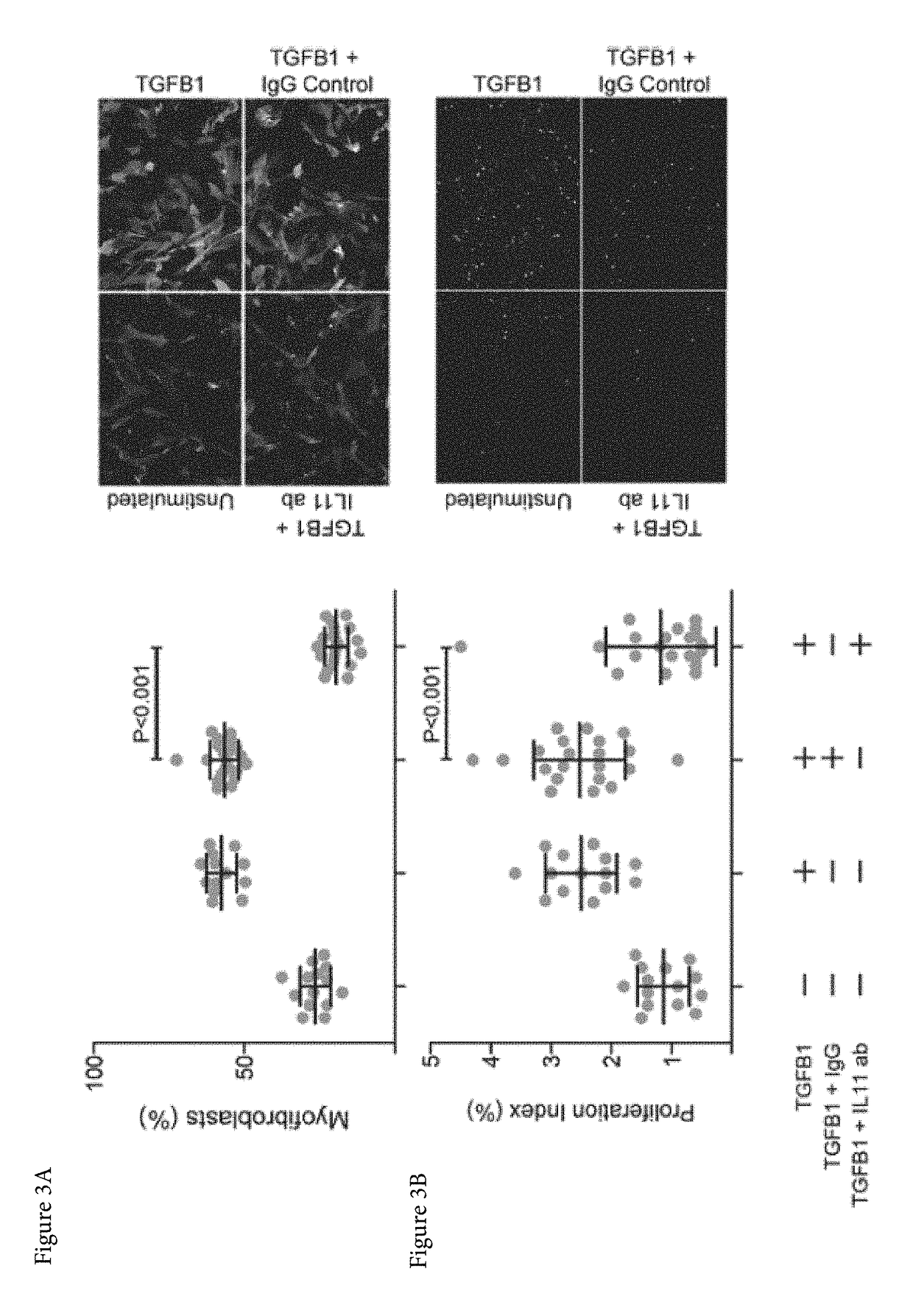
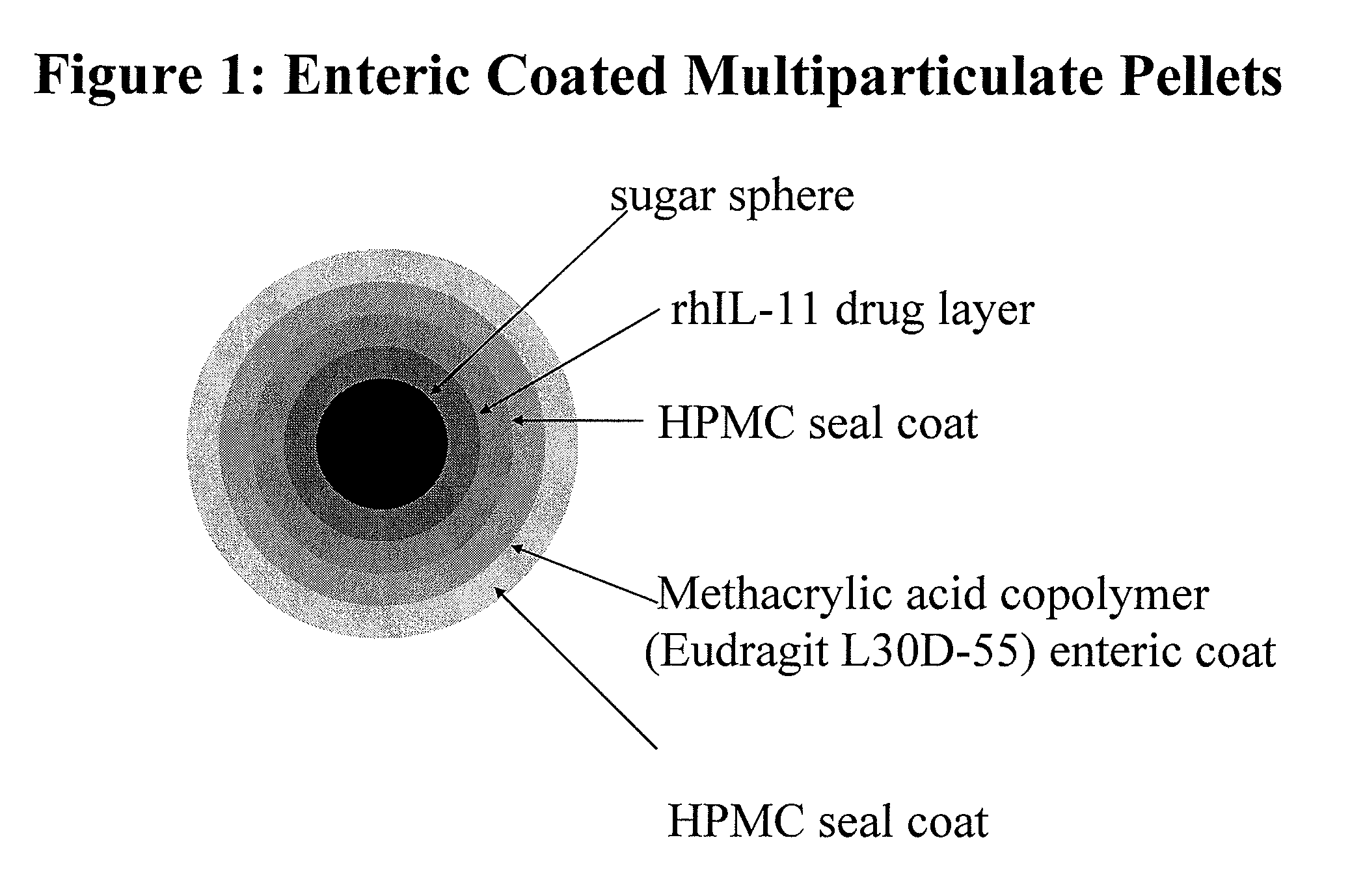
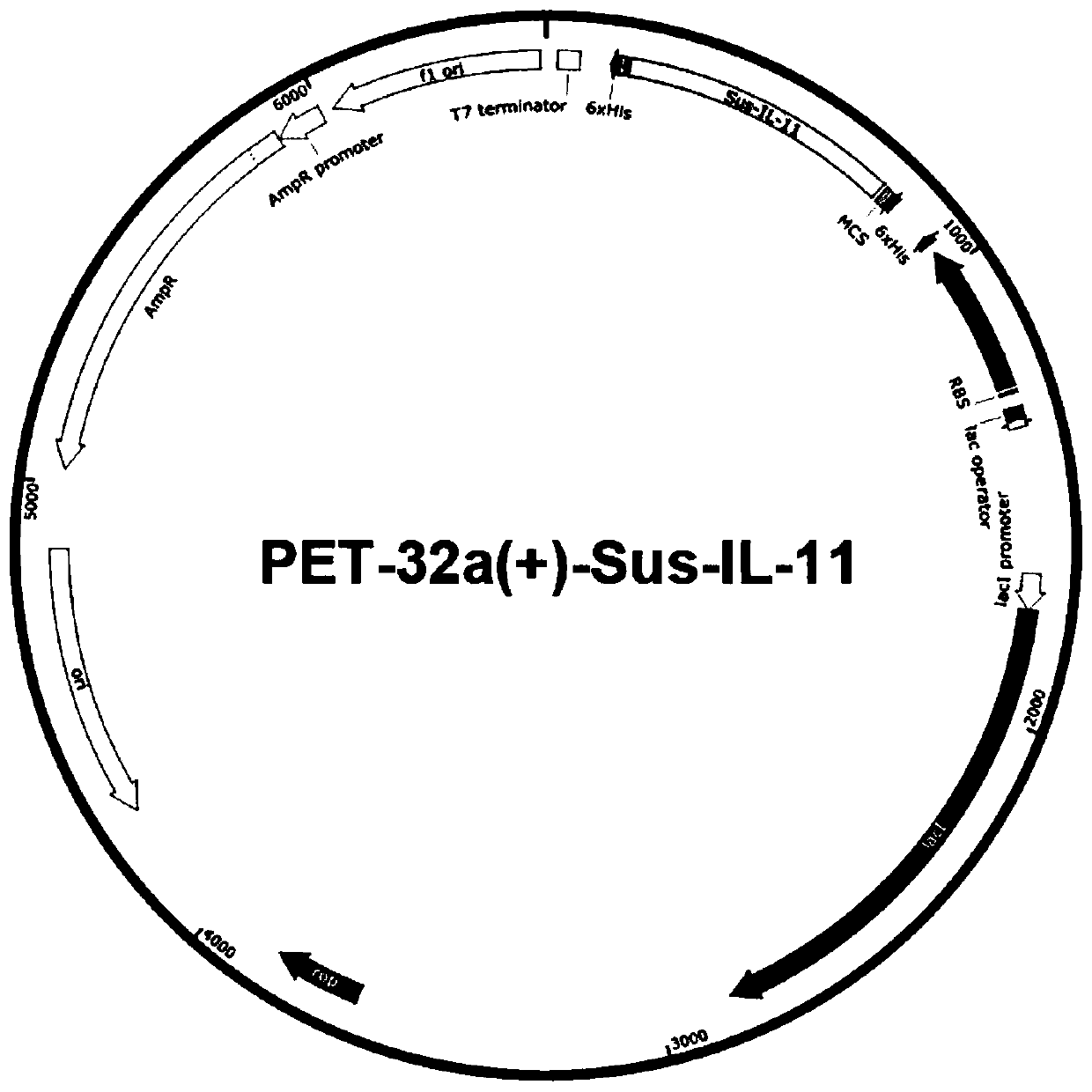
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Interleukin 11" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 11 (IL-11) is a protein that in humans is encoded by the IL11 gene. IL-11 is a cytokine and first isolated in 1990 from bone marrow-derived fibrocyte-like stromal cells. It was initially thought to be important for hematopoiesis, notably for megakaryocyte maturation, but subsequently shown to be redundant for platelets, and for other blood cell types, in both mice and humans. It is also