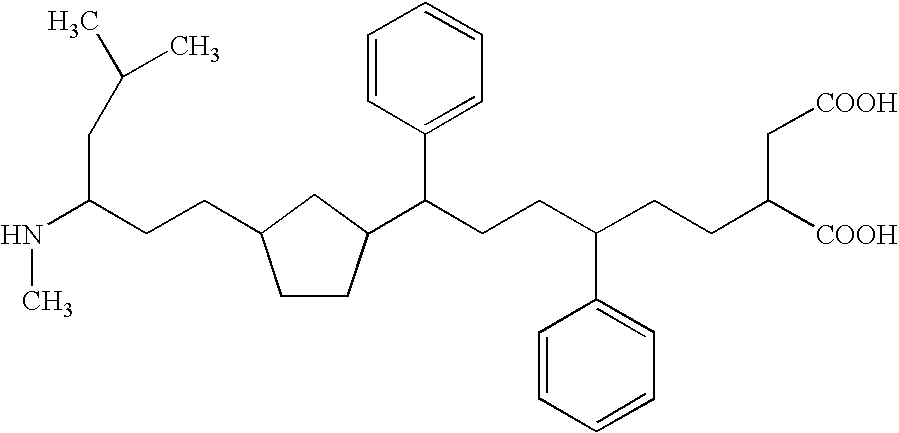
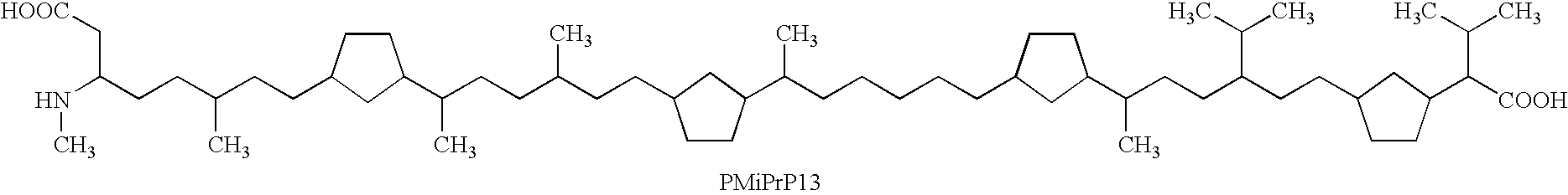
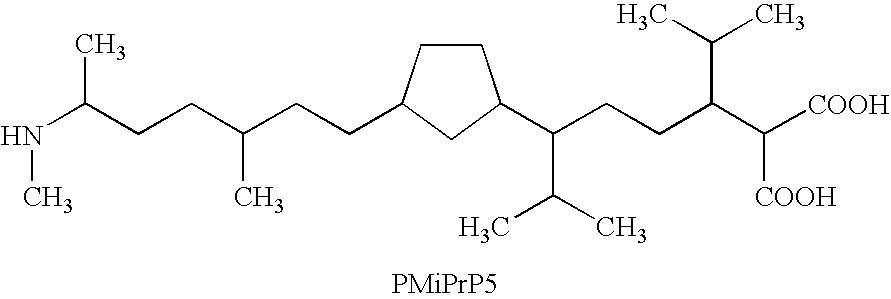
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Beta sheet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
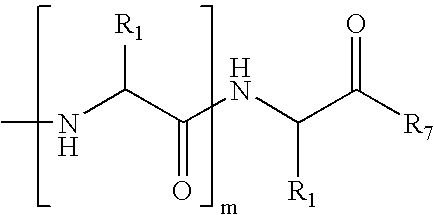
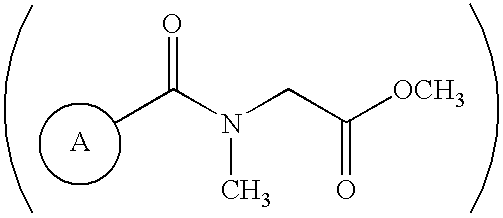
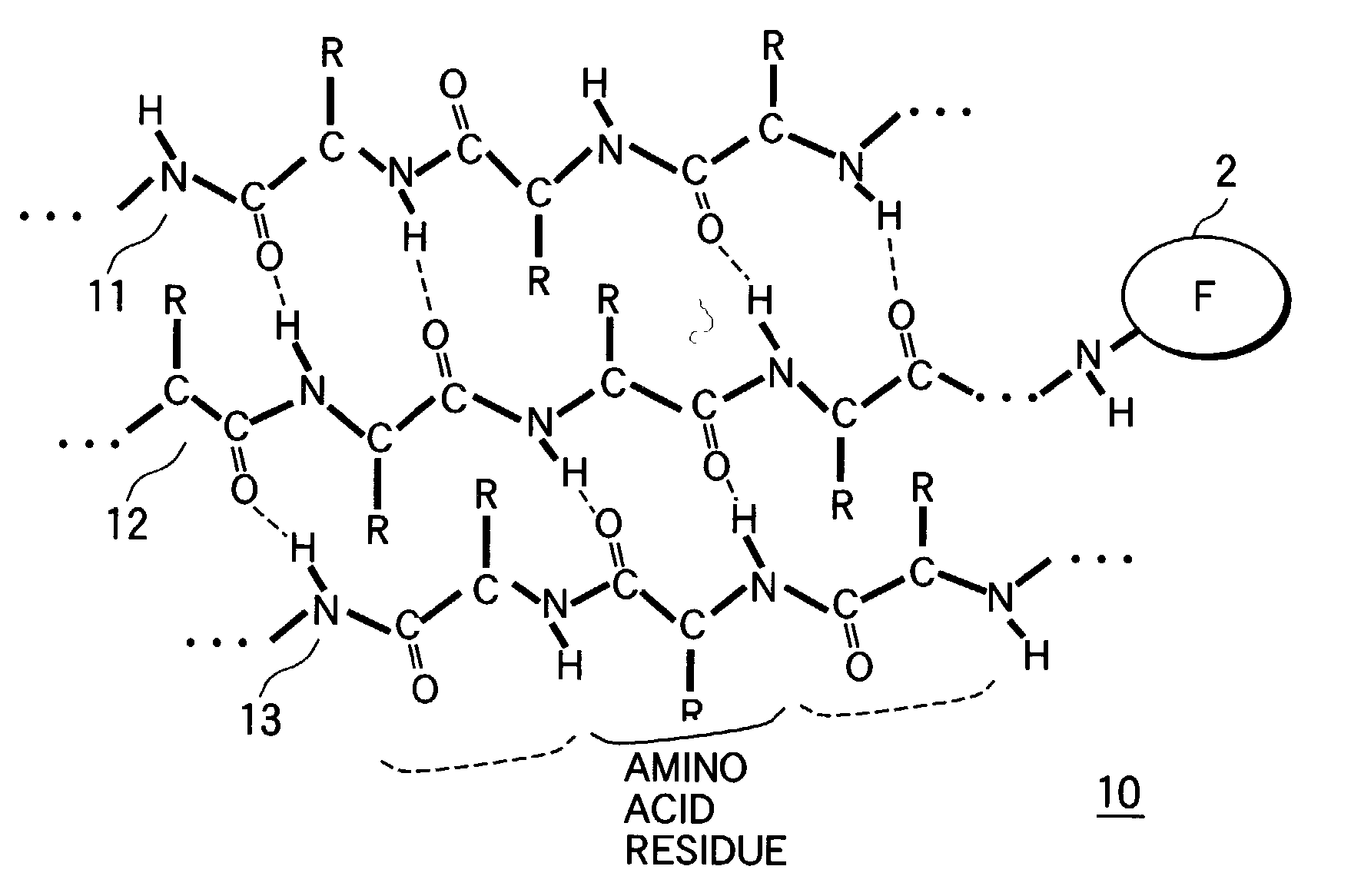
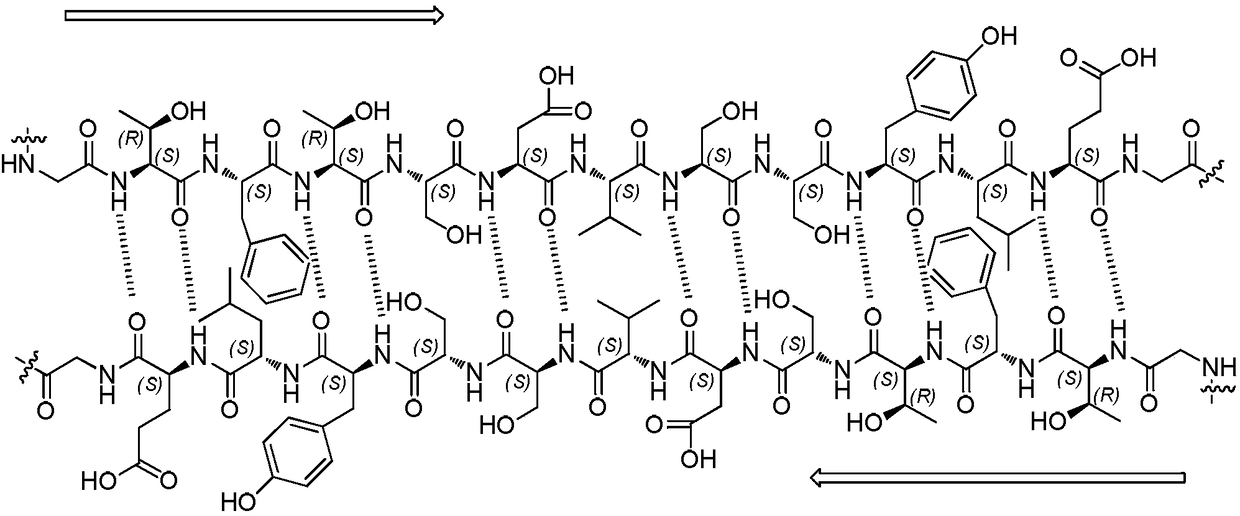
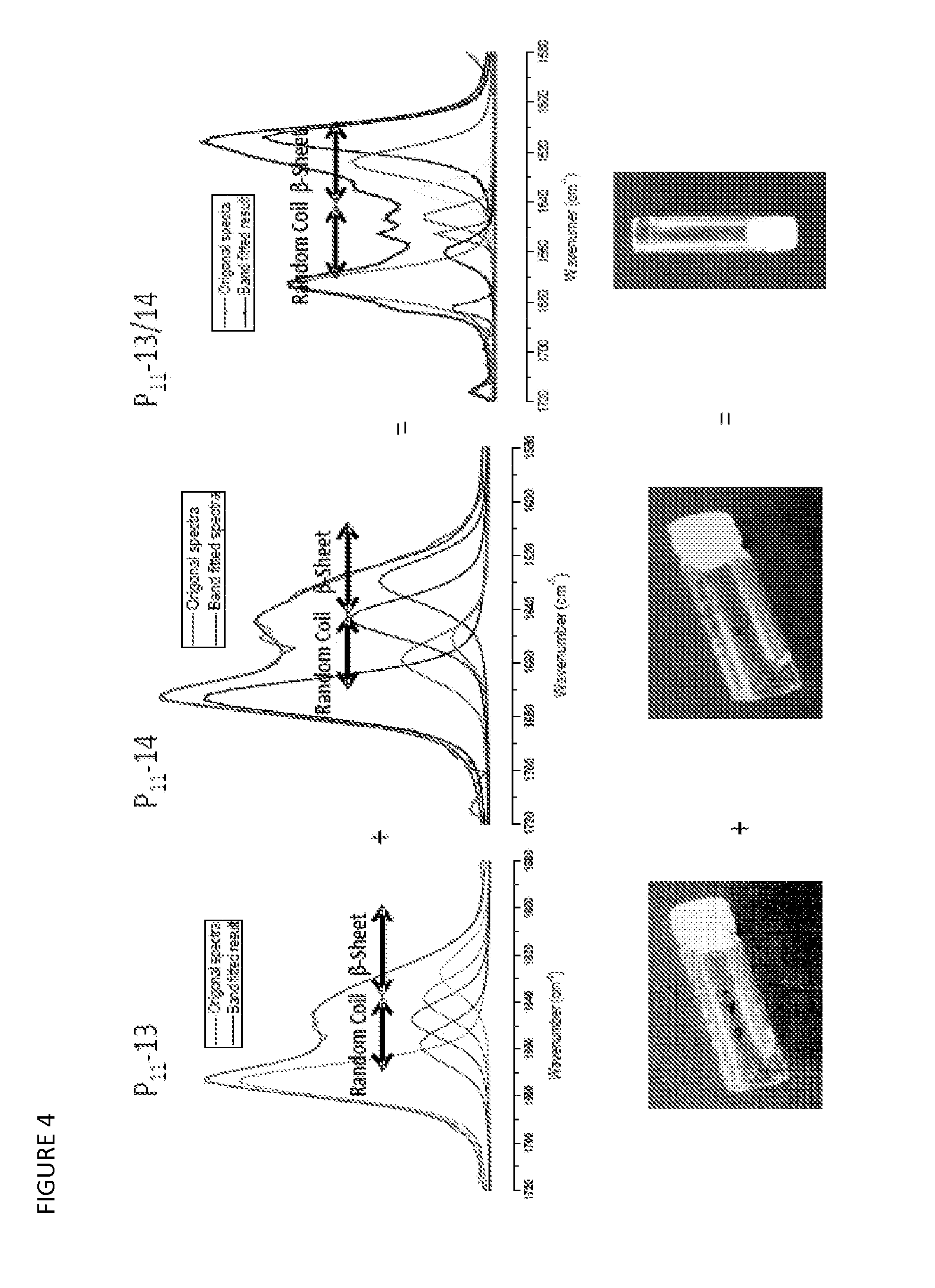
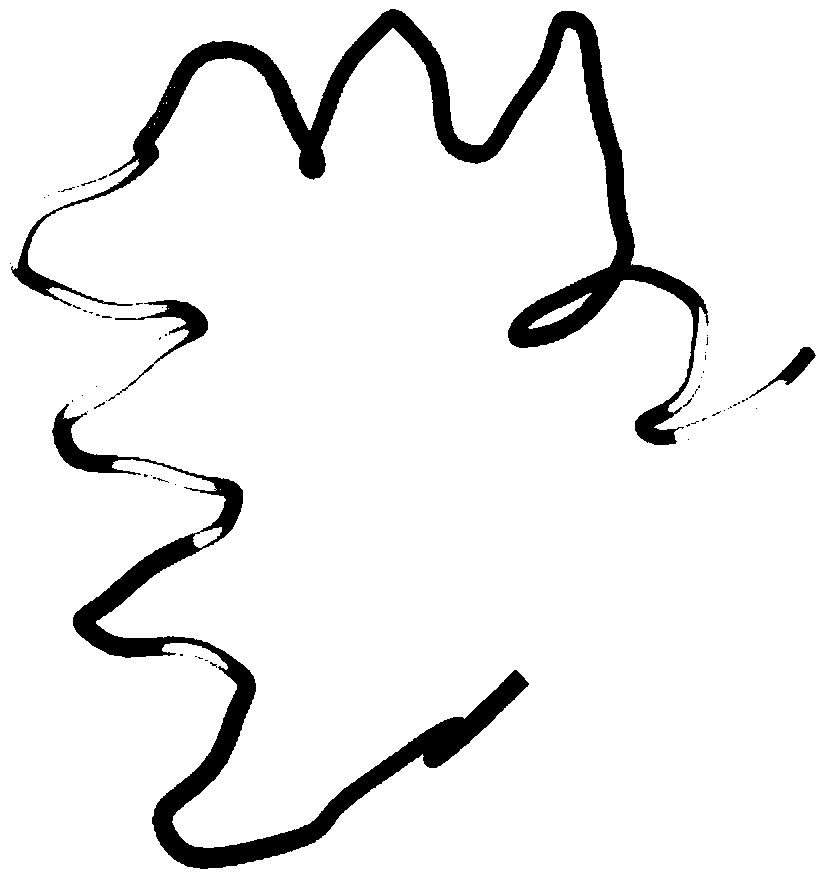
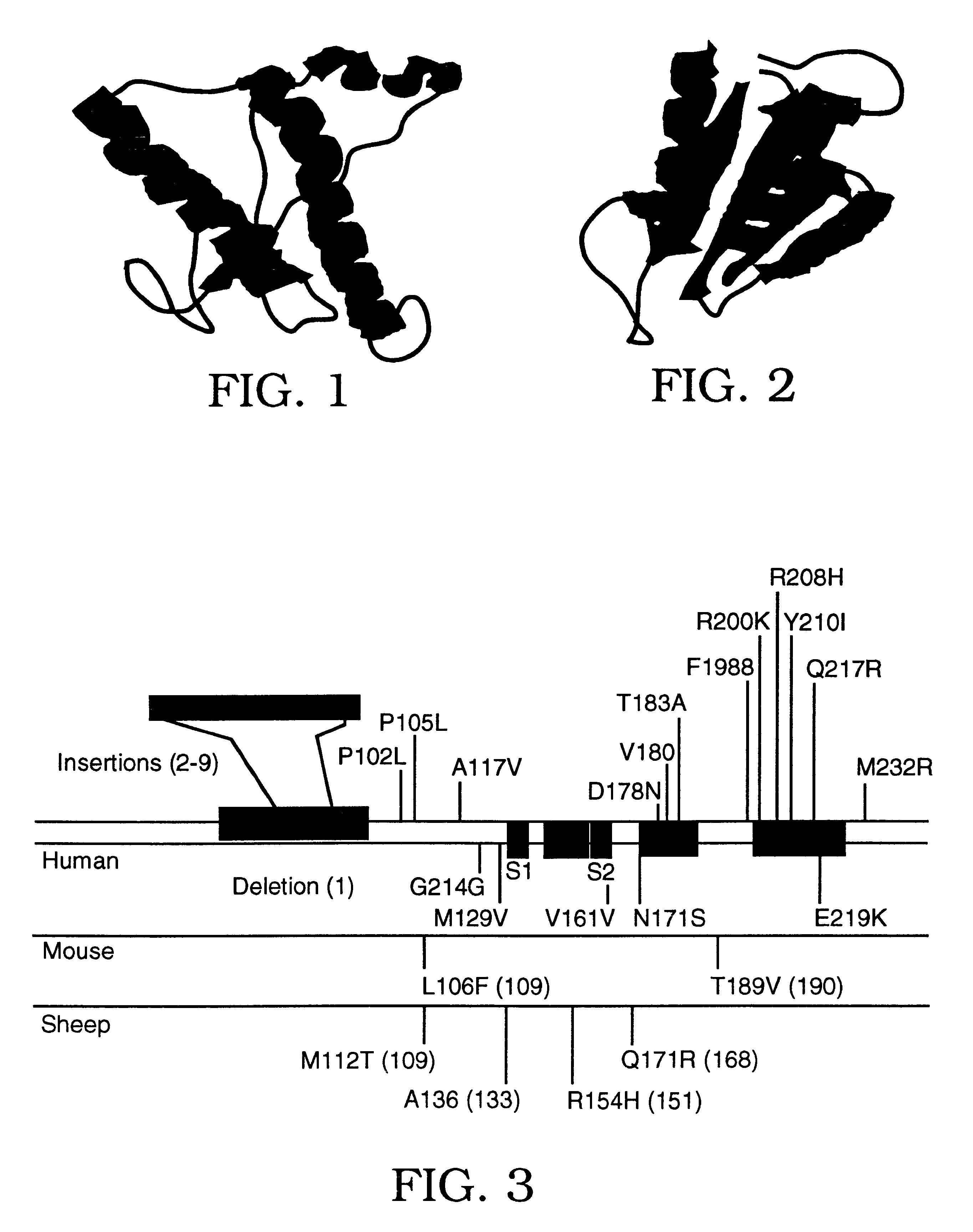
The β-sheet (also β-pleated sheet) is a common motif of regular secondary structure in proteins. Beta sheets consist of beta strands (also β-strand) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in formation of the protein aggregates and fibrils observed in many human diseases, notably the amyloidoses such as Alzheimer's disease.
