Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Glucose low" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low blood sugar is a condition that occurs when the body's blood sugar (glucose) decreases and is too low. Blood sugar below 70 mg/dL (3.9 mmol/L) is considered low. Blood sugar at or below this level can be harmful.
Method, system, and computer program product for the processing of self-monitoring blood glucose(smbg)data to enhance diabetic self-management
ActiveUS20050214892A1Easy to monitorContinuous informationMedical simulationMicrobiological testing/measurementAcute hyperglycaemiaOptimal control
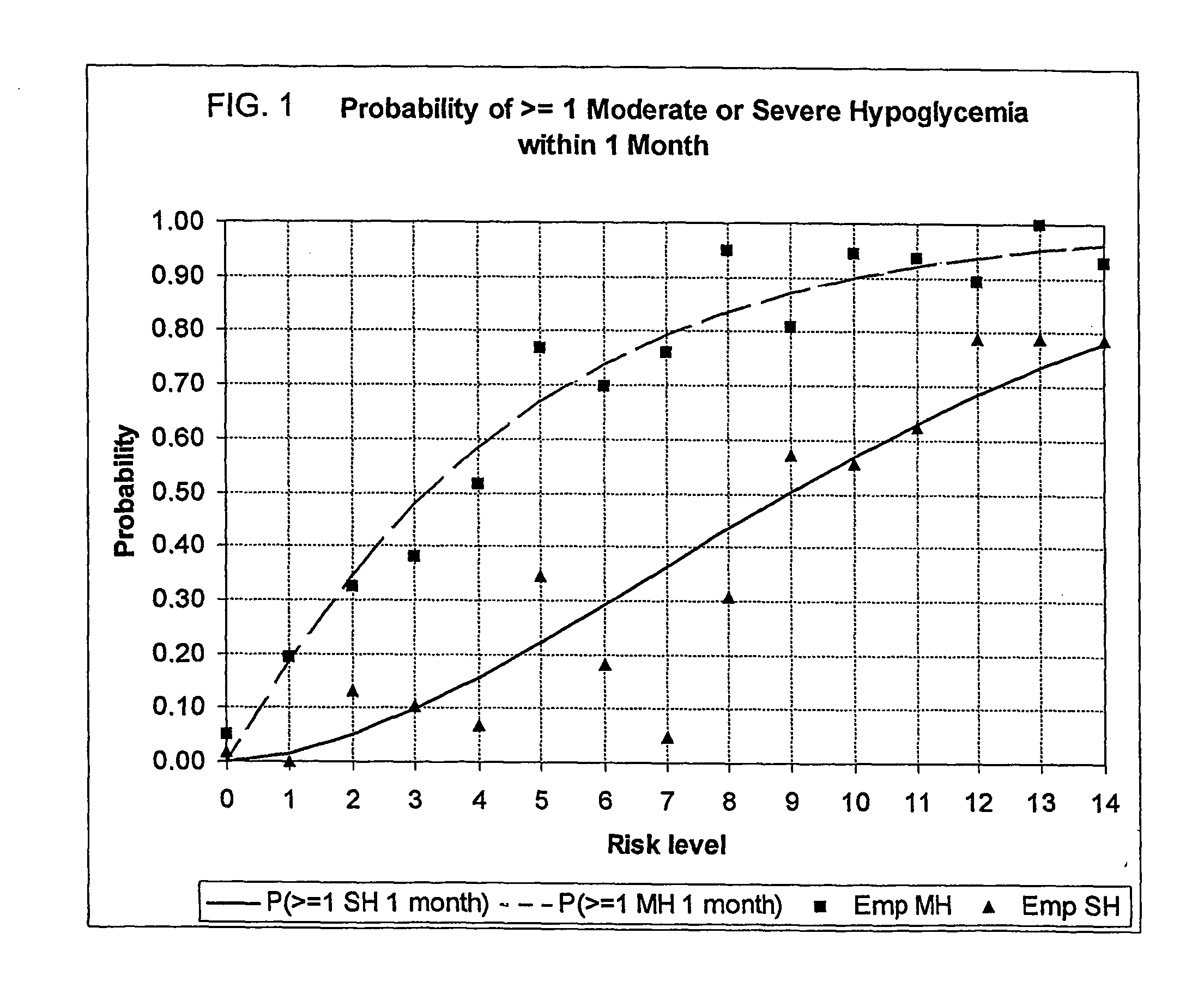
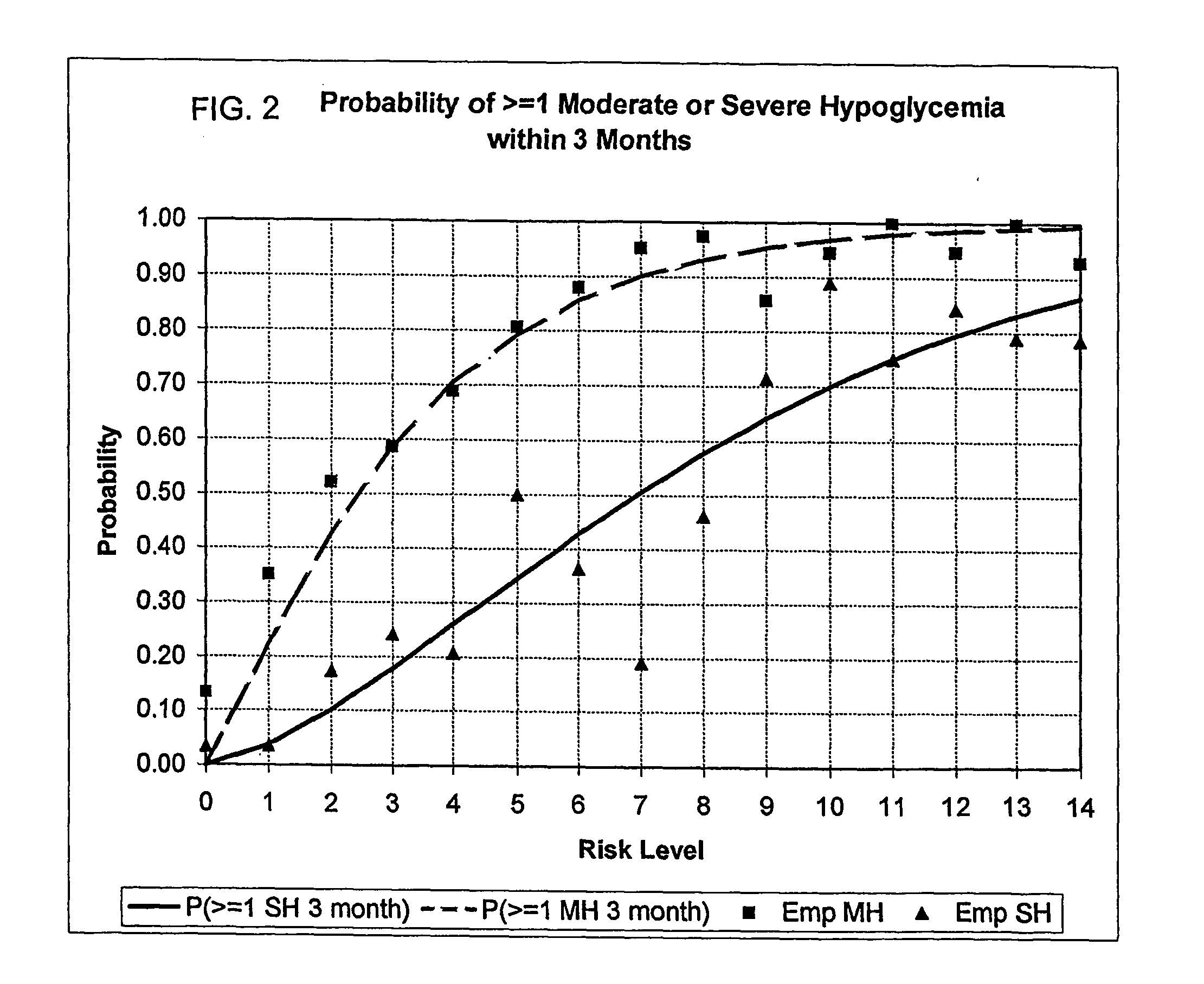
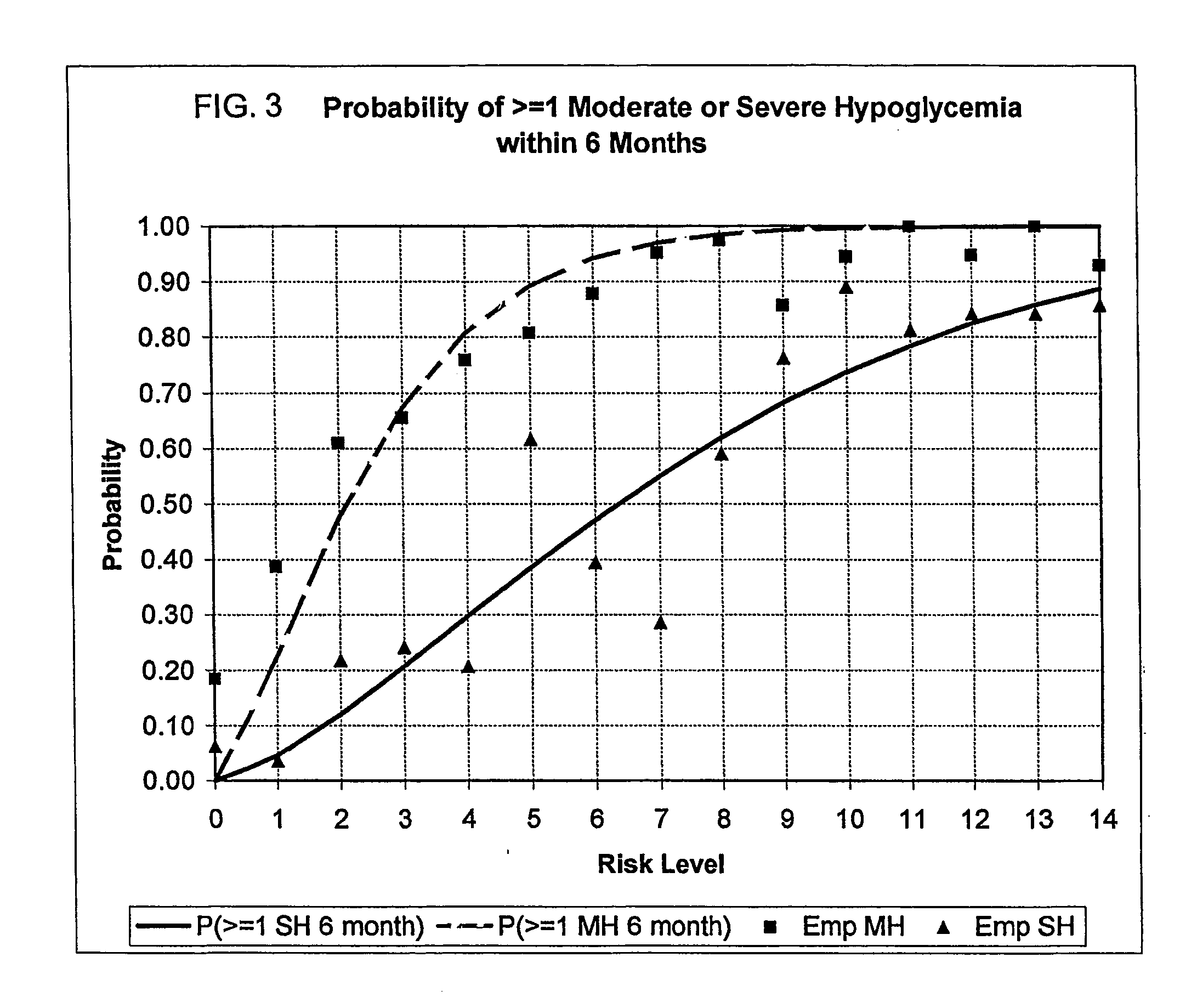
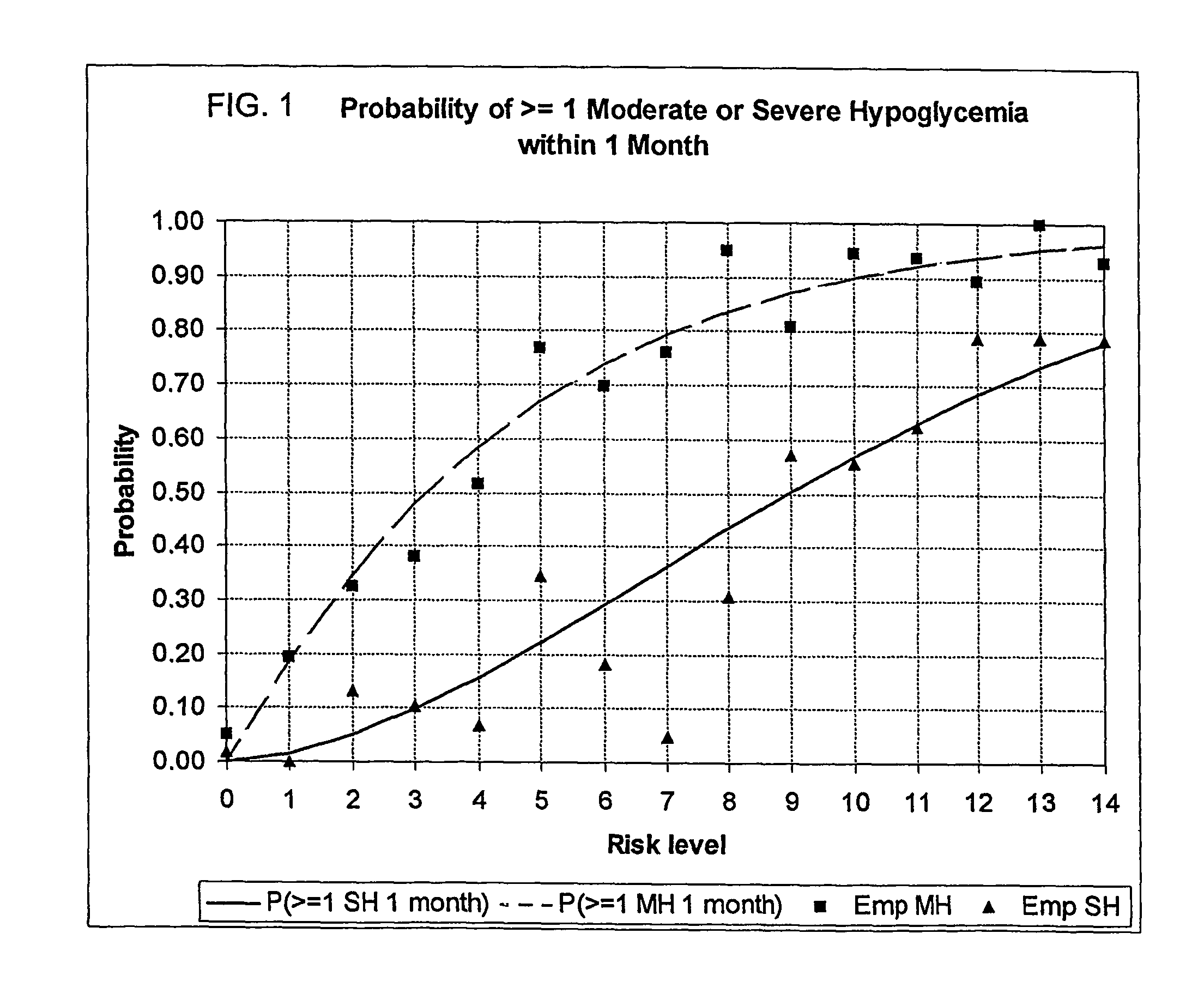
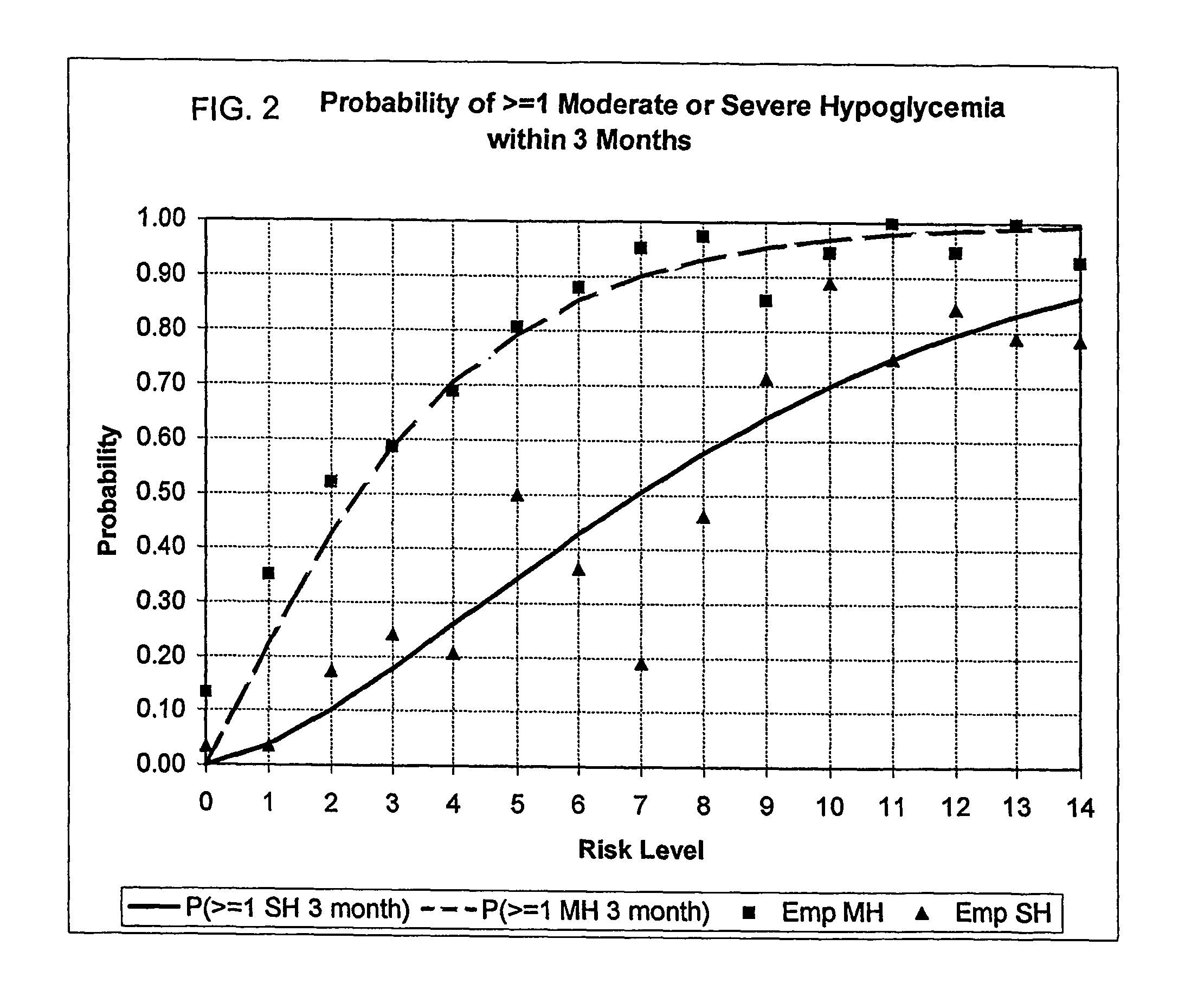
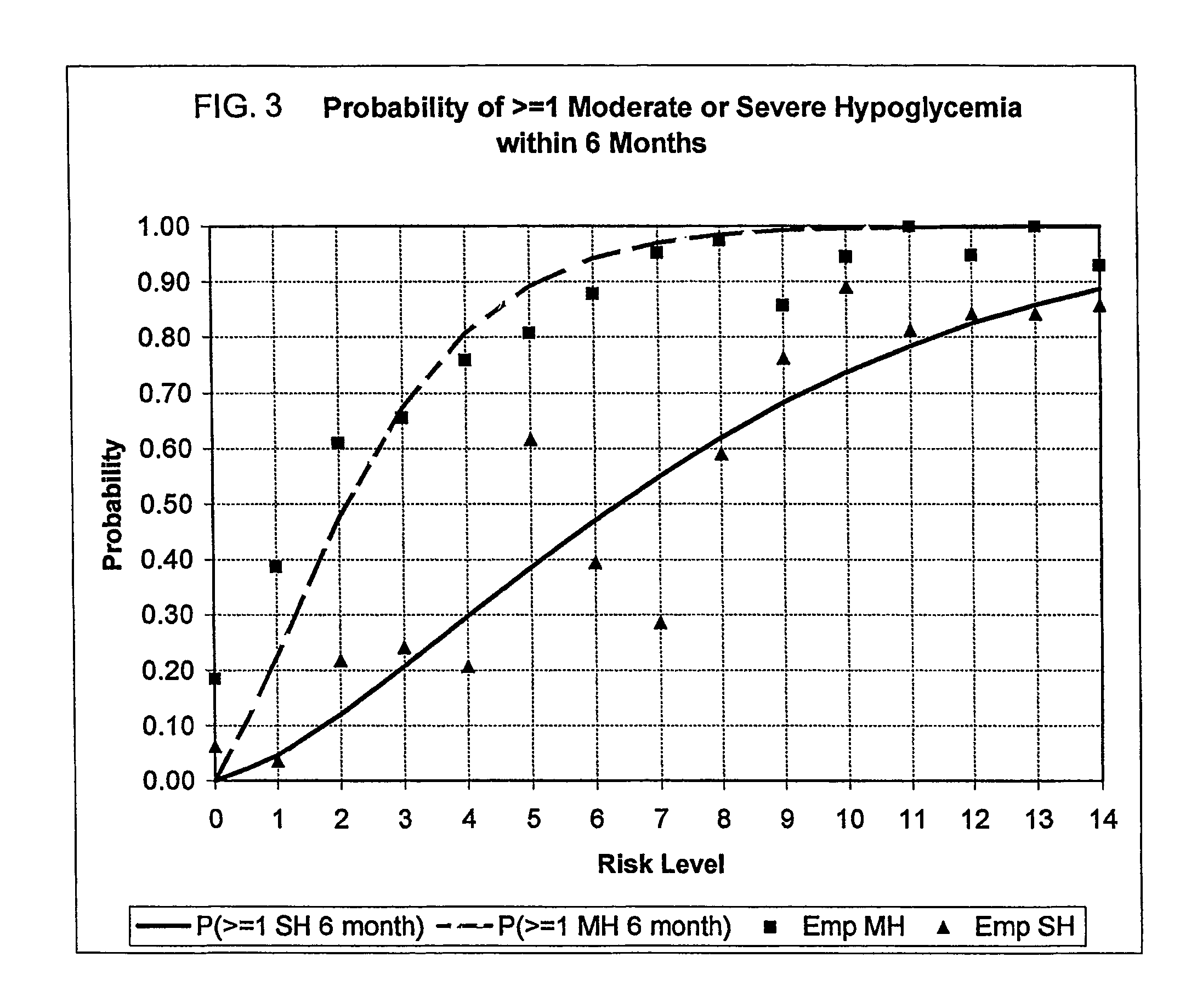
A method, system, and computer program product related to the maintenance of optimal control of diabetes, and is directed to predicting the long-term exposure to hyperglycemia, and the long-term and short-term risks of severe or moderate hypoglycemia in diabetics, based on blood blucose readings collected by a self-monitoring blood glucose device. The method, system, and computer program product pertain directly to the enhancement of existing home blood glucose monitoring devices, by introducing an intelligent data interpretation component capable of predicting both HbA1c and periods of increased risk of hypoglycemia, and to the enhancement of emerging continuous monitoring devices by the same features. With these predictions the diabetic can take steps to prevent the adverse consequences associated with hyperglycemia and hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Glycemic control monitoring using implantable medical device
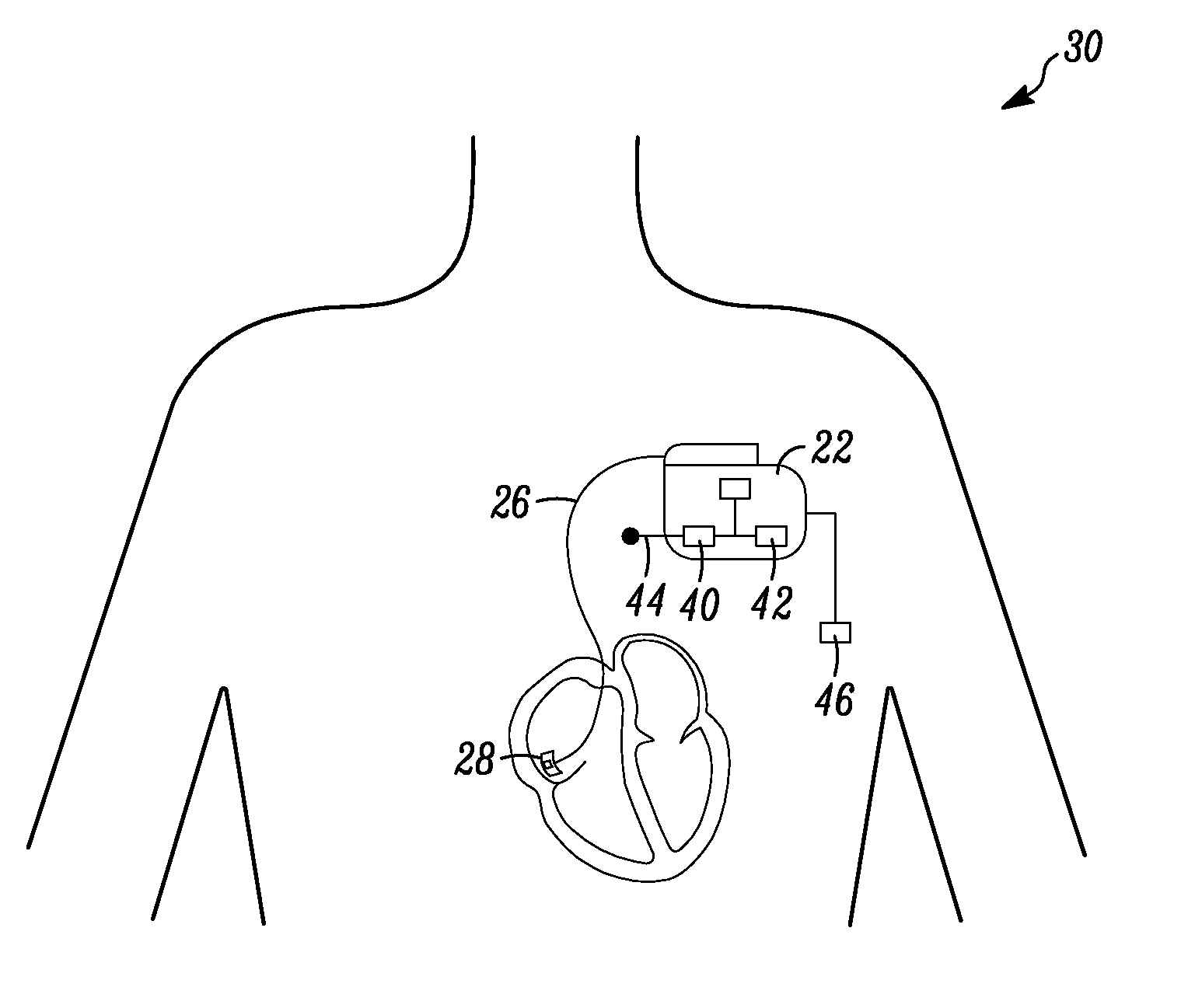
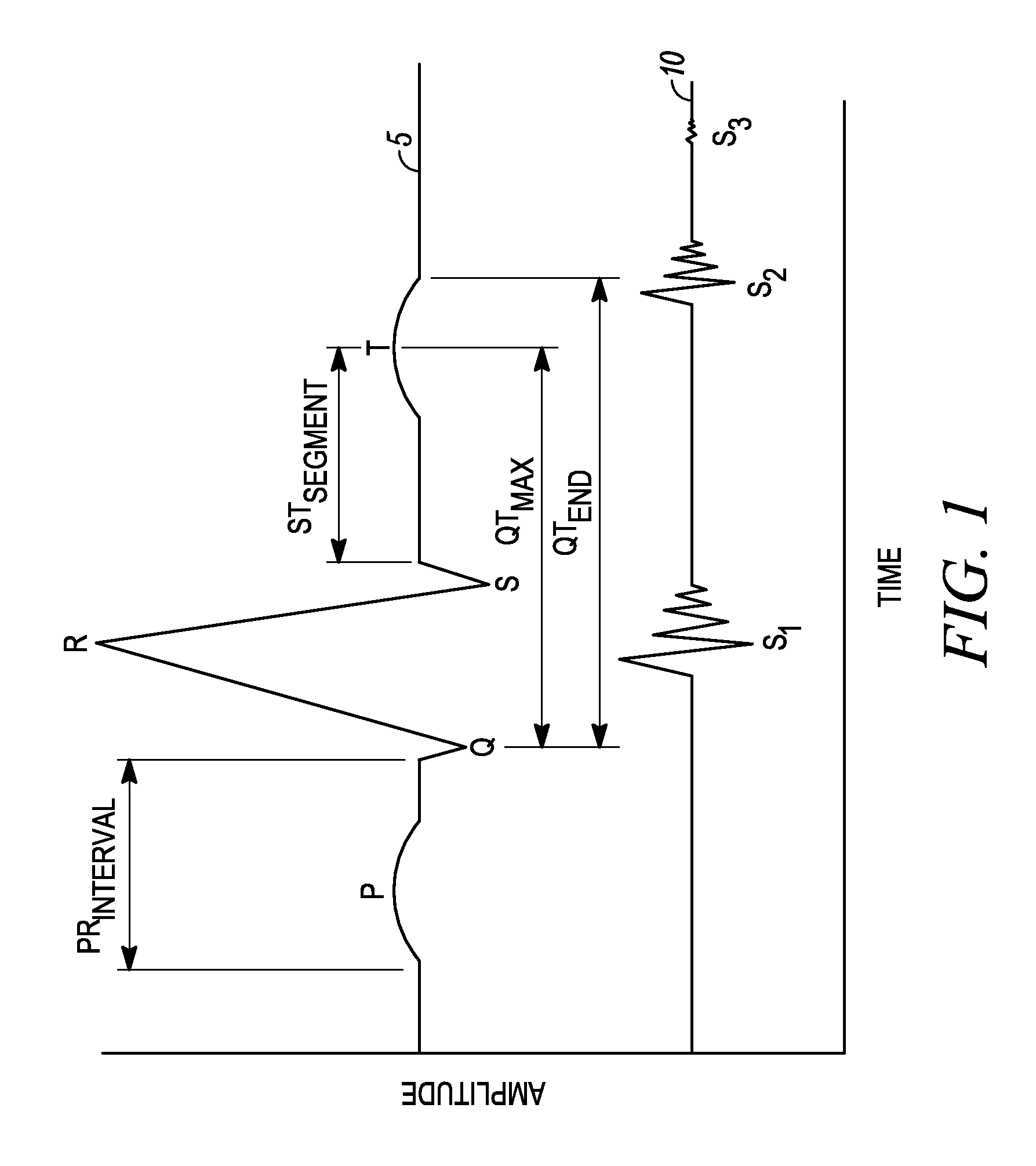
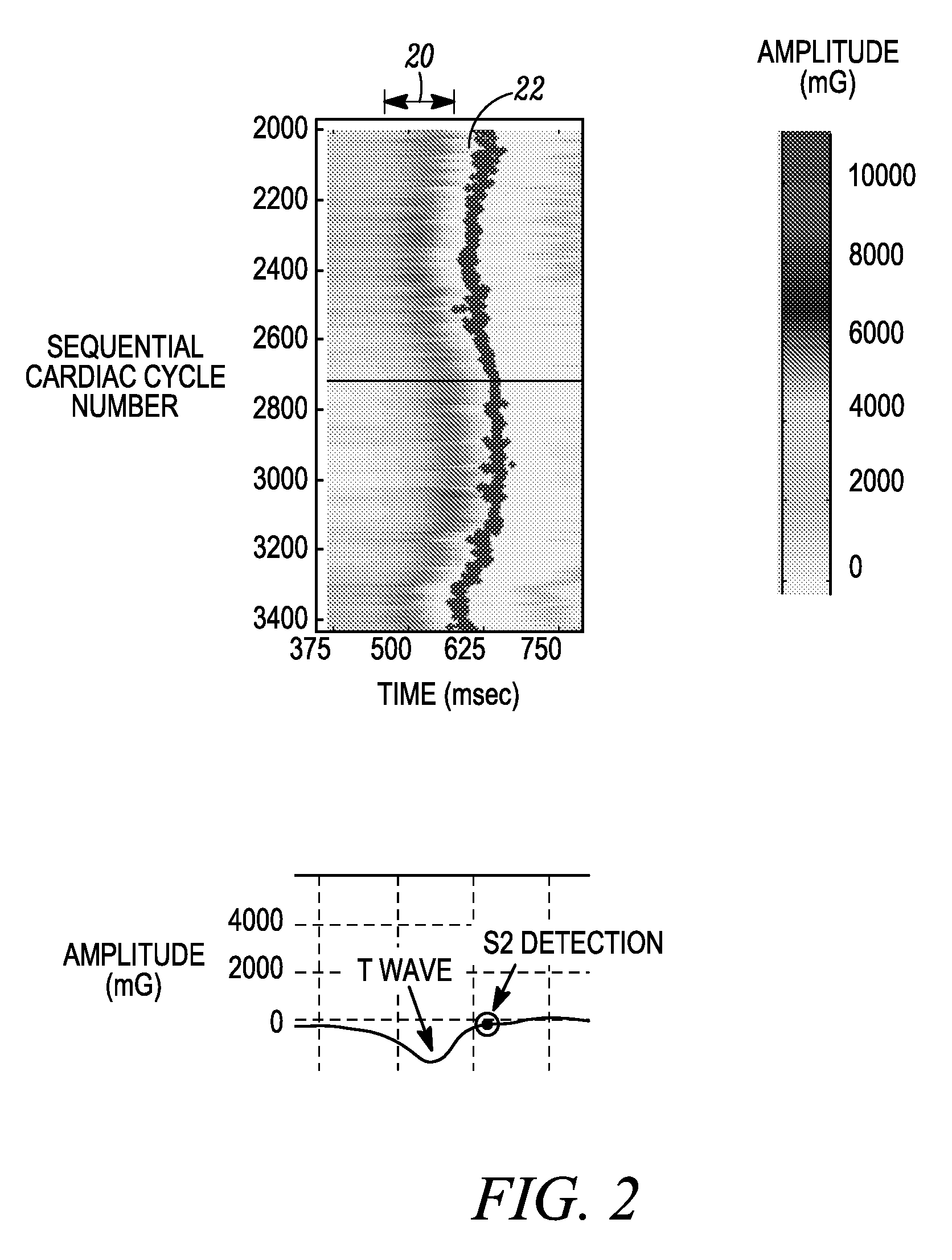
An apparatus for monitoring a patient's blood glucose level. The apparatus includes an implantable medical device having a controller and an implantable heart sounds sensor configured to transmit signals to the controller of the implantable medical device. The controller is configured to determine if a patient is hypoglycemic or hyperglycemic based on the signals from the heart sounds sensor. A method is also disclosed that includes sensing the patient's heart sounds, determining the amplitude of the S2 heart sound, determining the length of the interval from the S1 heart sound to the S2max heart sound, determining the length of the interval from the S1 heart sound to the S2end heart sound, and determining the patient's blood glucose status based on the patient's heart sounds.