known under the names adipogenesis inhibitory factor (AGIF) and was developed as a recombinant protein (rhIL-11) as the drug substance oprelvekin.
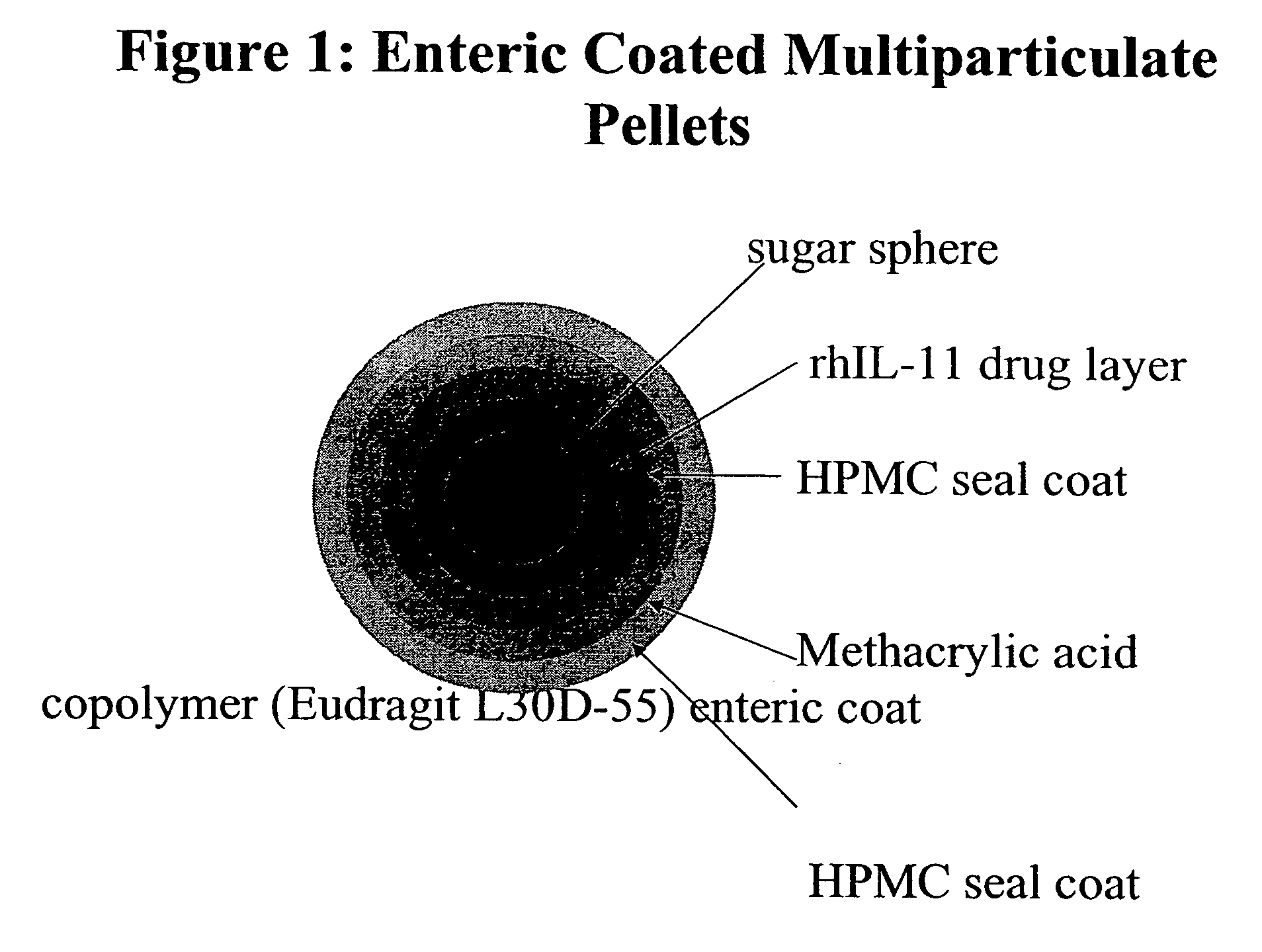
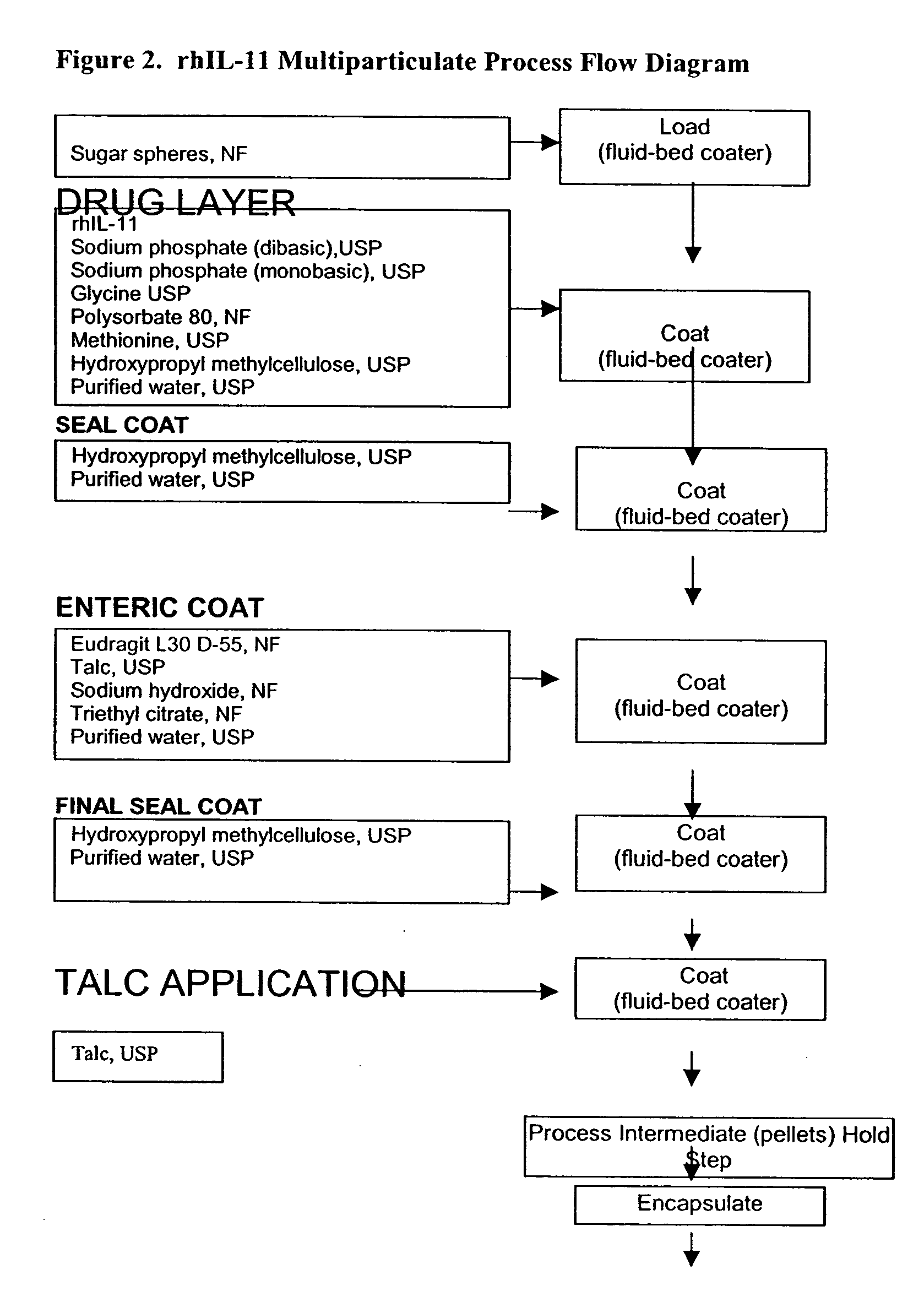
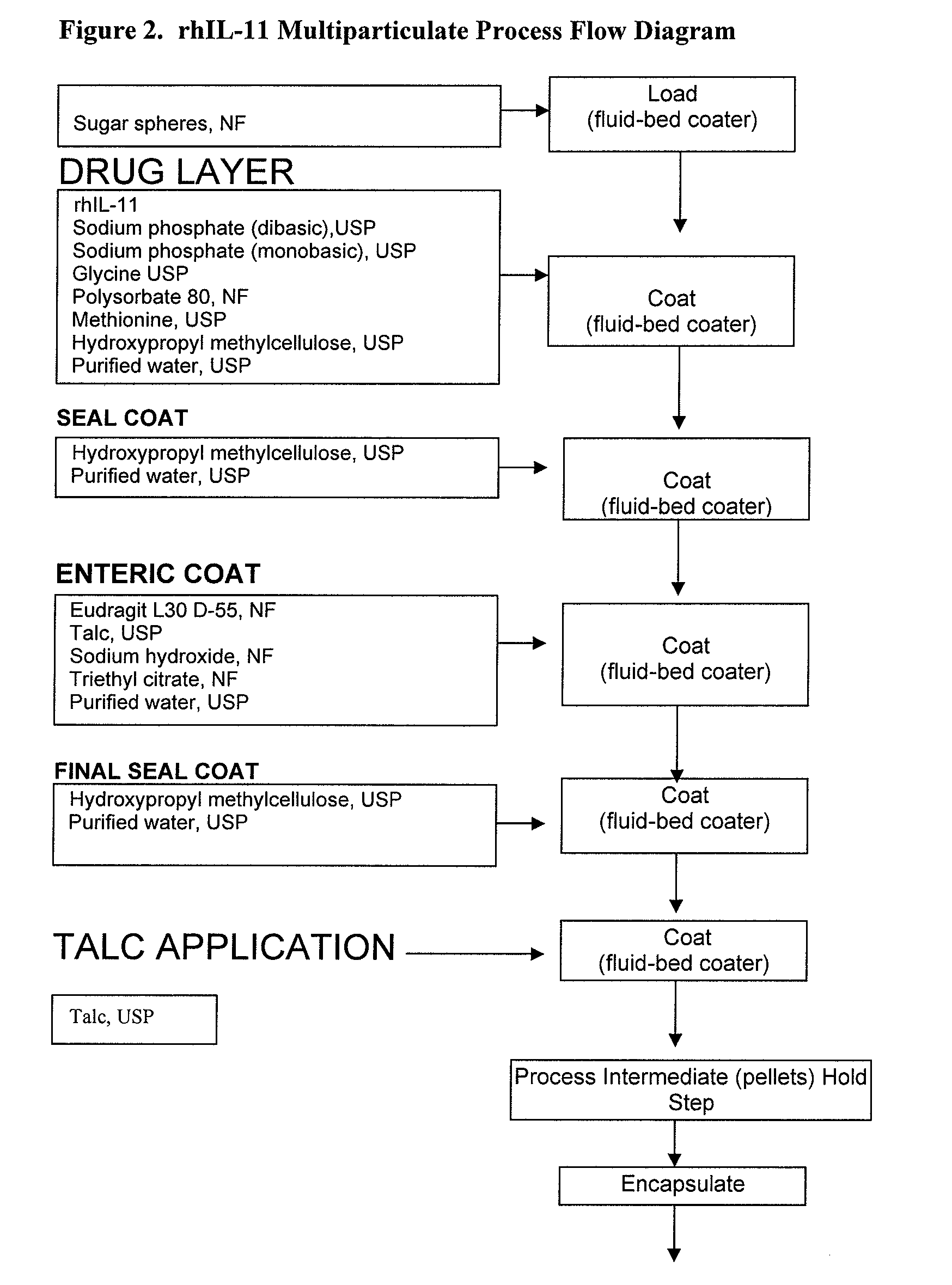
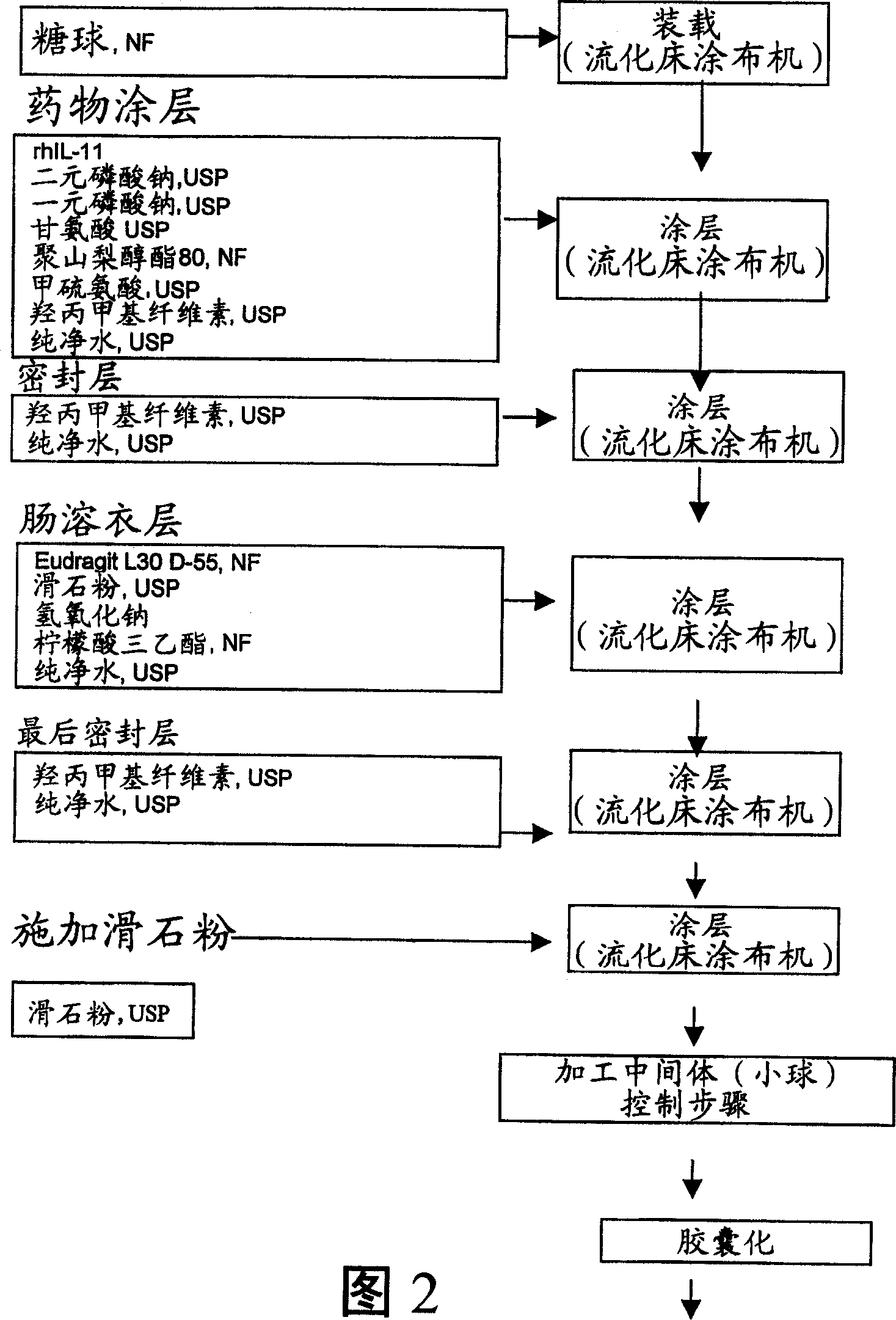
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
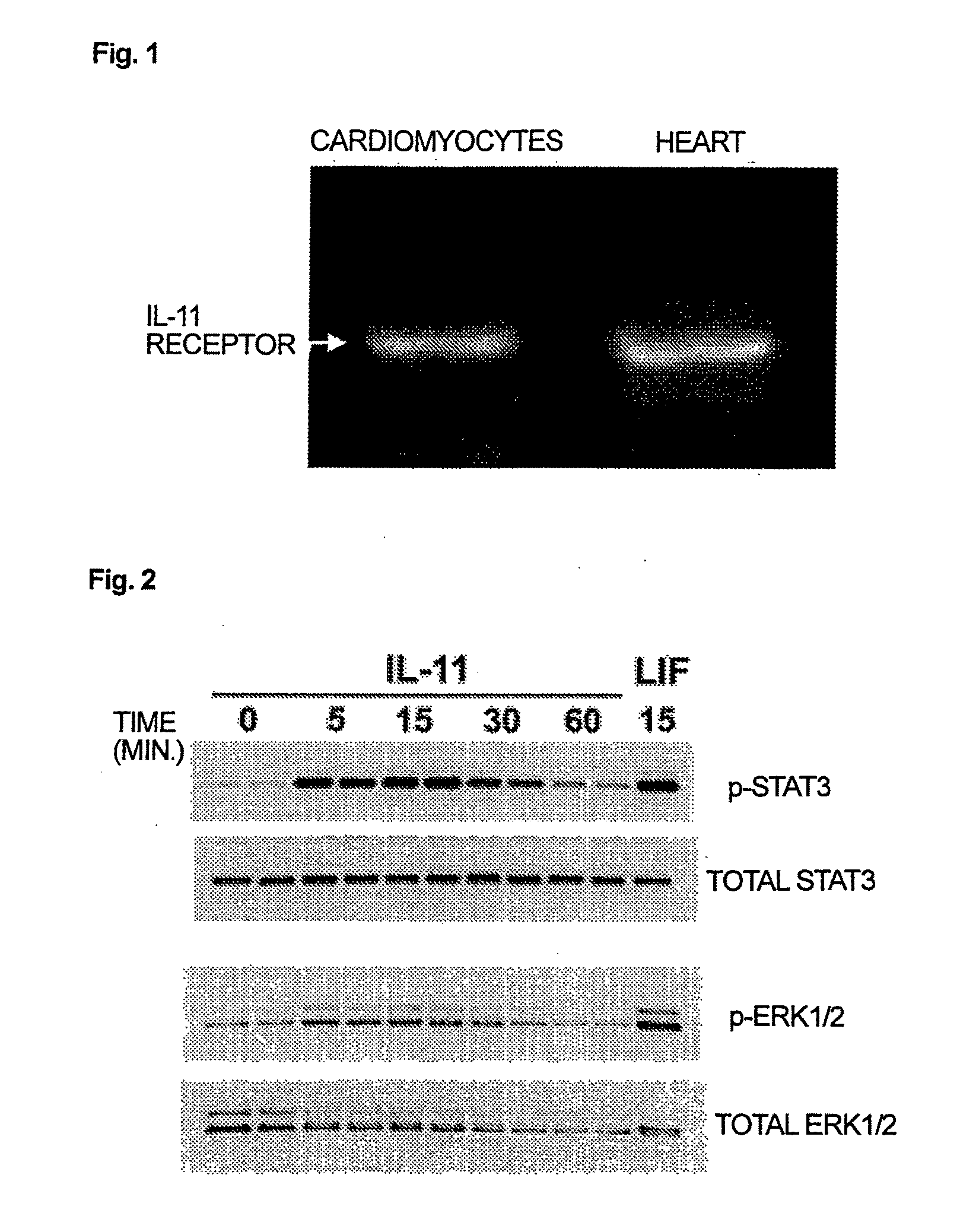
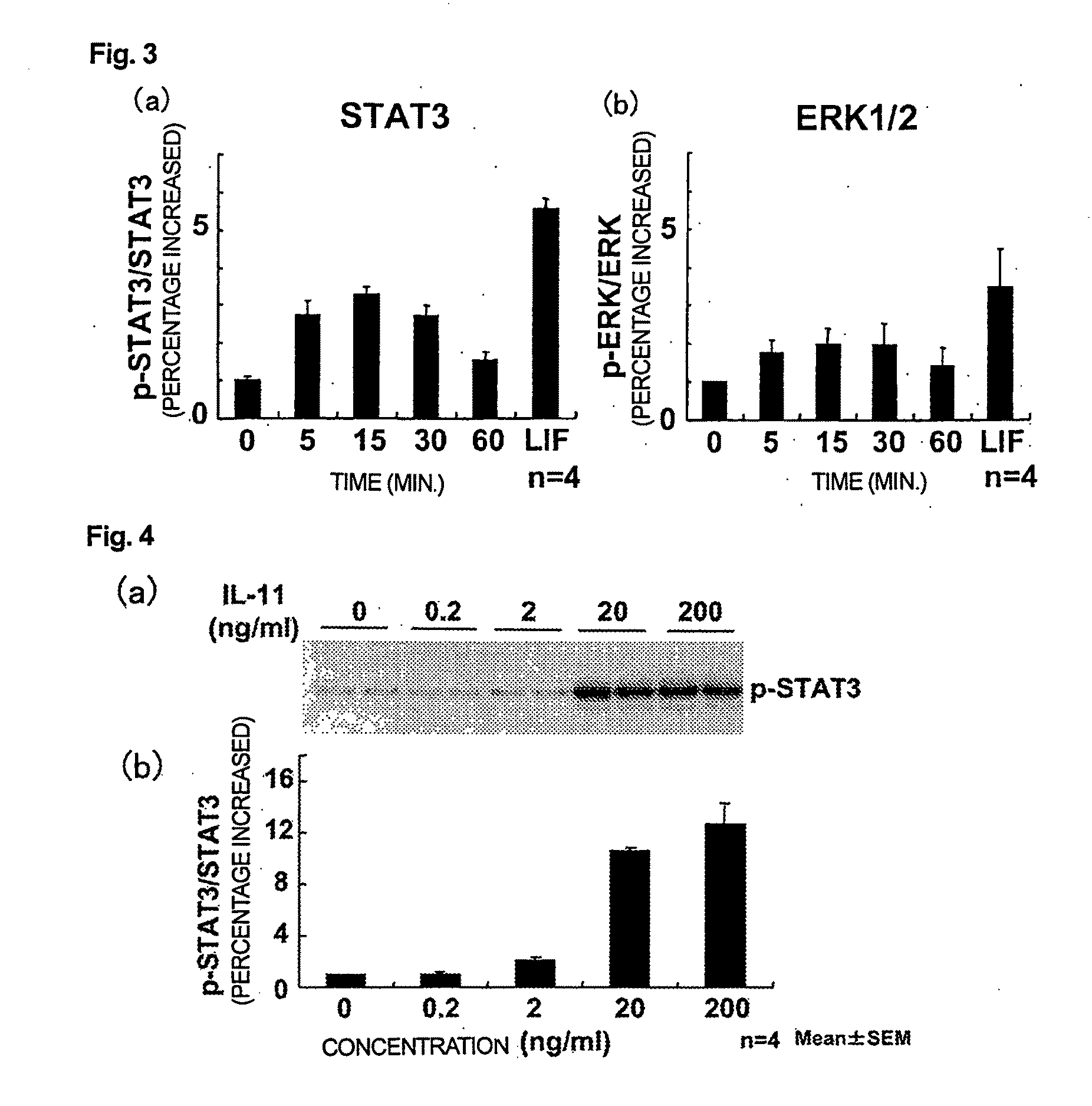
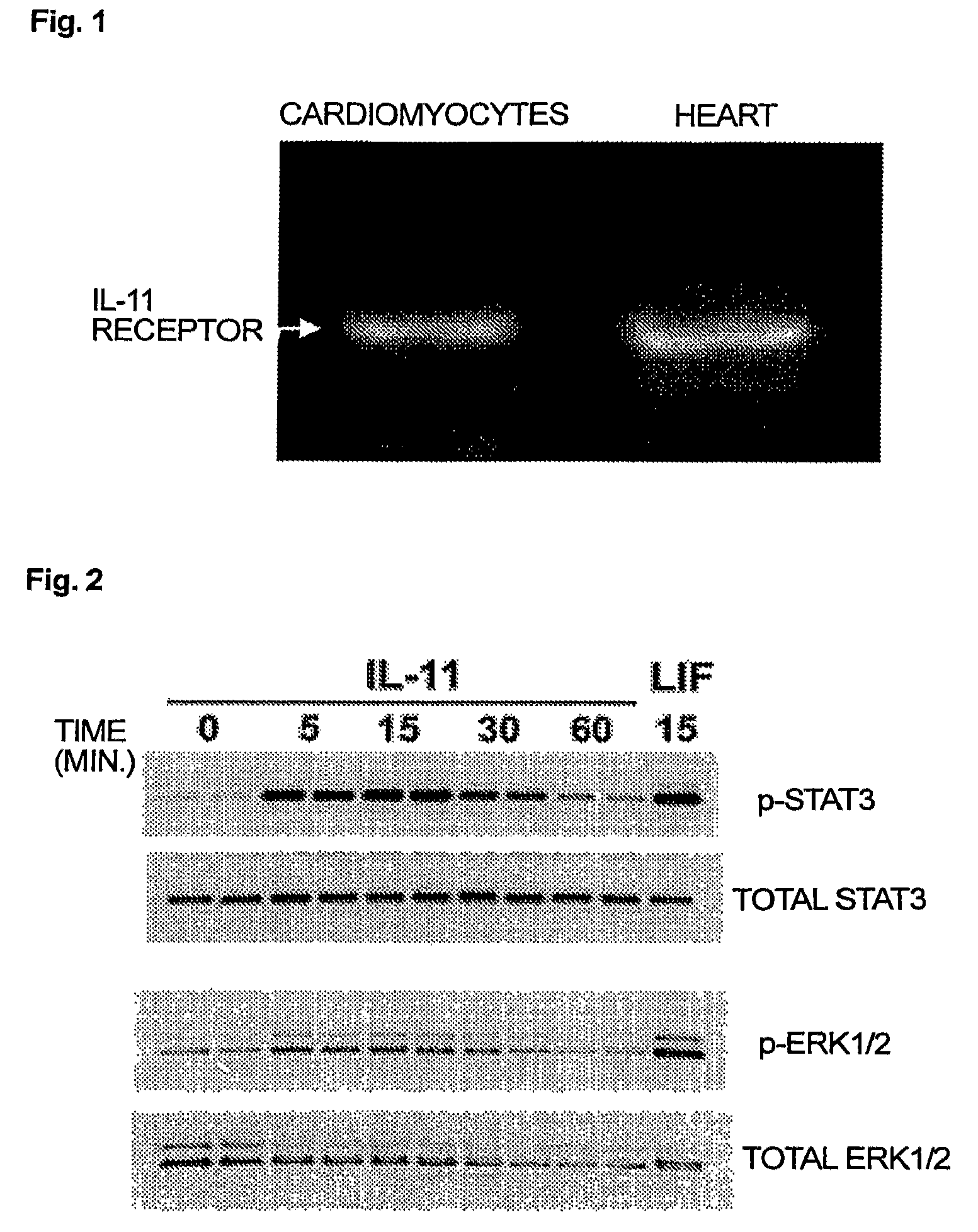
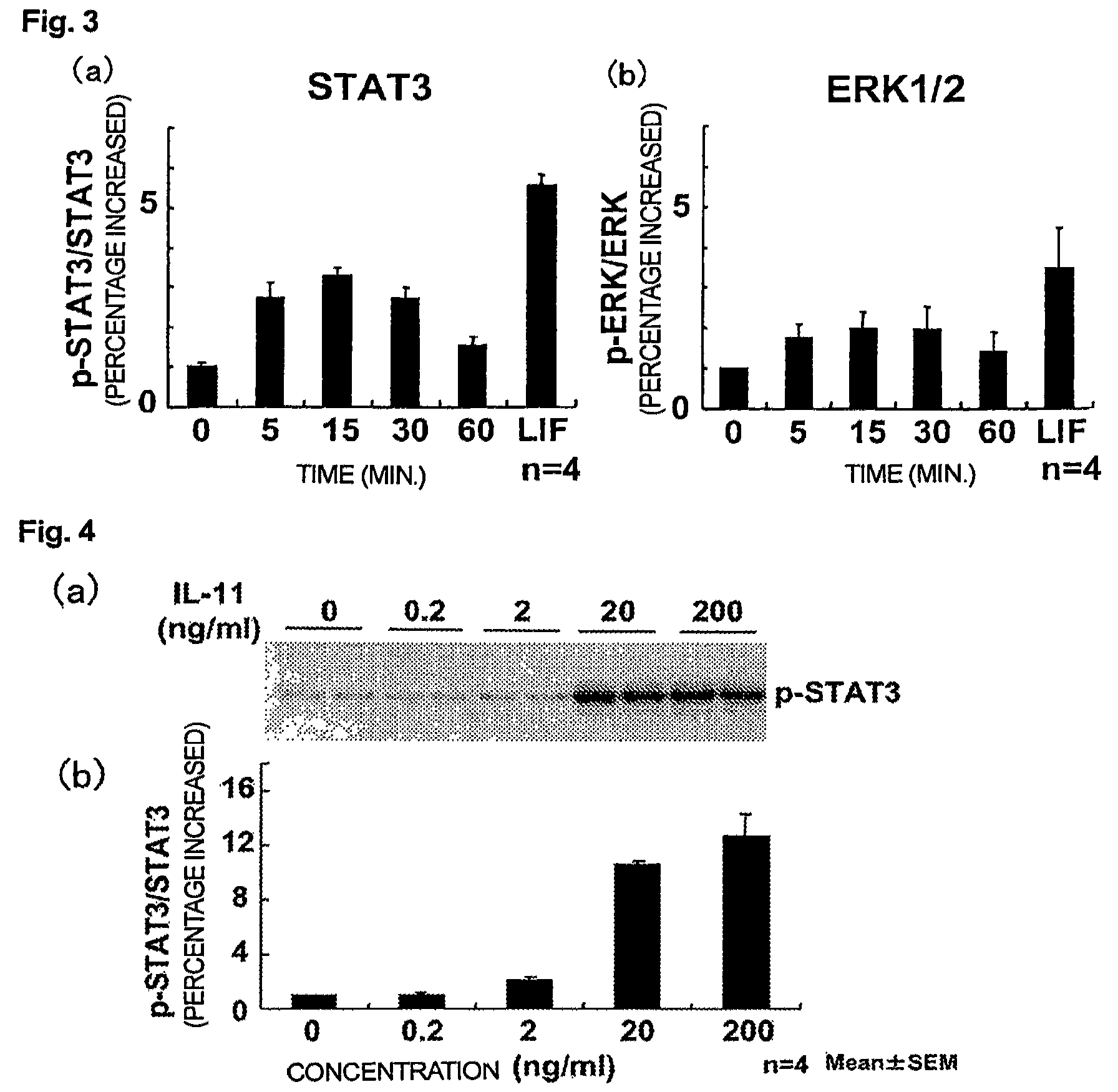
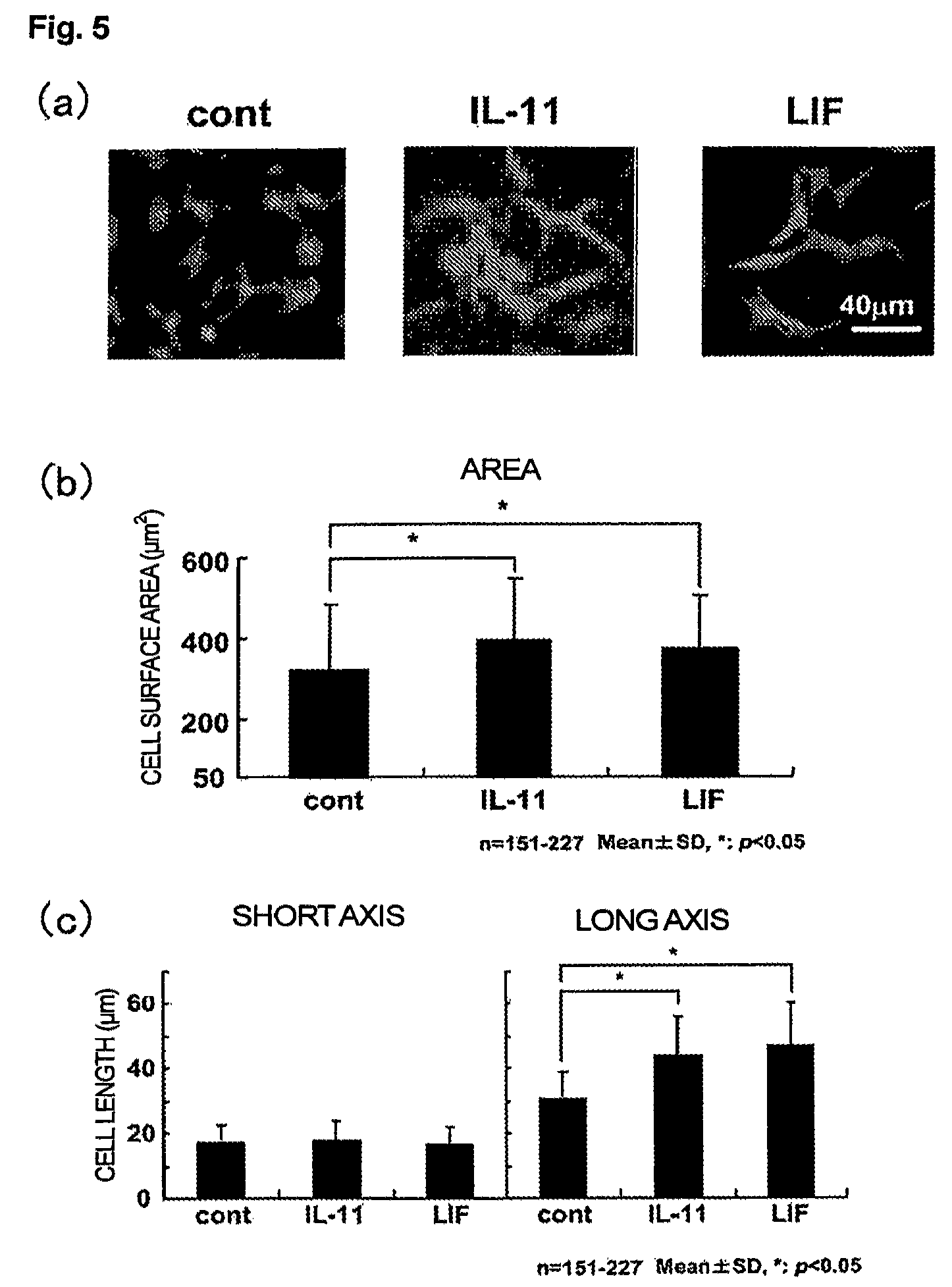
Use of interleukin-11 as therapeutic agent for heart disease