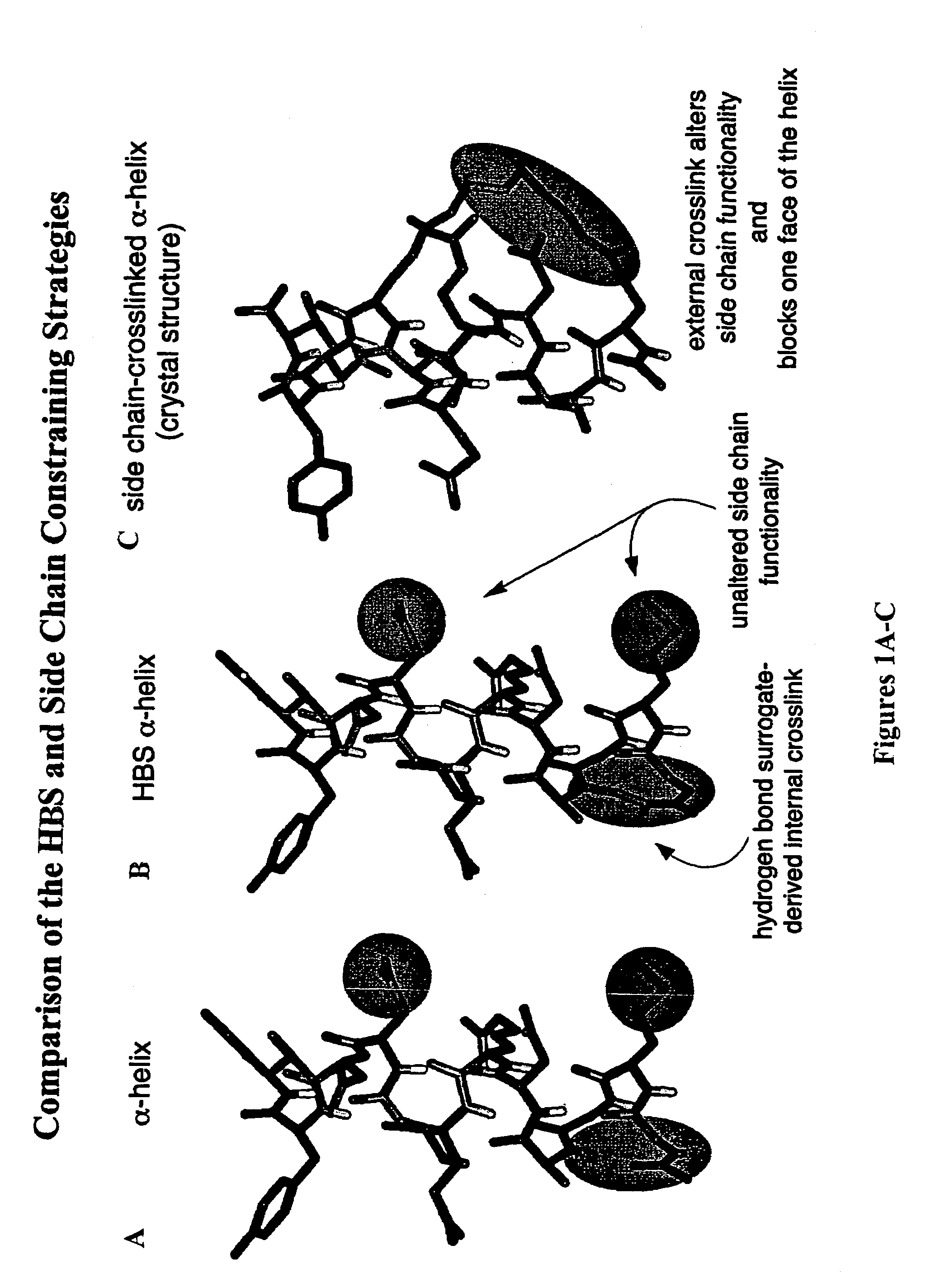
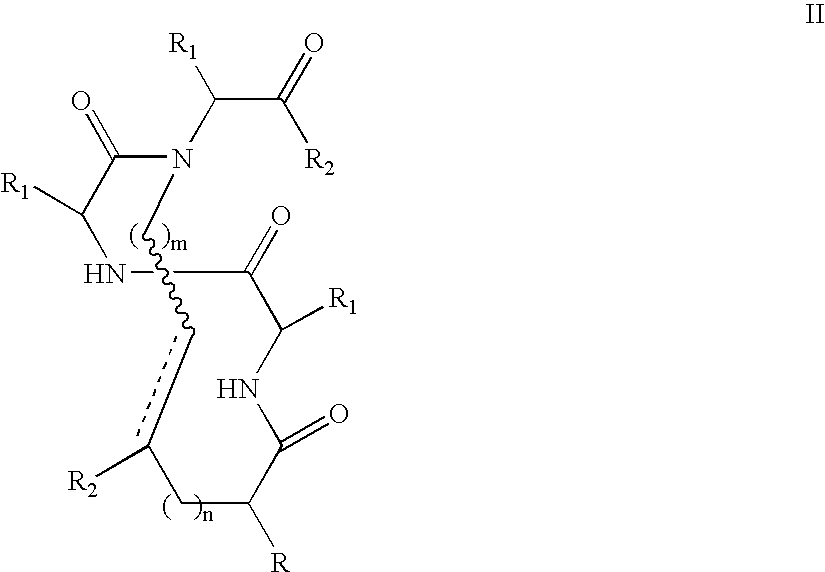
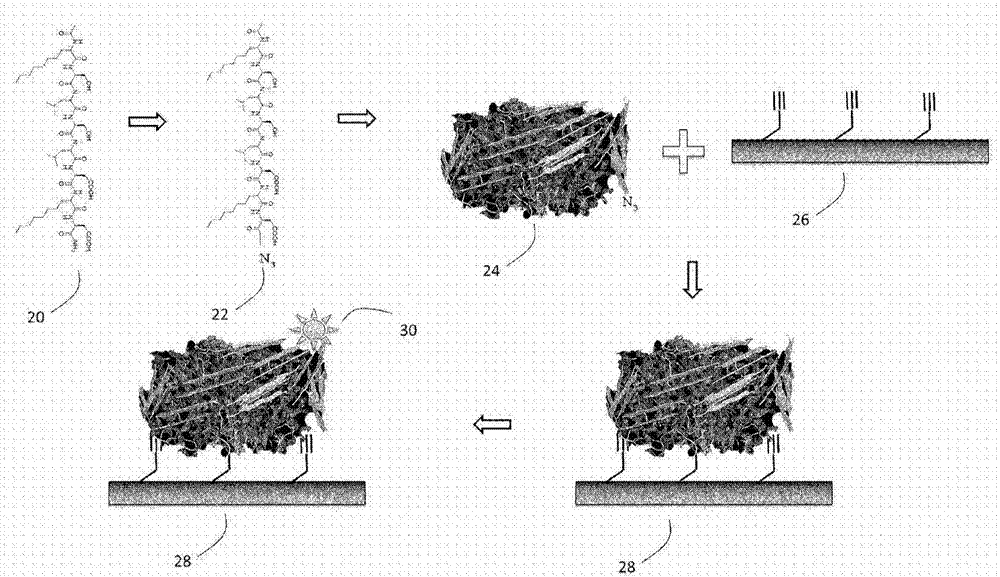
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS7202332B2Stable and irreversible bondPeptide preparation methodsCyclic peptide ingredientsBeta sheetHelix
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS20060014675A1Promote cell deathAvoid interactionPeptide preparation methodsImmunoglobulinsGreek letter betaBeta sheet
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Purified amphiphilic peptide compositions and uses thereof
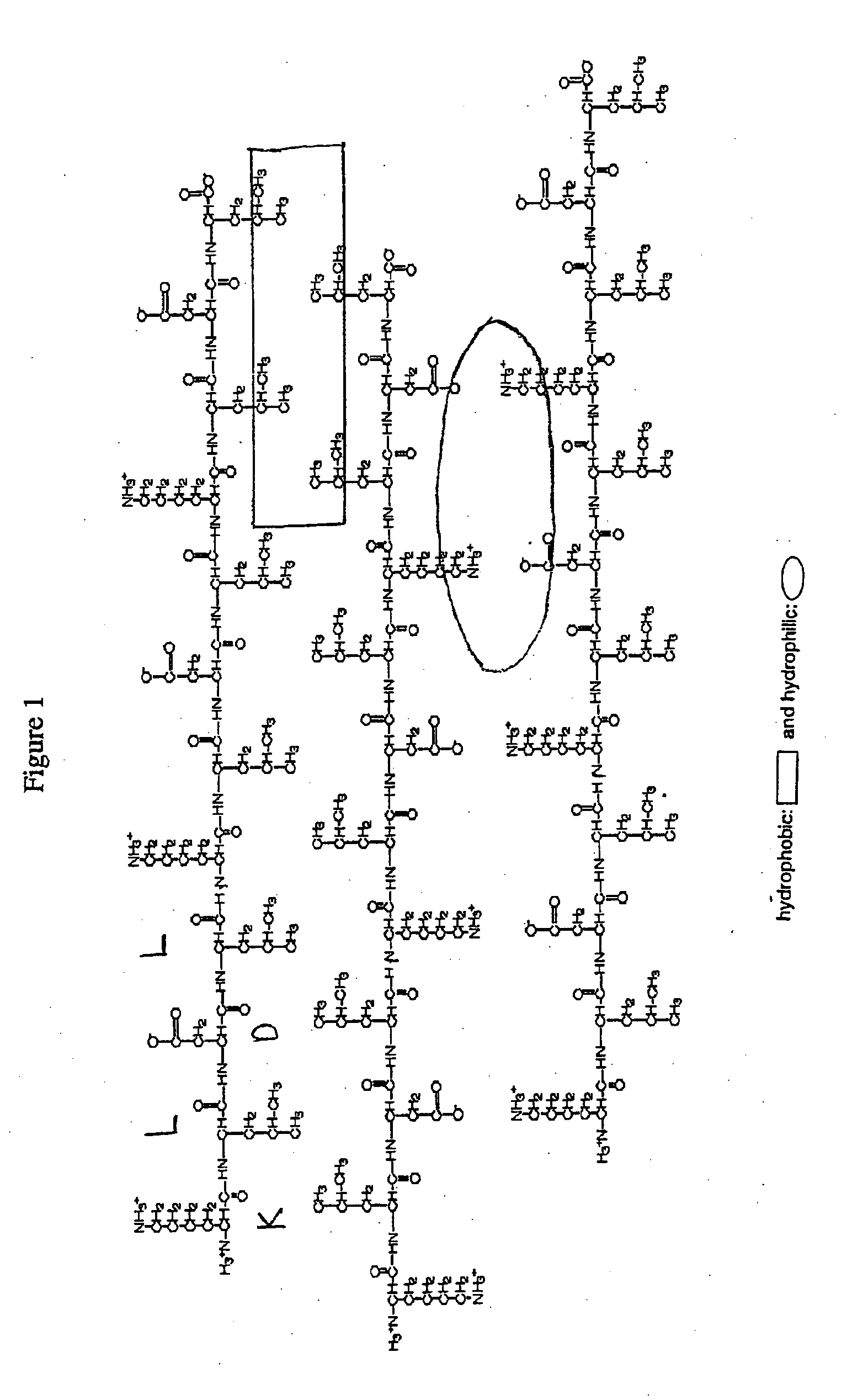
A plurality of amphiphilic peptide chains having alternating hydrophilic and hydrophobic amino acids, wherein the peptide contains at least 8 amino acids, are complementary and structurally compatible, and self-assemble into a beta-sheet macroscopic scaffold wherein peptide at least about 75% of the chains have the same sequence.
Owner:SPIRIO LISA +1
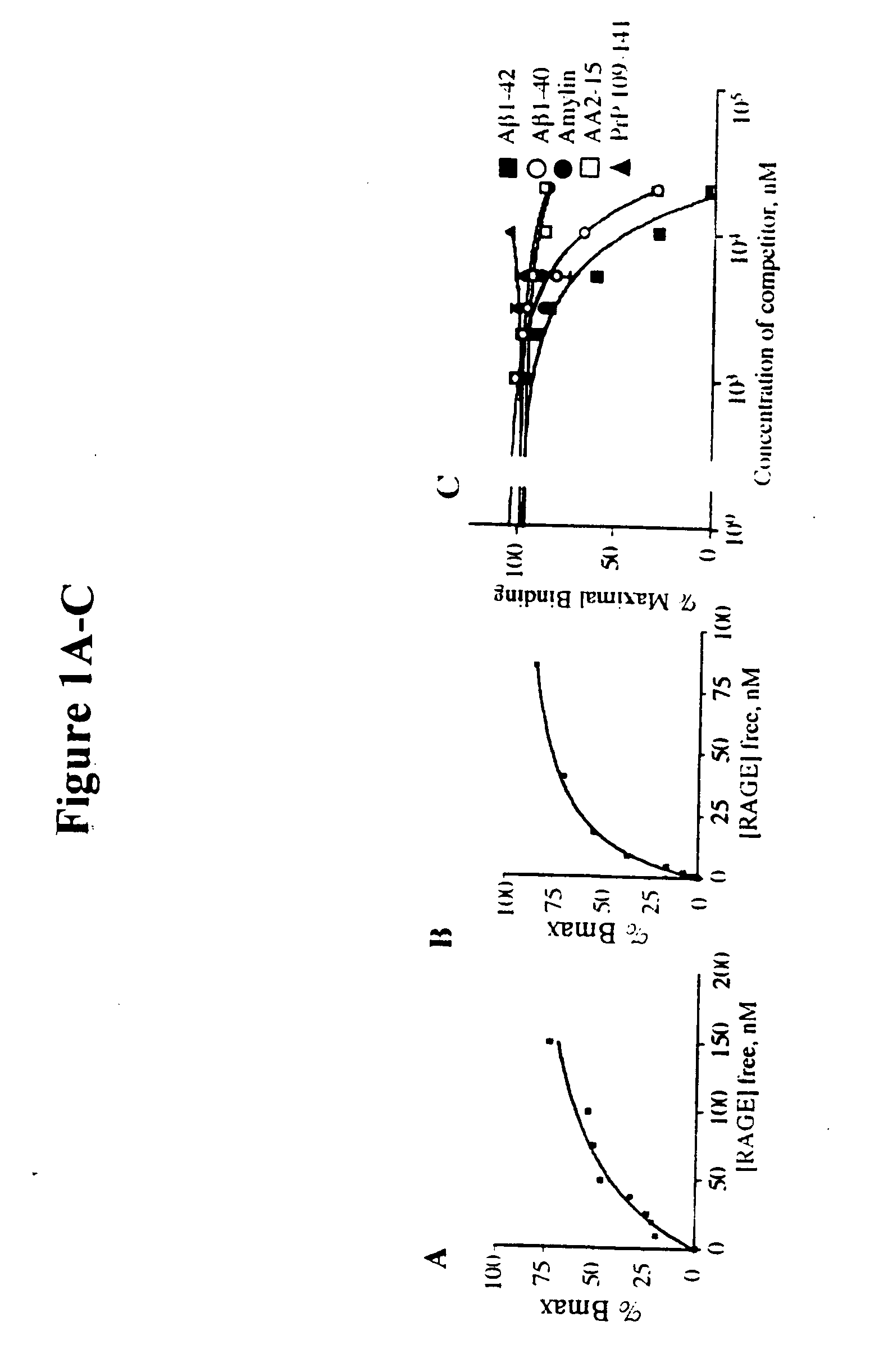
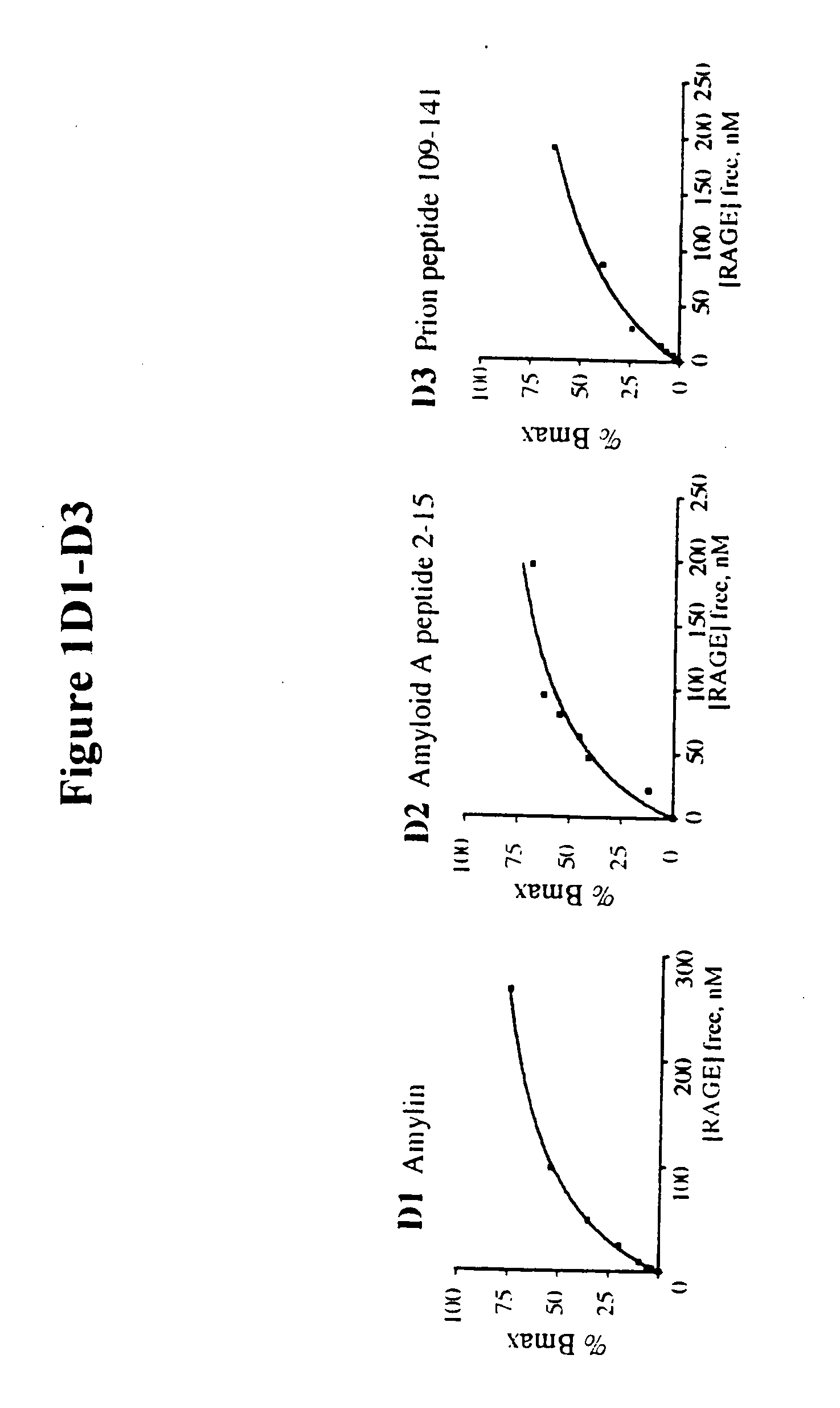
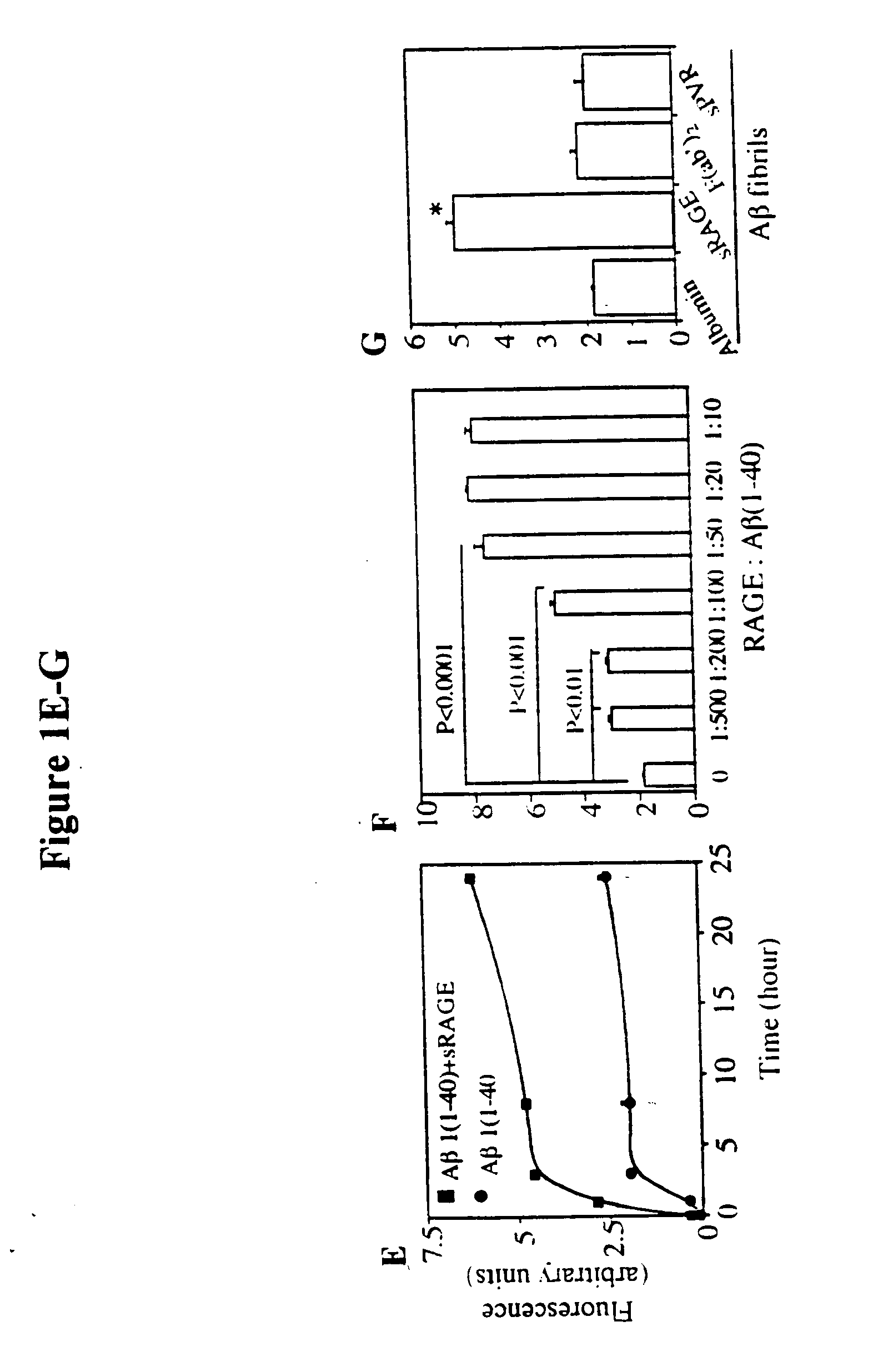
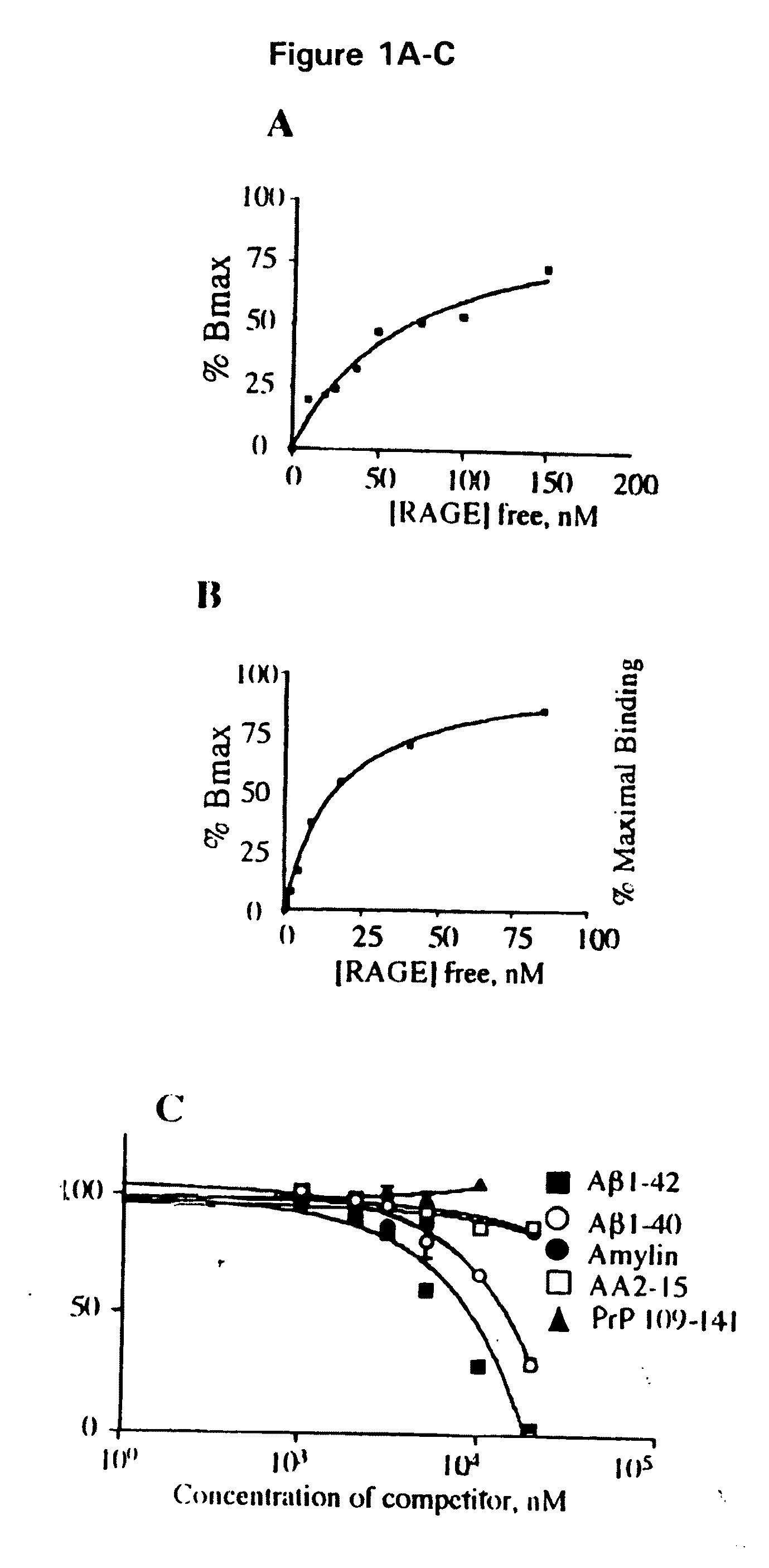
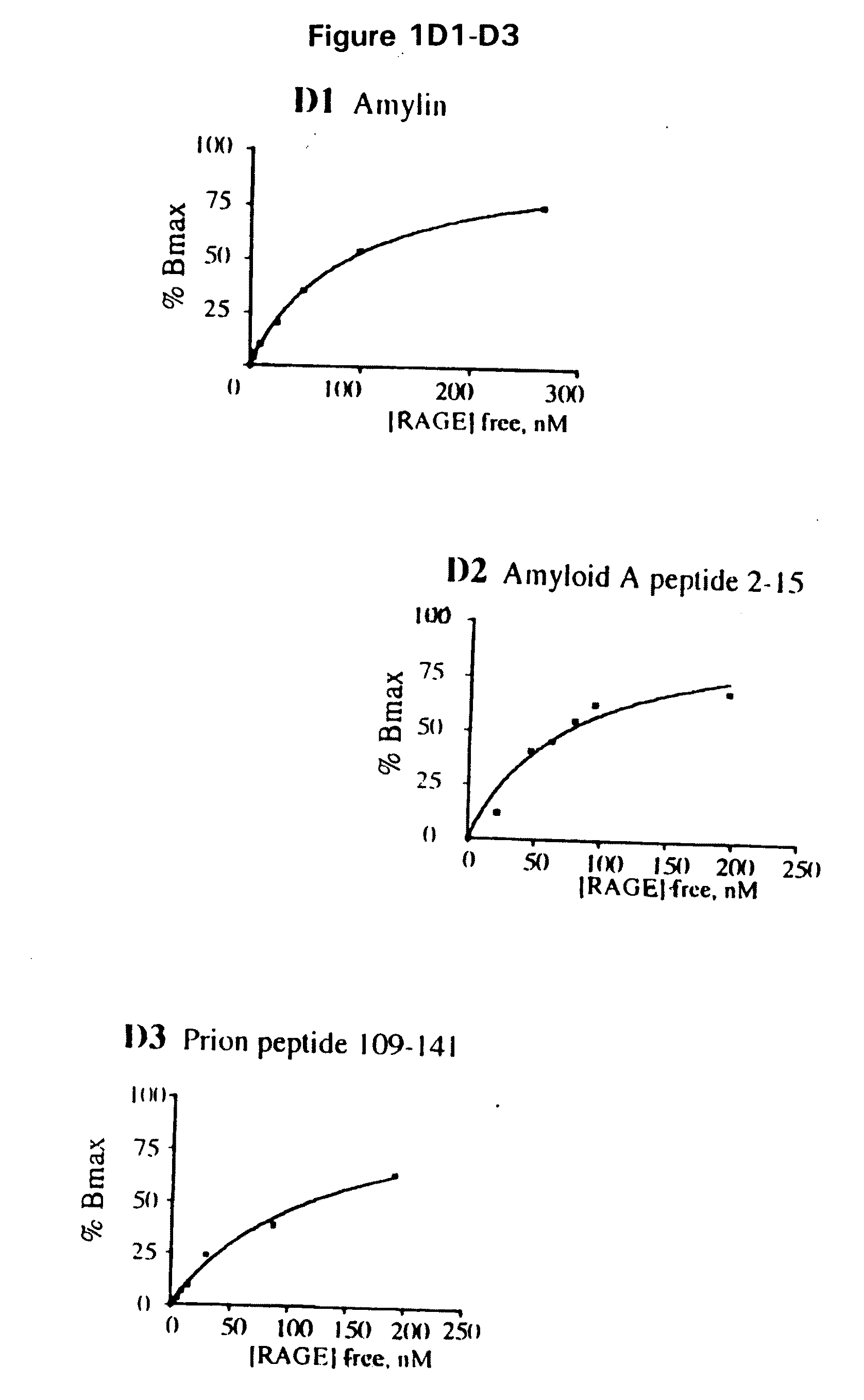
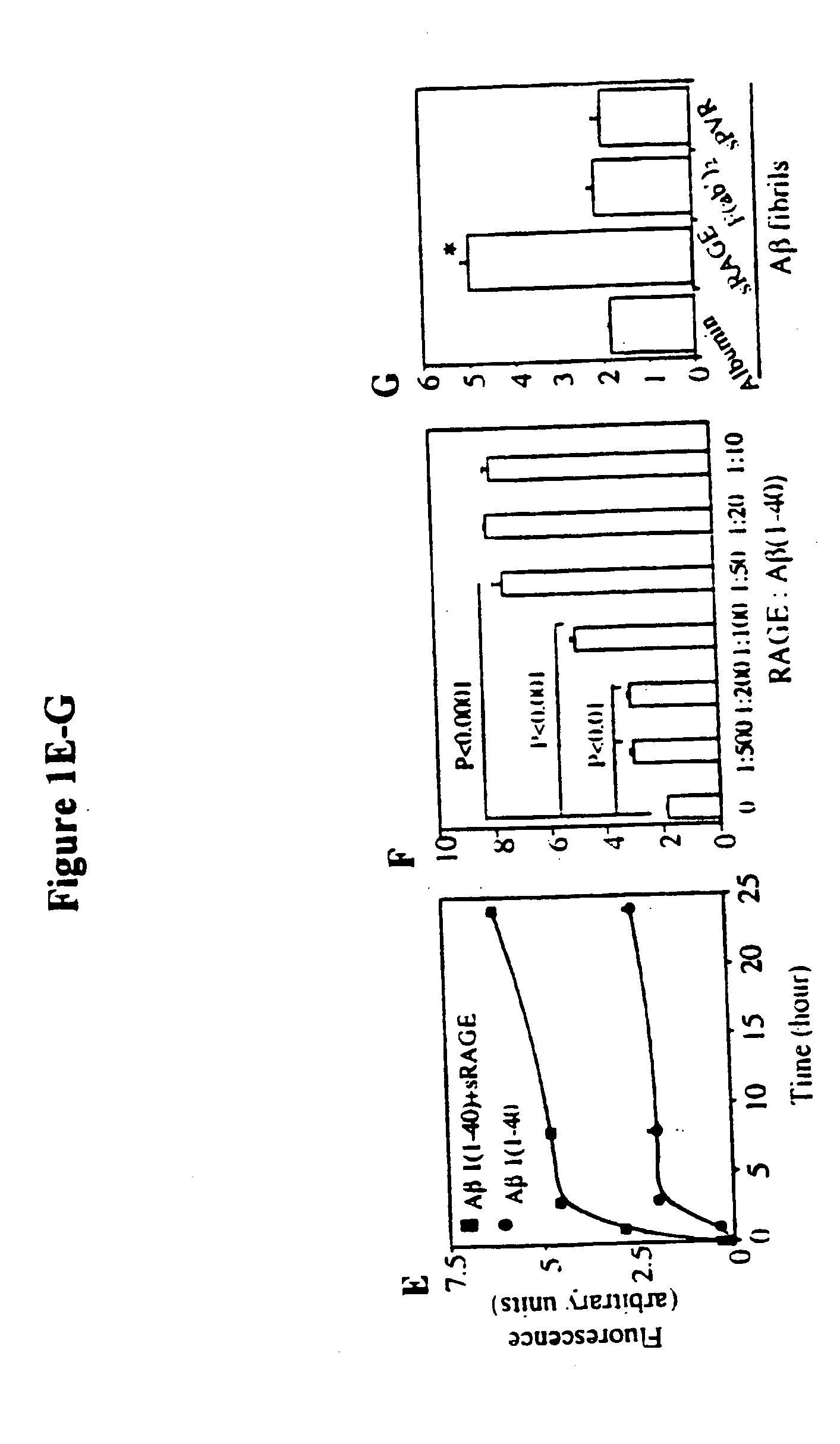
Methods of inhibiting binding of beta-sheet fibril to rage and consequences thereof
This invention provides a method of inhibiting the binding of a β-sheet fibril to RAGE on the surface of a cell which comprises contacting the cell with a binding inhibiting amount of a compound capable of inhibiting binding of the β-sheet fibril to RAGE so as to thereby inhibit binding of the β-sheet fibril to RAGE. In one embodiment the β-sheet fibril is amyloid fibril. In one embodiment, the compound is sRAGE or a fragment thereof. In another embodiment, the compound is an anti-RAGE antibody or portion thereof. This invention provides the above method wherein the inhibition of binding of the β-sheet fibril to RAGE has the consequences of decreasing the load of β-sheet fibril in the tissue, inhibiting fibril-induced programmed cell death, inhibiting fibril-induced cell stress. This invention also provides methods of determining whether a compound inhibits binding of a β-sheet fibril to RAGE on the surface of a cell.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Beta sheet breaker peptide analogs that inhibit beta pleated sheet formation in amyloid beta-peptide
The present invention provides peptide analogs and peptide mimetics that inhibit pleated sheet formation in amyloid beta-peptide, pharmaceutical compositions thereof and their therapeutic use. The inhibitory peptides possess activity as inhibitors in the formation of amyloid-like deposits and are useful in the treatment of Alzheimer's Disease.
