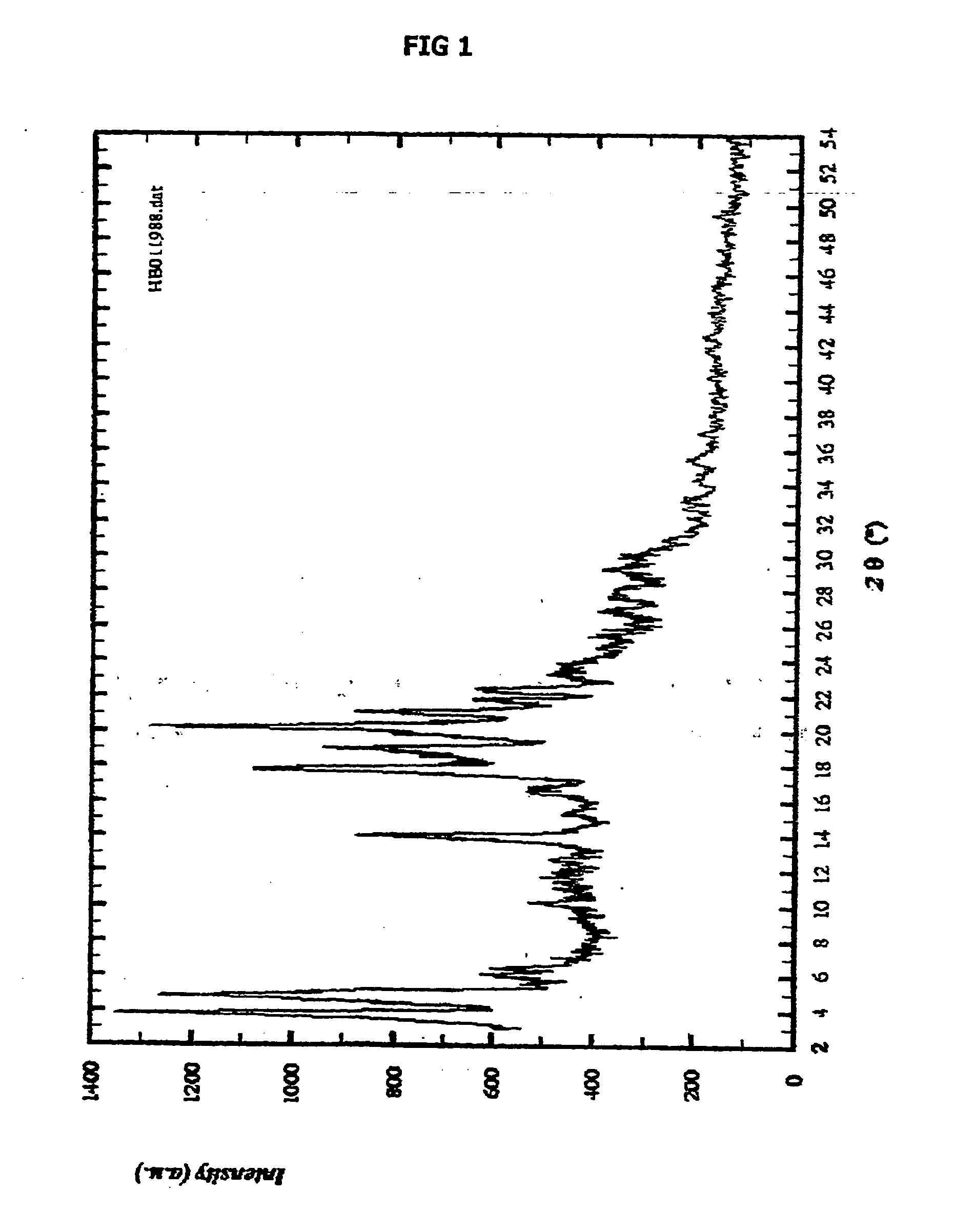
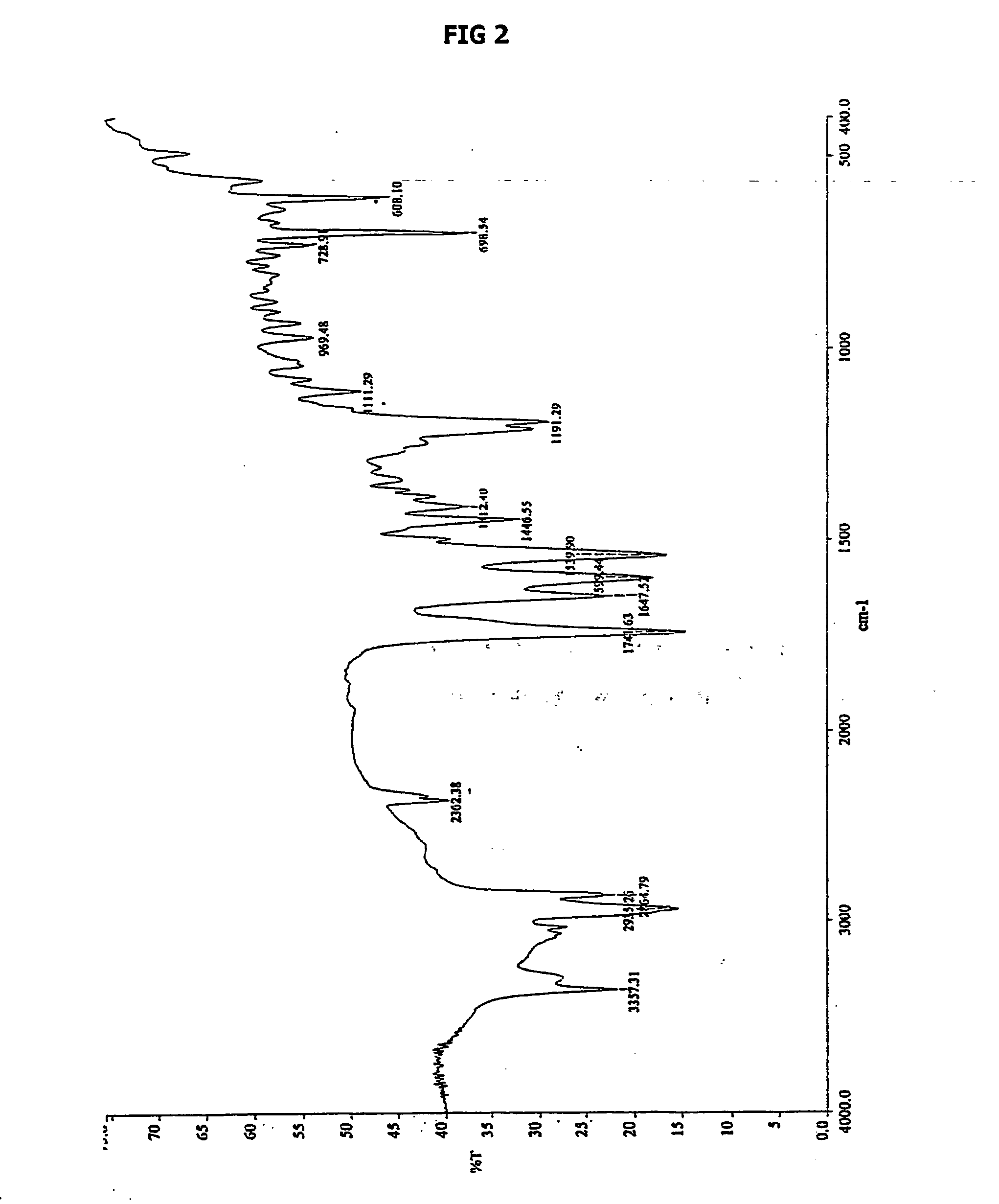
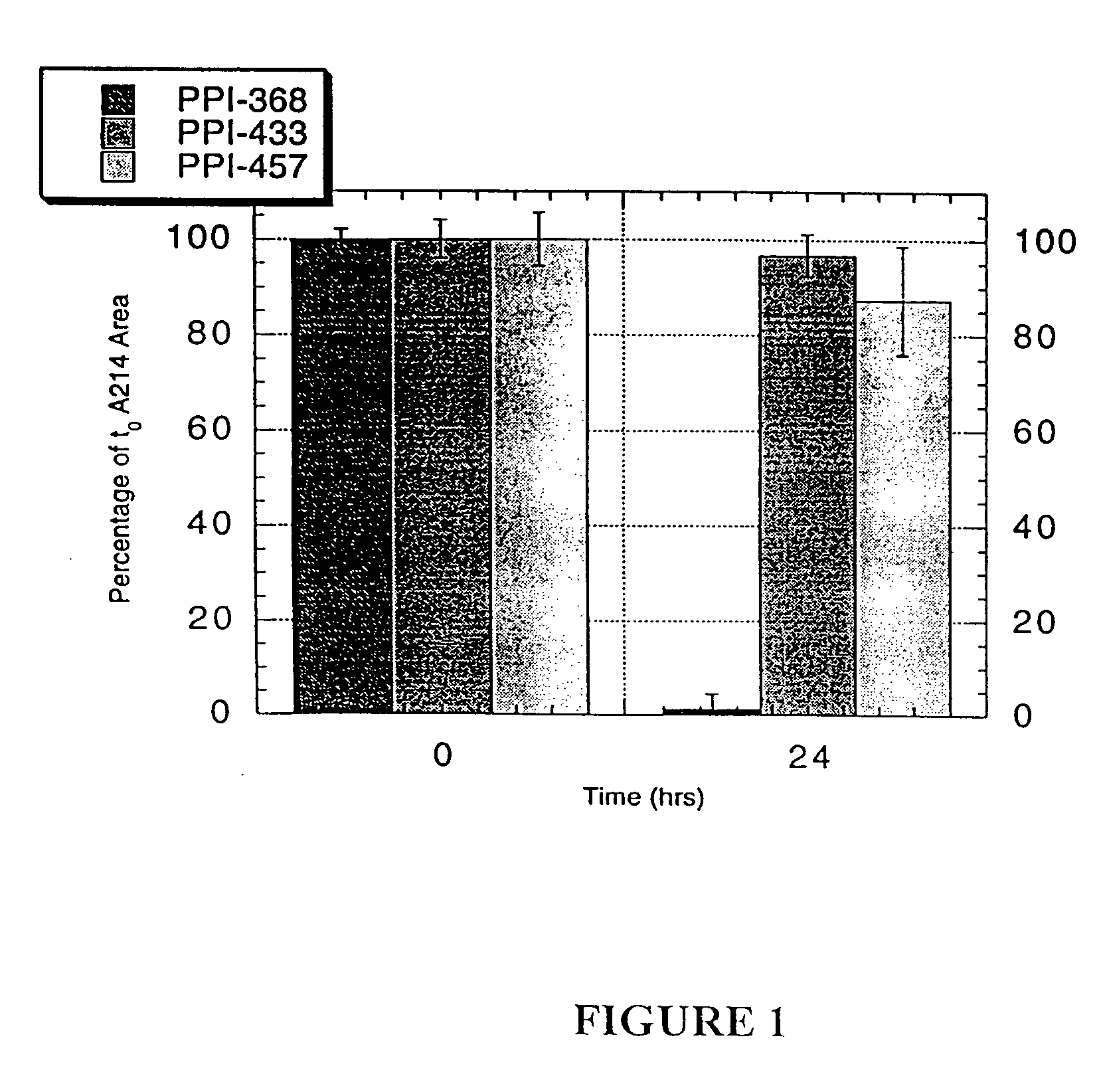
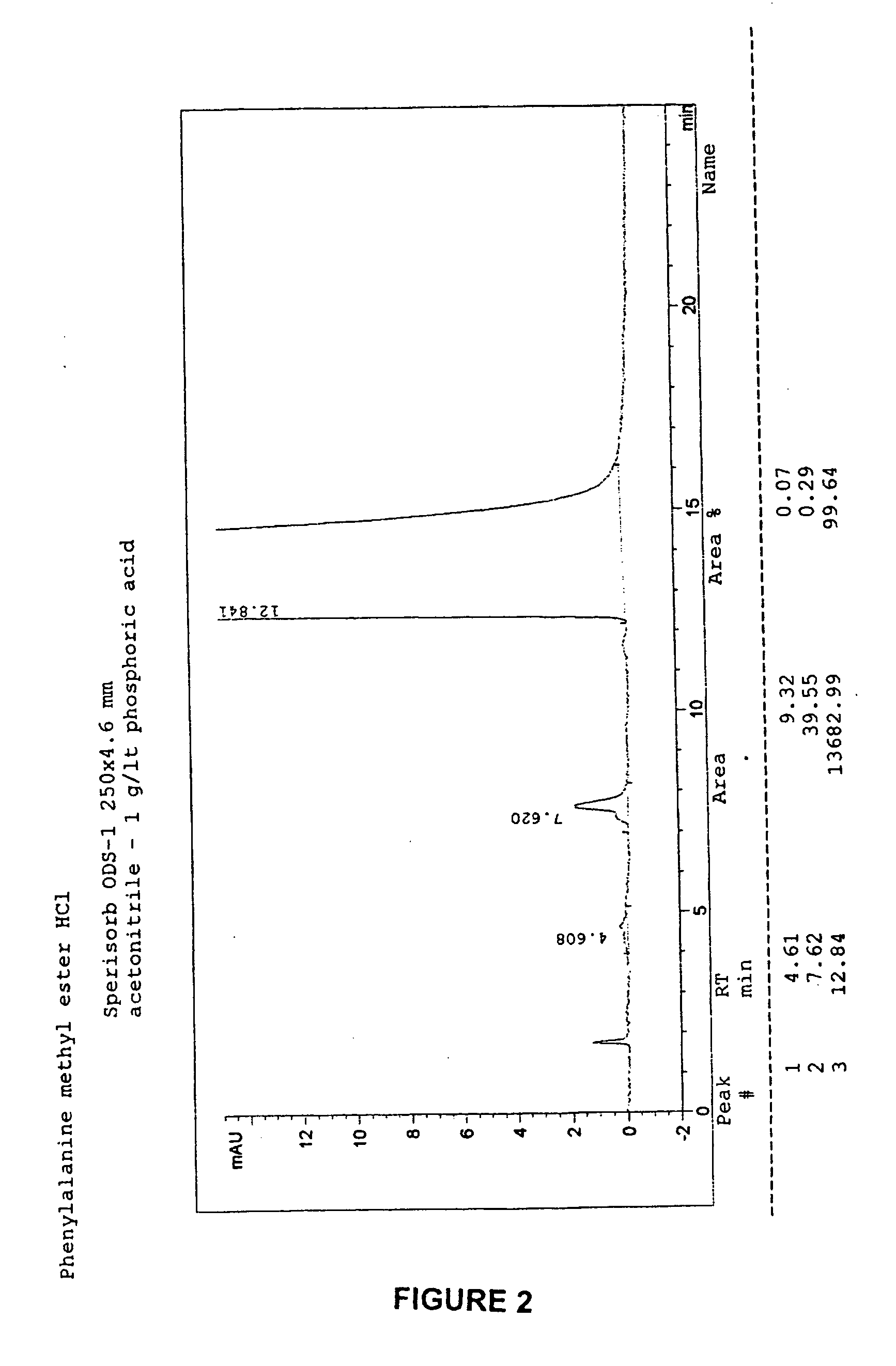
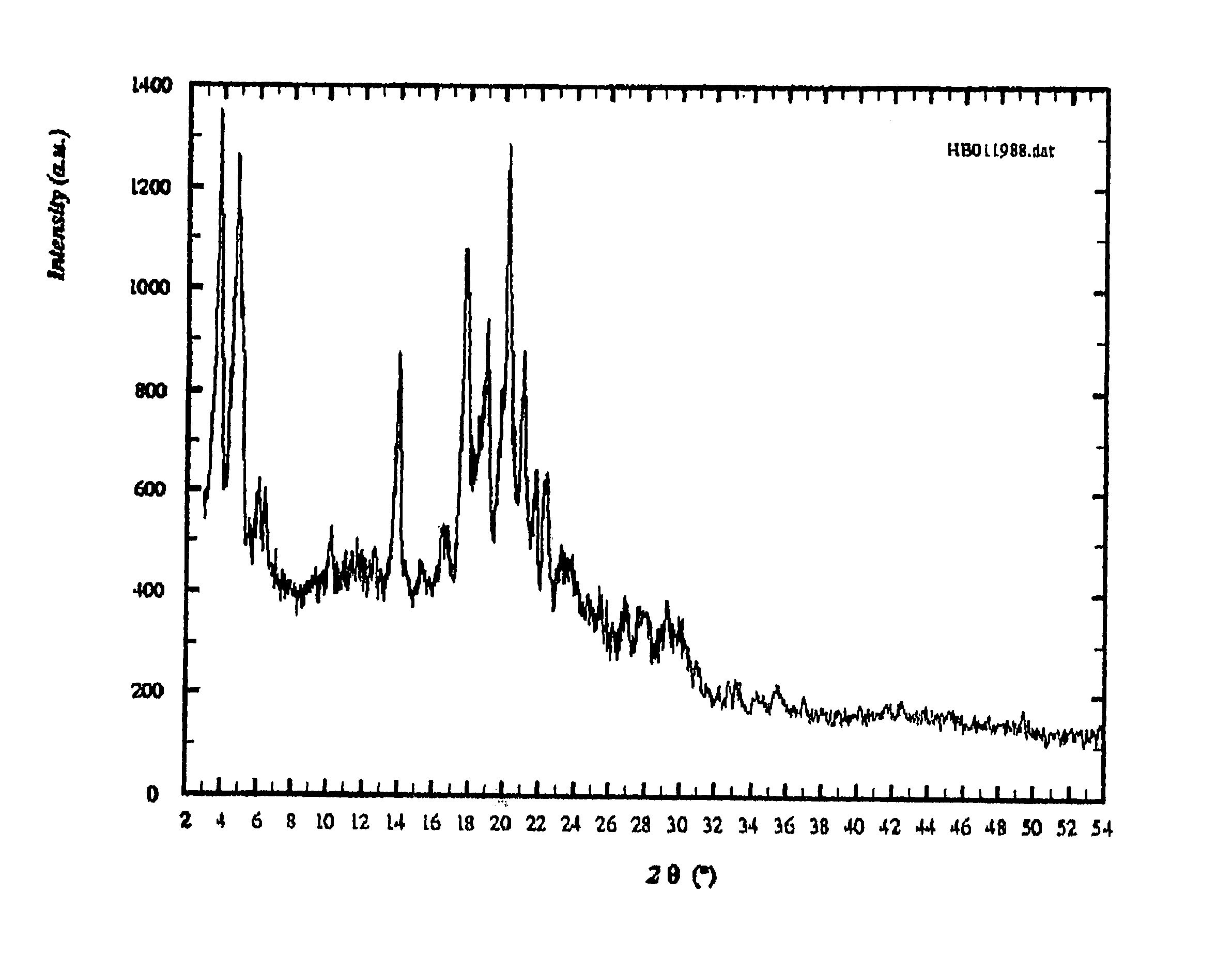
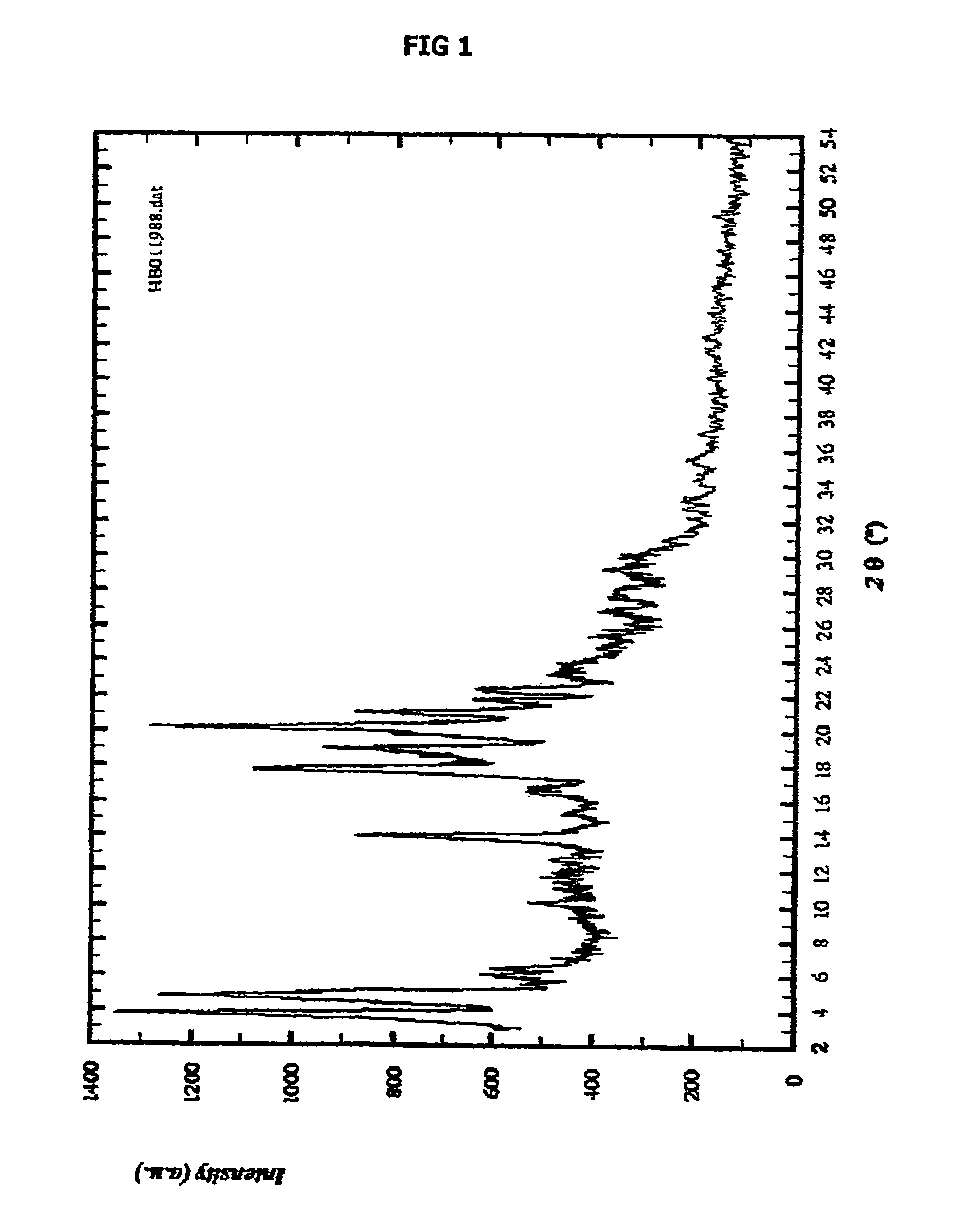
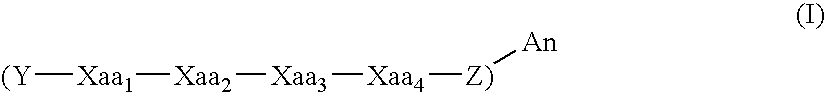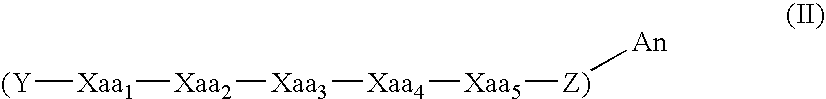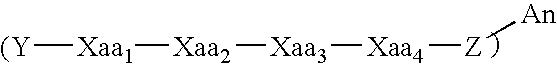Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "D-Phenylalanine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
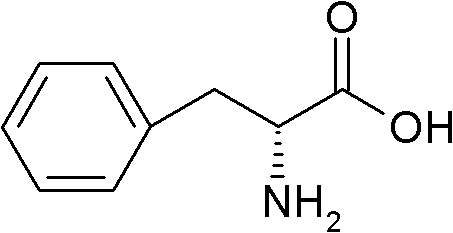
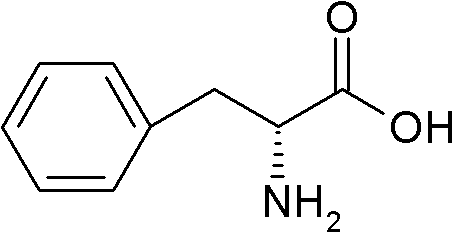
The synthetic dextro isomer of phenylalanine, an essential amino acid with anti-depressant and analgesic activities. D-Phenylalanine is converted into tyrosine and tyrosine in turn is converted into L-dopa, norepinephrine, and epinephrine, three key neurotransmitters. As a result this agent is associated with elevated levels of the neurotransmitters dopamine and norepinephrine in the brain, which may alleviate symptoms of depression. In addition, as an inhibitor of enkephalinase, which metabolizes endorphins, D-phenylalanine may be used to treat chronic pain through blocking the break down of endorphins (natural pain killers).