ActiveUS20100093976A1Long lastingHigh expressionPeptide/protein ingredientsAntinoxious agentsCardiac muscleIn vivo
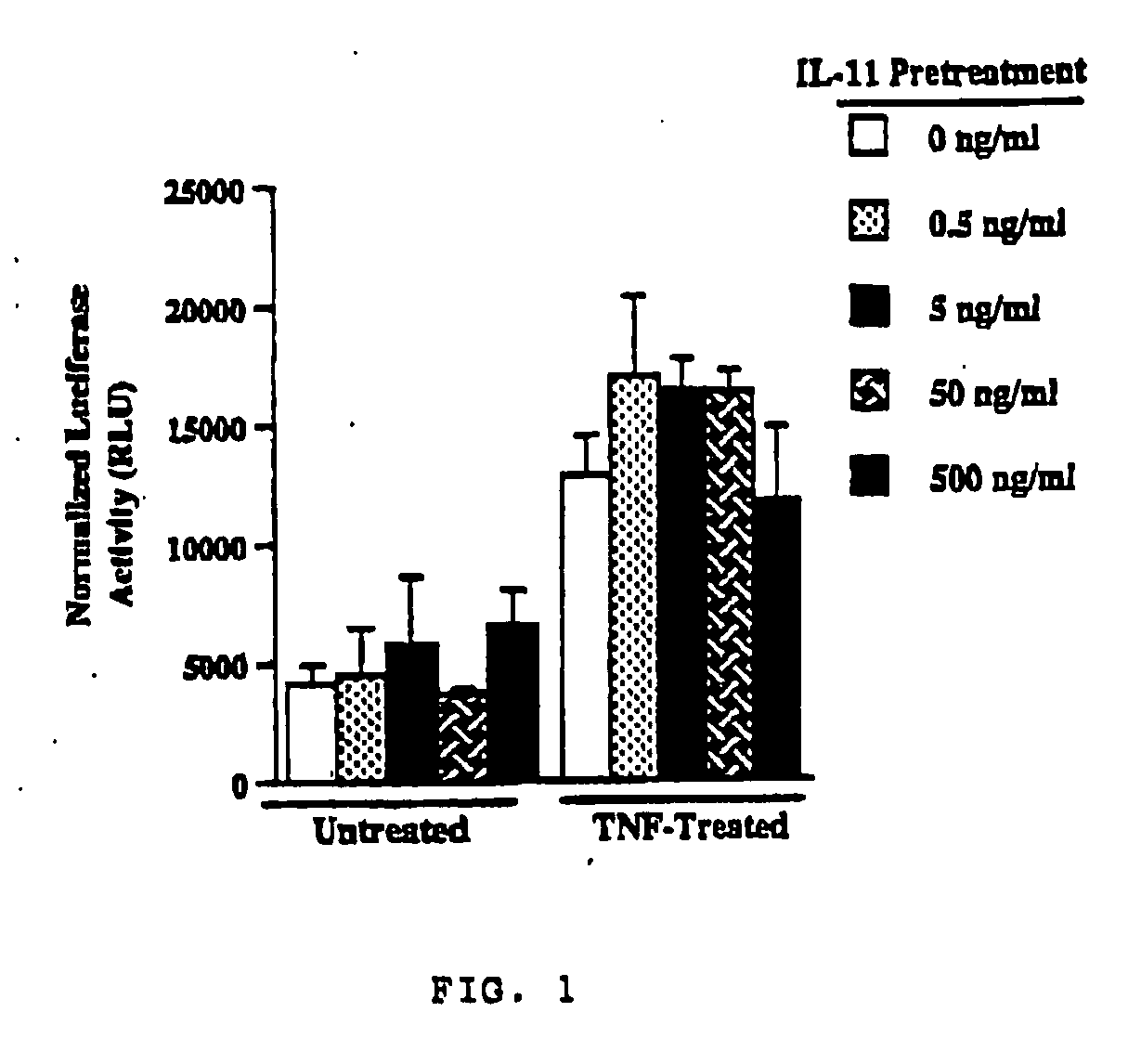
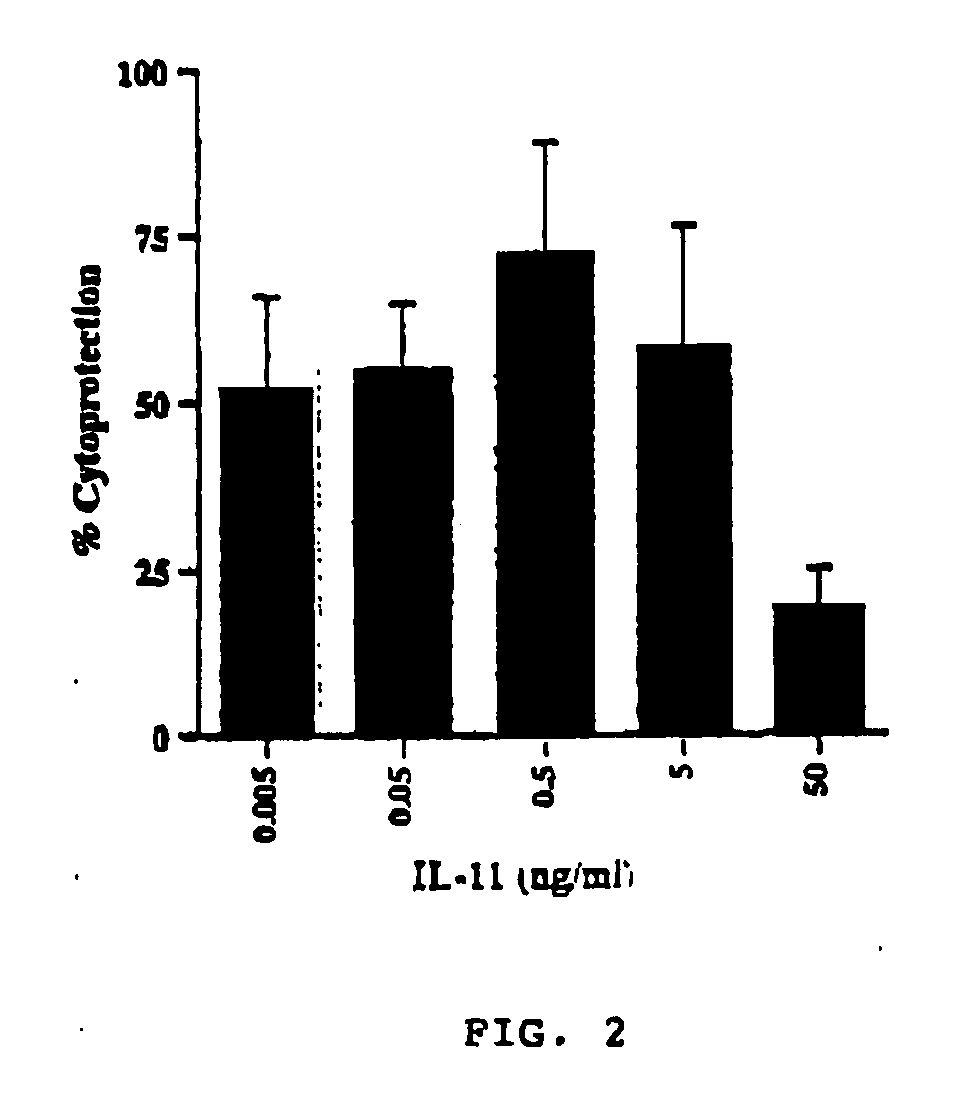
The present invention demonstrates the myocardial protective effects of interleukin 11 in vivo, and provides preventive, therapeutic, or other types of drugs for heart disease using interleukin 11 as the active ingredient. The present invention, by utilizing the myocardial protective effects of interleukin 11, can suppress the progress of myocardial injury, prevent the onset of heart failure, or suppress the progress of heart failure.