Owner:AXONYX INC
Triazole macrocycle systems
InactiveUS7981999B2Simple structureExhibit biological activityPeptide-nucleic acidsTumor rejection antigen precursorsΑ helicalCombinatorial chemistry
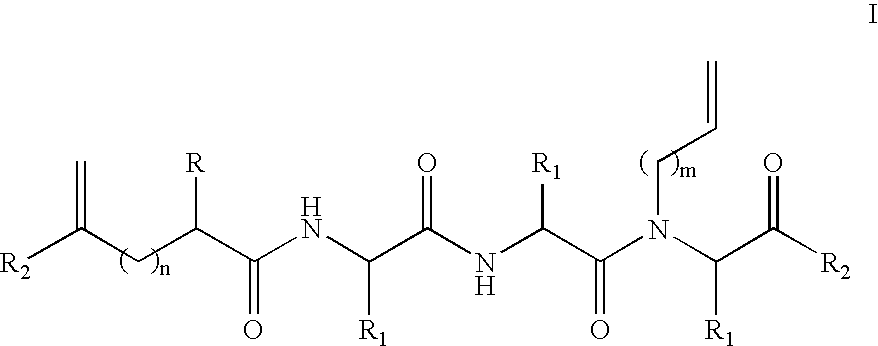
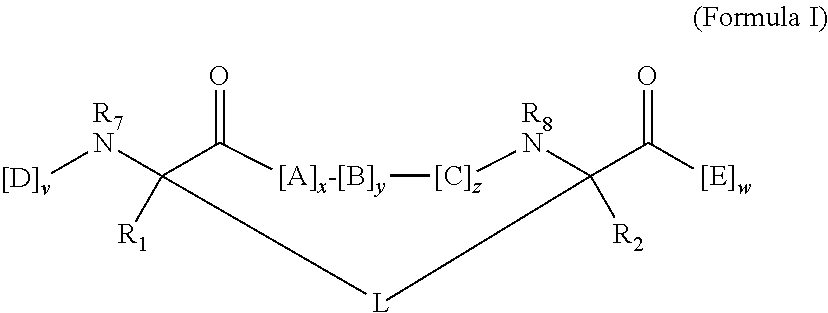
The present invention provides novel peptidomimetic macrocycles and methods for their preparation and use, as well as amino acid analogs and macrocycle-forming linkers, and kits useful in their production. In various embodiments, the peptidomimetic macrocycles are of Formula I:The linker L includes a triazole moiety. Peptidomimetic macrocycles according to the invention may exhibit increased α-helical or beta sheet structure in aqueous solution compared to a corresponding non-macrocyclic polypeptide.
Owner:AILERON THERAPEUTICS INC
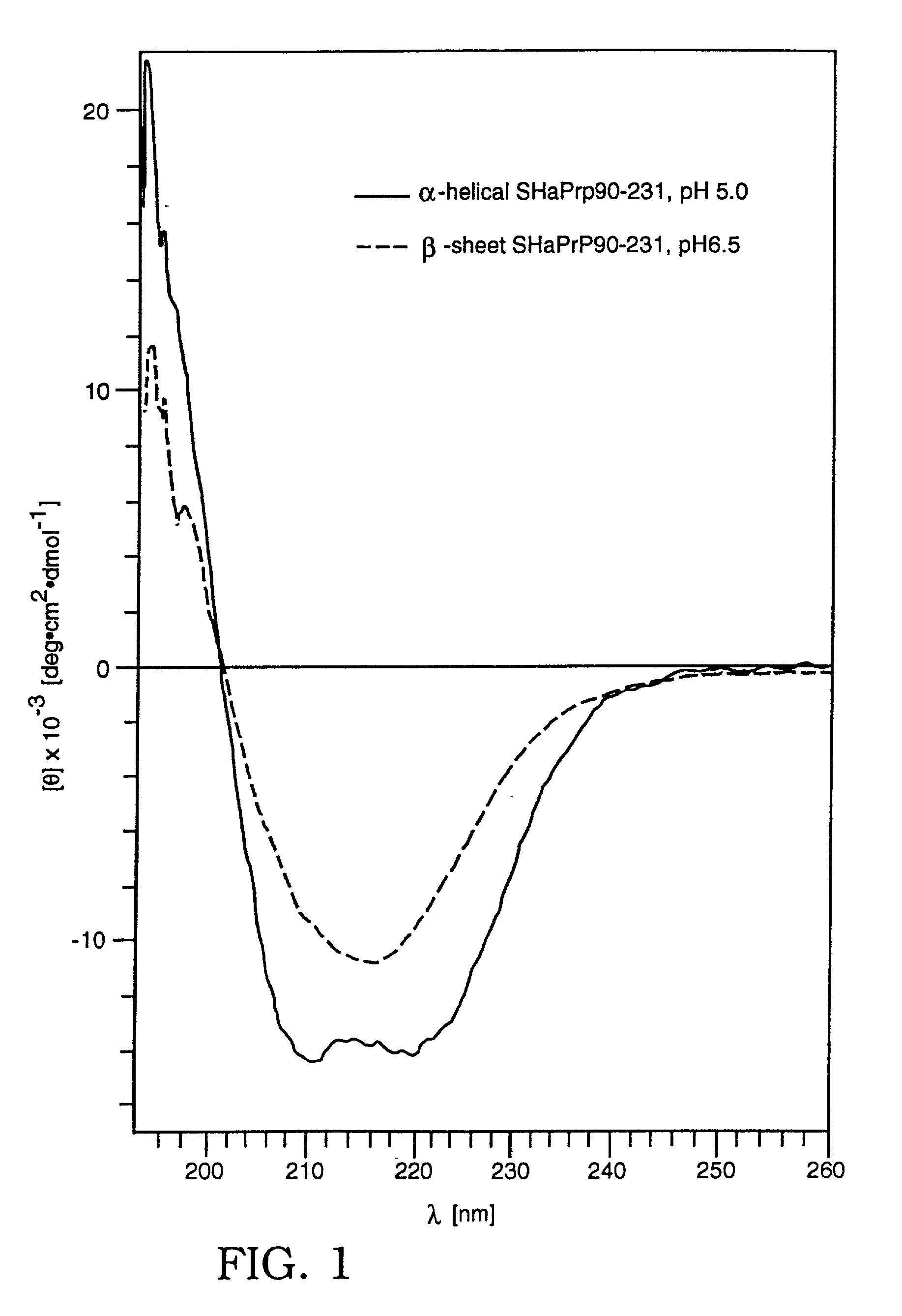
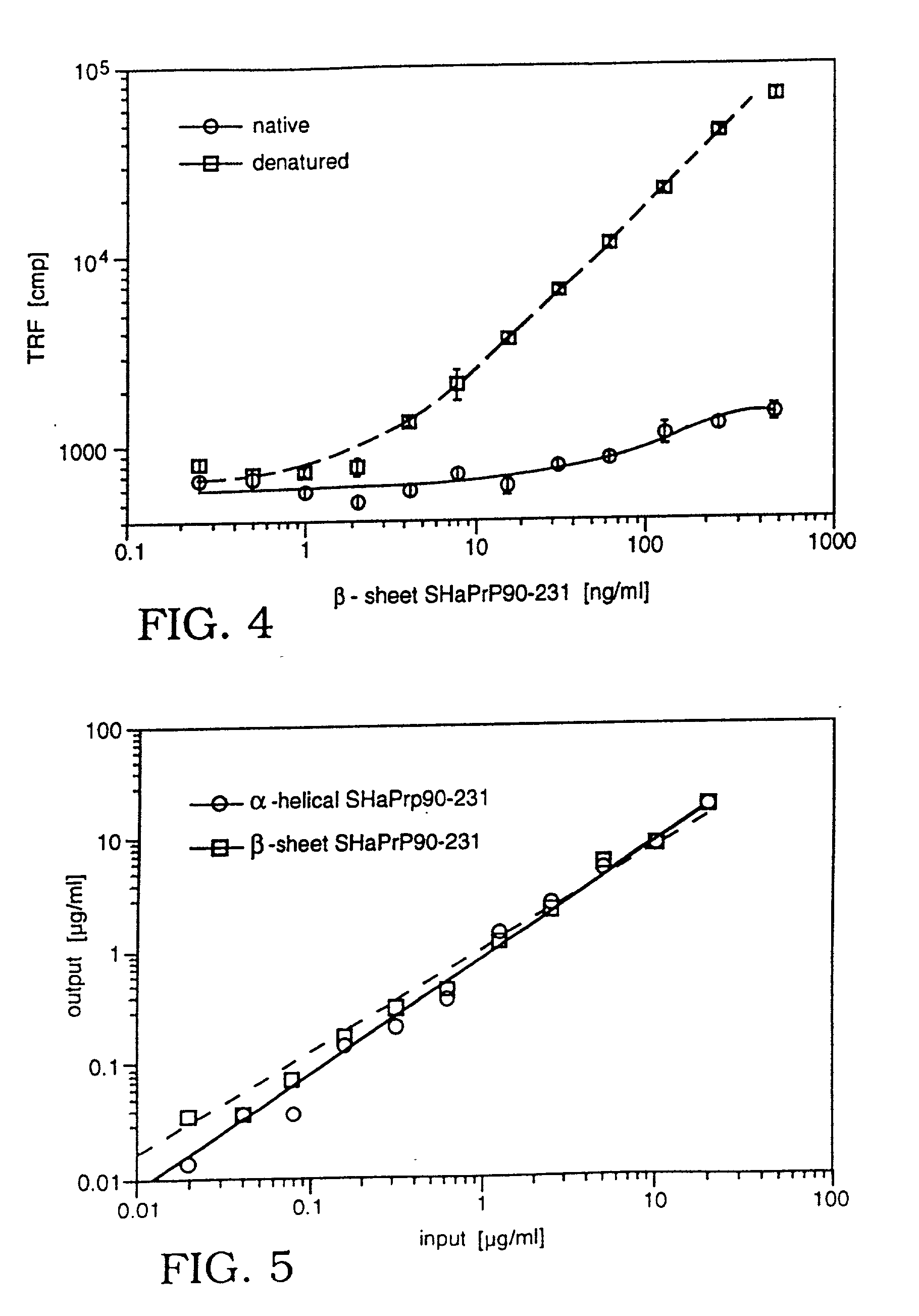
Assay for disease related conformation of a protein
InactiveUS20010001061A1Quickly and accurately presenceSignal obtained can be enhancedCompound screeningApoptosis detectionCross-linkBeta sheet
An assay method is disclosed which makes it possible to determine the presence of a diseased related conformation of a protein (e.g., PrPSc or the beta-sheet form of betaA4) in a sample. A sample is divided into two portions and the first portion is cross-linked to a first solid support and then contacted with a labeled antibody which binds to a non-disease form of the protein with a higher degree of affinity (e.g., 4 to 30 fold higher) than to the disease form of the protein. The second portion is treated in a manner which causes any disease form of the protein to change conformation to a form with a higher binding affinity for the labeled antibody. The treated second portion is then bound to a second solid support and contacted with labeled antibody. The level of labeled antibody binding to a protein in the first and second portions is determined and the amounts measured in each are compared. The difference between the two measurements is an indication of whether the disease related conformation of the protein was present in the sample. The method can also determine the concentration of the disease related conformation and the particular strain present.