Peptide, a method for its preparation and a pharmaceutical composition containing the peptide
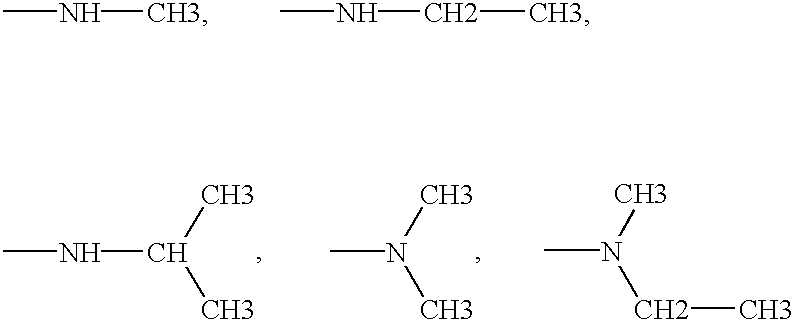
A peptide of the formula Iwherein X is hydrogen, glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophan, gamma-aminobutyric acid or ζ-aminocaproic acid; A is D-gluptamic acid or D-y-glutamic acid; and Y is glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophn, gamma-aminobutyric acid, ζ-aminocaproic acid, hydroxyl, or an amide group.
Owner:IMMUNOTECH DEV
Peptide, a method for its preparation and a pharmaceutical composition containing the peptide
A peptide of the formula IX-A-D-Trp-Ywherein X is hydrogen, glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophan, gamma -aminobutyric acid or xi -aminocaproic acid; A is D-gluptamic acid or D-y-glutamic acid; and Y is glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophn, gamma -aminobutyric acid, xi -aminocaproic acid, hydroxyl, or an amide group.