Owner:OSAKA UNIV
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20120283128A1Eliminate needEasy to adaptBioreactor/fermenter combinationsBiological substance pretreatmentsMatrilysinInterleukin-1beta
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a plurality of assays, one or more of which is configured to detect a kidney injury marker selected from the group consisting of Hyaluronic acid, Immunoglobulin A, Immunoglobulin G1, Immunoglobulin G2, Insulin-like growth factor-binding protein 7, Alpha-1 antitrypsin, Serum amyloid P component, Metalloproteinase inhibitor 2, Hepatocyte growth factor, Intercellular adhesion molecule 1, Beta-2-glycoprotein 1, Interleukin-1 beta, Neutrophil Elastase, Tumor necrosis factor receptor superfamily member 11B, Interleukin-11, Cathepsin D, C—C motif chemokine 24, C—X—C motif chemokine 6, C—C motif chemokine 13, C—X—C motif chemokines -1, -2, and -3, Matrilysin, Interleukin-2 receptor alpha chain, Insulin-like growth factor-binding protein 3, and Macrophage colony-stimulating factor 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Interleukin-11 compositions and methods of use
InactiveUS20070160577A1Prevents thrombocytopeniaReduces thrombocytopeniaPeptide/protein ingredientsAntipyreticWhite blood cellInterleukin 11
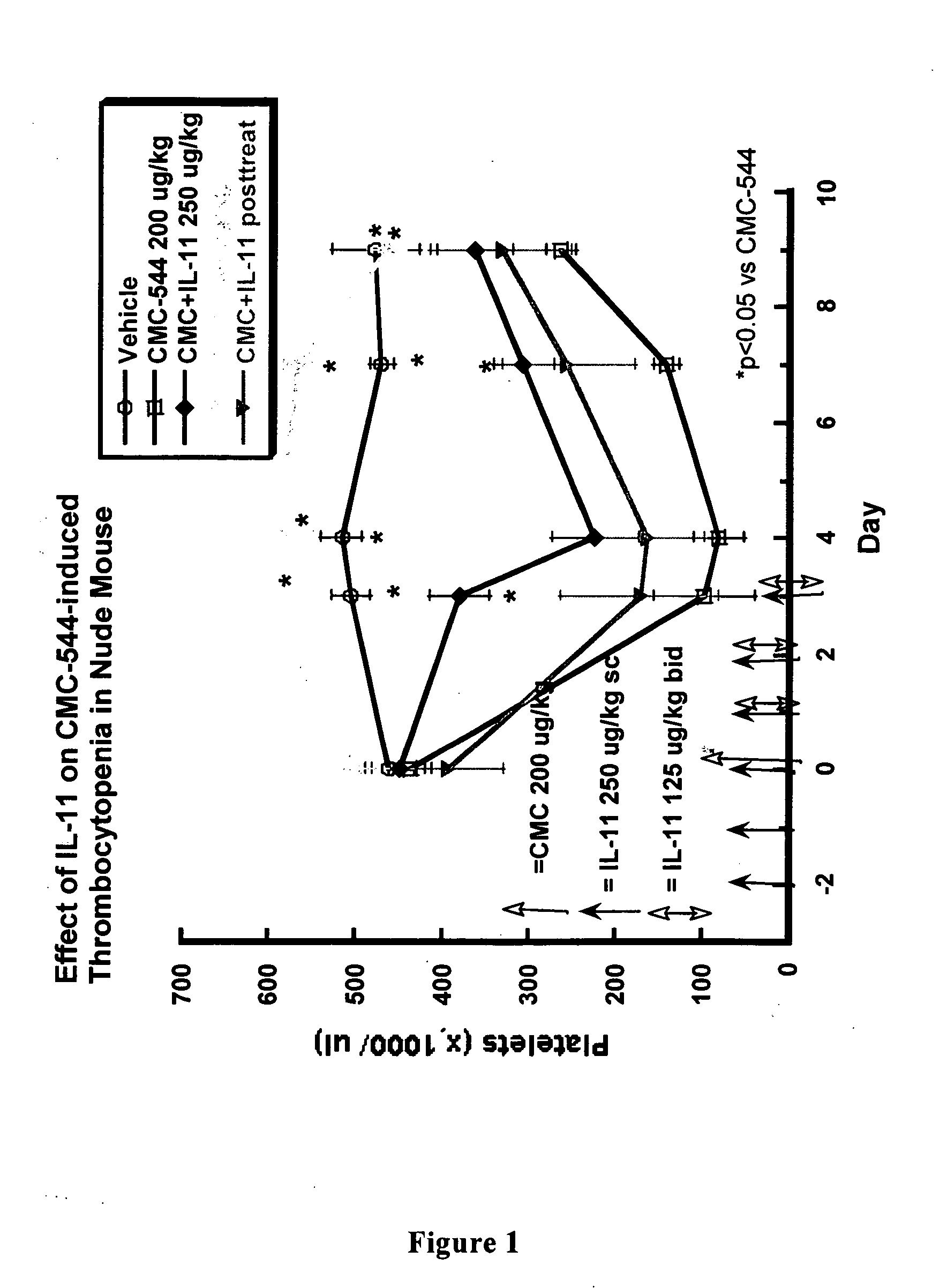
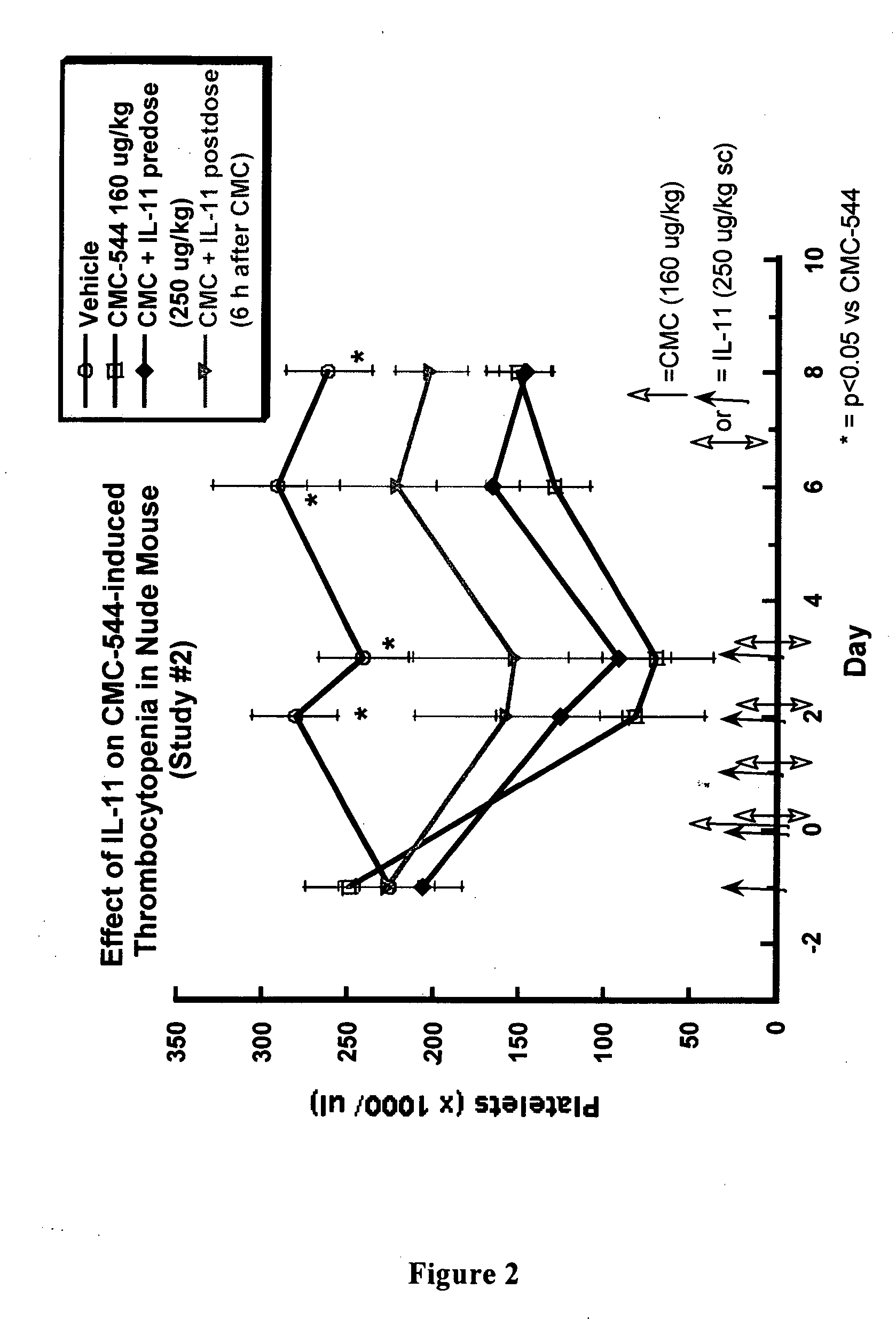
The present invention provides methods for the treatment and / or prevention of thrombocytopenia including thrombocytopenia associated with drug-induced liver damage and thrombocytopenia associated with drug-induced bone marrow destruction. The methods of treatment of the invention include administration of interleukin-11 to a subject suffering from or susceptible to thrombocytopenia and / or receiving or about to receive a treatment involving a conjugate therapeutic agent whose administration results in thrombocytopenia. Also provided are pharmaceutical compositions and kits useful for carrying out such methods of treatment.
Owner:WYETH
Cysteine muteins in the C-D loop of human interleukin-11
InactiveUS7495087B2Peptide/protein ingredientsMicrobiological testing/measurementWhite blood cellADAMTS Proteins
Disclosed are cysteine variants of interleukin-11 (IL-11) and methods of making and using such proteins in therapeutic applications.
Owner:BOLDER BIOTECH
Use of interleukin-11 to prevent immune-mediated cytotoxicity
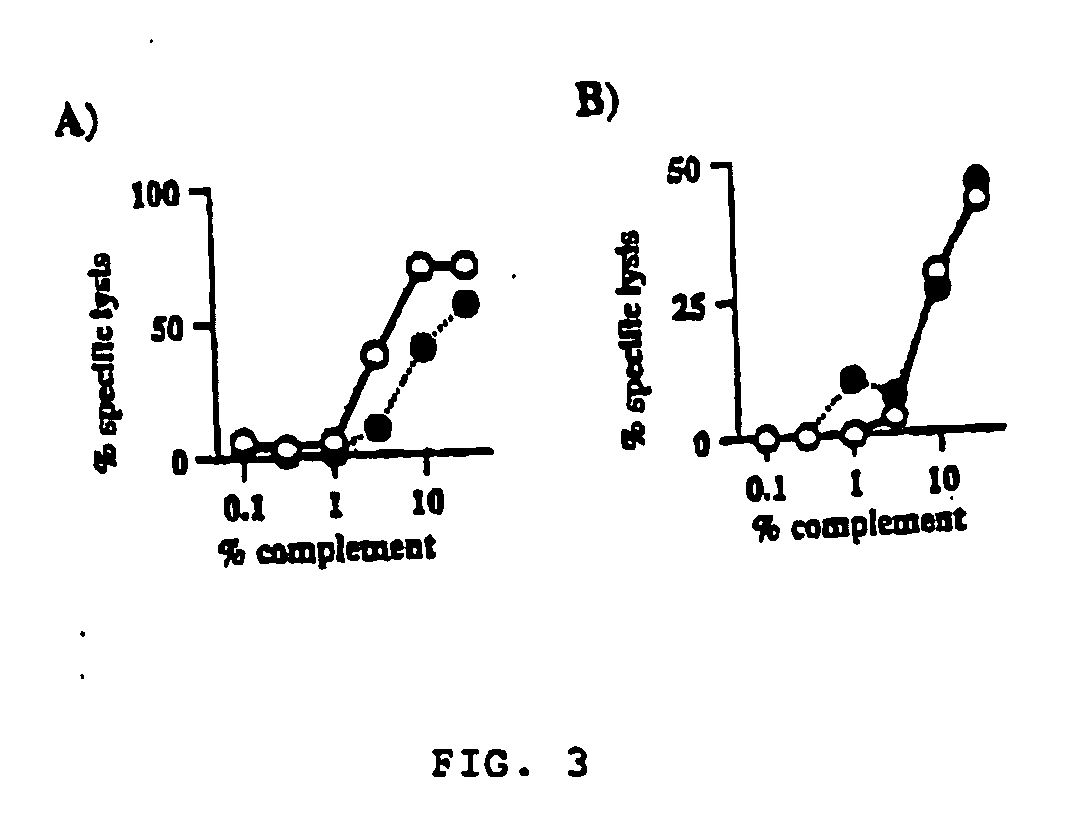
InactiveUS6953777B2Preventing CTL and complement-dependent rejectionLow cytotoxicityPeptide/protein ingredientsReceptors for hormonesCytotoxicityInterleukin 11
The use of interleukin-11 to prevent, to ameliorate, and to treat an immune-mediated disease in a mammal in need of such treatment is disclosed.
Owner:YALE UNIV +1
Treatment of fibrosis
ActiveUS20170174759A1Prevent and reduce bindingAvoid actionOrganic active ingredientsSenses disorderFibrosisInterleukin 11
Aspects of the disclosure relate to the treatment, prevention or alleviation of conditions such as fibrosis in a subject. In some embodiments, the treatment, prevention or alleviation of fibrosis in a subject through the administration of an agent capable of inhibiting the action of Interleukin 11 (IL-11) is disclosed.
Owner:SINGAPORE HEALTH SERVICES PTE +1
Delayed Release Formulations for Oral Administration of a Polypeptide Therapeutic Agent and Methods of Using Same
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
Use of interleukin-11 as therapeutic agent for heart disease
ActiveUS8361966B2High expressionEffective in repair and regenerationPeptide/protein ingredientsAntinoxious agentsWhite blood cellCardiac muscle
The present invention demonstrates the myocardial protective effects of interleukin 11 in vivo, and provides preventive, therapeutic, or other types of drugs for heart disease using interleukin 11 as the active ingredient. The present invention, by utilizing the myocardial protective effects of interleukin 11, can suppress the progress of myocardial injury, prevent the onset of heart failure, or suppress the progress of heart failure.
Owner:OSAKA UNIV
Use of interleukin-11 to prevent immune-mediated cytotoxicity
InactiveUS20060062760A1Preventing CTL and complement-dependent rejectionLow cytotoxicityPeptide/protein ingredientsImmunological disordersCytotoxicityInterleukin 11
The use of interleukin-11 to prevent, to ameliorate, and to treat an immune-mediated disease in a mammal in need of such treatment is disclosed.
Owner:GENETICS INST INC +1
Preparation method and application of D-dencichine
PendingCN105439883AGood for treating thrombocytopeniaHigh yieldOrganic active ingredientsCarbamic acid derivatives preparationTherapeutic effectInterleukin 11
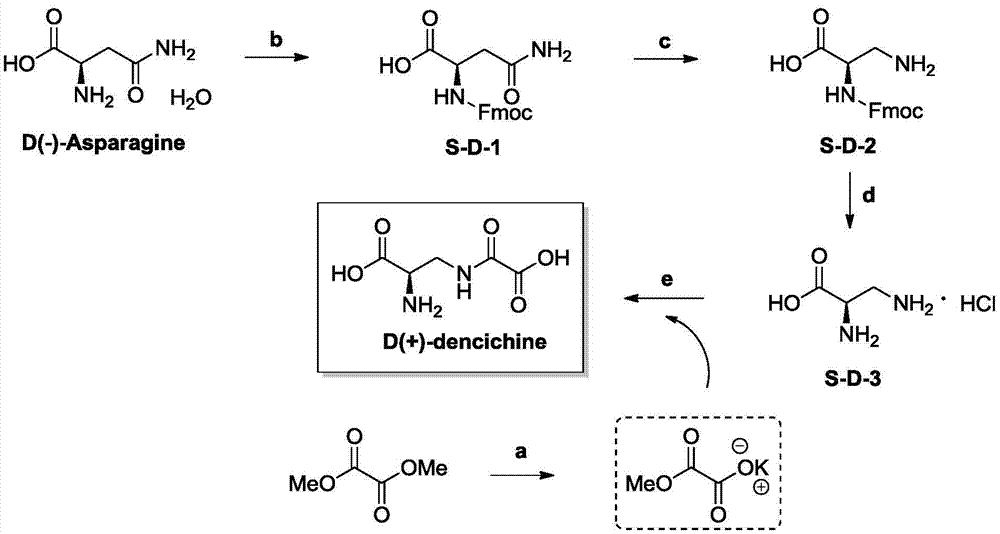
The invention discloses a preparation method and application of D-dencichine. The preparation method comprises the following steps: carrying out Fmoc protection on the amino group of D-asparagine so as to obtain a first intermediate; subjecting the first intermediate to Hoffman degradation reaction so as to obtain a second intermediate; subjecting the second intermediate to removal of Fmoc protection under the action of organic base so as to obtain a third intermediate; and subjecting the third intermediate and monomethyl oxalate to condensation reaction under a highly basic condition so as to obtain D-dencichine. D-dencichine is prepared by using the high-efficiency safe preparation method; the preparation method is simple and has high yield; the compound D-dencichine prepared by using the method has good treatment effect on thrombocytopenia and the effect is better than the effect of a clinical medicine interleukin-11, so D-dencichine can be used as a candidate medicine for treating thrombocytopenia.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Hydroxyethyl starch-containing polypeptide compositions
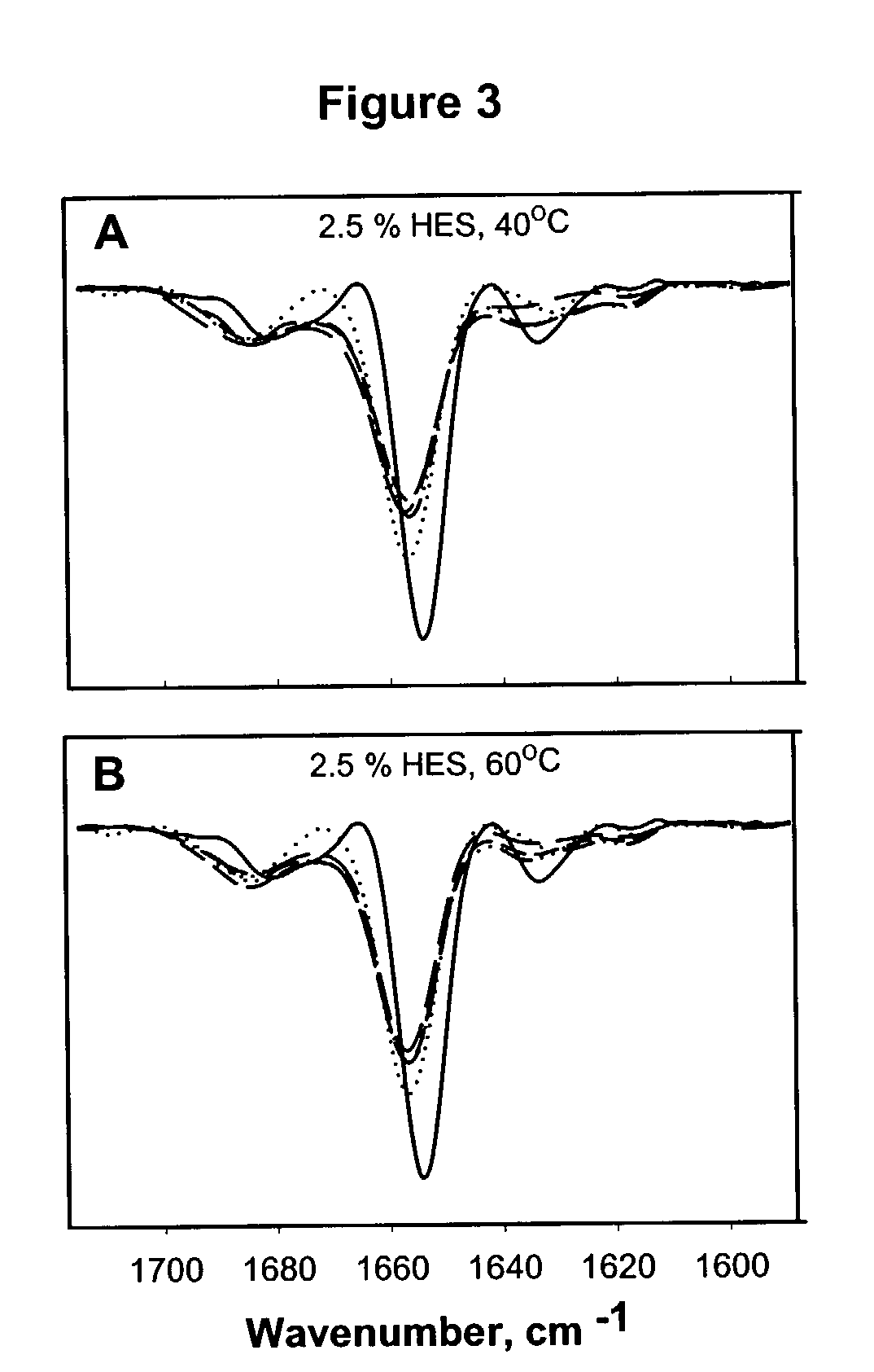
The invention provides compositions containing hydroxyethyl starch and polypeptides, including therapeutic polypeptides such as interleukin-11, that provide for enhanced stability of the polypeptide following storage at room temperature or elevated temperatures.
Owner:WYETH LLC +1
Production method for Pichia pastoris expression recombinant human interleukin 11
ActiveCN102329388AReduce preprocessingConvenient purification workPeptide preparation methodsInterleukinsPichia pastorisInterleukin 11
The invention discloses a production method for Pichia pastoris expression recombinant human interleukin 11, which comprises the step of combined purification sequentially through reverse phase column chromatography, hydrophobic column chromatography and gel column chromatography. By adopting the production method, high-purity medicinal recombinant human interleukin 11 proteins can be prepared ina large scale.