Owner:CARDIAC PACEMAKERS INC
Methods of monitoring glucose levels in a subject and uses thereof
Methods of frequently monitoring glucose amounts and / or concentrations in a subject who is at risk for hypoglycemia, hyperglycemia, and / or glucose level fluctuations that put the subject at risk are provided. Also provided are methods of monitoring the effects of one or more pharmaceutical compositions on the levels of glucose in a subject.
Owner:ANIMAS TECH +1
Methods of determining pre or post meal time slots or intervals in diabetes management
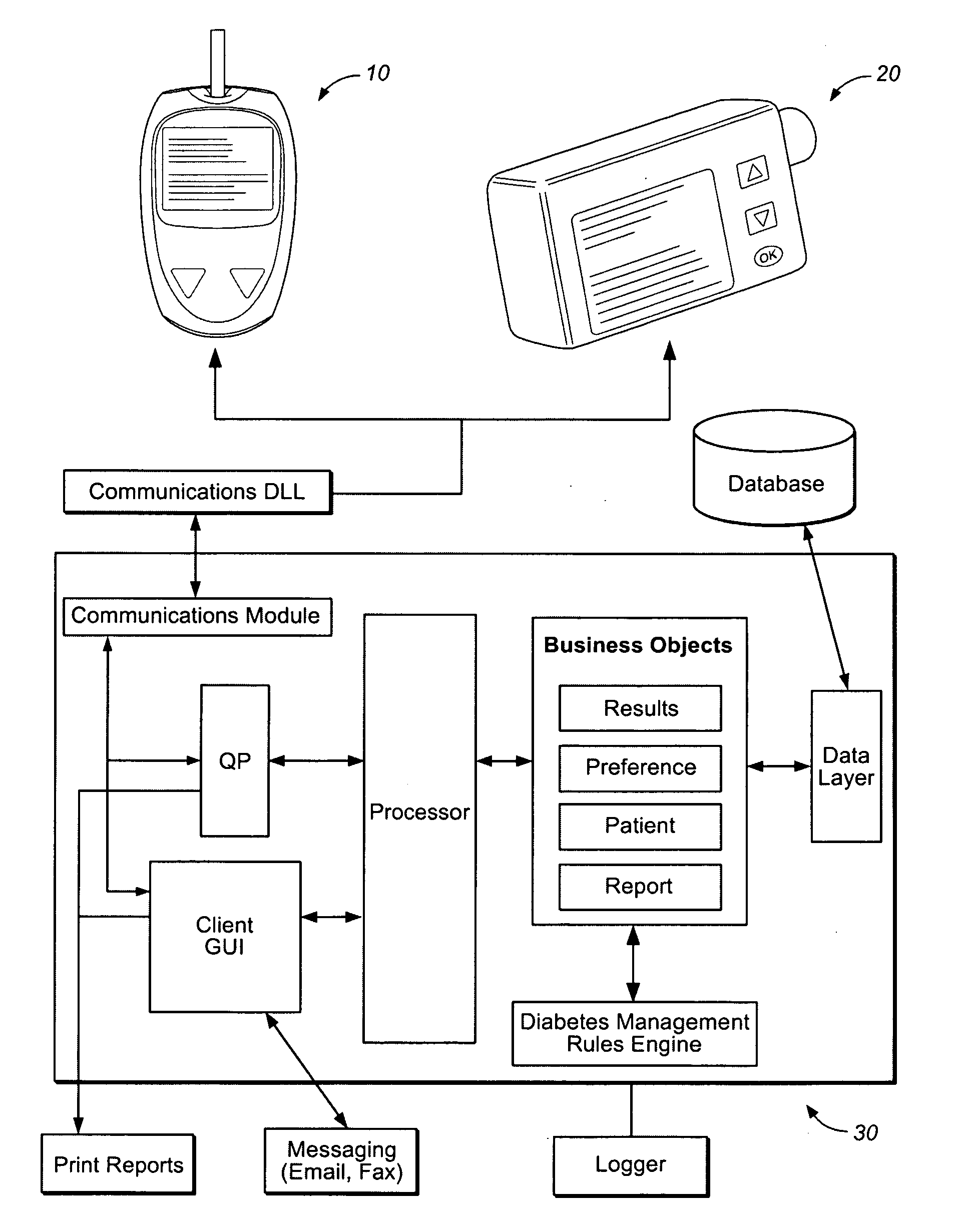
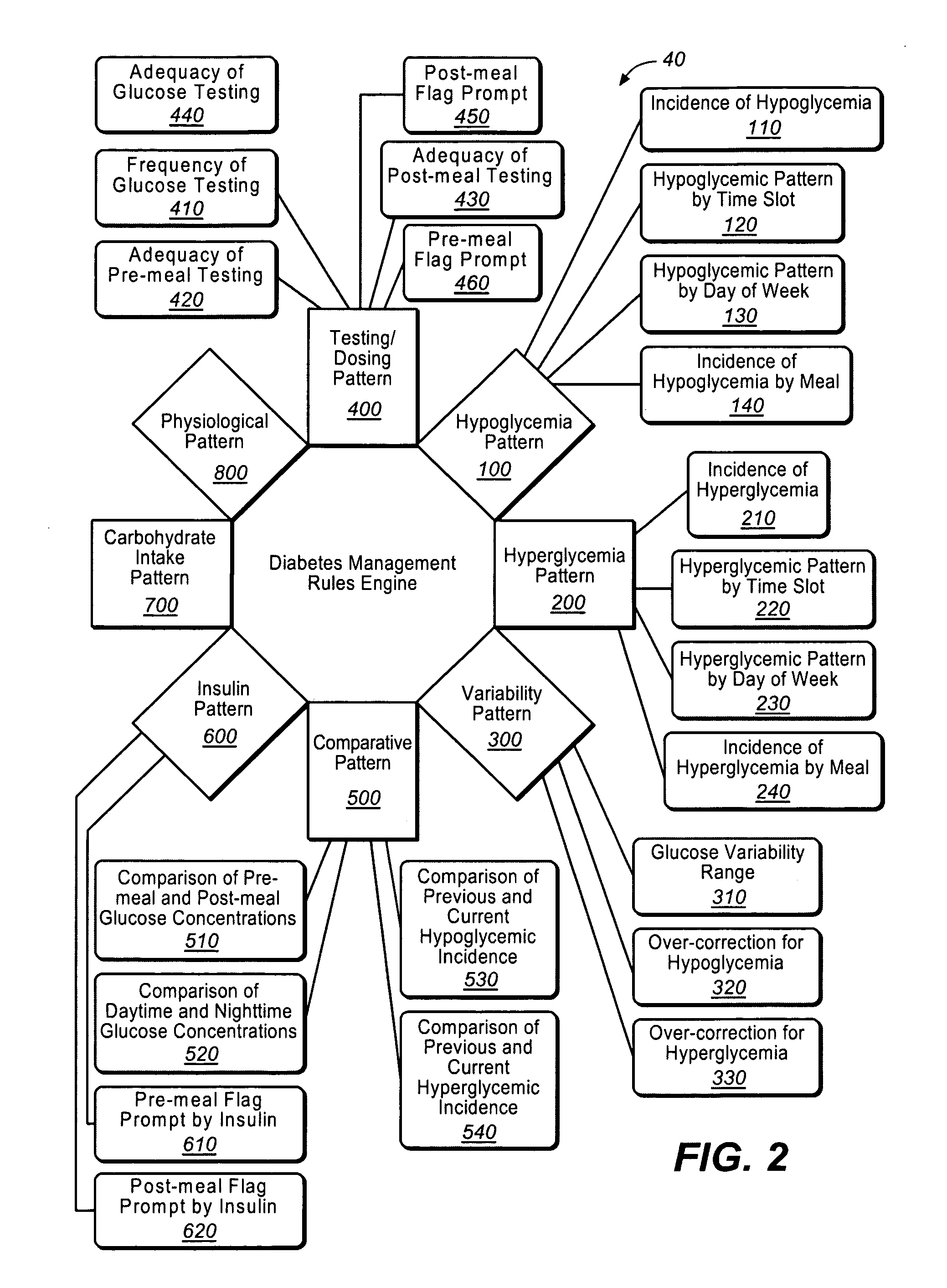
A diabetes management system or process is provided herein that may be used to analyze and recognize patterns for a large number of blood glucose concentration measurements and other physiological parameters related to the glycemia of a patient. In particular, a method of monitoring glycemia in a patient may include storing a patient's data on a suitable device, such as, for example, a blood glucose meter. The patient's data may include blood glucose concentration measurements. The diabetes management system or process may be installed on, but is not limited to, a personal computer, an insulin pen, an insulin pump, or a glucose meter. The diabetes management system or process may identify a plurality of pattern types from the data including a testing / dosing pattern, a hypoglycemic pattern, a hyperglycemic pattern, a blood glucose variability pattern, and a comparative pattern. After identifying a particular pattern with the data management system or process, a warning message may be displayed on a screen of a personal computer or a glucose meter. Other messages can also be provided to ensure compliance of any prescribed diabetes regiments or to guide the patient in managing the patient's diabetes.
Owner:LIFESCAN INC
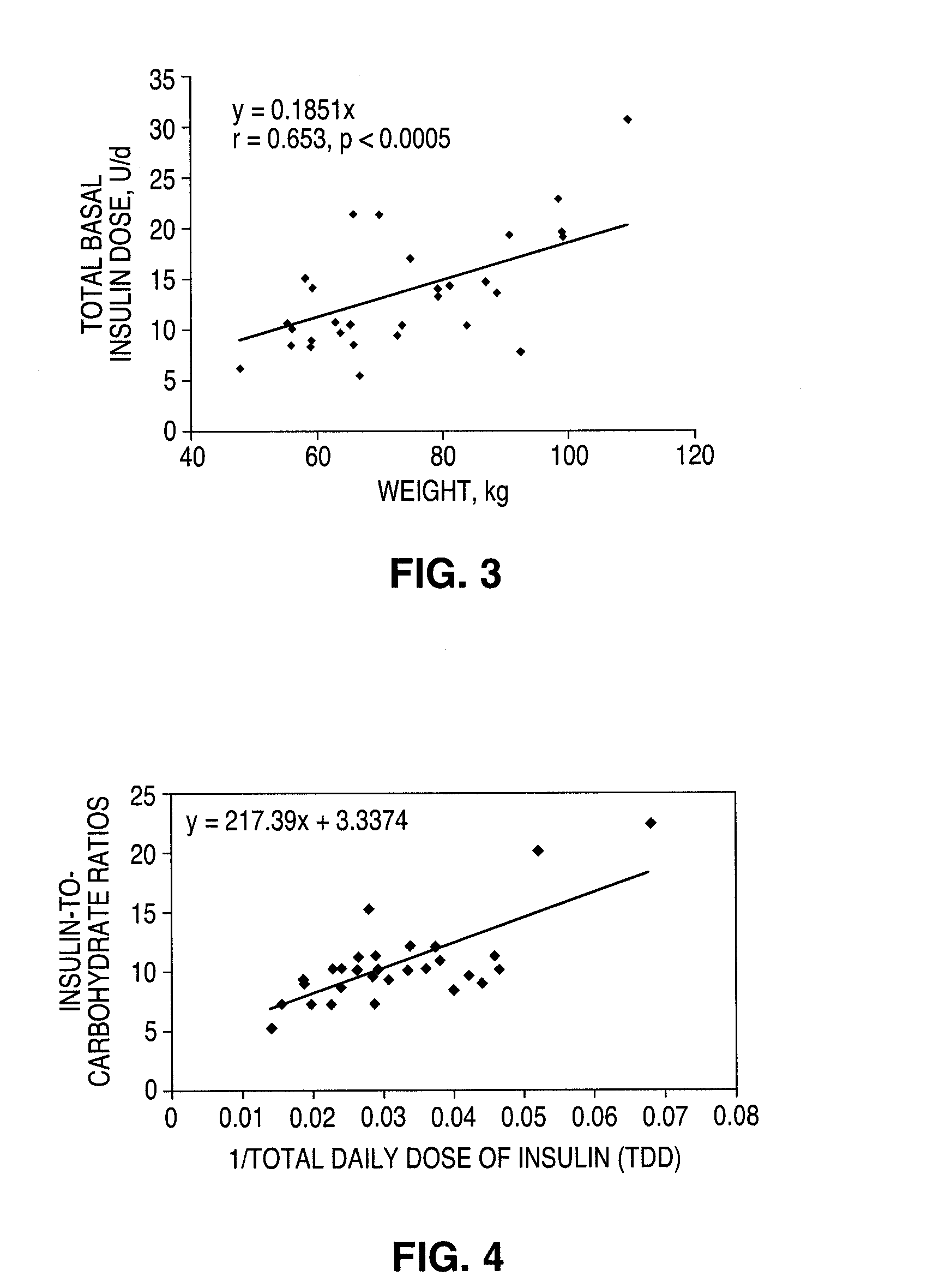
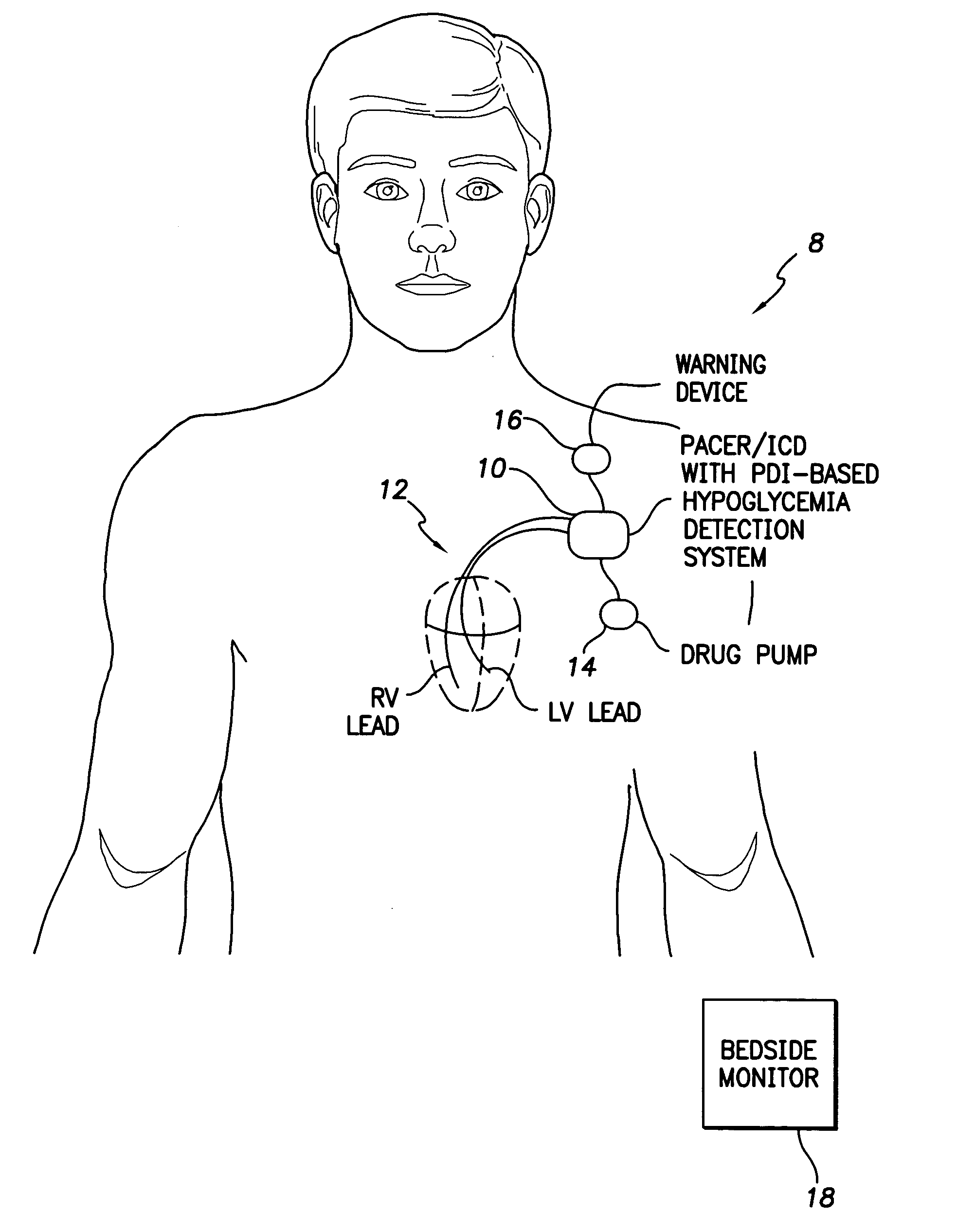
Continuous glucose monitoring-directed adjustments in basal insulin rate and insulin bolus dosing formulas
InactiveUS20090036753A1Accurate estimateAppropriate confidence and self-sufficiencySurgeryDiagnostic recording/measuringInsulin dependentHypoglycemic episodes
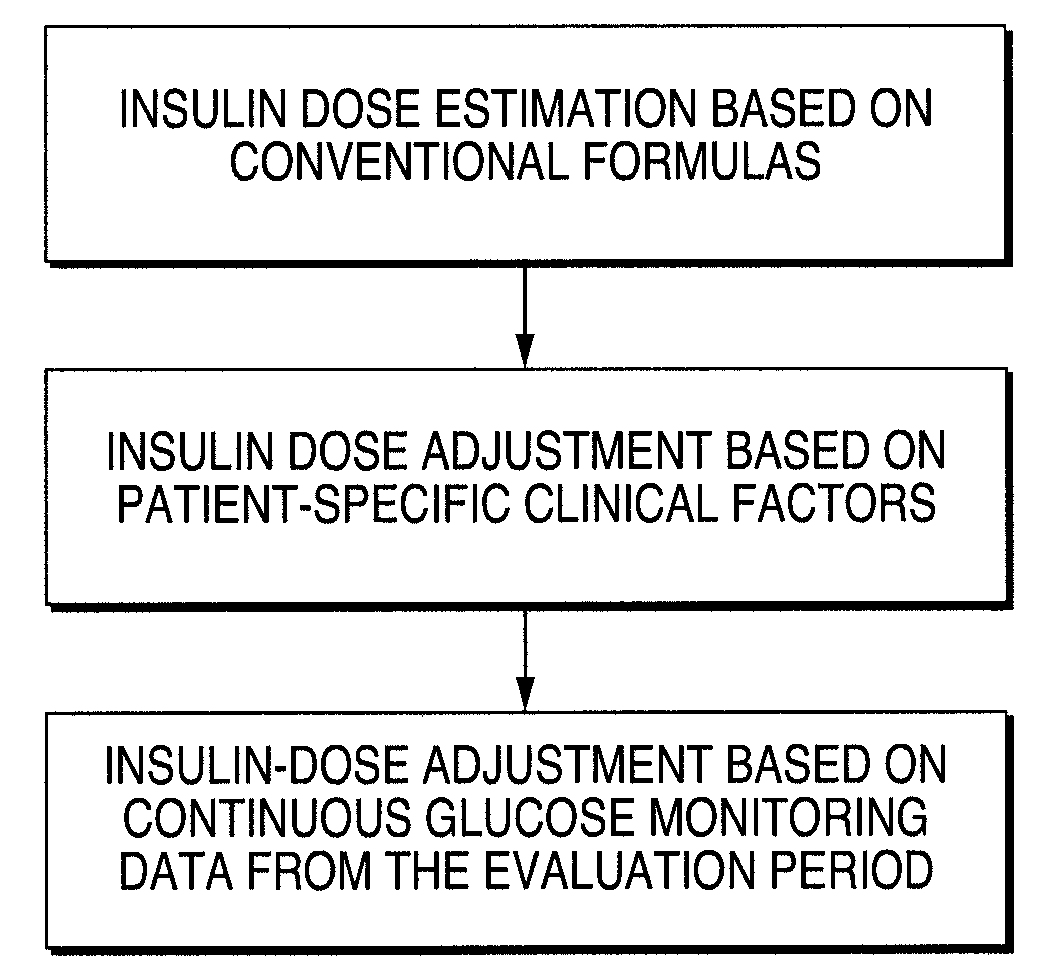
A method for individualized management of diabetes in insulin-dependent patients provides a period of evaluation as the patient adheres to a structured pattern of eating, sleeping, and physical activity. Glucose is monitored with a continuous glucose monitoring system, insulin doses are metered, carbohydrate consumption is quantified, and glucose, carbohydrate, and insulin data are collected and analyzed. Insulin dosage is adjusted in three steps: (1) an insulin dosage is estimated from conventional formulas, (2) adjustments are made according to the patient's clinical specifics, and (3) further insulin dose adjustments are made according to glucose data obtained during the evaluation period. By the end of the evaluation period, substantially normal glucose values are achieved, and quantitative relationships from data are calculated that are then applied to determine insulin dosages for an ensuing period of therapy. By this method, diabetic patients achieve a near normal glycemic profile, and without significant occurrence of hypoglycemic episodes.