Owner:IMMUNOTECH DEV
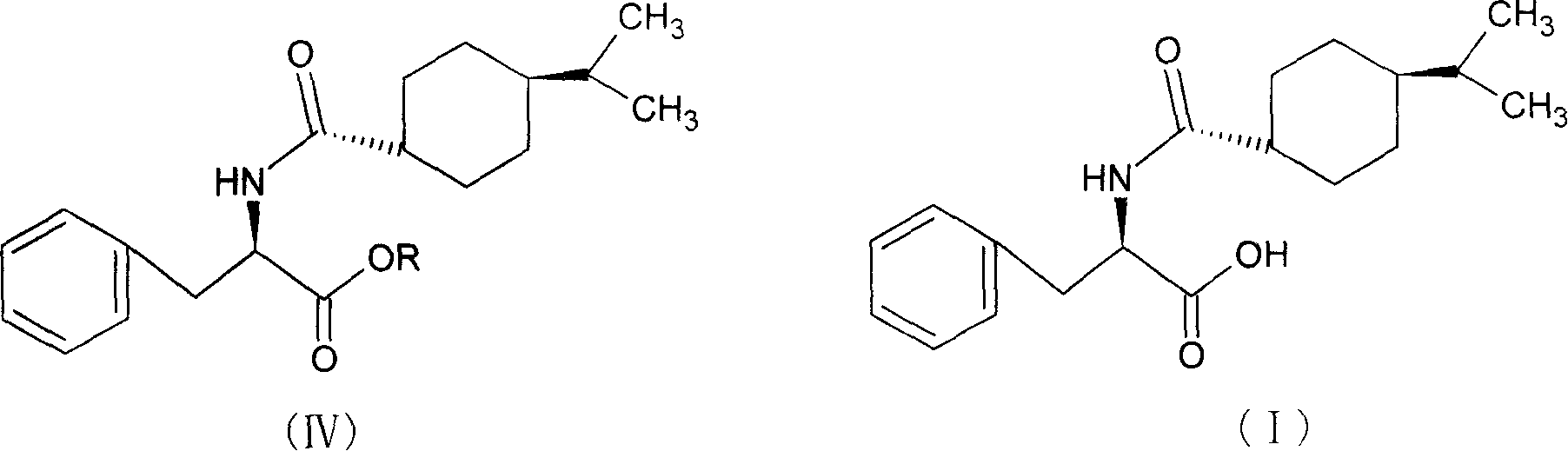
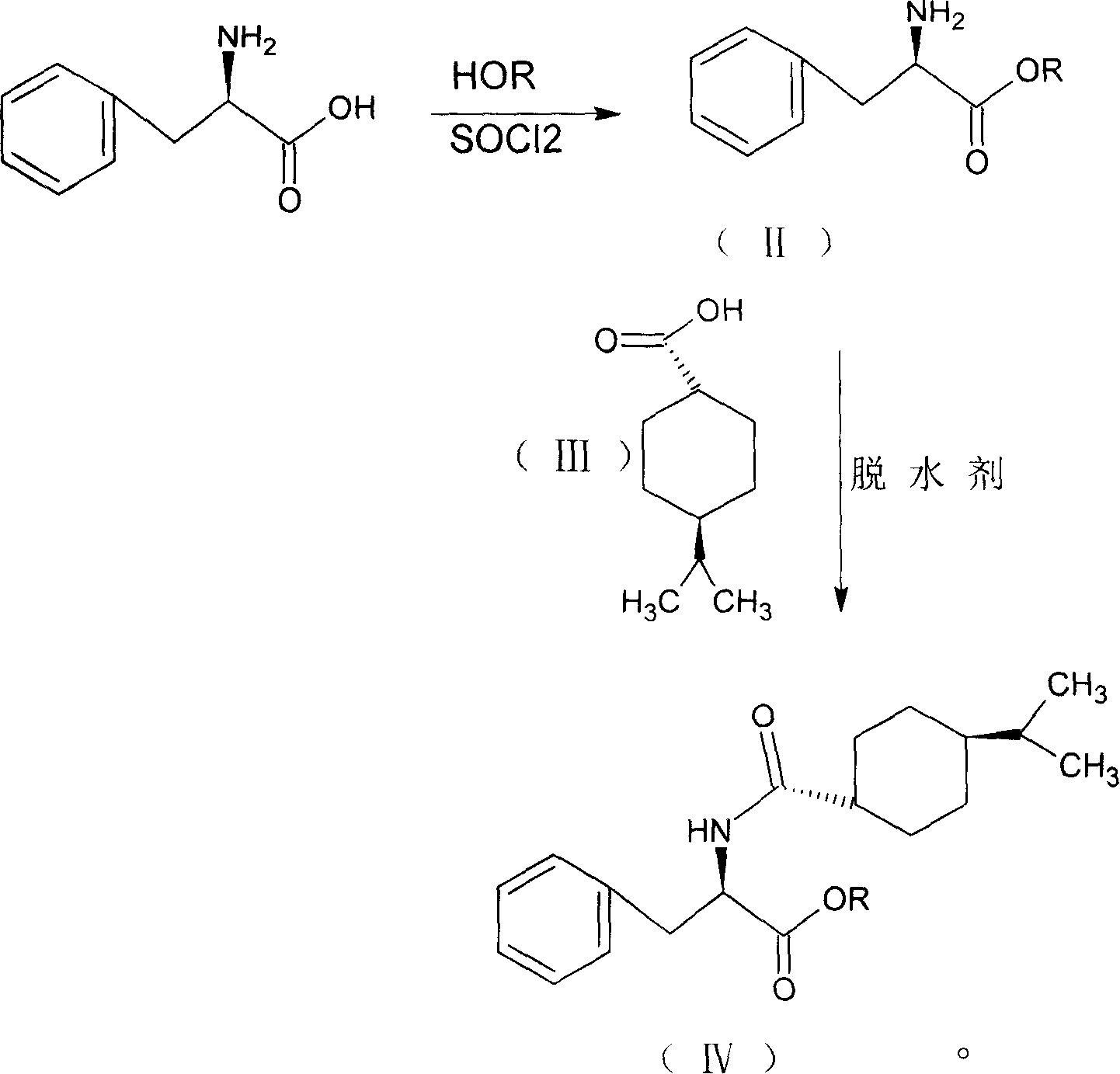
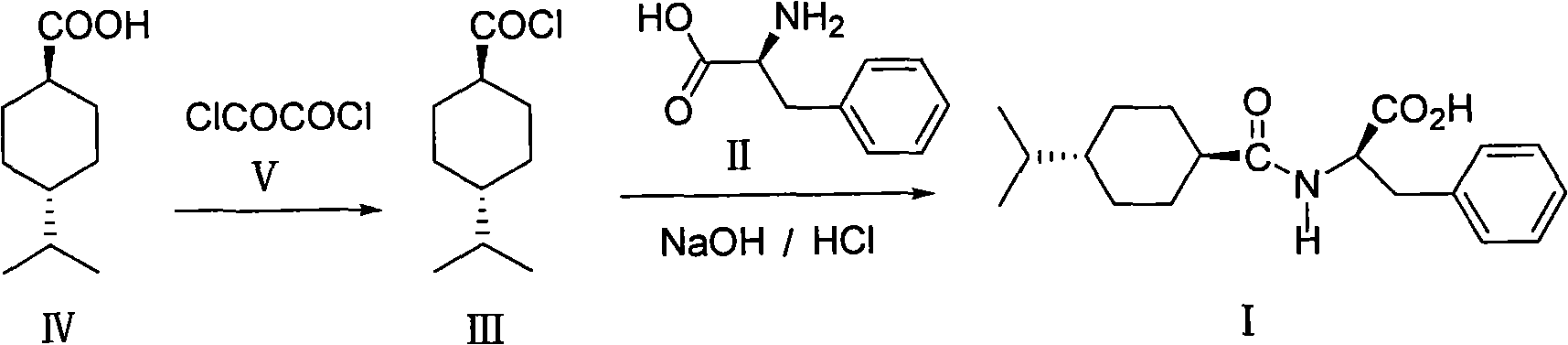
Process for preparing nateglinide and intermediates thereof
Provided is a process for preparation of an intermediate in the synthesis of nateglinide. Trans-4-isopropylcyclohexane acid chloride is formed by reacting 4-isopropylcyclohexanecarboxyl acid with thionyl chloride in the presence of an effective amount of an organic amide.Also provided are processes for preparation of nateglinide by acylation of a suitable salt of D-phenylalanine with trans-4-isopropylcyclohexane acid chloride in both a single and a two phase system, and in water free of a co-solvent.
Owner:TEVA PHARM USA INC
Method for preparing dextrorotary phenylalanine by asymmetric conversion method
InactiveCN1418862AAvoid entrained precipitationReduce usageOrganic compound preparationAmino-carboxyl compound preparationIce waterD-Phenylalanine
The method for preparing D-phenylalanine by using DL-phenylalanine includes the following steps: firstly, mixing DL-phenylalanine and D-tartaric acid according to the mole ratio of 1:1.5-1.5:1 and dissolving them in organic solvent, adding 1.0-5.0% of aromatic alolehyde catalyst and making reaction for 6-8 hr. at 70-90 deg.C, cooling by using ice water bath, filtering, using anhydrous ethyl etherto wash and drying to obtain D-tartaric acid: D-phenylalanine salt, then dissolving the above-mentioned product in solvent, adding ammonization agent to make ammonization while stirring, after 1 hr. cooling to 5 deg.C-10 deg.C, filtering and washing with solvent so as to obtain the invented D-phenylalanine.
Owner:SOUTHEAST UNIV
Cyclic pentapeptide as well as synthetic method and application thereof
ActiveCN103965299AAvoid ring closureImprove ring closure efficiencyDepsipeptidesPeptide preparation methodsChemical synthesisDipeptide
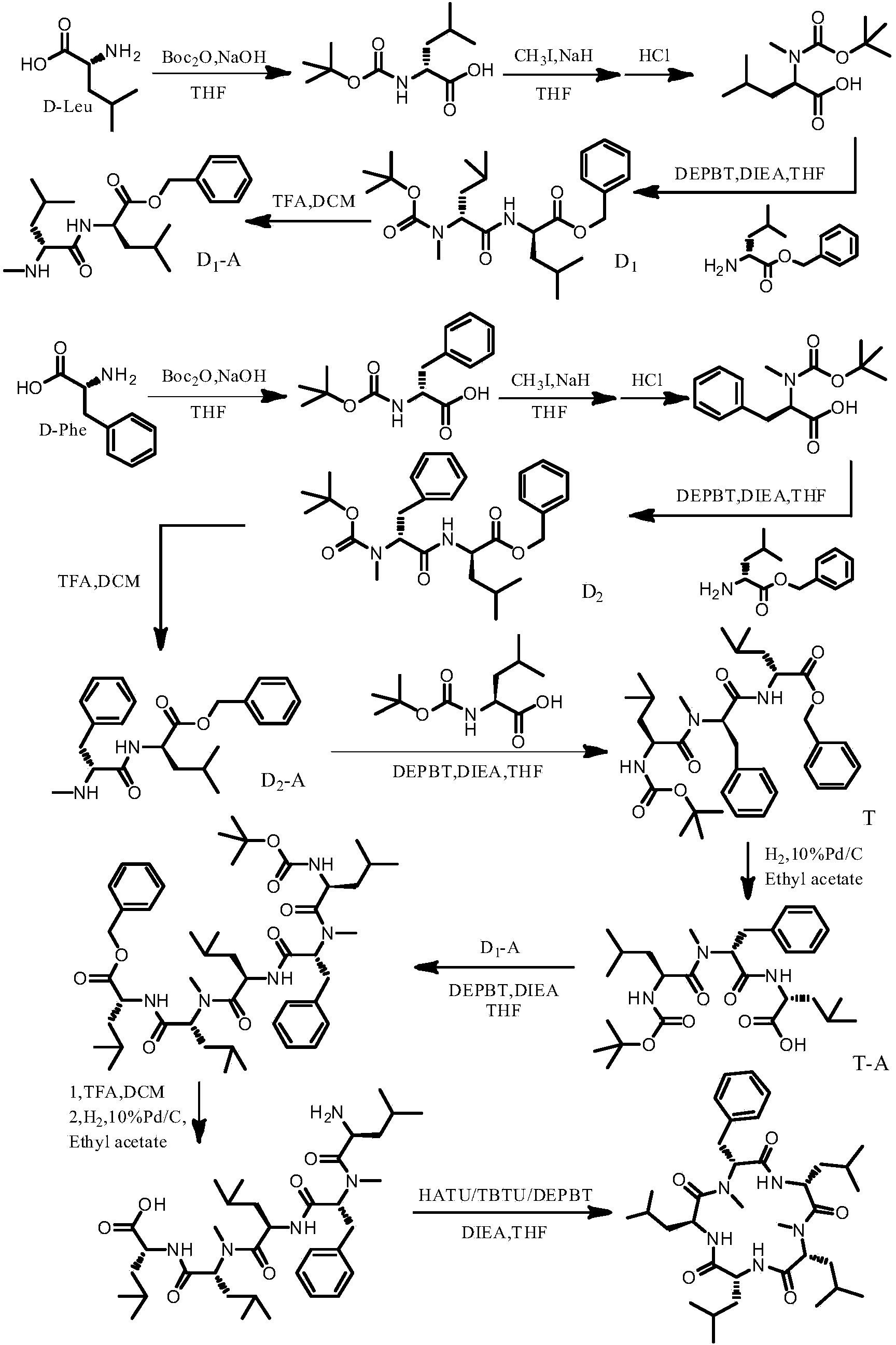
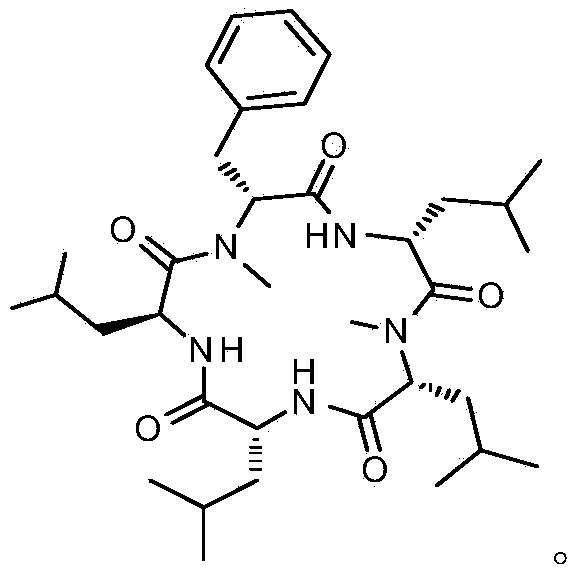
The invention belongs to the field of organic chemical synthesis, and discloses cyclic pentapeptide with a novel structure, as well as a synthetic method and application thereof. The cyclic pentapeptide with the novel structure is cyclo-(N-Me-D-Phe-D-Leu-N-Me-D-Leu-D-Leu-L-Leu), the molecular formula is C35H57N5O5, and the structural formula is shown in the description. The cyclic pentapeptide adopts D-Leu, L-Leu, D-Phe and D-Leu benzyl ester tosilate as raw materials, a tripeptide midbody and a dipeptide midbody are respectively prepared through a proper technology, and a cyclization locus is selected at the amido linkage position adjacent to D and L configurations, so that the cyclization efficiency is effectively increased. The cyclic pentapeptide has the advantages of simple synthetic process, low raw material cost, mild reaction conditions, high purity and easy industrial production, and can fully meet the requirements of medical experiments and clinical application.
Owner:JINAN UNIVERSITY
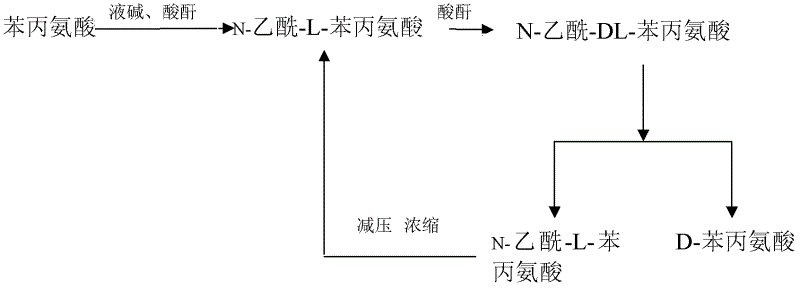
Method for producing D-phenylalanine by using L-phenylalanine as raw material
The invention relates to a method for producing D-phenylalanine by using L-phenylalanine as a raw material. The method comprises the following steps: preparing the L-phenylalanine into 0.3-0.4kg / L aqueous solution; adjusting the pH value to 8-13 with sodium hydroxide solution; dropping acetic anhydride for acylating; controlling the reaction temperature below 40DEG C until no color displays in a ninhydrin reaction; adjusting the pH value of the acylated acetyl-L-phenylalanine to 4-6 by using hydrochloric acid; when the temperature is raised to 60-90DEG C, dropping the anhydride for racemizing; when a rotation value of a racemic liquid is less than or equal to 0.1, adjusting the pH value of the racemic liquid to 1-4 with the hydrochloric acid and cooling and crystallizing to obtain an acetyl-DL-phenylalanine crystal; preparing the obtained acetyl-DL-phenylalanine crystal into 0.04kg / L resolution liquid, adjusting the pH value to 7.8-8.2, adding 15mg / L enzyme D, preserving the heat of 40DEG C for 24 hours and stopping resolution; and extracting the resolution liquid through cation exchange resin to obtain the D-phenylalanine. The method disclosed by the invention has the advantages of simple process flow, capability of obtain high-purity products and low cost.