Owner:HANGZHOU JIUYUAN GENE ENG
Biopolymer Conjugates Comprising an Interleukin-11 Analog
ActiveUS20100098658A1Improve the immunityImprove stabilityPeptide/protein ingredientsPeptide preparation methodsSerum igeWhite blood cell
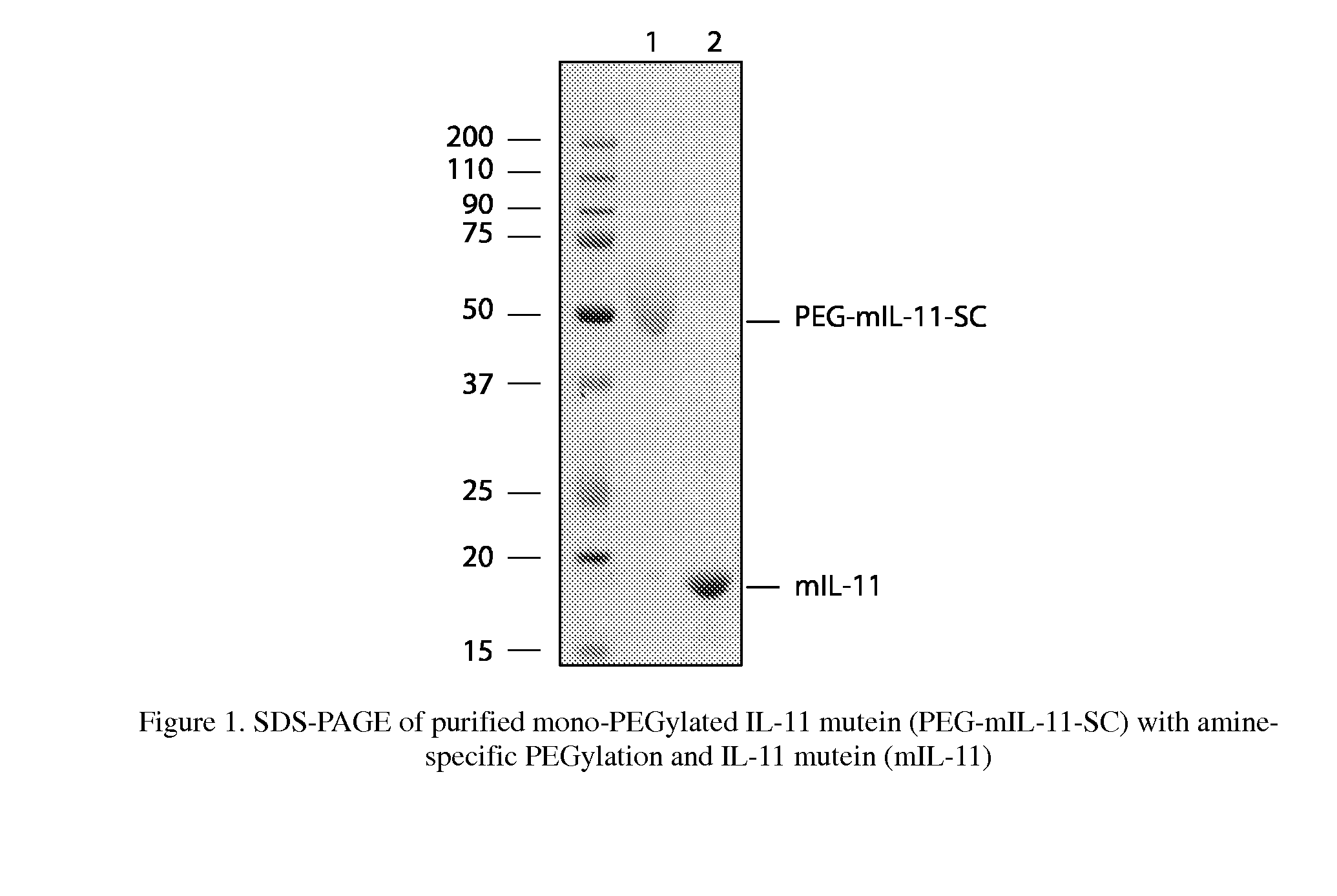
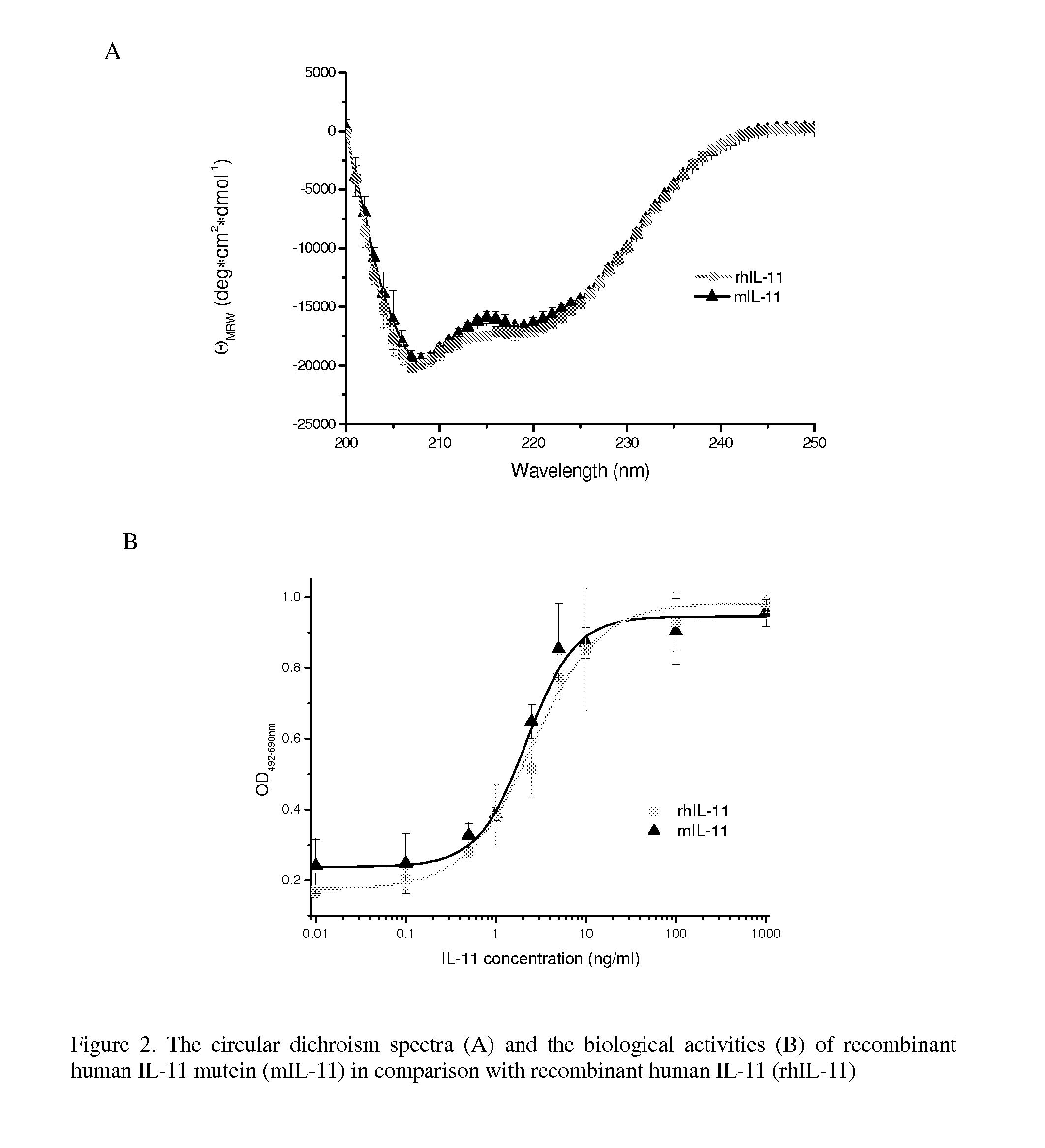
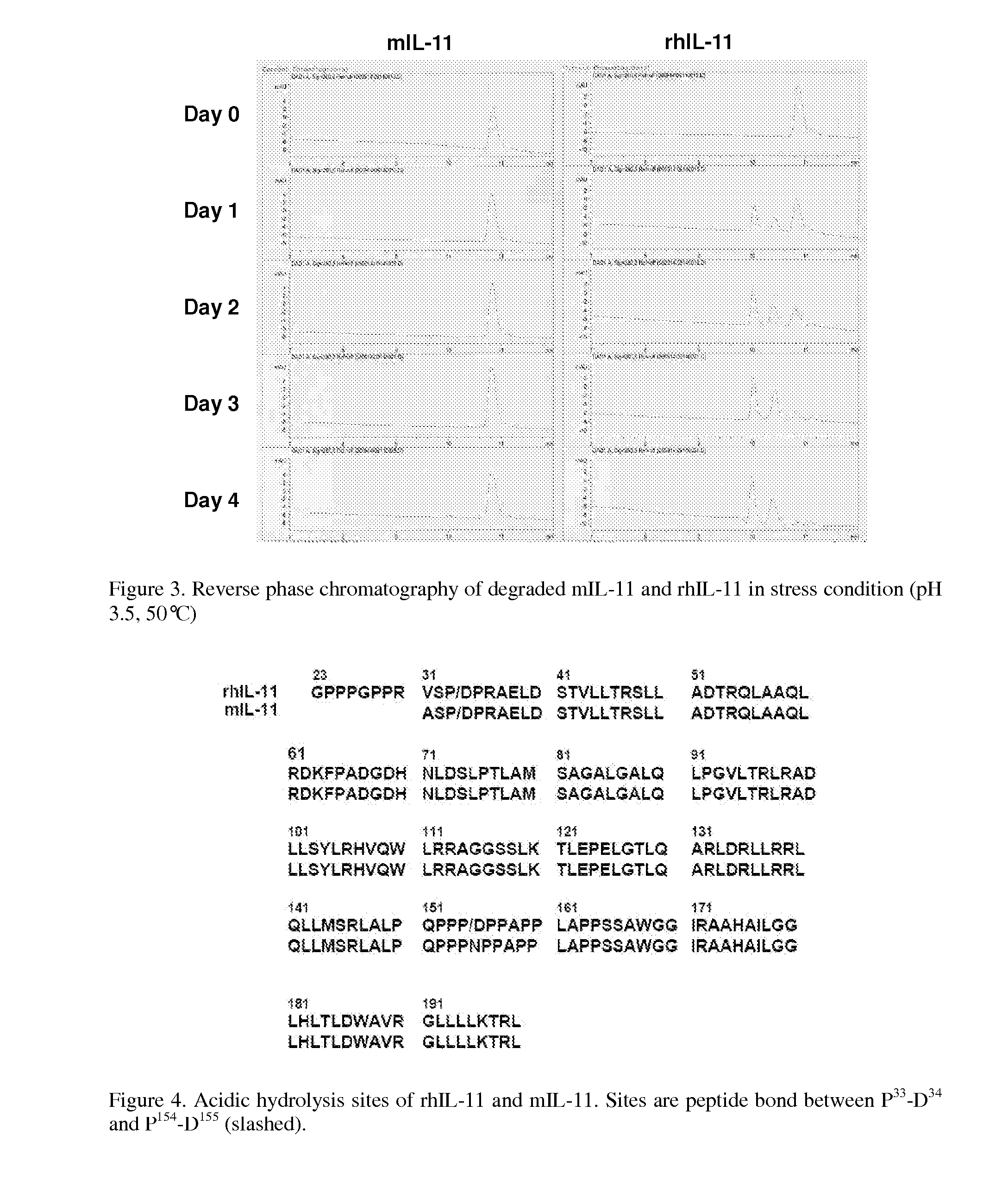
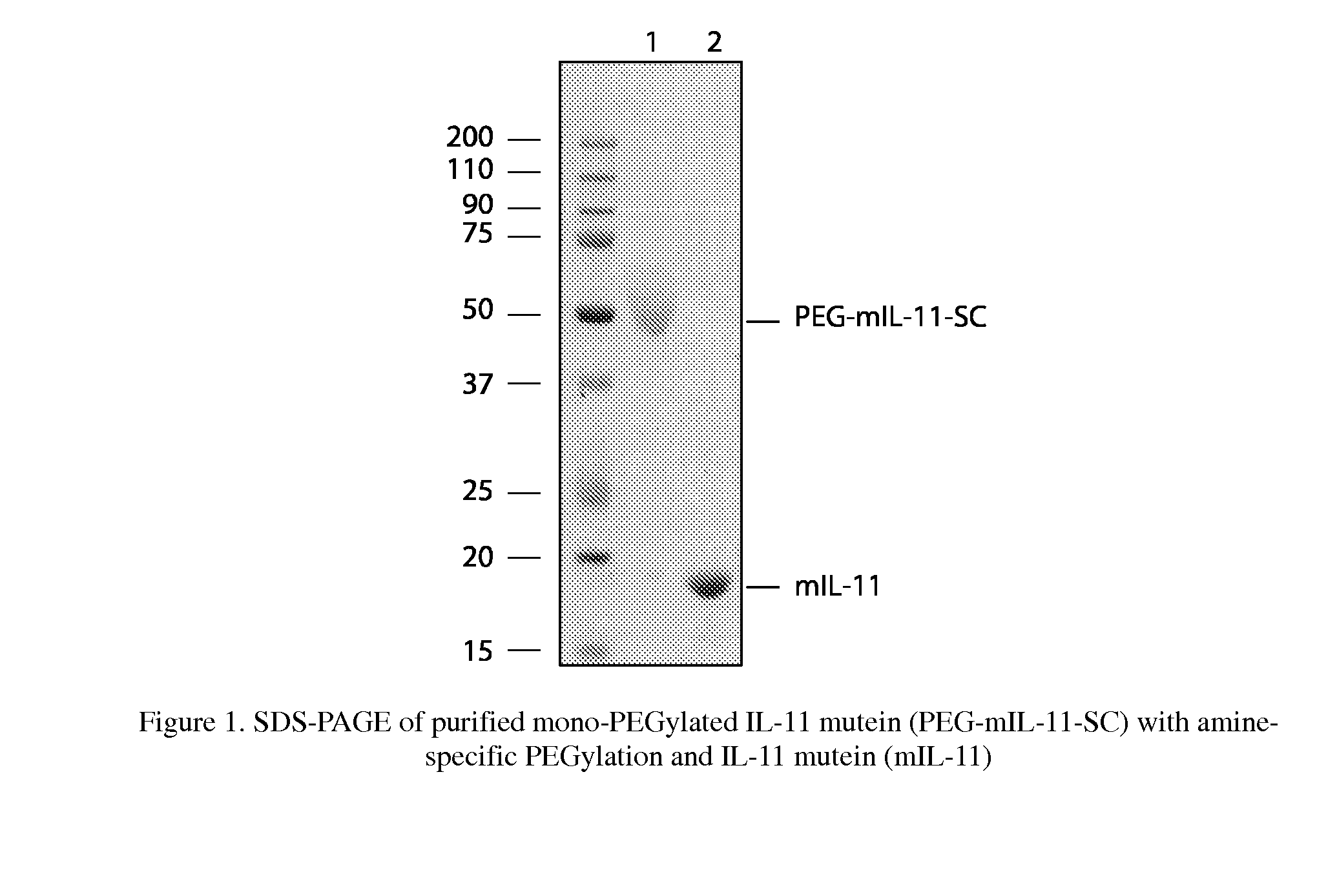
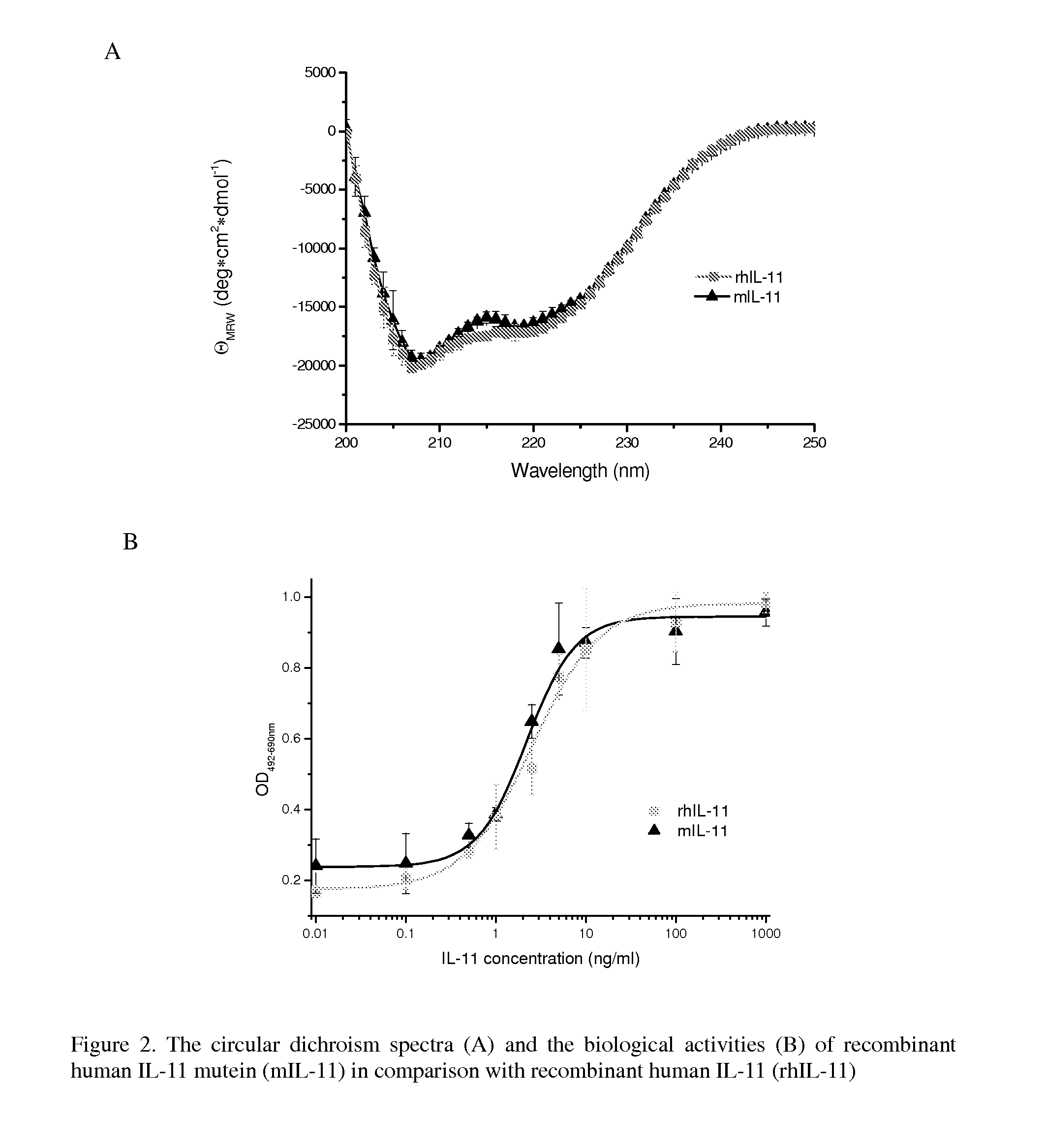
The present invention provides for biopolymer conjugates of an IL-11 analog (mIL-11) and a biocompatible polymer. The mIL-11 of the invention displays an enhanced resistance to acidolysis and shows increased stability as compared to rhIL-11. The conjugates of the present invention are characterized by a longer serum half-life and exhibit essentially no loss of activity as compared to the corresponding unconjugated mIL-11.