Owner:DIABETES CARE CENT
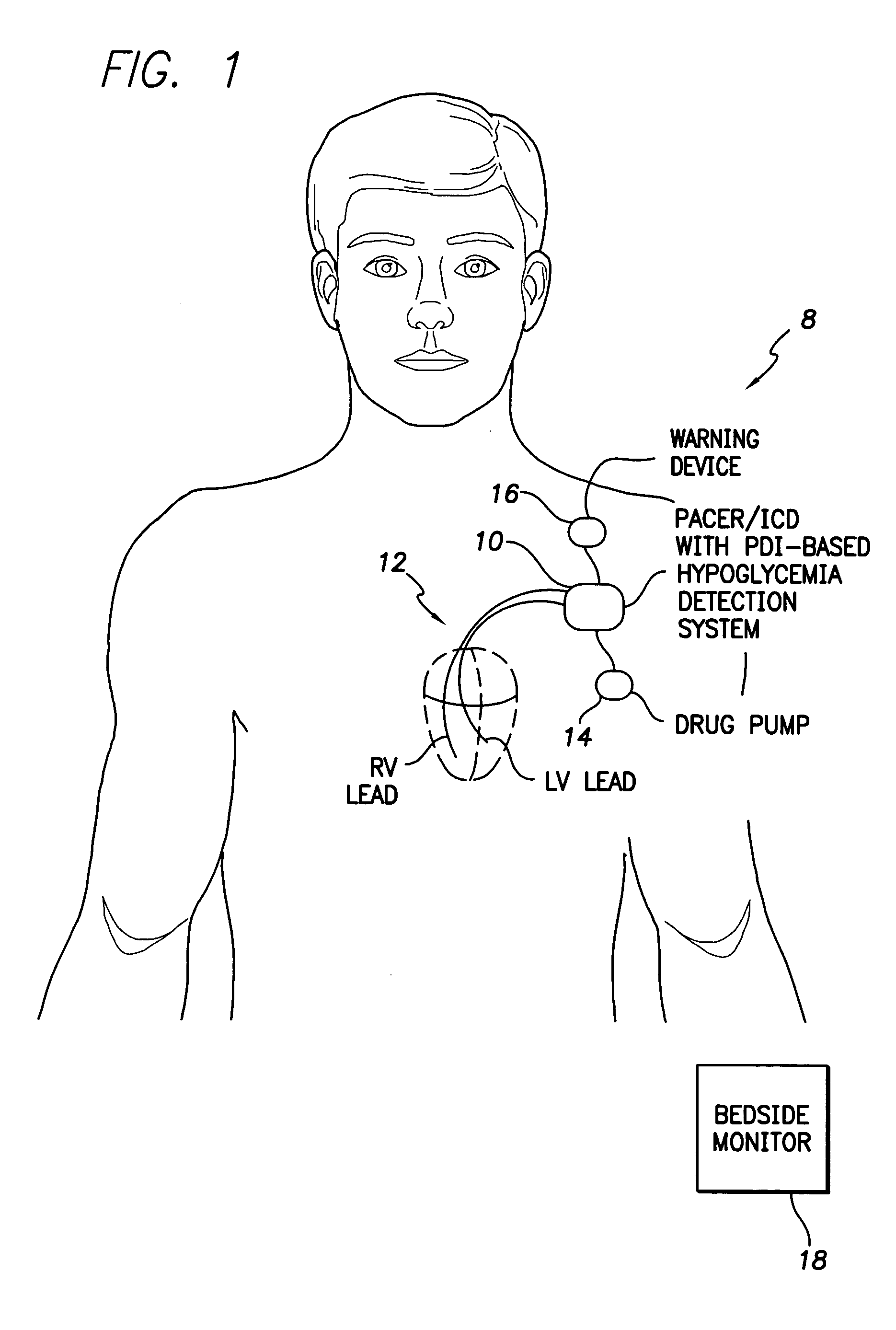
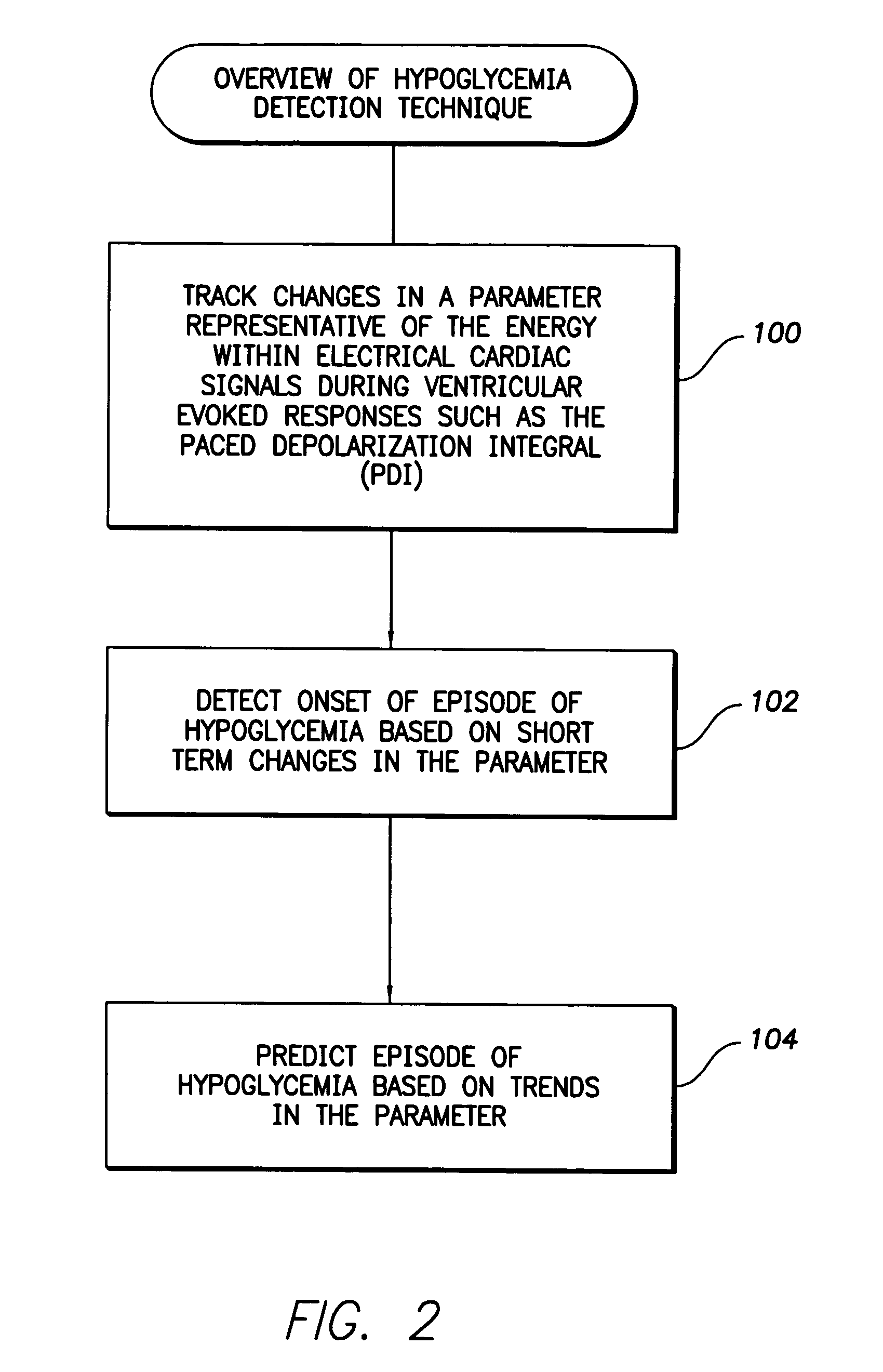
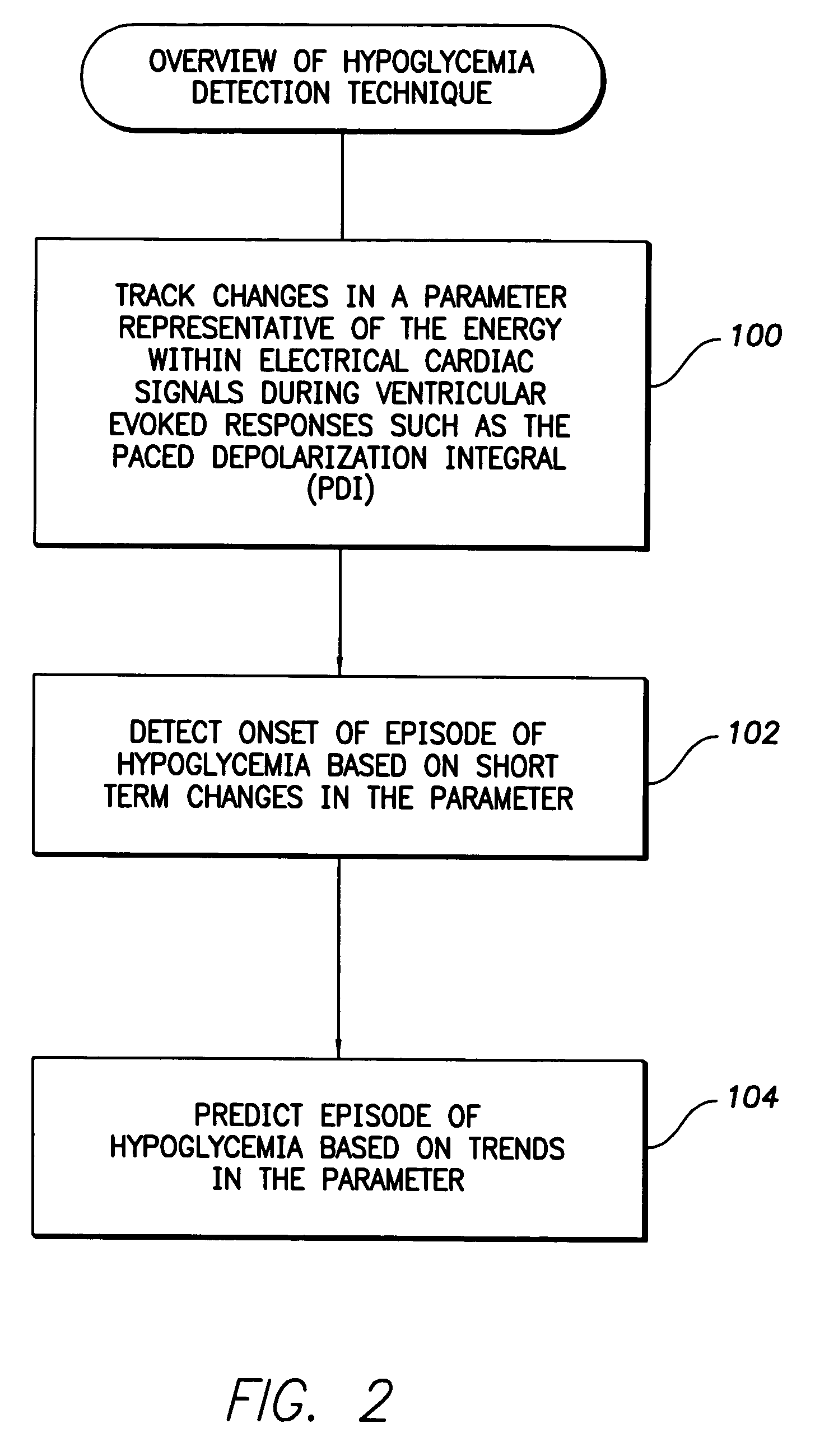
System and method for detecting hypoglycemia based on a paced depolarization integral using an implantable medical device
ActiveUS20060247685A1Improve blood sugar controlReduce deliveryElectrocardiographyMedical devicesCardiac pacemaker electrodeInsulin dependent
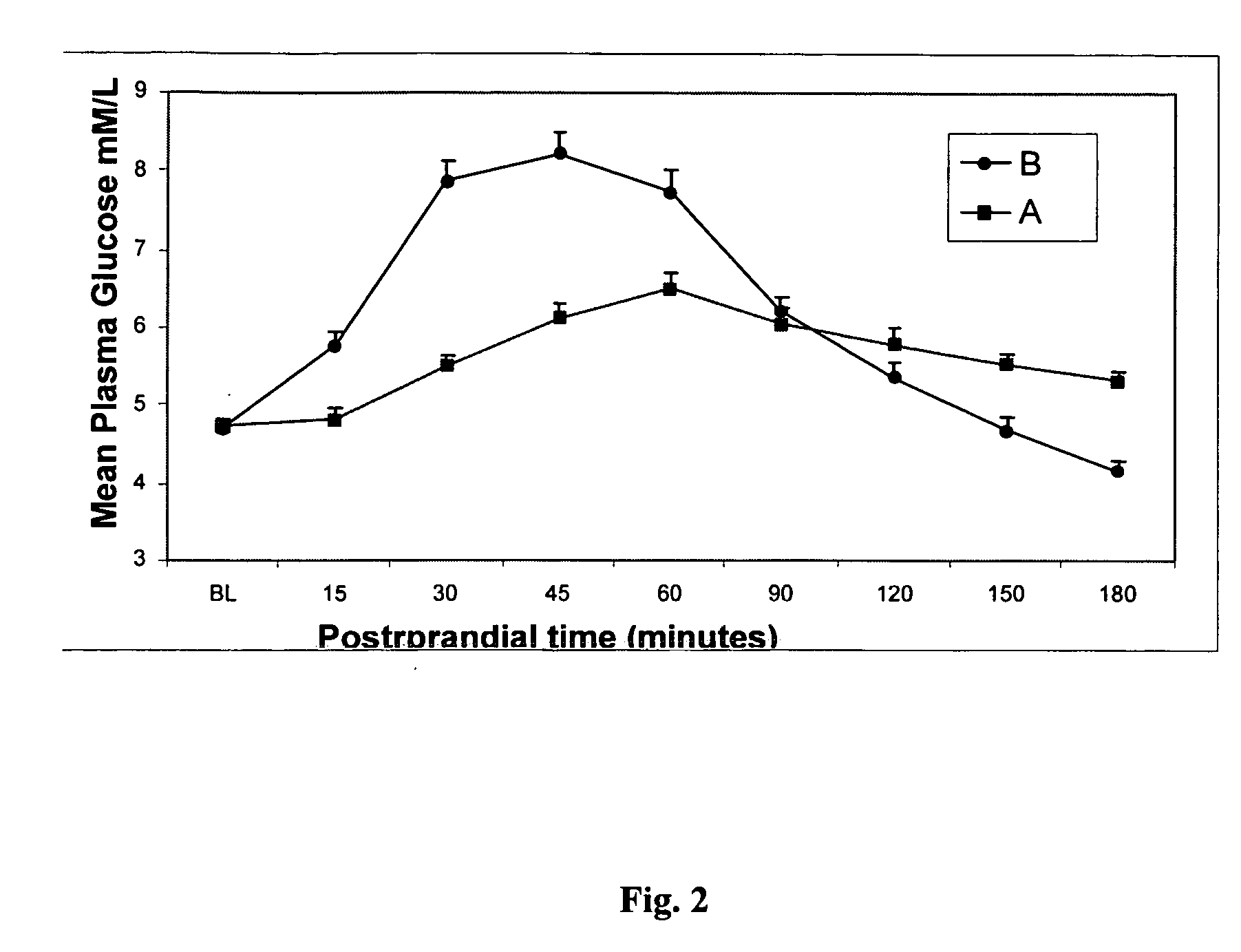
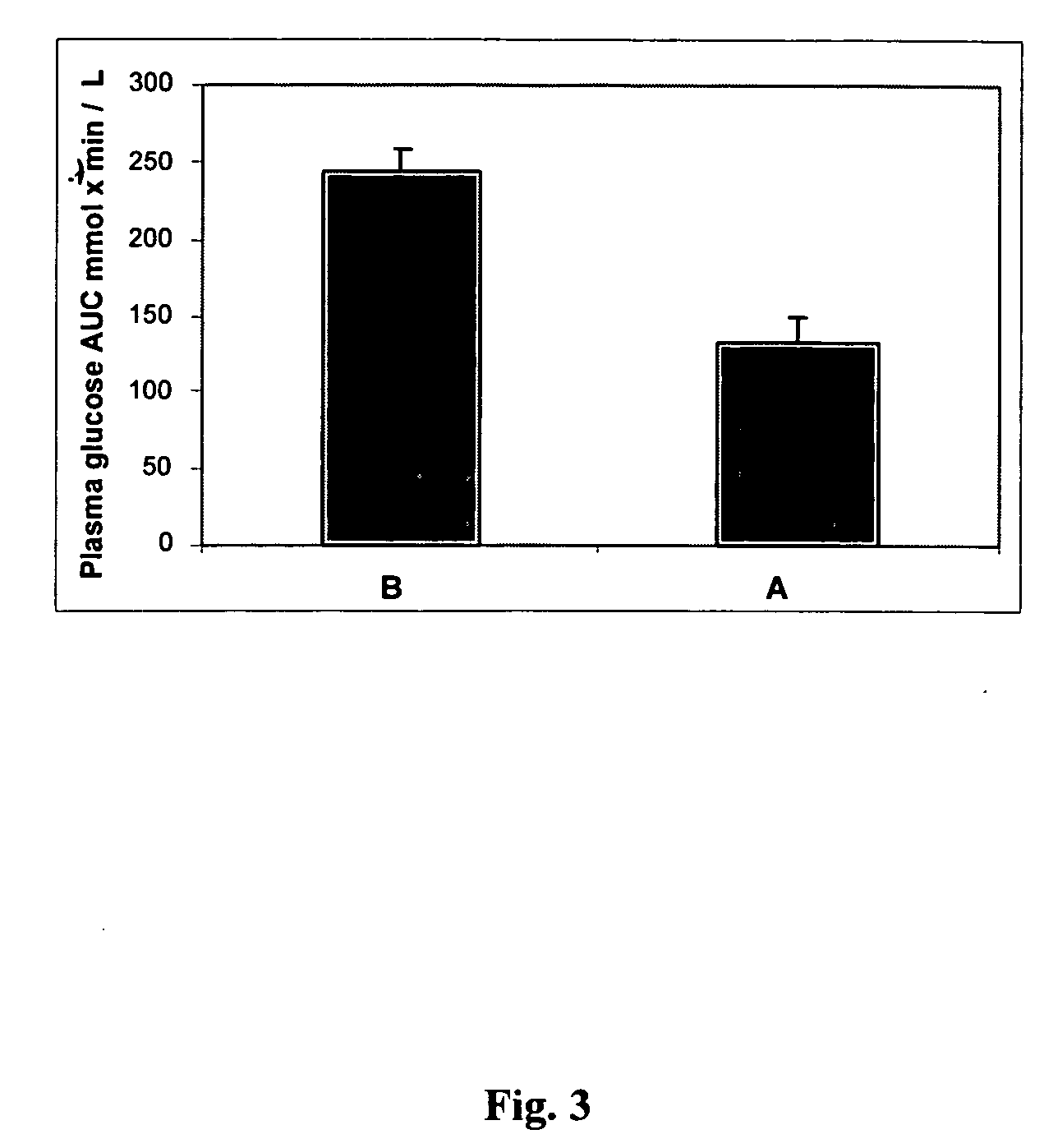
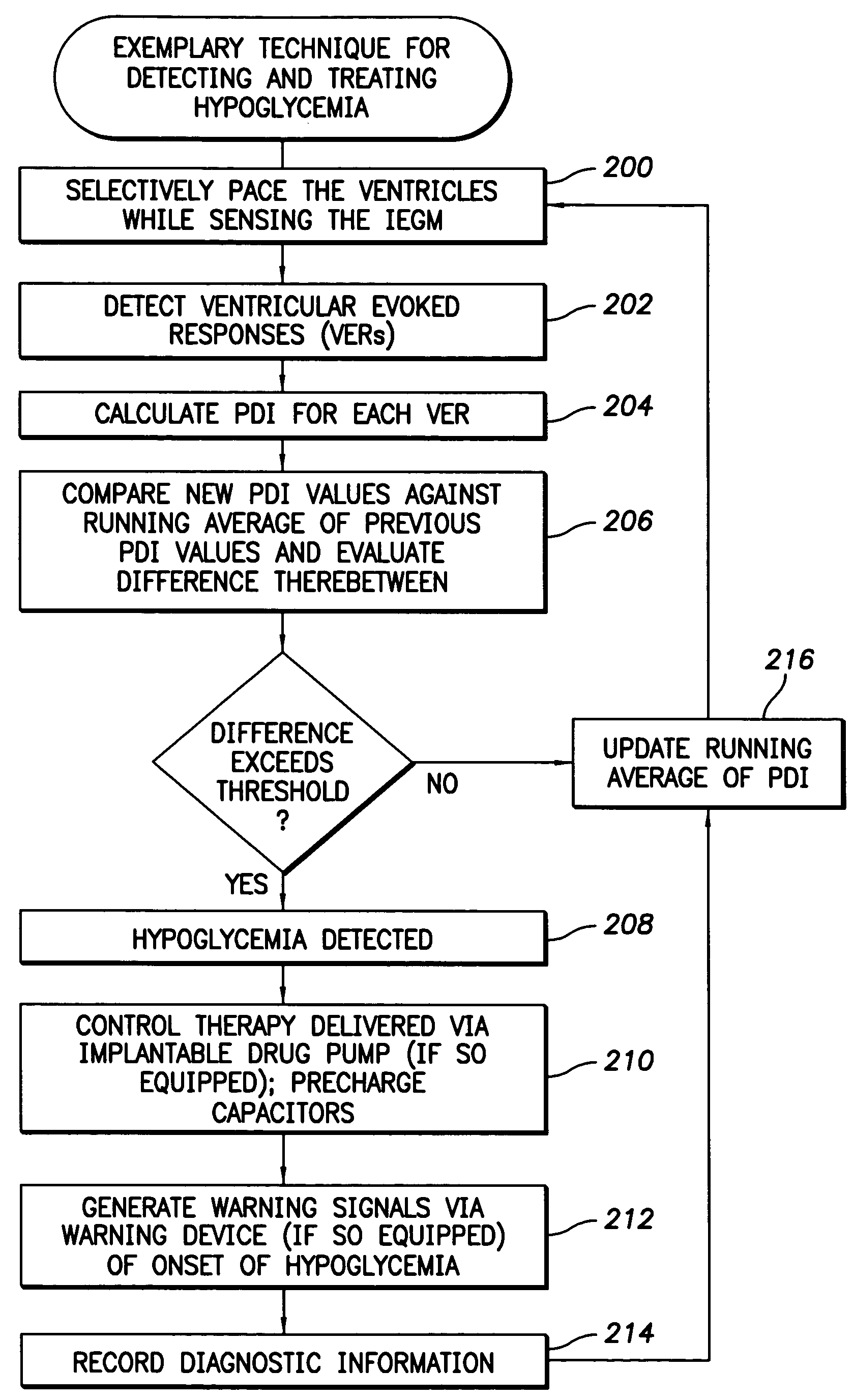
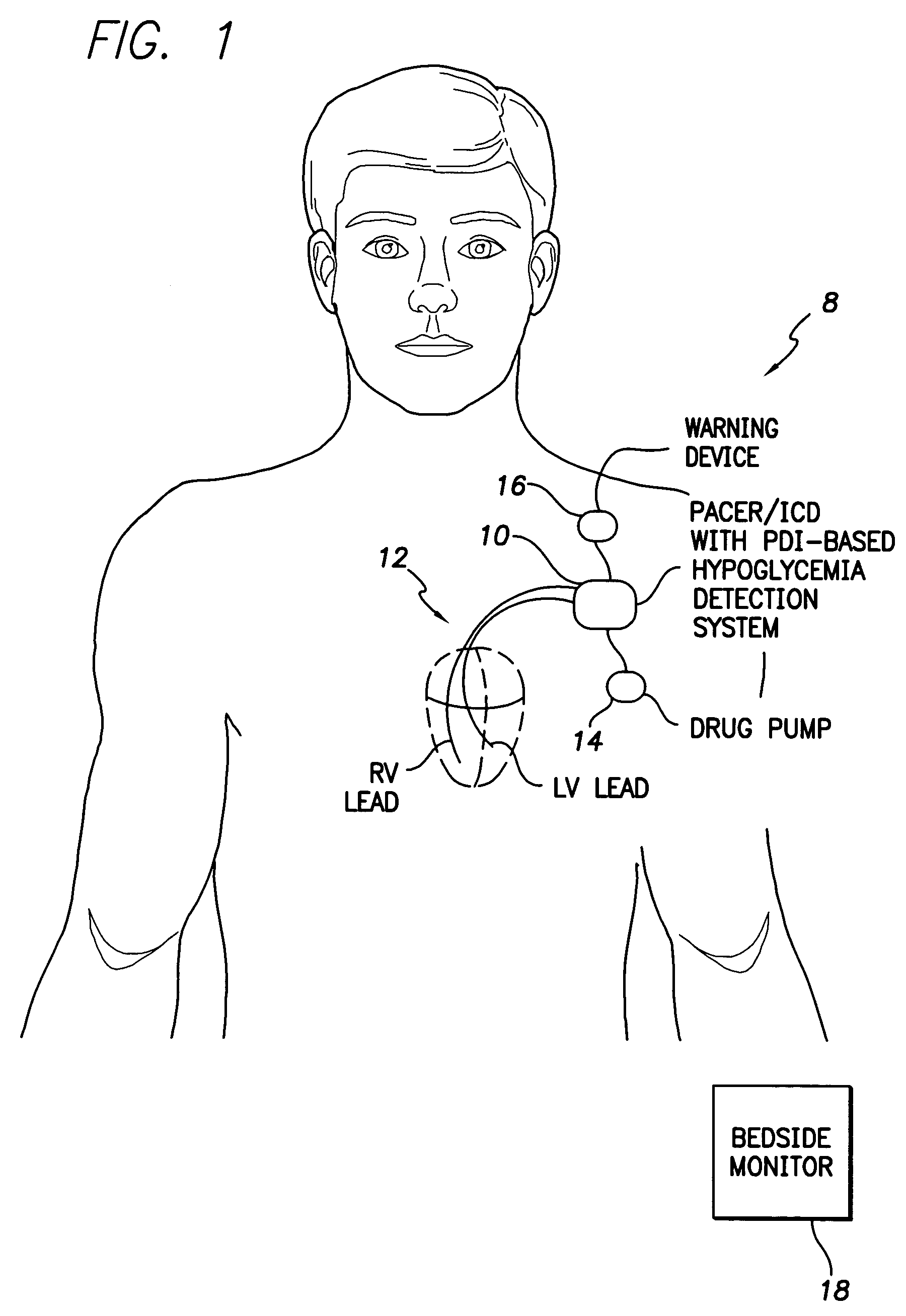
Techniques are provided for use with an implantable medical device such as a pacemaker or implantable cardioverter / defibrillator (ICD) for predicting and detecting hypoglycemia. In one example, the device tracks changes in a paced depolarization integral (PDI). A significant increase in PDI over a relatively short period of time indicates the onset of hypoglycemia (this can also be confirmed with QT changes). Upon detection of hypoglycemia, appropriate warning signals are generated to alert the patient. Certain therapies automatically provided by the implantable device may also be controlled in response to hypoglycemia. For example, if the patient is an insulin-dependent diabetic and the implantable device is equipped with an insulin pump capable of delivering insulin directly into the bloodstream, insulin delivery is automatically suspended until blood glucose levels return to acceptable levels. If the device is an ICD, it may be controlled to begin charging defibrillation capacitors upon detection of hypoglycemia so as to permit prompt delivery of a defibrillation shock, which may be needed if hypoglycemia triggers ventricular fibrillation. The detection techniques may be used in conjunction with other hypoglycemia detection techniques to improve detection specificity.