Owner:闫博
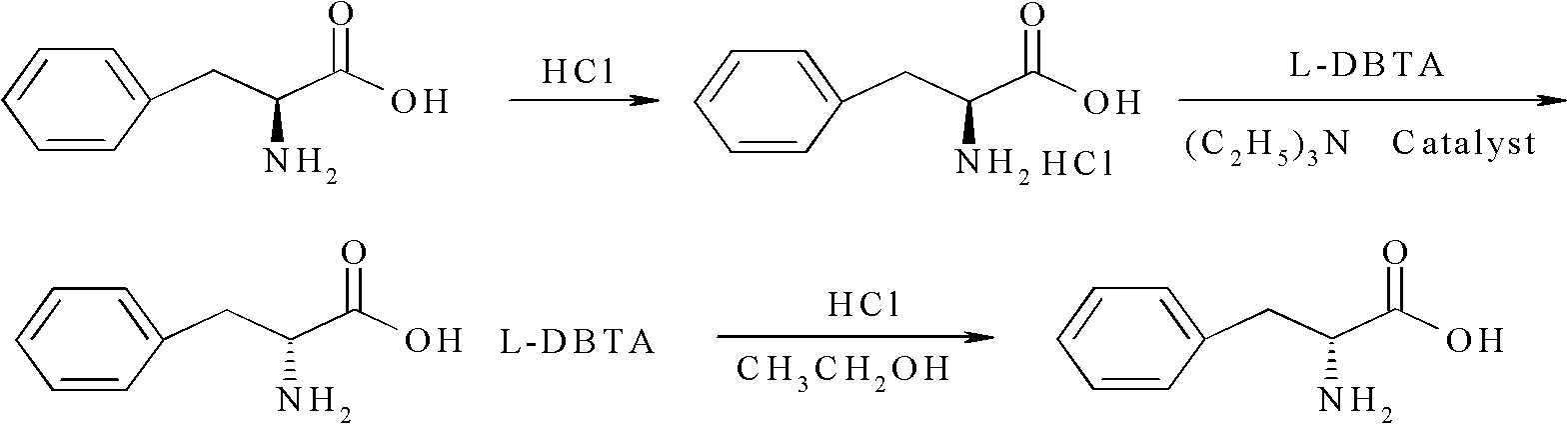
Method for preparing D-phenylalanine through dynamic kinetic resolution
InactiveCN102010345AHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSolventOperating cost
The invention discloses a method for preparing D-phenylalanine through dynamic kinetic resolution. The method comprises the following steps of: reacting L-phenylalanine serving as raw materials with hydrochloric acid to generate L-phenylalanine hydrochloride; racemizing and resolving under the action of a solvent and a racemization catalyst by taking the L-phenylalanine hydrochloride as a substrate and dibenzoyl tartaric acid (L-DBTA) as a resolving agent to obtain D-phenylalanine.L-DBTA disalt; and finally performing triethylamine resolution to prepare the target product D-phenylalanine. Compared with the prior art, the method directly takes the L-phenylalanine as the resolving substrate, mild alcohol as the solvent and an aldehyde pyridine compound as the racemization catalyst, racemizes and resolves the L-phenylalanine into the D-phenylalanine.L-DBTA disalt, has a theoretical conversion rate of about 100 percent, greatly improves the resolution yield, reduces the operating cost, ensures stable product quality and is suitable for industrial production.
Owner:SHANGHAI HUAYI GRP CO
Preparation method of mitiglinide calcium
The invention relates to a preparation method of a bulk drug of mitiglinide calcium used for treating type 2 diabetes mellitus. The mitiglinide calcium is prepared by a series of reaction steps comprising using D-phenylalanine as a starting material, esterification and cis-hexahydroisoindoline reaction. In the reaction, the problem of high cost of special equipment for high pressure hydrogenation and operation as well as chiral catalyst in the traditional process can be solved, the use of chiral resolution and a resolving agent is avoided, and the yield is improved.
Owner:DISHA PHARMA GRP +1
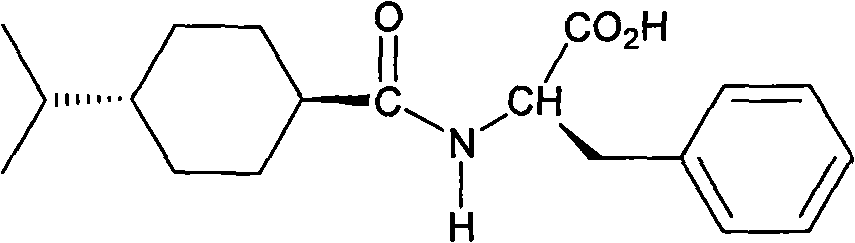
Novel form of n-(trans-4-isopropylcyclohexylcarbonyl)-d-phenylalanine
The present invention relates to a novel crystalline form of N-(trans-4-isopropylcyclohexylcarbonyl)-D-phenylalanine and methods for preparing the same.
Owner:BIOCON LTD
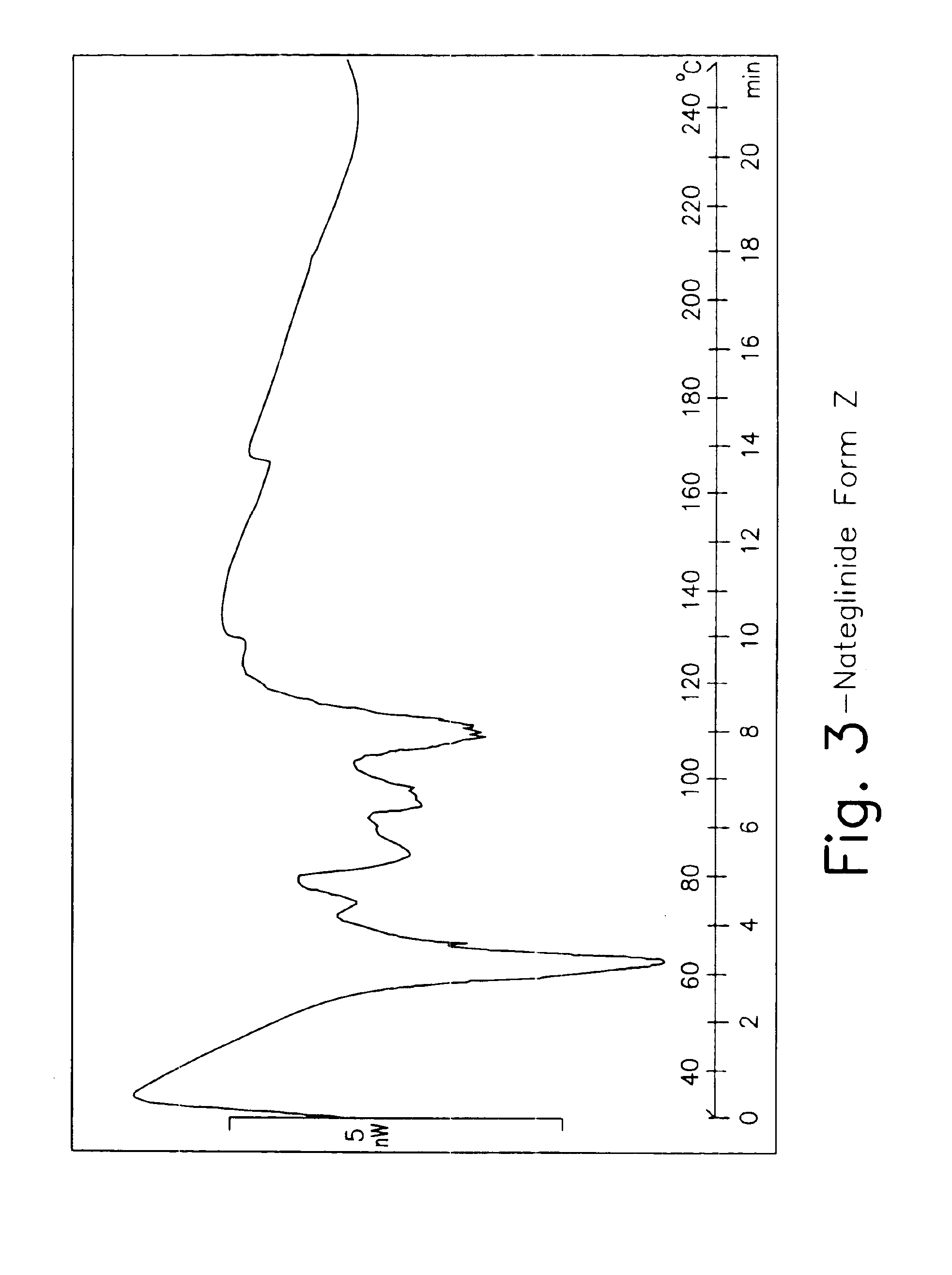
Methods for producing nateglinide crystals
InactiveUS7208622B2Organic compound preparationCarboxylic acid amide separation/purificationD-PhenylalanineKetone solvents
There is provided methods for producing nateglinide crystals, which comprises the steps of adding an acid(s) to a reaction mixture containing nateglinide to make it acidic, the reaction mixture being obtained by reacting trans-4-isopropylcyclohexylcarbonyl chloride with D-phenylalanine in a mixed solvent of ketone solvent and water in the presence of an alkali; and then adjusting the temperature of the mixture to 58° C. to 72° C. and the concentration of ketone solvent to more than 8 wt % and less than 22 wt % to conduct precipitation of nateglinide crystals. This producing method is the industrially beneficial methods for crystallization of nateglinide.
Owner:AJINOMOTO CO INC
Modulators of beta-amyloid peptide aggregation comprising D-amino acids
InactiveUS20060014696A1Improve abilitiesNervous disorderTetrapeptide ingredientsTert-leucineAmyloid disease
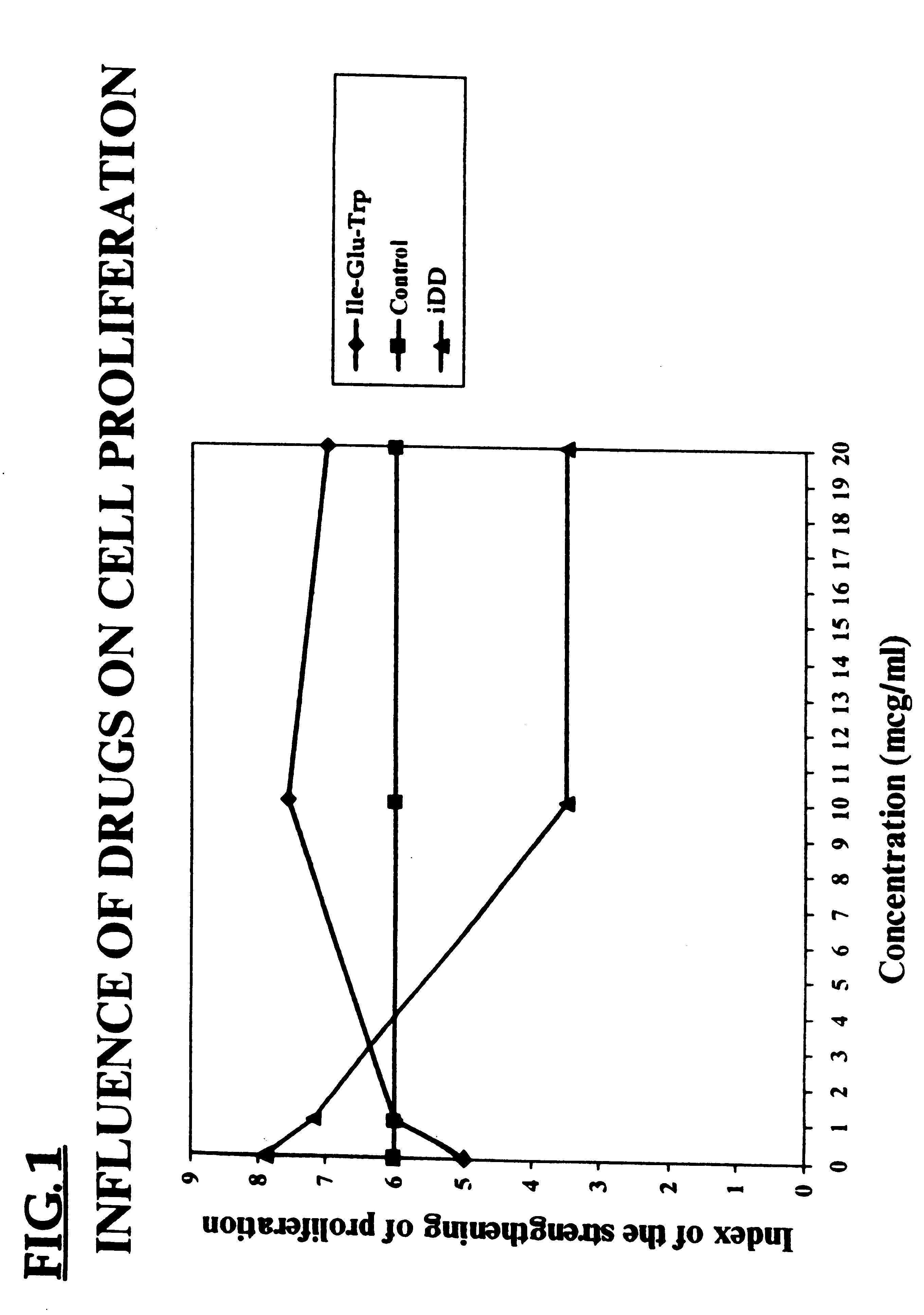
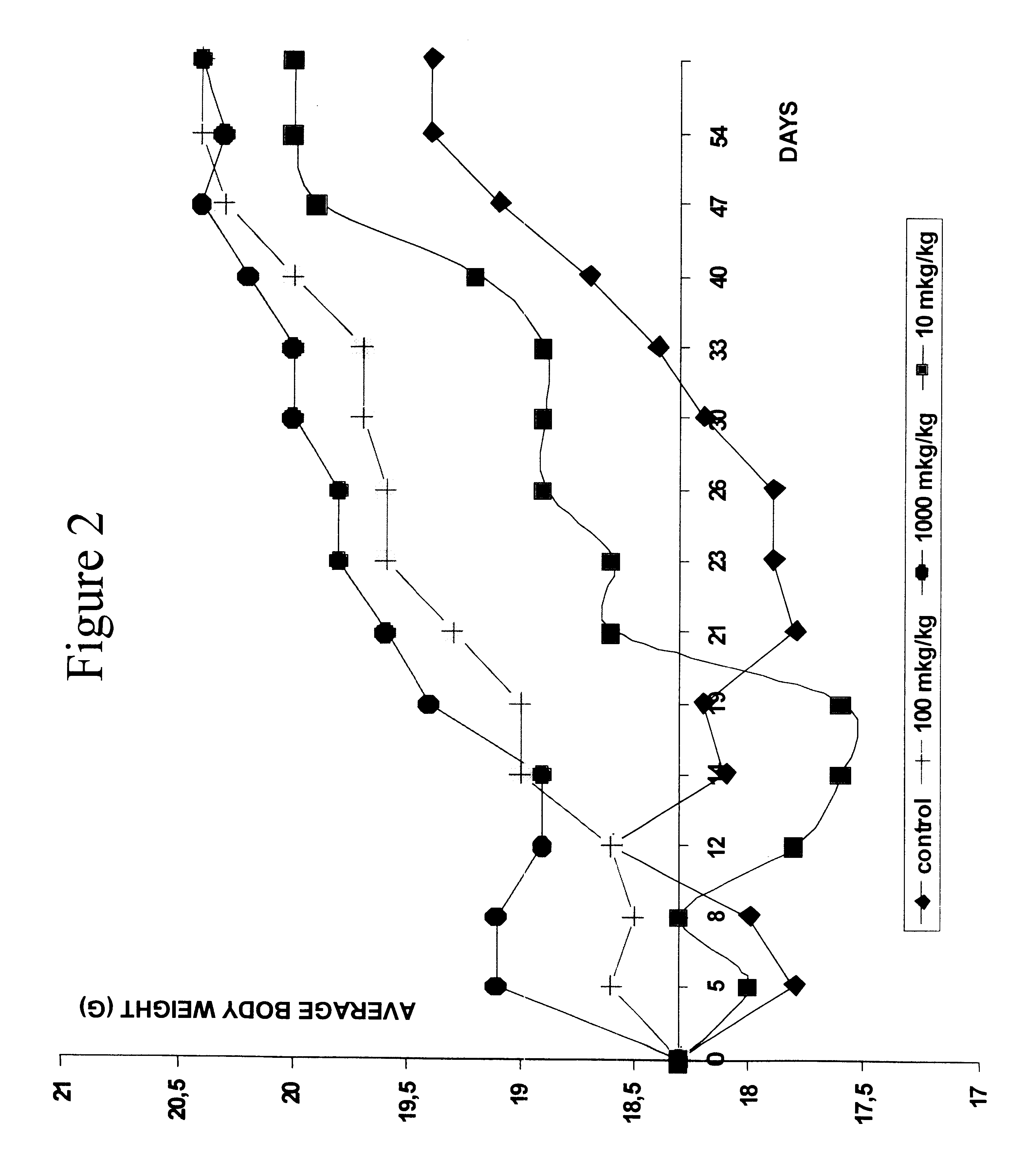
Compounds that modulate natural β amyloid peptide aggregation are provided. The modulators of the invention comprise a peptide, preferably based on a β amyloid peptide, that is comprised entirely of D-amino acids. Preferably, the peptide comprises 3-5 D-amino acid residues and includes at least two D-amino acid residues independently selected from the group consisting of D-leucine, D-phenylalanine and D-valine. In a particularly preferred embodiment, the peptide is a retro-inverso isomer of a β amyloid peptide, preferably a retro-inverso isomer of Aβ17-21. In certain embodiments, the peptide is modified at the amino-terminus, the carboxy-terminus, or both. Preferred amino-terminal modifying groups include cyclic, heterocyclic, polycyclic and branched alkyl groups. Preferred carboxy-terminal modifying groups include an amide group, an alkyl amide group, an aryl amide group or a hydroxy group. Pharmaceutical compositions comprising the compounds of the invention, and diagnostic and treatment methods for amyloidogenic diseases using the compounds of the invention, are also disclosed.