Owner:PRUSINER STANLEY B +1
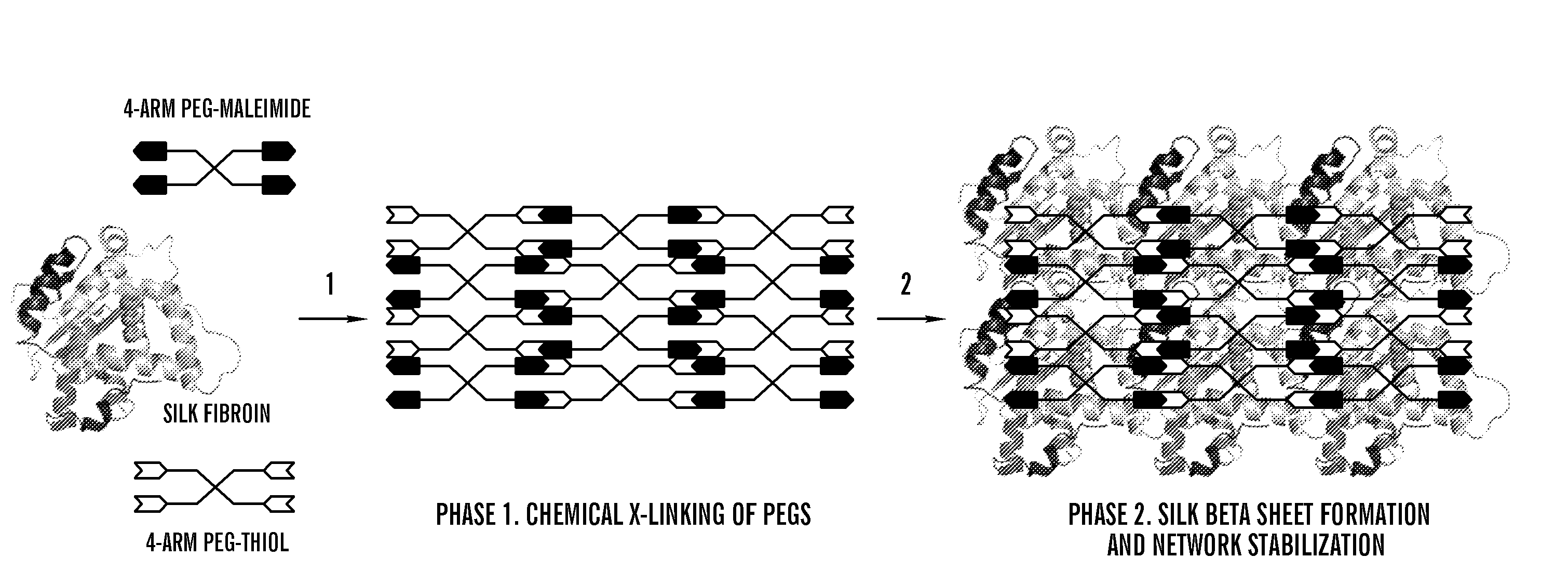
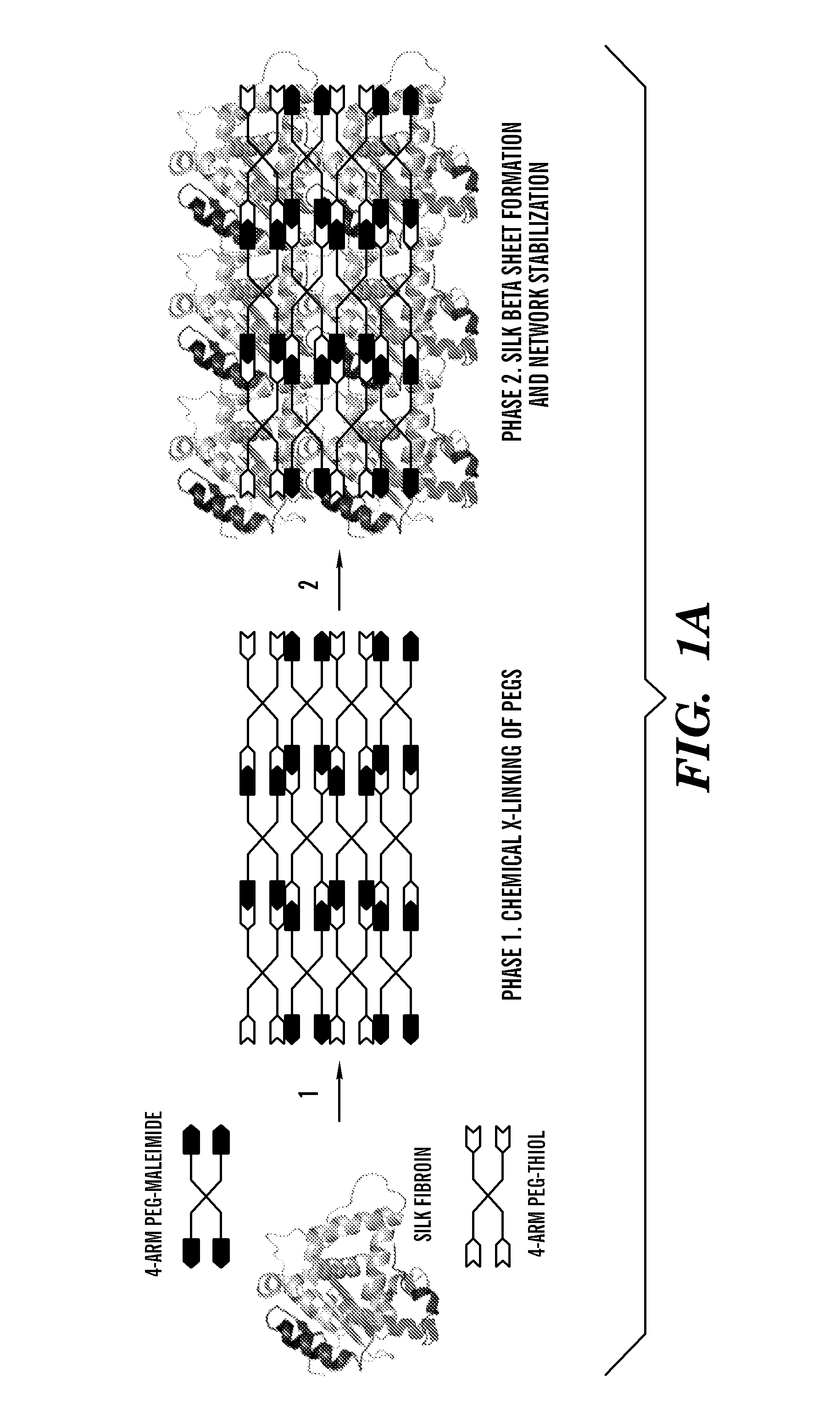
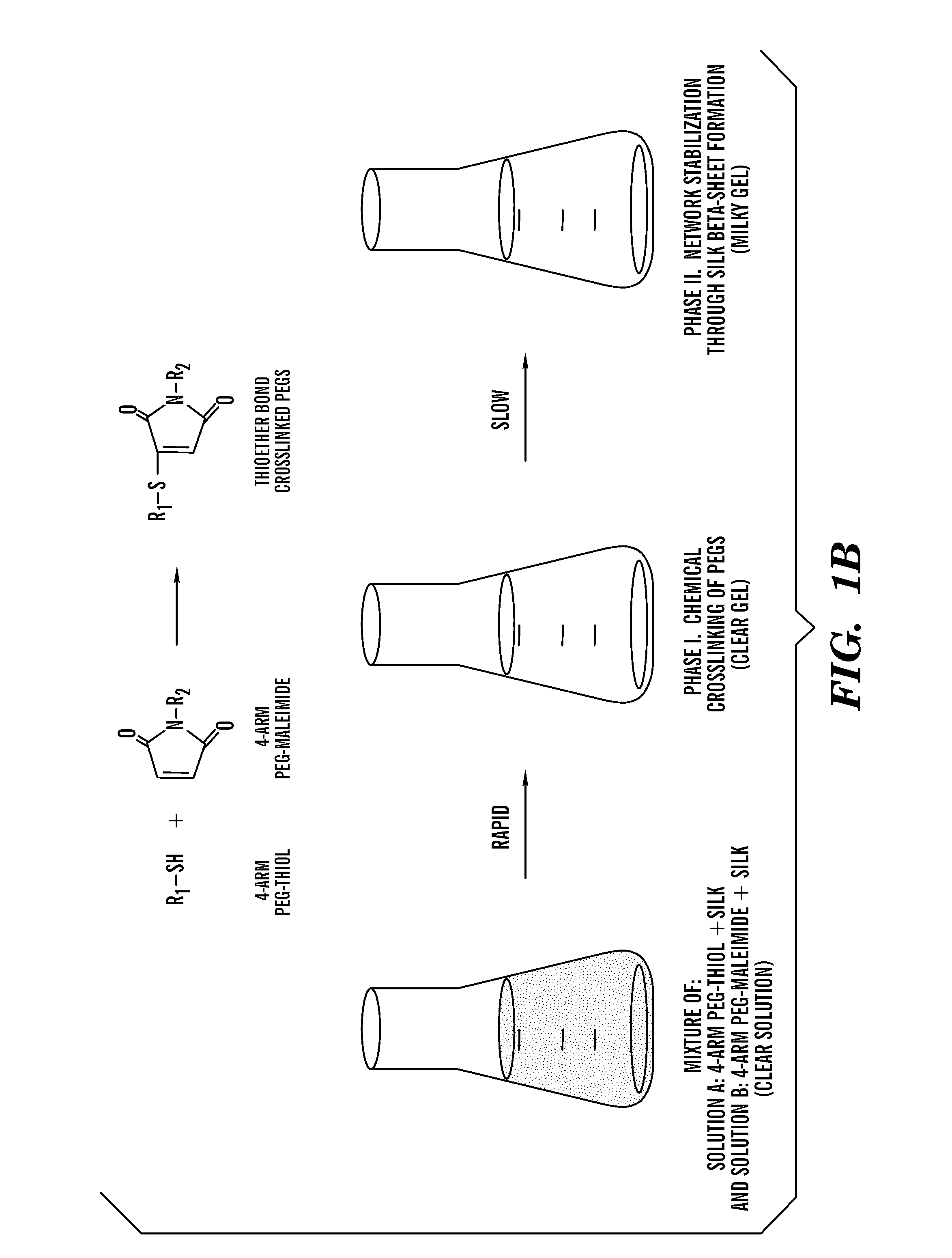
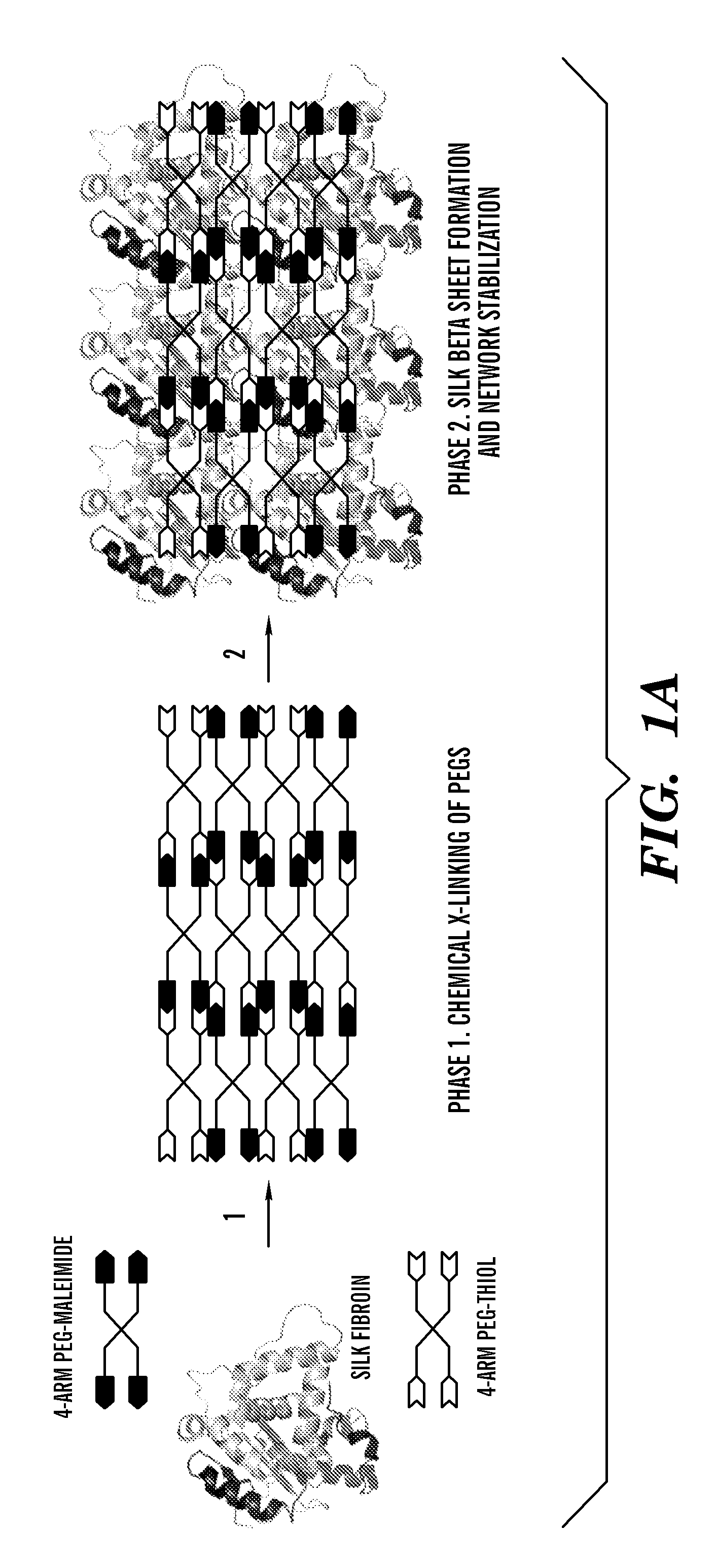
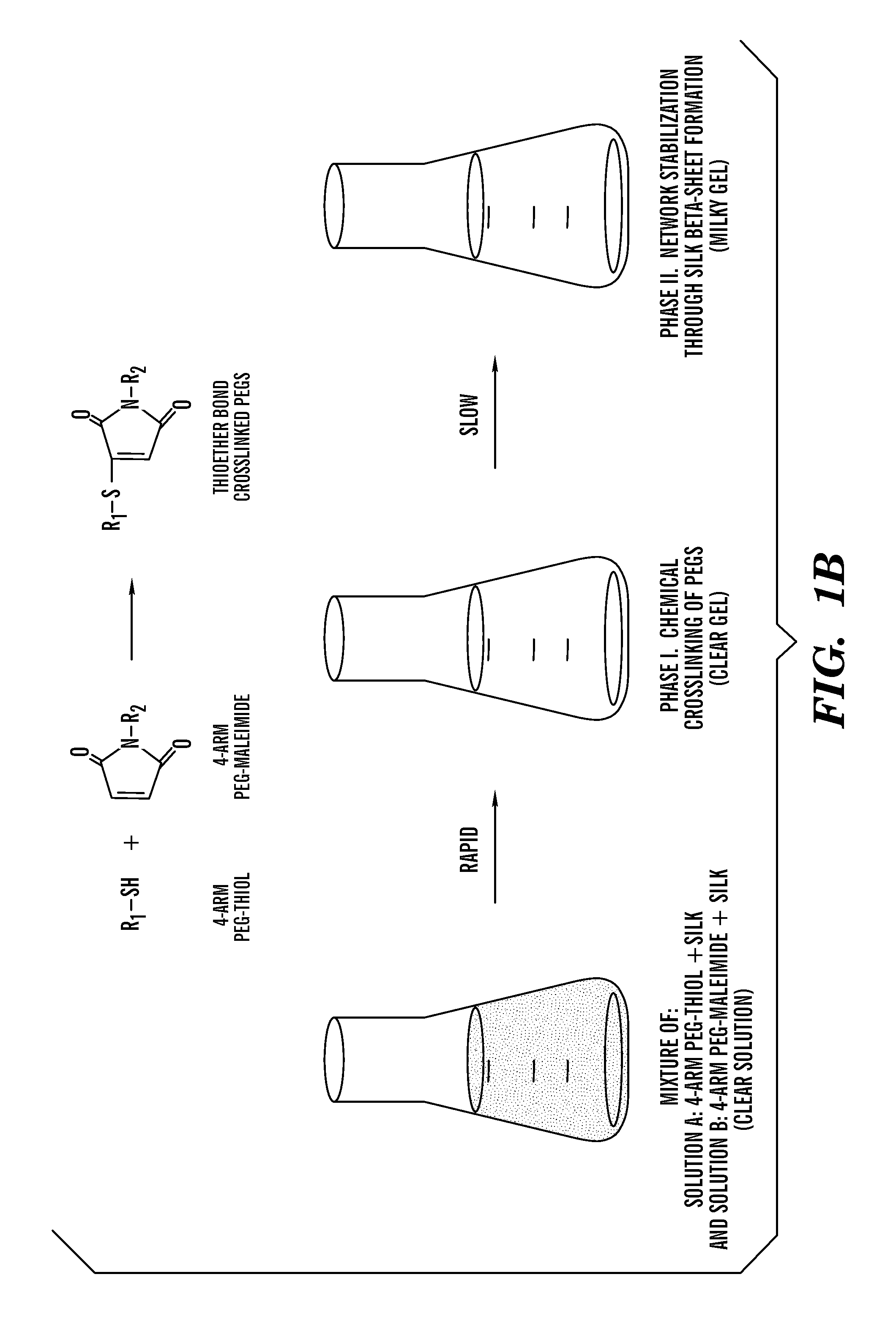
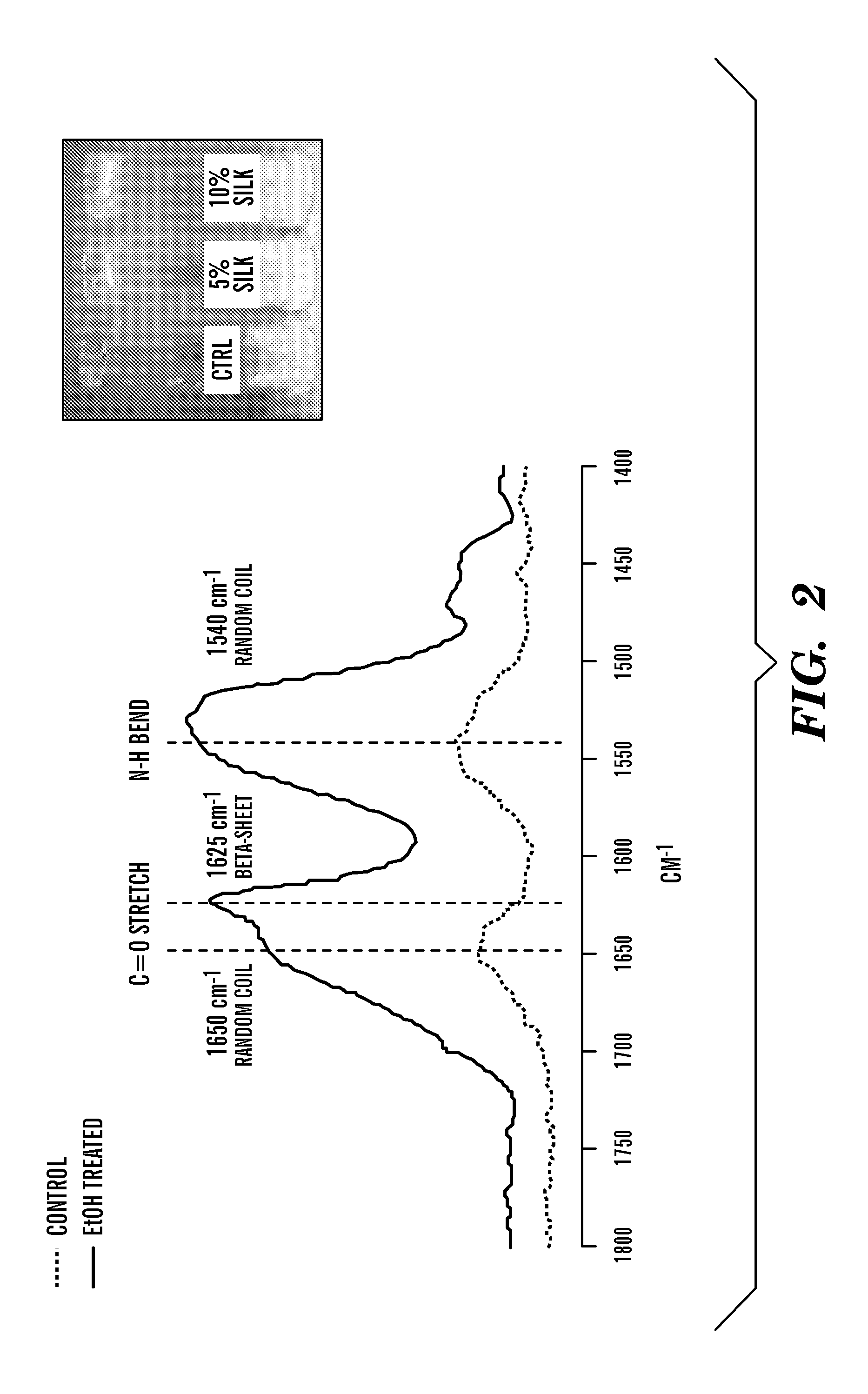
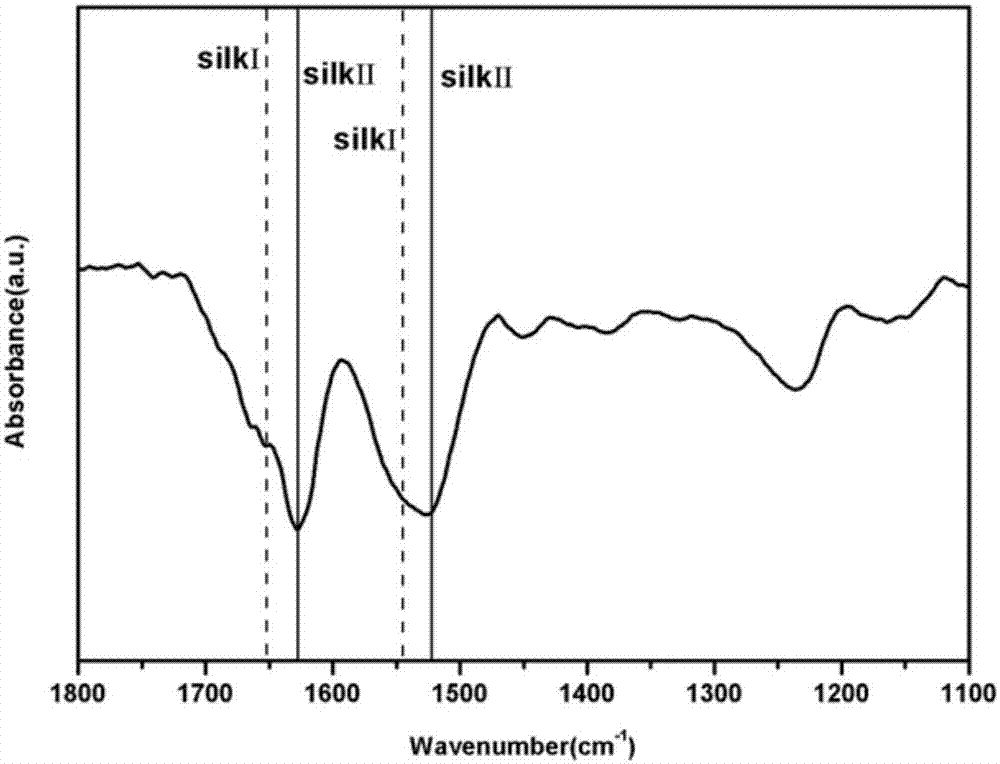
Silk fibroin and polyethylene glycol-based biomaterials
This invention relates to methods and compositions for preparation of silk-PEGs based biomaterials through crosslinking by chemically reacting active polyethylene glycols (PEGs) possessing different chemical groups (e.g., thiols and maleimides functionalized PEGs) that are additionally stabilized by the beta-sheet formation of silk fibroin. The crosslinked silk-PEGs biomaterials present strong adhesive properties, which are comparable to or better than the current leading PEG-based sealant, depending on the silk concentration in the silk-PEGs biomaterials. In addition, the silk-PEGs based biomaterials are cytocompatible, show decreased swelling behavior and longer degradation times, which make them suitable for hemostatic applications where the current available tissue sealant products can be contraindicated.