Owner:PACESETTER INC
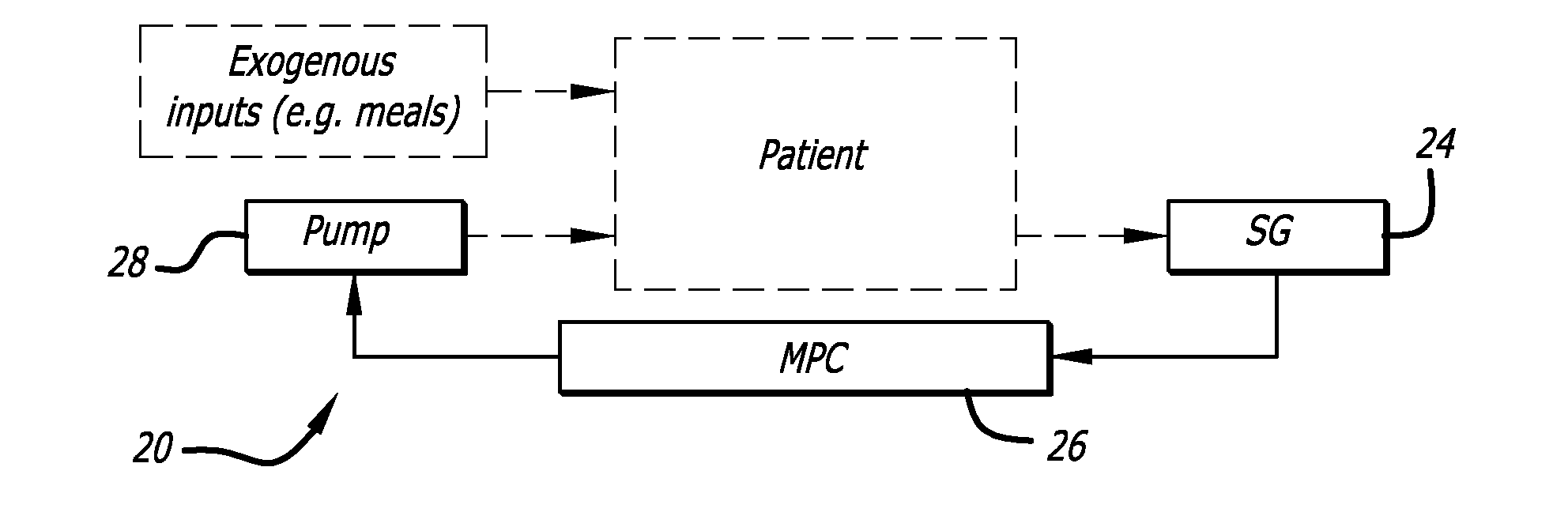
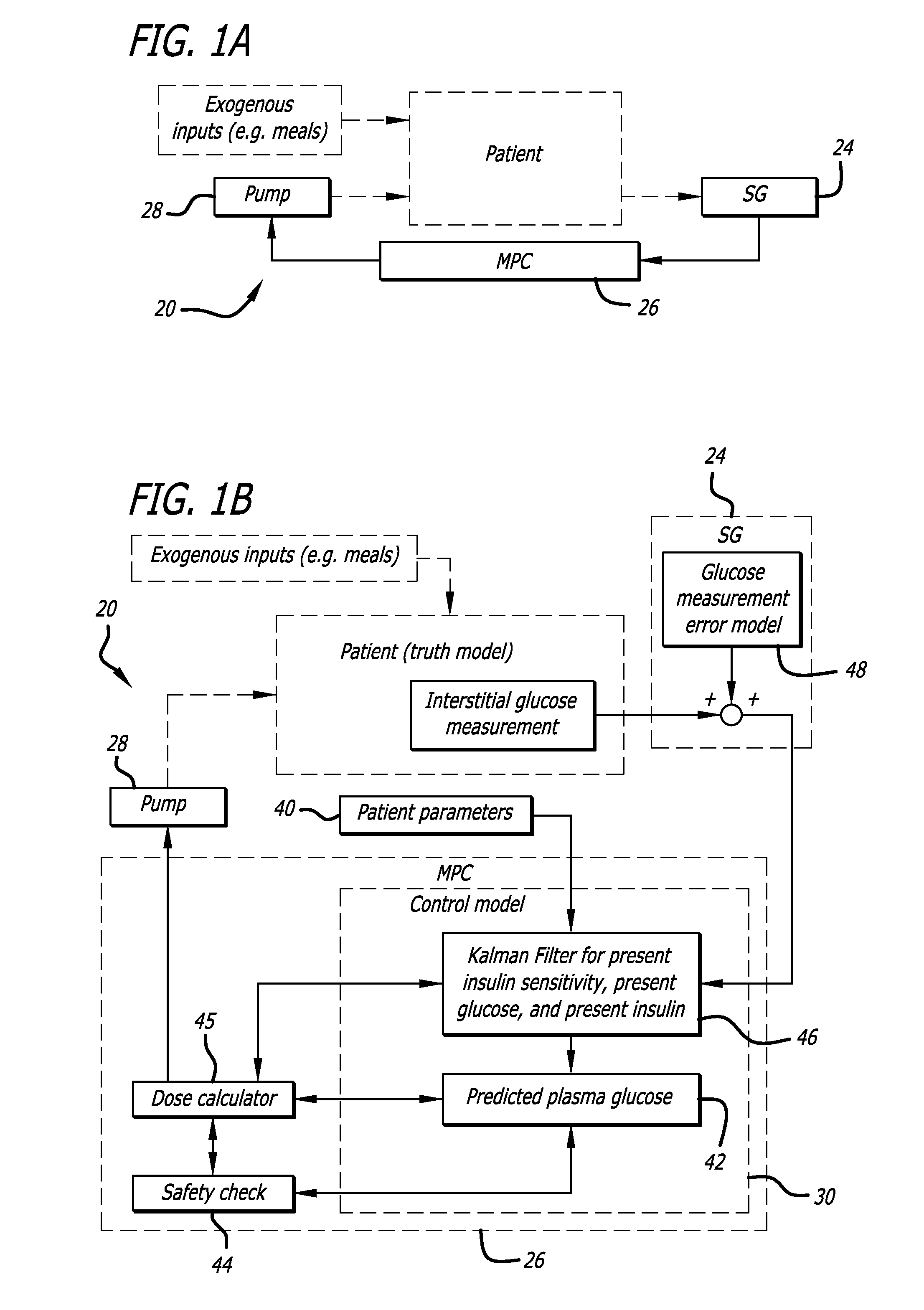
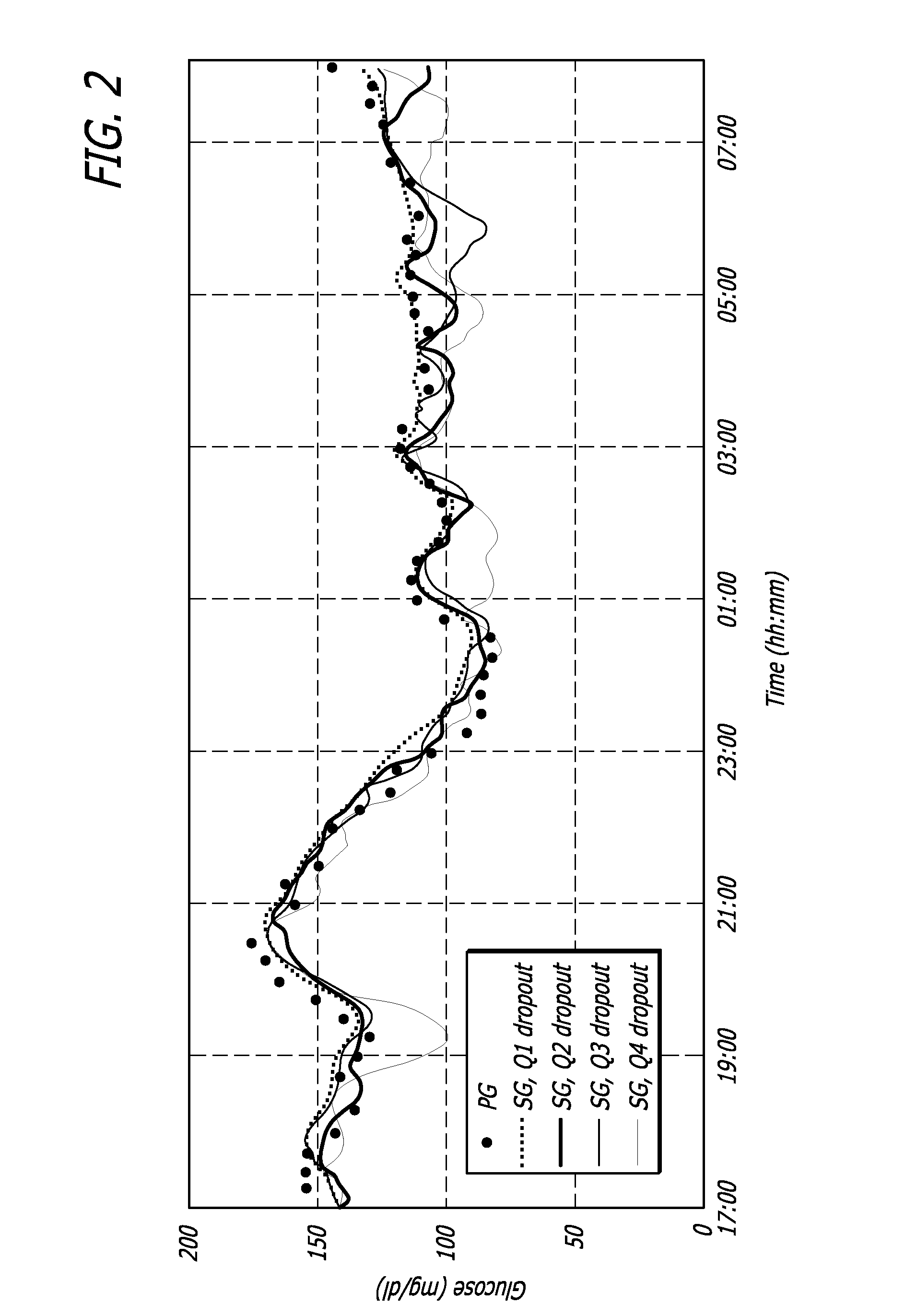
Overnight closed-loop insulin delivery with model predictive control and glucose measurement error model
ActiveUS20100280441A1Reducing insulin deliveryReduce deliveryDrug and medicationsMedical devicesGlucose sensorsClosed loop
A closed-loop system for insulin infusion overnight uses a model predictive control algorithm (“MPC”). Used with the MPC is a glucose measurement error model which was derived from actual glucose sensor error data. That sensor error data included both a sensor artifacts component, including dropouts, and a persistent error component, including calibration error, all of which was obtained experimentally from living subjects. The MPC algorithm advised on insulin infusion every fifteen minutes. Sensor glucose input to the MPC was obtained by combining model-calculated, noise-free interstitial glucose with experimentally-derived transient and persistent sensor artifacts associated with the FreeStyle Navigator® Continuous Glucose Monitor System (“FSN”). The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simulated overnight closed-loop control with the MPC algorithm using the glucose measurement error model suggesting that the continuous glucose monitoring technologies facilitate safe closed-loop insulin delivery.
Owner:CAMBRIDGE ENTERPRISE LTD +1
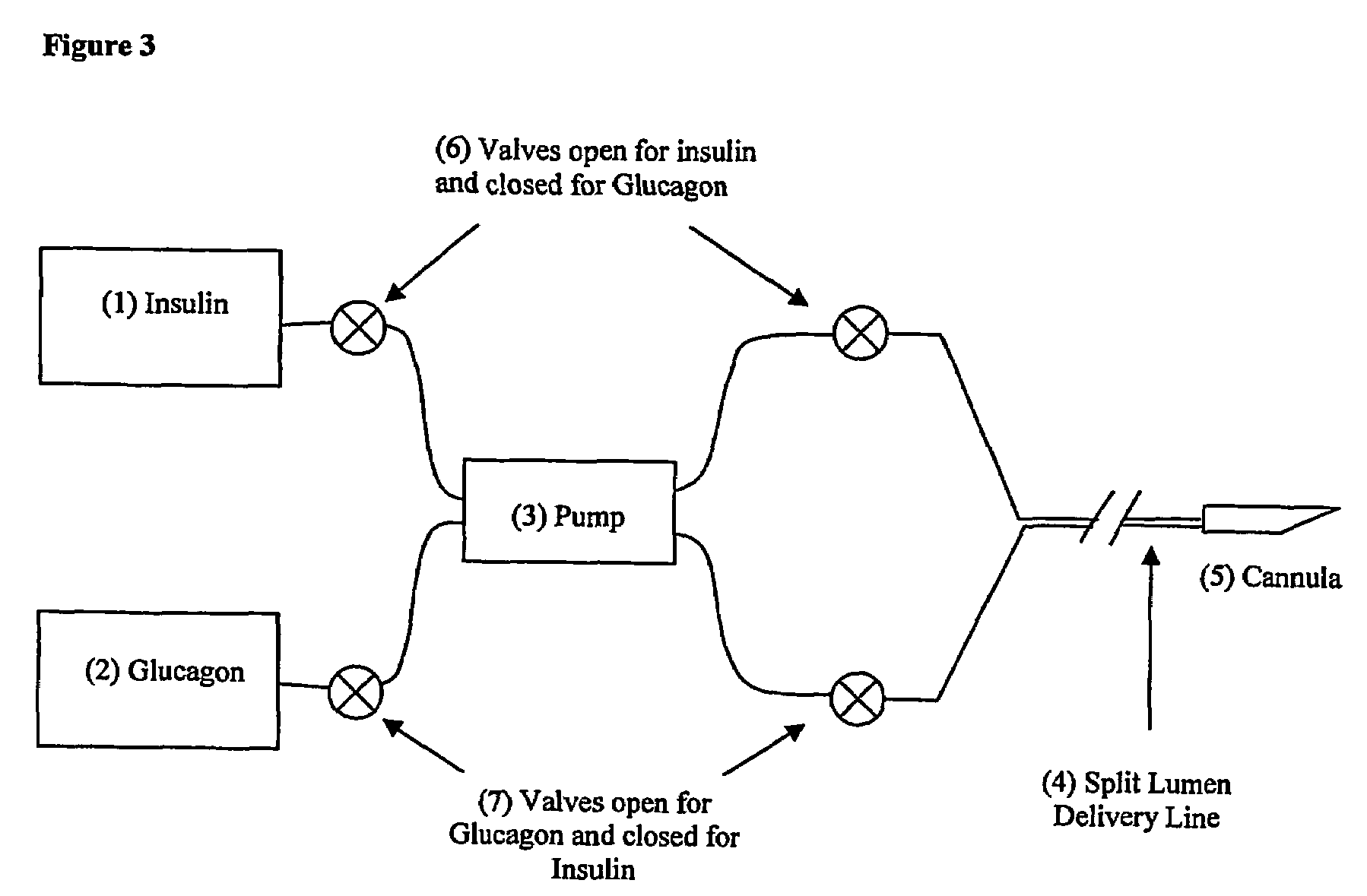
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS7314859B2Extended half-lifePowder deliveryDispersion deliveryInsulin induced hypoglycemiaCvd risk
Pharmaceutical composition comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:DIOBEX INC
Method and composition for enhanced parenteral nutrition
A composition and method for improved parenteral administration of nutrients is provided. The method comprises co-administration of nutrients, especially carbohydrates, and an insulinotropic peptide, or its derivatives, analogs, and fragments. The method and composition provide high carbohydrate nutrition while avoiding hyper- and hypoglycemia and their attendant deleterious effects.
Owner:AMYLIN PHARMA INC +1
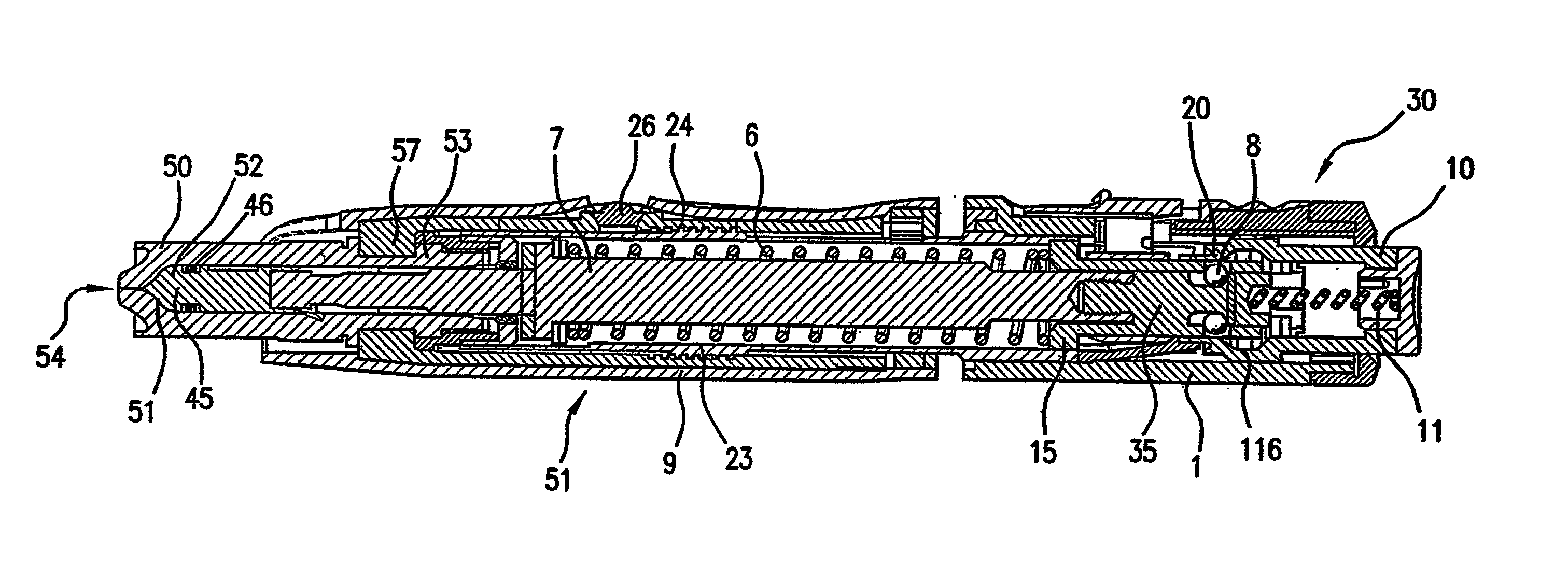
Administration of insulin by jet injection
InactiveUS20060106362A1Easily employedHigh level of skillJet injection syringesMetabolism disorderInsulin dependentJet injection
The invention relates to a method for minimizing mean blood glucose levels in an insulin dependent patient by administering insulin to the patient in a sufficiently fast manner to provide a difference of 50% or less between high and low blood glucose levels. Advantageously, the insulin is administered to the patient by jet injection and the high and low blood glucose levels differ by an amount that is less than that which would be obtained after injection of insulin by a conventional needle syringe. The invention also relates to a method for reducing mean blood glucose levels in an insulin dependent patient that is receiving insulin through a conventional syringe and needle arrangement. This method provides for administration of the insulin to the patient by jet injection rather than by the syringe by substituting a jet injector for the syringe.