Owner:PRAECIS PHARM INC
Process for the preparation of nateglinide, preferably in B-form
InactiveUS20060148902A1High-yieldIncrease volumeOrganic active ingredientsBiocideD-PhenylalanineChemistry
The present invention relates to a process for the preparation of nateglinide, preferably in B-form, substantially free from the H-form, comprising three steps starting from D-phenylalanine methyl ester or a salt thereof.
Owner:A M ANONIMA MATERIE SINT E
Form of N-(trans-4-isopropylcyclohexylcarbonyl)-D-phenylalanine
The present invention relates to a novel crystalline form of N-(trans-4-isopropylcyclohexylcarbonyl)-D-phenylalanine and methods for preparing the same.
Owner:BIOCON LTD
Chemical synthesis method of chiral D-phenylalanine
InactiveCN102234241AThe production process is simple to operateReduce manufacturing costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisD-Phenylalanine
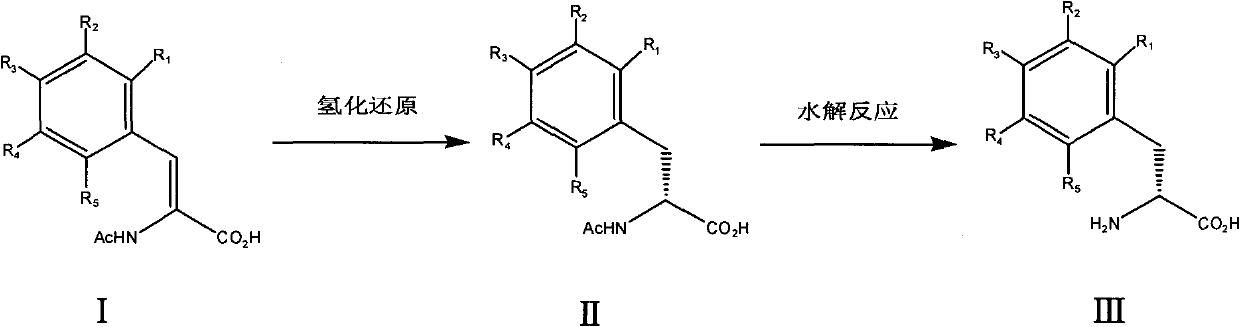
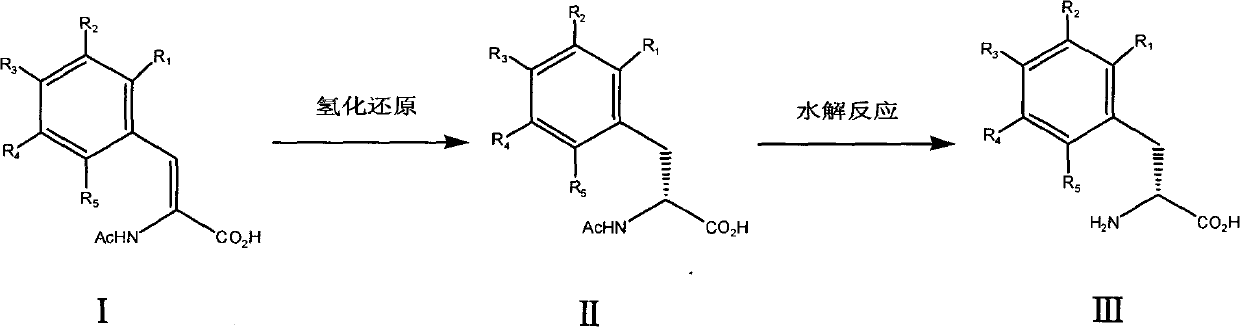
The invention relates to a chemical synthesis method for preparing chiral D-phenylalanine. The chemical synthesis method comprises the following reaction steps: (a) carrying out hydrogenation reduction on acetamidocinnamic acid and derivatives thereof (I) in the presence of a chiral catalyst to obtain acetamido-D-phenylalanine (II), and (b) carrying out hydrolysis reaction on the acetamido-D-phenylalanine to remove acetyl, thus obtaining D-phenylalanine (III).
Owner:北京金源化学集团有限公司
Bioactive peptide, preparation thereof and application thereof
InactiveCN101274958AEffective synthetic routeEnough raw materialsPeptide preparation methodsAntineoplastic agentsDipeptideTert-Butyloxycarbonyl protecting group
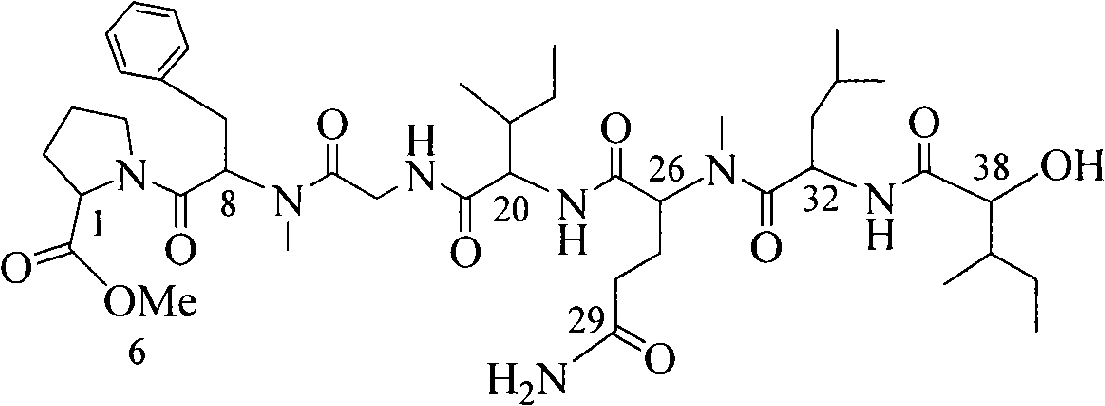
The invention relates to a biological bioactive peptide, and the molecular formula thereof is C42H67N7O10. When in preparation, tetrapeptide fragment N-(t-butoxycarbonyl)-L-isoleucine-N-methyl-D-phenylalanine-L-proline methyl ester is firstly prepared (t-butoxycarbonyl: BOC); then N-(9-fluorenylmethyloxycarbonyl)-N-methyl-glutamine is prepared (fluorenylmethyloxycarbonyl: Fmoc); then dipeptide fragment L-2-hydroxyl-3-methyl phocenic acid-O-benzyl salicylate-leucine is prepared; finally, the tetrapeptide fragment is detached from protecting groups and goes through condensation with N-(9-Fmoc)-N-methyl-glutamine, thus obtaining pentapeptide fragment N-(9-Fmoc)-N-methyl-glutamine-L-isoleucine-N-methyl-D-phenylalanine-L-proline methyl ester which goes through condensation after the dipeptide fragment and the pentapeptide fragment are detached from protecting groups. The biological bioactive peptide can provide sufficient raw materials and effective synthetic routes for the research of anti-tumor medicines and has a simple and convenient preparation method and easily controllable conditions.
Owner:OCEAN UNIV OF CHINA
Modulators of β-amyloid peptide aggregation comprising D-amino acids
Compounds that modulate natural β amyloid peptide aggregation are provided. The modulators of the invention comprise a peptide, preferably based on a β amyloid peptide, that is comprised entirely of D-amino acids. Preferably, the peptide comprises 3–5 D-amino acid residues and includes at least two D-amino acid residues independently selected from the group consisting of D-leucine, D-phenylalanine and D-valine. In a particularly preferred embodiment, the peptide is a retro-inverso isomer of a β amyloid peptide, preferably a retro-inverso isomer of Aβ17-21. In certain embodiments, the peptide is modified at the amino-terminus, the carboxy-terminus, or both. Preferred amino-terminal modifying groups include cyclic, heterocyclic, polycyclic and branched alkyl groups. Preferred carboxy-terminal modifying groups include an amide group, an alkyl amide group, an aryl amide group or a hydroxy group. Pharmaceutical compositions comprising the compounds of the invention, and diagnostic and treatment methods for amyloidogenic diseases using the compounds of the invention, are also disclosed.
Owner:PRAECIS PHARM INC
Method for preparing D-phenylalanine
ActiveCN101289410ANo pollution in the processSimple processOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisD-Phenylalanine
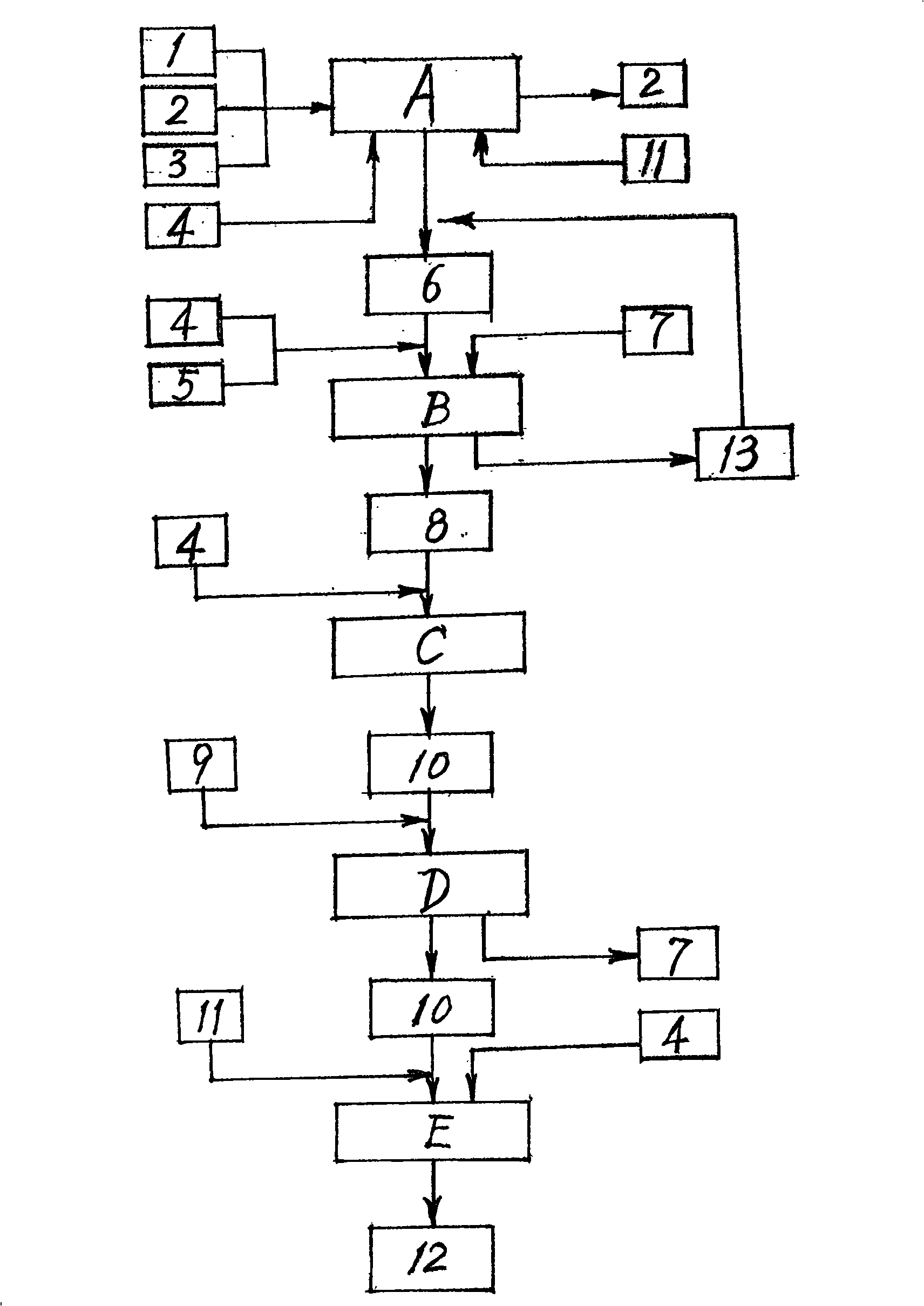
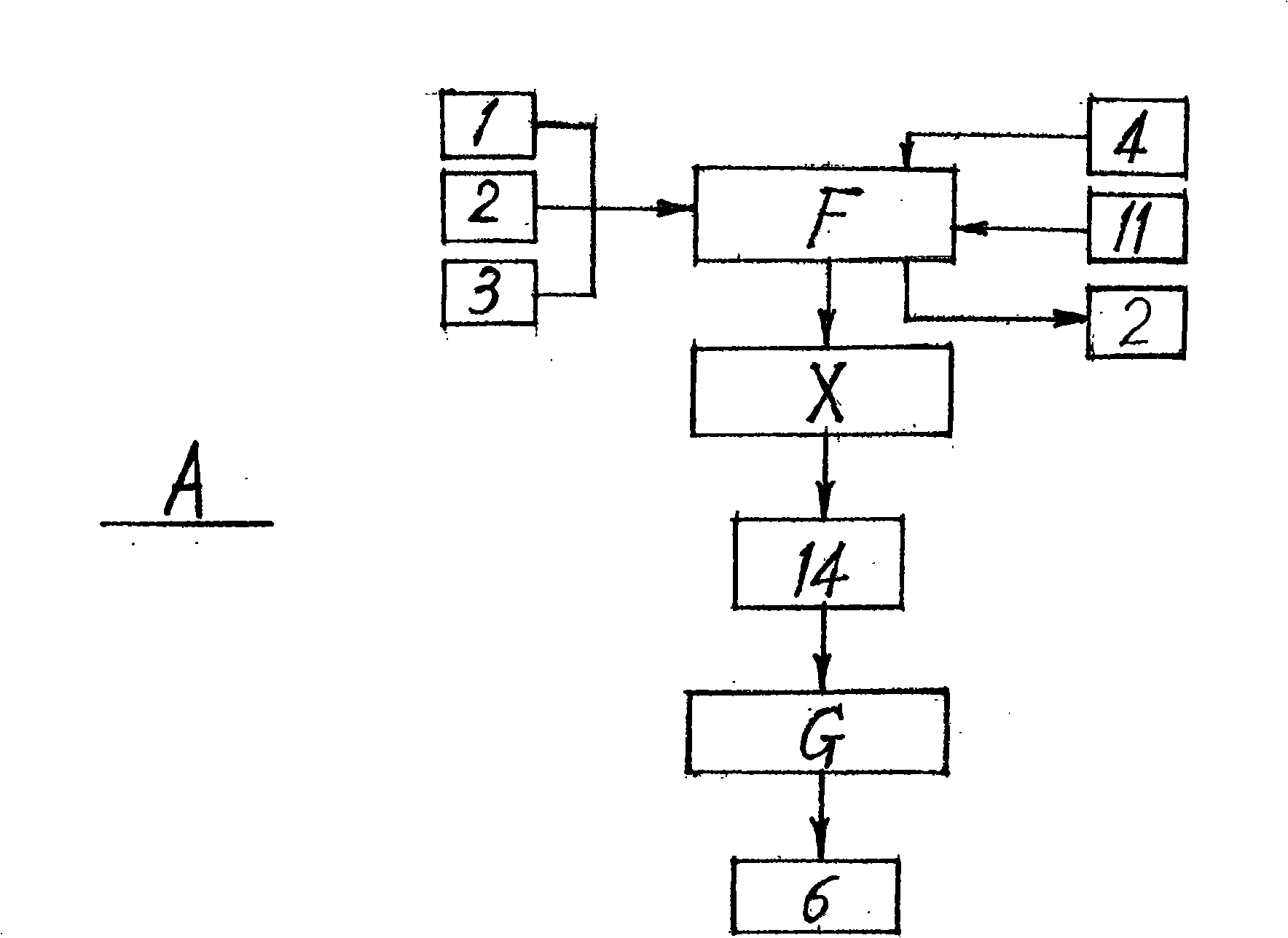
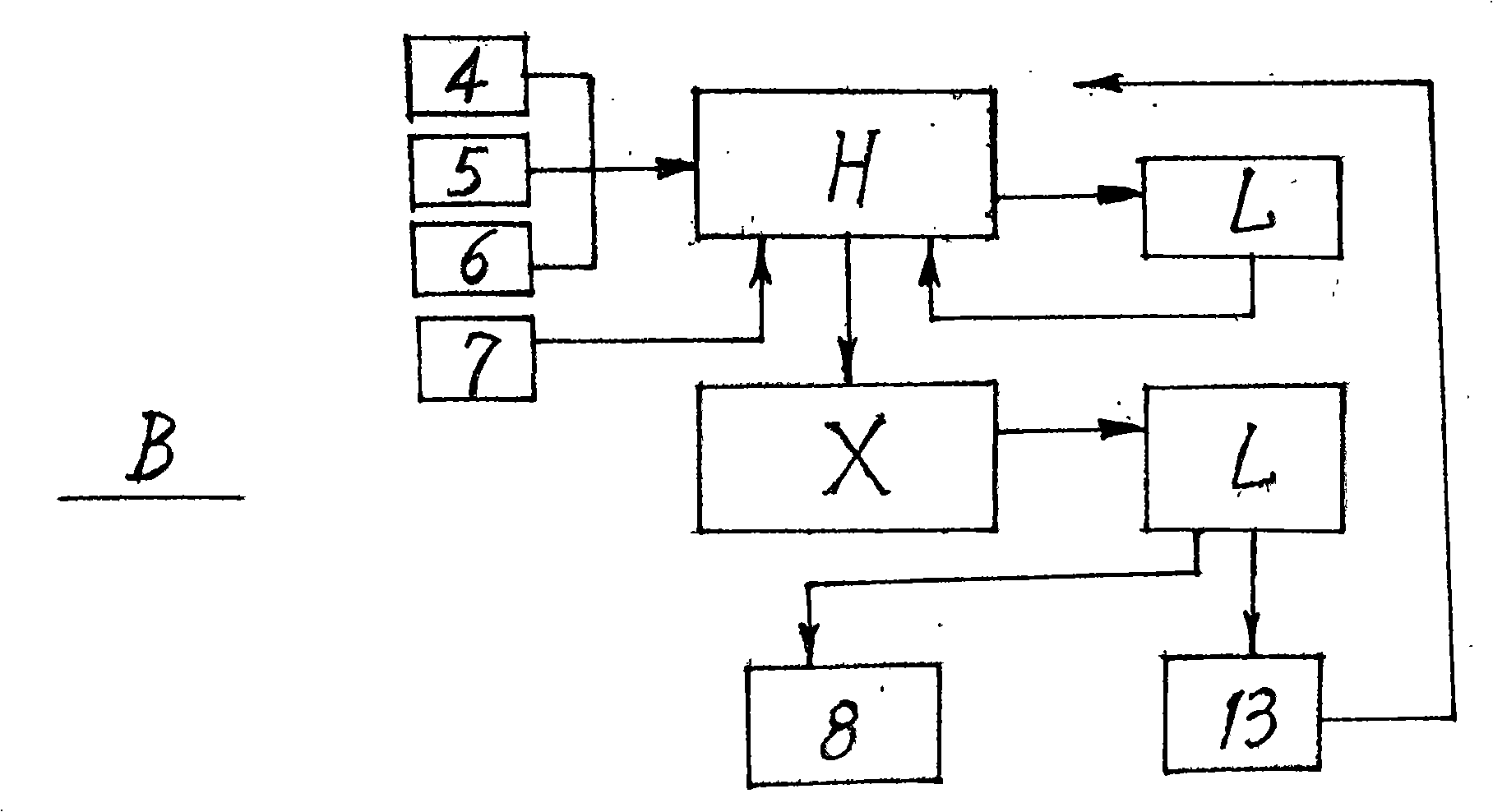