Owner:TRUSTEES OF TUFTS COLLEGE
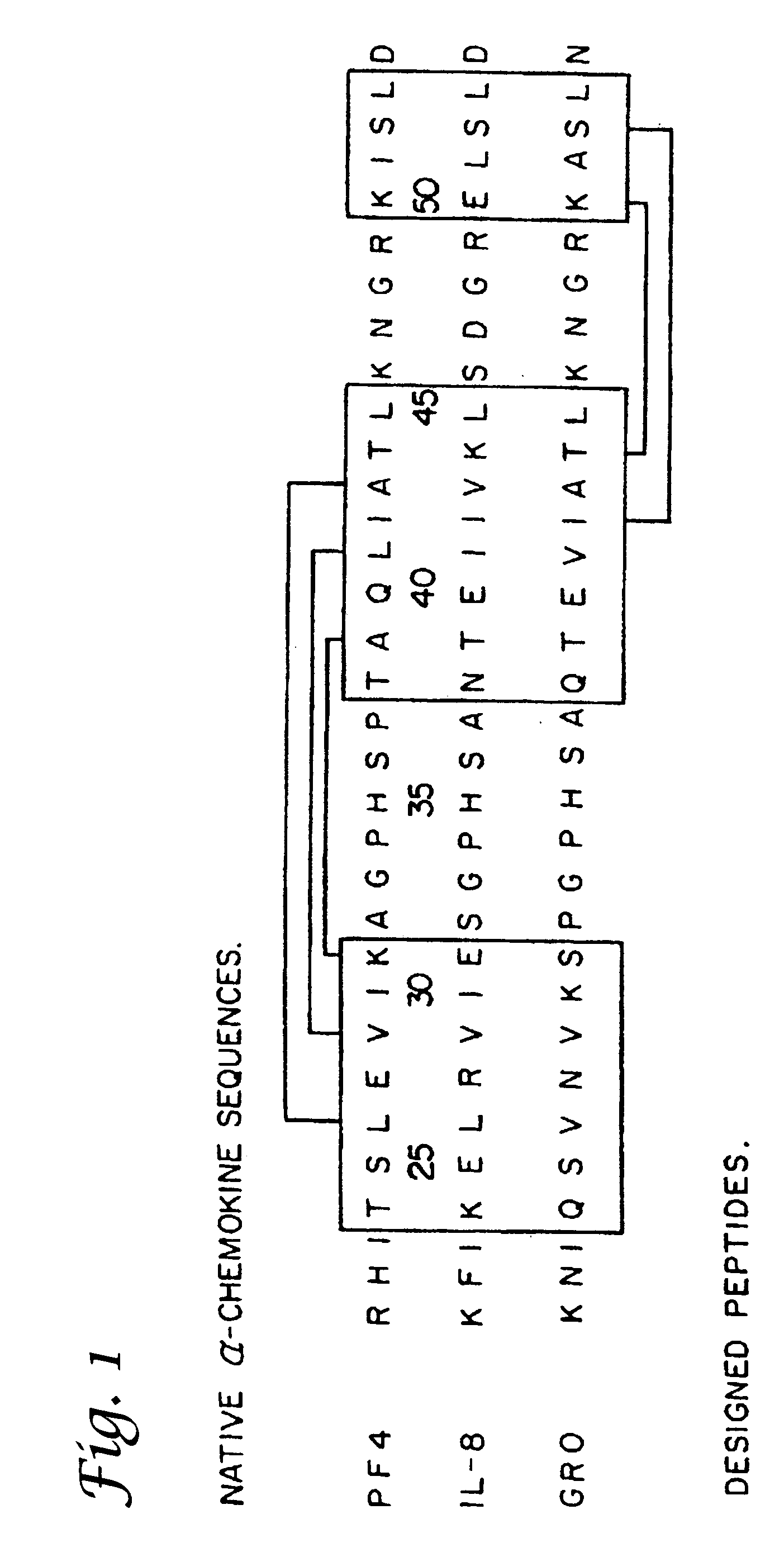
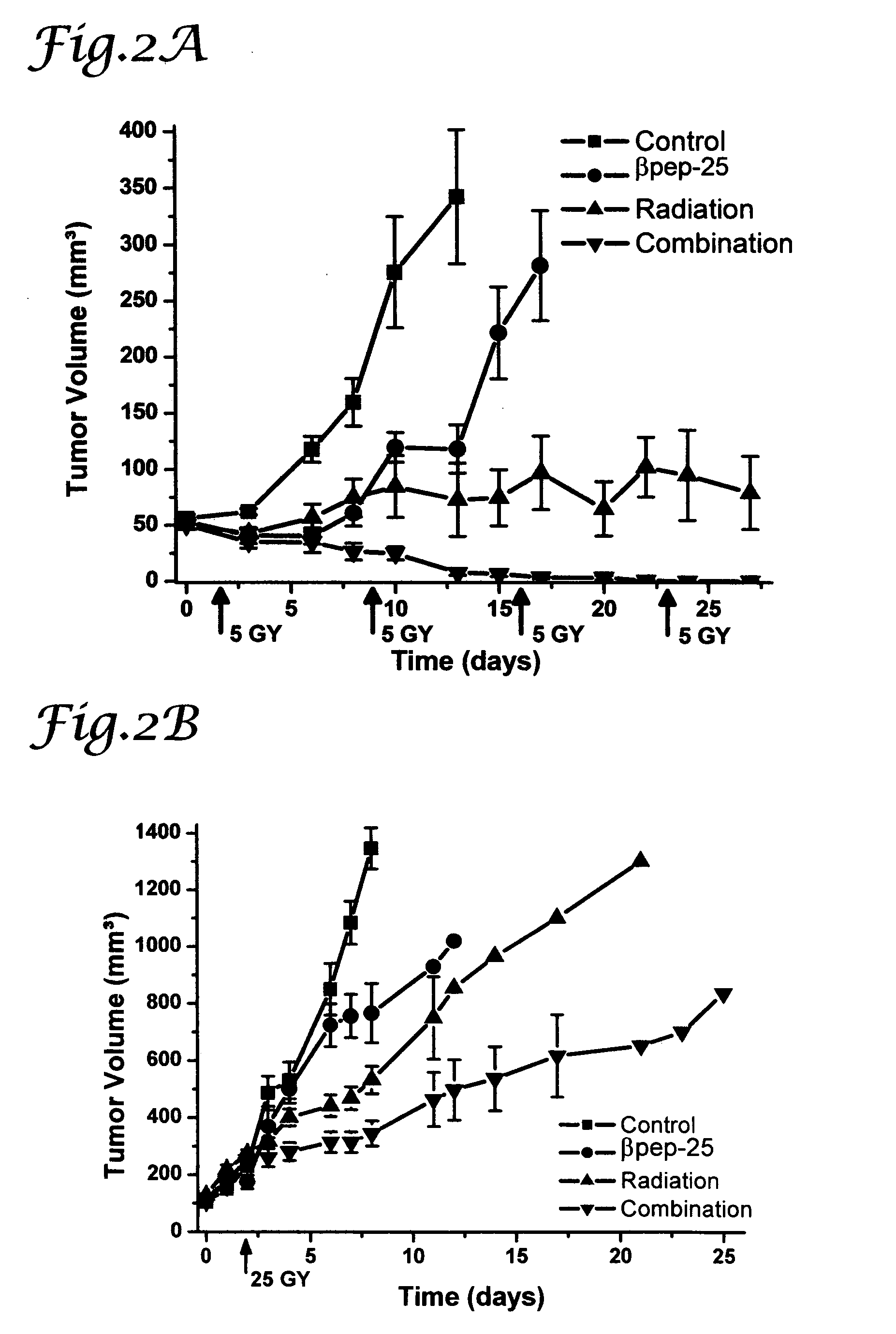
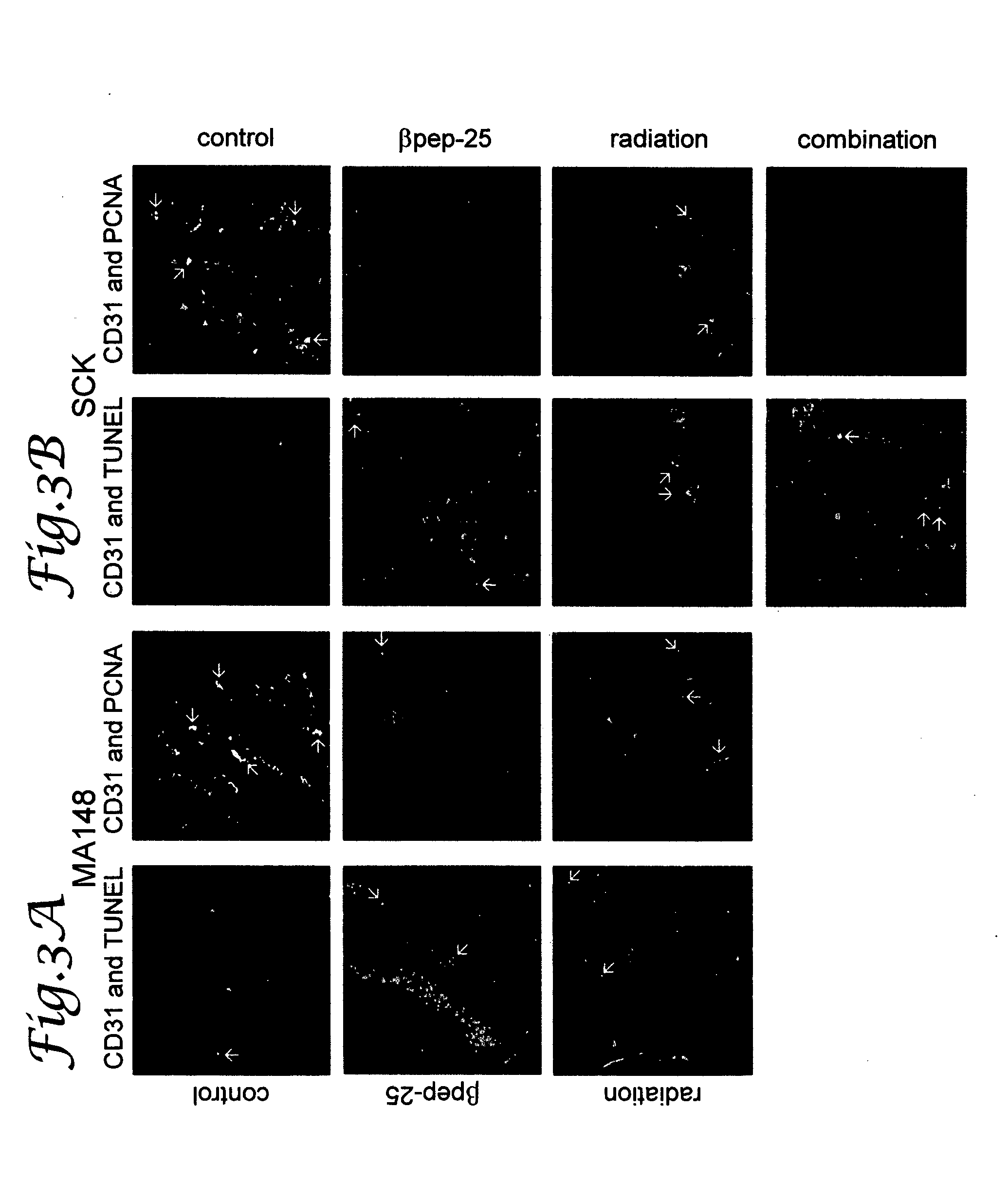
Tumor treatment using beta-sheet peptides and radiotherapy
InactiveUS20070010438A1Prolongs radiation-induced tumor growth delayEffective strategyPeptide/protein ingredientsDepsipeptidesAbnormal tissue growthTumor therapy
The invention relates to the use of designed β-sheet peptides together with radiation therapy for cancer treatment. The β-sheet peptides, which were designed using portions from several α-chemokines, exhibit activity as radiosensitizing agents, and have demonstrated synergism with radiation therapy for cancer treatment. The β-sheet peptides also exhibit activity as angiogenesis inhibitors.
Owner:RGT UNIV OF MINNESOTA
Fabrication of beta-pleated sheet proteins with specific binding properties
The present invention describes novel beta-sheet proteins having specific binding properties and catalytic properties and also methods for preparing these proteins.
Owner:SCIL PROTEINS
Functional peptide fiber, production method thereof and method for recovering peptide chains
InactiveUS20030162696A1Easy to modifyFirmly connectedPeptide-nucleic acidsSynthetic resin layered productsFiberBeta sheet
This invention provides a functional peptide fiber which comprises a plurality of peptide structure units each containing at least one peptide chain, wherein peptide chains contained in each adjacent peptide structure units do not form peptide bond but are structured into a fibrous form by taking a beta-sheet structure, and wherein at least one of the plurality of peptide structure units contains a peptide chain having a functional material connected thereto. Also disclosed are a method for producing the functional peptide fiber and a method for recovering peptide chains from the functional peptide fiber.
Owner:FUJIFILM BUSINESS INNOVATION CORP
Method used for preparing semaglutide
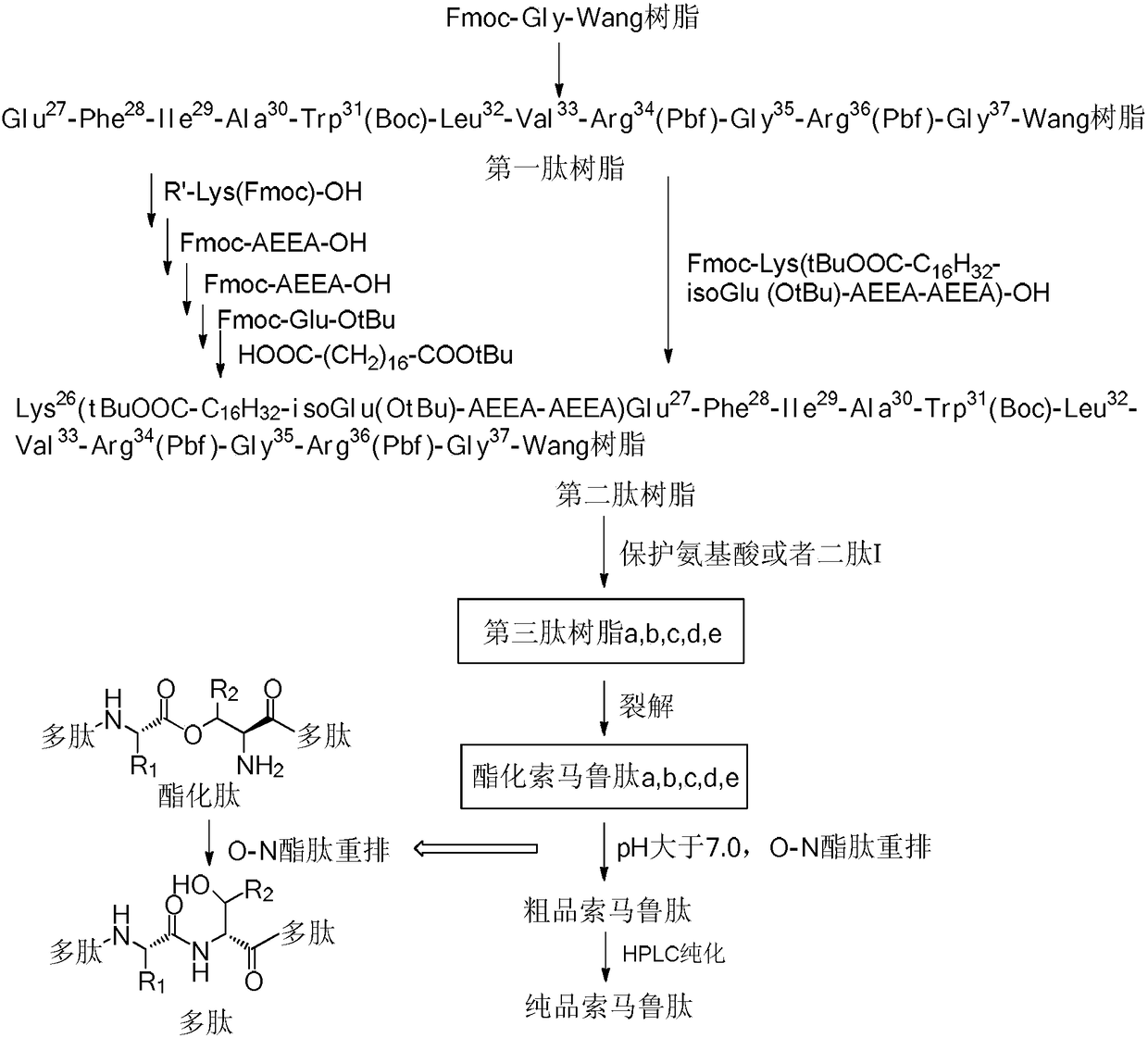
InactiveCN108203462AScale upPrevent polycondensationPeptide preparation methodsBulk chemical productionDipeptideHigh volume manufacturing
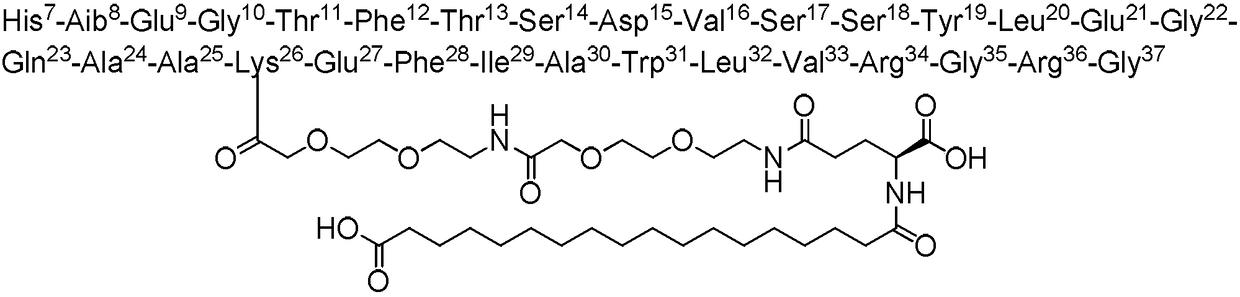
The invention belongs to the technical field of polypeptide synthesis, and especially relates to a synthesis method of semaglutide. The synthesis method comprises following steps; 1, prior coupling oflys26 side chain is carried out; and 2, O-iso-acylated dipeptide is adopted to break the secondary structure of beta-sheet in semaglutide synthesis process effectively so as to avoid polycondensationin polypeptide synthesis process, and then O to N transfer reaction is adopted to convert esterified semaglutide into semaglutide. The method is capable of reducing product synthesis difficulty, avoiding generation of a plurality of impurities, ensuring the product quality of semaglutide bulk drugs, increasing product yield, increasing the scale of single batch semaglutide production, and realizing mass production of semaglutide.
Owner:上海脉凯生物科技有限公司
Misfolded protein sensor method
ActiveUS20060286672A1Easy to detectRapid and cost-effectivePeptide preparation methodsDepsipeptidesCrystallographyDendrimer
A catalytic conformational sensor method for detecting abnormal proteins and proteinaceous particles. The method is based on the interaction of a peptide fragment or probe with an abnormal proteinaceous particle. The interaction catalyzes transformation of the probe to a predominately beta sheet conformation and allows the probe to bind to the abnormal proteinaceous particle. This in turn, catalyzes propagation of a signal associated with the test sample-bound probe. As a result signals can be propagated even from samples containing very low concentrations of abnormal proteinaceous particles. The peptide probes can be designed to bind to a desired peptide sequence or can even be based on dendrimer structure to control further aggregate propagation.
Owner:PRESYMPTO INC
Purified Amphiphilic Peptide Compositions and Uses Thereof
A plurality of amphiphilic peptide chains having alternating hydrophilic and hydrophobic amino acids, wherein the peptide contains at least 8 amino acids, are complementary and structurally compatible, and self-assemble into a beta-sheet macroscopic scaffold wherein peptide at least about 75% of the chains have the same sequence.
Owner:3D MATRIX
Self-regulated peptide hydrogel for insulin delivery
ActiveUS20150025005A1Avoiding both hyper- and hypoglycemiaStopping releasePeptide/protein ingredientsOintment deliveryConcentrations glucoseHypoglycemia
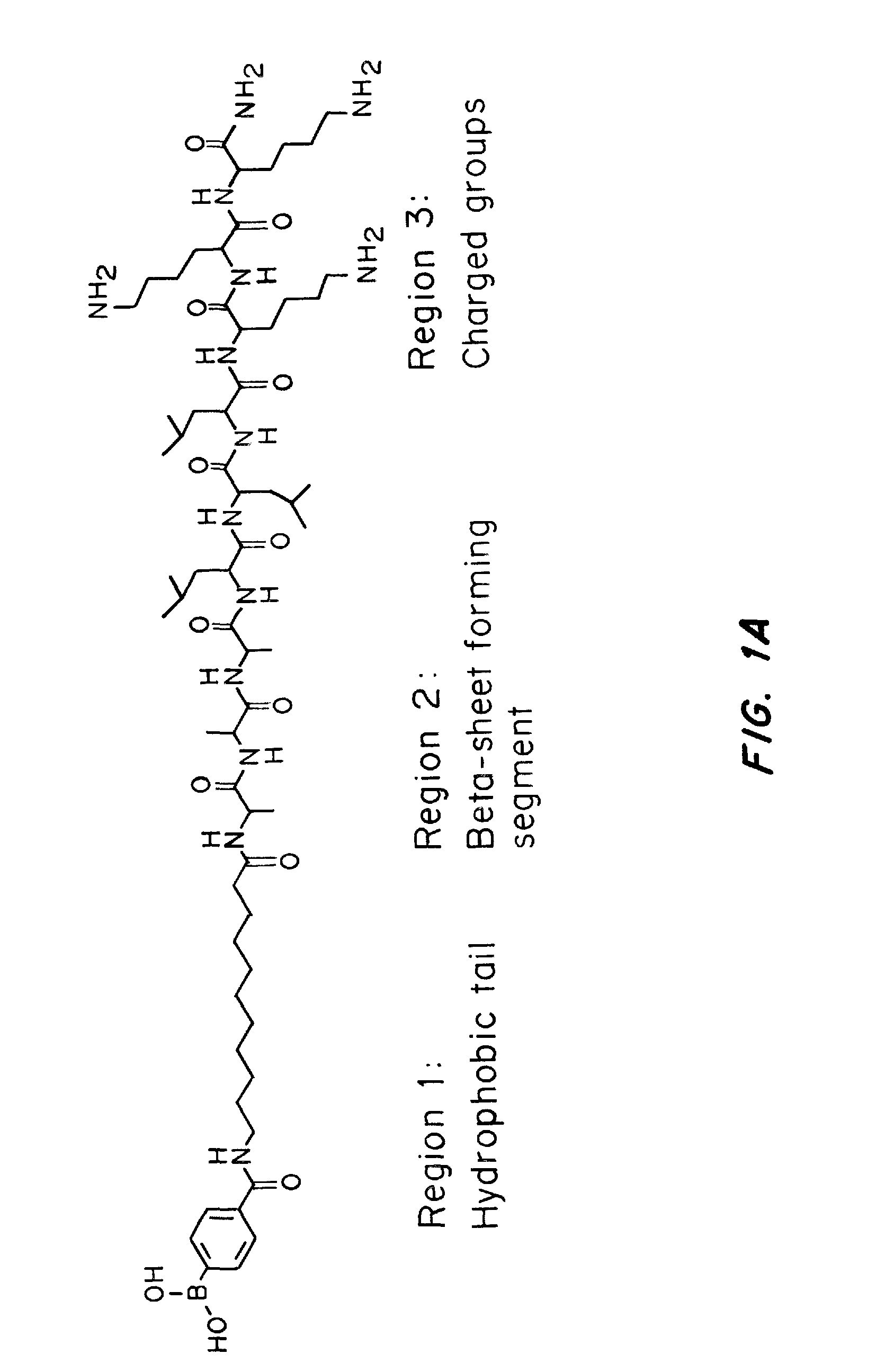
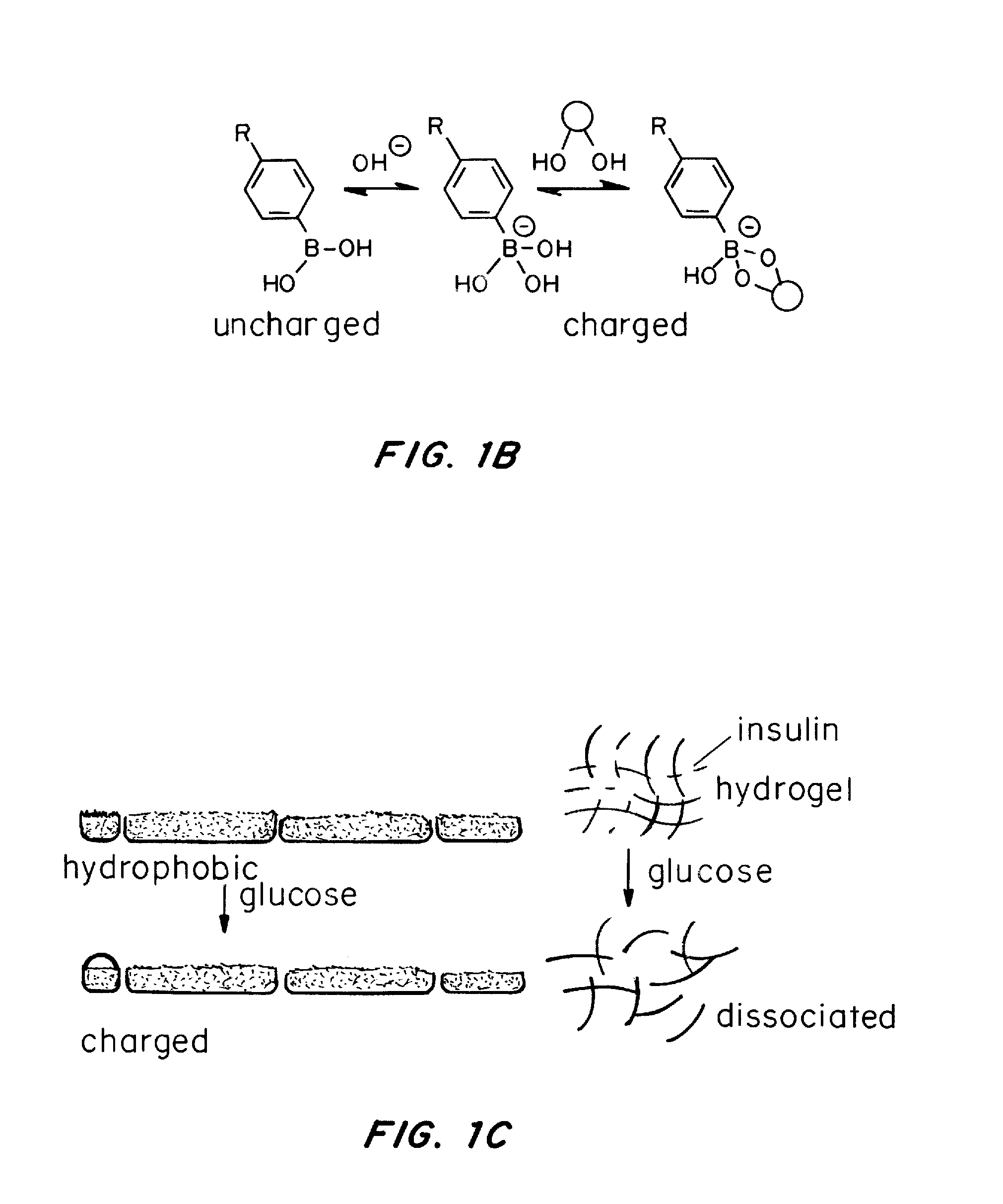
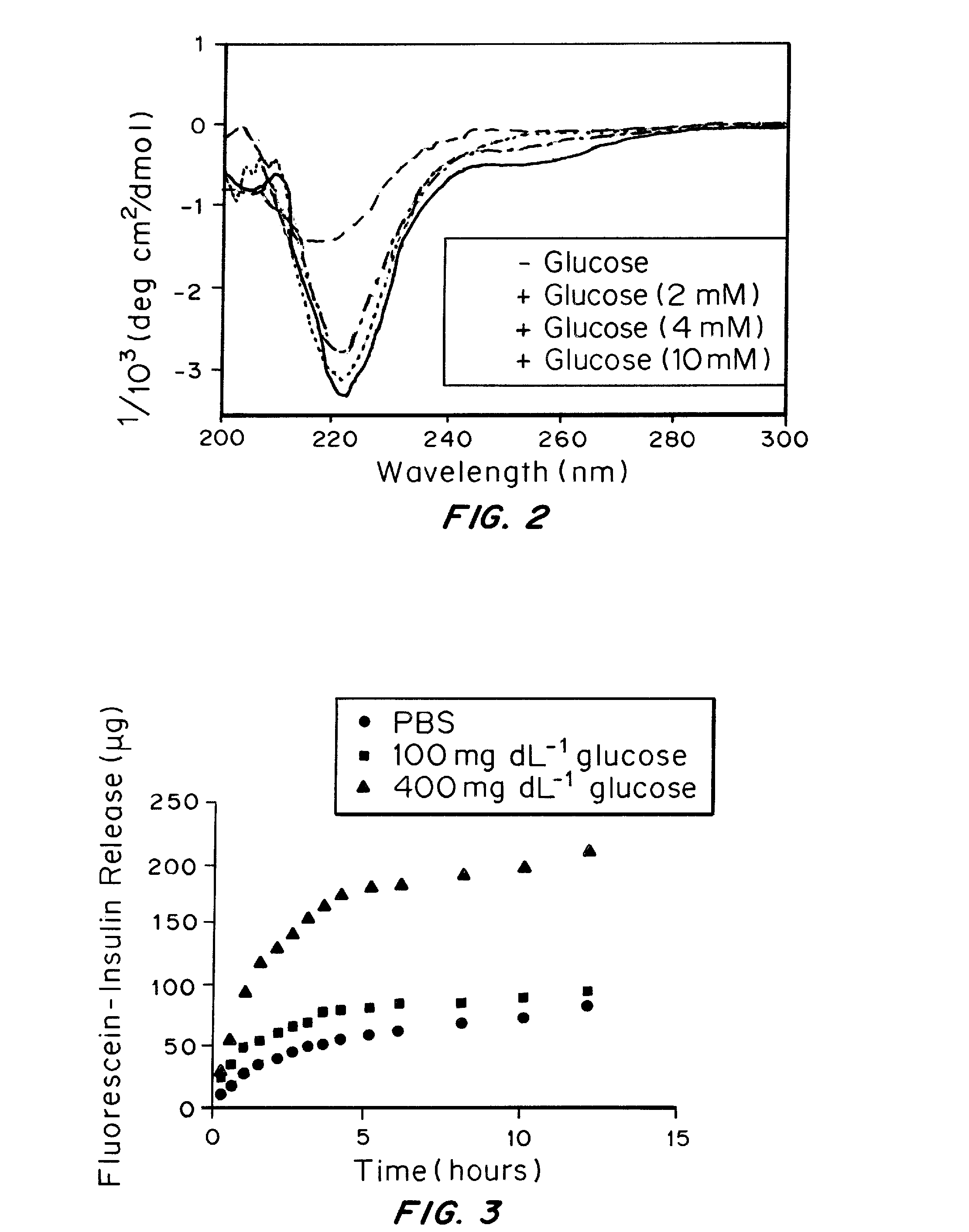
A glucose binding amphiphilic peptide hydrogel insulin delivery system that is responsive to glucose concentrations under physiological conditions is provided. Insulin is encapsulated in a glucose binding hydrogel, made from self-assembling amphiphilic peptides including a hydrophobic domain including a beta sheet forming region coupled to a charged hydrophilic domain modified to contain a glucose binding segment. The formulations are designed to release insulin as a function of blood glucose level, maintaining the patients' blood glucose level in an optimum range and avoiding both hyper- and hypoglycemia.