Owner:ANTARES PHARMA
Methods of monitoring glucose levels in a subject and uses thereof
Methods of frequently monitoring glucose amounts and / or concentrations in a subject who is at risk for hypoglycemia, hyperglycemia, and / or glucose level fluctuations that put the subject at risk are provided. Also provided are methods of monitoring the effects of one or more pharmaceutical compositions on the levels of glucose in a subject.
Owner:ANIMAS TECH
Potentiation of glucose elimination
InactiveUS20070020191A1Improve efficiencyEffective controlPowder deliveryOrganic active ingredientsInsulin activityPostprandial Hypoglycemia
Owner:MANNKIND CORP
Rapid acting and long acting insulin combination formulations
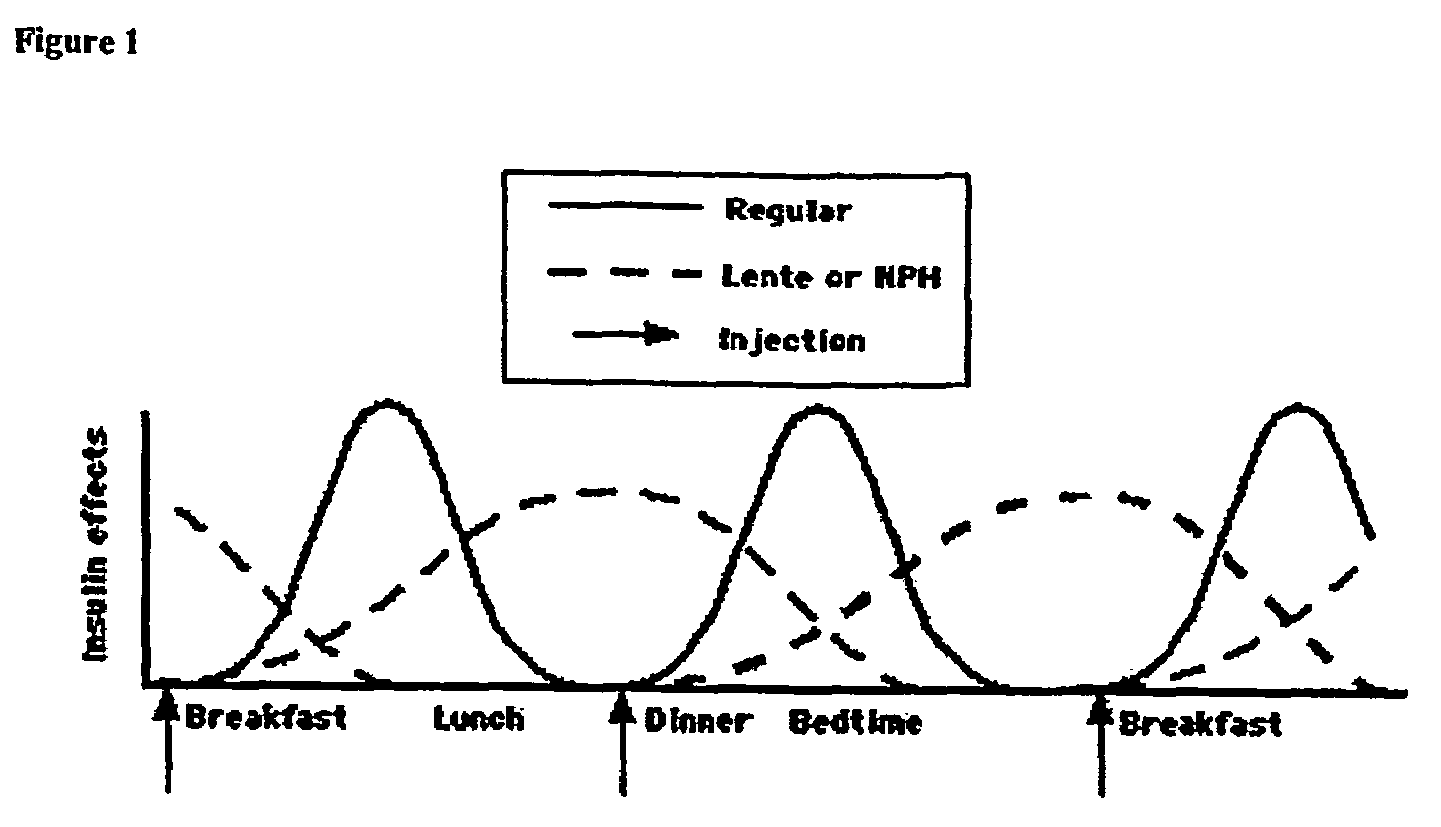
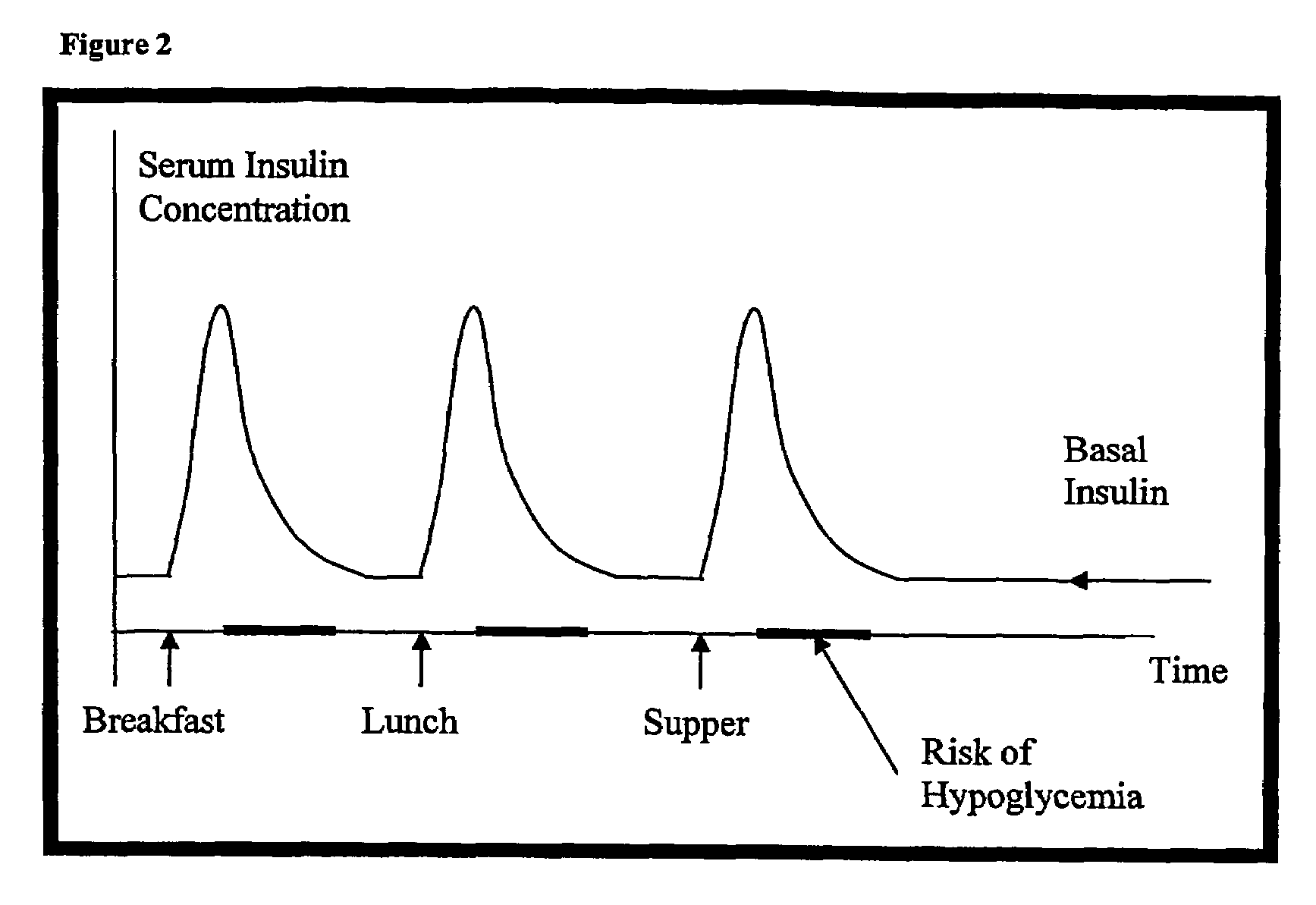
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
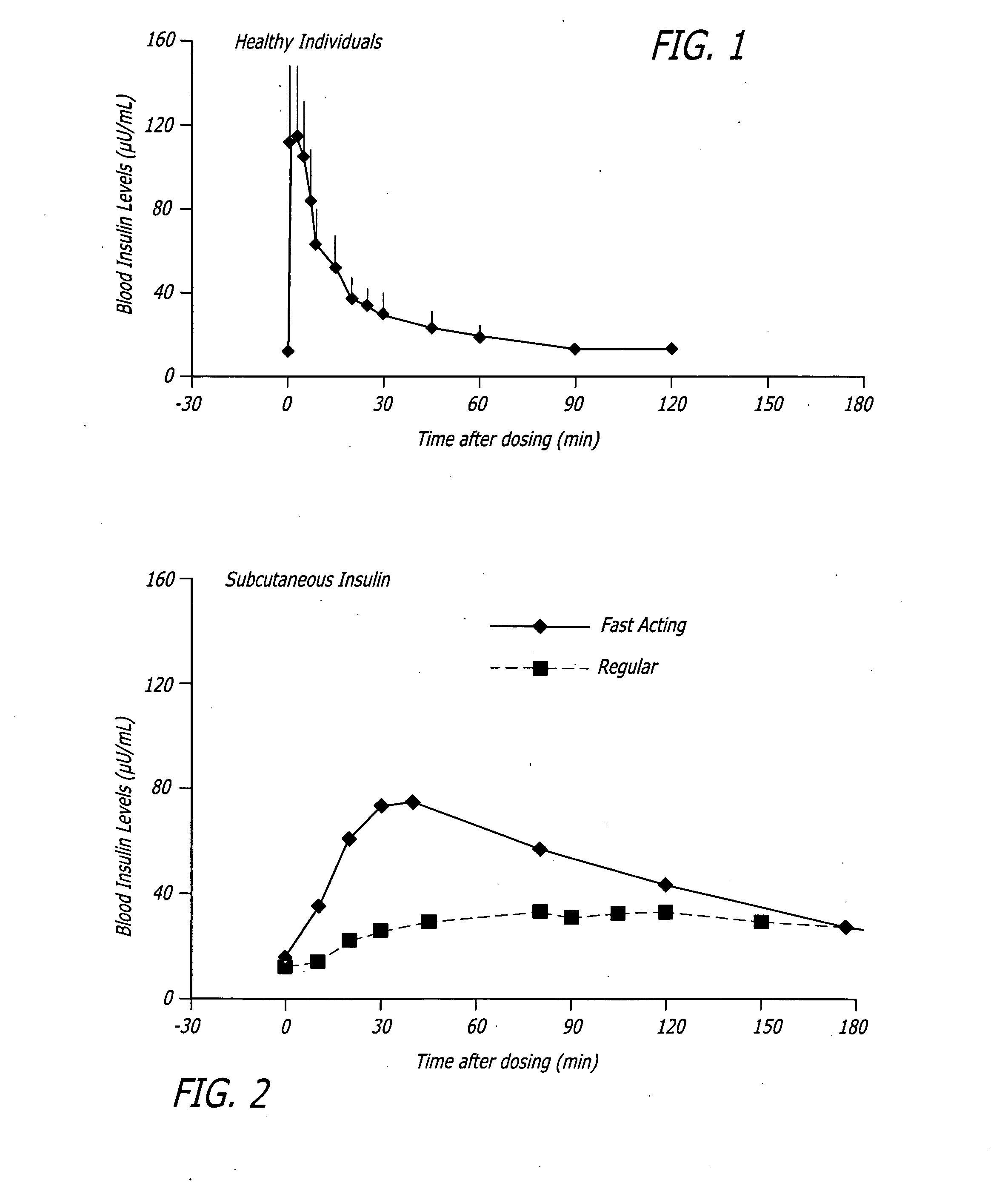
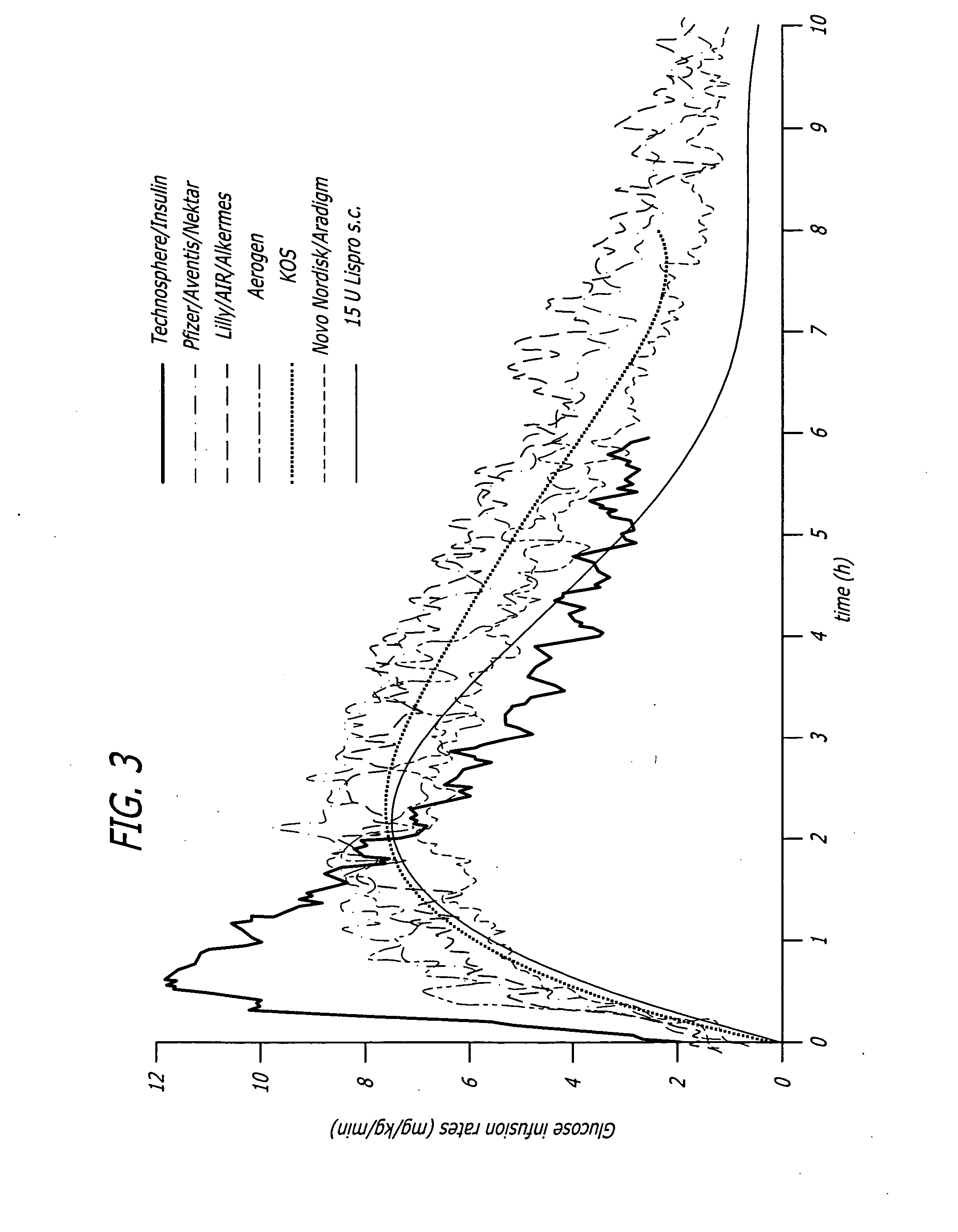
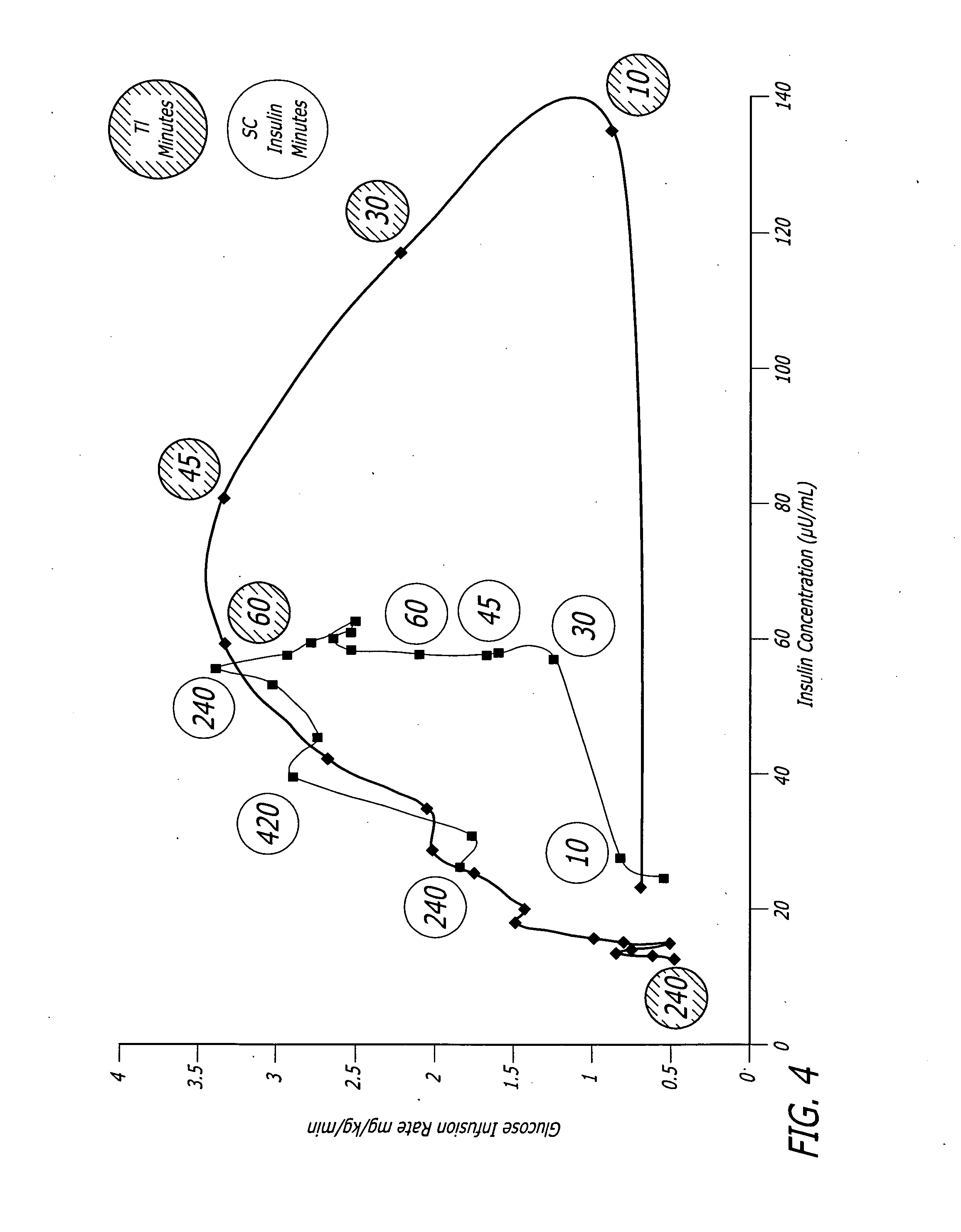
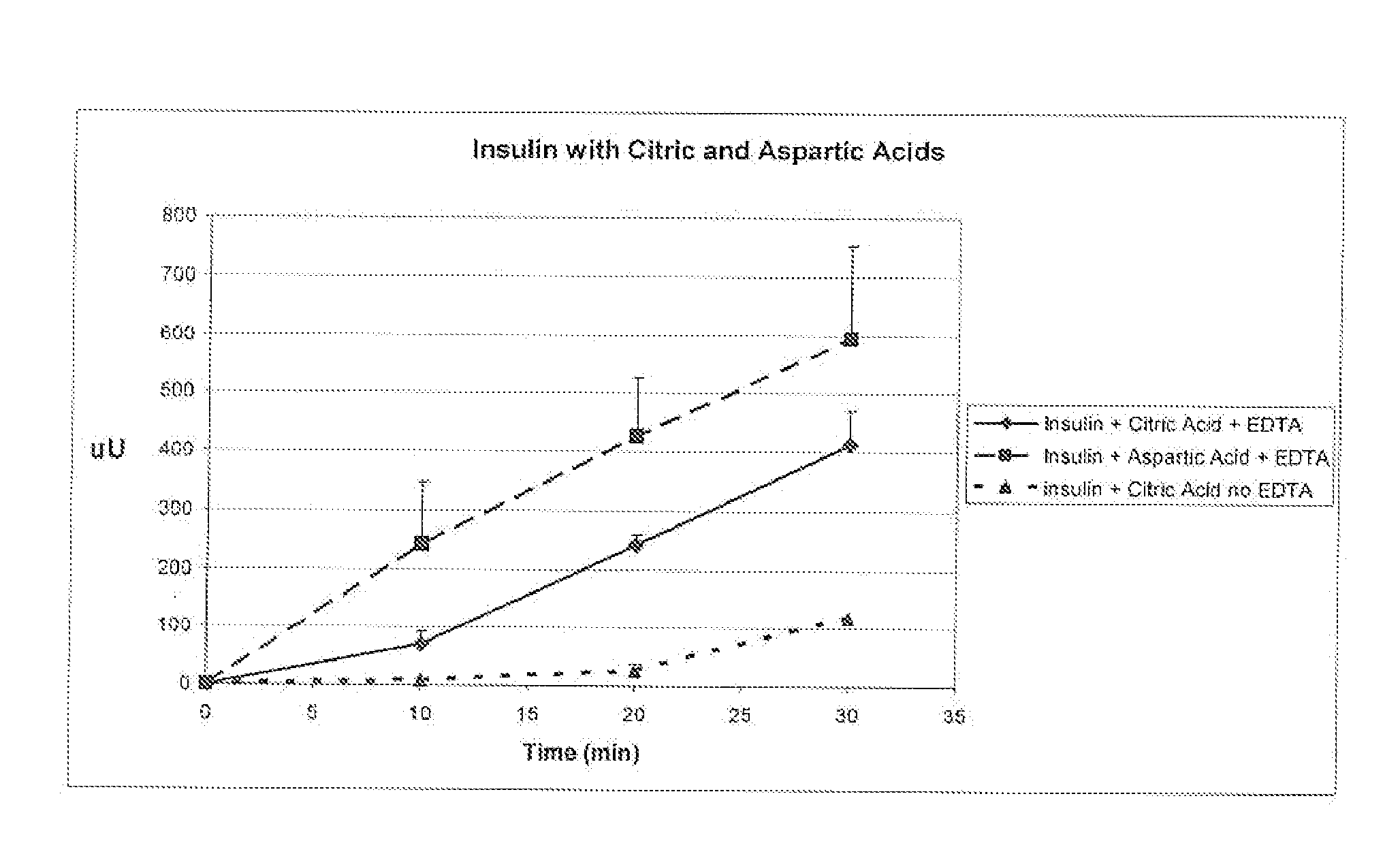
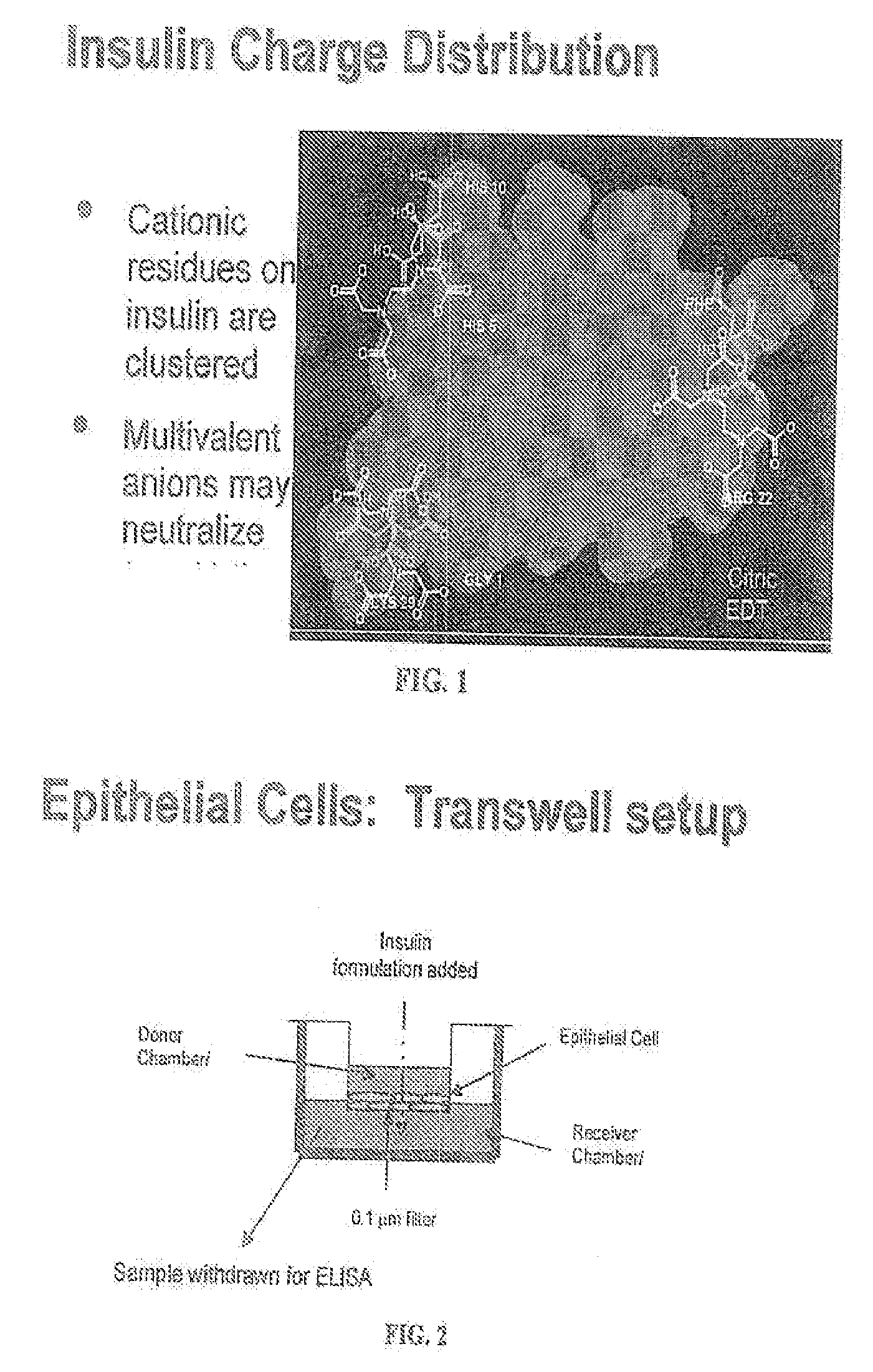
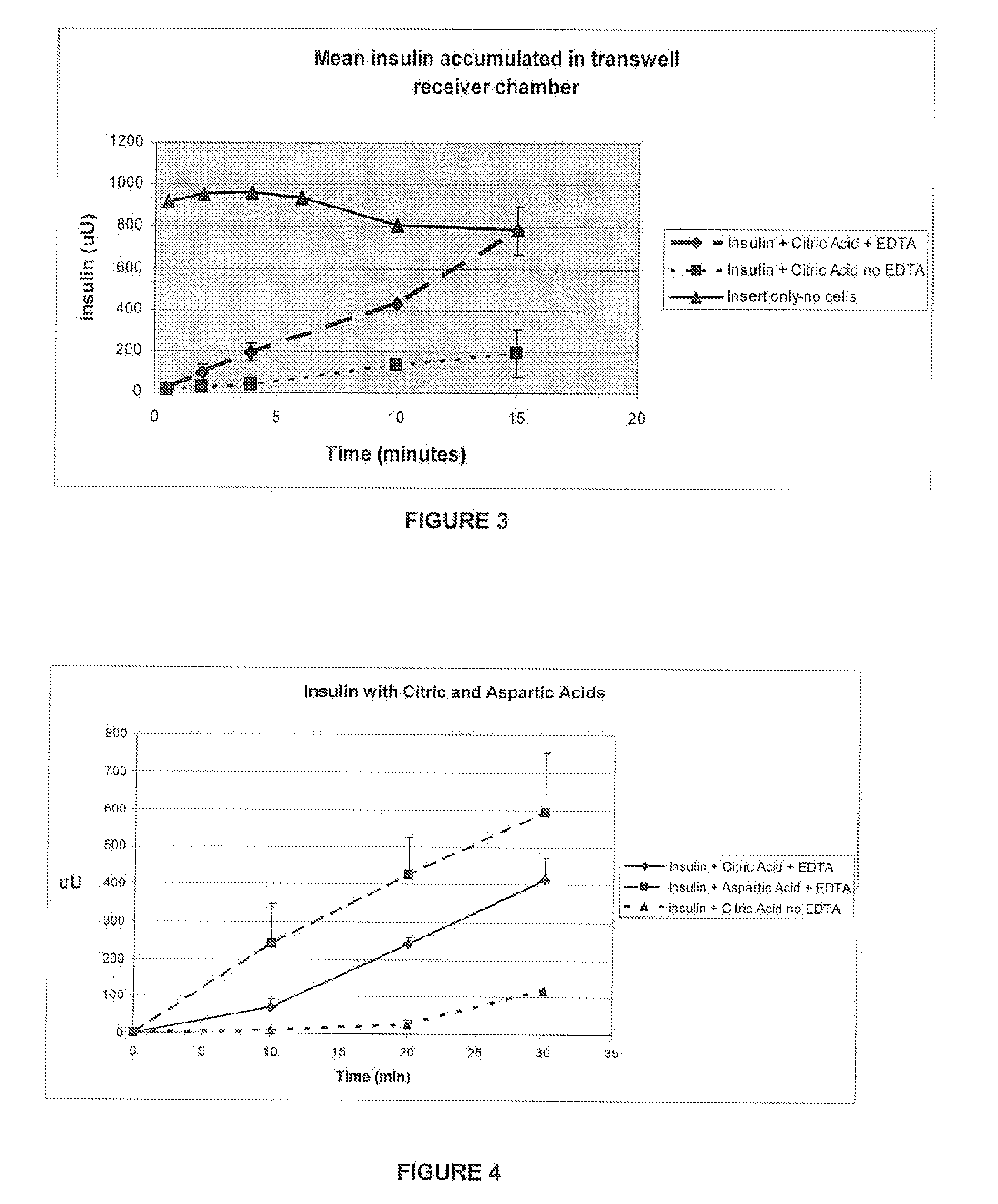
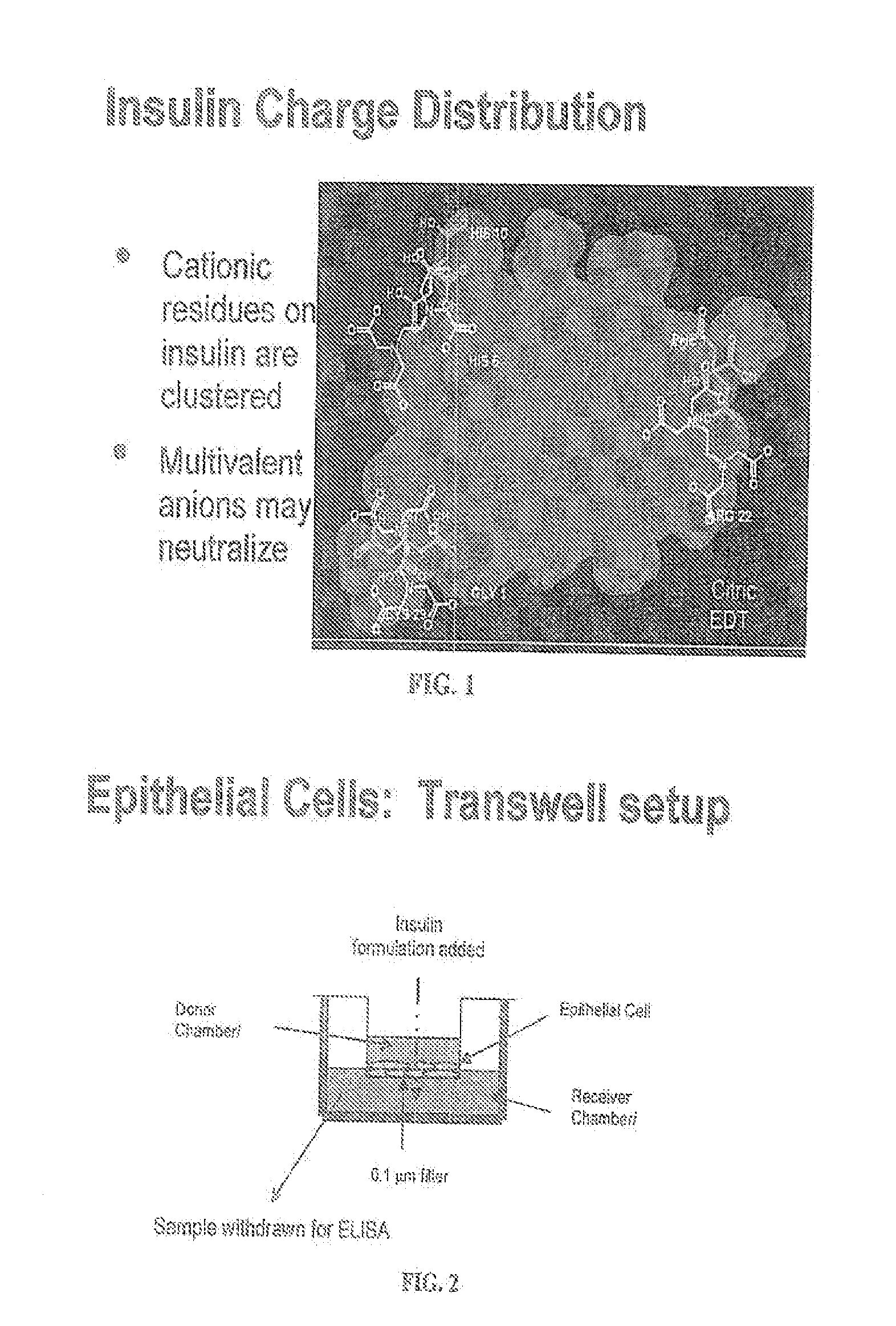
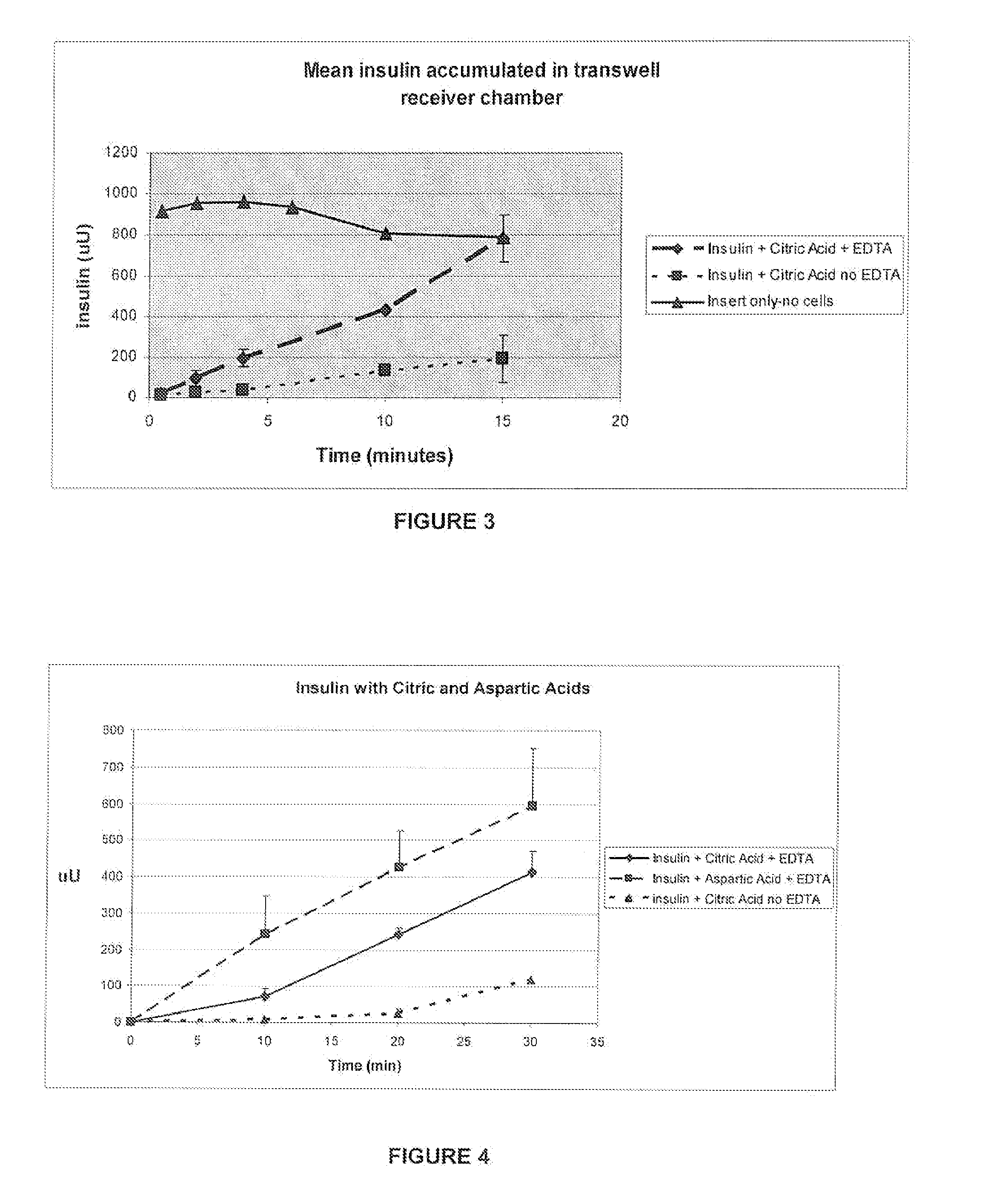
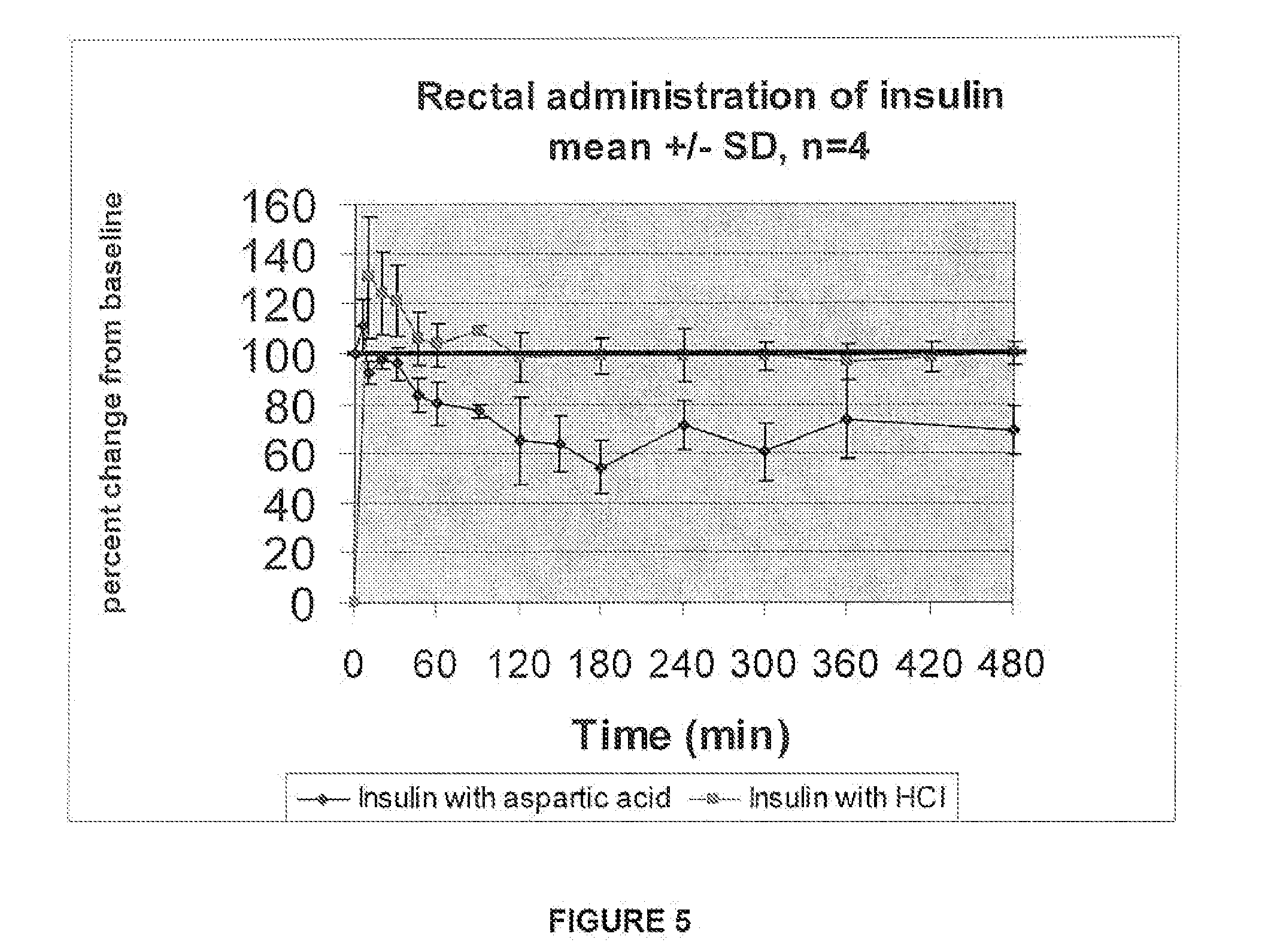
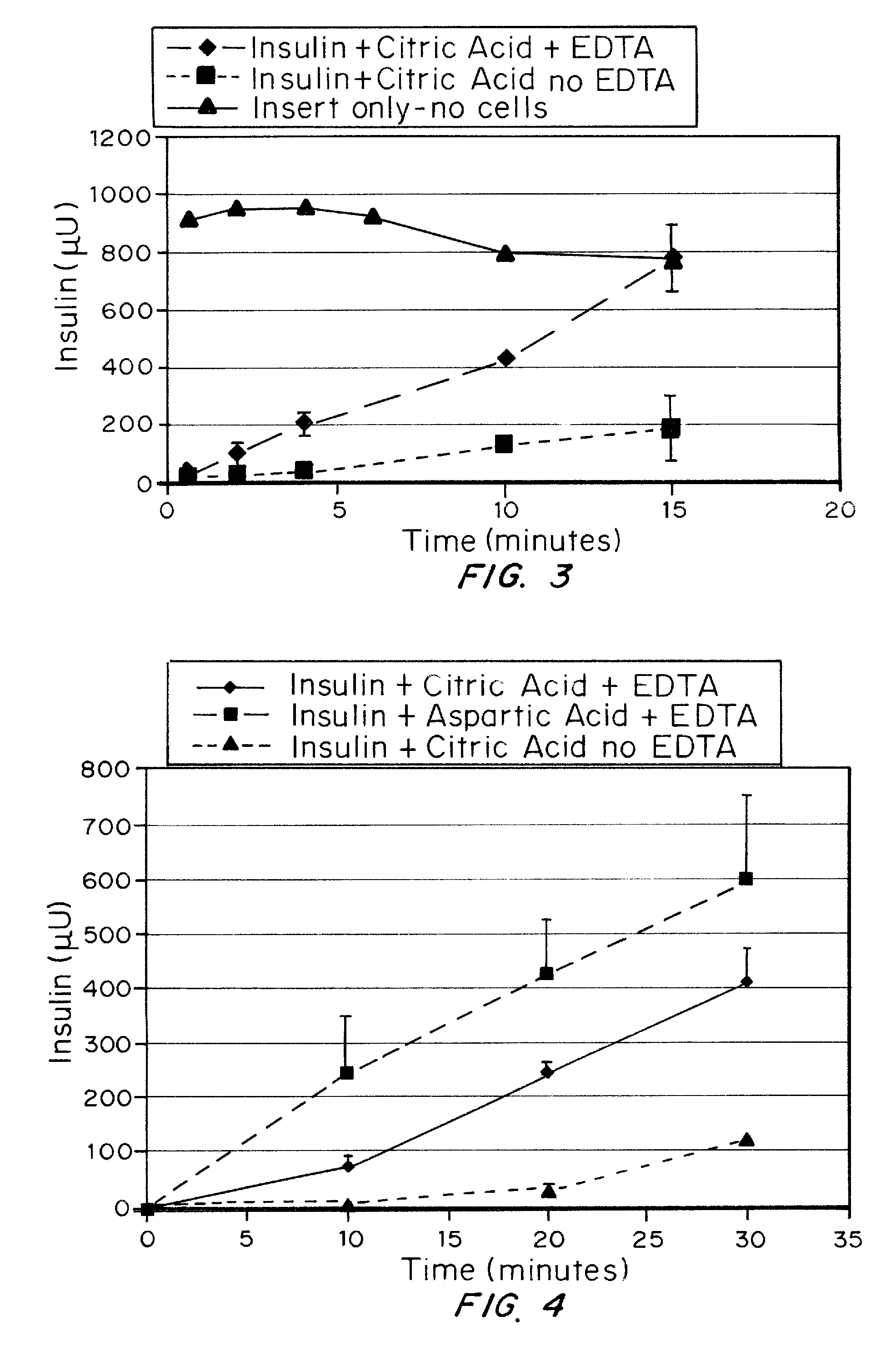
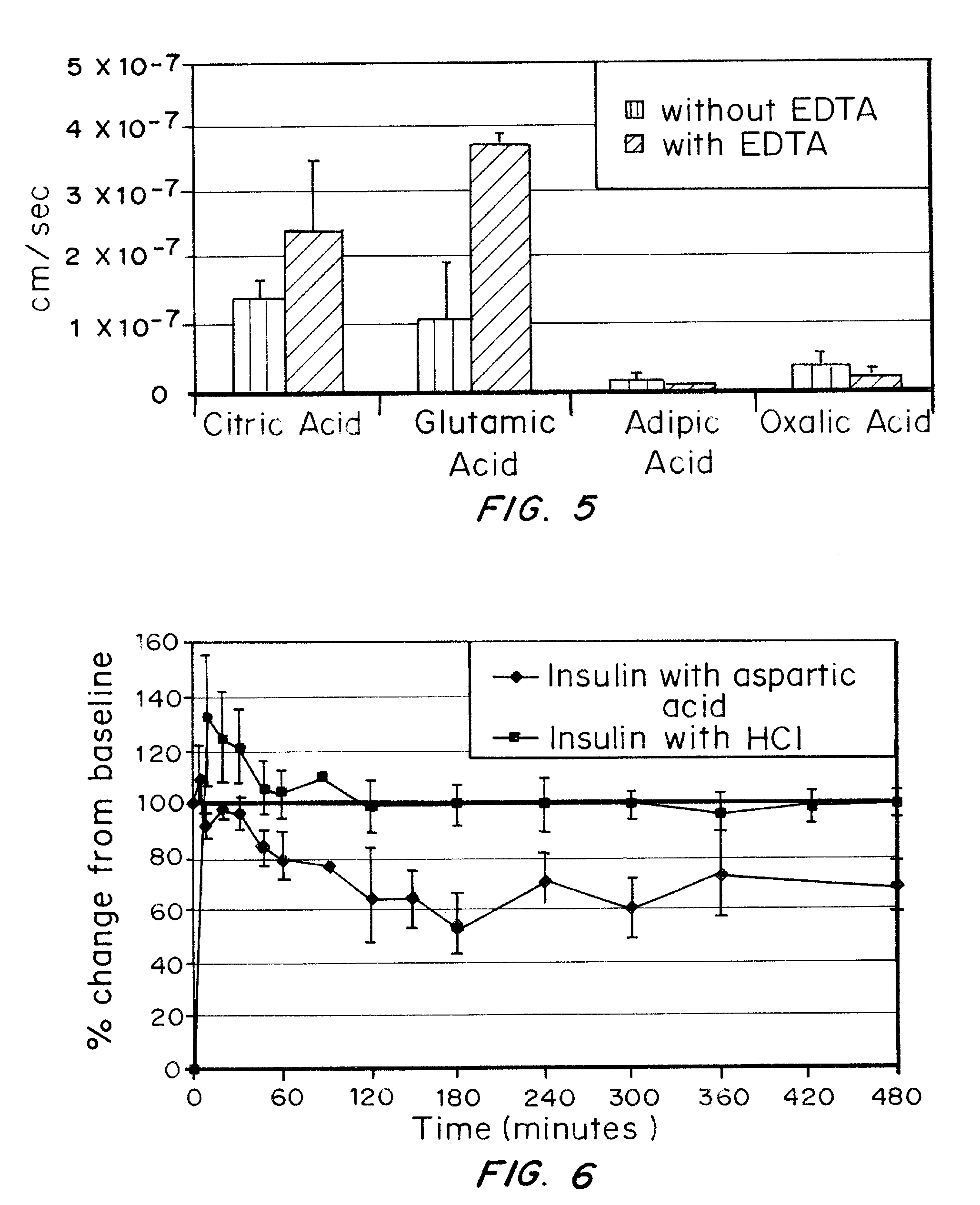
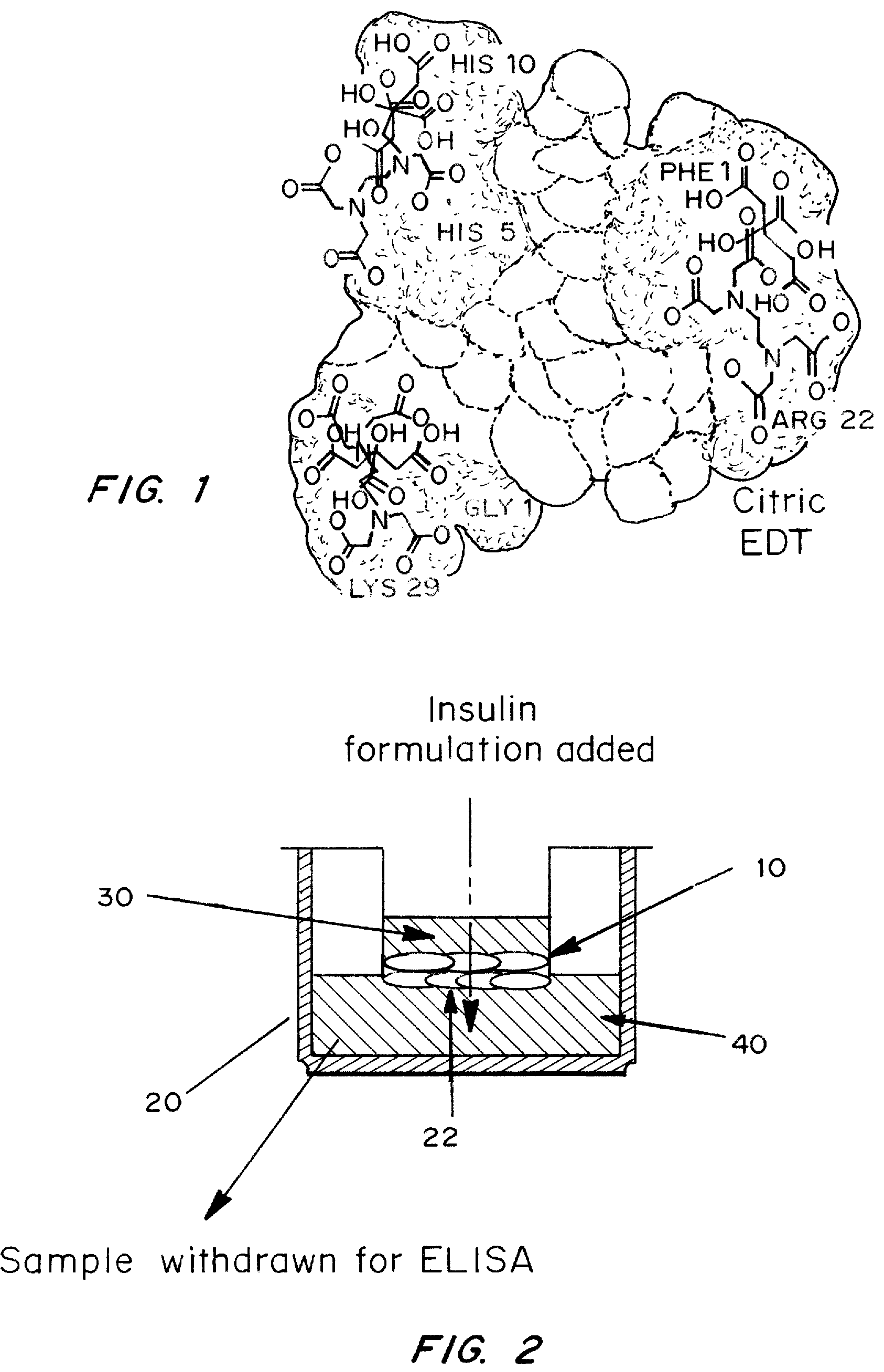
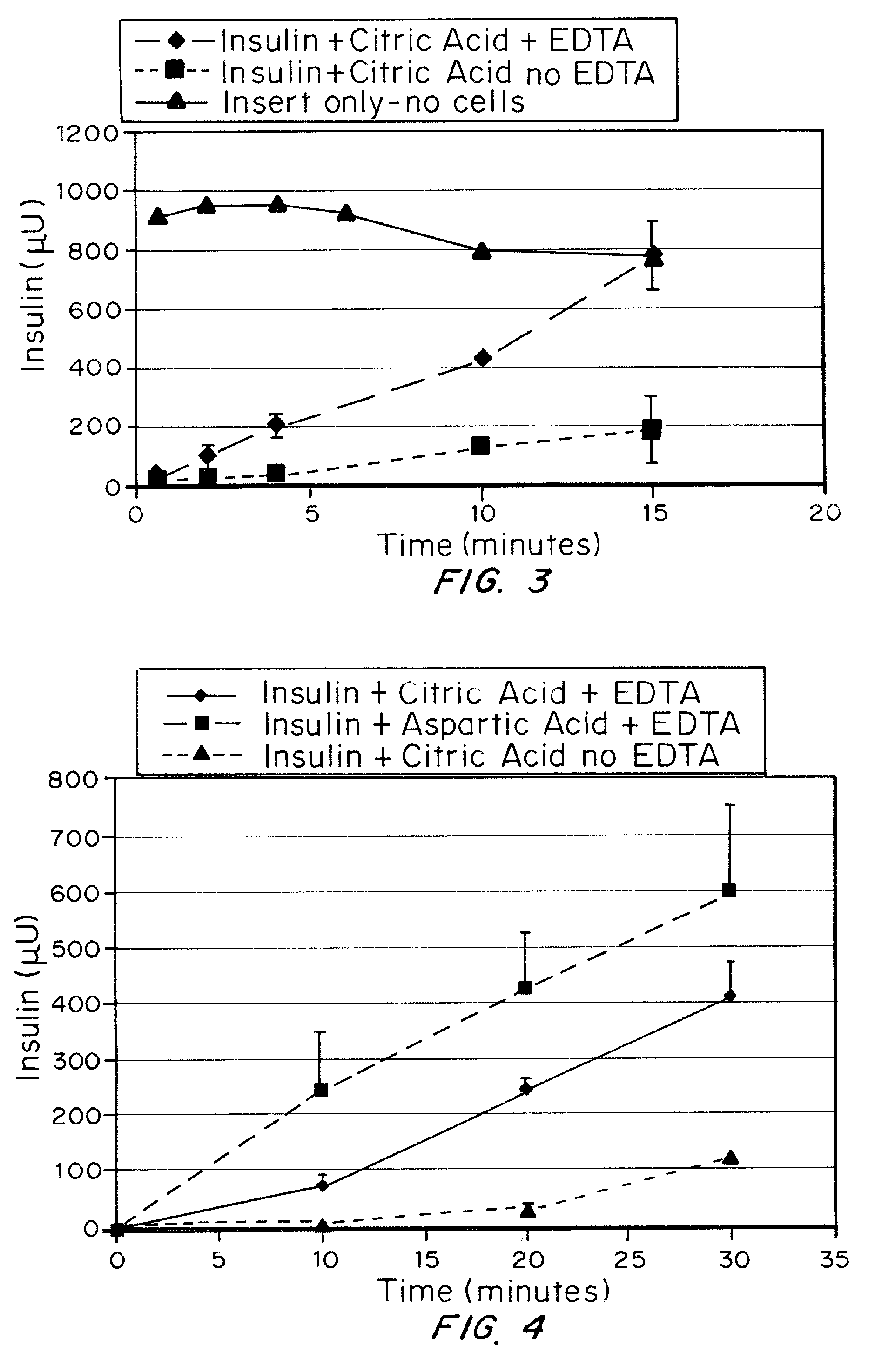
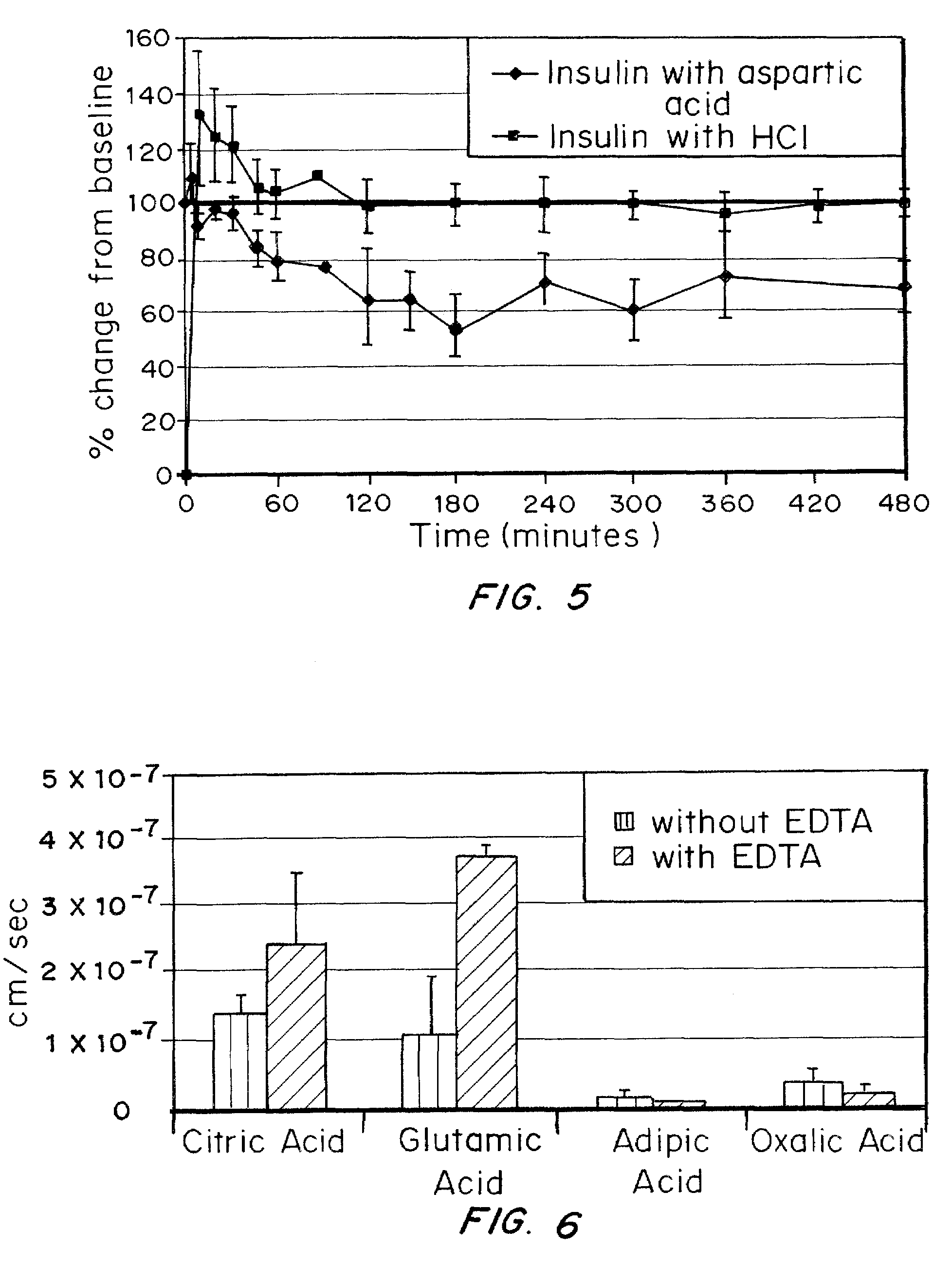
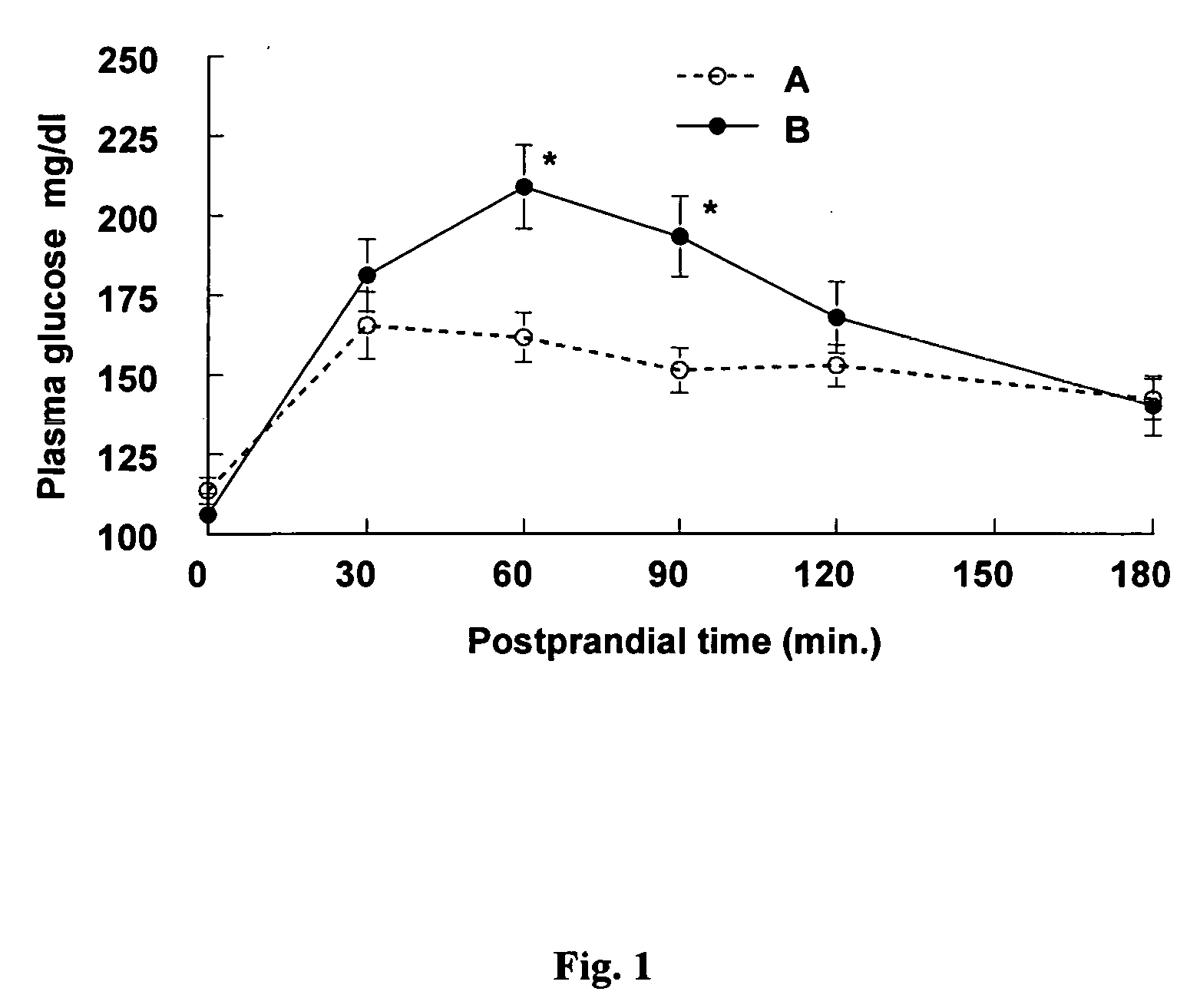
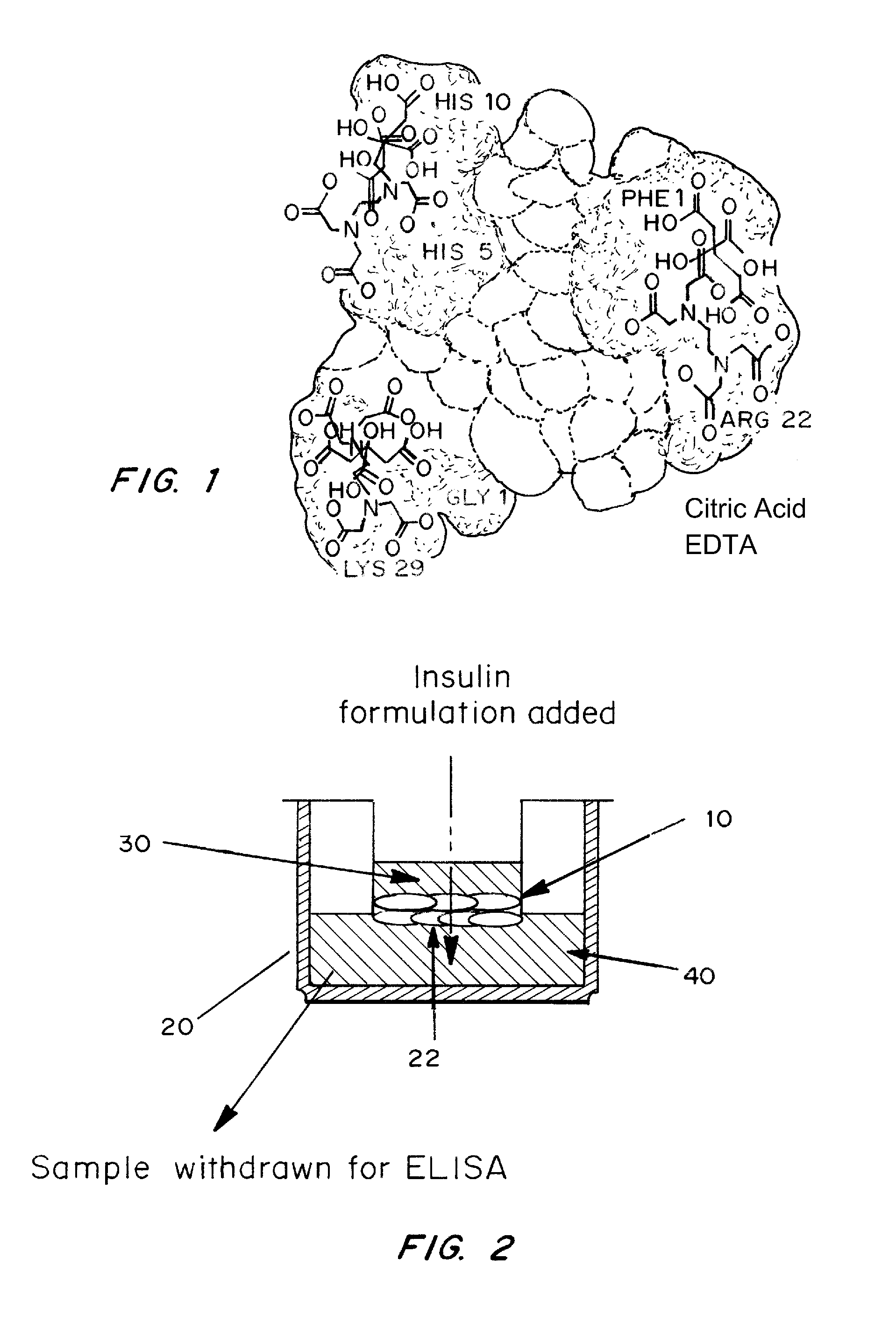
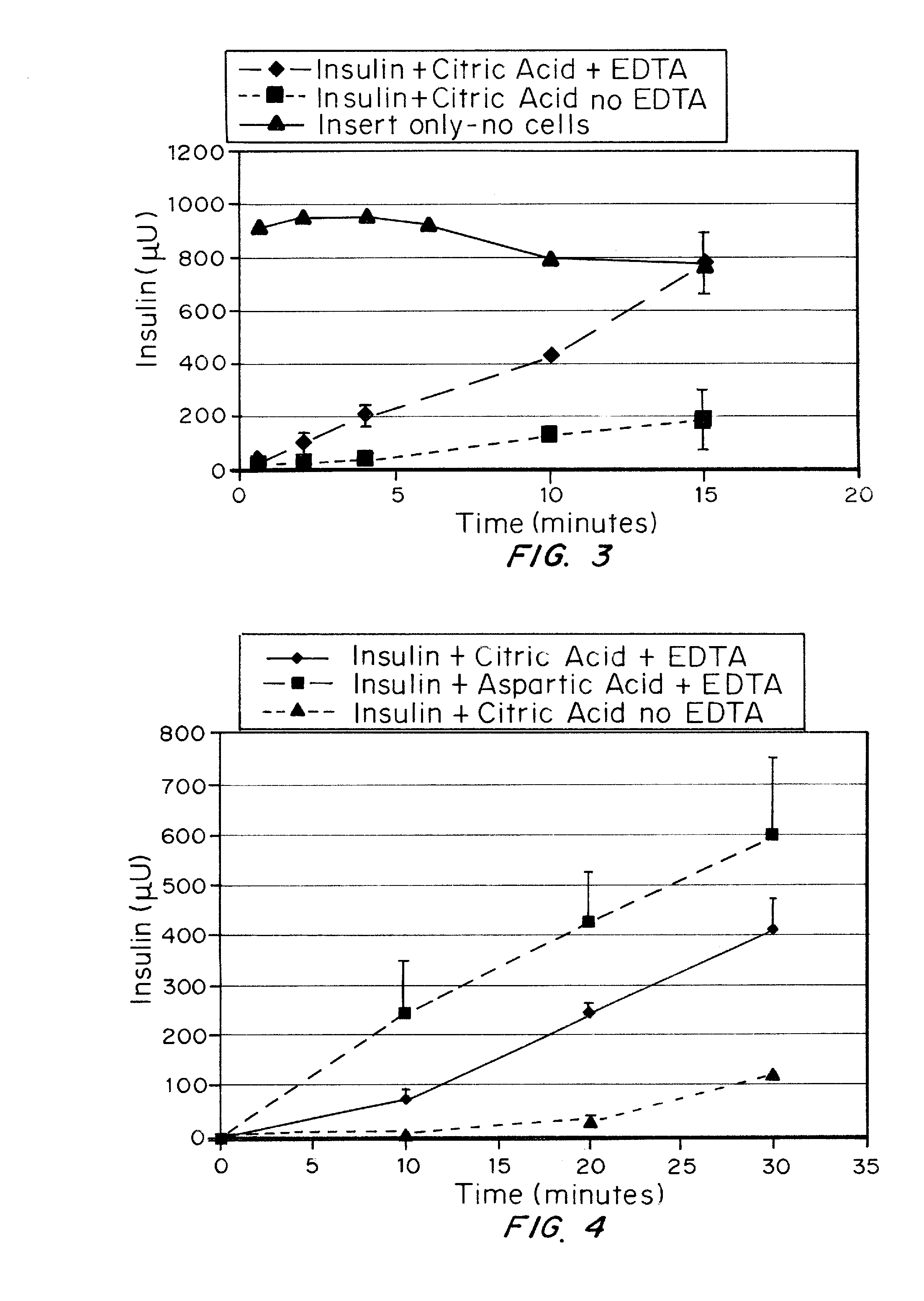
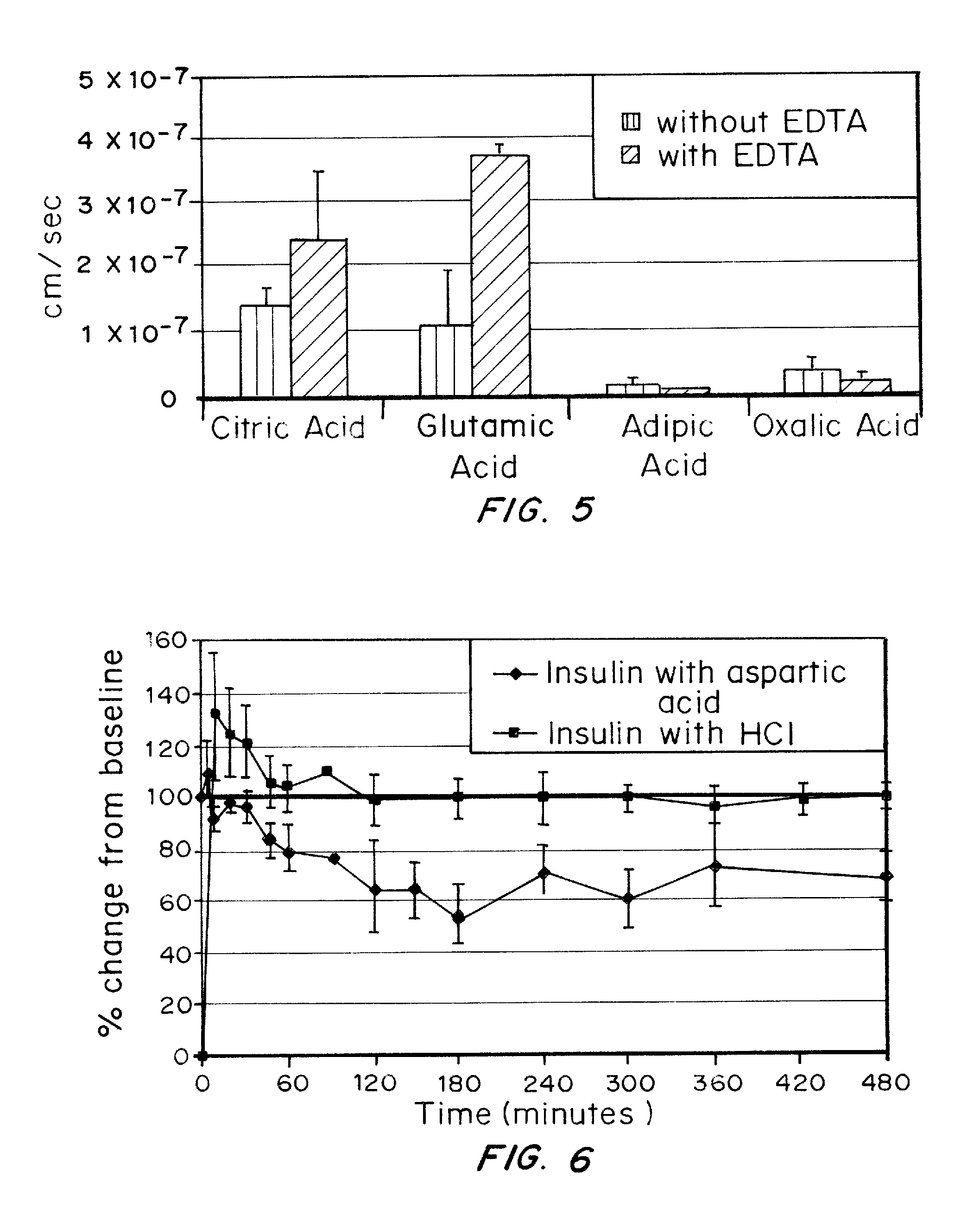
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Nutritional supplement for the management of weight
InactiveUS20060159724A1Increase satietyDelay returnBiocidePharmaceutical non-active ingredientsNutrition supplementationFood grade
Described herein is a low-glycemic nutritional supplement to be incorporated into the diet of an overweight or obese patient comprising low glycemic index ingredients including carbohydrate source, a source of protein, and a source of fat, and further comprising a source of green tea extract, a source of 5-hydroxytryptophan (5-HTP), and a source of chromium. The supplement provides active food-grade ingredients to improve the management weight loss, prevention of weight gain, and a feeling of satiety.
Owner:ADVANCED FUNCTIONAL FOODS INT
Rapid Acting and Long Acting Insulin Combination Formulations
InactiveUS20080039365A1Promote absorptionAct quicklyBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Drug composition for blood sugar control
InactiveUS20050267195A1Improve blood sugar controlInhibition is effectiveBiocideSenses disorderAcute hyperglycaemiaDisease
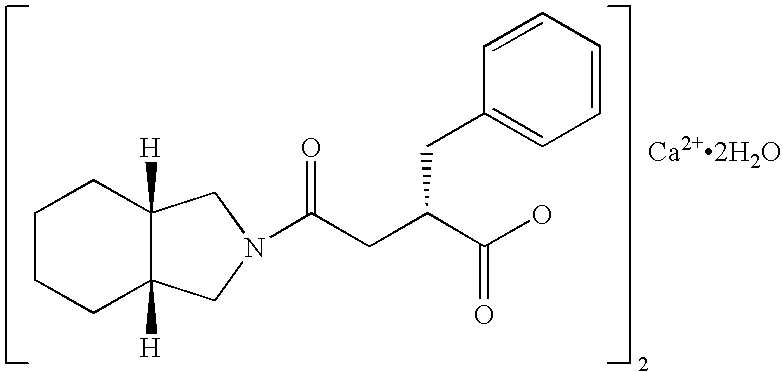
The present invention provides pharmaceutical compositions which can achieve good state of glycemic control and correct postprandial hyperglycemia and early morning fasting hyperglycemia. The present pharmaceutical composition is for administration before meal to control blood glucose, which comprises 5 to 45 mg, as a single dose, of mitiglinide or a pharmaceutically acceptable salt thereof, or a hydrate thereof (for example, mitiglinide calcium salt hydrate). And said compositions are extremely useful for prevention or treatment of, for example, type II diabetes, because the frequency of adverse drug reactions such as hypoglycemic symptoms and gastrointestinal disorders is low.