The invention relates to a preparation method of D-phenylalanine which takes L-phenylalanine (1) as the main raw material, adopts equipments, such as a reactor, a filter (L), a centrifuge (X), a vacuum drier (G), etc., adds solvent and chemical auxiliary materials according to the ratio of species and dose and then carries out chemosynthesis at the given temperature, environment and time. DL-phenylalanine (6) is generated by recemization technique (A). Complex salt crude product (8) of the D-phenylalanine is generated by splitting technique (B). Refined salt (10) of the D-phenylalanine is generated by refining technique (C) of the complex salt crude product. Splitting agent (7) is separated by the lysogenic response (D) of the refined salt. Finally, the D-phenylalanine (12) is made by the process flow of refined product technology. The method meets the needs of the environment protection without producing the pollution of three wastes, guarantees the quality, improves the yield and makes progress in research and development of chemical synthesis of the D-phenylalanine (12).
Owner:山东奥博生物科技有限公司
Preparation process for synthesizing D-phenylalanine by using high-activity bio-enzyme
The invention discloses a preparation process for synthesizing D-phenylalanine by using high-activity bio-enzyme, comprising the steps of: culturing Psudomonas No.1 strains by using a multivariant culture medium, flocculating and collecting mycelium, putting the collected mycelium in a transformation tank, and putting 5-benzyl hydantoin at the same time, wherein the weight of the 5-benzyl hydantoin is 1 / 5-1 / 6 of the total weight of fermentation liquor before the flocculation; protecting the transformation tank by using nitrogen, wherein the pressure is 0.1-0.2kg, the transformation temperature is 90DEG C, the stirring speed is 120RPM, the pH is kept at 8 and the transformation time is 24h; and conventionally collecting the transformed clear liquor, then refining, concentrating, evaporating, crystallizing, centrifuging and drying the obtain the finished product. In the invention, high-activity D-hydantoinase and N-carbamoyl enzyme can be generated after the fermentation of the strains. Through the transformation at proper transformation temperature, the transformation rate of the enzyme to a substrate is stabilized at 99-100%, so that the product yield of the D-phenylalanine can achieve 84-86%.
Owner:崔传僧
Preparation and application of graphene-ferrocene functionalized cyclodextrin chiral compound material
InactiveCN109251369AReduce agglomeration effectIncrease layer spacingMaterial electrochemical variablesD-PhenylalanineFerrocene
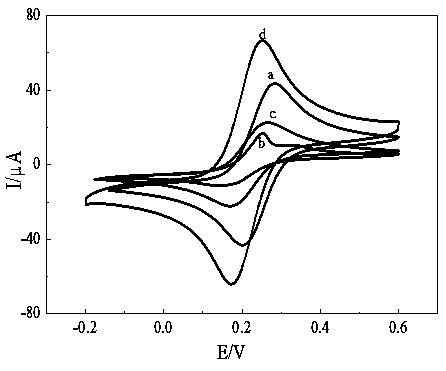
The invention discloses preparation and application of a graphene-ferrocene functionalized cyclodextrin chiral compound material. Firstly, graphene oxide (GO) is exfoliated by using ferrocene (Fc) through pi-pi action to reduce an aggregation effect of the GO and to increase interlayer spacing of the GO, so that functionalized GO-Fc is obtained; then under an alkaline condition, the GO-Fc and beta-cyclodextrin (beta-CD) are subjected to one-pot reaction to prepare the chiral compound material rGO-Fc-CD. A surface of a glassy carbon electrode (GCE) is modified with the chiral compound materialrGO-Fc-CD to form an electrochemical chiral sensing interface (rGO-Fc-CD / GCE), and the electrochemical chiral sensing interface (rGO-Fc-CD / GCE) has excellent conductivity. The chiral recognition material modified electrode rGO-Fc-CD / GCE is placed in a phenylalanine isomer solution, differential pulse voltammetry is used for scanning, and due to the fact that when L-phenylalanine and D-phenylalanine interact with the rGO-Fc-CD / GCE, peak current magnitude is different, a phenylalanine isomer can be identified fast and sensitively.
Owner:NORTHWEST NORMAL UNIVERSITY
Method for synthesizing AHU377 calcium salt
InactiveCN108299226ALow costMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationPhenylmagnesium bromideTert-Butyloxycarbonyl protecting group
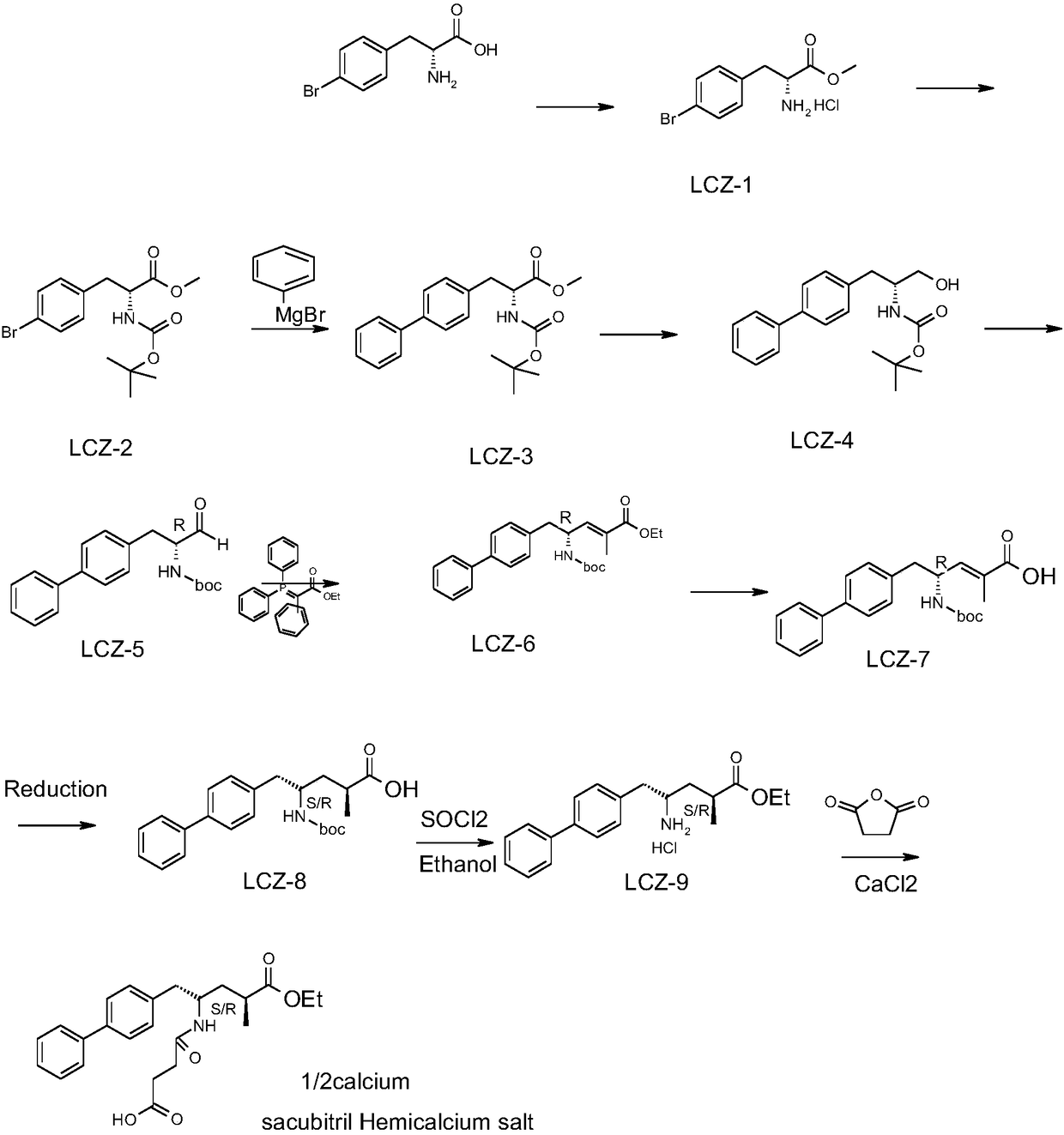
The invention discloses a method for synthesizing an AHU377 calcium salt. The method comprises the following steps: reacting 4-bromo-D-phenylalanine with thionyl chloride, reacting obtained methyl 4-bromo-D-phenylalaninate hydrochloride with BOC acid anhydride, reacting the obtained reaction product with phenylmagnesium bromide to obtain N-tert-butyloxycarbonyl-amino-4,4-biphenyl-R-alanine methylester, reacting the N-tert-butyloxycarbonyl-amino-4,4-biphenyl-R-alanine methyl ester with sodium borohydride, reacting the obtained reaction product with ethyl 2-(triphenylphosphoranylidene)propionate to obtain ethyl (4R)-5-[1,1'-biphenyl]-4-yl-4-[[tert-butoxycarbonyl]amino]-2-methyl-2-pentenoate, reacting the ethyl (4R)-5-[1,1'-biphenyl]-4-yl-4-[[tert-butoxycarbonyl]amino]-2-methyl-2-pentenoatewith lithium hydroxide, performing catalytic hydrogenation, reacting the obtained catalytic hydrogenation product with thionyl chloride to obtain ethyl (2R, 4S)-5- ([1,1-biphenyl)-4-amino-2-methylpentenoate hydrochloride, and stirring and reacting the ethyl (2R, 4S)-5- ([1,1-biphenyl)-4-amino-2-methylpentenoate hydrochloride, calcium chloride and succinic anhydride to obtain the target product.The method has the advantages of simple steps, mild reaction conditions, high purity and high yield.