Owner:MASSACHUSETTS INST OF TECH +1
Misfolded protein sensor method in body fluids
InactiveUS20060275910A1Easy to detectRapid and cost-effective analyticalDisease diagnosisBiological testingCrystallographyTest sample
A catalytic conformational sensor method for detecting abnormal proteins and proteinaceous particles. The method is based on the interaction of a peptide fragment or probe with an abnormal proteinaceous particle. The interaction catalyzes transformation of the probe to a predominately beta sheet conformation and allows the probe to bind to the abnormal proteinaceous particle. This in turn, catalyzes propagation of a signal associated with the test sample-bound probe. As a result signals can be propagated even from samples containing very low concentrations of abnormal proteinaceous particles as is the case in many body-fluid derived samples.
Owner:ADLYFE INC
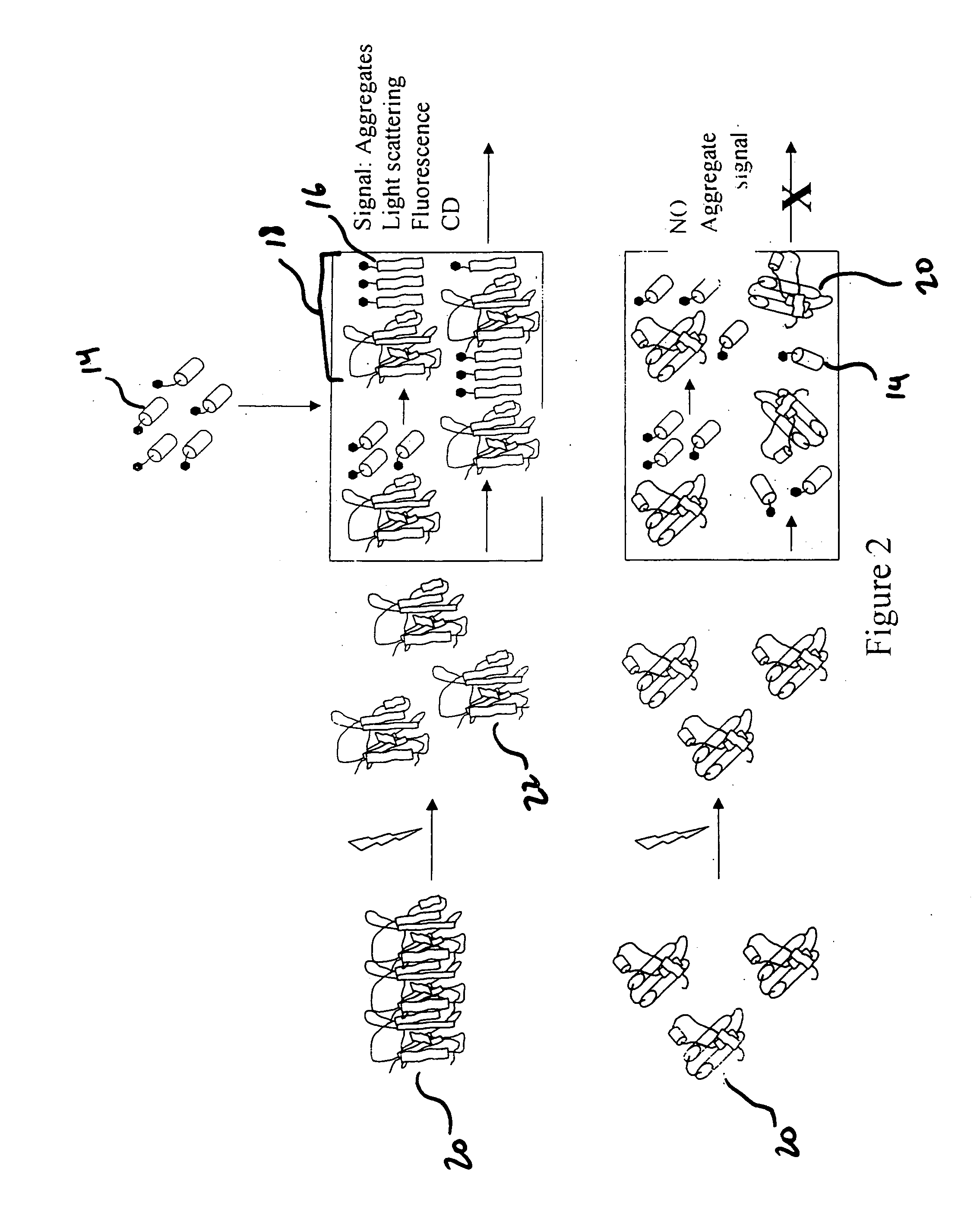
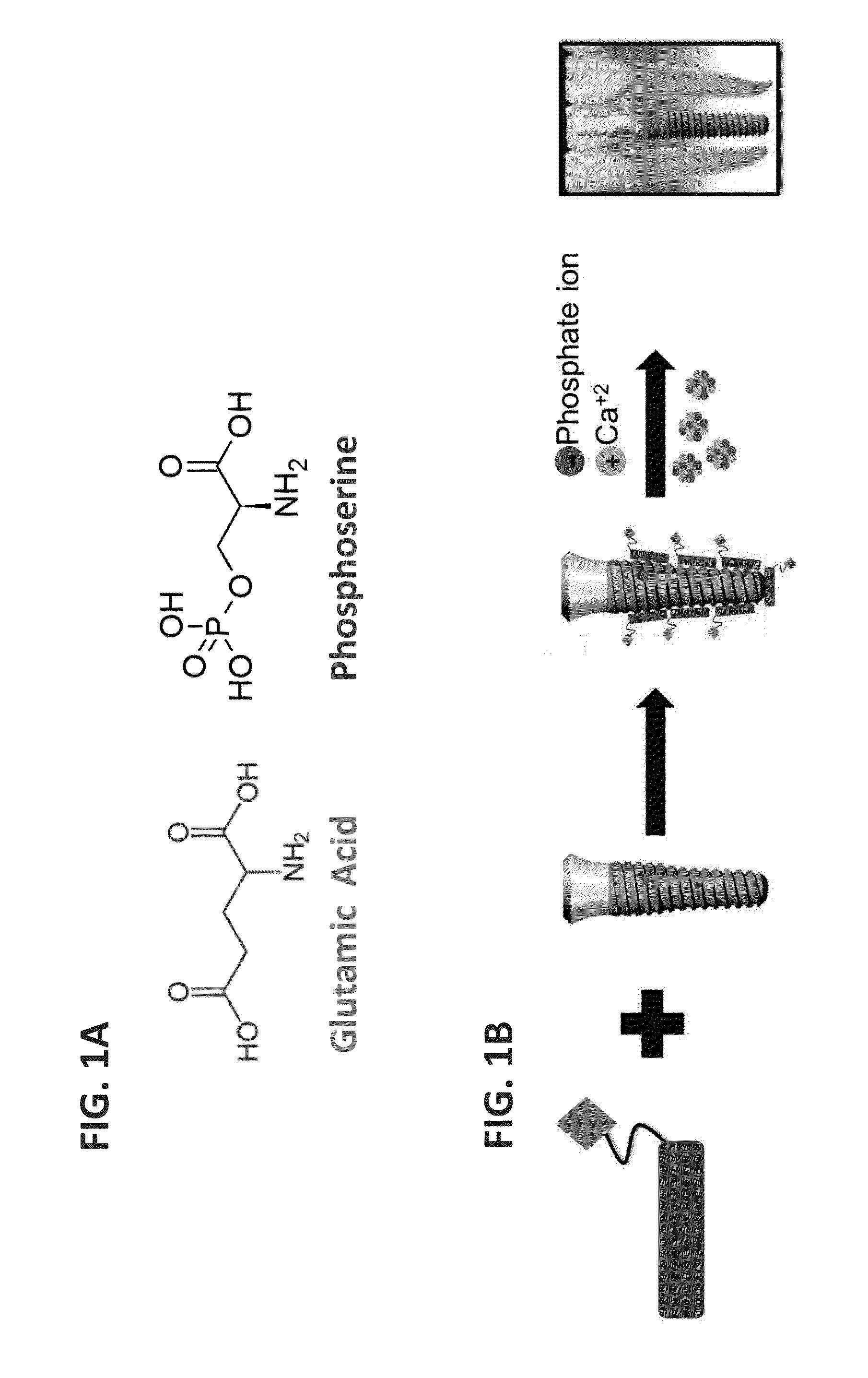
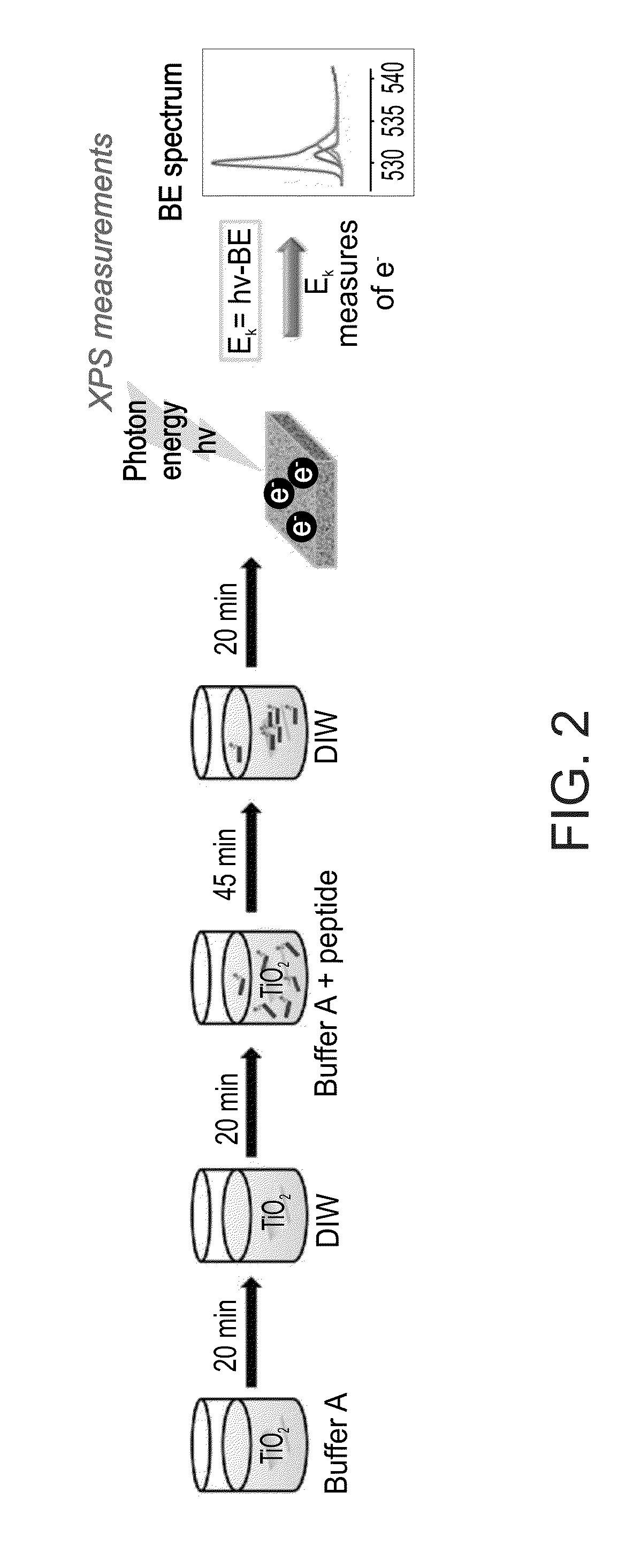
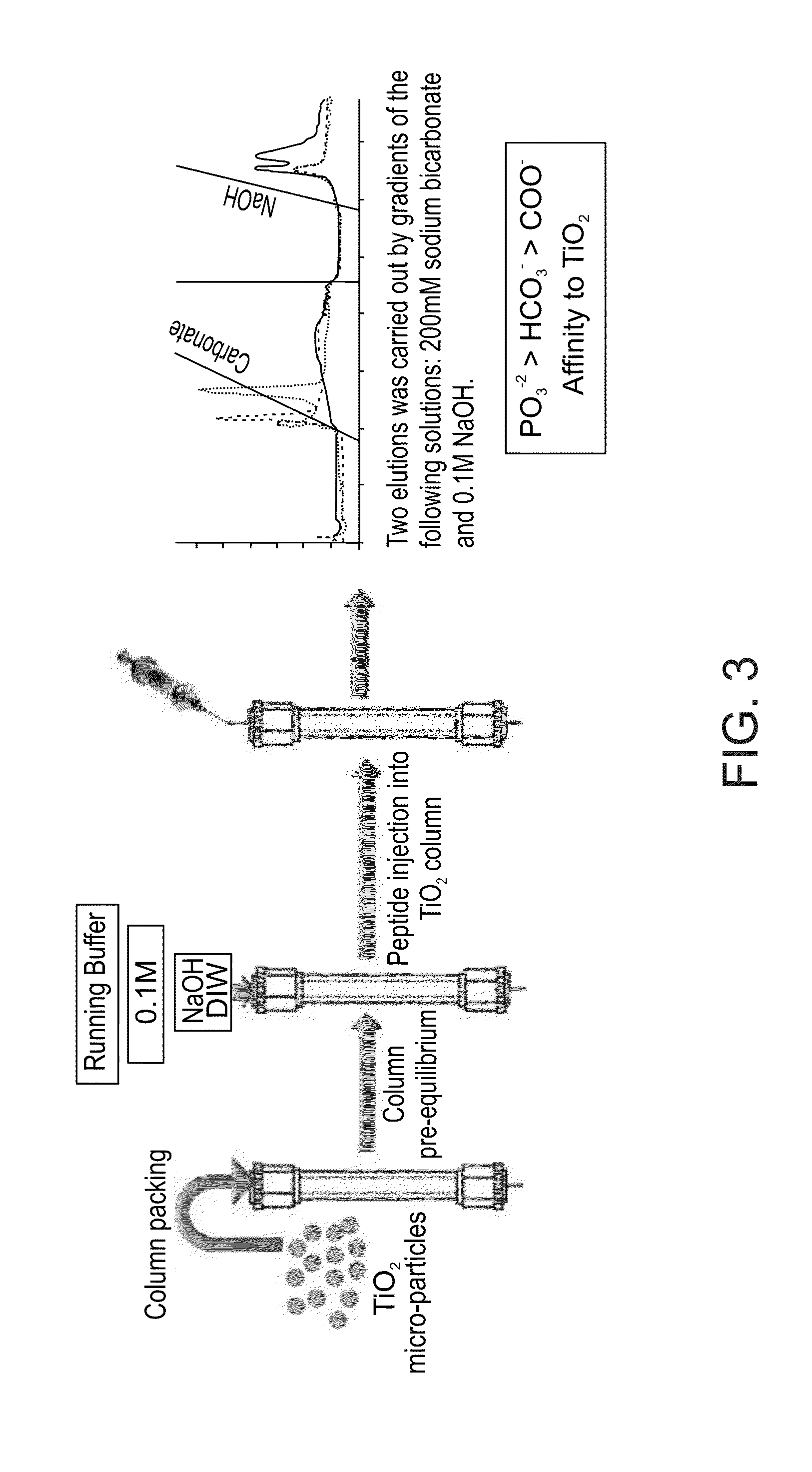
Functionalized titanium binding peptides and implants coated with same
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Methods of inhibiting binding of beta-sheet fibril to rage and consequences thereof
This invention provides a method of inhibiting the binding of beta-sheet fibril to RAGE on the surface of a cell which comprises contacting the cell with a binding-inhibiting amount of a compound capable of inhibiting binding of beta-sheet fibril to RAGE so as to thereby inhibit binding of beta-sheet fibril to RAGE.In one embodiment, the beta-sheet fibril is amyloid fibril. In one embodiment, the compound is sRAGE or a fragment thereof. In another embodiment, the compound is an anti-RAGE antibody or portion thereof.This invention provides the above method wherein the inhibition of binding of the beta-sheet fibril to RAGE has the consequences of decreasing the load of beta-sheet fibril in the tissue, inhibiting fibril-induced programmed cell death, and inhibiting fibril-induced cell stress.This invention also provides methods of determining whether a compound inhibits binding of a beta-sheet fibril to RAGE on the surface of a cell.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
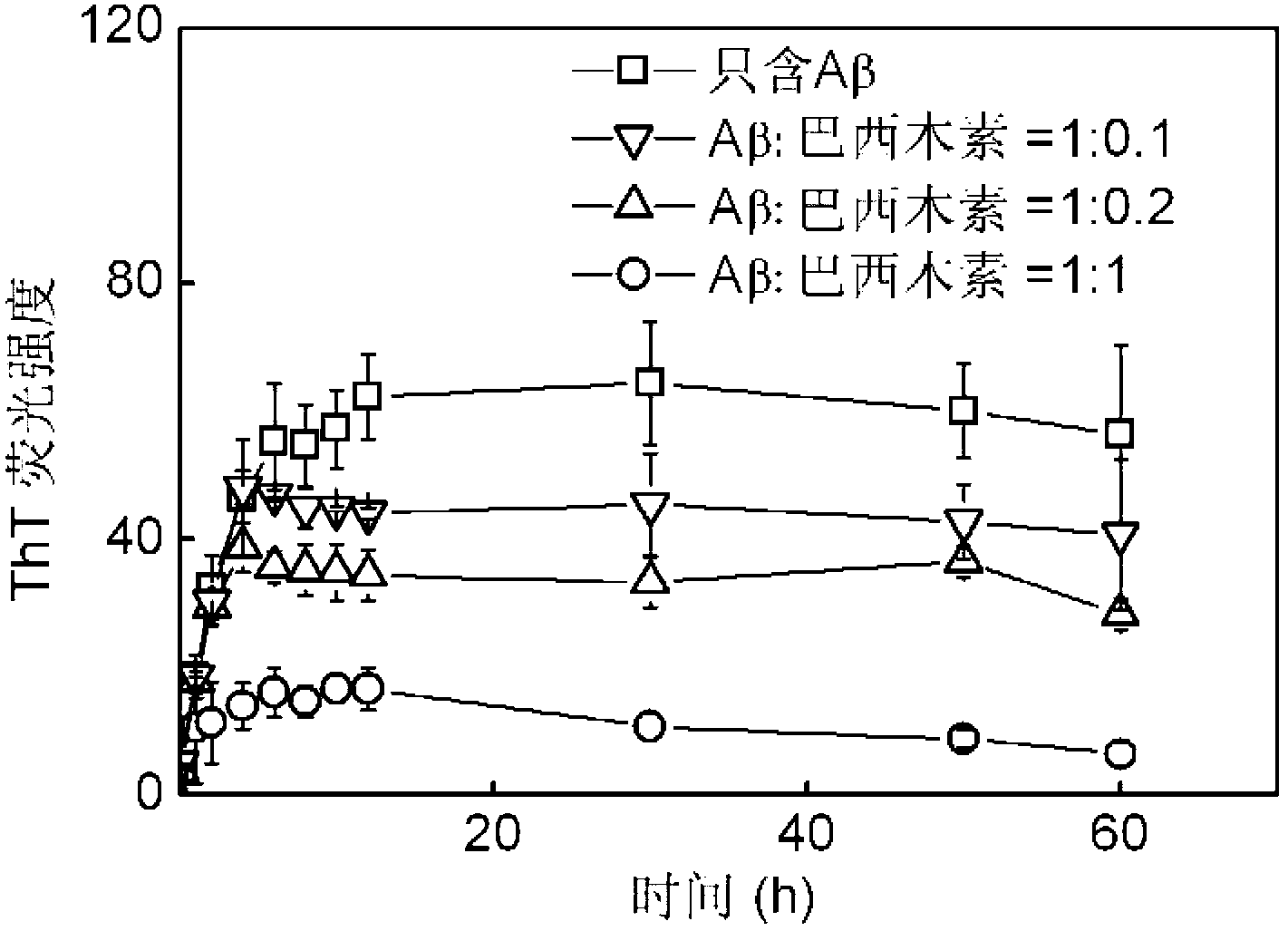
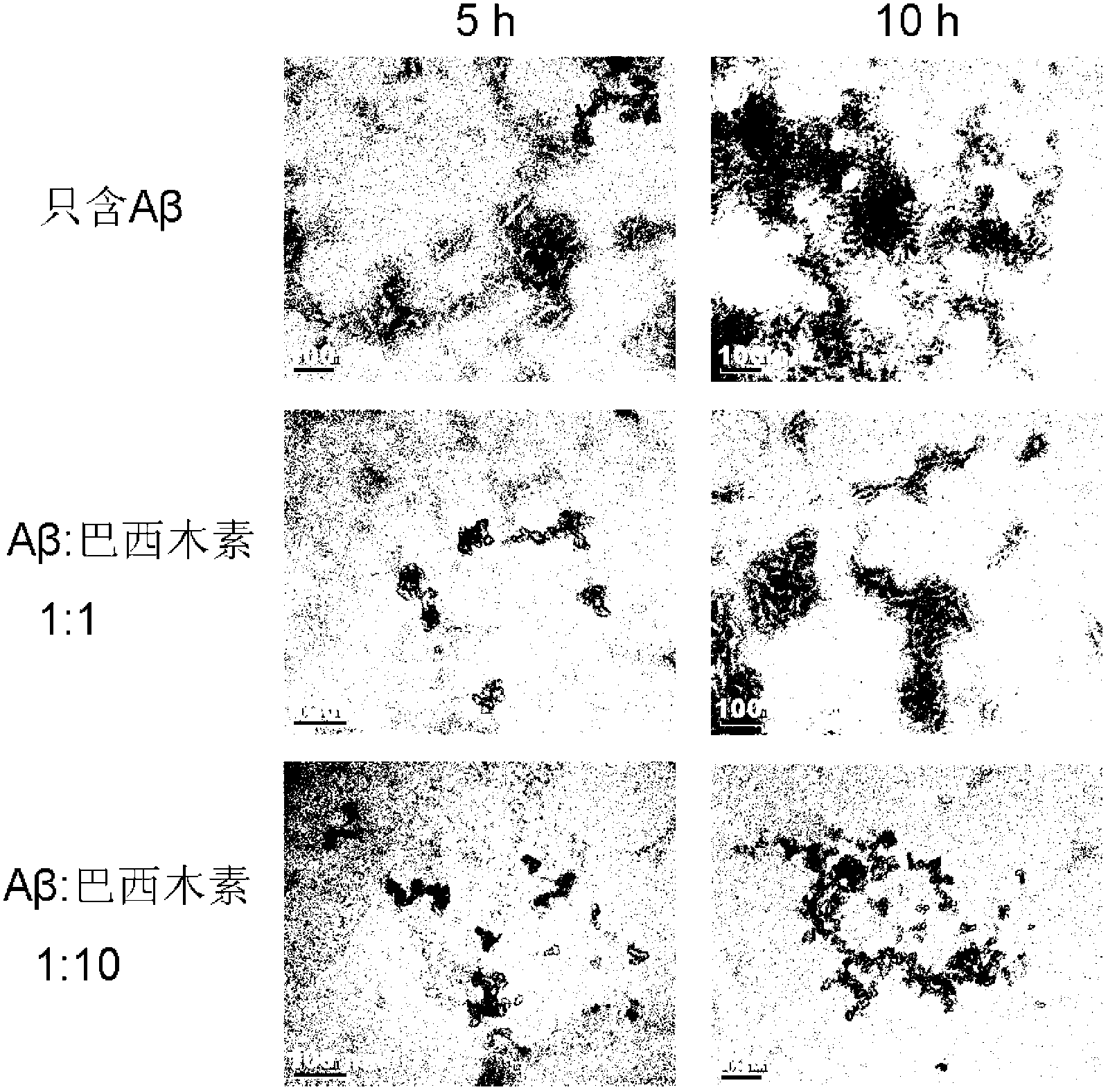
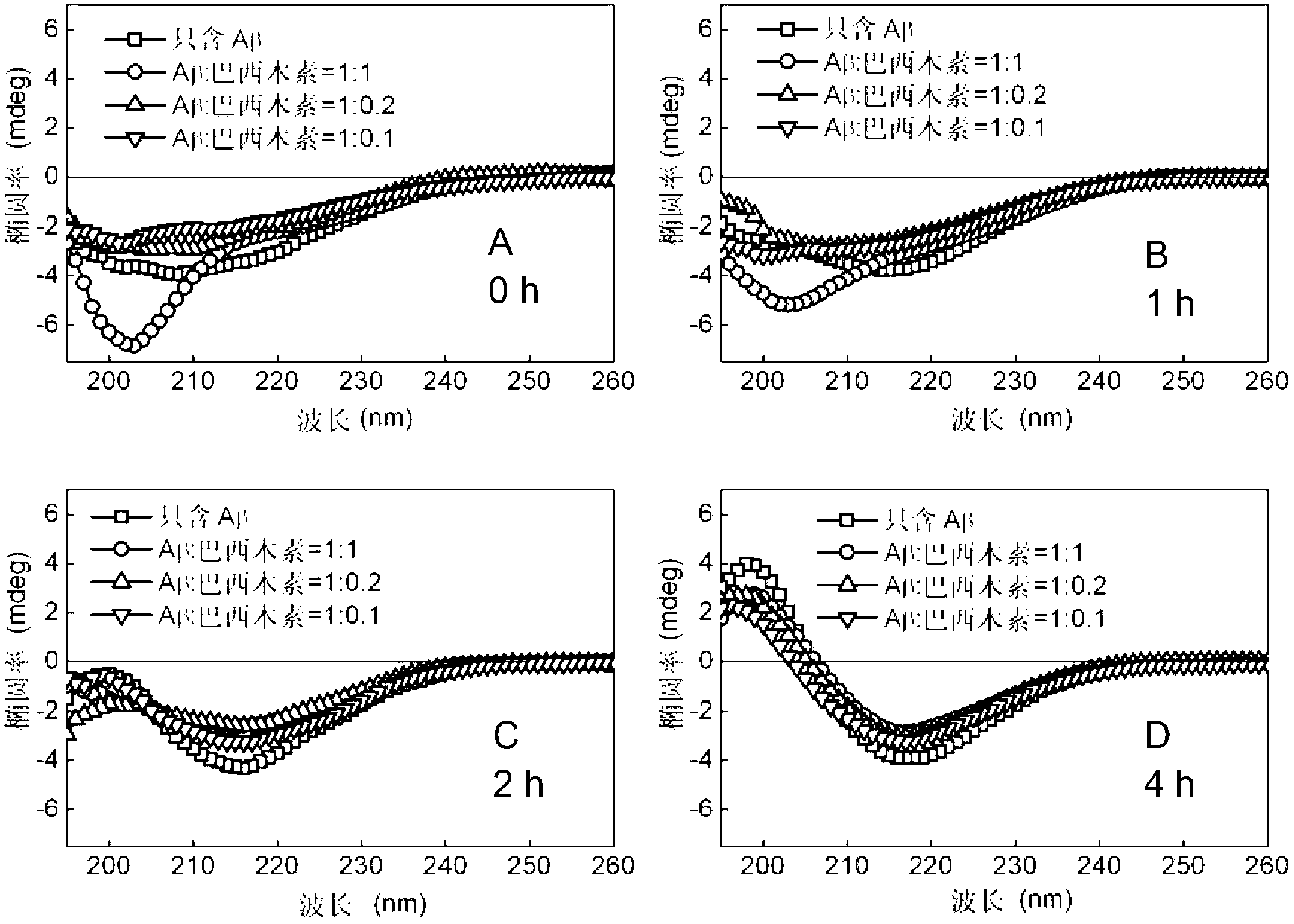
Application of brazilin to preparation of drug or health care product for inhibiting aggregation of beta-amyloid proteins
InactiveCN103006635AInhibit aggregationAvoid toxicityOrganic active ingredientsNervous disorderBrazilinCytotoxicity
The invention discloses an application of brazilin to preparation of drug or a health care product for inhibiting aggregation of beta-amyloid proteins. Various experimental means prove that brazilin can inhibit amyloid beta 42 aggregation, can change the appearance of the amyloid beta 42 aggregation, prevents and slows down the conversion of the appearance of the amyloid beta 42 aggregation into the fibrous appearance, slows down the process of converting a secondary structure of the amyloid beta 42 into a beta-sheet structure, decreases the content of the amyloid beta 42 converted into the beta-sheet in the solution, and inhibits the amyloid beta 42 aggregation from producing a toxic effect on cells effectively. Brazilin is considered as a potential new rug or health care product molecule, can inhibit amyloid beta 42 aggregation effectively and the comformational change process of the amyloid beta 42 aggregation, reduces the cytotoxic effect generated during the amyloid beta 42 aggregation process, and is an ideal inhibitor for amyloid beta 42 aggregation.