Owner:VIROMED CO LTD +1
Method for preparing autologous hematopoietic stem cells, kit, the stem cells and application
ActiveCN104419660AYield advantagePurity advantageAntinoxious agentsDead animal preservationInterleukin 6Culture fluid
Owner:深圳百年干细胞技术研究院有限公司
High efficiency finished purification method for colibacillus expression recombinant human interleukin-11
ActiveCN101143889AIncrease flow rateEase of preparation for productionPeptide preparation methodsInterleukinsProtein solutionEscherichia coli
The invention relates to an elaborate purification method of recombinant human interleukin 11 (rhIL-11). Fusion protein is desalinated by utilizing a dextran gel G-25 desalination column, the desalinated liquid is collected to carry out the cascade sample loading of negative and positive ion exchange chromatographic columns, after the sample loading is finished, the desalinated liquid is eluted by the specific Gly-NaOH buffer solution, and the collected main eluted protein solution is desalinated by the dextran gel G-25 desalination column, so that a high-purity protein sample is obtained. The method is suitable for large-scale production and is characterized in convenient operation, short production cycle, high purity of products, stable technique, etc.
Owner:QILU PHARMA CO LTD
Method for preparing recombinant human interleukin-11
InactiveCN102140487ATreatment safetySafe and effective treatmentPeptide preparation methodsFermentationWhite blood cellGradient elution
The invention provides a method for preparing recombinant human interleukin-11 (rhIL). The method comprises the following steps: 1) providing a fusion protein, wherein from the N-end to the C-end, the fusion protein is thioredoxin-(His)6-proteolytic enzyme recognition site-rhIL-11 or (His)6-thioredoxin-proteolytic enzyme recognition site-rhIL-11; 2) using the proteolytic enzyme to perform enzyme cutting to the fusion protein and obtain an enzyme cutting product, wherein the enzyme cutting product contains thioredoxin and rhIL-11; and 3) using the nickel ion chelate affinity column chromatography to purify the enzyme cutting product, and performing gradient elution to the column to obtain rhIL-11, wherein the thioredoxin and the rhIL-11 are both adsorbed on the column. By adopting the method provided by the invention, the rhIL-11 can be purified rapidly, conveniently and efficiently and the recovery rate can be greatly increased.
Owner:DONGGUAN TAILI BIOTECH
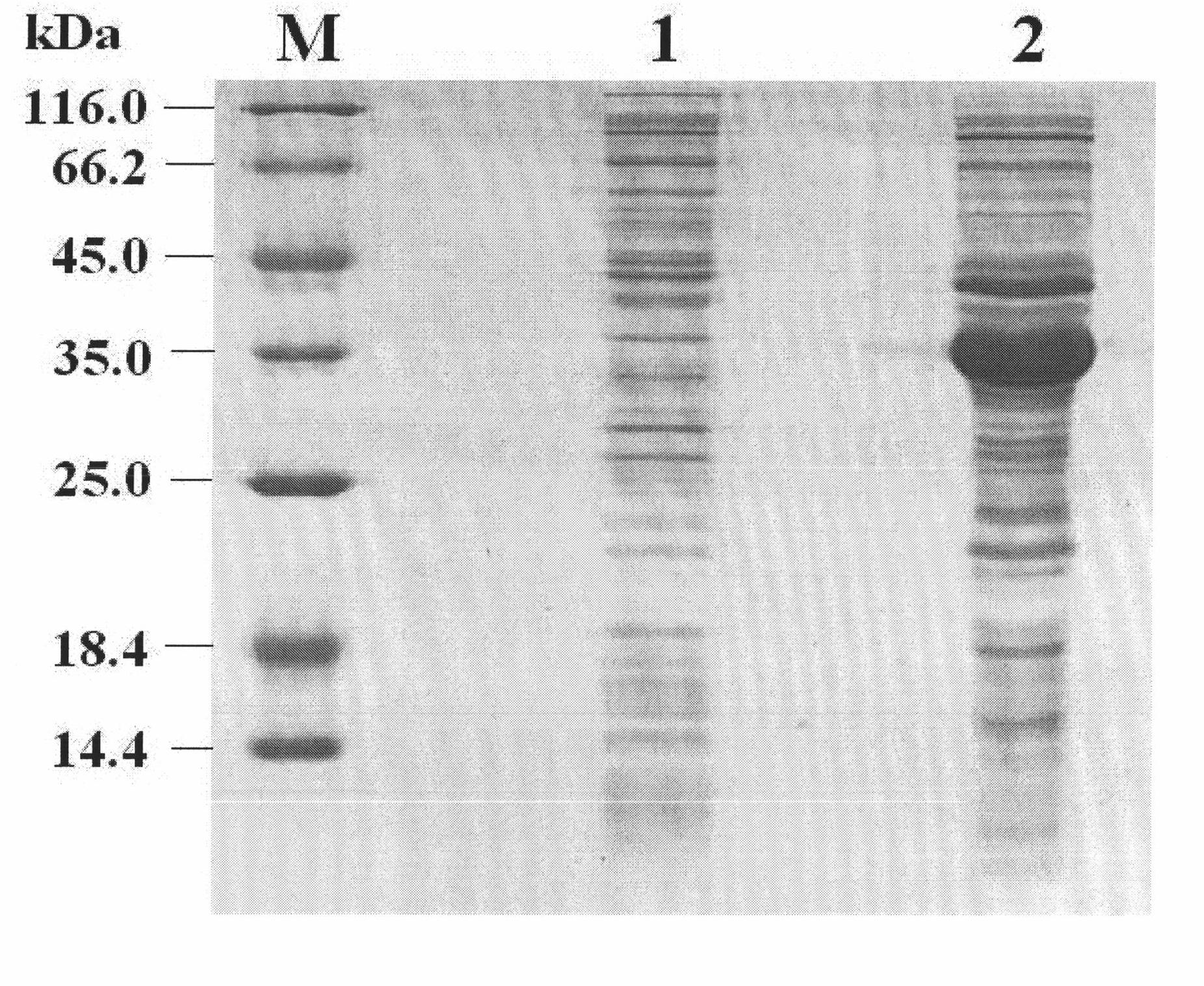
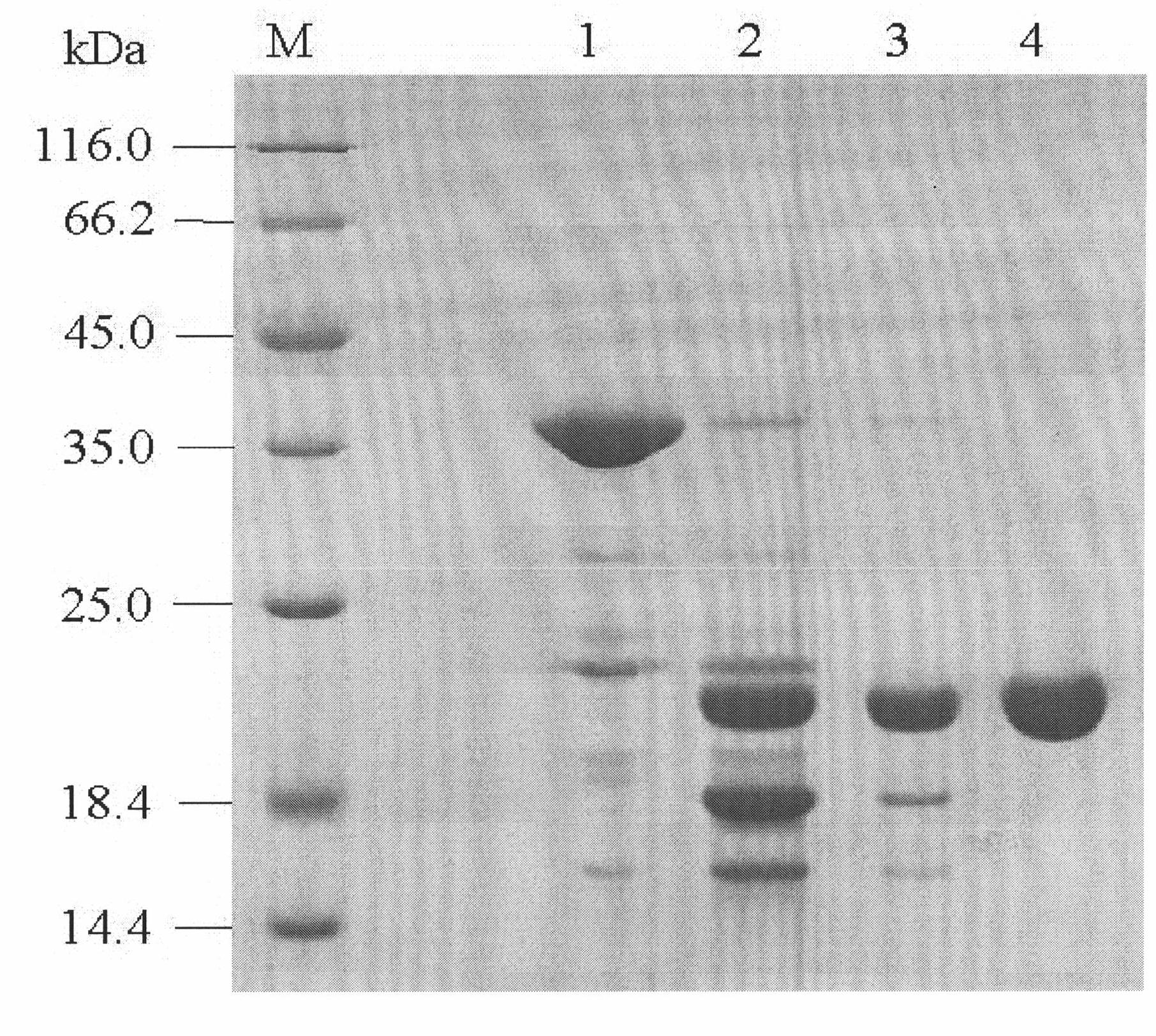
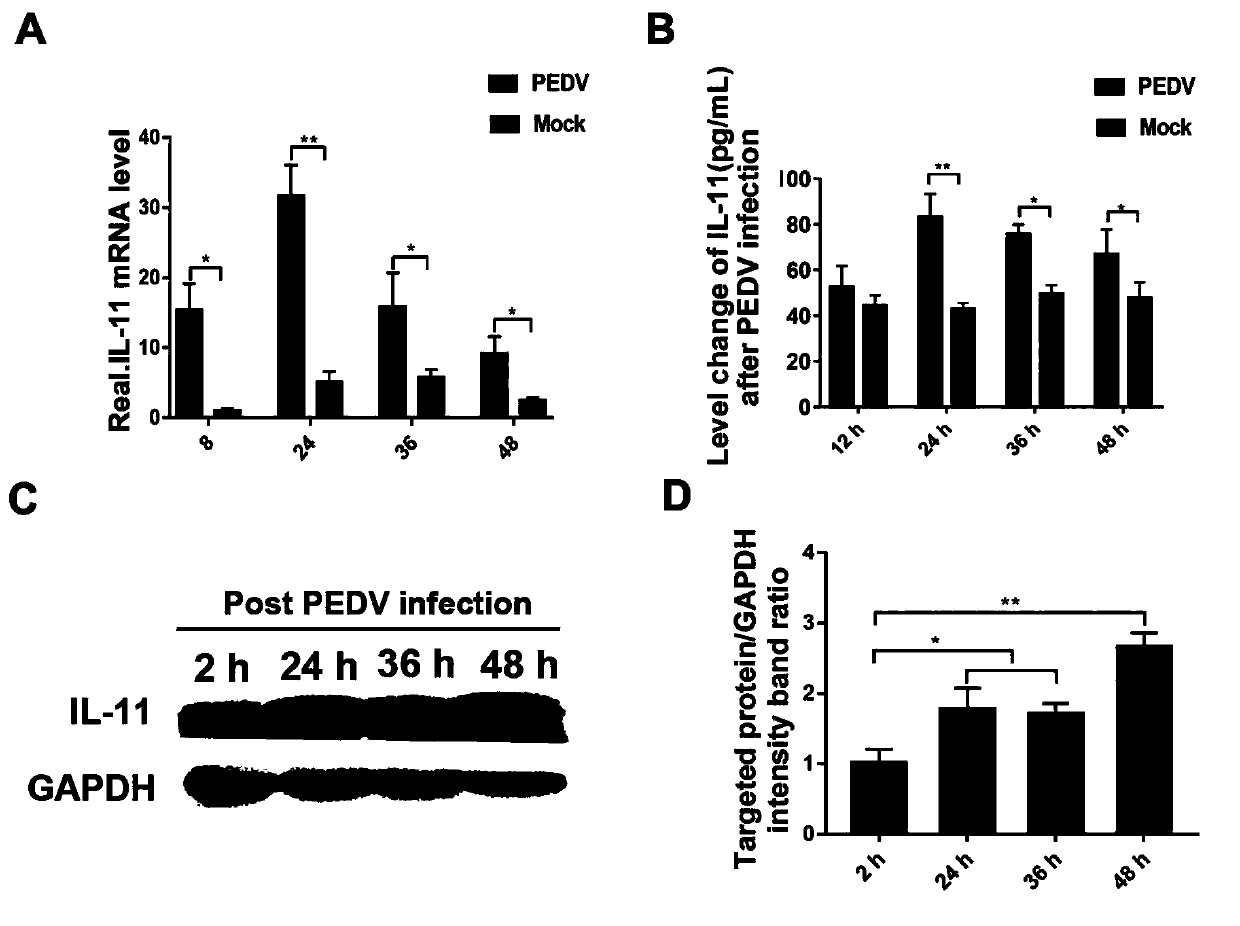
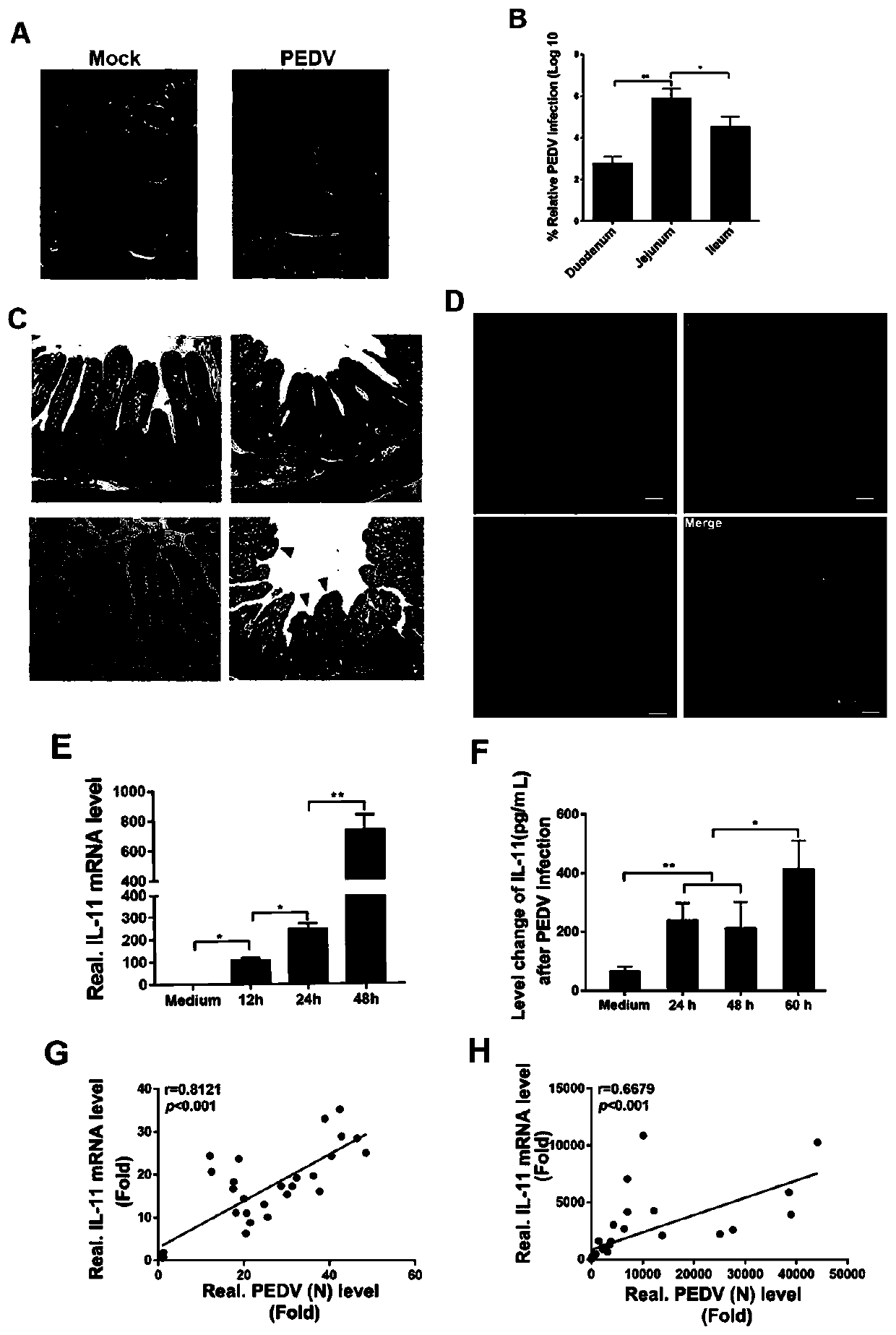
Purpose of porcine interleukin 11 in resisting porcine epidemic diarrhea virus infection
The invention relates to a porcine interleukin 11, and a purpose of the porcine interleukin 11 in resisting porcine epidemic diarrhea virus infection. After porcine epidemic diarrhea virus infection happens, a piglet intestinal tract secretes cell factor porcine pIL-11 which is obviously improved, and the expression of the cell factor exhibits high correlation with a virus infection level. The invention discloses a prokaryotic expression plasmid for constructing a recombinant pIL-11, a transformed escherichia coli BL21, and a specific method for obtaining a high-purity recombinant pIL-11 through purification. The recombinant pIL-11 can inhibit the infection of the PEDV (Porcine epidemic diarrhea virus) for a host cell, and has obvious dose dependency. The recombinant pIL-11 activates a STAT3 (signal transducer and activator of transcription 3) signal to inhibit host cell apoptosis induced after PEDV infection happens so as to perform a function of resisting virus infection. The porcinesource IL-11 has a function of resisting virus infection and alleviating piglet intestinal tract injury, and is expected to become an effective medicine for resisting PEDV intestinal tract infectionin the future.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for preparing megakaryocytic preparation by amplifying macronucleus ancestral cell and mature megacaryocyte and use
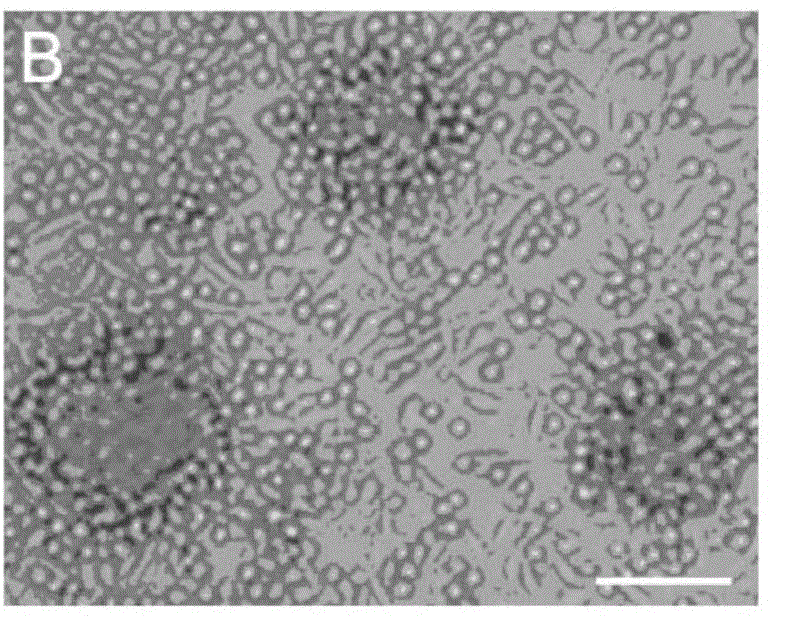
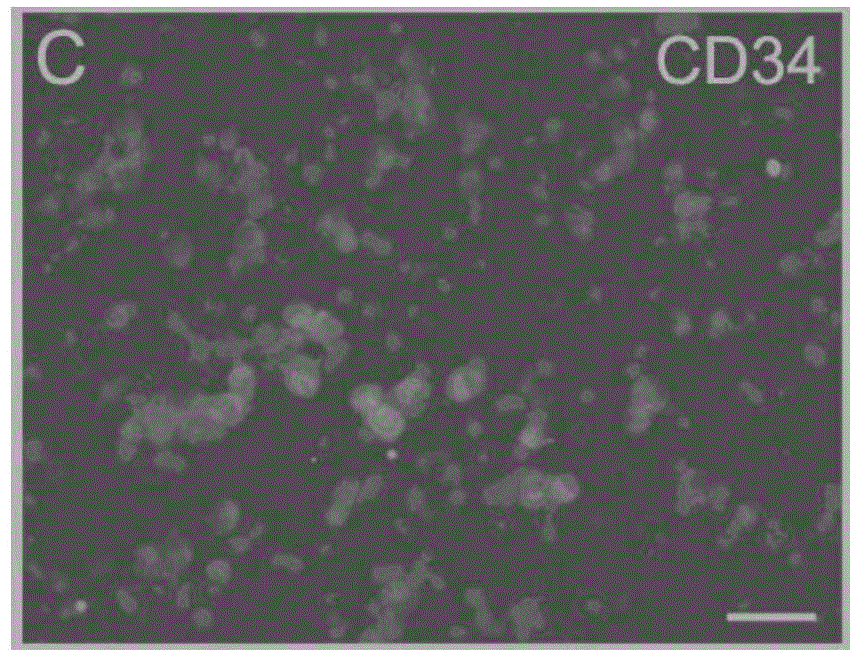
The present invention relates to mainly one system of efficient culturing and directive inducing stem cell differentiation for culturing and proliferating megakaryocyte. The system has umbilical cord blood and marrow stem cell as seed cell, and the serum-free culture medium comprising TPO, IL-11 and heparin as the main component for megakaryocyte proliferation. The megakaryocyte preparation prepared based on the present invention contains great amount of macronucleus ancestral cells and mature megakaryocyte as well as CD34+ cells, and has the functions of treating thrombocytopenia and improving the blood-forming function after stem cell transplantation. The present invention provides novel efficient cell treating preparation for various thrombocytopenia and hemocyitopenia.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Biopolymer conjugates comprising an interleukin-11 analog
ActiveUS8716446B2Improve the immunityNo loss of activityPeptide/protein ingredientsDepsipeptidesSerum igeHalf-life
Owner:VIROMED CO LTD +1
Method of using IL-11 for treating mucositis
Provided by the present invention are topical formulations of Interleukin-11 and methods for treating a variety of disorders, including inflammatory bowel diseases (e.g., Crohn's disease, ulcerative colitis, indeterminate colitis, and infectious colitis), mucositis (e.g., oral mucositis, gastrointestinal mucositis, nasal mucositis, and proctitis), necrotizing enterocolitis, inflammatory skin disorders (e.g., psoriasis, atopic dermatitis, and contact hypersensitivity), aphthous ulcers, pharyngitis, esophagitis, peptic ulcers, gingivitis, periodontitis, and ocular diseases (e.g., conjunctivitis, retinitis, and uveitis).