Owner:NANJING REDWOOD FINE CHEM CO LTD
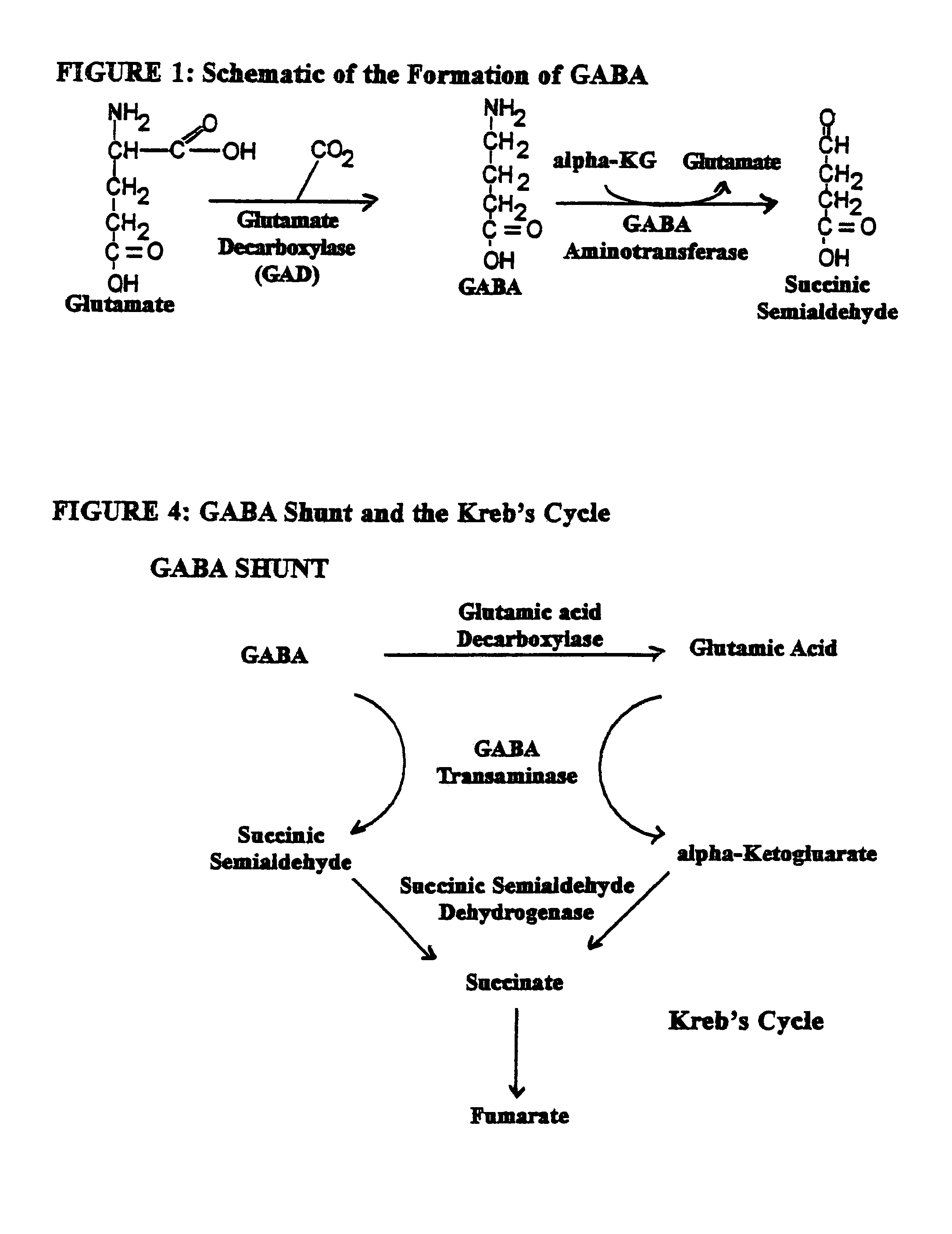
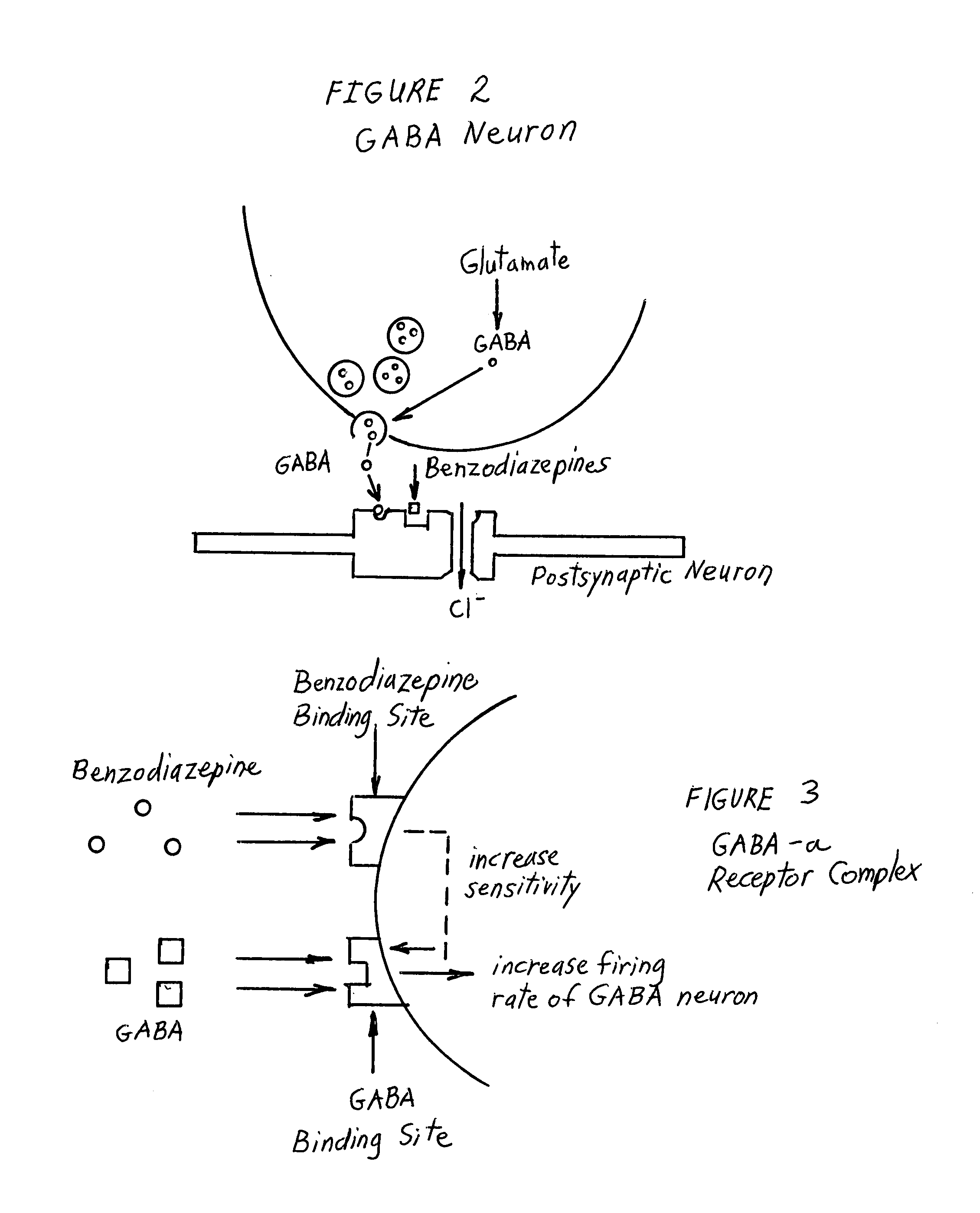
Enkephalinase inhibitor and GABA precursor loading as anti-anxiety compositions
ActiveUS7255882B2Improve the level ofPreventing significant perturbation and instabilityBiocideNervous disorderBenzodiazepinePanic
Benzodiazepine withdrawal induced anxiety is treated by administration of an enkephalinase inhibitor, and a gamma-aminobutyric acid (“GABA”) precursor, or an endorphin or enkephalin releaser. These components promote restoration of normal neurotransmitter function and are non-addictive. Use of the enkephalinase inhibitor D-phenylalanine, the GABA precursor glutamine, the serotonin precursor 5-HTP, glycine, and taurine, in combination with coenzymatic functionality of folic acid is preferred for activation of ligand-gated Cl− channel in the central nervous system. Food supplement embodiments provide the human body nutritional supplements to intake certain neurotransmitter precursor substrates, thereby enabling patients of all ages to self-regulate their ability to quell perturbations to equilibrium and simultaneously to adverse effects upon normal physiological and psychological functioning attributable to induced anxiety and panic associated with benzodiazepine withdrawal.
Owner:BIESER ALBERT HOWARD +1
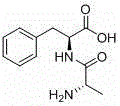
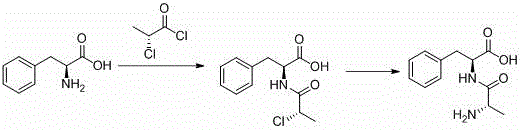
Preparation method for chiral L-alanyl-phenylalanine
The invention discloses a preparation method for chiral L-alanyl-phenylalanine, belonging to the technical field of synthesis of organic matters. The method comprises the following steps: subjecting L-phenylalanine, D-phenylalanine or DL-phenylalanine and D-2-chloropropionyl chloride to condensation so as to obtain 2-chloropropionyl-phenylalanine with a corresponding configuration; carrying out ammoniation on 2-chloropropionyl-phenylalanine so as to obtain a crude L-alanyl-phenylalanine product with a corresponding configuration; and purifying the crude product so as to obtain finished L-alanyl-L-phenylalanine, L-alanyl-D-phenylalanine or L-alanyl-DL-phenylalanine. The method provided by the invention is low in raw material consumption and high in yield; and prepared chiral L-alanyl-phenylalanine has high quality and can meet requirements of industrial production.
Owner:HUBEI HUNTIDE BIOTECH
Method for preparing D-phenylalanine through asymmetric transformation of L-phenylalanine
ActiveCN104926671AHigh yieldReduce manufacturing costOrganic compound preparationAmino-carboxyl compound preparationOrganic acidOrganic solvent
The invention discloses a method for preparing D-phenylalanine through asymmetric transformation of L-phenylalanine, and belongs to the technical field of chirality organic compounds. The method comprises steps: dissolving the L-phenylalanine and aldehyde catalysts in organic acid, adding D-tartaric acid, performing a reaction for 5-10 hours at the temperature of 60-90 DEG C, performing cooling and filtration, and washing a solid with an organic solvent so as to obtain D-tartaric acid .D-phenylalanine salt; and adding the obtained D-tartaric acid .D-phenylalanine salt into alkali liquid in batches, performing dissociation, performing a stirring reaction for 1-2 hours, and performing filtration, washing and drying so as to obtain the D-phenylalanine. The method disclosed by the invention solves the problems of poor stability, low resolution efficiency, poor product quality, high production cost and the like existing in the technology of preparing the D-phenylalanine through the L-phenylalanine. The method disclosed by the invention has the advantages of being high in yield, low in production cost, simple to operate, easy in industrialization and the like.
Owner:CHENGDU LIKAI CHIRAL TECH
Method for preparing dextrorotary phenylalanine by asymmetric conversion method
InactiveCN1226275CAvoid entrained precipitationReduce usageOrganic compound preparationAmino-carboxyl compound preparationOrganic solventIce water
The method for preparing D-phenylalanine by using DL-phenylalanine includes the following steps: firstly, mixing DL-phenylalanine and D-tartaric acid according to the mole ratio of 1:1.5-1.5:1 and dissolving them in organic solvent, adding 1.0-5.0% of aromatic alolehyde catalyst and making reaction for 6-8 hr. at 70-90 deg.C, cooling by using ice water bath, filtering, using anhydrous ethyl ether to wash and drying to obtain D-tartaric acid: D-phenylalanine salt, then dissolving the above-mentioned product in solvent, adding ammonization agent to make ammonization while stirring, after 1 hr. cooling to 5 deg.C-10 deg.C, filtering and washing with solvent so as to obtain the invented D-phenylalanine.
Owner:SOUTHEAST UNIV
Synthesis of pharmaceutical
InactiveCN101190887ALow costOrganic compound preparationCarboxylic acid amides preparationD-PhenylalanineSynthesis methods
The invention relates to a synthesis method of a nateglinide, in particular to the synthesis of the nateglinide by a one-pot method. Original material D-phenylalanine is used as raw material in the method, D-phenylalanine esterified substance (II) is obtained through esterification, a compound (IV) is obtained through acylation of isopropylcyclohexanecarboxylic acid (III), and then the compound (IV) is ester hydrolyzed so as to obtain the nateglinide. The technique of the method has simple technique process and easy and convenient post-treatment; meanwhile, the method has the advantages of strong operability, being easy to be industrialized and cost saving.