Owner:TIANJIN UNIV
Silk fibroin and polyethylene glycol-based biomaterials
This invention relates to methods and compositions for preparation of silk-PEGs based biomaterials through crosslinking by chemically reacting active polyethylene glycols (PEGs) possessing different chemical groups (e.g., thiols and maleimides functionalized PEGs) that are additionally stabilized by the beta-sheet formation of silk fibroin. The crosslinked silk-PEGs biomaterials present strong adhesive properties, which are comparable to or better than the current leading PEG-based sealant, depending on the silk concentration in the silk-PEGs biomaterials. In addition, the silk-PEGs based biomaterials are cytocompatible, show decreased swelling behavior and longer degradation times, which make them suitable for hemostatic applications where the current available tissue sealant products can be contraindicated.
Owner:TRUSTEES OF TUFTS COLLEGE
Compositions and methods related to 2 dimensional molecular composites
ActiveUS20190352430A1Polycrystalline material growthFrom normal temperature solutionsInorganic compoundOrganic layer
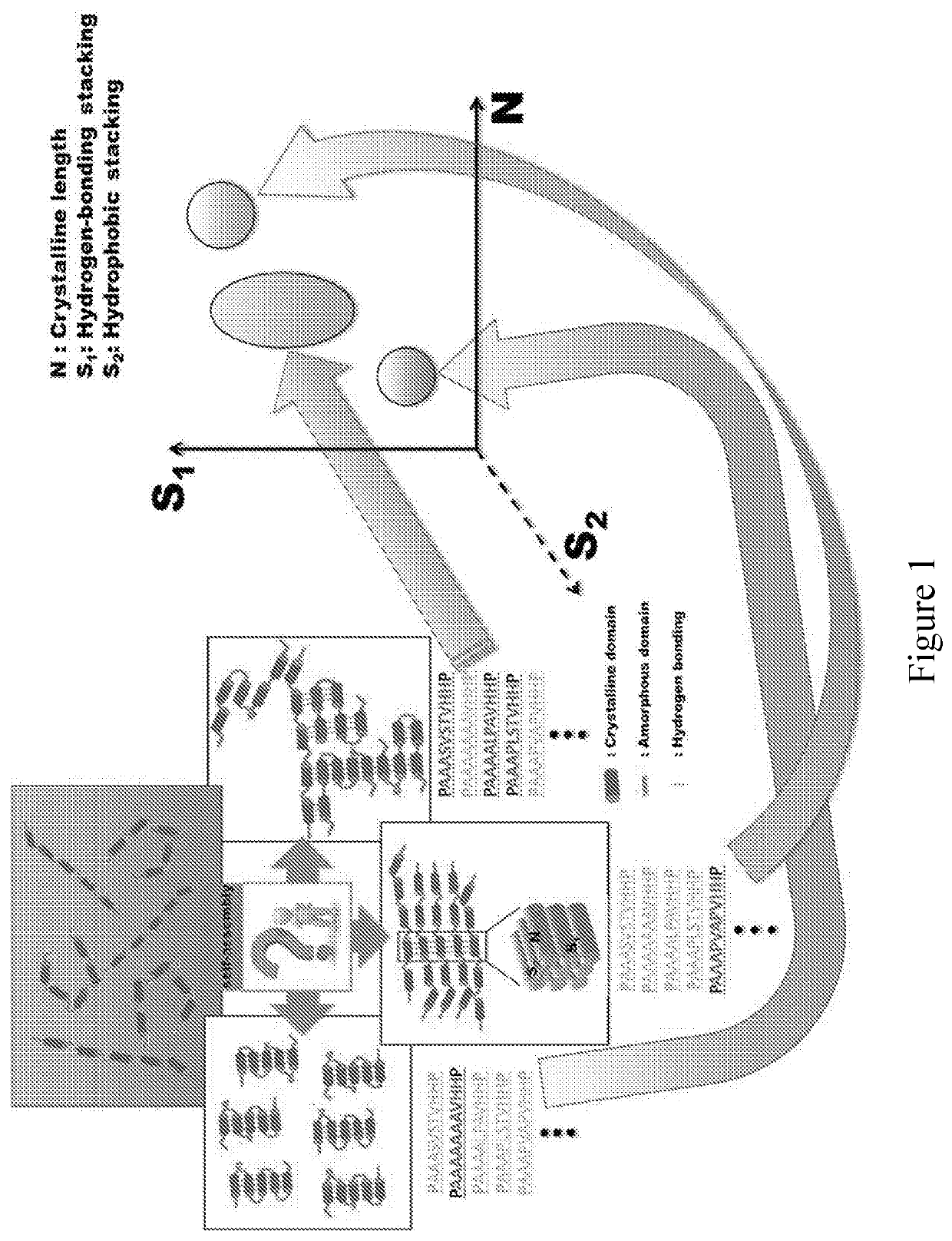
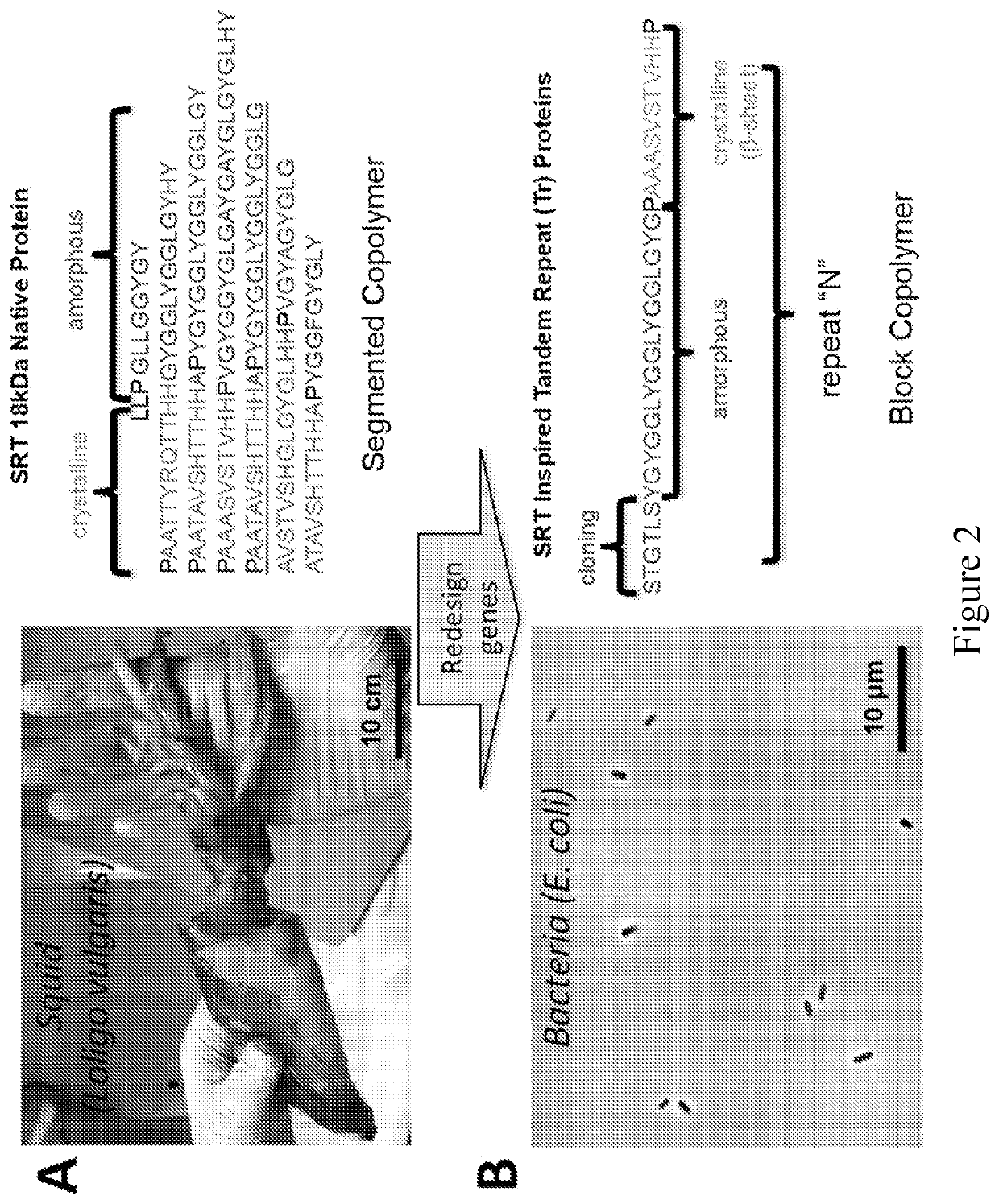
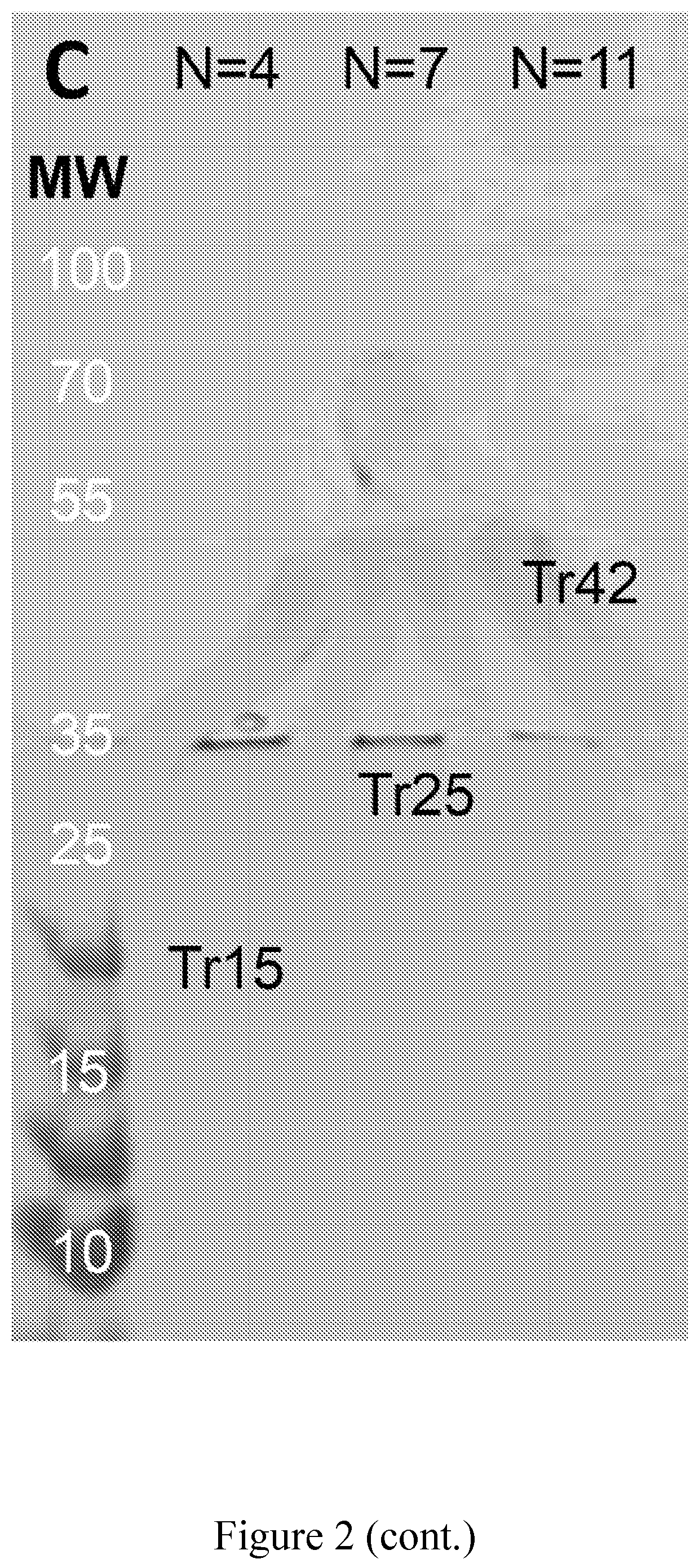
Provided are compositions that include at least one two-dimensional layer of an inorganic compound and at least one layer of an organic compound in the form of one or more polypeptides. Methods of making and using the materials are provided. The organic layer contains one or more polypeptides, each of which have alternating repeats of crystallite-forming subsequences and amorphous subsequences. The crystallite-forming subsequences form crystallites comprising stacks of one or more beta-sheets. The amorphous subsequences form a network of hydrogen bonds. A method includes i) combining one or more polypeptides with an inorganic material and an organic solvent, and ii) depositing one or more polypeptides, the inorganic material and the organic solvent onto a substrate. These steps can be repeated to provide a composite material that is a multilayer composite material. The composite materials can be used in a wide array of textile, electronic, semi-conducting, and other applications.
Owner:PENN STATE RES FOUND
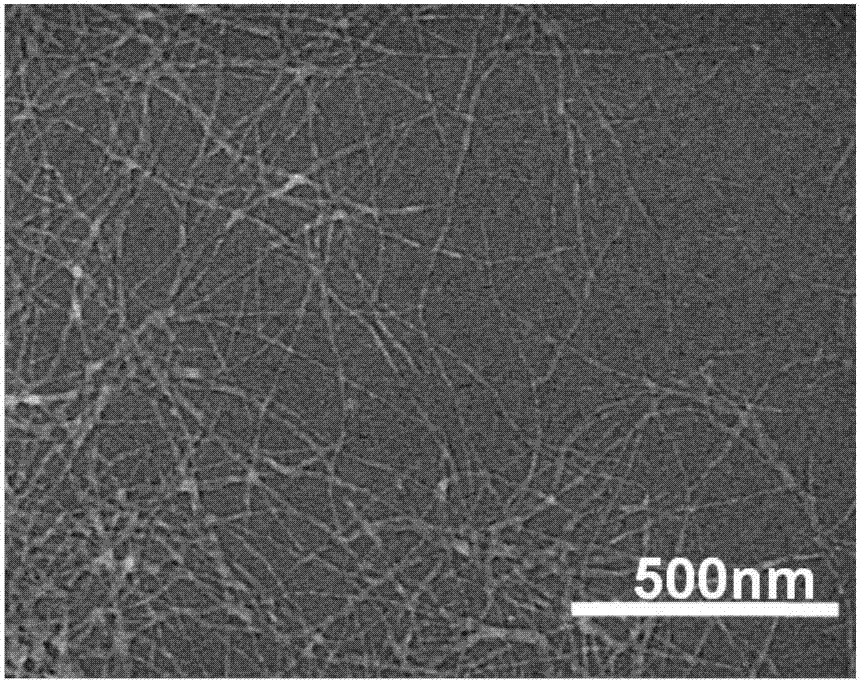
Silk fibroin sponge and preparation method thereof
ActiveCN107080859AReduce contentPharmaceutical delivery mechanismTissue regenerationFreeze-dryingBiocompatibility Testing
The invention discloses silk fibroin sponge. The content of beta-sheet is lower than 30 percent by weight; the silk fibroin sponge is insoluble in water. The invention also provides a preparation method of the silk fibroin sponge, comprising the following steps: A) sealing and cultivating a silk fibroin water solution, and then mixing with a high-crystallized silk fibroin nanofiber solution to obtain a mixed solution; B) freezing the mixed solution, and then freeze-drying to obtain the silk fibroin sponge. A little amount of high-crystallized silk fibroin nanofiber is taken as an inducer and is mixed with the treated silk fibroin water solution, and a silk fibroin porous sponge material insoluble to water is directly prepared from the water solution by freezing and freeze-drying in sequence. According to the method, high-crystallized silk fibroin nanofiber induction, temperature treatment and silk fibroin assembly rate regulation and control in the freezing process are combined, the porous sponge insoluble to the water is obtained without adding any other solvent or treatment process, the technology is simple and environment-friendly, and the biocompatibility is excellent.