Owner:GENETICS INST INC
Composition for regulating enteric microorganisms and preparation method thereof
InactiveCN107029214AIncrease lethalitySpeed up the removal processAntibacterial agentsPeptide/protein ingredientsIntestinal microorganismsAlbumin solution
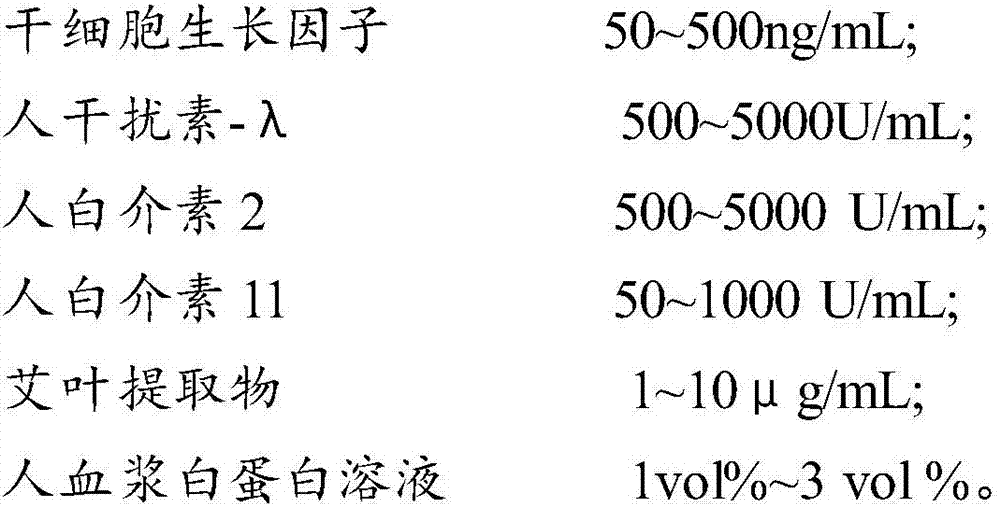
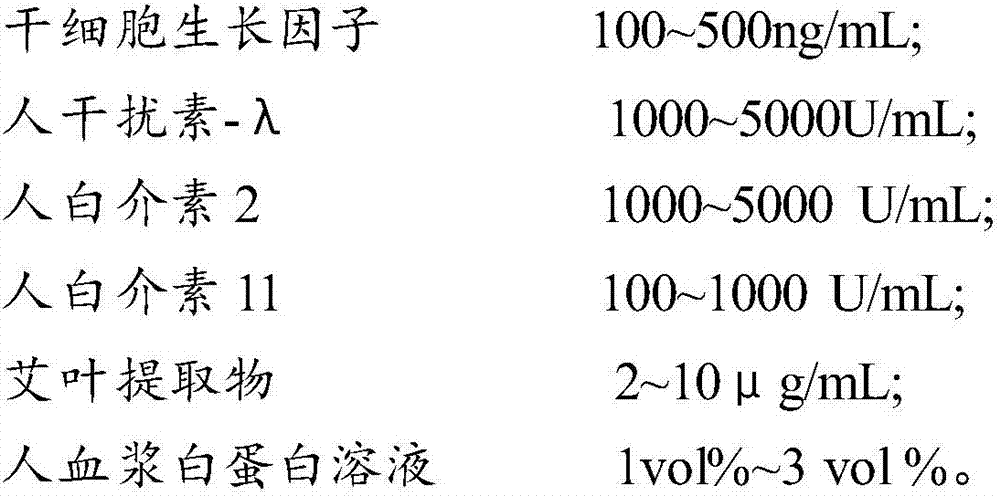
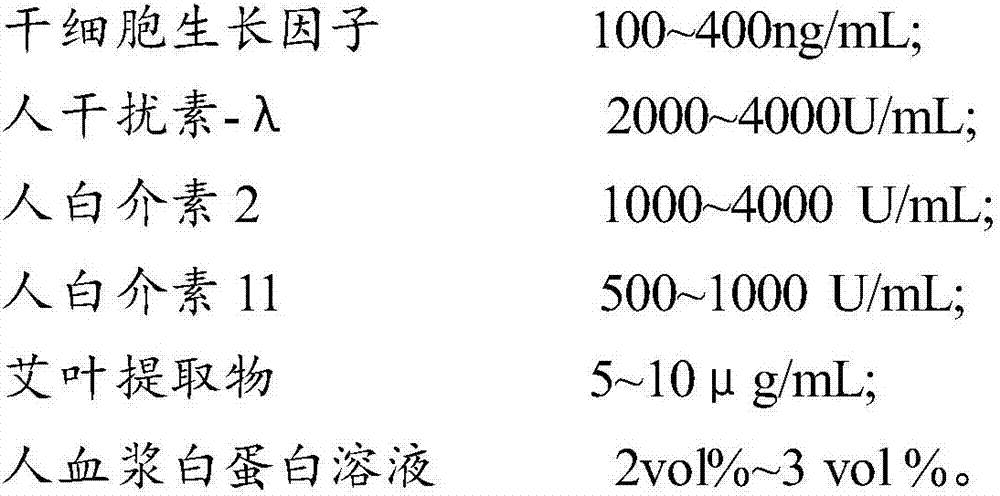
The invention provides a composition for regulating enteric microorganisms. The composition comprises solvent and components, such as, by weight, 50-500ng / mL of stem cell growth factor, 500-5000 U / mL of human interferon-Lambda, 500-5000 U / mL of human interleukin 2, 50-1000 U / mL of human interleukin 11, 1-10 mu g / mL of folium artemisiae argyi extractive, and 1vol%-3vol% of human plasma albumin solution. Through synergistic effect of the stem cell growth factor, the human interferon-Lambda, the human interleukin 2, the human interleukin 11, the human plasma albumin solution and the folium artemisiae argyi extractive, the composition can improve the whole immune system of a human body and strengthen the killing and removing ability of own immune cell to harmful flora; meanwhile, the composition can effectively regulate the intestinal microflora, increase probiotics, reduce harmful bacteria, and maintain a healthy environment of the whole intestinal canal.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130165344A1Eliminate needLibrary screeningDisease diagnosisInsulin-like growth factor bindingCancer antigen
Owner:ASTUTE MEDICAL
Compound stypticum
ActiveCN101138632AReduce dosageGuaranteed curative effectPeptide/protein ingredientsBlood disorderBone Marrow Stromal CellWhite blood cell
A compound medicine for arresting bleeding is characterized in that the compound medicine is a mixture of a batroxobin and an interleukin-11; the clinical dosage of the batroxobin is 0.01-10u and that of IL-11 is 0.06-50mg; the batroxobin is the batroxobin from snake venom of Brazilian spearhead adder, white-eyebrow adder or acutus adder, or the batroxobin from recombinant Brazilian spearhead adder, white-eyebrow adder or acutus adder obtained by genetic engineering; the interleukin-11 is produced by human bone-marrow stromal cells (fibroblasts) and interstitial cells, or the recombinant IL-11 by genetic engineering. The medicine for arresting bleeding can not only ensure the curative effect but also decrease the amount of protease so as to reduce the rate of adverse immune response.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WEISS INC
Topical formulation for delivery of interleukin-11
InactiveUS20050129658A1Peptide/protein ingredientsPharmaceutical delivery mechanismUveitisEnterocolitis
Provided by the present invention are topical formulations of Interleukin-11 and methods for treating a variety of disorders, including inflammatory bowel diseases (e.g., Crohn's disease, ulcerative colitis, indeterminate colitis, and infectious colitis), mucositis (e.g., oral mucositis, gastrointestinal mucositis, nasal mucositis, and proctitis), necrotizing enterocolitis, inflammatory skin disorders (e.g., psoriasis, atopic dermatitis, and contact hypersensitivity), aphthous ulcers, pharyngitis, esophagitis, peptic ulcers, gingivitis, periodontitis, and ocular diseases (e.g., conjunctivitis, retinitis, and uveitis).
Owner:WARNE NICK W +4
Method for preparing medicinal recombinant human interleukin-11
InactiveCN101892279AQuality is easy to controlNo pollution in the processPeptide preparation methodsFermentationWhite blood cellInterleukin 11
The invention discloses a method for preparing medicinal recombinant human interleukin-11, which adopts the following preparation steps of: 1) initial purification of a fusion protein in the interleukin-11, which comprises steps of crushing thalli and separating a chelate column; 2) digesting the fusion protein containing the interleukin-11 by enterokinase; and 3) fine purification of the interleukin-11, wherein Macro Cap SP column chromatography and Superdex-75 column chromatography are carried out to obtain the medicinal recombinant human interleukin-11. The method has the characteristics of simple process, high expression amount, high stability, uniform product, low production cost, environmental protection and high activity, improves the safety, controllability and validity of medicaments and is suitable for industrial production.
Owner:XIAMEN AMOYTOP BIOTECH
Interleukin-11 compositions and methods of use
InactiveCN101321538APeptide/protein ingredientsPharmaceutical non-active ingredientsInterleukin 11Hepatocyte
The present invention provides methods for the treatment and / or prevention of thrombocytopenia including thrombocytopenia associated with drug-induced liver damage and thrombocytopenia associated with drug-induced bone marrow destruction. The methods of treatment of the invention include administration of interleukin-11 to a subject suffering from or susceptible to thrombocytopenia and / or receiving or about to receive a treatment involving a conjugate therapeutic agent whose administration results in thrombocytopenia. Also provided are pharmaceutical compositions and kits useful for carrying out such methods of treatment.
Owner:WYETH LLC
Treatment and prevention of metabolic diseases
PendingUS20200384083A1High activityPromoting neurogenesisOrganic active ingredientsPeptide/protein ingredientsDiseaseWhite blood cell
Methods of treating and preventing metabolic disease through inhibiting interleukin 11 (IL-11)-mediated signalling are disclosed, as well as agents for use in such methods.
