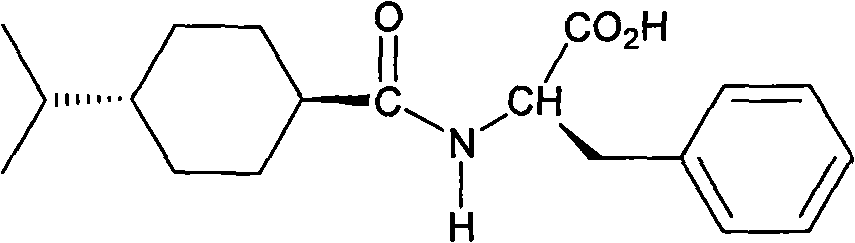
Preparation method of N-(trans-4-isopropylcyclohexyl-1-formyl)-D-phenylalanine
A technology of phenylalanine and isopropyl ring is applied in the field of preparation of phenylalanine derivative pharmaceutical intermediates and products, and can solve the problems of changing trans configuration, high toxicity of halogenating agents, troublesome post-processing and the like, Achieve the effect of small environmental impact, easy operation and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
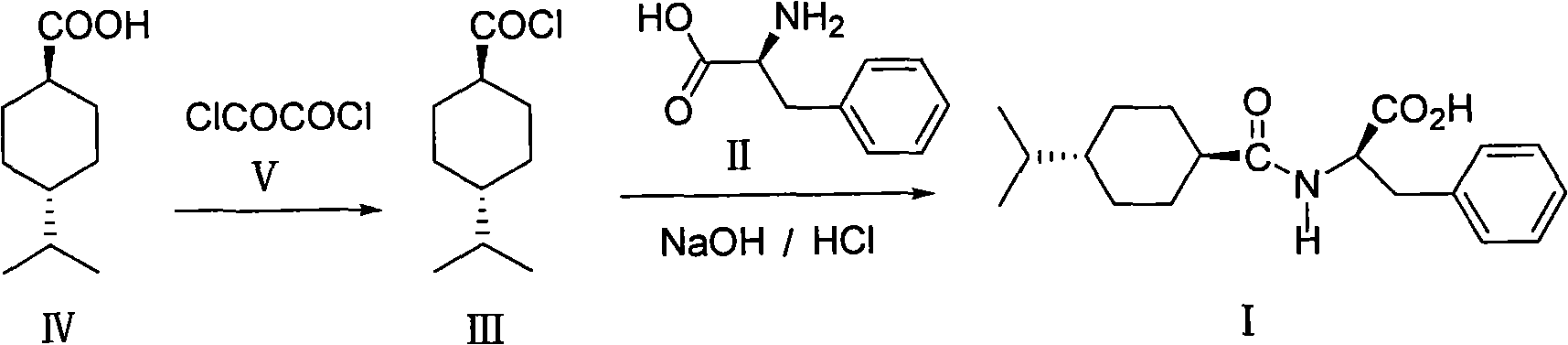
[0020] Add 6.8g (0.04mol) of trans-4-isopropylcyclohexanecarboxylic acid (IV) into a 150ml three-necked flask, add 60ml of n-hexane, stir and dissolve, then add 11.7g (0.09mol) of oxalyl chloride (V), The reaction was stirred at 25°C, and the hydrogen chloride gas generated was absorbed into a 20% sodium hydroxide solution through a calcium chloride drying tube. The reaction was stirred for about 16 h until almost no bubbles escaped, and the reaction was terminated. After the solvent and excess oxalyl chloride were distilled off by water pump decompression (external temperature<80°C), oil pump vacuum distillation collected 79~81°C / 3mmHg fraction to obtain colorless liquid trans-4-isopropylcyclohexylformyl chloride ( III), weighing 7.7g, yield 95.1%.
[0021] In a 150ml three-necked flask, 3.14g (0.02mol) of D-phenylalanine (II) was dissolved in 35g of a 2% sodium hydroxide solution by mass percentage, and 30ml of acetone was added, cooled in an ice-water bath to maintain the ...
Embodiment 2
[0023] Add 68.1g (0.4mol) of trans-4-isopropylcyclohexanecarboxylic acid (IV) into a 1-liter three-necked flask, add 600ml of n-hexane, stir and dissolve, then add 116.7g (0.92mol) of oxalyl chloride (V) , 50 ° C stirring reaction, the hydrogen chloride gas generated through the calcium chloride drying tube to the mass percent concentration of 20% sodium hydroxide solution to absorb. The reaction was stirred for about 10 h until almost no bubbles escaped, and the reaction was terminated. After the solvent and excess oxalyl chloride were distilled off by water pump decompression (external temperature<80°C), oil pump vacuum distillation collected 79~81°C / 3mmHg fraction to obtain colorless liquid trans-4-isopropylcyclohexylformyl chloride ( III), weight 68.2g, yield 90.3%.
[0024] In a 500ml three-necked flask, 31.4g (0.19mol) of D-phenylalanine (II) was dissolved in 78g of a 10% sodium hydroxide solution by mass percentage, 160ml of acetone was added, cooled in an ice bath, an...
Embodiment 3
[0026]Add 10.2g of trans-4-isopropylcyclohexanecarboxylic acid (IV) into a 150ml three-necked flask, add 100ml of cyclohexane, stir to dissolve, add 34.5g of oxalyl chloride (V), stir and react at 15°C, the resulting The hydrogen chloride gas is absorbed in a 30% sodium hydroxide solution through a calcium chloride drying tube. The reaction was stirred for about 20 h until almost no bubbles escaped, and the reaction was terminated. After the solvent and excess oxalyl chloride were distilled off by water pump decompression (external temperature<80°C), oil pump vacuum distillation collected 79~81°C / 3mmHg fraction to obtain colorless liquid trans-4-isopropylcyclohexylformyl chloride ( III), weighing 7.4g, yield 91.4%.
[0027] In a 150ml three-necked flask, dissolve 9.2g of D-phenylalanine (II) in 21.8g of 20% sodium hydroxide solution by mass percentage, add 110ml of acetone, cool in an ice-water bath, and maintain the temperature at about 0°C . Under vigorous stirring, 8.4 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com