Owner:KISSEI PHARMA
Rapid acting and long acting insulin combination formulations
ActiveUS7718609B2Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Method, system, and computer program product for the processing of self-monitoring blood glucose(SMBG)data to enhance diabetic self-management
ActiveUS8538703B2Easy to monitorContinuous informationMedical simulationMicrobiological testing/measurementAcute hyperglycaemiaOptimal control
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Rapid acting and long acting insulin combination formulations
InactiveUS7713929B2Rapid acting insulin isPromote absorptionBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Methods of using gamma cyclodextrin to control blood glucose and insulin secretion
InactiveUS20050215523A1Blood glucose levelProlonged glycemic responseOrganic active ingredientsBiocideCyclodextrinDuodenum length
Disclosed are methods of producing a blunted postprandial glycemic response in an individual, and / or reducing postprandial insulin secretion, said methods comprising administering to the individual a nutritional or other product comprising gamma-cyclodextrin. Also disclosed are similar other methods directed toward the use of such products to provide weight and appetite control, to normalize blood glucose levels in individuals with impaired glucose tolerance, to minimize nighttime hypoglycemia in diabetic and non-diabetic patients, to prevent reactive hypoglycemia in susceptible non-diabetics, to normalize blood glucose levels in individuals with gestational diabetes or impaired glucose tolerance during gestation, and / or to provide a prolonged glycemic response during exercise. The methods are based upon the discovery that gamma-cyclodextrins are rapidly metabolized and absorbed in the small intestine, but subsequently result in a surprisingly blunted postprandial glycemic response and reduced insulin secretion.
Owner:ABBOTT LAB INC
System and method for detecting hypoglycemia based on a paced depolarization integral using an implantable medical device
ActiveUS7590443B2Improve blood sugar controlReduce deliveryElectrocardiographyMedical devicesCardiac pacemaker electrodeInsulin dependent
Techniques are provided for use with an implantable medical device such as a pacemaker or implantable cardioverter / defibrillator (ICD) for predicting and detecting hypoglycemia. In one example, the device tracks changes in a paced depolarization integral (PDI). A significant increase in PDI over a relatively short period of time indicates the onset of hypoglycemia (this can also be confirmed with QT changes). Upon detection of hypoglycemia, appropriate warning signals are generated to alert the patient. Certain therapies automatically provided by the implantable device may also be controlled in response to hypoglycemia. For example, if the patient is an insulin-dependent diabetic and the implantable device is equipped with an insulin pump capable of delivering insulin directly into the bloodstream, insulin delivery is automatically suspended until blood glucose levels return to acceptable levels. If the device is an ICD, it may be controlled to begin charging defibrillation capacitors upon detection of hypoglycemia so as to permit prompt delivery of a defibrillation shock, which may be needed if hypoglycemia triggers ventricular fibrillation. The detection techniques may be used in conjunction with other hypoglycemia detection techniques to improve detection specificity.