Owner:丝纳特(苏州)生物科技有限公司
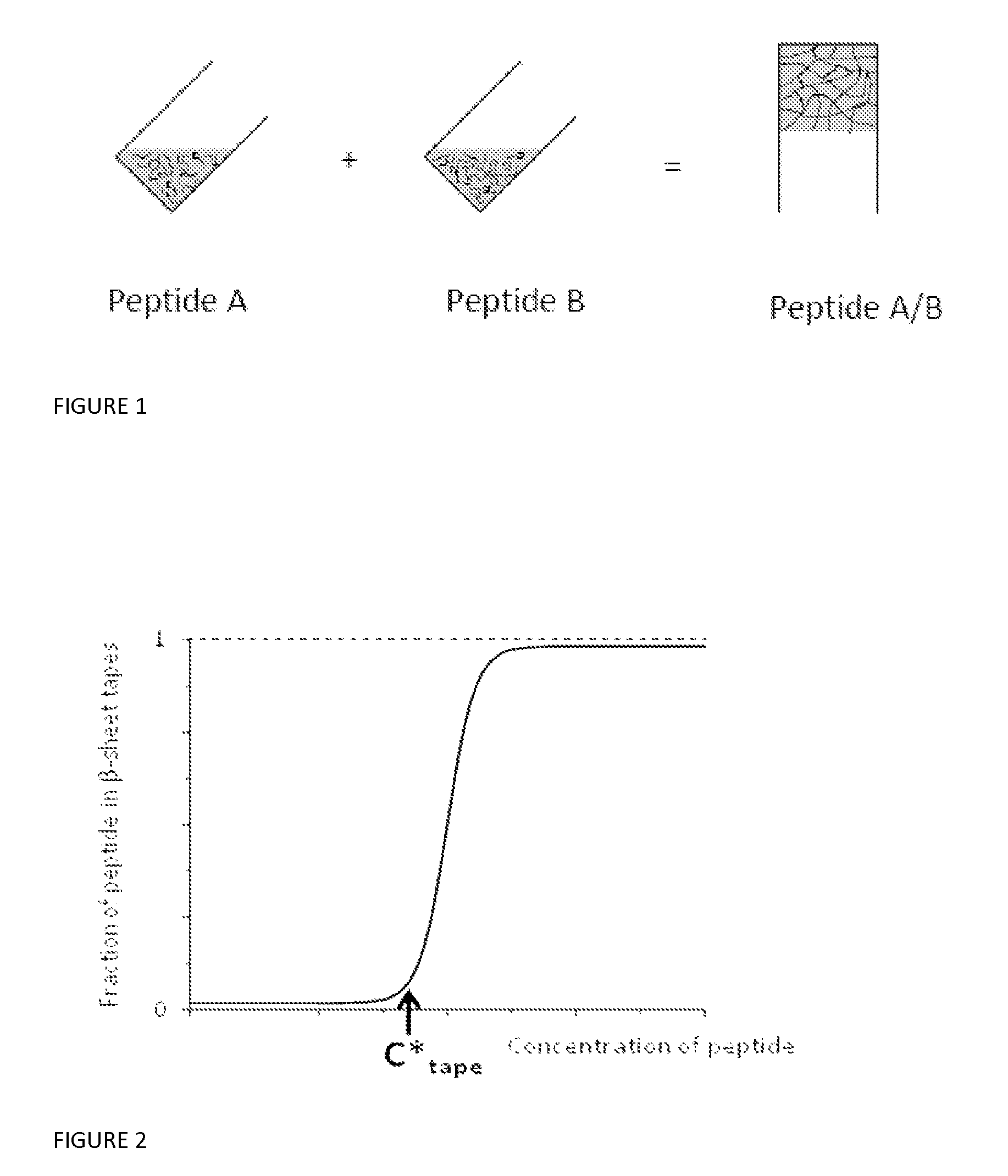
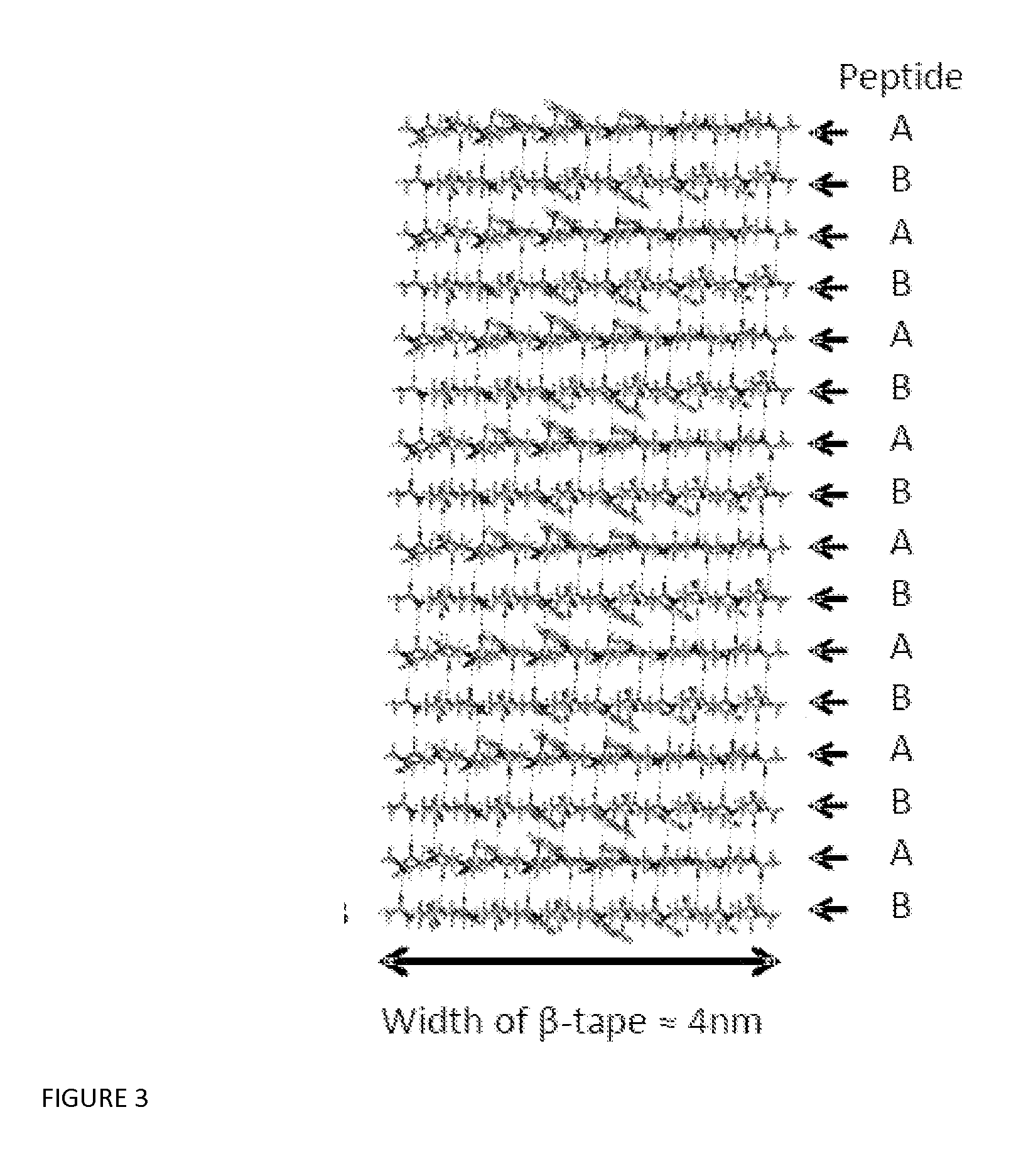
Beta sheet tapes ribbons in tissue engineering
InactiveUS20150299266A1Long lastingEffective presenceCosmetic preparationsImpression capsFiberFibril
There is described a material comprising tapes, ribbons, fibrils or fibres characterized in that each of the ribbons, fibrils or fibres have an antiparallel arrangement of peptides in a β-sheet tape-like substructure wherein the material comprises a pair of self assembling complementary polypeptides.
Owner:CREDENTIS AG
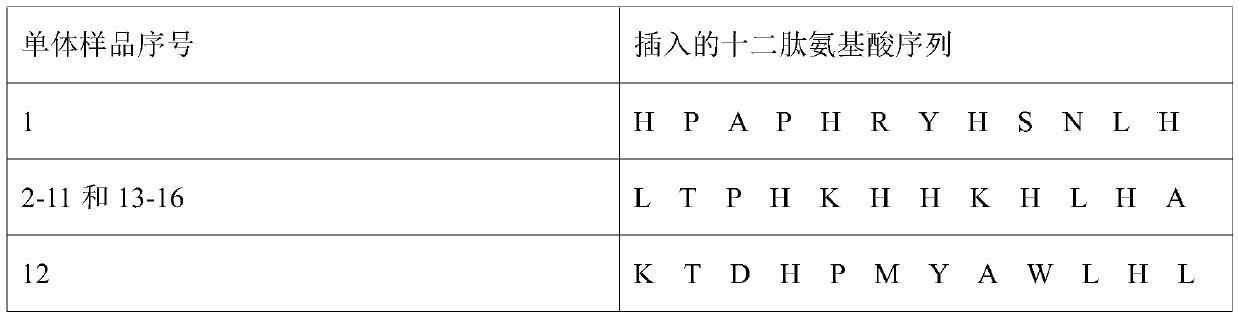
Amylin compatible polypeptide and application thereof
ActiveCN110240632AHigh affinityStrong characteristicMetabolism disorderPeptidesInsulin dependent diabetesFluorescence
The invention provides Amylin compatible polypeptide and an application thereof. The amino acid sequence of the compatible polypeptide is LTPHKHHKHLHA. Firstly the compatible polypeptide sequence of specific binding Amylin is obtained through a phage display library and a biopanning technique, then Amylin and compatible polypeptide are subjected to molecular simulation and constant temperature titer thermal analysis, which indicates that the interaction site of the compatible polypeptide and the Amylin comprises that Amylin aggregation forms hot spot sequence of beta-sheet, and the affinity and the combination specificity of the Amylin and the compatible polypeptide are high. The AFM and ThT fluorescence detection experiment indicates that the compatible polypeptide has obvious restraining effects on the process of forming fibers through Amylin aggregation, and certain theoretical basis and test basis are provided for development of Amylin aggregation inhibitors and drugs relevant with non-insulin dependent diabetes.
Owner:EAST CHINA UNIV OF SCI & TECH
Inhibitors of prion formation
InactiveUS6365359B1Increase infectivityOrganic active ingredientsNervous disorderCellular componentADAMTS Proteins
Molecules are disclosed that interact with the cellular components involved in conversion of PrPC to PrPSc. The molecules disclosed can be small molecules, peptides or protein analogs, e.g. analogs of PrPC. In one embodiment, these molecules interfere with prion formation and / or replication, e.g. by preventing interactions of proteins involved in a prion complex or by interfering with beta-sheet formation. In another embodiment, the molecules of the invention promote PrPC conversion to PrPSc, e.g. by binding to PrPC and facilitating a conformational change from PrPC to PrPSc.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing PDGF acceptor inhibitor through solid-phase synthesis
InactiveCN104513304ASolving the beta sheet problemAvoid situations that reduce reaction efficiencyPeptide preparation methodsAnimals/human peptidesDiseaseAbnormal tissue growth
The invention discloses a method for preparing a PDGF (platelet derived growth factor) acceptor inhibitor through solid-phase synthesis. The polypeptide is composed of 47 amino acids and is used to treatment on tumors, hepatic fibrosis and other diseases. The technical scheme comprises the following steps: taking 2-chlorotrityl chloride resin or wang resin with the substitution degree of 0.2 mmol / g-0.9 mmol / g as an initial raw material, and successively connecting protective amino acids according to the amino acid sequence of the polypeptide from the C terminal by using a solid-phase synthesis process; on the aspect of synthetic strategy, employing a special amino acid to enclose an active site, using a protection group with relatively large steric hindrance to process the side chain of a protective amino acid at a specific position so as to destroy beta-sheet in the peptide chain, and using other special synthetic manners; after synthesis is finished, cutting the synthesized polypeptide from resin by using a cutting fluid, and simultaneously cutting off the side-chain protection group of amino acids, and performing ether precipitation and washing, so as to obtain a high-purity polypeptide crude product. Through HPLC detection, the purity of the target polypeptide reaches 62.5% or more. The related synthetic method has the advantages of stable technology, reliable raw material source, high synthetic efficiency, stable product quality, low synthesis cost and simple operation, and is suitable for large-scale production.
Owner:WUXI MTLH BIOTECH
Functionalized beta-sheet peptide stabilized membrane proteins, constructs comprising same, and methods of forming and using same
InactiveCN107074916ASemi-permeable membranesCell receptors/surface-antigens/surface-determinantsBeta sheetProtein C
Owner:艾伯塔大学校董
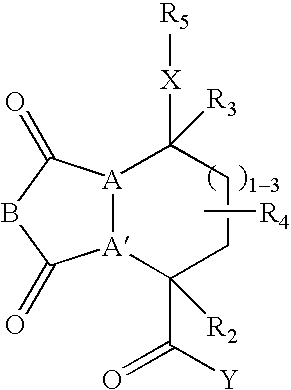
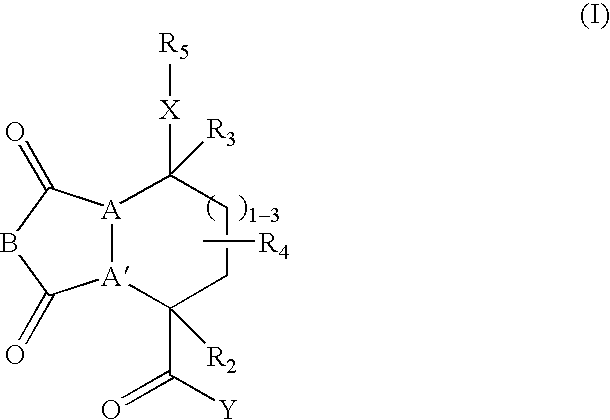
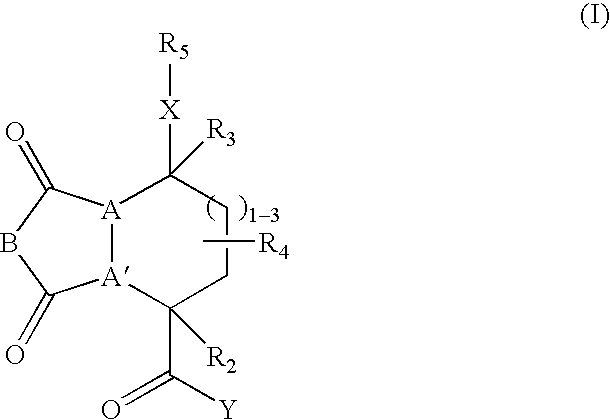
Beta-sheet mimetics and composition and methods relating thereto
Compounds having the following structure: including pharmaceutically acceptable salts and stereoisomers thereof, wherein A, A', B, X, Y, R2, R3, R4 and R5 are as defined herein. Such compounds have utility over a wide range of applications, including use as diagnostic and therapeutic agents. In particular, compounds of this invention, and pharmaceutical compositions containing such compounds, are tryptase antagonists.
Owner:PRISM BIOLAB
Beta Sheet Inhibiting Peptides For Preventing And/Or Treating Alzheimer`s Disease
ActiveUS20110105404A1Inhibition formationPrevention and/or treatment of ADNervous disorderPeptide/protein ingredientsHomologous sequenceMedicine
Provided are β-sheet breaker peptides binding to β amyloid peptide (Aβ1-42), which have the homologous sequence with Aβ 14-23. β-sheet breaker peptides can be used for the manufacture of a medicament for prophylactic and / or therapeutic treatment of Alzheimer's Disease.
Owner:TIANJIN MEDICAL UNIV
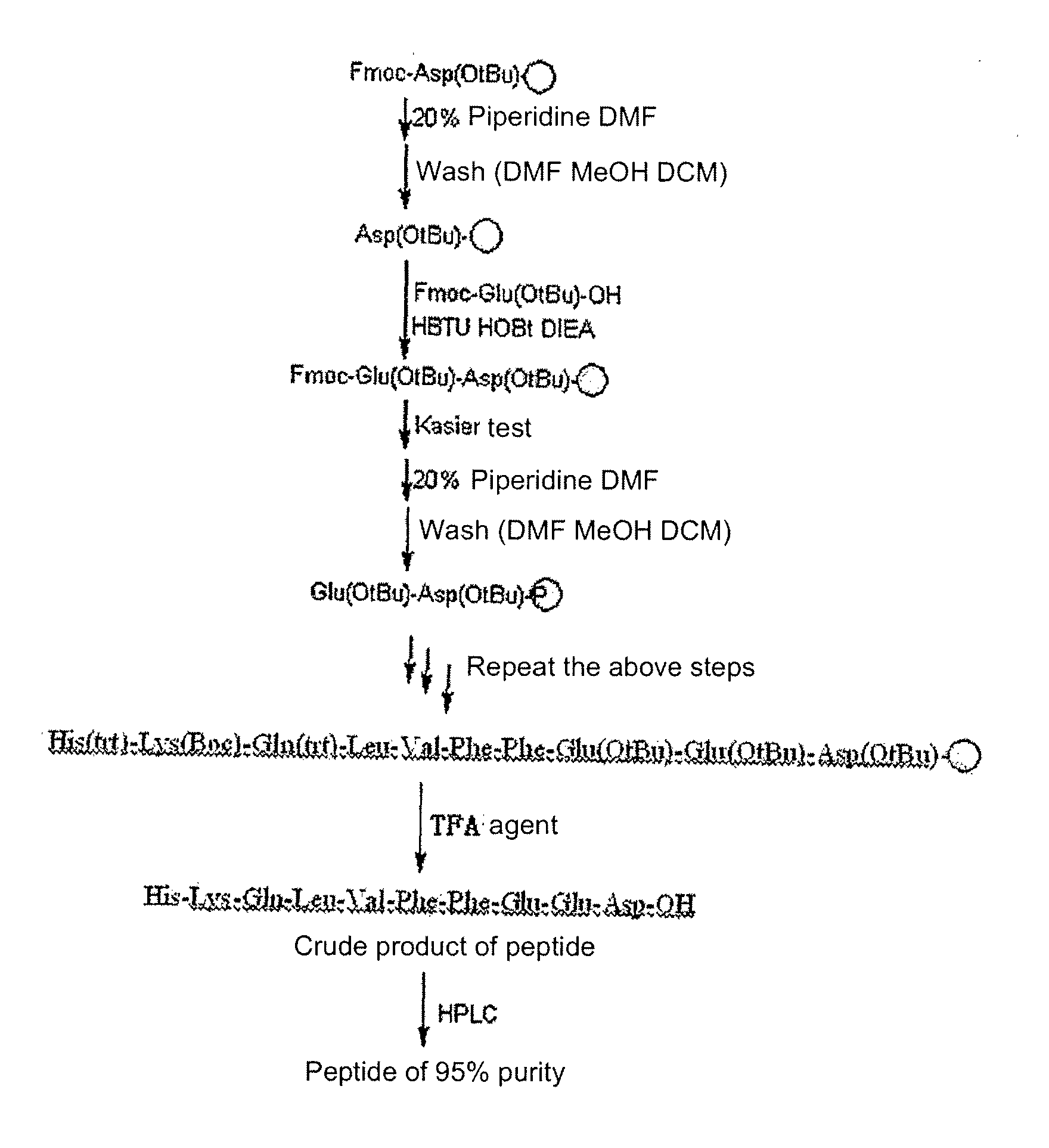
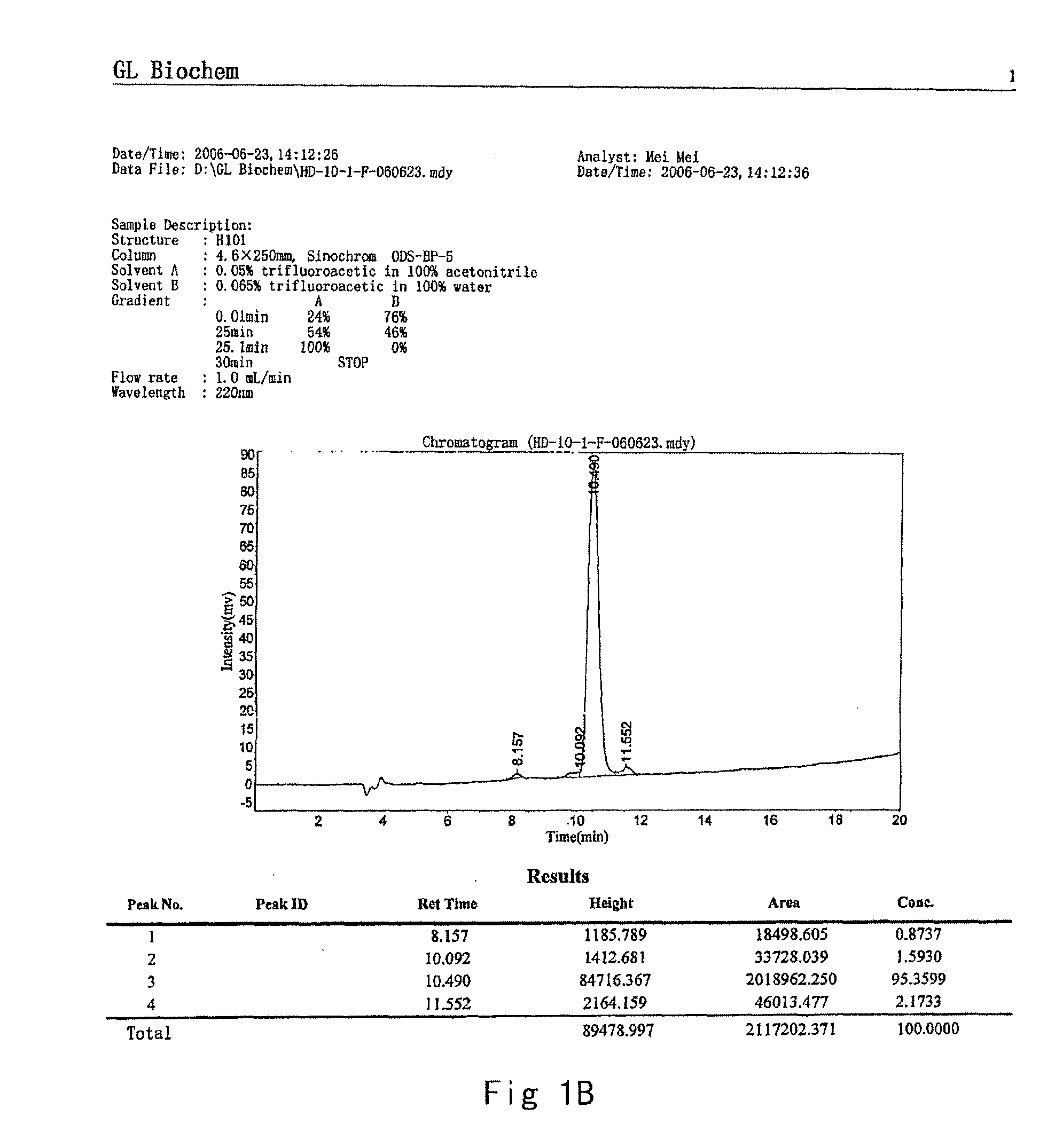
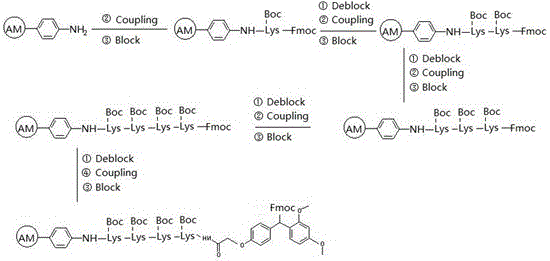
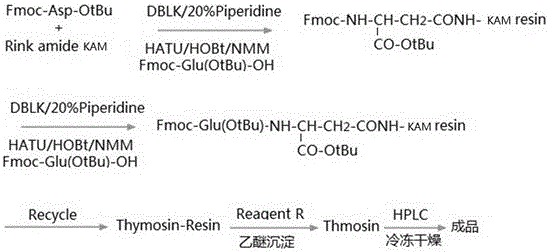
Synthesis process of thymalfasin
InactiveCN106543279AElimination of beta sheetsEasy to operateHormone peptidesPeptide preparation methodsAcetic anhydrideSide chain
The invention provides a synthesis process of thymalfasin and belongs to the field of solid phase polypeptide synthesis. The synthesis process of the thymalfasin is characterized in that firstly, AM resin is transformed, two to six side chain protection hydrophilic amino acid residues are coupled to the AM resin, and Lys, Arg, Glu, and Asp amino acid serve as the hydrophilic amino acid; and then, Rink amide Linker is coupled to the resin, and Fmoc-AAn...AA2-AA1-AM resin is prepared. The linear distance between synthetic polypeptide and the resin is prolonged, and hydrophilia of the resin is increased; the transformed resin is used for the synthesis of the thymalfasin, thymalfasin solid phase synthesis adopts the linear continuous synthesis from C terminal to N terminal; the best synthesis efficiency is obtained by controlling the amino acid excess multiple and the coupling time; after peptide chain synthesis is finished, Fmoc protection is removed; acetic anhydride acetylizes polypeptide N-terminal; decomposition is conducted through a cracking reagent; and the thymalfasin is obtained after diethyl ether precipitating. According to the synthesis process of the thymalfasin, the prepared resin performs efficiently when the thymalfasin synthesis is carried out, and the beta sheet of the thymalfasin is eliminated well in the process of the solid phase synthesis.
Owner:岳阳新华达制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com