Owner:TIANJIN PHARMA GROUP CORP
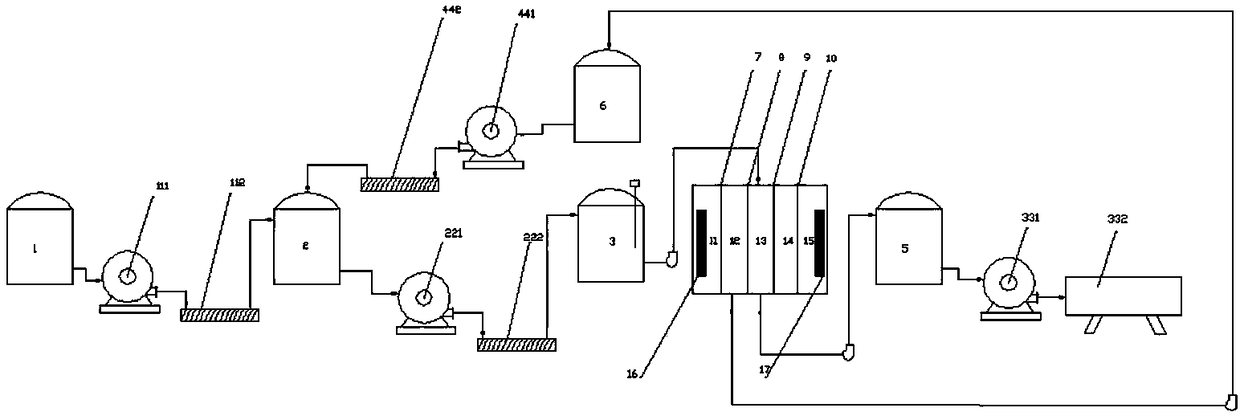
Method and system for producing D-phenylalanine
InactiveCN108299217AEasy to operateHigh yieldOrganic compound preparationOrganic chemistry methodsDouble saltElectrodialysis
The invention discloses a method and a system for producing D-phenylalanine. The method comprises the following steps: adding water to dissolve a D-phenylalanine and D-tartaric acid double salt, adding ammonia water to adjust the pH value to the isoelectric point of D-phenylalanine, adding the obtained solution into the raw water chamber of an electrodialysis device, introducing the aqueous solution of D-phenylalanine in the raw water chamber into a first concentrating crystallization kettle when the electric conductivity is about zero, performing concentrating crystallization, centrifuging and drying to obtain D-phenylalanine, introducing D-tartrate ions in a cathode concentrating chamber into a second concentrating crystallization kettle, performing concentrating crystallization and centrifuging to obtain D-tartaric acid, and returning the obtained D-tartaric acid into a second reaction vessel for reuse. Existing methods and systems are improved, and the electrodialysis device is used to separate the obtained double salt in order to obtain the D-phenylalanine, so the method and the system have the advantages of simplicity and easiness in operation, time saving, high yield reaching 85-90%, no use of methanol or triethylamine, low cost and environmental protection.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Method for preparing D-phenylalanine through dynamic kinetic resolution
InactiveCN102010345BHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSolventOperating cost
The invention discloses a method for preparing D-phenylalanine through dynamic kinetic resolution. The method comprises the following steps of: reacting L-phenylalanine serving as raw materials with hydrochloric acid to generate L-phenylalanine hydrochloride; racemizing and resolving under the action of a solvent and a racemization catalyst by taking the L-phenylalanine hydrochloride as a substrate and dibenzoyl tartaric acid (L-DBTA) as a resolving agent to obtain D-phenylalanine.L-DBTA disalt; and finally performing triethylamine resolution to prepare the target product D-phenylalanine. Compared with the prior art, the method directly takes the L-phenylalanine as the resolving substrate, mild alcohol as the solvent and an aldehyde pyridine compound as the racemization catalyst, racemizes and resolves the L-phenylalanine into the D-phenylalanine.L-DBTA disalt, has a theoretical conversion rate of about 100 percent, greatly improves the resolution yield, reduces the operating cost, ensures stable product quality and is suitable for industrial production.
Owner:SHANGHAI HUAYI GRP CO
Preparation method of N-(trans-4-isopropylcyclohexyl-1-formyl)-D-phenylalanine
InactiveCN101560169ARaw materials are cheap and easy to getFew reaction stepsMetabolism disorderOrganic compound preparationD-PhenylalanineDistillation
The invention relates to a preparation method of N-(trans-4-isopropylcyclohexyl-1-formyl)-D-phenylalanine, which comprises the following steps: step A: trans-4-isopropylcyclohexanecarboxylic acid, halogenating agent and reaction solvent are put into a reactor and stirred to react for 3-25hours when the temperature is controlled at 5-60 DEG C; trans-4-isopropylcyclohexanecarboxylic acyl chloride is obtained by reduced pressure distillation, fraction collection; step B: D-phenylalanine, alkali liquor and reaction solvent are put into a reaction bulb; trans-4-isopropylcyclohexanecarboxylic acyl chloride is added into the reaction bulb to react with the above mixture for 1-15 hours under the stirring state, when the controlling temperature is 0-50 DEG C; water is added for dissolving after the reaction is over, and N-(trans-4-isopropylcyclohexyl-1-formyl)-D-phenylalanine is obtained by filtering after acidification treatment is carried out with acid. The raw materials used in the invention are cheap and easy to be obtained. The method has the advantages of less reaction process, simple reaction condition, convenient operation, mild condition, and high yield and high purity, which is applicable to industrialized production.
Owner:NANJING UNIV OF TECH
Modulators of ß-amyloid peptide aggregation
InactiveUS20050137128A1Improve biostabilityProlonged elevated plasma levelNervous disorderIn-vivo radioactive preparationsTert-leucineValine
Compounds that modulate natural β amyloid peptide aggregation are provided. The modulators of the invention comprise a peptide, preferably based on a β amyloid peptide, that is comprised entirely of D-amino acids. Preferably, the peptide comprises 3-5 D-amino acid residues and includes at least two D-amino acid residues independently selected from the group consisting of D-leucine, D-phenylalanine and D-valine. In a particularly preferred embodiment, the peptide is a retro-inverso isomer of a β amyloid peptide, preferably a retro-inverso isomer of Aβ17-21. In certain embodiments, the peptide is modified at the amino-terminus, the carboxy-terminus, or both. Preferred amino-terminal modifying groups alkyl groups. Preferred carboxy-terminal modifying groups include an amide group, an acetate group, an alkyl amide group, an aryl amide group or a hydroxy group. Pharmaceutical compositions comprising the compounds of the invention, and diagnostic and treatment methods for amyloidogenic diseases using the compounds of the invention, are also disclosed.
Owner:GLAXO SMITHKLINE LLC
Antimicrobial peptide compositions for plants
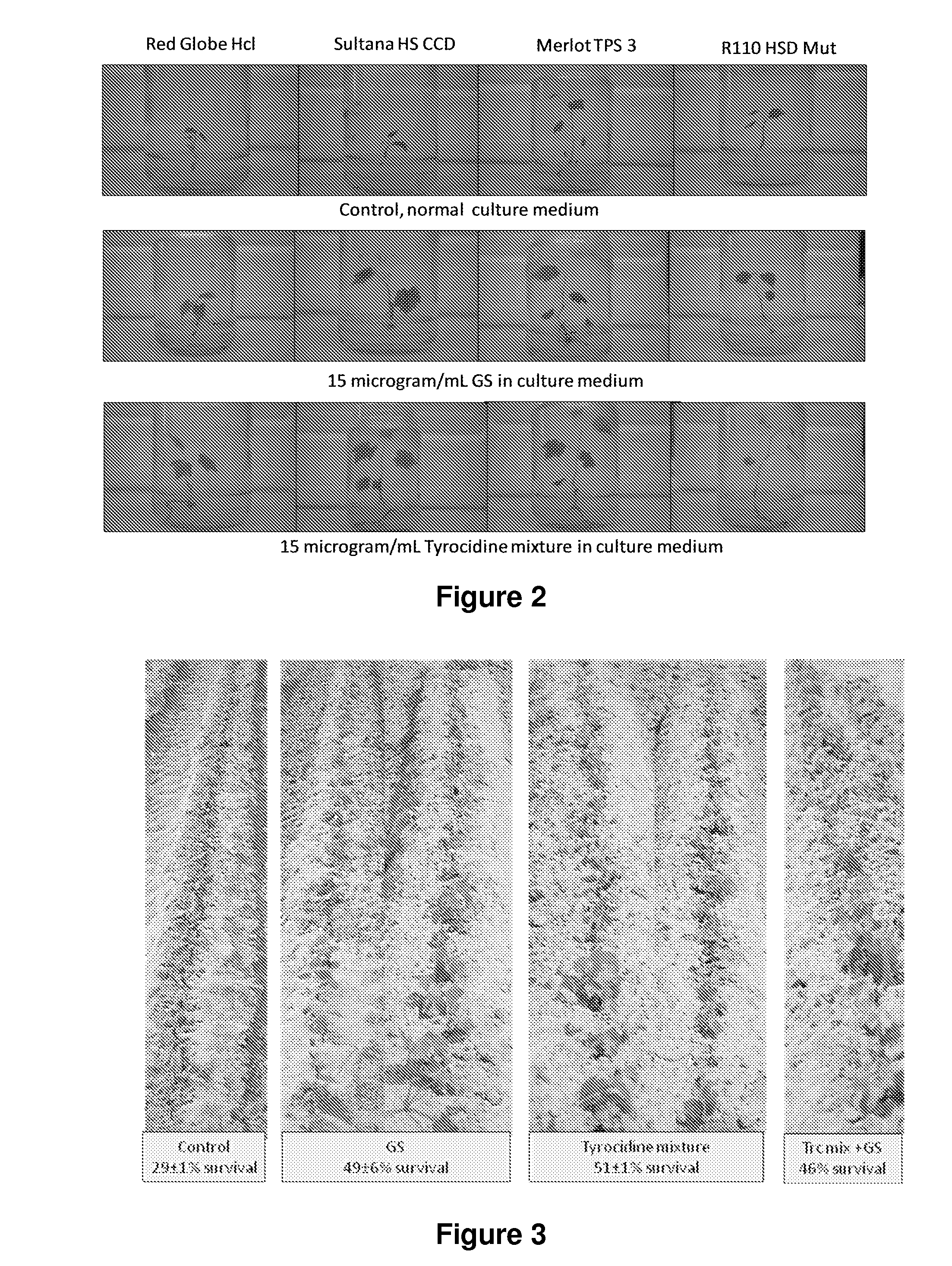
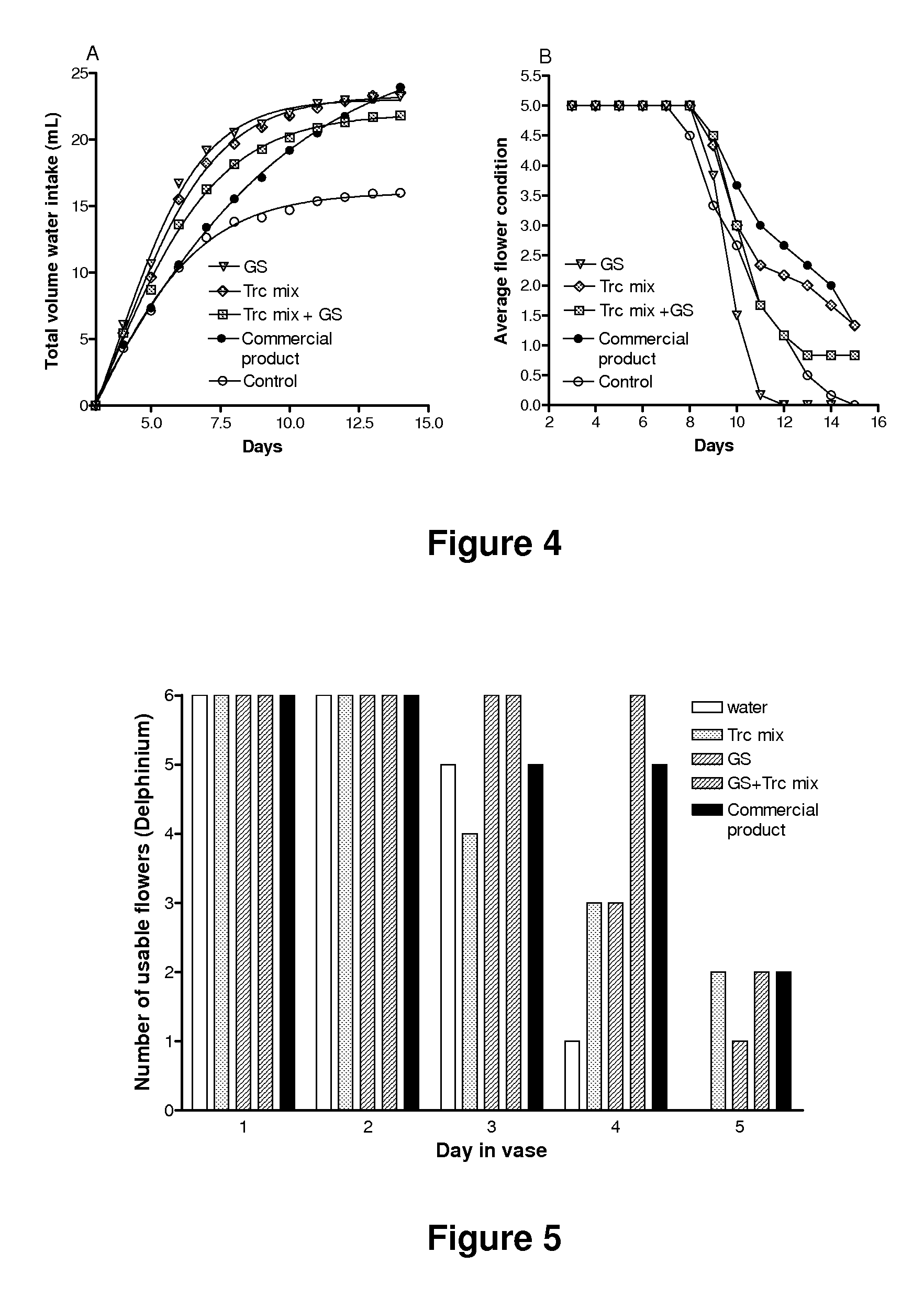
InactiveUS20150030566A1Improve solubilityImprove bio-availabilityBiocideAntimycoticsActive agentAntimicrobial peptides
Compositions containing one or more tyrocidines, tryptocidines, phenycidines and / or gramicidin S, or derivatives and analogues thereof, are described for controlling antimicrobial growth on plants, plant material or plant growth media, and methods for controlling or preventing the growth of microbial pathogens, and in particular fungal pathogens, on plants, plant parts and plant material are described herein. The active agents used to control these pathogens are tyrocidines, tryptocidines, phenycidines and / or gramicidin S, or derivatives, analogues or modifications thereof. The tyrocidines, tryptocidines, phenycidines and / or gramicidin S are cyclic decapeptides having the general amino acid sequence cyclo(valine-Xrleucine-D-phenylalanine-proline-X2-X3-X4-X5-X6) (SEQ ID NO: 1) or a derivative or analogue thereof, wherein X1 is ornithine or lysine, X2 is valine, leucine, isoleucine, phenylalanine, tryptophan or tyrosine; X3 is the D-isomer of valine, leucine, isoleucine, phenylalanine, tryptophan, tyrosine, ornithine, or lysine; X4 is asparagine, glutamine or leucine; X5 is glutamine, the D-isomer of valine, leucine, or isoleucine; and X6 is tyrosine, phenylalanine, tryptophan or proline.
Owner:STELLENBOSCH UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com