Owner:PACESETTER INC
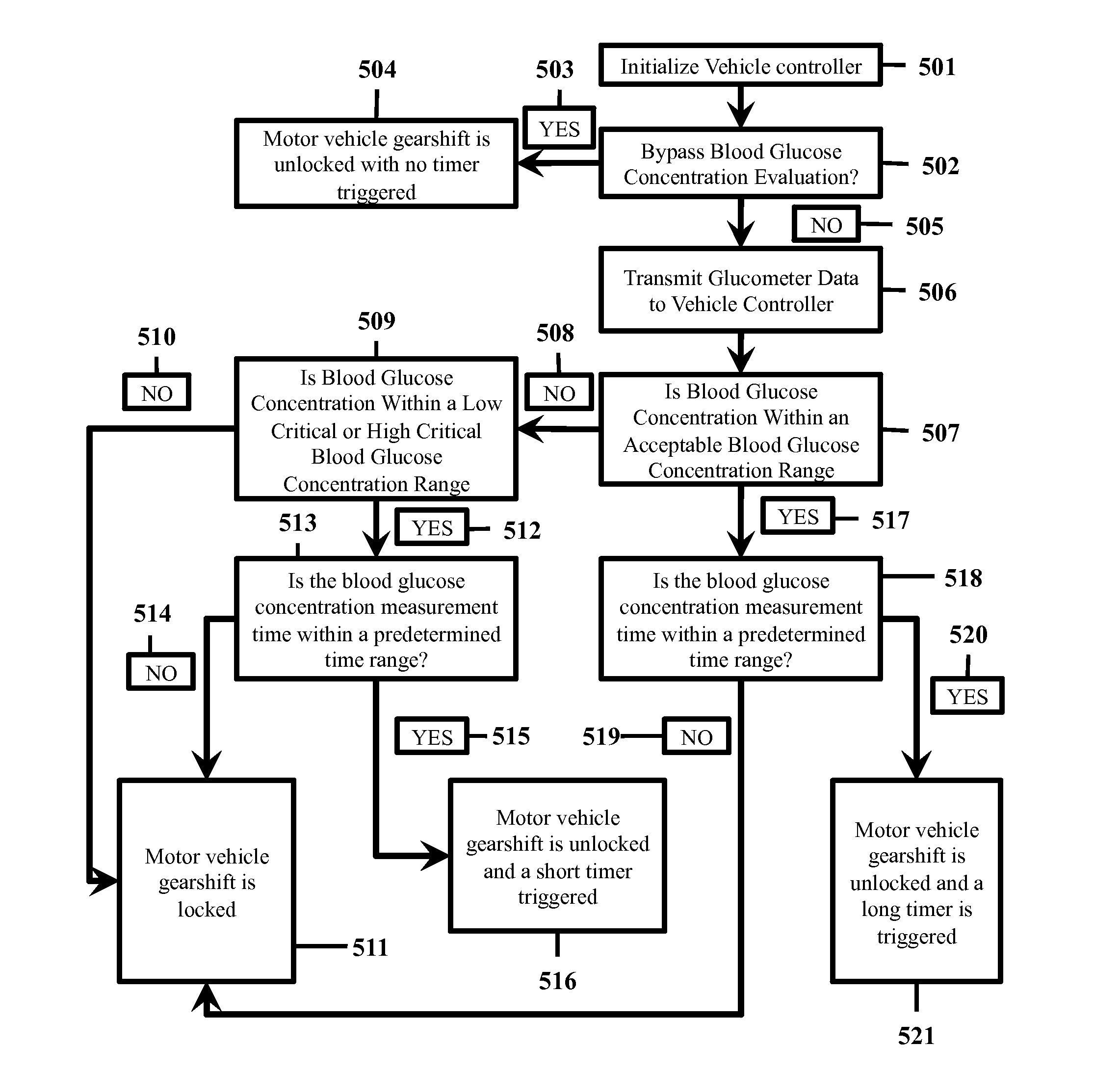
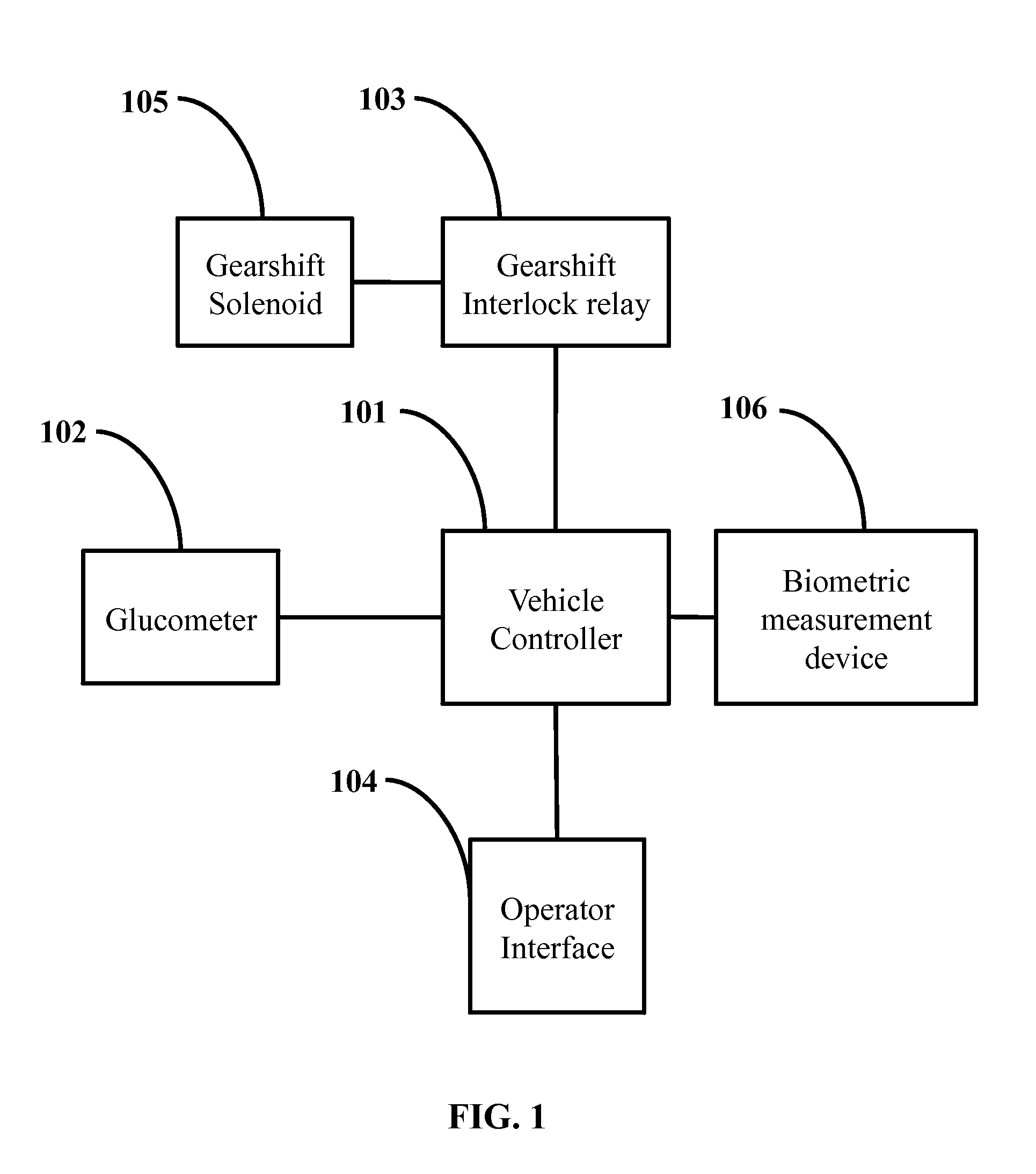
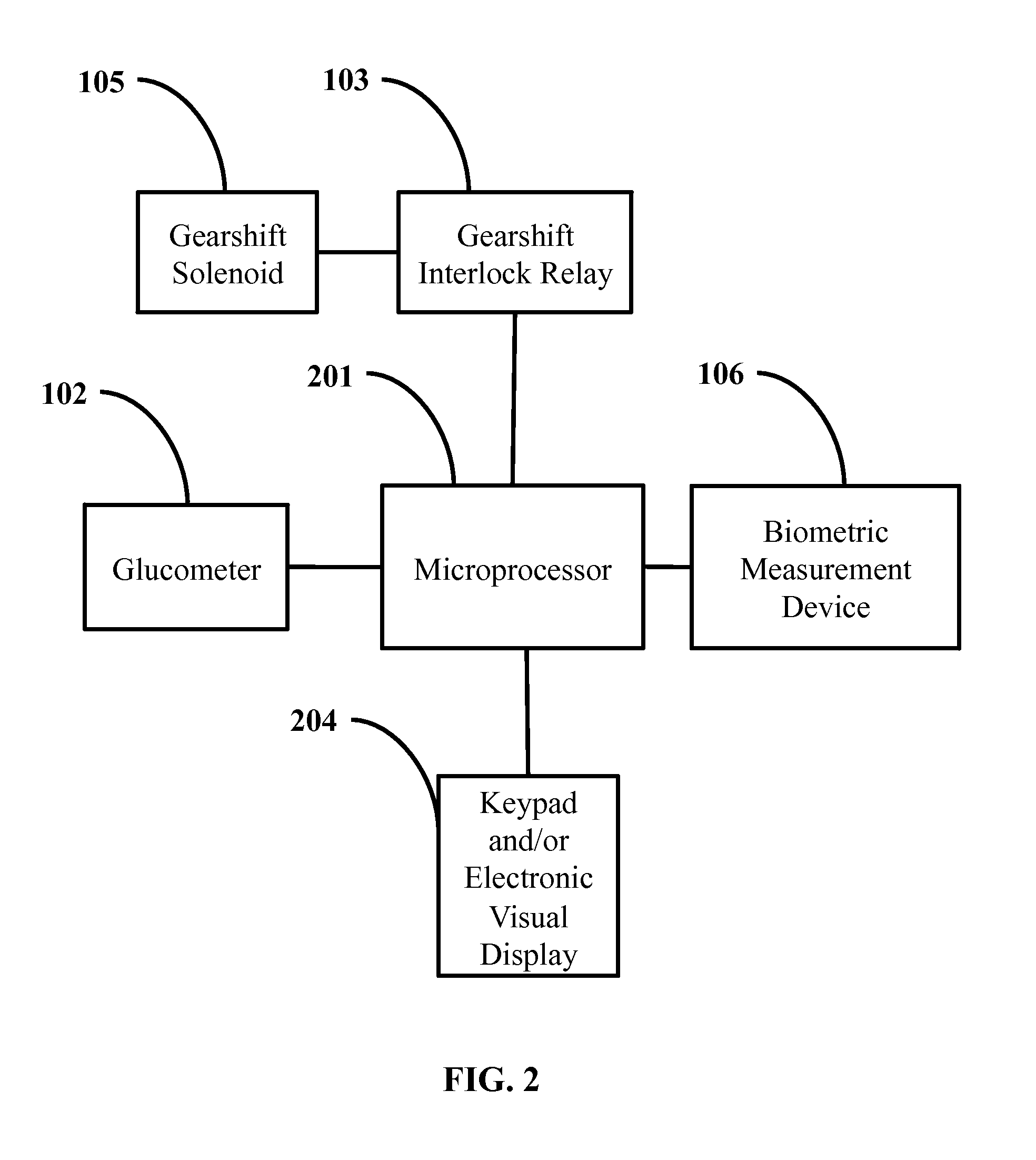
In vehicle glucose apparatus and vehicular operation inhibitor
When diabetics undergo a hyperglycemic or hypoglycemic event, their cognitive-motor function can be severely impaired. This has contributed to a positive correlation between diabetes and traffic incidences. Thus, there are ongoing efforts to improve blood glucose monitoring to improve safety of diabetic drivers.
Owner:AONEC
Carbohydrate composition for flat glucose response
ActiveUS20090215718A1The process is convenient and fastPrevent too low level of glucoseBiocideSugar food ingredientsTrehaloseInsulin resistance
A low-glycemic available carbohydrate composition of the invention contains the following components:(i) 5-60 wt. % of one or more monosaccharides selected from monosaccharides other than glucose and fructose, in particular galactose, ribose and mannose;(ii) 15-95 wt. % of oligosaccharides having a length of 2 to 20 anhydromonose units, at least half of which are anhydroglucose units linked by non-α-1,4 bonds; these oligosaccharides preferably comprising disaccharides such as palatinose, isomaltose and trehalose and / or non-α-1,4 linked higher glucose-containing oligosaccharides;(iii) 0-45 wt. % of other available carbohydrates, such as glucose and maltodextrins.This carbohydrate composition can be part of a foodcomposition for the treatment of diabetes, obesitas, insulin resistance, or for postprandial glucose response.
Owner:NUTRICIA
Compositions containing policosanol and chromium and/or chromium salts and their pharmaceutical uses
InactiveUS20060024383A1Increasing lean body massLower blood sugar levelsHeavy metal active ingredientsBiocideCoronary heart diseaseHypoglycemia
A composition is provided which contains policosanol and chromium and / or chromium salts and which may be used for treating, preventing and or reducing metabolic syndrome, hypercholesterolemia and hypoglycemia related diseases, total cholesterol, LDL-cholesterol, LDL / HDL ratio, triglycerides, coronary heart disease (heart attacks and strokes), inflammation, deep-vein thrombosis, immunoregulatory diseases, cardiovascular diseases, obesity, insulin resistance, dyslipidemia, raised blood pressure, fatigue, premenstrual syndrome, anxiety, depression and / or neurodegenerative disorders, and / or raising HDL cholesterol and / or lean body mass in humans and animals. The method comprises administering policosanol and chromium and / or chromium salts which together effectively lower blood glucose levels and lower the LDL / HDL cholesterol ratio. Typically, the administered composition includes about 0.1-10:1 parts by weight of policosanol to chromium and / or chromium salts.