Owner:NAT HEART CENT OF SINGAPORE +2
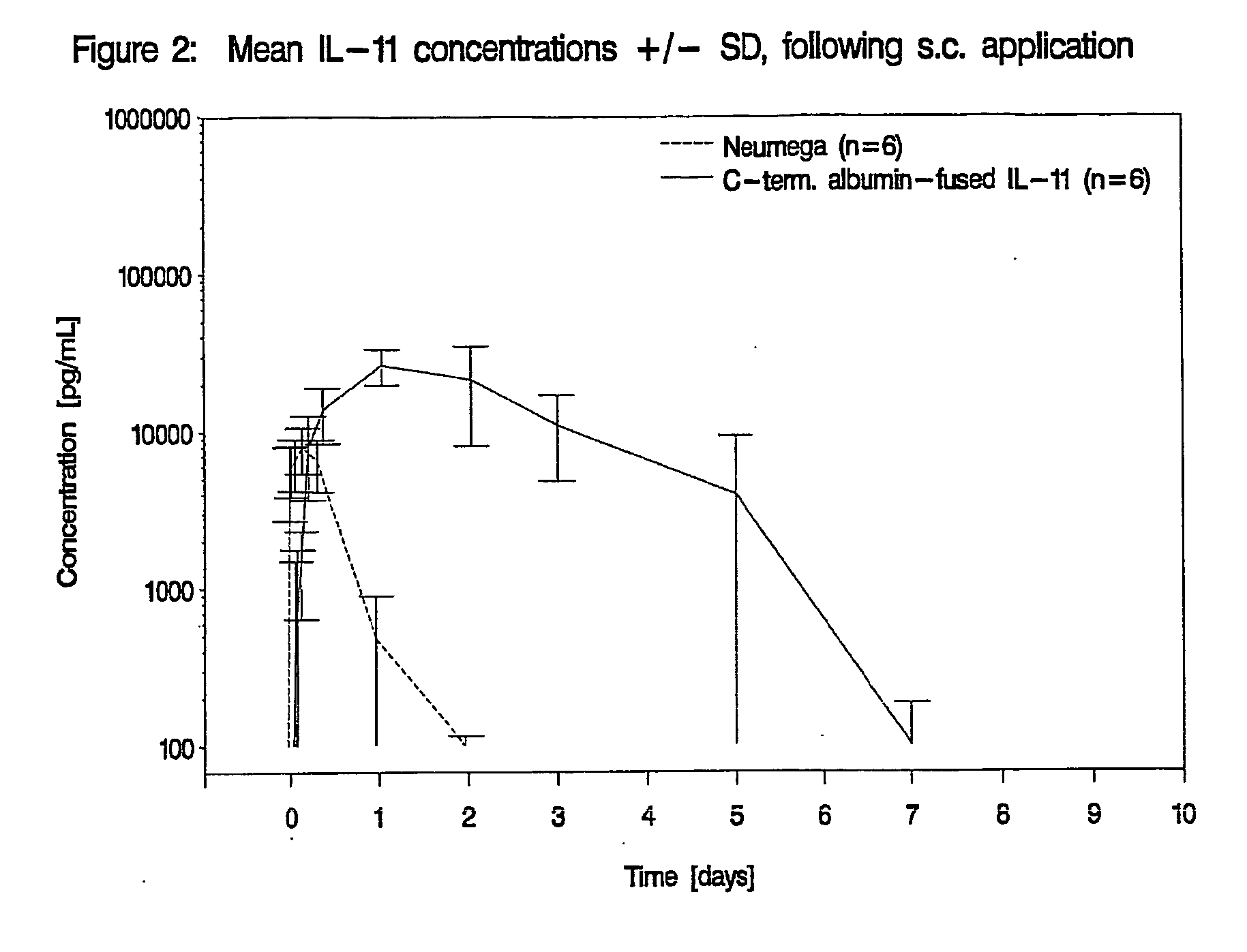
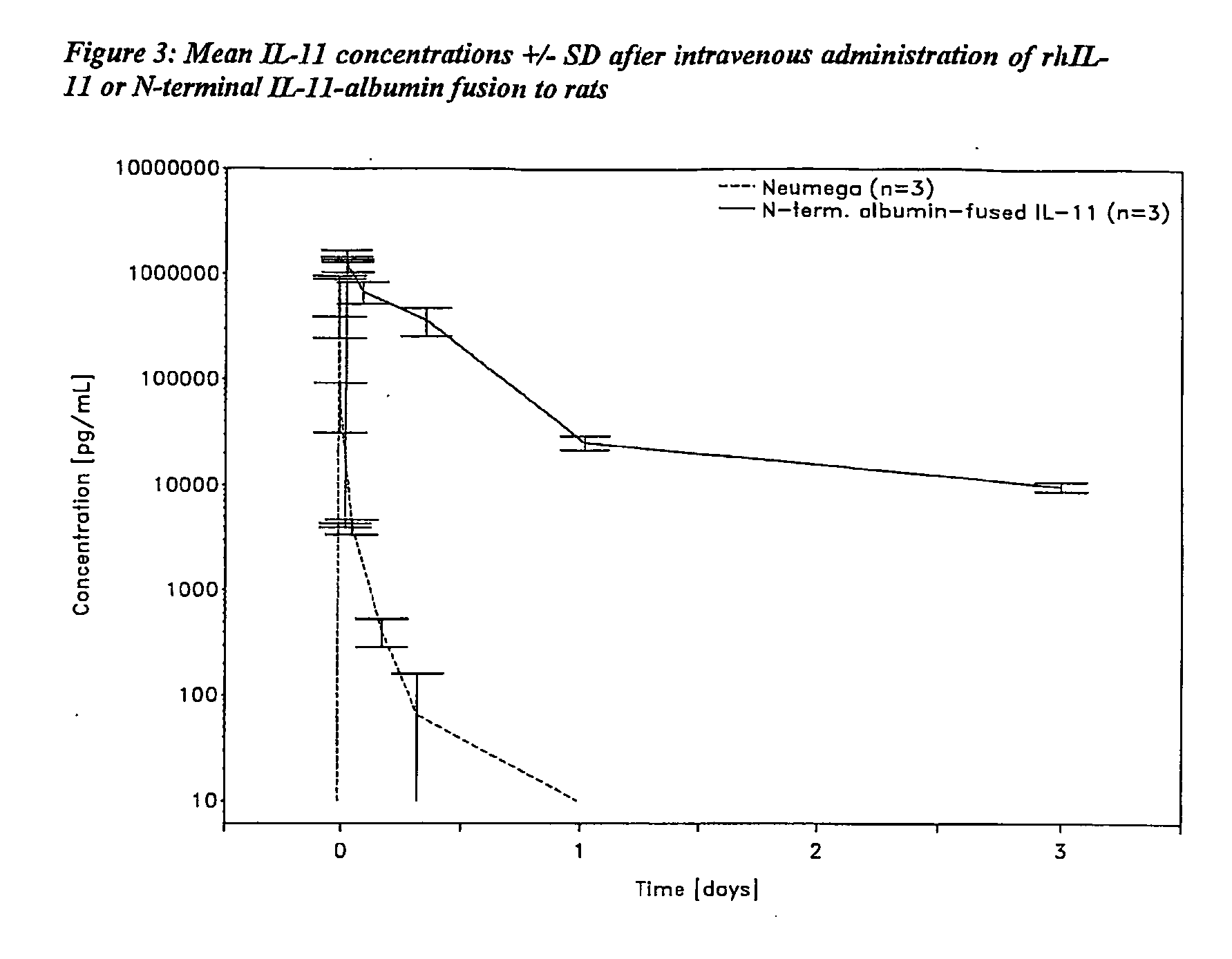
Interleukin-11 Fusion Proteins
InactiveUS20100143973A1Increased solubility and stability and circulating timeLow immunogenicityFungiBacteriaDiseaseWhite blood cell
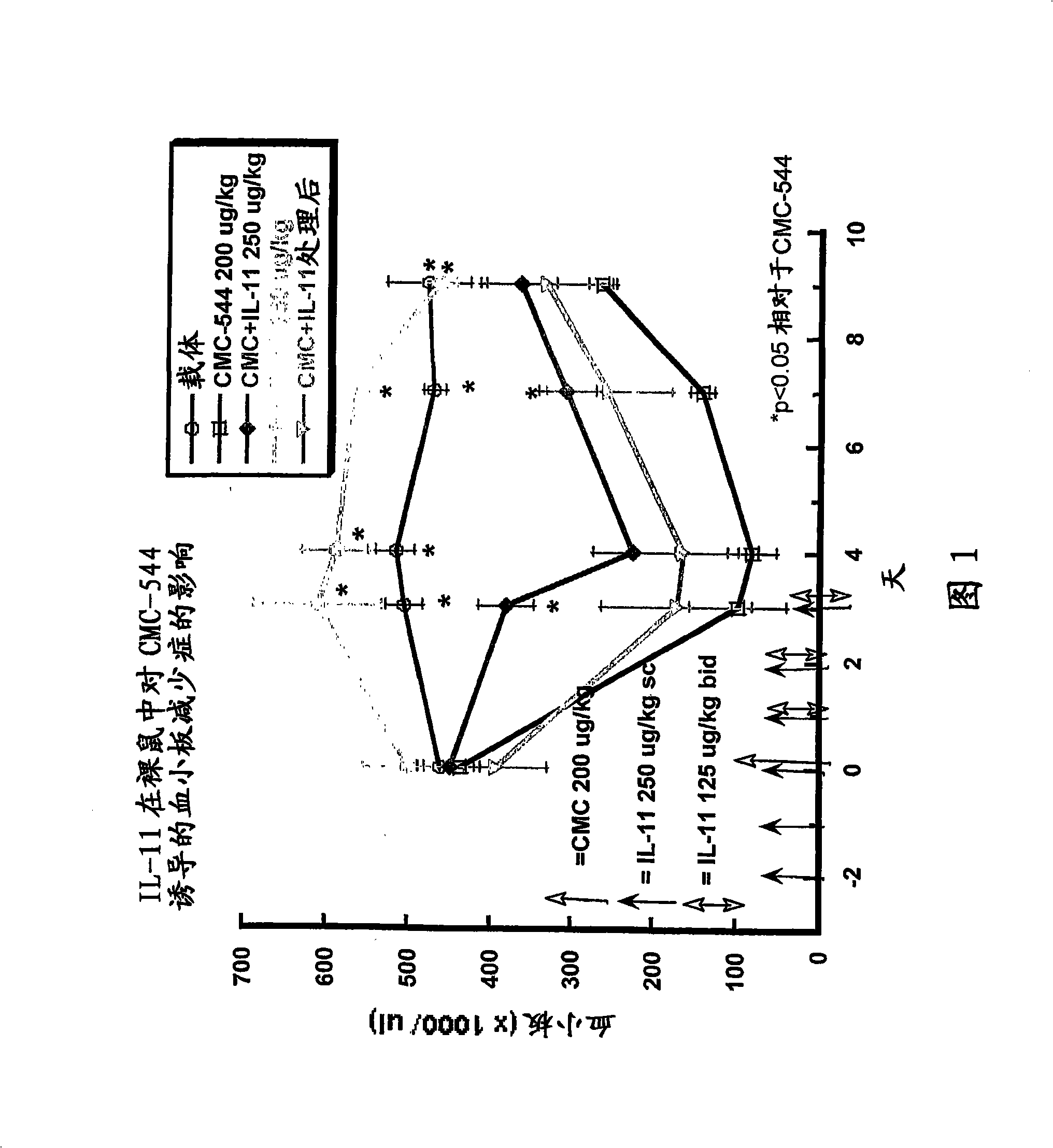
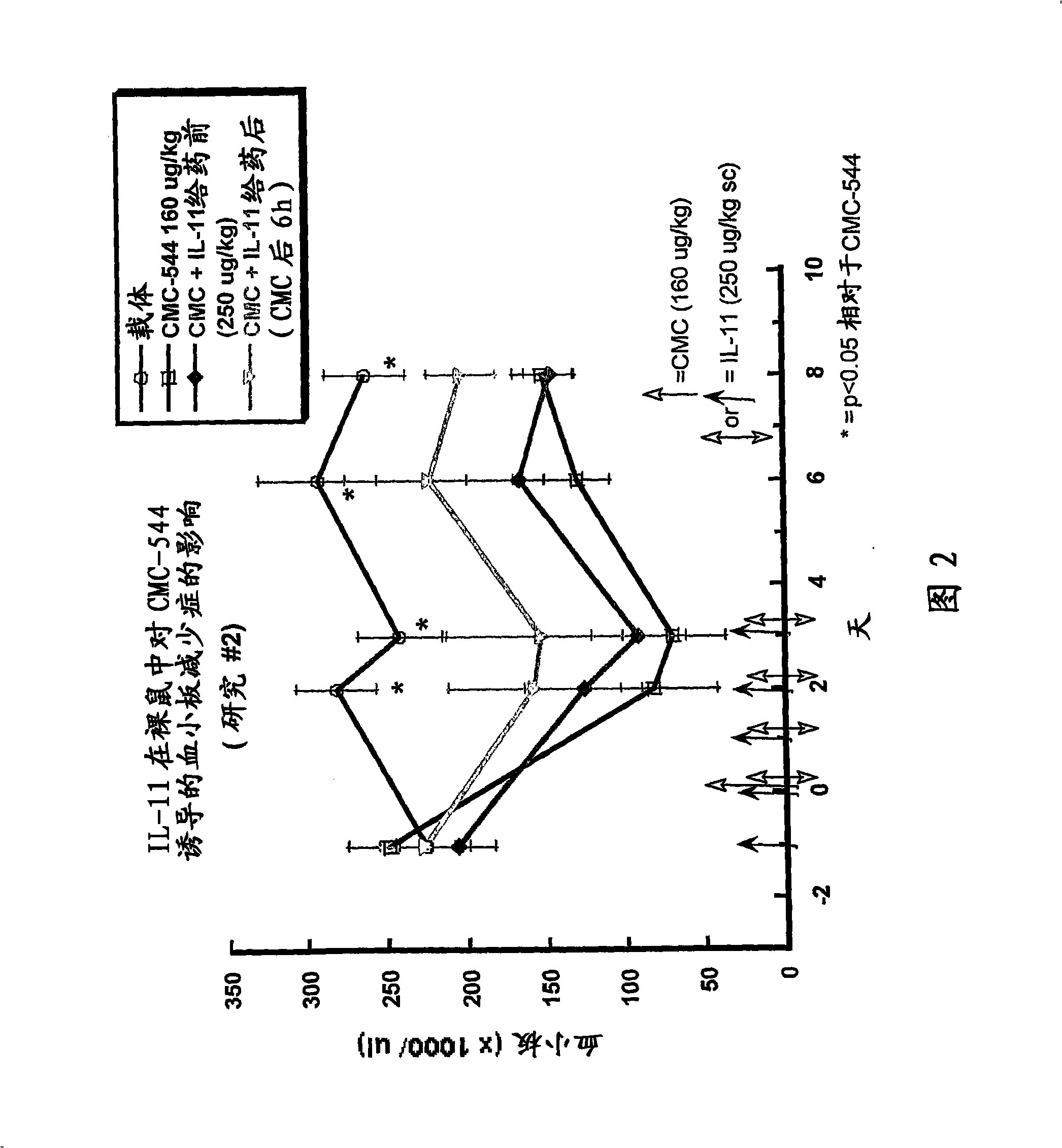
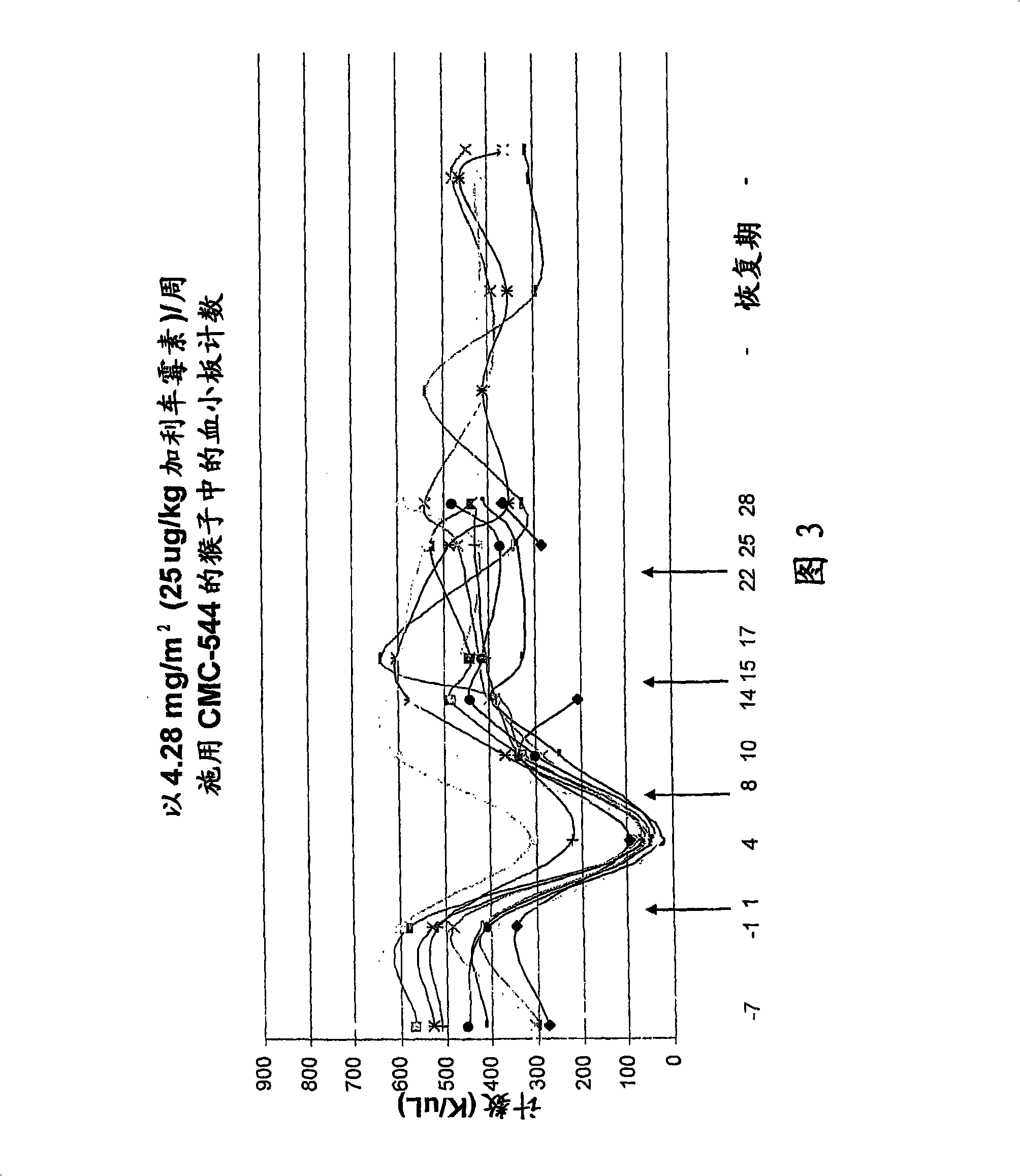
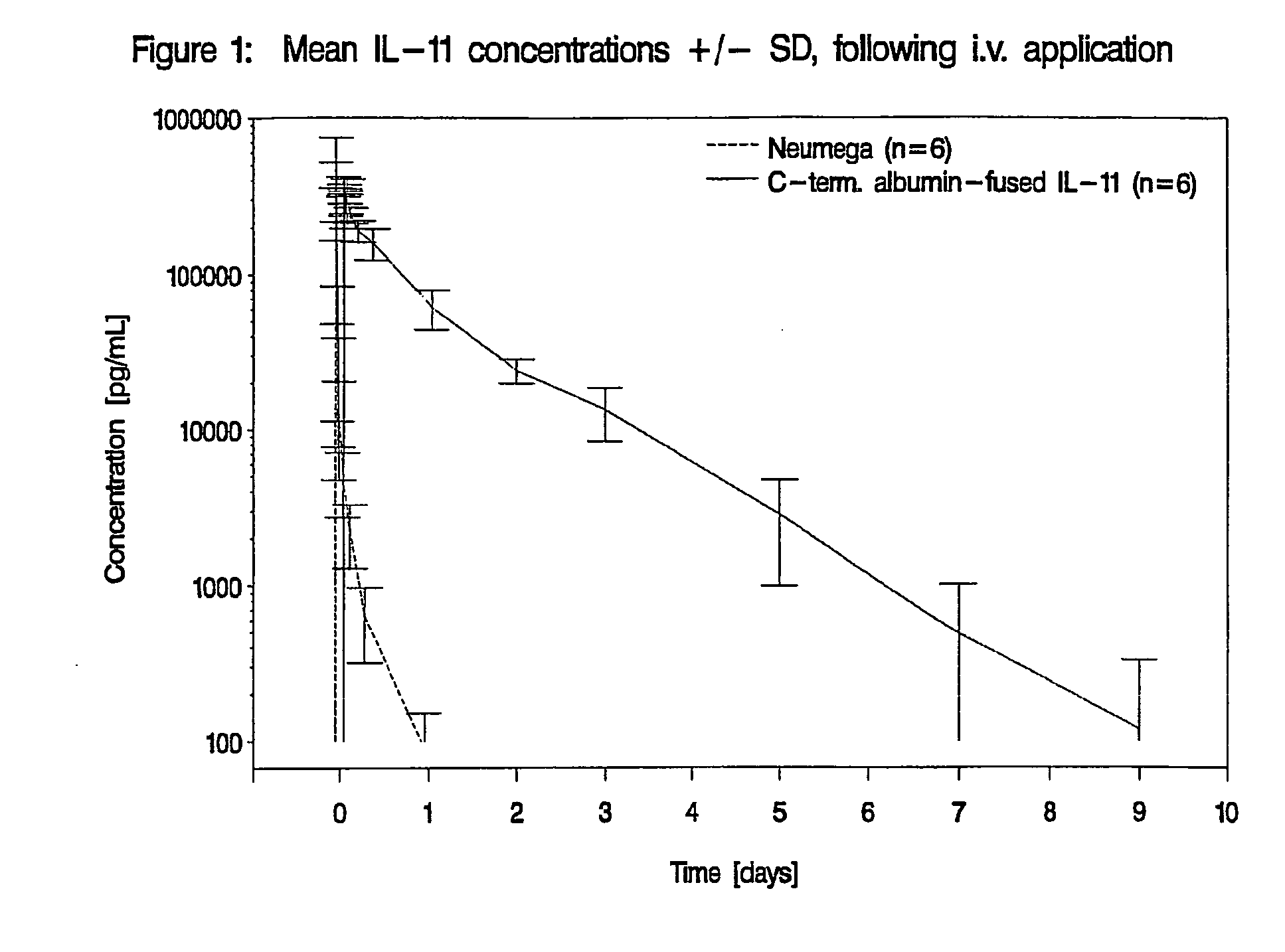
The invention relates to proteins comprising Interleukin 11 (IL-11) (including, but not limited to, fragments and variants thereof), which exhibit thrombopoietic or anti-inflammatory properties, fused to albumin (including, but not limited to, fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention”. These fusion proteins exhibit extended shelf-life and / or pharmacokinetic properties and / or extended or therapeutic activity. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acids, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for treatment or prophylaxis of thrombocytopenia or inflammatory diseases.
Owner:KROZ MONIKA +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com