Owner:WYETH
Methods for rapidly treating severe hypoglycemia
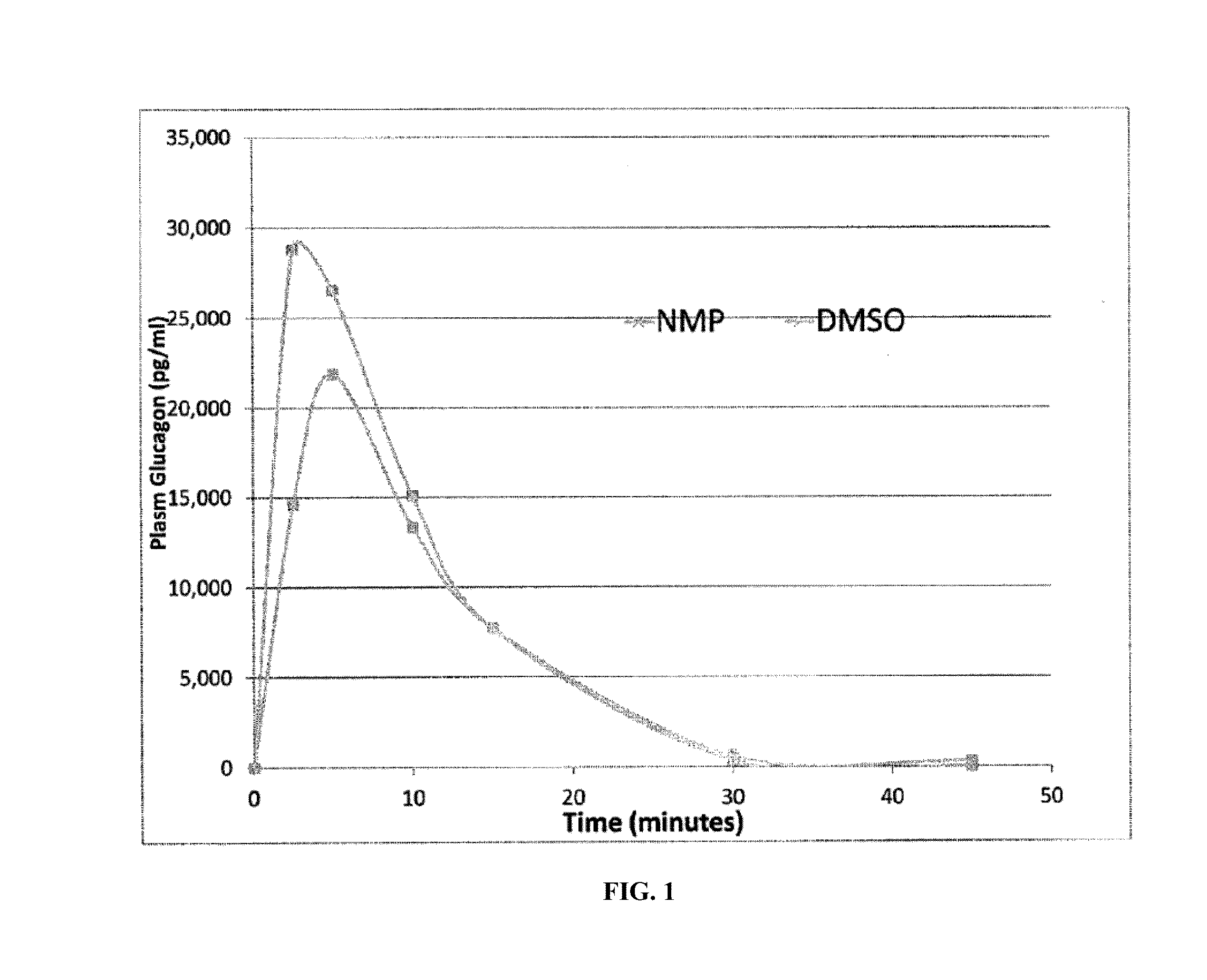
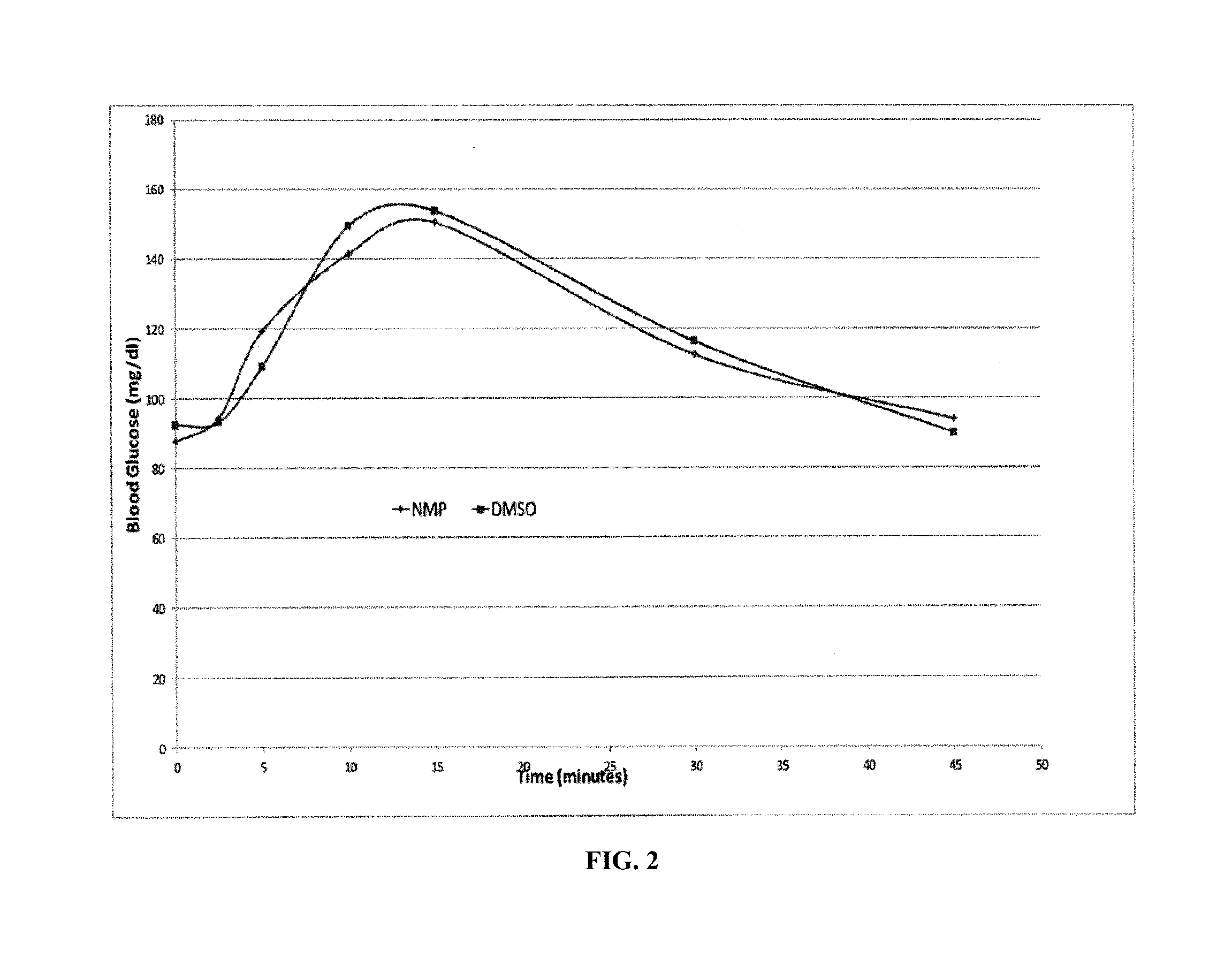
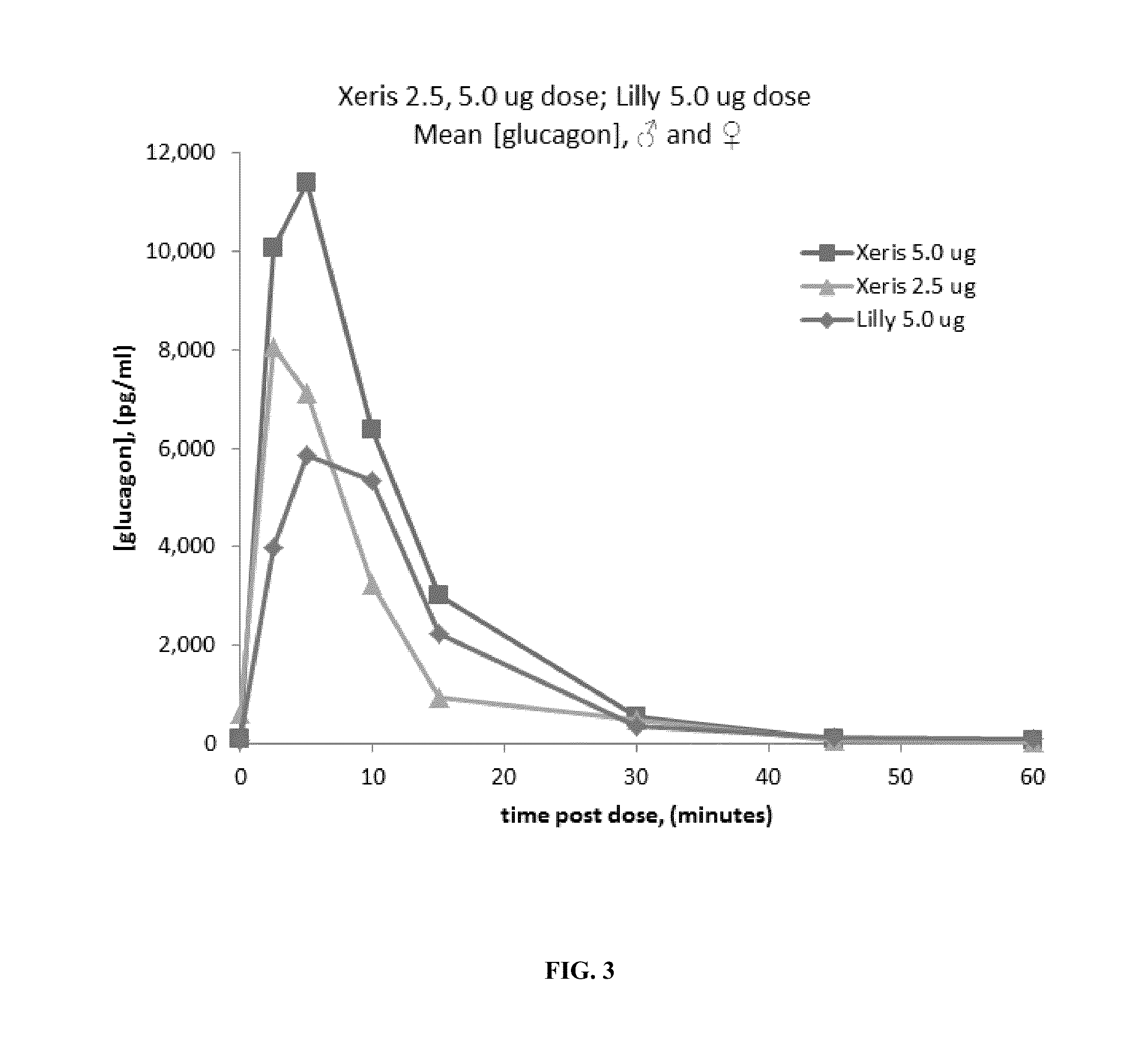
Disclosed is a method for treating or preventing hypoglycemia in a patient comprising administering an effective amount of a composition comprising a glucagon peptide which has been dried in a non-volatile buffer, and wherein the glucagon peptide has a pH memory that is about equal to the pH of the glucagon peptide in the non-volatile buffer, and an aprotic polar solvent, wherein the moisture content of the formulation is less than 5%, and wherein the dried glucagon peptide maintains the pH memory that is about equal to the pH of the glucagon peptide in the non-volatile buffer when the dried glucagon peptide is reconstituted in the aprotic polar solvent, wherein the patient has been diagnosed as having a blood glucose level between 0 mg / dL and less than 50 mg / dL or has an indication of impending hypoglycemia based on a blood glucose monitoring device before administration of the composition, and wherein the patient has a blood glucose level greater than 50 mg / dL to 180 mg / dL within 1 to 20 minutes after administration of the composition.
Owner:XERIS PHARMA
Rapid acting and long acting insulin combination formulations
ActiveUS20090137455A1Short durationImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
An injectable formulation containing a combination of a rapid acting insulin and a long acting insulin has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting insulin, e.g. insulin glargine, remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for up to 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. Alternatively, through adjustment of the ratio of rapid acting insulin to long acting insulin, the long acting insulin may be shortened to a 12 hour formulation. This rapid and long acting blend is re-administered to the patient at dinner time, providing a safe and effective basal insulin level until morning. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
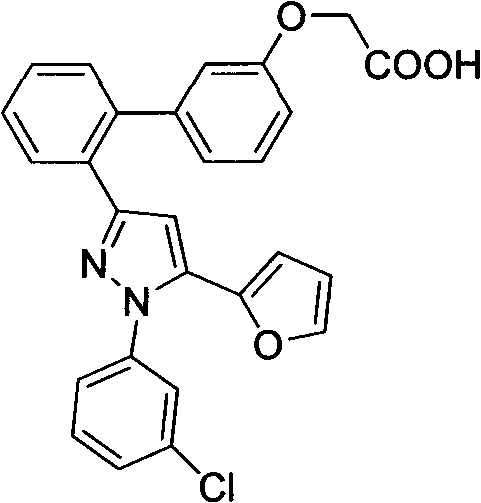
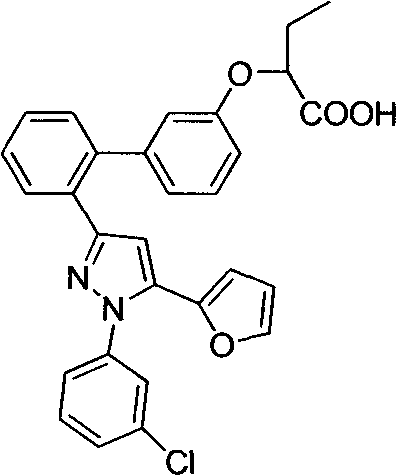
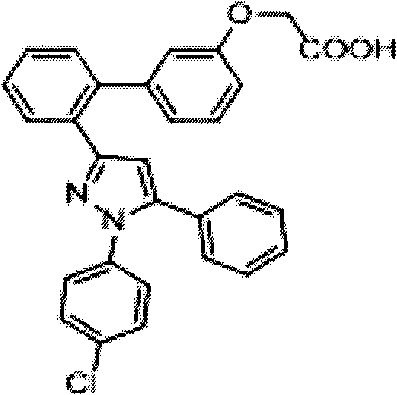
Pyrazole compound as well as composition and application thereof
The invention provides a compound and application of the compound and the pharmaceutically acceptable salt thereof or a stereo isomer or a prodrug molecule thereof in preparing a medicament for treating or preventing metabolic diseases, wherein the compound has the structural characteristic of a general expression I and is used as a novel fat cell type fatty acid binding protein FABP inhibitor. The compound having the structural characteristic of the general expression I can provide a new selection for the clinical prevention and treatment of the following diseases: 1. type II diabetes, 2. hyperglycemia, 3. hypoglycemia tolerance, 4. insulin resistance, 5. adiposity, 6. lipid turbulence, 7. blood-lipoid imbalance, 8. hyperlipaemia, 9. hypertriglyceridemia, 10. hypercholesterolemia, 11. low high-density protein level, 12. overhigh low-density protein level, 13. atherosclerosis and secondary diseases thereof, 14. hemadostenosis, 15. abdominal obesity, 16. a metabolic syndrome and 17. fatty liver.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Methods and kits for preventing hypoglycemia
InactiveUS7855177B1Reduce riskReduce incidencePeptide/protein ingredientsMetabolism disorderExcessive weight gainGlycemic
Improved methods and kits for treating the long-term complication of diabetes that reduce the risk of the patient developing hypoglycemia during C-peptide therapy. The use of such methods and kits, can also maintain good glycemic control, and avoid excessive weight gain that may otherwise be associated with excessive insulin administration or caloric intake during C-peptide therapy.
Owner:CEBIX
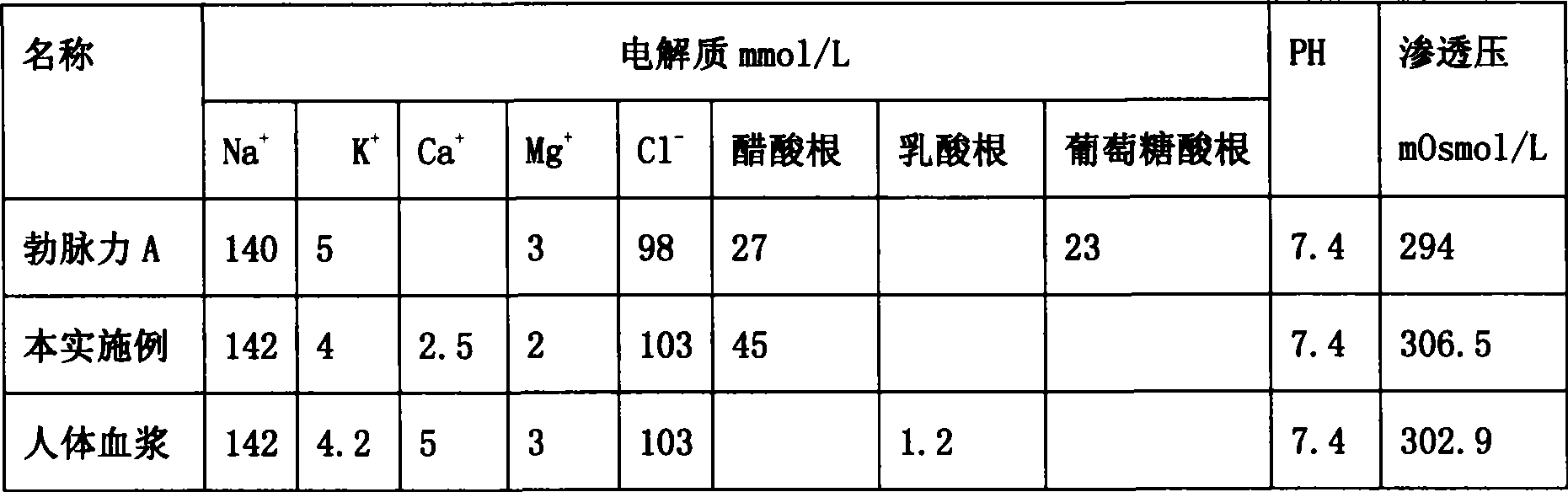
Compound sodium acetate injection
InactiveCN101474145AGood for contractilityReduce the burden onMetabolism disorderPharmaceutical delivery mechanismSodium acetateWater use
The invention relates to medical injection, in particular to a compound sodium acetate injection used for venoclysis. The injection is made of the materials with the weight ratio: 5.32g-5.88g of sodium chloride, 0.285g-0.315g of potassium chloride, 0.266g-0.294g of calcium chloride, 0.19g-0.21g of magnesium chloride and 5.035g-5.565g of sodium acetate; and the water used for injection is added up to 1000ml. Compared with the prior art, the pH value, the permeation pressure and the electrolyte of the compound sodium acetate injection are the closest to the pH value of blood plasma of human body, so that the compound sodium acetate injection can be widely suitable for various patients in a stress state, diabetes patients or hypoglycemia patients and children, and used for washing pipe when in blood transfusion. Furthermore, the compound sodium acetate injection has little incompatibility, low cost, wide application prospect and foreseeable great social and economic benefits.
Owner:沈七襄
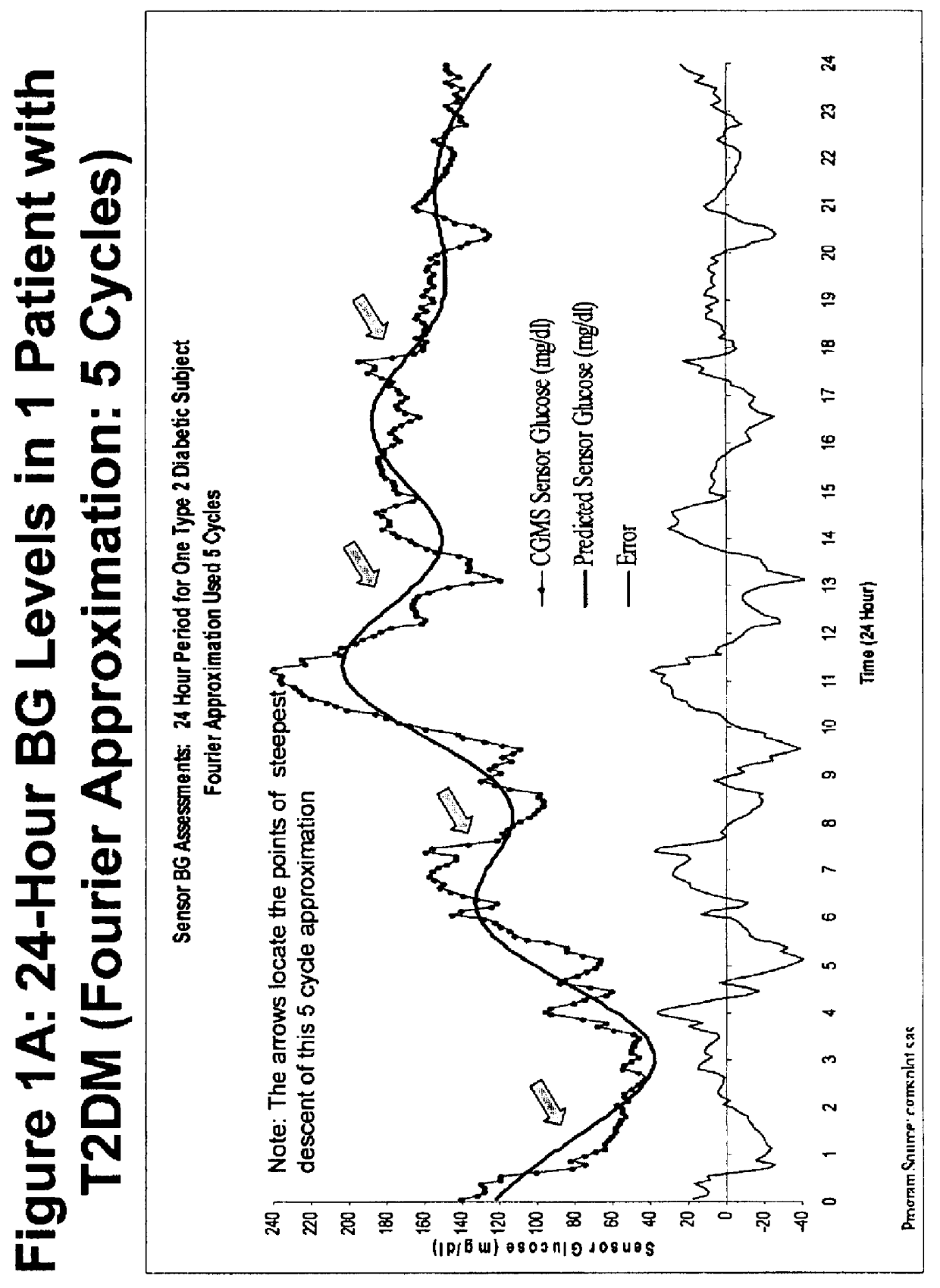
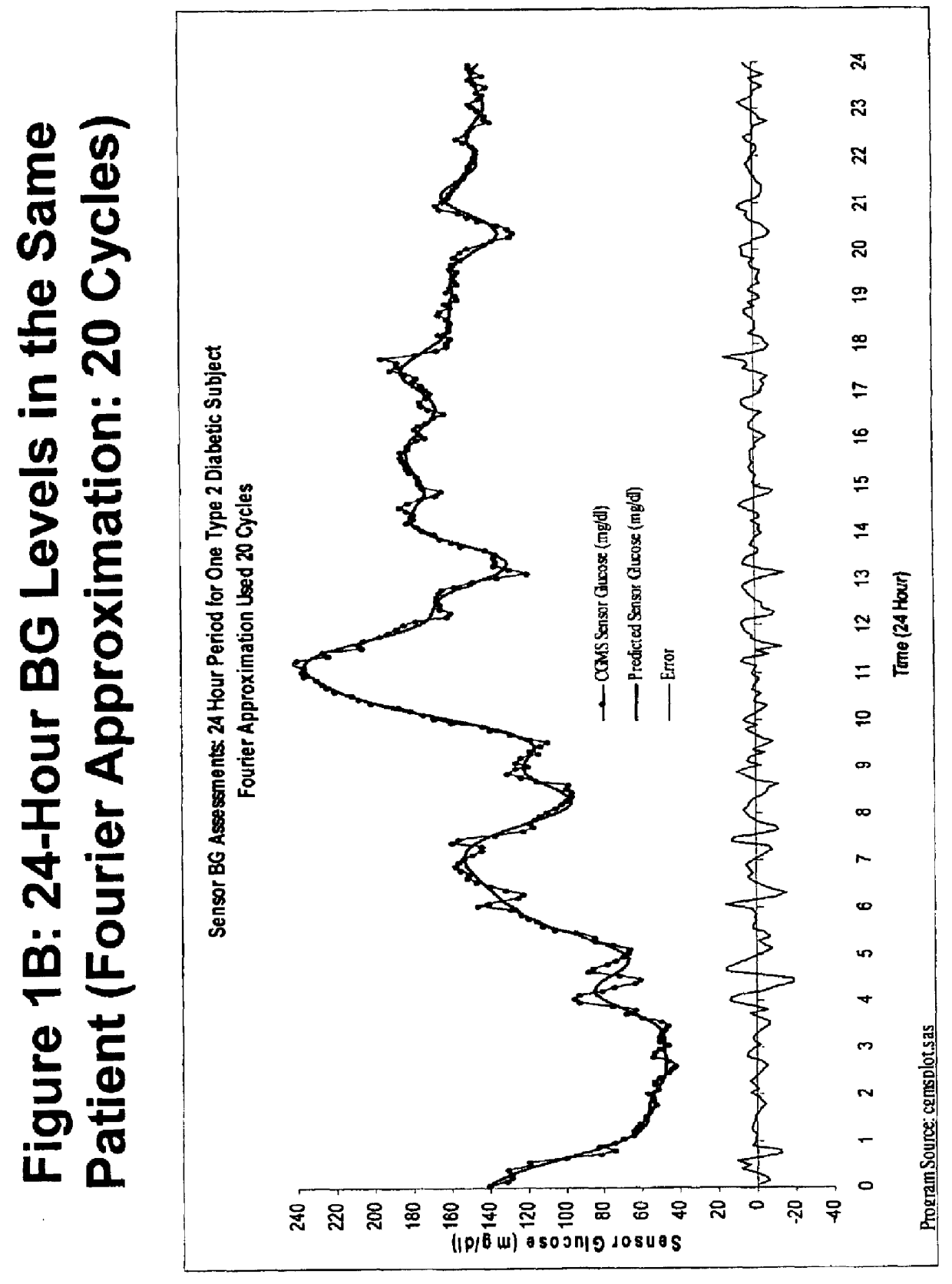
Method for analyzing continuous glucose monitoring data
ActiveUSH2246H1Increased susceptibilityMedical simulationDiagnostic recording/measuringMedicineContinuous glucose monitoring
A method for predicting the effectiveness of medication-based therapy in lowering average blood glucose levels in a diabetic patient is provided. This method may further comprise selectively recommending a medication-based therapy on the basis of the arithmetic average of the relative minima. A method for determining susceptibility to symptomatic hypoglycemia in a patient is provided. This method may further comprise selectively recommending a medication-based therapy on the basis of the arithmetic average of the relative minima. Provided also is a device for continuously monitoring blood glucose levels in a patient. The methods and device involve applying a Fourier approximation to blood glucose level data.
Owner:SANOFI SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com